Abstract
Background
Stem cell therapy (SCT) has been proposed as an alternative treatment for dilated cardiomyopathy (DCM), nonetheless its effectiveness remains debatable.
Objectives
To assess the effectiveness and safety of SCT in adults with non‐ischaemic DCM.
Search methods
We searched CENTRAL in the Cochrane Library, MEDLINE, and Embase for relevant trials in November 2020. We also searched two clinical trials registers in May 2020.
Selection criteria
Eligible studies were randomized controlled trials (RCT) comparing stem/progenitor cells with no cells in adults with non‐ischaemic DCM. We included co‐interventions such as the administration of stem cell mobilizing agents. Studies were classified and analysed into three categories according to the comparison intervention, which consisted of no intervention/placebo, cell mobilization with cytokines, or a different mode of SCT.
The first two comparisons (no cells in the control group) served to assess the efficacy of SCT while the third (different mode of SCT) served to complement the review with information about safety and other information of potential utility for a better understanding of the effects of SCT.
Data collection and analysis
Two review authors independently screened all references for eligibility, assessed trial quality, and extracted data. We undertook a quantitative evaluation of data using random‐effects meta‐analyses. We evaluated heterogeneity using the I² statistic. We could not explore potential effect modifiers through subgroup analyses as they were deemed uninformative due to the scarce number of trials available. We assessed the certainty of the evidence using the GRADE approach. We created summary of findings tables using GRADEpro GDT. We focused our summary of findings on all‐cause mortality, safety, health‐related quality of life (HRQoL), performance status, and major adverse cardiovascular events.
Main results
We included 13 RCTs involving 762 participants (452 cell therapy and 310 controls). Only one study was at low risk of bias in all domains. There were many shortcomings in the publications that did not allow a precise assessment of the risk of bias in many domains. Due to the nature of the intervention, the main source of potential bias was lack of blinding of participants (performance bias). Frequently, the format of the continuous data available was not ideal for use in the meta‐analysis and forced us to seek strategies for transforming data in a usable format.
We are uncertain whether SCT reduces all‐cause mortality in people with DCM compared to no intervention/placebo (mean follow‐up 12 months) (risk ratio (RR) 0.84, 95% confidence interval (CI) 0.54 to 1.31; I² = 0%; studies = 7, participants = 361; very low‐certainty evidence). We are uncertain whether SCT increases the risk of procedural complications associated with cells injection in people with DCM (data could not be pooled; studies = 7; participants = 361; very low‐certainty evidence). We are uncertain whether SCT improves HRQoL (standardized mean difference (SMD) 0.62, 95% CI 0.01 to 1.23; I² = 72%; studies = 5, participants = 272; very low‐certainty evidence) and functional capacity (6‐minute walk test) (mean difference (MD) 70.12 m, 95% CI –5.28 to 145.51; I² = 87%; studies = 5, participants = 230; very low‐certainty evidence). SCT may result in a slight functional class (New York Heart Association) improvement (data could not be pooled; studies = 6, participants = 398; low‐certainty evidence). None of the included studies reported major adverse cardiovascular events as defined in our protocol. SCT may not increase the risk of ventricular arrhythmia (data could not be pooled; studies = 8, participants = 504; low‐certainty evidence).
When comparing SCT to cell mobilization with granulocyte‐colony stimulating factor (G‐CSF), we are uncertain whether SCT reduces all‐cause mortality (RR 0.46, 95% CI 0.16 to 1.31; I² = 39%; studies = 3, participants = 195; very low‐certainty evidence). We are uncertain whether SCT increases the risk of procedural complications associated with cells injection (studies = 1, participants = 60; very low‐certainty evidence). SCT may not improve HRQoL (MD 4.61 points, 95% CI –5.62 to 14.83; studies = 1, participants = 22; low‐certainty evidence). SCT may improve functional capacity (6‐minute walk test) (MD 140.14 m, 95% CI 119.51 to 160.77; I² = 0%; studies = 2, participants = 155; low‐certainty evidence). None of the included studies reported MACE as defined in our protocol or ventricular arrhythmia.
The most commonly reported outcomes across studies were based on physiological measures of cardiac function where there were some beneficial effects suggesting potential benefits of SCT in people with non‐ischaemic DCM. However, it is unclear if this intermediate effects translates into clinical benefits for these patients.
With regard to specific aspects related to the modality of cell therapy and its delivery, uncertainties remain as subgroup analyses could not be performed as planned, making it necessary to wait for the publication of several studies that are currently in progress before any firm conclusion can be reached.
Authors' conclusions
We are uncertain whether SCT in people with DCM reduces the risk of all‐cause mortality and procedural complications, improves HRQoL, and performance status (exercise capacity). SCT may improve functional class (NYHA), compared to usual care (no cells).
Similarly, when compared to G‐CSF, we are also uncertain whether SCT in people with DCM reduces the risk of all‐cause mortality although some studies within this comparison observed a favourable effect that should be interpreted with caution. SCT may not improve HRQoL but may improve to some extent performance status (exercise capacity). Very low‐quality evidence reflects uncertainty regarding procedural complications. These suggested beneficial effects of SCT, although uncertain due to the very low certainty of the evidence, are accompanied by favourable effects on some physiological measures of cardiac function.
Presently, the most effective mode of administration of SCT and the population that could benefit the most is unclear. Therefore, it seems reasonable that use of SCT in people with DCM is limited to clinical research settings. Results of ongoing studies are likely to modify these conclusions.
Plain language summary
Bone marrow cells in non‐ischaemic dilated cardiomyopathy
Review question
Are bone marrow cells safe and effective as a treatment for non‐ischaemic dilated cardiomyopathy (DCM)?
Background
DCM is a disorder of the heart muscle with heart dilation (heart muscle becomes stretched) and impaired contraction, in the absence of high blood pressure, damaged or diseased heart valves, or heart disease present at birth or related to myocardial infarction (heart attack). The current standard of treatment is based on medicines and cardiac devices. However, DCM is still one of the leading causes of heart transplantation in adults.
Stem cells are special cells produced in the bone marrow that are able to develop into many different cell types. Giving stem cells directly into the heart muscle has been proposed as an alternative treatment to reduce or stop further deterioration in heart function in people with DCM.
Study characteristics
We selected randomized controlled trials (RCTs; clinical studies where people are randomly put into one of two or more treatment groups) comparing the infusion of bone marrow‐derived stem cells into the heart muscle with the usual‐care (control) treatment in people diagnosed with DCM. We searched multiple databases for trials up to 10 November 2020.
We included 13 RCTs involving 762 participants (452 receiving stem cell therapy and 310 controls). The trials included people with severe symptoms of ischaemic (following a heart attack) and non‐ischaemic DCM. We selected only the data from non‐ischaemic DCM.
The studies included an average of 60 people aged about 45 to 58.5 years and 50% to 89% men in each trial. Following therapy, the participants were assessed for six months to five years, with most at one year. One study declared a private funding whereas seven others had public or governmental funding, two had non‐profit funding, and four did not report this information.
Key results
SCT versus control: very low‐quality evidence reflects uncertainty regarding mortality, procedural complications, health‐related quality of life and exercise capacity. Low‐quality evidence suggests that SCT may slightly improve deterioration of heart function and may not increase the risk of abnormal heartbeats in people with DCM. No studies reported other relevant outcomes such as major cardiac adverse events.
STC plus cytokine versus control: very low‐quality evidence reflects uncertainty regarding mortality. Low‐quality evidence suggests that SCT plus cytokine may not improve health‐related quality of life but may improve exercise capacity as well as some physiological measures related to cardiac function (although it is unclear to what extent these latter outcomes are associated with relevant clinical benefits for patients). Hence, the results should be interpreted with caution. Very low‐quality evidence reflects uncertainty regarding procedural complications. No studies reported major cardiac adverse events or abnormal heartbeats.
Due to the limited number of studies we could not perform analyses to identify which specific features of SCT and clinical characteristics of patients are associated with better results. Thus, more research is needed to establish the role of SCT in the treatment of DCM and the most effective therapies.
Quality of evidence
The evidence in this review is of low to very low quality due to the small number of events, results not similar across studies, risk of bias, and issues with study design. Furthermore, the limitations in the reporting of most studies made it difficult to obtain and use the information to reach clearer conclusions.
Summary of findings
Background
Description of the condition
Dilated cardiomyopathy (DCM), also known as non‐ischaemic DCM, is a heart muscle disorder defined by the presence of left ventricular or biventricular systolic dysfunction and dilation in the absence of hypertension, valvular, congenital, or ischaemic heart disease (Bozkurt 2016; Pinto 2016). DCM is the most common form of non‐ischaemic cardiomyopathy worldwide (Jefferies 2010; McKenna 2017), and represents one of the leading causes for heart transplantation in adults (Merlo 2016; Stehlik 2011). DCM was first described by the World Health Organization (WHO) in 1980 (WHO/ISFC 1980), and its prevalence is estimated at 1 in 2500 people (Hershberger 2013). Most people with DCM present with symptoms of heart failure, including dyspnoea and fatigue on exertion, orthopnoea, ankle oedema, and excessive sweating (Dec 1994; Weintraub 2017). Survival in people with DCM is extremely poor after the diagnosis, and early studies have shown that most deaths occur within the first two years of follow‐up (Díaz 1987; Fuster 1981). Optimal medical therapy as a first‐line treatment, either with or without device therapy (Ponikowski 2016; Yancy 2013), has progressively and significantly improved the long‐term prognosis of DCM since the early 1990s (Merlo 2014). However, although a proportion of patients recover cardiac function, in the long term there is a trend towards worsening of left ventricular function (Merlo 2015). Cardiac transplantation is reserved for extremely ill patients and for those needing continuous intravenous inotrope support, mechanical ventilatory support or ventricular assist device support (Jefferies 2010). The use of stem cell therapy (SCT) may be an alternative treatment to reduce or stop further deterioration of left ventricular function in people with end‐stage DCM. However, two systematic reviews have shown some benefits in terms of systolic function and mortality but not in exercise tolerance (Lu 2016; Marquis‐Gravel 2014).
Description of the intervention
Stem cells are types of cells with special characteristics, such as proliferation, self‐renewal, regeneration, and the possibility of generating different lineages of differentiated progeny (Blau 2001). These features have prompted the development of SCT. The objective of SCT in the treatment of DCM is to achieve cardiac muscle regeneration and recovery of functional capacity, either by replacing the dead myocardium or by activating physiological repair mechanisms (Menasché 2018). The first description of cell transplantation into the human myocardium was a case report published in 2001 using skeletal myoblasts (Menasché 2001). Since then, cell‐based therapies have been used in different trials for treating ischaemic and non‐ischaemic heart failure (Fisher 2016a; Menasché 2018; Poglagen 2018; Vrtovec 2018a).
To date, several stem cell types, autologous and allogeneic, have been considered for the treatment of people with chronic heart failure secondary to ischaemic cardiomyopathy and DCM. These include skeletal myoblasts, haematopoietic stem cells, mesenchymal stem cells, cardiac stem cells, and cardiosphere‐derived cells (Menasché 2018). Haematopoietic stem cells may be collected from peripheral venous blood after a mobilization procedure involving injection of a growth‐stimulating factor (usually granulocyte colony‐stimulating factor, G‐CSF) over the previous days to increase the number of progenitor cells in the blood and to later culture these cells ex vivo. Bone marrow‐derived stem cells may also be isolated directly from bone marrow aspiration, a procedure in which a small sample of liquid bone is aspirated with a syringe under local anaesthesia, usually from the ilium of people receiving cell therapy (Strauer 2002). Afterwards, bone marrow mononuclear cells (BMMC) are separated from other bone marrow cells (BMC) by density gradient centrifugation (Assmus 2002; Erbs 2005). Stem cells are then administered to the patient using different delivery methods. The cells can be delivered through coronary arteries (Choudry 2016), coronary sinus (Patel 2015), or peripheral veins (Hare 2009). Alternatively, direct intramyocardial injection can be performed using a surgical approach (Stamm 2003), or transendocardial (Psaltis 2010).
How the intervention might work
Some authors have suggested favourable effects of SCT for non‐ischaemic DCM, such as improvement in ventricular function, functional capacity, and quality of life (Frljak 2018; Poglagen 2018). Although the mechanism of action of SCT is not completely understood, two main mechanisms may promote cardiac repair. The first is that transplanted cells are engrafted into the damaged myocardium, where they generate new myocardial tissue to replace the tissue that has been irreversibly lost. The second is that SCT acts by activating endogenous repair mechanisms (Menasché 2018). This paracrine mechanism may produce stimulatory cytokines that increase vascularity, promote cardiomyocyte proliferation, limit or reduce fibrosis, or activate endogenous resident stem cells (Behfar 2014). SCT may also modulate the immune system, improve endothelial function, and reverse ventricular remodelling (Hare 2017).
Why it is important to do this review
Both the European Society of Cardiology (ESC) in 2016 (Ponikowski 2016), and the American Heart Association (AHA) in 2013 (Yancy 2013), refer to this therapy as an evolving therapy, and more data are needed to establish a recommendation. In the last review published by the AHA regarding diagnosis and treatment of DCM, cell therapy is not supported for general management (Bozkurt 2016).
Despite several clinical trials since the early 2010s, controversy remains regarding the role of SCT in DCM. For instance, SCT has not been included in major clinical practice guidelines (Bozkurt 2016; Ponikowski 2016).
Systematic reviews of trials published before 2015 reported that, compared with conventional therapy, BMMC therapy had a moderate effect on left ventricular ejection fraction (LVEF) and left ventricular end‐systolic volume (LVESV) in non‐ischaemic DCM (Wen 2018). An earlier review concluded the bone marrow‐derived SCT may have some effect on mortality, a mild‐to‐moderate effect on LVEF increase within six months, but no improvement in functional capacity (Lu 2016).
Since then, additional trials have been conducted using cell‐based therapies for treating non‐ischaemic heart failure (Butler 2017; Chen 2008; Frljak 2018; Hare 2017; Vrtovec 2011; Vrtovec 2013; Vrtovec 2018b; Xiao 2017). The results of these trials provide a rationale for proposing this Cochrane Review to ascertain whether this intervention provides clinical benefits in people with DCM.
Objectives
To assess the efficacy and safety of SCT in adults with non‐ischaemic DCM.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐arm individually randomized controlled trials (RCTs). Due to the specific nature of this intervention, we deemed cluster‐RCTs not feasible or cross‐over RCTs not appropriate designs to assess mid‐ or long‐term clinical effects of SCT.
We did not restrict the study selection by publication status. We applied no restrictions on language of publication.
Types of participants
We included trials that evaluated adults aged 18 years or older with a diagnosis of non‐ischaemic DCM (as defined by the trial authors).
We also included trials that evaluated both ischaemic and non‐ischaemic disease when specific data for the participants with non‐ischaemic DCM was available and could be extracted.
Types of interventions
We included trials that compared:
any type or delivery modality of SCT versus no intervention, sham intervention, or placebo (comparison 1);
SCT versus therapy with G‐CSF or any other cytokine that stimulates the proliferation and differentiation of precursor cells in the bone marrow (but not comprising SCT) (comparison 2);
different types or delivery modalities of SCT against each other (comparison 3).
SCT in the context of this review may have consisted of a variety of modalities according to cell origin (autologous or heterologous), cell collection location (bone marrow‐derived cells or peripheral blood cells), type of cells infused (bone marrow‐derived mesenchymal stromal cells, mononuclear cells, myeloid cells, lymphoid cells, or mixed cells), delivery route (intracoronary, intramyocardial, or transendocardial), number of cell infusions (single or repeated infusions), volume of cells infused (high or low), and use of G‐CSF or cytokines for mobilization of stem cells. Although we had planned to take these variations into consideration by conducting subgroup analysis and investigation of heterogeneity, this was not done for the reasons noted below.
We accepted any type of co‐intervention (guideline‐recommended pharmacological and device therapy or G‐CSF) when such co‐intervention was provided similarly to the experimental and control groups.
Types of outcome measures
For trials that reported outcomes at several follow‐up points, we used the latest available time point for analysis of each outcome.
We had planned that if a published trial did not report any one of these outcomes, we would contact the trial authors to ascertain whether the outcomes were measured but not reported. This situation did not occur. All included trials measured and reported at least one of the below outcomes. For those outcomes that were not reported in a usable format, we presented the results in a narrative form.
When the information available in the article (main and secondary papers related to the same trial) was not detailed enough, whenever possible we used the information obtained in the ClinicalTrials.gov registry. Additionally, we contacted all study contact authors to complete information that was not available in other public sources.
Primary outcomes
All‐cause mortality.
Safety, as indicated by periprocedural complications occurring at the time of bone marrow aspiration or administration of SCT or control.
Safety, as documented adverse events (AE) (including tumorigenesis) within 30 days of treatment.
Secondary outcomes
Health‐related quality of life, as measured using a validated tool (e.g. Kansas City Cardiomyopathy Questionnaire (KCCQ), Minnesota Living with Heart Failure Questionnaire (MLHFQ), and EuroQol‐5D Questionnaire (EQ‐5D).
Performance status – functional class (New York Heart Association).
Performance status – exercise tolerance (6‐minute walk test).
Rehospitalizations.
Heart failure.
Ventricular arrhythmia.
Complete atrioventricular block.
Major adverse cardiovascular events (defined as non‐fatal stroke, non‐fatal myocardial infarction, or cardiovascular death).
Change in left ventricular ejection fraction (LVEF).
Change in left ventricular end‐systolic volume (LVESV).
Change in left ventricular end‐diastolic volume (LVEDV).
Change in plasma natriuretic peptide levels (brain natriuretic peptide [BNP] and N‐terminal pro b‐type natriuretic peptide [NT‐proBNP]).
Although the scope of this review was to assess the clinical effects of SCT in people with DCM, we retained the outcome of LVEF because it is a widely reported surrogate for cardiac function. We also included the surrogate outcomes of LVESV and LVEDV because we consider these to be more meaningful than LVEF in this context.
We have used one quality of life measure per study. For studies reporting two or more quality of life measures, we prioritized the measure with a specific instrument in people with cardiomyopathy over a generic one.
Search methods for identification of studies
Electronic searches
We performed systematic electronic searches of the following bibliographic databases on 10 November 2020:
Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library (2020, Issue 11);
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 9 November 2020);
Embase (Ovid, 1980 to 2020 week 45).
We also conducted a search in ClinicalTrials.gov (www.ClinicalTrials.gov), and the WHO International Clinical Trials Registry Platform (ICTRP) Search Portal (apps.who.int/trialsearch), for ongoing or unpublished trials in May 2020. For the search in both registries, the keywords were 'cell therapy' and 'dilated cardiomyopathy.'
We searched all databases from their inception to the present, with no restriction on language of publication or publication status. We found no retraction statements or errata for included studies.
We adapted the preliminary search strategy for MEDLINE for use in the other databases (Appendix 1). We applied the Cochrane sensitivity‐maximizing RCT filter to MEDLINE and adapted it to Embase, but not CENTRAL (Lefebvre 2011).
We did not perform a separate search for adverse effects of interventions used for the treatment of DCM. We considered adverse effects described in included studies only.
Searching other resources
We also:
searched the reference lists of all identified eligible papers and relevant systematic or narrative (or both) reviews as a complementary source for study identification and for validating our electronic search strategy;
searched in Epistemonikos in order to identify systematic reviews on the topic (www.epistemonikos.org), as well as all primary studies included in them by using the tool 'matrix of evidence;'
conducted a cross‐citation search in Google Scholar, using each included study as the index reference;
contacted all authors of the included studies to request additional information.
Data collection and analysis
Selection of studies
Three review authors (DP, FV, and GU) working in pairs independently screened the search results based on the title and abstract. At this stage, we coded decisions as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve.' We retrieved full‐text copied of the references deemed eligible (those coded as 'retrieve'), and the same authors independently confirmed eligibility based on the inclusion criteria. We resolved disagreements at either phase by consensus or by discussion with two other review authors (RD and EM). We coded reasons for exclusion of the ineligible studies.
For data management, we used Covidence as bibliographic management software to administer the results obtained from the search.
We collated multiple reports of the same study so that each trial, rather than each report, was the unit of interest in the review.
The search results as well as the decision made during the eligibility process are displayed in a PRISMA flow diagram (Liberati 2009; Figure 5).
Data extraction and management
We used a standardized data collection form to extract data from each study in sufficient detail to design a comprehensive characteristics of studies table, risk of bias table, and to obtain the outcome data for the meta‐analysis. We piloted the data collection form before we agreed the final version of it to be used in the review.
Three review authors (FV, DP, or GU) working in pairs independently extracted the data of each included study. In addition, a fourth author (RAD) checked all outcome data as well as risk of bias items of all studies. We resolved disagreements by consensus after discussion with participation of all members of the review team.
We extracted the following study characteristics.
Identification of the study and bibliographic references of all reports linked to the same study, as well as other secondary sources of relevant data (e.g. online supplements or trial registers).
Eligibility criteria, as stated in the included studies.
Participants: demographic (age, sex, and ethnicity), and relevant clinical data at baseline (those referred to severity of the disease and cardiac function, time from diagnosis to randomization, body mass index, smoking status, other relevant comorbidities, family history of DCM, and previous medical and device therapy). Also, the number of people randomized, the number who dropped out, and the number analyzed for each outcome.
Intervention (SCT): detailed description of SCT (including cells origin (autologous or heterologous), cell collection location (bone marrow‐derived cells or peripheral blood cells), type of cells infused (mesenchymal stromal cells, mononuclear cells, myeloid cells, lymphoid cells, or mixed), mobilization of stem cells with cytokines (yes or no), delivery route (intracoronary, transendocardial, or intramyocardial), volume of cells administered, and number of cell infusions (single or repeated)).
Control group: detailed description of the control group, and the corresponding category of the comparison of interest (comparison 1: no intervention, sham or placebo; comparison 2: treatment with cytokines (e.g. G‐CSF); and comparison 3: SCT).
Outcomes: primary and secondary outcomes planned, measured, and reported, specifying the instrument of measure used and time points reported. Also, we collected the outcomes reported in other secondary sources (e.g. clinical trials) to assess the risk of selective reporting bias.
Methods: study design, total duration of study, study setting and country, number of centres and location, period of study.
Risk of bias assessment: details on method of treatment allocation and concealment, blinding of the intervention or the outcome assessor (or both), and dropouts (number, distribution, and reasons) and study population of analysis.
Data on all relevant results reported (crude number of events or rates, mean values or mean change from baseline and the corresponding standard deviation (SD), and population analysed in each study arm).
Funding and other conflicts of interest.
We emailed all study contact authors requesting them to provide us with further details, as there were insufficiencies in all studies related to the outcome data or design features (or both).
One review author (EM) transferred data into Review Manager 5 (Review Manager 2014). Other members of the team (DP, FV, RAD, and GU) double‐checked data against the data extraction form and articles.
Assessment of risk of bias in included studies
Risk of bias in individual studies
Four review authors (FV, DP, GU and RAD) working in pairs independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). They resolved disagreements by discussion involving another two review authors (RD and EM).
For this purpose, we explored the six specific domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other potential sources of bias.
For each trial, we first described the design characteristics relating to each domain and then judged the risk of bias associated with the main outcome. We used a nominal scale for the judgement: 'low,' 'high,' or 'unclear' risk of bias according to the criteria described in additional Table 4.
2. The Cochrane tool for assessing risk of bias.
| Domain | Description |
| Random sequence generation |
|
| Allocation concealment |
|
| Blinding of participants and personnel |
|
| Blinding of outcome assessment |
|
| Incomplete outcome data |
|
| Selective outcome reporting |
|
| Other risks of bias |
|
Table adapted from Table 8.5.d: Criteria for judging risk of bias in the risk of bias assessment tool, in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017).
We contacted the principal investigator of all included studies to obtain or clarify key study features for a thorough risk of bias assessment. To June 2021, we obtained one response from Martino 2015 (MiHEART).
Overall risk of bias
Low risk of bias: we classified the outcome result at overall 'low risk of bias' only if all the bias domains were judged at low risk of bias. For objective outcomes (e.g. mortality), we considered whether blinding was of relevance, and still categorized this at overall low risk of bias if a lack of blinding was unlikely to introduce bias.
High risk of bias: we classified the outcome result at 'high risk of bias' if any bias domains (described above) were judged at 'unclear' or 'high risk of bias.'
We generated a risk of bias table specifying these judgements, and provided a detailed justification for each judgement so that it was transparent and reproducible. Where information on risk of bias related to unpublished data or correspondence with a trialist, we specified this in the risk of bias table.
When considering treatment effects across studies, we considered the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol (Diaz‐Navarro 2019), and reported deviations from it in the Differences between protocol and review section.
Measures of treatment effect
We expressed dichotomous data for each arm in a particular study as a proportion or risk and the treatment effect as a risk ratio (RR) with 95% confidence intervals (CIs), calculated using Mantel‐Haenszel methods.
We expressed continuous data for each arm in a particular study as a mean and SD, and the treatment effect as the mean difference (MD) if outcomes were measured in the same way across trials. We preferred the mean change difference over the difference in the final means if available. For studies that only reported baseline and endpoint data, when possible, we calculated the SD of the mean change from baseline based on reported CIs or P values, and used these values in the analysis. We presented studies with insufficient information to calculate the SD (e.g. studies that only report endpoint mean values) in combined analyses (assuming the differences in mean final values will on average be the same as the differences in mean change scores), as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
For studies that measured continuous outcomes using different tools, for example for health‐related quality of life, we expressed the treatment effect as the standardized mean difference (SMD). We have adopted the following arbitrary subjective terminology for the interpretation of SMD, based on seminal suggestions by Cohen 1988: if effect size is about 0.8 then 'large,' if effect size about 0.5 then 'medium,' and if effect size about 0.2 then 'small.'
Some studies presented results for continuous outcomes (especially LVEF, LVESV, and LVEDV) only in figures or graphs. To retrieve the raw data for use in the meta‐analyses, we used a specialized software (GetData Graph Digitizer 2.26).
Unit of analysis issues
We only included parallel‐group individually randomized RCTs.
Where multiple trial intervention groups were reported in a single trial, we included only the relevant groups. That was the case for Hamshere 2015, a four‐arm trial that contributed both to comparison 1 and 2 (see details of the arms used in each comparison in the Characteristics of included studies table). In Xiao 2017, we merged the two SCT arms for the comparison of SCT versus control for all‐cause mortality to avoid double‐counting of control group participants (Deeks 2017). Instead, for LVEF outcome, we used data from one of these arms as the study provided only specific data for each group (mean and SD). However, we also alternatively calculated the pooled effect using the other arm to ensure that the result did not change.
Dealing with missing data
We contacted the principal investigators of all studies to request mean change difference and SD for several relevant outcomes where these data were not available or could not be calculated. To June 2021, we had not received any response.
We used Review Manager 5 to calculate missing SDs using other data from the trial (Review Manager 2014), such as CIs or standard error (Henry 2014).
There were no major issues with missing data in the review. Therefore, we did not perform a sensitivity analysis as originally planned neither did we make any imputations on these data in our primary analysis.
Assessment of heterogeneity
We inspected forest plots visually to consider the direction and magnitude of effects and the degree of overlap between CIs. Second, we considered the P value from the Chi² test (threshold P < 0.10) to address the presence of statistical heterogeneity. We also used the I² statistic to quantify statistical heterogeneity not attributable to chance among the trials in each analysis but acknowledge that there is substantial uncertainty in the value of the I² statistic when there is a small number of studies. We followed the recommendations for thresholds in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017):
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100%: may represent considerable heterogeneity.
No meta‐analysis was avoided by this reason (Deeks 2017). In fact, there was considerable heterogeneity in most outcomes analyzed, which was considered when rating the quality of the available evidence of the effects of SCT.
Contrary to what was initially planned, we could not explore possible causes of considerable heterogeneity by prespecified subgroup analysis.
Assessment of reporting biases
As we were unable to pool more than 10 trials in the meta‐analyses, we did not use a funnel plot to explore possible small‐study biases for the primary outcomes, neither did we carried out the Harbord test to test asymmetry for dichotomous outcomes (Harbord 2006), or regression asymmetry test for continuous outcomes (Egger 1997).
Data synthesis
Whenever possible, we undertook meta‐analyses according to the recommendations stated in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2017). We used the Cochrane statistical software Review Manager 5 to analyse data (Review Manager 2014).
We assessed the intervention effects using random‐effects meta‐analyses due to the high heterogeneity we found in most outcomes (DerSimonian 1986). Even for those outcomes where we did not detect heterogeneity, we used this approach because it was more conservative.
Subgroup analysis and investigation of heterogeneity
Contrary to what we had initially planned, subgroup analyses were not carried out as they were considered uninformative. On the one hand, the absence of a global effect in almost all the outcomes analysed and, on the other, the small number of available studies (fewer than three) in some category of all the subgroup analyses, prevented this type of analysis.
Sensitivity analysis
We only carried out sensitivity analyses for an exploratory purpose to assess the influence of a particular study that showed very inconsistent results (outliers) with the rest in the combined analysis, and the degree of heterogeneity caused by it. These analyses are not shown in the review.
Summary of findings and assessment of the certainty of the evidence
We created a summary of findings table for comparison 1 (SCT versus control) and comparison 2 (SCT plus G‐CSF versus G‐CSF), using the following outcomes included in the review: all‐cause mortality; safety, as indicated by periprocedural complications; health‐related quality of life; performance status (exercise tolerance); ventricular arrhythmias; and MACE. For each outcome, we presented data at the longest follow‐up that was available for each study.
We have not created a summary of findings table for comparison 3 (different types or delivery modalities of SCT against each other) as this comparison included four RCTs that compared different aspects related to SCT delivery that could not be combined.
We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the evidence as it related to the studies that contributed data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2017), using GRADEpro GDT software (GRADEpro GDT).
We justified all decisions to downgrade the certainty of the evidence using footnotes and made comments to aid reader's understanding of the review where necessary.
Several review authors made judgements about the certainty of evidence after discussion among the team (DP, FV, GU, RAD, RD, EM, and SB). We justified, documented, and incorporated into reporting of results all judgements for each outcome.
Results
Description of studies
Results of the search
We identified 462 records from the electronic database searches and 39 records from other sources (clinical trials registers). Deduplication and removal of all clearly irrelevant references excluded 114 references. Initial screening of the remaining 387 citations against inclusion criteria excluded a further 337 references. Of the remaining 50 citations, we subsequently excluded 18 references, as they did not fully meet the inclusion criteria or they were abstracts of potentially relevant studies that never were published as a full paper after a sufficient time (see Excluded studies).
Four other relevant studies were identified in ClinicalTrials.gov that met the eligibility criteria. All of them are still ongoing or have been completed but not yet published (NCT01957826; NCT02033278; NCT02293603; NCT03797092), these are shown in the Characteristics of ongoing studies table.
The remaining 28 citations described 13 individual RCTs (see Characteristics of included studies table). Two identical phase II trials (Catheter‐DCM and IMPACT‐DCM) that run in parallel and compared SCT (a different modality each) versus control (same type) in a mixed population of people with ischaemic and non‐ischaemic DCM were published in the same article (Henry 2014). For the purpose of this review, both experimental (SCT) and control groups were respectively merged, and the study included as one study within comparison 1 (using only data of the people with non‐ischaemic DCM).
A summary of study classification is displayed in a PRISMA flow diagram (Figure 1).
1.
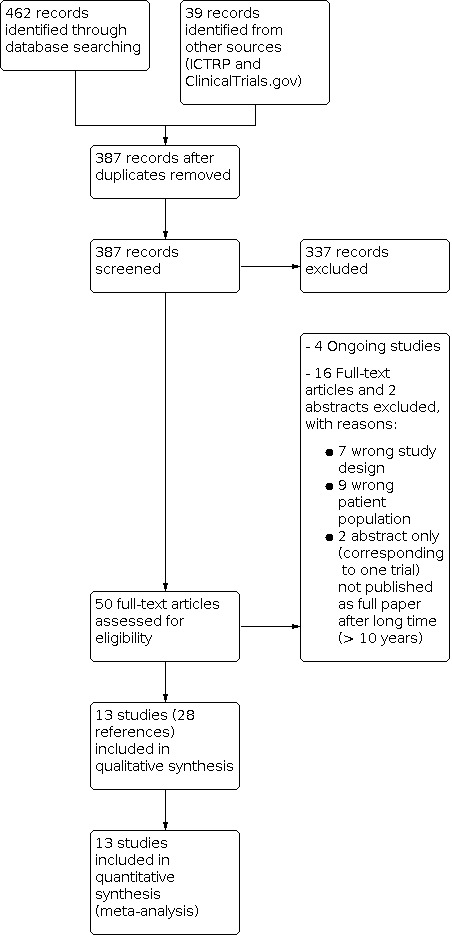
Included studies
Thirteen studies met the inclusion criteria for this review, including 762 randomized participants (452 bone marrow‐derived stem/progenitor cells and 310 controls) who were assessed for the primary outcomes of the study (Hamshere 2015; Hare 2017 (POSEIDON‐DCM); Henry 2014; Martino 2015 (MiHEART); Sant'Anna 2014 (INTRACELL); Seth 2010 (ABCD); Vrtovec 2011; Vrtovec 2013a (NOGA‐DCM); Vrtovec 2013b; Vrtovec 2018 (REMEDIUM); Wang 2006; Wu 2010; Xiao 2017). See Characteristics of included studies table for a summary of study participants and other characteristics of the studies.
Mean size of the included studies was 60 (ranging from 22 (Henry 2014) to 160 (Martino 2015 (MiHEART)), with a median of 55 participants. The mean age of participants ranged from 45 to 58.5 years, and the proportion of men ranged from 50% to 89%. All trials were presented as full journal articles, except one in a short format (Seth 2010 (ABCD)). Only two trials were multicentre (Henry 2014; Martino 2015 (MiHEART)). Studies were based worldwide, including the UK (Hamshere 2015), Slovenia (Vrtovec 2011; Vrtovec 2013a (NOGA‐DCM); Vrtovec 2013b; Vrtovec 2018 (REMEDIUM)), the US (Henry 2014; Hare 2017 (POSEIDON‐DCM)), Brazil (Martino 2015 (MiHEART); Sant'Anna 2014 (INTRACELL)), India (Seth 2010 (ABCD)), and China (Wang 2006; Wu 2010; Xiao 2017). All studies were published in English language except two that included publications in Chinese (Wang 2006; Wu 2010), which were translated into English for this review.
Twelve studies included participants with congestive heart failure (CHF) secondary to DCM where ischaemic aetiology was excluded. Henry 2014 included a mixed population but results were presented separately for participants with non‐ischaemic DCM. In all studies, participants remained with an optimal standard pharmacological treatment including beta‐blockers, angiotensin converting enzyme inhibitors, angiotensin II receptor blockers, spironolactone, digoxin, diuretics, and hydralazine plus nitrates.
Total duration of follow‐up was six months in two studies (Wang 2006; Vrtovec 2013b), 12 months in seven studies (Hamshere 2015; Hare 2017 (POSEIDON‐DCM); Martino 2015 (MiHEART); Sant'Anna 2014 (INTRACELL); Vrtovec 2011; Vrtovec 2018 (REMEDIUM); Xiao 2017), 24 months in two studies (Henry 2014; Wu 2010), three years in one study (Seth 2010 (ABCD)), and five years in one study (Vrtovec 2013a (NOGA‐DCM)).
The 13 included studies accounted for 29 study arms (11 RCTs with two arms, one with three arms, and one with four arms).
According to the funding source, only one study declared a private funding (Henry 2014) or a mixed public and private funding (Hamshere 2015), while six had public or governmental funding, and two non‐profit funding. The three studies set in China did not report this information (Wang 2006; Wu 2010; Xiao 2017).
All but four studies (Seth 2010 (ABCD); Wang 2006; Wu 2010; Xiao 2017) were registered in ClinicalTrials.gov.
According to the comparison group:
eight RCTs compared SCT versus no intervention or sham (comparison 1): Hamshere 2015; Henry 2014; Martino 2015 (MiHEART); Sant'Anna 2014 (INTRACELL); Seth 2010 (ABCD); Wang 2006; Xiao 2017; and Wu 2010;
three RCTs compared SCT plus G‐CSF versus G‐CSF (comparison 2): Hamshere 2015; Vrtovec 2011; and Vrtovec 2013a (NOGA‐DCM);
four RCTs compared SCT versus SCT (comparison 3): Hare 2017 (POSEIDON‐DCM); Vrtovec 2013b; Vrtovec 2018 (REMEDIUM), and Xiao 2017). A summary of the specific comparisons within this group is presented in Table 5.
3. Summary of comparison 3.
| Study | Details on the specific comparison |
| Hare 2017 (POSEIDON‐DCM) | Autologous vs allogeneic mesenchymal stem cells |
| Vrtovec 2013b | Intracoronary vs transendocardial delivery of stem cells |
| Vrtovec 2018 (REMEDIUM) | Repetitive vs single dose of stem cells |
| Xiao 2017 | Mononuclear vs mesenchymal bone marrow stem cells |
Excluded studies
Among the 18 papers excluded after reading the full text, 15 were studies with serious doubts and one was a trial registered in ClinicalTrials.gov. These were excluded due to a diversity or reasons:
wrong design (Bartolucci (INNOVA) 2015; Bocchi 2010; Butler 2017; Chen 2008; Fischer‐Rasokat 2009; Huang 2006; Tompkins 2018);
wrong population (Bartolucci (RIMECARD) 2017; Miyagawa 2017; NCT02256501; Perin (REVASCOR) 2015; Premer 2015; Xiao 2012a; Xiao 2012b; Yau 2019; Zemljic 2017).
One additional study (two references) was published in abstract format only, and although it appeared to meet the inclusion criteria, it did not contain sufficient data for inclusion. As it was published in 2011 and there has been no full paper published, we decided to exclude it from the review (Kakuchaya 2011).
Risk of bias in included studies
A summary of the risk of bias in individual studies is given below and in Figure 2. Judgements about each risk of bias item can be seen in Figure 3. Further details of our assessment of risk of bias can be found in the Characteristics of included studies table.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
We considered only one trial to have a low risk of bias across all domains (Martino 2015 (MiHEART)).
Due to the specific nature of the intervention (SCT), the most problematic item was related to selective reporting with major inconsistencies between the ClinicalTrials.org record and the publication, and with blinding of study personnel and participants (performance bias). Numerous shortcomings in the reporting of most of the included studies did not allow an adequate assessment of some of the following items. However, the additional information available in ClinicalTrials.gov and in online appendices or supplements, in some cases, allowed a more precise description and risk of bias assessment.
Frequently, the format of the continuous data available was not ideal for use in the meta‐analysis. We would have preferred to evaluate the mean change difference, since, as most of the studies were small, there were frequently baseline differences. However, limitations in the reporting of the studies made it impossible to obtain the necessary data to calculate this estimate, so that differences in the final means and difference in the mean changes were combined. Furthermore, in various studies, the final mean and the associated SD was obtained from graphs using specialized software (GetData Graph Digitizer 2.26).
Allocation
Only four studies provided details of randomization methods with a low risk of bias from random sequence generation (Hamshere 2015; Henry 2014; Martino 2015 (MiHEART); Sant'Anna 2014 (INTRACELL)). All four used randomization codes generated electronically. The remaining studies provided no details about the method used to generate the random sequence and, therefore, we judged risk of bias as unclear.
Only one study described appropriate methods of allocation concealment with a low risk of bias (Martino 2015 (MiHEART)), while all other trials had unclear allocation concealment.
Blinding
In four studies, participants randomized to the control group received a placebo injection (sham) (Hamshere 2015; Martino 2015 (MiHEART); Wang 2006; Xiao 2017). We judged these trials at a low risk of performance bias. We considered all other trials at high risk of performance bias due to the open‐label design.
For detection bias, 10 studies were at low risk of bias (four for using sham as a control (Hamshere 2015; Martino 2015 (MiHEART); Wang 2006; Xiao 2017), and six open‐label trials that reported blinding of outcome assessors (Hare 2017 (POSEIDON‐DCM); Henry 2014; Vrtovec 2011; Vrtovec 2013a (NOGA‐DCM); Vrtovec 2013b; Vrtovec 2018 (REMEDIUM)). The three other studies were at high risk of detection bias (Sant'Anna 2014 (INTRACELL); Seth 2010 (ABCD); Wu 2010).
Incomplete outcome data
Four trials had a high risk of attrition bias due to imbalances in the distribution of withdrawals and participants lost to follow‐up between groups, without a proper statistical handling of missing data in the analysis (Henry 2014; Sant'Anna 2014 (INTRACELL); Seth 2010 (ABCD); Xiao 2017). Five studies were at unclear risk of attrition bias, due to incomplete or confusing information about number of participants lost to follow‐up or the statistical methods used to deal with it (or both) (Hare 2017 (POSEIDON‐DCM); Vrtovec 2011; Vrtovec 2013a (NOGA‐DCM); Wang 2006; Wu 2010). Four studies were at low risk of attrition bias (Hamshere 2015; Martino 2015 (MiHEART); Vrtovec 2013b; Vrtovec 2018 (REMEDIUM)).
Selective reporting
Three studies were at high risk of reporting bias, but reasons differed. One study only specified the primary endpoint at ClinicalTrials.gov (Sant'Anna 2014 (INTRACELL)), while in two studies there were several important inconsistencies between the information provided in ClinicalTrials.gov and the final publication related to the number of participants, the time point for the outcomes, the completion date, and the outcomes that were planned and finally reported (Vrtovec 2011; Vrtovec 2013a (NOGA‐DCM)). We suspect that participants reported in Vrtovec 2011 were also included in the Vrtovec 2013a (NOGA‐DCM) analysis. We contacted the corresponding author by email but have received no response. Four trials were not previously registered in a public register and, therefore, the risk of reporting bias was unclear (Seth 2010 (ABCD); Wang 2006; Wu 2010; Xiao 2017). Finally, six studies were at low risk of reporting bias as they had been registered at ClinicalTrials.gov and all outcomes reported corresponded to those registered (Hamshere 2015; Hare 2017 (POSEIDON‐DCM); Henry 2014; Martino 2015 (MiHEART); Vrtovec 2013b; Vrtovec 2018 (REMEDIUM)).
Other potential sources of bias
There is an issue of lack of transparency that affects several of the studies that did not report the funding sources nor were registered in a public trial registry. However, in the specific context of cell therapy research, we think that even supposedly non‐commercial studies are also not free from bias. For this reason, this criterion was ultimately not used in the risk of bias summary and risk of bias tables.
Effects of interventions
Summary of findings 1. Stem cell therapy compared to control for dilated cardiomyopathy.
| SCT compared to control for DCM | ||||||
| Patient or population: DCM (non‐ischaemic) Setting: hospital for procedure; follow‐up ambulatory Intervention: SCT (any type) Comparison: Control (no intervention or sham intervention) | ||||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Comments | |
| Risk with control | Risk with SCT | |||||
|
All‐cause mortality Mean follow‐up 12 months |
361 (7 studies) | ⊕⊝⊝⊝ Very lowa,b |
RR 0.84 (0.54 to 1.31) | 196 per 1000 |
165 per 1000 (106 to 257) |
We are uncertain whether SCT reduces all‐cause mortality in people with DCM. We excluded 1 further study (29 participants) from the meta‐analysis because there were no events in either group (Henry 2014). See Table 2 for more details on mortality. |
|
Procedural complications Within 30 days |
361 (7 studies) |
⊕⊝⊝⊝ Very lowa,c |
Not estimable | See comment | See comment | We are uncertain whether SCT increases the risk of procedural complications in people with DCM. The definition of this outcome varied widely between studies. In some, it was included as an adverse event. For this reason, this outcome is only presented narratively. Overall, SCT is perceived as a safe intervention. |
|
Health‐related quality of life Mean follow‐up 12 months |
272 (5 studies) | ⊕⊝⊝⊝ Very lowd,e,f | — | — |
SMD 0.62 higher (0.01 higher to 1.23 higher) |
We are uncertain whether SCT improves health‐related quality of life in people with DCM. It was not possible to calculate mean value in the control group, considering that studies used different scales. Using Cohen 1988's approach, we interpret this SMD as a medium‐to‐large effect estimate. |
|
Performance status – 6MWT (m) Mean follow‐up 12 months |
230 (5 studies) | ⊕⊝⊝⊝ Very low a,b,e, |
— | 246.9 (SD 141.9) |
MD 70.12 m higher (5.28 lower to 145.51 higher) |
We are uncertain whether SCT improves performance status assessed by 6MWT in people with DCM. For the risk with control we used the mean average of the control groups of the included studies. 1 additional study assessed exercise time and found an improvement with SCT (P = 0.01) but a decrease in the control group after 1 year (Hamshere 2015). |
|
Performance status – change in functional class (NYHA) Mean follow‐up 12 months |
398 (6 studies) |
⊕⊕⊝⊝ Lowa,g |
Not estimable | See comment | See comment | SCT may result in a slight functional class (NYHA) improvement in people with DCM. 6 studies reported change in functional class (NYHA) in a diverse way that did not allow pooling of data. All studies reported a significant improvement with SCT. |
| MACE | 0 (0 studies) |
— | — | — | — | No studies reported MACE as defined in our protocol (Diaz‐Navarro 2019). |
|
Ventricular arrhythmia Mean follow‐up 12 months |
504 (8 studies) |
⊕⊕⊝⊝ Lowa,g |
Not estimable | See comment | See comment | SCT may not increase the risk of ventricular arrhythmia in people with DCM. Overall, ventricular arrhythmia rates were similar between groups. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWT: 6‐minute walk test; CI: confidence interval; DCM: dilated cardiomyopathy; MACE: major adverse cardiovascular events; MD: mean difference; NYHA: New York Heart Association; RCT: randomized controlled trial; RR: risk ratio; SCT: stem cell therapy; SD: standard deviation; SMD: standardized mean difference. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level due to unclear risk of bias related to randomization and allocation concealment and high risk of attrition bias and selective reporting. bDowngraded two levels for imprecision due to optimal information size not being met and confidence intervals including the threshold of null effect. cDowngraded two levels for inconsistency as the outcome had high heterogeneity within included studies. dDowngraded one level due to unclear risk of bias related to randomization and allocation, and high risk of bias regarding blinding. eDowngraded two levels due to inconsistency (I² = 80%). The substantial heterogeneity observed was attributable to Martino 2015 (MiHEART), a robust trial with a relatively large sample size, which did not show a beneficial effect of SCT on this outcome (measured using the Minnesota Living with Heart Failure Questionnaire), contrary to what is suggested by the other studies. fDowngraded one level for imprecision due to wide confidence intervals (reflecting both a null and a relevant effect). gDowngraded one level for indirectness due to different definitions of the outcome.
Summary of findings 2. Stem cell therapy (any type) compared to peripheral therapy with granulocyte colony‐stimulating factor for dilated cardiomyopathy.
| SCT (any type) compared to G‐CSF for DCM | ||||||
| Patient or population: DCM (non‐ischaemic) Setting: hospital for procedure, follow‐up ambulatory Intervention: SCT (any type) Comparison: G‐CSF (peripheral therapy with granulocyte colony‐stimulating factor) | ||||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Comments | |
| Risk with control | Risk with SCT | |||||
|
All‐cause mortality Follow‐up to 5 year |
195 (3 studies) | ⊕⊝⊝⊝ Very lowa,b | RR 0.46 (0.16 to 1.31) | 278 per 1000 |
128 per 1000 (45 to 365) |
We are uncertain whether SCT reduces all‐cause mortality in people with DCM. See Table 2 for more details on mortality. |
|
Procedural complications (safety) Follow‐up 1 year |
60 (1 study) |
⊕⊝⊝⊝ Very lowb,c | Not estimable | See comment | See comment | We are uncertain whether SCT increases the risk of procedural complications in people with DCM. Hamshere 2015 assessed the safety of the SCT infusion by measurement of creatine kinase and troponin T concentrations 12 hours after infusion. There were no cases of distal coronary artery occlusion, acute cardiac dysfunction, or significant creatine kinase or troponin T release. 1 participant experienced a localized coronary dissection during infusion. No complications or adverse events associated with G‐CSF therapy. |
|
Health‐related quality of life Follow‐up 1 year |
22 (1 study) | ⊕⊕⊝⊝ Lowc | — | 9.39 (SD 14.72) |
MD 4.61 higher (5.62 lower to 14.83 higher) | SCT may not improve health‐related quality of life in people with DCM. For the risk with control, we used the mean of the control group of the only included study. |
| Performance status – functional class (NYHA) | 0 (0 studies) |
— | — | — | — | No studies reported this outcome. |
|
Performance status – exercise tolerance (6MWT) (m) Follow‐up to 5 years |
155 (2 studies) | ⊕⊕⊝⊝ Lowa | — | 138.9 (SD 61.19) |
MD 140.14 m higher (119.51 higher to 160.77 higher) | SCT may improve performance status assessed by 6MWT in people with DCM. For the 2 studies included in this analysis, we obtained raw data from a figure by using a software. For the risk with control, we used the mean average of the control groups of the included studies. 1 additional study assessed exercise time and found an improvement with SCT (P = 0.01) but a decrease in the control group after 1 year (Hamshere 2015). |
| MACE | 0 (0 studies) |
— | — | — | — | No studies reported MACE as defined in our protocol (Diaz‐Navarro 2019). |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). 6MWT: 6‐minute walk test; CI: confidence interval; DCM: dilated cardiomyopathy; G‐CSF: granulocyte colony‐stimulating factor; MD: mean difference; NYHA: New York Heart Association; RR: risk ratio; SCT: stem cell therapy; SD: standard deviation. | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded two levels for risk of bias due to serious concerns about selective reporting with two studies. In Vrtovec 2011, there were inconsistencies between the trial register (NCT00629018) and the final publication regarding the total number of participants (110 vs 55), the time point for the outcomes (5 years vs 1 year) and some of the outcomes that were planned and finally reported, and there is a high suspicion that participants reported in Vrtovec 2011 were also included in the Vrtovec 2013a (NOGA‐DCM) analysis. For Vrtovec 2013a (NOGA‐DCM), the trial was registered as a phase II study to compare between intracoronary versus intramyocardial injection of SCT (NCT01350310), nevertheless the publication described a comparison between SCT and no cell therapy. We made several attempts to contact the authors with no reply. bDowngraded one level for indirectness due to concerns about the population. Vrtovec 2013a (NOGA‐DCM) has a ClinicalTrials.gov record (NCT01350310), which had an actual primary completion date of August 2014, but the study was published in 2013 after 5 years' follow‐up. We made many attempts to contact the author with no reply. cDowngraded two levels for imprecision due to optimal information size not being met.
The effects of the intervention (SCT) for each outcome analysed are presented below. Results are discussed separately depending on the type of comparison (control):
comparison 1: SCT versus no intervention or sham (Table 1);
comparison 2: SCT versus G‐CSF (Table 3).
Where possible, the results of the meta‐analysis are presented or, when not possible, narratively.
For comparison 3 (different types or delivery modalities of SCT against each other), the results are presented narratively, since the meta‐analysis did not apply as the they were very diverse and not comparable.
Primary outcomes
All‐cause mortality
Comparison 1: stem cell therapy versus control (no intervention or sham)
All studies comparing SCT versus control included mortality as an outcome, although it was not a primary outcome in any of them. We excluded one study that reported mortality with zero events in both arms in the non‐ischaemic population from the analysis (Henry 2014). Mortality rate was 16.2% (32/198) in participants who received SCT, similar to that observed in participants who received no cells (19.6%, 32/163) (RR 0.84, 95% CI 0.54 to 1.31; I² = 0%; studies = 7; participants = 361; very low‐certainty evidence; Analysis 1.1).
1.1. Analysis.

Comparison 1: Stem cell therapy (SCT; any type) versus control (no intervention or sham intervention), Outcome 1: All‐cause mortality
Despite the absence of statistical heterogeneity observed in the analysis, mortality at one year ranged from 0% (Hamshere 2015; Henry 2014) to 35.0% (Seth 2010 (ABCD)) in the control group, and from 0% (Henry 2014) to 36.8% (Sant'Anna 2014 (INTRACELL) in the SCT group. This variation suggests there were important differences in study population (baseline risk) across studies. For a better understanding of the differences observed among studies, mortality rates of all studies included in the review are displayed in Table 2.
1. Summary of mortality rates.
| Study (arm) | Follow‐up | SCT | No SCT | ||
| n | % | n | % | ||
| Hamshere 2015 | 12 m | 2/13 | 15.4% | 0/13 | 0% |
| Hare 2017 (POSEIDON‐DCM) (autologous) | 12 m | 2/16 | 12.5% | — | — |
| Hare 2017 (POSEIDON‐DCM) (allogeneic) | 12 m | 0/18 | 0% | — | — |
| Henry 2014a | 12 m | 0/18 | 0% | 0/11 | 0% |
| Martino 2015 (MiHEART) | 12 m | 13/61 | 21.3% | 11/54 | 20.4% |
| Sant'Anna 2014 (INTRACELL)b | 12 m | 7/19 | 36.8% | 1/9 | 11.1% |
| Seth 2010 (ABCD) | > 12 m (3 yr) | 10/41 | 24.4% | 14/40 | 35.0% |
| Vrtovec 2011 | 12 m | 2/28 | 7.1% | 8/27 | 29.6% |
| Vrtovec 2013a (NOGA‐DCM) | > 12 m (5 yr) | 8/55 | 14.5% | 19/55 | 34.5% |
| Vrtovec 2013b (IC) | 6 m | 0/20 | 0% | — | — |
| Vrtovec 2013b (TE) | 6 m | 0/20 | 0% | — | — |
| Vrtovec 2018 (REMEDIUM) (repetitive) | 12 m | 0/30 | 0% | — | — |
| Vrtovec 2018 (REMEDIUM) (single) | 12 m | 1/30 | 3.3% | — | — |
| Wang 2006 | 6 m | 1/12 | 8.3% | 2/12 | 16.7% |
| Wu 2010 | > 12 m (18 m) | 1/20 | 5.0% | 2/18 | 11.1% |
| Xiao 2017 | 12 m | 1/31 | 3.2% | 2/17 | 11.8% |
| Up to 12 m | 29/316 | 9.2% | 24/143 | 16.8% | |
| > 12 m | 19/116 | 16.4% | 35/113 | 31.0% | |
| TOTAL | 48/432 | 11.1% | 59/256 | 23.0% | |
IC: intracoronary; m: month; SCT: stem cell therapy; TE: transendocardial; yr: year.
aHenry 2014 studied mixed population. Data shown here correspond specifically to participants with non‐ischaemic dilated cardiomyopathy.
bSant'Anna 2014 (INTRACELL) assessed mortality at 6 months, but provides information on additional deaths observed up to 12 months only in the SCT group.
In summary, we are uncertain whether SCT may reduce mortality in people with DCM compared to control (no intervention or sham) due to the very low‐certainty evidence that is available.
Comparison 2: stem cell therapy versus granulocyte‐colony stimulating factor
The three studies comparing SCT versus G‐CSF assessed mortality as an outcome. One four‐arms study already included in comparison 1 is also included in this comparison (Hamshere 2015). For this comparison we used the data form 'peripheral G‐CSF' as the control group.
Mortality rate was 12.3% (12/98) in participants who received cell therapy plus G‐CSF, lower than that observed in participants who only received G‐CSF (30.3%, 27/97) (RR 0.46, 95% CI 0.16 to 1.31; I² = 39%; participants = 195; studies = 3; very low‐certainty evidence; Analysis 2.1). Although global heterogeneity is low, there is a discrepancy produced by Hamshere 2015 where two deaths were observed in the SCT group and none in the control, while in the two other studies mortality was reduced in the SCT group. Had we excluded this study from the meta‐analysis, the pooled estimate would clearly favour SCT.
2.1. Analysis.

Comparison 2: Stem cell therapy (SCT; any type) versus peripheral therapy with granulocyte colony‐stimulating factor (G‐CSF), Outcome 1: All‐cause mortality
In summary, we are uncertain whether SCT may reduce mortality in people with DCM compared to G‐CSF due to overall very low‐certainty evidence that is available.
Comparison 3: different types or delivery modalities of stem cell therapies against each other
Of the four studies comparing different types or delivery modalities of SCT, three assessed mortality at one year. In Hare 2017 (POSEIDON‐DCM), there were only two deaths among 34 participants treated with SCT (5.9%). These occurred in the group receiving autologous mesenchymal stem cells versus none in the allogeneic group. In Vrtovec 2018 (REMEDIUM), only 1/60 participant with SCT (mononuclear cells) died (1.7%). The participant was assigned to single SCT; the comparator group received repetitive SCT. In Xiao 2017, only 1/31 participants receiving SCT (3.2%) died at one year. The participant was assigned to BMMCs; the comparator group received bone marrow mesenchymal stem cells (BMSC).
Procedural complications (safety)
Ten studies assessed the composite outcome of procedural complications (safety). However, the definition of the outcome varied widely between studies. For this reason, we reported the outcome narratively.
Comparison 1: stem cell therapy versus control (no intervention or sham)
Hamshere 2015 assessed the safety of the SCT infusion by measurement of creatine kinase (CK) and troponin T concentrations 12 hours after infusion and procedural complications. There were no cases of distal coronary artery occlusion, acute cardiac dysfunction, ventricular arrhythmia, or significant CK or troponin T release occurred after the procedure. One participant experienced a localized coronary dissection during SCT infusion.
The primary objective of the studies run by Henry 2014 (IMPACT‐DCM and Catheter‐DCM) was to assess the safety of SCT (ixmyelocel‐T) administered via mini‐thoracotomy (IMPACT‐DCM) or intramyocardial catheter (Catheter‐DCM) injections. The study reported AEs per participant at days zero to five (perisurgical period), and at days six to 730 after the procedure. The five most common AEs in the SCT (ixmyelocel‐T) groups in the perisurgical period (day zero to five), according to what was stated by the trial authors, were hypotension, nausea, constipation, hyperglycaemia, and hypertension. Surgical delivery of SCT (ixmyelocel‐T) via mini‐thoracotomy was associated with a higher incidence of serious adverse events (SAEs) in the perisurgical period (day zero to five) (6.71 AEs/participant). This finding was not observed when SCT was delivered via catheter (0.93 AEs/participant). After the procedure (days six to 730), the number of AEs per participant in both SCT groups was comparable (8.21 via mini‐thoracotomy versus 6.27 AEs/participant via catheter).
Martino 2015 (MiHEART) reported no SAEs were directly related to cell injection during the trial. They provided no definition of AEs.
Sant'Anna 2014 (INTRACELL) assessed procedural safety as a secondary outcome. Authors reported that four participants died in the first month after the procedure. Two died of refractory cardiogenic shock in the first 72 hours postoperatively, one died on the 15th postoperative day due to incessant ventricular tachycardia, and one died on the 28th postoperative day due to heart failure that was refractory to treatment.
Seth 2010 (ABCD) stated "this study establishes the long‐term safety of this therapy in dilated cardiomyopathy." However, they provided no definition or specific data.
Wang 2006 concluded that cell transplantation was safe. There was no embolism, arrhythmia, or other negative clinical events throughout the study.
Wu 2010 observe no AEs such as fever, allergic reaction, myocardial infarction, embolism, and tumour formation related with the procedure.
Xiao 2017 defined procedural complications as any new‐onset ventricular arrhythmia, conduction disturbance, distal embolization, thrombus formation, and injury of the coronary artery related to the cell injection procedure. However, the study did not report this outcome.
Comparison 2: stem cell therapy versus granulocyte‐colony stimulating factor
Only one study out of three reported on this outcome within this comparison. In Hamshere 2015, one participant experienced a localized coronary dissection during STC infusion. There were no complications or AEs associated with G‐CSF therapy.
Comparison 3: different types or delivery modalities of stem cell therapies against each other
Vrtovec 2018 (REMEDIUM) defined SAE as "any serious event that may result in persistent or significant disability or incapacity and included death, heart transplantation, ventricular assist device implantation, sustained ventricular arrhythmia (ventricular tachycardia or ventricular fibrillation), and heart failure exacerbation requiring hospitalization." There were 10 SAEs in 60 participants, the most frequent being heart failure worsening (7%) and sustained ventricular arrhythmia (5%). The number of SAEs did not differ between the repetitive versus the single‐dose SCT groups.
In Hare 2017 (POSEIDON‐DCM), the primary safety endpoint was the incidence of any treatment‐emergent SAE occurring within 30 days of treatment. Secondary safety endpoints included other AEs. There were no 30‐day treatment‐emergent SAEs among the 34 study participants. At the end of follow‐up (one year), SAE incidence was 28.2% with allogeneic mesenchymal stem cells versus 63.5% with autologous mesenchymal stem cells.
Secondary outcomes
Health‐related quality of life
Six studies reported health‐related quality of life. Four used the MLHFQ (Hare 2017 (POSEIDON‐DCM); Henry 2014; Martino 2015 (MiHEART); Sant'Anna 2014 (INTRACELL)), and two used the KCCQ (Hamshere 2015; Seth 2010 (ABCD)). In addition, Hamshere 2015 also used the generic European Quality of Life‐5 Dimensions (EQ5D). For the pooled analysis, KCCQ was preferred over EQ5D. We used the KCCQ clinical summary score over the overall summary.
The pooled analysis (only for comparison 1) combines different HRQoL tools, and methods to estimate treatment effect (mean change differences and difference in final means (Seth 2010 (ABCD)). We obtained the raw data from figures using a specialized software for one study (Henry 2014). One study reported trimmed means instead of means (Martino 2015 (MiHEART)). In Sant'Anna 2014 (INTRACELL), the SD of the difference in mean change differences was imputed from Henry 2014 (baseline mean values were very similar in both studies).
In the analysis of quality of life outcomes, we converted MLHFQ scores to negative values in order to include these in a meta‐analysis with other measures on different scales using the SMD.
Comparison 1: stem cell therapy versus control (no intervention or sham)
We are uncertain whether SCT improves health‐related quality of life compared to control (no intervention or sham) (SMD 0.62, 95% CI 0.01 to 1.23; I² = 80%; studies = 5, participants = 272; very low‐certainty evidence; Analysis 1.2).
1.2. Analysis.

Comparison 1: Stem cell therapy (SCT; any type) versus control (no intervention or sham intervention), Outcome 2: Health‐related quality of life
The substantial heterogeneity observed in this analysis was attributable to Martino 2015 (MiHEART), a robust trial with a relatively large sample size, which found no beneficial effect of SCT on health‐related quality of life (measured with the MLHFQ), contrary to what is suggested by the remaining studies.
Comparison 2: stem cell therapy versus granulocyte‐colony stimulating factor
One four‐arm study comparing SCT versus G‐CSF assessed quality of life (Hamshere 2015). We used the data from 'peripheral G‐CSF' as the control group, and analysed KCCQ clinical summary score at one year (mean change difference). Small sample size lacked power to detect any difference between groups (SCT plus G‐CSF versus peripheral G‐CSF: MD 4.61 points, 95% CI −5.62 to 14.83; studies = 1, participants = 22). Other additional measures of quality of life used in this study (EQ5D Index score and visual analogue scale, and KCCQ Overall Summary) also found no differences between groups. Therefore, SCT may not improve health‐related quality of life in these participants.
Comparison 3: different types or delivery modalities of stem cell therapies against each other
In Hare 2017 (POSEIDON‐DCM), the MLHFQ improved in both SCT groups over 12 months (allogeneic mesenchymal group: P = 0.0022; autologous mesenchymal group: P = 0.1719). The data were shown in figure form only, but there were no differences between the groups. According to the authors, the improvement observed with either form of SCT was "clinically meaningful."
Performance status – functional class (NYHA)
Seven studies assessed the change in functional class (NYHA) from baseline. However, the diverse way the outcome was reported precluded pooling of data in a meta‐analysis. For this reason, we presented this outcome narratively.
Comparison 1: stem cell therapy versus control (no intervention or sham)
Hamshere 2015 reported the percentage of participants who showed an improvement in their NYHA classification from baseline. The percentage of participants who showed improvement in their NYHA classification at three months and one year was significantly higher in the SCT group (intracoronary BMCs) (P = 0.02). At one year, eight (66.7%) participants showed improvement in NYHA class with no participants demonstrating a deterioration in the SCT group versus one participant (8.3%) in the control group (peripheral placebo) showing improvement with three participants (23.1%) who worsened (P < 0.05).
Henry 2014 defined the outcome as the proportion of participants who achieved a NYHA class I/II at the end of the study. While there was a significant improvement in NYHA functional class with treatment with SCT (ixmyelocel‐T) compared to the control group in the ischaemic population (P < 0.05), this was not the case for the non‐ischaemic population (65% with SCT vs 41% with control, reported as "statistically non‐significant"; data presented only in a figure).
Martino 2015 (MiHEART) assessed changes in NYHA functional class but provided no raw data. The author declared: "Change in NYHA functional class differed significantly between groups at 6 (P = 0.003) but not at 12 months (P = 0.422)."
Sant'Anna 2014 (INTRACELL) assessed changes in NYHA class and concluded: "Functional class, evaluated using NYHA classification, showed (…) a statistically significant improvement in the BMMC group, no change in the control group. However, there were no statistically significant differences between groups."
The way Seth 2010 (ABCD) reported change in NYHA functional class is confusing as we could not determine how many participants in each group improved at the end of the study. In the SCT group, after three years, the percentage of participants in NYHA class III and IV had decreased, and 63.4% were in class I or II. In the control group, no participant reached a NYHA class I, and the percentage that was in class II had decreased (at baseline, around a third of all participants within this group was in this category, but none in the SCT group). The authors concluded that there was a significant improvement in functional status on long‐term follow‐up in the SCT group, being the greater effect for participants with class III compared to class IV.
Xiao 2017 assessed change in NYHA functional class at 12 months. In comparison with control, NYHA class improved significantly in the BMSC group at 12‐month follow‐up (P = 0.050), but not in the BMMC group.
In summary, data suggest that SCT may slightly improve NYHA functional class, but the magnitude of this effect is unclear.
Comparison 2: stem cell therapy versus granulocyte‐colony stimulating factor
No study assessed change in functional class (NYHA).
Comparison 3: different types or delivery modalities of stem cell therapies against each other
In Hare 2017 (POSEIDON‐DCM), at 12 months, the use of allogeneic mesenchymal stem cell was associated with a 66.7% improvement in NYHA functional class versus 27.3% for autologous mesenchymal stem cell (P = 0.0527).
Performance status – exercise tolerance – (6‐minute walk test)
Comparison 1: stem cell therapy versus control (no intervention or sham)
Five studies reported exercise tolerance. Due to the variable way the data were originally presented, we combined different measures of effect in the meta‐analysis has been also variable; in some cases, we used the difference of final means (Sant'Anna 2014 (INTRACELL); Wang 2006; Wu 2010), while in others, we used the mean change difference (Henry 2014; Martino 2015 (MiHEART)). We obtained raw data for Henry 2014 from a figure using specialized software.
The evidence was uncertain about the effect of SCT on exercise capacity as assessed by means of the 6MWT compared to control (no intervention or sham) (MD 70.12 m, 95% CI −5.28 to 145.51; I² = 87%; studies = 5, participants = 230; very low‐certainty evidence; Analysis 1.3).
1.3. Analysis.

Comparison 1: Stem cell therapy (SCT; any type) versus control (no intervention or sham intervention), Outcome 3: Performance status – 6‐minute walk test (m)
There was substantial heterogeneity across studies mainly due to two studies showing a beneficial effect for SCT at six (Wang 2006) and 24 months (Wu 2010). Baseline LVEF (%) in these two trials was greater than 30% as compared with the other three trials where participants were in a worse condition (LVEF 30% or less).
In addition, Hamshere 2015 also assessed exercise capacity by improvement in maximum rate of oxygen consumption (Opera), maximum exercise speed, and exercise time. At one year, there was a significant improvement in peak oxygen uptake (VO2peak) with SCT (mean change: 3.2 mL/kg/minute, 95% CI 0.69 to 5.71; P = 0.0179), but not with control (mean change: 0.35 mL/kg/minute, 95% CI −2.28 to 1.58; P = 0.6966). There was also an improvement in maximum exercise speed with SCT (1.28 miles per hour, 95% CI 0.30 to 2.26; P = 0.0164), but not with control (0.28 miles per hour, 95% CI −0.22 to 0.78; P = 0.1292). Exercise time was also increased with SCT (162.3 seconds, 95% CI 43.13 to 281.5; P = 0.0131), but there was a decrease with control (−4.385 seconds, 95% CI −61.08 to 52.31; P = 0.8545).
Comparison 2: stem cell therapy versus granulocyte‐colony stimulating factor
Two studies within this category reported exercise tolerance (Vrtovec 2011; Vrtovec 2013a (NOGA‐DCM)). In both cases, we obtained raw data from a figure using specialized software.
SCT may improve exercise capacity as assessed by the 6MWT compared to control (G‐CSF) (MD 140.14 m, 95% CI 119.51 to 160.77; I² = 0%; studies = 2, participants = 155; low‐certainty evidence; Analysis 2.3).
2.3. Analysis.

Comparison 2: Stem cell therapy (SCT; any type) versus peripheral therapy with granulocyte colony‐stimulating factor (G‐CSF), Outcome 3: Performance status – 6‐minute walk test (m)
In Hamshere 2015, in participants who received SCT, there was an improvement in exercise capacity measured by an improvement in VO2peak, maximum exercise speed, and exercise time. In neither of the two control groups that received only G‐CSF (peripheral G‐CSF and sham SCT plus G‐CSF) was there a favourable effect. The study authors judged these effects on exercise capacity as clinically meaningful.
Comparison 3: different types or delivery modalities of stem cell therapies against each other
At 12 months, Hare 2017 (POSEIDON‐DCM) found that 6MWT distance increased in participants receiving allogeneic mesenchymal cells by 37.0 m (95% CI 2.0 to 72.0; P = 0.04) compared with baseline, but did not significantly change in the autologous mesenchymal cells group (7.3 m, 95% CI 47.8 to 33.3; P = 0.71). The between‐group difference was 46.5 m (95% CI 5.5 to 98.5; P = 0.077) at 12 months.
Vrtovec 2013b found that 6MWT distance significantly increased at six months in participants assigned to the transendocardial SCT group compared to those in the intracoronary SCT group (mean change difference: 125 m (±33) in the transendocardial group versus 86 m (±13) in the intracoronary group; P = 0.03).
Vrtovec 2018 (REMEDIUM) observed no intergroup differences in change in 6MWT after one year between repetitive cell therapy versus single cell therapy (mean increased from 320 m (±92) to 434 m (±71) with repetitive SCT versus 341 m (±87) to 445 m (±96) with single dose SCT; P = 0.65). From baseline to six months, both groups LVEF improved (6.9% (±3.3) in repetitive SCT group; P =0.001; and 7.1% (±3.5) in single‐dose SCT; P = 0.001), but there were no changes between six months and one year.
Rehospitalizations
Henry 2014 and Hamshere 2015 assessed CHF exacerbations requiring hospitalization (e.g. acute heart failure), as part of the composite endpoint MACE.
Vrtovec 2018 (REMEDIUM) assessed heart failure exacerbation requiring hospitalization as part of the composite SAE. There were no specific data for this component.
In Hare 2017 (POSEIDON‐DCM), the 12‐month all‐cause rehospitalization rate was lower in the allogeneic mesenchymal cell group versus the autologous mesenchymal cell group (28.2% with allogeneic mesenchymal cell versus 70.0% with autologous mesenchymal cell; P = 0.0447).
Heart failure
No study assessed heart failure (instead, see 'Change in functional class (NYHA)' above).
Ventricular arrhythmia
Comparison 1: stem cell therapy versus control (no intervention or sham)
Henry 2014 assessed sustained ventricular arrhythmia as a component of the composite MACE. The study reported two events in the SCT group (one in the non‐ischaemic population) that were related with the surgical procedure.
Hamshere 2015 reported that no cases of ventricular arrhythmia occurred in participants receiving SCT.
Martino 2015 (MiHEART) found no increase in life‐threatening arrhythmias (no raw data provided).
Sant'Anna 2014 (INTRACELL) stated that 3/7 (35%) deaths observed in participants who received SCT were due to documented ventricular arrhythmia or sudden death.
Wang 2006 performed continuous ambulatory electrocardiograph (ECG) monitoring. There were no differences between SCT and control group at six months. No raw data of number of events were provided.
In Wu 2010, there were no differences in the 24‐hour ambulatory ECG monitoring between SCT and the control group. No raw data of number of events were provided.
Xiao 2017 assessed new‐onset ventricular arrhythmia as a component of procedural complications and as a component of MACE. No specific data were provided.
Vrtovec 2011 investigated the impact of transplanted cells on ventricular electrophysiology with high‐resolution ECG. The study found no effects of transplanted stem cells on QTc interval and QT interval variability, suggesting an absence of proarrhythmic effects of intracoronary BMC transplantation in people with DCM.
Comparison 2: stem cell therapy versus granulocyte‐colony stimulating factor
No study assessed ventricular arrhythmia.
Comparison 3: different types or delivery modalities of stem cell therapies against each other
Vrtovec 2018 (REMEDIUM) reported three events of sustained ventricular arrhythmia (ventricular tachycardia or ventricular fibrillation) among the 60 participants who received SCT.
Complete atrioventricular block
No study assessed complete atrioventricular block.
Major adverse cardiovascular events
None of the included studies assessed this composite outcome as defined in our protocol (Diaz‐Navarro 2019). For this reason, these results are not presented in our summary of findings tables. However, four studies reported MACE with a different definition.
Comparison 1: stem cell therapy versus control (no intervention or sham)
In Henry 2014, MACE included cardiac death, cardiac arrest, myocardial infarction, sustained ventricular arrhythmia (e.g. ventricular tachycardia or ventricular fibrillation), pulmonary oedema, heart failure exacerbation requiring hospitalization (e.g. acute heart failure), unstable angina, or major bleeding (defined as the need for 2 units of blood or greater within one week of the injection procedure or the need for operation because of bleeding). In this study, each participant was counted only once, regardless of the number of events experienced. In the SCT group, 8/18 (44.4%) participants presented an event versus 3/11 (27.3%) participants in the control group. All but one event in participants with non‐ischaemic DCM were due to CHF exacerbation.
Hamshere 2015 defined MACE as all‐cause death, myocardial infarction, hospitalization for heart failure, or major arrhythmias. There were two reports of MACE in the SCT group (intracoronary BMCs) and one report in the control group (peripheral placebo) at one year. The two events observed in the SCT group consisted of two deaths due to a non‐cardiac surgical procedure complication and bronchopneumonia. The event in the control group consisted of one arrhythmia.
In Xiao 2017, MACE consisted of procedural complications, any new‐onset arrhythmia, haemodynamic instability, and death by any cause. In the SCT group, 11/31 (35.5%) participants presented an event versus 7/17 (41.2%) participants in the control group. It was not clear from the paper if this study counted number of events or number of participants with an event. The events reported in the SCT group were haemodynamic instability (three), atrial fibrillation (three), right or left bundle branch block (four), and ventricular tachycardia (one). The events in the control group consisted of death (two), right bundle branch block (two), left bundle branch block (one), atrial fibrillation (one), and hemodynamic instability (one).
Comparison 2: stem cell therapy versus granulocyte‐colony stimulating factor
No study assessed MACE.
Comparison 3: different types or delivery modalities of stem cell therapies against each other
In Hare 2017 (POSEIDON‐DCM), MACE rate over 12 months was lower in the allogeneic mesenchymal group (20.3%, 95% CI 6.8% to 52.1%) compared with 57.1% (95% CI: 34.9% to 81.2%) in the autologous mesenchymal group (P = 0.0186).
Change in left ventricular ejection fraction
LVEF was the most commonly reported outcome by the studies included in the review.
Comparison 1: stem cell therapy versus control (no intervention or sham)
Eight studies comparing SCT versus control (no intervention or sham) reported changes in LVEF. Due to the variable way the data were originally presented, the measure of the effect we combined in the meta‐analysis was also variable; in some cases we used the difference of final means (Sant'Anna 2014 (INTRACELL); Seth 2010 (ABCD); Wang 2006; Wu 2010; Xiao 2017), while in others we used the mean change difference (Hamshere 2015; Henry 2014; Martino 2015 (MiHEART)). We obtained raw data for Henry 2014 from a figure using specialized software.
SCT did not improve LVEF in participants with DCM compared to control (no intervention or sham) (MD 5.41%, 95% CI −2.29 to 13.10; I² = 94%; studies = 8, participants = 353; Analysis 1.4). There was substantial heterogeneity across studies, that was due mainly to Wu 2010, where participants in the SCT group surprisingly reached a final mean value that doubled the baseline value, and, to a lesser extent, Seth 2010 (ABCD).
1.4. Analysis.

Comparison 1: Stem cell therapy (SCT; any type) versus control (no intervention or sham intervention), Outcome 4: Change in left ventricular ejection fraction
Comparison 2: stem cell therapy versus granulocyte‐colony stimulating factor
Three studies comparing STC versus G‐CSF reported changes in LVEF. For one study, we used difference in final means (Vrtovec 2013a (NOGA‐DCM)), while in the two other studies, we used mean change difference (Hamshere 2015; Vrtovec 2011). In addition, we obtained raw data for Vrtovec 2011 and Vrtovec 2013a (NOGA‐DCM) from a figure using specialized software.
SCT may improve LVEF in participants with DCM (MD 6.61%, 95% CI 5.61 to 7.62; I² = 0%; studies = 3; participants = 182; Analysis 2.4).
2.4. Analysis.

Comparison 2: Stem cell therapy (SCT; any type) versus peripheral therapy with granulocyte colony‐stimulating factor (G‐CSF), Outcome 4: Change in left ventricular ejection volume
Comparison 3: different types or delivery modalities of stem cell therapies against each other
Hare 2017 (POSEIDON‐DCM) found LVEF increased in participants receiving allogeneic mesenchymal cells by 8.0% (P = 0.004) compared with 5.4% in the autologous mesenchymal cells group at 12 months (P = 0.116) (allogeneic versus autologous group: between‐group P = 0.4887). This resulted in the LVEF rising above 40% in 46.7% of participants receiving allogeneic mesenchymal cells versus 22.2% of participants receiving autologous mesenchymal cells.
Vrtovec 2013b found LVEF increased in both groups; however, the change at six months was significantly higher in the transendocardial SCT group than in the intracoronary SCT group. According to the authors, the intracoronary group principally improved their LVEF within the first month, whereas in the transendocardial group the main improvement occurred between months one and three. Authors also note that "LVEF improvement correlated significantly with the percent of cells retained in the myocardium 18 hours after intracoronary or trans endocardial delivery (r=0.53, P < 0.001)."
Vrtovec 2018 (REMEDIUM) observed no intergroup differences in change in LVEF after one year between repetitive cell therapy versus single cell therapy (mean increased from 32.2% (±9.3) to 41.2% (±6.5) with repetitive cell therapy, and from 30.0% (±7.0) to 37.9% (±5.3) with single cell therapy; P = 0.40).
Change in left ventricular end‐systolic volume
Comparison 1: stem cell therapy versus control (no intervention or sham)
Four studies comparing SCT versus control (no intervention or sham) reported changes in LVESV. We used difference in final means in two studies (Hamshere 2015; Seth 2010 (ABCD)), while in two other cases, we used mean change difference (Henry 2014; Martino 2015 (MiHEART)). We obtained raw data for Henry 2014 from a figure using specialized software.
The combined meta‐analysis of these studies found a greater reduction in the LVESV between participants who received SCT and those who had not (MD −30.97 mL, 95% CI −54.18 to −7.75; I² = 0%; studies = 4, participants = 251; Analysis 1.5). However, the evidence was uncertain.
1.5. Analysis.

Comparison 1: Stem cell therapy (SCT; any type) versus control (no intervention or sham intervention), Outcome 5: Change in left ventricular end‐systolic volume
Comparison 2: stem cell therapy versus granulocyte‐colony stimulating factor
Only one study comparing SCT versus G‐CSF reported changes in LVESV (Hamshere 2015). There was no evidence of a difference observed with STC plus G‐CSF (intracoronary BMC) compared with peripheral G‐CSF (MD −71.30 mL, 95% CI −150.96 to 8.36; studies = 1, participants = 27; Analysis 2.5). There were no intragroup differences (pre–post) in either group.
2.5. Analysis.

Comparison 2: Stem cell therapy (SCT; any type) versus peripheral therapy with granulocyte colony‐stimulating factor (G‐CSF), Outcome 5: Change in left ventricular end‐systolic volume
Comparison 3: different types or delivery modalities of stem cell therapies against each other
Vrtovec 2013b reported data for changes in LVESV as a figure. There was no difference after six months between groups (−18 mL with transendocardial SCT versus −13 mL with intracoronary SCT; P = 0.10).
Vrtovec 2018 (REMEDIUM) assessed changes in LVESV but reported no data.
Change in left ventricular end‐diastolic volume
Comparison 1: stem cell therapy versus control (no intervention or sham)
Four studies comparing SCT versus control (no intervention or sham) reported changes in LVEDV. We used difference in final means in two studies (Hamshere 2015; Seth 2010 (ABCD)), while in two other cases, we used mean change difference (Henry 2014; Martino 2015 (MiHEART)). We obtained raw data for Henry 2014 from a figure using specialized software.
The combined meta‐analysis found a greater reduction in the LVEDV between participants who had received SCT than those who had not (MD −23.40 mL, 95% CI −49.74 to 2.94; I² = 0%; studies = 4, participants = 251; Analysis 1.6). However, the evidence was uncertain.
1.6. Analysis.

Comparison 1: Stem cell therapy (SCT; any type) versus control (no intervention or sham intervention), Outcome 6: Change in left ventricular end‐diastolic volume
Comparison 2: stem cell therapy versus granulocyte‐colony stimulating factor
One study comparing SCT versus G‐CSF reported change in LVEDV (Hamshere 2015). There was no evidence of a difference between STC plus G‐CSF (intracoronary BMC) and peripheral G‐CSF groups (MD −81.10 mL, 95% CI −175.54 to 13.34; studies = 1, participants = 27; Analysis 2.6). There were no intragroup differences (pre–post) between groups.
2.6. Analysis.

Comparison 2: Stem cell therapy (SCT; any type) versus peripheral therapy with granulocyte colony‐stimulating factor (G‐CSF), Outcome 6: Change in left ventricular end‐diastolic volume
Comparison 3: different types or delivery modalities of stem cell therapies against each other
One study comparing different types or delivery modalities of SCT assessed change in LVEDV; however, there were no data reported (Vrtovec 2018 (REMEDIUM)).
Change in plasma natriuretic peptide levels (BNP and NT‐proBNP)
Comparison 1: stem cell therapy versus control (no intervention or sham)
Four studies comparing SCT versus control (no intervention or sham) assessed BNP or NT‐proBNP levels, but we could not combine data in the meta‐analysis.
Henry 2014 measured changes in BNP levels but the paper only provides specific data for the SCT group and for the entire study population (not specifically for the participants with non‐ischaemic DCM).
Martino 2015 (MiHEART) measured changes in BNP concentrations, but provided no raw data. The authors stated: "Change in BNP concentrations did not differ significantly between the groups (P = 0.146 and 0.241 at 6 and 12 months, respectively)."
Hamshere 2015 measured changes in N‐terminal pro‐brain natriuretic peptide (NT‐proBNP) plasma levels and found decreased levels in participants who received SCT at one year (intragroup mean change difference −136.0 pg/mL, 95% CI −519.6 to 247.6; P = 0.0023), but not in those who had not (peripheral placebo) (−175.5 pg/mL, 95% CI −493.2 to 147.1; P = 0.2218). There was no P value for the between‐groups comparison provided.
Wang 2006 measured changes in plasma BNP levels and found a difference in final means between participants who had received SCT and those who had not (MD [final means] −124.20 ng/L, 95% CI −223.37 to −25.03).
Comparison 2: stem cell therapy versus granulocyte‐colony stimulating factor
Three studies comparing SCT versus G‐CSF reported NT‐proBNP levels that could be pooled in a meta‐analysis. We used difference in final means in one study (Vrtovec 2013a (NOGA‐DCM)), while in two other studies, we used mean change difference (Hamshere 2015; Vrtovec 2011). We obtained raw data for Vrtovec 2011 and Vrtovec 2013a (NOGA‐DCM)) from a figure using specialized software.
The meta‐analysis showed a greater reduction of NT‐proBNP levels with cell therapy (MD −1632.09 pg/mL, 95% CI −2180.18 to −1083.99; I² = 91%; studies = 3, participants = 181; Analysis 2.7). However, the evidence was uncertain. There was substantial heterogeneity across studies, which was due mainly to Vrtovec 2011.
2.7. Analysis.

Comparison 2: Stem cell therapy (SCT; any type) versus peripheral therapy with granulocyte colony‐stimulating factor (G‐CSF), Outcome 7: Change in plasma natriuretic peptide levels (BNP and NT‐proBNP)
Comparison 3: different types or delivery modalities of stem cell therapies against each other
Two studies comparing different types or delivery modalities of SCT assessed NT‐proBNP levels.
In Vrtovec 2013b, NT‐proBNP levels declined in both groups after six months; this was more in the transendocardial SCT group than in the intracoronary SCT group (−628 pg/mL with transendocardial SCT versus −315 pg/mL with intracoronary SCT; P = 0.04).
In Vrtovec 2018 (REMEDIUM), there were no intergroup differences in change in NT‐proBNP levels between repetitive cell therapy and single cell therapy (mean increased from 1525 pg/mL (±1030) to 732 pg/mL (±725) with repetitive cell therapy, and from 1753 pg/mL (±1008) to 1087 pg/mL (±978) with single cell therapy; P = 0.33). From baseline to six months, both groups displayed a significant improvement in decrease in NT‐proBNP, with no additional changes between six months and one year in either group.
Discussion
DCM is the most common form of non‐ischaemic cardiomyopathy worldwide (Jefferies 2010; McKenna 2017) and represents one of the leading causes of heart failure requiring heart transplantation in adults (Merlo 2016; Stehlik 2011). Since the early 1990s, optimal medical management for heart failure according to evidence‐based guidelines has improved the long‐term prognosis in people with DCM (Merlo 2014; Ponikowski 2016; Yancy 2013). Nevertheless, some patients experience a trend towards worsening of their left ventricular function (Merlo 2015), and most require continuous intravenous inotropic therapy, ventricular assist devices, and mechanical ventilation while awaiting a heart transplant (Jefferies 2010).
SCT has been proposed as a possible alternative therapy for DCM, after some studies suggested a favourable effect on functional status and mortality (Frljak 2018; Poglagen 2018). Although SCT has been reported to be safe (Menasché 2018), its efficacy in people with DCM is still controversial, and SCT is not included in the recommendations of the main clinical practice guidelines for heart failure (Ponikowski 2016; Yancy 2013). We reviewed the available information on the safety and efficacy of SCT administered to people with DCM and heart failure, based on RCTs.
Summary of main results
The review included 13 RCTs. Eight studies compared the effect of SCT with usual care or sham treatment; three studies compared the administration of stem cells after cell mobilization with G‐CSF injected subcutaneously with controls receiving subcutaneous G‐CSF but not cells (Vrtovec 2011; Vrtovec 2013a (NOGA‐DCM); Hamshere 2015); and four studies compared different modes of SCT delivery. Among these, one study compared the administration of autologous mesenchymal cells with allogenic mesenchymal cells delivered transendocardially (Hare 2017 (POSEIDON‐DCM)); one study compared intracoronary and transendocardial delivery of stem cells (Vrtovec 2013b); one study examined repetitive versus single‐infusion of stem cells (both groups receiving mobilization with G‐CSF) (Vrtovec 2018 (REMEDIUM)), and one study compared intracoronary administration of bone marrow mononuclear cells versus mesenchymal stem cells (Xiao 2017).
Participants were diagnosed with DCM after elimination of secondary causes of heart failure, and all were given optimal standard pharmacological treatment. Two studies had a six‐month follow‐up, seven reported follow‐up data for 12 months, and four had long‐term follow‐up of more than 12 months. Here, we define as primary outcomes all‐cause mortality, and safety both at the time of stem cell collection and administration, or within 30 days of treatment.
Our main findings for SCT compared with usual care or sham treatment (comparison 1) were as follows.
We are uncertain whether SCT reduces all‐cause mortality.
We are uncertain whether SCT increases the risk of procedural complications in people with DCM.
We are uncertain whether SCT improves health‐related quality of life.
We are uncertain whether SCT improves exercise capacity as assessed by the 6MWT.
SCT may slightly improve functional class as defined by the New York Association (NHYA).
No studies assessed MACE as defined in our protocol (Diaz‐Navarro 2019).
SCT may not increase the risk of ventricular arrhythmia.
Our main findings for SCT (any type) plus G‐CSF compared with G‐CSF (but no cells) (comparison 2) were as follows.
We are uncertain whether SCT reduces all‐cause mortality.
We are uncertain whether SCT increases the risk of procedural complications.
SCT may not improve health‐related quality of life.
SCT may improve exercise capacity as assessed by the 6MWT.
No studies assessed improvement in functional class as defined by NHYA.
No studies assessed MACE as defined in our protocol (Diaz‐Navarro 2019), or ventricular arrhythmia.
The evidence also suggests some effects of SCT on physiological outcomes such as change in LVEF, LVESV, and LVEDV, and to some extent in laboratory parameters (BNP) presumably related with cardiac function, but clinical implications of these observations remain unclear.
These results are somewhat paradoxical because, in comparison 2, the participants in the control group received active treatment (but without cells), that is considered superior to the control used in comparison 1. Consequently, the explanation for these apparently better results must lie in the nature of the experimental group (the specific mode of administration of SCT) or the characteristics of the study population, or both. This potential beneficial effect observed in a limited number of studies of low certainty suggests that the combination of G‐CSF with SCT compared with SCT alone might produce additional benefits. In an experimental model, therapy with G‐CSF has been reported to have a protective effect on people with heart failure following myocardial infarction (Harada 2005). However, these results must be treated with caution because the study on which they were based had major inconsistencies between the registry entry of the trial and its publication (i.e. different outcomes, interventions, and timing), and hence should be interpreted with caution.
There was a wide variation in mortality rates across studies. This variation could be due to large differences in follow‐up, or in baseline participant characteristics, among other factors. In addition, most studies did not describe the cause of death. This makes it difficult to draw meaningful conclusions with regard to the effects of SCT on all‐cause mortality.
There were substantial differences between the studies in terms of the characteristics of the study population and the cell therapy used (type and origin of the cells infused, cell dose, route, and cell delivery methods), that would have recommended performing subgroup analysis as was originally planned. However, the limited number of studies in each category prevented this.
Some additional studies have compared different cell therapy modalities with each other. Although they did not provide direct evidence of efficacy, they contributed to understanding the effects observed with specific modalities of SCT delivery. Limited and preliminary evidence suggests that repetitive infusions are no better than a single treatment with SCT, and that transendocardial injection is probably better than intracoronary delivery of cells.
In summary, this systematic review and meta‐analysis found no clear evidence that SCT is beneficial in terms of reducing all‐cause mortality, and improving health‐related quality of life and performance status in people with DCM. Thus, more research is needed to establish the role of SCT in the treatment of DCM and the most effective treatment modalities. New evidence from several ongoing studies may help determine the role of SCT in clinical practice.
Overall completeness and applicability of evidence
All the studies included in this review were conducted in cohorts of people with DCM. This diagnosis was made once any secondary causes of myocardial disease, including ischaemic heart disease, were excluded. However, some important inclusion criteria, such as LVEF, varied widely between the trials. Some authors considered LVEF less than 50%, while others used less than 45%, 40% or less, less than 35%, or 30% or less, and this may have affected the results. However, these studies remain as a representative population of people with DCM under optimal pharmacological medical treatment that might benefit from SCT.
Most included trials evaluated cell therapy versus non‐cell therapy, although there were some important differences in the specific modalities of cell therapy used. Most studies used BMMCs and delivered them via a single intracoronary infusion.
Trials included in this meta‐analysis evaluated a broad variety of outcomes and used diverse definitions, which represent a potential limitation of our review that precluded the possibility of evaluating the effects of SCT on clinically relevant outcomes. Beyond mortality, most of the outcomes evaluated were secondary and some were surrogate indices, such as the LVEF, LVEDV, LVESV, and plasma BNP levels.
Quality of the evidence
Overall, the studies included in this review were of a suboptimal quality, which in turn affects our assessment of the certainty of the evidence available from the analyzed results. Only one study qualified as 'low risk of bias' in all domains assessed, after we obtained further information from the study authors (Martino 2015 (MiHEART)). It is worth noting that insufficiencies were consistently detected in the quality of the reporting in most studies, whereby aspects key to assessing the risk of bias were not described in sufficient detail. It is important that medical journals that publish therapeutic research studies (RCTs) adhere to international reporting guidelines, such as CONSORT (Schulz 2010), so that the practical utility of the information published is increased. Moreover, only some, but not all, of the studies included in this review were registered in a public registry, which precluded us from obtaining complementary data or further verifying the information in this review. It is important that all RCTs are registered as a guarantee of transparency and to provide another source of information beyond any related publication. We wrote to the authors of each trial to gather additional information that might modify our conclusions about the risk of bias and the certainty of evidence. As of June 2021, only one of the 10 authors has responded (Martino 2015 (MiHEART)).
It is important to consider the variability in the numerical reporting of the results of the RCTs included in this review. In some cases, the format of the data was not useful for a meta‐analysis because of a lack of key details. In some cases, the observed differences between treatments were only supported by a P value, which does not allow their translation into a measure of the effect. In other cases, these data were only reported graphically without the corresponding raw data. The use of specialized software (GetData Graph Digitizer 2.26) allowed us to retrieve the original data from graphs in some studies. In addition, some reported data were incomplete, particularly continuous variables where the mean of the change (final versus basal) and its SD were required. This issue is particularly relevant in this review since the original studies were mostly small trials, and despite randomization there were baseline differences between the groups in most of the variables analyzed. This shortcoming has forced us to combine results in our analysis where effects have been quantified using different methods, which adds uncertainty to the results obtained. In conclusion, we consider the certainty of the evidence in this review to be limited, although this may not affect our overall conclusions.
Potential biases in the review process
This review was based on an exhaustive search for RCTs and their careful selection. During this process, we identified other potentially relevant studies but their design was not adequate for our review. For example, we identified some controlled, but not randomized, studies and their inclusion would have added risk of bias and confusion to our review.
Agreements and disagreements with other studies or reviews
In this systematic review, we focused on the effects of SCT in people with DCM based on the main outcomes of mortality and complications of the procedures, as well as other surrogate variables of secondary interest. While some earlier systematic reviews informed this approach, they are fundamentally different from our review (Jiao 2014; Lu 2016; Marquis‐Gravel 2014; Rong 2019; Wen 2018). It should be noted that none has a previous protocol record. Only one (Rong 2019) addressed the assessment of risk of bias by strictly adopting the methodology proposed by Cochrane (Higgins 2011). In general, the evaluation of the risk of bias or of the quality of the studies carried out in these reviews is very lenient, considering as adequate the simple fact of stating (although without giving details to verify them) some key characteristics of the study design. However, none of these reviews integrated quality assessment into the interpretation of their results.
Our conclusions about mortality were consistent with those of some previous systematic reviews (Rong 2019; Wen 2018), but disagree with those expressed by others (Jiao 2014; Lu 2016). All of these reviews, in addition to using eligibility criteria differing from ours (in some cases they included observational studies), did not consider the separate analysis of studies where mobilization with cytokines was used in the control group. This means that, when all the studies in the same pool are combined, the results are more favourable to SCT given the influence that the studies of Vrtovec 2011 and Vrtovec 2013a (NOGA‐DCM) had on the overall results. As previously noted, we consider that these studies deserve separate consideration given their specific characteristics regarding the modality of administration of cell therapy. Otherwise, analysing them with the rest of the studies will lead to inappropriate conclusions about the benefits of SCT in this pathology as observed by these reviews, which we believe are not supported by the available data.
A similar situation occurs when observing the effect of SCT on the surrogate variables of secondary interest that are based on cardiac volumes. All the aforementioned non‐Cochrane reviews support an increase in LVEF after SCT therapy but none of them reported an increase of 5%, a value associated with a reduction in mortality in the follow‐up of people with heart failure with reduced ejection fraction (Dunlay 2012). This is consistent with a study in which, through a sequential analysis based on two Cochrane systematic reviews where SCT was used as therapy in acute myocardial infarction and heart failure, there was no clinically relevant difference in LVEF observed after treatment (Fisher 2016b).
Other Cochrane Reviews addressing mortality reduction and the adverse effects of SCT in people with heart failure secondary to ischaemic cardiomyopathy support the possibility of success (Fisher 2015; Fisher 2016a). This suggests that the treatment in question could provide greater benefit to this group of patients. Nonetheless, no studies to date have shown that people with ischaemic heart failure have better response to cell therapy compared to people with DCM.
Authors' conclusions
Implications for practice.
This systematic review and meta‐analysis found that stem cell transplantation (SCT) appears to have little favourable effects in the treatment of people with dilated cardiomyopathy (DCM), while very low‐quality evidence reflects uncertainty regarding procedural complications. It is possible that some benefits might be obtained if SCT is administered in combination with peripheral administration of granulocyte‐colony stimulating factor (G‐CSF), but more robust information and data are needed to draw a clear conclusion. Specific aspects related to the modality of cell therapy or its delivery (type and origin of cells, dose, route of administration, number of infusions, etc.), or both, remain uncertain. There are several ongoing trials that could provide new evidence and modify our conclusions.
Implications for research.
Most studies included in this review administered bone marrow‐derived mononuclear cells. The lack of a clear benefit of SCT observed in this review may be explained by the use of adult stem cells delivered in their native state, as these cells have a limited capacity for differentiation. Future randomized trials using stem cells should take into account guiding criteria to identify the best candidates for this therapy (Blau 2019; Chien 2019; Menasché 2018), including identification of the best responders, choosing the proper cell phenotype, the best dose of and delivery route for the cells, and considering repetitive dosing.
A different stem cell phenotype, cardiopoietic cells, has been demonstrated to be safe and effective in people with ischaemic cardiomyopathy, and this approach represents a rationale to be considered for use in a broader spectrum of cardiovascular disorders including DCM (Terzic 2016).
The benefit of SCT appears to depend on left ventricular dilation, so people with an initial left ventricular end‐diastolic volume of 200 mL to 370 mL have a greater probability of a better response to SCT, while those with less than 200 mL or greater than 370 mL do not seem to respond to SCT (Bartunek 2017). Therefore, the selection of patients for SCT should include people with an LVEDV of 200 mL to 370 mL, which may be considered a predictor of beneficial cardiopoietic SCT response (Bartunek 2018). This benefit has also been demonstrated experimentally (Yamada 2020). Hence, a baseline enlargement of the left ventricle of 200 mL to 370 mL is a key factor that may influence the therapeutic responsiveness to SCT.
Establishing an evidence‐based posology paradigm is also required to ensure accurate titration of regenerative therapies and advance the science of regenerative medicine (Terzic 2017).
The use of repeat dosing of bone marrow‐derived stem cells three to six months after the initial intracoronary infusion of cells has been associated with improved clinical outcomes compared with a single treatment at two years of follow‐up (Assmus 2016). Repetitive cell dosing suggests pharmacodynamic synergism to rescue regenerative cell reserve and represents an emerging paradigm in regenerative medicine (Behfar 2016).
Beyond cell quantity per se, the delivery method is another factor that may influence the effectiveness of SCT. Intracoronary cell injections require cells to migrate into the myocardium, which may result in lower engraftment rates than intramyocardial injections (Terzic 2017). Transendocardial administration appears to be a better approach than intracoronary delivery, as injections can be targeted using electromechanical mapping to identify areas of viable and dysfunctional myocardium (Bartunek 2013). Moreover, novel catheters with incremental side holes for the transendocardial administration of stem cells can be used, because they enhance myocardial stem cell retention and improve delivery (Bartunek 2016).
In summary, new studies are needed to explore the effects of specific modalities of SCT in well‐selected specific subgroups of people with DCM who have a greater probability of obtaining a clinically relevant benefit. These studies must have a rigorous design, be transparent, with long‐term follow‐up, be focused on measuring effects on clinically relevant variables, have clear definitions of events, and provide a complete report according to the CONSORT guidelines. Until then, this intervention should remain within the scope of clinical research.
History
Protocol first published: Issue 9, 2019
Acknowledgements
We thank Cochrane Heart for their expert assistance in creating the search strategy and the provision of a template protocol.
We thank Bojan Vrtovec for his comments to the review in their role as peer reviewer, and also Aparna Kulkarni for her support as a contact editor. We are grateful to one other peer reviewer who wishes to remain anonymous.
We thank Yang Song (PhD student at the Iberoamerican Cochrane Centre) for her assistance in deciding about eligibility of some Chinese studies and for translating and performing data extraction of included studies written in Chinese.
Appendices
Appendix 1. Search strategies
CENTRAL #1 MeSH descriptor: [Cell‐ and Tissue‐Based Therapy] explode all trees #2 MeSH descriptor: [Stem Cells] explode all trees #3 MeSH descriptor: [Bone Marrow Cells] explode all trees #4 hematopoietic #5 haematopoietic #6 hemopoietic #7 haemopoietic #8 ((stem or progenitor or precursor or mesenchymal or stromal) NEAR/2 (cell or cells or marrow)) #9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 #10 MeSH descriptor: [Cardiomyopathy, Dilated] this term only #11 (dilat* NEAR/2 cardiomyop*) #12 (congestiv* NEAR/2 cardiomyop*) #13 (familial idiopath* NEAR/2 cardiomyop*) #14 DCM #15 #10 or #11 or #12 or #13 or #14 #16 #9 and #15 MEDLINE Ovid 1 exp "Cell‐ and Tissue‐Based Therapy"/ 2 exp Stem Cells/ 3 exp bone marrow cells/ 4 hematopoietic.tw. 5 haematopoietic.tw. 6 hemopoietic.tw. 7 haemopoietic.tw. 8 ((stem or progenitor or precursor or mesenchymal or stromal) adj2 (cell or cells or marrow)).tw. 9 or/1‐8 10 Cardiomyopathy, Dilated/ 11 (dilat* adj2 cardiomyop*).tw. 12 (congestiv* adj2 cardiomyop*).tw. 13 (familial idiopath* adj2 cardiomyop*).tw. 14 DCM.tw. 15 or/10‐14 16 9 and 15 17 randomized controlled trial.pt. 18 controlled clinical trial.pt. 19 randomized.ab. 20 placebo.ab. 21 drug therapy.fs. 22 randomly.ab. 23 trial.ab. 24 groups.ab. 25 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 26 exp animals/ not humans.sh. 27 25 not 26 28 16 and 27 Embase Ovid 1 exp biological therapy/ 2 exp stem cell/ 3 exp bone marrow cell/ 4 hematopoietic.tw. 5 haematopoietic.tw. 6 hemopoietic.tw. 7 haemopoietic.tw. 8 ((stem or progenitor or precursor or mesenchymal or stromal) adj2 (cell or cells or marrow)).tw. 9 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 10 congestive cardiomyopathy/ 11 (dilat* adj2 cardiomyop*).tw. 12 (congestiv* adj2 cardiomyop*).tw. 13 (familial idiopath* adj2 cardiomyop*).tw. 14 DCM.tw. 15 10 or 11 or 12 or 13 or 14 16 9 and 15 17 random$.tw. 18 factorial$.tw. 19 crossover$.tw. 20 cross over$.tw. 21 cross‐over$.tw. 22 placebo$.tw. 23 (doubl$ adj blind$).tw. 24 (singl$ adj blind$).tw. 25 assign$.tw. 26 allocat$.tw. 27 volunteer$.tw. 28 crossover procedure/ 29 double blind procedure/ 30 randomized controlled trial/ 31 single blind procedure/ 32 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 33 (animal/ or nonhuman/) not human/ 34 32 not 33 35 16 and 34
Data and analyses
Comparison 1. Stem cell therapy (SCT; any type) versus control (no intervention or sham intervention).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 All‐cause mortality | 7 | 361 | Risk Ratio (IV, Random, 95% CI) | 0.84 [0.54, 1.31] |
| 1.2 Health‐related quality of life | 5 | 272 | Std. Mean Difference (IV, Random, 95% CI) | 0.62 [0.01, 1.23] |
| 1.3 Performance status – 6‐minute walk test (m) | 5 | 230 | Mean Difference (IV, Random, 95% CI) | 70.12 [‐5.28, 145.51] |
| 1.4 Change in left ventricular ejection fraction | 8 | 353 | Mean Difference (IV, Random, 95% CI) | 5.41 [‐2.29, 13.10] |
| 1.5 Change in left ventricular end‐systolic volume | 4 | 251 | Mean Difference (IV, Random, 95% CI) | ‐30.97 [‐54.18, ‐7.75] |
| 1.6 Change in left ventricular end‐diastolic volume | 4 | 251 | Mean Difference (IV, Random, 95% CI) | ‐23.40 [‐49.74, 2.94] |
Comparison 2. Stem cell therapy (SCT; any type) versus peripheral therapy with granulocyte colony‐stimulating factor (G‐CSF).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 All‐cause mortality | 3 | 195 | Risk Ratio (IV, Random, 95% CI) | 0.46 [0.16, 1.31] |
| 2.2 Health‐related quality of life | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 4.61 [‐5.62, 14.83] |
| 2.3 Performance status – 6‐minute walk test (m) | 2 | 155 | Mean Difference (IV, Random, 95% CI) | 140.14 [119.51, 160.77] |
| 2.4 Change in left ventricular ejection volume | 3 | 182 | Mean Difference (IV, Random, 95% CI) | 6.61 [5.61, 7.62] |
| 2.5 Change in left ventricular end‐systolic volume | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐71.30 [‐150.96, 8.36] |
| 2.6 Change in left ventricular end‐diastolic volume | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐81.10 [‐175.54, 13.34] |
| 2.7 Change in plasma natriuretic peptide levels (BNP and NT‐proBNP) | 3 | 181 | Mean Difference (IV, Random, 95% CI) | ‐1632.09 [‐2180.18, ‐1083.99] |
2.2. Analysis.

Comparison 2: Stem cell therapy (SCT; any type) versus peripheral therapy with granulocyte colony‐stimulating factor (G‐CSF), Outcome 2: Health‐related quality of life
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hamshere 2015.
| Study characteristics | ||
| Methods |
Study design: RCT Parallel groups (4; arm 1: peripheral placebo; arm 2: peripheral G‐CSF; arm 3: IC serum; and arm 4: IC BMC) Single centre Country: UK Duration: 3 months (endpoint) and 1 year |
|
| Participants | 60 participants randomized (15 in each arm) Period (recruitment): July 2010 to April 2012 Inclusion criteria: diagnosis of NIDCM with no secondary cause found, LVEF < 45% (assessed by echocardiography at referral), symptoms classed as NYHA ≥ 2 and on optimal medical treatment (established for ≥ 6 months) Baseline characteristics
|
|
| Interventions |
Intervention group: IC BMC group (SCT+G‐CSF)
Details of SCT
Control group 1: peripheral placebo group (sham G‐CSF)
Control group 2: peripheral G‐CSF group (G‐CSF)
Control group 3: IC serum group (sham SCT+G‐CSF)
Details of the intervention (SCT)
Study included in Comparison 1 (STC vs IC serum) and Comparison 2 (STC vs peripheral G‐CSF). |
|
| Outcomes |
Outcomes included in review
Other outcomes reported
|
|
| Notes | Registered in ClinicalTrials.gov: NCT01302171 Funding: supported by unrestricted grants from the Heart Cells Foundation and Barts and the London Charity. Chugai Pharmaceutical donated supplies of G‐CSF and pharmaceutical costs. Funding to pay the Open Access publication charges for the article provided by the Barts Cardiovascular Biomedical Research Unit. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised using a dedicated trial software system (IHD Clinical Bishops Stortford, Hertfordshire, UK) in a 1:1:1:1 simple randomisation to one of our groups." |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "It was not possible for the study to be blinded across all four groups due to the invasive nature of the IC arm. However, participants and investigators were blinded within the IC arm between the IC BMC group and IC serum groups and in the peripheral arm between saline and G‐CSF… Data analysers were entirely masked to group assignment in both trial arms." Comparison 1 (SCT vs control), where control group consisted of sham SCT, risk of bias was low. Comparison 2 (SCT vs G‐CSF), where the control group only received a peripheral SC treatment, risk of bias was unclear due to the subjective nature of some outcomes (i.e. HRQoL). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "It was not possible for the study to be blinded across all four groups due to the invasive nature of the IC arm. However, participants and investigators were blinded within the IC arm between the IC BMC group and IC serum groups and in the peripheral arm between saline and G‐CSF… Data analysers were entirely masked to group assignment in both trial arms." Comparison 1 (SCT vs control), where control group consisted of sham SCT, risk of bias was low. Comparison 2 (SCT vs G‐CSF), where the control group only received a peripheral SC treatment, risk of bias was unclear due to the subjective nature of some outcomes (i.e. HRQoL). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 3 months (primary endpoint), 1 participant in each peripheral SC group was lost to follow‐up. At 1 year, 13 participants were available in each study arm (i.e. 2 losses per arm). Paper stated that participants who did not reach the primary and secondary endpoints were not included in all analyses. However, due to the equal distribution of missing participants, we deemed attrition bias risk low. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in ClinicalTrials.gov were latter reported in the paper. |
Hare 2017 (POSEIDON‐DCM).
| Study characteristics | ||
| Methods |
Study design: RCT Parallel groups (2; arm 1: autologous stem cells; arm 2: allogeneic stem cells) Single centre Country: USA Duration: 30 days (primary analysis: safety), and 12 months (secondary outcomes) |
|
| Participants | 37 participants randomized, but 3 did not receive the intervention leaving 34 valid cases (16 in arm 1 and 18 in arm 2). Period (recruitment): December 2011 to July 2015. Inclusion criteria: eligibility determined after confirmation of NIDCM diagnosis with an EF < 40% and either a left ventricular end‐diastolic diameter > 5.9 cm in males or > 5.6 cm in females, or an LV end‐diastolic volume index > 125 mL/m². Baseline characteristics
|
|
| Interventions | Intervention SCT arm 1 (19 participants): autologous‐hMSCs
Intervention SCT arm 2 (18 participants): allogeneic‐hMSCs
Details of SCT
Study included in Comparison 3 (STC vs STC) |
|
| Outcomes |
Outcomes included in review
Other outcomes reported
|
|
| Notes | Registered in ClinicalTrials.gov: NCT01392625 Funding: NIH grant 5R01HL110737 This was conceived as a pilot study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This was an open‐label trial. However, it was unclear how this could affect the way participants were managed since all received an active form of treatment consisting of SCT (whether allogeneic or autologous stem cells). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Although this was an open‐label study, all data analysis was masked to those assessing all study endpoints." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants in arm 2 were lost to follow‐up for the primary analysis at 3 months. 5 participants were lost to follow‐up at 1 year (arm 1: 3 vs arm 2: 2), with no clear reasons provided except for 2 deaths in arm 2 (allogeneic‐hMSCs). Unclear which was the population of analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in ClinicalTrials.gov were later reported in the paper. |
Henry 2014.
| Study characteristics | ||
| Methods |
Study design: RCT Henry 2014 consisted of two phase IIA trials (Catheter‐DCM and IMPACT‐DCM) that ran in parallel, both assessing the safety and efficacy of ixmyelocel‐T administered via mini‐thoracotomy (IMPACT‐DCM) or intramyocardial catheter injections (Catheter‐DCM). Parallel groups (2) Multicentre Country: USA Duration: 12 months for efficacy and 24 months for safety |
|
| Participants |
Catheter‐DCM 22 participants randomized in a 2:1 ratio (15 SCT and 7 control). Period (recruitment): April 2010 to March 2013. IMPACT‐DCM 39 participants randomized in a 3:1 ratio (25 SCT and 14 control). (Note: the allocation does not seem to correspond to a 3:1 ratio as stated by the authors.) Period (recruitment): November 2008 to September 2012 Inclusion criteria: high‐risk population with ischaemic or NIDCM based on the following criteria: WHO definitions and classifications, symptomatic HF, NYHA class III or IV, LVEF ≤ 30% by echocardiogram, and ineligibility for percutaneous or surgical revascularization. Results provided separately for NIDCM subgroup (18 participants in the intervention arm and 11 in control group). Baseline characteristics(both studies, only participants with NIDCM)
|
|
| Interventions |
Intervention group: STC Details of SCT
Control group: standard of care
Study included in Comparison 1 (STC vs control). |
|
| Outcomes |
Outcomes included in review
Other outcomes reported in protocol
|
|
| Notes |
Catheter‐DCM trial registered in ClinicalTrials.gov: NCT01020968 IMPACT‐DCM trial registered in ClinicalTrials.gov: NCT00765518 Funding: Aastrom Biosciences, Inc, Ann Arbor, MI, USA. Both studies reported the results in one publication (Henry TD, Traverse JH, Hammon BL, East CA, Bruckner B, Remmers AE, et al. Safety and efficacy of ixmyelocel‐T: an expanded, autologous multi‐cellular therapy, in DCM. Circulation Research 2014;115(8):730‐7). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A computer‐generated randomization schedule was used to assign patients within each stratum." |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "MACE were adjudicated in a blinded fashion by the Principal Investigators. In addition, changes from baseline in LVEF, LV dimensions and volumes were assessed by a blinded assessor." |
| Incomplete outcome data (attrition bias) All outcomes | High risk |
Catheter‐DCM: 1 participant in each group withdrew before 12 months (unclear if they were participants with ischaemic or NIDCM). There was no imputation for missing data. IMPACT‐DCM: 3/25 (12%) participants in the intervention group and 7/14 (50%) from the control group withdrew before 12 months (unclear if they were participants with ischaemic or NIDCM). There was no imputation for missing data. The distribution of withdrawals, greater in the control group, possibly biased the results against SCT. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in ClinicalTrials.gov were later reported in the paper. |
Martino 2015 (MiHEART).
| Study characteristics | ||
| Methods |
Study design: RCT Parallel groups (2) Double‐blind Multicentre (11) Country: Brazil Duration: 6 months (endpoint) and 12 months |
|
| Participants | 160 participants randomized in a 1:1 ratio (82 SCT and 78 control) Period (recruitment): January 2006 to December 2012 Inclusion criteria: previous diagnosis of HF according to Framingham criteria; HF symptoms for ≥ 1 year, with aetiological diagnosis of NIDCM according to WHO criteria; aged 18–75 years; NYHA functional class III or IV; appropriate drug therapy following the 4‐week optimization period and an echocardiogram showing EF < 35%. Baseline characteristics
|
|
| Interventions |
Intervention group: STC Details of SCT
Control group: placebo (sham SCT)
Study included in Comparison 1 (STC vs control). |
|
| Outcomes |
Outcomes included in the review
Other outcomes reported
|
|
| Notes | Registered in ClinicalTrials.gov: NCT00333827 Funding: Brazilian Ministry of Health, through the Brazilian Agency for Innovation (FINEP) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Software was created in R version 1.9.0 to specifically generate the randomization sequence for the study." In addition, blocks of variable size (2, 4 or 6 participants) were used. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised after bone marrow aspiration was performed." Information provided by the contacting author at our request: "Once randomised, the group allocation was available online (accessible by login and password) just for the person in charge of preparing the mononuclear fraction in each centre. This person then prepared the syringes containing either cells or placebo. Contents of the syringe could not be seen because it was involved with black insufilm. The PI [principal investigator] and the interventional cardiologist at each center were blinded to the randomisation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind trial. Quote: "According to the assigned group, darkened syringes containing the mononuclear cell fraction or saline with 5% autologous serum are then prepared and sent for implant into the patients." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind trial. Quote: "According to the assigned group, darkened syringes containing the mononuclear cell fraction or saline with 5% autologous serum are then prepared and sent for implant into the patients." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 45 participants (28%) (21 (25.6%) in the intervention group and 24 (30.8%) in the control group) abandoned the study or withdraw the consent. Used an intention‐to‐treat analysis with imputation of missing values using the "worst value recorded for that variable." |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in ClinicalTrials.gov were later reported in the paper. |
Sant'Anna 2014 (INTRACELL).
| Study characteristics | ||
| Methods |
Study design: RCT Parallel groups (2) Open label Single centre Country: Brazil Duration: 12 months |
|
| Participants | 30 participants randomized in a 2:1 ratio (20 SCT and 10 control) Period (recruitment): recruitment started in 2005 Inclusion criteria: people with HF; LVEF < 35% by echocardiogram; NYHA functional class III or IV, despite full medical treatment; aged 20–65 years; diagnosis of NIDCM for ≥ 12 months before enrolment. Baseline characteristics
|
|
| Interventions |
Intervention group: STC Details of SCT
Control group
Study included in Comparison 1 (STC vs control) |
|
| Outcomes |
Outcomes included in review
Other outcomes reported
|
|
| Notes | Registered in ClinicalTrials.gov: NCT00743639 Funding: financial support from Brazilian government agencies National Council for Scientific and Technological Development, e Committee for Postgraduate Courses in Higher Education, Ministry of Health, and FAPERGS |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomized using computer software for simple randomization. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "In the first three months of follow‐up, 25% (5 out of 20) of the patients from the BMMC group had either died or been withdrawn from the study, resulting in a decrease in the number of treated cases available for late follow‐up. Since those patients had a lower mean ejection fraction than the whole group (18.26% vs 21.75%), we excluded them from comparative analysis, in order to avoid overestimation of treatment effect. In other words, outcome analysis was performed in as‐treated basis, not as intention‐to‐treat." Contrary to what the authors commented, we consider that the exclusion of these participants from the SCT group poses a risk of bias in favour of treatment. |
| Selective reporting (reporting bias) | High risk | ClinicalTrials.gov register only specifies the primary endpoint (increase of the ejection function of the LV) but not the secondary outcomes. |
Seth 2010 (ABCD).
| Study characteristics | ||
| Methods |
Study design: RCT Parallel groups (2) Open label Single centre Country: India Duration: 3 years (mean follow‐up 28 months) |
|
| Participants | 85 participants randomized in a 1:1 ratio (45 SCT and 40 control), but 81 analyzed (41 SCT and 40 control). Period (recruitment): not reported Inclusion criteria: aged 15–70 years with idiopathic DCM with normal coronary arteries, EF < 40%, and no other severe comorbidities (e.g. chronic renal failure, liver failure, or any malignancy). Baseline characteristics
|
|
| Interventions |
Intervention group: STC Details of SCT
Control group: control
Study included in Comparison 1 (STC vs control). |
|
| Outcomes |
Outcomes included in the review
|
|
| Notes | Not registered on ClinicalTrials.gov. Funding: research funds from the All India Institute of Medical Sciences (AIIMS) under the Stem Cell Research Program. Study reported in a brief format as two research correspondence papers. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2 participants in the treatment arm were lost to follow‐up, and another 2 participants underwent biventricular pacing. These participants were excluded from the analysis, as well as 1 participant in the control group. |
| Selective reporting (reporting bias) | Unclear risk | Study was not registered on ClinicalTrials.gov. |
Vrtovec 2011.
| Study characteristics | ||
| Methods |
Study design: RCT Parallel groups (2) Open label Single centre Country: Slovenia Duration: 1 year |
|
| Participants | 55 participants randomized in a 1:1 ratio (28 SCT and 27 control) Period (recruitment): January 2008 to September 2008 Inclusion criteria: aged ≥ 18 years, diagnosis of DCM, optimal medical management for ≥ 6 months, marked ventricular systolic dysfunction (LVEF < 30%), and NYHA functional class III or IV for ≥ 3 months before referral. DCM defined based on the absence of any stenotic lesions on coronary angiography, no congenital heart disease, no primary valve disease on echocardiography, and no history of hypertension or alcohol abuse. Baseline characteristics
|
|
| Interventions |
Intervention group: STC Details of SCT
Control group: control
Study included in Comparison 2 (STC vs G‐CSF) |
|
| Outcomes |
Outcomes included in the review
Other outcomes reported
|
|
| Notes | Registered in ClinicalTrials.gov: NCT00629018 Funding: not reported Study conceived as a pilot study. There are some inconsistencies between the information provided by ClinicalTrials.gov and the final publication regarding the total number of participants (110 in ClinicalTrials.gov vs 55 in publication), the time point for the outcomes (5 years in ClinicalTrials.gov vs 1 year in publication) and some of the outcomes that were planned and finally reported. Besides, the results posted in ClinicalTrials.gov seem to correspond to Vrtovec 2013a (NOGA‐DCM), a study (110 participants) performed by the same author and team. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Echocardiography data were recorded and analyzed at 1 year by an independent echocardiographer who was blinded to treatment allocation. Similarly, 6MWT was performed by a blinded observer. These were the primary outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In the intervention group, 26/28 participants were analysed (2 deaths) whereas in the control group the number of participants analyzed was 19/27 (3 participants died and 5 underwent heart transplantation before 1 year). Except for the combined endpoint of mortality and heart transplantation, in all other outcomes used the 'last observation carried forward' strategy to include participants who died or received transplantation. Due to the distribution of participants who did not reach 1 year of follow‐up, we assumed that the effect estimates for SCT were likely underestimated to some extent. |
| Selective reporting (reporting bias) | High risk | There are some inconsistencies between the trial register (NCT) and the final publication regarding the total number of participants (110 in ClinicalTrials.gov vs 55 in publication), the time point for the outcomes (5 years in ClinicalTrials.gov vs 1 year in publication) and some of the outcomes that were planned and finally reported. Study was a pilot of Vrtovec 2013a (NOGA‐DCM). High suspicion that participants reported in Vrtovec 2011 were also included in the Vrtovec 2013a (NOGA‐DCM) analysis. |
Vrtovec 2013a (NOGA‐DCM).
| Study characteristics | ||
| Methods |
Study design: RCT Parallel groups (2) Open label Single centre Country: Slovenia Duration: 5 years |
|
| Participants | 110 participants randomized in a 1:1 ratio (55 SCT and 55 control) Period (recruitment): January 2005 to May 2006 Inclusion criteria: aged 18–65 years, diagnosis of NIDCM according to European Society of Cardiology position statement, optimal medical management for ≥ 6 months, marked ventricular systolic dysfunction (LVEF < 30%), and NYHA functional class III for ≥ 3 months before referral Baseline characteristics
|
|
| Interventions |
Intervention group: STC Details of SCT
Control group: control
Study included in Comparison 2 (STC vs G‐CSF) |
|
| Outcomes |
Outcomes included in the review
Other outcomes reported
|
|
| Notes | Registered in ClinicalTrials.gov: NCT01350310 Funding: Ministry of Health, Republic of Slovenia, Tertiary Care Scientific grants (20110130 and 20100368), Slovenian Research Agency, Slovenian‐US Collaborative Research grant (430‐11/2009), and Stanford Cardiovascular Institute Seed grants (JCW, FH). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The echocardiography data and 6‐minute walk test were performed by a blinded observer." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study did not specify the number of losses or provide a flow chart. Unclear if they performed an intention‐to‐treat analysis (most probably not). At 5 years, 8 participants in SCT arm and 19 in control arm had died. Unclear how many participants were analysed at 5 years for the remaining outcomes. |
| Selective reporting (reporting bias) | High risk | There were some inconsistencies between the ClinicalTrials.gov register and final publication regarding the total number of participants (110 in ClinicalTrials.gov vs 55 in publication), the time point for the outcomes (5 years in ClinicalTrials.gov vs 1 year in publication) and some of the outcomes that were planned and finally reported. Study performed after a previous pilot trial (Vrtovec 2011). High suspicion that participants reported in Vrtovec 2011 were also included in the Vrtovec 2013a (NOGA‐DCM) analysis. |
Vrtovec 2013b.
| Study characteristics | ||
| Methods |
Study design: RCT Parallel groups (2) Open label Single centre Country: Slovenia Duration: 6 months |
|
| Participants | 40 participants randomized, in a 1:1 ratio (20 IC and 20 TE delivery) Period (recruitment): January 2011 to January 2012 Inclusion criteria: aged 18–65 years, diagnosis of DCM according to European Society of Cardiology position statement, optimal medical management for ≥ 6 months, LVEF < 40%, and NYHA functional class III for ≥ 3 months before referral. Baseline characteristics
|
|
| Interventions |
Intervention group 1: IC delivery group (20 participants)
Intervention group 2: transendocardial delivery group (20 participants)
Details of SCT (same in both study groups)
Study included in Comparison 3 (STC vs SCT) |
|
| Outcomes |
Outcomes included in review
Other outcomes reported in protocol
|
|
| Notes | Registered in ClinicalTrials.gov: NCT01350310. Funding: Ministry of Health (Republic of Slovenia) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated in 1:1 ratio." No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The echocardiogram data were recorded and analysed by an independent echosonographer who was blinded to the intervention allocated. Change in LVEF from baseline was the primary outcome. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote from flow chart: "All patients were followed for 6 months." |
| Selective reporting (reporting bias) | Low risk | Paper reported all outcomes specified in ClinicalTrials.gov. |
Vrtovec 2018 (REMEDIUM).
| Study characteristics | ||
| Methods |
Study design: RCT Parallel groups (2; arm 1: repetitive doses, arm 2: single dose) Single centre Open label Country: Slovenia Duration: 12 months |
|
| Participants | 60 participants randomized, ratio 1:1 (30 arm 1; 30 arm 2) Period (recruitment): January 2014 to September 2017 Inclusion criteria: aged 18–70 years, diagnosis of DCM according to European Society of Cardiology position statement, optimal medical management for ≥ 3 months, LVEF < 40%, and NYHA functional class III for ≥ 3 months before referral Baseline characteristics
|
|
| Interventions |
Intervention group 1: repetitive doses
Intervention group 2: single dose
Details of SCT
Study included in Comparison 3 (STC vs SCT) |
|
| Outcomes |
Outcomes included in review
Other outcomes reported
|
|
| Notes | Registered in ClinicalTrials.gov: NCT02248532 Funding: Slovenian Research Agency grant # J3‐7312‐0381. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly allocated in 1:1 ratio." No further details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Echocardiography data recorded and analyzed at end of study by an independent echocardiographer who was blinded to the participant's treatment status. 6MWT assessed by a blinded outcome assessor. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants were lost to follow‐up at 1 year (2 heart transplants and 1 death) (1 in arm 1; 2 in arm 2). |
| Selective reporting (reporting bias) | Low risk | No changes from protocol as reported in ClinicalTrials.gov. |
Wang 2006.
| Study characteristics | ||
| Methods |
Study design: RCT Parallel groups (2) Open label Single centre Country: China Duration: 6 months |
|
| Participants | 24 participants randomized in a 1:1 ratio (12 SCT and 12 control) Period (recruitment): January 2002 to October 2004 Inclusion criteria: aged ≤ 70 years; DCM according to the WHO/International Society and Federation of Cardiology Task Force definition; LVEF < 45% Exclusion criteria: people with secondary myocardiopathy Baseline characteristics
|
|
| Interventions |
Intervention group: STC Details of SCT
Control group: placebo
Study included in Comparison 1 (STC vs control) |
|
| Outcomes |
Outcomes included in review
Other outcomes reported
|
|
| Notes | Not registered at ClinicalTrials.gov Study published in Chinese in short format. Funding: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled (control group received same volume of saline). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not mentioned if the outcome assessors were blinded but the use of a sham procedure as a placebo suggests that risk of detection bias was low. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No lost to follow‐up or flow chart reported. |
| Selective reporting (reporting bias) | Unclear risk | No protocol reported. |
Wu 2010.
| Study characteristics | ||
| Methods |
Study design: RCT Parallel groups (2) Open label Single centre Country: China Duration: 24 months |
|
| Participants | 38 participants (20 SCT vs 18 control) Period (recruitment): March 2004 to October 2006 Inclusion criteria: diagnosis of DCM by clinical manifestations, echocardiography, and coronary angiography, that excluded coronary atherosclerosis; LVEF < 50% Exclusion criteria: people with secondary DCM. Baseline characteristics
|
|
| Interventions |
Intervention group: STC Details of SCT
Control group:
Study included in Comparison 1 (STC vs control) |
|
| Outcomes |
Outcomes included in the review
Other outcomes reported
Study did not specify which was the primary outcome. |
|
| Notes | Not registered at ClinicalTrials.gov Study published in Chinese language. Funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly assigned at the intervention and control group." No further details are provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Apparently, there were no losses or withdrawals. No flow chart provided. |
| Selective reporting (reporting bias) | Unclear risk | No protocol reported. |
Xiao 2017.
| Study characteristics | ||
| Methods |
Study design: RCT Parallel groups (3; arm 1: IC administration of BMMC, arm 2: IC administration of BMSC, and arm 3: equal volume normal saline) Open label Single centre Country: China Duration: 12 months |
|
| Participants | 55 participants (BMMC arm 16, BMSC arm 17, control arm 20) Period (recruitment): March 2010 through June 2011 Inclusion criteria: people with DCM and reduced LVEF < 40%, aged 18–75 years, NYHA functional class II–IV, proportion of fixed defects < 40%, normal coronary arteries, and signed informed consent Exclusion criteria: coronary artery disease based on coronary angiography prior to cell delivery, ventricular arrhythmias, and any comorbidities with an impact on survival. Baseline characteristics
|
|
| Interventions |
Intervention group 1: BMMC
Intervention group 2: BMSC
Control group: placebo
Study included in Comparison 1 (STC vs control) and Comparison 3 (STC vs SCT) |
|
| Outcomes |
Outcomes included in review
Other outcomes reported
|
|
| Notes | Not registered at ClinicalTrials.gov. Funding: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Control group consisted of sham SCT. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Echocardiography examinations were performed at baseline and the 3‐month and 12‐month follow‐ups by an independent echocardiographer who was blinded to the participant grouping. Similarly, NYHA data were accessed by a blinded observer according to the standard clinical protocol." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "One patient in the BMMC group and 1 in the BMSC group were lost to follow‐up, and 3 cases were lost to follow‐up in the control group." According to Table II, at 3 months, 15 (1 lost to follow‐up) in BMMC arm, 16 (1 lost to follow‐up) in BMSC arm, and 18 (2 lost to follow‐up) in placebo arm were analysed, and at 12 months there were 14 (2 lost to follow‐up) in BMMC arm, 16 (1 lost to follow‐up) in BMSC arm, and 15 (5 lost to follow‐up) in placebo arm. The paper also stated that data from participants who were lost during follow‐up were excluded from the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not reported. |
6MWT: 6‐minute walk test; BMA: bone marrow aspiration; BMC: bone marrow cell; BMMC: bone marrow mononuclear cell; BNP: brain natriuretic peptide; CK: creatine kinase; DCM: dilated cardiomyopathy; EF: ejection fraction; EQ5D: European Quality of Life‐5 Dimensions; FDG‐PET: fluorodeoxyglucose‐positron emission tomography; G‐CSF: granulocyte‐colony stimulating factor; h: hour; HF: heart failure; hMSC: human mesenchymal stem cell; HRQoL: health‐related quality of life; IC: intracoronary; KCCQ: Kansas City Cardiomyopathy Questionnaire; LV: left ventricle; LVEDD: left ventricular end‐diastolic diameter; LVEDV: left ventricular end‐diastolic volume; LVESV: left ventricular end‐systolic volume; LVEF: left ventricular ejection fraction; MACE: major adverse cardiovascular event; min: minute; MLHFQ: Minnesota Living with Heart Failure Questionnaire; mph: miles per hour; NIDCM: non‐ischaemic dilated cardiomyopathy; NT‐proBNP: N‐terminal pro‐brain natriuretic peptide; NYHA: New York Heart Association; RCT: randomized controlled trial; SC: subcutaneous; SD: standard deviation; VE/VCO2: ventilation/volume of exhaled carbon dioxide; VO2peak: peak oxygen uptake; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bartolucci (INNOVA) 2015 | Design: non‐randomized prospective controlled trial (participants assigned in order of entry into experimental or control group). Population: included a mixed population (13/23 idiopathic DCM in experimental arm and 10/23 ischaemic DCM in control arm). No specific data for participants with non‐ischaemic DCM provided. |
| Bartolucci (RIMECARD) 2017 | Population: included a mixed population (quote: "ischaemic cardiomyopathy was the predominant pathogenesis of HFrEF [heart failure with reduced ejection fraction] (21 patients, 70%"). No specific data for participants with non‐ischaemic DCM provided. |
| Bocchi 2010 | Design: although the study randomized participants to 2 different groups of SCT (immediate or deferred administration of G‐SCF), the results presented in the paper compared SCT vs a non‐randomized external control group. |
| Butler 2017 | Design: placebo‐controlled cross‐over randomized trial. |
| Chen 2008 | Design: not an RCT (non‐randomized controlled trial). |
| Fischer‐Rasokat 2009 | Design: not an RCT (prospective single cohort study). Corresponded to the pilot study (TOPCARE‐DCM). |
| Huang 2006 | Design: not an RCT (non‐randomized controlled trial). |
| Kakuchaya 2011 | Abstract only (no full paper was even though abstract published in 2011). |
| Miyagawa 2017 | Wong population. |
| NCT02256501 | Population: children. |
| Perin (REVASCOR) 2015 | Population: included a mixed population (participants had either non‐ischaemic or ischaemic cardiomyopathy; more participants with ischaemic cardiomyopathy (77%). No specific data for participants for non‐ischaemic DCM provided. |
| Premer 2015 | Wrong population. |
| Tompkins 2018 | Wrong design. |
| Xiao 2012a | Population: people with ischaemic DCM. |
| Xiao 2012b | Population: people with ischaemic DCM. |
| Yau 2019 | People undergoing a left ventricular assist device implant |
| Zemljic 2017 | Mixed population; wrong outcomes. |
DCM: dilated cardiomyopathy; G‐SCF: granulocyte‐colony stimulating factor; RCT: randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
NCT01957826.
| Study name | Mesenchymal stem cells for idiopathic dilated cardiomyopathy (MYOCYTE) |
| Methods | RCT |
| Participants | Adults with dilated idiopathic cardiomyopathy |
| Interventions |
SCT group: transendocardial injection of 30–40 million bone marrow‐derived MSCs with the NOGA XPTM platform. 15 injections in the anterior wall of the left ventricle. Control: placebo (transendocardial injection of placebo solution) |
| Outcomes |
|
| Starting date | Completed but not yet published |
| Contact information | Contact: Dr Ricardo Sanz |
| Notes | Completed in 2018 and not yet published. It is expected to publish final results during 2020 (email contact with principal investigator: Dr Sanz). |
NCT02033278.
| Study name | Infusion intracoronary of mononuclear autologous adult no expanded stem cells of bone marrow on functional recovery in participants with idiopathic dilated cardiomyopathy and heart failure |
| Methods | Placebo‐controlled, double‐blind phase IIb RCT Follow‐up: 24 months |
| Participants | Adults aged 18–70 years with established non‐ischaemic idiopathic DCM (minimum evolution since diagnosis of 6 months) Expected size: 51 participants |
| Interventions |
SCT group: infusion of autologous mononuclear bone marrow cells plus conventional medical treatment (as indicated by clinician) Control: placebo infusion plus conventional medical treatment (as indicated by clinician) |
| Outcomes | Changes in ventricular function measured angiographically Degree of clinical improvement based on the absence of MACE during follow‐up Clinical and analytical progress (NYHA grade and BNP) Time of evolution since diagnosis of idiopathic DCM prior to study entry Functional recovery measured with ergometry Echocardiography and electrocardiography variables |
| Starting date | |
| Contact information | |
| Notes | Sponsor: Andalusian Initiative for Advanced Therapies ‐ Fundación Pública Andaluza Progreso y Salud ONGOING (recruiting March 2018). Expected date of finalization: 2022 |
NCT02293603.
| Study name | Dilated cardiomYopathy iNtervention With Allogeneic MyocardIally‐regenerative Cells (DYNAMIC) |
| Methods |
Phase Ia: open‐label, single‐arm, dose escalation of allogeneic cardiosphere‐derived cells (14 participants) Phase Ib: double‐blind, randomized, placebo‐controlled study design (28 participants) |
| Participants | Major inclusion criteria: DCM with LVEF ≤ 35% as determined by a historical TTE within the previous 6 months; NYHA class III or ambulatory class IV heart failure; use of evidence based medical‐therapy (beta‐blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, aldosterone antagonist) and with or without device‐therapy (implantable cardioverter‐defibrillator or cardiac resynchronizing therapy), in accordance with the American College of Cardiology/American Heart Association guidelines for the management of heart failure, for ≥ 3 months prior to enrolment or documented contraindication or intolerance or participant preference Note: unclear if the study includes people with ischaemic cardiomyopathy, or a mixed population. |
| Interventions |
SCT group: allogeneic cardiosphere‐derived cells Intracoronary delivery of CAP‐1002 Control: placebo |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes | Sponsor: Capricor Inc. Ongoing (not recruiting). Expected date of finalization: 2020. |
NCT03797092.
| Study name | Stem cell therapy in non‐ischaemic non‐treatable dilated cardiomyopathies II: a pilot study |
| Methods | Open (2:1) RCT Follow‐up: 6 months |
| Participants | Adults aged 30–80 years with non‐ischaemic DCM |
| Interventions |
SCT group: allogeneic adipose‐derived stromal cells (CSCC_ASC) Control: no treatment |
| Outcomes | Left ventricle end‐systolic volume (echocardiography) Allogeneic antibodies Changes in LVEF Change in echocardiogram‐measured global myocardial mass NYHA Kansas City Cardiomyopathy Questionnaire EQ‐5D3L Questionnaire 6‐minute walking test |
| Starting date | |
| Contact information | Principal investigator: Jens Kastrup, MD, DMSc |
| Notes | Study completion date: 1 September 2021 |
AE: adverse event; BNP: brain natriuretic peptide; DCM: dilated cardiomyopathy; EQ‐5D3L: EQ‐5D three level version; LVEF: left ventricular ejection fraction; MACE: major adverse cardiovascular event; MRI: magnetic resonance imaging; MSC: mesenchymal stem cell; NYHA: New York Heart Association; RCT: randomized controlled trial; SAE: serious adverse event; SCT: stem cell therapy; SPECT: single‐photon emission computed tomography; VO2: oxygen uptake.
Differences between protocol and review
We made the following changes from the protocol (Diaz‐Navarro 2019).
Search
We did not handsearch the conference abstracts from relevant heart or stem cell (or both) conferences (American Heart Association, European Society of Cardiology, and the International Society of Stem Cell Research, from 2000 onwards). Given the specific nature of cell therapy and the fact that it is a relatively recent intervention over time, we believe that our search in bibliographic databases and clinical trial registries allowed us to identify all relevant studies on this topic.
Study selection
Although we planned not to exclude studies based on publication status, we decided to exclude one study (two references) as it was published in abstract format only, and although it appeared to meet the inclusion criteria, it did not contain sufficient data for inclusion. It was published in 2011 and there has been no full paper published. We therefore decided to exclude it from the review.
We have renamed the outcome Change in blood natriuretic peptide level by the more inclusive Change in plasma natriuretic peptide levels (brain natriuretic peptide [BNP] and N‐terminal pro b‐type natriuretic peptide [NT‐proBNP]). The reason for this is the similar clinical implications of both measures.
Data synthesis
We assessed the intervention effects using random‐effects meta‐analyses due to the high heterogeneity for most outcomes. In those outcomes where there was no heterogeneity (mortality, left ventricular end‐systolic volume, and left ventricular end‐diastolic volume), we also used this method because it was more conservative.
Subgroup analyses
We did not perform subgroup analyses to explore the role of potential effect modifiers related with some clinical characteristics of participants or specific features related with SCT. The limited number of studies available within each comparison, as well as the very low number of studies (fewer than three) that were available in some categories precluded us from doing this as we considered these analyses were not informative.
Dealing with missing data
As this was not a major issue in this review, we did not explore the impact of including studies with high rate of missing data using a sensitivity analysis, using the software SAMURAI (Kim 2014), available in R software (R).
Sensitivity analysis
We did not carry out a sensitivity analysis excluding trials with high risk of bias from the analysis.
Summary of findings table
We created summary of findings tables for comparison 1 (stem cell therapy versus control (no intervention or sham)) and 2 (stem cell therapy versus granulocyte‐colony stimulating factor), but not for comparison 3 different types or delivery modalities of stem cell therapies against each other). This comparison included four randomized controlled trials that specifically compared different aspects related to stem cell transplantation delivery that could not be combined.
Contributions of authors
RD: draft of the initial protocol (Diaz‐Navarro 2019); arbiter at the screening and risk of bias phase; discussion of the analyses; draft of the review; approval of the final manuscript.
GU: draft of the initial protocol (Diaz‐Navarro 2019); screening of the searches; data extraction; risk of bias assessment; statistical analysis; certainty of the evidence; draft of the review; approval of the final manuscript.
JC: approval of the final manuscript.
DP: screening of the searches; data extraction; risk of bias assessment; discussion of the analyses; certainty of the evidence; summary of findings tables; PRISMA flow chart; draft of the review; approval of the final manuscript.
FV: screening of the searches; data extraction; risk of bias assessment; discussion of the analyses; certainty of the evidence; summary of findings tables; PRISMA flow chart; draft of the review; approval of the final manuscript.
RAD: data extraction; risk of bias assessment; statistical analysis; certainty of the evidence; draft of the review; approval of the final manuscript.
SB: discussion of the analyses; draft of the review; approval of the final manuscript.
GR: assistance in the searches for additional studies; approval of the final manuscript.
EM: draft of the initial protocol (Diaz‐Navarro 2019); arbiter at the screening and risk of bias phase; statistical analyses; certainty of the evidence; summary of findings tables; draft of the review; approval of the final manuscript.
Sources of support
Internal sources
New Source of support, UK
External sources
-
NIHR, UK
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Heart Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service, or the Department of Health and Social Care.
-
This project was supported by the CONICYT (grant num. MEC80170060), Chile
The contribution of Dr Gerard Urrútia to this review was supported by a grant by the CONICYT (government of Chile).
Declarations of interest
The performance of this review was free of any real or perceived bias introduced by receipt of any benefit in cash or kind, or any subsidy derived from any source that may have or be perceived to have an interest in the outcomes of the review.
RD: none.
GU: has participated in several educational activities (workshops) and has provided consultancy to several laboratories, but has not been paid for it (money was paid to the institution where he works: Clinical Epidemiology Service, at the Hospital de la Santa Creu i Sant Pau).
JC: has consulted widely with other healthcare companies (Amgen, Novartis, Stealth Biopharmaceuticals, Bayer, Philips, Servier, Pharma Nord, Pharmacosmos, Vifor, HeartFelt Technologies, PharmaIn and Viscardia) but not on the topic of stem cells. He also received payments from the European Society of Cardiology for the development of educational presentations.
DP: none.
FV: none.
RAD: none.
SB: none.
GR: none.
EM: none.
New
References
References to studies included in this review
Hamshere 2015 {published data only}
- Arnous S, Mozid A, Mathur A. The Bone Marrow Derived Adult Stem Cells for Dilated Cardiomyopathy (REGENERATE-DCM) trial: study design. Regenerative Medicine 2011;6(4):525-33. [DOI] [PubMed] [Google Scholar]
- EUCTR2009-013112-12-GB. Randomised controlled trial to compare the effects of G-CSF(Granocyte™) and autologous bone marrow progenitor cells on quality of life and left ventricular function In patients with idiopathic dilated cardiomyopathy – REGENERATE-DCM. ddrare.nibiohn.go.jp/cgi-bin/disease_who_e.cgi?id=57 (accessed prior to 1 July 2021).
- Hamshere S, Arnous S, Choudhury T, Choudry F, Mozid A, Yeo C, et al. Randomized trial of combination cytokine and adult autologous bone marrow progenitor cell administration in patients with non-ischaemic dilated cardiomyopathy: the REGENERATE-DCM clinical trial. European Heart Journal 2015;36(44):3061-9. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01302171. Bone Marrow Derived Adult Stem Cells for Dilated Cardiomyopathy (REGEN-DCM). clinicaltrials.gov/ct2/show/NCT01302171 (first received 24 February 2011).
Hare 2017 (POSEIDON‐DCM) {published data only}
- Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. Journal of the American College of Cardiology 2017;69(5):526-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mushtaq M, DiFede DL, Golpanian S, Khan A, Gomes SA, Mendizabal A, et al. Rationale and design of the Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis in Dilated Cardiomyopathy (the POSEIDON-DCM study): a phase I/II, randomized pilot study of the comparative safety and efficacy of transendocardial injection of autologous mesenchymal stem cell vs. allogeneic mesenchymal stem cells in patients with non-ischemic dilated cardiomyopathy. Journal of Cardiovascular Translational Research 2014;7(9):769-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramireddy A, Brodt CR, Mendizabal AM, DiFede DL, Healy C, Goyal V, et al. Effects of transendocardial stem cell injection on ventricular proarrhythmia in patients with ischemic cardiomyopathy: results from the POSEIDON and TAC-HFT Trials. Stem Cells Translational Medicine 2017;6:1366-72. [DOI: 10.1002/sctm.16-0328] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henry 2014 {published data only}
- Bruckner BA, Ghodsizad A, Hamman BL, Bull DA, Lattouf OM, Smedira NG, et al. IMPACT-DCM: a randomized, controlled, multi-center phase II trial utilizing expanded autologous bone marrow as sole therapy for dilated cardiomyopathy study update. Journal of Heart and Lung Transplantation 2011;30:79. [Google Scholar]
- Bunge RR, Patel AN, Hamman BL, Lattouf OM, Smedira NG, Bartel RL, et al. Safety and efficacy of ixmyelocel-t, an expanded patient-specific mixed cell product, in dilated cardiomyopathy (IMPACT-DCM). Journal of Heart and Lung Transplantation 2012;31:72-3. [Google Scholar]
- Henry TD, Traverse JH, Hammon BL, East CA, Bruckner B, Remmers AE, et al. Safety and efficacy of ixmyelocel-T: an expanded, autologous multi-cellular therapy, in dilated cardiomyopathy. Circulation Research 2014;115(8):730-7. [DOI: 10.1161/CIRCRESAHA.115.304554] [DOI] [PubMed] [Google Scholar]
- Patel AN, Hamman BL, Bruckner B, Lattouf OM, Smedira NG, East C, et al. Safety and efficacy of ixmyelocel-T, an expanded patient-specific mixed cell product, in dilated cardiomyopathy (IMPACT-DCM). Journal of Cardiac Failure 2011;17:58. [Google Scholar]
Martino 2015 (MiHEART) {published data only}
- Martino H, Brofman P, Greco O, Bueno R, Bodanese L, Clausell N, et al. Multicentre, randomized, double-blind trial of intracoronary autologous mononuclear bone marrow cell injection in non-ischaemic dilated cardiomyopathy (the dilated cardiomyopathy arm of the MiHeart study). European Heart Journal 2015;36(42):2898-904. [DOI] [PubMed] [Google Scholar]
- Tura BR, Martino HF, Gowdak LH, dos Santos RR, Dohmann HF, Krieger JE, et al. Multicenter randomized trial of cell therapy in cardiopathies MiHeart study. Trials 2007;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sant'Anna 2014 (INTRACELL) {published data only}
- Sant'Anna RT, Fracasso J, Valle FH, Castro I, Nardi NB, Sant'Anna JR, et al. Direct intramyocardial transthoracic transplantation of bone marrow mononuclear cells for non-ischemic dilated cardiomyopathy: INTRACELL, a prospective randomized controlled trial. Revista Brasileira de Cirurgia Cardiovascular 2014;29(3):437-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seth 2010 (ABCD) {published data only}
- Seth S, Bhargava B, Narang R, Mohanty S, Ray R, Gulati G, et al. A randomised trial of Autologous Bone marrow Cells in Dilated cardiomyopathy (ABCD). European Heart Journal 2009;30:501. [Google Scholar]
- Seth S, Bhargava B, Narang R, Ray R, Mohanty S, Gulati G, et al. The ABCD (Autologous Bone Marrow Cells in Dilated Cardiomyopathy) trial: a long-term follow-up study. Journal of the American College of Cardiology 2010;55(15):1643-4. [DOI] [PubMed] [Google Scholar]
- Seth S, Narang R, Bhargava B, Ray R, Mohanty S, Gulati G, et al. Percutaneous intracoronary cellular cardiomyoplasty for nonischemic cardiomyopathy: clinical and histopathological results: the first-in-man ABCD (Autologous Bone marrow Cells in Dilated cardiomyopathy) trial. Journal of the American College of Cardiology 2006;48(11):2350-1. [DOI] [PubMed] [Google Scholar]
Vrtovec 2011 {published data only}
- Vrtovec B, Poglajen G, Sever M, Lezaic L, Domanovic D, Cernelc P, et al. Effects of intracoronary stem cell transplantation in patients with dilated cardiomyopathy. Journal of Cardiac Failure 2011;17(4):272-81. [DOI: ] [DOI] [PubMed] [Google Scholar]
Vrtovec 2013a (NOGA‐DCM) {published data only}
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, et al. Effects of intracoronary CD34+ stem cell transplantation in non-ischemic dilated cardiomyopathy patients: 5-year follow up. Circulation Research 2012;112:165-73. [DOI: 10.1161/circresaha.112.276519] [DOI] [PubMed] [Google Scholar]
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, et al. Effects of intracoronary CD34+stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circulation Research 2013;112(1):165-73. [DOI] [PubMed] [Google Scholar]
- Vrtovec B, Sever M, Domanovic D, Lezaic L, Poglajen G, Cernelc P, et al. Long-term effects of stem cell transplantation in heart failure. Zdravniski Vestnik 2012;81(Suppl II):373-83. [Google Scholar]
Vrtovec 2013b {published data only}
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, et al. Comparison of clinical effects of transendocardial vs. intracoronary stem cell transplantation in dilated cardiomyopathy. Circulation 2012;126:21. [Google Scholar]
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Socan A, Domanovic D, et al. Comparison of transendocardial and intracoronary CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation 2013;128(11):S42-9. [DOI] [PubMed] [Google Scholar]
Vrtovec 2018 (REMEDIUM) {published data only}
- Frljak S, Jaklic M, Zemljic G, Cerar A, Poglajen G, Vrtovec B. CD34(+) cell transplantation improves right ventricular function in patients with nonischemic dilated cardiomyopathy. Stem Cells Translational Medicine 2018;7:168-72. [DOI: 10.1002/sctm.17-0197] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrtovec B, Poglajen G, Sever M, Zemljic G, Frljak S, Cerar A, et al. Effects of repetitive transendocardial CD34+ cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation Research 2018;123(3):389-96. [DOI] [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang JA, Xie XJ, He H, Sun Y, Jiang J, Luo RH, et al. A prospective, randomized, controlled trial of autologous mesenchymal stem cells transplantation for dilated cardiomyopathy. Chung Hua Hsin Hsueh Kuan Ping Tsa Chih 2006;34(2):107-10. [PubMed] [Google Scholar]
Wu 2010 {published data only}
- Wu ZH, Yuan MY, Li HM, Qiu JJ, Lao HZ, Wu XY, et al. Autologous peripheral blood stem cells transplantation for the treatment of dilated cardiomyopathy: a 24-month follow-up in 38 cases. Journal of Clinical Rehabilitative Tissue Engineering Research 2010;14(1):121-25. [DOI: ] [Google Scholar]
Xiao 2017 {published data only}
- Xiao W, Guo S, Gao C, Dai G, Gao Y, Li M, et al. A randomized comparative study on the efficacy of intracoronary infusion of autologous bone marrow mononuclear cells and mesenchymal stem cells in patients with dilated cardiomyopathy. International Heart Journal 2017;58(2):238-44. [DOI: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bartolucci (INNOVA) 2015 {published data only}
- Bartolucci J, Verdugo FJ, Carrion F, Abarzúa E, Goset C, Lamich R, et al. Long-term results of intracoronary transplantation of autologous bone marrow cells in dilated cardiomyopathy [Resultados a largo plazo deltrasplante intracoronario de células mononucleares de médula ósea autólogas enpacientes con cardiopatía dilatada de diversa etiología]. Revista Médica de Chile 2015;143(4):415-23. [DOI] [PubMed] [Google Scholar]
Bartolucci (RIMECARD) 2017 {published data only}
- Bartolucci J, Verdugo FJ, González PL, Larrea RE, Abarzua E, Goset C, et al. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD trial [Randomized clinical trial of intravenous infusion umbilical cord mesenchymal stem cells on cardiopathy]). Circulation Research 2017;121(10):1192-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bocchi 2010 {published data only}
- Bocchi EA, Bacal F, Guimaraes G, Mendroni A, Mocelin A, Filho AE, et al. Granulocyte-colony stimulating factor or granulocyte-colony stimulating factor associated to stem cell intracoronary infusion effects in non ischemic refractory heart failure. International Journal of Cardiology 2010;138(1):94-7. [DOI: ] [DOI] [PubMed] [Google Scholar]
Butler 2017 {published data only}
- Butler J, Epstein SE, Greene SJ, Quyyumi AA, Sikora S, Kim RJ, et al. Intravenous allogeneic mesenchymal stem cells for nonischemic cardiomyopathy: safety and efficacy results of a phase II – a randomized trial. Circulation Research 2017;120(2):332-40. [DOI] [PubMed] [Google Scholar]
Chen 2008 {published data only}
- Chen Y, Gao EM, Gao CY, Xu Y, Huang KJ, Niu ZM, et al. Effects of intracoronary autologous bone marrow mononuclear cells transplantation in patients with dilated cardiomyopathy. Zhonghua Xin Xue GuanBing Za Zhi 2008;36(12):1087-91. [PubMed] [Google Scholar]
Fischer‐Rasokat 2009 {published data only}
- Fischer-Rasokat U, Assmus B, Seeger FH, Honold J, Leistner D, Fichtlscherer S, et al. A pilot trial to assess potential effects of selective intracoronary bone marrow-derived progenitor cell infusion in patients with nonischemic dilated cardiomyopathy: final 1-year results of the transplantation of progenitor cells and functional regeneration enhancement pilot trial in patients with nonischemic dilated cardiomyopathy. Circulation. Heart Failure 2009;2(5):417-23. [DOI] [PubMed] [Google Scholar]
Huang 2006 {published data only}
- Huang RC, Yao K, Li YL, Zhang YQ, Xu SK, Shi HY, et al. Transplantation of autologous bone marrow mononuclear cells on patients with idiopathic dilated cardiomyopathy: early results on effect and security. Zhonghua Xin Xue Guan Bing Za Zhi 2006;34(2):111-3. [PubMed] [Google Scholar]
Kakuchaya 2011 {published data only}
- Kakuchaya T, Golukhova E, Eremeeva M, Chigogidze N, Aslanidi I, Nikitina T, et al. Bone-marrow progenitor stem cells for the treatment of patients with congestive heart failure of different etiology in a placebo controlled clinical trial. Interactive Cardiovascular and Thoracic Surgery 2011;12:568. [Google Scholar]
- Kakuchaya T, Golukhova E, Eremeeva M, Chigogidze N, Aslanidi I, Shurupova I, et al. Accurate design of randomized placebo-controlled clinical trials for assessment of stem cell effects on cardiac regeneration. European Heart Journal 2011;32:290. [Google Scholar]
Miyagawa 2017 {published data only}
- Miyagawa S, Domae K, Yoshikawa Y, Fukushima S, Nakamura T, Saito A, et al. Phase I clinical trial of autologous stem cell-sheet transplantation therapy for treating cardiomyopathy. Journal American Heart Association 2017;6(4):e003918. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT02256501 {unpublished data only}
- NCT02256501. Intracoronary transplantation of bone marrow derived mononuclear cells in pediatric cardiomyopathy. clinicaltrials.gov/ct2/show/NCT02256501 (first received 3 October 2014).
Perin (REVASCOR) 2015 {published data only}
- Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, et al. A phase II dose-escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circulation Research 2015;117(6):576-84. [DOI] [PubMed] [Google Scholar]
Premer 2015 {published data only}
- Premer C, Blum A, Bellio MA, Schulman IH, Hurwitz BE, Parker M, et al. Allogeneic mesenchymal stem cells restore endothelial function in heart failure by stimulating endothelial progenitor cells. EBioMedicine 2015;2(5):467-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tompkins 2018 {published data only}
- Tompkins BA, Rieger AC, Florea V, Banerjee MN, Natsumeda M, Nigh ED, et al. Comparison of mesenchymal stem cell efficacy in ischemic versus nonischemic dilated cardiomyopathy. Journal of the American Heart Association 2018;7(14):e008460. [DOI] [PMC free article] [PubMed] [Google Scholar]
Xiao 2012a {published data only}
- Xiao WT, Gao LJ, Gao CY, Gao YJ, Dai GY, Li MW, et al. Comparative study on the efficacy of intracoronary infusion with various types of autologous bone marrow stem cells for patients with dilated cardiomyopathy. Zhonghua Xin XueGuan Bing Za Zhi 2012;40(7):575-8. [PubMed] [Google Scholar]
Xiao 2012b {published data only}
- Xiao WT, Gao CY, Dai GY, Li MW, Wang XP, Liu HZ, et al. Autologous bone marrow mesenchymal stem cells for myocardial renewal and repair. Chinese Journal of Tissue Engineering Research 2012;16(27):5081-6. [Google Scholar]
Yau 2019 {published data only}
- Yau TM, Pagani FD, Mancini DM, Chang HL, Lala A, Woo YJ, et al. Intramyocardial injection of mesenchymal precursor cells and successful temporary weaning from left ventricular assist device support in patients with advanced heart failure: a randomized clinical trial. JAMA 2019;321(12):1176-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zemljic 2017 {published data only}
- Zemljic G, Poglajen G, Sever M, Cukjati M, Frljak S, Androcec V, et al. Electroanatomic properties of the myocardium predict response to CD34+ cell therapy in patients with ischemic and nonischemic heart failure. Journal of Cardiac Failure 2017;23(2):153-60. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01957826 {published data only}
- NCT01957826. Mesenchymal stem cells for idiopathic dilated cardiomyopathy. clinicaltrials.gov/ct2/show/NCT01957826 (first received 8 October 2013).
NCT02033278 {published data only}
- NCT02033278. Infusion intracoronary of mononuclear autologous adult no expanded stem cells of bone marrow on functional recovery in patients with idiopathic dilated cardiomyopathy and heart failure. clinicaltrials.gov/ct2/show/NCT02033278 (first received 10 January 2014).
NCT02293603 {published data only}
- NCT02293603. Dilated cardiomYopathy iNtervention With Allogeneic MyocardIally-regenerative Cells (DYNAMIC). clinicaltrials.gov/ct2/show/NCT02293603 (first received 18 November 2014).
NCT03797092 {published data only}
- NCT03797092. Stem cell therapy in non-ischemic non-treatable dilated cardiomyopathies II: a pilot study. clinicaltrials.gov/ct2/show/NCT03797092 (first received 8 January 2019).
Additional references
Assmus 2002
- Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, et al. Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI). Circulation 2002;106(24):3009-17. [DOI] [PubMed] [Google Scholar]
Assmus 2016
- Assmus B, Alakmeh S, De Rosa S, Bönig H, Hermann E, Levy WC, et al. Improved outcome with repeated intracoronary injection of bone marrow-derived cells within a registry: rationale for the randomized outcome trial REPEAT. European Heart Journal 2016;37(21):1659-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartunek 2013
- Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, et al. Cardiopoietic stem cell therapy in heart failure: the C-CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage-specified biologics. Journal of the American College of Cardiology 2013;61(23):2329-38. [DOI] [PubMed] [Google Scholar]
Bartunek 2016
- Bartunek J, Davison B, Sherman W, Povsic T, Henry TD, Gersh B, et al. Congestive Heart Failure Cardiopoietic Regenerative Therapy (CHART-1) trial design. European Journal of Heart Failure 2016;18(2):160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartunek 2017
- Bartunek J, Terzic A, Davison BA, Filippatos GS, Radovanovic S, Beleslin B, et al. Cardiopoietic cell therapy for advanced ischaemic heart failure: results at 39 weeks of the prospective, randomized, double blind, sham-controlled CHART-1 clinical trial. European Heart Journal 2017;38(9):648-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartunek 2018
- Bartunek J, Terzic A, Behfar A, Wijns W. Clinical experience with regenerative therapy in heart failure: advancing care with cardiopoietic stem cell interventions. Circulation Research 2018;122(10):1344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Behfar 2014
- Behfar A, Crespo-Diaz R, Terzic A, Gersh BJ. Cell therapy for cardiac repair-lessons from clinical trials. Nature Reviews. Cardiology 2014;11:232-46. [DOI] [PubMed] [Google Scholar]
Behfar 2016
- Behfar A, Gersh B, Terzic A. Repetition rescues regenerative reserve. European Heart Journal 2016;37(21):1667-70. [DOI] [PubMed] [Google Scholar]
Blau 2001
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell 2001;105(7):829-41. [DOI] [PubMed] [Google Scholar]
Blau 2019
- Blau HM, Daley GQ. Stem cells in the treatment of disease. New England Journal of Medicine 2019;380(18):1748-60. [DOI] [PubMed] [Google Scholar]
Bozkurt 2016
- Bozkurt B, Colvin M, Cook J, Cooper JT, Deswal A, Fonarow GC, et al. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation 2016;134:e579-646. [DOI] [PubMed] [Google Scholar]
Chien 2019
- Chien KR, Frisén J, Fritsche-Danielson R, Melton DA, Murry CE, Weissman IL. Regenerating the field of cardiovascular cell therapy. Nature Biotechnology 2019;37(3):232-7. [DOI] [PubMed] [Google Scholar]
Choudry 2016
- Choudry F, Hamshere S, Saunders N, Veerapen J, Bavnbek K, Knight C, et al. A randomized double-blind control study of early intra-coronary autologous bone marrow cell infusion in acute myocardial infarction: the REGENERATE-AMI clinical trial. European Heart Journal 2016;37(3):256-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioural Sciences. 2nd edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc, 1988. [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, accessed prior to 1 July 2021. Available at covidence.org.
Dec 1994
- Dec GW, Fuster V. Idiopathic dilated cardiomyopathy. New England Journal of Medicine 1994;331:1564-75. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG, on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [DOI] [PubMed] [Google Scholar]
Díaz 1987
- Díaz RA, Obasohan A, Oakley CM. Prediction of outcome in dilated cardiomyopathy. British Heart Journal 1987;58(4):393-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dunlay 2012
- Dunlay SM, Roger VL, Weston SA, Jiang R, Redfield MM. Longitudinal changes in ejection fraction in heart failure patients with preserved and reduced ejection fraction. Circulation Heart Failure 2012;5(6):720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Christoph M. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Erbs 2005
- Erbs S, Linke A, Adams V, Lenk K, Thiele H, Diederich KW, et al. Transplantation of blood-derived progenitor cells after recanalization of chronic coronary artery occlusion: first randomized and placebo-controlled study. Circulation Research 2005;97(8):756-62. [DOI] [PubMed] [Google Scholar]
Fisher 2015
- Fisher SA, Zhang H, Doree C, Mathur A, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD006536. [DOI: 10.1002/14651858.CD006536.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2016a
- Fisher SA, Doree C, Mathur A, Taggart DP, Martin-Rendon E. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database of Systematic Reviews 2016, Issue 12. Art. No: CD007888. [DOI: 10.1002/14651858.CD007888.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 2016b
- Fisher SA, Doree C, Taggart DP, Mathur A, Martin-Rendon E. Cell therapy for heart disease: trial sequential analyses of two Cochrane reviews. Clinical Pharmacology and Therapeutics 2016;100(1):88-101. [DOI] [PubMed] [Google Scholar]
Frljak 2018
- Frljak S, Jaklic M, Zemljic G, Cerar A, Poglajen G, Vrtovec B. CD34+ cell transplantation improves right ventricular function in patients with nonischemic dilated cardiomyopathy. Stem Cells Translational Medicine 2018;7:168-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fuster 1981
- Fuster V, Gersh BJ, Tajik AJ, Brandenburg RO, Frye RL. The natural history of idiopathic dilated cardiomyopathy. American Journal of Cardiology 1981;47(3):525-31. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed prior to 11 September 2019. Available at gradepro.org.
Harada 2005
- Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, et al. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nature Medicine 2005;11:305-11. [DOI] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443-57. [DOI] [PubMed] [Google Scholar]
Hare 2009
- Hare JM, Traverse JH, Henry TD, Dib N, Strumpf RK, Schulman SP, et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. Journal of the American College of Cardiology 2009;54(24):2277-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hare 2017
- Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El-Khorazaty J, et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. Journal of the American College of Cardiology 2017;69:526-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hershberger 2013
- Hershberger RE, Hedges DJ, Morales A. Dilated cardiomyopathy: the complexity of a diverse genetic architecture. Nature Reviews. Cardiology 2013;10(9):531-47. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Jefferies 2010
- Jefferies JL, Towbin JA. Dilated cardiomyopathy. Lancet 2010;375(9716):752-62. [DOI] [PubMed] [Google Scholar]
Jiao 2014
- Jiao R, Liu Y, Yang WJ, Zhu XY, Li J, Tang QZ. Effects of stem cell therapy on dilated cardiomyopathy. Saudi Medical Journal 2014;35(12):1463-8. [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2016
- Lu Y, Wang Y, Lin M, Zhou J, Wang Z, Jiang M, et al. A systematic review of randomised controlled trials examining the therapeutic effects of adult bone marrow-derived stem cells for non-ischaemic dilated cardiomyopathy. Stem Cell Research & Therapy 2016;7(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marquis‐Gravel 2014
- Marquis-Gravel G, Stevens LM, Manosur S, Avram R, Noiseux N. Stem cell therapy for the treatment of nonischemic cardiomyopathy: a systematic review of the literature and meta-analysis of randomized controlled trials. Canadian Journal of Cardiology 2014;30(11):1378-84. [DOI] [PubMed] [Google Scholar]
McKenna 2017
- McKenna WJ, Maron BJ, Thiene G. Classification, epidemiology, and global burden of cardiomyopathies. Circulation Research 2017;121:722-30. [DOI] [PubMed] [Google Scholar]
Menasché 2001
- Menasché P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, et al. Myoblast transplantation for heart failure. Lancet 2001;357(9252):279-80. [DOI] [PubMed] [Google Scholar]
Menasché 2018
- Menasché P. Cell therapy trials for heart regeneration-lessons learned and future directions. Nature Reviews. Cardiology 2018;15(11):659-71. [DOI] [PubMed] [Google Scholar]
Merlo 2014
- Merlo M, Pivetta A, Pinamonti B, Stolfo D, Zecchin M, Barbati G, et al. Long-term prognostic impact of therapeutic strategies in patients with idiopathic dilated cardiomyopathy: changing mortality over the last 30 years. European Journal of Heart Failure 2014;16(3):317-24. [DOI] [PubMed] [Google Scholar]
Merlo 2015
- Merlo M, Stolfo D, Anzini M, Negri F, Pinamonti B, Barbati G, et al. Persistent recovery of normal left ventricular function and dimension in idiopathic dilated cardiomyopathy during long-term follow-up: does real healing exist? Journal of the American Heart Association 2015;4(1):e001504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merlo 2016
- Merlo M, Cannatá A, Vitagliano A, Zambon E, Lardieri G, Sinagra G. Clinical management of dilated cardiomyopathy: current knowledge and future perspectives. Expert Review of Cardiovascular Therapy 2016;14(2):137-40. [DOI] [PubMed] [Google Scholar]
Patel 2015
- Patel AN, Mittal S, Turan G, Winters AA, Henry TD, Ince H, et al. REVIVE trial: retrograde delivery of autologous bone marrow in patients with heart failure. Stem Cells Translational Medicine 2015;4(9):1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pinto 2016
- Pinto YM, Elliot PM, Arbustini E, Adler Y, Anastasakis A, Böhm M, et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. European Heart Journal 2016;37(23):1850-8. [DOI] [PubMed] [Google Scholar]
Poglagen 2018
- Poglajen G, Zemljič G, Frljak S, Cerar A, Andročec V, Sever M, et al. Stem cell therapy in patients with chronic nonischemic heart failure. Stem Cells International 2018;2018:6487812. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ponikowski 2016
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal 2016;37:2129-200. [DOI] [PubMed] [Google Scholar]
Psaltis 2010
- Psaltis PJ, Zannettino AC, Gronthos S, Worthley SG. Intramyocardial navigation and mapping for stem cell delivery. Journal of Cardiovascular Translational Research 2010;3:135-46. [DOI] [PubMed] [Google Scholar]
R [Computer program]
- R Foundation for Statistical Computing R: a language and environment for statistical computing. Version 3.4.2. Vienna, Austria: R Foundation for Statistical Computing, 2017. Available at www.R-project.org.
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rong 2019
- Rong SL, Wang ZK, Zhou XD, Wang XL, Yang ZM, Li B. Efficacy and safety of stem cell therapy in patients with dilated cardiomyopathy: a systematic appraisal and meta-analysis. Journal of Translational Medicine 2019;17(1):221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D. CONSORT Group. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2017
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Stamm 2003
- Stamm C, Westphal B, Kleine HD, Petzsch M, Kittner C, Klinge H, et al. Autologous bone-marrow stem-cell transplantation for myocardial regeneration. Lancet 2003;361:45-6. [DOI] [PubMed] [Google Scholar]
Stehlik 2011
- Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dobbels F, et al. The registry of the International Society of Heart and Lung Transplantation: twenty-eight adult heart transplantation report. Journal of Heart and Lung Transplantation 2011;30(10):1078-94. [DOI] [PubMed] [Google Scholar]
Strauer 2002
- Strauer BE, Brehm M, Zeus T, Köstering M, Hernadez A, Sorg RV, et al. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation 2002;106:1913-8. [DOI] [PubMed] [Google Scholar]
Terzic 2016
- Terzic A, Behfar A. CardioPulse: regenerative medicine in the practice of cardiology. European Heart Journal 2016;37(14):1089-90. [PubMed] [Google Scholar]
Terzic 2017
- Terzic A, Behfar A. Posology for regenerative therapy. Circulation Research 2017;121(11):1213-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vrtovec 2013
- Vrtovec B, Poglajen G, Lezaic L, Sever M, Domanovic D, Cernelc P, et al. Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow-up. Circulation Research 2013;112:165-73. [DOI] [PubMed] [Google Scholar]
Vrtovec 2018a
- Vrtovec B. Cell therapy for nonischemic cardiomyopathy: current status and future perspectives. Circulation Research 2018;5(122):28-30. [DOI] [PubMed] [Google Scholar]
Vrtovec 2018b
- Vrtovec B, Poglajen G, Sever M, Zemljic G, Frljak S, Cerar A, et al. Effects of repetitive transendocardial CD34(+) cell transplantation in patients with nonischemic dilated cardiomyopathy. Circulation Research 2018;123:389-96. [DOI] [PubMed] [Google Scholar]
Weintraub 2017
- Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet 2017;390(10092):400-41. [DOI] [PubMed] [Google Scholar]
Wen 2018
- Wen Y, Ding J, Zhang B, Gao Q. Bone marrow-derived mononuclear cell therapy for nonischaemic dilated cardiomyopathy. A meta-analysis. European Journal of Clinical Investigation 2018;48(4):e12894. [DOI] [PubMed] [Google Scholar]
WHO/ISFC 1980
- World Health Organization/International Society and Federation of Cardiology. Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. British Heart Journal 1980;44(6):672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamada 2020
- Yamada S, Arrell DK, Rosenow CS, Bartunek J, Behfar A, Terzic A. Ventricular remodeling in ischemic heart failure stratifies responders to stem cell therapy. Stem Cells Translational Medicine 2020;9(1):74-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yancy 2013
- Yancy C, Jessup M, Bozkurt B, Butler J, Casey D, Drazner M, et al. ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;128(16):e240-27. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Diaz‐Navarro 2019
- Diaz-Navarro R, Urrútia G, Cleland JG, Poloni D, Villagran F, Bangdiwala S, et al. Stem cell therapy for dilated cardiomyopathy. Cochrane Database of Systematic Reviews 2019, Issue 9. Art. No: CD013433. [DOI: 10.1002/14651858.CD013433] [DOI] [PMC free article] [PubMed] [Google Scholar]


