Abstract
Background
Non‐bismuth quadruple sequential therapy (SEQ) comprising a first induction phase with a dual regimen of amoxicillin and a proton pump inhibitor (PPI) for five days followed by a triple regimen phase with a PPI, clarithromycin and metronidazole for another five days, has been suggested as a new first‐line treatment option to replace the standard triple therapy (STT) comprising a proton pump inhibitor (PPI), clarithromycin and amoxicillin, in which eradication proportions have declined to disappointing levels.
Objectives
To conduct a meta‐analysis of randomised controlled trials (RCTs) comparing the efficacy of a SEQ regimen with STT for the eradication of H. pylori infection, and to compare the incidence of adverse effects associated with both STT and SEQ H. pylori eradication therapies.
Search methods
We conducted bibliographical searches in electronic databases, and handsearched abstracts from Congresses up to April 2015.
Selection criteria
We sought randomised controlled trials (RCTs) comparing 10‐day SEQ and STT (of at least seven days) for the eradication of H. pylori. Participants were adults and children diagnosed as positive for H. pylori infection and naïve to H. pylori treatment.
Data collection and analysis
We used a pre‐piloted, tabular summary to collect demographic and medical information of included study participants as well as therapeutic data and information related to the diagnosis and confirmatory tests.
We evaluated the difference in intention‐to‐treat eradication between SEQ and STT regimens across studies, and assessed sources of the heterogeneity of this risk difference (RD) using subgroup analyses.
We evaluated the quality of the evidence following Cochrane standards, and summarised it using GRADE methodology.
Main results
We included 44 RCTs with a total of 12,284 participants (6042 in SEQ and 6242 in STT). The overall analysis showed that SEQ was significantly more effective than STT (82% vs 75% in the intention‐to‐treat analysis; RD 0.09, 95% confidence interval (CI) 0.06 to 0.11; P < 0.001, moderate‐quality evidence). Results were highly heterogeneous (I² = 75%), and 20 studies did not demonstrate differences between therapies.
Reporting by geographic region (RD 0.09, 95% CI 0.06 to 0.12; studies = 44; I² = 75%, based on low‐quality evidence) showed that differences between SEQ and STT were greater in Europe (RD 0.16, 95% CI 0.14 to 0.19) when compared to Asia, Africa or South America. European studies also showed a tendency towards better efficacy with SEQ; however, this tendency was reversed in 33% of the Asian studies. Africa reported the closest risk difference (RD 0.14 , 95% 0.07 to 0.22) to Europe among studied regions, but confidence intervals were wider and therefore the quality of the evidence showing SEQ to be superior to STT was reduced for this region.
Based on high‐quality evidence, subgroup analyses showed that SEQ and STT therapies were equivalent when STT lasted for 14 days. Although, overall, the mean eradication proportion with SEQ was over 80%, we noted a tendency towards a lower average effect with this regimen in the more recent studies (2008 and after); weighted linear regression showed that the efficacies of both regimens evolved differently over the years, having a higher reduction in the efficacy of SEQ (‐1.72% yearly) than in STT (‐0.9% yearly). In these more recent studies (2008 and after) we were also unable to detect the superiority of SEQ over STT when STT was given for 10 days.
Based on very low‐quality evidence, subgroup analyses on antibiotic resistance showed that the widest difference in efficacy between SEQ and STT was in the subgroup analysis based on clarithromycin‐resistant participants, in which SEQ reached a 75% average efficacy versus 43% with STT.
Reporting on adverse events (AEs) (RD 0.00, 95% CI ‐0.02 to 0.02; participants = 8103; studies = 27; I² = 26%, based on high‐quality evidence) showed no significant differences between SEQ and STT (20.4% vs 19.5%, respectively) and results were homogeneous.
The quality of the studies was limited due to a lack of systematic reporting of the factors affecting risk of bias. Although randomisation was reported, its methodology (e.g. algorithms, number of blocks) was not specified in several studies. Additionally, the other 'Risk of bias' domains (such as allocation concealment of the sequence randomisation, or blinding during either performance or outcome assessment) were also unreported.
However, subgroup analyses as well as sensitivity analyses or funnel plots indicated that treatment outcomes were not influenced by the quality of the included studies. On the other hand, we rated 'length of STT' and AEs for the main outcome as high‐quality according to GRADE classification; but we downgraded 'publication date' quality to moderate, and 'geographic region' and 'antibiotic resistance' to low‐ and very low‐quality, respectively.
Authors' conclusions
Our meta‐analysis indicates that prior to 2008 SEQ was more effective than STT, especially when STT was given for only seven days. Nevertheless, the apparent advantage of sequential treatment has decreased over time, and more recent studies do not show SEQ to have a higher efficacy versus STT when STT is given for 10 days.
Based on the results of this meta‐analysis, although SEQ offers an advantage when compared with STT, it cannot be presented as a valid alternative, given that neither SEQ nor STT regimens achieved optimal efficacy ( ≥ 90% eradication rate).
Plain language summary
First‐line sequential versus standard triple therapies for Helicobacter pylori eradication
Review question
To estimate the difference in cure rates between both treatments, and to identify factors that may improve or reduce the cure rate for both treatments.
Background
Gastric ulcer and cancer are mainly caused by infection with the bacteria Helicobacter pylori, a harmful micro‐organism able to colonise the human stomach. Published data seem to indicate that this bacteria is present in nearly half of the world's population. The bacterial colonisation leads to a chronic infection that, over time, may alter the stomach's function, tissue structure, and even cell cycle, being able to produce a variety of symptoms and diseases.
Although this micro‐organism may respond to traditional antibiotics, it has a strong resistance to treatment, and in a high percentage of cases can survive most single and double therapies. Different combinations of antibiotics have therefore been used, and the best treatment is still unclear. The most commonly recommended one is the standard triple therapy (STT), containing two antibiotics (clarithromycin, and a nitroimidazole or amoxicillin) and a stomach protector (omeprazole). However, several studies have demonstrated that STT fails in more than one in five people, so investigators proposed replacing it with a non‐bismuth quadruple sequential sequential (SEQ) treatment, containing a first phase with a dual therapy (amoxicillin and omeprazole), followed by a triple‐therapy phase (nitroimidazole, clarithromycin and omeprazole).
Study characteristics
We searched electronic databases and conference abstracts to identify any relevant studies. We include 44 studies, which tested and compared the cure rates of SEQ therapy against STTs. Our review covers research up to April 2015.
Key results
The review indicates that before 2008 the cure rate for SEQ was higher than for STT. However, the cure rate of both treatments is lower than we would wish. The review found that effectiveness depended on several factors, including the geographic region of the study, bacterial resistance, and the date of the study. For example, we found a reduction in the cure rate over time in both STT and SEQ therapies, with a stronger reduction for SEQ. This meant that in the studies published after 2008, SEQ was not more effective than triple therapy when they were both given for 10 days.
The evidence collected and combined in this review does not support the use of SEQ therapy, as its effectiveness can be matched and even improved on by better STTs (given for 10 or 14 days, or high acid inhibition). Results for SEQ were only partially successful. We need to find another form of therapy to provide the best treatment for patients.
Quality of the evidence
The studies included in this review were of mixed quality, but our analyses do not suggest that study quality was influencing cure rates.
Summary of findings
Summary of findings for the main comparison. Is 10‐day SEQ efficacy superior to STT?
| Is 10‐day SEQ efficacy superior to STT? | ||||||
| Patient or population: participants with Helicobacter pylori infection Settings: participants naïve to eradication treatment Intervention: 10‐day sequential regimen Comparison: standard triple therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Standard triple therapy | 10‐day sequential regimen | |||||
| Eradication proportion | Study population | RD 0.09, 95% CI 0.06 to 0.11 | 12,701 (44 studies) | ⊕⊕⊕⊝ moderate1,2,4 | Results were highly heterogeneous (I² = 75%), and 20 studies did not demonstrate differences between therapies | |
| 751 per 1000 | 833 per 1000 (811 to 863) | |||||
| Moderate | ||||||
| 750 per 1000 | 832 per 1000 (810 to 862) | |||||
| Geographic region | Study population | RD 0.09, 95% CI 0.06 to 0.12 | 12284 (44 studies) | ⊕⊕⊝⊝ low3 | The Latin American subgroup showed no consistent results with the remaining subgroups and there was a tendency to better efficacy with STT than with SEQ in all three included studies although two did not demonstrate differences between therapies | |
| 749 per 1000 | 839 per 1000 (809 to 869) | |||||
| Moderate | ||||||
| 749 per 1000 | 839 per 1000 (809 to 869) | |||||
| Publication date | Study population | RD 0.08, 95% CI 0.06 to 0.11 | 12751 (44 studies) | ⊕⊕⊕⊝ moderate1,2,4,5 | Results were more heterogeneous (69%) in the "after 2008" subgroup | |
| 750 per 1000 | 833 per 1000 (811 to 863) | |||||
| Moderate | ||||||
| 750 per 1000 | 832 per 1000 (810 to 862) | |||||
| STT length | 7 days | RD 0.14, 95% CI 0.12 to 0.17 | 5439 (22 studies) | ⊕⊕⊕⊕ high1 | Six out of 22 studies did not demonstrate differences when 7 days STT was compared to 10 days SEQ. Results for this comparison were consistent (I² = 38%) | |
| Study population | ||||||
| 725 per 1000 | 870 per 1000 (848 to 892) | |||||
| Moderate | ||||||
| 720 per 1000 | 864 per 1000 (842 to 886) | |||||
| 10 days | RD 0.06, 95% CI 0.02 to 0.10 | 3967 (19 studies) | ⊕⊕⊕⊕ high1,2 | In this subgroup 10 days SEQ was better than 10 days STT however heterogeneity between studies was greater (I² = 62%) than in the 7 days STT subgroup analysis. One study out of 19 demonstrated 10 days STT was superior to 10 days SEQ. Eleven studies could not demonstrate differences between therapies | ||
| Study population | ||||||
| 732 per 1000 |
791 per 1000 (754 to 835) |
|||||
| Moderate | ||||||
| 722 per 1000 |
780 per 1000 (744 to 823) |
|||||
| 14 days | RD 0.02, 95% CI ‐0.02 to 0.06 | 3831 (8 studies) | ⊕⊕⊕⊕ high1,2 | 14 days STT did not demonstrate differences with 10 days SEQ | ||
| Study population | ||||||
| 803 per 1000 | 811 per 1000 (795 to 827) | |||||
| Moderate | ||||||
| 811 per 1000 | 819 per 1000 (803 to 835) | |||||
| Bacterial antibiotic resistance | Study population | RD 0.13, 95% CI 0.03 to 0.24 | 832 (8 studies) | ⊕⊝⊝⊝ very low2,4,5,6,7,8 | SEQ was superior to STT in those patients with primary clarithromycin resistant strains only | |
| 672 per 1000 | 807 per 1000 (699 to 914) | |||||
| Moderate | ||||||
| 550 per 1000 | 660 per 1000 (572 to 748) | |||||
| Adverse events rate | Study population | RD 0.00, 95% CI ‐0.02 to 0.02 | 8103 (27 studies) | ⊕⊕⊕⊕ high5,9 | No differences were reported between treatment arms | |
| 195 per 1000 | 199 per 1000 (176 to 215) | |||||
| Moderate | ||||||
| 187 per 1000 | 191 per 1000 (168 to 206) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RD: Risk difference | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Different STT lengths (different total doses) modify RDs. 2There is moderate to substantial unexplained heterogeneity. 3Small number of studies and wider confidence intervals in the South American subgroup. 4Confidence intervals overlap. 5Wide confidence intervals in some subgroups. 6Lack of reporting in most of the studies. 7Small number of studies in some subgroups. 8Metronidazole resistance is dose‐dependent. 9Longer treatments (higher total dose) led to higher rates of AEs.
Background
Description of the condition
Helicobacter pylori (H. pylori) infects over 50% of the adult population globally (De Martel 2006) and is known to be associated with a wide range of upper gastrointestinal diseases including gastritis, peptic ulcer disease (PUD) and gastric cancer. The latest Maastricht IV Consensus (Malfertheiner 2012) has strongly recommended H. pylori eradication for people with PUD, mucosa‐associated lymphoid tissue (MALT), lymphoma and atrophic gastritis, post‐gastric cancer resection, in people who are first‐degree relatives of people with gastric cancer and in people with a preference to treat a known H pylori infection after consultation with physicians. It is also suggested thatH. pylori eradication is appropriate for people infected with H pylori, investigated for non‐ulcer dyspepsia (NUD). Treatment of H. pylori may also prevent PUD, bleeding or both in naïve users of non‐steroidal anti‐inflammatory drugs (NSAIDs) (Malfertheiner 2012). The treatment for H. pylori infection is therefore intended to eradicate the bacteria to stop progression of gastric lesions (atrophy, ulcers and cancer), and not to resolve dyspeptic symptoms, as in most cases those will remain after treatment.
Description of the intervention
Since 1997, a worldwide panel of experts, the European Helicobacter Study Group (EHSG) has recommended in its consensus conferences a triple therapy comprising a proton‐pump inhibitor (PPI) plus two antibiotics used twice daily as the first‐line H. pylori eradication regimen (Malfertheiner 1997; Malfertheiner 2002; Malfertheiner 2007; Malfertheiner 2012). Commonly, clarithromycin is used together with amoxicillin or nitroimidazole (metronidazole or tinidazole) (Gisbert 2007; Malfertheiner 2007). However, sequential therapy (SEQ), based on a PPI plus amoxicillin twice daily for the first five days followed by PPI plus clarithromycin together with nitroimidazole twice daily for the following five days, has been suggested as an alternative treatment to replace the standard triple therapy (STT) (De Francesco 2001; Zullo 2000). PPIs are used to protect the lining of the stomach against ulcerogenic effects and have a bactericidal action (McNicholl 2012).
Additionally, in this context of short‐term drug regimens (two weeks or less) where the main objective is to eradicate the bacteria, the benefits or harms of treatments and patients' satisfaction is somewhat limited.
How the intervention might work
The efficacy of STT is inversely related to the bacterial load, and higher eradication proportions are achieved in those with a low bacterial density in the stomach (Lai 2004; Perri 1998). It has therefore been suggested that the short initial dual therapy used in SEQ treatment with amoxicillin lowers the bacterial load in the stomach in order to improve the efficacy of the immediately subsequent short course of triple therapy (Moshkowitz 1995; Zullo 2007). In other words, it acts as an induction phase that may amplify the efficacy. The first five days of amoxicillin and PPI thus result in a marked reduction of H. pylori and even eradication in at least 50% of people (Marshall 2008; Moshkowitz 1995). The second stage of the regimen (clarithromycin and a nitroimidazole) would act to eradicate a rather small residual population of viable organisms (Marshall 2008).
Moreover, it has been suggested that the initial use of amoxicillin may offer another essential advantage in the eradication of H. pylori (Zullo 2007). It has been found that regimens containing amoxicillin prevent the selection of secondary clarithromycin resistance (Murakami 2002). The most accepted candidate theory suggests that SEQ might therefore improve eradication proportions as the initial phase of treatment with amoxicillin weakens bacterial cell walls, preventing the development of drug efflux channels involved in the reduction of clarithromycin and other drug concentrations inside the bacteria, although this has not yet been demonstrated. This may allow higher concentrations of antibiotics in the cytoplasm during the second phase of treatment that would facilitate the binding of clarithromycin to the ribosomes.
The sequential administration of antibiotics is not generally recommended because of concerns about promoting drug resistance (Graham 2007a). However, the dual therapy of SEQ uses a drug (amoxicillin) that rarely results in resistance, such that the outcomes should be either cure of the infection or a marked reduction in bacterial load, making the presence of a pre‐existing small population of resistant organisms less likely (Graham 2007b).
Why it is important to do this review
Standard triple therapy is the most commonly used treatment in clinical practice. However, a critical fall in the H. pylori eradication proportion following this therapy has been observed since the discovery of H. pylori. From 2007, STT eradication rates were below 80%, an efficacy which was defined as disappointing for any antimicrobial infection (Graham 2007a). Two double‐blind, US multicentre studies both found disappointingly low eradication proportions with STT (77%) (Laine 2000; Vakil 2004). Two meta‐analyses including more than 53,000 participants have shown that the cure proportion is below 80% (Janssen 2001; Laheij 1999). Also, a prospective study (Mégraud 2013), conducted in the European framework to assess H. pylori resistance to antibiotics and its relationship to antibiotic consumption, showed that because of the high clarithromycin resistance, empirical STT should not be used. Therefore, the ethics of continued use of STT have recently been questioned and the use of alternative therapy has been recommended in its place (Graham 2007c).
The SEQ regimen is an alternative therapeutic approach, but eradication efficacy must be confirmed now that the resistance proportion for clarithromycin has increased (Moayyedi 2007). Almost all studies using the SEQ regimen published during 2008, 2009 and 2010 had lower than 90% eradication proportions and in some cases rates of 80% or less have been reported (Park 2009). Moreover, the most commonly used SEQ therapy uses tinidazole, whilst in some studies metronidazole has been used. A recent review of SEQ therapy (Vaira 2009) showed that the eradication proportion achieved with metronidazole‐based regimens was significantly lower than that achieved with a tinidazole‐based regimen. Indeed, tinidazole has a markedly longer half‐life compared to metronidazole and this could be a cause for concern for successful H. pylori therapy. It is also important to mention that most of the studies considered in the previous pooled analyses and meta‐analyses were performed in Italy (Jafri 2008; Tong 2009; Vaira 2009). Some of the more recent studies, including other regions, have not demonstrated a beneficial effect of SEQ therapy when compared with STT but have instead shown equivalent eradication proportions (Gatta 2009; Gisbert 2010).
Previous meta‐analyses have compared STT with SEQ therapy (Gatta 2009; Jafri 2008; Tong 2009). In our preliminary search, we identified several randomised controlled trials (RCTs) which were not included in the previous meta‐analyses. We have therefore conducted a systematic review of RCTs comparing SEQ therapy versus STT for H. pylori eradication, using more databases, optimised search strategies and applying the rigorous techniques recommended by Cochrane. This systematic review was developed from a Cochrane review of all H. pylori eradication therapies (Forman 2000).
Objectives
Primary objective
To conduct a meta‐analysis of randomised controlled trials (RCTs) comparing the efficacy of a SEQ regimen with STT for the eradication of H. pylori infection.
Secondary objective
To compare the incidence of adverse effects associated with both STT and SEQ H. pylori eradication therapies.
Methods
Criteria for considering studies for this review
Types of studies
Only parallel‐group, randomised controlled trials were eligible for inclusion in the review. We included only those trials comparing a 10‐day SEQ versus a STT for H. pylori eradication, as defined in the headings below. We excluded studies that were not assessing an H. pylori treatment or that focused on other gastrointestinal conditions. We excluded non‐randomised studies, case reports, letters, editorials, commentaries and reviews. Abstracts and full‐text forms were eligible for inclusion. There were no restrictions by date of publication or by language.
Types of participants
Inclusion criteria
Randomised trials were eligible for inclusion if the study population included adults or children diagnosed as positive for H. pylori (with at least one confirmatory test) on the basis of monoclonal stool antigen test, rapid urease test (RUT), histology or culture of an endoscopic biopsy sample, or by urea breath test (UBT). Study participants had to be naïve to H. pylori eradication treatment.
Exclusion criteria
We excluded trials in which participants were diagnosed as H. pylori‐positive solely on the basis of serology or polymerase chain reaction (PCR), or who had previously been treated with an eradication therapy. Study participants could not present with serious comorbidities such as HIV infection, malignancy, etc.
Types of interventions
Sequential therapy
The 10‐day SEQ comprised a PPI and amoxicillin 1 g twice daily, all taken orally for the first five days, followed by PPI twice daily, clarithromycin 500 mg twice daily and a nitroimidazole (tinidazole or metronidazole at either 400 mg or 500 mg) twice daily, all taken orally for the following five days.
We included only trials assessing SEQ therapies lasting 10 days. Studies were subject to exclusion if there were any variations in the intervention schedule regarding the length of the SEQ treatment.
Standard triple therapy
The STT consisted of a PPI, clarithromycin 500 mg twice daily and amoxicillin 1 g twice daily, all taken orally and lasting at least seven days.
Types of outcome measures
We included all relevant trials, even if they did not report evidence of eradication of H. pylori as their primary outcome.
Primary outcomes
We considered reported efficacy, defined as the eradication/cure proportion/rate, to be the primary outcome.
Trials were included if they reported the number of participants with H. pylori eradication. If percentages were reported rather than numbers, then we derived the proportion of participants cured from the intention‐to‐treat (ITT) randomised sample size for each treatment arm.
Trials were eligible if H. pylori eradication was confirmed using RUT or histology of an endoscopic biopsy sample, or by a UBT or a monoclonal stool antigen test, at least four weeks after completion of treatment.
We excluded trials in which assessments were by serology test alone or by culture alone.
Secondary outcomes
Reported incidence of adverse events (AEs) was also included.
AEs incidence was recorded as the number of participants reporting: any type of AE; any gastrointestinal disturbance such as nausea or vomiting; any dermatological problem; any systemic effect (fever, headache or dizziness); or any serious AE.
We defined a serious AE as the occurrence of any undesirable and important medical event, such as, for example, death, a life‐threatening situation, hospitalisation, permanent damage associated with any medical drug. We distinguish between a serious AE and a severe AE, i.e. an intense form of AE that usually incapacitates an individual's normal life. Reported severe AEs were also collected.
We define treatment compliance (or adherence) as the extent to which a participant fulfilled the requirements of the prescribed treatment in terms of drug type, dosage and length of treatment.
We collected the reported proportion of participant withdrawals, defined as the number of participants discontinuing treatment due to AEs.
Search methods for identification of studies
Electronic searches
We conducted bibliographical searches in the Cochrane Central Register of Controlled Trials (CENTRAL) through the Cochrane Library (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3) and CINAHL (Appendix 4) electronic databases.
We combined search terms to capture two components of the study question: the disease (H. pylori infection) and the intervention of interest (the comparison of STT versus SEQ therapy). We used the following combination of terms (all fields): (Helicobacter OR pylori) AND sequential AND (triple OR “standard regimen” OR “standard therapy”). We adapted and conducted handsearches using the same syntax.
The design of the search was refined by the Trials Search Co‐ordinator at the Cochrane Upper Gastrointestinal and Pancreatic Diseases Review Group.
We ran the electronic search up to April 2015.
Searching other resources
We performed additional handsearches of websites in order to retrieve additional publications not captured by the electronic searches. The manual search aimed to identify abstracts of RCTs that might not have been published in peer‐reviewed journals but only as part of conference proceedings, specialised journals or international congresses such as the International Workshop of the European Helicobacter Study Group (EHSG), the American Digestive Disease Week (DDW) and the United European Gastroenterology Week (UEGW).
We reviewed each of the abstracts identified as potentially eligible and included only those meeting the inclusion criteria.
We conducted detailed cross‐referencing from the bibliographies of the included studies as well as from other systematic reviews, in order to identify further relevant trials.
Data collection and analysis
Selection of studies
Prior to the selection of studies phase, most duplicates were automatically removed when studies were imported to the citation manager. We removed the remaining duplicates manually during the first screening phase.
We conducted the selection of retrieved studies from the searches in two phases. We undertook an initial screening of titles and abstracts (first screening phase) against the inclusion criteria, to identify potentially relevant publications. Following this step, we checked of the full papers (second screening phase) of the studies identified as potentially eligible for inclusion during the first screening phase.
In the case of abstracts or articles with insufficient detail to meet the inclusion criteria, we contacted the authors.
Based on the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) approach (www.prisma‐statement.org), we developed a diagram to schematise the steps used for the identification and selection of studies. We specified the number of studies considered at each step and the reason for exclusion of each of the excluded studies.
Two review authors (OPN and AGM) carried out both the first and second screenings independently, resolving any discrepancies by discussion and consulting a third review author (JPG) for unresolved disagreements.
Data extraction and management
During the protocol phase we developed a pre‐tested data extraction form to record data from the selected papers. We collected the following fields during the data extraction process:
first author’s name and year of publication;
country;
format of publication (abstract versus journal article);
age of the population (adult versus children);
medical condition (PUD or NUD or other);
number of participants in each treatment group;
name, dose and timing of antibiotic administration;
length of STT;
eradication proportion per treatment regimen (ITT and per‐protocol (PP)): if only the PP sample was reported, we calculated the ITT sample on the basis of the randomisation and dropout information;
definition of compliance and the level of compliance in the ITT sample;
details of the method of assessment of H. pylori infection both before and after treatment;
whether the antibiotic sensitivity and resistance were tested before and after eradication; if so, the primary and secondary antibiotic resistance;
incidence, type and severity of AEs;
study quality: generation of the treatment allocation, concealment of the treatment allocation at randomisation, implementation of masking, completeness of follow‐up and use of ITT analysis.
We contacted study authors for any missing data.
Two review authors (OPN and AGM) carried out data extraction independently, resolving any discrepancies by discussion and consulting a third review author (JPG) for unresolved disagreements.
Assessment of risk of bias in included studies
We assessed four components of quality following the quality checklist recommended in the Cochrane Handbook for Systematic Reviews and Interventions (Higgins 2011). We assessed the quality according to the information available in the published trials, mindful of the risk of overestimating intervention effects in RCTs with inadequate methodological quality (Kjaergard 2001). We contacted authors for any missing information. Items assessed are described and listed in the headings below.
Two review authors (OPN and AGM) independently assessed the methodological quality of all the included studies. As in previous phases, we sought the opinion of a third review author (JPG) in case of disagreement.
Generation of the treatment allocation
We considered a study to be a RCT if it was explicitly described as ‘randomised’. This should include the use of words such as ‘randomly’, ‘random’ or ‘randomisation’. We then rated the randomised trial as truly random, pseudo‐random, non‐random, not stated, or unclear.
We defined a trial as ‘truly random’ if the allocation sequence was computer‐generated or generated by a random‐number table, coin toss, shuffles or throwing dice. The person involved in the recruitment of participants should not be the one performing the procedure.
If the selection was based on patient hospital numbers, birth dates, visit dates, alternate allocation or other method not involving a defined random mechanism but likely to produce an unpredictable sequence of numbers, we considered the trial to be ‘pseudo random’.
We excluded studies in which the selection was based on participant or clinical preference, or any selection mechanism that could not be described as random. We also excluded studies that did not state whether the treatment was randomly allocated.
We classified studies which were identified as randomised trials, but which did not describe how the treatment allocation was generated, as having an 'unclear' generation of treatment allocation.
Concealment of the treatment allocation at randomisation
A study was classified as concealed, unconcealed or unclear in the following situations (Haynes 2006):
We rated a study ‘concealed’ if the trial investigators were unaware of the allocation of each participant before they were entered into the trial. Adequate methods included central telephone randomisation schemes, pharmacy‐based schemes, sequentially‐numbered, opaque, sealed envelopes, sealed envelopes from a closed bag, or the use of numbered or coded bottles or containers.
We rated the allocation as ‘unconcealed’ when trial investigators were aware of the allocation of each participant before they entered the trial. For example, when it was based on participant data, such as the date of birth or hospital case‐note number, visit dates, sealed envelopes that were not opaque, or a random‐number table that was not concealed from the investigator.
If authors did not report or provide a description of an allocation concealment approach that allowed for classification as concealed or not concealed, then we categorised the study as ‘unclear allocation concealment’.
Implementation of masking
A trial could be considered double‐blinded, single‐blinded, not blinded or unclear, and was to be classified within a 'Risk of bias' table into one of three categories: low risk, unclear risk and high risk (Higgins 2011).
We judged a study as ‘not blinded’ if the authors defined it as an open‐label study, and we rated it at ‘high risk’. We classified studies as ‘unclear risk’ if no blinding information was reported.
If a trial was simply described as 'single‐blind', we recorded the degree of masking as ‘unclear’ for clinician and outcome assessor, while participants were presumed to be blinded.
If a trial was reported as ‘double‐blind’, it had to be rated as ‘low risk’. Double‐blinding, however, was unlikely, as the type of treatment administration could not easily allow the simultaneous blinding of the clinician, the outcome assessor, the participant and the pharmacist.
Completeness of follow‐up and use of intention‐to‐treat (ITT) analysis
We noted the proportion of participants for which there were missing outcome data and/or who were excluded from the analysis for each arm of the trial. For the ITT analyses we assumed that these participants had failed therapy. We stated whether the analysis included all randomised participants, i.e. whether an ITT approach was undertaken.
We recorded the authors’ definitions when they reported an ITT analysis. Due to the varied definitions of ITT used by authors, we favoured the most widely‐accepted definition of the ITT approach. All participants were to be analysed in the groups to which they were originally randomly assigned, regardless of whether they satisfied the entry criteria, which treatment they received, or subsequent withdrawal or deviation from the protocol (Hollis 1999).
We reported all available information for all randomised participants. We included studies reporting either ITT or PP analysis alone. We contacted authors of those studies either using a different ITT approach from the one used in this review, or reporting only a PP analysis, in order to obtain our preferred ITT analysis approach.
We included in our ITT meta‐analysis studies reporting an ITT analysis, but requiring participants to have the second test confirming their H. pylori infection status after randomisation in order to be included in the analysis.
These four quality components are part of the key methodological features that are important to the validity and interpretation of included trials as mentioned above (Moyer 2005). We did not score the quality of the studies, and did not exclude studies classified as ‘low quality’. We used the individual quality assessment items to explore heterogeneity. If we found significant heterogeneity between studies (details below), we explored it by using subgroup analysis with pooled effect‐size estimates, and discuss them when interpreting the results.
Measures of treatment effect
Given that the outcome was common, that is that 'H pylori eradication' was usually expected after treatment, and that the treatment and follow‐up themselves were fixed for each arm, the odds ratio (OR), would produce a biased effect estimate. We therefore expressed dichotomous outcomes of individual studies using the risk difference (RD) together with the 95% confidence interval (CI), taking 'H. pylori eradication' as the primary outcome. The RD describes the difference in the risk of observing an event in the SEQ treatment group versus the STT comparison group, for which a value of 0 indicates that the estimated effects are the same for both interventions. In the clinical context, generally the terms "odds" and "risks" are interchangeable, however; the term "risk" suits better our medical question as it defines the probability of an event will occur as opposite to the term "odd" which enhances the idea of the ratio of the probability that a particular event will occur to the probability that it will not occur (Higgins 2011).
We have treated the SEQ arm as the intervention group and the STT arm as the control group.
Unit of analysis issues
We included only standard design, parallel, randomised controlled trials. Our interest was only in the direct comparison between the two treatment regimens (10‐day SEQ and 7‐ to 14‐day STT). We did not include multiple groups in a single pair‐wise comparison, so that the same participant was not used twice in the same analysis.
However, multiple‐group comparisons are usual across treatment arms in clinical trials. For instance, the ITT population could be randomised into three different treatment arms (or schedules): STT lasting 7 days, STT lasting 14 days and SEQ therapy lasting 10 days. In such cases, for the purpose of the overall analysis, we combined the different arms of the same treatment (i.e. 7‐day STT and 14‐day STT) by summarising the number of participants in each arm. Afterwards, we undertook the corresponding subgroup meta‐analyses using the separate arms for STT treatment duration.
We assessed the different treatment schedules within the same treatment arm through standard single pair‐wise comparisons, as specified under the subgroup analyses section.
Dealing with missing data
We contacted authors for any incomplete outcome data from included studies. We considered those participants for whom outcome data were still missing (due to dropout or incomplete records) to have failed eradication for the primary outcome.
Assessment of heterogeneity
In order to identify the possible diversity in trial characteristics, we analysed the clinical, methodological and statistical components.
We performed the Chi² test for heterogeneity for each combined analysis, where P < 0.10 indicated significant heterogeneity between studies (Higgins 2002). The I² statistic was reported, which quantifies heterogeneity by calculating the percentage of total variation across studies that is due to heterogeneity (an approach that has been endorsed by Cochrane). We define significant heterogeneity as I² > 25%, based on the judgement that I² values below 25%, 50% and 75% represent low, moderate and high heterogeneity, respectively (Higgins 2003).
We used graphical methods (forest plots) to complete the Chi² test assessment. When we identified heterogeneity, we investigated the source using additional techniques, such as subgroup analyses or funnel plots, to work out whether particular characteristics of studies were related to the sizes of the treatment effect, in accord with the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011).
Assessment of reporting biases
To assess publication bias, we checked for funnel plot asymmetry by examining the relationship between the treatment effects and the standard error of the estimate.
We produced funnel plots for the principal outcome for each comparison (plots of risk difference (RD) against the standard error (log of RD)).
Data synthesis
In order to collate, combine and summarise the information from the included studies, we decided to undertake a quantitative (meta‐analytic) approach. If there were insufficient trials (two or fewer) reporting for the same comparison, then we would conduct a qualitative evaluation (narrative).
As the first step for the data synthesis, we present an initial overview of results referring generally to all included studies. We give these overall findings in a descriptive fashion, in terms of geographic region, target populations, sample sizes, age of the population, medical condition at baseline and treatment schedules assessed (Description of studies).
The second step in the evidence synthesis consisted of summarising the information related to the size of the effect for all studies, as well as for each different participant group, comparison or outcome measure undertaken. We also report results from subgroup analyses as well as sensitivity analyses.
We performed meta‐analysis combining the RDs for the individual studies in a global RD using a random‐effect method for dichotomous outcomes (Mantel‐Haenszel). Additional sensitivity analyses were performed to check the robustness of the results (DerSimonian 1986; Egger 1997). We conducted pooled analyses using Review Manager 5.3 software (RevMan 2014).
We performed subgroup analyses to identify sources of heterogeneity and report summary estimate of the RD within subgroups of these identified sources.
There are several methods to calculate the number needed to treat for an additional beneficial outcome (NNTB) and some have limitations (Altman 2002; Cates 2002; Moore 2002). Many published meta‐analyses do not provide the results or the methods used. In this review, we calculated the NNTB for efficacy and the number needed to treat for an additional harmful outcome (NNTH) for adverse events, by using the formula NNT = 1/ |RD| (Higgins 2011), where |RD| stands for the absolute value of the risk difference. The NNTB was always reported among those statistically significant comparisons.
Subgroup analysis and investigation of heterogeneity
We performed pre‐planned subgroup analyses, regardless of whether significant heterogeneity was present:
geographic region;
publication date;
age (children versus adults);
length of STT (7 versus 10 versus 14 days);
type of nitroimidazole (metronidazole versus tinidazole);
resistance of each antibiotic;
dosing for PPI (SEQ therapy versus STT);
type of disease at enrolment (PUD versus NUD).
Quality of the body of evidence (GRADE methodology)
We assessed the quality of the body of the evidence using GRADE methodology in those subgroup analyses where we found statistically significant differences between treatments for the main outcome. We have incorporated these outcomes into the 'Table 1 (SoF) for the SEQ versus STT comparison. We present GRADE quality assessments ranging from 'very low' to 'high' quality evidence alongside the effect estimates, and decisions made relating to downgrading (or upgrading) of evidence.
The GRADE approach uses five considerations: study limitations, consistency of effect, imprecision, indirectness and publication bias to assess the quality of the body of evidence for each outcome. The evidence was downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments of risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Sensitivity analysis
No arbitrary inclusion or exclusion criteria were established for the search strategy. If during the review process we identified sensitivity issues (missing data, individual peculiarities of the studies), we repeated the meta‐analysis to test for differences. We conducted sensitivity analyses to test the robustness of the review: using a random‐effects model instead of a fixed‐effect model; excluding trials with no or unclear allocation concealment; excluding trials where the method of randomisation was unclear; or excluding trials where masking was unclear.
Results
Description of studies
We retrieved 5889 citations from the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE (Ovid), EMBASE, and CINAHL; we found 15 additional references through handsearches and from the International Workshop of the European Helicobacter Study Group, the American Digestive Disease Week (DDW) and the United European Gastroenterology Week (UEGW) Congresses, up to April 2015.
After removal of duplicates, we initially screened 5228 citations resulting from the electronic searches. Based on consideration of their titles and abstracts we excluded 5111 citations, while 117 papers were targeted for full‐article review, either because they were potentially relevant, or because not enough information was reported in the title and abstract to make a final decision regarding the inclusion of the paper in the review.
After review of the full papers, we finally included 44 publications in the review. All of them were randomised controlled trials (RCTs).
A description of the process followed for the identification and selection of studies, and the number of studies identified through each step, is presented as part of the PRISMA diagram (Figure 1).
1.
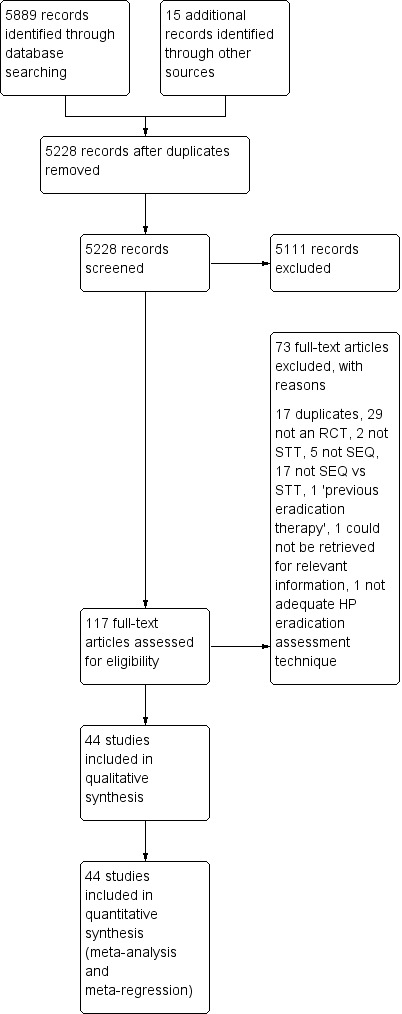
Study flow diagram.
Results of the search
We include 44 RCTs with a standard parallel‐group design. See the Characteristics of included studies for full details.
The primary objective of almost all the included studies was very similar, and aimed to assess the efficacy of the 10‐day SEQ therapy versus STT.
Two references reported different primary objectives: De Francesco 2004b aimed to identify predictive factors for the outcome of H. pylori eradication using two therapeutic schemes (STT and SEQ), and Molina‐Infante 2010, whose primary objective was to compare clarithromycin and levofloxacin in triple and SEQ first‐line regimens.
None of the included studies reported efficacy in groups of participants with another concomitant health condition.
For the purposes of the evidence synthesis, we categorised the included studies according to the relevant endpoint assessed, i.e. the overall eradication proportion with SEQ and STT, as well as the different variables evaluated within the subgroup analysis.
Included studies
Of the included studies, 11 were published in Italy (De Francesco 2004a; De Francesco 2004b; Focareta 2002; Focareta 2003; Franceschi 2011; Gatta 2011; Paoluzi 2010; Scaccianoce 2006; Vaira 2007; Zullo 2003; Zullo 2005), eight in Korea (Choi 2012; Chung 2012; Jeon 2013; Kim 2011; Lee 2014; Lee 2015; Oh 2012; Park 2012), eight in China (Gao 2010; Huang 2013; Liou 2013; Liou 2014; Lu 2010; Wu 2011; Yan 2011; Zhou 2014), two in India (Javid 2013; Nasa 2013), two in Morocco (Lahbabi 2013; Seddik 2013), one each in Iran (Aminian 2010), Spain (Molina‐Infante 2010), Latin‐America (Greenberg 2011), Poland (Albrecht 2011), Puerto‐Rico (Lopez‐Román 2011), Belgium (Bontems 2011), Slovenia (Tepes 2012), Kenya (Laving 2013), Saudi Arabia (Ali Habib HS 2013), Brazil (Eisig 2014), Japan (Hsu 2014), Turkey (Rakici 2014) and Singapore (Ang 2015). Eight of the included studies were published before 2008.
Six studies (Albrecht 2011; Ali Habib HS 2013; Bontems 2011; Huang 2013; Laving 2013; Lu 2010) published between 2010 and 2013 assessed the efficacy of 10‐day SEQ versus STT in children.
Twelve studies (Aminian 2010; Chung 2012; De Francesco 2004a; Greenberg 2011; Javid 2013; Kim 2011; Liou 2013; Molina‐Infante 2010; Scaccianoce 2006; Zhou 2014; Zullo 2003; Zullo 2005) assessed the efficacy of SEQ versus STT in either or both NUD and PUD participant groups. Eradication was reported for each of the groups independently, and the studies were pooled within the corresponding subgroup analysis.
The sample sizes across the included studies varied considerably, ranging from nine participants within both the STT and SEQ arms in Ali Habib HS 2013 to 522 participants within the SEQ arm and 527 participants in the STT arm in Zullo 2003.
Based on the eligibility criteria, all studies compared 10‐day SEQ versus STT. STT included different regimen lengths (7, 10 and 14 days) and different antibiotic doses (high and standard doses). The SEQ utilised different nitroimidazole types (metronidazole and tinidazole), and both regimens varied the type and dosage of PPIs: omeprazole, lansoprazole, pantoprazole, rabeprazole, or esomeprazole. One study (Gatta 2011) used double‐dose PPI and another (Chung 2012) low‐dose PPI in both treatment arms.
H. pylori eradication proportion with SEQ therapy ranged from 42% in Laving 2013 to 96% in the Italian study Focareta 2002.
Excluded studies
The total number of studies excluded after the first screening was 5111. We then excluded 73 studies during the full‐text review. See Characteristics of excluded studies tables for further details. One study was selected for potential inclusion, but although we contacted the authors in order to retrieve relevant information, we finally excluded it due to ineligibility.
Risk of bias in included studies
In the overall comparison 'Eradication proportion of SEQ versus STT', five studies (Albrecht 2011; Ang 2015; Huang 2013; Vaira 2007; Zhou 2014) were categorised as ‘low risk of bias’ in all four domains of the checklist assessing the quality of the methodology (Figure 2).
2.
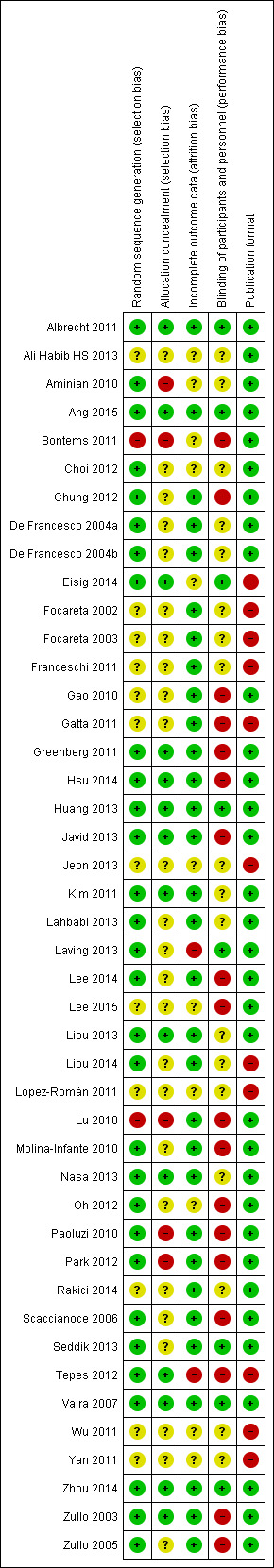
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Bontems 2011; Lu 2010 were categorised as ‘high risk’ in the items relating to randomisation, allocation and blinding. Three other studies (Aminian 2010; Paoluzi 2010; Park 2012) were likewise rated as having poor allocation concealment and blinding, with both items flagged as 'high risk', or at least one of them (Chung 2012; Gao 2010; Gatta 2011; Lee 2015; Zullo 2005).
A lack of comprehensive reporting of outcomes, as well as scarcity of information related to the assessed quality items within the aforementioned studies, made both selection and performance biases a threat to the validity of the review (Figure 2 and Figure 3).
3.
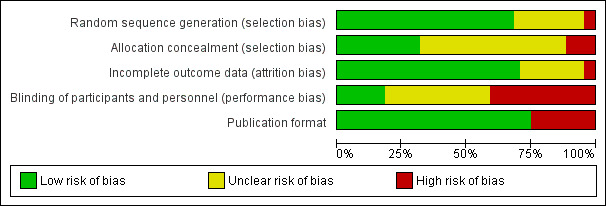
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
However, regardless of the potential biases, the subgroup analyses confirmed a significant gain in the overall ITT eradication proportion with 10‐day SEQ compared to STT. Most of the studies (72%) were reported to be ‘truly randomised’ (as defined in Assessment of risk of bias in included studies) and therefore were unlikely to have been subject to selection bias due to a lack of randomisation through sequence generation.
Performance bias due to lack of blinding of study participants and personnel was, a priori, the domain that was more likely to influence the review’s findings, since over 50% of studies were flagged as being at ‘high risk’. However, the importance of this finding in the context of H. pylori eradication is low, as addressed in the Discussion.
Allocation
In five (12%) studies (Aminian 2010; Bontems 2011; Lu 2010; Paoluzi 2010; Park 2012) the method of allocation was not concealed. Ten (23%) studies reported that allocation was concealed and the remaining ones did not report any information on allocation of the sequence generation, and were therefore flagged as unclear (Figure 2).
In order to generate an unpredictable and unbiased sequence, 10 (23%) studies reported ‘adequate’ concealment of the allocation sequence, mainly using opaque sealed envelopes and by involving personnel in the enrolment phase that were unaware of upcoming assignment of participants to treatments.
Albrecht 2011 reported that the intervention sets were prepared by the hospital’s pharmacy and by independent personnel not involved in the study. Similarly, in Kim 2011 only the independent staff could manage a matching list between study identification number and hospital number, and the data were only revealed to other investigators once recruitment and data collection were completed.
Blinding
We judged 17 (38%) studies to be at ‘high risk’, as authors reported either that the trial was not blinded or the design of the study was open‐label. Similarly, 18 studies, rated as ‘unclear risk’, either did not report any information regarding masking, or authors stated that only the investigators (but not the participants), were blinded to the treatment allocation, in which case we categorised the studies as single‐blinded (Figure 2).
We rated Albrecht 2011 and Vaira 2007 as ‘low risk’, given that the authors stated that a ‘double‐blind’ design was used with placebo during three days after completion of STT. With only two studies reported as double‐blinded, we could not conduct the planned subgroup meta‐analysis indicated in the protocol. The eradication proportions were 89% and 86% in the SEQ therapy arms and 77% and 69% in the STT therapy arms in Vaira 2007 and Albrecht 2011 respectively.
It should nonetheless be noted that the number of studies that were not blinded was due to the design of the SEQ regimen, where usually two drugs were used in the initial phase and three drugs during the second phase of treatment (as per protocol). Due to the manner in which the drugs were administered, participants could not be easily blinded to their assigned treatment.
Incomplete outcome data
Primary outcomes were correctly and consistently reported in the majority (75%) of the studies (Figure 3). Attrition bias was reported in three of the nine studies in abstract form (Eisig 2014; Lopez‐Román 2011; Wu 2011), accounting for around 386 participants, which represented 3% of the total randomised population in our meta‐analysis.
Indeed, information related to the medical condition at baseline, sex ratio, average age of the population, per protocol sample size, incidence of AEs or antibiotic resistance were scarcely described in the reports of abstracts of Congresses.
One study (Laving 2013) was rated at 'high risk' for the reporting of outcomes, as data regarding eradication were reported as the number of participants eradicated separately by stool antigen negative and histology negative. Also authors did not provide eradication proportions by ITT analysis.
We noted no differences in the number of excluded participants or dropouts between arms across the included studies.
Selective reporting
Eight (18%) studies reported H. pylori eradication proportions for those people with bacterial antibiotic resistance: four studies in people with clarithromycin bacterial resistance; seven studies in people with nitroimidazole bacterial resistance and six studies in people with both bacterial resistances. Five of these six studies reported the different cut‐off points for isolates assessed for nitroimidazole, clarithromycin, and amoxicillin; where minimal inhibitory concentrations to consider resistance were reported as ≥ 8 µg/mL for metronidazole, ≥ 1 µg/mL for clarithromycin and between 0.5 and 1 µg/mL for amoxicillin. The remaining study was an abstract and the information was not available and could not be retrieved from the authors.
Bias associated with selective reporting of this outcome measure therefore seemed likely.
Other potential sources of bias
Thirty‐five (81%) studies were in complete article form, indicating no bias due to publication status.
Studies were of mixed quality. Eradication was evaluated in subgroup analyses and the evidence was further assessed using GRADE. We include those subgroups in which eradication was found to be significantly different among groups or where subgroups were thought to influence H. pylori treatment efficacy in Table 1. We downgraded the quality of the RCT evidence for the following outcomes: publication date (moderate quality), geographic region (low quality) and antibiotic resistance (very low quality). The analyses based on STT length and the adverse event rate were rated as high quality.
Effects of interventions
See: Table 1
Overall H. pylori eradication
We include 44 studies in the overall analysis comparing SEQ versus STT.
Note that for the overall analysis, when combining data and only within the STT arm, several studies randomised participants into up to three different STT arms (7, 10 and 14 days). In order to preserve randomisation and weight among included studies, we present the final overall proportion of people cured with STT as a single figure, by adding the number of people cured in each of the three STT arms (as stated in the section Unit of analysis issues). The total of events (total STT eradication) was divided over the total of people assessed within the three STT arms.
The meta‐analysis showed that in an ITT analysis, the overall eradication proportion was higher with SEQ compared to STT (P < 0.001; Analysis 1.1). The risk difference (RD) for the overall ITT eradication of H. pylori was 0.09, 95% confidence interval (CI) 0.06 to 0.11; participants = 12,701; 44 studies; and the NNTB was 13 with a 95% CI of 11 to 16.
1.1. Analysis.
Comparison 1 Sequential therapy versus standard triple therapy, Outcome 1 Eradication proportion.
Results were highly heterogeneous (I² = 75%), so we considered a random‐effects model to be more appropriate to combine the dichotomous outcomes of the different studies.
Two studies (Aminian 2010; Greenberg 2011) demonstrated a significantly higher efficacy with STT. Both of the studies assessed adults: Aminian 2010 from Iran reported an ITT cure proportion of 91% and 80% with STT and SEQ respectively. Greenberg 2011, a multicentre trial in Latin America, reported an ITT cure proportion of 82% and 76% with STT and SEQ respectively. Two other studies (Lopez‐Román 2011; Zhou 2014) showed better efficacy of STT compared to SEQ, although differences between therapies were not statistically significant.
One included study (Laving 2013) reported the same ITT eradication in both treatment arms. The reason is that the test for assessment of H. pylori eradication was not performed in several participants allocated to the SEQ treatment arm. The per protocol analysis reported that 22 of 26 participants were cured in the SEQ arm while 22 of 45 were cured in the STT arm.
Twenty of the included studies did not demonstrate any clinical benefit for one regimen over the other. Thirteen of the studies (Ang 2015; Choi 2012; Gao 2010; Hsu 2014; Jeon 2013; Lee 2014; Lee 2015; Liou 2013; Liou 2014; Nasa 2013; Wu 2011; Yan 2011; Zhou 2014) were performed in Asia (mainly China and Korea but one in Japan, one in Singapore and one in India). The remaining studies were performed in South America (Lopez‐Román 2011; Eisig 2014), Africa (Laving 2013), Saudi Arabia (Ali Habib HS 2013), Turkey (Rakici 2014) or Europe (Bontems 2011; Franceschi 2011). All of them were conducted between 2011 and 2015.
Subgroup analyses: effects of different variables on the efficacy of both eradication treatments
Geographic region
Over half (n = 23) of the included studies were conducted in Asia, over one‐third (n = 15) were conducted in Europe and three each in South America and Africa (Analysis 1.2).
1.2. Analysis.
Comparison 1 Sequential therapy versus standard triple therapy, Outcome 2 Geographic region.
Studies published in Europe had the greatest risk difference for SEQ versus STT (RD 0.16, 95% CI 0.14 to 0.19; 3796 participants; 15 studies; I² = 0%) when SEQ and STT were compared by subgroup analysis. Among the studies conducted in Europe (n = 15), most of them (n = 11) were conducted in Italy and the rest in Spain, Belgium, Poland and Slovenia. All but one of these studies (Bontems 2011) showed significant differences between therapies, and people given SEQ reported greater cure proportions than those given STT. Also, Molina‐Infante 2010 reported differences between SEQ and STT at a borderline statistical level (RD 0.12, 95% CI 0.00 to 0.24). Note that the four studies published outside Italy had a tendency towards lower efficacy with SEQ compared to STT than those studies conducted in Italy.
Studies conducted in Asia had a smaller risk difference for SEQ versus STT (RD 0.05, 95% CI 0.02 to 0.08; 6728 participants; 23 studies; I² = 60%) than those in Europe, Africa or South America. Most of the studies were conducted in China or Korea. Fifteen of them did not show significant differences between SEQ and STT, and results were heterogeneous. Among these 15 studies, five reported better efficacy with STT than with SEQ. The previous tendency for better efficacy with SEQ shown in the European studies was reduced in the Asian studies.
Among the studies conducted in Africa, the risk difference for SEQ versus STT was 0.14, 95% CI 0.07 to 0.22; 604 participants; 3 studies; I² = 25%. One study (Laving 2013) did not show a significant difference between SEQ and STT. Note that only three studies were included in this subgroup analysis and the reported confidence internal was wide; however, people tended to benefit more from SEQ than from STT.
The last subgroup analysis included studies conducted in South America, with a risk difference for SEQ versus STT reported as ‐0.06, 95% CI ‐0.10 to ‐0.01; 1156 participants; 3 studies; I² = 0%. One study (Eisig 2014) did not show a significant difference between SEQ and STT. The remaining two studies reported better cure proportions with STT than with SEQ, showing than participants in this subgroup could benefit more from STT than with SEQ, contrary to the results for other subgroups.
All subgroup analyses evaluating the geographic region presented significant differences between SEQ and STT showing SEQ was superior to STT but for the South American region where STT was significantly better than SEQ(test for subgroup differences: Chi² = 84.36, df = 3 (P < 0.001; I² = 96.4%).
Publication date
Included studies were published between 2002 and 2015. Given the evolution in the H. pylori resistance to antibiotics, which has been reported as increasing over the years, we planned a subgroup analysis in order to explore heterogeneity with respect to the year the study was conducted/published. SEQ was reported significantly superior to STT in both before and after 2008 subgroups and the treatment difference was supported by the test for subgroup differences (Chi² = 24.28, df = 1 (P < 0.001; I² = 95.9%).
To evaluate the time trend and explore potential cut‐off points for this tendency, we generated a linear weighted regression model (Figure 4). The regression was controlled by each study weight (measured using a random‐effects model) following the statistical assumptions of the rest of the meta‐analysis. This model showed a tendency towards a lower efficacy through the years in the overall mean eradication proportion for both therapies.
4.
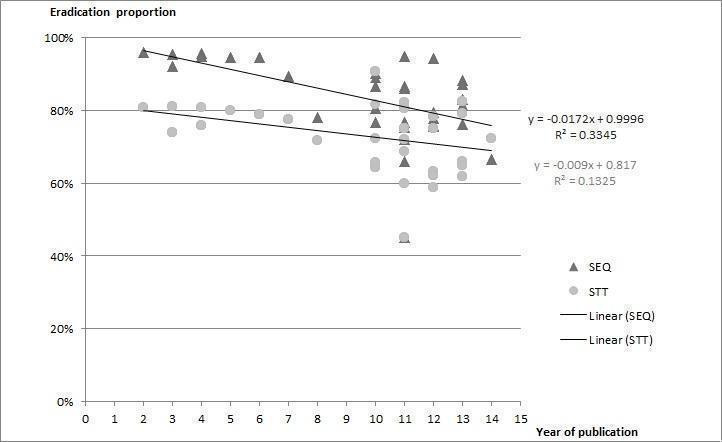
Weighted linear regression line in SEQ and STT by year of publication
As an exploratory model, it allowed us to identify a clear cut‐off point for subgrouping. Before 2008 the number of included studies per year was small and offered equivalent results (note that all the studies published before 2008 were of Italian origin); however, after 2009 the number of included trials per year increased and came from other countries and regions, and started to offer more heterogeneous results. No studies published in 2008 or 2009 met the inclusion criteria for our review.
Furthermore, as shown in the radar chart in Figure 5, both STT and SEQ eradication appeared constant (or similar) between studies before 2008 but after this year eradication was shown to be irregular over time, as represented by the various plots around the tendency lines between 2008 and 2015. We therefore used the lapsus years 2008 and 2009 as a cut‐off point for the forest plot analyses.
5.
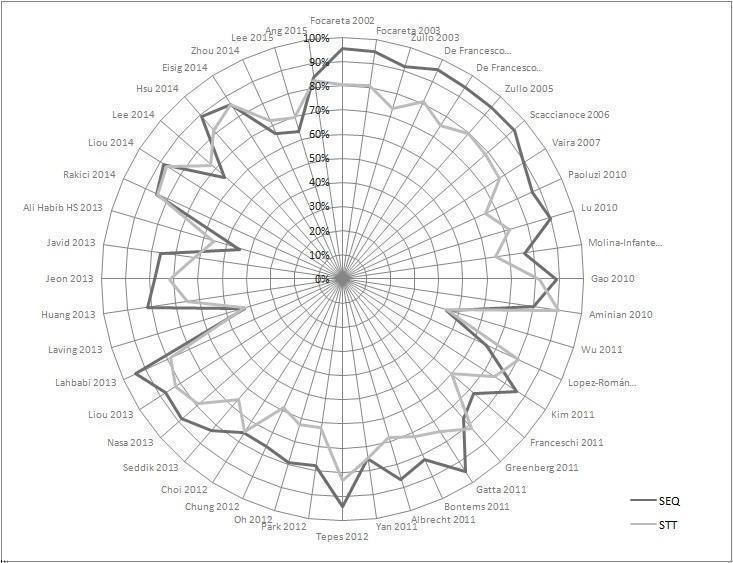
Radar chart depicting the eradication proportion for SEQ and STT in each included study
The forest plot (Figure 6; Analysis 1.3) presented differences in the eradication proportions between SEQ and STT among the studies performed before and after the year 2008. The risk difference for SEQ versus STT for the studies published before 2008 was 0.16, 95% CI 0.14 to 0.19; 2730 participants; 8 studies; I² = 0%, and the NNTB was 6 with a 95% CI of 5 to 7. The risk difference for SEQ versus STT for the studies published after 2008 was 0.06, 95% CI 0.03 to 0.09; participants = 10,021; studies = 36; I² = 69%. The NNTB was 18 and the 95% CI 14 to 26. Before 2008, studies reported higher eradication proportions and the RD was almost three times greater compared to studies published after 2008 (test for subgroup differences: Chi² = 24.28, df = 1 (P < 0.001); I² = 95.9%).
6.
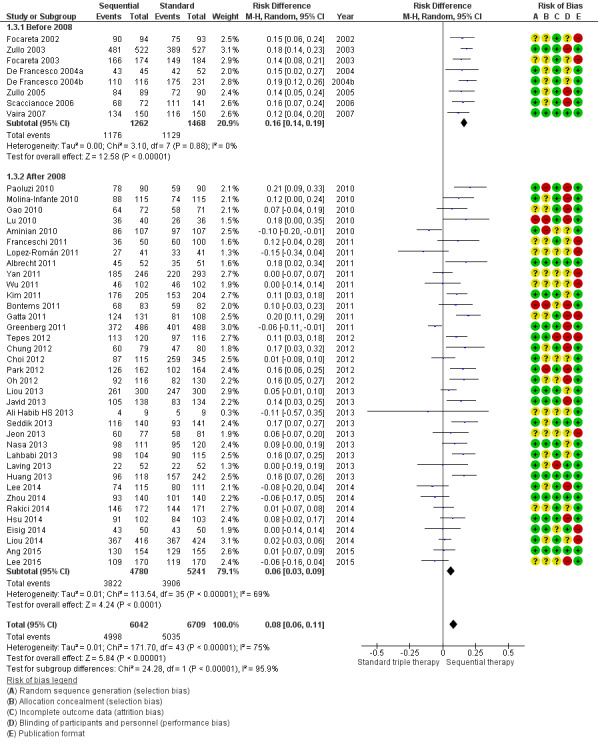
Forest plot of comparison: 1 Sequential therapy versus standard triple therapy, outcome: 1.3 Publication date.
1.3. Analysis.
Comparison 1 Sequential therapy versus standard triple therapy, Outcome 3 Publication date.
Two Italian studies (Gatta 2011; Paoluzi 2010) reported significantly larger risk differences for SEQ versus STT in the ‘after 2008’ subgroup. There is a decrease in SEQ eradication proportions below 90% starting in year 2008, except for four studies in which cure proportions were greater than or equal to 90% (Gatta 2011; Lahbabi 2013; Lu 2010; Tepes 2012).
As previously noted in our weighted regression model, a decreased efficacy over the years was shown for both therapies; however, this trend was more pronounced for SEQ (‐1.79% per year) than for STT (‐0.9% per year), which coincides with the lower RD obtained in the 'after 2008' subgroup.
Age of the population
All but six included studies were conducted in adults, with studies conducted in children (Albrecht 2011; Ali Habib HS 2013; Bontems 2011; Huang 2013; Laving 2013; Lu 2010) first published from 2010 onwards.
The pooled risk difference for eradication of H. pylori with SEQ compared to STT in children was reported to be slightly higher than in adults (Analysis 1.4). The risk difference in the children subgroup was 0.13, 95% CI 0.07 to 0.19; participants = 826; studies = 6; I² = 0%, and for adults RD 0.08, 95% CI 0.05 to 0.11; participants = 11,356; studies = 38; I² = 77%. However, the test for subgroup differences was not significant (Chi² = 2.18, df = 1; P = 0.14, I² = 54.1%) and differences between subgroups could not be clearly supported.
1.4. Analysis.
Comparison 1 Sequential therapy versus standard triple therapy, Outcome 4 Age of the population.
The NNTB in children was 8, with a 95% CI of 5 to 17, and in adults the NNTB was 13 with a 95% CI of 11 to 17.
Medical condition: non‐ulcer disease (NUD) versus peptic ulcer disease (PUD)
Twelve studies reported the baseline medical condition of participants. The risk difference for SEQ versus STT in the PUD group was 0.07, 95% CI ‐0.01 to 0.15; participants = 1822; studies = 9; I² = 81%, and in the NUD group the RD was 0.08, 95% CI ‐0.01 to 0.17; participants = 2293; studies = 8; I² = 87%. Differences between therapies were not significant (Analysis 1.5, test for subgroup differences: Chi² = 0.02, df = 1; P = 0.89, I² = 0%).
1.5. Analysis.
Comparison 1 Sequential therapy versus standard triple therapy, Outcome 5 Medical condition.
Length of the standard triple therapy (STT)
Analysis 1.6 compares 10‐day SEQ versus 7‐day (22 studies), 10‐day (19 studies) and 14‐day (eight studies) STT. SEQ was significantly better than 7‐day and 10‐day STT, but we found no significant differences between 14‐day STT and 10‐day SEQ (P = 0.32).
1.6. Analysis.
Comparison 1 Sequential therapy versus standard triple therapy, Outcome 6 STT length.
In the subgroup analysis, the H. pylori eradication proportions among the different STT lengths were compared with 10‐day SEQ. The risk difference in the 7‐day STT group was 0.14, 95% CI 0.12 to 0.17; participants = 5439; studies = 22; I² = 38%. In the 10‐day STT group, the risk difference was 0.06, 95% CI 0.02 to 0.10; participants = 3967; studies = 19; I² = 62%, and in the 14‐day STT group the RD was 0.02, 95% CI ‐0.02 to 0.06; participants = 3831; studies = 8; I² = 62%. The test for subgroup differences was significant (Chi² = 27.54, df = 2; P < 0.001; I² = 92.7%), supporting a clear difference between subgroups.
The NNTB when STT lasted seven days was 7, with a 95% CI of 6 to 8, and the NNTB when STT lasted 10 days was 20, with a 95% CI of 13 to 42.
Type of nitroimidazole
We included 43 studies in this subgroup meta‐analysis, with Liou 2014 not providing information on antibiotics or PPIs. Although we contacted the authors the information was not supplied.
Twenty‐one and 22 studies used metronidazole and tinidazole respectively in people treated with SEQ therapy. Both subgroups of people showed better results with SEQ than with STT (Analysis 1.7).
1.7. Analysis.
Comparison 1 Sequential therapy versus standard triple therapy, Outcome 7 Nitroimidazole type.
In the metronidazole group, the risk difference for SEQ versus STT was 0.07, 95% CI 0.03 to 0.11; participants = 6088; studies = 21; I² = 74%. The NNTB was 17 with a 95% CI of 13 to 27. In the tinidazole group the risk difference for SEQ versus STT was 0.11, 95% CI 0.08 to 0.15; participants = 5356; studies = 22; I² = 64%. The NNTB was 9 with a 95% CI of 7 to 11.
However, differences between these two subgroups of people treated with different nitroimidazole types were not significant for H.pylori eradication. Individual study risk differences did not particularly overlap, and heterogeneity was therefore substantial (test for subgroup differences: Chi² = 2.23, df = 1; P = 0.14, I² = 55.2%).
Acid inhibition with proton‐pump inhibitor (PPI)
Both STT and SEQ regimens used different PPIs (omeprazole, lansoprazole, pantoprazole, rabeprazole, or esomeprazole), as well as different PPI doses among the included studies. We performed a subgroup analysis to compare the efficacy of adjuvant medication within both treatment regimens. Acid inhibition was classified according to the type and dose of the PPI following the equivalences generally accepted (omeprazole 20 mg = pantoprazole 40 mg, lansoprazole 30 mg, rabeprazole and esomeprazole 20 mg).
We included 42 studies within this subgroup meta‐analysis. Two studies (Ang 2015; Liou 2014) were excluded, as they did not report data for PPI. In Ang 2015, we contacted the first author for the PPI information, who reported that most of the participants were given omeprazole standard doses, although some of them had rabeprazole or esomeprazole. We therefore decided not to include these data in the subgroup analysis, for consistency with the remaining included studies. We also excluded studies using paediatric injection formulations by participants' weight (Huang 2013; Lu 2010), as they cannot be pooled together with adult fixed‐tablet doses.
Only one study (Franceschi 2011) evaluated low acid inhibition with lansoprazole 15 mg twice a day, yielding a RD for SEQ versus STT of 0.24 (95% CI 0.05 to 0.43; 100 participants) for higher efficacy in SEQ. The majority of studies (n = 36) evaluated standard doses of the PPI, showing a marginally significant advantage for the use of SEQ versus STT (RD 0.09, 95% CI 0.06 to 0.12; 9794 participants). However, there was no increase in efficacy for SEQ in the three studies using potent acid inhibition (RD 0.02, 95% CI ‐0.17 to 0.21; 805 participants; Analysis 1.8).
1.8. Analysis.
Comparison 1 Sequential therapy versus standard triple therapy, Outcome 8 PPI acid inhibition.
We found no differential effect based on levels of acid inhibition with PPI (test for subgroup differences: Chi² = 2.96, df = 2; P = 0.23, I² = 32.4%)
Bacterial antibiotic resistance
Most of the studies did not perform prior antibiotic susceptibility testing, and eight out of 44 (18%) studies reported eradication by bacterial antibiotic resistance (Analysis 1.9).
1.9. Analysis.
Comparison 1 Sequential therapy versus standard triple therapy, Outcome 9 Bacterial antibiotic resistance.
We conducted subgroup meta‐analyses including separating H.pylori eradication for people with bacterial clarithromycin resistance, nitroimidazole resistance and dual resistance.
In the subgroup meta‐analyses, people with bacterial clarithromycin‐resistance eradication were significantly better when treated with SEQ therapy than with STT. The risk difference among this subgroup of participants was 0.33, 95% CI 0.13 to 0.54; participants = 214; studies = 8; I² = 64%. The NNTB was 3 with a 95% CI of 2 to 5. Although there seemed to be a similar trend for nitroimidazole resistance (87% versus 84%) and dual resistance (68% versus 63%), these apparent differences did not reach statistical significance.
Differences between all subgroups were significant, although heterogeneity in results was substantial (test for subgroup differences: Chi² = 7.12, df = 2; P = 0.03, I² = 71.9%). Additionally, the RD for SEQ versus STT within the clarithromycin‐resistance subgroup analysis was also greater compared to the risk difference of the overall eradication analysis (0.33 versus 0.13, respectively), meaning differences between treatment arms were even greater among those people with primary resistances.
Adverse events
Twenty‐seven studies (61%) described common adverse events (AEs) such as abdominal pain, diarrhoea, nausea, glossitis and vomiting, giving their incidence by treatment arms (Analysis 1.10). The trial reports did not mention whether there were any serious AEs.
1.10. Analysis.
Comparison 1 Sequential therapy versus standard triple therapy, Outcome 10 Adverse events rate.
Within the SEQ arms the incidence of AEs ranged from 2% in Aminian 2010 to 57% in Lee 2015. In the STT arm the incidence ranged from 2% in Aminian 2010 to 55% in Liou 2013.
In the ITT meta‐analysis, the overall adverse event proportions showed no significant differences between SEQ and STT (20.4% versus 19.5%, respectively). None of the studies was able to demonstrate differences between the side effects of the therapies. The risk difference was RD 0.00, 95% CI ‐0.01 to 0.02; participants = 8103; studies = 27. Results were homogeneous (I² = 26%), so we used a fixed‐effect model as appropriate. The NNTH was 105 with a 95% CI of 37 to 126.
Compliance
Compliance rates were reported in 21 studies. However, compliance definitions varied across studies, being defined as "good compliance" in most of the studies if participants had taken between 90 and 95% of the pills. In one study (Greenberg 2011), authors did not specified a minimum intake and reported compliance rates at different levels: when participants had taken all pills (100%), nearly all (defined as more than 80%), most of the pills (between 50 and 80%), less than the half of the pills (less than 50%), undetermined (but not all) and none of the pills.
For instance, in the study by Park 2012 compliance proportions were lower than in the other studies in both treatment arms: 72% and 58% with SEQ and STT respectively. In the study by Aminian 2010, compliance was reported as 100% in both treatment arms.
Sensitivity analysis
Risk of bias
We conducted sensitivity analyses on the 'Risk of bias' items assessed during the review process, in order to see whether our findings were robust. The table below summarises the risk difference in the overall eradication proportion when studies categorised as 'unclear' or 'high risk' for each domain were excluded from the overall meta‐analysis.
| Risk of bias item | RD (95% CI) in sensitivity analysis | Impact on the overall eradication |
| Randomisation (n = 12 excluded studies) | *0.09 (0.05 to 0.12) | Differences between therapies are significant |
| Allocation concealment (n = 30 excluded studies) | 0.08 (0.03 to 0.13) | Differences between therapies are significant |
| Blinding (n = 35 excluded studies) | 0.08 (0.03 to 0.10) | Differences between therapies are significant |
| Incomplete outcome data (n = 12 excluded studies) | *0.11 (0.08 to 0.14) | Differences between therapies are significant |
| Publication format (n = 35 excluded studies) | *0.09 (0.05 to 0.12) | Differences between therapies are significant |
When we compared the different RDs with the overall pooled RD 0.09 95% CI 0.06 to 0.11 (Analysis 1.1), none of the items appeared to reduce the absolute risk of one treatment over the other, and for some of the domains (*) the absolute risk increased when we excluded the poorest‐quality studies.
Also, differences between treatment arms remained significant as in the overall analysis. The 'Risk of bias' items therefore do not appear to influence the overall results when we compare SEQ to STT.
Year of publication
Given the strong differences we found regarding the year of publication, we repeated all subgroup analyses separating publications by the year published (before or after 2008 ‐ 2009). This sensitivity analysis, summarised in the table below, showed effect difference in only two subgroup analyses.
In the subgroup analysis by baseline medical condition, the non‐significant tendencies towards the superiority of SEQ compared to STT, in both NUD and PUD people found using all time‐span studies (Analysis 1.5), were reduced and nearly eliminated in studies performed after 2008 or 2009.
For the length of the STT regimen, the previously reported benefit of SEQ when compared to 10‐day STT (Analysis 1.6), could not be demonstrated in the most recent studies (2010 onward), in which the efficacy of SEQ was equivalent to that of 10‐day STT.
| Subgroups by year of publication (after 2008 or 2009) | RD (95% CI) in sensitivity analyses | Impact on the overall eradication |
| Baseline medical condition ‐ PUD people | 0.02 (‐0.07 to 0.12) | Tendency towards lower/no differences between therapies |
| Baseline medical condition ‐ NUD people | 0.01 (‐0.09 to 0.11) | Tendency towards lower/no differences between therapies |
| Length of STT regimen ‐ 10 days | 0.04 (‐0.01 to 0.09) | Tendency towards lower/no differences between therapies |
Length of STT
As previously mentioned, the length of the regimen is a major factor affecting the efficacy of antibiotic treatments, especially in the case of H. pylori. As shown in our length‐dependent subgroup analysis, the differences between SEQ and STT is reduced the longer the STT regimen is prescribed. Since STT is usually recommended as a 10‐day regimen, the same number of days of SEQ are given. For these reasons and to try and maintain fair comparisons, we confined our subgroup analyses to those studies comparing arms lasting 10 days.
| Subgroups by STT length 10 days | RD (95% CI) in sensitivity analyses | Impact on the overall eradication |
| Baseline medical condition ‐ PUD people | 0.02 (‐0.10 to 0.13) | Tendency towards lower/no differences between therapies |
| Baseline medical condition ‐ NUD people | 0.10 (‐0.06 to 0.26) | Tendency towards higher differences between therapies |
| Clarithromycin resistance | 0.54 (0.33 to 0.75) | Tendency towards higher differences between therapies |
| Nitroimidazole resistance | 0.01 (‐0.08 to 0.11) | Tendency towards lower/no differences between therapies |
| Dual resistance | ‐0.12 (‐0.32 to 0.08) | Tendency shift towards higher efficacy with STT |
| PPI dose ‐ standard acid inhibition | 0.06 (0.00 to 0.12) | Tendency towards lower/no differences between therapies |
| PPI dose ‐ high acid inhibition | ‐0.06 (‐0.16 to 0.04) | Tendency shift towards higher efficacy with STT |
| Geographic region ‐ Latin America | ‐0.06 (‐0.20 to 0.09) | Tendency towards lower/no differences between therapies |
| Geographic region ‐ Africa | 0.00 (‐0.19 to 0.19) | Tendency towards lower/no differences between therapies |
| Geographic region ‐ Asia | 0.03 (‐0.03 to 0.10) | Tendency towards lower/no differences between therapies |
Discussion
Multiple treatments have been suggested for H. pylori infection and have been discussed in the literature. Despite the large number of studies performed in the last two decades, no optimal first‐line eradication regimen has yet been defined.
There could be many explanations, but mainly efficacy, cost and ease of administration of drugs, as well as antibiotic resistance, have been reported among current challenges that need to be overcome.
Summary of main results
Our primary aim was to evaluate the efficacy of 10‐day SEQ versus STT from available published RCTs. The secondary objective was to compare the incidence of adverse events.
The screening and full‐text assessment of citations resulting from both the electronic and handsearches yielded 44 included RCTs. All studies addressed treatment and compared 10‐day SEQ versus 7‐, 10‐ or 14‐day STT.
From the included studies, 11 (25%) were published as abstracts from Congresses or Conferences; the sensitivity analyses showed that no effect modification was associated with the format of the publication, nor with the quality items assessed for all included studies. This ensures the robustness of the findings in this systematic review.
Among the other subgroup analyses, 25% of studies were published in Italy (n = 11), and among those eight were published before 2008. Many others were published after 2008 (n = 33), with very little of the evidence addressing children (n = 6). Efficacy was only given by pre‐treatment antibiotic susceptibility in eight studies, although antimicrobial resistance was discussed in almost all of the studies.
Overall, the efficacy of 10‐day SEQ was higher than treatment with 7‐or 10‐day STT, but we found no differences when 10‐day SEQ was compared with 14‐day STT. Moreover, the alleged superiority of SEQ and 10‐day STT disappeared when we used only recent studies (after 2009).
Overall efficacy of SEQ versus STT
Our efficacy endpoint of interest was the H. pylori ITT eradication proportion. From the 44 included studies covering 12,751 participants the overall meta‐analysis showed a significantly higher efficacy for 10‐day SEQ over the 7‐ or 10‐day STT. However, eradication in both SEQ and STT arms still remained lower (83% versus 75% respectively) than the optimal eradication levels (≥ 90%) generally required for microbial infections.
Our findings also showed that the efficacy of both regimens is decreasing over time.
Lack of optimal treatment effect has been mainly attributed to antibiotic resistance.The efficacy of SEQ was much less affected by clarithromycin resistance (‐8% eradication) than STT (‐32% eradication), which may indicate a beneficial effect of using SEQ versus STT in those areas in which clarithromycin resistance is high (> 20%).
Additionally, in previous studies, the success or failure of antibiotic regimens has been associated with a number of different factors, such as: number of antibiotics used, poor compliance, type of underlying disease such as PUD or NUD, shorter versus longer STT duration (7 versus 10 versus 14 days), drug‐related AEs, PPI type and dosage, previous stomach bacterial load, bacterial virulence (Cag A status), tobacco use, age of the population, geographical region, or any other variable that could predict or influence the treatment outcome (Vilaichone 2006).
We therefore decided to review each of the above variables that were suggested to potentially affect the efficacy of the therapeutical regimen.
Subgroup analyses: variables influencing efficacy of both treatments
Geographic region
A previous review (Gisbert 2010) showed that almost all studies comparing SEQ and STT therapies were performed in Italy, contributing to a lack of validation of findings in other settings. This limitation has been overcome in our meta‐analysis, with 11 studies performed in Italy and all of them showing a significant and clear advantage of SEQ over STT. The majority of studies from other European countries also identified this advantage, although with lower differences in eradication between arms.
The advantage of SEQ was also observed, with lower risk differences, in Asia and Africa; but STT offered higher eradication proportions versus SEQ in Latin America. As others have already noted, geographic location may be a surrogate factor for a given pattern of efficacy (or resistance) rather than a direct predictor of efficacy outcome (Graham 2011; Moayyedi 2009).
Publication date
We noted a trend toward a lower efficacy for both STT and SEQ in studies published after the year 2008 (Figure 4; Figure 5)
Published literature on the topic argues antibiotic resistance might be one of the most relevant factors mediating the trend of decreased efficacy of treatments over time, and a growing increase in clarithromycin resistance could explain the lower efficacy for both regimens. It is important to mention that if we consider the most recent publications (2010 onward) we found no differences when comparing SEQ with STT when the latter was used for 10 or 14 days.
Effect modifiers over time
It is worth noting that from the results of this meta‐analysis, we could not determine why studies published before 2008 resulted in higher treatment efficacy following SEQ (93%) compared with those published after 2008 (80%). This finding could depend on the modulating effect of either the geographic region or on some unevaluated variables associated with the publication date of the included studies, such as an increase in resistance/resilience proportions of the strains, migrant population, etc.
As mentioned, only Italian studies were published before the year 2008, and treatment success or failure was measured by these published studies; factors other than the publication date related to the Italian setting may contribute to the observed change in efficacy over time. Another major effect modifier is the length of STT, which must clearly be taken in consideration in sensitivity analyses.
As we have identified or proposed several modulators in this review, a meta‐regression analysis focusing on these factors should be performed as a follow‐up, which would enhance the evidence base on the best conditions for the use of SEQ treatment over STT.
Age of the population
Six RCTs assessed SEQ versus STT in children. Treatment with SEQ was more beneficial than with STT (76% versus 64%), but lower than in the adult population (83% versus 75%, respectively). Data from previous meta‐analyses showed similar results (Gatta 2009; He JD 2013; Horvath 2012), although as for adults, eradication rates with SEQ in children did not achieved the desired level of success.
Medical condition
Dyspepsia (functional or uninvestigated) is a common condition, and no therapy has yet been identified to effectively treat this disorder.
Findings of our review suggest that the eradication proportion following SEQ was similar for NUD and PUD people (85% and 83%, respectively), and that the previously reported differences for STT were not demonstrated in this review (76% versus 77%). Additionally, we found no differences for PUD or NUD people. However, we noted a tendency towards an increased benefit of SEQ over STT in both subgroups of people.
As reported in previous studies (De Francesco 2004a; Tong 2009) the fact that eradication proportions in both PUD and NUD people following SEQ were similar suggests that the SEQ scheme might overcome differences in participants' baseline medical conditions in a similar manner, or that the underlying disease itself is not a moderator nor a predictor of the treatment outcome.
STT length
In order to support and reinforce the curative effect of STT, some studies focused on investigating treatment duration. It has been postulated that longer treatment duration, for example extending STT to 14 days, might result in higher efficacy (Calvet 2000; Ford 2003; Fuccio 2007; Gisbert 2013).
In our review, 10‐day SEQ was more effective than 7‐ and 10‐day STT. We found no differences in efficacy between 10‐day SEQ and STT lasting 14 days. However, our sensitivity analysis did not support the superiority of SEQ over 10‐day STT in studies published from 2010 onward, where SEQ and 10‐day STT efficacies were reported to be equivalent.
Acid inhibition with PPI
Efficacy for SEQ was higher than with STT, regardless of the PPI dose used, although this benefit was unclear and nonsignificant when using high potent inhibition (double‐dose PPI). SEQ and STT showed a trend towards smaller differences in efficacy when the PPI coadjuvation was more potent (RD 0.24 for low inhibition, 0.09 for standard, and 0.02 for high).
When including only studies where SEQ and STT were both given for 10 days, the alleged benefit offered by SEQ was reduced to marginal significance in the standard inhibition studies. In the case of studies using double‐dose PPI (high acid inhibition) this benefit shifted, offering a better result with STT (only one study, Lee 2015; RD ‐0.06, 95% CI ‐0.16 to 0.04).
Bacterial antibiotic resistance
Eradication within antimicrobial‐resistant strains was reported in only eight studies. This represents a major limitation of our review, due to the lack of reporting of reliable, consistent and updated information on the prevalence of antibiotic susceptibility and resistance within the included RCTs.
Antimicrobial resistance has been considered the main factor responsible for the low efficacy of STT and for the decrease in eradication over time for SEQ (Mégraud 2004; Mégraud 2007a; Mégraud 2007b; Mégraud 2013).
In our review, SEQ was significantly more beneficial than STT only in those people with bacterial resistance to clarithromycin. This advantage was even more evident when both treatments were given for the same number of days. STT seems in any event to be more affected by resistance to clarithromycin (‐34% in efficacy) than SEQ (‐13%).
The benefit of SEQ over STT was not demonstrated for nitroimidazole or dual‐resistant strains. It is important to mention that efficacy for nitroimidazole‐resistance strains seems to be higher than the overall analysis, both for SEQ (87% verus 82%) and for STT (84% versus 75%). This counterintuitive improvement is due to the variation in efficacy in the studies reporting eradication by antimicrobial resistance. If we consider only studies reporting efficacy by antimicrobial resistance, the overall eradication is 88% for SEQ and 77% for STT.
Dual resistance had a strong impact on both SEQ and STT, which showed efficacies of 67% and 63% respectively. This tendency towards superiority (+4%) of SEQ treatment in dual‐resistant strains was reversed when we looked at treatment arms lasting the same number of days (10‐day STT), in which 10‐day STT offered higher efficacy (+7%) than SEQ.
We could not conduct a meta‐analysis of susceptible strains, due to a lack of reporting from the included studies.
Safety
Safety was assessed through the incidence of AEs in included studies. The main category reported was gastrointestinal distress, such as abdominal pain, diarrhoea, nausea, glossitis and vomiting.
From the studies addressing tolerance and compliance, the overall incidence of AEs with SEQ and STT was reported to be similar (20% and 19.5%, P = 0.72). The interruption of treatment due to AEs was also similar between treatment arms (near 1% with SEQ and 1.5% with STT).
Our findings support data from previous meta‐analyses (Gatta 2009; Gisbert 2010; Jafri 2008), where AEs as well as compliance were found to be comparable between both regimens.
Overall completeness and applicability of evidence
There is a noticeable absence in the included RCTs of any systematic assessment of antibiotic susceptibility or bacterial resistance. The RCTs failed to systematically report eradication by groups of people with different underlying diseases (PUD and NUD, mainly). There are also very few studies in children. Almost 30% of studies failed to systematically report data on safety, compliance or withdrawals due to treatment side effects. These factors limit the completeness and ultimately the generalisability of the evidence to wider H. pylori‐infected populations.
However, the large number of included studies were sufficient to address the main objective, and to cover the interventions, participants and outcomes of interest. Results were validated through previous research and, most importantly, findings helped to inform clinical practice and generate further evidence‐based supported research .
The identified factors affecting the relative efficacy between the treatments, including resistance, region, year of publication, length of treatment, etc., should be taken into consideration by clinicians deciding between these two regimens. For this, it is important to clarify that subgroup analyses may be misleading if they are not treated with caution. Although the risk difference may be higher in some subgroup analyses, indicating a higher support for SEQ treatment, this does not mean that the efficacy of SEQ for that subgroup analysis has improved beyond its overall efficacy, and therefore does not mean that SEQ should be the treatment of choice in that context. For example, although the highest RD was found for clarithromycin‐resistance strains, the actual efficacy in that context for SEQ was much lower than the overall efficacy for SEQ. In this context, SEQ would offer significantly better results than STT, but these results would be strongly suboptimal, and other treatments (bismuth quadruple or non‐bismuth quadruple concomitant regimens) should be pursued instead, if available.
Quality of the evidence
Included studies were of mixed quality. Usually, randomisation was not preserved at the allocation or concealment levels, and sequence generation was inadequate in 30% of the studies. Outcomes based on the length of STT or the rate of AEs were categorised as high quality; however, we downgraded the quality of the evidence for following outcomes: publication date (moderate quality), geographic region (low quality) and antibiotic resistance (very low quality). Results for these outcomes should therefore be interpreted cautiously.
Intention‐to‐treat reporting
All analyses were based on risk differences using the ITT approach.
In the Methods section we observed that the proportion of participants for which there were missing outcome data and/or who were excluded from the analysis should be noted for each arm of the trial, and for the ITT analyses these participants were assumed to have failed therapy. This assumption is likely to have resulted in frequent misclassification of the outcome, eradication, in the individual studies, and this could lead to information bias for the estimates of the RD. There is a trade‐off with maintaining the randomisation in the analysis (ITT analysis) to maximise comparability of the groups, with the cost of doing so being to increase the risk of information bias.
For the meta‐analysis, ITT eradication was based on the study authors' statements; that is, all people after randomisation were accounted for in the analysis, and any complications such as non‐compliance, withdrawals, protocol deviations and anything happening after randomisation were not considered (Gupta 2011).
For our review, complete outcome data were available in all included studies except for two. Firstly, in Lopez‐Román 2011 and Wu 2011 the number of participants randomised to each of the treatment arms was not provided, so we had to estimate the ratio specifying the number of people cured over the total number of participants randomised to the treatment arm from the percentage of people cured. The estimated numbers did not always exactly match the percentages.
Secondly, we noted that although ITT analyses were used as in the definition above, retrieving data on the flow of participants in the different phases of the trial was often challenging. Sometimes RCTs failed to report reliable, complete and uniform definitions of participation proportions within the study flow diagram. Proportions of participants allocated to one treatment arm or another might therefore be responding to different participation definitions. On the other hand, authors of trials might be reporting proportions without explicitly specifying to which participation definition they were referring.
Reporting of baseline characteristics by treatment arm versus not reporting findings by treatment arm
Four studies (Huang 2013; Lopez‐Román 2011; Paoluzi 2010; Yan 2011) did not mention the medical condition of the included participants at baseline. One study (Gao 2010) reported the number of people with ulcers at baseline but reported no information on the eradication for these people by treatment arm, but only the ulcer cicatrisation proportion. Two other studies (Gatta 2011; Wu 2011) mentioned that participants with a medical condition were included, but gave no further detail. The remaining studies (except those included in Analysis 1.5) reported the number of people with NUD or PUD at baseline but did not report their H. pylori eradication by treatment arm. In total, 21 studies (47%) failed to report the eradication by medical condition after treatment with SEQ or STT. We contacted authors but could not obtain these results, either because we failed to reach the authors or because the requested information was not available.
Masking of personnel and participants
Most of the studies were not blinded (neither single‐ nor double‐blinded) and this could be construed as considerably reducing their quality. However, it is generally accepted that H. pylori eradication is not affected by blinding, as it is unlikely that the placebo effect would have an effect on the tests performed to confirm eradication, nor on the bacteria itself.
Furthermore, unmasked studies are thought to give a better estimation of the efficacy in clinical practice, as it is feasible that the more complex SEQ regimen may affect compliance and therefore treatment success (Gisbert 2010).
Sample size
For meta‐analysis, larger sample sizes increase our confidence in the estimate. In our review, 16 studies (36%) had a sample size of fewer than 100 people at randomisation in each treatment arm. Post hoc sensitivity analyses did not show an improvement in the overall effect size of SEQ when sample sizes were doubled in each of the arms. This confirmed the robustness of the results of the meta‐analyses.
Recommendations, other treatments for H. pylori eradication and further research
STT has been endorsed as a first‐line therapy for the eradication of H. pylori in several countries (Malfertheiner 2012). On the other hand, many studies have reported better efficacy for SEQ, especially when compared to 7‐ and 10‐day STT. As previously mentioned, SEQ showed encouraging results when used among clarithromycin‐resistant populations compared to STT, but its efficacy is currently at a suboptimal level.
STT can easily be converted into a non‐bismuth 'concomitant' quadruple therapy by adding a nitroimidazole to the regimen. A recent review evaluated the findings of previous RCTs that had compared non‐bismuth quadruple therapies with STT. Results showed that non‐bismuth quadruple concomitant therapy is as well tolerated as STT, yet is more effective (Gisbert 2011). Further to this research, a meta‐analysis of RCTs comparing concomitant and STT demonstrated that non‐bismuth quadruple (concomitant) therapy appeared to be an effective, safe, and well‐tolerated alternative to STT and was reported to be less complex than SEQ (Gisbert 2012). However, the majority of the studies testing the non‐bismuth quadruple concomitant regimen have been conducted in middle‐ and high‐income countries. More studies using this regimen are needed in low‐income countries where the burden of H. pylori infection is greatest.
Furthermore, this meta‐analysis needs to be updated. More recent studies have evaluated the use of non‐bismuth quadruple therapies (both SEQ and concomitant regimens) in clinical settings with increased clarithromycin‐resistance proportions, and although differences did not reach statistical significance, there was a tendency towards better efficacy with concomitant therapy (Huang 2013; Lim 2013; McNicholl 2012; Wu 2011). When SEQ and concomitant regimens are compared with studies using specifically the same length and dosage, efficacy is reported to be higher with concomitant regimens than with SEQ (McNicholl 2014).
It is clear from our review that, overall, SEQ was a better strategy than STT prior to 2008 in the majority of the settings assessed. However, further robust assessment should focus on investigating the higher efficacy of 10‐day SEQ compared with 14‐day STT, of SEQ versus non‐bismuth concomitant quadruple therapy, and of 14‐day SEQ therapy. For instance, in one of the included studies (Liou 2013) 10‐day SEQ and 14‐day SEQ were compared with each other and with STT. The 14‐day SEQ yielded higher efficacy than 10‐day SEQ and than STT. SEQ 14‐day eradicated more than 90% of H. pylori infections. The overall efficacy obtained with 10‐day SEQ treatment in our meta‐analysis was clearly suboptimal at only 83% overall. Moreover, as with the STT regimen, there was a trend towards a reduction in efficacy of SEQ over the years, which does not bode well for this strategy.
It is important to identify the fairness or unfairness of comparisons, and it seems unethical to evidence base the clinical decisions on outdated comparisons (prior to 2008), or on those using suboptimal regimens as the control (7‐day STT) (Graham 2012). The tendency towards the evidece‐based supporting improved STT regimens (high acid inhibition, longer treatment durations) (Gisbert 2013) should set these improved regimens as the threshold for comparisons; on this principle, 10‐day SEQ has been unable to demonstrate consistent superiority.
Potential biases in the review process
There were adequate data from the trials on the efficacy in the different treatment arms, although some (usually those in abstract form) tended to report percentages rather than the number cured in each regimen, requiring some basic calculations to estimate the number of people whose infection was eradicated. In such cases, eradication proportions were obtained straightforwardly, but some potential bias due to outcome reporting must be acknowledged. Statistically, pooling of the data in the meta‐analyses was clear and transparent.
The methodology used throughout this systematic review strictly followed Cochrane standards. Three review authors (JPG, AGM and OPN) conducted constant and comprehensive searches of journal and conference databases, to ensure that we had identified all published and unpublished trials. However, it may be relevant to note that the electronic searching was performed in three stages through the years that the review was in preparation, and this led to additional work in de‐duplicating references or identifying different published citations for the same study under different first authors' names. We would not recommend re‐running searches whenever possible, although this may be generally accepted among review authors, and is understandable in some situations due to the time taken to complete the review. We applied no language restrictions. We usually contacted authors to ascertain data or ask for relevant information, although some were not accessible within the time available.
Agreements and disagreements with other studies or reviews
Our systematic review supports previous findings which affirm that SEQ therapy is more beneficial than STT when given for seven or 10 days and where antimicrobial resistances are low.
Findings from previously published pooled data analyses (Chen 2009; Gatta 2009; Gisbert 2010; Horvath 2012; Jafri 2008; Moayyedi 2007; Tong 2009; Zullo 2007) also found a significantly higher efficacy for 10‐day SEQ over the STT. Furthermore, substantially decreased eradication (lower than 80%) by triple therapies has been reported in Europe (De Francesco 2004b; Janssen 2001; Laheij 1999), Asia (Wong 2000), United Sates (Laine 2000) and Canada (Hunt 2004).
Also, many studies addressing eradication therapies for H. pylori infection have been published and included in new systematic reviews and meta‐analyses (Gisbert 2011; McLoughlin 2005).
Authors' conclusions
Implications for practice.
Our review provides further robust assessments across a much broader range of people comparing SEQ versus STT than in previously published reviews.
Findings showed a clear benefit of 10‐day SEQ over 7‐day STT in treatment‐naïve H. pylori‐infected people overall. Although 10‐day SEQ seemed to achieve higher eradication proportions than 10‐day STT, this benefit was not found in the most recent studies (from 2010 and later).
We observed a higher efficacy of SEQ versus STT among people with clarithromycin‐resistant strains.
Neither SEQ nor STT were able to achieve optimal results and therefore the evidence base does not support the use of either treatments except in those settings in which over 90% eradication proportions were achieved.
Implications for research.
Given the results of our meta‐analysis, 10‐day SEQ has inadequate efficacy to be favoured as an alternative first‐line therapy for H. pylori infection. More importantly, the efficacy obtained with other proposed treatments, such as non‐bismuth quadruple concomitant regimen and 14‐day SEQ, should be explored further, especially in low‐income countries where the burden of infection is greatest.
Safety, compliance and withdrawals due to adverse events were usually under‐reported in the included studies, and need to be considered more fully and systematically in future primary studies.
Notes
This review is the result of splitting a previously published Cochrane protocol (Forman 2000) into several reviews which are complementary and smaller in scope.
Acknowledgements
We would like to thank Professors Stephanie J Taylor and Khalid S Khan based at the Centre for Primary Care and Pubic Heatlh at Queen Mary University of London for their role as independent supervisors of this review, which was part of a PhD dissertation with international mentorship. We also thank Professor Khalid S Khan for his participation during the Thesis' Steering Group Committee at the Faculty of Medicine (UAM), Madrid.
We would like to thank referees for their feedback: Sarah Rhodes, Gyorgy Buzas and Frances Kellie in their role of Statistical Editor, Clinical Reviewer and Consumer Reviewer respectively, during the external peer‐review process.
Appendices
Appendix 1. EBM Reviews ‐ Cochrane Central Register of Controlled Trials search strategy
Via OVID platform
1. Helicobacter pylori/
2. pylori.mp. [mp=title, original title, abstract, mesh headings, heading words, keyword]
3. Helicobacter Infections/
4. or/1‐3
5. ((triple or standard) adj2 (regimen or therapy or treatment)).tw.
6. (sequential adj2 (regimen or therapy or treatment)).tw.
7. PPI.mp.
8. Proton Pump Inhibitors/
9. (Clarithromycin or biaxin or Claripen or Claridar or clarith or Crixan or Clacid or Fromilid or infex or klaricid or Klabax or Klacid or Vikrol).mp.
10. (amoxicillin or amoxycillin or actimoxi or almodan or amix or amox or amopen or amoram or amoxicot or amoxil or amrit or biomox or clamoxyl or dispermox or galenamox or larotid or moxatag or moxilin or p‐hydroxyampicillin or penamox or polymox or respillin or rimoxallin or senox or sumox or Tormoxin or trimox or utimox or wymox or zoxycil).mp.
11. (Alphamox or Amocla or Amoksibos or Amoxiclav Sandoz or Amoxidal or Amoxin or Amoksiklav or Amoxibiotic or Amoxicilina or Apo‐Amoxi or Augmentin or Bactox or Betalaktam or Cilamox or Curam or Dedoxil or Duomox or E‐Mox or Enhancin or Gimalxina or Geramox or Hiconcil or Isimoxin or Klavox or Lamoxy or Moxilen or Moxypen or Moxyvit or Nobactam or Novamoxin or Ospamox or Panklav or Pamoxicillin or Panamox or Samthongcillin or Sinacilin or Tolodina or Yucla or Zerrsox or Zimox).mp.
12. nitroimidazoles/ or metronidazole/ or tinidazole/
13. nitroimidazole*.tw.
14. (Metronidazole or nabact or clont or danizol or edg dentalgel or elyzol or flagyl or gineflavir or metrocream or metrodzhil or metrogel or metrolotion or metrolyl or metronizole or metrotop or metrovex or metrozol or metryl or noritate or norzol or nydamax or obagi or protostat or rozex or satric or trichopol or tricom or trivazol or vandazole or vitazol or zadstat or zidoval).mp.
15. (Tinidazole or bioshik or fasigin or fasigyn* or tindamax or tricolam).tw.
16. or/5‐15
17. 4 and 16
Appendix 2. MEDLINE search strategy
Via OVID platform
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. clinical trials as topic.sh. 6. randomly.ab. 7. trial.ti. 8. or/1‐7 9. exp animals/ not humans.sh. 10. 8 not 9 11. Helicobacter pylori/ 12. pylori.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 13. Helicobacter Infections/ 14. or/11‐13 15. ((triple or standard) adj2 (regimen or therapy or treatment)).tw. 16. (sequential adj2 (regimen or therapy or treatment)).tw. 17. PPI.mp. 18. Proton Pump Inhibitors/ 19. (Clarithromycin or biaxin or Claripen or Claridar or clarith or Crixan or Clacid or Fromilid or infex or klaricid or Klabax or Klacid or Vikrol).mp. 20. (amoxicillin or amoxycillin or actimoxi or almodan or amix or amox or amopen or amoram or amoxicot or amoxil or amrit or biomox or clamoxyl or dispermox or galenamox or larotid or moxatag or moxilin or p‐hydroxyampicillin or penamox or polymox or respillin or rimoxallin or senox or sumox or Tormoxin or trimox or utimox or wymox or zoxycil).mp. 21. (Alphamox or Amocla or Amoksibos or Amoxiclav Sandoz or Amoxidal or Amoxin or Amoksiklav or Amoxibiotic or Amoxicilina or Apo‐Amoxi or Augmentin or Bactox or Betalaktam or Cilamox or Curam or Dedoxil or Duomox or E‐Mox or Enhancin or Gimalxina or Geramox or Hiconcil or Isimoxin or Klavox or Lamoxy or Moxilen or Moxypen or Moxyvit or Nobactam or Novamoxin or Ospamox or Panklav or Pamoxicillin or Panamox or Samthongcillin or Sinacilin or Tolodina or Yucla or Zerrsox or Zimox).mp. 22. nitroimidazoles/ or metronidazole/ or tinidazole/ 23. nitroimidazole*.tw. 24. (Metronidazole or nabact or clont or danizol or edg dentalgel or elyzol or flagyl or gineflavir or metrocream or metrodzhil or metrogel or metrolotion or metrolyl or metronizole or metrotop or metrovex or metrozol or metryl or noritate or norzol or nydamax or obagi or protostat or rozex or satric or trichopol or tricom or trivazol or vandazole or vitazol or zadstat or zidoval).mp. 25. (Tinidazole or bioshik or fasigin or fasigyn* or tindamax or tricolam).mp. 26. or/15‐25 27. 14 and 26 28. 10 and 27
Appendix 3. EMBASE search strategy
Via OVID platform
1. Clinical trial/
2. Randomized controlled trial/
3. Randomization/
4. Single‐Blind Method/
5. Double‐Blind Method/
6. Cross‐Over Studies/
7. Random Allocation/
8. Placebo/
9. Randomi?ed controlled trial$.tw.
10. Rct.tw.
11. Random allocation.tw.
12. Randomly allocated.tw.
13. Allocated randomly.tw.
14. (allocated adj2 random).tw.
15. Single blind$.tw.
16. Double blind$.tw.
17. ((treble or triple) adj blind$).tw.
18. Placebo$.tw.
19. Prospective study/
20. or/1‐19
21. Case study/
22. Case report.tw.
23. Abstract report/ or letter/
24. or/21‐23
25. 20 not 24
26. Helicobacter pylori/
27. pylori.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer]
28. Helicobacter Infections/
29. or/26‐28
30. ((triple or standard) adj2 (regimen or therapy or treatment)).tw.
31. (sequential adj2 (regimen or therapy or treatment)).tw.
32. PPI.mp.
33. Proton Pump Inhibitors/
34. (Clarithromycin or biaxin or Claripen or Claridar or clarith or Crixan or Clacid or Fromilid or infex or klaricid or Klabax or Klacid or Vikrol).mp.
35. (amoxicillin or amoxycillin or actimoxi or almodan or amix or amox or amopen or amoram or amoxicot or amoxil or amrit or biomox or clamoxyl or dispermox or galenamox or larotid or moxatag or moxilin or p‐hydroxyampicillin or penamox or polymox or respillin or rimoxallin or senox or sumox or Tormoxin or trimox or utimox or wymox or zoxycil).mp.
36. (Alphamox or Amocla or Amoksibos or Amoxiclav Sandoz or Amoxidal or Amoxin or Amoksiklav or Amoxibiotic or Amoxicilina or Apo‐Amoxi or Augmentin or Bactox or Betalaktam or Cilamox or Curam or Dedoxil or Duomox or E‐Mox or Enhancin or Gimalxina or Geramox or Hiconcil or Isimoxin or Klavox or Lamoxy or Moxilen or Moxypen or Moxyvit or Nobactam or Novamoxin or Ospamox or Panklav or Pamoxicillin or Panamox or Samthongcillin or Sinacilin or Tolodina or Yucla or Zerrsox or Zimox).mp.
37. nitroimidazoles/ or metronidazole/ or tinidazole/
38. nitroimidazole*.tw.
39. (Metronidazole or nabact or clont or danizol or edg dentalgel or elyzol or flagyl or gineflavir or metrocream or metrodzhil or metrogel or metrolotion or metrolyl or metronizole or metrotop or metrovex or metrozol or metryl or noritate or norzol or nydamax or obagi or protostat or rozex or satric or trichopol or tricom or trivazol or vandazole or vitazol or zadstat or zidoval).mp.
40. (Tinidazole or bioshik or fasigin or fasigyn* or tindamax or tricolam).tw.
41. or/30‐40
42. 29 and 41
43. 25 and 42
Appendix 4. CINAHL search strategy
Via OVID platform
S12 (S1 and S11)
S11 S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10
S10 Tinidazole
S9 Metronidazole
S8 nitroimidazole*
S7 amoxicillin
S6 Clarithromycin
S5 Proton Pump Inhibitors
S4 PPI
S3 sequential and ( (regimen or therapy or treatment) )
S2 ( (triple or standard) ) and ( (regimen or therapy or treatment) )
S1 Helicobacter pylori
Data and analyses
Comparison 1. Sequential therapy versus standard triple therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Eradication proportion | 44 | 12701 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.06, 0.11] |
| 2 Geographic region | 44 | 12284 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.06, 0.12] |
| 2.1 Europe | 15 | 3796 | Risk Difference (M‐H, Random, 95% CI) | 0.16 [0.14, 0.19] |
| 2.2 Asia | 23 | 6728 | Risk Difference (M‐H, Random, 95% CI) | 0.05 [0.02, 0.08] |
| 2.3 Africa | 3 | 604 | Risk Difference (M‐H, Random, 95% CI) | 0.14 [0.07, 0.22] |
| 2.4 South America | 3 | 1156 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.10, ‐0.01] |
| 3 Publication date | 44 | 12751 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.06, 0.11] |
| 3.1 Before 2008 | 8 | 2730 | Risk Difference (M‐H, Random, 95% CI) | 0.16 [0.14, 0.19] |
| 3.2 After 2008 | 36 | 10021 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.03, 0.09] |
| 4 Age of the population | 44 | 12284 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.06, 0.12] |
| 4.1 Children | 6 | 826 | Risk Difference (M‐H, Random, 95% CI) | 0.13 [0.07, 0.19] |
| 4.2 Adults | 38 | 11458 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.05, 0.11] |
| 5 Medical condition | 12 | 4115 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [0.02, 0.13] |
| 5.1 PUD only | 9 | 1822 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [‐0.01, 0.15] |
| 5.2 NUD only | 8 | 2293 | Risk Difference (M‐H, Random, 95% CI) | 0.08 [‐0.01, 0.17] |
| 6 STT length | 44 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 6.1 STT 7 days | 22 | 5439 | Risk Difference (M‐H, Random, 95% CI) | 0.14 [0.12, 0.17] |
| 6.2 STT 10 days | 19 | 3967 | Risk Difference (M‐H, Random, 95% CI) | 0.06 [0.02, 0.10] |
| 6.3 STT 14 days | 8 | 3831 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.02, 0.06] |
| 7 Nitroimidazole type | 43 | 11444 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.06, 0.12] |
| 7.1 Metronidazole | 21 | 6088 | Risk Difference (M‐H, Random, 95% CI) | 0.07 [0.03, 0.11] |
| 7.2 Tinidazole | 22 | 5356 | Risk Difference (M‐H, Random, 95% CI) | 0.11 [0.08, 0.15] |
| 8 PPI acid inhibition | 40 | 10699 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.06, 0.12] |
| 8.1 Low acid inhibition | 1 | 100 | Risk Difference (M‐H, Random, 95% CI) | 0.24 [0.05, 0.43] |
| 8.2 Standard acid inhibition | 36 | 9794 | Risk Difference (M‐H, Random, 95% CI) | 0.09 [0.06, 0.12] |
| 8.3 High acid inhibition | 3 | 805 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.17, 0.21] |
| 9 Bacterial antibiotic resistance | 8 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Clarithromycin resistance | 8 | 214 | Risk Difference (M‐H, Random, 95% CI) | 0.33 [0.13, 0.54] |
| 9.2 Nitroimidazole resistance | 7 | 413 | Risk Difference (M‐H, Random, 95% CI) | 0.04 [‐0.06, 0.14] |
| 9.3 Dual resistance | 6 | 205 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.14, 0.19] |
| 10 Adverse events rate | 27 | 8103 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.01, 0.02] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albrecht 2011.
| Methods | Double‐blind randomised trial Dates the study was conducted: from December 2006 to September 2009 Funding sources and potential conflicts of interest: funded by a research grant from the Ministry of Science and Higher Education (grant number 3D144; contract N 404 051 32/1330) The authors declare no conflicts of interest Definition of compliance: drug intake > 95% was considered good compliance |
|
| Participants | Number and type of participants: 107 H.pylori‐positive children were enrolled in the study Participants were randomised to 2 different treatment groups: a 7‐day standard triple regimen and a 10‐day sequential regimen Number of participants randomised: 107 (ITT sample) Number of participants in the 7‐day STT arm, ITT analysis: 51 Number of participants in the 10‐day SEQ arm, ITT analysis: 52 103 children (96%) were included in the final analysis at 6 ‐ 8 weeks. Four children were excluded from analysis for not initiating the therapy. The number of participants at PP analysis by treatment arm is not available Country: Poland Average age (standard deviation) of the population in years reported by treatment group:
Sex proportions (%) as M/F per treatment group:
Medical condition at baseline: Not reported H. pylori diagnostic method: ¹³C‐UBT, histopathology and RUT. At least 2 of the 3 tests had to be positive to consider the participants infected |
|
| Interventions |
Name, dose timing of antibiotics in 7‐day STT: omeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 7 days) + placebo (during the following 3 days, i.e. days 8 to 10) Name, dose timing of antibiotics in 10‐day SEQ: omeprazole 20 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and omeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + tinidazole 500 mg twice a day (during 5 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status after treatment: ¹³C‐UBT Time for assessment of H. pylori status after treatment: 6 ‐ 8 weeks |
|
| Outcomes | ITT eradication rate (%) (95%CI) by treatment group:
Number needed to treat for an additional beneficial outcome (NNTB) (95%CI): 6 (3 to 5) Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance in ITT sample SEQ / STT: > 95 % in all participants in both groups Incidence rate (%) of AEs:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study was truly randomised. Authors reported that a block randomisation was done using a computer‐generated random number list prepared by an investigator with no clinical involvement in the trial |
| Allocation concealment (selection bias) | Low risk | The allocation was concealed. To ensure blinding and independence of the drug manufacturers, the study products, in an appropriate dosage for a given participant, were crushed with a pestle and mortar by a hospital pharmacist. The study was conducted double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants and all personnel involved in the study were unaware of treatment assignments. The intervention sets were reported to have been prepared by the hospital pharmacy and by independent personnel not involved in the conduct of the study. Participants were given placebo during 3 days after the 7‐day STT |
| Publication format | Low risk | Full article |
Ali Habib HS 2013.
| Methods | Prospective randomised trial Dates the study was conducted: not reported Funding sources and potential conflicts of interest: funded by the Deanship of Scientific Research, (DSR), King Abdulaziz University (KAU), Jeddah, under grant number (332/140/1431). The authors, therefore, acknowledge with thanks DSR for technical and financial support. The authors declare no conflicts of interest Definition of compliance: evaluated by counting the number of tablets returned |
|
| Participants | Number and type of participants: 18 H.pylori‐positive children were enrolled in the study Participants were randomised to 4 different treatment groups (groups A to D). Group A was administered a quadruple therapy and group C a quinolone‐based triple therapy (which were not of interest in this review) Groups B and D were of interest. Group B consisted of a 10‐day standard triple regimen and group D consisted of a 10‐day sequential regimen Number of participants randomised: 18 (each group had the same number of participants) Number of participants in the 10‐day STT arm, ITT analysis: 9 Number of participants in the 10‐day SEQ arm, ITT analysis: 9 Number of participants in the 10‐day STT arm, PP analysis: 9 Number of participants in the 10‐day SEQ arm, PP analysis: 7 Country: Saudi Arabia Average age (standard deviation) of the population in years reported by treatment group: not reported ‐ but authors mentioned adults participants were included Sex (M/F) per treatment group, n (%): only men and boys in both groups Medical condition at baseline: all participants were NUD H. pylori diagnostic method: culture of endoscopy biopsies (both from the antrum ant corpus). RUT was applied to the biopsies |
|
| Interventions |
Name, dose timing of antibiotics in 10‐day STT: rabeprazole 20 mg twice a day + clarithromycin 250 mg twice a day + amoxicillin 500 mg twice a day (during 10 days) Name, dose timing of antibiotics in 10‐day SEQ: rabeprazole 20 mg twice a day + amoxicillin 500 mg twice a day (during 5 days) and rabeprazole 20 mg twice a day + clarithromycin 250 mg twice a day + tinidazole 500 mg twice a day (during 5 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status after treatment: UBT Time for assessment of H. pylori status after treatment: 6 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group:
PP eradication rate (%) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance in ITT sample SEQ/STT: 2 boys from the SEQ group were poorly compliant, considered drop‐outs and excluded Incidence of AEs per treatment group: not reported WIthdrawals due to AEs: not reported. Incidence (%) serious AEs SEQ/STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomised in 1:1 groups; the method of randomisation is not reported |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation is not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary outcomes are not clearly reported in an ITT analysis |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information regarding blinding |
| Publication format | Low risk | Full article |
Aminian 2010.
| Methods | Prospective randomised trial Dates the study was conducted: not reported Funding sources and potential conflicts of interest: No information reported Definition of compliance: drug intake > 85% was considered good compliance. It was defined by a questionnaire and assessed by the physician |
|
| Participants | Number and type of participants: 428 NUD and H.pylori‐positive participants were enrolled in the study Participants were randomised to 4 different treatment groups (groups A to D). Group A was administered a quadruple therapy and group C a quinolone‐based triple therapy (which were not of interest in this review) Groups B and D were of interest. Group B consisted of a 10‐day standard triple regimen and group D consisted of a 10‐day sequential regimen. Number of participants randomised: 428 (each group had the same number of participants) Number of participants in the 10‐day STT arm, ITT analysis: 107 Number of participants in the 10‐day SEQ arm, ITT analysis: 107 Number of participants in the 10‐day STT arm, PP analysis: 107 Number of participants in the 10‐day SEQ arm, PP analysis: 106 Country: Iran Average age (SD) of the population in years reported by treatment group:
Sex (M/F) per treatment group, n (%)
No significant differences in age and gender were reported between the treatment groups Medical condition at baseline: all participants were NUD H. pylori diagnostic method: culture of endoscopy biopsies (both from the antrum ant corpus). RUT was applied to the biopsies |
|
| Interventions |
Name, dose timing of antibiotics in 10‐day STT: omeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 10 days) Name, dose timing of antibiotics in 10‐day SEQ: omeprazole 20 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and omeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (during 5 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status after treatment: stool antigen test (using the HpSA enzyme‐linked immunosorbent assay (ELISA)) Time for assessment of H. pylori status after treatment: 8 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group:
PP eradication rate (%) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance in ITT sample SEQ / STT:100% in all treatment groups Incidence rate (%) of AEs per treatment group:
WIthdrawals due to AEs were not reported Incidence (%) serious AEs SEQ / STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Truly random as authors reported participants were randomised to 4 treatment groups using computer‐generated tables of random numbers |
| Allocation concealment (selection bias) | High risk | It seems the random‐number table was not concealed from the investigator |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information is reported regarding the masking |
| Publication format | Low risk | Full article |
Ang 2015.
| Methods | Prospective randomised controlled study Dates the study was conducted: from December 2011 to March 2014 Funding sources and potential conflicts of interest: study partially supported by research grant from Changi General Hospital. The authors declare no conflicts of interest Definition of compliance: not defined |
|
| Participants | Number and type of participants: 462 H.pylori‐positive participants were enrolled in the study Participants were randomised to 3 different treatment groups: 10‐day standard triple regimen, 10‐day sequential regimen and concomitant therapy Only data regarding standard triple and sequential therapies are reported Country: Singapore Number of participants randomised: 462 (ITT sample) Number of participants in the 10‐day STT arm: 155 Number of participants in the 10‐day SEQ arm: 154 Mean age (SD) of the population reported as the number of participants by treatment group:
Sex ratio (Male/out of total) (%) per treatment group
Medical condition at baseline, as the presence of ulcer out of the total (%):
H. pylori diagnostic methods in all treatment arms: ¹³C‐UBT, rapid urease test or histology |
|
| Interventions |
Name, dose timing of antibiotics in 10‐day STT: PPI* + clarithromycin 500 mg twice a day, amoxicillin 1g twice a day Name, dose timing of antibiotics in 10‐day SEQ: PPI* + amoxicillin 1 g twice a day (during 5 days) and PPI, clarithromycin 500 mg twice a day+ metronidazole 400 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status after treatment: ¹³C‐UBT Time for assessment of H. pylori status after treatment: 4 weeks |
|
| Outcomes | ITT eradication rate (%) (95% CI) by treatment group :
PP eradication rate (%) (95% CI) by treatment group:
Compliance rate (%) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported No data on antibiotic resistance profile and treatment arm were reported, given the small number per treatment arm did not allow further subgroup analysis of treatment outcome. However, cure proportions among the total number of participants treated reported as the rate (%) were given, stratified by antibiotic resistance:
Incidence of AEs by treatment group (n, %): not reported Incidence (%) serious AEs, SEQ/STT: not reported Adverse events causing termination of the study occurred in 1 participant on triple therapy who developed vomiting and severe abdominal discomfort |
|
| Notes | We contacted the first author for the PPI use: most of the participants were given omeprazole standard doses although some of them rabeprazole or esomeprazole. We decided not to include these data in the subgroup analysis, for consistency with the remaining included studies | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed in blocks of 15 |
| Allocation concealment (selection bias) | Low risk | All randomisation codes were placed into sealed opaque envelopes and kept by an independent research assistant |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcomes were reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The technician who performed the antibiotic susceptibility test was blinded to treatment allocation |
| Publication format | Low risk | Full article |
Bontems 2011.
| Methods | Large, multicentre, open‐label, randomised controlled trial Definition of compliance: consumption of > 90% of the prescribed drugs, determined by pill counts and determined by a close interview Dates the study was conducted: from October 2007 to June 2009 Funding sources and potential conflicts of interest: No funding sources reported.The authors report no conflicts of interest AEs were assessed by interview in all centres plus a specific questionnaire |
|
| Participants | Number and type of participants: 165 H.pylori‐positive consecutive children were enrolled in the study Participants were randomised to 3 different treatment groups: 2 tailored 7‐day standard triple regimens or a 10‐day sequential regimen. The triple therapy was tailored according to previously tested antimicrobial susceptibility: those participants in whom H.pylori strains were found susceptible to clarithromycin were administered clarithromycin, whereas those susceptible to metronidazole and clarithromycin‐resistant were given metronidazole. Results are only presented for the STT group where clarithromycin was administered Number of participants randomised: 165 (ITT sample) Number of participants in the 10‐day SEQ arm, ITT analysis: 83 Number of participants in the 7‐day STT arm, ITT analysis: 82 Number of participants lost to follow‐up: 15 (6 in the SEQ group and 9 in the STT group) PP sample: 150 participants Number of participants in the 10‐day SEQ arm, PP analysis: 77 Number of participants in the 7‐day STT arm, PP analysis: 71 Countries: Belgium, France, Italy Number (n) of children included by centre:
Average age (range) of the ITT population in years: 10.4 (2.7 to 17) Sex (M/F) of the ITT population: 70/95 There were no significant differences between the 2 treatment groups in gender (M/F ratio) or age Chronic relevant diseases were present in 56 children (> 1 in some children) Number of participants (%) of the ITT population per treatment group with a medical condition (according to the endoscopy): ‐ Erosive oesophagitis
‐ Nodular gastritis
‐ Gastric erosions or ulcer
‐ Duodenal erosions or ulcer
|
|
| Interventions |
Name, dose and timing of antibiotics in 7‐day STT: omeprazole 20 mg twice a day + amoxicillin 1 g twice a day + clarithromycin 500 mg twice a day (during 7 days) Name, dose and timing of antibiotics in 10‐day SEQ: omeprazole 20 mg twice a day + amoxicillin 1 g twice a day (5 days) + omeprazole 15 mg twice a day, clarithromycin 500 mg twice a day + metronidazole 20 mg twice a day (5 days) Note: omeprazole was administered as 10 mg twice a day below 30 kg bodyweight or 20 mg twice a day above 30 kg. Amoxicillin was administered 50 mg/kg twice a day ‐max 2 g/day. Metronidazole was administered 20 mg/kg twice a day ‐max 1.5 g/day Sensitivity test (yes/no) to antibiotics before/after treatment: yes Antimicrobial susceptibility testing by treatment group: Clarythromicin resistance, n (%):
Metronidazole resistance, n (%):
Amoxicillin resistance, n (%):
Susceptible both to clarithromycin and metronidazole, n (%):
Method of assessment of H. pylori status after treatment: ¹³C‐UBT Time for assessment of H. pylori status after treatment: 8 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group:
PP eradication (%) by treatment group:
ITT eradication rate (%) clarithromycin resistant strains:
PP eradication rate (%) clarithromycin resistant strains:
ITT eradication rate (%) metronidazole resistant strains:
PP eradication rate (%) metronidazole resistant strains:
ITT eradication rate to strains susceptible to both metronidazole and clarithromycin (P = 0.01):
PP eradication to strains susceptible to both metronidazole and clarithromycin (P = 0.01):
Compliance rates were not reported AEs incidence (%) by type and treatment group: ‐ Abdominal pain (difference not significant)
‐ Diarrhoea:
‐ Nausea:
‐ Vomiting:
Incidence of serious AEs SEQ/STT: Not reported 1 participant in the STT stopped the treatment prematurely. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No information was given regarding the method of randomisation |
| Allocation concealment (selection bias) | High risk | No information was given regarding the concealment of the sequence allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded as it was defined as "open‐label" |
| Publication format | Low risk | Full article |
Choi 2012.
| Methods | Prospective, randomised controlled study Dates the study was conducted: from March 2008 to August 2011 Funding sources and potential conflicts of interest: No information reported Definition of compliance: intake of > 95% of the pills |
|
| Participants | Number and type of participants: 460 H.pylori‐positive participants were included in the study from March 2008 to August 2011 Participants were equally randomised to 4 different treatment groups, each with 115 participants: 3 different triple regimens and a sequential regimen Number of participants randomised: 460 (ITT sample) Number of participants in the 7‐day STT arm: 115 Number of participants in the 10‐day STT arm: 115 Number of participants in the 14‐day STT arm: 115 Number of participants in the 10‐day SEQ arm: 115 Country: Korea Average age of the population in years: 46.8 Sex (M/F) of the population: 221/239 Medical condition at baseline reported as n (%) by treatment group: ‐ Gastritis as NUD:
‐ Gastric ulcer:
‐ Duodenal ulcer:
H. pylori diagnostic method, n (%) in the population: not reported |
|
| Interventions |
Name, dose timing of antibiotics in 7‐day STT: rabeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 7 days) Name, dose timing of antibiotics in 10‐day STT: rabeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 10 days) Name, dose timing of antibiotics in 14‐day STT: rabeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 14 days) Name, dose timing of antibiotics in 10‐daySEQ: rabeprazole 20 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and rabeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + tinidazole 500 mg twice a day (during 5 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status after treatment: ¹³C‐UBT (in 408/427 participants) and histopathology (in 19/427 participants) Time for assessment of H. pylori status after treatment: 4 weeks |
|
| Outcomes | The study flow chart showed that 8, 10 and 6 participants did not complete the study in the 7‐day STT, 10‐day STT and 14‐day STT respectively, whereas 9 participants dropped out of the study in the 10‐day SEQ Eradication could be confirmed in 427 participants ITT eradication rate (%) by treatment group:
PP eradication rate (%) by treatment group:
Metronidazole resistance (%) before treatment, SEQITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance in ITT sample was reported as > 95% in all treatment groups. Incidence rate of AEs in the ITT sample and by treatment group:
Incidence (%) serious AEs SEQ / STT: not reported |
|
| Notes | We selected 10‐day STT ITT eradication for inclusion in the overall eradication meta‐analysis as per equivalence of comparator treatment, i.e. 10‐day SEQ duration. The other ITT eradication proportions corresponding to both the 7‐day STT and the 14‐day STT therapies have been reported under the subgroup meta‐analysis "STT length". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | No information on the allocation concealment was provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary outcome were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on the masking was provided |
| Publication format | Low risk | Full article |
Chung 2012.
| Methods | Prospective, randomised, controlled, open‐label study Dates the study was conducted: from November 2010 to August 2011 Funding sources and potential conflicts of interest: investigator‐initiated study funded partly by Jeil Pharma. The authors declare no conflicts of interest Definition of compliance: > 90% |
|
| Participants | Number and type of participants: 159 H.pylori‐positive participants were enrolled in the study Participants were randomised to 2 different treatment groups: 1 group received a 10‐day sequential therapy and the other group received a 10‐day triple therapy Number of participants randomised: 159 (ITT sample) Number of participants in the 10‐day SEQ arm: 79 Number of participants in the 10‐day STT arm: 80 PP sample: 68 participants in each arm. In the SEQ arm, the study flow diagram reported 3 dropouts due to side effects and 9 lost to follow‐up. In the 10‐day STT arm, 3 dropouts due to side effects were reported and 8 lost to follow‐up. Country: Korea Average age (SD) of the population in years: 49.6 (11.1) Sex (M/F) per treatment group
Medical condition at baseline: all participants were PUD participants ‐ Gastric ulcer:
‐ Duodenal ulcer:
‐ Gastric + duodenal ulcer:
|
|
| Interventions |
Name, dose timing of antibiotics in 10‐day STT: lansoprazole 30 mg twice a day + amoxicillin 1 g twice a day + clarithromycin 500 mg twice a day Name, dose timing of antibiotics in 10‐day SEQ: lansoprazole 30 mg twice a day + amoxicillin 1 g twice a day (5 days) and esomeprazole 30 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (5 days) Length of STT (days): 10 days Sensitivity test (yes/no) to antibiotics before/after treatment: no Method of assessment of H. pylori status after treatment: ¹³C‐UBT Time for assessment of H. pylori status after treatment: 4 ‐ 6 weeks |
|
| Outcomes | ITT eradication rate (%) (95% CI) by treatment group: (P = 0.01)
PP eradication rate (%) (95%CI) by treatment group: (P < 0.01)
Overall, bacterial culture was successful in 93 out of 159 participants. rate (%) of participants resistant to each antibiotic are reported:
Resistance was not reported by treatment groups. Compliance rate (%) reported as < 90% in the ITT sample and by treatment group:
Incidence rate (%) of AEs: reported by treatment group 10‐day SEQ/10‐day STT respectively and by type of AE:
Incidence (%) serious AEs SEQ/STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study was truly random, as authors used computer‐generated randomisation sequences table with block sizes 4 or 6 |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded as it was reported as open‐label |
| Publication format | Low risk | Full article |
De Francesco 2004a.
| Methods | Randomised controlled trial Dates the study was conducted: not reported Funding sources and potential conflicts of interest: No information reported Definition of compliance: > 90% intake was considered as 'acceptable' |
|
| Participants | Number and type of participants: 97 H.pylori‐positive participants were included in the study Number of participants randomised: 97 (ITT sample) Number of participants in the 10‐day SEQ arm: 45 (group A) Number of participants in the 10‐day STT arm: 52 (group B) Country: Italy Average age (SD) of the ITT population in years, by treatment group:
Sex (M/F) per treatment group
Number of participants (rate) of the ITT population per treatment group with a medical condition: ‐ NUD
‐ PUD
At baseline, the groups were reported homogeneous with regard to sex, age, smoking habit and endoscopic findings (PUD/NUD) |
|
| Interventions |
Name, dose timing of antibiotics in 10‐day STT (group B): rabeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 10 days) Name, dose timing of antibiotics in 10‐day SEQ (group A): rabeprazole 20 mg twice a day + amoxicillin 1 g twice a day (5 days) + rabeprazole 20 mg twice a day, clarithromycin 500 mg twice a day,tinidazole 500 mg twice a day (5 days) Sensitivity test (yes/no) to antibiotics before/after treatment: no Method of assessment of H. pylori status after treatment: ¹³C‐UBT Time for assessment of H. pylori status after treatment: 6 ‐ 8 weeks |
|
| Outcomes | 95 participants completed the study: 2/45 (2%) did not return after therapy for unreported reasons (1 PUD in group A and 1 NUD in group B) ITT eradication rate (%) by treatment group and PP:
PP eradication rate (%) by treatment group:
ITT eradication rate (%) in NUD participants: 60/70 (85.7) ITT eradication rate (%) in PUD participants: 25/25 (100) Metronidazole resistance (%) before treatment, SEQ ITT/PP:not reported Metronidazole resistance (%) before treatment, STT ITT/PP:not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP:not reported Clarithromycin resistance (%) before treatment, STT ITT/PP:not reported Compliance (%) in ITT sample SEQ/STT: not reported Incidence (%) of AEs SEQ/7‐day STT ‐ 10‐day STT: not reported Incidence (%) serious AEs SEQ/STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Truly randomised study by a computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | No information was reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information was reported |
| Publication format | Low risk | Full article |
De Francesco 2004b.
| Methods | Prospective randomised study Dates the study was conducted: not reported Funding sources and potential conflicts of interest: No funding sources reported.The authors report no conflicts of interest Definition of compliance: >95% intake |
|
| Participants | Number and type of participants: 347 H.pylori‐positive participants were enrolled and 342 completed the study Number of participants randomised: 347 (ITT sample) Number of participants in the 7‐day STT arm: 115 Number of participants in the 10‐day STT arm: 116 Number of participants in the 10‐day SEQ arm: 116 Average age (SD) of the population in years reported by treatment group:
Country: Italy Number of participants of the ITT population per treatment group with a medical condition: ‐ NUD, n = 228 (65.7%)
‐ PUD, n = 119 (34.3%)
Sex proportions as M/F per treatment group
|
|
| Interventions |
Name, dose timing of antibiotics in STT: rabeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day Name, dose timing of antibiotics in SEQ: rabeprazole 20 mg twice a day + amoxicillin 1 g twice a day (5 days) + rabeprazole 20 mg twice a day, clarithromycin 500 mg twice a day,tinidazole 500 mg twice a day (5 days) Sensitivity test (yes/no) to antibiotics before/after treatment: no Method of assessment of H. pylori status after treatment: ¹³C‐UBT, RUT, histology Time for assessment of H. pylori status after treatment: 6 ‐ 8 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group:
PP eradication rate (%) by treatment group:
Reported PP eradication rate (%) in NUD participants:
Reported PP eradication rate (%) in PUD participants:
Calculated ITT eradication rate (%) in NUD participants:
Calculated ITT eradication (%) in PUD participants:
Overall PP eradication rate (%) for both the NUD and PUD groups respectively: 177/224 (79) and 108/118 (91.5) Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance (%) in ITT sample SEQ/STT: reported as > 95% in all treatment groups Incidence (%) of AEs 10‐day SEQ/7‐day STT/10‐day STT: 10.3/6/7.7 Incidence (%) serious AEs SEQ / STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Truly randomised study by a computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | No information was reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information was reported |
| Publication format | Low risk | Full article |
Eisig 2014.
| Methods | Prospective, randomised, double‐bind, placebo‐controlled study Dates the study was conducted: not reported Funding sources and potential conflicts of interest: no information reported Definition of compliance: not reported |
|
| Participants | Number and type of participants: 100 functional dyspeptic H.pylori‐positive participants were enrolled in the study. Number of participants randomised: 100 in 2 treatment groups
Number of participants lost to follow‐up: not reported ITT sample: 50 PP sample: 97 (1 lost from the SEQ arm and 2 lost from the STT arm) Country: Brazil Median age of the ITT population in years: 47.2 Sex (M/F) of the ITT population: 29/71 Number of participants (%) of the ITT population with a medical condition: not reported |
|
| Interventions |
Name, dose and timing of antibiotics in 10‐day STT: lansoprazole 30 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1g, twice a day during 10 days Name, dose and timing of antibiotics in 10‐day SEQ: lansoprazole 30 mg twice a day + amoxicillin 1 g twice a day + placebo twice a day (5 days) + lansoprazole 30 mg twice a day + clarithromycin 500 mg twice a day + tinidazole 500 mg twice a day (5 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status before treatment: rapid urease and/or histology Method of confirmation of H. pylori eradication after treatment: ¹³C‐UBT Time for assessment of H. pylori status after treatment: 30 days |
|
| Outcomes | ITT eradication rate (%) by treatment group:
PP eradication rate (%) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance rates were not reported Incidence (number of participants) of AEs: not reported Incidence of serious AEs SEQ/STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Assumed |
| Allocation concealment (selection bias) | Low risk | Assumed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Given the amount of information given is scarce |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blindly, equally, randomly allocated into 2 groups |
| Publication format | High risk | Abstract |
Focareta 2002.
| Methods | Prospective randomised controlled trial Dates the study was conducted: not reported Funding sources and potential conflicts of interest: no information reported Definition of compliance: not reported |
|
| Participants | Number and type of participants: 187 H.pylori‐positive participants were enrolled in the study Number of participants randomised: 187 in 2 treatment groups
Number of participants lost to follow‐up: None. All patients completed the therapeutic schedules ITT sample: 187 PP sample: 187 (as authors mentioned all participants randomised completed the treatment in both arms) Country: Italy Average age (SD) of the ITT population in years: 46 Sex proportions as the number of M/F out of the total ITT population: 114/73 Number of participants (%) of the ITT population with a medical condition:
|
|
| Interventions |
Name, dose and timing of antibiotics in 7‐day STT: omeprazole 20 mg twice a day + amoxicillin 1 g twice a day + clarithromycin 500 mg twice a day during 7 days Name, dose and timing of antibiotics in 10‐day SEQ: omeprazole 20 mg twice a day + amoxicillin 1 g twice a day (5 days) and omeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (5 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status before treatment: RUT on 4 biopsy specimens Method of confirmation of H. pylori eradication after treatment: HpSA and ¹³C‐UBT Time for assessment of H. pylori status after treatment: 6 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group:
PP eradication rates were the same as ITT rates in both groups Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance rates were not reported Incidence rate (%) of AEs: Not reported Incidence rate (%) of serious AEs SEQ/STT: Not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial seems pseudo‐random as authors do not report a clear random mechanism likely to produce an unpredictable sequence of numbers, but the trial list seems to be based on participant's consecutive visit numbers only |
| Allocation concealment (selection bias) | Unclear risk | Authors did not provide clear allocation information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Publication format | High risk | Abstract |
Focareta 2003.
| Methods | Prospective randomised controlled trial Dates the study was conducted: patients were enrolled in 6‐months time but dates not reported Funding sources and potential conflicts of interest: no information reported Definition of compliance: not reported |
|
| Participants | Number and type of participants: 358 H.pylori‐positive participants were enrolled in the study Number of participants randomised: 358 in 2 treatment groups
Number of participants lost to follow‐up: not reported ITT sample: 358 participants PP sample: not available Country: Italy Average age of the ITT population in years: 44 Sex proportions as the number of M/F out of the ITT population: 217/141 Number of participants (%) of the ITT population with a medical condition:
|
|
| Interventions |
Name, dose and timing of antibiotics in 7‐day STT: esomeprazole 20 mg twice a day + amoxicillin 1 g twice a day+ clarithromycin 500 mg twice a day Name, dose and timing of antibiotics in 10‐day SEQ: esomeprazole 20 mg twice a day + amoxicillin 1 g twice a day (5 days) + rabeprazole 20 mg twice a day, clarithromycin 500 mg twice a day + tinidazole 500 mg twice a day (5 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status before treatment: endoscopy with biopsies and ¹³C‐UBT Method of confirmation of H. pylori eradication after treatment: HpSA and ¹³C‐UBT Time for assessment of H. pylori status after treatment: 6 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance, SEQ/STT: not reported Incidence (number of participants) of AEs: Not reported Incidence of serious AEs SEQ / STT: Not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial seems pseudo‐random as authors do not report a clear random mechanism likely to produce an unpredictable sequence of numbers, but the trial list seems to be based on participant's consecutive visit numbers only |
| Allocation concealment (selection bias) | Unclear risk | Authors did not provide clear allocation information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear blinding |
| Publication format | High risk | Abstract |
Franceschi 2011.
| Methods | Randomised clinical trial Dates the study was conducted: not reported Funding sources and potential conflicts of interest: no information reported Definition of compliance: not reported |
|
| Participants | Number and type of participants: 150 H.pylori‐positive participants were enrolled in the study Participants were randomised to 3 different treatment groups: 7‐day standard triple regimen (LCA), 7‐day high‐dose amoxicillin standard triple regimen (HDLCA) and 10‐day sequential regimen (LACT) Withdrawals: 2 dropouts in the LCA, 2 in the HDLCA, and 3 in the LACT Number of participants randomised: 150 (ITT sample) Number of participants in the 7‐day STT arm: 50 Number of participants in the high‐dose amoxicillin 7‐day STT arm: 50 Number of participants in the 10‐day SEQ arm: 50 PP sample: not reported Country: Italy Average age (SD) of the population in years: 64 (9) years Sex (M/F) of the population: 53%/47% Authors stated that the population was sex‐ and age‐matched for all 3 treatment arms Medical condition at baseline reported for the 7‐day STT, high dose amoxicillin 7‐day STT and 10‐day SEQ respectively:
Differences between groups were not significant H. pylori diagnostic methods in all treatment arms: not reported |
|
| Interventions |
Name, dose timing of antibiotics in 7‐day STT: lansoprazole 15 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day Name, dose timing of antibiotics in the high‐dose amoxicillin 7‐day STT: lansoprazole 15 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g three times a day Name, dose timing of antibiotics in 10‐day SEQ: lansoprazole 15 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and lansoprazole 15 mg twice a day + clarithromycin 500 mg twice a day + tinidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status both before and after treatment: ¹³C‐UBT Time for assessment of H. pylori status after treatment: 6 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group:
PP eradication rate (%) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance rate and incidence rate of AEs was reported similar in all treatment groups Incidence rate (%) of mild AEs SEQ/STT: diarrhoea, dysgeusia, headache, nausea
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is pseudo‐random as no clear statement has been reported on how the randomisation has been performed |
| Allocation concealment (selection bias) | Unclear risk | The sequence of randomisation was not explained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information was provided regarding the masking |
| Publication format | High risk | Abstract |
Gao 2010.
| Methods | Prospective, parallel, open‐label, randomised study Dates the study was conducted: from January 2005 to December 2009 Funding sources and potential conflicts of interest: no information reported Definition of compliance: > 95% |
|
| Participants | Number and type of participants: 215 H.pylori‐positive participants were enrolled in the study and all completed the treatment Participants were randomised to 3 different treatment groups: group A received a 10‐day bismuth pectin quadruple therapy, group B received a sequential therapy and group C received a standard 1‐week triple therapy. Note: The groups of interest for this review are B and C. Data from group A are not summarised. The participant groups did not differ in age, sex, gastritis distribution and location, and number of peptic ulcers in gastric mucosa Number of participants randomised: 215 (ITT sample) Number of participants in the 10‐day SEQ arm (group B): 72 Number of participants in the 7‐day STT arm (group C): 71 Country: China Sex (M/F) per treatment group
Medical condition at baseline per treatment group: ‐ Gastric ulcer
‐ Duodenal bulb ulcer
‐ Compound ulcers
Average age (SD) of the population in years reported by treatment group:
Number of participants per treatment group (B and C respectively) and medical condition: ‐ Antral gastritis: 61 in group B and 57 in group C ‐ Pangastritis: 15 in group B and 19 in group C ‐ Intestinal metaplasia: 21 in group B and 17 in group C ‐ Duodenitis: 13 in group B and 10 in group C ‐ Gastric ulcer: 42 in group B and 39 in group C ‐ Duodenal bulb ulcer: 12 in group B and 10 in group C ‐ Compound ulcers:7 in group B and 4 in group C |
|
| Interventions |
Name, dose timing of antibiotics in 7‐day STT: omeprazole 20 mg twice a day + amoxicillin 1 g twice a day + clarithromycin 500 mg twice a day Name, dose timing of antibiotics in 10‐day SEQ: omeprazole 20 mg twice a day + amoxicillin 1 g twice a day (5 days) and omeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + tinidazole 500 mg twice a day (5 days) Sensitivity test (yes/no) to antibiotics before/after treatment: no Method of assessment of H. pylori status after treatment: ¹³C‐UBT, histology and RUT Time for assessment of H. pylori status after treatment: 4 ‐ 6 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group:
ITT ulcer cicatrisation rate (%) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance (%) in ITT sample SEQ/ STT: > 95% / > 95% Incidence of AEs (%) by type and treatment group:
Incidence rate (%) of serious AEs SEQ/STT: not reported All side effects were self‐limiting after the therapy was ended |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was not specified how the randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | No information regarding the allocation concealment was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | We consider the study to be unblinded, as it was defined as 'open‐label' |
| Publication format | Low risk | Full article |
Gatta 2011.
| Methods | Prospective, open‐label, randomised study Dates the study was conducted: not reported Funding sources and potential conflicts of interest: no information reported Definition of compliance: not defined |
|
| Participants | Number and type of participants: 239 naïve H.pylori‐infected participants who underwent an EGDS were enrolled in the study Participants were randomised to 2 different treatment groups: 7‐day standard triple regimen or 10‐day sequential regimen. 1 participant in the 7‐day STT dropped out of the study Country: Italy Number of participants randomised: 239 (ITT sample) Number of participants in the 7‐day STT arm: 108 Number of participants in the 10‐day SEQ arm: 131 Median age of the population in years reported by treatment group:
Sex (M/F) per treatment group
Medical condition at baseline: dyspepsia or PUD participants H. pylori diagnostic methods in all treatment arms: ¹³C‐urea breath test, RUT, histology, bacterial culture with antibiotic resistance to clarithromycin |
|
| Interventions |
Name, dose timing of antibiotics in 7‐day STT: esomeprazole 40 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 7 days) Name, dose timing of antibiotics in 10‐day SEQ: esomeprazole 40 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and esomeprazole 40 mg twice a day + clarithromycin 500 mg twice a day + tinidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: yes, to clarithromycin Method of assessment of H. pylori status after treatment: ¹³C‐urea breath test Time for assessment of H. pylori status after treatment: 4 weeks |
|
| Outcomes | ITT eradication rate (%) (95% CI) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported ITT Clarithromycin resistance rate (%) by treatment group before treatment:
ITT eradication rate (%) (95% CI) by treatment group among the strains resistant to clarithromycin:
Differences: P = 0.004 Compliance in ITT sample SEQ/STT: not reported Incidence of AEs per treatment group: not reported Incidence (%) serious AEs SEQ/STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was not specified how the randomisation was performed |
| Allocation concealment (selection bias) | Unclear risk | No information regarding the allocation concealment was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcomes were clearly reported. Secondary outcome data were reported as percentages. Cure proportions reported under the study characteristics table were calculated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded as it was reported open‐label |
| Publication format | High risk | Abstract |
Greenberg 2011.
| Methods | Randomised trial Dates the study was conducted: from September 2009 to June 2010 Funding sources and potential conflicts of interest: study funded by Bill & Melinda Gates Foundation, US National Institutes of Health. One of this authors (Douglas R Morgan) submitted a patent application through the University of North Carolina for a technique using molecular endoscopy to detect cancer in the gastrointestinal tract, and did receive funding from Axcan for his participation in a speakers’ bureau; he also received a research grant from AstraZeneca, for a proton‐pump inhibitor study in Hispanic populations in the USA, and from Given Imaging, for ongoing efficacy studies of colon endocapsule efficacy. All other authors declare no conflicts of interest Definition of compliance: minimum intake was not specified, although compliance was defined by a questionnaire |
|
| Participants | Number and type of participants: 1463 H.pylori‐positive participants were enrolled in the study Participants were randomised to 3 different treatment groups: 14‐day standard triple regimen, 5‐day concomitant therapy and 10‐day sequential regimen. According to our study question, only the data referring to both the 14‐day STT and the 10‐day SEQ are relevant. Country: Latin America (7 sites: Chile (Santiago), Colombia (Túquerres), Costa Rica (Guanacaste), Honduras (Santa Rosa de Copán), Mexico (Ciudad Obregón and Tapachula), and Nicaragua (León)). Number of participants randomised: 1463 (ITT sample) Number of participants in the 14‐day STT arm: 488 Number of participants in the 10‐day SEQ arm: 486 Age range of the population reported as the number of participants by treatment group, 14‐day STT/10‐day SEQ:
Sex (M / F) per treatment group (n = 861 women and 602 men in the total sample)
Medical condition at baseline reported as the number of participants in the 14‐day STT and 10‐day SEQ respectively: 373 (25%) participants had chronic dyspeptic symptoms as classified by the Rome III criteria
H. pylori diagnostic methods in all treatment arms: ¹³C‐urea breath test |
|
| Interventions |
Name, dose timing of antibiotics in 14‐day STT: lansoprazole 30 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 14 days) Name, dose timing of antibiotics in 10‐day SEQ: lansoprazole 30 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and lansoprazole 30 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not performed Method of assessment of H. pylori status after treatment: ¹³C‐urea breath test Time for assessment of H. pylori status after treatment: 6 ‐ 8 weeks |
|
| Outcomes | The urea breath test at follow‐up was obtained in 1414 (97%) of 1463 participants ITT eradication rate (%) (95% CI) by treatment group :
PP eradication rate (%) by treatment group:
Definitive 6‐week UBT proportion rate (%) (95% CI) by treatment group: (n = 1414)
ITT eradication rate (%) in NUD participants:
Adherence rate (%) (95% CI) to therapy by treatment group: (n = 1314)
Difference rate (%) of 10‐day SEQ from the standard group 14‐day STT (adjusted 95% CI):
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported ITT adherence rate (%) by amount of drugs taken and by treatment group 14‐day STT/10‐day SEQ respectively:
Incidence rate (%) of AEs by treatment group:
Incidence (%) serious AEs SEQ/STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was implemented centrally through a dynamic balancing procedure at the SWOG Statistical Center to ensure balance within centre by age and sex across the 3 regimens. |
| Allocation concealment (selection bias) | Low risk | A web‐based data entry system was used to enter data on potentially eligible and consented individuals |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported. PP eradication (%) was calculated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded |
| Publication format | Low risk | Full article |
Hsu 2014.
| Methods | Open‐label, randomised controlled trial (registration no. NCT1769365) Dates the study was conducted: not reported Funding sources and potential conflicts of interest: funded by research grant NSC 99‐2314‐B‐075B‐009‐MY2 from the National Science Council. Authors declare no conflicts of interest Definition of compliance: assessed by pill counts. Good compliance was defined as taking 80% of the total medication |
|
| Participants | umber and type of participants: 307 H.pylori‐positive participants were enrolled in the study Participants were randomised to 3 different treatment groups: 7‐day standard triple regimen, 10‐day sequential therapy and 7‐day concomitant therapy. Only data related to STT and SEQ are relevant Country: Japan Number of participants randomised: 307 (ITT sample) Number of participants in the ITT 7‐day STT arm: 103 Number of participants in the ITT 10‐day SEQ arm: 102 Number of participants in the PP sample: 303 Number of participants in the PP 7‐day STT arm: 101 (1 insufficient compliance and 1 lost to follow‐up) Number of participants in the PP 10‐day SEQ arm: 100 (2 insufficient compliance) Mean age of the population reported as the number of participants by treatment group:
Sex (M/F) per treatment group
Medical condition at baseline (endoscopic findings) reported as n (%) in STT and SEQ respectively:
H. pylori diagnostic methods in all treatment arms: by RUT, histology or culture |
|
| Interventions |
Name, dose timing of antibiotics in 7‐day STT: pantoprazole 40 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (7 days) Name, dose timing of antibiotics in 10‐day SEQ: pantoprazole 40 mg twice a day + amoxicillin 1 g twice a day (5 days) followed by pantoprazole 40 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (5 days) Sensitivity test (yes/no) to antibiotics before/after treatment: Yes. Antibiotic susceptibility was determined by Etest (AB Biodisk, Solna, Sweden). H. pylori strains were tested for clarithromycin, amoxicillin, and metronidazole susceptibilities using the Etest (AB Biodisk). H. pylori strains with MICs of 1 g/ml, 0.5 g/ml, and 8 g/ml were considered to be resistant to clarithromycin, amoxicillin, and metronidazole, respectively A total of 127 (elsewhere reported 129 ‐ not clear) strains were isolated of the 160 participants receiving endoscopy and bacterial culture on enrolment Results are reported as (n of susceptible/n of resistant) by antibiotic and treatment arms respectively:
Method of assessment of H. pylori status after treatment: Eradication defined as the negative results of all RUT, histology, and culture, or (ii) a negative result from the UBT Time for assessment of H. pylori status after treatment: 6 weeks |
|
| Outcomes | ITT eradication rate (%) (95% CI) by treatment group:
PP eradication rate (%) (95% CI) by treatment group:
Eradication (%) in SEQ and STT according to antibiotic resistances: Metronidazole‐resistant strains:
Claritromycin‐resistant strains rate (%) by treatment group:
Dual resistant strains rate (%) by treatment group:
Adherence: not reported. Compliance rate (%) (95% CI) by treatment group: (n = 1314)
Incidence rate (%) (95% CI) of AEs by treatment group :
Incidence (%) serious AEs SEQ/STT: not reported Outcome related to adherence was not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated number sequence was used |
| Allocation concealment (selection bias) | Low risk | An independent research assistant assigned the therapies according to the treatment allocations kept in the envelopes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Publication format | Low risk | Full article |
Huang 2013.
| Methods | Prospective, multicentre, randomised controlled trial Dates the study was conducted: from January 2008 to December 2010 Funding sources and potential conflicts of interest: study funded in full by Key Projects of the National Science & Technology Pillar Program of China, No. 2007BAI04B02. Authors declare no conflicts of interest Definition of compliance: not defined Adherence was monitored by telephone interview of the participant or parent and review of daily reporting card |
|
| Participants | Number and type of participants: 360 H.pylori‐positive participants (all children) were enrolled in the study Participants were randomised to 3 different treatment groups: 7‐day standard triple regimen, 10‐day standard triple regimen and 10‐day sequential regimen Country: China Number of participants randomised: 360 (ITT sample) Number of participants in the 7‐day STT arm: 118 Number of participants in the 10‐day STT arm: 124 Number of participants in the 10‐day SEQ arm: 118 Mean age of the population reported as the number of participants by treatment group, 7‐day STT, 10‐day STT / 10‐day SEQ:
Sex (M/F) per treatment group
Medical condition at baseline was not detailed; just confirmed H.pylori gastritis H. pylori diagnostic methods in all treatment arms: RUT, HpSA, culture and histology |
|
| Interventions |
Name, dose timing of antibiotics in 7‐day STT: omeprazole 0.8 – 1.0 mg/kg/d + clarithromycin 20 mg/kg/d + amoxicillin 30 mg/kg/d Name, dose timing of antibiotics in 10‐day STT: omeprazole 0.8 – 1.0 mg/kg/d + clarithromycin 20 mg/kg/d + amoxicillin 30 mg/kg/d Name, dose timing of antibiotics in 10‐daySEQ: omeprazole 0.8 – 1.0 mg/kg/d + amoxicillin 30 mg/kg/ (during 5 days) and omeprazole 0.8 – 1.0 mg/kg/d + clarithromycin 20 mg/kg/d + metronidazole 20 mg/kg/d (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not performed Method of assessment of H. pylori status after treatment: by a negative H. pylori stool antigen test Time for assessment of H. pylori status after treatment: 4 weeks |
|
| Outcomes | ITT eradication (%) (95% CI) by treatment group:
PP eradication (%) by treatment group:
Adherence (%) (95% CI) to therapy by treatment group: (n = 1314)
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported ITT adherence by amount of drugs taken and by treatment group 14‐day STT/10‐day SEQ:
Incidence of AEs by treatment group (n, %):
Incidence (%) serious AEs SEQ/STT: not reported Outcome related to adherence was not reported |
|
| Notes | The 10‐day sequential regimen was significantly more effective than standard 7‐day or 10‐day triple regimens in eradicating H. pylori infection in Chinese children | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Restricted randomisation using block randomisation |
| Allocation concealment (selection bias) | Low risk | The randomisation code was developed by using a computer random‐number generator to select random permuted blocks, with a varied block size of 4, 8 or 10 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported, however authors failed to report outcomes related to adherence |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The details of treatment assignments were unknown to any of the investigators, study co‐ordinators or participants and were contained in a set of sealed envelopes |
| Publication format | Low risk | Full article |
Javid 2013.
| Methods | Prospective, comparative, open‐label, randomised trial Dates the study was conducted: from 2010 to 2011 Funding sources and potential conflicts of interest: no information reported Definition of compliance: determined by the recovery of empty medicine strips and questioning |
|
| Participants | Number and type of participants: 272 H.pylori‐positive participants with gastro‐duodenal ulcers were enrolled in the study Participants were randomised to 2 treatment groups: 10‐day standard triple regimen, 10‐day sequential regimen Country: India Number of participants randomised: 272 (ITT sample) Number of participants in the 10‐day STT arm: 134 Number of participants in the 10‐day SEQ arm: 138 Mean age (SD) of the population reported by treatment group:
Sex ratio (M/F) by treatment group:
Number of participants with a medical condition at baseline, by treatment group: Duodenal ulcer
Gastric ulcer
Number (%) of participants with gastric ulcer, by treatment group:
Number (%) of participants with NUD, by treatment group:
H. pylori diagnostic methods in all treatment arms: H. pylori infection in all PUD participants was determined by RUT and histological examination. Participants had to test positive for both tests in order to be included |
|
| Interventions |
Name, dose timing of antibiotics in 10‐day STT: pantoprazole 40 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 10 days) Name, dose timing of antibiotics in 10‐day SEQ: pantoprazole 40 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and pantoprazole 40 mg twice a day + clarithromycin 500 mg twice a day + tinidazole 500 mg twice a day (during 5 days) After treatment, pantoprazole was continued for a period of 8 weeks in both groups Sensitivity test (yes/no) to antibiotics before/after treatment: pre‐treatment cultures were not performed Method of assessment of H. pylori status after treatment: histology and RUT Time for assessment of H. pylori status after treatment: 4 weeks Participants were considered cured when both tests did not show H. pylori |
|
| Outcomes | ITT eradication (%) by treatment group :
Difference 95% CI: 14.1 (6.5 to 19), P = 0.005 PP eradication (%) by treatment group:
Difference 95%CI: 17.2 (8.2 to 23.5), P = 0.002 Adherence (%) to therapy by treatment group: participants were asked for strict medical adherence in both groups Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Incidence of (total) AEs by treatment group:
1 participant in standard therapy discontinued treatment due to uncontrolled diarrhoea. None of the differences was statistically significant Incidence (%) serious AEs SEQ/STT: not reported |
|
| Notes | Sequential therapy was significantly more effective than standard therapy for eradicating H. pylori infection in peptic ulcer disease in Asian participants in both ITT (76.0% vs 61.9%, P = 0.005) and PP (84.6% vs 67.4%, P = 0.002) analyses Side effects were similar in both treatment groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was carried out in the endoscopy laboratory by opening an opaque sealed numbered envelope by the senior endoscopy technologist |
| Allocation concealment (selection bias) | Low risk | Treatment assignments were made based on random numbers derived from a table of random numbers in blocks of 4 by using central computer‐generated block randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The authors stated the trial was open‐label |
| Publication format | Low risk | Full article |
Jeon 2013.
| Methods | Randomised controlled trial Dates the study was conducted: not reported Funding sources and potential conflicts of interest: no information reported Definition of compliance: intake > 90% |
|
| Participants | Number and type of participants: 158 H.pylori‐positive participants were enrolled in the study Participants were randomised to 2 different treatment groups: 7‐day standard triple regimen and 10‐day sequential regimen Number of participants randomised: 158 (ITT sample) Number of participants in the 7‐day STT arm: 81 Number of participants in the 10‐day SEQ arm: 77 PP sample: 146 participants Number of participants in the 7‐day STT arm: 76 Number of participants in the 10‐day SEQ arm: 70 Country: Korea Average age (SD) of the population in years reported by treatment group: not reported but assumed all adults Sex (M/F) per treatment group: not reported Medical condition at baseline reported as number of participants (%) per treatment group: not reported H. pylori diagnostic methods in both treatment arms: Not reported, but it was assumed methods used were the same as in the study published by Choi 2008 (previous study excluded as Jeon 2013 updated Choi 2008) |
|
| Interventions |
Name, dose timing of antibiotics in 7‐day STT: omeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day Name, dose timing of antibiotics in 10‐day SEQ omeprazole 20 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and omeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status both before and after treatment: Not reported, but it was assumed methods used were the same as in the study published by Choi 2008 Time for assessment of H. pylori status after treatment: 8 weeks |
|
| Outcomes | ITT eradication (%) by treatment group:
PP eradication (%) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Adherence: not reported Incidence of AEs at PP analysis: Figures were not reported but authors sated there were no significant differences and treatment compliance between the 2 groups Incidence (%) serious AEs SEQ/STT: not reported |
|
| Notes | The 10‐day SEQ did not achieve higher eradication proportions than 7‐day STT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information is reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary outcomes were clearly reported; however methods used for HP diagnosis and assessment of HP eradication after therapy were not stated. It was assumed same methods were used as in previous study by Choi 2008 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Publication format | High risk | Abstract (Choi 2008) |
Kim 2011.
| Methods | Prospective, randomised, single‐blinded, controlled trial Dates the study was conducted: from October 2008 to February 2009 Funding sources and potential conflicts of interest: study was not funded. Authors declare no conflicts of interest Definition of compliance: intake > 90% |
|
| Participants | Number and type of participants: 409 H.pylori‐positive PUD and NUD participants were enrolled in the study Participants were randomised to 2 different treatment groups: 14‐day standard triple regimen and 10‐day sequential regimen Number of participants randomised: 409 (ITT sample) Number of participants in the 14‐day STT arm: 204 Number of participants in the 10‐day SEQ arm: 205 PP sample: 370 participants Number of participants in the 14‐day STT arm: 180 Number of participants in the 10‐day SEQ arm: 190 Country: Korea Average age (SD) of the population in years reported by treatment group:
Sex (M/F) per treatment group
Medical condition at baseline reported as number of participants (%) per treatment group: ‐ Peptic ulcer:
‐ Non‐ulcer dyspepsia:
H. pylori diagnostic methods in both treatment arms: at least 1 test had to be positive in order to consider participants H. pylori‐positive
|
|
| Interventions |
Name, dose timing of antibiotics in 14‐day STT: pantoprazole 40 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day Name, dose timing of antibiotics in 10‐day SEQ: pantoprazole 40 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and pantoprazole 40 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (during 5 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status both before and after treatment: 1 of following: ¹³C‐UBT, RUT or histology in both treatment groups Time for assessment of H. pylori status after treatment: 4 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group:
PP eradication rate (%) by treatment group :
Eradication rate (%) in the PUD group:
Eradication rate (%) in the NUD group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported PP adherence rate (%) to the therapy: i.e. over 90% of medication taken
Incidence rate (%) of AEs at PP analysis:
Incidence (%) serious AEs SEQ / STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors reported that a randomisation sequence was prepared by independent staff, which was accomplished through block randomisation by using a block design and a block size of 4. Randomisation of block was done by means of the random‐number chart |
| Allocation concealment (selection bias) | Low risk | Only the independent staff could manage a matching list between study identification number and hospital number. The data were revealed to other investigators once recruitment and data collection were completed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome date were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded as participants were not blinded to randomisation |
| Publication format | Low risk | Full article |
Lahbabi 2013.
| Methods | Randomised trial Dates the study was conducted: from June 2009 to August 2011 Funding sources and potential conflicts of interest: No information reported on funding Authors declare no conflicts of interest Definition of compliance: evaluated by pill count. Compliance was considered good if ≥ 90% and poor if ≤ 60% |
|
| Participants | Number and type of participants: 327 H.pylori‐positive participants were enrolled in the study Participants were randomised to 3 different treatment groups: 2 groups with 7‐day standard triple regimens (different antibiotic amoxicillin versus metronidazole) and 1 group with10‐day sequential regimen. According to our study question, only the data referring to the 7‐day STT (amoxicillin group) and the 10‐day SEQ are relevant Country: Morocco Number of participants randomised: 323 (ITT sample) Number of participants in the 7‐day STT arm: 115 Number of participants in the 10‐day SEQ arm: 104 Mean age (SD) of the population reported by treatment group,
Sex ratio (M / F) by treatment group
Number (%) of PUD participants at baseline, by treatment group:
Number (%) of participants with gastric ulcer, by treatment group:
Number (%) of participants with NUD, by treatment group:
H. pylori diagnostic methods in all treatment arms: H. pylori infection was determined by at least 1 of the following tests: histology and/or PCR |
|
| Interventions |
Name, dose timing of antibiotics in 7‐day STT: omeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day Name, dose timing of antibiotics in 10‐day SEQ: omeprazole 20 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and omeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (during 5 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not performed Method of assessment of H. pylori status after treatment: ¹³C‐urea breath test Time for assessment of H. pylori status after treatment: 3 months |
|
| Outcomes | ITT eradication rate (%) by treatment group:
PP eradication rate (%) by treatment group:
Adherence rate (%) as > 90% to therapy by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Incidence (%) rate of (total) AEs by treatment group:
Incidence (%) serious AEs SEQ/STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was made by a computer‐generated list drawn up by a statistician |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blinded |
| Publication format | Low risk | Full article |
Laving 2013.
| Methods | Double‐blinded, randomised, controlled trial Format of publication: journal article Dates the study was conducted: from March 2007 to October 2007 Funding sources and potential conflicts of interest: No information of funding nor conflicts of interest reported Definition of compliance: not reported,although authors mentioned that parents were asked 2 weeks after treatment about the treatment schedule |
|
| Participants | Number and type of participants: 71 H.pylori‐positive children were enrolled in the study Participants were randomised to 2 different treatment groups: 10‐day standard triple regimen and 10‐day sequential regimen Number of participants randomised: 104 (ITT sample) Number of participants in the ITT 10‐day STT arm: 52 Number of participants in the ITT 10‐day SEQ arm: 52 PP sample: 71 Number of participants in the PP 10‐day STT arm: 45 Number of participants in the PP 10‐day SEQ arm: 26 Country: Kenya Average age (SD) of the population in years reported by treatment group: participants included in either group were under the age of 16 Sex (M/F) per treatment group:
H. pylori diagnostic methods in both treatment arm: stool antigen test and/or a repeat histology obtained at repeated endoscopy |
|
| Interventions |
Name, dose timing of antibiotics in 10‐day STT: pantoprazole 40 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 14 days) Name, dose timing of antibiotics in 10‐day SEQ: 1 mg/kg/day omeprazole + 50 mg/ kg/ day amoxicillin (during 5 days) and 1 mg/ kg/ day omeprazole + 15 mg/kg/day + clarithromycin 20 mg/kg/day + tinidazole (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status after treatment: repeated endoscopy and stool H. pylori antigen testing Time for assessment of H. pylori status after treatment: 6 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group:
PP eradication rate (%) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported PP adherence to the therapy: not reported Incidence of AEs at PP analysis: not reported Incidence (%) serious AEs SEQ/STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer programme was used to generate random numbers to assign participants to either of the 2 arms as they were recruited |
| Allocation concealment (selection bias) | Unclear risk | No information stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Data regarding eradication are confusing as authors reported the number of participants eradicated separately by stool antigen negative and histology negative testing |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both the study physicians and the participants were blinded |
| Publication format | Low risk | Full article |
Lee 2014.
| Methods | Prospective, randomised, open‐label, controlled clinical trial Dates the study was conducted: from May 2010 to September 2013 Funding sources and potential conflicts of interest: study supported by a grant from the National Research Foundation of Korea funded by the Korean Government (NRF‐2012R1A1B5002680). Authors declare no financial arrangements and no conflicts of interest Definition of compliance: assessed by a physician’s direct questioning and considered to be satisfactory when drug intake exceeded 85% |
|
| Participants | Number and type of participants: 332 participants with H. pylori infection were randomly assigned to receive either 7‐day PPI triple therapy, 10‐day sequential therapy, or 15‐day sequential therapy. For our review, only data referring to 7‐day STT and 10‐day SEQ are relevant Country: Korea Number of participants randomised: 332 (ITT total sample) Number of participants in the 7‐day STT arm: 115 (ITT sample) Number of participants in the 10‐day SEQ arm: 111 (ITT sample) Mean age (SD) of the population reported by treatment group,
Sex ratio (M/F) by treatment group
Number (%) of participants with PUD at baseline, by treatment group:
Number (%) of participants with non‐ulcer dyspepsia by treatment group:
Number (%) of participants with gastric cancer or dysplasia by treatment group:
H. pylori diagnostic methods in all treatment arms: H. pylori infection was confirmed by RUT by gastric mucosal biopsy or ¹³C‐UBT |
|
| Interventions | Participants randomised to the SEQ group were administered metronidazole Name, dose timing of antibiotics in 7‐day STT: esomeprazole 40 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 7 days) Length of STT (days): 7 Name, dose timing of antibiotics in 10‐day SEQ: esomeprazole 40 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and esomeprazole 40 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: Yes Method of assessment of H. pylori status after treatment: ¹³C‐urea breath or a combination of the RUT, Giemsa staining and culture when follow‐up endoscopy was necessary Time for assessment of H. pylori status after treatment: 4 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group :
PP eradication rate (%) by treatment group:
Adherence was not reported. Metronidazole resistance (%) before treatment, SEQ ITT/PP: 0/1 (0) Metronidazole resistance (%) before treatment, STT ITT/PP: 1/1 (100) Clarithromycin resistance (%) before treatment, SEQ ITT/PP: 0/2 (0) Clarithromycin resistance (%) before treatment, STT ITT/PP: 0/3 (0) Incidence rate (%) of total AEs by treatment group:
Three participants discontinued sequential treatment due to AEs Incidence (%) serious AEs SEQ/STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a computer‐generated table |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcomes are clearly reported. However, eradication proportions by antibiotic resistances are not reported and only cure proportions by antibiotic susceptibility are given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Publication format | Low risk | Full article |
Lee 2015.
| Methods | Prospective, multicentre, randomised controlled clinical trial Dates the study was conducted: from July 2013 to March 2014 Funding sources and potential conflicts of interest: study supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science and Technology, No. 2013R1A1A2062603. No conflicts of interest reported Definition of compliance: Compliance was considered good if ≥ 80%, otherwise participants were excluded from the PP analysis. Participants were asked to count the remaining pills |
|
| Participants | Number and type of participants: 680 H.pylori‐positive participants were enrolled in the study Participants were randomised to 4 different treatment groups: 2 groups to standard triple regimens (but different antibiotics used metronidazole instead of clarithromycin in 1 of them), 1 group with10‐day sequential regimen and 1 other group with concomitant therapy. According to our study question, only the data referring to the 7‐day STT therapy using clarithromycin and the 10‐day SEQ are relevant Country: Korea Number of participants randomised: 680 (ITT total sample) Number of participants in the 7‐day STT arm: 170 (ITT sample) Number of participants in the 10‐day SEQ arm: 170 (ITT sample) Mean age (SD) of the population reported by treatment group:
Sex ratio (M/F) by treatment group:
Number (%) of participants with gastric ulcer at baseline, by treatment group:
Number (%) of participants with duodenal ulcer by treatment group:
Number (%) of participants with gastritis by treatment group:
H. pylori diagnostic methods in all treatment arms: H. pylori infection was confirmed by endoscopy using histology (Warthin‐Starry stain) |
|
| Interventions |
Name, dose timing of antibiotics in 7‐day STT: rabeprazole 40 mg twice a day + clarithromycin 1g twice a day + amoxicillin 1 g twice a day (during 7 days) Name, dose timing of antibiotics in 10‐day SEQ: rabeprazole 40 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and rabeprazole 40 mg twice a day + clarithromycin 1g twice a day + metronidazole 500 mg twice a day (during 5 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not performed Method of assessment of H. pylori status after treatment: ¹³C‐urea breath test Time for assessment of H. pylori status after treatment: 6 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group :
PP eradication rate (%) by treatment group:
Adherence was not reported Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Incidence rate (%) of total AEs by treatment group:
Incidence (%) serious AEs SEQ/STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | The method of allocation was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary outcomes were clearly reported; however the frequency of antibiotics used was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Publication format | Low risk | Full article |
Liou 2013.
| Methods | Randomised, open‐label, multicentre trial. Dates the study was conducted: from December 2009 to September 2011 Funding sources and potential conflicts of interest: study funded by the National Taiwan University Hospital and National Science Council. Authors declare no conflicts of interest. Definition of compliance: defined as low when < 80% of pills were taken |
|
| Participants | Number and type of participants: 900 H.pylori‐positive participants were enrolled in the study Participants were randomised to 3 different treatment groups: 14‐day standard triple regimen, 10‐day sequential regimen and 14‐day sequential regimen. Only data referring to the 7‐day STT and 10‐day SEQ are relevant Number of participants randomised: 900 (ITT sample) Number of participants in the 14‐day STT arm, ITT analysis: 300 Number of participants in the 10‐day SEQ arm, ITT analysis: 300 Number of participants in the 14‐day STT arm, PP analysis: 279 Number of participants in the 10‐day SEQ arm, PP analysis: 285 Country: China Average age (SD) of the population in years reported by treatment group:
Sex proportions as the number of M/F in the ITT population by treatment group:
Medical condition (PUD participants) at baseline reported as number of participants (%) in the
Baseline resistance for every antibiotic as the number of participants (%) or participants positive/participants tested (%): Metronidazole ITT resistance (%) before treatment, 10‐day SEQ: 46/192 (24%) Metronidazole ITT resistance (%) before treatment, 14‐day STT: 48/183 (26%) Clarithromycin ITT resistance (%) before treatment, 10‐SEQ: 21/183 (11%) Clarithromycin ITT resistance (%) before treatment, 14‐day STT: 18/192 (9%) Amoxicillin ITT resistance (%) before treatment, 10‐day SEQ: 4/192 (2%) Amoxicillin ITT resistance (%) before treatment, 14‐day STT: 5/183 (3%) Levofloxacin ITT resistance (%) before treatment, 10‐day SEQ: 22/183 (12%) Levofloxacin ITT resistance (%) before treatment, 14‐day STT: 22/192 (11%) H. pylori diagnostic methods in both treatment arms: serology, RUT, histology, culture, UBT Participants with positive results in at least 2 of these tests were eligible for enrolment |
|
| Interventions | Participants randomised to the SEQ group were administered metronidazole Name, dose timing of antibiotics in 14‐day STT: lansoprazole 30 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 14 days) Length of STT (days): 14 Name, dose timing of antibiotics in 10‐day SEQ: lansoprazole 30 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and lansoprazole 30 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: Yes. probabilistic sensitivity tests were performed to investigate the effects of changes in the prevalence of the antibiotic resistant strains Method of assessment of H. pylori status after treatment: ¹³ C‐urea breath test Time for assessment of H. pylori status after treatment: 6 weeks |
|
| Outcomes | The study flow chart showed that after randomisation, in the 14‐day STT arm there were 21 dropouts, whereas in the 10‐day SEQ there were 15 dropouts ITT eradication rate (%) (95% CI) by treatment group:
PP eradication rate (%) (95%CI) by treatment group :
ITT eradication rate (%) in PUD participants:
ITT eradication rate (%) in NUD participants:
Univariate analysis of post‐treatment antibiotic susceptibilities and resistances in the 10‐day SEQ and 14‐day STT respectively: Clarithromycin resistance rates (%) (resistant ‐R and susceptible ‐S strains) (phenotypic) in 10‐day SEQ and 14‐STT groups respectively
Metronidazole resistance rates (%) (resistant ‐R and susceptible ‐S strains) (phenotypic) in 10‐day SEQ and 14‐STT groups respectively:
Amoxicillin resistance rates (%) (resistant ‐R and susceptible ‐S strains) (phenotypic) in 10‐day SEQ and 14‐STT groups respectively
Clarithromycin (Cla) and metronidazole (Met) resistance rates (%) (resistant ‐R and susceptible ‐S strains) (phenotypic) in 10‐day SEQ and 14‐STT groups respectively
Compliance rate (%) in ITT sample (took at least 80% of the drugs)
Number of patients (%) that did not take the 80% of the drugs:
Type and Incidence rate (%) of AEs reported in the 10‐day SEQ/14‐day STT arms respectively: Diziness: 31/295 (11)/19/299 (6) Skin rash: 9/295 (3)/7/299 (2) Headache: 9/295 (3)/16/299 (5) Taste distortion: 58/295 (20)/76/299 (25) Abdominal pain: 19/295 (6)/31/299 (10) Nausea: 23/295 (8)/11/299 (4) Diarrhoea: 48/295 (16)/62/299 (21) Constipation: 9/295 (3)/11/299 (4) Bloating: 21/295 (7)/17/299 (6) Any type of adverse events: 142/294 (48)/164/298 (55) Number of patients out of the total (%) that discontinued drugs because of AEs in the 10‐day SEQ/14‐day STT arms respectively: 6/295 (2)/13/297 (4) Incidence rate (%) of serious AEs SEQ / STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial was truly randomised. A permuted block randomisation method with a block size of 6 was used. An independent research assistant at the National Taiwan University Hospital generated the computerised random number sequence |
| Allocation concealment (selection bias) | Low risk | The sequence was concealed in an opaque envelope until the intervention was assigned. After the written informed consents were obtained from eligible participants, the independent research assistant telephoned study staff to give them each participant’s treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | All investigators were masked to the randomisation sequence |
| Publication format | Low risk | Full article |
Liou 2014.
| Methods | Randomised, open‐label, multicentre trial Dates the study was conducted: from February 2012 to April 2013 Funding sources and potential conflicts of interest: no information reported Definition of compliance: not reported |
|
| Participants | Number and type of participants: 1088 H.pylori‐positive participants were enrolled in the study Participants were randomised to 2 different treatment groups: 14‐day standard triple regimen and 10‐day sequential regimen Number of participants randomised: 840 (ITT sample) Number of participants in the 14‐day STT arm, ITT analysis: 424 Number of participants in the 10‐day SEQ arm, ITT analysis: 416 Number of participants in the 14‐day STT arm, PP analysis: 407 Number of participants in the 10‐day SEQ arm, PP analysis: 401 Country: China Average age (SD) of the population in years: not reported but authors mentioned they included adults Sex (M/F) by treatment group: not reported Medical condition (PUD participants) at baseline as number of participants (%): not reported Baseline resistance for antibiotic were performed using minimum inhibition concentrations determined by agar dilution test. 23S rRNA mutation was detected by PCR followed by direct sequencing H. pylori diagnostic methods in both treatment arms: not reported, but it was assumed method used was the same as in Liou 2013 |
|
| Interventions |
Name, dose timing of antibiotics in 14‐day STT: not reported Name, dose timing of antibiotics in 10‐day SEQ: not reported It was assumed authors used same antibiotics, PPIs and doses as in Liou 2013. However we decided not to include these data in the subgroup analysis by PPI and nitroimidazole types, for consistency with remaining included studies. Sensitivity test (yes/no) to antibiotics before/after treatment: Yes. Method of assessment of H. pylori status after treatment: Not reported; we contacted authors but not reached. For our purposes we assumed methods used were the same as in Liou 2013 Time for assessment of H. pylori status after treatment: Not reported; we contacted authors but not reached. For our purposes we assumed methods used were the same as in Liou 2013 |
|
| Outcomes | ITT eradication rate (%) by treatment group:
PP eradication rate (%) by treatment group:
Incidence (%) of AEs in the 10‐day SEQ/14‐day STT arms respectively: not reported Adverse events or serious adverse events were not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | Authors state the sequence was randomly allocated but did not specify if it was concealed |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcomes are reported clearly. However, efficacy data regarding antimicrobial resistance are missing |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No reported |
| Publication format | High risk | Abstract |
Lopez‐Román 2011.
| Methods | Prospective, randomised, Phase IIB clinical trial (interim analysis) Dates the study was conducted: not reported Funding sources and potential conflicts of interest: no information reported Definition of compliance: not reported |
|
| Participants | Number and type of participants: 123 H.pylori‐positive participants were enrolled in the study Participants were randomised to 3 different treatment groups: 10‐day standard triple regimen, 10‐day sequential regimen and 14‐day sequential regimen Number of participants randomised: 123 (ITT sample) Number of participants in the 10‐day STT arm: 41 Number of participants in the 10‐day SEQ arm: 41 PP sample: not reported Country: Puerto Rico Average age (SD) of the population in years: 64.7 (9.7) Sex (M/F) of the population: 120/3; i.e. 97.5% were men Medical condition at baseline: not reported H. pylori diagnostic methods in all treatment arms: CLO test and histology |
|
| Interventions | Participants randomised to the SEQ group were administered metronidazole Length of STT (days): 10 Name, dose timing of antibiotics in 10‐day STT: omeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 10 days) Name, dose timing of antibiotics in 10‐day SEQ: omeprazole 20 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and omeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status both before and after treatment: ¹³C‐UBT Time for assessment of H. pylori status after treatment: 8 weeks |
|
| Outcomes | ITT cure proportion (%) by treatment group:
PP cure (%) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance was > 99.5% in both treatment arms (10‐day SEQ and 10‐day STT) Incidence (%) of AEs in the total population: 25.2% Incidence (%) serious AEs SEQ / STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study is pseudo‐random as no clear statement on how the randomisation has been performed |
| Allocation concealment (selection bias) | Unclear risk | No clear information was provided on the sequence of randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary outcomes were reported as percentages. The number of participants randomised to each treatment arm was not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information was provided regarding the masking |
| Publication format | High risk | Abstract |
Lu 2010.
| Methods | Randomised controlled trial Dates the study was conducted: from September 2006 to September 2009 Funding sources and potential conflicts of interest: no information reported Definition of compliance: N/A |
|
| Participants | Number and type of participants: 76 H.pylori‐positive consecutive children (new‐born) were enrolled in the study Participants were randomised to 2 different treatment groups: 10‐day sequential regimen and 10‐day standard triple therapy. Number of participants randomised: 76 (ITT sample) Number of participants in the 10‐day SEQ arm, ITT analysis: 40 Number of participants in the 7‐day STT arm, PP analysis: 36 Number of participants lost to follow‐up: 2 from the SEQ and 3 from the STT PP sample: 71 Number of participants in the 10‐day SEQ arm, PP analysis: 38 Number of participants in the 7‐day STT arm, PP analysis: 33 Country: China Average age (SD) of the ITT population in years by treatment group:
Sex (M/F) of the ITT population by treatment group:
There were no significant differences between the 2 treatment groups in gender (M/F ratio) or age Chronic relevant diseases were present in all participants Number of participants (%) of the ITT population per treatment group with a medical condition: ‐ chronic active inflammation
‐ Gastric ulcer:
‐ Duodenal ulcer:
Ulcers were reported in 9 participants in the control group (10‐day STT) |
|
| Interventions | Participants randomised to the SEQ group were administered tinidazole Name, dose and timing of antibiotics in 10‐day SEQ: omeprazole 0.8 mg/kg/day , amoxicillin 40 mg/kg/day (5 days) + omeprazole 0.8 mg/kg/day, clarithromycin 15 mg/kg/day and tinidazole 15 mg/kg/day (5 days) Name, dose and timing of antibiotics in 10‐day STT: omeprazole 0.8 mg/kg/day , amoxicillin 40 mg/kg/day, clarithromycin 15 mg/kg/day (during 10 days) Length of STT (days): 10 Sensitivity test (yes/no) to antibiotics before/after treatment: N/R Method of assessment of H. pylori status after treatment: ¹³C‐UBT, blood test, RUT Time for assessment of H. pylori status after treatment: 4 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group:
ITT eradication proportions in the SEQ group were higher than in the STT group. The differences between treatment groups were statistically different (Chi² = 3.99, P < 0.05) PP eradication rate (%) by treatment group:
PP eradication rate in the SEQ group was higher than in the STT group. The differences between treatment groups were statistically different (Chi² = 4.06, P < 0.05) AEs rate (%) by treatment group:
Incidence of serious AEs SEQ/STT: Not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No information was given regarding the method of randomisation |
| Allocation concealment (selection bias) | High risk | No information was given regarding the concealment of the sequence allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study was not blinded |
| Publication format | Low risk | Full article |
Molina‐Infante 2010.
| Methods | Prospective, open‐label, single‐centre, randomised trial Dates the study was conducted: from January 2008 to August 2009 Funding sources and potential conflicts of interest: CIBEREHD is funded by the Instituto de Salud Carlos III. No conflicts of interest reported Definition of compliance: defined by a questionnaire |
|
| Participants | Number and type of participants: 460 H.pylori‐positive participants were included in the study from January 2008 to August 2009 Participants were equally randomised to 4 different treatment groups, each with 115 participants: 2 different triple regimens and 2 other sequential regimens Note: Only the data referring to the standard and the sequential regimens as described in the protocol are summarised below (that is. those regimens containing clarithromycin instead of levofloxacin) Number of participants randomised:460 (ITT sample) Number of participants in the 10‐day STT arm: 115 Number of participants in the 10‐day SEQ arm: 115 Country: Spain Average age (range) of the population in years reported by treatment group:
Sex proportions (%) reported as M/F per treatment group
Medical condition at baseline reported as n (%) in both regimens
H. pylori diagnostic method, n (%) in the 10‐day STT regimen/n (%) in the 10‐day SEQ regimen
|
|
| Interventions | Participants randomised to the SEQ group were administered metronidazole Name, dose timing of antibiotics in 10‐day STT: omeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 10 days) Length of STT (days): 10 Name, dose timing of antibiotics in 10‐day SEQ: omeprazole 20 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and omeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not specified Method of assessment of H. pylori status after treatment: ¹³C‐UBT except for participants requiring a follow‐up endoscopy because of gastric ulcer, in which histological examination of 4 samples taken from the body and the antrum stained with Giemsa was the diagnostic test Time for assessment of H. pylori status after treatment: 8 weeks |
|
| Outcomes | The study flow chart showed that in the 10‐day STT arm there was 1 dropout and 1 participant reported poor compliance, whereas in the 10‐day SEQ there was 1 dropout and 4 participants reported poor compliance. ITT eradication rate (%) by treatment group (95% CI) :
PP eradication rate (%) by treatment group (95% CI):
Eradication rate (%) in NUD participants reported by treatment group:
‐ Functional dyspepsia rate (%):
Eradication rate (%) in PUD participants reported by treatment group
Calculated cure proportions (%) in NUD (accounting for non‐investigated dyspepsia and functional dyspepsia) participants by treatment group:
Calculated cure proportions as rates in PUD (accounting for peptic ulcer participants only) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance rate in ITT sample, by treatment group: defined as poor
Incidence rate (%) of AEs in the overall cohort of participants (n = 460): 129 (28%) Incidence rate (%) of AEs by treatment group:
Incidence (%) serious AEs SEQ/STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done using a computer‐generated numeric sequence |
| Allocation concealment (selection bias) | Unclear risk | No further information was given regarding the sequence allocation concealment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | We considered the study not to be blinded as it was defined as 'open‐label' |
| Publication format | Low risk | Full article |
Nasa 2013.
| Methods | Prospective, open‐label, randomised, controlled clinical trial Dates the study was conducted: from July 2011 to June 2012 Funding sources and potential conflicts of interest: no information reported Definition of compliance: not defined Adherence was defined as consumption of more than 90% of the prescribed drugs and was determined by pill counts. Side effects were self‐reported |
|
| Participants | Number and type of participants: 231 H.pylori‐positive participants were enrolled in the study Participants were randomised to 2 different treatment groups: 14‐day standard triple regimen and 10‐day sequential regimen Country: India Number of participants randomised: 231 (ITT sample) Number of participants in the 14‐day STT arm:120 Number of participants in the 10‐day SEQ arm: 111 Mean age of the population (SD) reported as the number of participants by treatment group:
Sex ratio (M/F) per treatment group
Medical condition at baseline: antral gastritis, pangastritis, gastric ulcer, duodenal ulcer, normal H. pylori diagnostic methods in all treatment arms: gastroscopy and RUTor biopsy |
|
| Interventions | Participants randomised to the SEQ group were administered tinidazole Length of STT (days): 14 days Name, dose timing of antibiotics in 14‐day STT: pantoprazole 40 mg twice a day, clarithromycin 500 mg twice a day, amoxicillin 1g twice a day Name, dose timing of antibiotics in 10‐day SEQ: pantoprazole 40 mg twice a day + amoxicillin 1g twice a day (during 5 days) and pantoprazole 40 mg twice a day, clarithromycin 500 mg twice a day+ tinidazole 500mg twice a day (during 5 days) (Total: 10 days) Participants with gastric or duodenal ulcer were continued on pantoprazole for 1 month following completion of eradication regimen Sensitivity test (yes/no) to antibiotics before/after treatment: not performed Method of assessment of H. pylori status after treatment: RUT Time for assessment of H. pylori status after treatment: 4 weeks |
|
| Outcomes | ITT eradication rate (%) (95% CI) by treatment group :
PP eradication rate (%) by treatment group:
Adherence (%) (95% CI) to therapy by treatment group: Over 95% of the participants reported 100% adherence to the treatment, and none discontinued treatment due to adverse events. Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Incidence of AEs by treatment group (n, %):
Incidence (%) serious AEs SEQ/STT: none |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence generation was determined through a computer‐generated randomisation chart stratified according to centre |
| Allocation concealment (selection bias) | Low risk | Randomisation used a block design with a block size of 4 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcomes were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label study |
| Publication format | Low risk | Full article |
Oh 2012.
| Methods | Randomised, open‐label, double‐arm trial Dates study was conducted: from December 2009 to December 2010 Funding sources and potential conflicts of interest: no information reported Definition of compliance: intake > 90% |
|
| Participants | Number and type of participants: 246 H.pylori‐positive participants were enrolled in the study Participants were randomised to 2 different treatment groups: 7‐day standard triple regimen and 10‐day sequential regimen Number of participants randomised: 246 (ITT sample) Number of participants in the 7‐day STT arm, ITT analysis: 130 Number of participants in the 10‐day SEQ arm, ITT analysis: 116 Number of participants in the 7‐day STT arm, PP analysis: 127 Number of participants in the 10‐day SEQ arm, PP analysis: 111 Country: Korea Average age (SD) of the population in years reported by treatment group:
Sex proportions (M/F) by treatment group:
Medical condition at baseline reported as number of participants (%) in the 7‐day STT regimen/10‐day SEQ regimen:
H. pylori diagnostic methods in both treatment arms: both tests had to be positive for the participant to be classified as H. pylori‐positive
A gastric biopsy from the corpus was also taken |
|
| Interventions | Participants randomised to the SEQ group were administered metronidazole Name, dose timing of antibiotics in 7‐day STT: rabeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 7 days) Length of STT (days): 7 Name, dose timing of antibiotics in 10‐day SEQ: rabeprazole 20 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and rabeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status after treatment: ¹³C‐urea breath test Time for assessment of H. pylori status after treatment: 4 weeks |
|
| Outcomes | The study flow chart showed that after randomisation, in the 7‐day STT arm there were 3 dropouts, whereas in the 10‐day SEQ there were 2 dropouts ITT eradication rate (%) by treatment group :
PP eradication rate (%) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance in ITT sample SEQ/STT: 5 participants were excluded from the analysis as they took < 90% of the drugs Incidence and type of AEs reported as the number of participants (%) in the SEQ/STT arms respectively: Bitter taste: 6 (18.7)/6 (16.6), P = 1.000 Nausea 5 (15.6)/1 (3.2), P = 0.196 Epigastric soreness: 7 (21.8)/7 (19.3), P = 1.000 Diarrhoea: 7 (21.8)/6 (16.6), P = 1.000 Headache: 2 (6.2)/2 (6.4), P = 1.000 Dyspepsia: 1 (3.1)/5 (16.1), P = 0.104 Constipation: 1 (3.1)/1 (3.2), P = 1.000 Bloating: 2 (6.2)/2 (6.4), P = 1.000 Oral mucositis: 1 (3.1)/0 Dizziness: 0/1 (3.2) Total of AEs: 27.5 (32/116)/23.8 (31/130), P = 0.559 Incidence (%) of serious AEs SEQ/STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial was truly randomised as individuals were assigned into treatment groups by using a random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Concealment allocation was not defined |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded as it was reported open‐label |
| Publication format | Low risk | Full article |
Paoluzi 2010.
| Methods | Prospective, open‐label, randomised single study Dates study was conducted: not reported Funding sources and potential conflicts of interest: Authors declare no conflicts and do acknowledge no grant Definition of compliance: evaluated by counting the number of pills left in the packages returned at post‐therapy control and was defined as good if more than 90% of the tablets had been taken |
|
| Participants | Number and type of participants: 270 H.pylori‐positive participants were included in the study Participants were randomised into to 3 different treatment groups: 7‐day STT regimen, 8‐day SEQ regimen and 10‐day SEQ regimen Note: Only the data referring to the 7‐day STT and 10‐day SEQ regimens (as those described in the protocol) are summarised below Country: Italy Number of participants randomised: 270 (ITT sample) Number of participants in the 7‐day STT arm: 90 Number of participants in the 10‐day SEQ arm: 90 The medical condition at baseline was not reported in the 7‐day STT regimen, nor in the 10‐day SEQ regimen Average age (SD, range) of the population in years reported by treatment group:
Sex (M/F) per treatment group
The overall of participants were comparable in terms of age and sex distribution |
|
| Interventions | Participants randomised to the SEQ group were administered tinidazole Name, dose timing of antibiotics in 7‐day STT: esomeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 7 days) Length of STT (days): 7 Name, dose timing of antibiotics in 10‐day SEQ: esomeprazole 20 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and esomeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + tinidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status after treatment: UBT, stool antigen assay, RUT, and histology. The participant was considered H. pylori‐positive if 2 out of the 3 tests were positive Time for assessment of H. pylori status after treatment: 8 weeks |
|
| Outcomes | The study flow chart showed that in the 7‐day STT arm there were 12 dropouts, 7 participants were lost to follow‐up, 3 participants reported low compliance and 2 participants reported severe side effects. In the 10‐day SEQ arm, there were 3 dropouts due to severe side effects ITT eradication rate (%) by treatment group : (P < 0.05)
PP eradication by treatment group, n (%): (P < 0.05)
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance in ITT sample: good in both regimens with the exception of the participants who experienced severe side effects in both 7‐day STT and 10‐day SEQ arms Incidence rate (%) of AEs:
Nausea and taste perversion were the most frequently reported symptoms by participants on 10‐day SEQ and 7‐day STT, respectively. Incidence rate of serious AEs by treatment group dealing to drop‐out.
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐generated randomisation list. |
| Allocation concealment (selection bias) | High risk | Allocation was unconcealed as all investigators were also informed regarding the kind of therapy assigned to each participant enrolled in the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded as it was reported as open‐label |
| Publication format | Low risk | Full article |
Park 2012.
| Methods | Prospective, open‐label, multicentre, randomised, controlled trial Dates the study was conducted: from May 2009 to December 2010 Funding sources and potential conflicts of interest: Authors declare no funding and no personal interests Definition of compliance: intake > 90% and determined by pill counts and the medication personal diary |
|
| Participants | Number and type of participants: 326 H.pylori‐positive adults were enrolled in the study Participants were randomised into to 2 different treatment groups: 7‐day standard triple regimen and 10‐day sequential regimen Average age (SD) of the population in years, reported by treatment group:
Medical condition at baseline reported as number of participants (%) in the 7‐day STT regimen/10‐day SEQ regimen:
Country: Korea Number of participants randomised: 326 Number of participants in the 7‐day STT arm: 164 Number of participants in the 10‐day SEQ arm: 162 Sex (M/F) per treatment group, n (%)
H. pylori diagnostic methods in both treatment arms: ¹³C‐UBT |
|
| Interventions | Participants randomised to the SEQ group were administered metronidazole Name, dose timing of antibiotics in 7‐day STT: rabeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 7 days) Length of STT (days): 7 Name, dose timing of antibiotics in 10‐day SEQ: rabeprazole 20 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and rabeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not performed Method of assessment of H. pylori status after treatment: ¹³C‐UBT Time for assessment of H. pylori status after treatment: 4 weeks |
|
| Outcomes | The study flow chart showed that in the 7‐day STT arm there were 39 losses and exclusions (27 lost to follow‐up; 10 low compliance and 2 discontinued intervention due to adverse events), whereas in the 10‐day SEQ there were 30 losses and exclusions (19 lost to follow‐up; 9 low compliance and 2 discontinued intervention due to adverse events)
ITT eradication (%) (95% CI) by treatment group:
PP eradication (%) (95% CI) by treatment group:
Difference sequential vs triple therapy eradication: ITT: P = 0.002 PP = P= 0.013 Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance rate (%) in ITT sample by treatment group SEQ/STT:
Number of participants (n,(%) reporting adherence > 90% in ITT sample: (P = 0.738)
Incidence of AEs per type and number of participants n (%), by treatment group:
Withdrawals rate (%) due to AEs:
Incidence (n, %) serious AEs, SEQ/STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a computer‐generated randomisation list by an external statistician |
| Allocation concealment (selection bias) | High risk | The allocation was unconcealed as the treatment assignment was ascertained by the study investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The trial was not blinded as it was reported as open‐label |
| Publication format | Low risk | Full article |
Rakici 2014.
| Methods | Randomised, controlled trial Dates the study was conducted: from January 2010 to December 2011 Funding sources and potential conflicts of interest: no funding reported. Authors declare no conflicts of interest Definition of compliance: Non‐compliance was defined as participants who were reluctant to take the drugs due to nausea, diarrhoea, a bitter taste in the mouth, an allergic reaction and feeling ill |
|
| Participants | umber and type of participants: 514 H.pylori‐positive were enrolled in the study Participants were randomised into to 3 different treatment groups: 14‐day STT, 10‐day SEQ and a modified triple therapy (with moxifloxacin and metronidazole). For our review purpose, only data related to STT and SEQ are relevant. Country: Turkey Number of participants randomised: 514 Number of participants in the 7‐day STT arm: 171 Number of participants in the 10‐day SEQ arm: 172 Average age (range) of the population in years, reported by treatment group:
Medical condition at baseline reported as number of participants (%) in the 14‐day STT regimen/10‐day SEQ regimen:
Sex (M/F) per treatment group:
H. pylori diagnostic methods in both treatment arms: histological examination and stool antigen tests |
|
| Interventions | Participants randomised to the SEQ group were administered metronidazole Name, dose timing of antibiotics in 14‐day STT: lansoprazole 30 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 7 days) Length of STT (days): 14 Name, dose timing of antibiotics in 10‐day SEQ: lansoprazole 30 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and lansoprazole 30 mg twice a day + clarithromycin 500 mg twice a day + metronidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not performed Method of assessment of H. pylori status after treatment: stool antigen testing Time for assessment of H. pylori status after treatment: 4 ‐ 6 weeks |
|
| Outcomes | The study flow chart showed that in the 14‐day STT arm there were 2 losses, and in the 10‐day SEQ there were 2 losses ITT eradication rate (%) by treatment group:
PP eradication rate (%) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance (%) in ITT sample SEQ/STT: not reported Adherence > 90% (n, (%)) in ITT sample: not reported Incidence n (%) of AEs, by treatment group: not reported Incidence (n, %) serious AEs, SEQ / STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were reported to be randomised but method of randomisation given |
| Allocation concealment (selection bias) | Unclear risk | Concealment of the sequence was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcomes were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Publication format | Low risk | Full article |
Scaccianoce 2006.
| Methods | Prospective, parallel, open‐label, 2‐centre, randomised study Dates the study was conducted: not reported Funding sources and potential conflicts of interest: no information reported Definition of compliance: intake > 90% of prescribed drugs and determined by pills count at the follow‐up visit |
|
| Participants | Number and type of participants: 213 NUD and H.pylori‐positive participants were enrolled in the study Participants were randomised to 3 different treatment groups: 7‐day standard triple regimen, 10‐day standard triple regimen and 10‐day sequential regimen Number of participants randomised: 213 Number of participants in the 7‐day STT arm: 70 Number of participants in the 10‐day STT arm: 71 Number of participants in the 10‐day SEQ arm: 72 Country: Italy Average age (SD) of the population in years reported by treatment group:
Sex (M/F) per treatment group
Medical condition at baseline reported as number of participants per treatment group, 7‐day STT/10‐day STT/10‐day SEQ: All participants were NUD
Bacterial density per treatment group, 7‐day STT/10‐day STT/10‐day SEQ:
H. pylori diagnostic methods in all treatment arms: all participants were considered H. pylori‐positive if both of the following tests were positive:
|
|
| Interventions | Participants randomised to the SEQ group were administered tinidazole Length of STT (days): 7 and 10 days Name, dose timing of antibiotics in 7‐day STT: esomeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 7 days) Name, dose timing of antibiotics in 10‐day STT: esomeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 10 days) Name, dose timing of antibiotics in 10‐day SEQ: esomeprazole 20 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and esomeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + tinidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not specified Method of assessment of H. pylori status after treatment: ¹³C‐urea breath test Time for assessment of H. pylori status after treatment: 4 ‐ 6 weeks The breath samples were considered positive if there was > 5 per 1000 of ¹³CO₂ difference over baseline |
|
| Outcomes | Overall, 6 participants (2 in each treatment group) stopped the treatment and did not undergo the ¹³C‐urea breath test. The final PP population consisted of 207 participants ITT eradication rate (%) (95% CI) by treatment group:
PP eradication rate (%) (95% CI) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance in ITT sample SEQ/STT: reported as 'good' in all groups (> 95%) but for 6 participants who stopped the treatment due to side effects Incidence of AEs per type and number of participants (%) per treatment group:
Incidence (%) serious AEs SEQ / STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | This is a truly randomised trial where treatments were assigned by a computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | The allocation concealment is unclear as no information with a description of the allocation was provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This is not a blinded study as it was reported open‐label |
| Publication format | Low risk | Full article |
Seddik 2013.
| Methods | Prospective, randomised, controlled clinical trial Dates the study was conducted: from June 2011 to August 2012 Funding sources and potential conflicts of interest: No information on funding reported. Authors declared no conflict of interest. Compliance was defined as the consumption of > 90 % of the prescribed drugs and was determined by pill counts at the follow‐up visit Side effects were evaluated using a structured questionnaire by personal interview |
|
| Participants | Number and type of participants: 281 H.pylori‐positive participants were enrolled in the study Participants were randomised to 2 different treatment groups: 7‐day standard triple regimen and 10‐day sequential regimen Country: Morocco Number of participants randomised: 281 (ITT sample) Number of participants in the 7‐day STT arm: 141 Number of participants in the 10‐day SEQ arm: 140 Mean age of the population reported as the number of participants by treatment group:
Sex ratio (M/F) per treatment group
Medical condition at baseline: NUD (n, %)
PUD (n, %)
H. pylori diagnostic methods in all treatment arms: endoscopy with biopsies, 2 samples from the antrum ant 2 samples from the corpus |
|
| Interventions | Participants randomised to the SEQ group were administered tinidazole Length of STT (days): 7 days Name, dose timing of antibiotics in 14‐day STT: omeprazole 20 mg twice a day, clarithromycin 500 mg twice a day, amoxicillin 1g twice a day Name, dose timing of antibiotics in 10‐day SEQ: omeprazole 20 mg twice a day + amoxicillin 1g twice a day (during 5 days) and omeprazole 20 mg twice a day, clarithromycin 500 mg twice a day+ tinidazole 500mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: Performed but method not specified Method of assessment of H. pylori status after treatment: ¹³C‐urea breath test Time for assessment of H. pylori status after treatment: 4 ‐ 6 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group :
PP eradication rate (%) by treatment group:
Adherence (%) [95%CI] to therapy by treatment group: not reported Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Incidence rate (%) of AEs by treatment group:
Incidence (%) serious AEs SEQ / STT: not reported, but 1 participant in the SEQ group and 2 in the STT group discontinued treatment because of severe diarrhoea. All side effects were self‐limiting after therapy ended |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by a computer‐generated list |
| Allocation concealment (selection bias) | Unclear risk | Risk is unclear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcomes are reported clearly. However, adherence and eradication by underlying disease were not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Investigators were blind to the treatment group for the confirmation of HP eradication |
| Publication format | Low risk | Full article |
Tepes 2012.
| Methods | Multicentre, prospective, randomised, controlled clinical trial Dates the study was conducted: from 2011 to 2014 Funding sources and potential conflicts of interest: study co‐funded by Slovenian Association for Gastroenterology and Hepatology and a grant from KRKA Pharmaceuticals. No information of conflicts of interest reported Definition of compliance: not defined |
|
| Participants | Number and type of participants: 356 H.pylori‐positive participants were enrolled in the study Participants were randomised to 3 different treatment groups: 7‐day standard triple regimen, 10‐day sequential regimen and concomitant therapy for 10 days. For our review only data for the standard and the sequential therapies are relevant Country: Slovenia Number of participants randomised: 356 (ITT sample) Number of participants in the 7‐day STT arm: 116 Number of participants in the 10‐day SEQ arm: 120 Number of participants in the PP analysis: 344 Number of participants in the 7‐day STT arm (PP analysis): 110 Number of participants in the 10‐day SEQ arm (PP analysis): 117 Mean age of the population reported as the number of participants by treatment group: not reported Sex ratio (M/F) per treatment group:
Medical condition at baseline by treatment group as n (%) Functional dyspepsia:
Duodenal ulcer:
Gastric ulcer:
H. pylori diagnostic methods in all treatment arms: ¹³C‐urea breath test, rapid urease test, histology and H pylori culture. 2 tests had to be positive for definite diagnosis Sensitivity test (yes/no) to antibiotics before/after treatment: Culture positive biopsy specimens were phenotypically tested for susceptibility to amoxicillin, clarithromycin and metronidazole using gradient diffusion method Metronidazole resistance (%) before treatment:
Clarithromycin resistance (%) before treatment
Dual resistance (%) before treatment
|
|
| Interventions | Participants randomised to the SEQ group were administered metronidazole. Length of STT (days): 7 days Name, dose timing of antibiotics in 7‐day STT: esomeprazole 20 mg twice a day, clarithromycin 500 mg twice a day, amoxicillin 1g twice a day Name, dose timing of antibiotics in 10‐day SEQ: esomeprazole 20 mg twice a day + amoxicillin 1g twice a day (during 5 days) and esomeprazole 20 mg twice a day, clarithromycin 500 mg twice a day+ metronidazole 400 mg twice a day (during 5 days) (Total: 10 days) Method of assessment of H. pylori status after treatment: ¹³C‐urea breath test Time for assessment of H. pylori status after treatment: 4 weeks |
|
| Outcomes | ITT eradication rate (%) (95% CI) by treatment group :
Metronidazole resistance rate (%) after treatment, ITT analysis
Clarithromycin resistance rate (%) after treatment, ITT analysis
PP eradication rate (%) (95% CI) by treatment group: not reported
Metronidazole resistance rate (%) after treatment, PP analysis
Clarithromycin resistance (%) after treatment, PP analysis
Adherence rate (%) to therapy by treatment group: not reported Compliance rate (%) by treatment group: reported as "very good" in all treatment arms Incidence rate (%) of AEs by treatment group:
Incidence (%) serious AEs SEQ/STT: not reported |
|
| Notes | Author was contacted for further details on methods | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random‐number table |
| Allocation concealment (selection bias) | Low risk | Investigators in the centres did not know the details of the allocation sequence |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Eradication rate by treatment group was not reported in the abstract. However, first author provided detailed data when contacted |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Treatment was clearly explained to all participants by investigating physician in each participating centre. Study drugs were handed to patients with a day‐by‐ day intake scheme and diagram. Drugs were self‐administered orally at home 30 minutes before meals |
| Publication format | High risk | Abstract |
Vaira 2007.
| Methods | Randomised, double‐blind, placebo‐controlled trial Dates the study was conducted: from September 2003 to April 2006 Funding sources and potential conflicts of interest: Dr. Vakil was paid at a conference by Altana Pharma (Nicomed) (manufacturer of pantoprazole). Authors declare potential financial conflicts: grant received, consultancies and stock ownership Definition of compliance: > 90% |
|
| Participants | Number and type of participants: 300 participants with dyspepsia or peptic ulcers Participants were randomised to 2 different treatment groups: 10‐day standard triple regimen and 10‐day sequential regimen Number of participants randomised: 300 Number of participants in the 10‐day SEQ arm: 150 Number of participants in the 10‐day STT arm: 150 ITT sample: 300 participants PP sample: 289 participants ‐ Number of participants in the 10‐day SEQ arm: 143 ‐ Number of participants in the 10‐day STT arm: 146 Country:Italy Average age (SD) of the population in years by treatment group:
Sex proportions (%) reported as M/F, by treatment group:
Medical condition at baseline reported as the proportion of participants by treatment group: ‐ peptic ulcer:
‐ antral gastritis:
‐ intestinal metaplasia:
|
|
| Interventions | Participants randomised to the SEQ group were administered tinidazole Name, dose timing of antibiotics in 10‐day STT: pantoprazole 40 mg + amoxicillin 1 g + clarithromycin 500 mg Name, dose timing of antibiotics in 10‐day SEQ: pantoprazole 40 mg twice a day + amoxicillin 1 g twice a day + placebo twice a day (5 days) and pantoprazole 40 mg twice a day + clarithromycin 500 mg twice a day + tinidazole 500 mg twice a day (5 days) Length of STT (days): 10 days Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status after treatment: ¹³C‐UBT Time for assessment of H. pylori status after treatment: at both 4 and 8 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group:
PP eradication rate (%) by treatment group:
Regarding the influence of resistance, data for 246 participants, including 127 who were treated with 10‐day SEQ and 119 who were treated with the 10‐day STT, were available for the PP analysis: metronidazole resistance rate (%) before treatment:
*metronidazole‐susceptible proportion (%) before treatment: (P = 0.009)
*clarithromycin‐resistance proportion (%) before treatment: (P = 0.0034)
*Differences between groups were statistically significant PP clarithromycin‐susceptible proportion (%) before treatment:
PP both clarithromycin and metronidazole resistance (%):
Adherence to treatment < 90% reported as the number of participants (%) by treatment group:
Incidence rate (%) of minor AEs:
The most frequent side effects in both groups were epigastric pain (5.6% vs 4.8%; P = 0.902) and mild diarrhoea (4.8% vs 2.8%; P = 0.54) Incidence rate (%) of serious AEs by treatment group SEQ/STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation chart was used to determine allocation, which was stratified according to centre by using a block design and a block size of 4 |
| Allocation concealment (selection bias) | Low risk | Participant allocation was determined with a random‐number chart that was concealed from investigators and participants by using numbered blister packs of the study medication that corresponded to the random‐number chart Allocation was concealed with an opaque envelope, which contained a number that corresponded to the numbered blister packs |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | A placebo that was identical in colour and shape to the clarithromycin capsule was administered during the first 5 days of sequential therapy to maintain blinding |
| Publication format | Low risk | Full article |
Wu 2011.
| Methods | Randomised clinical trial Dates the study was conducted: not reported Funding sources and potential conflicts of interest: no information reported Definition of compliance: not reported |
|
| Participants | Number and type of participants: 102 H.pylori‐infected participants were enrolled in the study Participants were randomised to 2 different treatment groups: 14‐day standard triple regimen and 10‐day sequential regimen Number of participants randomised: 102 (ITT sample) Number of participants in the 14‐day STT arm: not reported Number of participants in the 10‐day SEQ arm: not reported PP sample: not reported Country: China Average age (SD) of the population in years: not reported Sex (M/F) of the population: not reported Medical condition at baseline: participants diagnosed with chronic gastritis and PUD H. pylori diagnostic methods in all treatment arms: not reported |
|
| Interventions | Participants randomised to the SEQ group were administered tinidazole Length of STT (days): 14 Name, dose timing of antibiotics in 14‐day STT: esomeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 14 days) Name, dose timing of antibiotics in 10‐day SEQ: esomeprazole 20 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and esomeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + tinidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status both before and after treatment: ¹³C‐UBT or endoscopy Time for assessment of H. pylori status after treatment: 4 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group:
PP eradication rate (%) by treatment group) were not reported Metronidazole resistance rate (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance rate (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance rate (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance rate (%) before treatment, STT ITT/PP: not reported Compliance rate (%): not reported incidence rate (%) of AEs:
Incidence (%) serious AEs: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on the method of randomisation was reported |
| Allocation concealment (selection bias) | Unclear risk | No information on the allocation concealment was reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Eradication proportions were reported as percentages. The number of participants randomised to each of the treatment groups was not reported, so ITT cure proportions were calculated but PP cure proportions could not be calculated |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information on the masking was reported |
| Publication format | High risk | Abstract |
Yan 2011.
| Methods | Multiple‐centre (Beijing, Shanghai, Wuhan and Guangzhou), prospective, randomised, controlled trial Dates the study was conducted: not reported Funding sources and potential conflicts of interest: no information reported Definition of compliance: not reported |
|
| Participants | Number and type of participants: 624 H.pylori‐positive participants were enrolled in the study Participants were randomised to 2 different treatment groups: 10‐day standard triple regimen and 10‐day sequential regimen Number of participants randomised: 622 (ITT sample) Number of participants in the 10‐day STT arm: 281 Number of participants in the 10‐day SEQ arm: 341 PP sample: not reported Country: China Average age (SD) of the population in years: not reported Sex (M/F) of the population: not reported Authors reported that there were no differences in age or BMI between treatment groups Medical condition at baseline: not reported H. pylori diagnostic methods in all treatment arms: histopathology Warthin‐Starry (WS) stain and RUT |
|
| Interventions | Participants randomised to the SEQ group were administered tinidazole Length of STT (days): 10 Name, dose timing of antibiotics in 10‐day STT: esomeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 10 days) Name, dose timing of antibiotics in 10‐day SEQ: esomeprazole 20 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and esomeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + tinidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status both before and after treatment: ¹³C‐UBT and histology WS stain Time for assessment of H. pylori status after treatment: 4 ‐ 12 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group:
PP eradication (%) by treatment group were not reported Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance: not reported Incidence of AEs at PP analysis in the total population: not reported Incidence (%) serious AEs SEQ / STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study was reported as "randomised", no additional information was given |
| Allocation concealment (selection bias) | Unclear risk | Risk is unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Risk is unclear |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Risk is unclear |
| Publication format | High risk | Abstract |
Zhou 2014.
| Methods | Prospective, randomised, controlled clinical trial Dates the study was conducted: from March 2008 to December 2010. Funding sources and potential conflicts of interest: study supported by the National Science & Technology Pillar Program of 11th Five‐Year Plan in China (2007BAI04B02). Authors declare no conflicts of interest Definition of compliance: Compliance, determined by pill counts, was defined as good when > 90 % of the prescribed drugs were taken. Participants who had taken 80% of the treatment drugs were considered to show poor compliance and were excluded from the PP analysis Adverse events were evaluated by using open‐ended questions, by participant self reports, and from physical examinations |
|
| Participants | Number and type of participants: 280 H.pylori‐positive participants were enrolled in the study Participants were randomised to 2 different treatment groups: 10‐day standard triple regimen and 10‐day sequential regimen Country: China Number of participants randomised: 280 (ITT sample) Number of participants in the 10‐day STT arm: 140 (ITT analysis) Number of participants in the 10‐day STT arm: 128 (PP analysis) Number of participants in the 10‐day SEQ arm: 140 (ITT analysis) Number of participants in the 10‐day SEQ arm: 132 (PP analysis) Mean age of the population (SD) reported as the number of participants by treatment group:
Sex ratio (M/F) per treatment group
Medical condition at baseline, endoscopic findings (NUD/PUD):
Antibiotic susceptibility was tested in vitro by the E‐tes from collected H pylori strains. Breakpoints were ≥ 1.0 μg/ml for amoxicillin and clarithromycin and ≥ 8 μg/ml for metronidazole. Isolated CLA‐R was defined as clarithromycin resistance and susceptibility to metronidazole. Isolated MET‐R was defined as metronidazole resistance and susceptibility to clarithromycin. Number of participants with clarithromycin resistance before treatment, n (%):
Number of participants with metronidazole resistance before treatment, n (%):
H. pylori diagnostic methods in all treatment arms: endoscopy with a gastric biopsy taken from the antrum was subjected to a RUT. If the result was positive, 2 additional specimens (from the antrum and corpus) were obtained for H. pylori culture. Participants with positive cultures were classified as being infected with H. pylori |
|
| Interventions | Participants randomised to the SEQ group were administered tinidazole Length of STT (days): 10 days Name, dose timing of antibiotics in 10‐day STT: esomeprazole 20 mg twice a day, clarithromycin 500 mg twice a day, amoxicillin 1g twice a day Name, dose timing of antibiotics in 10‐day SEQ: esomeprazole 20 mg twice a day + amoxicillin 1g twice a day (during 5 days) and esomeprazole 20 mg twice a day, clarithromycin 500 mg twice a day+ tinidazole 500mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: Performed but method not specified Method of assessment of H. pylori status after treatment: ¹³C‐urea breath test Time for assessment of H. pylori status after treatment: 8 ‐ 12 weeks |
|
| Outcomes | ITT eradication rate (%) by treatment group :
PP eradication rate (%) by treatment group:
Adherence rate (%) (95% CI) to therapy by treatment group: not reported Effect of antibiotic resistances on H pylori eradication proportions in the PP population, by treatment arm: ‐ Clarithromycin resistance rate (%) (amoxicillin and metronidazole susceptible):
‐ Metronidazole resistance rate (%) (amoxicillin and clarithromycin susceptible):
‐ Dual resistance rate (%) (clarithromycin and metronidazole resistance and amoxicillin susceptible):
Incidence of AEs by treatment group (n, %): not reported Incidence (%) serious AEs SEQ / STT: not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer‐generated randomisation scheme (SAS version 8.0; SAS Institute, Cary, NC), with stratification by centre, was constructed using a block design (block size of 4) by an independent statistician and was used to determine treatment allocation |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed by the use of opaque envelopes that were opened by the investigator when the participant was eligible for the study and had provided written informed consent. The endoscopists, pathologists, and technicians who performed RUT and UBT were all blinded to the treatment group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The participants were randomly assigned to treatment in a 1:1 ratio within 2 weeks of a positive culture result |
| Publication format | Low risk | Full article |
Zullo 2003.
| Methods | Large, multicentre, open‐label, randomised controlled trial Dates the study was conducted: from January 2001 to December 2001 Funding sources and potential conflicts of interest: no information reported Definition of compliance: consumption of > 90% of the prescribed drugs, determined by pill counts at the follow‐up visit |
|
| Participants | Number and type of participants: 1049 H.pylori‐positive participants were enrolled in the study Participants were randomised to 2 different treatment groups: 7‐day standard triple regimen and 10‐day sequential regimen Number of participants randomised: 1049 Number of participants in the 10‐day SEQ arm: 522 Number of participants in the 7‐day STT arm: 527 Number of participants lost to follow‐up: 36 (16 from the study group and 20 from the control group) ITT sample: 1049 participants PP sample: 1013 participants Country: Italy Average age (SD) of the ITT population in years: 53 (13) Average age (SD) of the ITT population in years, reported by treatment group:
Sex (M/F) of the ITT population per treatment group
Number of participants (%) of the ITT population per treatment group with a medical condition: ‐ Non‐ulcer dyspepsia
‐ Peptic ulcer disease
Among those with PUD: ‐ Number of participants with gastric ulcer
‐ Number of participants with duodenal ulcer
|
|
| Interventions | Participants randomised to the SEQ group were administered tinidazole Name, dose and timing of antibiotics in 10‐day SEQ: Rabeprazole 20 mg twice a day, amoxycillin 1 g twice a day (5 days) + rabeprazole 20 mg twice a day, clarithromycin 500 mg twice a day and tinidazole 500 mg twice a day (5 days) Name, dose and timing of antibiotics in 7‐day STT: rabeprazole 20 mg twice a day, amoxycillin 1 g twice a day, clarithromycin 500 mg twice a day during 7 days Length of STT (days): 7 Sensitivity test (yes/no) to antibiotics before/after treatment: yes Method of assessment of H. pylori status after treatment: endoscopy with biopsies (as at baseline) and 24 hours after ¹³C‐UBT Time for assessment of H. pylori status after treatment: minimum of 6 weeks |
|
| Outcomes | ITT eradication rate (%) (95% CI) by treatment group:
PP eradication rate (%) (95% CI) by treatment group:
ITT eradication rate (%) (95% CI) in the PUD group:
IPP eradication rate (%) (95% CI) in the PUD group:
ITT eradication rate (%) (95% CI) in the NUD group:
PP eradication rate (%) (95% CI) in the NUD group:
Clarithromycin resistance rate (%) (95% CI) by treatment group:
Nitroimidazole (tinidazole) resistance rate (%) (95% CI) by treatment group:
Both clarithromycin and metronidazole resistance rate (%) (95% CI) by treatment group:
Compliance rate (%) in ITT sample:
‐ 50 participants in the 10‐day SEQ and 36 in the 7‐day STT consumed > 50% but < 90% of the prescribed pills Incidence rate (%) of AEs:
The most frequent side effects in the 2 treatment arms were diarrhoea (39% vs 35%, P = 0.8, for the new regimen and standard therapy, respectively) and abdominal pain (22% vs 29%, P = 0.4, for the new regimen and standard therapy,respectively) Incidence of serious AEs SEQ/STT: Not reported |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | This study is a truly randomised trial as authors reported that the determination of whether a participant would be treated by 1 treatment or another was made on the basis of a computer‐generated randomisation list drawn by a statistician |
| Allocation concealment (selection bias) | Low risk | It appears there was concealment of the allocation as the details of the series were unknown to any of the investigators and were contained in a set of opaque, sealed envelopes with only the name and number of the hospital on the outside |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcomes were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study appears not to be blinded as it is defined as an open‐label study |
| Publication format | Low risk | Full article |
Zullo 2005.
| Methods | Prospective, open‐label, 3‐centre, randomised trial Dates the study was conducted: not reported Funding sources and potential conflicts of interest: no information reported Definition of compliance: intake > 90% |
|
| Participants | Number and type of participants: 179 H.pylori‐positive and PUD disease geriatric participants were enrolled in the study Participants were randomised to 2 different treatment groups: 7‐day standard triple regimen and 10‐day sequential regimen Number of participants randomised: 179 Number of participants in the 7‐day STT arm: 90 Number of participants in the 10‐day SEQ arm: 89 ITT sample: 179 participants PP sample: 174 participants Country: Italy (3 centres: Rome, Foggia, Bologna) Average age (range) of the population in years reported by treatment group:
Sex (M/F) per treatment group
Medical condition at baseline reported as number of participants per treatment group: ‐ Duodenal ulcer:
‐ Gastric ulcer:
H. pylori diagnostic methods in both treatment arms: both tests had to be positive in order to consider participants H. pylori‐positive
|
|
| Interventions | Participants randomised to the SEQ group were administered tinidazole Name, dose timing of antibiotics in 7‐day STT: rabeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + amoxicillin 1 g twice a day (during 7 days) Length of STT (days): 7 Name, dose timing of antibiotics in 10‐day SEQ: rabeprazole 20 mg twice a day + amoxicillin 1 g twice a day (during 5 days) and rabeprazole 20 mg twice a day + clarithromycin 500 mg twice a day + tinidazole 500 mg twice a day (during 5 days) (Total: 10 days) Sensitivity test (yes/no) to antibiotics before/after treatment: not reported Method of assessment of H. pylori status after treatment: both RUT and histology Time for assessment of H. pylori status after treatment: 4 ‐ 6 weeks |
|
| Outcomes | The study flow chart showed that in the 7‐day STT arm there were 3 dropouts, whereas in the 10‐day SEQ there were 2 dropouts ITT eradication rate (%) with (95% CI) by treatment group :
PP eradication rate (%) with (95% CI) by treatment group:
Metronidazole resistance (%) before treatment, SEQ ITT/PP: not reported Metronidazole resistance (%) before treatment, STT ITT/PP: not reported Clarithromycin resistance (%) before treatment, SEQ ITT/PP: not reported Clarithromycin resistance (%) before treatment, STT ITT/PP: not reported Compliance in ITT sample SEQ / STT: reported high in both groups (>95%) Incidence rate (%) of AEs in the 7‐day STT and 10‐day SEQ regimens: 10 / 90 (11.5%) and 9 / 89 (10.3%).
Incidence (%) serious AEs SEQ / STT: not reported Side effects were evaluated using a structured questionnaire by personal interview |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study is truly randomised as authors reported the use of a computer‐generated list to perform the randomisation |
| Allocation concealment (selection bias) | Unclear risk | There is no information regarding the concealment of the allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary outcome data were clearly reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The study is defined as not blinded as it is an open‐label study |
| Publication format | Low risk | Full article |
BMI: body mass index CLO: Campylobacter‐like organism EGDS: oesophagogastroduodenoscopy ITT: intention‐to‐treat NUD: non‐ulcer dyspepsia PCR: polymerase chain reaction PP: per protocol PUD: peptic ulcer disease RUT: rapid urease test SD: standard deviation UBT: urea breath test
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Francavilla 2005 | Not STT ‐ use of metronidazole instead of clarithromycin |
| Hu 2009 | Not SEQ vs STT |
| Huang 2012a | We contacted the authors but could not retrieve the relevant information |
| Huang 2012b | Not SEQ vs STT ‐ but vs concomitant therapy |
| Kadayifci 2008 | Not SEQ 10 days but 14 days |
| Kim 2013 | The confirmation test for eradication was not reported |
| Nagahara 2001 | Not SEQ vs STT |
| Neville 1999 | Not STT ‐ lasting just 5 days |
| Ntouli 2013 | Not SEQ as per protocol, or not clearly reported in abstract |
| Ruiz‐Obaldía 2008 | No adequate time of HP eradication assessment ‐ just 15 days after finalisation of therapy |
| Torres 2012 | Not the outcome of interest |
| Urgesi 2011 | Previous eradication therapy |
| Uygun 2008 | Not SEQ as per protocol ‐ tetracycline 500 mg was used |
| Valooran 2011 | Not SEQ as per protocol ‐ amoxicillin was used instead of metronidazole or tinidazole |
| Zhao 2009 | Not SEQ vs STT |
Differences between protocol and review
In the protocol, we stated that an odds ratio (OR) would be used as the effect estimate. However, we felt that the OR would be biased away from the null as the probability of the outcome was certain (eradication was the normal and subsequent event to happen after treatment). The treatment was fixed and the follow‐up period was fixed and ranged. We chose the risk difference (RD) as the appropriate estimate of the effect. The calculations such as the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) were therefore modified accordingly.
Likewise, we stated in the protocol a fixed‐effect model would be used for interpretation and data analysis. However, while doing the review, we realised that a part from the high heterogeneity found among studies (where a fixed model could also have been used) authors wanted to make an unconditional inference about the average outcome in a typical hypothetical population of studies from which the 44 studies included in our meta‐analysis were representative of this random sample. By leaving a fixed‐effect model, the inference would be confined to our sample only and conclusions would be confined to the results of these 44 studies. The suggestion was to change the model post‐hoc not to modify how data was to be read but to broaden its interpretation. The use of fixed effect model in this review was therefore unadvisable as the authors could not assume that the estimate of the effect was fixed between variations of treatment or different population; and therefore, the random effects model was chosen.
Contributions of authors
All authors participated in developing the protocol. Olga P Nyssen (OPN) was the lead review author, performed the data extraction, wrote the first draft and performed all the meta‐analyses. Adrian G McNicholl (AGM) was the second review author, duplicated all phases of the review: first, second screenings and data extraction as well as reviewing all meta‐analyses. Javier P Gisbert (JPG) was the review author reaching consensus when needed and acting as the principal supervisor of all phases of the review. The remaining authors critically reviewed and commented on both the protocol the analyses performed, and on the final manuscript.
Sources of support
Internal sources
Centro de Investigación Biomédica en Red en el Área temática de Enfermedades Hepáticas y Digestivas (CIBERehd), Spain.
External sources
No sources of support supplied
Declarations of interest
Centro de Investigación Biomédica en Red en el Área temática de Enfermedades Hepáticas y Digestivas (CIBERehd) is funded by Instituto de Salud Carlos III.
OPN: none known.
AGMcN: Dr McNicholl received fees from Allergan form speaking in 2016.
FM: Dr Mégraud's Institution received grants from Aphtalis Pharma.
VS: Prof. Vincenzo Savarino has received honoraria for speaker in medical congresses and consulting work from; Takeda Italia, Alfa Wassermann, Almirall, MSD, Abbvie, Reckitt Benckiser and Pfizer. All honoraria were received more than three years ago.
GO: none known.
CAF: Dr Fallone has done consulting work in the past three years for Pendopharm, Canada, Takeda, Canada, Janssen, Canada, Forest Laboratories, Canada and Actavis, Canada.
LF: Dr Fischbach was paid by Axcan Pharma to participate in an educational programme on treatment for H. pylori more than five years ago.
FB: Dr Bazzoli has received fees from Allergan for consulting work done in the past three years.
JPG: Dr. Gisbert has served as a speaker, a consultant and advisory member for, or has received research funding from, MSD, Abbvie, Hospira, Kern Pharma, Biogen, Takeda, Janssen, Pfizer, Ferring, Faes Farma, Shire Pharmaceuticals, Dr. Falk Pharma, Chiesi, Casen Fleet, Gebro Pharma, Otsuka Pharmaceutical, Vifor Pharma, Almirall, Nycomed, AstraZeneca, Casen Recordati, Allergan. Dr Gisbert is an editor with the Cochrane Upper GI and Pancreatic Diseases group. Dr Gisbert was not involved in the editorial processing of this review.
New
References
References to studies included in this review
Albrecht 2011 {published data only}
- Albrecht P, Kotowska M, Szajewska H. Sequential therapy compared with standard triple therapy for Helicobacter pylori eradication in children: a double‐blind, randomized, controlled trial. Journal of Pediatrics 2011;159(1):45‐9. [DOI] [PubMed] [Google Scholar]
Ali Habib HS 2013 {published data only}
- Ali Habib HS, Murad HA, Amir EM, Halawa TF. Effect of sequential versus standard Helicobacter pylori eradication therapy on the associated iron deficiency anemia in children. Indian Journal of Pharmacology 2013;45(5):470‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aminian 2010 {published data only}
- Aminian K, Farsad F, Ghanbari A, Fakhreih S, Hasheminasab SM. A randomized trial comparing four Helicobacter pylori eradication regimens: standard triple therapy, ciprofloxacin based triple therapy, quadruple and sequential therapy. Tropical Gastroenterology 2010;31(4):303‐7. [PubMed] [Google Scholar]
Ang 2015 {published data only}
- Ang TL, Fock KM, Ang D. A randomized controlled trial of triple therapy versus sequential therapy versus concomitant therapy as first line treatment for H. Pylori infection. Gastroenterology. Conference: Digestive Disease Week 2013, DDW 2013 Orlando, FL United States 2013;144(5 Suppl 1):S53. [Google Scholar]
- Ang TL, Fock KM, Song M, Ang D, Kwek AB, Ong J, et al. Ten‐day triple therapy versus sequential therapy versus concomitant therapy as first‐line treatment for Helicobacter pylori infection. Journal of Gastroenterology and Hepatology 2015;30(7):1134‐9. [DOI] [PubMed] [Google Scholar]
- Morse AL, Goodman KJ, Munday R, Chang HJ, Morse JW, Keelan M, et al. A randomized controlled trial comparing sequential with triple therapy for Helicobacter pylori in an Aboriginal community in the Canadian north. Canadian Journal of Gastroenterology 2013;27(12):701‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Ang TL, Fock KM. An update: a randomized controlled trial of triple therapy versus sequential therapy versus concomitant therapy as first line treatment for H. Pylori infection in Singapore. Gastroenterology. Digestive Disease Week 2014, DDW 2014 2014;146(5 SUPPL. 1):S104‐5. [Google Scholar]
Bontems 2011 {published data only}
- Bontems P, Kalach N, Oderda G, Salame A, Muyshont L, Miendje D, et al. Sequential therapy vs. tailored triple therapies for Helicobacter pylori infection in children: A prospective, open‐label, multi‐center study. Journal of Pediatric Gastroenterology and Nutrition 2011;53(6):646‐50. [DOI] [PubMed] [Google Scholar]
Choi 2012 {published data only}
- Choi HS, Chun HJ, Park SH, Keum B, Seo YS, Kim YS, et al. Comparison of sequential and 7‐, 10‐, 14‐d triple therapy for Helicobacter pylori infection. World Journal of Gastroenterology 2012;21(18/19):2377‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chung 2012 {published data only}
- Chung JW, Jung YK, Kim YJ, Kwon KA, Kim JH, Lee JJ, et al. Ten‐day sequential versus triple therapy for Helicobacter pylori eradication: a prospective, open‐label, randomized trial. Journal of Gastroenterology and Hepatology 2012;27(11):1675‐80. [DOI] [PubMed] [Google Scholar]
De Francesco 2004a {published data only}
- Francesco V, Zullo A, Margiotta M, Marangi S, Burattini O, Berloco P, et al. Sequential treatment for Helicobacter pylori does not share the risk factors of triple therapy failure. Alimentary Pharmacology & Therapeutics 2004;19(4):407‐14. [DOI] [PubMed] [Google Scholar]
De Francesco 2004b {published data only}
- Francesco V, Zullo A, Hassan C, Della VN, Pietrini L, Minenna MF, et al. The prolongation of triple therapy for Helicobacter pylori does not allow reaching therapeutic outcome of sequential scheme: a prospective, randomised study. Digestive & Liver Disease 2004;36(5):322‐6. [DOI] [PubMed] [Google Scholar]
Eisig 2014 {published data only}
- Eisig JN, Navarro‐Rodriguez T, Teixeira AC, Silva FM, Mattar R, Chinzon D, et al. Standard triple therapy for Helicobacter pylori is still the best first line treatment in Brazil, compared with sequential therapy: a randomized, prospective, double‐blind, placebo‐controlledStudy. Gastroenterology. Conference: Digestive Disease Week (DDW) 2014;146(5 Suppl 1):S‐390. [Google Scholar]
Focareta 2002 {published data only}
- Focareta R, Forte G, Ciarlegio A. Helicobacter pylori eradication: one week triple therapy versus 10‐day sequential regimen introduction. Digestive Liver Disease 2002;34:A17. [Google Scholar]
Focareta 2003 {published data only}
- Focareta R, Forte G, Forte F, Ciarleglio A, Grimaldi E, Ievoli F, et al. Could the 10‐days sequential therapy be considered a first choice treatment for the eradication of Helicobacter pylori infection?. Digestive and Liver Disease 2003;33:C091. [Google Scholar]
Franceschi 2011 {published data only}
- Franceschi F, Campanale M, Finizio R, Barbaro F, Tortora A, Gigante G, et al. High dose amoxicillin‐based first line regimen is equivalent to sequential therapy in the eradication of H. pylori infection. Gastroenterology 2011;140(5):S‐149. [PubMed] [Google Scholar]
Gao 2010 {published data only}
- Gao XZ, Qiao XL, Song WC, Wang XF, Liu F. Standard triple, bismuth pectin quadruple and sequential therapies for Helicobacter pylori eradication. World Journal of Gastroenterology 2010;16(34):4357‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gatta 2011 {published data only}
- Gatta L, Vakil N, Leandro G, Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta‐analysis of randomized controlled trials in adults and children. The American Journal of Gastroenterology 2009;104(12):3069‐79. [DOI] [PubMed] [Google Scholar]
Greenberg 2011 {published data only}
- Ferreccio C, Anderson GL, Morgan DR, Torres J, Bravo LE, Dominguez R, et al. A randomized trial comparing 14‐day triple, 10‐day sequential, and 5‐day concomitant therapy to eradicate Helicobacter pylori in seven Latin American populations. AGA Abstracts 2011;NA:S‐137. [Google Scholar]
- Greenberg ER, Anderson GL, Morgan DR, Torres J, Chey WD, Bravo LE, et al. 14‐day triple, 5‐day concomitant, and 10‐day sequential therapies for Helicobacter pylori infection in seven Latin American sites: a randomised trial. Lancet 2011;378(9790):507‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hsu 2014 {published data only}
- Hsu PI, Wu DC, Chen WC, Tseng HH, Yu HC, Wang HM, et al. Randomized controlled trial comparing 7‐day triple, 10‐day sequential, and 7‐Day concomitant therapies for Helicobacter pylori infection. Antimicrobial Agents and Chemotherapy 2014;58(10):5936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2013 {published data only}
- Huang J, Zhou L, Geng L, Yang M, Xu XW, Ding ZL, et al. Randomised controlled trial: sequential vs. standard triple therapy for Helicobacter pylori infection in Chinese children‐a multicentre, open‐labelled study. Alimentary Pharmacology & Therapeutics 2013;38(10):1230‐5. [DOI] [PubMed] [Google Scholar]
Javid 2013 {published data only}
- Javid G, Zargar SA, Bhat K, Khan BA, Yatoo GN, Gulzar GM, et al. Efficacy and safety of sequential therapy versus standard triple therapy in Helicobacter pylori eradication in Kashmir India: a randomized comparative trial. Indian Journal of Gastroenterology 2013;32(3):190‐4. [DOI] [PubMed] [Google Scholar]
Jeon 2013 {published data only}
- Choi WH, Park DI, Oh SJ, Baek YH, Hong CH, Hong EJ, et al. Effectiveness of 10 day‐sequential therapy for Helicobacter pylori eradication in Korea. Taehan Sohwagi Hakhoe Chi [The Korean Journal of Gastroenterology] 2008;51(5):280‐4. [PubMed] [Google Scholar]
- Jeon WK, Park D. Song C. Effectiveness of 10 day‐sequential treatment for Helicobacter pylori eradication in Korea. Gastroenterology 2013;144(5 Suppl. 1):S567‐8. [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim YS, Kim SJ, Yoon JH, Suk KT, Kim JB, Kim DJ, et al. Randomised clinical trial: the efficacy of a 10‐day sequential therapy vs. a 14‐day standard proton pump inhibitor‐based triple therapy forHelicobacter pylori in Korea. Alimentary Pharmacology & Therapeutics 2011;34:1098‐105. [DOI] [PubMed] [Google Scholar]
Lahbabi 2013 {published data only}
- Lahbabi M, Alaoui S, Rhazi K, Abkari M, Nejjari C, Amarti A, et al. Sequential therapy versus standard triple‐drug therapy for Helicobacter pylori eradication: Result of the HPFEZ randomised study. Clinics and Research in Hepatology and Gastroenterology 2013;37(4):416‐21. [DOI] [PubMed] [Google Scholar]
Laving 2013 {published data only}
- Laving A, Kamenwa R, Sayed S, Kimang’a AN, Revathi G. Effectiveness of sequential v. standard triple therapy for treatment of Helicobacter pylori infection in children in Nairobi, Kenya. South African Medical Journal 2013;103(12):921‐4. [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee JW, Kim N, Kim JM, Nam RH, Kim JY, Lee JY, et al. A comparison between 15‐day sequential, 10‐day sequential and proton pump inhibitor‐based triple therapy for Helicobacter pylori infection in Korea. Scandinavian Journal of Gastroenterology 2014;49(8):917‐24. [DOI] [PubMed] [Google Scholar]
Lee 2015 {published data only}
- Kim J, Kim J, Kim B, Kim H, Bang B, Kim C, et al. Triple therapy, sequential therapy, and concomitant therapy for Helicobacter pylori infection in Korea: A multicenter, randomized controlled trial. Helicobacter. 27th International Workshop on Helicobacter and Microbiota in Chronic Digestive Inflammation and Gastric Cancer Rome Italy. 2014; Vol. 19:80.
- Lee HJ, Kim JI, Lee JS, Jun EJ, Oh JH, Cheung DY, et al. Concomitant therapy achieved the best eradication rate for Helicobacter pylori among various treatment strategies. World Journal of Gastroenterology 2015;21(1):351‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim KJ, Kim JS, Kim BW, Kim CH, Kim HG, Bhang BW, et al. Triple therapy, sequential therapy, and concomitant therapy for Helicobacter pylori infection in Korea: A multicenter, randomized controlled trial. Journal of Gastroenterology and Hepatology. 2014; Vol. 29:230.
Liou 2013 {published data only}
- Liou JM, Chen CC, Chen MJ, Chang CY, Fang YJ, Lee JY, et al. Sequential versus triple therapy for the first‐line treatment of Helicobacter pylori: a multicentre, open‐label, randomised trial. Lancet 2013;381(9862):205‐13. [DOI] [PubMed] [Google Scholar]
Liou 2014 {published data only}
- Liou JM, Chen CC, Chang CY, Wu JY, Fang YJ, Luo JC, et al. Sequential therapy for 10 days versus triple therapy for 14 days in the first‐line treatment of Helicobacter pylori infection— a multicenter, open‐label, randomized trial. Clinical Gastroenterology and Hepatology 2013;12(1):161‐2. [Google Scholar]
Lopez‐Román 2011 {published data only}
- Lopez‐Román O, Warrington E, Cruz‐Correa MR, Toro DH. 10‐day and 14‐day sequential therapy vs. standard triple therapy for Helicobacter pylori infection in a Puerto Rican treatment‐naive population: an interim analysis. Gastroenterology 2011;140(5 Suppl 1):S149. [Google Scholar]
Lu 2010 {published data only}
- Lu JH, Xu MY, Sheng Y, Yang WX. Comparison of the efficacy of 10‐day sequential therapy and conventional triple therapy for Helicobacter pylori eradication in children. Zhongguo Dang Dai Er Ke Za Zhi 2010;12:988–90. [PubMed] [Google Scholar]
Molina‐Infante 2010 {published data only}
- Molina‐Infante J, Perez‐Gallardo B, Fernandez‐Bermejo M, Hernandez‐Alonso M, Vinagre G, Dueñas C, et al. Clinical trial: clarithromycin vs. levofloxacin in first‐line triple and sequential regimens for Helicobacter pylori eradication. Alimentary Pharmacology & Therapeutics 2010;31(10):1077‐84. [DOI] [PubMed] [Google Scholar]
Nasa 2013 {published data only}
- Nasa M, Choksey A, Phadke A, Sawant P. Sequential therapy versus standard triple‐drug therapy for Helicobacter pylori eradication: a randomized study. Indian Journal of Gastroenterology 2013;32(6):392‐6. [DOI] [PubMed] [Google Scholar]
Oh 2012 {published data only}
- Oh HS, Lee DH, Seo JY, Cho YR, Kim N, Jeoung SH, et al. Ten‐day sequential therapy is more effective than proton pump inhibitor‐based therapy in Korea: A prospective, randomized study. Journal of Gastroenterology and Hepatology 2012;27(3):504‐9. [DOI] [PubMed] [Google Scholar]
Paoluzi 2010 {published data only}
- Paoluzi OA, Visconti E, Andrei F, Tosti C, Erboso M, Lionetti RT, et al. Sequential regimens have greater efficacy and better tolerability than standard triple therapy in the eradication of Helicobacter pylori. Digestive Disease Week (AGA abstracts) 2008;M1065:A‐331. [Google Scholar]
- Paoluzi OA, Visconti E, Andrei F, Tosti C, Lionetti R, Grasso E, et al. Ten and eight‐day sequential therapy in comparison to standard triple therapy for eradicating Helicobacter pylori infection: a randomized controlled study on efficacy and tolerability. Journal of Clinical Gastroenterology 2010;44(4):261‐6. [DOI] [PubMed] [Google Scholar]
Park 2012 {published data only}
- Kim S. Park H, Jung M, Huh J, Jeon S. Ten‐day sequential therapy is a promising therapeutic approach for helicobacter pylori infection in naive patients: a randomized multicenter trial. Helicobacter. 25th International Workshop on Helicobacter and Related Bacteria in Chronic Digestive Inflammation and Gastric Cancer: European Helicobacter Study Group Ljubljana Slovenia. 2012; Vol. 17:99‐100.
- Park HG, Jung MK, Jung JT, Kwon JG, Kim EY, Seo HE, et al. Randomised clinical trial: a comparative study of 10‐day sequential therapy with 7‐day standard triple therapy for Helicobacter pylori infection in naïve patients. Alimentary Pharmacology & Therapeutics 2012;35(1):56‐65. [DOI] [PubMed] [Google Scholar]
- Park HG, Jung MK, Jung JT, Kwon JGK, Kim EY, Eun SHE, et al. Ten‐day sequential therapy is a promising therapeutic approach for Helicobacter pylori infection in naïve patients: a randomized multicenter trial. Gut 2011;60 (Suppl 3):A61. [Google Scholar]
Rakici 2014 {published data only}
- Rakici H, Akdoğan RA, Bedir R, Copur A, Yilmaz A. Comparison of standard triple therapy, sequential therapy and moxifloxacin‐based triple therapy for Helicobacterpylori infection: Patients’ compliance and bacterial eradication rates. Journal of Digestive Diseases 2014;15(9):508‐13. [DOI] [PubMed] [Google Scholar]
Scaccianoce 2006 {published data only}
- Scaccianoce G, Hassan C, Panarese A, Piglionica D, Morini S, Zullo A. Helicobacter pylori eradication with either 7‐day or 10‐day triple therapies, and with a 10‐day sequential regimen.. Canadian Journal of Gastroenterology 2006;20(2):113‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Seddik 2013 {published data only}
- Seddik H, Ahid S, Adioui T, Hamdi FZ, Hassar M, Abouqal R, et al. Sequential therapy versus standard triple‐drug therapy for Helicobacter pylori eradication: a prospective randomized study. European Journal of Clinical Pharmacology 2013;69(9):1709‐15. [DOI] [PubMed] [Google Scholar]
Tepes 2012 {published data only}
- Tepes B, Vujasinovic M, Seruga M, Stefanovic M, Jeverica S. Sequential and quadruple therapies for Helicobacter Pylori eradication compared with triple therapy in Slovenia: a multicenter, prospective, randomized, controlled trial. Helicobacter 2012;17:73. [Google Scholar]
Vaira 2007 {published data only}
- Vaira D, Zullo A, Vakil N, Gatta L, Ricci C, Perna F, et al. Sequential therapy versus standard triple‐drug therapy for Helicobacter pylori eradication: a randomized trial. Annals of Internal Medicine 2007;146(8):556. [DOI] [PubMed] [Google Scholar]
Wu 2011 {published data only}
- Wu GL, Lan Y, Zhang XJ. Sequential therapy versus standard triple therapy for Helicobacter pylori eradication. World Chinese Journal of Digestology 2011;19(29):3100‐3. [Google Scholar]
Yan 2011 {published data only}
- Yan X, Zhou L, Song Z, Xue Y, Wang Y, Bai P, et al. Sequential therapy for helicobacter pylori eradication in adults compared with triple therapy in China: a multiple‐ centre, prospective, randomized, controlled trial. Helicobacter 2011;16(Suppl 1):77‐143. Abtract WS145.142. [Google Scholar]
Zhou 2014 {published data only}
- Zhou L, Zhang J, Chen M, Hou X, Li Z, Song Z, et al. A comparative study of sequential therapy and standard triple therapy for Helicobacter pylori infection: a randomized multicenter trial. American Journal of Gastroenterology 2014;109(4):535‐41. [DOI] [PubMed] [Google Scholar]
Zullo 2003 {published data only}
- Zullo A, Hassan C, Lorenzetti R, Winn S, Morini S. A clinical practice viewpoint: to culture or not to culture Helicobacter pylori?. Digestive and Liver Disease 2003;35(5):357‐61. [DOI] [PubMed] [Google Scholar]
Zullo 2005 {published data only}
- Zullo A, Gatta L, Francesco V, Hassan C, Ricci C, Bernabucci V, et al. High rate of Helicobacter pylori eradication with sequential therapy in elderly patients with peptic ulcer: a prospective controlled study. Alimentary Pharmacology & Therapeutics 2005;21(12):1419‐24. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Francavilla 2005 {published data only}
- Francavilla R, Lionetti E, Castellaneta SP, Magista AM, Boscarelli G, Piscitelli D, et al. Improved efficacy of 10‐day sequential treatment for Helicobacter pylori eradication in children: a randomized trial. Gastroenterology 2005;129(5):1414‐9. [DOI] [PubMed] [Google Scholar]
Hu 2009 {published data only}
- Hu SQ, Zhang M. A 10‐day sequential therapy for Helicobacter pylori‐infected patients: an analysis of 39 cases. Shi Jie Hua Ren Xiao Hua Za Zhi [World Chinese Journal of Digestology] 2009;17(16):1693‐5. [Google Scholar]
Huang 2012a {published data only}
- Huang J, Gong ST, Ou WJ, Pan RF, Geng LL, Huang H, et al. A 10‐day sequential therapy for eradication of Helicobacter pylori infection in children. Zhonghua Er Ke za Zhi. [Chinese Journal of Pediatrics] 2012;50(8):563‐7. [PubMed] [Google Scholar]
Huang 2012b {published data only}
- Huang YK, Wu MC, Wang SS, Kuo CH, Lee YC, Chang LL, et al. Lansoprazole‐based sequential and concomitant therapy for the first‐line Helicobacter pylori eradication. Journal of Digestive Diseases 2012;13(4):232‐8. [DOI] [PubMed] [Google Scholar]
Kadayifci 2008 {published data only}
- Kadayifci A, Uygun A, Elcin CN, Kantarcioglu M, Toros AB, Polat Z, et al. Sequential treatment regimens of H. pylori in patients with non‐ulcer dyspepsia. Helicobacter 2008;13:392–479. [Google Scholar]
Kim 2013 {published data only}
- Kim JS, Kim B, Ji J, Lee B, Choi H. Sequential versus triple therapy for the treatment of Helicobacter pylori: A nationwide study. Helicobacter. 26th International Workshop on Helicobacter and Related Bacteria in Chronic Digestive Inflammation and Gastric Cancer of the European Helicobacter Study Group Madrid Spain. 2013; Vol. 18:83‐4.
Nagahara 2001 {published data only}
- Nagahara A, Miwa H, Yamada T, Kurosawa A, Ohkura R, Sato N. Five‐day proton pump inhibitor‐based quadruple therapy regimen is more effective than 7‐day triple therapy regimen for Helicobacter pylori infection. Alimentary Pharmacology & Therapeutics 2001;15:417‐21. [DOI] [PubMed] [Google Scholar]
Neville 1999 {published data only}
- Neville PM, Everett S, Langworthy H, Tompkins D, Mapstone NP, Axon AT, et al. The optimal antibiotic combination in a 5‐day Helicobacter pylori eradication regimen. Alimentary Pharmacology & Therapeutics 1999;13(4):497‐501. [DOI] [PubMed] [Google Scholar]
Ntouli 2013 {published data only}
- Ntouli V, Brakas S, Zeglinas C, Charalampopoulos S, Labrinakos S, Michalopoulos G, et al. Sequential versus classical triple treatment study in a Greek population. Helicobacter 2013;18(Suppl 1):130. [Google Scholar]
Ruiz‐Obaldía 2008 {published data only}
- Ruiz‐Obaldía JR, Torrazza EG, Carreno NO. Helicobacter pylori eradication with either Conventional 10‐day triple therapy or 10‐day modified sequential regimen (preliminary report). Gastroenterology 2008;134(4 Suppl 1):A‐24. [Google Scholar]
Torres 2012 {published data only}
- Torres J, Morgan DR, Greenberg ER, Salazar‐Martinez E, Dominguez R, Ferreccio C, et al. One‐year effectiveness and costs of six alternative H. Pylori test/treat and retest/retreat strategies using triple, concomitant or sequential drug regimens in seven Latin American Sites (SWOG Trial S0701). Gastroenterology. Digestive Diease Week 2012, DDW 2012 San Diego, CA United States 2012;142(5 Suppl 1):S115‐6. [Google Scholar]
Urgesi 2011 {published data only}
- Urgesi R, Pelecca G, Cianci R, Masini A, Zampaletta C, Riccioni ME, et al. Helicobacter pylori infection: is sequential therapy superior to standard triple therapy? A single‐centre Italian study in treatment‐naive and non‐treatment‐naive patients. Canadian Journal of Gastroenterology 2011;25(6):315‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Uygun 2008 {published data only}
- Uygun A, Kadayifci A, Yesilova Z, Safali M, Ilgan S, Karaeren N. Comparison of sequential and standard triple‐drug regimen for Helicobacter pylori eradication: a 14‐day, open‐label, randomized, prospective, parallel‐arm study in adult patients with nonulcer dyspepsia. Clinical Therapeutics 2008;30(3):528‐34. [DOI] [PubMed] [Google Scholar]
Valooran 2011 {published data only}
- Valooran GJ, Kate V, Jagdish S, Basu D. Sequential therapy versus standard triple drug therapy for eradication of Helicobacter pylori in patients with perforated duodenal ulcer following simple closure. Scandinavian Journal of Gastroenterology 2011;46(9):1045‐50. [DOI] [PubMed] [Google Scholar]
Zhao 2009 {published data only}
- Zhao QX, Huang DY. Efficacy of tinidazole‐containing sequential therapy in the eradication of Helicobacter pylori infection. [Chinese] [62]. [World Chinese Journal of Digestology] 2009;17(35):3666‐9. [Google Scholar]
Additional references
Altman 2002
- Altman D, Deeks J. Meta‐analysis, Simpson's paradox, and the number needed to treat. BMC Medical Research Methodology 2002;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Calvet 2000
- Calvet X, Garcia N, Lopez T, Gisbert JP, Gene E, Roque M. A meta‐analysis of short versus long therapy with a proton pump inhibitor, clarithromycin and either metronidazole or amoxycillin for treating Helicobacter pylori infection. Alimentary Pharmacology & Therapeutics 2000;14(5):603‐9. [DOI] [PubMed] [Google Scholar]
Cates 2002
- Cates CJ. Simpson's paradox and calculation of number needed to treat from meta‐analysis. BMC Medical Research Methodology 2002;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2009
- Chen Y, Wu LH, He XX. Sequential therapy versus standard triple therapy for Helicobacter pylori eradication in Chinese patients: A meta‐analysis. [Chinese]. World Chinese Journal of Digestology 2009;17(32):3365‐9. [Google Scholar]
De Francesco 2001
- Francesco V, Zullo A, Hassan C, Faleo D, Ierardi E, Panella C, et al. Two new treatment regimens for Helicobacter pylori eradication: a randomised study. Digestive & Liver Disease 2001;33(8):676‐9. [DOI] [PubMed] [Google Scholar]
De Martel 2006
- Martel C, Parsonnet J. Helicobacter pylori infection and gender: a meta‐analysis of population‐based prevalence surveys. Digestive Diseases and Sciences 2006;51(12):2292‐301. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐8. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Phillips AN. Meta‐analysis: principles and procedures. BMJ 1997;315(7121):1533‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ford 2003
- Ford A, Moayyedi P. How can the current strategies for Helicobacter pylori eradication therapy be improved?. Canadian Journal of Gastroenterology 2003;17 (Suppl B):36B‐40B. [DOI] [PubMed] [Google Scholar]
Forman 2000
- Forman D, Bazzoli F, Bennett C, Broutet N, Calvet‐Calvo X, Chiba N, et al. Therapies for the eradication of Helicobacter pylori. Cochrane Database of Systematic Reviews 2000, Issue 3. [DOI: 10.1002/14651858.CD003840.pub5] [DOI] [Google Scholar]
Fuccio 2007
- Fuccio L, Minardi ME, Zagari RM, Grilli D, Magrini N, Bazzoli F. Meta‐analysis: duration of first‐line proton‐pump inhibitor based triple therapy for Helicobacter pylori eradication. Annals of Internal Medicine 2007;147(8):553‐562. [DOI] [PubMed] [Google Scholar]
Gatta 2009
- Gatta L, Vakil N, Leandro G, Mario F, Vaira D. Sequential therapy or triple therapy for Helicobacter pylori infection: systematic review and meta‐analysis of randomized controlled trials in adults and children. American Journal of Gastroenterology 2009;104(12):3069‐79. [DOI] [PubMed] [Google Scholar]
Gisbert 2007
- Gisbert JP, Pajares R, Pajares JM. Evolution of Helicobacter pylori therapy from a meta‐analytical perspective. Helicobacter 2007;12(Suppl 2):50‐8. [DOI] [PubMed] [Google Scholar]
Gisbert 2010
- Gisbert JP, Calvet X, O´Connor A, Mégraud F, O´Morain CA. Sequential therapy for Helicobacter pylori eradication. A critical review. Journal of Clinical Gastroenterology 2010;44(5):313‐25. [DOI] [PubMed] [Google Scholar]
Gisbert 2011
- Gisbert JP, Calvet X. Review article: the effectiveness of standard triple therapy for Helicobacter pylori has not changed over the last decade, but it is not good enough. Alimentary Pharmacology & Therapeutics 2011;34(11‐12):1255‐68. [DOI] [PubMed] [Google Scholar]
Gisbert 2012
- Gisbert JP, Calvet X. Update on non‐bismuth quadruple (concomitant) therapy for eradication of Helicobacter pylori. Clinical and Experimental Gastroenterology 2012;5:23‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gisbert 2013
- Gisbert JP, Calvet X, Bermejo F, Boixeda D, Bory F, Bujanda L, et al. [III Spanish Consensus Conference on Helicobacter pylori infection]. Gastroenterologia y Hepatologia 2013;36(5):340‐74. [DOI] [PubMed] [Google Scholar]
Graham 2007a
- Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter 2007;12:275‐8. [DOI] [PubMed] [Google Scholar]
Graham 2007b
- Graham DY, Yamaoka Y. Ethical considerations of comparing sequential and traditional anti Helicobacter pylori therapy. Annals of Internal Medicine 2007;147(6):434‐5. [DOI] [PubMed] [Google Scholar]
Graham 2007c
- Graham DY, Lu H, Yamaoka Y. Therapy for Helicobacter pylori infection can be improved: sequential therapy and beyond. Drugs 2008;68(6):725‐36. [DOI] [PubMed] [Google Scholar]
Graham 2012
- Graham DY, Fischbach LA. Letter: the ethics of using inferior regimens in H. pylori randomised trials. Alimentary Pharmacology & Therapeutics 2012;35(7):852‐4. [DOI] [PubMed] [Google Scholar]
Gupta 2011
- Gupta SK. Intention‐to‐treat concept: A review. Perspectives in Clinical Research 2011;2(3):109‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haynes 2006
- Haynes RB, Sackett DL, Guyatt GH, Tugwell P. Clinical Epidemiology: How to Do Clinical Practice Research. 3rd Edition. Philadelphia: Lippincott Williams & Wilkins, 2006. [Google Scholar]
He JD 2013
- He JD, Liu L, Zhu YJ. Ten‐day sequential therapy for Helicobacter pylori eradication in children: a systematic review of randomized controlled trials. Zhonghua Yi Xue Za Zhi 2013;93(44):3500‐5. [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from cochrane.handbook.org.
Hollis 1999
- Hollis S, Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Horvath 2012
- Horvath A, Dziechciarz P, Szajewska H. Meta‐analysis: sequential therapy for Helicobacter pylori eradication in children. Alimentary Pharmacology & Therapeutics 2012;36(6):534‐41. [DOI] [PubMed] [Google Scholar]
Hunt 2004
- Hunt R, Fallone C, Veldhuyzan van Zanten S, Sherman P, Smaill F, Flook N, et al. Canadian Helicobacter Study Group Consensus Conference: update on the management of Helicobacter pylori‐‐an evidence‐based evaluation of six topics relevant to clinical outcomes in patients evaluated for H pylori infection. Canadian Journal of Gastroenterology 2004;18(9):547‐54. [DOI] [PubMed] [Google Scholar]
Jafri 2008
- Hornung CA, Howden CW. Meta‐analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Annals of Internal Medicine 2008;148(12):923‐31. [DOI] [PubMed] [Google Scholar]
Janssen 2001
- Janssen MJ, Oijen AH, Verbeek AL, Jansen JB, Boer WA. A systematic comparison of triple therapies for treatment of Helicobacter pylori infection with proton pump inhibitor/ranitidine bismuth citrate plus clarithromycin and either amoxicillin or a nitroimidazole. Alimentary Pharmacology & Therapeutics 2001;15:613‐24. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐98. [DOI] [PubMed] [Google Scholar]
Laheij 1999
- Laheij RJ, Rossum LG, Jansen JB, Straatman H, Verbeek AL. Evaluation of treatment regimens to cure Helicobacter pylori infection ‐ a meta‐analysis. Alimentary Pharmacology & Therapeutics 1999;13:857‐64. [DOI] [PubMed] [Google Scholar]
Lai 2004
- Lai YC, Yang JC, Huang SH. Pre‐treatment urea breath test results predict the efficacy of Helicobacter pylori eradication therapy in patients with active duodenal ulcers. World Journal of Gastroenterology 2004;10(7):991‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Laine 2000
- Laine L, Fennerty MB, Osato M, Sugg MSJ, Suchower L, Probst P, et al. Esomeprazole‐based Helicobacter pylori eradication therapy and the effect of antibiotic resistance: results of three US multicenter, double‐blind trials. American Journal of Gastroenterology 2000;95:3393‐8. [DOI] [PubMed] [Google Scholar]
Lim 2013
- Lim JH, Lee DH, Choi C, Lee ST, Kim N, Jeong SH, et al. Clinical outcomes of two‐week sequential and concomitant therapies for Helicobacter pylori eradication: a randomized pilot study. Helicobacter 2013;18(3):180‐6. [DOI] [PubMed] [Google Scholar]
Malfertheiner 1997
- Malfertheiner P. Current European concepts in the management of Helicobacter pylori infection. The Maastricht consensus report. Gut 1997;41(1):8‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malfertheiner 2002
- Malfertheiner P, Megraud F, O'Morain C, Hungin AP, Jones R, Axon A, et al. Current concepts in the management of Helicobacter pylori infection‐‐the Maastricht 2‐2000 Consensus Report. Alimentary Pharmacology & Therapeutics 2002;16(2):167‐80. [DOI] [PubMed] [Google Scholar]
Malfertheiner 2007
- Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El‐Omar E, Graham D, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut 2007;56(6):772‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malfertheiner 2012
- Malfertheiner P, Megraud F, O'Morain CA, Atherton J, Axon AT, Bazzoli F, et al. Management of Helicobacter pylori infection‐‐the Maastricht IV/ Florence Consensus Report. Gut 2012;61(5):646‐64. [DOI] [PubMed] [Google Scholar]
McLoughlin 2005
- McLoughlin RM, O'Morain CA, O'Connor HJ. Eradication of Helicobacter pylori: recent advances in treatment. Fundamental and Clinical Pharmacology 2005;19(4):421‐7. [DOI] [PubMed] [Google Scholar]
McNicholl 2012
- McNicholl AG, Linares PM, Nyssen OP, Calvet X, Gisbert JP. Meta‐analysis: esomeprazole or rabeprazole vs. first‐generation pump inhibitors in the treatment of Helicobacter pylori infection. Alimentary Pharmacology & Therapeutics 2012;36(5):414‐25. [DOI] [PubMed] [Google Scholar]
McNicholl 2014
- AG McNicholl, OP Nyssen, JP Gisbert. Sequential and concomitant treatments in H. pylori eradication: a network meta‐analysis. United European Gastroenterology Journal 2014;2(1S):A64. [Google Scholar]
Moore 2002
- Moore RA, Gavaghan DJ, Edwards JE, Wiffen P, McQuay HJ. Pooling data for number needed to treat: no problems for apples. BMC Medical Research Methodology 2002;2:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moshkowitz 1995
- Moshkowitz M, Konikoff FM, Peled Y, Santo M, Hallak A, Bujanover Y, et al. High Helicobacter pylori numbers are associated with low eradication rate after triple therapy. Gut 1995;36(6):845‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moyer 2005
- Moyer A, Finney JW. Rating methodological quality: toward improved assessment and investigation. Accountability in Research 2005;12(4):299‐313. [DOI] [PubMed] [Google Scholar]
Murakami 2002
- Murakami K, Fujioka T, Okimoto T, Sato R, Kodama M, Nasu M. Drug combinations with amoxycillin reduce selection of clarithromycin resistance during Helicobacter pylori eradication therapy. International Journal of Antimicrobial Agents 2002;19(1):67‐70. [DOI] [PubMed] [Google Scholar]
Mégraud 2004
- Mégraud F. H pylori antibiotic resistance: Prevalence, importance, and advances in testing. Gut 2004;53(9):1374‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mégraud 2007a
- Mégraud F. Helicobacter pylori and antibiotic resistance. Gut 2007;56(11):1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mégraud 2007b
- Mégraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clinical Microbiology Reviews 2007;20(2):280‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mégraud 2013
- Mégraud F, Coenen S, Versporten A, Kist M, Lopez‐Brea M, Hirschl AM, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013;62(1):34‐42. [DOI] [PubMed] [Google Scholar]
Park 2009
- Park S, Chun HJ, Kim ES, Park SC, Jung ES, Lee SD, et al. The 10‐day sequential therapy for Helicobacter pylori eradication in Korea: less effective than expected. Gastroenterology 2009;136:M1053. [Google Scholar]
Perri 1998
- Perri F, Clemente R, Festa V, Quitadamo M, Conoscitore P, Niro G, et al. Relationship between the results of pre‐treatment urea breath test and efficacy of eradication of Helicobacter pylori infection. Italian Journal of Gastroenterology and Hepatology 1998;30(2):146‐50. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, 2014.
Vakil 2004
- Vakil N, Lanza F, Schwartz H, Barth J. Seven‐day therapy for Helicobacter pylori in the United States. Alimentary Pharmacology & Therapeutics 2004;20(1):99‐107. [DOI] [PubMed] [Google Scholar]
Vilaichone 2006
- Vilaichone RK, Mahachai V, Graham DY. Helicobacter pylori diagnosis and management. Gastroenterology Clinics of North America 2006;35(2):229‐47. [DOI] [PubMed] [Google Scholar]
Wong 2000
- Wong BCY, Chang FY, Abid S, Abbas Z, Lin BR, Van RC, et al. Triple therapy with clarithromycin, omeprazole, and amoxicillin for eradication of Helicobacter pylori in duodenal ulcer patients in Asia and Africa. Alimentary Pharmacology & Therapeutics 2000;14(11):1529‐35. [DOI] [PubMed] [Google Scholar]
Zullo 2000
- Zullo A, Rinaldi V, Winn S, Meddi P, Lionetti R, Hassan C, et al. A new highly effective short‐term therapy schedule for Helicobacter pylori eradication. Alimentary Pharmacology & Therapeutics 2000;14(6):715‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Moayyedi 2007
- Moayyedi P. Sequential regimes for Helicobacter pylori eradication. Lancet 2007;370(9592):1010‐2. [DOI] [PubMed] [Google Scholar]
Nyssen 2011
- Nyssen OP, McNicholl AG, Megraud F, Savarino V, Oderda G, Fallone C, et al. Sequential versus standard triple therapy for Helicobacter pylori eradication. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD009034] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tong 2009
- Tong JL, Ran ZH, Shen J, Xiao SD. Sequential therapy vs. standard triple therapies for Helicobacter pylori infection: a meta‐analysis. Journal of Clinical Pharmacy and Therapeutics 2009;34:41‐53. [DOI] [PubMed] [Google Scholar]
Zullo 2007
- Zullo A, Francesco V, Hassan C, Morini S, Vaira D. The sequential therapy regiment for Helicobacter pylori eradication: a pooled data analysis. Gut 2007;56(10):1353‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]


