Abstract
Background
Postpartum anaemia is associated with breathlessness, tiredness, palpitations and maternal infections. Blood transfusions or iron supplementation have been used in the treatment of iron deficiency anaemia. Recently other anaemia treatments, in particular erythropoietin therapy, have also been used.
Objectives
To assess the clinical effects of treatments for postpartum anaemia, including oral, intravenous or subcutaneous iron/folate supplementation and erythropoietin administration, and blood transfusion.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (30 May 2004), the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 1, 2003), MEDLINE (1966 to March 2003), EMBASE (1980 to March 2003), Current Contents and ACP Journal Club (from inception to March 2003). We updated this search on 7 June 2012 and added the results to the awaiting classification section.
Selection criteria
Randomised controlled trials (RCTs) comparing therapy for postpartum iron deficiency anaemia (oral, intravenous or subcutaneous administration of iron, folate, erythropoietin or blood transfusion) with placebo, another treatment or no treatment.
Data collection and analysis
Two reviewers independently assessed trial quality and extracted data.
Main results
Six included RCTs involving 411 women described treatment with erythropoietin or iron as their primary interventions. No RCTs were identified that assessed treatment with blood transfusion. Few outcomes relating to clinical maternal and neonatal factors were reported: studies focused largely on surrogate outcomes such as haematological indices. Overall, the methodological quality of the included RCTs was reasonable; however, their usefulness in this review is restricted by the interventions and outcomes reported.
When compared with iron therapy only, erythropoietin increased the likelihood of lactation at discharge from hospital (1 RCT, n = 40; relative risk (RR) 1.90, 95% confidence interval (CI) 1.21 to 2.98). No apparent effect on need for blood transfusions was found, when erythropoietin plus iron was compared to treatment with iron only (2 RCTs, n = 100; RR 0.20, 95% CI 0.01 to 3.92), although the RCTs may have been of insufficient size to rule out important clinical differences. Haematological indices (haemoglobin and haemocrit) showed some increases when erythropoietin was compared to iron only, iron and folate, but not when compared with placebo.
Authors' conclusions
There is some limited evidence of favourable outcomes for treatment of postpartum anaemia with erythropoietin. However, most of the available literature focuses on laboratory haematological indices, rather than clinical outcomes. Further high‐quality trials assessing the treatment of postpartum anaemia with iron supplementation and blood transfusions are required. Future trials may also examine the significance of the severity of anaemia in relation to treatment, and an iron‐rich diet as an intervention.
[Note: The 27 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Keywords: Female; Humans; Anemia, Iron‐Deficiency; Anemia, Iron‐Deficiency/blood; Anemia, Iron‐Deficiency/drug therapy; Erythropoietin; Erythropoietin/therapeutic use; Iron; Iron/therapeutic use; Puerperal Disorders; Puerperal Disorders/blood; Puerperal Disorders/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Treatment for women with postpartum iron deficiency anaemia
Erythropoietin, a hormone, may help to treat women who develop anaemia after giving birth, but there may be rare adverse events.
Women with anaemia after childbirth may feel tired and breathless and are at risk of infection. Traditional treatments include iron supplementation and blood transfusion for severe anaemia. A hormone, erythropoietin, may help improve iron levels in the blood and the woman's ability to lactate. However, rare adverse events (damage to red blood cells) have been reported. No studies examined the effects of oral iron supplementation alone, the most common treatment for this type of anaemia, or blood transfusions as treatments for women with anaemia after childbirth. More research, particularly of simple interventions such as oral iron supplementation, is required.
Background
Anaemia after the birth of a baby (postpartum anaemia) is a common problem throughout the world and for most women is self limiting, resolving within a week (Atkinson 1994). For some women however, particularly in resource‐poor countries, it is a major cause of maternal morbidity (poor health) and mortality (Ekanem 1996; Harrison 1989; Kumar 1989; Rosenfield 1989). In this setting, anaemia may result from inadequate dietary intake, parasitic infection or malaria, and may be exacerbated by the physiological effects of pregnancy and blood loss at the time of birth (WHO 1999). Worldwide, anaemia contributed to approximately 20% of the 515,000 maternal deaths in 1995 (WHO 1999). Anaemia is often associated with other markers on blood testing of low iron stores in the body. During pregnancy most women show a fall in haemoglobin concentration as part of a normal response to pregnancy, where there is an increase in plasma and the circulating blood volume, which protects the woman from the blood loss associated with birth. The generally accepted threshold for anaemia in nonpregnant women is a haemoglobin concentration of less than 12 g/dL (WHO 2001). However, it should be noted that this is a value statistically derived from deviations from the population mean, and does not necessarily mean that the woman will have clinical symptoms associated with anaemia (WHO 2001).
Anaemia in the postpartum period may be associated with an increased prevalence of breathlessness, tiredness, palpitations and maternal infections, particularly of the urinary tract (Gibbs 1980; Vora 1998). Such symptoms may cause women to experience difficulty caring for their baby, and may influence the emotional bond the mother has with her baby (Gilbert 1987). Blood transfusions have been used in the treatment of postpartum anaemia, but there are risks associated with its use. These include reactions secondary to contamination (most commonly with leukocytes or red blood cells), infections (particularly with hepatitis, Human Immunodeficiency Virus (HIV) and cytomegalovirus), fluid overload, allergic reactions, lung injury and air embolism (Klapholz 1990; Naef 1995a; Nolan 1991; Skolnick 1992; Waymack 1990). Immunological reactions may be 'minor' and include fever, chills, urticaria (skin rash and/or hives), or more severe, including acute haemolysis (breakdown of red blood cells) arising from administration of incompatible blood (Naef 1995b). Hepatitis C infection is estimated to occur in approximately 0.1% of all patients who receive blood. The cost of blood transfusions includes extensive costs of screening the blood for infection, storage and sterile administration of blood products, all of which may generate increased financial burden, particularly in under‐resourced countries (Ekanem 1996).
Given the risks of blood transfusion and financial constraints, attention has been directed towards other forms of treatment of anaemia such as iron supplements and erythropoietin therapy, both oral (by mouth) and parenteral (by intravenous, intramuscular or subcutaneous injection). Erythropoietin is a hormone that is produced by the body and acts to stimulate red blood cell production.
Oral iron therapy has been used for centuries as a treatment of iron deficiency anaemia (Dudrick 1986), and has been used to treat iron deficiency anaemia during pregnancy (Mahomed 2003). The use of oral iron therapy is associated with some side‐effects, including constipation, nausea and gastric irritation. When given by injection, iron has been associated with pain and redness (erythema) at the injection site, and rarely anaphylactic reaction, characterised by itching, redness and in severe cases angioedema (swelling), vascular collapse, bronchospasm (constriction of the airways) and shock. Erythropoietin (EPO) therapy is a relatively recently identified alternative to blood transfusions for the treatment of iron deficiency anaemia, and has been used extensively in the treatment of anaemia associated with renal (kidney) disease. There are a few case reports of EPO therapy in people who have refused blood transfusions on religious grounds, with positive outcomes (Davis 1990), thus highlighting the potential use of EPO in the treatment of other forms of iron deficiency anaemia. Adverse effects of EPO treatment include mild flu‐like symptoms such as sore throat, cough, fever, muscle pains and weakness, headache and fatigue. Uncommon, but more serious adverse effects include hypertension (high blood pressure) and seizures and, more recently, pure red‐cell aplasia (Casadevall 2002).
Objectives
To evaluate the effects of treatments for postpartum anaemia, including oral, intramuscular, intravenous or subcutaneous iron/folate supplementation and erythropoietin administration, and blood transfusion.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomised controlled trials with reported data which compared outcomes for women who were administered therapy for postpartum iron deficiency anaemia with outcomes in women who were given a placebo, another treatment or no treatment.
Types of participants
Women with a haemoglobin value of less than 12 g/dl (WHO 2001) up to six weeks after birth.
Types of interventions
The administration of therapy (iron, folate, erythropoietin or blood transfusion) by oral, intravenous, intramuscular or subcutaneous routes for postpartum iron deficiency anaemia when compared with placebo, another treatment or no treatment, started in the first six weeks after birth.
Types of outcome measures
Maternal outcomes
(1) Use of blood transfusion(s) ‐ when the treatment has been with iron, folate or erythropoietin. (2) Fatigue (as reported by the women ‐ verbalisation of fatigue or lack of energy and inability to maintain usual routines; and as defined by trial authors). (3) Tolerance for physical load (as defined by the trial authors). (4) Dyspnoea (as reported by the women ‐ distressful sensation of uncomfortable breathing; and as defined by the trial authors). (5) Tachypnoea (increase in respiratory rate as defined by trial authors). (6) Tachycardia (heart rate greater than 100 beats per minute or as defined by trial authors). (7) Palpitations (as reported by women ‐ pounding or racing of the heart; and as defined by trial authors). (8) Orthostatic dizziness (as reported by the women ‐ a sensation on standing of faintness and whirling or inability to maintain balance in a standing or seated position; and as defined by trial authors). (9) Syncope (transient loss of consciousness and postural tone caused by reduced cerebral blood flow, and often preceded by a sensation of light headedness, as defined by trial authors). (10) Headache (as defined by trial authors). (11) Not breastfeeding: (a) at hospital discharge; (b) six weeks postpartum; (c) six months postpartum. (12) Infection up to six weeks postpartum: (a) urinary tract infection requiring treatment; (b) endometritis requiring treatment. (13) Psychological wellbeing (measured by the 'Blues Questionnaire', 'Self‐report symptom inventory 90 [SCL‐90‐R]' or similar questionnaire).
Use of health resources
(1) Length of postnatal hospital stay. (2) Readmission to hospital after primary hospital discharge. (3) Costs of treatment: (a) for the woman; (b) for the health service.
Maternal satisfaction with care
Woman satisfied with care.
Adverse effects of treatment
(1) Thromboembolic complications: (a) deep venous thrombosis (blood clots in the veins of the leg) requiring treatment; (b) pulmonary embolism (blood clots in the lung). (2) Anaphylactic reactions (characterised by itching, angioedema (swelling) and in severe cases, vascular collapse, bronchospasm (constriction of the airways) and shock). (3) Gastrointestinal symptoms when the treatment is iron supplementation (diarrhoea, constipation, nausea, heartburn and upper abdominal discomfort). (4) When the intervention has been treatment with erythropoietin: mild flu‐like symptoms (sore throat, cough, fever, muscular pains and weakness, chills, respiratory symptoms, headache, and fatigue), hypertension (blood pressure persistently exceeding 140/90 mm Hg), hypertensive encephalopathy (headache, convulsions and coma), seizures (focal or generalised), hyperkalaemia (plasma concentration > 4.5 mmol/L or serum concentration > 4.9 mmol/L) and hyperphosphataemia (> 1.50 mmol/L). (5) Viral infection ‐ as shown by positive serology on blood testing.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (30 May 2004). We updated this search on 7 June 2012 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched the Cochrane Controlled Trials Register (March 2003), MEDLINE (1966 to March 2003), EMBASE (1980 to March 2003), Current Contents and ACP journal club (1991 to March 2003) using the MeSH headings 'anemia', 'postpartum', 'puerperium', 'treatment', 'treatment ‐ outcome', 'therapy', 'drug ‐ therapy', 'transfusion'.
Searching other resources
We also searched the citation lists of relevant publications, review articles and included studies.
We did not apply any language restrictions.
Data collection and analysis
Two reviewers (Jodie Dodd and Marianna Dare) performed the search and selection of trials for inclusion in the review, with discrepancies resolved by discussion. Excluded studies are detailed in the 'Characteristics of excluded studies' table. Included studies were assessed for quality and methodological details without consideration of the results. Data were extracted separately by two of the three authors and double entered. Discrepancies were resolved by discussion. There was no blinding of authorship.
For all included randomised trials, we assigned quality scores for concealment of allocation to each trial, as described in section VI of the Cochrane Reviewers' Handbook (Clarke 2003): A = adequate; B = unclear; C = inadequate; D = not used.
Completeness of follow up was assessed for each included study as follows: A = less than 3% of participants excluded; B = 3% to 9.9% of participants excluded; C = 10% to 19.9% of participants excluded; D = 20% or more of the participants excluded; E = unclear.
For blinding of assessment of outcome: A = neither the investigator (outcome assessor) nor participant knew or were likely to guess the allocated allotment; B = either the investigator (outcome assessor) or participant knew the allocation. Or neither knew, but the outcome means that it is likely a significant portion of the participant's allocation could be easily identified; C = no blinding ‐ investigator (outcome assessor) and participant knew (or were likely to guess) the allocated treatment; D = unclear.
Descriptive data included the authors, year of publication, setting, country, time span of trial, pretrial calculation of sample size and the number of participants randomised and analysed.
For dichotomous data, results for each study were expressed as relative risks with 95% confidence intervals and combined for meta‐analysis with the RevMan Manager software (RevMan 2004). For continuous data we expressed results for each study as weighted mean differences with 95% confidence intervals. We used a random effects model.
We investigated heterogeneity in the data using the I2 statistic and cautiously explored it using sensitivity analyses (I2 > 50% was regarded as statistically significant heterogeneity).
Planned subgroup analyses were:
dose administered;
frequency of administration;
duration of treatment;
study setting ‐ resource‐poor versus resource‐rich countries;
severity of anaemia at trial entry;
concurrent disease at trial entry (including HIV, sickle cell disease, thalassaemia).
We were unable to perform most of the planned subgroup analyses due to the smallnumber of included trials. We explored results associated with route of treatment administration and duration of treatment for some outcomes when iron was compared with erythropoietin (see 'Graphs and tables'). Timing of outcome measurement varied across trials. To facilitate analyses, we grouped the results into two measurement periods, reflecting short‐ and longer‐term effects: within the first two weeks after treatment, and between two weeks and six weeks after treatment.
Results
Description of studies
Thirteen studies describing treatment of postpartum anaemia were identified, with seven not meeting the selection criteria. (Twenty‐seven reports from an updated search in June 2012 have been added to Studies awaiting classification.) We excluded two trials because of inadequate randomisation (Danko 1990; Huch 1992), one because it did not report on interventions defined as appropriate for inclusion in the review (Osmond 1953), two reported results on women who were not anaemic (Mara 2001; Picha 1975), one combined pregnant and postpartum women with anaemia in the study population (Casparis 1996) and one summarised the results of trials that were reported in included studies (Zimmermann 1995).
The six included randomised controlled trials (RCTs) involved 411 women (Breymann 1996; Breymann 2000; Lebrecht 1995; Makrydimas 1998; Meyer 1995; Zimmermann 1994). Some of the RCTs reported on more than one intervention. Four RCTs compared treatment with erythropoietin and iron to treatment with iron only (Breymann 1996; Breymann 2000; Makrydimas 1998; Lebrecht 1995), one RCT compared treatment with erythropoietin to treatment with placebo (Meyer 1995), two RCTs compared different routes of erythropoietin administration (intravenous versus subcutaneous) (Breymann 1996; Zimmermann 1994), and one RCT compared erythropoietin given as one dose with that given as two doses (Zimmermann 1994). No RCTs were identified that described results of folate therapy or blood transfusion as an intervention.
In Breymann 1996, 300 units/kg of erythropoietin (EPO) were administered to women in the EPO group once, and in Breymann 2000, this dose was administered daily for four days. In Lebrecht 1995, 20,000 units of EPO were given to women as a single dose. In Makrydimas 1998, 200 units/kg of EPO were administered to women in the EPO group daily for 15 days. Meyer 1995 administered 10,000 units of EPO twice. In Zimmermann 1994, two doses of 150 units/kg were compared with a single dose of 300 units/kg of EPO.
All RCTs focused on haematological indices in their results, with limited information regarding clinical outcomes. Of the clinical outcomes described, use of blood transfusions was described by two RCTs (Breymann 2000; Makrydimas 1998), not lactating by one RCT (Makrydimas 1998), thromboembolic complications by two RCTs (Lebrecht 1995; Makrydimas 1998), serious reactions by two RCTs (Breymann 1996; Breymann 2000), and side‐effects for erythropoietin by two RCTs (Lebrecht 1995; Zimmermann 1994).
Details of each RCT are given in the 'Characteristics of included studies' table.
Risk of bias in included studies
Three trials reported using sealed envelopes to allocate women to treatment groups (Breymann 1996; Breymann 2000; Zimmermann 1994). The remaining three studies, though stated to be randomised studies, were unclear in their method of concealment of allocation. All six trials were coded B for allocation concealment.
Blinding to intervention for either investigators or women did not appear to have occurred in any of the studies. Insufficient information was provided to determine whether blinding of outcome assessors had occurred in any of the studies.
No losses to follow up were reported, except for Meyer 1995 who described a drop‐out rate of over 20% (with no differences in dropout rate between the intervention and control groups).
Effects of interventions
Included studies described treatment with erythropoietin or iron as their primary interventions. Women in both the intervention and control groups received iron supplementation in all studies except one (Meyer 1995). No studies were identified that described treatment with blood transfusion, and for each intervention there were only one or two studies that reported the same outcomes and thus had results that could be combined.
Few of the prespecified outcomes were reported by the studies regarding 'maternal outcomes', 'use of health resources', 'maternal satisfaction with care', and 'side‐effects of treatment'.
The remainder of the results reported in the studies were blood indices. While these were not prespecified in the protocol as outcome measures that were of relevance to the population, we decided that to omit haematological outcomes altogether when reporting the results of the review would mean that much of the data reported by the studies would be lost. Thus we have presented the outcomes for the haematological studies as subsidiary outcomes reported by the reviewers in each section. The results are presented in dot point form to allow easier interpretation. The small number of women in the groups studied meant the confidence intervals were wide. Larger groups may have provided statistically significant results. Further details are provided in 'Graphs and tables'.
(1) Any treatment versus placebo
Intravenous (i.v.) erythropoietin (EPO) versus i.v. placebo
Maternal outcomes (see Other data tables)
For the Blues Questionnaire (used to assess postpartum depression), statistically significant increases were seen for the items 'able to concentrate', ' elated', 'happy', 'confident' and 'calm' when EPO was compared with placebo (Meyer 1995).
Use of health resources
No data were provided for these outcomes.
Maternal satisfaction with care
No data were provided for these outcomes.
Adverse effects
No data were provided for these outcomes.
Subsidiary outcomes ‐ (as reported by the authors, not prespecified by reviewers)
No significant difference was observed in haemoglobin (weighted mean difference (WMD) 0.40 g/dL 95% confidence interval (CI) ‐0.26 to 1.06) or haematocrit (WMD 1.60% 95% CI ‐0.42 to 3.62) within two weeks after treatment (Meyer 1995; 71 women).
(2) EPO versus any other treatment
EPO plus iron versus iron alone
Maternal outcomes
The relative risk (RR) for needing blood transfusion for EPO plus iron compared with iron only was 0.20; 95% CI 0.01 to 3.92 (100 women in two trials (Breymann 2000; Makrydimas 1998)).
Women treated with EPO plus iron were more likely to be lactating when discharged from hospital than women treated with iron only (RR 1.90; 95% CI 1.21 to 2.98). This was reported by one trial describing 40 women (Makrydimas 1998).
Use of health resources
No data were provided for these outcomes.
Maternal satisfaction with care
No data were provided for these outcomes.
Adverse effects
No adverse effects of treatment were seen in either the EPO + iron or iron alone groups (no thromboembolic complications reported in two trials (Breymann 2000; Makrydimas 1998; 96 women) and no anaphylactic reactions reported in three trials (Breymann 1996; Breymann 2000; Lebrecht 1995; 186 women).
Subsidiary outcomes (as reported by the authors ‐ not prespecified by reviewers)
Haemoglobin within two weeks after treatment
No difference was seen in haemoglobin within two weeks after treatment when EPO i.v. + iron was compared with oral or i.v. iron only (WMD 0.45 g/dL 95% CI ‐0.16 to 1.06; Breymann 1996; 60 women) or with oral and i.v. iron only (WMD 0.40 g/dL 95% CI ‐0.22 to 1.02; Lebrecht 1995; 36 women); or with i.v. iron (WMD 0.20% increase 95% CI ‐0.27% to 0.67%; Breymann 2000; 40 women).
When i.v. EPO + iron was compared with oral iron only, however, there was an increase in haemoglobin (WMD 0.70% increase 95% CI 0.23 to 1.17; Breymann 2000; 40 women).
When subcutaneous (s.c.) EPO + iron was compared with oral or i.v. iron only, there was a decrease in haemoglobin (‐0.55 g/dL 95% CI ‐0.99 to ‐0.11; Breymann 1996; 60 women).
Haemoglobin >2 weeks to 6 weeks after treatment
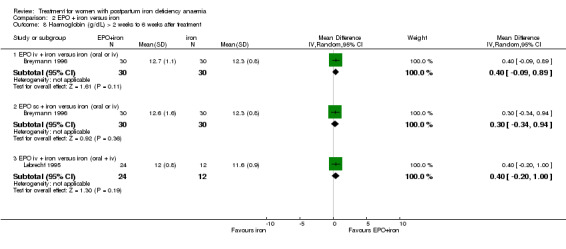
No differences were seen for EPO + iron versus iron only regardless of route ‐ i.v. EPO + iron compared with oral or i.v. iron only (WMD 0.40 g/dL 95% CI ‐0.09 to 0.89; Breymann 1996; 60 women); s.c. EPO + iron compared with oral or i.v. iron only (WMD 0.30 g/dL 95% CI ‐0.34 to 0.94; Breymann 1996; 60 women); or i.v. EPO + iron compared with oral and i.v. iron (WMD 0.40 g/dL 95% CI ‐0.20 to 1.00).
Haematocrit (%) within 2 weeks after treatment
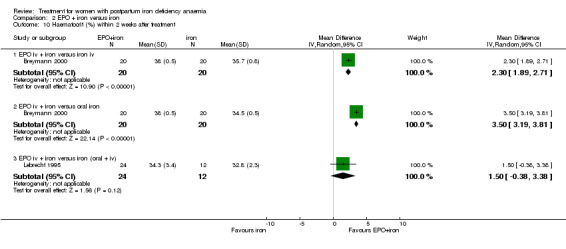
Haematocrit values were greater with EPO + iron compared with iron (either i.v. or oral) in Breymann 2000: WMD 2.30% 95% CI 1.89 to 2.71 compared with i.v. iron (40 women) and WMD 3.50% 95% CI 3.19 to 3.81 compared with oral iron (40 women).
In contrast, no significant difference was seen when EPO + iron was compared with oral and i.v. iron (WMD 1.50% 95% CI ‐0.38 to 3.38; Lebrecht 1995; 36 women).
Haematocrit (%) between two and six weeks after treatment
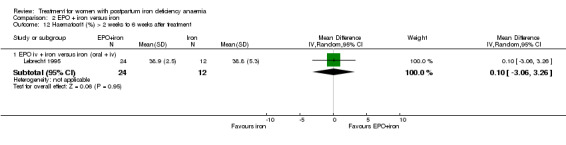
No significant difference was seen when EPO + iron was compared with oral and i.v. iron (WMD 0.10% 95% CI ‐3.06 to 3.26; Lebrecht 1995; 36 women)
(3) Comparing routes of EPO administration ‐ subcutaneous versus intravenous
Maternal outcomes
No data were available for these outcomes.
Use of health resources
No data were available for these outcomes.
Maternal satisfaction
No data were available for these outcomes.
Adverse effects
No adverse effects were reported when comparing different routes of erythropoietin administration in Zimmermann 1994 (95 women).
Subsidiary outcomes (as reported by authors, not an outcome prespecified by reviewers)
Haemoglobin (g/dL) within two weeks after treatment:
No differences were seen between i.v. and s.c. routes within two weeks after treatment (WMD ‐0.34 g/dL 95% CI ‐0.94 to 0.26; Breymann 1996; Zimmermann 1994; total of 145 women). However statistically significant heterogeneity was noted in the i.v. versus s.c. comparison when EPO was given as one dose (I2 = 70.7%). This may have been due to all women in Breymann 1996 receiving iron i.v. whereas in Zimmermann 1994, women received only oral iron supplementation.
Haemoglobin (g/dL) between two weeks to six weeks after treatment:
Similarly no differences were seen later (WMD 0.02 g/dL 95% CI ‐0.27 to 0.32; Breymann 1996; Zimmermann 1994; total of 145 women).
Haematocrit (%) within two weeks after treatment:
No difference was seen in the haematocrit value (WMD ‐0.48% 95% CI ‐1.83 to 0.86; Zimmermann 1994; 95 women).
Haematocrit (%) between two weeks to six weeks after treatment:
Similarly no differences were seen later (WMD ‐0.72% 95% CI ‐1.66 to 0.21; Zimmermann 1994; 95 women).
Discussion
The methodological quality of the included studies is generally reasonable; however their usefulness in this review is restricted by the interventions and outcomes reported. There is very limited information relating to clinical outcomes in the included studies, despite extensive reporting of haematological indices.
Laboratory haemoglobin values may not directly reflect the woman's clinical state. It is unclear whether the women involved in the original studies were clinically symptomatic, and treatment of a woman's symptoms would seem more appropriate than treatment determined by an arbitrary level of haemoglobin or values from other haematological indices. A haemoglobin of less than 12 g/dL is a very conservative marker for iron deficiency anaemia, and many women may not experience symptoms (and therefore will not require treatment) at this level. Most studies in the review reported results for women with an haemoglobin of 10 g/dL or less, but did not categorise the severity of anaemia for analysis. Future studies would benefit from further assessment of the results of treatment according to the severity of anaemia.
The availability of blood transfusion and issues related to safety and sterility mean that it has a limited role in treatment for women in low‐ and middle‐income countries. Similarly, erythropoietin is expensive and therefore not a realistic treatment option for those women at greatest risk of postpartum anaemia. Pure red cell aplasia has been reported as a rare adverse effect of erythropoietin, which is thought to be a consequence of antibody formation (Casadevall 2002). One of the companies marketing erythropoietin (as EPREX) has recommended that it should be given intravenously where possible, since this may reduce the risk of antibody formation (AADRB 2002).
As women in resource‐poor countries are more likely to suffer morbidity and/or mortality secondary to postpartum anaemia, future trials should address the role of simple and cost‐effective strategies to improve clinical outcomes for these women, in particular the role of oral iron therapy.
Authors' conclusions
Implications for practice.
There is limited evidence for favourable outcomes for treatment of postpartum anaemia with erythropoietin, with small studies suggesting improved lactation, without an increase in side‐effects. Haematological indices also appear to be favourably affected by treatment with erythropoietin, although how this relates to clinical practice is uncertain.
Information regarding the route of administration of erythropoietin is poor, as is information relating to outcomes following treatment with iron and blood transfusions. The possibility of rare adverse effects (such as red cell aplasia) needs to be monitored.
Implications for research.
Further high quality trials are required that assess the treatment of postpartum anaemia with oral and parental iron and blood transfusions and focus on clinically relevant outcomes such as maternal outcomes, safety and use of health resources. The effectiveness of common interventions such as iron‐rich diet should also be assessed.
[Note: The 27 citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
What's new
| Date | Event | Description |
|---|---|---|
| 9 September 2015 | Amended | Text has been added to Published notes to explain that this review will not be updated and has been superseded by Markova 2015. |
History
Protocol first published: Issue 2, 2003 Review first published: Issue 4, 2004
| Date | Event | Description |
|---|---|---|
| 7 June 2012 | Amended | Search updated. Twenty‐seven reports added to Studies awaiting classification (Backe 2009; Beard 2005; Bhandal 2004; Bhandal 2006; Breymann 2007; Breymann 2008; Daniilidis 2011; Dede 2005; Haidar 2005; Hashmi 2006; Jansen 2007; Krafft 2011; Murray‐Kolb 2009; Palacio 2007; Perez 2005; Prick 2010; Prick 2012; Seid 2007; Seid 2008; Tam 2005; Van der Woude 2011; Van Rhenen 2005; Van Wyck 2007; Verma 2011; Wagstrom 2007; Westad 2008; Westad 2009). Information about the updating of this review has been added to Published notes. |
| 20 September 2008 | Amended | Converted to new review format. |
Notes
This review will no longer be updated by the current review team and has been superseded by a new review on this topic, see Markova 2015.
Acknowledgements
As part of the pre‐publication editorial process, this review has been commented on by two peers (an editor and referee who are external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
Jacci Parsons (Department of Public Health, University of Adelaide) and Professor Crowther (Department of Obstetrics and Gynaecology, University of Adelaide) for their help designing and developing the protocol and the review.
Data and analyses
Comparison 1. EPO i.v. versus placebo i.v.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Self‐Report Symptom Inventory 90 Items ‐ Revised (SCL‐90‐R) | Other data | No numeric data | ||
| 2 Items of the Blues Questionnaire showing statistically significant difference by day 5 | Other data | No numeric data | ||
| 3 Haemoglobin (g/dL) within 2 weeks after treatment | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.26, 1.06] |
| 4 Haematocrit (%) within 2 weeks after treatment | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 1.60 [‐0.42, 3.62] |
1.1. Analysis.
Comparison 1 EPO i.v. versus placebo i.v., Outcome 1 Self‐Report Symptom Inventory 90 Items ‐ Revised (SCL‐90‐R).
| Self‐Report Symptom Inventory 90 Items ‐ Revised (SCL‐90‐R) | |||
|---|---|---|---|
| Study | Item number | Erythropoietin | Placebo |
| Meyer 1995 | 20 ‐ crying easily | Factor value ‐ 1.4 | Factor value ‐ 1.47 |
| Meyer 1995 | 24 ‐ temper outbursts | Factor value ‐ 0.65 | Factor value ‐ 0.61 |
| Meyer 1995 | 30 ‐ feeling blue | Factor value ‐ 0.8 | Factor value ‐ 0.85 |
| Meyer 1995 | 14 ‐ low in energy | Factor value ‐ 1.10 | Factor value ‐ 1.19 |
| Meyer 1995 | 71 ‐ feeling everything is an effort | Factor value ‐ 0.72 | Factor value ‐ 0.80 |
| Meyer 1995 | |||
| Meyer 1995 | |||
| Meyer 1995 | |||
| Meyer 1995 | |||
| Meyer 1995 | |||
1.2. Analysis.
Comparison 1 EPO i.v. versus placebo i.v., Outcome 2 Items of the Blues Questionnaire showing statistically significant difference by day 5.
| Items of the Blues Questionnaire showing statistically significant difference by day 5 | |||
|---|---|---|---|
| Study | Item number | Erythropoietin | Placebo |
| Meyer 1995 | Item 3 ‐ able to concentrate | % yes ‐ 40 | % yes ‐ 31 |
| Meyer 1995 | Item 5 ‐ elated | % yes ‐ 20 | % yes ‐ 19 |
| Meyer 1995 | Item 8 ‐ alert | % yes ‐ 33 | % yes ‐ 35 |
| Meyer 1995 | Item 12 ‐ relaxed | % yes ‐ 20 | % yes ‐ 33 |
| Meyer 1995 | Item 18 ‐ happy | % yes ‐ 40 | % yes ‐ 27 |
| Meyer 1995 | Item 19 ‐ confident | % yes ‐ 29 | % yes ‐ 28 |
| Meyer 1995 | Item 24 ‐ lively | % yes ‐ 16 | % yes ‐ 16 |
| Meyer 1995 | Item 28 ‐ calm | % yes ‐ 29 | % yes ‐ 28 |
| Meyer 1995 | |||
| Meyer 1995 | |||
1.3. Analysis.
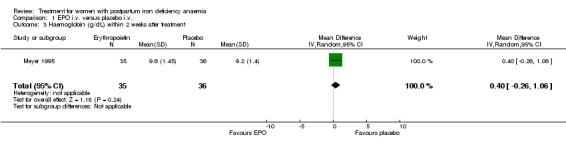
Comparison 1 EPO i.v. versus placebo i.v., Outcome 3 Haemoglobin (g/dL) within 2 weeks after treatment.
1.4. Analysis.
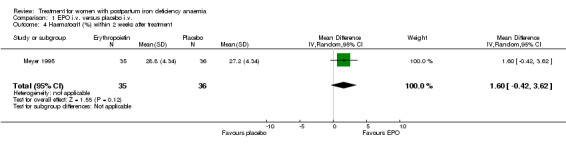
Comparison 1 EPO i.v. versus placebo i.v., Outcome 4 Haematocrit (%) within 2 weeks after treatment.
Comparison 2. EPO + iron versus iron.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Use of blood transfusions | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 0.2 [0.01, 3.92] |
| 2 Lactating | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 1.9 [1.21, 2.98] |
| 3 Thromboembolic complications | 2 | 96 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Anaphylactic/serious reaction | 3 | 186 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Length of postnatal hospital stay, median (days) | Other data | No numeric data | ||
| 6 Haemoglobin (g/dL) within 2 weeks after treatment | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 EPO iv + iron versus iron (oral or iv) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.45 [‐0.16, 1.06] |
| 6.2 EPO sc + iron versus iron (oral or iv) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.99, ‐0.11] |
| 6.3 EPO iv + iron versus iron (oral + iv) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.22, 1.02] |
| 7 Haemoglobin increase (%) within 2 weeks after treatment | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 EPO iv + iron versus iron iv | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.27, 0.67] |
| 7.2 EPO iv + iron versus oral iron | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.70 [0.23, 1.17] |
| 8 Haemoglobin (g/dL) > 2 weeks to 6 weeks after treatment | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 8.1 EPO iv + iron versus iron (oral or iv) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.09, 0.89] |
| 8.2 EPO sc + iron versus iron (oral or iv) | 1 | 60 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.34, 0.94] |
| 8.3 EPO iv + iron versus iron (oral + iv) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.20, 1.00] |
| 9 Haemoglobin (g/dL) median | Other data | No numeric data | ||
| 10 Haematocrit (%) within 2 weeks after treatment | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 10.1 EPO iv + iron versus iron iv | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 2.30 [1.89, 2.71] |
| 10.2 EPO iv + iron versus oral iron | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 3.5 [3.19, 3.81] |
| 10.3 EPO iv + iron versus iron (oral + iv) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 1.5 [‐0.38, 3.38] |
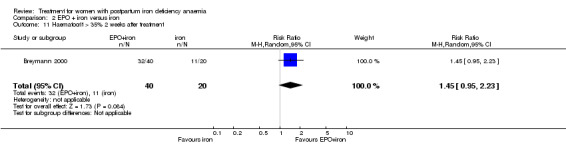
| 11 Haematocrit > 35% 2 weeks after treatment | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.45 [0.95, 2.23] |
| 12 Haematocrit (%) > 2 weeks to 6 weeks after treatment | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 12.1 EPO iv + iron versus iron (oral + iv) | 1 | 36 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐3.06, 3.26] |
| 13 Haematocrit (median %) | Other data | No numeric data |
2.1. Analysis.
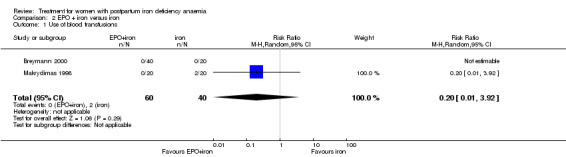
Comparison 2 EPO + iron versus iron, Outcome 1 Use of blood transfusions.
2.2. Analysis.
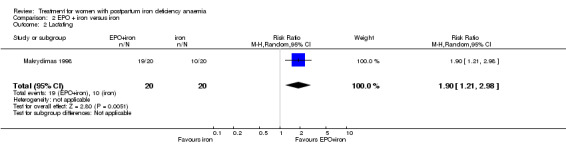
Comparison 2 EPO + iron versus iron, Outcome 2 Lactating.
2.3. Analysis.
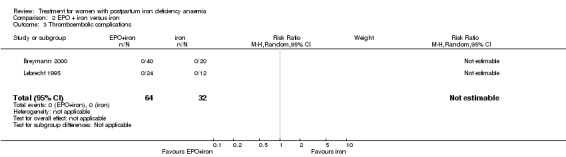
Comparison 2 EPO + iron versus iron, Outcome 3 Thromboembolic complications.
2.4. Analysis.
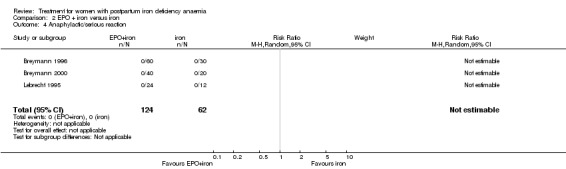
Comparison 2 EPO + iron versus iron, Outcome 4 Anaphylactic/serious reaction.
2.5. Analysis.
Comparison 2 EPO + iron versus iron, Outcome 5 Length of postnatal hospital stay, median (days).
| Length of postnatal hospital stay, median (days) | ||
|---|---|---|
| Study | EPO+iron+folate (n=2 | iron+folate (n=20) |
| Makrydimas 1998 | 11 (range 10‐16) | 14 (range 11‐19) |
2.6. Analysis.
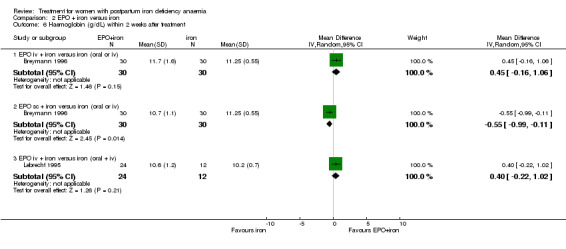
Comparison 2 EPO + iron versus iron, Outcome 6 Haemoglobin (g/dL) within 2 weeks after treatment.
2.7. Analysis.
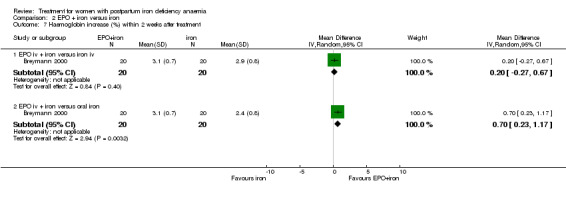
Comparison 2 EPO + iron versus iron, Outcome 7 Haemoglobin increase (%) within 2 weeks after treatment.
2.8. Analysis.
Comparison 2 EPO + iron versus iron, Outcome 8 Haemoglobin (g/dL) > 2 weeks to 6 weeks after treatment.
2.9. Analysis.
Comparison 2 EPO + iron versus iron, Outcome 9 Haemoglobin (g/dL) median.
| Haemoglobin (g/dL) median | |||
|---|---|---|---|
| Study | Days after | EPO+iron+folate | Iron+folate |
| Makrydimas 1998 | 2d | 7.8 | 7.3 |
| Makrydimas 1998 | 4d | 8.4 | 7.6 |
| Makrydimas 1998 | 14d | 10.3 | 8.9 |
| Makrydimas 1998 | 39d | 12.2 | 11.6 |
2.10. Analysis.
Comparison 2 EPO + iron versus iron, Outcome 10 Haematocrit (%) within 2 weeks after treatment.
2.11. Analysis.
Comparison 2 EPO + iron versus iron, Outcome 11 Haematocrit > 35% 2 weeks after treatment.
2.12. Analysis.
Comparison 2 EPO + iron versus iron, Outcome 12 Haematocrit (%) > 2 weeks to 6 weeks after treatment.
2.13. Analysis.
Comparison 2 EPO + iron versus iron, Outcome 13 Haematocrit (median %).
| Haematocrit (median %) | |||
|---|---|---|---|
| Study | Days after | EPO+iron+folate | Iron+folate |
| Makrydimas 1998 | 2 | 25 | 22 |
| Makrydimas 1998 | 4 | 27 | 24 |
| Makrydimas 1998 | 14 | 32 | 27 |
| Makrydimas 1998 | 39 | 37 | 35 |
Comparison 3. EPO s.c. versus EPO i.v.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse effects reported for EPO | 1 | 95 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Haemoglobin (g/dL) within 2 weeks after treatment | 2 | 155 | Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.94, 0.26] |
| 2.1 EPO given as 2 doses over 2 days | 1 | 51 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.55, 0.55] |
| 2.2 EPO given as one dose | 2 | 104 | Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.42, 0.34] |
| 3 Haemoglobin (g/dL) > 2 weeks to 6 weeks after treatment | 2 | 155 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.27, 0.32] |
| 3.1 EPO given as 2 doses over 2 days | 1 | 51 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.51, 0.31] |
| 3.2 EPO given as one dose | 2 | 104 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.27, 0.58] |
| 4 Haematocrit (%) within 2 weeks after treatment | 1 | 95 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.83, 0.86] |
| 4.1 EPO given as 2 doses over 2 days | 1 | 51 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.94, 0.94] |
| 4.2 EPO as one dose | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.87, 1.87] |
| 5 Haematocrit (%) > 2 weeks to 6 weeks after treatment | 1 | 95 | Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.66, 0.21] |
| 5.1 EPO given as 2 doses over 2 days | 1 | 51 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐2.10, 0.10] |
| 5.2 EPO as one dose | 1 | 44 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.77, 1.77] |
3.1. Analysis.
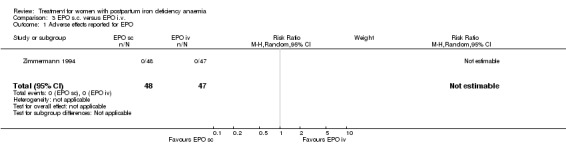
Comparison 3 EPO s.c. versus EPO i.v., Outcome 1 Adverse effects reported for EPO.
3.2. Analysis.
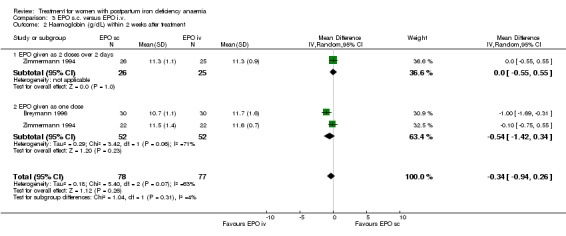
Comparison 3 EPO s.c. versus EPO i.v., Outcome 2 Haemoglobin (g/dL) within 2 weeks after treatment.
3.3. Analysis.
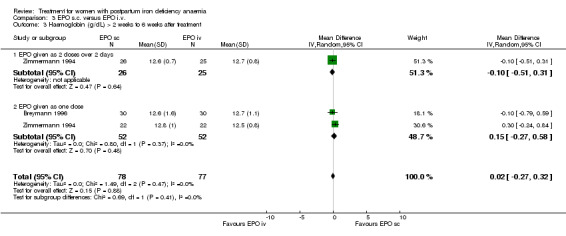
Comparison 3 EPO s.c. versus EPO i.v., Outcome 3 Haemoglobin (g/dL) > 2 weeks to 6 weeks after treatment.
3.4. Analysis.
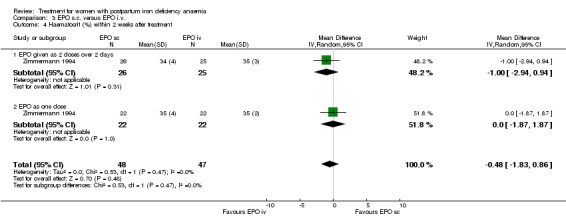
Comparison 3 EPO s.c. versus EPO i.v., Outcome 4 Haematocrit (%) within 2 weeks after treatment.
3.5. Analysis.
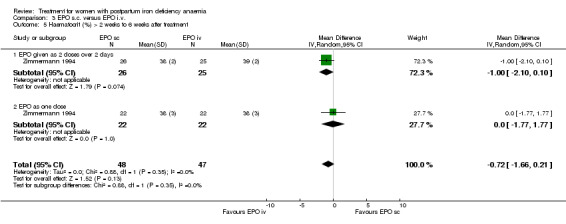
Comparison 3 EPO s.c. versus EPO i.v., Outcome 5 Haematocrit (%) > 2 weeks to 6 weeks after treatment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Breymann 1996.
| Methods | Randomised single centre study. Allocation concealment: sealed envelopes. | |
| Participants | 90 women. Inclusion criteria: postpartum haemoglobin < 10.0 g/ dL 48‐72 h after delivery, normal cardiac and renal function, oral iron substitution during pregnancy. Exclusion criteria: pregnancy anaemia, peripartal infection, peripartal blood transfusion, haematological disease, previous myelosuppressive medications, history of thromboembolism, haemosiderosis, iron intolerance or rheumatoid polyarthritis. | |
| Interventions | Group 1 (n = 30): saccharated iron 100mg, once i.v. Oral iron sulphate (160 mg elemental iron per day) and folic acid 0.7 mg per day for 6 weeks. Group 2 (n = 30): rhEPO 300 U/kg once s.c. Saccharated iron 100 mg once i.v. and oral iron sulphate (160 mg elemental iron per day) and folic acid 0.7 mg per day for 6 weeks. Group 3 (n = 30): rhEPO 300 U/kg once i.v., saccharated iron 100 mg once i.v., oral iron sulphate (160 mg elemental iron per day) and folic acid (0.7 mg/day) for 6 weeks. Treatment started 48‐72 h after birth. | |
| Outcomes | Blood samples taken on days 1, 4, 14 and 42 after the start of therapy. Vital signs recorded daily until discharge. Blood indices: haemoglobin, packed cell volume, reticulocyte count, serum iron and transferrin concentrations, serum ferritin concentration, C‐reactive protein concentration. | |
| Notes | Some results have been extracted from graphs presented in the paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Breymann 2000.
| Methods | Randomised, placebo‐controlled single‐centre study. Allocation concealment: sealed envelopes containing numbers allocated to one of three groups. | |
| Participants | 60 postpartum women (divided into 3 groups of 20). Inclusion criteria: haemoglobin < 10 g/ dL 24‐72 h after delivery. Exclusion criteria: pregnancy anaemia or anaemia prior to delivery, peripartal blood transfusion, anaemia as a result of causes other than blood loss, history of thromboembolism, signs of infection with a rectal temperature > 38.5 degrees, history of seizures, alcohol and/or drug abuse, renal or hepatic dysfunction, previous myelosuppressive medication, haemosiderosis, history of iron intolerance, and rheumatoid polyarthritis. | |
| Interventions | Placebo refers to rhEPO only, all women received iron therapy. Group 1 (n = 20): rhEPO 300 U/kg body weight daily i.v. on days 1‐4 and iron sucrose 200 mg i.v. daily. Group 2 (n = 20): rhEPO placebo (saline i.v.) and iron sucrose as per group 1. Group 3 (n = 20); (control): oral elemental iron sulphate (80 mg) and folic acid one hour before meals on an empty stomach. | |
| Outcomes | Blood indices (taken prior to treatment and on days 4, 7 and 14): absolute reticulocyte count, haematocrit and haemoglobin and red cell indices, iron status markers (serum ferritin, iron, transferrin), serum EPO, CRP, vitamin B12 and folic acid levels. Other: vital signs and the incidence and severity of serious or unusual adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lebrecht 1995.
| Methods | Randomised, placebo‐controlled single centre study. Allocation concealment: not stated. | |
| Participants | 36 women. Inclusion criteria: Hb < 9 g/dL on day two postpartum, after birth of a healthy child at least 38 weeks gestation. Exclusion criteria: anaemia from other causes, caesarean at delivery, cardiovascular illness, history of thromboembolic disease, infection, alcohol or drug dependence, blood transfusions, and renal or hepatic impairment. | |
| Interventions | Group 1 (n = 24): given 20,000 IE rHuEPO i.v.. Group 2 (n = 12): placebo. For both groups, injections were administered on the second day postpartum. Both groups received iron i.v. on day 2, and oral iron for 4 weeks thereafter. | |
| Outcomes | Blood samples taken immediately before therapy and on days 3, 4, 7, 14 and 28. Haemoglobin, haematocrit, erythrocyte count, reticulocytes, WCC, platelets, ferritin, transferrin saturation, and biochemical parameters were measured. On days 2, 7, 14 and 28 a survey regarding quality of life was filled out by participants. On days 2 and 28 the following clinical parameters were analysed: blood pressure, pulse, temperature. | |
| Notes | It is unclear from the text of the article whether the results have been expressed as means and standard deviations, however, for the purpose of this review, we have interpreted the results as means and standard deviations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Makrydimas 1998.
| Methods | Randomised, single centre study. Allocation concealment: not stated. | |
| Participants | 40 women on the first day following birth. Inclusion criteria: Hb levels < 10 g/dl on day 1 postpartum, age 19‐44 years, absence of serious illness including pre‐eclampsia. | |
| Interventions | Group 1 (n = 20): rHuEPO 200 IU/kg/day s.c. for 15 days, oral iron 200 mg/day for 40 days and folic acid 5 mg/day for 40 days. Group 2 (n = 20): Iron 200 mg/day and folic acid 5 mg/day for (both orally) for 40 days. | |
| Outcomes | Blood samples were taken before birth and on days 1, 3, 5, 10, 15 and 40 postdelivery. Blood indices measured: haemoglobin, haematocrit, platelets, electrolytes, creatinine, serum iron, ferritin, total iron binding capacity, B12, folic acid, liver and renal function tests. Serum EPO levels. Clinical indices: temperature, blood pressure, subjective symptoms (side‐effects ‐ flu‐like symptoms) and ECG (on days 1, 15 and 40). Ability to lactate and psychological wellbeing were noted. | |
| Notes | Many of the results have been expressed as medians. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Meyer 1995.
| Methods | Randomized, double‐blind, placebo‐controlled multicentre study. Allocation concealment: not stated. | |
| Participants | 90 (71) women postpartum. Inclusion criteria: women with a haemoglobin of less than 10 g/dl. | |
| Interventions | Intervention referred to rhEPO. Intervention group (n = 35): EPREX 10,000 IU i.v. at 24 hours. Control group (n = 36): placebo i.v. at 24 hours. | |
| Outcomes | On day 5 postpartum the haemoglobin level was measured. Psychopathology was measured using two questionnaires; the "Blues Questionnaire" during the first five consecutive days postpartum and the "SCL‐90‐R", used on the 5th day postpartum, before discharge from the hospital and at the time of presumed peak of mood changes shortly after delivery. | |
| Notes | There was a relatively high drop‐out rate of more than 20% (number of women analysed was 71). Drop‐outs were due to withdrawal of consent or transferral of the child to an intensive care unit. Some data were extracted from graphs. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Zimmermann 1994.
| Methods | Randomised study. Allocation concealment: sealed envelopes. | |
| Participants | 95 women postpartum. Inclusion criteria: Hb < 10 g/dl within 3 days after birth, stable cardiac function and normal renal function (urine output > 1 ml per hour). Exclusion criteria: high BP, pre‐eclampsia or eclampsia, fever > 38.0 degrees, peripartum blood transfusions, oncologic or haematologic disease, myelosuppressive medications, history of thromboembolic events, treatment with other cytokines. | |
| Interventions | Group 1 (n = 26): rHuEPO 150 U/kg body weight s.c. once daily for two consecutive days. Group 2 (n = 25): rHuEPO 150 U/kg body weight i.v. once daily for two consecutive days. Group 3 (n = 22): rHuEPO 300 U/kg body weight s.c. once only. Group 4 (n = 22): rHuEPO 300 U/kg body weight i.v. once only. All women received oral iron supplements (80 mg ferrous sulphate) and folic acid (0.35 mg) twice daily, regardless of iron supplementation during pregnancy. Therapy was begun 72 hours after birth at the latest. | |
| Outcomes | Blood was taken at day 0, day 4, day 14 and day 42. Blood indices measured: haemoglobin, haematocrit, platelets, reticulocytes, CRP, ferritin. Clinical measurements: blood pressure, temperature, lactation during the first 5 days. | |
| Notes | Reports results of different concentrations and routes of administration of erythropoietin rather than EPO versus other methods of treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
BP: blood pressure CRP: C‐reactive protein ECG: electrocardiogram EPO: erythropoietin h: hours Hb: haemoglobin IU (and IE): international units i.v.: intravenous rhEPO or rHuEPO: recombinant human erythropoietin s.c.: subcutaneous WCC: white cell count
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Casparis 1996 | Population combined women with anaemia during pregnancy and postpartum anaemia. |
| Danko 1990 | Trial was quasi‐randomised. |
| Huch 1992 | Trial was quasi‐randomised. |
| Mara 2001 | Population reported in the study included women who were not anaemic. |
| Osmond 1953 | The intervention focuses on crude liver extract, an intervention not prespecified for inclusion. |
| Picha 1975 | The study assessed the usefulness of iron therapy in prevention, not treatment, of postpartum anaemia. |
| Zimmermann 1995 | All three trials reported by the trials are reported in other included studies. |
Contributions of authors
Jodie Dodd (JD) and Marianna Dare (MD) formulated and wrote the protocol; retrieved the papers and applied the study selection criteria. JD, MD and Philippa Middleton (PM) extracted the data; MD compiled the review and, together, JD, MD and PM analysed the data and wrote the review.
Sources of support
Internal sources
Department of Obstetrics and Gynaecology, University of Adelaide, Australia.
External sources
Department of Health and Ageing, Australia.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Breymann 1996 {published data only}
- Breymann C, Zimmermann R, Huch R, Huch A. Use of recombinant human erythropoietin in combination with parenteral iron in the treatment of postpartum anaemia. European Journal of Clinical Investigation 1996;26:123‐30. [DOI] [PubMed] [Google Scholar]
- Krafft A, Breymann C, Huttner C, Huch R, Huch A. Erythropoietic quality of maternal milk. Lancet 1999;354(9180):778. [DOI] [PubMed] [Google Scholar]
Breymann 2000 {published data only}
- Breymann C, Richter C, Huttner C, Huch A. Effectiveness of recombinant erythropoietin and iron sucrose vs. iron therapy only, in patients with postpartum anaemia and blunted erythropoiesis. European Journal of Clinical Investigation 2000;30:154‐61. [DOI] [PubMed] [Google Scholar]
Lebrecht 1995 {published data only}
- Lebrecht A, Haberlin F, Eberhard J. Anemia in puerperium: parenteral iron substitution renders erythropoietin therapy dispensable [Anamie im Wochenbett: parenterale Eisensubstitution macht Erythropoetin‐Therapie entbehrlich]. Geburtshilfe und Frauenheilkunde 1995;55(3):167‐70. [DOI] [PubMed] [Google Scholar]
Makrydimas 1998 {published data only}
- Makrydimas G, Lolis D, Lialios G, Tsiara S, Georgiou I, Bourantas K. Recombinant human erythropoietin treatment of postpartum anaemia, preliminary results. European Journal of Obstetrics & Gynecology and Reproductive Biology 1998;81:27‐31. [DOI] [PubMed] [Google Scholar]
Meyer 1995 {published data only}
- Meyer J, Eichhorn K, Vetter K, Christen S, Schleusner E, Klos A, et al. Does recombinant human erythropoietin not only treat anemia but reduce postpartum (emotional) distress as well?. Journal of Perinatal Medicine 1995;23:99‐109. [DOI] [PubMed] [Google Scholar]
Zimmermann 1994 {published data only}
- Zimmermann R, Breymann C, Huch R, Huch A. rHuEPO in the treatment of postpartum anemia: subcutaneous versus intravenous administration. Clinical Investigation 1994;72(6 Suppl):S25‐30. [PubMed] [Google Scholar]
References to studies excluded from this review
Casparis 1996 {published data only}
- Casparis D, Carlo P, Branconi F, Grossi A, Merante D, Gafforio L. Effectiveness and tolerance of oral doses of liquid ferrous gluconate in iron deficiency anaemia during pregnancy and in the immediate post‐natal period: comparisons with other liquid or solid formulations containing bivalent or trivalent iron. Minerva Ginecologica 1996;48:511‐8. [PubMed] [Google Scholar]
Danko 1990 {published data only}
- Danko J, Huch R, Huch A. Epoetin alfa for treatment of postpartum anaemia. Lancet 1990;335:737‐8. [DOI] [PubMed] [Google Scholar]
Huch 1992 {published data only}
- Huch A, Eichhorn K, Danko J, Lauener P, Huch R. Recombinant human erythropoietin in the treatment of postpartum anemia. Obstetrics & Gynecology 1992;80:127‐31. [PubMed] [Google Scholar]
Mara 2001 {published data only}
- Mara M, Zivny J, Eretova V, Kvasnicka J, Kuzel D, Umlaufova A, et al. Changes in markers of anemia and iron metabolism and how they are influenced by antianemics in postpartum period. Acta Obstetrica Gynecologica Scandinavica 2001;80:142‐8. [PubMed] [Google Scholar]
Osmond 1953 {published data only}
- Osmond TG. Post‐partum anaemia, a practical treatment. Practitioner 1953;171:77‐80. [PubMed] [Google Scholar]
Picha 1975 {published data only}
- Picha E. A new direction for iron therapy [Ein neuer Weg der Eisentherapie]. Geburtshilfe und Frauenheilkunde 1975;35(10):792‐5. [PubMed] [Google Scholar]
Zimmermann 1995 {published data only}
- Zimmermann R, Breymann C, Richter C, Huch R, Huch A. rhEPO treatment of postpartum anaemia. Journal of Perinatal Medicine 1995;23:111‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Backe 2009 {published data only}
- Backe B. A 6‐week randomised, open comparative, multi‐centre study of intravenous ferric carboxymaltose (ferinject) and oral iron (duroferon) for treatment of post partum anemia. http://clinicaltrials.gov/ct2/show/record/NCT00929409 (accessed 5 June 2012) 2009.
Beard 2005 {published data only}
- Beard JL, Hendricks MK, Perez EM, Murray‐Kolb LE, Berg A, Vernon‐Feagans L, et al. Maternal iron deficiency anemia affects postpartum emotions and cognition. Journal of Nutrition 2005;135(2):267‐72. [DOI] [PubMed] [Google Scholar]
Bhandal 2004 {published data only}
- Bhandal N, Russell R. Intravenous versus oral iron therapy for postpartum anaemia. International Journal of Obstetric Anesthesia 2004;13(3):S7. [DOI] [PubMed] [Google Scholar]
Bhandal 2006 {published data only}
- Bhandal N, Russell R. Intravenous versus oral iron therapy for postpartum anaemia. BJOG: an International Journal of Obstetrics and Gynaecology 2006;113(11):1248‐52. [DOI] [PubMed] [Google Scholar]
Breymann 2007 {published data only}
- Breymann C, Seefried B, Stahel M, Geisser P, Canclini C. Milk iron content in breast‐feeding mothers after administration of intravenous iron sucrose complex. Journal of Perinatal Medicine 2007;35(2):115‐8. [DOI] [PubMed] [Google Scholar]
Breymann 2008 {published data only}
- Breymann C, Gliga F, Bejenariu C, Strizhova N. Comparative efficacy and safety of intravenous ferric carboxymaltose in the treatment of postpartum iron deficiency anemia. International Journal of Gynecology & Obstetrics 2008;101(1):67‐73. [DOI] [PubMed] [Google Scholar]
Daniilidis 2011 {published data only}
- Daniilidis A, Giannoulis C, Pantelis A, Tantanasis T, Dinas K. Total infusion of low molecular weight iron‐dextran for treating postpartum anemia. Clinical and Experimental Obstetrics and Gynecology 2011;38(2):159‐61. [PubMed] [Google Scholar]
Dede 2005 {published data only}
- Dede A, Uygur D, Yilmaz B, Mungan T, Ugur M. Intravenous iron sucrose complex vs. oral ferrous sulfate for postpartum iron deficiency anemia. International Journal of Gynecology & Obstetrics 2005;90(3):238‐9. [DOI] [PubMed] [Google Scholar]
Haidar 2005 {published data only}
- Haidar J, Umeta M, Kogi‐Makau W. Effect of iron supplementation on serum zinc status of lactating women in Addis Ababa, Ethiopia. East African Medical Journal 2005;82(7):349‐52. [PubMed] [Google Scholar]
Hashmi 2006 {published data only}
- Hashmi Z, Bashir G, Azeem P, Shah S. Effectiveness of intra‐venous iron sucrose complex versus intra‐muscular iron sorbitol in iron deficiency anemia. Annals of Pakistan Institute of Medical Sciences 2006;2(3):188‐91. [Google Scholar]
Huch 1990 {published data only}
- Huch A, Huch R. EPO (rHuEPO) use in obstetrics. Proceedings of Ross Laboratories Special Conference, Hot Topics '90 in Neonatology; 1990 Dec 9‐11; Washington DC, USA. 1990:79‐80.
Jansen 2007 {published data only}
- Jansen AJG, Duvekot JJ, Essink‐Bot ML, Hop WCJ, Rhenen DJ. Multicentre clinical study into the optimal blood transfusion policy in patients with postpartum haemorrhage: The 'Wellbeing of obstetric patients on minimal blood transfusions' (WOMB) study [Een klinisch multicentrisch onderzoek naar het optimale bloedtransfusiebeleid bij patienten met een fluxus post partum: De 'Wellbeing of obstetric patients on minimal blood transfusions'(WOMB)‐studie]. Nederlands Tijdschrift voor Geneeskunde 2007;151(39):2170‐2. [PubMed] [Google Scholar]
Krafft 2011 {published data only}
- Krafft A, Breymann C. Iron sucrose with and without recombinant erythropoietin for the treatment of severe postpartum anemia: a prospective, randomized, open‐label study. Journal of Obstetrics and Gynaecology Research 2011;37(2):119‐24. [DOI] [PubMed] [Google Scholar]
Krauss 1972 {published data only}
- Krauss V, Nowotny D, Scharifzadeh AH. Effect and tolerance of oral iron therapy in the puerperium. Munchener Medizinische Wochenschrift 1972;114(43):1877‐9. [PubMed] [Google Scholar]
Mara 1999 {published data only}
- Mara M, Eretova V, Zivny J, Kvasnicka J, Umlaufova A, Marova E. Anemia and its treatment with peroral anti‐anemia agents in women during the postpartum period. [Czech]. Ceska Gynekologie 1999;64:153‐8. [PubMed] [Google Scholar]
Murray‐Kolb 2009 {published data only}
- Murray‐Kolb LE, Beard JL. Iron deficiency and child and maternal health. American Journal of Clinical Nutrition 2009;89(3):946S‐50S. [DOI] [PubMed] [Google Scholar]
Palacio 2007 {published data only}
- Palacio M. Intravenous iron versus oral iron for severe postpartum anemia randomized trial. http://clinicaltrials.gov/ct2/show/record/NCT00660933 (accessed 5 June 2012) 2007.
Perez 2005 {published data only}
- Perez EM, Hendricks MK, Beard JL, Murray‐Kolb LE, Berg A, Tomlinson M, et al. Mother‐infant interactions and infant development are altered by maternal iron deficiency anemia. Journal of Nutrition 2005;135(4):850‐5. [DOI] [PubMed] [Google Scholar]
Prick 2010 {published data only}
- Prick BW, Steegers EAP, Jansen AG, Hop WCJ, Essink‐Bot M‐L, Peters NCJ, et al. Well being of obstetric patients on minimal blood transfusions (WOMB trial). BMC Pregnancy and Childbirth 2010;10:83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prick 2012 {published data only}
- Prick BW, Jansen AJG, Steegers EAP, Hop WCJ, Essink‐Bot ML, Uyl‐de Groot CA, et al. RBC transfusion leads to an improvement of physical fatigue in women with acute postpartum anemia: the WOMB study (NCT00335023). American Journal of Obstetrics and Gynecology 2012;206(Suppl 1):S41‐2. [Google Scholar]
Seid 2007 {published data only}
- Seid MH, Rogers R, Dinh Q. Treating postpartum anemia with intravenous ferric carboxymaltose in a randomized controlled study. American Journal of Obstetrics and Gynecology 2007;197(6 Suppl 1):S26, Abstract no: 55. [Google Scholar]
Seid 2008 {published data only}
- Seid MH, Derman RJ, Baker JB, Banach W, Goldberg C, Rogers R. Ferric carboxymaltose injection in the treatment of postpartum iron deficiency anemia: a randomized controlled clinical trial. American Journal of Obstetrics and Gynecology 2008;199(4):435.e‐1‐435.e7. [DOI] [PubMed] [Google Scholar]
Tam 2005 {published data only}
- Tam KF, Lee CP, Pun TC. Mild postnatal anemia: is it a problem?. American Journal of Perinatology 2005;22(7):345‐9. [DOI] [PubMed] [Google Scholar]
Van der Woude 2011 {published data only}
- Woude D. Iron and folic acid v.s. iron solely in the treatment of post partum anaemia; effects on haemoglobin and health status. Netherlands Trial Register (http://www.trialregister.nl) (accessed 8 July 2011) 2011.
Van Rhenen 2005 {published data only}
- Rhenen DJ. Wellbeing of obstetric patients on minimal bloodtranfusions study ‐ WOMB study. Netherlands Trial Register (http://www.trialregister.nl) (accessed 1 November 2005)) 2005.
Van Wyck 2007 {published data only}
- Wyck DB, Martens MG, Seid MH, Baker JB, Mangione A. Intravenous ferric carboxymaltose compared with oral iron in the treatment of postpartum anemia: a randomized controlled trial. Obstetrics & Gynecology 2007;110(2 Pt 1):267‐78. [DOI] [PubMed] [Google Scholar]
Verma 2011 {published data only}
- Verma S, Inamdar SA, Malhotra N. Intravenous iron therapy versus oral iron in postpartum patients in rural area. Journal of SAFOG 2011;3(2):67‐70. [Google Scholar]
Wagstrom 2007 {published data only}
- Wagstrom E, Akesson A, Rooijen M, Larson B, Bremme K. Erythropoietin and intravenous iron therapy in postpartum anaemia. Acta Obstetricia et Gynecologica Scandinavica 2007;86(8):957‐62. [DOI] [PubMed] [Google Scholar]
Westad 2008 {published data only}
- Westad S, Backe B, Salvesen KA, Nakling J, Okland I, Borthen I, et al. A 12‐week randomised study comparing intravenous iron sucrose versus oral ferrous sulphate for treatment of postpartum anemia. Acta Obstetricia et Gynecologica Scandinavica 2008;87(9):916‐23. [DOI] [PubMed] [Google Scholar]
Westad 2009 {published data only}
- Westad S, Backe B, Salvesen K, Nakling J, Okland I, Borthen I, et al. A 12‐week randomised, multi‐centre study comparing intravenous iron sucrose versus oral ferrous sulphate for treatment of postpartum anaemia. International Journal of Gynecology & Obstetrics 2009;107(Suppl 2):S377. [Google Scholar]
Additional references
AADRB 2002
- Epoetin alfa and pure red cell aplasia. Australian Adverse Drug Reactions Bulletin; www.health.gov.au/tga/adr/aadrd/aadr0208.htm [accessed 16 December 2003] August 2002.
Atkinson 1994
- Atkinson L, Baxley E. Post partum fatigue. American Family Physician 1994;50(1):113‐8. [PubMed] [Google Scholar]
Casadevall 2002
- Casadevall N, Nataf J, Viron B, Kolta A, Kiladjian J‐J, Martin‐Dupont P, et al. Pure red‐cell aplasia and antierythropoietin antibodies in patients treated with recombinant erythropoietin. New England Journal of Medicine 2002;346(7):469‐75. [DOI] [PubMed] [Google Scholar]
Clarke 2003
- Clarke M, Oxman A, editors. Cochrane Reviewers' Handbook 4.2.0 [updated March 2003]. Review Manager (RevMan) [computer program]. Oxford, UK: The Cochrane Collaboration, 2003. [Google Scholar]
Davis 1990
- Davis HP. Erythropoietin for patient refusing blood transfusion. Lancet 1990;336:384‐5. [DOI] [PubMed] [Google Scholar]
Dudrick 1986
- Dudrick S, O'Donnell J, Matheny R, Unkel S, Raleigh D. Stimulation of hematopoiesis as an alternative to transfusion. Southern Medical Journal 1986;79(6):669‐73. [DOI] [PubMed] [Google Scholar]
Ekanem 1996
- Ekanem A, Etuk S, Samson‐Akpan U. The influence of cultural practice on puerperal anemia. International Journal of Gynecology & Obstetrics 1996;55:169‐70. [DOI] [PubMed] [Google Scholar]
Gibbs 1980
- Gibbs R. Clinical risk factors for puerperal infection. Obstetrics & Gynecology 1980;55(5 Suppl):178S‐84S. [DOI] [PubMed] [Google Scholar]
Gilbert 1987
- Gilbert L, Porter W, Brown VA. Postpartum haemorrhage ‐ a continuing problem. British Journal of Obstetrics and Gynaecology 1987;94:67‐71. [DOI] [PubMed] [Google Scholar]
Harrison 1989
- Harrison K. Maternal mortality in developing countries. British Journal of Obstetrics and Gynaecology 1989;96:1‐3. [DOI] [PubMed] [Google Scholar]
Klapholz 1990
- Klapholz H. Blood transfusion in contemporary obstetric practice. Obstetrics & Gynecology 1990;75(6):940‐3. [PubMed] [Google Scholar]
Kumar 1989
- Kumar R, Sharma A, Barik S, Kumar V. Maternal mortality inquiry in a rural community of North India. International Journal of Gynecology & Obstetrics 1989;29:313‐9. [DOI] [PubMed] [Google Scholar]
Mahomed 2003
- Mahomed K. Iron supplementation in pregnancy (Cochrane Review). The Cochrane Library 2003, Issue 4. [Google Scholar]
Markova 2015
- Markova V, Norgaard A, Jørgensen KJ, Langhoff‐Roos J. Treatment for women with postpartum iron deficiency anaemia. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD010861.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Naef 1995a
- Naef R, Washburne J, Martin R, Magann E, Scanlon P, Morrison J. Hemorrhage associated with cesarean delivery: when is transfusion needed. Journal of Perinatology 1995;15(1):32‐5. [PubMed] [Google Scholar]
Naef 1995b
- Naef R, Morrison J. Transfusion therapy in pregnancy. Clinical Obstetrics and Gynecology 1995;38(3):547‐57. [DOI] [PubMed] [Google Scholar]
Nolan 1991
- Nolan T, Gallup D. Massive transfusion: a current review. Obstetrical and Gynecological Survey 1991;46(5):289‐95. [DOI] [PubMed] [Google Scholar]
RevMan 2004 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2.3. Oxford, UK: The Cochrane Collaboration, 2004.
Rosenfield 1989
- Rosenfield A. Maternal mortality in developing countries ‐ an ongoing but neglected 'epidemic'. JAMA 1989;262(3):376‐9. [PubMed] [Google Scholar]
Skolnick 1992
- Skolnick A. Transfusion medicine faces time of major 'challenges and changes'. JAMA 1992;268(6):697‐700. [PubMed] [Google Scholar]
Vora 1998
Waymack 1990
- Waymack J, Yurt R. The effect of blood transfusions on immune function. Journal of Surgical Research 1990;48:147‐53. [DOI] [PubMed] [Google Scholar]
WHO 1999
- World Health Organization. Reduction of maternal mortality. A joint WHO/UNFPA/UNICEF/World Bank statement. Geneva: WHO, 1999. [Google Scholar]
WHO 2001
- World Health Organization, United Nations Children's Fund, United Nations University. Iron deficiency anaemia; Assessment, Prevention and Control; A guide for programme managers. Geneva: World Health Organization, 2001. [Google Scholar]


