Abstract
Background
Cartilage degeneration is assessed using various methods. Although macroscopic evaluation can directly measure cartilage degeneration, it cannot accurately assess cartilage properties. Histological examination is one of the most accurate methods for evaluating cartilage degeneration. However, it is invasive and requires collection of cartilage tissue. In contrast, the Arthro-BST™ probe can assess cartilage properties noninvasively. This study aimed to evaluate the effectiveness of the Arthro-BST in assessing cartilage degeneration by comparing macroscopic (International Cartilage Repair Society [ICRS] classification) and histological evaluations (modified Mankin score and Osteoarthritis Research Society International [OARSI] histological grade).
Methods
Fourteen femoral heads were excised from 13 patients during surgery to treat hip osteoarthritis or femoral fracture. The ICRS score was used for macroscopic evaluation of cartilage degeneration. The Arthro-BST was applied at sites matching the areas of cartilage damage. The sites assessed using the ICRS classification and Arthro-BST were evaluated histologically (modified Mankin score and OARSI histological grade), and these were compared with the Arthro-BST results.
Results
The ICRS classification identified significant differences between grades 1 and 3 (p < 0.01), between grades 1 and 4 (p < 0.01), between grades 2 and 3 (p < 0.01), and between grades 2 and 4 (p < 0.01). Significant correlations were observed between the Arthro-BST results and the ICRS score, modified Mankin score (structure, cellularity, matrix staining, total score), and OARSI histological grade.
Conclusions
In the assessment of hip osteoarthritis, the Arthro-BST results correlated with those of macroscopic and histological evaluations. The Arthro-BST is useful for assessing hip osteoarthritis and may be helpful for noninvasive assessment of cartilage degeneration.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13075-021-02611-x.
Keywords: Osteoarthritis, Arthro-BST, ICRS classification, Modified Mankin score, OARSI histological system
Background
Osteoarthritis (OA) is a common form of arthritis among older people, and the number of patients with OA is increasing worldwide. Although OA is not a life-threatening disease, it affects daily activities and quality of life. Cartilage is one of the most difficult tissues to regenerate because of its avascular nature. Therefore, detecting early degeneration of articular cartilage is necessary for the prevention and treatment of OA. Various diagnostic methods, such as radiography [1], macroscopic imaging [2], magnetic resonance imaging (MRI) [3], and histological evaluation [4, 5], are used for evaluating OA.
Although radiography is the most frequently used technique for evaluating OA through assessment of joint space and osteophyte formation, this method cannot be used to evaluate the macroscopic changes and properties of articular cartilage. Arthroscopy is used widely for treating cartilage degeneration, and although it can be used to assess cartilage degeneration directly, it cannot be used to evaluate cartilage properties. MRI has recently been used for assessing cartilage degeneration. We have focused on the noninvasive assessment of cartilage degeneration and have reported the usefulness of MRI for assessing knee OA using T2 mapping and diffusion tensor imaging [3]. Although MRI can be used to assess cartilage degeneration noninvasively, it is difficult to assess cartilage degeneration when the cartilage damage is of a mixed nature. Histological evaluation can be used to assess cartilage degeneration accurately, but it involves the destruction of normal cartilage.
To overcome these disadvantages, nondestructive devices have been developed to assess cartilage degeneration [6–21]. We explored the literature on the assessment of cartilage degeneration that used laser-induced photoacoustic measurement (LIPA) instruments [21]. These devices can assess cartilage degeneration without the need to collect tissue samples. We have previously compared LIPA with histological evaluation and reported that LIPA can be used to assess cartilage degeneration and viscoelastic properties [21].
Articular cartilage contains proteoglycans that have electromechanical properties [22]. Interstitial water contains positive mobile ions, such as Na+ and K+, which balance the fixed negative electric charge from proteoglycans [22]. The mechanical compression of articular cartilage generates streaming potentials induced by water flowing out from articular cartilage [23], and these streaming potentials reflect cartilage integrity and degeneration [24–27]. Taking advantage of this property, the Arthro-BST instrument (Biomomentum, Laval, QC, Canada) was invented for assessing the streaming potentials of articular cartilage. This apparatus is used during arthroscopy and induces streaming potentials using 37 microelectrodes with the help of the spherical indenter of the tip. The effectiveness of the Arthro-BST for assessing the properties of articular cartilage of the knee has been reported previously [28–31]. However, no study has evaluated the effectiveness of the Arthro-BST for assessing the hip by evaluating the macroscopic and histological properties simultaneously.
This study aimed to evaluate the effectiveness of the Arthro-BST for evaluating the hip by comparing its findings with macroscopic findings. We used the International Cartilage Repair Society (ICRS) classification system [2] and histological findings such as the modified Mankin histological score [32] and Osteoarthritis Research Society International (OARSI) histopathology assessment system [5].
Methods
Sample source
Tissue was obtained from patients who had been diagnosed with OA or femoral fracture of the hip and who underwent total hip arthroplasty or bipolar hip arthroplasty at the authors’ institution. After resection of the femoral head of the hip, 14 femoral heads of 13 patients were evaluated (Table 1). This study was performed after approval from the research review committee at the author’s institution (approval number: 18R-187). All patients provided written informed consent.
Table 1.
Patient demographics
| Patient number | Age (years) | Sex | Body mass index (kg/m2) | Diagnosis | Kellgren–Lawrence classification |
|---|---|---|---|---|---|
| 1 | 72 | F | 26.2 | Osteoarthritis | 3 |
| 2 | 57 | F | 24.4 | Osteoarthritis | 3 |
| 3 | 65 | F | 22 | Osteoarthritis | 3 |
| 4 | 60 | F | 34.9 | Osteoarthritis | 4 |
| 5 | 83 | F | 18.6 | Osteoarthritis | 4 |
| 6 | 74 | F | 23.7 | Osteoarthritis | 4 |
| 7 | 82 | F | 17.2 | Femoral neck fracture | 1 |
| 8 | 67 | F | 26 | Osteoarthritis | 3 |
| 9 | 84 | F | 23 | Femoral neck fracture | 1 |
| 10 | 64 | F | 23.1 | Osteoarthritis | 4 |
| 11 | 54 | M | 24.8 | Osteoarthritis | 4 |
| 12 | 70 | F | 27.4 | Osteoarthritis | 4 |
| 13 | 71 | F | 23.2 | Femoral neck fracture | 1 |
| 14 | 55 | F | 24.4 | Osteoarthritis | 3 |
Assessment procedure
After resection of the femoral head, cartilage lesions were assessed macroscopically using the ICRS classification [2]. The same sites of cartilage lesions were assessed histologically using the modified Mankin score [32] and OARSI grade [5].
Macroscopic assessment
After dissection of the femoral head, the assessment points were marked with the help of photographs (Fig. 1). Two experienced orthopedic surgeons separately performed a macroscopic assessment of 14 femoral heads. Sixty-one locations of the femoral head were used to assess the ICRS classification (Table 2). Each femoral head was assessed for cartilage degeneration on the day of surgery.
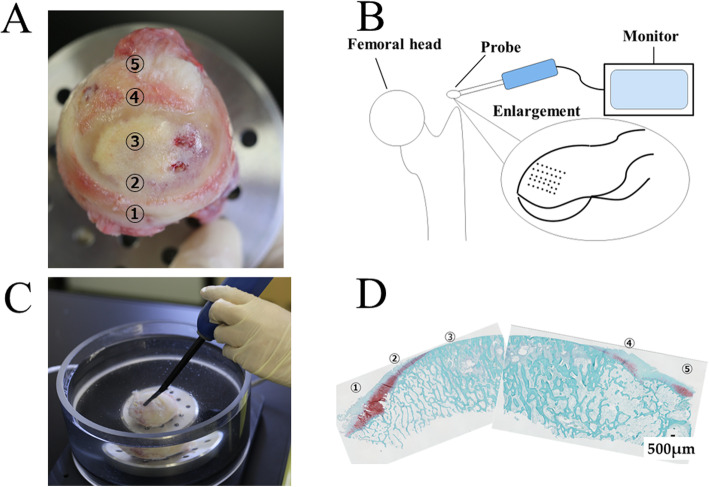
Fig. 1.
Cartilage tissue assessment. A The ICRS classification was used to assess the cartilage of the femoral head (see Table 1 for the definition of the grades). B Schematic drawing of the Arthro-BST. The Arthro-BST induces streaming potentials using 37 microelectrodes. C The Arthro-BST was used to assess the same points assessed by the ICRS classification. D Cartilage sections were made by cutting perpendicular to the same points assessed using the ICRS classification and Arthro-BST
Table 2.
ICRS classification as described by Mainil-Varlet et al. [2]
| Grade | Property |
|---|---|
| 1 | Superficial lesions, fissures and cracks, soft indentation |
| 2 | Defects that extend to less than 50% in depth |
| 3 | Defects that extend to more than 50% in depth |
| 4 | Complete loss of cartilage thickness, bone only |
Electromechanical assessment
The Arthro-BST measures streaming potentials generated during rapid compression of the articular cartilage with an array of 37 microelectrodes lying on a hemispherical indenter (effective radius of the tip = 3.18 mm, 5 microelectrodes/mm2) [29]. The device measures a quantitative parameter (QP), which corresponds to the number of microelectrodes in contact with the cartilage when the sum of all electrode potentials reaches 100 mV [30]. A high QP indicates strong electromechanical properties and normal cartilage, and a low QP indicates weak electromechanical properties and degenerated cartilage.
The resected femoral heads were placed onto a cylindrical platform and assembled into a testing chamber using screws. A single electromechanical measurement was performed manually at each position of the grid using the Arthro-BST. Measurements were performed on the day of surgery (Fig. 1).
Histological assessment
Twenty-five samples were used for histological assessment. Two experienced orthopedic surgeons separately performed a histological assessment of each sample. The tissue samples were cut into sections by making cuts perpendicular to the cartilage surface and then fixed in 4% paraformaldehyde for 1 month. After decalcification for 2 months using distilled water (pH 7.4) containing 10% ethylenediaminetetraacetic acid, the tissue was embedded in paraffin wax and sectioned perpendicularly through the center of the cartilage damage. Each section was stained with Safranin O dye for histological evaluation of glycosaminoglycans [33]. For histological assessment, sections stained with Safranin O were assessed using the modified Mankin score [32] and the OARSI histopathology assessment system (grade 0, surface intact, cartilage morphology intact; grade 1, surface intact; grade 2, surface discontinuity; grade 3, vertical fissures (clefts); grade 4, erosion; grade 5, denudation; grade 6, deformation) [5]. Two orthopedic surgeons of the Japanese Orthopedic Association independently performed the histological assessment. The modified Mankin score was determined by adding the scores of the parameters (structure, cellularity, matrix staining, and tidemark integrity), with 0 as the lowest score and 15 as the highest score (Table 3).
Table 3.
Modified Mankin score for evaluation of articular cartilage degeneration as described by Henson and Vincent [32]
| Score | Structure | Cellularity | Matrix staining | Tidemark integrity |
|---|---|---|---|---|
| Score 0 | Smooth surface | Normal arrangement | Normal staining | Normal and intact |
| Normal appearance | ||||
| Score 1 | Roughened surface | Clustering in the superficial layer or loss of cells up to 10% | Slight loss of stain | Disrupted |
| Single crack or area of delamination | ||||
| Score 2 | Multiple cracks | Disorganization or loss up to 25% | Moderate loss of stain | |
| Moderate delamination | ||||
| Score 3 | Fragmentation in cartilage or severe delamination | Cell rows absent or loss up to 50% | Severe loss of stain | |
| Score 4 | Loss of fragments | Very few cells present | No stain present | |
| Score 5 | Complete erosion to tidemark | |||
| Score 6 | Erosion beyond tidemark |
Statistical analysis
Kruskal–Wallis followed by the Bonferroni post hoc test was used to compare the ICRS grade and Arthro-BST result (QP). Spearman’s rank correlational analysis was used to identify significant relationships between the Arthro-BST QP and the ICRS grade, modified Mankin score, and OARSI histological grade. Interobserver reliability was tested using intraclass correlation coefficients (ICCs), and their 95% confidence intervals (CIs) were used to assess the reliability of the macroscopic and histological assessments. All tests were performed at a significance level of p < 0.05. Analyses were performed using IBM SPSS Statistics for Windows version 26 (IBM Corp., Armonk, NY, USA).
Results
Comparison between the Arthro-BST and the macroscopic assessment results
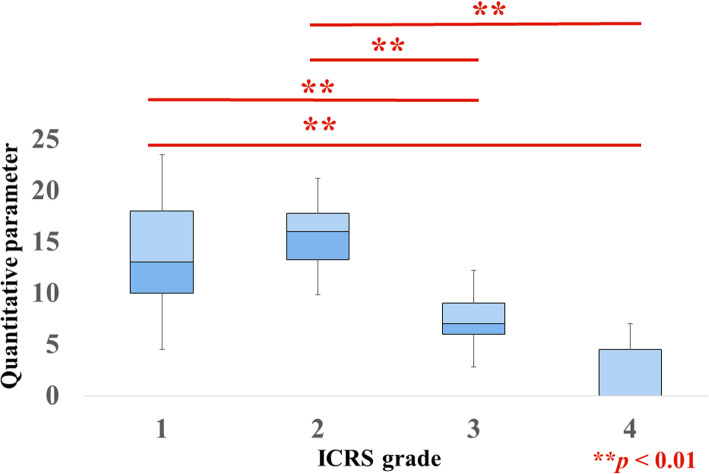
The ICRS grades of the cartilage lesion for all samples were grade 1 (n = 23), grade 2 (n = 18), grade 3 (n = 13), and grade 4 (n = 7). The interobserver reliability of the ICRS classification was 0.973 (95% CI, 0.956–0.984) (Additional file 1). The QPs for the grades were as follows: grade 1, 14.1 ± 5.5; grade 2, 15.6 ± 3.4; grade 3, 7.8 ± 3.2; and grade 4, 2 ± 2.5. Significant differences were observed between grades 1 and 3 (p < 0.01), between grades 1 and 4 (p < 0.01), between grades 2 and 3 (p < 0.01), and between grades 2 and 4 (p < 0.01) (Fig. 2).
Fig. 2.
Arthro-BST assessment and ICRS classification. The QP decreased as the ICRS grade increased. Significant differences were observed for ICRS grades 1 and 3 (p < 0.01), grades 1 and 4 (p < 0.01), grades 2 and 3 (p < 0.01), and grades 2 and 4 (p < 0.01)
Relationship between the Arthro-BST QP and the macroscopic assessment
The QP decreased as the ICRS grade increased, and the correlation between these variables was significant (p < 0.01) (Fig. 3).
Fig. 3.
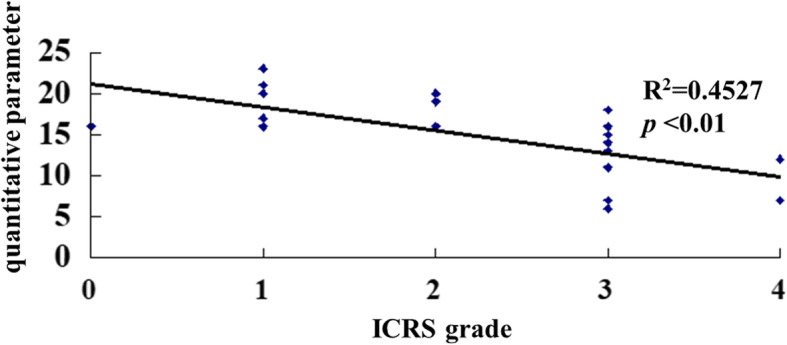
Scatter plot of the correlation between the Arthro-BST results and the ICRS classification. Each diamond represents the QP according to the ICRS grade in an individual hip. A significant strong correlation was observed between the Arthro-BST and the ICRS classification (R2 = 0.4527)
Relationship between the Arthro-BST and the histological assessment
Modified Mankin score: structure
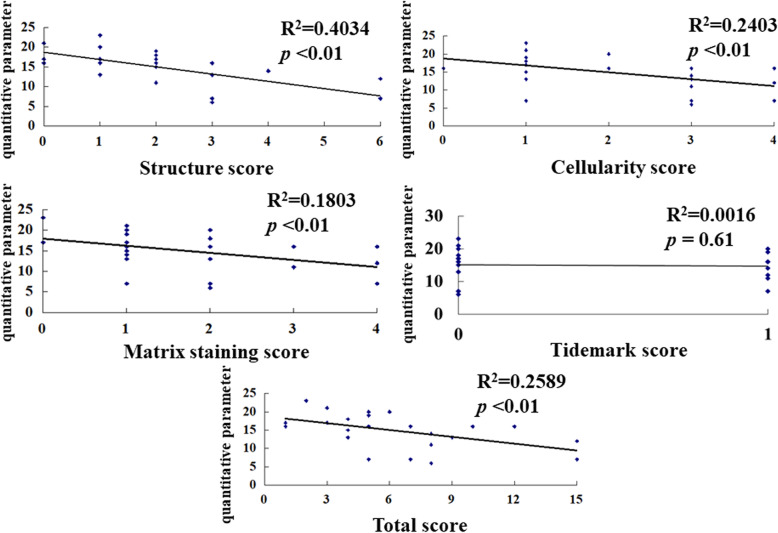
The values for the structure parameter of the modified Mankin score were 0 (n = 3), 1 (n = 8), 2 (n = 6), 3 (n = 5), 4 (n = 1), 5 (n = 0), and 6 (n = 2). The interobserver reliability of the score for structure was 0.877 (95% CI, 0.725–0.946) (Additional file 2). The QPs for the scores were as follows: score 0, 18 ± 2.6; score 1, 17.9 ± 3.3; score 2, 16 ± 2.6; score 3, 9.8 ± 4.4; score 4, 14; and score 6, 9.5 ± 3.5. The QP decreased as the structure score increased, and the correlation between these was significant (p < 0.01) (Fig. 4).
Fig. 4.
Scatter plot of the correlations between the Arthro-BST and the modified Mankin score. Each diamond represents the QP values for the structure, cellularity, matrix staining, tidemark, and total score in an individual hip. The Arthro-BST result correlated significantly with structure (R2 = 0.4034), cellularity (R2 = 0.2403), matrix staining (R2 = 0.1803), and total score (R2 = 0.2589)
Cellularity
The values for the cellularity parameter of the modified Mankin score were 0 (n = 1), 1 (n = 10), 2 (n = 5), 3 (n = 6), and 4 (n = 3). The interobserver reliability of the cellularity score was 0.9 (95% CI, 0.775–0.956) (Additional file 2). The QPs for the scores were as follows: score 0, 16; score 1, 16.7 ± 4.4; score 2, 17.6 ± 2.2; score 3, 11.1 ± 4; and score 4, 11.7 ± 4.5. The QP decreased as the cellularity score increased, and the correlation between these was significant (p < 0.01) (Fig. 4).
Matrix staining
The values for the matrix staining parameter of the modified Mankin score were 0 (n = 2), 1 (n = 10), 2 (n = 8), 3 (n = 2), and 4 (n = 3). The interobserver reliability for the matrix staining score was 0.874 (95% CI, 0.717–0.944) (Additional file 2). The QPs for the score were as follows: score 0, 20 ± 4.2; score 1, 15.9 ± 4; score 2, 14 ± 5; score 3, 13.5 ± 3.5; and score 4, 11.7 ± 4.5. The QP decreased as the matrix staining score increased, and the correlation between these was significant (p < 0.01) (Fig. 4).
Tidemark
The values for the tidemark parameter of the modified Mankin score were 0 (n = 15) and 1 (n = 10). The interobserver reliability for the tidemark score was 0.868 (95% CI, 0.704–0.942) (Additional file 2). The QPs for the scores were as follows: score 0, 15.1 ± 5.1; score 1, 14.7 ± 3.9. The correlation between the QP and the tidemark score was not significant (Fig. 4).
Total score
The interobserver reliability of the total score was 0.93 (95% CI, 0.844–0.969) (Additional file 2). The QP decreased as the total modified Mankin score increased, and the correlation between these was significant (p < 0.01) (Figs. 4 and 5).
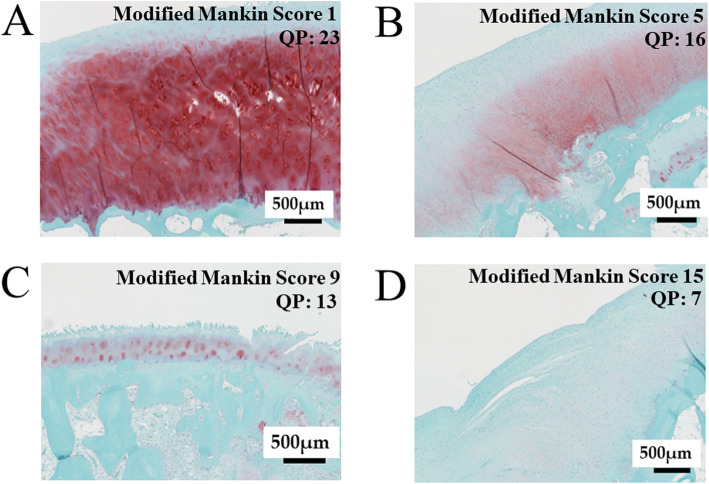
Fig. 5.
Histological assessment: representative Safranin O-stained sections for four parameters of the modified Mankin score and the corresponding QP. A The modified Mankin score of the cartilage section was 1 (structure, 1; cellularity, 0; matrix staining, 0; tidemark, 0; total, 1). The QP of the same point was 23. B The modified Mankin score of the cartilage section was 5 (structure, 0; cellularity, 2; matrix staining, 2; tidemark, 1; total, 5). The QP of the same point was 16. C The modified Mankin score of the cartilage section was 9 (structure, 4; cellularity, 3; matrix staining, 2; tidemark, 0; total, 9). The QP of the same point was 13. D The modified Mankin score of the cartilage section was 15 (structure, 6; cellularity, 4; matrix staining, 4; tidemark, 1; total, 15). The QP of the same point was 7
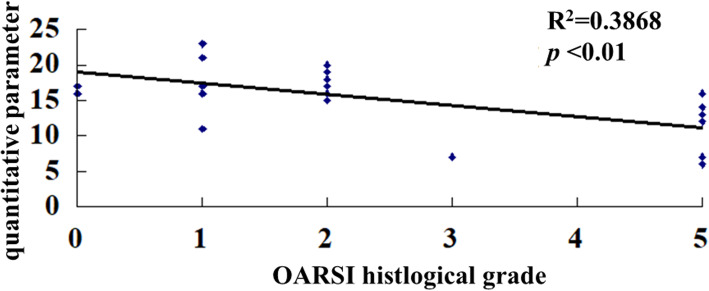
OARSI histological grade
The OARSI grades of cartilage lesions were as follows: grade 0 (n = 2), grade 1 (n = 6), grade 2 (n = 8), grade 3 (n = 1), grade 4 (n = 0), and grade 5 (n = 8). The interobserver reliability for the OARSI histological grade was 0.903 (95% CI, 0.782–0.957) (Additional file 3). The QPs for the grades were as follows: grade 0, 16.5 ± 0.7; grade 1, 17.3 ± 4.2; grade 2, 17.6 ± 1.9; grade 3, 7; and grade 4, 11 ± 3.8. The QP decreased as the OARSI grade increased, and the correlation between these was significant (p < 0.01) (Fig. 6).
Fig. 6.
Scatter plot of the correlation between the Arthro-BST results and the OARSI histopathological score. Each diamond represents the QP according to the OARSI grade in an individual hip. A significant correlation was observed between the Arthro-BST results and the OARSI histopathological score (R2 = 0.3868)
Discussion
Our results demonstrated that the Arthro-BST can distinguish between ICRS grades 1 and 3, between grades 1 and 4, between grades 2 and 3, and between grades 2 and 4. There was a significant correlation between the Arthro-BST QP and the ICRS grades as well as between the Arthro-BST QP and the modified Mankin scores (structure, cellularity, matrix staining, total score) and OARSI histological grade.
Various methods are used for the evaluation of cartilage degeneration. MRI has recently been used for the evaluation of OA. We have previously reported that Outerbridge grades correlate with MRI variables such as diffusion tensor imaging and T2 mapping [3]. Although the biggest advantage of MRI is its ability to assess cartilage degeneration noninvasively, it is not capable of assessing cartilage properties; hence, other methods that can evaluate cartilage properties without the need to sample cartilage tissue are needed.
Some studies have reported that the Arthro-BST QP correlates with the ICRS grade [29, 30, 34, 35] and Mankin score [29, 30]. Although the Arthro-BST could not distinguish ICRS grade 1 from 2 and grade 3 from 4 in our results, the QP correlated significantly with the ICRS grade. Similarly, Hadjab et al. [28] reported that the QP did not correlate significantly between ICRS grades 0 and 2, which seems reasonable because the ICRS grading system cannot distinguish small differences in cartilage damage [36] and because tissues affected by OA can have lesions with mixed grades of cartilage degeneration. We did not measure cartilage thickness in this study, and we found no significant differences between ICRS grades 1 and 2 or between grades 3 and 4. However, a comparison of the Arthro-BST results and the ICRS score is important. The Arthro-BST must be used under arthroscopy because the embedded sensors must be in direct contact with the cartilage. Although the Arthro-BST could not distinguish between ICRS grades 1 and 2 or between grades 3 and 4 in our study, we consider that this apparatus would be useful for hip OA because it can distinguish early OA (ICRS grade 1) from moderate OA (ICRS grade 2), early OA from severe OA (ICRS grade 3 or 4), and moderate OA from severe OA.
Cartilage tissue is classified into articular or hyaline cartilage and fibrous cartilage. Healthy articular cartilage comprises mainly hyaline cartilage, fibrous cartilage comprises mainly type I collagen, and hyaline cartilage comprises mainly type II collagen. Fibrous cartilage is frequently seen after injury and in degenerated cartilage tissue. The deposition of fibrous cartilage in place of articular cartilage is an inferior change that can lead to secondary OA. Distinguishing between fibrous and hyaline cartilage is difficult when using only the ICRS classification, but assessing the composition of regenerative tissue is necessary to prevent secondary OA. Chondrocyte proliferation appears during the early stages of OA [37], and the proteoglycan content decreases before collagen content decreases [37–39]. These changes induce fibrillation and fissures, which affect the mechanical strength of articular cartilage [40, 41]. Therefore, it is important to determine whether the Arthro-BST results correlate with the histological data.
As for the results of the histological assessment, some authors have reported that the QP correlates significantly with the total Mankin score [29, 30]. The Mankin score includes five parameters (structure, cellularity, matrix staining, tidemark, total score), and most reports have assessed only the QP and total score. We found that the total score and other parameters correlated significantly with the QP (Fig. 4). We have previously studied the effectiveness of LIPA [21]. LIPA is based on the use of photoacoustic waves and can assess viscoelastic properties without sampling cartilage tissue. We previously reported that the LIPA results correlated strongly with the ICRS grade, although we found no correlation between the LIPA results and the overall Mankin score [21]. Compared with LIPA, the Arthro-BST QP had stronger correlations with the total score as well as with structure, cellularity, and matrix staining. Therefore, we believe that the Arthro-BST is preferable to the LIPA for assessing cartilage degeneration.
Pritzker et al. [5] were the first to report the OARSI histological system. Some studies have reported excellent correlations between the Mankin score and the OARSI histological grade [42–46]. Compared with the modified Mankin score, the OARSI histological grade focuses more on structural parameters. The Mankin score does not have a staging component [42] and has limited usefulness for assessing mild and moderate OA [47]. In contrast, the OARSI system can distinguish between early and moderate OA [2]. Subchondral bone changes occur during the first stage of OA [48, 49], and these changes affect the pathogenesis of OA [50–53]. Finnilä et al. [54] reported a high correlation between the OARSI grade and subchondral plate thickness. Therefore, we used both histological scores in this study to assess each stage of OA accurately. We found a significant correlation between the QP and the OARSI histological grade and that the Arthro-BST was able to assess all grades of OA. Therefore, we believe that the Arthro-BST is useful for the simultaneous evaluation of macroscopic and cartilage properties. This device may also help in decision-making about the best method for cartilage regeneration and for assessing regenerating tissue.
There are some limitations to this study. First, some authors have already reported an association between the Arthro-BST results and the measures of OA. However, some of these authors have used cartilage from goats [31] or from human knees for evaluation [27–30]. To our knowledge, no studies have reported on the associations between the Arthro-BST results and OA measures in human cartilage of the hip. In addition, other authors have used human cartilage that underwent freeze–thaw cycles, which may have influenced the macroscopic appearance and histological properties of the cartilage tissue. Second, the assessments of the ICRS classification score, modified Mankin score, and OARSI histological score varied somewhat, which may have affected the results. However, the assessments were performed by two different evaluators, and all ICCs were > 0.7, which indicates high reproducibility.
Conclusions
The Arthro-BST can distinguish between ICRS grades 1 and 3, grades 1 and 4, grades 2 and 3, and grades 1 and 3. The Arthro-BST findings correlated with the macroscopic and histological assessment results. This apparatus may be helpful for performing macroscopic and histological assessments simultaneously, which may be useful for the noninvasive diagnosis of OA.
Supplementary Information
Additional file 1. Individual scoring data of the ICRS classification.
Additional file 2. Individual scoring data of the modified Mankin score.
Additional file 3. Individual scoring data of the OARSI histopathological system.
Acknowledgements
The authors wish to acknowledge the Support Center for Medical Research and Education of Tokai University Hospital for technical assistance. The authors wish to acknowledge Professor Hiroyuki Kobayashi for statistical analysis advice.
Abbreviations
- CI
Confidence interval
- ICC
Intraclass correlation coefficient
- ICRS
International Cartilage Repair Society
- LIPA
Laser-induced pulse acoustic
- MRI
Magnetic resonance imaging
- OA
Osteoarthritis
- OARSI
Osteoarthritis Research Society International
- QP
Quantitative parameter
Authors’ contributions
MS contributed to the conception and design of the study. TU, SW, and HO acquired and analyzed the data. TU and SW performed the orthopedic surgery and graded the cartilage damage. SW and TT operated the Arthro-BST. TU, SW, and TT contributed to the interpretation of the data. TU wrote the first version of the manuscript, and all other authors revised it critically for important intellectual content. All authors have read and approved the final manuscript. All authors agreed to be accountable for all aspects of the work.
Funding
This work was supported by a grant from the Japan Orthopaedics and Traumatology Research Foundation (no. 458).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was performed in accordance with the Declaration of Helsinki and its later amendments. The study was approved by the ethics committee of the Institutional Review Board (18R-187). Written informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Altman RD, Fries JF, Bloch DA, Carstens J, Cooke TD, Genant H, et al. Radiographic assessment of progression in osteoarthritis. Arthritis Rheum. 1987;30(11):1214–1225. doi: 10.1002/art.1780301103. [DOI] [PubMed] [Google Scholar]
- 2.Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS) J Bone Jt Surg Am. 2003;85-A(Suppl 2):45–57. doi: 10.2106/00004623-200300002-00007. [DOI] [PubMed] [Google Scholar]
- 3.Ukai T, Sato M, Yamashita T, Imai Y, Mitani G, Takagaki T, Serigano K, Mochida J. Diffusion tensor imaging can detect the early stages of cartilage damage: a comparison study. BMC Musculoskelet Disord. 2015;16(1):35. doi: 10.1186/s12891-015-0499-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53(3):523–537. doi: 10.2106/00004623-197153030-00009. [DOI] [PubMed] [Google Scholar]
- 5.Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, et al. Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil. 2006;14(1):13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 6.Ishihara M, Sato M, Sato S, Kikuchi T, Fujikawa K, Kikuchi M. Viscoelastic characterization of biological tissue by photoacoustic measurement. Jpn J Appl Phys. 2003;42(5B):L556–L558. doi: 10.1143/JJAP.42.L556. [DOI] [Google Scholar]
- 7.Ishihara M, Sato M, Sato S, Kikuchi T, Mitani G, Kaneshiro N, et al. Usefulness of the photoacoustic measurement method for monitoring the regenerative process of full-thickness defects in articular cartilage using tissue-engineering technology. Proc SPIE. 2005;5695. 10.1117/12.591890.
- 8.Ishihara M, Sato M, Sato S, Kikuchi T, Mochida J, Kikuchi M. Usefulness of photoacoustic measurements for evaluation of biomechanical properties of tissue-engineered cartilage. Tissue Eng. 2005;11(7–8):1234–1243. doi: 10.1089/ten.2005.11.1234. [DOI] [PubMed] [Google Scholar]
- 9.Ishihara M, Sato M, Ishihara M, Mochida J, Kikuchi M. Multifunctional evaluation of tissue engineered cartilage using nano-pulsed light for validation of regenerative medicine. In: Magjarevic R, Nagel JH, editors. World Congress on Medical Physics and Biomedical Engineering 2006. IFMBE Proceedings. Berlin: Springer; 2007. pp. 3319–3321. [Google Scholar]
- 10.Ishihara M, Sato M, Kaneshiro N, Mitani G, Sato S, Ishihara M, et al. Development of a noninvasive multifunctional measurement method using nanosecond pulsed laser for evaluation of regenerative medicine for articular cartilage. Proc SPIE. 2006;6084:60840V. doi: 10.1117/12.645888. [DOI] [Google Scholar]
- 11.Ishihara M, Sato M, Kaneshiro N, Mitani G, Nagai T, Kutsuna T, et al. Usefulness and limitation of measurement methods for evaluation of tissue-engineered cartilage function and characterization using nanosecond pulsed laser. Proc SPIE. 2007;6439:643909. doi: 10.1117/12.701489. [DOI] [Google Scholar]
- 12.Ishihara M, Sato M, Mitani G, Mochida J, Kikuchi M. Monitoring of extracellular matrix formation using nanosecond pulsed laser. J Inst Elect Engnr Jpn. 2007;127(12):2166–2170. [Google Scholar]
- 13.Ishihara M, Sato M, Mochida J, Kikuchi M. Measurement and image engineering for fundamental technology of regenerative medicine. In: Akaike T, editor. Regeneration medicine 4: bioengineering for regeneration medicine. Tokyo: Corona Publishing; 2007. pp. 147–167. [Google Scholar]
- 14.Ishihara M, Sato M, Mochida J, Kikuchi M. Noninvasive measurement for the evaluation and validation of regeneration medicine. J Biosci Biotechnol. 2007;85:438–441. [Google Scholar]
- 15.Ishihara M, Sato M, Kutsuna T, Ishihara M, Mochida J, Kikuchi M. Modification of measurement methods for evaluation of tissue engineered cartilage function and biochemical properties using nanosecond pulsed laser. Proc SPIE. 2008;6858:685805. doi: 10.1117/12.762481. [DOI] [Google Scholar]
- 16.Ishihara M, Sato M, Kaneshiro N, Mitani G, Sato S, Mochida J. Development of a photoacoustic measurement method for the evaluation of regenerative medicine and tissue engineering for articular cartilage. J Jpn Soc Laser Surg Med. 2005;26(1):53–59. doi: 10.2530/jslsm.26.53. [DOI] [Google Scholar]
- 17.Ishihara M, Sato M, Kaneshiro N, Mitani G, Sato S, Mochida J, Kikuchi M. Development of a diagnostic system for osteoarthritis using a photoacoustic measurement method. Lasers Surg Med. 2006;38(3):249–255. doi: 10.1002/lsm.20285. [DOI] [PubMed] [Google Scholar]
- 18.Kutsuna T, Sato M, Ishihara M, Furukawa KS, Nagai T, Kikuchi M, Ushida T, Mochida J. Noninvasive evaluation of tissue-engineered cartilage with time-resolved laser-induced fluorescence spectroscopy. Tissue Eng. 2010;16(3):365–373. doi: 10.1089/ten.tec.2009.0008. [DOI] [PubMed] [Google Scholar]
- 19.Changoor A, Fereydoonzad L, Yaroshinsky A, Buschmann MD. Effects of refrigeration and freezing on the electromechanical and biomechanical properties of articular cartilage. J Biomech Eng. 2010;132(6):064502. doi: 10.1115/1.4000991. [DOI] [PubMed] [Google Scholar]
- 20.Changoor A, Coutu JP, Garon M, Quenneville E, Hurtig MB, Buschmann MD. Streaming potential-based arthroscopic device is sensitive to cartilage changes immediately post-impact in an equine cartilage injury model. J Biomech Eng. 2011;133(6):061005. doi: 10.1115/1.4004230. [DOI] [PubMed] [Google Scholar]
- 21.Ukai T, Sato M, Ishihara M, Yokoyama M, Takagaki T, Mitani G, et al. Usefulness of using laser-induced photoacoustic measurement and 3.0 Tesla MRI to assess knee cartilage damage: a comparison study. Arthritis Res Ther. 2015;17:383. doi: 10.1186/s13075-015-0899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frank EH, Grodzinsky AJ. Cartilage electromechanics—1. Electrokinetic transduction and the effects of electrolyte pH and ionic strength. J Biomech. 1987;20(6):615–627. doi: 10.1016/0021-9290(87)90282-X. [DOI] [PubMed] [Google Scholar]
- 23.Buschmann MD, Grodzinsky AJ. A molecular model of proteoglycan-associated electrostatic forces in cartilage mechanics. J Biomech Eng. 1995;117(2):179–192. doi: 10.1115/1.2796000. [DOI] [PubMed] [Google Scholar]
- 24.Bonassar LJ, Jeffries KA, Paguio CG, Grodzinsky AJ. Cartilage degradation and associated changes in biochemical and electromechanical properties. Acta Orthop Scand Suppl. 1995;66(Suppl 266):38–44. doi: 10.3109/17453679509157645. [DOI] [PubMed] [Google Scholar]
- 25.Frank EH, Grodzinsky AJ, Koob TJ, Eyre DR. Streaming potentials: a sensitive index of enzymatic degradation in articular cartilage. J Orthop Res. 1987;5(4):497–508. doi: 10.1002/jor.1100050405. [DOI] [PubMed] [Google Scholar]
- 26.Légaré A, Garon M, Guardo R, Savard P, Poole AR, Buschmann MD. Detection and analysis of cartilage degeneration by spatially resolved streaming potentials. J Orthop Res. 2002;20(4):819–826. doi: 10.1016/S0736-0266(02)00002-5. [DOI] [PubMed] [Google Scholar]
- 27.Sim S, Chevrier A, Garon M, Quenneville E, Lavigne P, Yaroshinsky A, Hoemann CD, Buschmann MD. Electromechanical probe and automated indentation maps are sensitive techniques in assessing early degenerated human articular cartilage. J Orthop Res. 2017;35(4):858–867. doi: 10.1002/jor.23330. [DOI] [PubMed] [Google Scholar]
- 28.Hadjab I, Sim S, Karhula SS, Kauppinen S, Garon M, Quenneville E, Lavigne P, Lehenkari PP, Saarakkala S, Buschmann MD. Electromechanical properties of human osteoarthritic and asymptomatic articular cartilage are sensitive and early detectors of degeneration. Osteoarthr Cartil. 2018;26(3):405–413. doi: 10.1016/j.joca.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Sim S, Chevrier A, Garon M, Quenneville E, Yaroshinsky A, Hoemann CD, Buschmann MD. Non-destructive electromechanical assessment (Arthro-BST) of human articular cartilage correlates with histological scores and biomechanical properties. Osteoarthr Cartil. 2014;22(11):1926–1935. doi: 10.1016/j.joca.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 30.Sim S, Hadjab I, Garon M, Quenneville E, Lavigne P, Buschmann MD. Development of an electromechanical grade to assess human knee articular cartilage quality. Biomed Eng. 2017;45(10):2410–2421. doi: 10.1007/s10439-017-1879-4. [DOI] [PubMed] [Google Scholar]
- 31.Mickevicius T, Pockevicius A, Kucinskas A, Gudas R, Maciulaitis J, Noreikaite A, Usas A. Impact of storage conditions on electromechanical, histological and histochemical properties of osteochondral allografts. BMC Musculoskelet Disord. 2015;16(1):314. doi: 10.1186/s12891-015-0776-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Henson FMD, Vincent TA. Alterations in the vimentin cytoskeleton in response to single impact load in an in vitro model of cartilage damage in the rat. BMC Musculoskelet Disord. 2008;9(1):94. doi: 10.1186/1471-2474-9-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takaku Y, Murai K, Ukai T, Ito S, Kokubo M, Satoh M, Kobayashi E, Yamato M, Okano T, Takeuchi M, Mochida J, Sato M. In vivo cell tracking by bioluminescence imaging after transplantation of bioengineered cell sheets to the knee joint. Biomaterials. 2014;35(7):2199–2206. doi: 10.1016/j.biomaterials.2013.11.071. [DOI] [PubMed] [Google Scholar]
- 34.Abedian R, Willbold E, Becher C, Hurschler C. In vitro electro-mechanical characterization of human knee articular cartilage of different degeneration levels: a comparison with ICRS and Mankin scores. J Biomech. 2013;46(7):1328–1334. doi: 10.1016/j.jbiomech.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 35.Becher C, Ricklefs M, Willbold E, Hurschler C, Abedian R. Electromechanical assessment of human knee articular cartilage with compression-induced streaming potentials. Cartilage. 2016;7(1):62–69. doi: 10.1177/1947603515599191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spahn G, Klinger HM, Baums M, Pinkepank U, Hofmann GO. Reliability in arthroscopic grading of cartilage lesions: results of a prospective blinded study for evaluation of inter-observer reliability. Arch Orthop Trauma Surg. 2011;131(3):377–381. doi: 10.1007/s00402-011-1259-8. [DOI] [PubMed] [Google Scholar]
- 37.Pearle AD, Warren RF, Rodeo SA. Basic science of articular cartilage and osteoarthritis. Clin Sports Med. 2005;24(1):1–12. doi: 10.1016/j.csm.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 38.Lark MW, Bayne EK, Flanagan J, Harper CF, Hoerrner LA, Hutchinson NI, Singer II, Donatelli SA, Weidner JR, Williams HR, Mumford RA, Lohmander LS. Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest. 1997;100(1):93–106. doi: 10.1172/JCI119526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martel-Pelletier J, Boileau C, Pelletier JP, Roughley PJ. Cartilage in normal and osteoarthritis conditions. Best Pract Res Clin Rheumatol. 2008;22(2):351–384. doi: 10.1016/j.berh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 40.Poole AR, Kobayashi M, Yasuda T, Laverty S, Mwale F, Kojima T, et al. Type II collagen degradation and its regulation in articular cartilage in osteoarthritis. Ann Rheum Dis. 2002;61(Suppl 2):ii78–ii81. doi: 10.1136/ard.61.suppl_2.ii78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silver FH, Bradica G, Tria A. Elastic energy storage in human articular cartilage: estimation of the elastic modulus for type II collagen and changes associated with osteoarthritis. Matrix Biol. 2002;21(2):129–137. doi: 10.1016/S0945-053X(01)00195-0. [DOI] [PubMed] [Google Scholar]
- 42.Pauli C, Whiteside R, Heras FL, Nesic D, Koziol J, Grogan SP, Matyas J, Pritzker KPH, D’Lima DD, Lotz MK. Comparison of cartilage histopathology assessment systems on human knee joints at all stages of osteoarthritis development. Osteoarthr Cartil. 2012;20(6):476–485. doi: 10.1016/j.joca.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Custers RJ, Creemers LB, Verbout AJ, van Rijen MH, Dhert WJA, Saris DBF. Reliability, reproducibility and variability of the traditional Histologic/Histochemical Grading System vs the new OARSI Osteoarthritis Cartilage Histopathology Assessment System. Osteoarthr Cartil. 2007;15(11):1241–1248. doi: 10.1016/j.joca.2007.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Pearson RG, Kurien T, Shu KS, Scammell BE. Histopathology grading systems for characterisation of human knee osteoarthritis–reproducibility, variability, reliability, correlation, and validity. Osteoarthr Cartil. 2011;19(3):324–331. doi: 10.1016/j.joca.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Rout R, McDonnell S, Benson R, Athanasou N, Carr A, Doll H, Gill HS, Murray DW, Hulley PA, Price AJ. The histological features of anteromedial gonarthrosis—the comparison of two grading systems in a human phenotype of osteoarthritis. Knee. 2011;18(3):172–176. doi: 10.1016/j.knee.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 46.Waldstein W, Perino G, Gilbert SL, Maher SA, Windhager R, Boettner F. OARSI osteoarthritis cartilage histopathology assessment system: a biomechanical evaluation in the human knee. J Orthop Res. 2016;34(1):135–140. doi: 10.1002/jor.23010. [DOI] [PubMed] [Google Scholar]
- 47.Ostergaard K, Andersen CB, Petersen J, Bendtzen K, Salter DM. Validity of histopathological grading of articular cartilage from osteoarthritic knee joints. Ann Rheum Dis. 1999;58(4):208–213. doi: 10.1136/ard.58.4.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burr DB, Gallant MA. Bone remodelling in osteoarthritis. Nat Rev Rheumatol. 2012;8(11):665–673. doi: 10.1038/nrrheum.2012.130. [DOI] [PubMed] [Google Scholar]
- 49.Radin EL, Paul IL, Tolkoff MJ. Subchondral bone changes in patients with early degenerative joint disease. Arthritis Rheum. 1970;13(4):400–405. doi: 10.1002/art.1780130406. [DOI] [PubMed] [Google Scholar]
- 50.Burr DB. Anatomy and physiology of the mineralized tissues: role in the pathogenesis of osteoarthrosis. Osteoarthr Cartil. 2004;12(Suppl A):S20–S30. doi: 10.1016/j.joca.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 51.Goldring MB, Goldring SR. Articular cartilage and subchondral bone in the pathogenesis of osteoarthritis. Ann N Y Acad Sci. 2010;1192(1):230–237. doi: 10.1111/j.1749-6632.2009.05240.x. [DOI] [PubMed] [Google Scholar]
- 52.Castañeda S, Roman-Blas JA, Largo R, Herrero-Beaumont G. Subchondral bone as a key target for osteoarthritis treatment. Biochem Pharmacol. 2012;83(3):315–323. doi: 10.1016/j.bcp.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 53.Li G, Yin J, Gao J, Cheng TS, Pavlos NJ, Zhang C, Zheng MH. Subchondral bone in osteoarthritis: insight into risk factors and microstructural changes. Arthritis Res Ther. 2013;15(6):223. doi: 10.1186/ar4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finnilä MAJ, Thevenot J, Aho OM, Tiitu V, Rautiainen J, Kauppinen S, Nieminen MT, Pritzker K, Valkealahti M, Lehenkari P, Saarakkala S. Association between subchondral bone structure and osteoarthritis histopathological grade. J Orthop Res. 2017;35(4):785–792. doi: 10.1002/jor.23312. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Individual scoring data of the ICRS classification.
Additional file 2. Individual scoring data of the modified Mankin score.
Additional file 3. Individual scoring data of the OARSI histopathological system.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.