Abstract
Understanding how older people respond to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is critical if we are to confront the coronavirus disease 2019 (COVID-19) pandemic and establish effective vaccination strategies. Immunosenescence reduces the ability to respond to neoantigens and may compromise the life of infected individuals. Here, we analyzed the immunological memory to SARS-CoV-2 in 102 recovered patients aged over 60 years several months after the infection had been resolved. Specific memory T lymphocytes against the virus were measured by interferon-γ (IFN-γ) and granzyme B release by ELISpot; memory B-lymphocyte responses were quantified by detection of anti-S IgG1 producer cells by ELISpot and anti-S and anti-N antibodies were determined by enzyme-linked immunosorbent assay (ELISA). Memory T lymphocytes were found in peripheral blood of most of the studied donors, more than 7 months after the infection in some of them. Fewer patients maintained memory B lymphocytes, but antibodies, mainly anti-S, were highly durable and positively correlated with T responses. More robust humoral responses were found in patients who had more severe symptoms and had been admitted to hospital. We concluded that specific immunity against SARS-CoV-2 is effectively preserved regardless of age, despite the great heterogeneity of their immune responses, and that memory T lymphocytes and anti-S IgG might be more durable than memory B cells and anti-N IgG.
Keywords: COVID-19, memory B lymphocytes, memory T lymphocytes, specific antibodies
The emergence and rapid global expansion of the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and the resulting coronavirus disease 2019 (COVID-19) has been an example of what may happen when a new pathogen is introduced into a nonimmunized population. Since SARS-CoV-2 is a novel pathogen with no prior immune response, the entire population is susceptible to infection (1). Usually, the exposure to a pathogen or to its dominant antigens through infection and vaccination induces an immune response in the host and provides protection through the existence of immunological memory. In this context, generating a controlled and strong humoral and cellular memory that can not only resolve the infection effectively but also protect the patient from future encounters with the virus, would be extremely significant.
The maintenance of an effective immune memory response against SARS-CoV-2 over time has been a matter of considerable interest. Many group s have studied the antibody response against the virus and its kinetics (2–4). However, immunological memory depends not only on the maintenance of elevated antibody titers but also on the generation of memory T and B lymphocytes that can respond rapidly to the virus in future encounters. In fact, in a reinfection, specific memory B lymphocytes proliferate and differentiate into immunoglobulin (Ig)-secreting plasmablasts. Meanwhile, memory T CD4+ lymphocytes proliferate, regulate memory B-lymphocyte activation, and secrete cytokines such as interferon-γ (IFN-γ) (5), while memory T CD8+ lymphocytes secrete cytokines and kill virus-infected cells by releasing cytolytic molecules such as granzyme B and perforin (6,7). Some groups have studied the response of T cells to SARS-CoV-2 during the infection or in convalescent patients (6,8–12). Currently, descriptions of medium-term memory T-lymphocyte responses and antibody levels in recovered patients are emerging, but no special attention has been paid to older people, the population group most affected by the infection.
The efficacy of the immune memory could be reduced in older individuals in whom the adaptive response to new pathogens may be compromised due to the age-associated changes that take place in the immune system (13–16). The set of such changes, which affects the innate and adaptive immune branches, is collectively termed immunosenescence. It includes clonal expansion of T and B lymphocytes, compromised cytokine and specific antibody production, and a chronic low-grade inflammation called inflammaging (17,18). It has been proposed that the compromised immune system of older people may be unable to generate a strong immunological memory against the novel SARS-CoV-2 and vaccines might have a reduced ability to generate immune response (1,19). In light of the development of new vaccines against SARS-CoV-2, it is important to investigate whether older patients are able to maintain an effective memory response over time, so that we can predict their capacity to generate a strong immune memory after vaccination.
In the present study, we analyzed the cellular and humoral immunological memory against SARS-CoV-2 in 102 surviving patients over 60 years of age. We established that most of the patients maintained both types of response several months after infection resolution.
Patients and Methods
Study Participants
For this study, 102 volunteers with a polymerase chain reaction positive for SARS-CoV-2 were recruited by the Emergency Service of the Hospital Universitario Central de Asturias (Oviedo, Spain). Patients had been diagnosed with SARS-CoV-2 infection between 3.2 and 7.5 months before the study began. Peripheral blood samples were drawn from all participants for hematological and immunological analyses. Informed consent was obtained from all volunteers before they participated in the study. The study was approved by the ethics committee of the Hospital Central de Asturias (Oviedo, Spain) (nº 2020.269).
Isolation and Cell Culture
Peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood anticoagulated with ethylenediaminetetraacetic acid on Ficoll-Hypaque gradients (Lymphoprep; Nycomed, Oslo, Norway). Cultures were performed in RPMI 1640 medium supplemented with 10% fetal calf serum (ICN Flow, Costa Mesa, CA) and antibiotics. Cells were incubated at 37°C under 5% carbon dioxide. In some patients insufficient cells were isolated for it to be possible to perform all the proposed assays. Priority was given to analyzing the production of IFN-γ, then to memory B lymphocytes, and finally to granzyme B production. When there were not enough cells to quantify memory B lymphocytes, the release of granzyme B was studied since this requires fewer cells.
Immunophenotyping
For flow cytometry analysis isolated PBMCs were surface-stained with CD45-FITC/(CD56+CD16)-RD1/CD19-ECD/CD3-PC5 (Beckman-Coulter, Brea, CA). One hundred thousand cells were stained for 20 min at 4°C, washed twice in phosphate-buffered saline, and analyzed using Kaluza software in a Navios cytometer (Beckman-Coulter). Appropriate isotype control monoclonal antibodies were used for marker settings.
ELISpot Assay
To quantify IFN-γ- and granzyme B-producing T cells, PBMCs (2.5 × 105/well) were cultured for 18 hours on a filter plate (Millipore, Billerica, MA) previously coated with anti-IFN-γ or anti-granzyme B antibodies (15 μg/mL) (Mabtech, Nacka Strand, Sweden) and cultured in medium, in the presence of anti-CD3 (1 ng/mL), or of S1, S2, and N SARS-CoV-2 peptide pools (2 μg/mL) (Mabtech). IFN-γ or granzyme B captured by the plate-bound antibody was detected by biotinylated anti-IFN-γ or anti-granzyme B antibody (1 μg/mL) (Mabtech), followed by streptavidin-horseradish peroxidase (Streptavidin-HRP) (Mabtech). Spots were developed using tetramethylbenzidine (TMB) substrate (Mabtech) and counted with ImageJ software.
To quantify IgG1 anti-S-producing B lymphocytes, PBMCs were stimulated with interleukin-2 (10 ng/mL) and R-848 (1 μg/mL) (Mabtech) for 5 days. Cells were then washed in PBS and cultured for 18 hours at a concentration of 2.5 × 105/well in a filter plate that was either previously uncoated (negative control), or coated with anti-human-IgG (15 μg/mL) (Mabtech) or with conformational SARS-CoV-2 S protein (1 μg/mL) (Sino Biological, Wayne, PA). IgG1 was captured and detected in the same way as explained above for IFN-γ and granzyme B detection.
Quantification of Anti-SARS-CoV-2 S and N Antibodies
Levels of anti-SARS-CoV-2-specific IgG antibodies were determined with a Human anti-SARS-CoV2(S) IgG ELISA kit and a Human anti-SARS-CoV2(N) IgG ELISA kit (Fine Test, Wuhan, China), following the manufacturer’s specifications.
Statistical Analysis
Results are expressed as means and standard deviations (SDs). Correlations between variables were assessed using the Pearson test (r). Group differences for quantitative variables were compared using Student’s unpaired samples t test. Analyses were performed using PASW Statistics (IBM SPSS, NY, USA). Values of p < .05 were considered statistically significant. Images were created using GraphPad Prism 8.0.2.
Results
Features of Enrolled Donors
Patients were recruited a mean of 5.5 months after their SARS-CoV-2 infection, which had occurred between March and May 2020. All donors were more than 60 years old, with a mean age of 73.2 years (SD: 11.7 years), 58 (56.9%) were female and 44 (43.1%) were male. Sixty patients (58.8%) were asymptomatic or exhibited mild symptoms, whereas 42 (41.2%) required hospital admission, 13 (21.7%) of them to the intensive care unit (Table 1).
Table 1.
Characteristics of Participants (frequencies and, in parentheses, percentages), by Nonadmitted or Admitted to Hospital Group
| Nonadmitted (n = 60) | Admitted (n = 42) | |
|---|---|---|
| Age ± SD (years) | 72.7 ± 12.3 | 74.3 ± 10.8 |
| Gender | ||
| Male | 20 (33.3) | 24 (57.1) |
| Female | 40 (66.7) | 18 (42.9) |
| Risk factors | ||
| Hypertension | 28 (46.7) | 20 (47.6) |
| Diabetes mellitus type 2 | 14 (23.3) | 6 (14.3) |
| Asthma | 8 (13.3) | 3 (7.1) |
| COPD | 0 | 3 (7.1) |
| Cardiopathy | 14 (23.3) | 9 (21.4) |
| Smoking status | 3 (5.0) | 1 (2.4) |
| Drink status | 4 (6.7) | 5 (11.9) |
| Neoplasm | 0 | 3 (7.1) |
| Renal pathology | 4 (6.7) | 4 (9.5) |
| RNA copies ± SD (log) | 8.8 ± 9.58 | 8.2 ± 8.7 |
| Symptoms | ||
| Fever | 26 (43.3) | 29 (69.0) |
| Cough | 26 (43.3) | 30 (71.4) |
| Dyspnea | 10 (16.7) | 23 (54.8) |
| Ageusia | 10 (16.7) | 2 (4.8) |
| Anosmia | 11 (18.3) | 2 (4.8) |
| Mean days with symptoms (SD)* | 2.3 (2.8) | 7.6 (5.5) |
| Mean length of hospital stay (days) (SD) | NA | 28.0 (50.0) |
| ICU admission | NA | 13 (30.9) |
Notes: COPD = chronic obstructive pulmonary disease; ICU = intensive care unit; NA = not applicable; SD = standard deviation.
*Days of symptoms in hospital-admitted patients are those before admission to the hospital.
Quantification of Specific Memory T Lymphocytes Against SARS-Cov-2
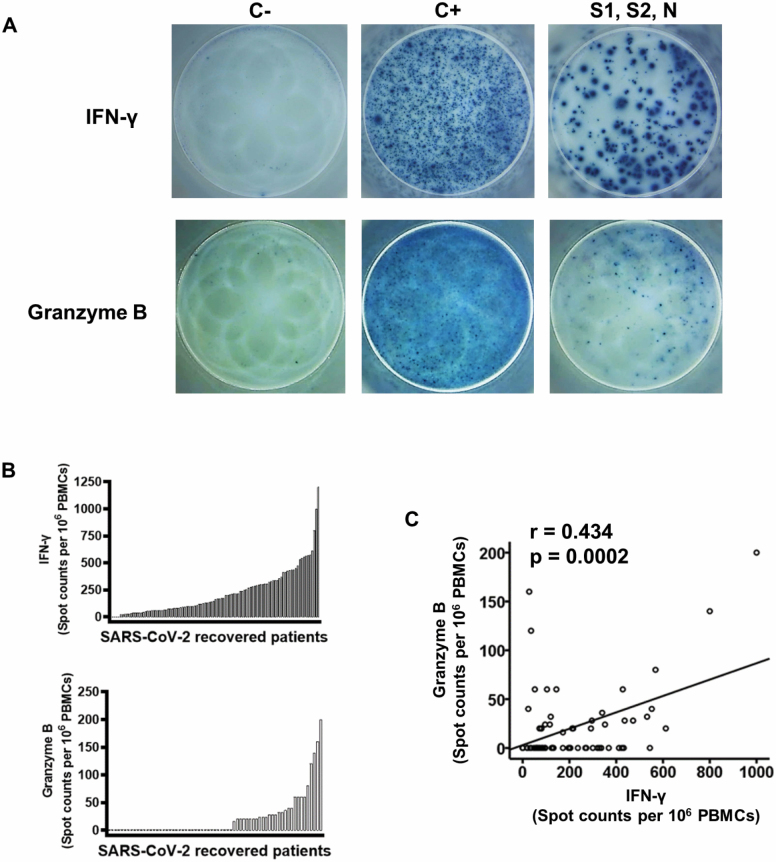
The ability to maintain an effective immune memory response against SARS-CoV-2 over time is a matter of considerable interest. The efficacy of such a response could be reduced in older individuals whose adaptive response to new pathogens may be compromised. To evaluate specific memory T lymphocytes against SARS-CoV-2, PBMCs were isolated from the recruited patients and cultured in the simultaneous presence of virus peptide pools of S1, S2, and N proteins, thereby to cover a broad range of putative antigens and to increase the level of detection. Anti-virus-specific T lymphocytes were quantified by an IFN-γ ELISpot assay (Figure 1A). No IFN-γ-producing cells were detected in 5 (4.9%) of the individuals tested. The frequency of specific T lymphocytes in patients in whom a response was detected ranged from 20 to 1200 per 106 PBMCs, with a mean of 229 cells per 106 PBMCs (SD ± 212 cells per 106 PBMCs). Individual responses of the 102 patients are shown in Figure 1B.
Figure 1.
Specific memory T-cell response to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 102 patients who had recovered from SARS-CoV-2 infection. (A) interferon-γ (IFN-γ) (n = 102) and granzyme B (n = 68) production in response to anti-CD3 (positive control, C+) and to SARS-CoV-2 were measured by ELISpot assay. Peripheral blood mononuclear cells (PBMCs) were stimulated with anti-CD3 (1 ng/mL) or SARS-Cov-2 peptides (S1, S2, and N peptide pools) (2 µg/mL) for 18 hours at 37°C. Examples of spots generated in negative controls (C−), positive controls (C+), and in the presence of SARS-CoV-2 are shown. (B) Histograms represent the number of antigen-specific spot-forming cells producing IFN-γ and granzyme B after background subtraction of control wells with no antigen in each patient. (C) Relationship between IFN-γ and granzyme B production. Pearson correlation coefficients and probabilities are shown in the upper left-hand corner.
In response to specific antigens, CD8+ T lymphocytes release cytotoxic proteins stored in cytoplasmic granules outside the cell. To quantify specific CD8+ T lymphocytes, granzyme B was also detected by ELISpot in response to the same SARS-CoV-2 peptide pools (Figure 1A). Although we found patients with granzyme B- and IFN-γ-producing cells, a large proportion of patients had no detectable granzyme B-releasing cells. Only 67 patients could be studied, since not enough cells could be isolated from 35 individuals to be able to perform granzyme B ELISpot. It is important to bear in mind that the patients not studied were significantly older (mean age = 77.1 years; SD = 11.8 years) than those who could be analyzed (mean age = 71.2 years; SD = 11.2 years) (Student’s t test, p = .014) and 25 (71.4%) of the patients excluded on this occasion had required hospitalization.
Granzyme B was not detected in 39 of the 67 (58.2%) patients studied, while a median of 51 cells per 106 PBMCs (range: 16–200, interquartile range: 40 per 106 PBMCs) was detected in the other 28 (41.8%) patients (Figure 1B). All 28 patients with a positive granzyme B ELISpot result also produced IFN-γ. Neither IFN-γ- nor granzyme B-producing cells were detected in another 3 patients (Supplementary Figure S1). There was a positive correlation between the amount of IFN-γ and the frequency of granzyme B T lymphocytes specific to SARS-CoV-2 peptides (Pearson test; r = 0.434, p = 2.4 × 10−4) (Figure 1C).
Quantification of Memory B-Lymphocyte Producers of Anti-S-IgG1
When naïve B lymphocytes meet their antigen, the immunoglobulin isotype switch operates and they differentiate into antibody-producing plasma cells and memory B lymphocytes. IgG1 is the most frequent IgG subclass produced against viral antigens. Memory B-lymphocyte producers of anti-S protein IgG1 were quantified. PBMCs were activated for 5 days in the presence of IL-2 (10 ng/mL) and R-848 (1 μg/mL), washed, and then cultivated in ELISpot plates for a further 18 hours. Wells were coated with conformational S protein (S1+S2), with anti-human-IgG in the positive control well and no coat in the negative control well. Again, the 20 patients in whom the ELISpot could not be performed were significantly older than those that could be studied (77.9 years, SD = 11.1 years vs 72.1 years, SD = 11.6 years; Student’s t test, p = .031). In this case, the proportions of patients requiring hospital admission were similar in the 2 groups.
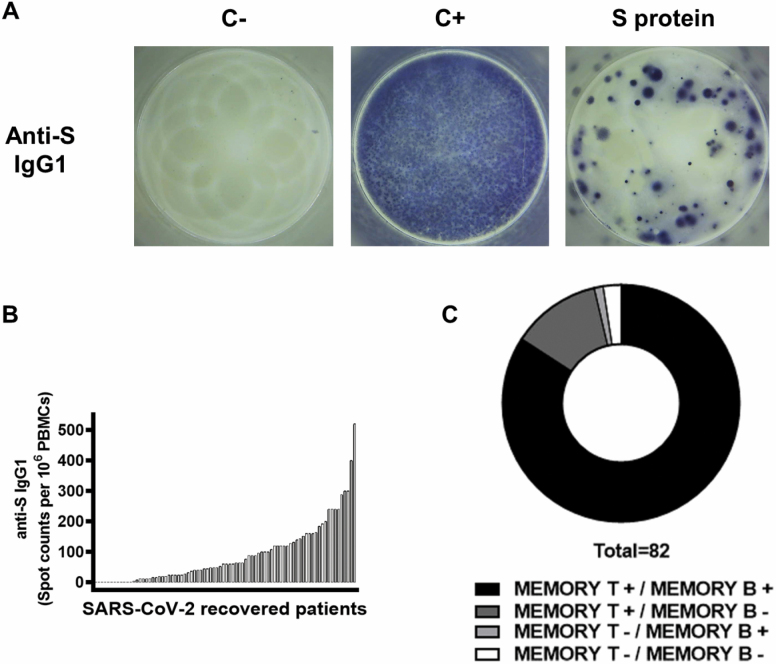
Memory B-lymphocyte producers of specific IgG1 anti-S protein were detected in 71 of the 82 (86.6%) patients tested (Figure 2A). The mean number of memory B lymphocytes in the responder patients was 104.5 cells per 106 (SD: 99.4 cells per 106, range: 4–520 cells per 106 PBMCs) (Figure 2B).
Figure 2.
Memory B-cell response specific to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 82 patients who had recovered from SARS-CoV-2 infection. (A) Anti-S IgG1-producing B cells were measured by ELISpot assay. Peripheral blood mononuclear cells (PBMCs) were stimulated with IL-2 (10 ng/mL) and R-848 (1 μg/mL) for 5 days and cultured on a plate coated with anti-human IgG (15 μg/mL; C+) or with conformational SARS-CoV-2 S protein (1 μg/mL) for 18 hours. Examples of spots generated in negative controls (C−), positive controls (C+), and in the presence of SARS-CoV-2 S protein are shown. (B) Histogram represents the number of antigen-specific spot-forming cells producing anti-S IgG1 after background subtraction of control wells with no antigen in each patient. (C) Distribution of patients according to their SARS-CoV-2-specific memory T and B lymphocytes.
The abundance of memory T, IFN-γ and granzyme B producers, was not correlated with the frequency of memory B lymphocytes in the patients (Supplementary Figure S2). IgG1 producer anti-S B lymphocytes were detected in one of the patients with no T-lymphocyte response, whereas 2 patients showed no cellular response, and solely memory T lymphocytes were detected in 10 cases (Figure 2C).
Serum Anti-S and Anti-N Antibody Titers
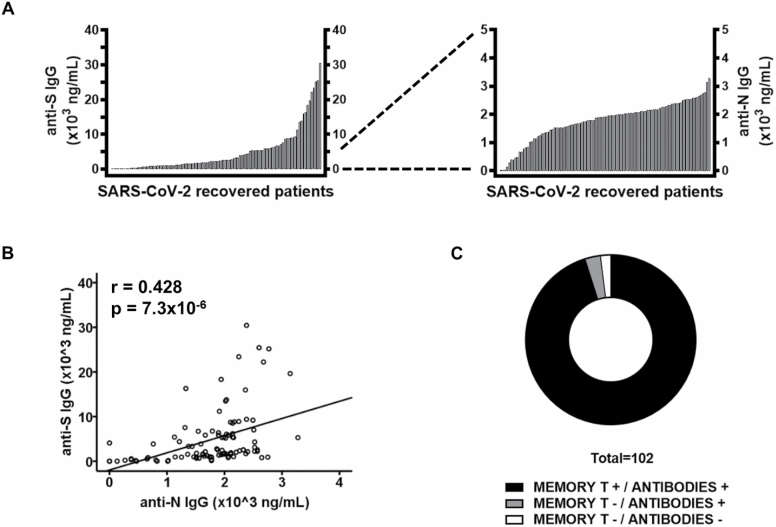
Antibody titers were measured in the serum of all 102 patients (Figure 3). Anti-S IgG antibodies were detected in 96 (94.1%) donors, whereas 6 were negative (5.9%). The mean titer was 5100 ng/mL (SD: 6336 ng/mL; range: 145–30 434 ng/mL) (Figure 3A). Anti-N IgG1 was detected in all but 3 (2.9%) individuals, but their antibody titers were significantly lower than those of anti-S, with a mean of 1812 ng/mL (SD: 634 ng/mL; range: 132–3276 ng/mL) (Figure 3A). The antibody titers against S and N proteins were positively correlated (Pearson test, r = 0.428, p = 7.3 × 10−6) (Figure 3B). Titers of both anti-S and anti-N antibodies were undetectable in 2 patients, who did not show a cellular response either. Both were completely asymptomatic. Moreover, 3 other patients produced anti-SARS-CoV-2 antibodies in the absence of a T-lymphocyte cellular response (Figure 3C).
Figure 3.
Anti-S and anti-N-specific antibody titers in 102 patients who had recovered from severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. (A) Serum titers of anti-S and anti-N IgG in 102 patients who had recovered from SARS-CoV-2. Data are shown as histograms. (B) The relationship between anti-S and anti-N IgG plasma titers was analyzed. Pearson correlation coefficients and probabilities are shown in the upper left-hand corner. (C) Distribution of patients according to their SARS-CoV-2-specific cellular and humoral memory.
Unexpectedly, anti-S memory B-lymphocyte levels were not related to anti-S antibody levels, but were significantly positively correlated with anti-N antibody titers (Pearson test, r = 0.294, p = .007) (Supplementary Figure S3A and B). Conversely, the frequency of memory IFN-γ–T lymphocytes that responded to the mixed S1, S2, and N peptide pool was associated with levels of antibodies against both proteins S (Pearson test, r = 0.202, p = .041) and N (Pearson test; r = 0.338, p = .001) (Supplementary Figure S3C and D).
Relationship Between Disease Severity and Duration of the Immune Response
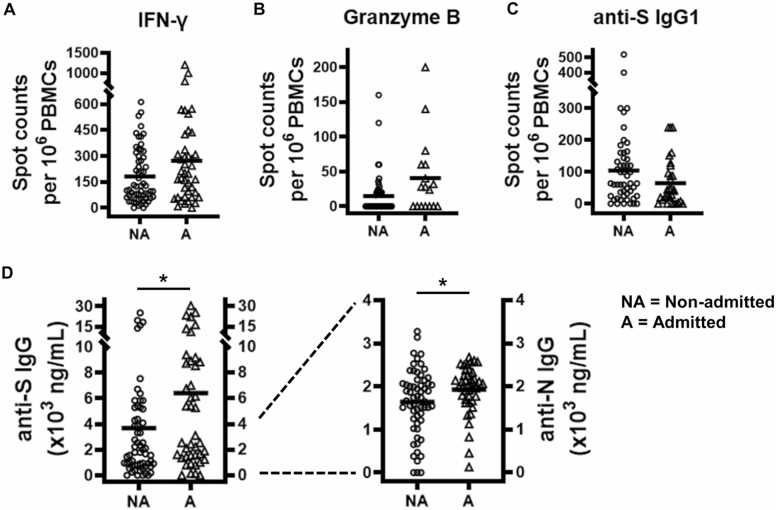
The magnitude of the humoral and specific T-lymphocyte response has been reported to be higher in patients with more severe symptoms (4,8,20). To examine the effect of disease severity on maintaining these responses over time in older patients, we compared the characteristics of those who were seriously ill (requiring hospital admission) with those of patients who were asymptomatic or had mild symptoms (not requiring hospitalization). The total number of T and B lymphocytes in ELISpot cultures did not differ between the 2 groups (Supplementary Figure S4). Differences in the frequency of SARS-CoV-2-specific T and B lymphocytes were not significant between admitted and nonadmitted patients (Figure 4A–C). Moreover, there was a trend toward higher and lower frequencies of T and B responder lymphocytes, respectively, in patients with more severe disease. Anti-S and anti-N antibody titers were significantly elevated in patients who required admission compared with those who were not admitted (mean: 3674 ng/mL, SD: 5076 ng/mL vs mean: 6410 ng/mL, SD: 7418 ng/mL, anti-S; mean: 1641 ng/mL, SD: 764 ng/mL vs 1928 ng/mL, SD: 551 ng/mL) (Student’s t test, p = .042 and p = .03, respectively).
Figure 4.
Cellular and humoral memory to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in 102 patients who had recovered from SARS-CoV-2 infection in nonadmitted (NA) and hospital-admitted (A) patients. Scatter plots show the number of antigen-specific spot-forming cells producing (A) interferon-γ (IFN-γ), (B) granzyme B, or (C) anti-S IgG1. (D) Serum titers of anti-S and anti-N IgG in nonadmitted (NA) and hospital-admitted (A) patients. Data are shown as scatter plots; horizontal lines indicate mean values. Significant differences between groups are indicated (Student’s unpaired samples t test). *p < .05.
We also studied the association of other related parameters with the response to virus infection. Age, gender, time since infection, and number of SARS-Cov-2 RNA copies showed no significant association with any of the immune characteristics analyzed (Supplementary Figure S5).
Discussion
In this study, we have measured the immunological memory to SARS-CoV-2 in recovered patients older than 60 years several months after the resolution of the infection. Our results show that, even at advanced ages, an immunological memory to this virus had been properly developed and remained active in most of the patients months after they had recovered from the infection.
Several studies have already demonstrated that the immune response to SARS-CoV-2 may last for several months, but none has considered this specifically in older individuals. It is essential to study this group of people, on the one hand, to help predict the duration of immunization after infection or vaccination, and on the other, to identify possible alterations in their specific responses to the virus that could be related to greater susceptibility to infection. Since the beginning of the SARS-CoV-2 pandemic, older age has been recognized as the main risk factor for severe illness, although some pathologies, such as diabetes, cardiovascular diseases, and obesity, are also associated with greater severity and mortality of COVID-19 (21). Older age is a risk not only in itself, but also because of the greater prevalence of many comorbidities in this group, including some of these mentioned above, which probably contribute to the high mortality and severity of symptoms more frequently noted in older people. Several changes take place in the immune system over time that may cause it to function poorly later in the life of some individuals. These changes affect the innate and the adaptive immune responses. Collectively, they contribute to the process of immunosenescence (13–16), and may influence the effectiveness of the response and thereby a person’s capacity to resolve a SARS-CoV-2 infection (22). Immunosenescence also affects responses to vaccination in older people (23), which may compromise the effectiveness of SARS-CoV-2 vaccines.
The importance of the cellular immune response in resolving viral infections and generating long-term immunity to the virus has prompted some groups, including ours, to study the B and T cellular memory to SARS-CoV-2. To characterize the immunological memory to SARS-CoV-2 in older people, we examined T- and B-specific lymphocytes against the virus. Some studies have already described medium-term immunological memory to SARS-CoV-2 for a wide range of patient ages, but no attention has previously been paid specifically to older people (24–26). Our results show that specific responses to SARS-CoV-2 are also persistent, to varying degrees, in almost all the older people studied, for up to 7 months in some cases. Moreover, we found the levels of T-lymphocyte response to be similar to those noted by Zuo et al in individuals younger than 65 years of age (12). Similar findings were also described by Peng et al in individuals between 45 and 75 years (8) and by Sherina et al in individuals between 29 and 89 years of age (26). It is not surprising that young people develop effective protection. In fact, it has been previously reported that specific memory T cells to SARS, the coronavirus most closely related to SARS-CoV-2, remain detectable in convalescent patients 17 years after the SARS epidemics (27). These findings are encouraging for older people, since there has been considerable doubt about the ability of the immune system to develop a memory of the virus. However, it must be borne in mind that all the participants analyzed in our study survived their SARS-CoV-2 infection and that the development and effectiveness of the immune response to SARS-CoV-2 might be more restricted in nonsurviving patients (28–31).
Our results show a robust T-lymphocyte response, mainly represented by IFN-γ-producing cells, whereas cytotoxic memory CD8+ T lymphocytes had been detected to a lesser extent and in fewer patients in response to stimulation with SARS-CoV-2 peptides. Other groups have reported findings that are consistent with ours, and the responses of CD4+ T cells to SARS-CoV-2 seem to be more noteworthy than those of CD8+ T cells (32,33). These differences might be more pronounced in older people since important changes may have occurred in the CD8+ T-lymphocyte compartment. With thymus involution and repeated encounters with antigens throughout life, accumulation of memory and reduction in naïve CD8+ T lymphocytes are typical features of an immunosenescent immune system (13). These changes are also observed in CD4+ T lymphocytes with age, but in a much slower and less pronounced fashion. Reduction of naïve CD8+ T-lymphocyte capacity to generate responses to new antigens may partially account for the underrepresentation of memory CD8+ T lymphocytes, although the number of cytotoxic cells is very closely correlated with that of IFN-γ-producing T lymphocytes. Additionally, the presence of SARS-CoV-2-specific CD4+ T lymphocytes is more strongly associated with lower COVID-19 severity than are the antibodies and CD8+ T cells (22). In parallel, the absence of SARS-CoV-2-specific CD4+ T cells is associated with severe or fatal COVID-19 (22,34,35), but the importance of these memory T cells and their possible role in future reinfections with SARS-CoV-2 are currently unknown and therefore require further study.
Specific memory B cells against SARS-CoV-2 have been detected in SARS-CoV-2-recovered patients up to 6–8 months after the virus infection (26). The detection of anti-S IgG1, a subclass that represents one of the predominant antibodies produced during viral infections, showed that, even in older people, memory B cells remain present in a large proportion of recovered patients. However, they might be less durable than memory T lymphocytes. This leads us to recall what happens in SARS-recovered patients, whose specific memory B response lasts for a significantly shorter time compared with T lymphocytes (36). Moreover, very low levels of memory B lymphocytes may be produced in older people. B lymphocytes are produced in the bone marrow and there is a reduction in lymphoid precursors with age that also affects T lymphocytes, causing a drop in the absolute number of early B-cell progenitors and generating a compartment rich in memory B lymphocytes in older age (14). The number of plasma cells does not increase, and the percentage of switched memory B cells, the predictors of optimal antibody responses, also decreases with age, as does the production of low-affinity antibodies (37,38).
We did not find any association between anti-S IgG1 producer B lymphocytes and anti-S IgG antibodies in serum. At first glance, the absence of such a correlation may seem puzzling, but it must be remembered that the 2 IgGs have different origins: the former are produced by the memory B lymphocytes, while the latter are secreted by the bone marrow-resident plasmatic cells. In fact, Turner et al have recently described a robust antigen-specific, long-lived humoral immune memory in convalescent individuals who had experienced mild SARS-CoV-2 infections, with anti-S antibody titers correlating the frequency of S-specific plasma cells in bone marrow aspirates (39). We must also consider that other subclasses of IgG may be detected, since high levels mainly of IgG1 but also of IgG3 are produced in response to the virus. Unlike SARS-CoV-2-specific memory B cells, which have been little studied, the antibodies generated against this virus have already been analyzed in depth. The best studied of these are the ones that react against S and N proteins. Anti-N and anti-S IgG titers are highly correlated in SARS-CoV-2-infected patients (20). Here we corroborate that this also happens in older people who have recovered from the infection. Antibodies were detected in most patients, but the levels differed markedly between donors. Related to this, it has been reported that although the SARS-CoV-2-specific T-cell response remains stable after the resolution of the infection, antibody titers against the virus decrease (40). Many studies have shown how a substantial proportion of COVID-19-recovered patients do not maintain high levels of circulating SARS-CoV-2-neutralizing antibodies (4,25,41). However, antibodies to SARS-CoV-2 were detected in recovered patients 8 months after their infection had resolved (25,26), and SARS-neutralizing antibodies could also be detected in recovered patients for up to 17 years after the end of the SARS epidemics (42). Some factors may affect the levels and duration of anti-SARS-Cov-2 antibodies and cellular responses. When we classified patients according to the severity of their disease, we found that those who had a SARS-CoV-2 infection with more severe clinical manifestations had a stronger response. This is consistent with what happens in other coronavirus infections, such as SARS and Middle East respiratory syndrome, in which patients with more severe symptoms had higher levels of specific memory T cells against the virus (36,43). In fact, other researchers have described neutralizing antibody titers and total anti-S-specific antibody titers correlates with COVID-19 disease severity (4,20,28), and stronger SARS-CoV-2-specific T-cell responses have been found in patients who have recovered from severe COVID-19 (8,44). Patients with worse disease evolution might have an inadequate early specific response, whereby they cannot control the spread of the virus into the organism. Delayed viral clearance could be responsible for the more intensive memory response, together with an excessive inflammation, which could be a compensatory mechanism. Although increased numbers of SARS-CoV-2-specific memory B cells have been found in hospitalized cases after the recovery from the infection (25), we did not find this association, once again possibly due to a defect in the ability to generate memory B lymphocytes in these older patients. Moreover, none of the variables analyzed were influenced by age, sex, RNA copy number, or time since infection. These results do not suggest that the humoral response decline over time, as other authors have proposed (40). Instead, it may be that the reduction in the antibody titer had slowed down during the first months after the infection had resolved, and all the patients had been cured between 3.2 and 7 months before their inclusion in the study.
These differences in time since infection from patient to patient may be considered a limitation of the study, although time does not seem to be a determining factor. However, the main limitation of our study was the availability of donor cells, which meant that not all parts of the study could be carried out in all patients, thereby limiting the information obtained and the conclusions that we can draw with confidence.
Nevertheless, our results show that specific immunity against SARS-CoV-2 develops regardless of age, even though the immune responses among these patients are highly variable. These findings suggest that memory to SARS-CoV-2 is maintained and that vaccines might also be able to trigger the development of effective immunological memory in older people. More studies are needed to understand the long-term immunity to SARS-CoV-2, the immunological response to SARS-CoV-2 vaccines and the influence of age on the development of this immunity.
Supplementary Material
Acknowledgments
The authors are grateful to the Instituto de Salud Carlos III for supporting the study and to the donors for their selfless contribution to help us better understand immunity to SARS-CoV-2 infection.
Funding
This research was partially supported by the Instituto de Salud Carlos III (COV20/00968) and by grant PI17/00714 from the Spanish I+D+i 2013–2016 State Program, which was cofounded by the Instituto de Salud Carlos III and the European Regional Development Fund (ERDF). E.B.-G. is sponsored by the Instituto de Investigación Sanitaria del Principado de Asturias (ISPA).
Conflict of Interest
None declared.
Author Contributions
The authors’ responsibilities were as follows: R.A.-A., M.A.M.-G., and S.A.-A. designed the study; A.G.-T., E.B.-G., R.L.-M., and B.R. prepared protocols, collected and processed all the samples, performed or oversaw the experimental protocols, and analyzed data; R.A.-A. and E.B.-G. wrote the manuscript; A.L.-G., A.S.-F., M.F.-G., L.C.-R., C.C.-C., A.B.P.-F., A.A.-R., C.M.-P., L.M.-P., N.G.-A., E.F.-D., A.F.-L., A.F.-M., C.F.-I., J.A.R., C.P.-F., E.U.-R., M.D.-F., H.M.-M., and P.H.-P. selected and recruited volunteers and organized their blood extractions and collected their clinical information; M.A.M.-G. and S.A.-A. reviewed the manuscript.
References
- 1.Chen Y, Klein SL, Garibaldi BT, et al. . Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65:101205. doi: 10.1016/j.arr.2020.101205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seow J, Graham C, Merrick B, et al. . Longitudinal observation and decline of neutralizing antibody responses in the three months following SARS-CoV-2 infection in humans. Nat Microbiol. 2020;5:1598–1607. doi: 10.1038/s41564-020-00813-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Röltgen K, Powell AE, Wirz OF, et al. . Defining the features and duration of antibody responses to SARS-CoV-2 infection associated with disease severity and outcome. Sci Immunol. 2020;5(54):abe0240. doi: 10.1126/sciimmunol.abe0240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robbiani DF, Gaebler C, Muecksch F, et al. . Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruterbusch M, Pruner KB, Shehata L, Pepper M. In vivo CD4+ T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu Rev Immunol. 2020;38:705–725. doi: 10.1146/annurev-immunol-103019-085803 [DOI] [PubMed] [Google Scholar]
- 6.Rodda LB, Netland J, Shehata L, et al. . Functional SARS-CoV-2-specific immune memory persists after mild COVID-19. Cell. 2021;184:1–15. doi: 10.1016/j.cell.2020.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt ME, Varga SM. The CD8 T cell response to respiratory virus infections. Front Immunol. 2018;9:678. doi: 10.3389/fimmu.2018.00678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng Y, Mentzer AJ, Liu G, et al. ; Oxford Immunology Network Covid-19 Response T cell Consortium; ISARIC4C Investigators. Broad and strong memory CD4+ and CD8+ T cells induced by SARS-CoV-2 in UK convalescent individuals following COVID-19. Nat Immunol. 2020;21(11):1336–1345. doi: 10.1038/s41590-020-0782-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ni L, Ye F, Cheng ML, et al. . Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity. 2020;52(6):971–977.e3. doi: 10.1016/j.immuni.2020.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neidleman J, Luo X, Frouard J, et al. . SARS-CoV-2-specific T cells exhibit phenotypic features of helper function, lack of terminal differentiation, and high proliferation potential. Cell Rep Med. 2020;1(6):100081. doi: S2666-3791(20)30102-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weiskopf D, Schmitz KS, Raadsen MP, et al. . Phenotype and kinetics of SARS-CoV-2-specific T cells in COVID-19 patients with acute respiratory distress syndrome. Sci Immunol. 2020;5(48):1–14. doi: 10.1126/SCIIMMUNOL.ABD2071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zuo J, Dowell A, Pearce H, et al. . Robust SARS-CoV-2-specific T-cell immunity is maintained at 6 months following primary infection. Nat Immunol. 2021;22(5):620–626. doi: 10.1038/s41590-021-00902-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moro-García MA, Alonso-Arias R, López-Vázquez A, et al. . Relationship between functional ability in older people, immune system status, and intensity of response to CMV. Age (Dordr). 2012;34:479–495. doi: 10.1007/s11357-011-9240-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciabattini A, Nardini C, Santoro F, Garagnani P, Franceschi C, Medaglini D. Vaccination in the elderly: the challenge of immune changes with aging. Semin Immunol. 2018;40:83–94. doi: 10.1016/j.smim.2018.10.010 [DOI] [PubMed] [Google Scholar]
- 15.Alpert A, Pickman Y, Leipold M, et al. . A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat Med. 2019;25(3):487–495. doi: 10.1038/s41591-019-0381-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weinberger B, Herndler-Brandstetter D, Schwanninger A, Weiskopf D, Grubeck-Loebenstein B. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis. 2008;46(7):1078–1084. doi: 10.1086/529197 [DOI] [PubMed] [Google Scholar]
- 17.Akbar AN, Henson SM, Lanna A. Senescence of T lymphocytes: implications for enhancing human immunity. Trends Immunol. 2016;37(12):866–876. doi: 10.1016/j.it.2016.09.002 [DOI] [PubMed] [Google Scholar]
- 18.Fulop T, Larbi A, Dupuis G, et al. . Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol. 2017;8:1960. doi: 10.3389/fimmu.2017.01960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pietrobon AJ, Teixeira FME, Sato MN. Immunosenescence and inflammaging: risk factors of severe COVID-19 in older people. Front Immunol. 2020;11:579220. doi: 10.3389/fimmu.2020.579220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piccoli L, Park YJ, Tortorici MA, et al. . Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell. 2020;183(4):1024–1042.e21. doi: 10.1016/j.cell.2020.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banerjee A, Pasea L, Harris S, et al. . Estimating excess 1-year mortality associated with the COVID-19 pandemic according to underlying conditions and age: a population-based cohort study. Lancet. 2020;395(10238):1715–1725. doi: 10.1016/S0140-6736(20)30854-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rydyznski Moderbacher C, Ramirez SI, Dan JM, et al. . Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183(4):996–1012.e19. doi: 10.1016/j.cell.2020.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tu W, Rao S. Mechanisms underlying T cell immunosenescence: aging and cytomegalovirus infection. Front Microbiol. 2016;7(Dec):2111. doi: 10.3389/fmicb.2016.02111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breton G, Mendoza P, Hägglöf T, et al. . Persistent cellular immunity to SARS-CoV-2 infection. J Exp Med. 2021;218(4):e20202515. doi: 10.1101/2020.12.08.416636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dan JM, Mateus J, Kato Y, et al. . Immunological memory to SARS-CoV-2 assessed for up to eight months after infection. Science (80-). 2021;371(6529):eabf4063. doi: 10.1126/science.abf4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sherina N, Piralla A, Du L, et al. . Persistence of SARS-CoV-2 specific B- and T-cell responses in convalescent COVID-19 patients 6–8 months after the infection. Med. 2021;2(3):281–295.e4. doi: 10.1016/j.medj.2021.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Bert N, Tan AT, Kunasegaran K, et al. . SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584(7821):457–462. doi: 10.1038/s41586-020-2550-z [DOI] [PubMed] [Google Scholar]
- 28.Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184(4):861–880. doi: 10.1016/j.cell.2021.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oja AE, Saris A, Ghandour CA, et al. . Divergent SARS-CoV-2-specific T- and B-cell responses in severe but not mild COVID-19 patients. Eur J Immunol. 2020;50(12):1998–2012. doi: 10.1002/eji.202048908 [DOI] [PubMed] [Google Scholar]
- 30.Szabo PA, Dogra P, Gray JI, et al. . Analysis of respiratory and systemic immune responses in COVID-19 reveals mechanisms of disease pathogenesis. medRxiv. Published online October 18, 2000. doi: 10.1101/2020.10.15.20208041 [Google Scholar]
- 31.Liao M, Liu Y, Yuan J, et al. . Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med. 2020;26(6):842–844. doi: 10.1038/s41591-020-0901-9 [DOI] [PubMed] [Google Scholar]
- 32.Sekine T, Perez-Potti A, Rivera-Ballesteros O, et al. ; Karolinska COVID-19 Study Group . Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell. 2020;183(1):158–168.e14. doi: 10.1016/j.cell.2020.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grifoni A, Weiskopf D, Ramirez SI, et al. . Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7):1489–1501.e15. doi: 10.1016/j.cell.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braun J, Loyal L, Frentsch M, et al. . SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587(7833):270–274. doi: 10.1038/s41586-020-2598-9 [DOI] [PubMed] [Google Scholar]
- 35.Tan AT, Linster M, Tan CW, et al. . Early induction of functional SARS-CoV-2-specific T cells associates with rapid viral clearance and mild disease in COVID-19 patients. Cell Rep. 2021;34(6):108728. doi: 10.1016/j.celrep.2021.108728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang F, Quan Y, Xin ZT, et al. . Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011;186(12):7264–7268. doi: 10.4049/jimmunol.0903490 [DOI] [PubMed] [Google Scholar]
- 37.Gibson KL, Wu YC, Barnett Y, et al. . B-cell diversity decreases in old age and is correlated with poor health status. Aging Cell. 2009;8(1):18–25. doi: 10.1111/j.1474-9726.2008.00443.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frasca D, Landin AM, Lechner SC, et al. . Aging down-regulates the transcription factor E2A, activation-induced cytidine deaminase, and Ig class switch in human B cells. J Immunol. 2008;180(8):5283–5290. doi: 10.4049/jimmunol.180.8.5283 [DOI] [PubMed] [Google Scholar]
- 39.Turner JS, Kim W, Kalaidina E, et al. . SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature 2021;595(7867):421–425. doi: 10.1038/s41586-021-03647-4 [DOI] [PubMed] [Google Scholar]
- 40.Bonifacius A, Tischer-Zimmermann S, Dragon AC, et al. . COVID-19 immune signatures reveal stable antiviral T cell function despite declining humoral responses. Immunity. 2021;54(2):340–354.e6. doi: 10.1016/j.immuni.2021.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wajnberg A, Amanat F, Firpo A, et al. . Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370(6521):1227–1230. doi: 10.1126/science.abd7728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tan CW, Chia WN, Qin X, et al. . A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat Biotechnol. 2020;38(9):1073–1078. doi: 10.1038/s41587-020-0631-z [DOI] [PubMed] [Google Scholar]
- 43.Sariol A, Perlman S. Lessons for COVID-19 immunity from other coronavirus infections. Immunity. 2020;53(2):248–263. doi: 10.1016/j.immuni.2020.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim DS, Rowland-Jones S, Gea-Mallorquí E. Will SARS-CoV-2 infection elicit long-lasting protective or sterilising immunity? Implications for vaccine strategies (2020). Front Immunol. 2020;11:571481. doi: 10.3389/fimmu.2020.571481 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.