Abstract
Background
The Recipient Epidemiology and Donor Evaluation Study-IV-Pediatric (REDS-IV-P) Epidemiology, Surveillance and Preparedness of the Novel SARS-CoV-2 Epidemic (RESPONSE) seroprevalence study conducted monthly cross-sectional testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibodies in blood donors in 6 US metropolitan regions to estimate the extent of SARS-CoV-2 infections over time.
Methods
During March–August 2020, approximately ≥1000 serum specimens were collected monthly from each region and tested for SARS-CoV-2 antibodies using a well-validated algorithm. Regional seroprevalence estimates were weighted based on demographic differences compared with the general population. Seroprevalence was compared with reported coronavirus disease 2019 (COVID-19) case rates over time.
Results
For all regions, seroprevalence was <1.0% in March 2020. New York, New York, experienced the biggest increase (peak seroprevalence, 15.8% in May). All other regions experienced modest increases in seroprevalence (1%–2% in May–June to 2%–4% in July–August). Seroprevalence was higher in younger, non-Hispanic black, and Hispanic donors. Temporal increases in donor seroprevalence correlated with reported case rates in each region. In August, 1.3–5.6 estimated cumulative infections (based on seroprevalence data) per COVID-19 case were reported to the Centers for Disease Control and Prevention.
Conclusions
Increases in seroprevalence were found in all regions, with the largest increase in New York. Seroprevalence was higher in non-Hispanic black and Hispanic than in non-Hispanic white blood donors. SARS-CoV-2 antibody testing of blood donor samples can be used to estimate the seroprevalence in the general population by region and demographic group. The methods derived from the RESPONSE seroprevalence study served as the basis for expanding SARS-CoV-2 seroprevalence surveillance to all 50 states and Puerto Rico.
Keywords: COVID-19, COVID-19 srological testing, SARS-CoV-2, seroprevalence
SARS-CoV-2 serosurveillance of blood donors in 6 US regions enabled population weighted seroprevalence estimates. Seroprevelance rates were higher in younger, non-Hispanic Black, and Hispanic donors and correlated with regional case rates. The study has expanded to a national serosurveillance program.
Globally, as of May 2021, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has caused >150 million diagnosed cases of coronavirus disease 2019 (COVID-19), >3 million deaths, and a substantial number of infections that are either asymptomatic or mildly symptomatic [1–3]. With application of sensitive and specific serological assays and algorithms to representative populations, SARS-CoV-2 serosurveys are critical for estimating total infection rates, infection fatality rates, the extent of herd immunity, and the effect of epidemic mitigation policies [4]. Blood-donor-based serosurveillance is a powerful and cost-effective strategy that has provided valuable insights on infection prevalence and incidence for past emerging infectious threats, including West Nile, dengue, chikungunya, and Zika virus infections [5-10]. Choice of assays for serosurveillance should be determined by intended purpose [11, 12] and assay performance, which can be influenced by antigen and immunoglobulin targets and assay configuration [13].
In response to the emergence of COVID-19 in the United States in early 2020, the National Heart, Lung, and Blood Institute (NHLBI) Recipient Epidemiology and Donor Evaluation Study-IV-Pediatric (REDS-IV-P) program developed and implemented molecular and serological surveillance for SARS-CoV-2 in 6 metropolitan regions, called the REDS-IV-P Epidemiology, Surveillance and Preparedness of the Novel SARS-CoV-2 Epidemic (RESPONSE) study. The RESPONSE project aims included conducting testing for SARS-CoV-2 antibodies to estimate seroprevalence, to evaluate trends in seroprevalence, and to compare the observed seroprevalence with reported case data.
METHODS
Study Sites and Donation Sampling
The RESPONSE study tested for SARS-CoV-2 antibodies in 3 early-outbreak regions starting in March 2020 (Seattle, Washington, New York, New York, and San Francisco, California), and 3 initially low-prevalence regions in April 2020 (Boston, Massachusetts, Los Angeles, California, and Minneapolis, Minnesota) (see Table 1 for donor characteristics and Figure 1 for testing algorithm). About 1000 serum specimens were randomly selected monthly from allogeneic blood donors from March/April through August 2020. In July and August, monthly sampling increased to 2000–4000 per region as the study transitioned into the expanded Multistate Assessment of SARS-CoV-2 Seroprevalence in Blood Donors Study [14]. Blood collection organizations provided routinely collected, deidentified demographic information for each blood donation, including donor age, sex, race/ethnicity, blood type, and zip code of residence.
Table 1.
Demographic Characteristics of Donors Who Provided Specimens, Overall and by US Metropolitan Region, March–August 2020
| Donations, % | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| New York, NY | San Francisco, CA | Seattle, WA | Boston, MA | Los Angeles, CA | Minneapolis. MN | All Regions | ||||||||
| Characteristic | Total (n = 131 622)) |
(n = 9132) | Total (n = 28 758) | Sampled (n = 7986) |
Total (n = 76 209) | Sampled (n = 8019) |
Total (n = 47 437) | Sampled (n = 6999) |
Total (n = 11 4692) | Sampled (n = 11 000) |
Total (n = 98 010) | Sampled (n = 7000) |
Total (n = 496 728) | Sampled (n = 50 156) |
| Sex | ||||||||||||||
| Female | 46.5 | 47.5 | 51.6 | 50.8 | 56.7 | 55.7 | 53.6 | 53.8 | 54.7 | 54.1 | 57.5 | 57.8 | 53.1 | 53.1 |
| Male | 53.5 | 52.5 | 48.4 | 49.2 | 43.3 | 44.3 | 46.4 | 46.2 | 45.3 | 45.9 | 42.5 | 42.2 | 46.9 | 46.9 |
| Age, y | ||||||||||||||
| 16–29 | 18.0 | 20.5 | 12.5 | 11.8 | 14.4 | 13.1 | 13.6 | 14.4 | 16.3 | 16.4 | 10.8 | 9.8 | 14.9 | 14.7 |
| 30–49 | 30.2 | 31.2 | 30.8 | 32.0 | 34.2 | 32.6 | 28.5 | 29.1 | 35.5 | 36.3 | 28.2 | 29.0 | 31.5 | 32.1 |
| 50–64 | 38.8 | 34.5 | 36.9 | 36.1 | 32.2 | 32.6 | 40.3 | 39.2 | 34.5 | 34.3 | 36.1 | 36.7 | 36.3 | 35.4 |
| ≥65 | 13.0 | 13.8 | 19.8 | 20.2 | 19.2 | 21.7 | 17.6 | 17.2 | 13.7 | 13.1 | 24.9 | 24.5 | 17.3 | 17.9 |
| Race/ ethnicity | ||||||||||||||
| White | 78.5 | 77.3 | 71.3 | 71.3 | 81.2 | 83.8 | 92.6 | 92.7 | 62.4 | 60.5 | 97.0 | 97.0 | 79.8 | 78.6 |
| Black | 3.6 | 3.8 | 1.4 | 1.4 | 0.9 | 0.7 | 1.0 | 1.0 | 2.1 | 2.2 | 0.3 | 0.3 | 1.8 | 1.7 |
| Hispanic | 8.9 | 8.0 | 8.2 | 8.4 | 2.4 | 2.3 | 2.0 | 2.2 | 19.9 | 20.8 | 0.8 | 0.7 | 8.1 | 8.1 |
| Other | 8.9 | 10.9 | 19.2 | 18.8 | 15.6 | 13.2 | 4.4 | 4.1 | 15.7 | 16.4 | 1.9 | 2.0 | 10.3 | 11.6 |
| Blood group | ||||||||||||||
| O | 50.9 | 49.4 | 48.6 | 48.2 | 50.4 | 52.0 | 51.2 | 51.6 | 50.7 | 50.6 | 47.5 | 47.3 | 50.0 | 49.9 |
| A | 32.6 | 33.5 | 33.7 | 34.4 | 35.3 | 34.9 | 33.2 | 33.1 | 32.8 | 32.4 | 37.3 | 37.0 | 34.1 | 34.1 |
| B | 12.3 | 12.5 | 12.2 | 12.4 | 10.3 | 9.9 | 11.2 | 10.7 | 11.9 | 12.3 | 10.6 | 11.0 | 11.4 | 11.6 |
| AB | 4.2 | 4.5 | 5.5 | 5.0 | 4.1 | 3.1 | 4.4 | 4.6 | 4.6 | 4.7 | 4.6 | 4.6 | 4.4 | 4.4 |
| Rh type | ||||||||||||||
| Positive | 83.3 | 84.2 | 83.0 | 83.5 | 79.9 | 79.2 | 80.7 | 80.5 | 84.7 | 84.5 | 78.5 | 77.9 | 81.9 | 81.9 |
| Negative | 16.7 | 15.8 | 17.0 | 16.5 | 20.1 | 20.7 | 19.3 | 19.4 | 15.3 | 15.5 | 21.4 | 22.1 | 18.1 | 18.0 |
| Donor status | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| First time | 14.6 | 14.8 | 28.4 | 18.0 | 21.1 | 16.2 | 22.8 | 21.8 | 30.9 | 27.3 | 18.0 | 16.2 | 21.6 | 19.5 |
| Repeat | 85.4 | 85.2 | 71.6 | 82.0 | 78.9 | 83.8 | d77.2 | 78.2 | 69.1 | 72.7 | 82.0 | 83.8 | 78.4 | 80.5 |
Abbreviation: Rh, rhesus factor.
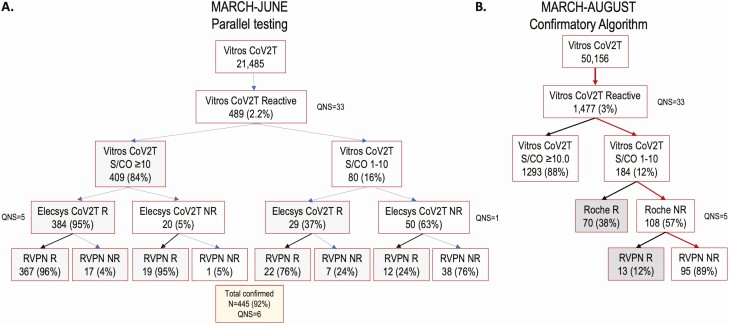
Figure 1.
Flow charts of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) serologic testing results for 6 US metropolitan regions. A, Parallel testing using the Roche Elecsys Nucleocapsid Anti-SARS-CoV-2 Total Immunoglobulin test (Elecsys CoV2T) and the pseudovirus reporter virus particle neutralization (RVPN) assay on samples reactive to the Ortho VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total test (Vitros CoV2T), collected during March–June 2020. B, Results from March–August 2020, combining the initial and revised supplementary testing algorithms. Abbreviations: NR, nonreactive; QNS, quantity not sufficient; R, reactive; S/CO, signal-to-cutoff ratio.
Beginning in June 2020, the blood collection organizations associated with 4 regions (San Francisco, Los Angeles, Minneapolis, and Boston) began screening all blood donors for SARS-CoV-2 antibodies [15]. In July and August in these regions, antibody data were extracted from donation records, whereas for Seattle and New York, study-initiated testing continued. For all months, donations made specifically to provide COVID-19 convalescent plasma were excluded. The study was determined to meet the definition of research but did not involve human subjects based on anonymization of data and routine consent for blood donation testing that includes use of residual samples for research purposes consistent with applicable federal law and Centers for Disease Control and Prevention (CDC) policy (45 CFR part 46; 21 CFR part 56; 42 USC §241[d], 5 USC §552a, 44 USC §3501). We used the STROBE cross sectional checklist when writing our report [16].
Screening and Supplemental Serology Assays and Establishing a Testing Algorithm
Initially, the serologic screening and supplemental testing algorithm consisted of screening all samples with the Ortho VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total test (Vitros CoV2T). Reactive samples were confirmed by parallel testing by both a nucleocapsid (NC)–based total immunoglobulin assay (Roche Elecsys NC Anti-SARS-CoV-2 Total Ig [Elecsys CoV2T]) and a pseudovirus reporter virus particle neutralization (RVPN) test (Appendix A in Supplementary Materials). Screened-positive specimens were considered confirmed if reactive by either Elecsys CoV2T or RVPN test. The Vitros CoV2T and Elecsys CoV2T assays were selected based on their double antigen-sandwich design, which enables durable detection of total immunoglobulin and used as an orthogonal algorithm to detect antibodies to different SARS-CoV-2 antigens (S1 and NC, respectively). Food and Drug Administration emergency use authorization instructions for use [17] and other reports have noted excellent sensitivity of both assays during acute infection and stability of antibody reactivity on serial samples collected >120 days after COVID-19 symptom onset [18–20].
Statistical Methods to Extrapolate Donor Seroprevalence to the General Population
The geographic distribution and demographic composition of sampled donors varied monthly. To ensure that sample populations represented a consistent geographic area over the course of the study, donations were restricted to zip codes in which ≥80% of donors resided, referred to in this study as the donor catchment regions (DCRs). Donations from donors that resided outside of the DCR were excluded (Supplementary Table 3). Monthly sample donor demographics were compared with monthly total donation demographics at each blood center, using χ2 statistics (without accounting for a multiple comparison adjustment), to ensure that sampled donations were representative of general donor populations.
To estimate the monthly seroprevalence in the general population based on blood donor seroprevalence, monthly estimation weights were created that accounted for demographic difference between the blood donor sample and general population. The 2018 American Community Survey estimates [21] for the age, sex, and race/ethnicity compositions of the DCRs were used to standardize DCR sample totals by raking. In addition to these estimation weights, monthly sets of 50 pseudo-replicate weights were created to compute weighted seroprevalence standard errors. Because seroprevalence in the US population is known to vary by location and time, a stratified (by blood center and month) logistic regression model was developed to assess the association between seropositivity and demographic characteristics.
Blood donation DCRs were defined by zip codes, but case reporting by state and local health departments to the CDC is reported by county. Therefore, to compare the number of cumulative infections estimated from seroprevalence with the number of cumulative cases reported to CDC by each region, we created county-based DCRs. The number of total cumulative infections in a DCR was estimated by multiplying the weighted seroprevalence by the total population in the DCR. See Supplementary Figure 1 and Appendix B (Supplementary Materials) for detailed statistical methods. For each county-based DCR, the number of cumulative infections based on seroprevalence was divided by the number of reported cases.
RESULTS
Validation of Supplemental Testing Algorithm
During March–June 2020, a total of 21 485 donations were screened with Vitros CoV2T, of which 489 reactive specimens were tested in parallel by the Elecsys CoV2T and the RVPN test (Figure 1A). Specimens were stratified based on Vitros CoV2T signal-to-cutoff (S/CO) ratios: specimens with S/COs 1–10 and those with S/COs ≥10. Parallel testing of all screened reactive specimens demonstrated that among the 404 specimens with Vitros CoV2T S/COs ≥10.0 and available Elecsys CoV2T results, 384 were Elecsys CoV2T reactive and 19 reactive by RVPN assay; thus, >99% of specimens with Vitros CoV2T S/COs ≥10 were confirmed reactive by either Elecsys CoV2T or the RVPN test. In contrast, of 79 screened reactive specimens with Vitros CoV2T S/COs 1–10 and available Elecsys CoV2T results, 29 were Elecsys CoV2T reactive and 12 were RVPN reactive; only 51% of specimens with S/COs 1–10 were confirmed reactive (Figure 1A and Supplementary Figure 3).
Thus, beginning in July we modified the supplemental testing algorithm to be more cost-effective while maintaining high sensitivity and specificity for July and August (Figure 1B), so that specimens were considered “confirmed antibody positive” if (1) they had an S/CO ≥10 on Vitros CoV2T screening assay (ie, no supplemental testing was performed) or (2) if the Vitros CoV2T S/CO was 1–10 and were reactive with either the Elecsys CoV2T or the RVPN assay. Details and results of application of this testing algorithm for the entire study interval (March–August) are presented in Appendix C (Supplementary Materials).
Seroprevalence Estimates Over Time, With and Without Supplemental Testing and Population Weighting
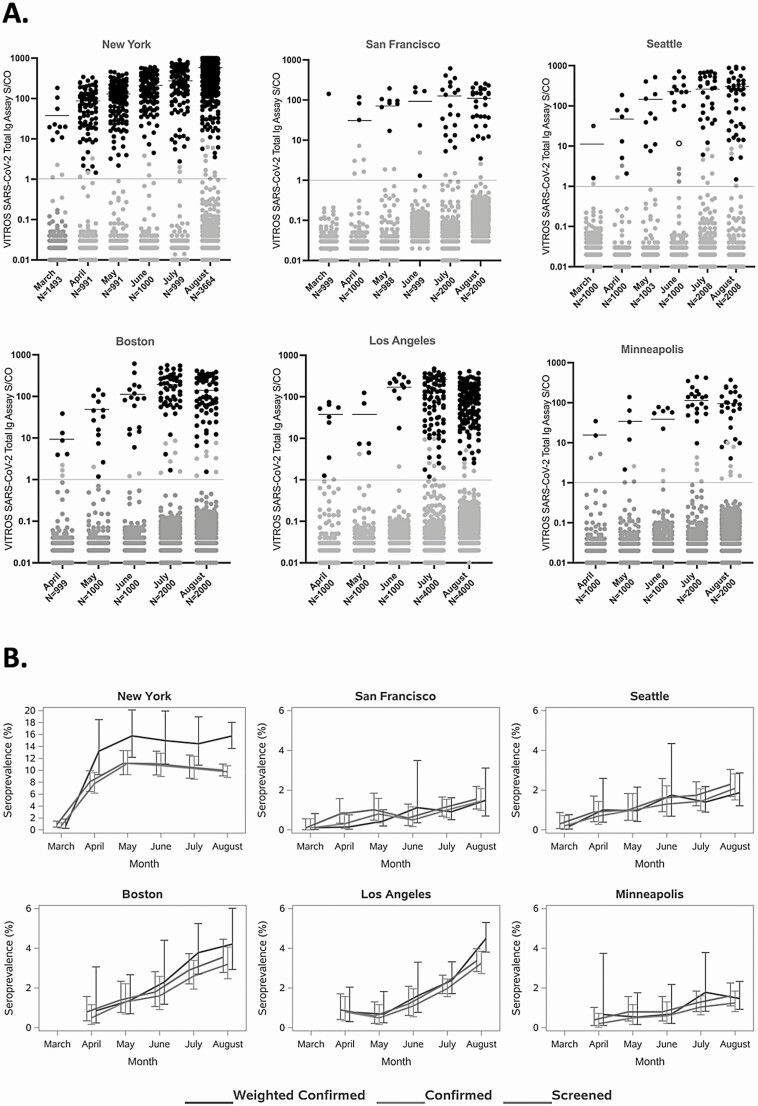
In total, 499 476 donations (excluding COVID-19 convalescent plasma donations) were collected in all participating regions during the study period, of which 50 156 (10%) were included in the study. The monthly distributions of Vitros CoV2T reactivity, supplemental testing status, and number of tested specimens are shown in Figure 2A, and seroprevalence by month and site are presented in Supplementary Table 2. Low rates of unweighted confirmed seroreactivity (<1%) were observed for all regions at the beginning of the testing period in March 2020, with variable increases over the 5–6-month serosurveillance period. The greatest increase in seroprevalence was seen in New York (from 0.7% to 15.7%), followed by Los Angeles (from 0.8% to 4.5%) and Boston (from 0.9% to 4.2%). Mean Vitros CoV2T signal intensity increased from an S/CO of 37.8 (range, 1.1–182.4) in March to 308.9 (1.0–1380.0) in August, demonstrating that both proportions of confirmed seropositive donations and mean signal intensities increased over time in each region.
Figure 2.
Monthly distribution of all Ortho VITROS Immunodiagnostic Products Anti-SARS-CoV-2 Total test (Vitros CoV2T) values (A) and unadjusted and weighted cumulative seroprevalence (B), for 6 US metropolitan regions in March–August 2020. A, Red lines indicate the Vitros CoV2T signal-to-cutoff (S/CO) value for reactivity (S/CO ratio, 1.0; log10 S/CO ratio, 0); black symbols, Black symbols, samples confirmed reactive based on the study algorithm; black lines, mean signal intensity of the Vitros CoV2T–reactive samples by region for each month of the study; gray symbols above the Vitros CoV2T cutoff threshold, samples that were reactive by the Vitros CoV2T screening assay but that were not confirmed using the study algorithm; gray symbols below the red line, samples that were nonreactive with the Vitros CoV2T assay; open black symbol (Seattle panel, June column), the only sample with Vitros CoV2T signal-to-cutoff ratio (S/CO) >10 that was not confirmed. Numbers represent number of sampled donations for each month. B, Screened and confirmed seroprevalence for each region, and confirmed seroprevalence restricted to zip code of residence.
In Figure 2B, the screening and confirmed seroprevalence data over time are presented for each DCR. A high proportion of screen-reactive donations confirmed, particularly in later months as seroprevalence increased; in July and August, 81%–96% of specimens that screened reactive for anti-S antibodies by Vitros CoV2T were also reactive for anti-NC antibodies by Elecsys CoV2T. The median weighted confirmed seroprevalence was 1.3 times higher than unweighted confirmed seroprevalences (interquartile range, 1.02–1.44).
Demographic, Blood Group, and Donation Status Associations With Weighted Seroprevalence Estimates
The confirmed, weighted seroprevalence estimates by donor demographic subcategories (sex, age, race/ethnicity) and by blood groups (ABO and Rh) presented in Table 2 were restricted to August as the most recent findings in this study. For New York, Los Angeles, and Boston, sites with sufficient donations from racial and ethnic minority donors for meaningful comparison, seroprevalence was higher among younger age groups and among non-Hispanic blacks and Hispanics compared with non-Hispanic whites. In New York in August, the seroprevalence was 28.6% among Hispanics, 16.0% among non-Hispanic blacks, and 8.4%.among non-Hispanic whites.
Table 2.
Weighted Confirmed Seroprevalence by Demographic Characteristics in 6 US Metropolitan Regions, August 2020
| Variable | Seroprevalence (95% CI) by Region | |||||
|---|---|---|---|---|---|---|
| New York, NY | San Francisco, CA | Seattle, WA | Los Angeles, CA | Boston, MA | Minneapolis, MN | |
| All donors | 15.7 (13.6–18.0) | 1.5 (.6–3.1) | 1.9 (1.2–2.9) | 4.5 (3.8–5.3) | 4.2 (2.8–6.0) | 1.5 (.9–2.3) |
| Age, y | ||||||
| 16–29 | 18.4 (13.9–23.7) | 4.1 (.7–12.2) | 1.9 (.4–5.2) | 8.0 (5.9–10.7) | 5.6 (2.5–10.3) | 2.1 (.5–5.7) |
| 30–49 | 17.4 (13.7–21.6) | 0.8 (.2–2.2) | 1.7 (.6–3.9) | 4.8 (3.4–6.6) | 3.4 (1.1–7.7) | 1.7 (.7–3.6) |
| 50–64 | 14.9 (10.8–19.8) | 0.5 (.1–1.6) | 2.5 (1.0–5.2) | 2.4 (1.4–3.8) | 5.1 (3.2–7.8) | 1.2 (.4–2.5) |
| ≥65 | 10.2 (5.3–17.3) | 0.9 (.1–3.3) | 1.2 (.3–3.3) | 1.2 (.4–2.8) | 2.6 (1.2–5.1) | 0.6 (.1–2.1) |
| Sex | ||||||
| Female | 16.8 (13.8–20.2) | 1.9 (.4–5.5) | 2.0 (1.1–3.6) | 5.6 (4.4–7.0) | 4.2 (2.2–7.2) | 0.9 (.3–1.8) |
| Male | 14.5 (11.6–17.8) | 1.1 (.5–2.1) | 1.7 (.8–3.2) | 3.4 (2.3–4.7) | 4.2 (2.6–6.4) | 2.1 (1.1–3.6) |
| Race/ethnicity | ||||||
| White | 8.4 (7.1–9.8) | 1.6 (.8–3.0) | 2.5 (1.5–3.9) | 2.1 (1.5–2.8) | 2.9 (2.1–3.8) | 1.8 (1.1–2.8) |
| Black | 16.0 (9.6–24.4) | 0.0 (.0–15.8) | 0.0 (.0–20.0) | 3.1 (.5–9.2) | 16.6 (3.8–40.5) | 0.0 (.0–60.0) |
| Hispanic | 28.6 (22.1–35.8) | 2.8 (.2–11.3) | 0.0 (.0–5.8) | 6.9 (5.2–8.9) | 8.7 (2.1–22.3) | 0.0 (.0–21.4) |
| Other | 13.0 (9.7–16.9) | 0.6 (.0–2.7) | 0.5 (.0–3.3) | 3.8 (2.3–6.0) | 5.6 (1.1–16.0) | 0.0 (.0–8.8) |
| Blood type | ||||||
| A | 12.4 (8.9–16.7) | 2.2 (.2–8.7) | 1.6 (.8–3.1) | 3.1 (2.1–4.4) | 4.0 (2.3–6.5) | 1.6 (.7–3.1) |
| AB | 23.1 (11.4–38.9) | 3.1 (.1–16.7) | 2.4 (.1–10.4) | 4.0 (1.0–10.3) | 5.5 (.3–22.7) | 2.0 (.0–10.8) |
| B | 15.8 (9.2–24.6) | 1.2 (.1–4.3) | 2.7 (.3–9.3) | 4.1 (2.2–6.9) | 4.9 (1.6–11.2) | 2.7 (.4–8.5) |
| O | 17.1 (13.5–21.2) | 1.0 (.3–2.3) | 1.8 (.8–3.4) | 5.4 (4.3–6.6) | 4.0 (2.2–6.6) | 1.0 (.4–2.1) |
| Rh type | ||||||
| Rh positive | 16.1 (13.6–18.7) | 1.6 (.6–3.4) | 1.9 (1.1–3.1) | 4.9 (4.1–5.8) | 4.4 (2.9–6.4) | 1.3 (.7–2.4) |
| Rh negative | 13.4 (8.9–19.0) | 0.9 (.0–3.9) | 1.7 (.3–5.1) | 1.6 (.5–3.7) | 3.1 (.9–7.7) | 2.1 (.9–4.1) |
Abbreviations: CI, confidence interval; Rh, rhesus factor.
In a logistic regression model that included results from all regions and months, seroprevalence was associated with younger age (P < .001): compared to persons aged 50–64 years, those aged 16–29 years had 1.31 (95% confidence interval [CI], 1.1–1.6) times the odds of seropositivity. Both non-Hispanic blacks (odds ratio [OR], 2.2 [95% CI, 1.6–2.9]) and Hispanics (2.6 [2.2–3.1]) had greater odds of seropositivity than non-Hispanic whites (Table 3). Sex and blood types were not significantly associated with seroprevalence. First-time donors had increased seroprevalence compared with repeat donors (OR, 2.2 [95% CI, 1.6–3.2])). In the 4 regions where donors in July and August were universally tested for SARS-CoV-2 antibodies, first-time donors had 2.2 (95% CI, 1.8–2.6) times the odds of being seropositive compared to repeat donors. In the 2 regions where blood donors were not being offered antibody testing, first-time donors had only 1.2 (95% CI, 1.0–1.5) times the odds of repeat donors.
Table 3.
Characteristics Associated With Severe Acute Respiratory Syndrome Coronavirus 2 Seropositivity in Blood Donors From 6 US Metropolitan Regions, March–August 2020
| Characteristic | OR (95% CI) | P Value |
|---|---|---|
| Sex | ||
| Female | 1 (Reference) | .22 |
| Male | 0.93 (.82–1.05) | |
| Age, y | ||
| 16–29 | 1.31 (1.11–1.55) | <.001 |
| 30–49 | 1.12 (.97–1.30) | |
| 50–64a | 1 (Reference) | |
| ≥65 | 0.66 (.53–.83) | |
| Race/ethnicity | <.001 | |
| White | 1 (Reference) | |
| Black | 2.16 (1.64–2.85) | |
| Hispanic | 2.57 (2.17–3.05) | |
| Other | 1.16 (.96–1.41) | |
| Blood type | ||
| A | 1.11 (.97–1.26) | .29 |
| AB | 1.21 (.91–1.61) | |
| B | 1.00 (.82–1.21) | |
| O | 1 (Reference) | |
| Rh type | ||
| Rh negative | 1.03 (.88–1.22) | .69 |
| Rh positive | 1 (Reference) | |
| Donor type | ||
| First time | 2.24 (1.58–3.16) | <.001 |
| Repeat | 1 (Reference) |
Abbreviations: CI, confidence interval; OR, odds ratio; Rh, rhesus factor.
aThe 50–64-year age group had the highest frequency of donations.
Comparison of Monthly Seroprevalence (as Calculated From Donor Serosurveillance) With Reported COVID-19 Case Rates
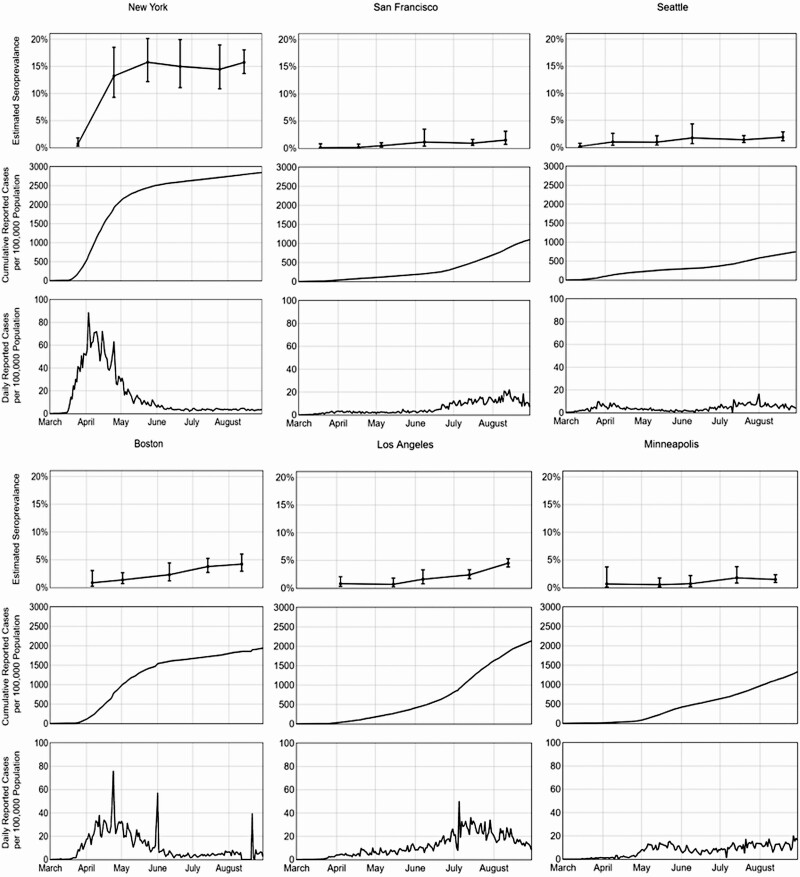
For each region, the monthly confirmed, weighted seroprevalence was juxtaposed with the weekly and cumulative COVID-19 case counts. Seroprevalence and cumulative COVID-19 case rates increased in all regions from March–April through August (Figure 3). New York reported the highest seroprevalence, increasing from 0.7% in March to 13.2% in April, corresponding with the sharp rise in reported New York COVID-19 cases. Coincident with a decrease in daily reported cases from May through July, the seroprevalence in New York stabilized at approximately 15%–16% during this time, with smaller increases in other regions. The cumulative case incidence for Boston and Los Angeles in July was similar to that incidence for New York in April (approximately 2000 cumulative reported cases per 100 000 population), but seroprevalences for Boston and Los Angeles remained substantially lower than for New York.
Figure 3.
Weighted confirmed severe acute respiratory syndrome coronavirus 2 seroprevalence derived from blood donors, coronavirus disease 2019 (COVID-19) case rates per 100 000 population (reported to the Centers for Disease Control and Prevention [CDC]), and daily COVID-19 case rates per 100 000 (as reported to the CDC) in 6 US metropolitan regions, March–August 2020.
The number of estimated cumulative infections, based on the adjusted donor seroprevalence and population sizes, was larger than the number of cumulative reported infections for all regions (Table 4). However, the ratio varied by region and over time. For all cities except New York, much higher numbers of estimated infections per reported case occurred in the first month of blood donor screening compared with later months. The highest reported ratio of estimated infections to reported cases occurred in Minneapolis in April (42 infections per reported case). By August 2020, all regions other than New York had 1.6–3.2 estimated infections per reported case. From May through August, New York had the highest number of estimated infections per reported case (5.3–6.4 infections per reported case).
Table 4.
Monthly Seroprevalence, Estimated Number of Cumulative Infections, Cumulative Reported Coronavirus Disease 2019 Cases, and Estimated Number of Cumulative Infections per Reported Case in 6 US Metropolitan Regions, March–August 2020
| Region and Month | Seroprevalence (95% CI) | Cumulative Infections, Estimated No.a (95% CI) |
Cumulative Reported Cases, No.b |
Infections per Reported Case, Estimated No.c (95% CI) |
|---|---|---|---|---|
| New York, NY | ||||
| March | 0.71 (.27–1.82) | 129 184 (49 126–331 148) | 34 562 | 3.7 (1.4–9.6) |
| April | 13.22 (9.27–18.5) | 2 405 373 (1 686 672–3 366 067) | 34 9083 | 6.9 (4.8–9.6) |
| May | 4 (12.19–20.14) | 2 867 525 (2 217 965–3 664 465) | 44 5202 | 6.4 (5.0–8.2) |
| June | 14.97 (11.08–19.93) | 2 723 785 (2 016 001–3 626 255) | 47 3521 | 5.8 (4.3–7.7) |
| July | 14.45 (10.87–18.96) | 2 629 171 (1 977 792–3 449 764) | 49 4591 | 5.3 (4.0–7.0) |
| August | 15.73 (13.68–18.03) | 2 862 067 (2 489 070–3 280 551) | 50 8746 | 5.6 (4.9–6.4) |
| San Francisco, CA | ||||
| March | 0.11 (.01–.82) | 8513 (773–63 462) | 624 | 13.6 (1.2–101.7) |
| April | 0.15 (.03–.76) | 11 609 (2321–58 819) | 6035 | 1.9 (.4–9.7) |
| May | 0.44 (.19–1.01) | 34 053 (14 704–78 167) | 8980 | 3.8 (1.6–8.7) |
| June | 1.13 (.36–3.49) | 87 454 (27 861–270 104) | 15 829 | 5.5 (1.8–17.1) |
| July | 0.91 (.51–1.62) | 70 428 (39 470–125 377) | 38 667 | 1.8 (1.0–3.2) |
| August | 1.48 (.7–3.11) | 114 542 (54 175–240 694) | 65 403 | 1.8 (.8–3.7) |
| Seattle, WA | ||||
| March | 0.18 (.04–.77) | 8824 (1961–37 751) | 262 | 33.7 (7.5–144.1) |
| April | 1.01 (.39–2.6) | 49 517 (19 120–127 471) | 6410 | 7.7 (3.0–19.9) |
| May | 0.97 (.43–2.16) | 47 556 (21 081–105 899) | 12 618 | 3.8 (1.7–8.4) |
| June | 1.75 (.69–4.34) | 85 798 (33 828–212 779) | 14 973 | 5.7 (2.3–14.2) |
| July | 1.4 (.9–2.17) | 68 638 (44 124–106 389) | 23 920 | 2.9 (1.8–4.4) |
| August | 1.87 (1.22–2.87) | 91 681 (59 813–140 708) | 35 170 | 2.6 (1.7–4.0) |
| Boston, MA | ||||
| April | 0.86 (.24–3.06) | 54 284 (15 149–193 152) | 12 677 | 4.3 (1.2–15.2) |
| May | 1.37 (.7–2.67) | 86 476 (44 185–168 534) | 63 678 | 1.4 (.7–2.6) |
| June | 2.3 (1.19–4.41) | 145 179 (75 114–278 366) | 101 040 | 1.4 (.7–2.8) |
| July | 3.78 (2.71–5.25) | 238 599 (171 059–331 388) | 108 209 | 2.2 (1.6–3.1) |
| August | 4.21 (2.93–6.02) | 265 741 (184 946–379 992) | 117 279 | 2.3 (1.6–3.2) |
| Los Angeles, CA | ||||
| April | 0.79 (.3–2.04) | 147 820 (56 134–381 713) | 7303 | 20.2 (7.7–52.3) |
| May | 0.68 (.25–1.82) | 127 237 (46 778–340 548) | 50 077 | 2.5 (.9–6.8) |
| June | 1.61 (.78–3.3) | 301 254 (145 949–617 477) | 87 888 | 3.4 (1.7–7.0) |
| July | 2.39 (1.71–3.32) | 447 203 (319 965–621 219) | 211 358 | 2.1 (1.5–2.9) |
| August | 4.5 (3.81–5.3) | 842 014 (712 905–991 706) | 347 083 | 2.4 (2.1–2.9) |
| Minneapolis, MN | ||||
| April | 0.67 (.12–3.74) | 36 579 (6551–204 192) | 875 | 41.8 (7.5–233.4) |
| May | 0.54 (.16–1.76) | 29 482 (8735–96 090) | 12 357 | 2.4 (.7–7.8) |
| June | 0.69 (.22–2.17) | 37 671 (12 011–118 475) | 25 883 | 1.5 (.5–4.6) |
| July | 1.78 (.83–3.79) | 97 182 (45 315–206 921) | 40 927 | 2.4 (1.1–5.1) |
| August | 1.47 (.93–2.33) | 80 257 (50 775–127 210) | 60 502 | 1.3 (.8–2.1) |
Abbreviation: CI, confidence interval.
aConfirmed seroprevalence multiplied by the region population size.
bNumber reported to the Centers for Disease Control and Prevention.
cNumber of estimated infections divided by the number of cumulative reported cases.
DISCUSSION
The use of blood donor populations with broad national representativeness provides a surveillance tool to monitor seroprevalence and to impute infection rates within communities, track outbreaks, and potentially correlate evolving infection rates with pandemic mitigation measures. Critical to the success of serosurveillance programs is the choice of SARS-CoV-2 antibody assays and the development and validation of supplemental testing algorithms. Antibody persistence or waning has been shown to be assay dependent [22], so it is essential to select assays demonstrating durable antibody reactivity to accurately estimate cumulative incidence based on serial cross-sectional seroprevalence data. Also important is the assay’s ability to sensitively detect antibodies after asymptomatic and mildly symptomatic infections, which may produce weak systemic antibody responses [23].
The Vitros CoV2T and Elecsys CoV2T assays used in this study satisfy many of these criteria for serosurveillance assays: They have stable S/CO values over at least 4–5 months after seroconversion [20, 24] and have wide dynamic ranges, enabling implementation of a screening assay S/CO threshold-based supplementary testing algorithm. By demonstrating that >99% of specimens that were screened with Vitros CoV2T and had S/COs ≥10 were also reactive by the Elecsys CoV2T or the RVPN assay, we were able to adopt a robust and lower-cost testing algorithm, limiting supplemental testing to screened specimens with S/Cos of 1–10. This algorithm is now being used by CDC’s nationwide seroprevalence blood donor study. To differentiate between natural infection–induced and vaccine-induced seropositivity, the nationwide study is testing all anti–S-reactive specimens with an NC-based assay beginning in January 2021 [25].
Seroprevalence was higher in non-Hispanic blacks and Hispanics than in non-Hispanic whites in most regions, but this difference was particularly notable in New York. These racial disparities in seroprevalence are consistent with other reports [15, 26], potentially because racial and ethnic minority groups experience inequities in access to healthcare, quality housing, the ability to work from home, and reliable transportation [27]. Increased risk for infection has been associated with younger age, possibly owing to lack of adherence to mitigation measures [15, 28]. Future analyses will include comparing region-, age-, and race/ethnicity-specific seroprevalence rates with the number of demographic group–specific cumulative reported cases.
In the current study, seroprevalence trends were consistent with the pattern of cumulative reported COVID-19 cases. For most regions, the ratio of estimated infections to reported cases was higher during March–April 2020 than in subsequent months. This suggests that underreporting of COVID-19 cases to CDC was more severe during the earliest months of the pandemic. Lack of available testing and avoiding medical care to obtain testing because of COVID-19–related concerns might also have contributed [29]. From May through August, the calculated seroprevalence predicted 1.6–3.2 SARS-CoV-2 infections per cumulative case reported to CDC for all regions except New York, which predicted 5.3–6.4 infections per reported case.
Compared with the other large seroprevalence survey conducted by the CDC using commercial laboratory specimens, the current study generally showed lower seroprevalence estimates [30]. A national seroprevalence study of dialysis patients with blood specimens collected during July 2020 also reported generally higher seroprevalence estimates [31]. Differences in the geographic distribution of participants, serologic assays used, and assumptions made when extrapolating seroprevalence estimates to the general population may explain these differences. Several local seroprevalence studies conducted in regions similar to the 6 regions in this study have calculated similar or higher seroprevalence estimates [32–34]. However, many of these collected specimens were from healthcare workers or hospitalized patients, who may be at higher risk of SARS-CoV-2 infection.
This study could have underestimated seroprevalence for several reasons. First, blood donors may represent a population less likely to be exposed to SARS-CoV-2 than the general population [35]. Moreover, blood donors tend to be in better health than the general population, and recruitment practices and eligibility criteria for blood donations may bias the donor sample toward lower-risk individuals; this may explain the lower rates of antibody positivity in repeat donors (who provide >80% of donations), compared with first-time donors. Second, many higher-risk populations cannot or do not donate, including persons who are acutely febrile or ill, children aged <16 years, and institutionalized persons, such as those residing in nursing homes or prison. Third, compared with the general population, relatively few ethnic and racial minorities donate, and these groups are at increased risk for SARS-CoV-2 infection; this bias is partially compensated for because our results were adjusted by weighting for race/ethnicity. Fourth, there is growing evidence that approximately 5%–10% of infected persons do not seroconvert [23]. We did not adjust our results to account for such “serosilent” infections.
Our results may overestimate seroprevalence because some blood collection organizations began SARS-CoV-2 antibody screening of all blood donations in the summer of 2020. These blood centers publicly advertised availability of this screening, which could have led to test seeking by prospective donors with increased concern over exposure to the virus. However, our analysis of relative seroprevalence before and after implementation of such “universal screening” in first-time donors, who give 15%–20% of total donations, indicates that although the OR was greater for those donors, the impact of such test seeking was small relative to the expanding pandemic. Finally, although there was no formal process for randomization, comparison of monthly samples with monthly donations (Supplementary Table 1) demonstrated only sporadic differences that are adjusted for in weighting of seroprevalence estimates to the general population.
Building on the approach developed in the RESPONSE seroprevalence study, in July 2020 the US CDC funded a nationwide blood donor seroprevalence program that expanded this surveillance program from 6 regions for 6 months to >60 US regions with monthly collections of 2000–6000 samples per region from July 2020 to December 2021 (Supplementary Figure 2). Similar to RESPONSE, changes in overall SARS-CoV-2 seroprevalence specific to geographic region, age, sex, and race/ethnicity will be calculated monthly over the course of the study and compared with clinical cases, deaths, and community serosurvey data.
In conclusion, serial serosurveillance studies of SARS-CoV-2 using blood donor populations, which are now being implemented in many countries [36], provide a powerful adjunct to standard public health case reporting. Although serosurveillance data from asymptomatic blood donors may lag behind viral transmission and case reporting by up to several weeks, if appropriately designed, executed, analyzed, and interpreted, these studies will provide urgently needed data to inform our understanding of the epidemiology and effectiveness of responses to this unprecedented pandemic.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. The authors thank C. Cassetti of the National Institute of Allergies and Infectious Diseases (NIAID), National Institutes of Health, and S. Gerber, M. Patton, F. Havers, and S. Basavaraju of the Centers for Disease Control and Prevention (CDC) for their technical support; A. E. Williams and S. Anderson of the US Food and Drug Administration and J. Haynes of the American Red Cross for their contribution of data from the Transfusion-Transmissible Infections Monitoring System; and L. McCain, A. Hui, C. Samuels, H. Tanner, and Z. Kaidarova of Vitalant Research Institute for their technical assistance.
The National Heart, Lung, and Blood Institute (NHLBI) Recipient Epidemiology and Donor Evaluation Study-IV-Pediatric (REDS-IV-P) Epidemiology, Surveillance and Preparedness of the Novel SARS-CoV-2 (RESPONSE) study is the responsibility of the following: M. P. B., P. J. Norris, and M. S., Data Coordinating Center, S. M. Mathew, Westat, Rockville, Maryland; Blood collection organizations: S. Stramer, American Red Cross, Gaithersburg, Maryland; D. Kessler, New York Blood Center, New York, New York; B. A. Konkle, Blood Works Northwest, Seattle, Washington; B. Custer, Vitalant Research Institute; Publications Committee chairman: P. M. Ness, Johns Hopkins University, Baltimore, Maryland; and Steering Committee chairpersons: S. H. Kleinman, University of British Columbia, Victoria, Canada, C. D. Josephson, Emory University, Atlanta, Georgia, and S. A. Glynn and K. Malkin, NHLBI.
Disclaimer. The content is solely the responsibility of the authors and does not represent the policy of the National Institutes of Health or the US Department of Health and Human Services. Any specific brand names included in this article are for identification purposes only and are not intended to represent an endorsement by the CDC. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Financial support. This work was supported by the NHLBI and the NIAID (research contracts HHSN 75N92019D00032 and HHSN 75N92019D00033).
Potential conflicts of interest. R. V. F. reports employment by Westat. Westat received funding to conduct this research under subcontract to Vitalant Research Institute, during the conduct of the study. P. S. and S. L. S. report laboratory support from Grifols, Roche, and Abbott, outside the submitted work. P. C. W. reports payment/honoraria from Roche Molecular Systems and Grifols. S. K. reports payments for multiple research activities related to the current work and to other related projects through a multicenter research contract by NHLBI, during the conduct of the study. M. P. B. also reports serving as president elect for the International Society of Blood Transfusion and serving on the TTID committee for AABB, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Contributor Information
NHLBI Recipient Epidemiology and Donor Evaluation Study-IV-Pediatric (REDS-IV-P):
C Cassetti, S Gerber, M Patton, F Havers, S Basavaraju, A E Williams, S Anderson, J Haynes, L McCain, A Hui, C Samuels, H Tanner, Z Kaidarova, M P B, P J Norris, M S, S M Mathew, S Stramer, D Kessler, B A Konkle, B Custer, P M Ness, S H Kleinman, C D Josephson, S A Glynn, and K Malkin
References
- 1. European Centre for Disease Prevention and Control. COVID-19 situation update worldwide, as of 12 May 2021. Available at: https://www.ecdc.europa.eu/en/geographical-distribution-2019-ncov-cases. Accessed 12 May 2021.
- 2. Long QX, Tang XJ, Shi QL, et al. . Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med 2020; 26:1200–4. [DOI] [PubMed] [Google Scholar]
- 3. Rothe C, Schunk M, Sothmann P, et al. . Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N Engl J Med 2020; 382:970–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Busch MP. Unprecedented nationwide blood studies seek to track U.S. coronavirus spread. In: Cohen J. Science, 2020. Available at: https://www.sciencemag.org/news/2020/04. [Google Scholar]
- 5. Williamson PC, Linnen JM, Kessler DA, et al. . First cases of Zika virus-infected US blood donors outside states with areas of active transmission. Transfusion 2017; 57:770–8. [DOI] [PubMed] [Google Scholar]
- 6. Stone M, Bakkour S, Lanteri MC, et al. ; NHLBI Recipient Epidemiology Donor Evaluation Study REDS-III Program . Zika virus RNA and IgM persistence in blood compartments and body fluids: a prospective observational study. Lancet Infect Dis 2020; 20:1446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Busch MP, Sabino EC, Brambilla D, et al. ; International Component of the NHLBI Recipient Epidemiology and Donor Evaluation Study-III (REDS-III) . Duration of dengue viremia in blood donors and relationships between donor viremia, infection incidence and clinical case reports during a large epidemic. J Infect Dis 2016; 214:49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lanteri MC, Lee TH, Wen L, et al. . West Nile virus nucleic acid persistence in whole blood months after clearance in plasma: implication for transfusion and transplantation safety. Transfusion 2014; 54:3232–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Simmons G, Bres V, Lu K, et al. . High incidence of chikungunya virus and frequency of viremic blood donations during epidemic, Puerto Rico, USA, 2014. Emerg Infect Dis 2016; 22:1221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saá P, Proctor M, Foster G, et al. . Investigational testing for Zika virus among U.S. blood donors. N Engl J Med 2018; 378:1778–88. [DOI] [PubMed] [Google Scholar]
- 11. Sethuraman N, Jeremiah SS, Ryo A. Interpreting diagnostic tests for SARS-CoV-2. JAMA 2020; 323:2249–51. [DOI] [PubMed] [Google Scholar]
- 12. Guo L, Ren L, Yang S, et al. . Profiling early humoral response to diagnose novel coronavirus disease (COVID-19). Clin Infect Dis 2020; 71:778–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Che XY, Qiu L, Liao ZY, et al. . Antigenic cross-reactivity between severe acute respiratory syndrome-associated coronavirus and human coronaviruses 229E and OC43. J Infect Dis 2020; 191:2033–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Centers for Disease Control and Prevention. Multistate assessment of SARS-CoV-2 seroprevalence in blood donors. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/blood-bank-serosurvey.html. Accessed 12 May 2021. [Google Scholar]
- 15. Dodd RY, Xu M, Stramer SL. Change in donor characteristics and antibodies to SARS-CoV-2 in donated blood in the US, June–August 2020. JAMA 2020; 324:1677–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61:344–9. [DOI] [PubMed] [Google Scholar]
- 17. US Food and Drug Administration. EUA authorized serology test performance. Available at: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/eua-authorized-serology-test-performance. Accessed 9 February 2021.
- 18. Zilla M, Wheeler BJ, Keetch C, et al. . Variable performance in 6 commercial SARS-CoV-2 antibody assays may affect convalescent plasma and seroprevalence screening. Am J Clin Pathol 2020. doi:10.1093/ajcp/aqaa228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Grandjean L, Saso A, Ortiz A, et al. . Humoral response dynamics following infection with SARS-CoV-2. medRxiv [Preprint: not peer reviewed]. March 26, 2021. Available from: https://www.medrxiv.org/content/10.1101/2020.07.16.20155663v2. [Google Scholar]
- 20. Clara Di Germanio CD, Simmons G, Kelly K, et al. . SARS-CoV-2 antibody persistence in COVID-19 convalescent plasma donors. medRxiv. [Preprint]. 2021. July 22, 2020. Available from: https://www.medrxiv.org/content/10.1101/2021.03.24.21254260v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. United States Census Bureau, American Community Survey. Available at: https://www.census.gov/programs-surveys/acs. Accessed 12 January 2021.
- 22. Buss LF, Prete CA Jr, Abrahim CMM, et al. . Three-quarters attack rate of SARS-CoV-2 in the Brazilian Amazon during a largely unmitigated epidemic. Science 2021; 371:288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petersen LR, Sami S, Vuong N, et al. . Lack of antibodies to SARS-CoV-2 in a large cohort of previously infected persons. Clin Infect Dis 2021; 73:e3066–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Peluso MJ, Takahashi S, Hakim J, et al. . SARS-CoV-2 antibody magnitude and detectability are driven by disease severity, timing, and assay. medRxiv [Preprint: not peer reviewed]. March 5, 2021. Available from: https://www.medrxiv.org/content/10.1101/2021.03.03.21251639v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Moore JP, Offit PA. SARS-CoV-2 vaccines and the growing threat of viral variants. JAMA 2021; 325:821–2. [DOI] [PubMed] [Google Scholar]
- 26. Mackey K, Ayers CK, Kondo KK, et al. . Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths: a systematic review. Ann Intern Med 2021; 174:362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Centers for Disease Control and Prevention. COVID-19 racial and ethnic health disparities. Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/health-equity/racial-ethnic-disparities/index.html. Accessed 12 January 2021. [Google Scholar]
- 28. Centers for Disease Control and Prevention. COVID-19 mitigation behaviors by age group—United States, April–June 2020. Morb Mortal Wkly Rep (MMWR) 2020; 69:1584–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Czeisler MÉ, Marynak K, Clarke KEN, et al. . Delay or avoidance of medical care because of COVID-19-related concerns—United States, June 2020. MMWR Morb Mortal Wkly Rep 2020; 63:1250–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Centers for Disease Control and Prevention. Commercial laboratory seroprevalence survey data. Accessed 12 January 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/commercial-lab-surveys.html#surveymap.
- 31. Anand S, Montez-Rath M, Han J, et al. . Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet 2020; 396:1335–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mansour M, Leven E, Muellers K, Stone K, Mendu DR, Wajnberg A. Prevalence of SARS-CoV-2 antibodies among healthcare workers at a tertiary academic hospital in New York City. J Gen Intern Med 2020; 35:2485–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Morcuende M, Guglielminotti J, Landau R. Anesthesiologists’ and intensive care providers’ exposure to COVID-19 infection in a New York City academic center: a prospective cohort study assessing symptoms and COVID-19 antibody testing. Anesth Analg 2020; 131:669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stadlbauer D, Tan J, Jiang K, et al. . Seroconversion of a city: longitudinal monitoring of SARS-CoV-2 seroprevalence in New York City. medRxiv [Preprint: not peer reviewed]. June 29, 2020. Available from: https://www.medrxiv.org/content/10.1101/2020.06.28.20142190v1. [Google Scholar]
- 35. Havers FP, Reed C, Lim T, et al. . Seroprevalence of antibodies to SARS-CoV-2 in 10 sites in the United States, March 23–May 12, 2020. JAMA Internal Med 2020; 180:1576–86. [DOI] [PubMed] [Google Scholar]
- 36. Erikstrup C, Hother CE, Pedersen OBV, et al. . Estimation of SARS-CoV-2 infection fatality rate by real-time antibody screening of blood donors. Clin Infect Dis 2020; 72:249–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.