Abstract
Background
The objectives were to assess the excess deaths among Nursing Home (NH) residents during the first wave of the COVID-19 pandemic, to determine their part in the total excess deaths and whether there was a mortality displacement.
Methods
We studied a cohort of 494,753 adults in 6,515 NHs in France exposed to COVID-19 pandemic (from 1 March to 31 May 2020) and compared with the 2014–2019 cohorts using data from the French National Health Data System. The main outcome was death. Excess deaths and standardized mortality ratios (SMRs) were estimated.
Result
There were 13,505 excess deaths. Mortality increased by 43% (SMR: 1.43). The mortality excess was higher among males than females (SMR: 1.51 and 1.38) and decreased with increasing age (SMRs in females: 1.61 in the 60–74 age group, 1.58 for 75–84, 1.41 for 85–94 and 1.31 for 95 or over; males: SMRs: 1.59 for 60–74, 1.69 for 75–84, 1.47 for 85–94 and 1.41 for 95 or over). No mortality displacement effect was observed up until 30 August 2020. By extrapolating to all NH residents nationally (N = 570,003), we estimated that they accounted for 51% of the general population excess deaths (N = 15,114 out of 29,563).
Conclusion
NH residents accounted for half of the total excess deaths in France during the first wave of the COVID-19 pandemic. The excess death rate was higher among males than females and among younger than older residents.
Keywords: COVID-19, excess deaths, older adults, nursing home
Key Points
During the first wave of the COVID-19 pandemic in France, the mortality among nursing home residents increased by 43%.
Nursing home residents accounted for 51% of the total excess deaths in France.
The excess mortality was higher among younger residents than among older residents.
The excess mortality was higher among males than among females.
We did not observe any mortality displacement during the study period (ending on 30 August 2020, i.e. three months after the end of the first wave).
Introduction
In high-income countries, almost all deaths cause by coronavirus disease 2019 (COVID-19) have occurred in older adults. In the United States, 80% of the deaths occurred among people aged 65 or over [1, 2]. In France, 71% of the in-hospital deaths between 1 March and 2 June 2020, concerned patients aged 75 or over [1]. In China, the case fatality rate was 2% overall, 8% in patients aged 70 to 79, and 15% in patients aged 80 and over [3]. In a meta-analysis of 58 studies covering 122,191 hospitalized patients worldwide, COVID-19 lethality was more strongly associated with age over 65 than with comorbidities, obesity, hypertension, diabetes, cardiovascular disease or cancer [4, 5].
Soon after the start of the pandemic, there were concerns about nursing homes (NHs) as setting at increased risk of infection and mortality [6]. Several European governments were criticized for reporting only in-hospital deaths because it soon became clear that deaths in NHs accounted for a non-negligible proportion of deaths—between 25 and 66% in the United States and in European countries [7–9]. Accordingly, the surveillance systems built to count daily COVID-19 deaths started to distinguish between hospitals, NHs and private homes [10, 11]. However, these systems give a fragmented view of the overall impact of the pandemic on NH residents, since some of the latter died in their NH and others died in hospital.
Hence, reporting COVID-19-related deaths alone gives a partial view of the pandemic’s impact. In contrast, the estimation of excess deaths takes account of the pandemic’s negative (mortality increasing) and positive (mortality decreasing) direct and indirect effects [12, 13].
We hypothesized that the overall impact of the first wave of the COVID-19 pandemic on NH residents in France could be assessed by leveraging the national NH administrative database (RESID-EHPAD, part of the French National Health Data System) [14]. A recent similar, but at a regional level, was conducted in Wales [15]. The primary objective of the present study was to assess the magnitude of excess mortality among NH residents in France during the first wave of the COVID-19 pandemic overall and by age, sex and territorial unit. The secondary objectives were to (i) determine excess deaths in NHs as a proportion of total excess deaths, (ii) assess changes over time of excess mortality, and determine whether a mortality displacement was present.
Methods
Setting
Throughout this article, the term ‘nursing homes’ refers in the French context to the Établissements d’hébergement pour personnes âgées dependants (EHPADs). EHPADs provide accommodation, non-medical services (such as meals and laundry), medical, nursing and social care to dependent residents who require regular medical and nurse attention.
Residents in EHPADs accounted for 80% of the population living in residential establishments for the older people [16]. Others establishments such as independent-living facilities (residences services or residences autonomy) and long-term care units (Unités de soins de longue durée) are not included. Independent-living facilities offer accommodation and access to a range of non-medical services but basically no medical care. Long-term care units are for heavily dependent patients with severe illnesses who need constant medical treatment and supervision. For the most part, these units are part of the hospital system. The average age of NH resident is 86 [16]. About, 74% of residents are women; 93% of residents require assistance with bathing; 86% require assistance with dressing and 70% require assistance with feeding. Coherence and orientation problems are reported in 77 and 83% of residents, respectively. Medical expenditure in NHs is entirely covered by the national-level health insurance, without any charge for the residents. Accommodation, non-medical services and social care services are partly charged to the residents through co-payments. Social benefits delivered by the departmental councils cover on average 64% of social care expenditures and 17% of accommodation expenditures [17]. The median co-payments paid for NHs resident were 1850€ per month in 2016 [18].
Design
This was a nationwide cohort study of individuals who were resident in NH in mainland France on March 1st of each year from 2014 to 2020.
Participants
There were two groups comprising an exposed group (present by 1 March 2020) and an unexposed group (combined data from 6 year period (2014–19); individuals enter the cohort on 1 March). The six-year reference period was used to smooth out annual variations in mortality [12, 19]. Residents can contribute follow-up time for each year in which they were registered in the NH between 1 January and 28/29 February i.e. residents who entered the care home between 1 March and 31 December were not included in the follow-up for that year.
Follow-up
We defined two periods of follow-up: the first period (from 1 March to 31 May 2020) covered the first wave of the COVID-19 pandemic in France and was compared with the same period in the previous 6 years (2014–2019). The second period ran from 1 June 2020 to 30 August 2020 to analyze a potential mortality displacement. Each week in this period was compared with the same week in the previous 6 years [19].
Residents could only contribute follow-up time for each year in which they were present in the NH on 1 March; therefore, residents who entered the care home between 1 March and 31 December were excluded from follow-up of the year in the main analysis.
By sensitivity analysis, we included new admissions during follow-up.
Data sources
RESID-EHPAD is a French national online database of administrative data of NH residents in France. NHs managed by the agricultural branch of the health social security are not included in the RESIDEHPAD database. Following French administrative data (CNSA), out of a total of 7,495 NHS and 570,003 NH residents in France, data from 6,515 NHs (87%) and 494,753 residents (87%) are available in RESID-EHPAD database.
All NH admissions and discharges are recorded in this dataset; this information is held electronically by each NH and integrated to the French National Health Data System on a monthly basis.
Although the RESID-EHPAD database is part of the French National Health Data System (Système National des Données de Santé, SNDS), it cannot be accessed routinely by researchers [14, 20]. The SNDS contains extensive individual data on all expenditure covered by France’s mandatory public health insurance system, together with the dates of deaths. The standard, anonymized SNDS data extracts made available to authorized researchers do not contain the RESID-EHPAD variables. Analyses of French National Health Data System were performed with the permission of the independent French data protection authority (Commission Nationale Informatique et Libertes) and by decree (articles R. 1461‐12 and seq. of the French Public Health Code).
Outcome
The main outcome was death, regardless of where it reportedly occurred. It was based on death records provided by the French National Statistics Office (Institut National de la Statistique et des Etudes Economiques, INSEE) through local health insurance offices.
Variables
We analyzed sex, age and the NH’s geographic location. Four age groups were pre-specified: 60–74, 75–84, 85–94 and 95 or over. Geographic locations were classified according to the third level of the Nomenclature of Territorial Units for Statistics (NUTS 3), corresponding to the département (of which there are 96 in mainland France).
Statistical analysis
The baseline characteristics of the NHs and their residents were described as the frequency (percentage) or the mean (standard deviation (SD)). Two indicators were used to summarize observed mortality, the total number of deaths, and the cumulative mortality rates over the whole period and on a weekly basis. For calculation of the mortality rates, the observation time per person present at the beginning of period (i.e. present on 1 March or at the beginning of a specific week) was calculated up to the date of death if this event occurred or till the end of the follow-up (discharge from the NH or 30 August 2020). For illustrative purpose, we represented also the mortality rates of the January–February period of each year.
Firstly, expected numbers of deaths were estimated as counterfactual values by applying the 5-year age group- and sex-specific mortality rates observed for the 2014–2019 cohorts to the 2020 cohort. Secondly, numbers of excess deaths were calculated as observed deaths minus expected deaths for the first-wave period and per week. Thirdly, standardized mortality ratios (SMRs) were obtained by computing the ratio between observed and expected deaths [19]. Excess mortality patterns were studied according to age, sex and geographical (NUTS3) location. Lastly, to estimate the excess death among the whole NH population, the SMR was applied to residents of missing NH from the same NUTS3 unit. We assume that the impact of pandemic was similar in residents of missing NH than residents of available NH of the same geographic area (NUTS units). Data on the numbers of missing NH residents were obtained from the French national registry of healthcare and social establishments (Fichier national des établissements sanitaires et sociaux, FINESS) as of 29 March 2020. FINESS is a dataset produced by the Ministry of Health and Social Affairs. It collects administrative information for all the structures and equipment in the health, medico-social and social sector subject to prior authorization by the public authorities. For NHs, FINESS records information related to the geographical localisation, the number of beds, the legal status of the structures and others administrative data. However, no information is collected on the resident’s characteristics. We then computed excess deaths among NH residents as a proportion of those in the whole population (according to data from INSEE) for 2020 vs. 2014–2019. Statistical analyses were performed with SAS Enterprise Guide software (version 4.3, SAS Institute Inc. Cary, NC). Results were reported according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines.
Results
Baseline characteristics
The 2020 cohort included 494,753 residents from 6,515 NHs in mainland France. The baseline characteristics of the residents and NHs are summarized in Table 1. About, 74% of the residents were female. The mean (SD) age was 89 (8) years for females and 84 (9) years for males. The mean number of residents per NH was 83 (43).
Table 1 .
Baseline characteristics of the residents and the NHs
| Variable | Item | n (%) or mean (SD) | |
|---|---|---|---|
| Residents | N = 494,753 | ||
| Sex | Females | Males | |
| 368,165 (74) | 126,588 (26) | ||
| Age (year), mean (SD) | 89 (8) | 84 (9) | |
| Age groups (years) | 60–74 | 22,755 (6) | 24,099 (19) |
| 75–84 | 60,376 (16) | 30,957 (25) | |
| 85–94 | 201,898 (55) | 57,161 (45) | |
| 95 or over | 83,136 (23) | 14,371 (11) | |
| NHs | N = 6,515 | ||
| For-profit | 3,797 (58) | ||
| Number of residents, mean (SD) | 83 (43) | ||
| Number of residents (classes) | <50 | 892 (14) | |
| 50–74 | 1,898 (29) | ||
| 75–99 | 2,442 (37) | ||
| 100–149 | 917 (14) | ||
| 150 or more | 366 (6) | ||
NH: nursing home.
Mortality rates and excess deaths
A total of 44,666 NH residents died between 1 March and 31 May 2020, versus an average of 30,647 over the comparable time period in the reference cohorts. The cumulative mortality rate in NHs during the first wave was 9.5, versus 6.4% during the same period in the previous 6 years. In all age groups, the mortality rate was higher in males than in females (Table 2). The mortality rate increased with age (Table 2). There were an estimated 13,505 excess deaths among NH residents during the first wave of pandemic, giving an SMR of 1.43; hence, mortality increased by 43% in 2020, relative to the previous 6 years.
Table 2 .
Mortality during the first wavea of the COVID-19 epidemic in France, by sex and by age group
| Sex | Age group (years) | Number of residents | Number of deaths | Cumulative mortality rate (%)b | Number of excess deaths | SMRc |
|---|---|---|---|---|---|---|
| Both | All | 494,753 | 44,666 | 9.5 | 13,505 | 1.43 |
| males | All | 126,588 | 14,445 | 12.1 | 4,905 | 1.51 |
| 60–74 | 24,099 | 1,180 | 5.0 | 437 | 1.59 | |
| 75–84 | 30,957 | 3,132 | 10.7 | 1,282 | 1.69 | |
| 85–94 | 57,161 | 7,680 | 14.5 | 2,473 | 1.47 | |
| 95 or over | 14,371 | 2,453 | 18.8 | 714 | 1.41 | |
| Females | All | 368,165 | 30,221 | 8.6 | 8,599 | 1.38 |
| 60–74 | 22,755 | 851 | 3.8 | 322 | 1.61 | |
| 75–84 | 60,376 | 3,606 | 6.2 | 1,325 | 1.58 | |
| 85–94 | 201,898 | 16,230 | 8.4 | 4,684 | 1.41 | |
| 95 or over | 83,136 | 9,534 | 12.2 | 2,268 | 1.31 |
aBetween 1 March and 31 May 2020.
bNumber of deaths divided by the number of person-times between 1 March and 31 May 2020.
cSMR: standardized mortality ratio.
Extending our results to the residents of missing NH, we estimated that there were 15,114 excess deaths among the entire population of NH residents. There were 29,563 excess deaths in the general population in 2020 during the first wave of pandemic. According to these estimations, NH residents may have accounted for 51% of the excess deaths in the France’s population.
Changes over time in the death rate
The observed number of deaths per week exceeded the expected number of deaths from week 11 to week 26, with a peak in weeks 14 (5,486 deaths) and 15 (5,474 deaths) (Figure 1A). The weekly mortality rate reached 1.16% during week 15 (Figure 1B). More than 3,000 excess deaths per week were observed in weeks 14 and 15 (SMR: 2.37) (Figure 1C and D). We did not observe a mortality displacement at any time during the study period (ending in week 35, August 2020; Figure 1). By sensitivity analysis including new admissions in NH during follow-up, excess deaths estimates were similar (Figure S1).
Figure 1 .
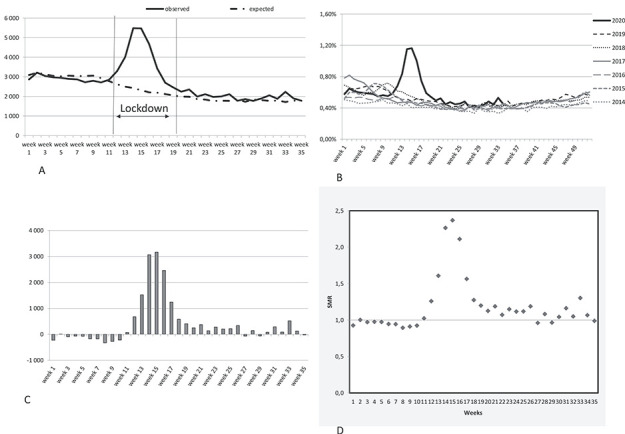
The weekly observed and expected deaths (A), mortality rates (B), weekly excess death (C) and standardized mortality ratios (D).
Age- and sex-specific mortality rates and excess deaths
The changes over time were similar in the four age groups and in both sexes (Women: Figure 2 and men: Figure S2). The excess mortality rate was higher among males than among females (Table 2) and decreased with age in females (Table 2 and Figure 2). In males, the SMR was higher in the 75–84 age group than in the 60–74 age group and then decreased again for older age groups (Table 2 and Figure S2).
Figure 2 .
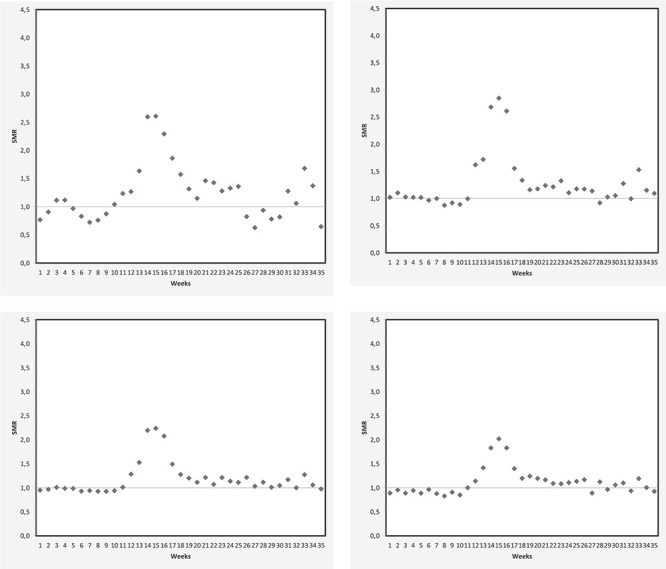
The weekly standardized mortality ratio in females aged 60–74 (A), 75–84(B), 85–94(C), and 95 or over (D).
Geographic distribution of mortality rates and excess deaths
At the NUTS3 level, the mortality rate among NH residents during the first wave of the pandemic ranged from 5.3% in Lozère (a rural département with the lowest population density) to 22.2% in the socially deprived Paris suburb of Seine-Saint-Denis. Accordingly, the SMR ranged from 0.92 in Lozère to 3.47 in Seine-Saint-Denis (Figure 3).
Figure 3 .
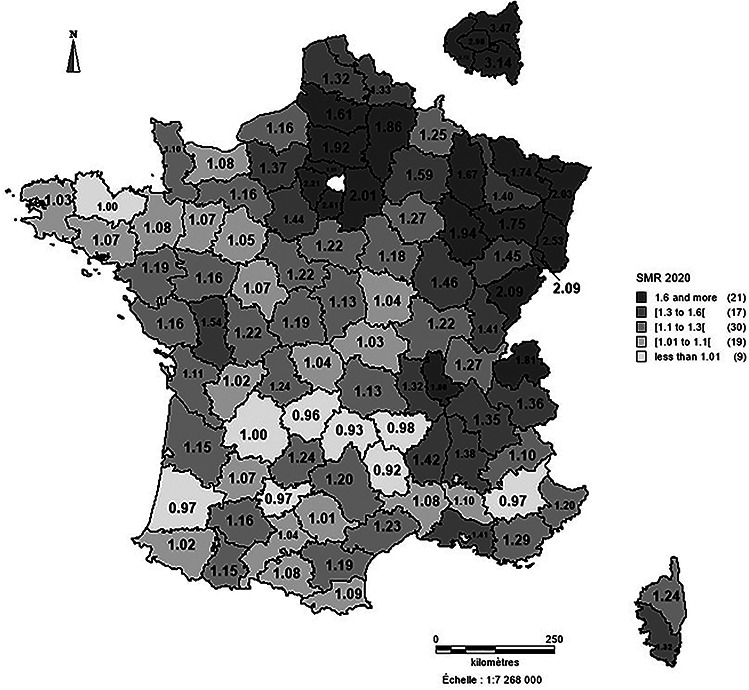
Standardized mortality ratios per level of territorial unit (defined according to the Nomenclature of Territorial Unit for Statistics).
Discussion
Among 494,753 residents of 6,515 NHs in mainland France, there was an estimated 13,505 excess deaths during the first wave of the COVID-19 pandemic (1 March to 31 May 2020), relative to the previous 6 years. The mortality increased by 43%. The excess mortality was higher in males than in females (51 and 38%, respectively) and decreased with age. The changes over time in excess mortality were similar in all age groups and in both sexes. At the peak of the first wave (weeks 14 and 15), there were more than 3,000 excess deaths (corresponding with SMRs of 2.26 and 2.37, respectively). The excess mortality varied greatly from one territorial unit to another (from −8 to +247%). We did not observe any mortality displacement at any time during the study period, which ended in week 35, 30 August 2020 (i.e. 3 months after the end of the first wave). After applying these estimates to the whole populations of NH residents, we estimate that there were 15,114 excess deaths. NH residents may have accounted for 51% of the excess mortality in the general population during the first wave of the COVID-19 pandemic.
The number of excess death estimated here (15,000) was slightly higher than the nationally reported number of COVID-19-related deaths among NH residents (14,000) [1]. The majority of studies in the literature reported on COVID-19-related deaths rather than excess deaths. In France and in England & Wales, respectively, 10,264 and 9,039 COVID-19-related deaths in NHs were reported between March and May 2020. Furthermore, 3,615 and 3,444 in-hospital COVID-19-related deaths of NH residents were, respectively, reported [1, 11]. A lack of PCR tests at the beginning of the pandemic, atypical clinical presentations in older people, reports of both suspected and confirmed cases to the health surveillance system, and (in France) erroneous reporting of the place of death by municipal offices of vital records, all led to uncertainty regarding deaths attributed to COVID-19 in NHs [21].
Moreover, as mentioned above, reporting at the start of the health crisis typically included in-hospital deaths but not deaths in NHs, and only the place of death (rather than the place of residence at the time of death) was taken into account [10, 11]. In England & Wales, the United Kingdom’s Office for National Statistics reported 57,074 excess deaths between March and May 2020, of which 26,211 (46%) occurred in care homes [10, 11]. In France, the INSEE estimated that the number of deaths in NHs increased by 54% between March and April 2020, compared with the previous year. However, these figures did not take account of NH residents who died after being transferred to a hospital. Moreover, there was a degree of uncertainty regarding the reporting on the place of death by municipal offices of vital records [10].
The mortality increase of 43%, i.e. substantially lower than the one observed in Wales care home, i.e. 72% [15]. This difference is in accordance with relative excess deaths increase observed in United Kingdom versus France in the general population [22].
The steep increase and then decrease in excess deaths during the first wave were similar to those observed in 65 NHs in New York City [23]. In France, however, the decrease in excess deaths began 4 weeks after visits with NH residents were prohibited (week 11) and became much more pronounced 3 weeks after the start of the nationwide period of lockdown (week 12). The mortality rate among NH residents in New York City decreased 5 weeks after visits were prohibited [23].
We also found an inverse relationship between the relative magnitude of excess deaths and increasing age in females. In males, the relative excess death rate was consistently lower in older age groups than in younger age groups, but the relationship was not linear: the relative number of excess deaths was higher in the 75–84 age group than in the 60–74 age group. Similar results have been observed in the general population [21]. In Italy, for instance, Conti and colleagues found a lower excess mortality in nonagenarians than in their younger counterparts [24]. This might have been due to a healthy survivor effect. Younger NH residents might have more comorbidities—notably stroke and diabetes, which are risk factors for COVID-19 severity and lethality—than older residents [5, 24–27]. Interestingly, a multicenter retrospective cohort study of 821 older COVID-19 patients (average age of 86 years) hospitalized in acute geriatric ward did not evidence an age-group-related difference in mortality after adjustment for comorbidities and dependency [27]. The excess mortality was higher among males than among females; this is in line with the well-known excess mortality among males in other settings [25]. This sex difference might also be due to the clinical profile of males in NHs, who are younger but have more cardiometabolic risk factors than females in NHs [20].
An acute health crisis can produce a mortality displacement as was suggested during the 2016 influenza A epidemics [28]. We did not observe a mortality displacement during the study period (ending in August 2020, i.e. three months after the end of the first wave). A similar observation was made in France after the August 2003 heatwave; 15,000 excess deaths were not followed by a lack of deaths until the following year [29]. This finding may indicate that the deaths observed during first wave of the COVID-19 pandemic had not affected only those whose health was already so compromised that they ‘would have died in the short term anyway’ but also compromised the health of the survivors. Another explanation might be the persistence of COVID-19 until August—the very beginning of the second wave. Lastly, COVID-19 survivors from the first wave may have been weakened and may have died afterwards.
In line with previous studies, we observed marked spatial heterogeneity in excess deaths among NH residents [23, 30]. This heterogeneity might be due to differences in the regional distribution of SARS-CoV-2 infections (notably in northeast France), urban density and other environmental factors for infection risk [23, 31, 32].
The pandemic and the corresponding public health responses had both direct and indirect effects on the general population by increasing social isolation, reducing the frequency of road traffic accidents and occupational injuries, and restricting access to care for health conditions other than COVID-19 [12, 13, 22, 24, 32, 33]. Similarly, the pandemic and the corresponding public health responses might have had both direct and indirect effects on NHs. The present study cannot draw firm conclusions about the direct link between SARS-COV-2 pandemic and excess deaths in NH. However, the number of excess deaths close from number of COVID-19-related deaths among NH residents, the changes over time in excess deaths and the concentration of these deaths in the territorial units most strongly affected by SARS-CoV-2 argue in favour of a direct effect.
The present study had several strengths. Firstly, the nationwide setting enabled us to extrapolate our results to the entire population of NH residents. Secondly, access to reference data from the previous 6 years in the same population enabled us to smooth out year-on-year variations in mortality. However, given that the impact of influenza epidemics on NH mortality varies widely from one year to the next, it would be interesting to compare 2020’s excess deaths with each single reference year as a function of the influenza epidemic at that time.
The present study has several limitations. Firstly, 13% of NH residents were missing in RESID-EHPAD database leading to an estimation of national excess deaths. Secondly, causes of deaths were not available. Thirdly, individual characteristics of residents except age and sex (e.g. comorbidities, frailty, dependency) were not analyzed.
Conclusion
NH residents may have accounted for half of the total excess deaths in France during the first wave of the COVID-19 pandemic. The excess death rate was higher among males than females and among younger than older residents.
Real-time mortality surveillance in both NHs and hospitals is critically important for monitoring epidemics and other health crises and for guiding public health decisions [34]. The analysis of causes of death (when available) should help to differentiate between deaths directly vs. indirectly caused by COVID-19. A refined analysis is required to identify individual and environmental risk factors associated with SARS-CoV-2 infections and COVID-19 lethality. In particular, the roles of comorbidities, old age, dependency, the resident: staff ratio, the use of personal protective equipment, NH crowding, population density and other socio-economic factors must be characterized before models of care and nursing for dependent older adults are designed [35].
Supplementary Material
Contributor Information
Florence Canouï-Poitrine, Univ Paris Est Creteil, Inserm, IMRB U955, CEpiA Team, F-94000 Creteil, France; Public Health Department, APHP, Henri-Mondor Hospital, F-94000 Creteil, France.
Antoine Rachas, Direction de la Stratégie, des Etudes et des Statistiques, CNAM, F-75000 Paris, France.
Martine Thomas, Direction de la Stratégie, des Etudes et des Statistiques, CNAM, F-75000 Paris, France.
Laure Carcaillon-Bentata, Santé Publique France (SpF), F-94410 Saint-Maurice, France.
Roméo Fontaine, INED, Mortality, Health and Epidemiology (UR5), F-93300 Aubervilliers, France.
Gaëtan Gavazzi, Geriatric Department, Grenoble Alpes University Hospital, F-38000 Grenoble, France; University of Grenoble-Alpes, GREPI TIMC-IMAG, CNRS UMR 552, F-38000 Grenoble, France.
Marie Laurent, Univ Paris Est Creteil, Inserm, IMRB U955, CEpiA Team, F-94000 Creteil, France; Geriatric Department, APHP, Henri-Mondor Hospital, F-94000 Creteil, France.
Jean-Marie Robine, INED, Mortality, Health and Epidemiology (UR5), F-93300 Aubervilliers, France; Univ Paris, INSERM, CNRS, EHSS, CERMES3, F-75000 Paris, France; Univ Montpellier, EPHE, INSERM, MMDN, F-34000 Montpellier, France; PSL Research University, F-75000 Paris, France.
Declaration of Conflict of Interest
None.
Declaration of Sources of Funding
None.
References
- 1.Santé Publique France (National Public Health Agency) , COVID-19—Point Epidemiologique hebdomadaire du 4 juin 2020 (COVID-19- Weekly epidemiological point, 4 June 2020). 2020. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-4-juin-2020(accessed 25 November 2020).
- 2.Severe Outcomes Among Patients with Coronavirus Disease . 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep 2020; 69: 343–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Z, McGoogan JM. Characteristics of and Important Lessons From the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases From the Chinese Center for Disease Control and Prevention. JAMA 2020; 323: 1239–42. [DOI] [PubMed] [Google Scholar]
- 4.Noor FM, Islam MM. Prevalence and Associated Risk Factors of Mortality Among COVID-19 Patients: A Meta-Analysis. J Community Health 2020; 45: 1270–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ioannou GN, Locke E, Green Pet al. . Risk Factors for Hospitalization, Mechanical Ventilation, or Death Among 10131 US Veterans With SARS-CoV-2 Infection. JAMA Netw Open 2020; 3: e2022310. doi: 10.1001/jamanetworkopen.2020.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnett M.L, Grabowski D.C. Nursing Homes Are Ground Zero for COVID-19 Pandemic. 2020. https://jamanetworkcom/channels/health-forum/fullarticle/2763666(accessed 25 November 2020). [DOI] [PubMed]
- 7.O’Dowd A. Care home deaths in England and Wales rise sharply. 2020. https://www.bmj.com/content/369/bmj.m1727(accessed 25 November 2020). [DOI] [PubMed]
- 8.INED Institut National des Etudes Démographiques , National Demographic Studies Institute, https://dc-covid.site.ined.fr/en/presentation/(accessed 25 November 2020).
- 9.Team EPHE, Danis K, Fonteneau Let al. . High impact of COVID-19 in long-term care facilities, suggestion for monitoring in the EU/EEA, May 2020. Euro Surveill 2020; 25. doi: 10.2807/1560-7917.ES.2020.25.22.2000956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.INSEE (Institut National de la Statistique et des Etudes Economiques-National Institute for Statistic and Economic Studies) . Évolution du nombre de décès entre le 1er mars et le 30 avril 2020 (Evolution of deaths number between 1st march and 30 April 2020). 2020. https://www.insee.fr/fr/statistiques/4500439?sommaire=4487854#consulter-sommaire(accessed 25 November 2020).
- 11.ONS (Office National of Statistic) . Deaths involving COVID-19 in the care sector, England and Wales: deaths occurring up to 12 June 2020 and registered up to 20 June 2020 (provisional). 2020. https://www.ons.gov.uk/peoplepopulationandcommunity/birthsdeathsandmarriages/deaths/articles/deathsinvolvingcovid19inthecaresectorenglandandwales/deathsoccurringupto12june2020andregisteredupto20june2020provisional(accessed 25 November 2020).
- 12.Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L. Excess Deaths From COVID-19 and Other Causes, March-April 2020. JAMA 2020; 324: 510–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolf SH, Chapman DA, Sabo RT, Weinberger DM, Hill L, Taylor DDH. Excess Deaths From COVID-19 and Other Causes, March-July 2020. JAMA 2020; 324: 1562–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atramont A, Bourdel-Marchasson I, Bonnet-Zamponi D, Tangre I, Fagot-Campagna A, Tuppin P. Impact of nursing home admission on health care use and disease status elderly dependent people one year before and one year after skilled nursing home admission based on 2012-2013 SNIIRAM data. BMC Health Serv Res 2017; 17: 667. doi: 10.1186/s12913-017-2620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollinghurst J, Lyons J, Fry Ret al. . The impact of COVID-19 on adjusted mortality risk in care homes for older adults in Wales, UK: a retrospective population-based cohort study for mortality in 2016-2020. Age Ageing 2021; 50, 25–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Müller M.L’accueil des personnes âgées en établissements: entre progression et diversification de l’offre. Les Dossiers de la Drees. Septembre 2017. Numéro 20. https://drees.solidarites-sante.gouv.fr/sites/default/files/2020-08/dd20_resultats_ehpa_2015.pdf(accessed 20 March 2021).
- 17.Fizzala A. Dépendance des personnes âgées: qui paie quoi? Les dossiers de la Drees. 2016. Numéro 1. https://www.epsilon.insee.fr/jspui/bitstream/1/63436/1/dossiers_1.pdf(accessed 20 March 2021). [Google Scholar]
- 18.Besnard X, Zakri M. Comment les seniors financent-ils leur maison de retraite. Etudes et Résulats. 2018 Numéro 1095. https://www.epsilon.insee.fr/jspui/bitstream/1/87433/1/er1095.pdf(accessed 20 March 2021).
- 19.Cilek L, Chowell G, Ramiro Fariñas D. Age-Specific Excess Mortality Patterns During the 1918-1920 Influenza Pandemic in Madrid, Spain. Am J Epidemiol 2018; 187: 2511–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Atramont A, Bonnet-Zamponi D, Bourdel-Marchasson I, Tangre I, Fagot-Campagna A, Tuppin P. Health status and drug use 1 year before and 1 year after skilled nursing home admission during the first quarter of 2013 in France: a study based on the French National Health Insurance Information System. Eur J Clin Pharmacol 2018; 74: 109–18. [DOI] [PubMed] [Google Scholar]
- 21.Fouillet, A. Surveillance de la mortalité au cours de l'épidémie de COVID-19 du 2 mars au 31 mai 2020 en France. Surveillance of mortality during COVID-19 epidemics from March, 2 to May, 31th, 2020 in France. 2020. https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/rapport-synthese/surveillance-de-la-mortalite-au-cours-de-l-epidemie-de-covid-19-du-2-mars-au-31-mai-2020-en-france(accessed 25 November 2020).
- 22.Kontis V, Bennett JE, Rashid Tet al. . Magnitude, demographics and dynamics of the effect of the first wave of the COVID-19 pandemic on all-cause mortality in 21 industrialized countries. Nat Med 2020. doi: 10.1038/s41591-020-1112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnett ML, Hu L, Martin T, Grabowski DC. Mortality, Admissions, and Patient Census at SNFs in 3 US Cities During the COVID-19 Pandemic. JAMA 2020; 324: 507–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Conti S, Ferrara P, Mazzaglia Get al. . Magnitude and time-course of excess mortality during COVID-19 outbreak: population-based empirical evidence from highly impacted provinces in northern Italy. ERJ Open Res 2020; 6. doi: 10.1183/23120541.00458-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fang X, Li S, Yu Het al. . Epidemiological, comorbidity factors with severity and prognosis of COVID-19: a systematic review and meta-analysis. Aging (Albany NY) 2020; 12: 12493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller LE, Bhattacharyya R, Miller AL. Diabetes mellitus increases the risk of hospital mortality in patients with Covid-19: Systematic review with meta-analysis. Medicine (Baltimore) 2020; 99: e22439. doi: 10.1097/md.0000000000022439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zerah L, Baudouin E, Pepin Met al. . Clinical Characteristics and Outcomes of 821 Older Patients with SARS-Cov-2 Infection Admitted to Acute Care Geriatric Wards. J Gerontol A Biol Sci Med Sci 2020. doi: 10.1093/gerona/glaa210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lytras T, Pantavou K, Mouratidou E, Tsiodras S. Mortality attributable to seasonal influenza in Greece, 2013 to 2017: variation by type/subtype and age, and a possible harvesting effect. Euro Surveill 2019; 24. doi: 10.2807/1560-7917.ES.2019.24.14.1800118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fouillet A, Rey G, Laurent Fet al. . Excess mortality related to the August 2003 heat wave in France. Int Arch Occup Environ Health 2006; 80: 16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamidi S, Ewing R, Sabouri S. Longitudinal analyses of the relationship between development density and the COVID-19 morbidity and mortality rates: Early evidence from 1,165 metropolitan counties in the United States. Health Place 2020; 64: 102378. doi: 10.1016/j.healthplace.2020.102378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaudart J, Landier J, Huiart L, et al. Factors associated with the spatial heterogeneity of the first wave of COVID-19 in France: a nationwide geo-epidemiological study. Lancet Public Health 2021; 6: e222–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mesnier J, Cottin Y, Coste Pet al. . Hospital admissions for acute myocardial infarction before and after lockdown according to regional prevalence of COVID-19 and patient profile in France: a registry study. Lancet Public Health 2020; 5: e536–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.MacDonald DRW, Neilly DW, Davies PSEet al. . Effects of the COVID-19 lockdown on orthopaedic trauma: a multicentre study across Scotland. Bone Jt Open 2020; 1: 541–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanratty B, Burton JK, Goodman C, Gordon AL, Spilsbury K. Covid-19 and lack of linked datasets for care homes. BMJ 2020; 369: m2463. doi: 10.1136/bmj.m2463. [DOI] [PubMed] [Google Scholar]
- 35.Brown KA, Jones A, Daneman Net al. . Association Between Nursing Home Crowding and COVID-19 Infection and Mortality in Ontario, Canada. JAMA Intern Med 2020. doi: 10.1001/jamainternmed.2020.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


