Abstract
Background
Estimates of coronavirus disease 2019 (COVID-19) vaccine effectiveness under real-world conditions, and understanding of barriers to uptake, are necessary to inform vaccine rollout.
Methods
We enrolled cases (testing positive) and controls (testing negative) from among the population whose SARS-CoV-2 molecular diagnostic test results from 24 February to 29 April 2021 were reported to the California Department of Public Health. Participants were matched on age, sex, and geographic region. We assessed participants’ self-reported history of mRNA-based COVID-19 vaccine receipt (BNT162b2 and mRNA-1273). Participants were considered fully vaccinated 2 weeks after second dose receipt. Among unvaccinated participants, we assessed willingness to receive vaccination. We measured vaccine effectiveness (VE) via the matched odds ratio of prior vaccination, comparing cases with controls.
Results
We enrolled 1023 eligible participants aged ≥18 years. Among 525 cases, 71 (13.5%) received BNT162b2 or mRNA-1273; 20 (3.8%) were fully vaccinated with either product. Among 498 controls, 185 (37.1%) received BNT162b2 or mRNA-1273; 86 (16.3%) were fully vaccinated with either product. Two weeks after second dose receipt, VE was 87.0% (95% confidence interval: 68.6–94.6%) and 86.2% (68.4-93.9%) for BNT162b2 and mRNA-1273, respectively. Fully vaccinated participants receiving either product experienced 91.3% (79.3–96.3%) and 68.3% (27.9–85.7%) VE against symptomatic and asymptomatic infection, respectively. Among unvaccinated participants, 42.4% (159/375) residing in rural regions and 23.8% (67/281) residing in urban regions reported hesitancy to receive COVID-19 vaccination.
Conclusions
Authorized mRNA-based vaccines are effective at reducing documented SARS-CoV-2 infections within the general population of California. Vaccine hesitancy presents a barrier to reaching coverage levels needed for herd immunity.
Keywords: COVID-19, real-world evidence, test-negative design, vaccine effectiveness
Vaccination is preventing documented SARS-CoV-2 infection in California, with 68% and 91% effectiveness against asymptomatic and symptomatic infection, respectively. Vaccine effectiveness was equivalent for BNT126b2 and mRNA-1273. Only 66% of unvaccinated participants were willing to receive the vaccine when eligible.
After being found safe and efficacious in preventing coronavirus disease 2019 (COVID-19) in randomized controlled trials [1–3], vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are being administered to the general public under emergency use authorization. Two mRNA-based vaccines encoding the SARS-CoV-2 spike protein, BNT162b2 (Pfizer/BioNTech) and mRNA-1273 (Moderna), have been the main products in use since December 2020. By early May 2021, 40% of California residents were considered fully vaccinated [4].
Observational studies characterizing COVID-19 vaccine effectiveness (VE) are needed to understand performance under real-world conditions [5]—for instance, evaluating VE against clinical endpoints not addressed in trials and defining VE for alternative dosing schedules [6]. While many studies of real-world VE have followed healthcare workers and other essential or frontline personnel [7–9], vaccine eligibility rapidly expanded to include broader population groups during early 2021 throughout the United States. In California, vaccination was offered to healthcare workers beginning 14 December 2020, and expanded to persons at increased risk due to older age or occupation (including workers in emergency services, food and agriculture, or childcare and education) during January and February 2021. Eligibility was extended to persons aged 16–64 years with high-risk medical conditions in March 2021 and to all persons aged 16 years and older on 15 April 2021. To inform vaccination efforts, it is crucial to understand VE within the general population, and to identify reasons behind individuals’ decisions to delay or defer vaccination.
In conjunction with epidemiologic surveillance, we initiated a test-negative case-control study design to monitor VE within the general population of California in real time. Over the study period (24 February 2021 to 29 April 2021), sequenced SARS-CoV-2 isolates in California were predominantly identified as B.1.427/429 (50–60%) variants in February and March; by April, the B.1.1.7 variant overtook other lineages and accounted for 49% of sequenced SARS-CoV-2 isolates, as compared to 6% in February, while the proportion of B.1.427/429 variants declined to approximately 20% [10]. Here we provide an assessment of VE for authorized mRNA-based COVID-19 vaccines, and report data on the intentions of unvaccinated participants to receive vaccination.
METHODS
Design
All diagnostic tests in California for SARS-CoV-2 are reported by laboratories and medical providers to their local health jurisdiction (LHJ). Sixty of 61 LHJs report data directly to the California Department of Public Health (CDPH) via a web-based reporting system, while Los Angeles County transmits data daily via an electronic file. California residents with molecular SARS-CoV-2 test results (eg, polymerase chain reaction [PCR]) between 24 February and 29 April 2021 and a telephone number were eligible for participation in this study. Cases were defined as persons with positive molecular SARS-CoV-2 test results during the study time frame. Controls were persons with negative SARS-CoV-2 molecular test results during the same period.
Each day during the study period, we prospectively selected cases with a telephone number and newly reported positive molecular test result within each of 9 regions of the state, sampling cases at random with intent to enroll equally across regions (Supplementary Table 1). For each case who consented and completed the study interview, we attempted to enroll and interview 1 control from a sample of 30 controls randomly selected to match the case by age (18–39, 40–64, ≥65 years), sex, region, and week of SARS-CoV-2 test. Up to 2 call attempts were made for each case and control. Call shifts were scheduled to cover mornings, afternoons, and evenings each day.
To mitigate bias resulting from previous infection-derived immunity [6], participants who recalled receiving any previous positive test result for SARS-CoV-2 infection or seropositivity, prior to the reported test, were not eligible to continue the interview. This analysis excludes data from children aged 0–17 years, who were generally ineligible for COVID-19 vaccination over the study period, and participants who reported receiving COVID-19 vaccinations other than BNT162b2 or mRNA-1273 (due to limited coverage of a third authorized vaccine, Ad26.COV2.S, over the study period), or receipt of COVID-19 vaccination without knowledge of vaccination dates.
Exposures
We administered a standardized questionnaire via facilitated telephone interviews in English or Spanish to collect data on participant demographics, symptoms, and vaccination status. We asked participants to indicate whether they had received any COVID-19 vaccine, and to reference their COVID-19 vaccination card to report the manufacturer, number, and dates of doses received. We also asked unvaccinated participants whether they would be willing to receive a COVID-19 vaccine when eligible; if participants indicated they were not likely to receive a vaccine or were unsure, we asked them to state reasons behind their hesitancy. Additionally, we asked participants to indicate reasons they sought a COVID-19 test and presence of any COVID-19 symptoms within the 14 days prior to their test date (Supplementary Text 1).
The study protocol was granted a non-research determination by the State of California Health and Human Services Agency Committee for the Protection of Human Subjects (project number: 2021-034).
Statistical Analysis
Our primary objective was to estimate VE of 2 doses of BNT162b2 or mRNA-1273 against documented SARS-CoV-2 infection, 2 weeks or more after receipt of the second dose of either vaccine. To estimate VE, we calculated the Mantel Haenszel (matched) odds ratio (ORMH) of vaccination among cases relative to test-negative controls [5, 6]. We used conditional logistic regression models defining match strata by age group, sex, region, and testing week to estimate the ORMH (and accompanying 95% confidence interval [CI]). We defined fully vaccinated status as receipt of 2 doses of BNT162b2 or mRNA-1273 2 or more weeks before participants’ date of testing; unvaccinated status was the reference exposure. We calculated adjusted VE as (1 − ORMH) × 100%. We determined that analyses with 500 cases and 500 controls would provide 90% statistical power for estimating VE of 55% or greater at the 2-sided P < .05 confidence threshold, assuming 10% of controls were fully vaccinated. We did analyses in R software (version 3.6.1; R Foundation for Statistical Computing, Vienna, Austria).
As secondary analyses, we also aimed to assess VE for incomplete vaccination series, VE for each product, and VE against SARS-CoV-2 infection endpoints corresponding to differing levels of clinical severity. To determine VE for incomplete vaccination series, we defined exposures as receipt of 1 dose or 2 doses of BNT162b2 or mRNA-1273 within 1–7 or 8–14 days before participants’ testing date, or 1 dose of BNT162b2 or mRNA-1273 15 or more days before participants’ testing date. As described above, we used conditional logistic regression models to compute the ORMH comparing cases with controls.
To determine product-specific VE, we restricted the vaccinated population to participants who received 2 doses of either BNT162b2 or mRNA-1273 15 or more days before their date of testing. To determine VE against differing clinical endpoints, we conducted analyses restricting cases to participants testing positive with symptoms, without symptoms, who were hospitalized for COVID-19, who reported seeking healthcare or advice via outpatient or virtual interactions with healthcare providers, and who did not seek treatment or advice from a healthcare provider beyond receipt of a molecular SARS-CoV-2 diagnostic testing. Each of these groupings of cases was compared against match-eligible controls to compute the ORMH of vaccination (defined as 2 doses received ≥15 days prior vs no doses received), using the same conditional logistic regression framework described above. For these secondary analyses, sufficient counts were not available to further stratify VE estimates by doses received and time since receipt.
Last, to understand factors predicting vaccine hesitancy among participants who had not yet received COVID-19 vaccination, we fit logistic regression models defining hesitancy to receive vaccination as the outcome; covariates selected a priori for inclusion as potential causal factors were age group, region, sex, income, and race/ethnicity. Participants who reported being unwilling or unsure about receiving a COVID-19 vaccine when eligible were considered vaccine hesitant. As missing data were present in participants’ responses regarding income (189/656; 28.8%) and race (10/656; 1.5%), we conducted analyses of vaccine hesitancy across 5 datasets generated through multiple imputation by chained equations using the Amelia II package in R [11]. Under the assumption that data were missing conditionally at random, given observations of other covariates, all variables included in the analyses model were included in the imputation models. We compared measures of association with those resulting from complete-case analysis without imputation as a supplemental check.
RESULTS
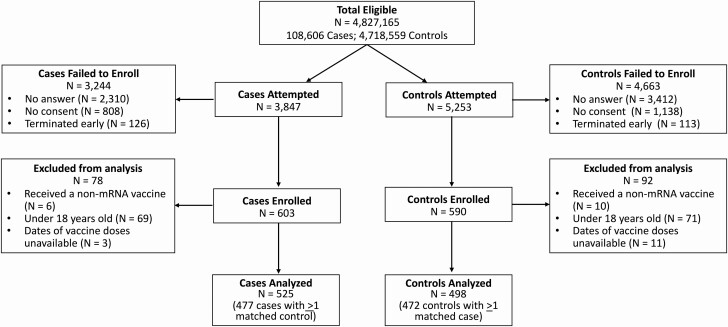
From 24 February to 29 April 2021, 4 827 165 SARS-CoV-2 molecular test results were reported to CDPH with a telephone number and indication of individuals’ age, sex, and region of residence (108 606 positive and 4 718 559 negative) (Figure 1, Supplementary Figures 1 and 2). We called 3847 cases and 5253 controls, among whom we enrolled 603 cases (15.7%) and 590 controls (11.2%). Among participants enrolled, 78 cases and 92 controls who were ineligible for the analyses reported here, including participants who were younger than 18 years old, received COVID-19 vaccines other than BNT162b2 or mRNA-1273 or were unable to provide precise dates of COVID-19 vaccine receipt. Our final study population included 525 cases and 498 controls, among these, 477 cases and 472 cases had eligible matches and thus contributed to conditional logistic regression analyses for VE estimation. While most strata included 1:1 (case:control) matches, 25 strata matched multiple controls to 1 case, and 33 strata matched multiple cases to 1 control (Table 1, Supplementary Table 2). Among participants enrolled, 20.9% (214/1023) and 98.3% (1006/1023) were contacted within 3 days and within 7 days of their test results being posted, respectively.
Figure 1.
Enrollment of participants in the California COVID-19 Case-Control Study. Data in the figure indicate numbers of tests reported, cases and controls for whom contact was attempted, and excluded and enrolled participants for this analysis. Abbreviation: COVID-19, coronavirus disease 2019.
Table 1.
Distribution of Cases and Controls
| Overall (N = 1023) | Cases (n = 525) | Controls (n = 498) | |
|---|---|---|---|
| Age | |||
| 18–29 y | 395 (38.6) | 200 (38.1) | 195 (39.2) |
| 30–49 y | 363 (35.5) | 188 (35.8) | 175 (35.1) |
| 50–64 y | 192 (18.8) | 100 (19.0) | 92 (18.5) |
| ≥65 y | 73 (7.1) | 37 (7.0) | 36 (7.2) |
| Region | |||
| Predominantly urban regions | |||
| San Francisco Bay area | 129 (12.6) | 66 (12.6) | 63 (12.7) |
| Greater Los Angeles area | 91 (8.9) | 48 (9.1) | 43 (8.6) |
| Greater Sacramento area | 115 (11.2) | 58 (11.0) | 57 (11.4) |
| San Diego and southern border | 110 (10.8) | 54 (10.3) | 56 (11.2) |
| Predominantly rural regions | |||
| Central Coast | 140 (13.7) | 74 (14.1) | 66 (13.3) |
| Northern Sacramento Valley | 116 (11.3) | 60 (11.4) | 56 (11.2) |
| San Joaquin Valley | 106 (10.4) | 54 (10.3) | 52 (10.4) |
| Northwestern California | 108 (10.6) | 55 (10.5) | 53 (10.6) |
| Sierras region | 108 (10.6) | 56 (10.7) | 52 (10.4) |
| Sex | |||
| Male | 519 (50.7) | 264 (50.3) | 255 (51.2) |
| Female | 504 (49.3) | 261 (49.7) | 243 (48.8) |
| Household income | |||
| Under $50 000 | 272 (26.6) | 153 (29.1) | 119 (23.9) |
| $50 000 to $100 000 | 220 (21.5) | 113 (21.5) | 107 (21.5) |
| $100 000 to $150 000 | 121 (11.8) | 45 (8.6) | 76 (15.3) |
| Over $150 000 | 135 (13.2) | 64 (12.2) | 71 (14.3) |
| Refused | 154 (15.1) | 86 (16.4) | 68 (13.7) |
| Not sure | 121 (11.8) | 64 (12.2) | 57 (11.4) |
| Race/ethnicity | |||
| White | 444 (43.4) | 217 (41.4) | 227 (45.6) |
| Hispanic | 286 (28.0) | 160 (30.5) | 126 (25.3) |
| Asian | 115 (11.3) | 58 (11.1) | 57 (11.4) |
| Black | 47 (4.6) | 30 (5.7) | 17 (3.4) |
| More than 1 race | 89 (8.7) | 36 (6.9) | 53 (10.6) |
| Native American | 16 (1.6) | 11 (2.1) | 5 (1.0) |
| Native Hawaiian | 10 (1.0) | 4 (0.8) | 6 (1.2) |
| Refused | 15 (1.5) | 8 (1.5) | 7 (1.4) |
| Vaccination | |||
| Unvaccinated | 767 (75.0) | 454 (86.5) | 313 (62.9) |
| Incompletely vaccinated | 150 (14.7) | 51 (9.7) | 99 (19.9) |
| Fully vaccinateda | 106 (10.4) | 20 (3.8) | 86 (17.3) |
Data are presented as n (%).
aAn individual was considered “fully vaccinated” >14 days after 2 doses of Pfizer/BioNTech BNT162b2 or Moderna mRNA-1273 and “incompletely vaccinated” if they received only 1 dose or 2 doses <14 days after the second dose
Among 525 cases, 288 (54.9%) indicated they were tested due to concerns about symptoms. Of these 288 symptomatic cases, 262 (91.0%) were unvaccinated and 26 (9.0%) had received 1 or more vaccine dose (Table 2). Among 498 controls, 56 (11.2%) sought testing due to symptoms, among whom 43 (76.8%) were unvaccinated and 13 (23.2%) had received 1 or more vaccine dose. The most common reason for testing among controls was routine screening required for work or school attendance (233/498; 46.8%), whereas the most common reasons for testing among cases were symptoms (288/525; 54.9%) and known contact with a positive case (173/525; 33.0%).
Table 2.
Reasons for Testing
| Controls | Cases | |||
|---|---|---|---|---|
| Reasonsa | Unvaccinated (n = 313) | Vaccinatedb (n = 185) | Unvaccinated (n = 454) | Vaccinated (n = 71) |
| Contact with positive case | 28 (8.9) | 8 (4.3) | 143 (31.5) | 30 (42.3) |
| Contact with symptomatic individual | 12 (3.8) | 4 (2.2) | 18 (4.0) | 2 (2.8) |
| Told by public health worker to get tested | 1 (0.3) | 1 (0.5) | 3 (0.7) | 0 (0.0) |
| Routine screening for my work or school | 120 (38.3) | 113 (61.1) | 29 (6.4) | 17 (23.9) |
| Test required for medical procedure or hospital admittance | 43 (13.7) | 25 (13.5) | 16 (3.5) | 5 (7.0) |
| Someone in household had contact with a positive case | 4 (1.3) | 0 (0.0) | 11 (2.4) | 0 (0.0) |
| Test required to attend public event/share public space | 2 (0.3) | 0 (0.0) | 1 (0.5) | 0 (0.0) |
| I just wanted to see if I was infected | 71 (22.7) | 18 (9.7) | 43 (9.5) | 4 (5.6) |
| Concerned about symptoms | 43 (13.7) | 13 (7.0) | 262 (57.7) | 26 (36.6) |
| Pre- or post-travel screening | 21 (6.7) | 7 (3.8) | 17 (3.7) | 4 (5.6) |
Data are presented as n (%).
Abbreviation: SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aSince interviewers indicated all reasons listed by participants, reasons will not sum to the total sample size.
bAn individual is considered vaccinated if they have had at least 1 dose of an SARS-CoV-2 mRNA vaccine.
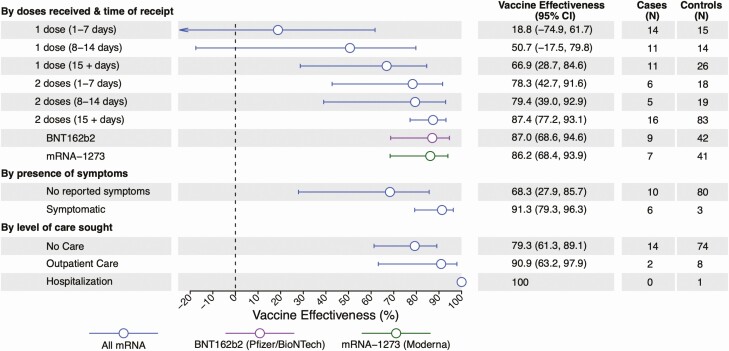
Among 525 cases, 43 (8.2%) and 28 (5.3%) reported receiving 1 or more dose of BNT162b2 and mRNA-1273, respectively (Figure 2, Table 1, Supplementary Table 3). Among 498 controls, 98 (19.7%) and 87 (17.5%) had received 1 or more dose of BNT162b2 and mRNA-1273, respectively. Twenty cases (3.8% of 525) and 86 (17.3% of 498) controls were fully vaccinated with either product, with 15 or more days passing from receipt of their second dose to their testing date. A majority of both vaccinated and unvaccinated participants agreed with the importance of masking and social distancing to prevent COVID-19, and vaccinated and unvaccinated participants were equally likely to report feeling anxious about COVID-19 (Supplementary Table 4). For fully vaccinated participants receiving either BNT162b2 or mRNA-1273, VE was 87.4% (95% confidence interval [CI]: 77.2–93.1%).
Figure 2.
COVID-19 vaccine effectiveness, by doses received and time since last dose. Lines denote 95% confidence intervals, respectively, for estimates of vaccine effectiveness. Estimates were calculated via conditional logistic regression. Estimates for the presence of symptoms and level of care sought compare fully vaccinated versus unvaccinated participants only. Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019.
We did not identify protection within the first 7 days after receipt of a first BNT162b or mRNA-1273 dose (VE: 18.8%; 95% CI: –74.9 to 61.7%). Within the second week after receipt of a first dose for either vaccine, VE was 50.7% (–17.5 to 79.8%); 15 or more days after receipt of a first dose, and before receipt of a second dose, VE was 66.9% (28.7–84.6%). Following receipt of a second dose, VE was 78.3% (42.7–91.6%) at days 1–7 and 79.4% (39.0–92.9%) at days 8–14. Vaccine effectiveness estimates were similar in analyses that restricted or did not restrict the sample to participants who reported consulting their vaccination cards or calendars during the telephone interview to confirm dates of receipt of each dose (Supplementary Figure 3).
Protection among fully vaccinated participants did not differ according to the product received; among recipients of BNT162b and mRNA-1273, VE was 87.0% (95% CI: 68.6–94.6%) and 86.2% (68.4–93.9%), respectively (Figure 2).
Among fully vaccinated cases, 45.0% (9/20) reported experiencing 1 or more symptom, in contrast to 78.0% (354/454) of unvaccinated cases, 66.7% (34/41) of partially vaccinated cases, and 13.7% (68/498) of controls (Supplementary Table 5). For symptomatic and asymptomatic infection endpoints, VE was 91.3% (95% CI: 79.3–96.3%) and 68.3% (27.9–85.7%), respectively, at 15 or more days after the second dose (Figure 2).
Eighteen (3.4%) of 525 cases were hospitalized by the time of our telephone interview, among whom 15 (83.3%) were unvaccinated and 3 (16.7%) were partially vaccinated (Supplementary Table 5). Among all 525 cases, 150 (28.6%) sought treatment, care, or advice via outpatient or virtual interactions with healthcare providers, among whom 132 (25.1%) were unvaccinated, 15 (2.9%) were incompletely vaccinated, and 3 (0.6%) were fully vaccinated. Among 128 cases who did not experience symptoms, 103 (80.4%) did not seek care. Considering these differing levels of care sought for SARS-CoV-2 infection, VE was 79.3% (95% CI: 61.3–89.1%) against episodes for which cases did not seek treatment or advice, 90.9% (63.2–97.9%) against episodes for which cases sought healthcare through outpatient or virtual interactions, and 100% (with undefined confidence limits) against hospitalized illness (Figure 2).
Overall, 226 (34.5%) of 656 unvaccinated participants (including 139/403 [34.5%] unvaccinated cases and 87/253 [34.4%] unvaccinated controls) indicated they were unlikely to receive or were unsure about receiving COVID-19 vaccination when eligible (Table 3, Supplementary Tables 6 and 7). Residents of rural regions had 2.42-fold (1.66- to 3.52-fold) higher adjusted odds of reporting hesitancy to receive vaccination, when eligible, whereas hesitancy to receive vaccination was not independently associated with age or household income. Adjusted odds of reporting hesitancy to receive vaccination were 1.47-fold (1.04- to 2.08-fold) higher among females than males. In comparisons by participants’ race/ethnicity, adjusted odds of reporting hesitancy to receive vaccination were 2.54-fold (1.24- to 5.15-fold) higher among non-Hispanic Black participants than non-Hispanic Whites; in contrast, adjusted odds of vaccine hesitancy were .72-fold (.46- to 1.12-fold) as high among Hispanic participants as among non-Hispanic Whites. Point estimates of odds ratios were similar in complete-case analyses without imputation (Supplementary Table 8). Fears over vaccine side effects (66/219 [30.1%]) or safety (60/219 [27.4%]) were the most common concerns among participants expressing hesitancy to receive vaccination (Table 4). No participants cited cost, inconvenience, or inability to access a COVID-19 vaccination site as a reason for not receiving vaccination.
Table 3.
Predictors of Vaccine Hesitancy
| Enthusiasm to Receive Vaccination, n (%) | Odds Ratio (95% CI) | |||
|---|---|---|---|---|
| Participant Characteristics | Not Willing/Unsure (n = 226) | Willing (n = 430) | Unadjusted | Adjusted |
| Case statusa | ||||
| Case with SARS-CoV-2 infection | 139 (61.5) | 264 (61.4) | N/A | N/A |
| Uninfected control | 87 (38.5) | 166 (38.6) | N/A | N/A |
| Age | ||||
| 18–29 y | 82 (36.3) | 189 (44.0) | Ref | Ref |
| 30–49 y | 93 (41.2) | 147 (34.2) | 1.45 (1.01, 2.10) | 1.45 (.97, 2.16) |
| 50–64 y | 34 (15.0) | 76 (17.7) | 1.03 (.64, 1.66) | .77 (.46, 1.28) |
| ≥65 y | 17 (7.5) | 18 (4.2) | 2.20 (1.07, 4.40) | 1.66 (.77, 3.57) |
| Region | ||||
| Predominantly urban regionsb | 67 (29.6) | 214 (49.8) | Ref | Ref |
| Predominantly rural regionsc | 159 (70.4) | 216 (50.2) | 2.35 (1.66, 3.29) | 2.42 (1.66, 3.52) |
| Sex | ||||
| Male | 107 (47.3) | 236 (54.9) | Ref | Ref |
| Woman | 119 (52.7) | 194 (45.1) | 1.35 (.97, 1.87) | 1.47 (1.04, 2.08) |
| Incomed | ||||
| Under $50 000 | 55 (24.3) | 132 (30.7) | Ref | Ref |
| $50 000 to $100 000 | 49 (21.7) | 98 (22.8) | 1.20 (.76, 1.91) | 1.17 (.73, 1.86) |
| $100 000 to $150 000 | 28 (12.4) | 39 (9.1) | 1.72 (.98, 3.07) | 1.4 (0.81,2.41) |
| Over $150 000 | 22 (9.7) | 44 (10.2) | 1.20 (.66, 2.18) | 1.25 (.7, 2.28) |
| Racee | ||||
| White | 104 (46.0) | 163 (38.0) | Ref | Ref |
| Hispanic | 53 (23.5) | 146 (34.0) | .57 (.38, .85) | .72 (.46, 1.12) |
| Asian | 7 (3.1) | 58 (13.5) | .19 (.08, .44) | .24 (.1, .55) |
| Black | 20 (8.8) | 18 (4.2) | 1.74 (.88, 3.44) | 2.54 (1.24, 5.15) |
| More than 1 race | 26 (11.5) | 36 (8.4) | 1.13 (.64, 1.97) | 1.4 (.78, 2.51) |
| Native American or Alaskan Native | 6 (2.7) | 4 (0.9) | 2.34 (.64, 8.48) | 2.02 (.54, 7.53) |
| Native Hawaiian or Pacific Islander | 3 (1.3) | 1 (0.2) | 4.73 (.48, 42.82) | 4.64 (.46, 45.74) |
Logistic regression models adjusting for age, region, sex, income, and race predicted the likelihood an individual was vaccine hesitant. Missing values of income and race were multiply imputed using the Amelia II package.
Abbreviations: CI, confidence interval; N/A, not applicable; Ref, reference; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aCase status is presented here for context but was not included in regression analyses as it could be considered an outcome of willingness to receive vaccination; thus, odds ratio estimates are N/A for this characteristic.
bPredominantly urban regions include San Francisco Bay area, Greater Los Angeles area, Greater Sacramento area, San Diego, and the Southern border. We tabulate regions of residence for individuals who were hesitant or willing to receive vaccination in Supplementary Table 1.
cPredominantly rural regions include Central Coast, Northern Sacramento valley, San Joaquin Valley, Northwestern California, and the Sierras region. We tabulate regions of residence for individuals who were hesitant or willing to receive vaccination in Supplementary Table 1.
dFor regression analyses, values were imputed for individuals who did not share income data due to refusal (43 [19.0%] among hesitant and 66 [15.3%] among nonhesitant participants) or those who did not know their income (29 [12.8%] among hesitant and 51 [11.9%] among nonhesitant participants).
eFor regression analyses, values were imputed for individuals who did not share race data (7 [3.1%] among hesitant and 3 [0.7%] among nonhesitant participants).
Table 4.
Reasons for Vaccine Hesitancy Among Individuals Not Yet Vaccinated
| Stated Reason | No. (%) Among 219 Respondents Reporting Hesitancy to Receive Vaccinationa |
|---|---|
| Concerned about any vaccine side effects | 66 (30.0) |
| Concerned about long-term vaccine side effects | 60 (27.4) |
| Concerned about COVID-19 vaccine safety | 60 (27.4) |
| Waiting to see more research on COVID-19 vaccines | 40 (18.3) |
| I have not yet thought about whether I want the COVID-19 vaccine | 24 (11.0) |
| Currently infected with SARS-CoV-2 | 23 (10.5) |
| Concerned about safety for vaccines generally | 22 (10.0) |
| Do not believe vaccination against COVID-19 is important | 20 (9.1) |
| Not at high risk for COVID-19 | 17 (7.8) |
| Currently pregnant | 9 (4.1) |
| Do not trust the government | 9 (4.1) |
| Negative reaction to prior vaccinations | 5 (2.3) |
| Lack of trust in the medical system | 5 (2.3) |
| Would only get vaccine if required by school/work | 5 (2.3) |
| Contraindicated medical condition | 5 (2.3) |
| Afraid of getting SARS-CoV-2 from the vaccine | 3 (1.4) |
| Depends on the vaccine product offered | 2 (0.9) |
| Object to vaccination due to religious reasons | 2 (0.9) |
| Afraid of needles | 1 (0.5) |
Abbreviations: COVID-19, coronavirus diseasea 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
aCalculated out of N = 219 because 7 individuals declined to answer.
DISCUSSION
Among a sample of the general population of Californians and during a period when 10 653 334 (27%) California residents became fully vaccinated, available mRNA-based COVID-19 vaccines demonstrated robust protection against documented SARS-CoV-2 infection under real-world conditions. While we identified partial protection before 2 weeks from receipt of the second dose, similar to other published estimates [7, 9], the increase in VE from 67% following the first dose to 87% at more than 15 days after receipt of the second dose indicated a robust 59% incremental reduction in risk. We also found that mRNA-based COVID-19 vaccines elicited substantial protection against both symptomatic illness and infections for which participants reported healthcare-seeking, with 91% VE against each of these endpoints. No hospitalizations were observed among fully vaccinated cases within our study, consistent with findings of other published studies demonstrating strong protection against clinically severe COVID-19 endpoints [12]. Our results closely resemble the estimated efficacy of mRNA-based COVID-19 vaccines in trials that monitored for symptomatic COVID-19 endpoints [1, 2]. The low frequency of postvaccination infections, and our estimate of 68% VE against infections for which participants did not report symptoms, together indicate vaccination may substantially reduce SARS-CoV-2 circulation within the community.
Our finding that 66% of as-yet unvaccinated participants in this early period of vaccine rollout were willing to receive COVID-19 vaccination aligns with national estimates of COVID-19 vaccine confidence [13]. We further identified rural–urban divides in vaccine enthusiasm, in addition to lower vaccine confidence among female and Black participants. Concerns over vaccine safety and side effects were reported by only a minority of all participants who expressed hesitancy about receiving COVID-19 vaccination (27–30%), but these were the most commonly cited reasons for hesitancy. Recent studies have documented emerging differences in acceptance of COVID-19 vaccination associated with region of residence, educational background, employment status, and ideological factors [14–16]. Differing messaging and outreach strategies will thus be needed to address barriers to vaccine acceptance across communities, including people whose hesitancy to receive vaccination stems from mistrust or adverse experiences within US healthcare systems [17]. Prior studies have demonstrated that a provider’s recommendation is a key determinant of vaccine acceptance [18]. As healthcare providers in California and other settings have generally reported high (although not universal) enthusiasm around receiving COVID-19 vaccination [19, 20], they may serve as important advocates to encourage vaccine uptake in their communities.
Our study has limitations. While observational studies face risks of bias (due, for instance, to differences in risk behavior between vaccinated and unvaccinated individuals), the similarity of our estimates to those of other studies, stepwise increases in VE with time since receipt of each dose, and the absence of apparent protection immediately following first-dose receipt each support the external validity of our findings [7, 8, 21]. Reliance on participants being available and willing to answer the phone is a limitation, although this applied to both cases and controls who received SARS-CoV-2 testing. Nonetheless, our study may have under-enrolled participants experiencing very severe illness (eg, who were hospitalized, had died, or were unable to participate in the phone interview due to sickness), who would be unable to answer the phone. As such, our findings should be interpreted as estimates of VE against a primarily mild to moderate spectrum of illness. We did not identify differential willingness to participate in the study among persons who tested positive and negative, provided contact was made. While misclassification of self-reported vaccination is possible, we did not find significant differences in VE estimates between analyses that did or did not restrict data to include participants who referenced a vaccine card. We did not re-contact cases to verify that cases who reported no symptoms remained asymptomatic over the course of their infection, or to confirm that cases who were not hospitalized or had not sought advice from healthcare providers at the time of their interview did not subsequently receive such care. Last, it is possible that certain participants were unaware of prior SARS-CoV-2 infections they may have experienced, particularly if these infections were mildly symptomatic or asymptomatic. Immunity resulting from such infections could lead to lower estimates of VE under our study design [6, 22].
Our findings indicate that vaccine rollout is preventing COVID-19 in the general population of California and significantly reducing the risk of both asymptomatic and symptomatic SARS-CoV-2 infection. Vaccine hesitancy among historically marginalized and rural populations, which account for a substantial proportion of all COVID-19 cases in California to date [4], presents a barrier to reaching coverage levels needed for herd immunity.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the California Department of Public Health. J. P., J. O., and J. F. M. were supported by a grant from the National Institutes of Health (NIH)/CDC Epidemiology and Laboratory Capacity for Infectious Disease (ELC), grant number 5–NU50CK000539 program of the US Centers for Disease Control and Prevention (program number 0187.0150). J. A. L. and N. P. J. were supported by the NIH/National Institute of Allergy and Infectious Diseases (grant number R01-AI14812701).
Potential conflicts of interest. J. A. L. discloses receipt of grants and honoraria from Pfizer, Inc, outside the submitted work. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
California COVID-19 Case-Control Study Team Members. Helia Samani, Sophia S. Li, Camilla M. Barbaduomo, Nikolina Walas, Christine Wan, Anna T. Fang, Timothy Ho, Vivian H. Tran, Erin Xavier, Mahsa H. Javadi, Diana J. Poindexter, Najla Dabbagh, Michelle M. Spinosa, Nozomi Birkett, Paulina M. Frost, Zheng N. Dong, Shrey Saretha, Adrian F. Cornejo, Jennifer L. DeGuzman, Miriam I. Bermejo, Hyemin Park, and Amanda Lam.
Contributor Information
California COVID-19 Case-Control Study Team:
Helia Samani, Sophia S Li, Camilla M Barbaduomo, Nikolina Walas, Christine Wan, Anna T Fang, Timothy Ho, Vivian H Tran, Erin Xavier, Mahsa H Javadi, Diana J Poindexter, Najla Dabbagh, Michelle M Spinosa, Nozomi Birkett, Paulina M Frost, Zheng N Dong, Shrey Saretha, Adrian F Cornejo, Jennifer L DeGuzman, Miriam I Bermejo, Hyemin Park, and Amanda Lam
References
- 1. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baden LR, El Sahly HM, Essink B, et al. ; COVE Study Group. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021; 384:403-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sadoff J, Le Gars M, Shukarev G, et al. Interim results of a phase 1–2a trial of Ad26.COV2.S COVID-19 vaccine. N Engl J Med 2021; 384:1824-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. State of California. Tracking COVID-19 in California—coronavirus COVID-19 response. Available at: Covid19.ca.gov. Accessed 13 July 2021.
- 5. Patel MM, Jackson ML, Ferdinands J. Postlicensure evaluation of COVID-19 vaccines. JAMA 2020; 324:1939-40. [DOI] [PubMed] [Google Scholar]
- 6. Lewnard JA, Patel MM, Jewell NP, et al. Theoretical framework for retrospective studies of the effectiveness of SARS-CoV-2 vaccines. Epidemiology 2021; 32:508-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amit S, Regev-Yochay G, Afek A, Kreiss Y, Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet 2021; 397:875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thompson MG, Burgess JL, Naleway AL, et al. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers—eight US locations, December 2020–March 2021. MMWR Morb Mortal Wkly Rep 2021; 70:495-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hall VJ, Foulkes S, Saei A, et al. ; SIREN Study Group. COVID-19 vaccine coverage in health-care workers in England and effectiveness of BNT162b2 mRNA vaccine against infection (SIREN): a prospective, multicentre, cohort study. Lancet 2021; 397:1725-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. State of California. Tracking variants. 2021. Available at: https://www.cdph.ca.gov/Programs/CID/DCDC/Pages/COVID-19/COVID-Variants.aspx. Accessed 13 July 2021.
- 11. Honaker J, King G, Blackwell M. Amelia II: a program for missing data. J Stat Soft 2011; 45:1-47. [Google Scholar]
- 12. Tenforde MW. Effectiveness of Pfizer-BioNTech and Moderna vaccines against COVID-19 among hospitalized adults aged ≥65 years—United States, January–March 2021. MMWR Morb Mortal Wkly Rep 2021; 70:674-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reiter PL, Pennell ML, Katz ML. Acceptability of a COVID-19 vaccine among adults in the United States: how many people would get vaccinated? Vaccine 2020; 38:6500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Khubchandani J, Sharma S, Price JH, Wiblishauser MJ, Sharma M, Webb FJ. COVID-19 vaccination hesitancy in the United States: a rapid national assessment. J Community Health 2021; 46:270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fridman A, Gershon R, Gneezy A. COVID-19 and vaccine hesitancy: a longitudinal study. PLoS One 2021; 16:e0250123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McCabe SD, Hammershaimb EA, Cheng D, et al. Unraveling attributes of COVID-19 vaccine hesitancy in the US: a large nationwide study. medRxiv [Preprint]. 2021. doi: 10.1101/2021.04.05.21254918. Available at: https://www.medrxiv.org/content/10.1101/2021.04.05.21254918v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Momplaisir F, Haynes N, Nkwihoreze H, Nelson M, Werner RM, Jemmott J. Understanding drivers of coronavirus disease 2019 vaccine hesitancy among blacks. Clin Infect Dis 2021; 73:1784–9. doi: 10.1093/cid/ciab102. Available at: https://jamanetwork.com/journals/jamanetworkopen/fullarticle/2777776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Paterson P, Meurice F, Stanberry LR, Glismann S, Rosenthal SL, Larson HJ. Vaccine hesitancy and healthcare providers. Vaccine 2016; 34:6700-6. [DOI] [PubMed] [Google Scholar]
- 19. Grumbach K, Judson T, Desai M, et al. Association of race/ethnicity with likeliness of COVID-19 vaccine uptake among health workers and the general population in the San Francisco Bay Area. JAMA Intern Med 2021; 181:1008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meyer MN, Gjorgjieva T, Rosica D. Trends in health care worker intentions to receive a COVID-19 vaccine and reasons for hesitancy. JAMA Netw Open 2021; 4:e215344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dagan N, Barda N, Kepten E, et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med 2021; 384:1412-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lewnard JA, Tedijanto C, Cowling BJ, Lipsitch M. Measurement of vaccine direct effects under the test-negative design. Am J Epidemiol 2018; 187:2686-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.