Abstract
Previous studies have shown that certain vaccines induce suboptimal responses in people living with human immunodeficiency virus (HIV, PLWH). However, responses to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccines have not been fully characterized in these patients. Here we show that the BNT162b2 vaccine induces robust immune responses comparable to responses in healthy donors.
Keywords: COVID, mRNA vaccine, PLWH, SARS-CoV-2
The BNT162b2 messenger RNA (mRNA) vaccine induces robust and protective humoral and cellular response to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein [1] and provides protection from infection with SARS-CoV-2 [2]. However, prior studies have shown suboptimal responses to some vaccines in people living with human immunodeficiency virus (HIV, PLWH) [3]. A recent study demonstrated that the ChAdOx1 nCoV-19 (AZD1222) vaccine was effective at inducing humoral and cellular immune responses in PLWH [4], but few studies have addressed the immunogenicity of mRNA vaccines in these patients [5, 6]. Here we determined the capacity of the BNT162b2 mRNA vaccine to induce effective cellular and humoral immune responses in PLWH.
METHODS
We obtained blood between 7 and 17 days after the second vaccine dose from 12 PLWH (7 women, 5 men) and 17 healthy donors (7 women, 10 men). None of these individuals had evidence of prior SARS-CoV-2 infection by history or by serology as described below. Informed consent was obtained from all study participants. All PLWH were on antiretroviral therapy (ART) and had a median CD4 + T cell count of 913 cells/uL (range of 649 to 1678 cells/uL). Eleven of the 12 PLWH were African American. Three participants had low level viremia despite being on ART (Supplementary Table 1). Peripheral blood mononuclear cells (PBMCs) and plasma were isolated from whole blood using ficoll centrifugation. We determined cellular immunity to the SARS-CoV-2 spike protein by performing an interferon-gamma (IFN-γ) Elispot assay with unfractionated PBMCs that were stimulated with a pool of overlapping SARS-CoV-2 spike peptides as previously described [7]. The assay was also performed with CD8 + T cell depleted PBMCs to determine the relative contribution of CD4 + T cells and CD8 + T cells to the cellular immune response. The titer of SARS-CoV-2 spike binding antibodies was determined with the Euroimmun Anti-SARS-CoV-2 immunoglobulin G (IgG) ELISA (Mountain Lakes, New Jersey, USA). Antibodies to the nucleocapsid protein were measured with the Bio-Rad Platelia SARS-CoV-2 Total Ab assay (Marnes-la-Coquette, France) and used to rule out natural infection with SARS-CoV-2 as mRNA for the nucleocapsid protein is not included in the vaccine. Measurement of antibodies in plasma that block SARS-CoV-2 Spike binding to ACE2 was performed with the MSD V-PLEX SARS-CoV-2 Panel 6 kit from Meso Scale Diagnostics (Rockville, Maryland, USA) using a 1:100 dilution of plasma. Differences in Elispot and Euroimmun values were assessed using a 2-tailed t test. Differences in ACE2 blocking between groups was determined by a 2-tailed Wilcoxon-Mann-Whitney test with a Bonferroni correction, employing R version 4.05. P-values < .05 were considered significant.
RESULTS
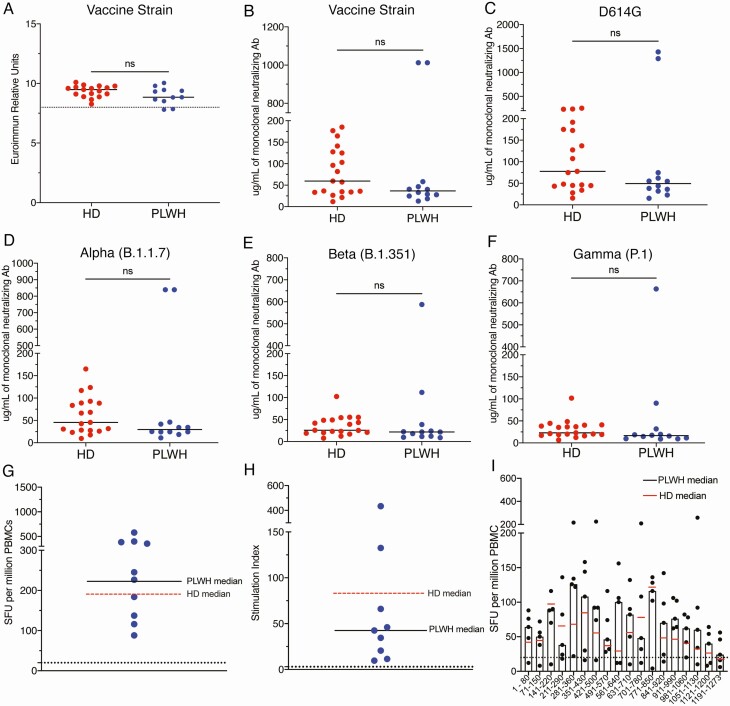
There was no significant difference in titers of SARS-CoV-2 spike binding antibodies in healthy donors (median value of 9.49) and PLWH (median value of 8.84 P = .07) (Figure 1A). Furthermore, healthy donors and PLWH had similar levels of neutralizing antibodies to the vaccine strain spike protein (Figure 1B) and spike proteins from variants of concern (VOC) including the D614G, alpha (B.1.1.7), beta (B.1.351), and gamma (P.1) strains (Figures 1B–1F). We next compared the cellular responses elicited by overlapping peptides from the vaccine strain spike protein in PLWH, to responses we obtained in healthy donors from a prior study [7]. There was no significant difference in the number of IFN-γ spot forming units or in the stimulation index (values normalized to media alone) between healthy donors and PLWH in unfractionated PBMCs (Figure 1G, 1H) or with CD8 + T cell depleted PBMCs (Supplementary Figure 1). Finally, the breadth of the T-cell response was comparable in the 2 groups, and the similar peptide pools were targeted by the 2 study groups (Figure 1I).
Figure 1.
Titer of SARS-CoV-2 spike binding antibodies from HD and PLWH (A). The horizontal line represents the 90th percentile titer in patients with natural infection. Titer of neutralizing antibodies to spike proteins from vaccine strains SARS-CoV2 (B) and variants of concern (C–F). SFUs (G) and SIs (H) to SARS-CoV-2 spike peptide pools from PBMCs from vaccinated PLWH. Black horizontal bars represent the median value for PLWH. Dashed red horizontal bar represents the median value for vaccinated HD from a prior study [7]. Dashed black horizontal lines denote a significant response (SFU > 20 and SI > 3). Breadth of CD8-depleted T-cell responses from PLWH to pools of 10 peptides that sequentially cover the entire spike protein (E). Horizontal bars represent the median values for PLWH; red horizontal line represents the HD median value. Abbreviations: HD, healthy donors; PBMC, peripheral blood mononuclear cells; PLWH, people living with human immunodeficiency virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SFU, spot-forming units; SI, stimulation index.
DISCUSSION
Our study is limited by the relatively small number of participants in both cohorts. Although we screened participants for antibodies to nucleocapsid to rule out prior natural infection, the half-life of antibodies to this protein is relatively short [8]. Thus, we may have missed cases of prior SARS-CoV-2 infection. However, our data confirm a prior study showing that mRNA vaccines induce antibody responses in PLWH [5] and extend the findings by showing that the level of binding antibodies is not significantly different from that produced in healthy donors. These data are similar to results obtained in a phase 2/3 clinical trial in which the ChAdOx1 nCoV-19 (AZD1222) vaccine was shown to elicit strong SARS-CoV-2 specific antibody and T cell responses in PLWH [4]. Of note, in a prior study of naturally infected individuals, antibody titers based on Euroimmun values above 8 were only seen in the top 10% of individuals and were highly correlated with the highest levels of neutralizing titers based on a microneutralization assay [9]. We also demonstrate that neither the magnitude and breadth of vaccine elicited T-cell responses nor the breadth of neutralizing antibodies, as determined by responses to spike proteins from wild-type virus and VOCs, is significantly different between PLWH and healthy donors. These findings are particularly impressive as the PLWH study participants (median age 52 years, range 25–59) were older than the healthy donors (median age 41 years, range 24–59), and the BNT162b2 vaccine induces a lower antibody titer in older individuals [10]. However, this vaccine also elicits a higher antibody titer in women compared to men [10], and our PLWH cohort had a higher frequency of female participants. Of note, prior vaccine studies in PLWH focused mainly [6] or exclusively on men [4]. Data from our balanced cohort strongly suggest that the BNT162b2 vaccine will lead to protection from COVID-19 in men and women living with HIV. Further studies will be needed to determine whether PLWH with lower CD4 T cell counts have the same robust humoral and cellular responses to the vaccine.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the Johns Hopkins COVID-19 Vaccine-related Research Fund, the Johns Hopkins Center for AIDS Research, and the National Cancer Institute (grant number U54CA260491). Additional support was provided by the Division of Intramural Research, National Institute Allergy and Infectious Diseases.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Sahin U, Muik A, Derhovanessian E, et al. . COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature 2020; 586:594-9. [DOI] [PubMed] [Google Scholar]
- 2. Polack FP, Thomas SJ, Kitchin N, et al. ; C4591001 Clinical Trial Group. . Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020; 383:2603–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tebas P, Frank I, Lewis M, et al. ; Center for AIDS Research and Clinical Trials Unit of the University of Pennsylvania. . Poor immunogenicity of the H1N1 2009 vaccine in well controlled HIV-infected individuals. AIDS 2010; 24:2187–92. [DOI] [PubMed] [Google Scholar]
- 4. Frater J, Ewer KJ, Ogbe A, et al. . Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine against SARS-CoV-2 in HIV infection: a single-arm substudy of a phase 2/3 clinical trial. Lancet HIV 2021. doi:10.1016/S2352-3018(21)00103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ruddy JA, Boyarsky BJ, Werbel WA, et al. . Safety and antibody response to the first dose of SARS-CoV-2 messenger RNA vaccine in persons with HIV. AIDS 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levy I, Wieder-Finesod A, Litchevski V, et al. . Immunogenicity and safety of the BNT162b2 mRNA COVID-19 vaccine in people living with HIV-1. doi:10.2139/ssrn.3829650. [DOI] [PMC free article] [PubMed]
- 7. Woldemeskel BA, Garliss CC, Blankson JN. SARS-CoV-2 mRNA vaccines induce broad CD4+ T cell responses that recognize SARS-CoV-2 variants and HCoV-NL63. J Clin Invest 2021; 131:e149335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lumley SF, Wei J, O’Donnell D, et al. . The duration, dynamics and determinants of SARS-CoV-2 antibody responses in individual healthcare workers. Clin Infect Dis 2021; 73:e699–709. doi:10.1093/cid/ciab004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patel EU, Bloch EM, Clarke W, et al. . Comparative performance of five commercially available serologic assays to detect antibodies to SARS-CoV-2 and identify individuals with high neutralizing titers. J Clin Microbiol 2021;59:e02257-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pellini R, Venuti A, Pimpinelli F, et al. . Initial observations on age, gender, BMI and hypertension in antibody responses to SARS-CoV-2 BNT162b2 vaccine. EClinicalMedicine 2021; 36:100928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.