Abstract
Background
Neutropenia is commonly encountered in cancer patients. Recombinant human granulocyte colony-stimulating factor (G-CSF, filgrastim), a cytokine that initiates proliferation and differentiation of mature granulocytes, is widely given to oncology patients to counteract neutropenia, reducing susceptibility to infection. However, the clinical impact of neutropenia and G-CSF use in cancer patients with coronavirus disease 2019 (COVID-19) remains unknown.
Methods
An observational cohort of 379 actively treated cancer patients with COVID-19 was assembled to investigate links between concurrent neutropenia and G-CSF administration on COVID-19-associated respiratory failure and death. These factors were encoded as time-dependent predictors in an extended Cox model, controlling for age and underlying cancer diagnosis. To determine whether the degree of granulocyte response to G-CSF affected outcomes, the degree of response to G-CSF, based on rise in absolute neutrophil count (ANC) 24 hours after growth factor administration, was also incorporated into a similar Cox model.
Results
In the setting of active COVID-19 infection, outpatient receipt of G-CSF led to an increased number of hospitalizations (hazard ratio [HR]: 3.54, 95% confidence interval [CI]: 1.25–10.0, P value: .017). Furthermore, among inpatients, G-CSF administration was associated with increased need for high levels of oxygen supplementation and death (HR: 3.56, 95% CI: 1.19–10.2, P value: .024). This effect was predominantly seen in patients that exhibited a high response to G-CSF based on their ANC increase post-G-CSF administration (HR: 7.78, 95% CI: 2.05–27.9, P value: .004).
Conclusions
The potential risks versus benefits of G-CSF administration should be considered in neutropenic cancer patients with COVID-19, because G-CSF administration may lead to worsening clinical and respiratory status.
Keywords: COVID-19, granulocyte colony stimulating factor, cancer, neutropenia
The safety profile of G-CSF in patients with severe SARS-CoV2 infection is unclear. Here, we systematically characterized the effect G-CSF on the risk for respiratory decompensation and death in cancer patients who were infected with COVID-19. “
Neutropenia is a common side-effect of many anti-cancer therapies due to their effects on rapidly dividing hematopoietic cells. Recombinant human granulocyte colony stimulating factor (G-CSF, filgrastim) is often given to cancer patients for ongoing or impending neutropenia. During the ongoing coronavirus disease 2019 (COVID-19) pandemic, there has been uncertainty about the effect of commonly used medications, such as G-CSF, on clinical outcomes. The stimulatory effects of G-CSF on cytokine and neutrophil production [1], the association of G-CSF with acute lung injury (ALI) or adult respiratory distress syndrome (ARDS) [2, 3], and observations of COVID-19-associated cytokine storm in severely ill patients, have raised concerns about the safety profile of G-CSF in COVID-19 patients. Moreover, autopsies of COVID-19 patients have shown neutrophil extravasation in the alveolar spaces of lungs [4–6], raising concerns that G-CSF administration and the resulting neutrophil expansion could lead to exaggerated neutrophil responses, worsening respiratory function in COVID-19 patients.
Our group published a case series describing the rapid clinical deterioration of 3 COVID-19 patients soon after receiving G-CSF [7]. Herein we sought to determine the clinical effects of G-CSF in a larger cohort of cancer patients with COVID-19 illness, in order to investigate whether receipt of G-CSF in cancer patients with COVID-19 illness would increase risk of adverse outcomes.
METHODS
Study Population
We performed a retrospective cohort study of 379 cancer patients on active cancer treatment with COVID-19 infection, evaluated between 11 March 2020 and 16 November 2020 at Memorial Sloan Kettering Cancer Center (MSKCC). All patients in our cohort were diagnosed with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection using real-time reverse transcription polymerase chain reaction (RT-PCR) for the purpose of both diagnostic (patient presented with compatible symptoms) and surveillance testing [8, 9].
Of these, 130 patients were admitted to Memorial Hospital during this time period. Active cancer treatment was defined as receipt of systemic anti-cancer therapy within 30 days of COVID-19 diagnosis (day 0). We defined our primary composite endpoints as respiratory failure and death, widely accepted clinical metrics for COVID-19 clinical research. As commonly described in other studies, we defined respiratory failure as the requirement for high-flow nasal oxygen, non-rebreather, bilevel positive airway pressure (BIPAP), and mechanical ventilation [10]. Patients with a diagnosis of acute myeloid leukemia or myelodysplastic syndrome were excluded from our analysis, as these patients are generally not candidates for G-CSF administration at our institution. G-CSF is usually given at the discretion of the provider, and typically used in patients with cancer treatment-related neutropenia (either observed or anticipated).
We further grouped inpatients that received G-CSF into high responders and low responders based on the level of rise in ANC. The high response was defined as a >50th percentile increase in ANC (equivalently, a fold change of ≥4) on day 1 post G-CSF administration compared to ANC values taken immediately prior to G-CSF administration (day 0). We defined G-CSF administration as the use of filgastrim or pegfilgastrim at any dose. The MSKCC institutional review board approved the study.
Laboratory Methods
COVID-19 infection was diagnosed using a nasopharyngeal swab to determine the presence of virus specific RNA (MSKCC Food and Drug Administration [FDA] emergency use authorization [EUA]-approved assay and Cepheid®). SARS-CoV-2 RNA was detected using the Centers for Disease Control and Prevention protocol targeting 2 regions of the nucleocapsid gene (N1 and N2) with modifications described elsewhere [11–13].
Statistical Analysis
In the primary analysis, we applied an extended Cox proportional hazards model using age (binned into ≤49, 50–59, 60–69, and ≥70 years old categories), receipt of cytotoxic (vs non-cytotoxic) chemotherapy within 30 days prior, and cancer type. We considered the top 5 cancer types in terms of prevalence and aggregated all other types into a sixth group. The first occurrence of neutropenia and the first administration of G-CSF (filgrastim or pegfilgrastim) following SARS-CoV-2 positivity were introduced as binary time-dependent covariates in the model, as these variables change over the follow-up period [14]. G-CSF events were encoded 1 day following actual administration to capture the timing of its expected effect (furthermore, the earliest evidence of ANC recovery we had was a day after G-CSF administration). Start time was defined as the date of COVID-19 diagnosis for each patient.
To determine whether G-CSF administration in the outpatient setting increased the risk of hospitalization, we applied the aforementioned extended Cox model to 379 analyzed patients with the endpoint defined as the date of admission/hospitalization.
The primary inpatient analysis was performed on the 130 hospitalized patients using a composite endpoint defined as the first occurrence of respiratory failure (defined above) or death following COVID-19 diagnosis. For the second analysis, to determine whether the degree of G-CSF response affected clinical outcomes, we categorized G-CSF recipients into high- and low-response groups, defined by the degree of ANC elevation (see Study Population), and introduced these as mutually exclusive binary time-dependent covariates.
All outpatient and inpatient patient events were right-censored (time-to-event) to 30 days, respectively, as events outside of this time window were considered unlikely to be related to COVID-19. All analyses were performed in R version 3.6.2 with the survival (version 3.1–11) [15].
RESULTS
To determine the relationship between G-CSF administration and clinical outcomes due to COVID-19, we assembled a cohort of 379 cancer patients at MSKCC who tested positive for COVID-19 and received cancer directed therapy within 30 days of their COVID-19 diagnosis between 11 March 2020 and 16 November 2020. These cases comprised a variety of cancer types, including breast cancer (n = 90; 24%), colorectal cancer (n = 41, 11%), lung cancer (n = 37, 10%), lymphoma (n = 30, 7%), prostate cancer (n = 27; 7%), and other cancers (n = 144, 41%). Of all patients with different cancer types, 227/379 (60%) of patients received cytotoxic chemotherapy within 30 days of their COVID-19 diagnosis. New symptoms at the time of testing are also shown: fever, cough, shortness of breath, and diarrhea (Table 1). No patients in our cohort required supplemental oxygen at home. The anticancer agents patients received within 30 days of their COVID-19 diagnosis and the COVID-19 therapies to treat them are shown in Supplementary Tables 1 and 2, respectively. Of the 379 outpatients enrolled in this study, 28 received either filgastrim or pegylated filgastrim (7.4%). In total, 130 of the 379 patients were hospitalized (34.3%) and confirmed by review of each patient’s medical record; it was determined that they were admitted to the hospital for the management of COVID-19 symptoms. Using an extended Cox model with G-CSF encoded as a time-dependent covariate, G-CSF administration was significantly associated with increased risk of hospitalization in patients with COVID-19 infection (hazard ratio [HR]: 3.54, 95% confidence interval [CI]: 1.25–10.0, P value: .017, Table 2, Supplementary Figure 1). Even among patients who were asymptomatic from COVID-19 infection, this association remained significant (HR: 18.31; 95% CI: 2.51–96.8, P value: .008, Supplementary Table 3).
Table 1.
Demographics and Baseline Characteristics of 379 Cancer Patients on Active Cancer Treatment (Received Chemotherapy Within 30 days of Their COVID-19 Diagnosis) Split by Those Who Were (n = 249) and Were Not Hospitalized (n = 130) Post-COVID 19 Diagnosis
| Characteristic | G-CSF NOT Given Outpatient N = 351 n (%) | G-CSF Given Outpatient N = 28 n (%) | Total N = 379 n (%) |
|---|---|---|---|
| Age, years | |||
| ≤49 | 87 (25%) | 7 (25%) | 94 (25%) |
| 50–59 | 89 (25%) | 7 (25%) | 96 (25%) |
| 60–69 | 88 (25%) | 7 (25%) | 95 (25%) |
| ≥70 | 87 (25%) | 7 (25%) | 94 (25%) |
| Sex | |||
| M | 167 (48%) | 13 (46%) | 180 (47%) |
| F | 184 (52%) | 15 (54%) | 199 (53%) |
| Race | |||
| White | 229 (65%) | 13 (46%) | 242 (64%) |
| Black | 59 (17%) | 6 (21%) | 65 (17%) |
| Asian | 23 (7%) | 2 (7%) | 25 (7%) |
| Other | 40 (11%) | 7 (25%) | 47 (12%) |
| Underlying cancer | |||
| Breast | 82 (23%) | 8 (29%) | 90 (24%) |
| Colorectal | 37 (11%) | 4 (14%) | 41 (11%) |
| Lung | 36 (10%) | 1 (4%) | 37 (10%) |
| Lymphoma | 26 (7%) | 4 (14%) | 30 (8%) |
| Prostate | 26 (7%) | 1 (4%) | 27 (7%) |
| Other∞ | 144 (41%) | 10 (36%) | 154 (41%) |
| Cytotoxic chemotherapy within 30 days | 202 (58%) | 25 (89%) | 227 (60%) |
| Clinical symptoms | |||
| Fever | 210 (60%) | 16 (57%) | 226 (60%) |
| Shortness of breath | 89 (25%) | 7 (25%) | 96 (25%) |
| Cough | 197 (56%) | 17 (61%) | 214 (56%) |
| Diarrhea | 63 (18%) | 9 (32%) | 72 (19%) |
| Hospitalization post-GCSF use | |||
| Was NOT hospitalized | 225 (78%) | 24 (86%) | 249 (66%) |
| Was hospitalized | 127 (36%) | 4 (14%) | 131 (34%) |
Other∞ :central nervous system cancers (ie, astrocytoma); genitourinary cancers (ie, bladder cancer); gynecologic malignancies (ie, cervical), acute (acute lymphoblastic leukemia), and chronic leukemias (chronic myeloid leukemia); plasma cell dyscrasias (ie, amyloidosis). Although there are no differences in rates of individual adverse events between case-control groups (patients that did and did not receive a G-CSF, respectively), there is a statistically significant difference in effect size of G-CSF administration on rates of inpatient hospitalization when incorporated as a time-dependent covariate in a survival analysis (Table 2).
Abbreviations: COVID-19, coronavirus disease 2019; G-CSF, granulocyte colony-stimulating factor.
Table 2.
Cox Proportional Hazards Regression Model Showing the Factors That Cause an Increased Risk of Hospitalization After Patients With COVID-19 Received G-CSF in the Outpatient Setting
| Variable | N | Hazard Ratio (95% CI) | P value |
|---|---|---|---|
| Outpatient G-CSF administration | 28 | 3.54 (1.25–10.0) | .017 |
| Age, years | |||
| 50–59 | 96 | 0.99 (.56–1.80) | .973 |
| 60–69 | 95 | 1.70 (1.01–2.80) | .044 |
| ≥70 | 94 | 1.95 (1.16–3.30) | .012 |
| Underlying cancer | |||
| Colorectal | 41 | 0.76 (.37–1.60) | .451 |
| Lung | 37 | 1.67 (.91–3.10) | .097 |
| Lymphoma | 30 | 1.51 (.7–2.90) | .227 |
| Prostate | 27 | 1.14 (.54–2.40) | .734 |
| Other∞ | 154 | 0.93 (.58–1.50) | .777 |
| Receipt of cyotoxic chemotherapy within 30 days | 227 | 1.23 (.84–1.80) | .285 |
G-CSF administration is incorporated as a time-dependent variable in this analysis.
Other∞: central nervous system cancers (ie, astrocytoma); genitourinary cancers (ie, bladder cancer); gynecologic malignancies (ie, cervical), acute and chronic leukemias (acute/chronic myeloid leukemia); plasma cell dyscrasias (ie, amyloidosis).
Abbreviations: CI, confidence interval; COVID-19, coronavirus disease 2019; G-CSF, granulocyte colony-stimulating factor.
Next, we considered whether inpatient administration of G-CSF was associated with respiratory compromise or death (ie, the primary composite endpoint) among cancer patients (Supplementary Figure 1). The patient characteristics of only hospitalized patients with COVID-19 that received G-CSF and had serial complete blood counts (CBCs) (n = 16) are shown in Table 3. Of note, 1 of these patients received G-CSF in both outpatient and inpatient settings. It was again confirmed by review of each patient’s medical record that the patients who reached the endpoint did so because of their COVID-19 infection and not for other causes.
Table 3.
Patient Characteristics of 16 Hospitalized Patients With COVID-19 That Received G-CSF and Had Serial CBCs Are Shown
| Characteristic | G-CSF Given as Inpatient N = 16, n (%)a |
|---|---|
| Age, years | |
| ≤49 | 4 (25%) |
| 50–59 | 6 (38%) |
| 60–69 | 4 (25%) |
| ≥70 | 2 (12%) |
| Sex | |
| M | 2 (12%) |
| F | 14 (88%) |
| Race | |
| White | 10 (62%) |
| Black | 5 (31%) |
| Asian | 0 |
| Other | 1 (6%) |
| Underlying cancer | |
| Breast | 4 (25%) |
| Colorectal | 1 (6%) |
| Lung | 2 (12%) |
| Lymphoma | 1 (6%) |
| Prostate | 0 |
| Otherb | 8 (50%) |
| Cytotoxic chemotherapy within 30 days | 15 (94%) |
| Clinical symptoms | |
| Fever | 13 (81%) |
| Shortness of breath | 6 (37%) |
| Cough | 11 (69%) |
| Diarrhea | 3 (19%) |
| Neutropenia before endpoint (respiratory failure/death) | |
| No | 1 (6%) |
| Yes | 15 (94%) |
| Inpatient G-CSF use prior to combined | 6 (38%) |
| No | 10 (62%) |
| Yes | 6 (38%) |
One patient received G-CSF in both outpatient and inpatient settings. Although there are no differences in rates of individual adverse events between case control groups (patients that did and did not receive a G-CSF, respectively), there is a statistically significant difference in effect size of G-CSF administration on inpatient outcomes when incorporated as a time-dependent covariate in a survival analysis (with respiratory failure and/or death as the primary endpoint; main analysis = Figure 1).
Abbreviations: CBC, complete blood count; COVID-19, coronavirus disease 2019; G-CSF, granulocyte colony-stimulating factor.
aFour patients received first dose of G-CSF as an outpatient, were subsequently admitted, and received another dose of G-CSF as an inpatient.
bOther central nervous system cancers (ie, astrocytoma); genitourinary cancers (ie, bladder cancer); gynecologic malignancies (ie, cervical), acute and chronic leukemias (acute/chronic myeloid leukemia); plasma cell dyscrasias (ie, amyloidosis).
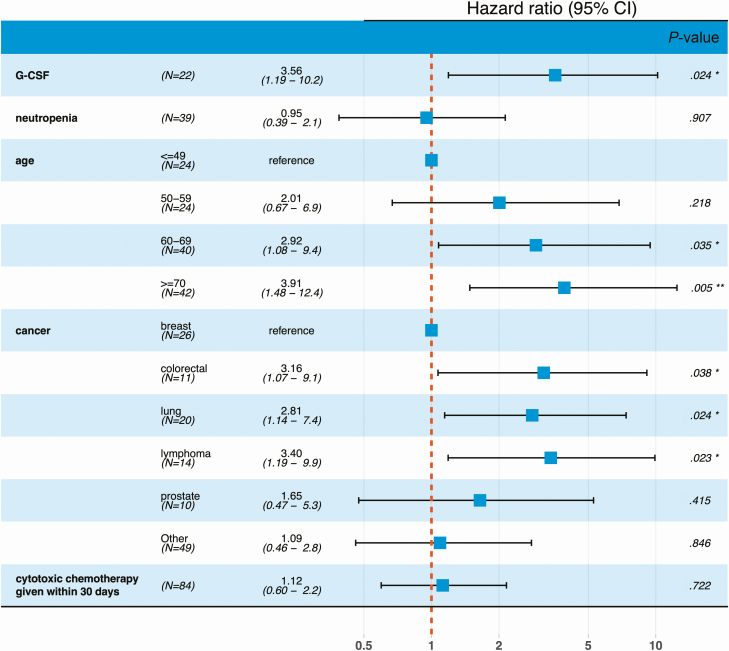
We evaluated the effect of neutropenia and G-CSF use on our primary composite endpoint. As expected, age was associated with significantly worse outcomes, with the effect most pronounced among the oldest individuals in our cohort (ages 60–69 and ≥70 years; HR 2.92 and 3.91 and P values .035 and .005, respectively, Figure 1). G-CSF use (HR: 3.56, 95% CI: 1.19–10.2, P value: .024), but not neutropenia (HR: 0.95, 95% CI: 0.39–2.1, P value: .907; Figure 1), portended significantly worse outcomes in our multivariate model. To rule out the possibility of G-CSF being reserved for cases with more severe neutropenia, we also show that the severity of neutropenia (ANC nadir in K/mcL) was not correlated with G-CSF use (Wilcoxon P value .55; Supplementary Figure 2).
Figure 1.
Forest plot showing the effect (HR) of (G-CSF) on the composite endpoint of the first occurrence of “respiratory failure” (defined in Methods) or death. HRs were computed with an extended Cox model, using binned ages and cancer type as time-independent covariates and neutropenia and G-CSF as time-dependent covariates. Abbreviations: CI, confidence interval; G-CSF, granulocyte colony-stimulating factor; HR, hazard ratio.
Outpatient and inpatient swimmer plots showing time-to-endpoint (hospitalization for outpatients and respiratory failure/death for inpatients) for each patient are shown in Supplementary Figures 3 and 4. Many outpatients and inpatients appeared to reach endpoint if G-CSF was given soon after COVID-19 diagnosis (day 0).
Ten of the 16 patients with serial CBCs that were neutropenic and received G-CSF had a pre- and post-chest radiograph, and 6/10 (60%) demonstrated radiologic worsening within 7 days of receiving G-CSF (Supplementary Table 4); 3 patients that developed radiographic deterioration post-G-CSF use are shown in Supplementary Figure 5 that correlated with an increase in oxygen requirements.
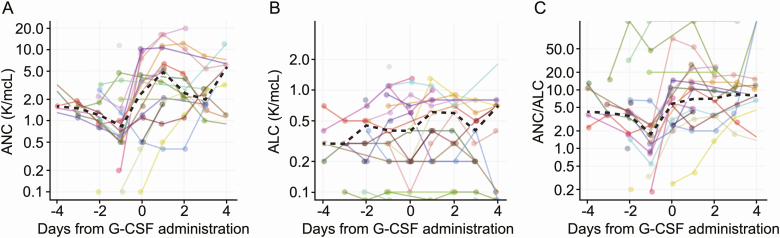
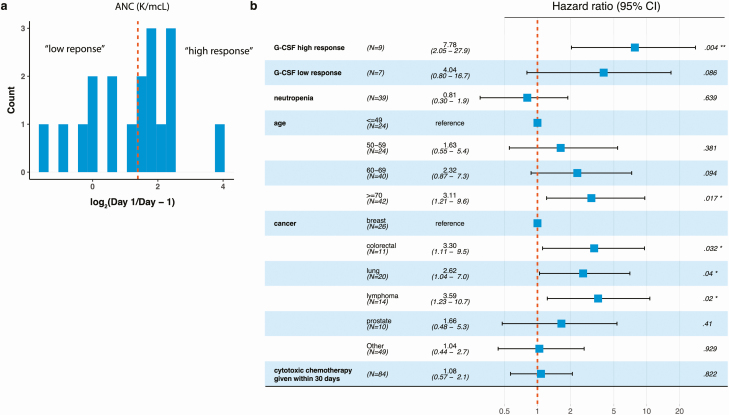
To determine whether the neutrophil-inducing properties of G-CSF may relate to the poor outcomes associated with G-CSF in COVID-19 infection, we further considered the neutrophil concentrations in peripheral blood prior to and immediately after G-CSF administration. As expected, ANC and ANC/ALC (ratio of absolute neutrophil count to absolute lymphocyte count) values increased after G-CSF administration. Changes in ANC values were primarily limited to the day after G-CSF administration (Figure 2A and 2C), whereas ALC values remained relatively constant (Figure 2B). We stratified the 16 inpatients that received G-CSF with serial CBCs based on their response to ANC, computed as the log fold-change between ANC values 1 day after the day of G-CSF administration (Figure 3A).
Figure 2.
Lab values of ANC (K/mcL) (A), ALC (K/mcL) (B), and their ratio, ANC/ALC (C) within a 4 day window for patients that received G-CSF around the time of G-CSF administration. Day 0 corresponds to the date of G-CSF administration. Black dashed line in each panel corresponds to the average lab value per day for all patients. Abbreviations: ALC, absolute lymphocyte count; ANC, absolute neutrophil count; G-CSF, granulocyte colony-stimulating factor.
Figure 3.
(a) Log-fold change values of ANC obtained 1 day after G-CSF administration and prior to G-CSF administration for the n = 16 patients that received G-CSF that were included in the survival analysis. Patients were stratified into “high” (to the right of the dashed orange line; defined as a >50th percentile increase in ANC (equivalently, a fold change of >=4) or “low” (to the left of the dashed red line) responders based on these values. (b) Forest plot showing the effect (HR) of high- and low-response to G-CSF on the first occurrence of “respiratory failure” (defined in Methods). HRs were computed with an extended Cox model, using binned ages and cancer type as time-independent covariates, and neutropenia and G-CSF (high- and low-response) as time-dependent covariates. Abbreviations: ANC, absolute neutrophil count; CI, confidence interval; G-CSF, granulocyte colony-stimulating factor; HR, hazard ratio.
Using response categories (“high” and “low”), we modified the extended Cox model shown in Figure 1 to categorize G-CSF events as high and low response; 9 patients and 7 patients were deemed to have a high and low response, respectively. There was a pronounced difference in the clinical outcomes of patients with low and high response to G-CSF. Although low response to G-CSF was not significantly associated with the primary composite endpoint (HR: 4.04, 95% CI: .80–16.7, P value: .086), high response to G-CSF was significantly associated with poorer outcomes (HR: 7.78, 95% CI: 2.05–27.9, P value: .004; Figure 3B). The difference between high- and low-response categories was not solely explained by steroid use (n = 5/9 of high- and n = 4/7 of low-response patients received steroids).
Discussion
The COVID-19 pandemic has complicated the delivery of effective cancer care, given the need to balance the competing risks of death from untreated cancer versus death or serious complications from COVID-19 infection [11, 16–20]. Although there have been some attempts to study the safety of administering chemotherapy to COVID-19 positive patients [11], this remains a complicated matter. In this study, we evaluated the potential impact of G-CSF use in cancer patients in the setting of neutropenia with concurrent COVID-19 infection. We observed a higher risk of hospitalization, as well as respiratory failure and death in patients that received G-CSF, particularly among patients that had a robust neutrophil response. Our observations on the inpatient side also suggest that neutropenia during COVID-19 illness itself was not an independent risk factor for adverse outcomes in COVID-19 illness.
There has recently been accumulating evidence of rapid clinical deterioration in some patients with COVID-19 due to the hyperactive immune response driving COVID-19 progression, causing extensive infiltration of myeloid cells into the lungs (particularly monocytes, macrophages, and neutrophils) [4, 21–23], leading to a cytokine storm. Zuo et al used cell-free DNA as a marker to detect neutrophil extracellular trap (NET) remnants in the blood, showing that these were strongly correlated with absolute neutrophil counts, which predict worse outcomes [6]. Lung injury is one consequence of the cytokine storm that can progress into its more severe form, ARDS [24]. Considering that neutrophil influx in the lung is a hallmark feature of ARDS [25], and that ALI has already been reported as a potential complication of G-CSF use [2, 3], administering G-CSF to all cancer patients with COVID-19 may have detrimental clinical consequences. Similar concerns exist for patients who receive chimeric antigen receptor T (CAR-T) cell therapy [26], where G-CSF administration is generally avoided to prevent immune system overactivation, given the increased risk of cytokine storm in these patients.
To best estimate the effects of neutropenia and G-CSF on respiratory function and death, we encoded G-CSF as a time-dependent covariate, while controlling for neutropenia as another time-dependent covariate. We also compared the clinical outcomes of patients that had different levels of response to G-CSF, finding that robust G-CSF neutrophil response was associated with substantially higher hazard (HR: 7.78, 95% CI: 2.05–27.9, P value: .004) for respiratory decompensation, compared to those that had less robust levels of response to G-CSF (HR: 4.04, 95% CI: .80–16.7, P value: .086). We also show that there is a substantial increase in the neutrophil to lymphocyte ratio (ANC/ALC) after G-CSF administration, previously shown to be an independent risk factor for mortality in hospitalized patients with COVID-19 [27]. Together, these results imply that the detrimental effects of G-CSF in newly COVID-19 positive cancer patients are primarily driven by patients with robust increases in ANC following G-CSF administration. Interestingly, neutropenia alone was not statistically associated with worse outcomes. Although our analysis solely considering neutropenic inpatients did not show a statistically significant effect of G-CSF on clinical outcomes, this is not unexpected given the small size of that cohort (n = 39).
To our knowledge, this is the first study describing the course of COVID-19 infection in selected cancer patients who received G-CSF for neutropenia. A randomized control study of G-CSF in non-cancer patients with lymphopenia was performed and found no difference in their primary endpoint (time to clinical improvement) [28, 29]. They did note a smaller number of patients progressing to critical illness in the G-CSF group, but no significant differences in the duration of hospitalization or supplemental O2 use were observed. However, the study cohort was limited (n = 200) and primarily composed of young patients with few or no medical comorbidities, who likely have good baseline pulmonary function and tend to have the best clinical outcomes with COVID-19, regardless of intervention.
This study has its limitations. First, the study ultimately included a modest number of patients that received G-CSF (n = 50). Some of our patients received pegfilgrastim instead of filgrastim, although we note that our results did not change when restricting to patients that only received filgrastim. In this observational cohort, unaccounted confounding factors are plausible. We also did not consider how inpatients with COVID-19 responded to G-CSF after the observation window (30 days after COVID-19 diagnosis).
This analysis attempted to assess G-CSF over a wide range of cancers; due to limitations in sample size, tumor-specific effects were difficult to ascertain. We also limited our primary analysis to the subset of patients who were hospitalized in order to assess our clinical endpoints of interest (respiratory failure or death). In both the inpatient and outpatient setting, adverse events (ie, hospitalization and severe respiratory failure, respectively) were only observed in patients receiving G-CSF within 10 days of COVID-19 diagnosis. The impact of G-CSF later in a patient’s course requires further study, as does the impact of G-CSF in those cancer patients who are persistently COVID-19 positive [30]. Although our analysis adjusts for the neutrophil count prior to G-CSF administration, we have not incorporated data on concurrent therapies such as chemotherapies or surgery prior to the diagnosis of COVID-19 in these patients.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Figure S1: Schematic diagram showing the experimental design and time-dependent survival modelling. Neutropenia and G-CSF are encoded as time-dependent covariates that are active after the time of occurrence (blue and red lines, respectively).
Figure S2: Boxplot showing the severity of neutropenia, as measured by the ANC nadir (K/mcL), in patients who did and did not receive G-CSF.
Figure S3: Swimmer plot showing each patient’s time to primary outpatient endpoint—in this case, inpatient hospitalization (“X) after receiving G-CSF (represented by pink line). Each bar represents one patient and bar length represents time to endpoint. Patients were more likely to reach this endpoint if G-CSF was given soon after COVID-19 diagnosis (day 0). Patients that did not meet endpoint had more patient-time to receive G-CSF.
Figure S4: Swimmer plot showing each patient’s time to primary inpatient endpoint--in this case, respiratory failure and death (“X”) after receiving G-CSF (represented by pink line). Each bar represents one patient and bar length represents time to endpoint. Patients were more likely to reach this endpoint if G-CSF was given soon after COVID-19 diagnosis (day 0). Patients that did not meet endpoint had more patient-time to receive G-CSF.
Figure S5: A i: Portable chest X-Ray performed the day of G-CSF administration demonstrating right basilar and left mid lung patch opacities. A ii: A day after the administration the airspace opacities increased bilaterally.
B i: Portable chest X-Ray performed two days prior to administration of G-CSF demonstrating bilateral predominantly bibasilar patchy opacities B ii and B iii: Axial and coronal images two days post G-CSF administration, demonstrating peripheral and peribronchovascular airspace opacities predominantly in the lower lobes.
C i: Portable chest X-ray at day 0 of G-CSF administration demonstrating faint right basilar opacity. C ii: day 4 post G-CSF administration, bilateral patchy opacities noted involving both upper and lower zones C iii: Day 13 post administration, increased bilateral diffuse reticular and airspace opacities.
Table S1: Listed chemotherapy agents given within 30 days prior to COVID-19 diagnosis. Our protocol to administer antineoplastic agents for symptomatic COVID-19+ patients is listed as a footnote.
Table S2: Therapies given to treat COVID-19 infection in the patient cohort (n = 379)
Table S3: The Cox proportional hazards regression model showing the factors that cause an increased risk of hospitalization in asymptomatic patients with COVID-19 who received G-CSF in the outpatient setting. G-CSF administration is incorporated as a time-dependent variable in this analysis.
Table S4: Radiologic evolution of patients receiving G-CSF. Baseline X-ray of patients was determined as normal or abnormal if airspace or reticulonodular opacities were noted. X-ray post G-CSF was compared to baseline assessing radiologic evolution and categorizing it as: unchanged, increased or decreased.
Notes
Acknowledgments. The authors would like to thank Susan Weill who works in the Design and Creative Services, Marketing and Communication Department at MSKCC who helped to create the figures for this manuscript.
Financial support. This research was supported in part by National Institutes of Health/National Cancer Institute (NIH/NCI) Cancer Center Support grant number P30 CA008748. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This study was supported by the Memorial Sloan Kettering Cancer Center K12 Paul Calabresi Career Development Award for Clinical Oncology (to A. F. D.) This work was further supported by the Parker Institute for Cancer Immunotherapy at Memorial Sloan Kettering Cancer Center, the Sawiris Foundation, the Society of Memorial Sloan Kettering Cancer Center; MSK Cancer Systems Immunology Pilot Grant, and Empire Clinical Research Investigator Program, the Memorial Sloan Kettering Cancer Center Department of Medicine and Weill Cornell Medicine.
Potential conflicts of interest. J. J. has a patent licensed by MDSeq, Inc. M. P. has received honoraria from Abbvie, Bellicum, Bristol-Myers Squibb, Incyte, Kite (Gliead), Merck, Novartis, Nektar Therapeutics, and Takeda; serves on DSMBs for Cidara Therapeutics, Servier, and Medigene, and the scientific advisory boards of MolMed and NexImmune; and has received research support for clinical trials from Incyte, Kite (Gilead) and Miltenyi Biotec. J. J. reports receiving am NIH training grant (funding from T32-CA009207). M. P. reports honoraria from Abbvie, Astellas, Bristol-Myers Squibb, Celgene, Equilium, Incyte, Karyopharm, Kite/Gilead, Merck, Miltenyi Biotec, MorphoSys, Novartis, Nektar Therapeutics, Omeros, Takeda, and VectivBio AG, Vor Biopharma. He serves on DSMBs for Cidara Therapeutics, Medigene, Sellas Life Sciences, and Servier, and the scientific advisory board of NexImmune. He has ownership interests in NexImmune and Omeros. He has received research support for clinical trials from Incyte, Kite/Gilead, Miltenyi Biotec, and Novartis. He serves in a volunteer capacity as a member of the Board of Directors of the American Society for Transplantation and Cellular Therapy (ASTCT) and Be The Match (National Marrow Donor Program, NMDP), as well as on the CIBMTR Cellular Immunotherapy Data Resource (CIDR) Executive Committee. A. D. is a coinventor of intellectual property related to CD371 CAR T-cell technology and field of use specific for allogeneic cell therapies, licensed by MSK to Caribou Biosciences. Caribou is a private biotechnology company that develops CRISPR technologies and allogenic cell therapies for oncology. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Martins A, Han J, Kim SO. The multifaceted effects of granulocyte colony-stimulating factor in immunomodulation and potential roles in intestinal immune homeostasis. IUBMB Life 2010; 62:611–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karlin L, Darmon M, Thiéry G, et al. . Respiratory status deterioration during G-CSF-induced neutropenia recovery. Bone Marrow Transplant 2005; 36:245–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiedermann FJ. Acute lung injury during G-CSF-induced neutropenia recovery: effect of G-CSF on pro- and anti-inflammatory cytokines. Bone Marrow Transplant 2005; 36:731. [DOI] [PubMed] [Google Scholar]
- 4.Barnes BJ, Adrover JM, Baxter-Stoltzfus A, et al. . Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J Exp Med 2020; 217:e20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox SE, Akmatbekov A, Harbert JL, et al. . Pulmonary and cardiac pathology in Covid-19: the first autopsy series from New Orleans. Lancet Resp Med 2021; 8:P681–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuo Y, Yalavarthi S, Shi H, et al. . Neutrophil extracellular traps in COVID-19. JCI Insight 2020; 5: e138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nawar T, Morjaria S, Kaltsas A, et al. . Granulocyte-colony stimulating factor in COVID-19: Is it stimulating more than just the bone marrow? Am J Hematol 2020. doi: 10.1002/ajh.25870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. Symptoms of COVID-19. Available at: https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html. Accessed 23 April 2021.
- 9.CDC. Overview of testing for SARS-CoV-2 (COVID-19). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/testing-overview.html. Accessed 23 April 2021.
- 10.WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis 2020; 20:e192–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robilotti EV, Esther Babady N, Mead PA, et al. . Determinants of COVID-19 disease severity in patients with cancer. Nat Med 2020; 26:1218–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.CDC. CDC 2019-novel Coronavirus (2019-nCoV) real-time RT-PCR diagnostic panel. Available at: https://www.fda.gov/media/134922/download. Accessed 23 April 2021. [DOI] [PMC free article] [PubMed]
- 13.CDC. Research use only 2019-novel Coronavirus (2019-nCoV) real-time RT-PCR primers and probes. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html. Accessed 22 June 2021.
- 14.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health 1999; 20:145–57. [DOI] [PubMed] [Google Scholar]
- 15.R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: http://www.R-project.org/. Accessed 22 June 2021.
- 16.Calabrò L, Peters S, Soria JC, et al. . Challenges in lung cancer therapy during the COVID-19 pandemic. Lancet Respir Med 2020; 8:542–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu J, Ouyang W, Chua MLK, Xie C. SARS-CoV-2 transmission in patients with cancer at a tertiary care hospital in Wuhan, China. JAMA Oncol 2020. doi: 10.1001/jamaoncol.2020.0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai M, Liu D, Liu M, et al. . Patients with cancer appear more vulnerable to SARS-COV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov 2020. doi: 10.1158/2159-8290.cd-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuderer NM, Choueiri TK, Shah DP, et al. ; COVID-19 and Cancer Consortium . Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet 2020; 395:1907–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang K, Sheng Y, Huang C, et al. . Clinical characteristics, outcomes, and risk factors for mortality in patients with cancer and COVID-19 in Hubei, China: a multicentre, retrospective, cohort study. Lancet Oncol 2020; 21:904–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Specialty Collaboration, UK . COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395:1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao M, Liu Y, Yuan J, et al. . Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat Med 2020; 26:842–4. [DOI] [PubMed] [Google Scholar]
- 23.Moore JB, June CH. Cytokine release syndrome in severe COVID-19. Science 2020; 368:473–4. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu M. Clinical Features of Cytokine Storm Syndrome. In: Cron RQ, Behrens EM, eds. Cytokine Storm Syndrome. vol. 69. Cham, Switzerland: Springer International Publishing, 2019:31–41. [Google Scholar]
- 25.Potey PM, Rossi AG, Lucas CD, Dorward DA. Neutrophils in the initiation and resolution of acute pulmonary inflammation: understanding biological function and therapeutic potential. J Pathol 2019; 247:672–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonifant CL, Jackson HJ, Brentjens RJ, Curran KJ.. Toxicity and management in CAR T-cell therapy. Mol Ther Oncolytics 2016; 3:16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Y, Du X, Chen J, et al. . Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect 2020; 81:e6–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheng L-L, Guan W-J, Duan C-Y, et al. . Effect of recombinant human granulocyte colony-stimulating factor for patients with Coronavirus disease 2019 (COVID-19) and lymphopenia: a randomized clinical trial. JAMA Intern Med 2020. doi: 10.1001/jamainternmed.2020.5503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer NJ, Lindell RB, John Wherry E. Immune stimulation with recombinant human granulocyte colony–stimulating factor for coronavirus disease 2019 (COVID-19): beware of blind spots. JAMA Int Med 2020. doi: 10.1001/jamainternmed.2020.5536. [DOI] [PubMed] [Google Scholar]
- 30.Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. . Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med 2020; 383:2586–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.