Abstract
Like other cellular models, endothelial cells in cultures stop growing when they reach confluence, even in the presence of growth factors. In this work, we have studied the effect of cellular contact on the activation of p42/p44 mitogen-activated protein kinase (MAPK) by growth factors in mouse vascular endothelial cells. p42/p44 MAPK activation by fetal calf serum or fibroblast growth factor was restrained in confluent cells in comparison with the activity found in sparse cells. Consequently, the induction of c-fos, MAPK phosphatases 1 and 2 (MKP1/2), and cyclin D1 was also restrained in confluent cells. In contrast, the activation of Ras and MEK-1, two upstream activators of the p42/p44 MAPK cascade, was not impaired when cells attained confluence. Sodium orthovanadate, but not okadaic acid, restored p42/p44 MAPK activity in confluent cells. Moreover, lysates from confluent 1G11 cells more effectively inactivated a dually phosphorylated active p42 MAPK than lysates from sparse cells. These results, together with the fact that vanadate-sensitive phosphatase activity was higher in confluent cells, suggest that phosphatases play a role in the down-regulation of p42/p44 MAPK activity. Enforced long-term activation of p42/p44 MAPK by expression of the chimera ΔRaf-1:ER, which activates the p42/p44 MAPK cascade at the level of Raf, enhanced the expression of MKP1/2 and cyclin D1 and, more importantly, restored the reentry of confluent cells into the cell cycle. Therefore, inhibition of p42/p44 MAPK activation by cell-cell contact is a critical step initiating cell cycle exit in vascular endothelial cells.
Cell proliferation in multicellular organisms is a highly regulated process with multiple levels of control. One of these mechanisms is the inhibition of cell growth by cellular contact, even in the presence of growth factors. In adult tissues, contact inhibition is thought to be continuously active, playing a critical role in the repression of somatic cell proliferation. Release from this state is associated with abnormal cell growth (i.e., cellular transformation) (5, 16). Vascular endothelial cells are particularly sensitive to cell contacts and undergo rapid and very tight cell cycle withdrawal at confluence both in vivo and in vitro (11, 31). These cells therefore represent an interesting model for studying the mechanisms implicated in the inhibition of cell growth by cellular confluence.
The membrane proteins implicated in growth arrest by cell-cell contact are relatively unknown. It has been suggested that cell surface adhesion molecules transmit growth-inhibitory signals. This role has been proposed for cadherins, which are transmembrane polypeptides that undergo homophilic binding in different cellular types, such as epithelial and endothelial cells (31). VE-cadherin, a specific vascular endothelial cell cadherin, has been shown to reduce cell growth when it is overexpressed in CHO cells (6). Other candidates shown to be implicated in the control of cell growth are the Drosophila tumor suppressor-like genes dlg and fat (34, 53). When these genes are mutated, they cause imaginal disc overgrowth due to greater cell proliferation. Dlg is a cytoplasmic protein with PDZ and SH3 domains and guanylate kinase activity, and it seems to be required for signal transduction processes. Fat is an enormous transmembrane protein containing 33 cadherin-like repeats of unknown function (34). Another protein implicated in the transduction of cell-cell contact signals is contactinhibin, a protein responsible for the density-dependent growth inhibition of normal human diploid fibroblasts (52). A receptor for this protein which is implicated in cell-cell contact-mediated arrest of human fibroblasts has been identified (19). All these molecules might be able to transduce growth-inhibitory signals, but the nature of these signals and the pathways involved are not yet known.
In fibroblasts, cellular confluence is accompanied by a lack of phosphorylation of the retinoblastoma product, a consequence of the inhibition of cyclin-dependent kinases 2 and 4/6 (13). Two cyclin-dependent kinase inhibitors, p27 and p16, have been shown to play a determinant role in controlling G0-G1-phase to S-phase progression by inhibiting cyclin-dependent kinases (26). In particular, studies have highlighted a critical role for p27, since p27 levels increase at confluence (21, 44). However, the increase in p27 levels at confluence might not be the cause of growth arrest but merely might be the consequence. Indeed, embryonic fibroblasts derived from p27-knockout mice still display contact inhibition of growth (38). Therefore, despite many attempts to understand the nature of the signals directly mediating growth arrest by cell-cell contact, the molecular bases of this regulation remain largely unknown.
The p42/p44 mitogen-activated protein kinase (MAPK) cascade is one of the most characterized signalling pathways that connects different types of membrane receptors to the nucleus after mitogenic stimulation (8, 46) or differentiation (36). The activation of the p42/p44 MAPK cascade involves the activation of low-molecular-weight GTP-binding proteins (Ras) at the plasma membrane and the sequential activation of a series of protein kinases: a MAPK kinase kinase (Raf-1) is activated and then activates by phosphorylation a MAPK kinase (consisting of MEK-1 and MEK-2 [MEK1/2]), which in turns phosphorylates p42/p44 MAPKs on threonine and tyrosine residues, leading to their activation. p42/p44 MAPKs are then able to phosphorylate cytoplasmic and nuclear targets (7, 18, 33). This pathway has been found to play a critical role in the control of cell proliferation via growth factor receptors and integrins (23). The objective of our work was to study the effect of cellular contact on p42/p44 MAPK activation in mouse vascular endothelial cells. We found that p42/p44 MAPK activation was indeed inhibited by confluence. However, the fact that the upstream activators Ras and MEK-1 were not affected by confluence suggests that specific MAPK phosphatases play a key role in cell-cell contact-mediated growth inhibition.
MATERIALS AND METHODS
Materials.
PD98059 was obtained from New England BioLabs, okadaic acid was obtained from BioMol, 4-hydroxytamoxifen was obtained from ICI Pharmaceuticals, fibroblast growth factor (FGF-2) was obtained from Pepro Tech Inc., and [γ-32P]ATP was obtained from ICN. Cell culture media, fetal calf serum (FCS), glutamine, and antibiotics were obtained from Gibco-BRL. Most commonly used chemicals were purchased from Sigma.
Cells and culture conditions.
Murine lung endothelial cells (1G11 cells) were obtained from Alberto Mantovani and Annunciata Vecchi (Instituto Ricerche Farmacologiche Mario Negri, Milan, Italy) (14). They were cultured in Dulbecco modified Eagle medium (DMEM) containing 20% inactivated FCS, 50 U of penicillin per ml, 50 μg of streptomycin sulfate per ml, 150 μg of endothelial cell growth supplement (Becton Dickinson) per ml, 100 μg of heparin per ml, 1% nonessential amino acids, and 2 mM sodium pyruvate. Cells were plated at a density sufficient to reach confluence in 2 days (50,000 cells/cm2) or at a density sufficient to maintain sparse-cell conditions (5,000 cells/cm2). After 3 days of culturing, cells were depleted for 24 h in a 1:1 mixture of DMEM and Ham’s F12 medium before stimulation with growth factors.
Mouse brain capillary endothelial cells (LIBE cells) were obtained from L. Claesson-Welsh (Ludwig Institute for Cancer Research, Uppsala, Sweden). These cells were established from transgenic mice expressing a temperature-sensitive (tsA58) variant of the simian virus 40 large T antigen under the control of a gamma interferon-responsive promoter (25). Cells were cultured in Ham’s F12 medium containing 20% inactivated FCS, 50 U of penicillin per ml, 50 μg of streptomycin sulfate per ml, 150 μg of endothelial cell growth supplement per ml, 10 ng of epidermal growth factor (EGF) (Sigma) per ml, 5 μg of insulin (Sigma) per ml, and 20 U of recombinant mouse gamma interferon (Sigma) per ml at 33°C. Cells were cultured for 2 days until they reached confluence or remained sparse and were depleted for 24 h in Ham’s F12 medium at 39°C before stimulation with growth factors.
Retroviral transfection and generation of 1G11-ΔRaf-1:ER cells.
Retroviral supernatants were generated by transient transfection of BOSC23 cells with plasmid pLNC ΔRaf-1:ER (45) and were used to infect 1G11 cells as previously described (42). Positive clones were selected on the basis of resistance to neomycin G418 (400 μg/ml) and morphology alterations in the presence of 1 μM estradiol in normal medium. Various studies were performed with two independent clones of 1G11-ΔRaf-1:ER cells (1G11 cells stably expressing ΔRaf-1:ER [45]). All the experiments were performed by adding 4-hydroxytamoxifen, an antiestrogen which binds and activates ΔRaf-1:ER, instead of estradiol to prevent nonspecific effects. Tamoxifen was dissolved in ethanol; therefore, the same concentrations of ethanol (0.1 to 1%) were added to control cells.
Thymidine incorporation.
1G11 cells were cultured in 24-well plates under conditions promoting confluence or sparseness and were deprived of growth factors for 24 h in a 1:1 mixture of DMEM and Ham’s F12 medium. Cells were then stimulated in fresh DMEM medium containing 20% FCS in the presence or absence of 1 μM 4-hydroxytamoxifen and 0.25 μCi of [methyl-3H]thymidine (Amersham) per ml (3 μM final concentration). After 20 h of incubation, cells were fixed and washed three times with ice-cold trichloroacetic acid (5%). Cells were then harvested with 0.1 N NaOH, and the incorporated radioactivity was counted by liquid scintillation.
BrdU.
DNA synthesis was measured by incorporation of bromodeoxyuridine (BrdU; Amersham) into DNA. Cells were cultured on glass coverslips for 72 h until they attained confluence or remained sparse. After 24 h of serum depletion, cells were stimulated with 20% FCS in the presence or absence of 1 μM 4-hydroxytamoxifen for 24 h. BrdU (10 μM) was added to the culture medium during the last 4 h. Cells were rinsed three times with phosphate-buffered saline (PBS) and fixed in 3% paraformaldehyde for 20 min. After four washes with PBS, cells were permeabilized with PBS–0.2% Triton X-100 for 5 min, treated with 2 N HCl for 10 min, and blocked for 45 min at room temperature in PBS containing 10% FCS (PBS/FCS). Incorporated BrdU was immunodetected by incubation with a mouse monoclonal anti-BrdU antibody (Amersham; diluted 1:1 in PBS/FCS) for 1 h at room temperature, followed by a Texas red-conjugated anti-mouse antibody (Molecular Probes; diluted 1/500 in PBS/FCS) for 45 min at room temperature. Three washes in PBS–0.1% Tween 20 followed each addition of antibody. Finally, cells were incubated with PBS–4′,6-diamidino-2′-phenylindole dihydrochloride (DAPI; Boehringer; 0.2 μg/ml) for 5 min at room temperature. Coverslips were then mounted with CITIFLUOR (UKC Chem Laboratory), and immunofluorescence was visualized with a Nikon Diaphot microscope (×40 lens).
Western blot analysis.
Cells were washed twice with cold PBS and lysed with Triton X-100 lysis buffer (50 mM Tris-HCl [pH 7.5], 100 mM NaCl, 50 mM NaF, 5 mM EDTA, 40 mM β-glycerophosphate, 200 μM sodium orthovanadate, 100 μM phenylmethylsulfonyl fluoride, 1 μg of leupeptin per ml, 1 μM pepstatin A, 4 μg of aprotinin per ml, 1% Triton X-100) for 15 min at 4°C. Insoluble material was removed by centrifugation at 12,000 × g for 5 min at 4°C. Proteins from cell lysates (between 25 and 75 μg) were separated on acrylamide-bisacrylamide (29:1; Gibco-BRL)–sodium dodecyl sulfate (SDS) gels and electrophoretically transferred to Immobilon-P membranes (Millipore) in 25 mM Tris-HCl–0.19 M glycine–20% ethanol. Membranes were blocked in PBS containing 5% nonfat dry milk (blocking solution) for 1 h at 37°C. The blots were then incubated with rabbit antiserum E1B (1:3,000), which specifically recognizes p42/p44 MAPK (37), rabbit antiserum Alb-1 (1:250), which specifically recognizes MAPK phosphatases 1 and 2 (MKP-1 and MKP-2, respectively) (2), monoclonal anti-cyclin D1 antibody (NeoMarkers; 1:300), polyclonal anti-Fos antibody (Santa Cruz; 1:1,000), polyclonal anti-MEK1 antibody (33), polyclonal anti-active p42/p44 MAPK antibody (Promega; 1:3,000), and polyclonal anti-active MEK1/2 antibody (New England BioLabs; 1:1,000) in blocking solution overnight at 4°C. After being washed in PBS–0.1% Tween 20, the blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin G (1:3,000) or anti-mouse immunoglobulin G (1:1,000) in blocking solution for 1 h and analyzed with an ECL kit (Amersham).
When needed the activity of p42/p44 MAPK was determined by a mobility shift assay in which, following cell lysis, proteins were separated by SDS-polyacrylamide gel electrophoresis (PAGE) with a 12.5% gel (acrylamide-bisacrylamide, 30:0.2) and Western blotting was performed with antiserum E1B.
Immune complex kinase assays. (i) p44 MAPK activity.
Cells were seeded in 6-cm plates and rendered quiescent by serum starvation for 24 h under conditions promoting confluence or sparseness. Cells were stimulated in DMEM with the appropriate agonist at 37°C for various times. Cells were then washed with ice-cold PBS and lysed with Triton X-100 lysis buffer for 15 min at 4°C. Insoluble material was removed by centrifugation at 12,000 × g for 5 min at 4°C. Proteins from lysates (150 μg) were incubated for 2 h at 4°C with a specific polyclonal anti-p44 MAPK antibody (Santa Cruz) preadsorbed to protein A-Sepharose beads (Pharmacia Biotech). Immune complexes were washed three times with Triton X-100 lysis buffer and twice with kinase buffer (20 mM HEPES [pH 7.4], 20 mM MgCl2, 1 mM dithiothreitol, 10 mM p-nitrophenyl phosphate [pNPP]). p44 MAPK activity was assayed by resuspending the final pellet in 40 μl of kinase buffer containing 50 μM [γ-32P]ATP (5,000 cpm/pmol) and 0.25 mg of myelin basic protein (MBP) per ml. The reaction was carried out for 30 min at 30°C and stopped by the addition of Laemmli sample buffer (30). The samples were separated on a 12% polyacrylamide gel and analyzed with a phosphorimager system.
(ii) MEK-1 activity.
Confluent or sparse 1G11 cells were serum starved for 24 h and treated with 25 ng of FGF-2 per ml for 5 min. When needed, 50 μM PD98059 was added 15 min before the addition of FGF-2. After two washes with cold PBS, cells were lysed with Triton X-100 lysis buffer. MEK-1 protein was immunoprecipitated from 1 mg of lysate by incubation for 4 h at 4°C with a specific anti–MEK-1 antibody, MKK16 (33), preadsorbed to protein A-Sepharose beads. Immune complexes were washed three times with Triton X-100 lysis buffer and twice with kinase buffer. MEK-1 activity was assayed by resuspending the final pellet in 40 μl of kinase buffer containing 50 μM [γ-32P]ATP (5,000 cpm/pmol) and 15 μg of GST–p44 MAPK-KAKA (a generous gift from S. Meloche, University of Montreal). The reaction was carried out for 30 min at 30°C and stopped by the addition of Laemmli sample buffer (30). The samples were separated on a 7.5% polyacrylamide gel and analyzed with a phosphorimager system.
p21ras activation assays.
A novel assay to measure the activity status of p21ras was used as described previously (12, 37a, 51). Briefly, confluent or sparse 1G11 cells were seeded in 15-cm plates and rendered quiescent by serum starvation for 24 h. Cells were stimulated in DMEM with 25 ng of FGF-2 per ml for 5 min at 37°C prior to being washed with ice-cold PBS and lysed with buffer A (50 mM Tris-HCl [pH 7.5], 15 mM NaCl, 20 mM MgCl2, 5 mM EGTA, 100 μM phenylmethylsulfonyl fluoride, 1 μg of leupeptin per ml, 1 μM pepstatin A, 1% Triton X-100, 1% N-octylglucoside) for 15 min at 4°C. Insoluble material was removed by centrifugation at 12,000 × g for 5 min at 4°C. Proteins from lysates (1 mg) were incubated for 2 h at 4°C with 30 μg of glutathione S-transferase (GST)–RBD fusion protein (where RBD is amino acids 51 to 131 of Raf-1 and is the minimal domain required for the binding of Ras-GTP) preadsorbed to glutathione-Sepharose beads. Precipitates were washed three times with buffer A. The presence of p21ras was detected by resuspending the final pellet in 25 μl of Laemmli sample buffer (30), followed by protein separation on 12.5% polyacrylamide gels and Western blotting with monoclonal antibody pan-Ras-Ab3, which specifically recognizes p21ras (Calbiochem). As a control, 25 μg of the supernatant was loaded to immunodetect total Ras.
Phosphatase activity.
Cell lysates were prepared as described for the Western blot protocol in the absence of phosphatase inhibitors. Fifty micrograms of lysate was incubated for 30 min at 37°C in phosphatase buffer (50 mM HEPES [pH 7.0], 60 mM NaCl, 60 mM KCl, 5 mM EDTA, 10 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 1 μg of leupeptin per ml, 1 μM pepstatin A, 5 μg of aprotinin per ml, 1 mg of bovine serum albumin per ml) and in the presence or absence of 200 μM sodium orthovanadate (final volume, 50 μl). The reaction was initiated by the addition of 10 mM pNPP. The reaction was stopped by the addition of 0.9 ml of 1 N NaOH. The results were quantified by spectrophotometry at 410 nm. The activity sensitive to orthovanadate was calculated by subtracting the activity in the presence of orthovanadate from the activity in the absence of orthovanadate.
Active p42 MAPK dephosphorylation.
Extracts from confluent or sparse 1G11 cells were obtained by lysis with Triton X-100 lysis buffer in the absence of phosphatase inhibitors. Five nanograms of bacterially expressed, dually phosphorylated active p42 MAPK (28) was incubated for 30 min at 37°C in the presence or absence of 50 μg of cell lysates in phosphatase buffer (final volume, 50 μl). The reaction was stopped by the addition of 1 mM sodium orthovanadate. To visualize the phosphorylation of MBP by active MAPK, 3× kinase buffer containing 50 μM [γ-32P]ATP (5,000 cpm/pmol) and 0.25 mg of MBP per ml was immediately added. The reaction mixture was incubated at 30°C for 30 min, and the reaction was stopped by the addition of Laemmli sample buffer (30). The samples were separated on a 12.5% polyacrylamide gel and analyzed with a phosphorimager system.
RESULTS
p42/p44 MAPK activation by growth factors is inhibited in confluent endothelial cells.
On culture plates, 1G11 endothelial cells grow until they form a perfect monolayer. At this stage, cells stop growing and become quiescent. This pattern can be easily shown by the overexpression of the cyclin-dependent kinase inhibitor p27 and the arrest of thymidine incorporation (data not shown). In order to evaluate the effect of cell confluence on p42/p44 MAPK activation, 1G11 cells were plated at two cell densities (50,000 and 5,000 cells/cm2) and grown for 3 days. At the higher density, cells formed a confluent monolayer in 2 days, whereas at the lower density, cells were still growing (sparseness). After a 24-h depletion of growth factors, confluent or sparse cells were stimulated for different times with 25 ng of FGF-2 per ml (Fig. 1A and B). In sparse 1G11 cells, FGF-2 induced transient activation of p42/p44 MAPK (measured by phosphorylation of the MBP), which peaked after 5 min and returned to near basal levels after 4 h (Fig. 1A). This transient activation of p42/p44 MAPK correlates well with the weak mitogenic effect of FGF-2 on 1G11 cells (data not shown). In contrast, stimulation of confluent cells by FGF-2 caused a much more moderate activation of p42/p44 MAPK. The activity also peaked after 5 min but was only 60% of the maximum effect obtained in nonconfluent cells. We also evaluated MAPK activation by monitoring the shift up of the hyperphosphorylated and therefore active forms of p42/p44 MAPK. The shift up of p42/p44 MAPK correlated perfectly with the level of activation obtained in kinase assays with MBP (compare Fig. 1A and B). In response to FGF-2, the shift up obtained in confluent cells was less marked than that obtained in sparse cells and declined very rapidly (compare the values at 30 min of stimulation).
FIG. 1.
Effect of confluence on p42/p44 MAPK activation by FGF-2 and FCS. Confluent or sparse 1G11 cells were rendered quiescent by 24 h of serum starvation. Cells were then stimulated or not stimulated with 25 ng of FGF-2 per ml (A and B) or 20% FCS (C and D) for the times indicated. Cells were rinsed three times with cold PBS, and lysates were obtained as described in Materials and Methods. (A) and (C) p44 MAPK was immunoprecipitated from lysates with a specific antibody. p44 MAPK activity was measured by the phosphorylation of MBP in the presence of [γ-32P]ATP. Proteins were separated on an SDS–12.5% polyacrylamide gel, and radioactivity was measured with a Fuji phosphorimager. Results are the means ± standard errors for five independent experiments and are expressed relative to the basal activity in the absence of stimulation. (B) and (D) Cell lysates were separated on an SDS–12.5% polyacrylamide gel with a special shift-up polyacrylamide (acrylamide-bisacrylamide, 30:0.2) and p42 MAPK and p44 MAPK were detected by immunoblotting with antiserum E1B. Hyperphosphorylated and active forms of p42 MAPK and p44 MAPK (indicated as pp42 and pp44, respectively) migrated more slowly than nonphosphorylated forms. Western blots representative of four experiments performed with identical results are shown.
We next studied the effect of a strong mitogen for 1G11 cells, 20% fetal calf serum (FCS) (Fig. 1C and D). In sparse 1G11 cells, p42/p44 MAPK was rapidly activated by 20% FCS, with a maximum at 10 min. This activation was sustained until 60 min, and after this time it declined to near basal levels by 8 h. Confluence reduced the capacity of FCS to activate p42/p44 MAPK in comparison with the activation obtained in sparse cells. In confluent cells, the activity at 10 min of stimulation was only 47% the maximal activity obtained in sparse cells. As with FGF-2, the same results were obtained in shift-up experiments (Fig. 1D). These results were not due to changes in the amount of p42/p44 MAPK present in confluent or sparse 1G11 cells (Fig. 1B and D). Moreover, the same “repression” of p42/p44 MAPK activity was observed in another endothelial cell model, LIBE cells (data not shown). These results suggest that the abrogation of p42/p44 MAPK activity by cellular contact could be a general mechanism in endothelial cells. In addition, we found that this restrictive activation of p42/p44 MAPK in confluent cells was reversible. When confluent cells were trypsinised and replated under conditions promoting sparseness, they recovered the capacity for stimulation of p42/p44 MAPK by FCS 2 h after trypsinisation (data not shown).
p42/p44 MAPK-dependent events are inhibited in confluent endothelial cells.
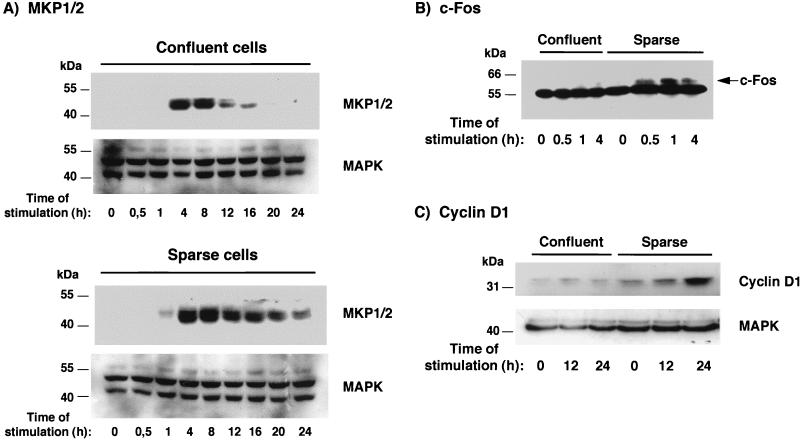
We next investigated the effect of confluence on the induction of proteins under the control of p42/p44 MAPK activation. We first examined the induction of MKP-1 and MKP-2 (MKP1/2) by FGF-2. MKP1/2 are two dual-specificity MAPK phosphatases that participate in the inactivation of MAPKs and that have been shown to be induced by p42/p44 MAPK activation (2). In sparse cells, activation by FGF-2 caused the induction of MKP1/2, with a maximum at 1 h, and this induction persisted at low levels until 24 h (Fig. 2A). In contrast, the evolution of MKP1/2 in confluent cells was less important and more transient, with the total loss of the signal at 20 h. The same result was obtained for the induction of c-Fos. It has been shown that the c-fos gene is under the control of a serum response element and that proteins which bind to this element and activate the promoter are targets of the Ras-p42/p44 MAPK pathway (22). Treatment of serum-starved sparse 1G11 cells with FGF-2 caused an increase in the levels of c-Fos protein, with maximal expression after 1 h of FGF-2 stimulation (Fig. 2B). In contrast, the same treatment of confluent cells induced very low levels of expression of c-Fos. Similarly, the induction of cyclin D1, a cyclin that is also under the control of the p42/p44 MAPK cascade (32), was barely detectable in confluent endothelial cells, whereas moderate induction occurred in sparse cells (Fig. 2C). These results indicate that, in accordance with the respective p42/p44 MAPK activity levels, all the effects which depend on the stimulation of p42/p44 MAPK become inhibited when endothelial cells attain confluence.
FIG. 2.
Cellular confluence inhibits MAPK-dependent signalling events. Confluent or sparse 1G11 cells were depleted of growth factors for 24 h and stimulated for the indicated times with 25 ng of FGF-2 per ml. Cells were lysed, and proteins were separated on a 10% polyacrylamide gel and Western blotted with anti-MKP1/2 and anti-MAPK (as a protein loading control) antisera (A), with an anti-Fos antibody (B), and with anti-cyclin D1 and anti-MAPK (as a control) antisera (C). Representative Western blots are shown.
Growth factor activation of Ras and MEK-1 is not sensitive to confluence.
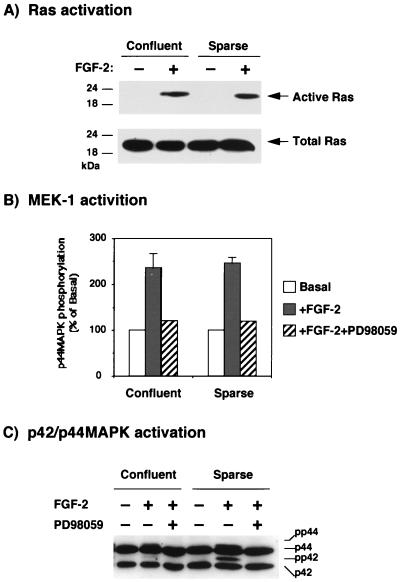
In order to determine whether cellular contacts affected upstream members of the p42/p44 MAPK cascade, we studied the effect of confluence on the activation of p21ras and MEK-1 by FGF-2. To study Ras activation, we used a new method that consists of the pull-down of active cellular p21ras by a GST fusion protein that contains the Ras-binding domain of Raf (12, 37a, 51). In this experiment, stimulation of 1G11 cells for 5 min with FGF-2 was followed by precipitation of active p21ras present in confluent and sparse cells. Using this method, we did not detect any difference in p21ras activation between confluent and sparse cells (Fig. 3A).
FIG. 3.
Cellular confluence does not affect the activation of upstream members of the p42/p44 MAPK cascade. (A) Confluent or sparse 1G11 cells were depleted of growth factors for 24 h and stimulated for 5 min with 25 ng of FGF-2 per ml. Cells were lysed and incubated with a GST-RBD fusion protein (where RBD is amino acids 51 to 131 of Raf-1 and is the minimal domain required for the binding of Ras GTP) preadsorbed to glutathione-Sepharose beads. The presence of active p21ras was detected by resuspending the final pellet in 25 μl of Laemmli sample buffer (30), followed by protein separation on 12.5% polyacrylamide gels and Western blotting with an antiserum specifically recognizing p21ras (Active Ras). The total amount of Ras present in the cells was detected by loading 25 μg of the total cell extract and performing Western blotting as indicated (Total Ras). An autoradiogram representative of four different experiments is shown. (B) Quiescent confluent or sparse cells were stimulated or not stimulated for 5 min with 25 ng of FGF-2 per ml in the presence or absence of 50 μM PD98059. After this time, cells were lysed and MEK-1 was immunoprecipitated by incubation with specific anti–MEK-1 antiserum preadsorbed to protein A-Sepharose beads. MEK-1 activity was assessed by incubation of the beads with [γ-32P]ATP and GST–p44 MAPK-KAKA as a substrate. After SDS-PAGE (8% polyacrylamide), the radioactivity incorporated was measured with a Fuji phosphorimager. The results are the means ± standard errors for three independent experiments and are expressed relative to the basal activity in the absence of stimulation. (C) The cell lysates used for measuring MEK-1 activity were loaded on a shift-up SDS–12.5% polyacrylamide gel as described in Materials and Methods, and p42/p44 MAPK was immunodetected by Western blotting with antiserum E1B. A representative Western blot is shown.
We next analyzed the effect of confluence on the activity of MEK-1, the immediate upstream activator of p42/p44 MAPK. After stimulation of confluent and sparse cells with FGF-2 for 5 min, MEK-1 was immunoprecipitated from lysates, and the capacity of MEK-1 to phosphorylate its natural substrate, p44 MAPK, was evaluated. As shown in Fig. 3B, the activation of MEK-1 in confluent and sparse 1G11 cells was identical, whereas preincubation of cells with the MEK-1 inhibitor PD98059 (15) completely abolished MEK-1 stimulation by FGF-2 in confluent and sparse cells. Under these conditions, the shift up of p42/p44 MAPK activation was markedly reduced in confluent cells, and as expected, preincubation with PD98059 completely abolished p42/p44 MAPK activation (Fig. 3C).
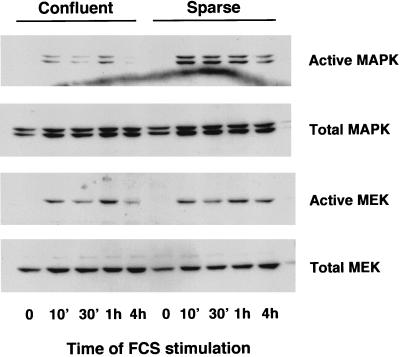
In order to confirm these results, we performed experiments with antibodies against the active forms of p42/p44 MAPK and MEK1/2 (47). Confluent and sparse 1G11 cells were stimulated for different times with 20% FCS, and the activities of p42/p44 MAPK and MEK1/2 were measured by Western blotting (Fig. 4). The results obtained confirmed the lack of effect of confluence on MEK1/2 activation under the same conditions in which the activation of p42/p44 MAPK was clearly reduced. Also, stimulation of phospholipase C activity by FCS, an effect that is independent of p42/p44 MAPK activation, was the same in confluent and sparse cells (data not shown). These results indicate that confluence specifically antagonizes p42/p44 MAPK activation and MAPK-dependent events, whereas the upstream portion of the signalling cascade (Ras and MEK-1) remains unaffected by the state of confluence.
FIG. 4.
MEK1/2 is normally activated in confluent cells. Confluent or sparse 1G11 cells were depleted of growth factors for 24 h and stimulated for the times indicated with 20% FCS. After lysis, 40 μg of protein was separated on an SDS–10% polyacrylamide gel, and active p42/p44 MAPK, total p42/p44 MAPK, active MEK1/2, and total MEK1/2 were immunodetected with specific antibodies. A representative Western blot is shown.
Sodium orthovanadate reverses the inhibition of p42/p44 MAPK by confluence, and phosphatase activity is increased in confluent cells.
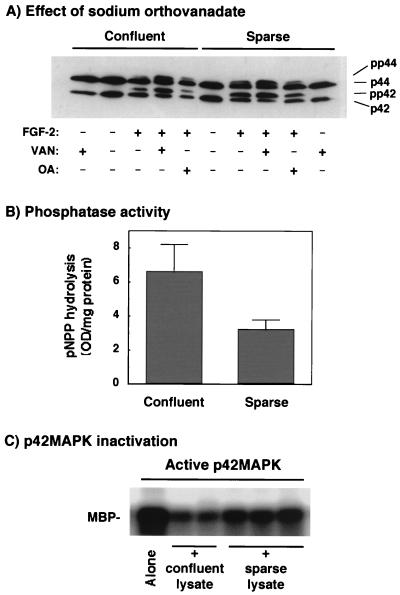
As indicated before, the activity of MAPKs is completely dependent on the state of phosphorylation (8, 46). Thus, another mechanism able to inhibit p42/p44 MAPK activation is the increased expression of a phosphatase able to dephosphorylate and inactivate MAPKs. To address the potential role of a phosphatase in the inhibition of p42/p44 MAPK activation by endothelial cell confluence, we used the protein tyrosine phosphatase inhibitor sodium orthovanadate (50). Preincubation of 1G11 cells for 15 min with 200 μM sodium orthovanadate before the addition of FGF-2 for an additional 10 min potentiated the effect of FGF-2 on the shift up of p42/p44 MAPK (Fig. 5A). Strikingly, the addition of orthovanadate completely abolished the MAPK inhibition triggered by confluence (Fig. 5A, compare FGF-2 plus orthovanadate in confluent and sparse cells) in the absence of any effect on MEK-1/2 activation (data not shown). In contrast, preincubation of cells with 1 μM okadaic acid, a specific serine/threonine phosphatase inhibitor (9), had no effect on the MAPK activity observed in confluent cells. All these results suggest the implication of a tyrosine phosphatase or a dual tyrosine/threonine phosphatase in the effect of confluence on p42/p44MAPK activation.
FIG. 5.
Sodium orthovanadate but not okadaic acid restores p42/p44 MAPK activation in confluent cells, and confluence affects phosphatase activity. (A) Quiescent confluent or sparse 1G11 cells were preincubated or not preincubated for 15 min in the presence of 200 μM sodium orthovanadate (VAN) or 1 μM okadaic acid (OA). After this time, cells were stimulated or not stimulated with 25 ng of FGF-2 per ml for 10 min in the presence or absence of orthovanadate or okadaic acid. After lysis, the level of activation of p42/p44 MAPK was determined by migration in a shift-up 12.5% polyacrylamide gel and Western blotting with antiserum E1B. A representative Western blot of three different experiments is shown. (B) Starved confluent or sparse cells were lysed in Triton X-100 lysis buffer in the absence of inhibitors of phosphatases. Cell lysates (50 μg) was incubated in the presence or absence of 200 μM sodium orthovanadate and 10 mM pNPP in phosphatase buffer for 30 min. The reaction was stopped by the addition of 0.9 ml of 1 N NaOH, and the absorbance of the samples was measured at 410 nm. The results shown are the means ± standard errors for three independent experiments showing orthovanadate-sensitive phosphatase activity. Phosphatase activities that were not sensitive to sodium orthovanadate and that were subtracted from the total activity were 5.8 ± 0.9 and 7.2 ± 1.7 optical density units/mg of protein for the confluent and sparse cell lysates, respectively. (C) Extracts from confluent or sparse 1G11 cells were obtained by lysis in Triton X-100 lysis buffer in the absence of phosphatase inhibitors. Active p42 MAPK (5 ng) was incubated for 30 min at 37°C in the presence or absence of 50 μg of cell lysates in phosphatase buffer (final volume, 50 μl). The reaction was stopped by the addition of 1 mM sodium orthovanadate. Immediately, the phosphorylation of MBP was determined to measure the activity status of p42 MAPK. The reaction mixture was incubated at 30°C for 30 min, and the reaction was stopped by the addition of Laemmli sample buffer (30). The samples were separated on a 12.5% polyacrylamide gel and revealed and quantified with a phosphorimager system.
We next evaluated orthovanadate-sensitive phosphatase activity (measured as the capacity to dephosphorylate the substrate pNPP) present in confluent and sparse endothelial cells. As shown in Fig. 5B, confluent 1G11 cells had two times more orthovanadate-sensitive phosphatase activity than sparse cells. Moreover, lysates from confluent 1G11 cells more effectively inactivated bacterially expressed, dually phosphorylated active p42 MAPK (28) than lysates from sparse cells (22 and 37% remaining p42 MAPK activity, respectively) (Fig. 5C). This result clearly suggests the existence of a MAPK phosphatase activity which is more important in confluent than in sparse endothelial cells. However, the MAPK phosphatase implicated does not seem to be MKP1/2, since Western blotting did not reveal any difference between confluent and sparse cells (Fig. 2).
Enforcing persistent activation of p42/p44 MAPK restores growth-signalling events at confluence.
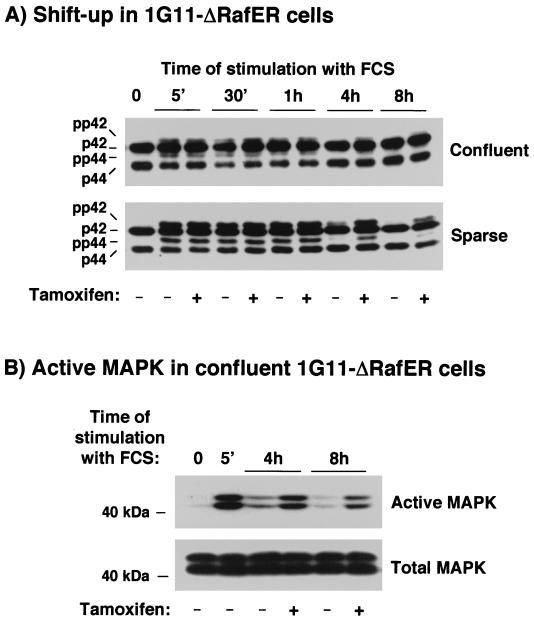
We wanted to evaluate the importance of p42/p44 MAPK inactivation caused by cell confluence in cell cycle withdrawal. For these experiments, we constructed 1G11 endothelial cells stably expressing the chimera ΔRaf-1:ER (45). This construct is a fusion between an oncogenic form of human Raf-1 and the steroid-binding domain of the human estrogen receptor. It can be simply activated by the addition of estradiol or of its antagonist 4-hydroxytamoxifen, leading to the stimulation of downstream components of the p42/p44 MAPK cascade. As expected, the addition of tamoxifen to parental untransfected cells had no effect on p42/p44 MAPK activation and did not cause any morphological change over 24 h of treatment (data not shown). In contrast, when clones of 1G11-ΔRaf-1:ER cells were treated with 20% FCS plus 1 μM tamoxifen, the time course of p42/p44 MAPK activation was more sustained than with FCS alone. Tamoxifen, however, did not modify the level of short-term activation (Fig. 6A, sparse cells).
FIG. 6.
Stimulation of p42/p44 MAPK by FCS and tamoxifen in confluent or sparse 1G11-ΔRaf-1:ER cells. Confluent or sparse 1G11-ΔRaf-1:ER cells were depleted of growth factors for 24 h. After this time, cells were stimulated or not stimulated with 20% FCS in the presence or absence of 1 μM tamoxifen for the times indicated. Cell extracts were prepared as described in the text. (A) Proteins were analyzed in a shift-up 12.5% polyacrylamide gel and blotted with p42/p44 MAPK antiserum E1B. (B) The same extracts from confluent cells stimulated for the times indicated were separated on an SDS–10% polyacrylamide gel, and active or total p42 MAPK and p44 MAPK were detected by immunoblotting. Representative autoradiograms of three Western blots are shown.
We therefore analyzed the effect of tamoxifen on FCS-stimulated confluent 1G11-ΔRaf-1:ER cells. As shown in Fig. 6A, confluence inhibited p42/p44 MAPK activation by FCS, as was the case for parental 1G11 cells (Fig. 6A, compare shift up of p42/p44 MAPK in confluent and sparse cells). The addition of 1 μM tamoxifen to the medium in the presence of 20% FCS did not change the inhibition of p42/p44 MAPK activation observed in confluent cells but caused a more sustained activation of p42/p44 MAPK in both confluent and sparse cells. To confirm the persistence of p42/p44 MAPK activation in confluent 1G11-ΔRaf-1:ER cells, the same extracts as those used in the experiment shown in Fig. 5A were analyzed with an antibody that recognizes active p42/p44 MAPK. As shown in Fig. 6B, we clearly detected the presence of the active forms of p42/p44 MAPK in confluent 1G11-ΔRaf-1:ER cells treated with FCS for 4 and 8 h. These active forms were enhanced by the addition of tamoxifen. These results indicate that the inhibitory effect caused by confluence is still present in confluent 1G11-ΔRaf-1:ER cells and that tamoxifen cannot reverse this inhibition but can maintain p42/p44 MAPK activation for a longer period of time.
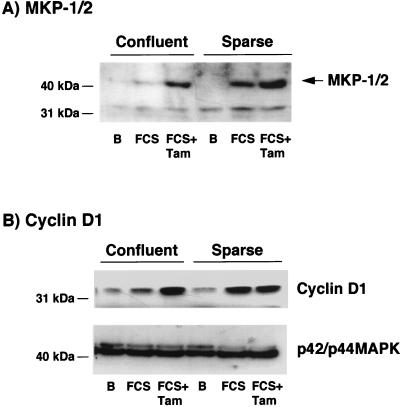
We next studied the consequence of the enhanced long-term p42/p44 MAPK activation on confluent 1G11-ΔRaf-1:ER cells. The induction of MKP1/2 (Fig. 7A) and cyclin D1 (Fig. 7B) by FCS was inhibited in confluent 1G11-ΔRaf-1:ER cells, as in parental 1G11 cells (Fig. 2). In contrast, the addition of 20% FCS plus 1 μM tamoxifen completely reversed the inhibition of MKP1/2 and cyclin D1 induction in confluent cells (Fig. 7, FCS+Tam), restoring the levels seen in sparse cells.
FIG. 7.
Enforced long-term activation of p42/p44 MAPK increases the expression of MKP1/2 and cyclin D1 at confluence. (A) Confluent or sparse 1G11-ΔRaf-1:ER cells were depleted of growth factors for 24 h. After this time, cells were stimulated or not stimulated (lane B) with 20% FCS (FCS) or 1 μM tamoxifen plus 20% FCS (FCS+Tam) for 4 h. (B) Quiescent 1G11-ΔRaf-1:ER cells were stimulated or not stimulated for 24 h with the same agonists as in panel A. Cells were lysed, and proteins were separated by SDS–12.5% PAGE. Western blot analysis was performed to immunodetect MKP1/2, cyclin D1, and p42/p44 MAPK as a control. Representative Western blots from three different experiments are shown.
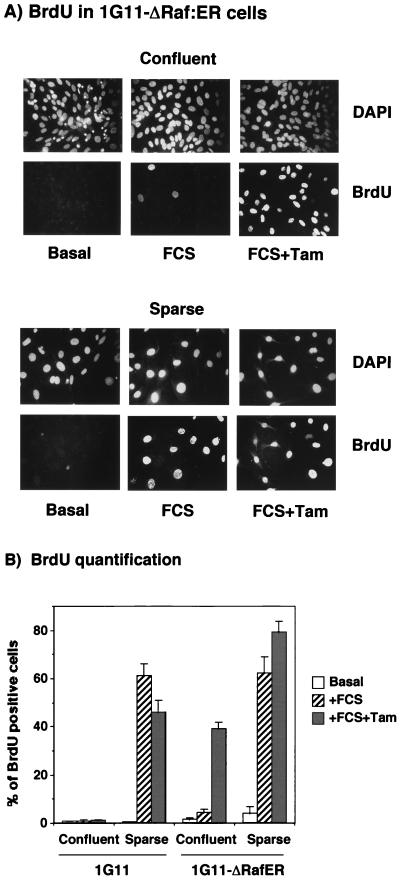
Finally, we evaluated whether the tamoxifen-induced increment in p42/p44 MAPK activation was sufficient to force the cells to reenter the cell cycle at confluence. We measured BrdU incorporation in confluent and sparse parental and 1G11-ΔRaf-1:ER cells in the presence of FCS alone or FCS plus 1 μM tamoxifen. As shown in Fig. 8, FCS alone increased BrdU incorporation in sparse cells only, with a negligible effect on confluent cells. The addition of FCS plus tamoxifen to sparse 1G11-ΔRaf-1:ER cells increased BrdU incorporation (25%) in the absence of any effect on parental cells (Fig. 8B), demonstrating that sustained activation of p42/p44 MAPK has a positive effect on DNA synthesis in nonconfluent cells. Furthermore, the addition of FCS plus tamoxifen to confluent 1G11-ΔRaf-1:ER cells drastically increased BrdU incorporation by more than eight times, compared to that in cells treated with FCS alone. These results indicate that the sustained p42/ p44 MAPK activation observed in 1G11-ΔRaf-1:ER cells stimulated with FCS plus tamoxifen was sufficient to force confluent cells to reenter the cell cycle. The same results were obtained with measurements of thymidine incorporation. Finally, and most importantly, cell numbers were doubled 3 days after the start of treatment with FCS plus tamoxifen (data not shown).
FIG. 8.
Enforced long-term activation of p42/p44 MAPK induces cell cycle reentry of confluent endothelial cells. Nontransfected parental 1G11 or 1G11-ΔRaf-1:ER cells were grown under conditions promoting sparseness or confluence and serum deprived for 24 h. Cells were stimulated or not stimulated (Basal) with 20% FCS (FCS) or 1 μM tamoxifen plus 20% FCS (FCS+Tam) for 24 h. During the last 4 h, cells were labelled with BrdU. DNA synthesis was assessed by immunodetection of cells that had incorporated BrdU. Nuclei were stained with DAPI. (A) Photographs of BrdU incorporation in sparse or confluent 1G11-ΔRaf-1:ER cells. (B) Quantification of the number of BrdU-positive nuclei in confluent or sparse parental and 1G11-ΔRaf-1:ER cells. Error bars show standard errors.
DISCUSSION
In this work, we have shown that (i) cellular confluence reduces p42/p44 MAPK activation by growth factors, while upstream members of the cascade are normally activated, (ii) p42/p44 MAPK activity is a limiting factor in the mitogenic response of mouse endothelial cells, and (iii) inhibition of p42/p44 MAPK activation by cellular confluence is sufficient to account for growth inhibition. Thus, the “repression” of MAPK activation by confluence appears to be an efficient mechanism for initiating cell cycle withdrawal of confluent vascular endothelial cells.
The p42/p44 MAPK cascade has been shown to be essential for the induction of proliferative responses in many cell types. This key role has been determined by experiments in which the interruption of the p42/p44 MAPK cascade by the expression of antisense p44 MAPK, the expression of a dominant negative p44 MAPK mutant (T192A), or the overexpression of a MAPK phosphatase (MKP-1) prevented quiescent fibroblasts from entering the S phase of the cell cycle in response to growth factors (3, 10, 39, 49). On the other hand, the expression of a constitutively active mutant of MEK-1 (S218D/S222D) in fibroblasts raised basal MAPK activity and induced oncogenicity (4, 10, 35). In endothelial cells, pretreatment with the MEK-1-specific inhibitor PD98059 inhibited the proliferation induced by vascular endothelial cell growth factor (29, 41).
In this work, we have shown that in capillary mouse endothelial cells, the p42/p44 MAPK cascade is also essential for mitogenicity, and if its activation is blocked, cells remain quiescent. This was the case when we preincubated cells with PD98059 before the addition of agonists (data not shown) as well as in a natural situation, such as cell-cell contact, in which p42/p44 MAPK activation is inhibited, as we have shown in this work. If p42/p44 MAPK is maintained in an active state in confluent cells (tamoxifen experiments), cells reenter the cell cycle and undergo DNA synthesis. Recently, similar results have been obtained with confluent NIH 3T3 cells (27). In these cells, hyperactivation of the p42/p44 MAPK cascade also reversed the quiescent state in cell-cell contact-inhibited cells. However, the authors correlated this result with a change in morphology that interrupts cellular contact and relieves antiproliferative signals mediated by cell-cell contact. In contrast, confluent 1G11-ΔRaf-1:ER cells stimulated by FCS in the presence of tamoxifen did not change their morphology during the first 48 h of treatment, probably due to the small increase in p42/p44 MAPK activity. Thus, the notion that a change in morphology could trigger the proliferation of contact-inhibited cells does not apply to our work.
It is important to stress that in confluent 1G11-ΔRaf-1:ER cells, a small sustained increase in p42/p44 MAPK activity is sufficient to stimulate an additional round of division. This result indicates that it is the sustained p42/p44 MAPK activity rather than the level of stimulation that is important for pushing endothelial cells into the cell cycle. For fibroblasts, it has been shown that sustained activation of p42/p44 MAPK is required for the cells to pass the G1 restriction point and enter the S phase (3, 39). This sustained activation of p42/p44 MAPK is always accompanied by the translocation of both isoforms into the nucleus (33). The same result has been obtained with confluent 1G11-ΔRaf-1:ER cells, for which the addition of FCS plus tamoxifen induced clear translocation of p42/p44 MAPK to the nucleus in more cells than did the addition of FCS alone (data not shown).
A striking finding is that cellular confluence specifically targets p42/p44 MAPK activation without affecting the activation of upstream members of this signalling cascade (Ras and MEK-1). This finding eliminates the possibility that transmembrane receptors do not signal as efficiently in confluent cells as in sparse cells. Thus, the pathway from cell surface receptors to MEK-1 is not subject to inhibition via cellular contact. Moreover, the activation of 1G11-ΔRaf-1:ER endothelial cells by FCS supplemented with tamoxifen induced more sustained p42/p44 MAPK activity but did not increase maximal activation, indicating that the mechanism causing the inhibition was still operating in these cell-cell contact-inhibited cells. In contrast, treatment of confluent endothelial cells with sodium orthovanadate completely reversed the inhibition, indicating that the full capacity to activate the p42/p44 MAPK cascade is still intact in confluent cells. This result is particularly important, as it suggests that the state of confluence has not sequestered p42/p44 MAPK out of the signalling module complex (Ras-MEK-MAPK) by changing, for example, the subcellular localization of the MAPK isoforms. Sodium orthovanadate is a known inhibitor of tyrosine phosphatases and dual-specificity phosphatases (24, 50). In this regard, it is interesting to recall that p42/p44 MAPK is activated by MEK-1 phosphorylation of two residues, Thr 183 and Tyr 185 (p42 MAPK sequence) (8, 46). Therefore, we suggest that orthovanadate-sensitive phosphatases participate in the inactivation of p42/p44 MAPK in confluent endothelial cells. In fact, a few reports have highlighted increases in both cytosolic and membrane-associated tyrosine phosphatase activities at high cell densities in osteoblast cells (48), in endothelial cells (17), and in Swiss 3T3 fibroblasts (40). In accord with these results, we have shown that the phosphatase activity sensitive to sodium orthovanadate is increased in confluent mouse endothelial cells compared to sparse cells. Moreover, extracts from confluent cells are more efficient at inactivating active p42 MAPK than are those from sparse cells. This cell density-dependent phosphatase activity represents a possible mechanism for maintaining a low level of p42/p44 MAPK activity in confluent endothelial cells.
The existence of this type of mechanism has been postulated to explain the inability of ΔRaf-1:ER to activate p42/p44 MAPK in Rat1 cells (45) and the capacity of extracts from nonstimulated PC12 cells to dephosphorylate and inactivate p44 MAPK (43). We have discarded the participation of PP2A, a serine/threonine phosphatase responsible for the rapid inactivation of p42/p44 MAPK in a number of cell models (1), since okadaic acid, a known inhibitor of PP2A (9), has no effect on p42/p44 MAPK activation in confluent cells. Phosphoamino acid analysis of p44 MAPK isolated from confluent and sparse cells reveals that the lower MAPK activity of confluent cells is not the result of specific dephosphorylation of one of the phosphoamino acid residues. Indeed, the stoichiometry of phosphotyrosine and phosphothreonine is 1:1 in both confluent and sparse cells (data not shown). This finding favors the hypothesis of the up-regulation of a dual-specificity tyrosine/threonine phosphatase. However, we cannot exclude the up-regulation of a limiting serine/threonine phosphatase that provides access to the action of a tyrosine phosphatase (1). We have shown that two dual-specificity MAPK phosphatases MKP1/2 (2) induced by growth factors were not particularly up-regulated in confluent cells. However, MKP1/2 is nuclear and could not account for the low p42/p44 MAPK activation during short-term stimulation. A possible role of other members of the MKP family, in particular, the cytoplasmic and p42/p44 MAPK-specific phosphatase MKP-3 (20), is very appealing. Preliminary results have not shown changes in the amount of MKP-3 protein in response to confluence; however, further experiments are required to validate or not validate this hypothesis.
ACKNOWLEDGMENTS
This work was supported by research grants from CNRS (Centre National de la Recherche Scientifique), INSERM (Institut National de la Santé et de la Recherche Medical), and ARC (Association pour la Recherche contre le Cancer). F.V. was the recipient of postdoctoral fellowships from ARC and from Ministerio de Educacion y Cultura (Spain) and of a Marie Curie reseach training grant (EC contract ERBFMBICT972706).
We thank A. Khokhlatchev and M. H. Cobb for the generous gift of active p42 MAPK, M. McMahon for pLNC ΔRaf-1:ER, A. Veri for 1G11 cells, L. Claesson-Welch for LIBE cells, Sylvain Méloche for GST–p44 MAPK-KAKA, Darren E. Richard for editorial support, Fergus McKenzie and Gilles L’Allemain for many helpful suggestions, Dominique Grall and Yan Fantei for excellent technical assistance, and all laboratory members for their support.
REFERENCES
- 1.Alessi D R, Gomez N, Moorhead G, Lewis T, Keyse S M, Cohen P. Inactivation of p42 MAP kinase by protein phosphatase 2A and a protein tyrosine phosphatase, but not CL100, in various cell lines. Curr Biol. 1995;5:283–295. doi: 10.1016/s0960-9822(95)00059-5. [DOI] [PubMed] [Google Scholar]
- 2.Brondello J M, Brunet A, Pouyssegur J, McKenzie F R. The dual specificity mitogen-activated protein kinase phosphatase-1 and -2 are induced by the p42/p44MAPK cascade. J Biol Chem. 1997;272:1368–1376. doi: 10.1074/jbc.272.2.1368. [DOI] [PubMed] [Google Scholar]
- 3.Brondello J M, McKenzie F R, Sun H, Tonks N K, Pouyssegur J. Constitutive MAP kinase phosphatase (MKP-1) expression blocks G1 specific gene transcription and S-phase entry in fibroblasts. Oncogene. 1995;10:1895–1904. [PubMed] [Google Scholar]
- 4.Brunet A, Pages G, Pouyssegur J. Constitutively active mutants of MAP kinase kinase (MEK1) induce growth factor-relaxation and oncogenicity when expressed in fibroblasts. Oncogene. 1994;9:3379–3387. [PubMed] [Google Scholar]
- 5.Bunge R, Glaser L, Lieberman M, Raben D, Salzer J, Whittenberger B, Woolsey T. Growth control by cell to cell contact. J Supramol Struct. 1979;11:175–187. doi: 10.1002/jss.400110207. [DOI] [PubMed] [Google Scholar]
- 6.Caveda L, Martin P I, Navarro P, Breviario F, Corada M, Gulino D, Lampugnani M G, Dejana E. Inhibition of cultured cell growth by vascular endothelial cadherin (cadherin-5/VE-cadherin) J Clin Investig. 1996;98:886–893. doi: 10.1172/JCI118870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen R H, Sarnecki C, Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992;12:915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cobb M H, Goldsmith E J. How MAP kinases are regulated. J Biol Chem. 1995;270:14843–14846. doi: 10.1074/jbc.270.25.14843. [DOI] [PubMed] [Google Scholar]
- 9.Cohen P, Holmes C F, Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Biochem Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-e. [DOI] [PubMed] [Google Scholar]
- 10.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 11.D’Amore P A. Mechanisms of endothelial growth control. Am J Respir Cell Mol Biol. 1992;6:1–8. doi: 10.1165/ajrcmb/6.1.1. [DOI] [PubMed] [Google Scholar]
- 12.De Rooij J, Bos J L. Minimal Ras-binding domain of Raf1 can be used as an activation-specific probe for Ras. Oncogene. 1997;14:623–625. doi: 10.1038/sj.onc.1201005. [DOI] [PubMed] [Google Scholar]
- 13.Dietrich C, Wallenfang K, Oesch F, Wieser R. Differences in the mechanisms of growth control in contact-inhibited and serum-deprived human fibroblasts. Oncogene. 1997;15:2743–2747. doi: 10.1038/sj.onc.1201439. [DOI] [PubMed] [Google Scholar]
- 14.Dong Q G, Bernasconi S, Lostaglio S, De C R, Martin P I, Breviario F, Garlanda C, Ramponi S, Mantovani A, Vecchi A. A general strategy for isolation of endothelial cells from murine tissues. Characterization of two endothelial cell lines from the murine lung and subcutaneous sponge implants. Arterioscler Thromb Vasc Biol. 1997;17:1599–1604. doi: 10.1161/01.atv.17.8.1599. [DOI] [PubMed] [Google Scholar]
- 15.Dudley D T, Pang L, Decker S J, Bridges A J, Saltiel A R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl Acad Sci USA. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fagotto F, Gumbiner B M. Cell contact-dependent signaling. Dev Biol. 1996;180:445–454. doi: 10.1006/dbio.1996.0318. [DOI] [PubMed] [Google Scholar]
- 17.Gaits F, Li R Y, Ragab A, Ragab T J, Chap H. Increase in receptor-like protein tyrosine phosphatase activity and expression level on density-dependent growth arrest of endothelial cells. Biochem J. 1995;311:97–103. doi: 10.1042/bj3110097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez F A, Seth A, Raden D L, Bowman D S, Fay F S, Davis R J. Serum-induced translocation of mitogen-activated protein kinase to the cell surface ruffling membrane and the nucleus. J Cell Biol. 1993;122:1089–1101. doi: 10.1083/jcb.122.5.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gradl G, Faust D, Oesch F, Wieser R J. Density-dependent regulation of cell growth by contactinhibin and the contactinhibin receptor. Curr Biol. 1995;5:526–535. doi: 10.1016/s0960-9822(95)00105-9. [DOI] [PubMed] [Google Scholar]
- 20.Groom L A, Sneddon A A, Alessi D R, Dowd S, Keyse S M. Differential regulation of the MAP, SAP and RK/p38 kinases by Pyst1, a novel cytosolic dual-specificity phosphatase. EMBO J. 1996;15:3621–3632. [PMC free article] [PubMed] [Google Scholar]
- 21.Hengst L, Dulic V, Slingerland J M, Lees E, Reed S I. A cell cycle-regulated inhibitor of cyclin-dependent kinases. Proc Natl Acad Sci USA. 1994;91:5291–5295. doi: 10.1073/pnas.91.12.5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill C S, Treisman R. Transcriptional regulation by extracellular signals: mechanisms and specificity. Cell. 1995;80:199–211. doi: 10.1016/0092-8674(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 23.Howe A, Aplin A E, Alahari S K, Juliano R L. Integrin signaling and cell growth control. Curr Opin Cell Biol. 1998;10:220–231. doi: 10.1016/s0955-0674(98)80144-0. [DOI] [PubMed] [Google Scholar]
- 24.Hunter T. Protein kinases and phosphatases: the yin and yang of protein phosphorylation and signaling. Cell. 1995;80:225–236. doi: 10.1016/0092-8674(95)90405-0. [DOI] [PubMed] [Google Scholar]
- 25.Kanda S, Landgren E, Ljungstrom M, Claesson W L. Fibroblast growth factor receptor 1-induced differentiation of endothelial cell line established from tsA58 large T transgenic mice. Cell Growth Differ. 1996;7:383–395. [PubMed] [Google Scholar]
- 26.Kato A, Takahashi H, Takahashi Y, Matsushime H. Inactivation of the cyclin D-dependent kinase in the rat fibroblast cell line, 3Y1, induced by contact inhibition. J Biol Chem. 1997;272:8065–8070. doi: 10.1074/jbc.272.12.8065. [DOI] [PubMed] [Google Scholar]
- 27.Kerkhoff E, Rapp U R. Induction of cell proliferation in quiescent NIH 3T3 cells by oncogenic c-Raf-1. Mol Cell Biol. 1997;17:2576–2586. doi: 10.1128/mcb.17.5.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khokhlatchev A, Xu S, English J, Wu P, Schaefer E, Cobb M H. Reconstitution of mitogen-activated protein kinase phosphorylation cascades in bacteria. Efficient synthesis of active protein kinases. J Biol Chem. 1997;272:11057–11062. doi: 10.1074/jbc.272.17.11057. [DOI] [PubMed] [Google Scholar]
- 29.Kroll J, Waltenberger J. The vascular endothelial growth factor receptor KDR activates multiple signal transduction pathways in porcine aortic endothelial cells. J Biol Chem. 1997;272:32521–32527. doi: 10.1074/jbc.272.51.32521. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Lampugnani M G, Dejana E. Interendothelial junctions: structure, signalling and functional roles. Curr Opin Cell Biol. 1997;9:674–682. doi: 10.1016/s0955-0674(97)80121-4. [DOI] [PubMed] [Google Scholar]
- 32.Lavoie J N, L’Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- 33.Lenormand P, Sardet C, Pages G, L’Allemain G, Brunet A, Pouyssegur J. Growth factors induce nuclear translocation of MAP kinases (p42mapk and p44mapk) but not of their activator MAP kinase kinase (p45mapkk) in fibroblasts. J Cell Biol. 1993;122:1079–1088. doi: 10.1083/jcb.122.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahoney P A, Weber U, Onofrechuk P, Biessmann H, Bryant P J, Goodman C S. The fat tumor suppressor gene in Drosophila encodes a novel member of the cadherin gene superfamily. Cell. 1991;67:853–868. doi: 10.1016/0092-8674(91)90359-7. [DOI] [PubMed] [Google Scholar]
- 35.Mansour S J, Matten W T, Hermann A S, Candia J M, Rong S, Fukasawa K, Vande W G, Ahn N G. Transformation of mammalian cells by constitutively active MAP kinase kinase. Science. 1994;265:966–970. doi: 10.1126/science.8052857. [DOI] [PubMed] [Google Scholar]
- 36.Marshall C J. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- 37.McKenzie F R, Pouyssegur J. cAMP-mediated growth inhibition in fibroblasts is not mediated via mitogen-activated protein (MAP) kinase (ERK) inhibition. cAMP-dependent protein kinase induces a temporal shift in growth factor-stimulated MAP kinases. J Biol Chem. 1996;271:13476–13483. doi: 10.1074/jbc.271.23.13476. [DOI] [PubMed] [Google Scholar]
- 37a.McKenzie, F. R., and J. Pouysségur. Unpublished data.
- 38.Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh D Y, Nakayama K. Mice lacking p27(Kip1) display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell. 1996;85:707–720. doi: 10.1016/s0092-8674(00)81237-4. [DOI] [PubMed] [Google Scholar]
- 39.Pages G, Lenormand P, L’Allemain G, Chambard J C, Meloche S, Pouyssegur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci USA. 1993;90:8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pallen C J, Tong P H. Elevation of membrane tyrosine phosphatase activity in density-dependent growth-arrested fibroblasts. Proc Natl Acad Sci USA. 1991;88:6996–7000. doi: 10.1073/pnas.88.16.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parenti A, Morbidelli L, Cui X L, Douglas J G, Hood J D, Granger H J, Ledda F, Ziche M. Nitric oxide is an upstream signal of vascular endothelial growth factor-induced extracellular signal-regulated kinase1/2 activation in postcapillary endothelium. J Biol Chem. 1998;273:4220–4226. doi: 10.1074/jbc.273.7.4220. [DOI] [PubMed] [Google Scholar]
- 42.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peraldi P, Scimeca J C, Filloux C, Van O E. Regulation of extracellular signal-regulated protein kinase-1 (ERK-1; pp44/mitogen-activated protein kinase) by epidermal growth factor and nerve growth factor in PC12 cells: implication of ERK1 inhibitory activities. Endocrinology. 1993;132:2578–2585. doi: 10.1210/endo.132.6.8389283. [DOI] [PubMed] [Google Scholar]
- 44.Polyak K, Kato J Y, Solomon M J, Sherr C J, Massague J, Roberts J M, Koff A. p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev. 1994;8:9–22. doi: 10.1101/gad.8.1.9. [DOI] [PubMed] [Google Scholar]
- 45.Samuels M L, Weber M J, Bishop J M, McMahon M. Conditional transformation of cells and rapid activation of the mitogen-activated protein kinase cascade by an estradiol-dependent human raf-1 protein kinase. Mol Cell Biol. 1993;13:6241–6252. doi: 10.1128/mcb.13.10.6241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seger R, Krebs E G. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 47.Shapiro P S, Vaisberg E, Hunt A J, Tolwinski N S, Whalen A M, McIntosh J R, Ahn N G. Activation of the MKK/ERK pathway during somatic cell mitosis: direct interactions of active ERK with kinetochores and regulation of the mitotic 3F3/2 phosphoantigen. J Cell Biol. 1998;142:1533–1545. doi: 10.1083/jcb.142.6.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Southey M C, Findlay D M, Kemp B E. Regulation of membrane-associated tyrosine phosphatases in UMR 106.06 osteoblast-like cells. Biochem J. 1995;305:485–490. doi: 10.1042/bj3050485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun H, Tonks N K, Bar S D. Inhibition of Ras-induced DNA synthesis by expression of the phosphatase MKP-1. Science. 1994;266:285–288. doi: 10.1126/science.7939666. [DOI] [PubMed] [Google Scholar]
- 50.Swarup G, Cohen S, Garbers D L. Inhibition of membrane phosphotyrosyl-protein phosphatase activity by vanadate. Biochem Biophys Res Commun. 1982;107:1104–1109. doi: 10.1016/0006-291x(82)90635-0. [DOI] [PubMed] [Google Scholar]
- 51.Taylor S J, Shalloway D. Cell cycle-dependent activation of Ras. Curr Biol. 1996;6:1621–1627. doi: 10.1016/s0960-9822(02)70785-9. [DOI] [PubMed] [Google Scholar]
- 52.Wieser R J, Schutz S, Tschank G, Thomas H, Dienes H P, Oesch F. Isolation and characterization of a 60-70-kD plasma membrane glycoprotein involved in the contact-dependent inhibition of growth. J Cell Biol. 1990;111:2681–2692. doi: 10.1083/jcb.111.6.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woods D F, Bryant P J. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]