Abstract
Background
Any form of screening aims to reduce disease‐specific and overall mortality, and to improve a person's future quality of life. Screening for prostate cancer has generated considerable debate within the medical and broader community, as demonstrated by the varying recommendations made by medical organizations and governed by national policies. To better inform individual patient decision‐making and health policy decisions, we need to consider the entire body of data from randomised controlled trials (RCTs) on prostate cancer screening summarised in a systematic review. In 2006, our Cochrane review identified insufficient evidence to either support or refute the use of routine mass, selective, or opportunistic screening for prostate cancer. An update of the review in 2010 included three additional trials. Meta‐analysis of the five studies included in the 2010 review concluded that screening did not significantly reduce prostate cancer‐specific mortality. In the past two years, several updates to studies included in the 2010 review have been published thereby providing the rationale for this update of the 2010 systematic review.
Objectives
To determine whether screening for prostate cancer reduces prostate cancer‐specific mortality or all‐cause mortality and to assess its impact on quality of life and adverse events.
Search methods
An updated search of electronic databases (PROSTATE register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CANCERLIT, and the NHS EED) was performed, in addition to handsearching of specific journals and bibliographies, in an effort to identify both published and unpublished trials.
Selection criteria
All RCTs of screening versus no screening for prostate cancer were eligible for inclusion in this review.
Data collection and analysis
The original search (2006) identified 99 potentially relevant articles that were selected for full‐text review. From these citations, two RCTs were identified as meeting the inclusion criteria. The search for the 2010 version of the review identified a further 106 potentially relevant articles, from which three new RCTs were included in the review. A total of 31 articles were retrieved for full‐text examination based on the updated search in 2012. Updated data on three studies were included in this review. Data from the trials were independently extracted by two authors.
Main results
Five RCTs with a total of 341,342 participants were included in this review. All involved prostate‐specific antigen (PSA) testing, with or without digital rectal examination (DRE), though the interval and threshold for further evaluation varied across trials. The age of participants ranged from 45 to 80 years and duration of follow‐up from 7 to 20 years. Our meta‐analysis of the five included studies indicated no statistically significant difference in prostate cancer‐specific mortality between men randomised to the screening and control groups (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.86 to 1.17). The methodological quality of three of the studies was assessed as posing a high risk of bias. The European Randomized Study of Screening for Prostate Cancer (ERSPC) and the US Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial were assessed as posing a low risk of bias, but provided contradicting results. The ERSPC study reported a significant reduction in prostate cancer‐specific mortality (RR 0.84, 95% CI 0.73 to 0.95), whilst the PLCO study concluded no significant benefit (RR 1.15, 95% CI 0.86 to 1.54). The ERSPC was the only study of the five included in this review that reported a significant reduction in prostate cancer‐specific mortality, in a pre‐specified subgroup of men aged 55 to 69 years of age. Sensitivity analysis for overall risk of bias indicated no significant difference in prostate cancer‐specific mortality when referring to the meta analysis of only the ERSPC and PLCO trial data (RR 0.96, 95% CI 0.70 to 1.30). Subgroup analyses indicated that prostate cancer‐specific mortality was not affected by the age at which participants were screened. Meta‐analysis of four studies investigating all‐cause mortality did not determine any significant differences between men randomised to screening or control (RR 1.00, 95% CI 0.96 to 1.03). A diagnosis of prostate cancer was significantly greater in men randomised to screening compared to those randomised to control (RR 1.30, 95% CI 1.02 to 1.65). Localised prostate cancer was more commonly diagnosed in men randomised to screening (RR 1.79, 95% CI 1.19 to 2.70), whilst the proportion of men diagnosed with advanced prostate cancer was significantly lower in the screening group compared to the men serving as controls (RR 0.80, 95% CI 0.73 to 0.87). Screening resulted in a range of harms that can be considered minor to major in severity and duration. Common minor harms from screening include bleeding, bruising and short‐term anxiety. Common major harms include overdiagnosis and overtreatment, including infection, blood loss requiring transfusion, pneumonia, erectile dysfunction, and incontinence. Harms of screening included false‐positive results for the PSA test and overdiagnosis (up to 50% in the ERSPC study). Adverse events associated with transrectal ultrasound (TRUS)‐guided biopsies included infection, bleeding and pain. No deaths were attributed to any biopsy procedure. None of the studies provided detailed assessment of the effect of screening on quality of life or provided a comprehensive assessment of resource utilization associated with screening (although preliminary analyses were reported).
Authors' conclusions
Prostate cancer screening did not significantly decrease prostate cancer‐specific mortality in a combined meta‐analysis of five RCTs. Only one study (ERSPC) reported a 21% significant reduction of prostate cancer‐specific mortality in a pre‐specified subgroup of men aged 55 to 69 years. Pooled data currently demonstrates no significant reduction in prostate cancer‐specific and overall mortality. Harms associated with PSA‐based screening and subsequent diagnostic evaluations are frequent, and moderate in severity. Overdiagnosis and overtreatment are common and are associated with treatment‐related harms. Men should be informed of this and the demonstrated adverse effects when they are deciding whether or not to undertake screening for prostate cancer. Any reduction in prostate cancer‐specific mortality may take up to 10 years to accrue; therefore, men who have a life expectancy less than 10 to 15 years should be informed that screening for prostate cancer is unlikely to be beneficial. No studies examined the independent role of screening by DRE.
Plain language summary
Screening for prostate cancer
Prostate cancer is one of the most prevalent forms of cancer in men worldwide. Screening for prostate cancer implies that diagnostic tests be performed in the absence of any symptoms or indications of disease. These tests include the digital rectal examination (DRE), the prostate‐specific antigen (PSA) blood test and transrectal ultrasound (TRUS) guided biopsy. Screening aims to identify cancers at an early and treatable stage, therefore increasing the chances of successful treatment while also improving a patient's future quality of life. This review identified five relevant studies, comprised of 341,342 participants in total. Two of the studies were assessed to be of low risk of bias, whilst the remaining three had more substantive methodological weaknesses. Meta‐analysis of all five included studies demonstrated no statistically significant reduction in prostate cancer‐specific mortality (risk ratio (RR) 1.00, 95% confidence interval (CI) 0.86 to 1.17). Meta‐analysis of the two low risk of bias studies indicated no significant reduction in prostate cancer‐specific mortality (RR 0.96, 95% CI 0.70 to 1.30). Only one study included in this review (ERSPC) reported a significant 21% relative reduction (95% CI 31% to 8%) in prostate cancer‐specific mortality in a pre‐specified subgroup of men. These results were primarily driven by two countries within the ERSPC study that had very high prostate cancer mortality rates and unusually large reduction estimates. Among men aged 55 to 69 years in the ERSPC study, the study authors reported that 1055 men would need to be screened to prevent one additional death from prostate cancer during a median follow‐up duration of 11 years. Harms included overdiagnosis and harms associated with overtreatment, including false‐positive results for the PSA test, infection, bleeding, and pain associated with subsequent biopsy.
Summary of findings
Summary of findings for the main comparison. Screening for prostate cancer.
| Screening for prostate cancer | ||||||
| Patient or population: adult male patients Settings: primary or secondary care Intervention: screening for prostate cancer | ||||||
| Outcomes1 | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Screening | |||||
| All‐cause mortality | 21 per 100 | 21 per 100 (20 to 22) | RR 1 (0.96 to 1.03) | 294856 (4 studies2,3) | ⊕⊕⊕⊝ moderate4,5,6 | |
| Prostate cancer‐specific mortality | 7 per 1000 | 7 per 1000 (6 to 8) | RR 1 (0.86 to 1.17) | 341342 (5 studies2,3) | ⊕⊕⊕⊝ moderate6,7,8,9 | |
| Prostate cancer diagnosis | 68 per 1000 | 88 per 1000 (69 to 112) | RR 1.3 (1.02 to 1.65) | 294856 (4 studies2,3) | ⊕⊕⊝⊝ low4,9,10,11 | |
| Tumour stage (localised T1‐T2, N0, M0) | 6 per 100 | 10 per 100 (7 to 15) | RR 1.79 (1.19 to 2.7) | 247954 (3 studies12,13) | ⊕⊕⊝⊝ low9,14,15,16 | |
| Tumour stage (advanced T3‐4, N1, M1) | 11 per 1000 | 9 per 1000 (8 to 9) | RR 0.8 (0.73 to 0.87) | 247954 (3 studies12,13) | ⊕⊕⊕⊝ moderate14,15,17 | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Information on costs, quality of life, metastatic disease at follow up, and harms of screening was limited and could not be meta‐analysed; available information is summarised in the text.
2 ERSPC study data includes all ages (not just 'core' age group defined by trialists). 3 PLCO study data is at 10 years of follow‐up for this outcome. 4 Risk of bias was 'high' or 'unclear' for allocation concealment in 3 studies; 'high' or 'unclear' for random sequence generation in 2 studies; 'low' for blinding in all 4 studies; 'unclear' for incomplete outcome data in 2 studies; 'unclear' for selective reporting in 1 study; and 'high' or 'unclear' for other bias in 2 studies. 5 I2 = 62%; Chi2 = 7.99 (P = 0.05). 6 Norrkoping study data for this outcome only included men who had been diagnosed with prostate cancer up to 12/31/1999, in whom mortality was then followed until 12/31/2008. 7 Risk of bias was 'high' or 'unclear' for allocation concealment in 4 studies; 'high' or 'unclear' for random sequence generation in 3 studies; 'unclear' for blinding of outcome assessment in 1 study; 'unclear' for incomplete outcome data in 2 studies; 'unclear' for selective reporting in 2 studies; and 'high' or 'unclear' for other bias in 3 studies. 8 I2 = 46%; Chi2 = 7.40 (P = 0.12). 9 Wide 95% CI. 10 I2 = 98%; Chi2 = 162.78 (P < 0.00001). 11 Screening intervention and screening interval varied between and even within some studies; the method of diagnosis also varied. 12 PLCO study data is provided at 13 years of follow‐up for this outcome. 13 ERSPC study data includes only 'core' age group, as defined by trialists. 14 Risk of bias was 'high' or 'unclear' for allocation concealment in 2 studies; 'high' for random sequence generation in 1 study; 'low' for blinding in all 3 studies; 'unclear' for incomplete outcome data in 2 studies; 'low' for selective reporting in all 3 studies; and 'high' or 'unclear' for other bias in 2 studies. 15 Tumour stage was unknown for some participants diagnosed with prostate cancer in all 3 studies. 16 I2 = 99%; Chi2 = 288.85 (P < 0.00001). 17 I2 = 0%; Chi2 = 1.34 (P = 0.51).
Background
Description of the condition
Adenocarcinoma of the prostate is common, with it being the second most prevalent cancer in men worldwide and the sixth leading cause of death in men (Jemel 2011). Prostate cancer is the most commonly diagnosed cancer in developed countries and the third leading cause of death in men in those countries (Jemel 2011). It is the sixth most commonly diagnosed cancer in developing countries (Jemel 2011). Advanced age is the primary risk factor, and it is more common in black men and those with a first degree relative who has had prostate cancer (Grönberg 2003). Prostate cancer is most commonly diagnosed in ageing men, with more than 75% of all prostate cancers diagnosed in men aged 65 years and over (Parkin 2005). Prostate cancer incidence is highest in Australia, North America, Northern and Western Europe, as well as the Caribbean (Jemel 2011). Conversely, incidence rates are lowest in South‐Eastern and South‐Central Asia, including China (Jemel 2011). This geographic variation may be attributed to racial, dietary, and environmental factors as well as differences in the intensity of cancer detection efforts.
Prostate cancer can cause haematuria or urinary obstruction due to local progression. Cancer that spreads outside the gland may result in lower extremity oedema from regional lymphatic obstruction or pain from bone metastasis. However, the vast majority of men with prostate cancer have no symptoms and their tumours are detected by routine testing. Bothersome lower urinary tract symptoms due to benign prostatic obstruction are common in elderly men and may result in increased concentrations of prostate‐specific antigen (PSA) but are not associated with an increased prostate cancer incidence (Jones 2010). For most men prostate cancer is slow growing and does not result in clinical signs or symptoms during their lifetime (Berry 1984;Holman 1999). However, in some men prostate cancer progresses and is a leading cause of cancer morbidity and mortality. Efforts to accurately determine prognosis have been problematic. However, high histologic grade, high PSA values, and larger tumour size are associated with worse disease‐specific prognosis (Partin 1993).
Description of the intervention
The PSA test and digital rectal examination (DRE) are used as primary screening tools in the early detection of prostate cancer. Transrectal ultrasound (TRUS) and TRUS‐guided needle biopsies are performed to confirm diagnosis following PSA or DRE testing, or both. These screening techniques aim to reduce overall and disease‐specific morbidity and mortality by identifying prostate cancer more frequently and earlier, and thus they hopefully lead to early treatment regimens that may be more effective when applied to cancer confined to the prostate gland.
How the intervention might work
Screening for any type of cancer aims to increase the chances of successful treatment through early detection of the disease. Screening may be performed by one of three methods, mass (that is large scale screening of an entire population); selective (that is screening high‐risk populations); or opportunistic (for example incorporated as part of a medical consultation). Testing for, or diagnosing of, a disease differs from screening. Diagnostic testing attempts to identify the disease in the presence of symptoms, whilst screening is offered to symptom‐free individuals. In the case of prostate cancer screening, the presence of lower urinary tract symptoms (LUTS), typically due to benign prostatic obstruction, are very common in the ageing male and are not considered to increase prostate cancer risk (Jones 2010). Therefore, PSA testing or DRE in men with LUTS is also considered screening.
Why it is important to do this review
Prostate cancer is common and a leading cause of morbidity and mortality. Prostate cancer rarely produces reliable early warning clinical signs or symptoms while still confined to the prostate gland. Preventive strategies, such as oral 5‐alpha reductase inhibitors, are not widely utilised or effective curative treatments, and do not work for disease that has spread beyond the prostate gland (Wilt 2008). Therefore, effective early detection and treatment strategies in asymptomatic men could potentially provide a large benefit to many men. While the intention of screening for prostate cancer is to decrease mortality and increase quality of life, the true benefit of screening for prostate cancer remains uncertain. Use of the DRE as a screening tool is limited due to poor reliability, sensitivity, and the inability to palpate the entire prostate gland, especially for small tumours that have not reached the prostatic capsule (Gambert 2001). However, it has the potential advantage of limiting overdiagnosis by detecting tumours that have grown in size to be detected on physical examination and that may progress to cause clinical signs or symptoms if left untreated. The PSA test produces high false‐negative and false‐positive results, depending on the thresholds utilised to define abnormality, and may detect prostate cancers that are unlikely to cause future health problems even if left untreated (overdiagnosis) (Gambert 2001). Recent data from a nested case‐control study, which assessed the validity standards of the PSA test, concluded that the PSA test does not attain the likelihood ratios (that is the likelihood of a given test result in a person with the disease compared to the likelihood that the same result would be apparent in a person without the disease) suitable for a screening test, regardless of what cut‐off value for the PSA is assigned (Holmström 2009).
Additional causes for concern include the cost of follow‐up tests, the potentially invasive nature of these tests, and the subsequent use of treatment regimens that may provide additional adverse events. Although a man's risk of prostate cancer diagnosis increases with age, many men will live with undiagnosed prostate cancer only to die from another disorder, as has been confirmed in unselected autopsies (Berry 1984; Holman 1999). Screening for prostate cancer in this scenario results in overdiagnosis, thereby exposing a patient to unnecessary treatment (Draisma 2003). Additionally, the long‐term prognosis for most men (especially elderly men) with PSA‐detected prostate cancer is excellent, even among those treated conservatively, and is superior to that for men diagnosed prior to PSA testing. This may be due in part to additional lead time, overdiagnosis related to PSA testing, grade migration, or advances in other medical care (Lu‐Yao 2009). It has been estimated that screening for prostate cancer includes a lead‐time bias (that is advancing the time of diagnosis) between five to 13 years (Draisma 2003).
The uncertainty about the effectiveness of prostate cancer screening has been further highlighted by the conflicting recommendations made by various medical entities (ACS 2010; Burford 2010; AUA 2009; RACGP 2012; USPSTF 2012). Screening for prostate cancer may reduce both morbidity and mortality, yet the best method of screening (if any) is unknown. Equally, screening may promote treatment procedures that are unwarranted or may adversely affect the health outcomes of the patient, resulting in no net benefit or even net harm. The cost‐benefits associated with screening and potential follow‐up tests and treatment may be justified; however, the economic implication of prostate cancer screening remains unknown. Additionally, only a single trial of treatment versus observation for early stage, screen‐detected prostate cancer has been reported (Wilt 2012).
Evidence on the effectiveness of treatment for prostate cancer is conflicting. An evaluation of radical prostatectomy versus watchful waiting in early prostate cancer in the Scandinavian Prostate Cancer Group Study Number 4 (SPCG‐4) identified that, after 15 years, radical prostatectomy reduced disease‐specific mortality, overall mortality, and risk of metastasis (Bill‐Axelson 2011). Reductions in overall and disease‐specific mortality and metastatic disease were limited to men less than 65 years of age. Conversely, a similar trial evaluating radical prostatectomy versus observation in localised prostate cancer reported that radical prostatectomy did not significantly reduce all‐cause or prostate cancer‐specific mortality when compared to observation (Wilt 2012). Sakr and colleagues have estimated that close to half of men aged over 50 years have histological evidence of prostate cancer, with this figure rising to close to 80% of men aged up to 80 years (Sakr 1996). Despite this high prevalence, prostate cancer is not commonly diagnosed as the primary cause of mortality in these men (Parkin 2005; Sakr 1996).
In addition, there have been a number of population‐based studies to examine the potential impact of prostate cancer screening that are frequently cited in favour of prostate cancer screening (Bartsch 2001; Jacobsen 1998; Kopec 2005; van Leeuwen 2010). Findings of these studies are not temporally or geographically consistent with a screening effect; for example, the decline in prostate cancer mortality seen in the United States that began shortly after the initiation of widespread PSA screening is likely to predate any plausible impact due to PSA testing given the long time to any potential benefit (that is 10 years). These studies are at high risk for confounding, most notably selection bias and lead and length‐time bias, which can only be adequately controlled for in a randomised controlled trial. These factors emphasize the importance of a systematic review of randomised trials for guiding individual patient, provider, and health policy decision‐making.
The first version of this Cochrane review (published in 2006) concluded that there was insufficient evidence to either support or refute the use of routine mass, selective, or opportunistic screening for prostate cancer. An update of the review in 2010 identified three additional trials, which were included in the review. Meta‐analysis of the five studies included in the 2010 review concluded that screening did not significantly reduce prostate cancer‐specific mortality. Since the last update, several studies have provided follow‐up data. This 2012 version of the review incorporates the latest updates to the literature to examine the current evidence and evaluate the absolute benefits and harms associated with screening for prostate cancer. We have rated the quality of the evidence by outcome according to GRADE and include a summary of findings table.
Objectives
The primary objective of this review was to determine the efficacy of screening men for prostate cancer in reducing prostate cancer‐specific and all‐cause mortality.
The secondary objectives of this review were to:
determine the impact of prostate cancer screening on quality of life and adverse effects; and
document the costs of screening for prostate cancer.
Methods
Criteria for considering studies for this review
Types of studies
All randomised, and quasi‐randomised, controlled trials of screening versus no screening for prostate cancer were eligible for this review. No language restrictions were placed on studies considered for inclusion in this review, and published or unpublished sources were considered.
Types of participants
All men enrolled in studies of prostate cancer screening were eligible for this review, with no exclusions based on ethnicity, age, or presence of lower urinary tract symptoms. Studies including men with a previous diagnosis and treatment of prostate cancer were excluded.
Types of interventions
Studies that used any of the following screening procedures, individually or in combination, were included:
digital rectal examination (DRE);
prostate‐specific antigen (PSA) test (including total, velocity, density, and percentage free and complex); and
transrectal ultrasound (TRUS)‐guided biopsy.
Types of outcome measures
The following outcomes were measured.
Primary outcomes
Primary outcome measures for this review were prostate cancer‐specific and all‐cause mortality.
Secondary outcomes
Secondary outcome measures included:
incident prostate cancers by stage and grade at diagnosis;
metastatic disease at follow‐up;
quality of life;
harms of screening (including both adverse outcomes from false‐positive or false‐negative results and their impact upon resulting treatment procedures); and
costs associated with screening programs.
Search methods for identification of studies
A combination of electronic and manual searches were conducted for this review.
Electronic searches
Electronic searches of the PROSTATE register (made available by the Cochrane Prostatic Diseases and Urologic Cancers Group), the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CANCERLIT, and the NHS EED. For the original version of this review the PROSTATE register was initially searched in November 2004, with the remaining databases searched for studies published between 1966 and January 2006. There was no restriction on language in any searches. The search strategy is provided in 'Appendix 1' and was adapted for each electronic database.
An updated search of the electronic databases was previously performed with the existing search strategy in July 2010. A further search of the electronic databases was performed for this current version of the review in June 2012.
Searching other resources
Handsearching for reviews and technical reports with regard to prostate cancer screening in specialist journals, as shown below, and grey literature was conducted in the original version of the review.
The following journals were handsearched until March 2005:
BJU International (2000 to 2005);
European Urology (2002 to 2005);
The Prostate (1998 to 2005);
The Journal of Urology (1996 to 2005);
Urology (2002 to 2005);
Cancer (1998 to 2005).
Authors of studies that were included in this review were contacted in order to request additional study information, as needed.
The authors of a 2010 BMJ (Djulbegovic 2010) systematic review of screening for prostate cancer performed a manual search of abstracts presented at the following meetings (from 2005 to 2010):
American Urological Association (AUA);
European Association of Urology (EAU);
American Society of Clinical Oncology (ASCO).
This present review contains authors from both the original Cochrane review and the BMJ review. As such, it was decided that handsearching of the grey literature would continue based on the proceedings from the AUA, EAU, and ASCO meetings. For the purposes of this update, handsearching was performed for abstracts presented at the AUA, EAU, and ASCO meetings from 2010 to 2012.
Data collection and analysis
The authors followed the recommended strategies for data collection and analysis as documented in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Two of the authors (DI and PD) independently selected trials for possible inclusion against a pre‐determined checklist of inclusion criteria. Studies were initially categorized into the following groups:
possibly relevant, studies that met the inclusion criteria and studies for which it was not possible to determine whether they met the criteria either from their title or abstract;
excluded, those clearly not meeting the inclusion criteria.
If a title or abstract appeared to meet the eligibility criteria for inclusion in the review, or we could not determine eligibility, a full‐text version of the article was obtained and assessed by two authors (DI and PD) in order to determine whether it met the inclusion criteria. Discrepancies between the authors were resolved via discussion.
Data extraction and management
Two authors (DI and MMN) independently extracted data using a standard data extraction form. Any discrepancies between the review authors were resolved by consensus. The data extraction form was pilot tested and modified accordingly before use. In addition to the quality characteristics and the results of the trial, the following details were recorded:
participant details, including demographic information and inclusion and exclusion criteria;
types of screening interventions used and their comparison;
outcomes reported, including the types of measure used to record the outcome.
Assessment of risk of bias in included studies
In the original review, the risk of bias was assessed by reporting the trial's conduct against the following key criteria:
randomisation;
allocation concealment, as coded according to the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2005);
blinding of participants (graded as yes, no, or unclear);
blinding of outcome assessors (graded as yes, no, or unclear);
completeness of the follow‐up, i.e. description of any numbers of participants lost to follow‐up (graded as yes, no, or unclear); and
whether or not an intention‐to‐screen analysis was performed (graded as yes, no, or unclear).
Trials were categorized as attributing a 'low', 'moderate', or 'high' risk of bias (Higgins 2005).
In this updated review, assessment of risk of bias was made using the Cochrane Collaboration's tool for assessing risk of bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Two authors (DI and MMN) independently assessed the susceptibility to bias of the selected trials. Risk of bias in this review was assessed by reporting the trial's conduct against the following key criteria:
sequence generation;
allocation concealment;
blinding of participants, personnel, and outcome assessors;
incomplete outcome data;
selective outcome reporting; and
other sources of bias (defined as inappropriate data analysis).
Each criterion was assessed by a question‐based entry, with the judgement being 'yes' indicating 'low' risk of bias, 'no' indicating a 'high' risk of bias, and 'unclear' (Higgins 2011). Overall risk of bias was summarised with consideration to the relative importance of domains and empirical evidence of bias. Risk of bias for each study was summarised as: (i) 'low' risk of bias, when a low risk of bias was described for all key domains; (ii) 'unclear' risk of bias, when the bias was deemed to be unclear in one or more of the domains; and (iii) 'high' risk of bias, when one or more domains were judged to be of a high risk of bias (Higgins 2011). Included studies were abstracted independently by the two authors using an abstraction form detailing the above mentioned criteria. Any discrepancies were discussed between the authors.
Additionally, the GRADE framework was applied to rate the quality of evidence for each outcome, with results reported in a summary of findings table (Guyatt 2011; Schünemann 2011). Evidence rated as 'high' quality means that further research is very unlikely to change our confidence in the estimate of effect, while a 'moderate' quality rating means further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Similarly, an evidence rating of 'low' quality means that further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. A 'very low' quality evidence rating means that we are very uncertain about the estimate.
Measures of treatment effect
Statistical analysis was performed according to the statistical guidelines referenced in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For dichotomous outcomes, the measure of effect is expressed as a risk ratio (RR) and absolute risk (AR) with 95% confidence intervals (CI); and for continuous outcomes, the measure of effect is expressed as a weighted mean difference with 95% CI. In the event that continuous data were reported on different continuous scales, outcomes were standardised, where possible, to calculate the standardised mean difference. Where data were available, and if the trial did not report intention‐to‐screen analysis results, we performed intention‐to‐screen analyses using the groups to which the participants were originally randomised (that is screening versus control).
Dealing with missing data
Any missing data were dealt with by contacting the original study investigators to request the missing data. In the event that missing data were not available to the review authors, analysis was performed on the available data.
Assessment of heterogeneity
Heterogeneity was analysed by graphical interpretation of the forest plot and with the I2 statistic. An I2 value above 75% was considered to be an indicator of considerable heterogeneity (Higgins 2011). We also evaluated studies for clinical heterogeneity, focusing primarily on patient characteristics (for example age) and screening and subsequent treatment protocols (for example PSA screening intervals and thresholds for additional evaluation).
Assessment of reporting biases
Funnel plots were used in exploratory data analyses to assess for possible reporting and small study biases. There are a number of explanations for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to study size, poor methodological design of small studies, and publication bias (Sterne 2001).
Data synthesis
We used the random‐effects model to determine the effect of screening on prostate cancer mortality using the Cochrane Collaboration's RevMan 5.1 software (RevMan 2011).
Subgroup analysis and investigation of heterogeneity
Due to the nature of the studies, we were not able to do a subgroup analysis based on screening intervention (DRE versus TRUS versus PSA). Since the prevalence of prostate cancer increases with age and the potential effectiveness of screening may also vary according to age, a subgroup analysis exploring screening of men aged greater than or equal to 45, 50, and 55 years of age was performed for this updated review.
Sensitivity analysis
Sensitivity analyses were performed to investigate the impact of risk of bias (for sequence generation and allocation concealment) of included studies on robustness of results. Sensitivity analyses were also performed to assess overall risk of bias by outcome. A post hoc sensitivity analysis excluding the Stockholm study was performed as the external validity of the Stockholm study was assessed as low since patients were only screened once. Additionally, the screening process and thresholds used in the study are not currently employed in clinical practice. A post hoc sensitivity analysis was also performed including the French centre in the European Randomised Study of Screening for Prostate Cancer (ERSPC) data for the outcomes of prostate cancer diagnosis, localised tumour stage, and advanced tumour stage, as mortality data from the French centre were not available. The ERSPC study did not include data from the French study centre either in mortality analyses, due to short duration of follow‐up, or in primary analyses of other outcomes.
Results
Description of studies
Five randomised controlled trials (RCTs) (ERSPC; Norrkoping; PLCO; Quebec; Stockholm) comparing mass screening for prostate cancer to no screening were identified as meeting the inclusion criteria for this review. All studies reported on prostate cancer‐specific mortality as the primary outcome. Additional reported outcomes included prostate cancer diagnosis, all‐cause mortality, clinical stage, Gleason score, and treatment follow‐up. The ERSPC and Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial studies provided some data on number of biopsies performed and harms associated with screening (for example infection and bleeding from TRUS‐guided biopsies). For further descriptive information about the studies, refer to the 'Characteristics of included studies' table.
Results of the search
The search in the original review returned 1965 citations identified by the search of MEDLINE (1966 to October 2006), of which 98 were selected for full‐text review. Searches of EMBASE, CANCERLIT, PROSTATE, NHS EED, the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2006, Issue 1), and bibliographies of reviewed articles did not reveal any further relevant studies that were not previously identified through the MEDLINE search. Handsearching of identified journals revealed one relevant study not identified through the electronic searches (Norrkoping). Of the 99 studies selected for further review, 52 were cohort studies, 19 were narrative reviews or commentaries, and five were studies reporting data from pilot studies, or associated data, from the ongoing multi‐centre ERSPC trial. Two studies ultimately met the selection criteria and were included in the original 2006 review (Norrkoping; Quebec).
The updated search in July 2010 yielded 366 citations, of which 106 were selected for full‐text review. Three new studies met the selection criteria and were included in the 2010 updated review (ERSPC; PLCO; Stockholm). The remaining studies consisted of 17 RCTs on topics incorporating elements of prostate cancer but not investigating the effect of screening on mortality; 31 cohort and case‐control or other comparative studies; 25 reviews, guidelines, or protocols; one editorial; and 29 studies associated with either the ERSPC or PLCO studies included in this review. An additional longer‐term follow‐up, site‐specific report on the Swedish arm of the ERSPC study was also included in the 2010 updated review, and the results incorporated with the other sites that formed the ERSPC study.
The updated search in June 2012 yielded 855 citations, of which 31 were selected for full‐text review. Twenty studies were excluded, with 10 of these reporting an outcome not relevant to the aims of this systematic review, and 10 reporting findings that were incorporated in other publications of the ERSPC. Three studies reporting updates of the ERSPC, PLCO, and Norrkoping studies were included, along with two studies referring to the ongoing Comparison Arm for ProtecT (CAP) study. A further four studies were included as part of the ERSPC study, with two further studies included as part of the PLCO study.
Included studies
Five RCTs were included in the review, with significant differences in the methodological design between them. The Norrkoping study recruited men 50 to 69 years of age in Sweden and screened every three years. During the initial phase of the study, only the DRE was offered, however the screening regimen later evolved to include DRE and PSA. A PSA level greater than 4.0 ng/mL was deemed the cut‐off for biopsy. Participants were followed up over a 20‐year period. The Quebec study recruited men 45 to 80 years of age in Canada and provided annual screening with combination DRE and PSA. A PSA greater than 3.0 ng/mL was deemed the cut‐off for biopsy. Participants were followed up over an 11‐year period. The Stockholm study recruited men aged 55 to 70 years in Sweden for a one‐time screening using DRE, PSA, and TRUS. A PSA greater than 10.0 ng/mL was deemed the cut‐off for biopsy, with repeat TRUS performed for PSA greater than 7.0 ng/mL. Participants were followed up over a 15‐year period. The PLCO study recruited men aged 55 to 74 years in the United States for annual screening with DRE and PSA. A PSA greater than 4.0 ng/mL was deemed possibly indicative of prostate cancer and patients were advised to seek diagnostic evaluation. Participants were followed up over a 10‐ to 13‐year period. The ERSPC recruited men ranging in age from 50 to 74 years across nine European countries. Screening regimens varied across participating sites, with cut‐off values for biopsy ranging from a PSA greater than 2.5, to 3.0, 4.0, and 10.0 ng/mL. Screening interval in six of the sites was every four years. For further information on included studies, see the 'Characteristics of included studies' table for further details.
Excluded studies
Studies were primarily excluded because they were not RCTs, or because they did not provide data specific to the primary and secondary outcomes of this systematic review. See the 'Characteristics of excluded studies' section for further information.
Risk of bias in included studies
Assessment for risk of bias of each included study is described in the 'Characteristics of included studies' section. Risk of bias is also represented graphically in 'Figure 1'. The risk of bias as determined for each included study was as follows.
1.
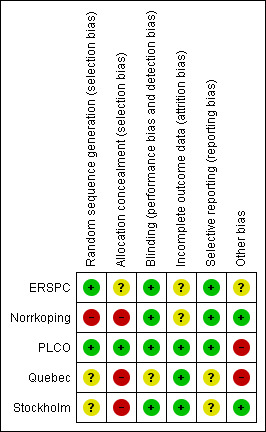
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
ERSPC: low risk of bias (majority of domains were low risk of bias, with the exception of the allocation concealment domain (unclear risk of bias), incomplete outcome data domain (unclear risk of bias), and other bias domain (unclear risk of bias)). Although an early pilot study indicated that adequate allocation concealment was used during the study, limited data were given on the details of allocation concealment across participating study sites (Schröder 1996).
Norrkoping: high risk of bias (due to high risk associated with the allocation sequence generation and allocation concealment, as well as uncertainty about incomplete outcome data).
PLCO: low risk of bias (majority of domains were low risk of bias, with the exception of the other bias domain due to high control group contamination (high risk of bias)).
Quebec: high risk of bias (due to high risk of bias associated with allocation concealment and analysing data not using the intention‐to‐treat principle, as well as uncertainty about random sequence generation, blinding of outcome assessors, and selective reporting).
Stockholm: high risk of bias (due to high risk associated with allocation concealment, and uncertainty with sequence generation and selective reporting). This study also had low external validity as it had a one‐time screen for prostate cancer, with biopsy only performed if the PSA value was greater than 10 ng/mL.
Authors were contacted via e‐mail to assist with the assessment for risk of bias of the included studies, as needed. Sensitivity analysis was performed on all outcomes to account for overall study risk of bias.
The quality of the evidence was rated as 'moderate' according to GRADE for all‐cause mortality, prostate cancer‐specific mortality, and advanced tumour stage; and 'low' for prostate cancer diagnosis and localised tumour stage (Guyatt 2011; Schünemann 2011) ('Table 1').
Allocation
Sequence generation was clearly described in the ERSPC and PLCO studies. The ERSPC trial used random number generators, while the PLCO study used a computerised randomisation scheme. The method of concealment was unclear for the ERSPC study. It also was not clear whether the method of concealment was uniform across all participating sites. The PLCO study achieved concealment through use of a central system. The method of sequence generation was unclear for the Quebec and Stockholm studies as the authors did not mention what process of sequence generation was used. The Norrkoping study did not have adequate sequence generation, as men were randomised according to a list of dates of births.
As the ERSPC and PLCO studies were assessed as at overall low risk of bias, no other additional sensitivity analysis (beyond that for overall risk of bias) was performed specifically for individual criteria such as risk of bias in the generation of the random sequence (where both studies were the only studies to be rated as low risk of bias) or allocation concealment (where only the PLCO study was assessed as being low risk of bias).
Blinding
Participants and clinicians were not blinded to the screening intervention. Methods to blind outcome assessment were adequately described for all but one study (Quebec).
Incomplete outcome data
The Quebec, Stockholm, and PLCO studies provided complete data, with any withdrawal cited and explained. Withdrawals were cited for the Norrkoping study, however it was unclear how data for men who participated but migrated out of the catchment area were obtained. The ERSPC study consisted of nine study centres, but it did not include data from the French study centre either in mortality analyses, due to short duration of follow‐up, or in primary analyses of other outcomes; and the Portuguese centre was excluded due to discontinuation. The ERSPC data in this review were therefore based on seven ERSPC centres.
Selective reporting
The ERSPC, Norrkoping, and PLCO studies were determined to be at low risk of bias for selective reporting as determined by comparisons between previously published protocols for the respective studies and the current published data. It was not possible to assess selective reporting for the remaining two studies due to insufficient information.
Other potential sources of bias
A preliminary article on the ERSPC study reported that a consensus workshop was formed to structure specific components of the study. It was decided that an age range of 55 to 70 years was determined as being the 'core' age group for participants, with the inclusion of higher or lower age groups, or both, being left to the discretion of the participating centres (Schröder 2003). Another preliminary paper also stated that the primary endpoint of the ERSPC study will be the prostate cancer mortality rate in the total study arm compared with the control arm; with one analysis to be conducted for the 'core' age group (men aged 55 to 69 years at entry to the trial) and another for all ages at entry (de Koning 2003). It was also described that the ERSPC study had sufficient power to detect a significant difference in prostate cancer mortality between the total study arm compared with the control arm if the true reduction in mortality by screening was 25% or more, or if contamination was limited to 10% if the true effect is 20% or more (de Koning 2002b; Schröder 2003). It has since been estimated that the contamination rate in the ERSPC study was 30.7%, accounting for 27,431 out of 89,353 men in the control group having at least one PSA test (Roobol 2009). Similarly, the PLCO study reported that 45% of participants entered the study with a history of PSA screening in the three years prior to randomisation, with subsequently 52% of men assigned to the control group undertaking some form of screening during the study period.
There were also changes to the screening protocol of the ERSPC study, where both the DRE and TRUS ceased to be used as screening tests in 1997 (Schröder 2003). The PSA cut‐off value was also reduced to 3.0 ng/mL during this time, however several centres continued to use a PSA value of 4.0 ng/mL as the cut‐off, or applied ancillary tests if PSA test values were within a certain range (for example men in the Italian centre with a PSA value of 2.5 to 3.9 ng/mL underwent DRE and TRUS) (ERSPC; Schröder 2003).
Data were not analysed according to the intention‐to‐screen principle in the Quebec study. From a total of 31,133 men randomised to the screening group, only 7348 (23.6%) were actually screened (that is all 31,133 men were invited to be screened but only 23.6% took up the invitation and actually were screened). Similarly, of the 15,353 men randomised to the control group, 1122 (7.3%) were screened for prostate cancer at the study site. The data were extracted and re‐analysed for this review according to the intention‐to‐screen principle by the authors of this review.
Funnel plots for all outcomes were symmetrical; however, the results using this tool were still interpreted with caution.
Effects of interventions
See: Table 1
Prostate cancer‐specific mortality
Results of meta‐analysis
Prostate cancer screening did not result in a statistically significant reduction in prostate cancer‐specific mortality when all populations of all studies were analysed according to intention‐to‐screen analysis. Meta‐analysis of the five included trials identified the risk ratio of prostate cancer‐specific mortality to be 1.00 (95% CI 0.86 to 1.17) ('Figure 2'). Our analysis of the five studies showed no statistically significant reduction in prostate cancer‐specific or all‐cause mortality among the whole population of men randomised to screening versus controls. The ERSPC demonstrated a marginally significant benefit for screening in reducing prostate cancer‐specific mortality among a 'core' subgroup of men aged 55 to 69 years at baseline (RR 0.79, 95% CI 0.69 to 0.92) over a median follow‐up duration of 11 years ('Analysis 1.2'). The other 'low' risk of bias study, PLCO, demonstrated no significant benefit for screening through 10 years of follow‐up (RR 1.15, 95% CI 0.86 to 1.54). A meta‐analysis incorporating the 'core age group' in the ERSPC study identified the RR of prostate cancer‐specific mortality to be 1.00 (95% CI 0.83 to 1.19) ('Analysis 1.2'). Sensitivity analysis demonstrated no significant difference on results with the inclusion or exclusion of the Stockholm study.
2.
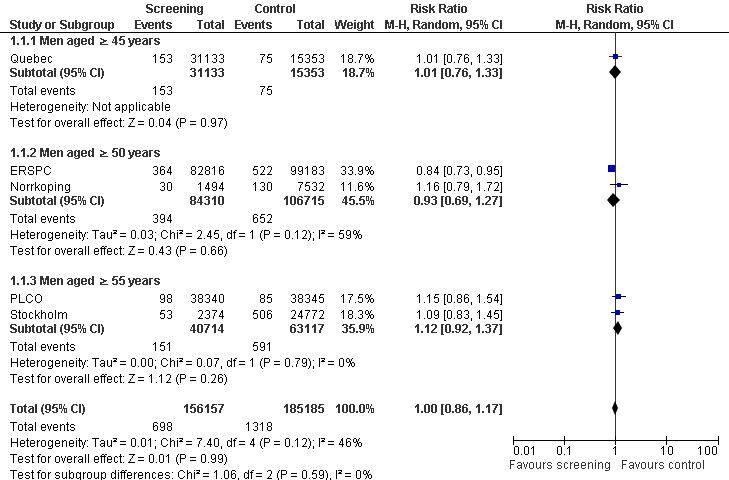
Forest plot of comparison: 1 Screening versus control, outcome: 1.3 Prostate cancer‐specific mortality (subgroup analysis age)
1.2. Analysis.
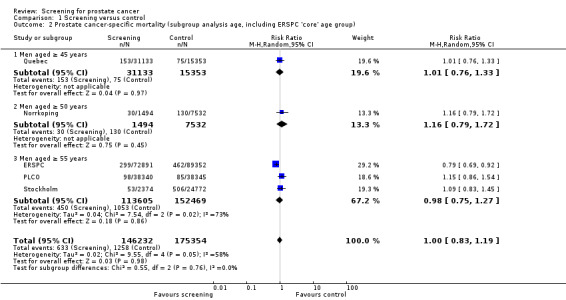
Comparison 1 Screening versus control, Outcome 2 Prostate cancer‐specific mortality (subgroup analysis age, including ERSPC 'core' age group).
Risk of bias sensitivity analysis
The quality of evidence was rated as moderate for this outcome ('Table 1'). Both the ERSPC and the PLCO studies were assessed as at low risk of bias, whilst the Norrkoping, Quebec, and Stockholm studies were assessed as high risk of bias. Meta‐analysis of the two low risk of bias studies produced a RR of 0.96 (95% CI 0.70 to 1.30) ('Figure 3'). Using data from the 'core age group' of the ERSPC study produced a RR of 0.94 (95% CI 0.65 to 1.35) ('Analysis 1.4').
3.
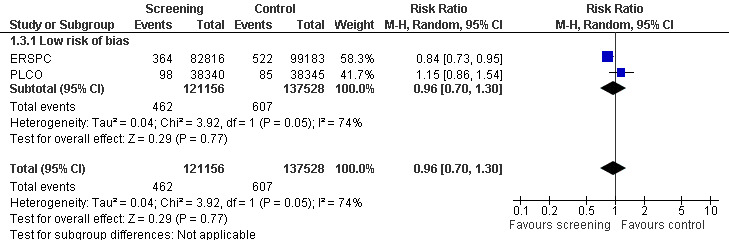
Forest plot of comparison: 1 Screening versus control, outcome: 1.3 Prostate cancer‐specific mortality (sensitivity analysis overall risk of bias).
1.4. Analysis.
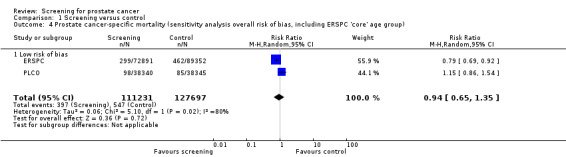
Comparison 1 Screening versus control, Outcome 4 Prostate cancer‐specific mortality (sensitivity analysis overall risk of bias, including ERSPC 'core' age group).
Subgroup analysis
Subgroup analysis explored prostate cancer‐specific mortality according to age. It identified no significant difference in prostate cancer‐specific mortality when men were screened from 45 years of age (RR 1.01, 95% CI 0.76 to 1.33), 50 years of age (RR 0.93, 95% CI 0.69 to 1.27), or 55 years of age (RR 1.12, 95% CI 0.92 to 1.37) ('Figure 2'). A second meta‐analysis was performed, which incorporated the 'core age group' of men from the ERSPC study (that is men aged 55 to 69 years). Conducting a meta‐analysis using this approach demonstrated no significant difference in prostate cancer‐specific mortality across any of the age groups ('Analysis 1.2').
Participant characteristics including race or ethnicity; family history of prostate cancer; enlarged prostate (or BPH); previous prostate biopsy, PSA, or DRE were only reported in the PLCO study.
All‐cause mortality
Results of meta‐analysis
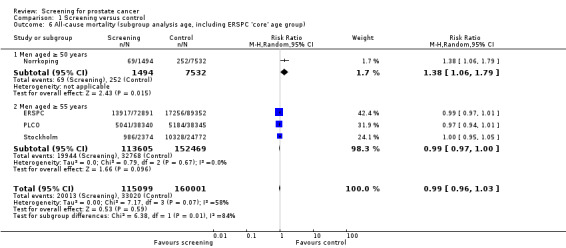
Prostate cancer screening did not result in a statistically significant reduction in all‐cause mortality. A meta‐analysis of four studies (ERSPC; Norrkoping; PLCO; Stockholm) demonstrated no difference in all‐cause mortality between the screening and control groups (RR 1.00, 95% CI 0.96 to 1.03) ('Figure 4'). This result did not differ when the data from the 'core age group' of the ERSPC study were used (RR 0.99, 95% CI 0.96 to 1.03) ('Analysis 1.6'). Sensitivity analysis demonstrated no significant difference in results with the inclusion or exclusion of the Stockholm study.
4.
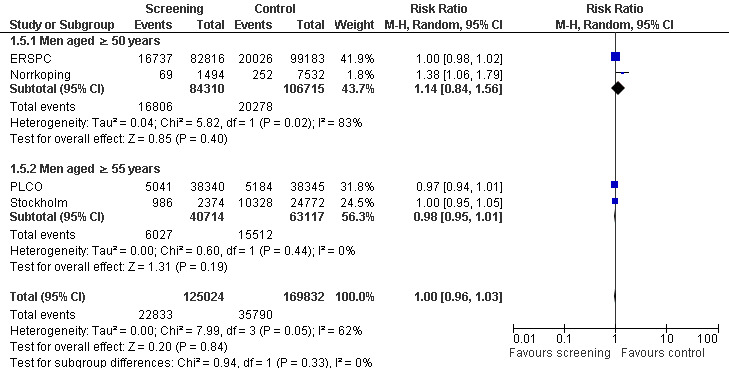
Forest plot of comparison: 1 Screening versus control, outcome: 1.5 All‐cause mortality (subgroup analysis age).
1.6. Analysis.
Comparison 1 Screening versus control, Outcome 6 All‐cause mortality (subgroup analysis age, including ERSPC 'core' age group).
Risk of bias sensitivity analysis
The quality of evidence was rated as moderate for this outcome ('Table 1'). The ERSPC and PLCO studies were assessed as at low risk of bias. Conversely, the Stockholm and Norrkoping studies were graded as at high risk of bias. Sensitivity analysis demonstrated no significant difference in results with the inclusion or exclusion of the Stockholm and Norrkoping studies ('Analysis 1.7'; 'Analysis 1.8').
1.7. Analysis.
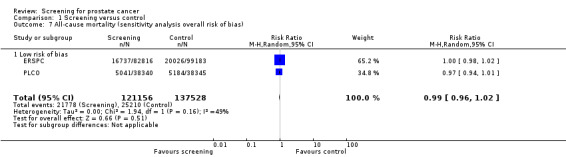
Comparison 1 Screening versus control, Outcome 7 All‐cause mortality (sensitivity analysis overall risk of bias).
1.8. Analysis.
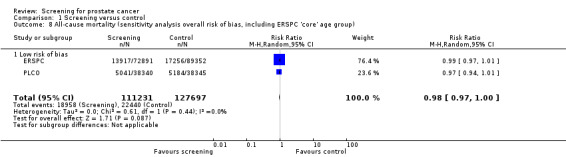
Comparison 1 Screening versus control, Outcome 8 All‐cause mortality (sensitivity analysis overall risk of bias, including ERSPC 'core' age group).
Subgroup analysis
Subgroup analysis explored all‐cause mortality according to age ('Figure 4'). It identified no significant difference in all‐cause mortality in men aged 50 years and above (RR 1.14, 95% CI 0.84 to 1.56) or men aged 55 years and above (RR 0.98, 95% CI 0.95 to 1.01). A second meta‐analysis incorporating the 'core age group' from the ERSPC study demonstrated a significant difference in all‐cause mortality only in men aged 50 years and above (RR 1.38, 95% CI 1.06 to 1.79), and this was based on the Norrkoping study alone ('Analysis 1.6').
Diagnosis of prostate cancer (as determined by study)
Results of meta‐analysis
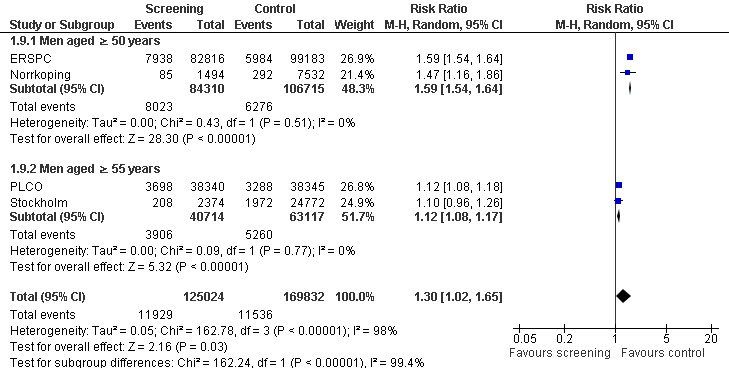
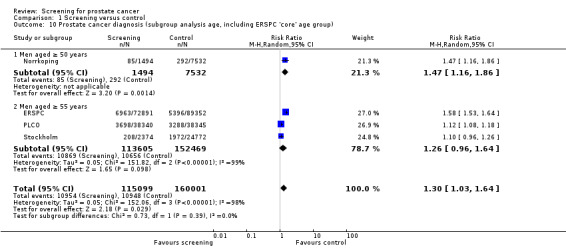
Prostate cancer screening increased the number of men diagnosed with prostate cancer. The number of men diagnosed with prostate cancer across both the screening and control groups was reported by four of the included studies. Meta‐analysis of the ERSPC, Norrkoping, PLCO and Stockholm trials indicated that screening was associated with a 30% increase in the number of men diagnosed with prostate cancer (RR 1.30, 95% CI 1.02 to 1.65) ('Figure 5'; 'Analysis 1.10'). Incorporating data from the French site of the ERSPC study resulted in no change in those findings (RR 1.26, 95% CI 1.06 to 1.51).
5.
Forest plot of comparison: 1 Screening versus control, outcome: 1.9 Prostate cancer diagnosis (subgroup analysis age).
1.10. Analysis.
Comparison 1 Screening versus control, Outcome 10 Prostate cancer diagnosis (subgroup analysis age, including ERSPC 'core' age group).
In the ERSPC study, a total of 16.6% of screening tests were assessed as positive in the 'core age group', with 85.9% of men with positive tests undergoing a biopsy. In the PLCO study, a total of 7.5% of men tested positive for a DRE and 7.9% for a PSA test, with 74% undertaking further diagnostic evaluation and 31.5% of men undergoing a biopsy within one year of screening.
Statistical heterogeneity was high for this outcome. Sensitivity analysis (using a fixed‐effect model for the meta‐analysis) demonstrated no significant difference in results (RR 1.40, 95% CI 1.37 to 1.44). Clinical heterogeneity was apparent with the Stockholm study, as the screening procedures adopted in that study differed considerably from the other included studies. Sensitivity analysis demonstrated no significant difference in results with the inclusion or exclusion of the Stockholm study.
Significant heterogeneity was associated with the meta‐analyses for prostate cancer diagnosis. Performing a meta‐analysis only according to age group significantly reduced the heterogeneity (see below).
Risk of bias sensitivity analysis
The quality of evidence was rated as low for this outcome ('Table 1'). Both the ERSPC and PLCO studies were assessed as at low risk of bias. The Norrkoping and Stockholm studies were graded as at high risk of bias. Sensitivity analysis demonstrated no meaningful difference in results with the exclusion of the Norrkoping and Stockholm studies.
Subgroup analysis
A subgroup analysis was performed with respect to the age at which men were first screened for prostate cancer. A meta‐analysis of the ERSPC and Norrkoping studies, for men screened aged 50 years or older, provided a RR of 1.59 (95% CI 1.54 to 1.64), with an I2 of 0% ('Figure 5'). A meta‐analysis of the PLCO and Stockholm studies, for men screened aged 55 years or older, provided a RR of 1.12 (95% CI 1.08 to 1.17), with an I2 of 0% ('Figure 5').
Prostate tumour stage
Results of meta‐analysis
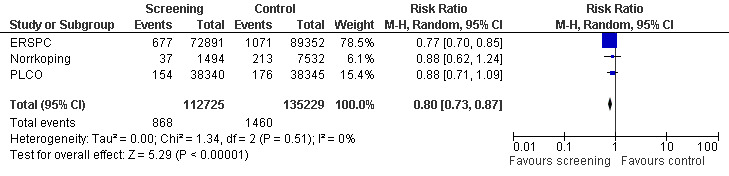
A meta‐analysis of the ERSPC, Norrkoping, and PLCO studies indicated that the proportion of men diagnosed with localised prostate cancer was significantly greater in the screening group compared to the control group (RR 1.79, 95% CI 1.19 to 2.70) ('Figure 6'). Incorporating data from the French site of the ERSPC study resulted in no change in these findings (RR 1.66, 95% CI 1.22 to 2.27).
6.
Forest plot of comparison: 1 Screening versus control, outcome: 1.11 Tumour stage (localised T1‐T2, N0, M0).
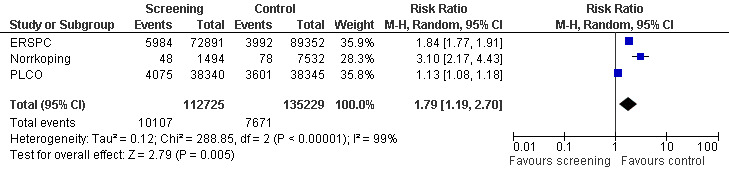
Conversely, the proportion of men diagnosed with advanced prostate cancer was significantly lower in the screening group compared to men in the control group (RR 0.80, 95% CI 0.73 to 0.87) ('Figure 7'). Incorporating data from the French site of the ERSPC study resulted in no change in these findings (RR 0.77, 95% CI 0.71 to 0.83). The eight‐year follow‐up publication of the Quebec study reported stage distribution in the screened cohort at the first and follow‐up visit ('Table 2').
7.
Forest plot of comparison: 1 Screening versus control, outcome: 1.12 Tumour stage (advanced T3‐4, N1, M1).
1. Stage of prostate cancer in the screening group (Quebec).
| Clinical stage | Number of men (%) at 1st visit | Number of men (%) at follow up |
| A2 | 1 (0.4) | 0 |
| A3 | 2 (0.8) | 0 |
| B0 | 15 (6.4) | 21 (17.9) |
| B1 | 86 (36.4) | 63 (53.9) |
| B2 | 69 (29.2) | 22 (18.8) |
| C1 | 28 (11.9) | 10 (8.5) |
| C2 | 20 (8.5) | 1 (0.8) |
| D1 | 3 (1.3) | 0 |
| D2 | 12 (5.1) | 0 |
| N/A | 8 | 6 |
| Total | 244 | 123 |
Risk of bias sensitivity analysis
The quality of evidence was rated as low for localised prostate cancer and moderate for advanced prostate cancer ('Table 1'). Both the ERSPC and PLCO studies were assessed as at low risk of bias, whereas the Norrkoping study was graded as at high risk of bias. Sensitivity analysis, with the exclusion of the Norrkoping study, demonstrated a reduction in the effectiveness of screening in detecting localised prostate cancer (RR 1.44, 95% CI 0.90 to 2.32) but no effect on advanced cancer.
Harms of screening
Prostate cancer screening resulted in a range of harms that can be considered minor to major in severity and duration. Common minor harms from screening include bleeding, bruising, and short‐term anxiety. Common major harms include overdiagnosis and overtreatment, including infection, blood loss requiring transfusion, pneumonia, erectile dysfunction, and incontinence.
In total, 26,492 positive PSA tests were recorded in the ERSPC study, with a further 22,699 biopsies performed. No deaths were reported as a direct complication (from issues such as septicaemia or bleeding) from the biopsy procedure. Causes of death in the 14 men who were biopsied and subsequently died within 120 days included intercurrent death not as a result of biopsy (2), ischaemic heart disease (6), lung cancer (1), pancreatitis and myocarditis (1), subdural haematoma (1), basilar artery thrombosis (1), unknown (1), and a combination of issues (1) (ERSPC). The most common complications assessed as 'minor' were haematospermia and haematuria for greater than three days, whilst the most common side effects assessed as 'major' complications were pain after biopsy and fever (Raaijmakers 2002). Based on these biopsies, 7938 (9.6%) of 82,816 men in the screening group were diagnosed with prostate cancer; with 2483 (31.3%) of 7938 biopsied men diagnosed with prostate cancer outside of the screening protocol. The false‐positive rate for men who had an elevated PSA value (different PSA thresholds were used to define elevated but typically the threshold was defined as > 3.0 ng/mL) was 17.8% for men screened at least once in the ERSPC study, compared to a detection rate of 3.4% to 3.6% (ERSPC). The rate of overdiagnosis in the screening group was estimated to be up to 50% (ERSPC).
The PLCO study similarly reported on adverse events for screening and treatment, with a false‐positive rate of 10.4% and 15.0% for screening with the PSA test and DRE, respectively (PLCO). Pain or bleeding was associated with a rate of 0.3 per 10,000 screenings with DRE. The PSA test had a complication rate of 26.2 per 10,000 screenings (primarily dizziness, bruising, and haematoma; with three episodes of fainting). Medical complications from the diagnostic procedures occurred in 68 of 10,000 evaluations after a positive result from screening. These complications were primarily infection, bleeding, clot formation, and urinary difficulties.
The ongoing CAP study also reported a variety of harms associated with screening (Rosario 2012). Immediate short‐term adverse events (< 30 days) include mild or no pain (85%), dizziness (3%), and haematuria (7%). Moderate adverse events (up to 35 days post‐biopsy) include pain (44%), fever (20%), haematuria (66%), haematochezia (37%), and haemoejaculate (90%). Long‐term adverse events (≥ 2 weeks post‐biopsy) include pain (15%), fever (3%), haematuria (20%), haematochezia (5%), and haemoejaculate (60%).
Prostate grade distribution
The grade of prostate cancer cases was reported in both the control and screening groups for the PLCO ('Table 3'), Norrkoping ('Table 4'), and ERSPC ('Table 5') studies. There were very limited data on metastatic disease.
2. Prostate tumour grade (PLCO).
| Tumour Grade | Number of men (%) in control | Number of men (%) in screened |
| G2‐4 | 137 (4.6) | 222 (6.4) |
| G5‐6 | 1,656 (55.0) | 2,047 (58.9) |
| G7 | 779 (25.9) | 815 (23.4) |
| G8‐10 | 377 (12.5) | 315 (9.1) |
| Unknown | 61 (2.0) | 79 (2.3) |
| Total | 3,010 | 3,478 |
3. Prostate tumour grade (Norrkoping).
| Tumour Grade | # (%) in control | # (%) in screened |
| G1 | 94 (32.2) | 43 (50.6) |
| G2 | 149 (51.0) | 31 (36.5) |
| G3 | 43 (14.7) | 11 (12.9) |
| GX/tumour grade not recorded | 6 (2.1) | 0 (0) |
| Total | 292 | 85 |
4. Prostate tumour grade (ERSPC).
| Tumour Grade | # (%) in control | # (%) in screened |
| G2‐6 | 2,564 (47.5) | 4,528 (65.0) |
| G7 | 1,488 (27.6) | 1,433 (20.6) |
| G>7 | 857 (15.9) | 574 (8.2) |
| GX/tumour grade not recorded | 487 (9.0) | 428 (6.2) |
| Total | 5,396 | 6,963 |
Quality of life and cost of screening
None of the studies provided a complete assessment of the effect of screening on quality of life. Both the ERSPC and PLCO studies are currently assessing measures relating to quality of life. Authors from the ERSPC have published quality of life effects based on two participating sites in the study, which were modelled on the presence and absence of annual screening over the lifetime of 1000 men aged between 55 and 69 years (Heijnsdijk 2012). The model predicted a total of 73 life‐years gained, with a relative increase of 40% of prostate cancer diagnoses, and relative decrease of 28% of prostate cancer deaths; with harms including 247 additional negative biopsies and 41 additional men receiving treatment. The number of quality‐adjusted life‐years (QALYs) gained was 56 (range ‐21 to 97). Results relating to quality of life from both studies are expected to be published upon completion of the analysis and will be included in future updates of this review. None of the included studies provided a comprehensive assessment of resource utilization associated with screening for prostate cancer. However, estimates on the cost‐effectiveness of PSA screening from data extrapolated from the ERSPC study have been published (Shteynshlyuger 2011). Estimates from an earlier ERSPC publication reported that 1410 men would need to be screened (with the number of biopsies needed being 413 and subsequent number needed to treat of 48) to prevent one death from prostate cancer. Based on these figures, it has been estimated that it would cost between USD 262,758 and USD 347,549 per life‐year saved (Shteynshlyuger 2011), which is not indicative of cost‐effective care or high‐value care even if overall mortality was reduced to the same magnitude as prostate cancer‐specific mortality, an assumption that is unlikely.
Discussion
Summary of main results
A total of five studies were included in this review. The studies differed considerably in their design, screening methodologies, frequencies, thresholds, and analysis, thus limiting the value of strict reliance on pooled estimates. We therefore provide an overview of the individual studies and an overall assessment of their results and potential patterns of findings. Based on evidence from five RCTs, prostate cancer screening that included PSA testing increased the number of men diagnosed with prostate cancer but did not reduce prostate cancer‐specific or overall mortality. Findings from a 'core' subgroup enrolled in the ERSPC study indicated a 21% relative reduction in prostate cancer‐specific mortality among men aged 55 to 69 years. The absolute effect was 1 per 936 screened and was not observed in other studies of men this age nor in other men enrolled in the ERSPC study. The relative reduction in risk was observed in two of the seven trials that participated in the ERSPC study, which had large effects that may have driven the findings. When performing a meta‐analysis on the only two studies that were assessed to have 'low' risk of bias (ERSPC; PLCO), there was no significant difference in prostate cancer‐specific mortality observed (RR 0.94, 95% CI 0.65 to 1.35). Screening led to diagnostic procedure‐related harms that were generally minor but included pain, infection, and bleeding.
The ERSPC study consisted of seven sites that varied in the selection of participants with respect to age and length of follow‐up. Differences were also apparent in the screening intervention. Sites differed in their use of the PSA test, DRE, and TRUS biopsies; either as standalone tests, or in combination. PSA cut‐off values for biopsy also varied (ranging from 2.5 ng/mL to 4.0 ng/mL), along with the number of core biopsies. Screening interval differed between the sites, ranging from every two years, every four years, or between four to seven years. On average, each participant in the ‘core age group’ had 2.27 screening tests. Previous publications of the ERSPC study have reported a benefit for screening for a 'core' group of men. In updated publications, statistically significant results are not only in a 'core' group of men aged 55 to 69 years but are also present when all men that were randomised were evaluated for prostate cancer‐specific mortality (RR 0.84, 95% CI 0.73 to 0.95) (ERSPC). It should be noted that the variations in the screening and follow‐up methodologies employed across the eight participating sites (although results from the French site were not included in this analysis due to short duration of follow‐up) may influence the results. During the 11‐year median follow‐up duration, it was estimated that a total of 1055 men would need to be invited to undergo screening, and 37 prostate cancers detected, in order to prevent one death from prostate cancer (ERSPC). Quality of life effects were modelled on two participating sites in the study, which calculated that the number of QALYs gained was 56 (range ‐21 to 97). The authors concluded that any benefit of screening was diminished by the loss of QALYs due to post‐diagnosis effects including overdiagnosis. The QALY data should be interpreted with caution as the modelling was based on annual screening, whilst the ERSPC study sites used a variety of screening intervals (two years +).
The Norrkoping study did not provide a comparison of socio‐demographic data between the screening and control groups. It also reported that information regarding the study was distributed through newspaper, radio, and television broadcasting. This raises the potential for contamination and self‐selection bias, with participants in the control group choosing to be screened for prostate cancer. Furthermore, the quasi‐random method of allocation, lack of allocation concealment, and potentially incomplete outcome data for men who migrated increase the risk of bias of the trial. This study failed to demonstrate a reduction in prostate cancer‐specific mortality due to screening (RR 1.16, 95% CI 0.79 to 1.72).
The PLCO study was conducted at 10 sites across the USA. The methodological approach was uniform across all sites, with men aged 55 to 74 years recruited for the trial and the screening group offered annual DRE and PSA testing (with the cut‐off being 4 ng/mL). Participants in the screening group were offered annual PSA testing for six years and annual DRE for four years. Totals of 85% and 86% of men randomised to the screening group complied with the screening protocol for PSA testing and DRE, respectively, whereas 52% of men assigned to the control group underwent screening. The PLCO study reports on 10‐ and 13‐year follow‐up of participants; however, for the purposes of this review, the 10‐year data were abstracted since this captures follow‐up of 92% of participants compared to 57% at 13 years. With the exception of the analyses regarding tumour stage, which incorporated data from the 13‐year follow up, all other analyses including the PLCO study utilised the 10‐year follow‐up data. Findings from this study did not identify a significant reduction in prostate cancer‐specific mortality (RR 1.15, 95% CI 0.86 to 1.54), with results at 10 years of follow‐up indicating no statistically significant increase in prostate cancer‐specific mortality among screened individuals. While the high crossover rate is of concern in the PLCO study, the detection of prostate cancer in the screening group was 12% higher relative to the control group and the RRs for prostate cancer‐specific mortality remained greater than 1.0 (that is higher in the screened versus the control group) even at 10 years after randomisation. These facts argue against crossover being a major reason why the PLCO study did not find a reduction in prostate cancer‐specific mortality due to screening.
The Quebec study was limited by the lack of adherence to screening from participants randomised to the screening group. Although 31,133 men were randomised to receive screening for prostate cancer, only 23.6% of participants in this group actually complied with the randomisation and were screened. Similarly, approximately 7% of men randomised to the control group were screened for prostate cancer. Therefore, crossover between groups was an issue in this pragmatic trial. Data analysis was compromised as mortality data were not analysed according to the intention‐to‐screen principle. The authors of the trial reported a reduction in prostate cancer‐specific mortality by comparing mortality in men who were screened to that of men who were not screened, regardless of their initial randomisation. Conversely, our analysis of the data, according to the intention‐to‐screen principle, showed no significant difference in mortality between the two groups (RR 1.01, 95% CI 0.76 to 1.33).
The Stockholm study allocated 2374 men to be screened, whilst 24,772 served as controls (that is not invited for screening). Men assigned to be screened received a one‐time combination of DRE, PSA test, and TRUS biopsy. A PSA greater than 10.0 ng/mL was deemed the cut‐off for biopsy, with repeat TRUS performed for PSA greater than 7.0 ng/mL. This study did not identify a significant reduction in prostate cancer‐specific mortality (RR 1.09, 95% CI 0.83 to 1.45). Only three cancers were detected after repeat TRUS or after biopsies following increased PSA. Thus, this study is likely to have low applicability to current clinical practice.
Overall, reductions in prostate cancer‐specific and overall mortality were not observed. Four of the five included studies in this review reported no significant benefit of screening for prostate cancer when all men that were randomised were analysed. Meta‐analysis of eligible studies indicated no significant reduction in prostate cancer‐specific mortality, regardless of whether men were screened from 45, 50, or 55 years of age. Both the whole randomised population and the subgroup of men aged 55 to 69 years that were enrolled in the ERSPC were found to have a significant decrease in prostate cancer‐specific mortality following screening. Furthermore, even if assuming an actual overall benefit based on only the findings reported from the ERSPC (while ignoring the other RCT findings), the absolute magnitude of benefit is small, takes many years to accrue, and is accompanied by considerable overdetection. Any potential benefit of screening needs to be balanced with known harms associated with screening and with subsequent treatment. Several reports have quantified that the risks of screening and follow‐up biopsy, while typically transient, are not infrequent and include pain, bleeding, and infection. For any benefit of screening to occur, treatment must be effective. While the SPCG‐4 study demonstrated a reduction in prostate cancer‐specific mortality and morbidity among men with prostate cancer detected primarily by methods other than PSA testing, the magnitude of benefit for mortality was about 5% and was confined to men aged < 65 years (Bill‐Axelson 2008). Furthermore, several studies have reported on treatment‐related morbidity that includes urinary, bowel, and sexual dysfunction (Johansen 2008; Wilt 2008a).
A meta‐analysis of eligible studies indicated that screening was associated with an increase in the number of men diagnosed with prostate cancer (RR 1.30, 95% CI 1.02 to 1.65). Similarly, the proportion of localised prostate cancer was significantly greater in the screening group, with the proportion of advanced prostate cancer significantly higher in the control group. Despite this difference, a significant decrease in mortality was not demonstrated. Significant heterogeneity was associated with meta‐analysis of these outcomes, which may be attributed to the varying PSA test cut‐off levels, contamination in the control groups, or follow‐up biopsy procedures across the various included studies. There were very limited data on metastatic disease, quality of life, or cost‐effectiveness; however, a single quality of life derived model based on the ERSPC study suggests, at best, a small improvement in QALY that is not cost‐effective.
Overall completeness and applicability of evidence
There were several gaps in the reporting of criteria required for assessing the risk of bias of studies. Authors of studies with information gaps were contacted. Additional information about methodological details was obtained from authors of the ERSPC, PLCO, and Norrkoping studies. Both the Quebec and Stockholm studies provided insufficient information to determine how sequence generation was performed. The Quebec study additionally did not provide clear information about how blinding of outcome assessment was achieved. The Norrkoping study provided incomplete data about withdrawals from the study.
Three of the studies were performed across European countries, whilst the remaining two were performed in North America. None of the studies were conducted in Asian, African, or other low‐to‐middle income countries.
Quality of the evidence
The quality of the evidence was assessed using the approach outlined in 'Characteristics of included studies'. The body of evidence was classified as high, unclear, or low risk of bias for each outcome. Risk of bias was assessed as high for the majority of outcomes, as only the ERSPC and PLCO studies were assessed to have a low risk of bias. Additionally, the GRADE framework was applied to rate the quality of the evidence, which was assessed as 'moderate' for mortality.
Potential biases in the review process
This review primarily consisted of published data. Unpublished data on all‐cause mortality were obtained from the PLCO study. Future updated versions of the review will include more detailed analysis on primary and secondary outcomes as they become available through the continuing publications of the included studies.
Agreements and disagreements with other studies or reviews
The U.S. Preventive Services Task Force published an updated recommendation statement on screening for prostate cancer in 2012 (USPSTF 2012). The U.S. Preventive Services Task Force recommended against PSA‐based screening (grade D recommendation). This clinical guideline based its recommendation largely on data from the PLCO and ERSPC studies as well as a comprehensive review of the evidence examining the potential benefits and harms of prostate cancer screening.
The European Association of Urology (EAU) 2012 Clinical Practice Guidelines have included information from the ERSPC, PLCO, and Quebec studies (EAU 2012; Heidenreich 2011). The EAU guidelines suggest that a baseline PSA determination at age 40 years might be beneficial for risk‐stratification of patients, upon which further follow‐up intervals can then be based. It states that a screening interval of eight years may be sufficient in men with a baseline PSA of 1 ng/mL or less. The statement concludes that PSA testing is not recommended in men older than 75 years.
The American Cancer Society practice guidelines, published in 2010, recommend that asymptomatic men who have at least a 10‐year life expectancy should make an informed decision with their healthcare provider about screening for prostate cancer after they receive information about the uncertainties, risks, and potential benefits associated with prostate cancer screening. Men at average risk should receive this information beginning at age 50 years, whilst men in higher risk groups should receive this information before age 50 years (ACS 2010). This guideline included updated data from the ERSPC and PLCO studies, but not the Stockholm study.
The National Screening Committee in the United Kingdom (UK) has incorporated data from the ERSPC and PLCO studies (Burford 2010). It states that there are significant gaps in knowledge about the PSA test, prostate cancer, and treatment options. The National Screening Committee does not recommend a prostate cancer screening programme in the UK.
The Japanese Guideline for Prostate Cancer Screening, published in 2009, did not recommend population‐based screening for prostate cancer. It also recommended that individual patients requesting screening be given appropriate information about the benefits and limitations of screening to assist their choice. Their recommendations only included preliminary data from the ERSPC study and did not include data from the Stockholm or PLCO studies (Hamashima 2009). The Japanese Urological Association recommends PSA screening for men at risk of prostate cancer. This guideline, published in 2010, includes data from the ERSPC and PLCO studies (JUA 2010).
The American Urological Association (AUA) published their Best Practice Statement in 2009 recommending that, given the uncertainty, patients need to be informed of the risks and benefits of testing for prostate cancer (AUA 2009). The AUA also recommends PSA screening only for well‐informed patients who wish to pursue early diagnosis. This guideline incorporated results from the ERSPC and PLCO studies but not the Stockholm study.
The Royal Australian College of General Practitioners (RACGP) includes data from the ERSPC and PLCO studies but not the Stockholm study, and also cites the Djulbegovic 2010 systematic review and meta‐analysis and the 2010 version of this review in their guidelines for preventive activities in general practice (RACGP 2012). The RACGP does not recommend routine screening for prostate cancer with DRE, PSA test, or transabdominal ultrasound. Rather, the RACGP concludes that patients should make their own decisions about being tested for prostate cancer after being fully informed of the potential benefits, risks, and uncertainties of prostate cancer testing.
Several systematic reviews have been published. As previously mentioned, a 2010 review by Djulbegovic concluded that there was no evidence to support routine use of screening for prostate cancer (Djulbegovic 2010). A 2012 systematic review also reported that screening demonstrated no significant benefit on reducing prostate cancer‐specific mortality (Lumen 2012).
Authors' conclusions
Implications for practice.
A pooled meta‐analysis of the five included studies in this review identified that screening does not significantly decrease prostate cancer‐specific mortality and is associated with a high degree of overdiagnosis, treatment and screening‐related harms. Given the variation in study design and quality across the five included studies, it could be argued that pooling studies is not appropriate. However, assessment of four of the five studies individually using intention‐to‐screen analysis also indicated no decrease in prostate cancer‐specific mortality. The only exception was the ERSPC study, which reported, in a pre‐specified subgroup of men, that 1055 men needed to be invited to screening and 37 additional men subsequently diagnosed with prostate cancer needed to receive early intervention to prevent one additional prostate cancer death at a median follow‐up duration of 11 years. The known harms associated with screening (false‐positives with PSA testing, complications associated with TRUS‐guided biopsies, overdiagnosis and treatment‐related harms) suggest that any small mortality benefit of screening at 11 years would be challenged by the occurrence of these harms that occur early and may persist.
For men who express an interest in prostate cancer testing, including those with risk factors such as family history of prostate cancer and African ethnicity, clinicians should adopt a shared, informed approach to decision‐making. Men should be informed of the lack of benefit to at least 10 years, and demonstrated adverse effects, when deciding whether or not to undertake screening for prostate cancer. Any benefits from prostate cancer screening may take up to 10 years to accrue (Bill‐Axelson 2005; Bill‐Axelson 2008; Bill‐Axelson 2011; Johansson 2009). Men who have an anticipated life expectancy less than 10 to 15 years (either due to age or co‐morbid conditions) should be informed that testing for prostate cancer is unlikely to be beneficial given harms associated with testing.
It should be noted that the available evidence from randomised trials summarised in this review is largely based on men of European descent. In the United States, black men appear to have a higher incidence and higher risk of dying from prostate cancer that is approximately twice that of other men (Howlader 2012); the reasons for this are unknown. Only 4% of men in the PLCO trial were non‐Hispanic black men and, although no specific information is available, it can be assumed that few men in the ERSPC trial were non‐white. Therefore, there is uncertainty about the benefits and harms of prostate cancer screening in black men and men with a family history of prostate cancer.
Several fundamental issues must be addressed when considering screening for prostate cancer. Screening for prostate cancer is primarily performed using the DRE and PSA test, yet the specificity and sensitivity of both of these modalities are not ideal (Holmström 2009). The consequences of heightened anxiety, further examinations through biopsies, and the considerable side effects associated with various prostate cancer treatments must be appreciated. This predicament is further compounded by the inability to understand whether identified neoplasms are clinically significant. Some slow growing tumours may never threaten a man's life, as is represented by the discrepancy between the incidence and deaths attributed to prostate cancer (Parkin 2005).
A number of principles have been proposed, including the burden of the disease and the effectiveness of diagnostic tests and treatments, to assess whether a screening program is successful (WHO 1968). Prostate cancer is accepted as an important health problem; however, uncertainty exists over the effectiveness of diagnostic tests and treatments available. Much debate exists about use of the PSA test and the implications of potential false‐positive and false‐negative results. Similarly, although various treatments for prostate cancer are available (for example watchful waiting, radical prostatectomy, hormone and radiotherapy), high quality evidence is still developing (Bill‐Axelson 2008; Bill‐Axelson 2011; Wilt 2012). In future years, findings from the current screening RCTs will shed further light on longer‐term outcomes from screening for prostate cancer and will document quality of life outcomes. However, until such data is available greater emphasis will be placed on patient and doctor communication. Many medical organisations currently support the concept of patient‐informed, shared decision‐making, regardless of whether they support or reject screening for prostate cancer. In the absence of definitive evidence from RCTs, a shared approach to decision‐making between doctors and patients should be encouraged for men who inquire about prostate cancer screening or who have previously undergone prostate cancer screening. Facilitating this process with the aid of appropriate patient education materials will promote informed patient choice (O'Connor 2009) while minimizing workload burden among primary care providers and permitting primary care clinicians to focus on other preventive healthcare strategies of proven effectiveness for other health conditions.
Prior to obtaining a PSA test, men should be informed about the known harms that are frequent, both in the immediate‐ and long‐term, versus the potential for a benefit that may occur many years in the future. Clinicians may adopt either a 'reactive' or 'proactive' method of counselling patients on prostate cancer screening, depending on their attitudes to screening; that is clinicians in favour of screening men of a certain age will adopt a 'proactive' nature to counselling as opposed to those that wait for the patient to raise the topic of screening. We believe that rather than counselling all men (proactive), counselling should be targeted to men who ask about screening, or those who have previously screened, in order to provide updated information. This approach permits clinicians to focus time, effort, and resources on areas of greatest concern to their patients and where there is greatest evidence of effectiveness. Mass screening, selective and opportunistic screening in the absence of patient knowledge and consent should not be performed.
Implications for research.
Findings from this review support further research across a variety of health disciplines. Further long‐term follow‐up from existing trials is required to gain a better understanding of the adverse events, quality of life, and economic impact of screening. A longer follow‐up period of existing trials with respect to prostate cancer‐specific mortality will also provide more robust evidence that can better inform any net benefit of screening for prostate cancer. Future research could incorporate time‐to‐event analysis to account for the longer duration of follow‐up from the included trials. Any additional trials should aim to provide high quality data on the impact of prostate cancer screening on quality of life, potential harms, adverse events, and an economic evaluation in addition to mortality across different populations (for example Asia). Additionally, such studies should be conducted using appropriate, or justified, selection of participants, adequate allocation concealment, adequate blinding of assessors, completeness of follow‐up, and analysis of data according to intention‐to‐screen principles when possible. Prostate cancer‐specific mortality is also highly dependent on the effectiveness of treatment regimens. Greater research is required from long‐term, high quality trials to inform the effectiveness of current treatment regimens including radical prostatectomy, radiotherapies and active surveillance.
Evidence suggests that the PSA test does not have the required characteristics to be used as a widespread screening test for prostate cancer (Holmström 2009). If the PSA test is to be used as a screening tool, greater evidence is needed to establish cut‐off values for 'negative' and 'positive' test results to ensure that patients do not undergo unnecessary invasive investigations and, similarly, are able to be referred for further investigations when warranted. A systematic review of diagnostic test accuracy synthesising the current evidence would greatly inform the broader understanding of the PSA test, its characteristics and its value as a screening and diagnostic tool. Whilst the PSA test may be prostate‐specific, it is not specific to prostate cancer. Therefore, continued research into alternative prostate‐specific markers is required.
Additional research is also required to further identify the psychosocial aspect of screening, patient knowledge and uptake (or tendency for uptake) of screening, as well as clinician perspectives and needs on prostate cancer screening. Such research should include evidence‐based strategies for communicating the evidence on the merits of prostate cancer screening to patients and clinicians given the current barriers to uptake of patient and clinician education on this issue.
What's new
| Date | Event | Description |
|---|---|---|
| 20 November 2012 | New search has been performed | The review was updated to include a June 2012 search for published and unpublished studies. New outcomes data were abstracted from three studies and incorporated in the meta‐analysis. This update was completed with revised authorship. |
| 20 November 2012 | New citation required but conclusions have not changed | The review was updated and the conclusions did not change. |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 22 September 2010 | Amended | Under 'electronic searches' we changed the sentence " An updated search of the electronic databases was performed with the existing search strategy on the 10 June, 2009" to " An updated search of the electronic databases was performed with the existing search strategy in July 2010." |
| 10 June 2009 | New search has been performed | Search strategy re‐run and review updated. |
Acknowledgements
We thank the referees and editors of the Prostatic Diseases and Urologic Cancers Group for their comments and valuable assistance.
Appendices
Appendix 1. Electronic database search strategy
The following search strategy was used for MEDLINE, PROSTATE register and CANCERLIT:
Prostate‐Specific Antigen/
prostate specific antigen.mp
psa.mp.
digital rectal examination.mp.
dre.mp.
transrectal ultrasound$.mp.
TRUS.mp.
or/1‐7
Mass Screening/
screening.mp
or/9‐10
Prostatic Neoplasms/pc, di [Prevention & Control, Diagnosis]
prostat$ cancer.mp
or/12‐13
clinical trial.pt.
random$.mp
((single or double) adj (Blind$ or mask$)).mp
placebo$.mp
or/14‐18
8 and 11 and 14 and 19
The following search strategy was used for EMBASE:
Prostate‐Specific Antigen/
prostate specific antigen.mp
psa.mp.
digital rectal examination.mp.
dre.mp.
transrectal ultrasound$.mp.
TRUS.mp.
1 or 2 or 3 or 4 or 5 or 6 or 7
Mass Screening/
screening.mp
9 or 10
Prostate Tumor/pc, di [Prevention, Diagnosis]
prostat$ cancer.mp
12 or 13
clinical trial.pt.
random$.mp
((single or double) adj (Blind$ or mask$)).mp
placebo$.mp
or/14‐18
8 and 11 and 14 and 19
The following search strategy was used for the Cochrane Central Register of Controlled Trials (CENTRAL) and the NHS EED:
PROSTATE‐SPECIFIC ANTIGEN
(prostate next specific next antigen)
psa
(digital next rectal next examination)
dre
(transrectal next ultrasound*)
trus
(#1 or #2 or #3 or #4 or #5 or #6 or #7)
MASS SCREENING
screening
(#9 or #10)
PROSTATIC NEOPLASMS
(prostat* next cancer)
(#12 or #13)
(#8 and #11 and #14)
Data and analyses
Comparison 1. Screening versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Prostate cancer‐specific mortality (subgroup analysis age) | 5 | 341342 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.86, 1.17] |
| 1.1 Men aged ≥ 45 years | 1 | 46486 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.76, 1.33] |
| 1.2 Men aged ≥ 50 years | 2 | 191025 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.69, 1.27] |
| 1.3 Men aged ≥ 55 years | 2 | 103831 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.92, 1.37] |
| 2 Prostate cancer‐specific mortality (subgroup analysis age, including ERSPC 'core' age group) | 5 | 321586 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.83, 1.19] |
| 2.1 Men aged ≥ 45 years | 1 | 46486 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.76, 1.33] |
| 2.2 Men aged ≥ 50 years | 1 | 9026 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.79, 1.72] |
| 2.3 Men aged ≥ 55 years | 3 | 266074 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.75, 1.27] |
| 3 Prostate cancer‐specific mortality (sensitivity analysis overall risk of bias) | 2 | 258684 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.30] |
| 3.1 Low risk of bias | 2 | 258684 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.70, 1.30] |
| 4 Prostate cancer‐specific mortality (sensitivity analysis overall risk of bias, including ERSPC 'core' age group) | 2 | 238928 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.65, 1.35] |
| 4.1 Low risk of bias | 2 | 238928 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.65, 1.35] |
| 5 All‐cause mortality (subgroup analysis age) | 4 | 294856 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.96, 1.03] |
| 5.1 Men aged ≥ 50 years | 2 | 191025 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.84, 1.56] |
| 5.2 Men aged ≥ 55 years | 2 | 103831 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.95, 1.01] |
| 6 All‐cause mortality (subgroup analysis age, including ERSPC 'core' age group) | 4 | 275100 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.96, 1.03] |
| 6.1 Men aged ≥ 50 years | 1 | 9026 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.06, 1.79] |
| 6.2 Men aged ≥ 55 years | 3 | 266074 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.97, 1.00] |
| 7 All‐cause mortality (sensitivity analysis overall risk of bias) | 2 | 258684 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.96, 1.02] |
| 7.1 Low risk of bias | 2 | 258684 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.96, 1.02] |
| 8 All‐cause mortality (sensitivity analysis overall risk of bias, including ERSPC 'core' age group) | 2 | 238928 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.97, 1.00] |
| 8.1 Low risk of bias | 2 | 238928 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.97, 1.00] |
| 9 Prostate cancer diagnosis (subgroup analysis age) | 4 | 294856 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.02, 1.65] |
| 9.1 Men aged ≥ 50 years | 2 | 191025 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [1.54, 1.64] |
| 9.2 Men aged ≥ 55 years | 2 | 103831 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [1.08, 1.17] |
| 10 Prostate cancer diagnosis (subgroup analysis age, including ERSPC 'core' age group) | 4 | 275100 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.03, 1.64] |
| 10.1 Men aged ≥ 50 years | 1 | 9026 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [1.16, 1.86] |
| 10.2 Men aged ≥ 55 years | 3 | 266074 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [0.96, 1.64] |
| 11 Tumour stage (localised T1‐T2, N0, M0) | 3 | 247954 | Risk Ratio (M‐H, Random, 95% CI) | 1.79 [1.19, 2.70] |
| 12 Tumour stage (advanced T3‐4, N1, M1) | 3 | 247954 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.73, 0.87] |
1.1. Analysis.
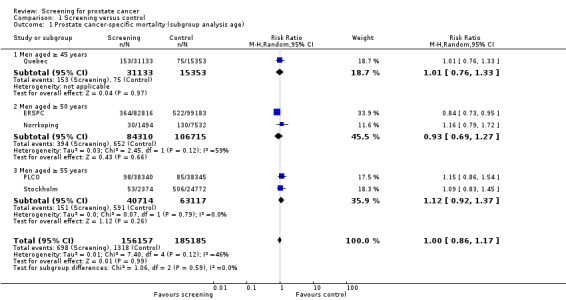
Comparison 1 Screening versus control, Outcome 1 Prostate cancer‐specific mortality (subgroup analysis age).
1.3. Analysis.
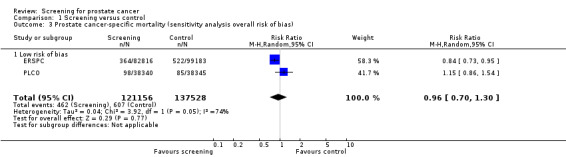
Comparison 1 Screening versus control, Outcome 3 Prostate cancer‐specific mortality (sensitivity analysis overall risk of bias).
1.5. Analysis.
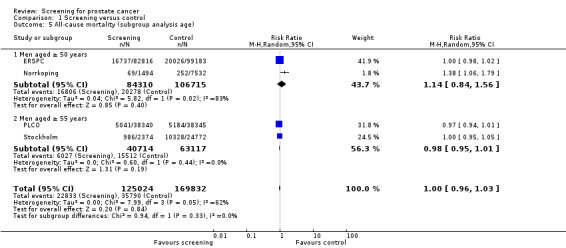
Comparison 1 Screening versus control, Outcome 5 All‐cause mortality (subgroup analysis age).
1.9. Analysis.
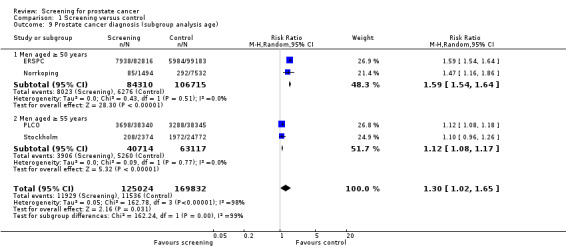
Comparison 1 Screening versus control, Outcome 9 Prostate cancer diagnosis (subgroup analysis age).
1.11. Analysis.
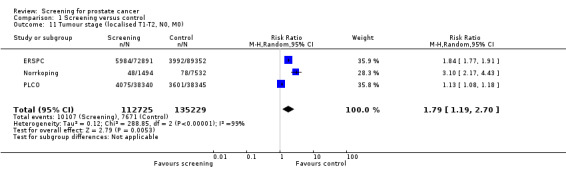
Comparison 1 Screening versus control, Outcome 11 Tumour stage (localised T1‐T2, N0, M0).
1.12. Analysis.
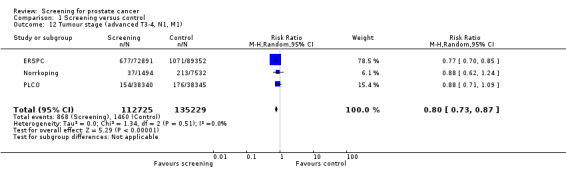
Comparison 1 Screening versus control, Outcome 12 Tumour stage (advanced T3‐4, N1, M1).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ERSPC.
| Methods | The ERSPC program was a randomised, multi‐centre trial across 9 European countries (The Netherlands, Belgium, Sweden, Finland, Italy, Spain, Switzerland, Portugal, and France). Each country used different recruitment and randomisation procedures. Participants were randomised 1:1 in all sites apart from Finland, which undertook a 2:3 randomisation process. Length of follow‐up was dependent on site of randomisation. The trial reports data on 8 countries (data from Portugal was not included in this follow‐up period). | |
| Participants | The core age group for male participants was 55 to 69 years. In Sweden, study investigators included men between the ages of 50 and 54 years, and investigators in the Netherlands, Italy, Belgium, and Spain included men up to the age of 74 years at entry. In Switzerland, men between the ages of 55 and 69 years were included, with screening up to the age of 75 years. In Finland, men were recruited at the ages of 55, 59, 63, and 67 years. Men with a diagnosis of prostate cancer were ineligible for the study. Numbers include: screening group ‐ 112,569 (total) 72,891 ('core' age group); control group ‐ 128,688 (total) 89,352 ('core' age group). |
|
| Interventions | Participants in the screening group were offered a combination of PSA testing, DRE and TRUS biopsy. Most sites used a PSA value of 3.0 ng/mL as the cut‐off and indication for biopsy. In Finland, a PSA value of 4.0 ng/mL was used for cut‐off ‐ men with a value between 3.0 to 3.9 ng/mL underwent a DRE until 1998. In Italy, a PSA value of 4.0 ng/mL was the defined cut‐off, but men with a PSA between 2.5 to 3.9 ng/mL underwent a DRE and TRUS. In the Belgian and Dutch sites, a combination of DRE, TRUS and PSA (with a cut‐off of 4.0 ng/mL) was used until 1997 ‐ from which PSA testing alone was used. In Belgium, the PSA cut‐off value was 10.0 ng/mL initially. The screening interval at 6 of the 7 sites was 4 years ‐ Sweden used a 2 year interval. There was a 7‐year interval between 1st and 2nd screening rounds in Belgium. | |
| Outcomes | Primary outcome was prostate cancer mortality. Also reported were all‐cause mortality, number of prostate cancers diagnosed, clinical stage, Gleason score, and risk. | |
| Notes | A total of 82.2% of men in the screening group were screened at least once. The mean and median durations of follow‐up were 10.5 and 11 years, respectively (core groups). No deaths were reported as a direct complication from the biopsy procedure. The rate of overdiagnosis in the screening group was estimated to be up to 50%. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The study was a multi‐centre trial across 9 European countries that randomly assigned men to screening or control groups. "Within each country, men were assigned to either the screening group or the control group... on the basis of random number generators." |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described in the publication. It was also unclear whether method of concealment differed among study sites given that different randomisation procedures were implemented across the different sites. "...randomization procedures differed among countries and were developed in accordance with national regulations." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It is not possible to blind participants and clinicians to the screening intervention. Causes of death were evaluated in a blinded manner. Causes of death were obtained from registries and individual chart reviews. A committee analysed causes of death at each centre, with an independent data and safety committee reviewing the trial. There was no information on blinding for other outcome measures (e.g. diagnosed cancers). "Causes of death were evaluated in a blinded fashion... or on the basis of official causes of death. The causes were classified by the independent committees." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data from the Portugal study centre were excluded from all analyses due to discontinuation. Data from the France centre of the trial were not included in mortality analyses due to short duration of follow‐up, and were not included in primary analyses of additional outcomes ‐ although data were provided. "the primary analysis was planned at the outset on the basis of follow‐up of at least 10 years, which was reached with data through 2008. The current analyses include follow‐up data through 2008…regarding the core age group analysis." |
| Selective reporting (reporting bias) | Low risk | Objectives of the ERSPC include cancer‐specific mortality and quality of life outcomes. Mortality is reported but quality of life is not descriptively reported in this publication. Measures relating to quality of life are currently being reviewed and will form the basis of future publications. "…an evaluation of the effect on quality of life is pending." |
| Other bias | Unclear risk | Main data analysis is based on the core age group (55‐69 years). There are differing age groups across the 8 reported sites. "The benefit of screening was restricted to the core age group of subjects who were between the ages of 55 and 69 years at the time of randomizations" |
Norrkoping.
| Methods | Randomised controlled trial in Norrkoping, Sweden. Participants were men residing in the city of Norrkoping identified from a national population register. The study reports on a 20‐year follow‐up of participants on prostate cancer outcome. | |
| Participants | Participants were male inhabitants of Norrkoping aged 50‐69 years. Every sixth man was randomly allocated to the screening group from a list of dates of births obtained from the national population register. The remaining men served as controls. Only men 69 or younger were invited to the fourth screening round in 1996. There was no mention of any other specific exclusion criteria (e.g. previous diagnosis of prostate cancer or with symptoms). Numbers include: screening group ‐ 1494; control group ‐ 7532. |
|
| Interventions | Interventions were screening every three years versus control (not invited for screening). The first and second rounds of screening were performed only using a DRE. The first screening round DREs were performed by a general practitioner and a urologist. In the second and subsequent rounds, the DRE was performed by a general practitioner only. The third and fourth rounds of screening included a DRE and a PSA test. TRUS biopsy was performed if the DRE was deemed abnormal or if PSA was greater than 4.0 ng/mL. | |
| Outcomes | Primary outcome was prostate cancer mortality at 20 years follow‐up. Also reported were all‐cause mortality, clinical stage and choice of therapy in men diagnosed with prostate cancer across both screened and control groups, and number of prostate cancers diagnosed. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Men were randomised to the screening group from a list of dates of birth. "... men were randomly allocated to be screened by including every sixth man from a list of dates of birth." |
| Allocation concealment (selection bias) | High risk | There was no description of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It is not possible to blind participants and clinicians to the screening intervention. Prostate cancer mortality was obtained from a national cancer registry and cross‐referenced against patient notes. There is no clear description of blinding during outcome assessment, however the outcomes (mortality, diagnosis) are unlikely to be influenced by lack of blinding. "In September 2009 cause of death was registered in a blinded review of the patients' records for all men who died." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawals were cited, but it is unclear how the data for those men who migrated were available. There were no missing data for mortality, but some for number of men diagnosed, due to migration and death. "The screened cohort diminished from 1492 men at the start of the study to 1118 in 1996 due to migration and death." |
| Selective reporting (reporting bias) | Low risk | Outcomes presented in publications correlate to study protocol obtained from the authors. |
| Other bias | Low risk | Data presented to allow analysis according to intention‐to‐screen principle. "All analyses were performed based on intention to screen comparisons." |
PLCO.
| Methods | The PLCO study was a randomised controlled trial across 10 study centres in the United States of America (USA). Each study centre used recruitment sources and strategies appropriate to the local situation. Participants were randomised 1:1. The study reports on a 10‐ to 13‐year follow‐up of participants regarding prostate cancer outcome. | |
| Participants | Participants were males aged 55 to 74 years. Men with a history of prostate, lung or colorectal cancer were excluded, along with participants currently receiving cancer treatment except non‐melanoma skin cancer. In 1995, men who had undertaken more than one PSA blood test in the previous three years were also excluded. Numbers include: screening group ‐ 38,340; control group ‐ 38,345. |
|
| Interventions | Participants in the screening group were offered annual PSA testing for six years and annual DRE for four years. A PSA value of 4.0 ng/mL was determined to be positive for prostate cancer. DREs were performed by physicians, qualified nurses or physician assistants. Men with positive PSA results, or abnormal DRE, were advised to seek diagnostic evaluation. Both participants and health‐care providers received the results, and they decided upon the method of evaluating abnormal screening results. | |
| Outcomes | Primary outcome was prostate cancer mortality at 10 years (92% follow up) and 13 years (57% follow up). Also reported were number of prostate cancers diagnosed, clinical stage and Gleason scores. | |
| Notes | All‐cause data provided in the trial report does not include deaths from prostate, lung, or colorectal (PLC) cancers – therefore not truly ‘all‐cause’. Authors were contacted, and updated information on all‐cause mortality data inclusive of deaths from PLC cancers was provided for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Individual randomisation was performed within blocks stratified according to centre, age and sex. Atlhough the method used to generate allocation sequence was not mentioned in the trial report, it was provided in an earlier publication (PLCO ‐ Prorock). "The randomization scheme uses blocks of random permutations of varying lengths and is stratified by SC (study centre), gender and age. Random assignment is implemented using compiled software and encrypted files loaded on SC microcomputers." |
| Allocation concealment (selection bias) | Low risk | Concealment was achieved through a central system. "As each person is successfully randomized into the trial, data including name, gender, date of birth and study arm are automatically stored in encrypted data tables." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It is not possible to blind participants and clinicians to the screening intervention. Data on diagnosed cancers and mortality were obtained by patient reported questionnaire and followed up by telephone (unblinded). This data was supplemented by linkage to the National Death Index. Death certificates were obtained to confirm deaths and determine cause. Possible cancer‐specific deaths were reviewed by blinded reviewers. "Reviewers of these deaths were unaware of study‐group assignments for deceased subjects." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data on mortality and diagnosis are available for the 10‐year follow up, but follow‐up data on 13‐year outcomes are not complete. "As of December 31, 2009 (the cutoff date for this analysis), the vital status of 92% of the trial participants was known at 10 years and of 57% of the participants at 13 years." |
| Selective reporting (reporting bias) | Low risk | Study protocol is available and the study's pre‐specified outcomes have been reported. Outcomes, such as harms, are to be reported in future publications. "...there is evidence of harms, in part associated with the false‐positive tests, but also with the overdiagnosis inseparable from PSA screening, especially in older men." |
| Other bias | High risk | Data were analysed according to the intention‐to‐screen principle. Data on contamination were also provided (estimated to be 40‐52%). |
Quebec.
| Methods | Randomised controlled trial in Quebec, Canada. Participants were men identified from electoral rolls and allocated 2:1 in favour of screening. The study reports on an 11‐year follow‐up of participants on prostate cancer outcome. | |
| Participants | Participants were male inhabitants of Quebec city aged 45 to 80 years. Men with a previous diagnosis of prostate cancer or previously screened and referred to the study clinic for consultation were not eligible. Numbers include: screening group ‐ 31,133; control group ‐ 15,353. |
|
| Interventions | Interventions were annual screening versus control (not invited for screening). The first screening round included a PSA test and a DRE. TRUS biopsy was performed in cases with PSA > 3.0 ng/mL and/or abnormal DRE (except for first 1002 men who had all three procedures performed). Follow‐up screening rounds included a PSA test. TRUS biopsy was only performed if PSA was above 3.0 ng/mL for the first time or increased by more than 20% from last measurement. | |
| Outcomes | Primary outcome was prostate cancer mortality at 11 years follow‐up. Also reported were prostate cancer death incidence rates in screened versus unscreened cohorts, and clinical stage and choice of therapy in men diagnosed with prostate cancer. | |
| Notes | Crossover and contamination were issues for this pragmatic trial. The compliance and contamination rate within both the screening and control groups was described. From a total of 31,133 men randomised to the screening group, 7348 (23.6%) were actually screened (i.e. all 31,133 men were invited to be screened, but only 23.6% took up the invitation and actually were screened). Similarly, of the 15,353 randomised to the control group, 1122 (7.3%) were screened for prostate cancer at the study site. There was no report of any other withdrawals or whether participants in the control group were screened somewhere other than the study site; hence it is possible that more than 7.3% of the control group were actually screened. The data were re‐analysed by the authors of this review according to the intention‐to‐screen principle. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No sequence generation process is mentioned. Authors only state that men were randomly assigned to groups. "... men were randomly allocated either to the group invited for annual screening or to the control group not invited for screening at a ratio of 2:1 in favor of screening." |
| Allocation concealment (selection bias) | High risk | No mention of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | It is not possible to blind participants and clinicians to the screening intervention. Blinding of outcome assessment was not clearly described. "The information on cause‐specific death was obtained from the Death Registry of the Health Department of the Province of Quebec." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawals from both the screening and control groups were cited. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | High risk | Data were not analysed according to the intention‐to‐screen principle. A total of 31,133 men were randomised to receive screening for prostate cancer, but only 23.6% of participants in this group actually complied with the randomisation and were screened. Similarly, approximately 7% of men randomised to the control group were screened for prostate cancer. "... all screened men were compared to all unscreened men irrespective of the original randomization group." |
Stockholm.
| Methods | Randomised controlled trial in Stockholm, Sweden. Male participants living in the catchment area of Stockholm South Hospital were identified through census records. The study reports on a 15‐year follow‐up of participants on prostate cancer outcome. | |
| Participants | Participants were all men aged between 55 to 70 years living in the catchment area of Stockholm South Hospital. Men with an earlier diagnosis of prostate cancer were excluded from the study. Numbers include: screening group ‐ 2374; control group ‐ 24,772. |
|
| Interventions | Inverventions were one‐time screening versus control (not invited for screening). The screening consisted of DRE, PSA test and TRUS. TRUS‐guided biopsies were performed if abnormal findings occurred during the DRE and/or TRUS. A repeat TRUS was performed if the PSA was greater than 7 ng/mL. Randomized quadrant biopsies were taken if the PSA was greater than 10 ng/mL. | |
| Outcomes | Primary outcome was prostate cancer mortality at 15 years follow‐up. Also reported was "any" cause mortality (including attendees and non‐attendees), "other" cause mortality (including attendees and non‐attendees), and number of prostate cancers diagnosed. | |
| Notes | Median follow‐up time was 12.9 years overall. Mean years follow up for the screened group was 12.9 years (0.2 to 15.7). Mean years follow up for the control group was 13.0 years (0.7 to 15.7). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The authors only state that patients were randomly selected for screening. No additional information is provided on the method of randomisation. "... 2,400 (men) were randomly selected and invited to participate in a prostate cancer screening study...The 24,202 remaining men served as a control group." |
| Allocation concealment (selection bias) | High risk | No method of allocation concealment was described. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | It is not possible to blind participants and clinicians to the screening intervention. There was no specific mention of blinding of outcome assessors. Outcomes (mortality and diagnosis) and outcome measurement are unlikely to be influenced by lack of blinding. Outcomes were obtained from a national cancer registry, with urologists independently reviewing medical records to assign cause of death. "We collected information on prostate cancer diagnosis and the date of diagnosis from the Swedish Cancer Register for the entire source population. From the Cause of Death register we collected information on date of death and the underlying cause of death... three senior urologists independently reviewed the medical records and assigned the cause of death." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data for mortality or number diagnosed. However, there was discrepancy between population sizes from Swedish census records in 1988 and Statistics Sweden records. "The file containing the registration number of the original 26,602 men...could not be retrieved due to a change of record holders. Therefore, we reconstructed the cohort...This comprised 27,204 men, that is, 602 (2%) more than in the original source population." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement. |
| Other bias | Low risk | Data were analysed according to the intention‐to‐screen principle. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agalliu 2007 | Case control study |
| Ahmed 2008 | Cohort study ‐ survey of a cohort of men regarding prostate screening awareness |
| Anonymous 2000 | Descriptive study ‐ report on a screening program in the USA |
| Anonymous 2008 | Narrative review |
| Aus 2001 | Cohort study ‐ cancer detection rate via biopsy in men with an elevated PSA level in the screening group of the Swedish arm of the ERSPC |
| Aus 2004 | Cohort study ‐ explored the cumulative risk of cancer detection in a cohort of men from the screening group of the Swedish arm of the ERSPC |
| Aus 2005 | Cohort study ‐ reported the cumulative prostate cancer risk in men with different PSA levels within the Swedish arm of the ERSPC |
| Aus 2007 | Preliminary results from the ERSPC |
| Auvinen 1996 | Finnish pilot study for the ERSPC, with a 2 year follow‐up and no results report for the control group |
| Auvinen 2009 | Results from the ERSPC, but reported outcomes are not relevant to the systematic review |
| Bangma 1995a | Cohort study ‐ evaluation of the diagnostic value of volume adjusted PSA values in a screening population of the Dutch feasibility study for the ERSPC |
| Bangma 1995b | Cohort study ‐ explored the value of the f/t PSA ratio for cancer detection in the screening group of the Dutch arm of the ERSPC |
| Bangma 1995c | Cohort study ‐ explored the value of the f/t PSA ratio, PSAD and PSA age references for cancer detection in the screening group of the Dutch arm of the ERSPC |
| Bangma 1997 | Descriptive study ‐ report on the design and features of the Dutch arm of the ERSPC |
| Beemsterboer 1999 | Cohort study ‐ explored the incidence of prostate cancer in the screening group of the Dutch arm of the ERSPC |
| Beemsterboer 2000 | Survey ‐ identified the rate of PSA testing before and during the Dutch arm of the ERSPC |
| Bergstralh 2007 | Case control study |
| Boevee 2010 | Results are available in the main article of ERSPC study |
| Borre 2007 | Narrative review |
| Bul 2011 | Results only from one arm of the ERSPC study |
| Bunker 2007 | RCT ‐ exploring lycopene supplementation for prostate cancer |
| Candas 2000 | Cohort study ‐ incidence of prostate cancer in screening cohort of the Quebec trial along with a cost assessment |
| Carlsson 2007 | Study associated with the ERSPC study |
| Carlsson 2011 | Results only from one arm of the ERSPC study |
| Carriere 2007 | Ecological study |
| Chavarro 2008 | Case control study |
| Ciatto 1993 | Cohort study ‐ Italian pilot feasibility study for the ERSPC with preliminary results |
| Ciatto 1994 | Cohort study ‐ Italian pilot feasibility study for the ERSPC with preliminary results |
| Ciatto 2002 | Cohort study ‐ analysis of PSA velocity in 'healthy' participants in the screening cohort within the Italian arm of the ERSPC |
| Ciatto 2003b | Descriptive study ‐ report on the issue of screening within the control group of the Italian arm of the ERSPC |
| Ciatto 2008 | Cohort study ‐ relating to results from ERSPC |
| Collin 2008 | Ecological study of prostate cancer mortality |
| Concato 2009 | Narrative review |
| Cusan 1994 | Descriptive study ‐ report on the preliminary results of the Quebec trial |
| D'Amico 2007 | RCT ‐ exploring Finasteride for hair loss |
| de Koning 2002 | Descriptive study ‐ report on the preliminary results of the ERSPC and PLCO trials |
| Draisma 2009 | Additional results from the ERSPC study, reporting on cancer lead time |
| Driscoll 2008 | RCT ‐ patient education trial |
| Döbrőssy 2007 | Narrative review |
| Ellison 2008 | RCT ‐ study of patient education materials |
| Essink‐Bot 1998 | Cohort study ‐ explored the health status of men randomised to screening in the Dutch arm of the ERSPC |
| Etzioni 2008 | Narrative review |
| Fenton 2008 | Cohort study ‐ treatment of prostate cancer patients |
| Finne 2002 | Cohort study ‐ explored the diagnostic value of PSA properties in diagnosing prostate cancer in the screening group of the Finnish arm of the ERSPC |
| Finne 2010 | Results from the ERSPC, but reported outcomes are not relevant to the systematic review |
| Fitzpatrick 2009 | Narrative review |
| Fleshner 2007 | Study protocol |
| Fleshner 2009 | Narrative review |
| Ford 2003 | Descriptive study ‐ described the demographic details of the AAMEN project, which attempts to recruit African American men to the PLCO |
| Ford 2008 | RCT ‐ exploration of a case management intervention for prostate cancer patients |
| Frosch 2008 | RCT ‐ study exploring patient education materials |
| Gohagan 1994a | Descriptive study ‐ report on the design of the PLCO |
| Gohagan 1994b | Editorial on the issue of screening for prostate cancer |
| Gohagan 1995 | Descriptive study ‐ report on the design of the PLCO |
| Gohagan 2000 | Descriptive study ‐ report on the design of the PLCO |
| Gonzalgo 2007 | Narrative review |
| Gosselaar 2008 | RCT ‐ reporting associated findings from the ERSPC study |
| Gosselaar 2008b | Associated study within the ERSPC |
| Grosclaude 2008 | Narrative review |
| Grubb 2008 | RCT ‐ preliminary results from the PLCO study |
| Grubb 2009 | Cohort study ‐ additional data relating to the PLCO study |
| Gustafsson 1992 | Cohort study ‐ explored the correlation between PSA measurement to prostate cancer in a randomly selected cohort of men screened for prostate cancer in Sweden |
| Gustafsson 1995 | Cohort study ‐ explored the cost effectiveness of prostate cancer screening in a randomly selected cohort of men in Sweden |
| Gustafsson 1998 | Cohort study ‐ explored the effectiveness of prostate cancer screening in a randomly selected cohort of men in Sweden |
| Heijnsdijk 2009 | Results from the ERSPC, but reported outcomes are not relevant to the systematic review |
| Hoedemaeker 1999 | Cohort study ‐ explored the prostate cancer characteristics in a cohort of the screening group of the Dutch arm of the ERSPC |
| Hoedemaeker 2001 | Cohort study ‐ explored the frequency of PIN in prostate biopsies within the Dutch arm of the ERSPC |
| Horinaga 2007 | Cohort study ‐ diagnostic study |
| Hosseini 2007 | Cohort study ‐ mass screening of a cohort of men |
| Imamura 2008 | Economic evaluation and review |
| Janes 2008 | Cohort study ‐ investigating epidemiological properties of screening |
| Jegu 2009 | Results only from one arm of the ERSPC study |
| Johansen 2008 | Cohort study ‐ examination of hormone treatment and prostate cancer survival |
| Kawamura 2008 | Cohort study ‐ development of a nomogram for PSA testing |
| Kerfoot 2008 | RCT ‐ study of teaching materials |
| Kerfoot 2009 | RCT ‐ exploring patient education on prostate cancer screening |
| Kerkhof 2010 | Results only from one arm of the ERSPC study |
| Kerns 2008 | RCT ‐ study of patient education materials |
| Khatami 2007 | Preliminary results from the ERSPC study |
| Khatami 2009 | Cohort study ‐ exploring PSA doubling time |
| Kiemeny 2008 | Cohort study ‐ exploring family history of prostate cancer |
| Kilpeläinen 2010 | Results only from one arm of the ERSPC study |
| Klotz 2008 | Narrative review |
| Kramer 2009 | Clinical guideline on prostate cancer treatment |
| Kramer 2009b | Clinical guideline on prostate cancer treatment |
| Kripalani 2007 | RCT ‐ exploring patient education materials |
| Krist 2007 | RCT ‐ exploring physician‐patient discussion |
| Labrie 1992 | Cohort study ‐ explored the effectiveness of prostate cancer screening in a randomly selected cohort of men in Quebec, Canada |
| Labrie 1996 | Cohort study ‐ explored the stage and grade of prostate cancer in men within the screening arm of the Quebec study |
| Laurila 2010 | Results are available in the main article of ERSPC study |
| Leitzmann 2008 | Reports outcomes other than prostate cancer from the PLCO study |
| Leoni 2008 | Cohort study ‐ estimating measures of PSA testing |
| Lim 2008 | Position statement |
| Lin 2006 | Narrative review |
| Lin 2008 | Guideline for prostate cancer screening |
| Lobel 2007 | Narrative review |
| Lodding 1998 | Cohort study ‐ explored the biopsy characteristics in a cohort of the screening group from the Swedish arm of the ERSPC |
| Lucia 2008 | Provided additional information for the PLCO study |
| Lujan 2004 | Cohort study ‐ explored the detection rates and clinical characteristics of cancers in a cohort of the screening group from the Spanish arm of the ERSPC |
| Makinen 2001 | Cohort study ‐ explored the detection rate in a cohort of the screening group from the Finnish arm of the ERSPC |
| Makinen 2002 | Cohort study ‐ explored the association between family history and diagnosis of prostate cancer in the screening group from the Finnish arm of the ERSPC |
| Makinen 2004 | Cohort study ‐ reported intermediate screening efficacy indicators within the Finnish arm of th ERSPC |
| Mao 2007 | RCT ‐ exploring prostate cancer treatments |
| Marcella 2008 | Case control study |
| Meeks 2008 | Cohort study ‐ exploring PSA velocity |
| Mitterberger 2007 | RCT ‐ exploring ultrasound detection in prostate cancer patients |
| Määttänen 1999 | Cohort study ‐ explored the detection rate in a cohort of the screening group from the Finnish arm of the ERSPC |
| Määttänen 2001 | Descriptive study ‐ report on the preliminary results of the Finnish arm of the ERSPC |
| Määttänen 2007 | Preliminary results of the ERSPC |
| Nanri 2007 | Cohort study |
| Nelen 2010 | Results from the ERSPC, but reported outcomes are not relevant to the systematic review |
| Norming 1991 | Cohort study ‐ explored the effectiveness of prostate cancer screening in a randomly selected cohort of men in Sweden |
| Otto 2003 | ERSPC ‐ explored the extent of opportunistic screening in the Dutch arm of the ERSPC |
| Otto 2010 | Results are available in the main article of ERSPC study |
| Pedersen 1990 | Cohort study ‐ explored the effectiveness of prostate cancer screening in a randomly selected cohort of men in Sweden |
| Pienta 2009 | A narrative review |
| Pinksy 2007 | Associated study with the PLCO study |
| Pinsky 2010 | Associated study with the PLCO study |
| Postma 2004 | Cohort study ‐ explored the incidence and circumstances of advanced prostate cancer in a cohort of the screening group from the Dutch arm of the ERSPC |
| Postma 2007 | Preliminary results from the ERSPC |
| Prorok 1994 | Descriptive study ‐ report on the design of the PLCO |
| Raaijmakers 2004a | Cohort study ‐ explored the cancer detection rate of men in a cohort from the screening group from the Dutch arm of the ERSPC |
| Raaijmakers 2004b | Cohort study ‐ explored the indicators of prostate cancer from changes in PSA characteristics in a cohort from the screening group from the Dutch arm of the ERSPC |
| Raaijmakers 2007 | Cohort study associated with the ERSPC |
| Rauscher 2008 | Review |
| Recker 2001 | Cohort study ‐ explored the cancer detection rate of men in a cohort from the screening group from the Swiss arm of the ERSPC |
| Richard 2009 | Commentary on the ERSPC study |
| Rietbergen 1997a | Cohort study ‐ explored the cancer detection rates and characteristics within the screening group from the Dutch arm of the ERSPC |
| Rietbergen 1997b | Cohort study ‐ explored the risk factors associated with performing a biopsy in a cohort of the screening group from the Dutch arm of the ERSPC |
| Rietbergen 1998a | Cohort study ‐ explored the discriminating potential of the PSA test in a cohort of the screening group from the Dutch arm of the ERSPC |
| Rietbergen 1998b | Cohort study ‐ explored the yield of serial screening in a cohort of the screening group from the Dutch arm of the ERSPC |
| Rietbergen 1999 | Preliminary results of the ERSPC exploring the cancer detection rate and clinical features of men in the screening group from the Dutch arm of the ERSPC to the incidental cases in a region where no screening was performed |
| Roemeling 2007 | Study associated with the ERSPC study |
| Roemeling 2007b | Study associated with the ERSPC study |
| Roemeling 2007c | Study associated with the ERSPC study |
| Romero 2008 | Cohort study ‐ exploring patient perception of DRE |
| Roobol 2004 | Cohort study ‐ explored possible predictors of prostate cancer in the screening group from the Dutch arm of the ERSPC |
| Roobol 2007 | Study associated with the ERSPC study |
| Roobol 2007b | Study associated with the ERSPC study |
| Roobol 2009 | Results from the ERSPC, but reported outcomes are not relevant to the systematic review |
| Rosser 2008 | Editorial |
| Scattoni 2008 | Descriptive study relating to ERSPC |
| Schröder 1995 | Descriptive study ‐ report on the pilot studies of the ERSPC |
| Schröder 1996 | Dutch pilot studies to establish the feasibility of the ERSPC |
| Schröder 1997 | Descriptive study ‐ report on the design and features of the ERSPC |
| Schröder 1998 | Cohort study ‐ explored the value of the DRE as a stand alone test in the screening group from the Dutch arm of the ERSPC |
| Schröder 1999 | Descriptive study ‐ report on the design and features of the ERSPC |
| Schröder 2000 | Cohort study ‐ explored the diagnostic value of tests in a cohort of the screening group from the Dutch arm of the ERSPC |
| Schröder 2001a | Cohort study ‐ explored the diagnostic value of tests in a cohort of the screening group from the Dutch arm of the ERSPC |
| Schröder 2001b | Descriptive study ‐ commentary on the issue of screening for prostate cancer |
| Schröder 2005a | Cohort study ‐ explored the PSA progression within a specified period of screened men |
| Schröder 2008 | Interim findings of the ERSPC study |
| Schröder 2008b | Associated findings from the ERSPC study |
| Schröder 2009 | Narrative review |
| Schröder 2009b | Reporting on a cohort of men from the ERSPC |
| Shteynshlyuger 2011 | Study on cost‐effectiveness related to PLCO ‐ but does not provide results related to objectives of this systematic review |
| Sieverding 2008 | Cohort study ‐ survey results from a cohort of participants undergoing screening |
| Sotelo 2007 | Cohort study ‐ exploring assays for PSA testing |
| Stamatiou 2008 | RCT ‐ relating to patient education |
| Standaert 1997 | Descriptive study ‐ report on the progress of the ERSPC |
| Stephens 2008 | Cohort study ‐ exploring patient education on prostate cancer screening |
| Steyerberg 2007 | Cohort study ‐ exploring use of nomograms |
| Taha 2005 | Cohort study ‐ explored the effectiveness of prostate cancer screening in a randomly selected cohort of men in Saudi Arabia |
| Tarhan 2007 | Cohort study ‐ exploring PSA measurement |
| Thompson 2007 | RCT ‐ associated with the Prostate Cancer Prevention Trial |
| Thompson 2008 | Cohort study ‐ exploring the performance of PSA testing in the Prostate Cancer Prevention Trial |
| Thompson 2008b | Cohort study ‐ exploring the effects of finasteride |
| Tornblom 2001 | Cohort study ‐ explored the correlation between PSA measurement to prostate cancer in a randomly selected cohort of men screened for prostate cancer in Sweden |
| Tornblom 2004 | Cohort study ‐ explored the lead time for prostate cancer detection in Sweden |
| Torres Zambrano 2007 | Preliminary results from the ERSPC study |
| USPSTF 2008 | Guideline and recommendation for prostate cancer screening |
| van den Bergh 2008 | RCT ‐ results from the Prostate Cancer Prevention Trial |
| van Leeuwen 2012 | Results from the ERSPC, but reported outcomes are not relevant to the systematic review |
| van Weerden 2008 | Narrative review |
| Varenhorst 1989 | Cohort study ‐ explored the effectiveness of prostate cancer screening in a randomly selected cohort of men in Sweden. Pilot results for the Norrkoping study. No data is given on the controls. |
| Varenhorst 1991 | Cohort study ‐ explored the effectiveness of prostate cancer screening in a randomly selected cohort of men in Sweden. Pilot results for the Norrkoping study. No data is given on the controls. |
| Verratti 2007 | Cohort study |
| Vickers 2008 | Cohort study ‐ associated findings from the ERSPC study |
| Villers 2008 | RCT ‐ results from the Prostate Cancer Prevention Trial |
| Vis 2001a | Cohort study ‐ explored the categorization of cancer in a select cohort of men from the screening group of the Dutch arm of the ERSPC |
| Vis 2001b | Cohort study ‐ explored the value of differing screening protocols within the ERSPC |
| Vis 2002 | Cohort study ‐ explored the magnitude of prostate cancer detection by serendipity within the Dutch arm of the ERSPC |
| Vis 2007 | Study associated with the ERSPC study |
| Wallner 2008 | Cohort study ‐ reporting psychosocial factors in screening |
| Weinrich 2007 | RCT ‐ exploring patient education materials |
| Weiss 2008 | Case control study nested in the PLCO study |
| Wilbur 2008 | Narrative review |
| Wilt 2008 | Systematic review on five alpha reductase |
| Wolters 2008 | Cohort study ‐ additional information from the ERSPC study |
| Wolters 2010 | Results from the ERSPC, but reported outcomes are not relevant to the systematic review |
| Yang 2008 | Cohort study ‐ exploring prostate cancer treatment |
| Yasunaga 2008 | Cohort study ‐ survey of men regarding screening issues |
| Zackrisson 2003 | Cohort study ‐ explored the value of serial screening in a cohort of the screening group from the Swedish arm of the ERSPC |
| Zackrisson 2004 | Cohort study ‐ explored the clinical and pathological cancer characteristics within the Swedish arm of the ERSPC |
| Zhu 2011 | Results from the Rotterdam arm of the ERSPC |
Characteristics of ongoing studies [ordered by study ID]
CAP.
| Trial name or title | Comparison Arm for ProtecT (CAP) |
| Methods | The CAP study cluster‐randomised primary care (general practice) centres located in and around eight United Kingdom (UK) cities. |
| Participants | Participants are men 50 to 69 years of age without diagnosed prostate cancer attending one of the over 550 cluster‐randomised primary care centres. Estimated enrolment: Intervention arm – 225,000 men Comparison arm – 225,000 men |
| Interventions | Intervention group receives a single round of PSA testing (total PSA threshold ≥ 3.0 ng/mL) as part of the ProtecT study. Comparison group receives the UK National Health Service Prostate Cancer Risk Management advice. |
| Outcomes | Primary outcomes are “prostate cancer or intervention‐related specific mortality at an average of 10 years following randomization.” The study is also evaluating prostate cancer diagnosis, death, and clinical and resource use data. |
| Starting date | 2002. |
| Contact information | J.A Lane (athene.lane@bristol.ac.uk). |
| Notes | “Findings for both trials (CAP and ProtecT) regarding prostate cancer‐specific mortality will be published in around 2016.” |
Differences between protocol and review
Assessment for study risk of bias has been performed using the Cochrane Collaboration's risk‐of‐bias tool in this review. The GRADE framework has been applied in this review to assess the quality of the evidence as reported in the summary of findings table.
Contributions of authors
Dragan Ilic initiated the review and wrote the initial protocol. He conducted the literature search, reviewed abstracts and full‐text studies for inclusion, performed quality assessment, data extraction, analysis, and writing of the review.
Molly M Neuberger performed data extraction, quality assessment, analysis, and reviewed the full‐text of the review.
Mia Djulbegovic contributed to the literature search, reviewed abstracts for inclusion, performed data extraction and quality assessment.
Philipp Dahm co‐initiated the review update, reviewed abstracts and full‐text studies for inclusion, performed quality assessment, and contributed to the writing of the review.
Sources of support
Internal sources
Department of Urology, College of Medicine, University of Florida, USA.
Malcom Randall Veterans Affairs Medical Center, Gainesville, Florida, USA.
External sources
Dennis W. Jahnigen Career Development Scholars Award by the American Geriatrics Society, USA.
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
ERSPC {published and unpublished data}
- Carlsson SV, Holmberg E, Moss SM, Roobol MJ, Schröder FH, Tammela TLJ, et al. No excess mortality after prostate biopsy: results from the European Randomized Study of Screening for Prostate Cancer. BJU International 2011;107(12):1912‐7. [DOI] [PubMed] [Google Scholar]
- Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, et al. Mortality results from the Göteborg randomised population‐based prostate‐cancer screening trial. Lancet Oncology 2010;11(8):725‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpeläinen TP, Auvinen A, Määttänen L, Kujala P, Ruutu M, Stenman U‐H, et al. Results of the three rounds of the Finnish Prostate Cancer Screening Trial ‐ the incidence of advanced cancer is decreased by screening. International Journal of Cancer 2010;127(7):1699‐705. [DOI] [PubMed] [Google Scholar]
- Kilpeläinen TP, Tammela TLJ, Roobol M, Hugosson J, Ciatto S, Nelen V, et al. False‐positive screening results in the European randomized study of screening for prostate cancer. European Journal of Cancer 2011;47(18):2698‐705. [DOI] [PubMed] [Google Scholar]
- Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, et al. Prostate‐cancer mortality at 11 years of follow‐up. The New England Journal of Medicine 2012;366(11):981‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, et al. Screening and prostate‐cancer mortality in a randomized European study. The New England Journal of Medicine 2009;360(13):1320‐8. [DOI] [PubMed] [Google Scholar]
- Zhu X, Leeuwen PJ, Bul M, Bangma CH, Roobol MJ, Schröder FH. Identifying and characterizing "escapes" ‐ men who develop metastases or die from prostate cancer despite screening (ERSPC, section Rotterdam). International Journal of Cancer 2011;129(12):2847‐54. [DOI] [PubMed] [Google Scholar]
Norrkoping {published and unpublished data}
- Carlsson P, Pedersen KV, Varenhorst E. Costs and benefits of early detection of prostatic cancer. Health Policy 1990;16:241‐53. [DOI] [PubMed] [Google Scholar]
- Sandblom G, Varenhorst E, Lofman O, Rosell J, Carlsson P. Clinical consequences of screening for prostate cancer: 15 years follow‐up of a randomised controlled trial in Sweden. European Urology 2004;46:717‐24. [DOI] [PubMed] [Google Scholar]
- Sandblom G, Varenhorst E, Rosell J, Lofman O, Carlsson P. Randomised prostate cancer screening trial: 20 year follow‐up. BMJ 2011;342:d1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varenhorst E, Carlsson P, Capik E, Lofman O, Pedersen K. Repeated screening for carcinoma of the prostate by digital rectal examination in a randomly selected population. Acta Oncologica 1992;31(8):815‐21. [DOI] [PubMed] [Google Scholar]
PLCO {published and unpublished data}
- Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al. Mortality results from a randomized prostate‐cancer screening trial. The New England Journal of Medicine 2009;360(13):1310‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andriole GL, Crawford ED, Grubb RL 3rd, Buys SS, Chia D, Church TR, et al. Prostate cancer screening in the randomized Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial: mortality results after 13 years of follow‐up. Journal of the National Cancer Institute 2012;104(2):125‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford ED, Grubb R 3rd, Black A, Andriole GL Jr, Chen M‐H, Izmirlian G, et al. Comorbidity and mortality results from a randomized prostate cancer screening trial. Journal of Clinical Oncology 2011;29(4):355‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croswell JM, Kramer BS, Kreimer AR, Prorok PC, Xu J‐L, Baker SG, et al. Cumulative incidence of false‐positive results in repeated, multimodal cancer screening. Annals of Family Medicine 2009;7(3):212‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prorok PC, Andriole GL, Bresalier RS, Buys SS, Chia D, Crawford ED, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Controlled Clinical Trials 2000;21 (6 Suppl):273S‐309S. [DOI] [PubMed] [Google Scholar]
Quebec {published data only (unpublished sought but not used)}
- Labrie F, Candas B, Cusan L, Gomez JL, Belanger A, Brousseau G, et al. 11‐year follow‐up of the 1988 Quebec prospective randomized controlled trial. Prostate 2004;59(3):311‐8. [DOI] [PubMed] [Google Scholar]
- Labrie F, Candas B, Dupont A, Cusan L, Gomez JL, Suburu R, et al. Screening decreases prostate cancer death: first analysis of the 1988 Quebec prospective randomized controlled trial. Prostate 1999;38(2):83‐91. [DOI] [PubMed] [Google Scholar]
- Labrie F, Cusan L, Gomez JL, Levesque J, Candas B. Screening and treatment of localized prostate cancer decreases mortality: first analysis of the first prospective and randomized study on prostate cancer screening. The Aging Male 1999;2(1):33‐43. [Google Scholar]
Stockholm {published data only}
- Kjellman A, Akre O, Norming U, Tornblom M, Gustafsson O. 15‐Year follow up of a population based prostate cancer screening study. The Journal of Urology 2009;181:1615‐21. [DOI] [PubMed] [Google Scholar]
- Kjellman A, Akre O, Norming U, Tornblom M, Gustafsson O. Dihydrotestosterone levels and survival in screening‐detected prostate cancer: A 15‐yr follow‐up study. European Urology 2007;53:106‐11. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agalliu 2007 {published data only}
- Agalliu I, Weiss NS, Lin DW, Stanford JL. Prostate cancer mortality in relation to screening by prostate‐specific antigen testing and digital rectal examination: a population‐based study in middle‐aged men. Cancer Causes & Control 2007;18(9):931‐7. [DOI] [PubMed] [Google Scholar]
Ahmed 2008 {published data only}
- Ahmed FS, Borrell LN, Spencer BA. Health risk behaviors and prostate specific antigen awareness among men in California. The Journal of Urology 2008;180(2):658‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 2000 {published data only}
- Anonymous. From the Centers for Disease Control and Prevention. Screening with the prostate‐specific antigen test ‐ Texas, 1997. JAMA 2000;284(18):2313‐4. [PubMed] [Google Scholar]
Anonymous 2008 {published data only}
- Anonymous. Prostate cancer screening. Medical Letter on Drugs & Therapeutics 2008;50:85‐6. [PubMed] [Google Scholar]
Aus 2001 {published data only}
- Aus G, Bergdahl S, Hugosson J, Lodding P, Pihl CG, Pileblad E. Outcome of laterally directed sextant biopsies of the prostate in screened males aged 50 ‐ 66 years. Implications for sampling order. European Urology 2001;39(6):655‐60. [DOI] [PubMed] [Google Scholar]
Aus 2004 {published data only}
- Aus G, Becker C, Franzen S, Lilja H, Lodding P, Hugosson J. Cumulative prostate cancer risk assessment with the aid of the free‐to‐total prostate specific antigen ratio. European Urology 2004;45(2):160‐5. [DOI] [PubMed] [Google Scholar]
Aus 2005 {published data only}
- Aus G, Damber J‐E, Khatami A, Lilja H, Stranne J, Hugosson J. Individualized screening interval for prostate cancer based on prostate‐specific antigen level: results of a prospective, randomized, population‐based study. Archives of Internal Medicine 2005;165(16):1857‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Aus 2007 {published data only}
- Aus G, Bergdahl S, Lodding P, Lilja H, Hugosson J. Prostate cancer screening decreases the absolute risk of being diagnosed with advanced prostate cancer‐‐results from a prospective, population‐based randomized controlled trial. European Urology 2007;51(3):659‐64. [DOI] [PubMed] [Google Scholar]
Auvinen 1996 {published data only}
- Auvinen A, Tammela T, Stenman U‐H, Uusi‐Erkkilä I, Leinonen J, Schröder FH, et al. Screening for prostate cancer using serum prostate‐specific antigen: a randomised, population‐based pilot study in Finland. British Journal of Cancer 1996;74(4):568‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Auvinen 2009 {published data only}
- Auvinen A, Raitanen J, Moss S, Koning HJ, Hugosson J, Tammela T, et al. Test sensitivity in the European prostate cancer screening trial: results from Finland, Sweden, and the Netherlands. Cancer Epidemiology, Biomarkers & Prevention 2009;18(7):2000‐5. [DOI] [PubMed] [Google Scholar]
Bangma 1995a {published data only}
- Bangma CH, Grobbee DE, Schröder FH. Volume adjustment for intermediate prostate‐specific antigen values in a screening population. European Journal of Cancer 1995;31A(1):12‐4. [DOI] [PubMed] [Google Scholar]
Bangma 1995b {published data only}
- Bangma CH, Kranse R, Blijenberg BG, Schröder FH. The value of screening tests in the detection of prostate cancer. Part II: Retrospective analysis of free/total prostate‐specific analysis ratio, age‐specific reference ranges, and PSA density. Urology 1995;46(6):779‐84. [DOI] [PubMed] [Google Scholar]
Bangma 1995c {published data only}
- Bangma CH, Kranse R, Blijenberg BG, Schröder FH. The value of screening tests in the detection of prostate cancer. Part I: Results of a retrospective evaluation of 1726 men. Urology 1995;46(6):773‐8. [DOI] [PubMed] [Google Scholar]
Bangma 1997 {published data only}
- Bangma CH, Rietbergen JB, Schröder FH. Prostate‐specific antigen as a screening test. The Netherlands experience. Urologic Clinics of North America 1997;24(2):307‐14. [DOI] [PubMed] [Google Scholar]
Beemsterboer 1999 {published data only}
- Beemsterboer PMM, Kranse R, Koning HJ, Habbema JDF, Schröder FH. Changing role of 3 screening modalities in the European Randomized Study of Screening for Prostate Cancer (Rotterdam). International Journal of Cancer 1999;84(4):437‐41. [DOI] [PubMed] [Google Scholar]
Beemsterboer 2000 {published data only}
- Beemsterboer PMM, Koning HJ, Kranse R, Trienekens PH, Maas PJ, Schröder FH. Prostate specific antigen testing and digital rectal examination before and during a randomized trial of screening for prostate cancer: European Randomized Study of Screening for Prostate Cancer, Rotterdam. The Journal of Urology 2000;164(4):1216‐20. [PubMed] [Google Scholar]
Bergstralh 2007 {published data only}
- Bergstralh EJ, Roberts RO, Farmer SA, Slezak JM, Lieber MM, Jacobsen SJ. Population‐based case‐control study of PSA and DRE screening on prostate cancer mortality. Urology 2007;70(5):936‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boevee 2010 {published data only}
- Boevee SJ, Venderbos LDF, Tammela TLJ, Nelen V, Ciatto S, Kwiatkowski M, et al. Change of tumour characteristics and treatment over time in both arms of the European Randomized study of Screening for Prostate Cancer. European Journal of Cancer 2010;46(17):3082‐9. [DOI] [PubMed] [Google Scholar]
Borre 2007 {published data only}
- Borre M, Iversen P. Screening for prostate cancer‐what does the evidence show?. Ugeskrift for Laeger 2007;169:1887‐8. [PubMed] [Google Scholar]
Bul 2011 {published data only}
- Bul M, Leeuwen PJ, Zhu X, Schröder FH, Roobol MJ. Prostate cancer incidence and disease‐specific survival of men with initial prostate‐specific antigen less than 3.0 ng/ml who are participating in ERSPC Rotterdam. European Urology 2011;59(4):498‐505. [DOI] [PubMed] [Google Scholar]
Bunker 2007 {published data only}
- Bunker CH, McDonald AC, Evans RW, Rosa N, Boumosleh JM, Patrick AL. A randomized trial of lycopene supplementation in Tobago men with high prostate cancer risk. Nutrition and Cancer 2007;57:130‐7. [DOI] [PubMed] [Google Scholar]
Candas 2000 {published data only}
- Candas B, Cusan L, Gomez JL, Diamond P, Suburu RE, Levesque J, et al. Evaluation of prostatic specific antigen and digital rectal examination as screening tests for prostate cancer. Prostate 2000;45(1):19‐35. [DOI] [PubMed] [Google Scholar]
Carlsson 2007 {published data only}
- Carlsson S, Aus G, Wessman C, Hugosson J. Anxiety associated with prostate cancer screening with special reference to men with a positive screening test (elevated PSA) ‐ Results from a prospective, population‐based, randomised study. European Journal of Cancer 2007;43:2109‐16. [DOI] [PubMed] [Google Scholar]
Carlsson 2011 {published data only}
- Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, Stranne J, et al. The excess burden of side‐effects from treatment in men allocated to screening for prostate cancer. The Göteborg randomised population‐based prostate cancer screening trial. European Journal of Cancer 2011;47(4):545‐53. [DOI] [PubMed] [Google Scholar]
Carriere 2007 {published data only}
- Carriere P, Baade P, Newman B, Aitken J, Janda M. Cancer screening in Queensland men. Medical Journal of Australia 2007;186:404‐7. [DOI] [PubMed] [Google Scholar]
Chavarro 2008 {published data only}
- Chavarro JE, Stampfer MJ, Campos H, Kurth T, Willett WC, Ma J. A prospective study of trans‐fatty acid levels in blood and risk of prostate cancer. Cancer Epidemiology, Biomarkers & Prevention 2008;17:95‐101. [DOI] [PubMed] [Google Scholar]
Ciatto 1993 {published data only}
- Ciatto S, Bonardi R, Mazzotta A, Lombardi C, Zappa M. Clinico‐echographic screening in the early diagnosis of prostatic carcinoma. Preliminary results of a feasibility study of a randomized trial. La Radiologia Medica 1993;85(4):430‐3. [PubMed] [Google Scholar]
Ciatto 1994 {published data only}
- Ciatto S, Bonardi R, Mazzotta A, Lombardi C, Santoni R, Cardini S, et al. Comparison between 2 techniques of screening for prostatic carcinoma. Rectal exploration and transrectal ultrasonography vs. prostate specific antigen. La Radiologia Medica 1994;88(4):453‐7. [PubMed] [Google Scholar]
Ciatto 2002 {published data only}
- Ciatto S, Bonardi R, Lombardi C, Zappa M, Gervasi G, Cappelli G. Analysis of PSA velocity in 1666 healthy subjects undergoing total PSA determination at two consecutive screening rounds. International Journal of Biological Markers 2002;17(2):79‐83. [DOI] [PubMed] [Google Scholar]
Ciatto 2003b {published data only}
- Ciatto S, Zappa M, Villers A, Paez A, Otto SJ, Auvinen A. Contamination by opportunistic screening in the European Randomized Study of Prostate Cancer Screening. BJU International 2003;2:97‐100. [DOI] [PubMed] [Google Scholar]
Ciatto 2008 {published data only}
- Ciatto S, Rubeca T, Martinelli F, Pontenani G, Lombardi C, Lollo S. PSA doubling time as a predictor of the outcome of random prostate biopsies prompted by isolated PSA elevation in subjects referred to an outpatient biopsy facility in a routine clinical scenario. International Journal of Biological Markers 2008;23:187‐91. [DOI] [PubMed] [Google Scholar]
Collin 2008 {published data only}
- Collin SM, Martin RM, Metcalfe C, Gunnell D, Albertsen PC, Neal D, et al. Prostate‐cancer mortality in the USA and UK in 1975‐2004: an ecological study. Lancet Oncology 2008;9:445‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Concato 2009 {published data only}
- Concato J. What will the emperor say? Screening for prostate cancer as of 2008. Cancer Journal 2009;15:7‐12. [DOI] [PubMed] [Google Scholar]
Cusan 1994 {published data only}
- Cusan L. Prostate cancer screening with PSA, DRE and TRUS. Canadian Journal of Oncology 1994;1:63‐4. [PubMed] [Google Scholar]
D'Amico 2007 {published data only}
- D'Amico AV, Roehrborn CG. Effect of 1 mg/day finasteride on concentrations of serum prostate‐specific antigen in men with androgenic alopecia: a randomised controlled trial. Lancet Oncology 2007;8:21‐5. [DOI] [PubMed] [Google Scholar]
de Koning 2002 {published data only}
- Koning HJ, Auvinen A, Berenguer Sanchez A, Calais da Silva F, Ciatto S, Denis L, et al. Large‐scale randomized prostate cancer screening trials: program performances in the European Randomized Screening for Prostate Cancer trial and the Prostate, Lung, Colorectal and Ovary cancer trial. International Journal of Cancer 2002;97(2):237‐44. [DOI] [PubMed] [Google Scholar]
Döbrőssy 2007 {published data only}
- Döbrőssy L, Kovács A, Budai A, Cornides A. Is the mass screening for prostate cancer justifiable? [Indokolt‐e a népegészségügyi prosztataszűrés?]. Orvosi Hetilap 2007;148(26):1213‐6. [DOI] [PubMed] [Google Scholar]
Draisma 2009 {published data only}
- Draisma G, Etzioni R, Tsodikov A, Mariotto A, Wever E, Gulati R, et al. Lead time and over diagnosis in prostate‐specific antigen screening: importance of methods and context. Journal of the National Cancer Institute 2009;101:374‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Driscoll 2008 {published data only}
- Driscoll DL, Rupert DJ, Golin CE, McCormack LA, Sheridan SL, Welch BM, et al. Promoting prostate‐specific antigen informed decision‐making. Evaluating two community‐level interventions. American Journal of Preventive Medicine 2008;35:87‐94. [DOI] [PubMed] [Google Scholar]
Ellison 2008 {published data only}
- Ellison GL, Weinrich SP, Lou M, Xu H, Powell IJ, Baquet CR. A randomized trial comparing web‐based decision aids on prostate cancer knowledge for African‐American men. Journal of the National Medical Association 2008;100:1139‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Essink‐Bot 1998 {published data only}
- Essink‐Bot M‐L, Koning HJ, Nijs HGT, Kirkels WJ, Maas PJ, Schröder FH. Short‐term effects of population‐based screening for prostate cancer on health‐related quality of life. Journal of the National Cancer Institute 1998;90(12):925‐31. [DOI] [PubMed] [Google Scholar]
Etzioni 2008 {published data only}
- Etzioni R. Statistical issues in the evaluation of screening and early detection modalities. Urologic Oncology 2008;26:308‐15. [DOI] [PubMed] [Google Scholar]
Fenton 2008 {published data only}
- Fenton JJ, Franks P, Reid RJ, Elmore JG, Baldwin LM. Continuity of care and cancer screening among health plan enrollees. Medical Care 2008;46:58‐62. [DOI] [PubMed] [Google Scholar]
Finne 2002 {published data only}
- Finne P, Auvinen A, Aro J, Juusela H, Maattanen L, Rannikko S, et al. Estimation of prostate cancer risk on the basis of total and free prostate‐specific antigen, prostate volume and digital rectal examination. European Urology 2002;41(6):619‐26. [DOI] [PubMed] [Google Scholar]
Finne 2010 {published data only}
- Finne P, Fallah M, Hakama M, Ciatto S, Hugosson J, Koning H, et al. Lead‐time in the European Randomised Study of Screening for Prostate Cancer. European Journal of Cancer 2010;46(17):3102‐8. [DOI] [PubMed] [Google Scholar]
Fitzpatrick 2009 {published data only}
- Fitzpatrick JM, Banu E, Oudard S. Prostate‐specific antigen kinetics in localized and advanced prostate cancer. BJU International 2009;103:578‐87. [DOI] [PubMed] [Google Scholar]
Fleshner 2007 {published data only}
- Fleshner N, Gomella LG, Cookson MS, Finelli A, Evans A, Taneja SS, et al. Delay in the progression of low‐risk prostate cancer: rationale and design of the Reduction by Dutasteride of Clinical Progression Events in Expectant Management (REDEEM) trial. Contemporary Clinical Trials 2007;28:763‐9. [DOI] [PubMed] [Google Scholar]
Fleshner 2009 {published data only}
- Fleshner NE, Lawrentschuk N. Risk of developing prostate cancer in the future: overview of prognostic biomarkers. Urology 2009;73(Suppl 5A):S21‐7. [DOI] [PubMed] [Google Scholar]
Ford 2003 {published data only}
- Ford ME, Havstad SL, Tilley BC. Recruiting older African American men to a cancer screening trial (the AAMEN Project). Gerontologist 2003;43(1):27‐35. [DOI] [PubMed] [Google Scholar]
Ford 2008 {published data only}
- Ford ME, Havstad SL, Fields ME, Manigo B, McClary B, Lamerato L. Effects of baseline comorbidities on cancer screening trial adherence among older African American men. Cancer Epidemiology, Biomarkers & Prevention 2008;17:1234‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frosch 2008 {published data only}
- Frosch DL, Bhatnagar V, Tally S, Hamori CJ, Kaplan RM. Internet patient decision support: a randomized controlled trial comparing alternative approaches for men considering prostate cancer screening. Archives of Internal Medicine 2008;168:363‐9. [DOI] [PubMed] [Google Scholar]
Gohagan 1994a {published data only}
- Gohagan JK, Prorok PC, Kramer BS, Cornett JE. Prostate cancer screening in the prostate, lung, colorectal and ovarian cancer screening trial of the National Cancer Institute. The Journal of Urology 1994;152(5 Pt 2):1905‐9. [DOI] [PubMed] [Google Scholar]
Gohagan 1994b {published data only}
- Gohagan JK, Kramer BS, Greenwald P. Screening for prostate cancer. American Journal of Preventive Medicine 1994;10(4):245‐6. [PubMed] [Google Scholar]
Gohagan 1995 {published data only}
- Gohagan JK, Prorok PC, Kramer BS, Hayes RB, Cornett J. The Prostate, Lung, Colorectal, and Ovarian cancer screening trial of the National Cancer Institute. Cancer 1995;75(7 Suppl):1869‐73. [Google Scholar]
Gohagan 2000 {published data only}
- Gohagan JK, Prorok PC, Hayes RB, Kramer BS, for the PLCO Project Team. The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Controlled Clinical Trials 2000;21(6 Suppl 1):251S‐72S. [DOI] [PubMed] [Google Scholar]
Gonzalgo 2007 {published data only}
- Gonzalgo ML, Carter HB. Update on PSA testing. Journal of the National Comprehensive Cancer Network 2007;5:737‐42. [DOI] [PubMed] [Google Scholar]
Gosselaar 2008 {published data only}
- Gosselaar C, Kranse R, Roobol MJ, Roemeling S, Schröder FH. The interobserver variability of digital rectal examination in a large randomized trial for the screening of prostate cancer. Prostate 2008;68:985‐93. [DOI] [PubMed] [Google Scholar]
Gosselaar 2008b {published data only}
- Gosselaar C, Roobol MJ, Roemeling S, Wolters T, Leenders GJ, Schröder FH. The value of an additional hypoechoic lesion‐directed biopsy core for detecting prostate cancer. BJU International 2008;101:685‐90. [DOI] [PubMed] [Google Scholar]
Grosclaude 2008 {published data only}
- Grosclaude P. Prostate cancer: an update on screening. Bulletin de l'Acadmie Nationale de Medicine 2008;192:1013‐8. [PubMed] [Google Scholar]
Grubb 2008 {published data only}
- Grubb RL 3rd, Pinsky PF, Greenlee RT, Izmirlian G, Miller AB, Hickey TP, et al. Prostate cancer screening in the Prostate, Lung, Colorectal and Ovarian cancer screening trial: update on findings from the initial four rounds of screening in a randomized trial. BJU International 2008;102:1524‐30. [DOI] [PubMed] [Google Scholar]
Grubb 2009 {published data only}
- Grubb RL, Black A, Izmirlian G, Hickey TP, Pinsky PF, Mabie JE, et al. Serum prostate‐specific antigen hemodilution among obese men undergoing screening in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Epidemiology, Biomarkers & Prevention 2009;18:748‐51. [DOI] [PubMed] [Google Scholar]
Gustafsson 1992 {published data only}
- Gustafsson O, Norming U, Almgard LE, Fredriksson A, Gustavsson G, Harvig B, et al. Diagnostic methods in the detection of prostate cancer: a study of a randomly selected population of 2,400 men. The Journal of Urology 1992;148(6):1827‐31. [DOI] [PubMed] [Google Scholar]
Gustafsson 1995 {published data only}
- Gustafsson O, Carlsson P, Norming U, Nyman CR, Svensson H. Cost‐effectiveness analysis in early detection of prostate cancer: an evaluation of six screening strategies in a randomly selected population of 2,400 men. Prostate 1995;26(6):299‐309. [DOI] [PubMed] [Google Scholar]
Gustafsson 1998 {published data only}
- Gustafsson O, Mansour E, Norming U, Carlsson A, Tornblom M, Nyman CR. Prostate‐specific antigen (PSA), PSA density and age‐adjusted PSA reference values in screening for prostate cancer ‐ a study of a randomly selected population of 2,400 men. Scandinavian Journal of Urology and Nephrology 1998;32(6):373‐7. [DOI] [PubMed] [Google Scholar]
Heijnsdijk 2009 {published data only}
- Heijnsdijk E, Kinderen A, Wever E, Draisma G, Roobol M, Koning H. Overdetection, overtreatment and costs in prostate‐specific antigen screening for prostate cancer. British Journal of Cancer 2009;101:1833‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoedemaeker 1999 {published data only}
- Hoedemaeker RF, Kranse R, Rietbergen JB, Kruger AE, Schroder FH, Kwast TH. Evaluation of prostate needle biopsies in a population‐based screening study: the impact of borderline lesions. Cancer 1999;85(1):145‐52. [PubMed] [Google Scholar]
Hoedemaeker 2001 {published data only}
- Hoedemaeker RF, Kwast TH, Boer R, Koning HJ, Roobol M, Vis AN, et al. Pathologic features of prostate cancer found at population‐based screening with a four‐year interval. Journal of the National Cancer Institute 2001;93(15):1153‐8. [DOI] [PubMed] [Google Scholar]
Horinaga 2007 {published data only}
- Horinaga M, Kitamura K, Saito S, Ukimura O, Nakanoma T, Okihara K, et al. Prostate cancer screening with prostate‐specific antigen in hemodialysis patients. Urologia Internationalis 2007;78:334‐7. [DOI] [PubMed] [Google Scholar]
Hosseini 2007 {published data only}
- Hosseini SY, Moharramzadeh M, Ghadian AR, Hooshyar H, Lashay AR, Safarinejad MR. Population‐based screening for prostate cancer by measuring total serum prostate‐specific antigen in Iran. International Journal of Urology 2007;14:406‐11. [DOI] [PubMed] [Google Scholar]
Imamura 2008 {published data only}
- Imamura T, Yasunaga H. Economic evaluation of prostate cancer screening with prostate‐specific antigen. International Journal of Urology 2008;15:285‐8. [DOI] [PubMed] [Google Scholar]
Janes 2008 {published data only}
- Janes H, Pepe MS. Adjusting for covariates in studies of diagnostic, screening, or prognostic markers: an old concept in a new setting. American Journal of Epidemiology 2008;168:89‐97. [DOI] [PubMed] [Google Scholar]
Jegu 2009 {published data only}
- Jegu J, Tretarre B, Grosclaude P, Rebillard X, Bataille V, Malavaud B, et al. Results and participation factors to the European Randomized study of Screening for Prostate Cancer (ERSPC) with Prostate Specific Antigen: French departments of Tarn and Herault [État des lieux et facteurs de participation à l’étude européenne ERSPC de dépistage randomisé du cancer de la prostate par dosage sérique de l’antigène prostatique spécifique : départements français du Tarn et de l’Hérault]. Progrès en Urologie 2009;19(7):487‐98. [DOI] [PubMed] [Google Scholar]
Johansen 2008 {published data only}
- Johansen TE, Berg C. Hormone treatment of prostate cancer in Norway [Hormonbehandling av prostatakreft i Norge]. Tidsskrift for Den Norske Laegeforening 2008;128(22):2558‐62. [PubMed] [Google Scholar]
Kawamura 2008 {published data only}
- Kawamura K, Suzuki H, Kamiya N, Imamoto T, Yano M, Miura J, et al. Development of a new nomogram for predicting the probability of a positive initial prostate biopsy in Japanese patients with serum PSA levels less than 10 ng/mL. International Journal of Urology 2008;15:598‐603. [DOI] [PubMed] [Google Scholar]
Kerfoot 2008 {published data only}
- Kerfoot BP. Interactive spaced education versus web based modules for teaching urology to medical students: a randomized controlled trial. The Journal of Urology 2008;179:2356‐7. [DOI] [PubMed] [Google Scholar]
Kerfoot 2009 {published data only}
- Kerfoot BP, Brotschi E. Online spaced education to teach urology to medical students: a multi‐institutional randomized trial. American Journal of Surgery 2009;197:89‐95. [DOI] [PubMed] [Google Scholar]
Kerkhof 2010 {published data only}
- Kerkhof M, Roobol MJ, Cuzick J, Sasieni P, Roemeling S, Schröder FH, et al. Effect of the correction for noncompliance and contamination on the estimated reduction of metastatic prostate cancer within a randomized screening trial (ERSPC section Rotterdam). International Journal of Cancer 2010;127(11):2639‐44. [DOI] [PubMed] [Google Scholar]
Kerns 2008 {published data only}
- Kerns JW, Krist AH, Woolf SH, Flores SK, Johnson RE. Patient perceptions of how physicians communicate during prostate cancer screening discussions: a comparison of residents and faculty. Family Medicine 2008;40:181‐7. [PubMed] [Google Scholar]
Khatami 2007 {published data only}
- Khatami A, Aus G, Damber JE, Lilja H, Lodding P, Hugosson J. PSA doubling time predicts the outcome after active surveillance in screening‐detected prostate cancer: results from the European randomized study of screening for prostate cancer, Sweden section. International Journal of Cancer 2007;120:170‐4. [DOI] [PubMed] [Google Scholar]
Khatami 2009 {published data only}
- Khatami A, Hugosson J, Wang W, Damber JE. Ki‐67 in screen‐detected, low‐grade, low‐stage prostate cancer, relation to prostate‐specific antigen doubling time, Gleason score and prostate‐specific antigen relapse after radical prostatectomy. Scandinavian Journal of Urology and Nephrology 2009;43(1):12‐8. [DOI] [PubMed] [Google Scholar]
Kiemeny 2008 {published data only}
- Kiemeney LA, Broeders MJ, Pelger M, Kil PJ, Schröder FH, Witjes JA, et al. Screening for prostate cancer in Dutch hereditary prostate cancer families. International Journal of Cancer 2008;122:871‐6. [DOI] [PubMed] [Google Scholar]
Kilpeläinen 2010 {published data only}
- Kilpeläinen TP, Tammela TLJ, Määttänen L, Kujala P, Stenman U‐H, Ala‐Opas M, et al. False‐positive screening results in the Finnish prostate cancer screening trial. British Journal of Cancer 2010;102(3):469‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Klotz 2008 {published data only}
- Klotz L. Active surveillance for prostate cancer: trials and tribulations. World Journal of Urology 2008;26:437‐42. [DOI] [PubMed] [Google Scholar]
Kramer 2009 {published data only}
- Kramer BS, Hagerty KL, Justman S, Somerfield MR, Albertsen PC, Blot WJ, et al. Use of 5alpha‐reductase inhibitors for prostate cancer chemoprevention: American Society of Clinical Oncology/American Urological Association 2008 Clinical Practice Guideline. The Journal of Urology 2009;181:1642‐57. [DOI] [PubMed] [Google Scholar]
Kramer 2009b {published data only}
- Kramer BS, Hagerty KL, Justman S, Somerfield MR, Albertsen PC, Blot WJ, et al. Use of 5‐alpha‐reductase inhibitors for prostate cancer chemoprevention: American Society of Clinical Oncology/American Urological Association 2008 Clinical Practice Guideline. Journal of Clinical Oncology 2009;27:1502‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kripalani 2007 {published data only}
- Kripalani S, Sharma J, Justice E, Justice J, Spiker C, Laufman LE, et al. Low‐literacy interventions to promote discussion of prostate cancer: a randomized controlled trial. American Journal of Preventive Medicine 2007;33:83‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krist 2007 {published data only}
- Krist AH, Woolf SH, Johnson RE. How physicians approach prostate cancer screening before and after losing a lawsuit. Annals of Family Medicine 2007;5:120‐5. [PMC free article] [PubMed] [Google Scholar]
Labrie 1992 {published data only}
- Labrie F, Dupont A, Suburu R, Cusan L, Tremblay M, Gomez JL, et al. Serum prostate specific antigen as pre‐screening test for prostate cancer. The Journal of Urology 1992;147(3 Pt 2):846‐51. [DOI] [PubMed] [Google Scholar]
Labrie 1996 {published data only}
- Labrie F, Candas B, Cusan L, Gomez JL, Diamond P, Suburu R, et al. Diagnosis of advanced or noncurable prostate cancer can be practically eliminated by prostate‐specific antigen. Urology 1996;47(2):212‐7. [DOI] [PubMed] [Google Scholar]
Laurila 2010 {published data only}
- Laurila M, Kwast T, Bubendorf L, Lollo S, Pihl C‐G, Ciatto S, et al. Detection rates of cancer, high grade PIN and atypical lesions suspicious for cancer in the European Randomized Study of Screening for Prostate Cancer. European Journal of Cancer 2010;46(17):3068‐72. [DOI] [PubMed] [Google Scholar]
Leitzmann 2008 {published data only}
- Leitzmann MF, Ahn J, Albanes D, Hsing AW, Schatzkin A, Chang SC, et al. Diabetes mellitus and prostate cancer risk in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Cancer Causes & Control 2008;19:1267‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leoni 2008 {published data only}
- Leoni M, Falcini F, Ravaioli A, Foca F, Andalo G, Benini F, et al. Estimating standard performance measures of opportunistic screening for prostate cancer [Stima di misure di processo standard dell’attività spontanea di screening per il cancro prostatico]. Epidemiologia e Prevenzione 2008;32(6):285‐93. [PubMed] [Google Scholar]
Lim 2008 {published data only}
- Lim LS, Sherin K, ACPM Prevention Practice Committee. Screening for prostate cancer in U.S. men: ACPM position statement on preventive practice. American Journal of Preventive Medicine 2008;34(2):164‐70. [DOI] [PubMed] [Google Scholar]
Lin 2006 {published data only}
- Lin AM, Small EJ. Prostate cancer update: 2006. Current Opinion in Oncology 2006;19:229‐33. [DOI] [PubMed] [Google Scholar]
Lin 2008 {published data only}
- Lin K, Lipsitz R, Miller T, Janakiraman S, U.S. Preventive Services Task Force. Benefits and harms of prostate‐specific antigen screening for prostate cancer: an evidence update for the U.S. Preventive Services Task Force. Annals of Internal Medicine 2008;149:192‐9. [DOI] [PubMed] [Google Scholar]
Lobel 2007 {published data only}
- Lobel B. Does localized prostate cancer exist?. Recent Results in Cancer Research 2007;175:101‐7. [DOI] [PubMed] [Google Scholar]
Lodding 1998 {published data only}
- Lodding P, Aus G, Bergdahl S, Frosing R, Lilja H, Pihl CG, et al. Characteristics of screening detected prostate cancer in men 50 to 66 years old with 3 to 4 ng/ml. Prostate specific antigen. The Journal of Urology 1998;159(3):899‐903. [PubMed] [Google Scholar]
Lucia 2008 {published data only}
- Lucia MS, Darke AK, Goodman PJ, Rosa FG, Parnes HL, Ford LG, et al. Pathologic characteristics of cancers detected in The Prostate Cancer Prevention Trial: implications for prostate cancer detection and chemoprevention. Cancer Prevention Research 2008;1:167‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lujan 2004 {published data only}
- Lujan M, Paez A, Miravalles E, Fernandez I, Llanes L, Berenguer A. Prostate cancer detection is also relevant in low prostate specific antigen ranges. European Urology 2004;45(2):155‐9. [DOI] [PubMed] [Google Scholar]
Määttänen 1999 {published data only}
- Määttänen L, Auvinen A, Stenman U‐H, Rannikko S, Tammela T, Aro J, et al. European randomized study of prostate cancer screening: first‐year results of the Finnish trial. British Journal of Cancer 1999;79(7‐8):1210‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Määttänen 2001 {published data only}
- Määttänen L, Auvinen A, Stenman U‐H, Tammela T, Rannikko S, Aro J, et al. Three‐year results of the Finnish prostate cancer screening trial. Journal of the National Cancer Institute 2001;93(7):552‐3. [DOI] [PubMed] [Google Scholar]
Määttänen 2007 {published data only}
- Määttänen L, Hakama M, Tammela TLJ, Ruutu M, Ala‐Opas M, Juusela H, et al. Specificity of serum prostate‐specific antigen determination in the Finnish prostate cancer screening trial. British Journal of Cancer 2007;96:56‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Makinen 2001 {published data only}
- Makinen T, Tammela TL, Hakama M, Stenman UH, Rannikko S, Aro J, et al. Prostate cancer screening within a prostate specific antigen range of 3 to 3.9 ng./ml.: a comparison of digital rectal examination and free prostate specific antigen as supplemental screening tests. The Journal of Urology 2001;166(4):1339‐42. [PubMed] [Google Scholar]
Makinen 2002 {published data only}
- Makinen T, Tammela TL, Stenman UH, Maattanen L, Rannikko S, Aro J, et al. Family history and prostate cancer screening with prostate‐specific antigen. Journal of Clinical Oncology 2002;20(11):2658‐63. [DOI] [PubMed] [Google Scholar]
Makinen 2004 {published data only}
- Makinen T, Tammela T, Stenman U, Maattanen L, Aro J, Juusela H, et al. Second round results of the Finnish population based prostate cancer screening trial. Clinical Cancer Research 2004;10:2231‐6. [DOI] [PubMed] [Google Scholar]
Mao 2007 {published data only}
- Mao S, Daliani DD, Wang X, Thall PF, Do KA, Perez CA, et al. Employing the treatment‐free interval of intermittent androgen ablation to screen candidate prostate cancer therapies. Prostate 2007;67:1677‐85. [DOI] [PubMed] [Google Scholar]
Marcella 2008 {published data only}
- Marcella SW, Rhoads GG, Carson JL, Merlino F, Wilcox H. Prostate‐specific antigen screening and mortality from prostate cancer. Journal of General Internal Medicine 2008;23:248‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meeks 2008 {published data only}
- Meeks JJ, Thaxton CS, Loeb S, Roehl KA, Helfand BT, Catalona WJ. Comparison of prostate specific antigen velocity in screened versus referred patients with prostate cancer. The Journal of Urology 2008;179:1340‐3. [DOI] [PubMed] [Google Scholar]
Mitterberger 2007 {published data only}
- Mitterberger M, Horninger W, Pelzer A, Strasser H, Bartsch G, Moser P, et al. A prospective randomized trial comparing contrast‐enhanced targeted versus systematic ultrasound guided biopsies: impact on prostate cancer detection. Prostate 2007;67:1537‐42. [DOI] [PubMed] [Google Scholar]
Nanri 2007 {published data only}
- Nanri M, Nanri K, Fujiyama C, Tokuda Y, Nakamura K, Uozumi J. Prostate‐specific antigen assay using whole blood samples spotted on filter paper and its application to mass screening for prostate cancer. International Journal of Urology 2007;14:505‐9. [DOI] [PubMed] [Google Scholar]
Nelen 2010 {published data only}
- Nelen V, Thys G, Hermans A, D'Hooge K, Dourcy‐Belle‐Rose B, Coebergh J‐W, et al. Interval cancers in the Antwerp European randomised study of screening for prostate cancer study, using a 6 year screening interval. European Journal of Cancer 2010;46(17):3090‐4. [DOI] [PubMed] [Google Scholar]
Norming 1991 {published data only}
- Norming U, Gustafsson O, Nyman CR, Almgard L, Fredriksson A, Gustafsson G, et al. Digital rectal examination versus transrectal ultrasound in detection of prostate cancer. Preliminary results from a study of a randomly selected population. Acta Oncologica 1991;30(2):277‐9. [DOI] [PubMed] [Google Scholar]
Otto 2003 {published data only}
- Otto SJ, Cruijsen IW, Liem MK, Korfage IJ, Lous JJ, Schröder FH, et al. Effective PSA contamination in the Rotterdam section of the European Randomized Study of Screening for Prostate Cancer. International Journal of Cancer 2003;105(3):394‐9. [DOI] [PubMed] [Google Scholar]
Otto 2010 {published data only}
- Otto SJ, Moss SM, Määttänen L, Roobol M, Zappa M, Nelen V, et al. PSA levels and cancer detection rate by centre in the European Randomized Study of Screening for Prostate Cancer. European Journal of Cancer 2010;46(17):3053‐60. [DOI] [PubMed] [Google Scholar]
Pedersen 1990 {published data only}
- Pedersen KV, Carlsson P, Varenhorst E, Lofman O, Berglund K. Screening for carcinoma of the prostate by digital rectal examination in a randomly selected population. BMJ 1990;300(6731):1041‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pienta 2009 {published data only}
- Pienta KJ. Critical appraisal of prostate‐specific antigen in prostate cancer screening: 20 years later. Urology 2009;73(5 Suppl):S11‐20. [DOI] [PubMed] [Google Scholar]
Pinksy 2007 {published data only}
- Pinsky PF, Crawford ED, Kramer BS, Andriole GL, Gelmann EP, Grubb R, et al. Repeat prostate biopsy in the Prostate, Lung, Colorectal and Ovarian cancer screening trial. BJU International 2007;99(4):775‐9. [DOI] [PubMed] [Google Scholar]
Pinsky 2010 {published data only}
- Pinsky P, Blacka A, Kramer B, Miller A, Prorok P, Berg C. Assessing contamination and compliance in the prostate component of the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial. Clinical Trials 2010;7:303‐11. [DOI] [PubMed] [Google Scholar]
Postma 2004 {published data only}
- Postma R, Roobol M, Schröder FH, Kwast TH. Potentially advanced malignancies detected by screening for prostate carcinoma after an interval of 4 years. Cancer 2004;100(5):968‐75. [DOI] [PubMed] [Google Scholar]
Postma 2007 {published data only}
- Postma R, Schröder FH, Leenders GJ, Hoedemaeker RF, Vis AN, Roobol MJ, et al. Cancer detection and cancer characteristics in the European Randomized Study of Screening for Prostate Cancer (ERSPC) ‐ Section Rotterdam. A comparison of two rounds of screening. European Urology 2007;52:89‐97. [DOI] [PubMed] [Google Scholar]
Prorok 1994 {published data only}
- Prorok P. The National Cancer Institute Multi‐Screening Trial. Canadian Journal of Oncology 1994;1:98‐9. [PubMed] [Google Scholar]
Raaijmakers 2004a {published data only}
- Raaijmakers R, Blijenberg BG, Finlay JA, Rittenhouse HG, Wildhagen MF, Roobol MJ, et al. Prostate cancer detection in the prostate specific antigen range of 2.0 to 3.9 ng/ml: value of percent free prostate specific antigen on tumor detection and tumor aggressiveness. The Journal of Urology 2004;171(6 Pt 1):2245‐9. [DOI] [PubMed] [Google Scholar]
Raaijmakers 2004b {published data only}
- Raaijmakers R, Wildhagen MF, Ito K, Paez A, Vries SH, Roobol MJ, et al. Prostate‐specific antigen change in the European Randomized Study of Screening for Prostate Cancer, section Rotterdam. Urology 2004;63(2):316‐20. [DOI] [PubMed] [Google Scholar]
Raaijmakers 2007 {published data only}
- Raaijmakers R, Vries SH, Blijenberg BG, Wildhagen MF, Postma R, Bangma CH, et al. hK2 and free PSA, a prognostic combination in predicting minimal prostate cancer in screen‐detected men within the PSA range 4‐10 ng/ml. European Urology 2007;52:1358‐64. [DOI] [PubMed] [Google Scholar]
Rauscher 2008 {published data only}
- Rauscher GH, Johnson TP, Cho YI, Walk JA. Accuracy of self‐reported cancer‐screening histories: a meta‐analysis. Cancer Epdemiology, Biomarkers & Prevention 2008;17:748‐57. [DOI] [PubMed] [Google Scholar]
Recker 2001 {published data only}
- Recker F, Kwiatkowski MK, Huber A, Stamm B, Lehmann K, Tscholl R. Prospective detection of clinically relevant prostate cancer in the prostate specific antigen range 1 to 3 ng./ml. combined with free‐to‐total ratio 20% or less: the Aarau experience. The Journal of Urology 2001;166(3):851‐5. [PubMed] [Google Scholar]
Richard 2009 {published data only}
- Richard F. Screening for prostate cancer: results from the first screening round in the French population of the ERSPC trial [Dépistage du cancer de la prostate : les données du premier tour de dépistage de la population française participant à l’essai européen ERSPC]. Progrès en Urologie 2009;19(7):499‐500. [DOI] [PubMed] [Google Scholar]
Rietbergen 1997a {published data only}
- Rietbergen JBW, Kranse R, Kirkels WJ, Koning HJ, Schröder FH. Evaluation of prostate‐specific antigen, digital rectal examination and transrectal ultrasonography in population‐based screening for prostate cancer: improving the efficiency of early detection. British Journal of Urology 1997;79 Suppl 2:57‐63. [DOI] [PubMed] [Google Scholar]
Rietbergen 1997b {published data only}
- Rietbergen JB, Kruger AE, Kranse R, Schröder FH. Complications of transrectal ultrasound‐guided systematic sextant biopsies of the prostate: evaluation of complication rates and risk factors within a population‐based screening program. Urology 1997;49(6):875‐80. [DOI] [PubMed] [Google Scholar]
Rietbergen 1998a {published data only}
- Rietbergen JB, Kranse R, Hoedemaeker RF, Kruger AE, Bangma CH, Kirkels WJ, et al. Comparison of prostate‐specific antigen corrected for total prostate volume and transition zone volume in a population‐based screening study. Urology 1998;52(2):237‐46. [DOI] [PubMed] [Google Scholar]
Rietbergen 1998b {published data only}
- Rietbergen JB, Kruger AE, Hoedemaeker RF, Bangma CH, Kirkels WJ, Schröder FH. Repeat screening for prostate cancer after 1‐year followup in 984 biopsied men: clinical and pathological features of detected cancer. The Journal of Urology 1998;160(6 Pt 1):2121‐5. [DOI] [PubMed] [Google Scholar]
Rietbergen 1999 {published data only}
- Rietbergen JB, Hoedemaeker RF, Kruger AE, Kirkels WJ, Schröder FH. The changing pattern of prostate cancer at the time of diagnosis: characteristics of screen detected prostate cancer in a population based screening study. The Journal of Urology 1999;161(4):1192‐8. [PubMed] [Google Scholar]
Roemeling 2007 {published data only}
- Roemeling S, Roobol MJ, Kattan MW, Kwast TH, Steyerberg EW, Schröder FH. Nomogram use for the prediction of indolent prostate cancer: impact on screen‐detected populations. Cancer 2007;110:2218‐21. [DOI] [PubMed] [Google Scholar]
Roemeling 2007b {published data only}
- Roemeling S, Roobol MJ, Otto SJ, Habbema DF, Gosselaar C, Lous JJ, et al. Feasibility study of adjustment for contamination and non‐compliance in a prostate cancer screening trial. Prostate 2007;67:1053‐60. [DOI] [PubMed] [Google Scholar]
Roemeling 2007c {published data only}
- Roemeling S, Roobol MJ, Vries SH, Wolters T, Gosselaar C, Leenders GJ, et al. Active surveillance for prostate cancers detected in three subsequent rounds of a screening trial: characteristics, PSA doubling times, and outcome. European Urology 2007;51:1244‐50. [DOI] [PubMed] [Google Scholar]
Romero 2008 {published data only}
- Romero FR, Romero AW, Brenny Filho T, Bark NM, Yamazaki DS, Oliveira FC. Patients' perceptions of pain and discomfort during digital rectal exam for prostate cancer screening. Archivos Españoles de Urología 2008;61(7):850‐4. [DOI] [PubMed] [Google Scholar]
Roobol 2004 {published data only}
- Roobol MJ, Kranse R, Koning HJ, Schröder FH. Prostate‐specific antigen velocity at low prostate‐specific antigen levels as screening tool for prostate cancer: results of second screening round of ERSPC (Rotterdam). Urology 2004;63(2):309‐13. [DOI] [PubMed] [Google Scholar]
Roobol 2007 {published data only}
- Roobol MJ, Grenabo A, Schröder FH, Hugosson J. Interval cancers in prostate cancer screening: comparing 2‐ and 4‐year screening intervals in the European Randomized Study of Screening for Prostate Cancer, Gothenburg and Rotterdam. Journal of the National Cancer Institute 2007;99:1296‐303. [DOI] [PubMed] [Google Scholar]
Roobol 2007b {published data only}
- Roobol MJ, Zappa M, Maattanen L, Ciatto S. The value of different screening tests in predicting prostate biopsy outcome in screening for prostate cancer data from a multicenter study (ERSPC). Prostate 2007;67:439‐46. [DOI] [PubMed] [Google Scholar]
Roobol 2009 {published data only}
- Roobol MJ, Kerkhof M, Schröder FH, Cuzick J, Sasieni P, Hakama M, et al. Prostate cancer mortality reduction by prostate‐specific antigen‐based screening adjusted for nonattendance and contamination in the European Randomised Study of Screening for Prostate Cancer (ERSPC). European Urology 2009;56(4):584‐91. [DOI] [PubMed] [Google Scholar]
Rosser 2008 {published data only}
- Rosser CJ. Prostate cancer ‐ to screen, or not to screen, is that the question?. BMC Urology 2008;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Scattoni 2008 {published data only}
- Scattoni V. Words of wisdom. Re: Is prostate‐specific antigen velocity selective for clinically significant prostate cancer in screening? European randomized study of screening for prostate cancer (Rotterdam). European Urology 2008;54:945‐6. [DOI] [PubMed] [Google Scholar]
Schröder 1995 {published data only}
- Schröder FH, Denis LJ, Kirkels W, Koning HJ, Standaert B. European randomized study of screening for prostate cancer. Progress report of Antwerp and Rotterdam pilot studies. Cancer 1995;76(1):129‐34. [DOI] [PubMed] [Google Scholar]
Schröder 1996 {published data only}
- Schröder FH, Damhuis RAM, Kirkels WJ, Koning HJ, Kranse R, Nus HGT, et al. European Randomized Study of Screening for Prostate Cancer ‐ the Rotterdam pilot studies. International Journal of Cancer 1996;65(2):145‐51. [DOI] [PubMed] [Google Scholar]
Schröder 1997 {published data only}
- Schröder FH, Bangma CH. The European Randomized Study of Screening for Prostate Cancer (ERSPC). British Journal of Urology 1997;1:68‐71. [DOI] [PubMed] [Google Scholar]
Schröder 1998 {published data only}
- Schröder FH, Maas P, Beemsterboer P, Kruger AB, Hoedemaeker R, Rietbergen J, et al. Evaluation of the digital rectal examination as a screening test for prostate cancer. Rotterdam section of the European Randomized Study of Screening for Prostate Cancer. Journal of the National Cancer Institute 1998;90(23):1817‐23. [DOI] [PubMed] [Google Scholar]
Schröder 1999 {published data only}
- Schröder FH, Kranse R, Rietbergen J, Hoedemaeke R, Kirkels W. The European Randomized Study of Screening for Prostate Cancer (ERSPC): an update. Members of the ERSPC, Section Rotterdam. European Urology 1999;35(5‐6):539‐43. [DOI] [PubMed] [Google Scholar]
Schröder 2000 {published data only}
- Schröder FH, Cruijsen‐Koeter I, Koning HJ, Vis AN, Hoedemaeker RF, Kranse R. Prostate cancer detection at low prostate specific antigen. The Journal of Urology 2000;163(3):806‐12. [PubMed] [Google Scholar]
Schröder 2001a {published data only}
- Schröder FH, Roobol‐Bouts M, Vis AN, Kwast T, Kranse R. Prostate‐specific antigen‐based early detection of prostate cancer ‐ validation of screening without rectal examination. Urology 2001;57(1):83‐90. [DOI] [PubMed] [Google Scholar]
Schröder 2001b {published data only}
- Schröder FH, Wildhagen MF, Rotterdam Study Group of the 'European Randomised Study of Screening for Prostate Cancer' (ERSPC). Screening for prostate cancer: evidence and perspectives. BJU International 2001;88(8):811‐7. [DOI] [PubMed] [Google Scholar]
Schröder 2005a {published data only}
- Schröder F, Raaijmakers R, Postma R, Kwast T, Roobol M. 4 year prostate specific antigen progression and diagnosis of prostate cancer in the European Randomized Study of Screening for Prostate Cancer, section Rotterdam. The Journal of Urology 2005;174:489‐94. [DOI] [PubMed] [Google Scholar]
Schröder 2008 {published data only}
- Schröder F. Screening for prostate cancer (PC) ‐ an update on recent findings of the European Randomized Study of Screening for Prostate Cancer (ERSPC). Urologic Oncology 2008;26:533‐41. [DOI] [PubMed] [Google Scholar]
Schröder 2008b {published data only}
- Schröder FH, Bangma CH, Roobol MJ. Is it necessary to detect all prostate cancers in men with serum PSA levels <3.0 ng/ml? A comparison of biopsy results of PCPT and outcome‐related information from ERSPC. European Urology 2008;53:901‐8. [DOI] [PubMed] [Google Scholar]
Schröder 2009 {published data only}
- Schröder FH. Review of diagnostic markers for prostate cancer. Recent Results in Cancer Research 2009;181:173‐82. [DOI] [PubMed] [Google Scholar]
Schröder 2009b {published data only}
- Schröder FH, Roobol MJ, Andriole GL, Fleshner N. Defining increased future risk for prostate cancer: evidence from a population based screening cohort. The Journal of Urology 2009;181:69‐74. [DOI] [PubMed] [Google Scholar]
Shteynshlyuger 2011 {published data only}
- Shteynshlyuger A, Andriole GL. Cost‐effectiveness of prostate specific antigen screening in the United States: extrapolating from the European study of screening for prostate cancer. The Journal of Urology 2011;185(3):828‐32. [DOI] [PubMed] [Google Scholar]
Sieverding 2008 {published data only}
- Sieverding M, Matterne U, Ciccarello L, Luboldt HJ. Early detection of prostate cancer in Germany. A study of a representative random sample of the population. Der Urologe 2008;47:1233‐8. [DOI] [PubMed] [Google Scholar]
Sotelo 2007 {published data only}
- Sotelo RJ, Mora KE, Perez LH, Novoa J, Carmona O, Andrade R, et al. Assay standardization bias: different prostate cancer detection rates and clinical outcomes resulting from different assays for free and total prostate‐specific antigen. Urology 2007;69:1143‐6. [DOI] [PubMed] [Google Scholar]
Stamatiou 2008 {published data only}
- Stamatiou K, Skolarikos A, Heretis I, Papadimitriou V, Alevizos A, Ilias G, et al. Does educational printed material manage to change compliance with prostate cancer screening?. World Journal of Urology 2008;26:365‐73. [DOI] [PubMed] [Google Scholar]
Standaert 1997 {published data only}
- Standaert B, Denis L. The European Randomized Study of Screening for Prostate Cancer: an update. Cancer 1997;80(9):1830‐4. [PubMed] [Google Scholar]
Stephens 2008 {published data only}
- Stephens RL, Xu Y, Volk RJ, Scholl LE, Kamin SL, Holden EW, et al. Influence of a patient decision aid on decisional conflict related to PSA testing: a structural equation model. Health Psychology 2008;27:711‐21. [DOI] [PubMed] [Google Scholar]
Steyerberg 2007 {published data only}
- Steyerberg EW, Roobol MJ, Kattan MW, Kwast TH, Koning HJ, Schröder FH. Prediction of indolent prostate cancer: validation and updating of a prognostic nomogram. The Journal of Urology 2007;177:107‐12. [DOI] [PubMed] [Google Scholar]
Taha 2005 {published data only}
- Taha S, Kamal B. Screening program for prostate cancer at a university hospital in eastern Saudi Arabia. Saudi Medical Journal 2005;26:1104‐6. [PubMed] [Google Scholar]
Tarhan 2007 {published data only}
- Tarhan F, Orcun A, Kucukercan I, Camursoy N, Kuyumcuoglu U. Effect of hemodialysis on serum complexed prostate‐specific antigen levels. Scandinavian Journal of Urology and Nephrology 2007;41:382‐6. [DOI] [PubMed] [Google Scholar]
Thompson 2007 {published data only}
- Thompson IM, Tangen CM, Goodman PJ, Lucia MS, Parnes HL, Lippman SM, et al. Finasteride improves the sensitivity of digital rectal examination for prostate cancer detection. The Journal of Urology 2007;177:1749‐52. [DOI] [PubMed] [Google Scholar]
Thompson 2008 {published data only}
- Thompson IM, Tangen CM, Ankerst DP, Chi C, Lucia MS, Goodman P, et al. The performance of prostate specific antigen for predicting prostate cancer is maintained after a prior negative prostate biopsy. The Journal of Urology 2008;180:544‐7. [DOI] [PubMed] [Google Scholar]
Thompson 2008b {published data only}
- Thompson IM, Tangen CM, Parnes HL, Lippman SM, Coltman CA Jr. Does the level of prostate cancer risk affect cancer prevention with finasteride?. Urology 2008;71:854‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tornblom 2001 {published data only}
- Tornblom M, Norming U, Becker C, Lilja H, Gustafsson O. Variation in percentage‐free prostate‐specific antigen (PSA) with prostate volume, age and total PSA level. BJU International 2001;87(7):638‐42. [DOI] [PubMed] [Google Scholar]
Tornblom 2004 {published data only}
- Tornblom M, Eriksson H, Franzen S, Gustafsson O, Lilja H, Norming U, et al. Lead time associated with screening for prostate cancer. International Journal of Cancer 2004;108(1):122‐9. [DOI] [PubMed] [Google Scholar]
Torres Zambrano 2007 {published data only}
- Torres Zambrano G, Lujan Galán M, Pascual Mateo C, García Tello A, Rodríguez N, Berenguer Sánchez A. Preliminary data of the Spanish contribution to the European Randomized Study on Screening of Prostate Cancer (ERSPC) [Datos preliminares de la contribución Española al Estudio Randomizado Europeo de Screening del Cáncer de Próstata (ERSPC)]. Archivos Españoles de Urología 2007;60(7):737‐43. [DOI] [PubMed] [Google Scholar]
USPSTF 2008 {published data only}
- U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine 2008;149(3):185‐91. [DOI] [PubMed] [Google Scholar]
van den Bergh 2008 {published data only}
- Bergh RC, Roobol MJ, Wolters T, Leeuwen PJ, Schröder FH. The Prostate Cancer Prevention Trial and European Randomized Study of Screening for Prostate Cancer risk calculators indicating a positive prostate biopsy: a comparison. BJU International 2008;102:1068‐73. [DOI] [PubMed] [Google Scholar]
van Leeuwen 2012 {published data only}
- Leeuwen PJ, Roobol MJ, Kranse R, Zappa M, Carlsson S, Bul M, et al. Towards an optimal interval for prostate cancer screening. European Urology 2012;61(1):171‐6. [DOI] [PubMed] [Google Scholar]
van Weerden 2008 {published data only}
- Weerden WM, Schröder FH. The use of PSA as biomarker in nutritional intervention studies of prostate cancer. Chemico‐Biological Interactions 2008;171:204‐11. [DOI] [PubMed] [Google Scholar]
Varenhorst 1989 {published data only}
- Varenhorst E, Enlund AL, Herder A, Malmquist E, Berglund K, Löfman O, et al. Prostatic cancer screening by rectal palpation can be organized with consideration to cost effectiveness. Läkartidningen 1989;86(41):3475‐7. [PubMed] [Google Scholar]
Varenhorst 1991 {published data only}
- Varenhorst E, Pedersen KV, Carlsson P, Berglund K, Löfman O. Screening for carcinoma of the prostate in a randomly selected population using duplicate digital rectal examination. Acta Oncologica 1991;30(2):273‐5. [DOI] [PubMed] [Google Scholar]
Verratti 2007 {published data only}
- Verratti V, Giulio C, Francesco S, Berardinelli F, Pellicciotta M, Gidaro S, et al. Chronic hypoxia, physical exercise and PSA: correlation during high‐altitude trekking (2004 K2 expedition). Urologia Internationalis 2007;78:305‐7. [DOI] [PubMed] [Google Scholar]
Vickers 2008 {published data only}
- Vickers AJ, Cronin AM, Aus G, Pihl CG, Becker C, Pettersson K, et al. A panel of kallikrein markers can reduce unnecessary biopsy for prostate cancer: data from the European Randomized Study of Prostate Cancer Screening in Goteborg, Sweden. BMC Medicine 2008;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Villers 2008 {published data only}
- Villers A. Practical follow‐up of a patient treated with finasteride in screening for prostate cancer [Suivi pratique d’un patient traité par finastéride pour le dépistage de cancer de prostate]. Progrès en Urologie 2008;18 Suppl 3:S58‐62. [DOI] [PubMed] [Google Scholar]
Vis 2001a {published data only}
- Vis AN, Hoedemaeker RF, Kwast TH, Schröder FH. Defining the window of opportunity in screening for prostate cancer: validation of a predictive tumor classification model. Prostate 2001;46(2):154‐62. [DOI] [PubMed] [Google Scholar]
Vis 2001b {published data only}
- Vis AN, Hoedemaeker RF, Roobol M, Kwast TH, Schröder FH. Tumor characteristics in screening for prostate cancer with and without rectal examination as an initial screening test at low PSA (0.0‐3.9 ng/ml). Prostate 2001;47(4):252‐61. [DOI] [PubMed] [Google Scholar]
Vis 2002 {published data only}
- Vis AN, Kranse R, Roobol M, Kwast TH, Schröder FH. Serendipity in detecting disease in low prostate‐specific antigen ranges. BJU International 2002;89(4):384‐9. [DOI] [PubMed] [Google Scholar]
Vis 2007 {published data only}
- Vis AN, Roemeling S, Kranse R, Schröder FH, Kwast TH. Should we replace the Gleason score with the amount of high‐grade prostate cancer?. European Urology 2007;51:931‐9. [DOI] [PubMed] [Google Scholar]
Wallner 2008 {published data only}
- Wallner LP, Sarma AV, Lieber MM, Sauver JL, Jacobson DJ, McGree ME, et al. Psychosocial factors associated with an increased frequency of prostate cancer screening in men ages 40 to 79 years: the Olmsted County study. Cancer Epidemiology, Biomarkers & Prevention 2008;17:3588‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinrich 2007 {published data only}
- Weinrich SP, Seger R, Curtsinger T, Pumphrey G, NeSmith EG, Weinrich MC. Impact of pretest on posttest knowledge scores with a Solomon Four research design. Cancer Nursing 2007;30:E16‐28. [DOI] [PubMed] [Google Scholar]
Weiss 2008 {published data only}
- Weiss JM, Huang WY, Rinaldi S, Fears TR, Chatterjee N, Hsing AW, et al. Endogenous sex hormones and the risk of prostate cancer: a prospective study. International Journal of Cancer 2008;122:2345‐50. [DOI] [PubMed] [Google Scholar]
Wilbur 2008 {published data only}
- Wilbur J. Prostate cancer screening: the continuing controversy. American Family Physician 2008;78:1377‐84. [PubMed] [Google Scholar]
Wilt 2008 {published data only}
- Wilt TJ, MacDonald R, Hagerty K, Schellhammer P, Kramer BS. 5‐alpha‐reductase inhibitors for prostate cancer prevention. Cochrane Database of Systematic Reviews 2008, Issue 2. [DOI: 10.1002/14651858.CD007091] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolters 2008 {published data only}
- Wolters T, Roobol MJ, Schröder FH, Kwast TH, Roemeling S, Cruijsen‐Koeter IW, et al. Can non‐malignant biopsy features identify men at increased risk of biopsy‐detectable prostate cancer at re‐screening after 4 years?. BJU International 2008;101:283‐8. [DOI] [PubMed] [Google Scholar]
Wolters 2010 {published data only}
- Wolters T, Kwast TH, Vissers CJ, Bangma CH, Roobol M, Schröder FH, et al. False‐negative prostate needle biopsies: frequency, histopathologic features, and follow‐up. The American Journal of Surgical Pathology 2010;34(1):35‐43. [DOI] [PubMed] [Google Scholar]
Yang 2008 {published data only}
- Yang Y. Treatment of the positive surgical margin following radical prostatectomy. Chinese Medical Journal 2008;121(4):375‐9. [PubMed] [Google Scholar]
Yasunaga 2008 {published data only}
- Yasunaga H. Willingness to pay for mass screening for prostate cancer: a contingent valuation survey. International Journal of Urology 2008;15:102‐5. [DOI] [PubMed] [Google Scholar]
Zackrisson 2003 {published data only}
- Zackrisson B, Aus G, Lilja H, Lodding P, Pihl CG, Hugosson J. Follow‐up of men with elevated prostate‐specific antigen and one set of benign biopsies at prostate cancer screening. European Urology 2003;43(4):327‐32. [DOI] [PubMed] [Google Scholar]
Zackrisson 2004 {published data only}
- Zackrisson B, Aus G, Bergdahl S, Lilja H, Lodding P, Pihl CG, et al. The risk of finding focal cancer (less than 3 mm) remains high on re‐biopsy of patients with persistently increased prostate specific antigen but the clinical significance is questionable. The Journal of Urology 2004;171(4):1500‐3. [DOI] [PubMed] [Google Scholar]
Zhu 2011 {published data only}
- Zhu X, Leeuwen PJ, Bul M, Otto SJ, Koning HJ, Bangma CH, et al. Disease‐specific survival of men with prostate cancer detected during the screening interval: results of the European Randomized Study of Screening for Prostate Cancer ‐ Rotterdam after 11 years of follow‐up. European Urology 2011;60(2):330‐6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
CAP {published data only}
- Lane JA, Hamdy FC, Martin RM, Turner EL, Neal DE, Donovan JL. Latest results from the UK trials evaluating prostate cancer screening and treatment: the CAP and ProtecT studies. European Journal of Cancer 2010;46(17):3095‐101. [DOI] [PubMed] [Google Scholar]
- Pashayan N, Duffy SW, Pharoah P, Greenberg D, Donovan J, Martin RM, et al. Mean sojourn time, overdiagnosis, and reduction in advanced stage prostate cancer due to screening with PSA: implications of sojourn time on screening. British Journal of Cancer 2009;100:1198‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
ACS 2010
- Wolf AMD, Wender RC, Etzioni RB, Thompson IM, D'Amico AV, Volk RJ, et al. American Cancer Society guideline for the early detection of prostate cancer (update 2010). CA: A Cancer Journal for Clinicians 2010;60(2):70‐98. [DOI] [PubMed] [Google Scholar]
AUA 2009
- American Urological Association. Prostate‐specific antigen best practice statement: 2009 update. American Urological Association Education and Research, Inc.; 2009; http://www.auanet.org/content/media/psa09.pdf?CFID=1939643&CFTOKEN=68944383&jsessionid=84301df90d486270f1d9119276a176173111 (accessed 12/05/2010).
Bartsch 2001
- Bartsch G, Horninger W, Klocker H, Reissigl A, Oberaigner W, Schonitzer D, et al. Prostate cancer mortality after introduction of prostate‐specific antigen mass screening in the federal state of Tyrol, Austria. Urology 2001;58:417‐24. [DOI] [PubMed] [Google Scholar]
Berry 1984
- Berry S, Coffey D, Walsh P, Ewing L. The development of human benign prostatic hyperplasia with age. The Journal of Urology 1984;132:474‐9. [DOI] [PubMed] [Google Scholar]
Bill‐Axelson 2005
- Bill‐Axelson A, Holmberg L, Ruutu M, Haggman M, Andersson SO, Bratell S, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. The New England Journal of Medicine 2005;352:1977‐84. [DOI] [PubMed] [Google Scholar]
Bill‐Axelson 2008
- Bill‐Axelson A, Holmber L, Filen F, Ruutu M, Garmo H, Busch C, et al. Radical prostatectomy versus watchful waiting in localized prostate cancer: the Scandinavian Prostate Cancer Group‐4 Randomized Trial. Journal of the National Cancer Institute 2008;100:1144‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bill‐Axelson 2011
- Bill‐Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, et al. Radical prostatectomy versus watchful waiting in early prostate cancer. The New England Journal of Medicine 2011;364:1708‐17. [DOI] [PubMed] [Google Scholar]
Burford 2010
- Burford DC, Kirby M, Austoker J. Prostate Cancer Risk Management Programme information for primary care; PSA testing in asymptomatic men. Evidence document. NHS Cancer Screening Programmes; 2010 Jan.; http://www.cancerscreening.nhs.uk/prostate/pcrmp‐guide‐2.html (accessed 12/05/2010).
de Koning 2002b
- Koning HJ, Liem MK, Baan CA, Boer R, Schröder FH, Alexander FE. Prostate cancer mortality reduction by screening: power and time frame with complete enrollment in the European Randomised Screening for Prostate Cancer (ERSPC) trial. International Journal of Cancer 2002;98(2):268‐73. [DOI] [PubMed] [Google Scholar]
de Koning 2003
- Koning HJ, Hakulinen T, Moss SM, Adolfsson J, Smith PH, Alexanders FE, et al. Monitoring the ERSPC trial. BJU International 2003;92 Suppl 2:112‐4. [DOI] [PubMed] [Google Scholar]
Djulbegovic 2010
- Djulbegovic M, Beyth RJ, Neuberger MM, Stoffs TL, Vieweg J, Djulbegovic B, et al. Screening for prostate cancer: systematic review and meta‐analysis of randomised controlled trials. BMJ 2010;341:c4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
Draisma 2003
- Draisma G, Boer R, Otto SJ, Cruijsen IW, Damhuis RAM, Schröder F, et al. Lead time and over detection due to prostate‐specific antigen screening: Estimates from the European Rondomized Study of Screening for Prostate Cancer. Journal of the National Cancer Institute 2003;95(12):868‐78. [DOI] [PubMed] [Google Scholar]
EAU 2012
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, Mason MD, et al. Guidelines on prostate cancer. European Association of Urology; 2012 Feb.; http://www.uroweb.org/gls/pdf/08%20Prostate%20Cancer_LR%20March%2013th%202012.pdf (accessed 1 November 2012).
Gambert 2001
- Gambert SR. Screening for prostate cancer. International Urology and Nephrology 2001;33(2):249‐57. [DOI] [PubMed] [Google Scholar]
Grönberg 2003
- Grönberg P. Prostate cancer epidemiology. Lancet 2003;361(9360):859‐64. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Hamashima 2009
- Hamashima C, Nakayama T, Sagawa M, Saito H, Sobue T. The Japanese guideline for prostate cancer screening. Japanese Journal of Clinical Oncology 2009;39(6):339‐51. [DOI] [PubMed] [Google Scholar]
Heidenreich 2011
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, et al. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. European Urology 2011;59(1):61‐71. [DOI] [PubMed] [Google Scholar]
Heijnsdijk 2012
- Heijnsdijk EAM, Wever EM, Auvinen A, Hugosson J, Ciatto S, Nelen V, et al. Quality‐of‐life effects of prostate‐specific antigen screening. The New England Journal of Medicine 2012;367(7):595‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2005
- Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions 4.2.5. In: The Cochrane Library, Issue 2, 2005. Chichester, UK: John Wiley & Sons, Ltd, updated May 2005. [Google Scholar]
Higgins 2011
- Higgins J, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2011. [Google Scholar]
Holman 1999
- Holman C, Wisniewski Z, Semmens J, Rouse I, Bass A. Mortality and prostate cancer risk in 19598 men after surgery for benign prostatic hyperplasia. BJU International 1999;84:37‐42. [DOI] [PubMed] [Google Scholar]
Holmström 2009
- Holmström B, Johansson M, Bergh A, Stenman U‐H, Hallmans G, Stattin P. Prostate specific antigen for early detection of prostate cancer: longitudinal study. BMJ 2009;339:b3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howlader 2012
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Altekruse SF, et al. SEER Cancer Statistics Review, 1975‐2009 (Vintage 2009 Populations), National Cancer Institute, Bethesda, MD. http://seer.cancer.gov/csr/1975_2009_pops09/index.html (accessed 15 November 2012).
Jacobsen 1998
- Jacobsen SJ, Bergstralh EJ, Katusic SK, Guess HA, Darby CH, Silverstein MD, et al. Screening digital rectal examination and prostate cancer mortality: a population‐based case‐control study. Urology 1998;52:173‐9. [DOI] [PubMed] [Google Scholar]
Jemel 2011
- Jemel A, Bray F, Center M, Ferlay J, Ward E, Forman D. Global Cancer Statistics. CA Cancer Journal for Clinicians 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
Johansson 2009
- Johansson E, Bill‐Axelson A, Holmberg L, Onelov E, Johansson J, Steineck G. Time, symptom burden, androgen deprivation, and self‐assessed quality of life after radical prostatectomy or watchful waiting: The randomized Scandinavian Prostate Cancer Group Study Number 4 (SPCG‐4) clinical trial. European Urology 2009;55:422‐32. [DOI] [PubMed] [Google Scholar]
Jones 2010
- Jones C, Hill J, Chappele C. Management of lower urinary tract symptoms in men: summary of NICE guidance. BMJ 2010;340:c2354. [DOI] [PubMed] [Google Scholar]
JUA 2010
- The Committee for Establishment of the Guidelines on Screening for Prostate Cancer, Japanese Urological Association. Updated Japanese Urological Association Guidelines on prostate‐specific antigen‐based screening for prostate cancer in 2010. International Journal of Urology 2010;17(10):830‐8. [DOI] [PubMed] [Google Scholar]
Kopec 2005
- Kopec JA, Goel V, Bunting PS, Neuman J, Sayre EC, Warde P, et al. Screening with prostate specific antigen and metastatic prostate cancer risk: a population based case‐control study. The Journal of Urology 2005;174:495‐9. [DOI] [PubMed] [Google Scholar]
Lu‐Yao 2009
- Lu‐Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Outcomes of localized prostate cancer following conservative management. JAMA 2009;302(11):1202‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lumen 2012
- Lumen N, Fonteyne V, Meerleert G, Ost P, Villeirs G, Mottrie A, et al. Population screening for prostate cancer: An overview of available studies and meta‐analysis. International Journal of Urology 2012;19(2):100‐8. [DOI] [PubMed] [Google Scholar]
O'Connor 2009
- O’Connor A, Bennett C, Stacey D, Barry M, Col N, Eden K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD001431.pub2] [DOI] [PubMed] [Google Scholar]
Parkin 2005
- Parkin M, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians 2005;55(2):74‐108. [DOI] [PubMed] [Google Scholar]
Partin 1993
- Partin A, Yoo J, Carter H, Pearson J, Chan D, Epstein J, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. The Journal of Urology 1993;150:110‐4. [DOI] [PubMed] [Google Scholar]
Raaijmakers 2002
- Raaijmakers R, Kirkels WJ, Roobol MJ, Wildhagen MF, Schröder FH. Complication rates and risk factors of 5802 transrectal ultrasound‐guided sextant biopsies of the prostate within a population‐based screening program. Urology 2002;60(5):826‐30. [DOI] [PubMed] [Google Scholar]
RACGP 2012
- The Royal Australian College of General Practitioners. Guidelines for preventive activities in general practice, 8th edition. East Melbourne: The Royal Australian College of General Practitioners; 2012; http://www.racgp.org.au/your‐practice/guidelines/redbook/ (accessed 1 November 2012).
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rosario 2012
- Rosario DJ, Lane JA, Metcalfe C, Donovan JL, Doble A, Goodwin L, et al. Short term outcomes of prostate biopsy in men tested for cancer by prostate specific antigen: prospective evaluation within ProtecT study. BMJ 2012;344:d7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sakr 1996
- Sakr W, Grignon D, Haas G, Heilbrum L, Pontes J, Crissman J. Age and racial distribution of prostatic intraepithelial neoplasia. European Urology 1996;30:138‐44. [DOI] [PubMed] [Google Scholar]
Schröder 2003
- Schröder FH, Denis LJ, Roobol M, all participants of the ERSPC. The story of the European Randomized Study of Screening for Prostate Cancer. BJU International 2003;92 Suppl 2:1‐13. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Sterne 2001
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. BMJ 2001;323:101‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
USPSTF 2012
- Moyer VA, on behalf of the U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine 2012;157(2):120‐34. [DOI] [PubMed] [Google Scholar]
van Leeuwen 2010
- Leeuwen PJ, Connolly D, Gavin A, Roobol MJ, Black A, Bangma CH, et al. Prostate cancer mortality in screen and clinically detected prostate cancer: estimating the screening benefit. European Journal of Cancer 2010;46:377‐83. [DOI] [PubMed] [Google Scholar]
WHO 1968
- Wilson J, Jungner G. Principles and practice of screening for disease. World Health Organization 1968.
Wilt 2008a
- Wilt T, MacDonald R, Rutks I, Shamliyan T, Taylor B, Kane R. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Annals of Internal Medicine 2008;148:435‐48. [DOI] [PubMed] [Google Scholar]
Wilt 2012
- Wilt T, Brawer M, Jones K, Barry M, Aronson W, Fox S, et al. Radical prostatectomy versus observation for localized prostate cancer. The New England Journal of Medicine 2012;367:203‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Ilic 2006
- Ilic D, O'Connor D, Green S, Wilt T. Screening for prostate cancer. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD004720.pub2] [DOI] [PubMed] [Google Scholar]
Ilic 2010
- Ilic D, O'Connor D, Green S, Wilt TJ. Screening for prostate cancer. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD004720.pub2] [DOI] [PubMed] [Google Scholar]


