Abstract
Background
Ketotifen is an antihistamine which may be used to treat asthma. Since administering inhaled therapy to younger children can be difficult, an oral agent such as ketotifen offers potential advantages.
Objectives
The objective of this review is to determine, whether ketotifen alone or in combination with other co‐interventions results in better control of asthma in children with asthma and/or wheezing and examine its safety profile.
Search methods
We searched the Cochrane Airways Group Specialised Register of trials, CENTRAL and reference lists of articles. The latest search was carried out in May 2010.
Selection criteria
Clinical studies had to be randomised‐controlled and double‐blinded, comparing oral ketotifen with placebo in children with asthma and/or wheeze for at least eight weeks at a dose not less than one mg daily.
Data collection and analysis
Two reviewers independently performed selection of trials, quality assessment and data extraction; a third reviewer was included in the consensus process if necessary.
Main results
A total of 26 relevant studies involving 1826 participants were included in this review. Children's age ranged from 4 months to 18 years and ketotifen was given between 10 and 32 weeks. The proportion of children able to reduce or stop their bronchodilator use within 12 to 16 weeks of treatment was significantly higher in the ketotifen group (relative risk 2.39, 95% CI 1.64 to 3.48) based on four trials; this result was statistically significant in a subgroup of two trials with well described and adequate method of blinding. Statistically significant beneficial effects of ketotifen were also observed in the following secondary outcomes: efficacy evaluated by physician (10 trials) and parents/patients (7 trials), asthma symptom score (4 trials), asthma exacerbations (2 trials), and reduction in use of oral steroids (4 trials). However, sub‐group analyses of trials with well described and adequate method of blinding was only significant for the outcome asthma symptom score and non‐significant for the remaining secondary outcomes. Reported side effects were more frequent in the ketotifen group (sedation: 21%, weight gain: 27%) than in the placebo group (sedation: 12%, weight gain: 17%).
Authors' conclusions
Evidence from randomised controlled trials indicates that ketotifen alone or in combination with other co‐interventions improves control of asthma and wheezing in children with mild and moderate asthma. However due to the high proportion of children with atopy in some trials the results cannot necessarily be generalised to all asthmatic children. The benefit is obtained at the cost of minor side effects, namely sedation and weight gain. The validity of this conclusion is limited by the low reported, methodological quality of included trials.
Plain language summary
Ketotifen alone or as additional medication for long‐term control of asthma and wheeze in children
Children with asthma can find using inhaled treatments medication difficult and so oral medication such as ketotifen, which is an antihistamine, can be used to help control symptoms. The review found that mild asthma symptoms were well‐controlled in the studies of 4 to 32 week duration with reduction in use of rescue bronchodilator, rescue oral steroids and in exacerbations as well as clear perception of effectiveness from physicians, parents and children.
Background
With the recognition of the chronic inflammation of the airways present in asthma, anti‐inflammatory medication has assumed increasing importance in asthma therapy over recent years. Of these medications inhaled steroids are the most potent and are widely used all over the world (Pao 2002; Suissa 2002). However, since administering inhaled therapy to younger children can be difficult, an oral agent offers potential advantages. Furthermore inhaled steroids are not completely innocuous: potential serious side effects include suppression of adrenal function, retardation in growth and bone formation (Pauwels 2003).
Ketotifen is an oral medication that may be used for maintenance treatment of asthma. It is an antihistamine whose mechanism of action is not fully understood, but it may inhibit the release of inflammatory mediators, inhibit bronchospasm by reducing calcium uptake in mast cells and in smooth muscle (Volovitz 1988). As an oral preparation, ketotifen might be useful in managing children with asthma, especially in the pre‐school age. However, because of conflicting trial results, it remains unclear whether oral ketotifen is effective either alone or in combination with other therapies in the management of pediatric asthma.
Objectives
The objective of this review is to determine whether ketotifen alone, or in combination with other co‐interventions, results in better control of asthma in children with asthma and/or wheezing and examine its safety profile.
Methods
Criteria for considering studies for this review
Types of studies
The clinical studies had to be randomised, double‐blind, and placebo‐controlled.
Types of participants
Children aged 0 to 18 years with chronic or recurrent (two or more) episodes of wheezing, in whom there is no other chronic pulmonary diseases, such as bronchopulmonary dysplasia or cystic fibrosis.
Types of interventions
In the intervention group, ketotifen was given orally, either alone or in combination with other asthma‐medication for at least eight weeks at a dose not less than one mg daily. The intervention, given for a minimum of eight weeks, must have been compared to placebo.
Types of outcome measures
All clinical outcomes were considered for inclusion in the review.
Primary outcomes
The primary outcome is the reduction in the use of rescue bronchodilators.
Secondary outcomes
Secondary clinical endpoints include the overall efficacy of treatment ‐ either evaluated by physicians or by patients/parents; symptom scores; number of asthma exacerbations; use of rescue oral steroids and theophylline; and side effects of the treatment.
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials which is derived from systematic searching of bibliographic databases including CENTRAL, MEDLINE, EMBASE and CINAHL, and handsearching of respiratory journals and meeting abstracts (please see the Airways Group Module for further details). All records in the Specialised Register coded as 'asthma' were searched using the following terms:
(ketotif* OR zaditen OR "hc20‐511" ) AND (child* or paediat* or pediat* or adolesc* or infan* or toddler* or bab* or young* or preschool* or "pre school*" or pre‐school* or newborn* or "new born*" or new‐born* or neo‐nat* or neonat*)
In addition we carried out an advanced search of CENTRAL using these terms. The latest searches were carried out in May 2010.
Searching other resources
We considered studies not yet registered in the Airways Specialised Register, which were identified by handsearching for this review. Four German pediatric journals (Monatsschrift Kinderheilkunde, Klinische Pädiatrie, Pädiatrische Praxis, Kinderarzt) were handsearched from 1970 to 1999. Five German pulmonary, allergology and internal medicine journals (Pneumologie from 1970 to 2002, Atemwegs‐ und Lungenkrankheiten from 1975 to 1997, Allergologie from 1978 to 2002, Internist from 1960 to 1995, Internistische Praxis from 1961 to 1997) were handsearched for clinical trials comparing ketotifen with placebo in asthmatic and/or wheezing children.
We checked reference lists of each relevant trial and review articles to identify additional potentially relevant citations.
We contacted colleagues, collaborators and other investigators working in the field of asthma and asked for further trials.
Data collection and analysis
Selection of studies
Two review authors assessed studies for inclusion in the review based on the titles and abstracts retrieved from electronic and hand searches.
Data extraction and management
For each included trial, two reviewers (DB, GS) independently extracted data for all outcome variables; disagreements were resolved by discussion. One reviewer (GS) entered data into RevMan 4.2 and another reviewer (DB) cross‐checked data.
Assessment of risk of bias in included studies
We assessed the risk of bias for each study based on the degree of protection against bias provided by randomisation and blinding procedures. We have judged the risk of bias as being of low risk, high risk and unclear. The judgements and the information on which they are predicated are provided alongside the characteristics of each study in Included studies.
In addition, each study was assessed using the Jadad scale (Jadad 1996):
Was the study described as randomised? (1 = yes; 0 = no)
Was the study described as double‐blind? (1 = yes; 0 = no)
Was there a description of withdrawals and dropouts? (1 = yes; 0 = no)
Was the method of randomisation well described and appropriate? (1 = yes; 0 = no)
Was the method of double blinding well described and appropriate? (1 = yes; 0 = no)
Deduct 1 point if methods for randomisation or blinding were inappropriate.
The Jadad scale was used as a summary measure of trial quality. If necessary, authors of relevant publications were contacted to provide additional information on allocation concealment, blinding and randomisation.
Data synthesis
We conducted statistical analyses with RevMan 5 and R, Version 1.7.1 (Ihaka 1996). A random effects model was used in all meta‐analyses. For dichotomous outcomes, the relative risk (RR) was used to evaluate the treatment effect. Continuous outcomes (i.e. asthma symptom score and bronchodilator consumption) were measured on different scales, therefore, the standardised mean difference (SMD) was used in meta‐analyses of continuous outcomes. We examined heterogeneity for all outcomes; a p value less than 0.05 was considered indicative of heterogeneity.
Subgroup analysis and investigation of heterogeneity
In meta‐analyses with at least four trials, subgroup analyses were conducted for the following age groups (Infant‐Preschool: 4 months to 6 years, School to Adolescents: 5 to 25 years, Overlapping: 1 to 16 years). Initially, a cut‐off of less than six years was defined for the age group Infant to Preschool; after study identification, the cut‐off was changed to include six years to better reflect the trials' population. The following studies were included in the Infant to Preschool group: Loftus 1987 (2 to 6 years, mean age: 3.8 years), Reid 1989 (2 to 6 years, 4.5 years), White 1988 (1.3 to 5.9 years, 4.1 years). The following studies were included in the School to Adolescent group even though the lower limit was five years: Dawson 1989 (5 to 13 years, 7.0 years), Kabra 2000 (5 to 15 years, 8.8 years), Rackham 1989 (5 to 17 years, 10.1 years), Salmon 1982 (5 to 11 years, 8.5 years).
Sensitivity analysis
Sensitivity analyses were predetermined for concealment of treatment allocation, appropriateness of randomisation (item 4 in Jadad scale) and appropriateness of blinding (item 5 in Jadad scale). Furthermore, in meta‐analyses with at least four trials, a funnel plot was generated and a linear regression test was performed (Egger 1997) to examine the likely presence of bias in meta‐analysis. A p‐value less than 0.1 was considered indicative of bias in meta‐analysis for the linear regression test.
Results
Description of studies
Results of the search
We identified 334 abstracts from the Cochrane Airways Group trials register and we obtained 113 full text versions in the first version of the review. Reference lists of these 113 publications were checked and led to another 49 potentially relevant publications which also were obtained in full text. Two independent reviewers (DB, AM) selected eligible trials for inclusion; disagreement was resolved by discussion. If necessary, a third reviewer (JF) was included in the consensus process. An update search conducted in May 2010 did not identify any new trials.
Included studies
Initially we included 27 trials based on criteria for study design, participants and interventions: 24 trials identified from the Cochrane Airways Group trials register and three trials identified by reference checking. Handsearching in nine German medical journals did not reveal any further trials. All trials were described as placebo‐controlled, double‐blind and randomised but often the method of randomisation and double blinding was not clearly stated. We tried to contact 17 authors to get additional information. Nine (53%) replied to our inquiry: Kelly 1982; Poder 1982; Loftus 1987; Dawson 1989; Rackham 1989; Myloma 1990; Van Asperen 1992; Varsano 1993; Canny 1997. One trial (Poder 1982) was excluded from the analysis since it was not double‐blind (personal communication, Dr. Poder). Accordingly, quality assessment (allocation concealment and Jadad score), data extraction and analysis were done for the remaining 26 trials. The trials have been published between 1982 and 2000 (with 17 publications (65%) before 1990) in 17 different countries (Europe: 14, North America: two, Middle America: one, South America: two, Oceania: three, Asia: four).
Participants
The trials were heterogeneous with respect to the age of the participants. All except one trial included patients aged between four months and 18 years. The study of Croce 1995 included patients between six and 25 years. We decided to include this study due to a mean age of 11.9 years.
Overall, 12 trials (46%) reported data on atopy/extrinsic asthma however with different levels of atopy. Most data were in respect to performed skin and IgE tests and/or a history of atopic symptoms (e.g. urticaria, allergic rhinitis, eczema).
A total of 13 trials (50%) of trials reported data on the duration of illness prior to study entry. However, reported data were either vague (just stating "chronic asthma") or differed widely across trials (with a range of four weeks to four years duration of asthma).
With respect to severity, criteria differed also widely across trials: three trials reported the reversibility in lung function parameters after inhalation of beta‐2 agonists (15% to 30% in fixed expiratory flow rate in one second (FEV1), peak expiratory flow rate (PEFR)) as a criteria of severity. These data suggest that children included suffered from mild to moderate asthma. Five trials reported the concomitant medication as a criteria of severity. Most of the five trials excluded children on inhaled or systemic steroids, while others required medication with beta‐2 agonists and/or xanthines. The concomitant medication allowed in the studies reflects well the time the studies were performed (e.g. none of the studies included anti‐leukotrienes) and did not allow to separate between mild, moderate or severe asthma. Other authors just described the children included in their studies as suffering from "mild" or "moderate" asthma. Overall, by the description of studies it is obvious, that from today's point of view, most of the children included in this review suffered from mild to moderate asthma.
Interventions
The dose of ketotifen administered ranged from 1 mg/d to 4 mg/d; in most trials (16 of 26) 2 mg/d of ketotifen were administered at a uniform dose to all participants. In three trials, 1 mg/d of ketotifen was administered and in the remaining seven trials the dose of ketotifen varied, depending on body weight, age or protocol.
Six trials did not have a run‐in phase. In the remaining trials, the run‐in period ranged from one to four weeks: one trial had a run‐in period of one week, nine trials of two weeks, and 10 trials of four weeks.
The duration of treatment ranged from 10 and 32 weeks: two trials had a treatment period of less than 12 weeks, 15 trials of 12 weeks, five trials of 16 weeks, and four trials had a treatment period of more than 16 weeks.
Concomitant medication
A total of 18 trials reported that beta‐2 agonists were allowed as co‐intervention; in only one of these 18 trials (Van Asperen 1992) beta‐2 agonists were the only allowed agent for control of asthma, in all other trials further agents were allowed as co‐interventions. Furthermore, in four trials (Montoya 1988; Neijens 1988; Chay 1992; Santos 1999), the use of bronchodilators was allowed without giving detail on specific drugs. In four trials (Kelly 1982; Mulhern 1982; Salmon 1982; White 1988) only vague information on allowed co‐interventions was given. Due to insufficient reporting, it was not possible to distinguish between trials giving beta‐2 agonists as maintenance and rescue treatment but it can be assumed that beta‐2 agonists were given as rescue medication in most trials.
In 15 trials, theophylline was administered additionally to beta‐2 agonists. Inhaled corticosteroids were allowed as additional intervention in eight trials, oral steroids were allowed in 10 trials and cromoglycate was allowed in four trials.
Outcomes
Trials differed widely in the number of reported outcomes and the definition of outcomes. Accordingly, some of the 12 outcomes considered in this systematic review are either based on a limited number of trials and patients or consist of trials with different outcome definitions.
The main outcome, reduction in bronchodilator use (outcome 01), pertains to the number of participants reducing or discontinuing bronchodilator consumption at the end of the treatment. Four trials provided data this outcome; three trials reported both reduction and discontinuation and one trial only reported reduction of bronchodilator consumption, after 12 weeks (three trials) or 16 weeks (one trial) of treatment. One trial reported reduction in beta‐2 agonists (Spicak 1983) while the other three trials reported reduction in bronchodilator use which may include other drugs than beta‐2 agonists. Only one trial (Chay 1992) had a run‐in period of two weeks to compare the use of bronchodilators to, the other three trials did not have a run‐in period. Thus, it remains unclear how assessments of reduction in bronchodilators have been done in these three trials.
Other means of reporting the consumption of bronchodilator use were considered as secondary outcomes, namely:
"Use of bronchodilators" (outcome 04) summarises the number of participants on concomitant bronchodilator therapy was reported in five trials; three trials contained information on the number of patients using bronchodilators after 12 weeks (two trials) or 16 weeks (one trial) of treatment; the other two trials reported the number of participants using bronchodilators between week 13 and 16 and week 8 and 12 of treatment, respectively. Two trials reported use of beta‐2 agonists (Van Asperen 1992; Varsano 1993) while the other three trials reported use of bronchodilators.
"Bronchodilator consumption" (outcome 07) aggregates trials reporting either number of days of bronchodilator use or doses of bronchodilators, which included three trials defining bronchodilator consumption in very different ways: average percentage of days on which a participant took salbutamol up to week 26, number of doses of salbutamol per month after 16 weeks of treatment, difference of the number of times bronchodilators were used at week 4 and 20.
Various side effects were reported in the publications. Sedation, weight gain and withdrawals due to side effects were considered to evaluate the safety of ketotifen. Other side effects like dryness of mouth, increased appetite and somnolence were only reported in two trials. Information on side effects given in three trials (Kelly 1982; Mulhern 1982; Van Asperen 1992) could not be considered in the analysis since the number of side effects not the number of participants with these side effects was reported.
Five trials are not included in any analysis of this systematic review: two publications (Graff Lonnevig 1985; Rebmann 1987) solely reported outcomes not considered in the review (lung function parameters, sensitivity to metacholine), one publication (Dawson 1989) did not report the necessary measures of variability (e.g. standard errors), two cross‐over trials (Loftus 1987; Volovitz 1988) reported neither the results of the first treatment period separately (required to include the studies as parallel group design) nor the necessary measures of variability, i.e. the standard error of the patient‐specific treatment differences (required to include the studies as cross‐over design). Contacting the authors did not reveal additional information.
Excluded studies
Risk of bias in included studies
Although all 26 included studies were described as randomised, double‐blind trials, the details of reporting were insufficient in 25 trials to confirm the appropriateness of concealment of allocation at randomisation and blinding post‐randomisation (Table 1 and Characteristics of included studies); consequently 8/26 trials had a reported high methodology score (Jadad 4 or 5). Most trials have been published prior to the CONSORT statement (Begg 1996), i.e. before 1990, when reporting of methods were not as stringent as they are now, which may lead to inadequate reporting of good methods rather than bad methods per se.
1. Quality assessment (Concealment of treatment allocation and Jadad score items).
| Author | Allocation Conceal. | Jadad score | Randomisation | Blinding | Adequate Random. | Adequate Blinding | Withdrawals |
| Broberger 1986 | B | 4 | 1 | 1 | 0 | 1 | 1 |
| Canny 1997 | B | 3 | 1 | 1 | 0 | 0 | 1 |
| Chay 1992 | A | 4 | 1 | 1 | 0 | 1 | 1 |
| Croce 1995 | B | 3 | 1 | 1 | 0 | 0 | 1 |
| Dawson 1989 | B | 2 | 1 | 1 | 0 | 0 | 0 |
| de Benedictis 1990 | B | 3 | 1 | 1 | 0 | 0 | 1 |
| Graff Lonnevig 1985 | B | 3 | 1 | 1 | 0 | 0 | 1 |
| Kabra 2000 | B | 3 | 1 | 1 | 0 | 0 | 1 |
| Kelly | B | 3 | 1 | 1 | 0 | 0 | 1 |
| Loftus 1987 | B | 4 | 1 | 1 | 0 | 1 | 1 |
| Longo 1986 | B | 3 | 1 | 1 | 0 | 0 | 1 |
| Montoya 1988 | B | 2 | 1 | 1 | 0 | 0 | 0 |
| Mulhern 1982 | B | 3 | 1 | 1 | 0 | 0 | 1 |
| Myloma 1990 | B | 4 | 1 | 1 | 0 | 1 | 1 |
| Neijens 1988 | B | 3 | 1 | 1 | 0 | 0 | 1 |
| Rackham 1989 | B | 4 | 1 | 1 | 0 | 1 | 1 |
| Rebmann 1987 | B | 2 | 1 | 1 | 0 | 0 | 0 |
| Reid 1989 | B | 3 | 1 | 1 | 0 | 0 | 1 |
| Salmon 1982 | B | 3 | 1 | 1 | 0 | 0 | 1 |
| Santos 1999 | B | 2 | 1 | 1 | 0 | 0 | 0 |
| Simons 1982 | B | 3 | 1 | 1 | 0 | 0 | 1 |
| Spicak 1983 | B | 3 | 1 | 1 | 0 | 0 | 1 |
| Van Asperen 1992 | B | 5 | 1 | 1 | 1 | 1 | 1 |
| Varsano 1993 | B | 4 | 1 | 1 | 0 | 1 | 1 |
| Volovitz 1988 | B | 4 | 1 | 1 | 0 | 1 | 1 |
| White 1988 | B | 3 | 1 | 1 | 0 | 0 | 1 |
Concealment of treatment allocation was judged to be adequate in one trial and uncertain in the remaining 25 trials. The method of randomisation was well described and adequate in one trial, and undescribed in the remaining 25 trials. The method of blinding was well described and adequate in eight trials, for the remaining trials the method of blinding was not reported in sufficient detail and therefore classified as unclear. The trials with adequate blinding are those eight trials scored 4 or 5 on the Jadad scale.
The number of withdrawals and dropouts was reported in 22 trials. Inclusion and exclusion criteria were not clearly reported in most trials. Four authors (Kelly 1982; Loftus 1987; Van Asperen 1992; Varsano 1993) provided additional information on randomisation and/or blinding.
Sensitivity analyses were done for appropriateness of blinding. No sensitivity analyses were conducted for appropriateness of randomisation and concealment of treatment allocation since these quality measures were judged to be adequately reported only in one trial.
Effects of interventions
Primary outcome: Reduction in the use of bronchodilators
A total of 12 trials with 820 participants reported reduction in the use of bronchodilators, but data were presented in different ways. Although three outcomes on the use of bronchodilators were defined in this review, the primary outcome was the reduction in bronchodilator use after 12 to 16 weeks of treatment. This particular outcome was reported in four trials. Participants treated with ketotifen are more than twice likely to reduce or stop bronchodilator use (relative risk (RR) 2.39, 95% confidence interval (CI) 1.64 to 3.48, Figure 1). No heterogeneity was observed. The effect was similar across age groups (Analysis 1.2) and appropriateness of blinding (Analysis 1.3). Visual inspection of funnel plot did not suggest any significant bias.
1.
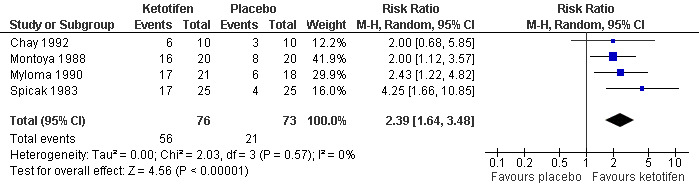
Forest plot of comparison: 1 Ketotifen versus Placebo, outcome: 1.1 Reduction of bronchodilator use.
1.2. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 2 Reduction of bronchodilator use (subgroup analysis: age).
1.3. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 3 Reduction of bronchodilator use (sensitivity analysis: blinding).
Secondary outcomes
Other measures of bronchodilator use were reported and show a similar treatment effect with ketotifen. The number of participants on concomitant bronchodilator therapy (Outcome 04) was reported in five trials; significantly less participants in the ketotifen group used bronchodilators after 8 to 16 weeks of treatment (five trials, RR 0.61, 95% CI 0.41 to 0.92). Heterogeneity was apparent (p = 0.0022) and the funnel plot suggested publication bias (p = 0.085). Subgroup and sensitivity analyses show that heterogeneity might be explained either by age (outcome 05, p = 0.0054, chi squared test of between‐group heterogeneity) or appropriateness of blinding (outcome 06, p = 0.0007). More specifically, the effect of ketotifen was stronger in school children (RR 0.25, 95% CI 0.12 to 0.53], one trial) than in infants/pre‐school (RR 0.72, 95% CI 0.51 to 1.01], four trials). The effect of ketotifen disappeared in trials with adequate blinding (RR 0.93, 95% CI 0.71 to 1.22), two trials) as compared to trials with unclear reporting of blinding methods (RR 0.45, 95% CI 0.30 to 0.68), three trials). A post‐hoc analysis examined the possibility of a differential effect when ketotifen was used as a single versus add‐on agent. In one trial (Van Asperen 1992) rescue beta‐2 agonists were the only concomitant drug permitted. Additional medication was reported in three studies (theophylline, de Benedictis 1990; steroids, Santos 1999; theophylline and steroids, Varsano 1993) and remained unclear for one study (Neijens 1988). The study by Van Asperen 1992 showed the smallest treatment effect. However, a subgroup analysis of this study versus the three studies with additional medication (de Benedictis 1990; Varsano 1993;Santos 1999) was not significant.
No meta‐analysis was conducted for bronchodilator consumption (outcome 07), because of the various reporting of this variable as average percentage of days on which a participant took salbutamol up to week 26, number of doses of salbutamol per month after 16 weeks of treatment, and difference of the number of times bronchodilators were used at week four and 20. A tendency for a decreased consumption in the ketotifen group was suggested as all three trials point in this direction.
Efficacy evaluated by physician or parents/children
The overall efficacy was evaluated by physicians in 10 trials with a total of 625 participants (outcome 08). In all but one trial, the estimated relative risk was less than one indicating a better perceived efficacy for ketotifen than placebo: the pooled relative risk for ineffectiveness is 0.60, 95% CI 0.46 to 0.79). Some heterogeneity between trials was apparent (p = 0.021) which could not be explained by differences between age groups (outcome 9: p = 0.13) and appropriateness of blinding (outcome 10: p = 0.15). Furthermore, the funnel plot suggested bias (p = 0.068)
Overall efficacy assessed either by participants or parents was reported in seven trials with a total of 599 participants (outcome 11). The estimated relative risk of perceived effectiveness was in favour of the ketotifen group in five trials and in favour of the placebo group in only one trial. Overall, ketotifen shows a significantly favourable protection against perceived ineffectiveness (RR 0.71, 95% CI 0.52 to 0.96). No indication for heterogeneity between trials (p = 0.088) or publication bias in meta‐analysis (p = 0.28) is present.
Asthma symptoms/asthma exacerbations
Four trials with a total of 148 participants reported results on an asthma symptom score (outcome 14); all results were in favour of ketotifen. The pooled standardised mean difference shows a significant and beneficial effect of ketotifen (SMD ‐0.49, 95% CI ‐0.82 to ‐0.16). Neither between‐trial heterogeneity (p = 0.61) nor publication bias was present (p = 0.62).
Participants with asthma exacerbations were reported in two trials (outcome 17). Both trials report very large and similar treatment effects. The overall relative risk is 0.31 (95% CI 0.19 to 0.59) indicating a highly significant beneficial effect of ketotifen in preventing exacerbations. However both trials included a high proportion of children with atopy (nasal symptoms in 70%; eczema in 31%; family history of asthma in 55%).
Additional medication
Four trials with a total of 306 participants evaluated the use of oral steroids (outcome 18); all results were in favour of ketotifen. The result indicates a significant protective effect of ketotifen against the use of rescue oral steroids (RR 0.28, 95% CI 0.13 to 0.58). Neither the test for heterogeneity (p = 0.16) nor the test for bias in meta‐analysis (p = 0.89) is significant. Nevertheless, the treatment effect appeared weaker for trials with adequate blinding as compared to trials with unclear reporting of blinding methods (outcome 20).
Only two trials with 246 participants reported results on the use of theophylline (outcome 21). The overall relative risk is not significant 0.79 (95% CI 0.50 to 1.27).
Safety of ketotifen (analysis of side effects)
Ketotifen increased the risk of sedation (seven trials; 439 participants; outcome 22; RR 1.69, 95% CI 1.11 to 2.59). No information was available on the time lag between onset of treatment with ketotifen and sedation to confirm resolution of sedation with use.
Weight gain was more common in the ketotifen group compared with placebo (five trials; 283 participants; outcome 25, RR 1.42, 95% CI 1.02 to 1.99). The magnitude of weight gain could not be determined because of reporting issues. However, quantitative information on weight gain is available in three trials. Simons 1982 reported an average increase in weight of 2.1 kg for participants taking ketotifen and 1.6 kg for those treated with placebo after 12 weeks of treatment (mean weight of all children: 35 kg). Varsano 1993 reported an average increase in weight after 12 weeks of treatment in the ketotifen and control group of 1.11 kg and 0.67 kg, respectively (mean weight of all children: 10.5 kg). Canny 1997 reported a difference in weight gain of 1 kg or 0.5 kg/m2 body mass for ketotifen compared to placebo after 32 weeks of treatment (mean age: 8.6 years). The study of Loftus 1987 not included in this meta‐analysis due to missing information reported an average weight gain of 1.56 kg with ketotifen versus 1.11 kg with placebo (mean age: 3.8 years). There is no indication of heterogeneity between trials or bias in meta‐analysis for sedation and weight gain.
Only three trials with 238 participants reported the number of participants that withdrew due to side‐effects. No evidence is available for an increased risk of withdrawal in the ketotifen group (RR 1.22, 95% CI 0.30 to 4.92, Analysis 1.28) suggesting that side effects were considered minor.
1.28. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 28 Side effects ‐ withdrawal.
Discussion
Ketotifen (administered between 10 and 32 weeks at a daily dose of at least mg) used alone or in combination with other co‐interventions (cromoglycate (DSCG), theophylline, inhaled and/or oral steroids) results in better control of asthma as attested by the clear decrease in bronchodilator use with no heterogeneity or suggestion of bias. This is supported by the number of patients remaining on bronchodilator at the end of treatment, physician and patient or parent preference as well as the relief of asthma symptoms and the decreased need of rescue oral steroids. The reported side effects (sedation, weight gain) are minor.
The trials included in this review are very heterogeneous on age, baseline asthma severity, type of asthma (atopic/non‐atopic), use of ketotifen as single versus add‐on agent. For most outcomes, subgroup and sensitivity analyses as well as tests of bias in meta‐analysis had low power since only a limited number of trials could be combined in the analyses of this systematic review.
Age was not an important effect modifier for the main outcome. It appeared as an important co‐variate, however, for the use of bronchodilators at the end of treatment (outcome 05). However, appropriateness of blinding is another possible explanation for heterogeneity between trials. With bias suggested by the funnel plot, it is questionable whether the effect of ketotifen is truly age‐dependent.
As most of the trials were published before 1990, a definition of asthma according to current international guidelines was not provided. A retrospective classification is not possible due to lacking or inconsistent information. An attempt was made to sort the studies included in the primary outcome according to the baseline severity of the participants included. Severity was measured as three criteria: number of asthma attacks per month, additional medication allowed (especially oral steroids) and classification of asthma as "mild", "moderate" or "severe" provided by the authors of the studies. For the main outcome, patients reducing or discontinuing bronchodilator use, the study of Spicak 1983 that included most severely affected patients as determined by the use of steroids and frequency of asthma attacks, showed the larger treatment effect of ketotifen. However, this finding should be interpreted with caution in this post‐hoc analysis.
Overall, 46% of trials reported data on atopy/extrinsic asthma however with different levels of atopy. Most data are in respect to performed skin and IgE tests and/or a history of atopic symptoms (e.g. urticaria, allergic rhinitis, eczema). Some studies included a high proportion of children with atopy. Therefor the results of the review cannot necessarily be generalized to include all asthmatic children but may apply particularly to those who are atopic as well. As Ketotifen interferes with histamine receptors this is physiologically plausible even if it is a post‐hoc finding and further research should be done.
Additional medication was definitely administered in two (theophylline, Myloma 1990; theophylline and steroids, Spicak 1983) of the four trials contributing data to the main outcome: it remained unclear for the other two studies (Chay 1992, Montoya 1988). Therefore, no subgroup analysis comparing ketotifen as monotherapy versus add‐on therapy could be performed. For the use of bronchodilators at the end of treatment (outcome 04), a post‐hoc analysis failed to reveal difference in treatment effect associated with use of ketotifen as monotherapy versus add‐on therapy. Imprecise reporting also prevented the documentation of any corticosteroid‐sparing effect of ketotifen, when used as add‐on to inhaled corticosteroids. As inhaled corticosteroids have proven to be effective and are the gold standard for therapy of children with moderate asthma today, this is one of the most important question that should be addressed in future studies.
We have assessed the risk of bias based on the degree to which the design of the study protects against bias. Our assessments were hampered due to poor reporting of study design in the publications (most trials were published long before the CONSORT statement, Begg 1996, which may lead to inadequate reporting of good methods rather than bad methods). Only eight trials have a Jadad score of 4 or 5 and the remaining trials have Jadad scores of 2 and 3 which is very low considering that randomisation and blinding were inclusion criteria for trials. Concealment of treatment allocation is adequate in a single trial and uncertain in the remaining trials. Similarly, the method of randomisation is well described and adequate in only one trial. Therefore, sensitivity analyses were only feasible for the method of double blinding which is well described and adequate in eight trials. This sensitivity analysis is identical to an analysis comparing high (Jadad score 4 or 5) and low (Jadad score 2 or 3) quality trials, since a significant proportion of the Jadad score is based on blinding (2 out of 5 points). Sensitivity analysis with respect to blinding/quality revealed no significant group difference for the main outcome, the magnitude of effect remained highly significant for trials with adequate blinding.
There was some indication that the treatment effect weakened or disappeared with adequate blinding for some but not all secondary outcomes. In fact, the effect of blinding was only apparent for use of bronchodilators at the end of treatment (outcome 06) and was suspected because of heterogeneity. For other outcomes with no apparent heterogeneity, treatment effect seemed to weaken with adequate reporting of blinding for evaluation of treatment efficacy by physicians (outcome 10) or patients/parents (outcome 13), and the use of oral steroids (outcome 20). In absence of heterogeneity, the interpretation of these findings is unclear.
Overall, results of this systematic review show a common pattern indicating clearly that ketotifen alone or in combination with other co‐interventions results in a better control of asthma and/or wheezing in children than placebo. The validity of this conclusion is limited by the small number of participants for the assessed outcomes, the clinical heterogeneity and the low reported methodological quality of the included trials.
Authors' conclusions
Implications for practice.
Ketotifen (at a dose not less than one mg daily for at least eight weeks) alone or in combination with other co‐interventions results in better control of asthma and/or wheezing in children with a decrease in the consumption of bronchodilators, symptom score, number of exacerbations and the use of rescue oral steroids. This is further supported by a greater perception of the efficacy by physicians and parents/patients. Use of ketotifen appears safe being associated only with minor side effects (weight gain, transient sedation). Therefore, according to the existing evidence it seems reasonable to treat children with mild or moderate asthma with ketotifen especially if there is a history of atopy. No head‐to head comparison of ketotifen with other commonly used medications in childhood asthma (e.g. inhaled steroids) is included in this review. Therefore, no recommendation on the indication of ketotifen in comparison to alternative medications in children with asthma and/or wheezing can be given. No evidence was found to answer the questions whether the effect of ketotifen is different when used as monotherapy versus add‐on therapy and if the effect of ketotifen is depending on the severity level of asthma.
Implications for research.
Based on the facts that the results of this systematic review indicate that ketotifen is more effective than placebo in all available outcomes and that placebo controlled trials in children with asthma are considered unethical in many countries, we recommend that future research should focus on comparisons of ketotifen to other drugs. Especially, the following investigations of ketotifen are important:
as monotherapy versus low dose of inhaled steroids, leukotriene antagonists, or cromolyn;
as add‐on therapy, to low dose of inhaled steroids with the recent recognition of the flat dose‐response curve of inhaled steroids in mild and moderate asthma;
Further research should also concentrate on the subgroup of children (atopic versus non‐atopic) that is most likely to benefit from Ketotifen.
A first step would be to address these topics in systematic reviews. Randomised controlled trials should be performed if systematic reviews do not provide definite answers on the efficacy of ketotifen. The design of the trials should meet the following requirements:
design: adequately concealed, randomised and double‐blind;
intervention: ketotifen given at a daily dose not less than one mg for at least eight weeks;
co‐interventions: rescue beta‐2 agonists and systemic steroids;
inclusion criteria: children suffering from mild to moderate asthma defined according to established international guidelines;
primary outcome: change from baseline in the consumption of rescue medications;
reporting of trial results according to CONSORT (Moher 2001).
What's new
| Date | Event | Description |
|---|---|---|
| 13 May 2010 | New search has been performed | New literature search run: no new studies identified. |
History
Protocol first published: Issue 2, 1998 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 12 May 2008 | Amended | Converted to new review format. |
| 1 November 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We would like to thank the Cochrane Child Health Field who provided the authors with a bursary to complete this review. Thanks to Gerd Antes, Steve Milan, Toby Lasserson, Chris Cates, Anna Barra, Jane Dennis, Karen Blackhall, Mark Everard, the Cochrane Airways Group, the Cochrane Child Health Field and all people handsearching at the German Cochrane Centre. Furthermore, we would like to thank all authors who provided additional information: Dr. McKay (Van Asperen 1992), Dr. Canny, Mrs. Mogridge (Dawson 1989), Dr. Kelly, Dr. Loftus, Dr. Mylona Karayianni, Dr. Poder, Mrs. Godin (Rackham 1989) and Prof. Varsano (Varsano 1993).
Data and analyses
Comparison 1. Ketotifen versus Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reduction of bronchodilator use | 4 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 2.39 [1.64, 3.48] |
| 2 Reduction of bronchodilator use (subgroup analysis: age) | 4 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 2.39 [1.64, 3.48] |
| 2.1 Infants ‐ Preschool | 1 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.68, 5.85] |
| 2.2 Overlapping | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [1.70, 5.13] |
| 2.3 School ‐ Adolescents | 1 | 40 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [1.12, 3.57] |
| 3 Reduction of bronchodilator use (sensitivity analysis: blinding) | 4 | 149 | Risk Ratio (M‐H, Random, 95% CI) | 2.39 [1.64, 3.48] |
| 3.1 Adequate blinding | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [1.29, 4.09] |
| 3.2 Method of blinding unclear | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 2.68 [1.26, 5.71] |
| 4 Use of bronchodilators | 5 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.41, 0.92] |
| 5 Use of bronchodilators (subgroup analysis: age) | 5 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.41, 0.92] |
| 5.1 Infants ‐ Preschool | 4 | 429 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.51, 1.01] |
| 5.2 School ‐ Adolescents | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.25 [0.12, 0.53] |
| 6 Use of bronchodilators (sensitivity analysis: blinding) | 5 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.41, 0.92] |
| 6.1 Adequate blinding | 2 | 220 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.22] |
| 6.2 Method of blinding unclear | 3 | 271 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.30, 0.68] |
| 7 Bronchodilator consumption | 3 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 8 Poor overall efficacy ‐ evaluated by physician | 10 | 625 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.46, 0.79] |
| 9 Poor overall efficacy ‐ evaluated by physician (subgroup analysis: age) | 10 | 625 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.46, 0.79] |
| 9.1 Infant ‐ Preschool | 3 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.13, 1.09] |
| 9.2 Overlapping | 3 | 117 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.43, 0.81] |
| 9.3 School ‐ Adolescents | 4 | 310 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.54, 1.01] |
| 10 Poor overall efficacy ‐ evaluated by physician (sensitivity analysis: blinding) | 10 | 625 | Risk Ratio (M‐H, Random, 95% CI) | 0.60 [0.46, 0.79] |
| 10.1 Adequate blinding | 3 | 256 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.52, 1.09] |
| 10.2 Method of blinding unclear | 7 | 369 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.39, 0.80] |
| 11 Poor overall efficacy ‐ evaluated by patients or parents | 7 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.52, 0.96] |
| 12 Poor overall efficacy ‐ evaluated by patients or parents (subgroup analysis: age) | 7 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.52, 0.96] |
| 12.1 Infant ‐ Preschool | 3 | 311 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.49, 1.27] |
| 12.2 Overlapping | 1 | 35 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.18, 0.88] |
| 12.3 School ‐ Adolescents | 3 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.39, 1.20] |
| 13 Poor overall efficacy ‐ evaluated by patients or parents (sensitivity analysis: blinding) | 7 | 599 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.52, 0.96] |
| 13.1 Adequate blinding | 3 | 260 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.60, 1.08] |
| 13.2 Method of blinding unclear | 4 | 339 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.34, 0.97] |
| 14 Asthma symptom score | 4 | 148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.82, ‐0.16] |
| 15 Asthma symptom score (subgroup analysis: age) | 4 | 148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.82, ‐0.16] |
| 15.1 Overlapping | 2 | 69 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐1.10, 0.20] |
| 15.2 School ‐ Adolescents | 2 | 79 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.98, ‐0.07] |
| 16 Asthma symptom score (sensitivity analysis: blinding) | 4 | 148 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.82, ‐0.16] |
| 16.1 Adequate blinding | 2 | 66 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.65 [‐1.14, ‐0.15] |
| 16.2 Method of blinding unclear | 2 | 82 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.82, 0.07] |
| 17 Asthma exacerbations | 2 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.16, 0.59] |
| 18 Use of oral steroids | 4 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.13, 0.58] |
| 19 Use of oral steroids (subgroup analysis: age) | 4 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.13, 0.58] |
| 19.1 Infants ‐ Preschool | 1 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.18, 3.31] |
| 19.2 School ‐ Adolescents | 3 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.10, 0.50] |
| 20 Use of oral steroids (sensitivity analysis: blinding) | 4 | 306 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.13, 0.58] |
| 20.1 Adequate blinding | 2 | 137 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.16, 2.22] |
| 20.2 Method of blinding unclear | 2 | 169 | Risk Ratio (M‐H, Random, 95% CI) | 0.21 [0.07, 0.60] |
| 21 Use of theophylline | 2 | 246 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.50, 1.27] |
| 22 Side effects ‐ sedation | 7 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.11, 2.59] |
| 23 Side effects ‐ sedation (subgroup analysis: age) | 7 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.11, 2.59] |
| 23.1 Infant ‐ Preschool | 3 | 165 | Risk Ratio (M‐H, Random, 95% CI) | 1.82 [0.94, 3.52] |
| 23.2 Overlapping | 1 | 50 | Risk Ratio (M‐H, Random, 95% CI) | 5.0 [0.25, 99.16] |
| 23.3 School ‐ Adolescents | 3 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [0.71, 2.77] |
| 24 Side effects ‐ sedation (sensitivity analysis: blinding) | 7 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [1.11, 2.59] |
| 24.1 Adequate blinding | 3 | 266 | Risk Ratio (M‐H, Random, 95% CI) | 1.55 [0.56, 4.34] |
| 24.2 Method of blinding unclear | 4 | 173 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.91, 3.02] |
| 25 Side effects ‐ weight gain | 5 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.02, 1.99] |
| 26 Side effects ‐ weight gain (subgroup analysis: age) | 5 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.02, 1.99] |
| 26.1 Infant ‐ Preschool | 2 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.88, 3.60] |
| 26.2 School ‐ Adolescents | 3 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.91, 1.96] |
| 27 Side effects ‐ weight gain (sensitivity analysis: blinding) | 5 | 283 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [1.02, 1.99] |
| 27.1 Adequate blinding | 3 | 197 | Risk Ratio (M‐H, Random, 95% CI) | 1.92 [0.98, 3.76] |
| 27.2 Method of blinding unclear | 2 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.88, 1.90] |
| 28 Side effects ‐ withdrawal | 3 | 238 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [0.30, 4.92] |
1.1. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 1 Reduction of bronchodilator use.
1.4. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 4 Use of bronchodilators.
1.5. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 5 Use of bronchodilators (subgroup analysis: age).
1.6. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 6 Use of bronchodilators (sensitivity analysis: blinding).
1.7. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 7 Bronchodilator consumption.
1.8. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 8 Poor overall efficacy ‐ evaluated by physician.
1.9. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 9 Poor overall efficacy ‐ evaluated by physician (subgroup analysis: age).
1.10. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 10 Poor overall efficacy ‐ evaluated by physician (sensitivity analysis: blinding).
1.11. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 11 Poor overall efficacy ‐ evaluated by patients or parents.
1.12. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 12 Poor overall efficacy ‐ evaluated by patients or parents (subgroup analysis: age).
1.13. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 13 Poor overall efficacy ‐ evaluated by patients or parents (sensitivity analysis: blinding).
1.14. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 14 Asthma symptom score.
1.15. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 15 Asthma symptom score (subgroup analysis: age).
1.16. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 16 Asthma symptom score (sensitivity analysis: blinding).
1.17. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 17 Asthma exacerbations.
1.18. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 18 Use of oral steroids.
1.19. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 19 Use of oral steroids (subgroup analysis: age).
1.20. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 20 Use of oral steroids (sensitivity analysis: blinding).
1.21. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 21 Use of theophylline.
1.22. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 22 Side effects ‐ sedation.
1.23. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 23 Side effects ‐ sedation (subgroup analysis: age).
1.24. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 24 Side effects ‐ sedation (sensitivity analysis: blinding).
1.25. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 25 Side effects ‐ weight gain.
1.26. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 26 Side effects ‐ weight gain (subgroup analysis: age).
1.27. Analysis.
Comparison 1 Ketotifen versus Placebo, Outcome 27 Side effects ‐ weight gain (sensitivity analysis: blinding).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Broberger 1986.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described; children were stratified in matched pairs with regard to the degree of sensitivity to birch tree pollen, as determined by bronchial challenge, duration of asthma, number of pollen seasons with clinical symptoms from asthma, additional allergic symptoms during the pollen season, age and sex; placebo tablets contained lactose and were indistinguishable | |
| Participants | N = 32 (fulfilled criteria of the study) N = 27 (completed) WITHDRAWAL/DROPOUT: 3 ("did not comply with the study procedure"), 2 in placebo group due to severe asthma symptoms AGE: 6‐15 years (mean 10,5 years) SEVERITY: pollen induced bronchial asthma; none was on local steroids or DSCG | |
| Interventions | DOSE: 1 mg twice daily RUN‐IN: 2 weeks TREATMENT: 10 weeks ADDITIONAL MEDICATION: only beta‐2 agonists and theophylline allowed; 2 patients in the placebo group received oral and local steroids due to severe asthma symptoms, none in the ketotifen group received steroids | |
| Outcomes | symptom score for asthma, additional medication for asthma and rhino‐conjunctivitis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Specific method of randomisation not described; children stratified in matched pairs based on sensitivity to birch tree pollen, duration of asthma, number of pollen seasons with clinical symptoms from asthma, additional allergic symptoms during the pollen season, age and sex |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Placebo tablets contained lactose and were indistinguishable |
Canny 1997.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: ketotifen and placebo were supplied by Sandoz Canada Inc. | |
| Participants | N = 66 (randomised) N = 52 (completed) WITHDRAWAL/DROPOUT: 10 in ketotifen group (unstable asthma: 6, poor compliance: 2, side effects: 2), 4 in placebo group (unstable asthma: 2, poor compliance: 1, side effects: 1) AGE: 6‐13 years (mean 8,65 years) SEVERITY: asthma diagnosis based on a compatible clinical history and the presence of bronchial hyperreactivity; required continuous treatment with an inhaled steroid preparation on < 1 mg/day for at least one month | |
| Interventions | DOSE: 2 mg/day RUN‐IN: 4 weeks TREATMENT: 10 weeks ADDITIONAL MEDICATION: beta‐2 agonists, inhaled steroids | |
| Outcomes | reductions of ICS dosage (defined as average daily dose of ICS used during the follow‐up phase, expressed as a percentage of the average daily dose required during the baseline phase); FEV1, diary card data (symptom score, PEFR diurnal variability, number of doses of inhaled beta‐2 agonists irrespective of dosage or delivery system), logarithm of PC20, adverse effects | |
| Notes | purpose of study was to evaluate a steroid sparing effect of Ketotifen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Ketotifen and placebo were supplied by Sandoz Canada Inc. |
Chay 1992.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: coding and uncoding of drugs were done by Sandoz, and not known to parents and doctors until study completion; placebo was ethanol free with sugar replaced by Lycasin and banana flavour | |
| Participants | N = 26 (randomised) N = 20 (completed) WITHDRAWAL/DROPOUT: 6 (default: 5, taking traditional medicine: 1) AGE: 6‐36 months SEVERITY: at least 2 episodes of wheezing over 8 weeks or persistent wheeze over 4 weeks | |
| Interventions | DOSE: 0,5 mg/day for 2 weeks followed by 1 mg twice daily for 10 weeks RUN‐IN: 2 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: "bronchodilator therapy"; patients on prophylactic agents or steroids were excluded from the study | |
| Outcomes | overall efficacy (evaluated by investigators and parents), reduction or discontinuation of bronchodilator therapy, performance indices (night cough, wheeze, sputum production), global evaluation, adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Low risk | Coding and uncoding of drugs were done by Sandoz, and not known to parents and doctors until study completion |
| Blinding? All outcomes | Low risk | Identical presentation of Ketotifen and placebo |
Croce 1995.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described | |
| Participants | N = 75 (randomised) N = 66 (completed) WITHDRAWAL/DROPOUT: 5 in Ketotifen group (adverse reaction: 1, non cooperation: 1, severe concurrent illness: 1, deterioration of asthma: 2), 4 in placebo group (deterioration of asthma: 3, non cooperation:1) AGE: 6‐25 years (mean 11,9 years) SEVERITY: moderate asthma defined as reversibility in FEV1 of at least 15% following inhaled beta‐2 agonists; oral steroids were not permitted in the month preceding the study | |
| Interventions | DOSE: 1 mg twice daily; dose dependent on body weight RUN‐IN: 0 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: beta2‐agonists, theophylline, inhaled corticosteroids | |
| Outcomes | patient's and doctor's opinion of treatment effect, withdrawal due to worsening of asthma, lung function (FEV1, FVC), clinical assessment of asthma severity | |
| Notes | three‐arm trial comparing DSCG, Ketotifen and placebo; only data of Ketotifen and placebo group extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Dawson 1989.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described; first 4 weeks of the study were regarded as "run in" period and their results were excluded from the final analysis. In the final phase of the study, existing treatment was withdrawn from both the active and the placebo series. | |
| Participants | N = 60 (randomised) WITHDRAWAL/DROPOUT: no information AGE: 5‐13 years (mean 7 years) SEVERITY: children included in the study had a minimum of four episodes per month, a perennial pattern and a FEV1 of greater than 50% of predicted | |
| Interventions | DOSE: 2 mg a day RUN‐IN: 4 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: inhaled beta‐2 agonists, oral theophylline, inhaled sodium cromoglycate, inhaled beclomethasone less than 400µg/day | |
| Outcomes | PEF, symptoms, restricted activity, asthma attacks, hospital admission, school absence | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
de Benedictis 1990.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described | |
| Participants | N = 79 (randomised) N = 75 (completed) WITHDRAWAL/DROPOUT: 2 in Ketotifen group for "personal reasons", 2 in placebo group due to incomplete data and no success in therapy AGE: 4‐23 months SEVERITY: wheezing or cough, diagnosed as asthma | |
| Interventions | DOSE: 0,5 mg twice daily RUN‐IN: 2 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: beta‐2 agonists, theophylline | |
| Outcomes | Number of patients with cough, with wheezing with severe asthma, using bronchodilators; side effects and treatment efficacy rated by physicians | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Graff Lonnevig 1985.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described; placebo tablets contained lactose | |
| Participants | N = 18 (randomised and completed) AGE: 6,9 ‐13,2 years (mean 9,6 years) SEVERITY: level of basal bronchial obstruction not lower than 30% of predicted values and a reversibility of not more than 30% after inhalation of ß2‐receptor stimulants; metacholine challenge should produce a lowering of FEV1 of at least 20% in the metacholine concentration interval 0,25 to 4,0 mg/ml; exclusion of patients with severe asthma and patients receiving steroid treatment, prophylactic treatment with disodium cromoglycate, hyposensitization treatment for mould or mite allergy | |
| Interventions | DOSE: 1 mg twice daily RUN‐IN: 2 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: beta‐2 receptor stimulants, theophylline, antihistamines, nasal congestants | |
| Outcomes | decreased sensitivity to metacholine, FEV1, PC20 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Kabra 2000.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described | |
| Participants | N = 120 (randomised) N = 107 (completed) WITHDRAWAL/DROPOUT: 4 in Ketotifen group (development of hepatitis B: 1, bronchiectasis: 1, not taking > 75% of trial drug: 2), 9 in placebo group (development of hepatitis B: 1, pulmonary tuberculosis: 1, not taking > 75% of trial drug: 3, dropout: 4) AGE: 5‐15 years SEVERITY: reversible bronchospasm; improvement in PEFR > 20% after inhalation of salbutamol; duration of asthma more than 1 year; no history of taking steroids, ketotifen, or sodium cromoglycate in the previous 3 months | |
| Interventions | DOSE: 1mg twice daily RUN‐IN: 4 weeks TREATMENT: 20 weeks ADDITIONAL MEDICATION: salbutamol, theophylline, inhaled steroids, oral prednisolone | |
| Outcomes | symptom score, symptom free days, lung function parameters, additional medication, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Kelly 1982.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described | |
| Participants | N = 60 (randomised) N = 58 (completed) WITHDRAWAL/DROPOUT: 2 in Ketotifen group (0,5 mg bd) due to side effects AGE: 31‐172 months (mean 102, 6 months) SEVERITY: asthmatic children not requiring long‐term oral steroids; skin testing against common allergens was performed on 45 patients and all had at least 2 significant reactions (weal greater than 3 mm diameter); all 60 children had elevated serum igE and 53 had eosinophilia (greater than 500 eosinophils/ml) | |
| Interventions | DOSE: 0,5 and 1,0 mg twice daily RUN‐IN: 4 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: treatment with antihistamines and DSCG stopped at start of run‐in period | |
| Outcomes | diary card (night wheeze, night cough, day activity, day wheeze, nasal symptoms, peak flow, other treatment), frequency of side effects, withdrawals due to adverse effects, morning and evening peak flow | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Loftus 1987.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled, CROSSOVER TRIAL (4‐week washout period) RANDOMISATION: method of randomisation not described; medication was prepared to look and taste identically | |
| Participants | N = 61 (randomised) N = 47 (completed) WITHDRAWAL/DROPOUT: 14 (move out of district: 2, non‐compliance: 2, not going to follow‐up visits: 7, side effects: 1, deterioration in symptoms: 2) AGE: 2‐6 years (mean 3,8 years) SEVERITY: requiring continuous treatment due to frequent symptoms (one bad attack per month or symptoms on most days), or taking prophylactic treatment daily; children on oral steroids were excluded; 42 children had elevated IgE levels; 42 of 52 tested children had positive results in skin prick tests of common allergens; multiple reactions in 36 children; active or previous eczema in 32 children, allergic rhinitis in 40 children | |
| Interventions | DOSE: 1 mg twice daily RUN‐IN: 4 weeks TREATMENT: 2x24 weeks ADDITIONAL MEDICATION: beta‐2 agonists, theophylline, cromoglycate, inhaled and oral steroids | |
| Outcomes | symptom score (day and night asthma), day eczema, night eczema, day rhinitis, night rhinitis, daily medication score | |
| Notes | data not reported separately by treatment group and treatment period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical presentation of Ketotifen and placebo |
Longo 1986.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described | |
| Participants | N = 41 (randomised) N = 36 (completed) WITHDRAWAL/DROPOUT: 5 (deterioration: 3, non‐compliance: 2) AGE: 4‐14 years (mean 9,8 years) SEVERITY: allergic asthma with a minimum of 2 asthma attacks per month | |
| Interventions | DOSE: 1 mg twice daily RUN‐IN: 0 weeks TREATMENT: 16 weeks ADDITIONAL MEDICATION: continuous antiasthmatic treatment stopped at first visit, thereafter, beta‐2 receptor stimulants, steroids allowed if needed | |
| Outcomes | clinical assessment (parents and physicians), PEFR, bronchodilator consumption | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Montoya 1988.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described; placebo tablets contained lactose | |
| Participants | N = 40 (randomised and completed) WITHDRAWAL/DROPOUT: exclusion of patients never going to follow‐up visits, these cases were replaced AGE: 6‐12 years (mean 9,4 years) SEVERITY: minimum of 2 years of evolution of sickness, showing low to moderate crisis, non corticosteroid‐dependent with equal intervals or less than 15 days; inclusion of patients with allergic asthma defined by coexistence of other allergic sickness (rhinitis, conjunctivitis, eczema), by the child's family history of allergic problems and because they had positive results in skin tests; exclusion of patients requiring frequent hospitalization, being in danger, with intrinsic asthma | |
| Interventions | DOSE: 1 mg twice daily RUN‐IN: 0 weeks TREATMENT: 16 weeks ADDITIONAL MEDICATION: "bronchodilators" | |
| Outcomes | reduction on crisis frequency, crisis intensity, time crisis lasted, reduction on bronchodilator consumption, treatment results (evaluated by parents) and global treatment results | |
| Notes | three arm trial comparing nifedipine, ketotifen and placebo; only data of ketotifen and placebo group extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Placebo tablets contained lactose |
Mulhern 1982.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: number (with corresponding treatment) was allotted to each patient according to trial entry | |
| Participants | N = 33 (randomised) N = 31 (completed) WITHDRAWAL/DROPOUT: 2 dropped out during run‐in period AGE: 4‐11 years (mean 6,9 years) SEVERITY: clinical diagnosis of atopic asthma and no other serious illness; no intercurrent respiratory infection at beginning of study | |
| Interventions | DOSE: 1 mg/d for 1 month and 2 mg/d for 2 months RUN‐IN: 2 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: "concomitant drug therapy (if any)" | |
| Outcomes | effectiveness of drug, side effects, average symptom score, average lung function readings, cough and wheeze (day and night) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Number (with corresponding treatment) was allotted to each patient according to trial entry |
| Blinding? All outcomes | Unclear risk | Information not available |
Myloma 1990.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described; ketotifen and placebo were indistinguishable with equal volume of oral solution | |
| Participants | N = 40 (randomised) N = 39 (completed) WITHDRAWAL/DROPOUT: 1 in placebo group did not complete the trial AGE: 3‐14 years (mean 7 years) SEVERITY: average duration of 4 years of asthma; positive family history of atopy in 34 children, history of rhinitis and /or eczema in 32 children inclusion criteria: at least 1 year asthma; moderate severity based on clinical evaluation (1‐2 attacks/month with night symptoms and/or reduced activity for at least once a week or less than 50% of the days); asthma attacks unrelated to respiratory infection; positive skin prick test for one or more common inhalant allergens; frequent or chronic use of non‐steroid medication | |
| Interventions | DOSE: 1 mg twice daily RUN‐IN: 0 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: inhaled beta‐2 agonists, oral solutions of salbutamol and theophylline, subcutaneous epinephrine | |
| Outcomes | asthma severity, weight gain, insomnia/somnolence, dryness of the mouth, bronchodilator use, asthma attacks, pulmonary physical findings | |
| Notes | only first part of study (randomised controlled trial) included in this review; second part (open study) not considered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical presentation of Ketotifen and placebo |
Neijens 1988.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described | |
| Participants | N = 142 (randomised) N = 134 (completed) WITHDRAWAL/DROPOUT: 8 due to protocol violations AGE: 4 months‐ 4 years (mean 22,3 months) SEVERITY: wheezing for at least 8 weeks; wheezing or coughing at entry into study; presence of allergy and nasal discharge (optional); exclusion of patients with any serious or other chronic disorder | |
| Interventions | DOSE: 0,5 mg twice daily RUN‐IN: 2 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: "bronchodilator use" | |
| Outcomes | asthmatic attacks, wheeze on auscultation, bronchodilator use, nasal symptoms, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Rackham 1989.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described; identical placebo tablet or syrup used | |
| Participants | N = 191 (randomised) N = 138 (completed) WITHDRAWAL/DROPOUT: 43 exclusions (non‐compliance or excessive missing data); 5 drop‐outs in Ketotifen group (sedation: 2, treatment failure: 3), 5 drop‐outs in placebo group (sedation: 1, treatment failure: 4) AGE: 5 ‐ 17 years (mean 10,1 years) SEVERITY: chronic asthma symptoms requiring daily medications (beta‐agonists and/or xanthines), having a documented history of extrinsic (atopic) asthma and having demonstrated reversible bronchoconstriction; patients had to show a response to a metered dose of inhaled beta‐agonists; exclusion of patients requiring treatment with steroids or DSCG at trial entry | |
| Interventions | DOSE: 1 mg twice daily RUN‐IN: 4 weeks TREATMENT: 26 weeks ADDITIONAL MEDICATION: beta‐2 agonists; theophylline and steroids allowed during treatment period (indication of lack of asthma control) | |
| Outcomes | average daily doses of theophylline and beta‐agonists, FEV1, FVC, PEFR, diurnal variability in peak flow, asthma symptom score, lung ausculation score, FEF (25‐75), number of patients using theophylline, patient global evaluation, physician's evaluation, intercurrent illness (hospital visits due to asthma or URTI), side effects (rush or urticaria, sedation, weight gain, diarrhoea, nausea, vomiting, increased appetite, abdominal pain) | |
| Notes | authors' comment on excluded patients: "Review of the data of the 43 patients that dropped out because of noncompliance did not reveal any outcome difference between these patients and the fully analyzed patients." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical presentation of Ketotifen and placebo |
Rebmann 1987.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described | |
| Participants | N = 29 (randomised) WITHDRAWAL/DROPOUT: no information AGE: 6‐16 years (mean 10,9 years) SEVERITY: allergic (extrinsic) or mixed asthma; skin and IgE tests were performed prior to study entry | |
| Interventions | DOSE: 1 mg twice daily RUN‐IN: 4 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: DSCG, theophylline, inhaled salbutamol, ipratropium bromide, beclomethasone, hyposensibilisation | |
| Outcomes | FEF(25‐75%), pO2, FEV1, additional medication, asthma severity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Reid 1989.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described | |
| Participants | N = 214 (randomised) N = 189 (completed) WITHDRAWAL/DROPOUT: 12 in Ketotifen group (deterioration of symptoms or adverse effects: 4, reasons unrelated to treatment, i.e. poor compliance, concurrent illness: 8), 13 in placebo group (deterioration of symptoms or adverse effects: 3, reasons unrelated to treatment: 10) AGE: 2‐6 years (mean 54 months) SEVERITY: symptoms of chronic recurrent cough and/or wheeze and evidence of airway hyperreactivity (symptoms present for at least 4 weeks); alternatively, history of 3 or more episodes of at least 5 days of cough and/or wheeze over a 2 month period or 5 or more episodes over a 3 month period;exclusion of patients taking inhaled or systemic steroids at study entry | |
| Interventions | DOSE: 1 mg/d for 1 week followed by 2 mg/d RUN‐IN: 4 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: beta‐2 agonists, xanthines, steroids | |
| Outcomes | parents' opinion, day and night cough, day and night wheeze, exacerbations, housebound days, medication score, average behavior score | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Salmon 1982.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described | |
| Participants | N = 63 (randomised) N = 61 (completed) WITHDRAWAL/DROPOUT: 2 because of drowsiness AGE: 5‐11 years (mean 8,5 years) SEVERITY: extrinsic asthma with reversible bronchospasm; history of attacks following exposure to known allergens; positive skin prick tests, otherwise evidence of atopy, e.g. urticaria, seasonal rhinitis, eczema or increased levels of IgE; no patient with serious disease of other body systems or intercurrent RTI at study entry | |
| Interventions | DOSE: 1 mg twice daily RUN‐IN: 1 weeks TREATMENT: 11 weeks ADDITIONAL MEDICATION: "concomitant medication used varied quite widely between patients" | |
| Outcomes | increased concomitant medication, overall efficacy, side effects, mean symptom score (day‐ and night‐time) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Santos 1999.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described | |
| Participants | N = 62 (randomised) WITHDRAWAL/DROPOUT: no information AGE: 6‐15 years SEVERITY: allergic asthmatic children | |
| Interventions | DOSE: 1 mg twice daily RUN‐IN: 0 weeks TREATMENT: 16 weeks ADDITIONAL MEDICATION: bronchodilators, steroids | |
| Outcomes | lung function, bronchodilator use, use of steroids | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Simons 1982.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not clearly described (drugs used were coded according to the route of administration and the pharmacological class and were converted into number of units per day) | |
| Participants | N = 21 (randomised) N = 20 (completed) WITHDRAWAL/DROPOUT: 1 patient left for personal reasons in week 2 AGE: 7,4‐12,7 years (mean 10,4 years) SEVERITY: history of intermittent wheezing; responsive to bronchodilator; atopic as documented by eosinophilia, elevated immunoglobulin E for age and at least three positive prick tests to common inhalant antigens; at least 20% improvement in FEV1 after salbutamol inhalation; all children required theophylline, beta‐adrenergic drugs or sodium cromoglycate treatment regularly for asthma prophylaxis at study entry; none ever required corticosteroid treatment between episodes of acute asthma | |
| Interventions | DOSE: 1 mg twice daily RUN‐IN: 2 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: no change in usual treatment during the first 2 weeks (run‐in), i.e. theophylline, beta‐adrenergic drug, sodium cromoglycate; "concomitant medication" measured after 2 weeks | |
| Outcomes | admission to hospital for acute asthma; emergency room visits, increase other anti‐asthma medications, adverse effects, weight increase, dry mouth, increased appetite, asthma symptom score (day‐time, night‐time), PEF, pulmonary function, mean pulse rate, mean systolic blood pressure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Drugs used were coded according to the route of administration and the pharmacological class and were converted into number of units per day |
| Blinding? All outcomes | Unclear risk | Information not available |
Spicak 1983.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described | |
| Participants | N = 50 (randomised and completed) AGE: 1‐8 years (mean 5,2 years) SEVERITY: allergic bronchial asthma with positive skin tests; desensitization performed without great benefit | |
| Interventions | DOSE: 1, 1,5 or 2 mg/d according to body weight RUN‐IN: 0 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: symptomatic anti‐asthmatic treatment with beta‐2 stimulants, theophylline or corticosteroids was continued and then gradually reduced until it was withdrawn or symptoms recurred | |
| Outcomes | overall assessment of efficacy by physician, total duration and frequency of asthmatic episodes per observation period, safety and side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
Van Asperen 1992.
| Methods | DESIGN: multicenter, prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: randomisation by computer generated code; parallel group design with infants stratified by age groups, gender and symptoms; placebo syrup was identical in appearance | |
| Participants | N = 113 (randomised) N = 97 (completed) WITHDRAWAL/DROPOUT: 1 (insufficient symptoms to continue treatment), 7 (loss to follow‐up or unwillingness to continue study), 4 adverse events (diarrhoea, vomiting or rush, each 2 in Ketotifen and placebo group), 2 (protocol violation), 2 (treatment failure) AGE: 6‐36 months (mean 22 months) SEVERITY: history of cough and/or wheeze for at least 3 months and occurring on at least 50% of the days; exclusion of infants with any serious chronic illness, previous long‐term use of sodium cromoglycate, currently use of theophylline or sodium cromoglycate or use of steroids for more than 5 days in the last 3 months | |
| Interventions | DOSE: 0,5 mg twice a day RUN‐IN: 4 weeks TREATMENT: 16 weeks ADDITIONAL MEDICATION: beta‐2 agonists, antibiotics | |
| Outcomes | treatment efficacy (assessed by investigators and parents) tolerability, beta‐agonist usage | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical presentation of Ketotifen and placebo |
Varsano 1993.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: randomisation by clinical trial department of Sandoz; study drugs (active and placebo) were supplied by Sandoz in bottles coded with serial numbers | |
| Participants | N = 117 (randomised) N = 108 (completed) WITHDRAWAL/DROPOUT: 9 patients excluded (non‐compliance or incomplete data) AGE: 6 months to 3 years (mean 17 months) SEVERITY: history of 3 or more wheezing episodes during the 12 weeks preceding the study; exclusion of infants with a history of chronic or congenital lung disease, using sodium cromoglycate for 4 weeks during the 2 months preceding the trial | |
| Interventions | DOSE: 0,5 or 1 mg twice daily depending on age RUN‐IN: 2 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: inhaled beta‐2 agonists, theophylline, oral steroids | |
| Outcomes | asthmatic symptomatology, use of additional drugs, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical presentation of Ketotifen and placebo |
Volovitz 1988.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled CROSSOVER TRIAL (2 week washout period) RANDOMISATION: method of randomisation not described; ketotifen and placebo syrup of similar consistency and appearance and almost identical in taste | |
| Participants | N = 31 (randomised) N = 30 (completed) WITHDRAWAL/DROPOUT: 1 patient excluded (non‐compliance) AGE: 12‐35 months (mean 22,6 months) SEVERITY: mild or moderate asthma | |
| Interventions | DOSE: 1 mg twice daily RUN‐IN: 2 weeks TREATMENT: 12 weeks ADDITIONAL MEDICATION: beta‐2 agonists, theophylline, burst of prednisone | |
| Outcomes | asthmatic symptomology, concomitant medication | |
| Notes | only data from first period of trial considered in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Low risk | Identical presentation of Ketotifen and placebo |
White 1988.
| Methods | DESIGN: prospective, randomised, double blind, placebo‐controlled trial RANDOMISATION: method of randomisation not described; patients were matched for age and severity of symptoms (as judged by need for inhaled or oral steroids) | |
| Participants | N = 42 (randomised) N = 37 (completed) WITHDRAWAL/DROPOUT: 2 in Ketotifen group (default within 2 months of starting treatment: 2), 3 in placebo group (default within 2 months of starting treatment: 3, drowsiness: 1) AGE: 1,3‐5,9 years (mean age 4,1) SEVERITY: atopic and allergic rhinitis; eczema in 15 children | |
| Interventions | DOSE: 1 mg twice daily (< 5 years) or 2 mg twice daily (> 5 years) RUN‐IN: 4 weeks TREATMENT: 16 weeks ADDITIONAL MEDICATION: DSCG was only drug forbidden during trial | |
| Outcomes | symptom score, bronchodilator use, side effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; patients were matched for age and severity of symptoms (as judged by need for inhaled or oral steroids) |
| Allocation concealment? | Unclear risk | Information not available |
| Blinding? All outcomes | Unclear risk | Information not available |
DSCG: cromoglycate FEF: forced expiratory flow FEV1: forced expiratory volume at 1 minute FVC: forced vital capacity ICS: inhaled corticosteroids pO2: local partial pressure for O2 PC20:provocative concentration for 20% fall in PEF: peak expiratory flow PEFR: peak expiratory flow rate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Botey 1983 | Dose of ketotifen less than 1mg/day for some children |
| Gonzalez 1988 | Not described as randomised |
| Göbel 1979 | Not placebo‐controlled |
| Hoshino 1997 | Age: 16‐50 years; no separate data for children/adolescents |
| Huerta 1985 | Dose of ketotifen less than 1mg/day for some children |
| Mathov 1983 | Age: 10‐55 years; no separate data for children/adolescents |
| Medici 1989 | Age: 6‐51 years; no separate data for children/adolescents |
| Miraglia 1986 | Dose of ketotifen less than 1mg/day for some children |
| Naspitz 1988 | Not decribed as randomised |
| Poder 1982 | Not double blinded |
| Podleski 1984 | Age: 12‐66 years; no separate data for children/adolescents |
| Polverino 1994 | Not described as placebo‐controlled (the control group was treated on an "as needed basis") |
| Tinkelmann 1985 | Only 15% of participants aged between 12‐18 years, no separate data for these participants |
| Weheba 1979 | Open study |
| Wenz 1986 | Not described as randomised; no information on age of participants |
Contributions of authors
Two reviewers (DB, AM) independently selected trials for inclusion. Disagreements were solved by discussion involving a third reviewer (JF). For each included trial, two reviewers (DB, GS) independently extracted data for all outcome variables. Data were entered into RevMan 5 by one reviewer (GS) and cross‐check by another reviewer (DB). The review was edited and supervised by a fifth reviewer (FD).
Sources of support
Internal sources
German Cochrane Centre (funded by the German Ministry of Health/University of Freiburg), Germany.
Bursary Award from the Cochrane Child Health Field, Canada.
External sources
No sources of support supplied
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Broberger 1986 {published data only}
- Broberger U, Graff‐Lonnevig V, Lilja G, Rylander E. Ketotifen in pollen‐induced asthma: a double blind placebo‐controlled study. Clinical Allergy 1986;16(2):119‐27. [DOI] [PubMed] [Google Scholar]
Canny 1997 {published data only}
- Canny GJ, Reisman J, Levison H. Does ketotifen have a steroid‐sparing effect in childhood asthma?. European Respiratory Journal 1997;10(1):65‐70. [DOI] [PubMed] [Google Scholar]
Chay 1992 {published data only}
- Chay OM, Foo AL, Ho L. Efficacy of Zaditen (ketotifen) in wheezy infants and young children. Journal of the Singapore Paediatric Society 1992;34(3‐4):235‐9. [PubMed] [Google Scholar]
Croce 1995 {published data only}
- Croce J, Negreiros EB, Mazzei JA, Isturiz G. A double‐blind, placebo‐controlled comparison of sodium cromoglycate and ketotifen in the treatment of childhood asthma. Allergy 1995;50(6):524‐7. [DOI] [PubMed] [Google Scholar]
Dawson 1989 {published data only}
- Dawson KP, Fergusson DM, Horwood LJ, Mogridge N. Ketotifen in asthma. Australian Paediatric Journal 1989;25(2):89‐92. [DOI] [PubMed] [Google Scholar]
de Benedictis 1990 {published data only}
- Benedictis FM, Giorgi G, Ceppi S, Falorni A. Prophylactic treatment of wheezy infants with ketotifen. A multicenter controlled study [Il trattamento dell'asma della prima infanzia con chetotifene. Studio multicentrico controllato]. Rivista italiana di pediatria (The Italian journal of pediatrics) 1990;16:8‐12. [Google Scholar]
- Benedictis FM, Todisco T, Dottorini M, Paglino A. Prophylactic treatment of wheezy infants with ketotifen. European Respiratory Journal 1988;1(Suppl 2):336s. [Google Scholar]
Graff Lonnevig 1985 {published data only}
- Graff‐Lonnevig V, Hedlin G. The effect of ketotifen on bronchial hyperreactivity in childhood asthma. Journal of Allergy & Clinical Immunology 1985;76(1):59‐63. [DOI] [PubMed] [Google Scholar]
Kabra 2000 {published data only}
- Kabra SK, Pandey RM, Singh R, Seth V. Ketotifen for asthma in children aged 5 to 15 years: a randomized placebo‐controlled trial. Annals of Allergy, Asthma and Immunology 2000;85:46‐52. [DOI] [PubMed] [Google Scholar]
Kelly 1982 {published and unpublished data}
- Kelly P, Taylor M. Interim report of a double‐blind controlled study of ketotifen in childhood asthma. Research and Clinical Forums 1982;4(1):35‐43. [Google Scholar]
Loftus 1987 {published and unpublished data}
- Loftus BG, Price JF. Long‐term, placebo‐controlled trial of ketotifen in the management of preschool children with asthma. Journal of Allergy & Clinical Immunology 1987;79(2):350‐5. [DOI] [PubMed] [Google Scholar]
Longo 1986 {published data only}
- Longo G, Strinati R, Poli F. Double‐blind controlled study by Ketotifen in childhood asthma [Valutazione in doppio cieco del Chetotifene verso placebo nell'asma allergico infantile]. Rivista italiana di pediatria 1986;12:568‐71. [Google Scholar]
Montoya 1988 {published data only}
- Montoya F. Asthma and nifedipine. Comparison of nifedipine, ketotifen and placebo in the prophylaxis of childhood extrinsic asthma. Allergologia et Immunopathologia 1988;16(4):253‐8. [PubMed] [Google Scholar]
- Montoya F. Asthma and nifedipine: comparative study of the profilactic effect of nifedipine against ketotifen and placebo in child's allergic asthma [Asma y nifedipina: estudio comparativo del efecto profilactico de la nifedipina frente al ketotifeno y placebo en asma alergica infantil]. Iatreia 1988;1(2):82‐90. [Google Scholar]
Mulhern 1982 {published data only}
- Mulhern B, O'Connor N, Hillery M, Ward OC. A double blind placebo controlled study on the use of ketotifen in childhood asthma. Irish Medical Journal 1982;75(1):25‐8. [PubMed] [Google Scholar]
Myloma 1990 {published data only}
- Mylona‐Karayanni C, Hadziargurou D, Liapi‐Adamidou G, Anagnostakis I, Sinaniotis C, Saxoni‐Papageorgiou F. Effect of ketotifen on childhood asthma: a double‐blind study. Journal of Asthma 1990;27(2):87‐93. [DOI] [PubMed] [Google Scholar]
Neijens 1988 {published data only}
- Neijens HJ, Knol K. Oral prophylactic treatment in wheezy infants. Immunology & Allergy Practice 1988;X(1):4/17‐10/23. [Google Scholar]
Rackham 1989 {published data only}
- Rackham A, Brown CA, Chandra RK, Ho P, Hoogerwerf PE, Kennedy RJ, et al. A Canadian multicenter study with Zaditen (ketotifen) in the treatment of bronchial asthma in children aged 5 to 17 years. Journal of Allergy & Clinical Immunology 1989;84(3):286‐96. [DOI] [PubMed] [Google Scholar]
Rebmann 1987 {published data only}
- Rebmann H. Ketotifen in a combination therapy of bronchial asthma in children: influence on lung‐function parameters of small airways [Ketotifen in der Kombinationstherapie des kindlichen Asthma bronchiale: Einfluß auf Lungenfunktionsparameter für kleine Atemwege]. Allergologie 1987;10(2):60‐3. [Google Scholar]
Reid 1989 {published data only}
- Reid JJ. Double‐blind trial of Ketotifen in childhood chronic cough and wheeze. Immunology & Allergy Practice 1989;XI(4):143/13‐20/150. [Google Scholar]
Salmon 1982 {published data only}
- Salmon MA. Multicentre study for the evaluation of ketotifen in the prophylaxis of extrinsic asthma in children. Research and Clinical Forums 1982;4(1):45‐50. [Google Scholar]
Santos 1999 {published data only}
- Santos OR. Double blind study with ketotifen and placebo in asthmatic children [Estudio doble ciego comparando ketotifeno y placebo en ninos asmaticos]. Pediatrika 1999;19(9):41‐5. [Google Scholar]
Simons 1982 {published data only}
- Simons FE, Luciuk GH, Becker AB, Gillespie CA. Ketotifen: a new drug for prophylaxis of asthma in children. Annals of Allergy 1982;48(3):145‐50. [PubMed] [Google Scholar]
Spicak 1983 {published data only}
- Spicak V. The prophylactic treatment of bronchial asthma in children with ketotifen: a double‐blind comparison with placebo. Journal of International Medical Research 1983;11(3):173‐8. [DOI] [PubMed] [Google Scholar]
Van Asperen 1992 {published and unpublished data}
- Asperen PP, McKay KO, Mellis CM, Loh RK, Harth SC, Thong YH, et al. A multicentre randomized placebo‐controlled double‐blind study on the efficacy of Ketotifen in infants with chronic cough or wheeze. Journal of Paediatrics & Child Health 1992;28(6):442‐6. [DOI] [PubMed] [Google Scholar]
Varsano 1993 {published and unpublished data}
- Varsano I, Volovitz B, Soferman R, Tal A, Schlessinger M, Rotchild M, et al. Multicenter study with ketotifen (Zaditen) oral drop solution in the treatment of wheezy children aged 6 months to 3 years. Pediatric Allergy & Immunology 1993;4(1):45‐50. [DOI] [PubMed] [Google Scholar]
Volovitz 1988 {published data only}
- Volovitz B, Varsano I, Cumella JC, Jaber L. Efficacy and safety of ketotifen in young children with asthma. Journal of Allergy & Clinical Immunology 1988;81(3):526‐30. [PubMed] [Google Scholar]
White 1988 {published data only}
- White MP, MacDonald TH, Garg RA. Ketotifen in the young asthmatic ‐ a double‐blind placebo‐controlled trial. Journal of International Medical Research 1988;16(2):107‐13. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Botey 1983 {published data only}
- Botey J, Eseverri JL, Malet A, Ibero M, Marin A. Ketotifen in childhood asthma [El ketotifeno en el asma infantil]. Allergologia et Immunopathologia 1983;11(5):329‐33. [PubMed] [Google Scholar]
Gonzalez 1988 {published data only}
- Gonzalez R, Girardi G. A clinical trial of Ketotifen in the management of asthma in infants. Immunology and Allergy Practice 1988;10(6):15‐9. [Google Scholar]
Göbel 1979 {published data only}
- Göbel P. Efficacy and tolerability of oral ketotifen in the long‐term prophylactic treatment of bronchial asthma in children. Pharmatherapeutica 1979;2(3):153‐8. [Google Scholar]
Hoshino 1997 {published data only}
- Hoshino M, Nakamura Y, Shin Z, et al. Effects of ketotifen on symptoms and on bronchial mucosa in patients with atopic asthma. Allergy 1997;52:814‐20. [DOI] [PubMed] [Google Scholar]
Huerta 1985 {published data only}
- Huerta JG. Ketotifen in the prophylaxis of child asthma. A controlled clinical test [Ketotifeno en la profilaxis del asma infantil. Ensayo clinico controlado]. Allergologia et Immunopathologia 1985;13(I):1‐7. [PubMed] [Google Scholar]
Mathov 1983 {published data only}
- Mathov E, Grinstein M, Greiding L, et al. A long‐term treatment of bronchial.asthma with ketotifen. Relationships with bronchial hypersensitivity [Atamiento del asma bronquial Ketotifeno a largo plazo laciones con la hiperirritabilidad bronquial]. Alergia 1983;30(I):13‐27. [PubMed] [Google Scholar]
Medici 1989 {published data only}
- Medici TC, Radielovic P, Morley J. Ketotifen in the prophylaxis of extrensic asthma. A multicenter double‐blind study with a modified‐release formulation. Chest 1989;96:1252‐7. [DOI] [PubMed] [Google Scholar]
Miraglia 1986 {published data only}
- Miraglia del Giudice M, Capristo AF, Maiello M, Decimo F, Bevacqua GA, Miraglia del Giudice JRM, et al. Study of the efficacy of Ketotifen treatment in asthmatic children under 3 years of age. Current Therapeutic Research 1986;40(4):685‐93. [Google Scholar]
Naspitz 1988 {published data only}
- Naspitz CK, Freire CAR. Evaluation of ketotifen in the prophylactic treatment of bronchial asthma in children. Allergologia et Immunopathologia 1988;16(1):27‐31. [PubMed] [Google Scholar]
Poder 1982 {published and unpublished data}
- Poder G, Cserhati E, Kelemen J, Mezei G, Romhanyi I. Development of clinical symptoms in asthmatic children under long‐term treatment of ketotifen [Asztmas gyermekek klinikai tüneteinek alakulasa tartos Ketotifen kezeles alatt]. Pneumonologia Hungarica 1982;35:561‐5. [Google Scholar]
- Poder G, Cserhati E, Kelemen J, Mezei G, Romhanyi I. Development of clinical symptoms in asthmatic children under long‐term treatment of ketotifen [Entwicklung der klinischen Symptome bei asthmatischen Kindern unter Langzeitbehandlung mit Ketotifen]. Pädiatrie und Grenzgebiete 1985;24(2):175‐80. [PubMed] [Google Scholar]
Podleski 1984 {published data only}
- Podleski WK, Zelenak TM, Schmidt JL. Long term trial of Ketotifen in bronchial asthma. Annals of Allergy 1984;52:406‐10. [PubMed] [Google Scholar]
Polverino 1994 {published data only}
- Polverino M. A correct diagnosis for a correct treatment: preventive therapy in asthma [Una corretta diagnosi per un corretto trattamento: terapia preventiva nell'asma]. Italian Journal of Chest Diseases 1994;Suppl B 48(3‐4):109‐15. [Google Scholar]
Tinkelmann 1985 {published data only}
- Tinkelman DG, Moss BA, Bukantz SC, et al. A multicenter trial of the prophylactic effect of ketotifen, theophylline, and placebo in atopic asthma. Journal of Allergy and Clinical Immunology 1985;76:487‐97. [DOI] [PubMed] [Google Scholar]
Weheba 1979 {published data only}
- Weheba AS. Prophylactic treatment of bronchial asthma in children with a new, orally active agent, ketotifen. Pharmatherapeutica 1979;2(3):147‐52. [Google Scholar]
Wenz 1986 {published data only}
- Wenz W, Mohorn M, Jäger L. Experiences with Ketotifen in diseases of the respiratory tract [Erfahrungen mit Ketotifen bei Erkrankungen des Respirationstrakttes]. Allergie und Immunologie 1986;32:215‐22. [PubMed] [Google Scholar]
Additional references
Begg 1996
- Begg C, Cho M, Eastwood S, Horton R, Moher D, Olkin I, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA 1996;276(8):637‐9. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ihaka 1996
- Ihaka R, Gentleman R. A language for data analysis and graphics. Journal of Computational and Graphical Statistics 1996;5(3):299‐314. [Google Scholar]
Jadad 1996
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman DG (for the CONSORT Group). The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. Annals of Internal Medicine 2001;134:657‐62. [DOI] [PubMed] [Google Scholar]
Pao 2002
- Pao CS, McKenzie SA. Randomized controlled trial of fluticasone in pre school children with intermittenet wheeze. American Journal of Respiratory and Critical Care Medicine 2002;166:945‐9. [DOI] [PubMed] [Google Scholar]
Pauwels 2003
- Pauwels RA, Petersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budenoside in mild persistent asthma: a randomised, double‐blind trial. Lancet 2003;361:1071‐6. [DOI] [PubMed] [Google Scholar]
Suissa 2002
- Suissa S, Ernst P, Kezouh A. Regular use of inhaled corticosteroids and the long term prevention of hospitalisation of asthma. Thorax 2002;57:880‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]


