Abstract
Background
Preparations of green tea are used as aids in weight loss and weight maintenance. Catechins and caffeine, both contained in green tea, are each believed to have a role in increasing energy metabolism, which may lead to weight loss. A number of randomised controlled trials (RCTs) evaluating the role of green tea in weight loss have been published; however, the efficacy of green tea preparations in weight loss remains unclear.
Objectives
To assess the efficacy and safety of green tea preparations for weight loss and weight maintenance in overweight or obese adults.
Search methods
We searched the following databases from inception to specified date as well as reference lists of relevant articles: The Cochrane Library (Issue 12, 2011), MEDLINE (December 2011), EMBASE (December 2011), CINAHL (January 2012), AMED (January 2012), Biological Abstracts (January 2012), IBIDS (August 2010), Obesity+ (January 2012), IPA (January 2012) and Web of Science (December 2011). Current Controlled Trials with links to other databases of ongoing trials was also searched.
Selection criteria
RCTs of at least 12 weeks' duration comparing green tea preparations to a control in overweight or obese adults.
Data collection and analysis
Three authors independently extracted data, assessed studies for risk of bias and quality, with differences resolved by consensus. Heterogeneity of included studies was assessed visually using forest plots and quantified using the I2 statistic. We synthesised data using meta‐analysis and descriptive analysis as appropriate; subgroup and sensitivity analyses were conducted. Adverse effects reported in studies were recorded.
Main results
Due to the level of heterogeneity among studies, studies were divided into two groups; those conducted in Japan and those conducted outside Japan. Study length ranged between 12 and 13 weeks. Meta‐analysis of six studies conducted outside Japan showed a mean difference (MD) in weight loss of ‐0.04 kg (95% CI ‐0.5 to 0.4; P = 0.88; I2 = 18%; 532 participants). The eight studies conducted in Japan were not similar enough to allow pooling of results and MD in weight loss ranged from ‐0.2 kg to ‐3.5 kg (1030 participants) in favour of green tea preparations. Meta‐analysis of studies measuring change in body mass index (BMI) conducted outside Japan showed a MD in BMI of ‐0.2 kg/m2 (95% CI ‐0.5 to 0.1; P = 0.21; I2 = 38%; 222 participants). Differences among the eight studies conducted in Japan did not allow pooling of results and showed a reduction in BMI ranging from no effect to ‐1.3 kg/m2 (1030 participants), in favour of green tea preparations over control. Meta‐analysis of five studies conducted outside Japan and measuring waist circumference reported a MD of ‐0.2 cm (95% CI ‐1.4 to 0.9; P = 0.70; I2 = 58%; 404 participants). Differences among the eight studies conducted in Japan did not allow pooling of results and showed effects on waist circumference ranging from a gain of 1 cm to a loss of 3.3 cm (1030 participants). Meta‐analysis for three weight loss studies, conducted outside Japan, with waist‐to‐hip ratio data (144 participants) yielded no significant change (MD 0; 95% CI ‐0.02 to 0.01). Analysis of two studies conducted to determine if green tea could help to maintain weight after a period of weight loss (184 participants) showed a change in weight loss of 0.6 to ‐1.6 kg, a change in BMI from 0.2 to ‐0.5 kg/m2 and a change in waist circumference from 0.3 to ‐1.7 cm. In the eight studies that recorded adverse events, four reported adverse events that were mild to moderate, with the exception of two (green tea preparations group) that required hospitalisation (reported as not associated with the intervention). Nine studies reported on compliance/adherence, one study assessed attitude towards eating as part of the health‐related quality of life outcome. No studies reported on patient satisfaction, morbidity or cost.
Authors' conclusions
Green tea preparations appear to induce a small, statistically non‐significant weight loss in overweight or obese adults. Because the amount of weight loss is small, it is not likely to be clinically important. Green tea had no significant effect on the maintenance of weight loss. Of those studies recording information on adverse events, only two identified an adverse event requiring hospitalisation. The remaining adverse events were judged to be mild to moderate.
Plain language summary
Green tea for weight loss and weight maintenance in overweight or obese adults
Green tea has a long history of many uses, one of which is helping overweight people to lose weight and to maintain weight loss. Believed to be able to increase a person's energy output, green tea weight loss preparations are extracts of green tea that contain a higher concentration of ingredients (catechins and caffeine) than the typical green tea beverage prepared from a tea bag and boiling water. This review looked at 15 weight loss studies and three studies measuring weight maintenance where some form of a green tea preparation was given to one group and results compared to a group receiving a control. Neither group knew whether they were receiving the green tea preparation or the control. A total of 1945 participants completed the studies, ranging in length from 12 to 13 weeks. In summary, the loss in weight in adults who had taken a green tea preparation was statistically not significant, was very small and is not likely to be clinically important. Similar results were found in studies that used other ways to measure loss in weight (body mass index, waist circumference). Studies examining the effect of green tea preparations on weight maintenance did not show any benefit compared to the use of a control preparation.
Most adverse effects, such as nausea, constipation, abdominal discomfort and increased blood pressure, were judged to be mild to moderate and to be unrelated to the green tea or control intervention. No deaths were reported, although adverse events required hospitalisation. One study attempted to look at health‐related quality of life by asking participants about their attitudes towards eating. Nine studies tracked participants' compliance with green tea preparations. Studies did not include any information about the effects of green tea preparations on morbidity, costs or patient satisfaction.
Summary of findings
Summary of findings for the main comparison. Green tea for overweight or obese adults.
| Green tea for overweight or obese adults | ||||||
|
Patient or population: overweight or obese adults
Settings: primary care and research centres
Intervention: green tea preparations Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Green tea | |||||
| All‐cause mortality | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Morbidity | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Health‐related quality of life | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Patient satisfaction | See comment | See comment | Not estimable | See comment | See comment | Not investigated |
| Adverse effects 12 to 13 weeks follow‐up | See comment | See comment | Not estimable | See comment | ⊕⊝⊝⊝ very low1 | 6 studies did not report on the presence or absence of adverse events, 8 reported that there were none and 4 reported the presence of adverse events. Most adverse effects, such as nausea, constipation, abdominal discomfort and increased blood pressure, were judged to be mild to moderate |
| Weight loss (change in kg) 12 to 13 weeks follow‐up | The mean weight loss in the control groups ranged from 4.2 kg lost to 0.08 kg gained | The mean weight loss in the intervention groups was 0.04 kg greater | ‐0.04 (0.5 to 0.43) | 532 (6 studies) | ⊕⊕⊕⊝ moderate2 | Although there was a very small effect with green tea compared to control, it was not statistically significant and may not be clinically relevant |
| Costs | See comment | See comment | Not estimable | See comment | See comment | Not reported |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Short follow‐up and non‐systematic reporting of adverse effects.
2Downgraded one level because all six studies were assessed as having unclear risk of bias in at least one domain. Five of six studies had random sequence generation assessed as unclear risk of bias. Four of six had both allocation concealment and blinding assessed as unclear risk of bias.
Background
Description of the condition
Obesity has been defined by the World Health Organization (WHO) as "abnormal or excessive fat accumulation that may impair health" (WHO 2011) and has reached epidemic proportions in the western world. Canadian statistics, for example, revealed that in 2004, 59% of adult Canadians were overweight with 23% of these adults classified as obese. The most common classification of the severity of obesity is made based on body mass index (BMI) measurements. According to WHO, an adult with a BMI of 25 kg/m2 or greater is classified as overweight and an adult with a BMI of 30 kg/m2 or greater is considered to be obese (Eckel 2008; WHO 2011). In some Asian countries, the minimum BMI to be considered overweight is often lower (Auvichayapat 2008). Addressing the epidemic of obesity is complex, with many physiological, psychological, and environmental factors to consider. The direct and indirect costs to the healthcare system that can be attributed to obesity are staggering. Changes in eating habits, coupled with lack of exercise, are often implicated in the increase in body weight experienced by many people. The majority of overweight and obese adults are well aware of the need to lose weight and the health risks associated with remaining overweight. However, just as the cause of excess weight gain can be complex, so can the remedy. Many overweight people have tried a variety of methods such as prescription and over‐the‐counter medications, diets and exercise programmes to lose weight, often with limited or short‐term success. Obesity is not an easy condition to manage because, although there are medications available to help with weight loss, significant changes in 'lifestyle' are also required for weight loss to be successful in the long term. It is important to note that research shows that even a modest weight loss of 5% to 10% of body weight is considered sufficient to have beneficial effects on cardiovascular (CV) risk factors associated with being overweight (Wing 2011).
The promise of taking something 'natural' to help lose weight is appealing to consumers. For consumers who have tried many treatments, a natural alternative that they have not tried previously and one that is easily available offers new hope. Weight loss products made from natural sources, such as green tea, are being used by increasing numbers of overweight adults who hope that because the product is 'natural' it will be safer than prescription drugs and will be effective in helping them lose weight. Healthcare providers and consumers need to know how effective green tea products actually are in weight management before deciding if green tea is an appropriate choice for weight loss therapy. This systematic review of studies that utilise green tea preparations for weight loss or weight maintenance fills a need for evidence‐based information about this commonly used natural product.
Description of the intervention
Green tea, made from the dried, non‐fermented leaves of the plant Camellia sinensis, is a natural product that has been enjoyed for centuries as a social beverage and to increase mental alertness (NCCAM 2011). More recently, preparations of green tea have been used as a treatment for a variety of conditions ranging from arthritis to weight loss, as well as a preventive measure for diseases such as cancer, although the supporting clinical evidence for the majority of these conditions is weak or lacking (Gregory 2011).
Green tea preparations used by consumers for weight loss and maintenance of weight loss are not usually the green tea beverages or tea bags for brewing that are marketed as foods and social drinks but rather are processed, more concentrated formulations of green tea, often referred to as green tea extracts (GTE) (Seeram 2006). The plant from which green tea is prepared contains hundreds of different chemicals that may contribute to its pharmacological activity. A complex mixture of polyphenolic compounds, known as catechins, account for up to 30% of the dry weight of Cameillia sinensis leaves and are believed to be responsible for most of green tea's pharmacological activity (Goto 1996; Sloan‐Kettering 2009). Epigallocatechin‐3‐gallate (EGCG), the most abundant catechin found in green tea, makes up almost 40%, by weight, of the mixture of catechins (Seeram 2006). Caffeine occurs naturally in green tea, making up 2% to 5% of the water‐extractable solids from green tea leaves and can account for some of the pharmacological activity of green tea (Sang 2011).
The qualitative and quantitative content of chemical compounds (catechins, caffeine, and others) found in green tea and its products are profoundly affected by a number of factors, some of which are difficult to control. As with all natural products, the chemical content of green tea is influenced by growing, harvesting and drying conditions (Busse 2000). Additionally, the way in which the green tea leaves are processed from the dried leaf into the formulation that is consumed can have a significant impact on the type and amount of pharmacologically active compounds that are present in the final preparation (Busse 2000; Gregersen 2009). For example, the traditional method of steeping dried leaves of green tea in boiling water for several minutes results in a relatively low concentration of catechins and caffeine being extracted from the leaves into the water that is then consumed. A higher concentration of catechins, caffeine, or both can result when green tea products are prepared by soaking dried leaves in water, alcohol, or both, then filtering and evaporating the liquid to produce a dried, concentrated extract, which is then put into a capsule. Some commercial green tea products are prepared using individual catechins that occur naturally in green tea and are isolated and purified. For example, a green tea product may be made by encapsulating purified EGCG, other catechins, or both that occur naturally in green tea. Products prepared in this manner may be different pharmacologically from products made from an extract of green tea that contains the complete array of catechins and caffeine in amounts that may be closer to their natural abundance. Health Canada has recognised the importance of content and to be approved by Health Canada as a green tea product for weight management, green tea products must contain EGCG and caffeine within the following range: 126 to 300 mg EGCG and 75 to 150 mg caffeine, with an EGCG:caffeine ratio of 1.8:1 to 4:1 per day (NHPD 2009).
In summary, the chemical content (both types and amounts of individual chemicals) of green tea preparations can vary significantly. This variation in chemical content can have a direct effect on the pharmacological activity of the green tea preparation. Therefore, when evaluating studies of the effects of green tea on weight loss and weight maintenance, it is essential to know the exact content of the specific green tea preparation being tested in each study.
Adverse effects of the intervention
Reported adverse effects (Gregory 2011) of green tea (as beverage, unless otherwise stated) include.
Gastrointestinal (GI) (high doses of beverage or extract equivalent to 5 to 6 L of beverage/day): nausea/vomiting, diarrhoea, flatulence, abdominal bloating and dyspepsia.
Hepatotoxicity (extract in pill form).
Central nervous system (CNS): dizziness, insomnia, fatigue, tremors, agitation, restlessness, confusion.
CV: tachycardia, palpitations.
Allergy.
How the intervention might work
Although its role in weight loss is unclear, GTE have been shown to increase both energy expenditure and fat oxidation (Dulloo 1999; Rains 2011). Catechins contained in green tea have been shown in vitro to inhibit catechol‐O‐methyltransferase (COMT), resulting in a decrease in the metabolism of norepinephrine (noradrenaline). The resulting increased levels of norepinephrine are believed to be responsible for an increase in energy expenditure and fat oxidation, which may lead to weight loss (Dulloo 1999; Hursel 2009a; Phung 2010). Additionally, results of several studies suggest that glucose metabolism may be improved with the consumption of catechins (Nagao 2009). Along with catechins, green tea also contains caffeine, which has been shown to increase energy metabolism in a dose‐dependent manner (Rains 2011). Caffeine inhibits phosphodiesterase, resulting in increased levels of cyclic adenosine monophosphate, which can stimulate the sympathetic nervous system. It has been shown that caffeine alone cannot account for the total increase in energy metabolism exhibited by green tea and so it is possible that catechins and caffeine, acting by different mechanisms, may have a synergistic effect (Gregersen 2009; Hursel 2009a; Rains 2011).
Why it is important to do this review
The rationale for undertaking this systematic review is that green tea products are promoted to aid in weight loss and weight maintenance and therefore both healthcare providers and consumers need reliable information as to whether green tea products are useful in aiding and maintaining weight loss in overweight and obese adults.
A scan of resources available to healthcare providers and consumers revealed that, in general, recommendations regarding the efficacy of green tea products in weight management are vague. One source stated that there was "insufficient evidence to rate its efficacy in obesity" (Gregory 2011), while another said that "there is not enough reliable data to determine whether green tea can aid in weight loss" (NCCAM 2011). Despite the lack of conclusive recommendations regarding the use of green tea in weight loss, numerous animal and human studies have been conducted in attempts to determine whether green tea preparations have an effect on weight loss. Many of these studies have been examined in a variety of types of reviews including a number of narrative reviews (Cabrera 2006; Chacko 2010; Clement 2009; Grove 2010; Kao 2006; Schneider 2009; Thielecke 2010; Wolfram 2006) and two systematic reviews (Hursel 2009a; Phung 2010) including meta‐analyses; however, the recommendations remain inconclusive.
Eight of the published narrative reviews on green tea and weight loss were examined to determine the basis for their individual conclusions. Three of the reviews (Cabrera 2006; Chacko 2010; Wolfram 2006) included a mixture of animal and human studies, as well as a variety of outcomes and each concluded with a positive statement about green tea being able to contribute to weight loss. Four narrative reviews (Clement 2009; Grove 2010; Kao 2006; Thielecke 2010) limited their conclusions to results of human studies and, in keeping with the three previous reviews, were also positive in their conclusions with phrases such as " may have utility in weight reduction in obese patients" (Clement 2009). Schneider 2009 was the only narrative review that concluded that there was no demonstrated persistent weight loss benefit from green tea.
There have been two systematic reviews of green tea and weight loss published, neither of which used Cochrane methodology (Hursel 2009a; Phung 2010). The first review included a meta‐analysis of 11 RCTs on green tea in weight loss; these authors concluded that catechins or an EGCG‐caffeine mixture contained in green tea had a "modest but significant effect" on weight loss and weight maintenance (Hursel 2009a). There were, however, several important limitations to the review. The first limitation was that all 11 trials included in the review were identified by searching only one database (PubMed), using text words only and did not include medical subject headings (MeSH). It is possible that studies were missed as a consequence of the incomplete search strategy. It is important to note that the search end date used in this review was July 2008. A second limitation was that there appeared to be incomplete reporting of the analysis of risk of bias, an analysis that is recommended in the 'preferred reporting items for systematic reviews and meta‐analyses' (PRISMA) statement (Liberati 2009). A systematic review of 15 trials, published in 2010, had a more comprehensive search strategy (ending in April 2009), utilising MEDLINE, EMBASE, CENTRAL, and the Natural Medicines Comprehensive Database (Phung 2010). The Phung 2010 review included only studies that examined green tea and weight loss (not weight maintenance) and concluded that green tea catechins with caffeine may positively affect weight loss, and reduction in BMI and waist circumference, and noted that the effect is small and not likely to be clinically relevant. The authors of the review identified the fact that the systematic review included studies with heterogeneous populations as a limitation.
Our Cochrane review adds to the results of previous systematic reviews by including several specific items in the methodology that increase the reliability of the conclusions of the review. In addition to including the assessment of risk of bias, this review is based on studies identified using an exhaustive search strategy that is not limited by language and on a clearly defined study population. A strength of this Cochrane review is that the impact of the chemical content of the green tea preparation used in each study was considered when comparing data between studies. In summary, this Cochrane review of the effects of green tea preparations on weight loss and weight maintenance is a significant and comprehensive contribution to the scientific literature and subsequently contributes to information available to healthcare providers and consumers.
Objectives
To assess efficacy and safety of green tea preparations for weight loss and weight maintenance in overweight or obese adults.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials.
Types of participants
Participants were otherwise healthy male or female adults (18 years of age and older), who were classified by study investigators as overweight or obese (as defined by accepted standards such as: BMI or percentage excess weight compared with ideal weight tables) at the beginning of the trial. Acceptable BMI values varied depending on the definition of overweight and obese in the country in which the study was conducted.
Studies were excluded if more than 25% of participants reported a co‐morbidity requiring drug therapy, such as diabetes or CV disease, or were taking medications (other than those in the study) that might have affected weight gain or loss.
Types of interventions
Intervention
A formulation composed exclusively of green tea. This included commercial products, dried alcoholic extracts, aqueous beverages (provided the chemical composition of resulting preparation was given) or a formulation made from the purified natural constituents of green tea (i.e. catechins). Studies were excluded if they used a green tea preparation of unspecified chemical composition or a green tea preparation that contained ingredients in addition to green tea or its natural constituents of catechins and caffeine (i.e. a combination preparation).
Control
Placebo or active weight loss medication amenable to blinding (e.g. trials using exercise solely as a control and not as part of the intervention were excluded).
Types of outcome measures
Primary outcomes
For inclusion in the review, a trial had at least one of the following primary outcomes.
Change in body weight or mass measure (absolute or percentage change in body weight or reduction in BMI; reduction in waist circumference or waist‐to‐hip ratio).
Mortality (any cause).
Health‐related quality of life.
Secondary outcomes
Compliance/adherence.
Patient satisfaction.
Morbidity.
Adverse effects of treatment (description, frequency, severity and outcome).
Costs.
Possible co‐variates, effect modifiers, confounders
Mean duration of obesity for participants in each study was recorded, if stated (duration of obesity has been found to be associated with difficulty in losing weight (Elfhag 2005)).
Compliance and attrition rates for each trial were recorded.
All other weight loss interventions, such as exercise, that participants were using were recorded.
Variation in chemical content of green tea preparation.
Timing of outcome measurement
Only trials of at least 12 weeks' duration were included. Outcome measurements were considered to be medium‐term (12 to 24 weeks) and long‐term (greater than 24 weeks).
Search methods for identification of studies
Electronic searches
We searched the following sources for the identification of studies.
The Cochrane Central Register of Controlled Trials (The Cochrane Library Issue 12, 2011).
MEDLINE (1950 to December 2011).
EMBASE (1980 to December 2011).
CINAHL (1982 to January 2012).
AMED (1985 to January 2012).
Biological Abstracts (1926 to January 2012).
IBIDS (1986 to August 2010 [now defunct]).
Obesity+ (2003 to January 2012).
IPA (1970 to January 2012).
Web of Science (1900 to December 2011).
We also searched databases of ongoing trials: 'Current Controlled Trials' (www.controlled‐trials.com ‐ with links to other databases of ongoing trials).
For detailed search strategies please see Appendix 1.
Studies published in any language were included. All duplicate reports of studies were removed.
Searching other resources
Reference lists of included studies, all types of reviews and health technology assessment reports were searched to identify additional studies.
Data collection and analysis
Selection of studies
To identify studies for further assessment, three review authors (TMJ, AMW, LK) independently scanned the title, abstract or both of every record retrieved. Full text was obtained of all potentially relevant records and assessed for relevance independently by three review authors (TMJ, AMW, LK) using a relevance assessment form developed specifically for this review. English abstracts of non‐English articles were used when possible. In cases where no English abstract was available, articles were translated in sufficient detail to complete the relevance assessment form. Results of relevance assessments were compared and differences of opinion were resolved by discussion and through contacting study authors for clarification, if required. An adapted PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow‐chart of study selection is included as Figure 1 (Liberati 2009).
1.
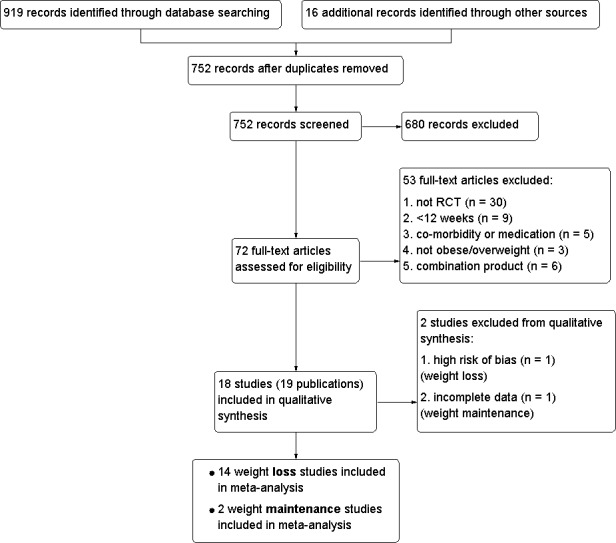
Study flow diagram.
Data extraction and management
For studies fulfilling selection criteria, three review authors (TMJ, AMW, LK) independently extracted relevant population and intervention characteristics using standard data extraction templates (for details see 'Characteristics of included studies, Table 2; Appendix 2; Appendix 3; Appendix 4; Appendix 5; Appendix 6) with any disagreements resolved by discussion. The description of the green tea preparation used in each study was also extracted and evaluated using an adapted version of an instrument designed specifically for natural products (Appendix 7; Appendix 8) (Jurgens 2009). Original author(s) of the article were contacted to obtain missing data, if required. All relevant non‐English articles were translated, in most cases independently by two translators.
1. Overview of study populations.
|
Characteristic Study ID |
Intervention(s) and control(s) | [N] screened | [N] randomised | [N] safety | [N] ITT | [N] finishing study | [%] of randomised participants finishing study |
| Auvichayapat 2008 1 (weight loss study) | I1: green tea extract C1: placebo |
I1: ‐ C1: ‐ T: 73 |
I1: 30 C1: 30 T: 60 |
I1: 30 C1: 30 T: 60 |
‐ | I1: 30 C1: 30 T: 60 |
I1: 100 C1: 100 T: 100 |
| Diepvens 2005 (weight loss study) | I1: green tea C1: placebo |
I1: ‐ C1: ‐ T: 57 |
I1: 23 C1: 23 T: 46 |
I1: 23 C1: 23 T: 46 |
‐ | I1: 23 C1: 23 T: 46 |
I1: 100 C1: 100 T: 100 |
| Hill 2007 (weight loss study) | I1: green tea extract C1: placebo |
‐ | I1: ‐ C1: ‐ T: 42 |
I1: ‐ C1: ‐ T: 42 |
‐ | I1: 19 C1: 19 T: 38 |
I1: ‐ C1: ‐ T: 91 |
| Hsu 2008 (weight loss study) | I1: green tea extract C1: placebo |
I1: ‐ C1: ‐ T: 336 |
I1: 50 C1: 50 T: 100 |
‐ | ‐ | I1: 41 C1: 37 T: 78 |
I1: 82 C1: 74 T: 78 |
| Hursel 2009 (weight maintenance study) | I1: green tea‐caffeine mixture + AP diet I2: green tea‐caffeine mixture + HP diet C1: placebo + AP diet C2: placebo + HP diet |
I1: ‐ I2: ‐ C1: ‐ C2: ‐ T: 100 |
I1: 20 I2: 20 C1: 20 C2: 20 T: 80 |
I1: 20 I2: 20 C1: 20 C2: 20 T: 80 |
‐ | I1: 20 I2: 20 C1: 20 C2: 20 T: 80 |
I1: 100 I2: 100 C1: 100 C2: 100 T: 100 |
| Kajimoto 2005 (weight loss study) | I1: catechin drink – low‐dose group I2: catechin drink – high‐dose group C1: placebo drink |
‐ | I1: ‐ I2: ‐ C1: ‐ T: 197 |
I1: ‐ I2: ‐ C1: ‐ T: 197 |
‐ | I1: 65 I2: 64 C1: 66 T: 195 |
I1: ‐ I2: ‐ C1: ‐ T: 99 |
| Kataoka 2004 (weight loss study) | I1: catechin beverage 278 mg I2: catechin beverage 570 mg I3: catechin beverage 845 mg C1: control beverage |
‐ | I1: 25 I2: 71 I3: 25 C1: 71 T: 192 |
I1: 25 I2: 71 I3: 25 C1: 71 T: 192 |
‐ | I1: 25 I2: 71 I3: 25 C1: 71 T: 192 |
I1: 100 I2: 100 I3: 100 C1: 100 T: 100 |
| Kovacs 2004 2 (weight maintenance study) | I1: green tea C1: placebo |
I1: ‐ C1: ‐ T: 140 |
I1: ‐ C1: ‐ T: 120 |
I1: 51 C1: 53 T: 104 |
‐ | I1: 51 C1: 53 T: 104 |
I1: ‐ C1: ‐ T: 87 |
| Kozuma 2005 3 (weight loss study) | I1: catechin beverage C1: placebo beverage |
I1: ‐ C1: ‐ T: 254 |
I1: ‐ C1: ‐ T: 234 |
I1: ‐ C1: ‐ T: 234 |
‐ | I1: 107 C1: 119 T: 226 |
I1: ‐ C1: ‐ T: 97 |
| Maki 2009 4 (weight loss study) | I1: green tea beverage C1: control beverage |
I1:‐ C1:‐ T: 337 |
I1: 67 C1: 65 T: 132 |
I1: 66 C1: 63 T: 129 |
I1: 65 C1: 63 T: 128 |
I1: 56 C1: 51 T: 107 |
I1: 84 C1: 79 T: 81 |
| Nagao 2007 (weight loss study) | I1: brewed green tea beverage with added catechins C1: brewed green tea beverage with no added catechins |
‐ | I1: 135 C1: 135 T: 270 |
‐ | ‐ | I1: 123 C1: 117 T: 240 |
I1: 91 C1: 87 T: 89 |
| Suzuki 2009 (weight loss study) | I1: tea catechin C1: placebo |
‐ | I1: 20 C1: 21 T: 41 |
‐ | ‐ | I1: 18 C1: 20 T: 38 |
I1: 90 C1: 95 T: 93 |
| Takase 2008 5 (weight loss study) | I1: catechin beverage C1: placebo beverage |
‐ | I1: 49 C1: 52 T: 101 |
I1: 48 C1: 46 T: 94 |
‐ | I1: 46 C1: 47 T: 93 |
I1: 94 C1: 90 T: 92 |
| Takashima 2004 (weight loss study) | I1: catechin beverage C1: control beverage |
‐ | I1: 10 C1: 9 T: 19 |
I1: 10 C1: 9 T: 19 |
‐ | I1: 10 C1: 9 T: 19 |
I1: 100 C1: 100 T: 100 |
| Takeshita 2008 (weight loss study) | I1: catechin beverage C1: placebo beverage |
‐ | I1: 40 C1: 41 T: 81 |
I1: 40 C1: 41 T: 81 |
‐ | I1: 40 C1: 41 T: 81 |
I1: 100 C1: 100 T: 100 |
| Tsuchida 2010 (weight loss study) | I1: catechin beverage C1: control beverage |
‐ | I1: 39 C1: 41 T: 80 |
I1: 39 C1: 41 T: 80 |
‐ | I1: 39 C1: 41 T: 80 |
I1: 100 C1: 100 T: 100 |
| Wang 2010 6 (weight loss study) | I1: green tea 1 I2: green tea 2 I3: green tea 3 C1: control drink |
I1: ‐ I2: ‐ I3: ‐ C1: ‐ T: 435 |
I1: ‐ I2: ‐ I3: ‐ C1: ‐ T: 205 |
‐ | ‐ | I1: ‐ I2: ‐ I3: ‐ C1: ‐ T: 192 |
I1: ‐ I2: ‐ I3: ‐ C1: ‐ T: 94 |
| Westerterp‐Plantenga 2005 (weight maintenance study) | I1: green tea‐caffeine capsule ‐ low habitual caffeine consumers I2: green tea‐caffeine capsule ‐ high habitual caffeine consumers C1: placebo ‐ low habitual caffeine consumers C2: placebo ‐ high habitual caffeine consumers |
I1: ‐ I2: ‐ C1: ‐ C2: ‐ T: 90 |
I1: 19 I2: 19 C1: 19 C2: 19 T: 76 |
I1: 19 I2: 19 C1: 19 C2: 19 T: 76 |
‐ | I1: 19 I2: 19 C1: 19 C2: 19 T: 76 |
I1: 100 I2: 100 C1: 100 C2: 100 T: 100 |
| Total7 | Weight loss studies | 1800 | 1685 | ||||
| Weight maintenance studies | 276 | 260 | |||||
| All studies | 2076 | 1945 | |||||
"‐" denotes not reported. 1 all participants were Khon Kaen University officers. 2 this was a two‐part study. Participants were given special diet (not including green tea) to lose weight for four weeks. They were then given green tea to see if it would help maintain weight loss. Sixteen participants dropped out during very‐low‐energy diet period before ever receiving the intervention or placebo. 3 254 participants "gave informed consent" – it is not clear if this was the actual number screened, but we think so. 4 this study's usage of ITT differs from the Cochrane definition. 5 "of these individuals, four whose insulin levels recorded at week 0 were abnormal (two in the placebo group and two in the catechin group) were excluded from analysis, leaving a group suitable for analysis of 45 individuals in the placebo group and 44 individuals in the catechin group." 6 although 192 completed the study 10 participants were excluded from analysis for various reasons; thus analysis was carried out for 182 participants. 7 not all studies specified how many participants were randomised into each group at the start, though totals are available for each study. In one case (Wang 2010), the number of participants in each group at the end of the study was also not given, though the total number of participants finishing was given.
C: control; I: intervention; ITT: intention to treat; T: total.
Assessment of risk of bias in included studies
Three review authors (TMJ, AMW, LK) assessed each trial independently. Risk of bias was assessed using The Cochrane Collaboration's tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
The following criteria were used.
Was the allocation adequately concealed?
Was the allocation sequence randomly generated?
Were participants and personnel blinded?
Was outcome assessment blinded?
Were incomplete outcome data adequately addressed?
Were reports of the study free of suggestion of selective outcome reporting?
Was the study apparently free of other bias?
We judged risk of bias criteria as 'low risk', 'high risk' or 'unclear risk'. Results for assessment of the risk of bias for each domain for each study were compared and disagreements resolved by discussion. A 'Risk of bias' figure (Figure 2) and a 'Risk of bias summary' figure (Figure 3) were produced.
2.
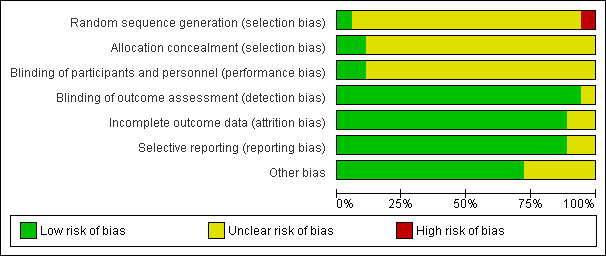
Risk of bias graph: review authors' judgements about each risk of bias domain presented as percentages across all included studies.
3.
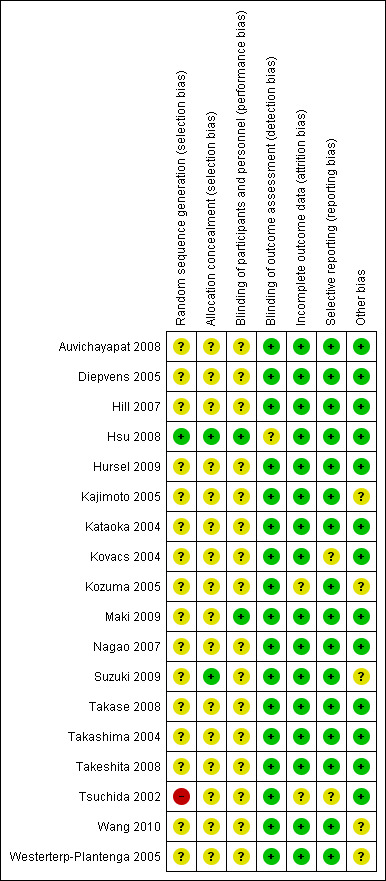
Risk of bias summary: review authors' judgements about each risk of bias domain for each included study.
Primary analyses for the review excluded studies judged to be of 'high' risk of bias, considering the domains relating to randomisation (sequence generation and allocation concealment) and blinding of participants, personnel and outcome assessors as these domains are likely to have the largest impact on bias in studies of green tea in weight loss and weight maintenance. If adequate data were available, we conducted a sensitivity analysis to assess the impact of individual bias domains on study results. A sensitivity analysis including all studies was performed to determine the impact that potentially high risk of bias studies may have had on the conclusions of the review.
Quality of reporting of adverse effects
The number of studies that assessed and reported adverse effects and the method used to gather the information was recorded (Appendix 6). The following information was documented: were all participants asked about adverse effects, were descriptions of adverse effects recorded, including their frequency, severity and outcome (whether they resolved, caused participant to withdraw from the study, caused hospitalisations or death).
Measures of treatment effect
All studies reported continuous data, in most cases as mean differences (MD) and standard deviations (SD). Studies focusing on weight loss were analysed separately from weight maintenance studies.
Unit of analysis issues
We have taken into account the level at which randomisation occurred, such as cross‐over studies and multiple observations for the same outcome.
Dealing with missing data
We attempted to contact 12 study authors to request relevant missing data, and we received responses from seven authors (Di Pierro 2009; Hill 2007; Hsu 2008; Kajimoto 2005; Kataoka 2004; Takase 2008; Takashima 2004). Responses were not received from five authors (Auvichayapat 2008; Kozuma 2005; Suzuki 2009; Takeshita 2008; Tsuchida 2002). For studies where difference in means for continuous outcomes was not provided, values were calculated as described in the Cochrane Handbook for Systematic Reviews of Interventions Section 9.2.3.1 (Higgins 2011). Similarly, if SD for changes from baseline were not reported, values were imputed using the method as described in section 16.1.3.2 in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Assessment of heterogeneity
We identified heterogeneity by visual inspection of forest plots, by using a standard Chi2 test and a significance level of α = 0.1, in view of the low power of this test. We quantified inconsistency across studies with the I2 statistic to assess the impact of heterogeneity on the meta‐analysis (Higgins 2002; Higgins 2003), where an I2 statistic of 75% and over indicates a considerable level of inconsistency (Higgins 2011). When heterogeneity was found, we attempted to determine potential reasons for it by examining individual studies and subgroup characteristics.
Assessment of reporting biases
We planned to use funnel plots in case we included 10 studies or more for a given outcome to assess small study effects. Due to several explanations for funnel plot asymmetry, we have carefully interpreted results (Sterne 2011). Reporting biases, including the potential impact that the size of the study had on results, was assessed as part of the risk of bias assessment described above under 'Assessment of risk of bias in included studies'.
Data synthesis
Data were synthesised using meta‐analysis, if data were available, sufficiently similar, and of sufficiently low risk of bias. We performed statistical analyses according to the statistical guidelines referenced in The Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Total daily dose of green tea catechins, individual catechins such as epigallocatechin‐3‐gallate (EGCG) or both, and caffeine were recorded for each study and used in assessment of whether the dose of specific constituents was directly related to effects produced in studies.
Subgroup analysis and investigation of heterogeneity
A subgroup analysis was conducted to assess whether interventions that contained caffeine, in addition to catechins, produced more weight loss than interventions containing only catechins. This was undertaken for the weight loss outcome, using weight loss (in kg) as the measurement. Studies were grouped based on documentation of caffeine content of green tea intervention and control. All studies that contained no caffeine in intervention or control or had caffeine content matched between intervention and control were grouped and analysed for mean weight loss. Similarly, studies that contained caffeine in the intervention only were grouped and analysed. Results of both analyses were compared to the mean weight loss produced by analysis of all studies, regardless of caffeine content. A subgroup analysis was also conducted on studies that were conducted in Japan to determine if results differed from studies conducted outside of Japan.
Sensitivity analysis
We performed sensitivity analyses in order to explore the influence of the following factors on effect size.
The effect of including studies with imputed results.
The effect of risk of bias, as specified above.
Results
Description of studies
See: Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We found 72 potentially eligible publications. Fifty‐three were excluded and 19 publications (18 studies) were included in the review for further analysis. A detailed flow chart of the process is presented as Figure 1.
Included studies
The 18 studies included in the review were all randomised controlled trials. Two thousand and seventy‐six participants were randomised in total, with 1945 participants finishing their respective study. The trial duration ranged between 84 and 91 days and study size ranged between 19 and 270 participants. Nine of the 18 studies took place in Japan (Kajimoto 2005; Kataoka 2004; Kozuma 2005; Nagao 2007; Suzuki 2009; Takase 2008; Takashima 2004; Takeshita 2008; Tsuchida 2002), and four were conducted in the Netherlands (Diepvens 2005; Hursel 2009; Kovacs 2004; Westerterp‐Plantenga 2005), while the rest took place in Australia (Hill 2007), China (Wang 2010), Taiwan (Hsu 2008), Thailand (Auvichayapat 2008), and the US (Maki 2009). Ten studies included both men and women, while five included only women (Diepvens 2005; Hill 2007; Hsu 2008; Suzuki 2009; Takase 2008) and three included only men (Kataoka 2004; Takashima 2004; Takeshita 2008). Fifteen studies were weight loss studies, while the remaining three were weight maintenance studies (Hursel 2009; Kovacs 2004; Westerterp‐Plantenga 2005). All studies used BMI as part of study inclusion criteria. In most cases, authors reported a BMI range that conformed with the WHO definition of overweight. Two studies reported the average BMI of participants but provided the BMI range of the group when contacted (Kataoka 2004; Takashima 2004). Lower and upper limits of acceptable BMI varied depending on the definition of overweight and obesity in the country in which the study was conducted. The range of BMI values for participant inclusion were noted for each study and are as follows: > 27 kg/m2 (Hsu 2008), > 25 kg/m2 (Auvichayapat 2008), 25 to < 30 kg/m2 (Takase 2008), 25 to 31 kg/m2 (Diepvens 2005), 25 to 35 kg/m2 (Hursel 2009; Kovacs 2004; Kozuma 2005; Takeshita 2008; Westerterp‐Plantenga 2005), 25 to 40 kg/m2 (Maki 2009), 25 to 39.9 kg/m2 (Hill 2007), 24 to 30 kg/m2 (Nagao 2007; Tsuchida 2002, 24 to 35 kg/m2 (Wang 2010), 23 to < 30 kg/m2 (Suzuki 2009), 22.5 to 30 kg/m2 (Kajimoto 2005), 22 to 30 kg/m2 (Takashima 2004) and 21 to 39 kg/m2 (Kataoka 2004).
Interventions
All 18 studies compared some form of green tea intervention to a control. All studies reported the doses given. An instrument designed to assess whether sufficient detail regarding the description of the identity and content of a natural product was provided in the report of a study was used (Appendix 7; Appendix 8) (Jurgens 2009).The manner in which the details of the chemical content of green tea were documented in each study ranged from the quantity (mg or %) of total catechins and caffeine contained in each dose to a more detailed description of the amount (mg or %) of individual catechins and caffeine contained in each dose (Appendix 2). The daily dose of green tea catechins used in the studies was as low as 140.85 mg and as high as 1206.9 mg, with 16 of the 18 studies ranging from 270 to 645.9 mg. The majority of studies had one intervention and one control group; however, three studies (Kajimoto 2005; Kataoka 2004; Wang 2010) each had more than one intervention group, with different doses of green tea administered to each. For the purposes of analysis, the multiple intervention groups in each of these three studies were pooled within each study, creating one green tea group and one control group for each study. Seven studies administered the intervention and placebo in capsule form (Auvichayapat 2008; Diepvens 2005; Hill 2007; Hsu 2008; Hursel 2009; Kovacs 2004; Westerterp‐Plantenga 2005), and the remaining 11 studies delivered the intervention and placebo as a beverage (Kajimoto 2005; Kataoka 2004; Kozuma 2005; Maki 2009; Nagao 2007; Suzuki 2009; Takase 2008; Takashima 2004; Takeshita 2008; Tsuchida 2002; Wang 2010).
Control
All studies included a placebo arm using preparations that closely matched the intervention in terms of dosage form and appearance. Three studies used a special diet along with green tea; however, the same diet was used for both the intervention and control groups (Diepvens 2005; Hursel 2009; Kovacs 2004). Three studies used some form of exercise in conjunction with both the intervention and control groups (Hill 2007; Maki 2009; Tsuchida 2002).
Types of outcome measures
The primary outcomes of interest in this review were: change in body weight or mass measure (absolute or percentage change in body weight or reduction in BMI; reduction in waist circumference or waist‐to‐hip ratio), mortality and health‐related quality of life.
To analyse the effect of green tea preparations on weight loss and weight maintenance, mean change scores and the associated SD for green tea and the control were required for each outcome in each study. Not all studies reported all required data. Ten studies provided mean change scores and SD for weight loss (Diepvens 2005; Hill 2007; Hsu 2008; Kataoka 2004; Kozuma 2005; Nagao 2007; Takase 2008; Takashima 2004; Takeshita 2008; Tsuchida 2002); with the exception of Diepvens 2005, the remaining nine studies provided mean and SD on baseline and final for three of the four measures used for the change in body mass outcome (weight loss, BMI, waist circumference). This allowed calculations of correlations between baseline and final measurements at end of study, which could be used to impute mean change scores and SDs. MDs and SDs for waist‐to‐hip ratio were reported for only two studies (Hill 2007; Tsuchida 2002).
Correlations of 0.965 to 0.996 were imputed in the green tea and control groups for change in weight. A conservative value of 0.975 was chosen as the correlation between weight at baseline and final measurement in studies without mean change score data. Similarly, correlations in the change in BMI ranged from 0.944 to 0.991 in both intervention and control, with a modest value of 0.95 selected to use in imputation methods. Correlations of 0.786 to 0.995 were seen in the change in waist circumference, with a value of 0.90 as a conservative estimate used in imputation. In the waist‐to‐hip ratio measurement, only one study provided baseline, follow up, and change data (Tsuchida 2002). The estimates of correlation were 0.986 and 0.897 in green tea and control groups respectively. Four studies provided baseline and follow‐up ratios. Mean change scores and SDs were imputed using a correlation of 0.9 in these four studies (Auvichayapat 2008; Diepvens 2005; Hill 2007; Kajimoto 2005).
In summary, for the weight loss outcome, using weight loss measured in kg, mean change scores were imputed in five weight loss studies (Auvichayapat 2008; Kajimoto 2005; Maki 2009; Suzuki 2009; Wang 2010) and two weight maintenance studies (Hursel 2009; Kovacs 2004). Insufficient data provided in the remaining weight maintenance study (Westerterp‐Plantenga 2005) made imputation impossible, therefore the study was not included in further analysis. The change in BMI measurements required imputation for four weight loss studies (Auvichayapat 2008; Diepvens 2005; Kajimoto 2005; Suzuki 2009) and two weight maintenance studies (Hursel 2009; Kovacs 2004); no data for BMI were reported for the remaining three studies (Maki 2009; Wang 2010; Westerterp‐Plantenga 2005). Seven studies required imputation of data for the change in waist circumference, five for weight loss (Auvichayapat 2008; Diepvens 2005; Kajimoto 2005; Suzuki 2009; Wang 2010) and two for weight maintenance (Hursel 2009; Kovacs 2004) with no data reported in the remaining studies (Maki 2009; Westerterp‐Plantenga 2005). The mean change in waist‐to‐hip ratio was imputed for three weight loss studies (Auvichayapat 2008; Diepvens 2005; Kajimoto 2005) and one weight maintenance study (Kovacs 2004) and no data for this measurement were reported for 12 studies (Hursel 2009; Hsu 2008; Kataoka 2004; Kozuma 2005; Maki 2009; Nagao 2007; Suzuki 2009; Takase 2008; Takashima 2004; Takeshita 2008; Wang 2010; Westerterp‐Plantenga 2005). A sensitivity analysis to determine the effect of including imputed data on results of meta‐analyses was conducted (Appendix 9).
No studies investigated mortality as an outcome, although eight studies had no drop‐outs and therefore it was assumed that there were no deaths during the study (Auvichayapat 2008; Diepvens 2005; Hursel 2009; Kataoka 2004; Takashima 2004; Takeshita 2008; Tsuchida 2002; Westerterp‐Plantenga 2005). No studies reported on health‐related quality of life as an outcome.
With regard to the secondary outcomes outlined for investigation in this review, nine studies reported on compliance/adherence, though usually not in great detail (Auvichayapat 2008; Hursel 2009; Kozuma 2005; Maki 2009; Nagao 2007; Suzuki 2009; Takase 2008; Takeshita 2008; Wang 2010). No studies reported on patient satisfaction or morbidity. There were eight studies that recorded adverse events as part of the study methodology (Hursel 2009; Kataoka 2004; Kozuma 2005; Takase 2008; Takeshita 2008; Tsuchida 2002; Wang 2010; Westerterp‐Plantenga 2010). Of these eight studies, four reported the occurrence of adverse events (Hsu 2008; Kajimoto 2005; Maki 2009; Suzuki 2009), and four stated that participants did not report any adverse effects. The remaining studies did not report on the presence or absence of adverse events. No studies reported on costs as an outcome.
Excluded studies
Fifty‐three studies were excluded from further analysis upon consideration of the full text for the following reasons (Characteristics of excluded studies): 30 studies were not randomised controlled trials (Batista 2009; Bayes 2005; Bolling 2009; Boon 2006; Boon 2008; Chantre 2002; Chou 2008; Di Pierro 2009; Diepvens 2007; Grove 2010; Gupta 2008; Harada 2005; Hardy 2008; Hursel 2009; Hursel 2010; Kuhad 2008; Lieberman 2003; Onakpoya 2010; Pittler 2005; Pittler 2006; Pittler 2007; Pittler 2009; Schneider 2009; Schulz 2009; Shixian 2006; Tian 2004; Westerterp‐Plantenga 2010; Wolfram 2006; Wolfram 2007; Yoneda 2009). Nine studies were less than 12 weeks in length (Bakker 2010; Basu 2010; Boschmann 2007; Brown 2009; Dalbo 2008; Dulloo 1999; Fukino 2005; He 2009; Thielecke 2010). Five studies involved a co‐morbidity or co‐medication (Chan 2006; Donovan 2009; Fukino 2008; Nagao 2009; Stendell‐Hollis 2010). In three studies, the participants were not obese or overweight (Belza 2009; Eichenberger 2009; Komatsu 2003). In six studies, the intervention was a combination preparation, containing at least one ingredient in addition to green tea and its natural constituents (Belza 2007; Berube‐Parent 2005; Nagao 2005; Rao 2006; Tsai 2009; Tucker 2008).
Risk of bias in included studies
Standardised risk of bias assessments were conducted for each of the 18 trials, assessing the following criteria: random sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting and other bias (Characteristics of included studies; Figure 2; Figure 3). Only one study was assessed as having a low risk of bias for six domains, with the one remaining domain being unclear (Hsu 2008). All other studies had at least one domain that was assessed as having an unclear or high risk of bias. One study (Tsuchida 2002) had a high risk of bias for the random sequence generation domain, a domain considered to have a large impact on bias in studies of green tea in weight loss and weight maintenance. This study was therefore excluded from the meta‐analysis of green tea in weight loss for all measurements. Sensitivity analyses were conducted to determine the impact that including studies with potentially high risk of bias in analyses may have had on the MD for each measurement for the weight loss outcome (Appendix 10). Results of these analyses showed that Tsuchida 2002 had a small impact on the MD in each outcome, in spite of the high risk of bias associated with randomisation in that study.
Allocation
The only study to describe the random sequence generation process clearly was Hsu 2008. In addition to Hsu 2008, Suzuki 2009 was judged to be at a low risk of allocation concealment selection bias (Characteristics of included studies; Figure 3). The remaining studies mentioned that allocation was concealed but did not comprehensively describe the methods used to ensure allocation concealment. Sensitivity analyses were conducted on weight loss studies for randomisation (allocation sequence generation) and allocation concealment domains including only studies assessed as having low risk of bias (Appendix 10). The analyses could not be performed on weight maintenance studies as no studies had a low risk of bias for these domains. Analysis of studies with loss risk of bias for randomisation showed a very small mean weight loss compared to analyses when all studies were included. The same was true when the measurement of weight loss was reduction in BMI. In Tsuchida 2002, one of the studies translated from Japanese, the allocation method was judged as having a high risk of bias.
Blinding
Two studies were judged to be at a low risk of both performance bias and detection bias with regard to blinding (Hsu 2008; Maki 2009), while it was unclear in the remaining studies whether blinding was satisfactorily achieved (Characteristics of included studies; Figure 3). Of the 18 studies, all but four (Auvichayapat 2008; Hill 2007; Kataoka 2004; Takashima 2004) described their work as blinded or double‐blinded, but complete details about the methods used to achieve blinding, and whether the blinding was successful, were frequently unavailable. All 18 studies delivered the control in a format (e.g. beverage, capsule) and administration schedule (e.g. once daily, three times daily with meals) identical to the intervention. With the exception of Hursel 2009, where it was unclear, all studies partially or completely matched the intervention to the control with regards to taste, smell, appearance, or a combination. Sensitivity analyses were conducted on weight loss studies for the blinding domain including only studies assessed as having low risk of bias (Appendix 10). Results of analysis of studies with low risk of bias produced a reduced amount of weight loss that was not statistically significant, while the effect on BMI reduction was relatively unchanged. The analyses could not be performed on weight maintenance studies as no studies had low risk of bias for this domain.
Incomplete outcome data
Eight studies had complete follow‐up of all participants (Auvichayapat 2008; Diepvens 2005; Hursel 2009; Kataoka 2004; Takashima 2004; Takeshita 2008; Tsuchida 2002; Westerterp‐Plantenga 2005), while the remainder reported some drop‐outs or loss of follow‐up. All studies addressed any incomplete outcome data or lack of follow‐up with the exception of two studies (Kozuma 2005; Tsuchida 2002) for which this was judged to be unclear (Characteristics of included studies; Figure 3). Two studies (Hsu 2008; Maki 2009) had higher attrition rates than the others, with Hsu reporting a 78% completion rate and Maki documenting a completion rate of 84% for the intervention group and 79% for the control group. The impact of attrition rate was not discussed by either author. Only one study (Maki 2009) stated that they used intention‐to‐treat (ITT) analyses; however, their analysis did not include four participants for whom post‐randomisation efficacy data were missing.
Selective reporting
All studies were judged to be free of selective reporting with the two exceptions (Kovacs 2004; Tsuchida 2002), where it was judged unclear if there was selective reporting (Characteristics of included studies; Figure 3). Kovacs 2004 was judged unclear because in the experimental design section, it was stated that adverse events were recorded, but these were not reported in the text, and because several measurements were not uniformly reported for all participants: resting energy expenditure, respiratory quotient and physical activity levels. Tsuchida 2002 was judged unclear because the authors did not explicitly state what they were looking for, so there is no way to determine if the results reported were for pre‐determined outcomes.
Given the small number of studies included in each meta‐analysis (14 for weight loss and two for weight maintenance), a funnel plot (Lau 2006; Sterne 2001; Sterne 2011) would be of limited use in identifying small study bias and so the potential effect that the lack of reporting of small negative studies might have had on results was not investigated further.
Other potential sources of bias
Similarity of baseline characteristics
Participant data for all 18 studies showed that baseline characteristics were similar between groups within studies, and most studies described efforts to ensure that there was a balance between groups with regard to characteristics such as age, weight and BMI.
Five of 18 studies (Kajimoto 2005; Kozuma 2005; Suzuki 2009; Wang 2010; Westerterp‐Plantenga 2005) were judged to have an unclear risk of bias based on reporting (or lack of reporting) of funding sources for studies (Characteristics of included studies; Figure 3)
Effects of interventions
See: Table 1
Primary outcomes
1. Change in body weight or mass measure
Body weight
Weight loss studies
Meta‐analysis of the 14 trials (Auvichayapat 2008; Diepvens 2005; Hill 2007; Hsu 2008; Kajimoto 2005; Kataoka 2004; Kozuma 2005; Maki 2009; Nagao 2007; Suzuki 2009; Takase 2008; Takashima 2004; Takeshita 2008; Wang 2010) that provided data for weight loss, using a random‐effects model, produced a statistically significant MD in body weight of ‐0.95 kg (95% CI ‐1.75 to ‐0.15; P = 0.02; I2 = 95%; 1562 participants; 14 studies) in favour of green tea preparations over control. However, the analysis revealed a considerable level of heterogeneity (I2 = 95%; P < 0.00001) and therefore possible sources of heterogeneity were explored as part of further analysis.
A sensitivity analysis to assess the impact of including studies that required MDs and SDs to be imputed was conducted by excluding the five trials (Auvichayapat 2008; Kajimoto 2005; Maki 2009; Suzuki 2009; Wang 2010) that required imputed data. The impact of imputed data on the MD was very small and results were statistically significant whether or not imputed data were used (Appendix 9).
The effect that all types of bias may have on results of studies was evaluated by conducting a sensitivity analysis. Figure 3 was used to identify studies that had been assigned a low risk of bias in at least one of the following domains: sequence generation, allocation concealment and blinding, as those domains were likely to have the largest impact on bias. A sensitivity analysis was conducted by performing a meta‐analysis on the three studies (Hsu 2008; Maki 2009; Suzuki 2009) determined to have low risk of bias. This analysis resulted in a reduction of the MD in weight loss from ‐0.95 kg (95% CI ‐1.75 to ‐0.15) to ‐0.41 kg (95% CI ‐0.98 to 0.17), a MD that was not statistically significant (Appendix 10). The effect that the number of participants in the study may have on the analysis was investigated by examining the forest plot of the meta‐analysis of all 14 studies. It showed that even though there was a range in the size of studies, all 14 studies contributed relatively evenly (6.3% to 8%) to the analysis, with the exception of Auvichayapat 2008 (3.1%), which was a small study of 60 participants. It appeared then, that all studies contributed similar weight to the analysis. Therefore, a sensitivity analysis conducted by removing the largest study to determine its impact was not done.
All 14 studies reported the total daily dose of catechins contained in each green tea preparation used as the intervention. This allowed an assessment of whether the total daily dose of green tea catechins used in each study was directly related to the effects attributed to the intervention. This was achieved by re‐plotting the meta‐analysis, and sorting the forest plot display by total daily dose of green tea catechins (highest to lowest) rather than effect size (Analysis 2.1). There did not appear to be a trend between the reported daily dose of the intervention and the effect size (Figure 4). For example, the study with the highest stated daily dose (Diepvens 2005) had a small, non‐significant effect size while two studies with relatively low declared total daily doses (Kozuma 2005; Takase 2008) had the highest effect sizes. With the exception of Wang 2010, all studies also reported the amount of EGCG, as a percentage of catechins contained in the total daily dose of green tea catechins that were taken in each study. As EGCG is thought to be the catechin most likely to be able to stimulate weight loss and its percentage in the catechin mixture can vary, the forest plot of the 13 studies (Analysis 3.1) was re‐plotted based on total daily dose of EGCG to see if a pattern of concentration of EGCG and effect size could be established (Figure 5). As with the total daily dose of catechins described above, the total percentage of EGCG administered daily did not appear to correlate with effect sizes observed in each study.
2.1. Analysis.
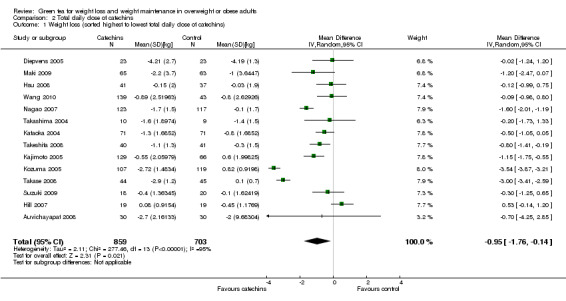
Comparison 2 Total daily dose of catechins, Outcome 1 Weight loss (sorted highest to lowest total daily dose of catechins).
4.
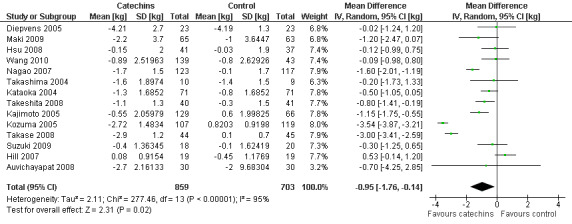
Forest plot of comparison: 2 Total daily dose of catechins: 2.1 Weight loss (weight loss studies) (sorted highest to lowest total daily dose of catechins).
3.1. Analysis.
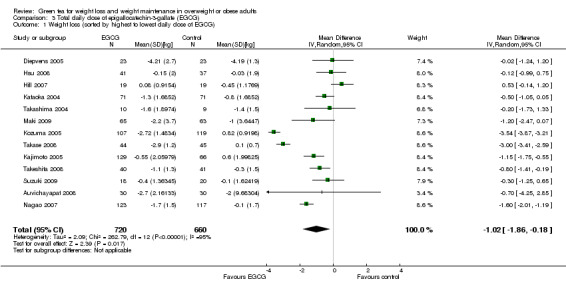
Comparison 3 Total daily dose of epigallocatechin‐3‐gallate (EGCG), Outcome 1 Weight loss (sorted by highest to lowest daily dose of EGCG).
5.
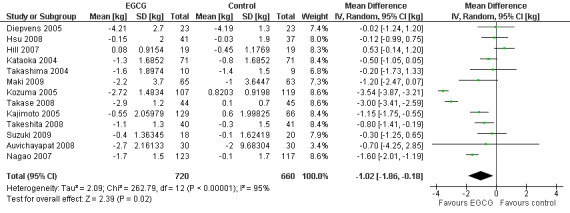
Forest plot of comparison: 3 Total daily dose of epigallocatechin‐3‐gallate (EGCG): 3.1 Weight loss (weight loss studies) (sorted by highest to lowest daily dose of EGCG).
A subgroup analysis, as specified in the protocol for this review, was conducted to assess the effect that the presence or absence of caffeine may have on weight loss induced by green tea preparations. Caffeine is a natural constituent of green tea and its role in weight loss as a result of green tea consumption is controversial (Rains 2011). All 14 weight loss studies included in the meta‐analysis reported the caffeine content of the intervention as well as the control. With the exception of three studies (Auvichayapat 2008; Diepvens 2005; Wang 2010), all studies either contained no caffeine in both the intervention and control, or the caffeine content was matched in both intervention and control, or the difference in caffeine content between intervention and control was very small (less than 28 mg). Many studies also documented, limited, or both, daily caffeine consumption in addition to that obtained through the green tea intervention. The subgroup analysis was conducted by excluding the three studies that had a significant difference in the amount of caffeine between the intervention and the control (Auvichayapat 2008; Diepvens 2005; Wang 2010). The resulting MD in weight for studies that contained either no caffeine in the intervention or control, or else caffeine was matched in intervention and control, was ‐1.11 kg (95% CI ‐1.99 to ‐0.24). When the subgroup analysis was repeated on the three studies with caffeine only in the intervention, the MD in weight was not significant at ‐0.10 kg (95% CI ‐0.80 to 0.60).
Of the 14 studies included in the meta‐analysis, eight were conducted in Japan. A subgroup analysis (not specified in protocol) was conducted to determine if the effect of green tea preparations on weight loss was affected by the country in which the study was conducted. Analysis 1.2 shows that when only the eight studies conducted in Japan (Kajimoto 2005; Kataoka 2004; Kozuma 2005; Nagao 2007; Suzuki 2009; Takase 2008; Takashima 2004; Takeshita 2008) were included in the meta‐analysis (Figure 6), the MD in weight loss increased from ‐0.95 kg (95% CI ‐1.75 to ‐0.15; P = 0.02) to ‐1.44 kg (95% CI ‐2.38 to ‐0.51; P = 0.002) (Analysis 1.2). This represents a larger, statistically significant loss in weight than that produced when the meta‐analysis was limited to the six studies (Auvichayapat 2008; Diepvens 2005; Hill 2007; Hsu 2008; Maki 2009; Wang 2010) conducted outside Japan (MD ‐0.04 kg; 95% CI ‐0.50 to 0.43; P = 0.88) (Analysis 1.2). Examination of the statistical measure of heterogeneity of these two meta‐analyses revealed that the studies conducted inside Japan were heterogeneous (I2 = 96%; P < 0.00001) while those conducted outside Japan were more similar to each other (I2 = 18%; P = 0.30).
1.2. Analysis.
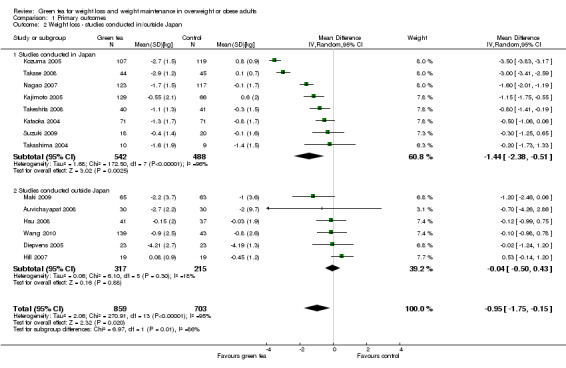
Comparison 1 Primary outcomes, Outcome 2 Weight loss ‐ studies conducted in/outside Japan.
6.
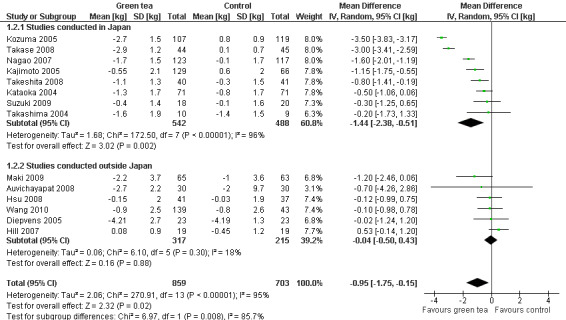
Forest plot of comparison: 1 Primary outcomes, outcome: 1.2 Weight loss studies conducted in/outside Japan.
All 14 studies were examined in an effort to identify sources of heterogeneity among the studies. Baseline characteristics of participants, including mean body weight and BMI, did not identify obvious sources of heterogeneity. We noted that there was, more often in studies conducted in Japan, a lack of detail provided as to how outcome measurements were made (i.e. how weight was measured, exactly where measurements of waist were taken etc.), which could lead to inaccurate outcome measurements. This could have contributed to the heterogeneity that was associated with the studies conducted in Japan as compared to those conducted outside. The composition and dose of the green tea preparation used in each study was compared. In general, although the composition and dose of green tea was different among the studies, the range was not large and did not appear to be substantially different between the two subgroups. The two studies that showed the highest MD in weight loss (Kozuma 2005; Takase 2008) were conducted in Japan and used green tea preparations of identical composition and daily dose; however, when the studies were analysed together, they showed heterogeneity (P = 0.06; I2 = 71%). When the three studies showing the most weight loss (Kozuma 2005; Nagao 2007; Takase 2008), all conducted within Japan, were removed from the meta‐analysis of the 14 studies, the heterogeneity was greatly reduced (P = 0.43; I2 = 0%). The source of heterogeneity remains unclear; however, it appears to be associated primarily with studies conducted within Japan.
Based on this subgroup analysis, it was decided that the analysis of studies should be conducted separately for the two groups; a meta‐analysis for studies conducted outside of Japan and a descriptive analysis stating the range of weight lost for the studies conducted in Japan. The weight loss experienced by participants in studies conducted outside Japan was not statistically significant, with a MD in weight loss of ‐0.04 kg (95% CI ‐0.50 to 0.43; P = 0.88; I2 = 18%; 532 participants; 6 studies) (Analysis 1.2) while the weight loss experienced by participants in the eight studies conducted in Japan ranged from a MD in weight loss of ‐0.20 kg to ‐3.5 kg (1030 participants) (Analysis 1.2).
Weight maintenance studies
There were three studies (Hursel 2009; Kovacs 2004; Westerterp‐Plantenga 2010) investigating the effects of green tea preparations on weight maintenance after weight loss. A meta‐analysis of two (Hursel 2009; Kovacs 2004) of the three studies providing data sufficient for imputing MD and SD, based on assumptions from weight loss studies, was conducted. The I2 statistic of the analysis showed considerable heterogeneity, therefore results are presented as a descriptive analysis. The MD in weight loss ranged from 0.6 to ‐1.6 kg.
Body mass index (BMI)
Weight loss studies
A meta‐analysis of the 12 trials (Auvichayapat 2008; Diepvens 2005; Hill 2007; Hsu 2008; Kajimoto 2005; Kataoka 2004; Kozuma 2005; Nagao 2007; Suzuki 2009; Takase 2008; Takashima 2004; Takeshita 2008) reporting data for BMI changes produced a MD in BMI of ‐0.47 kg/m2 (95% CI ‐0.77 to ‐0.17; P =0.002; I2 = 94%; 1252 participants) (Analysis 1.4) in favour of green tea preparations over control. As with the initial meta‐analysis of studies measuring loss of weight in kilograms, described in the previous section, this meta‐analysis showed a considerable level of heterogeneity. A sensitivity analysis was conducted to determine the impact of including studies where MDs and SDs were imputed. Excluding four studies (Auvichayapat 2008; Diepvens 2005; Kajimoto 2005; Suzuki 2009) with imputed values from the analysis resulted in very little change in effect size (Appendix 9). To assess the impact of bias on results, the original analysis was repeated including the two studies (Hsu 2008; Suzuki 2009) that had a low risk of bias for sequence generation, allocation concealment and blinding. The sensitivity analysis showed the mean reduction in BMI was relatively unchanged from the full analysis (Appendix 10). As Analysis 1.4 shows, all studies contributed relatively evenly (5.2% to 9.5% weight) to the analysis. This implies that the size of the study did not have an impact on the weight it was given in the analysis. Therefore, a sensitivity analysis was not conducted to determine the impact of the study size.
1.4. Analysis.
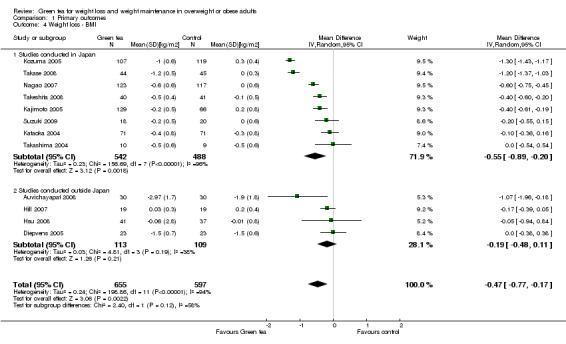
Comparison 1 Primary outcomes, Outcome 4 Weight loss ‐ BMI.
A subgroup analysis of the eight studies conducted in Japan (Kajimoto 2005; Kataoka 2004; Kozuma 2005; Nagao 2007; Suzuki 2009; Takase 2008; Takashima 2004; Takeshita 2008) showed a greater MD in BMI of ‐0.55 kg/m2 (95% CI ‐0.89 to ‐0.20; P = 0.002; I2 = 96%; 1030 participants) (Analysis 1.4) compared to a MD in BMI of ‐0.19 kg/m2 (95% CI ‐0.48 to 0.11; P = 0.21; I2 = 38%; 222 participants) in the four studies conducted outside of Japan (Auvichayapat 2008; Diepvens 2005; Hill 2007; Hsu 2008). The studies included in this analysis are 12 of the 14 studies described in the previous analysis and therefore, the same sources of heterogeneity are likely to apply. Therefore, the analysis of the effect of green tea preparations on BMI was conducted based on the two subgroups of studies conducted inside Japan (descriptive analysis) and those conducted outside Japan (a meta‐analysis). The four studies conducted outside Japan showed a MD in BMI of ‐0.19 kg/m2 (95% CI ‐0.48 to 0.11; P = 0.21; I2 = 38%; 222 participants), while studies conducted in Japan showed a reduction in BMI ranging from no effect to ‐1.30 kg/m2 (1030 participants).
All 12 studies reported total daily doses of green tea catechins as well as the percentage of EGCG contained in each of these total daily doses. The forest plot of the meta‐analysis of the 12 trials providing data about the effect of green tea catechins on BMI was re‐plotted, ordering studies by the largest to smallest total daily dose of catechins (Analysis 2.2) and total daily dose of EGCG (Analysis 3.2). As with weight loss described above, a relationship with total daily dose or total daily EGCG was not apparent for BMI changes.
2.2. Analysis.
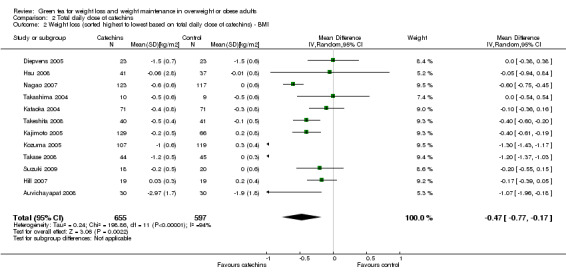
Comparison 2 Total daily dose of catechins, Outcome 2 Weight loss (sorted highest to lowest based on total daily dose of catechins) ‐ BMI.
3.2. Analysis.
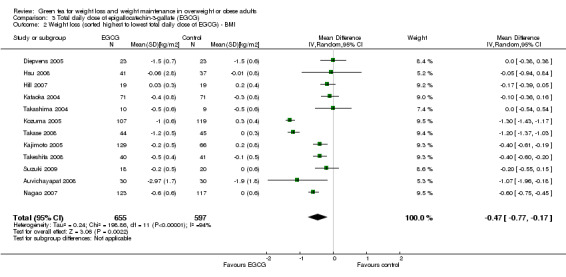
Comparison 3 Total daily dose of epigallocatechin‐3‐gallate (EGCG), Outcome 2 Weight loss (sorted highest to lowest total daily dose of EGCG) ‐ BMI.
Weight maintenance studies
As discussed above, the two studies (Hursel 2009; Kovacs 2004) that provided data that allowed imputation of MDs with SD were considerably heterogeneous (Analysis 1.5) and so a descriptive analysis was used. The MD in BMI observed during the weight maintenance was 0.2 to ‐0.5 kg/m2.
1.5. Analysis.
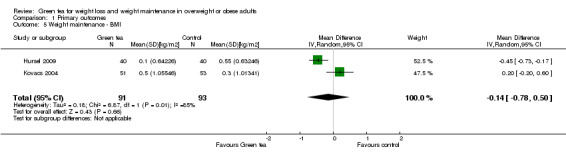
Comparison 1 Primary outcomes, Outcome 5 Weight maintenance ‐ BMI.
Waist circumference
Weight loss studies
Thirteen studies provided waist circumference data sufficient for meta‐analysis using a random‐effects model (Auvichayapat 2008; Diepvens 2005; Hill 2007; Hsu 2008; Kajimoto 2005; Kataoka 2004; Kozuma 2005; Nagao 2007; Suzuki 2009; Takase 2008; Takashima 2004; Takeshita 2008; Wang 2010). Analysis yielded a MD in waist circumference of ‐0.99 cm (95% CI ‐1.76 to ‐0.22; P = 0.01; I2 = 90%; 1434 participants; 13 studies) (Analysis 1.6) in favour of green tea over control. Re‐analysing the data and excluding five trials (Auvichayapat 2008; Diepvens 2005; Kajimoto 2005; Suzuki 2009; Wang 2010) with imputed data resulted in an increase in the effect size from a MD in waist circumference of ‐0.99 cm (95% CI ‐1.76 to ‐0.22) to a MD of ‐1.25 cm (95% CI ‐2.24 to ‐0.26) (Appendix 9). Sensitivity analysis restricting inclusion to the two studies (Hsu 2008; Suzuki 2009) that had a low risk of bias identified for sequence generation, allocation concealment and blinding, yielded a MD that now crossed zero and was no longer statistically significant (Appendix 10). The forest plot of the meta‐analysis (Analysis 1.6) showed that all studies contributed relatively evenly (4.3% to 9.9% weight) to the analysis. This implied that the size of the study did not have an impact on the weight it was given in the analysis. Therefore, a sensitivity analysis conducted by removing the largest study to determine its impact was not conducted.
1.6. Analysis.
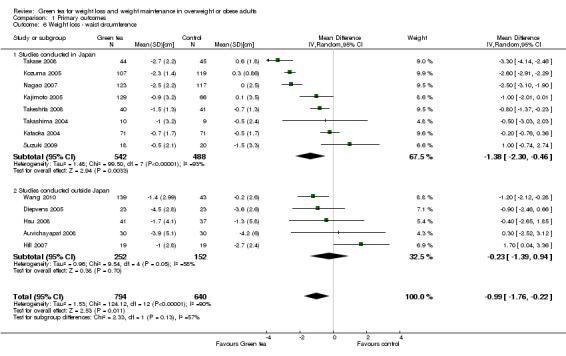
Comparison 1 Primary outcomes, Outcome 6 Weight loss ‐ waist circumference.
To assess whether the results were sensitive to the country in which the study was conducted, a subgroup analysis was conducted including the eight studies conducted in Japan (Kajimoto 2005; Kataoka 2004; Kozuma 2005; Nagao 2007; Suzuki 2009; Takase 2008; Takashima 2004; Takeshita 2008), followed by a separate analysis including only the five studies conducted outside Japan (Auvichayapat 2008; Diepvens 2005; Hill 2007; Hsu 2008; Wang 2010). The MD in waist circumference was ‐1.38 cm (95% CI ‐2.30 to ‐0.46; P = 0.003; I2 = 93%; 1030 participants), for the eight Japanese studies compared to ‐0.23 cm (95% CI ‐1.39 to 0.94; P = 0.07; I2 = 58%; 404 participants) for studies conducted outside Japan (Analysis 1.6). As with previous analyses with weight loss and decrease in BMI, the meta‐analysis of the studies conducted in Japan showed heterogeneity sufficient to warrant results being reported as a range rather than a meta‐analysis. Therefore, the MD in waist circumference was ‐0.23 cm (95% CI ‐1.39 to 0.94; P = 0.07; I2 = 58%; 404 participants) for studies conducted outside Japan and ranged from a gain of 1 cm to a loss of 3.30 cm (1030 participants) for studies conducted in Japan.
All 13 studies reported total daily doses of green tea catechins. The forest plot of the meta‐analysis of these studies was re‐done, ordering studies by the largest to smallest total daily dose of catechins (Analysis 3.3). No correlation between total daily dose and loss in waist circumference was observed. All but one study (Wang 2010) documented the amount of EGCG contained in each of the daily doses of green tea. The effect that the amount of EGCG in the green tea preparation tested had on waist circumference was assessed by re‐plotting the meta‐analysis of the 12 studies and ordering the forest plot by highest to lowest total daily dose of EGCG (Analysis 3.3). No trend between the amount of reduction in waist circumference and amount of EGCG contained in green tea preparation tested was observed.
3.3. Analysis.
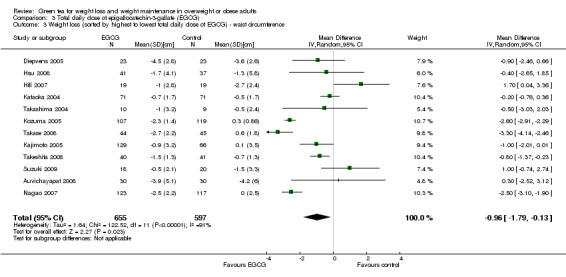
Comparison 3 Total daily dose of epigallocatechin‐3‐gallate (EGCG), Outcome 3 Weight loss (sorted by highest to lowest total daily dose of EGCG) ‐ waist circumference.
Weight maintenance studies
As discussed above, the two studies that provided sufficient data for analysis (Hursel 2009; Kovacs 2004) were substantially heterogeneous (Analysis 1.7) and so a descriptive analysis was used. The MD in waist circumference observed during the weight maintenance was 0.3 to ‐1.7 cm.
1.7. Analysis.
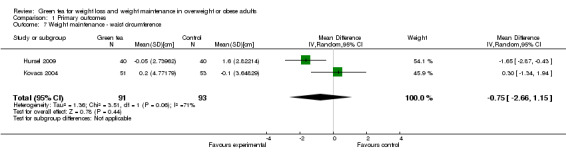
Comparison 1 Primary outcomes, Outcome 7 Weight maintenance ‐ waist circumference.
Waist‐to‐hip ratio
Weight loss studies
Four studies provided waist‐to‐hip ratio data sufficient for analysis (Auvichayapat 2008; Diepvens 2005; Hill 2007; Kajimoto 2005). Meta‐analysis, using a random‐effects model, yielded a MD in waist‐to‐hip ratio of ‐0 (95% CI ‐0.02 to 0.01; P = 0.69; I2 = 80%; 339 participants), which was not statistically significant (Analysis 1.8). A sensitivity analysis of the effect of using imputed data was conducted on the one study (Hill 2007) that did not require data to be imputed. The mean change in waist‐to‐hip ratio was relatively unchanged (Appendix 9). The forest plot of the meta‐analysis (Analysis 1.8) showed that all studies were not weighted evenly in the analysis. The impact that the size of the study had on results of the meta‐analysis was determined by examining the relative weight that was assigned to each study in Analysis 1.8. The study with the most participants (Kajimoto 2005) and the study with the fewest participants (Hill 2007) contributed similar weight (29.9% and 32.0%, respectively) to the meta‐analysis. The impact of the country in which the study was conducted was assessed by comparing the MD resulting from the analysis of the one study (Kajimoto 2005) conducted in Japan to the MD resulting from the analysis of the remaining three studies conducted outside Japan (Auvichayapat 2008; Diepvens 2005; Hill 2007) . Results were similar regardless of country in which the study was conducted (Analysis 1.8).
1.8. Analysis.
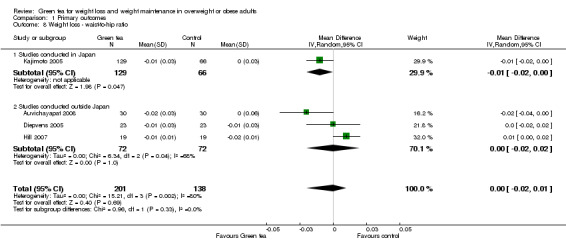
Comparison 1 Primary outcomes, Outcome 8 Weight loss ‐ waist‐to‐hip ratio.
The effect that the total daily dose of green tea catechins and the amount of EGCG contained in the catechins had on the waist‐to‐hip ratio was not investigated. This was because the meta‐analysis of the four studies showed a statistically insignificant effect of green tea on the waist‐to‐hip ratio, regardless of content of preparation tested.
Weight maintenance studies
One study (Kovacs 2004) provided data for weight maintenance using waist‐to‐hip ratio as one of the reported measurements. There was no difference in the calculated MD in waist‐to‐hip ratio between the group using green tea (MD 0; SD 0.039 (imputed)) and those using the control (MD 0; SD 0.035 (imputed)).
2. Mortality
There were no cases of mortality (any cause) reported in any included weight loss and weight maintenance studies.
3. Health‐related quality of life
This outcome was assessed in only one (Diepvens 2005) of 18 weight loss and weight maintenance studies where participants' attitude towards eating was assessed on days 4, 32 and 87 during the study using the three‐factor eating questionnaire.
Secondary outcomes
1. Compliance/adherence
Compliance/adherence to consuming the intervention or control according to study protocol was reported in nine weight loss and weight maintenance studies (Auvichayapat 2008; Hursel 2009; Kozuma 2005; Maki 2009; Nagao 2007; Suzuki 2009; Takase 2008; Takeshita 2008; Wang 2010). The methodology for tracking this outcome was provided in five of the nine studies (Kozuma 2005; Maki 2009; Nagao 2007; Takeshita 2008; Wang 2010).
2. Patient satisfaction
This outcome was not reported in any included studies.
3. Morbidity
This outcome was not reported in any included weight loss and weight maintenance studies.
4. Adverse effects of treatment
Adverse events that were experienced by participants in intervention and control groups were recorded in Appendix 6. In general, many studies did not record detailed information regarding adverse events. Ten studies reported no deaths, while eight studies did not mention any deaths. The reporting of all adverse events (whether associated with the intervention or control or not) was as follows: six studies did not report on the presence or absence of adverse events, eight reported that there were none and four reported the presence of adverse events (Hsu 2008; Kajimoto 2005; Maki 2009; Suzuki 2009). Regarding serious adverse events, 10 studies reported none, one study reported elevated blood pressure requiring hospitalisation (Maki 2009) and the remaining studies did not comment. Eleven studies reported on whether adverse events caused participants to drop out of the study and only one of these had one participant drop‐out (Maki 2009). Two studies each reported one hospitalisation of one participant in the intervention group (Hsu 2008; Maki 2009); one of the studies (Maki 2009) reported elevated blood pressure as the reason while the other study (Hsu 2008) did not provide the reason for hospitalisation. In both studies, the hospitalisations were judged by investigators as likely not to be associated with the intervention. Hospitalisations as a result of intervention or control were not reported in the remaining 16 studies. Adverse events requiring outpatient treatment were not recorded in any studies. Specific adverse events were listed in only two studies (Hsu 2008; Maki 2009). Fourteen adverse events were reported in the Maki 2009 study as follows: three cases of hypertension and one case each of dyspepsia and elevated liver enzymes in the intervention group and three cases of hypertension and one report each of constipation, nausea, pain, palpitation, elevated liver enzymes and ulcerative stomatitis in the control group.
5. Costs
This outcome was not reported in any included studies. Two preparations used in the studies were commercial products while the remaining were prepared specifically for each study.
Discussion
This review examined the effect of green tea preparations on weight loss and weight maintenance when used by overweight or obese adults. Data for the change in body weight or mass measure outcomes were reported in most studies as change in weight, BMI, waist circumference and waist‐to‐hip ratio. No studies reported on the length of time that participants had been overweight or obese, which is unfortunate as it has been shown that being overweight for a longer period of time could make it more difficult to lose weight (Elfhag 2005).
The risk of bias assessment tool was useful in evaluating the potential for a variety of types of biases to affect results of studies. Only one of the 18 studies was assessed as having a low risk of bias for six criteria and unclear risk for the remaining one criterion considered (Hsu 2008), while the study by Tsuchida 2002 was determined to have a high risk of bias for the random sequence generation domain, a domain considered to have a large impact on bias in studies of green tea in weight loss and weight maintenance. Due to its high risk of bias, the Tsuchida study was removed from the meta‐analysis. The remaining 17 studies were assessed as having a reasonably low risk of bias.
Change in body weight or mass measurement
Weight loss studies
Body weight
Meta‐analysis of six studies conducted outside Japan showed that preparations of green tea produced a statistically non‐significant MD in weight loss of approximately ‐0.04 kg, compared to control, during the 12‐week study period (Analysis 1.1). The lack of positive loss in weight is not surprising considering only one of the six studies showed a statistically significant weight loss. Of the eight studies conducted in Japan, five showed statistically significant results, yet as a group they were heterogeneous and showed a MD in weight loss ranging from ‐0.2 kg to ‐3.5 kg in favour of green tea preparations over control. Weight loss in both analyses was less than the 5% to 10% loss of body weight that is thought to be beneficial in reducing CV risk factors in overweight and obese adults (Wing 2011). Sensitivity analyses testing for the effects of inclusion of studies with imputed data, and size of study showed that these factors had limited impact on the results of the analysis. Meta‐analysis including only those studies with a low risk of bias, however, lowered the average weight loss to a very modest amount that was not statistically significant. This means that the weight loss reported in this meta‐analysis, which is already a modest sum, may be even less in reality if only those studies with the lowest risk of bias are considered. A striking trend emerged from analysis of the studies, a trend reported by other review authors (Hursel 2009a). In general, studies conducted in Japan reported larger losses in weight than those conducted in other countries. There are several possible explanations for this difference. For example, the definition of the level of BMI that is considered overweight/obese in different cultures can vary. In addition, the average levels of caffeine intake within cultures is different and that may affect response to caffeine in the green tea preparations. Another possible explanation for the difference in response to green tea preparations may be due to the documented genetic difference in the enzyme COMT between Japanese (Asians) and Caucasians (Hursel 2009a; Rains 2011). There is a known genetic variation in COMT, an enzyme responsible for metabolising norepinephrine, that can make it high‐activity or low‐activity. Those with low‐activity COMT have been shown to be more susceptible to weight gain. Asians are known to have a higher frequency of high‐activity COMT while Caucasians have a higher frequency of low‐activity COMT. It is proposed then that Asians, with the higher‐activity COMT, are more sensitive to the COMT inhibition effects of green tea than are Caucasians whose COMT is low‐activity (Rains 2011). As stated previously, catechins and caffeine are thought to work synergistically in promoting weight loss. Subgroup analysis of studies that contained either no caffeine in the intervention or control or else caffeine was matched in intervention and control gave a mean weight loss of ‐1.11 kg (95% CI ‐1.99 to ‐0.24), similar to the meta‐analysis of all studies regardless of the caffeine content. Analysis of studies that included caffeine along with catechins in the intervention and no caffeine in the control did not show an increased amount of weight loss compared to studies that had no difference in the amount of caffeine in intervention and control. These results do not support the theory of synergy with caffeine and catechins and weight loss; however, this is based on a small number of studies. This subgroup analysis suggests that the weight loss experienced with the use of green tea preparations was independent of the content of caffeine. Other factors in these studies would need to be explored before it could be determined if caffeine really does or does not have a role in weight loss produced by green tea.
1.1. Analysis.
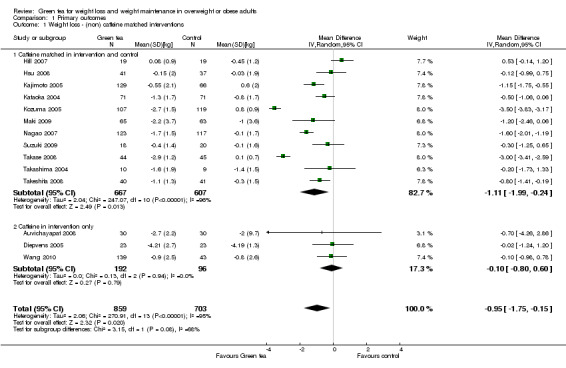
Comparison 1 Primary outcomes, Outcome 1 Weight loss ‐ (non) caffeine matched interventions.
Body mass index
Meta‐analysis of the four studies conducted outside Japan that used BMI as a measurement for weight loss revealed a statistically non‐significant MD in BMI of ‐0.19 kg/m2. Studies conducted in Japan were heterogeneous and produced MDs in BMI that ranged from no effect to ‐1.3 kg/m2 in favour of green tea preparations over control. Sensitivity analyses to determine effects of inclusion of studies requiring imputed data and including studies regardless of risk of bias failed to show that any of these factors had a measurable effect on results. The size of each study did not appear to affect the meta‐analysis as all studies were given similar weights in the analysis. Findings for country of study and content of preparation were similar to those reported for weight loss, as described above.
Waist circumference
Meta‐analysis of five studies conducted outside Japan showed a statistically non‐significant MD in waist circumference of ‐0.23 cm and ranged from a gain of 1 cm to a loss of 3.3 cm for studies conducted in Japan. The sensitivity analysis of the effect of using studies with imputed data and including studies with higher risk of bias did have modest effects on results. As described for other measurements, Japanese studies produced greater reductions than studies conducted elsewhere.
Waist‐to‐hip ratio
Meta‐analysis of the four studies providing data for waist‐to‐hip ratio did not provide statistically significant differences between intervention and control groups.
Weight maintenance studies
Meta‐analysis of two studies measuring the ability of green tea to help with weight maintenance showed a small, statistically non‐significant effect on weight and BMI. Not only were there few studies to evaluate, analysis required data to be imputed. Therefore, further study is required before it can be determined if green tea has a role in weight maintenance.
It is puzzling that studies that used the highest total daily doses of green tea catechins did not produce the greatest effect on weight loss; in fact several of the studies using the highest doses appeared to produce effects that were not statistically significant. There are several possible explanations for this finding: total daily doses of catechins were calculated by the authors of this review, based on information provided in the primary report of each study. The information may have not been accurate, either due to poor reporting in the article or because the study authors relied on stated label concentrations of catechins rather than on a chemical analysis of content of the preparation. The failure of effect to correspond to dose could also be a consequence of differences in study design. Linking total daily dose of EGCG, considered the most active catechin, to effect, was similar but not identical to the pattern seen with total daily dose of catechins and effect. As with the catechins, the studies that administered the highest total daily doses of EGCG did not produce statistically significant effects on measurements of weight loss, while the best effects were seen in the two studies that used products that were in the middle of the group, based on total daily dose of EGCG. The fact that the dose to effect was not identical for catechins and EGCG is not surprising as the amount of EGCG can vary from preparation to preparation. What is inexplicable, at least in principle, is that a higher dose does not produce a larger effect. This points to the effect that variations in study design can have on results. Further studies using chemically characterised products are required to establish the chemical content of green tea best able to aid in weight loss.
The effect that caffeine, a natural constituent of green tea, had on weight loss in studies is unclear. It was appropriate that the majority of studies documented, limited, or both the amount of caffeine participants consumed daily, as well as the amount of caffeine in the green tea preparation. Some studies, attempting to remove caffeine as a variable in the investigation, used caffeine‐free interventions and controls while others included caffeine in the intervention but cancelled out the effect of caffeine on weight loss in the study by adding the same amount of caffeine to the control. Analysis of these 'caffeine‐less' studies showed small, statistically significant reductions in weight, BMI and waist circumference, indicating that the catechins in green tea and not caffeine were likely to be responsible for the modest effect on weight loss. The issue was clouded when analysis of the other studies that did have a significantly higher level of caffeine in the intervention as compared to the control yielded a very small effect that was not statistically significant. It cannot be determined if the level of caffeine accounted for the low level of weight loss or if other factors were responsible. More research is required to clarify the role of caffeine in green tea as it applies to weight loss and weight maintenance.
Summary of main results
Meta‐analysis of six studies on 532 participants randomised to examine the effect of green tea preparations on weight loss when used for a minimum of 12 weeks, produced a very small, statistically non‐significant MD in weight loss. Eight studies conducted in Japan were too different from each other to be combined for analysis; however, they reported a MD in weight loss that ranged from ‐3.5 to ‐0.2 kg (1030 participants). Findings were similar for BMI and waist circumference, while there was no appreciable effect on waist‐to‐hip ratio, when compared to controls. The modest size of the reduction in weight produced by green tea preparations make them unlikely to be clinically relevant. These results were not substantially influenced by the use of imputed data in the analyses, or size of study. It is interesting to note that the country in which the study was conducted influenced the size of the effect. The meta‐analyses of studies conducted in Japan yielded a higher MD for all measurements of weight loss, with the exception of waist‐to‐hip ratio, than did the analyses of studies conducted outside of Japan.
Results of the effects of green tea preparations on 184 participants on weight maintenance after an initial weight loss were not statistically significant for any of the four measurements of weight change. This was likely to be a consequence of the small number of studies, in addition to the factors described above for the weight loss studies.
Overall completeness and applicability of evidence
The evidence provided by this systematic review is applicable to adult males and females who are overweight or obese, and have no contraindications to the use of green tea preparations. Data were sufficient for analysing one of the three primary outcomes of this review (change in body weight or mass measure). No studies were identified that aimed to investigate mortality or that provided data sufficient for addressing the remaining primary outcome of health‐related quality of life. The only secondary outcomes that could be addressed in the review were adverse effects of treatment and compliance. The adverse effects were not complete as many studies did not report whether participants were asked about adverse effects of green tea preparations or control. Similarly, half of the studies reported on compliance, with only five providing information on how compliance was tracked.
Quality of the evidence
In general, the studies were of reasonable quality. Most studies reported information regarding study design, with the exception of ITT analysis and adverse effects. Increasing the number of participants, documenting how long they had been overweight and using green tea preparations of defined chemical composition in a standardised dose would help to improve overall quality and reproducibility of evidence. Most of the meta‐analyses conducted in the review had I2 values that indicated a high level of heterogeneity. There were numerous potential reasons for heterogeneity among the studies, not the least of which was the significant variation in the content of the green tea preparation and in the doses used in studies. Another source of heterogeneity was the discrepancy between what investigators in each study set as the lowest BMI considered to be overweight (values varied from 21 to 25 kg/m2, although no documentation was provided to say how many participants were actually below 25 kg/m2).
Potential biases in the review process
We believe that the search strategies used in this systematic review were comprehensive but the possibility remains that a study could have been missed. Translations of the five Japanese studies were carried out independently by at least two volunteers for each study and the translations were compared for consistency; however it is possible that errors in translation were made. In some studies, there appeared to be incomplete reporting, and attempts were made to contact the study authors, with mixed success. We made conservative judgements with regards to the risk of bias criteria; for example, a number of study authors described their trials as "randomised", but if detail about how randomisation was achieved was not provided, we judged it to be "unclear" as to whether the study was truly randomised.
Agreements and disagreements with other studies or reviews
Two systematic reviews on green tea for the management of obesity have been published since July 2009 (Hursel 2009a; Phung 2010). The former, a meta‐analysis of 11 studies on green tea in weight loss, concluded that catechins or an EGCG‐caffeine mixture contained in green tea, had a small effect on weight loss and weight maintenance (Hursel 2009a). Using a random‐effects model, Hursel 2009 calculated that participants in intervention groups experienced an average weight change of ‐1.31 kg (95% CI ‐2.05 to ‐0.57; P < 0.001). Our meta‐analysis of 14 studies detected a high level of heterogeneity and so we separated our analysis into two groups: studies conducted in Japan and those conducted outside Japan. The Hursel 2009 review describes further analysis where they also divided studies into Asian studies and Caucasian studies, to examine differences in effects of green tea between these two groups. Hursel 2009 found that the average effect size with regard to weight loss for the eight Asian studies was ‐1.51 (95% CI ‐2.37 to ‐0.65), compared to ‐0.82 kg (95% CI ‐2.13 to 0.50) for the three Caucasian studies. Hursel 2009 noted that the difference in results between ethnic groups was not statistically significant. In this review, we compared Japanese (as opposed to Asian in Hursel 2009a) studies to studies conducted outside of Japan to determine if results between these groups differed. When the eight Japanese studies included in our review were analysed for weight loss, it was revealed that they were too heterogeneous to pool and so the range of weight loss was reported (MD of ‐3.5 to ‐0.20) (Analysis 1.1). Analysis of the six studies that were not conducted in Japan had a non‐significant MD of ‐0.04 kg (95% CI ‐0.50 to 0.43) (Analysis 1.1). Results of our analyses of the two groups suggested that there is potentially a difference in how the two ethnic groups responded to green tea preparations for weight loss, perhaps differently than when the comparison was made between Asians and Caucasians.
The more recently published systematic review, Phung 2010, looked at 15 studies and noted whether caffeine was included as part of the intervention or not. Phung 2010 calculated that participants in intervention groups who received green tea catechins in combination with caffeine had a decreased body weight (‐1.38 kg; 95% CI ‐1.7 to ‐1.06), BMI (‐0.55; 95% CI ‐0.65 to ‐0.4), and waist circumference (‐1.93 cm; 95% CI ‐2.82 to ‐1.04), compared with control groups containing similar amounts of caffeine. Although there was only overlap in eight of the 15 studies between the Phung 2010 systematic review and ours, our findings are in agreement with Phung when the quantity of caffeine was similar in the intervention and control. Phung also reported that in the studies where neither the green tea intervention nor control included caffeine (Hill 2007; Takeshita 2008), the intervention group did not have any significant advantage over the control in terms of changes in body weight, BMI or waist circumference, suggesting that caffeine needs to be present with catechins to have an effect on weight loss. Our findings were not in agreement with this. However, the effect of the presence of caffeine is not clear cut, based on just two studies, as we noted that Takeshita 2008 did actually have a small statistically significant effect on all three measurements of weight loss; weight loss (‐0.8 kg; 95% CI ‐1.41 to ‐0.19), BMI (‐0.3 kg/m2; 95% CI ‐0.50 to ‐0.10) and waist circumference (‐0.80 cm; 95% CI ‐1.37 to ‐0.23).
Authors' conclusions
Implications for practice.
Results from this review indicate that green tea has no significant effect on weight maintenance. Preparations of green tea used for 12 weeks in overweight/obese patients produced a very small, statistically non‐significant loss of weight, decrease in BMI, and decrease in waist circumference compared to a control substance. Adverse effects were minimal, with hypertension and constipation being most commonly reported. Many of the trials included in this review had methodological deficiencies that should be taken into account when considering the results of this review. Additionally, the clinical significance of the small changes seen in the parameters measured is likely to be minimal. However, even though the changes may be small, any small loss combined with minimal adverse effects may have an overall positive impact on an individual attempting to lose weight.
Implications for research.
A number of studies on the effectiveness of green tea preparations in weight loss and weight maintenance have been completed in the since the early 2000s, most of which are of reasonable design. What became clear when analysing studies was that there was qualitative and quantitative variation in chemicals contained in the green tea preparations being tested. Inconsistency in product content may account for the variation in results obtained. There are several factors that should be addressed if new studies on this topic are to increase our understanding of the value of green tea. To improve the quality of new studies, it is recommended that future studies: follow a standard reporting format, such as CONSORT, to ensure that all details of the study are available for assessment; perform an ITT and per protocol (PP) analysis to account for drop‐outs after randomisation; provide a complete quantitative description of the chemical content of the green tea preparations as well as the control; establish a standard dose of green tea, with a standardised content of catechins; and include outcomes such as health‐related quality of life and adverse effects.
Acknowledgements
The authors gratefully acknowledge the assistance of Randi Simons, Yvonne Rock and Saori Hamada, who, on a volunteer basis, translated studies published in Japanese into English. We also acknowledge the extensive and dedicated assistance of Sueko Matsumura Ng of the Cochrane Eyes and Vision Group, who volunteered to help us with initial assessments of Japanese language studies for relevancy to our review, and contacted researchers in Japan to request further information regarding specific studies. Thank you to Michelle Fiander, Cochrane Effective Practice and Organisation of Care (EPOC) Group for updating the search of AMED. We are grateful to Harriet Davies a member of our Advisory Committee, who assisted with developing the protocol and reviewed the final manuscript from both the consumer and the health provider point of view, and Dr. Jill Hayden (Advisory Committee), of the Nova Scotia Cochrane Resource Centre provided expert methodology advice throughout the review process.
Appendices
Appendix 1. Search strategies
| Search terms and databases |
| Unless otherwise stated, search terms are free text terms. Abbreviations: '$': stands for any character; '?': substitutes one or no character; adj: adjacent (i.e. number of words within range of search term); exp: exploded MeSH; MeSH: medical subject heading (MEDLINE medical index term); pt: publication type; sh: MeSH; tw: text word. |
| The Cochrane Library |
| #1 MeSH descriptor Obesity explode all trees #2 MeSH descriptor Weight gain explode all trees #3 MeSH descriptor Weight loss explode all trees #4 MeSH descriptor Body mass index explode all trees #5 MeSH descriptor Skinfold thickness explode all trees #6 MeSH descriptor Waist‐hip ratio explode all trees #7 MeSH descriptor Abdominal fat explode all trees #8 MeSH descriptor Overweight explode all trees #9 (overweight* in All Text or (over in All Text and weight* in All Text) ) #10 (fat in All Text and overload in All Text and syndrom* in All Text) #11 (overeat* in All Text or (over in All Text and eat* in All Text) ) #12 (overfeed* in All Text or (over in All Text and feed* in All Text) ) #13 (adipos* in All Text or obes* in All Text) #14 ( (weight in All Text near/3 cyc* in All Text) or (weight in All Text near/3 reduc* in All Text) or (weight in All Text near/3 los* in All Text) or (weight in All Text near/3 maint* in All Text) or (weight in All Text near/3 decreas* in All Text) or (weight in All Text near/3 watch* in All Text) or (weight in All Text near/3 control* in All Text) or (weight in All Text near/3 gain* in All Text) or (weight in All Text near/3 chang* in All Text) ) #15 ( (body in All Text and mass in All Text and ind* in All Text) or (waist‐hip in All Text and ratio* in All Text) ) #16 (skinfold in All Text and thickness* in All Text) #17 (abdominal in All Text and fat* in All Text) #18 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17) #19 MeSH descriptor Camellia sinensis explode all trees #20 (catechins in All Text or (epigallocatechin in All Text and gallate in All Text) or. EGCG in All Text) #21 (green in All Text and tea* in All Text) #22 (#19 or #20 or #21) #23 (#18 and #22) |
| MEDLINE |
| 1. exp obesity/ or exp obesity hypoventilation syndrome/ or exp obesity, abdominal/ or exp obesity, morbid/ or exp prader‐willi syndrome/ 2. exp Overweight/ 3. exp adipose tissue/ or exp adipose tissue, brown/ or exp adipose tissue, white/ or exp abdominal fat/ or exp intra‐abdominal fat/ or exp subcutaneous fat, abdominal/ or exp subcutaneous fat/ 4. exp weight gain/ or exp weight loss/ 5. exp body mass index/ or exp skinfold thickness/ or exp waist‐hip ratio/ 6. (overweight$ or over weight$).tw,ot. 7. fat overload syndrom$.tw,ot. 8. (overeat$ or over eat$).tw,ot. 9. (overfeed$ or over feed$).tw,ot. 10. (adipos$ or obes$).tw,ot. 11. (weight adj3 (cyc$ or reduc$ or los$ or maint$ or decreas$ or watch$ or control$ or gain$ or chang$)).tw,ot. 12. (body mass ind$ or waist‐hip ratio$).tw,ot. 13. skinfold thickness$.tw,ot. 14. abdominal fat$.tw,ot. 15. ((abdominal or subcutaneous or intra‐abdominal or visceral or retroperitoneal or retro peritoneal) adj3 fat*).tw,ot. 16. or/1‐15 17. Camellia sinensis.mp. or Camellia sinensis/ 18. Catechins.mp. 19. epigallocatechin gallate.mp. 20. EGCG.tw. 21. green tea*.mp. 22. or/17‐21 23. 16 and 22 24. randomised controlled trial.pt. 25. controlled clinical trial.pt. 26. randomi?ed.ab. 27. placebo.ab. 28. drug therapy.fs. 29. randomly.ab. 30. trial.ab. 31. groups.ab. 32. or/24‐31 33. Meta‐analysis.pt. 34. exp Technology Assessment, Biomedical/ 35. exp Meta‐analysis/ 36. exp Meta‐analysis as topic/ 37. hta.tw,ot. 38. (health technology adj6 assessment$).tw,ot. 39. (meta analy$ or metaanaly$ or meta?analy$).tw,ot. 40. ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systemat$)).tw,ot. 41. or/33‐40 42. 32 or 41 43. (comment or editorial or historical‐article).pt. 44. 42 not 43 45. 23 and 44 46. (animals not (animals and humans)).sh. 47. 45 not 46 |
| EMBASE |
| 1. exp Obesity/ 2. exp weight change/ or exp weight control/ or exp weight gain/ or exp weight reduction/ 3. exp body mass/ or exp waist circumference/ or exp waist hip ratio/ 4. (obes$ or overweight or over weight).ab,ti. 5. (overeat or over eat or overfeed or over feed or fat overload syndrom$).ab,ti. 6. (weight adj6 (cyc$ or reduc$ or los$ or maint$ or decreas$ or watch$ or control or chang$ or gain)).ab,ti. 7. (body mass ind$ or waist hip ratio or waist circumferenc$).ab,ti. 8. adipos$.ab,ti. 9. exp skinfold thickness/ 10. (abdominal fat or skinfold thickness).ab,ti. 11. or/1‐10 12. green tea*.mp. 13. Camellia sinensis.mp. 14. catechine*.mp. 15. epigallocatechin gallate.mp. 16. EGCG.tw,ot. 17. or/12‐16 18. 11 and 17 19. exp Randomized Controlled Trial/ 20. exp Controlled Clinical Trial/ 21. exp Clinical Trial/ 22. exp Comparative Study/ 23. exp Drug comparison/ 24. exp Randomization/ 25. exp Crossover procedure/ 26. exp Double blind procedure/ 27. exp Single blind procedure/ 28. exp Placebo/ 29. exp Prospective Study/ 30. ((clinical or control$ or comparativ$ or placebo$ or prospectiv$ or randomi?ed) adj3 (trial$ or stud$)).ab,ti. 31. (random$ adj6 (allocat$ or assign$ or basis or order$)).ab,ti. 32. ((singl$ or doubl$ or trebl$ or tripl$) adj6 (blind$ or mask$)).ab,ti. 33. (cross over or crossover).ab,ti. 34. or/19‐33 35. exp meta analysis/ 36. (metaanaly$ or meta analy$ or meta?analy$).ab,ti,ot. 37. ((review$ or search$) adj10 (literature$ or medical database$ or medline or pubmed or embase or cochrane or cinahl or psycinfo or psyclit or healthstar or biosis or current content$ or systematic$)).ab,ti,ot. 38. exp Literature/ 39. exp Biomedical Technology Assessment/ 40. hta.tw,ot. 41. (health technology adj6 assessment$).tw,ot. 42. or/35‐41 43. (comment or editorial or historical‐article).pt. 44. 34 or 42 45. 44 not 43 46. 18 and 45 47. limit 46 to human |
| CINAHL |
| S1 (MH "Obesity+") S2 (MH "Weight Gain+") S3 (MH "Weight Loss+") S4 (MH "Body Weight Changes+") S5 (MH "Body Mass Index") S6 (MH "Skinfold Thickness") S7 (MH "Waist‐Hip Ratio") S8 (MH "Abdominal Fat") S9 TX overweight* or TX over n3 weight* S10 TX fat n3 overload n3 syndrom* S11 TX overeat* or TX over n3 eat* S12 TX overfeed* or TX over n3 feed* S13 TX adipos* or TX obes* S14 TX weight n3 cyc* or weight n3 reduc* or weight n3 los* or weight n3 maint* or weight n3 decreas* or weight n3 watch* or weight n3 control* or weight n3 gain* or weight n3 chang* S15 TX body n3 mass n3 ind* or TX waist‐hip n3 ratio* S16 TX skinfold n3 thickness* S17 TX abdominal n3 fat* S18 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 S19 (MH "Green Tea") S20 TX catechin* or TX epigallocatechin n3 gallate or TX EGCG S21 TX green n3 tea* S22 s19 or s20 or s21 S23 s18 and s22 S24 (MH "Clinical Trials+") S25 (MH "Meta Analysis") S26 (MH "Comparative Studies") S27 (MH "Double‐Blind Studies") S28 (MH "Single‐Blind Studies") S29 (MH "Triple‐Blind Studies") S30 (MH "Prospective Studies") S31 TX clinical n3 trial* S32 TX random* S33 TX placebo* S34 TX meta analy* or TX meta*analy* S35 TX systematic n3 review S36 S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 S37 s23 and s36 S38 PT comment or PT editorial or PT historical S39 s37 not s38 S40 (MH "Animals+") S41 s39 not s40 |
| AMED |
| 1 exp Obesity/ 2 overweight.mp. 3 exp Adipose tissue/ 4 abdominal fat.mp. 5 weight gain.mp. 6 exp Weight loss/ 7 body mass.mp. 8 waist‐hip ratio.mp. 9 skinfold thickness.mp. 10 (weight adj3 (cyc* or reduc* or los* or maint* or decreas* or watch* or control* or gain* or chang*)).tw. 11 over weight.tw. 12 or/1‐11 13 exp camellia sinensis/ 14 Catechins.mp. 15 epigallocatechin gallate.mp. 16 EGCG.tw. 17 green tea*.tw. 18 or/13‐17 19 12 and 18 20 (animals not (animals and humans)).sh. 21 19 not 20 |
| Biological Abstracts |
| # 1 Topic=(obesity) # 2 Topic=(overweight*) OR Topic=(over weight*) # 3 Topic=(adipos*) AND Topic=(tissue) # 4 Topic=(weight gain) OR Topic=(weight loss) # 5 Topic=(fat overload syndrom*) OR Topic=(body mass index) # 6 Topic=(overeat*) OR Topic=(over eat*) # 7 Topic=(overfeed*) OR Topic=(over feed*) # 8 Topic=(waist hip ratio) # 9 Topic=(abdominal fat) # 10 Topic=(skinfold thickness*) # 11 #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 # 12 Topic=(green tea) # 13 Topic=(camellia sinensis) # 14 Topic=(catechin*) # 15 Topic=(epigallocatechin gallate) # 16 Topic=(EGCG) # 17 #16 OR #15 OR #14 OR #13 OR #12 # 18 Topic=(Clinical Trial) # 19 Topic=(Randomi*ed) AND Topic=(trial) # 20 Topic=(systematic) AND Topic=(review) # 21 Topic=(placebo) # 22 Topic=(Meta analysis) OR Topic=(Meta*analysis) # 23 Topic=(health technology assessment) # 24 Topic=(comparative stud*) # 25 Topic=(prospective stud*) # 26 #25 OR #24 OR #23 OR #22 OR #21 OR #20 OR #19 OR #18 # 27 #26 AND #17 AND #11 |
| IBIDS |
| +(obes* overweight "over weight" "adipose tissue" "abdominal fat" "weight gain" "weight loss" "body mass index" "waist hip ratio" "waist to hip ratio" "skinfold thickness") +(Catechin* "camellia sinensis" "green tea" "epigallocatechin gallate" "EGCG") +("controlled clinical trial" "clinical trial" "randomised clinical trial" "randomised clinical trial" placebo "meta analysis") |
| Obesity+ |
| green tea catechin* camellia sinensis epigallocatechin |
| IPA |
| 1 obes*.mp. 2 bmi.mp. 3 body mass index.mp. 4 weight gain.mp. 5 weight loss.mp. 6 adipos*.mp. 7 Skinfold thickness.mp. 8 waist hip ratio.mp. 9 waist circumference.mp. 10 Abdominal fat.mp. 11 overweight*.mp. 12 over weight*.mp. 13 fat overload syndrom*.mp. 14 overeat*.mp. 15 over eat*.mp. 16 overfeed*.mp. 17 over feed*.mp. 18 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19 green tea.mp. 20 egcg.mp. 21 (epigallocatechin and gallate).mp. 22 (Camellia and sinensis).mp. 23 catechin*.mp. 24 19 or 20 or 21 or 22 or 23 25 (Randomi*ed and controlled and trial).mp. 26 (controlled and clinical and trial).mp. 27 placebo.mp. 28 (double and blind).mp. 29 Meta analysis.mp. 30 25 or 26 or 27 or 28 or 29 31 18 and 24 and 30 |
| Web of Science |
| # 1 Topic=(obes*) # 2 Topic=(bmi) # 3 Topic=(body mass index) # 4 Topic=(weight gain) # 5 Topic=(weight loss) # 6 Topic=(adipos*) # 7 Topic=(Skinfold thickness) # 8 Topic=(waist hip ratio) # 9 Topic=(waist circumference) # 10 Topic=(Abdominal fat) # 11 Topic=(overweight*) # 12 Topic=(over weight*) # 13 Topic=(fat overload syndrom*) # 14 Topic=(overeat*) # 15 Topic=(over eat*) # 16 Topic=(overfeed*) # 17 Topic=(over feed*) # 18 #17 OR #16 OR #15 OR #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 OR #7 OR #6 OR #5 OR #4 OR #3 OR #2 OR #1 # 19 Topic=(green tea) # 20 Topic=(egcg) # 21 Topic=(epigallocatechin gallate) # 22 Topic=(Camellia sinensis) # 23 Topic=(catechin*) # 24 #23 OR #22 OR #21 OR #20 OR #19 # 25 Topic=(Randomi*ed controlled trial) # 26 Topic=(controlled clinical trial) # 27 Topic=(placebo) # 28 Topic=(double and blind) # 29 Topic=(Meta analysis) # 30 Topic=(HTA) # 31 Topic=(health technology assessment) # 32 #31 OR #30 OR #29 OR #28 OR #27 OR #26 OR #25 # 33 #32 AND #24 AND #18 |
Appendix 2. Description of interventions
|
Characteristic Study ID |
Intervention(s) [route, frequency, total dose/day] | Detailed chemical content of (daily) intervention [mg] | Control(s) [route, frequency, total dose/day] | All other weight loss interventions, such as exercise or diet, that participants may be using | Caffeine content [mg/d] |
| Auvichayapat 2008 | Oral, 3 capsules/d (1 after each meal);
total daily dose: 140.85 mg catechins 750 mg green tea extract (Herbal One brand) |
Caffeine 86.58 Total catechins 140.85 Catechin 12.27 Epicatechin gallate 27.84 Epigallocatechin gallate 100.74 also: gallic acid 0.72 daily – "gallate is a salt or ester of gallic acid" |
Oral, 3 capsules/d; total daily dose of 0 mg catechins | Note: participants consumed a regular diet (total energy, 8373.6 kJ/d) prepared by the Nutrition Unit of Srinagarind Hospital; participants were asked to maintain their regular exercise regimen | I1: 86.58 C1: 0 |
| Diepvens 2005 | Oral, 9 capsules/d (3 capsules at breakfast, lunch + dinner); total daily dose: 1206.9 mg catechins/d and 236.7 mg caffeine/d | Caffeine 236.7 Total catechins 1206.9 Catechin 31.5 Epicatechin 126 Gallocatechin 0 Epigallocatechin 240.3 Catechin gallate 0 Epicatechin gallate 212.4 Gallocatechin gallate 0 Epigallocatechin gallate 595.8 |
Oral, 9 capsules/d (3 capsules at breakfast, lunch + dinner); total daily dose of 0 mg catechins | I1: d 1‐3: standardised energy balance diet;
d 4 to 87 low‐energy diet
consumed (meal replacement diet plan of Slim‐Fast shake, soup, bar or pasta) at breakfast and lunch, and instructions about food allowed for dinner and snacks C1: d 1 to 3: standardised energy balance diet; d 4 to 87 low‐energy diet consumed (meal replacement diet plan of Slim‐Fast shake, soup, bar or pasta) at breakfast and lunch, and instructions about food allowed for dinner and snacks |
I1: 236.7 in 9 capsules + 300 mg/d (from coffee) = 536.7 mg C1: 300 mg/d (from coffee) Note: daily caffeine intake standardised for all participants to 300 mg/d |
| Hill 2007 | Oral, 2 capsules/d (1 before breakfast, 1 before evening meal on non‐exercise days; on exercise d 1 of the 2 capsules taken 1 h before exercise); total daily dose: 300 mg catechins | Caffeine 0 Total catechins 300 Epigallocatechin gallate 300 |
Oral, 2 capsules/d (1 before breakfast, 1 prior to evening meal on non‐exercise days; on exercise d 1 of the 2 capsules taken 1 h before exercise); total daily dose of 0 mg catechins | I1: run/walk for 45 min, 3 times/wk at HR corresponding to 75% of age
predicted maximum
in a protocol described previously C1: run/walk for 45 min, 3 times/wk at HR corresponding to 75% of age predicted maximum in a protocol described previously |
‐ *Note: participants excluded if they consumed ≥ 3 cups of green tea/d or had habitual caffeine intake of ≥ 300 mg (> 3 or 4 cups) from coffee/d |
| Hsu 2008 | Oral, 3 capsules/d (30 minutes after meals); total daily dose: 613.5 mg catechins |
Caffeine 27.3 Total catechins 613.5 Catechin 8.28 Epicatechin 70.33 Gallocatechin 61.58 Epigallocatechin 36.91 Catechin gallate 0 Epicatechin gallate 31.76 Gallocatechin gallate 27.48 Epigallocatechin gallate 377.15 |
Oral, 3 capsules/d; total daily dose of 0 mg catechins | Note: no other obesity management allowed; participants asked to maintain former diet during study period | I1: 27.3 C1: 0 |
| Hursel 2009 | I1: oral, 6 capsules/d (2 before each meal);
total daily dose: 270 mg EGCG + 150 mg caffeine I2: oral, 6 capsules/d (2 before each meal); total daily dose: 270 mg EGCG + 150 mg caffeine |
Caffeine 150 Total catechins 270 Epigallocatechin gallate 270 |
C1: oral, 6 capsules/d; total daily dose of 0 mg catechins C2: oral, 6 capsules/d; total daily dose of 0 mg catechins |
I1: AP diet (50‐60 g protein as 50 g calcium caseinate powder; ˜10% of energy from protein) I2: HP diet (100‐110 g protein as 50 g increments of calcium caseinate powder; ˜20% of energy from protein) C1: AP diet, same as I1 group C2: HP diet, same as I2 group |
I1: 150 I2: 150 C1: 0 C: 0 *Note: participants were allowed up to 100 mg caffeine/d from other sources |
| Kajimoto 2005 | I1: oral, 250 mL catechin drink twice/d
(at breakfast and dinner), placebo drink once daily at lunch;
total daily dose: 444.3 mg catechins + 50.2 mg caffeine I2: oral, 250 mL catechin drink 3 times/d at mealtimes; total daily dose: 665.9 mg catechins + 49.2 mg caffeine |
Low dose group numbers followed by
high dose group numbers: I1 (I2) Caffeine 50.2 (49.2) Total catechins 444.3 (645.9) Catechin 2.9 (2.7) Epicatechin 3.6 (4.8) Gallocatechin 3.6 (3.3) Epigallocatechin 3.9 (2.1) Catechin gallate 51.7 (73.5) Epicatechin gallate 68.2 (102.3) Gallocatechin gallate 158.1 (233.4) Epigallocatechin gallate 152.3 (223.8) |
Oral, 250 mL bottle 3 times/d at mealtimes; total daily dose of 41.1 mg catechins/d + 52.2 mg caffeine | Note: participants instructed not to change usual routine | I1: 50.2 mg I2: 49.2 mg C1: 52.2 mg |
| Kataoka 2004 | I1: oral, 500 mL once/d;
total daily dose of 277.9 mg catechins I2: oral, 500 mL once/d; total daily dose of 570.4 mg catechins I3: oral, 500 mL once/d; total daily dose of 844.7 mg catechins (also contained sweeteners, acidulants, electrolytes, antioxidants, and flavouring) |
Caffeine < 40 mg Total catechins Low/Med/High 277.9/570.4/844.7 Catechin 8.2/12.7/24.7 Epicatechin 24.7/49.7/73.9 Gallocatechin 17.7/36.0/53.4 Epigallocatechin 80.6/174.7/253.3 Catechin gallate 2.8/4.1/8.6 Epicatechin gallate 35.0/65.7/103.9 Gallocatechin gallate 3.3/9.1/11.1 Epigallocatechin gallate 105.6/218.4/315.8 |
Oral, 500 mL once/d; total daily dose of 0 mg catechins (also contained sweeteners, acidulants, electrolytes, antioxidants, and flavouring) |
Note: diet and food intake adjusted to constant levels; pedometer used to measure steps daily | I1: < 40 mg/d from beverage I2: < 40 mg/d from beverage I3: < 40 mg/d from beverage C1: < 40 mg/d from beverage Note: participants not allowed to consume catechin‐containing tea or caffeinated coffee beverages during study, or any food supplement that might affect energy metabolism (263) |
| Kovacs 2004 | Oral, 6 capsules/d (2 with breakfast, lunch + dinner); total daily dose: 572.76 mg catechins + 103.5 mg caffeine | Caffeine 103.5 Total catechins 572.76 Catechin 6.06 Epicatechin 34.56 Gallocatechin 6.66 Epigallocatechin 22.62 Catechin gallate 4.86 Epicatechin gallate 124.44 Gallocatechin gallate 50.7 Epigallocatechin gallate 322.86 |
Oral, 6 capsules/d (2 with breakfast, lunch + dinner); total daily dose of 0 mg catechins | I1: none C1: none Note: weight loss intervention used in 4‐week run‐in phase |
I1: 103.5 mg caffeine C1: 0 Note: habitual caffeine consumption of GT group was 369 (227) mg/d, with a range of 1 to 991 mg/d (N = 19 with < 300 mg caffeine/d; N = 32 with > 300 mg caffeine/d) Habitual caffeine consumption of placebo group was 358 (190) mg/d, with a range of 39 to 996 mg/d (N n= 19 with < 300 mg caffeine/d; N = 32 with > 300 mg caffeine/d) |
| Kozuma 2005 | Oral, 500 mL once/d; total daily dose of 539.7 mg catechins | Caffeine ‐ Total catechins 539.7 Catechin 14.0 Epicatechin 47.5 Gallocatechin 35.1 Epigallocatechin 166.9 Catechin gallate 8.3 Epicatechin gallate 58.9 Gallocatechin gallate 7.6 Epigallocatechin gallate 201.4 |
Oral, 500 mL once/d; total daily dose of 0 mg catechins | Note: participants were assisted by head physicians to maintain regular food intake and exercise Medications, special health products, supplements or dietary foods that might affect metabolism of sugar, fat, or energy were restricted Intake of fat was not restricted |
‐ |
| Maki 2009 | Oral, 500 mL once/d; total daily dose: 625 mg catechins (also contains water, sodium chloride, artificial citrus flavouring, glucose, erythritol, and sucralose; 15 kcal of energy and 250 mg of sodium) | Caffeine 39.5 Total catechins 625 Catechin 19.2 Epicatechin 53.9 Gallocatechin 51.8 Epigallocatechin 207.5 Catechin gallate 6.0 Epicatechin gallate 56.5 Gallocatechin gallate 15.4 Epigallocatechin gallate 214.4 |
Oral, 500 mL once/d; total daily dose of 0 mg catechins (containing water, sodium chloride, artificial citrus flavouring, glucose, erythritol, and sucralose; 15 kcal of energy and 250 mg of sodium) |
I1: exercise programme (at least 3 supervised exercise sessions per week with goal of ≥ 180 min/wk of moderate‐intensity
physical activity) C1: exercise programme (at least 3 supervised exercise sessions per week with goal of ≥ 180 min/wk of moderate‐intensity physical activity) |
I1: ˜ 39 C1: ˜ 39 Note: participants agreed: to record all caffeinated beverages consumed not to consume > 2 caffeinated beverages/d not to consume over the counter supplements containing caffeine not to consume any medications containing caffeine |
| Nagao 2007 | Oral, 340 mL once/d; total daily dose: 582.76 mg catechins, within 1 h of evening meal (steeped green tea used as base for both I and C) | Caffeine 72.42 Total catechins 583.1 Catechin 42.84 Epicatechin 32.3 Gallocatechin 127.5 Epigallocatechin 69.36 Catechin gallate 40.12 Epicatechin gallate 30.94 Gallocatechin gallate 139.74 Epigallocatechin gallate 100.3 |
Oral, 340 mL once/d; total daily dose: 96.3 mg catechins, within 1 h of evening meal (steeped green tea used as base for both I and C) |
Note: medications known to influence lipid or carbohydrate metabolism were prohibited | I1: 72.42 C1: 75.14 Note: tea and coffee were not limited though trial |
| Suzuki 2009 | Oral, 340 mL twice/d (1 with breakfast, 1 with dinner); total daily dose: 392.2 mg catechins + 85.4 mg caffeine | Caffeine 85.4 Total catechins 392.2 Catechin 0 Epicatechin 0 Gallocatechin 35.8 Epigallocatechin 16.6 Catechin gallate 31 Epicatechin gallate 45.2 Gallocatechin gallate 135.4 Epigallocatechin gallate 128.2 |
Oral, 340 mL twice/d (1 with breakfast, 1 with dinner); total daily dose: 65.4 mg catechins + 93 mg caffeine | Note: directed to maintain usual lifestyle patterns: Get adequate sleep Avoid excessive eating/drinking Avoid strenuous exercise Participants given identical meals to consume: Breakfast and dinner 2 and 3 d before examinations Breakfast, lunch and dinner the day before examinations |
I1: 85.4 C1: 93 |
| Takase 2008 | Oral, 500 mL once/d; total daily dose: 539.7 mg catechins | Caffeine 40 mg Total catechins 539.7 Catechin 14.0 Epicatechin 47.5 Gallocatechin 35.1 Epigallocatechin 166.9 Catechin gallate 8.3 Epicatechin gallate 58.9 Gallocatechin gallate 7.6 Epigallocatechin gallate 201.4 |
Oral, 500 mL once/d; total daily dose of 0 mg catechins | Note: participants were instructed to maintain diet and exercise levels, avoiding change |
I1: 40 C1: 0 |
| Takashima 2004 | Oral, 500 mL once/d; total daily dose: 570.4 mg catechins | Caffeine < 40 Total catechins 570.4 mg Catechin 12.7 Epicatechin 49.7 Gallocatechin 36.0 Epigallocatechin 174.7 Catechin gallate 4.1 Epicatechin gallate 65.7 Gallocatechin gallate 9.1 Epigallocatechin gallate 218.4 |
Oral, 500 mL once/d; total daily dose of 0 mg catechins | Note: participants were instructed to maintain diet and exercise levels, avoiding change |
I1: < 40 mg/d (stated that beverage contained < 8 mg/100 mL) C1: < 40 mg/d (stated that beverage contained < 8 mg/100 mL) Note: during the study participants were not allowed to consume any tea with catechins or coffee with caffeine |
| Takeshita 2008 | Oral, 500 mL once/d; total daily dose: 548 mg catechins | Caffeine 0 Total catechins 547.5 Catechin 17.5 Epicatechin 50.5 Gallocatechin 39.5 Epigallocatechin 282 Catechin gallate 0 Epicatechin gallate 18.5 Gallocatechin gallate 7 Epigallocatechin gallate 132.5 |
Oral, 500 mL once/d; total daily dose of 0 mg catechins | Note: foods or medicines that would affect the metabolism
of fat or sugar prohibited; participants asked to maintain previous level of physical fitness |
I1: 0 C1: 0 Note: tea beverages with catechins or caffeine prohibited; coffee with caffeine restricted to 2 cups/d maximum |
| Tsuchida 2010 | Oral, 340 mL once/d; total daily dose of 587.52 mg catechins and 82.96 mg caffeine | I1/C1: Caffeine 82.96/81.26 Total catechins 587.52/126.82 Catechin 39.44/7.82 Epicatechin 27.54/4.76 Gallocatechin 134.30/31.96 Epigallocatechin 79.56/18.70 Catechin gallate 34.68/ 5.78 Epicatechin gallate 30.60/5.44 Gallocatechin gallate 126.48/27.2 Epigallocatechin gallate 114.92/25.16 |
Oral, 340 mL once/d; total daily dose of 126.82 mg catechins and 81.26 mg caffeine | Note: participants were to maintain usual levels of food intake and exercise as much as possible Food products that might affect energy absorption (e.g. dietary fibre supplements, psyllium) were restricted, as were medications that affect lipid metabolism |
I1: 82.96 mg C1: 81.26 mg |
| Wang 2010 | I1: oral, 250 mL twice/d; total daily dose of 458 mg catechins and 104 mg caffeine I2: oral, 250 mL twice/d; total daily dose of 468 mg catechins and 126 mg caffeine I3: oral, 250 mL twice/d; total daily dose of 886 mg catechins and 198 mg caffeine All drinks consumed at least 4 h apart, following a meal: breakfast before first treatment, and lunch or dinner before second treatment |
Caffeine GT1: 104 GT2: 126 GT3: 198 Total catechins GT1: 458 GT2: 468 GT3: 886 |
Oral, 250 mL twice/d; total daily dose of 30 mg catechins and 10 mg caffeine All drinks were consumed at least 4 h apart, and following a meal: breakfast before first treatment, and lunch or dinner before second treatment |
Note: participants were not to consume other beverages with catechins or caffeine. Participants were asked to use the same mode of transportation to reach the research facility during the study period | I1: 104 I2: 126 I3: 198 C1: 10 Note: no other sources of caffeine were allowed |
|
Westerterp‐ Plantenga 2005 |
I1: oral, 6 capsules/d (2 capsules before each meal)
total daily dose: 270 mg EGCG + 150 mg caffeine I2: oral, 6 capsules/d (2 capsules before each meal) total daily dose: 270 mg EGCG + 150 mg caffeine |
Caffeine 150 Total catechins 270 Epigallocatechin gallate 270 |
C1: oral, 6 capsules/d; total daily dose of 0 mg catechins C2: oral, 6 capsules/d; total daily dose of 0 mg catechins |
‐ | I1: 150 mg + low habitual caffeine consumption I2: 150 mg + high habitual caffeine consumption C1: 0 + low habitual caffeine consumption C2: 0 + high habitual caffeine consumption |
|
Footnotes "‐" denotes not reported AP: adequate protein; C: control; EGCG: epigallocatechin‐3‐gallate ; GT: green tea; HP: high protein; HR: heart rate; I: intervention | |||||
Appendix 3. Matrix of study end points
|
Characteristic Study ID |
Intervention(s) and control(s) | Primarya end point(s) | Secondarya end point(s) | Otherb end point(s) |
| Auvichayapat 2008 | I1: green tea extract C1: placebo |
‐ | ‐ | Average left capsule (%)
Blood pressure BMI Body fat % Diastolic blood pressure Food intake Heart rate Height Hip circumference Leptin Physical activity Respiratory quotient Resting energy expenditure Satiety Systolic blood pressure Substrate oxidation Urine vanillylmandelic acid Waist circumference Waist‐to‐hip ratio Weight |
| Diepvens 2005 | I1: green tea C1: placebo |
‐ | ‐ | Appetite ratings Attitudes towards eating BMI Body fat % Caffeine intake Carbohydrate oxidation Cognitive restraint Diastolic blood pressure Disinhibition Eating behaviour (restrained/unrestrained) Emotional eating Energy expenditure Fat free mass Fat mass Fat oxidation Heart rate Height Hip circumference Hunger Mood Respiratory quotient (post‐absorptive) Respiratory quotient (post‐prandial) Resting energy expenditure Systolic blood pressure Tolerance of treatment Total body water Waist circumference Waist‐to‐hip ratio Weight |
| Hill 2007 | I1: green tea extract C1: placebo |
‐ | ‐ | Abdominal fat Adipocytokines Adiponectin Blood lipids Blood pressure (resting) BMI Body composition Body fat mass Body fat percentage Body lean mass Diastolic blood pressure Direct glyceryl trinitrate‐induced vasodilation Endothelium‐dependent flow‐mediated dilation Energy expenditure Energy intake Flow‐mediated dilation Follicle stimulating hormone Fructosamine Glucose Glyceryl trinitrate‐mediated dilation Haematology Heart rate Height Hip circumference Insulin Intra‐abdominal adipose tissue Large artery compliance Leptin Liver function Serum electrolytes Small artery compliance Subcutaneous adipose tissue Systolic blood pressure Total body fat Visceral fat area Waist circumference Waist‐to‐hip ratio Weight |
| Hsu 2008 | I1: green tea extract C1: placebo |
Note: "The outcome was evaluated
as % reduction in BW, BMI and WC
after 12 weeks of intervention" The word "primary" was not used |
‐ | Adiponectin Aminotransferase alanine Aminotransferases aspartate Blood sugar BMI Cholesterol Creatinine Ghrelin Glucose HDL‐cholesterol Height Hip circumference HOMA insulin resistance index Insulin LDL‐cholesterol Leptin Triglyceride Uric acid Waist circumference Weight |
| Hursel 2009 | I1: green tea‐caffeine mixture + AP diet I2: green tea‐caffeine mixture + HP diet C1: placebo + AP diet C2: placebo + HP diet |
‐ | ‐ | Attitudes towards eating Beta‐hydroxybutyrate BMI Body fat distribution Fat free mass Fat mass Free fatty acid concentrations Glucose Glycerol concentrations Height Hunger Insulin Leptin Physical activity level Post‐absorptive appetite profile *(VAS ) Respiratory quotient Resting energy expenditure Satiety Substrate oxidation Total body water Total energy expenditure Triacylglycerol Waist circumference Weight |
| Kajimoto 2005 | I1: catechin drink – low dose group I2: catechin drink – high dose group C1: placebo drink |
‐ | ‐ | Adverse events
Alanine aminotransferase
Albumin
Alcohol intake
Alkaline phosphatase
Amylase
Aspartate aminotransferase
BMI
Calcium
Carbohydrate intake
Chloride
Chlorine
Cholesterol intake
Cholinesterase
Creatinine
Creatinine phosphokinase
Dietary fibre intake
Direct bilirubins
Energy intake
Erythrocytes
Fasting glucose level
Fat intake
Ferritin
Gamma‐glutamyl transpeptidase
Glucose
Glycated haemoglobin
HDL‐cholesterol
Height
Haematocrit
Haemoglobin
Hip circumference
Inorganic Phosphate
Insulin
Iron
Ketone body fractions
Lactate dehydrogenase
LDL‐cholesterol
Leukocytes
Magnesium
Physical activity levels
Platelet counts
Potassium
Protein intake
Sodium
Subcutaneous fat area
Total bilirubins Total cholesterol Total fat area Total proteins Triacylglycerol Unsaturated iron binding capacity Urea nitrogen Uric acid Visceral fat area (VFA) Waist circumference Waist‐to‐hip ratio Weight |
| Kataoka 2004 | I1: catechin beverage 278 mg I2: catechin beverage 570 mg I3: catechin beverage 845 mg C1: control beverage |
‐ | ‐ | Abdominal fat area Albumin Alkaline phosphatase Blood sugar Blood urea nitrogen BMI Body fat ratio Calcium Chloride Creatinine Energy intake Fat intake Free fatty acid Gamma‐glutamyl transpeptidase GOT GPT HDL cholesterol Height Haematocrit Haemoglobin Hip circumference Inorganic phosphate Iron Lactate dehydrogenase LDL cholesterol Magnesium Number of steps Platelet count Potassium Red blood cell count Sodium Subcutaneous fat area Total cholesterol Total fat area Total plasminogen activator inhibitor‐1 Total protein Triacylglycerol Uric acid Visceral fat area Waist circumference Weight White blood cell count |
| Kovacs 2004 | I1: green tea C1: placebo |
‐ | ‐ | Adverse events Attitude towards eating Beta‐hydroxybutyrate BMI Body fat % Caffeine intake Cognitive restraint Disinhibition Fat free mass Fat mass Frequency of previous dieting Glucose Glycerol concentrations Height Hip circumference Hunger Insulin Leptin Non‐esterified fatty acids Physical activity level Post‐absorptive appetite profile Respiratory quotient Resting energy expenditure Satiety Substrate oxidation Total body water Total energy expenditure Triacylglycerol Waist circumference Waist‐to‐hip ratio Weight |
| Kozuma 2005 | I1: catechin beverage C1: placebo beverage |
‐ | ‐ | Acetoacetic acid Alanine aminotransferase Albumin Alkaline phosphatase Aspartate aminotransferase Beta‐hydroxybutyric acid Blood sugar Blood urea nitrogen BMI Body fat Calcium Chloride Creatinine Diastolic blood pressure Fasting blood sugar Gamma‐glutamyl transpeptidase HDL‐cholesterol Height Haematocrit Haemoglobin Hip circumference Inorganic phosphate Iron Lactate dehydrogenase LDL cholesterol Magnesium Non‐esterfied fatty acid Platelet counts Potassium Pulse rate Red blood cells Remnant lipoprotein cholesterol Sodium Subcutaneous fat area Systolic blood pressure Total cholesterol Total fat area Total proteins Triglycerides Uric acid Urine ketone bodies Urine protein Urine sugar Urine urobilinogen Visceral fat area Waist circumference Weight White blood cells |
| Maki 2009 | I1: green tea beverage C1: control beverage |
‐ | ‐ | Abdominal fat areas Beta‐hydroxybutyrate Blood pressure Body composition Caffeine intake Insulin (fasting) Free fatty acids Glucose (fasting) High‐sensitivity C‐reactive protein (hs‐CRP) Intra‐abdominal fat area MDA (plasma concentration) Maximal treadmill exercise testing Metabolic equivalent hours (MET‐hours) Pedometer readings Plasma high‐sensitivity C‐reactive protein (hs‐CRP) Serum FFA Serum HDL‐cholesterol Serum LDL‐cholesterol Serum lipids Serum total‐C concentrations Subcutaneous fat area Total fat mass Triglyceride concentrations Waist circumference Weight Whole blood glycosylated haemoglobin |
| Nagao 2007 | I1: brewed green tea beverage
with added catechins C1: brewed green tea beverage with no added catechins |
‐ | ‐ | Abdominal fat level Alkaline phosphatase BMI Body fat mass Body fat ratio Caffeine intake from tea and coffee Coffee intake Computed tomography scan Diastolic blood pressure Energy intake based on food intake Fat energy ratio Fat intake Free fatty acids Gamma glutamyl transferase Gamma glutamyl transpeptidase Glucose Glutamic‐oxaloacetic transaminase Glutamic‐pyruvic transaminase HDL cholesterol Height Haematocrit values Haemoglobin Hip circumference Lactate dehydrogenase LDL cholesterol Lean body mass Platelets Pulse rate Red blood cell count Serum triacylglycerol Subcutaneous fat area Systolic blood pressure Tannin intake from tea and coffee Tea intake Total cholesterol Total fat area Visceral fat area Waist circumference Weight White blood cell count |
| Suzuki 2009 | I1: tea catechin C1: placebo |
‐ | ‐ | Abdominal fat area Alcohol intake BMI Energy intake HDL cholesterol Height Hip circumference LDL cholesterol Number of steps Observable symptoms Patient complaints Remnant lipoprotein cholesterol Small dense LDL Subcutaneous fat area Total cholesterol Total fat area Triglycerides Visceral fat area Waist circumference Weight |
| Takase 2008 | I1: catechin beverage C1: placebo beverage |
‐ | ‐ | Acetic acid
Albumin
Alkaline phosphatase
Aspartate aminotransferase
Beta‐hydroxybutyric acid
BMI
Calcium
Calories
Carbohydrate (% of energy)
Chlorine
Cholesterol (total)
Creatine
Diastolic blood pressure
Energy intake based on food intake
Fasting blood sugar
Fat intake
Fibre
Free fatty acids
Gamma‐glutamyl transpeptidase HDL cholesterol Height Haematocrit values Haemoglobin Hip circumference Inorganic phosphate Insulin Iron ketone bodies (total) Lactate dehydrogenase LDL cholesterol Magnesium Neutral fats Nutritional content of food intake Platelets Potassium protein (total) Protein intake Red blood cell count Sodium Subcutaneous fat area Systolic blood pressure Total fat area Triglycerides Urea nitrogen Uric acid Urine glucose Urine ketones Urine protein Urobilinogen Visceral fat area Waist circumference Weight White blood cell count |
| Takashima 2004 | I1: catechin beverage C1: control beverage |
‐ | ‐ | Abdominal fat area Blood sugar BMI Body fat ratio Carbohydrate oxidation CO2 consumption Energy expenditure Exercise tolerance Fat free mass Fat oxidation Free fatty acid Height Haematocrit Haemoglobin Hip circumference Insulin Ketone body Lactic acid Number of daily steps Oxygen consumption Red blood cell count Respiratory exchange ratio Respiratory quotient Resting energy expenditure Subcutaneous fat area Total energy expenditure Total fat area Visceral fat area Waist circumference Weight |
| Takeshita 2008 | I1: catechin beverage C1: placebo beverage |
Note: "Measurement of the key factor of interest, the amount of abdominal fat (area of abdominal fat), occurred at the beginning of the study (baseline) and at its completion (the 12th week)" The word “primary” was not used | ‐ | Abdominal fat Alanine aminotransferase Albumin Alcohol intake (g/d) Alkaline phosphatase Aspartate aminotransferase Blood pressure Blood urea nitrogen BMI Body fat mass Body fat ratio Calcium Carbohydrate (% of energy) Chlorine Cholesterol Coffee intake (mg caffeine/d) Coffee intake (mL/d) Creatinine Diastolic blood pressure Dietary fibre (g/d) Dietary intake Energy (kcal/d) Fat (% of energy) Free fatty acids Gamma‐glutamyltranspeptidase Glucose Height Haematocrit Haemoglobin HDL‐cholesterol Hip circumference Inorganic phosphate Insulin Intake of beverage Iron How they were feeling Ketone bodies Lactate dehydrogenase LDL cholesterol Lean body mass Magnesium Neutral fat Pedometer steps (steps/d) Plasma glucose Platelets Potassium Protein (% of energy) Pulse rate Red blood cells Safety parameters Side effects Sodium State of health Subcutaneous fat area Systolic blood pressure Tannin intake from coffee (mg/d) Total cholesterol Total fat area Triacylglycerol Unsaturated iron‐binding capacity Uric acid Urine ketone bodies Urea nitrogen Urine protein Urine sediment Urine sugar Urine urobilinogen Visceral fat area Waist circumference Weight White blood cells |
| Tsuchida 2010 | I1: catechin beverage C1: control beverage |
‐ | ‐ | Albumin Alkaline phosphatase Blood sugar Body fat mass Body fat ratio BMI Calcium Chloride Creatinine Diastolic blood pressure Energy intake Fat energy ratio Fat intake Free fatty acids Gamma‐glutamyl transpeptidase Glucose GOT GPT HDL‐cholesterol Heart rate Height Haematocrit Haemoglobin Hip circumference Inorganic phosphate Iron Ketone bodies Lactate dehydrogenase LDL cholesterol Lean body mass Magnesium Phospholipids Platelet counts Potassium Protein Red blood cells Sodium Subcutaneous fat area Systolic blood pressure Total cholesterol Total fat area Total plasminogen activity inhibitor‐1 Total protein Triglyceride Urea nitrogen Uric acid Urine glucose Urine ketone bodies Urine protein Urine urobilinogen Visceral fat area Waist circumference Waist‐to‐hip ratio Weight White blood cells |
| Wang 2010 | I1: GT1 I2: GT2 I3: GT3 C1: C |
Note: “The response variable of main interest was body weight.” The word "primary was not used. | ‐ | Alanine aminotransferase Alkaline phosphatase Aspartate aminotransferase Blood glucose BMI Body fat % Dietary habits Energy intake Fat mass Gamma‐glutamyltransferase Glucose HDL cholesterol Height Hip circumference Intra‐abdominal fat Lactate dehydrogenase LDL cholesterol Lean mass Lifestyle habits Product liking Sagittal diameter Self‐perceived health status Total cholesterol Triglycerides Waist circumference Weight Whole body fat mass |
|
Westerterp‐ Plantenga 2005 |
I1: green tea‐caffeine capsule
‐ low habitual caffeine consumers I2: green tea‐caffeine capsule ‐ high habitual caffeine consumers C1: placebo ‐ low habitual caffeine consumers C2: placebo ‐ high habitual caffeine consumers |
‐ | ‐ | adverse effects Attitude towards eating Beta‐hydroxybutyrate BMI Body fat % Caffeine intake Cognitive restraint Disinhibition Fat distribution Fat free mass Fat mass Free fatty acids Frequency of previous dieting Glucose Glycerol Height Hunger Insulin Leptin Physical activity level Respiratory quotient Resting energy expenditure Satiety Substrate oxidation Total body water Total energy expenditure Triacylglycerol Triglycerides Waist circumference Weight |
|
Footnotes "‐" denotes not reported aAs stated in the publication bNot stated as primary or secondary end point(s) in the publication AP: adequate protein; BMI: body mass index; CO2: carbon dioxide; C: control; GOT: glutamic oxalacetic transaminase; GPT: glutamic‐pyruvic transaminase; GT: green tea; HP: high protein; HOMA: homeostasis model assessment; I: intervention; VAS: visual analogue scale | ||||
Appendix 4. Baseline characteristics (I)
|
Characteristic Study ID |
Intervention(s) and control(s) | Participating population | Sex [female %] | Age [mean years (SD)] | Body weight [mean, kg] | BMI [mean, kg/m2] | Ethnic groups [%] |
| Auvichayapat 2008 | I1: green tea extract C1: placebo |
I1: 30 C1: 30 |
I1: 70 C1: 70 |
I1: 49 (6) C1: 49 (5) |
I1: 69.30±9.54 C1: 71.90±11.70 |
I1: 27.42±3.26 C1: 28.00±3.51 |
Note: participants described as "Thai", but we cannot be sure of complete ethnic background of participants |
| Diepvens 2005 | I1: green tea C1: placebo |
I1: 23 C1: 23 |
I1: 100 C1: 100 |
I1: 42 (9) C1: 42 (10) |
I1: 76.4 (SD 6.3) C1: 76.3 (SD 6.6) |
I1: 27.7 (SD 1.8) C1: 27.7 (SD 1.8) |
‐ |
| Hill 2007 | I1: green tea extract C1: placebo |
I1: 19 C1: 19 |
I1: 100 C1: 100 |
‐ | I1: 79.92±1.73 C1: 81.05±2.01 |
I1: 30.65±0.59 C1: 31.39±0.73 |
‐ |
| Hsu 2008 | I1: green tea extract C1: placebo |
I1: 41 C1: 37 |
I1: 100 C1: 100 |
I1: 43 (11) C1: 44 (13) |
I1: 78.5 (SD 10.3) C1: 76.3 (SD 14.5) |
I1: 31.2 (SD 3.5) C1: 30.5 (SD 4.6) |
‐ |
| Hursel 2009 | I1: green tea‐caffeine mixture + AP diet I2: green tea‐caffeine mixture + HP diet C1: placebo + AP diet C2: placebo + HP diet |
I1: 20 I2: 20 C1: 20 C2: 20 |
I1: 55 I2: 55 C1: 55 C2: 55 |
I1: 44 (2) I2: 44 (2) C1: 44 (2) C2: 44 (2) |
I1: 85.1 ±7.6 I2: 85.0±7.4 C1: 85.1±7.8 C2: 85.0±7.3 |
I1: 29.6±2.1 I2: 29.5±2.0 C1: 29.6±2.1 C2: 29.5±1.9 |
‐ |
| Kajimoto 2005 | I1: catechin drink – low dose group I2: catechin drink – high dose group C1: placebo drink |
I1: 65 I2: 64 C1: 66 |
T: 50 | ‐ | I1: 68.4±1.1 I2: 68.4±1.1 C1: 68.7±1.1 |
I1: 25.6±0.2 I2: 25.7±0.2 C1: 25.7±0.3 |
Note: participants described as "Japanese", but we cannot be sure of complete ethnic background of all participants |
| Kataoka 2004 | I1: catechin beverage 278 mg I2: catechin beverage 570 mg: I3: catechin beverage 845 mg C1: control beverage |
I1: 25 I2: 71 I3: 25 C1: 71 |
I1: 0 I2: 0 I3: 0 C1: 0 |
I1: 40 (2) I2: 39 (1) I3: 39 (2) C1: 39 (1) |
I1: 72.6±0.9 I2: 73.8±1.1 I3: 71.8±2.3 C1: 73.4±1.0 |
I1: 24.3±0.4 I2: 25.1±0.3 I3: 24.6±0.7 C1: 24.9±0.3 |
‐ |
| Kovacs 2004 | I1: green tea C1: placebo |
I1: 51 C1: 53 |
I1: 71 C1: 79 |
‐ | I1: 85.1 (SD 11.5) C1: 85.4 (SD 10.3) |
I1: 29.7 (SD 2.9) C1: 29.7 (SD 2.3) |
‐ |
| Kozuma 2005 | I1: catechin beverage C1: placebo beverage |
I1: 107 C1: 119 |
I1: 50 C1: 54 |
I1: 56 (1) (female), 43 (2) (male) C1: 55 (1) (female), 40 (2) (male) |
I1: 63.42±0.79 C1: 5.04±0.92 |
I1: 26.73±0.26 C1: 27.12±0.29 |
Note: study took place in Japan, but we cannot be sure of complete ethnic background of all participants, as no information is provided |
| Maki 2009 | I1: green tea beverage C1: control beverage |
I1: 65 C1: 63 |
I1: 50.8/49.2 C1: 44.4/55.6 |
I1: 47 (1) C1: 49 (1) |
I1: 95.1± 1.7 C1: 95.1±1.7 |
I1: 32.2±0.5 C1: 32.2±0.5 |
I1: Non‐Hispanic White: (90.8) African‐American: (6.2) Hispanic: (3.1) Other: (0) C1: Non‐Hispanic White: (92.1) African‐American: (3.2) Hispanic: 3.2% Other: (1.6) |
| Nagao 2007 | I1: brewed green tea beverage with added catechins C1: brewed green tea beverage with no added catechins |
I1: 123 C1: 117 |
I1: 41.5/58.5 C1: 41.9/58.1 |
‐ T: 42 (10) |
I1: 73.3 (SD 9.7) C1: 72.1 (SD 10.0) |
I1: 26.9 (SD 1.9) C1: 26.7 (SD 2.1) |
Note: participants described as "Japanese", but we cannot be sure of complete ethnic background of all participants |
| Suzuki 2009 | I1: tea catechin C1: placebo |
I1: 18 C1: 20 |
I1: 100/0 C1: 100/0 |
I1: 45 (11) C1: 48 (10) |
I1: 64.5±5.6 C1: 63.3±7.2 |
I1: 26.2±1.5 C1: 25.8±1.7 |
Note: study took place in Japan, but we cannot be sure of complete ethnic background of all participants, as no information is provided |
| Takase 2008 | I1: catechin beverage C1: placebo beverage |
I1: 44 C1: 45 |
I1: 100/0 C1: 100/0 |
I1: 48 (6) C1: 48 (6) |
I1: 67.2±6.6 C1: 66.9±6.1 |
I1: 27.7±1.5 C1: 27.7±1.6 |
Note: participants described as "Japanese women", but we cannot be sure of complete ethnic background of all participants |
| Takashima 2004 | I1: catechin beverage C1: control beverage |
I1: 10 C1: 9 |
I1: 0/100 C1: 0/100 |
‐ T: 38 (1) |
I1: 71.4±2.3 C1: 72.5±2.5 |
I1: 23.7±0.6 C1: 24.3±0.8 |
‐ |
| Takeshita 2008 | I1: catechin beverage C1: placebo beverage |
I1: 40 C1: 41 |
I1: 0/100 C1: 0/100 |
I1: 40 (12) C1: 41 (9) |
I1: 82.3±9.9 C1: 82.8±10.1 |
I1: 27.8±2.3 C1: 28.0±2.6 |
Note: participants described as "Japanese males", but we cannot be sure of complete ethnic background of all participants |
| Tsuchida 2010 | I1: catechin beverage C1: control beverage |
I1: 39 C1: 41 |
I1: 48.7/51.3 C1: 43.9/56.1 |
Note: T: females 545, males 42 not divided by group: 43 males aged 30 ‐ 62 (mean age 42) 37 post‐menopausal women aged 43 ‐ 65 (mean age 55) |
I1: 70.68±1.85 C1: 70.36±2.00 |
I1: 26.43±0.37 C1: 26.06±0.31 |
‐ |
| Wang 2010 | I1: GT1 I2: GT2 I3: GT3 C1: C |
I1: 47 I2: 49 I3: 43 C1: 43 |
‐ T: 73.1/26.9 |
I1: 37 (9) I2: 37 (10) I3: 38 (9) C1: 37 (9) |
I1: 71.4 (SD 9.8) I2: 71.5 (SD 11.8) I3: 71.1 (SD 11.9) C1: 69.7 (SD 8.9) |
I1: 27.1 (SD 2.2) I2: 27.2 (SD 2.5) I3: 26.8 (SD 2.2) C1: 26.8 (SD 2.0) |
Note: participants described as "Chinese subjects", but we cannot be sure of complete ethnic background of all participants |
|
Westerterp‐ Plantenga 2005 |
I1: green tea‐caffeine capsule ‐ low habitual caffeine consumers I2: green tea‐caffeine capsule ‐ high habitual caffeine consumers C1: placebo ‐ low habitual caffeine consumers C2: placebo ‐ high habitual caffeine consumers |
I1: 19 I2: 19 C1: 19 C2: 19 |
‐ T: 69.7/30.3 |
‐ | I1: 85.1±9.5 I2: 85.0±8.5 C1: 85.1±9.6 C2: 85.0±8.3 |
I1: 29.6±2.6 I2: 29.5±2.4 C1: 29.6±2.7 C2: 29.5± 2.2 |
‐ |
|
Footnotes "‐" denotes not reported AP: adequate protein; BMI: body mass index; C: control; HP: high protein; I: intervention | |||||||
Appendix 5. Baseline characteristics (II)
|
Characteristic Study ID |
Intervention(s) and control(s) | Country | Duration of intervention | Duration of follow‐up | Co‐medications | Co‐morbidities |
| Auvichayapat 2008 | I1: green tea extract C1: placebo |
Thailand | 12 weeks | ‐ | Participants were excluded if taking prescribed medications, (e.g. antipsychotics, antidepressants, anti‐obesity medications, hormonal therapy) | Participants were excluded if they had a history of metabolic disease (e.g. diabetes mellitus, hyper‐ or hypothyroidism, Cushing's syndrome) or systemic disease, (e.g. heart, renal, liver disease) |
| Diepvens 2005 | I1: green tea C1: placebo |
The Netherlands | 84 days | ‐ | Excluded if on medications | Excluded if not healthy |
| Hill 2007 | I1: green tea extract C1: placebo |
Australia | 12 weeks | ‐ | Antihypertensive, lipid‐lowering or obesity medications not allowed | Note: participants excluded if had diabetes, liver, gastrointestinal or CV disease, or abnormal thyroid function |
| Hsu 2008 | I1: green tea extract C1: placebo |
Taiwan | 12 weeks | ‐ | Obesity medications not allowed – no other details provided | ‐ |
| Hursel 2009 | I1: green tea‐caffeine mixture + AP diet I2: green tea‐caffeine mixture + HP diet C1: placebo + AP diet C2: placebo + HP diet |
The Netherlands | 3 months | ‐ | Medication use not permitted | Participants had to be in good health, no further details were provided |
| Kajimoto 2005 | I1: catechin drink – low dose group I2: catechin drink – high dose group C1: placebo drink |
Japan | 12 weeks | 8‐week withdrawal period | Participants were excluded if taking medicines that might affect lipid metabolism | ‐ |
| Kataoka 2004 | I1: catechin beverage 278 mg I2: catechin beverage 570 mg: I3: catechin beverage 845 mg C1: control beverage |
Japan | 12 weeks | ‐ | No medications that affected weight were allowed | Participants with co‐morbidities were excluded |
| Kovacs 2004 | I1: green tea C1: placebo |
The Netherlands | 13 weeks | ‐ | Participants were excluded if taking any medications | Participants had to be in good health, no further details were provided |
| Kozuma 2005 | I1: catechin beverage C1: placebo beverage |
Japan | 12 weeks | ‐ | Medicine, specific health foods, beverages and supplements that had the possibility of influencing lipid and glucose metabolism and other energy levels were restricted | Note: participants were excluded if had serious liver, kidney, heart disease, or diabetes |
| Maki 2009 | I1: green tea beverage C1: control beverage |
USA | 12 weeks | ‐ | ‐ | ‐ |
| Nagao 2007 | I1: brewed green tea beverage with added catechins C1: brewed green tea beverage with no added catechins |
Japan | 12 weeks | ‐ | Medicines or foods known to influence lipid or carbohydrate metabolism were prohibited | Participants with serious liver or renal disease were excluded |
| Suzuki 2009 | I1: tea catechin C1: placebo |
Japan | 12 weeks | 4‐week post‐ consumption observation period | Note: use of pharmaceutical drugs or health
supplements that might affect fat metabolism
discouraged Use of over‐the‐counter drugs was not allowed |
Note: participants receiving medical treatment for obesity, hyperlipidaemia, diabetes or hypertension were excluded Participants with serious liver, kidney, heart, pulmonary, endocrine or metabolic disorder were excluded |
| Takase 2008 | I1: catechin beverage C1: placebo beverage |
Japan | 12 weeks | ‐ | Medicine, food, beverages and supplements with possibility of influencing lipid and glucose metabolism and other energy levels were restricted | Participants undergoing medical treatment, with severe kidney, liver, heart disease or diabetes, were excluded. No further information available |
| Takashima 2004 | I1: catechin beverage C1: control beverage |
Japan | 12 weeks | ‐ | Medications were not allowed | Participants with co‐morbidities were excluded |
| Takeshita 2008 | I1: catechin beverage C1: placebo beverage |
Japan | 12 weeks | ‐ | No treatment for high blood pressure, hyperlipidaemia or diabetes | Participants with severe liver, kidney, heart disease, or diabetes were excluded |
| Tsuchida 2010 | I1: catechin beverage C1: control beverage |
Japan | 12 weeks | 12 weeks | Food products and medications that might affect energy absorption or lipid metabolism (e.g. dietary fibre supplements, psyllium) were restricted | ‐ |
| Wang 2010 | I1: GT1 I2: GT2 I3: GT3 C1: control beverage |
China | 90 days | ‐ | No medications allowed except oral contraceptives and over‐the‐counter medications | Participants suffering from any medical or psychiatric condition were excluded |
|
Westerterp‐ Plantenga 2005 |
I1: green tea‐caffeine capsule ‐ low habitual caffeine consumers I2: green tea‐caffeine capsule ‐ high habitual caffeine consumers C1: placebo ‐ low habitual caffeine consumers C2: placebo ‐ high habitual caffeine consumers |
The Netherlands | 3 months | ‐ | Medications not allowed | ‐ |
|
Footnotes "‐" denotes not reported AP: adequate protein; C: control; GT: green tea; HP: high protein; I: intervention. | ||||||
Appendix 6. Adverse events
|
Characteristic Study ID |
Intervention(s) and control(s) | Deaths [N] | All adverse events [N / %] | Serious adverse events [N / %] | Left study due to adverse events [N / %] | Hospitalisation [N / %] | Outpatient treatment [N / %] | Symptoms [N / %] |
| Auvichayapat 2008 | I1: green tea extract C1: placebo |
I1: 0 C1: 0 T: 0 |
‐ | I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
‐ | ‐ | ‐ |
| Diepvens 2005 | I1: green tea C1: placebo |
I1: 0 C1: 0 T: 0 |
‐ | ‐ | I1: 0 C1: 0 T: 0 |
‐ | ‐ | ‐ |
| Hill 2007 | I1: green tea extract C1: placebo |
‐ | I1: ‐ (Liver function measurements indicated no adverse effects of treatment, however this info is incomplete) C1: ‐ |
‐ | ‐ | ‐ | ‐ | ‐ |
| Hsu 2008 | I1: green tea extract C1: placebo |
I1: 0 C1: 0 T: 0 |
I1: 5 C1: 3 T: 8 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
I1: 1a C1: 0 T: 1 |
‐ | I1: 4 C1: 1 T: 5 |
| Hursel 2009 | I1: green tea‐caffeine mixture + AP diet I2: green tea‐caffeine mixture + HP diet C1: placebo + AP diet C2: placebo + HP diet |
I1: 0 I2: 0 C1: 0 C2: 0 T: 0 |
I1: 0 I2: 0 C1: 0 C2: 0 T: 0 |
I1: 0 I2: 0 C1: 0 C2: 0 T: 0 |
I1: 0 I2: 0 C1: 0 C2: 0 T: 0 |
‐ | ‐ | ‐ |
| Kajimoto 2005 | I1: catechin drink – low dose group I2: catechin drink – high dose group C1: placebo drink |
‐ | I1: 17 I2: 11 C1: 17 T: 45 |
‐ | ‐ | ‐ | ‐ | ‐ |
| Kataoka 2004 | I1: catechin beverage 278 mg I2: catechin beverage 570 mg: I3: catechin beverage 845 mg C1: control beverage |
I1: 0 I2: 0 I3: 0 C1: 0 T: 0 |
I1: 0 I2: 0 I3: 0 C1: 0 T: 0 |
I1: 0 I2: 0 I3: 0 C1: 0 T: 0 |
I1: 0 I2: 0 I3: 0 C1: 0 T: 0 |
‐ | ‐ | ‐ |
| Kovacs 2004 | I1: green tea C1: placebo |
I1: 0 C1: 0 T: 0 |
‐ | ‐ | I1: 0 C1: 0 T: 0 |
‐ | ‐ | ‐ |
| Kozuma 2005 | I1: catechin beverage C1: placebo beverage |
‐ | I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
‐ | ‐ | ‐ | |
| Maki 2009 | I1: green tea beverage C1: control beverage |
‐ | I1: 67% C1: 71% T: ‐ |
I1: 2 / 3% (tooth disorder, and
elevated blood pressure) C1: 1 / 1.6% (tachycardia) T: 3 of 128 / 2.3% |
I1: 1 / 1.5% C1: 0 T: 1/128 (0.8%) |
I1: 1 / 1.5% C1: 0 T: 1 / 0.8% |
‐ | ‐ |
| Nagao 2007 | I1: brewed green tea beverage with added catechins C1: brewed green tea beverage with no added catechins |
‐ | I1: 0 C1: ‐ T: ‐ |
I1: 0 C1: ‐ T: ‐ |
I1: 0 C1: ‐ T: ‐ |
‐ | ‐ | ‐ |
| Suzuki 2009 | I1: tea catechin C1: placebo |
‐ | I1: 9 C1: 1 T: 10 |
‐ | ‐ | ‐ | ‐ | ‐ |
| Takase 2008 | I1: catechin beverage C1: placebo beverage |
‐ | I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
‐ | ‐ | ‐ |
| Takashima 2004 | I1: catechin beverage C1: control beverage |
I1: 0 C1: 0 T: 0 |
‐ | ‐ | I1: 0 C1: 0 T: 0 |
‐ | ‐ | ‐ |
| Takeshita 2008 | I1: catechin beverage C1: placebo beverage |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
‐ | ‐ | ‐ |
| Tsuchida 2010 | I1: catechin beverage C1: control beverage |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
I1: 0 C1: 0 T: 0 |
‐ | ‐ | ‐ |
| Wang 2010 | I1: GT1 I2: GT2 I3: GT3 C1: C |
‐ | I1: 0 I2: 0 I3: 0 C1: 0 T: 0 |
I1: 0 I2: 0 I3: 0 C1: 0 T: 0 |
I1: 0 I2: 0 I3: 0 C1: 0 T: 0 |
‐ | ‐ | ‐ |
|
Westerterp‐ Plantenga 2005 |
I1: green tea‐caffeine capsule ‐ low habitual caffeine consumers I2: green tea‐caffeine capsule ‐ high habitual caffeine consumers C1: placebo ‐ low habitual caffeine consumers C2: placebo ‐ high habitual caffeine consumers |
I1: 0 I2: 0 C1: 0 C2: 0 T: 0 |
I1: 0 I2: 0 C1: 0 C2: 0 T: 0 |
I1: 0 I2: 0 C1: 0 C2: 0 T: 0 |
I1: 0 I2: 0 C1: 0 C2: 0 T: 0 |
‐ | ‐ | ‐ |
|
Footnotes "‐" denotes not reported aOne participant was hospitalised but it is not clear if this was related to treatment and did not complete study AP: adequate protein; C: control; GT: green tea; HP: high protein; I: intervention; T: total | ||||||||
Appendix 7. Adapted Dalhousie assessment instrument for the critical appraisal of the content of natural products in randomised controlled trials (I)
Adapted Dalhousie assessment instrument for the critical appraisal of the content of natural products used in randomised controlled trials (Jurgens 2009).
| Evaluation statementa | |||||||||
| Genus and species | Part of the plant, used | How the plant was processed / extracted | Brand name, if preparation was a commercial product | Name of manufacturer, if preparation was a commercial product | Lot number(s), if preparation was a commercial product | Name of active or marker chemical(s) | Amount or percentage of active or marker chemical(s) | Preparation was analysed for chemical content | |
| Auvichayapat 2008 | N | Y | N | Y | Y | N | Y | Y | Y |
| Diepvens 2005 | Y | Y | N | Y | Y | N | Y | Y | N |
| Hill 2007 | N | N | N | Y | Y | N | Y | Y | N |
| Hsu 2008 | N | Y | P | U | U | U | Y | Y | Y |
| Hursel 2009 | N | N | N | U | U | U | Y | Y | N |
| Kajimoto 2005 | N | N | N | Y | Y | N | Y | Y | Y |
| Kataoka 2004 | Y | Y | Y | U | U | U | Y | Y | Y |
| Kovacs 2004 | N | N | N | U | U | U | Y | Y | Y |
| Kozuma 2005 | Y | Y | Y | U | U | U | Y | Y | Y |
| Maki 2009 | N | N | N | U | U | U | Y | Y | N |
| Nagao 2007 | N | Y | Y | U | U | U | Y | Y | N |
| Suzuki 2009 | N | N | N | U | U | U | Y | Y | Y |
| Takase 2008 | N | N | N | U | U | U | Y | Y | N |
| Takashima 2004 | Y | Y | Y | U | U | U | Y | Y | Y |
| Takeshita 2008 | Y | N | Y | U | U | U | Y | Y | Y |
| Tsuchida 2010 | Y | Y | Y | U | U | U | Y | Y | N |
| Wang 2010 | Y | Y | Y | U | U | U | Y | Y | Y |
|
Westerterp‐ Plantenga 2005 |
N | N | N | U | U | U | Y | Y | N |
|
Footnotes aCheck of the level of detail provided for each of the statements about the green tea preparation used in the study N: no; P: partially; U: unable to determine / not applicable; Y: yes |
|||||||||
Appendix 8. Adapted Dalhousie assessment instrument for the critical appraisal of the content of natural products in randomised controlled trials (II)
Adapted Dalhousie assessment instrument for the critical appraisal of the content of natural products used in randomised controlled trials (Jurgens 2009).
| Evaluation statementa | |||||||
|
|
Dosage form | Dose | Frequency of administration | Route of administration |
The natural product (NP)
under study was compared to: (A) placebo and / or (B) accepted treatment |
The content of the placebo or comparison treatment was stated |
The placebo/ comparison treatment
and NP under study were matched
in terms of:
(A) taste, smell and / or appearance (B) dosing regimen |
| Auvichayapat 2008 | Y | Y | Y | Y | A | Y | A: Y B: Y |
| Diepvens 2005 | Y | Y | Y | Y | A | Y | A: P B: Y |
| Hill 2007 | Y | Y | Y | Y | A | Y | A: P B: Y |
| Hsu 2008 | Y | Y | Y | Y | A | Y | A: P B: Y |
| Hursel 2009 | Y | Y | Y | Y | A | Y | A: U B: Y |
| Kajimoto 2005 | Y | Y | Y | Y | A | Y | A: P B: Y |
| Kataoka 2004 | Y | Y | Y | Y | A | Y | A: P B: Y |
| Kovacs 2004 | Y | Y | Y | Y | A | N | A: Y B: Y |
| Kozuma 2005 | Y | Y | Y | Y | A | Y | A: Y B: Y |
| Maki 2009 | Y | Y | Y | Y | A | Y | A: Y B: Y |
| Nagao 2007 | Y | Y | Y | Y | A | Y | A: P B: Y |
| Suzuki 2009 | Y | Y | Y | Y | A | Y | A: Y B: Y |
| Takase 2008 | Y | Y | Y | Y | A | P | A: Y B: Y |
| Takashima 2004 | Y | Y | Y | Y | A | Y | A: P B: Y |
| Takeshita 2008 | Y | Y | Y | Y | A | Y | A: Y B: Y |
| Tsuchida 2010 | Y | Y | Y | Y | A | Y | A: P B: Y |
| Wang 2010 | Y | Y | Y | Y | A | Y | A: Y B: Y |
|
Westerterp‐ Plantenga 2005 |
Y | Y | Y | Y | A | Y | A: P B: Y |
|
Footnotes aCheck of the level of detail provided for each of the statements about the green tea preparation used in the study. N: no; P: partially; Y: yes. | |||||||
Appendix 9. Sensitivity analysis 1 (the effect of including imputed data)
| All Studies | Studies with no imputed means (SD) | |||||
| Outcome | No. of studies | No. of participants | Effect size c | No. of studies | No. of participants | Effect size c |
| 1.1 Weight loss (WL) a | 14 | 1562 | ‐0.95 (95% CI ‐1.75 to ‐0.15) | 9 | 959 | ‐1.07 (95% CI ‐2.10 to ‐0.05) |
| 1.2 Weight loss (WM) b | 2 | 184 | ‐0.52 (95% CI ‐2.62 to 1.59) | 0 | 0 | Not measured ‐ all studies required imputation |
| 1.3 BMI (WL) a | 12 | 1252 | ‐0.47 (95% CI ‐0.78 to ‐0.16) | 8 | 913 | ‐0.52 (95% CI ‐0.91 to ‐0.13) |
| 1.4 BMI (WM) b | 2 | 184 | ‐0.14 (95% CI ‐0.78 to 0.50) | 0 | 0 | Not measured ‐ all studies required imputation |
|
1.5 Waist circumference (WL) a |
13 | 1434 | ‐0.99 (95% CI ‐1.75 to ‐0.22) | 8 | 913 | ‐1.25 (95% CI ‐2.24 to ‐0.26) |
|
1.6 Waist circumference (WM) 2 |
2 | 184 | ‐0.75 (95% CI ‐2.66 to 1.15) | 0 | 0 | Not measured ‐ all studies required imputation |
| 1.7 Waist‐to‐hip ratio (WL) a | 4 | 339 | ‐0.00 (95% CI ‐ 0.01 to 0.01) | 1 | 36 | 0.01 (95% CI 0.01 to 0.01) |
|
Footnotes aWL: weight loss bWM: weight maintenance cStatistical method: mean difference (IV, Random, 95% CI) | ||||||
Appendix 10. Sensitivity analysis 2 (the effect of risk of bias)
| Outcome | Effect size d (all studies) | Effect size d (all studies except Tsuchida 2010) | Effect size d Studies with low risk of bias (randomisation (allocation sequence generation)) | Effect size d Studies with low risk of bias (allocation concealment) | Effect size d Studies with low risk of bias (blinding) | Effect size d Studies with low risk of bias (combined randomisation, allocation concealment, blinding) |
| 1.1 Weight loss (WL) a kg | ‐0.97 (95% CI ‐1.73 to ‐0.21) | ‐0.95 (95% CI ‐1.75 to ‐0.15) | ‐0.1 (95% CI (‐0.99 to ‐0.75)e | ‐0.20 (95% CI ‐0.84 to 0.44)f | ‐0.56 (95% CI ‐1.59 to 0.48)g | ‐0.41 (95% CI ‐0.98 to 0.17)h |
| 1.2 Weight loss (WM) b kg | ‐0.52 (95% CI ‐2.62 to 1.59) | n/a3 | No studies with low risk of bias | No studies with low risk of bias | No studies with low risk of bias | No studies with low risk of bias |
| 1.3 BMI (WL) a kg/m2 | ‐0.47 (95% CI ‐0.77 to ‐0.18) |
‐0.47 (95% CI ‐0.78 to ‐0.16) | ‐0.05 (95% CI ‐0.85 to 0.84)e | ‐0.52 (95% CI ‐0.86 to ‐0.18)f | ‐0.05 (95% CI ‐0.85 to 0.84)f | ‐0.52 (95% CI‐0.86 to ‐0.18)f |
| 1.4 BMI (WM) b kg/m2 | ‐0.14 (95% CI ‐0.78 to 0.50) | n/a3 | No studies with low risk of bias | No studies with low risk of bias | No studies with low risk of bias | No studies with low risk of bias |
|
1.5 Waist circumference (WL) a cm |
‐0.97 (95% CI ‐1.70 to ‐0.23) |
‐0.99 (95% CI ‐1.75 to ‐0.22) | ‐0.40 (95% CI ‐2.65 to 1.85)e | 0.48 (95% CI ‐0.90 to 1.85)f | ‐0.40 (95% CI ‐2.65 to 1.85)f | 0.48 (95% CI ‐0.90 to 1.85)f |
|
1.6 Waist circumference (WM) b cm |
‐0.75 (95% CI ‐2.66 to 1.15) | n/ac | No studies with low risk of bias | No studies with low risk of bias | No studies with low risk of bias | No studies with low risk of bias |
| 1.7 Waist‐to‐hip ratio (WL) a | 0.00 (95% CI ‐0.01 to 0.01) | 0.00 (95% CI ‐0.02 to 0.01) |
No studies with low risk of bias | No studies with low risk of bias | No studies with low risk of bias | No studies with low risk of bias |
|
Footnotes aW: weight loss bWM: weight maintenance cn/:not applicable dStatistical method: mean difference (IV, Random, 95% CI) eHsu 2008 fHsu 2008; Suzuki 2009 gHsu 2008; Maki 2009 hHsu 2008; Maki 2009; Suzuki 2009 | ||||||
Appendix 11. Definition of end point measurement
|
Characteristic Study ID |
Weight | BMI | Waist circumference | Waist‐to‐hip ratio | Health‐related quality of life | Compliance / adherence | Patient satisfaction | Morbidity |
| Auvichayapat 2008 | Measured before breakfast and after voiding while wearing a hospital gown and using a digital scale | "Calculated as weight (kgs) divided by height (m2)". Height was measured using a "wall mounted ruler" | Measured "1 inch above umbilicus, in standing position" using a standardised tape by the same well‐trained staff. The tape that was calibrated before use | "Calculated by dividing the waist circumference by hip circumference". Hip circumference measured at the site of the largest circumference between waist and thighs | ‐ | No description of how measurement was made | ‐ | ‐ |
| Diepvens 2005 | "Measured using a digital balance accurate to 0.02 kg with subjects in underwear after voiding their bladder" | Does not say how BMI was calculated. Height "measured to the nearest 0.1 cm using a wall‐mounted stadiometer" | Measured with participant in the standing position "at the site of the smallest circumference between the rib cage and the iliac crest" | "Calculated by dividing the waist circumference by the hip circumference." Hip measured at "the site of the largest circumference between the waist and the thighs". Performed with participant in standing position |
"Attitude towards eating was analyzed using a validated Dutch translation of the Three Factor Eating Questionnaire", which asked about mood, tolerance and hunger profiles | ‐ | ‐ | ‐ |
| Hill 2007 | "Measured to nearest 200 grams using an electronic scale with subjects wearing minimal clothing and without shoes" | Does not say how BMI was calculated. "Height was measured to nearest 0.1 cm using a wall‐mounted telescopic stadiometer with subjects in stockinged or bare feet" | "Taken using a plastic fiber tape measure with subjects standing with arms relaxed by their side and balanced on both feet. The tape was held tight to the skin but without compression of tissue". "Measured just above the iliac crest as recommended in the National Institutes of Health Guidelines" | Does not say how waist‐to‐hip ratio was calculated. Hip circumference was measured according to International Society for the Advancement of Kinanthropometry protocol, taken at the greatest posterior protuberance of the buttocks |
‐ | ‐ | ‐ | ‐ |
| Hsu 2008 | "Measurements were done after an overnight fast using standardized methods" "Subjects were measured in their undergarments with hospital gown on" "using a calibrated balance beam scale to the nearest 0.1kg" | Calculated according to the formula: BMI = body weight/height (kg/m2). Height "was measured with a wall‐mounted stadiometer to the nearest 0.1 cm" | "Measured midway between the lateral lower rib margin and the iliac crest" | ‐ | ‐ | ‐ | ‐ | ‐ |
| Hursel 2009 | "Measured with a digital balance with subjects in underwear, in a fasted state and after emptying their bladder" | "BMI was calculated". "Height was measured using a wall‐mounted stadiometer" |
Measured "at the site of the smallest circumference between the rib cage and the iliac crest" with the participants in the standing position | ‐ | ‐ | ‐ | ‐ | ‐ |
| Kajimoto 2005 | "Was measured without shoes or heavy outer clothing and recorded to the nearest 0.1kg using a scale" | Does not say how BMI was calculated. "Height was measured without shoes to the nearest 0.1cm" | "Was measured as the smallest location of the midsection to nearest 0.1 cm" in standing position" | Does not say how waist‐to‐hip ratio was calculated. "Hip circumference was measured at the location of the greatest gluteal mass to nearest 0.1 cm" in standing position | ‐ | ‐ | ‐ | ‐ |
| Kataoka 2004 | No description other than "measured in standing position" | "Was calculated from height and body weight data" | No description other than "measured in standing position" | ‐ | ‐ | ‐ | ‐ | ‐ |
| Kovacs 2004 | "Measured on a digital balance with subjects in underwear, in fasted state after voiding their bladder" | "BMI was calculated as body weight/height2 (kg/m2)". "Height was measured using a wall‐mounted stadiometer" | "Measured at the site of the smallest circumference between the rib cage and ileac crest with the subjects in a standing position" | "Waist:hip ratio calculated by dividing the waist circumference by the hip circumference". "Hip circumference measured at the site of the largest circumference between the waist and the thighs" | ‐ | ‐ | ‐ | ‐ |
| Kozuma 2005 | No description of how measurement was made | Based on height and weight | Measured in the abdominal area | ‐ | ‐ | Measured by participants self‐reporting compliance in a journal | ‐ | ‐ |
| Maki 2009 | No description of how measurement was made | ‐ | "Measured on a horizontal plane at the level of the iliac crest using a nonstretch anthropometric tape at the end of a normal expiration" (but data were not provided) | ‐ | ‐ | Measured based on interview of participants and on counting unused servings | ‐ | ‐ |
| Nagao 2007 | No description of how measurement was made | "BMI calculated from height and body weight" | "Circumference at the umbilical level was measured as the waist circumference" | ‐ | ‐ | Measured by participants self‐reporting compliance in a journal | ‐ | ‐ |
| Suzuki 2009 | No description of how measurement was made | Height measurement from preparatory survey was used to calculate BMI | No description of how measurement was made | ‐ | ‐ | No description of how measurement was made | ‐ | ‐ |
| Takase 2008 | No description of how measurement was made | BMI calculated from height and weight | Measured at umbilicus | ‐ | ‐ | No description of how measurement was made | ‐ | ‐ |
| Takashima 2004 | Measured at the standing position | Was calculated from height (measured at the standing position) and body weight | Measured at the standing position | ‐ | ‐ | ‐ | ‐ | ‐ |
| Takeshita 2008 | No description of how measurement was made | Calculated from height and weight | Circumference of umbilical region | ‐ | ‐ | Test participants recorded their test beverage intake on a daily basis | ‐ | ‐ |
| Tsuchida 2002 | No description of how measurement was made | Determined from height and body weight | No description of how measurement was made | "Determined from waist and hip circumferences", no description of how hip measurement was made | ‐ | ‐ | ‐ | ‐ |
| Wang 2009 | No description of how measurement was made | ‐ | No description of how measurement was made | ‐ | ‐ | Investigator distributed each dose to measure compliance | ‐ | ‐ |
| Westerterp‐Plantenga 2005 | "Measured with a digital balance, weighing accuracy of 0.1 kg with subjects in underwear in a fasted state after voiding their bladder" | "BMI calculated as weight (kgs) divided by height (meters) squared". "Height was measured with a wall mounted stadiometer" | Measured "at the site of the smallest circumference between the rib cage and the ileac crest with subjects in a standing position" | ‐ | ‐ | ‐ | ‐ | ‐ |
|
Footnotes "‐" denotes not reported BMI: body mass index | ||||||||
Data and analyses
Comparison 1. Primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight loss ‐ (non) caffeine matched interventions | 14 | 1562 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.75, ‐0.15] |
| 1.1 Caffeine matched in intervention and control | 11 | 1274 | Mean Difference (IV, Random, 95% CI) | ‐1.11 [‐1.99, ‐0.24] |
| 1.2 Caffeine in intervention only | 3 | 288 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.80, 0.60] |
| 2 Weight loss ‐ studies conducted in/outside Japan | 14 | 1562 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.75, ‐0.15] |
| 2.1 Studies conducted in Japan | 8 | 1030 | Mean Difference (IV, Random, 95% CI) | ‐1.44 [‐2.38, ‐0.51] |
| 2.2 Studies conducted outside Japan | 6 | 532 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.50, 0.43] |
| 3 Weight maintenance | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐2.62, 1.59] |
| 4 Weight loss ‐ BMI | 12 | 1252 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.77, ‐0.17] |
| 4.1 Studies conducted in Japan | 8 | 1030 | Mean Difference (IV, Random, 95% CI) | ‐0.55 [‐0.89, ‐0.20] |
| 4.2 Studies conducted outside Japan | 4 | 222 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.48, 0.11] |
| 5 Weight maintenance ‐ BMI | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.78, 0.50] |
| 6 Weight loss ‐ waist circumference | 13 | 1434 | Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐1.76, ‐0.22] |
| 6.1 Studies conducted in Japan | 8 | 1030 | Mean Difference (IV, Random, 95% CI) | ‐1.38 [‐2.30, ‐0.46] |
| 6.2 Studies conducted outside Japan | 5 | 404 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐1.39, 0.94] |
| 7 Weight maintenance ‐ waist circumference | 2 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐2.66, 1.15] |
| 8 Weight loss ‐ waist‐to‐hip ratio | 4 | 339 | Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.02, 0.01] |
| 8.1 Studies conducted in Japan | 1 | 195 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.02, ‐0.00] |
| 8.2 Studies conducted outside Japan | 3 | 144 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.02, 0.02] |
1.3. Analysis.
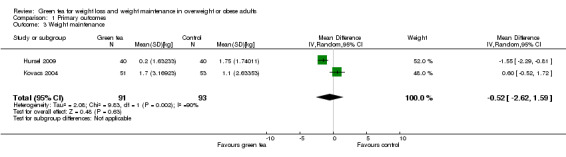
Comparison 1 Primary outcomes, Outcome 3 Weight maintenance.
Comparison 2. Total daily dose of catechins.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight loss (sorted highest to lowest total daily dose of catechins) | 14 | 1562 | Mean Difference (IV, Random, 95% CI) | ‐0.95 [‐1.76, ‐0.14] |
| 2 Weight loss (sorted highest to lowest based on total daily dose of catechins) ‐ BMI | 12 | 1252 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.77, ‐0.17] |
| 3 Weight loss ‐ waist circumference | 13 | 1434 | Mean Difference (IV, Random, 95% CI) | ‐0.99 [‐1.76, ‐0.22] |
2.3. Analysis.
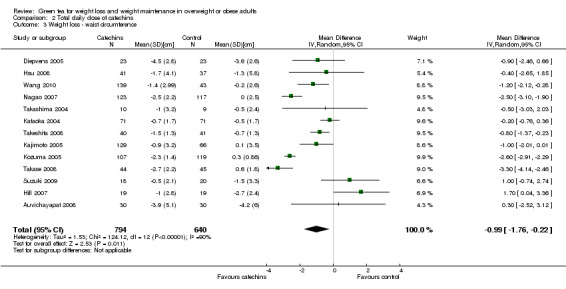
Comparison 2 Total daily dose of catechins, Outcome 3 Weight loss ‐ waist circumference.
Comparison 3. Total daily dose of epigallocatechin‐3‐gallate (EGCG).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Weight loss (sorted by highest to lowest daily dose of EGCG) | 13 | 1380 | Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐1.86, ‐0.18] |
| 2 Weight loss (sorted highest to lowest total daily dose of EGCG) ‐ BMI | 12 | 1252 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.77, ‐0.17] |
| 3 Weight loss (sorted by highest to lowest total daily dose of EGCG) ‐ waist circumference | 12 | 1252 | Mean Difference (IV, Random, 95% CI) | ‐0.96 [‐1.79, ‐0.13] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Auvichayapat 2008.
| Methods | This was a randomised, placebo‐controlled clinical trial with a treatment to control randomisation ratio of 1:1 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA: a history of:
DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: NR COUNTRY/ LOCATION: Thailand SETTING: NR TREATMENT BEFORE STUDY: unknown |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in English in Physiology & Behavior, a peer‐reviewed journal, and was supported by the Invitation Research Fund of the Faculty of Medicine of Khon Kaen University, Thailand | |
| Stated aim of study | Quote from study: "We therefore studied whether green tea reduces body weight in obese Thais" | |
| Notes | BMI: body mass index; NR: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: "the subjects were equally randomised into two groups, the green tea group and the placebo group" Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from study: "all subjects were blind to the aim of the study" "the subjects in the placebo group received cellulose capsules, which were indistinguishable from the green tea capsules" Comment: no mention of effectiveness of blinding efforts. No mention of whether researchers and staff were blinded to study components |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: investigators specified enrolment details and explained that there was no attrition of participants |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes specified at the beginning were adequately investigated and reported |
| Other bias | Low risk | Comment: appears to be free of other bias |
Diepvens 2005.
| Methods | This study was a randomised, double‐blind, placebo‐controlled, parallel trial with a randomisation ratio of 1:1 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA: NR DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: NR COUNTRY/ LOCATION: the Netherlands SETTING: NR TREATMENT BEFORE STUDY: 3 days of energy balanced diet before rest of study |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in English in The British Journal of Nutrition, a peer‐reviewed journal, and was commercially supported by Unilever food and Health Research Institute, Unilever R&D Vlaardingen, Vlaardingen, the Netherlands, with Slim‐Fast diet products donated by Unilever Bestfoods Nederland BV | |
| Stated aim of study | Quote from study: "the aim of the present study was therefore to investigate whether GT [green tea] ingestion, independent of habitual caffeine intake, increased REE [resting energy expenditure] and substrate oxidation, whether this effect was present after a 4‐week administration of GT along with a LED (meal replacement diet plan) and whether GT ingestion during the LED offset the expected reduction in REE. Furthermore, we investigated the effect of a 12‐week GT administration during the LED on body weight and fat loss. We hypothesised that GT might increase REE and fat oxidation compared with PLAC [placebo], and that this effect might be present after a 4‐week ingestion of GT along with a LED (meal replacement diet plan). We further hypothesised that GT might offset the reduction in REE that is expected to occur during the LED and that GT might stimulate the loss of body weight and fat" | |
| Notes | BMI: body mass index; NR: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: "the two groups were randomly assigned to the two treatments" Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from study: "a double‐blind, placebo‐controlled, parallel design was adopted" "In addition, the subjects ingested three capsules (hard gelatine, size no. 1) of PLAC (maltodextrin) or GT three times daily..." Comment: effort appears to have been made to make capsules uniform in appearance. No mention of effectiveness of blinding efforts. No mention of whether researchers and staff were blinded to study components |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: investigators specified enrolment details and there does not appear to have been any attrition of participants |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes specified at the beginning were adequately investigated and reported |
| Other bias | Low risk | Comment: study funded by Unilever, maker of meal replacement drink product. One author was affiliated with Unilever Food and Health Research Institute. Source of capsules with green tea or placebo is unclear. Results were not positive so we judged that there was a low risk of bias |
Hill 2007.
| Methods | This study was a randomised placebo controlled clinical trial with an unknown randomisation ratio | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA:
DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: NR COUNTRY/ LOCATION: Australia SETTING: NR TREATMENT BEFORE STUDY: unknown |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in English in The Journal of the American College of Nutrition (JACN), a peer‐reviewed journal, and was commercially supported by a collaborative agreement with DSM Nutritional Products; 1 author is an employee of DSM Nutritional Products | |
| Stated aim of study | Quote from study: "therefore, the aim of this study was to investigate the metabolic effects of a combination of EGCG and regular aerobic exercise in overweight/obese post‐menopausal women." | |
| Notes | BMI: body mass index; EGCG: epigallocatechin‐3‐gallate; CV: cardiovascular; NR: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: "the groups were then randomly assigned to either of the two treatments" Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote from study: "subjects were allocated to one of two groups which were balanced by age, BMI and fasting blood glucose" Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no mention of blinding efforts. No description of appearance of capsule product compared to placebo found |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: authors adequately accounted for attrition |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measurements stated in methods section were reported in the results |
| Other bias | Low risk | Comment: study funded by DSM Nutritional products, maker of test product. However, results were not positive so we judged that there was a low risk of bias |
Hsu 2008.
| Methods | This was a randomised, double‐blind, placebo‐controlled clinical trial with a randomisation ratio of 1:1 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA:
DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: 1 COUNTRY/ LOCATION: Taiwan/Taipei SETTING: hospital (outpatient clinic) TREATMENT BEFORE STUDY: note: in the lead‐in period of 2 weeks, the patients should maintain weight and weight control within 0.5% |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): per cent reduction of BMI and body weight SECONDARY OUTCOME(S): per cent reduction of: glucose, cholesterol, LDL, HDL and triglycerides, leptin, adiponectin and ghrelin comparisons and analysis OTHER OUTCOME(S): NR |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in English in Clinical Nutrition, a peer‐reviewed journal, and was supported by a grant from the National Science Council, Taiwan | |
| Stated aim of study | Quote from study: "thus, we conducted this randomised clinical trial to examine the effect of GTE [green tea extract] on obese women and to explore the relationship between GTE and obesity‐related hormone peptides." | |
| Notes | Our inclusion criteria state that we will only look at studies with participants over the age of 18. In this case, we contacted the author and learned that though the researchers would have accepted participants below the age of 18, no such participants were included in this study. BMI: body mass index; HDL: high‐density lipoprotein; LDL: low‐density lipoprotein; NR: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote from study: "a random number between 0.0 and 0.99 was generated by the computer for each subject. Subjects with a random number between 0.0 and 0.49 were assigned to the group with the GTE [green tea extract] while those with a random number between 0.50 and 0.99 were assigned to the placebo group with cellulose" Comment: sequence generation was appropriate |
| Allocation concealment (selection bias) | Low risk | Comment: numbers were computer generated as each patient was enrolled therefore the group they would be allocated to was concealed and depended upon the computer to generate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from study: "a randomised, double‐blind, placebo‐controlled clinical trial was conducted" "The same opaque capsules containing either dried powder GTE or placebo (cellulose) were administered to the subjects by a research assistant blinded to the contents in the capsules. All subjects were treated in the same fashion" Comment: no mention of effectiveness of blinding efforts; however, care was clearly taken to blind both staff and participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: study authors gave reasons that most but not all participants withdrew from the study. The number who did not complete the study was higher than for other studies: 9/50 in the intervention and 13/50 in the controlled group. It is unclear the effect that those who did not give reasons for withdrawal and those who withdrew because they did not see an effect may have had on results |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes specified at the beginning were adequately investigated and reported |
| Other bias | Low risk | Comment: appears to be free of other bias |
Hursel 2009.
| Methods | The was a randomised, placebo‐controlled, double‐blind parallel trial with a treatment to control randomisation ratio of 1:1 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA: NR DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: NR COUNTRY/ LOCATION: the Netherlands SETTING: NR TREATMENT BEFORE STUDY: participants were required to enter 4 weeks of weight loss before study for weight maintenance began |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in English in The American Journal of Clinical Nutrition, a peer‐reviewed journal, and was supported by NUTRIM, Maastricht University, Maastricht, Netherlands. | |
| Stated aim of study | Quote from study: "the following study addresses this research question, with the aim to investigate whether a green tea–caffeine mixture added to an HP [high protein] diet may improve weight maintenance by preventing or limiting weight regain after weight loss of 5–10% in moderately obese subjects with a low habitual caffeine intake" | |
| Notes | BMI: body mass index; NR: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: "subjects were randomly assigned to 4 groups" Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from study: "a randomised, placebo‐controlled, double‐blind parallel trial was conducted" "A double‐blind administration of the supplementation (green tea‐caffeine mixture or placebo) was performed" Comment: no description of capsule product compared to placebo found. No mention of effectiveness of blinding efforts |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: authors adequately accounted for attrition |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measurements stated in methods section were reported in the results |
| Other bias | Low risk | Comment: appears to be free of other bias |
Kajimoto 2005.
| Methods | This study was a double‐blind, placebo‐controlled randomised trial with a 3‐arm parallel design, with a low‐dose treatment to high‐dose treatment to control randomisation ratio of 1:1.015:0.97 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA:
DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: NR COUNTRY/ LOCATION: Japan SETTING: NR TREATMENT BEFORE STUDY: 2‐week run‐in period |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in English in the Journal of Health Science, a peer‐reviewed journal, and no sources of support were declared by the authors | |
| Stated aim of study | Quote from study: "in the present study, we investigated the effectiveness of 12 week consumption of a tea catechin‐containing drink with a little caffeine on body fat reduction and evaluated its safety in healthy adult men and women showing 22.5<body mass index (BMI)≤30kg/m2" | |
| Notes | We contacted the authors to ask if this trial was randomised, and received an affirmative reply BMI: body mass index; NR: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: "the subjects were divided into 3 groups based on the results of the preliminary screening so that the three groups were uniform in background, including age, BMI and waist/hip ratio." Comment: contact with authors confirmed that the study was randomised; however, the method of randomisation was not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from study: "we conducted a double‐blind study with three parallel arms" "Prior to initiation of the study, it was confirmed that the catechin drink could not be distinguished from the placebo drink by flavour, taste or packaging" Comment: no mention of effectiveness of blinding efforts. No mention of whether researchers and staff were blinded to study components |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: authors adequately accounted for attrition |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measurements stated in methods section were reported in the results |
| Other bias | Unclear risk | Comment: study drink produced by ITOEN Ltd, and 1 of the authors was employed by ITOEN Ltd.; nature of corporate support for study was unclear. Results were positive so we judged that there was an unclear risk of bias |
Kataoka 2004.
| Methods | The study was a randomised, placebo‐controlled trial with a low‐, medium‐ and high‐treatment group to control group randomisation ratio of 1:2.84:1:2.84 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA:
DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: NR COUNTRY/ LOCATION: Japan SETTING: NR TREATMENT BEFORE STUDY: note: 4‐week run‐in period |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in English in the journal Progress in Medicine and no sources of funding were reported; the corresponding author is affiliated with Health Care Products Research Laboratories No.1 Kao Corporation and no other affiliations were stated | |
| Stated aim of study | Quote from study: "in the present study, the effects of physical activity and exercise habits on reducing body fat by the long term intake of catechins was examined" | |
| Notes | BMI: body mass index; NR: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: participants were "randomly divided into four groups by a controller with similar age, anthropometric parameters (body weight, BMI, waist circumference, hip circumference and abdominal fat area measured by computer tomography" Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: both the test beverage and control beverage were formulated as sports drinks but no further detail was provided regarding their similarity or differences. No mention of whether researchers and staff were blinded to study components |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: investigators specified enrolment details and there was apparently no attrition of participants |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measurements stated in methods section were reported in the results |
| Other bias | Low risk | Comment: appears to be free of other bias |
Kovacs 2004.
| Methods | This study was a randomised, parallel, double‐blind, placebo‐controlled trial with a randomisation ratio of 1:1.04 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA: NR DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: 1 COUNTRY/ LOCATION: the Netherlands SETTING: university TREATMENT BEFORE STUDY: 4 weeks very‐low‐energy diet intervention |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in English in The British Journal of Nutrition, a peer‐reviewed journal, and no sources of support were declared by the authors | |
| Stated aim of study | Quote from study: "the aim of the present study was to investigate whether green tea may improve weight maintenance by preventing or limiting weight regain after weight loss of 5 to 10 % in overweight and moderately obese subjects" | |
| Notes | BMI: body mass index; NR: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: "subjects were randomised to an intensive (n=80) or an extensive group (n=40). The intensive group underwent all the measurements. The extensive group underwent the same measurements as the intensive group, except for resting energy expenditure (REE), substrate oxidation and physical activity" "for assessment during the weight‐maintenance period of 13 weeks, the subjects, stratified for sex, BMI, age, cognitive restraint and REE, were divided into two groups" Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from study: "the administration of the supplementation was double‐blind. The green tea capsules and the placebo capsules were indistinguishable" Comment: no mention of effectiveness of blinding efforts. No mention of whether researchers and staff were blinded to study components |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: authors adequately accounted for attrition |
| Selective reporting (reporting bias) | Unclear risk | Comment: authors noted that adverse events during treatment were recorded with a specific questionnaire tool, but do not include this information elsewhere in the study report. Physical activity levels (PAL) and total energy expenditure (TEE) were not reported for all participants |
| Other bias | Low risk | Comment: during the weight loss period preceding the weight maintenance period during which the green tea capsules or placebo were administered, a very‐low‐energy diet product, Modifast, was provided by Novartis Nutrition. Study authors do not mention any other financial or product‐based support that would indicate a probably risk of bias |
Kozuma 2005.
| Methods | The study was a randomised, placebo‐controlled, double‐blind parallel trial with an unknown treatment to control randomisation ratio | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA:
DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: 10 COUNTRY/ LOCATION: Japan/Isogo Chuo Hospital for Neurosurgery (Yokohama City), Kameido Minami Guchi Clinic (Tokyo), Kosei Hospital (Tokyo), Kodama Chuo Hospital (Kodama District), Central Hospital (Tokyo), Danduka Clinic (Inuma City), Nakayama Clinic of Obstetrics and Gynecology (Kumagaya City), Hisano Maynds Tower Clinic (Tokyo), Hiratsuka Gastroenterological Hospital (Tokyo) and Prima Clinic (Konosu City) SETTING: medical treatment facilities TREATMENT BEFORE STUDY: note: 2‐week preliminary observation period |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in Japanese in the journal Progress in Medicine and no sources of funding were declared; some authors were affiliated with Kao corporation | |
| Stated aim of study | Quote from study: "in this study, we investigated the effects and safety of continuous ingestion of a 500 ml beverage containing 540 mg catechins on the body composition of obese individuals over a 12 week period" | |
| Notes | BMI: body mass index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: "this trial was conducted as a multi‐site, randomised, double‐blind, placebo‐controlled, and parallel study..." Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from study: "this trial was conducted as a multi‐site, randomised, double‐blind, placebo‐controlled, and parallel study..." "The catechin and placebo drink both had the flavour of a sports drink and aside from the amount of catechins were regulated to have a similar makeup to that reported by the Takashima group" Comment: no mention of effectiveness of blinding efforts. Though beverages are described as similar, it is not clear if they are indistinguishable. No mention of whether researchers and staff were blinded to study components |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: authors adequately accounted for attrition, but did not clearly identify which group lost participants and when |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measurements stated in methods section were reported in the results |
| Other bias | Unclear risk | Comment: in "Methods" section, authors note that "all business relating to the conduct of the study was entrusted to the third party Contract Research Organization (CRO) and conducted under their capable supervision." No further information was provided, so we are uncertain about which aspects of the study were entrusted to the third party Comment: it is difficult to be sure of the study end points, as authors note: "if a significant [weight] difference was determined from the onset of the study, participants continued beverage ingestion until the following check‐up date (another 4 week period). The beverage ingestion period was from 12 weeks to at the most 24, and the study ended for each subject at the time beverage ingestion ended" |
Maki 2009.
| Methods | This was a randomised, placebo‐controlled, double‐blind trial, with a treatment to control randomisation ratio of 1:0.97 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA:
DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: 2 COUNTRY/ LOCATION: USA/Provident Clinical Research (Bloomington, IN) and Merithen Research (St Petersburg, FL) SETTING: clinical research sites TREATMENT BEFORE STUDY: unknown |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): changes in body fat mass SECONDARY OUTCOME(S): changes in body weight OTHER OUTCOME(S): NR |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in English in The Journal of Nutrition, a peer‐reviewed journal, and was supported by Kao Corporation in Tokyo, Japan | |
| Stated aim of study | Quote from study: "the present study was designed to evaluate the effects of a green tea catechin‐containing beverage on body composition and fat distribution in overweight and obese adults in the United States during exercise‐induced weight loss" | |
| Notes | BMI: body mass index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: "this was a randomised, double‐blind, controlled clinical trial" Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote from study: "study beverages were packaged in identical, single‐serving containers. The study products were labelled and coded in such a manner that subjects and staff were unaware of which product each participant was receiving. The beverages had similar sensory characteristics. Internal pretrial testing showed no difference in preference or palatability ratings between the active and control beverages" Comment: no mention of effectiveness of blinding efforts |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: the attrition in this study was higher than in most (84% in intervention and 79% in controlled group completed the study). Authors adequately accounted for attrition. However, the reasons for not completing the study were adequately described with the exception of participants who were lost to follow up (similar number in both groups). Therefore, we assessed the risk of bias as low. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes specified at the beginning were adequately investigated and reported |
| Other bias | Low risk | Comment: appears to be free of other bias |
Nagao 2007.
| Methods | This trial was a randomised, double‐blind, controlled parallel clinical study with a randomisation ratio of 1:1 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA:
DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: 7 COUNTRY/ LOCATION: Japan/ Kanto district SETTING: medical institutions TREATMENT BEFORE STUDY: note: 2‐week run‐in period |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in English in Obesity, a peer‐reviewed journal, and did not receive funding or outside support. The lead author is affiliated with Health Care Food Research Laboratories, Kao Corporation in Tokyo, Japan | |
| Stated aim of study | Quote from study: "therefore, the present trial was conducted to clarify the body fat reducing effect of the continuous ingestion of a green tea extract (GTE) high in catechins in more than 200 Japanese women and men who were maintaining their usual lifestyles. We also examined the effects of a GTE high in catechins on risk factors of cardiovascular disease" | |
| Notes | BMI: body mass index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: "this was a randomised double‐blind, controlled parallel multicenter trial" "randomisation was stratified by gender and BMI measured at the time of the run‐in period at each institution" Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Quote from study: "after the run‐in period, the subjects were allocated into two groups" Comment: allocation concealment not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from study: "this was a randomised double‐blind, controlled parallel multicenter trial" Comment: no description of GTE product compared to placebo found, other than that the base for both was brewed green tea. No mention of effectiveness of blinding efforts. No mention of whether researchers and staff were blinded to study components |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: authors adequately accounted for attrition |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes specified at the beginning were adequately investigated and reported |
| Other bias | Low risk | Comment: appears to be free of other bias |
Suzuki 2009.
| Methods | This study was a randomised double‐blind, placebo‐controlled trial with a parallel design with a treatment to control randomisation ratio of 1:1.05 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA:
DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: NR COUNTRY/ LOCATION: Japan SETTING: NR TREATMENT BEFORE STUDY: note: 2‐week observation period |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in Japanese in the journal Japanese Pharmacology and Therapeutics and no sources of support were declared by the study authors | |
| Stated aim of study | Quote from study: "our aim in this study was to measure visceral fat area in women of all ages and, with full regard for safety, establish whether there is an effect reducing body fat in women" | |
| Notes | BMI: body mass index; LDL: low density lipoprotein; NR: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: "this study was conducted as a placebo‐controlled randomised double‐blind trial run in parallel groups for comparison purposes" Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Quote from study: "a third party not directly involved in the study [StatCom Co. Ltd (Tokyo; President: Hirokuni Amari)] randomly divided qualifying participants into two groups" Comment: method of allocation concealment not reported; however, due to the use of a third party company to allocate participants into 2 groups, we judged there to be a low risk of bias |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from study: "this study was conducted as a placebo‐controlled randomised double‐blind trial run in parallel groups for comparison purposes" "IRB [review committee] verified prior to implementation of the study that there was no discernible difference between test beverages in appearance, taste or container (steel cans)." Comment: No mention of effectiveness of blinding efforts. No mention of whether researchers and staff were blinded to study components. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote from study: Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: authors adequately accounted for attrition |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measurements stated in methods section were reported in the results |
| Other bias | Unclear risk | Comment: 3 authors are affiliated with Soiken, Inc. and participants had previously registered as volunteers with Soiken, Inc; nature of corporate support for study is unclear |
Takase 2008.
| Methods | This was a randomised, double‐blind, placebo‐controlled, parallel‐group clinical trial with a randomisation ratio of 1:1.06 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA:
DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: NR ("multi‐facility") COUNTRY/ LOCATION: Japan SETTING: NR TREATMENT BEFORE STUDY: unknown |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in Japanese in the journal Japanese Pharmacology & Therapeutics, and all 6 authors are affiliated with Kao Corporation in the Health Care Food Research Laboratories; no sources of funding were reported | |
| Stated aim of study | Quote from study: "the present study used visceral fat area measured in CT scans as a criterion for the selection of study participants to investigate the effects of daily consumption of a high‐dose tea catechin beverage on visceral fat and metabolic syndrome in obese females with large accumulations of visceral fat" | |
| Notes | BMI: body Mass Index; CT: computerised axial tomography; NR: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: "this study was implemented as a multi‐facility, randomised, double‐blind, placebo‐controlled, parallel‐group trial" "based on the preliminary exam, participants were randomly assigned to either the catechin or the placebo group" "in this study, 101 individuals selected to participate as a result of prior screening were then randomly divided into two groups" Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from study: "the catechin beverage and placebo beverage were flavoured to taste like a sports drink and except for their catechin content were formulated in the same way" Comment: no mention of effectiveness of blinding efforts. No mention of whether researchers and staff were blinded to study components |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: authors adequately accounted for attrition |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes specified at the beginning were adequately investigated and reported |
| Other bias | Low risk | Comment: appears to be free of other bias |
Takashima 2004.
| Methods | This study was a randomised placebo‐controlled trial with a randomisation ratio of 1:0.9 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA:
DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: NR COUNTRY/ LOCATION: Japan SETTING: NR TREATMENT BEFORE STUDY: note: run‐in period of 4 weeks |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in English in the journal Progress in Medicine and no sources of funding were reported; the corresponding author was affiliated with Health Care Products Research Laboratories No.1 Kao Corporation and no other affiliations are stated | |
| Stated aim of study | Quote from study: "in the present study the effects of the long term intake of catechins on energy metabolism, including lipid catabolism, during exercise were examined to confirm the effects of a combination of exercise with catechin intake, using a beverage in the form of a sport drink with 570mg catechins" | |
| Notes | Authors were contacted for additional information BMI: body mass index; NR: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: "the subjects were randomly divided into two groups by a controller with similar age, anthropometric parameters, conditions of food intake and physical activity, and exercise tolerance test data..." Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: both the test beverage and control beverage were formulated as sports drinks but no further detail is provided regarding their similarity or differences. No mention of whether researchers and staff were blinded to study components |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: investigators specified enrolment details and explained that there was no attrition of participants |
| Selective reporting (reporting bias) | Low risk | Comment: all outcome measurements stated in methods section were reported in the results |
| Other bias | Low risk | Comment: appears to be free of other bias |
Takeshita 2008.
| Methods | This was a randomised, double‐blind, placebo‐controlled, parallel group trial with a treatment to control randomisation ratio of 1:1.025 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA:
DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: 8 COUNTRY/ LOCATION: Japan/Isoko Central Neurological Surgery Clinic (Yokohama City), Mizuno Internal Medicine Clinic (Tokorogawa City), Kameido Minamiguchi Clinic (Tokyo), Yuki Clinic (Tokyo), Kodama Central Hospital (Honjo City), Ishiguro Clinic (Gifu City), Dantsuka Clinic (Iruma City), Hiratsuka Hospital for Gastrointestinal Disease (Tokyo) SETTING: clinics/hospital TREATMENT BEFORE STUDY: note: 2‐week observation period before trial began |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in Japanese in the journal Japanese Pharmacology & Therapeutics, and 10 of the 13 authors are affiliated with Kao Corporation in the Health Care Food Research Laboratories of their Human Health Care Research Center; no sources of funding were reported | |
| Stated aim of study | Quote from study: "therefore, the objective of this study was to examine the effects of long‐term daily consumption of a non‐caffeinated beverage containing high concentrations of tea catechins on body fat, on factors relating to blood lipids, and on safety parameters in obese Japanese males" | |
| Notes | BMI: body mass index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: "this study was implemented as a multi‐facility, randomised, double‐blind, placebo‐controlled, parallel‐group trial for comparison purposes..." "Without bias with respect to age, facility, or measurements of height, weight, body fat, BMI, or waist size taken during the prior observation period, participants were randomly assigned either to the group that would consume the non‐caffeinated test beverage containing a high concentration of tea catechins (catechin beverage), or to the group that would consume the placebo beverage" Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from study: "both the catechin beverage and the placebo beverage were formulated like a sports drink similar to that in Kozuma et al's report in terms of electrolytes, sweetness, acidity and added flavour. Also, in the case of the placebo beverage, a colouring agent was added to the base mixture to achieve an appearance like that of the catechin beverage. Both beverages were sterilized and then bottled in polyethylene terephthalate containers" Comment: no mention of effectiveness of blinding efforts. No mention of whether researchers and staff were blinded to study components |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: authors adequately accounted for attrition |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes specified at the beginning were adequately investigated and reported |
| Other bias | Low risk | Comment: appears to be free of other bias |
Tsuchida 2002.
| Methods | The study was a randomised, placebo‐controlled, double‐blind trial with a treatment to control randomisation ratio of 1:1.05 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA: NR DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: 6 COUNTRY/ LOCATION: Japan/Kodama Central Hospital (Kodama County), Central Hospital (Tokyo), Hiratsuka Gastroenterological Hospital, (Tokyo), Kousei Medical Clinic (Tokyo), Takanashi Clinic (Tokyo) and Isogo Central Neurosurgical Hospital and Health Management Center (Yokohama) SETTING: clinics, hospitals, health management centre TREATMENT BEFORE STUDY: note: 2‐week run‐in period |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in Japanese in the journal Progress in Medicine and no sources of funding were reported | |
| Stated aim of study | Quote from study: "In the present study, we expanded the scale of investigation so as to verify the effect of catechins on body fat reduction in a participant group that included women" | |
| Notes | BMI: body mass index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote from study: "after a two‐week run‐in period, participants at each facility were randomly assigned, male and female by turn, into two groups" Comment: we judged this to be an inappropriate method of randomisation, and at a high risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from study: "a double blind study was then conducted over two 12‐week periods..." Comment: no mention of effectiveness of blinding efforts. No mention of whether researchers and staff were blinded to study components |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: recruitment and randomisation numbers are not given, so it is unclear whether there was any attrition |
| Selective reporting (reporting bias) | Unclear risk | Comment: authors did not clearly state what they would look for, so it is difficult to be sure that there was not selective reporting |
| Other bias | Low risk | Comment: appears to be free of other bias |
Wang 2010.
| Methods | This study was a randomised, double‐blind placebo‐controlled trial using a between‐subject design with a randomisation ratio of 1:1.04:0.91:0.91 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA:
DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: 1 COUNTRY/ LOCATION: China/Shanghai SETTING: study centre TREATMENT BEFORE STUDY: note: run‐in period of 2 weeks where participants drank 1 serving of control tea at study site to test adherence and protocol compliance |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in English in Obesity, a peer‐reviewed journal, and was supported by the Lipton Institute of Tea; all authors but 1 were employees of Unilever | |
| Stated aim of study | Quote from study: "in this large, double‐blind placebo‐controlled trial we investigated the effects of consuming GT [green tea] with different amounts of catechins on measures of body weight, total body fat mass and the distribution of fat between abdominal and other depots" | |
| Notes | BMI: body mass index | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: "subjects were randomly allocated to one of four groups. Randomization of subjects into experimental groups was based on stratification by BMI, waist circumference/height, and gender" Comment: Method of randomisation not reported. |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from study: "to ensure that subjects would not know they were consuming a control treatment, a small amount of unextracted green leaf (0.5 g) and tea power perfume were added" Comment: No mention of whether investigators were blinded as well. No mention of effectiveness of blinding efforts |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes on which the study reported were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: authors adequately accounted for attrition |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes specified at the beginning were adequately investigated and reported |
| Other bias | Unclear risk | Comment: authors are employees of Unilever or Lipton; nature of corporate support for study is unclear |
Westerterp‐Plantenga 2005.
| Methods | This study was a randomised, double‐blind, placebo‐controlled trial with a parallel design, with a treatment to control randomisation ratio of 1:1 | |
| Participants |
INCLUSION CRITERIA:
EXCLUSION CRITERIA:
DIAGNOSTIC CRITERIA:
|
|
| Interventions |
NUMBER OF STUDY CENTRES: NR COUNTRY/ LOCATION: Netherlands SETTING: NR TREATMENT BEFORE STUDY: 4 weeks' very‐low energy‐diet |
|
| Outcomes |
OUTCOME(S) (as stated in the protocol/registered trial documents): PRIMARY OUTCOME(S): unknown SECONDARY OUTCOME(S): unknown OTHER OUTCOME(S): unknown |
|
| Study details | STUDY TERMINATED BEFORE REGULAR END: no | |
| Publication details | This study was published in English in Obesity Research, a peer‐reviewed journal, and was commercially funded by Novartis Consumer Health, Nyon, Switzerland | |
| Stated aim of study | Quote from study: "therefore, we executed a follow‐up study on body weight maintenance with the aim of investigating whether the same green tea‐caffeine mixture may improve weight maintenance by preventing or limiting weight regain after weight loss of 5% to 10% in moderately obese subjects with a low or high habitual caffeine intake" | |
| Notes | BMI: body mass index; NR: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote from study: participants "were divided into two stratified groups according to sex, BMI, age, dietary restraint, resting energy expenditure (REE), and being either habitual low caffeine consumers or habitual high caffeine consumers.... Subsequently, within the low or high habitual caffeine consumption groups, subjects were further stratified according to the characteristics mentioned above and randomised to a prospective green tea‐caffeine mixture treatment group and a placebo group for the weight maintenance phase" "Within the low or high caffeine intake group, subjects were stratified according to sex, BMI, age, dietary restraint, and REE and divided into two groups" Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote from study: "a randomised placebo‐controlled double blind parallel trial in 76 overweight and moderately obese subjects, matched for sex, age, BMI, height, body mass, and habitual caffeine intake was conducted" Quote from study: "a double‐blind administration of the supplementation (green tea‐caffeine mixture or placebo) was carried out" Comment: no mention of effectiveness of blinding efforts. Intervention and placebo delivered as a capsule, capsules not described for appearance |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: all outcomes that study reported on were objective and measured by assessors. Therefore, whether or not assessors were blinded, risk of detection bias was assessed as low |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: there appears to have been no attrition of participants requiring explanation, or other incomplete outcome data |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes specified at the beginning were adequately investigated and reported |
| Other bias | Unclear risk | Comment: study funded by Novartis, maker of test product. Results were positive so we judged that there was an unclear risk of bias |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bakker 2010 | Study was less than 12 weeks in length |
| Basu 2010 | Study was less than 12 weeks in length |
| Batista 2009 | Study was not a randomised controlled clinical trial |
| Bayes 2005 | Study was not a randomised controlled clinical trial |
| Belza 2007 | Intervention was delivered as a combination product rather than just green tea or its components |
| Belza 2009 | Study participants were not classified as overweight or obese at start of trial |
| Berube‐Parent 2005 | Intervention was delivered as a combination product rather than just green tea or its components |
| Bolling 2009 | Study was not a randomised controlled clinical trial |
| Boon 2006 | Study was not a randomised controlled clinical trial |
| Boon 2008 | Study was not a randomised controlled clinical trial |
| Boschmann 2007 | Study was less than 12 weeks in length |
| Brown 2009 | Study was less than 12 weeks in length |
| Chan 2006 | Study participants were diagnosed with a co‐morbidity that might have affected weight loss |
| Chantre 2002 | Study was not a randomised controlled clinical trial |
| Chou 2008 | Study was not a randomised controlled clinical trial |
| Dalbo 2008 | Study was less than 12 weeks in length |
| Di Pierro 2009 | Study was not a randomised controlled clinical trial |
| Diepvens 2007 | Study was not a randomised controlled clinical trial |
| Donovan 2009 | Study participants were taking medications that might have affected weight loss |
| Dulloo 1999 | Study was less than 12 weeks in length |
| Eichenberger 2009 | Study participants were not classified as overweight or obese at start of trial |
| Fukino 2005 | Study was less than 12 weeks in length |
| Fukino 2008 | Study participants were taking medications that might have affected weight loss |
| Grove 2010 | Study was not a randomised controlled clinical trial |
| Gupta 2008 | Study was not a randomised controlled clinical trial |
| Harada 2005 | Study was not a randomised controlled clinical trial |
| Hardy 2008 | Study was not a randomised controlled clinical trial |
| He 2009 | Study was less than 12 weeks in length |
| Hursel 2009a | Study was not a randomised controlled clinical trial |
| Hursel 2010 | Study was not a randomised controlled clinical trial |
| Komatsu 2003 | Study participants were not classified as overweight or obese at start of trial |
| Kuhad 2008 | Study was not a randomised controlled clinical trial |
| Lieberman 2003 | Study was not a randomised controlled clinical trial |
| Nagao 2005 | Intervention was delivered as a combination product rather than just green tea or its components |
| Nagao 2009 | Study participants were taking medications that might have affected weight loss |
| Onakpoya 2010 | Study was not a randomised controlled clinical trial |
| Pittler 2005 | Study was not a randomised controlled clinical trial |
| Pittler 2006 | Study was not a randomised controlled clinical trial |
| Pittler 2007 | Study was not a randomised controlled clinical trial |
| Pittler 2009 | Study was not a randomised controlled clinical trial |
| Rao 2006 | Intervention was delivered as a combination product rather than just green tea or its components |
| Schneider 2009 | Study was not a randomised controlled clinical trial |
| Schulz 2009 | Study was not a randomised controlled clinical trial |
| Shixian 2006 | Study was not a randomised controlled clinical trial |
| Stendell‐Hollis 2010 | Study participants were diagnosed with a co‐morbidity that might have affected weight loss |
| Thielecke 2010 | Study was less than 12 weeks in length |
| Tian 2004 | Study was not a randomised controlled clinical trial |
| Tsai 2009 | Intervention was delivered as a combination product rather than just green tea or its components |
| Tucker 2008 | Intervention was delivered as a combination product rather than just green tea or its components |
| Westerterp‐Plantenga 2010 | Study was not a randomised controlled clinical trial |
| Wolfram 2006 | Study was not a randomised controlled clinical trial |
| Wolfram 2007 | Study was not a randomised controlled clinical trial |
| Yoneda 2009 | Study was not a randomised controlled clinical trial |
Differences between protocol and review
Why it is important to do this review: in the protocol section on 'Why it is important to do this review', three RCTs from 2009 were identified that were published after the last known meta‐analysis (Phung 2010). It was expected that these three RCTs (Gregersen 2009; Reinbach 2009; Wang 2010) would be included in the final review; however, the first two trials did not meet our inclusion criteria and were excluded at the abstract screening stage. The participants of Gregersen 2009 were of normal weight rather than overweight or obese at the start of the study, and the intervention preparation used in Reinbach 2009 was a combination preparation (green tea plus other ingredients). These factors made these two trials unsuitable for inclusion in the full review.
Objectives: the objective was changed from "To assess the effects of green tea products for weight loss and weight maintenance in overweight or obese adults" as stated in the protocol to "To assess efficacy and safety of green tea preparations for weight loss and weight maintenance in overweight or obese adults" in the review.
Searching: the search conducted for the review included one additional database, on the advice of the Trials Search Coordinator (TSC) of the Cochrane Metabolic and Endocrine Disorders Group (CMED): AMED (Allied and Complementary Medicine).
Data extraction: during the data extraction phase it became apparent that in Appendix 7, the item listed as number 4, 'full chemical name', was superfluous, as the data were captured in the item, 'name of active or marker chemical(s)'. Accordingly, 'full chemical name' does not appear in the version of Appendix 7 used in the review.
Subgroup analysis: two subgroup analyses that were listed in the protocol were not conducted. The first subgroup analysis that was not performed was 'trials that used green tea products of similar composition were analysed as a group and compared to all other trials using green tea products of varying content to see if content could be linked with weight loss'. Rather than conduct a subgroup analysis, this was done instead by re‐ordering the forest plot of effect size according to daily dose of catechins to see if trends were apparent. Similar analyses were conducted ordering trials by effect size and putting highest daily dose of ‐EGCG‐) at the top and lowest daily dose at the bottom and examining for trends. The second subgroup analysis that was not conducted was 'trials that included only obese participants and analysing them separately from trials that included anyone who was overweight'. This was not possible as there were no trials identified that included only obese participants. Subgroup analyses that were conducted in the review and not outlined in the protocol were undertaken for studies conducted in Japan versus those conducted elsewhere.
Sensitivity analysis: two of the three sensitivity analysis statements listed in the protocol were completed and are described in the review. The remaining statement "Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), country" was not conducted as planned. On the advice of the statistician who was added to our group after the protocol was published, we conducted a subgroup analysis, rather than a sensitivity analysis, for country where studies were conducted as the studies could be pooled into two groups‐conducted in Japan or outside Japan. Additionally, we did not conduct the planned sensitivity analysis of language of publication as the only two languages of the included studies were English and Japanese. The differences between the two groups were captured, for the most part, in the subgroup analysis of the country in which the studies were conducted, as described above. A sensitivity analysis of funding was not done in the review as the level of detail provided among the studies was insufficient. Finally, when designing the analysis of the studies, we thought it would be instructive to conduct a sensitivity analysis to see the effect that including studies that used different definitions of the BMI that was considered as overweight or obese would have. When the data were collected (see last sentence in “Results, Included studies”) it became apparent that there was a range in values, as well as some overlap in values and it was decided that analysis would not be meaningful and therefore was not included in the review.
Review authors: the review had two additional authors (S Doucette and L Killian) who were not part of the protocol.
Contributions of authors
Tannis Jurgens: co‐ordination and development of protocol, search strategy development, study selection, data extraction, data analysis, data interpretation, review development and writing.
Anne Marie Whelan: protocol draft, study selection, data extraction, data interpretation and contributing to writing of review.
Lara Killian: search strategy development, conducting and managing searches and results, study selection, contacting authors, co‐ordinating translations, data extraction and contributing to writing of review.
Steve Doucette: statistical expertise in data analysis, data interpretation, contributed to writing sections of the review related to statistical analysis and interpretation of results.
Sara Kirk: protocol draft, data interpretation, subgroup analyses and contributing to writing of review.
Elizabeth Foy: search strategy development, resource for research assistant in conducting searches, contributed to writing of searching section of review and overall editing.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Nova Scotia Health Research Foundation, Canada.
Knowledge Transfer/Exchange Program
Declarations of interest
None known.
New
References
References to studies included in this review
Auvichayapat 2008 {published data only}
- Auvichayapat P, Prapochanung M, Tunkamnerdthai O, Sripanidkulchai BO, Auvichayapat N, Thinkhamrop B, et al. Effectiveness of green tea on weight reduction in obese Thais: a randomized, controlled trial. Physiology & Behavior 2008;93(3):486‐91. [CENTRAL: 00637191; PUBMED: 18006026] [DOI] [PubMed] [Google Scholar]
Diepvens 2005 {published data only}
- Diepvens K, Kovacs EMR, Nijs IMT, Vogels N, Westerterp‐Plantenga MS. Effects of green tea on resting energy expenditure and substrate oxidation during weight loss in overweight females. British Journal of Nutrition 2005;94(6):1026‐34. [DOI: 10.1079/BJN20051580; EMBASE: 2006031819; PUBMED: 16351782] [DOI] [PubMed] [Google Scholar]
- Diepvens K, Kovacs EMR, Vogels N, Westerterp‐Plantenga MS. Metabolic effects of green tea and of phases of weight loss. Physiology & Behavior 2006;87(1):185‐91. [CENTRAL: 00552881; DOI: 10.1016/j.physbeh.2005.09.013; PUBMED: 16277999] [DOI] [PubMed] [Google Scholar]
Hill 2007 {published and unpublished data}
- Hill AM, Coates AM, Buckley JD, Ross R, Thielecke F, Howe PRC. Can EGCG reduce abdominal fat in obese subjects?. Journal of the American College of Nutrition 2007;26(4):396S‐402S. [CENTRAL: 00700928; PUBMED: 17906193] [DOI] [PubMed] [Google Scholar]
Hsu 2008 {published and unpublished data}
- Hsu CH, Tsai TH, Kao YH, Hwang KC, Tseng TY, Chou P. Effect of green tea extract on obese women: a randomized, double‐blind, placebo‐controlled clinical trial. Clinical Nutrition 2008;27(3):363‐70. [CENTRAL: 00639525; DOI: 10.1016/j.clnu.2008.03.007; PUBMED: 18468736] [DOI] [PubMed] [Google Scholar]
Hursel 2009 {published data only}
- Hursel R, Westerterp‐Plantenga MS. Green tea catechin plus caffeine supplementation to a high‐protein diet has no additional effect on body weight maintenance after weight loss. The American Journal of Clinical Nutrition 2009;89(3):822‐30. [CENTRAL: 00680355; DOI: 10.3945/%E2%80%8Bajcn.2008.27043; PUBMED: 19176733] [DOI] [PubMed] [Google Scholar]
Kajimoto 2005 {published and unpublished data}
- Kajimoto O, Kajimoto Y, Yabune M, Nakamura T, Kotani K, Suzuki Y, et al. Tea catechins with a galloyl moiety reduce body weight and fat. Journal of Health Science 2005;51(2):161‐71. [CENTRAL: 00575415; EMBASE: 2005209246] [Google Scholar]
Kataoka 2004 {published data only}
- Kataoka K, Takashima S, Shibata E, Hoshino E. Body fat reduction by the long term intake of catechins and the effects of physical activity. Progress in Medicine 2004;24(12):3358‐70. [Google Scholar]
Kovacs 2004 {published data only}
Kozuma 2005 {published data only}
- Kozuma K, Chikama A, Hishino E, Kataoka K, Mori K, Hase T, et al. Effect of intake of a beverage containing 540 mg catechins on the body composition of obese women and men. Progress in Medicine 2005;25:185‐97. [Google Scholar]
Maki 2009 {published data only}
Nagao 2007 {published data only}
Suzuki 2009 {published data only (unpublished sought but not used)}
- Suzuki Y, Nozawa A, Miyamoto S, Sagesaka YM, Azuma M, Kajimoto Y, et al. Reduction of visceral fat in overweight female volunteers by long‐term ingestion of tea catechins with a galloyl moiety: a randomised double‐blind placebo‐controlled study. Japanese Pharmacology and Therapeutics 2009;37(6):521‐7. [EMBASE: 2009395839] [Google Scholar]
Takase 2008 {published data only (unpublished sought but not used)}
- Takase H, Nagao T, Otsuka K, Meguro S, Komikado M, Tokimitsu I. Effects of long‐term ingestion of tea catechins on visceral fat accumulation and metabolic syndrome risk in women with abdominal obesity. Japanese Pharmacology and Therapeutics 2008;36(3):237‐45. [CENTRAL: 00707360; EMBASE: 2008211757] [Google Scholar]
Takashima 2004 {published data only}
- Takashima S, Kataoka K, Shibata E, Hoshino E. The long term intake of catechins improves lipid catabolism during exercise. Progress in Medicine 2004;24(12):3371‐9. [Google Scholar]
Takeshita 2008 {published data only (unpublished sought but not used)}
- Takeshita M, Takashima S, Harada U, Shibata E, Hosoya N, Takase H, et al. Effects of long‐term consumption of tea catechins‐enriched beverage with no caffeine on body composition in humans. Japanese Pharmacology and Therapeutics 2008;36(8):767‐76. [CENTRAL: 00708595; EMBASE: 2008454808] [Google Scholar]
Tsuchida 2002 {published data only (unpublished sought but not used)}
- Tsuchida T, Itakura H, Nakamura H. Reduction of body fat in humans by long‐term ingestion of catechins. Progress in Medicine 2002;22(9):2189‐203. [Google Scholar]
Wang 2010 {published data only}
- Wang H, Wen Y, Du Y, Yan X, Guo H, Rycroft JA, et al. Effects of catechin enriched green tea on body composition. Obesity 2010;18(4):773‐9. [DOI: 10.1038/oby.2009.256; PUBMED: 19680234] [DOI] [PubMed] [Google Scholar]
Westerterp‐Plantenga 2005 {published data only}
- Westerterp‐Plantenga MS, Lejeune MPGM, Kovacs EMR. Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obesity Research 2005;13(7):1195‐204. [DOI: 10.1038/oby.2005.142; PUBMED: 16076989] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bakker 2010 {published data only}
- Bakker GC, Erk MJ, Pellis L, Wopereis S, Rubingh CM, Cnubben NH, et al. An antiinflammatory dietary mix modulates inflammation and oxidative and metabolic stress in overweight men: a nutrigenomics approach. The American Journal of Clinical Nutrition 2010;91(4):1044‐59. [PUBMED: 20181810] [DOI] [PubMed] [Google Scholar]
Basu 2010 {published data only}
- Basu A, Sanchez K, Leyva MJ, Wu M, Betts NM, Aston CE, et al. Green tea supplementation affects body weight, lipids, and lipid peroxidation in obese subjects with metabolic syndrome. Journal of the American College of Nutrition 2010;29(1):31‐40. [PUBMED: 20595643] [DOI] [PubMed] [Google Scholar]
Batista 2009 {published data only}
- Batista GAP, Cunha CLP, Scartezini M, Heyde R, Bitencourt MG, Melo SF. Prospective double‐blind crossover study of Camellia sinensis (green tea) in dyslipidemias. Arquivos Brasileiros de Cardiologia 2009;93(2):128‐34. [PUBMED: 19838489] [DOI] [PubMed] [Google Scholar]
Bayes 2005 {published data only}
- Bayes M, Rabasseda X, Prous JR. Gateways to clinical trials. Methods and Findings in Experimental and Clinical Pharmacology 2005; Vol. 27, issue 6:411‐61. [PUBMED: 16179960] [PubMed]
Belza 2007 {published data only}
- Belza A, Frandsen E, Kondrup J. Body fat loss achieved by stimulation of thermogenesis by a combination of bioactive food ingredients: a placebo‐controlled, double‐blind 8‐week intervention in obese subjects. International Journal of Obesity (2005) 2007;31(1):121‐30. [PUBMED: 16652130] [DOI] [PubMed] [Google Scholar]
Belza 2009 {published data only}
- Belza A, Toubro S, Astrup A. The effect of caffeine, green tea and tyrosine on thermogenesis and energy intake. European Journal of Clinical Nutrition 2009;63(1):57‐64. [PUBMED: 17882140] [DOI] [PubMed] [Google Scholar]
Berube‐Parent 2005 {published data only}
- Berube‐Parent S, Pelletier C, Dore J, Tremblay A. Effects of encapsulated green tea and Guarana extracts containing a mixture of epigallocatechin‐3‐gallate and caffeine on 24 h energy expenditure and fat oxidation in men. The British Journal of Nutrition 2005;94(3):432‐6. [PUBMED: 16176615] [DOI] [PubMed] [Google Scholar]
Bolling 2009 {published data only}
- Bolling BW, Chen CY, Blumberg JB. Tea and health: preventive and therapeutic usefulness in the elderly?. Current Opinion in Clinical Nutrition and Metabolic Care 2009;12(1):42‐8. [PUBMED: 19057186] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boon 2006 {published data only}
- Boon G, Lockwood B. Could nutraceuticals be a further weapon in the battle for weight loss?. The Pharmaceutical Journal 2006;276(7382):15‐8. [Google Scholar]
Boon 2008 {published data only}
- Boon N. Health potential for functional green teas?. Internationale Zeitschrift fur Vitamin‐ und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition [International Journal for Vitamin and Nutrition research] 2008;78(6):275‐81. [PUBMED: 19685436] [DOI] [PubMed] [Google Scholar]
Boschmann 2007 {published data only}
- Boschmann M, Thielecke F. The effects of epigallocatechin‐3‐gallate on thermogenesis and fat oxidation in obese men: a pilot study. Journal of the American College of Nutrition 2007;26(4):389S‐95S. [PUBMED: 17906192] [DOI] [PubMed] [Google Scholar]
Brown 2009 {published data only}
- Brown AL, Lane J, Coverly J, Stocks J, Jackson S, Stephen A, et al. Effects of dietary supplementation with the green tea polyphenol epigallocatechin‐3‐gallate on insulin resistance and associated metabolic risk factors: randomised controlled trial. The British Journal of Nutrition 2009;101(6):886‐94. [PUBMED: 18710606] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chan 2006 {published data only}
- Chan CC, Koo MW, Ng EH, Tang OS, Yeung WS, Ho PC. Effects of Chinese green tea on weight, and hormonal and biochemical profiles in obese patients with polycystic ovary syndrome: a randomised placebo‐controlled trial. Journal of the Society for Gynecologic Investigation 2006;13(1):63‐8. [PUBMED: 16378915] [DOI] [PubMed] [Google Scholar]
Chantre 2002 {published data only}
- Chantre P, Lairon D. Recent findings of green tea extract AR25 (Exolise) and its activity for the treatment of obesity. Phytomedicine 2002;9(1):3‐8. [PUBMED: 11924761] [DOI] [PubMed] [Google Scholar]
Chou 2008 {published data only}
- Chou P. Authors' reply. Focus on Alternative and Complementary Therapies 2008;13(4):258‐9. [Google Scholar]
Dalbo 2008 {published data only}
- Dalbo VJ, Roberts MD, Stout JR, Kerksick CM. Acute effects of ingesting a commercial thermogenic drink on changes in energy expenditure and markers of lipolysis. Journal of the International Society of Sports Nutrition 2008;5:6. [DOI: 10.1186/1550-2783-5-6; PUBMED: 18289388] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diepvens 2007 {published data only}
- Diepvens K, Westerterp KR, Westerterp‐Plantenga MS. Obesity and thermogenesis related to the consumption of caffeine, ephedrine, capsaicin, and green tea. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology 2007;292(1):R77‐85. [PUBMED: 16840650] [DOI] [PubMed] [Google Scholar]
Di Pierro 2009 {published data only}
- Pierro F, Menghi AB, Barreca A, Lucarelli M, Calandrelli A. Greenselect Phytosome as an adjunct to a low‐calorie diet for treatment of obesity: a clinical trial. Alternative Medicine Review 2009;14(2):154‐60. [PUBMED: 19594224] [PubMed] [Google Scholar]
Donovan 2009 {published data only}
- Donovan JL. Prevention of weight gain and dyslipidemia by green tea in patients initiating therapy with olanzapine. www.clinicaltrials.gov/ct2/show/NCT00934908 (accessed 14 November 2012). [CENTRAL: CN‐00725384]
Dulloo 1999 {published data only}
- Dulloo AG, Duret C, Rohrer D, Girardier L, Mensi N, Fathi M, et al. Efficacy of a green tea extract rich in catechin polyphenols and caffeine in increasing 24‐h energy expenditure and fat oxidation in humans. The American Journal of Clinical Nutrition 1999;70(6):1040‐5. [PUBMED: 10584049] [DOI] [PubMed] [Google Scholar]
Eichenberger 2009 {published data only}
- Eichenberger P, Colombani PC, Mettler S. Effects of 3‐week consumption of green tea extracts on whole‐body metabolism during cycling exercise in endurance‐trained men. Internationale Zeitschrift fur Vitamin‐ und Ernahrungsforschung. Journal International de Vitaminologie et de Nutrition [International journal for vitamin and nutrition research] 2009;79(1):24‐33. [PUBMED: 19839000] [DOI] [PubMed] [Google Scholar]
Fukino 2005 {published data only}
- Fukino Y, Shimbo M, Aoki N, Okubo T, Iso H. Randomized controlled trial for an effect of green tea consumption on insulin resistance and inflammation markers. Journal of Nutritional Science and Vitaminology 2005;51(5):335‐42. [PUBMED: 16392704] [DOI] [PubMed] [Google Scholar]
Fukino 2008 {published data only}
- Fukino Y, Ikeda A, Maruyama K, Aoki N, Okubo T, Iso H. Randomized controlled trial for an effect of green tea‐extract powder supplementation on glucose abnormalities. European Journal of Clinical Nutrition 2008;62(8):953‐60. [PUBMED: 17554248] [DOI] [PubMed] [Google Scholar]
Grove 2010 {published data only}
- Grove KA, Lambert JD. Laboratory, epidemiological, and human intervention studies show that tea (Camellia sinensis) may be useful in the prevention of obesity. The Journal of Nutrition 2010;140(3):446‐53. [PUBMED: 20089791] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gupta 2008 {published data only}
- Gupta J, Siddique YH, Beg T, Ara G, Afzal M. A review on the beneficial effects of tea polyphenols on human health. International Journal of Pharmacology 2008;4(5):314‐38. [Google Scholar]
Harada 2005 {published data only}
- Harada U, Chikama A, Saito S, Takase H, Nagao T, Hase T, et al. Effects of the long‐term ingestion of tea catechins on energy expenditure and dietary fat oxidation in healthy subjects. Journal of Health Science 2005;51(2):248‐52. [Google Scholar]
Hardy 2008 {published data only}
- Hardy M. Study fails to demonstrate effect of a Camellia sinensis (green tea) extract on body‐weight, waist circumference or appetite‐related hormone levels in obese women. Commentary. Focus on Alternative and Complementary Therapies 2008;13(4):257‐8. [Google Scholar]
He 2009 {published data only}
- He RR, Chen L, Lin BH, Matsui Y, Yao XS, Kurihara H. Beneficial effects of oolong tea consumption on diet‐induced overweight and obese subjects. Chinese Journal of Integrative Medicine 2009;15(1):34‐41. [PUBMED: 19271168] [DOI] [PubMed] [Google Scholar]
Hursel 2009a {published data only}
- Hursel R, Viechtbauer W, Westerterp‐Plantenga MS. The effects of green tea on weight loss and weight maintenance: a meta‐analysis. International Journal of Obesity (2005) 2009;33(9):956‐61. [PUBMED: 19597519] [DOI] [PubMed] [Google Scholar]
Hursel 2010 {published data only}
- Hursel R, Westerterp‐Plantenga MS. Thermogenic ingredients and body weight regulation. International Journal of Obesity (2005) 2010;34(4):659‐69. [PUBMED: 20142827] [DOI] [PubMed] [Google Scholar]
Komatsu 2003 {published data only}
- Komatsu T, Nakamori M, Komatsu K, Hosoda K, Okamura M, Toyama K, et al. Oolong tea increases energy metabolism in Japanese females. The Journal of Medical Investigation 2003;50(3‐4):170‐5. [PUBMED: 13678386] [PubMed] [Google Scholar]
Kuhad 2008 {published data only}
- Kuhad A, Chopra K. Highlights from the 3rd international conference on polyphenols and health: current trends in polyphenol research: from Mother Nature to molecular mechanisms. Drugs of the Future 2008;33(3):249‐87. [Google Scholar]
Lieberman 2003 {published data only}
- Lieberman S. Natural methods for accelerating weight loss: the low glycemic index diet, green tea, chromium, and 5‐hydroxytryptophan. Alternative and Complementary Therapies 2003;9(6):307‐11. [DOI: 10.1089/107628003322658575] [DOI] [Google Scholar]
Nagao 2005 {published data only}
- Nagao T, Komine Y, Soga S, Meguro S, Hase T, Tanaka Y, et al. Ingestion of a tea rich in catechins leads to a reduction in body fat and malondialdehyde‐modified LDL in men. The American Journal of Clinical Nutrition 2005;81(1):122‐9. [PUBMED: 15640470] [DOI] [PubMed] [Google Scholar]
Nagao 2009 {published data only}
- Nagao T, Meguro S, Hase T, Otsuka K, Komikado M, Tokimitsu I, et al. A catechin‐rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity (Silver Spring, Md.) 2009;17(2):310‐7. [PUBMED: 19008868] [DOI] [PubMed] [Google Scholar]
Onakpoya 2010 {published data only}
- Onakpoya I. Supplement containing Camellia sinensis (green tea) may help treat obesity. Focus on Alternative and Complementary Therapies 2010;15(1):15‐6. [Google Scholar]
Pittler 2005 {published data only}
- Pittler MH, Nagao T. Encouraging effects of Camellia sinensis (oolong tea) ingestion for reducing body‐weight. Focus on Alternative & Complementary Therapies 2005;10(2):115‐7. [EMBASE: 2009010075] [Google Scholar]
Pittler 2006 {published data only}
- Pittler MH, Diepvens K. Similar reductions in body‐weight for placebo and green tea. Focus on Alternative & Complementary Therapies 2006;11(2):107‐8. [EMBASE: 2009229037] [Google Scholar]
Pittler 2007 {published data only}
- Pittler MH. Little effect of Camellia sinensis (green tea) extract on glucose abnormalities: commentary. Focus on Alternative and Complementary Therapies 2007;12(4):259‐60. [Google Scholar]
Pittler 2009 {published data only}
- Pittler MH. Camellia sinensis (green tea) catechins seem to affect body composition positively: commentary. Focus on Alternative and Complementary Therapies 2009;14(2):98‐9. [Google Scholar]
Rao 2006 {published data only}
- Rao AV, Andrews K, Logan A. A double‐blind, randomised‐controlled trial of a nutritional supplement (abs+) containing conjugated linoleic acid (CLA) and epigallocatechin‐gallate (EGCG) in human weight loss. Journal of Herbs, Spices and Medicinal Plants 2006;12(3):67‐76. [DOI: 10.1300/J044v12n03_05] [DOI] [Google Scholar]
Schneider 2009 {published data only}
- Schneider C, Segre T. Green tea: potential health benefits. American Family Physician 2009;79(7):591‐4. [PUBMED: 19378876] [PubMed] [Google Scholar]
Schulz 2009 {published data only}
- Schulz V. Green tea extract for weight reduction? Randomized double blind study cannot confirm positive preliminary results. Zeitschrift fur Phytotherapie 2009;30(2):74‐5. [Google Scholar]
Shixian 2006 {published data only}
- Shixian Q, VanCrey B, Shi J, Kakuda Y, Jiang Y. Green tea extract thermogenesis‐induced weight loss by epigallocatechin gallate inhibition of catechol‐O‐methyltransferase. Journal of Medicinal Food 2006;9(4):451‐8. [PUBMED: 17201629] [DOI] [PubMed] [Google Scholar]
Stendell‐Hollis 2010 {published data only}
- Stendell‐Hollis NR, Thomson CA, Thompson PA, Bea JW, Cussler EC, Hakim IA. Green tea improves metabolic biomarkers, not weight or body composition: a pilot study in overweight breast cancer survivors. Journal of Human Nutrition and Dietetics 2010;23(6):590‐600. [PUBMED: 20807303] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thielecke 2010 {published data only}
- Thielecke F, Rahn G, Bohnke J, Adams F, Birkenfeld AL, Jordan J, et al. Epigallocatechin‐3‐gallate and postprandial fat oxidation in overweight/obese male volunteers: a pilot study. European Journal of Clinical Nutrition 2010;64(7):704‐13. [PUBMED: 20372175] [DOI] [PubMed] [Google Scholar]
Tian 2004 {published data only}
- Tian WX, Li LC, Wu XD, Chen CC. Weight reduction by Chinese medicinal herbs may be related to inhibition of fatty acid synthase. Life Sciences 2004;74(19):2389‐99. [PUBMED: 14998716] [DOI] [PubMed] [Google Scholar]
Tsai 2009 {published data only}
- Tsai CH, Chiu WC, Yang NC, Ouyang CM, Yen YH. A novel green tea meal replacement formula for weight loss among obese individuals: a randomized controlled clinical trial. International Journal of Food Sciences and Nutrition 2009;60(S6):151‐9. [DOI: 10.1080/09637480903136667; PUBMED: 19736596] [DOI] [PubMed] [Google Scholar]
Tucker 2008 {published data only}
- Tucker LA, Cook AJ, Nokes NR, Adams TB. Telephone‐based diet and exercise coaching and a weight‐loss supplement result in weight and fat loss in 120 men and women. American Journal of Health Promotion 2008;23(2):121‐9. [PUBMED: 19004162] [DOI] [PubMed] [Google Scholar]
Westerterp‐Plantenga 2010 {published data only}
- Westerterp‐Plantenga MS. Green tea catechins, caffeine and body‐weight regulation. Physiology & Behavior 2010;100(1):42‐6. [PUBMED: 20156466] [DOI] [PubMed] [Google Scholar]
Wolfram 2006 {published data only}
- Wolfram S, Wang Y, Thielecke F. Anti‐obesity effects of green tea: from bedside to bench. Molecular Nutrition & Food Research 2006;50(2):176‐87. [PUBMED: 16470636] [DOI] [PubMed] [Google Scholar]
Wolfram 2007 {published data only}
- Wolfram S. Effects of green tea and EGCG on cardiovascular and metabolic health. Journal of the American College of Nutrition 2007;26(4):373S‐88S. [PUBMED: 17906191] [DOI] [PubMed] [Google Scholar]
Yoneda 2009 {published data only}
- Yoneda T, Shoji K, Takase H, Hibi M, Hase T, Meguro S, et al. Effectiveness and safety of 1‐year ad libitum consumption of a high‐catechin beverage under nutritional guidance. Metabolic Syndrome and Related Disorders 2009;7(4):349‐56. [PUBMED: 19558270] [DOI] [PubMed] [Google Scholar]
Additional references
Busse 2000
- Busse W. The significance of quality for efficacy and safety of herbal medicinal products. Drug Information Journal 2000;34(1):15‐23. [EMBASE: 2000222072] [Google Scholar]
Cabrera 2006
- Cabrera C, Artacho R, Giménez R. Beneficial effects of green tea: a review. Journal of the American College of Nutrition 2006;25(2):79‐99. [PUBMED: 16582024] [DOI] [PubMed] [Google Scholar]
Chacko 2010
- Chacko SM, Thambi PT, Kuttan R, Nishigaki I. Beneficial effects of green tea: a literature review. Chinese Medicine 2010;5(13):1‐9. [DOI: 10.1186/1749-8546-5-13; PUBMED: 20370896] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clement 2009
- Clement Y. Can green tea do that? A literature review of the clinical evidence. Preventive Medicine 2009;49(2‐3):83‐7. [DOI: 10.1016/j.ypmed.2009.05.005; PUBMED: 19465043] [DOI] [PubMed] [Google Scholar]
Eckel 2008
- Eckel RH. Nonsurgical management of obesity in adults. New England Journal of Medicine 2008;358(18):1941‐50. [DOI: 10.1056/NEJMcp0801652; PUBMED: 18450605] [DOI] [PubMed] [Google Scholar]
Elfhag 2005
- Elfhag K, Rossner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obesity Reviews 2005;6:67‐85. [DOI: 10.1111/j.1467-789X.2005.00170.x; EMBASE: 2005048588; PUBMED: 15655039] [DOI] [PubMed] [Google Scholar]
Goto 1996
- Goto T, Yoshida Y, Kiso M, Nagashima H. Simultaneous analysis of individual catechins and caffeine in green tea. Journal of Chromatography 1996;749:295‐9. [DOI: 10.1016/0021-9673(96)00456-6; EMBASE: 1996337480] [DOI] [Google Scholar]
Gregersen 2009
- Gregersen NT, Bitz C, Krog‐Mikkelsen I, Hels O, Kovacs EM, Rycroft JA, et al. Effect of moderate intakes of different tea catechins and caffeine on acute measures of energy metabolism under sedentary conditions. British Journal of Nutrition 2009;102(8):1187‐94. [DOI: 10.1017/S0007114509371779; PUBMED: 19445822] [DOI] [PubMed] [Google Scholar]
Gregory 2011
- Gregory P (editor). Natural Medicines Comprehensive Database: green tea. www.naturaldatabase.com. (accessed 14 November 2012).
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI: 10.1002/sim.1186; PUBMED: 12111919] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [DOI: 10.1136/bmj.327.7414.557; PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jurgens 2009
- Jurgens T, Whelan AM, MacDonald M, Lord L. Development and evaluation of an instrument for the critical appraisal of randomised controlled trials of natural products. BMC Complementary and Alternative Medicine 2009;9:11. [DOI: 10.1186/1472-6882-9-11; PUBMED: 19389240] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kao 2006
- Kao YH, Chang HH, Lee MJ, Chen CL, Kao YH, Chang HH, et al. Tea, obesity, and diabetes. Molecular Nutrition & Food Research 2006;50(2):188‐210. [DOI: 10.1002/mnfr.200500109; PUBMED: 16416476] [DOI] [PubMed] [Google Scholar]
Lau 2006
- Lau J, Ioannidis JPA, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. British Medical Journal 2006;333(7568):597‐600. [DOI: 10.1136/bmj.333.7568.597; PUBMED: 16974018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic and meta‐analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine 2009;6(7):1‐28. [DOI: 10.1371/journal.pmed.1000100; PUBMED: 19621070] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCCAM 2011
- NCCAM. Green tea (Camellia sinensis). nccam.nih.gov/health/greentea. (accessed 14 November 2012).
NHPD 2009
- Natural Health Products Directorate. Compendium of Monographs: green tea, 2009. www.hc‐sc.gc.ca/dhp‐mps/prodnatur/applications/licen‐prod/monograph/index‐eng.php. (accessed 14 November 2012).
Phung 2010
- Phung OJ, Baker WL, Matthews LJ, Lanosa M, Thorne A, Coleman CI. Effect of green tea catechins with or without caffeine on anthropometric measures: a systematic review and meta‐analysis. American Journal of Clinical Nutrition 2010;91(1):73‐81. [DOI: 10.3945/ajcn.2009.28157; PUBMED: 19906797] [DOI] [PubMed] [Google Scholar]
Rains 2011
- Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: a mechanistic review. Journal of Nutritional Biochemistry 2011;22:1‐7. [DOI: 10.1016/j.jnutbio.2010.06.006; EMBASE: 2010677882; PUBMED: 21115335] [DOI] [PubMed] [Google Scholar]
Reinbach 2009
- Reinbach HC, Smeets A, Martinussen T, Moller P, Westerterp‐Plantenga MS. Effects of capsaicin, green tea and CH‐19 sweet pepper on appetite and energy intakes in human in negative and positive energy balance. Clinical Nutrition 2009;28(3):260‐5. [DOI: 10.1016/j.clnu.2009.01.010; PUBMED: 19345452] [DOI] [PubMed] [Google Scholar]
Sang 2011
- Sang S, Lambert JD, Ho CT, Yang CS. The chemistry and biotransformation of tea constituents. Pharmacological Research 2011;64(2):87‐99. [DOI: 10.1016/j.phrs.2011.02.007; EMBASE: 2011346809; PUBMED: 21371557] [DOI] [PubMed] [Google Scholar]
Seeram 2006
- Seeram NP, Henning SM, Niu Y, Lee R, Scheuller HS, Heber D. Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. Journal of Agricultural Food Chemistry 2006;54:1599‐1603. [DOI: 10.1021/jf052857r; PUBMED: 16506807] [DOI] [PubMed] [Google Scholar]
Sloan‐Kettering 2009
- Memorial Sloan‐Kettering Cancer Center. Green tea. www.mskcc.org/cancer‐care/integrative‐medicine/about‐herbs. (accessed 14 November 2012).
Sterne 2001
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care; Meta‐analysis in Context. London: BMJ Publishing Group, 2001:189‐208. [Google Scholar]
Sterne 2011
- Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta‐analyses of randomised controlled trials. BMJ 2011;343:d4002. [DOI] [PubMed] [Google Scholar]
WHO 2011
- World Health Organization. Obesity and overweight, March 2011. http://www.who.int/mediacentre/factsheets/fs311/en/. (accessed 14 November 2012).
Wing 2011
- Wing RR, Lang W, Wadden TA, Safford M, Knowler WC, Bertoni AG, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011;34:1481‐6. [PUBMED: PMID: 21593294] [DOI] [PMC free article] [PubMed] [Google Scholar]


