Abstract
Background
Acute myocardial infarction (AMI) is the most important cause of morbidity from ischaemic heart disease, and is among the leading causes of death in the western world. Danshen, a Chinese herbal medicine, is widely used in China for treatment of several diseases, including AMI.
Objectives
To assess the effects (both benefits and harms) of danshen preparations for AMI.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library (issue 4, 2006), MEDLINE (1966‐2006), EMBASE (1980‐2006), and the Chinese Biomedical Database (CBM) (1982‐2006). We also handsearched 75 Chinese medical journals.
Selection criteria
Randomised controlled trials (RCTs) lasting at least 7 days were sought. Since it seemed evident that few RCTs were available, we also considered other controlled studies.
Data collection and analysis
Eligibility and trial quality were assessed by three reviewers.
Main results
Six studies comprised of 2368 participants were included. Only one trial was judged to be a genuine RCT and showed no statistically significant difference in reduction of total mortality (Peto OR 0.55, 95% CI 0.23 to 1.32), but a quasi‐RCT reported a reduced total mortality (Peto OR 0.42, 95% CI 0.23 to 0.77). Pooling these trials yielded an approximate halving of mortality in those patients treated with danshen preparations plus usual care compared with usual care alone (Peto OR 0.46, 95% CI 0.28 to 0.75).
Authors' conclusions
The evidence to support use of danshen preparations is too weak to make any judgement about its effects. Evidence from RCTs is insufficient and of low quality. The safety of danshen preparations is unproven, although some adverse events have been reported. More evidence from high quality trials is needed to support the clinical use of danshen preparations.
Plain language summary
Danshen (Chinese medicinal herb) preparations for acute myocardial infarction
Danshen ‐ a Chinese herbal treatment ‐ is widely used in China in addition to usual western forms of therapy in the treatment of acute myocardial infarction (AMI). However there is no strong evidence to support its use, and few rigorous studies have been conducted. Well designed and conducted randomised controlled trials are needed to provide adequate evidence of its role in the treatment of AMI.
Background
Description of the condition Acute myocardial infarction (AMI) is due to the formation of an area of necrosis in heart muscle caused by inadequate supply of blood to the muscle, usually as a result of occlusion of a coronary artery (Burton 1996). After approximately 3 to 6 hours the area of necrotic cardiac muscle reaches its largest size. AMI can lead to death from complications due to cardiogenic shock, cardiac perforation ‐ external or interventricular, embolism, heart failure, papillary muscle rupture, rhythm disturbances or autoimmune pericarditis (MVS 2002). AMI is the most common cause of morbidity from ischaemic heart disease and is the leading cause of death in the western world. Each year approximately 800,000 to 1.5 million people in the United States experience AMI, and about 213,000 of them die (MVS 2002, Herlitz 1989, NHLBI 1992). Accurate data on mortality and incidence of AMI in China are not available. Mortality from myocardial infarction varies according to the patient's sex and age. Younger women with AMI have higher 30‐day case fatality than men (e.g. < 55 years, women 6.5% versus 4.8% men, P < 0.0001), and men are more likely to die before hospitalisation (McCann 2001). Currently myocardial infarction can be accurately diagnosed on the basis of clinical history and electrocardiographic (ECG) evidence. If the ECG evidence is unclear then alterations in cardiac enzymes, such as troponin, are used to aid diagnosis. Other cardiac enzymes besides troponins, are used in helping with the diagnosis, e.g. creatine kinase (CK) and CK‐MB. Patients are usually admitted to hospital for acute treatment and monitoring, and the certainty of diagnosis of AMI is increased if cardiac enzyme levels show a rise over time. Typically, troponin levels are used with ECG evidence and clinical symptoms to make a diagnosis of AMI (Brooks 2001).
Treatments for the condition Drug therapy for AMI includes thrombolytic therapy, aspirin for its antiplatelet properties and morphine for pain relief as needed. In addition to pharmacotherapy aimed at improving outcomes, supportive actions including admission to a cardiac care unit, 24˜36 hours of bed‐rest, continuous ECG monitoring, and oxygen therapy are widely used. In some centres, percutaneous transcoronary angioplasty may be used as primary therapy (MVS 2002).
Danshen compound Danshen, a Chinese herbal medicine, is widely used in China for treatment of several diseases, including AMI. The main component of danshen compound is the roots of the danshen plant (Latin name Salvia miltiorrhiza). Three other plants are typically also included with it, Crataegus laevigata (hawthorn), Coleus forskohlii (an Indian herb) and Valeriana officinalis (valerian). Various formaulations are shown in Table 1. Typically, danshen is used as one ingredient together with other herbs and water which is then boiled for several hours resulting in a herbal soup, which can be administered as an injection.
1. Content of danshen compound formulations in included studies.
| Formulation | Contents | Method of using |
| Kangxingen Heji | Danshen, Cishao, Yujin, Huangqi, Dangshen, Huangjing | Oral intake while intravenous drip both Yiqi injection and Huoxue injection |
| Yiqi injection | Huangqi, Dangshen, Huangjing | Intravenous drip with Huoxue injection |
| Huoxue injection | Cishao, Danshen, Yujin | Intravenous drip with Yiqi injection |
| Fufang Danshen injection | Danshen, Jiangxiang | Intravenous drip |
| Huangqi injection | Huangqi | Intravenous drip |
| Shenmai injection | Hongshen, Maidong | intravenous drip |
| Quyu huatan xiezhuo fang | Jioudahuang 6‐10g, Quangualou 10‐15g, Jioubai 10g, Zhike 10g, Yujin 10g | Oral the water decoction, two times a day in one week |
| Yiqi Huoxue fang | Huangqi 10‐20g, Dangshen 10‐15g, Danshen 15‐20g, Cishao 10‐15g, Honghua 6‐10g, Chenpi 6‐10g | Oral the water decoction, two times a day in one week |
Salvia miltiorrhiza promotes blood circulation and relieves blood stasis (Bensky 1987). It also increases the activity of superoxide dismutase in platelets and inhibits platelet aggregation, thus providing protection against pulmonary embolism. Other effects include cholesterol lowering, reduction of endothelial damage, inhibition of lipid peroxidation, and inhibition of noradrenaline induced contraction of the aortic strips by reducing calcium ion mobilisation (Negard 1996; Wang 1996; Wu 1998). Of the other components, Crataegus laevigata lowers blood pressure but can lead to arrhythmia in the ischaemic myocardium (Garjani 2000); Valeriana officinalis is used as a sedative, lowers blood pressure and relieves angiospasm (BHMA 1983); and Coleus forskohlii inhibits platelet activation, increases the force of contraction of heart muscle and lowers blood pressure. The actions of Crataegus laevigata are thought to be enhanced by Salvia miltiorrhiza and Coleus forskohlii. The relaxing and stress reducing actions of Valeriana officinalis are thought to further enhance the overall effect of danshen compound.
Danshen compound has several functions: 1. dilation of the coronary arteries, increasing coronary arterial blood flow (Sang 1979; Zhen 1997); 2. increasing the deformability of white blood cell and reducing their adherence, thus improving the flow of blood (Liu 2006a) and protecting the cardiac muscle from ischaemia (Zhou 1991); and 3. constituent herbs act together to improve peripheral circulation and general well being. In addition, other herbs are sometimes used with danshen compound, for example huangqi (Table 1).
Table 2 provides a glossary of some of the traditional Chinese herbs mentioned in throughout this review.
2. Names of the herbs in three languages of included studies.
| Pingying | English | Latin |
| Danshen | Danshen Root | Radix Salviae Miltiorrhizae |
| Chishao | Red Paeony Root | Radix Paeoniae Rubra |
| Yujin | Turmeric Root‐tuber | Radix Curcumae |
| Huangqi | Mongolian Milkcetch Root | Radix Astragali |
| Dangshen | Pilose Asiabell Root | Radix Codonopsis |
| Huangjing | Manyflower Solomonseal Rhizome | Rhizoma Polygonati |
| Jiangxiang | Rosewood Heart Wood | Lignum Dalbergiae Odoriferae |
| Hongshen | Ginseng | Radix Ginseng |
| Maidong | Dwarf Lilyturf Tuber | Radix Ophiopogonis |
| Jioudahuang | Rhubarb | Radix et Rhizoma Rhei |
| Quangualou | Snakegourd Fruit | Fructus Trichosanthis |
| Xiebai | Longstamen Onion Bulb | Bulbus Allii Macrostemi |
| Zhiqiao | Bitter Orange | Fructus Aurantii |
| Yujin | Turmeric Root‐tuber | Radix Curcumae |
| Chenpi | Tangerine Peel | Pericarpium Citri Reticulatae |
Reported side effects of danshen We identified 840 reports about the side effects of danshen compound which include anaphylaxis and anaphylactic shock, stomach discomfort, loss of appetite, pruritus, low blood pressure, dizziness, headache and an increase in the risk of bleeding especially with excessive use. Bleeding may occur in the skin or mucous membranes or menorrhagia may occur. Increased in serum aminotransferase has also been reported (He 2006; Lu 2006; Zhen 1997; Xu 2006).
These herbs may also affect the action of other pharmacological agents:Salvia miltiorrhiza may exaggerate the anticoagulant effect of warfarin (Chan 2001) and Crataegus laevigata may act synergistically with digitalis, cardiac glycosides and hypotensive drugs. The dosage of medical drugs may need to be modified if used concurrently.
Up to now literally thousands of studies about the effects of danshen compound for AMI have been published, and many purport to show markedly beneficial effects of treatment. This is reflected in the fact that various danshen preparations have been widely used clinically. However, the quality of these studies and the results of clinical trials have not been systematically reviewed and assessed. Accordingly, this review aims to summarise the existing evidence concerning the comparative effects and safety of danshen preparations.
Objectives
To assess the effects (harms and benefits) of danshen compound preparations for AMI.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled studies lasting at least 7 days were sought. In the event that few RCTs were available, it was our intention to also consider other controlled studies.
Types of participants
Participants were adult men and women of any age or ethnic origin with AMI. Diagnosis of AMI was defined as the presence of unequivocal ECG changes and/or unequivocal enzyme changes. The medical history could be typical or atypical.
Types of interventions
Danshen compound defined as preparations containing danshen, in various forms (e.g. injection, tablet, decoction) to be compared with:
standard or usual care for acute myocardial infarction;
usual pharmacotherapy;
placebo; or
other Chinese herbs.
We also included those trials in which danshen preparations were used as control.
Types of outcome measures
Primary outcome measure 1. Mortality: death from AMI within 30 days.
Secondary outcome measures 1. Deterioration or improvement in symptoms of angina (such as chest pain, breathlessness, reduction in strength, etc). 2. Recurrent myocardial infarction. 3. Quality of life. 4. Readmission to hospital or use of revascularisation. Adverse event outcome measures Any adverse event as a result of treatment, including:
death;
life‐threatening reactions;
toxic responses;
anaphylaxis; or
anything that resulted in discontinuation of treatment.
Search methods for identification of studies
Electronic searches Searches were conducted to identify all published and unpublished randomised controlled trials (RCTs). Studies in any language were sought. Where necessary, non‐English‐language papers were translated so that they could be fully assessed for potential inclusion in the review.
Trials were identified by searching the Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library (Issue 4, 2006), MEDLINE (1966 to Dec., 2006), EMBASE (1980 ‐ Dec., 2006) and the Chinese Biomedical Base, CBM (1982 ‐ Dec., 2006). Search terms below were used for CENTRAL and adapted appropriately for other databases:
#1 DANSHEN #2 (DAN next SHEN) #3 SALVIA* #4 SAGE #5 (((#1 or #2) or #3) or #4) #6 MYOCARDIAL‐INFARCTION*:ME #7 (MYOCARDIAL next INFARCT*) #8 (ACUTE next CORONARY) #9 (UNSTABLE near ANGINA) #10 (HEART next ATTACK) #11 (CORONARY near DISEASE) #12 MYOCARDIAL‐ISCHEMIA*:ME #13 AMI #14 (((((((#6 or #7) or #8) or #9) or #10) or #11) or #12) or #13) #15 (#5 and #14)
Handsearches We also searched the reference lists of relevant trials and identified reviews, and handsearched 75 Chinese traditional medicine Journals (listed in Table 3)
3. Chinese medical journals handsearched.
| Acta Chinese Medicine and Pharmacology Beijing Journal of Traditional Chinese Medicine China Journal of Chinese Materia Medica China Journal of Basic Medicine in Traditional Chinese Medicine Chinese Journal of Integrated Traditional and Western Medicine Chinese Journal of Integrated Traditional and Western Medicine in Intensive and Critical Care Chinese Journal of Traditional Medical Science and Technology Chinese Journal of Traditional & Western Medicine Chinese Traditional Patent Medicine Chinese Traditional Patent Medicine Research Chinese Traditional Herbal Drags Chinese Pharmaceutical Abstracts Clinical Journal of Anhui Traditional Chinese Medicine Forum on Traditional Chinese Medicine Fujian Journal of Traditional Chinese Medicine Guang Ming Zhong Yi Journal of Traditional Chinese Medicine Gansu Journal of Traditional Chinese Medicine Guangxi Journal of Traditional Chinese Medicine Guangdong Journal of Traditional Chinese Medicine Hebei Integrated Traditional and Western Medicine Hebei Journal of Traditional Chinese Medicine Heilongjang Journal of Traditional Chinese Medicine Henan Journal of Traditional Chinese Medicine and Pharmacy Henan Journal of Traditional Chinese Medicine Hunan Journal of Traditional Chinese Medicine Information on Traditional Chinese Medicine Jiangxi Journal of Traditional Chinese Medicine Jiangshu Journal of Traditional Chinese Medicine Jilin Journal of Traditional Chinese Medicine Journal of Anhui College of Traditional Chinese Medicine Journal of Beijing University of Traditional Chinese Medicine Journal of Chengdu University of Traditional Chinese Medicine Journal of Chinese Medicinal Materials Journal of Emergency in Traditional Chinese Medicine Journal of Guangzhou University of Traditional Chinese Medicine Journal of HeNan College of Traditional Chinese Medicine Journal of Integrated Traditional and Western Medicine Journal of Practical Traditional Chinese Medicine Journal of Practical Chinese Traditional Internal Medicine Journal of Sichuan of Traditional Chinese Medicine Journal of Traditional Chinese Medicine Journal of Emergency Syndromes in Chinese Medicine Journal of Nanjing college of Traditional Chinese Medicine Journal of Hubei college of Traditional Chinese Medicine Journal of Guiyang college of Traditional Chinese Medicine Journal of Gansu college of Traditional Chinese Medicine Journal of Changchun college of Traditional Chinese Medicine Journal of Yunnan college of Traditional Chinese Medicine Journal of Tianjin college of Traditional Chinese Medicine Journal of Liaoning college of Traditional Chinese Medicine Journal of Hujian college of Traditional Chinese Medicine Journal of Jiangxi college of Traditional Chinese Medicine Journal of Shandong college of Traditional Chinese Medicine Journal of Hebei college of Traditional Chinese Medicine Journal of Zhejiang college of Traditional Chinese Medicine Journal of Shanghai University of Traditional Chinese Medicine Journal of the Traditional Chinese Medicine Journal of University of Traditional Chinese Medicine Liaoning Journal of Traditional Chinese Medicine Modern Journal of Integrated Chinese Traditional and Western Medicine Modern Traditional Chinese Medicine Neimongol Journal of Traditional Chinese Medicine New Journal of Traditional Chinese Medicine Pharmacology and Clinics of Chinese Materia Medica Research of Traditional Chinese Medicine Shanxi Journal of Traditional Chinese Medicine Shanxi Journal of Traditional Chinese Medicine Shandong Journal of Traditional Chinese Medicine Shanghai Journal of Traditional Chinese Medicine Shenzhen Journal of Integrated Traditional and Western Medicine Tianjin Journal of Traditional Chinese Medicine Traditional Chinese Medicine Research Xinjiang Journal of Traditional Chinese Medicine Yunnan Journal of Traditional Chinese Medicine and Materia Medica Zhejiang Journal of Traditional Chinese Medicine |
The International Standard Randomised Controlled Trial Number Register (ISRCTN) and ClinicalTrial.gov were also searched for ongoing trials.
Data collection and analysis
Trials selection TW and NJ scanned the results of the search relevant records were identified. Full articles for all potentially relevant trials were retrieved. Since all the publications were in Chinese, TW and NJ were able independently to interview by telephone many of the original authors to identify what method was used to generate the allocation sequence. TW selected the trials for inclusion. All identified reports were scrutinised to check for multiple publications of the same trials.
Quality assessment of trials We assessed the methodological quality of each trial in terms of generation of allocation sequence, allocation concealment, blinding, and loss to follow up. For each trial, we classified each quality component as 'adequate', 'inadequate', 'unclear' or 'not used' according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2006). After including all eligible studies in the primary analysis, we conducted sensitivity analyses for each of the quality factors using the subgroups adequate, inadequate, or unclear. There were no recorded disagreements between the three authors' assessments.
Data extraction We independently extracted data. A data abstraction form was specifically designed for this review, and included the following items:
General information: published/unpublished, title, authors, reference/source, contact address, country, urban/rural etc., language of publication, year of publication, duplicate publications, sponsor, setting.
Trial characteristics: design, duration of follow up, method of randomisation, allocation concealment, blinding (patients, people administering treatment, outcome assessors).
Intervention(s): intervention(s) (dose, route, timing), comparison intervention(s) (dose, route, timing), co‐medication(s) (dose, route, timing).
Patients: inclusion and exclusion criteria, total number, and number in comparison groups, age, baseline characteristics, diagnostic criteria, similarity of groups at baseline (including any co‐morbidity), assessment of compliance, withdrawals/losses to follow up (reasons/description), subgroups.
Outcomes: outcomes specified above, any other outcomes assessed, other events, length of follow up, quality of reporting of outcomes.
Results: for outcomes and times of assessment (including a measure of variation), where necessary these were converted to measures of effect specified below, and intention‐to‐treat analysis applied.
We also obtained more information e.g. on method of randomisation, allocation concealment from the original authors by telephone interview.
Data analysis We analysed mortality using Review Manager (version 4.2.9) to perform a limited pooled analyses for two studies (Chen 1984a and Chen 1984b), because no heterogeneity was detected we used a fixed‐effect model to pool the data. We conducted subgroup analysis based on gender, prospective versus retrospective studies, and different interventions, respectively, we did not combine data due to only one study included in these subgroups. The effects were presented as Peto odds ratio (Peto OR) together with 95% confidence intervals.
Results
Description of studies
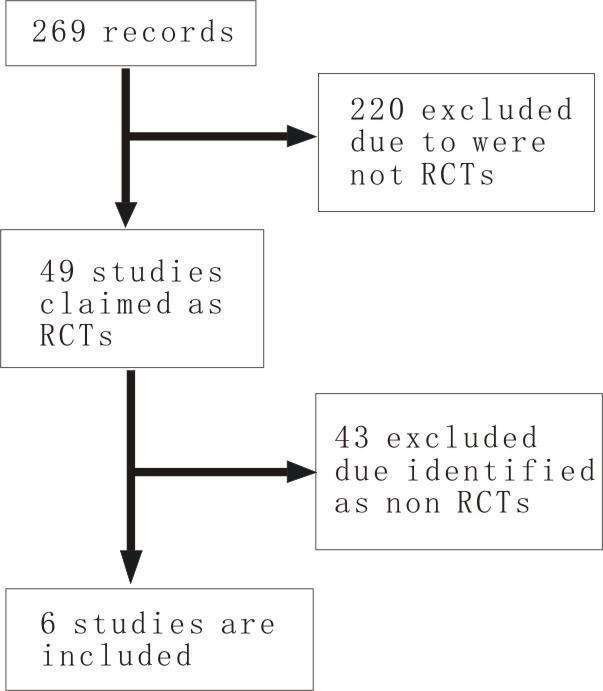
Studies identified The initial search yielded 269 records. After scanning the title and abstract, 49 of these appeared to be comparative studies of danshen preparations.
Excluded studies Of the 49 published articles initially identified, 19 articles were excluded as they were not randomised controlled comparisons (Chen 1996; Cui 2004; Dong 2002; Han 1992; Li 2003; Liang 2001; Liu 1998; Liu 2006b; Luo 2001; Sun 1992; Sun 1997; Wang 1995; Wang 2000; Xu 2003; Yang 1997; Yuan 2003; Zhang 1999; Zhou 1996; Zhu 2000), seven articles were excluded as the outcome of interest was not reported (Cui 1983; Liang 1999, Shi 2000, Wang 2003; Wu 2005; Zhang 2001, Zhong 2000); four studies were excluded one of which had an unclear description of interventions in the text (CYG 1981) and the remaining three were multiple versions of that study (Chen 1987; Chen 1988; GAM 1984); eight studies* were excluded as their aims of studies were assessment of other remedies for which danshen preparations served as control (Cha 1998; Chen 2004; He 2000; Li 1995; Tang 1999; Wang 1999; Xie 2001; Ye 2006), and five studies were excluded because they studied other remedies combined with danshen preparations (Deng 1998; Liang 2000; Qian 1996; Xu 2001; Zhou 1998). Details of excluded studies are given in the table of excluded studies.
*In this group, six authors (Cha 1998; Chen 2004; He 2000; Li 1995; Wang 1999; Ye 2006) were interviewed by telephone and were also excluded because the authors misunderstood the concept of randomisation. Included studies Only six studies (Chen 1984a; Chen 1984b; Chen 2001; Li 2004; Wang 1994; Zhang 1996) were included in the review (Figure 1).
1.
Chen 1984a was considered to be a quasi‐RCT due to the fact that allocation was performed by odd or even number of admission order. Nonetheless it was our decision to include this trial as it represents the first clinical trial of danshen compounds for AMI supported by the Beijing government, and was carried out in several leading institutions. We included Chen 1984a rather than CYG 1981 because we noted that they were conducted concurrently in the same hospital and have the same project name, the sole differences being the authors' name and sample size, with CYG 1981 reporting 276 cases and Chen 1984a reporting 430 cases. From these findings we surmised that CYG 1981 was included in Chen 1984a.
Chen 2001 was included as an RCT although there are still some uncertanties, for example, the authors were unable to remember the randomisation method, the paper was published 3 years after the study closed, and there were no usable data except the number of death. The reason for including this paper is pricipally to focus on discussing the shortcomings of trials of traditional Chinese medicine. Only Chen 1984b was considered an RCT (dependent on the description provided in the paper as "strictly random allocation") and as such was one of the most important studies of Danshen for AMI. We did not include other claimed RCTs or quasi‐RCTs for the reason of poor methodology.
Although Wang 1994 was not an RCT but rather a retrospective study, it was included because the randomisation to fufang danshen injection for treating AMI was felt to provide potentially important information, and the study was conducted in leading institutions under the support of the Shanghai government and with Huashan Hospital of Shanghai First University of Medical Sciences as a leader of this multicentre study. Patients were also treated in a standardised fashion and were monitored carefully, suggesting that the study might provide particularly worthwhile information. Li 2004 and Zhang 1996 were included becuase they appeared (from the information provided) to be RCTs; unfortunately we were not able to contact the authors by telephone to clarify this issue. All of the studies were published in Chinese.
A total of 2368 participants are included in the review. Numbers of participants in the individual studies ranged from 68 (Zhang 1996) to 1350 (Wang 1994).
All of patients had been diagnosed with AMI by using the WHO 1979 diagnosis criteria. Only one study (Chen 1984a) mentioned the traditional Chinese medicine (TCM) sign 'zheng' ‐ 232 patients were recognised as 'qixu xueyu zheng' and some were 'yingxu xueyu' and 'xueyu'. However no numerical information was provided. Interventions of included studies Two studies (Chen 1984a and Chen 1984b) used the same interventions, where danshen was the principle ingredient of a 'kangxingeng heji' decoction and 'yiqi huoxue' injection. Within the first 6 hours of enrolment, AMI patients were treated with a 10 mL 'yiqi huoxue' injection together with a 250 mL infusion of 5% glucose infusion given by intravenous drip, which 1 week later was changed to taking 'kangxingeng heji' orally.
Both the 'quyu huatan xiezhu' and 'yiqi huoxue' formulations in the study by Chen 2001 contain the basic elements danshen, chishao and honghua.
Li 2004 compared huangqi injection with danshen injection versus placebo, and shenmai injection with danshen injection versus placebo, respectively.
Wang 1994 used intravenous danshen injection. Zhang 1996 used isosorbide dinitrate in the experimental group, and isosorbide dinitrate plus danshen injection in the control group.
See the Characteristics of included studies table for additional details.
Outcome measures of included studies All studies reported mortality. Chen 2001 listed frequency of angina pectoris, change of lung moist rales, and change of arrhythmia as outcomes, but there were no data that could be used for analysis. Zhang 1996 reported on improvement of heart function, but the data was unusable.
Risk of bias in included studies
All of the six included trials were of low quality (see Characteristics of included studies table). Randomisation Although all of the studies mentioned random allocation of participants, overall reporting of methods was poor. In Chen 1984a it is mentioned that the patients with odd numbers were allocated to use 'yiqi huoxue' injection and to take 'kangxingeng heji' decoction, and the study was therefore considered as quasi‐randomised. The authors of Chen 2001 could not recall what method of randomisation was used. In Chen 1984b it is stated that investigators "collected the strictly randomly allocated patients' data for analysis", and the study was therefore considered to be a valid RCT. The description in Wang 1994 states that "patients were randomly allocated to danshen or control depending on whether they used danshen or not", and was considered to be a retrospective study. For Li 2004 and Zhang 1996 attempts to contact the authors were unsuccessful. None of the studies concealed allocation.
Blinding None of the studies used blinding.
Description of withdrawals and losses to follow up and intention‐to‐treat analysis None of the included studies mentioned drop‐outs or performed an intention‐to‐treat analysis.
Compliance assessment None of the studies mentioned any methods of ensuring or assessing compliance. Similarity of comparison groups at baseline Comparisons of groups at baseline was mentioned in all of the included studies, and included age, sex, degree of heart dysfunction, infarct lacations, and disease duration, at time of entry into the study. However in some studies baselines were dissimilar, for example in Chen 1984a there were 10 patients with the cardiogenic shock in the danshen group and 16 in the control group. In addition, 87 patients in the danshen group and 63 in the control group had heart failure.
Effects of interventions
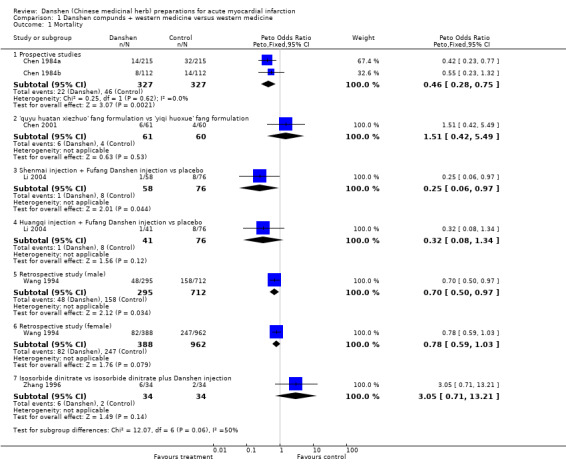
Mortality The results for mortality are shown in comparison 01. The quasi‐randomised study reported in Chen 1984a demonstrated a significant difference favouring treatment with danshen versus controls (Peto OR 0.42, 95% CI 0.23 to 0.77). The study reported in Chen 1984b, the sole investigation in this review that could confidently be considered an RCT, again showed fewer deaths in the group treated with danshen than the control group (8/112 versus 14/112, respectively), but in this case the difference was not statistically significant (Peto OR 0.55, 95% CI 0.23 to 1.32). However pooled analysis of these two studies using a fixed‐effect model did yield a significant difference favouring danshen preparations (Peto OR 0.46, 95% CI 0.28 to 0.75). Although the number of studies is small and the rigour of conducting the trials somewhat uncertain, the results suggest that danshen therapy may reduce the death rate in AMI compared with conventional western therapy alone. Chen 2001 concluded that the 'quyu huatan xiezhuo' formulation reduced both frequency of angina pectoris and onset of arrhythmia as compared with the 'yiqi huoxue' formulation (P < 0.01, 0.05, respectively), but otherwise the author did not provide any useful data. There was no statistically significant difference between the two groups in mortality, with six deaths in a group of 61 patients in the 'quyu huatan xiezhuo' group, and four deaths among 60 patients in the 'yiqi huoxue' group (Peto OR 1.51, 95% CI 0.42 to 5.49).
The study described in Li 2004 reported a single death in both the shenmai injection plus danshen injection group and the huangqi injection plus danshen groups, with eight deaths in each control group. There was a borderline statistically significant difference in the former (Peto OR 0.25, 95% CI 0.06 to 0.97), but not the latter (Peto OR 0.32, 95% CI 0.08 to 1.34).
In the retrospective study reported in Wang 1994, an analysis of total mortality did not show a significant advantage for danshen (Peto OR 0.78, 95% CI 0.59 to 1.03; data not shown), whereas analysis according to gender showed an advantage for Danshen in males but not females (Peto OR 0.70, 95% CI 0.50 to 0.97 for men; 0.78, 95% CI 0.59 to 1.03 for women). However these values lie very close to one another and are of doubtful clinical significance. The study reported in Zhang 1996 cites the number of deaths in the study groups in the results section, although mortality was not listed as an outcome. Deaths in the isosorbide dinitrate plus danshen group numbered six of 24 patients, and in the isosorbide dinitrate group two of 24 patients, a non‐significant difference (Peto OR 3.05, 95% CI 0.71 to 13.21).
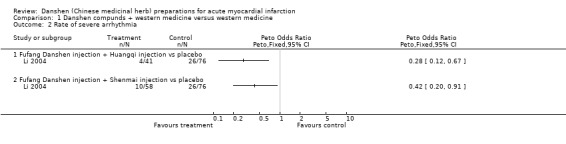
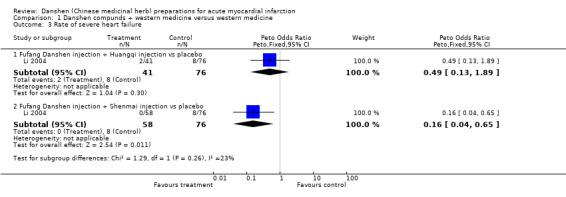
In summary, this group of studies, which are often questionable in both design and interpretation, also collectively do not offer strong support for the use of danshen in treating AMI. Rate of severe arrhythmia The study reported in Li 2004 indicated that the use of both fufang danshen injection plus huangqi injection and fufang danshen injection plus shenmai injection significantly reduced the rate of severe arrhythmia (Peto OR 0.28, 95% CI 0.12 to 0.67, and Peto OR 0.42, 95% CI 0.20 to 0.91, respectively). Rate of heart failure The study reported in Li 2004 indicated that the use of fufang danshen injection plus huangqi injection failed to reduce the rate of heart failure (Peto OR 0.49, 95% CI 0.13 to 1.89), whereas use of fufang danshen injection with shenmai injection did show a significant advantage (Peto OR 0.16, 95% CI 0.04 to 0.65).
Discussion
We found that studies of danshen preparations for AMI were inadequate to provide reliable estimates on the effects, due to poor study design and methodological quality of the research protocols. The following need to be considered:
1. All of the studies located were Chinese language publications, facilitating our telephone contact with many of the trial authors about the method of randomisation they used. Although a large number of the trials claimed to be RCTs, we found that few of the authors had described the concept of randomisation correctly. In addition to this, some of the studies were conducted several years prior to publication, and the trial authors in some instances seemed unfamiliar with the details of the methodology they employed. Even in some leading institutions such as those connected with the studies reported in Chen 1984a and Wang 1994, the description of the randomisation process sometimes appeared flawed. None of the trials used concealment of allocation. These two limitations may have resulted in a high risk of selection bias.
2. None of studies mentioned ethical issues or whether the participants gave informed consent.
3. We found that in all of the trials using danshen, the comparator treatment or 'basal or routine treatment' was often sub‐optimal with some patients receiving only nitrates, and not low molecular weight heparin or other effective remedies (ICSI 2005). Consequently, danshen may have shown exaggerated beneficial effects in such trials.
4. A large number of the trials used self‐prepared formulation or hospital‐made preparations for treating AMI. Although Chinese herbal medicine as a treatment for diseases and their method of manufacture are widely accepted in China, many of the constituents of the pharmacologically prepared drugs used in trials cannot be clearly specified. This is in marked contrast to pharmacological agents used in western medicine, in which the chemical constituents, their quantities, the percentage of any impurities or contaminants are precisely known and the variation between different production batches is kept within specified limits. Variation between formulations and batches of treatments are inevitable consequences of traditional Chinese medicine, though the Chinese government does specify the acceptable limits of variation. However this variation is a factor that may contribute to any heterogeneity between different study results.
One must accept the fact that the overall treatment concept for traditional Chinese medicine is different from that used in Western medicine. Nonetheless, when a study uses a self‐prepared herbal formulation, the quality of herbs and methods of preparation should be stated in detail, in order to achieve consistent effects.
5. It is noteworthy that in Chen 2001 an attempt was made to explore the effects of different Danshen compounds for AMI patients with different types of the traditional Chinese medicine sign 'zheng'. Sixty‐one AMI patients were in the 'quyu huatan xiezhuo' group, of these nine were 'qixu xueyu zheng', three with both 'qi and yin xu zheng', four with 'tanshi zhuci zheng'and 45 with 'tanyu huzhu zheng' (means both 'tan and yu' stasis and interacted each other). Of 60 in the 'yiqi huoxue' group, six were with "qixu xueyu zheng', three with both 'qi and yin xu zheng', three with 'tanshi zhucu zheng' and 48 with 'tanyu huzhu zheng'. The results appeared to suggest that 'quyu huatan xiezhuo' formulation is superior to 'yiqi huoxue' formulation in improving heart function and heart failure, and reducing angina attack and arrhythmia. This result alludes to that (1) traditional Chinese medicine preparations should be used according to the traditional Chinese medicine signs 'zheng' of patients strictly; and (2) most AMI patients are with 'tanyu huzhu zheng'. Regrettably the authors did not perform a stratification analysis by 'zheng'. There is a need to conduct more and better designed studies to test whether use of danshen preparations for treating AMI is dependent on the traditional Chinese medicine 'zheng' or not.
6. We also noted that if danshen was studied as the experimental drug, it tended to show a better result than the controls, and that conversely when danshen was an agent in the control group, the experimental drug tended to be superior to danshen. For example in Chen 1984a and Chen 1984b as well as in other excluded studies such as Han 1992 and Liang 2001 in which danshen preparations was used as the experimental intervention, positive effects of danshen were noted. In contrast, in Zhang 1996, in which isosorbide dinitrate was used as the experimental drug and the control group was given isosorbide dinitrate plus danshen injection, there was a trend for the former treatment to be superior to the latter treatment, albeit not significantly so. However these somewhat inconsistent results may result at least in part from selection bias or detection bias, and possibly even conflict of interest should be considered.
7. We did not find any studies that reported calculation of sample size requirements. This may explain the conduct of smaller studies with low power to detect clinically important differences. Any future studies should be adequately powered.
Authors' conclusions
Implications for practice.
From the six studies included in this review, it is concluded that, although danshen compound may possibly have beneficial effects on AMI mortality compared to routine treatments, the evidence must be considered inconclusive. This is because of the small number of available studies which were of poor quality. In addition, the safety of danshen preparation is unproven because of the lack of toxicological evidence, although adverse events have only rarely been reported.
Implications for research.
Further research should consider the following points: 1. The design and performance of trials should be greatly improved, and include adequate methods for randomisation. Attention should be given to the generation of allocation sequences, insuring that the person who generated the allocation sequence is not involved in patient recruitment, etc. 2. Reconciling the method of Danshen treatment with the traditional Chinese medicine 'zheng' of AMI patients, assuring that the control traditional Chinese medicine formulation should suit the type of 'zheng', and ensuring that the traditional Chinese medicine outcome is an endpoint outcome rather than simply an intermediate outcome. 3. If an active drug is used as control, its effect should be supported by reliable evidence. 4. Sample size calculations should be made, and should match the power requirement of study type. It should be specified whether the objective is to demonstrate equivalence, non‐inferiority, or superiority.
The reporting of the trials should focus in detail on how to achieve allocation concealment and blinding of participants, investigators and outcome assessors, according to CONSORT for traditional Chinese medicine (Wu 2007) and CONSORT (Moher 2001). In particular the statement should include: 1. Description of the components of the formulations, who provided or prepared (made) the medicine (including tablet, decoction, injection, or other forms of traditional Chinese medicine). 2. If a decoction was used as the intervention, a detailed statement of the quality of herbs, methods of preparation, and means of assuring that blinding is maintained. 3. The traditional Chinese medicine signs should be described and defined in detail, for example, what kinds of 'zheng' of participants were included? What diagnosis standard was used? 4. Assurance that those who evaluate traditional Chinese medicine signs for assessment of effects is done in a manner that preserves blinding. 5. Assurance that the quality assessment of traditional Chinese medicine outcomes is done in a way that preserves blinding.
What's new
| Date | Event | Description |
|---|---|---|
| 4 March 2013 | Review declared as stable | This is a low priority topic for the CHG. Review includes studies published between 1984 and 2004. No known ongoing studies. |
History
Protocol first published: Issue 3, 2003 Review first published: Issue 2, 2008
| Date | Event | Description |
|---|---|---|
| 7 July 2008 | Amended | Converted to new review format. |
Notes
Authors are not able to update the review.
Acknowledgements
This publication was made possible in part by Grant Number 1 R24 AT001293‐01 from the National Center for Complementary and Alternative Medicine (NCCAM). The contents of this review are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, or the National Institutes of Health.
We thank the Cochrane Heart Group editorial board for their kind help for writing this review.
We thank Zhou Likun, Duan Xin, Qiao Jieqi, Chen Xiaoyan, Zhen Jie, Wang Qin for their contribution to the handsearch.
Data and analyses
Comparison 1. Danshen compunds + western medicine versus western medicine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Prospective studies | 2 | 654 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.28, 0.75] |
| 1.2 'quyu huatan xiezhuo' fang formulation vs 'yiqi huoxue' fang formulation | 1 | 121 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.51 [0.42, 5.49] |
| 1.3 Shenmai injection + Fufang Danshen injection vs placebo | 1 | 134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.25 [0.06, 0.97] |
| 1.4 Huangqi injection + Fufang Danshen injection vs placebo | 1 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.32 [0.08, 1.34] |
| 1.5 Retrospective study (male) | 1 | 1007 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.50, 0.97] |
| 1.6 Retrospective study (female) | 1 | 1350 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.59, 1.03] |
| 1.7 Isosorbide dinitrate vs isosorbide dinitrate plus Danshen injection | 1 | 68 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.05 [0.71, 13.21] |
| 2 Rate of severe arrhythmia | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.1 Fufang Danshen injection + Huangqi injection vs placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Fufang Danshen injection + Shenmai injection vs placebo | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Rate of severe heart failure | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Fufang Danshen injection + Huangqi injection vs placebo | 1 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.49 [0.13, 1.89] |
| 3.2 Fufang Danshen injection + Shenmai injection vs placebo | 1 | 134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.16 [0.04, 0.65] |
1.1. Analysis.
Comparison 1 Danshen compunds + western medicine versus western medicine, Outcome 1 Mortality.
1.2. Analysis.
Comparison 1 Danshen compunds + western medicine versus western medicine, Outcome 2 Rate of severe arrhythmia.
1.3. Analysis.
Comparison 1 Danshen compunds + western medicine versus western medicine, Outcome 3 Rate of severe heart failure.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 1984a.
| Methods | "Random allocation" was mentioned, but the method of randomisation unclear. In CYG 1981, the randomisation was described as "patients with odds numbers were allocated to TCM with western medicine group". Hence, we considered it a quasi‐RCT. No blinding, baseline similar (P>0.05) | |
| Participants | 430 participants diagnosed by WHO 1979 criteria of AMI. Treatment group: 215 patients, M/F=150/65, age: 3 patients < 40 years; 45 patients = 41‐50 years; 78 patients = 51‐60 years; 57 patients = 61‐70 years; 32 patients > 70 years. Control group: 215 patients, M/F=133/82, age: 9 patients < 40 years; 54 patients = 41‐50 years; 61 patients = 51‐60 years; 49 patients = 61‐70 years; 42 patients >70 years. TCM signs were used to sort the type of patients. Of these patients, 232 were 'qixu xueyu zheng', some were 'yingxu xueyu' and 'xueyu', but the numbers were not mentioned. | |
| Interventions | 'Yiqi huoxue heji" decoction was taken orally, and water extraction of huangqi, danshen and huangjin named as 'yiqi' injection, water extraction of danshen, cishao and yujin called 'huoxue' injection were intravenous drip in the integrated TCM group. Both integrated TCM (group A) and western medicine (group B) groups were given routine treatment: monitor, isosorbide dinitrate 0.1g, dipyridamole 0.1g 3 times per day. No longer than six hours after enrolment, the patients in integrated TCM group were given both 'yiqi' injection and 'huoxue' injection by intravenous drip, and oral 'yiqi huoxue heji' decoction for 8 weeks. | |
| Outcomes | Mortality: 14 deaths in the integrated TCM and western medicine group during the trial, while 32 patients died in the control group. | |
| Notes | This was the second version of CYG 1981. This was the first ever study of danshen in the treatment of AMI. We decided to include this trial because of its position on the danshen therapeutic research for AMI although its randomisation may not be exactly correct that can be judged from its baseline not similar, for example, 10 patients complicated with the cardiogenic shock in the A group and 16 in the B group at the begining of treatment; 87 patients in the A group and 63 in control group complicated with heart failure; etc. In the other versions of this study, for example, Chen 1987 and Chen 1988, the method of randomisation was described as that "the odd number patients" were allocated to integrade TCM and western group, and the even numbers were allocated to the western medicine group. The number of patients in two groups were exactly equel: 215/215. So, this study was not considered as a RCT but a quasi‐RCT, and there was a high potential risk of selection bias. | |
Chen 1984b.
| Methods | It was described that the participants with AMI where allocated into two arms by critical randomisation. But the method was not mentioned. No blinding. | |
| Participants | 224 AMI patients diagnosed by WHO criteria and no longer than 72 hours after onset were included and equally allocated to two groups. Of these patients, M/F 159/65; 121 patients older than 60 years old; 168 patients with infarction at anterior or/and antesepterior or/and inferior myocardial infarction; 119 patients complicated with hypertension, some with cerebral disease or/and diabetes. There was no any significant difference between two groups on baseline. | |
| Interventions | 'Yiqi huoxue' injection was the water abstration of huangqi, danshen, huanjin, chishao, yujin. Routine treatment (western medicine) was given in both two groups, and the 'yiqi huoxue' injection was used by intravenous drip in a does of 10 mL in 250 mL 5% glucose infusion within 6 hours after enrolment. | |
| Outcomes | 1. Mortality: 8 participants died in integrated TCM and western group and 14 in control group; The causes of death also been reported; 2. The number of complications including hypotension, shock happend during treatment in integrated TCM and western medicine group was statistically significant fewer than that in the control group; but no data can be used; 3. the number of heart failure in integrated treatment group during the treatment was significantly fewer than in control group, but no data can be used. | |
| Notes | 1. This was a abstract only, no whole text of the paper can be found; 2. We did not interview the original author, the evaluating was made depends on the report; 3. The authors considered that 'yiqi huoxue zhusheye' can reduces the death rate in the first week of treatment, improved partial patients' prognosis, and may preventing AMI complications including hypotension, shock, heart failure. 4. The western medicine "routine treatment" not been specified in detail. | |
Chen 2001.
| Methods | "Patients were randomly allocated to two groups" was mentioned, but lack of description about the method. The original authors unable to provide the information of randomisation in detail. Parallel design. No blinding. | |
| Participants | All participants were enrolled within 2 days of the AMI onset. 61 in 'quyu huatan xiezhuo' group, M/F 46/15, 38 to 89 years, average 63.7. Of these patients, 9 were 'qixu xueyu zheng', 3 with 'both qi and yin xu zheng', 4 with 'tanshi zhuci zheng' and 45 with 'tanyu huzhu zheng'. 60 in 'yiqi huoxue' group, M/F 43/17, 34 to 90 years old, average 63.8. Of those patients, 6 with 'qixu xueyu zheng', 3 with 'both qi and yin xu zheng', 3 with 'tanshi zhucu zheng' and 48 with 'tanyu huzhu zheng'. Severity of AMI at baseline was similar between groups. | |
| Interventions | The basal treatment was same in two groups. All participants were treated by nitroglycerin and polarisation liquid by intravenous drip, and oral isosorbide dinitrate, captopril, aspirin enteric coated tablets, etc. Expectant treatment was used for those who with complications. In the experimental group, used 'quyu huatan xiezhuo fang'. In the control group, used 'yiqi huoxue fang'. | |
| Outcomes | 1. frequency of angina pectoris; 2. change of lung moist rales; 3. change of arrhythmia. | |
| Notes | 1. Wu TX telephed Professors Chen KY and Shi DZ in 23 Auguest, 2007. They don't remember the method of the randomisation and other information in detail due to the trial was conducted ten years ago. Prof. Chen said the allocation sequence seem was generated by statistician, "a random number table seem was took out from computer." 2. The paper was published in 2001, but the study was conducted during Jan. 1996 to Dec. 1998; 3. The number of death was reported in the 'Result' section although it did not be included in the 'outcomes' of 'Methods' section; 4. There is no any useful data for analysis, the description about the results were extremely simple. | |
Li 2004.
| Methods | "Randomly allocated patients into three groups" was mentioned, but no any description about the method of generation the sequence. TW tried to contact the authors for further information, but they could not be located. Parallel groups. No blinding. | |
| Participants | Total 175 AMI patients were included from 1997 to 2002. 41 participants in group 1, 58 in group 2 and 76 in group 3. Similar in baseline includes age, sex, position of AMI, type of TCM 'zheng'. | |
| Interventions | Same basal treatment for three groups. Group 1 used huangqi injection 20 mL and fufang danshen injection 20 mL intravenous drip twice daily for 2 weeks; Group 2 used shenmai injection 20mL and fufang danshen injection 20mL intravenouse drip twice daily for 2 weeks; Group 3 use basal treatment only (oxgen supply, antipaint, anticoagulation, etc, no description about the drugs in details. | |
| Outcomes | 1. Mortality. 2. Rate of severe arrhythmia. 3. Rate of heart failure. | |
| Notes | 1. Although this study is included in the review, but there are some doubtful points for its reliability of randomisation, for example, the arms were not balanced (41:58:76), no any explanation for this odd rate. 2. 76 patients were included in the control group, but in the Results section, the number was became '68 patients', no any explain for this change. | |
Wang 1994.
| Methods | "Random allocation" was mentioned but the method was not clear. Blinding not used. | |
| Participants | Danshen group included 388 participants, M/F=295/93, average age was 66+/‐9 years; control group included 962 patients, M/F=712/250. Average age was 67+/‐10 years. | |
| Interventions | Danshan group: danshen injection 16˜24 g with 500 mL 5% glucose injection or low molecular dextran by intravenous drip, one time a day, 7˜14 days a course. | |
| Outcomes | Mortality: Danshen group: M 48/295, F 34/93; Control group: M 158/712, F 89/250 | |
| Notes | 1. The description about the randomisation in the original text was not clear: "all of the patients were randomly allocated to danshen group and control group depends on whether they used danshen injection or not". Following a telephone interview with the original author the method was judged inadequate; it's clear that the patients were given danshen injection optionally by the doctors, and the allocation to intervention or control groups followed treatment; 2. Although it was judged as a retrospective study, we still included it as it is an important study in the history of danshen for AMI, and the member hospitals of this study are high level hospitals in Shanghai, included Huashan Hospital and Zhongshan Hospital of Fudan University, Shanghai Cardiavascular Diseases Institute, Shanghai Second University of Medical Sciences Affiliated Ruijin Hospital and Renji Hospital, Xinhua Hospital. There should be some value for reference. | |
Zhang 1996.
| Methods | "Randomly allocated patients to two groups" was mentioned, but lack of description about the method of randomisation. Parallel groups. No blinding. | |
| Participants | Participants with AMI were enrolled after 30 minutes to 6 hourses of AMI onset during 1991 to early 1995. 34 participants in isosorbide dinitrate group, M/F 25/9, 33 to 82 years old (average 64.2+/‐8.55), heart function I grade 29 and II grade 5 cases. 34 participants in control group, M/F 28/6, 40 to 82 years old (average 64.8+10.21), heart function I grade 30 and II grade 4 cases. | |
| Interventions | All participants were kept given strict bed rest. Isosorbide dinitrate was given in both groups. Isosorbide dinitrate group: 20 mg isosorbide dinitrate via intravenous drip 4˜7 hours a day for 2 weeks; after 2 weeks, oral 10 mg, three times a day. Control group: oral isosorbide dinitrate 10 mg, three times a day, fufang danshen 16 mL a day via intravenous drip for 2 weeks. | |
| Outcomes | 1. Heart function, used Killin grade. | |
| Notes | 1. TW tried to contact the original author but did not receive any further information. The impression from this paper is that it is a case records analysis not a trial due to its long duration and poor description. It is included because there is no evidence to demonstrate it is not an RCT since the original authors cannot be found; 2. The original study did not use mortality as the primary outcome, but in the Results, number of death was reported; 3. There was no description about the measure of heart function in detail, the result was reported as percentage only. | |
TCM ‐ traditional Chinese medicine 'zheng' ‐ a TCM sign
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cha 1998 | The aim of this article was to assess the effect of bothropsatrox antithrombusase but not danshen, the latter was used as control. The result was that the bothropsatrox antithrombusase much better than danshen on marked improvement and reducing death. The "randomly allocated the patients" was mentioned, but it was judged as optional allocation. Dr. Cha said that her assistant developed a protocol. For the reason of unbalanced arms (14 versus 8), she explained that most patients were loss of follow‐up. So, the study was judged as a non‐RCT for the reason of 1. only Dr Cha was the sole author, 2. the AMI patients unable to loss of follow‐up in a two weeks treatment duration. The author was interviewed in 18 June, 2007 by number 86‐0755‐26537163. |
| Chen 1987 | The fourth version of CYG 1981. |
| Chen 1988 | The fifth version of CYG 1981. |
| Chen 1996 | "Randomly allocation" mentioned. But in the description about the method of randomisation, the method was described as "those not willing to accept TCM or hypersusceptibility to TCM were allocated to control group". Thus, this study was not considered as RCT. |
| Chen 2004 | The aim of this article was to assess the effect of bothropsatrox antithrombusase but not danshen, the latter was used as control. And the patients included not only AMI (29 patients) but also included 163 patients with myocardial ischemia without AMI. The result was that the bothropsatrox antithrombusase was much better than danshen on marked improvement. |
| Cui 1983 | The outcomes of interest were not reported. |
| Cui 2004 | Randomly allocation was mentioned. But numbers of male and female in two groups were not balanced, in the danshen group, the number of male was 36/46, and in the control group it was 28/46, respectively. It thus be considered as an optional allocation study. |
| CYG 1981 | This was the first clinical study about danshen formulation preparation for AMI. We do not include this article due to it was included in Chen 1984, and the use of intervention did not be described clearly. |
| Deng 1998 | This study aims to assess combined shenmai injection with danshen injection in the treatment of AMI, so, the shenmai was a confounder factor for assessing danshen. Wu TX telephoned Dr. Deng, the first author, in 24 Aug. 2007, he said this paper was wrote when he was a residency ten years ago, and was a retrospective case records analysis. |
| Dong 2002 | Not an RCT |
| GAM 1984 | The third version of CYG 1981. |
| Han 1992 | Not an RCT. |
| He 2000 | The aim of this article was to assess the effect of bothropsatrox antithrombusase but not danshen, the latter was used as control. The result of reducing mortality was the bothropsatrox antithrombusase much better than danshen. The "randomly allocated patients" was mentioned. But it actually was a retrospective study. This was know by telephoning the third author of the paper. Dr. Li Yinjun answered my question and said "we did not develop a protocol previously, we allocated the patients not strictly, just by physician's selection and "time window" of disease. The author was interviewed in 18 June, 2007. |
| Li 1995 | The aim of this article was to assess the effect of bothropsatrox antithrombusase but not danshen, the latter was used as control. The result was that the bothropsatrox antithrombusase was much better than danshen on death from AMI. "Randomly allocated the patients" mentioned, but it was actually a retrospective study. This was known by telephoning the Dr. Li, the original author. He was interviewed in 20 June, 2007. |
| Li 2003 | Not an RCT |
| Liang 1999 | Outcome measures of interest not reported |
| Liang 2000 | This study aims to compare the effect of combined use of urokinase with shenmai injection and danshen injection versus urokinase alone. The interesting thing was that the former was much better than the latter on vessel recanalisation rate, reducing mortality, bleedding complication, and shock, heart failure. Wu TX telephoned Prof. Liang, the first author, in 24 Aug. 2007. Prof. Liang has promoted to as the director of Guangxi TCM Collage office. He said that this was a retrospective paper of case records analysis, no protocol was developed previously. |
| Liang 2001 | "Randomly allocation" was mentioned, but the original auhtor was interviewed by telephone and it was understood that the patients were actually allocated by optional selection. |
| Liu 1998 | "Large sample size, randomised, double blind controlled trial" was mentioned. But the "randomisation" was described as "randomly took drugs", of the 14,962 patients with AMI, 34.8% patients randomly used danshen injection. So, this study was considered a non‐randomised controlled study. |
| Liu 2006b | "Randomly allocation" was mentioned. But it was discovered telephone interview that this was not a real RCT. The patients were allocated based on patients' opinion but not by any random method. |
| Luo 2001 | The randomisation was described as "the patients were randomly allocated by the order of admission". So, it was considered as an optional allocating study. |
| Qian 1996 | This study aims to assess the effect of integrated TCM therapy includes aupuncture and danshen multiple compound decoction and western medicine versus western medicine alone. So, the effect of danshen compound cannot be assessed separately. Wu TX telephoned the second author, Dr. Tian ZZ on 23 Aug. 2007. Dr. Tian said that this was an analysis for case records data and no protocol was developed before study. |
| Shi 2000 | Outcome measures of interest not reported |
| Sun 1992 | The randomisation was described as that the patients were allocated by the order of admission. So, the method was considered as optional allocation. |
| Sun 1997 | The randomisation was descibed as that the patients were allocated by the order of admission. So, it was considered as an optional allocating study rather than RCT. |
| Tang 1999 | This study aims to compare the effect of shenmai injection to danshen injection. The result was that the former is much better than the latter on the reducing mortality and improving heart function, the general improvement rate also showed that the former much higher than the latter. Wu TX telephoned Dr. Tang, the original author, in 27 Aug. 2007. Dr. Tang did not develop a protocol previously for this study, because this was a data analysis for case records only. |
| Wang 1995 | The randomisation was described as that the patients were allocated according to the order of admission. So, it was considered as an optional allocation study ranther than RCT. |
| Wang 1999 | The aim of this article was to assess the effect of bothropsatrox antithrombusase but not danshen, the latter was used as control. The result was that the bothropsatrox antithrombusase was much better than danshen on mortality (rate of death from AMI). "Randomly allocated the patients" mentioned. But some of the patients used danshen injection, others used mailuoning injection in control group. It can be judged very clearly that the study did not well designed. It cannot be included because we cannot devide who was treated by danshen and who was by mailuoning, even if it is an authentic RCT. Taixiang Wu had tried to contact the author, but none of the authors can be found. |
| Wang 2000 | The randomisation was described as that the patients were allocated by the order of admission. Thus, we considered it an optional allocation study rather than a RCT. |
| Wang 2003 | Outcome measures of interest not reported |
| Wu 2005 | The outcomes did not match inclusion criteria of this review. |
| Xie 2001 | The aim of this study was assess for effect of dan ao, the fufang danshen injection was used as control. The effect of dan ao was better than the control. Wu TX telephoned Dr. Xie, the first author of the paper, in 23 Aug. 2007. Dr. Xie said that this was a retrospective study by collecting cases reports during last year. |
| Xu 2001 | This study aims to demonstrate the self‐prepared formulation "Wutou ChiShizhi Tang" decoction combined with Danshen injection. So, the decoction was a confunding factor. Wu TX telephoned Dr. Xu GH in 27 Aug. 2007. Dr. Xu said this was a case records analysis for inpatients, no protocol was developed previously. |
| Xu 2003 | Not an RCT |
| Yang 1997 | The randomisation was described as that the patients were allocated according to order of admission. So, the method was considered as optional allocation. |
| Ye 2006 | The aim of this article was to assess the effect of bothropsatrox antithrombusase but not danshen, the latter was used as control. The result was that the bothropsatrox antithrombusase was much better than danshen on marked improvement and reducing death from AMI. "Randomly allocated the patients" was mentioned. Taixiang Wu telephoned the original author, Director Ye, and understood that this was not a real RCT. The patients were allocated optionally by doctor, or depended on the price of drug, etc. The author sounds misunderstanding the concept of randomisation. |
| Yuan 2003 | Not an RCT |
| Zhang 1999 | The randomisation was described that the patients were allocated according to the order of admission. Thus, it was considered as an optional allocation study. |
| Zhang 2001 | Outcome measures of interest not reported |
| Zhong 2000 | The outcome measures not match the including criteria. |
| Zhou 1996 | The randomisation was described as that the patients were allocated according to the order of admission. Thus, it was considered as an optional allocation study. |
| Zhou 1998 | This study aims to assess the effect of integrated TCM and western medicine versus western medicine alone. TCM included danshen injection, huangqi injection and shexiang baoxin wan tablet. Wu TX telephoned Dr. Zhou. She said that this was a cases records analysis, no protocol was developed before the study. Date of interview is 23 Aug. 2007. |
| Zhu 2000 | Randomisation allocation was mentioned in the text, but it can be judged as a non‐RCT because the patients were allocated to the treatment group based on those who suit for thrombolytic thereapy, and if not, they were allocated to the control group. |
Contributions of authors
Taixiang, Wu Protocol development, searching for trials, quality assessment of trials, data extraction, data analysis, telephone interview the original authors, review development. Juan, Ni Protocol development, searching for trials, quality assessment of trials, data extraction, telephone interview the original author.. Jiafu, Wei Searching for trials, quality assessment of trials, data extraction.
Sources of support
Internal sources
Chinese Cochrane Centre, West China Hospital of Sichuan University, China.
External sources
National Center for Complementary and Alternative Medicine (NCCAM), USA.
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Chen 1984a {published data only}
- Chen DQ, Wong XZ, Zhao LY, Li MQ. Yiqi Huoxue mixture with Western medicine compared to Western medicine alone in the treatment of 430 patients with AMI and an animal research. Journal of Medical Research 1984, (9):20‐1. [Google Scholar]
Chen 1984b {published data only}
- Chen KJ, Qian ZH, Dong QZ, Tu XH, Tao SY, Tao SQ. A randomised controlled trial of Yiqi Huoxue Zhusheyie injection in the treatment of 224 patients with AMI. Chinese Journal of Integrated Traditional Chinese and Western Medicine 1984;4(7):416. [Google Scholar]
Chen 2001 {published data only}
- Chen KY, Wu DL, Chen XL, Wang XF, Shi DZ. A clinical observation for the effect of "Quyu Huatan Xiezhuo" formulation in the treatment of patients with AMI. China Journal of Traditional Chinese Medicine 2001;16(5):34‐6. [Google Scholar]
Li 2004 {published data only}
- Li Y, Chen LX, Liu QQ. Huangqi, Shenmai injection combined with Fufang danshen injection in the treatment of 99 patients with AMI. Journal of Traditional Chinese Medicine 2004;45(1):45. [Google Scholar]
Wang 1994 {published data only}
- Wang SY, Dai RH, Fan WF. The effect of "Dan Shen" injection for acute myocardial infection. Journal of Traditional Chinese Medicine 1994;35(1):29‐30. [Google Scholar]
Zhang 1996 {published data only}
- Zhang Y, Zhang KL. Influence of isosorbide dinitrate for heart function of AMI patients. Qingdao Medical Journal 1996;28(1):12‐4. [Google Scholar]
References to studies excluded from this review
Cha 1998 {published data only}
- Cha YX. Observation for the effect of Bothropsatrox antithrombusase in the treatment of acute myocardial infarction. Jiaotong Medicine 1998;12(1):30. [Google Scholar]
Chen 1987 {published data only}
- Chen DQ, Weng XZ, Zhao LY. Integrated TCM and Western medicine compared to Western medicine alone in the treatment of 233 patients with AMI complicated with arrhythmia. Shanxi TCM 1987;3(2):19‐21. [Google Scholar]
Chen 1988 {published data only}
- Chen DQ. Comparing TCM with Western medicine in the treatment of 181 patients with AMI complicated with heart failure. Journal of Practical Internal TCM 1988;2(1):17‐8. [Google Scholar]
Chen 1996 {published data only}
- Chen HT. Observation for effects of middle and low does urokinase in the treatment of 43 senior patients with AMI. Chinese Journal of Clinical Pharmacology and Therapeutic 1996;1(1):41‐3. [Google Scholar]
Chen 2004 {published data only}
- Chen SQ, Jiang H. Bothropsatrox antithrombusase in the treatment of patients with AMI. Journal of Microcycology 2004;14(3):87 and 90. [Google Scholar]
Cui 1983 {published and unpublished data}
- Cui ZC, Li QL, Yao JN, Wang M, Gu FS, Su CL, et al. The effect of blood serum enzyme, platelet congregate activity and blood rheology in "Kangxingeng Heji" for acute myocardial infarction. Integrate Chinese Traditional and Western Medicine Journal 1983;3(5):268‐9. [PubMed] [Google Scholar]
Cui 2004 {published data only}
- Cui CX, Wei XL. Obervation for the effects of integrated traditional Chinese and western medicine in the treatment of patients with AMI. Liaoning Journal of TCM 2004;31(10):868‐9. [Google Scholar]
CYG 1981 {published data only}
- Beijing Chao‐yang Hospital and TCM Institute Guang‐an Men Hospital coronary heart disease group. The primary observation for comparing of integrated Chinese traditional medicine and Western medicine to western medicine along in the treatment of 276 patients with acute myocardial infection. Chinese Journal of Integrated Traditonal Chinese and Western Medicine 1981;1(1):10‐1. [Google Scholar]
Deng 1998 {published data only}
- Deng XM, Tang AH. Observation for the effect of integrated traditional Chinese and western medicine in the treatment of 36 patients with AMI. Jounal of Guangxi College of TCM 1998;15(2):29‐30. [Google Scholar]
Dong 2002 {published and unpublished data}
- Dong SP, Shen J. The efficacy of "Huangqi", "Dan Shen" injection for acute myocardial infarction. Taishan Waisheng 2002;26(3):36. [Google Scholar]
GAM 1984 {published data only}
- Guang Anmen hospital, Chao Yang hospital, Beijing Red Cross Hospital heart group. "Yiqi Huoxue Heji" for acute myocardial infarction. Journal of Traditional Chinese Medicine 1984, (12):22‐3. [Google Scholar]
Han 1992 {published data only}
- Han GZ. Integrated traditional Chinese medicine with Western medicine in the treatment of 88 patients with acute myocardial infarction. Shanxi TCM 1992;13(4):152‐3. [Google Scholar]
He 2000 {published data only}
- He WG, Fang D, Li YJ, Liang FQ. Observation on the effect of defirase for acute myocardial infarction. Journal of Youjiang Medical College for Nationalities 2000;22(5):715‐6. [Google Scholar]
Li 1995 {published data only}
- Li ZS. Bothropsatrox antithrombusase in the treatment of patients with AMI. Shandon Medicine 1995;35(9):18. [Google Scholar]
Li 2003 {published and unpublished data}
- Li J, Jiang T. The effect of compound Danshen pill in the treatment of patients with acute myocardial infarction left ventrical reconstruction. Tian Jin Pharmacy 2003;15(1):36‐8. [Google Scholar]
Liang 1999 {published and unpublished data}
- Liang TJ, Gao SZ, Wang AW. The effect of blood serum muscle‐calcium protein in "Dan Shen" compound pill for acute myocardial infarction patients. Li Shizhen Medicine and Materia Medica Research 1999;10(2):90. [Google Scholar]
Liang 2000 {published data only}
- Liang J, Li QR, Wang LM. Comparing the effect of thrombolytic combined with TCM in the treatment of 32 patients with AMI. Anthology of Medicine 2000;19(6):868‐9. [Google Scholar]
Liang 2001 {published data only}
- Liang JL, Sun ZX, Qu F. A clinical observation on integrated traditional Chinese and western medicine in the treatment of patients with AMI. Journal of Tianjin College of Traditional Chinese Medicine 2001;20(3):24‐5. [Google Scholar]
Liu 1998 {published data only}
- Liu LS, Wang W, Fang WQ, Pan XW, Huang P, Ma FZ, et al. Effects of captopril combined with other medicines on clinical cardiovascular events among 14962 patients with acute myocardial infarction ‐ Chinese Cardiac Study (CCS‐1) subgroup report. Chinese Journal of Hypertension 1998;6(2):157‐60. [Google Scholar]
Liu 2006b {published data only}
- Liu DF, Liu L. Xiaogentang decoction with western medicine in the treatment of 30 patients with AMI. Shanxi TCM 2006;27(7):786‐7. [Google Scholar]
Luo 2001 {published data only}
- Luo B. Integrate traditional Chinese and western medicine for acute myocardial infarction complicated with heart failure. Hubei Journal of TCM 2001;23(4):13. [Google Scholar]
Qian 1996 {published data only}
- Qian KY, Tian ZZ. Integrated TCM and western medicine in the treatment of 50 patients with AMI. Chinese Journal of Integrated Traditional Chinese and Western Medicine 1996;16(5):308‐9. [Google Scholar]
Shi 2000 {published and unpublished data}
- Shi XY, Xia ZY, Fang JG, Gu JZ, Wu LW, Yu JP. Effects of salvia miltiorrhiza compound injection on serum endothelin, prostaglandin I2/thromboxane A2 atio alteration following myocardial ischemia‐reperfusion in patients undergoing intracardiac surgery. Chinese Journal of Integrate Chinese Traditional and Western Medicine 2000;20(12):896‐8. [PubMed] [Google Scholar]
Sun 1992 {published data only}
- Sun JQ, Wang SK, Cheng JL, Wu ST, Wang XJ, Huang JH. Observation for effect of integrated traditional Chinese medicine with western medicine in the treatment of 37 patients with acute myocardial infection. Acta Chinese Medicine and Pharmacology 1992, (1):34‐5. [Google Scholar]
Sun 1997 {published data only}
- Sun WF, Wang LL, Wang XM, Wang GL, Yin ZY. Integrated traditional Chinese and western medicine for acute myocardial infarction complicated with heart failure. Acta Chinese Medicine and Pharmacology 1997, (2):10. [Google Scholar]
Tang 1999 {published data only}
- Tang CS. Clinical observation on the effect of Shanmai injection in the treatment of 60 patients with AMI. Hainan Medicine 1999;10(6):54‐5. [Google Scholar]
Wang 1995 {published data only}
- Wang JK. Observation for the clinical effect of Danshen compound in the treatment of 51 patients with acute myocardial infarction in an hour. Ge Jiu Science and Technology 1995, (1):18‐21. [Google Scholar]
Wang 1999 {published data only}
- Wang ZF, Shu HQ, Wang LN. Bothropsatrox antithrombusase in the treatment of 80 patients with AMI. Journal of Snake 1999;11(4):63‐4. [Google Scholar]
Wang 2000 {published data only}
- Wang Q, Yin XZ, Zhao RJ, Chang YP, Jiang ZP. Observation for the effects of Shanmai injection combined with Danshen powder injection in the treatment of patients with AMI with conduction block. Chinese Journal of TCM Emergency 2000;9(6):261. [Google Scholar]
Wang 2003 {published and unpublished data}
- Wang YS, Zhang S, Zhang D, Liu ZM. Protective effects of Shenmai and salvia Miltiorrhiza injection on ischemia and reperfusion myocardium in acute myocardial infarction patients. Chin J Crit Care Med 2003;23(12):834‐5. [Google Scholar]
Wu 2005 {published data only}
- Wu ZD, Guo XQ. Point injecting by Danshan injection for the hiccup after AMI. Guangdo Medicine 2005;26(3):407‐8. [Google Scholar]
Xie 2001 {published data only}
- Xie J, Li SQ, Zhao L. A clinical study of Dan Ao in the treatment of patients with AMI. Clinical Medicine 2001;21(10):50‐1. [Google Scholar]
Xu 2001 {published data only}
- Xu GH, Zhang XS, Huang ZX, Liu M. Observation for the effect of "Wutou Cishitang" decoction with "Dan Shen" injection in the treatment of acute myocardial infarction. New Chinese Medicine 2001;33(9):30‐1. [Google Scholar]
Xu 2003 {published and unpublished data}
- Xu XL, Wang BW. The effect of re‐open rate with integrated Chinese traditional and western medicine for acute myocardial infarction. Chinese Journal of Practical Medicine 2003;3(9):831‐2. [Google Scholar]
Yang 1997 {published data only}
- Yang YS, Zhang H, Zhang RJ, Wu XX. Observation for effect of integrated traditional Chinese medicine in the treatment of patients with acute myocardial infection. Tianjin Journal of TCM 1997;14(3):105‐6. [Google Scholar]
Ye 2006 {published data only}
- Ye ZB. Bothropsatrox antithrombusase in the treatment of AMI. Chinese Medical Journal of Metallurgical Industry 2006;23(2):162‐3. [Google Scholar]
Yuan 2003 {published and unpublished data}
- Yuan ZM, Di RA, Zhao WY. "Dan Shen" injection with vienal dissolve embolism therapy for 46 patients with acute myocardial infection. Shanxi TCM 2003;24(2):107‐8. [Google Scholar]
Zhang 1999 {published data only}
- Zhang DJ, Tang BX, Xu NT, Yang RP, Chang BX, Yan JL. Comparing integrated Chinese traditonal medicine to western medicine in 52 patients with acute myocardial infarction. Clinical Report 1999, (5):16‐7. [Google Scholar]
Zhang 2001 {published and unpublished data}
- Zhang YH. The effect of "Dan Shen" compound pill for the QT period disperse degree of acute myocardial infarction. Chinese Journal of Cardiovasc Rehabil Medicine 201;10(4):371‐2. [Google Scholar]
Zhong 2000 {published and unpublished data}
- Zhong JB, Zhang JC, Miao Y, Tu XH, Qian HY, Xu MY. Observation on the therapeutic effect of " Phlegm‐Stasis Eliminating Formula" in the treatment of acute myocardial infarction in early stage. Shanghai Journal of Traditional Chinese Medicine 2000;34(4):26‐7. [ISSN:1007‐1334] [Google Scholar]
Zhou 1996 {published data only}
- Zhou YH. Observation for the effect of polarization fluid combined with Mg, low‐melocule dextran and "Dan Shen" compound injection for acute myocardial infarction. Journal of Emergency in Traditional Chinese and Westen Medicine 1996;3(9):397‐8. [Google Scholar]
Zhou 1998 {published data only}
- Zhou Y, You WQ, Xiou H. Danshen and Huangqi injection in the treatment of 50 patients with AMI complicated with heart function deficiency. Information on Traditional Chinese Medicine 1998, (2):24‐5. [Google Scholar]
Zhu 2000 {published data only}
- Zhu MJ, Shi XQ, Fan HL. Clinical observation for integrated traditional Chinese medicine and Western medicine in the treatment of 58 patients with AMI. Journal of Shanxi TCM 2000;16(4):30‐1. [Google Scholar]
Additional references
Bensky 1987
- Bensky D, Gamble A. Chinese Herbal Medicine: Materia Medica. Seattle: Eastland Press, 1987:384. [Google Scholar]
BHMA 1983
- British Herbal Medicine Association. British Herbal Pharmacopoeia. Bournemouth: BHMA, 1983. [Google Scholar]
Brooks 2001
- Brooks N. Guideline for the management of patients with acute coronary syndromes without persistent ECG ST segment elevation. British Cardiac Society guidelines and Medical Practice Committee and Royal College of Physicians Clinical Effectiveness and Evaluation Unit. Heart 2001;85(2):133‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Burton 1996
- Burton E.Sobel. Acute myocardial infarction. In: Bennet C J, Plum F editor(s). Cecil's Textbook of Medicine. 20th Edition. Philadelphia: WB Saunders, 1996. [o‐7216‐3561‐x] [Google Scholar]
Chan 2001
- Chan TY. Interaction between warfarin and danshen (Salvia miltiorrhiza). Annals of Pharmacotherapy 2001;35(4):501‐4. [DOI] [PubMed] [Google Scholar]
Garjani 2000
- Garjani A, Nazemiyeh H, Maleki N, Valizadeh H. Effects of extracts from flowering tops of Crataegus meyeri A. Pojark. on ischaemic arrhythmias in anaesthetized rats. Phytotherapy Research 2000;14(6):428‐31. [DOI] [PubMed] [Google Scholar]
He 2006
- He Q. Analysis for 25 patients with side effect caused by Fufang Danshen injection. Clinical Medicine 2006;26(12):91. [Google Scholar]
Herlitz 1989
- Herlitz J, Blohm M, Hartford M, Hjalmarsson A, Holmberg S, Karlson BW. Delay time in suspected acute myocardial infarction and the importance of its modification. Clinical Cardiology 1989;12(7):370‐4. [DOI] [PubMed] [Google Scholar]
Higgins 2006
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 4.2.6 [updated September 2006]. www.cochrane.org/resources/handbook/hbook.htm.
ICSI 2005
- Institute for Clinical Systems Improvement (ICSI). Diagnosis and treatment of chest pain and acute coronary syndrome (ACS). http://www.guideline.gov/summary/summary.aspx?doc_id=8362&nbr=4683 [accessed 15 Jan 2006].
Liu 2006a
- Liu Y, Song Y, Li M. Danshen's pharmaceutic function and its clinical use. Chinese Journal of Misdiagnosis 2006;6(24):4732‐3. [Google Scholar]
Lu 2006
- Lu JJ, Chen YH. Two cases of anaphylaxic shock caused by Fufang Danshen injection intravenous drip. Clinical Misdiagnosis & Mistherapy 2006;19(7):90. [Google Scholar]
McCann 2001
- MacIntyre K, Stewart S, Capewell S, Chalmers JW, Pell JP, Boyd J, et al. A population‐based study of 201,114 men and women following a first acute myocardial infarction. Journal of the American College of Cardiology 2001;38(3):729‐35. [DOI] [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D. The CONSORT Statement: Revised Recommendations for Improving the Quality of Reports of Parallel‐Group Randomized Trials. Journal of the American Medical Association 2001;285(15):1987‐91. [DOI] [PubMed] [Google Scholar]
MVS 2002
- MVS. MVS Pathophysiology: Myocardial Infarction. http://sprojects.mmip.mcgill.ca/mvs/PATHOS/MI.HTM#anchor175396 2002.
Negard 1996
- Nagai M, Noguchi M, Iizuka T, Otani K, Kamata K. Vasodilator effects of des(alpha‐carboxy‐3,4‐ dihydroxyphenethyl)lithospermic acid (8‐epiblechnic acid), a derivative of lithospermic acids in salviae miltiorrhizae radix. Biological and Pharmaceutical Bulletin 1996;19:228‐32. [DOI] [PubMed] [Google Scholar]
NHLBI 1992
- National Heart Lung and Blood Institute. Morbidity and Mortality: Chartbook on Cardiovascular, Lung, and Blood Diseases. Bethesda, Md, US: NHLBI, May 1992. [Google Scholar]
Sang 1979
- Sang GW. The investigation of pharmacological effect of Dan Shen and Dan Shen compound. ZheJiang medicine 1979;1(1):25‐9. [Google Scholar]
Wang 1996
- Wang X, Pang J, Shan C, Yang S, Zheng Y. Effect of danshen injection on pulmonary thromboembolism and platelet free radical levels in mice. Zhongguo Zhong Yao Za Zhi 1996;21:558‐60. [PubMed] [Google Scholar]
WHO 1979
- World Health Organization. Nomenclature and criteria for diagnosis of ischemic heart disease. Report of the Joint International Society and Federation of Cardiology/World Health Organization task force on standardization of clinical nomenclature. Circulation 1979;59(3):607‐9. [DOI] [PubMed] [Google Scholar]
Wu 1998
- Wu YJ, Hong CY, Lin SJ, Wu P, Shiao MS. Increase of vitamin E content in LDL and reduction of atherosclerosis in cholesterol‐fed rabbits by a water‐soluble antioxidant‐rich fraction of Salvia miltiorrhiza. Arteriosclerosis, Thrombosis & Vascular Biology 1998;18(3):481‐6. [DOI] [PubMed] [Google Scholar]
Wu 2007
- Wu TX, Li YP, Bian ZX, Li TQ, Li J, Dagenais S, et al. Consolidated Standards for Reporting Trials of Traditional Chinese Medicine (CONSORT for TCM). Journal of Evidence‐Based Medicine 2007;7(8):601‐5. [Google Scholar]
Xu 2006
- Xu MG, Luo YH. Analysis for side effects of 98 patients treated by Fufang Danshen injection. Journal of Hubei Institute for Nationalities. Medical Edition 2006;23(3):1. [Google Scholar]
Zhen 1997
- Zhen HZ, Dong ZH, She J. Danshen. Modern Study of Traditional Chinese Medicine. Vol. 2, Beijing: Xue Yuan Publish House, 1997:1093‐1193. [Google Scholar]
Zhou 1991
- W Zhou. Protective effect of Dan Shen on myocardial ischemia and reperfusion: An isolated rat heart study. American Journal of Chinese Medicine 1990;18:19‐21. [DOI] [PubMed] [Google Scholar]


