Abstract
Background
Interleukin 6 (IL‐6) blocking agents have been used for treating severe coronavirus disease 2019 (COVID‐19). Their immunosuppressive effect might be valuable in patients with COVID‐19 characterised by substantial immune system dysfunction by controlling inflammation and promoting disease tolerance.
Objectives
To assess the effect of IL‐6 blocking agents compared to standard care alone or with placebo on efficacy and safety outcomes in COVID‐19.
We will update this assessment regularly.
Search methods
We searched the World Health Organization (WHO) International Clinical Trials Registry Platform (up to 11 February 2021) and the L‐OVE platform, and Cochrane COVID‐19 Study Register to identify trials up to 26 February 2021.
Selection criteria
We included randomised controlled trials (RCTs) evaluating IL‐6 blocking agents compared with standard care alone or with placebo for people with COVID‐19, regardless of disease severity.
Data collection and analysis
We followed standard Cochrane methodology. The protocol was amended to reduce the number of outcomes considered. Two review authors independently collected data and assessed the risk of bias with the Cochrane Risk of Bias 2 tool. We rated the certainty of evidence with the GRADE approach for the critical outcomes such as clinical improvement (defined as hospital discharge or improvement on the scale used by trialists to evaluate clinical progression or recovery) (day (D) 28 / ≥ D60); WHO Clinical Progression Score of level 7 or above (i.e. the proportion of participants with mechanical ventilation +/‐ additional organ support OR death) (D28 / ≥ D60); all‐cause mortality (D28 / ≥ D60); incidence of any adverse events; and incidence of serious adverse events.
Main results
We identified 10 RCTs with available data including one platform trial comparing tocilizumab and sarilumab with standard of care. These trials evaluated tocilizumab (nine RCTs including two platform trials; seven were reported as peer‐reviewed articles, two as preprints; 6428 randomised participants); and two sarilumab (one platform trial reported as peer reviewed article, one reported as preprint, 880 randomised participants).
All trials included were multicentre trials. They were conducted in Brazil, China, France, Italy, UK, USA, and four were multi‐country trials. The mean age range of participants ranged from 56 to 65 years; 4572 (66.3%) of trial participants were male. Disease severity ranged from mild to critical disease. The reported proportion of participants on oxygen at baseline but not intubated varied from 56% to 100% where reported. Five trials reported the inclusion of intubated patients at baseline.
We identified a further 20 registered RCTs of tocilizumab compared to placebo/standard care (five completed without available results, five terminated without available results, eight ongoing, two not recruiting); 11 RCTs of sarilumab (two completed without results, three terminated without available results, six ongoing); six RCTs of clazakisumab (five ongoing, one not recruiting); two RCTs of olokizumab (one completed, one not recruiting); one of siltuximab (ongoing) and one RCT of levilimab (completed without available results). Of note, three were cancelled (2 tocilizumab, 1 clazakisumab). One multiple‐arm RCT evaluated both tocilizumab and sarilumab compared to standard of care, one three‐arm RCT evaluated tocilizumab and siltuximab compared to standard of care and consequently they appear in each respective comparison.
Tocilizumab versus standard care alone or with placebo
a. Effectiveness of tocilizumab for patients with COVID‐19
Tocilizumab probably results in little or no increase in the outcome of clinical improvement at D28 (RR 1.06, 95% CI 1.00 to 1.13; I2 = 40.9%; 7 RCTs, 5585 participants; absolute effect: 31 more with clinical improvement per 1000 (from 0 fewer to 67 more); moderate‐certainty evidence). However, we cannot exclude that some subgroups of patients could benefit from the treatment. We did not obtain data for longer‐term follow‐up (≥ D60).
The effect of tocilizumab on the proportion of participants with a WHO Clinical Progression Score of level of 7 or above is uncertain at D28 (RR 0.99, 95% CI 0.56 to 1.74; I2 = 64.4%; 3 RCTs, 712 participants; low‐certainty evidence). We did not obtain data for longer‐term follow‐up (≥ D60).
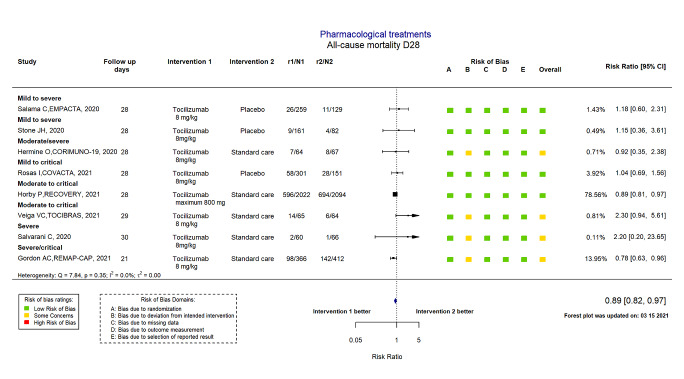
Tocilizumab reduces all‐cause mortality at D28 compared to standard care alone or placebo (RR 0.89, 95% CI 0.82 to 0.97; I2 = 0.0%; 8 RCTs, 6363 participants; absolute effect: 32 fewer deaths per 1000 (from 52 fewer to 9 fewer); high‐certainty evidence). There is uncertainty around the effect on mortality at ≥ D60 (RR 0.86, 95% CI 0.53 to 1.40; I2 = 0.0%; 2 RCTs, 519 participants; low‐certainty evidence).
b. Safety of tocilizumab for patients with COVID‐19
The evidence is very uncertain about the effect of tocilizumab on adverse events (RR 1.23, 95% CI 0.87 to 1.72; I2 = 86.4%; 7 RCTs, 1534 participants; very low‐certainty evidence). Nevertheless, tocilizumab probably results in slightly fewer serious adverse events than standard care alone or placebo (RR 0.89, 95% CI 0.75 to 1.06; I2 = 0.0%; 8 RCTs, 2312 participants; moderate‐certainty evidence).
Sarilumab versus standard care alone or with placebo
The evidence is uncertain about the effect of sarilumab on all‐cause mortality at D28 (RR 0.77, 95% CI 0.43 to 1.36; 2 RCTs, 880 participants; low certainty), on all‐cause mortality at ≥ D60 (RR 1.00, 95% CI 0.50 to 2.0; 1 RCT, 420 participants; low certainty), and serious adverse events (RR 1.17, 95% CI 0.77 to 1.77; 2 RCTs, 880 participants; low certainty). It is unlikely that sarilumab results in an important increase of adverse events (RR 1.05, 95% CI 0.88 to 1.25; 1 RCT, 420 participants; moderate certainty). However, an increase cannot be excluded
No data were available for other critical outcomes.
Authors' conclusions
On average, tocilizumab reduces all‐cause mortality at D28 compared to standard care alone or placebo and probably results in slightly fewer serious adverse events than standard care alone or placebo. Nevertheless, tocilizumab probably results in little or no increase in the outcome clinical improvement (defined as hospital discharge or improvement measured by trialist‐defined scales) at D28. The impact of tocilizumab on other outcomes is uncertain or very uncertain. With the data available, we were not able to explore heterogeneity. Individual patient data meta‐analyses are needed to be able to identify which patients are more likely to benefit from this treatment.
Evidence for an effect of sarilumab is uncertain and evidence for other anti‐IL6 agents is unavailable.
Thirty‐nine RCTs of IL‐6 blocking agents with no results are currently registered, of which nine are completed and seven trials were terminated with no results available. The findings of this review will be updated as new data are made available on the COVID‐NMA platform (covid-nma.com).
Plain language summary
Can medicines that block interleukin‐6 (a protein involved in immune responses) treat COVID‐19?
Key messages
Treating COVID‐19 with tocilizumab (a medicine that blocks interleukin‐6) reduces the numbers of people who die within 28 days of treatment, and probably results in fewer serious unwanted effects than placebo treatment.
Studies of other medicines that block interleukin‐6 to treat COVID‐19 are under way. We will update this review when results from them become available.
COVID‐19
COVID‐19 is an infectious respiratory disease caused by a type of virus called a coronavirus. If the infection becomes severe, people may need intensive care and support in hospital, including machines to help them breathe (mechanical ventilation). Medicines that are currently used to treat other diseases are being tested in the search to find effective treatments for COVID‐19.
Blocking interleukin‐6
An immune response is how the body recognises and defends itself against harmful substances, such as viruses. COVID‐19 can disrupt the immune system, causing it to over‐react and produce dangerously high levels of inflammation. Interleukin‐6 (IL‐6) is a protein involved in triggering inflammation. Blocking the production of interleukin‐6 could reduce inflammation and help the immune system to fight COVID‐19.
Why we did this Cochrane Review
Tocilizumab and sarilumab are two medicines that block interleukin‐6. They are used to treat other conditions that involve an "over‐reactive" immune system, such as rheumatoid arthritis. We wanted to find out if medicines that block interleukin‐6 can be used to treat COVID‐19, and whether they might cause any unwanted effects.
What did we do?
We searched for studies that tested if medicines that block interleukin‐6 could treat COVID‐19.
We looked for randomised controlled studies, in which the treatments people received were decided by chance. This type of study usually gives the most reliable evidence about the effects of a treatment.
Search date: we searched for trials up to 26 February 2021.
What we found
We found 10 studies in 6896 people with COVID‐19. The average age of people in the studies was 56 to 65 years, and 66% of the people enrolled were men. The studies took place in Brazil, China, France, Italy, the UK and the USA; four studies took place in more than one country. Three studies were funded by pharmaceutical companies.
The medicines tested were tocilizumab and sarilumab. Both medicines were compared against a placebo (a dummy treatment that appears identical to the medicine being tested but without any active medicine) or standard care. The results were measured 28 days after treatment and after 60 days or more.
We also found 41 more studies of medicines blocking interleukin‐6 to treat COVID‐19 that had not yet published any results. These included 20 studies of tocilizumab, 11 studies of sarilumab and 10 studies of other medicines. Some of those studies are still ongoing and we will update this review to include their results when published.
What are the main results of our review?
Compared with placebo treatment or standard treatment, treatment with tocilizumab:
· reduces the number of people who died, of any cause, after 28 days (evidence from 6363 people in 8 studies); on average, 32 fewer people per 1000 died when treated with tocilizumab plus standard care, compared with standard care alone or placebo.
· probably makes little or no difference to clinical improvement (which is defined as leaving hospital or improvement in COVID‐19 symptoms) at 28 days (evidence from 5585 people in 7 studies).
· probably reduces slightly the number of serious unwanted effects, such as life‐threatening conditions or death (evidence from 2312 people in 8 studies).
We are uncertain about the effects of tocilizumab treatment on:
‐ severity of COVID‐19; that is, how many patients died of COVID‐19 or needed a ventilator or additional organ support at 28 days (evidence from 712 people in 3 studies); or
‐ how many patients died, of any cause, after 60 days or more (evidence from 519 people in 2 studies).
No results were reported for tocilizumab after 60 days or more for improvement, or severity at 28 days of COVID‐19.
We are uncertain about how sarilumab treatment affected the:
‐ numbers of people who died (of any cause) at 28 days (evidence from 880 people in 2 studies) and after 60 days (evidence from 420 people in 1 study); or
‐ the numbers of serious unwanted effects, such as life‐threatening conditions or death (evidence from 880 people in 2 studies).
‐ Sarilumab probably does not cause more unwanted effects (of any type) than placebo treatment (evidence from 420 people in 1 study). No other results for sarilumab treatment were reported.
We were not able to explore which COVID‐19 patients are more likely to benefit from this treatment.
Our confidence in our results
We are confident that tocilizumab reduced the number of deaths (from any cause) at 28 days. Our confidence in the other results for tocilizumab is moderate to low; further evidence may change our results. Our confidence in the results for sarilumab is low; further evidence is likely to change these results. Our confidence was lowered because some of the studies did not report all their results.
Summary of findings
Background
Description of the condition
In December 2019, a novel coronavirus outbreak began in Wuhan, Hubei Province, China. Infection with this severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) spread rapidly. The World Health Organization (WHO) declared the coronavirus 19 (COVID‐19) disease a pandemic on 11 March 2020 (WHO 2020a). The COVID‐19 prevalence has increased exponentially in almost all countries during the first and subsequent waves (Worldometer 2020). The clinical spectrum of SARS‐CoV‐2 pneumonia ranges from mild to severe and critical manifestations. Approximately 15% of patients with SARS‐CoV‐2 infection develop severe COVID‐19 pneumonia (Guan 2020). Enormous efforts are focused on finding treatments to reduce the need of invasive mechanical ventilation and/or the risk of death in these patients.
Some authors have proposed that patients at high risk of COVID‐19 may experience a “cytokine storm”; a complex milieu of immune mis‐firing characterised by an early interferonopathy followed by hypercytokinaemia with high inflammatory markers and low reparative growth factors (Bastard 2020; Galani 2020; Lucus 2020; Mehta 2020; Pedersen 2020). In this milieu, Interleukin 6 (IL‐6) stands out as a particularly important biomarker (Chen 2020; Herold 2020; Laguna‐Goya 2020; Stukas 2020). IL‐6 levels or C‐reactive protein (CRP), a marker of IL‐6 driven inflammation, are associated with the severity of the disease (Caricchio 2020; Galvan‐Roman 2021; Knight 2020; Manson 2020; Webb 2020).
Description of the intervention
IL‐6 blocking agents are a class of therapeutic agents directed against the IL‐6 peptide or receptor. Available IL‐6 blocking agents are classified as anti‐IL‐6 receptor monoclonal antibodies (e.g. sarilumab, tocilizumab, levilimab) or anti‐IL‐6 monoclonal antibodies (siltuximab, olokizumab, clazakizumab).
How the intervention might work
IL‐6 blockers are beneficial in some hyperinflammatory diseases, such as rheumatoid arthritis (Scott 2017), giant cell arteritis (Stone 2017), and cytokine release syndrome induced by chimeric antigen receptor T‐cell therapy (Kotch 2019). SARS‐CoV‐2 infection induces a dose‐ and time‐dependent production of cytokines, including IL‐6 (Kang 2020).
The immunosuppressive effect of IL‐6 blockers might be valuable in patients with COVID‐19 who are characterised by substantial immune system dysfunction by controlling inflammation and promoting disease tolerance (Campochiaro 2020).
Why it is important to do this review
Given the urgent need for an effective treatment for COVID‐19 globally, patients have been treated with several costly immune‐modulating compounds including JAK (Janus kinase) inhibitors (Cao 2020; Kalil 2021), and specific cytokine blockers (Guaraldi 2020). The main immunomodulatory therapies that have been explored are JAK inhibition (broad suppression of inflammatory cytokines) and targeted inhibition of IL‐1 and IL‐6 (CORIMUNO‐19 Collaborative group 2021). Policymakers, scientific experts and the public need high‐quality, up‐to‐date evidence evaluating the effectiveness and safety of IL‐6 blocking agents for treating COVID‐19. This is a high‐priority question, for which the existing evidence is inconclusive (Solis‐García Del Pozo 2020). A living systematic review is an optimal approach to track and assess the effectiveness of IL‐6 blocking agents use in patients with COVID‐19.
This evidence synthesis will be updated weekly on the COVID‐NMA platform (covid-nma.com). This published Cochrane Review will be updated when new evidence emerges with potential to change the certainty of the evidence or the review authors’ conclusions, or at least every six months if new evidence is available. The process of the living systematic review is described in Appendix 1.
Objectives
To assess the effects of IL‐6 blocking agents compared with standard care alone or with placebo on effectiveness and safety outcomes in patients with COVID‐19.
This review is part of a larger project: the COVID‐NMA project (Boutron 2020a). The COVID‐NMA project provides decision‐makers with a complete, high‐quality and up‐to‐date mapping and synthesis of evidence on interventions for preventing and treating COVID‐19. We developed a master protocol on the effect of all interventions for preventing and treating COVID‐19 (Boutron 2020b). Our results are made available and updated weekly on the COVID‐NMA platform at covid-nma.com.
This living review focuses on SARS‐CoV‐2 and does not consider studies evaluating treatment with IL‐6 blocking agents for other coronavirus infections affecting humans.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) of any design with no restrictions on language. The following trial designs were eligible for inclusion: parallel group, cluster, cross‐over and factorial. Early‐phase clinical trials, single‐arm trials, non‐randomised studies and modeling studies of interventions for COVID‐19 were excluded as were prognosis studies, systematic reviews and meta‐analyses, and diagnostic test accuracy studies.
The protocol of this review is available on PROSPERO (CRD42020214700).
Types of participants
We included trials evaluating children or adults with suspected, probable or confirmed ambulatory or hospitalised COVID‐19 (see classification in Appendix 2; (WHO 2020b)).
Types of interventions
We included the following IL‐6 blocking agents with no restriction on dose, frequency, or mode of administration.
Tocilizumab (humanised monoclonal antibody against the IL‐6 receptor)
Sarilumab (human monoclonal antibody against the IL‐6 receptor)
Clazakizumab (humanised rabbit monoclonal antibody against IL‐6)
Olokizumab (humanised monoclonal antibody against IL‐6)
Siltuximab (chimeric monoclonal antibody against IL‐6)
Levilimab (human monoclonal antibody against the IL‐6 receptor)
Comparator(s)
We considered the following types of comparators in this review.
Standard care alone or with placebo.
Standard of care as defined by trialists.
Types of outcome measures
Our outcome selection was based on the CORE outcome sets developed by the WHO (WHO Working Group 2020), and advice from content experts.
We predefined the following critical and important outcome measures.
Critical outcomes
The following outcomes with related time points reported as days (D) of follow‐up were considered:
clinical improvement (D28 / ≥ D60) defined as a hospital discharge or improvement on the scale used by trialists to evaluate clinical progression and recovery. We recorded the scale and the threshold used by authors to define improvement as appropriate;
WHO Clinical Progression Score of level 7 or above (i.e. mechanical ventilation +/‐ additional organ support (extra corporeal membrane oxygenation (ECMO), vasopressors or dialysis) OR death (D28 / ≥ D60);
all‐cause mortality (D28 / ≥ D60);
We reported all assessments performed at D60 and later under ≥ D60
Safety outcomes
Incidence of any adverse events (AEs)
Incidence of serious AEs (SAEs)
For each time point, we considered time of randomization as D0. However, if not reported, we considered D0 as reported by the authors.
When outcomes are assessed at time points other than those selected by the review, we chose the closest (e.g. D15 for D28).
Important outcomes
Time to clinical improvement
Time to WHO Clinical Progression Score of level 7 or above
Time to death
We present all critical and important outcomes in Table 1; Table 2.
Summary of findings 1. Tociliuzumab compared to standard care/placebo for mild/moderate/severe/critical COVID‐19.
| Tociliuzumab compared to standard care/placebo for mild/moderate/severe/critical COVID‐19 | ||||||
|
Patient or population: participants with mild/moderate/severe/critical COVID‐19 Settings: Brazil, China, France, Italy, UK, USA Intervention: tociliuzumab Comparison: standard care/placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with standard care/placebo | Risk with tocilizumab | |||||
| Clinical improvement D28 | 515 per 1000 |
545 per 1000 (515 to 581) |
RR 1.06 (1.00 to 1.13) |
5585 (7 RCTs) | ⊕⊕⊕⊝ moderate1 | Data at D ≥ 60 was not available Clinical improvement was defined variably as an improvement from baseline in > 2 categories on a 7‐category ordinal scale (2 studies); a decrease of at least 2 points on an ordinal clinical improvement scale (1 study); or hospital discharge or ready to discharge (7 studies) |
|
WHO progression score (level 7 or above) D28 |
262 per 1000 |
260 per 1000 (147 to 457) |
RR 0.99 (0.56 to 1.74) |
712 (3 RCTs) | ⊕⊕⊝⊝ low2,3 | Data at D ≥ 60 was not available |
| All‐cause mortality D28 | 291 per 1000 |
259 per 1000 (239 to 283) |
RR 0.89 (0.82 to 0.97) |
6363 (8 RCTs | ⊕⊕⊕⊕ high4 | |
| All‐cause mortality D60 | 133 per 1000 |
114 per 1000 (70 to 186) |
RR 0.86 (0.53 to 1.40) |
519 (2 RCTs) | ⊕⊕⊝⊝ low5,6 | |
| Adverse events | 457 per 1000 |
562 per 1000 (397 to 786) |
RR 1.23 (0.87 to 1.72) |
1534 (7 RCTs) | ⊕⊝⊝⊝ very low7,8,9 | |
| Serious adverse events | 149 per 1000 |
132 per 1000 (111 to 157) |
RR 0.89 (0.75 to 1.06) |
2312 (8 RCTs) | ⊕⊕⊕⊝ moderate7 | |
|
Time to clinical improvement 28 to 90 days follow‐up |
High |
HR 1.23 (1.08 to 1.39) |
2118 (6 RCTs) | ⊕⊕⊕⊝ moderate1, 13 | ||
| 889 per 1000 |
933 per 1000 (917 to 957 |
|||||
|
Time to WHO progression score (level 7 and above) 28 to 90 days follow‐up |
Low |
HR 0.62 (0.42 to 0.91) |
762 (3 RCTs) | ⊕⊕⊕⊝ moderate10,11, 13 | ||
| 123 per 1000 |
78 per 1000 (54 to 113) |
|||||
|
Time to death follow‐up 28 to 90 days |
Low |
HR 0.65 (0.51 to 0.83) |
1152 (3 RCTs) | ⊕⊕⊝⊝ low2, 12, 13 | ||
| 37 per 1000 |
24 per 1000 (19 to 31) |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; HR: Hazard Ratio: WHO: World Health Organization | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Risk of bias downgraded by 1 level: some concerns due to deviation from intended interventions and outcome measurement
2 Risk of bias downgraded by 1 level: some concerns due to deviations from intended interventions
3 Imprecision downgraded by 1 level: due to wide confidence interval consistent with the possibility for benefit and the possibility for harm
4 Despite some concerns due to deviation from intended interventions, risk of bias was not downgraded because the studies at risk contributed < 20% weight to the effect estimate.
5 Despite some concerns due to deviation from intended intervention in 1 study, risk of bias was not downgraded because this study contributed only 30% weight to the effect estimate.
6 Imprecision downgraded by 2 levels: due to low number of events and a wide confidence interval consistent with the possibility for benefit and the possibility for harm
7 Risk of bias downgraded by 1 level: some concerns regarding randomisation, deviations from intended interventions, outcome measurement and selection of reported result
8 Inconsistency downgraded by 1 level: I² = 86.4%
9 Imprecision downgraded by 1 level: due to a wide confidence interval consistent with the possibility for no effect and the possibility for harm
10 Despite some concerns due to deviation from intended intervention in 2 studies, risk of bias was not downgraded.
11 Imprecision downgraded by 1 level: due to low number of events and a wide confidence interval consistent with the possibility for benefit and the possibility for little or no effect
12 Imprecision downgraded by 1 level: due to low number of events and participants
13 Control group risk at 28 days from Stone 2020
Summary of findings 2. Sarilumab compared to standard care for severe/critical COVID‐19.
| Sarilumab compared to standard care for severe/critical COVID‐19 | ||||||
|
Patient or population: participants with severe/critical COVID‐19 Settings: Brazil, China, France, Italy, UK, USA Intervention: sarilumab Comparison: standard care | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Clinical improvement D28 | ‐ | ‐ | ‐ | ‐ | ‐ | Outcome of interest not reported |
|
WHO progression score (level 7 or above) D28 |
‐ | ‐ | ‐ | ‐ | ‐ | Outcome of interest not reported |
| All‐cause mortality D28 | 299 per 1000 |
230 per 1000 (129 to 407) |
RR 0.77 (0.43 to 1.36) |
880 (2 RCTs) | ⊕⊕⊝⊝ low1,2 | |
| All‐cause mortality D60 or above | 105 per 1000 |
105 per 1000 (52 to 209) |
RR 1.0 (0.5 to 2.0) |
420 (1 RCT) | ⊕⊕⊝⊝ low2, 3 | |
| Adverse events | 640 per 1000 |
672 per 1000 (563 to 799) |
RR 1.05 (0.88 to 1.25) |
420 (1 RCT) | ⊕⊕⊕⊝ moderate4,5 | |
| Serious adverse events | 62 per 1000 |
73 per 1000 (48 to 110) |
RR 1.17 (0.77 to 1.77) |
880 (2 RCTs) | ⊕⊕⊝⊝ low2,4 | |
|
Time to clinical improvement follow‐up 90 days |
Moderate |
HR 1.28 (0.88 to 1.87) |
880 (2 RCTs) | ⊕⊕⊝⊝ low6,7,9 | Clinical improvement defined as hospital discharge | |
| 460 per 1000 |
546 per 1000 (419 to 684) |
|||||
|
Time to WHO progression score (level 7 and above) |
‐ | ‐ | ‐ | ‐ | ‐ | Outcome of interest not reported |
|
Time to death follow‐up 90 days |
Moderate |
HR 0.55 (0.33 to 0.91) |
460 (1 RCT) | ⊕⊕⊝⊝ low1, 5,8,9 | ||
| 330 per 1.000 |
198 per 1000 (124 to 305) |
|||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; HR: Hazard Ratio, WHO:World Health Organization | ||||||
|
GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Despite some concerns due to deviation from intended interventions, we did not downgrade for risk of bias
2 Imprecision downgraded by 2 levels: due to wide confidence interval consistent with the possibility for benefit and the possibility for harm and few events
3 Despite some concerns due to selection of the reported result, we did not downgrade for risk of bias
4 We presume that the adverse event rates and the corresponding relative risks, are similar across diverse settings; therefore not downgraded for indirectness.
5 Imprecision downgraded by 1 level: few events
6 Risk of bias downgraded by 1 level: some concerns due to deviations from intended intervention and outcome measurement
7 Imprecision downgraded by 1 level: wide confidence interval consistent with the possibility for benefit and the possibility for no effect
8 Indirectness downgraded by 1 level: single multicentre study only from high‐income countries, therefore results in this population might not be generalisable to other settings
9 Control group risk taken from Gordon REMAP‐CAP 2021 at 30 days
Search methods for identification of studies
The search relies on the search for the COVID‐NMA initiative (Boutron 2020a; Boutron 2020b)
The initial search strategy was developed with an information specialist from the Cochrane Editorial & Methods Department (Robin Featherstone).
We conducted an evaluation of two secondary sources the L‐OVE platform and the Cochrane COVID‐19 Study Register. We found that searching both secondary sources allowed identifying 100% of the reports of RCTs (preprint or peer‐reviewed publication) assessing treatment or preventive interventions for COVID‐19 (see Appendix 3). We updated our search 7 September 2020, and now only search the L‐OVE platform, the Cochrane COVID‐19 Study Register, the Retraction Watch Database and all other resources listed below. The last search date was 26 February 2021.
Electronic searches
We searched the following databases.
The L‐OVE platform (https://app.iloveevidence.com/covid19), every working day since 7 September 2020 (last search February 26, 2021).
The Cochrane COVID‐19 Study Register (https://covid-19.cochrane.org/), every working day since 7 September 2020 (last search February 26, 2021).
· PubMed every working day up to 7 September 2020.
MedRχiv (https://www.medrxiv.org). This is a free online archive and distribution server for complete but unpublished manuscripts (preprints) in the medical, clinical, and related health sciences. A curated list of records for COVID‐19 and SARS‐CoV‐2 is available at https://connect.biorxiv.org/relate/content/181. Note that this list also includes sources listed in bioRχiv, but we only screened the sources published on MedRχiv. We searched this archive every working day from 1 March 2020 to 7 September 2020.
CNKI (China National Knowledge infrastructure, https://www.cnki.net/), database and (http://journal.yiigle.com/). We searched on 17 April 2020.
Chinaχivhttp://chinaxiv.org/. This is a free online archive and distribution server for complete but unpublished manuscripts (preprints) in Chinese. We searched every working day from 1 March 2020 to 7 September 2020.
LitCOVID (https://www.ncbi.nlm.nih.gov/research/coronavirus/), is a curated database that tracks scientific evidence on COVID‐19 published in PubMed. The hub is updated daily and studies are categorised by domain (e.g. “transmission” or “treatment” (https://www.nature.com/articles/d41586-020-00694-1). We screened studies listed under “treatment” from 1 March 2020 to 1 June 2020. We decided to stop searching LitCOVID because it did not identify any trials that were not already identified in the primary source.
WHO database of publications on coronavirus disease (COVID‐19) (https://www.who.int/emergencies/diseases/novel-coronavirus-2019/global-research-on-novel-coronavirus-2019-ncov), from 1 March 2020 to 28 August 2020. We decided to stop searching these secondary sources because they did not identify any trials that were not already identified in the primary source.
We screened other sources such as the EPPI‐Centre living map of evidence (http://eppi.ioe.ac.uk/COVID19_MAP/COVID_map_v5.html), and Meta‐evidence, developed by Campbell UK & Ireland (http://meta-evidence.co.uk/), from 1 March 2020 to 28 August 2020. We decided to stop searching these secondary sources because they did not identify any trials that were not already identified in the primary source.
We also searched the Retraction Watch Database for retracted trials (https://retractionwatch.com/retracted-coronavirus-covid-19-papers/), (26 February 2021).
If no peer‐review publication was available, we extracted data from the preprint. We recognise that preprints are not peer reviewed and are living documents that can be updated or published. We developed a preprint tracker in collaboration with a research team from the French National Centre for Scientific Research, which systematically informs us when a preprint is updated or published. As soon as an update was identified, we checked the data for discrepancies against that already extracted and recorded the data not available in the initial report and updated the analysis if needed.
Searching other resources
We searched the following trial registries for unpublished and ongoing trials:
the WHO International Clinical Trials Registry Platform (ICTRP, https://www.who.int/ictrp/en/), to identify ongoing and completed clinical trials on COVID‐19. We used the List By Health Topic: 2019‐nCoV / COVID‐19 filter and retrieved all studies identified. (search 11 February 2021);
we intended to search the European Medicines Agency (EMA) clinical data website (https://clinicaldata.ema.europa.eu/web/cdp/home), to identify trials submitted to the EMA and searched for the Clinical Study Report of eligible trials (search 26 February 2021). However, the website was not accessible. There is currently some discussion between various stakeholders and the EMA to request for publication of clinical reports of COVID‐19 interventions.
we also searched the USFood and Drug Administration (FDA) website to identify FDA approval trials (https://www.fda.gov/emergency-preparedness-and-response/counterterrorism-and-emerging-threats/coronavirus-disease-2019-covid-19), (search 26 February 2021).
Data collection and analysis
As part of the COVID‐NMA living systematic review (Boutron 2020b), we search, screen, and extract data daily. An updated synthesis is reported online at least weekly.
Selection of studies
Two review authors screened all retrieved titles and abstracts for eligibility; all excluded abstracts were screened in duplicate. Two review authors independently screened full‐texts of reports. We resolved discrepancies on exclusion and screening of full texts by consensus between both reviewers or by involving a third reviewer. We recorded reasons for exclusion for all studies excluded after full‐text review.
We use an Excel spreadsheet to document search dates and numbers of citations identified. The screening of records and abstracts was done in duplicate independently using Rayyan (Ouzzani 2016). We resolved discrepancies any disagreements by involving a third reviewer .
Data extraction and management
Two review authors independently read each preprint, peer‐reviewed publication, protocol, or other study reports, evaluated the completeness of the data availability, and assessed the risk of bias. We used a specific structured online data extraction form. All discrepancies automatically identified by the online tool were discussed by both review authors involved in the data extraction to reach consensus.
The information we extracted included study characteristics (such as first author, publication year and journal, funding source), number of participants randomised, patient characteristics (e.g. severity of clinical presentation), comorbidities, cointerventions, intervention details (e.g. dose, schedule), outcome measures, and 'Risk of bias' assessment.
We systematically contacted the trial authors to ask them for supplementary information unavailable from the trial reports. These data were requested by a personalised email sent by the WHO as a partner in the COVID‐NMA project.
Disease severity was classified as described below according to the clinical status or clinical management of patients. This classification relies on existing classification and clinical expertise (WHO 2020c; WHO 2020b). We considered the description of eligibility criteria as well as the baseline characteristics of participants and classified the severity as follows:
mild disease ambulatory: "outpatients" whose clinical symptoms are mild with no sign of pneumonia on imaging;
mild disease: clinical symptoms requiring hospitalization but no need for supplemental oxygen;
moderate disease: fever and respiratory symptoms with radiological findings of pneumonia and requiring standard oxygen therapy O2 (3 to 5 L/min);
-
severe disease: meeting any of the following criteria:
respiratory distress (≧ 30 breaths/min);
oxygen saturation ≤ 93% at rest in ambient air or oxygen saturation ≤ 97% with O2> 5 L/min;
PaO2/FiO2 ≦ 300 mmHg (1 mmHg = 0.133 kPa). PaO2/FiO2 in high‐altitude areas (> 1000 metres above sea level) is corrected by the following formula: PaO2/FiO2 x (atmospheric pressure (mmHg)/760);
patients hospitalised on non‐invasive ventilation (NIV)/high flow nasal oxygen (HFNO);
-
critical disease: cases meeting the following criteria:
respiratory failure requiring invasive mechanical ventilation without or with vasopressor, dialysis, or extracorporeal membrane oxygenation (ECMO).
It is worth mentioning that since the classification of severity class was heterogenous among studies, we reclassified the participant disease severity based on the above severity criteria. Consequently, the severity reported by investigators might differ from the severity reported in this review. For example, Gordon REMAP‐CAP 2021 classified the included participants as critical, yet according to our definition we classified them as severe‐critical (patients who receive non‐invasive ventilation or high flow nasal cannula are considered as severe according to the classification detailed above).
When no data related to these classifications were available, we requested the information from authors.
Assessment of risk of bias in included studies
We assessed the trials using the Cochrane Risk of Bias 2 (RoB 2) tool for RCTs (Sterne 2019).
The Cochrane RoB 2 tool is structured into five domains:
risk of bias arising from the randomization process;
risk of bias due to deviations from intended interventions;
risk of bias due to missing outcome data;
risk of bias in measurement of the outcome;
risk of bias in the selection of the reported result.
A series of “signalling questions” elicit information relevant to 'Risk of bias' assessment within each domain. The response options to the signalling questions are: “yes”; “probably yes”; “probably no”; “no”; and “no information”. A 'Risk of bias' judgement for each domain is generated by an algorithm, based on answers to the signalling questions. Judgement can be “low”, “some concerns” or “high” risk of bias. Overall risk of bias is considered “low” if all domains are at “low risk”; “some concerns” if at least one domain has “some concern” and no domain at “high” risk of bias; and “high” if at least one domain is at “high risk”.
We assessed the risk of bias for all critical and important outcomes listed in the protocol of the living systematic review COVID‐NMA (Boutron 2020b).
In the context of this review, we are interested in quantifying the effect of assignment to the interventions at baseline, regardless of whether the interventions were received as intended (the Intention‐to‐treat (ITT) effect).
The Cochrane Bias Methods Group developed a training material on 'Risk of bias' assessment tool RoB2, which is used by the systematic reviewers participating in data extraction and 'Risk of bias' assessment for the COVID‐NMA platform (available upon request).
We recorded judgements for each domain and time point by using an online data extraction tool.
Two review authors independently assessed the risk of bias of each study. All 'Risk of bias' assessments were done at the outcome level by two independent review authors with consensus in case of disagreement. Review authors had epidemiological training or were members of the Cochrane Response team. They were trained using the material developed by the Cochrane Bias Methods Group. Each review author independently assessed the included manuscripts and used signalling questions for each domain of bias, which was fed into the related algorithm to obtain a judgement. Both review authors recorded their judgement and support for judgement. However, answers to signalling questions were not recorded. For the consensus, all disagreements in judgement were identified, discussed until consensus was achieved. If needed, a third review author was involved.
To ensure standardisation of judgement and justification, the review authors as well as the COVID‐NMA core team revised the assessments/support for judgement.
In the context of the COVID‐19 pandemic, we also standardized our assessment of some domains.
Domain 2. Risk of bias due to deviations from intended interventions.
In trials where participants and carers were not blinded, we specified some deviations that could arise because of the trial context and could affect the trial outcomes.
A. Cross‐over from the control group to the intervention group
When the number of patients in the control receiving the intervention was important, this domain was rated as ‘some concern’.
When the cross‐over was planned in the protocol for participants with clinical worsening, we decided to rate this domain as ‘some concern’ because the decision to provide the treatment could have been influenced by the trial context.
B. Cointerventions
The following cointerventions could affect the trial outcomes:
remdesivir and other antivirals;
corticosteroids;
biologics.
When these cointerventions were reported and balanced, this domain was assessed as ‘low’ risk of bias. When these cointerventions were reported but imbalanced, this domain was rated as ‘some concern’ and not ‘high risk’ of bias as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect.
Domain 2. Analysis to estimate the effect of assignment
Intention‐to‐treat analyses were considered appropriate.
When the analysis was not an ITT analysis the rating of this domain was made on a case‐by‐case basis according to:
the number of participants who crossed over and were not analyzed in the group allocated;
the number of participants excluded from the analysis for reason other than missing data as well as imbalance between arms in terms of number and reasons for exclusion.
Of note, for critical outcomes (i.e. binary outcomes), the analysis evaluated was usually based on our analysis where we considered all participants randomised as the denominator.
Domain 4. Risk of bias in measurement of the outcome
We prespecified the following rules.
Clinical Improvement (D28/ ≥ D60/time to event): assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
WHO clinical progression score level 7 or above (D28/ ≥ D60/time to event): assessment of this outcome is probably not influenced by knowledge of the intervention assignment.
All‐cause mortality (D28/ ≥ D60/time to event): assessment of this outcome is not influenced by knowledge of the intervention assignment.
-
Adverse events and serious adverse event:
when detection of events relies only on measures that cannot be influenced by judgement (e.g., laboratory detected events): assessment of this outcome is probably not influenced by knowledge of the intervention assignment;
when detection events rely only on measures that can be influenced by judgement (e.g., clinically and laboratory detected events): assessment of this outcome can be influenced by knowledge of the intervention assignment but is not likely in the context of a pandemic.
Measures of treatment effect
For dichotomous outcomes, we calculated the relative risk (RR) with 95% confidence intervals (CIs) as a measure of effect. We extracted the number of events and number of total participants in each trial arm. For time‐to‐event outcomes, we extracted the hazard ratio (HR) with 95% CIs. When these were not provided, we attempted to obtain them using the tools provided in Tierney 2007. When confidence intervals were not reported, but credible intervals were reported instead, we extracted the latter. In the absence of prior information these two are not expected to differ substantially numerically. For time to improvement, when available, we extracted the data with death treated as a competing risk. When several analyses were reported, we extracted results obtained from the ITT analysis whenever these were available. If ITT results were not available, results from any modified ITT analyses were extracted.
Unit of analysis issues
We treated comparisons from multi‐arm or platform trials as independent two‐arm trials since we did not pool comparisons of different drugs in the same meta‐analysis. We did not identify any cross‐over or cluster‐randomized trials. If we do identify eligible cluster‐randomized trials in future updates of the review, we will extract results that properly account for the cluster design (such as based on a multilevel model or on generalized estimating equations). If such an analysis is not reported, we will try to obtain an estimate of the intraclass correlation coefficient and calculate data required for the meta analyses, taking the design effect into consideration.
Dealing with missing data
For missing outcome data, we extracted the number of participants who dropped out before completing the trial and how trial authors handled missing outcome data. In our primary analysis for the critical outcomes, we followed a conservative approach assuming that participants with missing outcome data did not experience the event of interest. Hence, we calculated all RRs with the number of participants randomised in each group in the denominator. We also conducted sensitivity analyses to assess the potential impact of missing outcome data on the results by using an available‐case analysis with the number of participants analyzed (e.g. only participants without missing outcome data or only patients who received treatment) in the denominator (see Sensitivity analysis section).
Assessment of heterogeneity
We generated descriptive statistics for both the trial and population characteristics and examined the distribution of important clinical and methodological variables (e.g. age, disease severity, pre‐existing conditions and comorbidities, location). We used visual inspection of forest plots, the I2 statistic and the magnitude of between‐study variance (τ2) to estimate the level of heterogeneity. We did not conduct prediction intervals (the interval within which the effect of a future trial is expected to lie (Riley 2011)), and comparison of with appropriate empirical distributions (Turner 2012), in this review because of the small number of trials; however, these are planned for future updates if appropriate
Assessment of reporting biases
Assessing risk of bias due to missing results in the synthesis
We assessed the risk of bias due to missing results in the synthesis according to the framework proposed in Chapter 13 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020).
We used searches in trial registries to identify any initiated, ongoing, or completed, but not published trials meeting this review's eligibility criteria. We contacted all responsible parties to obtain an updated report of the results included in the trial registry. For published trials, we contacted the corresponding authors to obtain the missing data.
We checked whether the results of all our critical and important outcomes were reported as prespecified in the trial register. When registration was not prospective, we also checked the protocol or statistical analysis plan if available.
When any trial results were not available, we used a matrix indicating availability of study results as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020), and Kirkham 2018.
We checked whether results were unavailable because of the P value, magnitude, or direction of the result. We considered risk of bias due to missing results if one specified outcome of the registry was lacking in the main report because of these reasons.
Due to the small number of trials, we could not assess the potential for reporting bias across studies graphically or statistically.
Data synthesis
We have combined trials evaluating the same drug with standard care alone or with placebo comparators together under the same comparison. We included all eligible RCTs in the primary analysis, regardless of the 'Risk of bias' assessment.
For binary outcomes, we calculated the logRRs and their standard error using the number of events and the number of total participants in each arm. Then we pooled the trial‐specific effect sizes. For time‐to‐event outcomes, we directly extracted the HRs and the respective 95% CIs from the trial reports and subsequently these were pooled in the meta‐analysis.
For each direct comparison with at least two trials providing data, we presented effect estimates with 95% CIs. We used the random‐effects model to incorporate the anticipated clinical and methodological heterogeneity across trials. We treated comparisons from multi‐arm or platform trials as independent two‐arm trials since we did not pool comparisons of different drugs in the same meta‐analysis.
All analyses were conducted using our R‐shiny application (available from https://covid-nma.com/pairwise_meta_analysis/), which is based on the metafor package in R.
Subgroup analysis and investigation of heterogeneity
We carried out pre‐specified subgroup analyses to explore the impact of trial location (single countries versus multinational). Post‐hoc subgroup analyses included funding sources (private versus public/non‐profit versus mixed) and conflict of interests (conflict of interests declared versus no conflict of interests).
Sensitivity analysis
We performed sensitivity analyses by excluding trials with high overall risk of bias and RCTs reported as preprint only. In order to assess the potential impact of missing outcome data on the results by using an available‐case analysis with the number of participants analyzed, we also ran the analyses by using the number of participants analyzed, instead of those randomised, (Chaimani 2018; Mavridis 2015; Mavridis 2018; White 2008). A post‐hoc sensitivity analysis was carried out to check the robustness of results after excluding trials that involved participants with all types of severity.
Summary of findings and assessment of the certainty of the evidence
To evaluate the confidence in the results of the pairwise comparisons for critical and important outcomes, we used the GRADE approach (Schünemann 2019). We prepared two 'Summary of findings’ tables to present estimated relative and absolute risks. Overall certainty of the evidence for each outcome was assessed by one review author and cross‐checked by another review author using the GRADE classification (GRADEpro GDT).
Results
Description of studies
For a full description of studies please see the Characteristics of included studies; Characteristics of excluded studies; and the Characteristics of registered studies in Appendix 4.
Results of the search
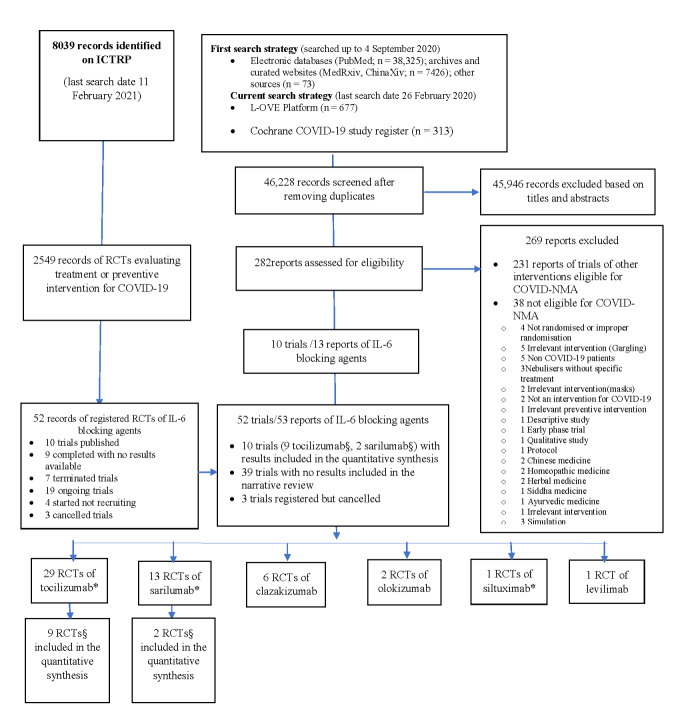
The results of our searches are detailed in Figure 1. On 26 February 2021, we retrieved a total of 46,814 references by searching electronic bibliographic databases. After excluding duplicates, we screened 46,228 records: 282 were eligible for full‐text screening. Key excluded studies are listed in Characteristics of excluded studies. Ten RCTs (seven published in peer‐reviewed journals and three reported as preprints) evaluating IL‐6 blocking agents were included in this review. Nine RCTs evaluated tocilizumab including one platform trial evaluating tocilizumab and sarilumab, and one three‐arm trial evaluated sarilumab.
1.
Flowchart of included RCTs of interleukin 6 (IL‐6) blocking agents (last search date 11 February 2021).
COVID‐NMA is a living systematic review of all trials assessing treatment and preventive interventions for COVID‐19 (Boutron 2020b). This review is a sub‐review of COVID‐NMA.
*two multiple‐arm RCTs evaluated both tocilizumab and sarilumab, one three‐arm RCT evaluated tocilizumab and siltuximab and consequently they appear twice. §one multi‐arm RCT evaluated both tocilizumab and sarilumab
We did not identify any retracted articles. The search of the US Food and Drug Administration website did not retrieve any reports. The search in ICTRP identified 39 registered trials with no results available and three cancelled registered trials (two evaluating tocilizumab and one clazakizumab).
We also contacted the named contacts for trials registered with no associated publication of results. The responses are detailed in Appendix 5 and Appendix 6 . We did not classify any trial as awaiting classification.
Overall, considering the data available in trial registries and the answers obtained from responsible parties, we identified 29 RCTs of tocilizumab (seven published in peer‐reviewed journals, two reported as preprints, five completed with no results available, five terminated with no results available, eight ongoing, two not recruiting); 13 RCTs of sarilumab (one published in peer‐reviewed journal, one published as preprint, two completed with no results available, three terminated with no results available, six ongoing); six RCTs of clazakisumab (five ongoing, one not recruiting); two RCTs of olokizumab (one completed with no results available, one not recruiting); one of siltuximab (ongoing); one RCT of levilimab (completed with no results available). Of note, two RCTs were multiple arm/platform trials evaluating both tocilizumab and sarilumab compared to standard of care (one published in a peer‐reviewed journal, one terminated with no results available), one three‐arm RCT evaluating tocilizumab and siltuximab compared to standard of care and consequently they appear in each respective comparison
Included studies
See: Characteristics of included studies
Source of the data
Reports of the 10 RCTs with results were published in peer‐reviewed journals (n = 7) (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Rosas COVACTA 2021; Salama EMPACTA 2020; Salvarani 2020; Stone 2020; Veiga TOCIBRAS 2021), or available as preprints (n = 3) (Horby RECOVERY 2021; Lescure 2021; Wang 2020). No results were posted on clinical trial registries. We contacted corresponding authors of nine trials to request for additional data; three provided information (Hermine CORIMUNO‐19 2020; Rosas COVACTA 2021; Salama EMPACTA 2020), and one agreed to provide data when the trial is published in a peer‐reviewed journal (Wang 2020. No answers were obtained from the rest of the trial authors. One included study was only recently published we have not yet contacted the authors (Horby RECOVERY 2021).
Study design
Eight trials used a two‐arm parallel‐group randomised design and three were platform trials/multiple arms, and one evaluated tocilizumab and sarilumab. Four were placebo‐controlled trials (Lescure 2021; Rosas COVACTA 2021; Salama EMPACTA 2020; Stone 2020). The median sample size was 315.5 participants (interquartile range (IQR): 128.25 to 545.5) (range 65 to 4116). Four trials did not achieve their target sample size; Salvarani 2020 achieved 32% (126/398) of the target population and the trial Scientific Committee decided to interrupt the trial for futility; Wang 2020 achieved only 35% (65 randomised/188 planned) of the sample size because of the rapid decline in the numbers of patients with COVID‐19 in China; Gordon REMAP‐CAP 2021 was stopped at a scheduled interim analysis following the decision of the Data Safety Monitoring Board; Veiga TOCIBRAS 2021 was terminated after the first interim analysis following the recommendations of the data monitoring committee, owing to an excess number of deaths at 15 days in the tocilizumab group. Further, results from Horby RECOVERY 2021 are results of a preliminary analysis and all patients’ follow‐up is not complete (results for primary outcome was available for 92% of patients but the full follow‐up form was only available for 79% of patients).
Study registration
All trial registration records were available. Five trials were retrospectively registered (Hermine CORIMUNO‐19 2020; Lescure 2021; Salvarani 2020; Stone 2020; Veiga TOCIBRAS 2021). The delay between the registration and the onset of the study was two days (Hermine CORIMUNO‐19 2020; Stone 2020), three days (Lescure 2021), 15 days (Salvarani 2020), and 19 days (Veiga TOCIBRAS 2021).
Settings
All trials included were multicentre trials (6 to 65 centres); they were conducted in Brazil (Veiga TOCIBRAS 2021), China (Wang 2020), France (Hermine CORIMUNO‐19 2020), Italy (Salvarani 2020), UK (Horby RECOVERY 2021), USA (Stone 2020), UK; and four were multi‐country trials (Gordon REMAP‐CAP 2021; Lescure 2021; Salama EMPACTA 2020; Rosas COVACTA 2021). They were performed between February 2020 and January 2021, with a mean duration of fifteen weeks (range three to 41). All participants were recruited from a hospital inpatient setting.
Characteristics of participants
We included a total of 6896 participants (10 RCTs) in the analysis of this review. Overall, 6428 participants (nine RCTs) were included in the analysis comparing tocilizumab with control; 880 participants (two RCTs) were included in the analysis comparing sarilumab with control. The mean age range varied from 56 to 65 years; 4572/6896 (66.3%) were men.
Participants had mild to critical disease in one RCT (N = 452) (Rosas COVACTA 2021), mild to severe diseases in two RCTs (N = 625) (Salama EMPACTA 2020; Stone 2020), moderate to severe disease in two RCTs (N = 196) (Hermine CORIMUNO‐19 2020; Wang 2020), moderate to critical disease in three RCTs (N = 4665) (Horby RECOVERY 2021; Lescure 2021; Veiga TOCIBRAS 2021), severe disease in one RCT (N = 158) (Salvarani 2020), and severe to critical disease in one RCT (N = 826) (Gordon REMAP‐CAP 2021). Inflammation makers varied but was high in most trials.
The percentage of participants on oxygen at baseline but not intubated was 56% (Rosas COVACTA 2021), 71% (Gordon REMAP‐CAP 2021), 84% (Stone 2020), 84% (Veiga TOCIBRAS 2021), 86% (Horby RECOVERY 2021), 87% (Lescure 2021), 88% (Salama EMPACTA 2020), 100% (Hermine CORIMUNO‐19 2020; Wang 2020). One trial did not provide this information (Salvarani 2020). Five trials reported the percentage of patients that were intubated at baseline: 12% (Lescure 2021), 14% (Horby RECOVERY 2021), 16% (Veiga TOCIBRAS 2021), 29% (Gordon REMAP‐CAP 2021) ,and 37% (Rosas COVACTA 2021). In the other trials, no patient was intubated at baseline (a single patient intubated at baseline in the control group in Stone 2020).
Details of the interventions
Eight trials assessed tocilizumab compared with standard of care alone or with placebo, one study assessed tocilizumab and sarilumab compared with standard of care, and one trial compared two regimens of sarilumab versus placebo. For the analysis, the two arms were merged.
Seven trials evaluated tocilizumab 8 mg/kg by infusion for one day (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Rosas COVACTA 2021; Salama EMPACTA 2020; Salvarani 2020; Stone 2020; Veiga TOCIBRAS 2021); the dose was adapted to patients’ weight according to an algorithm in one trial (Horby RECOVERY 2021), and one evaluated a lower dose of 400 mg by infusion for one day (Wang 2020). A second infusion was allowed in six trials (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Horby RECOVERY 2021; Rosas COVACTA 2021; Salvarani 2020; Wang 2020).
In Gordon REMAP‐CAP 2021, participants received sarilumab at 400 mg by infusion for one day. In Lescure 2021, participants received sarilumab at 200 mg or 400 mg by infusion for one day with an option for a second dose within 24 to 48 hours.
The comparator was standard care with placebo in four trials (Lescure 2021; Rosas COVACTA 2021; Salama EMPACTA 2020; Stone 2020), and the standard of care in the other six (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Horby RECOVERY 2021; Salvarani 2020; Veiga TOCIBRAS 2021; Wang 2020).
The use of steroids at baseline was reported in eight trials (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Horby RECOVERY 2021; Lescure 2021; Rosas COVACTA 2021; Salama EMPACTA 2020; Salvarani 2020; Stone 2020; Veiga TOCIBRAS 2021). Three trials reported that more participants received steroids in the control group (Hermine CORIMUNO‐19 2020; Rosas COVACTA 2021; Salama EMPACTA 2020). There was some cross‐over planned in the protocol in one trial (Salvarani 2020), with 22% of participants in the control arm receiving the experimental treatment.
Funding and conflict of interest
Three trials were funded by public/non‐profit sources (Hermine CORIMUNO‐19 2020; Horby RECOVERY 2021; Wang 2020), four received mixed funding (Gordon REMAP‐CAP 2021; Rosas COVACTA 2021; Salvarani 2020; Veiga TOCIBRAS 2021), and three were funded by the pharmaceutical industry (Lescure 2021; Salama EMPACTA 2020; Stone 2020). All authors reported their conflict of interests. The authors of seven trials declared conflicts of interest (Gordon REMAP‐CAP 2021; Lescure 2021; Rosas COVACTA 2021; Salama EMPACTA 2020; Salvarani 2020; Stone 2020; Veiga TOCIBRAS 2021), whilst in three studies (Hermine CORIMUNO‐19 2020; Horby RECOVERY 2021; Wang 2020), all authors declared that they had no conflicts.
Excluded studies
We excluded a total of 269 reports; 231 were RCTs evaluating other interventions and consequently included in the COVID‐NMA platform (covid‐nma.com); 38 full‐text reports (36 RCTs) were excluded from the COVID‐NMA platform. We provided details on the reasons for exclusions in Characteristics of excluded studies.
Ongoing studies
We identified 42 trials from registries, search data: up to 11 February 2021. After contacting the investigators, we were informed that three of them were cancelled (two evaluating tocilizumab and one clazakizumab). More details are available in Appendix 4 and Appendix 5
Tocilizumab
Of the 20 unpublished trials assessing tocilizumab, five trials were completed without results available (732 participants planned); five were terminated without results available, eight were ongoing (1976 participants), two are not yet recruiting (204 participants planned).
Sarilumab
We identified two completed trials without results available (859 participants planned), three terminated without results available and six ongoing trials (857 participants planned).
Clazakizumab
Five trials are ongoing (270 participants planned) and one is not recruiting (30 participants planned).
Olokizumab
We identified one completed trial without results available (372 participants planned) and one not recruiting (376 participants planned).
Siltuximab
We identified one ongoing trial (342 participants planned).
Levilimab
We identified one completed trial without results available (206 participants planned).
Risk of bias in included studies
The‘Risk of bias' assessment summarizes the 'Risk of bias' assessment by outcome.
The‘Risk of bias' assessments for each outcome are summarised in Table 3; Table 4; Table 5; Table 6; Table 7; Table 8; Table 9; Table 10; Table 11; Table 12; Table 13; Table 14; Table 15; Table 16 and Table 17.
1. ROB table: tocilizumab vs standard care(SC)/placebo. Clinical improvement (D28).
| Study | 1.Randomisation | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Hermine CORIMUNO‐19 2020 | Low | Some concerns1 | Low | Some concerns2 | Low | Some concerns |
| Rosas COVACTA 2021 | Low | Low | Low | Low | Low | Low |
| Salama EMPACTA 2020 | Low | Low | Low | Low | Low | Low |
| Salvarani 2020 | Low | Some concerns3 | Low | Some concerns4 | Some concerns5 | Some concerns |
| Stone 2020 | Low | Low | Low | Low | Low | Low |
| Horby RECOVERY 2021 | Low | Low | Low | Some concerns6 | Low | Some concerns |
| Veiga TOCIBRAS 2021 | Low | Some concerns7 | Low | Some concerns8 | Low | Some concerns |
1 Quote: “Open‐label study” Comment: unblinded study. Deviations from intended intervention arising because of the study context: three participants in the treatment group did not receive study drug. Administration of co‐interventions of interest (antivirals, corticosteroids and biologics) were reported. The proportions of participants receiving antivirals and steroids were imbalanced between two arms (> 10% solute difference between the two arms) for steroids. This deviation could affect the outcome and was not balanced. Nevertheless, this domain was rated as 'Some Concern' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
2 Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of clinical improvement probably does not differ between groups. Unblinded study. Assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
3 Quote: "the trial was open label" Comment: unblinded study. Deviations from intended intervention arising because of the study context: cross over: 15 (23%) participants in the standard care arm received the study treatment. For 12 (18%) the studied treatment was administered because of clinical worsening as planned in the protocol. Nevertheless, this decision could have been influenced by the trial context. Administration of co‐interventions of interest were reported and not balanced: antivirals (35% vs 47%) and corticosteroids (10% vs 10.6%). These deviations could affect the outcome. Nevertheless, this domain was rated as 'Some Concern' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
4 Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of clinical improvement probably does not differ between groups. Unblinded study. Assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
5 Comment: the protocol and statistical analysis plan were available. The outcomes 'Clinical improvement (defined as discharge)' is not present in the protocol or registry. No information on whether the results for these outcomes were selected from multiple outcome measurements or analyses of the data.
6Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of clinical improvement probably does not differ between groups. Unblinded study. Assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
7 Quote: “open label” trial. Comment: unblinded study. Deviations from intended intervention arising because of the study context: cross over: 2/64 (3%) of the control arm received tocilizumab. Co‐interventions of interest (corticosteroids and antivirals), were reported, but no information on another co‐intervention of interest: biologics. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
8 Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of clinical improvement probably does not differ between groups. Unblinded study. Assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
2. ROB table: tocilizumab vs standard care(SC)/placebo. WHO Clinical Progression Score level 7 or above (D28).
| Study | 1.Randomisation | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Hermine CORIMUNO‐19 2020 | Low | Some concerns 1 | Low | Low | Low | Some concerns |
| Rosas COVACTA 2021 | Low | Low | Low | Low | Low | Low |
| Veiga TOCIBRAS 2021 | Low | Some concerns 2 | Low | Low | Low | Some concerns |
1 Quote: “Open‐label study” Comment: unblinded study.
Deviations from intended intervention arising because of the study context: three participants in the treatment group did not receive study drug. Administration of co‐interventions of interest (antivirals, corticosteroids and biologics) were reported. The proportions of participants receiving antivirals and steroids were imbalanced between two arms (>10% absolute difference between the two arms) for steroids. This deviation could affect the outcome and was not balanced. Nevertheless, this domain was rated as 'Some Concern' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect.
Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
2 Quote: “open label” trial. Comment: unblinded study.
Deviations from intended intervention arising because of the study context: cross over: 2/64 (3%) of the control arm received tocilizumab. Co‐interventions of interest (corticosteroids and antivirals) were reported, but no information on another co‐intervention of interest: biologics. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
3. ROB table: tocilizumab vs standard care(SC)/placebo. All‐cause mortality (D28).
| Study | 1.Randomisation | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Hermine CORIMUNO‐19 2020 | Low | Some concerns1 | Low | Low | Low | Some concerns |
| Rosas COVACTA 2021 | Low | Low | Low | Low | Low | Low |
| Salama EMPACTA 2020 | Low | Low | Low | Low | Low | Low |
| Salvarani 2020 | Low | Some concerns2 | Low | Low | Low | Some concerns |
| Stone 2020 | Low | Low | Low | Low | Low | Low |
| Gordon REMAP‐CAP 2021 | Low | Some concerns3 | Low | Low | Low | Some concerns |
| Horby RECOVERY 2021 | Low | Low | Low | Low | Low | Low |
| Veiga TOCIBRAS 2021 | Low | Some concerns4 | Low | Low | Low | Some concerns |
1 Quote: “Open‐label study” Comment: unblinded study.
Deviations from intended intervention arising because of the study context: three participants in the treatment group did not receive study drug. Administration of co‐interventions of interest (antivirals, corticosteroids and biologics) were reported. The proportions of participants receiving antivirals and steroids were imbalanced between two arms (> 10% absolute difference between the two arms) for steroids. This deviation could affect the outcome and was not balanced. Nevertheless, this domain was rated as 'Some Concern' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
2 Quote: "the trial was open label" Comment: unblinded study.
Deviations from intended intervention arising because of the study context: cross over: 15 (23%) participants in the standard care arm received the study treatment. For 12 (18%) the studied treatment was administered because of clinical worsening as planned in the protocol. Nevertheless, this decision could have been influenced by the trial context. Administration of co‐interventions of interest were reported and not balanced: antivirals (35% vs 47%) and corticosteroids (10% vs 10.6%). These deviations could affect the outcome and were not balanced. Nevertheless, this domain was rated as 'Some Concern' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
3 Quote: “open‐label” Comment: unblinded study.
Deviations from intended intervention arising because of the study context: no participant cross‐over. Administration of co‐interventions of interest: antivirals (Remdesivir, 32.8%) and corticosteroids (> 80%) were administered, but numbers per group were not reported. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
4 Quote: “open label” trial. Comment: unblinded study.
Deviations from intended intervention arising because of the study context: cross over: 2/64 (3%) of the control arm received tocilizumab. Co‐interventions of interest (corticosteroids and antivirals), were reported, but no information on another co‐intervention of interest: biologics. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Data for the outcome were analysed using intention‐to‐treat analysis for this outcome. This method was considered appropriate to estimate the effect of assignment to intervention.
4. ROB table: tocilizumab vs standard care(SC)/placebo. All‐cause mortality (≥ D60).
| Study | 1.Randomisation | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Hermine CORIMUNO‐19 2020 | Low | Some concerns 1 | Low | Low | Low | Some concerns |
| Salama EMPACTA 2020 | Low | Low | Low | Low | Low | Low |
1 Quote: “Open‐label study” Comment: unblinded study.
Deviations from intended intervention arising because of the study context: three participants in the treatment group did not receive study drug. Administration of co‐interventions of interest (antivirals, corticosteroids and biologics) were reported. The proportions of participants receiving antivirals and steroids were imbalanced between two arms (> 10% absolute difference between the two arms) for steroids. This deviation could affect the outcome and was not balanced. Nevertheless, this domain was rated as 'Some Concern' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention
5. ROB table: tocilizumab vs standard care(SC)/placebo. Incidence of any adverse events.
| Study | 1.Randomisation | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Hermine CORIMUNO‐19 2020 | Low | Some concerns1 | Low | Some concerns2 | Low | Some concerns |
| Rosas COVACTA 2021 | Low | Low | Low | Low | Low | Low |
| Salama EMPACTA 2020 | Low | Low | Low | Low | Low | Low |
| Salvarani 2020 | Low | Some concerns3 | Low | Some concerns4 | Low | Some concerns |
| Stone 2020 | Low | Low | Low | Low | Low | Low |
| Wang 2020 | Some concerns5 | Some concerns6 | Low | Some concerns7 | Some concerns8 | High |
| Veiga TOCIBRAS 2021 | Low | Some concerns9 | Low | Some concerns10 | Low | Some concerns |
1 Quote: “Open‐label study” Comment: unblinded study.
Deviations from intended intervention arising because of the study context: three participants in the treatment group did not receive study drug. Administration of co‐interventions of interest (antivirals, corticosteroids and biologics) were reported. The proportions of participants receiving antivirals and steroids were imbalanced between two arms (> 10% absolute difference between the two arms) for steroids. This deviation could affect the outcome and was not balanced. Nevertheless, this domain was rated as 'Some Concerns' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
2 Comment: method of measuring the outcome probably appropriate. Measurement of outcome probably does not differ between groups. Unblinded study. The outcome may contain both clinically‐ and laboratory‐detected events. Assessment of this outcome can be influenced by knowledge of the intervention assignment but is not likely in the context of a pandemic.
3 Quote: "the trial was open label" Comment: unblinded study.
Deviations from intended intervention arising because of the study context: cross over: 15 (23%) participants in the standard care arm received the study treatment. For 12 (18%) the studied treatment was administered because of clinical worsening as planned in the protocol. Nevertheless, this decision could have been influenced by the trial context. Administration of co‐interventions of interest were reported and not balanced: antivirals (35% vs 47%) and corticosteroids (10% vs 10.6%). These deviations could affect the outcome and were not balanced. Nevertheless, this domain was rated as 'Some Concern' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
4 Comment: method of measuring the outcome probably appropriate. Measurement of outcome probably does not differ between groups. Unblinded study. The outcome may contain both clinically‐ and laboratory‐detected events. Assessment of this outcome can be influenced by knowledge of the intervention assignment but is not likely in the context of a pandemic.
5 Quote: "sation numbers were generated using SAS statistical software package (SAS Institute, Cary, USA). A computer‐ generated 1:1 block randomization scheme was used to assign participants to either treatment group or control one. Each consecutively coded participant was randomly enrolled by the sub‐site investigators until the total number of cases allocated to the site was reached." Comment: Allocation sequence random. Allocation concealment unclear.
6 Quote: "One case in the control group aggravated on day three after randomization was transferred to the tocilizumab group according to the rules of the study protocol." Comment: unblinded study. Deviations from intended intervention arising because of the study context: one participant cross‐over. No information on administration of any co‐interventions of interest: antivirals, corticosteroids, biologics. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Participants were not analysed according to their randomised groups for the outcome. Of note, 1 participant randomised to the control group was analysed in the intervention group. Nevertheless, we considered the analysis to be probably appropriate to estimate the effect of assignment to intervention.
7 Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study. The outcome contains both clinically‐ and laboratory‐detected events. Assessment of this outcome can be influenced by knowledge of the intervention assignment but is not likely in the context of a pandemic.
8 Comment: the protocol and statistical analysis plan were not available. The registry was available. Adverse events were not mentioned in the registry but reported in the paper. No information on whether results were selected from multiple outcome measurements or analyses of the data.
9 Quote: “open label” trial. Comment: unblinded study.
Deviations from intended intervention arising because of the study context: cross over: 2/64 (3%) of the control arm received tocilizumab. Co‐interventions of interest (corticosteroids and antivirals) were reported, but no information on another co‐intervention of interest: biologics. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported.
Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
10 Comment: method of measuring the outcome probably appropriate. Measurement of outcome probably does not differ between groups. Unblinded study. The outcome contains both clinically‐ and laboratory‐detected events. Assessment of this outcome can be influenced by knowledge of the intervention assignment but is not likely in the context of a pandemic.
6. ROB table: tocilizumab vs standard care(SC)/placebo. Incidence of serious adverse events.
| Study | 1.Randomisation |
2.Deviations from intervention |
3.Missing outcome data |
4.Measurement of the outcome |
5.Selection of the reported results |
Overall risk of bias |
| Hermine CORIMUNO‐19 2020 | Low | Some concerns 1 | Low | Some concerns 2 | Low | Some concerns |
| Rosas COVACTA 2021 | Low | Low | Low | Low | Low | Low |
| Salama EMPACTA 2020 | Low | Low | Low | Low | Low | Low |
| Salvarani 2020 | Low | Some concerns3 | Low | Some concerns4 | Low | Some concerns |
| Stone 2020 | Low | Low | Low | Low | Low | Low |
| Wang 2020 | Some concerns 5 | Some concerns 6 | Low | Some concerns 7 | Some concerns8 | High |
| Gordon REMAP‐CAP 2021 | Low | Some concerns 9 | Low | Some concerns10 | Low | Some concerns |
| Veiga TOCIBRAS 2021 | Low | Some concerns11 | Low | Some concerns 12 | Low | Some concerns |
1 Quote: “Open‐label study” Comment: unblinded study.
Deviations from intended intervention arising because of the study context: three participants in the treatment group did not receive study drug. Administration of co‐interventions of interest (antivirals, corticosteroids and biologics) were reported. The proportions of participants receiving antivirals and steroids were imbalanced between two arms (> 10% absolute difference between the two arms) for steroids. This deviation could affect the outcome and was not balanced. Nevertheless, this domain was rated as 'Some Concern' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
2 Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study. The outcome contains both clinically‐ and laboratory‐detected events which can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
3 Quote: "the trial was open label" Comment: unblinded study.
Deviations from intended intervention arising because of the study context: Cross over: 15 (23%) participants in the standard care arm received the study treatment. For 12 (18%) the studied treatment was administered because of clinical worsening as planned in the protocol. Nevertheless, this decision could have been influenced by the trial context. Administration of co‐interventions of interest were reported and not balanced: antivirals (35% vs 47%) and corticosteroids (10% vs 10.6%). These deviations could affect the outcome and were not balanced. Nevertheless, this domain was rated as 'Some Concern' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
4 Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study. The outcome contains both clinically‐ and laboratory‐detected events which can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
5 Quote: "Randomization numbers were generated using SAS statistical software package (SAS Institute, Cary, USA). A computer‐ generated 1:1 block randomization scheme was used to assign participants to either treatment group or control one. Each consecutively coded participant was randomly enrolled by the sub‐site investigators until the total number of cases allocated to the site was reached." Comment: allocation sequence random. Allocation concealment unclear.
6 Quote: "One case in the control group aggravated on day three after randomization was transferred to the tocilizumab group according to the rules of the study protocol." Comment: unblinded study. Deviations from intended intervention arising because of the study context: One participant cross‐over. No information on administration of any co‐interventions of interest: antivirals, corticosteroids, biologics. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Participants were not analysed according to their randomised groups for the outcome. Of note, 1 participant randomised to the control group was analysed in the intervention group. Nevertheless, we considered the analysis to be probably appropriate to estimate the effect of assignment to intervention.
7 Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study. The outcome may contain both clinically‐ and laboratory‐detected events. Assessment of this outcome can be influenced by knowledge of the intervention assignment but is not likely in the context of a pandemic.
8 The protocol and statistical analysis plan were not available. The registry was available. Serious adverse events were not mentioned in the registry but reported in the paper. No information on whether results were selected from multiple outcome measurements or analyses of the data.
9 Quote: “open‐label” Comment: unblinded study. Deviations from intended intervention arising because of the study context: No participant cross‐over. Administration of co‐interventions of interest: antivirals (Remdesivir, 32.8%) and corticosteroids (> 80%) were administered, but numbers per group were not reported. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
10 Comment: method of measuring outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study (outcome assessor). Outcome may contain both clinically‐ and laboratory‐detected events which can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
11 Quote: “open label” trial. Comment: unblinded study.
Deviations from intended intervention arising because of the study context: Cross over: 2/64 (3%) of the control arm received tocilizumab. Co‐interventions of interest (corticosteroids and antivirals), were reported, but no information on another co‐intervention of interest: biologics. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
12 Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study. The outcome contains both clinically‐ and laboratory‐detected events which can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic
7. ROB table: tocilizumab vs standard care(SC)/placebo. Time to clinical improvement.
| Study | 1.Randomisation | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Gordon REMAP‐CAP 2021 | Low | Some concerns[1] | Low | Some concerns[2] | Low | Some concerns |
| Hermine CORIMUNO‐19 2020 | Low | Some concerns[3] | Low | Some concerns[4] | Low | Some concerns |
| Rosas COVACTA 2021 | Low | Low | Low | Low | Low | Low |
| Salama EMPACTA 2020 | Low | Some concerns[5] | Low | Low | Low | Some concerns |
| Stone 2020 | Low | Low | Low | Low | Low | Low |
| Salvarani 2020 | Low | Some concerns6 | Low | Some concerns7 | Some concerns8 | Some concerns |
1 Quote: “open‐label” Comment: unblinded study. Deviations from intended intervention arising because of the study context: No participant cross‐over. Administration of co‐interventions of interest: antivirals (Remdesivir, 32.8%) and corticosteroids (> 80%) were administered, but numbers per group were not reported. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Participants were analysed according to their randomised groups for the outcome. Of note, 13 vs 10 participants were excluded from the analysis post‐randomisation for reasons related to missing data. This method was considered appropriate to estimate the effect of assignment to intervention.
2 Comment: method of measuring outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study (outcome assessor). Assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
3 Quote: “Open‐label study” Comment: unblinded study.
Deviations from intended intervention arising because of the study context: three participants in the treatment group did not receive study drug. Administration of co‐interventions of interest (antivirals, corticosteroids and biologics) were reported. The proportions of participants receiving antivirals and steroids were imbalanced between two arms (> 10% absolute difference between the two arms) for steroids. This deviation could affect the outcome and was not balanced. Nevertheless, this domain was rated as 'Some Concerns' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect.
Participants were analysed according to their randomized groups for the outcome. Of note, 1 vs 0 participants were excluded from the analysis because of consent withdrawal. Nevertheless, we consider the analysis appropriate to estimate the effect of assignment to intervention.
4 Comment: method of measuring the outcome probably appropriate.
Measurement or ascertainment of outcome probably does not differ between groups Unblinded study. Assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
5 Quote: “double‐blind, placebo‐controlled trial.” “A site blinding plan was established at each site to identify which personnel would be blinded or unblinded at a site level. A pharmacy manual and specific training in addition to completion of a site blinding plan was provided to each site. Each site had an unblinded pharmacist that randomized the participant and prepared and labeled study medication in the same method for both tocilizumab and placebo. The remainder of the study team was blinded to treatment assignment. There was no communication during the study between unblinded and blinded members. In addition, there was an unblinded medical monitor available to answer questions from the unblinded site staff. Placebo was not provided and consisted of an unaltered saline infusion bag, the same as would be used to prepare tocilizumab. The volume of tocilizumab diluted in saline appears colorless and matches saline.” Comment: blinded study. Participants were blinded. Carers were probably blinded.
Participants were analysed according to their randomised groups for the outcome. Of note, 10 vs 1 participants were excluded from the analysis post‐randomisation because they did not receive the drug. This method was considered inappropriate to estimate the effect of assignment to intervention for this time‐to‐event outcome. There was probably no substantial impact of failure to analyse participants according to their randomised groups.
6 Quote: "the trial was open label" Comment: unblinded study.
Deviations from intended intervention arising because of the study context: cross over: 15 (23%) participants in the standard care arm received the study treatment. For 12 (18%) the studied treatment was administered because of clinical worsening as planned in the protocol. Nevertheless, this decision could have been influenced by the trial context. Administration of co‐interventions of interest were reported and not balanced: antivirals (35% vs 47%) and corticosteroids (10% vs 10.6%). These deviations could affect the outcome and were not balanced. Nevertheless, this domain was rated as 'Some Concern' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. Data for the outcome were analyced using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
7 Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups Unblinded study. Assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
8 Comment: the protocol and statistical analysis plan were available. The outcome time to clinical improvement (defined as time to discharge) is not mentioned in the protocol or registry. No information on whether the results for these outcomes were selected from multiple outcome measurements or analyses of the data.
8. ROB table: tocilizumab vs standard care(SC)/placebo. Time to WHO score 7 or above.
| Study | 1.Randomisation |
2.Deviations from intervention |
3.Missing outcome data |
4.Measurement of the outcome |
5.Selection of the reported results |
Overall risk of bias |
| Hermine CORIMUNO‐19 2020 | Low | Some concerns 1 | Low | Low | Low | Some concerns |
| Salama EMPACTA 2020 | Low | Some concerns 2 | Low | Low | Low | Some concerns |
| Stone 2020 | Low | Low | Low | Low | Low | Low |
1 Quote: “Open‐label study” Comment: unblinded study.
Deviations from intended intervention arising because of the study context: 3 participants in the treatment group did not receive study drug. Administration of co‐interventions of interest (antivirals, corticosteroids and biologics) were reported. The proportions of participants receiving antivirals and steroids were imbalanced between 2 arms (> 10% absolute difference between the 2 arms) for steroids. This deviation could affect the outcome and was not balanced. Nevertheless, this domain was rated as Some Concerns as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. Participants were analysed according to their randomised groups for the outcome. Of note, 1 vs 0 participants were excluded from the analysis because of consent withdrawal. Nevertheless, we consider the analysis appropriate to estimate the effect of assignment to intervention.
2 Quote: “double‐blind, placebo‐controlled trial.” “A site blinding plan was established at each site to identify which personnel would be blinded or unblinded at a site level. A pharmacy manual and specific training in addition to completion of a site blinding plan was provided to each site. Each site had an unblinded pharmacist that randomized the participant and prepared and labeled study medication in the same method for both tocilizumab and placebo. The remainder of the study team was blinded to treatment assignment. There was no communication during the study between unblinded and blinded members. In addition, there was an unblinded medical monitor available to answer questions from the unblinded site staff. Placebo was not provided and consisted of an unaltered saline infusion bag, the same as would be used to prepare tocilizumab. The volume of tocilizumab diluted in saline appears colorless and matches saline.”
Comment: Blinded study. Participants were blinded. Carers were probably blinded. Participants were analysed according to their randomised groups for the outcome. Of note, 10 vs 1 participants were excluded from the analysis post‐randomisation because they did not receive the drug. This method was considered inappropriate to estimate the effect of assignment to intervention for this time‐to‐event outcome. There was probably no substantial impact of failure to analyse participants according to their randomised groups
9. ROB table: tocilizumab vs standard care(SC)/placebo. Time to death.
| Study | 1.Randomisation |
2.Deviations from intervention |
3.Missing outcome data |
4.Measurement of the outcome |
5.Selection of the reported results |
Overall risk of bias |
| Gordon REMAP‐CAP 2021 | Low | Some concerns 1 | Low | Low | Low | Some concerns |
| Hermine CORIMUNO‐19 2020 | Low | Some concerns 2 | Low | Low | Low | Some concerns |
| Stone 2020 | Low | Low | Low | Low | Low | Low |
1 Quote: “open‐label” Comment: unblinded study. Deviations from intended intervention arising because of the study context: No participant cross‐over. Administration of co‐interventions of interest: antivirals (Remdesivir, 32.8%) and corticosteroids (> 80%) were administered, but numbers per group were not reported. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Participants were analysed according to their randomised groups for the outcome. Of note, 13 vs 10 participants were excluded from the analysis post‐randomisation for reasons related to missing data. This method was considered appropriate to estimate the effect of assignment to intervention.
2 Quote: “Open‐label study” Comment: unblinded study.
Deviations from intended intervention arising because of the study context: three participants in the treatment group did not receive study drug. Administration of co‐interventions of interest (antivirals, corticosteroids and biologics) were reported. The proportions of participants receiving antivirals and steroids were imbalanced between 2 arms (> 10% absolute difference between the 2 arms) for steroids. This deviation could affect the outcome and was not balanced. Nevertheless, this domain was rated as Some Concerns as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. Participants were analysed according to their randomised groups for the outcome. Of note, 1 vs 0 participants were excluded from the analysis because of consent withdrawal. Nevertheless, we consider the analysis appropriate to estimate the effect of assignment to intervention.
10. ROB table: sarilumab vs standard care(SC). All‐cause mortality (D28).
| Study | 1.Randomisation | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Gordon REMAP‐CAP 2021 | Low | Some concerns 1 | Low | Low | Low | Some concerns |
| Lescure 2021 | Low | Low | Low | Low | Low | Low |
1 Quote: “open‐label” Comment: unblinded study. Deviations from intended intervention arising because of the study context: No participant cross‐over. Administration of co‐interventions of interest: antivirals (Remdesivir, 32.8%) and corticosteroids (> 80%) were administered, but numbers per group were not reported. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Participants were analysed according to their randomised groups for the outcome. Of note, 3 vs 15 participants were excluded from the analysis post‐randomisation for reasons related to missing data. This method was considered appropriate to estimate the effect of assignment to intervention.
11. ROB table: sarilumab vs standard care(SC). All‐cause mortality (≥ D60).
| Study | 1.Randomisation | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Lescure 2021 | Low | Low | Low | Low | Some concerns 1 | Some concerns |
1 Comment: the study registry was available. Mortality outcome was not pre‐specified for day 60 in the registry. No information whether the result was selected from multiple outcome measurements or analyses of the data.
12. ROB table: sarilumab vs standard care(SC). Incidence of adverse events.
| Study | 1.Randomisation | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Lescure 2021 | Low | Low | Low | Low | Low | Low |
13. ROB table: sarilumab vs standard care(SC). Incidence of serious adverse events.
| Study | 1.Randomisation | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Gordon REMAP‐CAP 2021 | Low | Some concerns 1 | Low | Some concerns 2 | Low | Some concerns |
| Lescure 2021 | Low | Low | Low | Low | Low | Low |
1 Quote: “open‐label” Comment: unblinded study. Deviations from intended intervention arising because of the study context: No participant cross‐over. Administration of co‐interventions of interest: antivirals (Remdesivir, 32.8%) and corticosteroids (> 80%) were administered, but numbers per group were not reported. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Participants were analysed according to their randomised groups for the outcome. Of note, 3 vs 10 participants were excluded from the analysis post‐randomisation for reasons related to missing data. This method was considered appropriate to estimate the effect of assignment to intervention.
2 Comment: method of measuring outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study (outcome assessor). Assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
14. ROB table: sarilumab vs standard care(SC). Time to clinical improvement.
| Study | 1.Randomisation | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Gordon REMAP‐CAP 2021 | Low | Some concerns 1 | Low | Some concerns2 | Low | Some concerns |
| Lescure 2021 | Low | Low | Low | Low | Low | Low |
1 Quote: “open‐label” Comment: unblinded study. Deviations from intended intervention arising because of the study context: No participant cross‐over. Administration of co‐interventions of interest: antivirals (Remdesivir, 32.8%) and corticosteroids (> 80%) were administered, but numbers per group were not reported. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Participants were analysed according to their randomised groups for the outcome. Of note, 3 vs 10 participants were excluded from the analysis post‐randomisation for reasons related to missing data. This method was considered appropriate to estimate the effect of assignment to intervention.
2 Comment: method of measuring outcome probably appropriate. Measurement or ascertainment of outcome probably does not differ between groups. Unblinded study (outcome assessor). Assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
15. ROB table: sarilumab vs standard care(SC). Time to death.
| Study | 1.Randomisation | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Gordon REMAP‐CAP 2021 | Low | Some concerns1 | Low | Low | Low | Some concerns |
1 Quote: “open‐label” Comment: unblinded study. Deviations from intended intervention arising because of the study context: No participant cross‐over. Administration of co‐interventions of interest: antivirals (Remdesivir, 32.8%) and corticosteroids (> 80%) were administered, but numbers per group were not reported. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Participants were analysed according to their randomised groups for the outcome. Of note, 3 vs 10 participants were excluded from the analysis post‐randomisation for reasons related to missing data. This method was considered appropriate to estimate the effect of assignment to intervention
Risk of bias arising from the randomization process
Randomisation was described adequately and was appropriate in nine trials (Hermine CORIMUNO‐19 2020; Gordon REMAP‐CAP 2021; Horby RECOVERY 2021; Lescure 2021; Rosas COVACTA 2021; Salama EMPACTA 2020; Salvarani 2020; Stone 2020; Veiga TOCIBRAS 2021). There were some concerns in one trial because the method used to conceal the allocation of treatment was unclear (Wang 2020). There was no imbalance in baseline data that indicate problem with the randomization process.
Risk of bias due to deviations from intended interventions
We judged the risk of bias due to deviation from intended interventions as low for all the outcomes reported in four blinded trials (Horby RECOVERY 2021; Lescure 2021; Rosas COVACTA 2021; Stone 2020).
However, this domain was rated as some concerns for all the outcomes reported in five unblinded trials (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Salvarani 2020; Veiga TOCIBRAS 2021; Wang 2020). In Hermine CORIMUNO‐19 2020, cointerventions were reported but not balanced. Particularly steroids were more frequently provided in the standard of care group. This deviation could affect the outcome. Nevertheless, this domain was rated as 'Some Concern' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. In Salvarani 2020, 23% of participants allocated to standard care arm received tocilizumab mainly because of clinical worsening. This decision was planned in the protocol. Nevertheless, it could have been influenced by the trial context and this domain was consequently rated as some concerns. These deviations would be responsible for an underestimation of the treatment effect. Other trials were rated as some concern because co‐interventions were not completely reported (Gordon REMAP‐CAP 2021; Veiga TOCIBRAS 2021; Wang 2020).
Finally, Salama EMPACTA 2020 was rated as some concern for important outcomes because participants who did not receive the drug (10 versus one) were excluded from the analysis post‐randomisation.
Of note, in Horby RECOVERY 2021, 17% of participants allocated to tocilizumab did not receive the treatment allocated. We considered this deviation probably did not arise because of the trial context and assessed the domain as low risk.
Risk of bias due to missing outcome data
We judged the risk of bias due to incomplete outcome data as low for all trials and all outcomes since there was no or a low amount of missing data in the included trials. We rated reports of preliminary analyses with missing information, because the follow‐up was not complete, as low risk of bias.
Risk of bias in the measurement of the outcome
We judged risk of bias as low for all outcomes in the four blinded trials (Lescure 2021; Rosas COVACTA 2021; Salama EMPACTA 2020; Stone 2020). In the six open trials, we considered risk of bias low for observer‐reported outcomes not involving clinical judgement (i.e. mortality, WHO score 7 and above, time to death and time to WHO score 7 and above) (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Horby RECOVERY 2021; Salvarani 2020; Veiga TOCIBRAS 2021; Wang 2020). In contrast, there were some concerns for the outcomes that could be potentially influenced by knowledge of the intervention assignment (i.e. clinical improvement, time to clinical improvement, adverse events and serious adverse events) (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Horby RECOVERY 2021; Salvarani 2020; Veiga TOCIBRAS 2021; Wang 2020).
Risk of bias in the selection of the reported results
The protocol was available in seven trials (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Horby RECOVERY 2021; Salama EMPACTA 2020; Salvarani 2020; Stone 2020; Veiga TOCIBRAS 2021). In three trials, neither the protocol or the statistical analysis plan was available (Lescure 2021; Rosas COVACTA 2021; Wang 2020).
Overall, seven trials were judged as low risk of bias in this domain for all outcomes (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Horby RECOVERY 2021; Rosas COVACTA 2021; Salama EMPACTA 2020; Stone 2020; Veiga TOCIBRAS 2021). This domain was rated as some concern for the outcome adverse event and serious adverse event in one trial (Wang 2020), clinical improvement and time to clinical improvement in one trial (Salvarani 2020), and for all‐cause mortality D60 in one trial (Lescure 2021).
Bias due to missing results in the synthesis
We present a matrix indicating the availability of trial results for critical and important outcomes of the review in Appendix 7. Eight trials reported or provided results of all the review outcomes as pre‐specified in the trial registry. We identified bias due to missing results in the synthesis of the tocilizumab comparison for the critical outcome all‐cause mortality at D28 (Wang 2020) and in the synthesis of the sarilumab comparison for the critical outcome clinical improvement at D28 (Lescure 2021), as the outcomes were specified in the registry but not reported in the corresponding trial report.
Nine registered trials are completed but not yet published, the dates of completion range between 24 July 2020, and 10 December 2020; one of these trials is completed according to the response received from the authors but we are unaware of the completion date (NCT04479358), two other trials are reported as completed in the registry, but date of completion was not reported. The delay of publication since study completion ranged between 63 days and 202 days.
Effects of interventions
Comparision 1. Tocilizumab versus standard of care/placebo
We report the certainty evidence for the critical and for important outcomes in Table 1
Critical outcomes
Clinical improvement
Clinical improvement was defined as hospital discharge (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Horby RECOVERY 2021; Salama EMPACTA 2020; Salvarani 2020; Veiga TOCIBRAS 2021), or as an improvement from baseline by at least two categories on a 7‐category ordinal scale (Rosas COVACTA 2021; Stone 2020).
The proportion of participants achieving improvement at D28 was reported in seven RCTs (5585 participants) (Hermine CORIMUNO‐19 2020; Horby RECOVERY 2021; Rosas COVACTA 2021; Salama EMPACTA 2020; Salvarani 2020; Stone 2020; Veiga TOCIBRAS 2021). Tocilizumab probably results in little or no increase in clinical improvement at D28 (risk ratio (RR) 1.06, 95% confidence interval (CI) 1.00 to 1.13; I2 = 40.9%; 7 RCTs; 5585 participants; absolute effect: 31 more per 1000 (from 0 fewer to 67 more); moderate‐certainty evidence) (Figure 2). However, we cannot exclude that some subgroup of patients could benefit from the treatment. We did not obtain data for longer‐term follow‐up (≥ D60).
2.

Tociliuzumab compared to standard care/placebo for mild/moderate/severe/critical COVID‐19: Clinical improvement D28
WHO Clinical Progression Score of level 7 or above (i.e. the proportion of participants with mechanical ventilation +/‐ additional organ support or death)
Three RCTs (712 participants) reported the proportion of participants with mechanical ventilation or death at D28 (Hermine CORIMUNO‐19 2020; Rosas COVACTA 2021; Veiga TOCIBRAS 2021). Overall, the evidence is uncertain for the effect of tocilizumab on the proportion of participants with a WHO Clinical Progression Score of level 7 or above at D28 (RR 0.99, 95% CI 0.56 to 1.74; I2 = 64.4 %; 3 RCTs, 712 participants; low‐certainty evidence) (Figure 3). We did not obtain data for longer‐term follow‐up (≥ D60).
3.

Tociliuzumab compared to standard care/placebo for mild/moderate/severe/critical COVID‐19: WHO progression score (level 7 or above) D28
All‐cause mortality
Eight RCTs (6363 participants) reported all‐cause mortality at D28 (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Horby RECOVERY 2021; Rosas COVACTA 2021; Salama EMPACTA 2020; Salvarani 2020; Stone 2020; Veiga TOCIBRAS 2021); two RCTs (518 participants) at ≥ D60 (Hermine CORIMUNO‐19 2020; Salama EMPACTA 2020).
Tocilizumab reduces all‐cause mortality at D28 compared with standard care alone or with placebo (RR 0.89, 95% CI 0.82 to 0.97; I2 = 0.0%; 8 RCTs, 6363 participants; absolute effect 32 fewer per 1000 (from 52 fewer to 9 fewer); high‐certainty evidence) (Figure 4).
4.
Tociliuzumab compared to standard care/placebo for mild/moderate/severe/critical COVID‐19: All‐cause mortality D28
The evidence of an effect of tocilizumab on all‐cause mortality is uncertain at ≥ D60 (RR 0.86, 95% CI 0.53 to 1.40; I2 = 0.0%; 2 RCTs; 519 participants; low‐certainty evidence) (Figure 5).
5.

Tociliuzumab compared to standard care/placebo for mild/moderate/severe/critical COVID‐19: All‐cause mortality D60 or above
Adverse events (AEs)
AEs were assessed by spontaneous reporting (Wang 2020), active monitoring (Salvarani 2020; Stone 2020), and unknown methods in five RCTs (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Rosas COVACTA 2021; Salama EMPACTA 2020; Veiga TOCIBRAS 2021).
AEs were reported in seven RCTs (1534 participants) (Hermine CORIMUNO‐19 2020; Rosas COVACTA 2021; Salama EMPACTA 2020; Salvarani 2020; Stone 2020; Veiga TOCIBRAS 2021; Wang 2020). The evidence comparing tocilizumab with standard care alone or with placebo on adverse events is very uncertain (RR 1.23, 95% CI 0.87 to 1.72; I2 = 86.4%; 7 RCTs, 1534 participants; very low‐certainty evidence). We explored the sources of heterogeneity in the sensitivity analysis (Figure 6).
6.

Tociliuzumab compared to standard care/placebo for mild/moderate/severe/critical COVID‐19: Adverse events
Serious adverse events (SAEs)
SAEs were reported in eight RCTs (2312 participants) (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Rosas COVACTA 2021; Salama EMPACTA 2020; Salvarani 2020; Stone 2020; Veiga TOCIBRAS 2021; Wang 2020). Tocilizumab probably results in slightly fewer SAEs than standard care alone or with placebo (RR 0.89, 95% CI 0.75 to 1.06; I2 = 0.0%; 8 RCTs, 2312 participants; moderate‐certainty evidence). However, the confidence intervals are consistent with no effect (Figure 7).
7.

Tociliuzumab compared to standard care/placebo for mild/moderate/severe/critical COVID‐19: Serious adverse events
Important outcomes
(Table of results for tocilizumab versus placebo or standard care: important outcomes reported in Appendix 8.)
Time to clinical improvement
This outcome was reported in six RCTs (1992 participants) (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Rosas COVACTA 2021; Salama EMPACTA 2020; Salvarani 2020; Stone 2020). Tocilizumab probably increases the number of people who achieve clinical improvement compared with standard care alone or with placebo at a specific time point up to D28 to 90 (HR 1.23, 95% CI 1.08 to 1.39; I2 = 28.3%; 6 RCTs, 2118 participants; moderate‐certainty evidence).
Time to WHO Clinical Progression Score of level 7 or above
This outcome was reported in three RCTs (762 participants) (Hermine CORIMUNO‐19 2020; Salama EMPACTA 2020; Stone 2020). Tocilizumab probably reduces the number of people who reach the WHO Clinical Progression Score of level 7 or above compared with standard care alone or with placebo at a specific time point up to D28 to 90 (HR 0.62, 95% CI 0.42 to 0.91; I2 = 0.0%; 3 RCTs, 762 participants; moderate‐certainty evidence).
Time to death
This outcome was reported in three RCTs (1152 participants) (Gordon REMAP‐CAP 2021; Hermine CORIMUNO‐19 2020; Stone 2020). The evidence for an effect of tocilizumab compared with standard care alone or with placebo on time to death is uncertain (HR 0.65, 95% CI 0.51 to 0.83; I2 = 0.0%; 3 RCTs; 1152 participants; low‐certainty evidence).
Comparison 2. Sarilumab versus standard of care/placebo
A three‐arm trial (n = 420) (Lescure 2021), and one platform trial reported on the comparison of sarilumab (n = 48) with standard care (n = 412) (Gordon REMAP‐CAP 2021). We report the certainty evidence for the critical and for important outcomes in Table 2.
Critical outcomes
No data are available for clinical improvement (D28, ≥ D60), or WHO Clinical Progression Score of level 7 or above (D28, ≥ D60).
All‐cause mortality
The evidence for an effect of sarilumab compared with standard care alone/with placebo on all‐cause mortality at D28 is uncertain (RR 0.77, 95% CI 0.43 to 1.33; 2 RCTs, 880 participants; low‐certainty evidence) (Figure 8).
8.

Sarilumab compared to standard care for severe/critical COVID‐19: All‐cause mortality D28
The evidence for an effect of sarilumab compared with standard care alone/with placebo on all‐cause mortality at ≥ D60 is uncertain (RR 1.00, 95% CI 0.50 to 2.00; 1 RCT, 420 participants; low‐certainty evidence) (Figure 9).
9.

Sarilumab compared to standard care for severe/critical COVID‐19:All‐cause mortality D60 or above
Adverse events
Sarilumab is not likely to results in an increase in adverse events (RR 1.05, 95% CI 0.88 to 1.25; 1 RCT, 420 participants; absolute effect 32 more per 1000 (from 77 fewer to 160 more) ; moderate‐certainty evidence). However, an important increase cannot be excluded (Figure 10).
10.

Sarilumab compared to standard care for severe/critical COVID‐19: Adverse events
Serious adverse events
The evidence for an effect of sarilumab compared with standard care alone on serious adverse events is uncertain (RR 1.17, 95% CI 0.77 to 1.77; 2 RCTs, 880 participants; low‐certainty evidence) (Figure 11).
11.

Sarilumab compared to standard care for severe/critical COVID‐19:Serious adverse events
Important outcomes
(Table of results for sarilumab versus placebo or standard care: important outcomes reported in Appendix 9.)
No data are available for time to WHO Clinical Progression Score of level 7 or above.
Time to clinical improvement
The evidence for an effect of sarilumab compared with standard care alone/placebo on time to clinical improvement is uncertain (hazard ratio (HR) 1.28, 95% CI 0.88 to 1.87; 2 RCTs, 880 participants; low‐certainty evidence).
Time to death
The evidence for an effect of sarilumab compared with standard care alone/placebo on time to death is uncertain (HR 0.55, 95% CI 0.33 to 0.91; 1 RCT, 460 participants; low‐certainty evidence).
Investigation of heterogeneity
The limited number of RCTs that provided results and the absence of variation across trials in some variables such as age and gender prevented us from performing all pre‐planned subgroup analyses (see Differences between protocol and review). Some subgroup analyses were possible only for the comparisons evaluating tocilizumab. The results are available at https://zenodo.org/record/4605399#.YE9-Oi3pOfQ. We were able to explore the impact of trial location (multi‐national/national) and we also considered two post‐hoc subgroup analyses based on the type of funding and the presence of conflict of interest. Two of these characteristics appeared to have a substantial effect on the results based on the visual inspection of the forest plots and the test for subgroup differences: the conflict of interest of the trials and the type of funding. However, it is unclear whether the two characteristics had a real impact on the results as the summary effect in the subgroup including the Horby RECOVERY 2021 trial was mainly driven by this trial.
Sensitivity analysis
Sensitivity analyses were only possible for the comparison tocilizumab versus controls. With the exception of mortality, results were consistent when considering only trials reported as peer‐reviewed article. As noted with subgroup analysis, the difference in effect estimates when restricting to peer reviewed articles likely reflects the dominance of Horby RECOVERY 2021 in the analysis. The exclusion of one trial conducted in China (Wang 2020), judged as high risk of bias did not change the results. However, it led to an important reduction of the heterogeneity for the outcome adverse events. We also decided post‐hoc to check the robustness of results after excluding the trial (Rosas COVACTA 2021), that involved participants ranging from mild to critical disease. No important discrepancies in the summary results were observed when we used the number analyzed in the RCTs instead of the number randomised as denominator.
Discussion
Summary of main results
This review aimed to assess the effectiveness and safety of IL‐6 blocking agents for COVID‐19. We identified 10 RCTs with reported results. Participants were mainly patients with moderate‐severe disease. Three trials were reported as preprints (Horby RECOVERY 2021; Lescure 2021; Wang 2020). Four trials did not achieve their targeted sample size (Gordon REMAP‐CAP 2021; Salvarani 2020; Veiga TOCIBRAS 2021; Wang 2020).
Our results suggest that on average tocilizumab reduces all‐cause mortality at D28 compared to standard care alone or placebo. Results of important outcomes (time to clinical improvement, time to WHO progression score level 7 or above and time to death) were consistent with a beneficial effect of tocilizumab. Nevertheless, tocilizumab probably results in little or no increase in the outcome clinical improvement defined as hospital discharge or improvement on the scale used by trialists at Day D28. The discrepancy in these results could be related to the large variation in the information size across the outcomes. The beneficial effect of tocilizumab has been debated because of the important discrepancies in trial results. Several explanations for these discrepancies were discussed, particularly differences in cointerventions, particularly steroid, timing of treatment, severity of the disease, participants pattern of immune reaction (McCreary 2021). With the data available, we were not able to explore heterogeneity. Individual patient data meta‐analyses are needed to be able to identify which patients are more likely to benefit from this treatment.
Regarding safety outcomes, tocilizumab probably slightly reduces serious adverse events. Evidence for its effect on all other critical outcomes was of low or very low certainty.
Evidence on the impact of sarilumab on critical outcomes was of low certainty for most outcomes.
Overall completeness and applicability of evidence
We identified 49 registered RCTs evaluating IL‐6 blocking agents; only 10 had results available. All RCTs with results were multicentre and three involved several countries. We identified nine completed trials (total planned sample size 2169 participants) without results available, seven terminated trials without results available, 19 ongoing trials and four not recruiting.
The interpretation of the results of this review should be made with caution. Although participants included in these trials required oxygen or were intubated, disease severity was were heterogeneous. Four trials involved participants with critical disease (Gordon REMAP‐CAP 2021; Horby RECOVERY 2021; Rosas COVACTA 2021; Veiga TOCIBRAS 2021). The severity of the disease could be an important effect modifier. Similarly, markers of inflammation (C‐reactive protein (CRP)) varied between trials. However, because of the limited number of trials, heterogeneity and the impact of effect modifiers could not be explored adequately through subgroup analysis or meta‐regression. There was also heterogeneity in the use of steroid that became standard care over time. In some trials the treatment effect could be underestimated because of imbalances in the use of steroids (Hermine CORIMUNO‐19 2020; Rosas COVACTA 2021; Salama EMPACTA 2020), or planned cross‐over from the control group to active treatment (Salvarani 2020). Individual participant data meta‐analysis would enable a more definitive investigation of heterogeneity and help to establish who would likely benefit most from interleukin 6 blocking agents.
Considering the amount of awaiting data, the conclusions of subsequently updated reviews may allow for a better judgement regarding the effectiveness and safety of IL‐6 blocking agents.
Quality of the evidence
Overall, for tocilizumab, the certainty of the evidence ranged from very low for one critical outcome (adverse events), low for two critical outcomes (WHO Clinical Progression Score (level 7 or above) at D28, all‐cause mortality at D60 or above) and one important outcome (time to death), moderate for two critical outcomes (clinical improvement at D28, serious adverse events) and two important outcomes time to clinical improvement and time to WHO Clinical Progression Score (level 7 or above)), and high for one critical outcome (all‐cause mortality D28).
For sarilumab the certainty of the evidence was low for all outcomes except adverse events (moderate‐certainty evidence).
Reasons for downgrading the certainty of evidence were risk of bias, primarily due to some concerns about deviation from intended interventions and outcome measurement; imprecision, and inconsistency (see Table 1; Table 2).
Potential biases in the review process
We followed the guidance of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). Several potential biases in the review process were minimised. First, the search strategy was peer‐reviewed. We initially performed a thorough search in several electronic databases. Then, we evaluated the L‐OVE platform and the Cochrane clinical trial registry and showed searches on these platforms provided 100% sensitivity on the identification of COVID‐19 RCTs with considerable reduction in workload. Our search strategy was consequently modified. Second, all data were extracted in duplicate with consensus. Third, to increase our review’s informative value, we are tracking all registered trials in a living mapping. This allows investigators to be contacted to obtain an update on their trial status and inform them of our outcomes of interest. Finally, the review is updated continually. We search for new trials every working day, collect data and update the syntheses once a week. All updates of this review will be available on the COVID‐NMA platform (covid-nma.com).
However, important methodological issues arose. Indeed, COVID‐19 is a novel disease, and new knowledge is produced daily. Consequently, the choice of critical and important outcomes and prespecified subgroup analyses can evolve over time. Due to the lack of understanding of the disease when trials were planned, we identified a lot of heterogeneity in the outcomes assessed and the definitions used. We updated the review protocol to reduce the number of outcomes considered (Boutron 2020b).
Another consideration for this rapidly evolving field is the availability of preprint articles that have not yet undergone peer review. In this review, we also included preprints. However, we are aware of these publications' potentially differing quality and that results could change once the peer‐reviewed journal publications are available (Oikonomidi 2020). To overcome this issue, we developed a preprint tracker to be informed of updates and update data collection and data analysis when a preprint is modified or published.
Furthermore, patient care and consequently the standard of care evolves over time. A given trial could be stopped early if the peak of the pandemic has passed, or with recruitment over two periods (first and second wave), management has considerably evolved.
Finally, several studies were terminated and we have no data on the number of patients included in these studies.
Agreements and disagreements with other studies or reviews
We identified 23 systematic reviews focusing on IL6‐blocking agents for COVID‐19. Of these, 21 systematic reviews included only observational studies or preclinical studies and two included RCTs (Khan 2021; Tleyjeh 2021); the latter is a living systematic review (Tleyjeh 2021). Further, there are currently two large ongoing network meta‐analyses of COVID‐19 drug treatment (Juul 2020a; Juul 2020b; Siemieniuk 2020).
Authors' conclusions
Implications for practice.
On average, tocilizumab reduces all‐cause mortality at day 28 (D28) and probably results in slightly fewer serious adverse events compared to standard care alone or placebo. It is likely that tocilizumab increases time to clinical improvement and decreases time to intubation or death. Nevertheless, tocilizumab probably results in little or no increase in the outcome clinical improvement (defined as hospital discharge or improvement on the scale used by trialists) at D28. The impact of tocilizumab on other outcomes is uncertain.
Evidence for an effect of sarilumab is uncertain and evidence for other anti‐IL6 agents are not available.
Implications for research.
With the data available, we were not able to explore heterogeneity. The severity of disease varied within the trials we included, and individual patient data meta‐analyses are needed to identify which patients are most likely to benefit from this treatment.
Thirty‐nine RCTs of IL‐6 blocking agents with no results are currently registered, of which nine are completed and seven trials were terminated with no results available. The findings of this review will be updated as soon as new data are available on the COVID‐NMA platform (http://covid-nma.com).
What's new
| Date | Event | Description |
|---|---|---|
| 18 March 2021 | Amended | Reporting of results for the outcome of time to clinical improvement and time to WHO clinical progression score (level 7 or above) amended in 'Effects of intervention' |
History
Review first published: Issue 3, 2021
Acknowledgements
We particularly thank Elise Diard for her help in the website and extraction tool development, we also thank Laura De Nale for her help in the project management and communication with authors.
We would like to thank Sara Abboud and Hillary Bonnet for conducting quality control on the data extraction.
We would like to thank the peer reviewers for their invaluable comments during the preparation of this systematic review: Luke YC Chen and Corrado Campochiaro; and the protocol: Jen Hilgart, Chantelle Garritty, Jessica J Manson, and Jaume Alijotas‐Reig.
The editorial process was managed by Cochrane's Central Editorial Service in collaboration with Cochrane Emergency and Critical Care (ECC).
We thank: Helen Wakeford (Executive Editor of Central Editorial Service) who managed the editorial process; Leticia Rodrigues (Central Editorial Service Administrative Assistant) who provided editorial support; Robin Featherstone (Central Editorial Service Information Specialist) who provided peer review of search; Liz Bickerdike (Associate Editor) who commented on the methods; Jane Cracknell, who transferred the manuscript from Word to RevMan (Consultancy basis for Central Editorial Service); and Heather Maxwell (Copy‐Editor) for copy‐editing the manuscript. In addition we thank the following people for their help and advice with the review's RoB2 methods: Theresa Moore (Methodologist), Kayleigh Kew (Senior Methods Editor), and Kerry Dwan (Methods Support Unit Lead and Statistical Editor).
We acknowledge and thank Harald Herkner (Co‐ordinating Editor, ECC) and Vernon Hedge (Managing Editor, ECC), for their contribution to the editorial process.
Members of the COVID‐NMA consortium are listed below by alphabetical order
Solaf Alawadhi1,2, Sihem Amer‐Yahia3, Chiara Arienti4, David Auber5, Camila Ávila6, Aïda Bafeta2, FulviaBaldassarre7, Rita Banzi8, Julien Barnier9, Julia Baudry10, Hanna Bergman11, Claudia Bollig12, Hillary Bonnet1,13, Marinette Bouet14, Mohand Boughanem15, Isabelle Boutron1,2,16, Brian Buckley11, Guillaume Cabanac15, Anna Chaimani1,16, Sarah Charpy2, David Chavalarias17, Yaolong Chen18, Astrid Chevance2, Sarah Cohen‐Boulakia19, Elise Cogo11, Françoise Conil20, Emmanuel Coquery20, Mauricia Davidson2,16, Laura De Nale16, Declan Devane21, Elise Diard16, Taoufiq Dkaki15, Bastien Doreau14, Merwan El Asri19, Theodoros Evrenoglou1,16, Alice Fabbri22, Gilles Feron23, Gabriel Ferrand16, Leopold Fezeu10, Mathilde Fouet24, Joly Ghanawi25, Lina Ghosn El Chall16, Robin Featherstone26, Carolina Graña2,16, Giacomo Grasselli27, François Grolleau1, Benoit Groz19, Mohand‐Saïd Hacid20, Candyce Hamel11, Camilla Hansen22, Nicholas Henschke11, Ameer Hohlfeld28, Asbjørn Hróbjartsson22, Philipp Kapp2,16, Chantal Julia10, Dimitris Mavridis29, Joerg J Meerpohl12,30, Sonia Menon2,16, Brice Meyer14, Silvia Minozzi31, Jose G. Moreno15, Nivantha Naidoo2, Van Thu Nguyen16, Theodora Oikonomidi1,16, Matthew Page32, Jennifer Petkovic11, Elizabeth Pienaar28, Olivier Pierre2, Katrin Probyn11, Fiona Quirke33, Gabriel Rada6,34, Philippe Ravaud1,2,16, Pierre Ripoll20, Carolina Riveros2,16, Philippe Rivière20, Marie Sauvant14, Jelena Savovic35, Christine Schmucker30, Yanina Sguassero11, Jonathan Sterne36, Farouk Toumani14, David Tovey16, Gemma Villanueva11, Romain Vuillemot20, Jun Xia37, Xuan Yu18, Emina Zoletic1,2, Pierre Zweigenbaum38 (See Appendix 10 for list of COVID‐NMA consortium's participating members affiliations.)
Appendices
Appendix 1. Living process of the review
Steering committee
We set up a steering committee of epidemiologists, methodologists, statisticians and clinicians with content expertise. This committee will meet regularly, discuss the conduct of the project, difficulties encountered and possible changes in the protocol according to new knowledge available on COVID‐19 disease. Changes in the protocol could consist for example of changes in the search strategy, eligibility criteria (e.g. study design), research questions for the pairwise meta‐analyses, outcomes.
Process and quality control
Our aim is to update the synthesis at least every week. For this purpose, we will search, screen and extract data every day. The updated synthesis will be reported online at least every week.
To standardise the process and ensure both rapidity and quality, we will proceed as follows.
We will separate the process into different tasks and set up a team for each task (i.e. a researcher/volunteer will be involved in a single task). Each team will be led by a senior researcher ensuring the quality and standardisation of the task.
-
For some tasks, we will develop a short training program for researchers/volunteers joining the team. This program will involve:
reading a manual detailing the task;
performing the task on a sample as an exercise (e.g. evaluating the risk of bias of three studies) and contacting the team leader to ask about difficulties; and
after a successful training, the newcomer will perform the double data extraction with a senior well‐trained researcher.
Each team will hold a weekly meeting to discuss difficulties and ensure standardization. All decisions and changes will be recorded.
We will set‐up an internal quality control process where a senior researcher, and former editor in chief of Cochrane, (D Tovey) will check the data extracted and reported on the website. All points will be discussed with the data extraction team and modifications recorded for transparency.
We will develop an external quality control process for data collection involving senior researchers who will check a random sample of the data collected (e.g. member of the Cochrane Bias Methods Group for risk of bias)
We will consider the following tasks:
research mapping: screening and extracting data from registries;
screening of databases from title/abstract to full text;
data extraction;
data analyses;
assessment of evidence certainty.
The core team will perform the analysis, presentation and interpretation of the results.
Evolution of the protocol over time
The process will also evolve over time according to the new knowledge available regarding COVID‐19.
The steering committee will systematically discuss and achieve consensus on the changes of protocol proposed.
Appendix 2. Case definitions
Suspect case
A. A patient with acute respiratory illness (fever and at least one sign/symptom of respiratory disease (e.g. cough, shortness of breath)), AND with no other etiology that fully explains the clinical presentation AND a history of travel to or residence in a country/area or territory reporting local transmission of COVID‐19 disease during the 14 days prior to symptom onset.
OR
B. A patient with any acute respiratory illness AND having been in contact with a confirmed or probable COVID‐19 case (see definition of contact) in the last 14 days before onset of symptoms.
OR
C. A patient with severe acute respiratory infection (fever and at least one sign/symptom of respiratory disease (e.g. cough, shortness breath)) AND requiring hospitalisation AND no other etiology that fully explains the clinical presentation.
Probable case
A suspect case for whom testing for COVID‐19 is inconclusive (inconclusive being the result of the test reported by the laboratory).
Confirmed case
A person with laboratory confirmation of COVID‐19 infection, regardless of clinical signs and symptoms.
Of note, when the definition used to classify cases was not clearly reported, we will rely on the classification provided by authors.
Appendix 3. Search strategies
Current Strategy (last updated 11 December 2020)
| Source | |
| PubMed (2019 nCoV[tiab] OR 2019nCoV[tiab] OR corona virus[tiab] OR corona viruses[tiab] OR coronavirus[tiab] OR coronaviruses[tiab] OR COVID[tiab] OR COVID19[tiab] OR nCov 2019[tiab] OR SARS‐CoV2[tiab] OR SARS CoV‐2[tiab] OR SARSCoV2[tiab] OR SARSCoV‐2[tiab] OR "COVID‐19"[Mesh] OR "COVID‐19 Testing"[Mesh] OR "COVID‐19 Vaccines"[Mesh] OR "Coronavirus"[Mesh:NoExp] OR "SARS‐CoV‐2"[Mesh] OR "COVID‐19"[nm] OR "COVID‐19 drug treatment"[nm] OR "COVID‐19 diagnostic testing"[nm] OR "COVID‐19 serotherapy"[nm] OR "COVID‐19 vaccine"[nm] OR "LAMP assay"[nm] OR "severe acute respiratory syndrome coronavirus 2"[nm] OR "spike protein, SARS‐CoV‐2"[nm]) NOT ("animals"[mh] NOT "humans"[mh]) NOT (editorial[pt] OR newspaper article[pt]) Embase.com ((('coronaviridae'/de OR 'coronavirinae'/de OR 'coronaviridae infection'/de OR 'coronavirus disease 2019'/exp OR 'coronavirus infection'/de OR 'SARS‐related coronavirus'/de OR 'Severe acute respiratory syndrome coronavirus 2'/exp OR '2019 nCoV':ti,ab,kw OR 2019nCoV:ti,ab,kw OR ((corona* OR corono*) NEAR/1 (virus* OR viral* OR virinae*)):ti,ab,kw OR coronavir*:ti,ab,kw OR coronovir*:ti,ab,kw OR COVID:ti,ab,kw OR COVID19:ti,ab,kw OR HCoV*:ti,ab,kw OR 'nCov 2019':ti,ab,kw OR 'SARS CoV2':ti,ab,kw OR 'SARS CoV 2':ti,ab,kw OR SARSCoV2:ti,ab,kw OR 'SARSCoV 2':ti,ab,kw) NOT (('animal experiment'/de OR 'animal'/exp) NOT ('human'/exp OR 'human experiment'/de))) NOT 'editorial'/it) NOT ([medline]/lim OR [pubmed‐not‐medline]/lim) AND [1‐12‐2019]/sd CENTRAL 1 ("2019 nCoV" OR 2019nCoV OR "corona virus*" OR coronavirus* OR COVID OR COVID19 OR "nCov 2019" OR "SARS‐CoV2" OR "SARS CoV‐2" OR SARSCoV2 OR "SARSCoV‐2"):TI,AB AND CENTRAL:TARGET 2 Coronavirus:MH AND CENTRAL:TARGET 3 Coronavirus:EH AND CENTRAL:TARGET 4 #1 OR #2 OR #3 5 2019 TO 2021:YR AND CENTRAL:TARGET 6 #5 AND #4 7 INSEGMENT 8 #6 NOT #7 ClinicalTrials.gov COVID‐19 OR 2019‐nCoV OR SARS‐CoV‐2 OR coronavirus WHO ICTRP We screen the entire COVID‐19.csv file available from https://www.who.int/emergencies/diseases/novel-coronavirus-2019 medRxiv We screen the entire COVID‐19 results identified by the Stephen B. Thacker CDC Library |
LOVE
| Source | Search strategy |
| Epistemonikos Database | coronavir* OR coronovirus* OR betacoronavir* OR "beta‐coronavirus" OR "beta‐coronaviruses" OR "corona virus" OR "virus corona" OR "corono virus" OR "virus corono" OR hcov* OR "covid‐19" OR covid19* OR "covid 19" OR "2019‐ncov" OR cv19* OR "cv‐19" OR "cv 19" OR "n‐cov" OR ncov* OR (wuhan* and (virus OR viruses OR viral)) OR sars* OR sari OR (covid* and (virus OR viruses OR viral)) OR "severe acute respiratory syndrome" OR mers* OR "middle east respiratory syndrome" OR "middle‐east respiratory syndrome" OR "covid‐19‐related" OR "2019‐ncov‐related" OR "cv‐19‐related" OR "n‐cov‐related" |
Appendix 4. Characteristics of registered studies
Characteristics of unpublished studies: completed studies
| NCT04315298 | |
| Trial name or title | Evaluation of the efficacy and safety of sarilumab in hospitalized patients With COVID‐19 |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: March 2020 Location: USA Phase 2/3 |
| Participants | Randomised: 1912 participants Inclusion criteria
Exclusion criteria
Phase 3 cohort 2 only
|
| Interventions |
Intervention: sarilumab (low, mild or high dose, dosage not stated) Control interventions: placebo |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: 18 March 2020 Study completion date: 2 September 2020 |
| Contact information | Regeneron Pharmaceuticals |
| Notes | Completed |
| EUCTR‐2020‐001162‐12‐FR | |
| Trial name or title | An adaptive phase 3, randomized, double‐blind, placebo‐controlled, study assessing efficacy and safety of sarilumab for hospitalized patients with COVID‐19 |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: March 2020 Location: Canada, France, Germany, Israel, Italy, Japan, Russian Federation, Spain Phase 2/3 |
| Participants | Randomised: 460 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: sarilumab (IV, 200 mg) Control interventions: placebo (IV) |
| Outcomes |
Primary outcome Secondary outcomes
|
| Starting date | Study start date: 26 March 2020 Study completion date: 29 September 2020 |
| Contact information | Sanofi‐Aventis France, Public‐Registry‐MA‐France@sanofi.com |
| Notes | Completed |
| NCT04380519 | |
| Trial name or title | Study of the efficacy and safety of a single administration of olokizumab and RPH‐104 with standard therapy in patients with severe severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection (COVID‐19) |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: May 2020 Location: Russian Federation Phase 2/3 |
| Participants | Randomised: 372 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention
Control interventions
|
| Outcomes |
Primary outcome Secondary outcomes
|
| Starting date | Study start date: 23 April 2020 Study completion date: 24 July 2020 |
| Contact information | R‐Pharm, Mikhail Samsonov |
| Notes | Completed |
| NCT04397562 | |
| Trial name or title | A clinical trial of the efficacy and safety of levilimab (BCD‐089) in patients with severe COVID‐19 |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: May 2020 Location: Russian Federation Phase 3 |
| Participants | Randomised: 206 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: levilimab (SC, 324 mg) Control interventions: standard of care |
| Outcomes |
Primary outcome Secondary outcomes
|
| Starting date | Study start date: 29 April 2020 Study completion date: 3 August 2020 |
| Contact information | Biocad |
| Notes | Completed |
| NCT04479358 | |
| Trial name or title | Low‐dose tocilizumab versus standard of care in hospitalized patients with COVID‐19 |
| Methods | RCT, active‐controlled, open‐label study Date of study: July 2020 Location: USA Phase 2 |
| Participants | Randomised: 332 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: ·tocilizumab (IV, 8 mg/kg, up to a maximum dose 800 mg, up to 1 additional dose may be given if clinical symptoms worsen or show no improvement) Control interventions: placebo |
| Outcomes |
Primary outcome Secondary outcomes
|
| Starting date | Study start date: 10 September 2020 |
| Contact information | University of Chicago Medicine, Pankti D Reid, pankti.reid@uchospitals.edu |
| Notes | Completed |
| IRCT20200525047570N1 | |
| Trial name or title | A comparative study of the effects of tocilizumab, interferon‐gamma and vitamin C on the recovery of critically ill Covid‐19 patients and cytokine storm |
| Methods | RCT, active‐controlled, open‐label study Date of study: July 2020 Location: Iran Phase 2 |
| Participants | Randomised: 60 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: tocilizumab (SC, 62 mg/0.9 mL, 1 time a week for 2 weeks) Control interventions: standard of care |
| Outcomes |
Primary outcome Secondary outcomes |
| Starting date | Study start date:‐ |
| Contact information | Tabriz University of Medical Sciences, Negin Hadisi, +98 44 4432 6311, nhadisi72@yahoo.com |
| Notes | Completed |
| NCT04690920 | |
| Trial name or title | Theranostic Implication of complementary medicines against interleukin receptors and Gp‐130 proteins |
| Methods | RCT, active‐controlled, open‐label study Date of study: December 2020 Location: Pakistan Phase ‐ |
| Participants | Randomised: 200 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention
Control interventions
|
| Outcomes |
Primary outcome Secondary outcomes
|
| Starting date | Study start date: 23 July 2020 |
| Contact information | The University of Lahore, Arif Malik |
| Notes | Completed |
| IRCT20200510047383N1 | |
| Trial name or title | Evaluation of the effect of tocilizumab on outcomes of the severe COVID‐19 patients |
| Methods | RCT, active‐controlled, double‐blind study Date of study: May 2020 Location: Iran Phase 3 |
| Participants | Randomised: 100 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: tocilizumab (8 mg per kilogram of body weight tocilizumab up to a maximum dose of 800 mg) Control interventions: standard of care |
| Outcomes |
Primary outcome
Secondary outcomes |
| Starting date | Study start date: 21 May 2020 |
| Contact information | Arak University of Medical Sciences, Mohammadreza Bozorgmanesh, +98 86 3223 1350, mhmmdrz_bzrgmnsh@yahoo.com |
| Notes | Completed |
| IRCT20081027001411N4 | |
| Trial name or title | Effect of TOCILIZUMAB (ACTEMRA) on treatment of COVID‐19 |
| Methods | RCT, active/placebo‐controlled, double‐blind study Date of study: June 2020 Location: Iran Phase 2 |
| Participants | Randomised: 40 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: tocilizumab (8 mg/kg, if the patient’s condition is not stable 2 doses by 12 hours will be administrated, maximum dose: 800 mg) Control interventions
|
| Outcomes |
Primary outcome
Secondary outcomes |
| Starting date | Study start date:‐ |
| Contact information | Tehran University of Medical Sciences, Ahmadreza Jamshidi, +98 21 8822 0065, jamshida@sina.tums.ac.ir |
| Notes | Completed |
Ongoing studies
| NCT04330638 | |
| Trial name or title | Treatment of COVID‐19 patients with anti‐interleukin drugs |
| Methods | RCT, active‐controlled, open‐label study Date of study: April 2020 Location: Belgium Phase 3 |
| Participants | Randomised: 342 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention
Control interventions Usual care |
| Outcomes |
Secondary outcomes
|
| Starting date | Study start date: 3 April 2020 |
| Contact information | University Hospital, Ghent, Anja Delporte, +32‐9‐3320228, anja.delporte@uzgent.be |
| Notes | Ongoing |
| NL8504 | |
| Trial name or title |
Pre‐emptive tocilizumab in hypoxic COVID‐19 patients, a prospective randomized trial |
| Methods | RCT, active‐controlled, open‐label study Date of study: April 2020 Location: the Netherlands |
| Participants | Randomised: 354 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: ·tocilizumab (IV, 8 mg/kg, maximum dose 800 mg, which can be repeated at the same dose after 8 hours if the hypoxia has not improved) Control interventions: standard of care (unclear) |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: 6 April 2020 |
| Contact information | UMCG, Margriet Dijkstra, +31 50 3610468, m.j.dijkstra‐tiekstra@umcg.nl |
| Notes | Ongoing |
| NCT04348500 | |
| Trial name or title | Clazakizumab (anti‐IL‐ 6 monoclonal) compared to placebo for COVID19 disease |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: April 2020 Location: USA Phase 2 |
| Participants | Randomised: 17 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: clazakizumab (IV, 25 mg in 50 cc NS x 1 dose) Control interventions: placebo |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: 24 April 2020 |
| Contact information | Cedars‐Sinai Medical Center, Stanley Jordan, MD |
| Notes | Ongoing |
| NCT04357808 | |
| Trial name or title | Efficacy of subcutaneous sarilumab in hospitalised patients with moderate‐severe COVID‐19 infection (SARCOVID) |
| Methods | RCT, active‐controlled, open‐label study Date of study: April 2020 Location: Spain Phase 2 |
| Participants | Randomised: 30 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: sarilumab (SC, 2 x 200 mg, single dose) Control interventions: standard of care |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: 13 April 2020 |
| Contact information | Fundación de Investigación Biomédica ‐ Hospital Universitario de La Princesa, Rosario Garcia de Vicuña, MD PhD |
| Notes | Ongoing |
| NCT04359901 | |
| Trial name or title | Sarilumab for patients with moderate COVID‐19 disease |
| Methods | RCT, active‐controlled, open‐label study Date of study: April 2020 Location: USA Phase 2 |
| Participants | Randomised: 120 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: sarilumab (SC,400 mg) Control interventions: standard of care |
| Outcomes |
Primary outcome · Intubation or death (within 14 days of enrolment) Secondary outcomes |
| Starting date | Study start date: 10 April 2020 |
| Contact information | Westyn Branch‐Elliman, VA Boston Healthcare System, Sara Schiller, MPH, 857‐364‐2012, Sara.Schiller1@va.gov |
| Notes | Ongoing |
| NCT04363502 | |
| Trial name or title | Use of the interleukin‐6 inhibitor clazakizumab in patients with life‐threatening COVID‐19 infection |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: April 2020 Location: USA Phase 2 |
| Participants | Randomised: 30 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: clazakizumab (IV, 25 mg, if the CRP does not decrease by 50% within 36 to 48 hours after the 1st dose, a 2nd dose of 25 mg clazakizumab will be given no later than day 3) Control interventions: placebo |
| Outcomes |
Primary outcome Secondary outcomes |
| Starting date | Study start date: 7 May 2020 |
| Contact information | Johns Hopkins University, Nada Alachkar, MD, 4106149225, nalachk1@jhmi.edu |
| Notes | Ongoing |
| NCT04377750 | |
| Trial name or title | The use of tocilizumab in the management of patients who have severe COVID‐19 with suspected pulmonary hyperinflammation |
| Methods | RCT, placebo‐controlled, open‐label study Date of study: May 2020 Location: Israel Phase 4 |
| Participants | Randomised: 500 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: tocilizumab(IV, 8 mg/kg up to total dose of 800 mg) Control interventions: placebo |
| Outcomes |
Primary outcome
Secondary outcomes |
| Starting date | Study start date: 8 April 2020 |
| Contact information | Hadassah Medical Orginisation, Reuven Pizov, Prof., 972‐50‐6265542, pizovr@hadassah.org.il |
| Notes | Ongoing |
| EUCTR2020‐002037‐15‐ES | |
| Trial name or title | Multicenter, randomized, open‐label study to evaluate the efficacy and safety of SOC + sarilumab versus standard of care for the early treatment of COVID‐19‐pneumonia in hospitalized patients |
| Methods | RCT, active‐controlled, open‐label study Date of study: May 2020 Location: Spain Phase 2 |
| Participants | Randomised: 200 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: sarilumab (IV, 200 mg) Control interventions: standard of care |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: 25 May 2020 |
| Contact information | Hospital Universitario Puerta de Hierro Majadahonda, 0034911917479, mariabelen.ruiz@salud.madrid.org |
| Notes | Ongoing |
| NCT04412291 | |
| Trial name or title | A study in patients with COVID‐19 and respiratory distress not requiring mechanical ventilation, to compare standard‐of‐care with anakinra and tocilizumab treatment the immunomodulation‐CoV assessment (ImmCoVA) study |
| Methods | RCT, active‐controlled, open‐label study Date of study: June 2020 Location: Sweden Phase 2 |
| Participants | Randomised: 120 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention
Control interventions
|
| Outcomes |
Primary outcome Secondary outcomes
|
| Starting date | Study start date: 11 June 2020 |
| Contact information | Karolinska University Hospital, Jonas Sundén‐Cullberg, +46‐8‐58580000, Jonas.sunden‐cullberg@sll.se |
| Notes | Ongoing |
| NCT04412772 | |
| Trial name or title | A RCT ‐ safety & efficacy of tocilizumab ‐ Tx of severe COVID‐19: ARCHITECTS |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: June 2020 Location: USA Phase 3 |
| Participants | Randomised: 300 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: tocilizumab (IV, 8 mg/kg for up to max 800 mg, single infusion, 1 additional dose may be given if clinical symptoms worsen) Control interventions: placebo |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: 12 June 2020 |
| Contact information | Queen's Medical Centre, Todd Seto, tseto@queens.org |
| Notes | Ongoing |
| EUCTR2020‐001390‐76‐IT | |
| Trial name or title | A phase 3, randomized, open‐labeled, multi‐center study comparing clinical efficacy and safety of intravenous sarilumab plus standard of care compared to standard of care, in the treatment of patients with severe COVID‐19 pneumonia. |
| Methods | RCT, active‐controlled, open‐label study Date of study: June 2020 Location: Italy Phase 3 |
| Participants | Randomised: 171 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: ·sarilumab (IV, 400 mg) Control interventions: standard of care |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: 27 April 2020 |
| Contact information | Istituto Nazionale per le Malattie Infettive Lazzaro Spallanzani, 0655170546, immunodeficienzevirali@inmi.it |
| Notes | Ongoing |
| CTRI/2020/05/025369 | |
| Trial name or title | A study on treatment of COVID‐19 patients with study drug along with standard of care |
| Methods | RCT, active‐controlled, open‐label study Date of study: May 2020 Location: India Phase 3 |
| Participants | Randomised: 180 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: tocilizumab (IV, 6 mg/kg for up to max 480 mg, single infusion) Control interventions: standard of care |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: 30 May 2020 |
| Contact information | Medanta Institute of Education and Research (MIER), 01244855100, pooja.sharma@medanta.org |
| Notes | Ongoing |
| NCT04494724 | |
| Trial name or title | Clazakizumab vs. placebo ‐ COVID‐19 infection |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: July 2020 Location: USA Phase 2 |
| Participants | Randomised: 60 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: clazakizumab (IV, 25 mg in 50 mL of 0.9% saline) Control interventions: placebo |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: 13 July 2020 |
| Contact information | Houston Methodist Hospital, Isioma Agboli, 713‐441‐6311, iagboli@houstonmethodist.org |
| Notes | Ongoing |
| NCT04577534 | |
| Trial name or title | Use of tocilizumab in the inflammatory phase of COVID‐19 / new coronavirus disease |
| Methods | RCT, active‐controlled, open‐label study Date of study: October 2020 Location: Finland Phase 3 |
| Participants | Randomised: 90 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: tocilizumab (IV, single infusion according to weight of patient) Control interventions: standard of care |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: 14 August 2020 |
| Contact information | Turku University Hospital, Jarmo Oksi, +358 40 5414813, jarmo.oksi@utu.fi |
| Notes | Ongoing |
| EUCTR2020‐001290‐74‐ES | |
| Trial name or title | Efficacy and safety of sarilumab in the early treatment of hospitalized patients with mild‐moderate pneumonia and COVID19 infection versus standard of care |
| Methods | RCT, active‐controlled, open‐label study Date of study: April 2020 Location: Spain Phase 3 |
| Participants | Randomised: 216 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: sarilumab Control interventions: usual care |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: 11 April 2020 |
| Contact information | Consorci PSMAR, Ana Aldea, +34933160490, aaldea@imim.es |
| Notes | Ongoing |
| EUCTR2020‐001767‐86‐IE | |
| Trial name or title | An open‐label, multi‐centre, randomised trial comparing different doses of single‐dose tocilizumab in adults with severe, non‐critical, PCR‐confirmed COVID‐19 infection with evidence of progressive |
| Methods | RCT, active‐controlled, open‐label study Date of study: April 2020 Location: Ireland Phase 2 |
| Participants | Randomised: 90 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: tocilizumab (IV, single dose, different doses) Control interventions: standard of care |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: 25 June 2020 |
| Contact information | University College Dublin, crc.monitoring@ucd.ie |
| Notes | Ongoing |
| NCT04659772 | |
| Trial name or title | A study to evaluate clazakizumab in patients with life‐threatening COVID‐19 infection. |
| Methods | RCT, placebo‐controlled, blind‐label study Date of study: December 2020 Location: USA Phase 2 |
| Participants | Randomised: 30 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: clazakizumab(IV, single dose, 25 mg, repeated dose for patients who fail expected decrease in inflammatory markers) Control interventions: placebo |
| Outcomes |
Primary outcome
Secondary outcomes |
| Starting date | Study start date: ‐ |
| Contact information | Mayo Clinic in Arizona, Ayan Sen |
| Notes | Ongoing |
| NCT04343989 | |
| Trial name or title | A randomized placebo‐controlled safety and dose‐finding study for the use of the IL‐6 inhibitor clazakizumab in patients with life‐threatening COVID‐19 Infection |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: April 2020 Location: USA Phase 2 |
| Participants | Randomised: 90 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: clazakizumab (IV, 25 mg/12.5 mg, the CRP does not decrease by 50% within 36 to 48 hours after the 1st dose, a 2nd dose of 25 mg/12.5 mg will be given no later than day 3) Control interventions: placebo |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: 31 March 2020 |
| Contact information | NYU Langone Health, Bonnie Lonze, MD, 212‐263‐8365, bonnie.lonze@nyulangone.org |
| Notes | Ongoing |
| NCT04357860 | |
| Trial name or title | Clinical trial of sarilumab in adults with COVID‐19 |
| Methods | RCT, active‐controlled, open‐label study Date of study: April 2020 Location: Spain Phase 2 |
| Participants | Randomised: 120 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: sarilumab (SC, 200 mg/400 mg, up to 14 days) Control interventions: standard of care |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: ‐ |
| Contact information | Hospital Universitario Reina Sofía, Antonio Luque, 0034671596070, uicec@imibic.org |
| Notes | Ongoing |
Studies that are registered but not recruiting
| NCT04381052 | |
| Trial name or title | Study for the use of the IL‐6 Inhibitor clazakizumab in patients with life‐threatening COVID‐19 infection |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: May 2020 Location: USA Phase 2 |
| Participants | Randomised: 30 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: clazakizumab (IV, 25 mg, if the CRP does not decrease by 50% within 36 to 48 hours after the 1st dose, a 2nd dose of placebo will be given no later than day 3) Control interventions: placebo |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date: ‐ |
| Contact information | Columbia University, David J. Cohen, MD, 212‐305‐3273, djc5@cumc.columbia.edu |
| Notes | Registered but not recruiting |
| NCT04452474 | |
| Trial name or title | Study of the efficacy and safety of a single administration of olokizumab vs. placebo in addition to standard treatment in patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection (COVID‐19) |
| Methods | RCT, placebo‐controlled, double‐blind study Date of study: June 2020 Location: USA Phase 2/3 |
| Participants | Randomised: 376 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: · olokizumab (SC, 64 mg, single dose on day 1) Control interventions: placebo |
| Outcomes |
Primary outcome
Secondary outcomes
|
| Starting date | Study start date:‐ |
| Contact information | R‐Pharm, Mikhail Samsonov |
| Notes | Registered but not recruiting |
| ACTRN12620000580976 | |
| Trial name or title | Tocilizumab for the treatment of COVID‐19 in intensive care patients: effect on days free of ventilatory support |
| Methods | RCT, active‐controlled, open‐label study Date of study: April 2020 Location: Australia Phase 1/2 |
| Participants | Randomised: 150 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: tocilizumab (IV, 400 mg, single dose) Control intervention: standard of care |
| Outcomes |
Primary outcome Secondary outcomes
|
| Starting date | Study start date: 19 October 2020 |
| Contact information | QIMR Berghofer Medical Research Institute, Bridget Barber, +61733620104, bridget.barber@qimrberghofer.edu.au |
| Notes | Registered but not recruiting |
| CTRI/2020/12/029793 | |
| Trial name or title | Efficacy and safety of tocilizumab in patients with severe COVID‐19 pneumonia on steroid therapy: a prospective, randomized, double blind placebo‐controlled trial |
| Methods | RCT, placebo‐controlled, blind‐label study Date of study: December 2020 Location: India Phase 3 |
| Participants | Randomised: 54 participants Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: tocilizumab (IV, single dose, 8 mg/kg body weight) Control interventions: placebo |
| Outcomes |
Primary outcome Secondary outcomes
· Change of ventilation mode and invasiveness |
| Starting date | Study start date: 31 December 2020 |
| Contact information | Nehru Hospital, Naveen Naik, navin_amc@yahoo.com |
| Notes | Registered but not recruiting |
ALT: alanine aminotransferase; ANC: absolute neutrophil count; anti‐HCV: HCV antibody test; ARDS: acute respiratory distress syndrome; AST: aspartate aminotransferase; BAL: bronchoalveolar lavage;BCRSS: Brescia COVID respiratory severity score; BP: blood pressure; CECT: contrast enhanced computerised tomography; CCL: creatinine clearance; COPD: chronic obstructive pulmonary disease; cPAP: continuous positive airway pressure; CRP: C‐reactive protein; CURB‐65: also known as the CURB criteria (clinical prediction rule); CT: computerised tomography; CXR: chest radiograph; DD: D‐Dimer; DMARDs: disease modifying antirheumatic drugs; ECMO: extracorporeal membrane oxygenation; eGFR: estimated glomerular filtration rate; EUA: emergency use authorisation; FDA: Food and Drug Agency; GI: gastro intestinal; HBV: hepatitis B; HCV: hepatitis C; Hgb: haemoglobin; IC: inclusion criteria; ICU: intensive care unit;IL: interleukin; IP: investigational product; IUD: intrauterine device; IUS: intrauterine hormone‐releasing system; IV: intravenous; JAKi: Janus kinase inhibitors; L: litre; LAR: legally acceptable representative; LDH: lactate dehydrogenase; LSN: lymphocyte:segmented neutrophils; MEWS: modified early warning score; MTX: methotrexate; NYHA: New York Heart Association; NIV: non invasive ventilation; NS: normal saline; NYU: New York University; PCR: polymerase chain reaction; PEEP: positive end‐expiratory pressure; PPD: purified protein derivative; qSOFA: quick sequential organ failure assessment score; RCT: randomised controlled trial; RNA: ribonucleic acid; RT‐PCR: real time polymerase chain reaction; SARS: severe acute respiratory syndrome; SARS‐CoV‐2: severe acute respiratory syndrome coronavirus 2; SBP: systolic blood pressure; SC: sub cutaneous; SGOT: serum glutamic‐oxaloacetic transaminase; SGPT: serum glutamic pyruvic transaminase; TB: tuberculosis; TCZ: tocilizumab; TNF: tumour necrosis factor; TNFα: tumour necrosis factor alpha; ULN: upper limit of normal; US: ultra sound; WBC: white blood count;WHO: World Health Organization
Appendix 5. Details on the request to authors of unpublished trials
(i.e. Update on the status of the study: If ongoing, communicate the expected completion date; If complete, request to share results before publications) for information sent to investigators of IL 6‐blocking agent trials registered.
| Study ID | Trialist’s contact name | Treatment | Date of contact | Reply (last check 28 January 2021) |
| EUCTR2020‐001275‐32‐DK / NCT04322773 | Dr Lars Erik Kristensen | Sarilumab/ Tocilizumab |
3 Nov 2020 | No response |
| NCT04333914 | Dr Virginie Avrillon | Tocilizumab | 3 Nov 2020 | Cancelled |
| NL8504 | Dr Margriet Dijkstra | Tocilizumab | 3 Nov 2020 | No response |
| NCT04335071 | Dr Peter M. Villiger | Tocilizumab | 3 Nov 2020 | Stopped Couldn’t include a sufficient number of patients |
| NCT04361552 | Dr Ajay K Nooka | Tocilizumab | 3 Nov 2020 | Cancelled |
| EUCTR2020‐001408‐41‐DE | Dr Tobias Wengenmayer | Tocilizumab | 3 Nov 2020 | Stopped |
| EUCTR2020‐001770‐30‐BE | Dr Camelia Rossi | Tocilizumab | 3 Nov 2020 | No response |
| NCT04377750 | Dr.Juli Benbenisty | Tocilizumab | 3 Nov 2020 | Required further details – willing to collaborate |
| IRCT20200510047383N1 | Dr Mohammadreza Bozorgmanesh | Tocilizumab | 3 Nov 2020 | No response |
| ACTRN12620000580976 | Prof Bridget Barber | Tocilizumab | 3 Nov 2020 | Not started yet Lack of patients in Australia |
| NCT04412291 | Dr Jon Lampa | Tocilizumab | 3 Nov 2020 | Recruiting The expected completion date is December 2021 |
| NCT04412772 | Dr Todd Seto | Tocilizumab | 3 Nov 2020 | No response |
| NCT04435717 | Dr Jose A Perez‐Molina | Tocilizumab | 3 Nov 2020 | Stopped Will share data on included patients |
| CTRI/2020/05/025369 | Dr Pooja Sharma | Tocilizumab | 3 Nov 2020 | No response |
| IRCT20081027001411N4 | Dr Mahdi Mahmoudi | Tocilizumab | 3 Nov 2020 | No response |
| NCT04479358 | Dr Pankti D Reid | Tocilizumab | 3 Nov 2020 | Completed |
| IRCT20200525047570N1 | Dr Negin Hadisi | Tocilizumab | 3 Nov 2020 | Completed |
| NCT04577534 | Dr Jarmo Oksi | Tocilizumab | 3 Nov 2020 | Ongoing The expected completion date is in 2 to 3 months. |
| EUCTR2020‐001767‐86‐IE | University College Dublin ‐ QRAM | Tocilizumab | 3 Nov 2020 | No response |
| NCT04690920 | Arif Malik | Tocilizumab | 3 Nov 2020 | No response |
| CTRI/2020/12/029793 | Naveen Naik B | Tocilizumab | 3 Nov 2020 | No response |
| NCT04315298 | Regeneron Pharmaceuticals | Sarilumab | 3 Nov 2020 | Required further details |
| EUCTR‐2020‐001246‐18‐FR | Dr Cécile Kedzia | Sarilumab/ Tocilizumab |
3 Nov 2020 | No response |
| NCT04345289 | Dr Thomas Benfield | Sarilumab | 3 Nov 2020 | Stopped |
| NCT04357808 | Dr Rosario Garcia de Vicuña | Sarilumab | 3 Nov 2020 | Ongoing The expected completion date is December 2020 |
| NCT04357860 | Dr Julián de la Torre Cisneros | Sarilumab | 03/11/2020 | Ongoing The expected completion date is in 6 to 7 months. |
| NCT04359901 | Dr Westyn Branch‐Elliman | Sarilumab | 3 Nov 2020 | Ongoing The expected completion date is not provided |
| EUCTR2020‐002037‐15‐ES | Dr Belen Ruiz Antorán | Sarilumab | 3 Nov 2020 | Recruiting The expected completion date is January 2021. |
| EUCTR2020‐001390‐76‐IT | Dr Giovanna Onnelli | Sarilumab | 3 Nov 2020 | Ongoing The expected completion date is May to June 2021. |
| EUCTR2020‐001290‐74‐ES | Dr Ana Aldea | Sarilumab | 3 Nov 2020 | No response |
| NCT04351724 | Dr Bernd Jilma | Clazakizumab | 3 Nov 2020 | Cancelled |
| NCT04343989 | Dr Bonnie Lonze | Clazakizumab | 3 Nov 2020 | No response |
| NCT04348500 | Dr Noriko Ammerman | Clazakizumab | 9 Nov 2020 | No response |
| NCT04363502 | Dr Nada Alachkar | Clazakizumab | 3 Nov 2020 | No response |
| NCT04381052 | Dr David J. Cohen | Clazakizumab | 3 Nov 2020 | No response |
| NCT04494724 | Dr Isioma Agboli | Clazakizumab | 3 Nov 2020 | No response |
| NCT04659772 | Dr Ayan Sen | Clazakizumab | 26 Jan2021 | No response |
| NCT04380519 | Dr Mikhail Samsonov | Olokizumab | 20 Nov 2020 | No response |
| NCT04452474 | Dr Mikhail Samsonov | Olokizumab | 3 Nov 2020 | No response |
| NCT04397562 | Biocad | Levilimab | Not yet contacted | No correspondence email |
| NCT04330638 | Dr Bart Lambrecht | Siltuximab/ Tocilizumab |
1 Dec 2020 | No response |
Appendix 6. Details on the request for information sent to authors of published IL 6‐blocking agent trials
| Study ID | Author’s contact name | Treatment | Requested information |
Date of contact |
Reply (last check 27/01/2021) |
| Rosas COVACTA 2021 | Dr Ivan O. Rosas | Tocilizumab | Study’s protocol and statistical plan + some missing data for: outcomes, co‐interventions, participant characteristics | 25 Sep 2020 | Missing data requested received on 7 December 2020. Protocol and statistical plan still not available. |
| Wang 2020 | Drs Xiaoling Xu and Xiaodong Mei | Tocilizumab | Study’s protocol and statistical plan +some missing data for: outcomes, co‐interventions, participant characteristics | 25 Sep 2020 | Cannot share before publication |
| Hermine CORIMUNO‐19 2020 | Dr Xavier Mariette | Tocilizumab | Request to share all required data | 9 Oct 2020 | Publication received+ all requested data 23 October 2020 |
| Salvarani 2020 | Dr Carlo Salvarani | Tocilizumab | Some missing data for: outcomes, co‐interventions, participant characteristics | 3 Nov 2020 | No response |
| Stone 2020 | Dr Stone | Tocilizumab | Some missing data for: outcomes, co‐interventions, participant characteristics | 3 Nov 2020 | No response |
| Salama EMPACTA 2020 | Dr Shalini V. Mohan | Tocilizumab | Study’s protocol and statistical plan + some missing data for: Outcomes, Co‐interventions, Participant characteristics | 3 Nov 2020 | e‐mail received with some of the data requested on 3 Dec 2020 |
| Gordon REMAP‐CAP 2021 | Dr Anthony Gordon | Sarilumab Tocilizumab |
Some missing data for: outcomes, co‐interventions, participant characteristics | 25 Jan 2021 | No response |
| Veiga TOCIBRAS 2021 | 1. Viviane C Veiga | Tocilizumab | Some missing data for: outcomes, co‐interventions, participant characteristics | 1 Feb 2021 | |
| Lescure 2021 | 2. Dr Francois X Lescure | Sarilumab | Some missing data for: outcomes, co‐interventions, participant characteristics | 12 Feb 2021 | No response |
| Horby RECOVERY 2021 | Professor Peter W Horby and Professor Martin J Landray | Tocilizumab | * | * | Not yet contacted |
Appendix 7. Matrices indicating availability of trial results for critical and important outcomes
Appendix 7.1 Matrix indicating availability of trial results for the critical and important outcomes of the review. Tocilizumab versus standard care/placebo
Appendix 7.1.1 Matrix indicating availability of trial results for the critical outcomes of the review. Tocilizumab versus standard care/placebo
| Critical outcomes | |||||||||||
| Trial ID | Study follow‐up (in days) |
TZ n |
Standard of care or Placebo n |
All‐cause mortality | Clinical improvement | WHO SCORE 7 and above | AE | SAE | |||
| Day 28 | Day ≥60 | Day 28 | Day ≥60 | Day 28 | Day ≥60 | ||||||
| Rosas COVACTA 2021 | 60 | 301 | 151 | ✓ | * | * | * | ✓ | * | ✓ | ✓ |
| Wang 2020 | 14 | 33 | 32 | X | * | * | * | ✓ | * | ✓ | ✓ |
| Hermine CORIMUNO‐19 2020 | 90 | 64 | 67 | ✓ | ✓ | ✓ | * | ✓ | * | ✓ | ✓ |
| Salvarani 2020 | 30 | 60 | 66 | ✓ | * | ✓ | * | * | * | ✓ | ✓ |
| Stone 2020 | 28 | 161 | 82 | ✓ | * | ✓ | * | * | * | ✓ | ✓ |
| Salama EMPACTA 2020 | 60 | 259 | 129 | ✓ | ✓ | * | * | * | * | ✓ | ✓ |
| Gordon REMAP‐CAP 2021 | 90 | 48 | 412 | ✓ | * | * | * | * | * | * | ✓ |
| Horby RECOVERY 2021 | 28 | 2022 | 2094 | ✓ | * | ✓ | * | ✓ | * | * | * |
Key: ✓ A study result is available for inclusion in the synthesis X No study result is available for inclusion, (probably) because the P value, magnitude or direction of the results generated were considered unfavourable by the study investigators – No study result is available for inclusion, (probably) because the outcome was not assessed, or for a reason unrelated to the P value, magnitude or direction of the results ? No study result is available for inclusion, and it is unclear if the outcome was assessed in the study
AE: adverse event; SAE: serious adverse event; TZ: tocilizumab
Appendix 7.1.2 Matrix indicating availability of trial results for important outcomes of the review: tocilizumab versus standard care/placebo
| Important outcomes | ||||||
| Trial ID | Study follow‐up (in days) | Sample size Tocilizumab | Sample size Standard of care or Placebo |
Time to death | Time to clinical improvement | Time to WHO score 7 and above |
| Rosas COVACTA 2021 | 60 | 301 | 151 | * | ✓ | * |
| Wang 2020 | 14 | 33 | 32 | * | * | * |
| Hermine CORIMUNO‐19 2020 | 90 | 64 | 67 | ✓ | ✓ | ✓ |
| Salvarani 2020 | 30 | 60 | 66 | * | ✓ | * |
| Stone 2020 | 28 | 161 | 82 | ✓ | ✓ | ✓ |
| Salama EMPACTA 2020 | 60 | 259 | 129 | ✓ | ✓ | * |
| Gordon REMAP‐CAP 2021 | 90 | 366 | 412 | ✓ | ✓ | * |
| Horby RECOVERY 2021 | 28 | 2022 | 2094 | * | * | * |
Key: ✓ A study result is available for inclusion in the synthesis X No study result is available for inclusion, (probably) because the P value, magnitude or direction of the results generated were considered unfavourable by the study investigators – No study result is available for inclusion, (probably) because the outcome was not assessed, or for a reason unrelated to the P value, magnitude or direction of the results ? No study result is available for inclusion, and it is unclear if the outcome was assessed in the study
AE: adverse event; SAE: serious adverse event; TZ: tocilizumab
Appendix 7.2. Matrix indicating availability of trial results for the critical and important outcomes of the review: sarilumab
Appendix 7.2.1 Matrix indicating availability of trial results for the critical outcomes of the review: sarilumab 400 mg versus standard care
| Critical outcomes | |||||||||||
| Trial ID | Study follow‐up (in days) |
SAR 400 mg n |
Standard of care or Placebo n |
All‐cause mortality | Clinical improvement | WHO SCORE 7 and above | AE | SAE | |||
| Day 28 | Day ≥60 | Day 28 | Day ≥60 | Day 28 | Day ≥60 | ||||||
| Gordon REMAP‐CAP 2021 | 90 | 48 | 412 | ✓ | * | * | * | * | * | * | ✓ |
| Lescure 2021 | 60 | 173 | 86 | ✓ | ✓ | X | * | * | * | ✓ | ✓ |
AE: adverse event; SAE: serious adverse event; SAR: sarilumab
Appendix 7.2.2 Matrix indicating availability of trial results for important outcomes of the review. Sarilumab 400 mg versus standard care
| Important outcomes | ||||||
| Trial ID | Study follow‐up (in days) | Sample size Sarilumab 400 mg | Sample size Standard of care or Placebo |
Time to death | Time to clinical improvement | Time to WHO score 7 and above |
| Gordon REMAP‐CAP 2021 | 90 | 48 | 412 | ✓ | ✓ | * |
| Lescure 2021 | 60 | 173 | 86 | * | ✓ | * |
Key: ✓ A study result is available for inclusion in the synthesis X No study result is available for inclusion, (probably) because the P value, magnitude or direction of the results generated were considered unfavourable by the study investigators – No study result is available for inclusion, (probably) because the outcome was not assessed, or for a reason unrelated to the P value, magnitude or direction of the results ? No study result is available for inclusion, and it is unclear if the outcome was assessed in the study
Appendix 7.2.3 Matrix indicating availability of trial results for the critical outcomes of the review: sarilumab 200 mg vs standard care
| Critical outcomes | |||||||||||
| Trial ID | Study follow‐up (in days) |
SAR 200 mg n |
Standard of care or Placebo n |
All‐cause mortality | Clinical improvement | WHO SCORE 7 and above | AE | SAE | |||
| Day 28 | Day ≥60 | Day 28 | Day ≥60 | Day 28 | Day ≥60 | ||||||
| Lescure 2021 | 60 | 161 | 86 | ✓ | ✓ | X | * | * | * | ✓ | ✓ |
AE: adverse event; SAE: serious adverse event; SAR: sarilumab
Appendix 7.2.4 Matrix indicating availability of trial results for important outcomes of the review: sarilumab 200 mg vs standard care
| Important outcomes | ||||||
| Trial ID | Study follow‐up (in days) | Sample size Sarilumab 200 mg | Sample size Standard of care or Placebo |
Time to death | Time to clinical improvement | Time to WHO score 7 and above |
| Lescure 2021 | 60 | 161 | 86 | * | ✓ | * |
Key: ✓ A study result is available for inclusion in the synthesis X No study result is available for inclusion, (probably) because the P value, magnitude or direction of the results generated were considered unfavourable by the study investigators – No study result is available for inclusion, (probably) because the outcome was not assessed, or for a reason unrelated to the P value, magnitude or direction of the results ? No study result is available for inclusion, and it is unclear if the outcome was assessed in the study
Appendix 8. Table of results for tocilizumab versus placebo or standard care: important outcomes.
| Outcome | No. of studies | No. of participants | Statistical method | Effect size |
| Time to clinical improvement | 6 | 2118 | Hazard Ratio (95% CI) | 1.23 (1.08 to 1.39) |
| Time to WHO progression score (level 7 and above) | 3 | 762 | Hazard Ratio (95% CI) | 0.62 (0.42 to 0.91) |
| Time to death | 3 | 1152 | Hazard Ratio (95% CI) | 0.65 (0.51 to 0.83) |
Appendix 9. Table of results for sarilumab versus placebo or standard care: important outcomes.
| Outcome | No. of studies | No. of participants | Statistical method | Effect size |
| Time to clinical improvement | 2 | 880 | Hazard Ratio (95% CI) | 1.28 (0.88 to 1.87) |
| Time to death | 1 | 460 | Hazard Ratio (95% CI) | 0.55 (0.33 to 0.91) |
Appendix 10. Affiliations of the COVID‐NMA consortium's participating members
Affiliations of the COVID‐NMA consortium's participating members listed in the Acknowledgment section
(See Acknowledgements)
Université de Paris, France
Centre of Research in Epidemiology and StatisticS (CRESS UMR1153), Methods team, France
Laboratoire d’Informatique de Grenoble (LIG), CNRS, France
IRCCS Fondazione Don Carlo Gnocchi, Italy
Laboratoire Bordelais de Recherche en Informatique (LaBRI), Université Bordeaux I, France
Epistemonikos Foundation, Chile
McMaster University, Canada
Center for Health Regulatory Policies, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Italy
Centre Max Weber, CNRS, France
Centre of Research in Epidemiology and StatisticS (CRESS UMR1153), Eren team, France
Cochrane Response
Cochrane Germany, Cochrane Germany Foundation, Freiburg, Germany
Bordeaux Pharmacoepi ‐ ADERA, France
Laboratoire d'Informatique, de Modélisation et d'Optimisation des Systèmes (LIMOS), CNRS, Université Clermont Auvergne
Université Toulouse 3 – Paul Sabatier ‐ Institut de Recherche en Informatique de Toulouse – IRIT UMR 5505, France
Cochrane France
Institut des Systèmes Complexes de Paris IDF (ISC‐PIF), CNRS, France
WHO Collaborating Centre for Guideline Implementation and Knowledge Translation & Chinese GRADE Centre, Lanzhou University, China
Laboratoire de recherche en Informatique (LRI), CNRS, Université Paris‐Saclay, France
Laboratoire d’InfoRmatique en Image et Systèmes d’information (LIRIS), CNRS, Université Claude Bernard Lyon 1, France
Evidence Synthesis Ireland, Cochrane Ireland and HRB‐Trials Methodology Research Network, National University of Ireland, Galway, Ireland
Centre for Evidence Based Medicine Odense (CEBMO), University of Southern Denmark and Odense University Hospital, Denmark
French National Research Institute for Agriculture, Food and Environment (INRAE), France
Service de Neurochirurgie, Hôpital d'Instruction des Armées Percy (HIA), France
The Collaborative Approach to Meta‐Analysis and Review of Animal Data from Experimental Studies (CAMARADES), Centre for Clinical Brain Sciences, University of Edinburgh, Scotland
Cochrane Editorial and Methods Department, Cochrane Central
Department of Anesthesia, Intensive Care and Emergency, Fondazione IRCCS Ca' Granda Ospedale Maggiore Policlinico, Department of Pathophysiology and Transplantation, University of Milan, Italy
Cochrane South Africa, South African Medical Research Council
Department of Primary Education, University of Ioannina, Greece
Institute for Evidence in Medicine, Medical Center & Faculty of Medicine, University of Freiburg, Freiburg, Germany
Cochrane Review Group on Drugs and Alcohol; International GRADE Working Group; Department of Epidemiology, Lazio Regional Health Service, Italy
Research Methodology Division, School of Public Health and Preventive Medicine, Monash University, Australia
Health Research Board‐Trials Methodology Research Network (HRB‐TMRN), NUI Galway, Ireland
UC Evidence Center, Cochrane Chile Associated Center, Pontificia Universidad Católica de Chile, Santiago, Chile
Population Health Sciences, Bristol Medical School, University of Bristol, UK; NIHR CLAHRC West, University Hospitals Bristol and Weston NHS Foundation Trust, UK
Bristol Medical School, Bristol Population Health Science Institute, University of Bristol, UK
Nottingham Ningbo GRADE Centre, The Nottingham China Health Institute, the University of Nottingham Ningbo, China
Laboratoire d'Informatique pour la Mécanique et les Sciences de l'Ingénieur (LIMSI), CNRS, France
Appendix 11. 'Risk of bias' assessments
Clinical improvement (D28)
| Study | 1.Randomisation | 2.Deviations from intervention | 3.Missing outcome data | 4.Measurement of the outcome | 5.Selection of the reported results | Overall risk of bias |
| Hermine 2020 | Low | Some concerns1 | Low | Some concerns2 | Low | Some concerns |
| Rosas 2021 | Low | Low | Low | Low | Low | Low |
| Salama 2020 | Low | Low | Low | Low | Low | Low |
| Salvarani 2020 | Low | Some concerns3 | Low | Some concerns4 | Some concerns5 | Some concerns |
| Stone 2020 | Low | Low | Low | Low | Low | Low |
| Horby 2021 | Low | Low | Low | Some concerns6 | Low | Some concerns |
| Veiga 2021 | Low | Some concerns7 | Low | Some concerns8 | Low | Some concerns |
[1] Quote: “Open‐label study” Comment: unblinded study. Deviations from intended intervention arising because of the study context: three participants in the treatment group did not receive study drug. Administration of co‐interventions of interest (antivirals, corticosteroids and biologics) were reported. The proportions of participants receiving antivirals and steroids were imbalanced between two arms (> 10% solute difference between the two arms) for steroids. This deviation could affect the outcome and was not balanced. Nevertheless, this domain was rated as 'Some Concern' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
2Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of clinical improvement probably does not differ between groups. Unblinded study. Assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
3 Quote: "the trial was open label" Comment: unblinded study. Deviations from intended intervention arising because of the study context: cross over: 15 (23%) participants in the standard care arm received the study treatment. For 12 (18%) the studied treatment was administered because of clinical worsening as planned in the protocol. Nevertheless, this decision could have been influenced by the trial context. Administration of co‐interventions of interest were reported and not balanced: antivirals (35% vs 47%) and corticosteroids (10% vs 10.6%). These deviations could affect the outcome. Nevertheless, this domain was rated as 'Some Concern' as it is impossible to distinguish deviation because of trial context and deviation because of intervention effect. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
4 Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of clinical improvement probably does not differ between groups. Unblinded study. Assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
5 Comment: the protocol and statistical analysis plan were available. The outcomes 'Clinical improvement (defined as discharge)' is not present in the protocol or registry. No information on whether the results for these outcomes were selected from multiple outcome measurements or analyses of the data.
6Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of clinical improvement probably does not differ between groups. Unblinded study. Assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
7 Quote: “open label” trial. Comment: unblinded study. Deviations from intended intervention arising because of the study context: cross over: 2/64 (3%) of the control arm received tocilizumab. Co‐interventions of interest (corticosteroids and antivirals), were reported, but no information on another co‐intervention of interest: biologics. Hence, this domain was rated as some concern as not enough information on deviations that arose because of the trial context were reported. Data for the outcome were analysed using intention‐to‐treat analysis. This method was considered appropriate to estimate the effect of assignment to intervention.
8 Comment: method of measuring the outcome probably appropriate. Measurement or ascertainment of clinical improvement probably does not differ between groups. Unblinded study. Assessment of this outcome requires clinical judgement and can be influenced by knowledge of the intervention assignment, but is not likely in the context of the pandemic.
.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gordon REMAP‐CAP 2021.
| Study characteristics | ||
| Methods | RCT‐ adaptive platform trial Blinding: unblinded Date of study: from 19 April 2020 to 19 November 2020 Location: multicentre: Australia, Ireland, the Netherlands, New Zealand, Saudi Arabia, UK Follow‐up duration (days): 90 | |
| Participants |
Population: patients with confirmed or suspected COVID‐19 (severe‐critical) Randomised: 826 participants (n1 tocilizumab arm = 366/ n2 sarilumab arm = n2 = 48/ n3 control arm = 412) Characteristics of participants
Inclusion criteria
Exclusion criteria
Dropouts and withdrawals: n = 34/826 (4%); withdrawal due to adverse events: NR |
|
| Interventions |
Interventions: tocilizumab (8 mg/kg infusion, maximum 800 mg), a 2nd infusion could be administered 12 to 24 hours after the 1st at the discretion of the treating clinician. 29% received a 2nd dose. Treatment initiated within 24 hours after starting organ support in the ICU. Sarilumab (400 mg, IV). 90% received the drug. Control: standard care Definition of standard care: other aspects of patient management were provided per each site's standard of care. Overall, > 80% of participants received corticosteroids. Remdesivir use was recorded in 33% (265/807) of patients. Co‐interventions: steroid use at baseline or any time during the study in > 80% of participants |
|
| Outcomes |
Primary outcome of the trial: respiratory and cardiovascular organ support‐free days up to day 21 Note: the definition of clinical improvement extracted is hospital discharge |
|
| Notes |
Funding: mixed (PREPARE consortium by the EU; FP7‐HEALTH‐2013‐INNOVATION‐1; RECOVER consortium by the EU's Horizon 2020 research & innovation programme; Australian National Health & Medical Research Council; Health Research Council of New Zealand, and the Canadian Institute of Health Research, the UK National, the Health Research Board of Ireland, the UPMC Learning While Doing Program, the Breast Cancer Research Foundation, the French Ministry of Health, the Minderoo Foundation and the Wellcome Trust Innovations Project.)
Conflict of interest: yes. (Quote:) “Dr. Gordon reports grants from NIHR, grants from NIHR Research Professorship (RP‐2015‐06‐18), non‐financial support from NIHR Clinical Research Network, non‐financial support from Roche Products Ltd, non‐financial support from Sanofi (Aventis Pharma)”
Protocol: yes, available. Statistical plan: yes, available Data‐sharing stated: yes, after submission of proposal to info@remapcap.org Overall comment: in addition to the pre‐print article, the study registry and protocol were used in data extraction and 'Risk of bias' assessment. Appendices were not available. The report contains early, preliminary results of tocilizumab and sarilumab from the Immune Modulation Therapy domain of the REMAP‐CAP clinical trial (an international, adaptive platform trial); further follow‐up and analysis are ongoing. As a result, long‐term outcomes were not reported. (Quote:) "At a scheduled interim analysis, the independent DSMB reported that tocilizumab had met the statistical trigger for efficacy (posterior probability 99.75%, odds ratio 1.87, 95%CrI 1.20, 2.76) based on an interim analysis of patients as of October 28. As per protocol, further assignment to control closed on November 19 with randomization continuing between different active immune modulation interventions (...) Following a subsequent interim analysis, the DSMB reported that sarilumab had also met the statistical trigger for efficacy and so these results are also reported" There were no important changes from the trial registration in the population, intervention, or control treatments. (Quote:) "Investigators at each site selected a priori at least two interventions, one of which had to be control, to which patients would be randomized...Randomization to the Corticosteroid domain for Covid‐19 closed on June 17, 2020.12 Thereafter, corticosteroids were allowed as per recommended standard of care." This trial was updated on 1 March 2021 after publication of the study report. |
|
Hermine CORIMUNO‐19 2020.
| Study characteristics | ||
| Methods | RCT Blinding: unblinded Date of study: from 31 March 2020 to 18 April 2020 Location: multicentre / France Follow‐up duration (days): 90 | |
| Participants |
Population: patients with COVID‐19 (moderate‐severe) Randomised: 131 participants (n1 tocilizumab arm = 64 / n2 control arm = 67) Characteristics of participants
Inclusion criteria
Exclusion criteria
Dropouts and withdrawals: 1/131(1%); 0 withdrawal due to AEs |
|
| Interventions |
Intervention: tocilizumab (8 mg/kg infusion) on day 1, an additional fixed dose of 400 mg IV on day 3 at physician discretion. The number of patients who received 2nd dose is not reported.
Control: standard care
Definition of standard care: usual care (antibiotic agents, antiviral agents, corticosteroids, vasopressor support, anticoagulants) was provided at the discretion of the clinicians. Co‐interventions Steroid use at baseline or any time during the study Tocilizumab: 21 (33%) Standard care: 41 (61%) |
|
| Outcomes |
Primary outcome of the trial:
Note: the definition of clinical improvement extracted is hospital discharge |
|
| Notes |
Funding: public/nonprofit. (This trial was publicly funded (Ministry of Health, Programme Hospitalier de Recherche Clinique, Foundation for Medical Research (FRM), AP‐HP Foundation and the Reacting program).)
Conflict of interest: declared. No conflict of interest. Quote: “Dr Tharaux has received honorarium fees for participation on advisory boards for Retrophin Inc not related to this work. No other disclosures are reported.”
Protocol: yes, available.
Statistical plan: yes, available. Data‐sharing stated: yes, with publication. philipperavaud@gmail.com Overall comment: in addition to the published article, the trial registry, protocol and supplemental materials and the reply provided by authors were used in data extraction and assessment of risk of bias. There were no major differences between trial registry, protocol and published article in procedures and outcomes, and no changes in treatments. Immunotherapy co‐interventions consisted of anakinra (1 participant in intervention group, 3 in control) and eculizumab (1 participant in control). Remdesivir was given to 1 participant in control group. On 23 October 2020, we received additional information from authors on this study. This study was updated with data from contact with authors. |
|
Horby RECOVERY 2021.
| Study characteristics | ||
| Methods | RCT Blinding: unblinded Date of study: from 14 April 2020‐to 24 January 2021 Location: multicentre (131 centres) / UK Follow‐up duration (days): 28 | |
| Participants |
Population: patients with suspected or confirmed COVID‐19 (moderate‐critical) admitted to 131 centres in the UK Randomised: 4116 participants (n1= 2022 / n2 = 2094) Characteristics of participants
Inclusion criteria
Exclusion criteria
Dropouts and withdrawals : 0% dropout, withdrawal due to AEs: NR |
|
| Interventions |
Intervention: tocilizumab (800 mg if weight > 90 kg; 600 mg if weight > 65 and ≤ 90 kg; 400 mg if weight > 40 and ≤ 65 kg; 8 mg/kg if weight ≤ 40 kg); a 2nd infusion could be administered 12 to 24 hours after the 1st)
Control: standard care Co‐interventions Steroid use at baseline or any time during the study Tocilizumab: 1664 (82%) Standard care: 1721 (82%) |
|
| Outcomes |
Primary outcome of the trial 28‐day mortality Note: the definition of clinical improvement extracted is discharged alive from hospital within 28 days. |
|
| Notes |
Funding: public/non profit (UK research and Innovation/National Institute for Health Research (NIHR); NIHR Oxford Biomedical Research Centre, Wellcome; Bill and Melinda Gates Foundation; Department for International Development; Health Data Research UK; Medical Research Council Population Health Research Unit; NIHR Clinical Trials Unit Support Funding; Abbvie (lopinavir‐ritonavir); Roche Products Ltd (tocilizumab); Regeneron (REGEN‐480 COV2))
Conflict of interest: yes, declared. The authors have no conflict of interest or financial relationships relevant to the submitted work to disclose
Protocol: yes. In English
Statistical plan: yes
Data‐sharing stated: yes, within 3 months of publication
Data accessibility: ndph.ox.ac.uk/data-access Overall comment: in addition to the pre‐print article, the study registry and protocol were used in data extraction and 'Rrisk of bias' assessment. This article is a preliminary report on the tocilizumab arm of the ongoing RECOVERY platform study after 28 days with the main analysis planned at 6 months post‐randomisation. As a result, the target sample size specified in the registry was not achieved. There is no change from the trial registration in the intervention and control treatments. |
|
Lescure 2021.
| Study characteristics | ||
| Methods | RCT Blinding: quadruple blinding Date of study: from 28 March 2020 to 3 July 2020 Location: multicentre (45 centres) / Argentina, Brazil, Canada, Chile, France, Germany, Israel, Italy, Japan, Russia, and Spain Follow‐up duration (days): 60 | |
| Participants |
Population: patients with confirmed (any specimen) COVID‐19 (moderate‐critical) admitted to 45 centres in Argentina, Brazil, Canada, Chile, France, Germany, Israel, Italy, Japan, Russia, and Spain. Randomised: 420 participants (n1 sarilumab 400 mg = 173/ n2 sarilumab 200 mg = 161/ n3 control = 86) Characteristics of participants
Inclusion criteria
Exclusion criteria
Dropouts and withdrawals: 0% dropout, withdrawal due to AEs: NR |
|
| Interventions |
Intervention
Control: placebo Definition of standard care: local standard of care Co‐interventions Steroid use at baseline or any time during the study Sarilumab 400 mg: 78 (45%) Sarilumab 200 mg: 58 (36%) Placebo: 39 (45%) |
|
| Outcomes |
Primary outcome of the trial Time from baseline to clinical improvement of ≥ 2 points on a 7‐point ordinal scale. Discharge prior to day 29 was considered as a 2‐point improvement. Note: the definition of clinical improvement extracted is improvement from baseline by at least 2 categories on a 7‐point ordinal scale |
|
| Notes |
Funding: private (Sanofi and Regeneron Pharmaceuticals, Inc)
Conflict of interest: yes, declared. F‐XL has received lecture fees from Merck Sharp & Dohme and Gilead Science. HH has nothing to disclose of relevance to this study. RF has no financial conflicts to disclose. JSL, GS, PW, NP, and OH are employees of Sanofi and may hold stock and/or stock options in the company.
Protocol: NR
Statistical plan: NR
Data‐sharing stated: yes, currently available
Data accessibility: clinicalstudydatarequest.com/ Overall comment: in addition to the pre‐print article, the supplementary materials, and the study registry were used in data extraction and r'Rsk of bias' assessment. Neither study protocol nor statistical analysis plan were available. There were no substantive differences between the prospective registry and the pre‐print article. The study was an adaptive design and any changes in protocol versions are reported with rationales in the article. The study achieved its pre‐stated sample size. As this study was conducted in 11 countries across 45 sites, standard of care may have differed (supported by concomitant medication use presented in Table S2). |
|
Rosas COVACTA 2021.
| Study characteristics | ||
| Methods | RCT Blinding: double‐blinding Date of study: from 3 April 2020 to 28 July 2020 Location: multicentre: Canada, Denmark, France, Germany, Italy, the Netherlands, Spain, UK, USA Follow‐up duration (days): 60 | |
| Participants |
Population: patients with confirmed COVID‐19 (mild to critical) Randomised: 452 participants (n1 tocilizumab arm = 301 / n2 control arm = 151) Characteristics of participants
Inclusion criteria Patients 18 years or older with severe COVID‐19 pneumonia confirmed by positive polymerase chain reaction test in any body fluid and evidenced by bilateral chest infiltrates on chest x‐ray or CT were enrolled. Eligible patients had blood oxygen saturation ≤ 93% or partial pressure of oxygen/fraction of inspired oxygen < 300 mm/Hg. Informed consent was obtained for all enrolled patients. Exclusion criteria Patients were excluded if the treating physician determined that death was imminent and inevitable within 24 hours or if they had active tuberculosis or bacterial, fungal, or viral infection other than SARS‐CoV‐2. Dropouts and withdrawals :14/452 (3%); 0 withdrawal due to AEs |
|
| Interventions |
Intervention: tocilizumab (8 mg/kg infusion, maximum 800 mg), a second infusion could be administered 8 to 24 hours after the first) Control: placebo Co‐interventions Steroid use at baseline or any time during the study Tocilizumab: 57 (19%) Placebo: 41 (28%) |
|
| Outcomes |
Primary outcome of the trial Clinical status assessed on a 7‐category ordinal scale at day 28 Note: the definition of clinical improvement extracted is improvement from baseline by at least 2 categories on the ordinal scale |
|
| Notes |
Funding: mixed (F. Hoffmann‐La Roche Ltd; Department of Health and Human Services, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority)
Conflict of interest: yes. (Quote:) “I.O.R. received a grant from Roche/Genentech during the conduct of the study; a grant and personal fees from Genentech outside the submitted work; and personal fees from Boehringer and Bristol‐Myers Squibb outside the submitted work. A.M.’s institution received grant support from Roche/Genentech during the conduct of the study; he has received funding from the National Institutes of Health outside the submitted work and medical education from Merck and Livanova outside the submitted work.” Protocol: yes, available Statistical plan: yes, available Data‐sharing stated: yes, through vivli.org Overall comment: in addition to all available versions of the pre‐print article, the study registry and supplementary appendix, as well as responses from contact with authors were used in data extraction and 'Risk of bias' assessment. The protocol and statistical analysis plan were not available although it was sent by authors after requested. The full data could not be accessed. Patients in the tocilizumab group received a 2nd dose only if their condition did not improve or worsened. The study achieved the target sample size prespecified in the registry. There is no change from the trial registration in the intervention and control treatments as well as primary outcome. Some secondary outcomes in the registry were not reported in the pre‐print article, particularly regarding the 60‐day time point as well. The sponsor (Hoffman‐La Roche Ltd.) played a prominent role, with writing support for the authors provided by Sara Duggan, Ph.D., of ApotheCom, funded by F. Hoffmann‐La Roche Ltd. 3 authors were employees of Roche Products Ltd. On 7 December 2020, we received additional information from authors on this study. This study was updated with data from contact with authors on 13 January 2021. This trial was updated on 1 March 2021 after publication of the study report. |
|
Salama EMPACTA 2020.
| Study characteristics | ||
| Methods | RCT Blinding: double‐blinding Date of study: from 14 May 2020 to 18 August 2020 Location: multicentre / Brazil, Kenya, Mexico, Peru, South Africa, USA Follow‐up duration (days): 60 | |
| Participants |
Population: patients with confirmed COVID‐19 (mild to severe) Randomised: 388 participants (n1 tocilizumab arm = 259 / n2 control arm = 129) Characteristics of participants
Inclusion criteria
Exclusion criteria
Dropouts and withdrawals: 11/388 (3%); 0 withdrawal due to AEs |
|
| Interventions |
Intervention: tocilizumab (8 mg/kg up to 800 mg max infusion)
Control: placebo Co‐interventions Steroid use at baseline or any time during the study Tocilizumab: 200 (77%) Placebo: 112 (87%) |
|
| Outcomes |
Primary outcome of the trial: Mechanical ventilation (invasive mechanical ventilation or extracorporeal membrane oxygenation) or death by day 28 Note: the definition of clinical improvement extracted is improvement from baseline by at least 2 categories on the ordinal scale |
|
| Notes |
Funding: private (Genentech, Inc.)
Conflict of interest: yes, declared. Quote “C.S. reports personal fees from Genentech, Inc. J.H., L.Y., W.G.R., B.K., and S.V.M are employees and shareholders of Genentech, Inc. and have filed a patent for a method of treating pneumonia, including COVID‐19 pneumonia, with an IL‐6 antagonist.”
Protocol: yes, available. Statistical plan: yes, available. Data‐sharing stated: yes, through vivli.org/ Overall comment: in addition to the published article, the pre‐print article, study registry, protocol, statistical analysis plan and supplementary appendix were used in data extraction and 'Risk of bias' assessment. The study achieved the target sample size specified in the trial registry. There is no change from the trial registration in the intervention and control treatments. The registry and protocol version 1 primary outcome (cumulative proportion of mechanical ventilation) does not reflect the primary outcome reported in the paper and protocol version 2 (cumulative proportion of mechanical ventilation or death). Some secondary outcomes reported in the registry were not reported in the manuscript. On 21 December 2020, we received additional information from authors on this study, we updated the study results based on authors reply. The study was also updated on 13 January 2021 with data from the New England Journal of Medicine publication. |
|
Salvarani 2020.
| Study characteristics | ||
| Methods | RCT Blinding: unblinded Date of study: from 31 March 2020 to 11 June 2020 Location: multicentre / Italy Follow‐up duration (days): 30 | |
| Participants |
Population: patients with confirmed COVID‐19 (severe) Randomised: 126 participants (n1 tocilizumab arm = 60 / n2 control arm = 66) Characteristics of participants
Inclusion criteria Patients 18 years and older, with an instrumental diagnosis of COVID‐19 pneumonia confirmed by a positive reverse‐transcriptase polymerase chain reaction assay for SARS‐CoV‐2 in a respiratory tract specimen. Other inclusion criteria were the presence of acute respiratory failure with a partial pressure of arterial oxygen to fraction of inspired oxygen (PaO2/FIO2) ratio between 200 mm Hg and 300 mm/Hg, an inflammatory phenotype defined by a temperature greater than 38 ‘C during the last 2 days, and/or serum C‐reactive protein (CRP) levels of 10mg/dL or greater and/or CRP level increased to at least 2 times the admission measurement. Exclusion criteria included
Dropouts and withdrawals: 3/126 (2%); 0 withdrawals due to AEs |
|
| Interventions |
Intervention: tocilizumab (8 mg/kg) on day 1 up to a maximum of 800 mg, followed by a 2nd dose after 12 hours Control: standard care Definition of standard care: supportive care following the treatment protocols of each centre. All drugs were allowed but IL‐1 blockers, Jak inhibitors, and tumour necrosis factor inhibitors. Steroids were allowed if already taken before hospitalization. In case of occurrence of documented clinical worsening, patients randomised in both arms could receive any therapy, including steroids, and, for patients randomised in the control arm, tocilizumab. Co‐interventions Steroid use at baseline or any time during the study Tocilizumab: 6 (10%) Standard care: 7 (11%) |
|
| Outcomes |
Primary outcome of the trial
Note: the definition of clinical improvement extracted is discharge. |
|
| Notes |
Funding: mixed (local resources, the Italian Ministry of Health and Roche)
Conflict of interest: yes, declared. Quote “Dr Costantini reported receiving nonfinancial support (provision of experimental drug and distribution to clinical sites) from Roche during the conduct of the study. Dr Angheben reported receiving grants from Italian Ministry of Health” Protocol: yes, available Statistical plan: yes, available Data‐sharing stated: yes, after approval of a proposal Overall comments: in addition to the published article, the trial registries, protocol and supplemental material were used in data extraction and assessment of risk of bias. The trial was terminated on the decision of the Scientific Committee due to lack of effect and poor enrolment because of the dramatic decrease in the incidence of the disease in Italy at the time. There were some differences between trial registration and published article in inclusion and exclusion criteria. There was no difference in study treatments between trial registration and published article.14 participants in the standard care group crossed over and received tocilizumab after clinical worsening |
|
Stone 2020.
| Study characteristics | ||
| Methods | RCT Blinding: double‐blinding Date of study: from 20 April 2020 to 15 June 2020 Location: multicentre / USA Follow‐up duration (days): 28 | |
| Participants |
Population: patients with COVID‐19 (mild to severe) Randomised 243 participants (n1 tocilizumab arm = 161 / n2 control arm = 82) Characteristics of participants
Inclusion criteria
Exclusion criteria Unable to provide verbal informed consent or have verbal agreement to participate through attestation and signature of a witness required, as outlined in the Partners IRB’s Table for Consenting in COVID Research that is More than Minimal Risk. Patients between the ages of 79 and 86 will be excluded if they have:
Dropouts and withdrawals: 1/243 (1%); 0 withdrawal due to AEs |
|
| Interventions |
Intervention: tocilizumab (8 mg/kg infusion up to 800 mg max) single dose Control: placebo |
|
| Outcomes |
Primary outcome of the trial: the primary outcome was intubation (or death, for patients who died before intubation) after administration of tocilizumab or placebo, assessed in a time‐to‐event analysis. Note: improvement was defined as an decrease in score by at least 2 points on the ordinal clinical improvement scale. |
|
| Notes |
Funding: private (supported by Genentech)
Conflict of interest: yes, declared. Quote: “Dr. Stone reports grants from Genentech, during the conduct of the study; grants and personal fees from Principia Biopharma and Roche, grants from Viela, personal fees from Sanofi, Chemocentryx, Celgene, Abbvie, Chugai, Grunenthal, Glaxo Smith Kline, InflaRx, INSmed, Regeneron, Roivant, outside of submitted work.” Protocol: yes, available. Statistical plan: yes, available. Data‐sharing stated: yes, following approval of proposal. Overall comment: in addition to the published article, the trial registry, study protocol and statistical analysis plan were used in data extraction and assessment of risk of bias. The study did not achieve the sample size recorded in the trial registry. There were no other notable differences in study population, procedures, treatments or outcomes between the published article and the trial registry, study protocol and statistical analysis plan. |
|
Veiga TOCIBRAS 2021.
| Study characteristics | ||
| Methods | RCT Blinding: unblinded Date of study: from 8 May 2020 to 17 July 2020 Location: multicentre (9 centres) / Brazil Follow‐up duration (days): 29 | |
| Participants |
Population: patients with confirmed COVID‐19 (moderate‐critical) Randomised: 129 participants (n1 tocilizumab arm = 65 / n2 control arm = 64) Characteristics of participants
Inclusion criteria
Exclusion criteria
Dropouts and withdrawals: (0%); 0 withdrawal due to AEs |
|
| Interventions |
Intervention: tocilizumab (8 mg/kg, IV) on day 1 up to a maximum of 800 mg.
Control: standard care
Definition of standard care: standard of care (best supportive care), according to the local protocol. The concomitant use of hydroxychloroquine, azithromycin, corticosteroids, and antibiotics was allowed according to standard care per local institutional guidelines for patients with covid‐19. Remdesivir was not available in Brazil. Co‐interventions Steroid use at baseline or any time during the study Tocilizumab: 56 (86%) Standard care: 55 (86%) |
|
| Outcomes |
Primary outcome of the trial Clinical status at 15 days evaluated with the use of a 7‐level ordinal scale Note: the definition of clinical improvement extracted is discharge alive |
|
| Notes |
Funding: mixed (the hospitals and research institutes participating in Coalition covid‐19 Brazil; Fleury Laboratory (laboratory analysis); Instituto Votorantim (donation for drug provision))
Conflict of interest: yes, declared. "Support from hospitals and research institutes participating in the Coalition covid‐19 Brazil, Fleury Laboratory in São Paulo, Brazil, and Instituto Votorantim for the submitted work. JAGGP reports support from Pfizer, Jansen, Sanofi,..."
Protocol: yes, available.
Statistical plan: yes, available. Data‐sharing stated: yes, 3 months after publication. Request to the corresponding author at viviane.veiga@bp.org.br Overall comment: in addition to the published article and its supplementary materials, the trial registry, published protocol and statistical analysis plan were used in data extraction and 'Risk of bias' assessment. Viral clearance was an exploratory outcome in the protocol but results were not reported. There were no other substantive differences between the protocol, registry and published report in study population, procedures or interventions. Unblinded study. The trial was terminated early after the first interim analysis owing to an excess number of deaths at 15 days in the tocilizumab group. Quote: "The trial registration on Clinicaltrials.gov was finalised only after enrolment of the first patient because of an administrative error by the research team. Thus, the study did not achieve the sample size recorded in the trial registry. On May 8th, an eligible patient was identified at our centre and enrolment offered to the patient. At the same day, the protocol was included in ClinicalTrials.gov but could not be registered. On May 11th, we received a response with a modified Protocol Registration and Results System for registration. On May 12th, we uploaded our protocol information in ClinicalTrials.gov as approved by the Brazilian Ethics authorities. As we did not receive a reply from ClinicalTrials.gov in subsequent days, a new contact was made on May 24th and the protocol as initially submitted was published." Quote. "In the first version of the trial protocol, need of mechanical ventilation was an exclusion criterion. On June 4th, 2020, after the study was initiated, an amendment was made to allow inclusion of patients under mechanical ventilation for less than 24 hours. On July 7th, 2020 chest X‐ray evidence of COVID‐19 was included as an alternative to computed tomography in the inclusion criteria" |
|
Wang 2020.
| Study characteristics | ||
| Methods | RCT Blinding: unblinded Date of study: from 13 February 2020 to 13 March 2020 Location: multicentre / China Follow‐up duration (days): 14 | |
| Participants |
Population: patients with confirmed COVID‐19 (moderate‐severe) to 6 Randomised: 65 participants (n1 Tocilizumab arm = 33 / n2 control arm = 32) Characteristics of participants
Inclusion criteria
Exclusion criteria
Dropouts and withdrawals: 0/65 (0%); 0 withdrawal due to AEs |
|
| Interventions |
Intervention: tocilizumab (400 mg infusion). Patients received a 2nd dose only if their condition did not improve or worsened. The number of patients received 2nd dose is not reported.
Control: standard care Definition of standard care: standard care was given according to the “Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (5th or update version)”. Co‐interventions Steroid use at baseline or any time during the study Tocilizumab: NR Standard care: NR |
|
| Outcomes |
Primary outcome of the trial:
|
|
| Notes |
Funding: public/nonprofit (Department of Science and Technology of Anhui Province and Health Commission of Anhui Province, China National Center for Biotechnology Development)
Conflict of interest: declared. No conflict of interest (quote:9 “We declare no competing interests.” Protocol: NR Statistical plan: NR Data‐sharing stated: Yes, to qualifying researchers who submit a proposal with a valuable research question. Overall comment: in addition to all available versions of the pre‐print article, the study registry was used in data extraction and 'Risk of bias' assessment. The study did not achieve the target sample size specified in the registry. Quote: "Because of the rapid decline in the number of COVID‐19 patients in China, finally a total of 65 pneumonia patients with laboratory confirmed SARS‐CoV‐2 infection underwent randomization." There is no change from the trial registration in the intervention and control treatments, nor in the primary outcome. Mortality was stated as a secondary outcome in the registry but not in the report. Conversely, some secondary outcomes in the report (recovery rate of hypoxia over 14 days and the time to negative virus load) were not in the registry. The study was judged to raise some concerns for 4 out of 5 domains which substantially lowered the confidence in the result, hence it was deemed an overall high risk of bias. |
|
AE: adverse event; ALT: alanine aminotransferase; AST::aspartate aminotransferase; CKD‐EPI score: Chronic Kidney Disease Epidemiology Collaboration; CT: computed tomographic; DSMB: Data and Safety Monitoring Board; EU: European Union; ICU: intensive care unit; IV: intravenous; IL: interleukin; LDH: lactate dehydrogenase; MDRD score: Modification of Diet in Renal Disease; NIHR: National Institute for Health Research; n1: n in experimental arm; n2: n in control arm; NIV: non‐invasive ventilation; NR: not reported; NYHA: New York Heart Association; RCT: randomised controlled trial; SAE: serious adverse event;SGOT: Serum glutamic oxaloacetic transaminase;SGPT; serum glutamic pyruvic transaminase; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abolghasemi 2020 | Not randomised or improper randomisation |
| Bandopadhyay 2020 | Descriptive study |
| Behzadnia 2020 | Non COVID‐19 patients |
| Burnett 2020 | Not an intervention for COVID‐19 |
| Chitra 2021 | Siddha medicine |
| Choudhury 2021 | Irrelevant intervention (gargling) |
| Dound 2021 | Herbal medicine |
| Duong‐Quy 2020 | Irrelevant intervention (masks) |
| Farnoosh 2020 | Irrelevant intervention |
| Gupta 2021 | Ayurvedic medicine |
| Guvenmez 2020 | Nebulisers without specific treatment |
| Huang 2020 | Not randomised or improper randomisation |
| Hyun 2020 | Not randomised or improper randomisation |
| Kemran 2020 | Not randomised or improper randomisation |
| Kimura 2020 | Qualitative study |
| Koshak 2020 | Homeopathic medicine |
| Liu 2021 | Chinese medicine |
| Malysz 2020 | Simulation study |
| Mohamed 2020 | Irrelevant intervention (gargling) |
| Mukhtar 2020 | Irrelevant intervention (gargling) |
| Noor Azhar 2020 | Simulation study |
| Onal 2021 | Homeopathic medicine |
| Painter 2020 | Early phase |
| Pizzoli 2020 | Non COVID‐19 patients |
| Saju 2020 | Non COVID‐19 patients |
| Schaller 2020 | Nebulisers without specific treatment |
| Schumacher 2020 | Simulation study |
| Seneviratne 2020 | Irrelevant intervention (gargling) |
| Shapira 2021 | Non COVID‐19 patients |
| Shaw 2020 | Irrelevant intervention (masks) |
| Simpson 2021 | Irrelevant preventive intervention |
| Tomazini 2020 | Protocol |
| Trieu 2021 | Chinese medicine |
| Ward 2021 | Not an intervention for COVID‐19 |
| Zhou 2020 | Herbal medicine |
| Zhou 2021 | Non COVID‐19 patients |
Differences between protocol and review
We made the following changes, in the review, to the protocol (Boutron 2020b).
Outcomes: to avoid multiplicity, we reduced the number of outcomes. For the selected outcome domains, we now consider only two time points (D28 and ≥ D60). We no longer evaluate the outcome domain WHO Clinical Progression Score level 6 or above as IL‐6 blocking agents as the definition used in the studies appears to be subject to variation due to local guidelines and resources. It is therefore an unreliable or inconsistent indicator when assessed across studies.
'Risk of bias' assessment: we did not consider anticoagulants as a relevant co‐intervention for assessing risk of bias in the domain deviations from intervention after discussion with content experts.
Subgroup analyses: the subgroup analyses planned to explore age, sex, severity of the disease, comorbidity status and time after the beginning of the outbreak were not conducted because of the limited number of RCTs providing relevant data and the absence of variation across trials in some variables such as age and gender. We decided to conduct post‐hoc subgroup analysis to explore the impact of the funding source (public or non‐profit/mixed or private) and conflict of interests
Contributions of authors
All authors contributed to the development of the review.
Sources of support
Internal sources
Cochrane France, France
Center of Research in Epidemiology and StatisticS (CRESS), France
Centre d’Epidémiologie Clinique (GHU Cochin, Hôtel Dieu), France
Assistance Publique Hôpitaux de Paris (APHP), France
Université de Paris, France
Centre National de la Recherche Scientifique (CNRS), France
External sources
-
Agence Nationale de la Recherche (ANR), France
Funding provided to produce review
-
World Health Organization (WHO), Switzerland
Funding provided to produce review
-
Federal Ministry of Education and Research, Germany
This review is supported through funding for the project COVID‐19 evidence eco‐system (COVID‐19 Evidenzökosystem“ (CEO‐sys)) under a funding scheme issued by the National Research Network of University Medical Centers on COVID‐19 (Nationales Forschungsnetzwerk der Universitätsmedizin zu Covid‐19) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung, BMBF).
Declarations of interest
Lina Ghosn has no interest to declare.
Anna Chaimani has no interest to declare.
Theodoros Evrenoglou has no interest to declare.
Mauricia Davidson has no interest to declare.
Carolina Graña has no interest to declare.
Christine Schmucker has no interest to declare.
Claudia Bollig has no interest to declare.
Nicholas Henschke has been an employee of Cochrane Response since 2016. Cochrane Response was commissioned by the WHO to perform parts of this systematic review.
Yanina Sguassero been an employee of Cochrane Response since 2019. Cochrane Response was commissioned by the WHO to undertake tasks relevant to this systematic review.
Camilla Hansen Nejstgaard has no interest to declare.
Sonia Menon works as a systematic reviewer for p95 consultancy company.
Thu Van Nguyen has no interest to declare.
Gabriel Ferrand has no interest to declare.
Philip Kapp has no interest to declare.
Carolina Riveros has no interest to declare.
Camila Ávila has no interest to declare.
Declan Devane is Principal Investigator for a grant from the Health Research Board (HRB, Ireland) and the Health and Social Care, Research and Development (HSC R&D) Division of the Public Health Agency in Northern Ireland to establish Evidence Synthesis Ireland within which Cochrane Ireland is hosted. The funds are received by his institution. Declan's position as Director of Cochrane Ireland and Director of Cochrane Ireland is paid 0.5FTE from this grant.
Joerg J Meerpohl has no interest to declare.
Gabriel Rada has no interest to declare.
Asbjørn Hróbjartsson has no interest to declare.
Giacomo Grasselli has received personal fees for lectures from Getinge, Fisher&Paykel, Draeger Medical, Biotest, Thermofisher and MSD; support for travel‐meeting expenses from Biotest and Getinge (all outside the present work). I also received an unrestricted research grant from Fisher&Paykel (unrelated to the present work).
David Tovey is a paid editorial advisor to Cochrane France.
Philippe Ravaud is minority shareholder of INATO and SAVANA. He is also principal investigator of the CORIMUNO platform (funding: French Ministry of Health, Programme Hospitalier de Recherche Clinique [PHRC COVID‐19‐20‐0143, PHRC COVID‐19‐20‐0029], Foundation for Medical Research (FRM), AP‐HP Foundation and the Reacting program).
Isabelle Boutron has no interest to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Gordon REMAP‐CAP 2021 {published data only}
- Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, et al, The REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically Ill patients with Covid-19. New England Journal of Medicine 2021;Feb 25:Epub ahead of print. [DOI: 10.1056/NEJMoa2100433] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, et al, The REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19 – preliminary report. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.01.07.21249390] [DOI] [PMC free article] [PubMed]
- NCT02735707. Randomized, embedded, multifactorial adaptive platform trial for community- acquired pneumonia (REMAP-CAP). clinicaltrials.gov/ct2/show/NCT02735707 (first received 13 April 2016). [DOI: 10.1101/2021.01.07.21249390] [DOI]
Hermine CORIMUNO‐19 2020 {published and unpublished data}
- Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P, et al, CORIMUNO-19 Collaborative Group. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia. A randomized clinical trial. JAMA Internal Medicine 2021;181(1):32-40. [DOI: 10.1001/jamainternmed.2020.6820] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04331808. CORIMUNO-19 - tocilizumab trial - TOCI (CORIMUNO-TOCI) (CORIMUNO-TOC). clinicaltrials.gov/ct2/show/NCT04331808 (first received 2 April 2020).
Horby RECOVERY 2021 {published data only}
- Horby PW, Pessoa-Amorim G, Peto L, Brightling CE, Sarkar R, Thomas K, et al. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): preliminary results of a randomised, controlled, open-label, platform trial. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.02.11.21249258] [DOI]
- ISRCTN50189673. A randomised trial of treatments to prevent death in patients hospitalised with COVID-19 (coronavirus). isrctn.com/ISRCTN50189673 (first received 30 March 2020).
- NCT04381936. Randomised evaluation of COVID-19 therapy (RECOVERY). clinicaltrials.gov/ct2/show/NCT04381936 (first received 11 May 2020).
Lescure 2021 {published data only}
- Lescure FX, Honda H, Fowler RA, Lazar JS, Shi G, Wung P, et al. Sarilumab treatment of hospitalised patients with severe or critical COVID-19: a multinational, randomised, adaptive, phase 3, double-blind, placebo-controlled trial. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.02.01.21250769] [DOI]
- NCT04327388. Sarilumab COVID-19. clinicaltrials.gov/ct2/show/NCT04327388 (first received 31 March 2020).
Rosas COVACTA 2021 {published and unpublished data}
- NCT04320615. A study to evaluate the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia (COVACTA) [A randomized, double-blind, placebo-controlled, multicenter study to evaluate the safety and efficacy of tocilizumab in patients with severe COVID-19 pneumonia]. clinicaltrials.gov/ct2/show/NCT04320615 (first received 25 March 2020).
- Rosas IO, Bräu N, Waters M, Go R, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with COVID-19 pneumonia. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.08.27.20183442] [DOI] [PMC free article] [PubMed]
- Rosas IO, Bräu N, Waters M, Go R, Hunter BD, Bhagani S, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. New England Journal of Medicine 2021;Feb 25:Epub ahead of print. [DOI: 10.1056/NEJMoa2028700] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salama EMPACTA 2020 {published and unpublished data}
- NCT04372186. A study to evaluate the efficacy and safety of tocilizumab in hospitalized participants with COVID-19 pneumonia (EMPACTA). clinicaltrials.gov/ct2/show/NCT04372186 (first received May 1 2020).
- Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in patients hospitalized with Covid-19 pneumonia. New England Journal of Medicine 2021;1(384):20-30. [DOI: 10.1056/NEJMoa2030340] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Salvarani 2020 {published and unpublished data}
- NCT04346355. Efficacy of early administration of tocilizumab in COVID-19 patients. clinicaltrials.gov/ct2/show/NCT04346355 (first received 15 April 2020).
- Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al, RCT-TCZ-COVID-19 Study Group. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia. A randomized clinical trial. JAMA Internal Medicine 2021;181(1):24-31. [DOI: 10.1001/jamainternmed.2020.6615] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stone 2020 {published data only}
- NCT04356937. Efficacy of tocilizumab on patients with COVID-19. clinicaltrials.gov/ct2/show/NCT04356937 (first received 22 April 2020).
- Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al, BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. New England Journal of Medicine 2020;383(24):2333-44. [DOI: 10.1056/NEJMoa2028836] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Veiga TOCIBRAS 2021 {published data only}
- NCT04403685. Safety and efficacy of tocilizumab in moderate to severe COVID-19 with inflammatory markers (TOCIBRAS). clinicaltrials.gov/ct2/show/NCT04403685 (first received 27 May 2020).
- Veiga VC, Prats JA, Farias DL, Rosa RG, Dourado LK, Zampieri FG, et al, Coalition covid-19 Brazil VI Investigators. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ 2021;372:n84. [DOI: 10.1136/bmj.n84] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2020 {published data only}
- ChiCTR2000029765. A multicenter, randomized controlled trial for the efficacy and safety of tocilizumab in the treatment of new coronavirus pneumonia (COVID-19). chictr.org.cn/showprojen.aspx?proj=49409 (first received 13 February 2020).
- Wang D, Fu B, Peng Z, Yang D, Han M, Li M, et al. Tocilizumab ameliorates the hypoxia in COVID-19 moderate patients with bilateral pulmonary lesions: a randomized, controlled, open-label, multicenter trial. Available at SSRN: papers.ssrn.com/sol3/papers.cfm?abstract_id=3667681 [Preprint with The Lancet] 2020. [DOI: 10.2139/ssrn.3667681] [DOI]
References to studies excluded from this review
Abolghasemi 2020 {published data only}
- Abolghasemi H, Eshghi P, Cheraghali AM, Imani Fooladi AA, Bolouki Moghaddam F, Imanizadeh S, et al. Clinical efficacy of convalescent plasma for treatment of COVID-19 infections: results of a multicenter clinical study. Transfusion and Apheresis Science 2020;59(5):102875. [DOI: 10.1016/j.transci.2020.102875] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bandopadhyay 2020 {published data only}
- Bandopadhyay P, D’Rozario R, Lahiri A, Sarif J, Ray Y, Paul SR, et al. Nature and dimensions of the cytokine storm and its attenuation by convalescent plasma in severe COVID-19. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.09.21.20199109] [DOI] [PMC free article] [PubMed]
Behzadnia 2020 {published data only}
- Behzadnia B, FatahModares S. Basic psychological need-satisfying activities during the COVID-19 outbreak. Applied Psychology. Health and Well Being 2020;12(4):115-39. [DOI: 10.1111/aphw.12228] [PMID: ] [DOI] [PubMed] [Google Scholar]
Burnett 2020 {published data only}
- Burnett GW, Zhou G, Fried EA, Shah RS, Park C, Katz D. Intraoperative aerosol box use: does an educational visual aid reduce contamination? Korean Journal of Anesthesiology 2020;Nov 2017:Epub ahead of print. [DOI: 10.4097/kja.20511] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chitra 2021 {published data only}
- Chitra SM, Mallika P, Anbu N, NarayanaBabu R, SugunaBai A, David Paul Raj RS, et al. An open clinical evaluation of selected siddha regimen In expediting the management of Covid-19 - a randomized controlled study. Journal of Ayurveda and Integrative Medicine 2021;Jan 21:Epub ahead of print. [DOI: 10.1016/j.jaim.2021.01.002] [PMID: 33519133] [DOI] [PMC free article] [PubMed] [Google Scholar]
Choudhury 2021 {published data only}
- Choudhury IM, Shabnam N, Ahsan T, Kabir S, Khan R, Abu Ahsan SM. Effect of 1% povidone iodine mouthwash/gargle, nasal and eye drop in COVID-19 patient. bioresearchcommunications.com/index.php/brc/article/view/176 (Accessed: 22 February 2021).
Dound 2021 {published data only}
- Dound YA, Mandlik S, Suryavanshi S, Sehgal R, Naik A. A randomized, comparative clinical study to evaluate the activity of CureqovitaaTM formulation for management of SARS-COV-2 infection (COVID-19). Available at: longdom.org/open-access/a-randomized-comparative-clinical-study-to-evaluate-the-activity-of-cureqovitaatm-formulation-for-management-of-sarscov2.pdf (accessed 19 February 2021) 2021.
Duong‐Quy 2020 {published data only}
- Duong-Quy S, Ngo-Minh X, Tang-Le-Quynh T, Tang-Thi-Thao T, Nguyen-Quoc B, Le-Quang K, et al. The use of exhaled nitric oxide and peak expiratory flow to demonstrate improved breathability and antimicrobial properties of novel face mask made with sustainable filter paper and Folium Plectranthii amboinicii oil: additional option for mask shortage during COVID-19 pandemic. Multidisciplinary Respiratory Medicine 2020;15(1):664. [DOI: 10.4081/mrm.2020.664] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Farnoosh 2020 {published data only}
- Farnoosh G, Akbariqomi M, Badri T, Bagheri M, Izadi M, Saeedi-Boroujeni A, et al. Efficacy of a low dose of melatonin as an adjunctive therapy in hospitalized patients with COVID-19: a randomized, double-blind clinical trial. Authorea Preprint 2020. [DOI: 10.22541/au.160734344.45295921/v1] [DOI] [PMC free article] [PubMed]
Gupta 2021 {published data only}
- Gupta A, Madan A, Yadav B, Mundada P, Singhal R, Pandey YK, et al. Chyawanprash for the prevention of COVID-19 infection among healthcare workers: a randomized controlled trial. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.02.17.21251899] [DOI]
Guvenmez 2020 {published data only}
- Guvenmez O. An effective inhaler medication In the treatment of COVID-19 associated pneumonia. Accessed 23 February 2021. Preprint removed: researchsquare.com/article/rs-53215/v2. [DOI: 10.21203/rs.3.rs-53215/v2 ] [CRSREF: 16705801]
- Guvenmez O. Novel treatment approach to the novel coronavirus (COVID-19) with a new inhaler therapeutic. Accessed 23 February 2021. Preprint removed: researchsquare.com/article/rs-53215/v1. [DOI: 10.21203/rs.3.rs-53215/v1] [DOI]
Huang 2020 {published data only}
- Huang M, Tang T, Pang P, Li M, Ma R, Lu J, et al. Treating COVID-19 with Chloroquine. Journal of Molecular Cell Biology 2020;12(4):322-5. [DOI: 10.1093/jmcb/mjaa014] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyun 2020 {published data only}
- Hyun M, Lee JY, Kwon YS, Kim JY, Park JS, Park S, et al. COVID-19: comparing the applicability of shared room and single room occupancy. Transboundary and Emerging Diseases 2020;Sept 2020:[Epub ahead of print. [DOI: 10.1111/tbed.13853] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kemran 2020 {published data only}
- Kamran SM, e Humayun Mirza Z, Naseem BA, Saeed F, Azam R, Ullah N, et al. Clearing the fog: is HCQ effective in reducing COVID-19 progression: a randomized controlled trial. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.07.30.20165365] [DOI] [PMC free article] [PubMed]
Kimura 2020 {published data only}
- Kimura KS, Freeman MH, Wessinger BC, Gupta V, Sheng Q, Huang LC, et al. Interim analysis of an open-label randomized controlled trial evaluating nasal irrigations in non-hospitalized patients with coronavirus disease 2019. International Forum of Allergy & Rhinology 2020;10(12):1325-8. [DOI: 10.1002/alr.22703] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Koshak 2020 {published data only}
- Koshak AE, Koshak EA, Mobeireek AF, Badawi MA, Wali SO, Malibary HM, et al. Nigella sativa supplementation accelerates recovery from mild COVID-19: first randomized controlled clinical trial (RCT). OSF [Preprint] 2020. [DOI: 10.31219/osf.io/urb6f] [DOI]
Liu 2021 {published data only}
- Liu J, Yang W, Liu Y, Lv C, Ruan L, Zhao C, et al. Chinese medicine (Q-14) in the treatment of patients with coronavirus disease 2019 (COVID-19): a single-center, open label, randomised controlled trial. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.01.25.21249417] [DOI] [PMC free article] [PubMed]
Malysz 2020 {published data only}
- Malysz M, Dabrowski M, Böttiger BW, Smereka J, Kulak K, Szarpak A, et al. Resuscitation of the patient with suspected/confirmed COVID-19 when wearing personal protective equipment: a randomized multicenter crossover simulation trial. Cardiology Journal 2020;27(5):497-506. [DOI: 10.5603/CJ.a2020.0068] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohamed 2020 {published data only}
- Mohamed NA, Baharom N, Sulaiman WS, Rashid ZZ, Ken WK, Ali UK, et al. Early viral clearance among covid-19 patients when gargling with povidone-iodine and essential oils – a clinical trial. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.09.07.20180448] [DOI]
Mukhtar 2020 {published data only}
- Mukhtar K, Qassim S, DanJuma MI, Mohamedali M, Al Farhan H, Mohammed F, et al. On the possible beneficial role for the regular use of potent mouthwash solutions as a preventive measure for COVID19 transmission; invoking the Evolutionary biology and Game Theory. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.11.27.20234997] [DOI]
Noor Azhar 2020 {published data only}
- Noor Azhar M, Bustam A, Poh K, Ahmad Zahedi AZ, Mohd Nazri MZ, Azizah Ariffin MA, et al. COVID-19 aerosol box as protection from droplet and aerosol contaminations in healthcare workers performing airway intubation: a randomised cross-over simulation study. Emergency Medicine Journal 2020;38(2):111-7. [DOI: 10.1136/emermed-2020-210514] [PMID: 33219133] [DOI] [PMC free article] [PubMed] [Google Scholar]
Onal 2021 {published data only}
- Onal H, Arslan B, Ergun NU, Topuz S, Semerci SY, Kurnaz M, et al. Treatment of COVID-19 patients with quercetin: a prospective, single - centre, randomized, controlled trial. Available at: https://d197for5662m48.cloudfront.net/documents/publicationstatus/56437/preprint_pdf/21303ff6e65f36cda1c1b68c2ed728fe.pdf (accessed 19 February 2021). [DOI] [PMC free article] [PubMed]
Painter 2020 {published data only}
- Painter WP, Holman W, Bush JA, Almazedi F, Malik H, Eraut NC, et al. Human safety, tolerability, and pharmacokinetics of a novel broad-spectrum oral antiviral compound, molnupiravir, with activity against SARS-CoV-2. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.12.10.20235747] [DOI] [PMC free article] [PubMed]
Pizzoli 2020 {published data only}
- Pizzoli SF, Marzorati C, Mazzoni D, Pravettoni G. Web-based relaxation intervention for stress during social isolation: randomized controlled trial. JMIR Mental Health 2020;7(12):e22757. [DOI: 10.2196/22757] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Saju 2020 {published data only}
- Saju MD, Scaria L, Shaju KK, Cheguvera N, Jospeh MK, Benny AM, et al. REaCH-Resiliency Engagement and Care in Health; a telephone befriending intervention to address the psycho-social challenges of vulnerable population in the context of COVID-19 pandemic: an exploratory trial in India. Research Square [Preprint] 2020. [DOI: 10.21203/rs.3.rs-72843/v1] [DOI]
Schaller 2020 {published data only}
- Schaller G, Nayar SK, Erotocritou M, Overton A, Stelzhammer T, Berber O. Efficacy of surgical helmet systems for protection against COVID-19: a double-blinded randomised control study. International Orthopaedics 2021;45(1):39-42. [DOI: 10.1007/s00264-020-04796-3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schumacher 2020 {published data only}
- Schumacher J, Arlidge J, Dudley D, Sicinski M, Ahmad I. The impact of respiratory protective equipment on difficult airway management: a randomised, crossover, simulation study. Anaesthesia 2020;75(10):1301-6. [DOI: 10.1111/anae.15102] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Seneviratne 2020 {published data only}
- Seneviratne CJ, Balan P, Ki KK, Udawatte NS, Lai D, Lin DN, et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. medRxiv [Preprint] 2020. [DOI: 10.1101/2020.09.14.20186494] [DOI] [PMC free article] [PubMed]
- Seneviratne CJ, Balan P, Ko KK, Udawatte NS, Lai D, Ng DH, et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection 2020;Dec(1-7):Epub ahead of print. [10.1007/s15010-020-01563-9] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shapira 2021 {published data only}
- Shapira S, Yeshua-Katz D, Cohn-Schwartz E, Aharonson-Daniel L, Sarid O, Clarfield AM. A pilot randomized controlled trial of a group intervention via Zoom to relieve loneliness and depressive symptoms among older persons during the COVID-19 outbreak. Internet interventions 2021;Jan 2021:Epub ahead of print. [DOI: 10.1016/j.invent.2021.100368] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaw 2020 {published data only}
- Shaw K, Butcher S, Ko J, Zello GA, Chilibeck PD. Wearing of cloth or disposable surgical face masks has no effect on vigorous exercise performance in healthy individuals. International Journal of Environmental Research and Public Health 2020;17(21):8110. [DOI: 10.3390/ijerph17218110] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simpson 2021 {published data only}
- Simpson R, Sandrin R. The use of personal protective equipment (PPE) by police during a public health crisis: an experimental test of public perception. Journal of Experimental Criminology [Epub ahead of print] 2021. [DOI: 10.1007/s11292-020-09451-w] [PMID: ] [DOI] [PMC free article] [PubMed]
Tomazini 2020 {published data only}
- Tomazini BM, Maia IS, Bueno FR, Silva MV, Baldassare FP, Costa EL, et al, for the COALITION COVID-19 Brazil III Investigators. COVID-19-associated ARDS treated with DEXamethasone (CoDEX): study design and rationale for a randomized trial. medRxiv [Preprint] 2020. [DOI: 10.5935/0103-507X.20200063] [DOI] [PMC free article] [PubMed]
Trieu 2021 {published data only}
- Trieu V, Saund S, Rahate PV, Barge VB, Nalk KS, Windlass H, et al. Targeting TGF-β pathway with COVID-19 drug candidate ARTIVeda/PulmoHeal accelerates recovery from mild-moderate COVID-19. medRxiv [Preprint] 2021. [DOI: 10.1101/2021.01.24.21250418] [DOI]
Ward 2021 {published data only}
- Ward BJ, Séguin A, Couillard J, Trépanier S, Landry N. Phase III: randomized observer-blind trial to evaluate lot-to-lot consistency of a new plant-derived quadrivalent virus like particle influenza vaccine in adults 18–49 years of age. Vaccine 2021;39(10):1528-33. [DOI: 10.1016/j.vaccine.2021.01.004] [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhou 2020 {published data only}
- Zhou WM, Zhao FM, Li BL. Clinical efficacy of diammonium glycyrrhizinate in the treatment of common type patients with novel coronavirus pneumonia. epistemonikos.org/en/threads/5f18204d7db23a1920da4374. [DOI: 10.13242/j.cnki.bingduxuebao.003679] [DOI]
- Zhou WM, Zhao FM, Li BL. Clinical value of glycyrrhizinate in the treatment of patients with common new coronavirus pneumonia. Chinese Journal of Virology [Epub ahead of print] 2020.
Zhou 2021 {published data only}
- Zhou S, Dong X, Liu F, Zhang Y, Yue D, Zhou Q, et al. A stepped wedge cluster randomized control trial to evaluate the implementation and effectiveness of optimized quality-improvement initiatives in improving quality of care for acute cardiac events in response to the COVID-19 outbreak. Research Square [Preprint] 2021. [DOI] [PMC free article] [PubMed]
Additional references
ACTRN12620000580976
- ACTRN12620000580976. Tocilizumab for the treatment of COVID-19 in intensive care patients: effect on days free of ventilatory support. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379640&isReview=true (first received 19 May 2020).
Bastard 2020
- Bastard P, Rosen LB, Zhang Q, Michailidis E, Hoffmann HH, Zhang Y, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science 2020;370(6515):eabd4585. [DOI: 10.1126/science.abd4585] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boutron 2020a
- Boutron I, Chaimani A, Meerpohl JJ, Hróbjartsson A, Devane D, Rada G, et al. The COVID-NMA project: building an evidence ecosystem for the COVID-19 pandemic. Annals of Internal Medicine 2020;173(12):1015-7. [DOI: 10.7326/M20-5261] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campochiaro 2020
- Campochiaro C, Dagna L. The conundrum of interleukin-6 blockade in COVID-19. Lancet. Rheumatology 2020;2(10):e579-e80. [DOI: 10.1016/S2665-9913(20)30287-3] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cao 2020
- Cao Y, Wei J, Zou L, Jiang T, Wang G, Chen L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. Journal of Allergy and Clinical Immunology 2020;146(1):137-46.e3. [DOI: 10.1016/j.jaci.2020.05.019] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Caricchio 2020
- Caricchio R, Gallucci M, Dass C, Zhang X, Gallucci S, Fleece D, et al, Temple University COVID-19 Research Group. Preliminary predictive criteria for COVID-19 cytokine storm. Annals of the Rheumatic Diseases 2021;80(1):88-95. [DOI: 10.1136/annrheumdis-2020-218323] [PMID: ] [DOI] [PubMed] [Google Scholar]
Chaimani 2018
- Chaimani A, Mavridis D, Higgins JP, Salanti G, White IR. Allowing for informative missingness in aggregate data meta-analysis with continuous or binary outcomes: extensions to metamiss. Stata Journal 2018;18(3):716-40. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Chen 2020
- Chen LY, Hoiland RL, Stukas S, Wellington CL, Sekhon MS. Confronting the controversy: Interleukin-6 and the COVID-19 cytokine storm syndrome. European Respiratory Journal 2020;56(4):2003006. [DOI: 10.1183/13993003.03006-2020] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CORIMUNO‐19 Collaborative group 2021
- CORIMUNO-19 Collaborative group. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomised controlled trial. Lancet Respiratory Medicine 2021;9(3):295-304. [DOI: 10.1016/S2213-2600(20)30556-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
CTRI/2020/05/025369
- CTRI/2020/05/025369. A study on treatment of COVID-19 patients with study drug along with standard of care [A multi‐center, randomized controlled, phase III study to evaluate the clinical outcomes and safety of tocilizumab along with standard of care in patients with cytokine release syndrome associated with COVID‐19 infection]. cochranelibrary.com/central/doi/10.1002/central/CN-02168097/full (first received 31 October 2020).
CTRI/2020/12/029793
- CTRI/2020/12/029793. Efficacy and safety of tocilizumab in patients with severe COVID-19 pneumonia on steroid therapy: A prospective, randomized, double blind placebo-controlled trial. ctri.nic.in/Clinicaltrials/showallp.php?mid1=50303&EncHid=&userName=Tocilizumab (first received 15 December 2020).
EUCTR‐2020‐001162‐12‐FR
- EUCTR-2020-001162-12-FR. An adaptive phase 2/3, randomized, double-blind, placebo-controlled, study assessing efficacy and safety of sarilumab for hospitalized patients with COVID19. clinicaltrialsregister.eu/ctr-search/trial/2020-001162-12/FR (first received 20 March 2020).
EUCTR2020‐001290‐74‐ES
- EUCTR2020-001290-74-ES. Efficacy and safety of sarilumab in the early treatment of hospitalized patients with mild-moderate pneumonia and COVID19 infection versus standard of care. clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-001290-74 (first received 11 April 2020).
EUCTR2020‐001390‐76‐IT
- EUCTR2020-001390-76-IT. A phase 3, randomized, open-labeled, multi-center study comparing clinical efficacy and safety of intravenous sarilumab plus standard of care compared to standard of care, in the treatment of patients with severe COVID-19 pneumonia. clinicaltrialsregister.eu/ctr-search/search?query=eudract_number:2020-001390-76 (first received 27 April 2020).
EUCTR2020‐001767‐86‐IE
- EUCTR2020-001767-86-IE. An open-label, multi-centre, randomised trial comparing different doses of single-dose tocilizumab in adults with severe, non-critical, PCR-confirmed COVID-19 infection with evidence of progressive. clinicaltrialsregister.eu/ctr-search/trial/2020-001767-86/IE (first received 15 April 2020).
EUCTR2020‐002037‐15‐ES
- EUCTR2020-002037-15-ES. Multicenter, randomized, open-label study to evaluate the efficacy and safety of SOC + sarilumab versus standard of care for the early treatment of COVID-19-pneumonia in hospitalized patients. clinicaltrialsregister.eu/ctr-search/search?query=Multicenter%2C+randomized%2C+open-label+study+to+evaluate+the+efficacy+and+safety+of+SOC+%2B+Sarilumab+versus+Standard+of+Care+for+the+Early+Treatment+of+COVID-19-pneumonia+in+Hospitalized+Patients (first received 25 May 2020.
Galani 2020
- Galani IE, Rovina N, Lampropoulou V, Triantafyllia V, Manioudaki M, Pavlos E, et al. Untuned antiviral immunity in COVID-19 revealed by temporal type I/III interferon patterns and flu comparison. Nature Immunology 2021;22(1):32-40. [DOI: 10.1038/s41590-020-00840-x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Galvan‐Roman 2021
- Galván-Román JM, Rodríguez-García SC, Roy-Vallejo E, Marcos-Jiménez A, Sánchez-Alonso S, Fernández-Díaz C, et al, REINMUN-COVID Group. IL-6 serum levels predict severity and response to Tocilizumab in COVID-19: an observational study. Journal of Allergy and Clinical Immunology 2021;147(1):72-80. [DOI: 10.1016/j.jaci.2020.09.018] [PMID: 33010257] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 21 February 2021. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Guan 2020
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al, China Medical Treatment Expert Group for Covid-19. Clinical characteristics of Coronavirus Disease 2019 in China. New England Journal of Medicine 2020;382(18):1708-20. [DOI: 10.1056/NEJMoa2002032] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guaraldi 2020
- Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, et al. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet. Rheumatology 2020;2(8):e474-84.Erratum in: Lancet Rheumatol. 2020 Oct;2(10):e591. [DOI: 10.1016/S2665-9913(20)30173-9.] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herold 2020
- Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, Bergwelt-Baildon M, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. Journal of Allergy and Clinical Immunology 2020;146(1):128-36.e4. [DOI: 10.1016/j.jaci.2020.05.008] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2020
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
IRCT20081027001411N4
- IRCT20081027001411N4. Effect of TOCILIZUMAB (ACTEMRA) on treatment of COVID-19 [Study of tocilizumab effect on treatment and clinical symptoms and laboratory signs of Iranian COVID-19 patients: a clinical trial study]. en.irct.ir/trial/48396 (first received 9 July 2020).
IRCT20200510047383N1
- IRCT20200510047383N1. Evaluation of the effect of Tocilizumab on outcomes of the severe COVID-19 patients. en.irct.ir/trial/48024 (first received 15 May 2020).
IRCT20200525047570N1
- IRCT20200525047570N1. A comparative study of the effects of tocilizumab, interferon-gamma and vitamin C on the recovery of critically ill Covid-19 patients and cytokine storm. en.irct.ir/trial/48583 (first received 30 July 2020).
Juul 2020a
- Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E, et al. Interventions for treatment of COVID-19: A living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). PLOS Medicine 2020;17(9):e1003293. Erratum in: PLoS Med. 2020 Dec 29;17(12):e1003517. [DOI: 10.1371/journal.pmed.1003293] [PMID: 32941437] [DOI] [PMC free article] [PubMed] [Google Scholar]
Juul 2020b
- Juul S, Nielsen EE, Feinberg J, Siddiqui F, Jørgensen CK, Barot E, et al. Interventions for treatment of COVID-19: second edition of a living systematic review with meta-analyses and trial sequential analyses (The LIVING Project). medRxiv [Preprint) 2020. [DOI: 10.1101/2020.11.22.20236448] [DOI] [PMC free article] [PubMed]
Kalil 2021
- Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, et al, ACTT-2 Study Group Members. Baricitinib plus remdesivir for hospitalized adults with Covid-19. New England Journal of Medicine 2021;384(9):795-807. [DOI: 10.1056/NEJMoa2031994] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kang 2020
- Kang S, Narazaki M, Metwally H, Kishimoto T. Historical overview of the interleukin-6 family cytokine. Journal of Experimental Medicine 2020;217(5):e20190347. Erratum in: J Exp Med. 2020 May 4;217(5). [DOI: 10.1084/jem.20190347] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2021
- Khan F, Stewart I, Fabbri L, Moss S, Robinson KA, Smyth A, et al. Systematic review and meta-analysis of anakinra, sarilumab, siltuximab and tocilizumab for COVID-19. Thorax 2021;Feb 2021:Epub ahead of print. [DOI: 10.1136/thoraxjnl-2020-215266] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kirkham 2018
- Kirkham JJ, Altman DG, Chan AW, Gamble C, Dwan KM, Williamson PR. Outcome reporting bias in trials: a methodological approach for assessment and adjustment in systematic reviews. BMJ 2018;362:k3802. [DOI: 10.1136/bmj.k3802] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Knight 2020
- Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Review of Clinical Immunology 2019;15(8):813-22. [DOI: 10.1080/1744666X.2019.1629904] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kotch 2019
- Kotch C, Barrett D, Teachey DT. Tocilizumab for the treatment of chimeric antigen receptor T cell-induced cytokine release syndrome. Expert Review of Clinical Immunology 2019;15(8):813-22. [DOI: 10.1080/1744666X.2019.1629904] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laguna‐Goya 2020
- Laguna-Goya R, Utrero-Rico A, Talayero P, Lasa-Lazaro M, Ramirez-Fernandez A, Naranjo L, et al. IL-6-based mortality risk model for hospitalized patients with COVID-19. Journal of Allergy and Clinical Immunology 2020;146(4):799-807. [DOI: 10.1016/j.jaci.2020.07.009] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2019
- Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biology of Blood and Marrow Transplantation 25;4:625-38. [DOI: 10.1016/j.bbmt.2018.12.758] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lucus 2020
- Lucas C, Wong P, Klein J, Castro TB, Silva J, Sundaram M, et al. Longitudinal analyses reveal immunological misfiring in severe COVID-19. Nature 2020;584(7821):463-9. [DOI: 10.1038/s41586-020-2588-y] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manson 2020
- Manson JJ, Crooks C, Naja M, Ledlie A, Goulden B, Liddle T, et al. COVID-19-associated hyperinflammation and escalation of patient care: a retrospective longitudinal cohort study. Lancet. Rheumatology 2020;2(10):e594-602. [DOI: 10.1016/S2665-9913(20)30275-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mavridis 2015
- Mavridis D, White IR, Higgins JP, Cipriani A, Salanti G. Allowing for uncertainty due to missing continuous outcome data in pairwise and network meta-analysis. Statistics in Medicine 2015;34(5):721-41. [DOI: 10.1002/sim.6365] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mavridis 2018
- Mavridis D, Chaimani A, Efthimiou O, Salanti G. Missing outcome data in meta-analysis. Evidence Based Mental Health 2018;21(3):123. [DOI: 10.1136/eb-2014-101899] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
McCreary 2021
- McCreary EK, Meyer NJ. Covid-19 controversies: the tocilizumab chapter. BMJ 2021;372:n244. [DOI] [PubMed] [Google Scholar]
Mehta 2020
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395(10229):1033-4. [DOI: 10.1016/S0140-6736(20)30628-0] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04315298
- NCT04315298. Evaluation of the efficacy and safety of sarilumab in hospitalized patients with COVID-19 [An adaptive phase 2/3, randomized, double-blind, placebo-controlled study assessing efficacy and safety of sarilumab for hospitalized patients with COVID-19]. clinicaltrials.gov/ct2/show/NCT04315298 (first received 19 March 2020).
NCT04330638
- NCT04330638. Treatment of COVID-19 patients with anti-interleukin drugs [A prospective, randomized, factorial design, interventional study to compare the safety and efficacy of combinations of blockade of interleukin-6 pathway and interleukin-1 pathway to best standard of care in improving oxygenation and short- and long-term outcome of COVID-19 patients with acute hypoxic respiratory failure and systemic cytokine release syndrome]. clinicaltrials.gov/ct2/show/NCT04330638 (first received 1 April 2020).
NCT04343989
- NCT04343989. A randomized placebo-controlled safety and dose-finding study for the use of the IL-6 inhibitor clazakizumab in patients with life-threatening COVID-19 Infection. clinicaltrials.gov/ct2/show/NCT04343989 (first received 14 April 2020).
NCT04348500
- NCT04348500. Clazakizumab (anti-IL- 6 monoclonal) compared to placebo for COVID19 disease [A phase II trial to evaluate the safety and tolerability of Clazakizumab® (anti-IL- 6 monoclonal) compared to placebo for the treatment of COVID-19 infection]. clinicaltrials.gov/ct2/show/NCT04348500 (first received 16 April 2020).
NCT04357808
- NCT04357808. Efficacy of subcutaneous sarilumab in hospitalised patients with moderate-severe COVID-19 infection (SARCOVID) [Randomized open pilot study to evaluate the efficacy of subcutaneous sarilumab in patients with moderate-severe COVID-19 infection]. clinicaltrials.gov/ct2/show/NCT04357808 (first received 22 April 2020).
NCT04357860
- NCT04357860. Clinical trial of sarilumab in adults with COVID-19. clinicaltrials.gov/ct2/show/NCT04357860 (first received 22 April 2020).
NCT04359901
- NCT04359901. Sarilumab for patients with moderate COVID-19 disease [Sarilumab for patients with moderate COVID-19 disease: a randomized controlled trial with a play-the-winner design]. clinicaltrials.gov/ct2/show/NCT04359901 (first received 24 April 2020).
NCT04363502
- NCT04363502. Use of the interleukin-6 inhibitor clazakizumab in patients with life-threatening COVID-19 infection [A randomized placebo-controlled safety and dose-finding study for the use of the IL-6 inhibitor clazakizumab in patients with life-threatening COVID-19 infection]. clinicaltrials.gov/ct2/show/NCT04363502 (first received 27 April 2020).
NCT04377750
- NCT04377750. The use of tocilizumab in the management of patients who have severe COVID-19 with suspected pulmonary hyperinflammation [The use of tocilizumab in the management of patients who have severe COVID-19 with suspected pulmonary hyperinflammation]. clinicaltrials.gov/ct2/show/NCT04377750 (first received 6 May 2020).
NCT04380519
- NCT04380519. Study of the efficacy and safety of a single administration of olokizumab and RPH-104 with standard therapy in patients with severe severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19) [An international, multicenter, randomized, double-blind, adaptive placebo-controlled study of the efficacy and safety of a single administration of olokizumab and RPH-104 with standard therapy in patients with severe SARS-CoV-2 infection (COVID-19)]. clinicaltrials.gov/ct2/show/NCT04380519 (first received 8 May 2020).
NCT04381052
- NCT04381052. Study for the use of the IL-6 Inhibitor clazakizumab in patients with life-threatening COVID-19 infection. clinicaltrials.gov/ct2/show/NCT04381052 (first received 8 May 2020).
NCT04397562
- NCT04397562. A clinical trial of the efficacy and safety of levilimab (BCD-089) in patients with severe COVID-19 [A multicenter, randomized, double-blind, placebo-controlled, adaptively designed clinical trial of the efficacy and safety of levilimab (BCD-089) in patients with severe COVID-19]. clinicaltrials.gov/ct2/show/NCT04397562 (first received 21 May 2020).
NCT04412291
- NCT04412291. A study in patients with COVID-19 and respiratory distress not requiring mechanical ventilation, to compare standard-of-care with anakinra and tocilizumab treatment the immunomodulation-CoV assessment (ImmCoVA) study [A multi-center, randomized, open-label study in patients with COVID-19 and respiratory distress not requiring mechanical ventilation, to compare standard-of-care with anakinra and tocilizumab treatment the immunomodulation-CoV assessment (ImmCoVA) study]. clinicaltrials.gov/ct2/show/NCT04412291 (first received 2 June 2020).
NCT04412772
- NCT04412772. A RCT - safety & efficacy of tocilizumab - Tx of severe COVID-19: ARCHITECTS. clinicaltrials.gov/ct2/show/NCT04412772 (first received 2 June 2020).
NCT04452474
- NCT04452474. Study of the efficacy and safety of a single administration of olokizumab vs. placebo in addition to standard treatment in patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19). clinicaltrials.gov/ct2/show/NCT04452474 (first received 30 June 2020).
NCT04479358
- NCT04479358. Low-dose tocilizumab versus standard of care in hospitalized patients with COVID-19 [A multi-center, randomized, controlled phase 2 trial comparing early administration of low-dose tocilizumab to standard of care in hospitalized patients with COVID-19 pneumonitis not requiring invasive ventilation]. clinicaltrials.gov/ct2/show/NCT04479358 (first received 21 July 2020).
NCT04494724
- NCT04494724. Clazakizumab vs. placebo - COVID-19 infection [A phase 2 trial to evaluate the safety and tolerability of Clazakizumab® [anti-interleukin (IL)-6 monoclonal] compared to placebo for the treatment of COVID-19 infection]. clinicaltrials.gov/ct2/show/NCT04494724 (first received 31 July 2020).
NCT04577534
- NCT04577534. Use of tocilizumab in the inflammatory phase of COVID-19 / new coronavirus disease. clinicaltrials.gov/ct2/show/NCT04577534 (first received 8 October 2020).
NCT04659772
- NCT04659772. A study to evaluate clazakizumab in patients with life-threatening COVID-19 infection. clinicaltrials.gov/ct2/show/NCT04659772 (first received 9 December 2020).
NCT04690920
- NCT04690920. Theranostic implication of complementary medicines against interleukin receptors and Gp-130 proteins [Theranostic implication of complimentary medicines against IL-6/Gp-130 in COVID-19: an in vitro and in silco approach]. clinicaltrials.gov/ct2/show/NCT04690920 (first received 31 December 2020).
NL8504
- NL8504. Pre-emptive tocilizumab in hypoxic COVID-19 patients, a prospective randomized trial. trialregister.nl/trial/8504 (first received 6 April 2020).
Oikonomidi 2020
- Oikonomidi T, Boutron I, Pierre O, Cabanac G, Ravaud P, COVID-19 NMA Consortium. Changes in evidence for studies assessing interventions for COVID-19 reported in preprints: meta-research study. BMC Medicine 2020;18(1):402. [DOI: 10.1186/s12916-020-01880-8] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ouzzani 2016
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan — a web and mobile app for systematic reviews. Systematic Reviews 2016;5(1):210. [DOI: 10.1186/s13643-016-0384-4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pedersen 2020
- Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. Journal of Clinical Investigation 2020;130(5):2202-5. [DOI: 10.1172/JCI137647] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ 2011;342:d549. [DOI: 10.1136/bmj.d549] [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons, Ltd, 2019. [Google Scholar]
Scott 2017
- Scott LJ. Tocilizumab: a review in rheumatoid arthritis. Drugs 2017;77(17):1865-79. Erratum in: Drugs. 2017 Dec 19. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Siemieniuk 2020
- Siemieniuk RA, Bartoszko JJ, Ge L, Zeraatkar D, Izcovich A, Kum E, et al. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ 2020;370:m2980. [DOI: 10.1136/bmj.m2980] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Solis‐García Del Pozo 2020
- Solis-García Del Pozo J, Galindo MF, Nava E, Jordán J. A systematic review on the efficacy and safety of IL-6 modulatory drugs in the treatment of COVID-19 patients. European Review for Medical and Pharmacological Sciences 2020;24(13):7475-84. [DOI: 10.26355/eurrev_202007_21916] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JA, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366:l4898. [DOI: 10.1136/bmj.l4898] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stone 2017
- Stone JH, Tuckwell K, Dimonaco S, Klearman M, Aringer M, Blockmans D, et al. Trial of tocilizumab in giant-cell arteritis. New England Journal of Medicine 2017;377(4):317-28. [DOI: 10.1056/NEJMoa1613849] [PMID: ] [DOI] [PubMed] [Google Scholar]
Stukas 2020
- Stukas S, Hoiland RL, Cooper J, Thiara S, Griesdale DE, Thomas AD, et al. The association of inflammatory cytokines in the pulmonary pathophysiology of respiratory failure in critically ill patients with Coronavirus Disease 2019. Critical Care Explorations 2020;2(9):e0203. [DOI: 10.1097/CCE.0000000000000203] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI: 10.1186/1745-6215-8-16] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tleyjeh 2021
- Tleyjeh IM, Kashour Z, Damlaj M, Riaz M, Tlayjeh H, Altannir M, et al. Efficacy and safety of tocilizumab in COVID-19 patients: a living systematic review and meta-analysis. Clinical Microbiology and Infection 2021;27(2):215-27. [DOI: 10.1016/j.cmi.2020.10.036] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818-27. [DOI: 10.1093/ije/dys041] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Webb 2020
- Webb BJ, Peltan ID, Jensen P, Hoda D, Hunter B, Silver A, et al. Clinical criteria for COVID-19-associated hyperinflammatory syndrome: a cohort study. Lancet. Rheumatology 2020;2(12):e754-63. [DOI: 10.1016/S2665-9913(20)30343-X] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2008
- White IR, Higgins JP, Wood AM. Allowing for uncertainty due to missing data in meta-analysis--part 1: two-stage methods. Statistics in Medicine 2008;27(5):711-27. [DOI: 10.1002/sim.3008] [PMID: ] [DOI] [PubMed] [Google Scholar]
WHO 2020a
- World Health Organization (WHO). Rolling updates on coronavirus diseases (COVID-19). Available from www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen.
WHO 2020b
- Clinical management of severe acute respiratory infection when COVID-19 is suspected. who.int/publications/i/item/10665-332299 2020 (accessed 24 February 2021).
WHO 2020c
- Report of the WHO-China Joint Mission on Coronavirus Disease 2019 (COVID-19). who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report.pdf 2020 (Accessed 24 February 2021).
WHO Working Group 2020
- WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infectious Diseases 2020;20(8):e192-e7. Erratum in: Lancet Infect Dis. 2020 Oct;20(10):e250. [DOI: 10.1016/S1473-3099(20)30483-7] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Worldometer 2020
- Coronavirus Symptoms (COVID-19). worldometers.info/coronavirus/coronavirus-symptoms/ Accessed 24 February 2021.
References to other published versions of this review
Boutron 2020b
- Boutron I, Chaimani A, Devane D, Meerpohl JJ, Rada G, Hróbjartsson A, et al. Interventions for the prevention and treatment of COVID‐19: a living mapping of research and living network meta‐analysis. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD013769. [DOI: 10.1002/14651858.CD013769] [DOI] [Google Scholar]
CRD42020214700
- CRD42020214700. Interleukin (IL)-6 blocking agents for the treatment of COVID-19. A living systematic review. crd.york.ac.uk/prospero/display_record.php?RecordID=214700 (first received 29 October 2020).