Abstract
Background
Anti‐leukotriene (AL) agents are being considered as 'add‐on' therapy to inhaled corticosteroids (ICS), in chronic asthma.
Objectives
To examine the safety and efficacy of daily AL plus ICS compared to ICS alone, and determine the corticosteroid‐sparing effect of AL when added to ICS in chronic asthma.
Search methods
We searched MEDLINE, EMBASE, CINAHL (until August 2003), reference lists of review articles and trials, contacted international headquarters of AL manufacturers and looked at American Thoracic Society and European Respiratory Society meeting abstracts (1998 to 2003).
Selection criteria
Randomised placebo‐controlled trials of asthmatics aged two years and older with at least one month intervention.
Data collection and analysis
Two reviewers assessed quality and extracted data independently. Trials were grouped by asthma control at baseline (symptomatic or well‐controlled) and dose of ICS in the control group (same or double).
Main results
Of 587citations, 27 (25 adult and 2 paediatric) trials met inclusion criteria. Sixteen trials were published in full‐text and 16 trials reported data in a way that allowed meta‐analysis. In symptomatic patients, addition of licensed doses of anti‐leukotrienes to ICS resulted in a non‐significant reduction in the risk of exacerbations requiring systemic steroids: Relative Risk (RR) 0.64; 95% Confidence Interval (CI) 0.38 to 1.07). A modest improvement group difference in PEF was seen (Weighted Mean Difference (WMD) 7.7 L/min; 95% CI 3.6 to 11.8 L/min) together with decrease in use of rescue short‐acting beta2‐agonist use (WMD 1 puff/week; 95%CI 0.5 to 2). With only 3 trials comparing the use of licensed doses of anti‐leukotrienes with increasing the dose of inhaled glucocorticoids, no firm conclusion can be drawn about the equivalence of both treatment options. In ICS‐sparing studies of patients who were well controlled at baseline, addition of anti‐leukotrienes produced no overall difference in dose of inhaled glucocorticoids (WMD ‐21 mcg/d, 95%CI ‐65, 23 mcg/d), but it was associated with fewer withdrawals due to poor asthma control (RR 0.63, 95% CI 0.42 to 0.95).
Authors' conclusions
The addition of licensed doses of anti‐leukotrienes to add‐on therapy to inhaled glucocorticoids brings modest improvement in lung function. Although addition of anti‐leukotrienes to inhaled glucocorticoids appears comparable to increasing the dose of inhaled steroids, the power of the review is insufficient to confirm the equivalence of both treatment options. Addition of anti‐leukotrienes is associated with superior asthma control after glucocorticoid tapering; although the glucocorticoid‐sparing effect cannot be quantified at present, it appears modest.
Plain language summary
The addition of anti‐leukotriene agents to inhaled corticosteroids versus placebo for chronic asthma
Inhaled steroids remain the cornerstone of asthma treatment. Anti‐leukotrienes constitute a new class of drugs that can be taken by mouth and do not have the side effects associated with steroids. We looked to see how effective these drugs were when they were added to the treatment of patients who needed steroid inhalers to control their asthma.In asthmatics that are not well controlled on inhaled steroids, the addition of anti‐leukotrienes brings modest improvement in asthma control but it remains unclear whether they are as effective as increasing the dose of inhaled steroids. Higher doses of anti‐leukotrienes are more effective, but associated with an increased risk of side effect that occurs with these particular drugs. In asthmatics that are well controlled on inhaled steroids, the addition of anti‐leukotrienes did not allow a reduction in the amount of inhaled steroid used but they seemed to have a beneficial effect on the asthma control. There are only two studies that have looked at these issues in children; this is insufficient to firmly conclude whether anti‐leukotrienes are useful in children.
Background
Since the mid 1980's, the understanding of the primarily inflammatory nature of asthma has tremendously changed its management (Murphy 1993). Infiltration of bronchial airways with eosinophils and neutrophils with production of inflammatory mediators is characteristic of asthma. The most potent inflammatory mediators may be the cysteinyl leukotrienes which are produced by the 5‐lipoxygenase pathway of the arachidonic acid metabolism. These mediators stimulate the production of airway secretions, cause microvascular leakage and enhance eosinophilic migration in the airways; thus, leukotrienes are believed to play a major role in mediating bronchoconstriction and inflammatory changes pivotal in the pathophysiology of asthma (Piper 1989). Treatment of airway inflammation is now advocated by all recent consensus statements on asthma (GINA 2002;BTS 2003;Australia 2002;Boulet 2001;CTS 1999). Although several drugs such as ketotifen, sodium cromoglycate and sodium nedocromil have anti‐inflammatory properties, inhaled glucocorticoids remain the cornerstone of asthma management because of their efficacy, tolerance, and rapid onset of action (CTS 1999; Spahn 1996). The administration of inhaled glucocorticoids is generally considered safe, unless the daily dose required for control of symptoms remains large for a prolonged period. In these conditions, adverse effects such as growth stunting in children (Wolthers 1996; Lipworth 1999; Kamada 1995), suppression of the adrenal axis (Wolthers 1998) and bone osteopenia may be observed (Efthimiou 1998; Bootsma 1997). To reduce the dose of glucocorticoids required for symptom control, other drugs may be added. Long‐acting beta‐2‐agonists, which have only mild anti‐inflammatory effect, are being used for this purpose (Bisgaard 2000; CTS 1999). Anti‐leukotrienes form a new class of anti‐inflammatory drugs which may have important glucocorticoids‐sparing effects. These drugs interfere either with leukotriene production (5‐ lipoxygenase inhibitors) or with leukotriene receptors (leukotriene receptors antagonists). Anti‐leukotrienes have the advantage of being administered orally in a single or twice daily dose and, importantly, seem to lack the adverse effects associated with long‐term systemic glucocorticoid therapy. However, safety remains an important issue particularly with elevation of hepatic enzymes and Churg‐Strauss syndrome reported in some patients treated with anti‐leukotrienes (Price 2000).
As relatively new therapeutic agents, it is important to determine the proper place of anti‐leukotrienes in the management of asthma. While the 2002 Global Initiative for Asthma guidelines classifies the role of anti‐leukotrienes as still under investigation,(GINA 2002) several national guidelines advocate its use as adjunct therapy to inhaled glucocorticoids in moderate to severe persistent asthma or as alternative single‐agent management in mild asthma (BTS 2003; CTS 1999; Australia 2002). Randomised controlled trials have provided evidence of the efficacy of anti‐leukotrienes as add‐on therapy to inhaled glucocorticoids in persistent asthma (Laviolette 1999; Lofdahl 1999; Tamaoki 1997; Virchow 2000); the evidence was summarized in a Cochrane review last updated in September 2001. With the publication of new trials, an update of the systematic review of the randomised controlled trials was indicated to review the potential benefit of anti‐leukotrienes as add‐on therapy to inhaled glucocorticoids and to provide better insight in the influence of study characteristics on results.
Objectives
In patients who were symptomatic, despite use of maintenance inhaled corticosteroids, we wished to determine whether the addition of anti‐leukotriene agents reduced the frequency and severity of exacerbations and improved chronic asthma control while maintaining a good safety profile. The addition of anti‐leukotriene agents to inhaled corticosteroids was compared to either the use of the same or double dose of inhaled corticosteroids.
In patients who were well controlled on their baseline dose of inhaled corticosteroids, we wished to quantify the magnitude of dose reduction in inhaled glucocorticoids (glucocorticoid‐sparing effect) that could be achieved with the addition of anti‐leukotrienes.
Secondary objectives were to determine whether patients' characteristics, such as dose and type of anti‐leukotrienes, dose of inhaled glucocorticoids required to control symptoms, age (adult versus child), severity of airway obstruction on baseline (% predicted FEV1), duration of intervention, and asthma triggers (allergens, viral infection, aspirin, or others), influenced the magnitude of effect attributable to anti‐leukotrienes.
Methods
Criteria for considering studies for this review
Types of studies
Randomised placebo controlled trials in adults and children in which anti‐leukotriene agents were added to inhaled glucocorticoid were considered for inclusion. Sensitivity analyses were performed based on the reported quality of randomisation, concealment of allocation, blind assessment of outcomes, and description of withdrawals and dropouts.
Types of participants
Children aged two to 17 years and adults with recurrent or chronic asthma, symptomatic or well‐controlled on daily maintenance inhaled glucocorticoids.
Types of interventions
Combination of daily oral anti‐leukotriene agents, including leukotriene synthesis inhibitors and leukotriene receptor antagonists, with inhaled glucocorticoids (treatment group) compared to inhaled glucocorticoids alone (control group).
Types of outcome measures
Primary outcomes
The primary outcome was the number of patients with exacerbations requiring use of systemic glucocorticoids when the intervention was compared to the same or increased dose of inhaled glucocorticoids. The change from the baseline dose of inhaled glucocorticoids required to maintain control was the main outcome when the intervention was aimed at establishing the steroid‐sparing effect.
Secondary outcomes
The following secondary outcomes were considered after various lengths of treatment:
Change in clinical or physiologic outcomes reflecting chronic asthma control (such as symptom score, night‐time awakenings, days without symptoms, quality of life, rescue short‐acting beta‐2‐agonist use, and pulmonary function tests)
Clinical or physiologic outcomes reflecting the frequency and severity of asthma exacerbations (such as hospital admissions)
Inflammatory markers (such as serum and sputum eosinophils, serum eosinophilic cationic protein (ECP), expired nitric oxide, etc)
Clinical and biochemical adverse effects (i.e. elevation of liver enzymes) as well as withdrawal rates were also examined.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Airways Group Asthma trials register on all fields for:
[leukotriene* OR anti‐leukotriene* OR *lukast* ] AND [[inhaled* AND [steroid* OR corticosteroid* OR fluticasone * OR triamcinolone* OR dexa* OR deca* OR gluticasone OR flovent OR beclomethasone* OR beclovent OR becloforte OR budesonide* OR pulmicort OR flunisolide OR aerobid OR bronalide OR azmacort OR vanceril OR becotide OR flixotide OR aerobec]].
We also completed an advanced search of CENTRAL, the Cochrane Central Register of Controlled Trials using the above search strategy.
Searching other resources
We then reviewed all abstracts and citations and annotated as a RCT, clearly not an RCT or unclear. We obtained and reviewed the publications of references identified as RCTs or unclear and identified trials as clearly or potentially relevant. Secondly, we checked reference lists of all identified RCTs to identify potentially relevant citations. Thirdly, we contacted the international headquarters of pharmaceutical companies producing anti‐leukotrienes and inhaled steroids. We also made enquiries regarding other published or unpublished studies known and/or supported by these companies or their subsidiaries. Finally, we searched the abstract books of the American Thoracic Society and the European Respiratory Society Meetings from 1998‐2003. The search was last updated in August 2003.
Data collection and analysis
Selection of studies
Two authors independently assessed the search results (FD or ZS). Studies were retrieved selected on the basis of relevance, and were selected for inclusion by the agreement of two authors.
Data extraction and management
Data were extracted independently by two reviewers (FMD and ZS, GH or RK) , blinded as above, and disagreement was dealt with by consensus.
Assessment of risk of bias in included studies
The methodological quality of the eligible controlled trials was assessed with a 5‐point scoring instrument (Jadad 1995). This instrument evaluates the reported quality of randomisation, blinding, and description of withdrawals and dropouts. The quality assessment was done independently by two reviewers (FMD and ZS, GH or RK). We dealt with disagreement by consensus. We sought confirmation of methodology for all included trials directly from the authors and/or the funding pharmaceutical companies.
Dealing with missing data
Confirmation of data extraction for all included trials was sought directly from the authors and/or the funding pharmaceutical companies.
Assessment of heterogeneity
Homogeneity of effect sizes between studies being pooled was tested with the DerSimonian & Laird method, with p < 0.05 being used as the cut‐off level for significance. If heterogeneity was suggested, the DerSimonian & Laird random‐effects model was applied to the summary estimates. Due to low power for detecting heterogeneity with few trials, any difference in the conclusions related to the choice of the (fixed or random) model were reported, when observed for the main outcomes. To detect possible biases, funnel plot symmetry was examined for trials contributing data to the main outcomes (Egger 1997). All estimates were reported with their 95% confidence interval.
Data synthesis
Treatment effects for dichotomous outcomes were reported as pooled relative risks with the fixed‐effect model (Greenland 1985) or, in case of heterogeneity, the random‐effects model (DerSimonian 1986). We summarised differences between groups in event rates, such as the number of participants with exacerbations in a specific period of time, using the Relative Risk of the event occurring. Equivalence was assumed if the relative risk estimate and its confidence interval were between 0.9 and 1.1. For continuous outcomes, such as pulmonary function tests or quality of life scores, we used a Weighted Mean Difference (WMD) or Standardised Mean Difference (SMD), where appropriate, to estimate individual and pooled effect sizes. Numbers Needed to Treat (NNT) were derived from the pooled Odds Ratios using Visual Rx (an online calculator at www.nntonline.net) as the results are not affected by the way in which the events are recorded (Cates 2002).
Trials were divided into three protocols, according to the relative dose of inhaled glucocorticoids used by the control group (same, double, tapered).
(1) Anti‐leukotrienes (AL) + inhaled glucocorticoids (ICS) versus SAME dose of ICS.
(2) Anti‐leukotrienes (AL) + inhaled glucocorticoids (ICS) versus DOUBLE dose of ICS.
(3) Anti‐leukotrienes (AL) + inhaled glucocorticoids (ICS) versus ICS (TAPERING protocol).
In all comparisons, the dose and type of anti‐leukotriene agents served as the primary stratifying variable.
Subgroup analysis and investigation of heterogeneity
The magnitude or direction of treatment response: (1) dose and anti‐leukotrienes used, (2) baseline dose of inhaled glucocorticoids, (3) age, (4) severity of baseline airway obstruction, (5) duration of intervention and (6) asthma triggers. Whenever possible, these factors were examined as possible source of heterogeneity. The mean baseline dose of inhaled glucocorticoids in the intervention group was recorded as 'User defined order' in mcg/day of chlorofluorocarbon (CFC)‐propelled beclomethasone dipropionate or equivalent x 0.1; where, irrespective of delivery system used, 1 mcg of beclomethasone = 1 mcg of budesonide = 0.5 mcg of fluticasone = 2 mcg of triamcinolone = 2 mcg of flunisolide (USA 2002). Usual licensed doses of leukotriene receptor antagonists are: montelukast 10 mg daily, pranlukast 450 mg daily, and zafirlukast 20 mg twice daily.For the primary outcome, sensitivity analyses were performed to determine the effect of publication status and methodological quality on results. We performed the meta‐analysis using RevMan software
Results
Description of studies
Results of the search
The search strategy last updated in August 2003 yielded additional 211 citations for a total of 587 citations.
A total of 27 (2 paediatric and 25 adult) trials, 16 of which were published in full text at the time of this report (Kanniess 2002; Laviolette 1999; O'Sullivan 2003; Price 2003; Riccioni 2001; Riccioni 2002; Shingo 2001; Simons 2001; Tamaoki 1997; Tohda 2002; Tomari 2001; Tomita 1999; Vaquerizo 2003 ;Virchow 2000; Wada 1999), met the inclusion criteria for this review. We grouped these 27 trials according to one of three protocols defining their specific objective and design.
Included studies
Anti‐leukotrienes + ICS versus SAME dose of inhaled corticosteroids (ICS)
DESIGN
Thirteen trials, including 10 full‐text publications (Laviolette 1999; O'Sullivan 2003; Riccioni 2001; Riccioni 2002; Simons 2001; Tamaoki 1997; Tomita 1999; Vaquerizo 2003; Virchow 2000; Wada 1999) and two abstracts (Finn 2000;Nishimura 1999) and an unpublished report (Hultquist 2000) evaluated the degree of asthma control achieved by the addition of anti‐leukotrienes to inhaled corticosteroids compared to the same dose of inhaled corticosteroids in the control group. All but three trials (Nishimura 1999; O'Sullivan 2003; Simons 2001) were parallel‐group randomised controlled trials. Eight trials (Finn 2000; Hultquist 2000; Laviolette 1999; Nishimura 1999; Simons 2001; Vaquerizo 2003; Virchow 2000; Wada 1999) enrolled participants who were clearly symptomatic at baseline; two trials enrolled well controlled adults (Riccioni 2001; Riccioni 2002); two trials (Tamaoki 1997; Tomita 1999) recruited well controlled participants in whom their usual dose of ICS required to maintain control was suddenly reduced by half on randomisation in an attempt to render them symptomatic; and the last trial (O'Sullivan 2003) enrolled well controlled mild asthmatics in a cross‐sectional study to determine the impact on inflammatory markers and lung function of the addition of leukotriene receptor antagonists. Finn 2000 randomised 479 children of whom only 98 received co‐treatment with inhaled glucocorticoids which were analysed separately (although it is unclear if the randomisation was stratified on the presence/absence of co‐treatment with inhaled glucocorticoids). Two trials (Tomita 1999; Wada 1999) were open label studies (no blinding) with no placebo use in the control group, while two other trials reported their study (Riccioni 2001; Riccioni 2001) as double‐blind for the patients and assessor but with no use of placebo.
PARTICIPANTS
All but two trials, (Finn 2000; Simons 2001), focused on adults; the mean age of participants varied between 38 to 50 years; the pediatric trials enrolled children with a mean age of 10 years for Simons 2001 and an age range of 5‐11 years in Finn 2000. There was relatively similar representation of genders. In adult trials, the average duration of asthma was greater than 10 years. The severity of airway obstruction on baseline was mild to moderate. In the trials that enrolled participants who were symptomatic on maintenance inhaled glucocorticoids, the baseline FEV1 varied between 63% to 81% of predicted (Virchow 2000; Finn 2000; Hultquist 2000;Laviolette 1999;Simons 2001;Vaquerizo 2003) while it was unreported in Wada 1999. In the trials that enrolled asthmatics who were well controlled prior to sudden decrease of the inhaled glucocorticoids dose, the FEV1 was 80% predicted in Tamaoki 1997 and 88% predicted in Tomita 1999. O'Sullivan 2003, Riccioni 2001 and Riccioni 2002 enrolled well controlled mild asthmatics with an average of 90% predicted FEV1. Allergic triggers were reported in over 45% to 100% of participants, when reported (Laviolette 1999;O'Sullivan 2003, Simons 2001; Tomita 1999;Virchow 2000;Wada 1999); allergic rhinitis was seldom mentioned.
INTERVENTION
The duration of trials was short, varying between four weeks (Nishimura 1999; Simons 2001; Wada 1999), six weeks (Finn 2000; Tamaoki 1997; Virchow 2000), eight weeks (Hultquist 2000; O'Sullivan 2003;Riccioni 2001 ;Tomita 1999), to 16 weeks (Laviolette 1999; Riccioni 2002;Vaquerizo 2003). The tested intervention drugs were all leukotriene receptor antagonists, namely pranlukast (Nishimura 1999;Tamaoki 1997; Tomita 1999; Wada 1999) at a daily dose of 450 mg/day (double dose in Tamaoki 1997), montelukast 5 mg (Simons 2001), montelukast 10 mg die (Laviolette 1999;O'Sullivan 2003;Riccioni 2002;Vaquerizo 2003), zafirlukast 10 mg bid (Finn 2000), zafirlukast 20 mg bid (Hultquist 2000;Riccioni 2001), and zafirlukast 80 mg bid (Virchow 2000). In all trials, the inhaled glucocorticoids was administered at the same daily dose in both the control and intervention groups. Most trials used beclomethasone dipropionate (BDP). Five trials added leukotriene receptor antagonists to low doses of inhaled glucocorticoids (<=400 mcg/day of BDP or equivalent) (Hultquist 2000; Laviolette 1999; O'Sullivan 2003; Simons 2001; Tomita 1999), four trials pertained to moderate doses of inhaled steroids (i.e., 401‐800 mcg/day of BDP or equivalent) (Tamaoki 1997;Riccioni 2001;Riccioni 2002;Vaquerizo 2003); three trials pertained high doses of inhaled steroids (i.e..,> 800 mcg/day of BDP or equivalent) (Nishimura 1999;Virchow 2000;Wada 1999)); while it remained unspecified in one trial (Finn 2000)
In five trials, Hultquist 2000, Laviolette 1999, Riccioni 2001,Riccioni 2002 and Vaquerizo 2003, no additional co‐treatment was permitted other than rescue inhaled beta‐2 agonists and systemic corticosteroids. In other trials, the following co‐treatments were permitted if maintained constant throughout the trial: oral beta‐2 agonists, theophylline and inhaled anticholinergics (Tamaoki 1997), maintenance oral corticosteroid therapy was permitted in five participants in Tomita 1999 and slow‐release theophylline in Wada 1999. No information regarding co‐intervention was provided for Simons 2001,Finn 2000,Nishimura 1999, and O'Sullivan 2003.
OUTCOMES
The pre‐designated primary outcome (the number of participants with exacerbations requiring systemic corticosteroids) was documented in only four trials (Laviolette 1999; Simons 2001;Tamaoki 1997; Virchow 2000). Six trials (Hultquist 2000; Laviolette 1999; Simons 2001; Tamaoki 1997; Virchow 2000; Vaquerizo 2003) reported other measures of asthma control (pulmonary function tests, symptoms scores, or functional status indices) as change from baseline. Five trials (Hultquist 2000;Laviolette 1999; Simons 2001; Virchow 2000;Vaquerizo 2003) performed intention‐to‐treat analyses. Five trials (Finn 2000; Nishimura 1999; O'Sullivan 2003; Tomita 1999; Wada 1999) contributed no data in the format required for the meta‐analysis.
Anti‐leukotrienes + ICS versus INCREASED dose of ICS
DESIGN
Seven trials, (Green 2002a; Nayak 1998; Nsouli 2000; Price 2003; Ringdal 1999; Tomari 2001; Yildirim 2001), compared the addition of anti‐leukotrienes to inhaled corticosteroids to increasing the dose of inhaled corticosteroids in symptomatic patients. All but one trial (Green 2002a) were parallel‐group, randomised trials, placebo‐controlled. In two trials (Nayak 1998;Ringdal 1999), various doses of anti‐leukotrienes were considered in a three‐arm trial design (see below). Only two trials are currently full text publications (Price 2003; Tomari 2001), while data for two additional trials (Nayak 1998;Ringdal 1999) were derived from abstracts and unpublished reports kindly provided by Astra‐Zeneca, the manufacturer of zafirlukast.
PARTICIPANTS
All trials focused on adults with a mean age of about 40 years, with balanced representation of genders (except in Nayak 1998 with 38% of males). The severity of airway obstruction on baseline was mild to moderate, with mean % FEV1 of 67% to 71% of predicted in 3 trials (Nayak 1998;Price 2003;Tomari 2001) and 85% predicted (Ringdal 1999) while on maintenance inhaled glucocorticoids dose (described below). Allergic triggers were not reported, although 94.2% of patients enrolled in Nayak 1998 had allergic rhinitis.
INTERVENTION
The duration of each trial was: 4 weeks (Green 2002a), 12‐13 weeks (Nayak 1998; Nsouli 2000; Price 2003; Ringdal 1999;Yildirim 2001) and 16 weeks (Tomari 2001). Licensed doses of leukotriene receptor antagonists were tested in 6 trials, namely montelukast 10 mg die (Green 2002a; Nsouli 2000; Price 2003; Yildirim 2001), pranlukast 450 mg die (Tomari 2001) and zafirlukast 20 mg bid (Ringdal 1999). Two trials also used higher than licensed doses of zafirlukast of 40 mg bid (Nayak 1998) and 80 bid (Nayak 1998, Ringdal 1999). In the intervention group, BDP or BUD was given at a dose of 200 mcg/day (Green 2002a), 400‐500 mcg/day (Nayak 1998; Ringdal 1999; Yildirim 2001) and 600‐800 mcg/day in the remaining 3 trials (Nsouli 2000; Price 2003; Tomari 2001). While the control groups all received double‐dose of inhaled glucocorticoids, the magnitude of the increased dose was of 400 mcg/day of beclomethasone or equivalent in 3 trials (Nayak 1998;Ringdal 1999; Yildirim 2001) and of 600 to 800 mcg/day in the remaining 4 trials (Green 2002a; Nsouli 2000; Price 2003; Tomari 2001). No additional co‐treatment was described other than rescue inhaled beta2‐agonists and systemic corticosteroids.
OUTCOMES
The primary outcome was the number of participants with exacerbations requiring systemic corticosteroids: this was documented in only three trials (Nayak 1998; Price 2003; Ringdal 1999). Other measures of asthma control (pulmonary function tests, symptoms scores, functional status indices), withdrawals and adverse effects were also considered. Intention‐to‐treat analyses were reported in 2 trials (Price 2003; Tomari 2001). Three trials (Green 2002a; Nsouli 2000; Yildirim 2001), all published in abstract form, contributed no data in the format required for the meta‐analysis.
Anti‐leukotrienes + ICS versus SAME dose of ICS (TAPERING ICS dose)
DESIGN
Seven trials included participants who were well controlled at baseline (Baba 1999;Bateman 1995;Kanniess 2002 ;Laitinen 1995; Lofdahl 1999; Shingo 2001; Tohda 2002), and assessed the magnitude of reduction in inhaled corticosteroids following the addition of anti‐leukotrienes. At the time of publication, four trials (Kanniess 2002;Lofdahl 1999; Shingo 2001;Tohda 2002) were published in full‐text; the data for Bateman 1995 and Laitinen 1995 were derived from abstracts and unpublished reports provided by Astra‐Zeneca; the data provided in one abstract (Baba 1999) was insufficient to be used in this review. All but one trial (Kanniess 2002) were parallel‐group trials; Kanniess 2002 described a cross‐over study where tapering (from 800 to 400 mcg/day of beclomethasone or equivalent) was initiated in the first period then, without a washout period, patients crossed‐over to the alternate treatment strategy in the second period where the dose of inhaled steroids was tapered (from 400 to 200 mcg/day of beclomethasone or equivalent). Because this unusual design did not allow merging of the two periods and because no significant change in asthma control occurred in the first period suggesting of over‐treatment at baseline, the second period was chosen for analysis. Use of an identical placebo was described in all but one trial (Baba 1999).
PARTICIPANTS
All trials focused on adults with a mean age of 40 years, with relatively balanced representation of genders and an asthma duration of 13 to 18 years. Participants were well controlled and no evidence of airway obstruction with mean baseline FEV1 of 2.5 to 2.6 L (Bateman 1995; Laitinen 1995) or 83 to 93% predicted FEV1 (Kanniess 2002; Lofdahl 1999;Shingo 2002;Tohda 2002). Allergic triggers were reported in 43 to 48% of enrolled participants, when reported (Bateman 1995; Laitinen 1995); the proportion of participants afflicted with allergic rhinitis was not mentioned. No participants' description was provided for one trial (Baba 1999)
INTERVENTION
Only two of the seven trials, (Laitinen 1995;Lofdahl 1999), had a dose optimisation period prior to randomisation during which the maintenance dose of inhaled corticosteroids was tapered according to a standard procedure over a period of two weeks to three months. The intervention period varied from six (Kanniess 2002), eight (Shingo 2002), 12 (Laitinen 1995; Lofdahl 1999), 20 weeks (Bateman 1995), and 24 weeks (Tohda 2002) during which the tapering of inhaled corticosteroids to the minimum effective dose was continued. Baba 1999 failed to describe the intervention duration.
The intervention drugs were: zafirlukast 20 mg bid (Bateman 1995; Laitinen 1995), montelukast 10 mg die (Kanniess 2002;Lofdahl 1999; Shingo 2002;Tohda 2002) and pranlukast at an unspecified dose (Baba 1999). The reported dose of inhaled corticosteroids at randomisation varied widely within and between trials: 400 mcg (Kanniess 2002), 300 to 3000 mcg (Lofdahl 1999), 400 to 750 mcg (Bateman 1995), 945 mcg on average (Tohda 2002), 800 to 2000 mcg (Laitinen 1995), 1600 mcg (Shingo 2002) while it was unreported in one trial (Baba 1999). Lofdahl 1999 and Shingo 2002 reported the use of several types of inhaled corticosteroids; Lofdahl 1999 reported use of 16% beclomethasone, 22% budesonide, 15% flunisolide, 7% fluticasone, and 40% triamcinolone, but the corticosteroid dose reduction could not be provided in 'beclomethasone‐equivalent' dose (TR Reiss, Personal communication, June 2000); Shingo 2002 reported the use of triamcinolone in 72%, flunisolide in 18% and beclomethasone in 10%. Baba 1999, Bateman 1995, Kanniess 2002 , Laitinen 1995, and Tohda 2002 reported the use of beclomethasone and/or budesonide.
OUTCOMES
The primary outcome with the tapering‐steroid studies was the change from baseline in the dose of inhaled corticosteroids needed to maintain asthma control. This was documented in sufficient detail to permit aggregation of data in four trials after 12 to 24 weeks of tapering (Bateman 1995; Laitinen 1995; Lofdahl 1999;Tohda 2002). In the study by Kanniess 2002, the tapering from 400 mcg to 200 mcg was forced and thus, could not served to document the minimal effective dose; instead the data was used to document maintenance of asthma control. The lowest tolerated dose reported by Lofdahl 1999 was assumed to have been observed after 12 ±4 weeks. Intention‐to‐treat analyses were used in four trials (Bateman 1995; Kanniess 2002;Lofdahl 1999; Shingo 2002). To confirm the stability of participants, Bateman 1995, Laitinen 1995 and Shingo 2002 reported the other measures of asthma control as final values measured at the endpoint (or end of study). Kanniess 2002, Lofdahl 1999, and Tohda 2002 reported change from baseline at the lowest tolerated ICS dose, irrespective of when this was achieved. One study (Baba 1999) published in abstract form contributed insufficient data to include in the meta‐analysis.
Excluded studies
Of these 560 citations were excluded for the following mutually exclusive reasons: (1) duplicate references (N = 115) , (2) not a randomised controlled trial (N = 231), ongoing trials (N = 3), or awaiting assessment (N = 3) (3) subjects were not asthmatics (N = 16), (4) the tested intervention was not anti‐leukotrienes (N = 15), (5) no consistent co‐treatment with inhaled glucocorticoids (N = 138), (6) the control intervention was not inhaled corticosteroids (N = 19), (7) use of non permitted drugs (N = 5), (8) the tested intervention was administered for less than 4 weeks (N = 7), (9) outcomes measures did not reflect asthma control (N = 7) and (10) acute care setting (N = 1). Due to the large number of citations considered, the references and reasons for exclusion are provided only for full‐text randomised controlled trials.
Risk of bias in included studies
Fifteen of the 27 trials had high reported methodological quality with a Jadad score >= 4 (Bateman 1995; Hultquist 2000;Kanniess 2002; Laitinen 1995; Laviolette 1999; Lofdahl 1999; Nayak 1998; Price 2003;Ringdal 1999; Shingo 2002; Simons 2001; Tamaoki 1997; Tohda 2002;Vaquerizo 2003;Virchow 2000); confirmation of methodology was confirmed in 15 trials (Bateman 1995;Hultquist 2000;Kanniess 2002;Laitinen 1995;Laviolette 1999;Lofdahl 1999;Nayak 1998;Price 2003;Ringdal 1999;Riccioni 2001;Riccioni 2002;Shingo 2001;Simons 2001;Tohda 2002;Virchow 2000).
Blinding of allocation: all but 7 trials (Baba 1999; Riccioni 2001; Riccioni 2002; Tomita 1999; Wada 1999; Nsouli 2000; Tomari 2001; Yildirim 2001) clearly reported convincing blinding of treatment. Poor reported methodological quality were often associated with abstract report.
Randomisation was clearly described and appropriate in 16 trials (Hultquist 2000; Bateman 1995; Kanniess 2002; Laitinen 1995; Laviolette 1999; Lofdahl 1999; Nayak 1998; Price 2003; Riccioni 2001; Riccioni 2002; Riccioni 2002; Ringdal 1999; Simons 2001; Shingo 2001; Tohda 2002; Vaquerizo 2003; Virchow 2000); reporting of methods of randomisation and mode of allocation not insufficiently described in the remaining trials.
Withdrawal rate was described in all but seven trials (Baba 1999; Finn 2000; Green 2002a; Nishimura 1999; Nsouli 2000; Tomita 1999; Yildirim 2001). Withdrawal rates varied from 2% (Simons 2001) to 27% (Lofdahl 1999) in the control group and from 2% (Tamaoki 1997) to 20% (Bateman 1995) in the intervention group.
Effects of interventions
The results are stratified within each study protocol on the use of usual, versus higher than licensed, doses of anti‐leukotrienes and anti‐leukotriene used.
(1) Anti‐leukotrienes + ICS vs. SAME dose of ICS
Of the thirteen trials using this protocol, five trials (2 abstracts Finn 2000; Nishimura 1999; and 3 full‐text publications O'Sullivan 2003, Tomita 1999, Wada 1999) reported data in a way that could not be used in this review. The meta‐analysis therefore pertains to 8 trials contributing data to the following outcomes.
Primary outcome: requirement for oral steroids
Although 6 trials (Laviolette 1999; Riccioni 2001; Riccioni 2002; Simons 2001; Tamaoki 1997; Virchow 2000) contributed data to the primary outcome, only four trials tested anti‐leukotrienes at licensed doses, namely zafirlukast (Riccioni 2001) and montelukast at adult (Laviolette 1999;Riccioni 2002) and paediatric (Simons 2001) doses. The addition of licensed doses of anti‐leukotrienes to 400 to 800 mcg/day of beclomethasone or equivalent resulted in a non‐significant reduction in the risk of exacerbations requiring systemic steroids (RR 0.64, 95% CI 0.38 to 1.07, Figure 1). When higher than licensed doses were examined, the addition of pranlukast or zafirlukast to high doses of inhaled corticosteroids clearly reduced by 66% the risk of exacerbations requiring systemic steroids (RR 0.34, 95% CI 0.13 to 0.88, Analysis 1.1). The number of patients needed to be treated with higher than licensed doses of anti‐leukotrienes to prevent one patient with exacerbation(s) requiring systemic corticosteroids is 22 (95% CI 17 to 117). Between and within each stratum (licensed versus higher than licensed doses of anti‐leukotrienes), the results were homogeneous despite the different doses and anti‐leukotrienes tested, age, baseline dose of inhaled glucocorticoids and duration of intervention. The individual effect of the type of anti‐leukotrienes, dose of beclomethasone (400 to 1600 mcg/day), duration of treatment (4 to 16 weeks), asthma triggers, and intention‐to‐treat (not reported for Tamaoki 1997) on this outcome could not be examined due to insufficient trials and/or inadequate reporting. Removal of the two trials with no convincing blinding (Riccioni 2001;Riccioni 2002) did not affect the results (RR 0.61, 95% CI 0.36 to 1.05). Due to the published status of all six contributing trials, no additional sensitivity analyses were performed.
1.
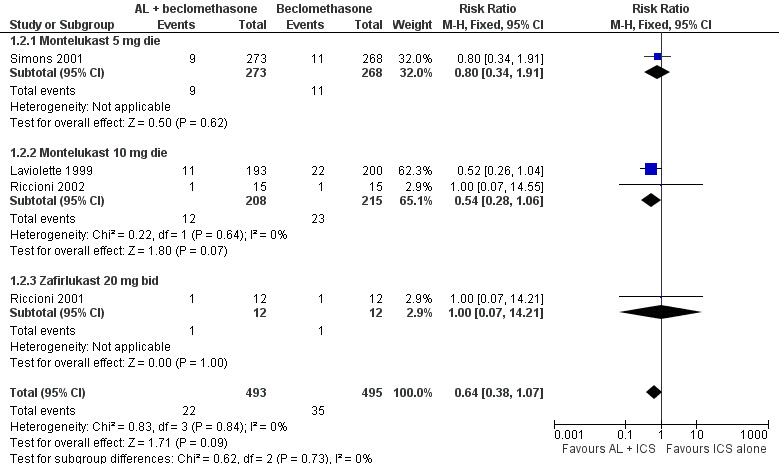
Forest plot of comparison: 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, outcome: 1.2 Patients with exacerbations requiring systemic steroids with licensed AL doses at 4 to 16 weeks.
1.1. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 1 Patients with exacerbations requiring systemic steroids with higher than licensed AL doses at 4 to 16 weeks.
Secondary outcomes
Pooling of the four trials testing the use of licensed doses of montelukast or zafirlukast for 4 (Simons 2001), 8 (Hultquist 2000), and 16 weeks (Laviolette 1999, Vaquerizo 2003) revealed significant, but modest, group differences in favour of anti‐leukotrienes in the change from baseline in morning peak expiratory flow rate (4 trials, WMD 7.65 L/min, 95% CI 3.55 to 11.75), ß2‐agonists use (4 trials, SMD ‐0.15, 95% CI ‐0.24 to ‐0.05) or (3 trials, WMD ‐1, 95% CI: ‐0.5 to ‐2 puffs/week), and eosinophil counts (N=2 trials, WMD ‐0.07 x 10e9/L, 95% CI ‐0.14 to 0.00, random effects model). No significant group difference was observed in the change in FEV1 (3 trials, WMD 0.06 L, 95% CI ‐0.01 to 0.14; random effects model), symptom score (2 trials, WMD = ‐0.10, 95% CI ‐0.24 to 0.03), nocturnal awakenings (2 trials, WMD ‐6.25, 95% CI ‐12.72 to 0.23) or quality of life (2 trials, WMD 0.08, 95% CI ‐0.03 to 0.20). No significant group differences were observed in the risk of overall withdrawals (3 trials, RR 0.97, 95% CI 0.69 to 1.37), withdrawal due to poor asthma control (3 trials, RR 0.46, 95% CI 0.16 to 1.31), withdrawals due to adverse effects (3 trials, RR 0.63, 95% CI 0.29 to 1.37), overall adverse effects (2 trials, RR 1.01, 95% CI 0.88 to 1.15), elevated liver enzymes (2 trials, RR 1.02, 95% CI 0.36 to 2.88), headache (3 trials, RR 1.15, 95% CI 0.89 to 1.49), and nausea (2 trials, RR 0.45, 95% CI 0.19 to 1.07),. There was no death. Insufficient number of trials prevented the pooling of data for nocturnal waking, markers of inflammation, and hospital admissions.
Pooling of the two trials using higher than licensed doses of pranlukast (Tamaoki 1997) or zafirlukast (Virchow 2000) for six weeks of treatment, revealed a significant group difference favouring the addition of anti‐leukotrienes to inhaled corticosteroids in the magnitude of improvement from baseline in FEV1 (WMD= 0.10 L, 95% CI 0.01 to 0.20), PEFR (WMD= 27.2 L/min, 95% CI: 18.6 to 35.8); asthma symptom scores (SMD= ‐0.46, 95% CI ‐0.25 to ‐0.66); use of rescue beta2‐agonists (SMD ‐0.43, 95% CI ‐0.22 to ‐0.63). No significant group difference was observed the change from baseline in night awakenings (WMD ‐0.52 episodes/week, 95% CI ‐1.35 to 0.32).
There was no significant group difference in the risk of overall withdrawals (2 trials, RR 0. 74 95% CI 0.39 to 1.39), withdrawals due to adverse effects (RR 0.73, 95% CI: 0.28 to 1.88), overall adverse effects (RR 1.02, 95% CI: 0.81 to 1.27) and nausea (RR 1.48, 95% CI 0.45 to 4.87). Insufficient data prevented pooling of trials for withdrawals due to poor asthma control, inflammatory markers, headache, elevated liver enzymes and death.
(2) Anti‐leukotrienes + ICS vs. INCREASED dose of ICS
Of the seven trials using this protocol, three trials (Green 2002a; Nsouli 2000; Yildirim 2001) all published in abstract form, reported data in a way that could not be used in this review. The meta‐analysis therefore pertains to 4 trials contributing data to the following outcomes. Three trials (Price 2003; Ringdal 1999; Tomari 2001) tested licensed doses of leukotriene receptor antagonist while two trials (Nayak 1998; Ringdal 1999) also tested higher than licensed doses.
Primary outcome: requirement for oral steroids
When comparing the combination of licensed doses of leukotriene receptor antagonist with 400 ‐ 800 mcg/day of BDP or equivalent to doubling the dose of inhaled steroids, there was no significant group difference in the risk of experiencing an asthma exacerbation requiring systemic steroids (2 trials, RR 0.92, 95% CI 0.56 to 1.51, Figure 2); this finding did not meet our criteria of equivalence.
2.
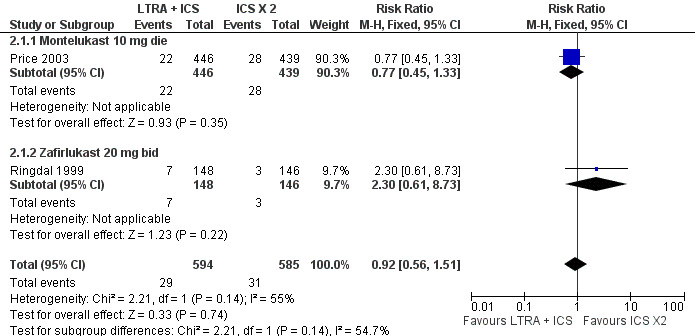
Forest plot of comparison: 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, outcome: 2.1 Patients with 1 or more exacerbations requiring systemic steroids at LICENSED DOSES.
When comparing the combination of two to four‐fold the licensed doses of leukotriene receptor antagonists with 400‐500 mcg/day of BDP or equivalent as opposed to doubling the dose of inhaled steroids, there was no significant group difference in the risk of experiencing an asthma exacerbation requiring systemic steroids (2 trials, RR 1.05 95% CI 0.55 to 2.00, Analysis 2.2); this finding did not met our criteria of equivalence.
2.2. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 2 Patients with 1 or more exacerbations requiring systemic steroids at HIGHER THAN LICENSED DOSES.
Secondary outcomes
There was also no significant group difference in the change from baseline in AM peak expiratory flow rate (2 trials, WMD 1.56, 95% CI ‐5.77 to 8.89; random effect model), in symptoms score (2 trials, WMD 0.01, 95% CI ‐0.09 to 0.10), in use of rescue B2‐agonists (2 trials, WMD ‐0.03 95% CI ‐0.24 to 0.18), withdrawals due to poor asthma control (2 trials, RR 0.49, 95% CI 0.15 to 1.63). Safety measures also show no significant group difference for overall withdrawals (2 trials, RR 0.99, 95% CI 0.63 to 1.55), withdrawals due to side effects (2 trials, RR 1.14, 95% CI 0.55 to 2.37), overall adverse effects (2 trials, RR 0.95, 95% CI 0.84 to 1.06), elevated liver enzymes (2 trials, RR 0.8 95% CI 0.34 to 1.92), headache (2 trials, RR 1.07, 95% CI 0.76 to 1.52), and nausea (2 trials, RR 0.63 95% CI 0.25 to 1.60). There was no death. The width of all these confidence interval exceeded our definition of equivalence. Insufficient data preventing pooling of trials for FEV1, diurnal variation in PEF, night waking, admission and moniliasis. The small number of trials prevented subgroup and sensitivity analyses.
The change from baseline in FEV1 in favour of anti‐leukotrienes reached our definition of equivalence (2 trials, RR 0.01, 95% CI ‐0.05 to 0.07). With regards to other outcomes, no significant group difference were observed in the chance from baseline in AM peak expiratory flow rate (3 trials, WMD 6.05, 95% CI ‐1.26 to 13.36), in change in diurnal variation in peak expiratory flow rate (3 trials, SMD ‐0.11%, 95% CI ‐0.23 to 0.03), in change in symptoms (3 trials, WMD ‐0.06, 95% CI ‐0.16 to 0.03), in use of rescue B2‐agonists (3 trials, WMD 0.00 95% CI ‐0.37 to 0.37), and in withdrawals due to poor asthma control (3 trials, RR 0.72 95% CI 0.29 to 1.76); the width of all these confidence intervals exceeded our definition of equivalence.
With regards to safety, use of higher than licensed doses of leukotriene receptor antagonist was associated with a five‐fold increased risk of liver enzyme elevation (3 trials, RR 4.97 95% CI 1.45 to 17), but has a marked protective effect on oral moniliasis (3 trials, RR 0.29 95% CI 0.10 to 0.81). Other safety measures show no significant group difference, namely for overall withdrawals (3 trials, RR 1.05 95% CI 0.73 to 1.50), withdrawals due to side effects (3 trials, RR 2.27 95% CI 0.95 to 5.45), overall adverse effects (3 trials, RR 0.98 95% CI 0.89 to 1.07), headache (3 trials, RR 1.14 95%CI 1.14 to 1.63), and nausea (3 trials, RR 1.77 95% CI 0.79 to 3.95). There was no death. The small number of trials prevented subgroup and sensitivity analyses.
(3) Anti‐leukotrienes + ICS vs. SAME dose of ICS (TAPERING ICS dose)
The data from six of the seven identified trials, all testing licensed doses of anti‐leukotrienes, were provided in sufficient details to be analysed.(Bateman 1995; Kanniess 2002; Laitinen 1995; Lofdahl 1999; Shingo 2001; Tohda 2002).
Assessment of the glucocorticoid‐sparing effect of anti‐leukotrienes rests on the demonstration that asthma control was adequate after tapering and comparable between the treatment and control groups.
Primary outcome: maintenance dose of inhaled corticosteroids
After 6 to 24 weeks of treatment, there was no significant group difference in the % change from baseline in the inhaled corticosteroid dose required to maintain asthma control (4 trials; WMD ‐3%, 95% CI: ‐7 to 2; Figure 3). When the lowest tolerated dose of inhaled glucocorticoids was considered, no significant group difference was observed either (4 trials, WMD ‐21 mcg/day, 95% CI:‐65 to 23; Analysis 3.2). There was no heterogeneity between the four trials contributing data to the glucocorticoids dose reduction although the trials differed not only in the dose and anti‐leukotriene used, but also in the baseline dose and inhaled glucocorticoid used, dose optimisation period, weaning protocol, and intention‐to‐treat analysis. When the two trials not analysed by intention‐to‐treat were excluded, the lowest tolerated dose of glucocorticoids remained not significantly different between groups (WMD ‐71,95% CI ‐284 to 141). The rate of complete glucocorticoid weaning was similar between groups (3 trials, RR 1.18, 95% CI 0.95 to 1.47; Analysis 3.4).
3.
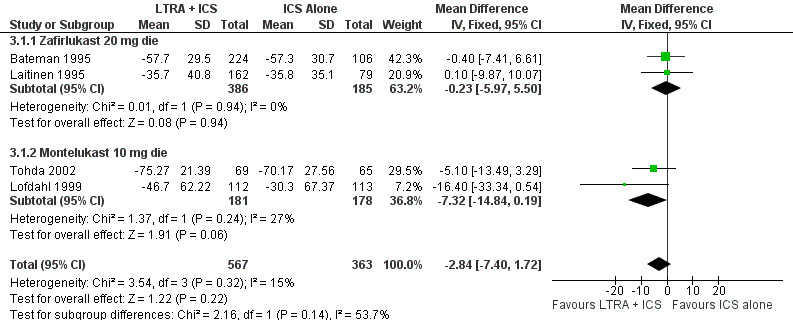
Forest plot of comparison: 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, outcome: 3.1 % Change from baseline ICS dose at 12 ‐24 weeks.
3.2. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 2 Last ICS dose tolerated (mcg) at 12 ‐24 weeks.
3.4. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 4 Complete withdrawal from ICS.
Secondary outcomes
After 12 to 20 weeks of treatment, two trials using zafirlukast (Bateman 1995; Laitinen 1995), reported no significant group difference in final FEV1 (WMD 0.13 L, 95% CI ‐0.02 to 0.27), final PEFR (WMD 17.98.0 L/min, 95% ‐1.68 to 37.64), final symptom scores (WMD ‐0.06, 95% CI ‐0.17 to 0.05), final ß2‐agonists use (WMD ‐0.2 puffs/day, 95% CI ‐0.7 to 0.3). More sensitive outcomes for insuring similar asthma control are the changes from baseline. When the lowest tolerated dose of inhaled glucocorticoids was reached, there was no significant group difference in the change from baseline in FEV1 (2 trials, WMD 0 L, 95% CI ‐0.10 to 0.09), in PEFR (2 trials, WMD 7.9 L/min, 95% CI: ‐1.61 to 17.4) and in ß2‐agonists use (2 trials, WMD ‐0.15 puffs/week; 95% CI ‐0.91, 0.61). Yet, pooling of the five trials revealed a marked reduction (RR 0.63, 95% CI 0.42 to 0.95) in the rate of withdrawals due to poor asthma control in the group treated with leukotriene receptor antagonists, suggesting better asthma control with the combination therapy. When only the trials using intention‐to‐treat analysis were considered, the rate of withdrawals due to poor asthma control was unaffected (RR 0.63, 95% CI 0.42 to 0.95). A trend favouring leukotriene receptor antagonists was also observed in the number of patients with exacerbations requiring systemic steroids (RR 0.47, 95% CI 0.20, 1.09).
Markers of inflammation such as serum eosinophils showed no group difference (WMD 0.18, 95% CI ‐1.13 to 1.50); nitric oxide concentration was only reported for one study, preventing pooling of data.
Less withdrawals due to any cause were observed in the leukotriene receptor antagonists group (6 trials, RR 0.77, 95% CI: 0.60 to 0.98), probably influenced by the marked reduction in withdrawals due to poor asthma control in the anti‐leukotriene group.
With regard to side effects, there was no group difference in the number of withdrawals due to adverse effects (RR 0.88; 95% CI 0.52 to 1.51) overall adverse effects (RR 0.95; 95% CI 0.83 to 1.08, random effect model), elevated liver enzymes (RR 1.67, 95% CI 0.86 to 3.21), headache (RR 0.79, 95% CI 0.58 to 1.08), nausea (RR 1.49, 95% CI 0.70 to 3.19). In contrast, there was a significant increased risk of serious adverse events defined in accordance to the U.S. Food and Drug Administration (FDA) criteria (Anonymous 2001), associated with zafirlukast (RR 2.47, 95% CI 1.53 to 3.97) (Bateman 1995; Laitinen 1995). Only one death, not related to asthma, was reported.
Discussion
In patients who were symptomatic on inhaled corticosteroids (ICS), two treatment alternatives were addressed in these studies: adding an anti‐leukotriene agent or increasing the dose of inhaled corticosteroids.
When compared to an unchanged dose of ICS, the addition of LICENSED doses of anti‐leukotrienes resulted in a non‐significant reduction in the risk of exacerbations requiring systemic glucocorticoids with modest group differences in peak expiratory flow (+8 L/min), ß2‐agonist use (‐0.3 puffs/day), and eosinophils counts (‐0.07 x 10e9) in favour of anti‐leukotrienes. The beneficial effect of anti‐leukotrienes was clearly apparent with HIGHER THAN LICENSED doses of pranlukast or zafirlukast where a 66% reduction in exacerbations requiring rescue glucocorticoids was firmly documented. Twenty two (95% Confidence interval: 17 to 117) patients would need to receive anti‐leukotrienes at unlicensed doses to prevent one patient having an exacerbation that required systemic corticosteroids during the six weeks of treatment. Group differences in the improvement from baseline in FEV1 (+100 mL), PEF (+27 L/min), symptoms (‐0.5 SD) and ß2‐agonist use (‐0.4 SD) in favour of the combination of anti‐leukotrienes and ICS are consistent with the reduction in exacerbations. When licensed and unlicensed doses of anti‐leukotrienes were combined, no statistical heterogeneity was observed despite variation in the dose and anti‐leukotrienes used, dose (750‐2000 mcg/day) of beclomethasone, age, duration of treatment and intention‐to‐treat analysis. The risk of side effects from anti‐leukotrienes was comparable to that of placebo. The available evidence thus suggests a modest effect of licensed doses of montelukast in symptomatic children and adults, but a clear beneficial effect of higher than licensed doses of pranlukast and zafirlukast, as add‐on therapy to inhaled glucocorticoids in adults.
In symptomatic patients, however, most physicians would be uncomfortable with status quo; the majority would increase the dose of inhaled glucocorticoids or consider additional therapy. Only three trials compared the combination of licensed doses of leukotriene receptor antagonists with 400 to 800 mcg/day of BDP (or equivalent) to increasing the dose of inhaled steroids by 400‐800 mcg/day. There was no significant group difference in the risk of experiencing a moderate exacerbations or any other outcomes, but due to the paucity of trials, equivalency could not be established. Both two options appears comparably safe.
When the combination of leukotriene receptor antagonists at higher than licensed doses with 400‐500 mcg/day of BDP (or equivalent) was compared to doubling the dose of inhaled steroids, the improvement in FEV1 was equivalent between the two treatment options, with a modest benefit in the diurnal variation of peak flow rates in favour of anti‐leukotrienes. However, no other group differences in exacerbations requiring systemic steroids, peak flow, symptoms, and use of rescue beta2‐agonists were observed: again, there was insufficient power to conclude to equivalency. The use of higher doses of zafirlukast was associated with a five‐fold increase in the risk of liver enzyme elevation, but was clearly protective for oral moniliasis, raising serious doubt about the safety of this strategy.
Also of interest is whether the addition of anti‐leukotrienes will allow a meaningful reduction in the dose of inhaled glucocorticoids required to maintain control. Data from six trials (Bateman 1995; Kanniess 2002;Laitinen 1995; Lofdahl 1999; Shingo 2001; Tohda 2002) all using licensed doses of anti‐leukotrienes, tested the inhaled corticosteroid sparing properties of anti‐leukotrienes. In adults well controlled on various doses (300 to 3000 mcg/day) of inhaled glucocorticoids, a 6 to 24‐week treatment combining daily oral anti‐leukotrienes with inhaled steroids did not result in a reduction in the dose of inhaled steroids any more than placebo (Bateman 1995; Laitinen 1995; Lofdahl 1999; Tohda 2002). No significant group differences were observed in the lowest tolerated dose of inhaled steroids or in the rate of patients with complete steroid withdrawal. Pooling of trials did not resulted any statistical heterogeneity.
To establish the overall or relative efficacy of anti‐leukotrienes, the level of asthma control achieved after glucocorticoid tapering must be similar among groups. In fact, patients treated with anti‐leukotrienes appeared to have better control than the placebo group, with a significant 37% reduction in withdrawals due to poor asthma control; yet there was no group difference in the change from baseline in FEV1 and PEFR after the minimal tolerated dose was achieved. The apparent discordant findings may be explained by the small number of trials (N=2) reporting change from baseline in FEV1 compared to the 5 trials reporting withdrawals and by the inconsistent use of intention‐to‐treat analyses. These observations may suggest that various factors such as trial‐specific designs may influence the level of asthma control after tapering. Possible explanations include differences in the dose optimisation period prior to randomisation, tapering protocols, baseline dose of inhaled glucocorticoids, anti‐leukotriene used, and intention‐to‐treat analysis. A longer tapering period for example, may have permitted greater reduction in the dose of inhaled corticosteroids or demonstrated better asthma control in favour of anti‐leukotriene agents. Although there are insufficient data to make a firm conclusion, based on the upper confidence limit for each anti‐leukotrienes, the maximal glucocorticoid‐sparing effect of anti‐leukotrienes would probably be less than 300 mcg/day. This is concordant with a previous systematic review demonstrating that the use of anti‐leukotrienes as single agent is less effective than 400 mcg/day of beclomethasone (Ducharme 2001). Although, there are insufficient data to make firm conclusions about the magnitude of the corticosteroid‐sparing effect of anti‐leukotriene agents, it is important to note that during the pre‐randomisation run‐in period of Lofdahl 1999, it was possible to taper the dose of inhaled corticosteroids by 500‐600 mcg, a third of the original dose. A reduction of similar magnitude was observed in the placebo group after randomisation. Similarly Kanniess 2002 reduced by 400 mcg the baseline dose of beclomethasone with no difference in asthma control. This attests to a general overdosing of enrolled patients. If the patients recruited to this study were typical of patients on moderate‐high dose inhaled corticosteroids, the level of dose reduction achievable, without any additional treatment, appears to far outweigh that achieved with anti‐leukotriene agents. The importance of repeated attempts at tapering the dose of inhaled corticosteroids in well‐controlled patients is clear. With only one paediatric trial contributing data and showing little benefit, extrapolation of data from any of the above protocols to children remains speculative. There is insufficient data to determine whether patients' characteristics such as age, severity of airway obstruction on baseline, asthma triggers, and baseline dose of inhaled glucocorticoids required to achieve control have any influence of the magnitude of response. With the lack of heterogeneity between trials, there is no evidence to suggest that the anti‐leukotriene used and duration of intervention affect the findings.
Like all systematic reviews, this meta‐analysis is limited by the quantity and quality of existing data (Khan 1996). Despite the abundance of literature on anti‐leukotrienes, few randomised controlled trials were designed to assess the role of anti‐leukotrienes as add‐on therapy to inhaled glucocorticoids; 67% of trials compared anti‐leukotrienes to placebo in groups of patients comprised of, or including, steroid naive patients. Most included trials (15/16) contributing data to the meta‐analysis were of high methodological quality (Jadad score >= 4). Data from 9 of the 11 trials with lower reported methodological quality (Jadad score <= 3) were not used in this review because they were not reported in a way that permitted aggregation and thus, could not bias the conclusions (Baba 1999; Finn 2000; Green 2002a; Nishimura 1999; Nsouli 2000; O'Sullivan 2003; Tomari 2001; Tomita 1999; Wada 1999; Yildirim 2001). Sensitivity analyses excluding the remaining 2 trials with Jadad score of 0 (Riccioni 2001; Riccioni 2002) failed to alter the findings. A thorough systematic search resulted in the identification of methodologically strong, unpublished trials, increasing the power and scope of the review (Cook 1993; Thornton 2000). The value of this review is strengthened by the direct confirmation of methodology and extracted data from the authors or sponsors of 15 of 27 trials, and the voluntary disclosure of data for five unpublished trials (Hultquist 2000; Nayak 1998; Ringdal 1999; Bateman 1995; Laitinen 1995). Because the number and size of studies pooled under each protocol was small, the robustness of the analyses could not be assessed. Furthermore, the influence on study results of different types of inhaled glucocorticoids and anti‐leukotrienes, doses, age, duration of intervention, severity of airway obstruction on baseline, and asthma triggers remain speculative. No trials have documented the known adverse effects associated with prolonged used of inhaled glucocorticoids such as osteopenia, adrenal suppression, and growth suppression in children, which would permit a fairer comparison between the safety profile of treatment options. Clearly, these preliminary conclusions may be modified with accumulating data from future well‐designed trials.
This review summarises the best evidence available until August 2003 and emphasises the ongoing shortage of relevant trials testing the role of licensed doses of anti‐leukotrienes as add‐on to inhaled glucocorticoids.
Authors' conclusions
Implications for practice.
The small number and short duration of trials pooled under each protocol preclude firm conclusions. However, the data currently available suggest that:
(1) In patients with chronic asthma who are symptomatic on≥400 mcg/day of inhaled beclomethasone, the addition of licensed doses of montelukast to inhaled glucocorticoids may improve lung function, symptoms, and use of relief beta2‐agonists by a modest amount. High dose of anti‐leukotrienes (2 to 4 times the licensed dose of pranlukast or zafirlukast) reduces the rate of exacerbations that require systemic corticosteroids; approximately 22 patients would have to be treated to achieve this effect. Use of higher than licensed doses of anti‐leukotrienes are also associated with significant improvement in lung function, symptoms, and use of relief beta‐2 agonists. (2) In patients treated with 400 to 800 mcg/day of beclomethasone‐equivalent of inhaled corticosteroids, use of licensed doses of leukotriene receptor antagonists are associated with improvement similar to that of dose doubling of inhaled glucocorticoids but there is insufficient power to conclude to equivalency. Use of higher than the licensed dose of zafirlukast also appears to have a similar, but not equivalent, effect to that of doubling the dose of inhaled steroids. However, this is associated with increased risk of liver enzyme elevation. With only three trials, there is still insufficient evidence to firmly recommend the use of licensed doses of anti‐leukotrienes as a substitute to increasing the dose of inhaled glucocorticoids.
(3) In well‐controlled patients, the addition of anti‐leukotrienes as compared to placebo is possibly associated with superior asthma control after glucocorticoids tapering. There is insufficient evidence to firmly quantify the corticosteroids‐sparing effect, which would appear to be less than 300 mcg/day of beclomethasone or equivalent. To date, there is no evidence to suggest that the anti‐leukotriene used (montelukast, zafirlukast, or pranlukast) influenced the response to treatment: the findings appear similar irrespective of the leukotriene receptor antagonists.
Implications for research.
Future studies should focus on children in whom few trials have been published. Two main protocols should be examined:
the addition of anti‐leukotriene agents to inhaled corticosteroids versus dose‐doubling of inhaled corticosteroids in symptomatic children and adults (grouped separately)
the corticosteroid‐sparing effect of anti‐leukotriene agents, after ensuring similar asthma control between the treatment and control groups, in well‐controlled children and adults (grouped separately).
In addition, the following issues should be considered: The best way to assess corticosteroids‐sparing effect of anti‐leukotrienes as 'add‐on' therapy to inhaled corticosteroids is to design trials with a prolonged run‐in period prior to randomisation, during which inhaled corticosteroids are tapered to the minimum effective dose. The run‐in period may need to be as long as 16 weeks as evidenced by the large reduction in maintenance dose of inhaled corticosteroids in the placebo groups both pre‐ and post‐randomisation. Documentation of comparable asthma control after tapering must be demonstrated, preferably with exacerbations requiring systemic steroids and change from baseline in lung function, asthma symptoms, and use of rescue beta‐2 agonists. The corticosteroids‐sparing effect should be reported at the lowest tolerated dose in an intention‐to‐treat analysis.
The trials should :
be double‐blind, randomised, with complete reporting of withdrawals and drop‐outs and intention‐to‐treat analysis
be parallel‐group
involve relatively homogeneous asthmatics in terms of severity of airway obstruction on baseline as evidenced by their lung function on a given dose of inhaled glucocorticoids.
have a minimal intervention period of >= 24‐52 weeks for assessing the corticosteroid‐sparing effect and long‐term side effects of both interventions (anti‐leukotrienes and inhaled corticosteroids)
provide complete reporting of continuous (N, mean change and mean standard deviation of change) and dichotomous (denominators and event rate) data.
report specifically the number of patients with exacerbations requiring systemic corticosteroids and those requiring hospital admissions
report specifically change from baseline (rather than final values at the end of the intervention period) in lung function, symptoms, functional status, use of rescue beta2‐ agonist.
systematically document reasons for withdrawals and adverse effects, including those associated with inhaled corticosteroids such as oral candidiasis, osteopenia, adrenal suppression, growth suppression, etc.
test anti‐leukotriene agents (synthesis inhibitor and receptor antagonists) at LICENSED DOSES
use more uniform doses and type of inhaled corticosteroids (reported in mcg/day of CFC‐beclomethasone‐ equivalent), whenever possible, within the same trial.
What's new
| Date | Event | Description |
|---|---|---|
| 18 July 2011 | Review declared as stable | This review is no longer being updated. It is being replaced by two new protocols/reviews. The review question will be split into adults and children. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 30 September 2009 | Amended | Change in contact details for Francine Ducharme |
| 16 May 2008 | Amended | Converted to new review format. |
| 13 October 2003 | New citation required and conclusions have changed | This review represents the second update since its initial publication in The Cochrane Library 2001, Issue 1, May 2001; the first update being in September 2001. The search strategy updated in August 2003 yielded 211 additional citations; 197 citations were excluded for the following mutually exclusive reasons: (1) Duplicate references (N = 91) (2) Not a randomised controlled trial (N = 44), ongoing trial (N=1), or awaiting assessment (N=3) (3) Subjects were not asthmatics (N =4) (4) The tested intervention was not anti‐leukotrienes (N =9) (5) No consistent co‐treatment with inhaled glucocorticoids (N =20) (6) The control intervention was not inhaled corticosteroids (N = 8) (7) Use of non permitted drugs (N=5) (8) The tested intervention was administered for less than 4 weeks (N =5 ) (8) Outcomes not reflective of asthma control (N = 6) (9) Acute care setting (N=1). Fourteen new trials were included in the updated review; ‐7 new trials (2 abstracts contributing little data, 4 full‐text publications and 1 full disclosure of unpublished trial) comparing anti‐leukotrienes versus placebo as add‐on to inhaled glucocorticoids for a total of 13 trials, of which 8 trials contributed data with sufficient details to be aggregated; ‐5 new trials (3 abstracts contributing little data and 2 full‐text publications) comparing anti‐leukotrienes as add‐on to inhaled glucocorticoids versus double‐dose of inhaled steroids for a total of 7 trials, of which 4 trials could be aggregated; ‐2 new trials (both full‐text publications) comparing anti‐leukotrienes versus placebo as add‐on to tapering doses of inhaled glucocorticoids for a total of 7 trials, with 6 trials contributing data that were aggregated. The updated review now comprised 27 trials comparing anti‐leukotrienes versus placebo as add‐on to inhaled glucocorticoids. The systematic review was re‐structured to clearly distinguish trials that use licensed, from those that used higher than licensed, doses of anti‐leukotrienes. The results of the review have not changed markedly as result of the 14 additional trials. The main new feature is the addition of 2 new trials for a total of 3 trials comparing the addition of leukotriene receptor antagonists at LICENSED dose to inhaled glucocorticoids as compared to double‐dose of inhaled glucocorticoids. The two options appear to provide similar benefit although the power is insufficient to assume equivalency. |
Notes
Several comments were made about an earlier version of this systematic review. They have been removed from this version of the review but remain listed on the comments and criticisms website. To read them and the author's response in full, please refer to: http://www.update.co.uk/feedback/introduction.htm.
Acknowledgements
We wish to thank Zachary Schwartz, Giselle Hicks, and Ritz Kakuma for their participation in the identification of eligible trials, assessment of methodology and data extraction, and diligent data entry. We are indebted to the following individuals who replied to our request for confirmation of methodology and data extraction, and graciously provided additional data whenever possible: Christopher Miller and Susan Shaffer from Astra‐Zeneca, USA; Ian Naya and Roger Metcalf for Astra‐Zeneca, Sweden ; Theodore F Reiss and GP Noonan from Merck Frosst, USA; Frank Kanniess from the Pulmonary Research Institute, Germany; Takaaki Ishine, PhD, Banyu Pharmaceutical Co, LTD, August 2003, D.P. Price UK., August 2003 and Graziano Riccioni, Italy, Sept‐Oct 2003 . We thank the Cochrane Airways Review Group, namely Toby Lasserson and Karen Blackhall, for the literature search and ongoing support, and Paul Jones and Christopher Cates for their constructive comments. We are indebted to Dr Keiji Hayashi for translating Japanese articles. Thanks also to Kirsty Olsen who copy edited this review.
Appendices
Appendix 1. Protocol for update of the review: Addition of anti‐leukotriene agents to inhaled corticosteroids for chronic asthma
Methods
Types of studies
Randomised placebo controlled trials in adults and children in which anti‐leukotriene agents are added to inhaled glucocorticoid were considered for inclusion. We will restrict the eligible studies to those of 4 weeks duration and longer.
Types of participants
Children aged two to 17 years and adults with recurrent or chronic asthma, symptomatic or well‐controlled on daily maintenance inhaled glucocorticoids. We will include studies where participants have to have been taking their inhaled steroids for a minimum of 4 weeks prior to study entry (inclusive of any run‐in period).
Types of interventions
Combination of daily oral anti‐leukotriene agents, including leukotriene synthesis inhibitors and leukotriene receptor antagonists, with inhaled glucocorticoids (treatment group) compared to inhaled glucocorticoids alone (control group). This review will consider three comparisons:
Anti‐leukotrienes versus placebo in addition to a stable dose of ICS with equal doses of ICS between treatment groups: Anti‐leukotrienes can be added to any dose of inhaled steroids which must be fixed. The dose of ICS in both groups must either be the same, or can be maintained from pre‐study entry. Details of the type, dose and delivery of inhaled steroid will be recorded. We will record whether the ICS and dose were maintained from pre‐study treatment, titrated during a run‐in period, or whether a standard ICS and dose were introduced.
Anti‐leukotrienes versus placebo in addition to a stable dose of ICS versus a higher dose of ICS in the control group: Anti‐leukotrienes can be added to any dose of inhaled steroids which must be fixed. The dose of ICS in the control must be higher than that of the ICS in the treatment group. Details of the type, dose and delivery of inhaled steroid will be recorded. We will record whether the ICS and dose were maintained from pre‐study treatment, titrated during a run‐in period, or whether a standard ICS and dose were introduced.
Studies assessing anti‐leukotrienes as an inhaled steroid sparing agent: Anti‐leukotrienes can be added to ICS and then the dose of ICS in both groups will be permitted to vary according to a pre‐specified protocol (such as symptoms, lung function, exacerbation history or a combination).
Types of outcome measures
Primary outcomes
Number of participants with exacerbations requiring use of systemic glucocorticoids for comparisons 1 and 2. For comparison 3, our primary endpoint will be the change from the baseline dose of inhaled glucocorticoids required to maintain control.
Secondary outcomes
The following secondary outcomes were considered after various lengths of treatment:
Change in clinical or physiologic outcomes reflecting chronic asthma control (such as symptom score, night‐time awakenings, days without symptoms, quality of life, rescue short‐acting beta‐2‐agonist use, and pulmonary function tests)
Clinical or physiologic outcomes reflecting the frequency and severity of asthma exacerbations (such as hospital admissions)
Inflammatory markers (such as serum and sputum eosinophils, serum eosinophilic cationic protein (ECP), expired nitric oxide, etc)
Clinical and biochemical adverse effects (i.e. elevation of liver enzymes) as well as withdrawal rates were also examined.
Data collection and analysis
Two authors will independently assess the search results (FD or TL). Studies will be retrieved selected on the basis of relevance, and will be selected for inclusion by the agreement of two authors.
Data extraction and management
Data will be extracted independently by two reviewers (FD and TL), and disagreement will be resolved by discussion.
Assessment of risk of bias in included studies
We will assess the risk of bias for each study according to the Risk of Bias tool outlined in Chapter 8 of the Cochrane Handbook. The domains we will assess for the risk of bias will be:
Allocation generation. The method used to allocate participants to treatment group (e.g. computer‐generated random number sequences)
Allocation concealment. The method used to conceal the sequence of treatment group assignment from study investigators and participants (e.g. assignment by date of birth, opaque sealed envelopes)
Blinding. The method by which knowledge of treatment group assignment was concealed from study investigators and participants after the study began (double‐blind, single‐blind).
Completeness of outcome data. The method for handling data from participants who withdrew from the study (e.g. intention to treat analysis, available case).
Selective reporting. Whether there is evidence that outcomes measured in the study are unreported (e.g. unreported harms, relevant efficacy data that are not available due for reporting and not statistical reasons).
Other sources of bias. Any other aspect of the study design which may be a source of bias.
We will collect and report information for each domain of bias and provide a judgment based on this information as high, low or unclear risk of bias.
We will seek confirmation of methodology for all included trials directly from the authors and/or the funding pharmaceutical companies.
Dealing with missing data
Confirmation of data extraction for all included trials will be sought directly from the authors and/or the funding pharmaceutical companies.
Assessment of heterogeneity
Homogeneity of effect sizes between studies being pooled will be assessed with the I square measurement. This estimates the amount of statistical variation between the studies above what would be expected with the play of chance (Cochrane Handbook).
Data synthesis
Treatment effects for dichotomous outcomes were reported as pooled Risk Ratios with a fixed‐effect model (RR). Equivalence was assumed if the RR estimate and its confidence interval were between 0.9 and 1.1.
For continuous outcomes, such as pulmonary function tests or quality of life scores, we used a Weighted Mean Difference (WMD) or Standardised Mean Difference (SMD), where appropriate, to estimate individual and pooled effect sizes. Numbers Needed to Treat (NNT) were derived from the pooled Odds Ratios using Visual Rx (an online calculator at www.nntonline.net) as the results are not affected by the way in which the events are recorded (Cates 2002).
Trials were divided into three protocols, according to the relative dose of inhaled glucocorticoids used by the control group (same, double, tapered).
(1) Anti‐leukotrienes (AL) + inhaled glucocorticoids (ICS) versus SAME dose of ICS.
(2) Anti‐leukotrienes (AL) + inhaled glucocorticoids (ICS) versus DOUBLE dose of ICS.
(3) Anti‐leukotrienes (AL) + inhaled glucocorticoids (ICS) versus ICS (TAPERING protocol).
In all comparisons, the dose and type of anti‐leukotriene agents served as the primary stratifying variable.
To detect possible biases, funnel plot symmetry was examined for trials contributing data to the main outcomes (Egger 1997). All estimates were reported with their 95% confidence interval.
We will complete a Summary of Findings (SoF) table using GRADEpro software. Our assessments of the overall quality of evidence will be made according to the recommendations of the GRADE working group . We will include outcome data for the primary outcomes of OCS‐treated exacerbations (comparisons 1 and 2), change from baseline in ICS dose (comparison 3), hospital admission and serious adverse events.
Subgroup analysis and investigation of heterogeneity
If the I square measurement exceeds 25% we will consider its possible causes and assess whether this affected the results of the analysis by incorporating the variation in a random effects model. Subgroup analysis is also planned based on the following aspects of the studies:
dose and anti‐leukotrienes used,
baseline dose of inhaled glucocorticoids,
age,
severity of baseline airway obstruction,
duration of intervention,
asthma triggers.
The mean baseline dose of inhaled glucocorticoids in the intervention group will be recorded in mcg/day of chlorofluorocarbon (CFC)‐propelled beclomethasone dipropionate or equivalent x 0.1; where, irrespective of delivery system used, 1 mcg of beclomethasone = 1 mcg of budesonide = 0.5 mcg of fluticasone = 2 mcg of triamcinolone = 2 mcg of flunisolide (USA 2002).
Usual licensed doses of leukotriene receptor antagonists are: montelukast 10 mg daily, pranlukast 450 mg daily, and zafirlukast 20 mg twice daily. For the primary outcome, sensitivity analyses will be performed to determine the effect of publication status and risk of bias on results. We will perform the meta‐analysis using RevMan 5.
Data and analyses
Comparison 1. Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with exacerbations requiring systemic steroids with higher than licensed AL doses at 4 to 16 weeks | 2 | 451 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.13, 0.88] |
| 1.1 Pranlukast 450 mg bid | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.01, 3.77] |
| 1.2 Zafirlukast 80 mg bid | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.14, 1.01] |
| 2 Patients with exacerbations requiring systemic steroids with licensed AL doses at 4 to 16 weeks | 4 | 988 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.38, 1.07] |
| 2.1 Montelukast 5 mg die | 1 | 541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.34, 1.91] |
| 2.2 Montelukast 10 mg die | 2 | 423 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.28, 1.06] |
| 2.3 Zafirlukast 20 mg bid | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.21] |
| 3 Patients with exacerbations requiring hospital admission using licensed AL doses | 2 | 934 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.43] |
| 3.1 Montelukast 5 mg die | 1 | 541 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Montelukast 10 mg die | 1 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.43] |
| 4 Change from baseline FEV1 (L) using higher than licensed LTRA doses at 6 weeks | 2 | 343 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.01, 0.20] |
| 4.1 Pranlukast 450 mg bid | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.41 [‐0.65, 1.47] |
| 4.2 Zafirlukast 80 mg bid | 1 | 264 | Mean Difference (IV, Fixed, 95% CI) | 0.1 [0.01, 0.19] |
| 5 Change from baseline FEV1 using licensed doses of LTRA (at 4 to 16 weeks) | 3 | 1123 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.01, 0.14] |
| 5.1 Montelukast 5 mg die | 1 | 502 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.01, 0.07] |
| 5.2 Montelukast 10 mg die | 1 | 389 | Mean Difference (IV, Random, 95% CI) | 0.12 [0.07, 0.17] |
| 5.3 Zafirlukast 20 mg bid | 1 | 232 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.13, 0.15] |
| 6 Change from baseline Am PEFR (L/min) using higher than licensed AL doses at 6 weeks | 2 | 382 | Mean Difference (IV, Fixed, 95% CI) | 27.19 [18.60, 35.78] |
| 6.1 Pranlukast 450 mg bid | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 51.0 [35.20, 66.80] |
| 6.2 Zafirlukast 80 mg bid | 1 | 303 | Mean Difference (IV, Fixed, 95% CI) | 17.2 [6.96, 27.44] |
| 7 Change from baseline AM PEFR using licensed doses of LTRA at 4‐16 weeks | 4 | 1681 | Mean Difference (IV, Fixed, 95% CI) | 7.65 [3.55, 11.75] |
| 7.1 Montelukast 5 mg die | 1 | 436 | Mean Difference (IV, Fixed, 95% CI) | 9.40 [‐4.61, 23.41] |
| 7.2 Montelukast 10 mg die | 2 | 1013 | Mean Difference (IV, Fixed, 95% CI) | 7.07 [2.63, 11.51] |
| 7.3 Zafirlukast 20 mg bid | 1 | 232 | Mean Difference (IV, Fixed, 95% CI) | 13.14 [‐3.22, 29.50] |
| 8 Change from baseline in mean asthma symptom using higher than licensed AL doses at 6 weeks | 2 | 381 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.66, ‐0.25] |
| 8.1 Pranlukast 450 mg bid | 1 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.93, ‐0.03] |
| 8.2 Zafirlukast 80 mg bid | 1 | 302 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.45 [‐0.68, ‐0.22] |
| 9 Change from baseline mean asthma symptom score using licensed doses using licensed doses of AL at 4 ‐ 16 wks | 2 | 1018 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.24, 0.03] |
| 9.1 Montelukast 5 mg die | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Montelukast 10 mg die | 2 | 1018 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐0.24, 0.03] |
| 10 Change from baseline mean daily use of B2‐agonists (puffs) using higher than licensed AL doses at 6 weeks | 2 | 382 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.63, ‐0.22] |
| 10.1 Pranlukast 450 mg bid | 1 | 79 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.73 [‐1.19, ‐0.27] |
| 10.2 Zafirlukast 80 mg bid | 1 | 303 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.35 [‐0.58, ‐0.12] |
| 11 Change from baseline mean daily use of B2‐agonists (puffs/day or %) using licensed doses of AL at 4 to 16 wee | 4 | 1699 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.24, ‐0.05] |
| 11.1 Montelukast 5 mg die | 1 | 450 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.15 [‐0.33, 0.04] |
| 11.2 Montelukast 10 mg die | 2 | 1018 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.26, ‐0.01] |
| 11.3 Zafirlukast 20 mg bid | 1 | 231 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.47, 0.05] |
| 12 Change in night‐time awakenings(episodes/week) using higher than licensed AL doses at 6 weeks | 2 | 382 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐1.35, 0.32] |
| 12.1 Pranlukast 450 mg bid | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐4.94, 3.14] |
| 12.2 Zafirlukast 80 mg bid | 1 | 303 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐1.35, 0.35] |
| 13 Change from baseline in night‐time awakenings(episodes/week) using licensed doses of LTRA | 1 | 393 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐1.68, 0.50] |
| 13.1 Montelukast 5 mg die | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 Montelukast 10 mg die | 1 | 393 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐1.68, 0.50] |
| 14 Change in quality of life using licensed doses of AL | 2 | 921 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.03, 0.20] |
| 14.1 Montelukast 5 mg die | 1 | 296 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.13, 0.31] |
| 14.2 Montelukast 10 mg die | 1 | 625 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.06, 0.22] |
| 15 Change in mean serum ECP concentration (ug/L) using higher than licensed AL doses at 6 +/‐ 4 weeks | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Pranlukast 450 mg bid | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Change from baseline NO concentration (ppb) using higher than licensed AL doses at 6 +/‐ 4 weeks | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.1 Pranlukast 450 mg bid | 1 | 79 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Change from baseline eosinophil counts using licensed AL doses at 4 to 16 weeks | 2 | 849 | Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.14, ‐0.00] |
| 17.1 Montelukast 5 mg die | 1 | 464 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.17, ‐0.05] |
| 17.2 Montelukast 10 mg die | 1 | 385 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.06, ‐0.02] |
| 18 Withdrawals due to poor asthma control/exacerbations | 5 | 1975 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.29, 1.39] |
| 18.1 Montelukast 5 mg die | 1 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.2 Montelukast 10 mg die | 2 | 1032 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.16, 1.31] |
| 18.3 Zafirlukast 80 mg bid | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.27, 4.11] |
| 18.4 Zafirlukast 20 mg bid | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 14.55] |
| 19 Overall withdrawals | 7 | 2082 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.24] |
| 19.1 Montelukast 5 mg die | 1 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.53, 4.08] |
| 19.2 Montelukast 10 mg die | 2 | 1032 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.63, 1.32] |
| 19.3 Pranlukast 450 mg bid | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.03, 2.86] |
| 19.4 Zafirlukast 80 mg bid | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.42, 1.58] |
| 19.5 Zafirlukast 20 mg bid | 2 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.27, 8.24] |
| 20 Withdrawals due to adverse effects | 6 | 2030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.37, 1.22] |
| 20.1 Montelukast 5 mg die | 1 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.04, 5.38] |
| 20.2 Montelukast 10 mg die | 3 | 1034 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.28, 1.48] |
| 20.3 Pranlukast 450 mg bid | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.4 Zafirlukast 80 mg bid | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.28, 1.88] |
| 21 Headache | 4 | 1933 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.92, 1.50] |
| 21.1 Montelukast 5 mg die | 1 | 547 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.58, 1.78] |
| 21.2 Montelukast 10 mg die | 2 | 1018 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.90, 1.59] |
| 21.3 Zafirlukast 80 mg bid | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.59, 3.49] |
| 22 Overall adverse effects | 4 | 1623 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.90, 1.13] |
| 22.1 Montelukast 5 mg die | 1 | 547 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.76, 1.12] |
| 22.2 Montelukast 10 mg die | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.91, 1.31] |
| 22.3 Pranlukast 450 mg bid | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.80 [0.12, 66.70] |
| 22.4 Zafirlukast 80 mg bid | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.81, 1.26] |
| 23 Elevated liver enzymes | 3 | 1301 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.50, 3.35] |
| 23.1 Montelukast 5 mg die | 1 | 547 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.18, 21.37] |
| 23.2 Montelukast 10 mg die | 1 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.27, 2.77] |
| 23.3 Zafirlukast 80 mg bid | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.22 [0.25, 108.01] |
| 24 Nausea | 4 | 1391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.34, 1.32] |
| 24.1 Montelukast 5 mg die | 1 | 547 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 1.99] |
| 24.2 Montelukast 10 mg die | 1 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.17, 1.33] |
| 24.3 Pranlukast 450 bid | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.80 [0.12, 66.70] |
| 24.4 Zafirlukast 80 mg bid | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.36, 4.78] |
| 25 Death | 3 | 1308 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.49] |
| 25.1 Montelukast 5 mg die | 1 | 547 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Montelukast 10 mg die | 1 | 393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Zafirlukast 80 mg bid | 1 | 368 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.49] |
| 26 % nocturnal awakenings | 2 | 856 | Mean Difference (IV, Fixed, 95% CI) | ‐6.25 [‐12.72, 0.23] |
| 26.1 Montelukast 10 mg die | 1 | 625 | Mean Difference (IV, Fixed, 95% CI) | ‐6.60 [‐15.51, 2.31] |
| 26.2 Zafirlukast 20 mg bid | 1 | 231 | Mean Difference (IV, Fixed, 95% CI) | ‐5.85 [‐15.26, 3.56] |
1.2. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 2 Patients with exacerbations requiring systemic steroids with licensed AL doses at 4 to 16 weeks.
1.3. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 3 Patients with exacerbations requiring hospital admission using licensed AL doses.
1.4. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 4 Change from baseline FEV1 (L) using higher than licensed LTRA doses at 6 weeks.
1.5. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 5 Change from baseline FEV1 using licensed doses of LTRA (at 4 to 16 weeks).
1.6. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 6 Change from baseline Am PEFR (L/min) using higher than licensed AL doses at 6 weeks.
1.7. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 7 Change from baseline AM PEFR using licensed doses of LTRA at 4‐16 weeks.
1.8. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 8 Change from baseline in mean asthma symptom using higher than licensed AL doses at 6 weeks.
1.9. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 9 Change from baseline mean asthma symptom score using licensed doses using licensed doses of AL at 4 ‐ 16 wks.
1.10. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 10 Change from baseline mean daily use of B2‐agonists (puffs) using higher than licensed AL doses at 6 weeks.
1.11. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 11 Change from baseline mean daily use of B2‐agonists (puffs/day or %) using licensed doses of AL at 4 to 16 wee.
1.12. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 12 Change in night‐time awakenings(episodes/week) using higher than licensed AL doses at 6 weeks.
1.13. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 13 Change from baseline in night‐time awakenings(episodes/week) using licensed doses of LTRA.
1.14. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 14 Change in quality of life using licensed doses of AL.
1.15. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 15 Change in mean serum ECP concentration (ug/L) using higher than licensed AL doses at 6 +/‐ 4 weeks.
1.16. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 16 Change from baseline NO concentration (ppb) using higher than licensed AL doses at 6 +/‐ 4 weeks.
1.17. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 17 Change from baseline eosinophil counts using licensed AL doses at 4 to 16 weeks.
1.18. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 18 Withdrawals due to poor asthma control/exacerbations.
1.19. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 19 Overall withdrawals.
1.20. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 20 Withdrawals due to adverse effects.
1.21. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 21 Headache.
1.22. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 22 Overall adverse effects.
1.23. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 23 Elevated liver enzymes.
1.24. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 24 Nausea.
1.25. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 25 Death.
1.26. Analysis.
Comparison 1 Leukotriene Receptor Antagonists (LTRA) + ICS vs. same dose of ICS in SYMPTOMATIC patients, Outcome 26 % nocturnal awakenings.
Comparison 2. Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients with 1 or more exacerbations requiring systemic steroids at LICENSED DOSES | 2 | 1179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.56, 1.51] |
| 1.1 Montelukast 10 mg die | 1 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.45, 1.33] |
| 1.2 Zafirlukast 20 mg bid | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.61, 8.73] |
| 2 Patients with 1 or more exacerbations requiring systemic steroids at HIGHER THAN LICENSED DOSES | 2 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.55, 2.00] |
| 2.1 Zafirlukast 40 mg bid | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.36, 2.77] |
| 2.2 Zafirlukast 80 mg bid | 2 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.47, 2.50] |
| 3 Patients with 1 or more exacerbations requiring hospital admission at LICENSED DOSES | 2 | 1179 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 Montelukast 10 mg die | 1 | 885 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Zafirlukast 20 mg bid | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Change from baseline FEV1(L) at LICENSED DOSES at 12 weeks | 2 | 225 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.12, 0.06] |
| 4.1 Montelukast 10 mg die | 1 | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Zafirlukast 20 mg bid | 1 | 223 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.12, 0.06] |
| 5 Change from baseline FEV1 (L) at HIGHER THAN LICENSED doses at 12 +/‐ 4 weeks | 2 | 638 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.05, 0.07] |
| 5.1 Zafirlukast 40 mg bid | 1 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.12, 0.10] |
| 5.2 Zafirlukast 80 mg bid | 2 | 432 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.05, 0.09] |
| 6 Change from baseline Am PEFR (L/min) at LICENSED DOSES at 12 weeks weeks | 2 | 1130 | Mean Difference (IV, Random, 95% CI) | 1.56 [‐5.77, 8.89] |
| 6.1 Montelukast 10 mg die | 1 | 889 | Mean Difference (IV, Random, 95% CI) | 3.40 [‐5.19, 11.99] |
| 6.2 Zafirlukast 20 mg bid | 1 | 241 | Mean Difference (IV, Random, 95% CI) | ‐3.40 [‐17.49, 10.69] |
| 7 Change from baseline Am PEFR (L/min) at HIGHER THAN LICENSED DOSES at 12 +/‐ 4 weeks | 2 | 663 | Mean Difference (IV, Fixed, 95% CI) | 6.05 [‐1.26, 13.36] |
| 7.1 Zafirlukast 40 mg bid | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | 7.60 [‐4.70, 19.90] |
| 7.2 Zafirlukast 80 mg bid | 2 | 455 | Mean Difference (IV, Fixed, 95% CI) | 5.21 [‐3.89, 14.30] |
| 8 % Change from baseline mean diurnal variation in PEF at LICENSED DOSES at 12 +/‐ 4 weeks | 1 | 241 | Mean Difference (IV, Random, 95% CI) | ‐6.40 [‐15.63, 2.83] |
| 8.1 Zafirlukast 20 mg bid | 1 | 241 | Mean Difference (IV, Random, 95% CI) | ‐6.40 [‐15.63, 2.83] |
| 9 Change (% or L/min) from baseline mean diurnal variation in PEF at HIGHER THAN LICENSED DOSES at 12 +/‐ 4 wee | 2 | 746 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.25, 0.03] |
| 9.1 Zafirlukast 40 mg bid | 1 | 251 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.37, 0.13] |
| 9.2 Zafirlukast 80 mg bid | 2 | 495 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.28, 0.07] |
| 10 Change from baseline mean symptom scores at LICENSED DOSES at 12 +/‐ 4 weeks | 2 | 1101 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.09, 0.10] |
| 10.1 Montelukast 10 mg die | 1 | 860 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.10, 0.12] |
| 10.2 Zafirlukast 20 mg bid | 1 | 241 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.18, 0.18] |
| 11 Change from baseline mean symptom scores at HIGHER THAN LICENSED DOSES at 12 +/‐ 4 weeks | 2 | 664 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.16, 0.03] |
| 11.1 Zafirlukast 40 mg bid | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.17, 0.15] |
| 11.2 Zafirlukast 80 mg bid | 2 | 456 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.21, 0.03] |
| 12 Change from baseline mean daily use of B2‐agonists at LICENSED DOSES at 12 +/‐ 4 weeks | 2 | 1015 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.24, 0.18] |
| 12.1 Montelukast 10 mg die | 1 | 774 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.25, 0.19] |
| 12.2 Zafirlukast 20 mg bid | 1 | 241 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.74, 0.74] |
| 13 Change from baseline mean daily use of B2‐agonists at HIGHER THAN LICENSED DOSES at 12 +/‐ 4 weeks | 2 | 663 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.37, 0.37] |
| 13.1 Zafirlukast 40 mg bid | 1 | 208 | Mean Difference (IV, Fixed, 95% CI) | 0.29 [‐0.34, 0.92] |
| 13.2 Zafirlukast 80 mg bid | 2 | 455 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.62, 0.31] |
| 14 Change in night‐time awakenings per week at LICENSED DOSES at 12 +‐4 weeks | 1 | 241 | Mean Difference (IV, Fixed, 95% CI) | 0.4 [‐0.60, 1.40] |
| 14.1 Zafirlukast 20 mg bid | 1 | 241 | Mean Difference (IV, Fixed, 95% CI) | 0.4 [‐0.60, 1.40] |
| 15 Change in night‐time awakenings per week at HIGHER THAN LICENSED DOSES at 12 +‐4 weeks | 1 | 236 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.73, 1.13] |
| 15.1 Zafirlukast 80 mg bid | 1 | 236 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.73, 1.13] |
| 16 Overall withdrawals at LICENSED DOSES | 2 | 1183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.63, 1.55] |
| 16.1 Montelukast 10 mg die | 1 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.43, 1.34] |
| 16.2 Zafirlukast 20 mg bid | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.74, 3.64] |
| 17 Withdrawals due to adverse effects at LICENSED DOSES | 2 | 1183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.55, 2.37] |
| 17.1 Montelukast 10 mg die | 1 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.31, 1.98] |
| 17.2 Zafirlukast 20mg bid | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.61, 8.73] |
| 18 Withdrawals due to poor asthma control/exacerbations at LICENSED DOSES | 2 | 1183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.15, 1.63] |
| 18.1 Montelukast 10 mg die | 1 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.06, 1.35] |
| 18.2 Zafirlukast 20 mg bid | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.18, 21.52] |
| 19 Overall adverse effects at LICENSED DOSES | 2 | 1183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.06] |
| 19.1 Montelukast 10 mg die | 1 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.76, 1.06] |
| 19.2 Zafirlukast 20 mg bid | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.19] |
| 20 Elevated liver enzymes at LICENSED DOSES | 2 | 1147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.34, 1.92] |
| 20.1 Montelukast 10 mg die | 1 | 853 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.18, 1.61] |
| 20.2 Zafirlukast 20 mg bid | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.37, 10.61] |
| 21 Headache at LICENSED DOSES | 2 | 1183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.76, 1.52] |
| 21.1 Montelukast 10 mg die | 1 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.72, 1.86] |
| 21.2 Zafirlukast 20 mg bid | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.60, 1.62] |
| 22 Nausea at LICENSED DOSES | 2 | 1183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.25, 1.60] |
| 22.1 Montelukast 10 mg die | 1 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.19, 2.31] |
| 22.2 Zafirlukast 20 mg bid | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.14, 2.43] |
| 23 Oral Moniliasis at LICENSED DOSES | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 2.00] |
| 23.1 Zafirlukast 20 mg bid | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.08, 2.00] |
| 24 Death at LICENSED DOSES | 2 | 1183 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.1 Montelukast 10 mg die | 1 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Zafirlukast 20 mg bid | 1 | 294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Patients with 1 or more exacerbations requiring hospital admission at HIGHER THAN LICENSED DOSES | 2 | 699 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.04] |
| 25.1 Zafirlukast 40 mg bid | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.2 Zafirlukast 80 mg bid | 2 | 439 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 73.04] |
| 26 Nightime awakenings/week at USUAL LICENSED DOSES at 12 weeks | 1 | 820 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.69, 4.69] |
| 26.1 Montelukast 10 mg die | 1 | 820 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.69, 4.69] |
| 27 Overall withdrawals at HIGHER THAN LICENSED DOSES | 2 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.73, 1.50] |
| 27.1 Zafirlukast 40 mg bid | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.57, 1.74] |
| 27.2 Zafirlukast 80 mg bid | 2 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.68, 1.72] |
| 28 Withdrawals due to adverse effects at HIGHER THAN LICENSED DOSES | 2 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.27 [0.95, 5.45] |
| 28.1 Zafirlukast 40 mg bid | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.99] |
| 28.2 Zafirlukast 80 mg bid | 2 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.77 [1.02, 7.58] |
| 29 Withdrawals due to poor asthma control/exacerbations at HIGHER THAN LICENSED DOSES | 2 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.29, 1.76] |
| 29.1 Zafirlukast 40 mg bid | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.46] |
| 29.2 Zafirlukast 80 mg bid | 2 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.25, 2.62] |
| 30 Overall adverse effects at HIGHER THAN LICENSED DOSES | 2 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.07] |
| 30.1 Zafirlukast 40 mg bid | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.77, 1.08] |
| 30.2 Zafirlukast 80 mg bid | 2 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.90, 1.12] |
| 31 Elevated liver enzymes at HIGHER THAN LICENSED DOSES | 2 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.97 [1.45, 17.00] |
| 31.1 Zafirlukast 40 mg bid | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.97] |
| 31.2 Zafirlukast 80 mg bid | 2 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.36 [1.40, 20.44] |
| 32 Headache at HIGHER THAN LICENSED DOSES | 2 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.79, 1.63] |
| 32.1 Zafirlukast 40 mg bid | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.65, 2.86] |
| 32.2 Zafirlukast 80 mg bid | 2 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.71, 1.62] |
| 33 Nausea at HIGHER THAN LICENSED DOSES | 2 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.79, 3.95] |
| 33.1 Zafirlukast 40 mg bid | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.62, 14.59] |
| 33.2 Zafirlukast 80 mg bid | 2 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.55, 3.66] |
| 34 Oral moniliasis at HIGHER THAN LICENSED DOSES | 2 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.10, 0.81] |
| 34.1 Zafirlukast 40 mg bid | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.15, 2.46] |
| 34.2 Zafirlukast 80 mg bid | 2 | 556 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.77] |
| 35 Death at HIGHER THAN LICENSED DOSES | 2 | 820 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 35.1 Zafirlukast 40 mg bid | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 35.2 Zafirlukast 80 mg bid | 2 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 1 Patients with 1 or more exacerbations requiring systemic steroids at LICENSED DOSES.
2.3. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 3 Patients with 1 or more exacerbations requiring hospital admission at LICENSED DOSES.
2.4. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 4 Change from baseline FEV1(L) at LICENSED DOSES at 12 weeks.
2.5. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 5 Change from baseline FEV1 (L) at HIGHER THAN LICENSED doses at 12 +/‐ 4 weeks.
2.6. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 6 Change from baseline Am PEFR (L/min) at LICENSED DOSES at 12 weeks weeks.
2.7. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 7 Change from baseline Am PEFR (L/min) at HIGHER THAN LICENSED DOSES at 12 +/‐ 4 weeks.
2.8. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 8 % Change from baseline mean diurnal variation in PEF at LICENSED DOSES at 12 +/‐ 4 weeks.
2.9. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 9 Change (% or L/min) from baseline mean diurnal variation in PEF at HIGHER THAN LICENSED DOSES at 12 +/‐ 4 wee.
2.10. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 10 Change from baseline mean symptom scores at LICENSED DOSES at 12 +/‐ 4 weeks.
2.11. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 11 Change from baseline mean symptom scores at HIGHER THAN LICENSED DOSES at 12 +/‐ 4 weeks.
2.12. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 12 Change from baseline mean daily use of B2‐agonists at LICENSED DOSES at 12 +/‐ 4 weeks.
2.13. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 13 Change from baseline mean daily use of B2‐agonists at HIGHER THAN LICENSED DOSES at 12 +/‐ 4 weeks.
2.14. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 14 Change in night‐time awakenings per week at LICENSED DOSES at 12 +‐4 weeks.
2.15. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 15 Change in night‐time awakenings per week at HIGHER THAN LICENSED DOSES at 12 +‐4 weeks.
2.16. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 16 Overall withdrawals at LICENSED DOSES.
2.17. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 17 Withdrawals due to adverse effects at LICENSED DOSES.
2.18. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 18 Withdrawals due to poor asthma control/exacerbations at LICENSED DOSES.
2.19. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 19 Overall adverse effects at LICENSED DOSES.
2.20. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 20 Elevated liver enzymes at LICENSED DOSES.
2.21. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 21 Headache at LICENSED DOSES.
2.22. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 22 Nausea at LICENSED DOSES.
2.23. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 23 Oral Moniliasis at LICENSED DOSES.
2.24. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 24 Death at LICENSED DOSES.
2.25. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 25 Patients with 1 or more exacerbations requiring hospital admission at HIGHER THAN LICENSED DOSES.
2.26. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 26 Nightime awakenings/week at USUAL LICENSED DOSES at 12 weeks.
2.27. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 27 Overall withdrawals at HIGHER THAN LICENSED DOSES.
2.28. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 28 Withdrawals due to adverse effects at HIGHER THAN LICENSED DOSES.
2.29. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 29 Withdrawals due to poor asthma control/exacerbations at HIGHER THAN LICENSED DOSES.
2.30. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 30 Overall adverse effects at HIGHER THAN LICENSED DOSES.
2.31. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 31 Elevated liver enzymes at HIGHER THAN LICENSED DOSES.
2.32. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 32 Headache at HIGHER THAN LICENSED DOSES.
2.33. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 33 Nausea at HIGHER THAN LICENSED DOSES.
2.34. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 34 Oral moniliasis at HIGHER THAN LICENSED DOSES.
2.35. Analysis.
Comparison 2 Leukotriene Receptor Antagonists (LTRA) + ICS vs. DOUBLE dose of ICS in SYMPTOMATIC PATIENTS, Outcome 35 Death at HIGHER THAN LICENSED DOSES.
Comparison 3. Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 % Change from baseline ICS dose at 12 ‐24 weeks | 4 | 930 | Mean Difference (IV, Fixed, 95% CI) | ‐2.84 [‐7.40, 1.72] |
| 1.1 Zafirlukast 20 mg die | 2 | 571 | Mean Difference (IV, Fixed, 95% CI) | ‐0.23 [‐5.97, 5.50] |
| 1.2 Montelukast 10 mg die | 2 | 359 | Mean Difference (IV, Fixed, 95% CI) | ‐7.32 [‐14.84, 0.19] |
| 2 Last ICS dose tolerated (mcg) at 12 ‐24 weeks | 4 | 932 | Mean Difference (IV, Random, 95% CI) | ‐40.65 [‐111.22, 29.93] |
| 2.1 Zafirlukast 20 mg die | 2 | 573 | Mean Difference (IV, Random, 95% CI) | 5.60 [‐47.22, 58.43] |
| 2.2 Montelukast 10 mg die | 2 | 359 | Mean Difference (IV, Random, 95% CI) | ‐103.32 [‐235.02, 28.37] |
| 3 Change from baseline in ICS dose (mcg) | 1 | 134 | Mean Difference (IV, Fixed, 95% CI) | 66.95 [‐25.41, 159.31] |
| 3.1 Montelukast 10 mg die | 1 | 134 | Mean Difference (IV, Fixed, 95% CI) | 66.95 [‐25.41, 159.31] |
| 4 Complete withdrawal from ICS | 3 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.95, 1.47] |
| 4.1 Zafirlukast 20 mg bid | 1 | 359 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.81, 1.40] |
| 4.2 Montelukast 10 mg | 2 | 248 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.97, 1.96] |
| 5 Final FEV1 (L) at lowest tolerated dose (at 12 ‐20 weeks) | 2 | 621 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.02, 0.27] |
| 5.1 Zafirlukast 20 mg bid | 2 | 621 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.02, 0.27] |
| 6 Change from baseline in FEV1 (L) at lowest tolerated dose (at 6‐24 weeks) | 2 | 164 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.10, 0.09] |
| 6.1 Montelukast 10 mg die | 2 | 164 | Mean Difference (IV, Fixed, 95% CI) | ‐0.00 [‐0.10, 0.09] |
| 7 Final Am PEFR (L/min) at 12 ‐20 weeks | 2 | 500 | Mean Difference (IV, Fixed, 95% CI) | 17.98 [‐1.68, 37.64] |
| 7.1 Zafirlukast 20 mg bid | 2 | 500 | Mean Difference (IV, Fixed, 95% CI) | 17.98 [‐1.68, 37.64] |
| 8 Change from baseline in morning PEFR (L/min) at lowest tolerated dose | 2 | 183 | Mean Difference (IV, Fixed, 95% CI) | 7.90 [‐1.61, 17.40] |
| 8.1 Montelukast 10 mg die | 2 | 183 | Mean Difference (IV, Fixed, 95% CI) | 7.90 [‐1.61, 17.40] |
| 9 Final mean symptom scores (episodes/week) at 12 ‐20 weeks | 2 | 487 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.17, 0.05] |
| 9.1 Zafirlukast 20 mg bid | 2 | 487 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.17, 0.05] |
| 10 Change in symptom scores at 6‐24 weeks | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐0.79, ‐0.21] |
| 10.1 Montelukast 10 mg bid | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐0.79, ‐0.21] |
| 11 Final mean daily use of B2‐agonists (puffs/day) at lowest tolerated dose ( at 12‐20 weeks) | 2 | 496 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.73, 0.32] |
| 11.1 Zafirlukast 20 mg bid | 2 | 496 | Mean Difference (IV, Fixed, 95% CI) | ‐0.21 [‐0.73, 0.32] |
| 12 Absolute change in mean daily use of B2‐agonists (puffs/week) at lowest tolerated dose (at 6‐24 weeks) | 3 | 409 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.76, 0.52] |
| 12.1 Montelukast 10 mg die | 3 | 409 | Mean Difference (IV, Fixed, 95% CI) | ‐0.12 [‐0.76, 0.52] |
| 13 Change in serum eosinophils at lowest tolerated dose (at 6‐ 24 weeks) | 2 | 167 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐1.13, 1.50] |
| 13.1 Montelukast 10 mg die | 2 | 167 | Mean Difference (IV, Fixed, 95% CI) | 0.18 [‐1.13, 1.50] |
| 14 Patients with 1 or more exacerbations requiring systemic steroids | 4 | 502 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.20, 1.09] |
| 14.1 Zafirlukast 20 mg bid | 1 | 262 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.10, 2.41] |
| 14.2 Montelukast 10 mg die | 3 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.17, 1.25] |
| 15 Patients with 1 or more exacerbations requiring hospital admission | 2 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 Montelukast 10 mg die | 2 | 231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Overall withdrawals | 6 | 1110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.60, 0.98] |
| 16.1 Zafirlukast 20 mg bid | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.66, 1.34] |
| 16.2 Montelukast 10 mg die | 4 | 489 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.45, 0.89] |
| 17 Withdrawals due to adverse effects | 6 | 1110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.52, 1.51] |
| 17.1 Zafirlukast 20mg bid | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.73, 3.82] |
| 17.2 Montelukast 10 mg die | 4 | 489 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.23, 1.05] |
| 18 Withdrawals due to poor asthma control/exacerbations | 6 | 1110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.42, 0.95] |
| 18.1 Zafirlukast 20 mg bid | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.21, 2.59] |
| 18.2 Montelukast 10 mg die | 4 | 489 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.40, 0.95] |
| 19 Overall adverse effects | 6 | 1100 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.83, 1.08] |
| 19.1 Zafirlukast 20 mg bid | 2 | 621 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.89, 1.09] |
| 19.2 Montelukast 10 mg die | 4 | 479 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.54, 1.25] |
| 20 Serious adverse events | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.53, 3.97] |
| 20.1 Zafirlukast 20 mg bid | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.47 [1.53, 3.97] |
| 21 Elevated liver enzymes | 6 | 1099 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.86, 3.21] |
| 21.1 Zafirlukast 20 mg bid | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.75, 10.68] |
| 21.2 Montelukast 10 mg die | 4 | 478 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.61, 2.79] |
| 22 Headache | 6 | 1100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.58, 1.08] |
| 22.1 Zafirlukast 20 mg bid | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.56, 1.28] |
| 22.2 Montelukast 10 mg die | 4 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.47, 1.16] |
| 23 Nausea | 6 | 1100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.70, 3.19] |
| 23.1 Zafirlukast 20 mg bid | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.52, 3.27] |
| 23.2 Montelukast 10 mg die | 4 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.50, 7.86] |
| 24 Death | 6 | 1100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.1 [0.13, 75.10] |
| 24.1 Zafirlukast 20 mg bid | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.2 Montelukast 10 mg die | 4 | 479 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.1 [0.13, 75.10] |
| 25 Patients unable to taper inhaled corticosteroids | 1 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.51, 1.11] |
| 25.1 Montelukast 10 mg | 1 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.51, 1.11] |
| 26 Change in expired NO concentration (ppb) at 6 weeks | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐3.45, 3.51] |
| 26.1 Montelukast 10 mg die | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐3.45, 3.51] |
3.1. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 1 % Change from baseline ICS dose at 12 ‐24 weeks.
3.3. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 3 Change from baseline in ICS dose (mcg).
3.5. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 5 Final FEV1 (L) at lowest tolerated dose (at 12 ‐20 weeks).
3.6. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 6 Change from baseline in FEV1 (L) at lowest tolerated dose (at 6‐24 weeks).
3.7. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 7 Final Am PEFR (L/min) at 12 ‐20 weeks.
3.8. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 8 Change from baseline in morning PEFR (L/min) at lowest tolerated dose.
3.9. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 9 Final mean symptom scores (episodes/week) at 12 ‐20 weeks.
3.10. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 10 Change in symptom scores at 6‐24 weeks.
3.11. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 11 Final mean daily use of B2‐agonists (puffs/day) at lowest tolerated dose ( at 12‐20 weeks).
3.12. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 12 Absolute change in mean daily use of B2‐agonists (puffs/week) at lowest tolerated dose (at 6‐24 weeks).
3.13. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 13 Change in serum eosinophils at lowest tolerated dose (at 6‐ 24 weeks).
3.14. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 14 Patients with 1 or more exacerbations requiring systemic steroids.
3.15. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 15 Patients with 1 or more exacerbations requiring hospital admission.
3.16. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 16 Overall withdrawals.
3.17. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 17 Withdrawals due to adverse effects.
3.18. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 18 Withdrawals due to poor asthma control/exacerbations.
3.19. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 19 Overall adverse effects.
3.20. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 20 Serious adverse events.
3.21. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 21 Elevated liver enzymes.
3.22. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 22 Headache.
3.23. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 23 Nausea.
3.24. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 24 Death.
3.25. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 25 Patients unable to taper inhaled corticosteroids.
3.26. Analysis.
Comparison 3 Leukotriene Receptor Antagonists (LTRA) + ICS vs. ICS (TAPERING protocol) in WELL CONTROLLED patients, Outcome 26 Change in expired NO concentration (ppb) at 6 weeks.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baba 1999.
| Methods | DESIGN: ‐parallel‐group
‐3 arm study:
a. pranlukast
b. seratrodast
c. placebo ALLOCATION ‐random (means of allocation un‐specified) BLINDING ‐not specified ‐placebo‐controlled WITHDRAWAL/DROPOUTS ‐not described JADAD's Quality Score = 1 Confirmation of methodology not obtained |
|
| Participants | WELL‐CONTROLLED PARTICIPANTS ‐RANDOMISED = 24 Pranlukast = 8/ Control = 9/ Seratrodast = 7 ‐WITHDRAWALS: not described ‐AGE: not described ‐GENDER: not described ‐SEVERITY: not described ‐Baseline FEV1(L) not described ‐ALLERGEN TRIGGERS: not described ‐ASTHMA DURATION: not described ELIGIBILITY CRITERIA ‐AGE: not described ‐well‐controlled on non‐described dose of beclomethasone |
|
| Interventions | PROTOCOL:
AL + ICS vs same dose
ICS (TAPERING ICS dose) DURATION ‐Dose optimisation period: not described ‐Intervention Period: not described TEST GROUP ‐Pranlukast (dose not specified) + beclomethasone (dose not specified) CONTROL GROUP ‐Placebo + beclomethasone (dose not specified) DEVICE ‐not specified ‐CO‐TREATMENT: not specified CRITERIA FOR TAPERING every 4 weeks by 50% the beclomethasone dose MINIMAL DOSE OF ICS ALLOWED: 1/3 to 1/4 of baseline dose |
|
| Outcomes | INTENTION‐TO‐TREAT ANALYSES
‐not specified PULMONARY FUNCTION TESTS ‐PEF rates ‐SYMPTOM SCORES not reported FUNCTIONAL STATUS ‐not reported ‐exacerbations ICS DOSE REDUCTION: ‐not reported ‐successful tapering INFLAMMATORY MARKERS: not reported ADVERSE EFFECTS ‐not reported WITHDRAWALS ‐not reported |
|
| Notes | ‐Abs (1999) ‐funding source (not specified) ‐No contact information provided in abstract. Unable to request confirmation of methodology and data extraction until publication in full‐text of the report ‐User‐defined order: unable to be determined (re: un‐specified mean daily ICS dose ) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Bateman 1995.
| Methods | DESIGN: ‐parallel‐group
‐multicentre trial ALLOCATION ‐Random: 2:1 ratio intervention:control ‐Computer‐generated BLINDING ‐double‐blind ‐placebo‐controlled ‐identical placebo WITHDRAWAL/DROPOUTS ‐described JADAD's Quality Score = 5 Confirmation of methodology obtained |
|
| Participants | WELL‐CONTROLLED PARTICIPANTS ‐RANDOMISED = 359 Zafurkast = 242/ Control = 117 ‐WITHDRAWALS: Zafirlukast: 20% Control: 22% ‐AGE: Zafirlukast:42.2 ± 14.8 (SD) years Control: 41.6 ± 14.2 years ‐GENDER: Zafirlukast: 45% male Control: 44% male ‐SEVERITY: mild asthma ‐Baseline FEV1(L) Zafirlukast: 2.64 ± 0.86 (SD) L Control: 2.63 ± 0.85 (SD) ALLERGEN TRIGGERS: Zafirlukast: 48% Control:49% ASTHMA DURATION: Zafirlukast: 13.1 ± 12.3 (SD) years Control: 14.1±13.0 years ELIGIBILITY CRITERIA ‐AGE: 12 to 70 years ‐reversibility >= 15% after inhaled beta2‐agonists ‐well‐controlled on ICS 400 to 750 mcg daily (Beclomethasone or BUDesonide) |
|
| Interventions | PROTOCOL:
AL + ICS vs same dose ICS (TAPERING ICS dose) DURATION ‐Run‐in Period: 1 week to confirm asthma control ‐Dose optimisation period: NONE ‐Intervention Period: 20 weeks TEST GROUP ‐ICI 204,219 = Zafirlukast 20 mg bid p.o. + ICS 400 to 750 ug/day (beclomethasone or BUDesonide) CONTROL GROUP ‐Placebo + ICS 400 to 750 ug/day (beclomethasone or BUDesonide) DEVICE ‐various devices used ‐CO‐TREATMENT: none reported CRITERIA FOR TAPERING ICS every 2 weeks: ‐ FEV1 >= 80% of predicted ‐ B2‐use <= 800 ug/day of salbutamol MINIMAL DOSE OF ICS ALLOWED: None |
|
| Outcomes | PER‐PROTOCOL (PP) ANALYSES
‐ some intention‐to‐treat (ITT) analysis
‐outcomes used at 6, 12, and 20 weeks PULMONARY FUNCTION TESTS (reported as cross‐sectional values not as change from baseline) ‐FEV1 (L) ‐ ITT ‐Am PEFR ‐PP (L/min) SYMPTOM SCORES (PP) ‐Mean daytime symptom scores (range 0 to 3) FUNCTIONAL STATUS (PP) ‐ Mean daily use of B2‐agonists at (puffs/day) averaged over a week ICS DOSE REDUCTION (PP) ‐**ICS dose reduction (%) ‐% complete ICS withdrawal INFLAMMATORY MARKERS ‐not reported ADVERSE EFFECTS ‐elevated liver enzymes, headache, nausea, death, etc. WITHDRAWALS ‐reported (** denotes primary outcomes) |
|
| Notes | ‐Abs (1995) and unpublished data graciously provided by Christopher Miller and Susan Shaffer from Astra‐Zeneca, USA
(Oct 2000) ‐funded by Zeneca ‐Confirmation of methodology and data extraction graciously received from M. Christopher Miller and Ms. Susan Shaffer, Astra‐Zeneca, Oct 2000 ‐User‐defined order: 54 (mean ICS dose of 540 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Finn 2000.
| Methods | DESIGN
‐parallel‐group
‐multicentre trial
‐Analysed by co‐treatment with ICS
ALLOCATION
‐Random
‐Methods of randomisation: not described
‐Means of treatment assignment: not described BLINDING ‐double‐blind ‐Means of concealment: not described WITHDRAWAL/DROPOUT ‐not described JADAD's Quality Score =2 ‐Confirmation of methodology: not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED N = 479 of which only 98 were co‐treated with inhaled glucocorticoids Zafirlukast + ICS = 56 ICS alone = 42 WITHDRAWALS AMONG CHILDREN WITH CO‐TX WITH ICS not described Zafirlukast: % Control: % AGE: 5‐11 years old Zafirlukast: years Control: years GENDER (% male) not described SEVERITY: mild‐moderate asthma BASELINE % predicted FEV1 Zafirlukast: 72 ± 12 (SD) Control: 71 ± 12 ALLERGIC RHINITIS: Zafirlukast: 76% Control: 74% EXERCISE‐INDUCED ASTHMA: Zafirlukast: 88% Control: 83% ASTHMA DURATION: Zafirlukast: 19 (0.5 to 62) years Control: 18 (0.5 to 59) years ELIGIBILITY CRITERIA ‐Age: > 15 years old ‐healthy, non‐smoking ‐history of >= one year of intermittent or persistent asthma symptoms ‐ICS treatment >= 6 wks prior to pre‐study visit (ICS dose comparable to beclomethasone 400 to 500 mcg) ‐50 to 85% FEV1 Pred ‐improvement > 15% FEV1 after B2‐agonist ‐ >= 1 puff/day of B2‐agonist EXCLUSION CRITERIA: ‐Upper respiratory tract infection < 3 weeks |
|
| Interventions | PROTOCOL:
AL + ICS vs SAME dose ICS DURATION: ‐Run‐in Period: 7‐14 days ‐Intervention Period: 10 weeks TEST GROUP Accolate 10 mg bid +/‐ ICS (dose not specified) CONTROL GROUP Placebo +/‐ ICS (dose not specified) DEVICE ‐not specified ‐CO‐TREATMENT: not described |
|
| Outcomes | INTENTION‐TO‐TREAT ANALYSES: not described PULMONARY FUNCTION TESTS ‐*change in AM PEF (L/min) SYMPTOM SCORES (PP) ‐not reported FUNCTIONAL STATUS (PP) ‐change in mean daily use of B2‐agonist (puffs/day) ‐change in nocturnal awakenings/week INFLAMATORY MARKERS ‐not described ADVERSE EFFECTS ‐not reported WITHDRAWALS ‐not reported * primary outcome |
|
| Notes | ‐Abstract 2000 ‐Funded by Astra‐Zenece ‐Confirmation of methodology and data extraction: not obtained. User‐defined order: not specified (mean intervention ICS dose in mcg/day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Green 2002a.
| Methods | DESIGN
‐cross‐over trial
‐4 weeks for each of the 4 periods
‐1‐month wash‐out between periods ALLOCATION ‐Random ‐Means of randomisation: not specified ‐Mode of allocation: not specified BLINDING ‐double‐blind ‐mode of blinding: not specified WITHDRAWL/DROPOUTS ‐not described JADAD's Quality score = 2 Confirmation of methodology ‐ not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED N = 49 for each of 4 periods WITHDRAWALS not reported AGE: not reported GENDER (% male) not reported SEVERITY: ?mild‐moderate asthma BASELINE % predicted FEV1 ± (SD) not reported BUD 800: BUD 200 + Montelukast: BUD 200 + Formoterol: BUD 200 + Placebo: ALLERGIC RHINITIS: ‐not reported ASTHMA DURATION: ‐not reported ELIGIBILITY CRITERIA ‐not reported EXCLUSION CRITERIA ‐not reported |
|
| Interventions | PROTOCOL:
AL + ICS vs HIGHER dose ICS DURATION: ‐Run‐in Period: not described ‐Intervention Period: 4 weeks ‐Wash‐out period: 4 weeks TEST GROUP 1 BUD 100 mcg bid + Montelukast 10 mg die TEST GROUP 2 (not used in this review) BUD 100 mcg bid + formoterol CONTROL GROUP 1 4 x ICS: BUD 400 mcg bid CONTROL GROUP 2 (not used in this review) BUD 100 mcg bid DEVICE ‐not specified CO‐TREATMENT: ‐not reported |
|
| Outcomes | INTENTION‐TO‐TREAT ANALYSES PULMONARY FUNCTION TESTS ‐change in FEV1 (L) ‐*change in AM PEF (L/min) SYMPTOM SCORES (PP) ‐change in symptom score (scale 0 to 6) FUNCTIONAL STATUS (PP) ‐change in mean daily use of B2‐agonist (puffs/day) ‐change in quality of life score (range 1‐7) ‐change in nocturnal awakenings INFLAMATORY MARKERS ‐change in peripheral blood eosinophil count ADVERSE EFFECTS ‐not reported WITHDRAWALS ‐not reported * primary outcome |
|
| Notes | ‐Abstract 2002 ‐Funding: not specified ‐Confirmation of methodology and data extraction: not obtained. User‐defined order: 20 (mean intervention BUD dose in mcg/day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Hultquist 2000.
| Methods | DESIGN
‐parallel‐group study
‐multicentre trial (49 centres in 6 countries) ALLOCATION ‐Random ‐Methods of randomisation: computer generated ‐means of assignment by opaque consecutive numbered envelopes containing assignment BLINDING ‐double‐blind ‐double‐dummy WITHDRAWAL/DROPOUT ‐ described by treatment groups JADAD's Quality Score = 5 Confirmation of methodology: received |
|
| Participants | INADEQUATELY controlled participants on inhaled glucocorticoids at baseline BASELINE INHALED STEROID DOSAGE: 400‐1000 ug of ICS (not specified)/day RANDOMISED ‐Anti‐leukotriene= 118 ‐Long‐acting beta2‐agonist = 118 ‐Bud=116 WITHDRAWALS: ‐Anti‐leukotriene= 19/118 (16%) ‐Long‐acting beta2‐agonist = 12/118 (10%) ‐BUD= 9/116 (8%) AGE in years: mean ± SD ‐Anti‐leukotriene= 38.3 ± NS ‐Long‐acting beta2‐agonist = 38.1 ± NS ‐BUD=38.1 ± NS GENDER (% male) ‐Anti‐leukotriene= 47 % ‐Long‐acting beta2‐agonist = 49% ‐BUD=53% SEVERITY: Not described BASELINE FEV1 (% pred) ‐Anti‐leukotriene= 72.03 ± SD ‐Long‐acting beta2‐agonist = 69.71 ± SD ‐BUD=72.12 ± SD ALLERGEN TRIGGERS: ‐Not reported ALLERGIC RHINITIS: ‐Not reported ASTHMA DURATION in years ‐Anti‐leukotriene= 10.1 ± SD ‐Long‐acting beta2‐agonist = 12.1 ± SD ‐BUD=10.6 ± SD ELIGIBILITY CRITERIA ‐male or female outpatient ‐age 12‐70 years ‐treated for at least 3 mo with 400‐1000 mcg of inhaled glucocorticoids ‐asthma diagnosis ‐FEV1 50‐80% predicted ‐>=12 % reversibility in FEV1 and at least 200 mL after inhalation of 1 mg of terbutaline ‐smoking history of <=10 pack years In the 7 days prior to randomisation one or more of the following: ‐ an symptom score of >=1 on 4 days ‐awakening on >= 1 night due to asthma symptoms ‐use of B2‐agonists >=10 puffs as weekly mean EXCLUSION CRITERIA: ‐Respiratory infection ‐clinical obstructive pulmonary disease, or pulmonary dysfunction other than asthma ‐pregnant or lactating women ‐use of long‐acting beta2‐agonist within 1 month prior to visit 1 ‐previous use ever of a leukotriene antagonist ‐known intolerance to study drugs or inhaled lactose SETTING: not described |
|
| Interventions | PROTOCOL:
AL + ICS vs SAME dose ICS
(Stable dose of ICS) DURATION: ‐Run‐in Period: not reported ‐Intervention Period: 8 weeks INTERVENTION GROUP 1 ‐AL = Zafirlukast 20 mg bid + Budesonide 200 mcg bid via Turbuhaler INTERVENTION GROUP 2 (not used) ‐LAB2 = Formoterol 9 ug bid, via Turbuhalor + Budesonide 200 mcg bid via Turbuhaler CONTROL: Budesonide 200 mcg bid via Turbuhaler ‐CO‐TREATMENT: None allow other than rescue LAB2 |
|
| Outcomes | Modified INTENTION‐TO‐TREAT ANALYSES
(on all patients who receive at least 1 dose of study medication) ‐outcomes used at endpoint or 8 weeks PULMONARY FUNCTION TESTS ‐**Change from baseline in AM PEFR ‐Change from baseline in PM PEFR ? SYMPTOM SCORES ‐Change from baseline OVERALL symptom scores? ‐Change from baseline DAYTIME symptom scores? ‐Change in symptom‐free days? ‐ Patient satisfaction? EXACERBATIONS Definition: Any worsening of asthma symptoms requiring treatment beyond the use of blinded study drug &/or supplemental albuterol. Patients who experienced an asthma exacerbation were withdrawn from the study ?? FUNCTIONAL STATUS ‐Change from baseline in mean OVERALL use of B2‐agonists (puffs/DAY)? ‐Change from baseline in mean DAYTIME use of B2‐agonists (puffs/DAY)? ‐Change from baseline in mean NIGHT‐TIME use of B2‐agonists (puffs/DAY)? ‐Change in rescue‐free days? ‐Change in night‐time awakenings ? INFLAMMATORY MARKERS: Not reported ADVERSE EFFECTS ‐drug related & non‐drug related ? WITHDRAWALS ‐due to adverse effects reported ? (** denotes primary outcome) |
|
| Notes | ‐ Unpublished data ‐Received full disclosure of unpublished data provided by Ian Naya and Roger Metcalf, AstraZeneca, Sept 2003 ‐Funded by Astra Zeneca Report #SD‐004CR‐0216 Confirmation with supportive documents received for methodology and data extraction (Sept 2003) User‐defined order: 40 (mean intervention BUD dose in mcg/day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Kanniess 2002.
| Methods | DESIGN
‐cross‐over trial
‐6 weeks for each period
‐no wash‐out between periods
‐dose reduction of 50% at beginning of each period ALLOCATION ‐Random: Computer‐generated random number ‐means of assignment: opaque consecutive numbered envelopes BLINDING ‐double‐blind ‐placebo‐controlled ‐identical placebo WITHDRAWL/DROPOUTS ‐described JADAD's Quality score = 5 Confirmation of methodology ‐ obtained |
|
| Participants | WELL‐CONTROLLED PARTICIPANTS RANDOMISED N = 50 ‐Montelukast = 26 ‐Control = 24 WITHDRAWALS: ‐Montelukast = 3 (11.54%) ‐Control = 2 (8.33%) AGE: ‐Montelukast: 38 ± 12 (SEM) years ‐Control: 43± 11 (SEM) years GENDER (% male): ‐Montelukast: 50% ‐Control: 46% SEVERITY: ‐moderate bronchial asthma BASELINE % PREDICTED FEV1: ‐Montelukast: 95.0 ± 2 (SEM) ‐Control: 92.3 ± 1.8 (SEM) ATOPY: not reported ASTHMA DURATION: not reported ELIGIBILITY CRITERIA ‐taking a dose of ICS (Beclomethasone or equivalent) 800 ug/day for more than 8 weeks prior to randomisation ‐FEV1 > 80% predicted ‐no oral corticosteroids within 6 months of entry to study ‐no oral antihistamines within 4 weeks of entry to study ‐provocation concentration of methacholine causing a 20% fall in FEV1 (PC20) <8mg/mL ‐FEV1 and PC20 be reproducible within 15% and 1.5 doubling concentrations, respectively, between visits one and two ‐ no smokers ‐no signs of an acute exacerbation or respiratory tract infection within 4 weeks prior to screening EXCLUSION CRITERIA ‐none mentioned |
|
| Interventions | PROTOCOL
‐AL + ICS vs same dose ICS (TAPERING ICS) DURATION ‐Run‐in Period: 1‐3 weeks ‐Dose optimisation period: none ‐Intervention Period: 6 weeks (X 2 periods) TEST GROUP Period 1 ‐Montelukast 10 mg once daily + ‐BDP 800 mcg/day or equivalent Period 2: Placebo + BDP‐eq 400 mcg/day CONTROL GROUP Period 1 ‐Placebo + BDP 800 mcg/day or equivalent Period 2 ‐Montelukast 10 mg die + BDP‐eq 400 mcg/day DEVICE ‐not reported CO‐TREATMENT: ‐none reported (need confirmation) CRITERIA FOR TAPERING ‐1st treatment period ICS reduced to 50% baseline (400 ug/day) ‐2nd treatment period ICS reduced to 50% of 1st treatment (25% of baseline or 200ug/day) * For purpose of the analysis, because this unusual design did not allow merging of the two periods and because no significant change in asthma control occurred in the first period suggesting of over‐treatment at baseline, the second period was arbitrarily chosen for analysis. |
|
| Outcomes | INTENTION‐TO‐TREAT ANALYSES
‐ yes PULMONARY FUNCTION TESTS ‐data are given as changes relative to baseline (1st period) and relative to each other (2nd period) ‐ change in FEV1 (L) ‐ change in PEF daytime ‐ change in PEF night‐time ‐ change in PC20 SYMPTOM SCORES ‐ change in daytime symptoms score (range 0 ‐ 4) ‐ change in night‐time symptoms score (range 0 ‐ 4) FUNCTIONAL STATUS ‐Use of rescue B2‐agonists (puffs/day) ICS DOSE REDUCTION: ‐ fixed by protocol INFLAMMATORY MARKERS: ‐data are given as changes relative to baseline (1st period) and relative to each other (2nd period) ‐exhaled NO ppb ‐% sputum eosinophils ADVERSE EFFECTS ‐reported (personal communication) WITHDRAWALS ‐reported |
|
| Notes | ‐Full‐text (2002) publication and unpublished data ‐Funds by an educational grant from MSD, Munich, Germany ‐Confirmation of data extraction and methodology graciously obtained from Dr Frank Kanniess, from the Pulmonary Research Institute, Germany, August 2003. ‐User defined number = 40 (mcg of beclomethasone‐equivalent at baseline of the 2nd period) (mean ICS dose of 400 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Laitinen 1995.
| Methods | DESIGN
‐parallel‐group
‐multicentre trial (83 centres) ALLOCATION ‐Random: 2:1 ratio intervention:control ‐Computer‐generated BLINDING ‐double‐blind ‐placebo‐controlled ‐identical placebo WITHDRAWAL/DROPOUT ‐described JADAD's Quality Score = 5 Confirmation of methodology obtained |
|
| Participants | WELL‐CONTROLLED PARTICIPANTS ‐RANDOMISED N = 262 Zafirlukast:175 Control:87 WITHDRAWALS Zafirlukast:15% Control:14% AGE: Zafirlukast: 45.5 ±13.6 (SD) years Control: 43.5 ± 13.4 years GENDER (% male) Zafirlukast:46% Control:34% SEVERITY: moderate asthma BASELINE FEV1 (L) Zafirlukast: 2.58 ± 0.89 (SD) Control: 2.42 ± 0.78 ALLERGEN TRIGGERS: Zafirlukast: 45% Control:40% ASTHMA DURATION: Zafirlukast: 14.1 ± 13.1 (SD) years Control: 14.3 ± 12.5years ELIGIBILITY CRITERIA ‐taking a dose of ICS (BUD or BDP) between 800 and 2000 ug/day ‐stable in the preceding month |
|
| Interventions | PROTOCOL:
AL + ICS vs same dose ICS
(TAPERING ICS dose) DURATION: ‐Run‐in Period: 1 week to confirm asthma control ‐Dose optimisation period: 2 weeks to 3 months Zafirlukast: 7.7 ± 4.0 weeks (SD) Control: 7.9 ± 4.0 weeks ‐Intervention Period: 12 weeks TEST GROUP ‐ICI 204219 = Zafirlukast 20 mg bid p.o. + ICS (BUD or BDP) 800 to 2000 ug/day CONTROL GROUP Placebo + ICS (BUD or BDP) 800 to 2000 ug/day DEVICE ‐various devices used ‐CO‐TREATMENT: none reported CRITERIA FOR TAPERING every 2 weeks by 200 to 250 mcg/day ‐FEV1>= 80% predicted ‐B2‐agonist use <= 800 ug/day of salbutamol MINIMAL DOSE OF ICS ALLOWED: 400 mcg/day CRITERIA TO INCREASE CORTICOSTEROIDS: ‐FEV1< 60% of predicted |
|
| Outcomes | PER‐PROTOCOL (PP) ANALYSES
‐ some intention‐to‐treat (ITT) analysis ‐outcomes used at 6 and 12 weeks PULMONARY FUNCTION TESTS (reported as cross‐sectional values not as change from baseline) ‐FEV1 (L)‐ ITT ‐Mean Am PEFR (L/min) ‐PP SYMPTOM SCORES ‐ PP ‐Mean daytime symptom scores FUNCTIONAL STATUS ‐ PP ‐Mean daily use of beta2‐agonist (puffs/day) averaged over a week ICS DOSE REDUCTION: (PP) ‐**ICS dose reduction (% change from baseline) INFLAMMATORY MARKERS ‐not reported ADVERSE EFFECTS ‐elevated liver enzymes, headache, nausea, death, etc. WITHDRAWALS ‐reported (** denotes primary outcomes) |
|
| Notes | ‐Abs (1995) and unpublished data graciously provided by Christopher Miller and Susan Shaffer from Astra‐Zeneca, USA (Oct 2000) ‐funded by Zeneca ‐Confirmation of methodology and data extraction received from M. Christopher Miller and Ms. Susan Shaffer, Astra‐Zeneca, Oct 2000 ‐User‐defined order: 114 (mean intervention ICS dose of 1137 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Laviolette 1999.
| Methods | DESIGN
‐parallel‐group
‐Multicentre trial (70 centres)
‐4‐arm study of which two are considered in this review ALLOCATION ‐Random ‐computer‐generated allocation ‐opaque consecutive‐numbered envelopes containing assignment BLINDING ‐double‐blind ‐double‐dummy ‐identical placebo WITHDRAWAL/DROPOUT ‐described JADAD's Quality Score = 5 ‐Confirmation of methodology obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED N = 393 Montelukast = 193 Control = 200 WITHDRAWALS Montelukast: 8% Control: 21% AGE: 15 to 78 years old Montelukast: 40 (range: 15 to 76) years Control:39(15 to 78) years GENDER (% male) Montelukast: 56% Control: 52% SEVERITY: mild‐moderate asthma BASELINE % predicted FEV1 Montelukast: 72 ± 12 (SD) Control: 71 ± 12 ALLERGIC RHINITIS: Montelukast: 76% Control: 74% EXERCISE‐INDUCED ASTHMA: Montelukast: 88% Control: 83% ASTHMA DURATION: Montelukast: 19 (0.5 to 62) years Control: 18 (0.5 to 59) years ELIGIBILITY CRITERIA ‐Age: > 15 years old ‐healthy, non‐smoking ‐history of >= one year of intermittent or persistent asthma symptoms ‐ICS treatment >= 6 wks prior to prestudy visit (ICS dose comparable to beclomethasone 400 to 500 mcg) ‐50 to 85% FEV1 Pred ‐improvement > 15% FEV1 after B2‐agonist ‐ >= 1 puff/day of B2‐agonist EXCLUSION CRITERIA: ‐Upper respiratory tract infection < 3 weeks |
|
| Interventions | PROTOCOL
AL + ICS vs SAME dose ICS DURATION ‐Run‐in Period: 4 weeks ‐Dose optimisation period: none ‐Intervention Period: 16 weeks TEST GROUP 1 ‐Montelukast 10 mg die + Inhaled Beclomethasone 200 ug bid TEST GROUP 2 ‐Montelukast 10 mg die + Placebo (not used in this review) CONTROL GROUP 1 ‐Placebo + Inhaled Beclomethasone 200 ug bid CONTROL GROUP 2 ‐Placebo + Placebo (not used in this review) DEVICE ‐metered‐dose aerosol inhalers ‐CO‐TREATMENT: none permitted (other than anti‐histaminic) |
|
| Outcomes | INTENTION‐TO‐TREAT ANALYSIS
‐outcomes used at 16 weeks PULMONARY FUNCTION TESTS ‐**Change from baseline FEV1 ‐Change from baseline AM PEFR SYMPTOM SCORES ‐**Change from baseline daytime symptom scores ‐Change from baseline nocturnal awakenings (nights/wk) FUNCTIONAL STATUS ‐Change from baseline mean daily B2‐agonist use (puffs/day) INFLAMMATORY MARKERS ‐Change from baseline in blood serum eosinophils ADVERSE EFFECTS ‐elevated liver enzymes, headache, nausea, death, etc. WITHDRAWALS ‐reported (** denotes primary outcomes) |
|
| Notes | ‐Full Text 1999 and unpublished data graciously provided by Theodore F Reiss and GP Noonan from Merck Frosst, USA ‐Funded by Merck ‐Confirmation of methodology received June 1999 ‐Confirmation of data extraction graciously provided by T.F Reiss and G.P. Noonan, Merck Frosst, USA, June 2000 and June 2001. ‐User‐defined order: 40 (mean intervention ICS dose of 400 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Lofdahl 1999.
| Methods | DESIGN
‐parallel‐group
‐multicentre trial ALLOCATION ‐Random ‐computer‐generated allocation ‐opaque consecutive‐numbered envelopes containing assignment BLINDING ‐triple‐blind ‐placebo‐controlled ‐identical placebo WITHDRAWAL/DROPOUTS ‐ described JADAD's Quality Score = 5 Confirmation of methodology obtained |
|
| Participants | WELL‐CONTROLLED PARTICIPANTS RANDOMISED N = 226 Montelukast = 113 Placebo = 113 WITHDRAWALS Montelukast: 16% Control: 27% AGE: 16 to 70 years old Montelukast: 40 years (mean) Control: 41 years GENDER:(% male) Montelukast: 42% Control: 45% SEVERITY: not described BASELINE FEV1 (% predicted): Montelukast: 84.4 ± 11.1 % (SD) Control: 82.3 ± 12.9 % ALLERGEN TRIGGERS: not reported ASTHMA DURATION: Montelukast: 18 ± 13.3 (SD) years Control: 19 ± 14 years ELIGIBILITY CRITERIA ‐non‐smoking adults ‐clinical history of asthma for at least one year ‐treatment with stable, ICS bid for at least 3 weeks prior to pre‐study visit ‐>= 70% FEV1 % Pred ‐>= 15% reversibility after inhaled B2‐agonist ‐after two ICS dose reductions: ‐FEV1 >= 90% of baseline value, ‐levels < baseline level in asthma symptoms and B2‐agonist use, ‐>= 65% of maximum peak flow, and ‐a required prespecified minimum ICS dose was met EXCLUSION: ‐emergency treatment in past 1 month ‐hospitalised in past 3 months ‐upper respiratory tract infection within 3 weeks |
|
| Interventions | PROTOCOL:
AL + ICS vs same dose
ICS (TAPERING ICS dose) DURATION ‐Dose optimisation period: <=7 weeks (ICS dose reduced to minimum dose necessary to maintain stability before randomisation) ‐Intervention Period: 12 weeks TEST GROUP ‐Montelukast 10 mg die p.o. + ICS 300 to 3000 ug/day CONTROL GROUP ‐Placebo + ICS 300 to 3000 ug/day (Various ICS including fluticasone 7%, beclomethasone 16%, BUDesonide 22%, flunisolide 15%, triamcinolone 40%) DEVICE ‐variety used ‐CO‐TREATMENT: none reported CRITERIA FOR TAPERING every 2 weeks by 25% of their ICS dose ‐FEV1 >= 90% of value at randomisation ‐B2‐agonist use <= 135% of pre‐allocation ‐Daytime symptoms score <= 120% of pre‐allocation baseline MINIMAL DOSE OF ICS ALLOWED: None |
|
| Outcomes | INTENTION‐TO‐TREAT ANALYSES
‐outcomes used at last tolerated dose or 12 weeks PULMONARY FUNCTION TESTS ‐Change from baseline FEV1 at visit of lowest tolerated ICS dose SYMPTOM SCORES ‐Change from baseline daily symptom scores at visit of lowest tolerated ICS dose FUNCTIONAL STATUS ‐Change from baseline mean daily use of B2‐agonists at visit of lowest tolerated ICS dose ICS DOSE REDUCTION: ‐**Mean % change from baseline ICS dose reduction ‐**Change from baseline ICS dose (mcg) INFLAMMATORY MARKERS: not reported ADVERSE EFFECTS ‐elevated liver enzymes, headache, nausea, death WITHDRAWALS ‐reported (** denotes primary outcomes) |
|
| Notes | ‐Full Text 1999 and unpublished data ‐Funded by Merck Frosst ‐Confirmation of methodology received June 1999 ‐Confirmation of data extraction graciously received from T.F Reiss and G.P. Noonan, June 2000. ‐User‐defined order: 98 (mean intervention ICS dose of 976 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Nayak 1998.
| Methods | DESIGN
‐parallel‐group
‐multicentre trial (41 centres)
‐3 arms study:
(1) Z80+ICS
(2) Z160+ICS
(c) 2 x ICS ALLOCATION ‐Random ‐Computer generated random numbers, ‐number coded solutions supplied by pharmacy BLINDING ‐triple‐blind ‐double‐dummy ‐identical placebo‐controlled WITHDRAWAL/DROPOUT ‐described JADAD's Quality Score = 5 ‐Confirmation of methodology obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED N = 394 Z80+ICS = 130 Z160+ICS = 134 2 x ICS = 130 WITHDRAWALS:Z40+ICS = 16% Z160+ICS = 12% 2 x ICS = 16% AGE MEAN (range) (1) Z80+ICS = 39.2 (12 to 78) years (2) Z160+ICS = 39.3 (12 to 79) years (3) 2 x ICS = 39.5 (12 to 74) years GENDER (% male) (1) Z80+ICS = 40% (2) Z160+ICS=37% (3) 2 x ICS=38% SEVERITY: mild to moderate BASELINE FEV1 (% predicted): (1) Z80+ICS = 68.3% (2) Z160+ICS=67.3% (3) 2 x ICS = 67.2% ‐ALLERGIC RHINITIS: 94.2% ELIGIBILITY CRITERIA ‐Age: >= 12 years old ‐symptomatic on low‐dose ICS ‐non‐smoker for at least 6 months preceding screening ‐FEV1 of 45 to 80% of predicted at least 6 hours after short‐acting inhaled beta2‐agonist and at least 48 hours after salmeterol or antihistamines ‐reversibility >= 15% after inhaled beta2‐agonists within 6 months of screening or non‐specific bronchial hyperreactivity to histamine or methacholine challenge between 0.25‐8.0 mg/ml within 12 months of screening ‐85% compliance with placebo tablets ‐no chronic diseases ‐no drug/alcohol abuse ‐has not taken other steroids besides BDP ‐no use of salmeterol 48 hours prior to, or astemizole 3 months prior screening ‐no recent upper respiratory tract infection ‐no seasonal asthma |
|
| Interventions | PROTOCOL:
AL + ICS vs DOUBLE dose ICS DURATION ‐Run‐in Period: 7‐14 days ‐Dose optimisation period: NONE ‐Intervention Period: 13 weeks TEST GROUP (Z80+ICS) ‐Zafirlukast 40 mg bid p.o. + Beclomethasone 400 ug/day TEST GROUP Z160+ICS ‐Zafirlukast 80 mg bid p.o. + Beclomethasone 400 ug/day CONTROL GROUP (2 X ICS) ‐Beclomethasone 800 ug/day DEVICE ‐metered‐dose aerosol inhaler ‐CO‐TREATMENT: none |
|
| Outcomes | PER‐PROTOCOL (PP) ANALYSES
‐outcomes used at 6‐8 and 13 weeks PULMONARY FUNCTION TESTS ‐Change from baseline FEV1 ‐**Change from baseline Am PEFR SYMPTOM SCORES ‐**Change from baseline daytime symptom scores (scale 0‐3) ‐Change from baseline nocturnal awakenings ‐Change from baseline mornings with asthma FUNCTIONAL STATUS ‐Change from baseline mean daily use of B2‐agonists INFLAMMATORY MARKERS: ‐not reported ADVERSE EFFECTS ‐elevated liver enzymes, oral moniliasis, headache, nausea, death WITHDRAWALS ‐reported (** denotes primary outcomes) |
|
| Notes | ‐Abs (1998) and unpublished report provided by Astra‐Zeneca (Oct 2000) ‐Funded by Astra‐Zeneca ‐Confirmation of methodology and data extraction graciously received from M. Christopher Miller and Ms. Susan Shaffer, Astra‐Zeneca, Oct 2000 ‐User‐defined order: 40 (mean intervention ICS dose of 400 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Nishimura 1999.
| Methods | DESIGN
‐Cross‐over trial ALLOCATION ‐Random ‐Mode of randomisation: not described ‐Means of assignment: not described BLINDING ‐double‐blind ‐means of blinding: not described WITHDRAWAL/DROPOUT ‐not described JADAD's Quality Score = ‐Confirmation of methodology obtained/not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED N = 27 WITHDRAWALS not described AGE: not described GENDER (% male) 56% male SEVERITY: moderate to severe persistent asthma BASELINE % predicted FEV1 not described ALLERGIC RHINITIS: not described EXERCISE‐INDUCED ASTHMA: not described ASTHMA DURATION: not described ELIGIBILITY CRITERIA ‐Adults: age not specified ‐asthma: not defined ‐improperly controlled on BDP 800‐1200 mcg/day for 3 months. EXCLUSION CRITERIA: ‐not described |
|
| Interventions | PROTOCOL
AL + ICS vs SAME dose ICS DURATION ‐Run‐in Period: 4 not described ‐Intervention period: 4 weeks ‐Wash‐out period:not described TEST GROUP ‐Pranlukast 225 mg twice daily + Inhaled BDP 800‐1200 mcg /day CONTROL GROUP ‐Placebo + Inhaled BDP 800‐1200 mcg /day DEVICE ‐not described ‐CO‐TREATMENT: ‐not described |
|
| Outcomes | INTENTION‐TO‐TREAT ANALYSIS
‐not described
‐outcomes reported at 4 weeks PULMONARY FUNCTION TESTS ‐*Change from baseline AM PEFR ‐Change from baseline in FEV1 SYMPTOM SCORES ‐Change from baseline daytime symptom scores ‐Change from baseline nighttime symptoms FUNCTIONAL STATUS ‐Change from baseline mean daily B2‐agonist use ‐Change from baseline in health‐related quality of life (Living with Asthma Questionnaire) INFLAMMATORY MARKERS ‐not reported ADVERSE EFFECTS ‐not reported WITHDRAWALS ‐not reported (* denotes primary outcomes) |
|
| Notes | ‐Abstract 1999 ‐Funding source: not reported ‐Confirmation of methodology and data extraction: not obtained. User‐defined order: not specified (mean intervention ICS dose in mcg/day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Nsouli 2000.
| Methods | DESIGN
‐parallel‐group ALLOCATION ‐Random ‐Methods of randomisation: not reported ‐means of assignment: not reported BLINDING ‐no blinding (open label) WITHDRAWAL/DROPOUT ‐not described JADAD's Quality Score = 1 ‐Confirmation of methodology not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED ‐ N= 30 ‐M10 + ICS = NR ‐2 X ICS= NR WITHDRAWALS ‐M10 + ICS =NR ‐2 X ICS= NR GENDER (% male) ‐M10 + ICS = NR ‐2 X ICS =NR AGE (SD) years ‐M10 + ICS = NR ‐2 X ICS= NR SEVERITY ‐not specified BASELINE PREDICTED FEV1 % (SD) ‐M10 + ICS = NR ‐2 X ICS = NR ALLERGEN TRIGGERS ‐not described ASTHMA DURATION (SD) years ‐ M10 + ICS = NR ‐2 X ICS = NR ELIGIBILITY CRITERIA ‐symptomatic on low dose of ICS (FP 100‐300 mcg; BDP 200 ‐ 1200 mcg; BUD 200‐400 mcg; flunisolide: 500‐1000 mcg; TAA:400‐1000 mcg) EXCLUSION CRITERIA ‐not reported |
|
| Interventions | PROTOCOL:
AL + ICS vs DOUBLE dose ICS DURATION ‐Run‐in Period: not described ‐Intervention period: 12 weeks TEST GROUP ‐Montelulast 10 mg die p.o. + Beclomethasone 200‐500/day or equivalent CONTROL GROUP (2 X ICS) ‐Beclomethasone 400‐1200 ug/day or equivalent DEVICE ‐not described ‐CO‐TREATMENT: not described |
|
| Outcomes | INTENTION‐TO‐TREAT ANALYSIS
‐not described
‐outcomes reported at 12weeks PULMONARY FUNCTION TESTS ‐Change from baseline AM PEFR ‐Change from baseline in FEV1 SYMPTOM SCORES ‐Change from baseline daytime symptom scores ‐Change from baseline nighttime symptoms FUNCTIONAL STATUS ‐Change from baseline mean daily B2‐agonist use INFLAMMATORY MARKERS ‐not reported ADVERSE EFFECTS ‐not reported WITHDRAWALS ‐not reported (* denotes primary outcomes) |
|
| Notes | ‐Abstract 2000 ‐Funding source: not reported ‐Confirmation of methodology and data extraction: not obtained. User‐defined order: 35 (mean intervention (350) dose in mcg/day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
O'Sullivan 2003.
| Methods | DESIGN
‐Cross‐over trial ALLOCATION ‐Random ‐Mode of randomisation: not described ‐Means of assignment: not described BLINDING ‐?triple‐blind ‐?double‐dummy ‐?identical placebo‐controlled WITHDRAWAL/DROPOUT ‐? described JADAD's Quality Score = ‐Confirmation of methodology obtained/not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 34 adults WITHDRAWALS: Montelukast: 3 (9%) Placebo: 2 (6%) + 1 no group specified AGE (Mean, SEM): ‐27.7 ± 1.1 years (range: 19 to 55) GENDER(% male): ‐53% Baseline mean FEV1 % Pred (± SD) ‐90.7± 3.4 % predicted ASTHMA SEVERITY: ‐mild ATOPY: ‐100 % atopic (one positive skin prick test to house dust mites or two other commonly inhaled allergens) ASTHMA DURATION: ‐not described ELIGIBILITY CRITERIA ‐Age: >=19 years ‐mild persistent asthma ‐FEV1 >=60% of predicted ‐change in FEV1 of 12% or more after salbutamol ‐provocative PD20% of 4 mg/mL or less ‐use of rescue B2‐agonists as needed ‐no inhaled steroids in previous 6 weeks ‐pre‐existing history of asthma Exclusion criteria: ‐not described |
|
| Interventions | PROTOCOL
AL + ICS vs ICS (same dose) Duration ‐Run‐in Period: 2 weeks ‐Intervention Period 2: 8 weeks ‐Washout period: not reported TEST GROUP ‐Montelukast 10 mg qd p.o. ‐FP 100 mcg bid CONTROL GROUP ‐FP 100 mcg bid ‐Placebo montelukast capsule die DEVICE ‐Diskus CO‐INTERVENTION: ‐not described |
|
| Outcomes | ANALYSES not detailed (ITT vs per protocol) PULMONARY FUNCTION TESTS ‐change in FEV1 (L) ‐*change in AM PEF (L/min) ‐change in PM PEF (L/min) ‐methacholine PC20 SYMPTOM SCORES (PP) ‐change in symptom score (on a visual analogue scale) FUNCTIONAL STATUS (PP) ‐change in mean daily use of B2‐agonist (puffs/day) ‐change in quality of life score (range 1‐7) ‐change in Juniper Asthma Quality of Life INFLAMATORY MARKERS ‐change in induced sputum ‐exhaled nitric oxide ADVERSE EFFECTS ‐not reported WITHDRAWALS ‐not reported * primary outcome |
|
| Notes | ‐Full text 2003 ‐Funding: GlaxoSmithKline R & D, UK ‐Confirmation of methodology and data extraction: not obtained ‐User‐defined order: 40 (intervention FP dose of 200 mcg/ day X 2) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Price 2003.
| Methods | DESIGN
‐multicentre ALLOCATION ‐random ‐computer generated random numbers ‐assignment by numbered coded Rx supplied by pharmacy BLINDING ‐triple blind ‐identical placebo ‐double ‐dummy WITHDRAWALS/ DROPOUT ‐described JADAD's Quality Score = 5 Confirmation of methodology confirmed |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED ‐ N= 889 ‐M10 + BUD = 448 ‐2 X ICS= 441 WITHDRAWALS ‐M10 + BUD 800 = 20 (4.5%) ‐2 X ICS= 26 (6%) GENDER (% male) ‐M10 + BUD= 41% ‐2 X ICS =39% AGE (SD) years ‐M10 + BUD = 43 ± 14 ‐2 X ICS= 43 ± 14 (SD) SEVERITY ‐not specified BASELINE PREDICTED FEV1 % (SD) ‐M10 + BUD = 69.0 ± 13.3 ‐2 X ICS = 68.3 ± 13.4 ALLERGEN TRIGGERS ‐not described ASTHMA DURATION (SD) years ‐ M10 + BUD = 18 ±14 ‐2 X ICS = 17 ± 15 ELIGIBILITY CRITERIA ‐non‐smokers or ex‐smokers ‐diagnosis of asthma > 1 year ‐age 15 to 75 ‐not optimally controlled on ICS 600 to 1200 mcg/day of BUD, BDP, triamcinolone, flunisolide, or 300‐800 mcg FP ‐FEV1 >= 50% predicted ‐>=12% improvement in FEV1 after B2‐agonist use ‐symptoms requiring at least 1 puff/day of B2‐agonist during the last 2 weeks of the run‐in EXCLUSION CRITERIA ‐other active pulmonary disorders ‐respiratory infection within 3 weeks of visit 1 or during the run‐in period ‐ER visit for asthma in past 2 months of visit 1 ‐systemic corticosteroid treatment within 1 month ‐cromones or leukotriene receptor antagonists within 2 weeks ‐long‐acting antihistamine within 1 week (astemizole 3 months) ‐long acting B2 agonist or anticholinergic agents within 24 hours |
|
| Interventions | PROTOCOL
‐AL + ICS vs. DOUBLE dose ICS DURATION ‐run‐in period = 4 weeks ‐treatment period = 12 weeks TEST GROUP ‐ICS (BUD 800mcg/day) + 10 mg/day montelukast CONTROL GROUP ‐ICS (BUD 1600mcg/day) + placebo DEVICE ‐Turbohaler CO‐TREATMENT ‐ none reported |
|
| Outcomes | INTENTION‐TO‐TREAT ANALYSES PULMONARY FUNCTION TESTS ‐change in FEV1 (L) ‐*change in AM PEF (L/min) SYMPTOM SCORES (PP) ‐change in symptom score (scale 0 to 6) FUNCTIONAL STATUS (PP) ‐change in mean daily use of B2‐agonist (puffs/day) ‐change in quality of life score (range 1‐7) ‐change in nocturnal awakenings INFLAMATORY MARKERS ‐change in peripheral blood eosinophil count ADVERSE EFFECTS ‐reported WITHDRAWALS ‐reported * primary outcome |
|
| Notes | ‐Full text 2003 ‐Funding: Merck & Co ‐Confirmation of methodology and data extraction obtained by DB Price, August 2003 ‐User‐defined order: 80 (mean intervention ICS dose of 800 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Riccioni 2001.
| Methods | DESIGN
‐parallel group ALLOCATION ‐Random ‐method of randomization: computer generated ‐method of assessment: not mentioned BLINDING ‐reported as double‐blind (patient and assessor) but without placebo WITHDRAWAL/DROPOUT ‐described JADAD's Quality Score = 2 ‐Confirmation of methodology: received (Oct 2003) |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED: N = 48 Z= 12 BUD= 12 Z+BUD= 12 Control= 12 WITHDRAWALS; Z=2 (16%) BUD= 1 (8%) Z+BUD= 2 (16%) AGE: mean (± SD) years Z: 33.75 (±11.24) BUD: 32.15 (±10.27) Z+BUD: 33.44 (±11.12) Control: 29.15 (±10.34) GENDER (% male): Z: 6 (50) BUD: 6 (50) Z+BUD: 6 (50) Control: 6 (50) BASELINE SEVERITY: ‐mild persistent % Predicted FEV1 (Mean +‐ SD) Z: 94.75 ± 7.68 BUD: 92.75 ± 9.87 Z+BUD: 92.16 ± 5.06 Control: 95.75 ± 5.84 ATOPY/ALLERGEN TRIGGERS: ‐not reported ASTHMA DURATION (years) ‐1 year ELIGIBILITY CRITERIA ‐1 year of mild persistent bronchial asthma ‐PEF >80% pred. and PEF daily variability in 20‐30% range with positive salbutamol reversibility test EXCLUSION: ‐URTI in last 3 weeks ‐hospitalization for asthma in the last 3 months ‐treatment with antihistamines, anticholinergics, theophylline drugs ‐presence of autoimmune, hepatic, or renal disorders ‐malabsorption, drug or alcohol addiction ‐pregnancy or lactation |
|
| Interventions | PROTOCOL Duration ‐run‐in Period: 2 weeks ‐Intervention: 8 weeks TEST GROUP: ‐Zafirlukast 20 mg bid TEST GROUP 2 ‐Zafirlukast 20 mg bid + Budesonide 400 g bid CONTROL GROUP ‐Budesonide 400 g bid CONTROL GROUP 2 ‐Placebo DEVICE: ‐not reported CO‐INTERVENTION: ‐not reported |
|
| Outcomes | ANALYSIS PER PROTOCOL
(not by ITT) OUTCOMES ‐reported at 8 weeks PULMONARY FUNCTION TEST ‐pre‐ and post FVC values ‐pre‐ and post FEV1 ‐pre‐ and post PC20 SYMPTOM SCORES ‐not reported FUNCTIONAL STATUS ‐not reported INFLAMMATORY MEDIATORS ‐not reported ADVERSE EVENTS ‐not mentioned WITHDRAWALS ‐not reported Primary outcome not specified |
|
| Notes | ‐Full‐text publication (2001) Funded by University ‐confirmation of methodology and data extraction: obtained from Graziano Riccioni, Oct 2003 USER‐DEFINED ORDER: 800 (daily dose of BDP‐equivalent=800 mcg ) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Study investigators aware as to treatment group assignment (Cochrane Grade C) |
Riccioni 2002.
| Methods | DESIGN
‐parallel‐group ALLOCATION ‐random ‐method of randomization: computer generated ‐method of assessment: not described BLINDING ‐reported as double‐blind (patient and assessor) but without placebo WITHDRAWAL/DROPOUT ‐described JADAD = 3 CONFIRMATION OF METHODOLOGY: obtained (Oct 2003) |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED (N=45) M=15 BUD=15 M+BUD=15 WITHDRAWALS: M=1 (7%) BUD=1 (7%) M+BUD=1 (7%) AGE (± SD) M= 26.7 ± 8.6 BUD=26.9 ± 12.3 M+BUD=28.2 ± 10 GENDER (% male) M= 9 (60) BUD=8 (53.3) M+BUD=5(33.7) ASTHMA SEVERITY: ‐mild ASTHMA DURATION: ‐1 year % Pred. FEV1 M=97 (85‐123) BUD=97 (76‐123) M+BUD=99 (84‐131) Mean (+‐SD) beta2agonist use (puffs per day) ‐not described ATOPY: ‐100% (Definition not mentioned) ELIGIBILITY CRITERIA ‐asthma as per ATS criteria ‐confirmation of the presence of BHR by methacholine on the initial visit ‐regular attendance of the outpatient clinic over 4 months from the initial visit ‐PEF >= 80% of predicted ‐PEF variability <=20% as per NIH criteria EXCLUSION: ‐ER visit for asthma exacerbation within 1 month ‐ URTI in the past 4 weeks ‐hospitalization for asthma in past 6 months ‐treatment with antihistamines, anticholinergics, theophylline and cromones, LABA, inhaled and oral corticosteroids ‐bronchiectasis ‐gastroesophageal reflux ‐poor knowledge of the Italian language |
|
| Interventions | PROTOCOL Duration ‐Run‐in: 4 weeks ‐Intervention: 16 weeks TEST GROUP 1 ‐10 mg montelukast once daily TEST GROUP 2 10 mg Montelukast once daily + 400 ug budesonide bid CONTROL GROUP ‐400 Budesonide bid DEVICE ‐turbohaler CRITERIA FOR WITHDRAWAL ‐not described CO‐INTERVENTION: ‐none permitted |
|
| Outcomes | ANALYSIS PER PROTOCOL
(not by ITT) OUTCOMES ‐reported at 16 weeks PULMONARY FUNCTION TEST ‐change from baseline in FVC ‐change from baseline in FEV1 ‐change from baseline in PC20 SYMPTOM SCORES ‐not reported FUNCTIONAL STATUS ‐Change in AQOL (Asthma Quality of Life Questionnaire ‐ Juniper) in each of 4 domains ‐Asthma exacerbations (defined as requiring systemic steroids or hospital admission or ED treatment for worsening asthma or decrease in morning PEF >25% INFLAMMATORY MEDIATORS ‐not reported ADVERSE EVENTS ‐not mentioned WITHDRAWALS ‐reported Primary outcome not specified |
|
| Notes | ‐Full‐text publication Funded by University ‐confirmation of methodology and data extraction: obtained by Graziono Riccioni, Oct 2003 ‐confirmation of methodology and data obtained USER‐DEFINED ORDER: 800 (daily dose of BDP‐equivalent=800 mcg ) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Study investigators aware as to treatment group assignment (Cochrane Grade C) |
Ringdal 1999.
| Methods | DESIGN
‐parallel‐group
‐multicentre
‐3‐arm study
(1) Z40 + ICS
(2) Z160 + ICS
(3) 2 x ICS ALLOCATION ‐Random ‐Computer generated random numbers ‐sealed envelopes containing allocation BLINDING ‐double‐blind ‐double‐dummy ‐identical placebo WITHDRAWAL/DROPOUT ‐described JADAD's Quality Score = 5 ‐Confirmation of methodology obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS Randomised N = 440 Z40+ICS = 148 Z160+ICS = 146 2 x ICS = 146 WITHDRAWALS Z40+ICS = 10% Z160+ICS = 12% 2 x ICS = 6% AGE: 12 to 70 years old Z40+ICS: 41.3 ± 14.36 years Z160 + ICS: 40.1 ± 13.46 years 2 x ICS: 42.2 ± 14.54 years GENDER (% male): Z40 + ICS: 49% Z160 + ICS: 48% 2 x ICS: 50% SEVERITY: mild‐ moderate BASELINE FEV1 (% Pred) Z40 + ICS: 84.1 ± 12.53 % Z160 + ICS: 85.0 ± 14.25 % 2 x ICS: 85.1 ± 15.02 % ALLERGEN TRIGGERS: not described ASTHMA DURATION ‐not described MEAN ICS DOSE AT ENTRY (mcg/day) Z40 + ICS:432 ± 63 Z160 + ICS:424 ± 63 2 x ICS:426 ± 54 ELIGIBILITY CRITERIA ‐treated with either BDP, BUD or FP and beta2‐agonist prn ‐FEV1 >= 60 at screening ‐ >= 15% improvement in clinic FEV1 or PEF in response to dose =< 400ug albuterol at screening ‐total asthma symptom score >= 10 (0 to 3 scale recorded daily) in the last 7days of screening period |
|
| Interventions | PROTOCOL:
AL + ICS vs DOUBLE dose ICS DURATION: ‐Run‐in Period: 2 week (fixed) ‐Dose optimisation period: none Intervention Period: 12 weeks TEST GROUP Z40 + ICS: Zafirlukast 20 mg bid + BDP 400 to 500 ug/day TEST GROUP ‐Z160 + ICS: Zafirlukast 80 mg bid + BDP 400 to 500 ug/day CONTROL GROUP 2 x ICS: BDP 800 to 1000 ug/day DEVICE ‐metered‐dose aerosol inhaler ‐CO‐TREATMENT: none reported |
|
| Outcomes | PER PROTOCOL
(PP) ANALYSES‐ ‐some Intention ‐to‐ treat (ITT) analyses available
‐outcomes used at 6 and 12 weeks PULMONARY FUNCTION TESTS (PP) ‐**Change in morning PEFR (L/min) ‐Change in evening PEFR SYMPTOM SCORES (PP) ‐Change in mean symptom scores ‐Change in night‐time awakenings per week FUNCTIONAL STATUS ‐Change in mean daily use of beta2‐ agonists (PP) ‐St‐Georges' QOL questionnaire (ITT) INFLAMMATORY MARKERS: ‐not reported ADVERSE EFFECTS ‐elevated liver enzymes, headache, nausea, death WITHDRAWALS ‐reported (** denotes primary outcomes) |
|
| Notes | ‐Abs (1999) and poster and unpublished report provided by Astra‐Zeneca (Oct 2000) ‐Funded by Astra‐Zeneca ‐Confirmation of methodology and data extraction graciously eceived from M. Christopher Miller and Ms. Susan Shaffer, Astra‐Zeneca, Oct 2000 ‐User‐defined order: 45 (mean intervention ICS dose of 450 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Shingo 2001.
| Methods | DESIGN
‐parallel‐group study
‐multicentre ALLOCATION ‐Random ‐computer‐generated ‐assignment by numbered coded solutions supplied by Merck BLINDING ‐double‐blind ‐identical placebo WITHDRAWAL/DROPOUT ‐described JADAD's Quality Score =5 Methodology confirmed |
|
| Participants | WELL‐CONTROLLED PARTICIPANTS N = 22 patients montelukast: 10 placebo: 12 WITHDRAWALS: Montelukast: 10% Placebo: 8% AGE (Mean, SD): Montelukast: 41.0 ± 11.03 years Placebo: 37.00 ± 8.64 years GENDER(% male): Montelukast: 60% Placebo: 25% BASELINE % PRED FEV1 mean (SD): Montelukast:84.48± 8.68 % Placebo: 84.68 ± 8.4 % ATOPY: not described BASELINE DOSE OF ICS: 793 beclo‐equivalent:1523 (536) mcg/day or 761 mcg/day of BPD‐equivalent montelukast: 1600 mcg/day or 800 mcg/day of BDP‐equivalent placebo: 1350 mcg/day or 675 mcg/day of BDP equivalent ELIGIBILITY CRITERIA ‐non‐smoking patients ‐age 15 to 70 years ‐Stable asthma ‐Baseline FEV1>= 75% of predicted ‐improvement in FEV1>= 15% after inhaled beta2‐agonist ‐daytime symptom score <= 7 averaged over the run‐in period ‐stable doses of inhaled glucocorticoids for >= 21 days, namely BDP (600 to 1600 mcg/day), flunisolide (1000 to 2000 mcg/day), or triamcinolone (1200 to 3200 mcg/day) Exclusion criteria: not described |
|
| Interventions | PROTOCOL
AL + ICS vs same dose
ICS (TAPERING ICS dose) Duration ‐Run‐in Period: 7 to 10 days ‐Intervention Period : 8 weeks TEST GROUP ‐Montelukast 10 mg qd p.o. ‐Inhaled glucocorticoids: dose (median: 761 mcg/day of BDP equivalent) CONTROL GROUP ‐Placebo die ‐Inhaled glucocorticoids: dose (median: 675 mcg/day of BDP equivalent) DEVICE ‐not specified CO‐INTERVENTION: theophylline (? %) of patients |
|
| Outcomes | INTENTION‐TO‐TREAT ANALYSIS OUTCOMES REPORTED AT 8 WEEKS PULMONARY FUNCTION TESTS (measured but not reported) ‐% Change from baseline FEV1 ‐Change in am and pm PEFR (L/min) averaged over ? weeks ‐ diurnal PEFR variation FUNCTIONAL STATUS (measured but not reported) ‐Use of rescue beta2‐agonist (puffs/days) ‐Change in daytime symptom score (0‐6) ‐night‐time awakening ‐exacerbations requiring systemic steroids or hospital admission (reported upon request) ICS DOSE REDUCTION: ‐Number of patients tapered off ICS (mcg) INFLAMMATORY MARKERS: ‐not reported ADVERSE EVENTS: reported upon request WITHDRAWALS: reported upon request Primary outcome: change in baseline dose of ICS |
|
| Notes | ‐Full‐text publication and unpublished data ‐Funded by Merck ‐Confirmation of methodology and data extraction graciously received from T.F Reiss and G.P. Noonan, Merck Frosst, USA, June 2001 user‐defined order: 80 (mean intervention ICS dose of 800 mcg/day of BDP‐equivalent X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Simons 2001.
| Methods | DESIGN
‐Cross‐over study ALLOCATION ‐Random ‐Computer‐generated random number ‐Assignment by numbered coded solutions supplied by Merck BLINDING ‐triple‐blind ‐identical placebo WITHDRAWAL/DROPOUT ‐described but not by group/period JADAD's Quality Score =5 ‐Methodology confirmed |
|
| Participants | SYMPTOMATIC PARTICIPANTS N = 279 children RANDOMISED: PERIOD 2: Montelukast: 146 Placebo: 133 PERIOD 3: Montelukast: 129 Placebo: 137 WITHDRAWALS: Montelukast: 3% Placebo: 2% AGE (Mean, SD):10.4 ± 2.2 years (range: 5 to 15 years GENDER(% male): 67 Baseline mean FEV1 % Pred (SD) = 77.7 (10.6) ATOPY: 72% allergic rhinitis ELIGIBILITY CRITERIA ‐Age: 6 to 14 years ‐persistent asthma ‐treatment with inhaled glucocorticoid for at least 6 weeks at 200 to 800 mcg/day of BUDesonide, beclomethasone, triamcinolone, flunisolide or 100 to 500 mcg/day of fluticasone ‐during run‐in on 200 mcg bid of BUDesonide: FEV1 between 60% to 85% of predicted; >= 12% improvement in FEV1 after beta2‐agonist, beta2‐agonist use >= 2 puffs/day Exclusion criteria: not described |
|
| Interventions | PROTOCOL
AL + ICS vs ICS (same dose) Duration ‐Run‐in Period 1: 2 weeks ‐Intervention Period 2: 4 weeks Period 3: 4 weeks No washout period TEST GROUP ‐Montelukast 5 mg qd p.o. ‐BUDesonide 200 mcg bid CONTROL GROUP ‐BUDesonide 200 mcg bid ‐Placebo die DEVICE ‐Turbuhaler CO‐INTERVENTION: not reported |
|
| Outcomes | INTENTION‐TO‐TREAT ANALYSIS
(adjusted for period effect) PULMONARY FUNCTION TESTS ‐% Change from baseline FEV1 at 4 weeks ‐Change in am and pm PEFR (L/min) averaged over 2 weeks ‐diurnal PEFR variation? FUNCTIONAL STATUS ‐Use of rescue beta2‐agonist (puffs/days) ‐Change in quality of life ‐Physician and parents global assessments ‐exacerbations days ‐Asthma exacerbations and attack rates (unscheduled visit to a physician, or Ed or admission to hospital or treatment with oral glucocorticoids) ‐Patients with exacerbations requiring systemic steroids (provided upon request) ADVERSE EVENTS: reported WITHDRAWALS: not reported |
|
| Notes | ‐Full Text 2001 and unpublished data ‐Funded by Merck ‐Confirmation of methodology and data extraction (published and unpublished data) graciously obtained from T.F Reiss and G.P. Noonan, Merck Frosst, USA, June 2001 User‐defined order = 40 (mean intervention ICS dose of 400 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Tamaoki 1997.
| Methods | DESIGN
‐parallel‐group
‐multicentre ALLOCATION ‐Random ‐blocks of four at each center BLINDING ‐double‐blind ‐placebo‐controlled ‐identical placebo WITHDRAWAL/DROPOUTS ‐described JADAD's Quality Score = 4 Confirmation of methodology not obtained |
|
| Participants | WELL CONTROLLED PARTICIPANTS‐ SUDDEN ICS DOSE REDUCTION ‐RANDOMISED N = 79 Pranlukast = 43 Control = 40 WITHDRAWALS: Pranlukast = 2% Control = 8% GENDER: 43 % male AGE Pranlukast: 49 ± 3 years (SEM) Control: 47 ± 3 years SEVERITY: not described BASELINE FEV1 (% Pred) Pranlukast: 79.1 ±2.6 (SEM) Control: 81.6 ± 2.3 (SEM) ALLERGEN TRIGGERS: not described ASTHMA DURATION: Pranlukast: 11.3 ± 4.5 years (SEM) years Control: 10.6 ± 3.9 years ELIGIBILITY CRITERIA ‐Age >= 21 years old ‐Am PEF > 70%, or FEV1 > 70% Pred ‐< 10 asthma symptom score during previous 2 weeks ‐no systemic steroid course during previous 8 weeks, or < 4 short courses during past year ‐symptoms well controlled on daily dose of inhaled beclomethasone >= 1500 mcg for at least 6 weeks |
|
| Interventions | PROTOCOL:
AL + ICS vs SAME dose ICS DURATION: ‐Run‐in Period: 2 weeks ‐Dose Optimisation period: none (sudden decrease in ICS at study onset) ‐Intervention Period: 6 weeks TEST GROUP ‐Pranlukast 450 mg bid p.o. + Beclomethasone dipropionate 1/2 of usual dose (? 750 ug/day) CONTROL GROUP ‐Placebo + Beclomethasone dipropionate 1/2 of usual dose(? 750 ug/day) DEVICE ‐metered‐dose aerosol inhaler ‐CO‐TREATMENT: ‐Oral beta2‐agonist (Pranlukast: 93%/Control:97%) ‐Oral theophylline: (Pranlukast:93%/Control:91%) ‐Inhaled anticholinergics (Pranlukast:50%/control:51%) |
|
| Outcomes | ANALYSES (intention‐to‐ treat not specified)
‐used at 6 weeks ‐PULMONARY FUNCTION TESTS ‐**Change from baseline FEV1 ‐Change from baseline Am PEF ‐Change from baseline Pm PEF ‐Change from baseline mean diurnal variation of PEF SYMPTOM SCORES ‐Change from baseline daytime asthma symptoms (episodes/week) ‐Change from baseline nighttime asthma symptoms (episodes/week) FUNCTIONAL STATUS ‐Change from baseline daytime use of B2‐agonists (puffs/weeks) ‐Change from baseline nighttime use of B2‐agonists (puffs/week) ‐Change in night wakings (episodes/week) ‐Number of patients experiencing exacerbations requiring systemic steroids INFLAMMATORY MARKERS ‐Change from baseline serum ECP (ug/L) ‐Change from baseline exhaled NO (ppb) ADVERSE EFFECTS ‐not reported WITHDRAWALS ‐reported (** denotes primary outcomes) |
|
| Notes | ‐Full Text (1997) ‐Funded by Japanese Ministry of Educ, Sci, & Culture and Glaxo/ONO? ‐Request for confirmation of methodology and data extraction (sent to Tamaoki 28 May 1999) ‐No reply as of Sept 2001 ‐User‐defined order: 75 (mean intervention ICS dose of 750 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Tohda 2002.
| Methods | DESIGN
‐parallel‐group
‐multicentre (16 study sites) ALLOCATION ‐Computer generated random allocation ‐Mode of allocation (not described) BLINDING ‐double‐blind ‐placebo‐controlled ‐identical placebo WITHDRAWL/DROPOUTS ‐described JADAD's Quality score = 5 Confirmation of methodology confirmed |
|
| Participants | WELL‐CONTROLLED PARTICIPANTS RANDOMISED N = 191 ‐Montelukast = 93 ‐Control = 98 WITHDRAWALS: ‐Montelukast = 14 (15%) ‐Control = 20 (20%) AGE (years): ‐Montelukast: 50.1 ± 14.5 (SD) ‐Control: 53.0 ± 13.2 (SD) GENDER (% male): ‐Montelukast: 58.3% ‐Control: 58.3% SEVERITY: ‐moderate‐to‐severe bronchial asthma BASELINE PREDICTED FEV1 (%): ‐Montelukast: 87.4 ± 18.4 (SD) ‐Control: 85.6 ± 24.8 (SD) ALLERGEN TRIGGERS ‐not reported ASTHMA DURATION ‐montelukast: < 10 years = 57 (67.9); >10 years = 27 (32.1) ‐ control: < 10 years = 56 (66.7); > 10 years = 28 (33.3) ELIGIBILITY CRITERIA ‐taking a dose of ICS 800‐1600 ug/day ‐PEFR >= 80% of patients best or predicted ‐diurnal variation PEFR of no more than 20% ‐asthma symptom score of no more than 5 points/week EXCLUSION CRITERIA ‐ use of following medication one month prior to run‐in period ‐ anti‐allergic drugs (disodium cromoglycate, ketotifen or pranlukast) ‐ oral corticosteroids at the start of the run‐in period ‐long‐acting corticosteroids (methylprednisolone acetate or triamcinolone acetonide) |
|
| Interventions | PROTOCOL:
AL + ICS vs same dose
ICS (TAPERING ICS dose) DURATION ‐4 week run‐in period ‐24 week treatment period with ICS titrated at weeks 8 and 16 TEST GROUP ‐Montelukast 10 mg film‐coated tablet once daily + BDP 800‐1600 mcg daily CONTROL GROUP ‐Placebo + + BDP 800‐1600 mcg daily DEVICE ‐ not reported CO‐TREATMENT: ‐ bronchodilators : xanthine derivatives, anticholinergics ‐antibiotics, antitussive and expectorants, chinese medicine, desensitization therapy CRITERIA FOR TAPERING ‐ mean PEFR observed during previous 2 weeks in not less than 90% of value in run‐in ‐ weekly mean symptom score during the previous 2 weeks is not 3 points or more higher than score at run‐in period ‐ mean inhaled B‐agonist use during the previous 2 weeks is less than twice use at run‐in period CRITERIA FOR MAINTAINING ICS DOSE ‐ 2 out of the 3 criteria for dose tapering mentioned above CRITERIA FOR INCREASING ICS DOSE ‐ 1 or none of the criteria for dose tapering mentioned above MINIMAL DOSE OF ICS ALLOWED: ‐ no minimal dose required |
|
| Outcomes | EFFICACY ANALYSIS ‐ NO INTENTION TO TREAT ANALYSIS
‐outcomes available at 4, 8, 16 and 24 weeks PULMONARY FUNCTION TESTS ‐% change in PEFR ‐% change in FVC ‐% change in FEV1 SYMPTOM SCORES ‐% change in breathlessness and wheezing (range 0‐9) ‐ % change in therapy score (range not reported, based on Japanese Society of Allergology) ‐ % change in asthmatic score (combining symptom score with therapy score) FUNCTIONAL STATUS ‐ not reported ICS DOSE REDUCTION ‐ INFLAMATORY MARKERS ‐ not reported ADVERSE EFFECTS ‐ alopecia areata, headache, bitter taste, stomach ache, heartburn and asthma in the montelukast group, and toxicoderma, papules, headache, diarrhoea, constipation, retching, palpitations and nocturia in the placebo group ****data not reported**** WITHDRAWLS ‐ reported |
|
| Notes | ‐Full text (2002) and unpublished data ‐Funded by Banyu Pharmaceutical Limited (makers of montelukast) ‐Confirmation of methodology and data extraction graciously obtained from Takaaki Ishine, PhD, Banyu Pharmaceutical Co, LTD, August 2003 ‐user‐defined order: 92.mcg/day (mean ICS dose of 925 mcg/ day x 0.1 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Tomari 2001.
| Methods | DESIGN
‐parallel‐group ALLOCATION ‐random: not specified ‐Mode of allocation (not described) BLINDING ‐not described WITHDRAWALS/ DROPOUTS ‐described JADAD's Quality Score = 2 Confirmation of methodology not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED ‐N=41 ‐BDP + pranlukast = 21 ‐2 X BDP = 20 WITHDRAWALS ‐none AGE years (SEM) ‐BDP + pranlukast = 53.7 (3.1) (SEM) ‐2 X BDP = 56.1 (3.2) (SEM) GENDER (% male) ‐BDP + pranlukast = 38% ‐2 X BDP = 45% SEVERITY ‐ moderate asthma BASELINE % PREDICTED FEV1 ‐not documented BASELINE % PREDICTED PEF ‐BDP + pranlukast = 72.1 ± 2.8 (SEM) ‐2 X BDP =69.5 ± 3.1 (SEM) ALLERGEN TRIGGERS ‐not reported ASTHMA DURATION years (SEM) ‐BDP + pranlukast = 14.8 (2.7) (SEM) ‐2 X BDP = 14.1 (2.7) (SEM) ELIGIBILITY CRITERIA ‐diagnosis of asthma ‐treated with 800 mcg/day of BDP ‐PEFR < 80% predicted EXCLUSION CRITERIA ‐pulmonary or bronchial diseases for 2 weeks before study |
|
| Interventions | PROTOCOL:
AL + ICS vs DOUBLE dose ICS DURATION ‐2 week run‐in period ‐16 week treatment period TEST GROUP ‐BDP 800 mcg/day + 450 mg/day pranlukast CONTROL GROUP ‐2 X BDP (1600 mcg/day) DEVICE ‐not specified CO‐TREATMENT ‐theophylline and B2‐agonists if previously in use |
|
| Outcomes | INTENTION‐TO‐TREAT ANALYSES
‐outcome reported at 16 weeks PULMONARY TESTS ‐pre and post treatment am PEFR (%) ‐pre and post treatment in daily variability of PEFR (%) SYMPTOM SCORES ‐pre and post treatment symptom score (sum of 7 symptoms scored from 0‐3) FUNCTIONAL STATUS ‐pre and post treatment B2‐agonists use/week INFLAMATORY MARKERS ‐not reported ADVERSE EFFECTS ‐reported WITHDRAWALS ‐no withdrawals * Primary outcome not mentioned |
|
| Notes | ‐Full text 2001 ‐Funding not specified (information requested) ‐Confirmation of methodology and data extraction not obtained. ‐User‐defined order: 80 (mean intervention ICS dose of 800 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Tomita 1999.
| Methods | DESIGN
‐parallel‐group ALLOCATION ‐Random (method of randomisation not described) ‐Mode of allocation (not described) BLINDING ‐none ‐no placebo WITHDRAWALS/DROP‐OUTS ‐not reported JADAD's Quality score = 1 Confirmation of methodology not obtained |
|
| Participants | WELL‐CONTROLLED PARTICIPANTS‐ SUDDEN DECREASE OF ICS DOSE RANDOMISED N = 41 pranlukast = 24 control = 17 WITHDRAWALS Pranlukast:? Placebo:? AGE Pranlukast: 56.7 ± 18.0 years (SD) Control: 42.2 ± 16.9 years GENDER (% male): Pranlukast: 62% Control: 59% SEVERITY: mild‐moderate asthma BASELINE FEV1 (% predicted FEV1/HT): Pranlukast: 90.3 ± 13.4 (SD) Control: 86.3 ± 19.0 ALLERGEN TRIGGERS (% atopic): Pranlukast: 67%/ Control: 65% ASTHMA DURATION: not specified ELIGIBILITY CRITERIA: Adults with stable asthma well controlled on 800 mcg/day of beclomethasone for 3 months EXCLUSION CRITERIA: ‐non described |
|
| Interventions | PROTOCOL:
AL + ICS vs SAME dose ICS DURATION ‐Run‐in Period (4 weeks) ‐Dose Optimisation Period: NONE ‐Intervention period ( 8 weeks) TEST GROUP: ‐Pranlukast 450 mg die p.o. + ICS 400 mcg/day of beclomethasone CONTROL GROUP: ‐400 mcg/day of beclomethasone ‐no placebo DEVICE: not described ‐CO‐TREATMENT: ? 2 patients on oral steroids (to be confirmed) |
|
| Outcomes | NO INTENTION‐TO‐TREAT ANALYSES
‐outcome used at 8 weeks PULMONARY FUNCTION TESTS (reported as cross‐sectional values not as change from baseline) ‐FEV1 ‐V50 ‐V25 ‐morning % PEF ‐evening % PEF ‐diurnal PEF variation SYMPTOM SCORES (reported as cross‐sectional values not as change from baseline) ‐symptom score ‐therapeutic score (both defined by the Japanese Society of Allergology) FUNCTIONAL STATUS ‐not reported (?) INFLAMMATORY MARKERS ‐not reported (?) ADVERSE EFFECTS ‐not reported (?) WITHDRAWALS ‐not reported (?) (unclear primary outcome) |
|
| Notes | ‐Full‐text (1999) Japanese paper with English abstract ‐Partial translation obtained from Cochrane Collaborator ‐Funding status (unknown) ‐Confirmation of methodology and data extraction (requested Jan 2001: pending) ‐User‐defined order: 40 (mean intervention ICS dose of 400 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Vaquerizo 2003.
| Methods | DESIGN
‐parallel‐group
‐multi‐centre (80 sites in Spain) ALLOCATION ‐random ‐central computer generated schedule ‐treatment assignments (1:1) stratified according to site and 3 BUD dose levels (400‐800 mcg/day ; 801‐1200 mcg/day ; and 1201‐1600 mcg/day) ‐Mode of treatment allocation not described WITHDRAWALS/ DROPOUTS ‐described JADAD's quality score = 5 Confirmation of methodology: requested but not obtained |
|
| Participants | WELL‐CONTROLLED PARTICIPANTS RANDOMISED ‐N = 639 ‐BUD + M = 326 ‐BUD + placebo = 313 WITHDRAWALS ‐BUD + M = 34 (10%) ‐BUD + placebo = 32 (10%) AGE years (SD) ‐range 18‐79 ‐BUD + M = 42 (15) years ‐BUD + placebo = 44 (16) years GENDER (% male) ‐BUD + M = 62% ‐BUD + placebo = 61% SEVERITY ‐mild to moderate asthma BASELINE % PREDICTED FEV1 (SD) ‐BUD + M = 81 (19) ‐BUD + placebo = 81 (21) ALLERGEN TRIGGERS ‐not described ELIGIBILITY CRITERIA ‐non‐smokers ‐aged 18‐70 (*age of participants outside of range*) ‐treated with 400‐1600mcg/day BUD for at least 8 weeks ‐FEV1 >= 55% predicted ‐reversible airway obstruction (increase >= 12% of baseline FEV1) ‐minimum total daytime asthma symptom score of 64 during the 2 week run‐in period ‐using a mean of at least 1 puff/day of B2‐agonist during run‐in period ‐negative pregnancy test (urine B‐human chorionic gonadotropin) ‐use of contraceptive 2 weeks prior to treatment and 2 weeks after study EXCLUSION: ‐not described |
|
| Interventions | PROTOCOL
‐AL + ICS vs. SAME dose ICS + placebo DURATION ‐2 week run‐in period ‐16 week treatment period TEST GROUP ‐montelukast (10mg/day) + BUD (400‐1600 mcg/day) CONTROL GROUP ‐placebo + BUD (400‐1600 mcg/day) DEVICE ‐Turbuhaler CO‐TREATMENT ‐use of B2‐agonist as needed |
|
| Outcomes | INTENTION‐TO‐TREAT ANALYSES PULMONARY FUNCTION TESTS ‐% change from baseline at end point ‐% change in am and pm PEFR (L/min) ‐% change in FEV1 (L) SYMPTOM SCORE ‐% change from baseline in daily daytime symptom score (range not specified) FUNCTIONAL STATUS ‐*% of asthma exacerbation days ‐% change in mean daily use of B2‐agonist (puffs/day) ‐change in nocturnal awakenings ‐change in asthma specific quality of life (32 questions, range 0‐6) INFLAMATORY MARKERS ‐none documented ADVERSE EFFECTS ‐described ‐influenza, headache, URI, worsening asthma, epigastric pain, urinary tract infections, rhinitis, pharyngitis, bronchitis WITHDRAWALS ‐reported * Primary outcome |
|
| Notes | ‐Full text (2003) ‐Funded by Merck Sharp and Dohme Spain ‐Confirmation of methodology and data extraction requested:not obtained ‐user‐defined order: 80 mcg/day (mean ICS dose about 800 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Virchow 2000.
| Methods | DESIGN
‐parallel‐group
‐multicentre (82 centres) ‐ALLOCATION ‐Random ‐Computer‐ generated BLINDING ‐double‐blind ‐placebo controlled ‐identical placebo ‐number coded solutions/boxes WITHDRAWAL/DROPOUT ‐described JADAD's Quality Score = 5 ‐Confirmation of methodology obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED N = 368 Zafirlukast = 180 Control = 188 WITHDRAWALS: Zafirlukast = 18% Control = 18% AGE: Zafirlukast: 49.2 ±12.9 (SD) years Control: 47.4 ± 12.6 years GENDER (% male) Zafirlukast : 48% Control: 53% SEVERITY: moderate/severe asthma BASELINE FEV1 (% pred): Zafirlukast: 64.3 ± 7.5 (SD) Control: 63.5 ±8.0 ALLERGEN TRIGGERS (%) Zafirulast: 41% Control:51% DURATION OF ASTHMA: Zafirlukast: 15.7 ± 12.61(SD) years Control: 17.5 ± 13.63 years ICS DOSE: Zafirlukast: 1598 ± 381 mcg/day (SD) Control: 1650 ± 456 mcg/day of beclomethasone‐equivalent ELIGIBILITY CRITERIA ‐50 to 75% FEV1 Pred ‐ICS dose >= 1200 mcg/day beclomethasone‐equivalent ‐mean FEV1/PEF reversibility >= 15% after salbutamol ‐symptomatic asthma ‐no smoking in preceding 6 months |
|
| Interventions | PROTOCOL:
AL + ICS vs SAME dose ICS DURATION: ‐Run‐in Period: not described ‐Dose optimisation period: NONE ‐Intervention Period: 6 weeks TEST GROUP ‐Zafirlukast 80 mg bid p.o. + Beclomethasone >= 1200 ug/day CONTROL GROUP ‐Placebo + Beclomethasone >= 1200 ug/day DEVICE ‐various devices used ‐CO‐TREATMENT: none reported |
|
| Outcomes | INTENTION‐TO‐TTREAT (ITT) ANALYSIS
(some per‐protocol (PP) analyses)
‐results used at 6 weeks PULMONARY FUNCTION TESTS ‐ ITT ‐Change from baseline FEV1 ‐**Change from baseline Am PEF FUNCTIONAL STATUS ‐ITT ‐Change in night wakings (episodes/week)‐Number of patients with exacerbations requiring additional treatment ‐Number of days off work ‐Change in use of rescue beta2‐agonist (puffs/day) INFLAMMATORY MARKERS ‐not reported ADVERSE EFFECTS ‐elevated liver enzymes, headache, nausea, death, etc. WITHDRAWALS ‐reported (** denotes primary outcomes) |
|
| Notes | ‐Full Text (2000) ‐Funded by Astra‐Zeneca ‐Confirmation of methodology and data extraction received from M. Christopher Miller and Ms. Susan Shaffer, Astra‐Zeneca, Oct 2000 ‐User‐defined order: 165 (mean intervention ICS dose of 1650 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Study investigators unaware as to order of treatment group assignment (Cochrane Grade A) |
Wada 1999.
| Methods | DESIGN:
‐parallel group ALLOCATION ‐Random ‐Method of randomisation not described ‐Mode of treatment allocation not described BLINDING ‐none WITHDRAWALS/DROPOUTS ‐Described JADAD's Quality Score=1 CONFIRMATION OF METHODOLOGY: ‐not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED N = 80 Pranlukast = 40 Control = 40 WITHDRAWAL Pranlukast: 8% Control: 18% AGE: pranlukast 50.8 years ±2.4 (SEM) Control: 48.4 years ± 2.2 (SEM) GENDER (% male): Pranlukast: 59% Control: 40% SEVERITY: moderate asthma BASELINE FEV1 (L): Pranlukast: 1.83 L Control: not reported ALLERGEN TRIGGERS: Pranlukast: 54% Control: 51% BASELINE BDP DOSE: Pranlukast: 1048.6 ± 39.1 mcg/day Control: 1127.3 ± 53.5 mcg/day ELIGIBILITY CRITERIA Adults not well controlled on: ‐ 800 mcg/day of beclomethasone ± slow‐release theophylline for 3 months ‐FEV1 > 50% of predicted ‐Reversibility in FEV1 > 15% post B2‐agonists on 800 mcg/day of beclomethasone for 3 months EXCLUSION CRITERIA: ‐no systemic steroids and no upper respiratory tract infection in past 4 weeks |
|
| Interventions | PROTOCOL:
AL + ICS vs SAME dose ICS DURATION: ‐Run‐in period: 2 weeks to establish baseline ‐Dose optimisation period: NONE ‐Intervention period: 4 weeks TEST GROUP: ‐Pranlukast 225 mg bid po + 800‐1200 mcg/day of beclomethasone CONTROL GROUP: ‐800‐1200 mcg/day of beclomethasone ‐no placebo DEVICE: ‐not described CO‐TREATMENT: unspecified % on slow‐release theophylline |
|
| Outcomes | ANALYSIS (intention‐to‐treat not specified)
‐outcomes used at 4 weeks PULMONARY FUNCTION TESTS ‐FEV1 (L) ‐morning PEF (L/min) ‐V25/HT (L/min) SYMPTOMS SCORES (reported as cross‐sectional values, not change from baseline) ‐symptom scores averaged over 2 weeks (scale 0‐9) FUNCTIONAL STATUS (reported as cross‐sectional values, not change from baseline) ‐use of rescue Beta2‐agonist (puffs/week) ‐exacerbations requiring systemic steroids INFLAMMATORY MARKERS (reported as cross‐sectional values, not change from baseline) ‐sputum eosinophils(%) ‐serum eosinophils (/uL) ‐serum ECP(ng/uL) ‐serum total IgE ‐serum Der f‐specific IgE ADVERSE EFFECTS ‐not reported WITHDRAWALS ‐reported Primary outcome not specified |
|
| Notes | ‐Full‐text (2000) paper ‐funding status (not reported) ‐confirmation of methodology and data extraction (pending) ‐User‐defined order: 100 (mean intervention ICS dose of 1000 mcg/ day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Yildirim 2001.
| Methods | DESIGN
‐parallel‐group ALLOCATION ‐Random ‐Methods of randomisation: not reported ‐means of assignment: not reported BLINDING ‐not reported WITHDRAWAL/DROPOUT ‐not described JADAD's Quality Score = 1 ‐Confirmation of methodology not obtained |
|
| Participants | SYMPTOMATIC PARTICIPANTS RANDOMISED ‐ N= 30 ‐M10 + ICS = NR ‐2 X ICS= NR WITHDRAWALS ‐M10 + ICS =NR ‐2 X ICS= NR GENDER (% male) ‐M10 + ICS = NR ‐2 X ICS =NR AGE (SD) years ‐M10 + ICS = NR ‐2 X ICS= NR SEVERITY ‐not specified BASELINE PREDICTED FEV1 % (SD) ‐M10 + ICS = NR ‐2 X ICS = NR ALLERGEN TRIGGERS ‐not described ASTHMA DURATION (SD) years ‐ M10 + ICS = NR ‐2 X ICS = NR ELIGIBILITY CRITERIA ‐not reported EXCLUSION CRITERIA ‐not reported |
|
| Interventions | PROTOCOL:
AL + ICS vs DOUBLE dose ICS DURATION ‐Run‐in Period: not described ‐Intervention period: 12 weeks TEST GROUP ‐Montelulast 10 mg die p.o. + BUD 400 mcg/day CONTROL GROUP (2 X ICS) ‐BDP 800 mcg/day DEVICE ‐not described ‐CO‐TREATMENT: none allowed |
|
| Outcomes | INTENTION‐TO‐TREAT ANALYSIS
‐not described
‐outcomes reported at 12 weeks PULMONARY FUNCTION TESTS ‐Change from baseline AM PEFR ‐Change from baseline in FEV1 SYMPTOM SCORES ‐Change from baseline daytime symptom scores ‐Change from baseline nighttime symptoms FUNCTIONAL STATUS ‐Change from baseline mean daily B2‐agonist use INFLAMMATORY MARKERS ‐serum eosinophil counts ADVERSE EFFECTS ‐not reported WITHDRAWALS ‐not reported (* denotes primary outcomes) |
|
| Notes | ‐Abstract 2001 ‐Funding source: not reported ‐Confirmation of methodology and data extraction: not obtained. User‐defined order: 40 (mean intervention (400) dose in mcg/day X 0.1) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available (Cochrane Grade B) |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Altman 1998 | No co‐treatment with inhaled corticosteroids |
| Barnes 1996 | Inconsistent co‐treatment with inhaled corticosteroids, and no tapering attempt Outcomes solely the result of provocation |
| Barnes 1997 | No co‐treatment with inhaled corticosteroids |
| Basyigit 2001 | No co‐treatment with inhaled corticosteroids |
| Baumgartner 1999 | No co‐treatment with inhaled corticosteroids |
| Becker 2000 | No co‐treatment with inhaled corticosteroids |
| Bisgaard 1999 | No systematic co‐treatment with inhaled corticosteroids Intervention administered for < 4 weeks Outcomes were solely the results of provocation tests |
| Bisgaard 2000 | No co‐treatment with inhaled corticosteroids Outcomes did not reflect chronic asthma control Intervention administered for < 4 weeks |
| Bjermer 2000 | Planned (ongoing) trial Control intervention was not placebo (long‐acting beta2‐agonist) |
| Bjermer 2002 | Use of other non permitted drug |
| Brabson 2002 | No co‐treatment with inhaled corticosteroids |
| Brannan 2001 | No co‐treatment with inhaled corticosteroids Intervention < 4 weeks Control intervention is not placebo (it is an anti‐histamine) |
| Brocks 1996 | Subjects were not asthmatic |
| Bronsky 1997 | Subjects were not asthmatic |
| Bruce 2002 | Outcome measures did not reflect asthma control |
| Busse 1999 | No systematic co‐treatment with inhaled corticosteroids Control intervention was not placebo (it's a long‐acting beta2‐agonist) |
| Cakmak 2000 | Outcome measures did not reflect asthma control |
| Calhoun 1997 | No co‐treatment with inhaled corticosteroids |
| Calhoun 2001 | Use of other non permitted drug |
| Camargo 2002 | Acute asthma setting |
| Capella 2001 | Subjects not asthmatic |
| Chuchalin 2002 | Intervention was not anti‐leukotrienes |
| Clifford 2000 | Duplicate reference |
| Cloud 1989 | No co‐treatment with inhaled corticosteroids |
| Currie 2003 | Duplicate reference |
| Currie 2003 (B) | Use of other non permitted drug |
| Cylly 2003 | Ongoing trial |
| Dahlen 2002 | No consistent co‐treatment with inhaled corticosteroids. Merck Frosst unable to provide subgroup analysis. |
| Daikh 2003 | Subjects were not asthmatics |
| Dempsey 1999 | Intervention administered for < 4 weeks. No consistent co‐treatment with inhaled glucocorticoids |
| Dempsey 2000 | Intervention administered for < 4 weeks (single dose study) |
| Dempsey 2000b | Control intervention was not placebo |
| Dempsey 2001 | No co‐treatment with inhaled glucocorticoids |
| Dempsey 2002 | Control intervention wase not placebo |
| Dessanges 1999 | Intervention administered for < 4 weeks (single dose study) Outcomes did not reflect control of chronic or acute asthma (solely the results of exercise challenge) No consistent co‐treatment with inhaled corticosteroids |
| Diamant 1999 | No co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period Outcomes solely the result of provocation |
| Dicpinigaitis 2002 | Outcome measures did not reflect asthma control |
| Dockhorn 2000 | Intervention administered for < 4 weeks (single dose study) No co‐treatment with inhaled corticosteroids |
| Eliraz 2001 | Tested intervention is not anti‐leukotrienes |
| Faul 2002 | Outcome measures did not reflect asthma control |
| Findlay 1992 | No co‐treatment of inhaled corticosteroids Intervention was administered for less than a four‐week period |
| Fischer 1995 | No co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period Outcomes were solely the result of provocation |
| Fischer 1997 | No co‐treatment with inhaled corticosteroids |
| Fish 1997 | No co‐treatment with inhaled corticosteroids |
| Flynn 2001 | Duplicate Reference |
| Fujimura 1993 | No co‐treatment with inhaled corticosteroids |
| Gaddy 1990 | No co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period, intraveneously |
| Galant 2001 | Duplicate reference |
| Geha 2001 | Tested intervention is not anti‐leukotrienes |
| Georgiou 1997 | No co‐treatment with inhaled corticosteroids Subjects were not asthmatic Intervention was administered for less than a four‐week period |
| Ghiro 2002 | Outcome measures did not reflect asthma control |
| Gold 2001 | Control intervention was not placebo |
| Green 2002b | Intervention was not anti‐leukotrien |
| Grossman 1995 | No co‐treatment with inhaled corticosteroids |
| Grossman 1997 | No co‐treatment with inhaled corticosteroids |
| Haahtela 1994 | Intervention was not anti‐leukotrienes |
| Hamilton 1998 | No co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period Outcomes were solely the result of provocation |
| Hassall 1998 | No co‐treatment with inhaled corticosteroids |
| Hood 1999 | Control subjects not asthmatics (normal subjects) |
| Howland 1994 | No co‐treatment with inhaled corticosteroids |
| Hsieh 1996 | Intervention was not anti‐leukotrienes |
| Hughes 1999 | No co‐treatment with inhaled corticosteroids |
| Hui 1991 | No co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period |
| Israel 1990 | No co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period Outcomes were solely the result of provocation |
| Israel 1992 | No co‐treatment with inhaled corticosteroids |
| Israel 1993 | No co‐treatment with inhaled corticosteroids |
| Israel 1996 | No co‐treatment with inhaled corticosteroids |
| Israel 2002 | No co‐treatment with inhaled corticosteroids |
| Jayaram 2002 | No co‐treatment with inhaled corticosteroids |
| Johnson 1999 | No co‐treatment with inhaled corticosteroids |
| Juniper 1995 | No co‐treatment with inhaled corticosteroids |
| Kalberg 1999 | No co‐treatment with inhaled corticosteroids |
| Kanniess 2002 (B) | No co‐treatment with inhaled corticosteroids |
| Kemp 1995 | No co‐treatment with inhaled corticosteroids |
| Kemp 1997 | No co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period Outcomes were solely the result of provocation |
| Kemp 1998 | No co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period |
| Kemp 1999 | Not a randomised controlled trial (meta‐analysis of RCTs) Subjects not all treated with inhaled corticosteroids |
| Kim 2000 | No co‐treatment with inhaled corticosteroids |
| Kips 1991 | No co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period, intravenously |
| Knorr 1997 | Inconsistent co‐treatment with inhaled corticosteroids, and no tapering attempt |
| Knorr 1998 | Inconsistent co‐treatment with inhaled corticosteroids, and no tapering attempt |
| Knorr 1999 | Control intervention were not placebo |
| Korenblat 1998 | No co‐treatment with inhaled corticosteroids |
| Kuna 1997 | Inconsistent co‐treatment with inhaled corticosteroids, and no tapering attempt |
| Kylstra 1998 | No co‐treatment with inhaled corticosteroids |
| Laitinen 1997 | No co‐treatment with inhaled corticosteroids |
| Leff 1998 | No co‐treatment with inhaled corticosteroids Outcomes solely the result of provocation |
| Leigh 2002 | Tested intervention administrated for less than 4 weeks |
| Leigh 2002 (B) | Use of other non permitted drug |
| Lipworth 1999 | Ongoing trial No co‐treatment with inhaled corticosteroids |
| Lipworth 2000 | Planned (or ongoing) trial Control intervention is not placebo (anti‐histamine) |
| Liu 1996 | No co‐treatment with inhaled corticosteroids |
| Lockey 1995 | No co‐treatment with inhaled corticosteroids |
| Malerba 2002 | No co‐treatment with inhaled corticosteroids |
| Malmstrom 1999 | No co‐treatment with inhaled corticosteroids |
| Margolskee 1991 | No co‐treatment with inhaled corticosteroids |
| Meltzer 2002 | No co‐treatment with inhaled corticosteroids |
| Micheletto 1997 | Interim report of an included eligible trial : Laitinen LA, Zetterstrom O, Holgate ST, Binks SM, Whitney JG. Effects of Accolate (zafirlukast: 20 mg bd) in permitting reduced therapy with inhaled steroids: a multicentre trial in patients with doses of inhaled steroids optimised between 800 and 2000 mcg per day. Allergy 1995: 50 suppl 26:320 (Abs) |
| Minkwitz 1998 | No co‐treatment with inhaled corticosteroids |
| Miyamoto 1999 | No co‐treatment with inhaled corticosteroids |
| Nathan 1998 | No co‐treatment with inhaled corticosteroids |
| Nathan 2001 | No co‐treatment with inhaled corticosteroids |
| Nelson 2001 | Control intervention were not placebo |
| Nishizawa 2002 | Intervention was not anti‐leukotrien |
| Noonan 1998 | No co‐treatment with inhaled corticosteroids |
| Nsouli 2001 | Control group were not placebo |
| O'Shaughnessy 1996 | Subjects were not asthmatic Outcomes were solely the result of provocation |
| Obase 2001 | Subjects received additional non‐permitted drugs (maintenance systemic steroids) |
| Overbeek 2002 | No co‐treatment with inhaled corticosteroids |
| Paterson 1999 | Tested intervention administrated for less than 4 weeks |
| Pearlman 1999 | No co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period Outcomes solely the result of provocation |
| Pearlman 2002 | No co‐treatment with inhaled corticosteroids |
| Pizzichini 1999 | No co‐treatment with inhaled corticosteroids |
| Pullerits 1999 | Subjects non asthmatics (allergic rhinitis) |
| Pullerits 2001 | Subjects were not asthmatics |
| Pullerits 2002 | Subjects were not asthmatics |
| Ramsay 1997 | No co‐treatment with inhaled corticosteroids |
| Ramsay 1998 | No co‐treatment with inhaled corticosteroids |
| Reiss 1996 | Inconsistent co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period |
| Reiss 1997a | Inconsistent co‐treatment with inhaled corticosteroids, and no tapering attempt |
| Reiss 1997b | Inconsistent co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period |
| Reiss 1998a | No co‐treatment with inhaled corticosteroids |
| Reiss 1998b | No co‐treatment with inhaled corticosteroids |
| Reiss 1998c | No co‐treatment with inhaled corticosteroids (Phase II) Not blinded (Phase IV) |
| Riccioni 2002 (B) | No co‐treatment with inhaled corticosteroids |
| Riccioni 2002 (c) | No co‐treatment with inhaled corticosteroids |
| Ringdal 1997 | No co‐treatment with inhaled corticosteroids |
| Robinson 2001 | Treatment for < 4 weeks |
| Rosenhall 2003 | Intervention was not anti‐leukotrienes |
| Sahn 1997 | No co‐treatment with inhaled corticosteroids No measure of efficacy or inflammatory markers. |
| Schwartz 1998 | No co‐treatment with inhaled corticosteroids |
| Sheth 2002 | No co‐treatment with inhaled corticosteroids |
| Skalky 1999 | No co‐treatment with inhaled corticosteroids |
| Smith 1993 | No co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period |
| Smith 1998 | No co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period Outcomes solely the result of provocation |
| Spector 1992 | No co‐treatment with inhaled corticosteroids |
| Spector 1994 | No co‐treatment with inhaled corticosteroids |
| Spector 1995 | No co‐treatment with inhaled corticosteroids |
| Stanford 2002 | No co‐treatment with inhaled corticosteroids |
| Stelmach 2002 | No co‐treatment with inhaled corticosteroids |
| Stelmach 2002 (b) | No co‐treatment with inhaled corticosteroids |
| Storms 2001 | Duplicate reference |
| Suissa 1997 | No co‐treatment with inhaled corticosteroids |
| Svensson 1994 | No co‐treatment with inhaled corticosteroids |
| Tashkin 1998 | No co‐treatment with inhaled corticosteroids |
| Terzano 2001 | Intervention was not anti‐leukotrien |
| Townley 1995 | No co‐treatment with inhaled corticosteroids |
| Tukiainen 2002 | Intervention was not anti‐leukotrien |
| Verhoeven 2001 | Subjects non‐asthmatics |
| Vethanayagam 2002 | Tested intervention administrated for less than 4 weeks |
| Vidal 2001 | No consistent co‐treatment with inhaled corticosteroids Intervention administered for < 4 weeks |
| Volovitz 1999 | Subjects not all treated with inhaled corticosteroids |
| Von Berg 2002 | Intervention was not anti‐leukotrien |
| Wahedna 1991 | Intervention was administered for less than a four‐week period Outcomes solely the result of provocation challenge |
| Weinberg 1998 | No co‐treatment with inhaled corticosteroids |
| Welch 1994 | No co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period |
| Wenzel 1994 | No co‐treatment with inhaled corticosteroids |
| Wenzel 1995 | Control subjects were not asthmatic Intervention was administered for less than a four‐week period |
| Wenzel 1997 | No co‐treatment with inhaled corticosteroids |
| Westbroek 1998 | No co‐treatment with inhaled corticosteroids |
| Westbroek 2000 | Intervention administered for < 4 weeks Subjects not all treated with inhaled corticosteroids |
| Williams 2000 | 3 studies: Study A :No co‐treatment with inhaled corticosteroids Study B: No consistent co‐treatment with inhaled corticosteroids Study C: No consistent co‐treatment with inhaled corticosteroids |
| Wilson 1999 | Ongoing trial Subjects not asthmatics (allergic rhinitis) |
| Wilson 1999 (b) | Control intervention were not placebo |
| Wilson 2001 | Outcome measures did not reflect asthma control |
| Wilson 2001 (c) | Subjects were not asthmatics |
| Wilson 2001a | No co‐treatment with inhaled corticosteroids control intervention is not placebo treatment < 4 weeks |
| Wilson 2001b | Control intervention is not placebo (it's a long‐acting beta2‐agonist) |
| Xiang 2001 | Use of other non permitted drug |
| Yamamoto 1994 | No co‐treatment with inhaled corticosteroids Intervention was administered for less than a four‐week period Outcomes solely the result of provocation |
| Yamauchi 2001 | No co‐treatment with inhaled corticosteroids |
| Yoo 2001 | Inconsistent co‐treatment with inhaled corticosteroids |
| Yoshida 2000 | Tested intervention asministrated for less than 4 weeks |
| Yoshida 2002 | Tested intervention asministrated for less than 4 weeks |
| Zhang 1999 | No co‐treatment with inhaled corticosteroids |
| Zorc 2003 | Intervention was not anti‐leukotrienes |
Contributions of authors
Dr Francine Ducharme conceived the protocol, requested the literature search, identified and contacted the corresponding authors and/or the pharmaceutical companies to solicit their collaboration in this review and in the identification of other possibly relevant trials, created the methodology and data extraction forms, reviewed all citations for relevance with research assistants, reviewed all included trials for methodology and data extraction, corresponded with authors or pharmaceutical companies to verify methodology and data extraction, verified all references, description of studies and data entry, analysed and interpreted results of the meta‐analysis.
Three research assistants participated in some aspects of the review. From November 1999 to February 2000, Giselle Hicks reviewed many citations for relevance, extracted the methodology and data and entered data for several trials.
Miss Ritz Kakuma, supported by The Canadian Cochrane Network, participated in the review from June 2000 to November 2001. She reviewed several included trials for methodology and data extraction, and entered data.
Mr Zachary Schwartz assisted in the August 2003 update. He extracted the methodology and data for the new identified trials, identified missing information for, and completed the table of characteristics of, included studies, and entered the references for all excluded studies with their reason for exclusion. Justin Grondines, data manager, tallied the reasons for exclusion for the literature search.
Sources of support
Internal sources
No sources of support supplied
External sources
Received research assistant support from the Canadian Cochrane Network, Canada.
Francine Ducharme was supported by a senior clinical scientist award from the Fonds de la Santé du Québec, Canada.
Declarations of interest
Francine Ducharme has received travel support, research funds and fees for speaking from Zeneca Pharma Inc. producer of zafirlukast and Merck Frosst Inc, producer of montelukast. She has received some travel support for meeting attendance, research grant and consulting fee from Glaxo Wellcome Inc, producer of some inhaled corticosteroids preparation to which anti‐leukotriene agents have been compared. Zachary Schwartz, Giselle Hicks, and Ritz Kakuma: None declared.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Baba 1999 {published data only}
- Baba K, Hattori T, Sakakibara A, Kobayashi T, Takagi K. The usefulness of pranlukast or seratrodast for step‐down of inhaled corticosteroid therapy in adult chronic asthma. American Journal of Respiratory & Critical Care Medicine 1999;159(3 (Part 2 of 2)):A 626. [Google Scholar]
Bateman 1995 {published and unpublished data}
- Bateman ED, Holgate ST, Binks SM, Tarna IP. A multicentre study to assess the steroid‐sparing potential of Accolate (zafirlukast; 20 mg bd). Allergy 1995;50(Suppl 26):320, Abs. P‐0709. [Google Scholar]
Finn 2000 {published data only}
- Finn AF, Bonuccelli CM, Traxler BM, Beatty SE. Zaifirlukast improves asthma control in children treated with and without inhaled corticosteroids. European Respiratory Journal 2000;16(Supplement 31):307. [Google Scholar]
Green 2002a {published data only}
- Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Wardlaw AJ, Pavord ID. A placebo controlled comparison of formoterol, montelukast or higher dose of inhaled corticosteroids in subjects with symptomatic asthma despite treatment with low dose inhaled corticosteroids. Thorax 2002;57(Supp III):iii11 (S31). [Google Scholar]
Hultquist 2000 {unpublished data only}
- Hultquist C, Domeij W, Kasak V, Laitinen L, O'Neill S. Oxis turbuhaler (formoterol), accolate (zafirlukast) or placebo as add‐on treatment to pulmicort turbuhaler (budesonide) in asthmatic patients on inhaled steroids. Astra‐Zeneca Report #: SD‐004CR‐0216 2000.
Kanniess 2002 {published and unpublished data}
- Kanniess F, Richter K, Janicki S, Schleiss MB, Jorres RA, Magnussen H. Dose reduction of inhaled corticosteroids under concomitant medication with montelukast in patients with asthma. Eur Respir J 2002;20:1080‐1087. [DOI] [PubMed] [Google Scholar]
Laitinen 1995 {published and unpublished data}
- Laitinen LA, Zetterstrom O, Holgate ST, Binks SM, Whitney JG. Effects of Accolate (zafirlukast; 20 mg bd) in permitting reduced therapy with inhaled steroids: a multicenter trial in patients with doses of inhaled steroid optimised between 800 and 2000 mcg per day. Allergy 1995;50 Suppl 26:320, Abs P‐0710. [Google Scholar]
Laviolette 1999 {published data only}
- Laviolette M, Malmstrom K, Lu S, Chervinsky P, Pujet J‐C, Peszek I, et al. Montelukast added to inhaled beclomethasone in treatment of asthma. American Journal of Respiratory & Critical Care Medicine 1999;160(6):1862‐8. [DOI] [PubMed] [Google Scholar]
Lofdahl 1999 {published and unpublished data}
- Lofdahl CG, Reiss TF, Leff JA, Israel E, Noonan MJ, Finn AF, et al. Randomized, placebo controlled trial of effect of leukotriene receptor antagonist, montelukast, on tapering inhaled corticosteroids in asthmatic patients. British Medical Journal 1999;319(7202):87‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nayak 1998 {published and unpublished data}
- Nayak AS, Anderson P, Charous BL, Williams K, Simonson S. Equivalence of adding zafirlukast versus double‐dose inhaled corticosteroids in asthmatic patients symptomatic on low‐dose inhaled corticosteroids. Journal of Allergy & Clinical Immunology 1998;101(1 part 2):S233, Abs 965. [Google Scholar]
Nishimura 1999 {published data only}
- Nishimura K, Hajiro T, Ishihara K, Hasegawa T, Taniguchi H, Katayama S, Ikeda K, Tomii K, Izumi T. Additive effect of pranlukast in combination with inhaled corticosteroid in the treatment of patients with chronic asthma. European Respiratory Journal 1999:P837.
Nsouli 2000 {published data only}
- Nsouli SM, McNutt WJ. The addition of montelukast to a low dose inhaled corticosteroid compared with a doubl‐dose of an inhaled corticosteroid in patients with persistent asthma. Annals of Allergy, Asthma & Immunology 2000;84:159. [Google Scholar]
O'Sullivan 2003 {published data only}
- O'Sullivan S, Akveld M, Burke C M, Poulter L W. Effect of the addition of montelukast to inhaled fluticasone propionate on airway inflammation. Am J Respir Crit Care Med 2003;167(5):745‐750. [DOI] [PubMed] [Google Scholar]
Price 2003 {published data only}
- Price D B, Hernandez D, Magyar P, Fiterman J, Beeh K M, James I G, Konstantopoulos S, Rojas R, Noord J A, Pons M, Gilles L, Leff J A. Randomised controlled trial of montelukast plus inhaled budesonide versus double dose inhaled budesonide in adult patients with asthma. Thorax 2003;58:0‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riccioni 2001 {published data only}
- Riccioni G, Castronuove M, Benedictis M, Pace‐Palitti V, Gioacchino M, Della Vecchia R, Schiavone C, Sensi S, Guagnano M.T. Zafirlukast versus budesonide on bronchial reactivity in subjects with mild‐persistent asthma. International Journal of Immunopathology and Pharmacology 2001;14(2):87‐92. [PubMed] [Google Scholar]
Riccioni 2002 {published data only}
- Riccioni G, Ballone E, D'Orazio N, Sensi S, Nicola M, Mascio R, Santilli F, Guagnano MT, Della Vecchia R. Effectiveness of montelukast versus budesonide on quality of life and bronchial reactivity in subjects with mild‐persistent asthma. Int J Immunopathol Pharmacol 2002;15(2):149‐155. [DOI] [PubMed] [Google Scholar]
Ringdal 1999 {published and unpublished data}
- Ringdal N, White M, Harris A. Addition of Zafirlukast (Accolate) compared with a double‐dose of inhaled corticosteroids in patients with reversible airways obstruction symptomatic on inhaled corticosteroids.. American Journal of Respiratory & Critical Care Medicine 1999; Vol. 159, issue 3 part 2:639 Abs.
Shingo 2001 {published and unpublished data}
- Shingo S, Zhang J, Noonan N, Reiss TF, Leff JA. A standardized composite clinical score for inhaled corticosteroids taper studies in asthma. Drug Inform J 2002;36:501‐508. [Google Scholar]
Simons 2001 {published and unpublished data}
- Simons FER, Villa JR, Lee BW, Teper AM, Lyttle B, Aristizabal G, et al. Montelukast added to budesonide in children with persistent asthma: a randomised, double‐blind, crossover study. Journal of Pediatrics 2001;138(5):694‐8. [DOI] [PubMed] [Google Scholar]
Tamaoki 1997 {published data only}
- Tamaoki J, Kondo M, Sakai N, Nakata J, Takemura H, Nagai A, et al. Leukotriene antagonist prevents exacerbation of asthma during reduction of high‐dose inhaled corticosteroid. American Journal of Respiratory & Critical Care Medicine 1997;155:1235‐40. [DOI] [PubMed] [Google Scholar]
Tohda 2002 {published and unpublished data}
- Tohda Y, Fujimura M, Taniguchi H, Takagi K, Igarashi T, Yasuhara H, Takahashi K, Nakajima S. Leukotriene receptor antagonist, montelukast, can reduce the need for inhaled steroid while maintaining the clinical stability of asthmatic patients. Clin Exp Allergy 2002;32:1180‐1186. [DOI] [PubMed] [Google Scholar]
Tomari 2001 {published data only}
- Tomari S, Shimoda T, Kawano T, Mitsuta K, Obase Y, Fukushima C, Matsuse H, Kohno S. Effects of pranlukast, a cysteinyl leukotriene receptor 1 antagonist, cobined with inhaaled beclomethasone in patients with moderate or severe asthma. Ann Allergy Asthma Immunol 2001;87:156‐161. [DOI] [PubMed] [Google Scholar]
Tomita 1999 {published data only}
- Tomita K, Hashimoto K, Matsumoto S, Nakamoto N, Tokuyasu H, Yamasaki A, et al. Pranlukast allows reduction of inhaled steroid dose without deterioration in lung function in adult asthmatics. Article in Japanese. Arerugi ‐ Japanese Journal of Allergology 1999;48(4):459‐65. [PubMed] [Google Scholar]
Vaquerizo 2003 {published data only}
- Vaquerizo MJ, Casan P, Castillo J, Perpina M, Sanchis J, Sobradillo V, Valencia A, Verea H, Viejo JL, Villasante C, Gonzalez‐Esteban J, Picado C. Effect of montelukast added to inhaled budesonide on control of mild to moderate asthma. Thorax 2003;58:204‐211. [DOI] [PMC free article] [PubMed] [Google Scholar]
Virchow 2000 {published and unpublished data}
- Virchow JC, Prasse A, Naya I, Summerton L, Harris A. Zafirlukast improves asthma control in patients receiving high‐dose inhaled corticosteroids. American Journal of Respiratory & Critical Care Medicine 2000;162:578‐85. [DOI] [PubMed] [Google Scholar]
Wada 1999 {published data only}
- Wada K, Minoguchi K, Kohno Y, Oda N, Matsuura T, Kawazu K, et al. Effect of a leukotriene receptor antagonist, pranlukast hydrate, on airway inflammation and airway hyperresponsiveness in patients with moderate to severe asthma. Allergology International 2000;49(1):63‐8. [Google Scholar]
Yildirim 2001 {published data only}
- Yildirim Z, Ozlu T, Bulbul Y. Montelukast and budesonide vs double dose budesonide in moderate asthma. European Respiratory Journal 2001;18(Supp 33):261s. [Google Scholar]
References to studies excluded from this review
Altman 1998 {published data only}
- Altman LC, Munk Z, Seltzer J, Noonan N, Shingo S, Zhang J, et al. A placebo‐controlled, dose‐ranging study of montelukast, a cysteinyl leukotriene‐receptor antagonist. Journal of Allergy & Clinical Immunology 1998;102:50‐6. [DOI] [PubMed] [Google Scholar]
Barnes 1996 {published data only}
- Barnes NC, Black B, Syrett N, Cohn J. Reduction of exacerbations of asthma in multi‐national clinical trials with zafirlukast (Accolate). Allergy 1996;51(Suppl 30):84. [Google Scholar]
Barnes 1997 {published data only}
- Barnes NC, Pujet J‐C. Pranlukast, a novel leukotriene receptor antagonist: results of the first european, placebo controlled, multicentre clinical study in asthma. Thorax 1997;52:523‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Basyigit 2001 {published data only}
- Basyigit IE, Yildiz F, Özkara SK, Boyaci H, Ilgazli A, Özkarakas O. The effects of inhaled steroids, leukotriene receptor antagonists and theophylline on induced sputum ECP levels mild persistent asthma. European Respiratory Journal 2001;18(Supp 33):261s. [Google Scholar]
Baumgartner 1999 {unpublished data only}
- Baumgartner RA, Polis A, Angner R, Bird S, Reiss TF. Comparison between montelukast and inhaled beclomethasone therapy in chronic asthma: a double‐blind, placebo‐controlled, parallel study in asthmatic patients. Merck Research Laboratories 1999.
Becker 2000 {published data only}
- Becker A. Leukotriene receptor antagonists: efficacy and safety in children with asthma. Pediatric Pulmonology 2000;30(2):183‐6. [DOI] [PubMed] [Google Scholar]
Bisgaard 1999 {published data only}
- Bisgaard H, Loland L, Anhoj J. NO in exhaled air of asthmatic children is reduced by the leukotriene receptor antagonist montelukast. American Journal of Respiratory & Critical Care Medicine 1999;160:1227‐31. [DOI] [PubMed] [Google Scholar]
Bisgaard 2000 {published data only}
- Bisgaard H, Nielsen KG. Bronchoprotection with a leukotriene receptor antagonist in asthmatic preschool children. American Journal of Respiratory & Critical Care Medicine 2000;162(1):187‐90. [DOI] [PubMed] [Google Scholar]
Bjermer 2000 {published data only}
- Bjermer L, Bisgaard H, Bousquet J, Fabbri LM, Greening A, Haahtela T, et al. Montelukast or salmeterol combined with an inhaled steroid in adult asthma: design and rationale of a randomized, double‐blind comparative study. Respiratory Medicine 2000;94(6):612‐21. [DOI] [PubMed] [Google Scholar]
Bjermer 2002 {published data only}
- Bjermer L, Greening A, Haahtela T, Bousquet J, Holgate ST, Picado C, Bisgaard H, Fabbri L, Menten J, Lef JA, IMPACT study group. Addition of montelukast or salmeterol to fluticasone in patients with uncontrolled asthma: results of the IMPACT trial.. Chest 2002:434. [Google Scholar]
Brabson 2002 {published data only}
- Brabson J H, Clifford D, Kerwin E, Raphael G, Pepsin P J, Edwards L D, Srebro S, Rickard K. Efficacy and safety of low‐dose fluticasone propionate compared with zafirlukast in patients with persistent asthma. American Journal of Medicine 2002;113(1):15‐21. [DOI] [PubMed] [Google Scholar]
Brannan 2001 {published data only}
- Brannan JD, Anderson SD, Gomes K, King GG, Chan HK, Seale JP. Fenofenadine decreases sensitivity to and montelukast improves recovery from inhaled manitol. American Journal of Respiratory & Critical Care Medicine 2001;163(6):1420‐5. [DOI] [PubMed] [Google Scholar]
Brocks 1996 {published data only}
- Brocks DR, Upward JW, Georgiou P, Stelman G, Doyle E, Allen E, et al. The single and multiple dose pharmacokinetics of pranlukast in healthy volunteers. European Journal of Clinical Pharmacology 1996;51:303‐8. [DOI] [PubMed] [Google Scholar]
Bronsky 1997 {published data only}
- Bronsky E, Grossman J, Nathan RA, Jong B. Pranlukast (Ultair) reduces symptoms of seasonal allergic rhinitis: results of the first US double‐blind, placebo controlled trial in 484 patients. SmithKline Beecham Pharmaceuticals 1997.
Bruce 2002 {published data only}
- Bruce C, Palmqvist M, Sjöstrand M, Aronsson B, Arvidsson P, Lotvall J. Greater attenuation of the late asthmatic reaction by fluticasone propionate compared to montelukast. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A215. [Google Scholar]
Busse 1999 {published data only}
- Busse W, Nelson H, Wolfe J, Kalberg C, Yancey SW, Rickard KA. Comparison of inhaled salmeterol and oral zafirlukast in patients with asthma. Journal of Allergy & Clinical Immunology 1999;103:1075‐80. [DOI] [PubMed] [Google Scholar]
Cakmak 2000 {published data only}
- Cakmak G, Demir T, Aydemir A, Serdaroglu E, Erginoz E, Donma O, Gemicioglu B. The effect of adding zafirlukast to budesonide treatment on total antoxydant capacity. European Respiratory Journal 2000;16(Supplement 31):457s. [Google Scholar]
Calhoun 1997 {published data only}
- Calhoun WJ, Weisberg SC, Faiferman I, Stober PW. Pranlukast (Ultair) is effective in improving asthma: results of a 12‐week, multicenter, dose‐range study. Journal of Allergy & Clinical Immunology 1997;99:S318, Abs 1305. [Google Scholar]
Calhoun 2001 {published data only}
- Calhoun W J, Nelson H S, Nathan R A, Pepsin P J, Kalberg C, Emmett A, Rickard K A, Dorinsky P. Comparison of fluticasone propionate‐salmeterol combination therapy and montelukast in patients who are symptomatic on short‐acting beta(2)‐agonists alone. American Journal of Respiratory & Critical Care Medicine. 2001; 164(5):759‐63. 2001;164(5):759‐763. [DOI] [PubMed] [Google Scholar]
Camargo 2002 {published data only}
- Camargo C A, Smithline H A, Marie‐Pierre M, Green S A, Reiss T F. A Randomized Controlled Trial of Intravenous Montelukast in Acute Asthma. Am J Respir Crit Care Med 2002. [DOI] [PubMed] [Google Scholar]
Capella 2001 {published data only}
- Capella GL, Frigerio E, Altomare G. A randomized trial of leukotriene receptor antagonist montelukast in moderate‐to‐severe atopic dermatitis of adults. European Journal of Dermatology 2001;11(3):209‐13. [PubMed] [Google Scholar]
Chuchalin 2002 {published data only}
- Chuchalin A G, Ovcharenko S I, Goriachkina L A, Sidorenko I V, Tsoi A N, Alekseev V G, Bart B Y, Borisova N K, Belousov Y B, Golovko M G, Goriachkina L A, Chereiskaya N K, Daniliak I G, Didkovsky N A, Dobrotina I S, Emelyanov A V, Korovina O V, Mamtsev B N, Sokurenko S I, Valekjanina T G, Zadionchenko V S. The safety and efficacy of formoterol OxisRTurbuhalerR plus budesonide PulmicortRTurbuhaler in mild to moderate asthma: A comparison with budesonide Turbuhaler alone and current non‐corticosteroid therapy in Russia. International Journal of Clinical Practice 2002;56(1):15‐20. [PubMed] [Google Scholar]
Clifford 2000 {published data only}
- Clifford D, Pepsin P, Srebro S, Edwards L, Rickard K. Fluticasone propionate 88mcg bid is superior to zafilukast 20mg bid in controlling persistent asthma [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A202. [Google Scholar]
Cloud 1989 {published data only}
- Cloud ML, Enas GC, Kemp J, Platts‐Mills T, Altman LC, Townley R, et al. A specific LTD4‐LTE4‐receptor antagonist improves pulmonary function in patients with mild, chronic asthma. American Review of Respiratory Disease 1989;140(5):1336‐9. [DOI] [PubMed] [Google Scholar]
Currie 2003 {published data only}
- Currie G P. Montelukast confers complimentary non‐steroidal anti‐inflammatory activity in asthmatics receiving fluticasone alone and fluticasone/salmeterol combination. J Allergy Clin Immunol 2003;111(2):Abstact 309. [Google Scholar]
Currie 2003 (B) {published data only}
- Currie G P, Lee D K C, Haggart K, Bates C E, Lipworth B J. Effects of montelukast on surrogate inflammatory markers in corticosteroid‐treated patients with asthma. American Journal of Respiratory and Critical Care Medicine 2003;167(9):1232‐1238. [DOI] [PubMed] [Google Scholar]
Cylly 2003 {published data only}
- Cylly A, Kara A, Ozdemir T, Ogus C, Gulkesen K H. Effects of oral montelukast on airway function in acute asthma. Respir Med 2003;97(5):533‐536. [DOI] [PubMed] [Google Scholar]
Dahlen 2002 {published and unpublished data}
- Dahlén SE, Malmstrom K, Nizankowska E, Dahlén B, Kuna P, Kowalski M, et al. Improvement of aspirin‐intolerant asthma by montelukast, a leukotriene antagonist. A randomised, double‐blind, placebo controlled trial. American Journal of Respiratory & Critical Care Medicine 2002;165(1):9‐14. [DOI] [PubMed] [Google Scholar]
Daikh 2003 {published data only}
- Daikh B E, Ryan C K, Schwartz R H. Montelukast reduces peripheral blood eosinophilia but not tissue eosinophilia or symptoms in a patient with eosinophilic gastroenteritis and esophageal stricture. Annals of Allergy, Asthma, & Immunology 2003;90(1):23‐27. [DOI] [PubMed] [Google Scholar]
Dempsey 1999 {published data only}
- Dempsey OJ, Wilson AM, Sims EJ, Lipworth BJ. A comparison of once daily topical budesonide (BUD) and oral montelukast (MON) in seasonal allergic rhinitis (SAR) and asthma. European Respiratory Society 1999. [DOI] [PubMed] [Google Scholar]
Dempsey 2000 {published data only}
- Dempsey OJ, Wilson AM, Sims EJ, Mistry C, Lipworth BJ. Additive bronchoprotective and bronchodilator effects with single doses of salmeterol and montelukast in asthmatic patientsreceiving inhaled corticosteroids. Chest 2000;117:950‐3. [DOI] [PubMed] [Google Scholar]
Dempsey 2000b {published data only}
- Dempsey OJ, Wilson AM, Sims EJ, Lipworth BJ. Additive anti‐inflammatory effects of montelukast but not salmeterol in asthmatics suboptimally controlled on inhaled steroids [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A198. [Google Scholar]
Dempsey 2001 {published data only}
- Dempsey OJ, Wilson AM, Lipworth BJ. Comparative efficacy and anti‐inflammatory profile of once daily low dose Hfa‐triamcinolone Acetonide (TAA) and Montelukast (ML) in patients with mild persistent atopic asthma. Journal of Allergy and Clinical Immunology 2001;107(2):S316. [DOI] [PubMed] [Google Scholar]
Dempsey 2002 {published data only}
- Dempsey OJ, Fowler SJ, Wilson A, Kennedy G, Lipworth BJ. Effects of adding either a leukotriene receptor antagonist or low‐dose theophylline to a low or medium dose of inhaled corticosteroid in patients with persistent asthma. Chest 2002;122(1):151‐159. [DOI] [PubMed] [Google Scholar]
Dessanges 1999 {published data only}
- Dessanges JF, Prefaut C, Taytard A, Matran R, Naya I, Compagnon A, et al. The effect of zafirlukast on repetitive exercise‐induced bronchoconstriction: The possible role of leukotrienes in exercise‐induced refractoriness. Journal of Allergy & Clinical Immunology 1999;104(6):1155‐61. [DOI] [PubMed] [Google Scholar]
Diamant 1999 {published data only}
- Diamant Z, Grootendorst DC, Veselic‐Charvat M, Timmers MC, Smet M, Leff JA, et al. The effect of montelukast (MK‐0476), a cysteinyl leukotriene receptor antagonist, on allergen‐induced airway responses and sputum cell counts in asthma. Clinical & Experimental Allergy 1999;29(1):42‐51. [DOI] [PubMed] [Google Scholar]
Dicpinigaitis 2002 {published data only}
- Dicpinigaitis P V, Dobkin J B, Reichel J. Antitussive effect of the leukotriene receptor antagonist zafirlukast in subjects with cough‐variant asthma. Journal of Asthma 2002;39(4):291‐297. [DOI] [PubMed] [Google Scholar]
Dockhorn 2000 {published data only}
- Dockhorn RJ, Baumgartner RA, Leff JA, Noonan M, Vandormael K, Stricker W, et al. Comparison of the effects of intravenous and oral montelukast on airway function: A double blind, placebo controlled, three period, crossover study in asthmatic patients. Thorax 2000;55(4):260‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eliraz 2001 {published data only}
- Eliraz A, Raminez‐Rivera A, Ferranti P, et al. Similar efficacy following four weeks treatment of asthmatics with formoterol 12 mcg bid delivered by two different dry powder inhalers: differences in inhaler handling. International Journal of Clinical Practice 2001;55(3):164‐70. [PubMed] [Google Scholar]
Faul 2002 {published data only}
- Faul JL, Canfield JC, Gould MK, Wilson SR, Kischner WG. A randomized, double‐blind, placebo‐controlled, crossover study comparing the effects of inhaled fluticasone propionate (880 Micrograms per day) and montelukast (10 MG per day) on glucose control patients with diabetes and asthma. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A217. [Google Scholar]
Findlay 1992 {published data only}
- Findlay SR, Barden JM, Easley CB, Glass M. Effect of the oral leukotriene antagonist, ICI 204,219, on antigen‐induced bronchoconstriction in subjects with asthma. Journal of Allergy & Clinical Immunology 1992;89:1040‐5. [DOI] [PubMed] [Google Scholar]
Fischer 1995 {published data only}
- Fischer AR, McFadden CA, Frantz R, Awni WM, Cohn J, Drazen JM, et al. Effect of chronic 5‐lipoxygenase inhibition on airway hyperresponsiveness in asthmatic subjects. American Journal of Respiratory & Critical Care Medicine 1995;152(4 Part 1):1203‐7. [DOI] [PubMed] [Google Scholar]
Fischer 1997 {published data only}
- Fischer AR, Rosenberg MA, Roth M, Loper M, Jungerwirth S, Israel E. Effect of a novel 5‐lipoxygenase activating protein inhibitor, BAYx 1005, on asthma induced by cold air. Thorax 1997;52:1074‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fish 1997 {published data only}
- Fish JE, Kemp JP, Lockey RF, Glass M, Hanby L, Bonuccelli CM. Zafirlukast for symptomatic mild‐to‐moderate asthma: a 13‐week multicenter study. Clinical Therapeutics 1997;19(4):675‐90. [DOI] [PubMed] [Google Scholar]
Flynn 2001 {published data only}
- Flynn David. N of 1 Trials of Montelukast (ML) or Singular (Trade Name) in Childhood Asthma. The Royal Free Hampstead NHS Trust. [Google Scholar]
Fujimura 1993 {published data only}
- Fujimura M, Sakamoto S, Kamio Y, Matsuda T. Effect of a leukotriene antagonist, ONO‐1078, on bronchial hyperresponsiveness in patients with asthma. Respiratory Medicine 1993;87:133‐8. [DOI] [PubMed] [Google Scholar]
Gaddy 1990 {published data only}
- Gaddy J, Bush RK, Margolskee D, Williams VC, Busse W. The effects of a leukotriene D4 (LTD4) antagonist (MK‐571) in mild to moderate asthma. Journal of Allergy & Clinical Immunology 1990;85:197, Abs. 216. [Google Scholar]
Galant 2001 {published data only}
- Galant S, Gode‐Sellers S, Kalberg C, Edwards L, Srebro S, Rickard K. Low dose inhaled Fluticasone Propionate provides greater improvement in pulmonary function as compared to Montelukast in patients with persistent asthma. Journal of Allergy and Clinical Immunology 2001;107(2):S106. [DOI] [PubMed] [Google Scholar]
Geha 2001 {published data only}
- Geha RS. Desloratadine: a new, nonsedating, oral antihistamine. Journal of Allergy & Clinical Immunology 2001;107(4):751‐62. [DOI] [PubMed] [Google Scholar]
Georgiou 1997 {published data only}
- Georgiou P, Compton C, Allen A, Hust R, Collie H. Pranlukast (Ultair) has no effect on cardiovascular parameters in healthy male subjects. ATS. 1997:Abs C49.
Ghiro 2002 {published data only}
- Ghiro L, Zanconato S, Rampon O, Piovan V, Pasquale M F, Baraldi E. Effect of montelukast added to inhaled corticosteroids on fractional exhaled nitric oxide in asthmatic children. Eur Respir J. 2002;20(3):630‐634. [DOI] [PubMed] [Google Scholar]
Gold 2001 {published data only}
- Gold M, Jõgi R, Mulder PGH, Akveld MLM. Salmeterol/fluticasone propionate combination 50/100µg bid is more effective than fluticasone propionate 100µg bid plus montelukast 10 mg once daily in reducing exacerbations. European Respiratory Journal 2001;18(Supp 33):262s. [Google Scholar]
Green 2002b {published data only}
- Green RH, Brightling CE, McKenna S, Hargadon B, Parker D, Bradding P, Wardlaw A J, Pavord I D. Asthma exacerbations and sputum eosinophil counts: A randomised controlled trial. Lancet 2002;360(9347):1715‐1721. [DOI] [PubMed] [Google Scholar]
Grossman 1995 {published data only}
- Grossman J, Bronsky E, Busse W, Montanaro A, Southern L, Tinkelman D, et al. A multicenter, double‐blind, placebo‐controlled study to evaluate the safety, tolerability and clinical activity of oral, twice‐daily LTA, Pranlukast (SB 205312) in patients with mild to moderate asthma. Journal of Allergy & Clinical Immunology 1995;95(1):352, Abs 846. [Google Scholar]
Grossman 1997 {published data only}
- Grossman J, Faiferman I, Dubb JW, Tompson DJ, Busse W, Bronsky E, et al. Results of the first U.S. double‐blind, placebo‐controlled, multicenter clinical study in asthma with pranlukast, a novel leukotriene receptor antagonist. Journal of Asthma 1997;34(4):321‐8. [DOI] [PubMed] [Google Scholar]
Haahtela 1994 {published data only}
- Haahtela T, Jarvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, et al. Effects of reducing or discontinuing inhaled budesonide in patients with mild asthma. New England Journal of Medicine 1994;331(11):700‐5. [DOI] [PubMed] [Google Scholar]
Hamilton 1998 {published data only}
- Hamilton AL, Faiferman I, Stober P, Watson RM, O'Byrne PM. Pranlukast, a cysteinyl leukotriene receptor antagonist, attenuates allergen‐induced early and late phase bronchoconstriction and airway hyperresponsiveness in asthmatic subjects. Journal of Allergy & Clinical Immunology 1998;102(2):177‐83. [DOI] [PubMed] [Google Scholar]
Hassall 1998 {published data only}
- Hassell SM, Miller C, Harris A. Zafirlukast (Accolate) reduces the need for oral steroid bursts. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411. [Google Scholar]
Hood 1999 {published data only}
- Hood PP, Cotter TP, Costello JF, Sampson AP. Effect of intravenous corticosteroid on ex vivo leukotriene generation by blood leucocytes of normal and asthmatic patients. Thorax 1999;54(12):1075‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Howland 1994 {published data only}
- Howland III W, Segal A, Glass M, Minkwitz MC. 6‐week therapy with the oral leukotriene‐receptor antagonist, ICI 204,219, in the treatment of asthma. Journal of Allergy & Clinical Immunology 1994;93(1):259, Abs. 581. [Google Scholar]
Hsieh 1996 {published data only}
- Hsieh, K‐H. Evaluation of efficacy of traditional chinese medicines in the treatment of childhood bronchial asthma: clinical trial, immunological tests and animal study. Pediatric Allergy & Immunology 1996;7:130‐40. [DOI] [PubMed] [Google Scholar]
Hughes 1999 {unpublished data only}
- Hughes GL, Edelman JM, Turpin JA, Liss C, Weeks K, Schweiger D, et al. Randomized, open‐label pilot study comparing the effects of montelukast sodium tablets, fluticasone aerosol inhaler, and budesonide dry powder inhaler on asthma control in mild asthmatics. Merck Research Laboratories 1999.
Hui 1991 {published data only}
- Hui K, Barnes NC. Lung function improvement in asthma with a cysteinyl‐leukotriene receptor antagonist. Lancet 1991;337:1062‐3. [DOI] [PubMed] [Google Scholar]
Israel 1990 {published data only}
- Israel E, Dermarkarkian R, Rosenberg M, Sperling R, Taylor G, Rubin P, et al. The effects of a 5‐lipoxygenase inhibitor on asthma induced by cold, dry air. New England Journal of Medicine 1990;323:1740‐4. [DOI] [PubMed] [Google Scholar]
Israel 1992 {published data only}
- Israel E, Drazen J, Pearlman H, Cohn J, Rubin P. A double‐blind multicenter study of zileuton, a potent 5‐lipoxygenase (5‐LO) inhibitor versus placebo in the treatment of spontaneous asthma in adults. Journal of Allergy & Clinical Immunology 1992;89:236, Abs. 368. [Google Scholar]
Israel 1993 {published data only}
- Israel E, Rubin P, Kemp JP, Grossman J, Pierson W, Siegel SC, et al. The effect of inhibition of 5‐lipoxygenase by zileuton in mild‐to‐moderate asthma. Annals of Internal Medicine 1993;119(11):1059‐66. [DOI] [PubMed] [Google Scholar]
Israel 1996 {published data only}
- Israel E, Cohn J, Dube L, Drazen JM. Effect of treatment with zileuton, a 5‐lipoxygenase inhibitor, in patients with asthma. The Journal of the American Medical Association 1996;275(12):931‐6. [PubMed] [Google Scholar]
Israel 2002 {published data only}
- Israel E, Chervinsky P S, Friedman B, Bavel J, Skalky C S, Ghannam A F, Bird S R, Edelman J M. Effects of montelukast and beclomethasone on airway function and asthma control. Journal of Allergy & Clinical Immunology 2002;110(6):847‐854. [DOI] [PubMed] [Google Scholar]
Jayaram 2002 {published data only}
- Jayaram L, Pizzichini MMM, Hussack P, Lemiere C, Cartier A, Man SFP, Pizzichini E, Hargreave FE. First line anti‐inflammatory treatment for asthma; inhaled steroid or leukotriene antagonist?. Respirology 2002;7(Supplement):A19. [Google Scholar]
Johnson 1999 {published data only}
- Johnson MC, Srebro S, Edwards L, Bowers B, Rickard K. Physician‐ and patient‐rated assessments correlate well with clinical efficacy measurements in a study comparing fluticasone and zafirlukast. Journal of Allergy and Clinical Immunology 1999;103(1 Part 2):Abs. 882. [Google Scholar]
Juniper 1995 {published data only}
- Juniper EF, Dube L, Swanson LJ, Zileuton Study Group. The effect of zileuton, a 5‐lipoxygenase inhibitor, on asthma quality of life. Asthma. 1995.
Kalberg 1999 {published data only}
- Kalberg CJ, Yancey S, Emmett AH, Rickard K. A comparison of salmeterol versus zafirlukast in patients using inhaled corticosteroids. Journal of Allergy & Clinical Immunology 1999;103(1 Part 2):abs 881. [DOI] [PubMed] [Google Scholar]
Kanniess 2002 (B) {published data only}
- Kanniess F, Richter K, Bohme S, Jorres R A, Magnussen H. Montelukast versus fluticasone: effects on lung function, airway responsiveness and inflammation in moderate asthma. Eur Respir J. 2002;20(4):853‐858. [DOI] [PubMed] [Google Scholar]
Kemp 1995 {published data only}
- Kemp JP, Glass M, Minkwitz MC. Onset of action of the leukotriene‐receptor antagonist, zafirlukast (Accolate), in patients with asthma. Journal of Allergy & Clinical Immunology 1995;95(1 Part 2):351, Abs. 844. [Google Scholar]
Kemp 1997 {published data only}
- Kemp JP, et al. Montelukast, a leukotriene receptor antagonist, inhibits exercise induced bronchoconstriction in 6‐14 year old children. Journal of Allergy & Clinical Immunology 1997.
Kemp 1998 {published data only}
- Kemp JP, Tinkelman D, Sublett J, Compton C, Georgiou P. Pranlukast (Ultair) pharmacokinetics in children consistent with that of adults. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411. [Google Scholar]
Kemp 1999 {published data only}
- Kemp JP, Minkwitz MC, Bonuccelli CM, Warren MS. Therapeutic effect of zafirlukast as monotherapy in steroid‐naive patients with severe persistent asthma. Chest 1999;115(2):336‐42. [DOI] [PubMed] [Google Scholar]
Kim 2000 {published data only}
- Kim KT, Ginchansky EJ, Srebro S, Pepsin PJ, Edwards L, Stanford RH, et al. Fluticasone propionate versus zafirlukast: effect in patients previously receiving inhaled corticosteroid therapy. Annals of Allergy, Asthma, & Immunology 2000;85(5):398‐406. [DOI] [PubMed] [Google Scholar]
Kips 1991 {published data only}
- Kips JC, Joos GF, Lepeleire I, Margolskee DJ, Buntinx A, Pauwels RA, et al. MK‐571, a potent antagonist of leukotriene D4‐induced bronchoconstriction in the human. American Review of Respiratory Disease 1991;144:617‐21. [DOI] [PubMed] [Google Scholar]
Knorr 1997 {published data only}
- Knorr BA, et al. Montelukast improves asthma in children 6‐14 years. American Thoracic Society 1997.
Knorr 1998 {published data only}
- Knorr B, Matz J, Bernstein JA, Nguyen H, Seidenberg BC, Reiss TF, et al. Montelukast for chronic asthma in 6‐ to 14‐year‐old children. The Journal of the American Medical Association 1998;279(15):1181‐6. [DOI] [PubMed] [Google Scholar]
Knorr 1999 {published data only}
- Knorr B, Nguycn HH, Seidenberg BC, Reiss TF, Montelukast Pediatric Study Group. Montelukast, a leukotriene receptor antaconist, provides additional clinical benefit in asthmatic children aged 6 to 14 years using inhaled corticosteroids. European Respiratory Society 1999;P363. [Google Scholar]
Korenblat 1998 {published data only}
- Korenblat P, Chervinsky P, Wenzel S, Faiferman I, Bakst A. Pranlukast (Ultair) reduces health care utilization and improves quality of life in adult patients with mild‐to‐moderate asthma. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411. [Google Scholar]
Kuna 1997 {published data only}
- Kuna P, Malmstrom K, Dahlen SE, Nizankowska E, Kowalski M, Stevenson D, et al. Montelukast (MK‐0476), a cysLT1 receptor antagonist, improves asthma control in aspirin‐intolerant asthmatic patients. American Journal of Respiratory & Critical Care Medicine 1997;155(4):A975. [Google Scholar]
Kylstra 1998 {published data only}
- Kylstra JW, Sweitzer DE, Miller CJ, Bonuccelli CM. Zafirlukast (Accolate) in moderate asthma: patient‐reported outcomes and peripheral eosinophil data from a 13‐week trial. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411. [Google Scholar]
Laitinen 1997 {published data only}
- Laitinen LA, Naya IP, Binks S, Harris A. Comparative efficacy of zafirlukast & low dose steroids in asthmatics on prn beta2‐agonists. European Respiratory Journal 1997;10(Suppl 25):419‐20s, Abs. 2716. [Google Scholar]
Leff 1998 {published data only}
- Leff JA, Busse WW, Pearlman D, Bronsky EA, Kemp J, Hendeles L, et al. Montelukast, a leukotriene‐receptor antagonist, for the treatment of mild asthma and exercise‐induced bronchoconstriction. New England Journal of Medicine 1998;339:147‐52. [DOI] [PubMed] [Google Scholar]
Leigh 2002 {published data only}
- Leigh R, Vethanayagam D, Yoshida M, Watson R M, Rerecich T, Inman M D, O'Byrne P M. Effects of montelukast and budesonide on airway responses and airway inflammation in asthma. Am J Respir Crit Care Med 2002;166(9):1212‐1217. [DOI] [PubMed] [Google Scholar]
Leigh 2002 (B) {published data only}
- Leigh R, Vethanayagam D, Yoshida M, Watson RM, Rererich T, Killian K, O'Byrne PM. Effects of montelukast and budesonide alone or in combination on allergen induced early and late asthmatic responses, and post‐allergen airway hyperresponsiveness [abstract]. American Journal of Respiratory and Critical Care Medicine 2002;165(8 Suppl):A216. [Google Scholar]
Lipworth 1999 {unpublished data only}
- Lipworth BJ. Comparative potency and anti‐inflammatory profile of monotherapy with either montelukast or zafirlukast, patients with mild‐moderate asthma. Academic Publications (Ongoing trial) 1999. [Google Scholar]
Lipworth 2000 {unpublished data only}
- Lipworth BJ. A comparison of anti‐histamine and leukotriene receptor antagonist as steroid sparing agents in patients with atopic asthma.. Ongoing trial 2000.
Liu 1996 {published data only}
- Liu MC, Dube LM, Lancaster J, Zileuton Study Group. Acute and chronic effects of a 5‐lipoxygenase inhibitor in asthma: a 6‐month randomized multicenter trial. Journal of Allergy & Clinical Immunology 1996;98:859‐71. [DOI] [PubMed] [Google Scholar]
Lockey 1995 {published data only}
- Lockey RF, Lavins BJ, Snader L. Effects of 13 weeks of treatment with ICI 204,219 (Accolate) in patients with mild to moderate asthma. Journal of Allergy & Clinical Immunology 1995;95(Part 2):350, Abs. 839. [Google Scholar]
Malerba 2002 {published data only}
- Malerba M, Radaeli A, Ceriani L, Amato M, Tomenzoli D, Nicolai P, Tantucci C, Grassi V. Comparison of oral montelukast and inhaled fluticasone in the treatment of asthma associated with chronic rhinopolyposis: A single‐blind, randomized, pilot study. Current Therapeutic Research, Clinical & Experimental 2002;63(6):355‐365. [Google Scholar]
Malmstrom 1999 {published data only}
- Malmstrom K, Rodriguez‐Gomez G, Guerra J, Villaran C, Pineiro A, Lynn X, et al. Oral montelukast, inhaled beclomethasone, and placebo for chronic asthma: a randomized, controlled trial. Annals of Internal Medicine 1999;130(6):487‐95. [DOI] [PubMed] [Google Scholar]
Margolskee 1991 {published data only}
- Margolskee D, Bodman S, Dockhorn R, Irael E, Kemp J, Mansmann H. The therapeutic effects of MK‐571, a potent and selective leukotriene (LT) D4 receptor antagonist, in patients with chronic asthma. Journal of Allergy & Clinical Immunology 1991;87(1 Part 2):309, Abs. 677. [Google Scholar]
Meltzer 2002 {published data only}
- Meltzer E O, Lockey R F, Friedman B F, Kalberg C, Goode‐Sellers S, Srebro S, Edwards L, Rickard K. Efficacy and safety of low‐dose fluticasone propionate compared with montelukast for maintenance treatment of persistent asthma. Mayo Clinic Proceedings 2002;77(5):437‐445. [PubMed] [Google Scholar]
Micheletto 1997 {unpublished data only}
- Micheletto C, Turco P, Dal Negro R. Accolate 20 mg works as steroid sparing in moderate asthma. American Journal of Respiratory & Critical Care Medicine 1997; Vol. 155, issue 4 Part 2:A664.
Minkwitz 1998 {published data only}
- Minkwitz MC t al. Zafirlukast (Accolate) response in severe persistent ?. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411. [DOI] [PubMed] [Google Scholar]
Miyamoto 1999 {published data only}
- Miyamoto T, Accolate™ clinical trial committee. Effects of zafirlukast on symptoms and pulmonary function of asthmatic patients with and without corticosteroids. European Respiratory Society 1999:P835. [Google Scholar]
Nathan 1998 {published data only}
- Nathan RA, Bernstein JA, Bielory L, Bonuccelli CM, Calhoun WJ, Galant SP, et al. Zafirlukast improves asthma symptoms and quality of life in patients with moderate reversible airflow obstruction. Journal of Allergy & Clinical Immunology 1998;102:935‐42. [DOI] [PubMed] [Google Scholar]
Nathan 2001 {published data only}
- Nathan RA, Bleecker ER, Kalberg C. A comparison of short‐term treatment with inhaled fluticasone propionate and zafirlukast for patients with persistent asthma. American Journal of Medicine 2001;111(3):195‐202. [DOI] [PubMed] [Google Scholar]
Nelson 2001 {published data only}
- Nelson H S, Nathan R A, Kalberg C, Yancey S W, Rickard K, A. Comparison of inhaled salmeterol and oral zafirlukast in asthmatic patients using concomitant inhaled corticosteroids. Medgenmed Computer File: Medscape General Medicine 2001;3(4):3. [PubMed] [Google Scholar]
Nishizawa 2002 {published data only}
- Nishizawa Y, Greicy‐Goto H, Tanigaki Y, Fushiki S. Sparing effect of Saibokuto inhalation on inhaled beclomethasone dipropionate to halved of reduction of inhaled beclomethasone dipropionate‐dose: Well‐controlled comparative study of Saiboku‐to‐inhalation and sodium cromoglycate‐inhalation. Japanese. Oto‐Rhino‐Laryngology Tokyo 2002;45(SUPPL. 1):8‐15. [Google Scholar]
Noonan 1998 {published data only}
- Noonan MJ, Chervinsky P, Brandon M, Zhang J, Kundu S, McBurney J, et al. Montelukast, a potent leukotriene recpetor antagonist, causes dose‐related improvements in chronic asthma. European Respiratory Journal 1998;11:1232‐9. [DOI] [PubMed] [Google Scholar]
Nsouli 2001 {published data only}
- Nsouli SM, McNutt WJ. The additive effects of montelukast and salmeterol in moderate asthmatics who are uncontrolled on a low dose on inhaled corticosteroids. Annals of Allergy, Asthma & Immunology 2001; 86:81.;86:81. [Google Scholar]
O'Shaughnessy 1996 {published data only}
- O'Shaughnessy TC, Georgiou P, Howland K, Dennis M, Compton CH, Barnes NC. Effect of pranlukast, an oral leukotriene receptor antagonist, on leukotriene D4 (LTD4) challenge in normal volunteers. Thorax 1996;52:519‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Obase 2001 {published data only}
- Obase Y, Shimoda T, Tomari S, Mitsuta K, Fukushima C, et al. Efficacy and safety of long‐term treatment of athma in patients with pranlukast, a cysteinyl‐leukotriene‐receptor antagonist: a four‐year follow‐up study. Annals of Allergy, Asthma, & Immunology 2001;87(1):43‐7. [DOI] [PubMed] [Google Scholar]
Overbeek 2002 {published data only}
- Overbeek SE, O'Sullivan S, Leman K, Mulder PGM, Hoogsteden, Prins JB. Treatment with montelukast is less effective in reducing eosinophilic airway inflammation than fluticasone propionate in atopic asthmatics. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A215. [Google Scholar]
Paterson 1999 {published data only}
- Paterson MC, Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. The effect of combination therapy with salmeterol and montelukast in asthmatic patients receiving inhaled corticosteroids. European Respiratory Society 1999:P3490. [Google Scholar]
Pearlman 1999 {published data only}
- Pearlman DS, Ostrom NK, Bronsky EA, Bonuccelli CM, Hanby LA. The leukotriene D4‐receptor antagonist zafirlukast attenuates exercise‐induced bronchoconstriction in children. Journal of Pediatrics 1999;134(3):273‐9. [DOI] [PubMed] [Google Scholar]
Pearlman 2002 {published data only}
- Pearlman D S, White M V, Lieberman A K, Pepsin P J, Kalberg C, Emmett A, Bowers B, Rickard K A, Dorinsky P. Fluticasone propionate/salmeterol combination compared with montelukast for the treatment of persistent asthma. Ann Allergy Asthma Immunol 2002;88(2):227‐35. [DOI] [PubMed] [Google Scholar]
Pizzichini 1999 {published data only}
- Pizzichini E, Leff JA, Reiss TF, Hendeles L, Boulet L‐P, Wei LX, Efthimiadis A, et al. Montelukast reduced airway eosinophilic inflammation in asthma: a randomized, controlled trial. European Respiratory Journal 1999;14(1):12‐28. [DOI] [PubMed] [Google Scholar]
Pullerits 1999 {published data only}
- Pullerits T, Praks L, Skoogh BE, Ani R, Lotvall J. Randomized placebo‐controlled study comparing a leukotriene receptor antagonist and a nasal glucocorticoid in seasonal allergic rhinitis. American Journal of Respiratory & Critical Care Medicine 1999;159(6):1814‐8. [DOI] [PubMed] [Google Scholar]
Pullerits 2001 {published data only}
- Pullerits T, Praks L, Baker R, Ani R, Lotvall J. Comparison of nasal fluticasone propionate, montelukast, and combined montelukast+loratadine in allergic rhinitis [abstract]. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A193. [Google Scholar]
Pullerits 2002 {published data only}
- Pullerits T, Praks L, Ristioja V, Lotvall J. Comparison of a nasal glucocorticoid, antileukotriene, and a combination of antileukotriene and antihistamine in the treatment of seasonal allergic rhinitis. Journal of Allergy & Clinical Immunology 2002;109(6):949‐955. [DOI] [PubMed] [Google Scholar]
Ramsay 1997 {published data only}
- Ramsay CF, Kan CI, Nieman RB, Wang J, Krieken JHJM, Willems LNA, et al. The effects of oral pranlukast on airway immunopathology and clinical parameters in patients with asthma. American Thoracic Society. 1997:Abs C21.
Ramsay 1998 {published data only}
- Ramsay CF, Kan CI, Sterk PJ, Barnes NC. Pranlukast improves spirometry and bronchial hyperresponsiveness (BHR) in patients with mild asthma. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411. [Google Scholar]
Reiss 1996 {published data only}
- Reiss TF, Altman LC, Chervinsky P, Bewtra A, Stricker WE, Noonan GP, et al. Effects of montelukast (MK‐0476), a new potent cysteinyl leukotriene (LTD4) receptor antagonist, in patients with chronic asthma. Journal of Allergy & Clinical Immunology 1996;98:528‐34. [DOI] [PubMed] [Google Scholar]
Reiss 1997a {published data only}
- Reiss TF, et al. Montelukast improves asthma outcomes over a 3‐month treatment period. American Thoracic Society. 1997.
Reiss 1997b {published data only}
- Reiss TF, Sorkness CA, Stricker W, Botto A, Busse WW, Kundu S, et al. Effects of montelukast (MK‐0476), a potent cysteinyl leukotriene receptor antagonist, on bronchodilation in asthmatic subjects treated with and without inhaled corticosteroids. Thorax 1997;52(1):45‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reiss 1998a {published data only}
- Reiss TF, White R, Noonan G, Korenblat P, Hess J, Shingo S. Montelukast (MK‐0476) a cys LT1 receptor antagonist improves the signs and symptoms of asthma over one year of treatment. Allergy & Asthma Proceedings 1998;19(4):205‐6. [Google Scholar]
Reiss 1998b {published data only}
- Reiss TF, Chervinsky P, Dockhorn RJ, Shingo S, Seidenberg B, Edwards TB. Montelukast, a once‐daily leukotriene receptor antagonist, in the treatment of chronic asthma. Archives of Internal Medicine 1998;158:1213‐20. [DOI] [PubMed] [Google Scholar]
Reiss 1998c {published data only}
- Reiss TF, White R, Noonan G, Korenblat P, Hess J, Shingo S. Montelukast (MK‐0476) a cys LT1 receptor antagonist improves the signs and symptoms of asthma over one year of treatment. European Respiratory Journal 1997;10 Suppl 25:437s. [Google Scholar]
Riccioni 2002 (B) {published data only}
- Riccioni G, D'Orazio N, Ilio C, Della Vecchia R, Lorenzo A. Effectiveness and safety of montelukast versus budesonide at different doses on bronchial reactivity in subjects with mild‐persistent asthma. Clinica Terapeutica 2002;153(5):317‐321. [PubMed] [Google Scholar]
Riccioni 2002 (c) {published data only}
- Riccioni G, Ballone E, D'Orazio N, Sensi S, Nicola M, Mascio R, Santilli F, Guagnano M T, Della Vecchia R. Effectiveness of montelukast versus budesonide on quality of life and bronchial reactivity in subjects with mild‐persistent asthma. International Journal of Immunopathology & Pharmacology 2002;15(2):149‐155. [DOI] [PubMed] [Google Scholar]
Ringdal 1997 {published data only}
- Ringdal N, Whitney JG, Summerton L. Problems with inhaler technique and patient preference for oral therapy tablet zafirlukast versus inhaled beclomethasone. European Respiratory Journal 1997;10(Suppl 25):4372, abs P2806. [Google Scholar]
Robinson 2001 {published data only}
- Robinson DS, Campbell D, Barnes PJ. Addition of leukotriene antagonists to therapy in chronic persistent asthma: a randomised double‐blind placebo‐controlled trial. Lancet 2001;357(9273):2007‐11. [DOI] [PubMed] [Google Scholar]
Rosenhall 2003 {published data only}
- Rosenhall L, Elvstrand A, Tilling B, Vinge I, Jemsby P, Stahl E, Jerre F, Bergqvist P B F. One‐year safety and efficacy of budesonide/formoterol in a single inhaler (Symbicort Turbuhaler ) for the treatment of asthma. Respiratory Medicine 2003;97(6):702‐708. [DOI] [PubMed] [Google Scholar]
Sahn 1997 {published data only}
- Sahn SA, Galant S, Murray J, Bronsky E, Spector S, Faiferman I, et al. Pranlukast (Ultair) improves FEV in patients with asthma: results of a 12‐week multicenter study versus nedocromil. American Thoracic Society. 1997:Abs C49.
Schwartz 1998 {published data only}
- Schwartz HJ, Petty T, Dube LM, Swanson LJ, Lancaster JF. A randomized controlled trial comparing zileuton with theophylline in moderate asthma. Archives of Internal Medicine 1998;158(2):141‐8. [DOI] [PubMed] [Google Scholar]
Sheth 2002 {published data only}
- Sheth K, Borker R, Emmett A, Rickard K, Dorinsky P. Cost‐Effectiveness Comparison of Salmeterol/Fluticasone Propionate versus Montelukast in the Treatment of Adults with Persistent Asthma. Pharmacoeconomics 2002;20(13):909‐918. [DOI] [PubMed] [Google Scholar]
Skalky 1999 {published data only}
- Skalky CS, Edelman JM, Polis A, Bird S, Gormley GJ, Israel E. Montelukast sodium (MK) compared to inhaled beclomethasone dipropionate (BD) in adult asthmatics: a randomized, clinical trial. Journal of Allergy & Clinical Immunology 1999;103(1 Part 2):Abs. 880, protocol # 070. [Google Scholar]
Smith 1993 {published data only}
- Smith LJ, Glass M, Minkwitz MC. Inhibition of leukotriene D4‐induced bronchoconstriction in subjects with asthma: a concentration‐effect study of ICI 204,219. Clinical Pharmacology & Therapeutics 1993;54(4):430‐6. [DOI] [PubMed] [Google Scholar]
Smith 1998 {published data only}
- Smith LJ, Hanby LA, Lavins BJ, Simonson SG. A single dose of zafirlukast reduces LTD4‐induced bronchoconstriction in patients on maintenance inhaled corticosteroid therapy. Annals of Allergy, Asthma, & Immunology 1998;81:43‐9. [DOI] [PubMed] [Google Scholar]
Spector 1992 {published data only}
- Spector SL, Glass M, Minkwitz MC, ICI Asthma Trial Group. The effect of six weeks of therapy with oral doses of ICI 204,219 in asthmatics. American Review of Respiratory Disease 1992;145(4):A16. [Google Scholar]
Spector 1994 {published data only}
- Spector SL, Smith LJ, Glass M. Effects of 6 weeks of therapy with oral doses of ICI 204,219, a leukotriene D4 antagonist, in subjects with bronchial asthma. American Journal of Respiratory & Critical Care Medicine 1994;150:618‐23. [DOI] [PubMed] [Google Scholar]
Spector 1995 {published data only}
- Spector S, Miller CJ, Glass M. 13‐week dose‐response study with Accolate (zafirlukast) in patients with mild to moderate asthma. American Journal of Respiratory & Critical Care Medicine 1995;151(4):A379. [Google Scholar]
Stanford 2002 {published data only}
- Stanford R H, Borker R, Dorinsky P, Pepsin P, Kalberg C, Emmett A, Rickard K. The costs and efficacy of fluticasone propionate/salmeterol combination versus montelukast in the treatment of adults with persistent asthma. Chest 2002:P422. [Google Scholar]
Stelmach 2002 {published data only}
- Stelmach I, Grzelewski T, Stelmach W, Majak P, Jerzynska J, Gorski P, Kuna P. Effect of triamcinolone acetonide, montelukast, nedocromil sodium and formoterol on eosinophil blood counts, ECP serum levels and clinical progression of asthma in children. Polski Merkuriusz Lekarski 2002;12(69):208‐213. [PubMed] [Google Scholar]
Stelmach 2002 (b) {published data only}
- Stelmach I, Jerzynska J, Kuna P. A randomized, double‐blind trial of the effect of glucocorticoid, antileukotriene and [beta]‐agonist treatment on IL‐10 serum levels in children with asthma. Clinical & Experimental Allergy 2002;32(2):264‐269. [DOI] [PubMed] [Google Scholar]
Storms 2001 {published data only}
- Storms W, Michele T M, Knorr B, Noonan G, Shapiro G, Zhang J, Shingo S, Reiss T F. Clinical safety and tolerability of montelukast, a leukotriene receptor antagonist, in controlled clinical trials in patients aged > or = 6 years. Clinical & Experimental Allergy 2001;31(1):77‐87. [DOI] [PubMed] [Google Scholar]
Suissa 1997 {published data only}
- Suissa S, Dennis R, Ernst P, Sheehy O, Wood‐Dauphinee S. Effectiveness of the leukotriene receptor antagonist zafirlukast for mild‐to‐moderate asthma: a randomized, double‐blind, placebo‐controlled trial. Annals of Internal Medicine 1997;126(3):177‐83. [DOI] [PubMed] [Google Scholar]
Svensson 1994 {published data only}
- Svensson C, Greiff L, Andersson M, Alkner U, Persson CGA. Bradykinin‐, leukotriene D4‐, and histamine‐induced mucosal exudation of plasma in human airways in vivo. Abbott Laboratories 1994:303.
Tashkin 1998 {published data only}
- Tashkin DP, Minkwitz MC, Bonuccelli CM. Zafirlukast (Accolate) treatment results in better asthma control in patients with more moderate disease. American Journal of Respiratory & Critical Care Medicine 1998;157(3):A411. [Google Scholar]
Terzano 2001 {published data only}
- Terzano C, Allegra L, Barkai L, Cremonesi G. Beclomethasone dipropionate versus budesonide inhalation suspension in children with mild to moderate persistent asthma. European Review for Medical & Pharmacological Sciences 2001. [PubMed] [Google Scholar]
Townley 1995 {published data only}
- Townley R, Glass M, Minkwitz MC. 6‐week, dose‐escalation study with Accolate (zafirlukast) in patients with mild to moderate asthma. American Journal of Respiratory & Critical Care Medicine 1995;151(4):A379. [DOI] [PubMed] [Google Scholar]
Tukiainen 2002 {published data only}
- Tukiainen H, Rytila P, Hamalainen K M, Silvasti M S L, Keski‐Karhu J. Safety, tolerability and acceptability of two dry powder inhalers in the administration of budesonide in steroid‐treated asthmatic patients. Respiratory Medicine 2002;96(4):221‐229. [DOI] [PubMed] [Google Scholar]
Verhoeven 2001 {published data only}
- Verhoeven GT, Garrelds IM, Hoogsteden HC, Zijlstra FJ. Effects of fluticasone propionate inhalation on levels of arachidonic acid metabolites in patients with chronic obstructive lung pulmonary disease. Mediators of Inflammation 2001;10(1):21‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vethanayagam 2002 {published data only}
- Vethanayagam D, Leigh R, Yoshida M, Watson RM, Rerecich T, Killian K, O'Byrne PM. Effects of montelukast and budesonide alone and together on allergen‐induced airway inflammation in asthma [abstract]. American Journal of Respiratory and Critical Care Medicine 2002;165(8 Suppl):A216. [DOI] [PubMed] [Google Scholar]
Vidal 2001 {published data only}
- Vidal C, Fernandez‐Ovide E, Pineiro J, et al. Comparison of montelukast versus budesonide in the treatment of exercise‐induced bronchoconstriction. Annals of Allergy, Asthma, & Immunology 2001;86(6):655‐8. [DOI] [PubMed] [Google Scholar]
Volovitz 1999 {published data only}
- Volovitz B, Tabachnik E, Nussinovitch M, Shtaif B, Blau H, Gii‐Ad I, et al. Montelukast, a leukotriene receptor antagonist, reduces the concentration of leukotrienes in the respiratory tract of children with persistent asthma. Journal of Allergy & Clinical Immunology 1999;104(6):1162‐7. [DOI] [PubMed] [Google Scholar]
Von Berg 2002 {published data only}
- Berg A, Albrecht B, Darlath W, Vo H W, Berdel D. Intraindividual, randomised, double‐blind comparison between sodium cromoglycate and reproterol to assess the protective effect of the single drugs and their combination in children with exercise‐induced asthma. Allergologie 2002;25(11):557‐564. [Google Scholar]
Wahedna 1991 {published data only}
- Wahedna I, Wisniewski AS, Tattersfield AE. Effect of RG 12525, an oral leukotriene D4 antagonist, on the airway response to inhaled leukotriene D4 in subjects with mild asthma. British Journal of Clinical Pharmacology 1991;32:512‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weinberg 1998 {published data only}
- Weinberg EG, Summerton L, Harris A. Assessment of preference for oral zafirlukast vs inhaled beclomethasone in adolescent asthmatics. ERS Annual Congress. 1998; Vol. 12, issue 28:abs P0333.
Welch 1994 {published data only}
- Welch MJ, Nelson HS, Paull BR, Smith JA, Feiss G, Tobey RE. Effect of RG 12525, a new leukotriene antagonist, on pulmonary function of asthmatic adults. Annals of Allergy 1994;72:348‐52. [PubMed] [Google Scholar]
Wenzel 1994 {published data only}
- Wenzel S, Cohn J, Trudeau J, Wilson W, Martin R, Westcott J. Zileuton (Leutrol) decreases urine LTE4, BALF LTB4 and improves lung function in nocturnal asthmatics. Abbott Laboratories 1994:302.
Wenzel 1995 {published data only}
- Wenzel SE, Trudeau JB, Kaminsky DA, Cohn J, Martin RJ, Westcott JY. Effect of 5‐lipoxygenase inhibition on bronchoconstriction and airway inflammation in nocturnal asthma. American Journal of Respiratory & Critical Care Medicine 1995;152(3):897‐905. [DOI] [PubMed] [Google Scholar]
Wenzel 1997 {published data only}
- Wenzel S, Chervinsky P, Kerwin E, Silvers W, Faiferman I, Dubb J. Oral pranlukast (Ultair) vs. inhaled beclomethasone: results of a 12‐week trial in patients with asthma. American Journal of Respiratory & Critical Care Medicine 1997;155:A203. [Google Scholar]
Westbroek 1998 {published data only}
- Westbroek J, Pasma HR, James MH, Thwaites RMA. Inhaled fluticasone propionate and oral zafirlukast in moderate asthma: a clinical and cost comparison [abstract]. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A416. [Google Scholar]
Westbroek 2000 {published data only}
- Westbroek J, Pasma HR. Effects of 2 weeks of treatment with fluticasone propionate 100 mcg b.d. by comparison with zafirlukast 20 mg b.d. on bronchial hyper‐responsiveness in patients with mild to moderate asthma. Respiratory Medicine 2000;94(2):112‐8. [DOI] [PubMed] [Google Scholar]
Williams 2000 {published data only}
- Williams B, Noonan G, Reiss TF, Knorr B, et al. Long‐term asthma control with oral montelukast and inhaled beclomethasone. Clinical & Experimental Allergy 2001;31:1‐10. [DOI] [PubMed] [Google Scholar]
Wilson 1999 {unpublished data only}
- Wilson AM. Comparative effects of oral montelukast and cetirizine versus intranasal mometasone furoate therapy in patients with seasonal allergic rhinitis. Ongoing trial 1999.
Wilson 1999 (b) {published data only}
- Wilson AM, Dempsey OJ, Sims EJ, Lipworth BJ. A comparison of salmeterol and montelukast as second‐line therapy in asthmatic patients receiving inhaled corticosteroids. European Respiratory Society 1999:P3486. [Google Scholar]
Wilson 2001 {published data only}
- Wilson AM, Sims EJ, Orr LC, Coutie WJ, White PS, Gardiner Q, Lipworth BJ. Effects of topical corticosteroid and combined mediator blockade on domiciliary and laboratory measurements of nasal function in seasonal allergic rhinitis. Annals of Allergy, Asthma, & Immunology 2001;87(4):344‐349. [DOI] [PubMed] [Google Scholar]
Wilson 2001 (c) {published data only}
- Wilson A M, Orr L C, Sims E J, Lipworth B J. Effects of monotherapy with intra‐nasal corticosteroid or combined oral histamine and leukotriene receptor antagonists in seasonal allergic rhinitis. Clinical & Experimental Allergy 2001;31(1):61‐68. [PubMed] [Google Scholar]
Wilson 2001a {published data only}
- Wilson Am, Orr LC, Sims EJ, Dempsey OJ. Antiasthmatic effects of mediator block versus topical corticosteroids in allergic rhinitis and asthma. American Journal of Respiratory & Critical Care Medicine 2001;162 (4 part 1):1297‐1301. [DOI] [PubMed] [Google Scholar]
Wilson 2001b {published data only}
- Wilson Am, Dempsey OJ, Sims EJ, Lipworth BJ. Evaluation of salmeterol or montelukast as second‐line therapy for asthma not controlled with inhaled corticosteroids. Chest 2001;119(4):1021‐6. [DOI] [PubMed] [Google Scholar]
Xiang 2001 {published data only}
- Xiang X D, Zhou R, Chen Y. Influence of zafirlukast on pulmonary functions and quality of life in patients with asthma. Bulletin of Hunan Medical University 2001;26(3):251‐253. [PubMed] [Google Scholar]
Yamamoto 1994 {published data only}
- Yamamoto H, Nagata M, Kuramitsu K, Tabe K, Kiuchi H, Sakamoto Y, et al. Inhibition of analgesic‐induced asthma by leukotriene receptor antagonist ONO‐1078. American Journal of Respiratory & Critical Care Medicine 1994;150:254‐7. [DOI] [PubMed] [Google Scholar]
Yamauchi 2001 {published data only}
- Yamauchi K, Tanifuji Y, Pan LH, et al. Effects of pranlukast, a leukotriene receptor antagonist, on airway inflammation in mild asthmatics. Journal of Asthma 2001;38(1):51‐7. [DOI] [PubMed] [Google Scholar]
Yoo 2001 {published data only}
- Yoo SH, Park SH, Song JS, et al. Clinical effects of pranlukast, an oral leukotriene receptor antagonist, in mild‐to‐moderate asthma: a 4‐week randomized multicentre controlled trial. Respirology 2001;6(1):15‐21. [DOI] [PubMed] [Google Scholar]
Yoshida 2000 {published data only}
- Yoshida S, Sakamoto H, Ishizaki Y, Onuma K, Shoji T, Nakagawa H, Hasegawa H, Nakabayashi M, Amayasu H. Efficacy of leukotriene receptor antagonist in bronchial hyperresponsiveness and hypersensitivity to analgesic in aspirin‐intolerant asthma. Clinical & Experimental Allergy 2000;30(1):64‐70. [DOI] [PubMed] [Google Scholar]
Yoshida 2002 {published data only}
- Yoshida M, Vethanayagam D, Leigh R, Matsumoto K, Watson RM, Reerich T, Killian KJ, O'Bryne PM. A comparison montelukast and budesonide effects on interleukin‐10 profiles in peripheral blood lymphocytes. American Journal of Respiratory and Critical Care Medicine 2002;165(Suppl 8):A217. [Google Scholar]
Zhang 1999 {unpublished data only}
- Zhang J, Chang Y, Reiss TF. Predicting future response using patient baseline variables and early responses to montelukast, a potent cysLT1 antagonist. Merck Research Laboratories 1999.
Zorc 2003 {published data only}
- Zorc J J, Scarfone R J, Li Y, Hong T, Harmelin M, Grunstein L, Andre J B. Scheduled follow‐up after a pediatric emergency department visit for asthma: A randomized trial. Pediatrics 2003;111(3):495‐502. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bilancia 2000 {published data only}
- Bilancia R, Margiotta D Balacco D. Low doses of budesonide (B) plus montelukast (M) versus high doses of budesonide in asthmatic patients [abstract]. American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A197. [Google Scholar]
Demuro‐Mercon 2001 {published data only}
- Demuro‐Mercon C, Turpin J, Santanello N, Firriolo K, Edelman J. Motelukast improves asthma quality of life when added to Fluticasone. Journal of Allergy and Clinical Immunology 2001;107(2):S248. [Google Scholar]
Li 2001 {published data only}
- Li J, Zhang DH, Yang JY. Evaluation of the therapeutic effects of zafirlukast in asthma patients during reduction of high‐dose inhaled corticosteroid. Journal of Clinical Lung Section 2001;6(4):9‐11. [Google Scholar]
References to ongoing studies
Barnes NC {unpublished data only}
- Barnes NC. A randomised multi‐centre, double‐blind study to evaluate the effect of adding montelukast sodium to inhaled budesonide compared to doubling the dose of inhaled budesonide in adult patients with asthma. Ongoing trial 2000.
Additional references
Anonymous 2001
- Anonymous. IND Safety Reports. Code of Federal Regulations‐ U.S.Government Printing Office via GPO Access 2001; Vol. Title 21,Volume 5; section 312.32:69‐71. Available from :http://www.fda.gov/cder/regulatory.
Australia 2002
- Asthma Management Handbook 2002. Vol. 5th edition, Melbourne: National Asthma Campaign, 2002. [Google Scholar]
Beveridge 1996
- Beveridge R, Grunfeld A, Hodder R, Verbeek P. Guidelines for the emergency management of asthma in adults. Canadian Medical Association Journal 1996;155(1):25‐37. [PMC free article] [PubMed] [Google Scholar]
Bootsma 1997
- Bootsma GP, Dekhuijzen PR, Festen J, Vanherwaarden CA. Effects of inhaled corticosteroids on bone [Review]. Netherlands Journal of Medicine 1997;50(6):254‐60. [DOI] [PubMed] [Google Scholar]
Boulet 2001
- Boulet LP, Bai TR, Becker A, Berube D, Beveridge R, Bowie DM, et al. What is new since the last (1999) Canadian Asthma Consensus Guidelines?. Can Respir J 2001;8(Suppl A):5a‐27a. [DOI] [PubMed] [Google Scholar]
BTS 2003
- Anonymous. British Guidelines on the Management of Asthma. Thorax 2003;58(Suppl):i1‐i94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cates 2002
- Cates CJ. Simpson's paradox and calculation of number needed to treat from meta‐analysis. Bio Med Central 2002;BMC Medical Research Methodology 2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cook 1993
- Cook DJ, Guyatt GH, Ryan G, Clifton J, Buckingham L, Willan A, et al. Should unpublished data be included in meta‐analyses? Current convictions and controversies. The Journal of the American Medical Association 1993;269:2749‐53. [PubMed] [Google Scholar]
CTS 1999
- Boulet LP, Becker A, Berube D, Beveridge R, Ernst P, et al. Canadian asthma consensus report, 1999. Canadian Medical Association Journal 1999;161(11 Suppl):S1‐S72. [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Ducharme 2001
- Ducharme FM, Hicks G. Anti‐leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma (Cochrane Review). The Cochrane Library 2001, Issue 2. [DOI] [PubMed] [Google Scholar]
Efthimiou 1998
- Efthimiou J, Barnes PJ. Effect of inhaled corticosteroids on bones and growth [Review]. The European Respiratory Journal 1998;11(5):1167‐77. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2002
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention. NIH Publication 02‐3659. Bethesda, MD: National Heart, Lung and Blood Institute, 2002. [Google Scholar]
Greenland 1985
- Greenland S, Robins JM. Estimation of a common effect parameter from sparse follow‐up data. Biometrics 1985;41:55‐68. [PubMed] [Google Scholar]
Jadad 1995
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized controlled trials: Is blinding necessary?. Controlled Clinical Trials 1995;134(17):1‐12. [DOI] [PubMed] [Google Scholar]
Kamada 1995
- Kamada AK, Szefler SJ. Glucocorticoids and growth in asthmatic children [Review]. Pediatric Allergy & Immunology 1995;6(3):145‐54. [DOI] [PubMed] [Google Scholar]
Khan 1996
- Khan KS, Daya S, Jahad AR. The importance of quality of primary studies in producing unbiased systematic reviews. Archives of Internal Medicine 1996;156:661‐6. [PubMed] [Google Scholar]
Mitchell 1992
- Mitchell EA. Consensus on acute asthma management in children. Ad Hoc Paediatric Group. The New Zealand Medical Journal 1992;105(941):353‐5. [PubMed] [Google Scholar]
Murphy 1993
- Murphy S, Kelly HW. Asthma, inflammation, and airway hyperresponsiveness in children. [Review]. Current Opinion in Pediatrics 1993;5(3):255‐65. [DOI] [PubMed] [Google Scholar]
Piper 1989
- Piper PJ. Leukotrienes and the airways. European Journal of Anaesthesiology 1989;6:241‐55. [PubMed] [Google Scholar]
Price 2000
- Price D. Tolerability of montelukast. Drugs 2000;59(suppl 1):35‐42. [DOI] [PubMed] [Google Scholar]
Rachelefsky 1993
- Rachelefsky GS, Warner JO. International consensus on the management of pediatric asthma: a summary statement. [Review]. Pediatric Pulmonology 1993;15(2):125‐7. [DOI] [PubMed] [Google Scholar]
Spahn 1996
- Spahn JD, Leung DYM. The role of glucocorticoids in the management of asthma. Allergy & Asthma Proceedings 1996;17(6):341‐50. [DOI] [PubMed] [Google Scholar]
Thornton 2000
- Thornton A, Lee P. Publication bias in meta‐analysis: its causes and consequences. Journal of Clinical Epidemiology 2000;53:207‐16. [DOI] [PubMed] [Google Scholar]
USA 2002
- National Asthma Education and Prevention Program. NAEPP Expert Panel Report Guidelines for the Diagnosis and Management of Asthma. NIH Publication 02‐5075. National Heart, Lung and Blood Institute. Bethesda, MD: National Institute of Health, 2002:1‐111. [Google Scholar]
Wolthers 1996
- Wolthers OD. Long‐, intermediate‐ and short‐term studies in asthmatic children treated with inhaled glucocorticoids [Review]. European Respiratory Journal 1996;9(4):821‐7. [DOI] [PubMed] [Google Scholar]
Wolthers 1998
- Wolthers OD, Honour JW. Hypothalamic‐pituitary‐adrenal function in children with asthma and rhinitis treated with topical glucocorticoids [Review]. Clinical & Experimental Allergy 1998;28(5):545‐50. [PubMed] [Google Scholar]


