Abstract
Background
Individuals dying of coronavirus disease 2019 (COVID‐19) may experience distressing symptoms such as breathlessness or delirium. Palliative symptom management can alleviate symptoms and improve the quality of life of patients. Various treatment options such as opioids or breathing techniques have been discussed for use in COVID‐19 patients. However, guidance on symptom management of COVID‐19 patients in palliative care has often been derived from clinical experiences and guidelines for the treatment of patients with other illnesses. An understanding of the effectiveness of pharmacological and non‐pharmacological palliative interventions to manage specific symptoms of COVID‐19 patients is required.
Objectives
To assess the efficacy and safety of pharmacological and non‐pharmacological interventions for palliative symptom control in individuals with COVID‐19.
Search methods
We searched the Cochrane COVID‐19 Study Register (including Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (PubMed), Embase, ClinicalTrials.gov, World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), medRxiv); Web of Science Core Collection (Science Citation Index Expanded, Emerging Sources); CINAHL; WHO COVID‐19 Global literature on coronavirus disease; and COAP Living Evidence on COVID‐19 to identify completed and ongoing studies without language restrictions until 23 March 2021.
We screened the reference lists of relevant review articles and current treatment guidelines for further literature.
Selection criteria
We followed standard Cochrane methodology as outlined in the Cochrane Handbook for Systematic Reviews of Interventions.
We included studies evaluating palliative symptom management for individuals with a confirmed diagnosis of COVID‐19 receiving interventions for palliative symptom control, with no restrictions regarding comorbidities, age, gender, or ethnicity. Interventions comprised pharmacological as well as non‐pharmacological treatment (e.g. acupressure, physical therapy, relaxation, or breathing techniques). We searched for the following types of studies: randomized controlled trials (RCT), quasi‐RCTs, controlled clinical trials, controlled before‐after studies, interrupted time series (with comparison group), prospective cohort studies, retrospective cohort studies, (nested) case‐control studies, and cross‐sectional studies.
We searched for studies comparing pharmacological and non‐pharmacological interventions for palliative symptom control with standard care.
We excluded studies evaluating palliative interventions for symptoms caused by other terminal illnesses. If studies enrolled populations with or exposed to multiple diseases, we would only include these if the authors provided subgroup data for individuals with COVID‐19. We excluded studies investigating interventions for symptom control in a curative setting, for example patients receiving life‐prolonging therapies such as invasive ventilation.
Data collection and analysis
We used a modified version of the Newcastle Ottawa Scale for non‐randomized studies of interventions (NRSIs) to assess bias in the included studies. We included the following outcomes: symptom relief (primary outcome); quality of life; symptom burden; satisfaction of patients, caregivers, and relatives; serious adverse events; and grade 3 to 4 adverse events.
We rated the certainty of evidence using the GRADE approach.
As meta‐analysis was not possible, we used tabulation to synthesize the studies and histograms to display the outcomes.
Main results
Overall, we identified four uncontrolled retrospective cohort studies investigating pharmacological interventions for palliative symptom control in hospitalized patients and patients in nursing homes. None of the studies included a comparator. We rated the risk of bias high across all studies. We rated the certainty of the evidence as very low for the primary outcome symptom relief, downgrading mainly for high risk of bias due to confounding and unblinded outcome assessors.
Pharmacological interventions for palliative symptom control
We identified four uncontrolled retrospective cohort studies (five references) investigating pharmacological interventions for palliative symptom control. Two references used the same register to form their cohorts, and study investigators confirmed a partial overlap of participants. We therefore do not know the exact number of participants, but individual reports included 61 to 2105 participants. Participants received multimodal pharmacological interventions: opioids, neuroleptics, anticholinergics, and benzodiazepines for relieving dyspnea (breathlessness), delirium, anxiety, pain, audible upper airway secretions, respiratory secretions, nausea, cough, and unspecified symptoms.
Primary outcome: symptom relief
All identified studies reported this outcome. For all symptoms (dyspnea, delirium, anxiety, pain, audible upper airway secretions, respiratory secretions, nausea, cough, and unspecified symptoms), a majority of interventions were rated as completely or partially effective by outcome assessors (treating clinicians or nursing staff). Interventions used in the studies were opioids, neuroleptics, anticholinergics, and benzodiazepines.
We are very uncertain about the effect of pharmacological interventions on symptom relief (very low‐certainty evidence). The initial rating of the certainty of evidence was low since we only identified uncontrolled NRSIs. Our main reason for downgrading the certainty of evidence was high risk of bias due to confounding and unblinded outcome assessors. We therefore did not find evidence to confidently support or refute whether pharmacological interventions may be effective for palliative symptom relief in COVID‐19 patients.
Secondary outcomes
We planned to include the following outcomes: quality of life; symptom burden; satisfaction of patients, caregivers, and relatives; serious adverse events; and grade 3 to 4 adverse events.
We did not find any data for these outcomes, or any other information on the efficacy and safety of used interventions.
Non‐pharmacological interventions for palliative symptom control
None of the identified studies used non‐pharmacological interventions for palliative symptom control.
Authors' conclusions
We found very low certainty evidence for the efficacy of pharmacological interventions for palliative symptom relief in COVID‐19 patients. We found no evidence on the safety of pharmacological interventions or efficacy and safety of non‐pharmacological interventions for palliative symptom control in COVID‐19 patients. The evidence presented here has no specific implications for palliative symptom control in COVID‐19 patients because we cannot draw any conclusions about the effectiveness or safety based on the identified evidence. More evidence is needed to guide clinicians, nursing staff, and caregivers when treating symptoms of COVID‐19 patients at the end of life. Specifically, future studies ought to investigate palliative symptom control in prospectively registered studies, using an active‐controlled setting, assess patient‐reported outcomes, and clearly define interventions.
The publication of the results of ongoing studies will necessitate an update of this review. The conclusions of an updated review could differ from those of the present review and may allow for a better judgement regarding pharmacological and non‐pharmacological interventions for palliative symptom control in COVID‐19 patients.
Plain language summary
Which treatments are best for symptoms in COVID‐19 patients at the end of life?
The burden of symptoms at the end of life of COVID‐19 patients and helpful treatments
COVID‐19 patients may show symptoms such as breathlessness or delirium at the end of life. The goal of palliative medicine is to relieve such symptoms with specific treatments. Treatments can be drugs, for example opioids, or non‐drugs, such as breathing techniques or relaxation.
What was the aim of our review?
To explore how well different interventions (drugs and non‐drugs) work for the treatment of palliative symptoms in COVID‐19 patients at the end of life. We included patients of all ages and with all comorbidities (additional medical conditions).
What type of studies did we search for?
We searched selected medical databases and trial registries until 23 March 2021. We included studies looking at how well different palliative treatments work to relieve COVID‐19‐associated symptoms at the end of life. We wanted to compare studies investigating different medicines or therapies, but we only found studies without a comparison group. Only one study reported the specific drugs used for individual symptoms.
Key results
We found four studies that were published in five papers. Individual papers included between 61 and 2105 participants, and two papers partially reported on the same participants. All of the included studies investigated different drug treatments for palliative symptom management in people with COVID‐19.
Drugs for symptom control at the end of life
All of the included studies reported on the effectiveness of palliative care for symptom relief. In all studies, clinicians or nursing staff rated symptom relief rather than the patients themselves. Since the quality of the evidence was very low, we do not know the true effect of drug treatments on symptom relief and have very low confidence in the results of the studies. We did not find any data on quality of life; symptom burden; satisfaction of patients, caregivers, and relatives; or safety of the drug treatments.
Non‐drug therapies for symptom control at the end of life
We did not find any data on the benefits and harms of non‐drug therapies for symptom control of COVID‐19 patients at the end of life.
Conclusions
Based on our findings, we could not draw any conclusions on palliative symptom control of people with COVID‐19. Future studies need to be designed better so that we can determine which treatments work for symptom control in people with COVID‐19.
Summary of findings
Summary of findings 1. Pharmacological interventions for palliative symptom control.
| Pharmacological interventions for palliative symptom control for patients with COVID‐19 | ||||
|
Patient or population: Patients with COVID‐19 Intervention: Pharmacological interventions Comparison: None Outcome: Palliative symptom control | ||||
| Outcome | Number of participants | Results | Certainty of the evidence | Plain text summary |
| Symptom relief | 4 studies (5 references) with 61 to 2105 participants in individual studies1 | All studies rated a majority of interventions as effective in relieving symptoms of breathlessness, agitation, dyspnea, delirium, pain, and others. |
Very low Due to study design2 and high risk of bias3 |
We are very uncertain about the effect of palliative care interventions including opioids, neuroleptics, anticholinergics, and benzodiazepines on symptom relief. |
| Quality of life | Based on data from 0 participants in 0 studies Follow‐up: none |
Outcome not reported. | N/A | Outcome not reported in any study. |
| Symptom burden | Based on data from 0 participants in 0 studies Follow‐up: none |
Outcome not reported. | N/A | Outcome not reported in any study. |
| Satisfaction of patients | Based on data from 0 participants in 0 studies Follow‐up: none |
Outcome not reported. | N/A | Outcome not reported in any study. |
| Satisfaction of caregivers | Based on data from 0 participants in 0 studies Follow‐up: none |
Outcome not reported. | N/A | Outcome not reported in any study. |
| Satisfaction of relatives | Based on data from 0 participants in 0 studies Follow‐up: none |
Outcome not reported. | N/A | Outcome not reported in any study. |
| Serious adverse events | Based on data from 0 participants in 0 studies Follow‐up: none |
Outcome not reported. | N/A | Outcome not reported in any study. |
| Grade 3 to 4 adverse events | Based on data from 0 participants in 0 studies Follow‐up: none |
Outcome not reported. | N/A | Outcome not reported in any study. |
| N/A: Not applicable | ||||
1Participants of the two cohorts reported on in Strang 2021 partially overlap, therefore we do not know the exact number of participants.
2 Initial rating of certainty of the evidence: Rated low because all identified studies had a retrospective and uncontrolled design. 3Risk of bias: high. Lack of blinding of participants and personnel, resulting in the potential for detection bias in all studies. Lack of blinding of outcome assessors, resulting in the potential for detection bias in all studies. Selective outcome reporting, follow‐up not reported, missing intention‐to‐treat analysis, use of unvalidated outcome measures in Alderman 2020, Lovell 2020, and Hetherington 2020, and use of subjective outcome measures in all studies. Imprecision: none. Publication bias: none. Inconsistency: difficult to assess. Indirectness of evidence: difficult to assess.
Background
This work is part of a series of Cochrane Reviews investigating treatments and therapies for COVID‐19. Reviews of this series share information in the Background section and methodology based on the first published reviews about monoclonal antibodies, Kreuzberger 2020, and convalescent plasma (Valk 2020a).
Description of the condition
COVID‐19 is a highly infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2; WHO 2020a). On 22 March 2020, the World Health Organization (WHO) declared the current COVID‐19 outbreak a pandemic. COVID‐19 is unprecedented in comparison to previous coronavirus outbreaks, such as severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS), with 813 and 858 deaths, respectively (WHO 2007; WHO 2019). Despite intensive international efforts to contain its spread, it has resulted in more than 180 million confirmed cases and more than 4 million deaths worldwide until July 2021 (WHO 2021a; WHO 2021b).
Several vaccines against COVID‐19 have been distributed across countries, and an additional hundred vaccine candidates are in development at the time of the writing of this review (WHO 2021d). However, the process is time‐consuming, and global access to vaccines differs widely (Wouters 2021). Moreover, the degree to which the vaccines can protect against variants of SARS‐CoV‐2 was still unclear at the date of publication (Forni 2021).
Specific risk factors for severe disease, hospitalization, and mortality have been identified: individuals aged 65 years or older, smokers, and those with certain underlying medical conditions, such as cancer, chronic kidney disease, chronic obstructive pulmonary disease (COPD), heart conditions, immunocompromised state, obesity, sickle cell disease, or diabetes mellitus are more likely to have a severe course of the disease (Huang 2020; Liang 2020; WHO 2020a; Williamson 2020). COVID‐19 case fatality varies widely between countries and reporting periods (from 1% to more than 19%; Johns Hopkins 2021). However, these numbers may be misleading due to varying testing frequency, lag in reporting dates, incomplete capturing of all cases, and variations in case definitions since the beginning of the pandemic (WHO 2020b).
Sore throat, cough, fever, headache, fatigue, and myalgia or arthralgia are the most commonly reported symptoms (Struyf 2020). Other symptoms include dyspnea, chills, nausea or vomiting, diarrhea, loss of taste and smell, and nasal congestion (WHO 2020a). The majority of people infected at the beginning of the pandemic had mild symptoms (approximately 80%, Wu 2020), or remained completely asymptomatic (Buitrago‐Garcia 2020). Early data from China show that a smaller proportion (approximately 14%) are affected by severe or critical disease with intensive care unit (ICU) admittance due to respiratory failure, septic shock, or multiple organ dysfunction (Wu 2020).
At the time of the writing of this review, treatment consisted of supportive care with extracorporeal membrane oxygenation (ECMO), invasive ventilation, and non‐invasive ventilation in severe cases, and oxygen supply in moderately severe cases (WHO 2021c). Few drugs were approved for the treatment of COVID‐19, such as corticosteroids, monoclonal antibodies, or convalescent plasma. Recommendations for the use of corticosteroids, Siemieniuk 2020, and tocilizumab, Taskforce NCCE 2021; WHO 2021e, were given in clinical guidelines, but only for patients with severe or critical COVID‐19 infection receiving oxygen. Other drugs, such as ivermectin and hydroxychloroquine, were not recommended for the treatment of COVID‐19 at the point of review publication (WHO 2021e). Guidelines for symptom control (e.g. dyspnea) were mostly informed by studies of cancer patients and patients with COPD (Barnes 2016). As the course of COVID‐19 is quite different from these diseases with respect to the rapid onset of symptoms (e.g. dyspnea) and the underlying cause of symptoms, evidence on interventions in COVID‐19 patients is needed.
In light of evolving variants of the virus with increased transmissibility and possibly higher mortality (Challen 2021), the number of COVID‐19‐associated deaths might increase the need for adequate symptom control in a palliative situation. Moreover, we do not know if the needs of patients with COVID‐19 will change with the appearance of new variants. The most prevalent symptoms in hospitalized patients with COVID‐19 are dyspnea, cough, fatigue, myalgia (muscle pain), and delirium, with dyspnea being the most significant symptom in the dying (Keeley 2020). Delirium might be more prevalent in patients suffering from COVID‐19 when compared to other diseases (Barron 2012; Kennedy 2020). Especially in the dying, multimodal (pharmacological and non‐pharmacological) interventions are needed to alleviate symptoms and thus achieve best possible quality of life. If possible, these interventions should be provided by multiprofessional palliative care teams, but also by all other disciplines, such as intensive care or general medicine.
Description of the intervention
According to a recent consensus‐based definition, palliative care is "the active holistic care of individuals across all ages with serious health‐related suffering due to severe illness and especially of those near the end of life (Radbruch 2020)." Adequate palliative symptom control can significantly improve the quality of life for individuals and their families. This is considered in the WHO 2020c definition of palliative care: "Palliative care is an approach that improves the quality of life of patients and their families facing the problems associated with life‐threatening illness, through the prevention and relief of suffering by means of early identification and impeccable assessment and treatment of pain and other problems, physical, psychosocial and spiritual." COVID‐19 may be incurable or curable, and the treatment goals for these patients may change within hours. Based on the definition of palliative care by Radbruch 2020, we only included studies for patients “near the end of life,” and excluded studies addressing patients with curative treatment goals. We define “palliative symptom control” as palliative (not curative) interventions that aim at amelioration of symptoms in advanced COVID‐19.
Palliative symptom control utilizes both pharmacological and non‐pharmacological interventions, which are often provided by multiprofessional teams. At present, it is unclear which specific palliative interventions should be used for symptom control for individuals with COVID‐19.
Pharmacological interventions might include the use of opioids and second‐line benzodiazepines for the relief of dyspnea, or antipsychotics to alleviate symptoms of delirium (Mottiar 2020). Anticholinergics are used in the dying to reduce airway secretions ('death rattle') (Mercadamte 2014). Non‐pharmacological interventions include alternative interventions (e.g. Traditional Chinese Medicine) and psychological support, but also measures such as the discontinuation of interventions (Mottiar 2020). Non‐pharmacological interventions can be a prelude to pharmacological interventions or can be used alongside pharmacological treatments. Even though research on symptom control is scarce, the National Institute for Health and Care Excellence (NICE) has published consensus‐based guidelines for managing COVID‐19‐associated symptoms at the end of life (NICE 2020). For example, pharmacological treatments such as codeine linctus, codeine phosphate tablets, or morphine sulfate oral solution are recommended to treat breathlessness and cough; non‐pharmacological treatments include controlled breathing techniques.
Palliative care interventions provided by palliative care teams decrease symptom intensity and improve quality of life among individuals with advanced cancer compared to standard cancer care alone (Gaertner 2017; Haun 2017). Similiar positive outcomes can be expected in COVID‐19 patients if treated by palliative care teams.
How the intervention might work
The subjective perception of dyspnea, the difficulty to breathe, can appear without hypoxia or hypercapnia, but may be mediated via blood gas abnormalities (increase of partial pressure of carbon dioxide, decrease of oxygen, or both), detected by central chemoreceptors and processed in the respiratory center in the medulla and by cortical structures (Buchanan 2009). Furthermore, the muscular respiratory effort, as well as emotional, social, and psychological factors may significantly contribute to the sensation of dyspnea (Crombeen 2020; von Leupoldt 2007). Endogenous opioids may modulate breathlessness perception to be less unpleasant (Johnson 2020; Mahler 2013). Opioids may alleviate dyspnea via an altered response of the central nervous system to blood gas changes, resulting in a reduction of the respiratory drive (Banzett 2000; Pattinson 2009). Opioid‐induced pain reduction and sedation decrease oxygen demand and carbon dioxide production. Benzodiazepines induce anxiolytic, sedative, and anti‐agitative effects by gamma‐aminobutyric acid (GABA)‐receptor modulation (Griffin 2013). Antipsychotics (neuroleptics) for treatment of (terminal) delirium may act through antagonism at the dopamine (D2)‐receptor (Hui 2020; Meagher 2018). Anticholinergics may reduce 'death rattle' through reducing airway secretions (Mercadamte 2014). Non‐pharmacological interventions include psychological interventions (e.g. relaxation, imagination practices, controlled breathing techniques). They have beneficial effects on dyspnea through the reduction of tachypnea (von Leupoldt 2007).
Why it is important to do this review
There is a clear, urgent need for more information to guide symptom control and end‐of‐life care in people with COVID‐19. Management of the symptoms most frequently encountered in COVID‐19 patients, such as dyspnea, cough, fatigue, myalgia and agitation and delirium, is a central component of palliative care and reduces suffering at the end of life. Adequate symptom relief is therefore of utmost importance for patients, but also for relatives, and loved ones. Importantly, palliative care in the context of a pandemic poses new challenges, as the care has to be delivered in a quarantined context and often without families visiting the patient. Various recommendations for the control of these symptoms have been made in expert opinion statements by professional societies (Nehls 2020; NICE 2020). In addition, the first studies investigating symptom control in COVID‐19 have been published. A systematic review and subsequent update of the available literature is needed to inform recommendations for action on symptom control of COVID‐19 in the palliative setting.
Objectives
To assess the efficacy and safety of pharmacological and non‐pharmacological interventions for palliative symptom control in individuals with COVID‐19.
Methods
Criteria for considering studies for this review
Types of studies
The main description of methods is based on the template for intervention reviews with non‐randomized studies of the Cochrane Haematology review group. The protocol for this review was registered with the international prospective register of systematic reviews (PROSPERO) (Andreas 2021).
We planned to include randomized controlled trials (RCTs), and, if these were not available, the following types of studies in a top‐down approach: quasi‐RCTs, controlled clinical trials, controlled before‐after studies, interrupted time series (with comparison group), prospective cohort studies, retrospective cohort studies, (nested) case‐control studies, and cross‐sectional studies.
As planned at the protocol stage, we included non‐comparative study designs because we did not expect any evidence from RCTs. Randomized controlled studies are challenging in the palliative care setting for multiple reasons. For example, using placebo (or other) controls might not be ethically justifiable, as it could lead to unnecessary suffering at the end of life. Furthermore, dying people are often unable to consent to studies, and consent by proxy is complicated by the grief of the relatives. Especially in the field of palliative care, such issues are of major importance, because the vulnerable population and the unstable nature of the underlying diseases are associated with unexpected recruitment and attrition problems that demand the conduction of thoroughly performed feasibility trials (Shelby‐James 2012).
We thus do not expect that any RCTs on this topic will be published soon. One controlled study investigating the effectiveness of morphine in the treatment of dyspnea in COVID‐19 is currently being conducted (NCT04522037). However, information on the most effective symptom control in individuals with COVID‐19 is acutely needed. For this reason, we decided to also include non‐controlled studies.
We included studies with one or more participant(s) with COVID‐19. We followed the suggestions specified in the Cochrane Handbook for Systematic Reviews of Interventions to the greatest degree possible and applied the methodology outlined in the following sections of this review (Higgins 2021). Further information on the methods we had planned should we have identified RCTs or non‐randomized studies of interventions (NRSIs) is provided in Appendix 1.
We included full‐text publications, preprints, abstract publications, results published in trials registries, and information received from personal communication with investigators if sufficient information was available on study design, characteristics of participants, interventions, and outcomes. We did not apply any limitations with respect to study setting (home‐based, hospital, hospices, or nursing homes).
Types of participants
We included individuals with a confirmed diagnosis of COVID‐19 receiving interventions for palliative symptom control with no restrictions regarding comorbidities, age, gender, or ethnicity.
We excluded studies evaluating palliative interventions for symptoms caused by other terminal illnesses. If studies enrolled populations with or exposed to diseases, we would only include such studies if the authors provided subgroup data for SARS‐CoV‐2 infection. We excluded studies investigating interventions for symptom control in a curative setting, for example patients receiving life‐prolonging therapies such as invasive ventilation.
Types of interventions
We defined 'palliative symptom control' as palliative (not curative) interventions that aim to ameliorate symptoms in advanced COVID‐19. We included the following interventions: palliative symptom control as a multidimensional and holistic approach, including:
pharmacological interventions (including but not limited to opioids, benzodiazepines, neuroleptics, and anticholinergics);
non‐pharmacological interventions (including but not limited to acupressure, music therapy, physical therapy, distraction, breathing techniques, and relaxation).
In future updates, we plan to include the following comparisons for studies with a control arm:
pharmacological intervention A (e.g. opioids) versus pharmacological intervention B or placebo for symptom control of dyspnea, cough, agitation/delirium;
specialized palliative care versus standard care (specialized palliative care is given by specialized palliative care teams (e.g. palliative care consultation services in hospitals) in contrast to general palliative care (e.g. given by general practitioners)).
Types of outcome measures
We planned to evaluate the following outcomes.
Primary outcomes
Symptom relief, comprising any change of subjective and potentially burdensome symptoms like dyspnea/shortness of breath, cough, anxiety, agitation, fatigue, myalgia, and delirium between baseline measurement before intervention and after intervention, measured with validated patient‐reported outcome measures (e.g. visual analogue scale (VAS)), or other standardized instruments reported by patients, family members, or caregivers.
Secondary outcomes
Quality of life, including fatigue and neurological functions, assessed with standardized scales (e.g. European Organisation for Research and Treatment of Cancer (EORTC), McGill Quality of Life Questionnaire).
Symptom burden (e.g. distress thermometer, IPOS).
Satisfaction of patients.
Satisfaction of caregivers and relatives.
Serious adverse events, defined as number of participants with event.
Grade 3 to 4 adverse events, defined as number of participants with event.
Search methods for identification of studies
Electronic searches
For the identification of studies on interventions for palliative symptom control of COVID‐19, we designed search strategies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Lefebvre 2021). KG developed the search strategy based on input by clinicians. The search strategy was peer‐reviewed by two Information Specialists experienced in the terminologies used in COVID‐19 research (Ina Monsef and Maria‐Inti Metzendorf). Due to the international urgency for research on COVID‐19, we assumed that the abstracts of clinical trials would have been published in English. If the full‐text publication was published in a language outside the abilities of our team, we would have involved Cochrane Task Exchange to identify people who were able to translate (taskexchange.cochrane.org).
Searches for evidence synthesis
We initially conducted a search for existing or planned evidence synthesis in the following sources.
Manual search
Evidence Aid Coronavirus (COVID‐19) (evidenceaid.org/evidence/coronavirus-covid-19/)
Coronavirus (COVID‐19) (the Cochrane Library) (www.cochranelibrary.com/covid-19) including Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (PubMed), Embase, ClinicalTrials.gov, World Health Organization International Clinical Trials Registry Platform (WHO ICTRP), medRxiv
Usher Network for COVID‐19 Evidence Reviews (www.ed.ac.uk/usher/uncover)
US Department of Veterans Affairs Evidence Synthesis Program (www.hsrd.research.va.gov/publications/esp/)
Australian guidelines for the clinical care of people with COVID‐19 (https://files.magicapp.org/guideline/8b6f065b-814f-41f0-a1a5-70279b722e19/published_guideline_4346-12_0.pdf)
Norwegian Institute of Public Health systematic and living map on COVID‐19 evidence (www.fhi.no/en/qk/systematic-reviews-hta/map/)
COVID‐19 Evidence Alerts from McMaster PLUS (www.evidencealerts.com/)
L*OVE (iloveevidence.com/)
TRIP (www.tripdatabase.com/)
ECRI COVID‐19 Resource Center (www.ecri.org/coronavirus-covid-19-outbreak-preparedness-center/)
JBI Evidence Synthesis COVID‐19 Collection (jbi.global/covid-19)
NICE Coronavirus (COVID‐19) (www.nice.org.uk/guidance/conditions-and-diseases/respiratory-conditions/covid19)
Database search
MEDLINE (Ovid)
Manual search (planned evidence synthesis)
Oxford COVID‐19 Evidence Service—Current questions under review (www.cebm.net/oxford-covid-19-evidence-service/)
Cochrane COVID Review Bank (covidreviews.cochrane.org/search/site)
PROSPERO (www.crd.york.ac.uk/prospero/)
These initial searches were conducted on 27 November 2020. The search strategies are documented in Appendix 2, Appendix 3, and Appendix 4.
Searches for primary studies
We searched the following databases and trials registries for primary studies without any language limits, initially on 8 January 2021 and updated on 23 March 2021.
Cochrane COVID‐19 Study Register (covid-19.cochrane.org/)
Web of Science (Science Citation Index/Emerging Sources)
CINAHL (via EBSCO) (Cumulative Index to Nursing and Allied Health Literature)
World Health Organization COVID‐19 Global literature on coronavirus disease (search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/)
COAP Living Evidence on COVID‐19 (zika.ispm.unibe.ch/assets/data/pub/search_beta/)
The search strategies are documented in Appendix 2, Appendix 3, and Appendix 5.
If results were uploaded into trials registries and had not yet been published elsewhere, we integrated these data for the current review, and will add or replace data in future updates of this review in the case of publication.
Searching other resources
We handsearched reference lists of any included articles in order to identify any further relevant studies.
We checked the reference lists of all identified studies and relevant review articles identified by an additional literature search (the initial search) and current treatment guidelines for further literature. Please see Appendix 4 for additional information on our strategy.
Data collection and analysis
Selection of studies
Three members of the review team (MA, CB, and LJ) independently screened the results of the search for eligibility by reading the abstracts. We coded the abstracts as either 'include' or 'exclude' using the software Rayyan (Ouzzani 2016). In the case of disagreement, or if it was unclear whether we should retrieve the abstract or not, we obtained the full‐text publication for further discussion. Two review authors (MA and CB) assessed the full‐text articles of selected studies. In case of disagreement, a third review author (VP) was consulted to reach a final decision.
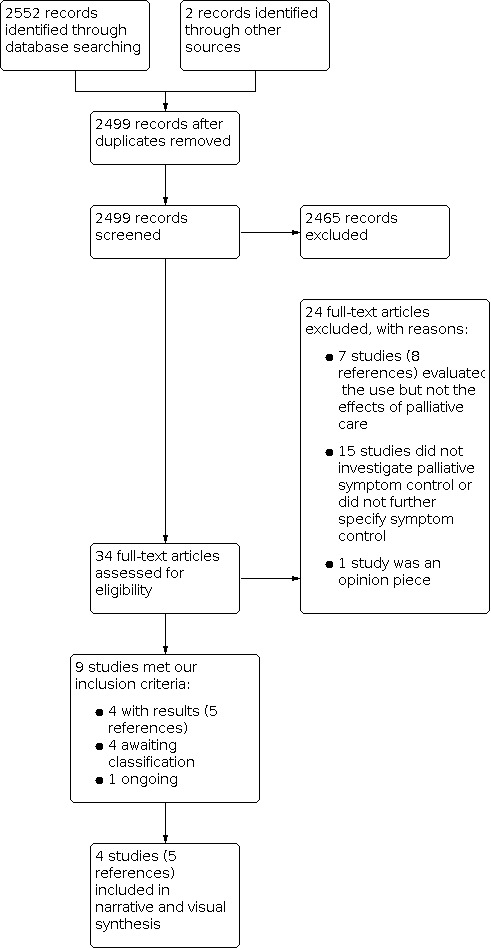
We documented the study selection process in a flow chart, as recommended in the PRISMA statement (Moher 2009), and show the total numbers of retrieved references and the numbers of included and excluded studies (see Figure 1). Articles excluded after full‐text assessment and the reasons for their exclusions are provided in Characteristics of excluded studies.
1.
Study flow diagram.
Data extraction and management
We conducted data extraction and assessments according to the guidelines proposed by Cochrane (Li 2021). Two out of three review authors (MA, MB, and CB) performed all data extraction and assessments. Two other review authors (VP and WM) verified the accuracy and (where applicable) the plausibility of data extraction and assessments. We collated multiple reports of one study so that each study, rather than each report, was the unit of analysis. We extracted data using a customized data extraction form developed in Microsoft Excel (Microsoft 2018), and extracted the following information.
General information: author, title, source, publication date, and country.
Quality assessment and risk of bias: study design, confounding, selection bias, attrition bias, detection bias, and reporting bias.
Study characteristics: study design, setting, and dates, source of participants, inclusion/exclusion criteria, comparability of groups, compliance with assigned treatment, and length of follow‐up.
Participant characteristics: age, gender, number of participants recruited/allocated/evaluated, disease, severity of disease, comorbidity, prevalence of symptoms and treated symptoms.
Interventions: pharmacological and non‐pharmacological treatment and mode of drug delivery.
Outcomes: as specified in Types of outcome measures.
Assessment of risk of bias in included studies
We planned that if RCT data were available, we would use the RoB 2 tool to analyze the risk of bias in the underlying study results (Sterne 2019). If NRSI data were available, we would use the Risk Of Bias In Non‐randomised Studies—of Interventions (ROBINS‐I) tool (Sterne 2016). Detailed information on how we had planned to assess risk of bias of RCTs and NRSIs is provided in Appendix 1.
To assess risk of bias in uncontrolled studies, we used a modified version of the Newcastle Ottawa Scale, provided by Mulder 2019.
As specified in Types of studies, we included uncontrolled studies only when we were unable to identify controlled studies.
Two review authors (MA and CB) assessed the included studies for methodological quality and risk of bias in accordance with the criteria outlined below and in Table 2. Any disagreements regarding the quality assessments were resolved by discussion, and two review authors (VP and WM) verified the accuracy and the plausibility of assessments. We performed and presented our judgements per outcome per study.
1. Risk of bias assessment criteria for observational studies.
| Heading | Internal validity | External validity |
| Study group |
Selection bias (unrepresentative study group)
The study group was considered representative,
or
|
Reporting bias (poorly defined study group)
The study group was considered as well defined,
and
|
| Follow‐up |
Attrition bias (incomplete outcome assessment/follow‐up ) The outcome assessment and follow‐up was considered as complete,
or
|
Reporting bias (poorly defined follow‐up) The follow‐up was considered as well defined,
|
| Outcome |
Detection bias (outcome assessors unblinded to investigated determinant) The detection bias was considered as low,
|
Reporting bias (poorly defined outcome) The outcome definition was considered as well defined,
|
| Risk estimation |
Confounding (important prognostic factors or follow‐up not adequately taken into account) Risk of confounding was considered as low,
|
Analyses (poorly defined risk estimates) The risk estimates were considered as well defined,
|
We assessed the following risk of bias domains.
Internal validity
Unrepresentative study group (selection bias)
Incomplete outcome assessment/follow‐up (attrition bias)
Outcome assessors unblinded to investigated determinants (detection bias)
Important prognostic factors or follow‐up not taken adequately into account (confounding)
External validity
Poorly defined study group (reporting bias)
Poorly defined follow‐up (reporting bias)
Poorly defined outcome (reporting bias)
Poorly defined risk estimates (analyses)
For every criterion, we made a judgement using one of three response options:
high risk of bias;
low risk of bias;
unclear risk of bias.
We used the highest rating to inform our overall risk of bias judgement per study outcome.
Measures of treatment effect
How we planned to measure the treatment effects of RCTs and NRSIs is discussed in Appendix 1.
For uncontrolled studies, we did not carry out an analysis using quantitative data from indirect controls, as we are aware of the difficulties of indirect comparisons of participant groups with varying baseline characteristics, especially in the absence of individual patient data. We did not meta‐analyze the data, but provided information from individual studies per outcome within tables.
Unit of analysis issues
We collated multiple reports of one study so that each study, rather than each report, was the unit of analysis. We did not combine any data from different study designs.
How we planned to resolve unit of analysis issues occurring in RCTs is discussed in Appendix 1.
Dealing with missing data
A number of potential sources of missing data are suggested in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions, which we took into account: at study level, at outcome level, and at summary data level (Higgins 2021). In the first instance, it is of the utmost importance to differentiate between data 'missing at random' and 'not missing at random.'
We requested missing data from the study authors. The authors of two studies provided us with missing data on interventions used and outcome assessment (Alderman 2020; Strang 2021). Additionally, we requested information on the exact numbers of participants in Strang 2021, as there was an overlap between the two cohorts reported on in the study.
Assessment of heterogeneity
As we identified uncontrolled studies only, meta‐analysis was not appropriate. Instead, we described and presented results per study in tables, and discussed potential heterogeneity based on the methodological and clinical components of each included study.
How we planned to assess heterogeneity in meta‐analysis is discussed in Appendix 1.
Assessment of reporting biases
As mentioned above, we searched trial registries to identify completed studies not published elsewhere, in order to minimize or determine whether there was publication bias.
How we plan to assess publication bias in future versions of this review is discussed in Appendix 6.
Data synthesis
We planned that if the clinical and methodological characteristics of individual studies were sufficiently homogeneous, we would pool data in meta‐analysis. We planned to perform analyses according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021). We would not conduct meta‐analyses that involved both RCTs and NRSIs. We planned to conduct separate meta‐analyses for each comparison. How we planned to synthesize data from RCTs and NRSIs is discussed in Appendix 1.
Meta‐analysis was not possible, therefore we synthesized study data without meta‐analysis, using the Synthesis Without Meta‐analysis (SWiM), Campbell 2020, guideline and Chapter 24 of the Cochrane Handbook for Systematic Reviews of Interventions, Reeves 2021, to inform our approach.
We presented outcome data individually per study within tables. For each table, we grouped studies per outcome and collated information on key study characteristics. We included information on study size, treated symptoms, and interventions for each included study. Furthermore, we visualized observed effects for each outcome and per study using histograms to portray the reported effects, so that outcome frequencies (e.g. for symptom relief) can be visually displayed and easily grasped by readers. We also noted risk of bias by color‐coding and signs to guide the reader's interpretation of our synthesis.
Subgroup analysis and investigation of heterogeneity
Lack of adequate data precluded subgroup analysis.
How we plan to conduct subgroup analysis in future versions of this review is discussed in Appendix 6.
Sensitivity analysis
Lack of adequate data precluded sensitivity analysis.
How we plan to conduct sensitivity analysis in future versions of this review is discussed in Appendix 6.
Summary of findings and assessment of the certainty of the evidence
We created one summary of findings table and evaluated the certainty of the evidence using the GRADE approach.
Summary of findings
We used the MAGICapp software to create a summary of findings tables (MAGICapp 2020).
According to Chapter 14 of the updated Cochrane Handbook for Systematic Reviews of Interventions, the “most critical and/or important health outcomes, both desirable and undesirable, limited to seven or fewer outcomes” should be included in the summary of findings table(s) (Schünemann 2021). We prioritized outcomes most relevant for individuals with terminal illness, as follows.
Symptom relief.
Quality of life, including fatigue and neurological functions, assessed with standardized scales (e.g. McGill Quality of Life Questionnaire).
Symptom burden (e.g. distress thermometer, IPOS).
Satisfaction of patients.
Satisfaction of caregivers and relatives.
Serious adverse events, defined as number of participants with event.
Grade 3 to 4 adverse events, defined as number of participants with event.
Assessment of the certainty in the evidence
We used the GRADE approach to assess the certainty of the evidence for the outcomes listed above.
The GRADE approach uses five domains (risk of bias, consistency of effect, imprecision, indirectness. and publication bias) to assess the certainty of the body of evidence for each prioritized outcome. According to GRADE guidance 18 (Schünemann 2019), the initial rating for randomized trials and NRSIs (the latter rated with ROBINS‐I) is high certainty. As reported in the GRADE guidance 3, uncontrolled studies start from low‐certainty evidence (Balshem 2011).
The certainty of the evidence can be downgraded for the following reasons:
serious (−1) or very serious (−2) study limitations; or moderate (−1), serious (−2), or critical (−3) study limitations for NRSIs;
serious (−1) or very serious (−2) inconsistency;
serious (−1) or very serious (−2) indirectness;
serious (−1) or very serious (−2) imprecise or sparse data;
serious (−1) or very serious (−2) publication bias.
The certainty of the evidence can be upgraded for uncontrolled studies for:
large effects;
dose‐response; and
plausible confounding.
The GRADE system uses the following criteria for assigning grades of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
We followed the current GRADE guidance for these assessments in its entirety as recommended in Chapter 14 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021). We used our overall risk of bias judgements to inform decisions on downgrading for study limitations. We phrased the findings and certainty of the evidence as suggested in the informative statement guidance of the GRADE guidance (Santesso 2020).
Results
Description of studies
Results of the search
We identified 2554 potentially relevant references (2552 from database searching and 2 from other sources). After removal of duplicates, we screened 2499 references based on their titles and abstracts, excluding 2465 references as irrelevant because they did not meet the prespecified inclusion criteria. We screened the full texts of the remaining 34 references, or, if these were not available, abstract publications or trial registry entries. We identified 9 eligible studies, four of which were assessed as awaiting classification as (complete) results had not yet been published (ChiCTR2000029994; Groninger 2021; Kelly 2020; Okuwoga 2020), and one study as ongoing as participants were still being recruited (NCT04522037).
The process and results of study selection are documented in the PRISMA flow diagram (Figure 1).
Included studies
Design and settings
An overview of the included studies and their characteristics is provided in Table 3.
2. Overview of included studies .
|
|
Alderman 2020 | Hetherington 2020 | Lovell 2020 | Strang 2021 |
| Study characteristics | ||||
| Setting |
|
|
|
|
| Design | Retrospective cohort study | Retrospective cohort study | Retrospective cohort study | Retrospective cohort study |
| Protocol | None published. | None published. | None published. | None published. |
| Number of participants | 61 | 186 | 101 | Unknown* |
| Symptoms treated | Shortness of breath, delirium, audible upper airway secretions, cough, pain, nausea | Not specified | Not specified | Cohort 1: breathlessness, anxiety, delirium, audible upper airway secretions, pain Cohort 2: breathlessness, anxiety, delirium, respiratory secretions |
| Outcome assessed | Symptom relief (assessed by clinicians) | Cinical impression of efficacy | Clinical impression of effectiveness | Symptom relief (assessed by clinicians) |
| Participant characteristics | ||||
| Age of participants | Median age 82 (IQR 53 to 98) years | Median age 76 (IQR 71 to 84) years | Median age 82 (IQR 72 to 89) years | First cohort: mean age 84.7 (range 47 to 104) Second cohort:
|
| Gender (male (n (%))) | 34 (55.5%) | 98 (52.6%) | 64 (45.5%) | Cohort 1: 201 (52%) Cohort 2:
|
| Comorbidities (%) | Dementia (13%), neurological disease (8%), cardiovascular disease (42.5%), hypertension (36%), respiratory disease (23%), renal disease (18%), diabetes mellitus (19.5%), cancer (24.5%) | Hypertension (31.2%), diabetes mellitus (28%), chronic obstructive pulmonary disease (26.9%) | Hypertension (53%), diabetes (35.6%), dementia (30.6%), advanced/metastatic cancer (24.7%), chronic pulmonary disease (21.7%), renal failure (20.7%), congestive heart failure (17.8%), stroke/neurological disorder (11.8%), peripheral vascular disorder (0.04%), liver disease (0.02%) | None reported. |
Abbreviations: IQR: interquartile range
*We do not know the exact number of participants since the cohorts reported on partially overlap. The first cohort included 390 participants, and the second cohort 2105 participants. It is unclear how big the overlap is between patients who died in hospitals in the first cohort (137) and nursing home residents who died in hospitals in the second cohort.
We included four uncontrolled retrospective cohort studies (five references). Three studies originated from the United Kingdom (Alderman 2020; Hetherington 2020; Lovell 2020), and one from Sweden (Strang 2021). All four studies investigated symptom relief in people with COVID‐19 in hospital palliative care. One study also included participants from nursing homes (Strang 2021). No study included a comparator.
Two references from Strang 2021 used the same register to form their cohorts, and study investigators confirmed a partial overlap of participants: the 253 participants who died in nursing homes reported on in the first cohort are a subset of 1903 participants who died in nursing homes included in the second cohort. It is unclear how big the overlap is between patients who died in hospitals in the first cohort (137) and nursing home residents who died in hospitals in the second cohort. However, the study authors estimate the overlap for the second group to be small. Consequently, we do not know the exact number of participants, but individual reports included 61 to 2105 participants.
The authors of two studies provided additional information upon request. Strang 2021 supplied us with information on the drugs prescribed for the study population and the size of the study population, and Alderman 2020 provided additional information on symptom measurement for shortness of breath and delirium.
Participants
Participants in the studies all had diagnosed COVID‐19. The age of participants ranged from 30 to 107 years. The studies reported that participants showed symptoms of dyspnea, delirium, agitation, pain, audible upper airway secretions, respiratory secretions, nausea, fatigue, fever, and cough. No study provided information on the severity of symptoms. One study reported that the Australian‐modified Karnofsky performance status of participants was 20, meaning that patients were bedfast and required extensive nursing care (Lovell 2020). Three studies reported comorbidities (Alderman 2020; Hetherington 2020; Lovell 2020). The most frequently reported comorbidities were hypertension, diabetes, COPD, respiratory diseases, dementia, and cancer. Strang 2021 did not investigate comorbidities. As older patients with comorbidities were included, we cannot be certain if COVID‐19 was the cause of death for participants in the included studies.
Interventions
All studies used pharmacological interventions for palliative symptom control, but the medications used differed between studies. Overall, opioids, benzodiazepines, anticholinergics, neuroleptics, or a combination were given for symptom relief. Two studies specified the dosing of the drugs (Alderman 2020; Lovell 2020). Only one study specified which drugs were prescribed for which symptom (Alderman 2020). Three studies reported the mode of drug delivery. In Lovell 2020, 58 participants were prescribed a continuous subcutaneous infusion. Continuous subcutaneous infusion was also mentioned as one mode of drug delivery in Hetherington 2020. In Alderman 2020 syringe pumps were utilized in 41 (67%) of participants. Strang 2021 did not specify the mode of drug delivery. None of the studies used non‐pharmacological interventions for palliative symptom control. None of the studies compared different interventions for palliative symptom control.
Treated symptoms included dyspnea, delirium, anxiety, pain, audible upper airway secretions, respiratory secretions, nausea, cough, and unspecified symptoms.
For details, see Characteristics of included studies.
Outcome measures
All studies assessed symptom relief. In Alderman 2020, symptom relief of shortness of breath was assessed by ward nurses noting whether the symptom was present every four hours. Symptom relief of delirium was assessed through modified Richmond Agitation and Sedation Scale (m‐RASS) scores every four hours. In Lovell 2020, symptom relief was assessed via clinical impression of effectiveness based on follow‐up documentation of symptoms. Possible answers were "yes," "no," and "unclear." Judgement of clinical effectiveness was made based on medical and nursing case notes. It is unclear who made the judgement. Likewise, in Hetherington 2020 symptom relief was assessed via clinical impression of efficacy. Possible answers were "effective," "partially effective," and "not effective." The judgement of clinical efficacy was made by specialist palliative care clinicians. Strang 2021 used data from the Swedish Register of Palliative Care (SRPC), which is a national quality register that focuses on palliative care in the last week of life. It is built on data assessed with an anonymized end‐of‐life questionnaire (ELQ). The ELQ is answered retrospectively by medical staff as soon as possible after a patient dies. The ELQ contains 30 questions and provides information on provided care content and quality during the last week of life, demographics, the occurrence of breakthrough symptoms (regardless of intensity), and, if symptoms occur, the degree of symptom alleviation during the last week of life (Svenska Palliativregistret 2021). Data in all studies were collected retrospectively.
No other predefined outcome was reported in the included studies.
For details, Characteristics of included studies.
Studies awaiting classification
We identified one RCT from a trial registry in China that might be relevant to this review (ChiCTR2000029994). We contacted the authors of the study to request missing information, but received no reply. In addition, we identified three relevant conference abstracts, but data for these studies had not yet been published (Groninger 2021; Kelly 2020; Okuwoga 2020). Data from these three studies would add a further 384 participants. Interventions investigated in these studies are Liu Zi Jue Qigong and acupressure (ChiCTR2000029994); pharmacological interventions such as morphine to manage dyspnea (Kelly 2020); injectable medications for symptom relief of agitation and delirium (Okuwoga 2020); and benzodiazepines and neuroleptics for not further classified symptoms (Groninger 2021).
Ongoing studies
We identified one ongoing study that is still recruiting participants (NCT04522037). This controlled study aims to investigate morphine to manage dyspnea in COVID‐19 patients.
Excluded studies
We excluded 23 studies (24 references), as follows.
Seven studies (eight references) evaluated the use but not the effects of palliative care (Haydar 2020; Heath 2020; Pavlu 2020; Rao 2021; Riva 2020; Sun 2020; Turner 2020).
Fifteen studies did not investigate palliative symptom control or did not further specify symptom control (ACTRN12620000443998p; Allande Cussó 2020; Anneser 2020; Bisson 2020; Cook 2020; Delisle 2020; Galazzi 2020; ISRCTN16561225; Johnston 2020; Lee 2020; Lopez 2020; Martinsson 2021; Mumoli 2021; Paice 2021; Ritchey 2020).
One study was an opinion piece (Mendoza 2020).
Risk of bias in included studies
Overall judgement
We rated the risk of bias within and across studies overall to be high. In addition to the high risk of bias related to the non‐randomised and uncontrolled study design, we assessed the internal and external validity as outlined in the 'risk of bias assessment criteria for observational studies' tool provided by the Cochrane Childhood Cancer Group (Table 2) (Mulder 2019). The full judgement per trial and category is presented in Figure 2, and the support for judgement in the Characteristics of included studies table.
2.

Summary of risk of bias.
Allocation
We considered all studies to be at low risk of selection bias since all studies were retrospective cohort studies that included all patients in palliative care in a certain time frame.
Blinding
All studies were unblinded to interventions and therefore at high risk of detection bias for subjective outcomes. The outcome symptom relief was assessed by physicians or hospital staff, thus all studies were at high risk of detection bias.
Incomplete outcome data
We assessed attrition bias in terms of whether studies (equally) assessed outcomes for all participants. We evaluated attrition bias for the outcome symptom relief. We rated attrition bias as high for one study (Lovell 2020), as 13 participants died before follow‐up. We rated attrition bias as low for Alderman 2020, Hetherington 2020, and Strang 2021, as the authors only measured symptom relief in participants that had died or had been discharged retrospectively. We considered outcome assessment to be complete for these three studies.
Selective reporting
We assessed reporting bias in terms of whether the study group and intervention were well defined and whether the outcomes were equally reported for all participants.
We evaluated reporting bias for the outcome symptom relief.
Poorly defined study group and intervention
We judged the risk of reporting bias to be high for all studies. While the study population was well defined in all studies, the interventions were not well described. Only Alderman 2020 specified which pharmacological treatment was used to treat which symptoms. Furthermore, only two studies listed the doses of the pharmacological interventions (Alderman 2020; Lovell 2020).
Poorly defined outcomes
We considered the risk of reporting bias to be high for three studies (Alderman 2020; Hetherington 2020; Lovell 2020), as symptom relief was measured subjectively by physicians and hospital staff responsible for palliative care. Symptom relief was measured on a validated scale in Strang 2021, resulting in a judgement of low risk of reporting bias for this study.
Poorly defined follow‐up
We considered the risk of reporting bias for follow‐up to be high for Hetherington 2020 and Lovell 2020, as the authors did not clearly define length of follow‐up. We judged the risk of reporting bias for follow‐up to be low for Alderman 2020 and Strang 2021, as participants were followed up until death.
Other potential sources of bias
Confounding
None of the included studies adjusted for confounding factors such as age, gender, or comorbidities of participants, therefore all studies were at high risk of confounding.
Poorly defined risk estimates
None of the studies performed any statistical analyses.
Effects of interventions
See: Table 1
Pharmacological interventions for palliative symptom control
We identified four uncontrolled retrospective cohort studies investigating pharmacological interventions for palliative symptom control in hospitalized patients and patients in nursing homes. None of the studies included a comparator. See Table 1.
Primary outcome
Symptom relief
All studies reported symptom relief. Statistical pooling of data was not possible due to heterogenous studies and participant and intervention characteristics. We summarized and visualized data per study in Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10, Figure 11 and below.
3.

Visual synthesis of Alderman 2020
4.

Visual synthesis for Alderman 2020
5.

Visual synthesis for Hetherington 2020
6.

Visual synthesis for Lovell 2020
7.

Visual synthesis for Strang 2021a
8.

Visual synthesis for Strang 2021a
9.
Visual synthesis for Strang 2021a
10.

Visual synthesis for Strang 2021b
11.

Visual synthesis for Strang 2021b
Alderman 2020 reported on 61 participants. Symptom relief for breathlessness was assessed by ward nurses during four‐hourly reviews, who indicated through a "yes/no" answer whether the symptom was present or not. A continuous subcutaneous infusion of morphine was applied to relieve shortness of breath. After four hours, the symptom was not present in 10 out of 16 participants. Relief for delirium was assessed by m‐RASS scores reported by nursing staff every four hours. Fourteen of 14 participants who were started on a continuous subcutaneous infusion either of haloperidol (first‐line treatment; n = 7), levomepromazine (second‐line treatment; n = 14), or levomepromazine and midazolam (third‐line treatment; n = 1) at the initial assessment had relief of agitation/delirium within four hours. Seven of these participants had no further episodes of agitation. In the last 72 hours of life, only one participant of the cohort had an m‐RASS score as high as three (very agitated), and no participant had an m‐RASS score of four (combative). One of 11 participants with persistent audible upper airway secretions did not respond to glycopyrronium, and two participants with nausea, one of whom was treated with haloperidol, did not have nausea at final assessment. One of four participants had pain at final assessment. For a visual synthesis, please see Figure 3 and Figure 4.
Hetherington 2020 reported on 186 participants. Symptom relief was not measured on a standardized scale, but assessed by a palliative care specialist in 126 participants to be "effective," "partially effective," or "not effective." The study authors described pharmacological treatment for symptom control as "effective" in 99 of 126 (79%) participants; "partially effective" in 24 of 126 (19%) participants; and "not effective" in 3 of 126 (2%) participants. No further information was provided with regard to the definition or assessment of effectiveness. For a visual synthesis, please see Figure 5.
Lovell 2020 reported on 101 participants. The clinical impression of effectiveness was determined based on documentation at follow‐up. Possible answers for the clinical impression of effectiveness were "yes," "no," and "unclear." The study authors described pharmacological treatment for symptom control as effective for 40 of 58 (69%) participants and not effective for 5 of 58 (9%) participants. Treatment effectiveness was rated as unclear for 13 (22%) participants who died before follow‐up. Effectiveness was not measured on a standardized scale. For a visual synthesis, please see Figure 6.
Strang 2021 reported on two cohorts extracted from the same registry. We do not know how many participants were reported on twice, and the study investigators were unable to provide this precise information. The first cohort encompassed 390 participants (data retrieved April 2020). Clinical impression of the effectiveness of symptom relief was rated on the ELQ for each participant by the nurse or physician responsible for palliative care. Answer alternatives were "completely relieved," "partly relieved," and "not relieved at all." Complete relief was reached in 53 of 173 (31%) participants with breathlessness, 131 of 197 (66%) participants with anxiety, 13 of 77 (17%) participants with delirium, 72 of 178 (40%) participants with death rattles, and 162 of 210 (77%) participants with pain. Partial relief was reached in 109 of 173 (63%) participants with breathlessness, 63 of 197 (32%) participants with anxiety, 47 of 77 (61%) participants with delirium, 99 of 178 (56%) of participants with death rattles, and 47 of 210 (22%) participants with pain. No relief was reached in 11 of 173 (6%) participants with breathlessness, 3 of 197 (2%) participants with anxiety, 17 of 47 (36%) participants with delirium, 7 of 178 (4%) participants with death rattles, and 1 of 210 (0.5%) participants with pain.
Additionally, Strang 2021 reported symptom relief separately for the subgroups of nursing home residents who died in nursing homes and those who died in the hospital in this cohort. For 253 participants who died in nursing homes, complete relief was reached in 35 of 84 (42%) participants with breathlessness, 96 of 131 (%) participants with anxiety, 7 of 38 (18%) participants with delirium, 54 of 118 (46%) participants with death rattles, and 122 of 147 (83%) participants with pain. Partial relief in this subgroup was reached in 45 of 84 (54%) participants with breathlessness, 33 of 131 (25%) participants with anxiety, 21 of 38 (55%) participants with delirium, 61 of 118 (52%) participants with death rattles, and 25 of 147 (17%) participants with pain. No relief was reached in 4 of 84 (5%) participants with breathlessness, 2 of 131 (2%) participants with anxiety, 10 of 38 (26%) participants with delirium, 3 of 118 participants (3%) with death rattles, and no participants with pain. For 137 participants who died in the hospital, complete relief was reached in 18 of 89 (20%) participants with breathlessness, 35 of 66 (53%) participants with anxiety, 6 of 39 (15%) participants with delirium, 18 of 60 (30%) participants with death rattles, and 40 of 63 (63%) participants with pain. Partial relief in this subgroup was reached in 64 of 89 (72%) participants with breathlessness, 30 of 66 (45%) participants with anxiety, 26 of 39 (67%) participants with delirium, 38 of 60 (63%) participants with death rattles, and 22 of 63 (35%) participants with pain. No relief was reached in 7 of 89 (8%) participants with breathlessness, 1 of 66 (2%) participants with anxiety, 7 of 39 (18%) participants with delirium, 4 of 60 (7%) participants with death rattles, and 1 of 63 (2%) participants with pain. For a visual synthesis, please see Figure 7, Figure 8 and Figure 9.
The second cohort reported on by Strang 2021 was retrieved in August 2020. The number of participants assessed was 2105. Complete relief was reached in 291 of 665 (44%) participants with breathlessness, 832 of 1117 (74%) participants with anxiety, 154 of 486 (32%) participants with delirium, and 559 of 1053 (53%) participants with respiratory secretions. Partial relief was reached in 352 of 665 (53%) participants with breathlessness, 277 of 1117 (25%) participants with anxiety, 230 of 486 (47%) participants with delirium, and 469 of 1053 (45%) participants with respiratory secretions. No relief was reached in 22 of 665 (3%) participants with breathlessness, 8 of 1117 (0.7%) participants with anxiety, 102 of 486 (21%) participants with delirium, and 25 of 1053 (2%) participants with respiratory secretions.
Additionally, Strang 2021 reported symptom relief separately for the subgroups of nursing home residents who died in nursing homes and those who died in the hospital in this cohort. In 1903 nursing home patients, complete relief was reached in 261 of 556 (47%) participants with breathlessness, 769 of 1015 (76%) participants with anxiety, 148 of 423 (35%) participants with delirium, and 530 of 956 (55%) participants with respiratory secretions. Partial relief was reached in 280 of 556 (50%) participants with breathlessness, 238 of 1015 (23%) participants with anxiety, 198 of 423 (47%) participants with delirium, and 408 of 956 (43%) participants with respiratory secretions. No relief was reached in 15 of 556 (3%) participants with breathlessness, 8 of 1015 (0.8%) participants with anxiety, 77 of 423 (18%) participants with delirium, and 18 of 956 (2%) participants with respiratory secretions. For 202 nursing home patients who died in the hospital, complete relief was reached in 30 of 109 (28%) participants with breathlessness, 63 of 102 (62%) participants with anxiety, 6 of 63 (10%) participants with delirium, and 29 of 97 (30%) participants with respiratory secretions. Partial relief was reached in 72 of 109 (66%) participants with breathlessness, 39 of 102 (38%) participants with anxiety, 32 of 63 (51%) participants with delirium, and 61 of 97 (63%) participants with respiratory secretions. No relief was reached in 7 of 109 (6%) participants with breathlessness, 0 of 102 (0%) participants with anxiety, 25 of 63 (40%) participants with delirium, and 7 of 97 (7%) participants with respiratory secretions. For a visual synthesis, please see Figure 10 and Figure 11.
In summary, all studies rated a majority of interventions as effective to relieve dyspnea, delirium, anxiety, pain, audible upper airway secretions, respiratory secretions, nausea, cough, and unspecified symptoms. We are very uncertain about the effect of opioids, neuroleptics, anticholinergics, and benzodiazepines on symptom relief in individuals with COVID‐19. The initial rating of the certainty of the evidence was low due to the non‐randomized study design. Additionally, our main reason for downgrading the certainty of the evidence was high risk of bias due to confounding and unblinded outcome assessors.
Secondary outcomes
We planned to assess the following secondary outcomes.
Quality of life, including fatigue and neurological functions, assessed with standardized scales (e.g. McGill Quality of Life Questionnaire).
Symptom burden (e.g. distress thermometer, IPOS).
Satisfaction of patients.
Satisfaction of caregivers and relatives.
Serious adverse events, defined as number of participants with event.
Grade 3 to 4 adverse events, defined as number of participants with event.
However, none of the included studies provided data for these outcomes, or any other information to describe the efficacy and safety of used interventions.
Non‐pharmacological interventions for palliative symptom control
None of the included studies used non‐pharmacological interventions for palliative symptom control.
Discussion
Summary of main results
The aim of this systematic review was to synthesize all available evidence on pharmacological and non‐pharmacological treatment options for palliative symptom control in people with COVID‐19. We identified four retrospective cohort studies from the United Kingdom and Sweden. None of the studies included a comparator.
The identified studies used different pharmacological treatments for symptom control; none of the studies used non‐pharmacological interventions. The treatments were opioids, neuroleptics, anticholinergics, and benzodiazepines. Pharmacological interventions were used to control dyspnea, delirium, anxiety, pain, audible upper airway secretions, respiratory secretions, nausea, cough, and unspecified symptoms. The results and the certainty of the evidence for the main outcomes are summarized in the summary of findings table (Table 1), the evidence synthesis table (Figure 3), and below for the outcome symptom relief.
Effects of interventions
Pharmacological interventions for palliative symptom control
Primary outcome: symptom relief
We identified four retrospective cohort studies (five references) reporting this outcome. Two references used the same register to form their cohorts, and study investigators confirmed a partial overlap of participants. We therefore do not know the exact number of participants, but individual reports included 61 to 2105 participants. For all symptoms (dyspnea, delirium, anxiety, pain, audible upper airway secretions, respiratory secretions, nausea, cough, and unspecified symptoms), a majority of interventions were rated as completely or partially effective by outcome assessors. Interventions were opioids, neuroleptics, anticholinergics, and benzodiazepines.
We are very uncertain about the effect of pharmacological interventions on symptom relief (very low‐certainty evidence). Based on the uncontrolled study design, we do not know whether one treatment worked better than other treatments for individuals with COVID‐19. The initial rating of the certainty of the evidence was low due to the non‐randomized study design. Additionally, our main reason for downgrading the certainty of the evidence was high risk of bias due to confounding and unblinded outcome assessors.
Secondary outcomes
We planned to include the following outcomes: quality of life; symptom burden; satisfaction of patients, caregivers, and relatives; serious adverse events; and grade 3 to 4 adverse events.
We did not find any data on these outcomes, or any other information on the efficacy and safety of used interventions.
Non‐pharmacological interventions for palliative symptom control
None of the included studies used non‐pharmacological interventions for palliative symptom control.
Overall completeness and applicability of evidence
The included studies reported data for one of our predefined outcomes. We found very low‐certainty evidence about the effect of multimodal pharmacological interventions (opioids, neuroleptics, anticholinergics, and benzodiazepines) on relieving dyspnea, delirium, anxiety, pain, audible upper airway secretions, respiratory secretions, nausea, cough, and unspecified symptoms, at the end of life. None of the studies provided information on quality of life; symptom burden; satisfaction of patients, caregivers, and relatives; or adverse events and serious adverse events.
The identified evidence has a limited informative value with regard to our review question because the described pharmacological interventions could not be matched to the reported outcomes, and there was no control for the interventions. Furthermore, outcomes for symptom relief were assessed by clinical staff and not by the patients themselves. Although it is not always possible for palliative patients to report their symptoms themselves, self‐reported outcome measures remain the gold standard and should be used whenever possible (Antunes 2014). If this is not feasible, for example for ethical reasons, the perspectives of family members, carers, or clinicians can be assessed. The average age of participants receiving the interventions was rather high, reflecting that COVID‐19‐related mortality increases with age (Williamson 2020). The pharmacological interventions used in the studies are commonly used in palliative care: opioids for relief of pain and dyspnea, neuroleptics for relief or prophylaxis of nausea and vomiting and relief of agitation/delirium, anticholinergics for relief of cough and death rattle, and benzodiazepines for relief of dyspnea, agitation, delirium, and for palliative sedation when necessary (Bausewein 2020). The included studies were not designed to provide detailed information on the effectiveness and safety of every used medication. We did not identify any study exploring non‐pharmacological interventions for palliative symptom control in people with COVID‐19. The review question could therefore not be properly answered by the available evidence.
We identified one controlled study investigating the effectiveness of morphine for dyspnea in individuals with COVID‐19 in a controlled setting that is still ongoing (NCT04522037), and one RCT investigating the effects of acupressure therapy and Liu Zi Jue Qigong exercises on dyspnea and quality of life in individuals with COVID‐19 that has not yet published results (ChiCTR2000029994). We further identified abstracts to three studies that might have relevant information on palliative symptom control once full texts are published (Groninger 2021; Kelly 2020; Okuwoga 2020). Results from these studies might add relevant information to this review and possibly necessitate an update.
Quality of the evidence
Certainty of the evidence
Pharmacological interventions for palliative symptom control
We assessed the certainty of evidence for the outcome symptom relief. None of the other prioritized outcomes of this review were reported in the included studies.
We have very low confidence in the identified evidence on symptom relief. All identified studies had a retrospective and uncontrolled design, thus the initial level of certainty was rated as low. Furthermore, we downgraded the level of certainty once because of study limitations due to risk of bias and confounding, reaching a very low level of certainty. Serious study limitations led to a judgement of high risk of detection and selection bias. Studies were not adjusted for potential confounders (e.g. age or gender of participants). It was difficult to assess imprecision and publication bias for the available studies, as results were not quantifiable.
We did not identify any studies reporting on the effects of pharmacological interventions on quality of life; satisfaction of patients, caregivers, or relatives; and symptom burden in people dying of COVID‐19, thus we cannot make a judgement on how to best address these outcomes. We also did not identify any studies reporting on adverse events or serious adverse events, and therefore do not know which adverse events, if any, are associated with pharmacological interventions.
Non‐pharmacological interventions for palliative symptom control
We do not know whether non‐pharmacological interventions are effective and safe for palliative symptom control in people with endstage COVID‐19, because we did not identify any studies investigating non‐pharmacological interventions.
Potential biases in the review process
To avoid potential bias in the review process, we were committed at all times to conduct a systematic review that followed published guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
While we published a protocol for this review beforehand (Andreas 2021), we did not publish a peer‐reviewed Cochrane Review protocol due to time constraints for this high‐priority review in this very critical area of research during the COVID‐19 pandemic. This review was based on a peer‐reviewed protocol of a series on interventions for COVID‐19 (Valk 2020b). However, since PICOs between the reviews differ, this could potentially lead to bias.
As COVID‐19 is a novel disease, results from RCTs are not yet available for palliative symptom control. Consequently, we included only data from retrospective cohort studies. As little guidance exists on how to synthesize studies where meta‐analysis is not possible, we had to develop an appropriate synthesis method. Our approach was informed by the SWiM (Synthesis Without Meta‐analysis) guideline, Campbell 2020, and Chapter 24 of the Cochrane Handbook for Systematic Reviews of Interventions (Reeves 2021). We used a mixed approach of visual and written presentation to provide the reader support in the interpretation of results; however, results without robust data might be misleading or overstated.
An experienced Information Specialist developed a sensitive search strategy to identify all ongoing and completed studies. The search strategy was peer‐reviewed by another experienced Information Specialist. We searched all relevant databases and trial registries, and two review authors conducted all review steps independently and in duplicate. In the case of missing data, we contacted study authors for additional data or relevant details as needed. We are confident that we identified all relevant studies, and will monitor ongoing studies as well as studies that have been completed but that are not yet published closely after the publication of this review.
We identified no other potential sources of bias in our review process.
Agreements and disagreements with other studies or reviews
We have identified no other systematic review assessing palliative symptom control in COVID‐19 patients. However, we did identify non‐systematic reviews addressing palliative symptom control in COVID‐19 patients. We agree with the review by Keeley 2020 that more information on palliative care for COVID‐19 patients is urgently needed. Symptoms addressed in other reviews and this review seem to be similar: cough, anxiety, pain, and dyspnea are reported to be prevalent among COVID‐19 patients (Mottiar 2020; Ting 2020).
Pharmacological interventions found in this review are similar to those that are recommended for the palliative care of cancer patients (Bausewein 2020). Furthermore, the pharmacological interventions used for symptom control found in this review are also recommended by the German Society for Palliative Medicine (Nehls 2020). We did not find any evidence on recommended non‐pharmacological interventions, as have been put forward by the National Institute for Health and Care Excellence (NICE) (NICE 2020).
More information is needed to investigate specific challenges for palliative care in the COVID‐19 pandemic.
Authors' conclusions
Implications for practice.
We did not find evidence to confidently support or refute whether pharmacological interventions may be effective for palliative symptom relief in COVID‐19 patients, and no evidence on the safety of pharmacological interventions, or effectiveness and safety of non‐pharmacological interventions for palliative symptom control in COVID‐19 patients. The evidence presented here has no specific implications for palliative symptom control in COVID‐19 patients because we cannot draw any conclusions about the effectiveness or safety based on the identified evidence. More evidence is needed to guide clinicians, nursing staff, and caregivers when treating symptoms of COVID‐19 patients at the end of life. Specifically, future studies ought to investigate palliative symptom control in prospectively registered studies, using an active‐controlled setting, assess patient‐reported outcomes, and clearly define interventions. The identified evidence does not rule out current practice for palliative symptom control, for example for cancer.
Implications for research.
The results of our systematic review show that randomized controlled trials (RCTs) on the palliative symptom management of COVID‐19 patients are needed. However, as RCTs in the palliative setting are complex due to ethical and logistic constraints, well‐conducted prospectively registered observational studies would help to fill the current research gap. Specifically, future research ought to investigate treatments in prospectively registered studies, using a controlled setting, assess patient‐reported outcomes, and clearly define interventions.
We identified one RCT that may be already completed but for which results are not yet available, investigating the effects of acupressure therapy and Liu Zi Jue Qigong exercises on dyspnea and quality of life in individuals with COVID‐19. We further identified abstracts to three studies that might have relevant information on palliative symptom control once full texts are published. In addition, we found one ongoing study investigating morphine in COVID‐19 patients with dyspnea in a controlled setting.
The publication of the results of these studies will necessitate an update of this review. The conclusions of the updated review could differ from those of the present review and may allow for a better judgement regarding pharmacological and non‐pharmacological interventions for palliative symptom control in COVID‐19 patients.
Acknowledgements
This review was published in collaboration with the Cochrane Pain, Palliative and Supportive Care group. We particularly thank Anna Erskine (Managing Editor), Neil O'Connell (Co‐ordinating Editor), Peter Tugwell (Sign‐off Editor) and Lisa Winer (Copy Editor) for their excellent support, valuable comments on the review, and timely management of the editorial process.
This work is part of a series of reviews investigating treatments and therapies for COVID‐19 as part of the project CEOsys. Text passages in the Background section (e.g. Description of the condition and Why it is important to do this review) are shared between reviews of this series. We thank the authors of the first published reviews of this series (Kreuzberger 2020 and Valk 2020a), for providing and sharing this information. Moreover, we thank the Cochrane Haematology working group for using the template for the description of methods.
The research was part of a project supported by the German Federal Ministry of Education and Research (NaFoUniMedCovid19, funding number: 01KX2021; part of the CEOsys project). The contents of this document reflect only the authors' views, and the German Ministry is not responsible for any use that may be made of the information it contains.
We thank the investigator team of Strang 2021 and Alderman 2020 for providing us with all the requested additional information and data.
The authors and editors thank peer reviewers Brian Duncan (Consumer Reviewer), Sarah Mitchell, Jennifer Hilgart, and Sarah Yardley.
Cochrane Review Group funding acknowledgement: this project was funded by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Pain, Palliative and Supportive Care Review Group (PaPaS). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
We thank Ina Monsef and Maria‐Inti Mezendorf for peer‐reviewing the search strategy.
Appendices
Appendix 1. Planned methodology for randomized controlled trials and non‐randomized studies of interventions
Types of studies
To assess the benefits and safety of palliative interventions for symptom control, we planned to include randomized controlled trials (RCTs) only, as such studies, if performed appropriately, currently give the best evidence for experimental therapies in highly controlled therapeutic settings. Had RCT data been available, we would have used the methods recommended by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), as specified in the description of the methods.
In the case of insufficient evidence (very low‐certainty evidence or no evidence) available from RCTs to answer our review question, we would include prospective controlled non‐randomized studies of interventions (NRSIs), including quasi‐RCTs (e.g. assignment to treatment by alternation or by date of birth), controlled before‐and‐after (CBA) studies, and interrupted time series (ITS) studies. In such a case we would use the methods proposed in the Cochrane Handbook for Systematic Reviews of Interventions for the inclusion of NRSIs in systematic reviews (Reeves 2021) .
In case of insufficient evidence (very low‐certainty evidence or no evidence) available from RCTs and NRSIs, we would include prospective observational studies with a control group and would adapt the methods for the inclusion of NRSIs in systematic reviews as specified in the Cochrane Handbook for Systematic Reviews of Interventions (Reeves 2021).
As there was no evidence from RCTs or NRSIs, and only one prospective observational study available, we included prospective non‐comparative study designs (e.g. case series) and followed the methodology as specified in the protocol (Andreas 2021).
Data extraction and management
Assessment of risk of bias in included studies
Randomized controlled trials
We planned to use the RoB 2 tool to analyze the risk of bias in the underlying study results (Sterne 2019). Of interest for this review was the effect of the assignment to the intervention (the intention‐to‐treat (ITT) effect), and we would perform all assessments with RoB 2 on this effect. We would address those outcomes specified for inclusion in Table 1. Accordingly, the outcomes had been prioritized according to the Core Outcome Measures in Effectiveness Trials Initiative for COVID‐19 patients (COMET 2020).
One review author would assess the risk of bias for each study result. A second review author would verify the accuracy and the plausibility. In case of discrepancies among their judgements or inability to reach consensus, a third review author would be consulted to reach a final decision. We would assess the following types of bias as outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021).
Bias arising from the randomization process
Bias due to deviations from the intended interventions
Bias due to missing outcome data
Bias in measurement of the outcome
Bias in selection of the reported result
To address these types of bias we planned to use the signalling questions recommended in RoB 2 and make a judgement using the following options:
'yes': if there is firm evidence that the question is fulfilled in the study (i.e. the study is at low or high risk of bias for the given the direction of the question);
'probably yes': a judgement has been made that the question is fulfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question);
'no': if there is firm evidence that the question is unfulfilled in the study (i.e. the study is at low or high risk of bias for the given the direction of the question);
'probably no': a judgement has been made that the question is unfulfilled in the study (i.e. the study is at low or high risk of bias given the direction of the question);
'no information' if the study report does not provide sufficient information to allow any judgement.
We planned to use the algorithms proposed by RoB 2 to assign each domain one of the following levels of bias:
low risk of bias;
some concerns;
high risk of bias.
We subsequently planned to derive a risk of bias rating for each prespecified outcome in each study in accordance with the following suggestions.
'Low risk of bias': the trial is judged to be at low risk of bias for all domains for this result.
'Some concerns': the trial is judged to raise some concerns in at least one domain for this result, but not to be at high risk of bias for any domain.
'High risk of bias': the trial is judged to be at high risk of bias in at least one domain for the result, or the trial is judged to have some concerns for multiple domains in such a way that substantially lowers confidence in the results.
Non‐randomized controlled studies
As reported above, we planned to include non‐randomized studies if there were insufficient evidence from RCTs.
One review author would assess eligible studies for methodological quality and risk of bias (using the Risk Of Bias In Non‐randomised Studies—of Interventions (ROBINS‐I) tool) (Sterne 2016). A second review author would verify the accuracy and the plausibility. The quality assessment strongly depends upon information on the design, conduct, and analysis of the trial. The two review authors would resolve any disagreements regarding the quality assessments by consulting a third review author until reaching consensus.
We planned to assess the following risk of bias domains.
Bias due to confounding
Bias in selection of participants into the study
Bias in classification of interventions
Bias due to deviations from intended interventions
Bias due to missing data
Bias in measurement of outcomes
Bias in selection of the reported result
For every criterion we planned to make a judgement using one of five response options.
Yes
Probably yes
Probably no
No
No information
Measures of treatment effect
Randomized controlled trials
For continuous outcomes, we planned to record the mean, standard deviation (SD), and total number of participants in both the treatment and control groups. For dichotomous outcomes, we planned to record the number of events and total number of participants in both the treatment and control groups.
For continuous outcomes using the same scale, we planned to perform analyses using the mean difference (MD) with 95% confidence intervals (CIs). For continuous outcomes measured with different scales, we planned to perform analyses using the standardized mean difference (SMD). In our interpretation of SMDs, we planned to re‐express the SMD in the original units of a particular scale with the most clinical relevance and impact.
We planned to extract and report hazard ratios (HRs) for time‐to‐event outcomes (overall survival, progression‐free survival) if these were available. Had HRs not been available, we would have made every effort to estimate as accurately as possible the HR using the available data and a purpose‐built method based on the Parmar and Tierney approach (Parmar 1998; Tierney 2007), in an update of this review. In the case of sufficient studies providing HRs, we would use HRs rather than risk ratios (RRs) or MDs in a meta‐analysis.
For dichotomous outcomes, we planned to report the pooled RR with a 95% CI (Deeks 2021). If the number of observed events was small (less than 5% of sample per group), and if studies had balanced treatment groups, we would report the Peto odds ratio (OR) with 95% CI (Deeks 2021).
For cluster‐randomized trials, we planned to extract and report direct estimates of the effect measure (e.g. RR with a 95% CI) from an analysis that accounts for the clustered design. We planned to obtain statistical advice to ensure the analysis was appropriate. Had appropriate analyses not been available, we would have made every effort to approximate the analysis following the recommendations in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021), in an update of this review.
For studies with a control group where meta‐analysis is not possible, we planned to perform analyses according to the recommendations in Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (McKenzie 2021). We will consider summarizing effect estimates when estimates of intervention effect are available, but the variances of the effects are not sufficiently reported. If P values, but no other information, are reported, we will combine P values using Fisher’s method to combine P values (see section 12‐2‐1‐2 of McKenzie 2021). We will employ forest plots to visualize results, as they allow a clear depiction of study results when meta‐analysis is not possible. In the case that these methods are not feasible, and if the direction of effect is reported, we will use vote counting based on the direction of effect. We will use harvest plots to visualize the effects of vote counting.
Non‐randomized controlled studies
For dichotomous outcomes, if available we planned to extract and report the RR with a 95% CI from statistical analyses adjusting for baseline differences (such as Poisson regressions or logistic regressions) or the ratio of RRs (i.e. the RR postintervention/RR pre‐intervention).
For continuous variables, if available we planned to extract and report the absolute change from a statistical analysis adjusting for baseline differences (such as regression models, mixed models, or hierarchical models), or the relative change adjusted for baseline differences in the outcome measures (i.e. the absolute postintervention difference between the intervention and control groups, as well as the absolute pre‐intervention difference between the intervention and control groups/the postintervention level in the control group) (EPOC 2017).
Unit of analysis issues
We followed the methods outlined in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions to identify the unit of analysis (Higgins 2021).
Repeated observations on participants
In case events were observed multiple times, we would consider the number of participants experiencing any event and not the number of experienced events for analysis.
Multiple treatment attempts
Palliative care is a multidimensional concept, and participants usually receive a combination of interventions. We planned to consider the number of participants and not the number of assigned treatments for analysis. In the case of cross‐over trials, we would consider results from the first cycle only, unless an appropriate wash‐out period had been applied.
Studies with multiple treatment groups
For studies with multiple treatment groups, we planned to combine arms as long as they could be regarded as subtypes of the same intervention. When arms could not be pooled in this way, we would compare each arm with the common comparator separately. For pair‐wise meta‐analysis, we would split the ‘shared’ group into two or more groups with smaller sample sizes, and include two or more (reasonably independent) comparisons. For this purpose, for dichotomous outcomes, both the number of events and the total number of participants would have been divided up, and for continuous outcomes, the total number of participants would have been divided up with unchanged means and SDs.
Cluster‐randomized trials
For cluster‐RCTs that did not make an allowance for the design effect, we planned to calculate the design effect based on a larger assumed intraclass correlation coefficient (ICC) of 0.10. We would follow the methods described in Section 23 of the Cochrane Handbook for Systematic Reviews of Interventions for this calculation (Higgins 2021).
Assessment of heterogeneity
We planned to assess heterogeneity of treatment effects between studies using a Chi2 test with a significance level at P < 0.1. We planned to use the I2 statistic to quantify possible heterogeneity (Higgins 2003), with an I2 > 30% signifying moderate heterogeneity and an I2 > 75% considerable heterogeneity (Deeks 2021). If heterogeneity was above 80%, we would explore potential causes through sensitivity and subgroup analyses. If we could not find a reason for heterogeneity, we would not perform a meta‐analysis, but would comment on results from all studies and present these in tables.
Data synthesis
If the clinical and methodological characteristics of individual studies were sufficiently homogeneous, we would pool data in meta‐analysis. We planned to perform analyses according to the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2021). We would not conduct meta‐analyses that involved both RCTs and non‐RCTs. We planned to conduct separate meta‐analyses for each comparison.
We planned to use Review Manager Web software for analyses (RevMan Web 2021). One review author would enter the data into the software, and a second review author would check the data for accuracy.
We planned to use the random‐effects model for all analyses, as we anticipate that true effects will be related but will not be the same for included studies. If we could not perform a meta‐analysis, we would comment on the results as a narrative, with the results from all studies presented in tables.
We planned that when meta‐analysis for RCTs was feasible, we would use the random‐effects model for pooling data. For binary outcomes, we planned to base the estimation of the between‐study variance using the Mantel‐Haenszel method. We planned to use the inverse‐variance method for continuous outcomes, outcomes that include data from cluster‐RCTs, or outcomes where HRs are available. We planned to explore heterogeneity above 80% with subgroup analyses. If we could not find a cause for the heterogeneity, then we would not perform a meta‐analysis, but comment on the results as a narrative with the results from all studies presented in tables.
Had meta‐analysis been feasible for non‐RCTs, CBA studies, ITS studies, and cohort studies, we would have analyzed the different types of studies separately. We planned to only analyze outcomes with adjusted effect estimates if these were adjusted for the same factors using the inverse‐variance method, as recommended in Chapter 24 of the Cochrane Handbook for Systematic Reviews of Interventions (Reeves 2021).
Appendix 2. Manual searches for evidence synthesis
1) Evidence Aid Coronavirus (COVID‐19) (evidenceaid.org/evidence/coronavirus-covid-19/)
search and screen: palliative care; terminal care; end of life care
2) Coronavirus (COVID‐19) (the Cochrane Library) (www.cochranelibrary.com/covid-19) – Special Collections“ + New & updated Cochrane Reviews ‐ screen: palliative care; terminal care; end of life care
3) Usher Network for COVID‐19 Evidence Reviews (https://www.ed.ac.uk/usher/uncover/register-of-reviews)
search and screen: palliative care; terminal care; end of life care
4) US Department of Veterans Affairs Evidence Synthesis Program (https://www.covid19reviews.org/)
Search Results Covid‐19 Reviews /reviews as of 11/25/20
search and screen: palliative care; terminal care; end of life care
5) Australian guidelines for the clinical care of people with COVID‐19 (https://covid19evidence.net.au/#living-guidelines)
screen: palliative care; terminal care; end of life care
6) Norwegian Institute of Public Health systematic and living map on COVID‐19 evidence (https://www.nornesk.no/forskningskart/NIPH_mainMap.html)
(Systematic reviews on COVID‐19):
www.fhi.no/en/sys/news/?blockId=90733&ownerPage=45271&language=en
select and screen: palliative (care); terminal (care); (end of life care) + screen reviews
7) COVID‐19 Evidence Alerts from McMaster PLUS (https://plus.mcmaster.ca/COVID-19)
search and screen: palliative care; terminal care; end of life care;
in „higher quality studies for clinical attention“
in ”studies currently under review”
8) L*OVE (iloveevidence.com/)
search and screen: (palliative care OR terminal care OR end of life OR EOL OR endstage)
+ limit to 1. systematic reviews
9) TRIP (www.tripdatabase.com/)
search and screen: (covid‐19 or "novel coronavirus") AND ("palliative care" OR "terminal care" OR "end of life" OR EOL) / + systematic reviews
10) ECRI COVID‐19 Resource Center (www.ecri.org/coronavirus-covid-19-outbreak-preparedness-center/)
screen
11) JBI Evidence Synthesis COVID‐19 Collection (https://journals.lww.com/jbisrir/pages/collectiondetails.aspx?TopicalCollectionId=15)
screen
12) NICE Coronavirus (COVID‐19) (https://www.nice.org.uk/guidance/conditions-and-diseases/respiratory-conditions/covid19)
screen
Appendix 3. Database searches for evidence synthesis
Medline (Ovid)
Database(s): Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily 1946 to November 25, 2020
(COVID‐19 or coronavirus or "Corona virus" or 2019‐nCoV or "novel CoV" or "novel coronavirus" or SARS‐CoV‐2 or sarscov2 or 2019nCoV or nCOV).mp.
((terminal* or end of life or EOL or palliati*) adj5 (care* or cari* or nurs* or surge* or therap* or treat*)).ti,ab.
exp "PALLIATIVE CARE"/
exp "TERMINAL CARE"/
exp "PALLIATIVE MEDICINE"/
exp "HOSPICE AND PALLIATIVE CARE NURSING"/
or/2‐6
cochrane database of systematic reviews.jn. or search*.tw. or review.pt. or meta analysis.pt. or medline.tw. or systematic review.tw. [8. Wong 2006 – systematic reviews filter –modified by adding review.pt]
1 AND 7
8 and 9
Complementary Search for Evidence Synthesis via PubMed „similar articles“
Export first 20 results, starting from:
Mitchell S, Maynard V, Lyons V, Jones N, Gardiner C. The role and response of primary healthcare services in the delivery of palliative care in epidemics and pandemics: A rapid review to inform practice and service delivery during the COVID‐19 pandemic. Palliat Med. 2020 Oct;34(9):1182‐1192. doi: 10.1177/0269216320947623. Epub 2020 Jul 31. PMID: 32736494; PMCID: PMC7528540.
Appendix 4. Manual searches for planned evidence synthesis
Oxford COVID‐19 Evidence Service – Current questions under review (www.cebm.net/oxford-covid-19-evidence-service/)
screen
Cochrane COVID Review Bank (covidreviews.cochrane.org/search/site)
screen
PROSPERO (www.crd.york.ac.uk/prospero/)
search and screen: (covid* AND (palliative care OR terminal care OR end of life))
Appendix 5. Database searches for primary studies
1) CCSRhttps://covid-19.cochrane.org
palliati* OR hospice* OR "terminal care" OR "terminal stage" OR "terminal disease" OR "terminally ill" OR "end stage" OR "end of life" OR "supportive care"
+ Filter by “Study design”:
Case Series/Case Control/Cohort
Parallel/Crossover
Cross‐sectional
Other
Unclear
2) Web of Science (Science Citation Index / Emerging Sources)
AB=((COVID OR "COVID‐19" OR COVID19) OR ("SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2") OR ("2019 nCoV" OR "2019nCoV" OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19") OR ("severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia") OR ("severe acute respiratory syndrome coronavirus 2")) OR TI=((COVID OR "COVID‐19" OR COVID19) OR ("SARS‐CoV‐2" OR "SARS‐CoV2" OR SARSCoV2 OR "SARSCoV‐2" OR "SARS coronavirus 2") OR ("2019 nCoV" OR 2019nCoV OR "2019‐novel CoV" OR "nCov 2019" OR "nCov 19") OR ("severe acute respiratory syndrome coronavirus 2" OR "novel coronavirus disease" OR "novel corona virus disease" OR "corona virus disease 2019" OR "coronavirus disease 2019" OR "novel coronavirus pneumonia" OR "novel corona virus pneumonia") OR ("severe acute respiratory syndrome coronavirus 2")) AND AB=((palliati* OR hospice OR “terminal care” OR "terminal stage" OR "terminal disease" OR "terminally ill" OR “end stage” OR “end of life” OR “supportive care” )) OR TI=((palliati* OR hospice OR “terminal care” OR "terminal stage" OR "terminal disease" OR "terminally ill" OR “end stage” OR “end of life” OR “supportive care”))
3) CINAHL (via EBSCO)
((TI ("SARS‐CoV‐2" OR "SARS‐CoV2" OR "SARSCoV‐2" OR SARSCoV2 OR "SARS‐CoV*" OR SARSCoV* OR "severe acute respiratory syndrome 2" OR "severe acute respiratory syndrome cov*" OR "Covid‐19" OR Covid19* OR Covid OR nCoV* OR 2019nCoV* OR 19nCoV* OR "HCoV‐19" OR coronavirus* OR "corona virus*") OR AB ("SARS‐CoV‐2" OR "SARS‐CoV2" OR "SARSCoV‐2" OR SARSCoV2 OR "SARS‐CoV*" OR SARSCoV* OR "severe acute respiratory syndrome 2" OR "severe acute respiratory syndrome cov*" OR "Covid‐19" OR Covid19* OR Covid OR nCoV* OR 2019nCoV* OR 19nCoV* OR "HCoV‐19" OR coronavirus* OR "corona virus*") OR SU ("SARS‐CoV‐2" OR "SARS‐CoV2" OR "SARSCoV‐2" OR SARSCoV2 OR "SARS‐CoV*" OR SARSCoV* OR "severe acute respiratory syndrome 2" OR "severe acute respiratory syndrome cov*" OR "Covid‐19" OR Covid19* OR Covid OR nCoV* OR 2019nCoV* OR 19nCoV* OR "HCoV‐19")) AND (DT 20191117‐3000)) AND ( MH ("Terminal Care" OR "Palliative Care" OR "Hospice Care" OR "Terminally Ill Patients" OR "Hospice Patients") OR TI (palliati* OR hospice OR “terminal care” OR "terminal stage" OR "terminal disease" OR "terminally ill" OR “end stage” OR “end of life” OR “supportive care”) OR AB (palliati* OR hospice OR “terminal care” OR "terminal stage" OR "terminal disease" OR "terminally ill" OR “end stage” OR “end of life” OR “supportive care”))
4) WHO COVID‐19 Global literature on coronavirus disease ‐ https://search.bvsalud.org/global-literature-on-novel-coronavirus-2019-ncov/
tw:((tw:(palliati* OR hospice* OR "terminal care" OR "terminal stage" OR "terminal disease" OR "terminally ill" OR "end stage" OR "end of life" OR "supportive care"))) AND mj:("Palliative Care" OR "Terminal Care" OR "Hospice and Palliative Care Nursing")
5) COAP Living Evidence on COVID‐19 (indexed: PubMed, EMBASE, medRxiv, bioRxiv) ‐ https://zika.ispm.unibe.ch/assets/data/pub/search_beta/
palliative OR hospice OR (terminal care) OR (terminal stage) OR (terminal disease) OR (terminally ill) OR (end stage) OR (end of life) OR (supportive care)
Appendix 6. Planned methodology for updates of this review
Publication bias
In an update of this review, we intend to explore potential publication bias by generating a funnel plot and statistically testing this by conducting a linear regression test for meta‐analyses involving at least 10 studies (Sterne 2019). We will consider P < 0.1 as significant for this test.
Subgroup analysis
For future updates, we plan subgroup analyses for the following characteristics:
different settings (e.g. hospital versus nursing homes versus hospices versus home palliative care);
different interventions (pharmacological versus non‐pharmacological);
analyses in subgroups of patients (different ages, comorbidities).
Sensitivity analysis
For future updates, we plan sensitivity analyses for the following:
risk of bias (low risk or some concerns versus high risk for RCTs, low risk or moderate risk versus serious risk for NRSIs (studies at critical risk would be excluded from all analyses));
influence of completed but not published studies.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alderman 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Primary review outcomes
Secondary review outcomes
Additional outcomes reported in the study
|
|
| Notes | Sponsor/funding: the authors received no financial support for the research, authorship, and/or publication of this article. COI: the authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias (unrepresentative study group) All outcomes | Low risk | Study group is representative of COVID‐19 patient population. |
| Attrition bias (incomplete outcome assessment/follow up) All outcomes | Low risk | Outcome assessed until participant death. |
| Detection bias (outcome detectors blinded to intervention) All outcomes | High risk | Not blinded |
| Confounding (important prognostic factors or follow‐up not taken adequately into account) All outcomes | High risk | Not adjusted for confounders |
| Reporting bias (poorly defined study group) All outcomes | Low risk | Criteria for inclusion is well described. |
| Reporting bias (poorly defined follow up) All outcomes | Low risk | Assessed until death |
| Reporting bias (poorly defined outcome) All outcomes | High risk | Effective if not present at subsequent assessments |
Hetherington 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
*IQR not reported |
|
| Outcomes | Primary review outcomes
Secondary review outcomes
Additional outcomes reported
|
|
| Notes | Sponsor/funding: the author(s) received no financial support for the research, authorship, and/or publication of this article. COIs: the author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias (unrepresentative study group) All outcomes | Low risk | Overall, data from 186 participants were captured. Clear inclusion criteria |
| Attrition bias (incomplete outcome assessment/follow up) All outcomes | Low risk | Reported until death or discharge |
| Detection bias (outcome detectors blinded to intervention) All outcomes | High risk | Not blinded |
| Confounding (important prognostic factors or follow‐up not taken adequately into account) All outcomes | High risk | Not adjusted for confounding factors |
| Reporting bias (poorly defined study group) All outcomes | Low risk | Study population well described, clear inclusion criteria. |
| Reporting bias (poorly defined follow up) All outcomes | High risk | Follow‐up period not defined. |
| Reporting bias (poorly defined outcome) All outcomes | High risk | Outcome was subjective and was not defined. Data collected retrospectively. Outcome assessment has not been validated. |
Lovell 2020.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
*IQR not reported |
|
| Outcomes | Primary review outcomes
Secondary review outcomes
Additional outcomes reported
|
|
| Notes | Sponsor/funding: the author(s) received no financial support for the research, authorship, and/or publication of this article. COIs: the author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias (unrepresentative study group) All outcomes | Low risk | Clear inclusion criteria |
| Attrition bias (incomplete outcome assessment/follow up) All outcomes | High risk | Unclear for how long data were collected, 13 participants died before follow‐up |
| Detection bias (outcome detectors blinded to intervention) All outcomes | High risk | Not blinded |
| Confounding (important prognostic factors or follow‐up not taken adequately into account) All outcomes | High risk | Not adjusted for confounding factors |
| Reporting bias (poorly defined study group) All outcomes | Low risk | Study group well‐defined. |
| Reporting bias (poorly defined follow up) All outcomes | High risk | Follow‐up period not defined. |
| Reporting bias (poorly defined outcome) All outcomes | High risk | Outcome was subjective and was not defined. Data collected retrospectively. Outcome assessment has not been validated. |
Strang 2021.
| Study characteristics | ||
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Primary review outcomes
Secondary review outcomes
Additional outcomes reported
|
|
| Notes | Participant data for the 2 cohorts were extracted from the same database, resulting in overlap between cohorts. We do not know how many participants are reported on twice. Sponsor/funding: supported by Region Stockholm (ALF) and the Stockholm Sjukhem Foundation’s Jubilee Fund. COI: no competing financial interests exist. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Selection bias (unrepresentative study group) All outcomes | Low risk | Clear inclusion criteria |
| Attrition bias (incomplete outcome assessment/follow up) All outcomes | Low risk | Only assessed when participant was dead |
| Detection bias (outcome detectors blinded to intervention) All outcomes | High risk | Not blinded |
| Confounding (important prognostic factors or follow‐up not taken adequately into account) All outcomes | High risk | Not controlled for confounders |
| Reporting bias (poorly defined study group) All outcomes | Low risk | Study group well defined. |
| Reporting bias (poorly defined follow up) All outcomes | Low risk | Assessed until death |
| Reporting bias (poorly defined outcome) All outcomes | Low risk | Validated questionnaire (ELQ) used. |
CSCI: continuous subcutaneous infusion
m‐RASS: modified Richmond Agitation‐Sedation Scale
COI: Conflict of Interest
IQR: Interquatile Range
NR: Not reported
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ACTRN12620000443998p | Does not investigate symptom control |
| Allande Cussó 2020 | Does not investigate symptom control |
| Anneser 2020 | Symptom control not specified. |
| Bisson 2020 | Does not investigate symptom control |
| Cook 2020 | Does not investigate symptom control |
| Delisle 2020 | Symptom control not specified. |
| Galazzi 2020 | No symptom control reported. |
| Haydar 2020 | Evaluated the use but not the effects of palliative care |
| Heath 2020 | Evaluated the use but not the effects of palliative care |
| ISRCTN16561225 | Does not investigate symptom control |
| Johnston 2020 | Does not investigate symptom control |
| Lee 2020 | Does not investigate symptom control |
| Lopez 2020 | Does not investigate symptom control |
| Martinsson 2021 | Does not investigate symptom control |
| Mendoza 2020 | Opinion piece; no data |
| Mumoli 2021 | Does not investigate symptom control |
| Paice 2021 | Symptom control not reported. |
| Pavlu 2020 | Evaluated the use but not the effects of palliative care |
| Rao 2021 | Evaluated the use but not the effects of palliative care |
| Ritchey 2020 | Does not investigate symptom control |
| Riva 2020 | Evaluated the use but not the effects of palliative care |
| Sun 2020 | Evaluated the use but not the effects of palliative care |
| Turner 2020 | Evaluated the use but not the effects of palliative care |
Characteristics of studies awaiting classification [ordered by study ID]
ChiCTR2000029994.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Notes | Sponsor/funding: this study is supported financially by the project of Emergency Scientific Research Project for prevention and control of new coronavirus (COVID‐19) by Shanghai University of Traditional Chinese Medicine (first batch) (no fund number) and High‐Level Innovation Team of “Peak and Plateau Discipline” in Traditional Chinese Medicine (30304114316). These two projects are funded by the Shanghai University of Traditional Chinese Medicine. The Shanghai further accelerates the 3‐year action plan for the development of Chinese medicine (ZY (2018‐2020)‐CCCX‐2004‐02), and the National Key Clinical Difficult Diseases Clinical Collaborative Pilot Construction Project—Osteoarticular Degenerative Disease (ZY (2018‐2020)‐FWTX‐2005) is funded by the Shanghai Government. The funders had no role in the design of the study; analysis, collection, and interpretation of the data; or the writing and decision for publication of the manuscript. This funding relates to a wider group of projects and applies to this study. The trial sponsor is the Shanghai Municipal Government and Shanghai University of Traditional Chinese Medicine. The final fund management is performed by the Yueyang Integrated Traditional Chinese and Western Medicine Hospital affiliated to Shanghai University of Traditional Chinese Medicine. Conflict of interest: none declared. Data have not yet been published. We have contacted the authors for more information. |
Groninger 2021.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Notes | Data have not yet been published. |
Kelly 2020.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Notes | Full data not yet published. |
Okuwoga 2020.
| Methods |
|
| Participants |
|
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Notes | Data not yet published. |
N/A: Not applicable
ADL: Activities of daily living
CT: Computed tomography
Characteristics of ongoing studies [ordered by study ID]
NCT04522037.
| Study name | Measurement of the efficacy of morphine in the early management of dyspnea in COVID‐19 positive patients (CODYS) |
| Methods |
|
| Participants | None yet, still recruiting |
| Interventions |
|
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | |
| Contact information | |
| Notes |
|
Differences between protocol and review
We included two further outcomes (adverse events and serious adverse events) after the protocol was published, as these were prioritized by the editorial team of the Cochrane Pain, Palliative and Supportive Care Group. We have also changed the order of the outcomes to reflect the relevance for individuals in palliative care.
Because of time constraints, three and not two review authors, as described in the protocol, screened the results of the search.
Contributions of authors
MA: methodological expertise; study selection; data extraction, assessment, and interpretation; and conception and writing of the review.
VP: methodological expertise, data assessment and interpretation, and conception and writing of the review.
NS: clinical and methodological expertise and advice.
KG: development of the search strategy.
MB: data extraction and assessment.
LJ: study selection.
GB: clinical expertise and advice.
WM: clinical expertise and advice and data assessment and interpretation.
CB: clinical expertise; study selection; data extraction, assessment, and interpretation; and conception and writing of the review.
Sources of support
Internal sources
-
University Hospital of Cologne, Germany
Evidence‐based Oncology, Department I of Internal Medicine, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf, Faculty of Medicine and University Hospital Cologne, University of Cologne, Cologne, Germany
External sources
-
Federal Ministry of Education and Research of Germany, Germany
This review is part of the CEOsys project funded by the Network of University Medicine (Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)) by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF)), grant number 01KX2021.
-
National Institute for Health Research (NIHR), UK
Cochrane infrastructure funding for the Cochrane Pain, Palliative and Supportive Care (PaPaS) Review Group
Declarations of interest
MA: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the CEOsys project), which was paid to the institution.
VP: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the CEOsys project), which was paid to the institution.
NS: has declared no conflict of interest.
KG: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the CEOsys project), which was paid to the institution.
MB: has declared no conflict of interest.
LJ: has declared no conflict of interest.
GB: has declared no conflict of interest. GB is a palliative care specialist.
WM: reports consultancy or talks for the following companies: Mundipharma, Grünenthal, Menarini, BioQPharma, Bionorica, Kyowa, TAD, Tilray, Sanofi, Septodont, and Northern Swan. He reports research funding from Pfizer, Mundipharma, and Grünenthal. WM is a palliative care specialist.
CB: is funded by the Federal Ministry of Education and Research, Germany (NaFoUniMedCovid19, funding number: 01KX2021; part of the CEOsys project), which was paid to the institution.
New
References
References to studies included in this review
Alderman 2020 {published and unpublished data}
- Alderman B, Webber K, Davies A. An audit of end-of-life symptom control in patients with corona virus disease 2019 (COVID-19) dying in a hospital in the United Kingdom. Palliative Medicine 2020;34(9):1249-55. [DOI: 10.1177/0269216320947312] [DOI] [PubMed] [Google Scholar]
Hetherington 2020 {published data only}
- Hetherington L, Johnston B, Kotronoulas G, Finlay F, Keeley P, McKeown A. COVID-19 and hospital palliative care—a service evaluation exploring the symptoms and outcomes of 186 patients and the impact of the pandemic on specialist hospital palliative care. Palliative Medicine 2020;34(9):1256-62. [DOI: 10.1177/0269216320949786] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lovell 2020 {published data only}
- Lovell N, Maddocks M, Etkind SN, Taylor K, Carey I, Vora V, et al. Characteristics, symptom management, and outcomes of 101 patients with COVID-19 referred for hospital palliative care. Journal of Pain and Symptom Management 2020;60(1):e77-81. [DOI: 10.1016/j.jpainsymman.2020.04.015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Strang 2021 {published data only}
- Strang P, Bergström J, Lundström S. Symptom relief is possible in elderly dying COVID-19 patients: a national register study. Journal of Palliative Medicine 2020;24(4):514-9. [DOI: 10.1089/jpm.2020.0249] [DOI] [PubMed] [Google Scholar]
- Strang P, Martinsson L, Bergström J, Lundström S. COVID-19: symptoms in dying residents of nursing homes and in those admitted to hospitals. Journal of Palliative Medicine 2021 Mar 5 [Epub ahead of print]. [DOI: 10.1089/jpm.2020.0688] [DOI] [PubMed]
References to studies excluded from this review
ACTRN12620000443998p {published data only}
- ACTRN12620000443998p. Home rehabilitation for people with COVID-19: implementing telehealth approaches to care. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=379528&isReview=true (first received 31 March 2020).
Allande Cussó 2020 {published data only}
- Allande Cussó R, Navarro Navarro C, Porcel Gálvez AM. Humanized care in a death for COVID-19: a case study [El cuidado humanizado en la muerte por COVID-19: a propósito de un caso]. Enfermería Clínica 2021;(31):62-7. [DOI: 10.1016/j.enfcli.2020.05.018] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anneser 2020 {published data only}
- Anneser J. Dying patients with COVID-19: what should hospital palliative care teams (HPCTs) be prepared for? Palliative & Supportive Care 2020;18(4):382-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisson 2020 {published data only}
Cook 2020 {published data only}
- Cook DJ, Takaoka A, Hoad N, Swinton M, Clarke FJ, Rudkowski JC, et al. Clinician perspectives on caring for dying patients during the pandemic: a mixed-methods study. Annals of Internal Medicine 2020 Dec 8 [Epub ahead of print]. [DOI: 10.7326/M20-6943] [DOI] [PMC free article] [PubMed]
Delisle 2020 {published data only}
- Delisle S, Heller FE, Blinderman CD. Prolonged critical illness and demoralization: curative factors in hospice care in the age of COVID-19. Journal of Hospice & Palliative Nursing 2020;22(6):428-31. [DOI: 10.1097/NJH.0000000000000689] [DOI] [PubMed] [Google Scholar]
Galazzi 2020 {published data only}
- Galazzi A, Brioni M, Mistraletti G, Roselli P, Abbruzzese C. The last farewell by video call. Minerva Anestesiologica 2020;86(11):1254-5. [DOI: 10.23736/S0375-9393.20.14906-X] [DOI] [PubMed] [Google Scholar]
Haydar 2020 {published data only}
- Haydar A, Lo KB, Goyal A, Gul F, Peterson E, Bhargav R, et al. Palliative care utilization among patients with COVID-19 in an underserved population: a single-center retrospective study. Journal of Pain and Symptom Management 2020;60(2):e18-21. [DOI: 10.1016/j.jpainsymman.2020.05.022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettus K, Lima L. Update to the fast tracked article entitled: palliative care utilization among patients with COVID-19 in an underserved population: a single-center retrospective study. Journal of Pain and Symptom Management 2020;60(4):e1. [DOI: 10.1016/j.jpainsymman.2020.07.017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heath 2020 {published data only}
- Heath L, Yates S, Carey M, Miller M. Palliative care during COVID-19: data and visits from loved ones. Journal of Hospice and Palliative Medicine 2020;37(11):988-91. [DOI: 10.1177/1049909120943577] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN16561225 {unpublished data only}
- ISRCTN16561225. CovPall: Improving palliative care for people affected by the COVID-19 pandemic by sharing learning—the national and international response. www.isrctn.com/ISRCTN16561225 (first received 5 May 2020). [DOI: 10.1186/ISRCTN16561225] [DOI]
Johnston 2020 {published data only}
- Johnston B, Blades S. COVID-19: using ‘knitted hearts’ in end-of-life care to enable continuing bonds and memory making. International Journal of Palliative Nursing 2020;26(8):391-3. [DOI] [PubMed] [Google Scholar]
Lee 2020 {published data only}
- Lee J, Abrukin L, Flores S, Gavin N, Romney ML, Blinderman CD, et al. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Internal Medicine 2020;180(9):1252-4. [DOI: 10.1001/jamainternmed.2020.2713] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lopez 2020 {published data only}
- Lopez S, Finuf KD, Marziliano A, Sinvani L, Burns EA. Palliative care consultation in hospitalized patients with COVID-19: a retrospective study of characteristics, outcomes, and unmet needs. Journal of Pain and Symptom Management 2021;62(2):276. [DOI: 10.1016/j.jpainsymman.2020.12.015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinsson 2021 {published data only}
- Martinsson L, Strang P, Bergström J, Lundström S. Were clinical routines for good end-of-life care maintained in hospitals and nursing homes during the first three months of the outbreak of COVID-19? A national register study. Journal of Pain and Symptom Management 2021;61(1):e11-9. [DOI: 10.1016/j.jpainsymman.2020.09.043] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendoza 2020 {published data only}
- Mendoza A. Palliative care during a pandemic. www.providermagazine.com/Monthly-Issue/2020/September/Pages/Palliative-Care-During-a-Pandemic.aspx.
Mumoli 2021 {published data only}
- Mumoli N, Florian C, Cei M, Evangelista I, Colombo A, Razionale G, et al. Palliative care in a COVID-19 internal medicine ward: a preliminary report. International Journal of Infectious Disease 2021;105:141-3. [DOI: 10.1016/j.ijid.2021.02.053] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paice 2021 {published data only}
- Paice JA, Wholihan D, Dahlin C, Rosa WE, Mazanec P, Long CO, et al. Palliative nursing: the core of COVID-19 care. Journal of Hospice & Palliative Nursing 2021;23(1):1-6. [DOI: 10.1097/NJH.0000000000000709] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pavlu 2020 {published data only}
- Pavlu D, DeMarco K, Sobrino-Bonilla Y. Field notes from the frontline of a COVID-19 outbreak: dyspnea management for hospitalized patients at end-of-life. Journal of Hospital & Palliative Nursing 2020;23(2):128-34. [DOI: 10.1097/NJH.0000000000000728] [DOI] [PubMed] [Google Scholar]
Rao 2021 {published data only}
- Rao A, Zaaqoq AM, Kang IG, Vaughan EM, Flores J, Avila-Quintero VJ, et al. Palliative care for patients on extracorporeal membrane oxygenation for COVID-19 infection. American Journal of Hospice and Palliative Medicine 2021 March 9 [Epub ahead of print]. [DOI: 10.1177/10499091211001009] [DOI] [PMC free article] [PubMed]
Ritchey 2020 {published data only}
- Ritchey KC, Foy A, McArdel E, Gruenewald DA. Reinventing palliative care delivery in the era of COVID-19: how telemedicine can support end of life care. American Journal of Hospice and Palliative Medicine 2020;37(11):992-7. [DOI: 10.1177/1049909120948235] [DOI] [PMC free article] [PubMed] [Google Scholar]
Riva 2020 {published data only}
Sun 2020 {published data only}
- Sun H, Lee J, Meyer BJ, Myers EL, Nishikawa MS, Tischler JL, et al. Characteristics and palliative care needs of COVID‐19 patients receiving comfort‐directed care. Journal of the American Geriatrics Society 2020;68(8):1162-4. [DOI: 10.1111/jgs.16507] [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2020 {published data only}
- Turner J, Eliot Hodgson L, Leckie T, Eade L, Ford-Dunn S. A dual-center observational review of hospital-based palliative care in patients dying with COVID-19. Journal of Pain and Symptom Management 2020;60(2):e75-8. [DOI: 10.1016/j.jpainsymman.2020.04.031] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
ChiCTR2000029994 {unpublished data only}
- ChiCTR2000029994. Liu-Zi-Jue qigong and acupressure therapy for pulmonary function and quality of life in patient with severe novel coronavirus pneumonia (COVID-19): A randomized controlled trial. Chinese Clinical Trial Registry 2020. [CHICTR: 2000029994] [URL: https://www.chictr.org.cn/showprojen.aspx?proj=49309] [DOI] [PMC free article] [PubMed]
- Zhang S, Zhu Q, Zhan C, Cheng W, Mingfang X, Fang M, et al. Acupressure therapy and Liu Zi Jue Qigong for pulmonary function and quality of life in patients with severe novel coronavirus pneumonia (COVID-19): a study protocol for a randomized controlled trial. Trials 2020;21(1):751. [DOI: 10.1186/s13063-020-04693-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Groninger 2021 {unpublished data only}
- Groninger H, McPherson A, Vaughan E, Kang IG, Kelemen A. Hospital-based palliative care experiences of patients with COVID-19. Journal of Pain and Symptom Management 2021;61(3):695. [ABSTRACT NUMBER: SCI947] [DOI: 10.1016/j.jpainsymman.2021.01.108] [DOI] [Google Scholar]
Kelly 2020 {unpublished data only}
- Kelly L, O’Shea N, Azhar M, Beatty S, Brennock J, Mannion E, et al. Symptomatology at the end of life with COVID-19. Abstracts of the 16th International E-Congress of the European Geriatric Medicine Society; 2020 Oct 7-9 2020 Apr 28 [Epub ahead of print]. [ABSTRACT NUMBER: 23] [DOI: 10.1007/s41999-020-00428-6] [DOI]
Okuwoga 2020 {unpublished data only}
- Okuwoga O, Picton S, Saber S, O’Sullivan C. Managing delirium in patients with COVID-19—lessons to learn. Abstracts of the 16th International E-Congress of the European Geriatric Medicine Society; 2020 Oct 7-9 2020 Apr 28 [Epub ahead of print]. [ABSTRACT NUMBER: 281] [DOI: 10.1007/s41999-020-00428-6] [DOI]
References to ongoing studies
NCT04522037 {published data only}
- NCT04522037. Measurement of the efficacy of morphine in the early management of dyspnea in COVID-19 positive patients (CODYS). clinicaltrials.gov/ct2/show/NCT04522037 (first received 21 August 2020).
Additional references
Antunes 2014
- Antunes B, Harding R, Higginson IJ. Implementing patient-reported outcome measures in palliative care clinical practice: a systematic review of facilitators and barriers. Palliative Medicine 2014;28(2):158-75. [DOI: 10.1177/0269216313491619] [DOI] [PubMed] [Google Scholar]
Balshem 2011
- Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 2011;64(4):401-6. [DOI: 10.1016/j.jclinepi.2010.07.015] [DOI] [PubMed] [Google Scholar]
Banzett 2000
- Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L. Breathlessness in humans activates insular cortex. Neuroreport 2000;11(10):2117-20. [DOI: 10.1097/00001756-200007140-00012] [DOI] [PubMed] [Google Scholar]
Barnes 2016
- Barnes H, McDonald J, Smallwood N, Manser R. Opioids for the palliation of refractory breathlessness in adults with advanced disease and terminal illness. Cochrane Database of Systematic Reviews 2016, Issue 3. Art. No: CD011008. [DOI: 10.1002/14651858.CD011008] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barron 2012
- Barron EA, Holmes J. Delirium within the emergency care setting, occurrence and detection: a systematic review. Emergency Medicine Journal 2013;30(4):263-8. [DOI: 10.1136/emermed-2011-200586] [DOI] [PubMed] [Google Scholar]
Bausewein 2020
- Bausewein C, Voltz R, Steffen S, Radbruch L. Evidence-based guideline: Palliative care for patients with incurable cancer. www.leitlinienprogramm-onkologie.de/fileadmin/user_upload/Downloads/Leitlinien/Palliativmedizin/Version_2/LL_Palliativmedizin_Langversion_2.2.pdf (accessed 26 July 2021). [AWMF-REGISTRATION NUMBER: 128/001OL]
Buchanan 2009
- Buchanan GF, Richerson GB. Role of chemoreceptors in mediating dyspnea. Respiratory Physiology and Neurobiology 2008;176(1):9-19. [DOI: 10.1016/j.resp.2008.12.002] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buitrago‐Garcia 2020
- Buitrago-Garcia D, Egli-Gany D, Counotte MJ, Hossmann S, Imeri H, Ipekci Aziz M, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARS-CoV-2 infections: a living systematic review and meta-analysis. PLOS Medicine 2020;17(9):e1003346. [DOI: 10.1371/journal.pmed.1003346] [DOI] [PMC free article] [PubMed] [Google Scholar]
Campbell 2020
- Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. BMJ 2020;368(l6890):1-6. [DOI: 10.1136/bmj.l6890] [DOI] [PMC free article] [PubMed] [Google Scholar]
Challen 2021
- Challen R, Brooks-Pollock E, Read JM, Dyson L, Tsaneva-Atanasova K, Danon L. Risk of mortality in patients infected with SARS-CoV-2 variant of concern 202012/1: matched cohort study. BMJ 2021;327(n579):1-10. [DOI: 10.1136/bmj.n579] [DOI] [PMC free article] [PubMed] [Google Scholar]
COMET 2020
- Core outcome set developers’ response to COVID-19 (2nd April 2020). www.comet-initiative.org/Studies/Details/1538 (accessed 9 April 2020).
Crombeen 2020
- Crombeen AM, Lilly EJ. Management of dyspnea in palliative care. Current Oncology 2020;27(3):142-5. [DOI: 10.3747/co.27.6413.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
EPOC 2017
- Cochrane Effective Practice and Organisation of Care (EPOC). What study designs can be considered for inclusion in an EPOC review and what should they be called? EPOC Resources for review authors. Available from epoc.cochrane.org/resources/epoc-resources-review-authors (accessed 23 April 2019).
Forni 2021
- Forni G, Mantovani A. COVID-19 vaccines: where we stand and challenges ahead. Cell Death & Differentiation 2021;28:626-39. [DOI: 10.1038/s41418-020-00720-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaertner 2017
- Gaertner J, Siemens W, Meerpohl JJ, Antes G, Meffert C, Xander C, et al. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: systematic review and meta-analysis. BMJ 2017;375:1-14. [DOI: 10.1136/bmj.j2925] [DOI] [PMC free article] [PubMed] [Google Scholar]
Griffin 2013
- Griffin CE 3rd, Kaye AM, Bueno FR, Kaye AD. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner Journal 2013;13(2):214-23. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Haun 2017
- Haun MW, Estel S, Rücker G, Friederich HC, Villalobos M, Thomas M, et al. Early palliative care for adults with advanced cancer. Cochrane Database of Systematic Reviews 2017, Issue 6. Art. No: CD011129. [DOI: 10.1002/14651858.CD011129.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327:557-60. [DOI: 10.1136/bmj.327.7414.557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook. [DOI] [PMC free article] [PubMed]
Huang 2020
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395(10223):497-506. [DOI: 10.1016/S0140-6736(20)30183-5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hui 2020
- Hui D, De La Rosa A, Wilson A, Nguyen T, Wu J, Delgado-Guay M, et al. Neuroleptic strategies for terminal agitation in patients with cancer and delirium at an acute palliative care unit: a single-centre, double-blind, parallel-group, randomised trial. Lancet Oncology 2020;21(7):989-98. [DOI: 10.1016/S1470-2045(20)30307-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johns Hopkins 2021
- Johns Hopkins University of Medicine Coronavirus Resource Center. Mortality analyses. coronavirus.jhu.edu/data/mortality (accessed 16 April 2021).
Johnson 2020
- Johnson MJ, Currow DC. Opioids for breathlessness: a narrative review. BMJ Supportive & Palliative Care 2020;10:287-95. [DOI: 10.1136/bmjspcare-2020-002314] [DOI] [PubMed] [Google Scholar]
Keeley 2020
- Keeley P, Buchanan D, Carolan C, Pivodic L, Tavabie S, Noble S. Symptom burden and clinical profile of COVID-19 deaths: a rapid systematic review and evidence summary. BMJ Supportive & Palliative Care 2020;10(4):381-4. [DOI: 10.1136/bmjspcare-2020-002368] [DOI] [PubMed] [Google Scholar]
Kennedy 2020
- Kennedy M, Helfand BKI, Gou RY, Gartaganis SL, Webb M, Moccia JM, et al. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Network Open 2020;3(11):e2029540. [DOI: 10.1001/jamanetworkopen] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kreuzberger 2020
- Kreuzberger N, Hirsch C, Chai KL, Piechotta V, Valk SJ, Estcourt LJ, et al. SARS-CoV-2-neutralising monoclonal antibodies for treatment of COVID-19. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013825. [DOI: 10.1002/14651858.CD013825] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lefebvre 2021
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Li 2021
- Li T, Higgins JPT, Deeks JJ. Chapter 5: Collecting data. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Liang 2020
- Liang W, Guan W, Chen R, Wang W, Li J, Xu K, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet 2020;21(3):335-7. [DOI: 10.1016/S1470-2045(20)30096-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
MAGICapp 2020 [Computer program]
- MAGIC Evidence Ecosystem Foundation MAGICapp: A digital authoring and publication platform for the evidence ecosystem. MAGIC Evidence Ecosystem Foundation, 2020. Available at app.magicapp.org/.
Mahler 2013
- Mahler DA, Gifford AH, Waterman LA, Ward J, Kraemer WJ, Kupchak BR, et al. Effect of increased blood levels of β-endorphin on perception of breathlessness. Chest 2013;143(5):1378-85. [DOI: 10.1378/chest.12-1541] [DOI] [PubMed] [Google Scholar]
McKenzie 2021
- McKenzie JE, Brennan SE. Chapter 12: Synthesizing and presenting findings using other methods. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Meagher 2018
- Meagher D, Agar MR, Teodorczuk A. Debate article: Antipsychotic medications are clinically useful for the treatment of delirium. International Journal of Geriatric Psychiatry 2018;33:1420-7. [DOI: 10.1002/gps.4759] [DOI] [PubMed] [Google Scholar]
Mercadamte 2014
- Mercadamte S. Death rattle: critical review and research agenda. Support Care Cancer 2014;22(2):571-5. [DOI: 10.1007/s00520-013-2047-5] [DOI] [PubMed] [Google Scholar]
Microsoft 2018 [Computer program]
- Excel. Microsoft Corporation. Microsoft Corporation, 2018. Available at office.microsoft.com/excel.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Journal of Clinical Epidemiology 2009;62(10):1006-12. [DOI] [PubMed] [Google Scholar]
Mottiar 2020
- Mottiar M, Hendin A, Fischer L, des Ordons AR, Hartwick M. End-of-life care in patients with a highly transmissible respiratory virus: implications for COVID-19. Canadian Journal of Anaesthesia 2020;67:1-7. [DOI: 10.1007/s12630-020-01699-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mulder 2019
- Mulder RL, Bresters D, Van den Hof M, Koot BG, Castellino SM, Loke YK, et al. Hepatic late adverse effects after antineoplastic treatment for childhood cancer. Cochrane Database of Systematic Reviews 2019, Issue 4. Art. No: CD008205. [DOI: 10.1002/14651858.CD008205.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nehls 2020
- Nehls W, Delis S, Haberland B, Maier BO, Sänger K, Tessmer G, et al. Management of patients with COVID-19—recommendations from a palliative care perspective. Pneumologie 2020;774(10):652-9. [DOI: 10.1055/a-1156-2759] [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2020
Ouzzani 2016
- Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Systematic Reviews 2016;5(1):210. [DOI: 10.1186/s13643-016-0384-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI: 10.1002/(SICI)1097-0258(19981230)] [DOI] [PubMed] [Google Scholar]
Pattinson 2009
- Pattinson KT, Governo RJ, MacIntosh BJ, Russell EC, Corfield DR, Tracey I, et al. Opioids depress cortical centers responsible for the volitional control of respiration. Journal of Neuroscience 2009;29(25):8177-86. [DOI: 10.1523/JNEUROSCI.1375-09.2009] [DOI] [PMC free article] [PubMed] [Google Scholar]
Radbruch 2020
- Radbruch L, De Lima L, Knaul F, Wenk R, Ali Z, Bhatnaghar S, et al. Redefining palliative care—a new consensus-based definition. Journal of Pain and Symptom Management 2020;60(4):754-64. [DOI: 10.1016/j.jpainsymman.2020.04.027] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reeves 2021
- Reeves BC, Deeks JJ, Higgins JPT, Shea B, Tugwell P, Wells GA. Chapter 24: Including non‐randomized studies on intervention effects. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
RevMan Web 2021 [Computer program]
- The Cochrane Collaboration Review Manager Web (RevMan Web). Version 3.6.0. The Cochrane Collaboration, 2021. Available at revman.cochrane.org.
Santesso 2020
- Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: Informative statements to communicate the findings of systematic reviews of interventions. Journal of Clinical Epidemiology 2020;1(119):126-35. [DOI: 10.1016/j.jclinepi.2019.10.014] [DOI] [PubMed] [Google Scholar]
Schünemann 2019
- Schünemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. Journal of Clinical Epidemiology 2019;111:105-14. [DOI: 10.1016/j.jclinepi.2018.01.012] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Shelby‐James 2012
- Shelby-James TM, Hardy J, Agar M, Yates P, Mitchell G, Sanderson C, et al. Designing and conducting randomized controlled trials in palliative care: a summary of discussions from the 2010 clinical research forum of the Australian Palliative Care Clinical Studies Collaborative. Palliative Medicine 2012;26(8):1042-7. [DOI: 10.1177/0269216311417036] [DOI] [PubMed] [Google Scholar]
Siemieniuk 2020
- Siemieniuk R, Rochwerg B, Agoritsas T, Lamontagne F, Leo YS, Macdonald H, et al. A living WHO guideline on drugs for covid-19. BMJ 2020;370:m3379. [DOI: 10.1136/bmj.m3379] [DOI] [PubMed] [Google Scholar]
Sterne 2016
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:1-7. [DOI: 10.1136/bmj.i4919] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2019
- Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019;366(l4898):1-8. [DOI: 10.1136/bmj.l4898] [DOI] [PubMed] [Google Scholar]
Struyf 2020
- Struyf T, Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Leeflang MM, et al. Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19 disease. Cochrane Database of Systematic Reviews 2020, Issue 7. Art. No: CD013665. [DOI: 10.1002/14651858.CD013665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Svenska Palliativregistret 2021
- The Swedish Register of Palliative Care (SRPC). www.palliativ.se (accessed prior to 9 August 2021).
Taskforce NCCE 2021
- Taskforce NCCE. Clinical Care Guidance. covid19evidence.net.au/ (accessed prior to 9 August 2021).
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ting 2020
- Ting R, Edmonds P, Higginson IJ, Sleeman KE. Palliative care for patients with severe covid-19. BMJ 2020;370:m2710. [DOI: 10.1136/bmj.m2710] [DOI] [PubMed] [Google Scholar]
Valk 2020a
- Valk SJ, Piechotta V, Chai KL, Doree C, Monsef I, Wood EM, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID‐19: a living systematic review. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD013600. [DOI: 10.1002/14651858.CD013600.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Valk 2020b
- Valk SJ, Piechotta V, Kimber C, Chai KL, Monsef I, Doree C, et al. Convalescent plasma and hyperimmune immunoglobulin to prevent infection with SARS-CoV-2. Cochrane Database of Systematic Reviews 2021, Issue 1. Art. No: CD013802. [DOI: 10.1002/14651858.CD013802] [DOI] [Google Scholar]
von Leupoldt 2007
- Leupoldt A, Dahme B. Psychological aspects in the perception of dyspnea in obstructive pulmonary diseases. Respiratory Medicine 2007;101(3):411-22. [DOI: 10.1016/j.rmed.2006.06.011] [DOI] [PubMed] [Google Scholar]
WHO 2007
- World Health Organization (WHO). Cumulative number of reported probable cases of SARS. www.who.int/csr/sars/country/2003_07_11/en/ (accessed 13 April 2020).
WHO 2019
- World Health Organization (WHO). Middle East respiratory syndrome coronavirus (MERS-CoV). www.who.int/emergencies/mers-cov/en/ (accessed 13 April 2020).
WHO 2020a
- World Health Organization (WHO). Report of the WHO‐China Joint Mission on coronavirus disease 2019 (COVID‐19). www.who.int/docs/default-source/coronaviruse/who-china-joint-mission-on-covid-19-final-report (accessed prior to 9 August 2021).
WHO 2020b
- World Health Organization (WHO). Estimating mortality from COVID-19—scientific brief. www.who.int/publications/i/item/WHO-2019-nCoV-Sci-Brief-Mortality-2020.1 (accessed 2 November 2020).
WHO 2020c
- World Health Organization (WHO). Palliative care. www.who.int/news-room/fact-sheets/detail/palliative-care (accessed 13 July 2021).
WHO 2021a
- World Health Organization (WHO). WHO Coronavirus Disease (COVID-19) Dashboard. covid19.who.int (accessed 14 July 2021).
WHO 2021b
- World Health Organization (WHO). Weekly epidemiological update—13 July. www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---13-july-2021 (accessed 14 July 2021).
WHO 2021c
- World Health Organization (WHO). COVID-19 Clinical management: living guidance. www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 (accessed 15 April 2021).
WHO 2021d
- World Health Organization (WHO). Draft landscape of COVID-19 candidate vaccines. www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed 15 April 2021).
WHO 2021e
- World Health Organization (WHO). Therapeutics and COVID-19: living guideline. Version 6.1. app.magicapp.org/#/guideline/nBkO1E (accessed 15 July 2021).
Williamson 2020
- Williamson E, Walker AJ, Bhaskaran KJ, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020;584:430-6. [DOI: 10.1038/s41586-020-2521-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wouters 2021
- Wouters OJ, Shadlen KC, Salcher-Konrad M, Pollard AJ, Larson HJ, Teerawattananon Y, et al. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet 2021;10278:1023-34. [DOI: 10.1016/S0140-6736(21)00306-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2020
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 2020;323(13):1239-42. [DOI: 10.1001/jama.2020.2648] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Andreas 2021
- Andreas M, Piechotta V, Becker G, Metzendorf M-I, Skoetz N, Meissner W, et al. Palliative symptom management in Covid-19 patients: a systematic review. PROSPERO. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021233630. 2021. [DOI] [PMC free article] [PubMed]


