Abstract
Averrhoa carambola L. (star fruit) is an edible fruit that is extensively cultivated in southern China, Southeast Asia, India, and northern South America. It has a sweet and juicy taste and is frequently used in fruit salads and fruit platters, as a garnish in cocktail drinks and beverages, or squeezed into juice and served as a beverage. Traditionally, it has been used for treating diabetes and diabetic nephropathy, arthralgia, vomiting, lithangiuria, coughing, hangovers, and chronic paroxysmal headache for thousands of years. Currently, approximately 132 compounds have been isolated from A. carambola. Among them, flavonoids, benzoquinone, and their glycosides have been considered as biologically active substances, which are responsible for various biological activities. Pharmacological studies have revealed that crude extracts or monomeric compounds from A. carambola exhibit multiple bioactivities, such as anti-oxidant, anti-hyperglycemic, anti-obesity, anti-hyperlipidemic, anti-tumor, anti-inflammatory, hepatoprotective, cardioprotective, anti-hypertensive, neuroprotective, and others. Thus, A. carambola is a valuable treatment in Chinese medicine with therapeutic potential for multiple diseases, especially diabetes and diabetes-related diseases. Even though it is a very promising candidate in the development of functional food and the pharmaceutical industry, reports on its bioactivities have only been conducted in vivo and in vitro and there is a gap in research regarding clinical settings and safety. This review therefore provides a comprehensive and systematic overview of current progress on botany, ethnopharmacology, phytochemistry, pharmacology, and toxicity of A. carambola, providing a valuable reference for further developments and applications of A. carambola in the pharmaceutical industry and functional food.
Keywords: Averrhoa carambola, chemical composition, antidiabetic, anticancer, toxicology
Introduction
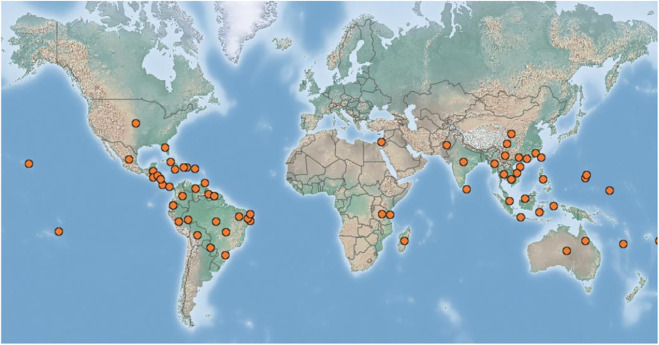
Averrhoa carambola L., commonly known as carambola or star fruit, is a perennial tree in the family Oxalidaceae (Figure 1). It is considered native to Malaysia, however, it is a tropical American species introduced to Asia by the Spanish galleons and mainly cultivated throughout tropical and warm subtropical areas (Figure 2). The fruit is of high commercial value and is specially and extensively distributed and cultivated in southern China, Southeast Asia, India, and northern South America (Saikia et al., 2015; Leivas et al., 2016b; Varela-Martínez et al., 2019; Zulfajri et al., 2019). A. carambola is fleshy, crunchy, juicy, slightly tart, acidic, and sweet in the taste. It is star-shaped and golden-yellow in appearance and is frequently used in the preparation of fruit salads and fruit platters, as a garnish in cocktails and beverages, or squeezed into a juice and served as a functional beverage. It is also used in jellies, ice creams, preserves, and sweets owing to its high moisture content and highly perishability, especially in tropical regions such as Malaysia, Singapore, and Indonesia (Valim et al., 2016; Chua et al., 2017; Huynh and Nguyen, 2017; Jia et al., 2018; Lu et al., 2018). For instance, in Malaysia star fruits are usually blended with apples and braised with cloves and sugars or cooked along with meat or seafood (Bhat et al., 2011). Generally, star fruits are regarded as an abundant source of various nutrients such as minerals, proteins, and vitamins, and also rich in natural phytochemicals such as flavonoids, terpenes, saponins, alkaloids, proanthocyanidins, vitamin C, tartaric acid, oxalic acid, α-ketoglutaric acid, citric acid, vitamin B1 and B2, carotene, pectin, cellulose, gallic acid, epicatechin, fatty acids, volatile flavors, fibers, hemicellulose, polysaccharides, and sterols (Shui and Leong, 2006; Benkeblia and Lopez, 2015; Leivas et al., 2015; Muthu et al., 2016; Yang et al., 2017; Zulfajri et al., 2019). Simultaneously, GC-MS analysis has demonstrated that the abundant fatty acids existing in A. carambola leaves were α-linolenic acid (62.04%) and oleic acid (55.44%) in fruits. Moreover, the proportion of total unsaturated fatty acids existing both in the fruits and leaves of A. carambola comprise more than 77% of total fatty acid (Wei et al., 2014). The fructose content (38–48%) and glucose content (21–25%) have predominantly sugar-based compositions in A. carambola ripe fruits, while sorbitol is also another major sugar alcohol (2.4–10.5%) in ripe fruits (Ramadan et al., 2020). Additionally, the presence of high amounts of fibers in this plant contributes to the absorption of glucose, restraint glucose diffusion into the bloodstream, and maintain normal blood glucose levels (Fan et al., 2020). Furthermore, the byproduct or pomace residue from A. carambola left after juice drink extraction contains more antioxidants than the extracted juice (Shui and Leong, 2006). Interestingly, incorporation of 4% A. carambola fruit juice and 6% Bambusa polymorpha Munro (Poaceae family) shoot extract, significantly prolonged the shelf life of pork nuggets by at least 2 weeks (Thomas et al., 2016). Recent studies found that an antifreeze protein purified from the cold acclimated leaves of Drimys angustifolia Miers (Winteraceae family) and synergistic pectin-maltodextrin-sodium chloride edible coating could dramatically increase the quality of frozen A. carambola (Provesi et al., 2019; Mohd Suhaimi et al., 2021).
FIGURE 1.
A. carambola: (A): whole plants; (B): fruits; (C): flowers and woods; (D): leaves (https://image.baidu.com/).
FIGURE 2.
Geographical distribution of A. carambola throughout the world (https://www.cabi.org/isc/datasheet/8082).
In recent years, phytochemical investigations have revealed that the major chemical components of A. carambola mainly include flavonoids, terpenes, and other phenolics. Among them, 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione (122, DMDD) is the most representative chemical compound with multiple biological activities (Gao et al., 2015; Xie et al., 2016; Chen et al., 2017b). Pharmacological studies have demonstrated that the crude extracts or active substances of A. carambola have multiple health-promoting effects, and many of the biological effects above mentioned have ethnomedicinal uses. Furthermore, the usable range of A. carambola is increasingly expanding from medicinal plants to ornamental plants in gardens. For instance, A. carambola is widely planted as a decorative tree in the streets of southern Chinese cities because of its beautiful appearance (Wu et al., 2020b). More importantly, it is reported that the total consumption per year of A. carambola in China is about 2.6 million tons, whereas the annual production of A. carambola is only two million tons (Wu et al., 2020b).
To date, there has been no authoritative published systematic and comprehensive review that focuses on all of the important aspects of A. carambola. In the present review, recent advances in traditional uses, botanical characteristics, distribution, taxonomy, phytochemical constituents, biological effects as well as the toxicities of A. carambola are comprehensively presented and critically evaluated. Furthermore, the underlying mechanism associated with the bioactivities of crude extracts or components from this plant is also well summarized. The review is helpful for researchers by providing a comprehensive understanding of this increasingly important herb and provides a scientific basis for further study and exploitation of medicinal agents or functional food from A. carambola in the future.
Material and Methods
This review collected, analyzed, summarized literature on the botanical description, traditional uses, chemical constituents, pharmacological activities, and toxicities of A. carambola. All information was systematically gathered from globally accepted scientific databases by Internet databases, including Elsevier, ScienceDirect, PubMed, Web of Science, Wiley, Springer, SciFinder, ACS Publications, CNKI, WanFang, Google Scholar, Baidu Scholar, The Plant List Database, and other literature sources (Ph.D. and MSc dissertations). All published contributions on A. carambola in different languages were included and cited. The identification and examination of the collected works were based on titles and abstracts. The reference lists of the retrieved publications were also checked to identify further relevant papers. The chemical structures of all isolated compounds were drawn by using ChemBioDraw Ultra 14.0 software.
Botanical Description, Geographic Distribution, and Taxonomy
Botanical Description
Botanically, A. carambola is a medium-sized tree reaching up to 3–15 m tall. The stem is gray bark. The leaf is odd-numbered compound leaves, alternate, leaflets 5-13, entire, ovoid, or elliptic, 3–7 cm long, acuminate at apex, with a round base, skewed on one side, sparsely pilose or glabrous underneath. The flower is cymes or panicles; sepals 5, about 5 mm long, arranged in imbricate shape, synthetic ring at the base; petals slightly dorsal, 0.8–1 cm long, purple-red, sometimes pink or white on the back; stamens 5-10; Ovary 5 compartments, many ovules in each compartment, style 5. The fruit is fleshy, drooping, with 5 edges, rarely 6 or 3 edges, star-shaped in cross section, 5–8 cm long, light green or waxy yellow, sometimes dark red. The seed is dark brown (Flora of China Editorial Committee, 1998).
Geographic Distribution
A. carambola is traditionally considered to originate from Malaysia, although it has also been speculated to be a tropical American species introduced to Asia by the Spanish galleons. A. carambola has a wider climate range and can grow within the latitudinal range from 32°N to 30°S and withstand growing in both the hot humid tropics and subtropical countries including Egypt and Israel, and can tolerate short periods of freezing temperatures as low as −3°C. It prefers well-drained soils ideally between pH 5.5–6.5 but can tolerate pH between 5 and 8.5 (Bircher and Bircher, 2000). In recent years, it has been extensively distributed and widely cultivated in most parts of the world (Figure 2), e.g., Asia countries including China and India, Africa countries including Madagascar and Tanzania, North America countries including Mexico and Honduras, Oceania countries including Australia and French Polynesia, South America countries including Brazil and Bolivia, etc. (https://www.cabi.org/isc/datasheet/8082).
Taxonomy
A. carambola belongs to the family Oxalidaceae, which consists of over 900 species belonging to seven genera, such as Dapania, Oxalis, Sarcotheca, Eichleria, Biophytum, Hypseocharis, and Averrhoa. Among them, the genus Averrhoa mainly includes three species, namely A. carambola, A. bilimbi L., and A. dolichocarpa Ruhayah and Sunart (https://www.cabi.org/isc/datasheet/8082; Moresco et al., 2012). Importantly, A. carambola is commonly known as star fruit or carambola, bearing deeply ridged, yellow-brown, edible fruit.
Traditional Uses
A. carambola has been traditionally used for thousands of years in treating diabetes and diabetic nephropathy (DN), arthralgia, vomiting, lithangiuria, coughing and hangovers, and chronic paroxysmal headache. The different medicinal organs of A. carambola including leaves, roots, flowers, and the fruits have been utilized as ethnomedicine in Chinese, Indian, Malaysian, and Brazilian medicine for a long time. For instance, the crushed shoots or leaves of A. carambola are commonly applied in traditional Malaysia medicine to treat headache, chicken-pox, and ringworm, while a decoction of the leaves and fruits of A. carambola is generally used for treating vomiting, fevers, aphthous stomatitis, and angina (Yang et al., 2020b). In Sri Lanka, A. carambola fruits are traditionally used to treat and prevent diabetes mellitus due to their excellent hypoglycemic effects (Abeysekera et al., 2015). In traditional Brazilian medicine, the fruit, juice, as well as tea made from leaves of A. carambola have been traditionally utilized to prevent and treat diabetes, high blood pressure, and urinary system diseases and A. carambola is also considered as a food supplement that can improve the appetite of people with poor appetite (Vasconcelos et al., 2006; Soncini et al., 2011). The traditional Indian medicine records that the ripe fruits of A. carambola can be used for effectively curing the hemorrhage of hemorrhoids and it is also regarded as a remedy for the treatment of eczema, fever, and diarrhea. Furthermore, the ripe fruit of A. carambola is mainly considered as digestive and tonic in Ayurveda (Vasant and Narasimhacharya, 2014). As a Traditional Chinese Medicine (TCM), the roots, fruits, and leaves of A. carambola have been increasingly recognized as an effective herbal medicine in invigorating kidney function and reinforcing Yang (it refers to the masculine, active and positive principle) and is used for the treatment of various ailments with a long history (World Health Organization and Regional Office for the Western Pacific, 2007; Wei et al., 2018). More specifically, the roots of A. carambola have been commonly accepted as a diuretic and appetite stimulant agent, it is also used as an antidiarrheal and febrifugal drug with a long history of medical use in TCM for the treatment of arthralgia, diabetes, DN, lithangiuria, and chronic paroxysmal headache in ancient times (Cabrini et al., 2011; Wen et al., 2013; Zheng et al., 2013; Chen et al., 2017b). At the same time, A. carambola leaves have been commonly utilized for alleviating vomiting, headaches, diabetes, coughing, and hangovers for a many years (Carolino et al., 2005; Ferreira et al., 2008). Furthermore, A. carambola fruits are frequently applied to effectively remedy malarial splenomegaly and food poisoning caused by meat sources (Pang et al., 2017). Overall, the leaves, roots, flowers, and fruits of this plant, might be used as a dietary supplement and should be further studied and developed as a functional food or therapeutic agent in the management of human health.
Nutritional and Phytochemical Composition
Nutritional Composition
Nutrient substances, such as minerals, vitamins, cellulose, hemicelluloses, pectin, and others are contained in the fruit of A. carambola. It has been reported that A. carambola contains cellulose (60%), hemicelluloses (27%), and pectin (13%), which may contribute to controlling blood sugar levels (Lakmal et al., 2021). Moreover, carotene, vitamins, and acids were found in the ripe fruit of A. carambola, with high levels of vitamin C (25.8 mg/100 g fruit), tartaric acid (4.37 mg/100 g fruit), vitamin B1 and B2 (0.12 mg/100 g fruit) (Muthu et al., 2016). Furthermore, mineral elements were also found to be contained in A. carambola, with high levels of potassium (167.13–168.0 mg/100 g fruit), phosphorous (17.87–17.88 mg/100 g fruit), magnesium (11.85–12.05 mg/100 g fruit), calcium (6.37–6.40 mg/100 g fruit), sodium (3.8–3.85 mg/100 g fruit), iron (0.34–0.45 mg/100 g fruit), zinc (0.29–0.51 mg/100 g fruit), copper (0.19–0.45 mg/100 g fruit), and manganese (0.04–0.52 mg/100 g fruit) (Muthu et al., 2016). These results indicate that A. carambola is low-calorie and may also have health-promoting properties.
Phytochemical Compounds
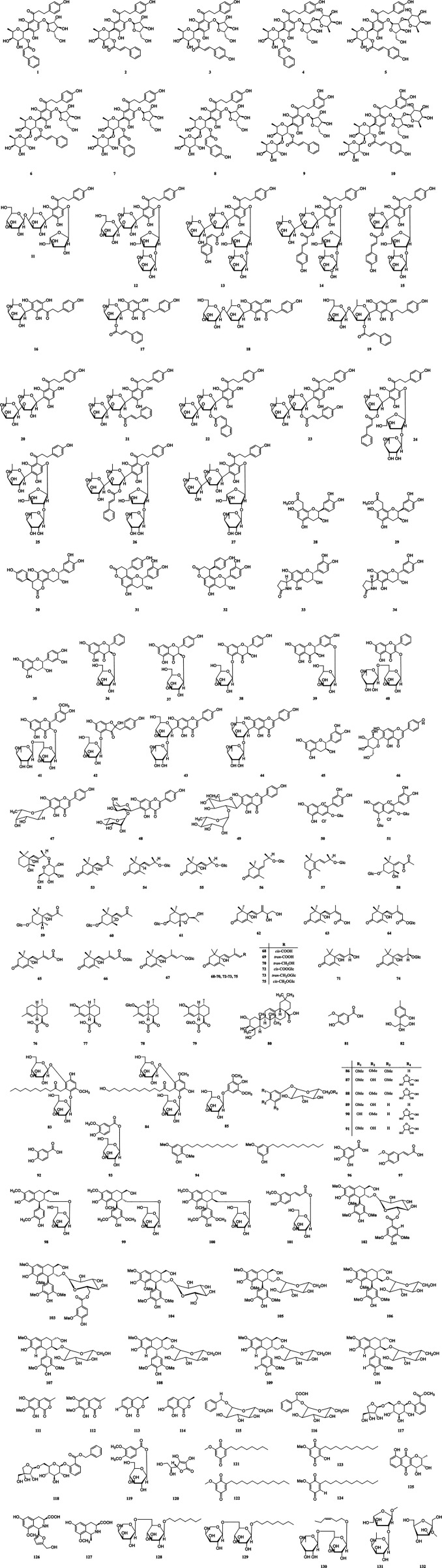
Currently, approximately 132 phytochemical compounds have been separated and identified from A. carambola, which contains flavonoids, terpenes, phenylpropanoids, and their glycosides, among others. These include flavonoids, benzoquinone and their glycosides, which have been considered as the biologically active components responsible for multiple bioactivities. The compounds isolated from A. carambola are documented and listed in Table 1 and the chemical structures are drawn and presented in Figure 3.
TABLE 1.
Chemical components isolated and structurally identified from A. carambola.
| No. | Chemical constituents | Molecular formula | Extracts | Parts | References |
|---|---|---|---|---|---|
| Flavonoids | |||||
| 1 | Carambolaside R1 | C35H38O14 | EtOH | Leaves | Yang et al. (2020a) |
| 2 | Carambolaside R2 | C35H38O14 | EtOH | Leaves | Yang et al. (2020a) |
| 3 | Carambolaside R3 | C35H38O15 | EtOH | Leaves | Yang et al. (2020a) |
| 4 | Carambolaside S1 | C41H48O18 | EtOH | Leaves | Yang et al. (2020a) |
| 5 | Carambolaside S2 | C41H48O18 | EtOH | Leaves | Yang et al. (2020a) |
| 6 | Carambolaside T1 | C41H48O18 | EtOH | Leaves | Yang et al. (2020a) |
| 7 | Carambolaside T2 | C41H48O18 | EtOH | Leaves | Yang et al. (2020a) |
| 8 | Carambolaside T3 | C41H48O19 | EtOH | Leaves | Yang et al. (2020a) |
| 9 | 3-Hydroxycarambolaside T1 | C41H48O19 | EtOH | Leaves | Yang et al. (2020a) |
| 10 | 3-Hydroxycarambolaside P | C47H58O24 | EtOH | Leaves | Yang et al. (2020a) |
| 11 | Carambolaside M | C32H42O18 | EtOH | Fruits | Jia et al. (2018) |
| 12 | Carambolaside N | C38H52O22 | EtOH | Fruits | Jia et al. (2018) |
| 13 | Carambolaside O | C47H58O23 | EtOH | Fruits | Jia et al. (2018) |
| 14 | Carambolaside P | C47H58O23 | EtOH | Fruits | Jia et al. (2018) |
| 15 | Carambolaside Q | C41H48O19 | EtOH | Fruits | Jia et al. (2018) |
| 16 | Carambolaside A | C21H24O9 | MeOH | Fruits | Yang et al. (2015) |
| 17 | Carambolaside B | C30H30O10 | MeOH | Fruits | Yang et al. (2015) |
| 18 | Carambolaside C | C27H34O14 | MeOH | Fruits | Yang et al. (2015) |
| 19 | Carambolaside D | C36H40O15 | MeOH | Fruits | Yang et al. (2015) |
| 20 | Carambolaside E | C27H34O13 | MeOH | Fruits | Yang et al. (2016) |
| 21 | Carambolaside F | C36H40O14 | MeOH | Fruits | Yang et al. (2016) |
| 22 | Carambolaside G | C36H40O14 | MeOH | Fruits | Yang et al. (2016) |
| 23 | Carambolaside H | C36H40O15 | MeOH | Fruits | Yang et al. (2016) |
| 24 | Carambolaside I | C41H48O18 | MeOH | Fruits | Yang et al. (2016) |
| 25 | Carambolaside Ia | C41H48O18 | MeOH | Fruits | Yang et al. (2016) |
| 26 | Carambolaside J | C47H58O22 | MeOH | Fruits | Yang et al. (2016) |
| 27 | Carambolaside Ja | C47H58O22 | MeOH | Fruits | Yang et al. (2016) |
| 28 | 8-carboxymethyl-(+)-epicatechin methyl ester | C18H18O8 | EtOH | Fruits | Jia et al. (2018) |
| 29 | (+)-Epicatechin | C15H14O6 | EtOH | Fruits | Jia et al. (2018) |
| 30 | Epicatechin-(5,6-bc)-4β-(p-hydroxyphenyl)-dihydro-2(3H)-pyranone | C24H20O8 | EtOH | Leaves | Yang et al. (2020b) |
| 31 | Epicatechin-(7,8-bc)-4α-(p-hydroxyphenyl)-dihydro-2(3H)-pyranone | C24H20O8 | EtOH | Leaves | Yang et al. (2020b) |
| 32 | Epicatechin-(7,8-bc)-4β-(p-hydroxyphenyl)-dihydro-2(3H)-pyranone | C24H20O8 | EtOH | Leaves | Yang et al. (2020b) |
| 33 | 6-(S-2-Pyrrolidinone-5-yl)-epicatechin | C19H19NO7 | EtOH | Leaves | Yang et al. (2020b) |
| 34 | 6-(R-2-pyrrolidinone-5-yl)-epicatechin | C19H19NO7 | EtOH | Leaves | Yang et al. (2020b) |
| 35 | (–)-Epicatechin | C15H14O6 | EtOAc | Fruits | Gunawardena et al. (2015) |
| 36 | Pinobanksin 3-O-β-D-glucoside | C21H22O10 | EtOH | Fruits | Jia et al. (2018) |
| 37 | Aromadendrin 3-O-β-D-glucoside | C21H22O11 | EtOH | Fruits | Jia et al. (2018) |
| 38 | Helicioside A | C21H22O12 | EtOH | Fruits | Jia et al. (2018) |
| 39 | Taxifolin 3′-O-β-D-glucoside | C21H22O12 | EtOH | Fruits | Jia et al. (2018) |
| 40 | Norathyriol | C13H8O6 | EtOH | Fruits | Jia et al. (2018) |
| 41 | Isorhamnetin 3-O-rutinoside | C28H32O16 | EtOH | Fruits | Jia et al. (2018) |
| 42 | Hovertichoside C | C36H46O14 | MeOH | Fruits | Yang et al. (2015) |
| 43 | Isovitexin 2″-O-α-L-rhamnopyranoside | C27H30O14 | MeOH | Fruits | Yang et al. (2015) |
| 44 | Carambolaflavone | C27H30O13 | MeOH | Fruits | Yang et al. (2015) |
| 45 | (+)-Catechin | C15H14O6 | Aqueous | Roots | Liao et al. (2019a) |
| 46 | Isovitexin | C21H22O10 | EtOH | Leaves | Araho et al. (2005) |
| 47 | Carambolaflavone A | C27H30O13 | EtOH | Leaves | Araho et al. (2005), Moresco et al. (2012), Wang et al. (2018) |
| 48 | Carambolaflavone B | C27H30O13 | EtOH | Leaves | Araho et al. (2005), Moresco et al. (2012), Wang et al. (2018) |
| 49 | Apigenin 6-C-(2′′-O-α-L-rhamnopyranosyl)-β-D-glucopyranoside | C36H37O18 | EtOH | Leaves | Moresco et al. (2012) |
| 50 | Cyanidin-3-O-β-D-glucoside | C21H21O11Cl | EtOH | Leaves | Gunasegaran (1992) |
| 51 | Cyanidin-3, 5-O-β-D-diglucoside | C27H37O16Cl | EtOH | Leaves | Gunasegaran (1992) |
| Terpenes | |||||
| 52 | (5R,6S,7E,9R)-5,6,9-trihydroxy-7-megastigmene 9-O-β-D-glucoside | C19H34O8 | EtOH | Fruits | Jia et al. (2019) |
| 53 | Drovomifoliol | C13H19O3 | EtOH | Fruits | Jia et al. (2019) |
| 54 | 3-oxo-α-ionol-9-O-β-D-glucoside | C19H31O7 | EtOH | Fruits | Jia et al. (2019) |
| 55 | Roseoside | C19H30O8 | EtOH | Fruits | Jia et al. (2019) |
| 56 | 3-oxo-9-O-β-D-glucosyloxy-4,6E-megastigmadien | C19H30O8 | EtOH | Fruits | Jia et al. (2019) |
| 57 | 4-oxo-β-ionol 9-O-β-D-glucoside | C19H31O8 | EtOH | Fruits | Jia et al. (2019) |
| 58 | Cannabiside D | C19H30O9 | EtOH | Fruits | Jia et al. (2019) |
| 59 | Dendranthemoside B | C19H32O8 | EtOH | Fruits | Jia et al. (2019) |
| 60 | Icariside | C27H30O11 | EtOH | Fruits | Jia et al. (2019) |
| 61 | Officinoside A | C19H32O8 | EtOH | Fruits | Jia et al. (2019) |
| 62 | 6S,7E,10S)-△9,15-10-hydroxyabscisic alcohol | C15H22O4 | EtOH | Fruits | Jia et al. (2019) |
| 63 | Abscisic acid | C15H20O4 | EtOH | Fruits | Jia et al. (2019) |
| 64 | Abscisyl β-D-glucoside | C21H31O10 | EtOH | Fruits | Jia et al. (2019) |
| 65 | 9E-abscisic acid | C15H20O4 | EtOH | Fruits | Jia et al. (2019) |
| 66 | 9E-abscisyl β-D-glucoside | C21H32O10 | EtOH | Fruits | Jia et al. (2019) |
| 67 | 9E-abscisic alcohol β-D-glucoside | C21H33O9 | EtOH | Fruits | Jia et al. (2019) |
| 68 | cis-abscisic acid | C15H20O4 | EtOAc | Fruits | Gunawardena et al. (2015) |
| 69 | trans-abscisic acid | C15H20O4 | EtOAc | Fruits | Gunawardena et al. (2015) |
| 70 | trans-abscisic alcohol | C15H22O3 | EtOAc | Fruits | Gunawardena et al. (2015) |
| 71 | (6S,9R)-vomifoliol | C13H20O3 | EtOAc | Fruits | Gunawardena et al. (2015) |
| 72 | cis-abscisic acid β-D-glucopyranosyl ester | C22H32O8 | EtOAc | Fruits | Gunawardena et al. (2015) |
| 73 | trans-abscisic alcohol β-D-glucopyranoside | C22H33O7 | EtOAc | Fruits | Gunawardena et al. (2015) |
| 74 | (6S,9R)-roseoside | C14H22O3 | EtOAc | Fruits | Gunawardena et al. (2015) |
| 75 | cis-abscisic alcohol β-D-glucopyranoside | C22H33O7 | EtOAc | Fruits | Gunawardena et al. (2015) |
| 76 | Artemisinic acid | C15H22O2 | EtOH | Fruits | Yang et al. (2012) |
| 77 | 3-β-hydroxyartemisinic acid | C15H22O3 | EtOH | Fruits | Yang et al. (2012) |
| 78 | Artemisinic acid 3-β-O-β-D-glucopyranoside | C21H32O8 | EtOH | Fruits | Yang et al. (2012) |
| 79 | 3-β-hydroxyartemisinic acid β-D-glucopyranosyl ester | C21H32O8 | EtOH | Fruits | Yang et al. (2012) |
| 80 | Arjunolic acid | C30H48O5 | MeOH | Fruits | Yang et al. (2014) |
| Phenolics | |||||
| 81 | Vanillic acid | C8H8O4 | MeOH | Fruits | Yang et al. (2014) |
| 82 | 8,9,10-trihydroxythymol | C10H10O4 | MeOH | Fruits | Yang et al. (2014) |
| 83 | Carambolaside K | C30H48O15 | EtOH | Fruits | Jia et al. (2017) |
| 84 | Carambolaside L | C32H52O16 | EtOH | Fruits | Jia et al. (2017) |
| 85 | Koaburaside | C14H20O9 | EtOH | Fruits | Jia et al. (2017) |
| 86 | 3,4,5-trimethoxyphenol-1-O-β-D-glucopyranoside | C15H22O9 | BuOH | Roots | Wen et al. (2012) |
| 87 | 3,5-dimethoxy-4-hydroxyphenyl 1-O-β-apiofuranosyl (1''→6')-O-β-D-glucopyranoside | C19H28O13 | BuOH | Roots | Wen et al. (2012) |
| 88 | 3,4,5-trimethoxyphenyl 1-O-β-apiofuranosyl (1''→6')-β-glucopyranoside | C20H30O13 | BuOH | Roots | Wen et al. (2012) |
| 89 | Methoxyhydroquinone-4-β-D-glucopyranoside | C13H18O18 | BuOH | Roots | Wen et al. (2012) |
| 90 | 3-hydroxy-4-methoxyphenol 1-O-β-D-apiofuranosyl-(1''→6')-O-β-D-glucopyranoside | C18H26O12 | BuOH | Roots | Wen et al. (2012) |
| 91 | 4-hydroxy-3-methoxyphenol 1-O-β-D-apiofuranosyl-(1''→6')-O-β-D-glucopyranoside | C18H26O12 | BuOH | Roots | Wen et al. (2012) |
| 92 | Protocatechuic acid | C7H6O4 | EtOH | Fruits | Jia et al. (2017) |
| 93 | 1-O-vanilloyl-β-D-glucose | C14H18O9 | EtOH | Fruits | Jia et al. (2017) |
| 94 | 2,5-dimethoxy-3-undecylphenol | C19H33O3 | EtOAc | Wood | Chakthong et al. (2010) |
| 95 | 5-methoxy-3-undecylphenol | C18H31O2 | EtOAc | Wood | Chakthong et al. (2010) |
| 96 | Gallic acid | C7H6O5 | Acetone | Fruits | Shui and Leong (2004) |
| Phenylpropanoids | |||||
| 97 | Ferulic acid | C10H10O4 | MeOH | Fruits | Yang et al. (2014) |
| 98 | (+)-isolariciresinol 9-O-β-D-glucoside | C27H36O12 | EtOH | Fruits | Jia et al. (2017) |
| 99 | (+)-lyoniresinol 9-O-β-D-glucoside | C28H38O13 | EtOH | Fruits | Jia et al. (2017) |
| 100 | (−)-lyoniresinol 9-O-β-D-glucoside | C28H38O13 | EtOH | Fruits | Jia et al. (2017) |
| 101 | 1-O-feruloyl-β-D-glucose | C16H20O9 | EtOH | Fruits | Jia et al. (2017) |
| 102 | Tarennanosides A | C37H46O17 | Aqueous | Roots | Liao et al. (2019a) |
| 103 | Fernandoside | C36H44O16 | Aqueous | Roots | Liao et al. (2019a) |
| 104 | 7α-[(β-glucopyranosyl) oxy]-lyoniresinol | C28H38O13 | Aqueous | Roots | Liao et al. (2019a) |
| 105 | (+)-lyoniresinol 3α-O-β-D-glucopyranoside | C28H38O13 | Aqueous | Roots | Liao et al. (2019a) |
| 106 | (−)-lyoniresinol 3α-O-β-D-glucopyranoside | C28H38O13 | Aqueous | Roots | Liao et al. (2019a) |
| 107 | (−)-5′-methoxy-isolariciresinol 3α-O-β-D-glucopyranoside | C27H36O12 | Aqueous | Roots | Liao et al. (2019a) |
| 108 | (+)-5′-methoxy-isolariciresinol 3α-O-β-D-glucopyranoside | C27H36O12 | BuOH | Roots | Wen et al. (2012) |
| 109 | (+)-isolariciresinol 3α-O-β-D-glucopyranoside | C26H34O11 | BuOH | Roots | Wen et al. (2012) |
| 110 | (−)-isolariciresinol 3α-O-β-D-glucopyranoside | C26H34O11 | BuOH | Roots | Wen et al. (2012) |
| 111 | Reticulol | C11H10O5 | EtOAc | Fruits | Sritharan et al. (2019) |
| 112 | 6-O-methyl-reticulol | C11H10O5 | EtOAc | Fruits | Sritharan et al. (2019) |
| 113 | 5-methylmellein | C11H12O3 | EtOAc | Fruits | Sritharan et al. (2019) |
| 114 | 7-hydroxy-5-methylmellein | C11H12O4 | EtOAc | Fruits | Sritharan et al. (2019) |
| Other constituents | |||||
| 115 | Benzyl-1-O-β-D-glucopyranoside | C13H18O6 | BuOH | Roots | Wen et al. (2012) |
| 116 | (2S)-2-O-β-D-glucopyranosyl-2-hydroxyphenyl-acetic acid | C14H18O8 | BuOH | Roots | Wen et al. (2012) |
| 117 | Methyl 2-β-D-apiofuranosyl-(1→6)-β-D-glucopyranosyloxybenzoate | C19H26O12 | EtOH | Leaves | Yang et al. (2020b) |
| 118 | Benzyl 2-β-D-apiofuranosyl-(1→6)-β-D-glucopyranosyloxybenzoate | C25H30O12 | EtOH | Leaves | Yang et al. (2020b) |
| 119 | Tecomin | C15H20O9 | EtOH | Fruits | Jia et al. (2017) |
| 120 | L-ascorbic acid | C6H8O6 | Acetone | Fruits | Shui and Leong (2004) |
| 121 | 2-methoxy-6-nonyl-cyclohexa-2,5-diene-1,4-dione | C16H24O3 | EtOH | Roots | Wen et al. (2014) |
| 122 | 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione | C19H30O3 | EtOH | Roots | Zhang et al. (2020) |
| 123 | 5-O-methylembelin | C18H28O4 | EtOAc | Woods | Chakthong et al. (2010) |
| 124 | 2-dehydroxy-5-O-methylembelin | C18H28O3 | EtOAc | Woods | Chakthong et al. (2010) |
| 125 | (+)-cryptosporin | C14H12O6 | EtOH | Fruits | Jia et al. (2017) |
| 126 | (1R*,3S*)-1-(5-hydroxymethylfuran-2-yl)-3-carboxy-6-hydroxy-8-methoxyl-1,2,3,4-tetrahydroisoquinoline | C16H17NO6 | MeOH | Fruits | Yang et al. (2014) |
| 127 | (1S*,3S*)-1-methyl-3-carboxy-6-hydroxy-8-methyoxyl-1,2,3,4-tetrahydroisoquinoline | C12H15NO4 | MeOH | Fruits | Yang et al. (2014) |
| 128 | Heptyl vicianoside | C18H34O10 | EtOH | Fruits | Yang et al. (2019b) |
| 129 | Octyl vicianoside | C21H30O11 | EtOH | Fruits | Yang et al. (2019b) |
| 130 | cis-3-hexenyl rutinoside | C34H43O16 | EtOH | Fruits | Yang et al. (2019b) |
| 131 | Methyl 2-O-β-D-fucopyranosyl-α-L-arabinofuranoside | C12H22O9 | EtOH | Fruits | Yang et al. (2019b) |
| 132 | Methyl α-D-fructofuranoside | C7H14O6 | EtOH | Fruits | Yang et al. (2019b) |
EtOH, ethanol; EtOAc, ethyl acetate; n-BuOH, n-butanol.
FIGURE 3.
Chemical structures of compounds isolated from A. carambola.
Flavonoids
Various studies have found that the flavonoids isolated from this plant possess excellent antioxidant and radical scavenging properties, which can be used to prevent and treat the occurrence of chronic and cardiovascular illness (Maqsood et al., 2020). Until now, 51 flavonoids (1–51) have been separated and characterized by nuclear magnetic resonance (NMR) and mass spectrometer (MS) technologies from the leaves, fruits, and roots of A. carambola. Among these are compounds (1–27) that are dihydrochalcone C-glycosides and other compounds (28–35) are flavan-3-ols, of which all exhibited significant radical scavenging activities against the DPPH and ABTS, while some compounds (36–51) are other types with multiple structures. Both the compounds carambolaflavone A (47) and carambolaflavone B (48) showed excellent antihyperglycemic activity both in hyperglycemic and diabetic rats’ model (Cazarolli et al., 2009; Cazarolli et al., 2012). Afterward, Wang et al. (2018) conducted the total synthesis of the enantiomers of carambolaflavone A (47) and found that structurally the β-fucosyl moiety absolute configuration was D instead of L. Information of these isolated flavonoids is listed in Table 1. Their chemical structures were drawn by ChemBioDraw Ultra 14.0 and are described in Figure 3.
Terpenes
Terpenes are a group of secondary metabolites in plants that consist of one or more isoprene subunits (Bahramsoltani et al., 2020). They have the function of promoting the coloring in many and various vegetables and fruits (Farias et al., 2020). To date, 29 terpenes (52–80) have been mainly separated and identified from the fresh fruits of A. carambola. Gunawardena et al. (2015) analyzed different terpenes in star fruits using NMR and MS methods, and the major terpenes identified were cis-abscisic acid (68, 12 mg), trans-abscisic acid (69, 3.5 mg), trans-abscisic alcohol (70, 12 mg), (6S,9R)-vomifoliol (71, 8.5 mg), cis-abscisic acid β-D-glucopyranosyl ester (72, 19 mg), trans-abscisic alcohol β-D-glucopyranoside (73, 12 mg), (6S,9R)-roseoside (74, 12 mg), and cis-abscisic alcohol β-D-glucopyranoside (75, 113 mg). Moreover, Jia et al. (2019) found that the terpenes-derived components from star fruits are primarily C13- and C15-norisoprenoids, which tremendously strengthen the flavor of A. carambola fruits. Information of these terpenes is listed in Table 1. The chemical structures were draw by ChemBioDraw Ultra 14.0 and are shown in Figure 3. However, the pharmacological activities of most terpenes is still unclear.
Phenolics
Phenolic compositions are represented as one of the major classes of plant secondary metabolites and extensively dispersed among plant parts, phytochemical studies have found that these compounds principally exist in the roots and fruits of A. carambola (Wen et al., 2012; Yang et al., 2014; Jia et al., 2017; Liao et al., 2019a). At present, 16 phenolic components (81–96) were isolated and characterized by FT-IR, 1H-NMR, and 13C-NMR, from the roots and fruits of A. carambola with excellent antioxidant properties. Among them, compounds 83, 84, 94, and 95 are alkyl phenols in structure. Pang et al. (2016) compared the phenolic and flavonoid compound content of four popular cultivars of A. carambola that originated from southern China and found that the contents of bound, free, and total phenolic for four cultivars were 6.4–19.7, 162.5–286.8, and 174.5–293.1 mg gallic acid equivalents per 100 g fresh weight, respectively. The contents of bound, free, and total flavonoid of the four cultivars were 1.1–7.8, 100.7–234.0, and 104.4–235.1 mg catechin equivalents per 100 g fresh weight, respectively, which indicated that a certain amount of non-flavonoid phenolic substances exist. These phenolic compositions are summarized in Table. Their chemical structures were drawn by ChemBioDraw Ultra 14.0 and are presented in Figure 3.
Phenylpropanoids
Phenylpropanoids are a kind of plant-derived organic compound, and these compounds are mainly derived from phenylalanine and tyrosine (Yao et al., 2020). At present, 18 phenylpropanoids (97–114) have been successfully separated and chemically identified by analyses of spectroscopic data including 1H-NMR and 13C-NMR, from the fruits and roots of A. carambola. These compounds can be classified as simple phenylpropanoids, lignans, and coumarins based on their substructure type. Among them, four simple phenylpropanoids (97–101) have been reported for the A. carambola fruit, twelve lignans (102–110) were primarily achieved and identified from A. carambola roots. Furthermore, four coumarins (111–114) have been found in A. carambola fruit, compounds reticulol (111) and 6-O-methyl-reticulol (112) are isocoumarins in structure, 5-methylmellein (113) and 7-hydroxy-5- methylmellein (114) are dihydroisocoumarins in structure. Reticulol (111) displayed moderate antioxidant capacity against DPPH with the IC50 value of 58 μg/ml (Sritharan et al., 2019). These phenylpropanoid constituents are summarized in Table 1 and the corresponding chemical structures were also draw by ChemBioDraw Ultra 14.0 and are presented in Figure 3.
Other Compounds
Up to date, apart from the chemical compounds listed above, few compounds (115–132) have been investigated and summarized in Table 1 and the corresponding chemical structures were presented in Figure 3. Briefly, compounds 117 and 118 are identified as benzoic acid, compounds 121–125 are quinones, compounds 126 and 127 are tetrahydroisoquinoline alkaloids, and components 128–132 are identified as alkyl glycosides. Among them, 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione (122) was obtained and characterized from A. carambola roots showing multiple bioactive properties both in cell and animal experimental studies, including anti-cancer (Gao et al., 2015; Chen et al., 2017b), anti-diabetic nephropathy (Lu et al., 2019), anti-obesity (Li et al., 2016), anti-hyperglycemic (Zhang et al., 2020), anti-inflammatory (Xie et al., 2016), and neuroprotective activities (Wei et al., 2018).
Pharmacological Activities
Antioxidant Activity
Among pharmacologically active substances, natural antioxidants have gained widespread attention because they are safe and have low toxicity, and promising biological functions (Rufino et al., 2011). A. carambola leaves (ACL) supposedly exhibit the most potent antioxidant activities determined by DPPH, FRAP, and TEAC assays (Table 2). Phytochemical studies have shown that these leaves are rich in phenolic and flavonoid components, which are closely associated with antioxidant effects; this indicates that ACL is potentially an abundant source of natural antioxidants and could help prevent and treat oxidative stress-related diseases (Chen et al., 2017a). In a systematic comparison between twenty locally available fruits planted in Sri Lanka, Silva and Sirasa (2018) demonstrated that A. carambola had the third most potent antioxidant properties based on assays against FRAP and DPPH activities, total flavonoid content, total phenolic content, and vitamin C content. Siddika et al. (2020) found that the methanol extract of A. carambola leaves (MEACL, at 50–375 μg/ml) demonstrated dose-dependent moderate antioxidant activity when assayed against DPPH and ABTS+, with IC50 values of 62.0 and 6.0 μg/ml, respectively. Other phytochemical investigations have shown MEACL is rich in phenolics, which could be the drive behind its radical scavenging activity. Using paper spray ionization (PSI) coupled to high-resolution mass spectrometry, one study revealed that a bioactive compound, norathyriol, isolated from ethanol extracts of the bark of A. carambola (EEBAC) had antioxidant properties. EEBAC (at concentrations of 1.0, 3.0, 10, 30, and 100 μg/ml) displayed concentration-dependent antioxidant characteristics via suppressing the activities of α-glucosidase, elastase, ABTS+, DPPH, and tyrosinase enzyme, with IC50 values of 7.15, 20.34, 26.29, 55.55, and 56.46 μg/ml, respectively (Islam et al., 2020). Compound (40) at 2.5, 5, and 10 μg/ml, has shown powerful antioxidant effects against DPPH and ABTS+, with corresponding IC50 values of 4.9 and 9.63 μg/ml (Islam et al., 2020). The efficiency and potency of crude extracts or bioactive ingredients from A. carambola suggest it is a promising antioxidant in the pharmaceutical and functional food industries.
TABLE 2.
Pharmacological effects of crude extracts and bioactive compounds of A. carambola.
| Pharmacological activity | Compounds/Extracts | Types | Testing subjects | Doses/Duration | Effects/Mechanisms | References |
|---|---|---|---|---|---|---|
| Antioxidant activity | ||||||
| MEACL | In vitro | DPPH and ABTS+ assays | 50–375 μg/ml | Showed moderate free radical scavenging activity against DPPH and ABTS+ free radical with the IC50 were 62.0 and 6.0 μg/ml, respectively | Siddika et al. (2020) | |
| PRAC | In vitro | DPPH and ABTS+ assays | 0.5–2.5 mg/ml | Showed significant free radical scavenging activity against DPPH and ABTS+ with the IC50 of 0.10 and 0.33 mg/ml, respectively | Liao et al. (2019b) | |
| EEBAC | In vitro | α-glucosidase, elastase, ABTS+, DPPH, and tyrosinase enzyme assays | 1.0, 3.0, 10, 30, and 100 μg/ml | Showed significant antioxidant activity, and the IC50 values were 7.15, 20.34, 26.29, 55.55 and 56.46 μg/ml, respectively | Islam et al. (2020) | |
| 40 | In vitro | DPPH and ABTS+ assays | 2.5, 5, and 10 μg/ml | Showed significant radical scavenging activity against the DPPH and ABTS+ with the IC50 values of 4.9 and 9.63 μg/ml, respectively | Islam et al. (2020) | |
| Anti-hyperglycemic activity | ||||||
| REAC | In vivo | STZ-induced diabetic mice | 150, 300, 600, and 1,200 mg/kg, daily for 21 days | Blood glucose, TC, TGs, and FFAs levels ↓; insulin level ↑; caspase-3, caspase-8, caspase-9, and Bax protein expressions ↓; Bcl-2 protein expression ↑ | Xu et al. (2014) | |
| REAC | In vivo | STZ-induced diabetic mice | 300, 600, and 1,200 mg/kg, i.g., daily for 14 days | FBG, IL-6 and TNF-α levels ↓; TLR4 and NF-κB mRNA and protein expression | Xu et al. (2015) | |
| REAC | In vivo | STZ-induced diabetic mice | 300, 600, and 1,200 mg/kg, i.g., daily for 42 days | FBG, Cr, BUN, and MDA levels ↓; SOD, GSH-Px, CAT activities ↑; Cyto-C, AIF, and caspase-3 protein expressions ↓ | Xu et al. (2017) | |
| FPAC | In vivo | Fluoride-induced hyperglycemia, hypercholesterolemia, and oxidative stress of rat’s model | 2.5, 5.0, and 10 g, i.g., for 30 days | Blood glucose, G-6-pase, SGOT, SGPT, ACP, and ALP levels ↓; hepatic glycogen and hexokinase, and FRAP activities ↑; plasma AIP, TL, TC, TG, LDL-C, VLDL-C, hepatic lipids-TL, TC and TG levels ↓; HDL-C level ↑; CAT, SOD, GPx, GSH, TAA activities ↑; MDA level ↓ | Vasant and Narasimhacharya (2014) | |
| JEAC | In vivo | STZ-induced diabetic mice | 25, 50, and 100 mg/kg, i.g., once a day for 21 days | FBG, FFA, TC, TG, Scr, BUN, and MDA levels ↓; SDH, cAMP, SOD, and insulin activities ↑; CTGF and TGF-β1 mRNA and protein expressions ↓ | Pham et al. (2017) | |
| JEAC | In vivo | STZ-induced diabetic mice | 5, 10, and 20 g/kg, orally, once a day for 14 days | FBG, blood glucose, area under curve, LDH, GC and pyruvate ↓; FINS level ↑ | Yang et al. (2019a) | |
| TFACL | In vivo | Alloxan-induced diabetic mice and STZ-induced diabetic rats | 0.2, 0.4, and 0.8 g/kg, i.g., daily for 7 days | FBG level ↓; glucose tolerance ↑ | Liu et al. (2013) | |
| EEACB | In vivo | STZ-induced diabetic mice | 50 and 100 mg/kg, i.g., for three successive days | Blood glucose level ↓ | Islam et al. (2020) | |
| 47 | In vivo | STZ-induced diabetic rats | 20 and 50 mg/kg, i.g., for 3 h | Serum blood glucose level ↓; insulin secretion ↑ | Cazarolli et al. (2012) | |
| 48 | ||||||
| 48 | In vitro | 14C-glucose uptake in rat soleus muscle | 50 and 100 μM for 1 h | Glucose uptake and glucose transport ↑ | Cazarolli et al. (2009) | |
| 105 | In vivo | STZ-induced diabetic mice | 20, 40, and 80 mg/kg, i.g., daily for 14 days | FBG, FINS, and ISI levels ↓; NF-κB, caspase-3, caspase-8, caspase-9, and Bax protein expressions ↓ | Wen et al. (2013) | |
| 106 | ||||||
| 121 | In vivo | STZ-induced diabetic mice | 30, 60, and 120 mg/kg, i.g., once daily, for 21 days | FBG, TC, TG, FFA, GHb, FINS, MCP-1, TNF-α, IL-6 and MDA levels ↓; SOD, GSH activities ↑ | Qin et al. (2019) | |
| 121 | In vivo | STZ-induced diabetic mice | 30, 60, and 120 mg/kg, i.g., once daily, for 21 days | TLR4, MyD88, p-NF-κB, TNF-α, and IL-6 mRNA and protein expression levels ↓ | Qin et al. (2020) | |
| 122 | In vivo | Type 2 diabetic KKAy mice | 12.5, 25, and 50 mg/kg, i.g., daily, for 56 days | FBG, AGEs glycosylated protein and TC levels ↓; albumin level ↑ | Zheng et al. (2013) | |
| 122 | In vivo | Type 2 diabetic KKAy mice | 12.5, 25, and 50 mg/kg, i.g., daily, for 56 days | FBG level ↓; RAGE, NF-B, TGF-β1 and CML protein expression levels ↓ | Zheng et al. (2021) | |
| 122 | In vivo | DN model established by STZ in TLR4 knockout mice and wild-type mice | 12.5, 25, and 50 mg/kg, i.g., daily for 28 days | TC, TG, HDL, LDL, Scr, BUN, and blood glucose ↓; IL-6 and TNF-α level ↓; TLR4, MyD88 and NF-κB mRNA and protein expressions ↓ | Lu et al. (2019) | |
| 122 | In vitro | HG-induced HK-2 cells | 30 μM for 48 h | Blood glucose ↓; Vimentin mRNA and protein level ↑; TLR4 and E-cadherin mRNA and protein levels ↓; BAMBI ↑; Smad2/3 ↓ | Zhang et al. (2019) | |
| 122 | In vivo | Diabetic kidney disease mice model induced by Wild type and TLR4 knockout | 12.5, 25, and 50 mg/kg, i.g., once daily, for 28 days | TC, TG, LDL-C, FBG, CysC, and urinary albumin levels ↓; TLR4, TGF-β1 and Smad2/3 mRNA and protein levels ↓ | Zhang et al. (2020) | |
| Antihyperlipidemic activity | ||||||
| IFRF | In vivo | Murine model | Diets formulations, i.g., for 30 days | TG, TC, HDL, and LDL levels ↓ | Herman-Lara et al. (2014) | |
| FF | ||||||
| MEACL | In vivo | Poloxamer-407-induced hyperlipidemic rat model | NM | TC, TG, LDL-C, VLDL-C and AI levels ↓ | Saghir et al. (2016) | |
| MEACL | In vivo | HFD-induced hyperlipidemic rats | 250, 500, and 1,000 mg/kg, i.g., daily, for 35 days | TC, TG, LDL-C, VLDL-C, and AI ↓; HDL-C ↑; GSH, GPx, SOD, CAT activities ↑; MDA level ↓ | Aladaileh et al. (2019) | |
| Anti-obesity activity | ||||||
| CEPAC | In vitro | 3T3-L1 preadipocytes | 10, 100, 500, and 1,000 mg/ml | TG accumulation ↓; PPAR-γ and C/EBPα mRNA expressions ↓; PPAR-α mRNA expression ↑ | Rashid et al. (2016) | |
| 122 | In vivo | high-fat diet (HFD) in mice | 12.5, 25, and 50 mg/kg, i.g., daily for 28 days | BW and adipose tissue weights, blood glucose, insulin, TC, TG, FFA, IL-6, TNF-α levels ↓; TLR4 and MyD88 expressions ↓; insulin secretion ↑ | Li et al. (2016) | |
| Antitumor activity | ||||||
| ACE | In vivo | DENA-induced and CCl4-promoted liver cancer in mice | 25 mg/kg, i.g., for five consecutive days | Tumor incidence, tumor yield, tumor burden ↓; LPO level ↓; GSH, SOD, CAT, total proteins content activities ↑ | Singh et al. (2014) | |
| MEACL | In vivo | EAC cell bearing mice | 25 and 50 mg/kg, i.g., for 5 days | viable cells and body weight ↓; survival time ↑; Hgb, WBC, RBC numbers ↑; p53 and Bax protein expression ↑ | Siddika et al. (2020) | |
| 122 | In vitro | Human breast cancer MCF-7 and BT20 cells | 10, 32, 100 μM for 24 h | Caspase-3/7, -8, and -9 activities ↑; TRAIL-R1, TRAIL-R2, Bad, and BID protein expressions ↑; cIAP, XIAP, and Survivin protein expressions ↓; G1 phase cell cycle arrest, ROS ↑; NF-κB ↓ | Gao et al. (2015) | |
| 122 | In vivo | Transplanted 4T1 breast cancer cells bearing mouse | 25, 50 and 100 mg/kg, i.g., for 14 days | Survival time ↑; tumor growth ↓; TNF-α, IL-6, IL-12, TGF-β, VEGF ↓; Bax, cleaved caspases-3 and -9 ↑; Bcl-2, MMP-2 and -9, NF-κB and IκBα ↓ | Chen et al. (2017b) | |
| 122 | In vitro | Radio-sensitivity of 4T1 breast carcinoma cell lines | 100 μM for 2 or 24 h | TIE, TRD ↓; radio-sensitivity of the 4T1 cells ↑ | Muhammed et al. (2019) | |
| 122 | In vitro | Lung cancer H1299 cells | 4.0, 6.0, and 8.0 μg/ml for 24 h | Cell apoptosis ↑; ERK/MAPK ↓; inhibition rates were 22.50, 30.13, and 58.87%, respectively | Zhou et al. (2019) | |
| 122 | In vivo | Hepatocarcinoma in nude mice | 25, 50, and 100 mg/kg, i.g., daily for 12 days | Tumor weight ↓; liver and spleen indexes ↓; IL-2 and IL-10 levels ↓; inhibition rates were 66.39, 63.11, and 47.33%, respectively; WBC, HGB, and PLT numbers ↑; TLR4, MyD88, and NF-κB expressions ↓ | Wu et al. (2020a) | |
| Anti-inflammatory activity | ||||||
| EEACL | In vivo | Croton oil-induced mouse ear edema | 0.03–1.0 mg/ear | Edema (IC50: 0.05) ↓; MPO activity (IC50: 0.22) ↓ | Cabrini et al. (2011) | |
| PFSCW | In vivo | Adult female Swiss mice | 100 and 300 mg/kg | Paw edema ↓ | Leivas et al. (2016a) | |
| 122 | In vivo | Pancreatic β-cell line Min6 cells | 10, 15, and 20 μmol/L | TNF-α, IL-6 and MCP-1 level ↓; cleaved-caspase-3, -8 and -9, TLR4, MyD88, and NF-KB expressions ↓; Bcl-2/Bax ratio ↑ | Xie et al. (2016) | |
| Hepatoprotective activity | ||||||
| FPEAC | In vivo | Leptin receptor- deficient (db/db) mice | 10, 20, and 30 g/kg, i.g., daily, for 8 weeks | TG, TC, LDL-C, and NEFA level ↓; AST and ALT activities ↓; HDL-C level ↑; p-AMPK protein expression ↑; SREBP-1c, FAS and SCD1 mRNA and protein expression levels ↓; mircoRNA-34a and mircoRNA-33 expression levels ↓ | Pang et al. (2017) | |
| FJAC | In vivo | STZ-induced diabetic mice | 5, 10, and 20 g/kg, i.g., daily for 14 days | MAD and cAMP levels ↓; SDH, MDA, and SOD activities ↑ | Yang et al. (2018) | |
| EACR | In vivo | CCl4-induced acute liver injury in mice | 0.3, 0.6, and 1.2 g/kg, i.g., dialy, for 7 days | f AST, ALT, IL-1, IL-6, MDA levels ↓; SOD, GSH, GSH-Px activities ↑; TNF-α, NF-κB, caspase-3 protein expression levels ↑ | Huang et al. (2019) | |
| EACR | In vivo | Liver fibrosis (HF) rats induced by CCl4 | 0.25, 0.5, and 1.0 g/kg, i.g., dialy, for 28 days | Albumin/globulin (A/G) ratio ↑; TBIL and TC levels ↓; NF-κB and Bax expression levels ↓; Bcl-2 expression level ↑ | Liang et al. (2020b) | |
| EACR | In vivo | CCl4-induced acute liver injury in rats | 0.25, 0.5, and 1.0 g/kg, i.g., dialy, for 8 weeks | AST, ALT, AKP, Hyp, HA, LN, Col III, Col IV, MDA levels ↓; SOD and GSH-Px activities ↑; COL-1a1, α-SMA, TIMP2, TGF-β1, Smad-2 and Smad-4 mRNA expression levels ↓; α-SMA, TIMP2, TGF-β1, Smad-2, Smad-3 and Smad-4, Bax and cleaved caspase-3 proteins expression levels ↓; Smad-7 mRNA expression and Smad-7 and Bcl-2 protein expression ↑ | Huang et al. (2020) | |
| Cardioprotective activity | ||||||
| AEAC | In vivo | Rats with ventricular remodelling induced by isoprenaline | 50, 100, and 200 mg/kg, i.g., daily, for 14 days | TGF-β, Ang II, iNOS, ECE, ET-1 levels and expressions ↓; VR index, CVF ↓; tNOS, eNOS protein expression levels ↑ | Liang et al. (2020a) | |
| Antihypertensive activity | ||||||
| AELAC | In vivo | Normotensive rats | 12.5–50.0 mg/kg, i.v. | MAP ↓; Ca2+-free medium ↓ | Soncini et al. (2011) | |
| EEACR | In vivo | Normal rats | 150, 300, and 600 mg/kg, i.g. | Blood pressure ↓ | Tang et al. (2017) | |
| FACF | In vivo | Normal rats and rats with hypertension induced by L-NAME | 300, 600, and 1,200 mg/kg, i.g., daily, for 5 weeks | SBP, DBP, MBP, blood pressure ↓ | Huang et al. (2017) | |
| Neuroprotective activity | ||||||
| 122 | In vivo | APP/PS1 transgenic AD mice | 12.5, 25, and 50 mg/kg, i.g., once a day for 21 days | Spatial learning and memory deficit, fear memory deficit apoptosis and loss of neuron ↑; Bcl-2/Bax ratio ↑ | Wei et al. (2018) | |
| 122 | In vitro | PC-12 cells | 5, 10, and 20 μmol/L | Bcl-2 mRNA and protein expressions ↑; Bax mRNA and protein expressions ↓; caspase-3 and caspase-9 expressions ↑; Bcl-2/Bax ratio ↑ | Wei et al. (2018) | |
| 122 | In vitro | SH-SY5Y cells induced by Aβ1-42 | 5, 10, and 20 μmol/L | cell viability loss and apoptosis ↓; Bax, caspase-3, caspase-8 and caspase-9 protein expression levels ↓; Bcl-2 protein expression ↑ | Lu et al. (2020) | |
| Reducing UVB-induced skin damage | ||||||
| EFAC | In vitro | Human HaCaT keratinocytes induced by UVB | 50, 100, and 250 µg/ml | Apoptotic cells number ↓; caspase 3 expression ↓; CPD ↓ | Ronpirin et al. (2016) | |
| AFAC | ||||||
NM, not mentioned; ACL, A. carambola leaves; MEACL, methanol extract of A. carambola leaves; EEBAC, ethanol extracts of bark from A. carambola; REAC, root extracts of A. carambola; FPAC, fruit powder of A. carambola; JEAC, juice extracts of A. carambola; TFACL, total flavones from A. carambola leaf; EEACB, ethanol extracts of A. carambola bark; MEACL, methanolic extract of A. carambola leaves; CEPAC, crude extract from peel of A. carambola; PEFAC, a homogenous polysaccharide extracted from the fruit of A. carambola; ACE, A. carambola extracts; EEACL, ethanol extract of A. carambola leaves; FPEAC, free phenolic extract from A. carambola; FJAC, fruit juice of A. carambola; EACR, extract of A. carambola root; AEAC, aqueous extract of A. carambola; AELAC, aqueous extract of leaves of A. carambola; EEACR, ethanol extracts of A. carambola root; FACF, flavonoids in A. carambola fruit; EFAC, ethanol fractions of A. carambola; AFAC, aqueous fractions of A. carambola; 40, Norathyriol; 47, Carambolaflavone A; 48, Carambolaflavone B; 105, (+)-lyoniresinol 3α-O-β-D-glucopyranoside; 106, (−)-lyoniresinol 3α-O-β-D-glucopyranoside; 121, 2-methoxy-6-nonyl-cyclohexa-2,5-diene-1,4-dione; 122, 2-dodecyl-6-methoxycyclohexa-2,5-diene-1,4-dione.
Anti-Hyperglycemic Activity
Diabetes mellitus is characterized by a chronic hyperglycemic condition possibly induced by insulin deficiency, damage to insulin signaling, or non-autoimmune etiology or caused by a remarkably diminished insulin sensitivity (Teng et al., 2018). This disorder poses a significant public health care problem, with its prevalence continuing to rise globally (Chen et al., 2019). Type 2 diabetes mellitus, in particular, is primarily distinguished by insulin resistance and β cell dysfunction, which cause insulin secretion reduction (Uuh-Narváez et al., 2021). Diabetes mellitus patients can die if they develop diabetic kidney disease.
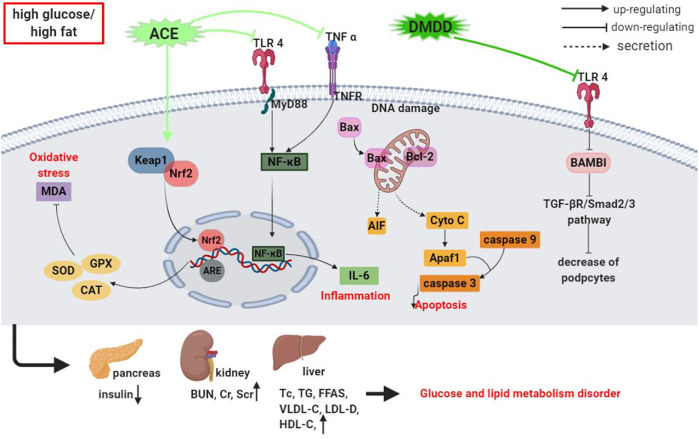
In the past 5 years, several studies have extensively explored the antidiabetic potential of A. carambola using various experimental models. The findings have revealed that A. carambola and its glycosides exhibit outstanding antidiabetic features, and insights into the underlying mechanisms have also been provided, although they are not yet fully understood. The underlying mechanism of the anti-hyperglycemic activity of crude extracts or bioactive substances from A. carambola is presented in Table 2 and Figure 4.
FIGURE 4.
Schematic representation of the underlying mechanism of anti-hyperglycemic activity of crude extracts or isolated compounds from A. carambola.
Crude Extract
Evaluations of the hypoglycemic activity of A. carambola crude extracts on rats and mice have been conducted with encouraging results. In one such assessment, mice with STZ-induced diabetes given root extracts of A. carambola (REAC at daily doses of 150, 300, 600, and 1,200 mg/kg for 21 days) orally had significantly decreased blood glucose, TC, TGs, and FFAs levels and elevated insulin content in their serum. Mechanically, REAC markedly downregulated pro-apoptosis caspase-3/8/9 and Bax protein expressions and upregulated anti-apoptotic Bcl-2 protein expression. Additionally, REAC prevented pancreatic β cell apoptosis in these mice (Xu et al., 2014). The findings of that investigation suggest that REAC possesses remarkable hyperglycemic abilities that could improve metabolic functions and suppress the apoptosis of pancreatic β cells.
Research into the anti-hyperglycemia activities of REAC on regulating the TLR4/NF-κB signaling pathway in STZ-induced diabetic mice showed that REAC (at daily doses of 300, 600, and 1,200 mg/kg, i.g. for 14 days) decreased the serum contents of fasting blood glucose (FBG), TNF-α, and IL-6 significantly and downregulated TLR4 and NF-κB protein and mRNA expressions in pancreatic tissue (Xu et al., 2015). In their assessment of the protective features of REAC on renal function injury in STZ-stimulated diabetic mice, Xu et al. (2017) found that REAC treatment reduced FBG, blood urea nitrogen (BUN), and creatinine levels in serum significantly, strengthened SOD, GSH-Px, and CAT activities considerably, and lessened MDA levels and Cyto-C, AIF, and caspase-3 protein expressions in the kidney tissues of the mice.
After orally treating fluoride-induced hyperglycemia, hypercholesterolemia, and oxidative stress in rats with fruit powder from A. carambola (FPAC, at daily doses of 2.5, 5.0, and 10 g for 30 days), the plasma glucose level and G-6-pase, SGOT, SGPT, ACP, and ALP activities reduced notably, while the activities of hepatic glycogen, hexokinase, and FPAC increased dose-dependently. Increments in the atherogenic index of plasma (AIP), total lipids (TL), TC, TG, LDL-C, and VLDL-C in plasma, TL, TC, and TG in the liver and the decrease in HDL-C were also reversed. Additionally, FPAC prompted an increase in CAT, SOD, GSH-Px, GSH, and total ascorbic acid (TAA) activities significantly, and a decline in MDA content in the hepatic and renal tissues of rats (Vasant and Narasimhacharya, 2014).
STZ-induced diabetic mice receiving juice extracts from A. carambola (JEAC) intragastrically (at daily doses of 25, 50, and 100 mg/kg for 21 days) have displayed markedly reduced FBG, FFA, MDA, TC, TG, serum creatinine (Scr), and BUN levels and significantly increased insulin, sorbitol dehydrogenase (SDH), cAMP, and SOD activities. In the same study, diabetes-instigated changes in kidney tissues, including thickened and tubular basement membranes and glomerular hypertrophy, greatly improved, and the expressions of related mRNAs and proteins, such as CTGF and TGF-β1, in kidney tissues markedly decreased after JEAC treatment (Pham et al., 2017). JEAC (at daily doses of 5, 10, and 20 g/kg, i.g. for 14 days) has also been proven to considerably decrease FBG levels, blood glucose, area under the curve, LDH, glucagon (GC), and pyruvate in serum and increase fasting insulin (FINS) levels in STZ-induced diabetic mice, indicating that this extract could lessen hyperglycemia and hyperlipidemia, suppressing DN progression and development; it is a potential candidate agent for treating or preventing DN (Yang et al., 2019a).
Liu et al. (2013) reported that the total flavones from A. carambola leaf (TFACL at daily doses of 0.2, 0.4, and 0.8 g/kg, i.g. for 7 days) significantly lowered FBG levels and enhanced glucose tolerance in mice and rats with diabetes mellitus. The STZ-induced diabetic mice also received ethanol extracts of A. carambola bark (EEACB) orally (at daily doses of 50 and 100 mg/kg for three successive days), which drastically decreased their level of blood glucose 150 min after orally receiving a single dose of glucose solution (1.0 g/kg); 100 mg/kg EEACB triggered a higher blood glucose level decline than 50 mg/kg EEACB (Islam et al., 2020). These results suggest that A. carambola is a potential hypoglycemic drug for the prevention and treatment of diabetes.
6.2.2 Isolated Compounds
As previously mentioned, some compounds, including 47, 48, 100, 101, 120, and 121, isolated and identified from A. carambola have been tested for their potential anti-hyperglycemic activity in vivo. Normal hyperglycemic rats receiving an oral treatment of flavonoid carambolaflavone A (at doses of 20 and 50 mg/kg) (47) and carambolaflavone B (at doses of 20 and 50 mg/kg) (48) extracted from A. carambola leaves demonstrated decreased glycemia levels, suggesting a potential hypoglycemic effect. Glycogen levels in the muscle and liver also increased sharply (Cazarolli et al., 2012). Another study by the same authors also found 50 mg/kg of carambolaflavone B (48) to notably decrease blood glucose levels in diabetic rats and provoke glucose-triggered insulin secretion after oral treatment of the hyperglycemic rats. Additionally, a noteworthy stimulatory function of compound 48 (at concentrations of 50 and 100 μM) on 14C-glucose uptake was observed, but treatment with inhibitors, including wortmannin, RO318220, and PD98059, reversed this activity. Interestingly, 100 μM of compound 48 and 10 nM of insulin had no synergistic effect on glucose uptake (Cazarolli et al., 2009).
Chiral lignan glucosides, (±)-lyoniresinol 3α-O-β-D-glucopyranoside (LGP1 (105) and LGP2 (106)), isolated from A. carambola root have been evaluated for health-promoting properties in LGP1 and LGP2-mediated hypoglycemia against renal injury in STZ-created diabetic mice. Diabetic mice that received LGP1 and LGP2 (at daily doses of 20, 40, and 80 mg/kg for 14 days) yielded contrasting outcomes. While LGP1 markedly alleviated the histopathological changes in the kidney and lowered FBG, FINS, and insulin sensitivity index (ISI) levels and caspase-3/8/9, Bax, and NF-κB protein expressions, LGP2 had no impact (Wen et al., 2013), suggesting that LGP1 could attenuate and treat the progression of DN through regulating several molecular targets.
Benzoquinone (121) isolated from the roots of A. carambola has also been analyzed, and, at doses of 30, 60, and 120 mg/kg, i.g. once a day for 21 days. It effectively decreased serum FBG, TC, TG, FFA, MCP-1, TNF-α, GHb, FINS, MDA, and IL-6 levels and increased SOD and GSH activities in STZ-induced diabetic rats. Mechanistically, the extract strikingly downregulated TNF-α, IL-6, MyD88, p-NF-κB, and TLR4 expressions in pancreatic tissues (Qin et al., 2019; Qin et al., 2020). These findings suggest that benzoquinone (121) exerts a protective effect against STZ-induced hyperglycemia, and the underlying molecular mechanism of its impact could be associated with the suppression of the activation of the TLR4/NF-κB signaling pathway.
Zheng et al. (2013), Zheng et al. (2021) reported that DMDD (122) isolated from A. carambola dry roots (at daily doses of 12.5, 25, and 50 mg/kg, i.g. for 56 days) to significantly reduce FBG, creatinine clearance, proteinuria, Scr, and serum urea-N contents and glomerular mesangial matrix expansion. It also markedly improved renal AGE formation, decreased AGE receptor, NF-κB, TGF-β1, and Nε-(carboxymethyl)lysine expressions in diabetic mice, and effectively alleviated DN in type 2 diabetic KKAy mice. However, the underlying mechanism of how DMDD alleviates DN has yet to be demonstrated; therefore, the precise action mechanism of DMDD against DN must be explored further (Lu et al., 2019). STZ-established DN in TLR4 knockout (TLR4−/−, KO) and wild-type (WT) mice treated orally with DMDD (at doses of 12.5, 25, and 50 mg/kg for 28 days) lowered serum TC, TG, HDL-C, and LDL-C, blood glucose content and kidney function markers, including Scr and BUN, significantly and increased the quantity and density of podocytes spectacularly, contributing to DN symptom alleviation. The treatment also markedly inhibited IL-6 and TNF-α levels and blocked the TLR4/MyD88/NF-κB signaling pathways. These effects, though, were notably different in TLR4−/− mice. In vitro, 30 μM DMDD radically subdued TLR4, Smad2, and Smad3 expressions in high glucose (HG)-stimulated HK-2 cells and diminished BMP and activin membrane-bound inhibitor (BAMBI) expressions (Zhang et al., 2019). Additionally, the increase in Smad2/3 expression and decrease in BAMBI expression in HG cultured cells were markedly annulled in cells treated with TAK-242 (a TLR4 signaling inhibitor). More importantly, BAMBI gene silencing extraordinarily enhanced the epithelial-mesenchymal transition (EMT) process and its over-expression did the opposite in HK-2 cells under HG conditions. EMT was eased with the pre-administration of DMDD to HK-2 cells, pointing to DMDD’s protective effects on HG-induced EMT in HK-2 cells via suppressing the TLR4/BAMBI/Smad2/3 signaling pathways (Zhang et al., 2019). These findings insinuate that DMDD isolated from A. carambola is a potential therapeutic drug for DN treatment.
In Zhang et al. (2020) study of the antidiabetic activity of DMDD isolated from A. carambola roots in Wild type and TLR4 knockout mice with diabetic kidney disease, DMDD (at daily doses of 12.5, 25, and 50 mg/kg, i.g. for 28 days) caused notable reductions in urinary albumin, TC, TG, LDL-C, FBG, and CysC levels. The extract also alleviated pathological changes and renal fibrosis clearly but silencing the TLR4 gene improved the pathology. Mechanistically, TLR4, TGF-β1, Smad2, and Smad3 protein expressions in renal tissues decreased pointedly after DMDD treatment. TGF-β1 and Smad2/3 genes and protein expressions also unusually declined in TLR4−/− mice than Wild type mice. Furthermore, according to IHC results, there were strong in situ expressions of TLR4, TGF-β1, Smad2, and Smad3 in kidney tissues, which were distinctly weakened after DMDD-treatment. Nevertheless, TGF-β1, Smad2, and Smad3 levels did not increase in the TLR4−/− mice with significance when compared with healthy mice (Zhang et al., 2020). These findings strongly suggest that TLR4 is critical for DMDD’s protective activity against renal insufficiency in diabetic mice, and hypoglycemic and anti-fibrosis properties could be regulated by the TLR4/TGF signaling pathway.
In summary, A. carambola could attenuate oxidative injury and inflammatory response in rat models by enhancing antioxidant enzyme activities and decreasing inflammatory cytokine levels, which result in insulin secretion, as well as blood glucose and blood lipid metabolism regulation. Some original findings on active compounds and their related mechanisms also exist; however, these investigations lack clear indications of whether these activities stem from a single substance or synergistic effects of several substances of A. carambola; therefore, before they can be advanced as bases for clinical research and exploration of the medicinal values of A. carambola, further evaluations must be performed.
Anti-Hyperlipidemic Activity
Cardiovascular diseases (CVDs) are among the most common causes of death globally, with hyperlipidemia representing one of its main risk factors (Mahdavi et al., 2020; Jayachandran and Qu, 2021). In Herman-Lara et al. (2014) evaluation of the protective activity of micronized insoluble fiber from the bagasse of A. carambola as an ingredient of a functional food (FF) or as micronized insoluble fiber-rich fraction (IFRF) and its effects in vivo on lipid metabolism in a murine model, serum TG, TC, HDL-C, and LDL-C decreased after a murine model was treated with IFRF and FF, with corresponding inhibition rates of 14.2, 25.4, 55.06, and 12.18% by IFRF, and 30.18, 39.47, 35.11, and 43.18% by FF. IFRF treatment also produced higher hypolipidemic activity and greatly avoided the occurrence of non-alcoholic fatty liver. Overall, IFRF and FF exerted hypolipidemic qualities, indicating that the star fruit’s insoluble fiber is potentially a component of FFs in the treatment and prevention of CVDs (Herman-Lara et al., 2014).
In Saghir et al. (2016) investigation of the anti-hyperlipidemic activity of methanolic and aqueous extracts from various A. carambola parts (such as leaves, stems, and ripe and unripe fruits), they found that the methanolic extract of A. carambola leaves (MEACL) had the most potent hypolipidemic effect in poloxamer-407-induced hyperlipidemic rats and effectively decreased serum TC, TG, LDL-C, VLDL-C, and atherogenic index (AI) levels. MEACL (at daily doses of 250, 500, and 1,000 mg/kg, i.g. for 35 days) significantly reduced serum and liver lipids levels, including TC, TG, LDL-C, VLDL-C, and AI, elevated HDL-C, and dose-dependently alleviated histopathological changes in the liver of HFD-induced hyperlipidemic rats. MEACL also considerably enhanced GSH, GSH-Px, SOD, and CAT activities and lowered MDA levels. Furthermore, MEACL treatment inhibited HMG-CoA reductase and lipase (Aladaileh et al., 2019). These results demonstrate that MEACL has a hypolipidemic activity it could exert by ameliorating lipid peroxidation and improving antioxidant defenses in HFD-fed rats (Table 2).
Anti-Obesity Activity
Obesity-related diseases are increasingly becoming common public health and social issues. They can cause a multitude of metabolic disorders closely associated with increased risk of many chronic diseases (Zhu et al., 2015; Li et al., 2016). One study found that some phytochemicals suppressed adipogenesis and obesity. According to Rashid et al. (2016), the crude extract from the peel of A. carambola (CEPAC) (at concentrations of 10, 100, 500, and 1,000 mg/ml) dose-dependently inhibited TG accumulation in their research. Additionally, 1,000 mg/ml of CEPAC substantially blocked most of the adipocyte differentiation in 3T3-L1 preadipocytes with no toxicity. The extract also notably reduced the mRNA expressions of two master adipogenic transcription factors, PPAR-γ and C/EBPα, and increased the PPAR-α receptor at the molecular level. In further phytochemical analyses, the authors recognized (-)-epicatechin as a natural bioactive molecule responsible for the features presented above (Rashid et al., 2016). These findings indicate that CEPAC is potentially promising in treating obesity and obesity-related disorders.
Per Li et al. (2016) inquiry into the benefits of DMDD against high-fat diet (HFD)-evoked obesity and insulin resistance in mice, DMDD (at daily doses of 12.5, 25, and 50 mg/kg, i.g. for 28 days) markedly decreased body and adipose tissue weights and reduced insulin, blood glucose, TC, TG, FFA, IL-6, and TNF-α levels in serum. DMDD also significantly downregulated TLR4 and MyD88 protein levels in epididymal adipose tissues, considerably enhanced insulin secretion, lowered the areas under the curve, notably heightened SOD and GSH-Px activities, and decreased MDA content in mice liver tissues (Li et al., 2016). Based on these results, DMDD possesses potential benefits that could be used to treat HFD-induced obesity and insulin resistance, potentially improving the lipid metabolism process and the suppression of TLR4 protein expression in adipose tissues (Table 2).
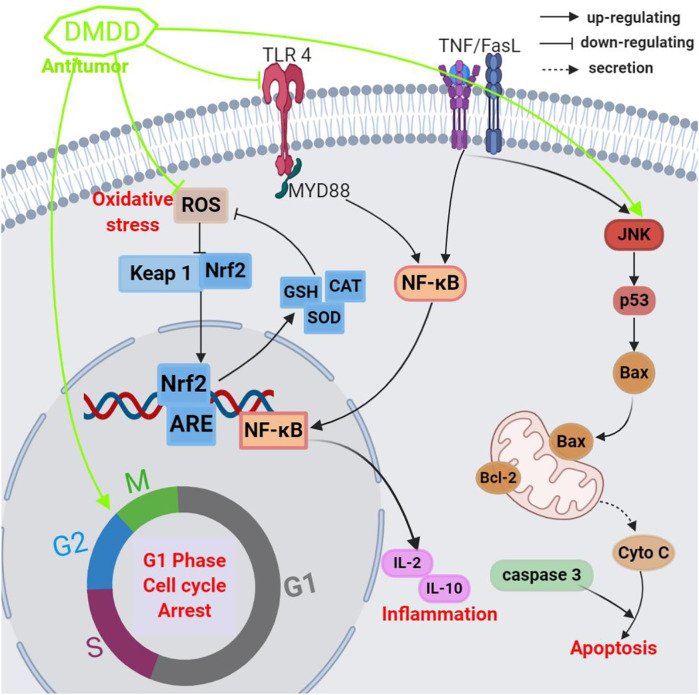
Antitumor Activity
Several studies have demonstrated the significant inhibitory activity of A. carambola against a variety of tumor cells in vivo and in vitro and the possible mechanism of the antitumor activity of crude extracts or isolated compounds from A. carambola (Table 2 and Figure 5). DENA-induced and CCl4-promoted liver cancer mice given A. carambola extracts (ACE, at a dose of 25 mg/kg for 5 days) orally resulted in ACE considerably lowering tumor incidence, tumor yield, and tumor burden. The treatment also significantly reduced lipid peroxidation (LPO) levels and elevated GSH, SOD, CAT activities (Singh et al., 2014). These findings hinted at ACE’s ability to exert a protective effect against liver cancer in mice and could be used as a good natural chemo-preventive product against cancer to further screen active components. Supposedly, MEACL (25 and 50 mg/kg, i.g. once a day for 5 days) significantly reduces body weight and cell viability, prolongs survival time, and improves altered hematological parameters (HGB, WBC, RBC) in Ehrlich ascites carcinoma (EAC) cell-bearing mice. MELA could induce cancer cell apoptosis through upregulating p53 and Bax protein expressions at the molecular level (Siddika et al., 2020).
FIGURE 5.
Schematic representation of the underlying mechanism of anti-tumor activity of crude extracts or isolated compounds from A. carambola.
Gao et al. (2015) also showed that DMDD significantly suppressed breast cancer MCF-7 and BT20 cell growth, with the IC50 values ranging from 3.13 to 5.57 μM; DMDD exerted anticancer traits by inducing apoptosis and blocking the cell cycle at the G1 phase, increasing the generation of intracellular ROS, which activated the extrinsic receptor and intrinsic mitochondrial pathways and inhibited NF-κB activation. One study using 4T1 breast cancer cell-bearing mice for in vivo research revealed that DMDD radically inhibited primary breast tumor growth and suppressed breast tumor metastasis in the lung and liver, as well as decreased inflammatory cytokine production, induced cell apoptosis, and prevented the activation of the TNF-α/NF-κB/MMPs pathways, prolonging the survival time of tumor-bearing mice (Chen et al., 2017b). These outcomes imply that DMDD has potent antitumor features that could help treat metastatic breast cancer.
Muhammed et al. (2019) explored the radio-sensitivity of 4T1 tumor cells treated with DMDD extracted from A. carambola roots, by analyzing the TIE and TRD of the in vivo 2 and 24 h treatment groups compared with the untreated control group. The threshold ionization energy (TIE) and threshold radiation dose (TRD) was determined utilizing a novel method that employs a laser trapping technique for single and multiple cell ionizations. Per the findings, treatment periods increased, and TIE and TRD notably decreased, pointing to DMDD’s powerful enhancement effect on 4T1 cell radio-sensitivity. However, TRD diminished with mass regardless of the intervention. TRD analyses of single vs multiple cells ionizations within each group continuously demonstrated the same behavior even after treatment. The underlying elements for these observed relations could be explained in terms of radiation, hyperthermia, and chemo effects (Muhammed et al., 2019). DMDD treatment (at 4.0, 6.0, and 8.0 μg/ml for 24 h) has been shown to dramatically and dose-dependently inhibit the proliferation, migration, and invasion of H1299 cells, with inhibition rates of 22.50, 30.13, and 58.87%, respectively. The same research determined that DMDD also induced the apoptosis of H1299 cells, with the mechanism possibly associated with the suppression of the ERK/MAPK pathway (Zhou et al., 2019).
Wu et al. (2020a) assessed the antitumor effect of DMDD on hepatocarcinoma in nude mice and its mechanism, the tumor weight in mice decreased significantly, with tumor inhibition rates for the three doses amounting to 66.39, 63.11, and 47.33%, respectively, after daily oral treatment with DMDD at 25, 50, and 100 mg/kg for 12 days. The tumor and organ indexes, including liver and spleen, and IL-2 and IL-10 serum levels also notably decreased, and the amounts of WBC, HGB, and PLT dramatically increased. Additionally, the expression of TLR4, MyD88, and NF-κB reduced markedly. These results suggest that DMDD has promising anticancer properties, with its mechanism of action is possibly linked to the repression of the TLR4/MyD88/NF-κB signaling pathways (Table 2).
Anti-Inflammatory Activity
The evaluation of the anti-inflammatory effects of the ethanol extract of A. carambola leaves (EEACL), its ethyl acetate, butanol, and hexane fractions, and two flavonoids against skin inflammation in mice with croton oil-stimulated ear edema revealed that topically used EEACL (at concentrations of 0.03–1.0 mg/ear) shrunk the edema dose-dependently, with an ID50 value of 0.05 at concentrations of 0.02–0.13 mg/ear and maximum inhibition of 73% at a concentration of 0.6 mg/ear. EEACL also suppressed myeloperoxidase (MPO) activity, with an ID50 value of 0.22 and a maximum inhibition of 61% at a concentration of 0.6 mg/ear. Furthermore, all the fractions examined markedly curbed edema formation and lowered MPO activity. Additional evaluations found that the ethyl acetate fraction of EEACL was the most effective inhibitor of MPO activity and edema formation, with inhibition rates of 54 and 75%, respectively (Cabrini et al., 2011). Unfortunately, EEACL bioactive substances against skin inflammation have not been tested extensively so far; therefore, they require further scrutiny to be optimized and developed into effective remedies for preventing and treating skin disorders.
GC-MS analyses of a homogenous polysaccharide extracted from the fruit of A. carambola (PFSCW, Mw 40 kDa) established that it consisted mainly of arabinose, galactose, and galacturonic acid in a molar ratio of 12.3:1.7:86.0. Methylation and NMR spectroscopy examination identified a substituted galacturonan composed of (1→4)-linked-α-D-Galp A units with a branch at O-2 by (1→5)-linked-α-L-Araf and terminal-α-L-Araf and α-D-Galp A units as components of PFSCW. PFSCW treatment (at doses of 100 and 300 mg/kg) drastically reduced intraplantar formalin-injected paw edema, with an inhibition rate of 53% at a dose of 300 mg/kg, suggesting that the extract has moderate anti-inflammatory properties (Leivas et al., 2016a). Xie et al. (2016) demonstrated that DMDD (at concentrations of 10, 15, and 20 μM) significantly inhibited palmitic acid (PA)-stimulated inflammation in pancreatic Min6 cells by strongly obstructing the TLR4/MyD88/NF-KB pathways, which lowered the generation of inflammatory cytokines (TNF-α, IL-6, and MCP-1), downregulating cleaved-caspase-3/8/9 protein expression, elevating the Bcl-2/Bax ratio, and decreasing TLR4, MyD88, and NF-KB protein expressions. These outcomes hint at DMDD's ability to reverse PA-stimulated Min6 cell dysfunction via alleviating cell apoptosis and inflammatory response, with the underlying mechanism of action almost certainly associated with the suppression of the TLR4/MyD88/NF-κB signaling pathways (Table 2).
Hepatoprotective Activity
An evaluation of the hepatic steatosis alleviating activity of a free phenolic extract of A. carambola (FPEAC) in leptin receptor-deficient (db/db) mice to clarify the underlying mechanisms of action for modulating hepatic lipogenesis found that FPEAC (at daily doses of 10, 20, and 30 g/kg, i.g. for 8 weeks) considerably lowered LDL-C, TG, TC, non-esterified fatty acid (NEFA) levels, and AST and ALT activities, elevated serum HDL-C level, and reduced TG content in the liver. Mechanically, FPEAC drastically downregulated sterol regulatory element-binding protein-1c (SREBP-1c), fatty acid synthase (FAS), and stearoyl-CoA desaturase 1 (SCD1) expressions in hepatic tissues and markedly upregulated p-AMPK α levels. More importantly, FPEAC significantly downregulated microRNA-34a and microRNA-33 expressions, which regulate the AMPK/SREBP-1c/FAS signaling pathway (Pang et al., 2017). These findings indicate that FPEAC has a compelling hepatic steatosis mitigating effect, partially through suppressing the signal transmission of hepatic lipogenesis. According to Yang et al. (2018), the fruit juice of A. carambola (FJAC, at daily doses of 5, 10, and 20 g/kg, i.g. for 14 days) showed potential hepatoprotective properties; it reduced MAD and cAMP levels and increased SDH, MDA, and SOD activities in the liver of mice with STZ-induced diabetes.
In one study, mice with acute liver injury given the extract of A. carambola roots (EACR, at daily doses of 0.3, 0.6, and 1.2 g/kg for 7 days) had significantly lower serum CCl4, AST, ALT, IL-1, and IL-6 levels and liver MDA levels but considerably increased SOD, GSH, and GSH-Px activities. At the molecular level, TNF-α, NF-κB, and caspase-3 protein expressions were significantly downregulated in the liver. HE staining showed that the liver injury was eased (Huang et al., 2019). In another investigation, EACR (at daily doses of 0.25, 0.5, and 1.0 g/kg, i.g. for 28 days) substantially increased the albumin/globulin (A/G) ratio, reduced total bilirubin (TBIL) and TC levels in the liver microstructure of rats with CCl4-induced liver fibrosis (HF), markedly lessened NF-κB and Bax protein expressions, and considerably augmented Bcl-2 protein expression (Liang et al., 2020b). Rats with CCl4-instigated chronic liver injury treated intragastrically with EACR (at doses of 0.25, 0.5, and 1.0 g/kg for 56 days) displayed drastically decreased serum AST, ALT, AKP, and Hyp content, serum hepatic fibrosis biomarkers (HA, LN, Col III, and Col IV), and liver tissue MDA content and increased SOD and GSH-Px capacities. EACR also considerably reversed the elevation in COL-1a1, α-SMA, TIMP2, TGF-β1, and Smad-2 and -4 mRNA expressions and inhibited α-SMA, TIMP2, TGF-β1, Smad-2, -3, -4, Bax, and caspase-3 levels in liver tissues. Additionally, the extract significantly increased Smad-7 mRNA expression and Smad-7 and Bcl-2 protein levels in the liver (Huang et al., 2020). These findings point to EACR being a promising remedy for liver fibrosis. However, further studies must be conducted urgently to identify the bioactive components and possible mechanism of EACR’s anti-fibrotic effects (Table 2).
Cardioprotective Activity
Ventricular remodeling (VR) results in changes in endothelial vasoactive substances, cardiomyocyte hypertrophy, myocardial fibrosis, and endothelial dysfunction. Per Liang et al. (2020a) investigation of the protective property of the aqueous extract of A. carambola (AEAC) on isoprenaline-stimulated endothelial function in rats with VR, AEAC (at daily doses of 50, 100, and 200 mg/kg, i.g. for 14 days) glaringly lowered serum levels of iNOS, TGF-β, Ang II, ECE, and ET-1 and their protein expressions and decreased the VR index and CVF but markedly elevated serum tNOS and eNOS levels and their protein expressions. Pathological evaluations demonstrated that AEAC radically alleviated inflammatory infiltration, apoptosis, fibrosis, and necrosis in rat myocardial tissues, suggesting that AEAC potentially mitigates the VR of rats and is, therefore, possibly associated with safeguarding the balance of vasoactive components and vascular endothelium function (Liang et al., 2020a). Another study showed that the high-glucose-high-fat diet combined with STZ induced the diabetes mellitus mice model administrated with DMDD (at doses of 12.5, 25, and 50, 100 mg/kg/day, i.g., for 21 days) markedly alleviated the myocardial tissues damage, inhibited the myocardial cell apoptosis, reduced the levels of FBG, LVEDP, ROS, MDA, Beclin-1, LC3II/I, and Atg5 as well as increased the SOD, p-PI3K/PI3K, p-Akt/Akt, and p-mTOR/mTOR, indicating that DMDD exerts cardioprotective activity via regulating the ROS-mediated PI3K/Akt/mTOR autophagy pathways (Table 2; Ma et al., 2021).
Anti-Hypertensive Activity
In Soncini et al. (2011) study of the anti-hypertensive effect of the aqueous extract of leaves of A. carambola (AELAC) in an isolated rat aorta and its possible mechanism, AELAC treatment (at doses of 12.5–50.0 mg/kg, i.v.) resulted in a remarkable dose-dependent decrease in mean arterial pressure (MAP) in normotensive rats. In vitro, AELAC reduced E max response to phenylephrine but triggered no sensitivity change. AEAC also suppressed CaCl2-stimulated aorta contractions and led to the rightward shift of the response curves of a depolarized Ca2+-free medium, suggesting that the extract repressed extracellular Ca2+ influx and caused vasoconstriction. These outcomes strongly support the traditional use of A. carambola leaves in hypertension.
Orally administered ethanol extract of A. carambola roots (EEACR, at 150, 300, and 600 mg/kg) has been shown to lower blood pressure in healthy rats at 300 and 600 mg/kg but have no noticeable effect on the heart rate (Tang et al., 2017). When Huang et al. (2017) explored the effect of flavonoids from A. carambola fruit (FACF) on healthy rats and rats with NG-nitro-L-arginine-methyl ester (L-NAME)-induced high blood pressure, they established that FACF (at daily doses of 300, 600, and 1,200 mg/kg, i.g. for 5 weeks) significantly lessened systolic blood pressure, diastolic blood pressure, and mean blood pressure in healthy rats. Moreover, the blood pressure of rats with hypertension was also drastically lowered by 600 and 1,200 mg/kg FAC treatments, suggesting that FACF contains an active ingredient that decreases blood pressure (Table 2; Huang et al., 2017).
Neuroprotective Activity
Alzheimer's disease (AD), characterized by the progressive deterioration of learning, memory, and cognition, is the most common, irreversible, and progressive neurodegenerative disease (Robins Wahlin and Byrne, 2011; Levenson et al., 2014). DMDD’s neuroprotective effect against memory deficits and neuron apoptosis in APP/PS1 transgenic AD mice has been reported (Wei et al., 2018). That research showed that mice receiving DMDD (at daily doses of 12.5, 25, and 50 mg/kg for 21 days) orally displayed significantly improved memory and spatial learning and inhibited neuron loss and apoptosis in APP/PS1 hippocampal tissues. In vitro, DMDD (at concentrations of 5, 10, and 20 μmol/L) radically suppressed Aβ1-42-stimulated apoptosis by upregulating Bcl-2 expression and downregulating Bax expression, increasing the mitochondria membrane potential (MMP) and activating PC-12 cell caspase-3 and caspase-9. Mice pretreated with DMDD also had drastically increased PC-12 cell Bcl-2/Bax ratio in vitro and in vivo (Wei et al., 2018). These findings indicated that DMDD has beneficial properties against the deficit of learning and memory in APP/PS1 transgenic AD mice through improving neuron apoptosis and suppressing Bax/Bcl-2-mediated loss of MMP (Table 2).
Recently, Lu et al. (2020) explored the neuroprotective activity of DMDD against Aβ1-42-evoked SH-SY5Y cell apoptosis and the underlying mechanism of DMDD’s protective function. Treatment with DMDD (at concentrations of 5, 10, and 20 μmol/L) significantly increased cell viability and inhibited Aβ1-42-induced SH-SY5Y cell apoptosis. Mechanistically, DMDD markedly downregulated Bax, caspase-3, caspase-8, and caspase-9 expressions, upregulated Bcl-2 levels, suppressed the release of cytochrome c, and the loss of MMP, and elevated the Bcl-2/Bax ratio (Lu et al., 2020). Overall, DMDD displayed excellent neuroprotective properties and is a promising ingredient for developing a neuroprotective drug for Alzheimer’s disease.
Reducing Ultraviolet B-Induced Skin Damage
Ultraviolet B (UVB) is a major causation factor of cell injury and skin cancer. It causes DNA damage, promotes apoptosis, and produces ROS. In Ronpirin et al. (2016) investigation of the protective effect of A. carambola on HaCaT keratinocytes caused by UVB, the ethanol and aqueous fractions of A. carambola (EFAC and AFAC, at a concentration of 250 µg/ml) significantly decreased cell apoptosis. Both fractions also ominously lowered caspase 3 protein expression. Additionally, EFAC (at concentrations of 100 and 250 µg/ml) and AFAC (at concentrations of 50, 100, and 250 µg/ml) drastically reduced the percentage of cyclobutane pyrimidine dimers (CPD), resulting in DNA repair (Ronpirin et al., 2016). These reports suggest that A. carambola extracts could be developed cosmetically to protect against UVB-induced skin damage (Table 2).
Antimicrobial Activity
The extract of the stem bark, leaves, and fruits of A. carambola supposedly display antibacterial and antifungal activity. Previous investigations demonstrated that the extracts of A. carambola fruits could suppress the growth of Staphylococcus aureus and Klebsiella spp., with the MBC of 15.62 and 125 mg/ml, respectively (Chang et al., 2000). Moreover, two compounds p-anisaldehyde and β-sitosterol (400 μg/disk) isolated from the bark of A. carambola strongly inhibited the growth of Escherichia coli with a zone of inhibition of 15 mm and had moderate inhibitory activity against fungi (Mia et al., 2007). Afterward, Wakte and Patil (2011) screened the antimicrobial activity of fruit extracts of star fruit at various stages against two Gram-positive bacteria (S. aureus ATCC 6538P and B. cereus ATCC 11778) and three Gram-negative bacteria (E. coli ATCC 25922, P. aeruginosa ATCC 19429, and S. typhimurium ATCC 23564), the results found that all the extracts show different degrees of activity against Gram-positive and Gram-negative bacteria, and the methanol and acetone extracts were considerably more effective than other solvent extracts in inhibiting the Gram-positive micro-organisms better than Gram negative microorganisms. In addition, the ethanolic extracts of A. carambola leaf at doses of 250 and 500 µg/disc were found to moderately suppress the growth of Shigella dysenteriae, Streptococcus pyogenes, Staphylococcus saprophyticus, Streptococcus agalactiae, and Pseudomonas spp. (Hossain et al., 2017).
Recently, Silva et al. (2021) found that the extracts of stem bark, leaves, and fruits from A. carambola perceptibly suppressed the growth of multi-resistant pathogenic bacteria and fungi with a minimal inhibitory concentration (MIC) of 100 μg/ml. Crude extracts of the leaf exhibited a broad spectrum of action against Gram-positive and Gram-negative bacteria, such as S. aureus 10 MRSA, S. aureus ATCC 29213 MRSA, S. aureus 12 MRSA, S. aureus 6 MRSA, K. pneumoniae 8 ESBL, E. faecalis ATCC 29212, and A. baumannii 2 MBL (Silva et al., 2021). Overall, the findings of these studies provide a research direction that points to A. carambola’s prospective therapeutic efficacy against bacterial and fungal infections.
Toxicity Assessment
Literature material on the assessment of the toxicity and safety of A. carambola is limited, although this plant is commonly utilized in TCM to treat numerous ailments. In recent years, several studies have found that the excessive consumption of A. carambola results in toxic effects. Aranguren et al. (2017) literature review revealed that 123 patients from eight nations developed acute renal injury after consuming this fruit. Forty-seven of the cases were registered in Brazil, the highest reported incidence, followed by Taiwan (36), Bangladesh (20), China and France (8 each), Sri Lanka (2), and Thailand and Colombia (1 each); 28 of the patients died. In the most recent investigation, Yasawardene et al. (2021) analyses of 10 case series and 28 case reports in humans (total number of individuals = 136) and eight animal studies for A. carambola nephrotoxicity and neurotoxicity, 94 (69.1%) patients had prior renal impairment, with renal histology revealing acute oxalate nephropathy with tubulointerstitial nephritis or tubular necrosis. Also, neurotoxicity performances ranged from hiccups to status epilepticus. The excessive uptake of A. carambola could lead to acute kidney injury, particularly in an empty stomach, or dehydration state, and the use of A. carambola as therapy for an elderly patient is not recommended on an empty stomach (Wijayaratne et al., 2018). In addition, A. carambola has threatened people's health despite the relatively low frequency of star fruit intake. Further clinical studies on the toxic ingredients, metabolites, and intake dose of star fruit toxicity must be carried out to provide clear indications of intake and prevent toxicity and mortality.
The results of animal experiments showed that the high dose of the different A. carambola extracts showed different degrees of toxicity or adverse reactions. Chen et al. (2001) found that an overdose intake of the fruit could result in nephrotoxicity and neurotoxicity in some individuals (Chen et al., 2001). Supposedly, the fruit of A. carambola could cause fatality in patients suffering from uremia, with oxalate identified as being a significant element in the toxicity of the fruit (Dembitsky et al., 2011). Furthermore, an acute toxicity study conducted in female albino rats revealed that oral administration of A. carambola juice extract (ACJE) at different doses (250, 500, 1,000, 2000, 4,000, and 5,000 mg/kg) was safe and did not lead to any poisonous reactions after 48 h of treatment, despite the dosage reaching up to 5,000 mg/kg. In the subacute evaluations, oral ACJE (at dosages of 200, 400, and 600 mg/kg for 28 consecutive days) treatment elicited no significant difference in total protein, albumin, hematological parameters, and globulin values between treatment-receiving and control groups. However, serum AST, ALT, and ALP levels and urea, creatinine, and MDA levels in the treat-receiving group dose-dependently increased significantly more than in the control group. Moreover, when compared with the control group, the histomorphology of the livers and kidneys of the rats treated with ACJE displayed lesions of degeneration and necrosis (Aba and Amadi, 2019). Recent investigations systematically revealed that the nephrotoxic effect of A. carambola stems primarily from oxalate deposition in renal tubules, causing interstitial nephritis and acute tubular necrosis (Yasawardene et al., 2020). These results indicate that excessive and long-term consumption of ACJE could be nephrotoxic and hepatotoxic. Therefore, it is very important to study the intake dose as well as effective and safe dose in the future.
Phytochemical studies have shown that A. carambola contains two poisonous substances, caramboxin, and oxalic acid. Caramboxin is a non-proteinogenic amino acid that stimulates the glutamate receptors in neurons. The chemical structure of caramboxin is similar to the amino acid phenylalanine, it is metabolized and excreted through the kidneys (Yasawardene et al., 2020). Caramboxin can effectively stimulate the central nervous system (CNS), resulting in symptoms of CNS disorder, including mental confusion, seizures, and status epilepticus (Yasawardene et al., 2020). It can cause belch, vomiting, confusion, consciousness disorders, and shock, etc. If normal people eat this fruit, caramboxin can be safely discharged, so normal people will not be damaged by the toxin when it is ingested. However, the patients with renal insufficiency, especially in patients undergoing peritoneal dialysis or hemodialysis can’t eat it. Huynh and Nguyen (2017) findings showed that the alcohol fermentation technologies efficaciously reduced oxalate contents in A. carambola, preventing the risk of kidney stone formation. However, the works on the dose of caramboxin and the bioavailability of oxalate and caramboxin after ingestion are still insufficient, and more comprehensive, systematic, and authentic studies are required to assess the dose of caramboxin of star fruit juice and its pharmacokinetic parameters in healthy individuals.
Future Outlooks
This review summarized the botany, ethnopharmacology, phytochemistry, pharmacology, and toxicity of A. carambola to explore this valuable fruit. The succulence and sweet taste of starfruit are of interest to the food industry. The pharmaceutical and health industries have been increasingly interested in A. carambola due to the nutritional properties as well as various health and pharmacological actions of this fruit. Phytochemical investigations have shown that 132 compounds have been mainly reported from A. carambola. The flavonoids represented by compounds 47 and 48 and benzoquinones represented by DMDD have been considered as the biologically active components with extensive biological properties, including anti-hyperglycemic, anti-hyperlipidemic, anti-obesity, anti-inflammatory, hepatoprotective, anti-tumor, cardioprotective, and neuroprotective activities. Numerous studies have revealed that A. carambola might be a promising candidate to treat diabetes mellitus and DN-related diseases. Furthermore, A. carambola can be employed in the prevention and treatment of ailments related to aging and oxidative stress. In summary, A. carambola, as a food and medicinal resource, has a good health care function and important edible and medicinal value, and thus has good prospects for utilization.
There are nevertheless still many problems in research that aims to bridge the gap between health and the bioactive substances of A. carambola, which need to be studied further: firstly, although there are a large number of in vitro and in vivo studies concentrated on the crude extracts of A. carambola using exorbitant doses, to the best of our knowledge, the active substances from these crude extracts with bioactivities are still unknown. Thus, research aiming to clarify the biological activities and the mechanism of active components found in A. carambola should be clearly conducted in further depth, as they will certainly collaborate to the establishment of safe dosages and accelerate the steps for the discovery of new molecules of biological interest. Secondly, the bioactive compounds and crude extracts of A. carambola have been increasingly and successfully utilized in diabetic disease prevention and health promotion in the last few decades. The documentary evidence points to a high number of bioactive compounds and crude extracts in star fruit that might be used as functional food sources against various illnesses including diabetes, cancer, cardiovascular disease, and other oxidative stresses or aging-induced chronic diseases. There is a need for further research to explore and utilize the processed products of this fruit, especially those phenolic extracts and dietary fibers in A. carambola for functional food formulation. Thirdly, although toxicity studies of A. carambola have been reported, the information related to its toxicity mechanism, especially nephrotoxicity and neurotoxicity, is still lacking. It is reported that A. carambola induced oxalate nephropathy remains an under-recognized cause for both acute and chronic kidney disease. Therefore, more public awareness about oxalate poisoning on uremic patients should be promoted. This will help to avoid adverse reactions to star-fruits in high uremic patients. The public must be well educated on the benefits as well as the hazardous effects of star fruits. Furthermore, toxicological studies are crucial for understanding the safety of herbal drugs. Therefore, to ensure the full use of its medicinal resources, further acute toxicity, and subacute toxicity, as well as the safety assessment studies of A. carambola, both in vitro and in vivo, should be carried out. Fourthly, continued efforts will be needed to clarify the pathways of pharmacokinetics including absorption, distribution, metabolism, and excretion, and to assess the long-term chronic toxicity and acute toxicity as well as the metabolites of phytochemicals formed in vivo of the bioactive compounds, especially for DMDD before proceeding to the development of pharmaceutical formulation. The pharmacokinetics parameters also contribute to facilitate the rational optimization of natural active substances, and increase therapeutic effects and reduce toxicities. Overall, in-depth research on clinical studies to justify their reported therapeutic potential, clinical efficacy, and safety of A. carambola is imminent.
Taken together, significant interest has been generated over A. carambola owing to the numerous beneficial effects. A. carambola appears to have great developing potential in the fields of functional food and modern medicine. Therefore, the opportunities and challenges co-exist. Meanwhile, it is an effective approach for TCM to get into the international market and an ideal choice for modern health care in developed countries for ongoing human health and thereby building a healthy community with a shared future for mankind. We believe that A. carambola will have an extensive international market and a broad prospect in terms of its applications in both medicine and functional food.
Author Contributions
FL obtained the literatures, wrote the manuscript, and finalized the paper. LP and ZL arranged the tables and designed the figures, XJ collected the pictures. JZ, YY, and XH retrieved relevant literature and discussed the manuscript layout. NZ and XH designed and revised the manuscript, checked the accuracy, and edited the final version. All authors read and approved the final version of the manuscript for publication.
Funding
This study was financially supported by the National Natural Science Foundation of China (Grant No. 82074094), the Open Research Fund of Chengdu University of Traditional Chinese Medicine Key Laboratory of Systematic Research of Distinctive Chinese Medicine Resources in Southwest China (Grant No. 2020XSGG002), the Xinglin Scholar Research Promotion Project of Chengdu University of Traditional Chinese Medicine (Grant No. CDTD2018014), the Science and Technology Plan Project of Guizhou Province (Grant No. Qian KeHe Platform Talents (2018)5772-074), and the Science and Technology Project of Zunyi (Grant No. ZSKH-HZ-(2020)-78).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
- ACP
acid phosphatase
- AI
atherogenic index
- AIP
atherogenic index of plasma
- AKP
alkaline phosphatase
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- AMPK α
AMP-activated protein kinase α
- Ang II
angiotensin II
- AST
aspartate aminotransferase
- BAMBI
BMP and activin membrane bound inhibitor
- Bax
bcl-associated X protein
- BUN
blood urea nitrogen
- cAMP
cyclic adenosine monophosphate
- CAT
catalase
- C/EBPα
CCAAT-enhancer binding protein α
- Col III
collagen type III
- Col IV
collagen type IV
- CTGF
connective tissue growth factor
- CVDs
cardiovascular diseases
- CVF
collagen volume fraction
- CysC
cystatin C
- DENA
diethylnitrosamine
- DMDD
2-dodecyl-6-methoxycyclohexa-2,5-diene- 1,4-dione
- DPPH
2,2-diphenyl-1-picrylhydrazyl radical scavenging activity
- eNOS
endothelial NO synthase
- ECE
endothelin-converting enzyme
- ET-1
endothelin 1
- FAS
fatty acid synthase
- FBG
fasting blood glucose
- FFA
free fatty acids
- FINS
fasting insulin
- FRAP
ferric reducing antioxidant power
- G-6-Pase
glucose-6-phosphatase
- GC-MS
gas chromatography mass spectrometry
- GHb
glucosylated hemoglobin
- GSH
reduced glutathione
- GSH-Px
glutathione peroxidase
- HA
hyaluronic acid
- HDL-C
high-density lipoprotein cholesterol
- Hyp
hydroxyproline
- iNOS
inducible NO synthase
- IL-6
interleukin-6
- ISI
insulin sensitivity index
- LDL-C
low-density lipoprotein cholesterol
- LN
laminin
- LPO
lipid peroxidation
- MDA
malondialdehyde
- MPO
myeloperoxidase
- Myd88
myeloid differentiation factor 88
- NEFA
non-esterified fatty acid
- NF-κB
nuclear factor-κB
- p-AMPK α
phospho-AMPK α
- PPAR-α/γ
peroxisome proliferator activated receptors-α/γ
- Scr
serum creatinine
- SCD1
stearoyl-CoA desaturase 1
- SDH
sorbitol dehydrogenase
- SGOT
serum glutamate oxaloacetate transaminase
- SGPT
serum pyruvate transaminase
- SOD
superoxide dismutase
- SREBP-1c
sterol regulatory element binding protein-1c
- STZ
streptozotocin
- TAA
total ascorbic acid
- TC
total cholesterol
- TEAC
trolox equivalent antioxidant capacity
- TG
triglycerides
- TGF-β1
transforming growth factor beta 1
- TL
total lipids
- TLR4
Toll-like receptors-4
- tNOS
total NO synthase
- TNF-α
tumor necrosis factor-α
- TQ
triglyceride
- VLDL-C
very low-density lipoprotein cholesterol
References
- Aba P. E., Amadi A. U. (2020). Evaluation of the Possible Hepatotoxic and Nephrotoxic Potentials of the Averrhoa carambola Juice Extract in Female Albino Rats. J. Basic Clin. Physiol. Pharmacol. 31 (1), 20190042. 10.1515/jbcpp-2019-0042 [DOI] [PubMed] [Google Scholar]
- Abeysekera R. A., Wijetunge S., Nanayakkara N., Wazil A. W., Ratnatunga N. V., Jayalath T., et al. (2015). Star Fruit Toxicity: a Cause of Both Acute Kidney Injury and Chronic Kidney Disease: a Report of Two Cases. BMC Res. Notes 8, 796. 10.1186/s13104-015-1640-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aladaileh S., Saghir S., Murugesu K., Sadikun A., Ahmad A., Kaur G., et al. (2019). Antihyperlipidemic and Antioxidant Effects of Averrhoa Carambola Extract in High-Fat Diet-Fed Rats. Biomedicines 7 (3), 72. 10.3390/biomedicines7030072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araho D., Miyakoshi M., Chou W. H., Kambara T., Mizutani K., Ikeda T. (2005). A New Flavone C-Glycoside from the Leaves of Averrhoa Carambola . Nat. Med. 59, 113–116. 10.1016/j.apsusc.2004.03.089 [DOI] [Google Scholar]
- Aranguren C., Vergara C., Rosselli D. (2017). Toxicity of Star Fruit (Averrhoa carambola) in Renal Patients: A Systematic Review of the Literature. Saudi J. Kidney Dis. Transpl. 28 (4), 709–715. [PubMed] [Google Scholar]
- Bahramsoltani R., Kalkhorani M., Abbas Zaidi S. M., Farzaei M. H., Rahimi R. (2020). The Genus Tamarix: Traditional Uses, Phytochemistry, and Pharmacology. J. Ethnopharmacol. 246, 112245. 10.1016/j.jep.2019.112245 [DOI] [PubMed] [Google Scholar]
- Benkeblia N., Lopez M. G. (2015). Saccharides and Fructooligosaccharides Composition of Green and Ripe Averrhoa carambola, Blighia sapida and Spondias dulcis Fruits. Food Chem. 176, 314–318. 10.1016/j.foodchem.2014.12.080 [DOI] [PubMed] [Google Scholar]
- Bhat R., Ameran S. B., Voon H. C., Karim A. A., Tze L. M. (2011). Quality Attributes of Starfruit (Averrhoa carambola L.) Juice Treated with Ultraviolet Radiation. Food Chem. 127 (2), 641–644. 10.1016/j.foodchem.2011.01.042 [DOI] [PubMed] [Google Scholar]
- Bircher A. G., Bircher W. H. (2000). Encyclopedia of Fruit Trees and Edible Flowering Plants in Egypt and the Subtropics. Cairo, Egypt: American University in Cairo Press, 596. [Google Scholar]
- Cabrini D. A., Moresco H. H., Imazu P., da Silva C. D., Pietrovski E. F., Mendes D. A., et al. (2011). Analysis of the Potential Topical Anti-inflammatory Activity of Averrhoa carambola L. in Mice. Evid. Based Complement. Alternat Med. 2011, 908059. 10.1093/ecam/neq026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carolino R., Beleboni R., Pizzo A., Vecchio F., Garciacairasco N., Moysesneto M., et al. (2005). Convulsant Activity and Neurochemical Alterations Induced by a Fraction Obtained from Fruit (Oxalidaceae: Geraniales). Neurochem. Int. 46 (7), 523–531. 10.1016/j.neuint.2005.02.002 [DOI] [PubMed] [Google Scholar]
- Cazarolli L. H., Folador P., Moresco H. H., Brighente I. M. C., Pizzolatti M. G., Silva F. R. M. B. (2009). Mechanism of Action of the Stimulatory Effect of Apigenin-6-C-(2″-O-α-l-Rhamnopyranosyl)-β-L-Fucopyranoside on 14C-Glucose Uptake. Chem. Biol. Interact. 179, 407–412. 10.1016/j.cbi.2008.11.012 [DOI] [PubMed] [Google Scholar]
- Cazarolli L. H., Kappel V. D., Pereira D. F., Moresco H. H., Brighente I. M. C., Pizzolatti M. G., et al. (2012). Anti-hyperglycemic Action of Apigenin-6-C-β-Fucopyranoside from Averrhoa Carambola. Fitoterapia 83, 1176–1183. 10.1016/j.fitote.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Chakthong S., Chiraphan C., Jundee C., Chaowalit P., Voravuthikunchai S. P. (2010). Alkyl Phenols from the wood of Averrhoa Carambola . Chin. Chem. Lett. 21, 1094–1096. 10.1016/j.cclet.2010.03.031 [DOI] [Google Scholar]
- Chang J. M., Hwang S. J., Kuo H. T., Tsai J. C., Guh J. Y., Chen H. C., et al. (2000). Fatal Outcome after Ingestion of Star Fruit (Averrhoa carambola) in Uremic Patients. Am. J. Kidney Dis. 35 (2), 189–193. 10.1016/s0272-6386(00)70325-8 [DOI] [PubMed] [Google Scholar]
- Chen C. L., Fang H. C., Chou K. J., Wang J. S., Chung H. M. (2001). Acute Oxalate Nephropathy after Ingestion of star Fruit. Am. J. Kidney Dis. 37 (2), 418–422. 10.1053/ajkd.2001.21333 [DOI] [PubMed] [Google Scholar]
- Chen C. X., Nong Z. H., Xie Q. Q., He J. H., Cai W. E., Tang X. N., et al. (2017b). 2-dodecyl-6-Methoxycyclohexa-2,5-Diene-1,4-Dione Inhibits the Growth and Metastasis of Breast Carcinoma in Mice. Sci. Rep. 7 (1), 6704. 10.1038/s41598-017-07162-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G. L., Zhang X., Chen S. G., Han M. D., Gao Y. Q. (2017a). Antioxidant Activities and Contents of Free, Esterified and Insoluble-Bound Phenolics in 14 Subtropical Fruit Leaves Collected from the South of China. J. Funct. Foods 30, 290–302. 10.1016/j.jff.2017.01.011 [DOI] [Google Scholar]
- Chen L., Lu X., El-Seedi H., Teng H. (2019). Recent Advances in the Development of Sesquiterpenoids in the Treatment of Type 2 Diabetes. Trends Food Sci. Technol. 88, 46–56. 10.1016/j.tifs.2019.02.003 [DOI] [Google Scholar]
- Chua C. B., Sun C. K., Tsui H. W., Yang P. J., Lee K. H., Hsu C. W., et al. (2017). Association of Renal Function and Symptoms with Mortality in star Fruit (Averrhoa carambola) Intoxication. Clin. Toxicol. 55 (7), 624–628. 10.1080/15563650.2017.1314490 [DOI] [PubMed] [Google Scholar]
- Dembitsky V. M., Poovarodom S., Leontowicz H., Leontowicz M., Vearasilp S., Trakhtenberg S., et al. (2011). The Multiple Nutrition Properties of Some Exotic Fruits: Biological Activity and Active Metabolites. Food Res. Int. 44, 1671–1701. 10.1016/j.foodres.2011.03.003 [DOI] [Google Scholar]
- Fan Y. Y., Sahu S. K., Yang T., Mu W. X., Wei J. P., Cheng L., et al. (2020). Dissecting the Genome of star Fruit (Averrhoa carambola L.). Hortic. Res. 7, 94. 10.1038/s41438-020-0306-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias D. P., Neri-Numa I. A., de Araújo F. F., Pastore G. M. (2020). A Critical Review of Some Fruit Trees from the Myrtaceae Family as Promising Sources for Food Applications with Functional Claims. Food Chem. 306, 125630. 10.1016/j.foodchem.2019.125630 [DOI] [PubMed] [Google Scholar]
- Ferreira E. B., Fernandes L. C., Galende S. B., Cortez A. G., Bazotte R. B. (2008). Hypoglycemic Effect of the Hydroalcoholic Extract of Leaves of Averrhoa carambola L. (Oxalidaceae). Rev. Bras. Farmacogn. 18 (3), 339–343. 10.1590/s0102-695x2008000300005 [DOI] [Google Scholar]
- Flora of China Editorial Committee (1998). Flora of China. Beijing, China: Science Press. [Google Scholar]
- Gao Y., Huang R., Gong Y., Park H. S., Wen Q., Almosnid N. M., et al. (2015). The Antidiabetic Compound 2-Dodecyl-6-Methoxycyclohexa-2,5-Diene-1,4-Dione, Isolated from Averrhoa carambola L., Demonstrates Significant Antitumor Potential against Human Breast Cancer Cells. Oncotarget 6 (27), 24304–24319. 10.18632/oncotarget.4475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunasegaran R. (1992). Flavonoids and Anthocyanins of Three Oxalidaceae. Fitoterapia 63, 89–90 . [Google Scholar]
- Gunawardena D. C., Jayasinghe L., Fujimoto Y. (2015). Phytotoxic Constituents of the Fruits of Averrhoa Carambola . Chem. Nat. Compd. 51 (3), 532–533. 10.1007/s10600-015-1332-6 [DOI] [Google Scholar]
- Herman-Lara E., Elvira-Torales L. I., Rodriguez-Miranda J., Torruco-Uco J. G., Carmona-García R., Mendoza-García P. G., et al. (2014). Impact of Micronized Starfruit (Averrhoa carambola L.) Fiber Concentrate on Lipid Metabolism in Mice. Int. J. Food Sci. Nutr. 65 (7), 862–867. 10.3109/09637486.2014.918590 [DOI] [PubMed] [Google Scholar]
- Hossain T., Barman A. K., Karmakar U. K., Bokshi B., Dev S., Biswas N. N. (2017). Phytochemical and Pharmacological Evaluation of Leaves of Averrhoa carambola Linn. (Family: Oxalidaceae). Biosci. Bioeng. Commun. 3 (1), 144–151. [Google Scholar]
- Huang W. S., Pham H. T. T., Xuan F. F., Li J. M., Huang R. B. (2017). Effects of Flavonoids Compound in Averrhoa carambola L. (Oxalidacaeae) Fruit (ACFTF) on Blood Pressure of Rats. China J. Tradit. Chin. Med. Pharm. 32 (4), 1786–1788. [Google Scholar]
- Huang X., Wang L., Meng M., Zhang S., Pham T. T. H., Jiang L., et al. (2020). Extract of Averrhoa carambola L. (Oxalidaceae) Roots Ameliorates Carbon Tetrachloride-Induced Hepatic Fibrosis in Rats. Biomed. Pharmacother. 121, 109516. 10.1016/j.biopha.2019.109516 [DOI] [PubMed] [Google Scholar]
- Huang X., Xie Q. Q., Ye F. X., Qin L. H., Huang R. B., Zhang S. J. (2019). Protective Effect of Extract of Averrhoa carambola L. Root on CCl4-Induced Acute Liver Injury in Mice. Chin. Pharmacol. Bull. 35 (1), 106–110. 10.3969/j.issn.1001-1978.2019.01.021 [DOI] [Google Scholar]
- Huynh N. K., Nguyen H. V. H. (2017). Effects of Juice Processing on Oxalate Contents in Carambola Juice Products. Plant Foods Hum. Nutr. 72 (3), 236–242. 10.1007/s11130-017-0615-4 [DOI] [PubMed] [Google Scholar]
- Islam S., Alam M. B., Ahmed A., Lee S., Lee S.-H., Kim S. (2020). Identification of Secondary Metabolites in Averrhoa carambola L. Bark by High-Resolution Mass Spectrometry and Evaluation for α-glucosidase, Tyrosinase, Elastase, and Antioxidant Potential. Food Chem. 332, 127377. 10.1016/j.foodchem.2020.127377 [DOI] [PubMed] [Google Scholar]
- Jayachandran M., Qu S. (2021). Harnessing Hyperuricemia to Atherosclerosis and Understanding its Mechanistic Dependence. Med. Res. Rev. 41, 616–629. 10.1002/med.21742 [DOI] [PubMed] [Google Scholar]
- Jia X., Xie H., Jiang Y., Wei X. (2018). Flavonoids Isolated from the Fresh Sweet Fruit of Averrhoa Carambola , Commonly Known as star Fruit. Phytochemistry 153, 156–162. 10.1016/j.phytochem.2018.06.007 [DOI] [PubMed] [Google Scholar]
- Jia X., Yang D., Xie H., Jiang Y., Wei X. (2017). Non-Flavonoid Phenolics from Averrhoa carambola Fresh Fruit. J. Funct. Foods 32, 419–425. 10.1016/j.jff.2017.03.025 [DOI] [Google Scholar]
- Jia X., Yang D., Yang Y., Xie H. (2019). Carotenoid-Derived Flavor Precursors from Averrhoa carambola Fresh Fruit. Molecules 24, 256. 10.3390/molecules24020256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakmal K., Yasawardene P., Jayarajah U., Seneviratne S. L. (2021). Nutritional and Medicinal Properties of Star Fruit (Averrhoa carambola L.): A Review. Food Sci. Nutr. 9 (3), 1810–1823. 10.1002/fsn3.2135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivas C. L., Iacomini M., Cordeiro L. M. C. (2016b). Pectic Type II Arabinogalactans from Starfruit (Averrhoa carambola L.). Food Chem. 199, 252–257. 10.1016/j.foodchem.2015.12.020 [DOI] [PubMed] [Google Scholar]
- Leivas C. L., Iacomini M., Cordeiro L. M. C. (2015). Structural Characterization of a Rhamnogalacturonan I-Arabinan-Type I Arabinogalactan Macromolecule from Starfruit (Averrhoa carambola L). Carbohydr. Polym. 121, 224–230. 10.1016/j.carbpol.2014.12.034 [DOI] [PubMed] [Google Scholar]
- Leivas C. L., Nascimento L. F., Barros W. M., Santos A. R. S., Iacomini M., Cordeiro L. M. C. (2016a). Substituted Galacturonan from Starfruit: Chemical Structure and Antinociceptive and Anti-Inflammatory Effects. Int. J. Biol. Macromol. 84, 295–300. 10.1016/j.ijbiomac.2015.12.034 [DOI] [PubMed] [Google Scholar]
- Levenson R. W., Sturm V. E., Haase C. M. (2014). Emotional and Behavioral Symptoms in Neurodegenerative Disease: a Model for Studying the Neural Bases of Psychopathology. Annu. Rev. Clin. Psychol. 10, 581–606. 10.1146/annurev-clinpsy-032813-153653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wei X., Xie Q., Hoa Pham T. T., Wei J., He P., et al. (2016). Protective Effects of 2-Dodecyl-6-Methoxycyclohexa-2,5 -Diene-1,4-Dione Isolated from Averrhoa Carambola L. (Oxalidaceae) Roots on High-Fat Diet-Induced Obesity and Insulin Resistance in Mice. Cell. Physiol. Biochem. 40 (5), 993–1004. 10.1159/000453156 [DOI] [PubMed] [Google Scholar]
- Liang C. X., Pham H. T. T., Zhang X. L., Liang X. M., Huang R. B., Huang X. (2020b). Effect of Extract of Averrhoa carambola L. Root on Liver Microstructure of Liver Fibrosis Rats. J. Guangxi Med. Univ. 37 (7), 1245–1249. 10.16190/j.cnki.45-1211/r.2020.07.008 [DOI] [Google Scholar]
- Liang X., Huang R., Huang J., Chen C., Qin F., Liu A., et al. (2020a). Effect of an Aqueous Extract of Averrhoa carambola L. on Endothelial Function in Rats with Ventricular Remodelling. Biomed. Pharmacother. 121, 109612. 10.1016/j.biopha.2019.109612 [DOI] [PubMed] [Google Scholar]
- Liao P. Y., Li D. P., Hu Z. Y., Chen H. P., Yang X. M., Wu Y. Y. (2019b). Extraction Process Optimization and In Vitro Activity Evaluation for Polysaccharides from Averrhoa carambola Roots. Chin. Tradit. Pat. Med. 41 (9), 2030–2034. 10.3969/j.issn.1001-1528.2019.09.002 [DOI] [Google Scholar]
- Liao P. Y., Zhou Z. Y., Chen Y. R., Chen H. P., Pan W. G., Yang X. M., et al. (2019a). Chemical Constituents of the Water Extracts of Averrhoa carambola L. Root and Their Biological Activities. Nat. Prod. Res. Dev. 31, 81–86. 10.16333/j.1001-6880.2019.1.013 [DOI] [Google Scholar]
- Liu F. Z., Song X. M., Wang X. L., Liu T., Liang R. F. (2013). Hypoglycemic Effect of Total Flavones from Averrhoa carambola L. Leaf. China J. Tradit. Chin. Med. Pharm. 19 (11), 279–281. 10.11653/syfj2013110279 [DOI] [Google Scholar]
- Lu S. Y., Wei X. J., Zhang H. L., Chen Z. F., Li J. M., Xu X. H., et al. (2020). Protective Effect of 2-Dodecyl-6-Methoxycyclohexa-2, 5-Diene-1, 4-Dione, Isolated from Averrhoa carambola L., Against Aβ1-42-Induced Apoptosis in SH-SY5Y Cells by Reversing Bcl-2/Bax Ratio. Psychopharmacology 238 (1), 193–200. 10.1007/s00213-020-05668-9 [DOI] [PubMed] [Google Scholar]
- Lu S., Zhang H., Wei X., Huang X., Chen L., Jiang L., et al. (2019). 2-Dodecyl-6-Methoxycyclohexa-2,5-Diene-1,4-Dione Isolated from Averrhoa carambola L. Root Ameliorates Diabetic Nephropathy by Inhibiting the TLR4/MyD88/NF-Κb Pathway. Diabetes Metab. Syndr. Obes. 12, 1355–1363. 10.2147/dmso.s209436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Tan C. W., Chen D., Liu S.-Q. (2018). Potential of Three Probiotic Lactobacilli in Transforming Star Fruit Juice into Functional Beverages. Food Sci. Nutr. 6 (8), 2141–2150. 10.1002/fsn3.775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Wu Y. N., Huang D. L., Zhou Y. P., Hou L. T., Li Y. K., et al. (2021). Averrhoa carambola L. Roots DMDD Alleviates Myocardial Injury in Diabetes Mellitus Mice by Regulating ROS-Mediated Autophagy Pathways. Chin. Pharmacol. Bull. 37 (6), 823–827. 10.3969/j.issn.1001-1978.2021.06.015 [DOI] [Google Scholar]
- Mahdavi A., Bagherniya M., Fakheran O., Reiner Ž., Xu S., Sahebkar A. (2020). Medicinal Plants and Bioactive Natural Compounds as Inhibitors of HMG-CoA Reductase: A Literature Review. Biofactors 46, 906–926. 10.1002/biof.1684 [DOI] [PubMed] [Google Scholar]
- Maqsood S., Adiamo O., Ahmad M., Mudgil P. (2020). Bioactive Compounds from Date Fruit and Seed as Potential Nutraceutical and Functional Food Ingredients. Food Chem. 308, 125522. 10.1016/j.foodchem.2019.125522 [DOI] [PubMed] [Google Scholar]
- Mia M., Rahman M., Begum K., Begum B., Rashid M. (2007). Phytochemical and Biological Studies of Averrhoa carambola . Dhaka Univ. J. Pharm. Sci. 6, 125–128. 10.3329/dujps.v6i2.688 [DOI] [Google Scholar]
- Mohd Suhaimi N. I., Mat Ropi A. A., Shaharuddin S. (2021). Safety and Quality Preservation of Starfruit (Averrhoa carambola) at Ambient Shelf Life Using Synergistic Pectin-Maltodextrin-Sodium Chloride Edible Coating. Heliyon 7 (2), e06279. 10.1016/j.heliyon.2021.e06279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moresco H. H., Queiroz G. S., Pizzolatti M. G., Brighente I. M. C. (2012). Chemical Constituents and Evaluation of the Toxic and Antioxidant Activities of Averrhoa carambola Leaves. Rev. Bras. Farmacogn. 22, 319–324. 10.1590/s0102-695x2011005000217 [DOI] [Google Scholar]
- Muhammed E., Chen L., Gao Y., Erenso D. (2019). Chemo-Treated 4T1 Breast Cancer Cells Radiation Response Measured by Single and Multiple Cell Ionization Using Infrared Laser Trap. Sci. Rep. 9 (1), 17547. 10.1038/s41598-019-53821-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthu N., Lee S. Y., Lee S. Y., Phua K. K., Bhore S. J. (2016). Nutritional, Medicinal and Toxicological Attributes of Star-Fruits (Averrhoa carambola L.): A Review. Bioinformation 12 (12), 420–424. 10.6026/97320630012420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang D., You L., Li T., Zhou L., Sun-Waterhouse D., Liu R. H. (2016). Phenolic Profiles and Chemical- or Cell-Based Antioxidant Activities of Four Star Fruit (Averrhoa carambola) Cultivars. RSC Adv. 6, 90646–90653. 10.1039/c6ra15692d [DOI] [Google Scholar]
- Pang D., You L., Zhou L., Li T., Zheng B., Liu R. H. (2017). Averrhoa Carambolafree Phenolic Extract Ameliorates Nonalcoholic Hepatic Steatosis by Modulating mircoRNA-34a, mircoRNA-33 and AMPK Pathways in Leptin Receptor-Deficient db/db Mice. Food Funct. 8 (12), 4496–4507. 10.1039/c7fo00833c [DOI] [PubMed] [Google Scholar]
- Pham H. T., Huang W., Han C., Li J., Xie Q., Wei J., et al. (2017). Effects of Averrhoa carambola L. (Oxalidaceae) Juice Mediated on Hyperglycemia, Hyperlipidemia, and its Influence on Regulatory Protein Expression in the Injured Kidneys of Streptozotocin-Induced Diabetic Mice. Am. J. Transl. Res. 9 (1), 36–49. [PMC free article] [PubMed] [Google Scholar]
- Provesi J. G., Valentim Neto P. A., Arisi A. C. M., Amante E. R. (2019). Extraction of Antifreeze Proteins from Cold Acclimated Leaves of Drimys Angustifolia and Their Application to star Fruit (Averrhoa carambola) Freezing. Food Chem. 289, 65–73. 10.1016/j.foodchem.2019.03.055 [DOI] [PubMed] [Google Scholar]
- Qin L. H., Wu X. C., Zhou X., Wu Y. N., Huang R. B., Zhang S. J. (2019). Effects of Benzoquinone of Averrhoa carambola L. Root on Glucose and Lipid Metabolism, Oxidative Stress and Inflammatory Injury in Diabetic Mice. Chin. Pharmacol. Bull. 35 (12), 1720–1725. 10.3969/j.issn.1001-1978.2019.12.019 [DOI] [Google Scholar]
- Qin L., Zhang X., Zhou X., Wu X., Huang X., Chen M., et al. (2020). Protective Effect of Benzoquinone Isolated from the Roots of Averrhoa carambola L. on Streptozotocin-Induced Diabetic Mice by Inhibiting the TLR4/NF-κB Signaling Pathway. Diabetes Metab. Syndr. Obes. 13, 2129–2138. 10.2147/dmso.s241998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadan N. S., Wessjohann L. A., Mocan A., C Vodnar D., H. El-Sayed N., A. El-Toumy S., et al. (2020). Nutrient and Sensory Metabolites Profiling of Averrhoa carambola L. (Starfruit) in the Context of its Origin and Ripening Stage by GC/MS and Chemometric Analysis. Molecules 25 (10), 2423. 10.3390/molecules25102423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid A. M., Lu K., Yip Y. M., Zhang D. (2016). Averrhoa carambola L. Peel Extract Suppresses Adipocyte Differentiation in 3T3-L1 Cells. Food Funct. 7 (2), 881–892. 10.1039/c5fo01208b [DOI] [PubMed] [Google Scholar]
- Robins Wahlin T.-B., Byrne G. J. (2011). Personality Changes in Alzheimer's Disease: a Systematic Review. Int. J. Geriat. Psychiatry 26 (10), 1019–1029. 10.1002/gps.2655 [DOI] [PubMed] [Google Scholar]
- Ronpirin C., Pattarachotanant N., Tencomnao T. (2016). Protective Effect of Mangifera indica Linn., Cocos nucifera Linn., and Averrhoa carambola Linn. Extracts against Ultraviolet B-Induced Damage in Human Keratinocytes. Evid. Based Compl. Alternat. Med. 2016, 1684794. 10.1155/2016/1684794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufino M. S. M., Alves R. E., Fernandes F. A. N., Brito E. S. (2011). Free Radical Scavenging Behavior of Ten Exotic Tropical Fruits Extracts. Food Res. Int. 44 (7), 2072–2075. 10.1016/j.foodres.2010.07.002 [DOI] [Google Scholar]
- Saghir S. A., Sadikun A., Al-Suede F. S., Majid A. M., Murugaiyah V. (2016). Antihyperlipidemic, Antioxidant and Cytotoxic Activities of Methanolic and Aqueous Extracts of Different Parts of Star Fruit. Curr. Pharm. Biotechnol. 17 (10), 915–925. 10.2174/1389201017666160603013434 [DOI] [PubMed] [Google Scholar]
- Saikia S., Mahnot N. K., Mahanta C. L. (2015). Optimisation of Phenolic Extraction from Averrhoa carambola Pomace by Response Surface Methodology and its Microencapsulation by spray and Freeze Drying. Food Chem. 171, 144–152. 10.1016/j.foodchem.2014.08.064 [DOI] [PubMed] [Google Scholar]
- Shui G., Leong L. P. (2004). Analysis of Polyphenolic Antioxidants in star Fruit Using Liquid Chromatography and Mass Spectrometry. J. Chromatogr. A 1022, 67–75. 10.1016/j.chroma.2003.09.055 [DOI] [PubMed] [Google Scholar]
- Shui G., Leong L. P. (2006). Residue from star Fruit as Valuable Source for Functional Food Ingredients and Antioxidant Nutraceuticals. Food Chem. 97, 277–284. 10.1016/j.foodchem.2005.03.048 [DOI] [Google Scholar]
- Siddika A., Zahan T., Khatun L., Habib M. R., Aziz M. A., Tareq A. R. M., et al. (2020). In Vivo the Antioxidative Extract of Averrhoa carambola Linn. Leaves Induced Apoptosis in Ehrilch Ascites Carcinoma by Modulating P53 Expression. Food Sci. Biotechnol. 29 (9), 1251–1260. 10.1007/s10068-020-00775-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva K. B., Pinheiro C. T. S., Soares C. R. M., Souza M. A., Matos-Rocha T. J., Fonseca S. A., et al. (2021). Phytochemical Characterization, Antioxidant Potential and Antimicrobial Activity of Averrhoa carambola L. (Oxalidaceae) against Multiresistant Pathogens. Braz. J. Biol. 81 (3), 509–515. 10.1590/1519-6984.220259 [DOI] [PubMed] [Google Scholar]
- Silva K. D. R. R., Sirasa M. S. F. (2018). Antioxidant Properties of Selected Fruit Cultivars Grown in Sri Lanka. Food Chem. 238, 203–208. 10.1016/j.foodchem.2016.08.102 [DOI] [PubMed] [Google Scholar]
- Singh R., Sharma J., Goyal P. K. (2014). Prophylactic Role of Averrhoa carambola (Star Fruit) Extract against Chemically Induced Hepatocellular Carcinoma in Swiss Albino Mice. Adv. Pharmacol. Sci. 2014, 158936. 10.1155/2014/158936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soncini R., Santiago M. B., Orlandi L., Moraes G. O. I., Peloso A. L. M., dos Santos M. H., et al. (2011). Hypotensive Effect of Aqueous Extract of Averrhoa carambola L. (Oxalidaceae) in Rats: an In Vivo and In Vitro Approach. J. Ethnopharmacol. 133 (2), 353–357. 10.1016/j.jep.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Sritharan T., Savitri Kumar N., Jayasinghe L., Araya H., Fujimoto Y. (2019). Isocoumarins and Dihydroisocoumarins from the Endophytic Fungus Biscogniauxia capnodes Isolated from the Fruits of Averrhoa carambola . Nat. Product. Commun. 14, 1934578X1985196. 10.1177/1934578X19851969 [DOI] [Google Scholar]
- Tang J. Z., Nong H. L., Liang X. M., Huang J. C., Qin F. Z., Huang R. B. (2017). Effect of the Ethanol Extracts of Averrhoa carambola Root on Blood Pressure in Normal Rats. West. China J. Pharm. Sci. 32 (2), 160–162. 10.1016/j.biopha.2019.109612 [DOI] [Google Scholar]
- Teng H., Yuan B., Gothai S., Arulselvan P., Song X., Chen L. (2018). Dietary Triterpenes in the Treatment of Type 2 Diabetes: To Date. Trends Food Sci. Technol. 72, 34–44. 10.1016/j.tifs.2017.11.012 [DOI] [Google Scholar]
- Thomas R., Jebin N., Saha R., Sarma D. K. (2016). Antioxidant and Antimicrobial Effects of Kordoi (Averrhoa carambola) Fruit Juice and Bamboo (Bambusa polymorpha) Shoot Extract in Pork Nuggets. Food Chem. 190, 41–49. 10.1016/j.foodchem.2015.05.070 [DOI] [PubMed] [Google Scholar]
- Uuh-Narváez J. J., González-Tamayo M. A., Segura-Campos M. R. (2021). A Study on Nutritional and Functional Study Properties of Mayan Plant Foods as a New Proposal for Type 2 Diabetes Prevention. Food Chem. 341, 128247. 10.1016/j.foodchem.2020.128247 [DOI] [PubMed] [Google Scholar]
- Valim F. d. P., Aguiar-Oliveira E., Kamimura E. S., Alves V. D., Maldonado R. R. (2016). Production of Star Fruit Alcoholic Fermented Beverage. Indian J. Microbiol. 56 (4), 476–481. 10.1007/s12088-016-0601-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Martínez D. A., González-Curbelo M. Á., González-Sálamo J., Hernández-Borges J. (2019). High-Throughput Analysis of Pesticides in Minor Tropical Fruits from Colombia. Food Chem. 280, 221–230. 10.1016/j.foodchem.2018.12.045 [DOI] [PubMed] [Google Scholar]
- Vasant R. A., Narasimhacharya A. V. R. L. (2014). Antidotal Activity of Averrhoa carambola (Star Fruit) on Fluoride Induced Toxicity in Rats. Interdiscip. Toxicol. 7 (2), 103–110. 10.2478/intox-2014-0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos C. M. L., Araújo M. S., Conde-Garcia E. A. (2006). Electrophysiological Effects of the Aqueous Extract of Averrhoa carambola L. Leaves on the Guinea Pig Heart. Phytomedicine 13 (7), 501–508. 10.1016/j.phymed.2005.01.013 [DOI] [PubMed] [Google Scholar]
- Wakte S. R., Patil D. A. (2011). Antimicrobial and Antioxidant Activity of Averrhoa Carambola L. Fruit at Various Stages of Ripening. J. Herb. Med. Toxicol. 5 (2), 121–129. [Google Scholar]
- Wang Y., Liu M., Liu L., Xia J.-H., Du Y.-G., Sun J.-S. (2018). The Structural Revision and Total Synthesis of Carambolaflavone A. J. Org. Chem. 83 (7), 4111–4118. 10.1021/acs.joc.8b00008 [DOI] [PubMed] [Google Scholar]
- Wei S.-D., Chen H., Yan T., Lin Y.-M., Zhou H.-C. (2014). Identification of Antioxidant Components and Fatty Acid Profiles of the Leaves and Fruits from Averrhoa carambola . LWT - Food Sci. Technol. 55, 278–285. 10.1016/j.lwt.2013.08.013 [DOI] [Google Scholar]
- Wei X. J., Xu X. H., Chen Z. F., Liang T., Wen Q. W., Qin N., et al. (2018). Protective Effects of 2-Dodecyl-6-Methoxycyclohexa-2,5-Diene-1,4-Dione Isolated from Averrhoa carambola L. (Oxalidaceae) Roots on Neuron Apoptosis and Memory Deficits in Alzheimer’s Disease. Cell. Physiol. Biochem. 49 (3), 1064–1073. 10.1159/000493289 [DOI] [PubMed] [Google Scholar]
- Wen Q., Lin X., Liu Y., Xu X., Liang T., Zheng N., et al. (2012). Phenolic and Lignan Glycosides from the Butanol Extract of Averrhoa carambola L. Root. Molecules 17, 12330–12340. 10.3390/molecules171012330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Q. W., Chen C. X., Liang X. M., Xu X. H., Huang R. B. (2014). Isolation, Identification and Determination for Benzoquinone Compounds from Averrhoa carambola Root. Chin. J. Exp. Tradit. Med. Formulae 20 (11), 70–73. 10.13422/j.cnki.syfjx.2014110070 [DOI] [Google Scholar]
- Wen Q. W., Liang T., Qin F. Z., Wei J. B., He Q. L., Luo X., et al. (2013). Lyoniresinol 3α-O-β-D-Glucopyranoside-Mediated Hypoglycaemia and its Influence on Apoptosis-Regulatory Protein Expression in the Injured Kidneys of Streptozotocin-Induced Mice. PLoS One 8 (12), e81772. 10.1371/journal.pone.0081772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijayaratne D. R., Bavanthan V., de Silva M. V. C., Nazar A. L. M., Wijewickrama E. S. (2018). Star Fruit Nephrotoxicity: a Case Series and Literature Review. BMC Nephrol. 19 (1), 288. 10.1186/s12882-018-1084-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization and Regional Office for the Western Pacific (2007). WHO International Standard Terminologies on Traditional Medicine in the Western Pacific Region. Manila: WHO Regional Office for the Western Pacific. [Google Scholar]
- Wu S. S., Sun W., Xu Z. C., Zhai J. W., Li X. P., Li C. R., et al. (2020b). The Genome Sequence of Star Fruit (Averrhoa carambola). Hortic. Res. 7, 95. 10.1038/s41438-020-0307-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X. C., Lu S. Y., Zhou X., Qin L. H., Jiang L. H., Li Y. C., et al. (2020a). Anti-hepatocarcinoma Effect of and its Mechanism of 2-Dodecyl-6-Methoxycyclohexa-2,5-Diene-1,4-Dione in Averrhoa carambola L. Roots. Chin. J. Hosp. Pharm. 40 (1), 42–47. 10.13286/j.1001-5213.2020.01.05 [DOI] [Google Scholar]
- Xie Q., Zhang S., Chen C., Li J., Wei X., Xu X., et al. (2016). Protective Effect of 2-Dodecyl-6-Methoxycyclohexa-2, 5-Diene-1, 4-Dione, Isolated from Averrhoa carambola L., Against Palmitic Acid-Induced Inflammation and Apoptosis in Min6 Cells by Inhibiting the TLR4-MyD88-NF-κB Signaling Pathway. Cell. Physiol. Biochem. 39 (5), 1705–1715. 10.1159/000447871 [DOI] [PubMed] [Google Scholar]
- Xu X. H., Phama H. T. T., Wei X. J., Qin N., Huang R. B. (2017). Study of the Extract of Averrhoa carambola L. Root on Renal Function in Diabetic Mice and its Anti-Oxidative Action. Chin. Pharmacol. Bull. 33 (1), 95–100. 10.3969/j.issn.1001-1978.2017.01.017 [DOI] [Google Scholar]
- Xu X., Liang T., Lin X., Wen Q., Liang X., Li W., et al. (2015). Effect of the Total Extract of Averrhoacarambola (Oxalidaceae) Root on the Expression Levels of TLR4 and NF-κB in Streptozotocin-Induced Diabetic Mice. Cell. Physiol. Biochem. 36 (6), 2307–2316. 10.1159/000430194 [DOI] [PubMed] [Google Scholar]
- Xu X., Liang T., Wen Q., Lin X., Tang J., Zuo Q., et al. (2014). Protective Effects of Total Extracts of Averrhoa carambola L. (Oxalidaceae) Roots on Streptozotocin-Induced Diabetic Mice. Cell. Physiol. Biochem. 33 (5), 1272–1282. 10.1159/000358695 [DOI] [PubMed] [Google Scholar]
- Yang D.-P., Liu X., Teng C. P., Owh C., Win K. Y., Lin M., et al. (2017). Unexpected Formation of Gold Nanoflowers by a Green Synthesis Method as Agents for a Safe and Effective Photothermal Therapy. Nanoscale 9 (41), 15753–15759. 10.1039/c7nr06286a [DOI] [PubMed] [Google Scholar]
- Yang D., Jia X., Xie H. (2019b). Heptyl Vicianoside and Methyl Caramboside from Sour star Fruit. Nat. Product. Res. 33 (8), 1233–1236. 10.1080/14786419.2018.1466123 [DOI] [PubMed] [Google Scholar]
- Yang D., Jia X., Xie H., Wei X. (2016). Further Dihydrochalcone C-Glycosides from the Fruit of Averrhoa carambola . LWT - Food Sci. Technol. 65, 604–609. 10.1016/j.lwt.2015.08.061 [DOI] [Google Scholar]
- Yang D., Xie H., Jia X., Wei X. (2015). Flavonoid C-Glycosides from Star Fruit and Their Antioxidant Activity. J. Funct. Foods 16, 204–210. 10.1016/j.jff.2015.04.048 [DOI] [Google Scholar]
- Yang D., Xie H., Yang B., Wei X. (2014). Two Tetrahydroisoquinoline Alkaloids from the Fruit of Averrhoa carambola . Phytochem. Lett. 7, 217–220. 10.1016/j.phytol.2013.12.007 [DOI] [Google Scholar]
- Yang L., Zhu J., Song L., Shi X., Li X., Yu R. (2012). Three Sesquiterpene Compounds Biosynthesised from Artemisinic Acid Using Suspension-Cultured Cells of Averrhoa carambola (Oxalidaceae). Nat. Product. Res. 26 (15), 1388–1394. 10.1080/14786419.2011.589055 [DOI] [PubMed] [Google Scholar]
- Yang Y., Jia X., Xie H., Wei X. (2020a). Dihydrochalcone C-Glycosides from Averrhoa carambola Leaves. Phytochemistry 174, 112364. 10.1016/j.phytochem.2020.112364 [DOI] [PubMed] [Google Scholar]
- Yang Y. X., Huang T. M., Huang R. B., Xu X. H. (2018). Study on the Liver Tissue Anti-Oxidative Stress and Hypoglycemic Effects of Averrhoa carambola Fruit Juice. Food Sci. Dev. 39 (9), 148–151. 10.3969/j.issn.1005-6521.2018.09.028 [DOI] [Google Scholar]
- Yang Y. X., Huang T. M., Huang R. B., Xu X. H. (2019a). The Effects and Mechanism of Averrhoa carambola Fruit Juice on Blood Glucose in Diabetic Mice. J. Guangxi Med. Univ. 36 (1), 7–10. 10.16190/j.cnki.45-1211/r.2019.01.002 [DOI] [Google Scholar]
- Yang Y., Xie H., Jiang Y., Wei X. (2020b). Flavan-3-ols and 2-Diglycosyloxybenzoates from the Leaves of Averrhoa carambola . Fitoterapia 140, 104442. 10.1016/j.fitote.2019.104442 [DOI] [PubMed] [Google Scholar]
- Yao Q., Gao Y., Lai C., Wu C., Zhao C. L., Wu J. L., et al. (2020). The Phytochemistry, Pharmacology and Applications of Melicope pteleifolia: A Review. J. Ethnopharmacol. 251, 112546. 10.1016/j.jep.2020.112546 [DOI] [PubMed] [Google Scholar]
- Yasawardene P., Jayarajah U., De Zoysa I., Seneviratne S. L. (2020). Mechanisms of Star Fruit (Averrhoa carambola) Toxicity: A Mini-Review. Toxicon 187, 198–202. 10.1016/j.toxicon.2020.09.010 [DOI] [PubMed] [Google Scholar]
- Yasawardene P., Jayarajah U., De Zoysa I., Seneviratne S. L. (2021). Nephrotoxicity and Neurotoxicity Following Star Fruit (Averrhoa carambola) Ingestion: A Narrative Review. Trans. R. Soc. Trop. Med. Hyg. 10.1093/trstmh/trab026 [DOI] [PubMed] [Google Scholar]
- Zhang H., Lu S., Chen L., Huang X., Jiang L., Li Y., et al. (2020). 2-Dodecyl-6-Methoxycyclohexa-2,5-Diene-1,4-Dione, Isolated from the Root of Averrhoa carambola L., Protects against Diabetic Kidney Disease by Inhibiting TLR4/TGFβ Signaling Pathway. Int. Immunopharmacol. 80, 106120. 10.1016/j.intimp.2019.106120 [DOI] [PubMed] [Google Scholar]
- Zhang H., Wei X., Lu S., Lin X., Huang J., Chen L., et al. (2019). Protective Effect of DMDD, Isolated from the Root of Averrhoa carambola L., on High Glucose Induced EMT in HK-2 Cells by Inhibiting the TLR4-BAMBI-Smad2/3 Signaling Pathway. Biomed. Pharmacother. 113, 108705. 10.1016/j.biopha.2019.108705 [DOI] [PubMed] [Google Scholar]
- Zheng N., Lin X., Wen Q., Kintoko S., Zhang J., et al. (2013). Effect of 2-Dodecyl-6-Methoxycyclohexa-2,5-Diene-1,4-Dione, Isolated from Averrhoa carambola L. (Oxalidaceae) Roots, on Advanced Glycation End-Product-Mediated Renal Injury in Type 2 Diabetic KKAy Mice. Toxicol. Lett. 219 (1), 77–84. 10.1016/j.toxlet.2013.03.001 [DOI] [PubMed] [Google Scholar]
- Zheng N., Lin X., Wen Q., Kintoko S., Zhang J., et al. (2021). Effect of 2-Dodecyl-6-Methoxycyclohexa-2, 5-Diene-1, 4-Dione, Isolated from Averrhoa carambola L. (Oxalidaceae) Roots, on Advanced Glycation End-Product-Mediated Renal Injury in Type 2 Diabetic KKAy Mice. Toxicol. Lett. 339, 88–96. 10.1016/j.toxlet.2020.11.022 [DOI] [PubMed] [Google Scholar]
- Zhou X., Qin L. H., Wu X. C., Wu Y. N., Chen L. X., Huang R. B. (2019). Inhibition Effect of 2-Dodecyl-6-Methoxycyclohexa-2,5-Diene-1,4-Dione on Lung Cancer H1299 Cells. Chin. J. Clin. Pharmacol. 35 (21), 2675–2678. [Google Scholar]
- Zhu C., Xiao Y., Liu X., Han J., Zhang J., Wei L., et al. (2015). Pioglitazone Lowers Serum Retinol Binding Protein 4 by Suppressing its Expression in Adipose Tissue of Obese Rats. Cell. Physiol. Biochem. 35 (2), 778–788. 10.1159/000369737 [DOI] [PubMed] [Google Scholar]
- Zulfajri M., Dayalan S., Li W. Y., Chang C. J., Chang Y. P., Huang G. G. (2019). Nitrogen-Doped Carbon Dots from Averrhoa carambola Fruit Extract as a Fluorescent Probe for Methyl Orange. Sensors 19 (22), 5008. 10.3390/s19225008 [DOI] [PMC free article] [PubMed] [Google Scholar]