Abstract
Background
Patients with acute asthma treated in the emergency department are frequently treated with intermittent inhaled beta‐agonists delivered by nebulisation. The use of continuous beta‐agonist (CBA) via nebulisation in the emergency setting may offer additional benefits in acute asthma.
Objectives
To determine the efficacy (e.g., reductions in admission, improvement in pulmonary functions) and risks (e.g., adverse events, effects on vital signs) of continuous versus intermittent inhaled beta‐agonists for the treatment of patients with acute asthma managed in the emergency department.
Search methods
Randomised controlled trials were identified from the Cochrane Airways Group Specialised Register of trials. In addition, primary authors and content experts were contacted to identify eligible studies. Bibliographies from included studies, known reviews and texts were also searched. The search is considered updated to February 2011.
Selection criteria
Only randomised controlled trials (RCTs) were eligible for inclusion. Studies were included if patients presented with acute asthma and were treated with either continuous or intermittent inhaled beta‐agonists early in the ED treatment. "Continuous" nebulisation was defined as truly continuous aerosol delivery of beta‐agonist medication (e.g., using a commercially available large‐volume nebuliser, or a small‐volume nebuliser with infusion pump) or sufficiently frequent nebulisations that medication delivery was effectively continuous (i.e., 1 nebulisation every 15 minutes or > 4 nebulisations per hour). Studies also needed to report either pulmonary function or admission results. Two reviewers independently selected potentially relevant articles and two additional reviewers independently selected articles for inclusion. Methodological quality was independently assessed by two reviewers.
Data collection and analysis
Data were extracted independently by two reviewers if the authors were unable to verify the validity of information. Missing data were obtained from authors or calculated from other data presented in the paper. The data were analysed using the Cochrane Review Manager (Version 4.1). Relative risks (RR), weighted mean differences (WMD) and standardized mean differences (SMD) are reported with corresponding 95% confidence intervals (CI); both peak expiratory flow rates (PEFR) and forced expiratory volume in one second (FEV‐1) data are reported.
Main results
165 trials were reviewed and eight were included; a total of 461 patients have been studied (229 with CBA; 232 with intermittent beta‐agonists). Overall, admission to hospital was reduced with CBA compared to intermittent beta‐agonists (RR: 0.68; 95% CI: 0.5 to 0.9); patients with severe airway obstruction at presentation appeared to benefit most from this intervention (RR: 0.64; 95% CI: 0.5 to 0.9). Patients receiving CBA demonstrated small but statistically significant improvements in pulmonary function tests when all studies were pooled. Patients receiving CBA had greater improvements in % predicted FEV‐1 (SMD: 0.3; 95% CI: 0.03 to 0.5) and PEFR (SMD: 0.33; 95% CI: 0.1 to 0.5); this effect was observed by 2‐3 hours. Continuous treatment was generally well tolerated, with no clinically important differences observed in pulse rate (WMD: ‐2.87; 95% CI: ‐6.0 to 0.3) or blood pressure (WMD: ‐1.75; 95% CI: ‐5.6 to 2.1) between the treatment groups. Tremor was equally common in both groups (OR: 0.81; 95% CI: 0.5 to 1.3) and potassium concentration was unchanged (WMD: 0.02; 95% CI: ‐0.2 to 0.2).
Authors' conclusions
Current evidence supports the use of CBA in patients with severe acute asthma who present to the emergency department to increase their pulmonary functions and reduce hospitalisation. Moreover, CBA treatment appears to be safe and well tolerated in patients who receive it.
Plain language summary
Continuous versus intermittent beta‐agonists for acute asthma
During acute asthma attacks, inhaled beta‐agonists (reliever medications) are used to treat spasm in the airways in the lungs. The medication can be administered by wet nebulisation or from an inhaler with a holding chamber; wet nebulisation may be delivered in a continuous or intermittent fashion. This review has collected information from randomised controlled trials comparing continuous to intermittent nebulised delivery methods in acute asthma attacks. Overall, differences were found between the two methods, with continuous nebulisers producing a modest reduction in admissions compared to intermittent beta‐agonist therapy. This finding was especially pronounced in severe acute asthma. Continuous nebuliser therapy may be more effective than intermittent nebulisers for delivering beta‐agonist drugs to relieve airway spasm in selected asthma populations.
Background
Acute asthma is a common presenting complaint to the emergency department (ED). In the United States, acute asthma accounts for approximately 2 million ED visits per year (Mannino 1998). Approximately 20‐30% of these patients will require admission to the hospital; of those discharged from the ED after apparently successful treatment, approximately 10‐20% will relapse within two weeks (Camargo 1998). The cost of asthma care is enormous, and care of the acute episode (e.g., emergency department and hospitalizations) represents approximately 25% of all care for this problem (Weiss 2001). The enormity of asthma as a health care problem worldwide has led to the creation of several national (Boulet 1999; NAEPP 1997; BTS 1997) and international (GINA 2002) asthma guidelines. These guidelines generally include sections that focus care in the acute setting. There is general agreement that beta‐agonists, such as albuterol (salbutamol), and corticosteroids, such as prednisone, are first‐line agents for acute asthma. Beta‐agonists are used to provide rapid symptom relief, whereas corticosteroids are used to counter airway inflammation and hasten resolution of the asthma exacerbation. Although both agents are widely used in the acute setting, there remain numerous controversies regarding the appropriate dose, frequency, and route of beta‐agonist delivery.
With regard to beta‐agonists, most experts (NAEPP 1997; Boulet 1999) suggest that the inhaled route is superior to parenteral routes; this assertion is the focus of another systematic review by the members of the Cochrane Airways Group (Travers 2001). Even among those who contend that inhaled therapy is superior, there are controversies concerning the route of inhaled beta‐agonist delivery. A recent systematic review found no material difference in the clinical efficacy of metered‐dose inhalers (MDIs) with holding chambers versus wet nebulisers in acute asthma (Cates 2006). Current recommendations suggest that either MDI or nebulisation may be used in acute asthma.
The focus of the present review is the assertion that "continuous" inhaled beta‐agonist therapy is more efficacious for acute asthma than the conventional (intermittent) method of beta‐agonist delivery (e.g., 2.5 mg albuterol nebulisations every 20‐30 minutes, or < or = to 3 nebulisations per hour). Given current practice patterns, the most important comparison is between continuous nebulisation and intermittent nebulisation. Continuous nebulisation may be delivered using commercially‐available machines or through nebulisation systems devised locally (e.g., using an infusion pump). Potential advantages of continuous nebulisation include reduced time and labor costs (by obviating multiple refills of the nebuliser) and more consistent medication delivery; the latter feature may allow deeper penetration into the patient's airways and greater reduction of bronchoconstriction. Moreover, continuous nebulisation may result in fewer side effects due to consistent (rather than bolus) beta‐agonist delivery.
To date, only a limited number of trials have examined continuous versus intermittent nebulisation and they have yielded inconsistent results. We are aware of one published systematic review on the role of continuous versus intermittent nebulisation in the treatment of acute asthma in the ED (Rodrigo 2002); however, our review includes other recent primary publications, uses Cochrane methods and was designed to further clarify the role of CBA in acute asthma.
Objectives
The objective of this review was to determine the effect (on pulmonary function, admission, and other outcomes) of treatment with continuous versus intermittent inhaled beta‐agonist therapy in the first two hours of ED treatment for acute asthma.
Specific Aims: To quantify the effect of continuous inhaled beta‐agonist therapy compared to the effect of these agents given intermittently. The specific outcomes included the effect of different beta‐agonist delivery techniques on: (1) pulmonary function (e.g., peak expiratory flow rate [PEFR] and forced expiratory volume in 1 second [FEV‐1]) (2) admission (e.g., time to decision, % admission). (3) other clinical outcomes (e.g., vital signs, symptom scores, adverse effects). (4) economic endpoints.
Methods
Criteria for considering studies for this review
Types of studies
To be considered, reported studies had to be randomised controlled clinical trials (RCT). We accepted blinded and unblinded trials, but not cohort or pseudo‐randomised trials.
Types of participants
Studies including only patients presenting to an ED or its equivalent were considered for inclusion in the review. If patients from other settings could be removed easily from the study (for example if stratified randomisation was employed) these data were also considered. Studies recruiting either children or adult patients were reviewed and patient age formed one of the subgroup analyses.
Types of interventions
Patients in studies had to be randomised to receive either continuous or intermittent inhaled beta‐agonists early in the ED treatment. "Continuous" nebulisation was defined as truly continuous aerosol delivery of beta‐agonist medication (e.g., using a commercially available large‐volume nebuliser, or a small‐volume nebuliser with infusion pump) or sufficiently frequent nebulisations that medication delivery was effectively continuous (i.e., 1 nebulisation every 15 minutes or > 4 nebulisations per hour). Since acute asthmatics require additional treatments (e.g., corticosteroids, ipratropium bromide, magnesium sulfate, etc.) data for any co‐interventions were recorded or requested from the authors when not reported in the studies.
Types of outcome measures
Primary outcomes
Change in pulmonary function
Secondary outcomes
Admission to the hospital or discharge from ED
Other clinical outcomes (e.g., vital signs, symptom scores)
Adverse effects (e.g., tremor, nausea, etc).
We searched for results from economic analyses; however, few were identified.
Search methods for identification of studies
Electronic searches
Trials were identified using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and handsearching of respiratory journals and meeting abstracts (please see the Airways Group Module for further details). The Register contains a variety of studies published in foreign languages, and we did not exclude trials on the basis of language. All records in the Specialised Register coded as 'asthma' were searched using the following terms:
(emerg* or acute or status or sever* or emerg* or exacerbat*) AND ("beta* agonist*" or bronchodilat* or albuterol or salbutamol or ventolin or proventil or metaproterenol or alupent or terbutaline or bricanyl or isoproterenol or epinephrine or adrenaline or isoprenaline or hexoprenaline or reproterol or broxaterol or carbuterol or fenoterol or formeterol or pirbuterol or rimiterol or salmeterol or tolubuterol or *terol)
The most recent serach was conducted in February 2011.
Searching other resources
Additional efforts to locate potential trials were as follows:
Reference lists of all available primary studies and review articles were reviewed to identify potentially relevant citations.
Inquiries were made regarding other published or unpublished trials known or supported by the authors of the primary studies so that these results could be included in this review.
The scientific advisors of the various pharmaceutical companies that manufacture aerosol delivery devices were contacted for any unpublished or interim results on relevant research.
Search of CENTRAL was completed using the following terms: asthma AND continuous AND (albuterol OR salbutamol OR ventolin OR proventil OR metaproterenol OR alupent OR terbutaline OR bricanyl).
Finally, personal contact with colleagues, collaborators and other trialists working in the field of asthma was made to identify potentially relevant studies.
Data collection and analysis
Selection of studies
From the title, abstract, or descriptors, two reviewers (CAC, BHR) independently reviewed literature searches to identify potentially relevant trials for full review. Searches of bibliographies and texts were conducted to identify additional studies. From the full text, using specific criteria, two reviewers (CAC, BHR) independently selected trials for inclusion in this review. Inter‐rater reliability was measured by using simple agreement and kappa statistics. Disagreement was resolved by consensus or third party adjudication (CHS).
Data extraction and management
Data for the trials were extracted by two reviewers (CAC, BHR) and entered into the Cochrane Collaboration software program (Review Manager). Primary study authors were requested to confirm data extraction and provide additional clarification and information for the review whenever there was a need. In some cases, expansion of graphic representations of data from the manuscripts were used to estimate missing data. All data, numeric calculations and graphic extrapolations were independently confirmed (CHS).
Assessment of risk of bias in included studies
Methodological quality assessment was performed by two reviewers working independently (CAC, BHR). Inter‐rater reliability was measured by using simple agreement and kappa statistics. Both reviewers used two approaches to grade quality:
1. Allocation concealment. Using the Cochrane (Shultz 1995) approach to assessment of allocation concealment, all trials were scored and entered using the following principles: Grade A: Adequate concealment Grade B: Uncertain Grade C: Clearly inadequate concealment
2. Quality assessment. Quality was assessed using a 5 part score (Jadad 1996) and summarised as follows: Was the study described as randomised (1=yes; 0=no) Was the study described as double‐blind (1=yes; 0=no) Was there a description of withdrawals and dropouts (1=yes; 0=no) Was the method of randomisation well described and appropriate (1=yes; 0=no) Was the method of double blinding well described and appropriate (1=yes; 0=no) Points were deducted for either inappropriate randomisation or blinding.
Dealing with missing data
Attempts were made to contact the primary investigators of included studies to obtain individual patient data, however this was largely unsuccessful.
When the standard deviation (SD) for a measure was missing from a study, an estimate was imputed. The estimate was based on the weighted average (by sample size) of the deviations from other included studies for that category. Data from several studies were not reported in tabular format; however, they were demonstrated in charts. To extract these data values the graphs were enlarged and data points were extracted using exact calipers; results were checked for reliability twice. Some graphs and tables displayed a point estimate measure with standard errors of the mean (SEM). SEM's for these trials were converted to SD's thus: SD(Xbar) = SEM(Xbar)*sqrt(n).
Assessment of heterogeneity
For pooled effects, heterogeneity was tested using the Breslow‐Day test; in settings where the chi‐square heterogeneity statistic revealed a P < 0.1, a random effects model was reported. Sensitivity analyses were conducted on fixed vs. random effects models and methodological quality (high vs. low).
Data synthesis
All trials were combined using the RevMan. For continuous outcomes, individual and pooled statistics were calculated as weighted mean differences (WMD) and 95% confidence intervals (CIs) using a fixed effect model. For dichotomous variables, individual and pooled statistics were calculated as relative risks (RR) with 95% CIs; again, a fixed effect model was used.
Subgroup analysis and investigation of heterogeneity
Three specific subgroups were planned a priori: (a) patient age (adults vs. children); (b) acute asthma severity (severe vs. not severe); and (c) type of continuous nebuliser (commercially available model vs. intermittent nebuliser with infusion pump). A two‐sided p‐value < 0.05 was considered statistically significant.
Results
Description of studies
Results of the search
The original search in 1999 revealed 165 abstracts from trials; all were reviewed and 26 potentially relevant studies were identified (kappa = 1.0). In addition, from the CCTR search, 42 abstracts, and 10 potentially relevant studies were identified (kappa = 0.94). In 2002 an updated search revealed two additional studies (Besbes‐Ouanes 2000; Innes 2002). One excluded study has been added to the review from searches between 2002 and 2009. The current systematic review includes Register search updates to February 2011. The 2011 update search returned a potentially eligible abstract and we await publication in full (Rose 2010).
Included studies
All studies were published after 1990; six were from centres in the United States, one each was from Canada and Tunisia. All studies were convenience or consecutive patients from a single centre. Overall, the studies enrolled small samples (range: 22‐170 patients) with all but 2 under 100 patients.
Populations: One study enrolled children (Khine 1996), one enrolled a mix of adults and children (Reisner 1995), and 6 enrolled adults only (Colacone 1990; Lin 1993; Rudnitsky 1993; Shrestha 1996; Besbes‐Ouanes 2000; Innes 2002). The populations varied from patients with mild‐moderate acute asthma (n = 3), to only those with "severe" attacks only (n = 5). Examination of the definitions used to designate the severe group reveals that patients had a combination of clinical findings, airflow measurements, or response to therapy that placed them in the severe category. The unadjusted admission proportion in the placebo group was 40% in the severe group vs. 11% in the mild‐moderate group, which serves to partially validate this subgroup designation.
Interventions: Continuous inhaled beta‐agonist treatment was administered early in the course of the ED treatment. One study used a small volume nebuliser and by increasing the amount of normal saline lengthened the nebulisation to 20 minutes, thereby creating back‐to‐back treatments and an effectively continuous nebulisation under these research conditions. The dosage of beta‐agonist varied across studies; however, the dose of continuous beta‐agonist (CBA) and intermittent beta‐agonists administered were equivalent over the course of the studies. The dose in adults ranged from 5 mg to 30 mg and was generally administered over 120 minutes. In the sole pediatric study, children received 15 mg (Khine 1996). The studies lasted from 110 minutes (Lin 1993) to 6 hours (Besbes‐Ouanes 2000).
Co‐interventions: Co‐interventions were reported in all studies. Theophylline administration was not permitted (n = 3) or not reported (n = 3) in most studies. Corticosteroids were routinely administered to all patients in most studies (n = 6; Besbes‐Ouanes 2000; Innes 2002; Reisner 1995; Khine 1996; Shrestha 1996), and at the discretion of physicians in older studies (n = 2; Colacone 1990; Lin 1993). Supplemental oxygen was routinely administered in two studies (Besbes‐Ouanes 2000; Shrestha 1996). Ipratropium bromide was administered at the discretion of the treating physician in one study (Besbes‐Ouanes 2000).
Outcomes: Outcomes were determined at variable times, but usually included a variety of pulmonary function results and admission to hospital or discharge to home. Short‐term follow‐up (up to 6 hours) was provided in all studies and at intermediate times (up to 24 hours) in several (Innes 2002; ) to determine the rate of relapse to additional care. However, the variability of treatment approaches following discharge makes comparisons problematic after discharge. Adverse effects and vital signs were reported frequently enough to permit pooling. There were limited alternative outcomes such as symptom scores and quality of life measure; no economic endpoints were reported.
Excluded studies
Risk of bias in included studies
A full manuscript was available from each study on which to base quality assessments. Overall, the methodological quality of the included studies was rated as poor. Randomisation was poorly described in most studies. No studies were double‐blind and only two were single‐blind (Besbes‐Ouanes 2000; Reisner 1995); all were controlled. However, few reported clearly on concealment of allocation, and many reported an insufficient number of outcomes. Four studies were rated as "strong" using the Jadad (scores = 3‐5) method (Lin 1993; Reisner 1995; Rudnitsky 1993; Besbes‐Ouanes 2000), and 4 were rated as "weak" (Colacone 1990; Khine 1996; Shrestha 1996; Innes 2002). Using the Cochrane methodology, 2 studies were rated as blinded allocation (Innes 2002; Besbes‐Ouanes 2000) while 5 studies were rated as having unclear allocation (Colacone 1990; Lin 1993; Reisner 1995; Rudnitsky 1993; Shrestha 1996). In one study, allocation was clearly unblinded (Khine 1996).
Effects of interventions
Results from this meta‐analysis are reported by outcome. The main results are reported as overall effects of continuous vs. intermittent inhaled beta‐agonist. In addition, the main subgroup based on asthma severity is also reported.
ED Pulmonary Function Results
A variety of short‐term pulmonary function tests were reported in the included trials. Most commonly, PEFR and FEV‐1 were reported at the completion of the trial (or as close to 6 hours as possible). Patients receiving CBA demonstrated significant improvements in PEFR (SMD = 0.33; 95% CI: 0.1 to 0.5, Figure 1), absolute FEV‐1 (WMD = 0.37; 95% CI: 0.1 to 0.6), and % predicted FEV‐1 (WMD = 0.28; 95% CI: 0.03 to 0.5) when all studies were pooled (Figure 2). No statistically significant heterogeneity was identified for the results when all studies were pooled using PEFR (P= 0.57), FEV‐1 (P = 0.61) or % predicted FEV‐1 (P = 0.45). Insufficient information was available to dissect these data to examine response based on severity of disease at presentation.
1.
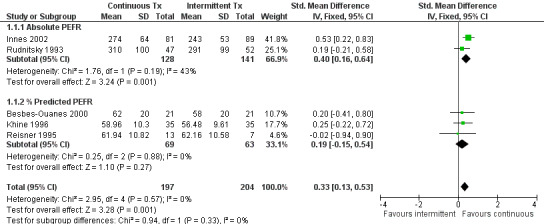
Absolute PEFR values (end of study)
2.
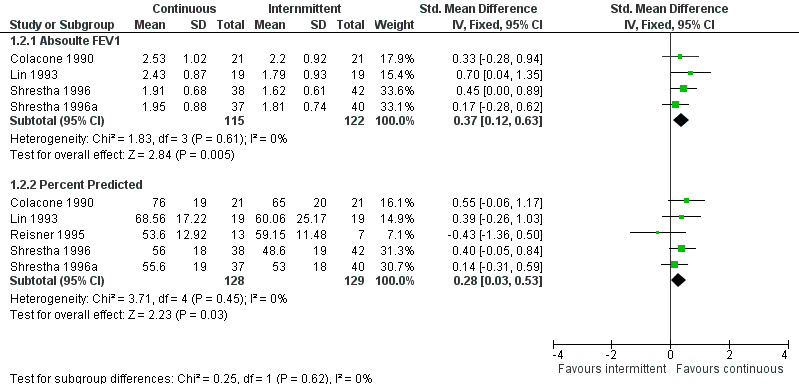
Absolute FEV1 values (end of study)
Time course of ED Pulmonary Function Improvements
Sufficient information was available to examine the time course for the changes observed at the end of the trial period reported above. In the first 60 minutes, differences between CBA and intermittent treatment were not obvious in PEFR (SMD: 0.14; 95% CI: ‐0.05 to 0.3) and were small when FEV‐1 was reported (SMD: 0.30; 95% CI: 0.05 to 0.5). By 2‐3 hours, the difference was significant in both measures, favouring treatment with CBA (PEFR SMD: 0.37; 95% CI: 0.2 to 0.6; FEV‐1 SMD: 0.33; 95% CI: 0.1 to 0.6). There were a limited number of studies (n = 3) which reported times beyond 4 hours, making results from this group less reliable. Nonetheless, the PEFR and FEV‐1 SMDs were insignificant at 4‐6 hours.
Admission to Hospital
A significant difference was identified between patients treated with CBA vs. intermittent beta‐agonist with respect to hospital admission at the end of the study period (RR: 0.68; 95% CI: 0.5 to 0.9). This result was similar if they were displayed as a odds ratio (OR: 0.57; 95% CI: 0.4 to 0.9) or number needed to treat (NNT: 10; 95% CI: 6, 34). This pooled result demonstrated no significant heterogeneity (chi square = 5.6; df = 7; P = 0.58). There was no apparent difference in hospitalisation for the studies where participants had mild‐moderate asthma (RR: 1.12; 95% CI: 0.4 to 2.9); however, the confidence intervals are wide and the sample is small. Patients with severe asthma had fewer hospital admissions when treated with CBA (RR: 0.64; 95% CI: 0.5 to 0.9). This pooled result did not demonstrate significant heterogeneity (chi square = 2.95; df = 4; P = 0.56).
Tolerability
Vital signs were recorded and reported in many of the included studies; pooled results did not demonstrate statistical or clinical heterogeneity. Continuous treatment was generally well tolerated, with no clinically important differences observed in pulse rate (WMD: ‐2.87; 95% CI: ‐6.1 to 0.3) or respiratory rate (WMD: 1.00; 95% CI: ‐1.6 to 3.6) between the treatment groups. Systolic blood pressure was similar in both groups (WMD: ‐1.75; 95% CI: ‐6.6 to 2.1); however, this result demonstrated heterogeneity and is reported using a random effects model. Overall, vital signs remained "stable" during the period immediately after treatment with CBA.
Side Effects
Some side effect monitoring was common in these studies, but comprehensive lists of adverse events were rarely reported. CBA appears to be a safe treatment approach for acute asthma; general adverse events were similarly rare across multiple trials (WMD = 0.2; 95% CI: 0.03 to 1.6). Four trials (n = 307 patients) reported tremor as a specific side effect; pooled results failed to demonstrate differences between treatment groups (RR: 0.81; 95% CI: 0.5 to 1.3). Nausea and palpitations were reported in only one trial each; however, in both cases, these side effects were more common in the intermittent beta‐agonists group than the CBA group. Potassium concentrations were reported in 3 trials and pooled results failed to demonstrate differences between treatment groups (WMD: 0.02; 95% CI: ‐0.2 to 0.2). However, an insufficient number of studies were available to provide meaningful sensitivity or sub‐group comparisons, or firm conclusions about side effects or adverse events.
Subgroup/Sensitivity Analyses
Due to an insufficient number of paediatric trials, a subgroup analysis based on age was not possible. The random (RE) and fixed effects (FE) models did not affect reported results; especially for the admission data where sufficient trials reported this outcome (FE RR: 0.68; 95% CI: 0.5 to 0.9 vs RE RR: 0.69; 95% CI: 0.5 to 0.9). Higher severity at initial presentation appeared to increase the response to CBA (RR: 0.64; 95% CI: 0.5 to 0.9). Finally, study quality was not found to influence pooled results for admissions. With all trials included in the analysis the OR = 0.57 (95% CI: 0.34 to 0.9) compared to OR = 0.55 (95% CI: 0.4 to 0.9) when the weakest trials were excluded.
Discussion
This systematic review examined the use of continuous nebulised beta‐agonists in the ED management of acute asthma. The pooled results demonstrate a modest (effect size = 0.3), yet statistically significant beneficial effect of CBA in terms of pulmonary functions by 2‐3 hours of therapy. The clinical significance of the magnitude of this pulmonary function improvement is difficult to determine, since the minimally clinically important difference for pulmonary functions in acute asthma have been infrequently studied. However, in chronic asthma an improvement of 12% predicted has been used (NAEPP 1997) and for acute studies, some have suggested an increase in PEFR of 30 L/min is clinically important (Tiffany 1993). The improvement of approximately 10% predicted FEV‐1 or 50 L/min PEFR represent what we believe are important PF changes, especially considering the severity at the start of therapy. Moreover, these PFT changes appear important enough to reduce admissions. The effect of CBA can be restated using NNT as follows: 10 patients would have to receive CBA to prevent 1 admission to hospital, compared to intermittent beta‐agonist nebulisation.
While the CBA delivery was well tolerated, with good efficacy in patients with severe asthma, its possible expense compared to other therapies and its more complex nature argues against its indiscriminate use in the emergency department treatment of acute asthma. Despite the lack of cost data, changing treatment approaches across EDs for all patients would not be appropriate; however, there is considerable evidence to suggest that the sub‐group of patients presenting with severe acute asthma respond differently to the use of CBA. Patients who presented with severe asthma benefit from the use of CBA, both in terms of pulmonary functions and admission rates.
LIMITATIONS
There is a possibility of publication bias in this meta‐analysis. For example, by missing unpublished negative trials we may be over‐estimating the efficacy of CBA treatment. However, a comprehensive search of the published literature for potentially relevant studies was conducted, using a systematic strategy to avoid bias. This was followed by attempts to contact corresponding and first authors. No unpublished trial was identified; however, several negative trials were uncovered. While we recognise that more of these types of trials may exist, the funnel plot for admission provide some additional reassurance that publication bias was not a threat to the conclusions reported here.
There is also a possibility of study selection bias. However, we employed two independent reviewers, and feel confident that the studies excluded were done so for consistent and appropriate reasons. Our search was comprehensive and has been updated, so it is unlikely that there are any trials in publication which were missed.
Finally, these results are discordant from the results presented in a published systematic review (Rodrigo 2002). These authors claimed equivalence of CBA and such a claim is difficult to understand given the wide confidence intervals and small number of trials they presented. Such evidence does not demonstrate "equivalence"; rather, it supports the conclusion of a "lack of evidence of benefit" for the treatment. Our systematic review included more studies (8 vs 6) and this may explain the benefit demonstrated here with respect to admissions (RR: 0.69; 95% CI: 0.52 to 0.93). This result is compared to the reported "equivalence" (RR = 0.68; 95% CI: 0.33 to 1.38) by the earlier review (Rodrigo 2002).
Authors' conclusions
Implications for practice.
Most patients who present for assessment and treatment with an asthmatic exacerbation do not benefit from early treatment with CBA compared to intermittent beta‐agonists in the emergency department.
In patients with "severe" acute asthma, benefits (e.g. pulmonary function improvements, reduced admissions) from treatment with CBA were observed. A practical clinical approach may be to identify those patients who fail to respond to initial beta‐agonists or who have severe airflow limitations in the ED as being candidates for CBA therapy.
In addition to the CBA intervention, standard acute asthma therapy must be administered to these patients early in the ED treatment (including corticosteroids, oxygen, ipratropium bromide, etc.).
In this review, CBA nebulisation was provided as 5‐15 mg over the first hour in adults, with lower doses in children.
Only 1 study was identified which examined the use of CBA in children. Given the small numbers involved in this study, readers should be cautious when extrapolating results to children, especially the very young.
Implications for research.
Many questions regarding the treatment of acute asthma with CBA remain unanswered.
Additional research is required to determine the optimal dose (high vs low) and duration of therapy.
Additional studies are needed to confirm the sub‐group findings from this review suggesting a beneficial effect of CBA in severe acute asthma. In future studies, severity must be clearly defined and based on presenting pulmonary function results AND response to initial beta‐agonist therapy whenever possible.
Studies involving very young children need to be performed to determine the effect of CBA in this age group.
Future research should examine the effect of CBA while maximising the use of known effective co‐intervention therapies in acute asthma (e.g., systemic corticosteroids, inhaled corticosteroids, IV MgSO4, ipratropium bromide, etc). The effect of CBA treatment may differ based on administration of these therapies.
Future research on acute asthma must concentrate on well defined outcomes which may lead to more informative reviews in the future. More specifically, criteria for admission/discharge and reporting of pulmonary function data in a systematic fashion would assist in further work. Finally, better description of the methodology would also be beneficial.
What's new
| Date | Event | Description |
|---|---|---|
| 9 March 2011 | New search has been performed | New literature search run. One abstract added to 'studies awaiting classification' (Rose 2010). |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 21 May 2009 | Amended | Change of contact details for primary author |
| 17 February 2009 | New search has been performed | Literature search run: no new studies identified. |
| 23 July 2008 | Amended | Converted to new review format. |
| 1 August 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to gratefully acknowledge the assistance provided by the members of the Cochrane Airways Group who helped with the protocol development, literature searches, and obtaining the studies (Steve Milan, Toby Lasserson and Karen Blackhall). We are grateful to Ms. Calacone for responding to our questions. Finally, we would also like to gratefully acknowledge the assistance of Dr. Chris Cates (ARG Technical and Criticism Editor) and Dr Paul Jones (ARG Coordinating Editor).
Data and analyses
Comparison 1. Continuous vs intermittent nebulisation (end of study).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 PEFR values (end of study) | 5 | 401 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.13, 0.53] |
| 1.1 Absolute PEFR | 2 | 269 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.40 [0.16, 0.64] |
| 1.2 % Predicted PEFR | 3 | 132 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.19 [‐0.15, 0.54] |
| 2 FEV1 values (end of study) | 5 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Absoulte FEV1 | 4 | 237 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.37 [0.12, 0.63] |
| 2.2 Percent Predicted | 5 | 257 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.28 [0.03, 0.53] |
| 3 Admission to hospital (end of observation period) | 6 | 461 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.51, 0.92] |
| 3.1 Moderate‐severe | 5 | 341 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.47, 0.87] |
| 3.2 Less severe | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.44, 2.85] |
| 4 Adverse effects | 4 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.03, 1.55] |
| 5 ED treatment time | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6 Respiratory therapist time | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Symptom scores | 2 | 112 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [0.05, 0.81] |
| 8 Potassium concentration | 3 | 206 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.16, 0.19] |
| 9 Pulse rate | 5 | 373 | Mean Difference (IV, Fixed, 95% CI) | ‐2.87 [‐6.07, 0.34] |
| 10 Respiratory rate (end of study) | 2 | 62 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐1.55, 3.55] |
| 11 Blood pressure (end of study) | 2 | 212 | Mean Difference (IV, Fixed, 95% CI) | ‐1.75 [‐5.55, 2.05] |
| 12 Tremor | 5 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.51, 1.28] |
| 13 Palpitations | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14 Nausea/vomiting | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
1.1. Analysis.
Comparison 1 Continuous vs intermittent nebulisation (end of study), Outcome 1 PEFR values (end of study).
1.2. Analysis.
Comparison 1 Continuous vs intermittent nebulisation (end of study), Outcome 2 FEV1 values (end of study).
1.3. Analysis.
Comparison 1 Continuous vs intermittent nebulisation (end of study), Outcome 3 Admission to hospital (end of observation period).
1.4. Analysis.
Comparison 1 Continuous vs intermittent nebulisation (end of study), Outcome 4 Adverse effects.
1.5. Analysis.
Comparison 1 Continuous vs intermittent nebulisation (end of study), Outcome 5 ED treatment time.
1.6. Analysis.
Comparison 1 Continuous vs intermittent nebulisation (end of study), Outcome 6 Respiratory therapist time.
1.7. Analysis.
Comparison 1 Continuous vs intermittent nebulisation (end of study), Outcome 7 Symptom scores.
1.8. Analysis.
Comparison 1 Continuous vs intermittent nebulisation (end of study), Outcome 8 Potassium concentration.
1.9. Analysis.
Comparison 1 Continuous vs intermittent nebulisation (end of study), Outcome 9 Pulse rate.
1.10. Analysis.
Comparison 1 Continuous vs intermittent nebulisation (end of study), Outcome 10 Respiratory rate (end of study).
1.11. Analysis.
Comparison 1 Continuous vs intermittent nebulisation (end of study), Outcome 11 Blood pressure (end of study).
1.12. Analysis.
Comparison 1 Continuous vs intermittent nebulisation (end of study), Outcome 12 Tremor.
1.13. Analysis.
Comparison 1 Continuous vs intermittent nebulisation (end of study), Outcome 13 Palpitations.
1.14. Analysis.
Comparison 1 Continuous vs intermittent nebulisation (end of study), Outcome 14 Nausea/vomiting.
Comparison 2. Continuous vs Intermittent PFT time line.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Early ( 1 hour or less) | 9 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 PEFR | 5 | 400 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.05, 0.34] |
| 1.2 FEV‐1 | 5 | 257 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.30 [0.05, 0.54] |
| 2 2‐3 hours | 9 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 PEFR | 5 | 400 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.37 [0.17, 0.57] |
| 2.2 FEV‐1 | 5 | 257 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.33 [0.08, 0.58] |
| 3 4‐6 hours | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 PEFR | 2 | 62 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.16 [‐0.34, 0.67] |
| 3.2 FEV‐1 | 1 | 20 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐1.31, 0.55] |
2.1. Analysis.
Comparison 2 Continuous vs Intermittent PFT time line, Outcome 1 Early ( 1 hour or less).
2.2. Analysis.
Comparison 2 Continuous vs Intermittent PFT time line, Outcome 2 2‐3 hours.
2.3. Analysis.
Comparison 2 Continuous vs Intermittent PFT time line, Outcome 3 4‐6 hours.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Besbes‐Ouanes 2000.
| Methods | Design: RCT. Method of randomisation: random numbers table, opaque envelopes. Blinding: patients and treating MDs blind to treatment. Objective: Safety & efficacy of continuous salbutamol in ED tx of acute adult asthma. Withdrawals: none described Baseline: groups similar at the start of the study. | |
| Participants | Location: one hospital ED, Tunisia. Recruitment: consecutive eligible pts. Sample size: 42 (CN=21, IN =21) Age: "adults" Gender: 60% Female. Inclusion: Dx asthma ‐ clinical definition; < 50% predicted PEFR plus 2 or more of: PR > 119/min, RR> 29/min, pulsus paradoxicus > 14, accessory muscle use, SaO2 < 92%, hypercapnia (>42 mm Hg). Exclusion: chronic cough, cardiac or hepatic disease, or pregnancy. Severity: moderate‐severe asthma. | |
| Interventions | Duration: 6 hrs. Intervention: Control: intermittent salbutamol 5mg q 20 minutes in 1st hour, then 2.5 mg hourly afterwards via pneumatic nebulization. Total given in 6 hrs = 27.5 mg salbutamol. CBA: salbutamol 15mg over 1st hour, repeated 2.5 mg/hr for 5 hours via continuous pneumatic nebulizer. Total given in 6 hrs = 27.5 mg salbutamol. Co‐intervention: 200 mg IV hydrocortisone q4 hrs and oxygen at 6 L/min for all pts. |
|
| Outcomes | Outcomes reported: Admission (to ICU or floor). Clinical: 5‐component scale. PEFR: at 0. 40 min, 1, 3 and 6 hours. Vital signs: PR, BP@ 60 & 120 min ADR: Potassium levels and ECG at 0 and 6 hrs. Arrhythmias (continuous monitoring) reported. | |
| Notes | Jadad Score: 3 Patients had to reach a set of criteria at 6 hours to achieve admission. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random numbers table |
| Allocation concealment? | Low risk | Opaque envelopes. |
Colacone 1990.
| Methods | Design: RCT. Method of randomisation: N/D Objective: Safety & efficacy of continuous albuterol in ED tx of acute adult asthma. Withdrawals: 4/46, described Baseline: BN group lower FEV1 values P=0.06) otherwise similar | |
| Participants | Location: General Hospital ED, Montreal, Canada. Recruitment: consecutive eligible pts. Sample size: 46 (CN=21, IN =21) Age: > 18 Gender: 22M, 20F Inclusion: Dx asthma ‐ ATS criteria, Exclusion: comorbidity: pneumonia, heart disease, diabetes, pregnant or nursing women Severity: not described | |
| Interventions | Duration: 2 hr. Intervention: Control: intermittent (BN)‐ albuterol 5.0mg (1.0mg diluted in 2.0mg saline) at time 0 & 1 hr. via facemask & Airlife‐Misty Neb., 100% O2 at 8L/min x 10‐15 min. Total given in 2 hrs = 10mg albuterol . CBA: albuterol 100mg (20ml) in 480 ml saline continuously via facemask & Jet neb, O2 5‐8 L/min. Total given in 2 hrs ˜ 10.07 +‐ 0.52mg albuterol . Co‐intervention: only IV aminophylline &/or corticosteroids allowed. |
|
| Outcomes | Outcomes reported: FEV1: absolute values 0‐120 min, % predicted 0‐120 min Vital signs: PR, RR, BP@30, 60, 90, 120 min ADR: Tremor | |
| Notes | Jadad Score: 2 Statistical issues: End of trial % predicted PFT calculated & adjusted for baseline differences. Used baseline SD Author contact: ADRs: none noted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not available |
| Allocation concealment? | Unclear risk | Information not available |
Innes 2002.
| Methods | Design: RCT. Method of randomisation: N/D Objective: Safety & efficacy of continuous albuterol in ED tx of acute adult asthma. Withdrawals: not well described Baseline: groups similar at the start of the study | |
| Participants | Location: Two Hospital EDs, Ipswich, UK. Recruitment: consecutive eligible pts. Sample size: 170 CN=81, IN =89 Age: 18‐64 Gender: 56% Female. Inclusion: Dx asthma ‐ clinical definition; < or = to 75% predicted PEFR. Exclusion: comorbidity: pregnancy, lung pathology, heart disease or arrhythmia, diabetes, status asthmaticus. Severity: not described | |
| Interventions | Duration: 2 hr. Intervention: Control: Oral prednisolone 40 mg; intermittent salbutamol 5.0mg (@ time 0) and 30 minutes) then q4 hourly nebulization. Total given in 2 hrs = 10 mg salbutamol. CBA: 60 mg oral prednisolone. Salbutamol 10mg over 1 hour, repeated over the second hour continuously via nebulizer. Total given in 2 hrs ˜ 20 mg albuterol . Co‐intervention: ipratropium bromide and IV beta‐agonists were allowed. |
|
| Outcomes | Outcomes reported: Admission, relapse if discharged, and length of stay is admitted. PEFR: change at 1 and 2 hours Vital signs: PR, BP@ 60 & 120 min ADR: Potassium levels and arrhythmias | |
| Notes | Jadad Score: 2 This study had an in‐patient component; however, we only examined the 2 hours data for this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised by blocking |
| Allocation concealment? | Low risk | 'sequential opaque sealed envelopes in which treatment allocation had been predetermined (...) by a statistician (EB) unconnected to the study.' |
Khine 1996.
| Methods | Design: RCT, SB (treating physician). Method of randomisation: block (poorly reported). Objective: Safety & efficacy of continuous albuterol in ED tx of acute asthma in children Withdrawals: 3/73 were excluded. Baseline groups similar. | |
| Participants | Location: Pediatric ED, Philadelphia, USA. Recruitment: children presenting to ED with mod ‐ severe asthma exacerbation. Sample size: 70 CN =35, IN =35 Age: ages 2‐18, mean = 7.6 y (SD 4). Gender: 52 M, 18 F Inclusion: At least 1 prior episode of wheezing Exclusion: hypersensitivity to albuterol, pre‐existing diagnosis with congenital heart disease, BPD, CF, sickle‐cell disease, possible foreign body aspiration, corticosteroid therapy in the past 6 hours, use of subQ epinephrine in preceding 20 min., concurrent stridor & wheeze, +ve RSV culture. Severity: clinical asthma score >=8 | |
| Interventions | All received corticosteroids at entry.
Intervention:
Control: Control: albuterol 0.15 mg/kg/dose (minimum of 2.5 mg/dose and a maximum of 7.5 mg/dose) q 30 min.
Total dose /hr over 2 hours equivalent in both groups.
Maximum of 2 hours of treatment and 30 minutes of observation. CBA: albuterol 0.3 mg/kg/hr (minimum 5 mg/hr, maximum 15mg/hr) achieved using facemask & HEART nebulizer Co‐intervention: oral or intravenous corticosteroid. O2 via neb if pulse symmetry showed hypoxemia. Theophylline allowed. |
|
| Outcomes | Outcomes reported: PEF: % mean change Admission to hospital: criteria ‐ persistent asthma score >=8 and/or oxygen saturation <94% on room air. Assessed independently by physician and investigator. Vital signs: PR, RR, BP. Mean increase ADR: tremor and vomiting Timing: time in ED | |
| Notes | Jadad Score: 2 Statistical issues: no baseline data to compute end of trial results Author contact: |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised by blocks. |
| Allocation concealment? | High risk | Single‐blind study; respiratory therapist administered treatment from behind curtain. |
Lin 1993.
| Methods | Design: RCT, Method of randomisation: Computer generated pseudo‐random numbers. Blinding: non‐blinded. Objective: Safety & efficacy of continuous vs intermittent albuterol in ED tx of acute adult asthma exacerbation. Withdrawals: none Baseline groups similar. | |
| Participants | Location: Public hospital ED, New York, USA. Recruitment: volunteers Sample size: 38 CN=19, IN=19 Age: 40.2 (SD 13.7) Gender: 20M, 18F Inclusion: Dx of asthma by ATS criteria, smoked < 20 pack yr of cigarettes, >=18yr. Severity: males with PEFR <300 L/min., females with PEFRs <250 L/min, pulse rate <=180 per min,BP <= 180/100 mm mercury. Exclusion: Symptomatic angina pectorus or atherosclerotic heart disease, pregnancy, | |
| Interventions | Intervention:
Control: albuterol 5mg/ml (1ml in 2 ml saline) over 10 min. q 20 min. x 6 tx periods via facemask & standard acorn‐type jet neb. Delivered by pressurized O2 at 6‐8 L/min. CBA: albuterol 5MG/ML (6 ml in 34 ml saline) via facemask & HEART system. Delivered by pressurized O2 at 10 L/min. Both groups received 30 mg of albuterol over 110 min. Co‐intervention: Corticosteroids allowed. Aminophylline not allowed. |
|
| Outcomes | Outcomes reported: FEV1: % predicted, absolute values 0 ‐110 min Admission to hospital: no criteria applied. Vital signs: PR. ADRs: tremor, palpitations, agitation, headache. | |
| Notes | Jadad Score: 3 Statistical issues: 1 pt in CN recommended for admission but refused. Included as an admission. Author contact: Correspondence pending from the author. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated pseudo‐random numbers |
| Allocation concealment? | Unclear risk | Information not available |
Reisner 1995.
| Methods | Design: RCT, SB (MD reading spirometry & ECG tracings) Method of randomisation: N/D Objective: Safety & efficacy of high dose CN neb albuterol vs high dose IN neb albuterol over 4 hrs Withdrawals: 2/22. 1 pt from each group. | |
| Participants | Location: ED Danbury, Conneticut, USA. Recruitment: adult asthmatics with acute asthma Sample size: 22 CN=14, IN=8 Age: mean 34.5 y (SD 15) Gender: 5M, 15F Inclusion: between 10‐65 yr., PEFR less than 60% predicted. Exclusions: Fever > 100 F, >10‐pack year history of smoking. Pregnant or nursing mothers, women in child‐bearing age. Intolerance to beta‐agonists: co‐morbid renal, cardiovascular, endocrine, hepatic, metabolic, neurologic or systemic disease prior to participation in this study. | |
| Interventions | Intervention:
Control: 2.5 mg albuterol at time zero then q 20 min. x 4 hrs. Total= 30 mg in 12 Tx periods. The solution was administered via an Airlife Misty Nebulizer driven by 100% at a flow rate of 8 L/min. CBA: 30 mg albuterol over 4 hours using a Travenol volumetric infusion pump. Nebulizer was driven by 100% oxygen at 8 L/ min. Co‐intervention: All received intravenous 125 mg of methyl‐ prednisolone on start of the study. No aminophylline. |
|
| Outcomes | Outcomes reported: PEF, FEV‐1: % change 0‐240 min. Vital signs: PR, RR, BP @ 240 min. | |
| Notes | Jadad Score: 3 Statistical issues: Calculated end of trial result % predicted from % change scores. Used baseline SD. 1 pt (IN) recommended for admission but refused. Included as an admission. Author contact: Pending author response. ADR: 1 pt had abdominal pain, Tx group not specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; no other information available |
| Allocation concealment? | Unclear risk | Information not available |
Rudnitsky 1993.
| Methods | Design: RCT, non‐blinded Method of randomisation: block in groups of 10 Objective: Safety & efficacy of continuous vs intermittent albuterol in ED tx of acute adult asthma exacerbation. Withdrawals: 8. 4= >1 enrolment, 4 lost to follow‐up. | |
| Participants | Location: ED Philadelphia, USA. Recruitment: Consecutive eligible pts. Sample size: 99. 47= CN, 52= IN. Age: mean 35.5 y (SD14.5) Gender: 30M, 69F Inclusion: Adults with an acute exacerbation of asthma (ATS criteria), & a non‐response to one tx of 2.5 mg neb albuterol Exclusion: COPD, T > 38.3C, evidence of pneumonia, heart failure, renal failure, pregnancy. Severity: no severity rating. | |
| Interventions | Intervention:
Control: 2.5 mg albuterol in 3ml saline via neb at time zero, 30, 60, 90 and 120 min. CBA: 10 mg albuterol in 70 ml saline via Vortran Heart Nebulizer Co‐intervention: All received 2.5 mg neb albuterol and 125 mg of IV methylprednisolone. No other meds allowed. |
|
| Outcomes | Outcomes reported: Clinical Scoring: official index at 120 minutes. Hospital Admission PEFR: absolute & SD @ 0, 30, 60, 90, 120 min. Vital signs: PR | |
| Notes | Jadad Score: 3 Statistical issues: none Author contact: Authors response still pending. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised by blocking groups of 10 |
| Allocation concealment? | Unclear risk | Information not available |
Rudnitsky 1993a.
| Methods | Results from severe cases | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | As for Rudnitsky 1993 |
| Allocation concealment? | Unclear risk | As for Rudnitsky 1993 |
Shrestha 1996.
| Methods | Design: RCT, DB Method of randomisation: N/D Objective: Safety & efficacy of high & low dose albuterol via CN & IN. Withdrawals: none mentioned Standard values | |
| Participants | Location: ED, U of Texas Medical Centre. USA Recruitment: Sample size: 157. CN: hi‐dose =37, Std‐dose =38, IN: hi‐dose = 40, std‐dose = 42 Age: mean 34.4 (SD 10.6) Gender: 77 M, 80 F Inclusion: Dx acute asthma: FEV1 < 40% predicted, Dx < 45 yrs.old, age =>=18, Exclusion: not pregnant, nursing or incarcerated, no Hx of allergy to albuterol, English speaking. | |
| Interventions | 4 groups Tx x 4 hr.
Intervention:
Control: hi‐dose: 7.5 mg q 1h (1.5 ml in 1.5 ml saline) @ 6 L/min.
Control standard: 2.5 mg q 1h (0.5 ml in 2.5 ml saline). CBA: hi‐dose: 7.5 mg/h albuterol (1.5 ml in 13.5 ml saline) CBA standard: 2.5 mg/h (0.5 ml in 14.5 ml saline) via Airlife Misty‐neb @ 6L/min Co‐intervention: O2 if O2 sat < 92% |
|
| Outcomes | Outcomes reported: FEV1: absolute, % predicted, % change @ 1 & 2 hr. | |
| Notes | Jadad Score: 2 Statistical issues: Author contact: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Described as randomised; other information not available |
| Allocation concealment? | Unclear risk | Information not available |
Shrestha 1996a.
| Methods | See Shrestha 1996 High dose‐values | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | As for Shrestha 1996 |
| Allocation concealment? | Unclear risk | As for Shrestha 1996 |
RCT = Randomised Control trial DB = double blind neb=nebuliser or nebulisation N/D = not described IN=intermittent nebulisation CBA=continuous nebulisation ADR = adverse reactions PEF =Peak expiry flow FEV1= forced expiratory flow in one second
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Andrews 2007 | Comparison of continuous racemic versus continuous levalbuterol. |
| Levitt 1995 | Continuous nebulization vs continuous metered dose inhaler with spacer in acute dyspnea. Mixed patient groups of COPD and asthma. |
| Moler 1988 | Case series; not a randomized controlled trial and has no control group. |
| Moler 1995 | In‐patient study of children with continuous agents delivered over 8 hours and more. |
| Nelson 1990 | No continuous arm of the trial; comparison of single to multiple doses. |
| Oshlaker 1993 | No control group; not a randomized controlled trial. |
| Papo 1993 | In‐patient study of pediatric patients admitted to the intensive care unit. |
| Portney 1988 | Case series; not a randomized controlled trial and has no controls. |
| Portney 1992 | Review article. |
| Schuh 1989 | High vs low dose "frequently" administered albuterol (not "continuous" vs intermittent) |
| Stein 2003 | Both groups received continuous nebulization. |
| Weber 1999 | Continuous nebulization of albuterol plus ipratropium bromide compared to albuterol alone. |
COPD = chronic obstructive pulmonary disease;
Contributions of authors
Camargo CA Jr: Protocol development, study selection, analyses and interpretation of data, and write‐up. Spooner CH: data checking and entry, quality scoring, conversion to RevMan 4.1. Rowe BH: Study selection, quality scoring, data extraction and entry, editing of manuscript, assigned editor.
Sources of support
Internal sources
Division of Emergency Medicine, University of Alberta, Edmonton, Canada.
External sources
National Institute of Health (CAC; NIH HL ‐ 03533), Bethesda, USA.
Canada Institute of Health Research (BHR), Ottawa, Canada.
Garfield Weston Foundation, UK.
Declarations of interest
The authors who have been involved in this review have done so without any known conflicts of interest. They are neither involved with the primary studies nor affiliated with any pharmaceutical company that produces any of the continuous nebulizers.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Besbes‐Ouanes 2000 {published data only}
- Besbes‐Ouanes L, Nouira S, Elatrous S, Knani J, Boussarsar M, Abroug F. Continuous versus intermittent nebulization of salbutamol in acute severe asthma: a randomized, controlled trial. Annals of Emergency Medicine 2000;36(3):236‐8. [DOI] [PubMed] [Google Scholar]
Colacone 1990 {published data only}
- Colacone A, Wolkove N, Stern E, Afilalo M, Rosenthal TM, Kreisman H. Continuous nebulization of albuterol (salbutamol) in acute asthma. Chest 1990;97(3):693‐7. [DOI] [PubMed] [Google Scholar]
Innes 2002 {published data only}
- Innes NJ, Stocking JA, Daynes TJ, Harrison BD. Randomised pragmatic comparison of UK and US treatments of acute asthma presenting to the hospital. Thorax 2002;57(12):1040‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khine 1996 {published data only}
- Khine H, Fuchs SM, Saville AL. Continuous vs intermittent nebulized albuterol for emergency management of asthma. Academic Emergeny Medicine 1996;3(11):1019‐24. [DOI] [PubMed] [Google Scholar]
Lin 1993 {published data only}
- Lin RY, Sauter D, Newman T, Sirleaf J, Walters J, Tavakol M. Continuous versus intermittent albuterol nebulization in the treatment of acute asthma. Annals of Emergency Medicine 1993;22(12):1847‐53. [DOI] [PubMed] [Google Scholar]
Reisner 1995 {published data only}
- Reisner C, Kotch A, Dworkin G. Continuous versus frequent intermittent nebulization of albuterol in acute asthma: a randomized, prospective study. Annals of Allergy Asthma and Immunology 1995;75:41‐7. [PubMed] [Google Scholar]
Rudnitsky 1993 {published data only}
- Rudnitsky GS, Eberlein RS, Schoffstall JM, Mazur JE, Spivey WH. Comparison of intermittent and continuously nebulized albuterol for treatment of asthma in an urban emergency department. Annals of Emergency Medicine 1993;22(12):1842‐6. [DOI] [PubMed] [Google Scholar]
Rudnitsky 1993a {published data only}
- Rudnitsky GS, Eberlein RS, Schoffstall JM, Mazur JE, Spivey WH. Comparison of intermittent and continuously nebulized albuterol for treatment of asthma in an urban emergency department. Annals of Emergency Medicine 1993;22(12):1842‐6. [DOI] [PubMed] [Google Scholar]
Shrestha 1996 {published data only}
- Shrestha M, Bidadi K, Gourlay S, Hayes J. Continuous vs intermittent albuterol, at high and low doses, in the treatment of severe acute asthma in adults. Chest 1996;110(1):42‐7. [DOI] [PubMed] [Google Scholar]
Shrestha 1996a {published data only}
- Shrestha M, Bidadi K, Gourlay S, Hayes J. Continuous vs intermittent albuterol, at high and low doses, in the treatment of severe acute asthma in adults. Chest 1996;110(1):42‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Andrews 2007 {published data only}
- Andrews T, McGintee E, Tyler L, Mittal M, Chew A, Pawlowski N, Zorc J. Efficacy of high dose continuous nebulized levalbuterol for pediatric status asthmaticus [Abstract 956]. Journal of Allergy and Clinical Immunology 2007;119(1 Suppl):S244. [Google Scholar]
Levitt 1995 {published data only}
- Levitt MA, Gambrioli EF, Fink JB. Comparative trial of continuous nebulization versus metered‐dose inhaler in the treatment of acute bronchospasm. Annals of Emergency Medicine 1995;26(3):273‐7. [DOI] [PubMed] [Google Scholar]
Moler 1988 {published data only}
- Moler FW, Hurwitz ME, Custer JR. Improvement in clinical asthma score and PaCO2 in children with severe asthma treated with continuously nebulized terbutaline. Journal of Allergy & Clinical Immunology 1988;81:1101‐9. [DOI] [PubMed] [Google Scholar]
Moler 1995 {published data only}
- Moler FW, Johnson CE, Laanen CV, Palmisano JM, Nasr SZ, Akingbola O. Continuous versus intermittent nebulized terbutaline: plasma levels and effects. American Journal of Respiratory and Critical Care Medicine 1995;151:602‐6. [DOI] [PubMed] [Google Scholar]
Nelson 1990 {published data only}
- Nelson MS, Hofstadter A, Parker J, Hargis C. Frequency of inhaled metaproterenol in the treatment of acute asthma exacerbation. Annals of Emergency Medicine 1990;19:21‐5. [DOI] [PubMed] [Google Scholar]
Oshlaker 1993 {published data only}
- Olshaker J, Jerrard D, Barish RA, Brandt G, Hooper F. The efficacy and safety of a continuous albuterol protocol for the treatment of acute adult asthma attacks. American Journal of Emergency Medicine 1993;11:131‐3. [DOI] [PubMed] [Google Scholar]
Papo 1993 {published data only}
- Papo MC, Frank J, Thompson AE. A prospective, randomized study of continuous versus intermittent nebulized albuterol for severe status asthmaticus in children. Critical Care Medicine 1993;21:1479‐86. [DOI] [PubMed] [Google Scholar]
Portney 1988 {published data only}
- Portnoy J, Aggarwal J. Continuous terbutaline nebulization for the treatment of severe exacerbations of asthma in children. Annals of Allergy 1988;60:368‐71. [PubMed] [Google Scholar]
Portney 1992 {published data only}
- Portnoy J, Nadel G, Amado M, Willsie‐Ediger S. Continuous nebulization for status asthmaticus. Annals of Allergy 1992;69:71‐9. [PubMed] [Google Scholar]
Schuh 1989 {published data only}
- Schuh S, Parkin P, Rajan A, Canny G, Healy R, Rieder M, et al. High‐ versus low‐dose, frequently administered, nebulized albuterol in children with severe, acute asthma. Pediatric 1989;83(4):513‐8. [PubMed] [Google Scholar]
Stein 2003 {published data only}
- Stein J, Levitt MA. A randomized controlled double‐blind trial of usual‐dose versus high‐dose albuterol via continuous nebulization in patients with acute bronchospasm. Academic Emergency Medicine 2003;10:31‐6. [DOI] [PubMed] [Google Scholar]
Weber 1999 {published data only}
- Weber EJ, Levitt MA, Covington JK, Gambrioli E. Effect of continuously nebulized ipratropium bromide plus albuterol on emergency department length of stay and hospital admission rates in patients with acute bronchospasm. A randomized controlled trial. Chest 1999;115(4):937‐44. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Rose 2010 {published data only}
- Rose JA, Cancelliere S, Matye P, Nair S, O'Riordan M. Comparison of a breath‐actuated nebulizer versus a conventional continuous‐output nebulizer in treating acute asthma in a pediatric emergency department: an ongoing randomized controlled trial [Abstract]. AAP National Conference and Exhibition. 2010:11795.
Additional references
Boulet 1999
- Boulet LP, Becker A, Berube D, Beveridge RC, Ernst P, on behalf of the Canadian Asthma Consensus Group. Canadian asthma consensus report 1999. Canadian Medical Association Journal 1999;161:S1‐61. [PMC free article] [PubMed] [Google Scholar]
BTS 1997
- BTS. The British guidelines on asthma management: 1995 review and position statement. Thorax 1997;52:152‐6. [Google Scholar]
Camargo 1998
- Camargo CA Jr, on behalf of the MARC Investigators. Management of acute asthma in US emergency departments: The Multicenter Asthma Research Collaboration [abstract]. American Journal of Respiratory and Critical Care Medicine 1998;157(3 Suppl):A623. [Google Scholar]
Cates 2006
- Cates C, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta‐agonist treatment of acute asthma (Cochrane Review). Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD000052.pub2] [DOI] [PubMed] [Google Scholar]
GINA 2002
- NHLBI/WHO Workshop Report. Global Initiative on Asthma (GINA): Management and Prevention (available at: www.ginasthma.com). 2nd Edition. Washington: NIH Publication, 2002. [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Mannino 1998
- Mannino DM, Homa DM, Pertowski, CA, Ashizawa A, Nixon LL, Johnson CA, et al. Surveillance for asthma‐‐United States, 1960‐1995. Morbidity and Mortality Weekly Report 1998;47:1‐27. [PubMed] [Google Scholar]
NAEPP 1997
- National Asthma Education and Prevention Program. Guidelines for the diagnosis and management of asthma. Expert Panel Report II. Bethesda, Md: National Institutes of Health, 1997. [Google Scholar]
Rodrigo 2002
- Rodrigo G, Rodrigo C. Continuous vs. intermittent B‐agonists in the treatment of acute severe asthma: A systematic review with meta‐analysis. Chest 2002;122:160‐5. [DOI] [PubMed] [Google Scholar]
Rowe 1992
- Rowe BH, Keller JL, Oxman AD. Steroid use in the emergency department treatment of asthma exacerbations: A meta‐analysis. American Journal of Emergency Medicine 1992;10:301‐10. [DOI] [PubMed] [Google Scholar]
Rowe 2007
- Rowe BH, Spooner CH, Ducharme FM, Bretzlaff JA, Bota GW. Corticosteroids for preventing relapse following acute exacerbations of asthma (Cochrane Review). Cochrane Database of Systematic Reviews 2007, Issue 3. [Art. No.: CD000195. DOI: 10.1002/14651858.CD000195.pub2.] [DOI] [PubMed] [Google Scholar]
Shultz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Tiffany 1993
- Tiffany BR, Berk WA, Todd IK, White SR. Magnesium bolus or infusion fails to improve expiratory flow in acute asthma exacerbations. Chest 1993;104:831‐4. [DOI] [PubMed] [Google Scholar]
Travers 2001
- Travers A, Jones AP, Kelly K, Barker SJ, Camargo CA Jr, Rowe BH. Intravenous beta2‐agonists for acute asthma in the emergency department (Cochrane Review). Cochrane Database of Systematic Reviews 2001, Issue 1. [Art. No.: CD002988. DOI: 10.1002/14651858.CD002988.] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weiss 2001
- Weiss KB, Sullivan SD. The health economics of asthma and rhinitis. I. As economic impact. Journal of Allergy & Clinical Immunology 2001;107:3‐8. [DOI] [PubMed] [Google Scholar]


