Abstract
Endoplasmic reticulum (ER) stress is essential in the development of obesity, insulin resistance, and hepatosteatosis. In the latest issue of Cell Metabolism, Tirosh et al. (2020) demonstrate that intracellular ER stress can be transmitted to neighboring hepatocytes via connexin 43, thus propagating ER stress and promoting hepatosteatosis and insulin resistance.
Obesity and type 2 diabetes enhance metabolic burden. As an adaptive response, the unfolded protein response (UPR) stimulates endoplasmic reticulum (ER) chaperones and maintains proper protein biosynthesis. Prolonged metabolic stress causes unfolded/misfolded protein accumulation in the ER, leading to a maladaptive ER stress response. Such a maladaptive ER stress response has been implicated in the pathogenesis of metabolic disorder, including obesity, insulin resistance, and fatty liver (Ozcan et al., 2006).
Metabolic and inflammatory stress dynamically alter cellular communication with neighboring and distant cells, which can occur either as an adaptive or maladaptive response. Secreted bioactive molecules, such as hormones, chemokines, cytokines, and lipids, mediate cell-to-cell communication. Recently, exosomes and their biologically active cargo have been implicated in the pathogenesis of metabolic syndrome, neurodegenerative diseases, and cancer. Therefore, both secreted bioactive molecules and exosomes play an important role in mediating cell-to-cell and organ-to-organ communication. Additionally, gap junctions and hemichannels contribute to intercellular communication via direct cell-to-cell contact. Adjacent cells share cytoplasmic contents, including ions, second messengers, and small metabolites, through gap junction channels. ER stress is known to stimulate the secretion of bioactive molecules and exosomes, thus affecting neighboring and distant cells in a paracrine or endocrine manner (Dasgupta et al., 2020). In the latest issue of Cell Metabolism, Tirosh et al. have shown that a maladaptive response to over-nutrition propagates ER stress to neighboring cells via connexin 43 (Cx43)-mediated gap junctions, thereby promoting hepatosteatosis and insulin resistance (Figure 1).
Figure 1. Intercellular Transmission of ER Stress via Connexin 43 Promotes Fatty Liver and Insulin Resistance.
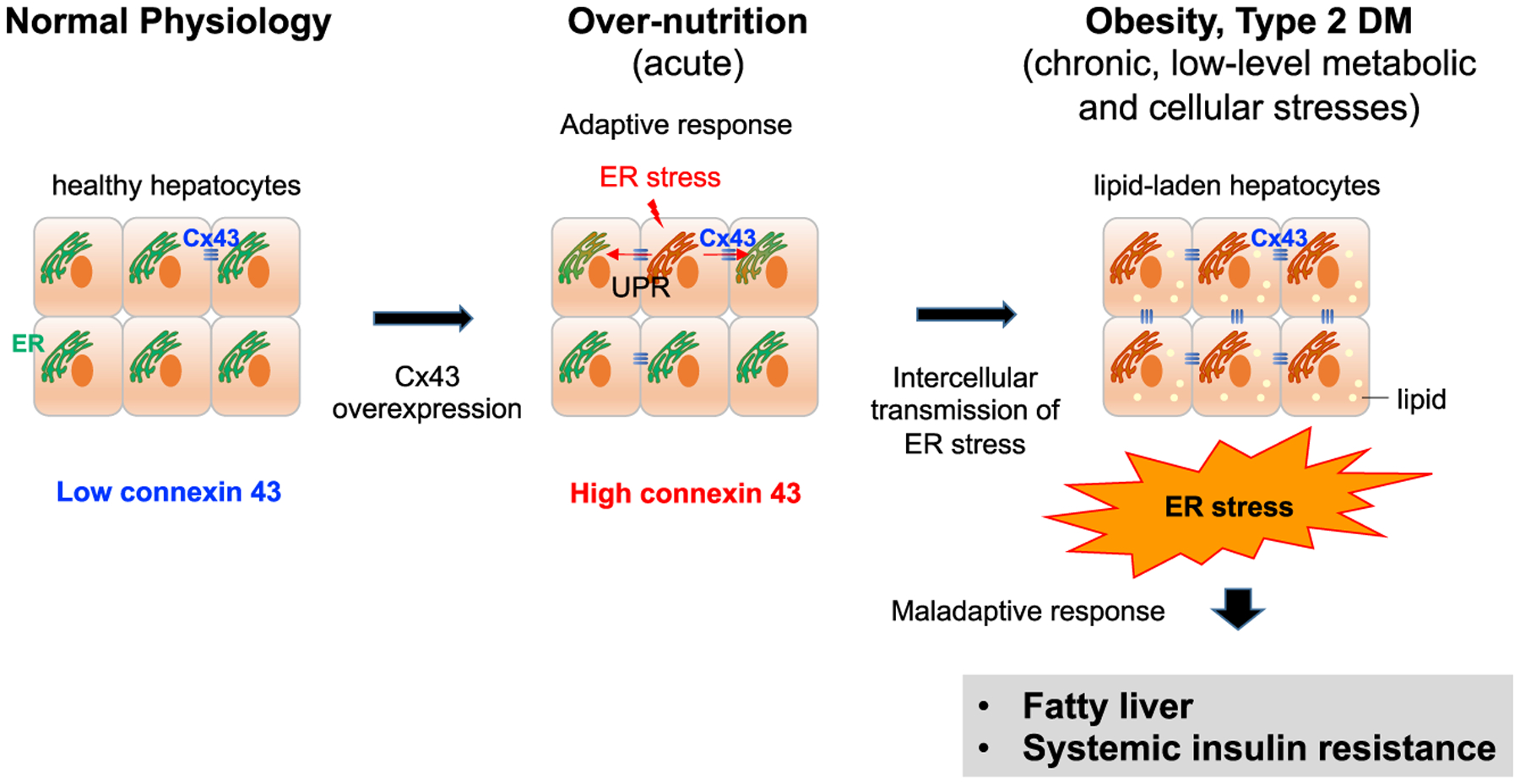
In normal hepatocytes, connexin 43 (Cx43) expression is low. Over-nutrition conditions increase the expression of hepatic Cx43 and ER stress. The unfolded protein response (UPR) can be transmitted to neighboring ER stress-naive cells via Cx43-mediated cell-to-cell contact. Although Cx43 plays a beneficial role under acute stress, intercellular propagation of ER stress via Cx43 promotes fatty liver and systemic insulin resistance under chronic metabolic stress.
Twelve connexin (Cx) proteins assemble to form gap junction channels, which are essential for direct cell-to-cell communication. The transmission of cellular contents between neighboring cells is most likely a protective adaptive response (Balasubramaniyan et al., 2013). Under pathological conditions, toxic substances, intracellular redox components, or signaling molecules can relay an inflammatory response to neighboring cells through these junctions. The Cx family consists of 21 human genes and mutation of these genes is associated with various human diseases, including sensorineural hearing loss and congenital cataracts (Laird et al., 2017).
Under physiological conditions, Cx32 and Cx26 are the primary isoforms expressed in hepatocytes. In contrast, Cx43 is not abundantly expressed in hepatocytes, but it is expressed in Kupffer cells, sinusoidal endothelial cells, hepatic stellate cells, and cholangiocytes (Hernández-Guerra et al., 2019). During cirrhosis and acute-on-chronic liver failure, enhanced hepatic Cx43 expression is associated with disease severity (Balasubramaniyan et al., 2013). Tirosh et al. have shown that a high-fat diet upregu lates hepatic Cx43 expression, but not Cx32 and Cx26 (Tirosh et al., 2020). Hepatocyte-specific Cx43 deletion ameliorates high-fat-induced hepatosteatosis and insulin resistance, whereas the opposite effect occurs with Cx43 overexpression. Furthermore, ER stress inducers, tunicamycin and thapsigargin, increase hepatic Cx43 expression. Although the mechanism of ER stress-induced Cx43 upregulation remains unclear, these findings suggest that Cx43 plays a role in the ER stress-mediated development of hepatosteatosis and insulin resistance.
The authors have found that transmission of the tunicamycin- or thapsigargin-induced UPR requires Cx43 and direct cell-to-cell contact and cannot be relayed to distant cells by conditioned medium, further supporting donor-recipient proximity as a prerequisite for Cx43-mediated ER stress transmission. ER stress induced by a chemical-free system is also transmitted intercellularly by Cx43. Subsequently, the authors have tried to determine whether the transmission of ER stress to neighboring cells causes either an adaptive or maladaptive response and have examined the aspects of ER function by monitoring the ER chaperone availability and ER folding capacity. Cx43-mediated intercellular transmission of ER stress results in decreased ER chaperone activity and ER folding capacity in recipient cells, indicating that ER stress signal transmission may be maladaptive. Consistently, hepatocyte-specific Cx43 deletion decreases hepatic ER stress, ER stress-induced hepatocyte-hepatocyte coupling, and intercellular transmission of ER stress, which mitigates hepatosteatosis and insulin resistance in mice. Thus, these findings confirm that Cx43 is responsible for the dissemination of deleterious signals under ER stress, which promote hepatosteatosis.
Interestingly, the diverse role of Cx43 depends on the context of liver diseases and whether the conditions are acute or chronic. In contrast to chronic ER stress, Cx43 plays a hepatoprotective role under acute ER stress. Others have demonstrated that Cx43-deficient mice show severe liver injury, inflammation, and oxidative stress due to acetaminophen overload compared with wild-type controls (Maes et al., 2016). These results support that Cx43 may be associated with an adaptive response during acute ER stress and liver injury. The regulatory mechanism of Cx43 in meditating beneficial adaptive and detrimental maladaptive responses remains unclear and further studies are warranted.
Cell-to-cell communication is particularly important for maintaining cellular and tissue homeostasis, which modulates the transmission of regulatory signals. Gap junction channels have a minimum width of 1.4 nm, allowing the intercellular exchange of molecules up to 1,000 Da in size, including ions, small metabolites, second messengers, microRNAs, and linear peptides (Bosco et al., 2011). In this context, one key issue is that the messenger of the Cx43-mediated ER stress remains unclear. In the study by Tirosh et al., quantitative, single-cell imaging of Ca2+ dynamics in ER-stressed donor and recipient cells has revealed that recipient cells show a similar intracellular Ca2+ concentration to that of the donor cell cytosol, and silencing of Cx43 in donor cells does not affect the intracellular Ca2+ status in recipient cells. Therefore, Ca2+ may act as a messenger of ER stress through Cx43 channels. However, it is possible that the increased Ca2+ levels in recipient cells adjacent to ER-stressed donor cells may be due to an indirect mechanism. The future application of single-cell level intracellular delivery of Ca2+ may fully elucidate the undefined conduit for Cx43-mediated intercellular communication (Yoon et al., 2020).
Overall, the authors have provided new evidence that an intracellular ER stress response can be transmitted to neighboring ER stress-naive cells via Cx43, which is overexpressed in response to over-nutrition and contributes to the pathogenesis of fatty liver and systemic insulin resistance. Because deletion of Cx43 in hepatocytes improves fatty liver and glucose metabolism under over-nutrition conditions, pharmacologically targeting intercellular communication using chemical-based inhibitors of connexin channels, neutralizing antibodies against Cx43, or peptide-based inhibitors may be potential therapeutic strategies for fatty liver and metabolic disorders (Willebrords et al., 2017).
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (R01DK085252 and P01CA233452) (E.S.) as well as a grant from the National Research Foundation of Korea (NRF) funded by the Korea government (MIST) (No. 2020R1C1C1004185) (Y.M.Y.).
REFERENCES
- Balasubramaniyan V, Dhar DK, Warner AE, Vivien Li WY, Amiri AF, Bright B, Mookerjee RP, Davies NA, Becker DL, and Jalan R (2013). Importance of connexin-43 based gap junction in cirrhosis and acute-on-chronic liver failure. J. Hepatol 58, 1194–1200. [DOI] [PubMed] [Google Scholar]
- Bosco D, Haefliger JA, and Meda P (2011). Connexins: key mediators of endocrine function. Physiol. Rev 91, 1393–1445. [DOI] [PubMed] [Google Scholar]
- Dasgupta D, Nakao Y, Mauer AS, Thompson JM, Sehrawat TS, Liao C-Y, Krishnan A, Lucien F, Guo Q, Liu M, et al. (2020). IRE1A stimulates hepatocyte-derived extracellular vesicles that promote inflammation in mice with steato-hepatitis. Gastroenterology 159, 1487–1503.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Guerra M, Hadjihambi A, and Jalan R (2019). Gap junctions in liver disease: implications for pathogenesis and therapy. J. Hepatol 70, 759–772. [DOI] [PubMed] [Google Scholar]
- Laird DW, Naus CC, and Lampe PD (2017). SnapShot: connexins and disease. Cell 170, 1260–1260.e1. [DOI] [PubMed] [Google Scholar]
- Maes M, McGill MR, da Silva TC, Abels C, Lebofsky M, Maria Monteiro de Araújo C, Tiburcio T, Veloso Alves Pereira I, Willebrords J, Crespo Yanguas S, et al. (2016). Involvement of connexin43 in acetaminophen-induced liver injury. Biochim. Biophys. Acta 1862, 1111–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Görgun CZ, and Hotamisligil GS (2006). Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313, 1137–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirosh A, Tuncman G, Calay ES, Rathaus M, Ron I, Tirosh A, Yalcin A, Lee YG, Livne R, Ron S, et al. (2020). Intercellular transmission of hepatic ER stress in obesity disrupts systemic metabolism. Cell Metab. 33, this issue, 319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willebrords J, Maes M, Crespo Yanguas S, and Vinken M (2017). Inhibitors of connexin and pannexin channels as potential therapeutics. Pharmacol. Ther 180, 144–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon S, Pan Y, Shung K, and Wang Y (2020). FRET-based Ca(2+) biosensor single cell imaging interrogated by high-frequency ultrasound. Sensors (Basel) 20, 4998. [DOI] [PMC free article] [PubMed] [Google Scholar]


