Abstract
Background
Asymptomatic bacteriuria is commonly detected in women aged up to 60 years, patients with diabetes, and the elderly. The benefit of antibiotic treatment for this condition is controversial.
Objectives
To assess the effectiveness and safety of antibiotics treatment for asymptomatic bacteriuria in adults. Specific objectives were to assess 1) the effectiveness of antibiotics for preventing development of symptomatic UTI, UTI‐related complications, overall mortality, UTI‐related mortality, and resolution of bacteriuria; 2) the development of resistance to antibiotic treatment by comparing resistance of grown bacteria in urine before and after therapy; and 3) the frequency of adverse events.
Search methods
We searched the Cochrane Renal Group's Specialised Register up to 24 February 2015 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review.
Selection criteria
Randomised controlled trials (RCTs) and quasi‐RCTs comparing antibiotics to placebo or no treatment for asymptomatic bacteriuria in adults were included. The outcomes of interest were the development of symptomatic urinary tract infection (UTI), complications, death, any adverse event, development of antibiotic resistance, bacteriological cure, and decline in kidney function.
Data collection and analysis
Two authors independently extracted the data and assessed study quality. Statistical analyses were performed using the random effects model and the results expressed as risk ratios (RR) with 95% confidence intervals (CI).
Main results
We included nine studies (1614 participants) in this review. Symptomatic UTI (RR 1.11, 95% CI 0.51 to 2.43), complications (RR 0.78, 95% CI 0. 35 to 1.74), and death (RR 0.99, 95% CI 0.70 to 1.41) were similar between the antibiotic and placebo or no treatment arms. Antibiotics were more effective for bacteriological cure (RR 2.67, 95% CI 1.85 to 3.85) but also more adverse events developed in this group (RR 3.77, 95% CI 1.40 to 10.15). No decline in the kidney function was observed across the studies; minimal data were available on the emergence of resistant strains after antimicrobial treatment.
The included studies were of medium and high quality, used different treatments for different durations of treatment and follow‐up, different populations, but this did not appear to influence the results of review.
Authors' conclusions
No differences were observed between antibiotics versus no treatment of asymptomatic bacteriuria for the development of symptomatic UTI, complications or death. Antibiotics were superior to no treatment for the bacteriological cure but with significantly more adverse events. There was no clinical benefit from treating asymptomatic bacteriuria in the studies included in this review.
Plain language summary
Antibiotic treatment for asymptomatic bacteriuria
Growth of bacteria in the urine without any complaints (asymptomatic bacteriuria) is commonly detected in women up to 60 years, people with diabetes and in the elderly. It is not clear whether antibiotic treatment for this condition is of benefit for non‐pregnant adults.
Nine studies of medium to high quality, enrolling 1614 institutionalised participants or outpatients, assigned to antibiotics or placebo/no treatment for treating asymptomatic bacteriuria for different durations of treatment and follow‐up were included in this review. The evidence is current to February 2015. No clinical benefit was found for antibiotic treatment. Antibiotics eradicated the growth of bacteria in more participants but at the cost of more adverse events than in the no treatment groups.
Summary of findings
for the main comparison.
| Antibiotics versus placebo or no treatment for asymptomatic bacteriuria in adults | ||||||
|
Patient or population: adults with asymptomatic bacteriuria Settings: outpatients or geriatric centres Intervention: antibiotics Comparison: placebo or no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| no treatment | antibiotics | |||||
|
Number of subjects who developed symptomatic UTI (6 months to 1 year) |
Medium risk population | RR 1.11 (0.51 to 2.43) | 1046 (5) | ⊕⊕⊕⊝ moderate | blinding methods not reported or not adequate | |
| 200 per 1000 | 222 per 1000 (102 to 486) | |||||
|
Number of subjects who developed complications (10 months to 3 years) |
Medium risk population | RR 0.80 (0.36 to 1.75) | 814 (3) | ⊕⊕⊕⊝ moderate | blinding methods not reported or not adequate | |
| 30 per 1000 | 24 per 1000 (11 to 52) | |||||
|
Death (6 months to 8 years) |
Medium risk population | RR 0.99 (0.70 to 1.41) | 761 (6) | ⊕⊕⊕⊝ moderate | included quasi‐randomised studies and studies with blinding method not reported or not adequate | |
| 140 per 1000 | 138 per 1000 (98 to 197) | |||||
|
Number of subjects who develop any adverse event (42 days to 10 months) |
Medium risk population | RR 3.77 (1.40 to 10.15) | 248 (3) | ⊕⊕⊕⊝ moderate | blinding method not reported or not adequate | |
| 40 per 1000 | 151 per 1000 (56 to 406) | |||||
|
Number of subjects with bacteriological cure (42 days to 4 years) |
Medium risk population | RR 2.32 (1.11 to 4.83) | 1154 (9) | ⊕⊕⊕⊝ moderate | included quasi‐randomised studies and studies with blinding method not reported or not adequate | |
| 430 per 1000 | 997 per 1000 (477 to 2077) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
The studies included mostly elderly men and women and one study included only diabetic patients
Background
Description of the condition
The prevalence of asymptomatic bacteriuria varies according to age, sex, sexual activity and the presence of genitourinary abnormalities. Asymptomatic bacteriuria is commonly detected in women aged up to 60 years at the rate of 3% to 5%. Asymptomatic bacteriuria is more common in patients with diabetes and the elderly (Lin 2008). As many as 25% to 50% of elderly women and 15% to 40% of elderly men in long‐term care facilities are bacteriuric. Asymptomatic bacteriuria is rare in healthy young men, but its prevalence increases substantially after the age of 60 years. Men with diabetes do not appear to have an increased prevalence of bacteriuria compared with non‐diabetic men (Nicolle 1997; Zhanel 1991). Causes of increased susceptibility to asymptomatic bacteriuria among older people can be attributed to declining cell‐mediated immunity, increased bacterial receptivity of uroepithelial cells, neurogenic bladder dysfunction, changed bladder defences from obstructive uropathy, reduced prostatic and vaginal antibacterial factors, urinary and vaginal pH, hormones, and urinary and faecal incontinence that favour bacteriuria (Nicolle 1987a; Nicolle 1988; Reid 1984; Sant 1987). The association of asymptomatic bacteriuria with symptomatic urinary tract infection (UTI) is likely attributable to host factors that promote both symptomatic and asymptomatic urinary infection, rather than symptomatic infection being attributable to asymptomatic bacteriuria. The risk factors for developing symptomatic UTI have not been well defined and the consequences of asymptomatic bacteriuria in diabetic patients are controversial (Ribera 2006). Glucosuria enhances bacterial growth in vitro, but this finding could not be confirmed in vivo in diabetic patients (Geerlings 1999). It is also unknown if asymptomatic bacteriuria precedes symptomatic bacteriuria in these patients (Geerlings 2000). It appears that in patients with diabetes, asymptomatic bacteriuria does not lead to severe complications, and it has therefore been recommended that screening for asymptomatic bacteriuria is unnecessary in diabetic patients (Nicolle 2005). Some studies have reported increased mortality associated with asymptomatic bacteriuria in the elderly (Dontas 1981; Evans 1982), but other studies did not confirm this finding. Clinical studies of older residents in long‐term care facilities have shown no benefits from screening or antimicrobial treatment for asymptomatic bacteriuria (Heinamaki 1986; Nicolle 1987a; Nordenstam 1986). Premenopausal, non‐pregnant women with asymptomatic bacteriuria experience no adverse effects and usually clear bacteriuria spontaneously. However, these women are more likely to experience subsequent symptomatic UTI than women who do not have asymptomatic bacteriuria (Hooton 2000).
Asymptomatic bacteriuria is characterised by the presence of a significant quantity of bacteria in a urine specimen properly collected from a person without symptoms or signs of UTI. Quantitative criteria for identifying significant bacteriuria in an asymptomatic person are at least 100,000 colony‐forming units (CFU)/mL of same species bacteria in midstream clean‐catch urine specimens in a single specimen for men or in two consecutive specimens for women, and at least 100 CFU/mL of same species from single catheterised urine specimens in men or women (Nicolle 2005). The leukocyte esterase and nitrite tests are often used in primary care settings to evaluate urinary symptoms; however, these tests are not useful in diagnosing asymptomatic bacteriuria because pyuria detection is not specific for UTIs. Urinalysis by microscopic examination for bacteria remains a useful test for the identification of bacteriuria (Colgan 2006).
Escherichia coli (E. coli) remains the most common organism isolated from patients with asymptomatic bacteriuria; coagulase‐negative staphylococci are common in men, as well as gram‐negative bacilli and Enterococcus species (Mims 1990). Patients with abnormalities of the genitourinary tract, including elderly institutionalised people, can have a wide variety of organisms isolated. In uncomplicated UTI, infecting E. coli have a number of virulence factors that assist in their colonisation of the urinary tract, including a variety of adhesions, iron sequestration systems and toxins (Zhang 2003); these strains are less virulent in patients with asymptomatic bacteriuria (Holden 2004; Hull 1999). Recent molecular studies demonstrate that some asymptomatic bacteriuria‐causing E. coli strains are non‐virulent commensal strains, whereas others were originally virulent strains that have evolved to commensalism (Klemm 2007; Zdziarski 2008). This low prevalence of virulence characteristics is consistent with previous reports among otherwise healthy individuals and in diabetic women with asymptomatic bacteriuria (Geerlings 2001; Vranes 2003). Bacteria that normally inhabit the bowel but do not invade the urinary tract under usual circumstances may be capable of migration in diabetic women; these infections can be persistent (Dalal 2009). The increased adherence of E. coli with type 1 fimbriae to diabetic uroepithelial cells, with lower cytokine secretion and leucocyte number, can partially explain the increased incidence and prevalence of asymptomatic bacteriuria in diabetic patients (Geerlings 2008).
Description of the intervention
A common dilemma in clinical practice is whether to treat asymptomatic patients who present with bacteria in their urine. Increasing antimicrobial resistance among bacteria is a major concern, and rational use of these agents requires identification of clinical situations in which antimicrobial therapy is not indicated. No consensus exists about treatment of asymptomatic bacteriuria in patients with diabetes (Zhanel 1990).
The Infectious Diseases Society of America (IDSA) recommends screening and treatment of asymptomatic bacteriuria in adults for pregnant women, before urological procedures where mucosal bleeding is anticipated, and among women with catheter‐acquired bacteriuria that persists 48 hours after removal of an indwelling catheter. No treatment is recommended for other groups of patients. No recommendations can be made for transplant recipients (Nicolle 2005).
How the intervention might work
Benefits and harms of treating or not treating asymptomatic bacteriuria are not clear. Screening and treatment of asymptomatic bacteriuria is appropriate if bacteriuria has adverse outcomes that can be prevented by antimicrobial therapy. There are a few scenarios in which antibiotic treatment of asymptomatic bacteriuria has been shown to improve patient outcomes, mainly in pregnancy. It was reported that treatment of asymptomatic bacteriuria neither decreases the frequency of symptomatic infections nor prevents further episodes of asymptomatic bacteriuria (Nicolle 2005). The eradication of microorganisms that cause UTI has been reported to be more difficult in diabetic patients because of an increased frequency of multidrug resistance (Wright 2000).
Why it is important to do this review
IDSA guidelines recommend further research and evaluation of asymptomatic bacteriuria in appropriately conducted clinical studies; the current guidelines were based on a review of published evidence that included studies of different qualities, with increased heterogeneity and controversial results (Nicolle 2005). No evidence based on a systematic review of randomised controlled trials (RCTs) exists to establish the need for screening and treatment of asymptomatic bacteriuria in adult non‐pregnant patients. Issues relating to pregnant women have been included in separate Cochrane reviews (Guinto 2010; Smaill 2007; Widmer 2011).
Objectives
To assess the effectiveness and safety of antibiotics treatment for asymptomatic bacteriuria in adults. Specific objectives were to assess the following.
The effectiveness of antibiotics for preventing development of symptomatic UTI, UTI‐related complications, overall mortality, UTI‐related mortality, overall and resolution of bacteriuria
The development of resistance to antibiotic treatment by comparing resistance of grown bacteria in urine before and after therapy
The frequency of adverse events.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs and quasi‐RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) looking at the use of antibiotics for the treatment of asymptomatic bacteriuria.
Types of participants
Inclusion criteria
Outpatients or institutionalised adults over 18 years of age with asymptomatic bacteriuria (no dysuria, suprapubic pain, frequency or urgency, fever, chills or flank pain) and with bacterial growth defined as at least 100,000 CFU/mL of same species bacteria in midstream clean‐catch urine specimens in a single specimen for men, or in two consecutive specimens for women, and at least 100 CFU/mL of same species from single catheterised urine specimens in men or women will be included.
Exclusion criteria
Pregnant women, catheterised participants (any type of catheter), patients with urinary stents, nephrostomy tubes, kidney or other transplant recipients, bacteriuria related to or close to urological procedures, spinal cord injury and hospitalised patients.
Studies were excluded if any of the following present: more than 10% participants were less than 18 years old, hospitalised, symptomatic UTI and no separate data for these groups will be available, a drop‐out rate of more than 30%.
Types of interventions
Antibiotic treatment of any type, dose or duration compared to placebo or no treatment.
Studies reporting combined interventions were included only if both treatment arms received the same co‐intervention.
Types of outcome measures
Primary outcomes
Proportion of patients who develop symptomatic UTI
Proportion of patients with complications: urosepsis, pyelonephritis
Death.
Secondary outcomes
Proportion of patients who develop any adverse event during treatment
Proportion of patients who develop resistance (grown bacteria in urine) during the treatment period, by comparing resistance of grown bacteria in urine before and after therapy
Proportion of patients with bacteriological cure
Proportion of patients with sepsis‐related mortality
Decline in kidney function as defined in the individual studies.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Renal Group's Specialised Register up to 24 February 2015 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Cochrane Renal Group’s Specialised Register contains studies identified from the following sources.
Quarterly searches of the Cochrane Central Register of Controlled Trials CENTRAL
Weekly searches of MEDLINE OVID SP
Handsearching of renal‐related journals and the proceedings of major renal conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected renal journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
See Appendix 1 for search terms used.
Searching other resources
Reference lists of review articles and relevant studies.
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. Titles and abstracts were screened independently by two authors, who discarded studies that are not applicable; however, studies and reviews that included relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and, if necessary the full text, of these studies to determine which satisfy the inclusion criteria.
Data extraction and management
Data extraction was performed independently by two authors using standard data extraction forms. There were not studies reported in non‐English language journals that had to be translated before assessment. Where more than one publication of one study exists, reports were grouped together and the publication with the most complete data was included. Where relevant outcomes are only published in earlier versions this data was used. Any discrepancy between published versions was highlighted. Any further information required from the original author was requested by written correspondence and any relevant information obtained in this manner was included in the review. Disagreements were resolved by consultation with all authors.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study (detection bias)?
Participants and personnel
Outcome assessors
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (all outcomes considered in the review) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement are used to assess the effects of treatment, the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales have been used.
Unit of analysis issues
For studies with multiple intervention groups, the numbers of participants of similar treatment groups were aggregated and considered as one treatment arm; the control group was considered only once in the analyses.
Dealing with missing data
We attempted to contact authors of the included studies to obtain missing data or for clarification if required.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi² test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance and with the I² test (Higgins 2003). I² values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
There were not sufficient data and studies for funnel plots to be constructed to estimate precision of studies (plots of RR for efficacy against the sample size) for potential asymmetry and publication bias.
Data synthesis
Data were pooled using the random‐effects model but the fixed‐effect model was also used to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
We anticipated heterogeneity between studies for different antibiotics, doses, qualities of studies, duration of treatment and follow‐up, random sequence generation and types of participants included: young, elderly, diabetic, those presenting with urinary tract abnormalities, immunosuppressed people, and among patients after removal of urinary catheters. Subgroup analyses were planned for these populations but could not be performed given the small number of studies included in the review and no separate data for these subgroups available. Because of the likelihood of differences among the various agents used, adverse effects were tabulated and assessed using descriptive techniques (Table 2).
1. Adverse events.
| Study | Treatment | Control | Comments |
| Giamarellou 1998 | 2 (vertigo, upper gastrointestinal symptoms) | 0 | Both discontinued treatment |
| Harding 2002 | 10 | 3 | No other information |
| Nicolle 1987a | 9 (rash, candidiasis, diarrhoea, swollen mouth) | 1 (dizziness) | 4 in treatment group discontinued treatment |
Sensitivity analysis
We conducted sensitivity analyses for studies that were found to include adequate concealment to allocation of treatment methodologies. We also compared high versus low risk random sequence generation.
Results
Description of studies
Results of the search
We identified 340 unique references of which we excluded 321 after inspection of the abstracts for the following reasons: not asymptomatic bacteriuria, not randomised or quasi‐randomised, observational studies, no intervention of interest or outcomes for our review, review articles, papers not fulfilling our inclusion criteria. We considered that 19 reports were potentially eligible for inclusion, but after inspection of the full papers, we excluded eight reports of seven studies (Figure 1).
1.
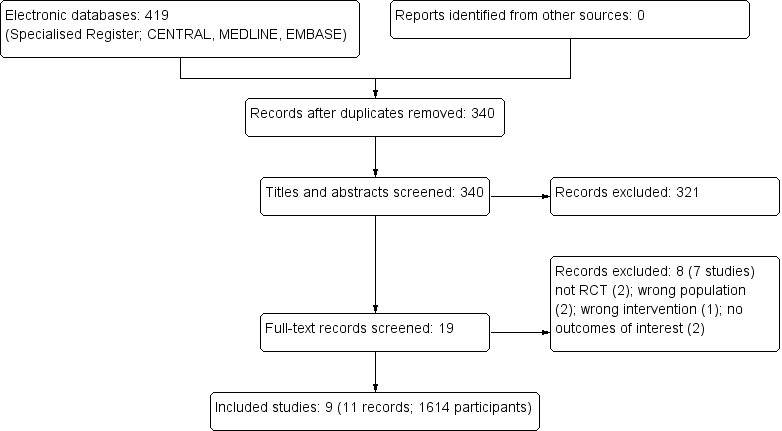
Study flow diagram
Included studies
Nine studies (11 reports) enrolling 1614 participants assigned to different antibiotics or placebo/no treatment met the pre‐stated inclusion criteria for this review. The studies were conducted in Europe, USA, and Canada. Different inclusion criteria were used in the studies, still the thresholds for considering positive urine culture were similar across the studies; different definitions for the bacteriological cure were used in the studies (Characteristics of included studies). Duration of treatment varied from single‐dose to up to six months treatment and follow‐up was from six months up to eight years across the studies. One study had two treatment arms and the numbers of participants were aggregated and considered as one treatment arm, the control group was considered only once in the analysis (Giamarellou 1998).
Participants
Participants included in the studies were men and women outpatient or from geriatric centres, independent or nursing home residents, with a diagnosis of asymptomatic bacteriuria. Four studies included participants younger than 65 years (Asscher 1969; Cai 2012; Harding 2002; Nicolle 1983); two of these studies gave no separate data for this group (Harding 2002; Nicolle 1983). One study included diabetic participants (Harding 2002).
Interventions
Four studies including 607 subjects compared antibiotics to placebo (Abrutyn 1994; Abrutyn 1996; Asscher 1969; Harding 2002). Eight studies including 1520 subjects compared antibiotics to no treatment (Abrutyn 1994, Abrutyn 1996, Boscia 1987, Cai 2012, Giamarellou 1998, Harding 2002, Nicolle 1983, Nicolle 1987). Three studies used placebo for the first part and no treatment for the second part of the study in the control group (Abrutyn 1994, Abrutyn 1996, Harding 2002). No other concomitant therapies were used in the studies.
Outcomes
All the studies reported at least one of the outcomes included in the review.
Excluded studies
Seven studies were excluded after inspecting the full papers as they did not fulfil the inclusion criteria of the review; not randomised (2), wrong population (2); wrong intervention (1); outcomes not relevant to this review (2) (Characteristics of excluded studies).
Risk of bias in included studies
See Characteristics of included studies, Figure 2 and Figure 3
2.
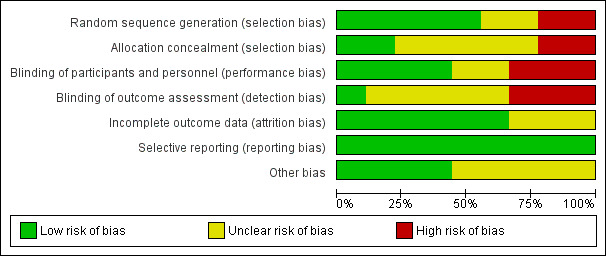
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
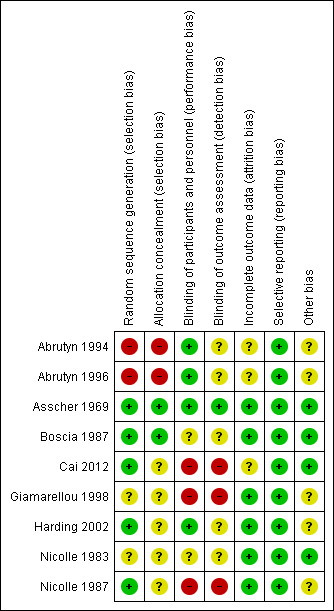
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Seven studies were RCTs and two were quasi‐randomised (Abrutyn 1994; Abrutyn 1996); all used a parallel group design. Two studies described the randomisation process and allocation concealment was adequate (Asscher 1969, Boscia 1987), three described the randomisation generation but concealment to allocated treatment was unclear (Cai 2012; Harding 2002; Nicolle 1987). Two studies reported randomisation but the method of randomisation and concealment of allocation were not mentioned (Giamarellou 1998; Nicolle 1983). Two were quasi‐randomised studies (Abrutyn 1994; Abrutyn 1996).
Blinding
Four studies were double‐blind (Abrutyn 1994; Abrutyn 1996; Asscher 1969; Harding 2002), one single‐blind (Boscia 1987), and three were open‐label studies (Cai 2012; Giamarellou 1998; Nicolle 1987). One study did not mention blinding (Nicolle 1983).
Incomplete outcome data
One study did not describe loss to follow‐up and performed intention to treat analyses (Abrutyn 1994). Loss to follow‐up was described in the other studies.
Selective reporting
No selective reporting was observed across the studies.
Other potential sources of bias
No other possible sources of bias were observed in the included studies, except funding for some studies.
Effects of interventions
See: Table 1
Symptomatic urinary tract infection
There was no difference in the number of symptomatic UTI between the antibiotic treatment and the placebo or no treatment arms (Analysis 1.1 (5 studies, 1046 participants): RR 1.11, 95% CI 0.51 to 2.43; I2 = 91%). Heterogeneity is attributed to the Cai 2012 study which included younger and higher risk patients for the development of symptomatic UTI (sexually active patients with recurrent symptomatic UTIs attending a STD clinic).
1.1. Analysis.
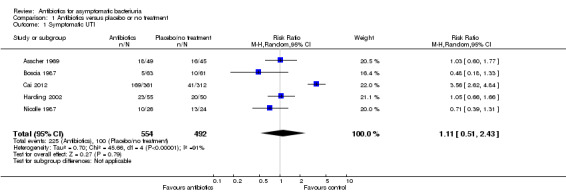
Comparison 1 Antibiotics versus placebo or no treatment, Outcome 1 Symptomatic UTI.
Complications
There was no difference in the number of complications between the antibiotic and placebo or no treatment arms (Analysis 1.2 (3 studies, 814 participants): RR 0.78, 95% CI 0.35 to 1.74; I2 = 0%).
1.2. Analysis.
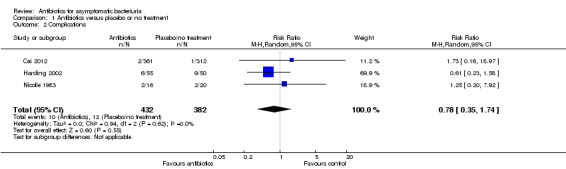
Comparison 1 Antibiotics versus placebo or no treatment, Outcome 2 Complications.
Death
There was no difference in the number of deaths between the antibiotic and placebo or no treatment arms (Analysis 1.3 (6 studies, 761 participants): RR 0.99, 95% CI 0.70 to 1.41; I2 = 0%).
1.3. Analysis.
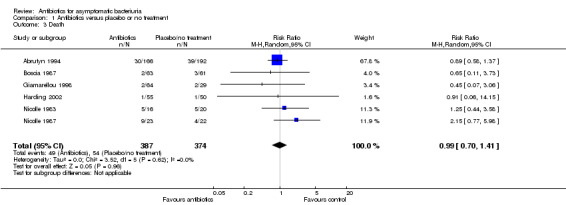
Comparison 1 Antibiotics versus placebo or no treatment, Outcome 3 Death.
Any adverse event during treatment
Significantly more adverse events were observed in the antibiotic treatment group compared to the placebo or no treatment group (Analysis 1.4 (3 studies, 248 participants): RR 3.77, 95% CI 1.40 to 10.15; I2 = 0%).
1.4. Analysis.
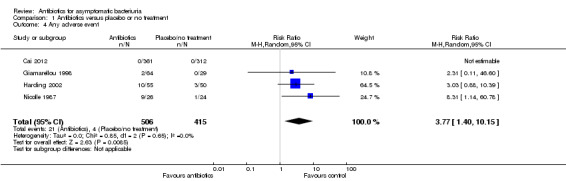
Comparison 1 Antibiotics versus placebo or no treatment, Outcome 4 Any adverse event.
Developed resistance (grown bacteria in urine) during treatment
One study reported resistant strains in 16 participants from the treatment arm after treatment compared to one participant in the no treatment arm. The number of evaluated participants for this outcome was not reported (Giamarellou 1998).
Bacteriological cure
Significantly more participants were cured in the antibiotic treatment arm compared to the placebo or no treatment arm (Analysis 1.5 (9 studies, 1154 participants): RR 2.67, 95% CI 1.85 to 3.85; I2 = 67%). Heterogeneity could be attributed to the different definitions of bacteriological cure and study design across the studies.
1.5. Analysis.
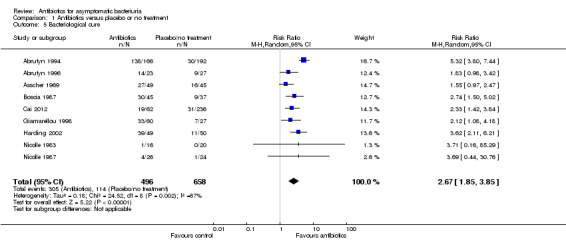
Comparison 1 Antibiotics versus placebo or no treatment, Outcome 5 Bacteriological cure.
Sepsis‐related mortality
One study reported 3.8% (1/26) and 4.1% (1/24) mortality in the treatment and no treatment arms respectively. Different pathogens were isolated from those causing bacteriuria in the control group; UTI may have contributed to one death due to hyperosmolar coma in the treatment group (Nicolle 1987).
Decline in kidney function
The mean serum creatinine at the end of the study was similar to the initial value for both groups and the post‐study creatinine concentration did not differ between groups in one study (Nicolle 1987a). There were non‐statistically significant differences in the serum creatinine levels between the treatment and no treatment arms in one study by the end of the study (P = 0.23) (Harding 2002). No decline in the kidney function was found in one study from mean laboratory values for serum creatinine (Giamarellou 1998). No data were available for performing a meta‐analysis for this outcome.
Sensitivity analyses
Sensitivity analyses that were performed by allocation concealment or randomisation process did not change the results (Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11).
1.6. Analysis.
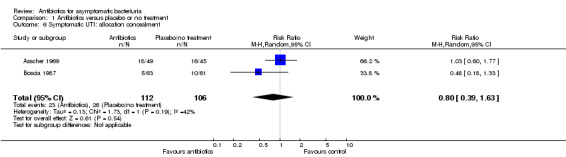
Comparison 1 Antibiotics versus placebo or no treatment, Outcome 6 Symptomatic UTI: allocation concealment.
1.7. Analysis.
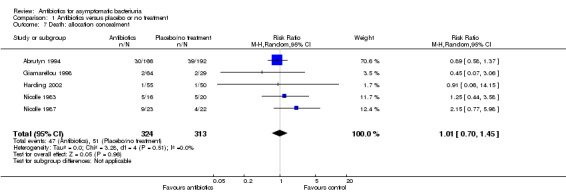
Comparison 1 Antibiotics versus placebo or no treatment, Outcome 7 Death: allocation concealment.
1.8. Analysis.
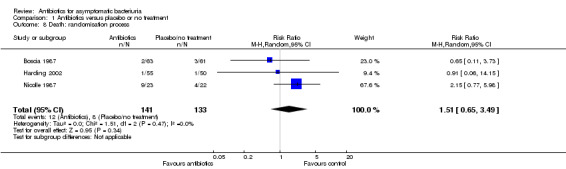
Comparison 1 Antibiotics versus placebo or no treatment, Outcome 8 Death: randomisation process.
1.9. Analysis.
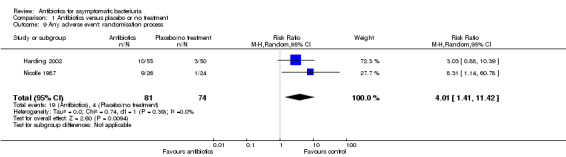
Comparison 1 Antibiotics versus placebo or no treatment, Outcome 9 Any adverse event: randomisation process.
1.10. Analysis.
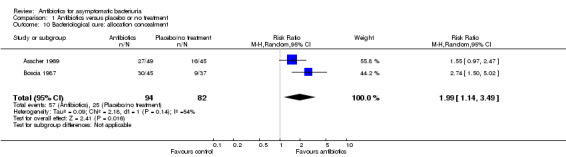
Comparison 1 Antibiotics versus placebo or no treatment, Outcome 10 Bacteriological cure: allocation concealment.
1.11. Analysis.
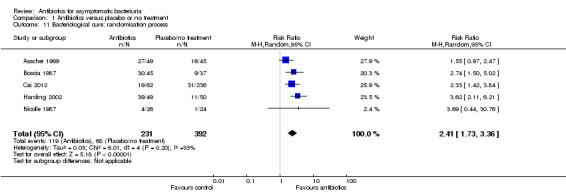
Comparison 1 Antibiotics versus placebo or no treatment, Outcome 11 Bacteriological cure: randomisation process.
The results of the study that included only diabetic participants did not differ from the results of the other studies that included non‐diabetic participants for the same outcomes by inspecting the graphs (Harding 2002).
Discussion
Summary of main results
Asymptomatic bacteriuria is common and screening for this condition in pregnant women is a well‐established, evidence‐based standard of current medical practice. Screening other groups of adults has not been shown to improve outcomes (Lin 2008).
Nine studies with 1614 participants were included in this review. Overall there was no evidence of any clinical benefit from treating asymptomatic bacteriuria for the categories of participants included. No differences between antibiotic treatment versus no treatment were observed for the development of symptomatic UTI, complications, mortality, decline in kidney function. More participants who received antibiotics were bacteriologically cured, but more adverse events were reported in this group mostly minor; six participants from two studies discontinued treatment because of adverse events (Table 2). Only one study reported sepsis related mortality in 3.8% (1/26) and 4.1% (1/24) in the treatment and no treatment groups, respectively; different pathogens from those causing bacteriuria were isolated in the control group and urinary infection may have contributed to one death due to hyperosmolar coma in the treatment group (Nicolle 1987). Mortality was not related to the asymptomatic bacteriuria in the other studies. Development of resistant urinary strains after treatment was reported in one study (Giamarellou 1998).
Overall completeness and applicability of evidence
The studies included young and elderly women and men outpatients or from geriatric centres. Overall, by inspecting the graphs, there were no significant differences between the results of the studies that included different populations, except for one study which included younger, sexually active women with recurrent UTI from a sexually transmitted disease centre; also, a definition for the bacteriological cure was not mentioned in this study (Cai 2012).
Quality of the evidence
The included studies were of medium and high quality (Risk of bias in included studies), used different treatments for different durations of treatment and follow‐up, different populations, but this did not seem to influence the results of the individual studies. Heterogeneity between the results of the studies was observed for the symptomatic cure cure, different populations, different durations of treatment and follow‐up across the studies and different methodology may have contributed to this finding. Less heterogeneity was observed when considering studies only by concealment. In one study participants developed more symptomatic UTI in the antibiotic treatment arm than in the no treatment arm as opposed to the results in the other studies included in the review; this could be attributed to the specific population included in this study (Cai 2012). Excluding this study from the meta‐analysis did not change the result.
Potential biases in the review process
Meta‐analyses were performed by using the random‐effects model and, for testing the robustness of the results the fixed‐effects model was used; no different results were obtained by using the two methods. Sensitivity analyses by allocation concealment and by randomisation process did not change the results.
Agreements and disagreements with other studies or reviews
Controversial results were found across different studies regarding the need for treatment of asymptomatic bacteriuria (Boscia 1986; Marketos 1969; Sourander 1972). The findings of our review are supported by current recommendations. Guidelines published by the IDSA in 2006 state that there is no measurable benefit to screen for or provide antibiotic treatment of asymptomatic bacteriuria in the following patients: premenopausal women who are not pregnant; patients with diabetes elderly patients living in the community and in long‐term care facilities; and in patients with spinal cord injury or indwelling bladder catheter. Exceptions occur when the patient is pregnant or when the urinary tract will be surgically manipulated (Nicolle 2005). The US Preventive Services Task Force has published recommendations similar to those of the IDSA (Lin 2008), based on evidence from systematic reviews, meta‐analyses, RCTs, cohort and case‐control studies and case series of large multi‐site databases. The incorrect management of asymptomatic bacteriuria is a worldwide problem. The Scottish Intercollegiate Guidelines Network, among others, has evaluated the issue thoroughly and has concluded that asymptomatic bacteriuria is a benign disorder for which treatment is not indicated (SIGN 2012). Reduction of indiscriminate use of antimicrobial therapy and of the appearance of multidrug‐resistant organisms is therefore recommended (Gross 2007).
Authors' conclusions
Implications for practice.
Treating asymptomatic bacteriuria with antibiotics did not show any clinical benefit in our review. More eradication of urinary pathogens was obtained but at the cost of significant more adverse events. Current recommendations for treating asymptomatic bacteriuria should be followed until proved otherwise.
Implications for research.
It is unlikely that more studies in the general population would change the results we show here. Studies on treatment of asymptomatic bacteriuria are needed in persons with diabetes.
Feedback
Reader comment, 22 April 2015
Summary
Thank you for your detailed systematic review and meta‐analysis of antibiotic use for asymptomatic bacteriuria. We have reviewed your article with interest, and have identified a few issues that we hoped to bring to your attention. Specifically, we identified significant heterogeneity observed in Analysis 1.1 and Analysis 1.5 (symptomatic UTI and bacteriologic cure, respectively). Furthermore, we had some concerns regarding the assessment of attrition bias within the risk of bias analysis. As described in your review, the heterogeneity in Analysis 1.1 is attributed to the Cai 2012 study. Indeed, we confirmed via sensitivity analysis that removal of the Cai 2012 study reduced I² to 0%. This result is unsurprising given the Cai 2012 study appears to be an outlier. Patients in the Cai 2012 study included younger and higher risk patients for the development of symptomatic UTI (sexually active patients with recurrent symptomatic UTIs attending a STD clinic) compared to the other studies in your analysis¹. With these differences in the Cai 2012 study, we believe it is more important to discuss the heterogeneity in the analysis instead of drawing global conclusions on heterogeneous pooled data. We would suggest either further sensitivity analysis exploring the heterogeneity, or modifying your exclusion criteria to exclude patients with recurrent UTIs from your meta‐analysis. Without the Cai 2012 study, the analysis would show a RR 0.88 (95% CI 0.65 to 1.17) for symptomatic UTI using a fixed‐effect model. If a sensitivity analysis is done, we suggest having a statement that further large, well‐designed studies may provide a more precise result as the sensitivity analysis shows the risk difference of symptomatic UTI could be either decreased by 13% or increased by 5% with antibiotics. The wide confidence interval suggests this decrease or increase is clinically meaningful and its discussion would be of benefit to the reader.
With regard to Analysis 1.5, we performed a sensitivity analysis to determine the source of heterogeneity: removing Abrutyn 1994 alone from the forest plot resulted in a reduction of I² to 89%, removing Cai 2012 alone resulted in a heterogeneity of 70%, while removal of both aforementioned studies drastically reduced the heterogeneity to 10%. In determining the reason for heterogeneity within these articles, we considered both the methodology and the population studies to deduce whether removal of these articles from the meta‐analysis would be appropriate. Abrutyn 1994 may have suffered from significant selection bias in its quasi‐RCT design nature, though Abrutyn 1996 possessed similar methodology despite having a significantly lesser impact on overall heterogeneity. However, Abrutyn 1994 appears to suffer from inconsistent data. In Analysis 1.5, the results from Abrutyn 1994 are 138 achieving bacteriological cure out of 166 patients in the treatment group versus 30 patients achieving bacteriological cure versus 192 patients in the placebo/no treatment group. Looking closer at the Abrutyn 1994 trial, there is a discrepancy in the reporting of their sample sizes. In the results section under the subheading “Controlled clinical trial”, Abrutyn 1994 states 192 patients were treated and 166 patients served as controls. However, in Table 6, the opposite is stated based on “Mean age”. Thus, there is uncertainty as to which values should be used as denominators in your analysis. Additional uncertainty exists for the numerator as well. The study does not report the numbers of patients achieving bacteriological cure from either group; instead, the study states that “overall cure rates during the placebo‐controlled portion of the trial were 82.9% in those given antimicrobial agents and 15.6% in those not given antimicrobials in an intention‐to‐treat analysis”. First, there is uncertainty whether these percentages should multiply 192 or 166 for either study group. Second, their statement describes overall cure rates during the placebo‐controlled portion of the trial. As described in the trial, control patients were given no therapy between October 10, 1983 and December 10, 1987. Patients were only given placebo after December 10, 1987 until February 1992. Thus, the quoted percentages above used to calculate your numerators for Analysis 1.5 may be incorrect, as an unknown proportion of the 358 total patients included in the study and your analysis were enrolled after December 10, 1987. Based on the current version of your review, we are unclear as to whether the original authors provided any insight into the bacteriologic cure rate used in your meta‐analysis: such clarification of author feedback could have some value in future revisions of this systematic review. With regards to the other outlying study (Cai 2012), the younger overall age of patients compared to the other trials as well as the history of recurrent UTIs makes this patient population quite different in terms of baseline characteristics. Likewise, this was the only study that showed a trend towards microbiological cure with placebo. It is our opinion that based on these findings, a meta‐analysis containing these two studies may not be appropriate due to both incomplete data reporting and heterogeneity in the study population. Though a trend towards significant increase in bacteriological cure was maintained even with the removal of the outlying studies (Abrutyn 1994 and Cai 2012), a qualitative description of the results may have served a better purpose. We found that several of the trials described as having a low or unclear risk of incomplete outcome data may have been better described as having a higher risk when considering Analysis 1.5: this included Abrutyn 1994, Boscia 1987, Cai 2012, Giamarellou 1998 and Nicolle 1987. The Abrutyn 1994 trial contained missing outcome reporting for the patients receiving no intervention (prior to implementation of a placebo) as mentioned previously. Similarly, the Cai 2012 study censored all patients who experienced a symptomatic UTI in terms of microbiological cure rather than assessing these patients as having persistent bacteriuria (i.e. microbiological failure). Sixty‐two total patients were assessed for microbiological cure at the end of 12 months (of which 43 were described as being cured in the meta‐analysis): had the total number of patients been assessed as the original number randomised instead of removing all censored patients (i.e. 361 vs. 62), the difference in effect size between intervention and control may have been significantly different. A more conservative approach might have been to use imputations for censored patients to observe the impact on the pooled effect size for the meta‐analysis. For instance, one could either assess all censored patients either bacteriologically cured or having persistent bacteriuria, thus incorporating these missing outcomes into the overall analysis. Likewise, we noticed that the total number of patients in the Boscia 1987 trial assessed for bacteriological cure was less than the total number of patients followed‐up to study completion. This appeared to be attributed to the fact that patients in either arm who received antibiotics outside of the study protocol were censored. One approach to rectifying this issue would be to assume bacteriuria persistence or apply the cure rate from those successfully followed‐up to estimate outcomes for missing patients in the Boscia 1987 trial rather than excluding them from the statistical analysis. After studying Giamarellou 2007, we could not determine how the event rates of 33/60 in the antibiotic group and 7/27 in the control group were chosen in Analysis 1.5. As above, if authors were contacted for clarity surrounding event rates, further transparency would remove ambiguity. A brief comment on how these values were chosen in the Characteristics of included studies section may be useful to the reader. Finally, the Nicolle 1987 study suffered from 18 and 8 lost to follow‐up in the antibiotic and control groups respectively, which may have significantly influenced the data given the low event rate in the study. If it were not possible to mitigate the attrition seen in the identified trials, we think the high or unclear risk of attrition bias may not be appropriate for meta‐analysis. A more conservative approach might be to state that there is uncertainty as to whether antibiotics lead to greater microbiological cure rather than the current conclusion of superiority of antibiotics for bacteriological cure. We thank you for the opportunity to provide feedback on this very relevant and interesting topic. Should you have any questions or comments regarding any of our analysis, we welcome you to contact us for further discussion. Reference: ¹ Hooton TM, Scholes D, Hughes JP, Winter C, Roberts PL, Stapleton a E, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New England Journal of Medicine 1996;335(7):468–74. I agree with the conflict of interest statement below: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
ANALYSIS 1.1 – changes have been made for bacteriological cure in the Cai 2012 study in the analysis and text and abstract. This did not change the conclusions for this outcome and thus heterogeneity between the studies was reduced. Risk difference could be considered in a future update.
ANALYSIS 1.5 – In the results section the reason for heterogeneity was mentioned. Also here there was an error when inserting the denominators for the bacteriological cure in the table for the Cai 2012 study. Numbers for the bacteriological failure were inserted instead of numbers for the bacteriological cure. The denominators should be 19 instead of 43 in the treatment group and 31 instead of 205 in the control group. This will be changed and then, when considering the per analysis numerator in this study, the results will not be different from the other studies, thus reducing heterogeneity. Heterogeneity will be maintained only when performing the ITT analysis, but even then this will not change the overall conclusion. As mentioned in the table of included studies this study did not report a definition for the bacteriological cure.
We noticed the errors on numbers in the text of Abrutyn 1994 study in the “Controlled clinical trial “ section, but considered the similarity of the numbers in the abstract, table and also calculated the numbers by using the percentages reported in the text in the results section under the heading “ Controlled clinical trial” (following the numbers 192 and 166 are mentioned percentages 18.1% and 20.3%). So the right numbers are 166 for the treatment group and 192 for the control group as we considered in the analyses. We calculated the denominators for the bacteriological cure from the percentages reported in the text (82.9% and 15%). Also for this study and this outcome it is mentioned in the text that ITT analysis was performed and these numbers were used for the numerators (all randomized patients). It is reported in the text that two separate analyses were done for the two study periods of treatment versus no treatment respectively placebo, results were very similar and this was the reason why why the two study periods were combined into an analysis of active treatment compared with a single control group.
Boscia 1987 – No data were reported in the text for the control group for the short‐term follow‐up period. For the long‐term follow‐up we considered the data as reported in the study in the table for patients that did not receive interim antimicrobial therapy and had no bladder catheterizations during the follow‐up.
Giamarellou 1998 – This study had three groups. We considered the two treatment groups (continuous and pulse treatment ) as one group and used only one control group. An explanation can be added in Notes in the table.
Nicolle 1987 – During the copy editing of the review when moving data between the tables, 18 lost to follow‐up was inserted instead of 12 in the antibiotic treatment group. The numbers should be 12 lost to follow‐up in the treatment group (3 discharged at 2,5 and 11 months and 9 dies – total 12) and 8 in the control group as we reported originally. When copy‐editing and moving data to another table 3 patients were considered by mistake for each time point.
Contributors
Connor Chan, BSc (Pharm) Torey Lau, BSc (Pharm) Aaron M. Tejani BSc (Pharm), PharmD Anca Zalmanovici Trestioreanu: review author
What's new
| Date | Event | Description |
|---|---|---|
| 18 June 2015 | Feedback has been incorporated | Minor amendments based on feedback ‐ no change to results |
| 16 June 2015 | Amended | Numbers for the bacteriological cure were changed as inserted by error for the bacteriological failure instead of cure in the original review |
Acknowledgements
We would like to thank the referees for their comments and feedback during the preparation of this review, Ruth Mitchell (Trials Search Coordinator) and Narelle Willis (Managing Editor) from the Renal Group for their support, Dr Lindsay Nicolle for her reply and information on missing data and peer referees Hashim U. Ahmed, David Mehr and Herney Andres Garcia‐Perdomo.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Antibiotics versus placebo or no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Symptomatic UTI | 5 | 1046 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.51, 2.43] |
| 2 Complications | 3 | 814 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.35, 1.74] |
| 3 Death | 6 | 761 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.70, 1.41] |
| 4 Any adverse event | 4 | 921 | Risk Ratio (M‐H, Random, 95% CI) | 3.77 [1.40, 10.15] |
| 5 Bacteriological cure | 9 | 1154 | Risk Ratio (M‐H, Random, 95% CI) | 2.67 [1.85, 3.85] |
| 6 Symptomatic UTI: allocation concealment | 2 | 218 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.39, 1.63] |
| 7 Death: allocation concealment | 5 | 637 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.70, 1.45] |
| 8 Death: randomisation process | 3 | 274 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.65, 3.49] |
| 9 Any adverse event: randomisation process | 2 | 155 | Risk Ratio (M‐H, Random, 95% CI) | 4.01 [1.41, 11.42] |
| 10 Bacteriological cure: allocation concealment | 2 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.14, 3.49] |
| 11 Bacteriological cure: randomisation process | 5 | 623 | Risk Ratio (M‐H, Random, 95% CI) | 2.41 [1.73, 3.36] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Abrutyn 1994.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Assigned to treatment by the last digit of a study number unrelated to the conduct of the study, even numbers (treatment), odd numbers (control) |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind, identical placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported; numbers evaluated same as numbers randomised by ITT analyses |
| Selective reporting (reporting bias) | Low risk | Not observed |
| Other bias | Unclear risk | Funding (grant support from the National Institutes of Health Teaching Nursing Home Award) |
Abrutyn 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Same regimen as used in Abrutyn 1994 | |
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Assigned to treatment by the last digit of a study number assigned before the study began |
| Allocation concealment (selection bias) | High risk | No allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported; number randomised same as number evaluated |
| Selective reporting (reporting bias) | Low risk | Not observed |
| Other bias | Unclear risk | Funding, support from the National Institutes of Health Teaching Nursing Home Award |
Asscher 1969.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised list of treatment to correspond to serial numbers on 1st attendance for each bacteriuric subject |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacist provided with a randomised list |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The nature of the treatment was unknown to patients, bacteriologists, or clinicians, and the code was not broken until after the conclusion of the whole trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The nature of the treatment was unknown to patients, bacteriologists, or clinicians, and the code was not broken until after the conclusion of the whole trial." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions, drop‐outs described |
| Selective reporting (reporting bias) | Low risk | Not observed |
| Other bias | Low risk | Not observed |
Boscia 1987.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised from code numbers prior to urine culture |
| Allocation concealment (selection bias) | Low risk | Assigned by an individual not associated with the study |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single blind |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions, drop‐out described: treatment group (died (2); incontinent (3); moved away (2); refused (1)); control group (3 died (3); incontinent (2); refused (1)) |
| Selective reporting (reporting bias) | Low risk | Not observed |
| Other bias | Low risk | Not observed |
Cai 2012.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned, 1:1 simple randomisation, computer generated schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐outs described (treatment group (8), control group (18)); reasons not reported |
| Selective reporting (reporting bias) | Low risk | Not observed |
| Other bias | Low risk | Not observed |
Giamarellou 1998.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up described; treatment groups (refused (3), adverse events (2)); control group (refused (1)) |
| Selective reporting (reporting bias) | Low risk | Not observed |
| Other bias | Unclear risk | Funding |
Harding 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions |
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned, computer generated list of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind for the placebo‐controlled period, matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs described; treatment group (early relapse discontinued (6), reduced medical/functional status (7), moved (6), refused (9), lost to follow‐up (4), death (1)); control group (reduced medical/ functional status (9), moved (3), refused (4), lost to follow‐up (9), death (1), pregnancy (1)) |
| Selective reporting (reporting bias) | Low risk | Not observed |
| Other bias | Unclear risk | Funding |
Nicolle 1983.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of treatment (mean ± SD): 7.1 ± 7.5 weeks |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, not described |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | Not observed |
| Other bias | Low risk | Not observed |
Nicolle 1987.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned, random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up described; treatment group (discharged at 2 (1), 5 (1) and 11 (1) months; died (9)); control group (uninterpretable urine specimens and excluded post‐randomisation (2); discharged at 10 months (1); long‐term indwelling catheter at 4 months of study (1); died (4)) |
| Selective reporting (reporting bias) | Low risk | Not observed |
| Other bias | Unclear risk | Funding |
ADL‐ activities of daily living; ASB ‐ asymptomatic bacteriuria; CFU ‐ colony forming units; ITT ‐ intention to treat; RCT ‐ randomised controlled trial; TMP ‐ trimethoprim; SMX ‐ sulfamethoxazole; UTI ‐ urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Butler 1995 | No data for our outcomes |
| Dalal 2009 | Not our outcomes |
| Freeman 1968 | Only 11% asymptomatic, 82% had instrumentation, no separate data for asymptomatic group and without instrumentation |
| Giamarellou 2007 | Not randomised or quasi‐randomised; author was sent e‐mail for information, no reply |
| Harding 1973 | No placebo or no treatment group |
| Nicolle 2006 | Not randomised or quasi‐randomised |
| Renneberg 1984 | Hospitalised participants |
Contributions of authors
Draft the protocol: AZ, MS, LL
Study selection: AZ, AL, MS
Extract data from studies: AZ, AL
Enter data into RevMan: AZ
Carry out the analysis: AZ
Interpret the analysis: AZ, AL, LL
Draft the final review: AZ, MS, LL
Disagreement resolution: LL
Update the review: AZ, AL
Declarations of interest
None known.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Abrutyn 1994 {published data only}
- Abrutyn E, Mossey J, Berlin JA, Boscia J, Levison M, Pitsakis P, et al. Does asymptomatic bacteriuria predict mortality and does antimicrobial treatment reduce mortality in elderly ambulatory women?. Annals of Internal Medicine 1994;120(10):827‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Abrutyn 1996 {published data only}
- Abrutyn E, Berlin J, Mossey J, Pitsakis P, Levison M, Kaye D. Does treatment of asymptomatic bacteriuria in older ambulatory women reduce subsequent symptoms of urinary tract infection?. Journal of the American Geriatrics Society 1996;44(3):293‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Asscher 1969 {published data only}
- Asscher AW, Sussman M, Waters WE, Evans JA, Campbell H, Evans KT, et al. Asymptomatic significant bacteriuria in the non‐pregnant woman. II. Response to treatment and follow‐up. British Medical Journal 1969;1(5647):804‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussman M, Asscher AW, Waters WE, Evans JA, Campbell H, Evans KT, et al. Asymptomatic significant bacteriuria in the non‐pregnant woman. I. Description of a population. British Medical Journal 1969;1(5647):799‐803. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boscia 1987 {published data only}
- Boscia JA, Kobasa WD, Knight RA, Abrutyn E, Levison ME, Kaye D. Therapy vs no therapy for bacteriuria in elderly ambulatory nonhospitalized women. JAMA 1987;257(8):1067‐71. [MEDLINE: ] [PubMed] [Google Scholar]
Cai 2012 {published data only}
- Cai T, Mazzoli S, Mondaini N, Meacci F, Nesi G, D'Elia C, et al. The role of asymptomatic bacteriuria in young women with recurrent urinary tract infections: to treat or not to treat?. Clinical Infectious Diseases 2012;55(6):771‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Cai T, Mazzoli S, Mondaini N, Meacci F, Nesi G, Luciani LG, et al. The role of asymptomatic bacteriuria in young women with recurrent urinary tract infections: To treat or not to treat [abstract]. European Urology, Supplements 2012;11(1):e188‐e188a. [EMBASE: 70863002] [DOI] [PubMed] [Google Scholar]
Giamarellou 1998 {published data only}
- Giamarellou H, Dontas AS, Zorbas P, Staszewska‐Pistoni M, Xirouchaki E, Petrikkos G. Asymptomatic bacteriuria in freely voiding elderly subjects. Long‐term continuous vs pulse treatment with ofloxacin. Clinical Drug Investigation 1998;15(3):187‐95. [EMBASE: 1998118841] [Google Scholar]
Harding 2002 {published data only}
- Harding GK, Zhanel GG, Nicolle LE, Cheang M, Manitoba Diabetes Urinary Tract Infection Study Group. Antimicrobial treatment in diabetic women with asymptomatic bacteriuria. New England Journal of Medicine 2002;347(20):1576‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nicolle 1983 {published data only}
- Nicolle LE, Bjornson J, Harding GK, MacDonell JA. Bacteriuria in elderly institutionalized men. New England Journal of Medicine 1983;309(23):1420‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nicolle 1987 {published data only}
- Nicolle LE, Mayhew WJ, Bryan L. Prospective randomized comparison of therapy and no therapy for asymptomatic bacteriuria in institutionalized elderly women. American Journal of Medicine 1987;83(1):27‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Butler 1995 {published data only}
- Butler P, Hamilton‐Miller JM, McIntyre N, Burroughs AK. Natural history of bacteriuria in women with primary biliary cirrhosis and the effect of antimicrobial therapy in symptomatic and asymptomatic groups. Gut 1995;36(6):931‐4. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalal 2009 {published data only}
- Dalal S, Nicolle L, Marrs CF, Zhang L, Harding G, Foxman B. Long‐term Escherichia coli asymptomatic bacteriuria among women with diabetes mellitus. Clinical Infectious Diseases 2009;49(4):491‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Freeman 1968 {published data only}
- Freeman RB, Bromer L, Brancato F, Cohen SI, Garfield CF, Griep RJ, et al. Prevention of recurrent bacteriuria with continuous chemotherapy. Annals of Internal Medicine 1968;69(4):655‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Freeman RB, Smith WM, Richardson JA, Hennelly PJ, Thurm RH, Urner C, et al. Long‐term therapy for chronic bacteriuria in men. U.S. Public Health Service cooperative study. Annals of Internal Medicine 1975;83(2):133‐47. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Giamarellou 2007 {published data only}
- Giamarellou H, Dontas AS, Petrikkos G, Gnardellis C, Zorbas P, Philippou P. Survival of elderly bacteriuric subjects following long‐term quinolone therapy. Journal of Chemotherapy 2007;19(2):185‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harding 1973 {published data only}
- Harding GK, Ronald AR. Clinical experiences: genitourinary infections. A. Infections of the urinary tract. Efficacy of trimethoprim‐sulfamethoxazole in bacteriuria. Journal of Infectious Diseases 1973;128(Suppl):641‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Nicolle 2006 {published data only}
- Nicolle LE, Zhanel GG, Harding GK. Microbiological outcomes in women with diabetes and untreated asymptomatic bacteriuria. World Journal of Urology 2006;24(1):61‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Renneberg 1984 {published data only}
- Renneberg J, Paerregaard A. Single‐day treatment with trimethoprim for asymptomatic bacteriuria in the elderly patient. Journal of Urology 1984;132(5):934‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Additional references
Boscia 1986
- Boscia JA, Kobasa WD, Abrutyn E, Levison ME, Kaplan AM, Kaye D. Lack of association between bacteriuria and symptoms in the elderly. American Journal of Medicine 1986;81(6):979‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Colgan 2006
- Colgan R, Nicolle LE, McGlone A, Hooton TM. Asymptomatic bacteriuria in adults. American Family Physician 2006;74(6):985‐90. [MEDLINE: ] [PubMed] [Google Scholar]
Dontas 1981
- Dontas AS, Kasviki‐Charvati P, Papanaylotou PC, Mareketos SG. Bacteriuria and survival in old age. New England Journal of Medicine 1981;304(16):939‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Evans 1982
- Evans DA, Kass EH, Hennekens CH, Rosner B, Miao L, Kendrick MI, et al. Bacteriuria and subsequent mortality in women. Lancet 1982;1(8264):156‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Geerlings 1999
- Geerlings SE, Brouwer EC, Gaastra W, Verhoel J, Hoepelman AI. Effect of glucose and pH on uropathogenic and non‐uropathogenic Escherichia coli: studies with urine from diabetic and non‐diabetic individuals. Journal of Medical Microbiology 1999;48(6):535‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Geerlings 2000
- Geerlings SE, Stolk RP, Camps MJ, Netten PM, Collet TJ, Hoepelman AI, et al. Risk factors for symptomatic urinary tract infection in women with diabetes. Diabetes Care 2000;23(12):1737‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Geerlings 2001
- Geerlings SE, Stolk RP, Camps MJ, Netten PM, Collet JT, Schneeberger PM, et al. Consequences of asymptomatic bacteriuria in women with diabetes mellitus. Archives of Internal Medicine 2001;161(11):1421‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Geerlings 2008
- Geerlings SE. Urinary tract infections in patients with diabetes mellitus: epidemiology, pathogenesis and treatment. International Journal of Antimicrobial Agents 2008;31 Suppl 1:S54‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gross 2007
- Gross PA, Patel B. Reducing antibiotic overuse: a call for a national performance measure for not treating asymptomatic bacteriuria. Clinical Infectious Diseases 2007;45(10):1335‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guinto 2010
- Guinto VT, Guia B, Festin MR, Dowswell T. Different antibiotic regimens for treating asymptomatic bacteriuria in pregnancy. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD007855.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Heinamaki 1986
- Heinamaki P, Haavisto M, Hakulinen T, Mattila K, Rajala S. Mortality in relation to urinary characteristics in the very aged. Gerontology 1986;32(3):167‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated February 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Holden 2004
- Holden NJ, Gally DL. Switches, cross‐talk and memory in Escherichia coli adherence. Journal of Medical Microbiology 2004;53(Pt 7):585‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hooton 2000
- Hooton TM, Scholes D, Stapleton AE, Roberts PL, Winter C, Gupta K, et al. A prospective study of asymptomatic bacteriuria in sexually active young women. New England Journal of Medicine 2000;343(14):992‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hull 1999
- Hull RA, Rudy DC, Donovan WH, Wieser IE, Stewart C, Darouiche RO. Virulence properties of Escherichia coli 83972, a prototype strain associated with asymptomatic bacteriuria. Infection & Immunity 1999;67(1):429‐32. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Klemm 2007
- Klemm P, Hancock V, Schembri MA. Mellowing out: adaptation to commensalism by Escherichia coli asymptomatic bacteriuria strain. Infection & Immunity 2007;75(8):3688‐95. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2008
- Lin K, Fajardo K, U.S. Preventive Services Task Force. Screening for asymptomatic bacteriuria in adults: evidence for the U.S. Preventive Services Task Force reaffirmation recommendation statement. Annals of Internal Medicine 2008;149(1):W20‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marketos 1969
- Marketos SG, Papanayiotou PC, Dontas AS. Bacteriuria and non‐obstructive renovascular disease in old age. Journal of Gerontology 1969;24(1):33‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mims 1990
- Mims AD, Norman DC, Yamamura RH, Yoshikawa TT. Clinically inapparent (asymptomatic) bacteriuria in ambulatory elderly men: epidemiological, clinical and microbiological findings. Journal of the American Geriatrics Society 1990;38(11):1209‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nicolle 1987a
- Nicolle LE, Henderson E, Bjornson J, McIntyre M, Harding GK, MacDonell JA. The association of bacteriuria with resident characteristics and survival in elderly institutionalized men. Annals of Internal Medicine 1987;106(5):682‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nicolle 1988
- Nicolle LE, Muir P, Harding GK, Norris M. Localization of urinary tract infection in elderly, institutionalized women with asymptomatic bacteriuria. Journal of Infectious Diseases 1988;157(1):65‐70. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nicolle 1997
- Nicolle LE. Asymptomatic bacteriuria in the elderly. Infectious Disease Clinics of North America 1997;11(3):647‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nicolle 2005
- Nicolle LE, Bradley S, Colgan R, Rice JC, Schaeffer A, Hooton TM. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clinical Infectious Diseases 2005;40(5):643‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nordenstam 1986
- Nordenstam GR, Brandberg CA, Oden AS, Svanborg Eden CM, Svanborg A. Bacteriuria and mortality in an elderly population. New England Journal of Medicine 1986;314(18):1152‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reid 1984
- Reid G, Zorzotto ML, Bruce AW. Pathogenesis of urinary tract infection in the elderly: the role of bacterial adherence to uroepithelial cells. Current Microbiology 1984;11(2):67‐72. [EMBASE: 1985159608] [Google Scholar]
Ribera 2006
- Ribera MC, Pascual R, Orosco D, Perez Barba C, Pedrera V, Gil V. Incidence and risk factors associated with urinary tract infection in diabetic patients with and without asymptomatic bacteriuria. European Journal of Clinical Microbiology & Infectious Diseases 2006;25(6):389‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sant 1987
- Sant GR. Urinary tract infection in the elderly. Seminars in Urology 1987;5(2):126‐33. [MEDLINE: ] [PubMed] [Google Scholar]
SIGN 2012
- Scottish Intercollegiate Guidelines Network (SIGN). Management of suspected bacterial urinary tract infection in adults: a national clinical guideline. 2012. www.sign.ac.uk/pdf/sign88.pdf (accessed 12 March 2015).
Smaill 2007
- Smaill FM, Vazquez JC. Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD000490.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sourander 1972
- Sourander LB, Kasanen A. A 5‐year follow‐up of bacteriuria in the aged. Gerontologia Clinica 1972;14(5):274‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vranes 2003
- Vranes J, Kruzic V, Sterk‐Kuzmanovic N, Schonwald S. Virulence characteristics of Escherichia coli strains causing asymptomatic bacteriuria. Infection 2003;31(4):216‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Widmer 2011
- Widmer M, Gülmezoglu AM, Mignini L, Roganti A. Duration of treatment for asymptomatic bacteriuria during pregnancy. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD000491.pub2] [DOI] [PubMed] [Google Scholar]
Wright 2000
- Wright SW, Wrenn KD, Haynes M, Haas DW. Prevalence and risk factors for multidrug resistant uropathogens in ED patients. American Journal of Emergency Medicine 2000;18(2):143‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zdziarski 2008
- Zdziarski J, Svanborg C, Wullt B, Hacker J, Dobrindt U. Molecular basis of commensalism in the urinary tract: low virulence or virulence attenuation?. Infection & Immunity 2008;76(2):695‐703. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhanel 1990
- Zhanel GG, Harding GK, Guay DR. Asymptomatic bacteriuria: which patients should be treated?. Archives of Internal Medicine 1990;150(7):1389‐96. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhanel 1991
- Zhanel GG, Harding GK, Nicolle LE. Asymptomatic bacteriuria in patients with diabetes mellitus. Reviews of Infectious Diseases 1991;13(1):150‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhang 2003
- Zhang L, Foxman B. Molecular epidemiology of Escherichia coli mediated urinary tract infections. Frontiers in Bioscience 2003;8:e235‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Zalmanovici Trestioreanu 2012
- Zalmanovici Trestioreanu A, Lador A, Sauerbrun‐Cutler MT, Leibovici L. Antibiotics for asymptomatic bacteriuria. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD009534] [DOI] [PMC free article] [PubMed] [Google Scholar]


