ABSTRACT
The antibiotic combination trimethoprim (TMP)-sulfamethoxazole (SMX) has a broad spectrum of activity and is used for the treatment of numerous infections, but pediatric pharmacokinetic (PK) data are limited. We previously published population PK (popPK) models of oral TMP-SMX in pediatric patients based on sparse opportunistically collected data (POPS study) (J. Autmizguine, C. Melloni, C. P. Hornik, S. Dallefeld, et al., Antimicrob Agents Chemother 62:e01813-17, 2017, https://doi.org/10.1128/AAC.01813-17). We performed a separate PK study of oral TMP-SMX in infants and children with more-traditional PK sample collection and independently developed new popPK models of TMP-SMX using this external data set. The POPS data set and the external data set were each used to evaluate both popPK models. The external TMP model had a model and error structure identical to those of the POPS TMP model, with typical values for PK parameters within 20%. The external SMX model did not identify the covariates in the POPS SMX model as significant. The external popPK models predicted higher exposures to TMP (median overprediction of 0.13 mg/liter for the POPS data set and 0.061 mg/liter for the external data set) and SMX (median overprediction of 1.7 mg/liter and 0.90 mg/liter) than the POPS TMP (median underprediction of 0.016 mg/liter and 0.39 mg/liter) and SMX (median underprediction of 1.2 mg/liter and 14 mg/liter) models. Nonetheless, both models supported TMP-SMX dose increases in infants and young children for resistant pathogens with a MIC of 1 mg/liter, although the required dose increase based on the external model was lower. (The POPS and external studies have been registered at ClinicalTrials.gov under registration no. NCT01431326 and NCT02475876, respectively.)
KEYWORDS: pediatric, population pharmacokinetics, trimethoprim, and sulfamethoxazole, pediatric, sulfamethoxazole
TEXT
Trimethoprim (TMP) and sulfamethoxazole (SMX) are two antifolate antibiotics with broad spectra of activity and wide tissue distribution. These characteristics allow the combination to be used for treating diverse bacterial and fungal infections in pediatric patients, including urinary tract infections, acute otitis media, shigellosis, Pneumocystis jirovecii pneumonia, and uncomplicated skin infections due to methicillin-resistant Staphylococcus aureus (1–4). For bacterial infections, the recommended dose is 160 to 320 mg (based on the TMP component) every 12 h for adults and 4 to 6 mg/kg of body weight every 12 h for pediatric patients older than 2 months (1, 2).
Pharmacokinetic (PK) studies in adults have reported that the absorptions of both TMP and SMX are rapid and complete following oral administration (1, 5). Approximately 42 to 46% of TMP and 70% of SMX are bound to plasma proteins (6). TMP is largely (61 to 85%) eliminated unchanged by the kidneys, with a small fraction metabolized by liver cytochrome P450 (CYP) 2C9 and CYP3A4 to inactive metabolites; in contrast, SMX is mainly metabolized by CYP2C9 and N-acetyltransferase (NAT) 1 and NAT2 to various metabolites, with only 10 to 12% excreted unchanged in urine (7). In adults, the apparent volumes of distribution (V/F) are 1.0 to 1.8 liters/kg for TMP and 0.17 to 0.27 liter/kg for SMX, and the apparent clearances (CL/F) are 0.071 to 0.11 liters/h/kg for TMP and 0.013 to 0.024 liters/h/kg for SMX (8–17).
TMP-SMX PK data for infants and children are relatively sparse (18), but an understanding of the underlying mechanism for elimination may provide some insights. For renally eliminated drugs, such as TMP, non-weight-adjusted clearance is expected to increase less than proportionally to weight and to increase sigmoidally with age, with most of the age-related change occurring in the first year of life, following renal function maturation (19). Weight-adjusted TMP clearance was lowest in neonates, at 1.84 ml/min/kg (20), and higher in infants than in older children (9, 21). Weight-adjusted volume of distribution data were conflicting, with one study suggesting lower values for younger children (9) and another study reporting a decrease with age (22). For SMX, CYP2C9 activity is known to rapidly increase to adult values after birth (23), but the ontogeny of the NATs has not been clearly elucidated, although some evidence suggested maturation around the age of 4 years (24). Based on studies with different median ages, weight-adjusted clearance and volume of distribution showed opposite trends, with neonates having the lowest clearance and highest volume of distribution, younger children having the highest clearance and lowest volume of distribution, and older children having a clearance and volume of distribution in between (20, 21, 25). A direct comparison of SMX PK from the same study was not available. Overall, both age and weight appeared to contribute to differences between adult and pediatric TMP-SMX PK.
Our group previously conducted a population PK (popPK) study of TMP-SMX, referred to below as the POPS (Pediatric Opportunistic PK Study) study (ClinicalTrials registration no. NCT01431326), which leveraged sparse opportunistically collected samples from pediatric patients treated for bacterial infections per standard of care (21). The dispositions of TMP and SMX were characterized using one-compartment PK models with first-order kinetics. After accounting for actual body weight (WT) using an allometric relationship, postnatal age (PNA) and serum creatinine level (SCR) were identified as significant covariates for TMP CL/F, while PNA and albumin concentration were identified as significant covariates for SMX CL/F. The POPS study aimed to achieve a free concentration at 50% of the dosing interval at steady state greater than the MIC of 0.5 or 1 mg/liter in the majority of each age cohort. The results suggested that for pathogens with a MIC of 1 mg/liter, a dose increase to 7.5 mg/kg TMP every 12 h for children 2 months to <6 years of age, and to 6 mg/kg TMP every 12 h for children 6 years of age or older, may be warranted. However, the POPS popPK models have not yet been externally evaluated.
External evaluation is an important component of popPK model evaluation to ensure the robustness and generalizability of the model (26), in particular for pediatric populations, where PK sampling is often sparser, and where there is substantial heterogeneity in disease severity and drug dosing. We have collected an independent data set for infants and children using a traditional, dedicated PK sampling strategy (ClinicalTrials.gov registration no. NCT02475876). Our objectives were to develop a new popPK model for TMP and SMX based on the new data set alone and to cross-evaluate the newly developed external popPK model and the POPS popPK model using the available data. Finally, we sought to use a simulation approach to evaluate TMP-SMX dosing for populations from infants to adolescents based on each popPK model.
RESULTS
Data set characteristics.
Demographic and clinical characteristics and dosing information for each data set are summarized in Table 1. Compared to subjects in the POPS data set, subjects in the external data set had more samples per person, had a narrower PNA, and received higher and more-frequent doses. Albumin concentrations were missing from a significant proportion of subjects in both data sets. SCR was lower in the external data set, but creatine clearance was comparable for the two data sets. Although the external study had a prospective design with protocol-specified doses, subjects who started TMP-SMX at a lower dose were eligible for enrollment in the external study, which led to variability in the dosing regimens. The concentrations from both data sets were dose-normalized to 4 mg/kg TMP and 20 mg/kg SMX and are plotted against time after the last dose in Fig. S1 in the supplemental material.
TABLE 1.
Population demographics, laboratory values, and drug dosing information for the POPSa and external data sets
| Characteristicb | POPS data | External data |
|---|---|---|
| No. of participants | 153 | 20 |
| No. of PK samples [no. missing]c | 240 [4] | 121 [0] |
| No. (%) of BLQ TMP samples | 22 (9.3) | 0 (0) |
| No. (%) of BLQ SMX samples | 15 (6.4) | 0 (0) |
| Median (range) value [no. of missing values] for: | ||
| No. of PK samples per subject | 1 (1–4) | 7 (2–7) |
| Gestational age (wks)d | 37 (30–39) [141] | 32 (25–41) [14] |
| Postnatal age (yrs) | 7.9 (0.055–20) [0] | 4.4 (0.23–15) [0] |
| Weight (kg) | 30 (2.3–150) [0] | 15 (1.9–65) [0] |
| Height (cm) | 130 (44–190) [3] | 98 (44–160) [0] |
| Albumin (g/dl) | 3.4 (1.7–4.8) [75] | 3.9 (3.1–4.2) [13] |
| Serum creatinine concn (mg/dl) | 0.50 (0.10–5.9) [33] | 0.32 (0.13–0.60) [0] |
| Creatinine clearance (ml/min/1.73m2)e | 100 (5–420) [0] | 120 (73–210) [0] |
| TMP dose (mg/kg)f | 2.5 (0.49–12) | 4.5 (2.1–6.6) |
| Dosing intervalf | 22 (6.3–84) | 12 (7.8–24) |
| Corrected dosing intervalf,g | 13 (6.3–49) | 12 (7.8–24) |
| No. (%) of subjects | ||
| Male | 82 (54) | 12 (60) |
| Caucasian | 109 (71) | 18 (90) |
| Obeseh | 53 (35) | 4 (20) |
POPS, Pediatric Opportunistic Pharmacokinetic Study.
Descriptive statistics for demographics and laboratory values are calculated on the basis of the value at the time of the first recorded dose. BLQ, below the limit of quantification; PK, pharmacokinetic; TMP, trimethoprim; SMX, sulfamethoxazole.
PK samples below the lower limit of quantification before the first dose were set as missing.
Gestational age information was collected for infants with a postnatal age of <120 days for the POPS data set and for infants with a PNA of <1 year for the external data set.
Calculated using the Bedside Schwartz formula.
Median dose information was first summarized for each individual patient before descriptive statistics were calculated. Three participants in the external data set received doses lower than the protocol-specified doses throughout their PK data.
Computed after excluding dose intervals of >60 h. A total of 99 dose intervals from the POPS study and 2 dose intervals from the external study were excluded. Extended dose intervals were likely to be due to separate dosing occasions for the same subject.
Defined as a body mass index in the 95th percentile or higher; not assessed for subjects <2 years old.
External TMP-SMX popPK model development.
Both TMP and SMX concentrations were adequately characterized using a one-compartment PK model with first-order absorption and elimination. For each drug, allometric scaling of total body WT using an exponent of 0.75 for CL/F and 1 for V/F was selected for inclusion in the base model, balancing practicality and improvement in objective function value.
For the TMP model, the interindividual variability (IIV) in the absorption rate constant (Ka) was fixed to zero because the shrinkage was large (99.6%), and the covariance between CL/F and V/F was fixed to zero because the estimated covariance was negligible with a very large relative standard error (RSE). PNA using a maximum-effect (Emax) maturation function and SCR using a power relationship were significant covariate relationships for CL/F. Therefore, the final external TMP model is as follows: Ka = 1.40, CL/F = 8.79 × (WT/70)0.75 × [PNA/(PNA + 0.91)] × (0.5/SCR)0.71, and V/F = 124 × (WT/70), where Ka is in unit 1/hour, CL/F is in unit of liters per hour, WT is in kilograms, PNA is in years, SCR is in milligrams per deciliter, and V/F is in unit of liters.
For the SMX model, the IIV for V/F was fixed to zero because it could not be precisely estimated (RSE, 170%) with high shrinkage (71.6%). The covariance between Ka and CL/F was fixed to zero because the estimated covariance was negligible, with an extremely large RSE, and the rationale for including covariance between CL/F and Ka was weak. No additional covariate effect was identified. The final SMX model is as follows: Ka = 1.10, CL/F = 1.17 × (WT/70)0.75, and V/F = 24 × (WT/70), where Ka is measured per hour, CL/F is measured in liters per hour, WT in kilograms, and V/F in liters.
Bias and precision for each popPK model with either data set.
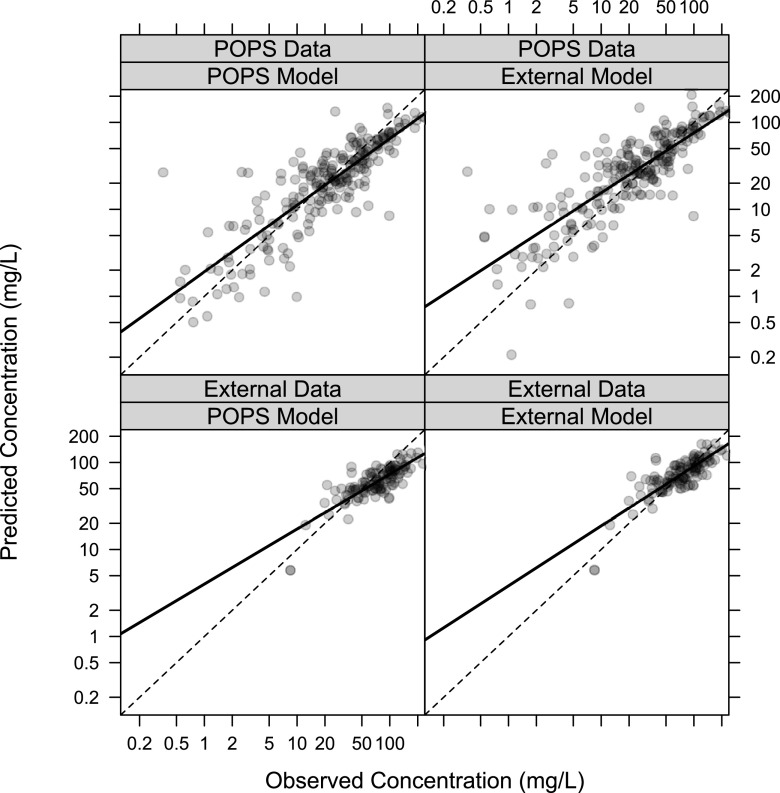
The POPS study model was associated with a negative median prediction error (PE) for both TMP and SMX for both data sets, while the external study model was associated with a positive median PE for both drugs for both data sets (Table S1). With both drugs, the POPS model better characterized the lower concentrations while the external model better characterized the higher concentrations, which were more prevalent in the external data set (Fig. 1 [TMP] and Fig. 2 [SMX]). The conditional weighted residuals (CWRES) plots demonstrated a roughly even distribution of the residuals around zero, with most CWRES falling between −2 and 2 (Fig. S2 to S5). External evaluations were associated with more positive residuals for the POPS model and more negative residuals for the external model.
FIG 1.
Goodness-of-fit plots comparing TMP PREDs with observations. PREDs were obtained by fixing the parameters in the published POPS model or the external model developed from the current study. The dashed line represents the line of unity; the solid line represents the best-fit line. We excluded 22 (9.3%) TMP samples and 15 (6.4%) SMX samples from the POPS data that were BLQ.
FIG 2.
Goodness-of-fit plots comparing SMX PREDs with observations. PREDs were obtained by fixing the model parameters for the published POPS model or the external model developed from the current study. The dashed line represents the line of unity; the solid line represents the best-fit line. We excluded 22 (9.3%) TMP samples and 15 (6.4%) SMX samples from the POPS data that were BLQ.
Reestimation and bootstrap analysis.
Each model was reestimated using either data set, and bootstrap analysis was performed to assess model stability and the precision of estimates for each model. The results for the estimation and bootstrap analysis of the POPS and external TMP models are combined in Table 2, given that the TMP models have identical structures. The estimation step and nearly all 1,000 bootstrap runs minimized successfully using either data set. The final estimates for the PK parameters were within 20% of each other. The 95% confidence intervals (CIs) for the covariate relationships overlapped significantly and did not include the no-effect threshold. The residual variability estimated for the POPS data set was greater than that in the external data set.
TABLE 2.
Parameter estimates and bootstrap analysis of the published POPS TMP model and the external TMP model developed from the current study using the POPS and external data setsa
| Parameterb | POPS data |
External data |
||
|---|---|---|---|---|
| Parameter value (% RSE)c | Bootstrap analysis (n = 1,000), 2.5th–97.5th percentiles | Parameter value (% RSE) | Bootstrap analysis (n = 1,000), 2.5th–97.5th percentiles | |
| Minimization successful | Yes | 998/1,000 | Yes | 999/1,000 |
| Fixed effects | ||||
| Ka (h–1) | 1.3 (36) | 0.57–2.5 | 1.4 (21) | 0.97–2.4 |
| CL/F (liters/h) | 11 (5.7) | 9.3–12.0 | 9.8 (10) | 7.9–13 |
| V/F (liters) | 150 (6.8) | 130–170 | 125 (7.4) | 110–150 |
| PNA50 (yr) | 0.24 (25) | 0.13–0.41 | 0.91 (41) | 0.35–2.7 |
| SCR exponent | 0.40 (20) | 0.22–0.56 | 0.71 (25) | 0.31–1.4 |
| Random effects | ||||
| IIV, CL/F (%) | 34 (18) | 12–48 | 31 (9.9) | 22–36 |
| IIV, V/F (%) | 21 (45) | 0.21–46 | 16 (45) | 0.16–29 |
| Proportional error (%) | 51 (7.2) | 43–58 | 19 (13) | 14–24 |
The Pediatric Opportunistic Pharmacokinetic Study (POPS) trimethoprim (TMP) model and the external TMP model have the same structural relationship: Ka (h–1) = θ1; ; V/F (liters) = θ5 × (WT/70), where θ is an estimated fixed effect, WT is the actual body weight in kilograms, and PNA is the postnatal age in years.
CL/F, apparent clearance; IIV, interindividual variability; Ka, absorption rate constant; PNA50, maturation half-life calculated as a function of postnatal age (in years); SCR, serum creatinine; V/F, apparent volume of distribution.
RSE, relative standard error.
The results of the reestimation and bootstrap analysis using the POPS SMX model with either data set are summarized in Table 3. When the POPS SMX model was reestimated and bootstrapped employing the data set used for its development, the results were similar to the results in the previous publication (21). However, the CIs for the Ka, V/F, the Hill coefficient on the maturation function with age, and the exponent on the albumin effect on clearance were wide, suggesting that these parameters could not be precisely identified. The reestimation and nearly half of the bootstrap analysis for the POPS SMX model did not minimize using the external data set, suggesting a lack of model stability. The bootstrap analysis yielded wide 95% CIs on the maturation half-life and on the albumin exponent, both of which included the no-effect threshold.
TABLE 3.
Parameter estimates and bootstrap analysis of the published POPS SMX model using the POPS and external data setsa
| Parameterb | POPS data |
External data |
||
|---|---|---|---|---|
| Parameter value (% RSE) | Bootstrap analysis (n = 1,000), 2.5th–97.5th percentiles | Parameter value (% RSE)c | Bootstrap analysis (n = 1,000), 2.5th–97.5th percentiles | |
| Minimization successful | Yes | 959/1,000 | No | 502/1,000 |
| Fixed effects | ||||
| Ka (h–1) | 0.58 (44) | 0.099 to 1.4 | 0.66 to 1.8 | |
| CL/F (liters/h) | 1.5 (5.1) | 1.3 to 1.8 | 1.0 to 6.0 | |
| V/F (liters) | 24 (10) | 6.4 to 28 | 20 to 28 | |
| PNA50 (yr) | 0.12 (17) | 0.051 to 0.19 | 3.8e−07 to 6.9e+5 | |
| PNA Hill | 2.1 (57) | 0.33 to 14 | 0.063 to 4.1 | |
| Albumin exponent | 0.77 (34) | 0.21 to 1.5 | −3.9 to 0.26 | |
| Random effects | ||||
| IIV, CL (%) | 36 (23) | 8.9 to 53 | 16 to 37 | |
| ρ (CL − V) | 0.68 (20) | −0.36 to 1.0 | −1.0 to 1.0 | |
| IIV, V (%) | 41 (21) | 13 to 140 | 0.44 to 30 | |
| Proportional error (%) | 47 (8.3) | 36 to 54 | 15 to 21 | |
| Additive error (mg/liter) | 0.071 (19)d | 0.00071 to 0.50 | 3.2e−5 to 6.2 | |
The structural relationship is given by the following equations: Ka (h−1) = θ1, , and V/F (liters) = θ6 × (WT/70), where θ is an estimated fixed effect, WT is actual body weight in kilograms, and PNA is postnatal age in years. POPS, Pediatric Opportunistic Pharmacokinetic Study; SMX, sulfamethoxazole.
CL/F, apparent clearance; IIV, interindividual variability; Ka, absorption rate constant; PNA50, maturation half-life calculated as a function of postnatal age (in years); PNA Hill, Hill coefficient in the maturation function; RSE, relative standard error; V/F, apparent volume of distribution.
Minimization terminated with the full external data set.
Different from the value for the publication by Autmizguine et al. (21), in which the authors neglected to calculate the square root of this variance estimate in order to transform it into concentration units.
The results of the reestimation and bootstrap analysis using the external SMX model with either data set are summarized in Table 4. The reestimated Ka using the POPS data set was smaller than the Ka based on the external data set, but the CL/F and V/F were within 20% of each other. More than 90% of the bootstrap minimized successfully using either data set, indicating reasonable model stability. The 95% CIs for CL/F were narrow in both bootstraps and narrower than that estimated for each respective data set using the POPS SMX model. The 97.5th percentile for the IIV on Ka using either data set exceeded 100%, so this parameter may not have been precisely estimated. Ka was larger in the external data set (1.1 h−1 versus 0.34 h−1), and IIV for Ka was large (55% and 110%) for both data sets. This is likely due to the paucity of samples during the absorption phase in both data sets.
TABLE 4.
Parameter estimates and bootstrap analysis of the external SMX model developed from the current study using the POPS and external data setsa
| Parameter | POPS data |
External data |
||
|---|---|---|---|---|
| Parameter value (% RSE) | Bootstrap analysis (n = 1,000), 2.5th–97.5th percentiles | Parameter value (% RSE) | Bootstrap analysis (n = 1,000), 2.5th–97.5th percentiles | |
| Minimization successful | Yes | 923/1,000 | Yes | 999/1,000 |
| Fixed effects | ||||
| Ka (h–1) | 0.34 (25) | 0.16–0.60 | 1.1 (29) | 0.66–3.2 |
| CL/F (liters/h) | 1.4 (5.0) | 1.3–1.5 | 1.2 (6.9) | 1.0–1.3 |
| V/F (liters) | 20 (8.5) | 14–23 | 24 (7.7) | 20–28 |
| Random effects (%) | ||||
| IIV, Ka | 110 (18) | 41–260 | 55 (26) | 0.55–160 |
| IIV, CL | 35 (20) | 20–56 | 29 (17) | 18–39 |
| Proportional error | 43 (10) | 33–52 | 18 (7.8) | 15–21 |
The structural relationship is given as follows: Ka (h–1) = θ1, CL/F (liters/h) = θ2 × (WT/70)0.75, and V/F (liters) = θ3 × (WT/70), where θ is an estimated fixed effect and WT is actual body weight in kilograms. CL/F, apparent clearance; IIV, interindividual variability; Ka, absorption rate constant; POPS, Pediatric Opportunistic Pharmacokinetic Study; RSE, relative standard error; SMX, sulfamethoxazole; V/F, apparent volume.
Pooled data analysis.
Data from both studies were combined, and the results for the pooled data popPK model development are presented in the supplemental material only (Table S2).
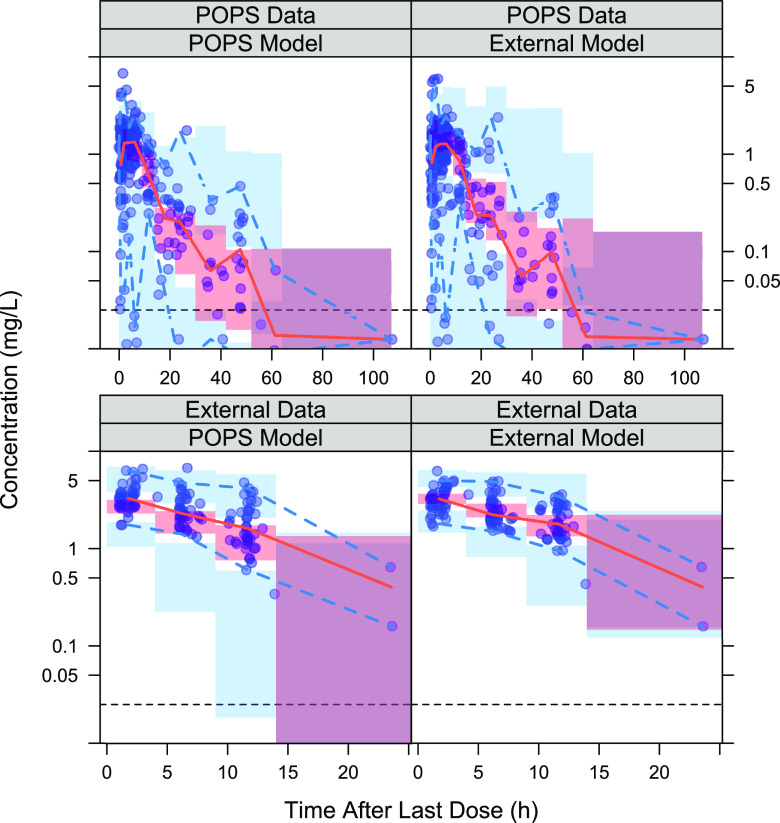
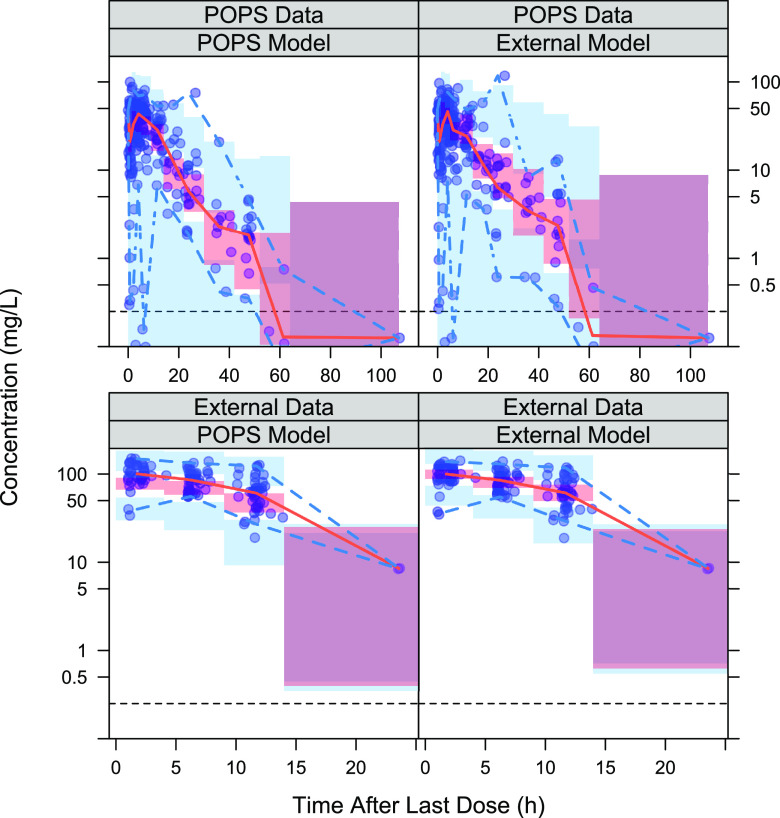
Simulation-based evaluation of each model’s predictive performance.
The prediction-corrected visual predictive checks (pcVPCs) of each model–data set combination are presented in Fig. 3 for TMP and Fig. 4 for SMX. For both TMP and SMX, the median percentile of the concentrations over time was well captured within the 95% CI in three of the four model–data set combinations, while underprediction was more apparent when the POPS model was applied to the external data. The prediction interval based on the validation data set was larger than the prediction interval based on the model development data set for both the POPS and external models. For each drug, the observed 2.5th and 97.5th percentiles were captured within the 95% confidence interval of the corresponding prediction interval for each model and its corresponding model development data set pairs, but the POPS model underpredicted the 2.5th percentile in the external data set while the external model had a larger confidence interval for the 97.5th percentile in the POPS data set. The external data set was tightly clustered and had only 20 subjects, so that underprediction of the lower bound may reflect the lack of heterogeneity in the external data set rather than overprediction of the variability in the POPS model. For SMX, the POPS model had an observed 97.5th percentile higher than the 95% confidence interval of the corresponding prediction. The high observation was much higher than the rest of the data and appeared to be a singular observation, so overall, the SMX POPS model still appeared to be adequate for predicting variability in the majority of the subjects. Overall, both models appeared to be acceptable for use in predicting exposure.
FIG 3.
pcVPCs for each TMP model–data set combination. The red shaded region represents the simulated 95% prediction interval for the median; the solid red line represents the observed median; the blue region represents the simulated 95% prediction interval for the 2.5th and 97.5th percentiles; the dashed blue lines represent the observed 2.5th and 97.5th percentiles; and the horizontal dashed black line represents the lower limit of quantification.
FIG 4.
pcVPCs for each SMX model–data set combination. The red shaded region represents the simulated 95% prediction interval for the median; the solid red line represents the observed median; the blue region represents the simulated 95% prediction interval for the 2.5th and 97.5th percentiles; the dashed blue lines represent the observed 2.5th and 97.5th percentiles; and the horizontal dashed black line represents the lower limit of quantification.
Simulations using the POPS and external TMP popPK models.
Dosing simulations showed that the external TMP model predicted higher exposure across all age groups (Fig. 5). For children below the age of 12 years, the dose that matched the area under the plasma concentration-versus-time curve in one dosing interval at steady state (AUCss) of adults taking the labeled dose of 160 mg every 12 h was 6 mg/kg every 12 h according to the POPS model and 4 mg/kg every 12 h according to the external model. In the cohort of individuals 12 to 18 years of age, most (88%) virtual subjects weighed 40 kg or more and received the standard adult dose of 160 mg every 12 h, so no difference between the dose levels was apparent. The POPS TMP model predicted slightly lower adult exposure than the literature adult AUCss range.
FIG 5.
Box plots of the AUCss (area under the plasma concentration-versus-time curve in one dosing interval at steady state) for TMP in virtual children (2 months to <2 years, 2 to <6 years, 6 to <12 years, and 12 to <18 years of age) compared to the exposure of adults taking 160 mg every 12 h. The mean ± twice the standard deviation for AUCss in one 12-h dosing interval at steady state based on seven studies of adults aged 18 to 60 years without significant renal or hepatic impairment taking 160 mg of TMP every 12 h (Q12h) is plotted in yellow (8–10, 12–15).
The proportion of subjects with concentrations above the MIC for more than half of the dosing interval at steady state is presented in Fig. S6. At each dose and MIC value, the external TMP model predicted a larger proportion than the POPS TMP model. At a MIC of 0.5 mg/liter, both models predicted that >90% of the virtual subjects in each age group achieved adequate time above the MIC at the labeled dose of 4 mg/kg every 12 h. However, when the MIC was increased to 1 mg/liter, only ≥41% based on the POPS model and ≥76% based on the external model had adequate exposure at 4 mg/kg every 12 h. In order for at least 90% of the subjects to achieve concentrations above 1 mg/liter for more than half of the dosing interval, the POPS model simulations suggested that a dose increase to 7.5 mg/kg every 12 h for infants and young children might be necessary. In the two cohorts above the age of 6 years, many subjects had doses capped at the adult dose of 160 mg every 12 h, which appeared to be subtherapeutic. In comparison, the external model suggested that a dose of 6 mg/kg every 12 h was likely adequate for all subjects, although only 88.6% of the virtual subjects in the adolescent cohort who predominantly received the adult dose of 160 mg every 12 h attained the specified target.
With WT-based dosing, the risk of supratherapeutic exposure is highest in the youngest cohort. The POPS TMP model predicts a minimal number of virtual subjects with an average simulated concentration at steady state (Cavg,ss) above 8 mg/liter at the tested doses of 4, 6, and 7.5 mg/kg every 12 h. The highest-risk cohort, 2-month-olds to <2-year-olds receiving a regimen of 7.5 mg/kg every 12 h, has 1.8% of subjects with Cavg,ss of >8 mg/liter. In contrast, the external TMP model predicts that a substantial proportion of the youngest cohort has supratherapeutic exposures, with 4%, 16%, and 26% of virtual subjects in the 2-month-old to <2-year-old cohort receiving 4, 6, and 7.5 mg/kg every 12 h, respectively, having Cavg,ss of >8 mg/liter.
DISCUSSION
This study is the first external evaluation of the initial popPK analysis of TMP-SMX administered by the oral route to infants and children (18). External evaluation elucidates the generalizability of the proposed model, which is important when the popPK model is used to assess exposure targets and make dosing recommendations, as with the POPS model. The newly collected external study data had much fewer subjects, though more samples per subject. In an exploratory analysis (results not shown), subjects with differing numbers of samples appeared to weigh equally in the parameter estimation, at least for a one-compartmental model. The decision was to emphasize the separate popPK model development and evaluation instead of the pooled data analysis, given that the more populous but sparse POPS study data strongly determine the outcome of the pooled model.
The independently developed external TMP model had a structure identical to that of the POPS TMP model. Therefore, the original model was reproducible with similar population estimates for the PK parameters. The external TMP model’s maturation half-life, calculated as a function of postnatal age in years (PNA50), was at nearly 1 year after birth (0.91 year), while the POPS TMP model had PNA50 at the age of ∼3 months (0.24 year). The external model’s PNA50 was likely overestimated, due to the lack of subjects below the age of 2.8 months in the external data set. Considering that TMP is mostly renally eliminated, the PNA Emax relationship likely described the effect of renal maturation on CL/F. Based on the work of Rhodin et al., 50% of the adult glomerular filtration rate is attained at a postmenstrual age (PMA) of 47.7 weeks, suggesting that the 3-month PNA50 estimate in the POPS TMP model has a stronger physiologic rationale (19). The inclusion of SCR as a covariate on CL/F further described the renal effect on TMP elimination. The exponent on the SCR was larger for the external TMP model (0.71) than for the POPS TMP model (0.40). For evaluating the exponent on the SCR, the external data set is limited by having renal impairment as an exclusion criterion, while the POPS data set included subjects with SCRs as high as 5.9 mg/dl. For subjects with normal SCR values, the two models predict similar effects of renal function on CL/F; for subjects with impaired renal function, the external TMP model predicts a more precipitous drop in CL/F than the POPS TMP model, and extrapolation of the external TMP model in these subjects may result in underprediction of TMP CL/F. Therefore, the covariate assessment based on the POPS TMP model may be more reliable.
In contrast, the external and POPS SMX models, though both one-compartment PK models, detected different covariate relationships and applied different residual error model structures. The POPS SMX model estimated a PNA50 of 0.12 year, which was less than the age of the youngest subject in the external data set. Assuming that the maturation effect in the POPS SMX model was accurate, the effect of age was expected to be negligible in the external data set, with the youngest two subjects most expected to be impacted, having only 20% and 3% decreases in CL/F. Given that TMP-SMX is usually contraindicated in pediatric patients below the age of 2 months due to the risk of kernicterus, the effect of age on clearance is unlikely to be relevant. The covariate effect of albumin was not assessed in external SMX model development, given that albumin data were not available from most subjects. The albumin level was also missing from nearly half of the subjects in the POPS study, and the imputation of missing albumin values based on age range could potentially confound the effects of age and albumin. For practical purposes, as well, it may be reasonable to exclude a covariate that is not routinely collected from patients. Although albumin may have an effect on protein binding and thus may affect the volume of distribution, SMX is only 70% protein bound, so alterations in albumin are expected to have limited clinical significance (27). While the independent external SMX model could not confirm the covariate relationships in the POPS SMX model, the difference likely reflected insufficient data in the external data set to evaluate the effects or overparameterization of the POPS model.
The bootstrap analysis of the POPS SMX model using either data set affirmed that the model was overparameterized, and the parameters were not precisely estimated. The other models of the POPS TMP model, external TMP model, and external SMX model had better model stability and narrower CIs. In the PE and pcVPC analyses for both drugs, the external model predicted higher exposure than the POPS model, and the POPS model predicted a larger prediction interval for the concentration ranges. Given that the external data set was composed of only 20 subjects, the possibility that it did not include enough data to represent the variabilities in the target population cannot be ruled out. Since the subjects in the POPS data set received lower doses and had a substantial fraction of concentrations below the limit of quantification (BLQ) (∼10% versus none in the external data set), it was also possible that the BLQ management choice in the POPS study (calculating the BLQ ceiling as the value of the lower limit of quantification divided by 2) biased the POPS model. However, this possibility was ruled out, because reestimation of both the POPS TMP and SMX models using the M3 method (which estimates the likelihood of a BLQ result at each measurement time) produced similar concentration predictions (results not shown), showing that the choice of BLQ management strategy was not important.
As in the previous publication, we focused the dosing simulation on the TMP component because the combination was available only in 1:5 fixed ratios, and the SMX concentration has not been correlated with efficacy or toxicity previously, since SMX has an active metabolite (21, 28). Simulations of the POPS and external TMP models at various dose levels were compared to adult steady-state exposure at 160 mg every 12 h, an exposure derived from several studies of healthy adults without apparent renal or hepatic impairment (8–10, 12–15). The external TMP model consistently predicted higher exposures than the POPS TMP model for all age cohorts. The most likely reason is that the external data set, being composed of only 20 subjects, does not capture the entire range of IIV in PK parameters. Based on the external TMP model, the original label dose of 4 mg/kg every 12 h was equivalent to the adult dose of 160 mg every 12 h, while the POPS TMP model implied that adolescents taking the adult dose had exposures at the lower end of the adult range.
Whether TMP-SMX exhibits time- or concentration-dependent antimicrobial killing has not been conclusively elucidated (29–32). A high maximum concentration was associated with increased rates of hematologic abnormalities, and dosing frequency was usually every 12 h, so the proportion of subjects with plasma drug concentrations above the MIC for >50% of the dosing interval at steady state was evaluated (33). For pathogens with a MIC of ≤0.5 mg/liter, the original label-recommended dose of 4 mg/kg every 12 h was appropriate based on either the POPS or the external TMP model. For pathogens with a MIC of 1 mg/liter, the POPS TMP model simulations suggested that the TMP dose must be increased to 7.5 mg/kg every 12 h, while the external TMP model suggested that a dose of 6 mg/kg every 12 h was appropriate. Therefore, both models implied that a dose increase was needed to counter increased resistance. On the other hand, the external TMP model had simulated concentrations that may suggest a greater risk of hematologic abnormalities (based on the use of a Cavg,ss value of >8 mg/liter as an upper exposure threshold) in the 2-month-old to <2-year-old cohort receiving a dose of ≥6 mg/kg every 12 h. For these subjects, a more conservative dosing approach or more-frequent laboratory monitoring may need to be considered.
While this is the first external evaluation analysis performed for pediatric TMP-SMX popPK models, several limitations must be considered. First, the external data set included only 20 subjects, which is unlikely to be a representative distribution of all children. Second, as discussed above, the external data set had a narrower age range, a narrower SCR range, and insufficient information on albumin levels, which limited its usefulness at evaluating all covariate effects in the POPS model. The covariate effects in the POPS TMP model were robust enough to be detected in the external data set, but the covariate effects in the POPS SMX model could not be evaluated, due to insufficient information in the external data set. With these limitations, a difference in conclusions based on either data set was unsurprising, and the conclusion based on the larger POPS study was considered to be more reliable.
MATERIALS AND METHODS
Study design.
Oral TMP-SMX PK data from two studies were available for analysis. Each study protocol was approved by the institutional review boards of participating institutions. Informed consent was obtained from the parent or guardian, and assent was obtained from the subject when appropriate. The first study is the Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care (POPS) trial (ClinicalTrials.gov registration no. NCT01431326), a multicenter (n = 16), open-label, prospective observational PK and safety study of understudied drugs administered to children (<21 years of age) per standard of care. Exclusion criteria included failure to obtain consent/assent or known pregnancy. Dosing differed between subjects, and PK samples were sparsely and opportunistically collected. The POPS study design has been described previously (21).
The external data study (ClinicalTrials.gov registration no. NCT02475876) was a multicenter (n = 3), open-label, interventional PK and safety study in which children between a postmenstrual age (PMA) of 36 weeks and the age of 16 years received either TMP-SMX or clindamycin at the discretion of the treating clinicians. Patients already receiving TMP-SMX were also allowed to be enrolled. Exclusion criteria included failure to obtain consent or assent, known pregnancy or breastfeeding, history of allergic reactions to study drugs, serum creatinine levels of >2 mg/dl, alanine aminotransferase concentrations of >250 U/liter or aspartate transaminase concentrations of >500 U/liter, or extracorporeal membrane oxygenation support. The protocol-specified doses were 6 mg/kg (based on the TMP component) every 12 h for subjects between the ages of 2 months and 12 years and 4 mg/kg every 12 h for subjects >12 to 16 years of age. PK samples were collected at protocol-specified times, which were 1 to 3 h and 6 to 8 h after the 1st and 6th dose and <30 min before the 2nd, 6th, and 7th dose.
Study data.
The POPS data set included 240 plasma samples from 153 patients. Among these samples, 26 (10.8% of the data) TMP concentrations and 19 (7.9%) SMX concentrations were BLQ. BLQ results that occurred at any time after the first dose were assigned a value of half the lower limit of quantification (LLOQ); 4 (1.7%) BLQ samples were collected before the first dose and treated as missing. The external data set included 121 plasma samples from 20 patients. None of the TMP or SMX concentrations was BLQ. One sample (0.8%) was suspected to be erroneous and was excluded from analysis because the TMP component indicated a trough level higher than the peak concentration.
The demographic characteristics, laboratory values, and dose information for each data set are presented in Table 1. Gestational age (GA) was collected for infants up to the age of ∼4 months for the POPS study and 1 year for the external data study; missing values were set to 40 weeks. The POPS study imputed missing height as the 50th percentile value of height for WT and sex, and it imputed missing SCR from PNA using linear regression as described previously (21). In the POPS data set, missing albumin measurements were set to the median albumin value for the age group (2.80 g/dl for ≤30 days, 3.30 g/dl for 31 days to <2 years, 3.35 g/dl for 2 to <13 years, 3.40 g/dl for 13 to <16 years, and 3.55 g/dl for 16 to <21 years). In the external data set, missing albumin measurements were set to a median albumin value of 3.35 g/dl from the overall POPS data set. A covariate correlation matrix plot is shown in Fig. S7 in the supplemental material.
The plasma samples of both studies were quantified at a single central laboratory (OpAns, LLC, Durham, NC, USA) using validated high-performance liquid chromatography–tandem mass spectrometry (HPLC–MS-MS) assays. The LLOQs were 0.025 mg/liter for TMP and 0.25 mg/liter for SMX. The analytical method has been described previously (21).
Population PK model development.
The POPS TMP and SMX popPK models were derived previously (21). In the current study, popPK modeling conducted using the merged data set is presented in the supplemental material, and independent popPK modeling using the external data set was performed to derive the external popPK models for TMP and SMX. The popPK modeling development followed a typical workflow of nonlinear mixed-effect modeling in NONMEM (version 7.4.3; Icon Development Solutions, Ellicott City, MD, USA) and a stepwise covariate modeling search. First-order conditional estimation with eta-epsilon interaction and log-normally distributed IIV in the PK parameters were assumed. One-, two-, and three-compartment PK models with linear kinetics were tested for both TMP and SMX. The correlations between random-effect parameters (ρ) were tested for each IIV pair in the model. The residual errors were explored using additive, proportional, or combined additive-plus-proportional error models. Total body WT scaled to a standard 70-kg adult with fixed allometric exponents of 0.75 for CL/F and 1 for V/F was assumed a priori (34, 35). Alternate size descriptors, including estimating the allometric WT, body mass index, body surface area, ideal body WT, adjusted body WT, lean body mass (3 different equations), fat-free mass, and normal fat mass, were also explored. The equations for the different size descriptors are summarized in Table S3.
Available covariates were tested for model inclusion using automated stepwise covariate modeling in the Perl-speaks-NONMEM (PsN) tool kit (version 4.7.0; Uppsala Pharmacometrics, Uppsala, Sweden) with a forward inclusion criterion of a P value of <0.05 (change in objective function value, >3.8 points) and backward elimination at a P value of <0.01 (change in objective function value, >6.6 points). The covariates of GA, PNA, PMA, SCR, and sex were tested in all parameter-covariate pairs. GA was not correlated to PMA, because there were only a few infants in our data set. PNA and PMA were highly correlated, but both were tested, because each had been used in ontogeny functions.
The effect of race was not explored because the data set consisted of predominantly Caucasian subjects. The effect of albumin was not explored because the data set did not have a sufficient number of albumin measurements. The effect of height was generally not explored in pediatric popPK studies that included infants, because height cannot be measured reliably in this population. The relationships tested included equation 1 for categorical covariates and equations 2 to 5 for continuous covariates, where COV denotes a covariate, COVmed indicates the median covariate value, PARCOV denotes the covariate effect on the parameter, θ is estimated, and θj denotes the θ for the jth unique categorical value.
| (1) |
| (2) |
| (3) |
| (4) |
| (5) |
Given that the covariate search was performed using an automated approach, failed individual model runs were manually repeated, and the final model was assessed for physiological plausibility.
External model evaluations.
Patient-level data sets from both the POPS and external studies were used to evaluate the POPS and external models. The stability of the parameter estimates and the predictive performance of the models were evaluated in multiple ways. First, the parameters in each of the models were fixed to evaluate the goodness-of-fit plots, which included the population prediction (PRED) versus observation, CWRES versus time after last dose, and CWRES versus PRED. Then the improvement in prediction error (PE) and the relative root mean-square error (rRMSE) were computed using equations 6 and 7, respectively:
| (6) |
| (7) |
where i represents the ith observation.
The parameter estimates of each model were reestimated using each data set and were bootstrapped 1,000 times using PsN to determine the 95% CI. The pcVPCs based on 1,000 simulations for each model and data set combination were generated using PsN.
Dosing simulations.
Four virtual pediatric populations with 500 subjects each were created in the software R for the age groups of 2 months to <2 years, 2 to <6 years, 6 to <12 years, and 12 to <18 years. Equal probability of male and female gender, as well as a uniform distribution for PNA, was assumed. The distribution of GAs was based on the most recent U.S. birth data at the time of analysis (36). WT was based on age- and sex-appropriate growth charts, which included the Fenton preterm growth chart for infants up to a PMA of 51 weeks, the World Health Organization growth chart for infants up to the age of 2 years, and the Centers for Disease Control and Prevention growth chart for children 2 years old and older (37–39). Age- and sex-appropriate serum creatinine values were simulated for each virtual subject (40). The simulated distributions of covariates are shown in Fig. S8 to S13.
Exposure was simulated based on the TMP component for both the POPS and the external TMP model. Simulation was conducted for doses of 4, 6, and 7.5 mg/kg of TMP every 12 h, with the maximum dose capped at the adult dose of 160 mg TMP every 12 h (21). Simulation results were assessed by (i) the percentage of subjects with free TMP concentrations above the MICs of relevant bacteria (Streptococcus pneumoniae, Escherichia coli, and community-acquired methicillin-resistant S. aureus [CA-MRSA]) for >50% of the dosing interval at steady state, assuming an unbound fraction of 56% (6); and (ii) AUCss compared to the exposure of adults taking 160 mg of TMP every 12 h (6, 21). The adult exposure was assessed from seven studies of adults aged 18 to 60 years without significant renal or hepatic impairment taking 160 mg of TMP every 12 h (8–10, 12–15).
Pooled data set analysis.
PopPK model development was also conducted with the pooled data set combining the POPS and external studies. The results are presented in the supplemental material (final model in Table S2; goodness of fit in Fig. S14).
ACKNOWLEDGMENTS
This Pediatric Trials Network (PTN) study was funded under National Institute of Child Health and Human Development (NICHD) contract HHSN275201000003I (Principal Investigator [PI], Daniel K. Benjamin, Jr.). The Best Pharmaceuticals for Children Act (BPCA) Data Coordinating Center was funded under contract HHSN275201700002C (PI, Ravinder Anand). Y.S.S.W. was supported through a University of North Carolina (UNC)/Nuventra Pharmacokinetics/Pharmacodynamics Fellowship. M.C.-W. received support for research from the National Institutes of Health (grants 1R01-HD076676-01A1 and 1K24-AI143971), the National Institute of Allergy and Infectious Diseases (grants HHSN272201500006I and HHSN272201300017I), the National Institute of Child Health and Human Development (NICHD) (grant HHSN275201000003I), the U.S. Food and Drug Administration (grant 5U18-FD006298), and industry for drug development in adults and children. C.P.H. received salary support for research from NICHD (grants 1K23HD090239 and R13HD102136), the National Heart Lung and Blood Institute (NHLBI) (grant R61/R33HL147833), and the U.S. Food and Drug Administration (grants 1R01-FD006099 [PI, Matthew Laughon] and 5U18-FD006298 [PI, Daniel K. Benjamin, Jr.]), and from the U.S. government for his work in pediatric clinical pharmacology (contract HHSN275201800003I [PI, Daniel K. Benjamin, Jr.] under the Best Pharmaceuticals for Children Act), from the nonprofit Burroughs Wellcome Fund, and from other sponsors for drug development in adults and children (https://dcri.org/about-us/conflict-of-interest/). J.G.G. received research support from the National Institute of General Medical Sciences (NIGMS)-funded T32 program (award 5T32GM122741), as well as the American Foundation for Pharmaceutical Education (AFPE). D.G. received research support from the NICHD (grants K23HD083465 and R01HD096435). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Y.S.S.W. and D.G. wrote the manuscript; Y.S.S.W. and D.G. designed the research; Y.S.S.W., M.C.-W., C.P.H., J.G.G., J.A., M.C., and D.G. performed the research; Y.S.S.W. and J.G.G. analyzed the data.
PTN Steering Committee Members include Daniel K. Benjamin, Jr., Christoph Hornik, Kanecia Zimmerman, Phyllis Kennel, and Rose Beci, Duke Clinical Research Institute, Durham, NC; Chi Dang Hornik, Duke University Medical Center, Durham, NC; Gregory L. Kearns, Texas Christian University and UNTHSC School of Medicine, Fort Worth, TX; Matthew Laughon, University of North Carolina at Chapel Hill, Chapel Hill, NC; Ian M. Paul, Penn State College of Medicine, Hershey, PA; Janice Sullivan, University of Louisville, Louisville, KY; Kelly Wade, Children's Hospital of Philadelphia, Philadelphia, PA; and Paula Delmore, Wichita Medical Research and Education Foundation, Wichita, KS.
The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) team members include Perdita Taylor-Zapata and June Lee.
The Emmes Company, LLC (Data Coordinating Center), team members include Ravinder Anand, Gaurav Sharma, Gina Simone, Kim Kaneshige, and Lawrence Taylor.
The PTN Publications Committee is chaired by Thomas Green, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL.
The Pediatric Trials Network trimethoprim-sulfamethoxazole POPS study team, principal investigators (PIs), and study coordinators (SCs) are as follows: at the Duke Clinical Research Institute, Tammy Day (clinical research associate [CRA]); at the Université de Montréal, Julie Autmizguine (PI); at The University of North Carolina at Chapel Hill, Matthew Laughon (site PI), Janice Bernhardt (SC), Ashley Mariconti (SC), and Daniel Gonzalez; at Ann and Robert H. Lurie Children’s Hospital, Ram Yogev (site PI), Laura Fearn (SC), Rohit Kalra (SC), Mayra Gomez (SC), and Bemajin Traisman (SC); at the University of Louisville, Norton Children’s Hospital, and Kosair Charities Pediatric Clinical Research Unit, Janice Sullivan (site PI), Jen Comings (SC), Jackie Perry (SC), and Michelle Wiseheart (SC); at the Medical University of South Carolina Children’s Hospital, Andrew Atz (site PI) and Layla Al Sarraf (SC); at the Oregon Health and Science University, Amira Al-Uzri (site PI), Kira Clark (SC), Carrie Farrar (SC), and Connie Swanson (SC); at the University of Maryland, Susan Mendley (site PI) and Donna Cannonier (SC); at the Riley Hospital for Children at Indiana University, Brenda Poindexter (site PI), Susan Gunn (SC), and Dianne Wilson (SC); at the Wesley Medical Center, Paula Delmore (site PI and SC); at Seattle Children’s Hospital, Joseph Flynn (site PI), Shannon Granillo (SC), Rob Johnson (SC), and Megan Kelton-Rehkopf (SC); at the University of California—San Diego Medical Center, Adriana Tremoulet (site PI) and Baharin Abdullah (SC); at Children’s Mercy Hospital, Kathy Neville (site PI) and Jaylene Weigel (SC); at Children’s National Medical Center, John van den Anker (site PI) and Elaine Williams (SC); at the University of Utah Hospitals and Clinics, Catherine Sherwin (site PI), Fumiko Alger (SC), Sharada Dixit (SC), JoAnn Narus (SC), Rebbecca Perez (SC), Priscilla Rosen (SC), and Yakub Salman (SC); at Axis Clinical Trials, Lydie Hazan (site PI) and Angelica Covarrubias (SC); at the Duke University Medical Center, Kevin Watt (site PI), Christie Milleson (SC), and Samantha Wrenn (SC); at the Alfred I. DuPont Hospital for Children, Marisa Meyer (site PI) and Ramany John (SC); at the Arkansas Children’s Hospital Research Institute, Laura James (site PI), Lee Howard (SC), D. Ann Pierce (SC), and Kristin Richmond (SC); and at the Yale University School of Medicine, Matthew Bizarro (site PI).
Study investigators for the external data study are as follows: at the Children's Hospital of Orange County Research Institute, Antonio Arrieta (site PI) and Claudia Enriquez (SC); at the Arkansas Children’s Hospital Research Institute, Laura James (site PI) and Howard Lee (SC); at the University of Michigan Health System, Varsha Bhatt-Mehta (site PI) and Chaandini Jayachandran (SC); at the Rady Children's Hospital and Health Center, John Bradley (site PI) and Sara Hingtgen (SC); at the Ann and Robert H. Lurie Childrens Hospital of Chicago, William Muller (site PI) and Laura Fearn (SC); at the Oregon Health and Science University, Amira Al-Uzri (site PI) and Kira Clark (SC); and at the Children’s Hospital of Philadelphia, Kevin Downes (site PI) and Shawn O’Connor (SC).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Sun Pharmaceutical Industries, Inc. 2009. Bactrim DS [package insert]. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0138a156-859a-48a3-bf5a-e2db0cc7f2f9.
- 2.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, J Rybak M, Talan DA, Chambers HF, Infectious Diseases Society of America. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 3.Miller LG, Daum RS, Creech CB, Young D, Downing MD, Eells SJ, Pettibone S, Hoagland RJ, Chambers HF, DMID 07-0051 Team. 2015. Clindamycin versus trimethoprim-sulfamethoxazole for uncomplicated skin infections. N Engl J Med 372:1093–1103. 10.1056/NEJMoa1403789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talan DA, Mower WR, Krishnadasan A, Abrahamian FM, Lovecchio F, Karras DJ, Steele MT, Rothman RE, Hoagland R, Moran GJ. 2016. Trimethoprim-sulfamethoxazole versus placebo for uncomplicated skin abscess. N Engl J Med 374:823–832. 10.1056/NEJMoa1507476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klepser ME, Zhu Z, Nicolau DP, Banevicius MA, Belliveau PP, Ross JW, Broisman L, Quintiliani R, Nightingale CH. 1996. Oral absorption of trimethoprim-sulfamethoxazole in patients with AIDS. Pharmacotherapy 16:656–662. [PubMed] [Google Scholar]
- 6.Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant R, Sajed T, Johnson D, Li C, Sayeeda Z, Assempour N, Iynnkaran I, Liu Y, Maciejewski A, Gale N, Wilson A, Chin L, Cummings R, Le D, Pon A, Knox C, Wilson M. 2017. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res 46:1074–1082. 10.1093/nar/gkx1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson EJ, Wu H, Maharaj A, Edginton AN, Balevic SJ, Cobbaert M, Cunningham AP, Hornik CP, Cohen-Wolkowiez M. 2019. Physiologically based pharmacokinetic modeling for trimethoprim and sulfamethoxazole in children. Clin Pharmacokinet 58:887–898. 10.1007/s40262-018-00733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stevens RC, Laizure SC, Williams CL, Stein DS. 1991. Pharmacokinetics and adverse effects of 20-mg/kg/day trimethoprim and 100-mg/kg/day sulfamethoxazole in healthy adult subjects. Antimicrob Agents Chemother 35:1884–1890. 10.1128/aac.35.9.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoppu K. 1987. Age differences in trimethoprim pharmacokinetics: need for revised dosing in children? Clin Pharmacol Ther 41:336–343. 10.1038/clpt.1987.36. [DOI] [PubMed] [Google Scholar]
- 10.Varoquaux O, Lajoie D, Gobert C, Cordonnier P, Ducreuzet C, Pays M, Advenier C. 1985. Pharmacokinetics of the trimethoprim-sulphamethoxazole combination in the elderly. Br J Clin Pharmacol 20:575–581. 10.1111/j.1365-2125.1985.tb05114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welling PG, Weinfeld RE, Craig WA, Amidon GL, Kunin CM. 1973. Pharmacokinetics of trimethoprim and sulfamethoxazole in normal subjects and in patients with renal failure. J Infect Dis 128(Suppl 3):S556–S566. 10.1093/infdis/128.Supplement_3.S556. [DOI] [PubMed] [Google Scholar]
- 12.Männistö PT, Mäntylä R, Mattila J, Nykänen S, Lamminsivu U. 1982. Comparison of pharmacokinetics of sulphadiazine and sulphamethoxazole after intravenous infusion. J Antimicrob Chemother 9:461–470. 10.1093/jac/9.6.461. [DOI] [PubMed] [Google Scholar]
- 13.Watson ID, Cohen HN, Stewart MJ, McIntosh SJ, Shenkin A, Thomson JA. 1982. Comparative pharmacokinetics of co-trifamole and co-trimoxazole to “steady state” in normal subjects. Br J Clin Pharmacol 14:437–443. 10.1111/j.1365-2125.1982.tb02004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ludwig E, Graber H, Csiba A. 1982. Pharmacokinetics of the sulphamethoxazole-trimethoprim combination in geriatric patients. Infection 10:315–316. 10.1007/BF01640884. [DOI] [PubMed] [Google Scholar]
- 15.Stevens RC, Laizure SC, Sanders PL, Stein DS. 1993. Multiple-dose pharmacokinetics of 12 milligrams of trimethoprim and 60 milligrams of sulfamethoxazole per kilogram of body weight per day in healthy volunteers. Antimicrob Agents Chemother 37:448–452. 10.1128/aac.37.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jelliffe RW, Gomis P, Tahani B, Ruskin J, Sattler FR. 1997. A population pharmacokinetic model of trimethoprim in patients with pneumocystis pneumonia, made with parametric and nonparametric methods. Ther Drug Monit 19:450–459. 10.1097/00007691-199708000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Alsaad N, Dijkstra JA, Akkerman OW, de Lange WCM, van Soolingen D, Kosterink JGW, van der Werf TS, Alffenaar JWC. 2016. Pharmacokinetic evaluation of sulfamethoxazole at 800 milligrams once daily in the treatment of tuberculosis. Antimicrob Agents Chemother 60:3942–3947. 10.1128/AAC.02175-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eunice Kennedy Shriver National Institute of Child Health and Human Development. 2019. Best Pharmaceuticals for Children Act (BPCA) priority list of needs in pediatric therapeutics. https://bpca.nichd.nih.gov/prioritization/working_groups/Pages/annual-prioritization.aspx.
- 19.Rhodin MM, Anderson BJ, Peters AM, Coulthard MG, Wilkins B, Cole M, Chatelut E, Grubb A, Veal GJ, Keir MJ, Holford NHG. 2009. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol 24:67–76. 10.1007/s00467-008-0997-5. [DOI] [PubMed] [Google Scholar]
- 20.Springer C, Eyal F, Michel J. 1982. Pharmacology of trimethoprim-sulfamethoxazole in newborn infants. J Pediatr 100:647–650. [DOI] [PubMed] [Google Scholar]
- 21.Autmizguine J, Melloni C, Hornik CP, Dallefeld S, Harper B, Yogev R, Sullivan JE, Atz AM, Al-Uzri A, Mendley S, Poindexter B, Mitchell J, Lewandowski A, Delmore P, Cohen-Wolkowiez M, Gonzalez D. 2017. Population pharmacokinetics of trimethoprim-sulfamethoxazole in infants and children. Antimicrob Agents Chemother 62:e01813-17. 10.1128/AAC.01813-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siber GR, Gorham CC, Ericson JF, Smith AL. 1982. Pharmacokinetics of intravenous trimethoprim-sulfamethoxazole in children and adults with normal and impaired renal function. Rev Infect Dis 4:566–578. 10.1093/clinids/4.2.566. [DOI] [PubMed] [Google Scholar]
- 23.Upreti VV, Wahlstrom JL. 2016. Meta-analysis of hepatic cytochrome P450 ontogeny to underwrite the prediction of pediatric pharmacokinetics using physiologically based pharmacokinetic modeling. J Clin Pharmacol 56:266–283. 10.1002/jcph.585. [DOI] [PubMed] [Google Scholar]
- 24.Saghir SA, Khan SA, McCoy AT. 2012. May. Ontogeny of mammalian metabolizing enzymes in humans and animals used in toxicological studies. Crit Rev Toxicol 42:323–357. 10.3109/10408444.2012.674100. [DOI] [PubMed] [Google Scholar]
- 25.Pressiat C, Mea-Assande V, Yonaba C, Treluyer J-M, Dahourou D-L, Amorissani-Folquet M, Blanche S, Eboua F, Ye D, Lui G, Malateste K, Zheng Y, Leroy V, Hirt D, MONOD Study Group. 2017. Suboptimal cotrimoxazole prophylactic concentrations in HIV-infected children according to the WHO guidelines. Br J Clin Pharmacol 83:2729–2740. 10.1111/bcp.13397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration. 1999. Population pharmacokinetics: guidance for industry. FDA, Silver Spring, MD. [Google Scholar]
- 27.Scheife RT. 1989. Protein binding: what does it mean? Ann Pharmacother 23:S27–S31. 10.1177/106002808902300706. [DOI] [PubMed] [Google Scholar]
- 28.Joos B, Blaser J, Opravil M, Chave JP, Lüthy R. 1995. Monitoring of co-trimoxazole concentrations in serum during treatment of Pneumocystis carinii pneumonia. Antimicrob Agents Chemother 39:2661–2666. 10.1128/aac.39.12.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wisell KT, Kahlmeter G, Giske CG. 2008. Trimethoprim and enterococci in urinary tract infections: new perspectives on an old issue. J Antimicrob Chemother 62:35–40. 10.1093/jac/dkn147. [DOI] [PubMed] [Google Scholar]
- 30.Close SJ, McBurney CR, Garvin CG, Chen DC, Martin SJ. 2002. Trimethoprim-sulfamethoxazole activity and pharmacodynamics against glycopeptide-intermediate Staphylococcus aureus. Pharmacotherapy 22:983–989. 10.1592/phco.22.12.983.33599. [DOI] [PubMed] [Google Scholar]
- 31.Brown GR. 2014. Cotrimoxazole—optimal dosing in the critically ill. Ann Intensive Care 4:13. 10.1186/2110-5820-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeldandi V, Strodtman R, Lentino JR. 1988. In-vitro and in-vivo studies of trimethoprim-sulphamethoxazole against multiple resistant Staphylococcus aureus. J Antimicrob Chemother 22:873–880. 10.1093/jac/22.6.873. [DOI] [PubMed] [Google Scholar]
- 33.Hughes WT, LaFon SW, Scott JD, Masur H. 1995. Adverse events associated with trimethoprim-sulfamethoxazole and atovaquone during the treatment of AIDS-related Pneumocystis carinii pneumonia. J Infect Dis 171:1295–1301. 10.1093/infdis/171.5.1295. [DOI] [PubMed] [Google Scholar]
- 34.Holford NA. 1996. Size standard for pharmacokinetics. Clin Pharmacokinet 30:329–332. 10.2165/00003088-199630050-00001. [DOI] [PubMed] [Google Scholar]
- 35.Holford N, Heo Y-A, Anderson B. 2013. A pharmacokinetic standard for babies and adults. J Pharm Sci 102:2941–2952. 10.1002/jps.23574. [DOI] [PubMed] [Google Scholar]
- 36.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK, Drake P. 2018. Births: final data for 2017. Natl Vital Stat Rep 67:1–50. [PubMed] [Google Scholar]
- 37.Fenton TR, Kim JH. 2013. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 13:59. 10.1186/1471-2431-13-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.WHO Multicentre Growth Reference Study Group. 2016. The WHO child growth standards. WHO, Geneva, Switzerland. https://www.who.int/childgrowth/standards/en/.
- 39.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11:1–190. [PubMed] [Google Scholar]
- 40.Savory DJ. 1990. Reference ranges for serum creatinine in infants, children and adolescents. Ann Clin Biochem 27(Pt 2):99–101. 10.1177/000456329002700201. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download AAC.02149-20-s0001.pdf, PDF file, 0.4 MB (399.3KB, pdf)