Abstract
BACKGROUND:
The safety and efficacy of brain parenchyma biopsy during minimally invasive (MIS) intracerebral hemorrhage (ICH) clot evacuation has not been previously reported. The objective of this study was to establish the safety and diagnostic efficacy of brain biopsy during MIS ICH clot evacuation and to validate the modified Boston criteria as a predictor of cerebral amyloid angiopathy (CAA) in this cohort.
METHODS:
From October 2016 to March 2018, superficial and perihematomal biopsies were collected for 40 patients undergoing MIS ICH clot evacuation and analyzed by the pathology department to assess for various ICH etiologies. Additionally, the admission magnetic resonance imaging or computed tomography scan of each patient was analyzed and evaluated for the likelihood of a CAA etiology based on the modified Boston criteria. Student t test was used to analyze intergroup differences in continuous variables, and a 2-tailed Fisher exact test was used to determine intergroup differences of categorical variables, with significance set at P < 0.05.
RESULTS:
Two of the 40 patients (5%) experienced postoperative rebleed. Four of the 40 patients (10%) had evidence of CAA on biopsy. Patients with CAA on biopsy were older (P = 0.005) and had a higher prevalence of parietal lobe (P = 0.02) and occipital lobe (P = 0.001) hemorrhage. The modified Boston criteria had a sensitivity of 100% (95% confidence interval [CI], 39.6%–100%) and a specificity of 72.2% (95% CI, 54.6%–84.2%) for predicting CAA on biopsy.
CONCLUSIONS:
Brain biopsy in MIS ICH clot evacuation is safe and allows for the diagnosis of various ICH etiologies.
Keywords: Biopsies, Cerebral amyloid angiopathy, Intracerebral hemorrhage, Minimally invasive surgery
INTRODUCTION
Intracerebral hemorrhage (ICH) is a devastating disease that accounts for 10%–15% of all stroke.1 Despite many new clinical trials in the past 2 decades, little to no improvement has been made in the mortality and morbidity of ICH.2,3 The 30-day mortality of ICH remains roughly 40%–50% with the proportion of patients dependent at 6 months at approximately 75%, making it the deadliest and most debilitating form of stroke.2,4,5
There are many documented etiologies of ICH, including hypertension, cerebral amyloid angiopathy (CAA), arteriovenous malformation, tumor, anticoagulant use, and illicit drug use.6–9 Of these, hypertension carries the strongest risk of ICH, with a documented odds ratio of 3.68 (95% confidence interval [CI], 2. 52–5.38).10 There is ample evidence that CAA is associated with lobar ICH because a recent systematic review found an odds ratio of 2.21 (95% CI, 1.09–4.45).11 Since the publication of the Surgical Trial in Lobar Intracerebral Haemorrhage trial in 2005, numerous clinical trials have evaluated the safety and efficacy of a minimally invasive (MIS) approach, including Intraoperative Stereotactic Computed Tomography-Guided Endoscopic Surgery, Minimally Invasive Surgery Plus rt-PA for Intracerebral Hemorrhage Evacuation, Minimally Invasive Surgery Plus rt-PA for Intracerebral Hemorrhage Evacuation III, and Early MiNimally-invasive Removal of IntraCerebral Hemorrhage.12–15 MIS surgery for ICH clot evacuation is now under active evaluation and can provide a source of brain biopsy in ICH evacuation surgery. In previous studies using an open biopsy technique, it has been advised that the site of the biopsy should be proximate to the site of the disease process evident on patient imaging, with data suggesting that diagnostic accuracy is improved when the leptomeninges, cortical, and subcortical regions are all sampled.16
However, similar criteria for MIS ICH biopsy have not been reported because of the novelty of the procedure. Tseng and Lin17 performed a hematoma biopsy of an ICH in stereotactic surgery; however, the authors note that no brain parenchyma was included. In addition, although MIS biopsy has been shown to cause fewer complications than open biopsy, hemorrhage is still a potentially devastating complication.18 In a cohort of patients with ICH, this consideration is salient because rebleeding is associated with poor outcome.19–21 It is therefore crucial that if biopsy is performed during the MIS ICH, it is performed without significant risk of intraoperative bleeding or postoperative rebleeding.
The purpose of this study is to establish the safety and efficacy of biopsy of brain parenchyma during MIS ICH clot evacuation, to determine the prevalence of CAA in a single-center MIS ICH cohort, and to validate the modified Boston criteria in this cohort. The modified Boston criteria is a set of demographic and imaging criteria to determine the probability of CAA etiology in a patient with ICH, including patient age of >55 years; evidence of lobar, cortical, or cortical/subcortical hemorrhage; evidence of cortical superficial siderosis; and absence of other causes.22,23
METHODS
From October 2016 to March 2018, 40 consecutive patients undergoing MIS endoscopic ICH evacuation via the stereotactic intracerebral hemorrhage underwater blood aspiration (SCUBA) technique were biopsied and retrospectively analyzed. Institutional review board study approval and patient consent were obtained. The SCUBA technique involves a 2-phase approach with the use of an aspiration device (Apollo or Artemis Device [Penumbra Inc., Alameda, California, USA]) inserted through the working channel of a Storz Lotta 3 port endoscope (KARL STORZ Endoscopy-America, Inc., El Segundo, California, USA). In the first phase, liquid and solid clot are aspirated with medium to high suction power beginning in the distal portion of the hematoma and retracting toward the operator. In the second phase, lactated ringer’s irrigation is infused continuously to prevent the hematoma cavity from collapsing to permit endoscopic exploration, direct visualization of all cavity walls, and cautery of bleeding vessels.24 The eligibility criteria for the SCUBA technique were as follows: a hematoma volume >20 cm3, a National Institutes of Health Stroke Scale score ≥6, a Glasgow Coma Scale score ≥4, and a baseline modified Rankin scale score of 0–3.24 All cases that were not deemed suitable for endoscopic MIS ICH evacuation but required evacuation because of neurologic impairment from mass effect were performed in an open approach and not included in this analysis. All surgeries were performed by cerebrovascular neurosurgeons (C.P.K. and/or J.M.).
Demographic and clinical data including age, sex, medical history, medications, urine toxicology, and image findings were collected prospectively. During the SCUBA procedure, one superficial and one perihematomal biopsy were collected for each patient and sent to pathology for analysis.
Biopsy Collection Using the MIS Approach
During the MIS endoscopic ICH evacuation procedures, a 1.5-cm-diameter burr hole was made (Figure 1A). Superficial and perihematomal biopsies were collected as follows. First, after the incision of the dura mater (Figure 1B), a 2 × 1 mm superficial biopsy was performed using a surgical Penfield Dissector #1 (Integra Life Sciences, Plainsboro, New Jersey, USA) (Figures 1C and 2). Second, during stereotactic passage of the sheath through the brain to the hematoma, passage was halted 5–10 mm from the hematoma edge along the trajectory line. The introducer was removed and the endoscopic biopsy tool was inserted through the working channel of the endoscope. A biopsy of nonvascular perihematomal white matter was obtained (Figure 3). Then the introducer was reinserted, an image of the stereotactic guidance screen was saved to mark the biopsy location, and the sheath passage was continued along the trajectory line into the hematoma (Figure 4).
Figure 1.
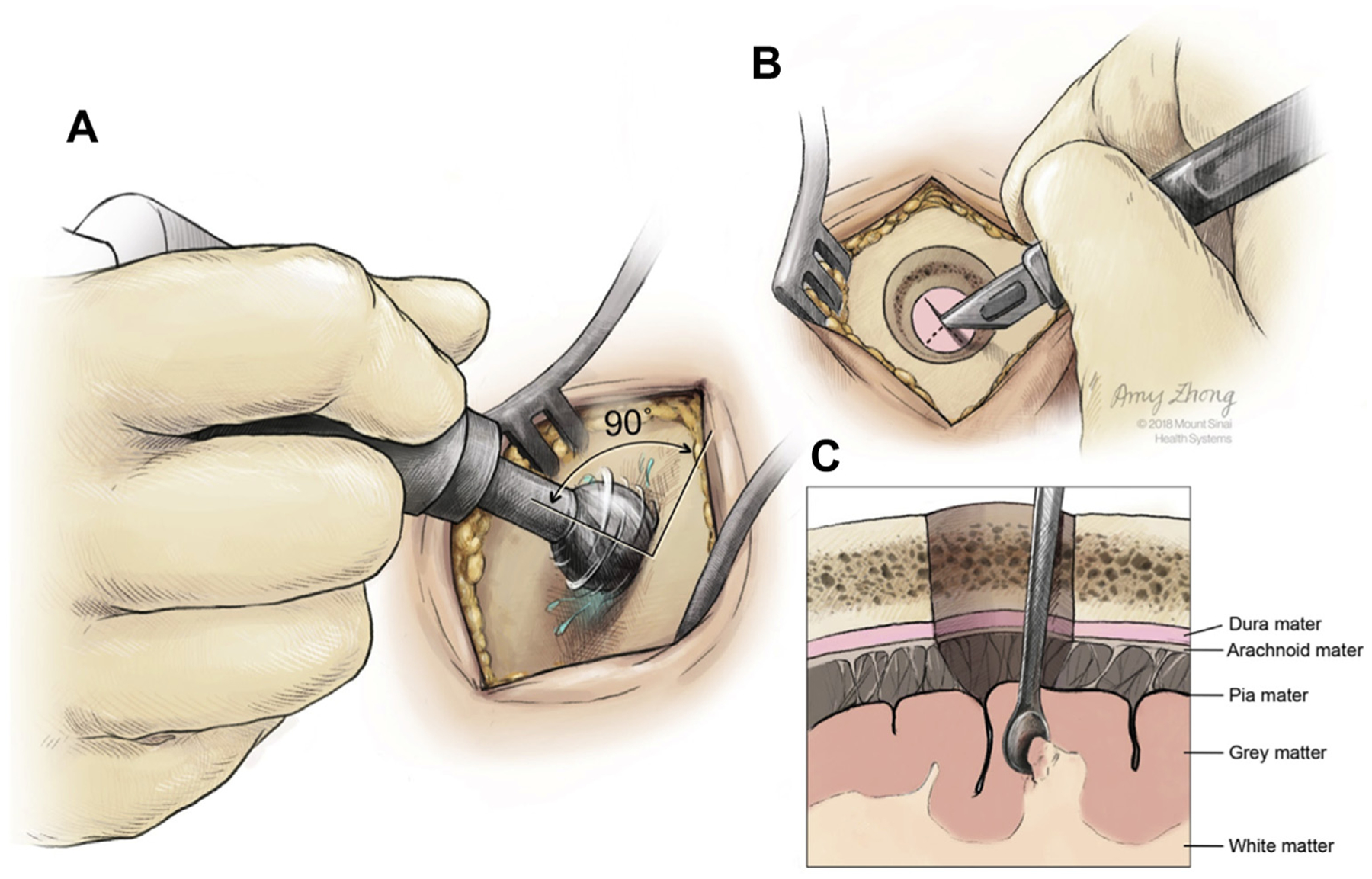
Schematic of the burr hole procedure (A), the incision of the dura (B), and the collection of a superficial biopsy at the gray/white matter junction (C). (Created by Amy Zhong at the Mount Sinai Hospital and reproduced with permission.)
Figure 2.
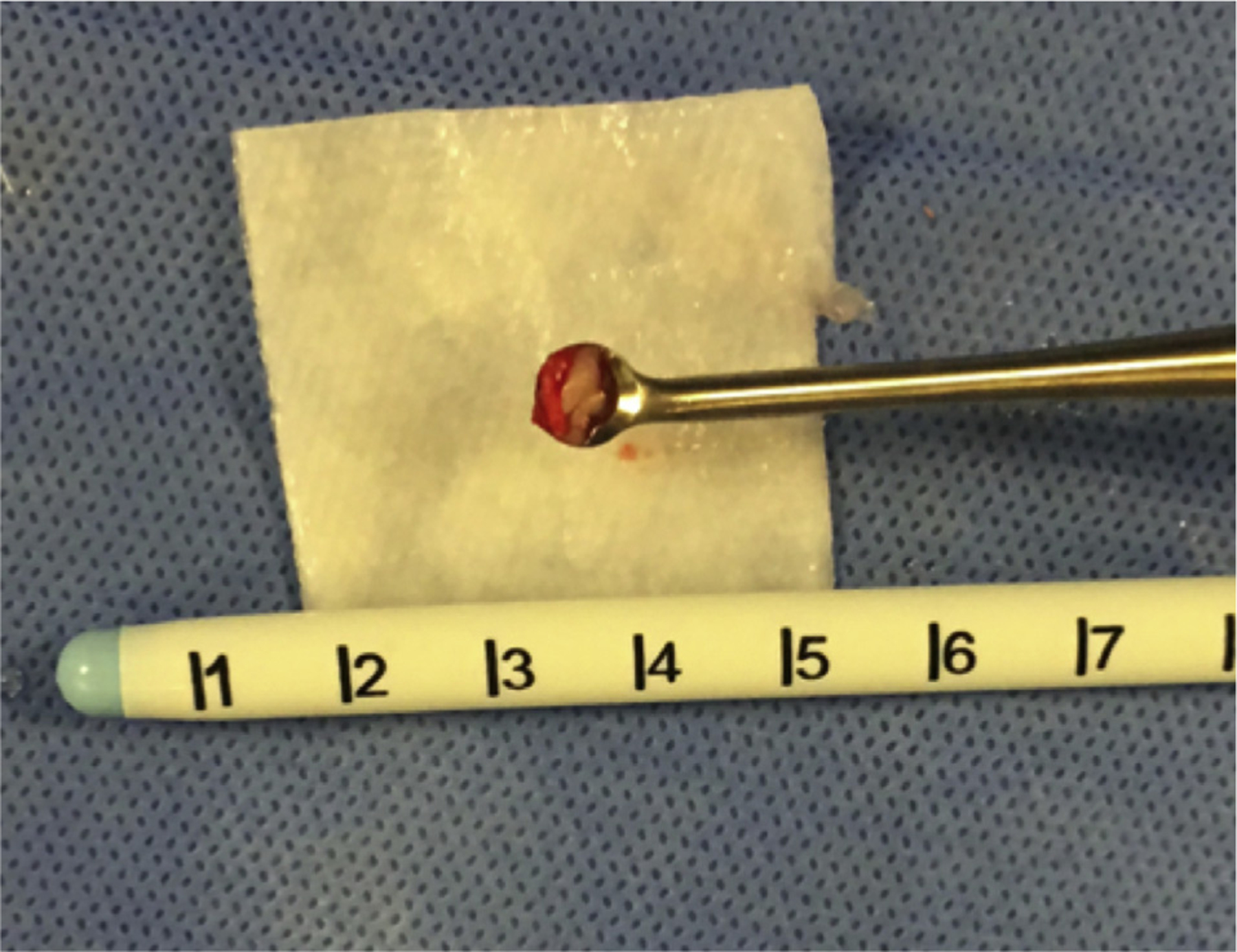
Photograph of a superficial biopsy collected for analysis during a minimally invasive stereotactic intracerebral hemorrhage underwater blood aspiration procedure.
Figure 3.
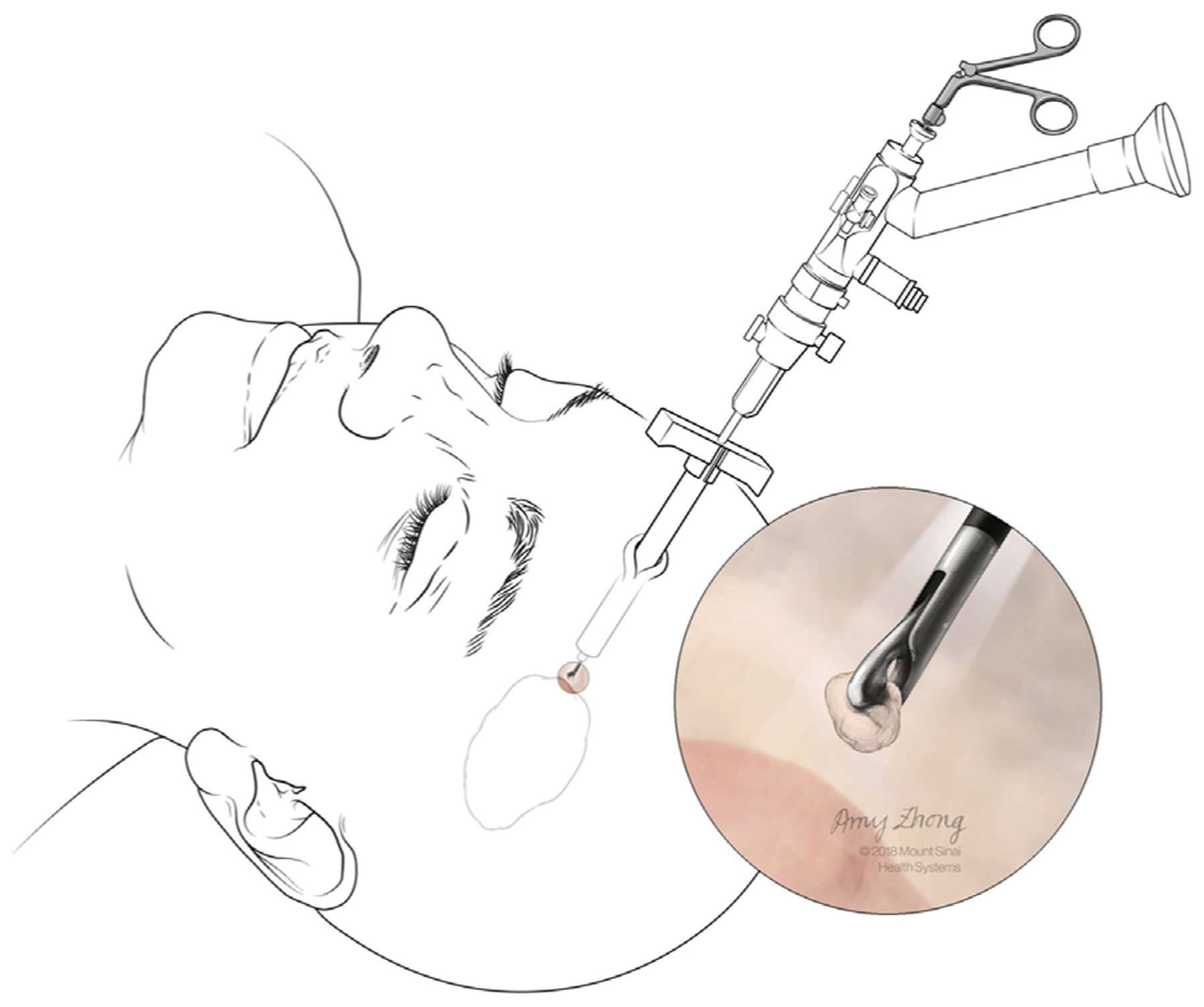
Collection of a deeper, perihematomal biopsy with the neuroendoscope in place. (Created by Amy Zhong at the Mount Sinai Hospital and reproduced with permission.)
Figure 4.
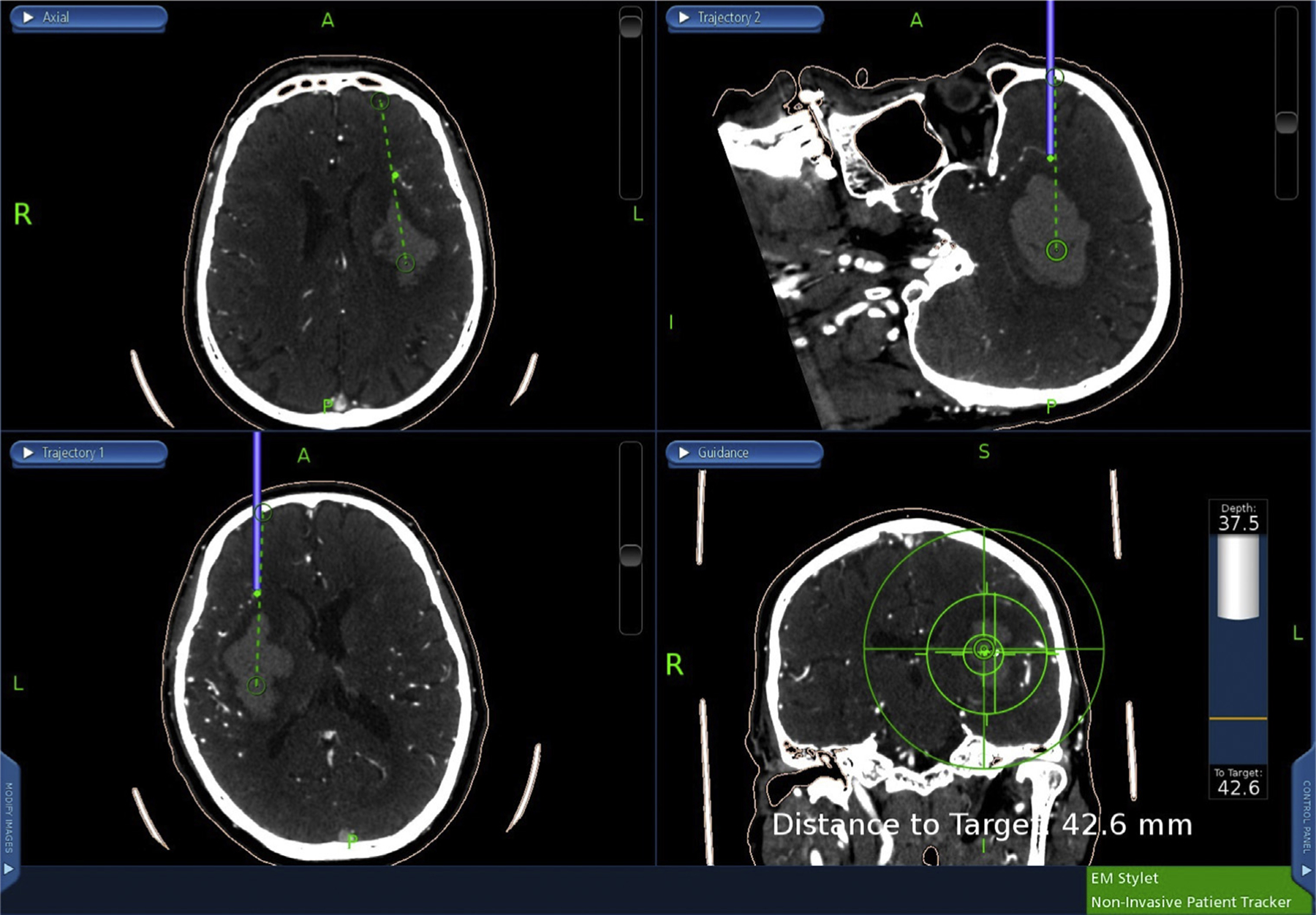
Perihematomal biopsy location is marked on the stereotactic guidance system (green dot), after which the sheath passage is continued along the trajectory line into the hematoma.
After the obtainment of the 2 samples, each specimen was immediately placed into saline on the operating field. Samples were then transferred off the operating field and placed in formalin for permanent sectioning analysis by the pathologist.
Pathologic Analysis
Immunohistochemical and histopathologic staining were performed to evaluate each sample for various causes of ICH, specifically hypertensive changes, amyloid deposition on vessel walls, tumor cells, vasculitic changes, and others. Evaluation for CAA included the use of Congo red stain with apple-green birefringence (Figure 5) and beta-amyloid immunohistochemical stain (Figure 6).
Figure 5.
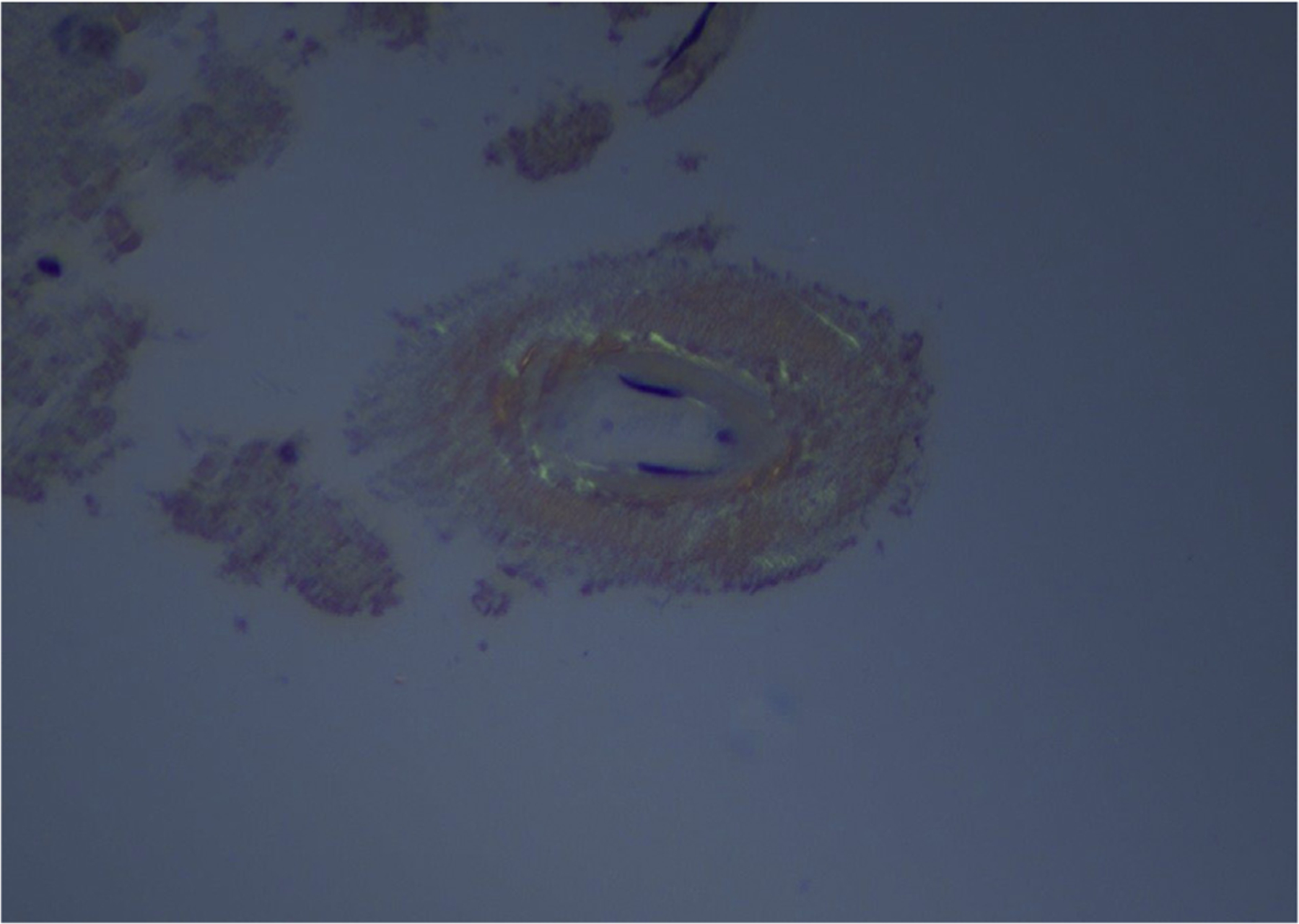
Congo red stain with apple-green birefringence (original magnification ×40).
Figure 6.
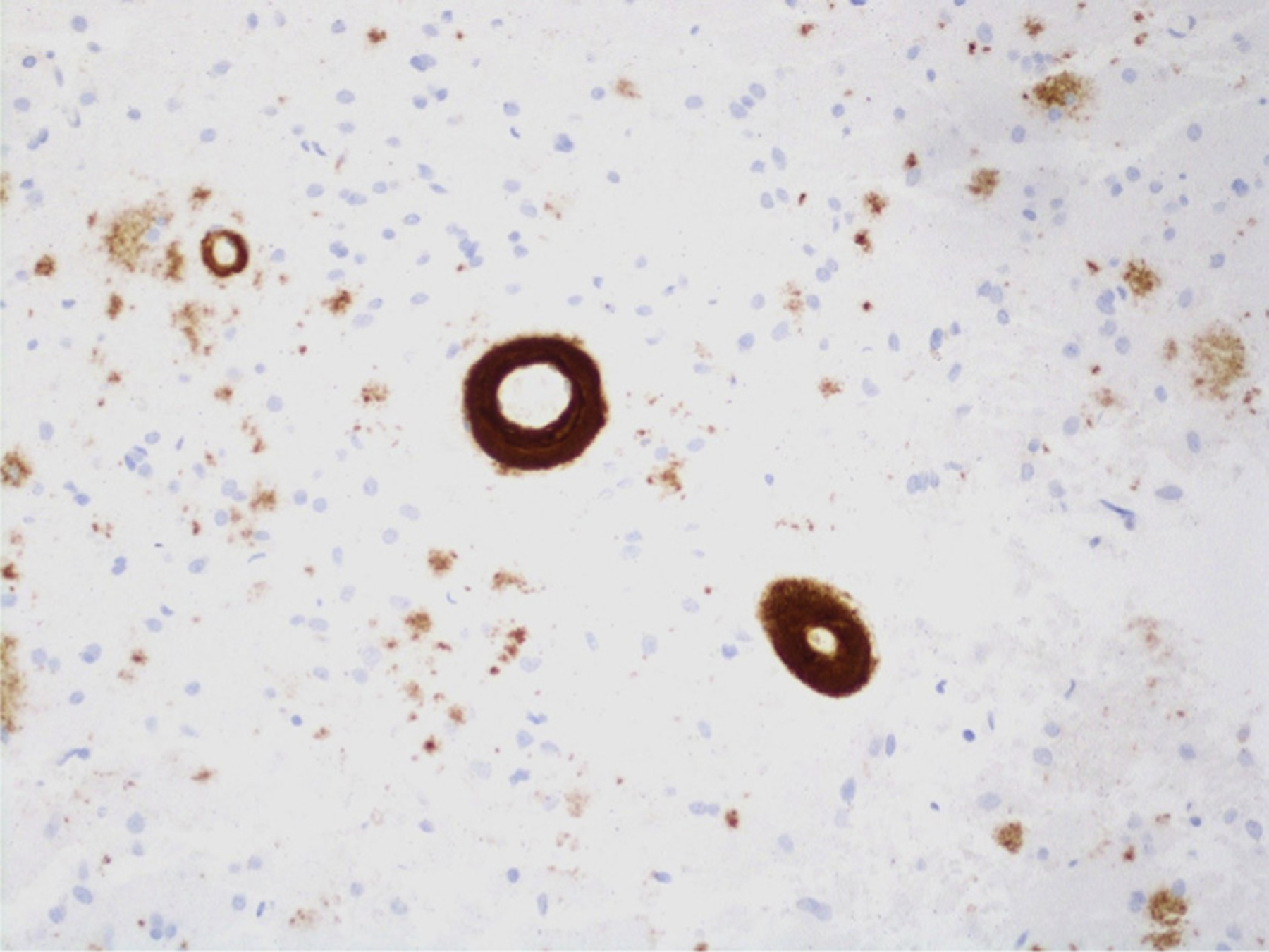
Beta-amyloid immunohistochemical staining (original magnification ×20).
Validation of the Modified Boston Criteria for Predicting CAA Etiology in the MIS ICH Patient
A blinded reviewer analyzed the magnetic resonance imaging (MRI) or computed tomography (CT) scan of each biopsied patient to determine the likelihood that the patient’s etiology was CAA. The criteria used to assess for probable CAA etiology consisted of the following checklist points adopted from the modified Boston criteria: age >55 years; single or multiple hemorrhages restricted to lobar, cortical, or cortico-subcortical regions; and focal (restricted to ≤3 sulci) or disseminated (affecting at least 4 sulci) superficial cortical siderosis. If an MRI was available, an MRI scan was used for the analysis. If not, a CT scan was used. Superficial cortical siderosis was defined as linear residues of blood in the superficial layers of the cerebral cortex.23,25,26 The first available scan following the patient’s ICH was reviewed, and no postoperative images were analyzed. The image reviewer was blinded to all of the identifying features of the patient, including name, date of birth, sex, medical record number, address, and clinical history.
Statistical Analysis
A Student t test was used to determine intergroup differences in continuous variables, such as age. A 2-tailed Fisher exact test was used to determine intergroup differences of categorical variables. Statistical significance was set at P < 0.05. Additionally, sensitivities and specificities were determined for the modified Boston criteria used with a CI set at 95%.
RESULTS
Characteristics
Forty patients were identified who underwent brain biopsy during MIS ICH procedures between October 2016 and March 2018. These patients had an average age of 62.7 years. There were 25 men (63%) and 15 women (37%) enrolled. Five patients (13%) had a history of prior ICH. Thirty-five patients (88%) had a history of hypertension, 10 patients (25%) had diabetes mellitus, 3 patients had renal disease (8%), and 1 patient had liver disease (3%). Eight patients (20%) were using at least 1 anticoagulant medication at baseline, 9 patients were using at least 1 antiplatelet medication (23%), and 14 patients (35%) were using at least 1 statin medication. Nine patients (23%) had a history of smoking, 3 patients had a history of alcohol abuse (8%), and 2 patients had a history of cocaine abuse (5%) (Table 1).
Table 1.
Demographics
| Characteristic | Total (N = 40) |
Non-CAA Group (n = 36) |
CAA Group (n = 4) |
P Value |
|---|---|---|---|---|
| Average age | 62.7 | 61 ± 11.3 | 78.3 ± 5.4 | 0.01 |
| Female sex | 15 (38) | 15 (42) | 0 (0) | 0.28 |
| Hypertension | 35 (88) | 32 (89) | 3 (75) | 0.42 |
| Diabetes mellitus | 10 (25) | 9 (25) | 1 (25) | >0.99 |
| EtOH abuse | 3 (8) | 2 (6) | 1 (25) | 0.28 |
| History of smoking | 9 (23) | 6 (17) | 3 (75) | 0.03 |
| Renal disease | 3 (8) | 2 (6) | 1 (25) | 0.28 |
| Liver disease | 1 (3) | 1 (3) | 0 (0) | >0.99 |
| Anticoagulant at baseline | 8 (20) | 6 (17) | 2 (50) | 0.17 |
| Antiplatelet at baseline | 9 (23) | 8 (22) | 1 (25) | >0.99 |
| Statin | 14 (35) | 12 (33) | 2 (50) | 0.60 |
| Cocaine | 2 (5) | 2 (6) | 0 (0) | >0.99 |
Values are number (%), mean ± SD, or as otherwise indicated.
CAA, cerebral amyloid angiopathy; EtOH, ethanol.
Blinded Image Assessment
A total of 23 patients (58%) had MRI scans available for analysis. The rest of the patients (n = 17, 43%) did not have an MRI performed on admission; therefore, the first available CT scan was analyzed. For both MRI and CT scan, the analyzed scan was pre-operative, with most scans performed at the central hospital and a minority of the scans performed at an outside hospital prior to transfer. The distribution of hemorrhage location was as follows: basal ganglia (n = 17, 43%), thalamic (n = 7, 18%), parietal (n = 3, 8%), temporal (n = 3, 8%), frontal (n = 3, 8%), occipital (n = 1, 3%), frontal and parietal (n = 1, 3%), fronto-temporo-parietal (n = 2, 5%), temporo-parieto-occipital (n = 1, 3%), parieto-occipital (n = 1, 3%), and intraventricular only (n = 1, 3%). Nineteen patients (48%) had intraventricular hemorrhage concomitant with a hemorrhage elsewhere. Fourteen patients (35%) had midline shift evaluated on CT scan. Eleven patients (28%) had superficial siderosis identified on MRI or CT scan, with 8 patients having focal superficial siderosis (≤3 affected sulci) and 3 patients having disseminated superficial siderosis (involving ≥4 sulci). Fifteen patients (38%) had concurrent subarachnoid hemorrhage identified on imaging, with 12 patients having subarachnoid hemorrhage that extended from the ICH, 2 patients having remote subarachnoid hemorrhage, and 1 patient having both. Finally, 16 patients (40%) had an irregular ICH border (Table 2).
Table 2.
Imaging Characteristics
| Characteristic | Total (N = 40) |
Non-CAA Group (n = 36) |
CAA Group (n = 4) |
P Value |
|---|---|---|---|---|
| Intraventricular hemorrhage | 19 (48) | 17 (47) | 2 (50) | >0.99 |
| Midline shift | 14 (35) | 13 (36) | 1 (25) | >0.99 |
| Superficial siderosis | 11 (28) | 8 (25) | 3 (75) | 0.06 |
| Focal (≤3 sulci) | 8 (20) | 7 (19) | 1 (25) | >0.99 |
| Disseminated (>3 sulci) | 3 (8) | 1 (3) | 2 (50) | 0.02 |
| Subarachnoid bleeding | 15 (38) | 12 (33) | 3 (75) | 0.14 |
| Irregular ICH border | 16 (40) | 13 (36) | 3 (75) | 0.28 |
| Postoperative bleeding | 2 (5) | 2 (6) | 0 (0) | >0.99 |
Values are number (%) or as otherwise indicated.
ICH, intracerebral hemorrhage.
Rebleeding
Thirty-eight out of the 40 patients (95%) experienced no rebleeding after MIS ICH and biopsy. Of the 2 patients (5%) who experienced postoperative rebleeding, the first patient’s rebleed was noted on routine postoperative day 1 imaging and did not result in any neurologic decline. The patient experienced a reaccumulation of hemorrhage in the deep extreme capsule in the posterior aspect of the hematoma cavity. The site of hematoma reaccumulation correlated with a bleeding vessel that was identified on endoscopic evaluation during the initial MIS procedure. The site of reaccumulated hemorrhage did not correlate with the site of the brain biopsy. We think the reaccumulation of hemorrhage for this patient was likely because of reactivation of the same vessel that was cauterized during the evacuation. Because the patient did not suffer a neurologic decline associated with this hemorrhage and it remained stable on follow-up imaging, the patient was managed medically and did not require an additional operation.
The second patient’s rebleed was noted on routine postoperative imaging and did not result in any change in neurologic examination. The patient was found to have a subgaleal collection on examination. After CTH evaluation, the hemorrhage was thought to originate from the subcutaneous tissue under the skin incision and had run into the evacuation cavity. Given that the blood products extended from an extracranial source into the hematoma cavity, it is unlikely that the bleeding originated from the brain biopsy site. There was no evidence of hematoma cavity expansion on follow-up imaging, and the patient was managed medically.
Histopathologic Sample Analysis
Four out of the 40 samples (10%) were determined to have amyloid on histopathologic sample analysis. All analyzed samples had some degree of gliosis, with 23% of cases estimated to have at least 50% gliosis. Additional pathologic biopsy results were as follows: ischemic change (n = 5, 13%), hypertensive vasculopathy (n = 3, 8%), and arteriosclerosis (n = 2, 5%).
CAA
Patients testing positive for CAA on biopsy had an average age of 78.3 ± 5.4 years compared with patients without CAA who had an average age of 61.0 ± 11.3 years (P = 0.005). Patients who were CAA positive had a higher prevalence of a previous cerebrovascular accident (75%) versus patients without CAA (11%) (P = 0.01). Additionally, patients who were CAA positive had a higher prevalence of hemorrhage involving the parietal lobe (P = 0.02) and hemorrhage involving the occipital lobe (P = 0.001) than patients without CAA. Fourteen out of 40 patients met possible CAA or higher requirements in the modified Boston criteria, with all 4 patients with pathologic evidence of CAA included in this group. Therefore, possible CAA of the modified Boston criteria had a sensitivity of 100% (95% CI, 39.6%–100%) and a specificity of 72.2% (95% CI, 54.6%–84.2%) for predicting a positive CAA biopsy result. None of the patients with pathologic evidence of CAA experienced rebleeding.
DISCUSSION
In this paper, we have demonstrated the safety and clinical utility of procuring superficial and perihematomal brain biopsies during MIS endoscopic ICH evacuation. Given the dearth of literature regarding the safety and efficacy of biopsy in this procedure, this study is helpful in establishing the minimal risk of rebleeding and other side effects when performing biopsies in this operative setting. Biopsy during ICH clot evacuation is an important tool that may be used to rule out pathology such as CAA, arteriovenous malformation, and tumor.27–29
We ultimately conclude that brain biopsy in our cohort is technically feasible with an acceptable safety profile. Biopsies were performed under direct visualization, and no patients had challenging intraoperative bleeding related to the biopsy. Although 2 patients (5%) suffered postoperative rebleeding, this phenomenon is well documented in surgery for ICH and is therefore more likely to be related to the underlying disease than the addition of the biopsy in this series. Morgenstern et al.,20 for example, reported a postoperative rebleeding rate of 40% in patients undergoing evacuation for ICH within 4 hours of the original hemorrhage and 12% when undergoing surgery within 12 hours of the hemorrhage. In phase II of the Minimally Invasive Surgery Plus Rt-PA for ICH Evacuation trial, which compared conservative management of ICH (medical group) with MIS surgery + alteplase (surgical group), patients in the surgical group had a rebleeding rate of 9% (5/54).12 In the Intraoperative Stereotactic Computed Tomography-Guided Endoscopic Surgery trial, 3 out of 14 (21.4%) surgically treated patients experienced rebleeding within 72 hours of the operation.14 In Miller et al.,30 stereotactic hematoma evacuation was compared with medical management for ICH, with 20% of the surgical group experiencing rebleeding or having hematoma expansion postoperatively. In a multicenter study of stereotactic aspiration of ICH, Teernstra et al.31 reported rebleeding rates of 21.9% of the 70 analyzed patients. Rebleeding is a major concern when performing surgery for ICH, particularly when using MIS techniques. This concern for rebleeding has informed device, technique, and clinical trial design for MIS ICH evacuation.
Endoscopic biopsies of intraventricular masses serve as a comparison for technique and complication rate. In 46 patients with intraventricular tumor of which 22 were biopsied, Schroeder et al.32 reported that 2 patients (4%) suffered hemorrhage during the procedure. However, it is unknown whether these 2 patients were among those biopsied.32 In the Peretta et al. study33 of intracranial fluid-filled cavities in pediatric patients, 2 out of 19 biopsied patients (11%) suffered hemorrhage. Finally, Depreitere et al.34 reported hemorrhage in 3 out of 31 patients (10%) undergoing endoscopic biopsy for intraventricular lesions. In these series, however, the biopsied tissue is pathologic and would be expected to have a higher rate of hemorrhage than the nonpathologic tissue biopsied during MIS ICH evacuation.
In this series of 40 MIS ICH patients, we found that 4 patients had evidence of CAA on neuropathologic report for a rate of 10%. The diagnosis of CAA can be useful for both assessing the intraoperative risk and postoperative risk of recurrent ICH. A previous study has shown an ICH recurrence rate of 31.7% in patients with CAA ICH, with an average follow-up time of 35.3 months, a rate much higher than that normally cited for ICH recurrence in the normal population.35 In our cohort, 0 out of the 4 patients with CAA (0%) rebled. Understanding patient populations at risk for rehemorrhage is an important tool for doctor-patient counseling and identifying which patients need closer postoperative management.
Although an amyloid diagnosis does not change treatment, it serves as a springboard in the patient-doctor conversation to motivate patients to make lifestyle changes, such as blood pressure control, smoking cessation, regular exercise, and abstinence from illicit drug abuse, and adhere to medication. In addition, patients may have concurrent neurologic problems unrelated to hemorrhage that benefit from biopsy, including various forms of dementia.
In our study, all 4 of the CAA ICH patients had lobar bleeds. We found that the most common hematoma sites were the parietal and occipital lobes, with 3 out of 4 CAA ICH patients (75%) having a bleed involving each respective location. Zhan et al.36 found the parietal lobe to be the most common site in 41 patients with amyloid angiopathy hemorrhage with a rate of 34%. However, one review suggested the occipital lobe to be the most frequent site of amyloid angiopathy hemorrhage.3 Additionally, in pathologic biopsies of 10 cases of CAA-related lobar hemorrhage, Li et al.37 found the most common hematoma site was the temporal lobe (5/10). Given the small number of patients in our cohort, it is difficult to draw any significant conclusions as to the prevalence of frontal, temporal, parietal, and occipital hemorrhage locations.
Limitations
Our study had several limitations. First, this study is a retrospective, single-center study. Second, the small number of patients of this series may overestimate the safety and/or clinical utility of performing biopsies during this procedure. Third, because inclusion in our study required that a surgeon obtained biopsies from the superficial and perihematomal regions for pathologic analysis, any patient with ICH who was not a surgical candidate or did not undergo biopsy were excluded from the study, potentially selecting only low-risk patients, favorable for biopsy, or those who were suspected to have an underlying pathologic diagnosis. Finally, no control group was used that did not undergo a biopsy, which prevents comparison between biopsied and nonbiopsied patients to evaluate the true risks and benefits of this procedure. However, we think that the low rebleeding rate in this series demonstrates an acceptable safety profile for biopsy in the context of existing literature on rebleeding in ICH and biopsy for brain masses.
CONCLUSIONS
In this study, we established the feasibility, safety, and utility of procuring diagnostically valuable brain parenchymal biopsies during MIS endoscopic ICH evacuation. We determined the prevalence of CAA to be 10% in this cohort, and the modified Boston criteria was found to be a reliable predictor of CAA when performing biopsies during this procedure.
Abbreviations and Acronyms
- CAA
Cerebral amyloid angiopathy
- CI
Confidence interval
- CT
Computed tomography
- ICH
Intracerebral hemorrhage
- MIS
Minimally invasive
- MRI
Magnetic resonance imaging
- SCUBA
Stereotactic intracerebral hemorrhage underwater blood aspiration
Footnotes
Conflict of interest statement: This research was supported in part by a grant from Arminio and Lucyna Fraga and by a grant from Mr. and Mrs. Durkovic.
REFERENCES
- 1.Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001;344:1450–1460. [DOI] [PubMed] [Google Scholar]
- 2.van Asch CJJ, Luitse MJA, Rinkel GJE, van der Tweel I, Algra A, Klijn CJM. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol. 2010;9:167–176. [DOI] [PubMed] [Google Scholar]
- 3.Attems J, Jellinger K, Thal DR, Van Nostrand W. Review: sporadic cerebral amyloid angiopathy. Neuropathol Appl Neurobiol. 2011;37:75–93. [DOI] [PubMed] [Google Scholar]
- 4.González-Pérez A, Gaist D, Wallander M-A, McFeat G, García-Rodríguez LA. Mortality after hemorrhagic stroke: data from general practice (The Health Improvement Network). Neurology. 2013;81:559–565. [DOI] [PubMed] [Google Scholar]
- 5.Sacco S, Marini C, Toni D, Olivieri L, Carolei A. Incidence and 10-year survival of intracerebral hemorrhage in a population-based registry. Stroke. 2009;40:394–399. [DOI] [PubMed] [Google Scholar]
- 6.Ikram MA, Wieberdink RG, Koudstaal PJ. International epidemiology of intracerebral hemorrhage. Curr Atheroscler Rep. 2012;14:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kase CS. Intracerebral hemorrhage: non-hypertensive causes. Stroke. 1986;17:590–595. [DOI] [PubMed] [Google Scholar]
- 8.Meretoja A, Strbian D, Putaala J, et al. SMASH-U: a proposal for etiologic classification of intracerebral hemorrhage. Stroke. 2012;43:2592–2597. [DOI] [PubMed] [Google Scholar]
- 9.Nolte KB, Brass LM, Fletterick CF. Intracranial hemorrhage associated with cocaine abuse: a prospective autopsy study. Neurology. 1996;46: 1291–1296. [DOI] [PubMed] [Google Scholar]
- 10.Ariesen MJ, Claus SP, Rinkel GJE, Algra A. Risk factors for intracerebral hemorrhage in the general population: a systematic review. Stroke. 2003; 34:2060–2065. [DOI] [PubMed] [Google Scholar]
- 11.Samarasekera N, Smith C, Al-Shahi Salman R. The association between cerebral amyloid angiopathy and intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry. 2012;83:275–281. [DOI] [PubMed] [Google Scholar]
- 12.Hanley DF, Thompson RE, Muschelli J, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol. 2016;15: 1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mayo S About MISTIE-III. Available at: http://braininjuryoutcomes.com/mistie-iii-about. AccessedNovember 14, 2018.
- 14.Vespa P, Hanley D, Betz J, et al. ICES (Intraoperative Stereotactic Computed Tomography-Guided Endoscopic Surgery) for brain hemorrhage: a multicenter randomized controlled trial. Stroke. 2016;47:2749–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.About ENRICH. Available at: https://www.enrichtrial.com/.AccessedNovember 14, 2018.
- 16.Filippi M, Stefano ND, Dousset V, McGowan JC, eds. MR Imaging in White Matter Diseases of the Brain and Spinal Cord. Springer-Verlag Berlin Heidelberg: Berlin, Germany; 2005. [Google Scholar]
- 17.Tseng J-H, Lin W-H. Glioblastoma multiforme hiding behind the intracerebral hematoma. Formosan Journal of Surgery. 2012;45:183–186. [Google Scholar]
- 18.Bernstein M, Parrent AG. Complications of CT-guided stereotactic biopsy of intra-axial brain lesions. J Neurosurg. 1994;81:165–168. [DOI] [PubMed] [Google Scholar]
- 19.Davis SM, Broderick J, Hennerici M, et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66:1175–1181. [DOI] [PubMed] [Google Scholar]
- 20.Morgenstern LB, Demchuk AM, Kim DH, Frankowski RF, Grotta JC. Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage. Neurology. 2001;56: 1294–1299. [DOI] [PubMed] [Google Scholar]
- 21.Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. [DOI] [PubMed] [Google Scholar]
- 22.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001; 56:537–539. [DOI] [PubMed] [Google Scholar]
- 23.van Rooden S, van der Grond J, van den Boom R, et al. Descriptive analysis of the Boston criteria applied to a Dutch-type cerebral amyloid angiopathy population. Stroke. 2009;40:3022–3027. [DOI] [PubMed] [Google Scholar]
- 24.Kellner CP, Chartrain AG, Nistal DA, et al. The Stereotactic Intracerebral Hemorrhage Underwater Blood Aspiration (SCUBA) technique for minimally invasive endoscopic intracerebral hemorrhage evacuation. J Neurointerv Surg. 2018;10: 771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenberg SM, Finklestein SP, Schaefer PW. Petechial hemorrhages accompanying lobar hemorrhage: detection by gradient-echo MRI. Neurology. 1996;46:1751–1754. [DOI] [PubMed] [Google Scholar]
- 26.Linn J, Herms J, Dichgans M, et al. Subarachnoid hemosiderosis and superficial cortical hemosiderosis in cerebral amyloid angiopathy. AJNR Am J Neuroradiol. 2008;29:184–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leclerc JL, Ahmad AS, Singh N, et al. Intracerebral hemorrhage outcomes following selective blockade or stimulation of the PGE2 EP1 receptor. BMC Neurosci. 2015;16:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lively S, Moxon-Emre I, Schlichter LC. SC1/hevin and reactive gliosis after transient ischemic stroke in young and aged rats. J Neuropathol Exp Neurol. 2011;70:913–929. [DOI] [PubMed] [Google Scholar]
- 29.Sukumari-Ramesh S, Alleyne CH, Dhandapani KM. Astrocyte-specific expression of survivin after intracerebral hemorrhage in mice: a possible role in reactive gliosis? J Neurotrama. 2012;29:2798–2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller CM, Vespa P, Saver JL, et al. Image-guided endoscopic evacuation of spontaneous intracerebral hemorrhage. Surg Neurol. 2008;69:441–446 [discussion: 446]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Teernstra OPM, Evers SMAA, Lodder J, Leffers P, Franke CL, Blaauw G. Multicenter randomized controlled trial (SICHPA): Stereotactic treatment of intracerebral hematoma by means of a plasminogen activator: a multicenter randomized controlled trial (SICHPA). Stroke. 2003;34:968–974. [DOI] [PubMed] [Google Scholar]
- 32.Schroeder HWS, Oertel J, Gaab MR. Incidence of complications in neuroendoscopic surgery. Childs Nerv Syst. 2004;20:878–883. [DOI] [PubMed] [Google Scholar]
- 33.Peretta P, Ragazzi P, Galarza M, et al. Complications and pitfalls of neuroendoscopic surgery in children. J Neurosurg. 2006;105(3 suppl):187–193. [DOI] [PubMed] [Google Scholar]
- 34.Depreitere B, Dasi N, Rutka J, Dirks P, Drake J. Endoscopic biopsy for intraventricular tumors in children. J Neurosurg. 2007;106(5 suppl):340–346. [DOI] [PubMed] [Google Scholar]
- 35.Hirohata M, Yoshita M, Ishida C, et al. Clinical features of non-hypertensive lobar intracerebral hemorrhage related to cerebral amyloid angiopathy. Eur J Neurol. 2010;17:823–829. [DOI] [PubMed] [Google Scholar]
- 36.Zhan R-Y, Tong Y, Shen J-F, et al. Study of clinical features of amyloid angiopathy hemorrhage and hypertensive intracerebral hemorrhage. J Zhejiang Univ Sci. 2004;5:1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li X-Q, Su D-F, Chen H-S, Fang Q. Clinical neuropathological analysis of 10 cases of cerebral amyloid angiopathy-related cerebral lobar hemorrhage. J Korean Neurosurg Soc. 2015;58:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]


