Abstract
Depression and anxiety have been linked to poor quality of life (QoL) – one’s subjective perception of relationships, physical health, daily functioning, general sense of well-being and life satisfaction. Elucidating abnormal white matter microstructure associated with mood and other symptoms and QoL is important to facilitate treatment. Ninety-six young adults (18–25 years old) seeking help for psychological distress, irrespective of presence or absence of psychiatric diagnosis completed diffusion weighted and anatomical scans, clinical and behavioral measures, and QoL assessment. We examined relationships between diffusion imaging properties in major white matter tracts involved in emotion processing and regulation, symptoms, and QoL. Depression and general distress levels fully mediated the relationship between fractional anisotropy (FA), an indirect index of fiber collinearity, and radial diffusivity (RD), an index sensitive to axonal/myelin damage, in right uncinate fasciculus and QoL. The relationship between reduced FA (and increased RD) in right uncinate fasciculus and poor QoL was explained by greater severity of depression and general distress. These findings underscore the role of white matter microstructure in right uncinate fasciculus in relation to depressive and general distress symptoms and, in turn, QoL. Importantly, they suggest that measures of white matter microstructure in this tract can be used as putative objective markers of emotion dysregulation, to inform and monitor the impact of interventions to reduce affective symptoms and improve QoL in young adults.
Keywords: Diffusion, Fractional anisotropy, Uncinate fasciculus, Mediation, Symptoms
1. Introduction
Depression and anxiety are prevalent in early adulthood and have been linked to poor quality of life (QoL) – one’s subjective perception of relationships, physical health, daily functioning, general sense of well-being and life satisfaction (Endicott et al., 1993). QoL has become an important target of treatment and criterion of treatment outcome (Dzevlan et al., 2019).
Elucidating abnormal neural function and structure associated with mood and other symptoms and poor QoL could facilitate treatment. However, few studies examined relationships between neural measures, clinical symptoms, and QoL. Previously, we reported that the relationship between amygdala and left ventrolateral prefrontal cortex activity during emotion processing and QoL is mediated by anxiety (Greenberg et al., 2017). These findings indicate that relationships between neural activity and QoL can be explained by anxiety severity and provide a first step towards identification of neural targets for intervention to reduce anxiety and improve QoL.
There is growing evidence for alterations in white matter microstructure in relation to affective symptoms. In general, studies have reported reductions in fractional anisotropy (FA), an indirect index of fiber collinearity, in symptomatic versus healthy individuals (Jenkins et al., 2016; van Velzen et al., 2020). For example, reduced FA in the uncinate fasciculus, a tract connecting the orbitofrontal cortex to anterior temporal lobes, and implicated in emotion processing and regulation, has been repeatedly observed in depressed individuals (Bracht et al., 2015; Dillon et al., 2018), and in anxious individuals (Phan et al., 2009).
In the current study, we aimed to extend our previous findings that linked neural activity and QoL (Greenberg et al., 2017) by examining directional relationships between white matter microstructure, behavioral/clinical measures, and QoL in young adults with a range of symptoms and problem behaviors (including depressive and anxiety symptoms and difficulties in coping with everyday stressors and interpersonal relationships). We used elastic-net penalized regressions and follow-up simple linear regressions to identify behavioral/clinical symptoms associated with both QoL and mean FA in major tracts involved in emotion processing and regulation (Lai and Wu, 2014; Versace et al., 2015). We then determined the extent to which these identified behavioral/clinical measures mediated the relationship between mean FA and QoL. Based on our previous work (Greenberg et al., 2017) and present literature, we hypothesized that:
Mean FA in key tracts involved in emotion processing and regulation would be negatively associated with depressive and/or anxiety symptoms and positively associated with QoL.
Depressive and/or anxiety symptoms would mediate the relationship between mean FA and QoL.
2. Methods
2.1. Participants
Participants were 96 individuals (70 females), 18–25 years old, seeking help for psychological distress including depressive and anxiety symptoms, irrespective of presence or absence of psychiatric diagnosis (Supplementary Table 1s). Exclusion criteria for the study were: history of head injury, neurological, pervasive developmental disorder or systemic medical disease, cognitive impairment (Mini-Mental State Examination (Folstein et al., 1975) score < 24), and premorbid North American Adult Reading Test (NAART; Blair and Spreen, 1989) IQ estimate < 85, visual disturbance (< 20/40 Snellen visual acuity), left or mixed handedness (Annett criteria; Annett, 1970), alcohol/substance use disorder (SUD, including nicotine) and/or illicit substance use (except cannabis) over the last 3 months determined by the Structured Clinical Interview for DSM-5, Research Version (First et al., 2015) and current use tested with urine and saliva tests. Additional MRI exclusion criteria were: positive pregnancy test/report for female participants, and any current psychotropic medication use for > 2 weeks. Previous medication was allowed, but individuals needed to be free from such medication for at least 3 months. The study was conducted in accordance with the Declaration of Helsinki and approved by the University of Pittsburgh Human Research Protection Office. All participants provided informed consent.
2.2. Behavioral/clinical measures
Participants were assessed on measures that examine symptoms and problem behaviors common in young adults (see Table 1): Hamilton Rating Scale for Depression (HAM-D; Hamilton, 1960); Mood and Anxiety Symptom Questionnaire, including the general distress anxiety subscale (MASQ-GA), general distress depression subscale (MASQ-DA), general distress mixed subscale (MASQ-GDM), anxious arousal subscale (MASQ-AA), and anhedonic depression subscale (Clark and Watson, 1991); the Hamilton Anxiety Rating Scale (HAM-A; Hamilton, 1959), Spielberger State-Trait Anxiety Inventory (Spielberger et al., 1970); Zuckerman sensation seeking scale (Zuckerman, 2007); Behavioral Inhibition and Activation System Scales BIS/BAS (Carver and White, 1994); Barratt Impulsiveness Scale (Patton et al., 1995); UPPS-P Impulsive Behavior Scale (Whiteside and Lynam, 2001); and Young Mania Rating Scale (Young et al., 1978). Participants also completed the Quality of life Enjoyment and Satisfaction Questionnaire - Short Form (Q-LES-Q-SF) to assess their perception of life enjoyment and satisfaction (Endicott et al., 1993).
Table 1.
Demographic and Clinical/Behavioral Data (n = 96; 70 females/26 males).
| Characteristic Mean (SD) | |
|---|---|
| Age (years) | 21.6 (2.1) |
| National Adult Reading Test score | 109.23 (7.08) |
| Clinical and behavioral measures a | |
| Hamilton Depression Rating Scale (17-item) | 14.65 (6.18) |
| Mood and Anxiety Symptom Questionnaire general distress mixed subscale | 41.22 (10.99) |
| Behavioral Inhibition and Activation System: Inhibition subscale | 23.37 (3.23) |
| Quality of life Enjoyment and Satisfaction Questionnaire - Short Form | 45.38 (7.41) |
Results for all 33 clinical and behavioral measures used in the elastic net analysis are in Supplementary Table 2s.
2.3. Image acquisition
Neuroimaging data (96 total) were collected on a 3T Siemens Prisma (n = 68) or a 3T Siemens Trio (n = 28) using the same imaging protocol, at the Magnetic Resonance Research Center (MRRC) in the University of Pittsburgh Medical Center. We acquired a single-shot spin-echo echo planar imaging (SE-EPI) sequence 197 diffusion-weighting gradient directions (32 vol with b = 700 s/mm2, 65 vol with b = 1000 s/mm2, 100 vol with b = 2500 s/mm2) and 13 reference volumes with b = 0 s/mm2 (repetition time (TR) = 3000 ms, echo time (TE) = 120 ms, flip angle = 90°, field-of-view (FOV) = 256 × 256, 2 mm3 isotropic voxel, simultaneous multi-slice (SMS) factor = 4, acquisition time = 630 s). The sequence was collected twice with opposite phase encoding directions (Posterior > Anterior and Anterior > Posterior) to reduce EPI distortions (Andersson and Sotiropoulos, 2015, 2016). We also acquired structural 3D axial MPRAGE images (TR = 1500 ms, TE = 3.19 ms, flip angle 8°, FOV = 256 × 256 mm, 1 mm3 isotropic voxels, 176 continuous slices).
2.4. Diffusion data analysis
Diffusion-weighted images were corrected for eddy current, subject motion and EPI distortion using topup and eddy (Andersson et al., 2003; Andersson and Sotiropoulos, 2015, 2016; Smith et al., 2004) within FMRIB’s Software Library (FSL 6.0). Using a triple-tensor model, as proposed in (Behrens et al., 2007; Jbabdi et al., 2012) and a global probabilistic tractographic approach, white-matter tracts were reconstructed with Tracts Constrained by Underlying Anatomy (TRACULA; Yendiki et al., 2011), the diffusion imaging toolbox of the FreeSurfer package (https://surfer.nmr.mgh.harvard.edu). For each participant, following visual inspection of reconstructed tracts, we extracted diffusion tensor measures estimated with dtifit, including mean fractional anisotropy (FA), radial diffusivity (RD), axial diffusivity (AD), and mean diffusivity (MD), in 13 white matter tracts implicated in emotion processing and regulation (Lai and Wu, 2014; Versace et al., 2015): forceps minor and right/left uncinate fasciculus, cingulum cingulate gyrus, cingulum angular bundle, superior longitudinal fasciculus - parietal endings, superior longitudinal fasciculus - temporal endings, and inferior longitudinal fasciculus (Supplementary Figure 2s).
2.5. Statistical analysis plan
We conducted elastic-net penalized least squares regression analyses (GLMNET package in R; Friedman et al., 2014) with α = 0.5 and 10-fold cross-validation, sex, scanner, age and National Adult Reading Test (NART) scores as covariates, and follow-up simple linear regressions, to identify variables for a mediation analysis using three steps: 1) FA measures (one per tract = 13 total) related to QoL. 2) behavioral/clinical measures (33 total) related to FA measures identified in step 1, and 3) behavioral/clinical measures (identified in step 2) related to QoL.
We then examined the extent to which behavioral/clinical measures associated with both QoL and FA measures mediated relationships between FA and QoL using PROCESS version 3.4 (Hayes and Little, 2018). We computed bootstrapped confidence intervals (BootCI) for all variables with 5000 samples and 95% level of confidence.
To aid FA findings interpretation, we conducted a secondary analysis using RD, AD, and MD measures in those tracts in which mean FA showed a significant relationship with QoL and behavioral/clinical measures.
3. Results
3.1. FA measures related to QoL (step 1)
One FA measure was identified as a non-zero predictor of poor QoL with elastic-net regression: lower FA in right uncinate fasciculus (i.e., lower FA in right uncinate fasciculus was associated with poorer QoL). The exponentiated coefficient (exp) = 2.017, which represents the ratio change in outcome variable (i.e., QoL) corresponding to one-unit change in predictor variable. A follow-up simple linear regression showed a significant positive association between right uncinate fasciculus FA and QoL (b = 1.87, F(1,94) = 5.82, p = 0.018; Supplementary Figure 2s).
3.2. Behavioral/clinical measures related to FA measures (step 2)
Three clinical measures were identified as non-zero predictors of lower FA in right uncinate fasciculus with elastic-net regression: greater HAM-D (exp = 0.974), MASQ-GDM (exp = 0.9995), and BIS (exp = 1.008). Namely, increased depressive symptoms, general distress, and behavioral avoidance (inhibition) were associated with lower FA in right uncinate fasciculus. Follow-up simple linear regressions, conducted for each of these clinical measures using Bonferroni adjusted alpha levels of .0167 per test (0.05/3), showed a significant negative association between HAM-D (b = −0.052, F(1,94) = 12.09, p = 0.001; Supplementary Figure 3s) and MASQ-GDM (b = −0.023, F(1,94) = 6.85, p = 0.01; Supplementary Figure 4s) and FA in right uncinate fasciculus, but not BIS (p = 0.217).
3.3. Behavioral/clinical measures related to QoL (step 3)
We conducted simple linear regressions with HAM-D and MASQ-GDM (the only clinical measures significantly associated with right uncinate fasciculus FA) as independent variables and QoL as the dependent variable using Bonferroni adjusted alpha levels of 0.025 per test (0.05/2).Increased depressive symptoms (b = −0.65, F(1,94) = 39.06, p < 0.001; Supplementary Figure 5s) and general distress levels (b = −0.383, F(1,94) = 44.81, p < 0.001; Supplementary Figure 6s), were associated with poorer QoL.
3.4. Testing regression assumptions and outliers for the mediation model
We tested normality of residuals, homoscedasticity, absence of multicollinearity and outlier tests of Mahalanobis, Cook’s, and Leverage values. Examination of Predicted Probability (P–P) plots showed normality of residuals and homoscedasticity. Residuals were independent, the Durbin Watson statistic (d) = 2.032, and the Variance Inflation Factor (VIF), a measure of multicollinearity was < 2. There were no cases that warranted exclusion (i.e. no cases had extreme values on two of the three outlier tests).
3.5. Mediation
We conducted a parallel mediation model (PROCESS version 3.4, model 4; Hayes and Little, 2018) to simultaneously test HAM-D and MASQ-GDM, the only clinical variables significantly associated with both QoL and white matter microstructure, as potential mediators of the relationship between right uncinate fasciculus FA and QoL. The coefficients in this multiple mediator model reflect the amount of mediation for each variable after accounting for the other mediator in the model.
HAM-D and MASQ-GDM fully mediated the positive relationship between right uncinate fasciculus FA and QoL [t(94) = 2.41, p = 0.018 (total effect) going to t(94) = 0.6302, p = 0.53 (direct effect); Fig. 1]. There were significant negative relationships between right uncinate fasciculus FA and HAM-D (t(94) = −3.48, p = 0.0008, BootCI = −3.53 to −0.848) and MASQ-GDM (t(94) = −2.62, p = 0.0103, BootCI = −5.38 to −0.748), and between HAM-D and MASQ-GDM and QoL (t(92) = −2.25, p = 0.027, BootCI = −0.576 to −0.064; t(92) = −3.18, p = 0.002, BootCI = −0.398 to −0.0998, respectively). The indirect (i.e. mediated) effects between right uncinate fasciculus FA and QoL, through HAM-D and MASQ-GDM, were significant (i.e. did not include zero; BootCI = 0.074 to 1.62 and BootCI = 0.139 to 1.58, respectively).
Fig. 1.
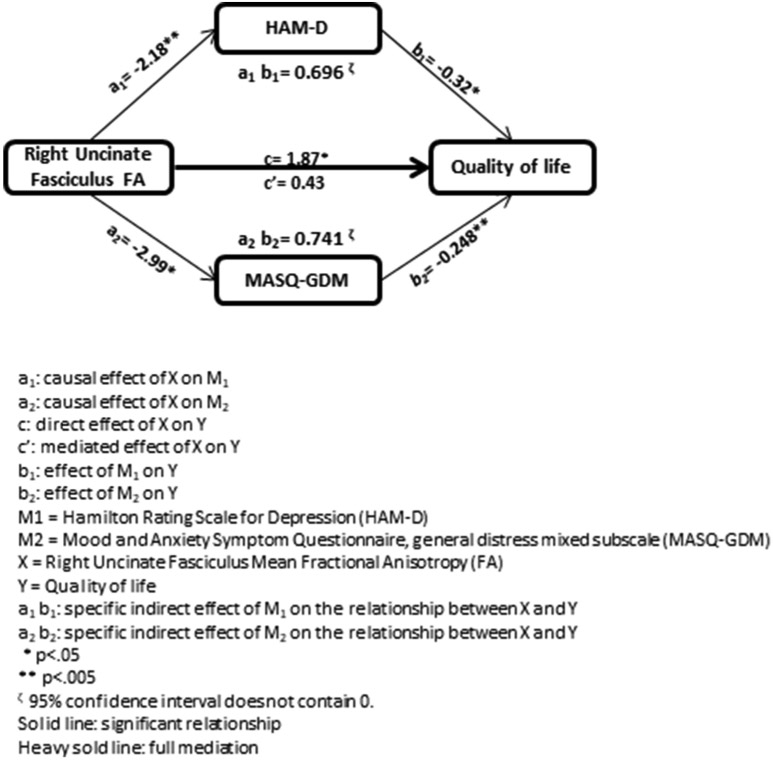
A parallel mediation model examining the effect of Hamilton Rating Scale for Depression (HAM-D) and the Mood and Anxiety Symptom Questionnaire, general distress mixed subscale (MASQ-GDM) scores on the relationship between mean fractional anisotropy (FA) in right uncinate fasciculus and quality of life (QoL).
3.6. Mediation: Alternative pathway
Right uncinate fasciculus FA did not mediate the relationship between HAM-D or MASQ-GDM, and QoL.
3.7. Relationships between RD, MD, and AD in right uncinate fasciculus and HAM-D, MASQ-GDM, and QoL
Higher right uncinate fasciculus RD was associated with increased HAM-D (r(96) = 0.405, p < 0.001) and MASQ-GDM (r(96) = 0.29, p = 0.004) scores. In addition, we observed a nonsignificant trend for an association between higher right uncinate fasciculus RD and poorer QoL (r(96) = −0.188, p = 0.066). A follow-up mediation analysis showed that HAM-D and MASQ-GDM fully mediated the negative relationship between right uncinate fasciculus RD and QoL (Supplementary Fig. 7s).
Higher right uncinate fasciculus MD was associated with increased HAM-D (r(96) = 0.33, p = 0.001) and MASQ-GDM (r(96) = 0.21, p = 0.043) scores. We did not observe a significant association between right uncinate fasciculus MD and QoL (r(96) = −0.117, p = 0.26).
There were no significant associations between right uncinate fasciculus AD and either HAM-D, MASQ-GDM, or QoL (all ps > 0.1).
4. Discussion
We show directional relationships between white matter microstructure, symptoms, and QoL in distressed young adults regardless of diagnosis. HAM-D and MASQ-GDM fully mediated the relationship between FA and RD in right uncinate fasciculus and QoL. Specifically, the relationship between reduced FA (and increased RD) in right uncinate fasciculus and poor QoL was explained by greater severity of depression and general distress.
Reduced FA in uncinate fasciculus has been observed most commonly in depression (Bracht et al., 2015), but also anxiety (Phan et al., 2009) and psychopathy (Motzkin et al., 2011). Here, we showed a negative (i.e. inverse) relationship between FA in right uncinate fasciculus (and a positive relationship for right uncinate fasciculus RD), and both depression and general distress levels (derived from depressive and anxiety symptoms). This supports a broader role for this tract in emotional dysregulation. Variations in uncinate fasciculus FA and RD, which are indirect indices of white matter integrity, suggest decreased connectivity that may disrupt prefrontal control over limbic regions, including the amygdala, thereby contributing to elevated affective symptoms, and in turn, poor QoL.
Participants’ levels of depression and general distress were most predictive of QoL consistent with findings demonstrating substantial QoL impairments in depressed and anxious individuals (Olatunji et al., 2007; Rapaport et al., 2005). QoL is an important measure of treatment outcome, that is related but not redundant with symptom severity (Endicott et al., 1993), and encompasses a range of psychosocial and physical factors of life satisfaction from the patient’s perspective (Dzevlan et al., 2019).
The strengths of this study are: the focus on early adulthood – an important neurodevelopmental period, and the transdiagnostic approach. The main limitation is the cross-sectional design that precludes causal inferences. Future studies should further examine relationships between white matter microstructure in uncinate fasciculus, symptoms, and QoL using measures that capture localized differences, and investigate whether developmental changes in this tract predict levels of QoL over time.
Together, our findings highlight the role of white matter microstructure in right uncinate fasciculus in relation to depressive and general distress symptoms, and, in turn, QoL. Furthermore, they suggest that measures of white matter microstructure in this tract can be used as putative objective markers of emotion dysregulation, to inform and monitor the impact of interventions to reduce mood and anxiety symptoms and improve QoL in young adults.
Supplementary Material
Acknowledgments
Funding
The study was supported by the National Institute of Mental Health at the National Institutes of Health (R01MH100041; MLP) and the Emmerling-Pittsburgh Foundation (MLP).
The funding sources had no role in the study design, data analysis, or manuscript preparation.
Footnotes
Declaration of competing interest
MLP received an honorarium from Sunovion Pharmaceuticals. All other authors report no financial relationships with commercial interests.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpsychires.2020.10.001.
References
- Andersson JLR, Skare S, Ashburner J, 2003. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 20 (2), 870–888. [DOI] [PubMed] [Google Scholar]
- Andersson JLR, Sotiropoulos SN, 2015. Non-parametric representation and prediction of single- and multi-shell diffusion-weighted MRI data using Gaussian processes. Neuroimage 122, 166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson JLR, Sotiropoulos SN, 2016. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annett M, 1970. A classification of hand preference by association analysis. Br. J. Psychol 61 (3), 303–321. London, England: : 1953. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW, 2007. Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34 (1), 144–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair JR, Spreen O, 1989. Predicting premorbid IQ: a revision of the national adult reading test. Clin. Neuropsychol 3 (2), 129–136. [Google Scholar]
- Bracht T, Linden D, Keedwell P, 2015. A review of white matter microstructure alterations of pathways of the reward circuit in depression. J. Affect. Disord 187, 45–53. [DOI] [PubMed] [Google Scholar]
- Carver CS, White TL, 1994. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment : the BIS/BAS scales. J. Pers. Soc. Psychol 67 (2), 319–333. [Google Scholar]
- Clark LA, Watson D, 1991. Tripartite model of anxiety and depression: psychometric evidence and taxonomic implications. J. Abnorm. Psychol 100 (3), 316–336. [DOI] [PubMed] [Google Scholar]
- Dillon DG, Gonenc A, Belleau E, Pizzagalli DA, 2018. Depression is associated with dimensional and categorical effects on white matter pathways. Depress. Anxiety 35 (5), 440–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzevlan A, Redzepagic R, Hadzisalihovic M, Curevac A, Masic E, Alisahovic-Gelo E, Merdzanovic E, Hadzimuratovic A, 2019. Quality of life assessment in antidepressant treatment of patients with depression and/or anxiety disorder. Mater. Sociomed 31 (1), 14–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J, Nee J, Harrison W, Blumenthal R, 1993. Quality of life enjoyment and satisfaction Questionnaire: a new measure. Psychopharmacol. Bull 29 (2), 321–326. [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, Spitzer RL, 2015. Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). American Psychiatric Association, Arlington, VA. [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 12 (3), 189–198. [DOI] [PubMed] [Google Scholar]
- Friedman J, Hastie T, Simon N, Tibshirani R, 2014. GLMNET, 2.0-2 ed. [Google Scholar]
- Greenberg T, Bertocci MA, Chase HW, Stiffler R, Aslam HA, Graur S, Bebko G, Lockovich JC, Phillips ML, 2017. Mediation by anxiety of the relationship between amygdala activity during emotion processing and poor quality of life in young adults. Transl. Psychiatry 7, e1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M, 1959. The assessment of anxiety states by rating. Br. J. Med. Psychol 32 (1), 50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M, 1960. A rating scale for depression. J. Neurol. Neurosurg. Psychiatry 23, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF, Little TD, 2018. Introduction to Mediation, Moderation, and Conditional Process Analysis : a Regression-Based Approach. The Guilford Press, New York. [Google Scholar]
- Jbabdi S, Sotiropoulos SN, Savio AM, Graña M, Behrens TE, 2012. Model-based analysis of multishell diffusion MR data for tractography: how to get over fitting problems. Magn. Reson. Med 68 (6), 1846–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins LM, Barba A, Campbell M, Lamar M, Shankman SA, Leow AD, Ajilore O, Langenecker SA, 2016. Shared white matter alterations across emotional disorders: a voxel-based meta-analysis of fractional anisotropy. Neuroimage Clin. 12, 1022–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CH, Wu YT, 2014. Alterations in white matter micro-integrity of the superior longitudinal fasciculus and anterior thalamic radiation of young adult patients with depression. Psychol. Med 44 (13), 2825–2832. [DOI] [PubMed] [Google Scholar]
- Motzkin JC, Newman JP, Kiehl KA, Koenigs M, 2011. Reduced prefrontal connectivity in psychopathy. J. Neurosci. : Off. J. Soc. Neurosci 31 (48), 17348–17357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olatunji BO, Cisler JM, Tolin DF, 2007. Quality of life in the anxiety disorders: a meta-analytic review. Clin. Psychol. Rev 27 (5), 572–581. [DOI] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES, 1995. Factor structure of the Barratt impulsiveness scale. J. Clin. Psychol 51 (6), 768–774. [DOI] [PubMed] [Google Scholar]
- Phan KL, Orlichenko A, Boyd E, Angstadt M, Coccaro EF, Liberzon I, Arfanakis K, 2009. Preliminary evidence of white matter abnormality in the uncinate fasciculus in generalized social anxiety disorder. Biol. Psychiatr 66 (7), 691–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapaport MH, Clary C, Fayyad R, Endicott J, 2005. Quality-of-life impairment in depressive and anxiety disorders. Am. J. Psychiatr 162 (6), 1171–1178. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM, 2004. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23, S208–S219. [DOI] [PubMed] [Google Scholar]
- Spielberger CD, Gorsuch RL, Lushene RE, 1970. Manual for the State-Trait Anxiety Inventory. Consulting Psychologists Press, Polo Alto, CA. [Google Scholar]
- van Velzen LS, Kelly S, Isaev D, Aleman A, Aftanas LI, Bauer J, Baune BT, Brak IV, Carballedo A, Connolly CG, Couvy-Duchesne B, Cullen KR, Danilenko KV, Dannlowski U, Enneking V, Filimonova E, Forster K, Frodl T, Gotlib IH, Groenewold NA, Grotegerd D, Harris MA, Hatton SN, Hawkins EL, Hickie IB, Ho TC, Jansen A, Kircher T, Klimes-Dougan B, Kochunov P, Krug A, Lagopoulos J, Lee R, Lett TA, Li M, MacMaster FP, Martin NG, McIntosh AM, McLellan Q, Meinert S, Nenadic I, Osipov E, Penninx B, Portella MJ, Repple J, Roos A, Sacchet MD, Samann PG, Schnell K, Shen X, Sim K, Stein DJ, van Tol MJ, Tomyshev AS, Tozzi L, Veer IM, Vermeiren R, Vives-Gilabert Y, Walter H, Walter M, van der Wee NJA, van der Werff SJA, Schreiner MW, Whalley HC, Wright MJ, Yang TT, Zhu A, Veltman DJ, Thompson PM, Jahanshad N, Schmaal L, 2020. White matter disturbances in major depressive disorder: a coordinated analysis across 20 international cohorts in the ENIGMA MDD working group. Mol. Psychiatr 25 (7), 1511–1525. 10.1038/s41380-019-0477-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Acuff H, Bertocci MA, Bebko G, Almeida JRC, Perlman SB, Leemans A, Schirda C, Aslam H, Dwojak A, Bonar L, Travis M, Gill MK, Demeter C, Diwadkar VA, Sunshine JL, Holland SK, Kowatch RA, Birmaher B, Axelson D, Horwitz SM, Frazier TW, Arnold LE, Fristad MA, Youngstrom EA, Findling RL, Phillips ML, 2015. White matter structure in youth with behavioral and emotional dysregulation disorders: a probabilistic tractographic study. JAMA Psychiatr. 72 (4), 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteside SP, Lynam DR, 2001. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers. Indiv. Differ 30 (4), 669–689. [Google Scholar]
- Yendiki A, Panneck P, Srinivasan P, Stevens A, Zöllei L, Augustinack J, Wang R, Salat D, Ehrlich S, Behrens T, Jbabdi S, Gollub R, Fischl B, 2011. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front. Neuroinf 5, 23–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA, 1978. A rating scale for mania: reliability, validity and sensitivity. Br. J. Psychiatry 133, 429–435. [DOI] [PubMed] [Google Scholar]
- Zuckerman M, 2007. The sensation seeking scale V (SSS-V): still reliable and valid. Pers. Indiv. Differ 43 (5), 1303–1305. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


