Abstract
Background
In the treatment of chronic heart failure, vasodilating agents, ACE inhibitors and beta‐blockers have shown an increase of life expectancy. Another strategy is to increase the inotropic state of the myocardium : phosphodiesterase inhibitors (PDIs) act by increasing intra‐cellular cyclic AMP, thereby increasing the concentration of intracellular calcium, and lead to a positive inotropic effect.
Objectives
This overview on summarised data aims to review the data from all randomised controlled trials of PDIs III versus placebo in symptomatic patients with chronic heart failure. The primary endpoint is total mortality. Secondary endpoints are considered such as cause‐specific mortality, worsening of heart failure (requiring intervention), myocardial infarction, arrhythmias and vertigos. We also examine whether the therapeutic effect is consistent in the subgroups based on the use of concomitant vasodilators, the severity of heart failure, and the type of PDI derivative and/or molecule. This overview updates our previous meta‐analysis published in 1994.
Search methods
Randomised trials of PDIs versus placebo in heart failure were searched using MEDLINE (1966 to 2004 January), EMBASE (1980 to 2003 December), Cochrane CENTRAL trials (The Cochrane Library Issue 1, 2004) and McMaster CVD trials registries, and through an exhaustive handsearching of international abstracting publications (abstracts published in the last 22 years in the "European Heart Journal", the "Journal of the American College of Cardiology" and "Circulation").
Selection criteria
All randomised controlled trials of PDIs versus placebo with a follow‐up duration of more than three months.
Data collection and analysis
Twenty one trials (8408 patients) were eligible for inclusion in the review. Four specific PDI derivatives and eight molecules of PDIs have been considered.
Main results
As compared with placebo, treatment with PDIs was found to be associated with a significant 17% increased mortality rate (The relative risk was 1.17 (95% confidence interval 1.06 to 1.30; p< 0.001). In addition, PDIs significantly increase cardiac death, sudden death, arrhythmias and vertigos. Considering mortality from all causes, the deleterious effect of PDIs appears homogeneous whatever the concomitant use (or non‐use) of vasodilating agents, the severity of heart failure, the derivative or the molecule of PDI used.
Authors' conclusions
Our results confirm that PDIs are responsible for an increase in mortality rate compared with placebo in patients suffering from chronic heart failure. Currently available results do not support the hypothesis that the increased mortality rate is due to additional vasodilator treatment. Consequently, the chronic use of PDIs should be avoided in heart failure patients.
Keywords: Humans; 3',5'‐Cyclic‐AMP Phosphodiesterases; 3',5'‐Cyclic‐AMP Phosphodiesterases/antagonists & inhibitors; Administration, Oral; Cyclic Nucleotide Phosphodiesterases, Type 3; Heart Failure; Heart Failure/drug therapy; Heart Failure/mortality; Phosphodiesterase Inhibitors; Phosphodiesterase Inhibitors/adverse effects; Phosphodiesterase Inhibitors/therapeutic use; Randomized Controlled Trials as Topic
Plain language summary
Phosphodiesterase III inhibitor class drugs taken orally and long term are associated with increased deaths in heart failure
A number of options are available to treat symptomatic chronic heart failure. These include ACE inhibitors, beta‐blockers and spironolactone, which result in an increase of life expectancy. Another strategy is to increase the strength of the pumping action of the heart as with digitalis and with phosphodiesterase III inhibitors. The review clearly showed evidence that people treated for chronic heart failure for three months or more with phosphodiesterase III inhibitors were more likely to die than people given an inactive placebo treatment.
Background
Heart failure leads to an impaired quality of life, morbidity requiring frequent hospitalizations, and shortened life expectancy. The treatment of chronic heart failure has evolved over the past 20 years, in particular with the introduction of vasodilators (Chatterjee 1980; Cohn 1973; Cohn 1977). Hydralazine and isosorbide dinitrate were first shown to reduce mortality (Cohn 1986), but have been superceded by angiotensin converting enzyme (ACE) inhibitors which have been evaluated in several clinical trials and meta‐analyses (Braunwald 1991; Cohn 1991; CONSENSUS 1987; Furberg 1988; Garg 1995; Mulrow 1988; SOLVD 1991). More recently, in conjunction with conventional treatment with diuretics and angiotensin‐converting enzyme inhibitors, beta blockers (bisoprolol, metoprolol and carvedilol) have been shown to improve myocardial function and survival when used in stable and euvolemic patients (CIBIS II 1999; MERIT‐HF 1999; Packer 2001).
Another therapeutic strategy involves the use of drugs which can enhance the inotropic state of the failing heart (i.e. to increase the strength of the pumping action of the heart), e.g. digitalis glycosides, sodium‐channel agonists, or drugs able to increase the concentration of intra‐cellular cyclic AMP (cAMP), either by promoting its synthesis (beta‐adrenergic agonists) or by retarding its degradation (phosphodiesterase inhibitors) (PDIs) (DiBianco 1991; Storstein 1988; Weber 1987). PDIs act by increasing intra‐cellular cyclic AMP, thereby increasing the concentration of intracellular calcium, and lead to a positive inotropic effect. PDIs also affect vascular smooth muscle causing vasodilation.
Because of their attractive mechanism of action and pharmacological profile many phosphodiesterase inhibitors have been synthesised with a variety of dissimilar structures. The development of amrinone, and its more potent analogue milrinone, (both pyridinone derivatives) was followed by the synthesis and pharmacological characterisation of many related positive inotropic agents (e.g. enoximone and piroximone derived from imidazolone, pimobendan, imazodan, indolidan and flosequinan derived from pyridazinone, OPC‐8212 and vesnarinone derived from quinolinone).
It is thought that these drugs, by means of their common acidic amide function, compete with the phosphate function of cAMP for binding to the esteratic site of Phosphodiesterase III (PDE III), thereby causing competitive inhibition of this enzyme and accumulation of intra‐cellular cAMP.
The efficacy on mortality of the PDIs was first evaluated in one clinical trial with sufficient statistical power in patients with stage III to IV heart failure. After a median follow‐up of 6.1 months (range 1 day to 20 months), a 28% increase in total mortality was observed in the group receiving milrinone compared with that receiving placebo and the trial was prematurely terminated (PROMISE 1991). Some previous trials (AMTG 1985; EMTG 1990; MMTG 1989; Packer 1984; Rettig 1986), several reviews (Hood 1989; Packer 1988a; Wood 1989), and one meta‐analysis (Yusuf 1990), have suggested a similar increased mortality rate of PDIs as compared to placebo.
However, after the publication of the results of three trials testing vesnarinone (Feldman 1991; OPC‐8212 MRG 1990; VSG 1993), a more recent meta‐analysis (Nony 1994) found a significant heterogeneity of the therapeutic effect due to the inclusion of the data of the vesnarinone trials. Vesnarinone is a drug with multiple mechanisms of action that appears to enhance contractility through ion‐channel effects that augment sodium‐calcium exchange. Short‐term administration of vesnarinone to patients with heart failure was associated with limited and variable haemodynamic effects, but small placebo‐controlled trials showed a favourable effect on the quality of life and morbidity (Feldman 1991). In a first multicenter study, initiated in 1990 (VSG 1993), a significant 50 percent reduction in the combined end point and a 61 % reduction in mortality from all causes were observed in the vesnarinone‐treated group. However the results were problematic (short duration of the trial and contradictory results between the two studied regimens) and the " Vesnarinone trial " (VEST 1998) was therefore initiated in 1995 to study the long‐term effects of 60 mg or 30 mg of vesnarinone daily. However results showed a dose‐related increase in mortality in response to vesnarinone.
Several possible explanations for the deleterious effects of PDIs on mortality have been proposed (Curfman 1991; Feldman 1987; Packer 1988b), including: 1) an excessive increase of intracellular cyclic AMP, which decreases in chronic heart failure patients, possibly as an adaptive response; 2) an arrhythmogenic effect; or 3) a potentiation of the deleterious effects of PDIs by concomitant treatment with vasodilators. This last phenomenon may account either for the observed increase in mortality rate or for the adverse effects, such as hypotension, blurred vision and syncope, reported under treatment. However, the increased mortality in the last vesnarinone trial was attributed to an increased incidence of sudden death (VEST 1998), which was seen in previous trials of milrinone and flosequinan. Whether these drugs exert direct arrhythmic effects or accelerate structural remodeling of the ventricle, thus providing the substrate for ventricular tachycardia and fibrillation, is unknown. The favourable effects of ACE inhibitors, the combination of isosorbide dinitrate and hydralazine, and beta‐blockers on the incidence of sudden death appear to be associated with the regression of structural ventricular remodeling, thus suggesting a link between ventricular dilatation and lethal arrhythmias. Unfortunately, the effect of vesnarinone on ventricular volume was not assessed in the last "vesnarinone trial". In view of these contradictory findings, and the likelihood that PDIs are harmful in chronic heart failure, a systematic review of all the evidence is needed. In addition, the PDIs III class effect will be revisited, in order to investigate for specific derivatives which are less dangerous than others. Lastly, such an overview will be an update of our previous published meta‐analysis (Nony 1994).
Objectives
The main aim is to systemically review the data from all randomised controlled trials of orally administered PDIs III versus placebo in symptomatic patients with chronic heart failure. The primary endpoint is total mortality. Secondary endpoints are considered such as cause‐specific mortality (cardiac or sudden death), worsening of heart failure, myocardial infarction, arrhythmias and vertigos. We also examine whether the therapeutic effect is consistent within the subgroups based on the use of concomitant vasodilators, the severity of heart failure, the type of molecule or derivative.
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials are included in our meta‐analysis if they satisfy the following inclusion criteria:
randomised, placebo‐controlled, double‐blind studies of orally administered PDI therapy;
trials with a well‐defined "a priori" end‐point;
trials having a minimum follow‐up of 3 months;
trials with data available using an intention to treat strategy;
No more than 10% loss to follow‐up for relevant clinical outcome.
Trials in which patients are not randomised are excluded from the meta‐analysis.
Types of participants
Patients with an overt chronic heart failure (class II to IV, NYHA scale), where the mention of age range, criteria for diagnosis of heart failure (i.e. clinical findings or objective indices) were included.
Types of interventions
Selected studies are those with the following comparisons :
PDIs III versus placebo;
4 specific derivatives and 9 molecules of PDIs were considered (Table 1);
subgroups investigated the effect of PDI on the concomitant use (or non‐use) of vasodilating agents, on the severity of heart failure (inclusion or non inclusion of NYHA class IV patients), on the type of molecules and derivatives.
1. Characteristics of the included trials.
| Id trial | Nb of patients | Follow‐up (months) | Severity of CHF | Molecule type | Derivative | Dose per day | Vasodilator used |
| AMTG 1985 | 99 | 3 | III, IV | Amrinone | Pyridinone | < 600 mg | YES |
| Berger‐Klein 1992 | 24 | 3 | II, III | Pimobendan | Pyridazinone | 10 mg | NO |
| Cowley 1993 | 135 | 4 | II, III | Flosequinan | Pyridazinone | 125 mg | NO |
| Cowley 1994 | 151 | 12 | III, IV | Enoximone | Imidazolone | 300 mg | YES |
| Dies 1989 | 74 | 3 | II, III | Indolidan | Pyridazinone | NA | YES |
| EMTG 1990 | 102 | 4 | II, III | Enoximone | Imidazolone | < 450 mg | NO |
| EPOCH 2002 | 298 | 12 | II, III | Pimobendan | Pyridazinone | 2.5 & 5 mg | YES |
| ESG 2000 | 105 | 3 | II, III | Enoximone | Imidazolone | 75 & 150 mg | YES |
| FACET 1993 | 322 | 4 | II, III | Flosequinan | Pyridazinone | 100 & 150 mg | YES |
| IRG 1991 | 147 | 3 | III, IV | Imazodan | Pyridazinone | 4, 10 & 20 mg | YES |
| Lardoux 1987 | 43 | 3 | NA | Enoximone | Imidazolone | 150 & 300 mg | NO |
| MMTG 1989 | 230 | 3 | II, III & IV | Milrinone | Pyridinone | < 40 mg | NO |
| OPC‐8212 MRG 1990 | 83 | 3 | II, III & IV | Vesnarinone | Quinolinone | 60 mg | YES |
| PICO 1996 | 331 | 6 | II, III | Pimobendan | Pyridazinone | 2.5 & 5 mg | YES |
| PMRG 1992 | 198 | 3 | II, III | Pimobendan | Pyridazinone | 2.5, 5 & 10 mg | YES |
| PROMISE 1991 | 1088 | 20 | III, IV | Milrinone | Pyridinone | 40 mg | YES |
| REFLECT 1993 | 193 | 3 | II, III | Flosequinan | Pyridazinone | 100 mg | NO |
| REFLECT II 1991 | 311 | 3 | II, III & IV | Flosequinan | Pyridazinone | 100 & 150 mg | NO |
| VEST 1998 | 3833 | 9 | III, IV | Vesnarinone | Quinolinone | 30 & 60 mg | YES |
| VSG 1993 | 477 | 6 | II, III & IV | Vesnarinone | Quinolinone | 60 mg | YES |
| WESG 1991 | 164 | 3 | II, III | Enoximone | Imidazolone | 150 & 300 mg | NO |
Types of outcome measures
Summarised data were collected where available in the publication, for each considered end‐point :
Primary endpoint: total mortality;
Secondary endpoints: cardiac death, sudden death, worsening of heart failure (requiring intervention like hospitalisation, modification of treatments, withdrawal from study), occurrence of myocardial infarction, cardiac transplantation, arrhythmias as defined by authors, and vertigos (dizziness, lipothymia or syncope).
Search methods for identification of studies
Relevant clinical trials were found:
by computerised searches of MEDLINE (1966 to 2004 February), EMBASE (1980 to 2004 February), Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library Issue 1, 2004 and McMaster CVD trials registries;
by scanning general reviews, overviews, guidelines, consensus conference reports;
by exhaustive handsearching of international abstracting publications of congress (abstracts published in the last 22 years from 1980 to September 2002 in the "European Heart Journal" for ECC, the "Journal of the American College of Cardiology" for ACC and "Circulation" for AHA);
by contacting experts, investigators, public research institutions and the manufacturers of PDIs;
No language restrictions were applied.
The computer search of the CENTRAL database (The Cochrane Library Issue 1, 2004) was performed using the following specific terms see Appendix 1: The search was adapted for use in other databases. Strategies used in MEDLINE and EMBASE are listed in Appendix 2 and Appendix 3.
Data collection and analysis
Data selection and extraction
All references identified were evaluated by two reviewers (CK, HG) to determine relevant articles for full text retrieval. The retrieved references were assessed for eligibility by two reviewers according to the pre‐specified inclusion criteria. For each trial fulfilling the inclusion criteria an assessment of methodological quality (allocation concealment and blinding) was done by two reviewers independently. Data extraction was also done independently by two reviewers using a standardised form and all extracted data were checked by the first author (EA). The information collected from each trial included study design, patient characteristics, interventions, outcomes. Any discordance between reviewers has been settled by a third reviewer (PN). Authors of studies were contacted when additional information was required.
Data analysis
Meta‐analytical techniques (Boissel 1989; Nony 1995; Nony 1997; Yusuf 1987) are used to increase the power of hypothesis tests and also to obtain information which is not directly available from individual trials. Since there was no way of deciding which effect model would be the most suitable (Nony 1994), the following methods are used for each analysis (additive, multiplicative or mixed models) :
risk difference method (additive model) (Yusuf 1987);
logarithm of relative risk (multiplicative model);
Greenland & Robins relative risk (multiplicative model);
logarithm of odds ratio method (multiplicative model) (Baptista 1977; Fleiss 1973);
Cochran method (Cochran 1954);
Mantel‐Haenszel method (Mantel 1959);
Peto method (Peto 1978).
The last three methods use a weighted function of the rate difference and are therefore different from the additive and multiplicative models. Using each method we calculated the value of the association statistic and thus the corresponding chi‐square statistic. The homogeneity chi‐square, calculated as the difference between the total chi‐square statistic and the association chi‐square statistic, enables the degree of homogeneity or equality among the values of association to be assessed. In case of non‐homogeneity (i.e. p<0.10), random effect models has been used (Der Simonian 1986). The results were expressed as the mean difference, the relative risk, the odds ratio, or the mean standardised difference (if appropriate), with a 95% confidence interval.
Significance level
association: to be cautious, only the "worst" (the least significant) p‐value for the association test should be taken into consideration for the conclusion, especially when there is no prior knowledge of the effect model the level of significance is set at p = 0.05;
homogeneity: since statistical tests lack power to measure heterogeneity, we use an empirical significance level at p = 0.10.
Results
Description of studies
From the 144 references of potential clinical trials initially selected, only 36 references (21 randomised controlled trials) satisfied our inclusion criteria and were included in this analysis. The main characteristics of these studies are summarised in Table 1 and the Characteristics of Included Studies table. The reasons for the exclusion of the remaining 108 references were :
narrative or review articles (23 references: 23 studies);
multiple publications (20 references: 15 studies);
uncontrolled trials or absence of placebo group (14 references: 14 studies);
more than 10% loss to follow up for relevant clinical outcome (13 references: 13 studies);
no relevant clinical data available and/or duration of treatment too short (13 references: 13 studies);
comparison with active treatment and/or acute IV administration of PDI and/or dose comparison of PDI (12 references: 12 studies);
withdrawal study (4 references: 4 studies);
intent to treat analysis impossible to perform (1 reference: 1 study);
studies awaiting assessment (8 references: 4 studies).
For the PROFILE (flosequinan) study, only an ancillary study has been published (PROFILE 1993) (Table 2) and we considered that a specific additional assessment was necessary. According to the above mentioned chemical structures of PDIs, we included the flosequinan studies in our meta‐analysis, whereas the most important pharmacologic activities of this drug consist in vasodilation. A further sensitivity analysis has been performed with the study of the subgroup of the flosequinan trials. As in the majority of studies on heart failure, the inclusion criteria for patients were : functional status (NYHA class II to IV); chest X‐ray film showing cardiac enlargement; impairment of ventricular performance (measured using non‐invasive techniques; and/or decreased duration of exercise).
2. Characteristics of studies awaiting assessment.
| Study ID | Description |
| Goldberg 1990 | 147 patients were randomized but the number of patients per group wasn't available |
| Kato 1991 | Article in Japanese. 100 patients were randomized and the duration of treatment was 4 weeks |
| Kato 1992 | Article in Japanese. 81 patients were randomized and the duration of treatment was 8 weeks |
| MIL 1077‐1078 | 254 patients were randomized but the number of patients per group wasn't available |
| Profile 1993 | 2345 patients were randomised and 431 deaths occurred (208 sudden death). On interim analyses (n=2304), flosequinan was significantly associated with an excess of mortality when compared with placebo (RR=1.43; p<0.05). Definite results were never published |
The exclusion criteria included obstructive valvular disease, active myocarditis, hypertrophic cardiomyopathy, or malfunctioning artificial heart valve. Patients were also usually excluded if they had severe : symptomatic ventricular arrhythmia; angina pectoris; primary pulmonary, renal, or hepatic disease; hypotension (systolic blood pressure below 85 mm Hg); or acute or recent myocardial infarction.
The characteristics of included patients in the trials are described in Table 3 and the Characteristics of Included Studies Table. On average, age of patients in the trials varied from 50 to 66.6 years. The proportion of men varied from 65.1% to 91.7% and the mean LVEF from 19% to 43.5% with most of values around 23%. The aetiology of heart failure was ischaemic for 16.7% to 78.1% of patients and non‐ischaemic for 21.9% to 83.3% of them. Concomitant therapy (Table 3 and the Characteristics of Included Studies table) generally consisted of digitalis and diuretics. In some trials particularly the earlier ones, no treatment with vasodilators was allowed, in particular no nitrate derivatives, hydralazine, alpha‐receptor blocking drugs or ACE inhibitors (Table 1).
3. Characteristics of patients included in the trials.
| Id trial | Age (mean: year) | Sex (%male) | LVEF (mean: %) | Etiology | NYHA Class | Treatments |
| AMTG 1985 | 55.5 | 87.9 | 21.5 | Ischemic: 52.5% Non‐ischemic: 47.5% | III: 90% IV: 10% | Diuretics: 100% Digitalics: 98% |
| Bergler‐Klein 1992 | 50 | 91.7 | 19 | Ischemic: 16.7% Non‐ischemic: 83.3% | II: 54.2% III: 45.8% | ACEi: 0% |
| Cowley 1993 | 62.9 | 66.7 | NS | Ischemic: 69.6% Non‐ischemic: 30.4% | II: 89.6% III: 10.4% | ACEi: 0% |
| Cowley 1994 | 66.6 | 80.8 | NS | Ischemic: 78.1% Non‐ischemic: 21.9% | III: 76.8% IV: 23.2% | Diuretics:100% ACEi: 94.7% |
| Dies 1989 | NS (not stated) | NS | NS | NS | NS | NS |
| EMTG 1990 | 61.5 | 72.5 | 21.5 | Ischemic: 49% Non‐ischemic: 51% | II: 35.3% III: 64.7% | Diuretics: 100% Digitalics: 100% ACEi: 0% |
| EPOCH 2002 | 63.9 | 73.6 | 32.9 | Ischemic: 33.7% Non‐ischemic: 66.3% | II: 66.3% III: 33.7% | Diuretics: 81.5% Digitalics: 59.1% ACEi: 33% |
| ESG 2000 | 59.5 | 76.2 | 23 | Ischemic: 56.2% Non‐ischemic: 43.8% | I: 1% II: 47.6% III: 51.4% | Diuretics: 93.3% Digitalics: 82.9% |
| FACET 1993 | 58 | 71 | 23 | Ischemic: 41% Non‐ischemic: 59% | II: 53% III: 44% IV: 3% | Diuretics: 96% Digitalics: 89% ACEi: 100% |
| IRG 1991 | 59 | 87.1 | 23 | Ischemic: 53.1% Non‐ischemic: 46.9% | I‐II: 46.2% III: 49% IV: 4.8% | Diuretics: 100% Digitalics: 84.4% ACEi: 55.1% |
| Lardoux 1987 | 61.2 | NS | NS | Ischemic: 48.8% Non‐ischemic: 51.2% | NS | ACEi: 0% |
| MMTG 1989 | 59.3 | 70.5 | 24 | Ischemic: 51% Non‐ischemic: 49% | II: 29.3% III: 66.7% IV: 4% | Digitalics: 53% ACEi: 23.5% |
| OPC‐8212 MRG 1990 | 58.5 | 65.1 | 43.5 | Ischemic: 16.9% Non‐ischemic: 83.1% | NS | Diuretics: 75.9% Digitalics: 74.7% |
| PICO 1996 | 65.5 | 80 | 27 | Ischemic: 69% Non‐ischemic: 31% | II: 52% III: 48% | Diuretics: 100% Digitalics: 59% ACEi: 100% |
| PMRG 1992 | 58 | 78.3 | 22 | Ischemic: 43.9% Non‐ischemic: 56.1% | III: 96% IV: 4% | Diuretics: 98% Digitalics: 88.4% ACEi: 79.8% |
| PROMISE 1991 | 63.7 | 78.4 | 21 | Ischemic: 54.2% Non‐ischemic: 45.8% | III: 58% IV: 42% | Diuretics: 100% Digitalics: 100% ACEi: 100% |
| REFLECT 1993 | 57.7 | 87 | 25.5 | Ischemic: 43.5% Non‐ischemic: 56.5% | II: 42% III: 58% | Diuretics: 100% Digitalics: 96% ACEi: 0% |
| REFLECT II 1991 | NS | NS | NS | NS | NS | ACEi: 0% |
| VEST 1998 | 63 | 76.3 | 20.9 | Ischemic: 58.2% Non‐ischemic: 41.8% | III: 84.9% IV: 15.1% | ACEi: 90% |
| VSG 1993 | 58.2 | 86.8 | 20 | Ischemic: 52.2% Non‐ischemic: 47.8% | I: 0.8% II: 18.7% III: 69.6% IV: 10.9% | Digitalics: 87.2% ACEi: 89.5% |
| WESG 1991 | 58.8 | 79.3 | 24.7 | Ischemic: 45.7% Non‐ischemic: 54.3% | II: 42.1% III: 57.9% | ACEi: 0% |
Risk of bias in included studies
Blinding
All included studies but one (Dies 1989: type of blinding not stated) were double‐blind placebo controlled trials.
Randomisation
The method of randomisation was concealed for five RCTs (AMTG 1985; EPOCH 2002; MMTG 1989; PROMISE 1991; VEST 1998; VSG 1993) that included 6025 patients (71.7% of all available patients). In one trial (AMTG 1985) the randomisations were stratified on concomitant treatment with ACE inhibitors or not, and therefore these subgroups of patients were included in the analyses examining concomitant vasodilators.
Type and language of publication
Four trials (Dies 1989; Lardoux 1987; PROFILE 1993; REFLECT II 1991) have only been published as an abstract. In three trials (Dies 1989; Lardoux 1987; REFLECT II 1991) , 428 patients were included. In the PROFILE 1993 trial 2345 patients were randomised and 431 deaths occurred (208 sudden death). On interim analyses (n=2304), flosequinan was significantly associated with an excess of mortality when compared with placebo (RR=1.43; p<0.05). Definite results were never published and no other information was available. Currently, this study is awaiting assessment before definite exclusion for unavailability of data. One trial (Bergler‐Klein 1992) included in this review was only published in german. This paper was translated before inclusion.
Loss to follow‐up
The proportion of participants lost to follow‐up was not stated in four trials (Cowley 1994; Dies 1989; EMTG 1990; ESG 2000) including 432 patients. In seven trials (Bergler‐Klein 1992; FACET 1993; IRG 1991; MMTG 1989; PROMISE 1991; VEST 1998; VSG 1993) no patient has been lost to follow‐up (6121 patients: 72,8% of all available patients). In the ten other trials (1855 patients) the proportion of patients loss to follow‐up was between 2.2% and 9.6%.
Primary outcome
A primary outcome was described in 14 trials and was clinically relevant in only four trials (EPOCH 2002; PROMISE 1991; VEST 1998; VSG 1993) including 5696 patients (67.7% of all available patients). In the remaining ten trials the primary outcome used was "exercise tolerance".
Effects of interventions
Description of search strategy results
Using MEDLINE and EMBASE databases, we found 1470 references, whereas 263 and 62 references were identified respectively with CENTRAL and the McMaster CVD trials registry.
Meta‐analysis results
Total mortality
Treatment with PDI was found to be associated with a significant increased mortality rate with relative risk 1.17 (95% Confidence Interval 1.06 to 1.30; p= 0.002) compared with placebo. However the test for heterogeneity was borderline significant (p=0.10) irrespective of the fixed relative risk effect model used see Comparison 01 01. For other statistical methods a significant heterogeneity has been observed: Log odds ratio, heterogeneity p=0.076; Mantel‐Haenzel odds ratio, heterogeneity p=0.031; Peto odds ratio, heterogeneity p=0.028; Cochran odds ratio, heterogeneity p=0.036; and Risk difference heterogeneity p= 0.0006.
The results from the various meta‐analysis techniques are shown in Table 4. Using the radial plot approach (Galbraith 1988) (Figure 1) the heterogeneity of the PDIs effect among trials seems essentially due to the results of the multicenter study of VSG 1993 (see Figure 1).
4. Summary of results (end point: total mortality).
| Statistical Method | Fixed Effect | Random Effect |
| Logarithm of the relative risk (RR) | 1.180 (95% CI 1.067 to 1.305); p<0.001; p‐hetero=0.10 | |
| Greenland & Robins (RR) | 1.175 (95% CI 1.062 to 1.299); p=0.002; p‐hetero=0.10 | |
| Logarithm of the odds ratio (OR) | 1.224 (95% CI 1.079 to 1.388); p=0.002; p‐hetero=0.076 | 1.201 (95% CI 0.946 to 1.526); p=0.13 |
| Mantel‐Haenzel (OR) | 1.223 (95% CI 1.079 to 1.386); p=0.002; p‐hetero=0.031 | 1.201 (95% CI 0.946 to 1.526); p=0.13 |
| Peto (OR) | 1.220 (95% CI 1.078 to 1.380), p=0.002; p‐hetero=0.028 | 1.201 (95% CI 0.946 to 1.526); p=0.13 |
| Cochrane (OR) | 1.215 (95% CI 1.075 to 1.374), p=0.002; p‐hetero=0.036 | 1.201 (95% CI 0.946 to 1.526); p=0.13 |
| Risk Difference (RD) | 2.58% (95% CI 1.01% to 4.16%), p = 0.001; p‐hetero=0.0006 | 1.17 (95% CI ‐0.86 to 3.19); p=0.24 |
1.
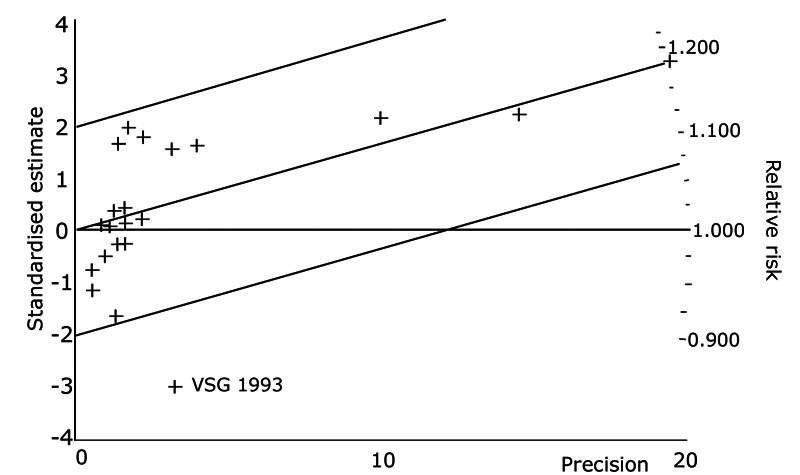
Exploration of heterogeneity between PDI trials for total mortality. Relative risk, Fixed model ‐ Radial plot . The VSG 1993 trial is outside of the limits of homogeneity of the treatment effect. This illustrates the heterogeneity introduced by this trial for total mortality.
Secondary endpoints
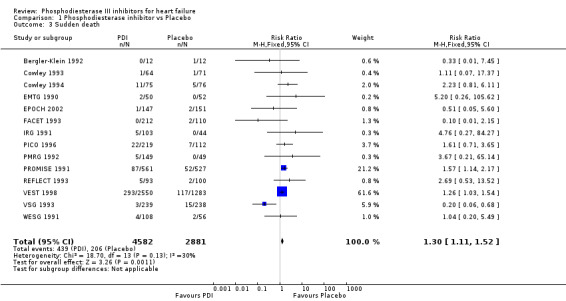
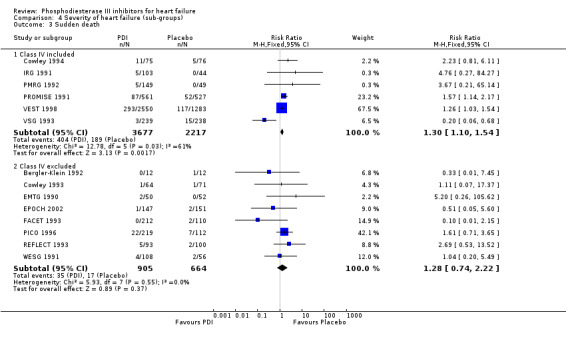
Compared with placebo PDIs significantly increased cardiac death with relative risk 1.16 (95% confidence interval 1.05 to 1.30; p=0.005), sudden death with relative risk 1.30 (95% confidence interval 1.11 to 1.52; p=0.001), arrhythmias with relative risk 1.25 (95% confidence interval 1.02 to 1.54; p=0.03) and vertigo with relative risk 1.81 (95% confidence interval 1.41 to 2.33; p<0.001). Again a significant heterogeneity was observed for cardiac death (p=0.008). The radial plot shown that this heterogeneity among trials on cardiac death is essentially due to the results of the study VSG 1993 (Figure 2). When a random effects model was used the effect of PDI on cardiac death was non significant and had a relative risk of 1.18 (95% confidence interval : 0.90 to 1.55; p=0.23).
2.
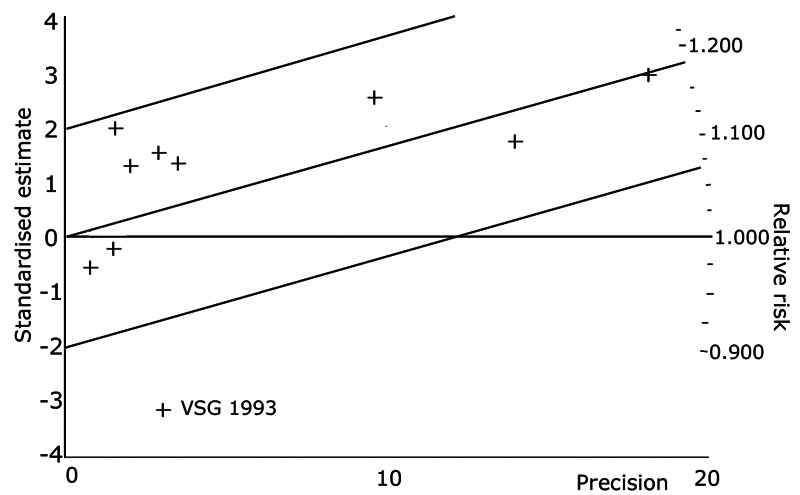
Exploration of heterogeneity between PDI trials for cardiac death. Relative risk, fixed model ‐ Radial plot. The VSG 1993 trial is outside of the limits of homogeneity of the treatment effect. This illustrates the heterogeneity introduced by this trial for cardiac death.
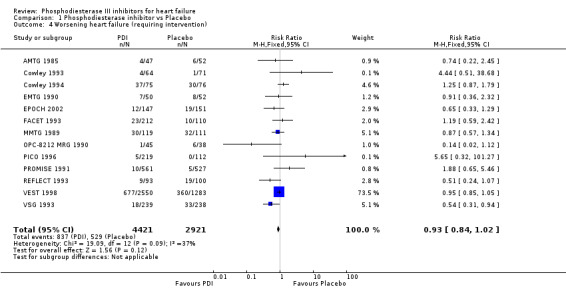
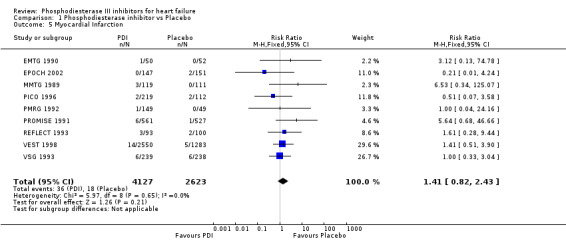
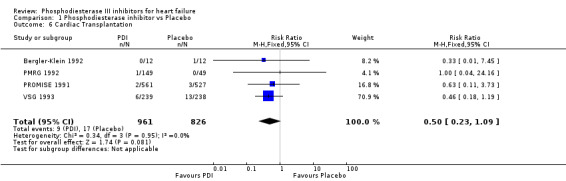
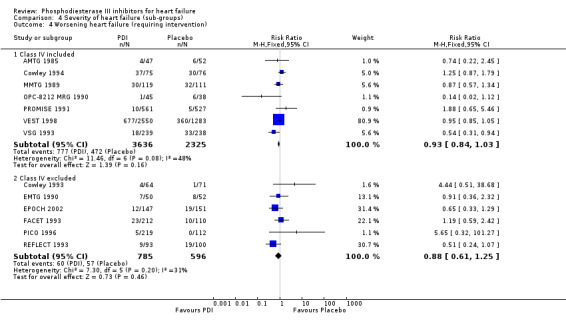
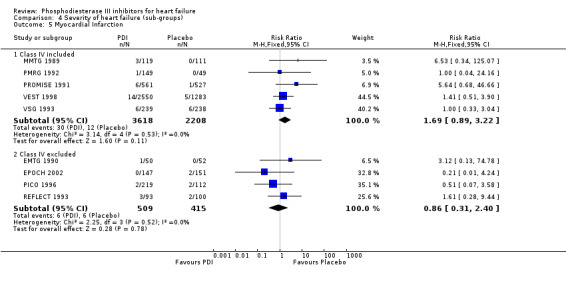
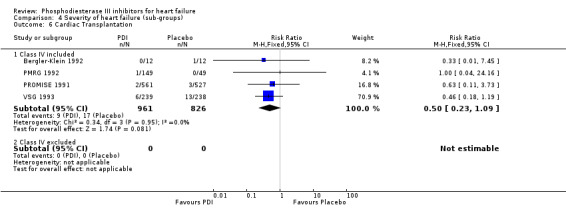
We identified a non significant decrease of worsening of heart failure with relative risk 0.93 (95% confidence interval 0.84 to 1.02; p=0.12) and cardiac transplantation with relative risk 0.50 (95% confidence interval 0.23 to 1.09; p=0.08). There was a non significant increase in myocardial infarction with relative risk 1.41 (95% confidence interval 0.82 to 2.43; p=0.21). Considering mortality from all causes, the deleterious effect of PDIs appeard homogeneous for the concomitant use or not of vasodilating agents, the severity of heart failure, the molecule or the derivative of PDI used (Table 5). No significant interaction was found.
5. Sub‐groups analysis for total mortality.
| Sub‐groups (SG) | Categories | Nb of trials | Nb of patients | RR (95% CI) | p‐hetero between SG |
| Molecule type | Amrinone | 1 | 99 | 1.105 (0.182 to 6.785) | 0.92 |
| Enoximone | 5 | 565 | 1.416 (0.953 to 2.105) | ||
| Flosequinan | 4 | 961 | 1.408 (0.771 to 2.570) | ||
| Imazodan | 1 | 147 | 1.091 (0.318 to 3.751) | ||
| Indolidan | 1 | 74 | 1.369 (0.266 to 7.048) | ||
| Milrinone | 2 | 1318 | 1.292 (1.065 to 1.567) | ||
| PImobendan | 4 | 851 | 1.331 (0.776 to 2.282) | ||
| Vesnarinone | 3 | 4393 | 1.086 (0.953 to 1.238) | ||
| Derivative | Imidazolone | 5 | 565 | 1.416 (0.953 to 2.105) | 0.47 |
| Pyridazinone | 10 | 2033 | 1.347 (0.926 to 1.957) | ||
| Pyridinone | 3 | 1417 | 1.289 (1.064 to 1.562) | ||
| Quinolinone | 3 | 4393 | 1.086 (0.953 to 1.238) | ||
| Severity of CHF | Class IV included | 10 | 6617 | 1.153 (1.038 to 1.280) | 0.33 |
| Class IV excluded | 10 | 1748 | 1.467 (1.005 to 1.301) | ||
| Vasodilator used | YES | 13 | 7337 | 1.157 (1.044 to 1.282) | 0.31 |
| NO | 7 | 972 | 1.669 (0.978 to 2.847) |
Sensitivity analysis
The substitution of a fixed effects model for a random effects model did not change our initial qualitative interpretation of the pooled treatment deleterious effect on total mortality or cardiac death. After exclusion of the VEST 1998 trial (3833 patients and 802 deaths, i.e. 45.6% of all patients included in the trials of this meta‐analysis and 58.3% of the deaths occurring within all trials) the deleterious effect of PDI on total mortality remained significant : the relative risk was 1.19 (95% confidence interval 1.02 to 1.38; p=0.025). The funnel plot (Egger 1997) did not show any asymmetry i.e. tendency for a potential publication bias.
Sub‐group analyses
All sub‐groups analyses were specified a priori in the protocol for this systematic review. They were:
Molecule type: Amrinone, Pimobendan, Flosiquinan, Enoximone, Milrinone and Vesnarinone.
Derivative type: Imidizolone, Pyridizanone, Pyridinone, Quinolinone.
Severity of heart failure: People with Class IV heart failure (NYHA scale) included and People with class IV heart failure excluded.
Concomitant use of vasodilators or not.
These analyses are only exploratory to document the main effect of PDI on outcomes (Table 5). No statistical difference between sub‐groups has been observed for any outcome (no significant interaction was defined as p>0.05). Consequently, the effect of PDI was homogeneous between molecules, derivatives, severity of heart failure, and vasodilator use for all outcomes. It was not possible to explore effect of PDI according of the aetiology of heart failure (ischaemic and non‐ischaemic), since data were not available.
Discussion
Overall results
This meta‐analysis clearly shows evidence for a deleterious effect of orally administered PDIs given to patients presenting with overt chronic heart failure. This deleterious effect is consistent for all the considered endpoints: total mortality, mortality from cardiac origins, and sudden death. The level of evidence is high, according to the significant results of the two largest trials in the field (PROMISE 1991; VEST 1998). In addition, the results were homogeneous according to the severity of heart failure, the PDI derivative or the molecule used, and the concomitant use or non‐use of vasodilating agents.
Homogeneity of results
Considering total mortality, the borderline significant results of some homogeneity tests have to be discussed. As already mentioned, such an interaction may be related to the results of only one trial (VSG 1993). In this trial initiated in 1990, 577 patients with NYHA class III or IV heart failure were randomly assigned to receive placebo or 60 mg of vesnarinone daily for six months (some patients were assigned to 120 mg of vesnarinone but this part of the trial was stopped early). A remarkable and significant 62 percent reduction in mortality from all causes was observed in the vesnarinone treated group. However, these results were problematic for several reasons. First, the duration of the trial was short (six months) and the total number of events was small (46 deaths). Second, two vesnarinone regimens (60 and 120 mg daily) were studied and the higher‐dose regimen was discontinued early by the data and safety monitoring committee because of a trend toward an adverse effect on mortality. The contradiction between the results of this study and those of our meta‐analysis does not have any obvious explanation. Examination of the patient populations reveals no differences that could reasonably account for the opposite response to the daily administration of 60 mg of vesnarinone. In the trial of VEST 1998, the progressive increase in mortality from placebo to 30 mg and to 60 mg of vesnarinone per day (and when combined with the striking increase in mortality with a daily dose of 120 mg in the trial of VSG 1993) indicates a nearly linear adverse dose‐response effect of vesnarinone. Consequently, the trials assessing mortality or mortality and morbidity in patients with heart failure should have sufficient power with enough events and long enough follow‐up to ensure that the results reliably reflect the long term effects of the tested drug as compared with placebo.
Other methodological considerations
One major bias inherent to meta‐analysis is the iceberg phenomenon or file drawer problem due to unpublished trials (Rosenthal 1979). These trials usually have nonsignificant or "negative" results, whereas published trials often have significant results and this therefore is a potential source of bias in the analysis. Moreover, some studies have been performed purely for registration purposes and have never been published, often because pharmaceutical companies regard the results as confidential. Despite exhaustive searching, including screening conference proceedings and abstract books and contacting several investigators no additional studies could be identified. We have therefore attempted to minimise this publication bias.
The numerical data considered in our meta‐analysis have not yet been checked by all the investigators of the selected trials, so that some data extraction bias (e.g. in case of printing errors) cannot be completely excluded.
The selected trials were all double‐blind, placebo‐controlled, randomised studies. In meta‐analysis on trials in heart failure patients, it is not possible to take into consideration the unavoidable differences in the patients' baseline characteristics, such as the aetiology (ischaemic / non ischemic) of heart failure, or the duration of the heart disease before inclusion. The percentage of patients with ischemic heart disease probably differed from one trial to another, and the percentage of patients who died under placebo, which is an indirect measure of the severity of the heart failure, only varied from 0 to 24% (results extrapolated to 3 months of follow‐up). The actual follow‐up in the studies varied from 3 to 52 months, but the meta‐analytical approach assumes a constant therapeutic effect and a relative stability of the odds ratio over time. As no well‐defined a priori effect model exists for our problem, we used different meta‐analytical methods which involve summary statistics. These methods are based on a statistical model of the difference between treated and control patients, with the value of association being based either on a multiplicative effect (risk ratio or odds ratio) or an additive effect (rate or risk differences). Since different techniques applied to the same data set can yield different results in terms of the statistical significance of association, it is usually recommended to use more than one technique to ensure that only conservative conclusions are drawn (Tukey 1977). In doing so, we found no major differences between the results obtained with these methods.
Potential mechanisms of toxicity
The mechanism by which PDIs increase mortality in heart failure patients has not yet been fully elucidated. Intra cellular levels of cyclic AMP are decreased in the failing heart (Katz 1990), and this abnormality in cellular metabolism was the reason for using PDIs in heart failure. It now appears more plausible that the reduction in cyclic AMP levels in the failing heart may be an adaptive response that protects myocardial cells from further injury (EMTG 1990). Agents that increase levels of cyclic AMP could, therefore, be expected to be harmful, perhaps by enhancing ventricular arrhythmia, as has been suggested by the results of some experimental and clinical studies (Chesebro 1988; MMTG 1989; Packer 1984).
The potential toxicity of the association of a PDI and an additional vasodilator had to be explored both on an experimental and a physiological basis. Amrinone, through its inhibitory action on phosphodiesterase, not only increases the rate of relaxation of the myocardium but also produces relaxation in the smooth muscle of gut, bronchi and blood vessel wall (Wilmshurst 1984). The vasodilator properties of amrinone have been detected at concentrations lower than those at which positive inotropic effects have been detected in guinea pig myocardium. The improvement in left ventricular performance induced by amrinone in patients with heart failure appears to result, in part, from a decrease in peripheral vascular resistance (LeJemtel 1980). This effect is due to a direct vasodilator effect on the systemic vessel resistance, and to an indirect vasodilation, secondary to the withdrawal of enhanced sympathetic constrictor tone consequent to the improvement in cardiac performance. This is also true for milrinone (Colucci 1991; Simonton 1985), for which an intra coronary injection demonstrated the direct and indirect inotropic effects and confirmed the reduction of the sympathetic tone (by measurement of the heart rate and norepinephrine level), due to reflex withdrawal (Ludmer 1986). It has been estimated that only one‐third of milrinone's effect on pump function can be attributed to its direct myocardial action (Ludmer 1986). It has also be observed that intravenous enoximone produced hypotension in 3% of the patients treated for heart failure (Gilfrich 1991). Artery vasodilators are associated with a reduction in both systolic and diastolic arterial pressure, and total systemic vascular and pulmonary vascular resistances (Mikulic 1977). Nevertheless, when arterial pressure falls to a dangerously low level during therapy, the irrigation of some ischaemic areas of myocardium may be altered. Hence, some of the potentiated effects of the PDI‐additional vasodilator combination offer possible benefits (Mancini 1985), whereas, the lowering of blood pressure with an increase of myocardial ischaemia can, in certain haemodynamic conditions, cause deleterious effects. Although clinical trials include in our meta‐analysis are not completely comparable, particularly in terms of exercise tolerance and haemodynamic effects during long term therapy (Hood 1989), we found that PDIs, with and without concomitant vasodilator, are responsible for an increase in mortality, compared with placebo, and thus concomitant vasodilator treatment cannot explain the deleterious effect of PDIs on survival.
Interaction between pathophysiology and clinical trials
Such results confirm the essential need for a constant interaction between pathophysiology and the results of clinical trials, in order to help in the understanding of the mechanism of action of drugs and the future development of new treatments. For PDIs, pathophysiology suggested first that the use of such agents could lead to clinical benefits in heart failure, but the results of the PROMISE study showed an increased total mortality under milrinone as compared to placebo. Then attention turned to the potential pathophysiological mechanisms of such deleterious effects : excessive increase in intracellular cyclic AMP, which decreases in chronic heart failure patients possibly as an adaptive response, or an arrhythmogenic effect. Vesnarinone was then developed and was supposed to be effective because of its multiple mechanisms of action : enhancement of contractility through ion‐channel effects that augment sodium‐calcium exchange, and inhibition of the production of cytokines. However, the last vesnarinone trial (VEST 1998) showed a dose‐related increase in mortality in response to the drug. Again, several pathophysiological explanations were proposed such as a direct proarrhythmic effect related to an accelerated structural remodeling of the ventricle, thus providing the substrate for ventricular tachycardia and fibrillation. But the effect of vesnarinone on ventricular volume in the patients included in this trial remains unknown. Such an example shows that the concepts useful for researchers (pathophysiological hypotheses) must be clearly differentiated from those useful to physicians (evidence‐based clinical data). In our opinion, the differences between these two ways of thinking should be made clearer in the fields of therapeutic information and "evidence based medicine" and especially during medical teaching (Nony 1999).
Authors' conclusions
Implications for practice.
Our results confirm that people with chronic heart failure given orally administered PDIs have increased mortality rates compared with those given an inactive placebo. Currently available results do not support the hypothesis that the increased mortality rate is due to additional vasodilator treatment. The chronic use of PDIs should be avoided in heart failure patients.
Implications for research.
Alternative strategies for the use of PDIs in patients with chronic heart failure could be considered (e.g. a discontinuous treatment regimen for use only during periods of functional worsening), such strategies have first to be carefully evaluated in further randomised controlled trials with suitable duration of follow up, outcomes and power.
What's new
| Date | Event | Description |
|---|---|---|
| 29 November 2012 | Review declared as stable | This review is no longer being updated due to an increase in mortality demonstrated. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 9 September 2008 | Amended | Converted to new review format. |
| 1 November 2004 | New citation required and conclusions have changed | Substantive amendment |
Notes
This review is no longer being updated because several trials and this meta‐analysis demonstrated an increase in mortality. Consequently, no additional clinical trial has been performed with phosphodiesterase III inhibitors in this indication.
Acknowledgements
We are grateful to Mrs. Rousselet for library assistance and Miss Gauthier for secretarial help. We also wish to thank J Brunelin and N Ouahdi for handsearching of abstracts.
Appendices
Appendix 1. CENTRAL search strategy
Appendix 2. MEDLINE search strategy
The computer search of the MEDLINE database (from 1966 to January 2004) was performed using the following specific terms: (1) (("controlled clinical trial"[Publication Type]) OR ("clinical trial") OR ("clinical trials") OR (random*) OR ("randomised controlled trial"[Publication Type])); (2) ("Phosphodiesterase Inhibitors"[Pharmacological Action]) OR ("Phosphodiesterase Inhibitors"[MeSH Terms]) OR (amrinone) OR (amrinon) OR (milrinone) OR (milrinon) OR (enoximone) OR (enoximon) OR (piroximone) OR (pimobendan) OR (imazodan) OR (sulmazole) OR (isomazole) OR (flosequinan) OR (indolidan) OR (quazinone) OR (adibendan) OR (pelrinone) OR (siguazodan) OR (cilostamide) OR (zardaverine) OR (alifedrine) OR (lixazinone); (3) ("Heart Failure, Congestive"[MeSH Terms]) OR ("heart failure") OR ("dilated cardiomyopathy") OR ("ventricular dysfunction") OR ("Ventricular Dysfunction"[MeSH Terms]) OR (cardiomyo*).
Appendix 3. EMBASE search strategy
The computer search of the EMBASE database (from 1980 to December 2003) was performed using the following specific terms : 1 exp heart failure/dt [drug therapy] (12588) 2 exp phosphodiesterase inhibitor/ct, ad, cm, dt [clinical trial, drug administration, drug comparison, drug therapy] (3606) 3 1 and 2 (1202) 4 3 (1202) 5 limit 4 to human (1135) 6 amrinone.mp. (1853) 7 milrinone.mp. (1836) 8 enoximone.mp. (965) 9 piroximone.mp. (229) 10 pimobendan.mp. (407) 11 imazodan.mp. (172) 12 sulmazole.mp. (407) 13 isomazole.mp. (69) 14 flosequinan.mp. (309) 15 indolidan.mp. (73) 16 quazinone.mp. (49) 17 adibendan.mp. (59) 18 pelrinone.mp. (17) 19 siguazodan.mp. (112) 20 cilostamide.mp. (157) 21 zardaverine.mp. (92) 22 alifedrine.mp. (20) 23 lixazinone.mp. (27) 24 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 (4556) 25 24 and 1 (1268) 26 25 and 5 (933) 27 25 or 5 (1470)
Data and analyses
Comparison 1. Phosphodiesterase inhibitor vs Placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 21 | 8408 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.06, 1.30] |
| 2 Cardiac death | 9 | 6708 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.05, 1.30] |
| 3 Sudden death | 14 | 7463 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.11, 1.52] |
| 4 Worsening heart failure (requiring intervention) | 13 | 7342 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.02] |
| 5 Myocardial Infarction | 9 | 6750 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.82, 2.43] |
| 6 Cardiac Transplantation | 4 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.23, 1.09] |
| 7 Arrhythmias | 13 | 3666 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.02, 1.54] |
| 8 Vertigo | 7 | 2216 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.41, 2.33] |
1.1. Analysis.
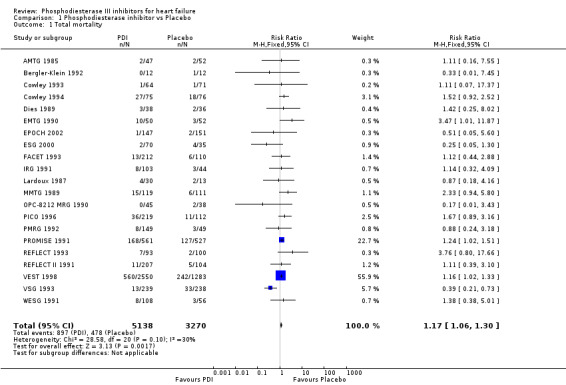
Comparison 1 Phosphodiesterase inhibitor vs Placebo, Outcome 1 Total mortality.
1.2. Analysis.
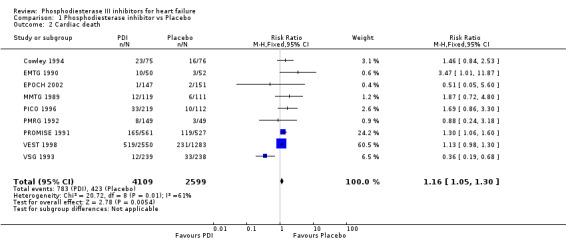
Comparison 1 Phosphodiesterase inhibitor vs Placebo, Outcome 2 Cardiac death.
1.3. Analysis.
Comparison 1 Phosphodiesterase inhibitor vs Placebo, Outcome 3 Sudden death.
1.4. Analysis.
Comparison 1 Phosphodiesterase inhibitor vs Placebo, Outcome 4 Worsening heart failure (requiring intervention).
1.5. Analysis.
Comparison 1 Phosphodiesterase inhibitor vs Placebo, Outcome 5 Myocardial Infarction.
1.6. Analysis.
Comparison 1 Phosphodiesterase inhibitor vs Placebo, Outcome 6 Cardiac Transplantation.
1.7. Analysis.
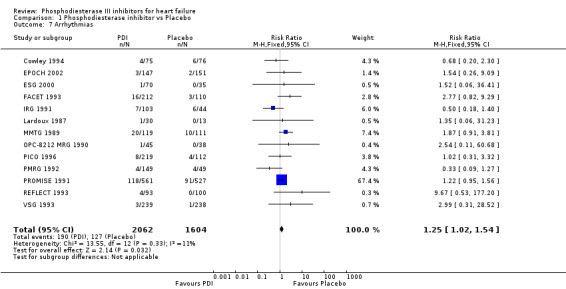
Comparison 1 Phosphodiesterase inhibitor vs Placebo, Outcome 7 Arrhythmias.
1.8. Analysis.
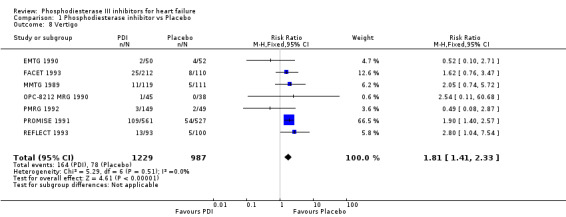
Comparison 1 Phosphodiesterase inhibitor vs Placebo, Outcome 8 Vertigo.
Comparison 2. Molecule type (sub‐groups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Amrinone | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.16, 7.55] |
| 1.2 Pimobendan | 4 | 851 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.78, 2.26] |
| 1.3 Flosequinan | 4 | 961 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.77, 2.57] |
| 1.4 Enoximone | 5 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.95, 2.10] |
| 1.5 Indolidan | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.25, 8.02] |
| 1.6 Imazodan | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.32, 4.09] |
| 1.7 Milrinone | 2 | 1318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.07, 1.57] |
| 1.8 Vesnarinone | 3 | 4393 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.95, 1.24] |
| 2 Cardiac death | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Pimobendan | 3 | 827 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.78, 2.44] |
| 2.2 Enoximone | 2 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.07, 2.92] |
| 2.3 Milrinone | 2 | 1318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.09, 1.62] |
| 2.4 Vesnarinone | 2 | 4310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.92, 1.21] |
| 3 Sudden death | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Pimobendan | 4 | 851 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.70, 2.87] |
| 3.2 Flosequinan | 3 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.38, 3.02] |
| 3.3 Enoximone | 3 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.90, 4.55] |
| 3.4 Imazodan | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.76 [0.27, 84.27] |
| 3.5 Milrinone | 1 | 1088 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.14, 2.17] |
| 3.6 Vesnarinone | 2 | 4310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.96, 1.42] |
| 4 Worsening heart failure (requiring intervention) | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Amrinone | 1 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.22, 2.45] |
| 4.2 Pimobendan | 2 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.43, 1.56] |
| 4.3 Flosequinan | 3 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.56, 1.45] |
| 4.4 Enoximone | 2 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.84, 1.66] |
| 4.5 Milrinone | 2 | 1318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.68, 1.50] |
| 4.6 Vesnarinone | 3 | 4393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.82, 1.01] |
| 5 Myocardial Infarction | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Pimobendan | 3 | 827 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.11, 1.84] |
| 5.2 Flosequinan | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.28, 9.44] |
| 5.3 Enoximone | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 74.78] |
| 5.4 Milrinone | 2 | 1318 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.94 [1.07, 33.08] |
| 5.5 Vesnarinone | 2 | 4310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.57, 2.57] |
| 6 Cardiac Transplantation | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Pimobendan | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.07, 4.75] |
| 6.2 Milrinone | 1 | 1088 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.11, 3.73] |
| 6.3 Vesnarinone | 1 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.18, 1.19] |
| 7 Arrhythmias | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Pimobendan | 3 | 827 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.37, 1.68] |
| 7.2 Flosequinan | 2 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.52 [1.18, 10.48] |
| 7.3 Enoximone | 3 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.28, 2.36] |
| 7.4 Indolidan | 1 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.18, 1.40] |
| 7.5 Milrinone | 2 | 1318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.02, 1.62] |
| 7.6 Vesnarinone | 2 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.45, 17.78] |
| 8 Vertigo | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Pimobendan | 1 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.08, 2.87] |
| 8.2 Flosequinan | 2 | 515 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.09, 3.62] |
| 8.3 Enoximone | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.71] |
| 8.4 Milrinone | 2 | 1318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.43, 2.56] |
| 8.5 Vesnarinone | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [0.11, 60.68] |
2.1. Analysis.
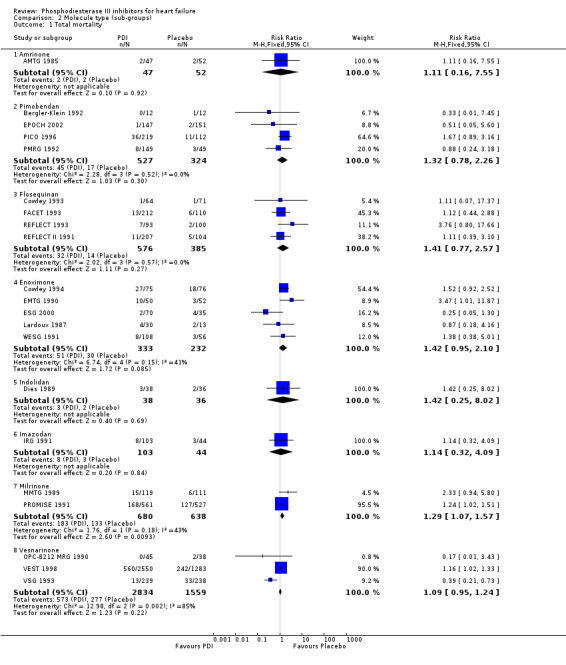
Comparison 2 Molecule type (sub‐groups), Outcome 1 Total mortality.
2.2. Analysis.
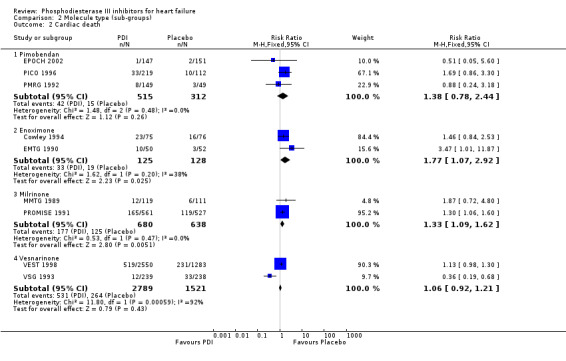
Comparison 2 Molecule type (sub‐groups), Outcome 2 Cardiac death.
2.3. Analysis.
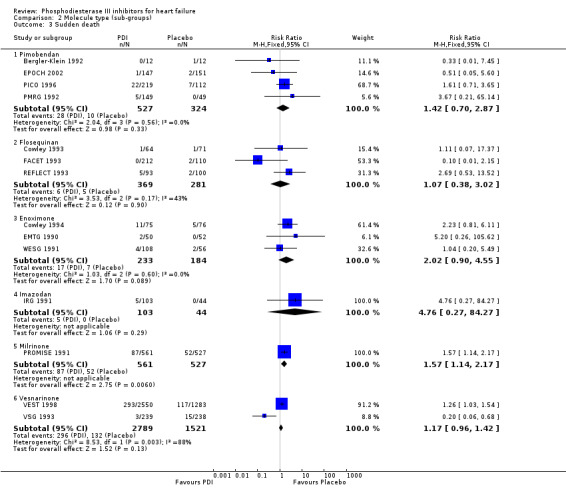
Comparison 2 Molecule type (sub‐groups), Outcome 3 Sudden death.
2.4. Analysis.
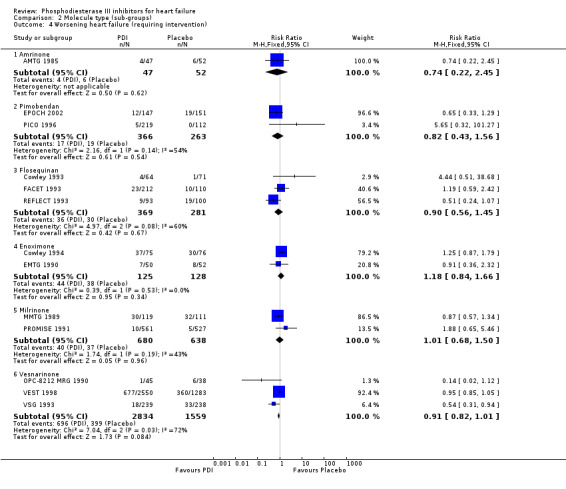
Comparison 2 Molecule type (sub‐groups), Outcome 4 Worsening heart failure (requiring intervention).
2.5. Analysis.
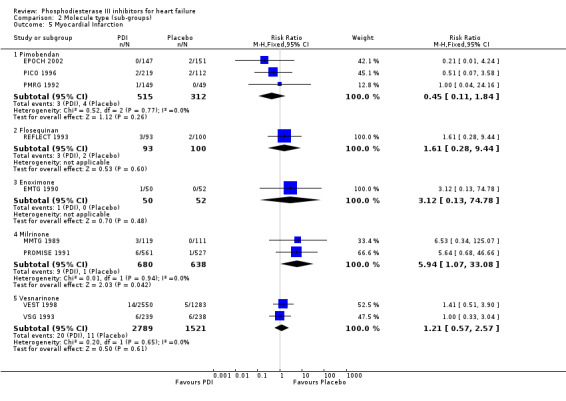
Comparison 2 Molecule type (sub‐groups), Outcome 5 Myocardial Infarction.
2.6. Analysis.
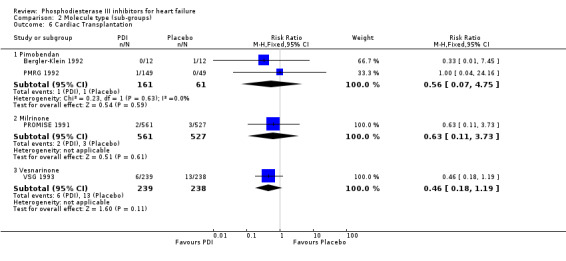
Comparison 2 Molecule type (sub‐groups), Outcome 6 Cardiac Transplantation.
2.7. Analysis.
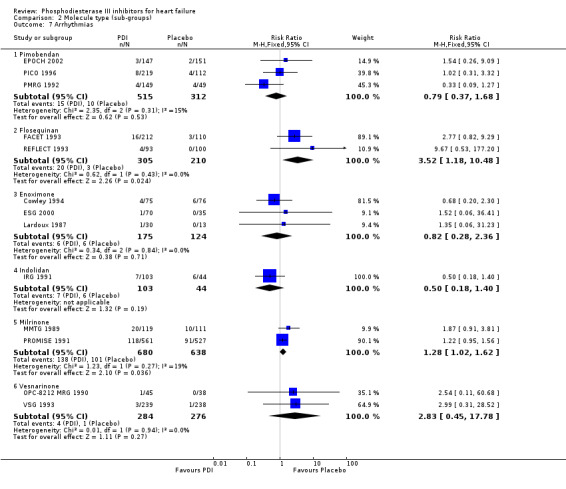
Comparison 2 Molecule type (sub‐groups), Outcome 7 Arrhythmias.
2.8. Analysis.
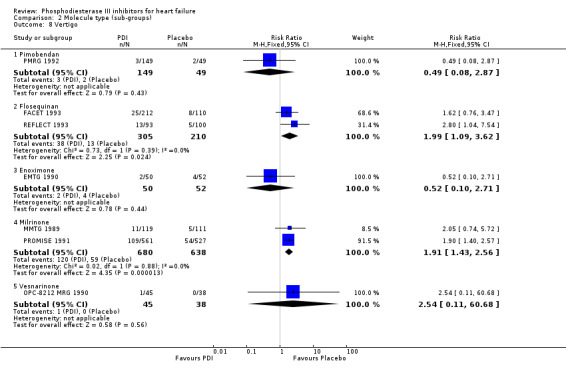
Comparison 2 Molecule type (sub‐groups), Outcome 8 Vertigo.
Comparison 3. Derivatives type (sub‐goups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Imidazolone | 5 | 565 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.95, 2.10] |
| 1.2 Pyridazinone | 10 | 2033 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.93, 1.95] |
| 1.3 Pyridinone | 3 | 1417 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.06, 1.56] |
| 1.4 Quinolinone | 3 | 4393 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.95, 1.24] |
| 2 Cardiac death | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Imidazolone | 2 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.07, 2.92] |
| 2.2 Pyridazinone | 3 | 827 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.78, 2.44] |
| 2.3 Pyridinone | 2 | 1318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [1.09, 1.62] |
| 2.4 Quinolinone | 2 | 4310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.92, 1.21] |
| 3 Sudden death | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Imidazolone | 3 | 417 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [0.90, 4.55] |
| 3.2 Pyridazinone | 8 | 1648 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.81, 2.52] |
| 3.3 Pyridinone | 1 | 1088 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.57 [1.14, 2.17] |
| 3.4 Quinolinone | 2 | 4310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.96, 1.42] |
| 4 Worsening heart failure (requiring intervention) | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Imidazolone | 2 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.84, 1.66] |
| 4.2 Pyridazinone | 5 | 1279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.59, 1.28] |
| 4.3 Pyridinone | 3 | 1417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.67, 1.42] |
| 4.4 Quinolinone | 3 | 4393 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.82, 1.01] |
| 5 Myocardial Infarction | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Imidazolone | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 74.78] |
| 5.2 Pyridazinone | 4 | 1020 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.26, 2.10] |
| 5.3 Pyridinone | 2 | 1318 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.94 [1.07, 33.08] |
| 5.4 Quinolinone | 2 | 4310 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.57, 2.57] |
| 6 Cardiac Transplantation | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Pyridazinone | 2 | 222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.07, 4.75] |
| 6.2 Pyridinone | 1 | 1088 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.11, 3.73] |
| 6.3 Quinolinone | 1 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.18, 1.19] |
| 7 Arrhythmias | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Imidazolone | 3 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.28, 2.36] |
| 7.2 Pyridazinone | 6 | 1489 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.70, 1.91] |
| 7.3 Pyridinone | 2 | 1318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [1.02, 1.62] |
| 7.4 Quinolinone | 2 | 560 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.45, 17.78] |
| 8 Vertigo | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Imidazolone | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.10, 2.71] |
| 8.2 Pyridazinone | 3 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.00, 3.04] |
| 8.3 Pyridinone | 2 | 1318 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.43, 2.56] |
| 8.4 Quinolinone | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [0.11, 60.68] |
3.1. Analysis.
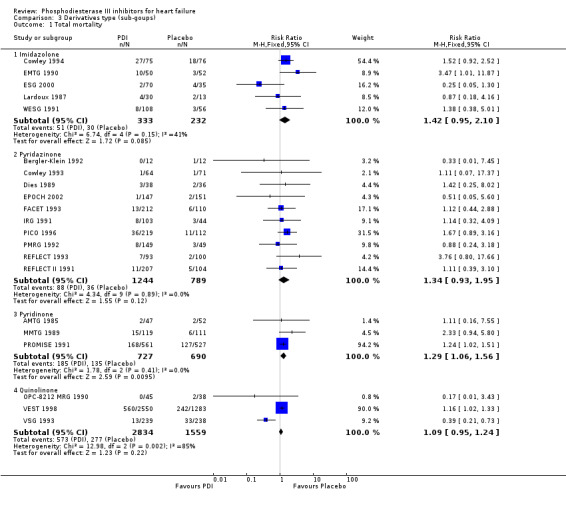
Comparison 3 Derivatives type (sub‐goups), Outcome 1 Total mortality.
3.2. Analysis.
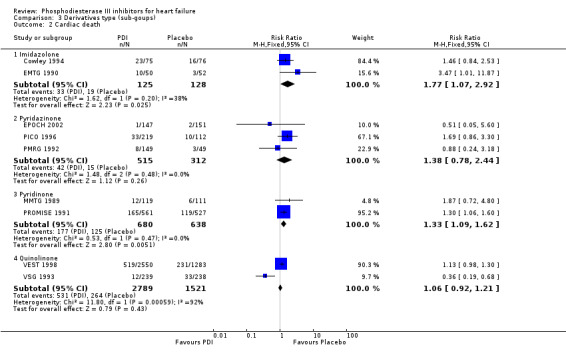
Comparison 3 Derivatives type (sub‐goups), Outcome 2 Cardiac death.
3.3. Analysis.
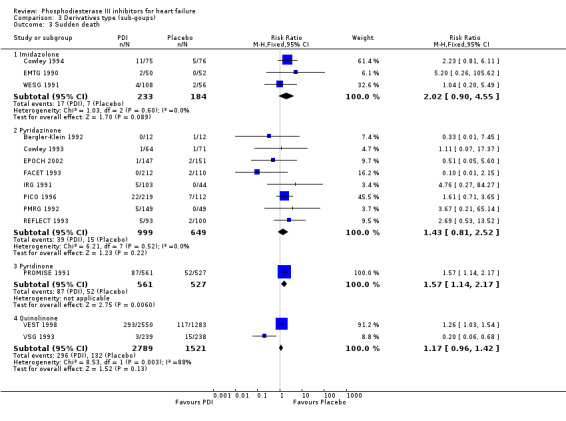
Comparison 3 Derivatives type (sub‐goups), Outcome 3 Sudden death.
3.4. Analysis.
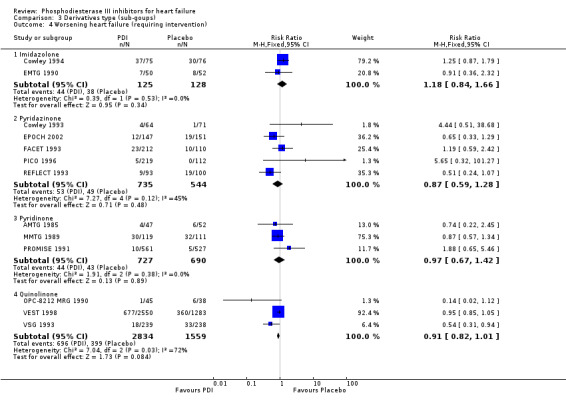
Comparison 3 Derivatives type (sub‐goups), Outcome 4 Worsening heart failure (requiring intervention).
3.5. Analysis.
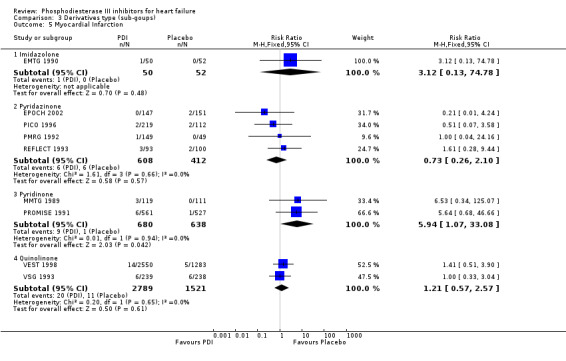
Comparison 3 Derivatives type (sub‐goups), Outcome 5 Myocardial Infarction.
3.6. Analysis.
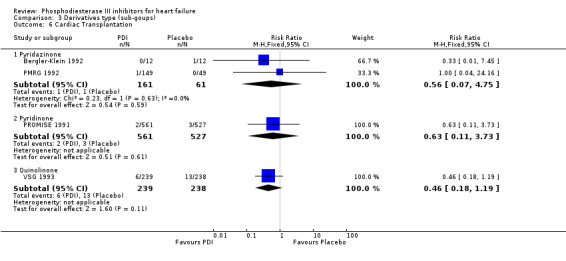
Comparison 3 Derivatives type (sub‐goups), Outcome 6 Cardiac Transplantation.
3.7. Analysis.
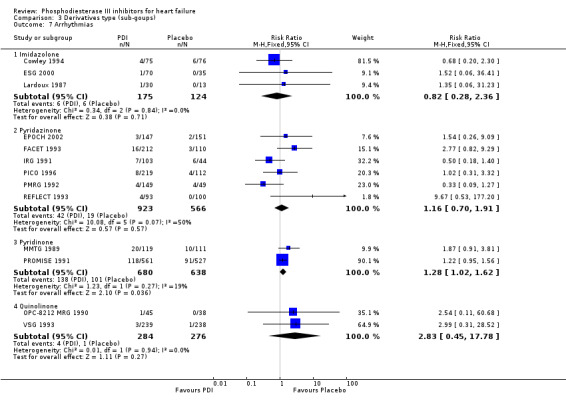
Comparison 3 Derivatives type (sub‐goups), Outcome 7 Arrhythmias.
3.8. Analysis.
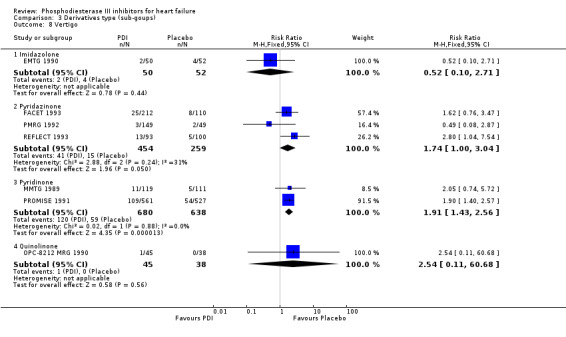
Comparison 3 Derivatives type (sub‐goups), Outcome 8 Vertigo.
Comparison 4. Severity of heart failure (sub‐groups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Class IV included | 10 | 6617 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.04, 1.28] |
| 1.2 Class IV excluded | 10 | 1748 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.00, 2.13] |
| 2 Cardiac death | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Class IV included | 6 | 5977 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.02, 1.27] |
| 2.2 Class IV included | 3 | 731 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.06, 3.23] |
| 3 Sudden death | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Class IV included | 6 | 5894 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.10, 1.54] |
| 3.2 Class IV excluded | 8 | 1569 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.74, 2.22] |
| 4 Worsening heart failure (requiring intervention) | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Class IV included | 7 | 5961 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.84, 1.03] |
| 4.2 Class IV excluded | 6 | 1381 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.61, 1.25] |
| 5 Myocardial Infarction | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Class IV included | 5 | 5826 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.89, 3.22] |
| 5.2 Class IV excluded | 4 | 924 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.31, 2.40] |
| 6 Cardiac Transplantation | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Class IV included | 4 | 1787 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.23, 1.09] |
| 6.2 Class IV excluded | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Arrhythmias | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Class IV included | 13 | 3666 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [1.02, 1.54] |
| 7.2 Class IV excluded | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Vertigo | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Class IV included | 7 | 2216 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.41, 2.33] |
| 8.2 Class IV excluded | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.
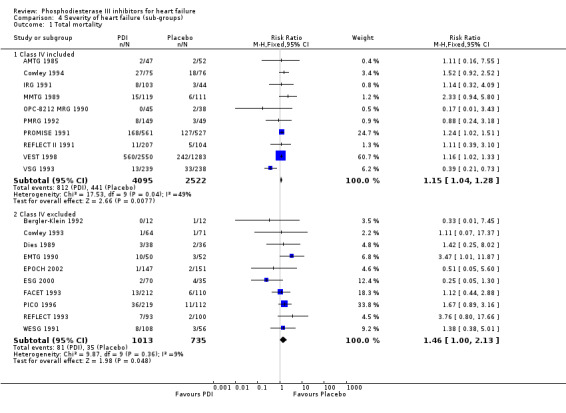
Comparison 4 Severity of heart failure (sub‐groups), Outcome 1 Total mortality.
4.2. Analysis.
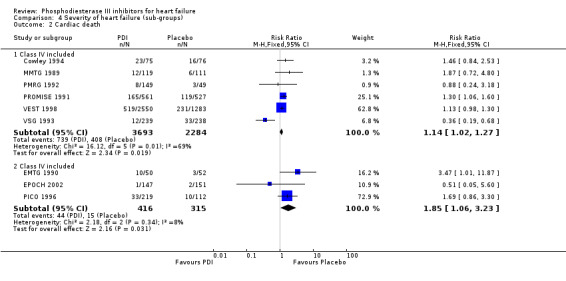
Comparison 4 Severity of heart failure (sub‐groups), Outcome 2 Cardiac death.
4.3. Analysis.
Comparison 4 Severity of heart failure (sub‐groups), Outcome 3 Sudden death.
4.4. Analysis.
Comparison 4 Severity of heart failure (sub‐groups), Outcome 4 Worsening heart failure (requiring intervention).
4.5. Analysis.
Comparison 4 Severity of heart failure (sub‐groups), Outcome 5 Myocardial Infarction.
4.6. Analysis.
Comparison 4 Severity of heart failure (sub‐groups), Outcome 6 Cardiac Transplantation.
4.7. Analysis.
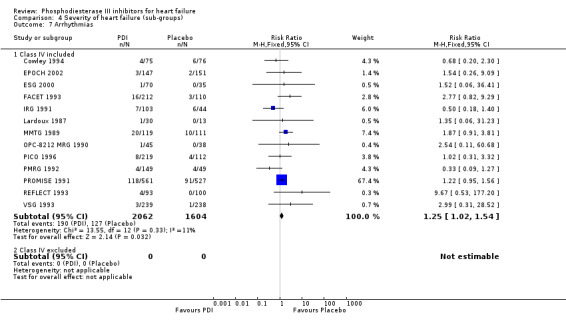
Comparison 4 Severity of heart failure (sub‐groups), Outcome 7 Arrhythmias.
4.8. Analysis.
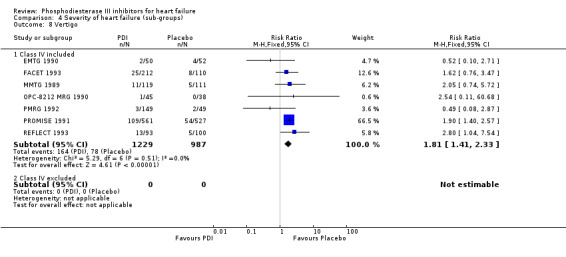
Comparison 4 Severity of heart failure (sub‐groups), Outcome 8 Vertigo.
Comparison 5. Vasodilators used (sub‐groups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 YES | 13 | 7337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [1.04, 1.28] |
| 1.2 NO | 7 | 972 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.97, 2.80] |
| 2 Cardiac death | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 YES | 8 | 6606 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.03, 1.28] |
| 2.2 NO | 1 | 102 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.47 [1.01, 11.87] |
| 3 Sudden death | 14 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 YES | 9 | 6845 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.10, 1.52] |
| 3.2 NO | 5 | 618 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.60 [0.65, 3.92] |
| 4 Worsening heart failure (requiring intervention) | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 YES | 9 | 6813 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.85, 1.03] |
| 4.2 NO | 3 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.45, 1.31] |
| 5 Myocardial Infarction | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 YES | 7 | 6455 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.76, 2.41] |
| 5.2 NO | 2 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [0.42, 8.86] |
| 6 Cardiac Transplantation | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 YES | 3 | 1763 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.23, 1.15] |
| 6.2 NO | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.45] |
| 7 Arrhythmias | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 YES | 11 | 3430 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.99, 1.50] |
| 7.2 NO | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.78 [0.67, 34.14] |
| 8 Vertigo | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 YES | 5 | 1921 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.39, 2.37] |
| 8.2 NO | 2 | 295 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [0.80, 3.93] |
5.1. Analysis.
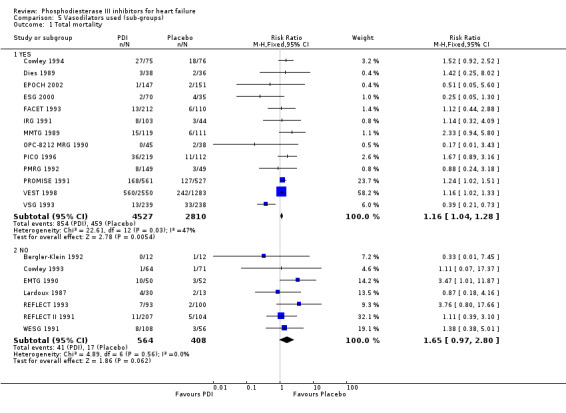
Comparison 5 Vasodilators used (sub‐groups), Outcome 1 Total mortality.
5.2. Analysis.
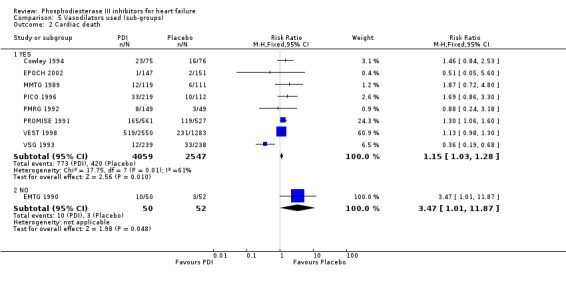
Comparison 5 Vasodilators used (sub‐groups), Outcome 2 Cardiac death.
5.3. Analysis.
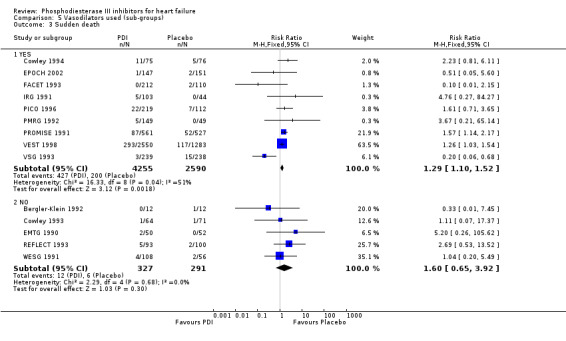
Comparison 5 Vasodilators used (sub‐groups), Outcome 3 Sudden death.
5.4. Analysis.
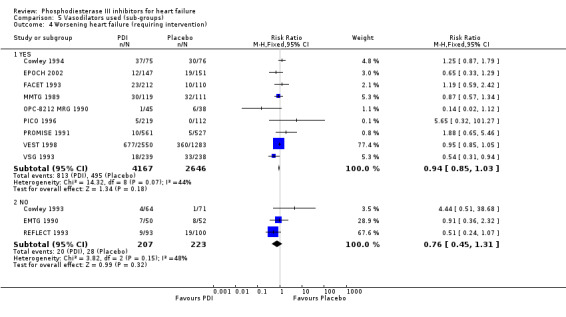
Comparison 5 Vasodilators used (sub‐groups), Outcome 4 Worsening heart failure (requiring intervention).
5.5. Analysis.
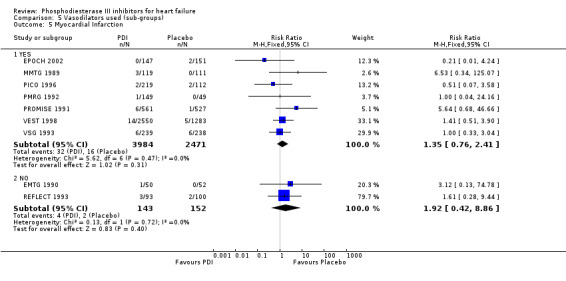
Comparison 5 Vasodilators used (sub‐groups), Outcome 5 Myocardial Infarction.
5.6. Analysis.
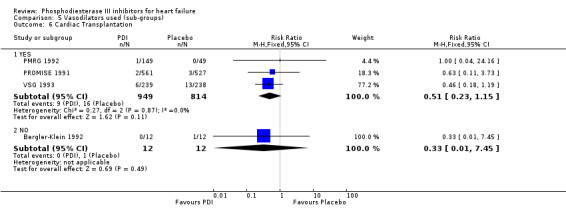
Comparison 5 Vasodilators used (sub‐groups), Outcome 6 Cardiac Transplantation.
5.7. Analysis.
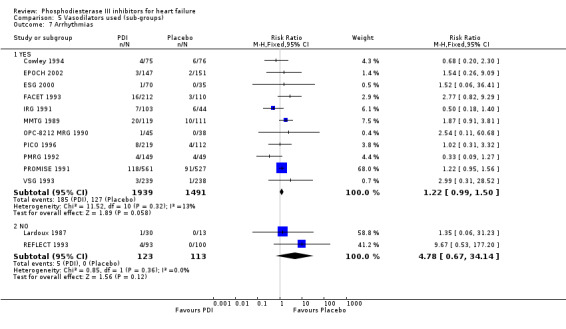
Comparison 5 Vasodilators used (sub‐groups), Outcome 7 Arrhythmias.
5.8. Analysis.
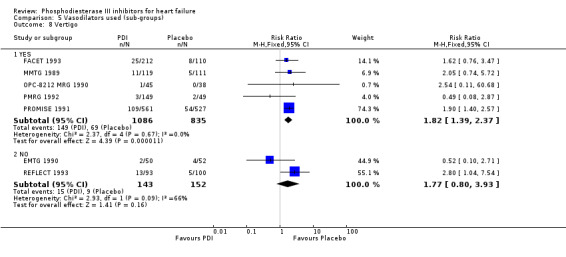
Comparison 5 Vasodilators used (sub‐groups), Outcome 8 Vertigo.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AMTG 1985.
| Methods | Double‐blinded placebo controlled randomised trial. Two groups of patients were recruited, one with concomitant vasodilator (group 1) and one without (group 2). Within each group patients were randomised between PDI or placebo. Follow‐up: 3 months. | |
| Participants | Severity of heart failure (NYHA class) III, IV. Group 1: No. of patients (treated/placebo) 15 / 16. Group 2: No. of patients (treated/placebo) 32 / 36. | |
| Interventions | Amrinone. Dose per day < 600 mg | |
| Outcomes | Changes in symptoms and NYHA classification, exercise tolerance, ejection fraction. Deaths, withdrawals due to treatment failure, adverse reactions. | |
| Notes | No primary outcome specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Bergler‐Klein 1992.
| Methods | Double blinded placebo controlled randomised trial. No concomitant vasodilator. Follow‐up: 6 months. | |
| Participants | Severity of heart failure (NYHA class) II, III. No. of patients (treated/placebo) 12 / 12. | |
| Interventions | Pimobendan. Dose per day: 10 mg | |
| Outcomes | Changes in NYHA classification, arrhythmias, cardiothoracic ratio, ejection fraction, clinical events | |
| Notes | No primary outcome specified. Trial only published in german | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cowley 1993.
| Methods | Double blinded placebo controlled randomised trial. No concomitant vasodilator . Follow‐up: 4 months. | |
| Participants | Severity of heart failure (NYHA class) II, III. No. of patients (treated/placebo) 64 / 71. | |
| Interventions | Flosequinan. Dose per day: 125 mg | |
| Outcomes | ‐ Primary: Exercise tolerance. ‐ Secondary: Changes in NYHA classification, quality of life score. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Cowley 1994.
| Methods | Double blinded placebo controlled randomised trial. With concomitant vasodilator . Follow‐up: 12 months. | |
| Participants | Severity of heart failure (NYHA class) III, IV. No. of patients (treated/placebo) 75 / 76. | |
| Interventions | Enoximone. Dose per day: 300 mg. | |
| Outcomes | ‐ Primary: Total mortality ‐ Secondary: Quality of life score, hospitalisation. | |
| Notes | The trial was stopped early because of an excess of mortality in the patients treated with enoximone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Dies 1989.
| Methods | Placebo controlled randomised trial. Blinding not stated With concomitant vasodilator. Follow‐up: 3 months. | |
| Participants | Severity of heart failure (NYHA class) II, III. No. of patients (treated/placebo) 38 / 36. | |
| Interventions | Indolidan. Data on dose not available | |
| Outcomes | Changes in symptoms and NYHA classification, ejection fraction, cardiothoracic ratio, total mortality, withdrawals, adverse reactions. | |
| Notes | Only published in an abstract. No primary outcome specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
EMTG 1990.
| Methods | Double blinded placebo controlled randomised trial. No concomitant vasodilator. Follow‐up: 4 months. | |
| Participants | Severity of heart failure (NYHA class) II, III. No. of patients (treated/placebo) 50 / 52. | |
| Interventions | Enoximone. Dose per day < 450 mg | |
| Outcomes | Changes in symptoms and NYHA classification, exercise tolerance, patient's daily activity score. Severity of symptoms, hospitalisation, ejection fraction, withdrawals, adverse reactions. | |
| Notes | No primary outcome specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
EPOCH 2002.
| Methods | Double blinded placebo controlled randomised trial. With concomitant vasodilator. Follow‐up: 12 months. | |
| Participants | Severity of heart failure (NYHA class) II, III. No. of patients (treated/placebo) 147 / 151. | |
| Interventions | Pimobendan. Dose per day 2.5 to 5 mg | |
| Outcomes | ‐ Primary: Adverse cardiac events (combined endpoint: death from heart failure, sudden death or hospitalisation for worsening heart failure) ‐ Secondary: Quality of life score, modification of treatment, death from other cardiac causes, death from non‐cardiac causes, changes in NYHA classification, ejection fraction. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
ESG 2000.
| Methods | Double blinded placebo controlled randomised trial. With concomitant vasodilator. Follow‐up: 3 months. | |
| Participants | Severity of heart failure (NYHA class) II, III. No. of patients (treated/placebo) 70 / 35. | |
| Interventions | Enoximone. Dose per day 75, 150 mg. | |
| Outcomes | ‐ Primary: Exercise tolerance ‐ Secondary: Adverse events, quality of life score, arrhythmias. | |
| Notes | Results presented for the first time in 1991. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
FACET 1993.
| Methods | Double blinded placebo controlled randomised trial. With concomitant vasodilator. Follow‐up: 4 months. | |
| Participants | Severity of heart failure (NYHA class) II, III. No. of patients (treated/placebo) 212 / 110. | |
| Interventions | Flosequinan. Dose per day 100, 150 mg | |
| Outcomes | ‐ Primary: Exercise tolerance. ‐ Secondary: Quality of life score, hospitalisation, hospitalisation for worsening heart failure, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
IRG 1991.
| Methods | Double blinded placebo controlled randomised trial. With concomitant vasodilator. Follow‐up: 3 months. | |
| Participants | Severity of heart failure (NYHA class) III, IV. No. patients (treated/placebo) 103 / 44. | |
| Interventions | Imazodan. Dose per day 4, 10, 20 mg | |
| Outcomes | ‐ Primary: Exercise tolerance. ‐ Secondary: Arrhythmias, total mortality. | |
| Notes | This trial was stopped early after an analysis indicated that a positive effect was unlikely to be achieved. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lardoux 1987.
| Methods | Double blinded placebo controlled randomised trial. No concomitant vasodilator. Follow‐up: 3 months. | |
| Participants | Severity of heart failure (NYHA class) not known. No. patients (treated/placebo) 30 / 13. | |
| Interventions | Enoximone. Dose per day: 150; 300 mg. | |
| Outcomes | Changes in NYHA classification, exercise tolerance, ejection fraction, total mortality. | |
| Notes | Only published in an abstract. No primary outcome specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
MMTG 1989.
| Methods | Double blinded placebo controlled randomised trial. No concomitant vasodilator . Follow‐up: 3 months. | |
| Participants | Severity of heart failure (NYHA class) II, III, IV. No. of patients (treated/placebo) 119 / 111. | |
| Interventions | Milrinone. Dose per day < 40 mg. | |
| Outcomes | Exercise tolerance, the need for cointervention for worsened heart failure, ejection fraction, total mortality, arrhythmias, adverse drug effects. | |
| Notes | No primary outcome specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
OPC‐8212 MRG 1990.
| Methods | Double blinded placebo controlled randomised trial. With concomitant vasodilator. Follow‐up: 3 months. | |
| Participants | Severity of heart failure (NYHA class) II, III, IV. No. of patients (treated/placebo) 45 / 38. | |
| Interventions | Vesnarinone. Dose per day: 60 mg. | |
| Outcomes | Quality of life score, withdrawals, changes in NYHA classification, cardiothoracic ratio, ejection fraction, total mortality | |
| Notes | No primary outcome specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
PICO 1996.
| Methods | Double blinded placebo controlled randomised trial. With concomitant vasodilator. Follow‐up: 6 months. | |
| Participants | Severity of heart failure (NYHA class) II, III. No. of patients (treated/placebo) 219 / 112. | |
| Interventions | Pimobendan. Dose per day 2.5, 5 mg. | |
| Outcomes | ‐ Primary: Exercise tolerance ‐ Secondary: total mortality, death or hospitalisation for cardiovascular cause, arrhythmias. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
PMRG 1992.
| Methods | Double blinded placebo controlled randomised trial. With concomitant vasodilator. Follow‐up: 3 months. | |
| Participants | Severity of heart failure (NYHA class) III, IV. No. patients(treated/placebo) 149 / 49. | |
| Interventions | Pimobendan. Dose per day 2.5, 5, 10 mg. | |
| Outcomes | ‐ Primary: Exercise tolerance, quality of life score, drug safety (arrhythmias and clinical adverse events). | |
| Notes | More than one primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
PROMISE 1991.
| Methods | Double blinded placebo controlled randomised trial. With concomitant vasodilator. Follow‐up: 20 months | |
| Participants | Severity of heart failure (NYHA class) III, IV. No. of patients (treated/placebo) 561 / 527. | |
| Interventions | Milrinone. Dose per day 40 mg. | |
| Outcomes | ‐ Primary: Total mortality ‐ Secondary: Cardiovascular mortality, hospitalisation for all causes, the need for cointervention for worsened heart failure, adverse events. | |
| Notes | The trial was stopped early because of an excess of mortality in the patients treated with milrinone. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
REFLECT 1993.
| Methods | Double blinded placebo controlled randomised trial. No concomitant vasodilator. Follow‐up: 3 months. | |
| Participants | Severity of heart failure (NYHA class) II, III. No. of patients (treated/placebo) 93 / 100. | |
| Interventions | Flosequinan. Dose per day 100 mg. | |
| Outcomes | ‐ Primary: Exercise tolerance ‐ Secondary: Changes in symptoms, changes in NYHA classification, cardiothoracic ratio, ejection fraction, withdrawals, total mortality. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
REFLECT II 1991.
| Methods | Double blinded placebo controlled randomised trial. No concomitant vasodilator. Follow‐up: 3 months. | |
| Participants | Severity of heart failure (NYHA class) II, III, IV. No. of patients (treated/placebo) 207 / 104. | |
| Interventions | Flosequinan. Dose per day 100 mg; 150 mg. | |
| Outcomes | ‐ Primary: Exercise tolerance | |
| Notes | Only published in an abstract. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
VEST 1998.
| Methods | Double blinded placebo controlled randomised trial. With concomitant vasodilator. Follow‐up: 9 months. | |
| Participants | Severity of heart failure (NYHA class) III, IV. No. of patients (treated/placebo) 2550 / 1283. | |
| Interventions | Vesnarinone. Dose per day 30, 60 mg. | |
| Outcomes | ‐ Primary: Total mortality ‐ Secondary: Combined endpoint (total mortality or major morbidity due to heart failure), quality of life, sudden death, hospitalisation for heart failure, adverse events. | |
| Notes | Major morbidity due to heart failure was defined as: hospitalisation for worsening heart failure that required treatment with intravenous inotropic or vasodilator drugs for at least four hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
VSG 1993.
| Methods | Double blinded placebo controlled randomised trial. With concomitant vasodilator. Follow‐up: 6 months. | |
| Participants | Severity of heart failure (NYHA class) II, III, IV. No. of patients (treated/placebo) 239 / 238. | |
| Interventions | Vesnarinone. Dose per day 60 mg. | |
| Outcomes | ‐ Primary: Combined endpoint of total mortality or major cardiovascular morbidity ‐ Secondary: Total mortality, quality of life score, withdrawals, transplantation | |
| Notes | The 120 mg study arm was discontinued early because of an excess of mortality after enrolment of 87 patients. Major cardiovascular morbidity was defined as: hospitalisation for worsening heart failure requiring treatment with intravenous inotropic agent for at least four hours. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
WESG 1991.
| Methods | Double blinded placebo controlled randomised trial. No concomitant vasodilator. Follow‐up: 3 months. | |
| Participants | Severity of heart failure (NYHA class) II, III. No. of patients (treated/placebo) 108 / 56. | |
| Interventions | Enoximone. Dose per day 150; 300 mg. | |
| Outcomes | ‐ Primary: Exercise tolerance ‐ Secondary: Quality of life, ejection fraction,arrhythmias, total mortality, clinical adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Assmann 1991 | More than 10% loss to follow up for relevant clinical outcome |
| Banning 1996 | More than 10% loss to follow up for relevant clinical outcome |
| Binkley 1989 | No relevant clinical data available and/or duration of treatment too short. |
| Corder 1992 | No relevant clinical data available and/or duration of treatment too short |
| Cowley 1989 | Comparison with active treatment and/or acute IV administration of PDI and/or dose comparison of PDI |
| Cowley 1991 | No relevant clinical data available and/or duration of treatment too short |
| Crawford 1989 | Comparison with active treatment and/or acute IV administration of PDI and/or dose comparison of PDI |
| DiBianco 1983 | Withdrawal study* |
| DiBianco 1984 | Withdrawal study |
| Dickstein 1990 | Comparison with active treatment and/or acute IV administration of PDI and/or dose comparison of PDI |
| DuBourg 1990 | More than 10% loss to follow up for relevant clinical outcome |
| Elborn 1989 | More than 10% loss to follow up for relevant clinical outcome |
| Elborn 1990 | More than 10% loss to follow up for relevant clinical outcome |
| Evans 1984 | Withdrawal study |
| Feldman 1991 | 7 patients were loss to follow‐up (7/80=8,8%) and no intent to treat analysis was possible |
| Galinier 1989 | Comparison with active treatment and/or acute IV administration of PDI and/or dose comparison of PDI |
| Gilbert 1991 | No relevant clinical data available and/or duration of treatment too short |
| Haas 1990 | More than 10% loss to follow up for relevant clinical outcome |
| Hori 1992 | More than 10% loss to follow up for relevant clinical outcome |
| Huanqlong 2000 | No relevant clinical data available and/or duration of treatment too short |
| Jondeau 1993 | More than 10% loss to follow up for relevant clinical outcome |
| Karlsberg 1992 | Comparison with active treatment and/or acute IV administration of PDI and/or dose comparison of PDI |
| Katz 1987 | No relevant clinical data available and/or duration of treatment too short |
| Katz 1992 | More than 10% loss to follow up for relevant clinical outcome |
| Khalife 1987 | More than 10% loss to follow up for relevant clinical outcome |
| Kneissl 1990 | Comparison with active treatment and/or acute IV administration of PDI and/or dose comparison of PDI |
| Konstam 1985 | Comparison with active treatment and/or acute IV administration of PDI and/or dose comparison of PDI |
| Leier 1987 | No relevant clinical data available and/or duration of treatment too short |
| Leier 1988 | No relevant clinical data available and/or duration of treatment too short |
| Leier 1989 | More than 10% loss to follow up for relevant clinical outcome |
| Likoff 1985 | More than 10% loss to follow up for relevant clinical outcome |
| Orie 1989 | Comparison with active treatment and/or acute IV administration of PDI and/or dose comparison of PDI |
| Pilcher 1985 | More than 10% loss to follow up for relevant clinical outcome |
| Raw 1989 | Comparison with active treatment and/or acute IV administration of PDI and/or dose comparison of PDI |
| Remme 1992 | Comparison with active treatment and/or acute IV administration of PDI and/or dose comparison of PDI |
| Rettig 1986 | Withdrawal study |
| Ruegg 1987 | No relevant clinical data available and/or duration of treatment too short |
| Schoeller 1986 | No relevant clinical data available and/or duration of treatment too short |
| Sridhara 1994 | Comparison with active treatment and/or acute IV administration of PDI and/or dose comparison of PDI |
| Voelker 1989 | Comparison with active treatment and/or acute IV administration of PDI and/or dose comparison of PDI |
| Weber 1990 | No relevant clinical data available and/or duration of treatment too short |
| Yoshikawa 2000 | No relevant clinical data available and/or duration of treatment too short |
| Zipperle 1987 | No relevant clinical data available and/or duration of treatment too short |
*: A withdrawal trial corresponds to a study in which all randomized patients have been taken an phosphodiesterase inhibitor (PDI) before randomization and then were randomized between placebo or PDI
Characteristics of ongoing studies [ordered by study ID]
EMOTE.
| Trial name or title | EMOTE |
| Methods | |
| Participants | Intravenous inotrope‐dependant subjects. |
| Interventions | Oral Enoximone. Study duration: 26 weeks. |
| Outcomes | Requirement for intravenous inotrope therapy |
| Starting date | |
| Contact information | Primary investigator: Ron Oren Heart Failure Treatment Programme. University of Iowa Hospitals and Clinics, 200 Hawkins Dr., S414GH. Iowa City, IA 52242. |
| Notes | A phase III, randomised, double‐blind, placebo‐controlled parallel study of oral Enoximone in intravenous inotrope‐dependant subjects. |
ESSENTIAL.
| Trial name or title | ESSENTIAL |
| Methods | |
| Participants | Advanced heart failure. N=1400 |
| Interventions | The studies of oral enoximone therapy in advanced heart failure. Study drug: enoximone 25 mg t.i.d, enoximone 50 mg t.i.d. Study Duration: 21 months on average |
| Outcomes | |
| Starting date | Study start date: February 2002. Estimated completion date: August 2004 |
| Contact information | Primary investigator: Phillip F Binkley. Protocol No. My021, My026 |
| Notes |
Contributions of authors
Dr E Amsallem Participated in the conception of the design of this review. Developed and ran search strategies, screened retrieved papers against inclusion criteria, appraised quality of studies. Entered data into RevMan, analysed data. Interpreted data and provided methodological perspective on the data Participated in writing the review
C Kasparian and Haddour G Undertook searches and screened search results. Organised retrieval of papers. Screened retrieved papers against inclusion criteria Abstracted data from papers
Pr JP Boissel, Dr P Nony Concieved and performed the previous version of this review. Prepared and designed the protocol of the present review. Coordinated the review process Participated in writing the review.
Sources of support
Internal sources
Clinical Phramacology Unit, EA 3736, APRET/EZUS, France.
External sources
No sources of support supplied
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
AMTG 1985 {published data only}
- Massie B, Bourassa M, Dibianco R, Hess M, Konstam M, Lifoff M et al for the Amrinone Multicenter Trial Group. Long‐term oral administration of amrinone for congestive heart failure: lack of efficacy in a muticenter controlled trial. Circulation 1985;71:963‐71. [DOI] [PubMed] [Google Scholar]
- Massie B, Bourassa M, Dibianco R, Hess M, Krebs C, Lifoff M et al for the Amrinone Multicenter Study Group. Multicenter controlled trial of oral amrinone in congestive heart failure : lack of benefit compared to placebo. Journal of the American College of Cardiology 1985;5:514 (abstr). [Google Scholar]
Bergler‐Klein 1992 {published data only}
- Bergler‐Klein J, Globits S, Stefenelli T, Mayr H, Porenta G, Sochor H, et al. Pimobendan (UDCG 115 BS) in long‐term therapy of chronic heart failure [Pimobendan (UDCG 115 BS) in der Langzeittherapie der chronischen Herzinsuffizienz]. Zeitschrift fur Kardiologie 1992;81(10):546‐52. [PubMed] [Google Scholar]
Cowley 1993 {published data only}
- Cowley AJ, McEntegart DJ. Placebo‐controlled trial of flosequinan in moderate heart failure. The possible importance of aetiology and method of analysis in the interpretation of the results of heart failure trials. International Journal of Cardiology 1993;38(2):167‐75. [DOI] [PubMed] [Google Scholar]
Cowley 1994 {published data only}
- Cowley AJ, Skene AM. Treatment of severe heart failure: quantity or quality of life? A trial of enoximone. Enoximone Investigators. British Heart Journal 1994;72(3):226‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dies 1989 {published data only}
- Dies F, Mcnay JL, Andrejasich CM, Burke PJ, Enas NH. Indolidan (a new phosphodiesterase inhibitor) in chronic heart failure. Circulation 1989;80(suppl II):II‐175 (abstr 0698). [Google Scholar]
EMTG 1990 {published data only}
- Uretsky BF, Jessup M, Konstam MA, Benotti JR, Sandberg JA, Dec GW et al for the Enoximone Study Group. Multicenter trial of oral enoximone in patients with moderately severe congestive heart failure: lack of benefit compared to placebo. Circulation 1989;80(suppl II):II‐174 (abstr 0694). [DOI] [PubMed] [Google Scholar]
- Uretsky BF, Jessup M, Konstam MA, Dec GW, Leier CV, Benotti J et al for the Enoximone Multicenter Trial Group. Multicenter trial of oral enoximone in patients with moderate to moderately severe congestive heart failure. Circulation 1990;82:774‐780. [DOI] [PubMed] [Google Scholar]
EPOCH 2002 {published data only}
- The EPOCH Study Group. Effects of pimobendan on adverse cardiac events and physical activities in patients with mild to moderate chronic heart failure: the effects of pimobendan on chronic heart failure study (EPOCH study). Circulation Journal 2002;66(2):149‐57. [DOI] [PubMed] [Google Scholar]
ESG 2000 {published data only}
- Lowes BD, Higginbotham M, Petrovich L, DeWood MA, Greenberg MA, Rahko PS, et al. Low‐dose enoximone improves exercise capacity in chronic heart failure. Enoximone Study Group. American Journal of Cardiology 2000;36(2):501‐8. [DOI] [PubMed] [Google Scholar]
- Schleman MM, for the enoximone study group. Double‐blind placebo‐controlled 12 week trial of low dose enoximone in patients with mild to moderate congestive heart failure. Circulation 1991;84(suppl II):II‐243 (abstr 0967). [Google Scholar]
FACET 1993 {published data only}
- Massie BM, Berk MR, Brozena SC, Elkayam U, Plehn JF, Kukin ML, et al. Can further benefit be achieved by adding flosequinan to patients with congestive heart failure who remain symptomatic on diuretic, digoxin, and an angiotensin converting enzyme inhibitor? Results of the flosequinan‐ACE inhibitor trial (FACET). Circulation 1993;88(2):492‐501. [DOI] [PubMed] [Google Scholar]
IRG 1991 {published data only}
- Goldberg AD, Nicklas J, Goldstein S. Effectiveness of imazodan for treatment of chronic congestive heart failure. The Imazodan Research Group. The American Journal of Cardiology 1991;68(6):631‐6. [DOI] [PubMed] [Google Scholar]
- Goldberg AD, Nicklas J, Goldstein S, Ford H. Multicenter trial of imazodan in patients with chronic congestive heart failure. European Heart Journal 1990;11:132 (abstr 677). [Google Scholar]
- Golderg AD, Goldstein S, Nicklas J. Mulicenter trial of imazadan in patients with chronic congestive heart failure. Circulation 1990;82(suppl III):III‐673 (abstr 2673). [Google Scholar]
Lardoux 1987 {published data only}
- Lardoux H, Trimarco B, Granier G, Marcadet O, Dubois‐Rande JL, Tarral A. Multicentric, double blind and controlled study of oral enoximone (MDL 17043) in chronic heart failure. Circulation 1987;76(suppl IV):IV‐179 (abstr 0711). [Google Scholar]
MMTG 1989 {published data only}
- Bianco R, Shabetai R, Kostuk W, Moran J, Schlant R, Wright R. Oral milrinone and digoxin in heart failure: results of a placebo‐controlled, prospective trial of each agent and the combination. Circulation 1987;76(suppl IV):IV‐256 (abstr 1020). [Google Scholar]
- DiBianco R, Shabetai R, Kostuk W, Moran J, Schlant RC, Wright R. A comparison of oral milrinone, digoxin, and their combination in the treatment of patients with chronic heart failure. New England Journal of Medicine 1989;320(11):677‐83. [DOI] [PubMed] [Google Scholar]
OPC‐8212 MRG 1990 {published data only}
- OPC‐8212 Multicenter Research Group. A placebo‐controlled, randomized, double‐blind study of OPC‐8212 in patients with mild chronic heart failure. Cardiovascular Drugs and Thearpy 1990;4:419‐26. [DOI] [PubMed] [Google Scholar]
PICO 1996 {published data only}
- Just H, Hjalmarsson AC, Remme WJ, Heinrich‐Nols J, Dumont JM, Seed P et al on behalf of the pimobendan in congestive heart failure (PICO) investigators. Pimobendan in congestive heart failure : results of the PICO trial. Circulation 1995;92(Suppl I):I‐722 (abstr 3469). [Google Scholar]
- Lubsen J, Just H, Hjalmarsson AC, Framboise D, Remme WJ, Heinrich‐Nols J, et al. Effect of pimobendan on exercise capacity in patients with heart failure: main results from the Pimobendan in Congestive Heart Failure (PICO) trial. Heart 1996;76(3):223‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
PMRG 1992 {published data only}
- Gollub SB, Kubo SH. Beneficial effects of pimobendan in patients with chronic heart failure: results of a multicenter trial. Circulation 1991;84(suppl II):II‐312 (abstr 1241). [DOI] [PubMed] [Google Scholar]
- Kubo SH, Gollub S, Bourge R, Rahko P, Cobb F, Jessup M et al for the Pimobendan Multicenter Research Group. Beneficial effects of pimobendan on exercise tolerance and quality of life in patients with heart failure. Results of a multicenter trial. Circulation 1992;85:942‐9. [DOI] [PubMed] [Google Scholar]
- Rector TS, Cohn JN. Assessment of patient outcome with the Minnesota Living with Heart Failure questionnaire: eliability and validity during a randomized, double placebo‐controlled trial of pimobendan. Pimobendan Multicenter Research Group. American Heart Journal 1992;124(4):1017‐25. [DOI] [PubMed] [Google Scholar]
PROMISE 1991 {published data only}
- Eichhorn EJ, Tandon PK, DiBianco R, Timmis GC, Fenster PE, Shannon J, et al. Clinical and prognostic significance of serum magnesium concentration in patients with severe chronic congestive heart failure: the PROMISE Study. Journal of the American College of Cardiology 1993;21(3):634‐40. [DOI] [PubMed] [Google Scholar]
- Packer M, Carver JR, Rodeheffer RJ, Ivanhoe RJ, DiBianco R, Zeldis SM et al for the PROMISE Study Research Group. Effect of oral milrinone on mortality in severe chronic heart failure. New England Journal of Medicine 1991;325:1468‐75. [DOI] [PubMed] [Google Scholar]
- Packer M, Francis GS, Abrams J, Cobb FR, Eichhorn EJ, Giles TD et al and the PROMISE investigators and coordinators. Oral milrinone increases the risk of sudden death in severe chronic heart failure : the PROMISE trial. Circulation 1991;84(suppl II):II‐310 (abstr 12434). [Google Scholar]
- Teerlink JR, Jalaluddin M, Anderson S, Kukin ML, Eichhorn EJ, Francis G, et al. Ambulatory ventricular arrhythmias in patients with heart failure do not specifically predict an increased risk of sudden death. PROMISE (Prospective Randomized Milrinone Survival Evaluation) Investigators. Circulation 2000;101(1):40‐6. [DOI] [PubMed] [Google Scholar]
REFLECT 1993 {published data only}
- Binkley PF, Nunziata E, Cody RJ. Influence of flosequinan on autonomic tone in congestive heart failure: implications for the mechanism of the positive chronotropic effect and survival influence of long‐term vasodilator administration. American Heart Journal 1994;128:1147‐56. [DOI] [PubMed] [Google Scholar]
- Packer M, Narahara KA, Elkayam U, Sullivan JM, Pearle DL, Massie BM, et al. Double‐blind, placebo‐controlled study of the efficacy of flosequinan in patients with chronic heart failure. Principal Investigators of the REFLECT Study. Journal of the American College of Cardiology 1993;22(1):65‐72. [DOI] [PubMed] [Google Scholar]
REFLECT II 1991 {published data only}
- Pitt B, on behalf of the REFLECT II Study Group. A randomized, multicenter, double‐blind placebo controlled study of the efficacy of Flosequinan in patients with chronic heart failure. Circulation 1991;84(suppl II):II‐311 (abstr 1238). [Google Scholar]
VEST 1998 {published data only}
- Cohn JN, Goldstein SO, Greenberg BH, Lorell BH, Bourge RC, Jaski BE, et al. A dose‐dependent increase in mortality with vesnarinone among patients with severe heart failure. Vesnarinone Trial Investigators. New England Journal of Medicine 1998;339(25):1810‐6. [DOI] [PubMed] [Google Scholar]
VSG 1993 {published data only}
- Carson PE, Colucci WS, Deedwania PC, Uresky BF, Bristow MR, Feldman AM, et al. Lack of proarrhythmia reduction by vesnarinone in advanced heart failure. Circulation 1993;88(suppl I):I‐602 (abstr 3240). [Google Scholar]
- Feldman AM, Bristow MR, Parmley WW, Carson PE, Pepine CJ, Gilbert EM et al for the vesnarinone study group. Effects of vesnarinone on morbidity and mortality in patients with heart failure. New England Journal of Medicine 1993;329:149‐55. [DOI] [PubMed] [Google Scholar]
WESG 1991 {published data only}
- Narahara KA, and the Western Enoximone Study Group. Enoximone versus placebo: a double‐blind trial in chronic congestive heart failure. Circulation 1989;80(suppl II):II‐175 (abstr 0695). [Google Scholar]
- Narahara KA, and the Western Enoximone Study Group. Oral enoximone therapy in chronic heart failure: a placebo‐controlled randomized trial. AmericanHeart Journal 1991;121:1471‐9. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Assmann 1991 {published data only}
- Assmann I, Kassel P, Duck HG, Fiehring H, Morgan P, Schmidt PKH, et al. Akut‐und langzeiteffekte von pimobendan (UD‐DG 115) bei herzinsuffizienz NYHA II und III. Ergebnisse einer randomisierten multizentrischen doppelblindstudie. Zeitschrift fur Kardiologie 1991;80:687‐94. [PubMed] [Google Scholar]
Banning 1996 {published data only}
- Banning AP, Ramsey MW, Jones EA, Evans W, Carolan G, Jones CH, et al. Flosequinan in chronic heart failure: how is exercise capacity improved?. European Journal of Clinical Pharmacology 1996;51:133‐8. [DOI] [PubMed] [Google Scholar]
Binkley 1989 {published data only}
- Binkley PF, Shaffer PB, Ryan JM, Leier CV. Augmentation of diastolic function with phosphodiesterase inhibition in congestive heart failure. The Journal of Laboratory and Clinical Medicine 1989;114(3):266‐71. [PubMed] [Google Scholar]
Corder 1992 {published data only}
- Corder CN, Puls A, Wilson M. Clinical effect of indolidan in congestive heart failure. International Journal of Clinical Pharmacology, Therapy, and Toxicology 1992;30:405‐9. [PubMed] [Google Scholar]
Cowley 1989 {published data only}
- Cowley AJ, Fullwood L, Stainer K, Hampton JR. Exercise tolerence in patients with heart failure ‐ how should it be measured?. International Journal of Cardiology 1989;24(3):311‐6. [Google Scholar]
Cowley 1991 {published data only}
- Cowley AJ, Fullwood L, Stainer K, Hampton JR. Exercise tolerance in patients with heart failure ‐ how should it be measured?. European Heart Journal 1991;12(1):50‐4. [DOI] [PubMed] [Google Scholar]
Crawford 1989 {published data only}
- Crawford MH, Deedwania P, Massie B, Rogers W, Smith G, Dae M, et al. Comparative efficacy of enoximone versus captopril in moderate heart failure. Circulation 1989;80(suppl II):II‐175 (abstr 0695). [Google Scholar]
DiBianco 1983 {published data only}
- Dibianco R, Shabetai R, Silverman B, Leier C, Benotti J, Silverman ME, et al. Oral amrinone for congestive heart failure : preliminary results of a multicenter double blind and placebo controlled withdrawal. Journal of the American College of Cardiology 1983;1:675 (abstr). [DOI] [PubMed] [Google Scholar]
DiBianco 1984 {published data only}
- DiBianco R, Shabetai R, Silverman BD, Leier CV, Benotti JR, with the Amrinone Multicenter Study Investigators. Oral amrinone for the treatment of chronic congestive heart failure: results of a multicenter randomized double‐blind and placebo‐controlled withdrawal study. Journal of the American College of Cardiology 1984;4:855‐66. [DOI] [PubMed] [Google Scholar]
Dickstein 1990 {published data only}
- Dickstein K, Gauthier DF, Hartley LH, Colucci WS. Effect of IV milrinone on submaximal exercise endurance in heart failure. Circulation 1990;82(suppl III):III‐324 (abstr 1285). [Google Scholar]
DuBourg 1990 {published data only}
- Dubourg O, Delorme G, Hardy A, Beauchet A, Tarral A, Bourdarias JP. Placebo‐controlled trial of oral enoximone in end‐stage congestive heart failure refractory to optimal treatment. International Journal of Cardiology 1990;28(suppl 1):S33‐42. [DOI] [PubMed] [Google Scholar]
Elborn 1989 {published data only}
- Elborn JS, Stanford CF, Nicholls DP. Effect of flosequinan on exercise capacity and symptoms in severe heart failure. British Heart Journal 1989;61(4):331‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elborn 1990 {published data only}
- Elborn JS, Riley M, Stanford CF, Nicholls DP. The effects of flosequinan on submaximal exercise in patients with chronic cardiac failure. British Journal of Clinical Pharmacology 1990;29(5):519‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Evans 1984 {published data only}
- Evans JR, Pacht K, Huss P, Unverferth DV, Bashore TM, Leier CV. Chronic oral amrinone therapy in congestive heart failure: a double‐blind placebo‐controlled withdrawal study. International Journal of Clinical Pharmacology Research 1984;4(1):9‐18. [PubMed] [Google Scholar]
Feldman 1991 {published data only}
- Feldman AM, Baughman KL, Lee WK, Gottlieb SH, Weiss JL, Becker LC, et al. Usefulness of OPC‐8212, a quinolinone derivative, for chronic congestive heart failure in patients with ischemic heart disease or idiopathic dilated cardiomyopathy. The American Journal of Cardiology 1991;68:1203‐10. [DOI] [PubMed] [Google Scholar]
Galinier 1989 {published data only}
- Galinier M, Massabuau P, Fourcade J, Rochiccioli JP, Puel J, Bounhoure JP. Hemodynamics effects of enoximone in severe congestive heart failure with low cardiac output. European Heart Journal 1989;10:76 (abstr P365). [Google Scholar]
Gilbert 1991 {published data only}
- Gilbert E. Double‐blind, placebo‐controlled (P), comparison of enoximone (E) and dobutamine (D) infusions in moderate to severe congestive heart failure patients (NYHA III‐IV). Journal of the American College of Cardiology 1991;17:274A (abstr 245). [DOI] [PubMed] [Google Scholar]
Haas 1990 {published data only}
- Haas GJ, Binkley PE, Leier CV. Chronic vasodilator therapy with flosequinan in congestive heart failure. Clinical Cardiology 1990;13(6):414‐20. [DOI] [PubMed] [Google Scholar]
Hori 1992 {published data only}
- Hori M, Sato H, Ozaki H, Inoue M, Naka M, Fukunami M, et al. Effect of flosequinan on exercise capacity and cardiac function in patients with chronic mild heart failure: a double‐blind placebo‐controlled study. Heart Vessels 1992;7(3):133‐40. [DOI] [PubMed] [Google Scholar]
Huanqlong 2000 {published data only}
- Huanqlong Z, Shan W, Dejla H, Xingbin L, Yuan F. Response to milrinone treatment in patients with chronic congestive heart failure. J WCUMS 2000;31:246‐7. [PubMed] [Google Scholar]
Jondeau 1993 {published data only}
- Jondeau G, Dubourg O, Delorme G, Chikli F, Dumas C, Bock F, et al. Is there any indication for oral phosphodiesterase inhibitor today?. Journal of the American College of Cardiology 1993;21:377A (abstr 800‐2). [Google Scholar]
Karlsberg 1992 {published data only}
- Karlsberg RP. Milrinone is superior to dobutamine in heart failure complicating myocardial infarction. Circulation 1992;86(suppl I):I‐808 (abstr 3214). [Google Scholar]
Katz 1987 {published data only}
- Katz SD, Forman R, Giustino SR, Sonnenblick EH, Jemtel TH. Double‐blind, randomized evaluation of pimobendan, a new inotrope and vasodilator agent for refractory heart failure. Circulation 1987;76(supll IV):IV‐178 (abstr 0707). [Google Scholar]
Katz 1992 {published data only}
- Katz SD, Kubo SH, Jessup M, Brozena S, Troha JM, Wahl J, et al. A multicenter, randomized, double‐blind, placebo‐controlled trial of pimobendan, a new cardiotonic and vasodilator agent, in patients with severe congestive heart failure. American Heart Journal 1992;123(1):95‐103. [DOI] [PubMed] [Google Scholar]
Khalife 1987 {published data only}
- Khalife K, Zannad F, Brunotte F, Belhadj K, Juilliere Y, Iannascoli F, et al. Placebo‐controlled study of oral enoximone in congestive heart failure with initial and final intravenous hemodynamic evaluation. American Journal of Cardiology 1987;60(5):75C‐79C. [DOI] [PubMed] [Google Scholar]
Kneissl 1990 {published data only}
- Kneissl GD, Scholz M, Dieterich HA, Bubmann WD. Efficacy of enoximone in comparison with captopril in congestive heart failure. European Heart Journal 1990;11:20 (abstr 117). [Google Scholar]
Konstam 1985 {published data only}
- Konstam MA, Benotti JR, Biddle T, Creager MA, Faxon DP, Firth BG, et al. Multicenter comparison of milrinone and dobutamine in congestive heart failure. Circulation 1985;72(supll III):III‐201 (abstr 803). [Google Scholar]
Leier 1987 {published data only}
- Leier CV, Meiller SEL, Binkley PF, Unverferth DV. Regional hemodynamic effects of enoximone in congestive heart failure. Circulation 1987;76(suppl IV):IV‐255 (abstr 1017). [Google Scholar]
Leier 1988 {published data only}
- Leier CV, Lima JJ, Meiler SE, Unverferth DV. Central and regional hemodynamic effects of oral enoximone in congestive heart failure: a double‐blind, placebo‐controlled study. American Heart Journal 1988;115(5):1051‐9. [DOI] [PubMed] [Google Scholar]
Leier 1989 {published data only}
- Leier CV, Binkley PF, Starling RC, Huss‐Randolph P. Disparity between improvement in left ventricular function and changes in clinical status and exercise capacity during chronic enoximone therapy. American Heart Journal 1989;117(5):1092‐8. [DOI] [PubMed] [Google Scholar]
Likoff 1985 {published data only}
- Likoff MJ, Weber KT, Andrews V, Janicki JS, Wilson H, Rocci ML Jr. Milrinone in the treatment of chronic cardiac failure: a controlled trial. American Heart Journal 1985;110(5):1035‐42. [DOI] [PubMed] [Google Scholar]
Orie 1989 {published data only}
- Orie JE, Rahko PS, Brookfield L, Gorwit J, McKiernan T. A double blind comparison of enoximone (Enox) and Captopril (Cap) in CHF. Circulation 1989;80(suppl II):II‐175 (abstr 0686). [Google Scholar]
Pilcher 1985 {published data only}
- Pilcher J, Choraria SK, Taylor DN. Enoximone (MDL 17043) and left ventricular function. A placebo controlled trial. Circulation 1985;72(suppl III):III‐404 (abstr 1616). [Google Scholar]
Raw 1989 {published data only}
- Raw K, Patel J, Mitha A, Chun R, Baragwanath JD. Amrinone vs dobutamine in congestive heart failure due to dilated cardiomyopathy ‐ differences in hemodynamic effects and therapeutic implications. European Heart Journal 1989;10:386 (abstr P2124). [Google Scholar]
Remme 1992 {published data only}
- Remme MW, Krayenbuhl HP, Frick H, Haehl M, Nehmiz G for the European Pimobendan‐Enalapril study group. Discordant hemodynamic and clinical efficacy of enalapril in moderate heart failure. Is pimobendan superior?. European Heart Journal 1992;13:345 (abstr P 1896). [Google Scholar]
Rettig 1986 {published data only}
- Rettig G, Sen S, Frohlig G, Schieffer H, Bette L. Withdrawal of long‐term amrinone therapy in patients with congestive heart failure: a placebo controlled trial. European Heart Journal 1986;7:628‐631. [DOI] [PubMed] [Google Scholar]
Ruegg 1987 {published data only}
- Ruegg PC, Salzmann R, Alther S. Assessment of a new inotropic agent, DPN 205‐734, by means of systolic time intervals (STIs) in healthy volunteers. European Heart Journal 1987;8(suppl 2):abstr 184. [Google Scholar]
Schoeller 1986 {published data only}
- Schoeller R, Bruggemann T, Vohringer H, Nothel T, Opitz C, Schroder K, et al. Long‐term oral administration of milrinone for dilated cardiomyopathy. Journal of the American College of Cardiology 1986;7:108A. [Google Scholar]
Sridhara 1994 {published data only}
- Sridhara BS, Senior R, Anagnostou E, Kinsey C, Lahiri A. Saterinone, a dual‐action drug ofr the management of congestive heart failure. European Heart Journal 1994:36 (abstr 393). [Google Scholar]
Voelker 1989 {published data only}
- Voelker W, Mauser M, Preisack M, Karsch KR. Acute hemodynamic effects of abidendan, a new phosphodiesteras inhibitor, in patients with severe heart failure. European Heart Journal 1989;10:387 (abstr 2126). [DOI] [PubMed] [Google Scholar]
Weber 1990 {published data only}
- Weber PW, Kupper W, Hamm CW, Heitmann NB, Heitmann NB, Bleifeld W. Acute hemodynamic effects of saterinone in patients with severe chronic heart failure. European Heart Journal 1990;11:19 (abstr 113). [Google Scholar]
Yoshikawa 2000 {published data only}
- Yoshikawa T, Baba A, Suzuki M, Yokozuka H, Okada Y, Nagami K, et al. Effectiveness of carvedilol alone versus carvedilol + pimobendan for severe congestive heart failure. For the Keio Interhospital Cardiology Study (KICS) Group. The American Journal of Cardiology 2000;85(12):1495‐7 (A7). [DOI] [PubMed] [Google Scholar]
Zipperle 1987 {published data only}
- Zipperle G, Butzer F, Dieterich HA, Heinrich F. A double‐blind dose response comparison of oral enoximone and placebo for congestive heart failure. The American Journal of Cardiology 1987;60(5):72C‐74C. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Goldberg 1990 {published data only}
- Golderg AD, Goldstein S, Nicklas J, for the Imazodan Study Group. Multicenter trial of imazadan in patients with chronic congestive heart failure. Circulation 1990;82(suppl III):III‐673 (abstr 2673)a. [Google Scholar]
Japanese's Studies {published data only}
- Kato K, Yasuda K, Isuka M, Sukimoto T, Kimata S, Taniguchi K, et al. Evaluation of the efficacy and safety of pimobendan (UD‐CG 115 BS) in treatment of chronic heart failure. A multicenter placebo‐controlled, double‐blind study. Rinsho Iyaku 1992;8:1311‐51b. [Google Scholar]
- Kato K, Yasuda K, Kawai C, Kumada T, Sugimoto T, Kimata S, et al. Clinical efficacy of pimobendan for patients with chronic heart failure. A dose‐finding study by a multicenter double‐blind comparison trial. Rinsho Iyaku 1991;7:2643‐9a. [Google Scholar]
MIL 1077‐1078 {published data only}
- Colucci WS, Sonnenblick EH, Adams KF, Berk M, Brozena SC, Cowley AJ, et al. Efficacy of phosphodiesterase inhibition with milrinone in combination with converting enzyme inhibitors in patients with heart failure. The Milrinone Multicenter Trials Investigators. Journal of the American College of Cardiology 1993;22(4 suppl A):113A‐8Aa. [DOI] [PubMed] [Google Scholar]
- Colucci WS, Sonnenblick EH, for the milrinone multicenter trials investigators. Oral milrinone as adjunctive therapy for CHF. Circulation 1991;84(suppl II):II‐243a. [Google Scholar]
PROFILE 1993 {published data only}
- Hansen MS, Stanton EB, Gaward Y, Packer M, Pitt B, Swedberg K et al for the Canadian PROFILE Investigators. Relation of circulating cardiac myosin light chain 1 isoform in stable severe congestive heart failure to survival and treatment with flosequinan. The American Journal of Cardiology 2002;90:969‐73. [DOI] [PubMed] [Google Scholar]
- Moe GW, Rouleau JL, Charbonneau L, Proulx G, Arnold JM, Hall C, et al. Neurohormonal activation in severe heart failure: relations to patient death and the effect of treatment with flosequinan. American Heart Journal 2000;139(4):587‐95. [DOI] [PubMed] [Google Scholar]
- Packer M, Rouleau J, Swedberg K, Pitt B, Fisher L, Klepper M and the PROFILE investigators & Coordinators. Effect of flosequinan on survival in chronic heart failure: preliminary results of the PROFILE study. Circulation 1993;88(suppl I):I‐301 (abstr 1612). [Google Scholar]
References to ongoing studies
EMOTE {published and unpublished data}
- EMOTE. http://investor.myogen.com/news/20040429‐134186.cfm Accessed November 2004.
- EMOTE. http://www.uihealthcare.com/depts/uiheartcare/clinicaltrials.html#emote [accessed on 04‐03‐2003].
ESSENTIAL {published and unpublished data}
- ESSENTIAL. http://clinicaltrials.gov/ct/gui/show/NCT00051285;jsessionid=9722D918B064FF37F8B0D5D470A1B3F1?order=3 [accessed on 04‐03‐2003].
Additional references
Baptista 1977
- Baptista J, Pike MC. Exact two‐sided confidence limits for the odds ratio in a 2 x 2 table. Algorithm AS 115. Applied statistics 1977;26:214‐20. [Google Scholar]
Boissel 1989
- Boissel JP, Blanchard J, Panak E, Peyrieux JC, Sacks H. Considerations for the meta‐analysis of randomized clinical trials. Summary of a panel discussion. Controlled Clinical Trials 1989;10:254‐81. [DOI] [PubMed] [Google Scholar]
Braunwald 1991
- Braunwald E. ACE inhibitors ‐ a cornerstone of the treatment of heart failure. New England Journal of Medicine 1991;325:351‐3. [DOI] [PubMed] [Google Scholar]
Chatterjee 1980
- Chatterjee K, Parmley WW. Vasodilator therapy for chronic heart failure. Annual Review of Pharmacology and Toxicology 1980;20:475‐80. [DOI] [PubMed] [Google Scholar]
Chesebro 1988
- Chesebro JH, Browne KF, Fenster PE, Garland WT, Konstam MA, for the Milrinone Research Group. The hemodynamic effects of chronic oral milrinone therapy: a multicenter controlled trial. Journal of the American College of Cardiology 1988;11:144A. [Google Scholar]
CIBIS II 1999
- Dargie HJ, Lechat P. The Cardiac Insufficiency Bisoprolol Study II (CIBIS‐II): a randomised trial. Lancet 1999;353:9‐13. [PubMed] [Google Scholar]
Cochran 1954
- Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10:101‐29. [Google Scholar]
Cohn 1973
- Cohn JN. Vasodilator therapy for heart failure. The influence of impedance on left ventricular performance. Circulation 1973;48:5‐8. [DOI] [PubMed] [Google Scholar]
Cohn 1977
- Cohn JN, Franciosa JA. Vasodilatator therapy of cardiac failure. New England Journal of Medcine 1977;297:254‐7. [DOI] [PubMed] [Google Scholar]
Cohn 1986
- Cohn JN, Archibald DG, Ziesche S, Franciosa JA, Harston WE, Tristani FE, et al. Effect of vasodilator therapy on mortality in chronic congestive heart failure. Results of a Veterans Administration Cooperative Study. New England Journal of Medicine 1986;314:1547‐52. [DOI] [PubMed] [Google Scholar]
Cohn 1991
- Cohn JN, Johnson G, Ziesche S, Cobb F, Francis G, Tristani F, et al. A comparison of enalapril with hydralazine‐isosorbide dinitrate in the treatment of chronic congestive heart failure. New England Journal of Medicine 1991;325:303‐10. [DOI] [PubMed] [Google Scholar]
Colucci 1991
- Colucci WS. Cardiovascular effects of milrinone. American Heart Journal 1991;121:1945‐7. [DOI] [PubMed] [Google Scholar]
CONSENSUS 1987
- The CONSENSUS Trial Study Group. Effects of Enalapril on mortality in severe congestive heart failure. New England Journal of Medicine 1987;316:1429‐35. [DOI] [PubMed] [Google Scholar]
Curfman 1991
- Curfman GD. Inotropic therapy for heart failure ‐ an unfulfilled promise. New England Journal of Medicine 1991;325:1509‐10. [DOI] [PubMed] [Google Scholar]
Der Simonian 1986
- Simonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
DiBianco 1991
- DiBianco R. Acute positic inotropic intervention: the phosphoesterase inhibitors. American Heart Journal 1991;121:1871‐5. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Feldman 1987
- Feldman MD, Copelas L, Gwathmey JK, Philips P, Warrn SE. Deficient production of cyclic AMP: pharmacologic evidence of an important cause of contractile dysfunction in patients with end‐stage heart failure. Circulation 1987;75:331‐9. [DOI] [PubMed] [Google Scholar]
Fleiss 1973
- Fleiss J. Statistical methods for rates and proportions. New York: Wiley, 1973. [Google Scholar]
Furberg 1988
- Furberg CD, Yusuf S. Effect of drug therapy on survival in chronic congestive heart failure. American Journal of Cardiology 1988;62:41A‐5A. [DOI] [PubMed] [Google Scholar]
Galbraith 1988
- Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Statistics in Medicine 1988;7(8):889‐94. [DOI] [PubMed] [Google Scholar]
Garg 1995
- Garg R, Yusuf S. Overview of randomized trials of angiotensin‐converting enzyme inhibitors on mortality and morbidity in patients with heart failure. Collaborative Group on ACE Inhibitor Trials. JAMA 1995;273:1450‐6. [PubMed] [Google Scholar]
Gilfrich 1991
- Gilfrich HJ, Dieterich HA. Tolerance of enoximone in patients with heart failure. Zeitschrift fur Kardiologie 1991;80(suppl 4):93‐7. [PubMed] [Google Scholar]
Hood 1989
- Hood WB. Controlled and uncontrolled studies of phosphodiesterase III inhibitors in contemporary cardiovascular medicine. American Journal of Cardiology 1989;63:46A‐53A. [DOI] [PubMed] [Google Scholar]
Katz 1990
- Katz AM. Cardiomyopathy of overload : a major determinant of prognosis in congestive heart failure. New England Journal of Medicine 1990;322:100‐10. [DOI] [PubMed] [Google Scholar]
LeJemtel 1980
- LeJemtel TH, Keung E, Ribner HS, Wexler J, Blaufox MD, Sonnenblick EH. Sustained beneficial effects of oral amrinone on cardiac and renal function in patients with severe congestive heart failure. American Journal of Cardiology 1980;45:123‐9. [DOI] [PubMed] [Google Scholar]
Ludmer 1986
- Ludmer PL, Wright RF, Arnold JML, Ganz P, Braunwald E, Colucci WS. Separation of the direct myocardial and vasodilator actions of milrinone administered by an intracoronary infusion technique. Circulation 1986;73:130‐7. [DOI] [PubMed] [Google Scholar]
Mancini 1985
- Mancini D, LeJemtel T, Sonnenblick E. Intravenous use of amrinone for the treatment of the failing heart. American Journal of Cardiology 1985;56:8B‐15B. [DOI] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22:719‐48. [PubMed] [Google Scholar]
MERIT‐HF 1999
- Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT‐HF). Effect of metoprolol CR/XL in chronic heart failure. Lancet 1999;353:2001‐7. [PubMed] [Google Scholar]
Mikulic 1977
- Mikulic E, Cohn JN, Franciosa JA. Comparative hemodynamic effects of inotropic and vasodilator drugs in severe heart failure. Circulation 1977;56:528‐33. [DOI] [PubMed] [Google Scholar]
Mulrow 1988
- Mulrow CD, Mulrow JP, Linn WD, Aguilar C, Ramirez G. Relative efficacy of vasodilator therapy in chronic congestive heart failure. Implications of randomized trials. JAMA 1988;259:3422‐6. [PubMed] [Google Scholar]
Nony 1995
- Nony P, Boissel JP, Lievre M, Cucherat M, Haugh MC, Dayoub G. Introduction à la méthodologie méta‐analytique. Revue de Medecine Interne 1995;16:536‐46. [DOI] [PubMed] [Google Scholar]
Nony 1997
- Nony P, Cucherat M, Haugh MC, Boissel JP. Standardization of terminology in meta‐analysis: a proposal for working definitions. Fundamental & Clinical Pharmacology 1997;11:481‐93. [DOI] [PubMed] [Google Scholar]
Nony 1999
- Nony P, Cucherat M, Boissel JP. Implication of evidence‐based medicine in prescription guidelines taught to French medical students: current status in the cardiovascular field. Clinical pharmacology and Therapeutics 1999;66:173‐84. [DOI] [PubMed] [Google Scholar]
Packer 1984
- Packer M, Medina N, Yushak M. Hemodynamic and clinical limitations of long‐term inotropic therapy with amrinone in patients with severe chronic heart failure. Circulation 1984;70:1038‐47. [DOI] [PubMed] [Google Scholar]
Packer 1988a
- Packer M. Effect of vasodilator and inotropic drugs on clinical symptoms and long‐term survival in chronic congestive heart failure. European Heart Journal 1988;9(suppl h):105‐8. [DOI] [PubMed] [Google Scholar]
Packer 1988b
- Packer M. Vasodilator and inotropic drugs for chronic heart failure: distinguishing hype from hope. The American Journal of Cardiology 1988;12:1299‐317. [DOI] [PubMed] [Google Scholar]
Packer 2001
- Packer M, Coats AJ, Fowler MB, Katus HA, Krum H, Mohacsi P, et al. Effect of carvedilol on survival in severe chronic heart failure. New England Journal of Medicine 2001;344:1651‐8. [DOI] [PubMed] [Google Scholar]
Peto 1978
- Peto R. Clinical trial methodology. Biomedicine 1978;28:24‐36. [PubMed] [Google Scholar]
Rosenthal 1979
- Rosenthal R. The "file drawer" problem and tolerance for null results. Psychol Bull 1979;86:638‐41. [Google Scholar]
Simonton 1985
- Simonton CA, Chatterjee K, Cody RJ, Kubo SH, Leonard D, Daly P, et al. Milrinone in congestive heart failure : acute and chronic hemodynamic and clinical evaluation. Journal of the American College of Cardiology 1985;6:453‐9. [DOI] [PubMed] [Google Scholar]
SOLVD 1991
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. New England Journal of Medicine 1991;325:293‐302. [DOI] [PubMed] [Google Scholar]
Storstein 1988
- Storstein L. Non‐receptor‐mediated inotropic drugs. European Heart Journal 1988;9:91‐3. [DOI] [PubMed] [Google Scholar]
Tukey 1977
- Tukey JW. Some thoughts on clinical trials, especially problems of multiplicity. Science 1977;198:679‐84. [DOI] [PubMed] [Google Scholar]
Weber 1987
- Weber KT, Gill SK, Janicki JS, Maskin CS, Jain MC. Newer positive inotropic agents in the treatment of chronic cardiac failure. Current status and future directions. Drugs 1987;33:503‐19. [DOI] [PubMed] [Google Scholar]
Wilmshurst 1984
- Wilmshurst PT, Walker JM, Fry CH, Mounsey JP, Twort CHC, Williams BT, et al. Inotropic and vasodilator effects of amrinone on isolated human tissue. Cardiovascular Research 1984;18:302‐9. [DOI] [PubMed] [Google Scholar]
Wood 1989
- Wood MA, Hess ML. Review: long‐term oral therapy of congestive heart failure with phosphodiesterase inhibitors. The American Journal of the Medical Sciences 1989;297:105‐13. [DOI] [PubMed] [Google Scholar]
Yusuf 1987
- Yusuf S, Simon R, Ellenberg S. Proceedings of "Methodological Issues in Overviews of Randomized Clinical Trials". Statistical Medicine 1987;6:217‐409. [Google Scholar]
Yusuf 1990
- Yusuf S, Teo K. Inotropic agents increase mortality in patients with congestive heart failure. Circulation 1990;82(suppl III):III‐673(abstr 2675). [Google Scholar]
References to other published versions of this review
Nony 1994
- Nony P, Boissel JP, Lievre M, Leizorovicz A, Haugh MC, Fareh S, et al. Evaluation of the effect of phosphodiesterase inhibitors on mortality in chronic heart failure patients: a meta‐analysis. European Journal of Clinical Pharmacology 1994;46:191‐6. [DOI] [PubMed] [Google Scholar]


