Abstract
Objective:
Although trials of neoadjuvant chemotherapy in ovarian cancer use 3 neoadjuvant cycles, real-world practice varies. We sought to evaluate the influence of increasing preoperative cycles on survival, accounting for surgical outcomes.
Methods:
We identified 199 women with newly diagnosed ovarian cancer recommended for neoadjuvant chemotherapy who underwent interval debulking surgery from July 2015 to December 2018. Non-parametric tests were used to compare clinical characteristics by neoadjuvant cycles. The Kaplan-Meier method was used to estimate differences in progression-free and overall survival. The Log-rank test was used to assess the relationship of covariates to outcome.
Results:
Median number of neoadjuvant cycles was 4 (range 3–8), with 56 (28%) women receiving ≥5 cycles. Compared with those receiving 3 or 4, women with ≥5 neoadjuvant cycles received less or no postoperative cycles (p<0.001) but had no other differences in clinical factors (p>0.05). Complete gross resection rates were similar among those receiving 3, 4 and ≥5 neoadjuvant cycles (68.5%, 70%, and 71.4% respectively, p=0.96). There were no significant differences in progression-free or overall survival when comparing 3 versus 4 neoadjuvant cycles. However, more cycles (≥5 vs. 4) was associated with worse progression-free survival, even after adjustment for BRCA status and complete gross resection (hazard ratio 2.20 95% confidence interval 1.45–3.33, p<0.001), and worse overall survival, even after adjustment for histology, response on imaging and complete gross resection rates (hazard ratio 2.78 95% confidence interval 1.37–5.63, p=0.016). The most common reason for receiving ≥5 cycles was extent of disease requiring more neoadjuvant chemotherapy.
Conclusions:
Despite maximal cytoreduction, patients receiving ≥5 neoadjuvant cycles have a poorer prognosis compared with those receiving 3–4 cycles. Future studies should focus on reducing surgical morbidity and optimizing novel therapies in this high-risk group.
Keywords: Ovarian cancer, Neoadjuvant chemotherapy, Preoperative cycles, Survival
INTRODUCTION
Use of neoadjuvant chemotherapy followed by interval debulking surgery in ovarian cancer has increased since 2010.1,2 The core rationale is the hypothesis that delivering chemotherapy prior to surgery may render initially inoperable patients operable by shrinking disease. As a result, much research has focused on delivery of neoadjuvant chemotherapy, including how best to optimize the number of preoperative cycles.3 Four large randomized studies comparing neoadjuvant chemotherapy followed by interval debulking surgery, with primary debulking surgery, have been conducted. In these studies, neoadjuvant chemotherapy was restricted to 3–4 cycles.4–7 However, real-world practice varies, and centers often give more than 4 preoperative cycles. Although multiple retrospective studies suggest that more neoadjuvant cycles may be associated with worse outcomes,8–11 many of these studies did not adjust for degree of cytoreduction or other clinical variables. Others have found that certain women may benefit from more than 4 cycles if complete cytoreduction can be achieved.12
The decision to proceed with interval debulking surgery is complex and influenced by multiple factors, including response to neoadjuvant chemotherapy (often assessed via interval computed tomography (CT) imaging after 2–3 cycles), ability to achieve complete cytoreduction, patient factors, and the individual surgeon’s philosophy. Multiple studies have emphasized the importance of optimal and complete cytoreduction on survival,13,14 and degree of surgery may influence the effects of neoadjuvant chemotherapy. Additionally, completion of neoadjuvant cycles may vary according to patient’s age and comorbidity.15
There is a need to describe real-world practices and characterize the influence of increased number of neoadjuvant cycles on outcomes. We sought to describe our institution’s practices in the delivery of neoadjuvant chemotherapy and to evaluate whether increasing number of preoperative cycles influenced survival, after adjusting for clinical variables and degree of cytoreduction.
METHODS
Patient Selection
From July 1st, 2015 until December 31st, 2018, we prospectively identified 199 women seeking medical care at our tertiary care institution for newly diagnosed, pathologically verified ovarian, fallopian tube or primary peritoneal epithelial cancer. Patients were identified prospectively at the point they initiate care at our institution via the center’s Ovarian Database, which tracks all patients seen with an ovarian complaint and is updated continuously. There was no selection, and all women who received care at our institution were included. Second opinion cases were excluded. All women were recommended to receive neoadjuvant chemotherapy and underwent interval debulking surgery.
Data Collection
Clinical data was abstracted from the electronic medical record by two independent reviewers (YL and KLR) from May 2019 to September 2019. Age was defined from date of pathological diagnosis. Histology was abstracted from pathological reports and stratified into high-grade serous carcinoma or other. Stage was defined at pathological diagnosis using the 2014 International Federation of Gynecology and Obstetrics (FIGO) staging system.16 BRCA testing status and results were abstracted from the medical record. Charlson comorbidity score, a composite score measuring comorbidity in 12 areas, and predictive of mortality,17,18 was calculated based on medical conditions present at pathological diagnosis.
Surgical and medical oncology notes were reviewed to determine the indication for neoadjuvant chemotherapy and were categorized into the following groups: 1) patient factors (Aletti Score19, comorbidity, venous thromboembolism, clinical trial, or other); or 2) disease factors (stage IV unresectable, extent of disease on imaging, extent of disease on laparoscopy, extent of disease requiring thoracic surgery).
Chemotherapy regimens were documented and categorized as: 1) weekly intravenous paclitaxel with carboplatin every 3 weeks; or 2) other, which included intravenous paclitaxel and carboplatin every 3 weeks, intraperitoneal chemotherapy, and clinical trials. Number of pre- and post-operative chemotherapy cycles were documented. The charts of patients receiving ≥5 neoadjuvant cycles were reviewed in detail, and the reasons for more cycles were characterized as follows: 1) surgical (disease too extensive for debulking); 2) medical (functional status or delayed chemotherapy recovery); 3) venous thromboembolism; 4) patient preferences; 5) logistics.
Radiologic reports from interval imaging (CT chest, abdomen and pelvis with contrast, or magnetic resonance imaging (MRI) pelvis with contrast and CT chest without contrast) obtained after 2–3 preoperative cycles were reviewed by investigators (YLL) to determine response. This was then categorized as: 1) response, defined as any amount of disease shrinkage on imaging; 2) no response or disease progression, defined as no change, or growth of disease on imaging. This dichotomous categorization was used as these were clinical exams and thus not scored using criteria such as RECIST. All patients underwent interval debulking surgery. An optimal debulking was defined as residual disease <1 cm. A complete gross resection was defined as no visible residual disease at the completion of surgery.
Statistical Analysis
Summary statistics were provided. The Fisher’s exact and Kruskal-Wallis tests were used to compare clinical characteristics by 3, 4 and ≥5 neoadjuvant cycles. Progression-free survival was defined from date of interval debulking surgery to date of disease progression, defined as clinical recurrence via pathologically confirmed biopsy (when available), or imaging showing disease recurrence per the clinical provider, or death in those without recurrence, or last follow-up in those without recurrence or death. Overall survival was defined from date of interval debulking surgery to date of death from all causes or last follow-up.
The Kaplan-Meier method was used to estimate progression-free and overall survival (median and rate at 1-year). The Log-rank test, and Cox Proportional Hazards model, were used to assess the relationship of covariates to outcome. Number of neoadjuvant chemotherapy cycles were recorded prior to interval debulking surgery and is therefore a known baseline covariate at the time of surgery. Landmark analysis was used for the time-dependent variable post-operative chemotherapy cycles, and only patients who received post-operative treatments were included in these analyses. Progression-free survival was plotted against neoadjuvant chemotherapy cycles as a continuous variable, and a weighted Kaplan-Meier survival function was obtained by smoothing to determine a smoothed median estimate across survival time.20 The maximally selected standardized log-rank statistic was then used to assess the predictive power of this relationship.21–23
This study was approved by our center’s Institutional Review Board.
RESULTS
Patient Characteristics
Among the 199 patients who received neoadjuvant chemotherapy and underwent interval debulking surgery, median age was 66.5 (mean 66.5) years, with a range of 43–88 years. Most (n=183, 92%) had high-grade serous carcinoma. Other histologies (n=16) included clear cell carcinoma (n=2), endometrioid carcinoma (n=1), carcinosarcoma/malignant mixed Müllerian tumor (n=2), low-grade serous (n=1), and Müllerian carcinoma non-specified (n=10). Most patients had stage IV disease (67%) at diagnosis; 15% had a BRCA 1/2 mutation, but 30 (15%) had not yet undergone testing. Median Charlson comorbidity score was 8, with a mean of 8.5 (range 6–12), representing intermediate peri-operative risk.24
Most patients received weekly intravenous paclitaxel with carboplatin every 3 weeks (72%); 53 (28%) patients received other regimens including intravenous paclitaxel and carboplatin every 3 weeks (n=34), combination intravenous-intraperitoneal chemotherapy (n=7), intravenous pegylated liposomal doxorubicin and carboplatin (n=1), and weekly intravenous paclitaxel and carboplatin with nivolumab every 3 weeks on a clinical trial (n=11). Twelve (6%) patients received bevacizumab with chemotherapy, and 16 (8%) received a PARP inhibitor.
Most patients received neoadjuvant chemotherapy due to disease factors (70%) and had some response after 2–3 cycles (91%). Patients who did not have a response on CT after 2–3 cycles did receive further imaging, and those who did not have a response sufficient for debulking surgery were not included in this study. The rate of optimal debulking was 91%, and the rate of complete gross resection was 70% (Table 1).
Table 1:
Patient characteristics by neoadjuvant cycles
| Variables | Whole Cohort | 3 Cycles | 4 Cycles | >=5 Cycles | p-value* |
|---|---|---|---|---|---|
| Patients (n) | 199 | 73 | 70 | 56 | |
| Age at Diagnosis | |||||
| Median(Mean) | 66.5(66.5) | 66(66.1) | 65.5(65) | 68.6(68.9) | 0.111 |
| Range | 43.1–87.9 | 43.1–86.4 | 43.2–87.2 | 45.1–87.9 | |
| Histology | |||||
| High Grade Serous | 183(92%) | 65(89%) | 66(94.3%) | 52(92.9%) | 0.516 |
| Others** | 16(8%) | 8(11%) | 4(5.7%) | 4(7.1%) | |
| Stage | |||||
| III | 66(33.2%) | 29(39.7%) | 23(32.9%) | 14(25%) | 0.226 |
| IV | 133(66.8%) | 44(60.3%) | 47(67.1%) | 42(75%) | |
| BRCA Status | |||||
| No mutation | 139(69.8%) | 48(65.8%) | 49(70%) | 42(75%) | 0.344 |
| BRCA1/2 Mutation | 30(15.1%) | 14(19.2%) | 12(17.1%) | 4(7.1%) | |
| Not tested | 30(15.1%) | 11(15.1%) | 9(12.9%) | 10(17.9%) | |
| Charlson score (12 missing) | |||||
| Median (Mean) | 8(8.5) | 8(8.4) | 8(8.4) | 9(8.6) | 0.451 |
| Range | 6–12 | 6–12 | 6–12 | 6–12 | |
| Chemotherapy Regimen (12 missing) | |||||
| Weekly Paclitaxel/Carboplatin | 134(71.7%) | 52(77.6%) | 49(73.1%) | 33(62.3%) | 0.17 |
| Others | 53(28.3%) | 15(22.4%) | 18(26.9%) | 20(37.7%) | |
| Neoadjuvant Indication *** | |||||
| Patient factors | 60(30.2%) | 28(38.4%) | 16(22.9%) | 16(28.6%) | 0.13 |
| Disease factors | 139(69.8%) | 45(61.6%) | 54(77.1%) | 40(71.4%) | |
| Response on Imaging after 2–3 Preoperative Cycles | |||||
| Response | 181(91%) | 68(93.2%) | 66(94.3%) | 47(83.9%) | 0.116 |
| No response/progression | 18(9%) | 5(6.8%) | 4(5.7%) | 9(16.1%) | |
| Optimal Debulking | |||||
| No | 18(9%) | 8(11%) | 5(7.1%) | 5(8.9%) | 0.745 |
| Yes | 181(91%) | 65(89%) | 65(92.9%) | 51(91.1%) | |
| Complete Gross Resection | |||||
| No | 60(30.2%) | 23(31.5%) | 21(30%) | 16(28.6%) | 0.962 |
| Yes | 139(69.8%) | 50(68.5%) | 49(70%) | 40(71.4%) | |
| Adjuvant Therapy (4 missing) | |||||
| No | 15(7.7%) | 1(1.4%) | 3(4.3%) | 11(20%) | <0.001 |
| Yes | 180(92.3%) | 69(98.6%) | 67(95.7%) | 44(80%) | |
| Postoperative Cycles | |||||
| Median (Mean) | 3(2.9) | 3(3.2) | 3(2.8) | 2(2.4) | <0.001 |
| Range | 1–6 | 2–6 | 1–4 | 1–4 | |
P-values are obtained using Kruskal-Wallis test for continuous variables and Fisher-Exact test for categorical variables.
Of the 16 women with other histologies, 2 had clear cell carcinoma, 1 had endometrioid, 2 had carcinosarcoma, 1 had low grade serous, and 10 had Mullerian carcinoma (non-specific).
Patient factors include Aletti Score, comorbidity, venous thromboembolism, clinical trial, or other. Disease factors include Stage IV unresectable, extent of disease on imaging, extent of disease on laparoscopy, extent of disease requiring thoracic surgery.
Neoadjuvant Chemotherapy Cycles
The number of neoadjuvant cycles ranged from 3 to 8, with a median of 4. Seventy-three (37%) patients received 3 cycles, 70 (35%) patients received 4 cycles, and 56 (28%) patients received ≥5 cycles. Number of post-operative cycles ranged from 0–6, with a median of 3. Fifteen patients received no post-operative chemotherapy. The number of neoadjuvant and post-operative chemotherapy cycles were significantly and negatively correlated: Pearson’s correlation coefficient −0.50 (p<0.001).
The median number of post-operative cycles for those receiving 3 and 4 neoadjuvant cycles was 3 (range 2–6) and 3 (range 1–4), respectively. Compared with patients receiving 3 or 4 neoadjuvant cycles, those receiving ≥5 cycles received fewer or no post-operative cycles (median 2, range 1–4, p<0.001) but had no differences in other clinical factors including age, stage (including more detailed staging, Table S1), comorbidity score, neoadjuvant indication, chemotherapy regimen, presence of response on imaging after 2–3 preoperative cycles, p>0.05. Of note, rates of optimal debulking and complete gross resection were similar among the groups (Table 1).
Progression-free Survival Analysis
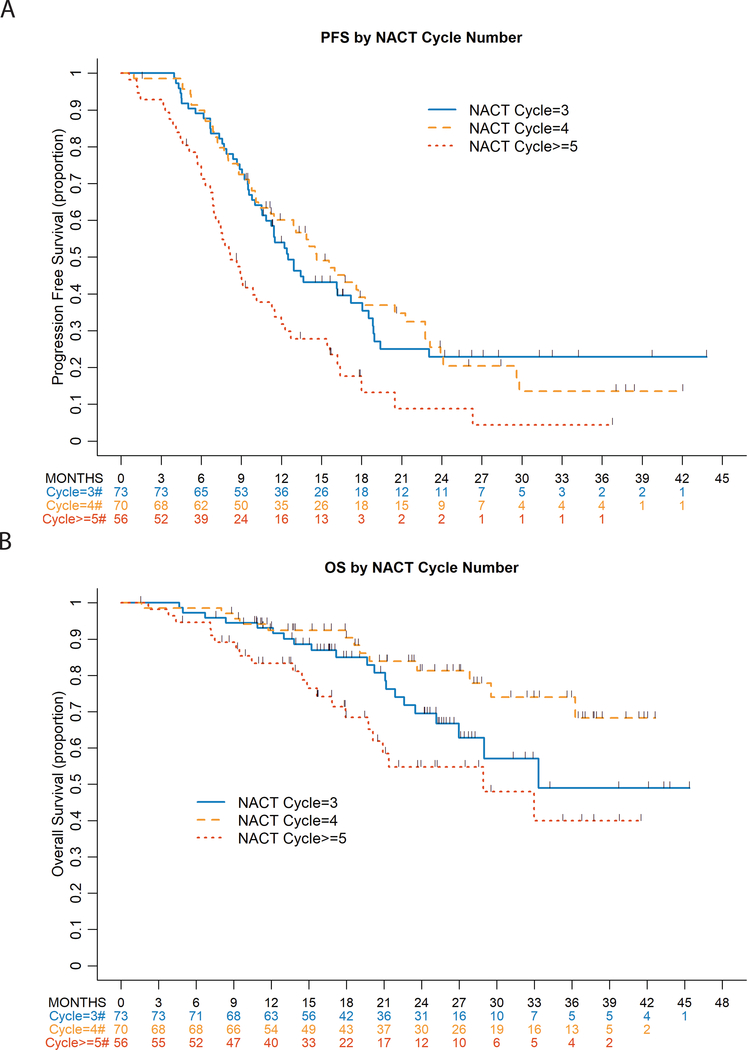
At a median follow-up of 15.7 months (range 1.6–43.8) for progression-free survivors, there were 142 (71%) recurrences; 2 (1%) deaths without recurrence were observed. Median progression-free survival was 12.0 months (95% CI 10.1–13.9) overall and 12.5 months (95% CI 10.6–17.2), 14.6 months (95% CI 11.2–18.3), and 8.2 months (95% CI 6.9–11.3) in those with 3, 4 and ≥5 neoadjuvant cycles respectively. On univariate analysis, receiving ≥5 neoadjuvant cycles was significantly associated with worse progression-free survival compared with 3 cycles (hazard ratio (HR) 1.97 95% confidence interval (CI) 1.32–2.96, p<0.001) and 4 cycles (HR 2.04 95% CI 1.36–3.08, p<0.001). There were no significant differences with respect to progression-free survival between 3 versus 4 neoadjuvant cycles. Presence of BRCA 1/2 mutations, achieving optimal debulking or complete gross resection, and receiving post-operative chemotherapy were associated with improved progression-free survival (p<0.05) on univariate analysis (Figure 1 and Table S2).
Figure 1:
Progression-Free and Overall Survival by Neoadjuvant Cycles
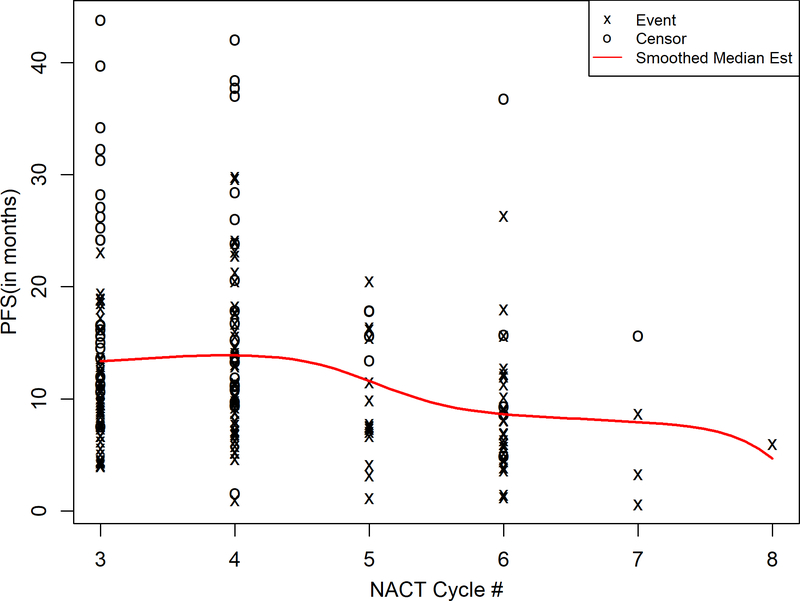
A smoothed survival function20 assessing the association of progression-free survival with neoadjuvant cycles as a continuous variable, found a cutoff at 4 cycles (Figure 2). The maximally selected rank statistics methodology21–23 suggests that receiving ≥5 neoadjuvant cycles was associated with worse progression-free survival (maximally selected log rank p=0.017). Given this, multivariate models of progression-free survival used 4 neoadjuvant cycles as the comparator. In multivariate models of progression-free survival, there were no significant differences between 3 and 4 cycles. Receiving ≥5 versus 4 cycles was associated with worse progression-free survival, even after adjustment for BRCA mutation status and complete gross resection (HR 2.20 95% CI 1.45–3.33, p<0.001) (Table 2).
Figure 2:
Smoothed Kaplan-Meier Survival Function of Progression-Free Survival and Neoadjuvant Cycles (Continuous)
Table 2:
Multivariate model of progression-free and overall survival
| Multivariate Model of Progression-Free Survival | ||||
| Variable | Hazard Ratio | 95%CI Lower Bound | 95%CI Upper Bound | p-value |
| Neoadjuvant Chemotherapy Cycle | <0.001 | |||
| 3 vs. 4 | 1.1 | 0.739 | 1.638 | |
| ≥5 vs. 4 | 2.196 | 1.45 | 3.325 | |
| BRCA Status | 0.006 | |||
| BRCA1/2 mutation vs None | 0.429 | 0.248 | 0.74 | |
| Not test vs No | 0.715 | 0.433 | 1.178 | |
| Complete Gross Resection: Yes vs. No | 0.397 | 0.278 | 0.566 | <0.001 |
| Multivariate Model of Overall Survival | ||||
| Variable | Hazard Ratio | 95%CI Lower Bound | 95%CI Upper Bound | p-value |
| Neoadjuvant Chemotherapy Cycle | 0.016 | |||
| 3 vs. 4 | 1.606 | 0.797 | 3.237 | |
| ≥5 vs. 4 | 2.775 | 1.369 | 5.625 | |
| Histology: High grade serous vs Other | 0.345 | 0.15 | 0.79 | 0.012 |
| Neoadjuvant Imaging: Response vs. None | 1.424 | 0.606 | 3.347 | 0.418 |
| Complete Gross Resection: Yes vs. No | 0.398 | 0.227 | 0.699 | 0.001 |
Overall Survival Analysis
At a median follow-up for survivors of 21.2 months (range 1.6–45.4), 55 deaths were observed. Median overall survival was not reached overall and was 33.3 months (95% CI 27-not reached), not reached, and 28.9 months (95% CI 19.7-not reached) in those with 3, 4 and ≥5 neoadjuvant cycles respectively. On univariate analysis, there were no significant differences between 3 and 4 neoadjuvant cycles. Receiving ≥5 cycles was significantly associated with worse overall survival compared with 3 cycles (HR 1.64 95% CI 0.89–3.0 p=0.008) and 4 cycles (HR 2.88 95% CI 1.43–5.77 p=0.008) (Figure 3 and Table S3). High-grade serous carcinoma, presence of BRCA 1/2 mutations, and achieving complete gross resection were significantly associated with improved overall survival; having no response on imaging after 2–3 cycles was significantly associated with worse overall survival on univariate analysis, p<0.05 (Table S3).
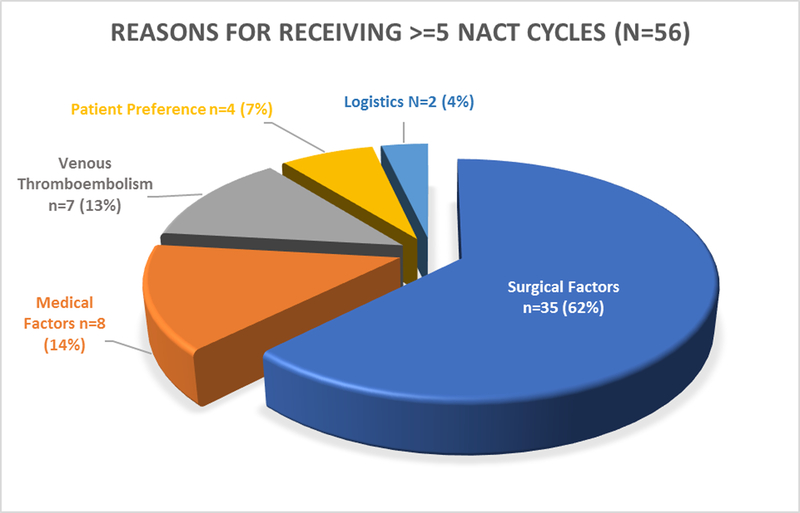
Figure 3:
Reasons for Receiving >= 5 Neoadjuvant Cycles
On multivariate analysis, receiving ≥5 versus 4 neoadjuvant cycles was significantly associated with worse overall survival, even after adjusting for high-grade serous carcinoma, presence of response on imaging after 2–3 preoperative cycles, and achieving complete gross resection (HR 2.78 95% CI 1.37–5.63, p=0.016). Of note, after adjustment for these factors, response on imaging after 2–3 cycles was no longer independently associated with overall survival (Table 2).
Reasons for Receiving ≥5 Neoadjuvant Chemotherapy Cycles
The most common reason cited for receiving ≥5 neoadjuvant cycles was surgical (62%) due to disease that was too extensive for interval debulking surgery after 2–3 cycles, as assessed by imaging, clinical exam, or both. Medical reasons (14%) for deferring interval debulking surgery included patient performance status and delayed recovery from chemotherapy. The presence of newly diagnosed venous thromboembolism, requiring delay of surgery, was cited in 13% of cases. Patient preferences and initial refusal of surgery, and logistics of scheduling, were cited in 7% and 4% of cases, respectively (Figure 3). In those receiving ≥5 cycles for surgical factors (n=35), median progression-free survival was 8.7 months (95% CI 6.7–12.0) and overall survival was 33.0 months (95% CI 19.7-not estimable). In those receiving ≥5 cycles for other reasons (n=21: medical factors (n=8), venous thromboembolism (n=7), patient preference (n=4), and logistics (n=2), median progression-free survival was 7.8 months (95% CI 4.4–12.3) and overall survival was 20.9 months (95% CI 10.4-not estimable) (Table S4).
DISCUSSION
In our single-center study of 199 women with newly diagnosed ovarian cancer receiving neoadjuvant chemotherapy who underwent interval debulking surgery, we found that the number of neoadjuvant cycles ranged from 3–8, with a median of 4 cycles. Although there were no differences in progression-free or overall survival between those receiving 3 versus 4 cycles, those receiving ≥5 neoadjuvant cycles (28%) had worse progression-free and overall survival, even after adjusting for clinical factors and complete gross resection rates. These data suggest that patients who remain unfit for interval debulking surgery after cycle 4 of neoadjuvant chemotherapy have a poorer prognosis, which is not mitigated by surgical effort.
Our study is consistent with multiple retrospective studies suggesting that a greater number of neoadjuvant chemotherapy cycles may be associated with worse outcomes. Bristow and Chi examined number of neoadjuvant cycles and survival, and found that each increase in the number of cycles above 3 was associated with a decrease in median survival time by 4.1 months.10 Altman et al. studied 403 women with ovarian cancer receiving neoadjuvant chemotherapy and found that those who received ≥4 cycles had worse outcomes, even after adjusting for degree of cytoreduction.8 Bogani et al. studied 193 women with ovarian cancer undergoing neoadjuvant chemotherapy and interval debulking surgery at four Italian centers and found that increased number of neoadjuvant cycles (4 vs. 3) was associated with worse overall survival (p=0.06), with significantly worse 10-year overall survival (26% vs. 18%, p=0.009).9 Others have corroborated these findings and have suggested that the number of neoadjuvant cycles should not exceed 4 (Table S5).11,25
Of note, we found no difference in complete gross resection rates, progression-free or overall survival between those receiving 3 versus 4 neoadjuvant cycles, suggesting that either 3 or 4 cycles is acceptable clinically. In practice, logistical constraints of scheduling often lead providers to administer an additional cycle of chemotherapy prior to interval debulking surgery. Interestingly, our Kaplan-Meier curve of overall survival shows some separation between 3 versus 4 cycles; although not statistically significant, there is a suggestion of potentially worse overall survival in patients undergoing 3 versus 4 cycles. Our small sample size, small number of deaths, and limited follow-up for assessment of overall survival make it difficult to draw conclusions. This is hypothesis generating, however, and should be studied in larger cohorts with longer follow-up.
Although some studies have suggested that achieving complete gross resection may negate the prognostic implications of receiving >4 neoadjuvant chemotherapy cycles,12 our study does not support this. In fact, our data show similar complete gross resection rates in those receiving 3, 4 and ≥5 cycles, and there does not appear to be an interaction between neoadjuvant cycles and complete gross resection. This implies that patients unfit for interval debulking surgery after 4 cycles have a worse prognosis that is not reversed by maximal cytoreduction. Limiting surgical morbidity in this patient population should remain a goal of care and warrants further study. However, these data do not suggest that interval debulking surgery should be withheld in those requiring >4 cycles, as some patients may still derive benefit. Careful consideration of the risks and benefits of cytoreduction should be addressed on an individual basis.
We found that patients receiving >4 neoadjuvant cycles received fewer post-operative cycles, and some received no adjuvant therapy. Those receiving 4 versus 3 neoadjuvant cycles did receive significantly fewer post-operative cycles (p<0.001), although both groups had a median of 3 post-operative cycles. Detailed review of the charts of women not receiving adjuvant therapy showed that most did not receive it due to poor tolerability or because the treating oncologist had finished the prescribed plan of 6 total cycles prior to surgery. Many oncologists will aim to deliver 6 total cycles, regardless of when they occur in relation to surgery. A small portion of these patients did die or progress shortly after surgery. Although differences in post-operative chemotherapy may be important and affect survival, our study showed only an association with progression-free and not overall survival, a finding supported by other studies.26 Additionally, number of pre- and post-operative cycles were highly correlated, and both variables likely reflect the underlying fitness of the patient and the aggressiveness of disease. In our analysis, only pre-operative cycles were associated with both progression-free and not overall survival, suggesting that pre-operative cycles may be a better prognostic indicator.
Although any response on imaging after 2 to 3 preoperative cycles was associated with overall survival on univariate analysis, this association disappeared in multivariate models adjusting for number of neoadjuvant cycles and surgical outcomes. McNulty et al. found that, although radiological assessment after neoadjuvant chemotherapy was correlated with chemotherapy response score on histopathologic review, it was not independently associated with survival.27 Our study corroborates these results and suggests that any response on imaging after 2 to 3 cycles was not an independent predictor of survival, after adjusting for neoadjuvant cycle number and surgical outcomes. This implies that the decision to proceed with more neoadjuvant cycles and interval debulking surgery is complex and should be based on clinical variables as well as imaging results. Given the high-risk nature of this group and high likelihood of recurrence, careful counseling, early integration of palliative care and individualization of care is particularly important.
Unlike our prior studies of patients with newly diagnosed ovarian cancer receiving neoadjuvant chemotherapy,28 age, stage and comorbidity score were not associated with survival and were not included in multivariate models. This may reflect a highly selected population that was considered fit to undergo debulking surgery.
The strengths of our retrospective study include utilization of a prospective Ovarian Database and inclusion of multiple clinically relevant variables. This is one of the first studies to assess the potential interaction of degree of cytoreduction and neoadjuvant chemotherapy cycles on survival. Limitations include our modest sample size and short follow-up of <2 years. Our characterization of response on imaging after 2 to 3 neoadjuvant cycles was dichotomous (yes/no), and patients who had any response represent a heterogeneous group, which could explain the lack of association with survival on multivariate models. This should be explored further in larger, imaging-based studies. In addition, although we collected detailed clinical information (Table S1), the prognosis of different subtypes of stage III and IV disease varies, and larger studies of neoadjuvant treatment should investigate the association of specific stage and outcomes.
In conclusion, women with newly diagnosed ovarian cancer receiving ≥5 neoadjuvant chemotherapy cycles may have a worse prognosis, despite maximal cytoreduction. Strategies to limit surgical morbidity and optimize patient selection, as well as novel systemic therapies, should be utilized in this high-risk population.
Supplementary Material
Supplementary Table S1: Detailed Staging by Number of Preoperative Cycles
Supplementary Table S2: Univariate Progression-Free Survival Analysis
Supplementary Table S3: Univariate Overall Survival Analysis
Supplementary Table S4: PFS and OS by Reason for Receiving >=5 NACT Cycles
Supplementary Table S5: Review of Studies Examining Role of NACT Cycles on Survival
HIGHLIGHTS.
Receiving >4 neoadjuvant cycles was associated with worse progression-free and overall survival
Negative prognosis associated with >4 neoadjuvant cycles was not alleviated by a complete gross resection
Disease extent precluding surgery was the most common reason for receiving >4 neoadjuvant cycles
ACKNOWLEDGEMENTS
Funding: This study was funded in part through the NIH/NCI Support Grant P30 CA008748.
Dr. Aghajanian reports personal fees from Tesaro (medical advisory board), Immunogen (medical advisory board), Mateon Therapeutics (steering committee) and Cerulean Pharma (medical advisory board), grants and personal fees from Clovis (medical advisory board) and Genentech (steering committee), and grants from AbbVie (steering committee) and Astra Zeneca, outside the submitted work.
Dr. Abu-Rustum reports grants from Stryker/Novadaq, grants from Olympus, grants from GRAIL, outside the submitted work.
Disclosures:
Dr. Liu reports a grant from AstraZeneca, outside the submitted work.
Dr. Iasonos reports personal fees from Mylan, outside the submitted work.
Dr. Chi reports personal fees from Bovie Medical Co. (medical advisory board and stock options), Verthermia Inc. (medical advisory board and stock options), C Surgeries (shareholder), and Intuitive Surgical Inc (stock ownership), outside the submitted work.
Dr. O’Cearbhaill reports personal fees from Clovis (medical advisory board) and Tesaro (medical advisory board), outside the submitted work.
Dr. Konner reports personal fees from Clovis (guest speaker), AstraZeneca (medical advisory board), and Immunogen (medical advisory board), outside the submitted work.
Dr. Long Roche reports personal fees from Intuitive Surgical (travel), outside the submitted work.
Footnotes
Conflict of Interest Statement: None declared.
REFERENCES
- 1.Mueller JJ, Zhou QC, Iasonos A, et al. Neoadjuvant chemotherapy and primary debulking surgery utilization for advanced-stage ovarian cancer at a comprehensive cancer center. Gynecologic oncology. 2016;140(3):436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melamed A, Hinchcliff EM, Clemmer JT, et al. Trends in the use of neoadjuvant chemotherapy for advanced ovarian cancer in the United States. Gynecologic oncology. 2016;143(2):236–240. [DOI] [PubMed] [Google Scholar]
- 3.Wright AA, Bohlke K, Armstrong DK, et al. Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian cancer: Society of Gynecologic Oncology and American Society of Clinical Oncology Clinical Practice Guideline. Gynecologic oncology. 2016;143(1):3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fagotti A, Ferrandina G, Vizzielli G, et al. Phase III randomised clinical trial comparing primary surgery versus neoadjuvant chemotherapy in advanced epithelial ovarian cancer with high tumour load (SCORPION trial): Final analysis of peri-operative outcome. European journal of cancer (Oxford, England : 1990). 2016;59:22–33. [DOI] [PubMed] [Google Scholar]
- 5.Kehoe S, Hook J, Nankivell M, et al. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet (London, England). 2015;386(9990):249–257. [DOI] [PubMed] [Google Scholar]
- 6.Onda T, Satoh T, Saito T, et al. Comparison of treatment invasiveness between upfront debulking surgery versus interval debulking surgery following neoadjuvant chemotherapy for stage III/IV ovarian, tubal, and peritoneal cancers in a phase III randomised trial: Japan Clinical Oncology Group Study JCOG0602. European journal of cancer (Oxford, England : 1990). 2016;64:22–31. [DOI] [PubMed] [Google Scholar]
- 7.Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. The New England journal of medicine. 2010;363(10):943–953. [DOI] [PubMed] [Google Scholar]
- 8.Altman AD, McGee J, May T, et al. Neoadjuvant chemotherapy and chemotherapy cycle number: A national multicentre study. Gynecologic oncology. 2017;147(2):257–261. [DOI] [PubMed] [Google Scholar]
- 9.Bogani G, Matteucci L, Tamberi S, et al. The Impact of Number of Cycles of Neoadjuvant Chemotherapy on Survival of Patients Undergoing Interval Debulking Surgery for Stage IIIC-IV Unresectable Ovarian Cancer: Results From a Multi-Institutional Study. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2017;27(9):1856–1862. [DOI] [PubMed] [Google Scholar]
- 10.Bristow RE, Chi DS. Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecologic oncology. 2006;103(3):1070–1076. [DOI] [PubMed] [Google Scholar]
- 11.Xu X, Deng F, Lv M, Chen X. The number of cycles of neoadjuvant chemotherapy is associated with prognosis of stage IIIc-IV high-grade serous ovarian cancer. Archives of gynecology and obstetrics. 2017;295(2):451–458. [DOI] [PubMed] [Google Scholar]
- 12.Phillips A, Sundar S, Singh K, et al. Complete cytoreduction after five or more cycles of neoadjuvant chemotherapy confers a survival benefit in advanced ovarian cancer. Eur J Surg Oncol. 2018;44(6):760–765. [DOI] [PubMed] [Google Scholar]
- 13.Chi DS, Eisenhauer EL, Lang J, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecologic oncology. 2006;103(2):559–564. [DOI] [PubMed] [Google Scholar]
- 14.du Bois A, Reuss A, Pujade-Lauraine E, Harter P, Ray-Coquard I, Pfisterer J. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the Arbeitsgemeinschaft Gynaekologische Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe d’Investigateurs Nationaux Pour les Etudes des Cancers de l’Ovaire (GINECO). Cancer. 2009;115(6):1234–1244. [DOI] [PubMed] [Google Scholar]
- 15.von Gruenigen VE, Huang HQ, Beumer JH, et al. Chemotherapy completion in elderly women with ovarian, primary peritoneal or fallopian tube cancer - An NRG oncology/Gynecologic Oncology Group study. Gynecologic oncology. 2017;144(3):459–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prat J Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124(1):1–5. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 18.Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. [DOI] [PubMed] [Google Scholar]
- 19.Aletti GD, Santillan A, Eisenhauer EL, et al. A new frontier for quality of care in gynecologic oncology surgery: multi-institutional assessment of short-term outcomes for ovarian cancer using a risk-adjusted model. Gynecologic oncology. 2007;107(1):99–106. [DOI] [PubMed] [Google Scholar]
- 20.Bowman A, Azzalini A. Applied Smoothing Techniques for Data Analysis: the Kernel Approach with S-Plus Illustrations. Oxford University Press, Oxford; 1997. [Google Scholar]
- 21.Lausen B, Hothorn T, Bretz F, Schumacher M. Assessment of optimally selected prognostic factors. Biometrical Journal. 2004;46(3):364–374. [Google Scholar]
- 22.Lausen B, Sauerbrei W, Schumacher M. Classification and regression tree(CART) used for the exploration of prognostic factors meansured on different scales in Dirschedl P and Ostermann R (Eds). Computational Statistics 1994:483–496. [Google Scholar]
- 23.Lausen B, Schumacher M. Maximally selected ranked statistics. Biometrics. 1992;48:73–85. [Google Scholar]
- 24.Di Donato V, Page Z, Bracchi C, et al. The age-adjusted Charlson comorbidity index as a predictor of survival in surgically treated vulvar cancer patients. Journal of gynecologic oncology. 2019;30(1):e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colombo PE, Labaki M, Fabbro M, et al. Impact of neoadjuvant chemotherapy cycles prior to interval surgery in patients with advanced epithelial ovarian cancer. Gynecologic oncology. 2014;135(2):223–230. [DOI] [PubMed] [Google Scholar]
- 26.Kim JS, Liang MI, Prendergast EN, et al. Clinical outcomes in ovarian cancer patients receiving three versus more cycles of chemotherapy after neoadjuvant treatment and interval cytoreductive surgery. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2019;29(7):1156–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNulty M, Das A, Cohen PA, Dean A. Measuring response to neoadjuvant chemotherapy in high-grade serous tubo-ovarian carcinoma: an analysis of the correlation between CT imaging and chemotherapy response score. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2019. [DOI] [PubMed] [Google Scholar]
- 28.Liu YL, Filippova OT, Zhou Q, et al. Characteristics and survival of ovarian cancer patients treated with neoadjuvant chemotherapy but not undergoing interval debulking surgery. Journal of gynecologic oncology. 2020;31(1):e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Detailed Staging by Number of Preoperative Cycles
Supplementary Table S2: Univariate Progression-Free Survival Analysis
Supplementary Table S3: Univariate Overall Survival Analysis
Supplementary Table S4: PFS and OS by Reason for Receiving >=5 NACT Cycles
Supplementary Table S5: Review of Studies Examining Role of NACT Cycles on Survival