Abstract
Background
Systemic antibiotics and antibiotic‐impregnated shunt systems are often used to prevent shunt infection.
Objectives
To evaluate the effectiveness of either prophylactic systemic antibiotics or antibiotic‐impregnated shunt systems for preventing infection in patients who underwent surgical introduction of intracranial ventricular shunts.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, LILACS and the meeting proceedings from the American Association of Neurological Surgeons and from the European Association of Neurosurgical Societies, until June 2005.
Selection criteria
We included randomized or quasi‐randomized controlled trials comparing the use of prophylactic antibiotics (either systemic or antibiotic‐impregnated shunt systems) in intracranial ventricular shunt procedures with placebo or no antibiotics.
Data collection and analysis
Two authors appraised quality and extracted data independently.
Main results
We included seventeen trials with overall 2134 participants. We performed two separate meta‐analyses: one that evaluated the use of systemic prophylactic antibiotics and another that evaluated the use of antibiotic‐impregnated systems. All studies included shunt infection in their primary outcome.
We could not analyse all‐cause mortality regarding systemic antibiotics due to lack of data. No significant differences were found (odds ratio (OR): 1.47, 95% confidence intervals (CI) 0.83 to 2.62) for this outcome regarding the use of antibiotic‐impregnated catheters compared with standard ones. The use of systemic antibiotic prophylaxis and the use of antibiotic‐impregnated catheters were associated with a decrease in shunt infection (OR: 0.52, 95% CI 0.36 to 0.74 and OR: 0.21, 95% CI 0.08 to 0.55 respectively). We found no significant benefit for shunt revision in both meta‐analyses that evaluated systemic antibiotics and impregnated‐shunt systems. We found no significant differences between the subgroups evaluated: type of shunt (internal/external, ventriculoperitoneal/ventriculoatrial), age and duration of the administration of antibiotics.
Authors' conclusions
We could demonstrate a benefit of systemic prophylactic antibiotics for the first 24 hours postoperatively to prevent shunt infection, regardless of the patient's age and the type of internal shunt used. The benefit of its use after this period remains uncertain. However this data derives from the rate of shunt infection, which is an intermediary outcome. Future trials should evaluate the effectiveness of different regimens of systemic antibiotics rather than placebo, and should include all‐cause mortality, shunt revision and adverse events as additional outcomes. Evidence suggests that antibiotic‐impregnated catheters reduce the incidence of shunt infection although more well‐designed clinical trials testing the effect of antibiotic‐impregnated shunts are required to confirm their net benefit.
Keywords: Humans, Antibiotic Prophylaxis, Anti‐Bacterial Agents, Anti‐Bacterial Agents/therapeutic use, Bacterial Infections, Bacterial Infections/prevention & control, Cerebrospinal Fluid Shunts, Cerebrospinal Fluid Shunts/adverse effects, Randomized Controlled Trials as Topic, Wound Infection, Wound Infection/prevention & control
Plain language summary
A review of the medical literature for evidence of whether the use of intravenous antibiotics, or devices impregnated with antibiotics, reduce the risks of infection during the surgical placement of catheters for the drainage of excess fluid from the brain
An intracranial ventricular shunt is a device (catheter/tube) used to drain an excess of cerebrospinal fluid from the brain. (Cerebrospinal fluid is a clear body fluid released into the subarachnoid space; the subarachnoid space surrounds the brain and the spinal cord.) Patients with intracranial ventricular shunts are prone to infection. Some doctors give either antibiotic drugs or use antibiotic‐impregnated devices to reduce the risk of infection. Our review included randomized controlled trials that compared the incidence of shunt infection in patients who were given preventive antibiotic therapy with those who did not receive these drugs. We also included trials comparing antibiotic‐impregnated shunt systems with those who received non‐antibiotic impregnated shunts. We included seventeen trials in our review. Although the available data does not provide much detail on mortality or the adverse events caused by antibiotics (an adverse event is an incident in which harm resulted to a person receiving the health care) it does support the use of preventative systemic prophylactic antibiotics for the first 24 hours postoperatively following an intracranial ventricular shunt operation or the use of antibiotic‐impregnated catheters. However this data was obtained from an intermediary outcome which is the rate of shunt infections. Therefore although the evidence suggests that the use of antibiotics is beneficial in reducing the incidence of shunt infection more research is needed to confirm their benefit.
Background
Intracranial ventricular shunts are silastic tubes that divert a pathologic excess of cerebrospinal fluid (CSF) from the ventricles of the brain either to the exterior (external ventricular shunts), or to other body cavities (internal ventricular shunts), where normal physiologic processes can absorb the fluid. Without these shunts, fluid can build up in spaces of the brain and cause damage to the brain tissue. External shunts are used in an emergency or potentially reversible hydrocephalus situations. Recommendations concerning routine changing of ventricular catheters in order to reduce the risk of infection remain controversial. Internal ventricular shunts are implanted for the life of the patient but as many as 30% of these will need to be replaced or modified at some point.
Ventriculo‐peritoneal shunts are the most commonly used internal shunts. Ventriculo‐atrial shunts, (which pass fluid to the superior cava), are an option when abdominal abnormalities are present. Ventriculo‐pleural shunts are another type of intracranial shunt. They may be used when other routes are not indicated. Placement of the distal end of the catheter has been reported in the gall bladder, ureter and bladder. Approximately 40 per 100,000 of people in the United States of America have shunts in place; the majority of these are children (Moss 1991). The most significant complication resulting from intracranial ventricular shunt is infection. This affects from 1.5 to 38% of patients (Claus 2004). Age seems to be an important risk factor, with infection rates amongst young children of up to 20% (Bondurant 1995). It is thought that younger people have higher levels of morbidity due to infection, which is of concern. The presenting symptoms of shunt infection are variable, they may be related to the type of infective organism and also age‐dependent. Shunt infection may be associated with increased mortality; increased risk of seizure disorders; and decreased intellectual performance. Treatment usually requires prolonged hospitalization for antibiotic administration and repeated surgery.
It is not clear what mechanism predisposes a shunt to infection (Borges 1982), since the device is recognized by the body as a foreign body. It is likely that the initial step in this process is bacterial colonization of the device during the surgical procedure. There is a reduction of normal defence mechanisms and local immunity caused by the foreign body, and from a complex and effective adherence process between bacteria and the device. These have a role in postoperative device‐related infections. However, the benefit of systemic antibiotic prophylaxis is not generally accepted and its use is frequently a matter of discussion. In fact, some authors found no relationship between infection and the use of systemic prophylactic antibiotics (Schmidt 1985; Stenager 1986). A meta‐analysis performed by Langley et al. showed a 50% reduction in the risk of infection for internal shunts if perioperative prophylaxis was used (Langley 1993); that is, if prophylaxis was maintained for 24 to 72 hours after surgery. Another meta‐analysis (Haines 1994) focused on data that were strongly correlated with a high baseline infection rate. The benefit associated with antibiotics was no longer apparent when the baseline infection was at or below about 5%. These studies did not comprise an extensive and comprehensive review of the literature and the papers included were searched until 1990 and 1993 respectively.
Nevertheless, continuous prophylactic antibiotics are widely administered to patients with external ventricular drains (EVD), despite a paucity of data supporting their use There is no evidence of a difference in the rate of CSF infection in patients with EVD who received continuous prophylactic antibiotics for the duration of drain placement, compared with those who received only periprocedural dosing (started immediately before placement of the drain and continued for three or fewer doses, or continued for less than 24 hours) (Alleyne 2000). The development and growing use of the newly developed antibiotic‐impregnated shunt systems also demand an evidence‐based investigation of their effectiveness and safety when compared with standard shunt systems.
In the light of increasing concern about antibiotic resistance (Frazee 1988), it is critical to search and analyse the evidence of effectiveness and safety of the use of prophylactic systemic antibiotics compared with placebo or no antibiotics; of the use of periprocedural versus continuous antibiotics; and of the use of antibiotic‐impregnated systems compared with standard systems for surgically introduced intracranial ventricular shunts.
Objectives
We examined the following hypotheses:
The incidence of shunt infection is less frequent with administration of systemic antibiotics beginning previously or at the time of the surgical procedure.
The incidence of shunt infection is less frequent when antibiotic‐impregnated shunt systems are used, considering that the eventual regimen of systemic antibiotics applied is the same.
We tested these hypotheses by performing a literature review with the following objectives:
Primary objective
Our primary objective was to establish the effectiveness and safety of the use of prophylactic antibiotics, whether systemic or catheter‐impregnated, for intracranial ventricular shunts.
We performed independent analyses for systemic antibiotics and antibiotic‐impregnated systems. Within each of the former analyses, we also performed separate analyses for external and internal intracranial shunts.
Secondary Objectives
We performed the following subgroup analyses if sufficient relevant information was available:
To determine the differences in the risk of developing an infection of external ventricular shunts versus internal ventricular shunts.
To determine the differences in the risk of developing an infection of ventriculoperitoneal shunts versus ventriculoatrial shunts.
To determine the differences in the risk of developing an infection in children versus all ages.
To determine the differences in the risk of developing an infection in using periprocedural antibiotics versus continuous antibiotics.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) comparing the use of prophylactic antibiotics in intracranial ventricular shunts procedures with placebo or the standard treatment.
We also included trials with poor allocation concealment (quasi‐randomized).
Types of participants
We included patients of any age with any type of intracranial ventricular cerebrospinal fluid shunt surgical procedure.
We excluded patients with either suspected or confirmed pre‐existing infection. We defined infection according to the study investigator's criteria.
Types of interventions
Systemic administration of any antibiotic(s), at any dosage, beginning previously or at the time of the surgical procedure and prolonged for any length of time, compared with placebo or no antibiotics.
Employment of antibiotic‐impregnated shunt systems compared with standard shunt systems.
We excluded trials comparing different antibiotics; different dosages; route of administration; or differences in timing or duration of administration.
Types of outcome measures
All‐cause mortality (death resulting from central nervous system infection or other cause mortality including progression of the primary disease process and multi‐system failure).
Shunt infection (evidence of infection in one or more of the following: shunt equipment, overlying wound, CSF or site related to distal drainage route: organism identification by tissue cultures from material in or around the shunt, or by cultures from fluid or CSF drawn from the shunt system itself).
Shunt revision (part or all of a shunt system).
Adverse events of antibiotics: number of patients with any adverse event, serious adverse events other than death, allergic reactions and other non‐allergic adverse effects. We considered the original author's categorization of the adverse events whenever stated it.
We considered disability, cognitive function and quality of life as additional outcome measures, whatever the management used in the individual trials. In addition, we also considered length of hospital stay as an additional outcome measure.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 3, 2005); MEDLINE (January 1966 to June 10th 2005, PubMed); EMBASE (1980 to June 10th 2005, SilverPlatter); and LILACS (1982 to June 10th 2005, Virtual Health Library).
Our search strategy for MEDLINE and CENTRAL will be found in Appendix 1. We combined this search with all three stages of the optimal search strategy devised for The Cochrane Collaboration (Dickersin 1994). We modified this search strategy for EMBASE (Appendix 2) and LILACS (Appendix 3).
Searching other resources
We screened titles, keywords and abstracts of the citations downloaded from the electronic searches.
We obtained full copies of reports of potentially suitable trials for further assessment.
We checked reference lists of located trials and review articles focusing on intracranial ventricular shunts. We also electronically searched meeting proceedings from the American Association of Neurological Surgeons (AANS) (1997 to June 10th 2005) and handsearched the abstracts from the meeting proceedings of the European Association of Neurosurgical Societies (EANS) (1995 to 2003).
We contacted researchers active in the field, for information regarding unpublished trials.
We also contacted authors of published trials for further information and unpublished data.
We did not apply any language restrictions.
Data collection and analysis
Two authors (BORL, JOAC) independently assessed the studies identified by the search strategy. We identified potentially suitable trials for the review, according to the criteria outlined above. We resolved disagreements by discussion with a third author (SCRI).
We independently assessed the full papers for the type of participants; the type and dose of antibiotic used; the methodological quality; the number of patients excluded or lost to follow‐up; and the outcome measures stated in the protocol. We searched for sources of bias.
We abstracted eligible data using standardized data extraction forms. We cross‐checked all data abstracted for accuracy before combining for analysis. We expressed the results as binary data. If data on disability, cognitive function, quality of life or length of hospital stay were available we provided a descriptive analysis. Additionally, if appropriate, we treated results as dichotomous, or handled them as continuous variables. When information on these outcomes was not available we reported their absence.
We analysed clinical heterogeneity qualitatively, taking into account factors such as the studied population, surgical procedure and technique. We made the decision to perform a meta‐analysis based on absence of considerable clinical heterogeneity. We provided a descriptive summary of the results where the outcome data from different studies cannot be combined.
We performed statistical analyses using the statistical software provided by the Cochrane Collaboration (RevMan 4.2). We investigated for statistical heterogeneity between trial results using the I2 statistic (Higgins 2005).
We used pooled summary estimates appropriate to the given data. We also used random‐effects models or fixed‐effect models as appropriate.
The number of patients who must be treated to prevent one adverse outcome (number needed to treat (NNT)) is a widely used measure. However, the NNTs derived from meta‐analysis may be misleading because of the variation in the event rates in trials; differences in the outcomes considered; effects of secular trends on disease risk, and differences in clinical setting (Smeeth 1999). There is debate concerning methods for calculating the NNTs from results of systematic reviews. We have calculated the NNTs from meta‐analysis estimates and have not treated the data as if they arose from a single trial since the latter approach is more prone to bias (Altman 2002). We assessed the difference between subgroups by calculating a two‐tailed z‐score using the following formulae for binary data:
z = (lnOR1 ‐ lnOR2 ) / √(var[lnOR1] + var[lnOR2]) where OR1 and OR2 are the combined odds ratios from each subgroup and var is the variance of each which was calculated from the 95% confidence intervals (Fleiss 1993).
Since there is evidence that the quality of allocation concealment particularly affects the results of studies (Schulz 1995), two authors (BORL, JOAC) scored this quality on the scale used by Schulz (Schulz 1995) as described below:
A = trials deemed to have taken adequate measures to conceal allocation (that is central randomization; serially numbered, opaque, sealed envelopes; or other description that contained elements convincing of concealment).
B = trials in which the authors either did not report an allocation concealment approach at all or reported an approach that did not fall into one of the other categories.
C = trials in which concealment was inadequate (such as alternation or reference to case record numbers or to dates of birth).
We performed a sensitivity analysis of class A trials. We used the graphical test proposed by Egger (Egger 1997) to evaluate publication bias.
Results
Description of studies
(See the Characteristics of included studies and Characteristics of excluded studies)
We identified twenty‐one potentially eligible trials, including one duplicate citation (Govender 2003). We excluded three studies (Epstein 1982; Shapiro 1986; Siqueira 1987), our reasons are listed in the table of 'Characteristics of Excluded Studies'.
We included seventeen trials in this systematic review. We were able to perform two meta‐analyses: one which evaluated the benefit of systemic prophylactic antibiotics for ventricular shunts included fifteen trials (Bayston 1975; Blomstedt 1985; Blum 1989; Bullock 1988; Djindjian 1986; Haines 1982; Odio 1984; Rieder 1987; Rocca 1992; Schmidt 1985; Walters 1992; Wang 1984; Yogev 1985; Young 1987; Zentner 1995). The second meta‐analysis investigated the effectiveness of antibiotic‐impregnated catheters compared with standard ones, and included two trials (Govender 2003; Zabramski 2003). All these trials had a randomized parallel design and were published between 1975 and 2003.
Entry criteria did not differ considerably between the included trials. Most trials enrolled patients undergoing a CSF shunting procedure, whether shunt insertion or shunt revision. The exceptions were the Djindjian 1986 and Rocca 1992 trials which included only patients receiving their first shunt implantation. There were eight trials that included only children (Bayston 1975; Blum 1989; Bullock 1988; Haines 1982, Odio 1984; Rieder 1987; Walters 1992;Wang 1984; Yogev 1985). Most trials evaluated patients who were given internal ventricular shunts, except Blomstedt 1985, (which evaluated a subgroup of patients given external ventricular drains) and Zabramski 2003 (which evaluated the effect of antibiotic‐impregnated catheters in external shunt procedure).
Shunt infection was a primary outcome in all included trials and was always based on clinical and laboratory grounds (CSF cell count/biochemistry suggestive of infection and/or positive CSF culture). From the trials that evaluated the effect of systemic antibiotics, only two reported data on outcomes of its safety and tolerability (Odio 1984; Zentner 1995).
In ten trials (Blomstedt 1985; Blum 1989; Bullock 1988; Haines 1982; Rieder 1987, Schmidt 1985; Wang 1984; Young 1987; Zentner 1995; Zabramski 2003) patients were generally well‐matched within studies between the treatment and control arms for demographics, etiology of hydrocephalus, type of shunting and duration of the operation. The remaining trials did not describe the characteristics of the population included in each group.
Description of withdrawals and dropouts sufficient to determine the number of patients in each treatment group entering and completing the trial were available in only four trials (Odio 1984; Schmidt 1985; Wang 1984; Young 1987).
Risk of bias in included studies
(See Characteristics of included studies) All studies stated that they randomized patients between the treatment and control options. The precise method of randomization and details of concealment of allocation was described and considered adequate in eight trials (Bullock 1988; Odio 1984; Rieder 1987; Schmidt 1985; Wang 1984; Young 1987; Zabramski 2003; Zentner 1995); considered unclear in seven trials (Bayston 1975; Blomstedt 1985; Djindjian 1986; Govender 2003; Rocca 1992; Walters 1992; Yogev 1985), and considered inadequate in two trials (Blum 1989; Haines 1982).
From the 15 studies evaluating the effect of the systemic antibiotics there were seven studies double blinded throughout using identical appearance interventions (Blomstedt 1985; Bullock 1988; Haines 1982; Odio 1984; Rieder 1987; Walters 1992; Wang 1984). Two other studies were also placebo controlled (Blum 1989; Yogev 1985); while the remaining six studies used no antibiotics as a control group (Bayston 1975; Djindjian 1986; Rocca 1992; Schmidt 1985; Young 1987; Zentner 1995) in which only Young 1987 described that outcome were also assessed blindly. Eleven studies reported the number and the reasons for patients leaving the trials (Blomstedt 1985; Bullock 1988; Govender 2003; Haines 1982; Odio 1984; Schmidt 1985; Walters 1992; Wang 1984; Young 1987; Zabramski 2003; Zentner 1995).
None of the studies reported effects from intention‐to‐treat (ITT) analysis; data was presented on a per‐protocol basis in all trials. Overall, 1736 patients were enrolled in the 15 studies that were included in the meta‐analysis that evaluated the effectiveness of systemic antibiotic prophylaxis, and 398 patients in the two studies that were included in the meta‐analysis that evaluated the use of antibiotic‐impregnated catheters for surgical introduction of ventricular drains.
Effects of interventions
We performed two separate meta‐analyses given the clinical heterogeneity resulting from two interventions that were fundamentally different.
The first meta‐analysis (15 RCTs) compared systemic prophylactic antibiotics with no antibiotic or placebo in patients with surgical introduction of ventricular drains.
The second meta‐analysis (two RCTs) compared the use of antibiotic‐impregnated shunt systems with standard shunt systems.
Missing data precluded the conduct of some planned analysis in this systematic review. We contacted the authors of the trials whenever we found this necessary, although no additional information was obtainable.
We tested heterogeneity between trials results using an I2 test. Assuming that significant statistic heterogeneity is absent if I2 is less than 50%, we found no evidence of heterogeneity for all groups in the measured outcomes. Therefore we used fixed‐effect models to synthesize data.
-
All cause mortality
Systemic antibiotic prophylaxis. We could not analyse this outcome since there was only one trial (Schmidt 1985) that reported this outcome and there were no events.
Antibiotic‐impregnated catheters. We could access relevant data from two trials (Govender 2003; Zabramski 2003) enrolling 398 patients. We found no significant differences (OR: 1.47, 95% CI 0.83 to 2.62). (See 'Comparisons and data' table 01/01)
-
Shunt infection
Systemic antibiotic prophylaxis . Analysis of the 15 included trials found a statistical significant difference for shunt infection that favoured treatment (OR: 0.52, 95% CI 0.36 to 0.74; NNT 12, 95% CI 7 to 30). Individually only one trial was statistically significant (Blomstedt 1985). Two trials favoured the control group, although not statistically significant (Rocca 1992; Schmidt 1985). (See 'Comparisons and data' table 02/01)
Antibiotic‐impregnated catheters. We found a statistical significant difference for shunt infection that favoured the use of antibiotic‐impregnated catheters (OR: 0.21, 95% CI 0.08 to 0.55; NNT 4, 95% CI 2 to 14). (See 'Comparisons and data' table 01/02)
-
Shunt revision
Systemic antibiotic prophylaxis. We could access relevant data for this outcome from three trials (Haines 1982; Rieder 1987; Wang 1984) involving 248 patients. We found there was no significant difference in shunt revision (OR: 0.76, 95% CI 0.42 to 1.36). (See 'Comparisons and data' table 02/02)
Antibiotic‐impregnated catheters. Govender 2003 was the only eligible trial that used antibiotic‐impregnated catheters for internal ventricular shunts (9/50 occurrences versus 15/60 controls). We found no significant difference between the groups (OR: 0.66, 95% CI 0.26 to 1.67). (See 'Comparisons and data' table 01/03)
Adverse events of antibiotics. Only two trials (Odio 1984; Zentner 1995) enrolling 163 patients reported the incidence of the adverse events of systemic antibiotics (8%, 7/87 occurrences). The eventual adverse effects of antibiotics from impregnated shunts were not reported in the eligible trials. (See 'Comparisons and data' table 02/03)
We were not able to study other proposed outcome measures such as disability, cognitive function, quality of life or length of hospital stay as no data were available.
Subgroup analyses
Data on mortality was too sparse to perform subgroup analyses. We found that it was appropriate to perform subgroup analyses for the most frequent outcome: shunt infection. Regarding systemic antibiotics we performed subgroup analyses according to the type of shunt (internal versus external, ventriculoperitoneal versus ventriculoatrial), age (children versus all ages), and length of the administration of prophylactic systemic antibiotics (periprocedural: started immediately before placement of the drain and continued for three or fewer doses, or continued for less than 24 hours versus continuous antibiotics for the duration of drain placement or longer than 24 hours) and to perform subgroup analysis for antibiotic‐impregnated systems according to the type of shunt (internal versus external).
-
1. Internal ventricular shunts versus external ventricular shunts
Systemic antibiotic prophylaxis. We could include all eligible trials in this subgroup analysis showing a statistical significant difference for internal shunt infection that favoured treatment (OR: 0.52, 95% CI 0.36 to 0.74). Only one trial (Blomstedt 1985) included a subgroup of patients with external shunts which was not statistically significant (OR: 1.08, 95% CI 0.06 to 18.30), with a weight of 1.05% in the total results. However no difference was found between the two subgroups (systemic antibiotics for internal versus external shunts; P = 0.2). (See 'Comparisons and data' table 02/01)
Antibiotic‐impregnated catheters. The Govender 2003 trial was the only RCT that evaluated infection of internal shunts and showed a non‐significant difference between trial groups (OR: 0.32, 95% CI 0.08 to 1.23). Zabramski 2003 evaluated external shunts and found a significant difference for this outcome (OR: 0.13, 95% CI 0.03 to 0.60). No difference was found between the two subgroups (antibiotic‐impregnated devices for internal versus external shunts; P = 0.3). (See 'Comparisons and data' table 01/02)
-
Ventriculoperitoneal (VP) shunts versus ventriculoatrial (VA) shunts
Systemic antibiotic prophylaxis. We were able to include 13 trials in this subgroup analysis that enrolled 1416 patients. Although the Djindjian 1986 trial did not detail the data, we included it in the subgroup analysis as a trial that enrolled only ventriculoperitoneal shunts (4 ventriculoatrial shunts out of 56 ventriculoperitoneal shunts). We excluded the Blum 1989 trial from this subgroup analysis because we could not discriminate the number of internal shunt type or the number of infections in each group. The author was contacted but could not provide additional information. Subgroup analyses for the effect of prophylactic systemic antibiotics of different type of internal shunts found a significant difference between treatment and control groups for both ventriculoatrial shunts (OR: 0.46, 95% CI 0.23 to 0.94) and ventriculoperitoneal shunts (OR: 0.54, 95% CI 0.34 to 0.85). No difference was found between the two subgroups (systemic antibiotics for ventriculoperitoneal versus ventriculoatrial shunts; P = 0.2). (See 'Comparisons and data' table 03/01)
Antibiotic‐impregnated catheters. We did not find any relevant data on antibiotic‐impregnated catheters.
-
Children (under 18 years) versus all ages
Systemic antibiotic prophylaxis. Subgroup analyses regarding the effect of prophylactic systemic antibiotics for internal shunts found a significant difference between treatment and control groups for both the subgroup of trials that included only children (OR: 0.52, 95% CI 0.33 to 0.83) and the subgroup of trials that included patients of all ages (OR: 0.52, 95% CI 0.30 to 0.90). No difference was found between the two subgroups (systemic antibiotics for children versus all ages; P = 0.06). (See 'Comparisons and data' table 04/01)
Antibiotic‐impregnated catheters. We did not find any relevant data on antibiotic‐impregnated catheters.
-
Periprocedural systemic antibiotics (fewer than 4 doses or for less than 24 hours postoperatively) versus continuous systemic antibiotics
Systemic antibiotic prophylaxis. We were able to include the 15 trials that used systemic prophylactic antibiotics for the subgroup analysis of prophylactic periprocedural systemic antibiotics versus continuous systemic antibiotics and found a significant difference (OR: 0.53, 95% CI 0.34 to 0.83; OR: 0.50, 95% CI 0.36 to 0.74 respectively). No benefit was found for the use of continuous antibiotics compared with periprocedural administration (P = 0.1). (See 'Comparisons and data' table 05/01)
Antibiotic‐impregnated catheters. Not applicable.
Sensitivity analysis
As planned in the protocol, we performed a sensitivity analysis for well allocated, versus poorly or unclearly allocated trials regarding the use of systemic antibiotics. We lacked data on mortality and so were unable to perform a sensitivity analyses on this outcome. We therefore performed these analyses for shunt infection which was the most frequently reported outcome. Class A trials: (Bullock 1988; Odio 1984; Rieder 1987; Schmidt 1985; Wang 1984; Young 1987; Zentner 1995) enrolled 736 patients (362 in the treatment group and 374 in the control group) and showed a non statistical significant effect for shunt infection regarding the use of systemic antibiotics (OR: 0.78, 95% CI 0.44 to 1.38). Class B and C trials: (Blomstedt 1985; Blum 1989; Djindjian 1986; Haines 1982; Rocca 1992; Walters 1992; Yogev 1985) enrolled 1000 patients (505 in the treatment group and 495 in the control group), found a statistical significant difference for shunt infection and for the other studied endpoints that favoured treatment group (OR: 0.41, 95% CI 0.26 to 0.65). (See 'Comparisons and data' table 02/04)
We also performed a separate analysis for placebo controlled trials versus standard care controlled trials for the most frequent outcome: shunt infection, regarding the use of systemic antibiotics in internal shunts, that showed a statistical significance only for placebo controlled trials (OR: 0.46, 95% CI 0.30 to 0.71; OR: 0.66, 95% CI 0.34 to 1.26 respectively). (See 'Comparisons and data' table 02/05)
Funnel plots for shunt infection regarding the use of systemic antibiotics did not suggest selective reporting of trials (Figure 1).
1.
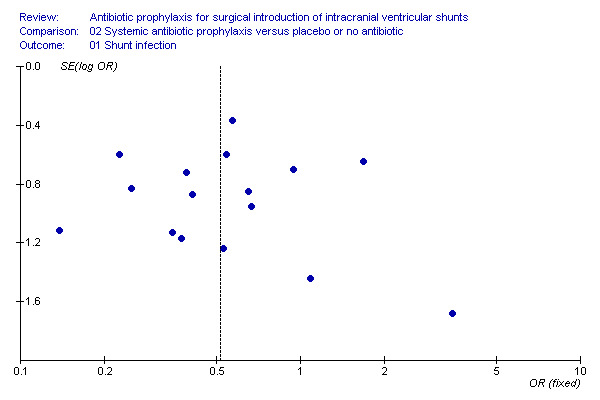
Funnel plot of trials of systemic antibiotics for shunt infection
Discussion
Quality of evidence
We included 17 RCTs that met our inclusion criteria that evaluated the use of prophylactic antibiotics in patients with surgical introduction of ventricular shunts. We have described above, the methodological shortcomings of these studies . For instance none of the included trials used an intention‐to‐treat analysis (ITT) and about half of the trials either did not describe the randomization method or it was inadequately described. Only seven of the studies were double‐blinded using identical appearance interventions (Blomstedt 1985; Bullock 1988; Haines 1982; Odio 1984; Rieder 1987; Walters 1992; Wang 1984). The attrition rate was about 5%. We were able to study a total of 2134 patients integrated in two meta‐analyses .
Systemic prophylactic antibiotics
We included 15 RCTs, that met our inclusion criteria, that supported the use of systemic prophylactic antibiotics for primary prevention of shunt infection. We were able to study a total of 1736 patients. Only one trial stated data relative to all‐cause mortality. Shunt infection was a primary outcome in all trials included. Only three trials evaluated shunt revision and two trials studied the adverse events of antibiotics.
Antibiotic‐impregnated catheters
Two trials compared antibiotic‐impregnated catheters with standard ones for primary prevention of shunt infections; one trial for external ventricular drains/shunts, and one for internal (ventriculoperitoneal) shunts. The difference in these two clinical situations is reflected in the duration of follow‐up: one week versus nine months, for the external versus the internal shunts respectively.
Methodology
We considered it appropriate to perform two meta‐analyses: one comparing systemic prophylactic antibiotic with placebo or no antibiotics and the second comparing antibiotic‐impregnated catheters with standard ones. Although we intended to test other endpoints beside shunt infection, such as all‐cause mortality, shunt revision, and adverse events of antibiotic, we were able only to include few trials in each one. We performed subgroup analyses for shunt infection whenever appropriate and where sufficient data was available.
We performed a sensitivity analyses that showed no significant differences for all endpoints when trials with poor or unclear allocation concealment were excluded. We combined trials that included different types of patients and with different follow‐up periods.
Findings
Only one trial evaluated the effect of systemic antibiotics in patients with external ventricular drains and included a small number of patients. No significant differences were found and no conclusions can be made for this particular type of ventricular shunt.
Based on subgroup analyses the benefit of systemic antibiotic prophylaxis was similar for children compared to a subgroup of patients constituted by patients of all ages, for ventriculoperitoneal shunts versus ventriculoatrial shunts and for periprocedural intravenous (IV) antibiotic therapy (given for less than 24 hours postoperatively), and continuous IV antibiotic therapy. Accordingly, administration of prophylactic antibiotics for more than 24 hours postoperatively does not have support. It would be desirable to have a head‐to‐head comparison of these two strategies. It should be emphasized that the lack of evidence of difference between the groups is consistent with there either being no difference between these groups in terms of effect size, or that there are too few people in the studies to allow a clear conclusion to be made due to lack of power.
The validity of the review may be weakened by the fact that when we performed the sensitivity analysis for adequate allocated trials regarding the use of systemic antibiotics that included seven RCTs, we found a non‐significant effect for shunt infection (OR: 0.78, 95% CI 0.44 to 1.38), as it occured with the remaining endpoints studied. However it is worth noticing the two subgroups maintain the same trend. The group of A trials is smaller which may account for the loss of statistical significance. In addition, all studies presented the data on a per‐protocol basis which may also inflate the effect size. This aspect may be particularly important in Walters 1992 and Rocca 1992. These two studies reported a high dropout rate (24/78 = 31% and 51/294 = 17% respectively) although the Rocca 1992 study had less weight (0.49%) and the Walters 1992 study is important but below the critical 20% level. Overall we believe that systemic antibiotics do have a beneficial effect but it is possible that the effect size is smaller than the one estimated in our review. We found two RCTs that compared the antibiotic‐impregnated shunts with the standard catheters.
Given the current data, it is possible to recommend the use of periprocedural systemic prophylactic antibiotic in patients undergoing surgical introduction of internal ventricular shunts; even though it was not possible to clearly evaluate the incidence of adverse effects of the antibiotics. The use of antibiotic‐impregnated catheters in ventricular shunts seems to be advantageous. This evidence is only limited by the low number of studies and participants; further well conducted RCTs are required to clarify this issue. However, our results suggest that the AIS are effective across populations: heterogeneity of populations across studies can make results more generalizable. The trials tested widely different antibiotics which have varied safety profiles. The impact of the actual antibiotics chosen could not be evaluated.
Authors' conclusions
Implications for practice.
This systematic review shows a significant difference between systemic prophylactic antibiotics and control for the prevention of shunt infection in patients with surgical introduction of internal ventricular shunts; regardless of the patient's age and the type of internal shunt used. Therefore its use in current practice is recommended even if there is sparse mortality data. Shunt infection was an intermediate outcome in this review and the type and dose of antibiotic needs to be optimized. Currently the available evidence only suggests a benefit for the use of prophylactic antibiotics for the first 24 hours postoperatively. The benefit of its use after the first 24 hours postoperatively remains uncertain.
No conclusions can be reached regarding the administration of prophylactic antibiotics for external ventricular drains. The evidence suggests that antibiotic‐impregnated catheters reduce the incidence of shunt infection although no strong recommendation can be made given the current data available.
Implications for research.
Future RCTs should study relevant outcomes such as mortality, antibiotics' adverse events and shunt revision. It should also be mandatory to evaluate the effectiveness of systemic prophylactic antibiotics for external ventricular drains, and the effectiveness of different regimens of systemic prophylactic antibiotics rather than placebo for internal ventricular shunts; namely the duration of the administration. More appropriately designed randomized controlled trials which test the effect of antibiotic‐impregnated shunts are required in order to establish whether or not there is a net benefit of this medication.
What's new
| Date | Event | Description |
|---|---|---|
| 1 September 2008 | Amended | Converted to new review format. |
Acknowledgements
We would like to thank Dr Harald Herkner, Jane Cracknell, Dr Andrea Nelson, Dr Jeffrey J. Olson, Dr Thomas Sycha, Dr Joseph M. Zabramski, Prof. Marcus Müllner, Janet Wale and Nete Villebro for their help and editorial advice during the preparation of this review and to Karen Hovhannisyan for conducting some of the electronic searches.
Appendices
Appendix 1. MEDLINE and CENTRAL
| Number | Search |
| 1. | exp Anti‐Infective Agents/ |
| 2. | Antibiotic Prophylaxis/ |
| 3. | antibiotic$.tw |
| 4. | antimicrob$.tw |
| 5. | antibacter$.tw |
| 6. | Bacteriocid$.tw |
| 7. | OR/1‐6 |
| 8. | (brain or intracran$ or CNS or (nerv$ and system)).tw |
| 9. | exp Central Nervous System Infections/ |
| 10. | exp Infection/ |
| 11. | infect$.tw |
| 12. | meningit$.tw |
| 13. | Empyem$ and subdura$.tw |
| 14. | Encephalit$.tw |
| 15. | Meningoencephalit$.tw |
| 16. | Encephalomyelit$.tw |
| 17. | Arachnoidit$.tw |
| 18. | Subdura$ and effusi$.tw |
| 19. | Ventriculit$.tw |
| 20. | Ventricul$ and debri$.tw |
| 21. | Peritonitis/ |
| 22. | Peritonit$.tw |
| 23. | Abdomin$ and abscess$.tw |
| 24. | Septicem$.tw |
| 25. | bacteriem$.tw |
| 26. | OR /8‐25 |
| 27. | Catheters, Indwelling/ |
| 28. | .Catheter$.tw |
| 29. | Impregnat$ and (cathet$ or devic$).tw |
| 30. | exp Cerebrospinal Fluid Shunts / |
| 31. | exp Cerebral Ventricles/ |
| 32. | Injections, Intraventricular/ |
| 33. | Cerebrospinal Fluid/ |
| 34. | exp Intracranial Hypertension/ |
| 35. | Hydrocephal$.tw |
| 36. | CSF and shunt$.tw |
| 37. | cerebrospin$ and Shunt$.tw |
| 38. | Ventriculostom$.tw |
| 39. | Ventricul$ and Shunt$.tw |
| 40. | Ventriculoperiton$ and Shunt$.tw |
| 41. | Ventriculoatria$ and Shunt$.tw |
| 42. | ventricul$ and drain$.tw |
| 43. | Ventriculocisternost$.tw |
| 44. | Ventriculopleura$.tw and Shunt$.tw |
| 45. | Bladder$.tw and Shunt$.tw |
| 46. | Ureter$.tw and Shunt$.tw |
| 47. | Gall bladder$.tw and Shunt$.tw |
| 48. | Sinus$.tw and Shunt$.tw |
| 49. | OR /27‐48 |
| 50. | 7 AND 26 AND 49 |
| 51. | limit 50 to human |
Appendix 2. EMBASE search strategy
| Number | Search terms |
| 1. | explode "antiinfective‐agent" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 2. | explode "antibiotic‐prophylaxis" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 3. | antibiotic* |
| 4. | antimicrob* |
| 5. | antibacter* |
| 6. | Bacteriocid* |
| 7. | #1 or #2 or #3 or #4 or #5 or #6 |
| 8. | explode "indwelling‐catheter" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 9. | Catheter* |
| 10. | Impregnat* and (cathet* or devic*) |
| 11. | brain or intracran* or CNS or (nerv* and system) |
| 12. | #8 or #9 or #10 |
| 13. | #11 and #12 |
| 14. | #7 or #13 |
| 15. | explode "central‐nervous‐system‐infection" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 16. | explode "infection‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 17. | infect* |
| 18. | meningit* |
| 19. | Empyem* and subdura* |
| 20. | Encephalit* |
| 21. | Meningoencephalit* |
| 22. | Encephalomyelit* |
| 23. | Arachnoidit* |
| 24. | Subdura* and effusi* |
| 25. | Ventriculit* |
| 26. | Ventricul* and debri* |
| 27. | "peritonitis‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 28. | Peritonit* |
| 29. | Abdomin* and abscess* |
| 30. | Septicem* |
| 31. | bacteriem* |
| 32. | #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 |
| 33. | explode "cerebrospinal‐fluid" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 34. | explode "brain‐ventricle" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 35. | "intracerebroventricular‐drug‐administration" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 36. | "cerebrospinal‐fluid" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 37. | explode "intracranial‐hypertension" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 38. | Hydrocephal* |
| 39. | CSF and shunt |
| 40. | cerebrospin* and Shunt* |
| 41. | Ventriculostom* |
| 42. | Ventricul* and Shunt* |
| 43. | Ventriculoperiton* and Shunt* |
| 44. | Ventriculoatria* and Shunt* |
| 45. | ventricul* and drain* |
| 46. | Ventriculocisternost* |
| 47. | Ventriculopleura* and Shunt* |
| 48. | Bladder* and Shunt* |
| 49. | Ureter* and Shunt* |
| 50. | Gall bladder* and Shunt* |
| 51. | Sinus* and Shunt* |
| 52. | #33 or #4 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 |
| 53. | #14 and #32 and #52 |
| 54. | explode shunt‐infection/ all subheadings |
| 55. | Shunt revision |
| 56. | infection* near ((shunt equipment*) or (overlying wound) or CSF or (distal drainage route)) |
| 57. | explode adverse‐drug‐reaction/ all subheadings |
| 58. | #54 or #55 or #56 or #57 |
| 59. | #53 and #58 |
| 60. | explode "randomized‐controlled‐trial" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 61. | (randomi?ed controlled trial*) in TI, AB |
| 62. | random* |
| 63. | explode "randomization‐" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 64. | randomi?ation |
| 65. | explode "clinical‐trial" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 66. | explode multicenter‐study / all subheadings |
| 67. | multi?cent* |
| 68. | explode phase‐4‐clinical‐trial / all subheadings or explode double‐blind‐procedure / all subheadings or explode single‐blind‐procedure / all subheadings |
| 69. | (RANDOM* or CROSS?OVER* or FACTORIAL* or PLACEBO* or VOLUNTEER*) in TI, AB, TW |
| 70. | ((SINGL* or DOUBL* or TREBL* or TRIPL*) near (BLIND* or MASK*)) in TI,AB |
| 71. | explode "follow‐up" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 72. | (follow?up near stud*) in TI, AB |
| 73. | evaluation stud* |
| 74. | explode "prospective‐study" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 75. | prospective?stud* |
| 76. | research near design* |
| 77. | explode "comparative‐study" / all SUBHEADINGS in DEM,DER,DRM,DRR |
| 78. | clinic* near trial* |
| 79. | #60 or #61 or #62 or #63 or #62 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 |
| 80. | (human) in DER |
| 81. | (animal or nonhuman) in DER |
| 82. | #80 and #81 |
| 83. | #81 not #82 |
| 84. | #79 not #83 |
| 85. | #84 and #59 |
Appendix 3. LILACS search strategy
| Number | key words |
| 1. | ant$ AND prophylaxis |
| 2. | ventriculoperit$ |
| 3. | cns AND infect$ |
| 4. | ventricul$ AND infect$ |
| 5. | hydroceph$ |
| 6. | impregnat$ |
| 7. | csf AND shunt$ |
| 8. | cerebrosp$ AND shunt$ |
| 9. | infect$ AND shunt |
| 10. | randomized OR randomised |
Data and analyses
Comparison 1. Antibiotic‐impregnated versus standard catheters.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality | 2 | 398 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.83, 2.62] |
| 1.1 Internal shunts | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.48, 9.31] |
| 1.2 External shunts | 1 | 288 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.74, 2.58] |
| 2 Shunt infection | 2 | 398 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.08, 0.55] |
| 2.1 Internal shunts | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.08, 1.23] |
| 2.2 External shunts | 1 | 288 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.03, 0.60] |
| 3 Shunt revision | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.26, 1.67] |
| 3.1 Internal shunts | 1 | 110 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.26, 1.67] |
1.1. Analysis.
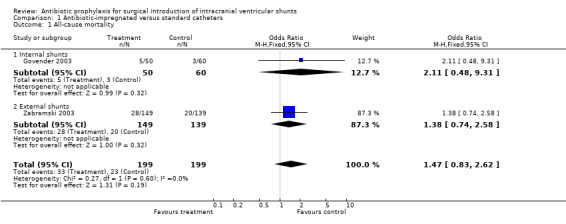
Comparison 1 Antibiotic‐impregnated versus standard catheters, Outcome 1 All‐cause mortality.
1.2. Analysis.

Comparison 1 Antibiotic‐impregnated versus standard catheters, Outcome 2 Shunt infection.
1.3. Analysis.

Comparison 1 Antibiotic‐impregnated versus standard catheters, Outcome 3 Shunt revision.
Comparison 2. Systemic antibiotic prophylaxis versus placebo or no antibiotic.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Shunt infection | 15 | 1736 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.36, 0.74] |
| 1.1 Internal shunts | 15 | 1684 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.36, 0.73] |
| 1.2 External shunts | 1 | 52 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.06, 18.30] |
| 2 Shunt revision | 3 | 248 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.42, 1.36] |
| 2.1 Internal shunts | 3 | 248 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.42, 1.36] |
| 3 Any adverse events | 2 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.44 [1.02, 371.30] |
| 3.1 Internal shunts | 2 | 166 | Odds Ratio (M‐H, Fixed, 95% CI) | 19.44 [1.02, 371.30] |
| 4 Shunt infection (sensitivity analysis) | 15 | 1736 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.37, 0.74] |
| 4.1 Class A studies | 7 | 736 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.44, 1.38] |
| 4.2 Class B + C studies | 8 | 1000 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.26, 0.65] |
| 5 Shunt infection (internal shunts) | 15 | 1684 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.36, 0.73] |
| 5.1 Placebo controlled trials | 9 | 1051 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.30, 0.71] |
| 5.2 Standard care controlled trials | 6 | 633 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.34, 1.26] |
2.1. Analysis.

Comparison 2 Systemic antibiotic prophylaxis versus placebo or no antibiotic, Outcome 1 Shunt infection.
2.2. Analysis.

Comparison 2 Systemic antibiotic prophylaxis versus placebo or no antibiotic, Outcome 2 Shunt revision.
2.3. Analysis.

Comparison 2 Systemic antibiotic prophylaxis versus placebo or no antibiotic, Outcome 3 Any adverse events.
2.4. Analysis.

Comparison 2 Systemic antibiotic prophylaxis versus placebo or no antibiotic, Outcome 4 Shunt infection (sensitivity analysis).
2.5. Analysis.

Comparison 2 Systemic antibiotic prophylaxis versus placebo or no antibiotic, Outcome 5 Shunt infection (internal shunts).
Comparison 3. VA versus VP shunts (subgroup analysis for systemic antibiotics).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Shunt infection | 12 | 1416 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.35, 0.76] |
| 1.1 Ventriculoatrial shunts | 3 | 328 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.23, 0.94] |
| 1.2 Ventriculoperitoneal shunts | 10 | 1088 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.34, 0.85] |
3.1. Analysis.

Comparison 3 VA versus VP shunts (subgroup analysis for systemic antibiotics), Outcome 1 Shunt infection.
Comparison 4. Children versus all ages (subgroup analysis for sytemic antibiotics).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Shunt infection | 15 | 1736 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.37, 0.74] |
| 1.1 Children | 8 | 957 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.33, 0.83] |
| 1.2 All ages | 7 | 779 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.30, 0.90] |
4.1. Analysis.

Comparison 4 Children versus all ages (subgroup analysis for sytemic antibiotics), Outcome 1 Shunt infection.
Comparison 5. Periprocedural versus continuous antibiotics (subgroup analysis for systemic antibiotics).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Shunt infection | 15 | 1736 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.36, 0.74] |
| 1.1 Periprocedural antibiotics | 12 | 1177 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.34, 0.83] |
| 1.2 Continuous antibiotics | 4 | 559 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.28, 0.90] |
5.1. Analysis.

Comparison 5 Periprocedural versus continuous antibiotics (subgroup analysis for systemic antibiotics), Outcome 1 Shunt infection.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bayston 1975.
| Methods | Randomized, controlled, not blinded. Method of randomization: not specified. Location: single centre in UK. Duration: Not specified. | |
| Participants | Inclusion criteria: patients (children) undergoing shunt surgery. Exclusion criteria: not specified. Characteristics of each group were not described. 132 patients completed the trial (treatment group n = 34+20, control group n = 78). Baseline for variables between groups were not performed. | |
| Interventions | Patients were randomized to one of two groups: 1st phase ‐ control group (no AB) and AB group (cloxacillin IM 1 hour before operation and 6 hours after); 2nd phase ‐ control group and AB group (gentamicin IM 1 hour before operation and 6 hours after). | |
| Outcomes | Primary outcome events considered were wound infection and colonized shunts (organisms isolated from wounds and shunts). Period of observation not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Blomstedt 1985.
| Methods | Randomized, placebo‐controlled, double‐blinded. Method of randomization: not specified. Location: single centre in Finland. Duration: 39 months. | |
| Participants | Inclusion criteria: All patients (above 12 years) undergoing a ventriculoatriostomy or an external ventriculostomy Exclusion criteria: patients under 12 years, allergy to sulfa or trimethoprim, patients who had received antibiotics during the preceding week, and patients who had already had a shunt in place. 174 patients were enrolled in the trial (62 in group 1, 60 in group 2, 25 in group 3 and 27 in group 4); 26 were excluded (19 regimen was not completed, 6 received other AB, 1 suspected allergic reaction). Age, sex, incidence of malignant tumours were similar in the two treatment groups. | |
| Interventions | For shunting procedures: Group 1 (trimethoprim 90 mg ‐sulphametoxazole 400 mg x 3 doses 12‐hourly; Group 2 (placebo) For external ventriculostomies: Group 3 (trimethoprim 90mg ‐sulphametoxazole 400mg 12‐hourly until drainage tube removal); Group 4 (placebo). | |
| Outcomes | Primary outcome event was shunt infection (bacteria isolated from cultures from blood, shunt catheter, or CSF whenever shunt infection was suspected). Minimum follow‐up period of 6 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Blum 1989.
| Methods | Randomized, placebo‐controlled, single blinded. Method of randomization: date of birth Location: single centre in Germany. Duration: 21 months. | |
| Participants | Inclusion criteria: All patients (children up to 14 years) operated on for hydrocephalus. Exclusion criteria: hypersensitivity to the drug selected, antibiotic therapy in the previous 7 days or another antibiotic in the posterior 10 days, concurrent infections, immunosuppressive therapy or immunodeficiency, agammaglobulinemia, agranulocytosis, leukemia, severe anemia, lymphoma, thymus aplasia, liver cirrhosis, burns, uremia, diabetes mellitus and factors which impairs wound healing. From 169 patients, 100 patients meet inclusion criteria (AB group n = 50, control group n = 50). Age and sex were similar in the two treatment groups. | |
| Interventions | Patients were randomized to one of two groups: treatment group (single‐dose prophylaxis with 50mg/kg body‐weight of cefazedone) or placebo group. | |
| Outcomes | Primary outcome event was shunt infection (diagnosis on microbiological and clinical observations and investigations, such as local signs of infection, pathogen detection, germ counts, and antibiograms. Classical infection parameters played only a secondary role.) Follow‐up period lasted 8 weeks. | |
| Notes | Author contacted but no further data was provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Bullock 1988.
| Methods | Randomized, placebo‐controlled, double‐blinded Method of randomization: random number list. Location: single centre in South Africa. Duration: 2 years. | |
| Participants | Inclusion criteria: patients (regardless of age) undergoing elective neurosurgical procedure. Exclusion criteria: known piperacillin hypersensitivity or allergy, neurosurgical operation in the previous month, any AB therapy within 7 days prior the study. 417 patients were enrolled in the trial in which 104 were ventriculoperitoneal shunts (treatment group n = 48, control group n = 56). Treatment and placebo groups were well matched for age, sex, hospital stay, operation time and duration of vacuum drainage. | |
| Interventions | Patients were randomized to one of two groups: treatment group (piperacillin 2g IV commenced between 30 and 60 minutes prior to the surgical incision and two further doses 6‐ hourly) and placebo group. | |
| Outcomes | Primary outcome events were sepsis (purulent discharge from a wound, meningitis verified by a low sugar and high polymorphonuclear lymphocyte count in the CSF or when pure culture of organisms from a wound exudate when redness was present). Follow‐up period of 90 days. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Djindjian 1986.
| Methods | Randomized, controlled, not blinded. Method of randomization: Not specified. Location: single centre in France Duration: 27 months. | |
| Participants | Inclusion criteria: patients (regardless of age) receiving first implantation of shunt. Exclusion criteria: no AB in the previous 14 days, previous ventricular drainage, replacement of a previously implanted shunt. Treatment group n = 30, control group n = 30. Baseline for variables between groups were not performed. | |
| Interventions | Patients were randomized to one of two groups: treatment group (oxacillin 200mg /kg/day with an IV administration every 4 hours for 24 hours (6 x 2g for an adult) and control group. | |
| Outcomes | Primary outcome event was CSF infection (meningitis or abscess of the wall with meningeal reaction and CSF cell count was clearly suggestive of infection, even when bacteria could not be isolated form the CSF or the shunt system). Minimum follow‐up period of 6 months. | |
| Notes | Author contacted but no further data provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Govender 2003.
| Methods | Randomized, controlled, single blind. Method of randomization: not specified. Location: 2 centre, South Africa and UK. Duration: Not specified. | |
| Participants | Inclusion criteria: Patients (regardless of age) with hydrocephalus who had been identified primarily as uninfected. Exclusion criteria: CSF infection before or at the time of shunt insertion, pregnancy or lactancy, known sensitivity to rifampicin or clindamycin, or open and uncorrected myelomeningocele or encephalocele. 153 patients were enrolled, 43 were later excluded (19 deaths before appropriate follow‐up period, 7 protocol violations, 2 CSF infection at the time of the shunt insertion, 15 did not return for follow‐up); AIS group n = 50, control group n = 60. No significant difference in the median follow‐up period between groups. | |
| Interventions | Patients were randomized to one of two groups: AIS group (patients receiving an AIS with clindamycin and rifampicin) or a control group (patients receiving a standard unishunt system that lacked any antibiotic agent). All patients were given cephalosporin IV at induction of anaesthesia and 3 postoperative doses given 8‐hourly. | |
| Outcomes | Primary outcome events were shunt infection (evidence of infection on shunt equipment, overlying wound, CSF, distal drainage route, or site related to the ventriculoperitoneal shunt) and shunt revision procedure (due to infection or non infectious causes). Median follow‐up period of 9 months (range 1‐20 months). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Haines 1982.
| Methods | Randomized, placebo‐controlled, double‐blinded. Method of randomization: coin toss. Location: single centre in USA. Duration: 1 year. | |
| Participants | Inclusion criteria: Children ( under 17 years) for insertion or revision of CSF shunts. Exclusion criteria: allergy to penicillin, patients who were on antibiotic therapy. 76 patients were enrolled on the trial. 2 were excluded due to CSF infection at the time of operation. Treatment group n = 35, placebo group n = 39. The 2 groups were well matched for age, sex, previous shunt infection, presence of spina bifida, having first shunt placement, CSF glucose, protein or WBC values. | |
| Interventions | Patients were randomized to one of two groups: antibiotic group (methicillin 12,5mg/kg every 6 hours beginning 6 hours before the operation, during the induction of anaesthesia, and continuing for a total of 72 hours) and placebo group (placebo in an identical regimen). | |
| Outcomes | Primary outcome events were shunt infection (required a positive CSF culture except when there was gross purulence along the shunt tract, organisms were seen on gram‐stain of CSF smears or the CSF protein, glucose and cell count were felt to be indicative of infection) and shunt malfunction (clinical criteria and almost always confirmed by CT scan). Follow‐up period of 6 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Odio 1984.
| Methods | Randomized, placebo‐controlled, double‐blinded. Method of randomization: central computer‐generated list of random numbers. Location: 2 centres, Costa Rica and USA. Duration: 5 months. | |
| Participants | Inclusion criteria: Children undergoing shunt procedures. Exclusion criteria: laboratory evidence of hepatic or renal disease, antibiotics received in the previous 72 hours. 37 patients were enrolled in the study. 2 were excluded because of adverse reactions to AB in the first dose. Treatment group n = 18, placebo group n = 17. Baseline for variables between treatment and placebo groups were not performed. | |
| Interventions | Patients were randomized to one of two groups: treatment group (vancomycin 15mg/kg IV one hour before surgery and 6 hours later) and placebo group (placebo in an equivalent volume given at the same time. | |
| Outcomes | Primary outcome events were shunt infection (based on examination of CSF that included a Gram‐stained smear, culture, cell count and protein and glucose determination whenever shunt infection was suspected on the basis of shunt malfunction, fever, nausea, or vomiting) and adverse effects of vancomycin. Follow‐up period of 7‐12 months. | |
| Notes | Author contacted but no further data provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rieder 1987.
| Methods | Randomized, placebo‐controlled, double‐blinded. Method of randomization: envelope draw system. Location: single‐centre in UK. Duration: 4 years. | |
| Participants | Inclusion criteria: Children who presented for elective ventriculoperitoneal shunt insertion. Exclusion criteria: evidence of active infection, history of shunt infection, immunosuppressed, corticosteroid therapy, allergies to penicillin or cephalothin, antibiotic therapy in the 4 weeks before shunt insertion. Treatment group n = 31, placebo group n = 32. The 2 groups were well matched for age, sex, history of CSF infection and duration of surgery. | |
| Interventions | Patients were randomised to one of two groups: treatment group (cephalothin 25mg/kg or 2g maximum IV before incision and 3 doses 6‐hourly) and placebo group (multivitamin preparation of the same colour and administered as the same regimen). | |
| Outcomes | Primary outcome events were shunt infection (if there were symptoms suggestive of shunt infection including irritability, reddening of the skin overlying the shunt tubing or unexplained fever and culture of CSF yielded bacterial growth) and shunt malfunction. Follow‐up period of 3 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Rocca 1992.
| Methods | Randomized, controlled. Method of randomization: not specified. Location: single centre in France. Duration: 2 years. | |
| Participants | Inclusion criteria: Patients (regardless of age) submitted to craniotomy or shunt placement. Exclusion criteria: infection or antibiotherapy in the previous 15 days, allergy to B‐lactams, urgent surgeries and reinterventions. 78 patients were enrolled in the study, in which 27 were shunt procedures (treatment group n = 13, control group n = 14). Baseline for variables between treatment and control groups were not performed. | |
| Interventions | Patients were randomized to one of two groups: treatment group (cefamandole 1.5g IV was given 1 hour before surgery and 3 doses 8‐hourly) and control group (no antibiotic). | |
| Outcomes | Primary outcome event were local and remote infections (wound discharge, leucytosis and fever associated with appropriate positive germ culture). Follow‐up period for 15 days. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Schmidt 1985.
| Methods | Randomized, controlled, not blinded. Method of randomization: random numbers Location: 2 centre in Denmark. Duration: 18 months. | |
| Participants | Inclusion criteria: Patients (regardless of age) who presented for shunt insertion or shunt revision. Exclusion criteria: no antibiotic had been given or signs of infection in the preceding 4 weeks. 152 patients were enrolled in the study, none were excluded (antibiotic group n = 79, control group n = 73) The 2 groups were well matched for age, sex, etiology of hydrocephalus, observation time, method of shunt and duration of the operation. | |
| Interventions | Patients were randomized to one of two groups: antibiotic group (methicillin totally 200mg/kg divided in 6 doses during 24 hours starting at the induction of anaesthesia) and control group (no antibiotic). | |
| Outcomes | Primary outcome event was shunt infection (when patient showed clinical signs of wound infection, septicaemia in patients with VA shunts, peritonitis in patients with VP shunts, or meningitis and if bacteria could be cultivated from blood, peritoneum, CSF, or the shunt system). Follow‐up period for at least 6 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Walters 1992.
| Methods | Randomized, placebo‐controlled, double‐blinded. Method of randomization: not specified. Location: single centre in Canada. Duration: 44 months. | |
| Participants | Inclusion criteria: Children undergoing CSF shunt procedure. Exclusion criteria: shunt infection, concomitant systemic infection, allergies to rifampicin or trimethoprim. 294 patients were enrolled in which 51 were withdrawal (15 lost to follow‐up and 36 infected at the time of initial surgery); Treatment group n = 130, placebo group n = 113. Baseline for variables between treatment and placebo groups were not performed. | |
| Interventions | Patients were randomized to one of two groups: treatment group (20mg/Kg rifampicin and 5mg/kg trimethoprim per day in divided doses, the first 2 hours preoperatively and 8‐hourly for further 48 hours) and placebo group. | |
| Outcomes | Primary outcome event was shunt infection (patients who required additional surgery for malfunction and were found at operation to have positive microbiological cultures from shunt tubing and/or CSF and those patients who presented with a reddened and oedematous shunt tract, pus from the wound, meningitis, or other systemic forms of infection and verified by positive cultures). Follow‐up period of 2 years. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wang 1984.
| Methods | Randomized, placebo‐controlled, double‐blinded. Method of randomization: table of random numbers. Location: single centre in Canada. Duration: 30 months. | |
| Participants | Inclusion criteria: Children undergoing ventriculoperitoneal shunt surgery. Exclusion criteria: shunt infection in the preceding month, antibiotic therapy within the previous week, allergy to trimethoprim or sulfamethoxazole. 127 patients were enrolled in the study. 7 withdrawals (unsuspected VP shunt infection diagnosed at the time of surgery); Treatment group n = 55, placebo group n = 65. The 2 groups were well matched for age, sex, history of shunt infection and duration of the operation. | |
| Interventions | Patients were randomized to one of two groups: treatment group (5mg/kg in combination with 25mg/kg of sulfamethoxazole IV during the hour preceding the surgery and 2 more doses 8‐hourly) and placebo group (equivalent volumes of 5%dextrose in water administered in an identical fashion. | |
| Outcomes | Primary outcome events were shunt infection (when bacteria were recovered from CSF or VP catheter specimens) or shunt malfunction. Duration of follow‐up was from the time of shunt surgery until the last patient contact. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Yogev 1985.
| Methods | Randomized, placebo‐controlled. Method of randomization: not specified. Location: single centre in USA. Duration: not specified. | |
| Participants | Inclusion criteria: all patients (children) undergoing ventriculoperitoneal shunt replacement or revision. Exclusion criteria: not specified. 190 patients were enrolled in the trial, treatment group n = 106 and placebo group n = 84). | |
| Interventions | Patients were randomized to one of two groups: treatment group (nafcillin alone or nafcillin and rifampicin 200mg/kg per day and 20mg/kg per day started 13 hours preoperatively and continued for 48 hours) and placebo group (saline placebo). | |
| Outcomes | Primary outcome event was shunt infection (shunt infection criteria was not described). Follow‐up period for 6 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Young 1987.
| Methods | Randomized, controlled, single blinded. Method of randomization: table random of numbers. Location: multicentre (3 centres), USA Duration: 56 months. | |
| Participants | Inclusion criteria: clean neurosurgical procedure (regardless age) Exclusion criteria: evidence of any concurrent infection, use of AB for any reason in the prior week, history of allergic reactions to cephalosporin or aminoglycosides. 846 patients were enrolled in the study, 16 were excluded (8 in each group and none had infection); reasons: 4 used AB in the prior week, 2 had inadvertent administration of AB following surgery, 4 had unsuspected brain abscess, 1 preoperative infection and 5 had inability to verify that the protocol was administered according to protocol. 133 patients underwent shunt placement (64 in the treatment group and 69 in the control group). The 2 groups were well matched for sex, and duration of the procedure. | |
| Interventions | Patients were randomized to one of two groups: treatment group (cefazolin 1g and gentamicin 80mg or 1mg/kg and 25mg/kg in paediatric patients respectively, IV, 1 hour before the incision and repeated every 6 hours as long as the operation procedure continued) and a control group (no AB). | |
| Outcomes | Primary outcome event was postoperative infection (wound infection, meningitis or ventriculitis confirmed with appropriate cultures). Minimum follow‐up period of 1 year. | |
| Notes | Author contacted but no further data was provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Zabramski 2003.
| Methods | Randomized, controlled. Method of randomization: central automated system. Location: multicentre (6 centres) in USA. Duration: 27 months. | |
| Participants | Inclusion criteria: All hospitalized patients (18 years or older) who required placement of external ventricular drain. Exclusion criteria: pregnancy, allergies to tetracycline or rifampicin, dermatitis or infection at the catheter insertion site, CSF shunt within the previous 30 days, known or suspected CSF infection, need for placement of more than one ventricular catheter, and uncorrected coagulopathy. 306 patients were enrolled in the study, in which 288 were included in the final analysis (14 excluded because ventricular catheter remained in place for less than 24 hours, 4 because of initial CSF culture revealed infection). Treatment group n = 149, control group n = 139. The 2 groups were well matched for age, sex, indication for catheter placement and length of time the catheter remained in place. | |
| Interventions | Patients were randomized to one of two groups: treatment group (catheter impregnated with minocycline and rifampicin) and control group (standard non‐impregnated silicone catheter). Most patients received a second‐generation cephalosporin begun before the catheter is placed and continued until the catheter is removed. | |
| Outcomes | Primary outcome event was CSF infection (positive CSF culture if the same organism grew on two different media or grew in the same medium twice). Follow‐up period of 1 week after catheter removal. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Zentner 1995.
| Methods | Randomized, controlled. Method of randomization: computerized lists. Location: single centre in Germany. Duration: 1 year. | |
| Participants | Inclusion criteria: All patients (regardless of age) undergoing CSF shunting. Exclusion criteria: Not specified. Treatment group n = 67, control group n = 62. The 2 groups were well matched for age, etiology of hydrocephalus, shunting procedure, shunt type, risk factors, duration of operation, and duration of preoperative hospitalization. | |
| Interventions | Patients were randomized to one of two groups: treatment group (cefotiam IV in a single dose 50mg/kg for children and 2g for adults after induction of anaesthesia and before the skin incision) and control group (no antibiotics). | |
| Outcomes | Primary outcome event was shunt infection (presence of clinical symptoms, elevated cell counts, and/or bacterial contamination of CSF requiring shunt removal and AB therapy). Follow‐up period of 6 months after surgery. | |
| Notes | Author contacted but no further data was provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
AB = antibiotic IM = intramuscularly mg = milligram g = gram IV = intravenously CSF = cerebrospinal fluid AIS = antibiotic‐impregnated system WBC = white blood cell kg = kilogram CT = computerized tomography VA = ventriculoatrial VP = ventriculoperitoneal
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Epstein 1982 | Randomized controlled trial comparing the use of both systemic and intraventricular cephalothin for 72 hours with placebo. 77 patients were enrolled in the trial. There were 1/39 shunt infections in the treatment group and 8/38 shunt infections in the control group. |
| Shapiro 1986 | Randomized controlled double‐blinded trial comparing the use of periprocedural systemic vancomycin and gentamycin with placebo in all patients undergoing neurosurgery. There was one case of infection in patients undergoing ventricular shunts but it was not specified in which group it occurred. Further data was unavailable from the author. |
| Siqueira 1987 | Randomized controlled trial comparing intra‐operative systemic antibiotics as a factor in reducing the incidence of wound infection in clean procedures. Author divided patients in four groups: cranial surgery with implants (71 patients); cranial surgery without implants (54 patients); spinal surgery (54 patients); and peripheral nerve surgery (23 patients). The study mentioned that the subgroup of patients submitted to CSF shunting was included in the cranial surgery with implants group but details on the number of patients, and the number of infections in this subgroup were not given. Further data were unavailable from the author. |
Contributions of authors
All correspondence: Bernardo Ratilal (BORL).
Drafting of review versions: BORL, Joao Costa (JOAC) and Cristina Sampaio (SCRI).
Searching for trials: BORL and JOAC.
Obtaining copies of trial reports: BORL and JOAC.
Selection of trials for inclusion and exclusion: BORL, JOAC and SCRI.
Extraction of data: BORL and JOAC.
Entry of data (in Review Manager (RevMan 4.2)): BORL and JOAC.
Interpretation of data analyses: BORL, JOAC and SCRI.
Sources of support
Internal sources
Clinical Therapeutics Institute, Lisbon Faculty of Medicine, Portugal.
External sources
No sources of support supplied
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Bayston 1975 {published data only}
- Bayston R. Antibiotic prophylaxis in shunt surgery. Developmental Medicine and Child Neurology Supplement 1975;35:99‐103. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blomstedt 1985 {published data only}
- Blomstedt GC. Results of trimethoprim‐sulfamethoxazole prophylaxis in ventriculostomy and shunting procedures. A double‐blind randomized trial. Journal of Neurosurgery 1985;62(5):694‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Blum 1989 {published data only}
- Blum J, Schwarz M, Voth D. Antibiotic single‐dose prophylaxis of shunt infections. Neurosurgical Review 1989;12(3):239‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bullock 1988 {published data only}
- Bullock R, Dellen JR, Ketelbey W, Reinach SG. A double‐blind placebo‐controlled trial of perioperative prophylactic antibiotics for elective neurosurgery. Journal of Neurosurgery 1988;69(5):687‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Djindjian 1986 {published data only}
- Djindjian M, Fevrier MJ, Otterbein G, Soussy JC. Oxacillin prophylaxis in cerebrospinal fluid shunt procedures: results of a randomized open study in 60 hydrocephalic patients. Surgical Neurology 1986;25(2):178‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Govender 2003 {published data only}
- Govender ST, Nathoo N, Dellen JR. Evaluation of an antibiotic‐impregnated shunt system for the treatment of hydrocephalus. Journal of Neurosurgery 2003;99(5):831‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Nathoo N, Govender ST, Barnett GH, Dellen JR. Evaluation of an antimicrobial impregnated shunt system for the treatment of hydrocephalus. Presented at the American Association of Neurological Surgeons 2004 Annual Meeting. 2004. [19604 (www.aans.org)]
Haines 1982 {published data only}
- Haines SJ, Taylor F. Prophylactic methicillin for shunt operations: effects on incidence of shunt malfunction and infection. Child's Brain 1982;9(1):10‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Odio 1984 {published data only}
- Odio C, Mohs E, Sklar FH, Nelson JD, McCracken GH. Adverse reactions to vancomycin used as prophylaxis for CSF shunts procedures. American Journal of Diseases of Children 1984;138(1):17‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rieder 1987 {published data only}
- Rieder MJ, Frewen TC, Maestro RF, Coyle A, Lovell S. The effect of cephalothin prophylaxis on postoperative ventriculoperitoneal shunt infections. Canadian Medical Association Journal 1987;36(9):935‐8. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Rocca 1992 {published data only}
- Rocca B, Mallet MN, Scemama F, Malca S, Chevalier A, Gouin F. Perioperative remote infections in neurosurgery. Role of antibiotic prophylaxis [Infections à distance du foyer opératoire en neurochirurgie. Rôle de l'antibioprophylaxie]. La Presse Médicale 1992;21(42):2037‐40. [MEDLINE: ] [PubMed] [Google Scholar]
Schmidt 1985 {published data only}
- Schmidt K, Gjerris F, Osgaard O, Hvidberg EF, Kristiansen JE, Dahlerup B, et al. Antibiotic prophylaxis in cerebrospinal fluid shunting: a prospective randomized trial in 152 hydrocephalic patients. Neurosurgery 1985;17(1):1‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Walters 1992 {published data only}
- Walters BC, Goumnerova L, Hoffman HJ, Hendrick EB, Humphreys RP, Levinton C. A randomized controlled trial of perioperative rifampin/trimethoprim in cerebrospinal fluid shunt surgery. Child's Nervous System 1992;8(5):253‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wang 1984 {published data only}
- Wang EE, Prober CG, Hendrick BE, Hoffman HJ, Humphreys RP. Prophylactic sulfamethoxazole and trimethoprim in ventriculoperitoneal shunt surgery. A double‐blind, randomized, placebo‐controlled trial. JAMA 1984;251(9):1174‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Yogev 1985 {published data only}
- Yogev R. Cerebrospinal fluid shunt infections: a personal view. The Pediatric Infectious Disease Journal 1985;4(2):113‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Young 1987 {published data only}
- Young RF, Lawner PM. Perioperative antibiotic prophylaxis for prevention of postoperative neurosurgical infections. A randomized clinical trial. Journal of Neurosurgery 1987;66(5):701‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zabramski 2003 {published data only}
- Zabramski JM, Whiting D, Darouiche RO, Horner TG, Olson J, Robertson C, et al. Efficacy of antimicrobial‐impregnated external ventricular drain catheters: a prospective, randomized, controlled trial. Journal of Neurosurgery 2003;98(4):725‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zentner 1995 {published data only}
- Zentner J, Gilsbach J, Felder T. Antibiotic prophylaxis in cerebrospinal fluid shunting: a prospective randomized trial in 129 patients. Neurosurgical Review 1995;18(3):169‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Epstein 1982 {published data only}
- Epstein MH, Kumor K, Hughes W, Lietman P. Use of prophylactic antibiotics in pediatric shunting operations ‐ a double‐blind prospective study. Presented at the American Association of Neurological Surgeons Annual Meeting. 1982.
Shapiro 1986 {published data only}
- Shapiro M, Wald U, Simchen E, Pomeranz S, Zagzag D, Michowiz SD, et al. Randomized clinical trial of intra‐operative antimicrobial prophylaxis of infection after neurosurgical procedures. Journal of Hospital Infection 1986;8(3):283‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Siqueira 1987 {published data only}
- Siqueira MG, Koury LS, Silva AD, Jabur A, Rezende Filho CP. Intraoperative antibiotic prophylaxis in neurosurgery: a randomized controlled clinical trial [Profilaxia antibiótica transoperatória em neurocirurgia: estudo prospectivo controlado]. Arquivos Brasileiros de Neurocirurgia 1987;6:5‐12. [Google Scholar]
Additional references
Alleyne 2000
- Alleyne CH, Hassan M, Zabramski JM. The efficacy and cost of prophylactic and perioprocedural antibiotics in patients with external ventricular drains. Neurosurgery 2000;47:1124‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Altman 2002
- Altman DG, Deeks JJ. Meta‐analysis, Simpson's paradox, and the number needed to treat. BMC Medical Research Methodology 2002;2:3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bondurant 1995
- Bondurant CP, Jimenez DF. Epidemiology of cerebrospinal fluid shunting. Pediatric Neurosurgery 1995;23:254‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Borges 1982
- Borges LF. Cerebrospinal fluid shunts interfere with hosts defenses. Neurosurgery 1982;10:55‐60. [MEDLINE: ] [PubMed] [Google Scholar]
Claus 2004
- Claus BC. Shunt Infection. In: H. Richard Winn editor(s). Youmans Neurological Surgery. 5th Edition. Vol. 3, Philadelphia: Saunders, 2004:3419‐25. [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐91. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fleiss 1993
- Fleiss JL Fleiss JL. The statistical basis of meta‐analysis.. Statistical Methods in Medical Research 1993;2(2):121‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frazee 1988
- Frazee RC, Mucha P Jr, Farnell MB, Ebersold MJ. Meningitis after basilar skull fracture. Does antibiotic prophylaxis help?. Postgraduate Medicine 1988;83(5):267‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haines 1994
- Haines SJ, Walters BC. Antibiotic prophylaxis for cerebrospinal fluid shunts: a metanalysis. Neurosurgery 1994;34:87‐92. [MEDLINE: ] [PubMed] [Google Scholar]
Higgins 2005
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Intervention 4.2.5 [updated in May 2005]. The Cochrane Library, Issue 3, 2005.
Langley 1993
- Langley JM, LeBlanc JC, Drake J, Milner R. Efficacy of antimicrobial prophylaxis in placement of cerebrospinal fluid shunts: meta‐analysis. Clinical infectious Diseases 1993;17:98‐103. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moss 1991
- Moss AJ, Hamburger S, Moore RM Jr, Jeng LL, Howie LJ. Use of selected medical device implants in the United States, 1988. Advance Data 1991;191(26):1‐24. [MEDLINE: ] [PubMed] [Google Scholar]
RevMan 4.2 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) [Computer program]. Version 4.2 for Windows. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2003.
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smeeth 1999
- Smeeth L, Haines A, Ebrahim S. Numbers needed to treat derived from meta‐analyses‐sometimes informative, usually misleading. BMJ 1999;318(7197):1548‐51. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stenager 1986
- Stenager E, Gerner‐Smidt P, Kock‐Jensen C. Ventriculostomy‐related infections: an epidemiological study. Acta Neurochirurgica 1986;83(1‐2):20‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]


