Abstract
Redirected chimeric antigen receptor (CAR) T-cells can recognize and eradicate cancer cells in a major histocompatibility complex independent manner. Genetic engineering of T cells through CAR expression has yielded great results in the treatment of hematological malignancies compared with solid tumors. There has been a constant effort to enhance the effectiveness of these living drugs, due to their limited success in targeting solid tumors. Poor T cell trafficking, tumor-specific antigen selection, and the immunosuppressive tumor microenvironment are considered as the main barriers in targeting solid tumors by CAR T-cells. Here, we reviewed the current state of CAR T-cell therapy in breast cancer, as the second cancer-related death in women worldwide, as well as some strategies adopted to keep the main limitations of CAR T-cells under control. Also, we summarized various approaches that have been developed to enhance the therapeutic outcomes of this treatment in solid tumors targeting.
Keywords: Adoptive immunotherapy, Breast neoplasms, Cell-based therapy, Chimeric antigen receptor
1. INTRODUCTION
Breast cancer is the most life-threatening malignancy and the second cause of cancer-related death in women worldwide. Over the past decades, continued investigations with the aim of prevention and screening pipelines, diagnostic approaches, and treatment strategies have led to a reduced breast cancer-related mortality rate. Recently, some novel precise therapeutic options for the targeted therapy of breast cancer including small molecule inhibitors, gene editing-based strategies, and immune-checkpoint blockade monoclonal antibodies are under intensive investigation. Some important advances in genomic techniques have led to recognize possible biomarkers involved in breast cancer development, progression, and invasion (1,2). Currently, personalized approaches towards the prediction and management of different subtypes of breast cancer have been developed based on omics data (3,4,5). Despite significant progress in treatment strategies, certain breast tumors remain resistant to the current treatments due to their heterogeneous nature. Therefore, new therapeutic strategies are needed, and the challenge for the future will be “improving the efficiency of targeted therapy”.
The potential role of T lymphocytes to successfully treat cancer is confirmed, and impressive advances in cancer immunotherapy indicate the cytotoxic power of T lymphocytes as a living drug. T-cell immunotherapy, as a personalized-cancer treatment strategy, is a realistic technique to recognize and eradicate cancer cells and has been recently verified as a beneficial strategy for hematological malignancies and solid tumors.
However, isolation and expansion of T cells restricted to tumor-associated antigens (TAA), remains a major constrain of this strategy. To overcome these obstacles, investigators have developed new methods to generate genetically engineered T lymphocytes to target tumor cells (6). In hematological malignancies, adoptive immunotherapy using T lymphocytes that genetically express chimeric antigen receptors (CARs) against TAA has shown promising antitumor activity. However, targeting solid tumors has encountered many problems, including lack of unique TAAs, poor T cell trafficking to the tumor site, and immunosuppressive microenvironments of tumors. Moreover, CAR T-cells-based therapies have been used to target the vast majority of solid tumors, such as colorectal, ovarian, breast, prostate, liver, and metastatic renal cell carcinoma. Nonetheless, the clinical efficacy of CAR T-cells in targeting solid tumors has not yet been elucidated (7).
Accordingly, it seems necessary to select an ideal TAA and improve the redirection of CAR T-cells to tumor microenvironment. Herein, we focused on the current state of CAR T-cell therapy regarding breast cancer and explored the investigations to overcome the limitations of targeting solid tumors by CAR T-cells.
2. DESIGN CONCEPTS OF CAR T CELLS
Lately, the adoptive transfer of CAR-expressing T cells has shown potential in the field of solid tumors immunotherapy. Compared with T cell receptor-modified cells, CARs recognize a wide range of TAAs in a major histocompatibility complex-independent manner. The production of CAR T-cells is based on efficient gene transfer to autologous T cells, which have been harvested through leukapheresis (8). CARs are special synthetic receptors composed of an extracellular ligand-binding ectodomain, hinge-transmembrane domain, and cytoplasmic signal transduction domain. The ectodomain, which plays a vital role in the efficacy and safety of CAR T-cells, is mostly a single-chain variable fragment (scFv) derived from a TAA-specific monoclonal antibody. It is responsible for recognizing tumor cell surface expressing TAAs and redirecting CAR T-cells to specific tumor cells. On the other hand, a hinge/spacer region, typically derived from a cluster of differentiation (CD) 8 or IgG4 molecules, provides flexibility and connects the binding and signaling modules of the CARs (9).
The intracellular signal transduction portion (endodomain) also contains immune-receptor tyrosine-based activating motif(s) derived from cytoplasmic CD3-ζ domain of the T cell receptor/CD3 complex, which is in turn responsible for T-cell activation. Further, the signaling module consists of one or two costimulatory domains, such as either CD28, OX40, or 4-1BB to enhance CAR T cell function as well as persistence (Fig. 1).
Fig. 1.
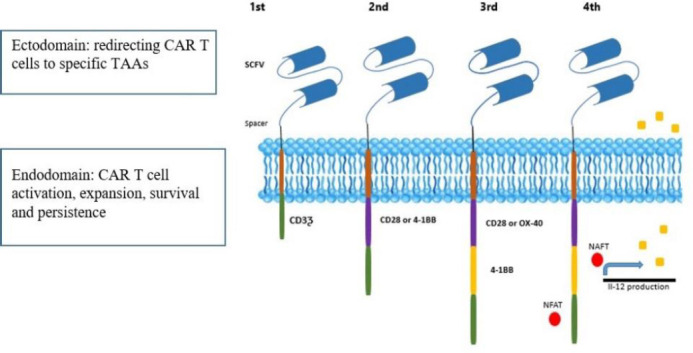
Schematic structure of CAR generations. First-generation CARs include an extracellular domain consisting of the scFv, a transmembrane domain; and an intracellular signaling module (endodomain). Additional one or two costimulatory endodomains such as CD28, CD137 (4-1BB), and CD134 (OX40) promote T cell activation and proliferation in second and third CAR generations respectively. CAR, Chimeric antigen receptor; NFAT; nuclear factor of activated T cells transcription factor; scFv, single-chain variable fragment; TAA, tumor-associated antigens.
Given that efficient T cell activation also requires immune-stimulatory cytokines, T cells redirected for antigen-unrestricted cytokine-initiated killing (TRUCK), also called 4th generation CAR T-cells, would comprise an additional cytokine signaling. Transgenic cytokine is released upon CAR engagement and contributes to optimal CAR T-cell activation. CAR T-cells engineered with transgenic interleukin (IL) 12 and IL-18 were effectively used in preclinical studies (10).
The antitumor effects of breast cancer-specific CAR T-cells have been shown in several preclinical and clinical studies. Recently, some of the breast cancer-associated tumor antigens, such as carbonic anhydrase IX, CD 133, ERbB2, mesothelin, epidermal growth factor receptor variant III, carcinoembryonic antigen, and mucin-1 (MUC-1) have been addressed using CAR technology in the clinical phase (Table 1) (11).
Table 1.
Recent clinical trials of chimeric antigen receptor -T cells for breast cancer and other solid tumors.
| Clinical trials government identifier | Target | Condition |
|---|---|---|
| NCT03696030 | HER2 | Malignant neoplasm metastatic, malignant neoplasm in the brain metastatic, malignant neoplasm in the leptomeninges, breast cancer, HER2-positive breast cancer |
| NCT04430595 | HER2, GD2, and CD44v6 | Breast cancer |
| NCT04107142 | Natural killer G2D | Colorectal cancer, triple-negative breast cancer, sarcoma, nasopharyngeal carcinoma, prostate cancer, gastric cancer. |
| NCT04348643 | Carcinoembryonic antigen | Solid-tumor lung cancer, colorectal cancer liver cancer, pancreatic cancer, gastric cancer, breast cancer |
| NCT02915445 | EpCAM | Malignant neoplasm of nasopharyngeal tumor/node/metastasis staging distant metastasis, breast cancer recurrent |
| NCT02792114 | Mesothelin | Breast cancer, metastatic HER2-negative breast cancer |
| NCT02706392 | Receptor tyrosine kinase-like orphan receptor 1 | Estrogen receptor-negative, HER2/Neu negative, progesterone receptor-negative, recurrent adult acute lymphoblastic leukemia, recurrent mantle cell lymphoma, refractory chronic lymphocytic leukemia, stage IV breast cancer AJCC v6 and v7Stage IV, non-small cell lung cancer AJCC v7, triple-negative breast carcinoma |
| NCT01837602 | cMet RNA | Metastatic breast cancer, triple-negative breast cancer |
| NCT03635632 | GD2 | Relapsed neuroblastoma, refractory neuroblastoma, relapsed osteosarcoma relapsed Ewing sarcoma, relapsed rhabdomyosarcoma, uveal melanoma, phyllodes breast tumor |
| NCT02541370 | CD133 | Liver cancer, pancreatic cancer, brain tumor, breast cancer, ovarian tumor, colorectal cancer, acute myeloid and lymphoid leukemia |
| NCT04025216 | TnMucin 1 | Non-small cell lung cancer, ovarian cancer, fallopian tube cancer, triple-negative breast cancer, multiple myeloma, pancreatic ductal adenocarcinoma |
HER2; Human epidermal growth factor receptor 2.
Recurrence of relapse and metastasis in patients with breast cancer indicates the role of a small subpopulation of undifferentiated cells, breast cancer stem cells or tumor-initiating cells, in several processes. Targeting cancer stem cells improves the clinical prognosis in several cancer types due to the major role of cancer stem cells in tumor re-growth and resistance to routine treatment strategies. Furthermore, mounting evidence has proved the critical role of cancer stem cells in the invasion, metastasis, and drug resistance of tumors. Although, only some of these breast cancer stem cell markers (CD24, CD44, CD133, and CD47) have been attended in the recent studies and the efficiency of CAR T-cells has been assessed in this area. Besides, the toxicities and antitumor activity of CAR-modified T-cell directed by CD133 were assessed by Wang et al. in a phase l clinical trial study on 23 patients. This immunohistochemistry analysis showed that CD133-expressing tumors were eradicated both in vitro and in xenograft tumor model after CAR-modified T-cell directed by CD133 infusions (12).
3. CAR T CELLS CHALLENGES TO TARGET SOLID TUMORS
To date, the adoptive transfer of CAR T-cells has shown huge success in eradicating hematologic malignancies (e.g. CD19 CARs in leukemia). However, clinical studies have demonstrated less promising results for targeting solid tumors using CAR T-cells because of some obstacles. At present, a growing number of clinical trials are in progress to overcome these barriers using new designs in CARs structure (13). In the following section, we have delineated some challenges and discussed new strategies to increase the outcomes of CAR T-cell therapy in breast cancer.
4. TUMOR ANTIGEN HETEROGENEITY
A crucial factor that restricts CAR T-cell therapy efficacy is on-target, off-tumor toxicities related to CAR activation against the targeted antigen which expresses on normal cells. On the other hand, tumor antigen heterogeneity and antigen escape due to antigen loss on tumor cells could also lead to an enhanced cancer relapse rate, metastasis, and resistance to treatment through CAR T-cell therapy.
Some unique investigational CAR structures are being designed to reduce cancer relapse rates related to tumor antigen heterogeneity. Moreover, the multi-antigen-targeted CAR T-cells are being considered as a strategy against solid tumors to overcome antigen heterogeneity and antigen loss, such as dual CAR T-cells, tandem CARs, and universal CARs (Fig. 2) (7,14). Dual CAR T-cells are distinct T cells that are genetically manipulated to co-express two separate CARs specific for two different TAAs, which can enhance the accuracy of tumor cell targeting and increase the antitumor activity of CAR T-cells. The potency of CAR T-cells with dual targeting of human epidermal growth factor receptor 2 (HER2) and the transmembrane glycoprotein mucin 1 (MUC1) was observed in breast cancer mice models. Wilkie et al. tested the cytotoxicity effect of co-expressing an ErbB2- and MUC1-specific CAR and they found that dual-targeted CARs led to CAR T-cells proliferation and have the power to kill tumor cells efficiently when target cells co-express MUC1 and ErbB2 (15).
Fig. 2.
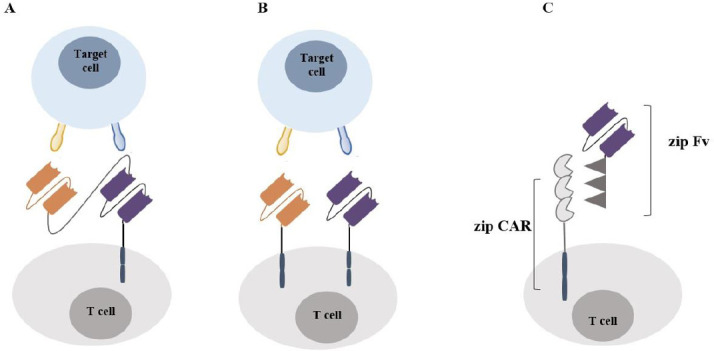
The advanced design of CAR T cells (A) Tandem CAR: A CAR structure which design to target two tumor antigens with a distinct ectodomain (scFv) linked sequentially with a single transduction domain; (B) dual CAR: CAR structure comprising two distinct antigen-recognition domains targeting different tumor antigens linked sequentially with a single transduction domain; (C) split, universal, and programmable CAR: a specific CAR model which has designed to improve CAR programmability comprising universal receptor zipCAR that allows a vast range of tumor antigens to be targeted via different zipFv. CAR, Chimeric antigen receptor; scFv, single-chain variable fragment.
Some dual CARs have also entered clinical trials such as targeting CD19/CD20 in patients with refractory and relapsed B-cell leukemia (NCT04260945), dual-specificity CD38, and the B cell maturation antigen CAR T-cells in patients with relapsed or refractory multiple myeloma (NCT03767751), and B cell maturation antigen/CD19 dual-target CAR T-cell immunotherapy in relapsed or refractory multiple myeloma (NCT04412889).
On the other hand, tandem CARs provide highly focused T-cell activation and propose that synergistic activation of two TAA-specific scFv with one intracellular signaling domain could effectively target the heterogeneous nature of solid tumors and diminish tumor resistance due to antigen escape (16). In a recent study, the generation of a tandem CAR molecule with two TAA-specific scFv that recognized two glioma-associated antigens (HER2 and IL13Rα2) led to effective targeting of either antigen with potent activity when both targets are encountered simultaneously. This newly generated CAR structure could target other tumor cells, including small-cell and non-small-cell lung cancers, colon cancer, and breast cancer (17).
Universal CARs are being designed as an approach to enhance the specificity, safety, and programmability of CAR T-cells. This generation of CAR T-cells uses a universal receptor that allows a vast panel of antigens to be targeted without re-engineering the immune cells (18). A split, universal, and programmable CAR system, is an example of universal CAR T-cells composed of a universal receptor zipCAR and zipFv fragments. The split, universal, and programmable CAR ectodomain made of a specific scFv with leucine zipper adaptor (zipFv) also fuses to a second leucine zipper and zipCAR intracellular signaling domains. One set of zipCAR T-cells can target different TAA without further modification of the CAR T-cells. In the mouse xenograft models of breast cancer, zipCAR T-cells showed a successful eradication of HER2-positive human breast cancer cells and a robust antitumor activity comparable to other conventional CAR-T cells (19).
5. INSUFFICIENT TRAFFICKING OF CAR T CELLS TO TUMOR SITES
Tumors often escape immune surveillance due to impairing in effector T cell recruitment and infiltration into the tumor foci. Trafficking of immune cells to tumor sites is a lively process controlled by complex interactions. During these processes, the CAR T-cells need to adhere to endothelial cells and initiate chemokine-chemokine receptor interactions to facilitate their extravasation into the tumor regions (20). Also, dysregulation in cytokine secretion from tumor cells and low chemokine receptors expression on CAR T-cells are the major factors responsible for the poor homing of CAR T-cells to the tumor site. Some different strategies to improve T cell trafficking are presented in Table 2 (21,22,23).
Table 2.
Different strategies to improve CAR T cell trafficking in solid tumors.
| Strategies | Mechanism of actions | Examples |
|---|---|---|
| Chemokine receptors expression on CAR T cells | Improved CAR T cell recruitment and migration | CCR2b, CCR4 (CCL17 receptor), CCL2, CCL17 and C-X-C motif chemokine receptor |
| Armed oncolytic adenovirus | Increase CAR T cell survival | Oncolytic virus engineered to express CCL5 and interleukin 15. |
| Engineered CAR T cells to secrete heparanase | Increase CAR T cell penetration into tumor cells | Degrade the extracellular matrix |
| Local delivery of CAR T cells | Direct delivery of CAR T cells into tumor site | Catheter infusion, intracranial, delivery, intracavitary, intraventricular and localized infusion through the hepatic artery |
CAR, Chimeric antigen receptor; CCR, C-C chemokine receptor type; CCL, C-C motif chemokine ligand;
Additionally, the safety, as well as the feasibility of treating metastatic breast cancer with intratumoral injection of c-Met-CAR T-cells, have been well-documented in the phase 1 clinical trial (NCT01837602).
Also, the robust therapeutic ability of the intratumoral/intracranial or intraventricular (intracerebroventricular) injection of second-generation HER2-specific CAR T-cell has been proved for the treatment of breast cancer metastasis to the brain using orthotopic human tumor xenograft models (24).
6. TUMOR IMMUNOSUPPRESSIVE MICROENVIRONMENT
The immunosuppressive microenvironment is a critical hurdle, which CAR T-cells face when invading the tumor site. Immune escape is mediated by suppressor cytokines, such as transforming growth factor beta, IL-10, IL-4, and also by co-stimulatory molecules of immune checkpoint inhibitors, including either programmed death-1 or programmed death-ligand 1, and the cytotoxic T-lymphocyte-associated antigen 4 (25). Different approaches have therefore been exploited to arm CAR T-cells in order to fight against tumor immunosuppressive microenvironment (26). The efficacy of CAR-T cells has improved via IL-12 secretion by engineered CARs and combination therapy through either activating cytokines, such as IL-2, IL-12, and IL-15, or monoclonal antibodies that block either cytotoxic T-lymphocyte-associated antigen 4 or programmed death-1 (27). Previous studies have shown that activation of adenosine 2A receptors generated by tumor cells effectively prevents endogenous antitumor T cell responses. Specifically in breast cancer, results showed that targeting the adenosine 2A receptor combined with blockade of programmed death-1 enhances CAR T-cells antitumor activity. For example, Beavis et al. found that targeting the adenosine 2A receptors increases CAR T-cell power with rising production of cytokines from CD8+ CAR T-cells and subsequently enhances activation of both CD8+ and CD4+ CAR T-cells (28).
7. CHIMERIC ANTIGEN-EXPRESSING NATURAL KILLER CELLS
Natural killer (NK) cells (CD56+ and CD3-) are a major subset of lymphocytes which originally identified to exhibit potent antitumor properties. CAR-engineered NK cells are known as ideal candidates for immunotherapy because of their short lifespan, superior safety, strong cytotoxicity, and major histocompatibility complex-independent recognition without the risk of inducing graft-versus-host disease compared to T cells (29). CAR-modified NK cells would be exhausted rapidly after destroying cancerous cells, with a turnover time of around 7 to 14 days. Limited in vivo persistence of these cells then excludes the need for an inducible suicide switch (30).
In this regard, several studies have been performed to target breast cancer using NK-CAR cells. Despite the promising data supporting the efficacy of activated primary NK-CAR cells, another different form of CAR-NK cell therapy has been carried out using permanent IL-2 dependent NK cell lines. The NK cell lines provide a new interesting source for generating NK-CAR cells because of the ease of maintenance in culture, large-scale expansions, and homogeneous characteristics.
Among several NK cell lines (such as HANK-1, NKG, NK-YS, YT, YTS cells, and NKL cells), NK-92 is the cell line mostly used in the field of cancer immunotherapy and has been effective in various clinical trials targeting solid tumors and hematological malignancies (31).
Uherek et al. and Liu et al. designed and constructed an ErbB2-specific scFv expressing NK92 cells which retarget NK cell cytolytic activity to ErbB2-expressing cancer cells. This function has led to significant enhancement in tumor cell-specific destruction and growth inhibition (32). Also, the efficacy of modified NK cells that express specific scFv against other breast cancer-associated antigens such as epidermal growth factor receptor, EpCAM, and MUC1 (NCT02839954) is well-documented, recently.
Future perspectives adoptive cell transfer with CAR-redirected T cells has shown remarkable success in hematological malignancies in preclinical and clinical studies. Nonetheless, this therapeutic strategy remains in its initial development steps in targeting solid tumors and urgent clinical approaches are required to enhance their efficacy in this field. Due to lack of ideal antigen targeting, poor trafficking, and tumor-related immunosuppressive microenvironments, driving CARs on the road of solid tumor therapy have encountered many hurdles. Currently, some works are ongoing to recognize and then overcome these barriers through different strategies and make CAR T-cells more effective in the treatment of solid tumors. In this review, we summarized the current state of CAR T-cell therapy in breast cancer, a life-threatening solid tumor, and discussed novel modifications in CAR T-cell generation to enhance the therapeutic power of these living drugs. Therefore, understanding the molecular pathways in tumorigenesis, tumor cell invasion and metastasis, tumor immune escape and the complex interaction between tumor foci and effector immune cells will help us to design appropriate and effective CAR T-cells to target solid tumors.
8. CONCLUSION
New strategies to address the challenges that have limited the efficacy of CAR T-cells in solid tumors are developing. Recent approaches enhance CAR T-cell activity through overcome antigen heterogeneity and immunosuppressive microenvironment of tumor cells. Based on recent reports, CAR T-cells can be regarded as a potential therapeutic tool for breast cancer and other solid tumors in the future.
Conflicts of interest statement
The authors declared no conflict of interest in this study.
Authors’ contribution
H. Khanahmad and I. Rahimmanesh conceived of the presented idea. I. Rahimmanesh developed the theoretical framework and wrote the article. H. Khanahmad revised the final version of the article.
9. REFERENCES
- 1.Bakhtiari H, Palizban AA, Khanahmad H, Mofid MR. Aptamer-based approaches for in vitro molecular detection of cancer. Res Pharm Sci. 2020;15(2):107–122. doi: 10.4103/1735-5362.283811. DOI: 10.4103/1735-5362.283811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300. doi: 10.1001/jama.2018.19323. DOI: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 3.Nagini S. Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem. 2017;17(2):152–163. doi: 10.2174/1871520616666160502122724. DOI: 10.2174/1871520616666160502122724. [DOI] [PubMed] [Google Scholar]
- 4.Low SK, Zembutsu H, Nakamura Y. Breast cancer: the translation of big genomic data to cancer precision medicine. Cancer Sci. 2018;109(3):497–506. doi: 10.1111/cas.13463. DOI: 10.1111/cas.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Darzi L, Boshtam M, Shariati L, Kouhpayeh S, Gheibi A, Mirian M, et al. The silencing effect of miR-30a on ITGA4 gene expression in vitro: an approach for gene therapy. Res Pharm Sci. 2017;12(6):456–464. doi: 10.4103/1735-5362.217426. DOI: 10.4103/1735-5362.217426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan CWH, Law BMH, So WKW, Chow KM, Waye MMY. Novel strategies on personalized medicine for breast cancer treatment: an update. Int J Mol Sci. 2017;18(11):2423. doi: 10.3390/ijms18112423. DOI: 10.3390/ijms1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Newick K, O’Brien S, Moon E, Albelda SM. CAR T cell therapy for solid tumors. Annu Rev Med. 2017;68:139–152. doi: 10.1146/annurev-med-062315-120245. DOI: 10.1146/annurev-med-062315-120245. [DOI] [PubMed] [Google Scholar]
- 8.Rahimmanesh I, Totonchi M, Khanahmad H. The challenging nature of primary T lymphocytes for transfection: effect of protamine sulfate on the transfection efficiency of chemical transfection reagents. Res Pharm Sci. 2020;15(5):437–446. doi: 10.4103/1735-5362.297846. DOI: 10.4103/1735-5362.297846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine BL, Miskin J, Wonnacott K, Keir C. Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev. 2016;4:92–101. doi: 10.1016/j.omtm.2016.12.006. DOI: 10.1016/j.omtm.2016.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chmielewski M, Abken H. TRUCKS, the fourth-generation CAR T cells: current developments and clinical translation. Adv Cell Gene Ther. 2020;3:e84–e92. DOI: 10.1002/acg2.84. [Google Scholar]
- 11.Xie Y, Hu Y, Zhou N, Yao C, Wu L, Liu L, et al. CAR T-cell therapy for triple-negative breast cancer: where we are. Cancer Lett. 2020;491:121–131. doi: 10.1016/j.canlet.2020.07.044. DOI: 10.1016/j.canlet.2020.07.044. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Chen M, Wu Z, Tong C, Dai H, Guo Y, et al. CD133-directed CAR T cells for advanced metastasis malignancies: a phase I trial. Oncoimmunology. 2018;7(7):e1440169,1–13. doi: 10.1080/2162402X.2018.1440169. DOI: 10.1080/2162402X.2018.1440169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abate-Daga D, Davila ML. CAR models: next-generation CAR modifications for enhanced T-cell function. Mol Ther Oncolytics. 2016;3:16014,1–7. doi: 10.1038/mto.2016.14. DOI: 10.1038/mto.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomark Res. 2017;5:22–27. doi: 10.1186/s40364-017-0102-y. DOI: 10.1186/s40364-017-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilkie S, van Schalkwyk MC, Hobbs S, Davies DM, van der Stegen SJ, Pereira AC, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol. 2012;32(5):1059–1070. doi: 10.1007/s10875-012-9689-9. DOI: 10.1007/s10875-012-9689-9. [DOI] [PubMed] [Google Scholar]
- 16.Han X, Wang Y, Wei J, Han W. Multi-antigen-targeted chimeric antigen receptor T cells for cancer therapy. J Hematol Oncol. 2019;12(1):128–137. doi: 10.1186/s13045-019-0813-7. DOI: 10.1186/s13045-019-0813-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hegde M, Mukherjee M, Grada Z, Pignata A, Landi D, Navai SA, et al. Tandem CAR T cells targeting HER2 and IL13Rα2 mitigate tumor antigen escape. J Clin Invest. 2016;126(8):3036–3052. doi: 10.1172/JCI83416. DOI: 10.1172/JCI83416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, Lin Q, Song Y, Liu D. Universal CARs, universal T cells, and universal CAR T cells. J Hematol Oncol. 2018;11(1):132–140. doi: 10.1186/s13045-018-0677-2. DOI: 10.1186/s13045-018-0677-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho JH, Collins JJ, Wong WW. Universal chimeric antigen receptors for multiplexed and logical control of t cell responses. Cell. 2018;173(6):1426–1438. doi: 10.1016/j.cell.2018.03.038. e11. DOI: 10.1016/j.cell.2018.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Di Stasi A, De Angelis B, Rooney CM, Zhang L, Mahendravada A, Foster AE, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113(25):6392–6402. doi: 10.1182/blood-2009-03-209650. DOI: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Craddock JA, Lu A, Bear A, Pule M, Brenner MK, Rooney CM, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother. 2010;33(8):780–788. doi: 10.1097/CJI.0b013e3181ee6675. DOI: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guedan S, Alemany R. CAR-T cells and oncolytic viruses: joining forces to overcome the solid tumor challenge. Front Immunol. 2018;9:2460–2469. doi: 10.3389/fimmu.2018.02460. DOI: 10.3389/fimmu.2018.02460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caruana I, Savoldo B, Hoyos V, Weber G, Liu H, Kim ES, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med. 2015;21(5):524–529. doi: 10.1038/nm.3833. DOI: 10.1038/nm.3833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Priceman SJ, Tilakawardane D, Jeang B, Aguilar B, Murad JP, Park AK, et al. Regional delivery of chimeric antigen receptor-engineered T cells effectively targets HER2(+) breast cancer metastasis to the brain. Clin Cancer Res. 2018;24(1):95–105. doi: 10.1158/1078-0432.CCR-17-2041. DOI: 10.1158/1078-0432.CCR-17-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labani-Motlagh A, Ashja-Mahdavi M, Loskog A. The tumor microenvironment: a milieu hindering and obstructing antitumor immune responses. Front Immunol. 2020;11:940–961. doi: 10.3389/fimmu.2020.00940. DOI: 10.3389/fimmu.2020.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Garcia A, Palazon A, Noguera-Ortega E, Powell DJ, Guedan S. CAR-T cells hit the tumor microenvironment: strategies to overcome tumor escape. Front Immunol. 2020;11:1109–1125. doi: 10.3389/fimmu.2020.01109. DOI: 10.3389/fimmu.2020.01109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chmielewski M, Hombach AA, Abken H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol Rev. 2014;257(1):83–90. doi: 10.1111/imr.12125. DOI: 10.1111/imr.12125. [DOI] [PubMed] [Google Scholar]
- 28.Beavis PA, Henderson MA, Giuffrida L, Mills JK, Sek K, Cross RS, et al. Targeting the adenosine 2A receptor enhances chimeric antigen receptor T cell efficacy. J Clin Invest. 2017;127(3):929–941. doi: 10.1172/JCI89455. DOI: 10.1172/JCI89455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19(1):120–145. doi: 10.1186/s12943-020-01238-x. DOI: 10.1186/s12943-020-01238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: a promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975,1–10. doi: 10.1016/j.ebiom.2020.102975. DOI: 10.1016/j.ebiom.2020.102975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Oberoi P, Oelsner S, Waldmann A, Lindner A, Tonn T, et al. Chimeric antigen receptor-engineered NK-92 cells: an off-the-shelf cellular therapeutic for targeted elimination of cancer cells and induction of protective antitumor immunity. Front Immunol. 2017;8:533–549. doi: 10.3389/fimmu.2017.00533. DOI: 10.3389/fimmu.2017.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uherek C, Tonn T, Uherek B, Becker S, Schnierle B, Klingemann HG, et al. Retargeting of natural killer-cell cytolytic activity to ErbB2-expressing cancer cells results in efficient and selective tumor cell destruction. Blood. 2002;100(4):1265–1273. PMID: 12149207. [PubMed] [Google Scholar]


