Abstract
Background and purpose:
The clinical use of the chemotherapeutic drug, doxorubicin (DXR), is significantly limited by its extensive multi-organ toxicity. Dipeptidyl peptidase-4 (DPP4) is over-expressed in oxidative stress, inflammation and apoptosis. DPP4 inhibitors have proven pleiotropic effects. The study investigates the protective effects of some DDP4 inhibitors; namely, saxagliptin (SAX) and vildagliptin (VIL) against DXR-induced nephrotoxicity in rats.
Experimental approach:
Forty rats were divided into 4 groups. Group I served as normal control. Nephrotoxicity was induced in the remaining 3 groups by single-DXR injection (15 mg/kg, i.p.). Groups III and IV administered oral SAX (10 mg/kg) and VIL (10 mg/kg) for 2 weeks.
Findings/Results:
DXR-control rats showed deteriorated renal functions, elevated renal inflammatory parameters (tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and inducible nitric oxide synthase (iNOS)), up-regulated nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3 (NLRP3) inflammasome and significant tubulointerstitial injury manifested by elevated neutrophil gelatinase-associated lipocalin concentration and distorted renal histopathological pictures. Immunohistochemical studies showed increased iNOS and Bax positivity in renal tissues of DXR-control rats. Treatment with SAX and VIL significantly attenuated DXR-induced nephrotoxicity via alleviation of all the above-mentioned parameters when compared to DXR-control rats.
Conclusion and implications:
The study elucidated the possible mechanisms beyond DXR-induced nephrotoxicity to be through inflammation plus tubulointerstitial injury. DXR nephrotoxicity has been linked to TNF-α, IL-1β, and NLRP3 inflammasome up-regulation and iNOS expression. The protective role of SAX and VIL in mitigating the tubular injury and inflammatory effects of DXR on renal tissues has been tested and proved.
Keywords: Doxorubicin, Saxagliptin, Vildagliptin, Nephrotoxicity, Inflammation, Tubulo-interstitial injury
INTRODUCTION
Dipeptidyl peptidase-4 (DPP4) inhibitors are mainly used for the treatment of non-insulin-dependent diabetes mellitus. DPP4 inhibitors exert their antidiabetic action via stimulation of insulin secretion from pancreatic beta cells and elevation of incretin hormones viz., type 1 glucagon-like peptide-1 (GLP-1) (1). DPP-4 is highly abundant in the proximal tubules as well as mesangial cells of the kidneys. Interestingly, DPP-4 is over-expressed in numerous disease conditions including obesity, oxidative stress, inflammation, and apoptosis (2). Pleiotropic effects of DPP4 inhibitors on cardiovascular and renal systems mainly via GLP-1 receptor (GLP-1R) have been proved. GLP-1R is significantly expressed in glomerular capillaries and proximal tubular cells in diabetic and non-diabetic renal tissues (3).
DPP4 inhibitors also exert anti-inflammatory and antifibrotic effects independently of GLP-1R (4). Many previous studies reported potential anti-inflammatory nephroprotective effects of DPP4 inhibitors in several non-diabetic animal models (5). Different DPP-4 inhibitors exert diverged actions on the kidney tissues. Some DPP-4 inhibitors (alogliptin and anagliptin) were reported to decrease glomerular injury as well as macrophage infiltration. Thus they exerted potent anti-inflammatory effects on kidney tissues.
Saxagliptin (SAX), along with its active metabolite, showed long-term inhibition of renal membrane-bound DPP-4 in comparison to other DDP-4 inhibitors. Luckily, SAX is well-tolerated in kidney patients (6). Vildagliptin (VIL) showed potential nephroprotective effects regardless of its antidiabetic effects (7).
Doxorubicin (DXR) has been extensively used as an antineoplastic anthracycline-derived antibiotic for treatment of many human cancers, including solid tumors, breast cancer as well as hematological malignancies (8). However, its clinical use as a chemotherapeutic agent is significantly limited due to its extensive multi-organ toxicity. DXR has been shown to cause extensive dose-dependent nephrotoxicity, cardiotoxicity, hepatotoxicity, hematological, pulmonary, and nervous system toxicity (9). The pathophysiological mechanisms underlying DXR-induced nephrotoxicity are multifactorial and not fully clear (10). These include apoptosis, oxidative stress and tubulointerstitial inflammation which eventually lead to tubular fibrosis and atrophy with significant proteinuria and hematuria (5). DXR stimulates cytokines and chemokines production e.g. tumor necrosis factor alpha (TNF-α), interleukin-1 beta (IL-1β), and inducible nitric oxide synthase (iNOS) among other inflammatory mediators in renal tubular cells (11). Moreover, DXR aggravates free radical production as well as lipid and protein oxidation, substantially leading to apoptosis and eventually abrupt decrease in glomerular number and tubular function. Apoptotic changes are usually accompanied by elevated Bax expression. Renal tubular injury is accompanied by the rise of neutrophil gelatinase-associated lipocalin (NGAL) concentrations (12). Therefore, it has been crucial to scout for new therapeutic agents that can decrease DXR-induced nephrotoxicity to enhance its clinical use (13).
Recent data demonstrate that nucleotide-binding oligomerization domain (NOD)-like receptor containing pyrin domain 3 (NLRP3) inflammasome is highly up-regulated in the pathogenesis of acute or chronic nephrotoxicity. However, the molecular mechanisms underlying this up-regulation are not well-elucidated (14). Interestingly, recent studies linked NLRP3 up-regulation to DXR-induced cardiotoxicity (15). However, to date, no data is present to link NLRP3 inflammasome up-regulation to DXR-induced nephrotoxicity.
Therefore, the current study aimed at scouting the potential anti-inflammatory effects of some DDP4 inhibitors; namely, SAX and VIL against DXR-induced nephrotoxicity in rats. The study also investigated the involvement of NLRP3 inflammasome up-regulation in DXR-induced nephrotoxicity and whether the selected DDP4 inhibitors could abate this up-regulation as well as the tubulointerstitial injury associated with DXR-induced nephrotoxicity in rats.
MATERIALS AND METHODS
Animals
Adult male albino Wistar rats, weighing 180-200 g, were utilized in the present study. Standard food pellets and tap water were supplied ad libitum. Animals and food pellets were obtained from the animal house colony of the National Research Centre (NRC, Egypt). The study was conducted in accordance with the National Research Centre-Medical Research Ethics Committee (Ethics No. NRC-MREC) for the use of animal subjects and following the recommendations of the National Institutes of Health Guide for Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978).
Preparation of drugs
DXR vials (Adricin®, Hikma Pharmaceuticals, Egypt) were used in the current study. SAX (Onglyza®, AstraZeneca, Canada) and VIL (Galvus®, Novartis, Switzerland) were orally administered in the current study. All other chemicals were of the highest commercial grade available.
Experimental design
Forty rats were weighed and randomly allocated into four groups (10 each). Rats of the 1st group i.p. injections of saline and served as the normal control group. Acute nephrotoxicity was induced in rats in the remaining three groups using DXR in a single nephrotoxic dose (15 mg/kg, i.p.) (16,17). Group II received only saline for two weeks and served as the DXR-control group. Groups III and IV received SAX (10 mg/kg, p.o.) and VIL (10 mg/kg, p.o.) on daily basis for two weeks, respectively. All animals were sacrificed 48 h after the last drug administration under sodium pentobarbital anesthesia (50 mg/kg, i.p.). Blood samples were taken, and renal tissues were isolated and weighed. The sera and kidneys were obtained for biochemical, histopathological, and immunohistochemical investigations.
Biochemical analysis
Serum was used for estimation of serum creatinine and blood urea nitrogen (BUN) levels, using specific diagnostic kits (Biodiagnostic, Egypt) according to the manufacturer’s instructions.
Immediately after blood sampling, animals were sacrificed under anesthesia. The two kidneys from each rat were immediately dissected out and rinsed with phosphate-buffered saline (PBS) to remove excess blood. Whole kidney weight was determined and weighed parts from both kidneys were homogenized (MPW-120 homogenizer, Med instruments, Poland) in PBS to obtain 20% homogenate that was stored overnight at -20 °C. After two freeze-thaw cycles, performed to break the cell membranes, the homogenates were centrifuged for 5 min at 5000 g using a cooling centrifuge (Sigma and Laborzentrifugen, 2k15, Germany). The supernatant was removed immediately and assayed for TNF-α (Biolegend Inc®, USA), IL-1β (Cohesion Biosciences, UK), neutrophil gelatinase-associated lipocalin (NGAL; Elabscience, USA), and NOD-like receptor containing pyrin domain 3 (NLRP3, Lifespan BioSciences Inc®, USA) using enzyme-linked immunosorbent assay (ELISA) kits according to the manufacturer’s instructions (Lifespan Biosciences, inc, USA; catalog No. LS-F39745).
Histopathological examination
Other parts of kidneys from all groups were fixed in 10% neutral buffered formalin for 72 h at least. The tissues were subsequently exposed to a series of histological processing, including dehydration, clearing, infiltration, and embedding in paraffin blocks. The tissues were cut into 4 μm thick sections and stained with hematoxylin and eosin (H&E).
For assessment of renal damage, a semi-quantitative scoring system scaled from 0 to 3 was used (18). A total of ten random low-power fields (10×) per group were examined. The pathological parameters used for this assessment were glomerular congestion, expansion of mesangial matrix, vacuolar degeneration with swelling of renal tubular epithelial cells intracytoplasmic aggregation of hyaline droplets, and apoptosis. The pathologic score was evaluated according to the percentage of tissue damage in the low power field as follow; 0, no definite histopathological lesion; 1, renal damage in less than 25% of tissue; 2, renal damage in 25-50% of tissue; and 3, renal damage in > 50% of tissue.
Glomerular histomorphometry
Histomorphometric analysis for estimation of glomerular tuft area was carried out using computer image analysis software (Image J). Images captured at 40× magnification power, showing glomerular tuft clearly, were collected and used for the analysis. The glomerular tuft area was measured in ten glomeruli per group and the obtained data was statistically analyzed.
Immunohistochemical analysis of Bax and iNOS expression
All the immunohistochemical procedures for the demonstration of Bax and iNOS expression in the renal tissues were carried out according to previously described methods (19). Precisely, the paraffin-embedded kidney sections were de-waxed and rehydrated in alcohol. The sections were then incubated with rabbit monoclonal anti-Bax (ab216985, abcam) and rabbit polyclonal anti-iNOS (ab3523, abcam) antibodies. The immune reaction was visualized using diaminobenzidine (DAB). A semi-quantitative grading system, scaled from 0 to 3, was used to assess the immune reactivity depending on the percentage of positive cells in ten high power fields (HPF; 40×) where 0 = no staining, 1 = positive staining in < 30% of cells/HPF, 2 = positive staining in 30-70% of cells/HPF, and 3 = positive staining in > 70% of cells/HPF (20).
Statistical analysis
All the values are presented as means ± standard error of means (SEM). Comparisons between different groups were carried out using one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison post hoc test. On the other hand, Bax and iNOS immunohistochemical staining assessment was analyzed by performing Kruskal-Wallis non-parametric ANOVA test followed by Mann-Whitney U test. In all cases, the difference was considered significant when P < 0.05. GraphPad Prism® software (version 6 for Windows, San Diego, California, USA) was used to carry out these statistical tests.
RESULTS
Effects of SAX and VIL on renal functions
DXR single i.p. injection (15 mg/kg) caused a significant increase in serum creatinine and BUN contents to 223% and 191%, respectively in comparison to the normal control rats. SAX treatment (10 mg/kg) significantly decreased serum creatinine and BUN contents to 69% and 75%, respectively; while VIL treatment (10 mg/kg) significantly decreased in serum creatinine and BUN contents to 52% and 62%, respectively compared to DXR-control rats (Table 1).
Table 1.
Effects of SAX and VIL on renal functions in DXR-induced nephrotoxicity. Data are presented as mean ± SEM, n = 10. Rats of the normal control group received intraperitoneal saline.
| Groups | Creatinine (mg/dL) | BUN (mg/dL) |
|---|---|---|
| Normal control | 0.71 ± 0.01 | 25.67 ± 0.45 |
| DXR-control (15 mg/kg, i.p) | 1.58a ± 0.05 | 49.11a ± 1.68 |
| DXR + SAX (10 mg/kg, p.o.) | 1.08ab ± 0.06 | 36.77ab ± 1.48 |
| DXR + VIL (10 mg/kg, p.o.) | 0.83b ± 0.05 | 30.53b ± 0.78 |
DXR, Doxorubicin; SAX, saxagliptin; VIL, vildagliptin; aP < 0.05 indicates significantly differences compared to the normal control group and bP < 0.05 versus DXR-control group.
Effects of SAX and VIL on inflammatory markers in renal tissue
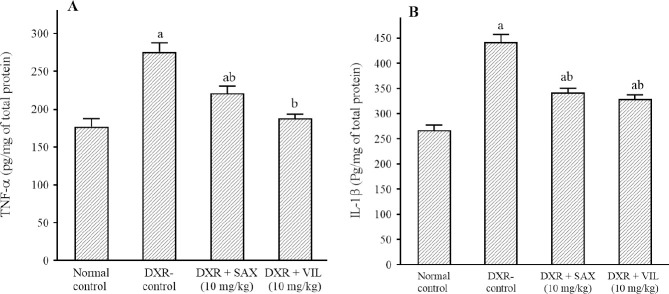
DXR injection caused a significant increment in renal tissue TNF-α and IL-1β contents to 163% and 153% respectively in comparison to normal control rats. SAX and VIL treatment significantly reduced TNF-α & IL-1β renal tissue contents to 82% & 83% and 69% & 75% respectively; in comparison to DXR-control rats (Fig. 1).
Fig. 1.
Effects of SAX and VIL on renal tissue contents of (A) TNF-α and (B) IL-1β in DXR-induced nephrotoxicity in rats. Rats of the normal control group received intraperitoneal saline. DXR-control group received only saline along with DXR. Data are presented as mean ± SEM, n = 10. aP < 0.05 indicates significant differences in comparison with the normal control group and bP < 0.05 versus DXR-control group. DXR, Doxorubicin; IL-1β, interleukin-1 beta; SAX, saxagliptin; TNF-α, tumor necrosis factor alpha; VIL, vildagliptin.
Effects of SAX and VIL on NLRP content in renal tissues
DXR use in the selected dose significantly elevated renal tissue NLRP3 content to 208% in comparison to the normal control rats. On the other hand, treatment with SAX and VIL significantly reduced renal tissue NLRP3 contents to 76% and 67%, respectively in comparison to DXR-control rats (Fig. 2).
Fig. 2.
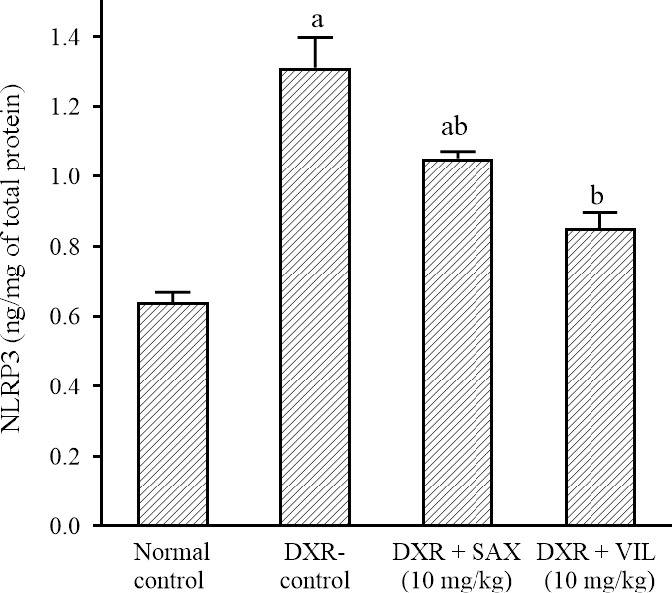
Effects of SAX and VIL on renal tissue NLRP3 content in DXR-induced nephrotoxicity in rats. Rats of the normal control group received intraperitoneal saline. DXR-control group received only saline along with DXR. Data are presented as mean ± SEM, n = 10. aP < 0.05 indicates significant differences in comparison with the normal control group and bP < 0.05 versus DXR-control group. DXR, Doxorubicin; NLRP3, nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3, SAX, saxagliptin; VIL, vildagliptin.
Effects of SAX and VIL on renal tubular injury marker
DXR use caused a significant increment in renal tissue NGAL concentration to 174% in comparison to the normal control rats. While, SAX and VIL significantly reduced renal tissue NGAL concentration to 78% and 70%, respectively in comparison to DXR-control rats (Fig. 3).
Fig. 3.
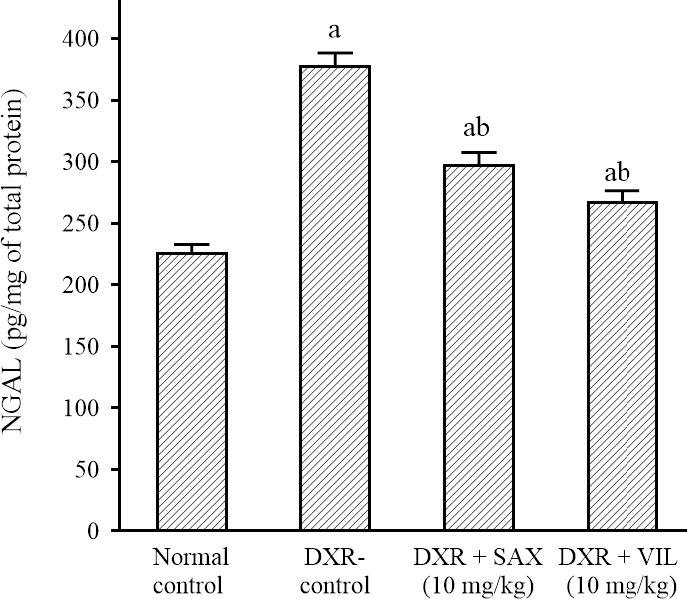
Effects of SAX and VIL on renal tissue NGAL concentration in DXR-induced nephrotoxicity in rats. Rats of the normal control group received intraperitoneal saline. DXR-control group received only saline along with DXR. Data are presented as mean ± SEM, n = 10. aP < 0.05 indicates significant differences in comparison with the normal control group and bP < 0.05 versus DXR-control group. DXR, Doxorubicin; NGAL, neutrophil gelatinase-associated lipocalin, SAX, saxagliptin; VIL, vildagliptin.
Histopathological examination and glomerular histomorphometry of renal tissue
Kidneys of the normal control group showed normal histological structures of renal glomeruli and tubules (Fig. 4A). Meanwhile, different histopathological lesions were demonstrated in the glomeruli and tubules of DXR-control group. Glomerular lesions were characterized by congestion of glomerular capillaries (Fig. 4B) and expansion of mesangial matrix (Fig. 4C) with pronounced increase of glomerular tuft area which is significantly different from the normal control group (Table 2). The renal tubules revealed pronounced swelling of their epithelial lining with vacuolization of their cytoplasm in addition to the presence of hyaline droplets in the cytoplasm (Fig. 4D) as well as numerous apoptotic figures. These histopathological lesions were markedly attenuated in the SAX-treated group, with decreased pathologic score of renal damage and decreased glomerular tuft area, which is significantly different from the DXR-control group (Table 2).
Fig. 4.
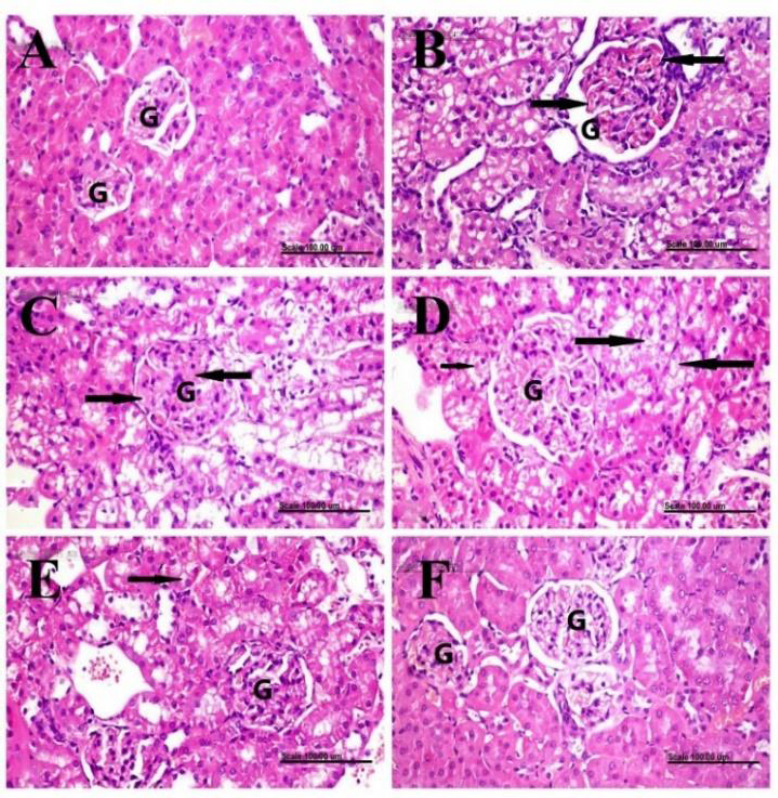
Histopathological examination and glomerular histomorphometry of renal tissue. Renal tissues of (A) normal control rats showing normal histology of renal glomeruli (G) and tubules; (B-D) doxorubicin-control rats showing glomerular congestion (arrows), mesangial matrix expansion (arrows), and pronounced swelling of the epithelial lining renal tubules with cytoplasmic vacuolization (long arrows) plus the presence of hyaline droplets (short arrow), respectively; (E) saxagliptin-treated group showing less swollen renal tubules with mild cytoplasmic vacuolization (arrow); and (F) vildagliptin-treated group showing a normal histological structure of glomeruli (G) and tubules. Stain, H&E, scale bar = 100 μm.
Table 2.
The mean pathologic score of renal damage and glomerular histomorphometry were recorded in the kidneys of normal and treated groups. Data are presented as mean ± SEM, n = 10. Rats of the normal control group received intraperitoneal saline. Different lowercase letters are significantly different (P < 0.05).
| Groups | Glomerular tuft area (μm2) | Mean pathologic score of renal damage |
|---|---|---|
| Normal control | 4545.32c ± 285.37 | 0.1 ± 0.10 |
| DXR-control (15 mg/kg, i.p) | 10048.81a ± 1158.94 | 2.8a ± 0.13 |
| DXR + SAX (10 mg/kg, p.o.) | 7206.09b ± 555.98 | 0.9b ± 0.23 |
| DXR + VIL (10 mg/kg, p.o.) | 6095.86b,c ± 507.78 | 0.4b ± 0.16 |
DXR, Doxorubicin; SAX, saxagliptin; VIL, vildagliptin.
The renal tubules appeared less swollen with mild vacuolization of some epithelial cells (Fig. 4E). Better improvement was demonstrated in VIL-treated group, with a significant reduction of glomerular tuft area, compared to DXR-control and SAX-treated groups (Table 2). The glomerular tuft area and tubules appeared closely similar to those of the normal group (Fig. 4F).
Immunohistochemical expression of Bax and iNOS in renal tissue
Kidneys of the normal control group showed no expression of Bax and iNOS in the renal tissue (Figs. 5A and 6A, respectively). In contrast, kidneys of DXR-control group showed increased expression of Bax and iNOS with a significant increase in the percentage of positively stained cells with strong and diffuse cytoplasmic staining of renal tubules (Figs. 5B and 6B; respectively). A significant decrease of Bax and iNOS expression with a pronounced decrease of the percentage of positively stained cells was recorded in the renal tissue of SAX-treated group which is significantly different from the DXR-control group (Figs. 5C and 6C; respectively). Similarly, a significant decrease of Bax and iNOS expression with few renal tubules with cytoplasmic staining was demonstrated in the renal tissue of VIL-treated group (Figs. 5D and 6D; respectively).
Fig. 5.
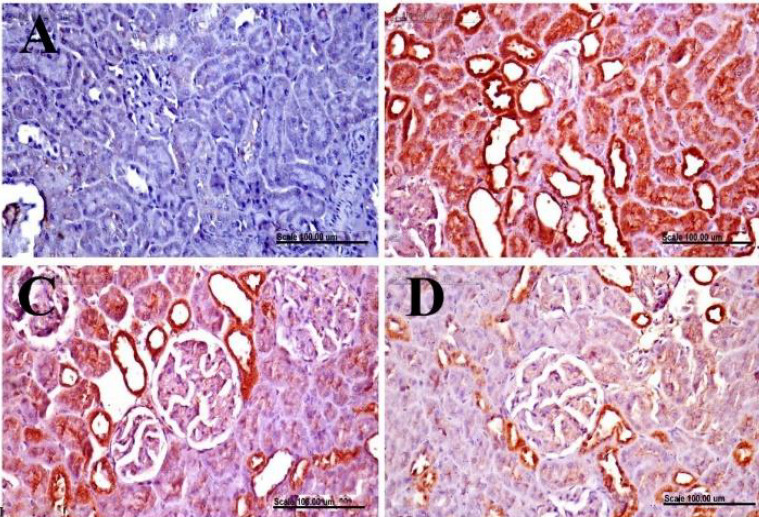
Immunohistochemical examination of Bax in renal tissue. Renal tissues, immunohistochemically stained with anti-Bax, from (A) normal control rats showing no expression of Bax in the renal tissue; (B) doxorubicin-control rats showing the increased percentage of Bax positively stained cells with diffuse and strong cytoplasmic staining of renal tubules; (C) saxagliptin-treated group showing a pronounced decrease of the percentage of positively stained cells; and (D) vildagliptin-treated group showing few renal tubules with cytoplasmic staining. (Bax immunohistochemical staining, scale bar = 100 μm).
Fig. 6.
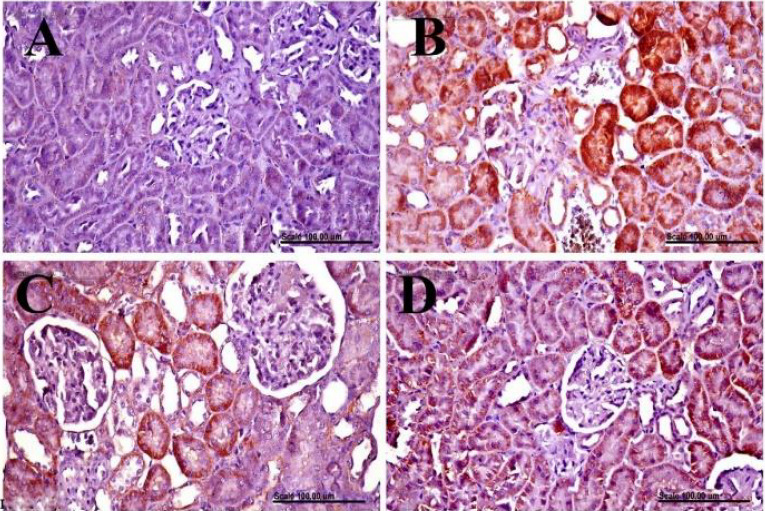
Immunohistochemical examination of iNOS in renal tissue. Renal tissues, immunohistochemically stained with anti-iNOS from (A) normal control rats showing no iNOS expression in the renal tissue; (B) doxorubicin-control rats showing the increased percentage of iNOS positively stained cells with diffuse cytoplasmic staining of renal tubules; (C) saxagliptin-treated group showing marked decrease of the percentage of iNOS positively stained cells, and (D) vildagliptin-treated group showing decreased of the percentage of iNOS positively stained cells with few renal tubules with cytoplasmic staining. iNOS immunohistochemical staining, scale bar = 100 μ. iNOS; Nitric oxide synthase.
The results of Bax and iNOS expression demonstrated in the renal tissue of normal and treated groups are illustrated in Table 3.
Table 3.
The results of Bax and iNOS expression recorded in the renal tissue of normal and treated groups. Data are presented as mean ± SEM, n = 10. Rats of the normal control group received intraperitoneal saline. Different lowercase letters are significantly different (P < 0.05).
| Groups | Bax expression (% of positive cells/HPF) | iNOS expression (% of positive cells/HPF) |
|---|---|---|
| Normal control | 0.10 ± 0.10 | 0.2 ± 0.13 |
| DXR-control (15 mg/kg, i.p) | 2.6a ± 0.16 | 2.7a ± 0.15 |
| DXR+SAX (10 mg/kg, p.o.) | 1.7b ± 0.21 | 1.4b ± 0.16 |
| DXR+VIL (10 mg/kg, p.o.) | 1.1b ± 0.10 | 1.1b ± 0.10 |
Bax, Bcl-2 Associated X-protein; DXR, doxorubicin; iNOS; nitric oxide synthase; SAX, saxagliptin; VIL, vildagliptin.
DISCUSSION
The clinical use of DXR; an antineoplastic agent; is hugely limited by its extensive multi-organ toxicity. DXR-induced nephrotoxicity has been commonly reported (9). However, the exact molecular and pathophysiological mechanisms underlying DXR-induced nephrotoxicity are not fully elucidated (10). Therefore, the current study tested the involvement of tubulointerstitial injury as well as up-regulation of NLRP3 inflammasome to DXR-induced nephrotoxicity in order to enhance its clinical use. Unfortunately, the kidneys are highly vulnerable to inflammatory as well as oxidative damage; mainly owing to the abundance of polyunsaturated fatty acids in the kidney tissues (21). The study also aimed at scouting for new therapeutic moieties; namely SAX and VIL, which may possess potential protective effects against DXR-induced nephrotoxicity in rats.
In the current work, DXR resulted in significant deterioration of renal functions as manifested by elevation of serum creatinine and BUN concentrations as compared to the normal control group. These data are in accordance with previous studies (9,13,17). Our results also demonstrated that DXR administration led to a significant increment in renal tissue concentration of inflammatory markers; TNF-α and IL-1β in comparison to the normal control group. These results are in accordance with that of Abd El-Aziz et al. who reported significant production of pro-inflammatory cytokines and chemokines as well as NF-kB stimulation post-DXR administration (22). Tu et al. reported induction of inflammatory response following DXR administration via macrophage stimulation that eventually resulted in the release of the inflammatory mediators IL-2 and TNF-α (23).
In the current work, DRX resulted in significant up-regulation of NLRP3 inflammasome as compared to normal control group. This is in line with previous data which stated that DXR administration was linked to activation and release of many inflammatory growth factors including IL-1β, IL-6, and TNF-α leading ultimately to up-regulation of NLRP3 inflammasome (24). DXR-induced inflammation and nephrotoxicity were found to be suppressed by agents that suppress NLRP3 inflammasome; e.g. N-acetyl cysteine and other inhibitors of reactive oxygen species (14,25). Previous studies showed that activation of NLRP3 occurs via macrophage priming which occurs through activation of Toll-like receptors that happens after tissue destruction and cellular death (26). The molecular mechanisms underlying these processes include the production of ribotoxic stress. Ribotoxic stressors inhibit protein translation and activate the release of p38 and MAP3K (27). DXR has been proved to be a well-characterized ribotoxic stressor because it inhibits protein translation as well as activates the release of p38 and MAP3K (28). Sauter et al. suggested that down-regulation of inflammasome NLRP3, as well as IL-1, may alleviate the inflammatory burden in cancer patients treated with DXR, thus may enhance its clinical use (24).
Moreover, DXR resulted in significant tubular injury as manifested by elevated NGAL as compared to the normal control group. Histopathological examination and glomerular histomorphometry showed significant glomerular lesions as manifested by elevated glomerular tuft area accompanied by swelling and distortion of renal tubules. NGAL is synthesized in the distal nephron in response to renal injury. NGAL is reported to be promptly and robustly secreted following acute nephrotoxicity and it is a sensitive marker for tubular injury and inflammation (29,30). Szalay et al. reported significant glomerular and tubular damage as well as tubulointerstitial inflammation post-DXR administration in rats. Damaged renal tubules resulted in elevated NGAL excretion (31). Li et al. also reported significant tubular injury following DXR administration in rats (14). NGAL concentrations are elevated significantly early in the setting of tubulitis and other tubular injuries (32). In agreement with our results, Jo et al. reported that DXR administration caused significant histopathological alterations as manifested by tubular dilation and atrophy accompanied by sloughing of the tubular epithelium (5).
Immunohistochemical examination of renal tissues of DXR-treated rats showed elevated-level of Bax and iNOS with a significant percentage increase of positively stained cells accompanied by strong and diffuse cytoplasmic staining of renal tubules as compared to the normal control group. Bax is a pro-apoptotic protein overexpressed post tubular cell death. Bax reflects the ongoing activation of mitochondrial apoptotic pathways (33). On the other hand, iNOS is a pro-inflammatory enzyme significantly expressed early in the setting of various tissue inflammations (34). El-Agamy et al. reported consistent results where DXR administration was linked to elevated immunohistochemical expression of Bax in cardiac cells, as compared to the normal group (8). Renal tissue apoptosis, manifested by increased immunohistochemical Bax expression accompanied by decreased Bcl-2 expression, was recorded in DXR-treated rats (35). In accordance with our results, recent studies reported increased immunohistochemical iNOS expression following DXR administration (10,36). Of note, increased immunohistochemical iNOS and cyclooxygenase-2 expression in renal tissues were recorded post-DXR administration; presumably due to oxidative stress as well as inflammation (37).
Therefore, searching for novel therapeutic moieties to abate DXR-induced toxicities has been mandatory in order to improve DXR clinical efficacy and tolerability (38). DPP4 inhibitors have proven-pleiotropic and anti-inflammatory actions on different biological systems independent of their antidiabetic effects. DPP-4 receptors were found to be highly expressed in kidney tissues. Moreover, DPP-4 receptors are over-expressed in response to oxidative stress, inflammation, and apoptosis (2). Many agents of the DPP4 inhibitor family have been tested to decrease the inflammatory changes accompanying chemotherapeutic agents’ use (8). Different DPP-4 inhibitors exhibit extremely different nephroprotective potential. Higashijima et al. reported that alogliptin and anagliptin decrease glomerular injury as well as macrophage infiltration, eventually lead to protective anti-inflammatory actions on kidney tissues (2).
This is by far the first study that investigated the protective anti-inflammatory effects of SAX and VIL against DXR-induced nephrotoxicity. Ribotoxic stress stimulates macrophages and initiates inflammation.
Renal inflammation results in elevation of renal tissue TNF-α, IL-1β, as well as iNOS renal expression. Renal inflammation further potentiates macrophage stimulation caused by DXR. Macrophage stimulation results in significant NLRP3 inflammasome up-regulation. Renal inflammation also stimulates tubulitis and tubular injury resulting in a significant elevation in NGAL concentrations. Eventually, renal inflammation leads to apoptosis as manifested by apoptotic renal histopathologi0cal changes as well as elevated Bax expression in renal tissues (Fig. 7).
Fig. 7.
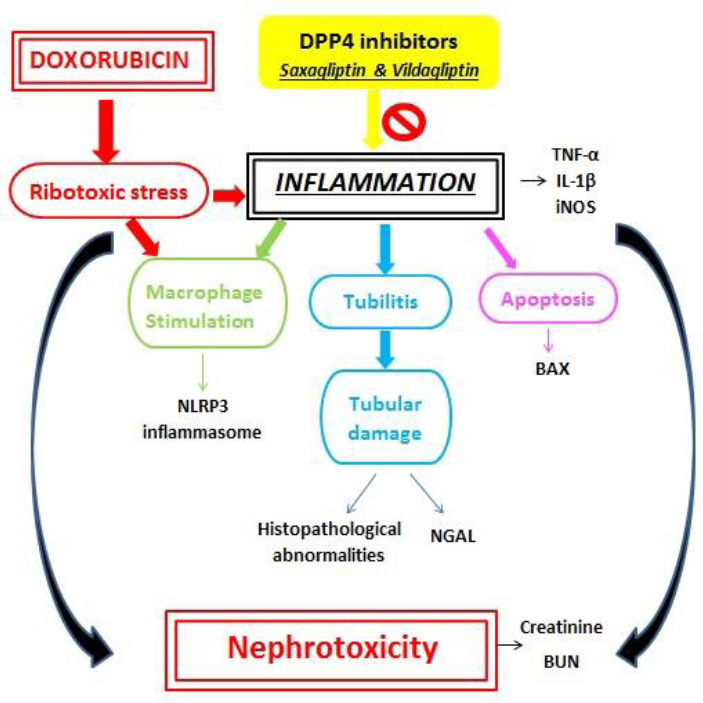
Schematic diagram of the mechanisms underlying doxorubicin-induced nephrotoxicity and the possible anti-inflammatory effects of saxagliptin and vildagliptin on renal tissue. IL-1β, Interleukin-1β; iNOS, inducible nitric oxide synthase; NGAL: Neutrophil gelatinase-associated lipocalin; NLRP3, NOD-like receptor containing pyrin domain 3; TNF-α, tumor necrosis factor-alpha.
Sax’s metabolism occurs mainly via cytochrome P450 3A4/5. 5-hydroxy saxagliptin, the major metabolite of SAX is also a member of DPP 4 family but it is only half potent as SAX. SAX is then excreted via renal and hepatic elimination (39). SAX is reported to be well-tolerated in kidney patients (6).
VIL undergoes extensive hepatic metabolism via at least four pathways prior to elimination. The major metabolite is not mediated via cytochrome P450. VIL undergoes extensive amide bond hydrolysis, glucuronidation, and oxidation (40). VIL has been reported as a potent nephron-protective agent regardless of its anti-diabetic properties (7).
The present data revealed that administration of both SAX and VIL abated all these abnormalities initiated by DXR use. SAX and VIL treatment resulted in significant improvement of renal function as was manifested by reduction of serum creatinine and BUN when compared to DXR-control group. Our data highlighted that administration of SAX and VIL in the selected doses caused a significant reduction of renal inflammation as reflected by a reduction of DXR-induced TNF-α, IL-1β, and NLRP3 inflammasome as well as immunohistochemical iNOS expression. Moreover, the use of SAX and VIL resulted in improvement of renal glomerular and tubular injuries as was reflected by reduced NGAL levels and improved histopathological pictures of renal tissues. Administration of SAX and VIL also resulted in down-regulation of Bax immunohistochemical expression as compared to the DXR-control group. The pathways demonstrating DXR-induced renal inflammation and nephrotoxicity as well as the anti -inflammatory reno-protective effects of SAX and VIL are presented in Fig. 7. Many studies reported similar results concerning the protective effects of various inhibitors of DPP4 family not only against DXR-induced renal injuries but also various chemotherapeutic-induced multi-system abnormalities. The molecular mechanisms beyond the pleiotropic effects of DPP4 inhibition are still not well-elucidated. However, these effects are hypothesized to be a mixture of their enzymatic as well as non-enzymatic properties on numerous signaling pathways (11). Both El-Agamy et al. and Kelleni et al. reported protective antioxidative, anti-apoptotic, and anti-inflammatory effects of the DPP4 inhibitor, sitagliptin, against DXR-induced cardiotoxicity in male Wistar rats (8,10). In accordance with our results, SAX use has been linked to improved renal hypertrophy, renal tissue macrophage infiltration, and histopathological markers of tubulointerstitial injury and thus it could presumably be used as an additional reno-protective drug in diabetic nephropathy (11). Also in agreement with our results, Jo et al. reported significant anti-inflammatory nephron-protective effects of sitagliptin as well as linagliptin in DXR-induced nephropathy. It has been reported that VIL exerted a cardioprotective effect against DXR-induced cardiomyopathy (41). Of note, sitagliptin and linagliptin were able to suppress NLRP3 inflammasome regardless of their ability to decrease oxidative stress (5). Our research is the first study that tested the protective anti-inflammatory effects of SAX and VIL against DXR-induced nephrotoxicity, so far.
CONCLUSION
In summary, we have elucidated the possible mechanisms beyond DXR-induced nephrotoxicity through tubulointerstitial injury as well as inflammation. DXR use has been linked to TNF-α, IL-1β, and NLRP3 inflammasome up-regulation as well as iNOS expression. The role of the selected DPP4 inhibitors; viz. SAX and VIL in mitigating the unfavorable tubular injury and inflammatory effects of DXR on the renal tissue have been thoroughly tested. Therefore, based on the current findings, we suggest further molecular and clinical studies on using SAX and VIL to abate DXR-induced nephrotoxicity.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Authors’ contribution
R.E. Mostafa participated in the practical experimentation, carried out the statistical analyses, and wrote the manuscript. G.F. Asaad participated in the practical experimentation and revised the final manuscript. A. Hassan carried out all the histopathological and immunohistochemical studies.
REFERENCES
- 1.Makino Y, Fujita Y, Haneda M. Dipeptidyl peptidase-4 inhibitors in progressive kidney disease. Curr Opin Nephrol Hypertens. 2015;24(1):67–73. doi: 10.1097/MNH.0000000000000080. DOI: 10.1097/MNH.0000000000000080. [DOI] [PubMed] [Google Scholar]
- 2.Higashijima Y, Tanaka T, Yamaguchi J, Tanaka S, Nangaku M. Anti-inflammatory role of DPP-4 inhibitors in a nondiabetic model of glomerular injury. Am J Physiol Renal Physiol. 2015;308(8):F878–F887. doi: 10.1152/ajprenal.00590.2014. DOI: 10.1152/ajprenal.00590.2014. [DOI] [PubMed] [Google Scholar]
- 3.Tanaka T, Higashijima Y, Wada T, Nangaku M. The potential for renoprotection with incretin-based drugs. Kidney Int. 2014;86(4):701–711. doi: 10.1038/ki.2014.236. DOI: 10.1038/ki.2014.236. [DOI] [PubMed] [Google Scholar]
- 4.Zoja C, Abbate M, Remuzzi G. Progression of renal injury toward interstitial inflammation and glomerular sclerosis is dependent on abnormal protein filtration. Nephrol Dial Transplant. 2015;30(5):706–712. doi: 10.1093/ndt/gfu261. DOI: 10.1093/ndt/gfu261. [DOI] [PubMed] [Google Scholar]
- 5.Jo CH, Kim S, Park JS, Kim GH. Anti-inflammatory action of sitagliptin and linagliptin in doxorubicin nephropathy. Kidney Blood Press Res. 2018;43(3):987–999. doi: 10.1159/000490688. DOI: 10.1159/000490688. [DOI] [PubMed] [Google Scholar]
- 6.Helal MG, Zaki MMAF, Said E. Nephroprotective effect of saxagliptin against gentamicin-induced nephrotoxicity, emphasis on anti-oxidant, anti-inflammatory and anti-apoptic effects. Life Sci. 2018;208:64–71. doi: 10.1016/j.lfs.2018.07.021. DOI: 10.1016/j.lfs.2018.07.021. [DOI] [PubMed] [Google Scholar]
- 7.Thongnak L, Chatsudthipong V, Lungkaphin A. Mitigation of renal inflammation and endoplasmic reticulum stress by vildagliptin and statins in high-fat high-fructose diet-induced insulin resistance and renal injury in rats. Biochim Biophys Acta, Mol Cell Biol Lipids. 2020;1865(9):158755. doi: 10.1016/j.bbalip.2020.158755. DOI: 10.1016/j.bbalip.2020. [DOI] [PubMed] [Google Scholar]
- 8.El-Agamy DS, Abo-Haded HM, Elkablawy MA. Cardioprotective effects of sitagliptin against doxorubicin-induced cardiotoxicity in rats. Exp Biol Med (Maywood) 2016;241(14):1577–1587. doi: 10.1177/1535370216643418. DOI: 10.1177/1535370216643418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demir F, Demir M, Aygun H. Evaluation of the protective effect of edaravone on doxorubicin nephrotoxicity by [99m Tc] DMSA renal scintigraphy and biochemical methods. Naunyn Schmiedebergs Arch Pharmacol. 2020;393(8):1383–1390. doi: 10.1007/s00210-020-01832-2. DOI: 10.1007/s00210-020-01832-2. [DOI] [PubMed] [Google Scholar]
- 10.Kelleni MT, Amin EF, Abdelrahman AM. Effect of metformin and sitagliptin on doxorubicin-induced cardiotoxicity in rats: impact of oxidative stress, inflammation, and apoptosis. J Toxicol 2015. 2015:1–8. doi: 10.1155/2015/424813. 2015:424813. DOI: 10.1155/2015/424813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gangadharan Komala M, Gross S, Zaky A, Pollock C, Panchapakesan U. Saxagliptin reduces renal tubulointerstitial inflammation, hypertrophy and fibrosis in diabetes. Nephrology (Carlton) 2016;21(5):423–431. doi: 10.1111/nep.12618. DOI: 10.1111/nep.12618. [DOI] [PubMed] [Google Scholar]
- 12.Nagai K, Fukuno S, Otani K, Nagamine Y, Omotani S, Hatsuda Y, et al. Prevention of doxorubicin-induced renal toxicity by theanine in rats. Pharmacology. 2018;101(3-4):219–224. doi: 10.1159/000486625. DOI: 10.1159/000486625. [DOI] [PubMed] [Google Scholar]
- 13.Demir F, Demir M, Aygun H. Evaluation of the protective effect of paricalcitol and vitamin D3 at doxorubicin nephrotoxicity in rats with 99mTechnetium-dimercaptosuccinic acid renal scintigraphy and biochemical methods. Hum Exp Toxicol. 2021;40(2):274–283. doi: 10.1177/0960327120950010. DOI: 10.1177/0960327120950010. [DOI] [PubMed] [Google Scholar]
- 14.Li W, He W, Xia P, Sun W, Shi M, Zhou Y, et al. Total extracts of Abelmoschus manihot L. attenuates adriamycin-induced renal tubule injury via suppression of ROS-ERK1/2-mediated NLRP3 inflammasome activation. Front Pharmacol. 2019;10:567–582. doi: 10.3389/fphar.2019.00567. DOI: 10.3389/fphar.2019.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maayah ZH, Takahara S, Dyck JR. The beneficial effects of reducing NLRP3 inflammasome activation in the cardiotoxicity and the anti-cancer effects of doxorubicin. Arch Toxicol. 2021;95(1):1–9. doi: 10.1007/s00204-020-02876-2. DOI: 10.1007/s00204-020-02876-2. [DOI] [PubMed] [Google Scholar]
- 16.Refaie MMM, Amin EF, El-Tahawy NF, Abdelrahman AM. Possible protective effect of diacerein on doxorubicin-induced nephrotoxicity in rats. J Toxicol 2016. 2016 doi: 10.1155/2016/9507563. 9507563,1-10. DOI: 10.1155/2016/9507563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rafiee Z, Moaiedi MZ, Gorji AV, Mansouri E. p-Coumaric acid mitigates doxorubicin-induced nephrotoxicity through suppression of oxidative stress, inflammation and apoptosis. Arch Med Res. 2020;51(1):32–40. doi: 10.1016/j.arcmed.2019.12.004. DOI: 10.1016/j.arcmed.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Altinkaynak Y, Kural B, Akcan BA, Bodur A, Özer S, Yulug E, et al. Protective effects of L-theanine against doxorubicin-induced nephrotoxicity in rats. Biomed Pharmacother. 2018;108:1524–1534. doi: 10.1016/j.biopha.2018.09.171. DOI: 10.1016/j.biopha.2018.09.171. [DOI] [PubMed] [Google Scholar]
- 19.El- Bialy BE, Abd Eldaim MA, Hassan A, Abdel- Daim MM. Ginseng aqueous extract ameliorates lambda- cyhalothrin-acetamiprid insecticide mixture for hepatorenal toxicity in rats: role of oxidative stress- mediated proinflammatory and proapoptotic protein expressions. Environ Toxicol. 2020;35(2):124–135. doi: 10.1002/tox.22848. DOI: 10.1002/tox.22848. [DOI] [PubMed] [Google Scholar]
- 20.Hassan NF, Nada SA, Hassan A, El-Ansary MR, Al-Shorbagy MY, Abdelsalam RM. Saroglitazar deactivates the hepatic LPS/TLR4 signaling pathway and ameliorates adipocyte dysfunction in rats with high-fat emulsion/LPS model-induced non-alcoholic steatohepatitis. Inflammation. 2019;42(3):1056–1070. doi: 10.1007/s10753-019-00967-6. DOI: 10.1007/s10753-019-00967-6. [DOI] [PubMed] [Google Scholar]
- 21.Jalili C, Moradi D, Roshankhah S, Salahshoor MR. Effect of pentoxifylline on kidney damage induced by nitrosamine in male rats. Res Pharm Sci. 2019;14(1):64–73. doi: 10.4103/1735-5362.251854. DOI: 10.4103/1735-5362.251854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abd El-Aziz TA, Mohamed RH, Pasha HF, Abdel-Aziz HR. Catechin protects against oxidative stress and inflammatory-mediated cardiotoxicity in adriamycin-treated rats. Clin Exp Med. 2012;12(4):233–240. doi: 10.1007/s10238-011-0165-2. DOI: 10.1007/s10238-011-0165-2. [DOI] [PubMed] [Google Scholar]
- 23.Tu Y, Sun W, Wan YG, Che XY, Pu HP, Yin XJ, et al. Huangkui capsule, an extract from Abelmoschus manihot (L.) medic, ameliorates adriamycin-induced renal inflammation and glomerular injury via inhibiting p38MAPK signaling pathway activity in rats. J Ethnopharmacol. 2013;147(2):311–320. doi: 10.1016/j.jep.2013.03.006. DOI: 10.1016/j.jep.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 24.Sauter KA, Wood LJ, Wong J, Iordanov M, Magun BE. Doxorubicin and daunorubicin induce processing and release of interleukin-1β through activation of the NLRP3 inflammasome: progress at a snail’s pace. Cancer Biol Ther. 2011;11(12):1008–1016. doi: 10.4161/cbt.11.12.15540. DOI: 10.4161/cbt.11.12.15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–677. doi: 10.1126/science.1156995. DOI: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. DOI: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jandhyala DM, Ahluwalia A, Obrig T, Thorpe CM. ZAK: a MAP3Kinase that transduces Shiga toxin-and ricin- induced proinflammatory cytokine expression. Cell Microbiol. 2008;10(7):1468–1477. doi: 10.1111/j.1462-5822.2008.01139.x. DOI: 10.1111/j.1462-5822.2008.01139.x. [DOI] [PubMed] [Google Scholar]
- 28.Sauter KA, Magun EA, Iordanov MS, Magun BE. ZAK is required for doxorubicin, a novel ribotoxic stressor, to induce SAPK activation and apoptosis in HaCaT cells. Cancer Biol Ther. 2010;10(3):258–266. doi: 10.4161/cbt.10.3.12367. DOI: 10.4161/cbt.10.3.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Devarajan P. Neutrophil gelatinase- associated lipocalin (NGAL): a new marker of kidney disease. Scand J Clin Lab Invest Suppl. 2008;241:89–94. doi: 10.1080/00365510802150158. DOI: 10.1080/00365510802150158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmidt-Ott KM, Mori K, Li JY, Kalandadze A, Cohen DJ, Devarajan P, et al. Dual action of neutrophil gelatinase-associated lipocalin. J Am Soc Nephrol. 2007;18(2):407–413. doi: 10.1681/ASN.2006080882. DOI: 10.1681/ASN.2006080882. [DOI] [PubMed] [Google Scholar]
- 31.Szalay CI, Erdélyi K, Kökény G, Lajtár E, Godó M, Révész C, et al. Oxidative/nitrative stress and inflammation drive progression of doxorubicin-induced renal fibrosis in rats as revealed by comparing a normal and a fibrosis-resistant rat strain. PLoS One. 2015;10(6):e0127090,1–17. doi: 10.1371/journal.pone.0127090. DOI: 10.1371/journal.pone.0127090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schaub S, Wilkins JA, Nickerson P. Proteomics and renal transplantation: searching for novel biomarkers and therapeutic targets. Contrib Nephrol. 2008;160:65–75. doi: 10.1159/000125934. DOI: 10.1159/000125934. [DOI] [PubMed] [Google Scholar]
- 33.Mostafa RE, Dalia OS, Dina FM. Cisplatin-induced nephrotoxicity in rats: modulatory role of simvastatin and rosuvastatin against apoptosis and inflammation. J Appl Pharm Sci. 2018;8(4):43–50. DOI: 10.7324/JAPS.2018.8406. [Google Scholar]
- 34.Salama AAA, Mostafa RE, Omara EA. Ameliorative effects of phosphodiesterase (PDE) inhibitors in potassium dichromate-induced acute renal failure in rats. Int J Pharm Sci Rev Res. 2016;36(2):40–46. [Google Scholar]
- 35.Liu LL, Li QX, Xia L, Li J, Shao L. Differential effects of dihydropyridine calcium antagonists on doxorubicin-induced nephrotoxicity in rats. Toxicology. 2007;231(1):81–90. doi: 10.1016/j.tox.2006.11.067. DOI: 10.1016/j.tox.2006.11.067. [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim MA, Ashour OM, Ibrahim YF, El-Bitar HI, Gomaa W, Abdel-Rahim SR. Angiotensin-converting enzyme inhibition and angiotensin AT1-receptor antagonism equally improve doxorubicin-induced cardiotoxicity and nephrotoxicity. Pharmacol Res. 2009;60(5):373–381. doi: 10.1016/j.phrs.2009.05.007. DOI: 10.1016/j.phrs.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Rehman MU, Tahir M, Khan AQ, Khan R, Oday-O-Hamiza , Lateef A, et al. D-limonene suppresses doxorubicin-induced oxidative stress and inflammation via repression of COX-2, iNOS, and NFκB in kidneys of Wistar rats. Exp Biol Med (Maywood) 2014;239(4):465–476. doi: 10.1177/1535370213520112. DOI: 10.1177/1535370213520112. [DOI] [PubMed] [Google Scholar]
- 38.Kumral A, Giriş M, Soluk-Tekkeşin M, Olgaç V, Doğru-Abbasoğlu S, Türkoğlu Ü, et al. Effect of olive leaf extract treatment on doxorubicin-induced cardiac, hepatic and renal toxicity in rats. Pathophysiology. 2015;22(2):117–123. doi: 10.1016/j.pathophys.2015.04.002. DOI: 10.1016/j.pathophys.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Boulton DW. Clinical pharmacokinetics and pharmacodynamics of saxagliptin, a dipeptidyl peptidase-4 inhibitor. Clin Pharmacokinet. 2017;56(1):11–24. doi: 10.1007/s40262-016-0421-4. DOI: 10.1007/s40262-016-0421-4. [DOI] [PubMed] [Google Scholar]
- 40.He H, Tran P, Yin H, Smith H, Batard Y, Wang L, et al. Absorption, metabolism, and excretion of [14C]vildagliptin, a novel dipeptidyl peptidase 4 inhibitor, in humans. Drug Metab Dispos. 2009;37(3):536–544. doi: 10.1124/dmd.108.023010. DOI: 10.1124/dmd.108.023010. [DOI] [PubMed] [Google Scholar]
- 41.Tarasova AP, Danilenko L, Tatarenkova IA, Khavanskii AV, Alena AA, Dovgan AP. Evaluation of cardioprotective effects of the incritin mimetics exenatide and vildagliptin in the experiment. Res Results Pharmacol. 2017;3(2):57–63. DOI: 10.18413/2313-8971-2017-3-2-57-63. [Google Scholar]