Abstract
Background and purpose:
Diabetes is a group of multifactorial disorders characterized by chronic-elevated blood glucose levels (hyperglycemia). Natural remedies are used as alternative medications to treat diabetes. Here, we tested the protective effect of the plant extracts of the Rosaceae family on improving insulin secretion and repairing the pancreatic beta cells in diabetic rats.
Experimental approach:
The oligosaccharide fraction was isolated from the Rosaceae family of herbs. LC-MS/MS was applied to characterize the isolated fractions. The male Wistar rats were randomly divided into six groups, 10 each, including the control group with no intervention, diabetic rats without treatment, diabetic rats that received the extract of Malus domestica (apple), Cydonia oblonga (quince), Prunus persica (nectarine), and Prunus persica (peach), separately. Rats were monitored for the weight, fasting plasma glucose, and insulin levels. The effect of extracts in streptozotocin (STZ)-induced diabetic rats on the pancreatic islets was evaluated by morphometric analysis.
Findings/Results:
LC-MS/MS results indicated a similar mass spectrum of isolated fractions from nectarine and peach with Rosa canina. Oral administration of nectarine and peach extracts to STZ-induced diabetic rats showed restoration of blood glucose levels to normal levels with a concomitant increase in insulin levels. Morphometric analysis of pancreatic sections revealed the increase in number, diameter, volume, and area of the pancreatic islets in the diabetic rats treated with extracts compared to the untreated diabetic rats.
Conclusion and implications:
Nectarine and peach extracts’ anti-diabetic properties improved insulin secretion and pancreatic beta-cell function and subsequently led to restoring pancreatic islet mass in STZ-induced diabetic rats.
Keywords: Diabetes, Insulin, Morphometric analysis, Nectarine, Pancreas, Peach, Streptozotocin
INTRODUCTION
Diabetes, a disease in which the body does not produce or properly use insulin, is a growing public health concern that affects many individuals worldwide. Diabetes has serious impacts on both patients and societies. The cost of its treatment has been estimated to be more than 825 billion USD per year (1). The incidence of diabetes worldwide was estimated at 382 million people and this number is expected to reach 439 million by the year 2030 (2). Currently, standard treatments for diabetes are injection of hypoglycemic agents (insulin, insulin analogs, etc.) and/or oral administration of synthetic hypoglycemic drugs, such as sulfonylureas, biguanides, glinides, glycosidase inhibitors, and thiazolidinedione derivatives. Owing to the side effects of medications and reduced sensitivity of insulin receptors due to long-term use of insulin, insulin resistance occurs and management of the conditions is failed (3).
A promising treatment of diabetes involves sustained glycemic control without hypoglycemia and other side effects.
Herbal remedies are among the best existing alternative therapies, which have been used since the ancient to treat diabetes. To this date, more than 400 traditional herbal treatments have been suggested to treat diabetes. However, some of plant-based treatments have been clinically assessed for their efficacy (4). Generally, the anti-hyperglycemic activity of the plants is mainly due to their ability to restore the function of pancreatic tissues by increasing insulin secretion or inhibiting the intestinal absorption of glucose or to the facilitation of metabolites in insulin-dependent processes (5).
Streptozotocin (STZ) is a compound that has preferential toxicity toward pancreatic β cells. Commonly, STZ injections have been used to induce diabetes in animal models. It binds to glucose transporter 2 in beta cell plasma membrane, enters cells and destroys them. The effect of STZ is observed by blood glucose-levels changes. A marked increase in blood glucose confirms the induction of diabetes and the effect of STZ (6,7).
In the previous work, we investigated the anti-diabetic activity and the mechanism of action of crude extract of Rosa canina as a member of the Rosaceae family in vitro. Our results confirmed that the isolated polysaccharide from Rosa canina can promote the proliferation of pancreatic beta cells providing a novel mechanism for the anti-diabetic effect of the plant (8). Also, we purified and evaluated a novel oligosaccharide with anti-diabetic activity from Rosa canina (9,10,11). We used high-performance liquid chromatography diode array detector tandem mass spectroscopy (HPLC-DAD MS/MS), infrared radiation (IR), and nuclear magnetic resonance (NMR) techniques and identified an oligosaccharide with a pectin structure composed of repeated tetrasaccharides ([C29H42O26] n) and several acetyl and methoxycarbonyl groups (Fig. 1) (10). In vitro study suggested that high concentrations of oligosaccharide increased cell death, while at low concentration protected beta-pancreatic cells from STZ-induced cell death. The anti-diabetic activity of this oligosaccharide was confirmed at the molecular level (10). Due to structural similarity between the Rosaceae family and the Rosa canina, we investigated the presence of any new oligosaccharide with anti-diabetogenic activity in the large family of Rosaceae.
Fig. 1.
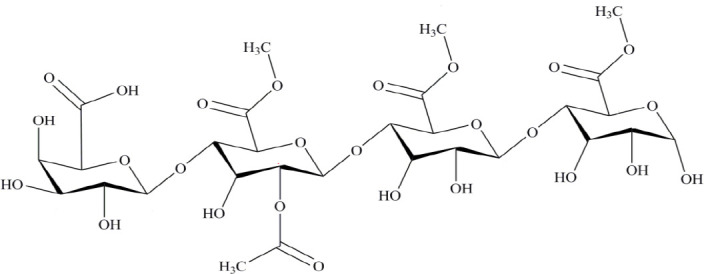
The structure of isolated oligosaccharide from Rosa canina. The chemical structure of oligosaccharide was characterized using HPLC, IR, NMR and MS analyses (8).
MATERIAL AND METHODS
Plant material collection and preparation of extracts
Plants were collected from different parts of Kermanshah province in western Iran during Nov-Dec (Table 1). After the separation of fruits, stems, and roots, they were dried and stored at appropriate temperatures and humidity. Next, the dried plants were cut into small pieces. To prepare the aqueous extract, 10 g of the plant material was boiled in 200 mL of deionized water to reach a final volume of 100 mL. The samples were then filtered using Whitman paper. During the purification step, the extract was precipitated by three times its volume of 96% ethanol and the resulting precipitate was dissolved in deionized water. The fractions containing the corresponding oligosaccharide were isolated according to the previous method described for the isolation of Rosa canina (10).
Table 1.
The intensity of the isolated oligosaccharide fraction of the plants detected using liquid chromatography-mass spectroscopy.
| Common name | Organ | Family | Scientific name | Intensity of peak corresponding to oligosaccharide |
|---|---|---|---|---|
| Blood peach | Roots, stems | Rosaceae | Prunus persicea | - |
| White nectarine | Roots, stems | Rosaceae | Prunus persicea | ++ |
| Cherry | Roots, stems | Rosaceae | Prunus cerasus | + |
| Red apple | Roots, stems | Rosaceae | Malus domestica | - |
| Reine Claude Verte | Stems, fruits | Rosaceae | Prunus cerasifera | -, Busy peak |
| Apricot | Roots, fruits | Rosaceae | Prunus armeniaca | - |
| Bitter cherry | Stems | Rosaceae | Prunus cerasus | - |
| Sour cherry | Stems | Rosaceae | Prunus cerasus | - |
| Yellow apple | Roots, stems | Rosaceae | Malus domestica | -and +, respectively |
| Green almond | Fruits | Rosaceae | Prunus cerasifera | Crowded spectrum |
| Quince | Roots, stems | Rosaceae | Cydonia oblonga | -and +, respectively |
| Rosa canina | Stems, fruits | Rosaceae | Rosa canina | +++ |
| Common medlar | Fruits | Rosaceae | Mespilus germanica | Crowded spectrum |
| Prune | Roots | Rosaceae | Prunus cerasifera | - |
| Strawberry | Fruits | Rosaceae | Fragaria ananassa | ++ |
-, Lack of the desired peak; +, weak peak; ++, strong peak.
Liquid chromatography-mass spectrometry
The isolated fractions collected from the purification process previously described (10) were filtered and further subjected to a liquid chromatography-mass spectrometry (LC-MS/MS) to examine and screen for the presence and quantity of oligosaccharide as one of the main antidiabetic constituents in the plant extracts. The active ingredient purified in the previous study (oligosaccharide from Rosa canina identified peak at 366 m/z) was used as the standard to identify the elution time of this compound.
Animal study
Adult male Wistar rats weighing 220-250 g provided by the Pasteur Institute of Iran (Tehran, I.R. Iran), maintained in a controlled environment with 12/12-h light/dark cycle, room temperature of 24-25 °C and humidity of 55% and had ad libitum access to dry laboratory food and water. Diabetes was induced via a single intraperitoneal injection of 45 mg/kg STZ (Sigma, Germany) dissolved in cold normal saline. Nondiabetic animals received intraperitoneal injection of normal saline only as the vehicle. The blood samples were withdrawn from the rat’s tail vein 48 h after the injection and their glucose levels were measured using a glucometer (GlucoDr, South Korea). Rats were considered diabetic if their blood glucose levels were greater than 200 mg/dL. Afterward, the rats were randomly divided into 6 groups of 10 each including the control group, STZ-induced diabetic group, and STZ-induced diabetic groups treated with the isolated fractions (2 mL of 40% by daily gavage) of Malus domestica (apple), Cydonia oblonga (quince), Prunus persica (nectarine), and Prunus persica (peach) for 4 weeks.
A
Measurement of insulin and fasting blood glucose
Blood samples were collected from the rat tail vein and the fasting blood glucose, as well as the blood insulin, were measured after 8 weeks using a glucometer (GlucoDr, South Korea) and enzyme-linked immunosorbent assay kit (insulin ELISA kit, Ab100578, Abcam, Cambridge, UK), respectively.
Immunohistochemical technique and hematoxylin and eosin staining
The rats were sacrificed at the end of the experiments. The pancreases were removed, fixed in 10% formalin, and after tissue processing (dehydration in ascending alcohols and clarification with xylem) were paraffin-embedded. Then, from each tissue sample, 5 μm sections were prepared with a microtome (Microm, EC350-2) on a slide. The slides were stained with insulin antibodies according to the Dako immunohistochemical kit (Denmark). Quantitative evaluation was performed to detect the regeneration and/or increase in the islet beta cells. Also, the slides were stained with the hematoxylin-eosin solution according to the standard method and histopathological changes were examined by light microscopy.
Statistical analysis
All data are presented as mean ± standard deviation (SD) of three independent experiments. Statistical evaluation was done using one-way analysis of variance (ANOVA) with SPSS version 21 (SPSS Inc., Chicago, IL, USA) software, and the level of significance was set at P <0.05.
RESULTS
Analysis of isolated fractions from Rosacea family plants for oligosaccharide
Owing to the promising anti-diabetic activity of an isolated oligosaccharide fraction with pectin structure from Rosa canina (Fig. 1), LC-MS/MS analyses were accomplished on isolated fractions to characterize whether the oligosaccharide is present in other Rosacea family of plants. The LC-MS/MS data showed that the roots of quince, prune, bitter cherry, red apple, rose apple, yellow apple, cherry, apricot, fig peach, blood peach, as well as the stems of Reine claude Verte, sour cherry, and bitter cherry lacked the desired oligosaccharide peak when compared to oligosaccharide standard. While the stems of yellow apple, quince, and cherry had a weak peak. The rest of the samples had stronger peaks of the active ingredient, but some of them including fruits of green almond, Reine claude Verte, common medlar, and unripe apricot had a crowded spectrum that could not be easily identified due to the presence of similar compounds of oligosaccharide derivatives. The peak intensity of the active ingredient in the LC-MS/MS showed that among all samples examined, the fruits of strawberry and the stem of Malus domestica (red apple), Prunus persica (nectarine), Prunus persica (peach), and Malus domestica (white nectarine) had the most amount and highest purity of the oligosaccharides (Table 1). Representative mass spectrum of Rosa canina fruit purified oligosaccharide (Fig. 2A) as the standard; and fractions isolated from some other plants such as Prunus cerasus (bitter cherry) stem (Fig. 2B), as the fraction lacking oligosaccharide); Prunus cerasus (cherry) stem (Fig. 2C), as the fraction with a low amount of oligosaccharide); Prunus armeniaca (apricot) fruit (Fig. 2D), as the fraction showing a crowded spectrum; and Prunus persica (Peach) stem (Fig. 2E), as the fraction containing a high quantity of oligosaccharide are illustrated in Fig. 2. Therefore, the stem of Prunus persica (peach), Malus domestica (apple), Prunus armeniaca (apricot), and Cydonia oblonga (quince) was adopted for further in vitro analysis.
Fig. 2.
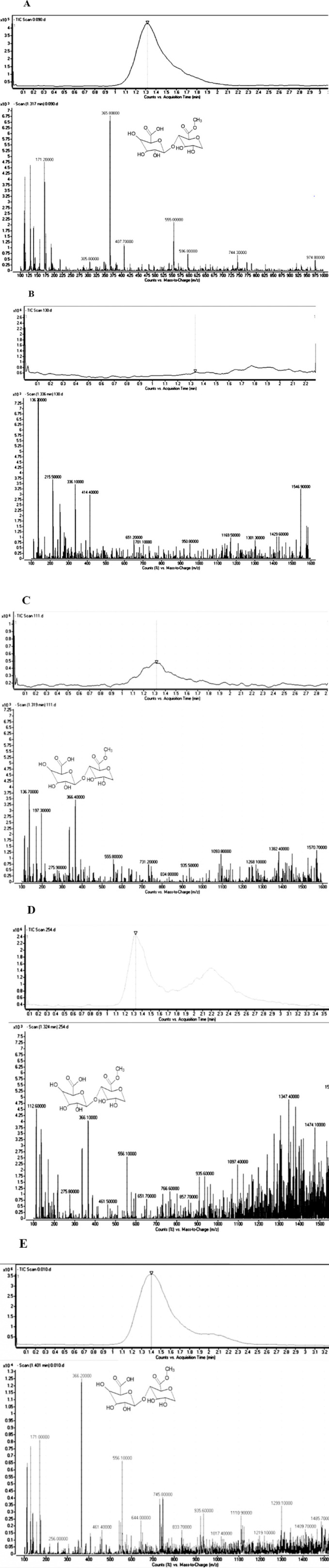
Representative mass spectrum of the isolated fractions of some specific plants. (A) Oligosaccharide purified from Rosa canina extract, (B) stem of Prunus cerasus (bitter cherry, lacking oligosaccharide), (C) stem of Prunus cerasus (cherry, weak presence of oligosaccharide), (D) fruit of Prunus armeniaca (apricot, crowded spectrum), and (E) stem of Prunus persicea (peach, presence of high quantity of oligosaccharide). The mass-to-charge ratio (366) was selected as the base peak.
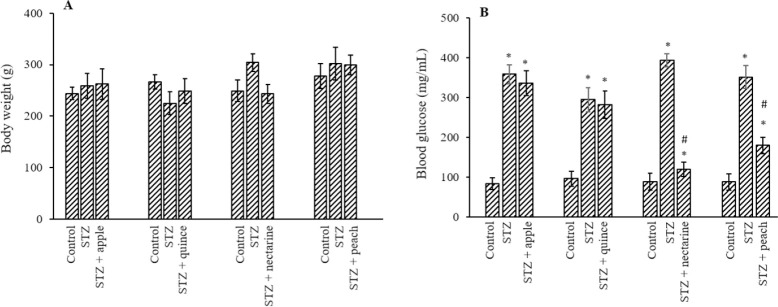
Effect of isolated fractions from nectarine and peach on body weight, blood glucose, and insulin level
The weight changes of rats were subtle (P > 0.05; Fig. 3A). The rats treated with the isolated fractions of nectarine and peach exhibited a drastic decrease in the fasting blood glucose (P < 0.05). The rats treated with apple and quince extract, on the other hand, did not show any significant changes in the blood glucose (P > 0.05; Fig. 3B). Therefore, we only used the extract of Prunus persica (nectarine) and Prunus persica (peach) for further analysis and experiments.
Fig. 3.
The effect of different isolated fractions (2 mL of 40% V/W) on (A) the body weight and (B) the blood glucose level of rats. The results show the mean ± SD. n = 3. *P < 0.05 Indicate significant differences compared with the control group and #P < 0.05 versus the STZ-treated group. STZ, Streptozotocin.
The blood insulin level of diabetic rats was lower than normal rats (P < 0.05). These decreased insulin levels were probably due to the damage to beta cells and the reduction of secreted insulin by these cells. On the other hand, symptoms of diabetes mellitus in rats were disappeared after 8 weeks of Prunus persica (nectarine) and Prunus persica (peach) extracts reception. The results showed that their blood insulin levels were significantly higher than the diabetic group (P < 0.05; Fig. 4).
Fig. 4.
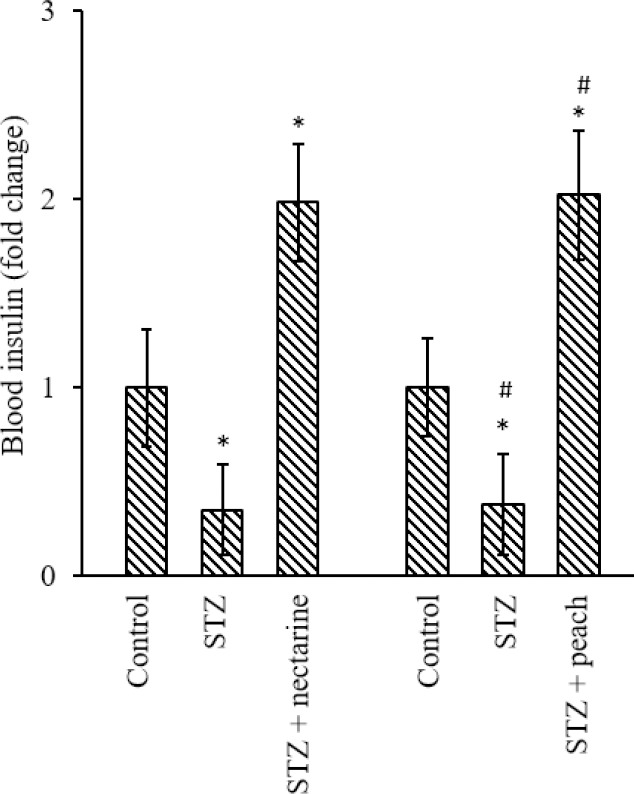
The effect of different isolated fractions (2 mL of 40% V/W) on the blood insulin level of rats. The results show the mean ± SD. n = 3. *P < 0.05 Indicates significant differences compared with the control group and #P < 0.05 versus the STZ-treated group. STZ, Streptozotocin.
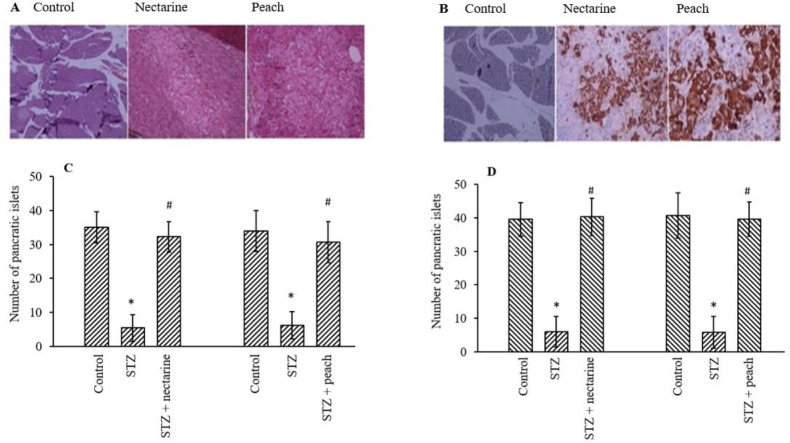
Effect of extracts on beta cells in the islands of Langerhans
Two methods of hematotoxin/eosin and insulin-specific antibody staining were used for pancreatic tissues (Fig. 5). Islet counts showed a significant increase in the mean number of islands in extracts-treated groups compared to the diabetic rats (P < 0.05). Also, the islets were approximately 2.5-fold larger in rats receiving both STZ and extracts than in rats receiving STZ. The volume of islands increased by 4 times as much as by the use of extracts.
Fig. 5.
The effect of nectarine and peach fractions on the regeneration of the Langerhans islets in diabetic animals. (A) hematoxylin and eosin and (B) immunohistochemical staining (magnification 40×) and (C and D) the number of the pancreatic islets from three independent experiments performed in triplicate. *P < 0.05 indicate significant differences compared with the control group and #P < 0.05 versus STZ group. STZ, Streptozotocin.
DISCUSSION
We showed that the plant extracts from the Rosaceae family have an anti-diabetic activity. Moreover, it has been indicated the extracts of Prunus persica (nectarine) and Prunus persica (peach) regenerated the pancreas by regeneration fashion. Given the promising anti-diabetic activities of Rosa canina extract and its isolated polysaccharide in our previous studies (8,10,12,13), it is prompted us to evaluate the hypoglycemic effects of other members of the Rosaceae family. To address this, the extract from fruits, stems, and roots of the plants was prepared and evaluated for the polysaccharide peak in the mass spectrum. According to the results, Prunus armeniaca (apricot), and Cydonia oblonga (quince) were considered for further in vitro stu dies.
Among the Rosaceae family, eight number of them including Rosa canina, Agrimonia eupatoria, Geum urbanum, Filipendula ulmaria, Crataegus monogyna, and Potentilla erecta were numerously used in pharmaceutical and medical applications (14,15). In this context, there is mounting evidence that the Rosaceae family possess anti-inflammatory effect (16,17), tumor growth inhibitory effects (18,19), liver protective activity (20), antioxidant (17), antiviral (17), and anti-diabetic properties. The anti-diabetic potential of Sorbus decora CK Schneid (Rosaceae) extract was confirmed in three models (pre-diabetes, types 1 and 2 diabetes) of an animal model (21). Sorbus decora CK Schneid extract also can potentiate uptake of glucose in a mouse myoblast cell line (22).
The lowered blood glucose levels in the rats treated with the isolated fractions from Prunus persica (nectarine) and Prunus persica (peach) extract are most likely due to a reduction in glucose uptake, glucose biosynthesis, and oral release of the stored glucose (e.g. glycogen). It can also be the result of an increase in glucose metabolism, storage of glucose in body organs such as liver and muscle, or the production of secreted hormones such as insulin. Considering the potency of all discussed factors, the production and secretion of insulin are more powerful regulators of glucose metabolism and therefore it is the main cause of improvements. Blood insulin levels were significantly decreased by STZ. Since both extracts increased the animals’ blood insulin levels, we investigated their effect on pancreatic islets. The number of islands increased significantly by extracts treatment. Thus, the increase in the number of pancreatic islets and beta cells may be a reason for the increase in blood insulin levels. Moreover, our microscopic qualitative examination of the island’s size confirmed these results. An increase in the size of the islets indicated an increase in the number of beta cells and suggesting the regeneration possibility. We plan in the future to further investigate this scenario by focusing on the stem/progenitors and cell cycle genes.
It has been suggested that the therapeutic effect of medicinal herbs is related to their active ingredients. Carbohydrates including oligosaccharides and polysaccharides extracted from herbs, fungi, and algae are effective in the treatment of several pathologies are used in the clinic (23,24,25,26,27). The prebiotic effect of arabinoxylan oligosaccharide isolated from wheat (28), antihyperglycemic effects of polysaccharide extracted from Memordica charantia (29), Inonotus obliquus (30), Hericium erinaceus (31), Ophiopogon japonicas (32), immune-modulatory effect of polysaccharide from Hibiscus sabdariffa L. (33), antioxidantive potential of polysaccharide from Euryale ferox Salisb. (34), antitumor effect of polysaccharide from Polygonum multiflorum (35) and other therapeutic efficacies of polysaccharides and oligosaccharides were investigated in the literature.
In line with the results of previous studies on the antidiabetic effects of the isolated fraction from Rosa canina, our results depicted that Rosacea plants with the same isolated fraction have the anti-diabetic potential compared to ones with no identified peak in the mass spectrum.
The antidiabetic effects of some of these plants were also reported in previous studies. For instance, quercetin-rich ethyl acetate fraction (PP-EtOAc) of leaves of Prunus persica was evaluated for antidiabetic activity. Results showed that at 200 mg/kg, significant anti-hyperglycemic activity (P < 0.05) was observed in the rat models (36). The antidiabetic ability of Cydonia oblonga and the underlying mechanism of action were investigated in L6 skeletal muscle cells. The results suggested that Cydonia oblonga promoted glucose metabolism by activating PI3K/AKT signaling pathway (37). Also, a study indicated promising antioxidant potentials of alcoholic extracts of apple (Malus domestica) manifested by elevation of reduced glutathione content (38).
Our data showed that the oligosaccharides identified in Rosa canina extract are probably responsible for their anti-diabetic properties in members of the Rosaceae family.
CONCLUSION
In conclusion, the Prunus persica (nectarine) and Prunus persica (peach) extracts significantly reduced blood glucose levels in STZ-induced diabetic rats. Blood insulin measurement of control and treated rats showed that the insulin level was significantly increased most likely due to the pancreatic islet beta cell regeneration. Overall, our data suggest that extracts have antidiabetic properties and that can mediate by enhancing insulin production in the pancreatic islet cells in diabetic models.
Conflict of interest statement
The authors declared no conflict of interest in this study.
Author’s contributions
Gh. Bahrami designed the experiments and supervised the research. B. Izadi and B. Mohammadi analyzed the data and co-authored the manuscript. M.T. Bahrami and S.Sh. Miraghaee performed all of the experiments and wrote the manuscript. S. Sajadimajd and A. Babaei edited the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This research was financially supported by Vice-Chancellor for Research of Kermanshah University of Medical Sciences, Kermanshah, the I.R. Iran under Grant No. 94195.
REFERENCES
- 1.Moucheraud C, Lenz C, Latkovic M, Wirtz VJ. The costs of diabetes treatment in low-and middle-income countries: a systematic review. BMJ Glob Health. 2019;4(1):e001258,1–12. doi: 10.1136/bmjgh-2018-001258. DOI: 10.1136/bmjgh-2018-001258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmet PZ, Magliano DJ, Herman WH, Shaw JE. Diabetes: a 21st century challenge. Lancet Diabetes Endocrinol. 2014;2(1):56–64. doi: 10.1016/S2213-8587(13)70112-8. DOI: 10.1016/S2213-8587(13)70112-8. [DOI] [PubMed] [Google Scholar]
- 3.Bai L, Li X, He L, Zheng Y, Lu H, Li J, et al. Antidiabetic potential of flavonoids from traditional Chinese medicine: a review. Am J Chin Med. 2019;47(5):933–957. doi: 10.1142/S0192415X19500496. DOI: 10.1142/S0192415X19500496. [DOI] [PubMed] [Google Scholar]
- 4.Khazaei M, Pazhouhi M. Protective effect of hydroalcoholic extracts of Trifolium pratense L. on pancreatic β cell line (RIN-5F) against cytotoxicity of streptozotocin. Res Pharm Sci. 2018;13(4):324–331. doi: 10.4103/1735-5362.235159. DOI: 10.4103/1735-5362.235159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel DK, Prasad SK, Kumar R, Hemalatha S. An overview on antidiabetic medicinal plants having insulin mimetic property. Asian Pac J Trop Biomed. 2012;2(4):320–330. doi: 10.1016/S2221-1691(12)60032-X. DOI: 10.1016/S2221-1691(12)60032-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Müller A, Schott-Ohly P, Dohle C, Gleichmann H. Differential regulation of Th1-type and Th2-type cytokine profiles in pancreatic islets of C57BL/6 and BALB/c mice by multiple low doses of streptozotocin. Immunobiology. 2002;205(1):35–50. doi: 10.1078/0171-2985-00109. DOI: 10.1078/0171-2985-00109. [DOI] [PubMed] [Google Scholar]
- 7.Sun N, Yang G, Zhao H, Savelkoul HF, An L. Multidose streptozotocin induction of diabetes in BALB/c mice induces a dominant oxidative macrophage and a conversion of TH1 to TH2 phenotypes during disease progression. Mediators Inflamm. 2005;2005(4):202–209. doi: 10.1155/MI.2005.202. DOI: 10.1155/MI.2005.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fattahi A, Niyazi F, Shahbazi B, Farzaei MH, Bahrami G. Antidiabetic mechanisms of Rosa canina fruits. An in vitro evaluation. J Evid Based Complementary Altern Med. 2017;22(1):127–133. doi: 10.1177/2156587216655263. DOI: 10.1177/2156587216655263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahrami G. Herbal extract composition for the treatment of diabetes and a method of extracting the same. Google Patents, 2016. No. US20140256673A1. https://patents.google.com/patent/US20140256673A1/en .
- 10.Rahimi M, Sajadimajd S, Mahdiand Z, Hemmati M, Malekkhatabi P, Bahrami G, et al. Characterization and anti-diabetic effects of the oligosaccharide fraction isolated from Rosa canina in STZ-induced diabetic rats. Carbohydr Res. 2020;489:107927. doi: 10.1016/j.carres.2020.107927. DOI: 10.1016/j.carres.2020. [DOI] [PubMed] [Google Scholar]
- 11.Bahrami G, Miraghaee SS, Mohammadi B, Bahrami MT, Taheripak G, Keshavarzi S, et al. Molecular mechanism of the anti-diabetic activity of an identified oligosaccharide from Rosa canina. Res Pharm Sci. 2020;15(1):36–47. doi: 10.4103/1735-5362.278713. DOI: 10.4103/1735-5362.278713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sajadimajd S, Bahrami G, Mohammadi B, Nouri Z, Farzaei MH, Chen JT. Protective effect of the isolated oligosaccharide from Rosa canina in STZ-treated cells through modulation of the autophagy pathway. J Food Biochem. 2020;44(10):e13404. doi: 10.1111/jfbc.13404. DOI: 10.1111/jfbc.13404. [DOI] [PubMed] [Google Scholar]
- 13.Bahrami G, Sajadimajd S, Mohammadi B, Hatami R, Miraghaee S, Keshavarzi S, et al. Anti-diabetic effect of a novel oligosaccharide isolated from Rosa canina via modulation of DNA methylation in Streptozotocin-diabetic rats. Daru. 2020;28(2):581–590. doi: 10.1007/s40199-020-00363-8. DOI: 10.1007/s40199-020-00363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castroviejo S. Flora Iberica. Madrid: Real Jardín Botánico. CSIC2012. 1986:1–8. 10-15, 17-18, 21. [Google Scholar]
- 15.Garcia-Oliveira P, Fraga-Corral M, Pereira A, Lourenço-Lopes C, Jimenez-Lopez C, Prieto M, et al. Scientific basis for the industrialization of traditionally used plants of the Rosaceae family. Food Chem. 2020;330:127197. doi: 10.1016/j.foodchem.2020.127197. DOI: 10.1016/j.foodchem.2020. [DOI] [PubMed] [Google Scholar]
- 16.Bae H, Kim HJ, Shin M, Lee H, Yin CS, Ra J, et al. Inhibitory effect of Agrimoniae Herba on lipopolysaccharide-induced nitric oxide and proinflammatory cytokine production in BV2 microglial cells. Neurol Res. 2010;32(Supp1):53–57. doi: 10.1179/016164109X12537002794002. DOI: 10.1179/016164109X12537002794002. [DOI] [PubMed] [Google Scholar]
- 17.Yoon SJ, Koh EJ, Kim CS, Zee OP, Kwak JH, Jeong WJ, et al. Agrimonia eupatoria protects against chronic ethanol-induced liver injury in rats. Food Chem Toxicol. 2012;50(7):2335–2341. doi: 10.1016/j.fct.2012.04.005. DOI: 10.1016/j.fct.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Tsirigotis-Maniecka M, Pawlaczyk-Graja I, Ziewiecki R, Balicki S, Matulová M, Capek P, et al. The polyphenolic-polysaccharide complex of Agrimonia eupatoria L. as an indirect thrombin inhibitor-isolation and chemical characterization. Int J Biol Macromol. 2019;125:124–132. doi: 10.1016/j.ijbiomac.2018.12.017. DOI: 10.1016/j.ijbiomac.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 19.Ad’hiah AH, Al-Bederi ON, Al-Sammarrae KW. Cytotoxic effects of Agrimonia eupatoria L. against cancer cell lines in vitro. J Assoc Arab Univ Basic Appl Sci. 2013;14(1):87–92. DOI: 10.1016/j.jaubas.2013.01.003. [Google Scholar]
- 20.Al-Snafi AE. The pharmacological and therapeutic importance of Agrimonia eupatoria-a review. Asian J Pharm Sci Technol. 2015;5(2):112–117. [Google Scholar]
- 21.Vianna R, Brault A, Martineau LC, Couture R, Arnason JT, Haddad PS. in vivo anti-diabetic activity of the ethanolic crude extract of Sorbus decora CK Schneid. (Rosacea): a medicinal plant used by Canadian James Bay Cree nations to treat symptoms related to diabetes. Evid Based Complement Alternat Med 2011. 2011;237941:1–7. doi: 10.1093/ecam/nep158. DOI: 10.1093/ecam/nep158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spoor DC, Martineau LC, Leduc C, Benhaddou-Andaloussi A, Meddah B, Harris C, et al. Selected plant species from the Cree pharmacopoeia of northern Quebec possess anti-diabetic potential. Can J Physiol Pharmacol. 2006;84(8-9):847–858. doi: 10.1139/y06-018. DOI: 10.1139/y06-018. [DOI] [PubMed] [Google Scholar]
- 23.Orhan DD, Hartevioglu A, Küpeli E, Yesilada E. In vivo anti-inflammatory and antinociceptive activity of the crude extract and fractions from Rosa canina L. fruits J Ethnopharmacol. 2007;112(2):394–400. doi: 10.1016/j.jep.2007.03.029. DOI: 10.1016/j.jep.2007.03.029. [DOI] [PubMed] [Google Scholar]
- 24.Uno K, Kosuna K, Sun B, Fujii H, Wakame K, Chikumaru S, et al. Active hexose correlated compound (AHCC) improves immunological parameters and performance status of patients with solid tumors. Biotherapy-Tokyo. 2000;14(3):303–307. [Google Scholar]
- 25.Kumosani TA, Balamash KS, Ghashlan H, Mohamed YA, Baothman OA, Zeyadi M, et al. Potential antioxidant and anti-proliferative activities of biologically active marine algae extracts. J Pharm Res Int. 2017;19(6):1–7. [Google Scholar]
- 26.Ribeiro DML, Carvalho Júnior AR, de Macedo GHR, Chagas VL, Silva LdS, Cutrim BdS, et al. Polysaccharide-based formulations for healing of skin-related wound infections: lessons from animal models and clinical trials. Biomolecules. 2019;10(1):63–78. doi: 10.3390/biom10010063. DOI: 10.3390/biom10010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wasser SP. Medicinal mushrooms in human clinical studies. Part I. Anticancer, oncoimmunological, and immunomodulatory activities: a review. Int J Med Mushrooms. 2017;19(4):279–317. doi: 10.1615/IntJMedMushrooms.v19.i4.10. DOI: 10.1615/IntJMedMushrooms.v19.i4.10. [DOI] [PubMed] [Google Scholar]
- 28.Neyrinck AM, Hiel S, Bouzin C, Campayo VG, Cani PD, Bindels LB, et al. Wheat-derived arabinoxylan oligosaccharides with bifidogenic properties abolishes metabolic disorders induced by western diet in mice. Nutr Diabetes. 2018;8(1):15–19. doi: 10.1038/s41387-018-0019-z. DOI: 10.1038/s41387-018-0019-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang C, Huang M, Hong R, Chen H. Preparation of a Momordica charantia L. polysaccharide-chromium (III) complex and its anti-hyperglycemic activity in mice with streptozotocin-induced diabetes. Int J Biol Macromol. 2019;122:619–627. doi: 10.1016/j.ijbiomac.2018.10.200. DOI: 10.1016/j.ijbiomac.2018.10.200. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Wang C, Li S, Li W, Yuan G, Pan Y, et al. Anti-diabetic effects of Inonotus obliquus polysaccharides in streptozotocin-induced type 2 diabetic mice and potential mechanism via PI3K-Akt signal pathway. Biomed Pharmacother. 2017;95:1669–1677. doi: 10.1016/j.biopha.2017.09.104. DOI: 10.1016/j.biopha.2017.09.104. [DOI] [PubMed] [Google Scholar]
- 31.Zhang C, Li J, Hu C, Wang J, Zhang J, Ren Z, et al. Antihyperglycaemic and organic protective effects on pancreas, liver and kidney by polysaccharides from Hericium erinaceus SG-02 in streptozotocin-induced diabetic mice. Sci Rep. 2017;7(1):10847,1–13. doi: 10.1038/s41598-017-11457-w. DOI: 10.1038/s41598-017-11457-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang LY, Wang Y, Xu DS, Ruan KF, Feng Y, Wang S. MDG-1, a polysaccharide from Ophiopogon japonicus exerts hypoglycemic effects through the PI3K/Akt pathway in a diabetic KKAy mouse model. J Ethnopharmacol. 2012;143(1):347–354. doi: 10.1016/j.jep.2012.06.050. DOI: 10.1016/j.jep.2012.06.050. [DOI] [PubMed] [Google Scholar]
- 33.Zheng D, Zou Y, Cobbina SJ, Wang W, Li Q, Chen Y, et al. Purification, characterization and immunoregulatory activity of a polysaccharide isolated from Hibiscus sabdariffa L. J Sci Food Agric. 2017;97(5):1599–1606. doi: 10.1002/jsfa.7908. DOI: 10.1002/jsfa.7908. [DOI] [PubMed] [Google Scholar]
- 34.Shang HM, Zhou HZ, Li R, Duan MY, Wu HX, Lou YJ. Extraction optimization and influences of drying methods on antioxidant activities of polysaccharide from cup plant (Silphium perfoliatum L.) PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0183001. e0183001,1-18. DOI: 10.1371/journal.pone.0183001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu W, Xue X, Zhang Z. Ultrasonic-assisted extraction, structure and antitumor activity of polysaccharide from Polygonum multiflorum. Int J Biol Macromol. 2016;91:132–142. doi: 10.1016/j.ijbiomac.2016.05.061. DOI: 10.1016/j.ijbiomac.2016.05.061. [DOI] [PubMed] [Google Scholar]
- 36.Sharma G, Kumar S, Sharma M, Upadhyay N, Ahmed Z, Mahindroo N. Anti-diabetic, anti-oxidant and anti-adipogenic potential of quercetin rich ethyl acetate fraction of Prunus persica. Pharmacogn J. 2018;10(3):463–469. DOI:10.5530/pj.2018.3.76. [Google Scholar]
- 37.Tang D, Xie L, Xin X, Aisa H. Anti-diabetic action of Cydonia oblonga seed extract: improvement of glucose metabolism via activation of PI3K/AKT signaling pathway. J Pharmacogn Phytochem. 2016;4(2):7–13. [Google Scholar]
- 38.Patel I, Padse O, Ingole Y. Comparative analysis of antioxidant and antidiabetic activity for apple (Malus domestica), banana (Musa paradisiaca) & kiwi (Actinidia deliciosa) Int J Res Advent Technol. 2015:28–31. Special Issue National Conference “ACGT 2015”. [Google Scholar]