Abstract
Background
This is one of a series of reviews of methods of cervical ripening and labour induction using standardized methodology.
Objectives
To determine the effects of oral prostaglandin E2 for third trimester induction of labour.
Search methods
The Cochrane Pregnancy and Childbirth Group's Trials Register (January 2007) and bibliographies of relevant papers. We updated this search on 8 June 2012 and added the results to the awaiting classification section of the review.
Selection criteria
Clinical trials comparing oral prostaglandin E2 used for third trimester cervical ripening or labour induction with placebo or no treatment or other methods listed above it on a predefined list of labour induction methods.
Data collection and analysis
A strategy was developed to deal with the large volume and complexity of trial data relating to labour induction. This involved a two‐stage method of data extraction.
Main results
There were 19 studies included in the review. Of these 15 included a comparison using either oral or intravenous oxytocin with or without amniotomy. The quality of studies reviewed was not high. Only seven studies had clearly described allocation concealment. Only two studies stated that providers or participants, or both, were blinded to treatment group.
For the outcome of vaginal delivery not achieved within 24 hours, in the composite comparison of oral PGE2 versus all oxytocin treatments (oral and intravenous, with and without amniotomy), there was a trend favoring oxytocin treatments (relative risk (RR) 1.97, 95% confidence interval (CI) 0.86 to 4.48).
For the outcome of cesarean section, in the comparison of PGE2 versus no treatment or placebo, PGE2 was favored (RR 0.54, 95% CI 0.29 to 0.98). Otherwise, there were no significant differences between groups for this outcome.
Oral prostaglandin was associated with vomiting across all comparison groups.
Authors' conclusions
Oral prostaglandin consistently resulted in more frequent gastrointestinal side‐effects, in particular vomiting, compared with the other treatments included in this review. There were no clear advantages to oral prostaglandin over other methods of induction of labour.
[Note: The six citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Keywords: Female; Humans; Pregnancy; Labor, Induced; Administration, Oral; Cervical Ripening; Dinoprostone; Dinoprostone/administration & dosage; Oxytocics; Oxytocics/administration & dosage; Pregnancy Trimester, Third
Plain language summary
Oral prostaglandin E2 for induction of labour
Oral prostaglandin E2 is no more effective than other methods of induction but has more adverse effects.
Induction of labour is sometimes considered beneficial in some clinical circumstances, e.g., when the baby is not growing properly, when there is pre‐eclampsia or when gestation goes beyond the normal length of pregnancy. There are many varying methods used to try to stimulate labour including administration of drugs, mechanical methods such as sweeping of the membranes, and more natural methods like nipple stimulation and having sex. Care needs to be taken to balance the stimulation of labour without over‐stimulating and causing the baby difficulties. Prostaglandin E2 (PGE2) is a hormone given either by mouth or by insertion through the vagina to prepare and stimulate the cervix and bring on labour. This review looked at oral PGE2 compared with no intervention, and compared with several other methods of induction. The review identified 19 studies involving 2688 women, looking at eight differing comparisons. The review found that none of the trials assessed the effectiveness of oral PGE2 in inducing labour, but overall the trials found that PGE2 caused more frequent gastrointestinal adverse effects, particularly vomiting. There were no clear advantages to oral PGE2 over other methods used to bring on labour, except that women may prefer a method that does not require an intravenous infusion. Over stimulation of the baby may possibly be a possible problem with PGE2, but the increased incidence of gastrointestinal side‐effects do not favor its use.
Background
Prostaglandins are hormones with a number of functions, and are normally produced at various sites in the body. They are derived from a fatty acid, arachidonic acid, which is generally available when needed. The role of endogenous prostaglandins in cervical ripening and initiation of labour was discovered in the 1960s.
Synthetically produced prostaglandins E2 (PGE2) and F2alpha (PGF2a) have been available for oral administration in several developed countries since the early 1970s. By 1977 five small trials had been published regarding use of PGE2 for cervical ripening. These were reviewed (Chalmers 1989) with the conclusion that the data failed to demonstrate suitability for this purpose. Vaginal administration has become the preferred route for the purpose of cervical ripening. PGE2 has also been studied as an agent for the induction of labour. The use of PGE2 as an agent for cervical ripening and labour induction is the subject of this review.
The gastrointestinal side‐effects of oral prostaglandins are important, and apparently more pronounced for PGF2a (Yeung 1977).
This review is one of a series of reviews of methods of labour induction using a standardized protocol. A review of the synthetic prostaglandin misoprostol is reviewed separately, seeAlfirevic 2006. For more detailed information on the rationale for this methodological approach, please refer to the currently published protocol (Hofmeyr 2000). For other currently published Cochrane Reviews on methods of induction of labour, seeAlfirevic 2006; Alfirevic 2009; Boulvain 2005; Boulvain 2008; Bricker 2000; Hapangama 2009; Hofmeyr 2010; Howarth 2001; Hutton 2001; Jozwiak 2012; Kavanagh 2001; Kavanagh 2005; Kavanagh 2006a; Kavanagh 2006b; Kelly 2001a; Kelly 2001b; Kelly 2009; Kelly 2011; Luckas 2000; Muzonzini 2004; Smith 2003; Smith 2004; Thomas 2001
Objectives
To determine, from the best available evidence, the effectiveness and safety of oral prostaglandin E2 for third trimester induction of labour.
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials comparing oral prostaglandin E2 for labour induction with placebo/no treatment or other methods of labour induction. This review includes only induction methods listed with lower numbers on a predefined list of methods of labour induction (see 'Methods of the review'). Studies comparing a low or constant dosing of oral prostaglandin with a high or incremental dosing regimen are also included. Additionally, studies comparing oral prostaglandin with oral oxytocin, with and without amniotomy, are also included. We have included only studies that have some form of random allocation of participants to study groups, and that report at least one of the prestated outcomes. Oral administration of the prostaglandin analogue misoprostol is reviewed separately (Alfirevic 2006).
Types of participants
Pregnant women due for third trimester induction of labour, carrying a viable fetus.
Predefined subgroup analyses were (see list below): previous cesarean section or not; nulliparity or multiparity; membranes intact or ruptured, and cervix unfavorable, favorable or undefined. Only those outcomes with data will appear in the analysis tables.
Types of interventions
Oral prostaglandin compared with placebo/no treatment or any other method above it on a predefined list of methods of labour induction.
Types of outcome measures
Clinically relevant outcomes for trials of methods of cervical ripening/labour induction have been prespecified by two authors of labour induction reviews (Justus Hofmeyr and Zarko Alfirevic). Differences were settled by discussion.
Five primary outcomes were chosen as being most representative of the clinically important measures of effectiveness and complications. Subgroup analyses are limited to the primary outcomes: (1) vaginal delivery not achieved within 24 hours; (2) uterine hyperstimulation with fetal heart rate (FHR) changes; (3) cesarean section; (4) serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood); (5) serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicemia).
Perinatal and maternal morbidity and mortality are composite outcomes. This is not an ideal solution because some components are clearly less severe than others. It is possible for one intervention to cause more deaths but less severe morbidity. However, in the context of labour induction at term this is unlikely. All these events will be rare, and a modest change in their incidence will be easier to detect if composite outcomes are presented. The incidence of individual components will be explored as secondary outcomes (see below).
Secondary outcomes relate to measures of effectiveness, complications and satisfaction.
Measures of effectiveness:
(6) cervix unfavorable/unchanged after 12 to 24 hours; (7) oxytocin augmentation.
Complications:
(8) uterine hyperstimulation without FHR changes; (9) uterine rupture; (10) epidural analgesia; (11) instrumental vaginal delivery; (12) meconium stained liquor; (13) Apgar score less than seven at five minutes; (14) neonatal intensive care unit admission; (15) neonatal encephalopathy; (16) perinatal death; (17) disability in childhood; (18) maternal side‐effects (all); (19) maternal nausea; (20) maternal vomiting; (21) maternal diarrhoea; (22) other maternal side‐effects; (23) postpartum hemorrhage (as defined by the trial authors); (24) serious maternal complications (e.g. intensive care unit admission, septicemia but excluding uterine rupture); (25) maternal death.
Measures of satisfaction:
(26) woman not satisfied; (27) caregiver not satisfied.
'Uterine rupture' will include all clinically significant ruptures of unscarred or scarred uteri. Trivial scar dehiscence noted incidentally at the time of surgery will be excluded.
Additional outcomes may appear in individual primary reviews, but will not contribute to the secondary reviews.
While all the above outcomes will be sought, only those with data will appear in the analysis tables.
The terminology of uterine hyperstimulation is problematic (Curtis 1987). In the reviews we have used the term 'uterine hyperstimulation without FHR changes' to include uterine tachysystole (more than 5 contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonus (a contraction lasting at least two minutes) and 'uterine hyperstimulation with FHR changes' to denote uterine hyperstimulation syndrome (tachysystole or hypersystole with FHR changes such as persistent decelerations, tachycardia or decreased short‐term variability).
Outcomes were included in the analysis: if reasonable measures were taken to minimise observer bias; and data were available for analysis according to original allocation.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (January 2007). We updated this search on 8 June 2012 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
The first search was performed simultaneously for all reviews of methods of inducing labour, as outlined in the generic protocol for these reviews (Hofmeyr 2000).
Searching other resources
we searched the reference lists of trial reports and reviews.
We did not apply any language restrictions.
Data collection and analysis
A strategy has been developed to deal with the large volume and complexity of trial data relating to labour induction. Many methods have been studied, in many different categories of women undergoing labour induction. Most trials are intervention‐driven, comparing two or more methods in various categories of women. Clinicians and parents need the data arranged by category of woman, to be able to choose which method is best for a particular clinical scenario. To extract these data from several hundred trial reports in a single step would be very difficult. We have therefore developed a two‐stage method of data extraction. The initial data extraction will be done in a series of primary reviews arranged by methods of induction of labour, following a standardized methodology. The data will then be extracted from the primary reviews into a series of secondary reviews, arranged by category of woman.
To avoid duplication of data in the primary reviews, the labour induction methods have been listed in a specific order, from one to 25. Each primary review includes comparisons between one of the methods (from two to 25) with only those methods above it on the list. Thus, the review of intravenous oxytocin (4) will include only comparisons with intracervical prostaglandins (3), vaginal prostaglandins (2) or placebo (1). Methods identified in the future will be added to the end of the list. The current list is as follows:
placebo/no treatment;
vaginal prostaglandins (Kelly 2009);
intracervical prostaglandins (Boulvain 2008);
intravenous oxytocin (Alfirevic 2009);
amniotomy (Bricker 2000);
amniotomy plus intravenous oxytocin (Howarth 2001);
vaginal misoprostol (Hofmeyr 2010);
oral misoprostol (Alfirevic 2006);
mechanical methods including extra‐amniotic Foley catheter (Jozwiak 2012);
membrane sweeping (Boulvain 2005);
extra‐amniotic prostaglandins (Hutton 2001);
intravenous prostaglandins (Luckas 2000);
oral prostaglandins (this review);
mifepristone (Hapangama 2009);
oestrogens alone of with amniotomy (Thomas 2001);
corticosteroids (Kavanagh 2006a);
relaxin (Kelly 2001b);
hyaluronidase (Kavanagh 2006b);
castor oil, bath and/or enema (Kelly 2001a);
acupuncture (Smith 2004);
breast stimulation (Kavanagh 2005);
sexual intercourse (Kavanagh 2001);
homeopathic methods (Smith 2003);
nitric oxide (Kelly 2011);
buccal or sublingual misoprostol (Muzonzini 2004);
hypnosis;
other methods for induction of labour.
The primary reviews are analysed by the following subgroups:
previous cesarean section or not;
nulliparity or multiparity;
membranes intact or ruptured;
cervix favorable, unfavorable or undefined.
The secondary reviews will include all methods of labour induction for each of the categories of women for which subgroup analysis has been done in the primary reviews, and will include only five primary outcome measures. There will thus be six secondary reviews of methods of labour induction in the following groups of women:
nulliparous, intact membranes (unfavorable cervix, favorable cervix, cervix not defined);
nulliparous, ruptured membranes (unfavorable cervix, favorable cervix, cervix not defined);
multiparous, intact membranes (unfavorable cervix, favorable cervix, cervix not defined);
multiparous, ruptured membranes (unfavorable cervix, favorable cervix, cervix not defined);
previous cesarean section, intact membranes (unfavorable cervix, favorable cervix, cervix not defined);
previous cesarean section, ruptured membranes (unfavorable cervix, favorable cervix, cervix not defined).
Each time a primary review is updated with new data, those secondary reviews which include data which have changed, will also be updated.
The trials included in the primary reviews first published in 2001 were extracted from an initial set of trials covering all interventions used in induction of labour (see above for details of search strategy). The data extraction process was conducted centrally. This was co‐ordinated from the Clinical Effectiveness Support Unit (CESU) at the Royal College of Obstetricians and Gynaecologists, UK, in co‐operation with the Pregnancy and Childbirth Group of The Cochrane Collaboration. This process allowed the data extraction process to be standardized across all the reviews.
The trials were initially reviewed on eligibility criteria, using a standardized form and the basic selection criteria specified above. Following this, data were extracted to a standardized data extraction form which was piloted for consistency and completeness. The pilot process involved the researchers at the CESU and previous review authors in the area of induction of labour.
Information was extracted regarding the methodological quality of trials on a number of levels. This process was completed without consideration of trial results. Assessment of selection bias examined the process involved in the generation of the random sequence and the method of allocation concealment separately. These were then judged as adequate or inadequate using the criteria described in Table 1 for the purpose of the reviews.
1. Methodological quality of trials.
| Methodological item | Adequate | Inadequate |
| Generation of random sequence. | Computer‐generated sequence, random‐number tables, lot drawing, coin tossing, shuffling cards, throwing dice. | Case number, date of birth, date of admission, alternation. |
| Concealment of allocation. | Central randomization, coded drug boxes, sequentially sealed opaque envelopes. | Open allocation sequence, any procedure based on inadequate generation. |
Performance bias was examined with regards to whom was blinded in the trials, i.e. woman, caregiver, outcome assessor or analyst. In many trials the caregiver, assessor and analyst were the same party. Details of the feasibility and appropriateness of blinding at all levels were sought.
Individual outcome data were included in the analysis if they met the prestated criteria in 'Types of outcome measures'. Included trial data were processed as described in The Cochrane Collaboration Handbook (Clarke 1999). Data extracted from the trials were analysed on an intention‐to‐treat basis (when this was not done in the original report, re‐analysis was performed if possible). Where data were missing, clarification was sought from the original authors. If the attrition was such that it might significantly affect the results, these data were excluded from the analysis. This decision rests with the review authors of primary reviews and has been clearly documented. Once missing data become available, they will be included in the analyses.
Data were extracted from all eligible trials to examine how issues of quality influence effect size in a sensitivity analysis. In trials where reporting is poor, methodological issues were reported as unclear or clarification sought.
Due to the large number of trials, double data extraction was not feasible and agreement between the three data extractors was therefore assessed on a random sample of trials.
Once the data had been extracted, they were distributed to individual review authors for entry onto the Review Manager computer software (RevMan 2003), checked for accuracy, and analysed as above using the RevMan software. For dichotomous data, relative risks and 95% confidence intervals were calculated, and in the absence of heterogeneity, results were pooled using a fixed‐effect model.
The predefined criteria for sensitivity analysis included all aspects of quality assessment as mentioned above, including aspects of selection, performance and attrition bias.
Primary analysis was limited to the prespecified outcomes and subgroup analyses. In the event of differences in unspecified outcomes or subgroups being found, these were analysed post hoc, but clearly identified as such to avoid drawing unjustified conclusions.
For this update, the additional reports identified from the updated search were assessed by one author.
Results
Description of studies
Excluded studies
There were 33 studies identified by the search strategy that were excluded. For details, see the table of 'Characteristics of excluded studies'. In four studies, allocation concealment was clearly inadequate due to randomization methods using alternation or odd or even medical record number. Six studies presented no outcomes of interest according to the review protocol. Six studies were not randomized trials. Two studies did not include oral prostaglandins. Two studies did not correspond to the topic of review, describing outcomes with policies of routine induction versus expectant management. One study was planned but not carried out. Insufficient data were available to assess the remainder.
Four studies were of two oral prostaglandin regimens that were considered too similar for meaningful comparison. Lorrain 1982 studied 'synthetic' versus 'natural' prostaglandin. Obel 1975 studied swallowed tablet versus oral solution. Thiery 1977 studied swallowed versus sublingual administration of tablets. In none of these studies was there any important difference described in outcomes. Davies 1991 used the same oral PGE2 regimen immediately versus the next morning at 9 a.m.
Somell 1983 considered both cervical ripening for two days and then induction with oral PGE2, compared with no treatment for ripening and intravenous (IV) oxytocin for induction. The combination comparison group (no treatment/IV oxytocin) is one not contemplated in the study protocol.
Included studies
There were 19 studies identified meeting the inclusion criteria. For details, see the table of 'Characteristics of included studies'. PGE2 was the oral prostaglandin used in all of these studies. The comparisons identified were:
oral PGE2 versus no treatment (n = three studies, 195 women);
oral PGE2 versus vaginal PGE2 (n = three studies, 108 women);
oral PGE2 versus cervical PGE2 (n = two studies, 80 women);
oral PGE2 versus IV oxytocin (n = seven studies, 779 women);
oral PGE2 versus IV oxytocin plus amniotomy (n = four studies, 435 women);
oral PGE2 versus oral oxytocin (n = four studies, 822 women);
oral PGE2 versus oral oxytocin plus amniotomy (n = two studies, 223 women);
oral PGE2 dose incremental or high dose versus oral PGE2 constant or low dose (n = two studies, 46 women).
A few studies included multiple (three or four) comparison groups.
The study by Somell 1987 included four groups. The women were first divided into two groups to receive oral prostaglandin or placebo for cervical ripening. Afterward, these two groups were further divided into induction groups to receive either oral prostaglandin or IV oxytocin. For this review, only the two groups receiving oral PGE2 and IV oxytocin without prior cervical ripening are included.
There are six studies currently excluded which may be included at a future date if the authors can supply missing data.
(Six reports from an updated search on 8 June 2012 have been added to Studies awaiting classification.)
Risk of bias in included studies
Randomization
Mathews 1976, Paul 1992, Ratnam 1974, and Westergaard 1983 used sealed envelopes. Golbus 1977 and Somell 1987 described their studies as double‐blind comparisons to placebo and with implicit indication that allocation was concealed. Hauth 1977 stated that allocation was concealed, but did not describe the method. In all other studies allocation concealment was not mentioned. A table of random numbers for allocation was used by Beard 1975. For all other included studies the specific methodology of randomization was not described.
Blinding
Only for the two studies with a double‐blind comparison to placebo was blinding described. In the case of Somell 1987, it is assumed that the blinding only applied to the cervical ripening phase of the study, and not the induction portion versus intravenous oxytocin. Lack of blinding certainly introduces some possibility of bias, which could go in favor of any arm of the study that participating clinicians or women were inclined to believe was better.
Intent to treat
A minority of studies stated that analysis was on an intent‐to‐treat (ITT) basis (Beard 1975; Lange 1981; Massil 1988; Mathews 1976; Ulstein 1979; Westergaard 1983a). For studies in which ITT was not mentioned, it was assumed that there were no exclusions after enrolment unless specified. It was possible to analyze all included studied by ITT with that assumption.
Effects of interventions
Primary outcomes
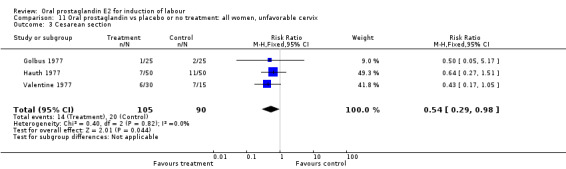
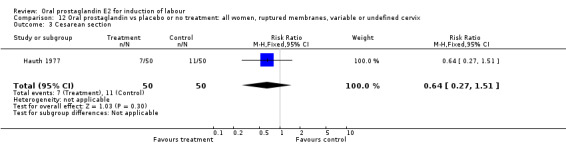
Of the five primary outcome measures, only cesarean section was consistently reported (in all but Mathews 1976). For the comparison versus no treatment or placebo, PGE2 was favored (relative risk (RR) 0.54, 95% confidence interval (CI) 0.29 to 0.98). Otherwise, there were no significant differences between groups for this outcome.
Vaginal delivery not achieved within 24 hours was included as an outcome measure in three studies (Lange 1981; Mathews 1976; Westergaard 1983a). Individual comparisons tended to favor other treatments without reaching statistical significance. In the composite of oral PGE2 versus all oxytocin treatments, there was a trend favoring other treatments (RR 1.97, 95% CI 0.86 to 4.48).
Uterine hyperstimulation with fetal heart rate (FHR) changes was an outcome reported in four studies (Massil 1988; Mathews 1976; Ulstein 1979; Westergaard 1983a). Of these, only one (Mathews 1976) reported cases in either group, all three of the cases in women receiving PGE2. The result was that in the composite of oral PGE2 versus all oxytocin treatments, there was a trend in favor of oxytocin treatments, though with a very wide confidence interval (RR 7.00 95% CI 0.37 to 132.10). Perinatal death was not consistently included as an outcome. Somell 1987 reported two deaths that were attributed to congenital malformations. The only study reporting what might be classified as severe perinatal morbidity, was the outcome called 'intrauterine asphyxia', by Ulstein 1979. There was no difference between the PGE2 and the oral oxytocin groups in that study.
Serious maternal morbidity or death was reported only by Paul 1992, which stated that there was none in either treatment group (oral PGE2 versus intravenous (IV) oxytocin).
Secondary outcomes
Gastrointestinal side‐effects
Gastrointestinal side‐effects were frequently reported in the studies reviewed. Often nausea and vomiting was reported as a composite outcome. In these cases, the data were entered under the variable vomiting. Gastrointestinal side‐effects were more frequent for women treated with oral PGE2 than with other treatments in all comparisons, most of which reached statistical significance.
Oxytocin augmentation
This outcome was reported in three studies (Hauth 1977; Mathews 1976; Westergaard 1983). It is not surprising that in Hauth's study of oral PGE2 versus no treatment for the first 12 hours, then oxytocin augmentation in both groups if needed, oxytocin was needed more often in the no treatment group. In the other two studies there was not a significant difference in use of oxytocin augmentation.
Uterine hyperstimulation without FHR changes
This outcome was reported in eight studies. No significant differences between comparison groups were observed.
Epidural analgesia
This outcome was reported in only three studies (Beard 1975; Lange 1981; Massil 1988). No significant differences between groups were observed.
Instrumental vaginal delivery
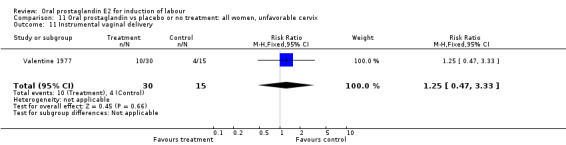
This outcome was reported in the majority (17) of studies. In none of the comparisons was there a statistically significant difference.
Meconium stained liquor
This outcome was reported in only two studies (Massil 1988; Mathews 1976). No significant differences were found in either study.
Apgar score less than seven at five minutes
This outcome was reported in seven studies (Beard 1975; Herabutya 1988Lange 1981; Paul 1992; Secher 1981; Somell 1987; Westergaard 1983). No significant differences were found between comparison groups.
Neonatal intensive care unit admission
This outcome was reported in only one study (Massil 1988) and there was not a significant difference between groups (oral PGE2 versus IV oxytocin).
Postpartum hemorrhage
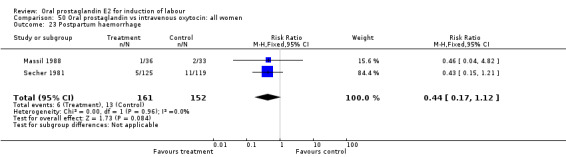
This outcome was reported in six studies (Beard 1975; Massil 1988; Mathews 1976; Read 1974; Secher 1981; Westergaard 1983a). No significant differences between comparison groups were found.
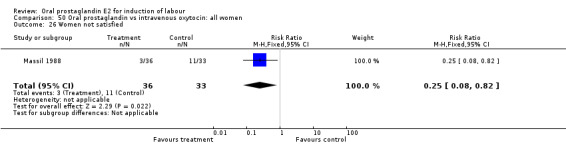
Women not satisfied
This outcome was reported in one (Massil 1988) small study. Women preferred an oral treatment versus IV (oxytocin) medication.
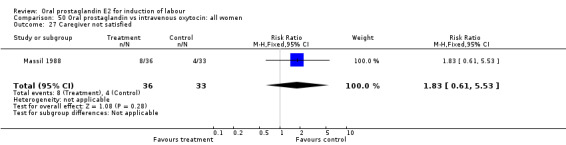
Caregiver not satisfied
In the same study (Massil 1988) caregivers did not have a clear preference for oral PGE2 or IV oxytocin.
Other outcomes
Maternal or neonatal infection requiring antibiotics
In the Hauth 1977 study of oral PGE2 versus no treatment for 12 hours, followed by oxytocin as needed in both treatment groups of women with ruptured membranes at term, there were trends favoring the oral PGE2 group for use of antibiotics in both mothers and infants. This may be attributable to more rapid delivery. Mean time to delivery was 11.6 versus 15.6 hours, (no standard deviation given).
Discussion
This review is limited by the quality of the available studies. Allocation concealment was not clearly described in most studies and, when it was, significant potential for bias still existed due to lack of blinding. A further limitation is that the number of participants in aggregate, is not large.
For the five primary outcome measures, only cesarean section was consistently reported. The results of this meta‐analysis trend in favor of oxytocin. This cannot be firmly concluded, however.
Of the secondary outcomes, results in favor of all oxytocin treatments regarding gastrointestinal side‐effects are consistent enough to be conclusive. Other secondary outcomes do not clearly favor oral prostaglandin or other treatments.
There were insufficient data to demonstrate superiority of high or incremental dosing regimens of oral PGE2 over low or constant dosing.
Authors' conclusions
Implications for practice.
Oral PGE2 is not more effective than oxytocin for achieving delivery within 24 hours of labor induction. Gastrointestinal side‐effects are more frequent with PGE2. There is no clear evidence favoring either oral PGE2 or oxytocin regimens regarding safety of women or their infants.
Implications for research.
Little further research is likely to be forthcoming comparing oral PGE2 to other regimens. Though some women may prefer an oral route of administration of agents for induction of labor, oral misoprostol (synthetic prostaglandin) is likely to supercede the oral preparations of PGE2 that are in current use.
[Note: The six citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
What's new
| Date | Event | Description |
|---|---|---|
| 8 June 2012 | Amended | Search updated. Six reports added to Studies awaiting classification (Bremme 1980a; Bremme 1980b; Bremme 1982; Bremme 1984a; Marzouk 1975; Murray 1975). |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2001
| Date | Event | Description |
|---|---|---|
| 12 May 2008 | Amended | Converted to new review format. |
| 25 January 2007 | New search has been performed | Search repeated. Four reports have been assessed and added to the list of excluded studies. |
Acknowledgements
None.
Data and analyses
Comparison 10. Oral prostaglandin vs placebeo or no treatment: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.29, 0.98] |
| 7 Oxytocin augmentation | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.10, 0.47] |
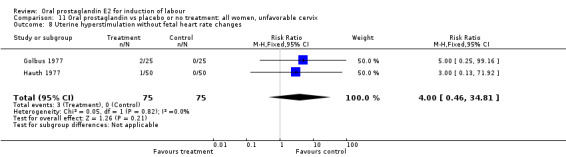
| 8 Uterine hyperstimulation without fetal heart rate changes | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.46, 34.81] |
| 11 Instrumental vaginal delivery | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.47, 3.33] |
| 20 Vomiting | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 21 Diarrhoea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 28 Maternal postpartum infections requiring antibiotics | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.34, 1.24] |
| 29 Neonatal infections requiring antibiotics | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.17, 1.21] |
10.3. Analysis.
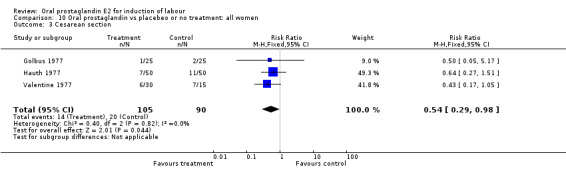
Comparison 10 Oral prostaglandin vs placebeo or no treatment: all women, Outcome 3 Cesarean section.
10.7. Analysis.
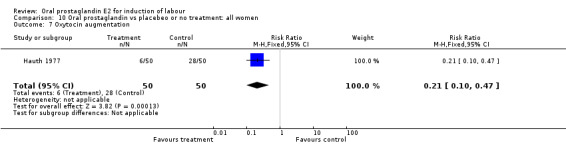
Comparison 10 Oral prostaglandin vs placebeo or no treatment: all women, Outcome 7 Oxytocin augmentation.
10.8. Analysis.
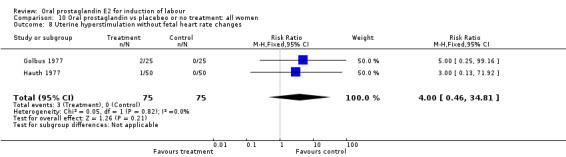
Comparison 10 Oral prostaglandin vs placebeo or no treatment: all women, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
10.11. Analysis.
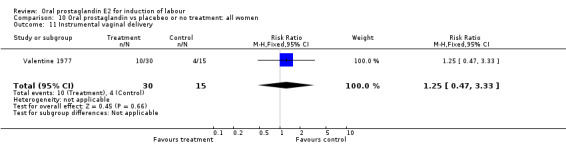
Comparison 10 Oral prostaglandin vs placebeo or no treatment: all women, Outcome 11 Instrumental vaginal delivery.
10.20. Analysis.
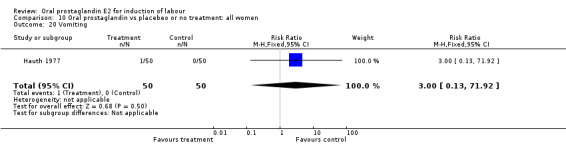
Comparison 10 Oral prostaglandin vs placebeo or no treatment: all women, Outcome 20 Vomiting.
10.21. Analysis.
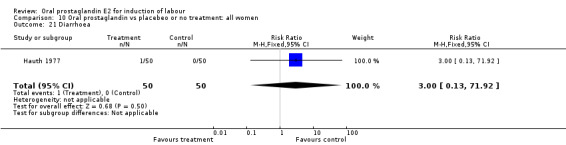
Comparison 10 Oral prostaglandin vs placebeo or no treatment: all women, Outcome 21 Diarrhoea.
10.28. Analysis.
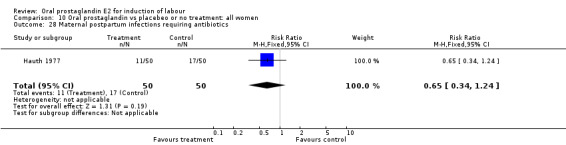
Comparison 10 Oral prostaglandin vs placebeo or no treatment: all women, Outcome 28 Maternal postpartum infections requiring antibiotics.
10.29. Analysis.
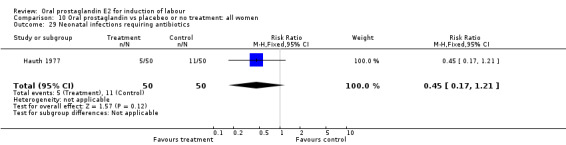
Comparison 10 Oral prostaglandin vs placebeo or no treatment: all women, Outcome 29 Neonatal infections requiring antibiotics.
Comparison 11. Oral prostaglandin vs placebo or no treatment: all women, unfavorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 3 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.29, 0.98] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 2 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.0 [0.46, 34.81] |
| 11 Instrumental vaginal delivery | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.47, 3.33] |
11.3. Analysis.
Comparison 11 Oral prostaglandin vs placebo or no treatment: all women, unfavorable cervix, Outcome 3 Cesarean section.
11.8. Analysis.
Comparison 11 Oral prostaglandin vs placebo or no treatment: all women, unfavorable cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
11.11. Analysis.
Comparison 11 Oral prostaglandin vs placebo or no treatment: all women, unfavorable cervix, Outcome 11 Instrumental vaginal delivery.
Comparison 12. Oral prostaglandin vs placebo or no treatment: all women, ruptured membranes, variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.27, 1.51] |
| 7 Oxytocin augmentation | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.10, 0.47] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 20 Vomiting | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 21 Diarrhoea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.92] |
| 28 Maternal postpartum infections requiring antibiotics | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.34, 1.24] |
| 29 Neonatal infections requiring antibiotics | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.17, 1.21] |
12.3. Analysis.
Comparison 12 Oral prostaglandin vs placebo or no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 3 Cesarean section.
12.7. Analysis.
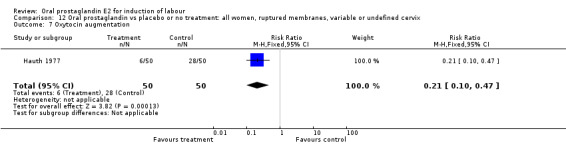
Comparison 12 Oral prostaglandin vs placebo or no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 7 Oxytocin augmentation.
12.8. Analysis.
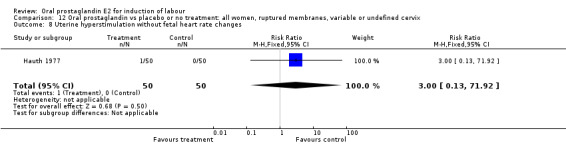
Comparison 12 Oral prostaglandin vs placebo or no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
12.20. Analysis.
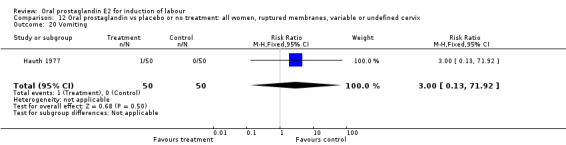
Comparison 12 Oral prostaglandin vs placebo or no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 20 Vomiting.
12.21. Analysis.
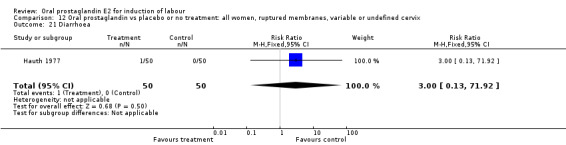
Comparison 12 Oral prostaglandin vs placebo or no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 21 Diarrhoea.
12.28. Analysis.
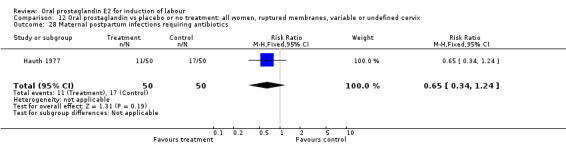
Comparison 12 Oral prostaglandin vs placebo or no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 28 Maternal postpartum infections requiring antibiotics.
12.29. Analysis.
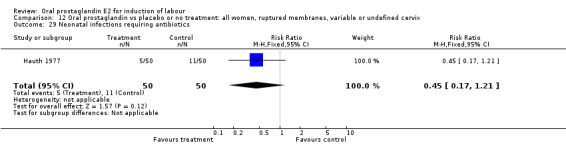
Comparison 12 Oral prostaglandin vs placebo or no treatment: all women, ruptured membranes, variable or undefined cervix, Outcome 29 Neonatal infections requiring antibiotics.
Comparison 13. Oral prostaglandin vs placebo or no treatment: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.30, 2.32] |
| 11 Instrumental vaginal delivery | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.42, 2.81] |
13.3. Analysis.
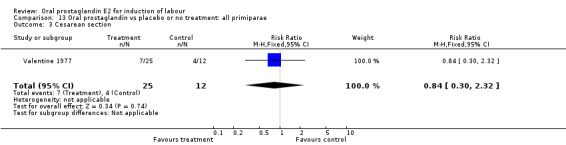
Comparison 13 Oral prostaglandin vs placebo or no treatment: all primiparae, Outcome 3 Cesarean section.
13.11. Analysis.
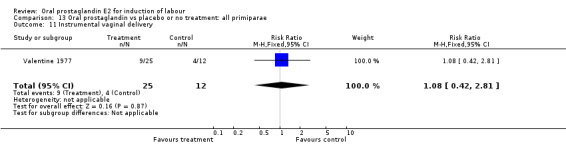
Comparison 13 Oral prostaglandin vs placebo or no treatment: all primiparae, Outcome 11 Instrumental vaginal delivery.
Comparison 14. Oral prostaglandin vs placebo or no treatment: all primiparae, unfavorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.30, 2.32] |
| 11 Instrumental vaginal delivery | 1 | 37 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.42, 2.81] |
14.3. Analysis.
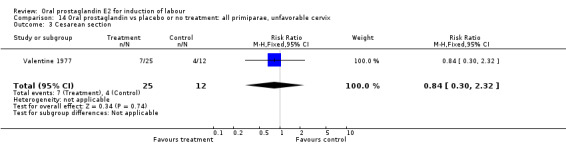
Comparison 14 Oral prostaglandin vs placebo or no treatment: all primiparae, unfavorable cervix, Outcome 3 Cesarean section.
14.11. Analysis.
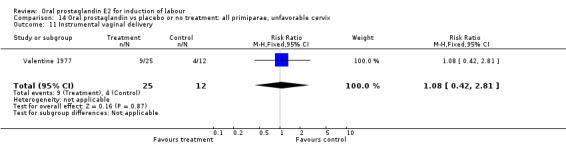
Comparison 14 Oral prostaglandin vs placebo or no treatment: all primiparae, unfavorable cervix, Outcome 11 Instrumental vaginal delivery.
Comparison 15. Oral prostaglandin vs placebo or no treatment: all multiparae (without previous cesarean section).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.20] |
| 11 Instrumental vaginal delivery | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.03, 9.46] |
15.3. Analysis.
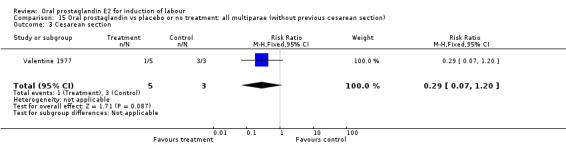
Comparison 15 Oral prostaglandin vs placebo or no treatment: all multiparae (without previous cesarean section), Outcome 3 Cesarean section.
15.11. Analysis.
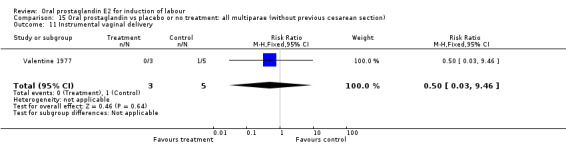
Comparison 15 Oral prostaglandin vs placebo or no treatment: all multiparae (without previous cesarean section), Outcome 11 Instrumental vaginal delivery.
Comparison 16. Oral prostaglandin vs placebo or no treatment: all multiparae, unfavorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.07, 1.20] |
| 11 Instrumental vaginal delivery | 1 | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.03, 9.46] |
16.3. Analysis.
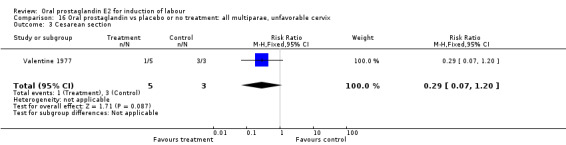
Comparison 16 Oral prostaglandin vs placebo or no treatment: all multiparae, unfavorable cervix, Outcome 3 Cesarean section.
16.11. Analysis.
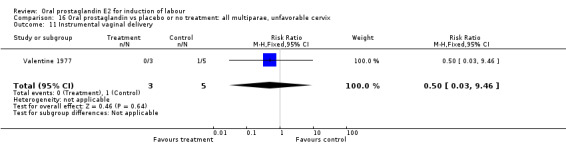
Comparison 16 Oral prostaglandin vs placebo or no treatment: all multiparae, unfavorable cervix, Outcome 11 Instrumental vaginal delivery.
Comparison 20. Oral prostaglandin vs vaginal prostaglandin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 2 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.33, 1.47] |
| 6 Cevrvix unfavorable/unchanged after12‐24 hours | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.21, 21.22] |
| 11 Instrumental vaginal delivery | 3 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.44, 1.54] |
20.3. Analysis.
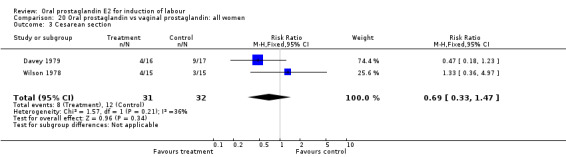
Comparison 20 Oral prostaglandin vs vaginal prostaglandin: all women, Outcome 3 Cesarean section.
20.6. Analysis.
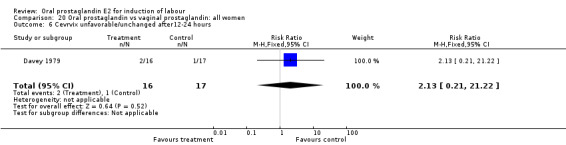
Comparison 20 Oral prostaglandin vs vaginal prostaglandin: all women, Outcome 6 Cevrvix unfavorable/unchanged after12‐24 hours.
20.11. Analysis.
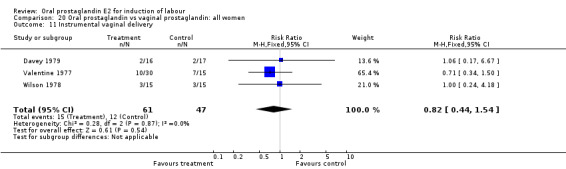
Comparison 20 Oral prostaglandin vs vaginal prostaglandin: all women, Outcome 11 Instrumental vaginal delivery.
Comparison 21. Oral prostaglandin vs vaginal prostaglandin: all women, unfavorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 2 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.33, 1.47] |
| 6 Cevrvix unfavorable/unchanged after12‐24 hours | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.13 [0.21, 21.22] |
| 11 Instrumental vaginal delivery | 3 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.44, 1.54] |
21.3. Analysis.
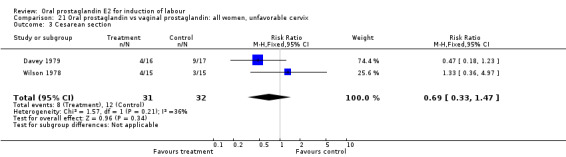
Comparison 21 Oral prostaglandin vs vaginal prostaglandin: all women, unfavorable cervix, Outcome 3 Cesarean section.
21.6. Analysis.
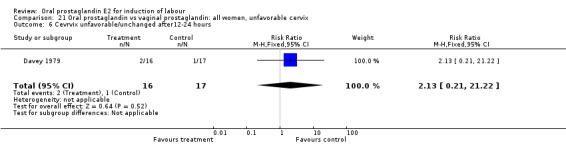
Comparison 21 Oral prostaglandin vs vaginal prostaglandin: all women, unfavorable cervix, Outcome 6 Cevrvix unfavorable/unchanged after12‐24 hours.
21.11. Analysis.
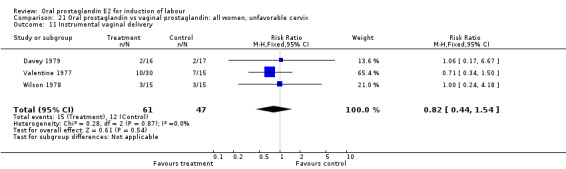
Comparison 21 Oral prostaglandin vs vaginal prostaglandin: all women, unfavorable cervix, Outcome 11 Instrumental vaginal delivery.
Comparison 22. Oral prostaglandin vs vaginal prostaglandin: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.36, 4.97] |
| 11 Instrumental vaginal delivery | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.24, 4.18] |
22.3. Analysis.
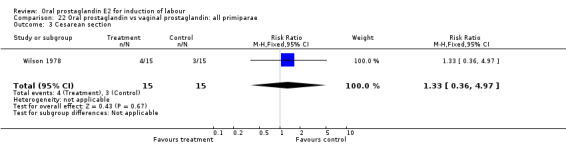
Comparison 22 Oral prostaglandin vs vaginal prostaglandin: all primiparae, Outcome 3 Cesarean section.
22.11. Analysis.
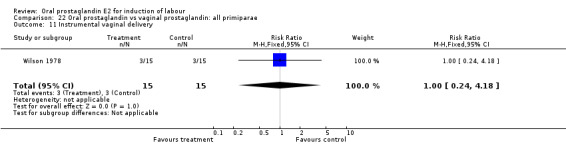
Comparison 22 Oral prostaglandin vs vaginal prostaglandin: all primiparae, Outcome 11 Instrumental vaginal delivery.
Comparison 23. Oral prostaglandin vs vaginal prostaglandin: all primiparae, unfavorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.36, 4.97] |
| 11 Instrumental vaginal delivery | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.24, 4.18] |
23.3. Analysis.
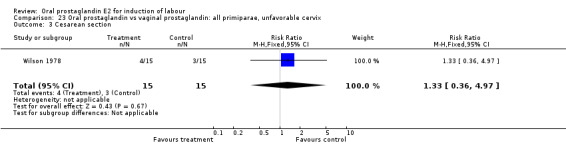
Comparison 23 Oral prostaglandin vs vaginal prostaglandin: all primiparae, unfavorable cervix, Outcome 3 Cesarean section.
23.11. Analysis.
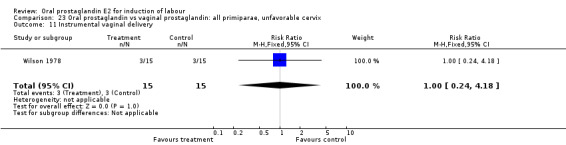
Comparison 23 Oral prostaglandin vs vaginal prostaglandin: all primiparae, unfavorable cervix, Outcome 11 Instrumental vaginal delivery.
Comparison 30. Oral prostaglandin vs intracervical prostaglandin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.24, 1.65] |
| 11 Instrumental vaginal delivery | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.38, 4.12] |
| 13 Apgar score < 7 at 5 minutes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
30.3. Analysis.
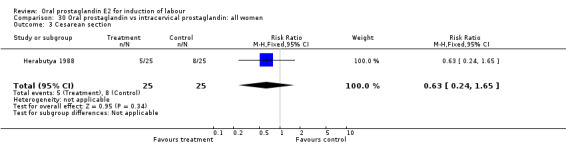
Comparison 30 Oral prostaglandin vs intracervical prostaglandin: all women, Outcome 3 Cesarean section.
30.11. Analysis.
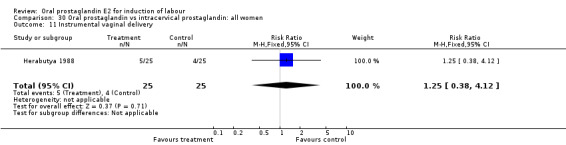
Comparison 30 Oral prostaglandin vs intracervical prostaglandin: all women, Outcome 11 Instrumental vaginal delivery.
30.13. Analysis.
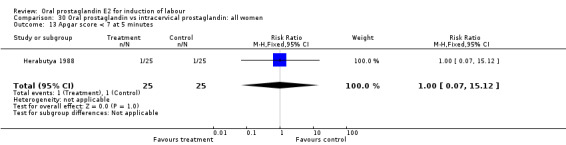
Comparison 30 Oral prostaglandin vs intracervical prostaglandin: all women, Outcome 13 Apgar score < 7 at 5 minutes.
Comparison 31. Oral prostaglandin vs intracervical prostaglandin: all women, unfavorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.24, 1.65] |
| 11 Instrumental vaginal delivery | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.38, 4.12] |
| 13 Apgar score < 7 at 5 minutes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
31.3. Analysis.
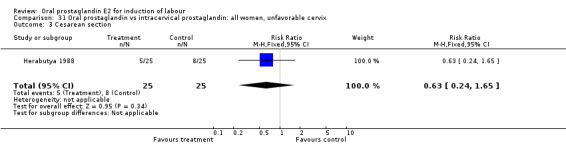
Comparison 31 Oral prostaglandin vs intracervical prostaglandin: all women, unfavorable cervix, Outcome 3 Cesarean section.
31.11. Analysis.
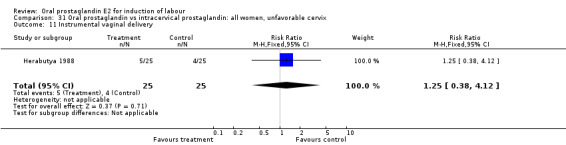
Comparison 31 Oral prostaglandin vs intracervical prostaglandin: all women, unfavorable cervix, Outcome 11 Instrumental vaginal delivery.
31.13. Analysis.
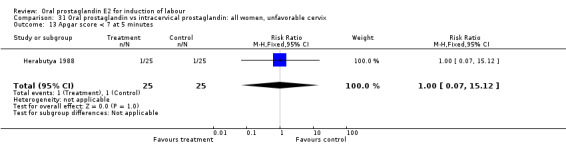
Comparison 31 Oral prostaglandin vs intracervical prostaglandin: all women, unfavorable cervix, Outcome 13 Apgar score < 7 at 5 minutes.
Comparison 32. Oral prostaglandin vs intracervical prostaglandin: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.24, 1.65] |
| 11 Instrumental vaginal delivery | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.38, 4.12] |
| 13 Apgar score < 7 at 5 minutes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
32.3. Analysis.
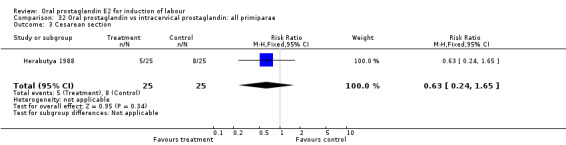
Comparison 32 Oral prostaglandin vs intracervical prostaglandin: all primiparae, Outcome 3 Cesarean section.
32.11. Analysis.
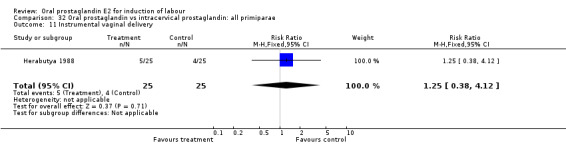
Comparison 32 Oral prostaglandin vs intracervical prostaglandin: all primiparae, Outcome 11 Instrumental vaginal delivery.
32.13. Analysis.
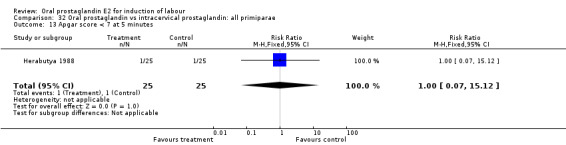
Comparison 32 Oral prostaglandin vs intracervical prostaglandin: all primiparae, Outcome 13 Apgar score < 7 at 5 minutes.
Comparison 33. Oral prostaglandin vs intracervical prostaglandin: all primiparae, unfavorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.24, 1.65] |
| 11 Instrumental vaginal delivery | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.38, 4.12] |
| 13 Apgar score < 7 at 5 minutes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.07, 15.12] |
33.3. Analysis.
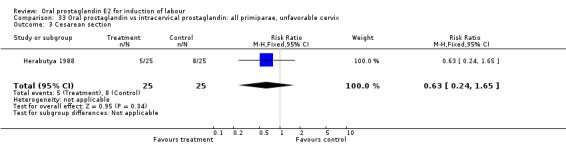
Comparison 33 Oral prostaglandin vs intracervical prostaglandin: all primiparae, unfavorable cervix, Outcome 3 Cesarean section.
33.11. Analysis.
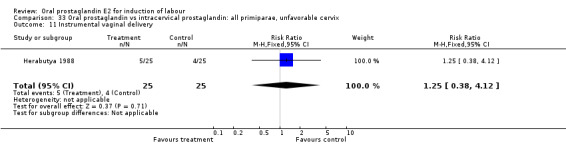
Comparison 33 Oral prostaglandin vs intracervical prostaglandin: all primiparae, unfavorable cervix, Outcome 11 Instrumental vaginal delivery.
33.13. Analysis.
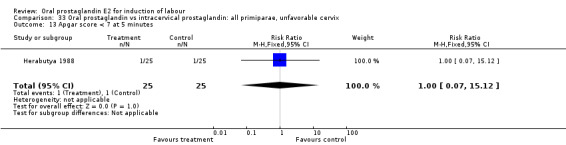
Comparison 33 Oral prostaglandin vs intracervical prostaglandin: all primiparae, unfavorable cervix, Outcome 13 Apgar score < 7 at 5 minutes.
Comparison 40. Oral prostaglandin vs all oxytocin regimens: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 3 | 494 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.86, 4.48] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 4 | 642 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.37, 132.10] |
| 3 Cesarean section | 14 | 2204 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.83, 1.59] |
40.1. Analysis.
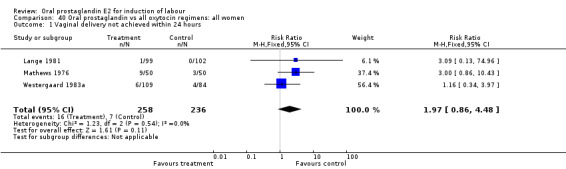
Comparison 40 Oral prostaglandin vs all oxytocin regimens: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
40.2. Analysis.
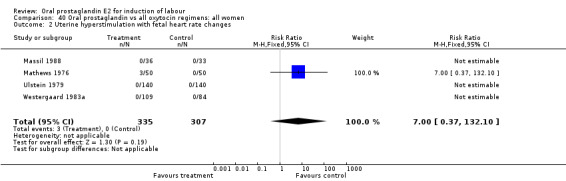
Comparison 40 Oral prostaglandin vs all oxytocin regimens: all women, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
40.3. Analysis.
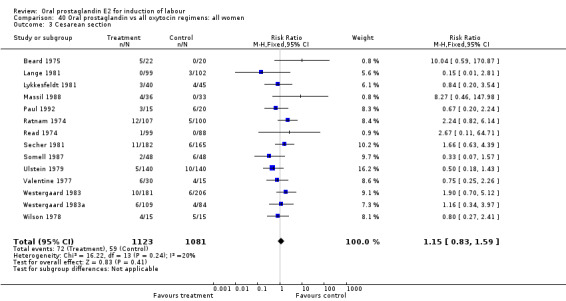
Comparison 40 Oral prostaglandin vs all oxytocin regimens: all women, Outcome 3 Cesarean section.
Comparison 50. Oral prostaglandin vs intravenous oxytocin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.13, 74.96] |
| 2 Uterine hyperstimulation with fetal heart rate chnages | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Cesarean section | 8 | 824 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.68, 1.68] |
| 5 Serious maternal morbidity or death | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 3 | 409 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.06] |
| 10 Epidural analgesia | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.56, 1.35] |
| 11 Instrumental vaginal delivery | 6 | 624 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.48, 1.02] |
| 12 Meconium‐stained liquor | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.30, 25.15] |
| 13 Apgar score < 7 at 5 minutes | 4 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.13, 1.97] |
| 14 Neonatal intensive care unit admission | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.21, 2.50] |
| 16 Perinatal death | 1 | 35 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Vomiting | 3 | 305 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.56 [2.15, 14.38] |
| 21 Diarrhoea | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.13 [1.03, 63.93] |
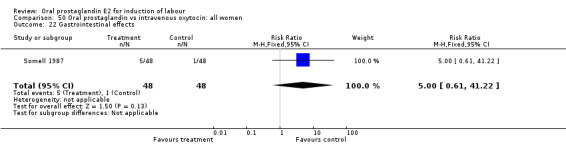
| 22 Gastrointestinal effects | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.61, 41.22] |
| 23 Postpartum haemorrhage | 2 | 313 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.17, 1.12] |
| 26 Women not satisfied | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.08, 0.82] |
| 27 Caregiver not satisfied | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [0.61, 5.53] |
50.1. Analysis.
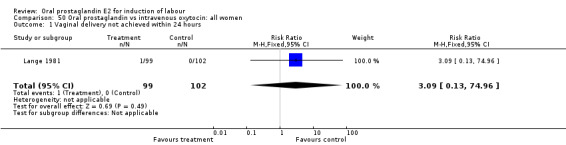
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
50.2. Analysis.
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 2 Uterine hyperstimulation with fetal heart rate chnages.
50.3. Analysis.
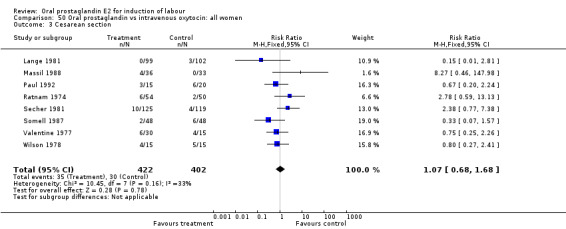
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 3 Cesarean section.
50.5. Analysis.
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 5 Serious maternal morbidity or death.
50.8. Analysis.
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
50.10. Analysis.
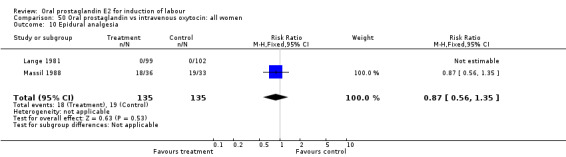
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 10 Epidural analgesia.
50.11. Analysis.
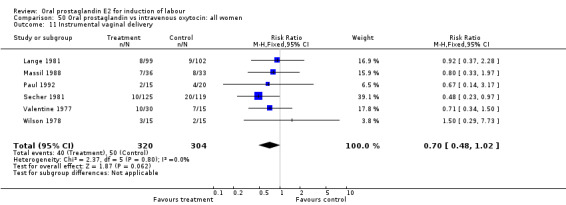
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 11 Instrumental vaginal delivery.
50.12. Analysis.
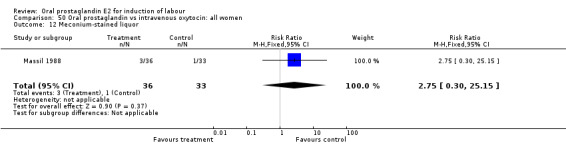
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 12 Meconium‐stained liquor.
50.13. Analysis.
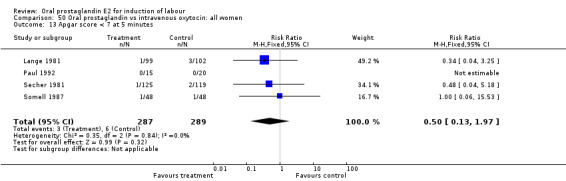
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 13 Apgar score < 7 at 5 minutes.
50.14. Analysis.
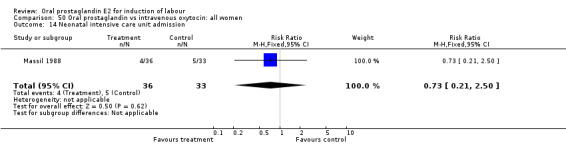
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 14 Neonatal intensive care unit admission.
50.16. Analysis.
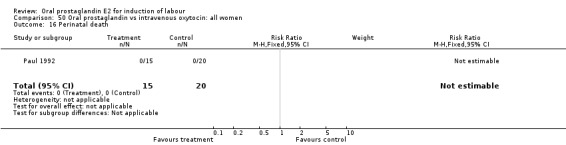
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 16 Perinatal death.
50.20. Analysis.
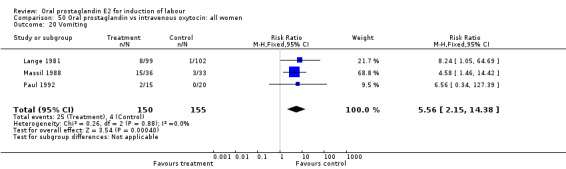
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 20 Vomiting.
50.21. Analysis.
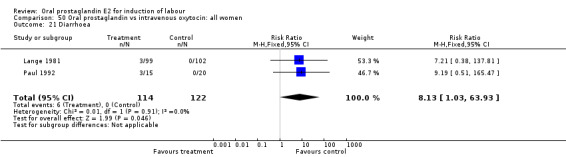
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 21 Diarrhoea.
50.22. Analysis.
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 22 Gastrointestinal effects.
50.23. Analysis.
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 23 Postpartum haemorrhage.
50.26. Analysis.
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 26 Women not satisfied.
50.27. Analysis.
Comparison 50 Oral prostaglandin vs intravenous oxytocin: all women, Outcome 27 Caregiver not satisfied.
Comparison 52. Oral prostaglandin vs intravenous oxytocin: all women, unfavorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 3 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.53, 2.09] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.06] |
| 11 Instrumental vaginal delivery | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.43, 1.68] |
| 13 Apgar scrore < 7 at 5 minutes | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.53] |
| 22 Gastrointestinal effects | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.61, 41.22] |
| 23 Postpartum haemorrhage | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.01] |
52.3. Analysis.
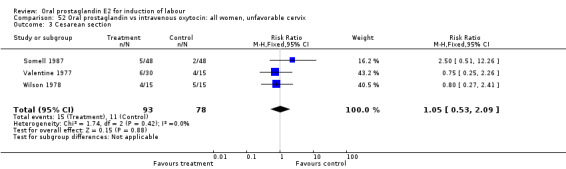
Comparison 52 Oral prostaglandin vs intravenous oxytocin: all women, unfavorable cervix, Outcome 3 Cesarean section.
52.8. Analysis.
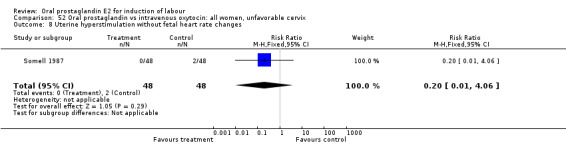
Comparison 52 Oral prostaglandin vs intravenous oxytocin: all women, unfavorable cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
52.11. Analysis.
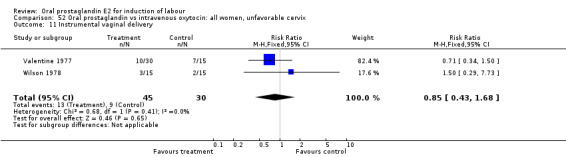
Comparison 52 Oral prostaglandin vs intravenous oxytocin: all women, unfavorable cervix, Outcome 11 Instrumental vaginal delivery.
52.13. Analysis.
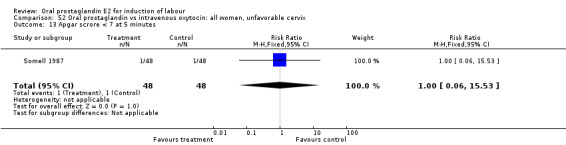
Comparison 52 Oral prostaglandin vs intravenous oxytocin: all women, unfavorable cervix, Outcome 13 Apgar scrore < 7 at 5 minutes.
52.22. Analysis.
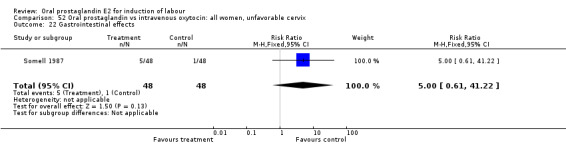
Comparison 52 Oral prostaglandin vs intravenous oxytocin: all women, unfavorable cervix, Outcome 22 Gastrointestinal effects.
52.23. Analysis.
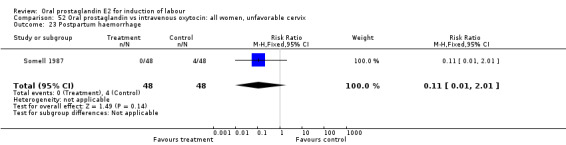
Comparison 52 Oral prostaglandin vs intravenous oxytocin: all women, unfavorable cervix, Outcome 23 Postpartum haemorrhage.
Comparison 54. Oral prostaglandin vs intravenous oxytocin: all women, intact membranes, variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.77, 7.38] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 1 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.23, 0.97] |
| 13 Apgar score < 7 at 5 minutes | 1 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.04, 5.18] |
| 23 Postpartum haemorrhage | 1 | 244 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.15, 1.21] |
54.3. Analysis.
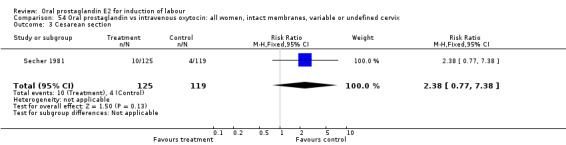
Comparison 54 Oral prostaglandin vs intravenous oxytocin: all women, intact membranes, variable or undefined cervix, Outcome 3 Cesarean section.
54.8. Analysis.
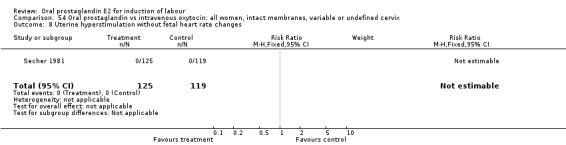
Comparison 54 Oral prostaglandin vs intravenous oxytocin: all women, intact membranes, variable or undefined cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
54.11. Analysis.
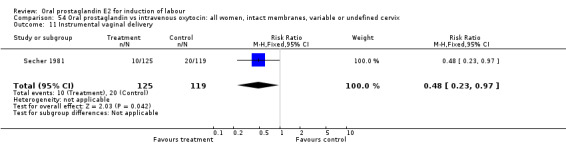
Comparison 54 Oral prostaglandin vs intravenous oxytocin: all women, intact membranes, variable or undefined cervix, Outcome 11 Instrumental vaginal delivery.
54.13. Analysis.
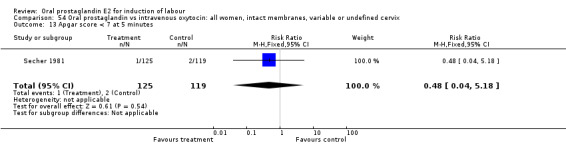
Comparison 54 Oral prostaglandin vs intravenous oxytocin: all women, intact membranes, variable or undefined cervix, Outcome 13 Apgar score < 7 at 5 minutes.
54.23. Analysis.
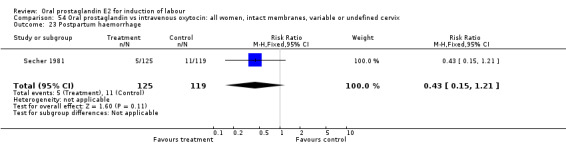
Comparison 54 Oral prostaglandin vs intravenous oxytocin: all women, intact membranes, variable or undefined cervix, Outcome 23 Postpartum haemorrhage.
Comparison 55. Oral prostaglandin vs intravenous oxytocin: all women, intact membranes, unfavorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 2 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.53, 3.12] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 4.06] |
| 11 Instrumental vaginal delivery | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.29, 7.73] |
| 13 Apgar scrore < 7 at 5 minutes | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.53] |
| 19 Postpartum haemorrhage | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.01] |
| 22 Gastrointestinal effects | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.61, 41.22] |
55.3. Analysis.
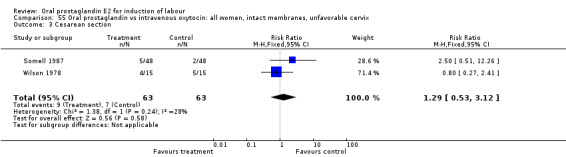
Comparison 55 Oral prostaglandin vs intravenous oxytocin: all women, intact membranes, unfavorable cervix, Outcome 3 Cesarean section.
55.8. Analysis.
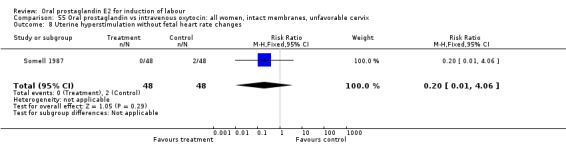
Comparison 55 Oral prostaglandin vs intravenous oxytocin: all women, intact membranes, unfavorable cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
55.11. Analysis.
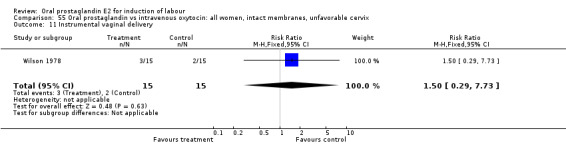
Comparison 55 Oral prostaglandin vs intravenous oxytocin: all women, intact membranes, unfavorable cervix, Outcome 11 Instrumental vaginal delivery.
55.13. Analysis.
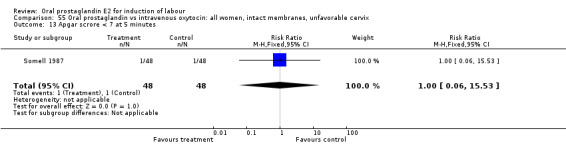
Comparison 55 Oral prostaglandin vs intravenous oxytocin: all women, intact membranes, unfavorable cervix, Outcome 13 Apgar scrore < 7 at 5 minutes.
55.19. Analysis.
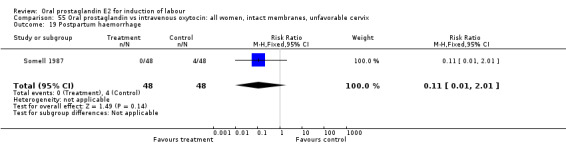
Comparison 55 Oral prostaglandin vs intravenous oxytocin: all women, intact membranes, unfavorable cervix, Outcome 19 Postpartum haemorrhage.
55.22. Analysis.
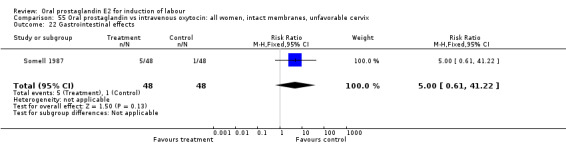
Comparison 55 Oral prostaglandin vs intravenous oxytocin: all women, intact membranes, unfavorable cervix, Outcome 22 Gastrointestinal effects.
Comparison 56. Oral prostaglandin vs intravenous oxytocin: all women, ruptured membranes, variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.09 [0.13, 74.96] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Cesarean section | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.32, 4.60] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.56, 1.35] |
| 11 Instrumental vaginal delivery | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.45, 1.63] |
| 12 Meconium‐stained liquor | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [0.30, 25.15] |
| 13 Apgar score < 7 at 5 minutes | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.25] |
| 14 Neonatal intensive care unit admission | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.21, 2.50] |
| 20 Vomiting | 2 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.46 [2.00, 14.88] |
| 21 Diarrhoea | 1 | 201 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.21 [0.38, 137.81] |
| 23 Postpartum haemorrhage | 1 | 69 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 4.82] |
56.1. Analysis.
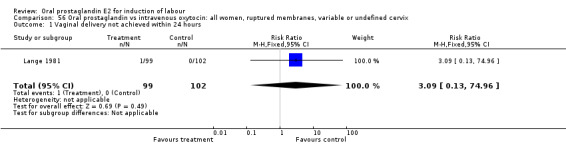
Comparison 56 Oral prostaglandin vs intravenous oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
56.2. Analysis.
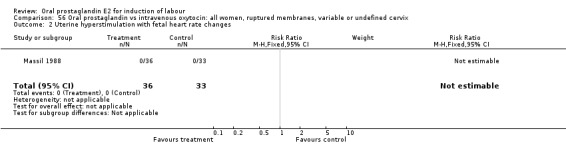
Comparison 56 Oral prostaglandin vs intravenous oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
56.3. Analysis.
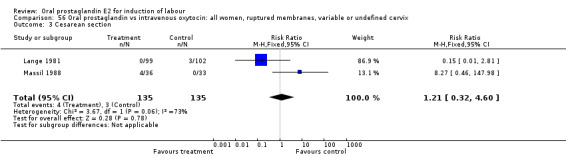
Comparison 56 Oral prostaglandin vs intravenous oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 3 Cesarean section.
56.8. Analysis.
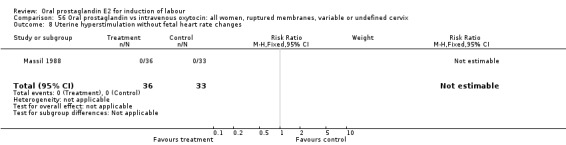
Comparison 56 Oral prostaglandin vs intravenous oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
56.10. Analysis.
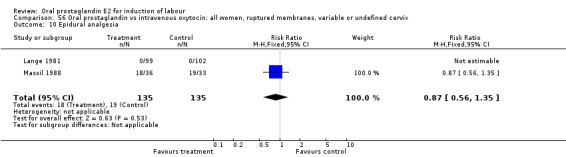
Comparison 56 Oral prostaglandin vs intravenous oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 10 Epidural analgesia.
56.11. Analysis.
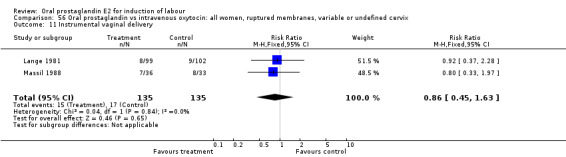
Comparison 56 Oral prostaglandin vs intravenous oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 11 Instrumental vaginal delivery.
56.12. Analysis.
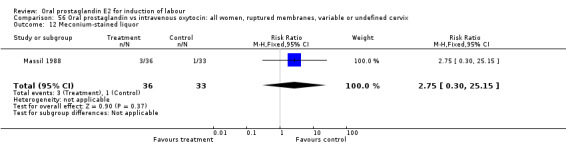
Comparison 56 Oral prostaglandin vs intravenous oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 12 Meconium‐stained liquor.
56.13. Analysis.
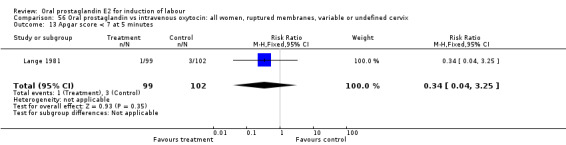
Comparison 56 Oral prostaglandin vs intravenous oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 13 Apgar score < 7 at 5 minutes.
56.14. Analysis.
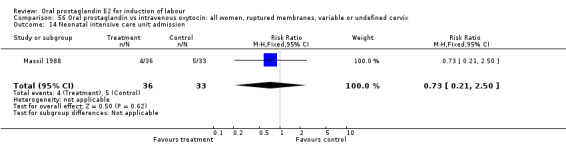
Comparison 56 Oral prostaglandin vs intravenous oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 14 Neonatal intensive care unit admission.
56.20. Analysis.
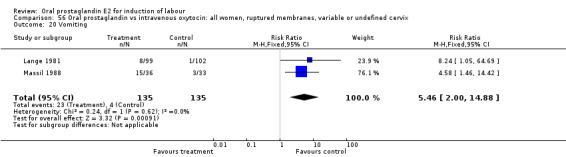
Comparison 56 Oral prostaglandin vs intravenous oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 20 Vomiting.
56.21. Analysis.
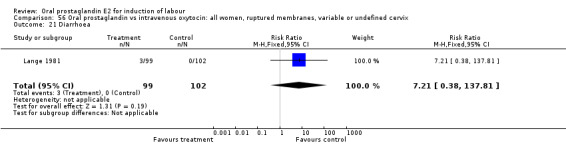
Comparison 56 Oral prostaglandin vs intravenous oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 21 Diarrhoea.
56.23. Analysis.
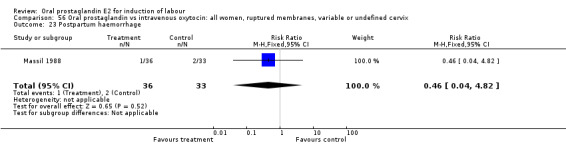
Comparison 56 Oral prostaglandin vs intravenous oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 23 Postpartum haemorrhage.
Comparison 57. Oral prostaglandin vs intravenous oxytocin: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Cesarean section | 4 | 136 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.63, 2.29] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.37] |
| 11 Instumental vaginal delivery | 3 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.29] |
| 12 Meconium‐stained liquor | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [0.28, 22.70] |
| 14 Neonatal intensive care unit admission | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.25, 2.00] |
| 20 Vomiting | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.92 [1.51, 23.27] |
| 23 Postpartum haemorrhage | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.37] |
57.2. Analysis.
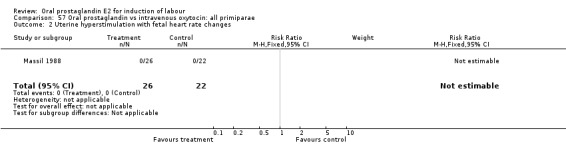
Comparison 57 Oral prostaglandin vs intravenous oxytocin: all primiparae, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
57.3. Analysis.
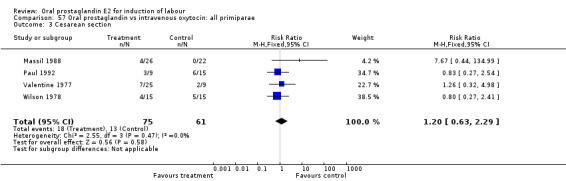
Comparison 57 Oral prostaglandin vs intravenous oxytocin: all primiparae, Outcome 3 Cesarean section.
57.8. Analysis.
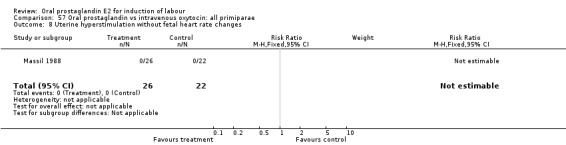
Comparison 57 Oral prostaglandin vs intravenous oxytocin: all primiparae, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
57.10. Analysis.
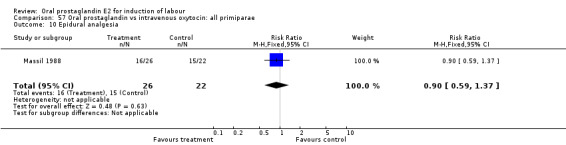
Comparison 57 Oral prostaglandin vs intravenous oxytocin: all primiparae, Outcome 10 Epidural analgesia.
57.11. Analysis.
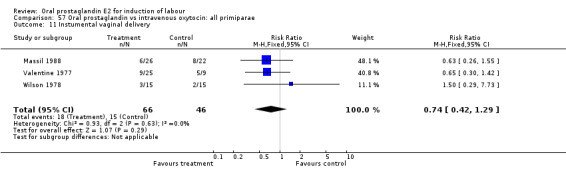
Comparison 57 Oral prostaglandin vs intravenous oxytocin: all primiparae, Outcome 11 Instumental vaginal delivery.
57.12. Analysis.
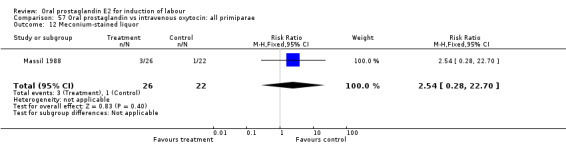
Comparison 57 Oral prostaglandin vs intravenous oxytocin: all primiparae, Outcome 12 Meconium‐stained liquor.
57.14. Analysis.
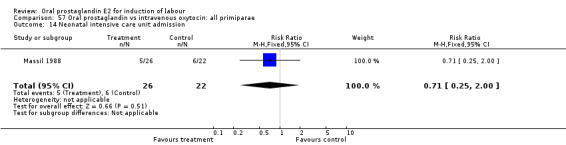
Comparison 57 Oral prostaglandin vs intravenous oxytocin: all primiparae, Outcome 14 Neonatal intensive care unit admission.
57.20. Analysis.
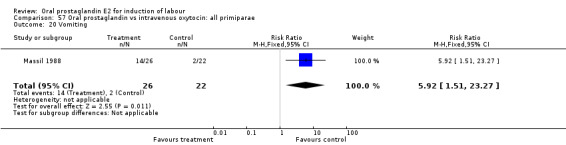
Comparison 57 Oral prostaglandin vs intravenous oxytocin: all primiparae, Outcome 20 Vomiting.
57.23. Analysis.
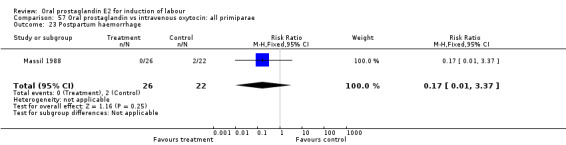
Comparison 57 Oral prostaglandin vs intravenous oxytocin: all primiparae, Outcome 23 Postpartum haemorrhage.
Comparison 58. Oral prostaglandin vs intravenous oxytocin: all primiparae, unfavorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.32, 4.98] |
| 11 Instrumental vaginal delivery | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.30, 1.42] |
58.3. Analysis.
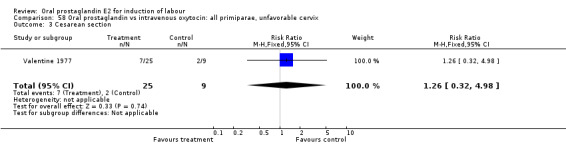
Comparison 58 Oral prostaglandin vs intravenous oxytocin: all primiparae, unfavorable cervix, Outcome 3 Cesarean section.
58.11. Analysis.
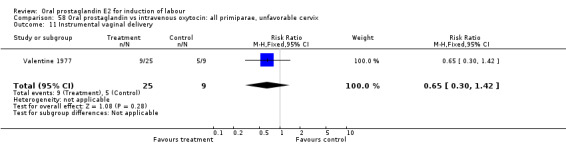
Comparison 58 Oral prostaglandin vs intravenous oxytocin: all primiparae, unfavorable cervix, Outcome 11 Instrumental vaginal delivery.
Comparison 60. Oral prostaglandin vs intravenous oxytocin: all primaparae, ruptured membranes, variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Cesarean section | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.67 [0.44, 134.99] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.59, 1.37] |
| 11 Instrumental vaginal delivery | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.26, 1.55] |
| 12 Meconium‐stained liquor | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [0.28, 22.70] |
| 14 Neonatal intensive care unit admission | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.25, 2.00] |
| 20 Vomiting | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.92 [1.51, 23.27] |
| 23 Postpartum haemorrhage | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.01, 3.37] |
60.2. Analysis.
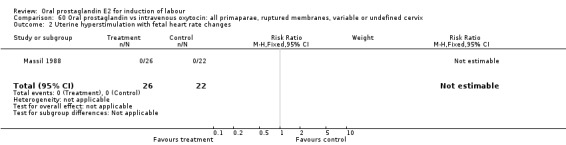
Comparison 60 Oral prostaglandin vs intravenous oxytocin: all primaparae, ruptured membranes, variable or undefined cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
60.3. Analysis.
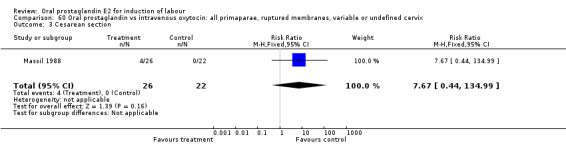
Comparison 60 Oral prostaglandin vs intravenous oxytocin: all primaparae, ruptured membranes, variable or undefined cervix, Outcome 3 Cesarean section.
60.8. Analysis.
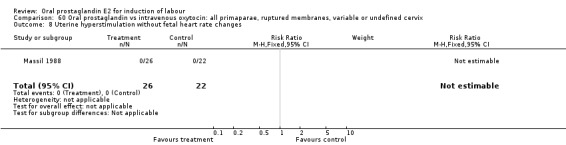
Comparison 60 Oral prostaglandin vs intravenous oxytocin: all primaparae, ruptured membranes, variable or undefined cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
60.10. Analysis.
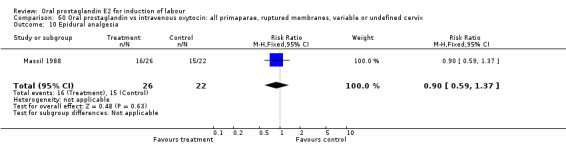
Comparison 60 Oral prostaglandin vs intravenous oxytocin: all primaparae, ruptured membranes, variable or undefined cervix, Outcome 10 Epidural analgesia.
60.11. Analysis.
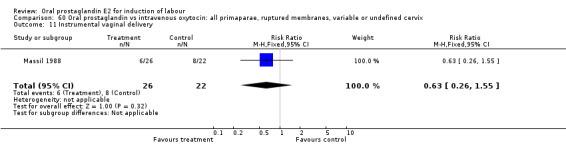
Comparison 60 Oral prostaglandin vs intravenous oxytocin: all primaparae, ruptured membranes, variable or undefined cervix, Outcome 11 Instrumental vaginal delivery.
60.12. Analysis.
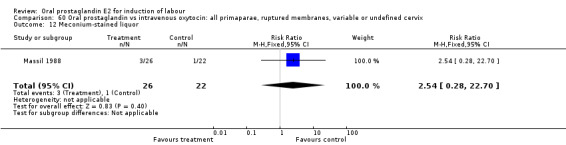
Comparison 60 Oral prostaglandin vs intravenous oxytocin: all primaparae, ruptured membranes, variable or undefined cervix, Outcome 12 Meconium‐stained liquor.
60.14. Analysis.
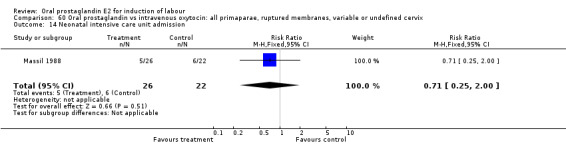
Comparison 60 Oral prostaglandin vs intravenous oxytocin: all primaparae, ruptured membranes, variable or undefined cervix, Outcome 14 Neonatal intensive care unit admission.
60.20. Analysis.
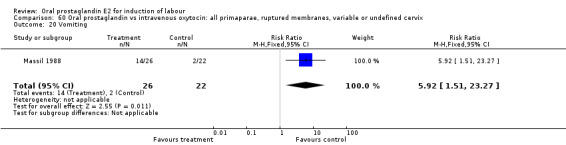
Comparison 60 Oral prostaglandin vs intravenous oxytocin: all primaparae, ruptured membranes, variable or undefined cervix, Outcome 20 Vomiting.
60.23. Analysis.
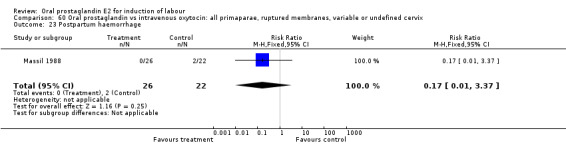
Comparison 60 Oral prostaglandin vs intravenous oxytocin: all primaparae, ruptured membranes, variable or undefined cervix, Outcome 23 Postpartum haemorrhage.
Comparison 62. Oral prostaglandin vs intravenous oxytocin: all multiparae (without previous cesarean section).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Cesarean section | 3 | 43 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.07, 4.83] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.07, 4.83] |
| 12 Meconium‐stained liquor | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Neonatal intensive care unit admission | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Vomiting | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.08, 15.36] |
| 23 Postpartum haemorrhage | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.15, 72.23] |
62.2. Analysis.
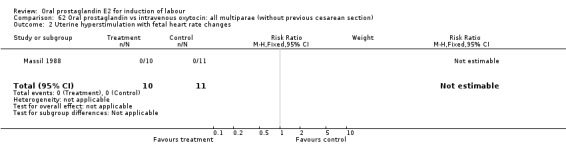
Comparison 62 Oral prostaglandin vs intravenous oxytocin: all multiparae (without previous cesarean section), Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
62.3. Analysis.
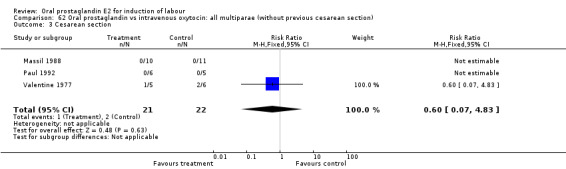
Comparison 62 Oral prostaglandin vs intravenous oxytocin: all multiparae (without previous cesarean section), Outcome 3 Cesarean section.
62.8. Analysis.
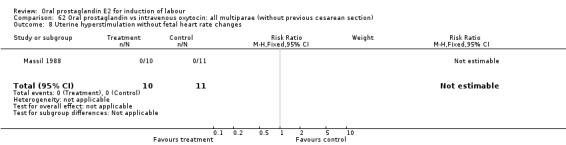
Comparison 62 Oral prostaglandin vs intravenous oxytocin: all multiparae (without previous cesarean section), Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
62.11. Analysis.
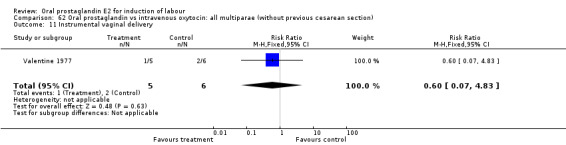
Comparison 62 Oral prostaglandin vs intravenous oxytocin: all multiparae (without previous cesarean section), Outcome 11 Instrumental vaginal delivery.
62.12. Analysis.
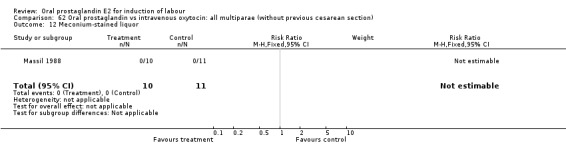
Comparison 62 Oral prostaglandin vs intravenous oxytocin: all multiparae (without previous cesarean section), Outcome 12 Meconium‐stained liquor.
62.14. Analysis.
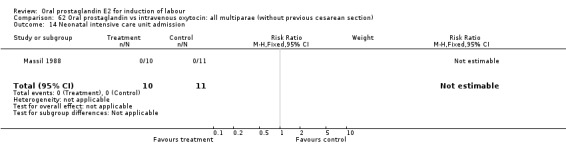
Comparison 62 Oral prostaglandin vs intravenous oxytocin: all multiparae (without previous cesarean section), Outcome 14 Neonatal intensive care unit admission.
62.20. Analysis.
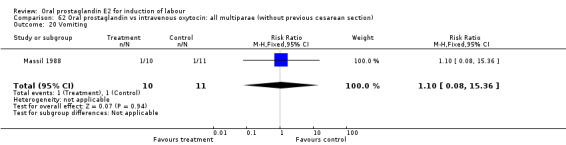
Comparison 62 Oral prostaglandin vs intravenous oxytocin: all multiparae (without previous cesarean section), Outcome 20 Vomiting.
62.23. Analysis.
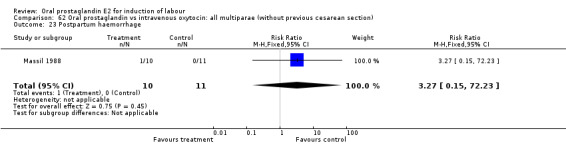
Comparison 62 Oral prostaglandin vs intravenous oxytocin: all multiparae (without previous cesarean section), Outcome 23 Postpartum haemorrhage.
Comparison 63. Oral prostaglandin vs intravenous oxytocin: all multiparae, unfavorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Cesarean section | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.07, 4.83] |
| 2 Instrumental vaginal delivery | 1 | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.07, 4.83] |
63.1. Analysis.
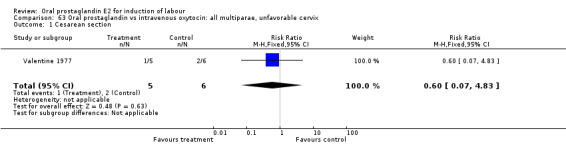
Comparison 63 Oral prostaglandin vs intravenous oxytocin: all multiparae, unfavorable cervix, Outcome 1 Cesarean section.
63.2. Analysis.
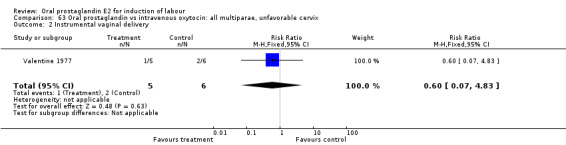
Comparison 63 Oral prostaglandin vs intravenous oxytocin: all multiparae, unfavorable cervix, Outcome 2 Instrumental vaginal delivery.
Comparison 65. Oral prostaglandin vs intravenous oxytocin; all multiparae, ruptured membranes, variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Cesarean section | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.13, 2.38] |
| 11 Instrumental vaginal delivery | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.15, 72.23] |
| 12 Meconium‐stained liquor | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Neonatal intensive care unit admission | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Vomiting | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.1 [0.08, 15.36] |
| 23 Postpartum haemorrhage | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.27 [0.15, 72.23] |
65.2. Analysis.
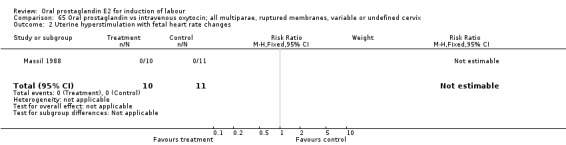
Comparison 65 Oral prostaglandin vs intravenous oxytocin; all multiparae, ruptured membranes, variable or undefined cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
65.3. Analysis.
Comparison 65 Oral prostaglandin vs intravenous oxytocin; all multiparae, ruptured membranes, variable or undefined cervix, Outcome 3 Cesarean section.
65.8. Analysis.
Comparison 65 Oral prostaglandin vs intravenous oxytocin; all multiparae, ruptured membranes, variable or undefined cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
65.10. Analysis.
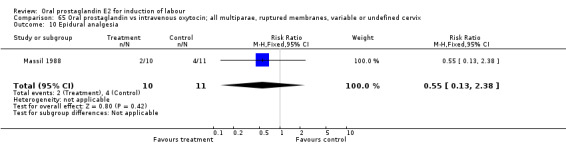
Comparison 65 Oral prostaglandin vs intravenous oxytocin; all multiparae, ruptured membranes, variable or undefined cervix, Outcome 10 Epidural analgesia.
65.11. Analysis.
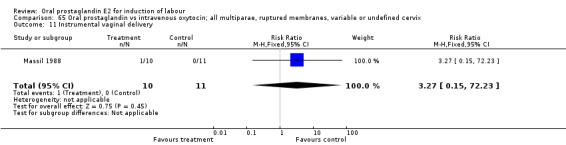
Comparison 65 Oral prostaglandin vs intravenous oxytocin; all multiparae, ruptured membranes, variable or undefined cervix, Outcome 11 Instrumental vaginal delivery.
65.12. Analysis.
Comparison 65 Oral prostaglandin vs intravenous oxytocin; all multiparae, ruptured membranes, variable or undefined cervix, Outcome 12 Meconium‐stained liquor.
65.14. Analysis.
Comparison 65 Oral prostaglandin vs intravenous oxytocin; all multiparae, ruptured membranes, variable or undefined cervix, Outcome 14 Neonatal intensive care unit admission.
65.20. Analysis.
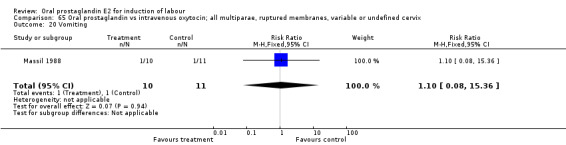
Comparison 65 Oral prostaglandin vs intravenous oxytocin; all multiparae, ruptured membranes, variable or undefined cervix, Outcome 20 Vomiting.
65.23. Analysis.
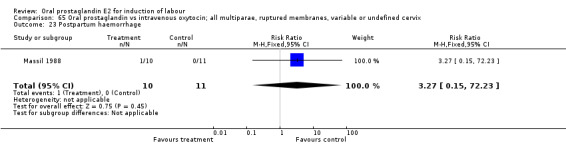
Comparison 65 Oral prostaglandin vs intravenous oxytocin; all multiparae, ruptured membranes, variable or undefined cervix, Outcome 23 Postpartum haemorrhage.
Comparison 67. Oral prostaglandin vs intravenous oxytocin with amniotomy: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 4 | 435 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.84, 5.31] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51, 1.21] |
| 11 Instrumental vaginal delivery | 3 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.43, 1.55] |
| 13 Apgar score < 7 at 5 minutes | 2 | 145 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.12, 6.12] |
| 20 Vomiting | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.60 [0.41, 31.59] |
| 21 Diarrhoea | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Postpartum haemorrhage | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
67.3. Analysis.
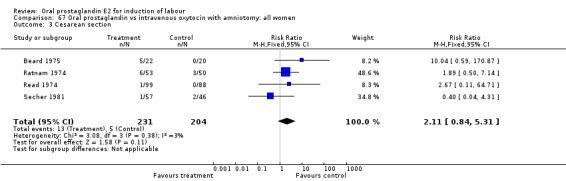
Comparison 67 Oral prostaglandin vs intravenous oxytocin with amniotomy: all women, Outcome 3 Cesarean section.
67.8. Analysis.
Comparison 67 Oral prostaglandin vs intravenous oxytocin with amniotomy: all women, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
67.10. Analysis.
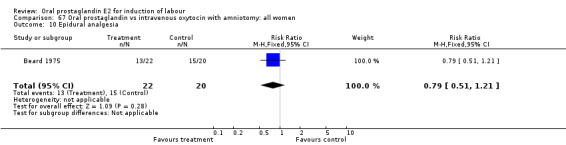
Comparison 67 Oral prostaglandin vs intravenous oxytocin with amniotomy: all women, Outcome 10 Epidural analgesia.
67.11. Analysis.
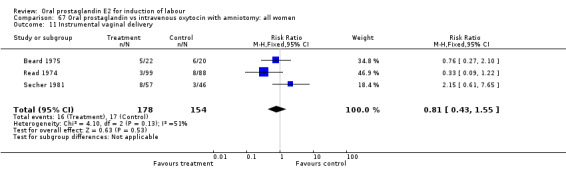
Comparison 67 Oral prostaglandin vs intravenous oxytocin with amniotomy: all women, Outcome 11 Instrumental vaginal delivery.
67.13. Analysis.
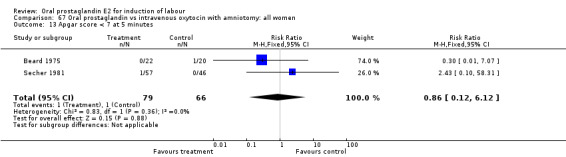
Comparison 67 Oral prostaglandin vs intravenous oxytocin with amniotomy: all women, Outcome 13 Apgar score < 7 at 5 minutes.
67.20. Analysis.
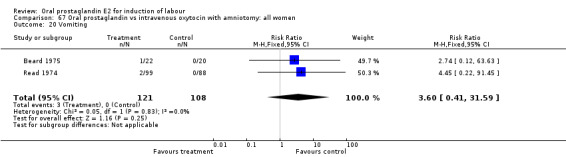
Comparison 67 Oral prostaglandin vs intravenous oxytocin with amniotomy: all women, Outcome 20 Vomiting.
67.21. Analysis.
Comparison 67 Oral prostaglandin vs intravenous oxytocin with amniotomy: all women, Outcome 21 Diarrhoea.
67.23. Analysis.
Comparison 67 Oral prostaglandin vs intravenous oxytocin with amniotomy: all women, Outcome 23 Postpartum haemorrhage.
Comparison 68. Oral prostaglandin vs intravenous oxytocin with amniotomy: all women, intact membranes, variable or undefined.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.04, 4.31] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.61, 7.65] |
| 13 Apgar score < 7 at 5 minutes | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.43 [0.10, 58.31] |
| 23 Postpartum haemorrhage | 1 | 103 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
68.3. Analysis.
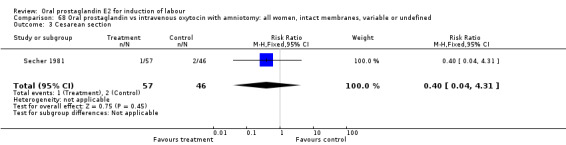
Comparison 68 Oral prostaglandin vs intravenous oxytocin with amniotomy: all women, intact membranes, variable or undefined, Outcome 3 Cesarean section.
68.8. Analysis.
Comparison 68 Oral prostaglandin vs intravenous oxytocin with amniotomy: all women, intact membranes, variable or undefined, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
68.11. Analysis.
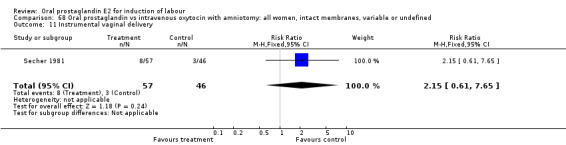
Comparison 68 Oral prostaglandin vs intravenous oxytocin with amniotomy: all women, intact membranes, variable or undefined, Outcome 11 Instrumental vaginal delivery.
68.13. Analysis.
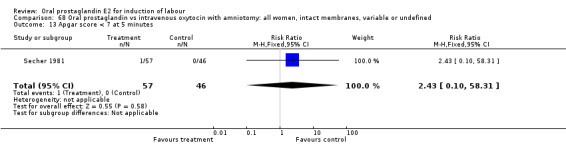
Comparison 68 Oral prostaglandin vs intravenous oxytocin with amniotomy: all women, intact membranes, variable or undefined, Outcome 13 Apgar score < 7 at 5 minutes.
68.23. Analysis.
Comparison 68 Oral prostaglandin vs intravenous oxytocin with amniotomy: all women, intact membranes, variable or undefined, Outcome 23 Postpartum haemorrhage.
Comparison 69. Oral prostaglandin vs intravenous oxytocin with amniotomy: all multiparae (without previous cesarean section).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.33 [0.81, 49.52] |
| 10 Epidural analgesia | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.51, 1.21] |
| 11 Instrumental vaginal delivery | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.23, 1.14] |
| 13 Apgar score < 7 at 5 minutes | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.01, 7.07] |
| 20 Vomiting | 2 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.60 [0.41, 31.59] |
| 21 Diarrhoea | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Postpartum haemorrhage | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
69.3. Analysis.
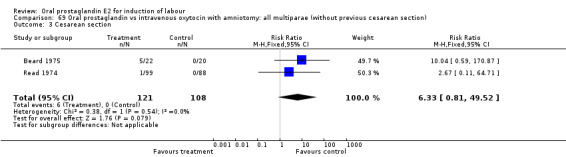
Comparison 69 Oral prostaglandin vs intravenous oxytocin with amniotomy: all multiparae (without previous cesarean section), Outcome 3 Cesarean section.
69.10. Analysis.
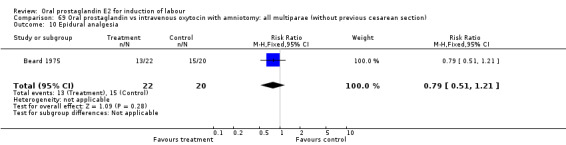
Comparison 69 Oral prostaglandin vs intravenous oxytocin with amniotomy: all multiparae (without previous cesarean section), Outcome 10 Epidural analgesia.
69.11. Analysis.
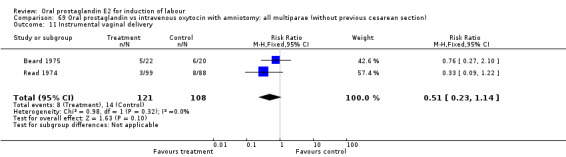
Comparison 69 Oral prostaglandin vs intravenous oxytocin with amniotomy: all multiparae (without previous cesarean section), Outcome 11 Instrumental vaginal delivery.
69.13. Analysis.
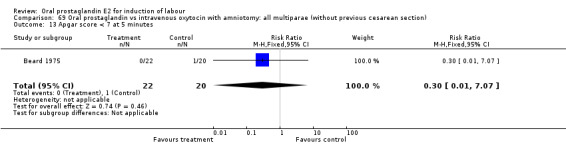
Comparison 69 Oral prostaglandin vs intravenous oxytocin with amniotomy: all multiparae (without previous cesarean section), Outcome 13 Apgar score < 7 at 5 minutes.
69.20. Analysis.
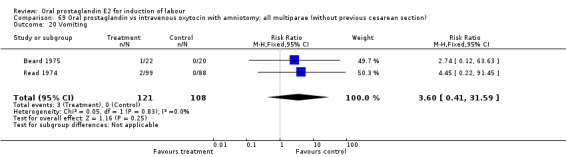
Comparison 69 Oral prostaglandin vs intravenous oxytocin with amniotomy: all multiparae (without previous cesarean section), Outcome 20 Vomiting.
69.21. Analysis.
Comparison 69 Oral prostaglandin vs intravenous oxytocin with amniotomy: all multiparae (without previous cesarean section), Outcome 21 Diarrhoea.
69.23. Analysis.
Comparison 69 Oral prostaglandin vs intravenous oxytocin with amniotomy: all multiparae (without previous cesarean section), Outcome 23 Postpartum haemorrhage.
Comparison 70. Oral prostaglandin vs oral oxytocin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.34, 3.97] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 2 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Cesarean section | 4 | 822 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.56, 1.77] |
| 4 Serious perinatal morbidity/perinatal death | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.25, 8.84] |
| 7 Oxytocin augmentation | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.28, 1.41] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 2 | 473 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.10, 56.20] |
| 11 Instrumental vaginal delivery | 4 | 822 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.91, 2.17] |
| 13 Apgar score < 7 at 5 minutes | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.18, 21.46] |
| 16 Perinatal death | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Nausea | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 23.0 [1.37, 386.56] |
| 20 Vomiting | 3 | 558 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.15 [2.35, 16.08] |
| 23 Postpartum haemorrhage | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.20, 6.76] |
70.1. Analysis.
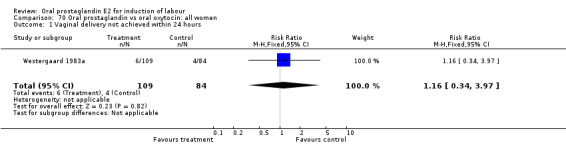
Comparison 70 Oral prostaglandin vs oral oxytocin: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
70.2. Analysis.
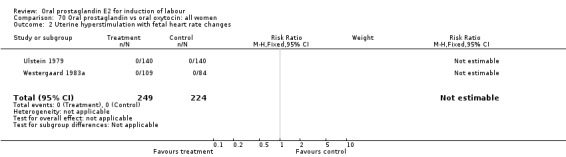
Comparison 70 Oral prostaglandin vs oral oxytocin: all women, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
70.3. Analysis.
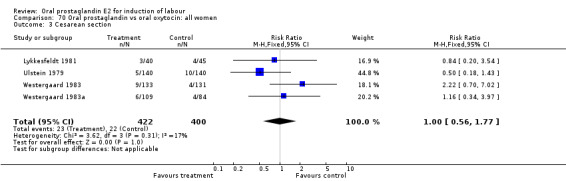
Comparison 70 Oral prostaglandin vs oral oxytocin: all women, Outcome 3 Cesarean section.
70.4. Analysis.
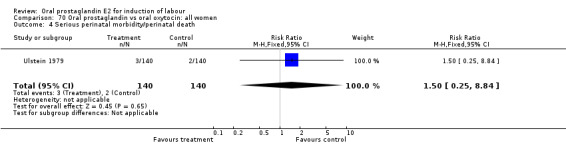
Comparison 70 Oral prostaglandin vs oral oxytocin: all women, Outcome 4 Serious perinatal morbidity/perinatal death.
70.7. Analysis.
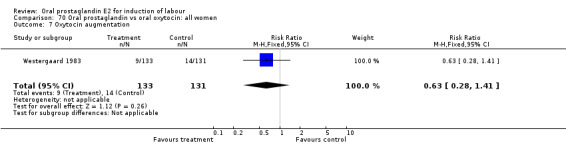
Comparison 70 Oral prostaglandin vs oral oxytocin: all women, Outcome 7 Oxytocin augmentation.
70.8. Analysis.
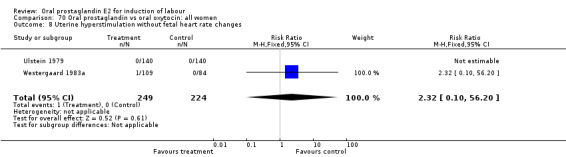
Comparison 70 Oral prostaglandin vs oral oxytocin: all women, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
70.11. Analysis.
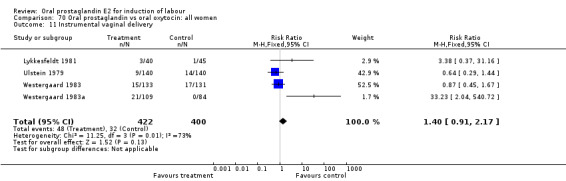
Comparison 70 Oral prostaglandin vs oral oxytocin: all women, Outcome 11 Instrumental vaginal delivery.
70.13. Analysis.
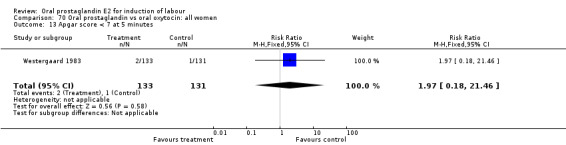
Comparison 70 Oral prostaglandin vs oral oxytocin: all women, Outcome 13 Apgar score < 7 at 5 minutes.
70.16. Analysis.
Comparison 70 Oral prostaglandin vs oral oxytocin: all women, Outcome 16 Perinatal death.
70.19. Analysis.
Comparison 70 Oral prostaglandin vs oral oxytocin: all women, Outcome 19 Nausea.
70.20. Analysis.
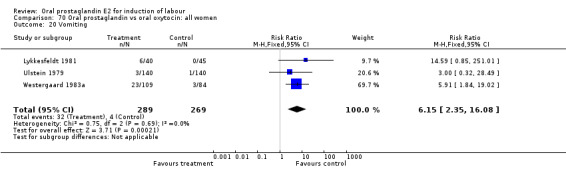
Comparison 70 Oral prostaglandin vs oral oxytocin: all women, Outcome 20 Vomiting.
70.23. Analysis.
Comparison 70 Oral prostaglandin vs oral oxytocin: all women, Outcome 23 Postpartum haemorrhage.
Comparison 71. Oral prostaglandin vs oral oxytocin:all women, unfavorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [0.70, 7.02] |
| 7 Oxytocin augmentation | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.28, 1.41] |
| 11 Instrumental vaginal delivery | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.45, 1.67] |
| 13 Apgar score < 7 at 5 minutes | 1 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.18, 21.46] |
71.3. Analysis.
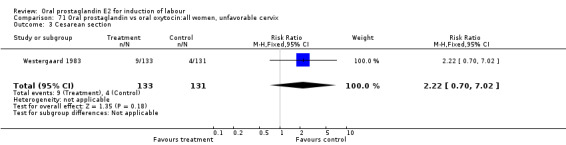
Comparison 71 Oral prostaglandin vs oral oxytocin:all women, unfavorable cervix, Outcome 3 Cesarean section.
71.7. Analysis.
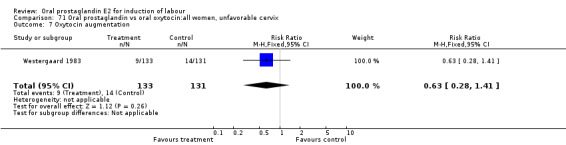
Comparison 71 Oral prostaglandin vs oral oxytocin:all women, unfavorable cervix, Outcome 7 Oxytocin augmentation.
71.11. Analysis.
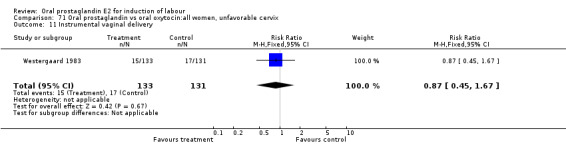
Comparison 71 Oral prostaglandin vs oral oxytocin:all women, unfavorable cervix, Outcome 11 Instrumental vaginal delivery.
71.13. Analysis.
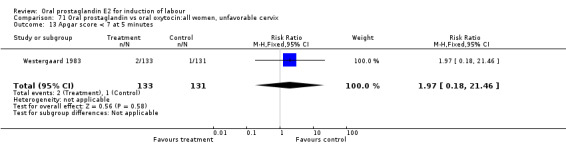
Comparison 71 Oral prostaglandin vs oral oxytocin:all women, unfavorable cervix, Outcome 13 Apgar score < 7 at 5 minutes.
Comparison 72. Oral prostaglandin vs oral oxytocin: all women, favorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Cesarean section | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.18, 1.43] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.29, 1.44] |
| 19 Nausea | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 23.0 [1.37, 386.56] |
| 20 Vomiting | 1 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 28.49] |
72.2. Analysis.
Comparison 72 Oral prostaglandin vs oral oxytocin: all women, favorable cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
72.3. Analysis.
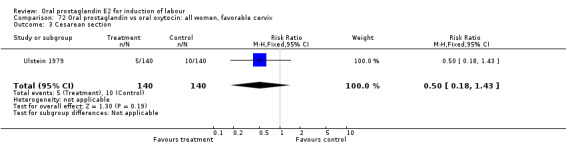
Comparison 72 Oral prostaglandin vs oral oxytocin: all women, favorable cervix, Outcome 3 Cesarean section.
72.8. Analysis.
Comparison 72 Oral prostaglandin vs oral oxytocin: all women, favorable cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
72.11. Analysis.
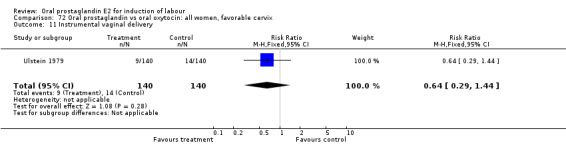
Comparison 72 Oral prostaglandin vs oral oxytocin: all women, favorable cervix, Outcome 11 Instrumental vaginal delivery.
72.19. Analysis.
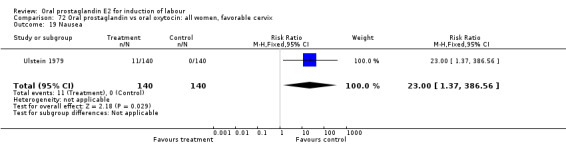
Comparison 72 Oral prostaglandin vs oral oxytocin: all women, favorable cervix, Outcome 19 Nausea.
72.20. Analysis.
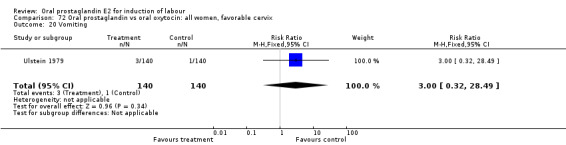
Comparison 72 Oral prostaglandin vs oral oxytocin: all women, favorable cervix, Outcome 20 Vomiting.
Comparison 73. Oral prostaglandin vs oral oxytocin: all women, ruptured membranes, variable or undefined cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.34, 3.97] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Cesarean section | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.43] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.10, 56.20] |
| 11 Instrumental vaginal delivery | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.87, 3.72] |
| 16 Perinatal death | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Vomiting | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.91 [1.84, 19.02] |
| 23 Postpartum haemorrhage | 1 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.20, 6.76] |
73.1. Analysis.
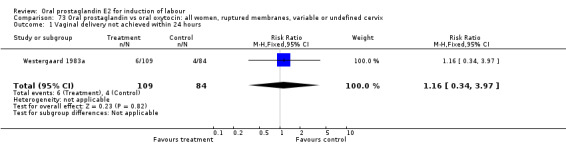
Comparison 73 Oral prostaglandin vs oral oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
73.2. Analysis.
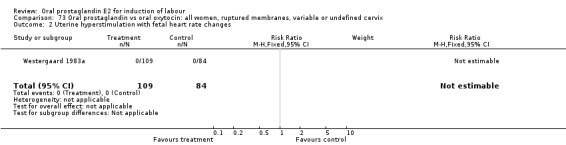
Comparison 73 Oral prostaglandin vs oral oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
73.3. Analysis.
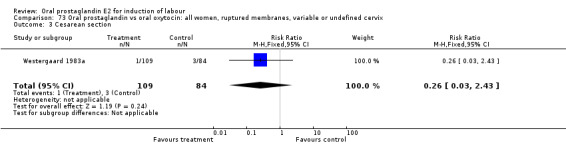
Comparison 73 Oral prostaglandin vs oral oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 3 Cesarean section.
73.8. Analysis.
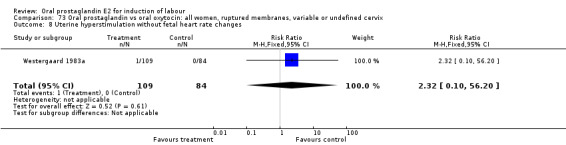
Comparison 73 Oral prostaglandin vs oral oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
73.11. Analysis.
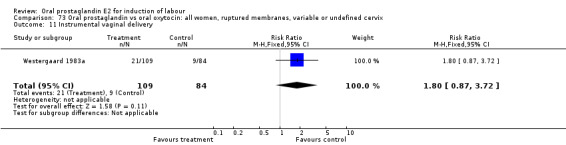
Comparison 73 Oral prostaglandin vs oral oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 11 Instrumental vaginal delivery.
73.16. Analysis.
Comparison 73 Oral prostaglandin vs oral oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 16 Perinatal death.
73.20. Analysis.
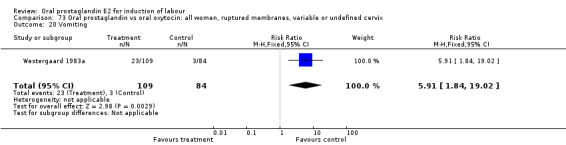
Comparison 73 Oral prostaglandin vs oral oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 20 Vomiting.
73.23. Analysis.
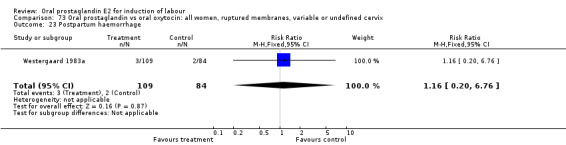
Comparison 73 Oral prostaglandin vs oral oxytocin: all women, ruptured membranes, variable or undefined cervix, Outcome 23 Postpartum haemorrhage.
Comparison 74. Oral prostaglandin vs oral oxytocin: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.20, 3.54] |
| 11 Instrumental vaginal delivery | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.38 [0.37, 31.16] |
| 20 Vomiting | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.59 [0.85, 251.01] |
74.3. Analysis.
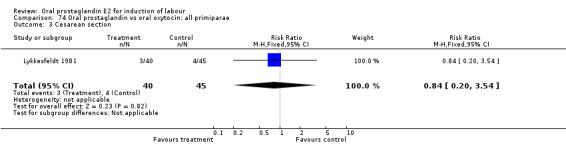
Comparison 74 Oral prostaglandin vs oral oxytocin: all primiparae, Outcome 3 Cesarean section.
74.11. Analysis.
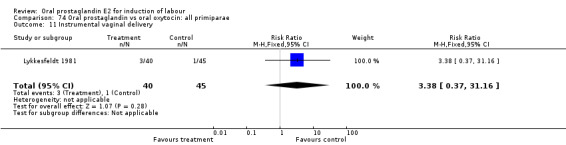
Comparison 74 Oral prostaglandin vs oral oxytocin: all primiparae, Outcome 11 Instrumental vaginal delivery.
74.20. Analysis.
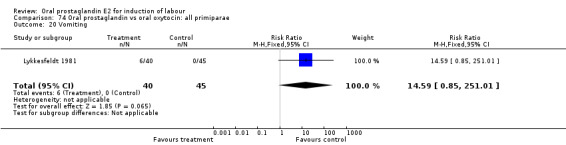
Comparison 74 Oral prostaglandin vs oral oxytocin: all primiparae, Outcome 20 Vomiting.
Comparison 75. Oral prostaglandin vs oral oxytocin: all primiparae, unfavorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.20, 3.54] |
| 11 Instrumental vaginal delivery | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.38 [0.37, 31.16] |
| 20 Vomiting | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 14.59 [0.85, 251.01] |
75.3. Analysis.
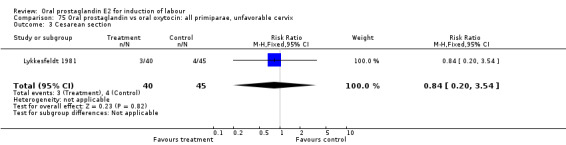
Comparison 75 Oral prostaglandin vs oral oxytocin: all primiparae, unfavorable cervix, Outcome 3 Cesarean section.
75.11. Analysis.
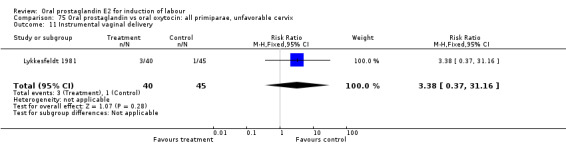
Comparison 75 Oral prostaglandin vs oral oxytocin: all primiparae, unfavorable cervix, Outcome 11 Instrumental vaginal delivery.
75.20. Analysis.
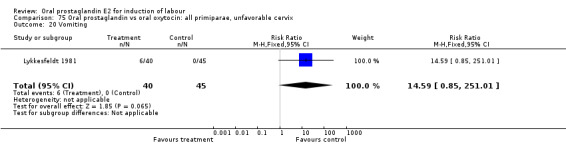
Comparison 75 Oral prostaglandin vs oral oxytocin: all primiparae, unfavorable cervix, Outcome 20 Vomiting.
Comparison 80. Oral prostaglandin vs oral oxytocin with amniotomy: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.86, 10.43] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.37, 132.10] |
| 3 Cesarean section | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.07, 8.38] |
| 6 Cervix unfavorable/unchanged after 12‐24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 27.87] |
| 7 Oxytocin augmentation | 2 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.36, 1.90] |
| 11 Instrumental vaginal delivery | 2 | 223 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.35, 3.75] |
| 12 Meconium‐stained liquor | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Apgar score < 7 at 5 minutes | 1 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.02, 12.44] |
| 20 Vomiting | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.65] |
| 21 Diarrhoea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 101.58] |
| 23 Postpartum haemorrhage | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.34] |
80.1. Analysis.
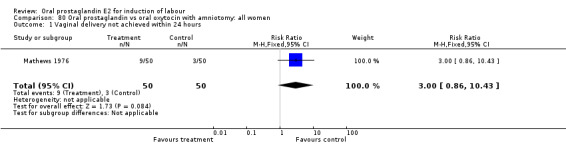
Comparison 80 Oral prostaglandin vs oral oxytocin with amniotomy: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
80.2. Analysis.
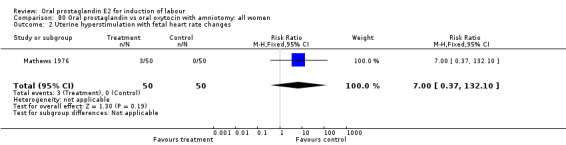
Comparison 80 Oral prostaglandin vs oral oxytocin with amniotomy: all women, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
80.3. Analysis.
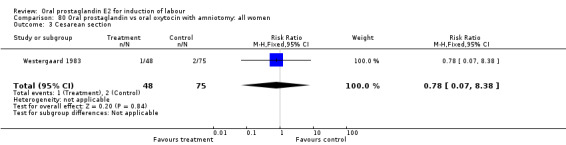
Comparison 80 Oral prostaglandin vs oral oxytocin with amniotomy: all women, Outcome 3 Cesarean section.
80.6. Analysis.
Comparison 80 Oral prostaglandin vs oral oxytocin with amniotomy: all women, Outcome 6 Cervix unfavorable/unchanged after 12‐24 hours.
80.7. Analysis.
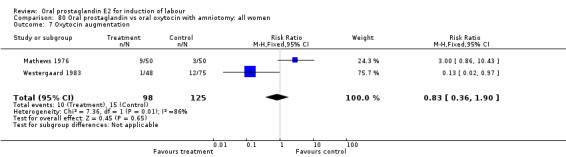
Comparison 80 Oral prostaglandin vs oral oxytocin with amniotomy: all women, Outcome 7 Oxytocin augmentation.
80.11. Analysis.
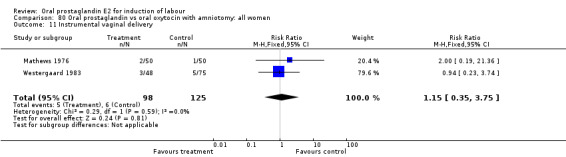
Comparison 80 Oral prostaglandin vs oral oxytocin with amniotomy: all women, Outcome 11 Instrumental vaginal delivery.
80.12. Analysis.
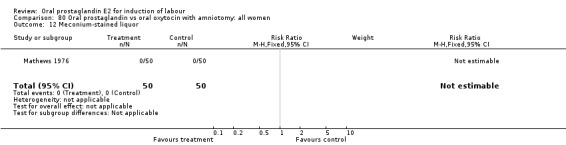
Comparison 80 Oral prostaglandin vs oral oxytocin with amniotomy: all women, Outcome 12 Meconium‐stained liquor.
80.13. Analysis.
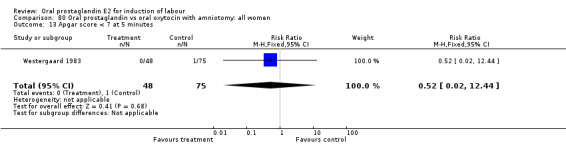
Comparison 80 Oral prostaglandin vs oral oxytocin with amniotomy: all women, Outcome 13 Apgar score < 7 at 5 minutes.
80.20. Analysis.
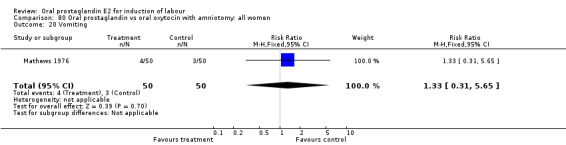
Comparison 80 Oral prostaglandin vs oral oxytocin with amniotomy: all women, Outcome 20 Vomiting.
80.21. Analysis.
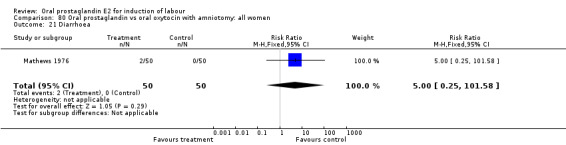
Comparison 80 Oral prostaglandin vs oral oxytocin with amniotomy: all women, Outcome 21 Diarrhoea.
80.23. Analysis.
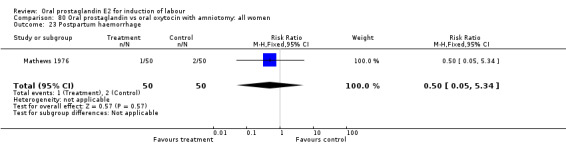
Comparison 80 Oral prostaglandin vs oral oxytocin with amniotomy: all women, Outcome 23 Postpartum haemorrhage.
Comparison 81. Oral prostaglandin vs oral oxytocin with amniotomy: all women, favorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.86, 10.43] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.37, 132.10] |
| 6 Cervix unfavorable/unchanged after 12‐24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 27.87] |
| 7 Oxytocin augmentation | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.86, 10.43] |
| 11 Instrumental vaginal delivery | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.36] |
| 12 Meconium‐stained liquor | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Vomiting | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.65] |
| 21 Diarrhoea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 101.58] |
| 23 Postpartum haemorrhage | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.34] |
81.1. Analysis.
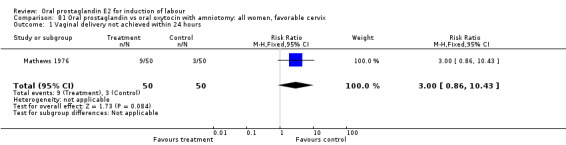
Comparison 81 Oral prostaglandin vs oral oxytocin with amniotomy: all women, favorable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
81.2. Analysis.
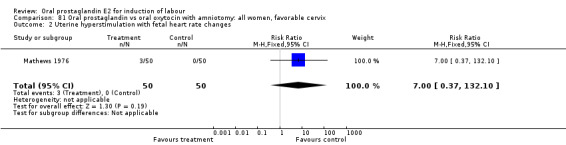
Comparison 81 Oral prostaglandin vs oral oxytocin with amniotomy: all women, favorable cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
81.6. Analysis.
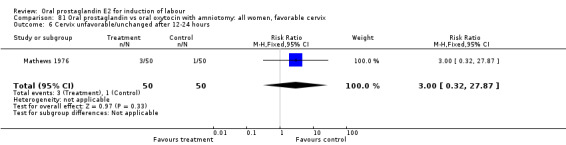
Comparison 81 Oral prostaglandin vs oral oxytocin with amniotomy: all women, favorable cervix, Outcome 6 Cervix unfavorable/unchanged after 12‐24 hours.
81.7. Analysis.
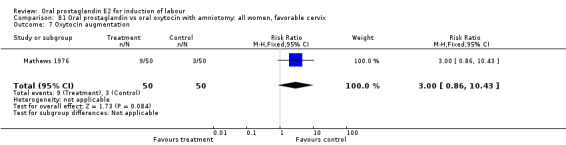
Comparison 81 Oral prostaglandin vs oral oxytocin with amniotomy: all women, favorable cervix, Outcome 7 Oxytocin augmentation.
81.11. Analysis.
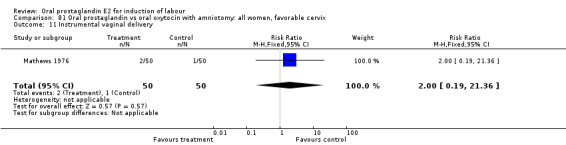
Comparison 81 Oral prostaglandin vs oral oxytocin with amniotomy: all women, favorable cervix, Outcome 11 Instrumental vaginal delivery.
81.12. Analysis.
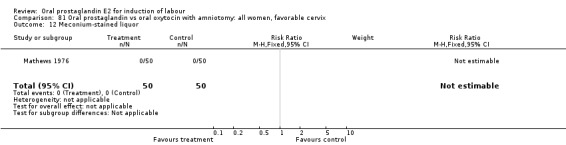
Comparison 81 Oral prostaglandin vs oral oxytocin with amniotomy: all women, favorable cervix, Outcome 12 Meconium‐stained liquor.
81.20. Analysis.
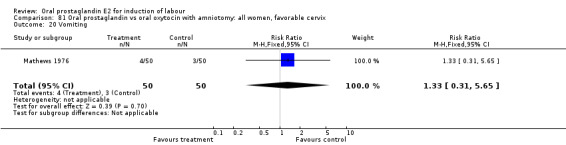
Comparison 81 Oral prostaglandin vs oral oxytocin with amniotomy: all women, favorable cervix, Outcome 20 Vomiting.
81.21. Analysis.
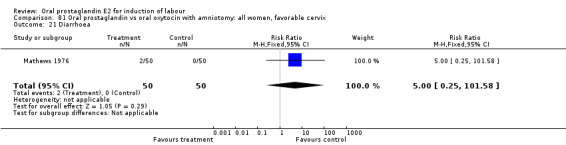
Comparison 81 Oral prostaglandin vs oral oxytocin with amniotomy: all women, favorable cervix, Outcome 21 Diarrhoea.
81.23. Analysis.
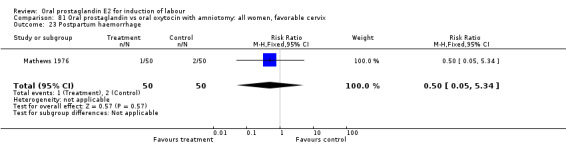
Comparison 81 Oral prostaglandin vs oral oxytocin with amniotomy: all women, favorable cervix, Outcome 23 Postpartum haemorrhage.
Comparison 82. Oral prostaglandin vs oral oxytocin with amniotomy: all women, intact membranes, favorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.86, 10.43] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.37, 132.10] |
| 6 Cervix unfavorable/unchanged after 12‐24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 27.87] |
| 7 Oxytocin augmentation | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.86, 10.43] |
| 11 Instrumental vaginal delivery | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.36] |
| 12 Meconium‐stained liquor | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Vomiting | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.65] |
| 21 Diarrhoea | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 101.58] |
| 23 Postpartum haemorrhage | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.34] |
82.1. Analysis.
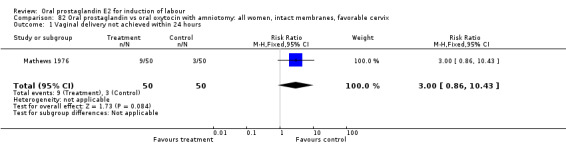
Comparison 82 Oral prostaglandin vs oral oxytocin with amniotomy: all women, intact membranes, favorable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
82.2. Analysis.
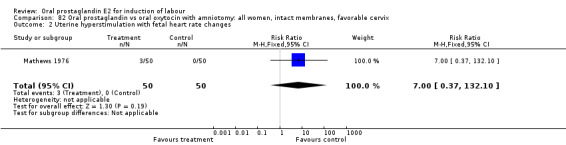
Comparison 82 Oral prostaglandin vs oral oxytocin with amniotomy: all women, intact membranes, favorable cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
82.6. Analysis.
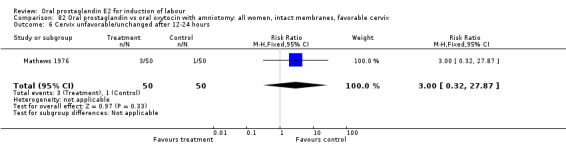
Comparison 82 Oral prostaglandin vs oral oxytocin with amniotomy: all women, intact membranes, favorable cervix, Outcome 6 Cervix unfavorable/unchanged after 12‐24 hours.
82.7. Analysis.
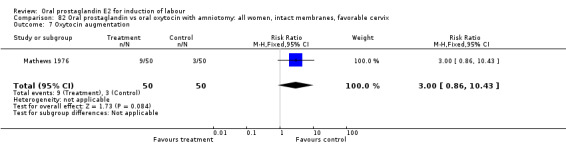
Comparison 82 Oral prostaglandin vs oral oxytocin with amniotomy: all women, intact membranes, favorable cervix, Outcome 7 Oxytocin augmentation.
82.11. Analysis.
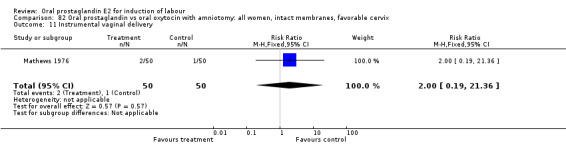
Comparison 82 Oral prostaglandin vs oral oxytocin with amniotomy: all women, intact membranes, favorable cervix, Outcome 11 Instrumental vaginal delivery.
82.12. Analysis.
Comparison 82 Oral prostaglandin vs oral oxytocin with amniotomy: all women, intact membranes, favorable cervix, Outcome 12 Meconium‐stained liquor.
82.20. Analysis.
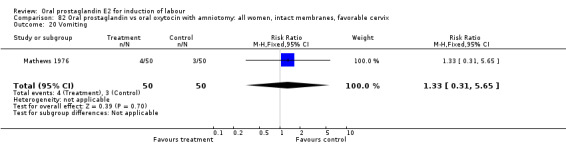
Comparison 82 Oral prostaglandin vs oral oxytocin with amniotomy: all women, intact membranes, favorable cervix, Outcome 20 Vomiting.
82.21. Analysis.
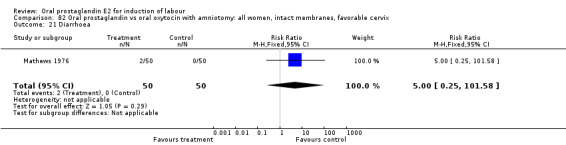
Comparison 82 Oral prostaglandin vs oral oxytocin with amniotomy: all women, intact membranes, favorable cervix, Outcome 21 Diarrhoea.
82.23. Analysis.
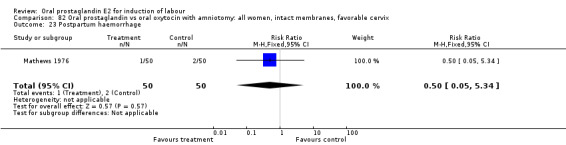
Comparison 82 Oral prostaglandin vs oral oxytocin with amniotomy: all women, intact membranes, favorable cervix, Outcome 23 Postpartum haemorrhage.
Comparison 83. Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.56, 7.12] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
| 20 Vomiting | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 26.92] |
| 21 Diarrhoea | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
| 23 Postpartum haemorrhage | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
83.1. Analysis.
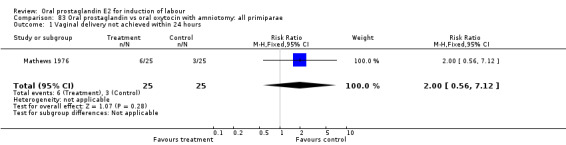
Comparison 83 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, Outcome 1 Vaginal delivery not achieved within 24 hours.
83.2. Analysis.
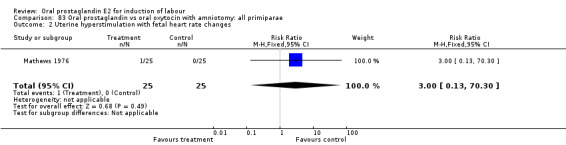
Comparison 83 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
83.20. Analysis.
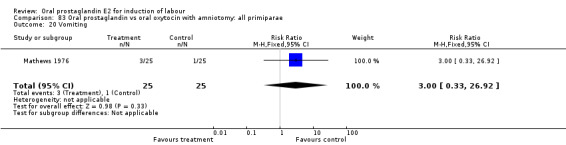
Comparison 83 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, Outcome 20 Vomiting.
83.21. Analysis.
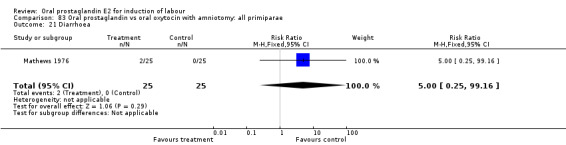
Comparison 83 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, Outcome 21 Diarrhoea.
83.23. Analysis.
Comparison 83 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, Outcome 23 Postpartum haemorrhage.
Comparison 84. Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, favorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.56, 7.12] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
| 20 Vomiting | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 26.92] |
| 21 Diarrhoea | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
| 23 Postpartum haemorrhage | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
84.1. Analysis.
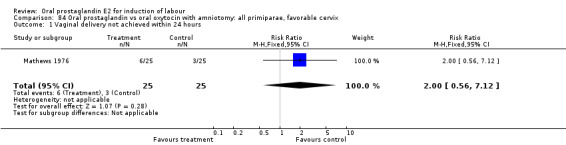
Comparison 84 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, favorable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
84.2. Analysis.
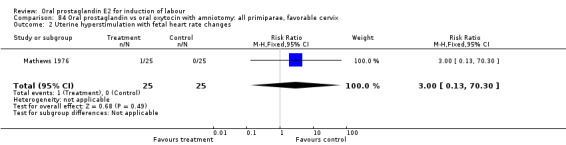
Comparison 84 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, favorable cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
84.20. Analysis.
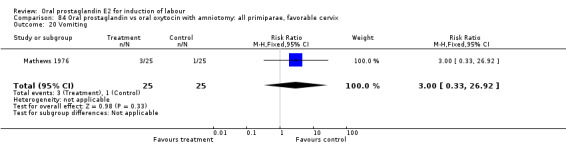
Comparison 84 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, favorable cervix, Outcome 20 Vomiting.
84.21. Analysis.
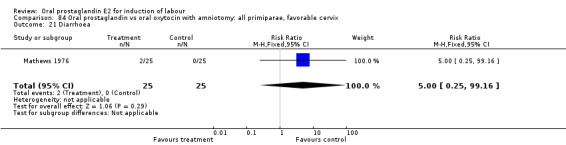
Comparison 84 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, favorable cervix, Outcome 21 Diarrhoea.
84.23. Analysis.
Comparison 84 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, favorable cervix, Outcome 23 Postpartum haemorrhage.
Comparison 85. Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, intact membranes, favorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.56, 7.12] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 70.30] |
| 20 Vomiting | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 26.92] |
| 21 Diarrhoea | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
| 23 Postpartum haemorrhage | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
85.1. Analysis.
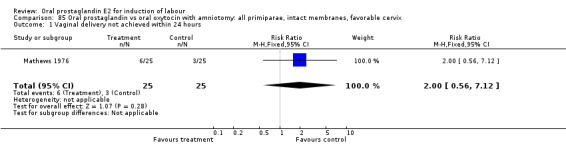
Comparison 85 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, intact membranes, favorable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
85.2. Analysis.
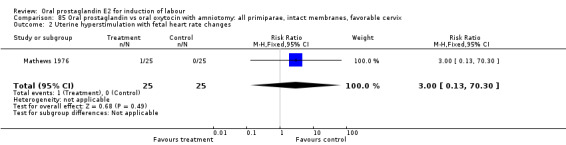
Comparison 85 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, intact membranes, favorable cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
85.20. Analysis.
Comparison 85 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, intact membranes, favorable cervix, Outcome 20 Vomiting.
85.21. Analysis.
Comparison 85 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, intact membranes, favorable cervix, Outcome 21 Diarrhoea.
85.23. Analysis.
Comparison 85 Oral prostaglandin vs oral oxytocin with amniotomy: all primiparae, intact membranes, favorable cervix, Outcome 23 Postpartum haemorrhage.
Comparison 86. Oral prostaglandin vs oral oxytocin with amniotomy : all multiparae (without previous cesarean section).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 128.87] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
| 20 Vomiting | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.17] |
| 21 Diarrhoea | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Postpartum haemorrhage | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.17] |
86.1. Analysis.
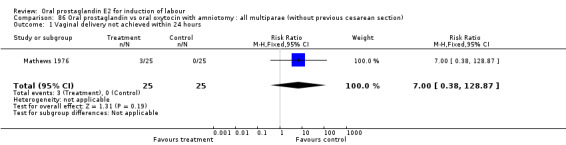
Comparison 86 Oral prostaglandin vs oral oxytocin with amniotomy : all multiparae (without previous cesarean section), Outcome 1 Vaginal delivery not achieved within 24 hours.
86.2. Analysis.
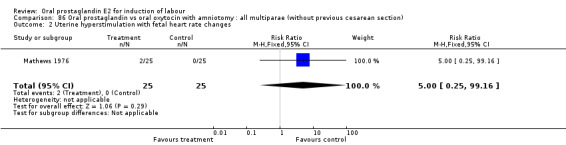
Comparison 86 Oral prostaglandin vs oral oxytocin with amniotomy : all multiparae (without previous cesarean section), Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
86.20. Analysis.
Comparison 86 Oral prostaglandin vs oral oxytocin with amniotomy : all multiparae (without previous cesarean section), Outcome 20 Vomiting.
86.21. Analysis.
Comparison 86 Oral prostaglandin vs oral oxytocin with amniotomy : all multiparae (without previous cesarean section), Outcome 21 Diarrhoea.
86.23. Analysis.
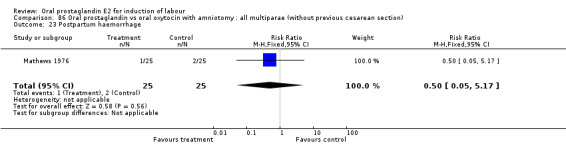
Comparison 86 Oral prostaglandin vs oral oxytocin with amniotomy : all multiparae (without previous cesarean section), Outcome 23 Postpartum haemorrhage.
Comparison 87. Oral prostaglandin vs oral oxytocin with amniotomy: all multiparae, favorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 128.87] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
| 20 Vomiting | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.17] |
| 21 Diarrhoea | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Postpartum haemorrhage | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.17] |
87.1. Analysis.
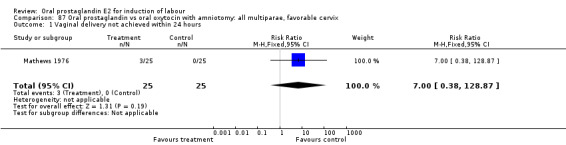
Comparison 87 Oral prostaglandin vs oral oxytocin with amniotomy: all multiparae, favorable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
87.2. Analysis.
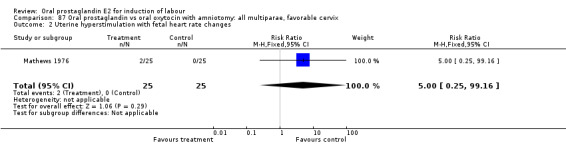
Comparison 87 Oral prostaglandin vs oral oxytocin with amniotomy: all multiparae, favorable cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
87.20. Analysis.
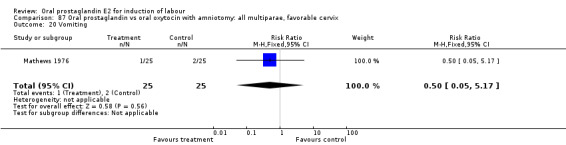
Comparison 87 Oral prostaglandin vs oral oxytocin with amniotomy: all multiparae, favorable cervix, Outcome 20 Vomiting.
87.21. Analysis.
Comparison 87 Oral prostaglandin vs oral oxytocin with amniotomy: all multiparae, favorable cervix, Outcome 21 Diarrhoea.
87.23. Analysis.
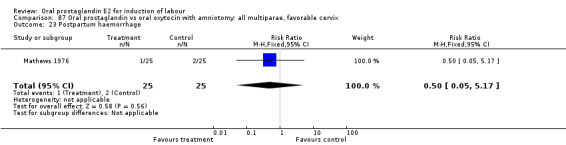
Comparison 87 Oral prostaglandin vs oral oxytocin with amniotomy: all multiparae, favorable cervix, Outcome 23 Postpartum haemorrhage.
Comparison 88. Oral prostaglandin vs oral oxytocin with amniotomy: all multiparae, intact membranes, favorable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 128.87] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 99.16] |
| 20 Vomiting | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.17] |
| 21 Diarrhoea | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Postpartum haemorrhage | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.17] |
88.1. Analysis.
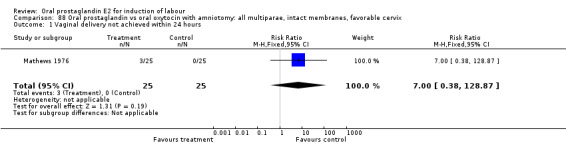
Comparison 88 Oral prostaglandin vs oral oxytocin with amniotomy: all multiparae, intact membranes, favorable cervix, Outcome 1 Vaginal delivery not achieved within 24 hours.
88.2. Analysis.
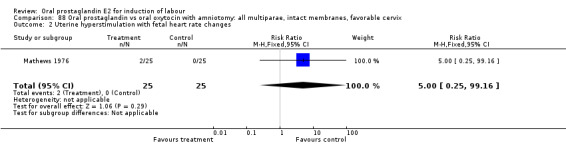
Comparison 88 Oral prostaglandin vs oral oxytocin with amniotomy: all multiparae, intact membranes, favorable cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
88.20. Analysis.
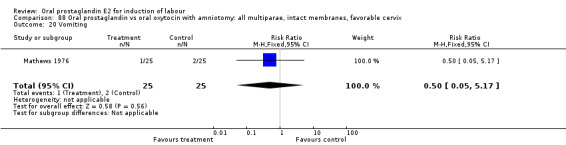
Comparison 88 Oral prostaglandin vs oral oxytocin with amniotomy: all multiparae, intact membranes, favorable cervix, Outcome 20 Vomiting.
88.21. Analysis.
Comparison 88 Oral prostaglandin vs oral oxytocin with amniotomy: all multiparae, intact membranes, favorable cervix, Outcome 21 Diarrhoea.
88.23. Analysis.
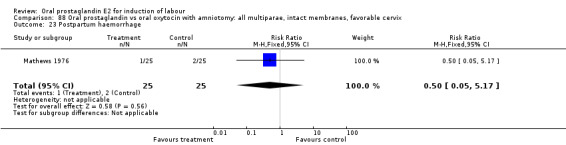
Comparison 88 Oral prostaglandin vs oral oxytocin with amniotomy: all multiparae, intact membranes, favorable cervix, Outcome 23 Postpartum haemorrhage.
Comparison 90. Oral prostaglandin high/incremental dose vs oral prostaglandin low dose: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Cesarean section | 2 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.33, 2.99] |
| 6 Cervix unfavorable/unchanged after 12‐24 hours | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.2 [0.01, 3.61] |
| 11 Instrumental vaginal delivery | 2 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.28, 1.92] |
90.3. Analysis.
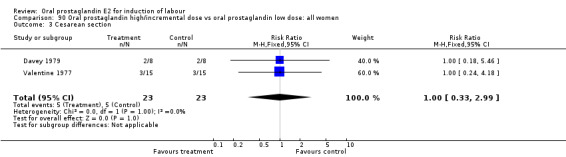
Comparison 90 Oral prostaglandin high/incremental dose vs oral prostaglandin low dose: all women, Outcome 3 Cesarean section.
90.6. Analysis.
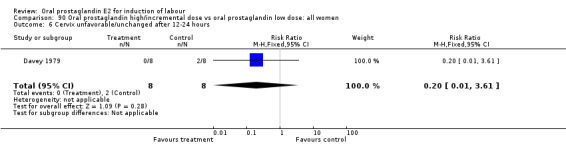
Comparison 90 Oral prostaglandin high/incremental dose vs oral prostaglandin low dose: all women, Outcome 6 Cervix unfavorable/unchanged after 12‐24 hours.
90.11. Analysis.
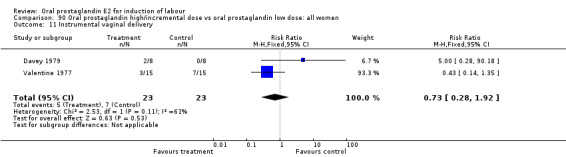
Comparison 90 Oral prostaglandin high/incremental dose vs oral prostaglandin low dose: all women, Outcome 11 Instrumental vaginal delivery.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Beard 1975.
| Methods | Allocation: list of random numbers. Blinding: none described. Study period: not stated. | |
| Participants | Inclusion criteria: multiparous women requiring induction of labor with Bishop score > 3. Setting: Maternity hospital, London, UK. Number of participants: n = 42. | |
| Interventions | Oral PGE2 solution 0.5 mg every 2 hours as needed with increases by 0.5 mg per dose 2 mg (n = 22) vs IV oxytocin (n = 20). All women had amniotomy at start of induction. | |
| Outcomes | Time of induction to delivery. Mode of delivery. Apgar score of infant. Umbilical artery pH. Hypertonus. Hypertonus with fetal bradycardia. Gastrointestinal side‐effects. | |
| Notes | The prostaglandin solution was acceptable to all women who received it. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Davey 1979.
| Methods | Allocation: "random", not further described. Blinding: none. Study period: not stated. | |
| Participants | Inclusion criteria: women with gestation of at least 36 weeks, indication for induction of labor, and Bishop score less than 6. Setting: University hospital, Cape Town, South Africa. Number of participants: n = 33. | |
| Interventions | Oral PGE2 1 mg hourly up to 5 mg (n = 16) vs intravaginal PGE2, 1 x 4 or 6 mg dose (n = 17). | |
| Outcomes | Bishop score after 12 hours. Cesarean sections. Instrumental vaginal deliveries. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Golbus 1977.
| Methods | Allocation: concealed. Blinding: double‐blind. Study period: not stated. | |
| Participants | Inclusion criteria: women with 36‐41 weeks' gestation with low or moderate Bishop score. Setting: University hospital, San Francisco, CA. Number of participants: n = 50. | |
| Interventions | Oral PGE2 1 mg every 3 hours for 3 doses (n = 25) vs placebo (n = 25). | |
| Outcomes | Mean change in Bishop score. Cesarean section. Uterine hypertonus. | |
| Notes | Oxytocin infusion was started 9‐11 hours after 3rd oral dose. If labor was not achieved, it was discontinued and spontaneous labor ensued 1‐18 days later. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Hauth 1977.
| Methods | Allocation: "randomized", not further described. Blinding: allocation stated to be concealed, otherwise no blinding described. Study period: not stated. | |
| Participants | Inclusion criteria: women with PROM at 38‐41 weeks' gestation and no uterine contractions 3 hours after rupture. Setting: University hospital Center, Dallas, Texas. Number of participants: n = 100. | |
| Interventions | Oral PGE2 0.5 mg every 30‐60 minutes starting 3 hours after PROM for up to 6 hours (n = 50) vs no treatment until 12 hours after PROM (n = 50). For both groups IV oxytocin was begun at 12 hours after PROM if not in active labor. | |
| Outcomes | Time from PROM to delivery. Fetal bradycardia. Mode of delivery. Gastrointestinal side‐effects. | |
| Notes | Subgroups were considered based on parity and Bishop score at 3 hours after PROM. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Herabutya 1988.
| Methods | Allocation: "random", not further described. Blinding: none. Study period: June 1986 to May 1987. | |
| Participants | Inclusion criteria: primiparous women with indication for induction of labor and Bishop score of 4 or less. Setting: University hospital, Bangkok, Thailand. Number of participants: n = 50. | |
| Interventions | Oral PGE2 0.5 mg hourly for 6 hours daily (n = 25) vs PGE2 intracervical gel, 3 mg for 1 dose per day (n = 25). | |
| Outcomes | Cesarean section. Instrumental vaginal delivery. Apgar score < 7 at 5 minutes. | |
| Notes | When Bishop score reached 6 or more oxytocin induction was performed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lange 1981.
| Methods | Allocation: "random", not further described. Blinding: none described. Study period: not stated. | |
| Participants | Inclusion criteria: women with PROM at or near term of at least 6 hours without labor activity and cervix less than 3 cm dilated. Setting: University Hospital, Odense, Denmark. Number of participants: n = 201. | |
| Interventions | Oral PGE2 0.5 mg hourly and with increases up to 1.5 mg per hour depending on response (n = 99) vs IV oxytocin up to 45 miliunits per minute (n = 102). | |
| Outcomes | Induction to delivery time. Mode of delivery. Apgar score less than 8. Gastrointestinal side‐effects. | |
| Notes | The Bishop scores of the oxytocin group were slightly higher overall and there were fewer women with scores of less than 6 (24 vs 28). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Lykkesfeldt 1981.
| Methods | Allocation: "randomized", not further described. Blinding: none described. Study period: not stated. | |
| Participants | Inclusion criteria: primiparous women with indication for induction of labor and Bishop score < 7. Setting: University Hospital, Copenhagen, Denmark. Number of participants: n = 132. | |
| Interventions | Oral PGE2 0.5 mg every 1/2 hour up to a dose of 5 mg in 24 hours vs demoxytocin 50 IU every 1/2 hour up to 500 IU in 24 hours vs demoxytocin in the 1st 24 hours and oral PGE2 the 2nd day. | |
| Outcomes | Successful induction within 48 hours. Mode of delivery. Gastrointestinal side‐effects. Uterine hyperstimulation. Apgar score < 7 at 1 minute. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Massil 1988.
| Methods | Allocation: "randomized", not further described. Blinding: none described. Study period: not stated. | |
| Participants | Inclusion criteria: women with PROM with gestation of at least 36 weeks and not in labor, stratified by parity. Setting: London, UK. Number of participants: n = 69. | |
| Interventions | Oral PGE2 0.5 mg per hour, increasing to 1.0 mg if needed (n = 36) vs IV oxytocin up to 32 miliunits per minute (n = 33). | |
| Outcomes | Successful stimulation defined as progression to active labor within 8 hours. Stimulation to delivery interval. Mode of delivery. Uterine hypertonus. Gastrointestinal side‐effects. Apgar scores. Woman's acceptability. | |
| Notes | More women who received oral PGE2 (91.6%) expressed satisfaction with the method of stimulation compared to 66.7% of those receiving IV oxytocin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Mathews 1976.
| Methods | Allocation: sealed envelopes. Blinding: none described. Study period: not stated. | |
| Participants | Inclusion criteria: women admitted for induction of labor with favorable cervix, singleton pregnancy, and clear amniotic fluid on amniotomy Setting: general hospital, Isle of Sheppey, UK. Number of participants: n = 100 (50 primigravidae, and 50 multigravidae). | |
| Interventions | Oral PGE2 0.5 mg initial dose increasing up to 2 mg if needed, total 5 mg maximum (n = 50) vs oral oxytocin starting at 100 IU and escalating at 1/2 hour intervals as needed up to 4400 IU total dose (n = 50). Both groups had amniotomy performed at the beginning of induction. | |
| Outcomes | Time to onset of labor. Induction to delivery interval. Duration of labor. Mode of delivery. Uterine hyperactivity. Gastrointestinal side‐effects. Apgar < 7 at 1 minute. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Paul 1992.
| Methods | Allocation: sealed envelopes. Blinding: none described. Study period: March through November 1992. | |
| Participants | Inclusion criteria: women presenting for induction of labor with fetus in cephalic position. Setting: University Hospital, Calcutta, India. Number of participants: n = 35. | |
| Interventions | Oral PGE2 0.5 mg hourly increasing to 1 mg hourly if needed (n = 15) vs IV oxytocin to a maximum of 20 miliunits per minute (n = 20). | |
| Outcomes | Mode of delivery. Induction to delivery interval. Gastrointestinal side‐effects. Uterine hyperstimulation. Apgar score. Fetal distress. Maternal satisfaction with induction method. | |
| Notes | 80% of women were satisfied with PGE2 vs 15% with IV oxytocin. Subgroup information is presented based on parity and Bishop score. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Ratnam 1974.
| Methods | Allocation: sealed envelopes. Blinding: none described. Study period: not stated. | |
| Participants | Inclusion criteria: women with indication for induction of labor without evidence of acute or chronic fetal distress. Setting: University hospital, Singapore. Number of participants: n = 207. | |
| Interventions | Oral PGE2 0.5 mg hourly increasing up to 2 mg per hour if needed (n = 107) vs IV oxytocin (no maximum infusion rate stated) (n = 100). Half of participants in each group underwent amniotomy at the start of induction. | |
| Outcomes | Time from start of treatment to establishment of labor. Labor to delivery interval. Mode of delivery. Gastrointestinal side‐effects. Uterine hypertonus. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Read 1974.
| Methods | Allocation: "random", not further described. Blinding: none described. Study period: not stated. | |
| Participants | Inclusion criteria: parous women admitted for induction of labor with membranes intact. Setting: general hospital, Manchester, UK. Number of participants: n = 187. | |
| Interventions | Oral PGE2 0.5 mg followed by 1.0 mg 1/2 hour later, then variable doses up to 2 mg at 2 hourly intervals (n = 99) vs IV oxytocin to a maximum of 80 miliunits per minute (n = 88). All women underwent amniotomy at the beginning of induction. | |
| Outcomes | Induction to delivery interval. Mode of delivery. Apgar score at 5 minutes. Gastrointestinal side‐effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Secher 1981.
| Methods | Allocation: "random", not further described. Blinding: none described. Study period: not stated. | |
| Participants | Inclusion criteria: women admitted for induction of labor with membranes intact, single live fetus in cephalic position. Setting: University hospital, Odense, Denmark. Number of participants: n = 471. | |
| Interventions | Women with ripe cervix and head engaged (n = 227) underwent amniotomy and observation for 4 hours. 124 were in labor. The remainder (n = 103) received oral PGE2 0.5 mg increasing to 1.5 mg per hour if needed (n = 57) vs IV oxytocin to a maximum infusion rate of 45 miliunits per minute (n = 46). Those with unfavorable cervix: oral prostaglandin as above (n = 125) vs IV oxytocin as above (n = 119). |
|
| Outcomes | Length of labor. Treatment time. Apgar score. Mode of delivery. Gastrointestinal side‐effects. | |
| Notes | IV oxytocin was given at a rapidly increasing rate, starting at 7.5 miliunits and increasing by 7.5 miliunits every 15 minutes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Somell 1987.
| Methods | Allocation: concealed. Blinding: double‐ blind. Study period: not stated. | |
| Participants | Inclusion criteria: women admitted for induction of labor with Bishop score less than 6 with membranes intact. Setting: University hospital, Huddinge, Sweden. Number of participants: n = 191. | |
| Interventions | For cervical ripening: oral PGE2 0.5 mg hourly for 12 hours for 2 days (n = 95) vs placebo (n = 96). For induction: oral PGE2 hourly with incremental dosing vs IV oxytocin. |
|
| Outcomes | Cesarean section. Serious neonatal morbidity/perinatal death. Uterine hyperstimulation without FHR changes. Apgar score < 7 at 5 minutes. Gastrointestinal side‐effects. Postpartum hemorrhage. | |
| Notes | The induction phase was carried out in 4 groups, based on primed or not primed, induction with PGE2 or IV oxytocin. Although the priming portion was double‐blind, it is not clear that the induction portion of the study was blinded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Ulstein 1979.
| Methods | Allocation: "random", not further described. Blinding: none described. Study period: not stated. | |
| Participants | Inclusion criteria: women undergoing induction of labor for gestation longer than 42 weeks. Setting: University Hospital, Bergen, Norway. Number of participants: n = 280. | |
| Interventions | Oral PGE2 0.5 mg per hour (n = 140) vs demoxytocin 50 IU every 1/2 hour (n = 140). | |
| Outcomes | Duration of labor. Mode of delivery. Mean Apgar scores. Gastrointestinal side‐effects. | |
| Notes | Amniotomy was performed when cervical dilatation reached 4‐5 cm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Valentine 1977.
| Methods | Allocation: "random", not further described. Blinding: none. Study period: not given. | |
| Participants | Inclusion criteria: women undergoing induction of labor with Bishop score of 4 or less. Setting: City Hospital, Nottingham, UK. Number of participants: n = 60. | |
| Interventions | Oral PGE2 0.5 mg hourly vs oral PGE2 1 mg hourly vs oxytocin IV vs no treatment. Each group n = 15. | |
| Outcomes | Cesarean section. Instrumental vaginal delivery. | |
| Notes | Once cervix was favorable, amniotomy was performed and IV oxytocin administered. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Westergaard 1983.
| Methods | Allocation: sealed envelopes. Blinding: None described. Study period: 24 months, specific dates not stated. | |
| Participants | Inclusion criteria: women admitted for induction of labor with single live fetus in cephalic position and membranes intact. Setting: University hospital, Odense, Denmark. | |
| Interventions | Women with unfavorable cervix (n = 264): oral PGE2 0.5 mg increasing to 1.5 mg per hour if needed (n = 133) vs demoxytocin 50 IU every 1/2 hour (n = 131). Women with favorable cervix underwent amniotomy (n = 259 of which 136 progressed into labour within 4 hours without medication). The remaining 123 women received oral PGE2 as above (n = 48) vs demoxytocin as above (n = 75). |
|
| Outcomes | Induction failure (defined as lack of regular contractions and cervical dilatation of 5 cm within 48 hours or 8 hours of spontaneous or artificial rupture of membranes). Treatment time. Duration of labor. Apgar score < 7. Mode of delivery. Gastrointestinal side‐effects. Uterine hypertonus. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Westergaard 1983a.
| Methods | Allocation: "randomized", not further described. Binding: none described. Study period: 24 months, specific dates not stated. | |
| Participants | Inclusion criteria: women presenting with PROM at 37 or more weeks' gestation and not in labour 6 hours after loss of amniotic fluid. Setting: University Hospital, Odense, Denmark. | |
| Interventions | Oral PGE2 0.5 mg hourly increasing to 1.5 mg if needed (n = 109) vs demoxitocin 50 IU every 1/2 hour (n = 84). | |
| Outcomes | Duration of labor. Stimulation to delivery interval. Failure (defined as lack of regular contractions and 2 cm progress in cervical dilatation within 8 hours of treatment). Apgar score < 7. Mode of delivery. Gastrointestinal side‐effects. Uterine hypertonus. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Wilson 1978.
| Methods | Allocation: "random", not further described. Blinding: none. Study period: not stated. | |
| Participants | Inclusion criteria: primigravid women with Bishop score of 4 or less and obstetric indication for induction of labor. Setting: District hospital, Alexandria, UK. Number of participants: n = 60. | |
| Interventions | Oral PGE2 1 mg hourly for 10 hours vs extra‐amniotic PGE2 gel (single dose) vs IV oxytocin for 8 hours vs PGE2 intravaginally. Each group n = 15. | |
| Outcomes | Mean change in Bishop score. Cesarean section. Instrumental vaginal delivery. | |
| Notes | Induction by amniotomy and IV oxytocin was started the following day if cervix was considered favourable. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
FHR: fetal heart rate IU: international units IV: intravenous PGE2: prostaglandins E2 PROM: premature rupture of the membranes vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amano 1999 | This is a study of routine policy of induction of labour at 39 weeks' gestation (without medical indication) vs expectant management, a situation not contemplated in this review. |
| Bloch 1975 | No randomization described. |
| Borisov 1985 | Insufficient information to assess the study. The review author will be writing to the trial authors for more information. |
| Bremme 1980 | Study of hormone levels in cord blood, without consideration of outcomes of interest to this review. |
| Bremme 1984 | Insufficient information to assess the study. The review author will be writing to the trial authors for more information. |
| Bremme 1987 | Study of hormone levels in cord blood, without consideration of outcomes of interest to this review. |
| Browne 1988 | Insufficient information to assess the study. The review author will be writing to the trial authors for more information. |
| Davies 1991 | Study of early vs delayed (till next day) induction using the same treatment regimen. The variable time frame of no treatment and lack of outcomes of interest for this review reported in the study during that time period, makes it difficult to include. |
| Friedman 1974 | This study allocated women to 3 different dosing regimens. Women received 1 of 3 oral PGE2 regimens by alternation rather than randomization. The 'control group' was by matching women who were not study participants. Thus, the study does not meet criteria for a RCT. |
| Friedman 1975a | Allocation by alternation, not concealed. |
| Friedman 1975b | No outcomes of interest as defined by this review. |
| Haeri 1976 | Allocation by odd or even record number, not concealed. |
| Ismail 1989 | No randomization method described. |
| Johnstone 1987 | Insufficient information to assess the study. The review author will be writing to the trial authors for more information. |
| Lange 1982 | Study of neonatal jaundice after induction with PGE2 vs oxytocin. No outcomes of interest to this review are described. |
| Lorrain 1982 | Study of 2 oral preparations of PGE2 'natural' vs 'synthetic'. These are not sufficiently different regimens. |
| Lykkesfeldt 1979 | Allocation not concealed. |
| Makary 1990 | Study planned but not undertaken. |
| Miller 1975 | Allocation by alternating time period, not concealed. |
| Mokgokong 1976 | No randomization described. |
| Nassief 1996 | Insufficient information to assess the study. The review author will be writing to the trial authors for more information. |
| Obel 1975 | Difference in treatment was only the method of oral administration of PGE2, tablet versus solution. |
| Pearce 1977 | No outcomes of interest reported. |
| Pulle 1986 | Oral prostaglandin was not used in this study. |
| Samal 2000 | No randomization described. |
| Sivasuriya 1978 | Study of neonatal bilirubin levels after induction. No outcomes of interest to this review were studied. |
| Somell 1983 | This study compared induction of labor with oral PGE2 vs IV oxytocin. However, the oral PGE2 group underwent cervical ripening for 2 days (with oral PGE2) prior to induction while the oxytocin group did not. Thus, there are 2 comparisons in the control arm, no treatment for ripening followed by IV oxytocin for induction. This situation was not contemplated in the protocol for the review. |
| Soni 2000 | No randomization described. |
| Suzuki 2000 | Study of elective induction vs expectant management of twin pregnancy. |
| Thiery 1977 | Not a difference in treatments, only the method of administration of oral PGE2 (sublingual vs swallowed). |
| Weiss 1975 | 4 of 60 participants enrolled were not analyzed in any of the 3 treatment groups, and no explanation was given. |
| Yacoob 1993 | Study does not include oral prostaglandin. |
| Yeung 1977 | Insufficient information to assess the study. The review author will be writing to the trial authors for more information. |
IV: intravenous PGE2: prostaglandins E2 RCT: randomized controlled trial vs: versus
Contributions of authors
L French prepared and maintains the review.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Beard 1975 {published data only}
- Beard RJ, Harrison R, Kiriakidis J, Underhill R, Craft I. A clinical and biochemical assessment of the use of oral prostaglandin E2 compared with intravenous oxytocin for labor induction in multiparous patients. European Journal of Obstetrics & Gynecology and Reproductive Biology 1975;5:203‐7. [Google Scholar]
Davey 1979 {published data only}
- Davey DA, MacNab M. Oral and intravaginal prostaglandin E2 for cervical ripening and induction of labour. South African Medical Journal 1979;55:837‐42. [PubMed] [Google Scholar]
Golbus 1977 {published data only}
- Golbus MS, Creasy RK. Uterine priming with oral prostaglandin E2 prior to elective induction with oxytocin. Prostaglandins 1977;14:577‐81. [DOI] [PubMed] [Google Scholar]
Hauth 1977 {published data only}
- Hauth JC, Cunningham FG, Whalley PJ. Early labor initiation with oral PGE2 after premature rupture of the membranes at term. Obstetrics & Gynecology 1977;49:523‐6. [PubMed] [Google Scholar]
Herabutya 1988 {published data only}
- Herabutya Y, O‐Prasertsawat P. A comparison of oral and intracervical prostaglandin E2 for ripening of the unfavourable cervix prior to induction of labour. Journal of the Medical Association of Thailand 1988;71:269‐73. [PubMed] [Google Scholar]
Lange 1981 {published data only}
- Lange AP, Secher NJ, Nielsen FH, Pedersen GT. Stimulation of labor in cases of premature rupture of the membranes at or near term. Acta Obstetricia et Gynecologica Scandinavica 1981;60:207‐10. [PubMed] [Google Scholar]
Lykkesfeldt 1981 {published data only}
- Lykkesfeldt G, Osler M. Induction of labor with oral prostaglandin E2 and buccal demoxytocin without amniotomy. Acta Obstetricia et Gynecologica Scandinavica 1981;60:429‐30. [DOI] [PubMed] [Google Scholar]
Massil 1988 {published data only}
- Massil HY, Baker AC, O'Brien PMS. A comparison of oral prostaglandin E2 tablets with intravenous oxytocin for stimulation of labor after premature rupture of membranes at term. Acta Obstetricia et Gynecologica Scandinavica 1988;67:703‐9. [DOI] [PubMed] [Google Scholar]
Mathews 1976 {published data only}
- Mathews DD, Hossain H, Bhargava S, D'Souza F. A randomised controlled trial of an oral solution of prostaglandin E2 and oral oxytocin used immediately after low amniotomy for induction of labour in the presence of a favourable cervix. Current Medical Research and Opinion 1976;4:233‐40. [DOI] [PubMed] [Google Scholar]
Paul 1992 {unpublished data only}
- Paul S, Bhowmick R. A randomised controlled trial of oral prostaglandin E2 (dinoprostone) and oxytocin infusion in induction of labour. Personal communication 1992.
Ratnam 1974 {published data only}
- Ratnam SS, Khew KS, Chen C, Lim TC. Oral prostaglandin E2 in induction of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1974;14:26‐30. [Google Scholar]
Read 1974 {published data only}
- Read MD, Martin RH. A comparison between intravenous oxytocin and oral prostaglandin E2 for the induction of labour in parous patients. Current Medical Research and Opinion 1974;2:236‐9. [DOI] [PubMed] [Google Scholar]
Secher 1981 {published data only}
- Secher NJ, Lange AP, Hassing Nielsen F, Thomson Pedersen G, Westergaard JG. Induction of labor with and without primary amniotomy. A randomized study of prostaglandin E2 tablets and intravenous oxytocin. Acta Obstetricia et Gynecologica Scandinavica 1981;60:237‐41. [DOI] [PubMed] [Google Scholar]
Somell 1987 {published data only}
- Somell C. Induction of labor and cervical ripening with oral PGE2 in risk pregnancies: a placebo‐controlled study. Acta Obstetricia et Gynecologica Scandinavica 1987;66:633‐7. [DOI] [PubMed] [Google Scholar]
Ulstein 1979 {published data only}
- Ulstein M, Sagen N, Eikhom SN. A comparative study of labor induced by prostaglandin E2 and buccal tablets of demoxytocin. International Journal of Gynecology & Obstetrics 1979;17:243‐5. [DOI] [PubMed] [Google Scholar]
Valentine 1977 {published data only}
- Valentine BH. Intravenous oxytocin and oral prostaglandin E2 for ripening of the unfavourable cervix. British Journal of Obstetrics and Gynaecology 1977;84:846‐54. [DOI] [PubMed] [Google Scholar]
Westergaard 1983 {published data only}
- Westergaard JG, Lange AP, Pedersen GT, Secher NJ. Oral oxytocics for induction of labor. Acta Obstetricia et Gynecologica Scandinavica 1983;62:103‐10. [DOI] [PubMed] [Google Scholar]
Westergaard 1983a {published data only}
- Westergaard JG, Lange AP, Pedersen GT, Secher NJ. Use of oral oxytocics for stimulation of labor in cases of premature rupture of the membranes at term. Acta Obstetricia et Gynecologica Scandinavica 1983;62:111‐6. [DOI] [PubMed] [Google Scholar]
Wilson 1978 {published data only}
- Wilson PD. A comparison of four methods of ripening the unfavourable cervix. British Journal of Obstetrics and Gynaecology 1978;85:941‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amano 1999 {published data only}
- Amano K, Saito K, Shoda T, Yoshihara H, Nishijima M. Elective induction of labor at 39 weeks gestation:a prospective randomized trial. Journal of Obstetrics and Gynaecology Research 1999;25(1):33‐7. [DOI] [PubMed] [Google Scholar]
Bloch 1975 {published data only}
- Bloch B. Induction of labour with prostaglandin E2 tablets. South African Medical Journal 1975;49:2001‐3. [PubMed] [Google Scholar]
Borisov 1985 {published data only}
- Borisov I, Starkalev I. Comparison of oral PGE2 and intravenous oxytocin for stimulation of labor in cases of premature rupture of the membranes. Archives of Gynecology 1985;237:305. [Google Scholar]
Bremme 1980 {published data only}
- Bremme K, Eneroth P. Changes in serum hormone levels during labor induced by oral PGE2 or oxytocin infusion. Acta Obstetricia et Gynecologica Scandinavica 1980;92:31‐43. [DOI] [PubMed] [Google Scholar]
Bremme 1984 {published data only}
- Bremme K, Nilsson B. Prediction of time to delivery from start of contractions in induced labor: a life table analysis approach. International Journal of Gynecology & Obstetrics 1984;22:225‐9. [DOI] [PubMed] [Google Scholar]
Bremme 1987 {published data only}
- Bremme K, Eneroth P, Kindahl H. 15‐keto‐13,14‐dihydroprostaglandin F2alpha and prolactin in maternal and cord blood during prostaglandin E2 or oxytocin therapy for labor induction. Journal of Perinatal Medicine 1987;15:143‐51. [DOI] [PubMed] [Google Scholar]
Browne 1988 {published data only}
- Browne MJ, Lang GD, Dougall A. Oral prostaglandin E2 in the management of patients with spontaneously ruptured membranes and no uterine activity. Proceedings of 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988:105.
Davies 1991 {published data only}
- Davies NJ, Martindale E, Haddad NG. Cervical ripening with oral PGE2 tablets and the effect of the latent period in patients with premature rupture of the membranes at term. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands. 1991:156.
- Davies NJ, Martindale E, Haddad NG. Cervical ripening with oral prostaglandin E2 tablets and the effect of the latent period in patients with premature rupture of the membranes at term. Journal of Obstetrics and Gynaecology 1991;11:405‐8. [Google Scholar]
Friedman 1974 {published data only}
- Friedman EA, Sachtleben MR. Oral prostaglandin E2 for induction of labor at term. Obstetrics & Gynecology 1974;43:178‐85. [PubMed] [Google Scholar]
Friedman 1975a {published data only}
- Friedman EA, Sachtleben MR, Green W. Oral prostaglandin E2 for induction of labor at term. II. Comparison of two low‐dosage regimens. American Journal of Obstetrics and Gynecology 1975;123:671‐4. [DOI] [PubMed] [Google Scholar]
Friedman 1975b {published data only}
- Friedman EA, Sachtleben MR. Preinduction priming with oral prostaglandin E2. American Journal of Obstetrics and Gynecology 1975;44(4):521‐3. [DOI] [PubMed] [Google Scholar]
Haeri 1976 {published data only}
- Haeri AD, Scher J, Davey DA, Leader M. Comparison of oral prostaglandin E2 and intravenous oxytocin for induction of labor. South African Medical Journal 1976;50:516‐8. [PubMed] [Google Scholar]
Ismail 1989 {published data only}
- Ismail AAA, Khowesah MMED, Shaala SA, Anwar MY, Darwish EA, Haiba NA. Induction of labor by oral prostaglandin E2 in protracted pregnancy. International Journal of Gynecology & Obstetrics 1989;29:325‐8. [DOI] [PubMed] [Google Scholar]
Johnstone 1987 {unpublished data only}
- Johnstone F. Oral prostaglandin E2 vs oxytocin in the management of prelabour rupture of the membranes at term. Personal communication 1987.
Lange 1982 {published data only}
- Lange AP, Secher NJ, Westergaard JG, Skovgard I. Neonatal jaundice after labour induced or stimulated by prostaglandin E2 or oxytocin. Lancet 1982;1:991‐4. [DOI] [PubMed] [Google Scholar]
Lorrain 1982 {published data only}
- Lorrain J, Beaumont L, Desaulniers G, Chemaly R. Comparative study of synthetic and natural prostaglandins in the induction of labour. Medical Union of Canada 1982;111:563‐8. [PubMed] [Google Scholar]
Lykkesfeldt 1979 {published data only}
- Lykkesfeldt G, Osler M. A comparison of three methods for inducing labor: oral prostaglandin E2, buccal desaminoxytocin, intravenous oxytocin. Acta Obstetricia et Gynaecologica Scandinavica 1979;58:321‐5. [DOI] [PubMed] [Google Scholar]
Makary 1990 {published data only (unpublished sought but not used)}
- Makary NA. Trial to investigate the effects of oral vs vaginal prostaglandin E2 on induction of labour in women with premature rupture of membranes at 37‐41 completed week's gestation with unfavourable cervix. Personal communication 1990.
Miller 1975 {published data only}
- Miller JF, Welply GA, Elstein M. Prostaglandin E2 tablets compared with intravenous oxytocin in induction of labour. BMJ 1975;1:14‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mokgokong 1976 {published data only}
- Mokgokong E. Induction of labour with oral prostaglandin E2. South African Medical Journal 1976;50:699‐701. [PubMed] [Google Scholar]
Nassief 1996 {published data only}
- Nassief SA, McFaul P, Rane A. Clinical trial comparing artificial rupture of membranes plus oral PGE2 tablets versus artificial rupture of membranes plus intravenous oxytocin for induction of labour in primigravid patients at term. Ulster Medical Journal 1996;65:145‐8. [PMC free article] [PubMed] [Google Scholar]
Obel 1975 {published data only}
- Obel EB, Larsen JF. A study of comparative efficacy of oral prostaglandin E2 as liquid formulation and tablets for induction of labour. Acta Obstetricia et Gynecologica Scandinavica 1975;37:35‐8. [DOI] [PubMed] [Google Scholar]
Pearce 1977 {published data only}
- Pearce DJ. Pre‐induction priming of the uterine cervix with oral prostaglandin E2 and a placebo. Prostaglandins 1977;14:571‐6. [DOI] [PubMed] [Google Scholar]
Pulle 1986 {published data only}
- Pulle C, Granese D, Panama S, Celona A. Cervical ripening and induction of labour by single intracervical PGE2‐gel application. Acta Therapeutica 1986;12:5‐12. [Google Scholar]
Samal 2000 {published data only}
- Samal S, Gupta U, Kumar SP, Agarwal P. A comparative study of oral prostaglandin (PGE2) & intravenous oxytocin in induction of labour. Journal of Obstetrics and Gynecology of India 2000;50(3):49‐51. [Google Scholar]
Sivasuriya 1978 {published data only}
- Sivasuriya M, Tan KL, Salmon YM, Karim SMM. Neonatal serum bilirubin levels in spontaneous and induced labour. British Journal of Obstetrics and Gynaecology 1978;85:619‐23. [DOI] [PubMed] [Google Scholar]
Somell 1983 {published data only}
- Somell C, Larsson B. Priming and induction of labor with oral PGE2 in patients with low Bishop score. Acta Obstetricia et Gynecologica Scandinavica 1983;62:315‐20. [DOI] [PubMed] [Google Scholar]
Soni 2000 {published data only}
- Soni M, Sachdeva L. Induction of labour with oral PGE2 and its comparison with intravenous oxytocin. Journal of Obstetrics and Gynecology of India 2000;50(6):51‐3. [Google Scholar]
Suzuki 2000 {published data only}
- Suzuki S, Otsubo Y, Sawa R, Yoneyama Y. Clinical trial of induction of labor versus expectant management in twin pregnancy. Gynecologic and Obstetric Investigation 2000;49:24‐7. [DOI] [PubMed] [Google Scholar]
Thiery 1977 {published data only}
- Thiery M, Benijts G, Martens G, Yo Le Sian A, Amy JJ, Derom R. A comparison of buccal (oromucosal) and oral prostaglandin E2 for the elective induction of labor. Prostaglandins 1977;14:371‐9. [DOI] [PubMed] [Google Scholar]
Weiss 1975 {published data only}
- Weiss RR, Tejani N, Israeli I, Evans MI, Bhakthavathsalan A, Mann LI. Priming of the uterine cervix with oral prostaglandin E2 in the term multigravida. Obstetrics & Gynecology 1975;46:181‐4. [PubMed] [Google Scholar]
Yacoob 1993 {published data only}
- Yacoob T, Lloyd M, Unwin A, Harrison RF. Intracervical prostaglandin E2, 0.5mg; gel or tablet for cervical ripening and induction of labour with an unfavourable cervix?. Journal of Obstetrics and Gynaecology 1993;13:167‐70. [Google Scholar]
Yeung 1977 {published data only}
- Yeung KK, Pang JCK. Oral prostaglandins E2 and F2alpha in the induction of labour. Australian and New Zealand Journal of Obstetrics and Gynaecology 1977;17:32‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bremme 1980a {published data only}
- Bremme K, Bygdeman M. A comparative study of uterine activity and fetal heart rate pattern in labor induced with oral prostaglandin E2 or oxytocin. Acta Anaesthesiologica Scandinavica. Supplementum 1980;92:23‐9. [DOI] [PubMed] [Google Scholar]
Bremme 1980b {published data only}
- Bremme K, Bygdeman M. Induction of labor by oxytocin or prostaglandin E2. Acta Obstetricia et Gynecologica Scandinavica Supplementum 1980;92:11‐21. [DOI] [PubMed] [Google Scholar]
Bremme 1982 {published data only}
- Bremme K, Nilsson B. Prediction of time to delivery in labour induced with oral prostaglandin E2 (PGE2) or intravenous oxytocin (OXY), both in combination with early amniotomy. Proceedings of 8th European Congress of Perinatal Medicine; 1982 Sept 7‐10; Brussels, Belgium. 1982:Abstract no: 86.
Bremme 1984a {published data only}
- Bremme K, Eneroth P, Samuelson K. Estriol and cholic acid in maternal serum in induced labor. Gynecologic and Obstetric Investigation 1984;17:120‐6. [DOI] [PubMed] [Google Scholar]
Marzouk 1975 {published data only}
- Marzouk AF. Oral and intravenous prostaglandin E2 in induction of labour. British Journal of Clinical Practice 1975;29:68‐70. [PubMed] [Google Scholar]
Murray 1975 {published data only}
- Murray CP, Clinch J. Comparative study of labour induced by oral prostaglandin E2 and intravenous syntocinon. Irish Medical Journal 1975;68(6):135‐9. [PubMed] [Google Scholar]
Additional references
Alfirevic 2006
- Alfirevic Z, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001338.pub2] [DOI] [PubMed] [Google Scholar]
Alfirevic 2009
- Alfirevic Z, Kelly AJ, Dowswell T. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003246.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulvain 2005
- Boulvain M, Stan CM, Irion O. Membrane sweeping for induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD000451.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulvain 2008
- Boulvain M, Kelly AJ, Irion O. Intracervical prostaglandins for induction of labour. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006971] [DOI] [PubMed] [Google Scholar]
Bricker 2000
- Bricker L, Luckas M. Amniotomy alone for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002862] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chalmers 1989
- Chalmers I, Enkin MW, Keirse MJNC. Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989. [Google Scholar]
Clarke 1999
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.0 [updated July 1999]. In: Review Manager (RevMan) [Computer program]. Version 4.0 Oxford, England: The Cochrane Collaboration, 1999.
Curtis 1987
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91‐5. [PubMed] [Google Scholar]
Hapangama 2009
- Hapangama D, Neilson JP. Mifepristone for induction of labour. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002865.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmeyr 2000
- Hofmeyr GJ, Alfirevic Z, Kelly T, Kavanagh J, Thomas J, Brocklehurst P, Neilson JP. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002074] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2010
- Hofmeyr GJ, Gülmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD000941.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Howarth 2001
- Howarth G, Botha DJ. Amniotomy plus intravenous oxytocin for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2001
- Hutton EK, Mozurkewich EL. Extra‐amniotic prostaglandin for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jozwiak 2012
- Jozwiak M, Bloemenkamp Kitty WM, Kelly AJ, Mol BWJ, Irion O, Boulvain M. Mechanical methods for induction of labour. Cochrane Database of Systematic Reviews 2012, Issue 3. [DOI: 10.1002/14651858.CD001233.pub2] [DOI] [PubMed] [Google Scholar]
Kavanagh 2001
- Kavanagh J, Kelly AJ, Thomas J. Sexual intercourse for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003093] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2005
- Kavanagh J, Kelly AJ, Thomas J. Breast stimulation for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003392.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2006a
- Kavanagh J, Kelly AJ, Thomas J. Corticosteroids for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003100.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2006b
- Kavanagh J, Kelly AJ, Thomas J. Hyaluronidase for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003097.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2001a
- Kelly AJ, Kavanagh J, Thomas J. Castor oil, bath and/or enema for cervical priming and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003099] [DOI] [PubMed] [Google Scholar]
Kelly 2001b
- Kelly AJ, Kavanagh J, Thomas J. Relaxin for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003103] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2009
- Kelly AJ, Malik S, Smith L, Kavanagh J, Thomas J. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD003101.pub2] [DOI] [PubMed] [Google Scholar]
Kelly 2011
- Kelly AJ, Munson C, Minden L. Nitric oxide donors for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD006901.pub2] [DOI] [PubMed] [Google Scholar]
Luckas 2000
- Luckas M, Bricker L. Intravenous prostaglandin for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002864] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muzonzini 2004
- Muzonzini G, Hofmeyr GJ. Buccal or sublingual misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2004, issue 4. [DOI: 10.1002/14651858.CD004221.pub2] [DOI] [PMC free article] [PubMed]
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
Smith 2003
Smith 2004
- Smith CA, Crowther CA. Acupuncture for induction of labour. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002962.pub2] [DOI] [PubMed] [Google Scholar]
Thomas 2001
- Thomas J, Kelly AJ, Kavanagh J. Oestrogens alone or with amniotomy for cervical ripening or induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003393] [DOI] [PMC free article] [PubMed] [Google Scholar]


