Abstract
Background
Rebleeding is an important cause of death and disability in people with aneurysmal subarachnoid haemorrhage. Rebleeding is probably related to dissolution of the blood clot at the site of aneurysm rupture by natural fibrinolytic activity. This review is an update of a previously published Cochrane review.
Objectives
To assess the effects of antifibrinolytic treatment in people with aneurysmal subarachnoid haemorrhage.
Search methods
We searched the Cochrane Stroke Group Trials Register (February 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 1), MEDLINE (1948 to December 2012), and EMBASE (1947 to December 2012). In an effort to identify further published, unpublished, and ongoing studies we searched reference lists and trial registers, performed forward tracking of relevant references and contacted drug companies.
Selection criteria
Randomised trials comparing oral or intravenous antifibrinolytic drugs (tranexamic acid, epsilon amino‐caproic acid, or an equivalent) with control in people with subarachnoid haemorrhage of suspected or proven aneurysmal cause.
Data collection and analysis
Two review authors independently selected trials for inclusion and extracted the data. Three review authors assessed trial quality. For the primary outcome we converted the outcome scales between good and poor outcome for the analysis. We scored death from any cause and rates of rebleeding, cerebral ischaemia, and hydrocephalus per treatment group. We expressed effects as risk ratios (RR) with 95% confidence intervals (CI). We used random‐effects models for all analyses.
Main results
We included 10 trials involving 1904 participants. The risk of bias was low in six studies. Four studies were open label and were rated as high risk of performance bias. One of these studies was also rated as high risk for attrition bias. Four trials reported on poor outcome (death, vegetative state, or severe disability) with a pooled risk ratio (RR) of 1.02 (95% confidence interval (CI) 0.91 to 1.15). All trials reported on death from all causes with a pooled RR of 1.00 (95% CI 0.85 to 1.18). In a trial that combined short‐term antifibrinolytic treatment (< 72 hours) with preventative measures for cerebral ischaemia the RR for poor outcome was 0.85 (95% CI 0.64 to 1.14). Antifibrinolytic treatment reduced the risk of re‐bleeding reported at the end of follow‐up (RR 0.65, 95% CI 0.44 to 0.97; 78 per 1000 participants), but there was heterogeneity (I² = 62%) between the trials. The pooled RR for reported cerebral ischaemia was 1.41 (95% CI 1.04 to 1.91, 83 per 1000 participants), again with heterogeneity between the trials (I² = 52%). Antifibrinolytic treatment showed no effect on the reported rate of hydrocephalus in five trials (RR 1.11, 95% CI 0.90 to 1.36).
Authors' conclusions
The current evidence does not support the use of antifibrinolytic drugs in the treatment of people with aneurysmal subarachnoid haemorrhage, even in those who have concomitant treatment strategies to prevent cerebral ischaemia. Results on short‐term treatment are promising, but not conclusive. Further randomised trials evaluating short‐term antifibrinolytic treatment are needed to evaluate its effectiveness.
Plain language summary
Drugs for preventing blood clot dissolution (antifibrinolytic therapy) to reduce the occurrence of rebleeding in aneurysmal subarachnoid haemorrhage
A subarachnoid haemorrhage (SAH) is a bleed into the small space between the brain and skull that contains blood vessels that supply the brain (the subarachnoid space). The cause of a bleeding here is usually a rupture of a weak spot in one of these vessels. A SAH is a relatively uncommon type of stroke, but it often occurs at a young age (half the patients are younger than 50 years). The outcome of SAH is often poor: one‐third of people die after the haemorrhage and of those who survive, one‐fifth will require help for everyday activities. An important cause of poor recovery after SAH is a second bleed from the weakened vessel (rebleeding). This is thought to be caused by the dissolving of the blood clot at the original bleeding site that results from natural blood clot dissolving (fibrinolytic) activity. Antifibrinolytic therapy that reduces this activity was introduced as a treatment for reducing rebleeding and therefore for improving recovery after SAH. This review included 10 trials, totaling 1904 participants that investigated the effect of these drugs in people with SAH. Antifibrinolytic treatment does indeed reduce the risk of rebleeding, but does not improve survival or the chance of being independent in everyday activities. This may be due to an increase in one of the other common complications of SAH. We conclude that antifibrinolytic treatment should not routinely be given to people with SAH, but new randomised trials are needed to establish if short‐term treatment might be beneficial.
Background
Description of the condition
In people with aneurysmal subarachnoid haemorrhage (SAH) rebleeding is an important cause of death and disability. Without aneurysm treatment approximately 30% of such people experienced a rebleed within one month of the initial haemorrhage (Locksley 1966). The rebleeding rate has now been reduced to about 15% because in most people the aneurysm is occluded early after admission (Roos 2000b). The risk of rebleeding is highest in the first 24 hours after SAH, with a peak in the first six hours (Guo 2011). The prognosis after rebleeding is poor: approximately 60% of people who rebleed die and another 30% remain dependent for activities of daily living (Naidech 2005).
Description of the intervention
Antifibrinolytic drugs were introduced to reduce the occurrence of rebleeding. These drugs are administered orally or intravenously and block endogenous fibrinolytic activity and could help prevent bleeding.
How the intervention might work
Dissolution of the blood clot at the site of the ruptured aneurysm is thought to be an important factor in the development of rebleeding. This dissolution probably results from endogenous fibrinolytic activity after SAH. Since antifibrinolytic agents reduce fibrinolytic activity, antifibrinolytic therapy may reduce the occurrence of rebleeding and thereby improve clinical outcome after SAH. However, reducing rebleeding might not automatically improve clinical outcome in all instances. Concerns have been raised that antifibrinolytic therapy might increase the occurrence of cerebral ischaemia. Cerebral ischaemia is a complication of SAH that occurs in about 30% to 40% of people between four and 14 days after SAH. In older trials the beneficial effect of antifibrinolytic treatment (reducing rebleeding) was offset by an increase in cerebral ischaemia, resulting in a neutral effect on outcome (Roos 2003).
Why it is important to do this review
In 1967 Gibbs and O'Gorman published the first report on antifibrinolytic treatment in people with SAH (Gibbs 1967). Since then, over 50 studies on antifibrinolytic therapy in aneurysmal SAH have been published. Unfortunately most of these studies are uncontrolled and only a minority of the controlled studies are randomised. Moreover, the results of some of the individual randomised studies contradict each other. Furthermore, as mentioned above, concerns have been raised that antifibrinolytic therapy might increase the occurrence of cerebral ischaemia. In a previous version of this review we found that even in people treated with measures to prevent and reverse cerebral ischaemia (such as administration of nimodipine), clinical outcome was not improved by antifibrinolytic treatment despite a reduction in rebleeding rate. The hypothesis has been posed that, when people with aneurysmal SAH are treated with antifibrinolytic agents, recovery from cerebral ischaemia is impeded by antifibrinolytic treatment. More recently it has been proposed that if antifibrinolytic treatment is discontinued before the period in which cerebral ischaemia typically occurs, the negative effect of antifibrinolytic therapy may be avoided. In this update of a previously published Cochrane review (Roos 2003), we sought to evaluate the effectiveness of short‐term antifibrinolytic treatment in people concomitantly treated with measures to prevent or reverse cerebral ischaemia.
Objectives
To assess the effects of antifibrinolytic treatment in people with aneurysmal SAH.
Methods
Criteria for considering studies for this review
Types of studies
All truly randomised unconfounded trials were eligible in which, after concealed allocation, antifibrinolytic drugs were compared with control treatment (open studies) or placebo (blind studies) in an intention‐to‐treat analysis. We excluded all trials in which allocation to treatment or control group was not concealed (e.g. trials in which people were allocated by means of an open random number list, alternation, or based on date of birth, days of the week, or hospital‐number), since foreknowledge of treatment allocation might lead to biased treatment allocation (i.e. selective enrolment). We also excluded trials in which an intention‐to‐treat analysis was not performed and could not be reconstructed on the basis of published data without a loss of 20% or more of all randomised participants.
Types of participants
Trials in which participants were included with clinical symptoms and signs of SAH from a ruptured aneurysm with confirmation of the diagnosis by the presence of subarachnoid blood on computed tomography (CT) scan or on cerebrospinal fluid (CSF) examination were eligible for this review.
Types of interventions
Antifibrinolytic drugs (e.g. tranexamic acid, epsilon amino‐caproic acid, or equivalent drugs), oral or intravenous, versus control treatment (open studies) or placebo treatment (blind studies). Since the risk of rebleeding is highest during the first two weeks after the initial bleeding, treatment had to start within two weeks after onset of the SAH. Distinction was made between early and short‐term (< 72 hours of onset of symptoms, i.e. before usual timing of onset of cerebral ischaemia) versus long‐term (> 72 hours) treatment duration.
Types of outcome measures
Primary outcomes
The primary outcome measure was 'poor outcome' (death, vegetative state, or severe disability, as assessed either with the Glasgow Outcome Scale or the Modified Rankin Scale) at the end of follow‐up at least three months after SAH. 'Death from all causes' at a minimum of at least three‐weeks follow‐up was our second primary outcome, since most studies only reported on case fatality.
Secondary outcomes
Secondary outcome measures were the effect of antifibrinolytic treatment on both the rates of reported and CT scan or autopsy confirmed (sensitivity analyses) episodes of rebleeding, cerebral ischaemia, and hydrocephalus. In this systematic review, we did not evaluate cerebral vasospasm without clinical signs of cerebral ischaemia.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We searched for trials in all languages and arranged translations of relevant reports published in languages other than English.
Electronic searches
We searched the Cochrane Stroke Group Trials Register (last searched February 2013), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 1), MEDLINE (1948 to December 2012) (Appendix 1) and EMBASE (1947 to December 2012) (Appendix 2).
We developed comprehensive search strategies with the help of the Cochrane Stroke Group Trials Search Co‐ordinator and adapted the MEDLINE strategy for CENTRAL.
We also searched the following major ongoing trial registers (December 2012): ClinicalTrials.gov (http://www.clinicaltrials.gov/), EU Clinical Trials Register (https://www.clinicaltrialsregister.eu), Stroke Trials Registry (www.strokecenter.org/trials/), Current Controlled Trials (www.controlled‐trials.com), and the WHO International Clinical Trials Registry Platform (http://apps.who.int/trialsearch/).
Searching other resources
In an effort to identify further published, unpublished, and ongoing studies:
we searched the list of references quoted in all included studies and reviews on antifibrinolytic therapy (Adams 1982; Adams 1987; Biller 1988; Carley 2005; Chawjol 2008; Connolly 2012; Fodstad 1982; Gaberel 2012; Lindsay 1987; Maira 2006; Mayberg 1994; Ramirez 1981; Rinkel 2008; Van Gijn 2001; Van Gijn 2007; Vermeulen 1980; Vermeulen 1996; Weaver 1994; Weir 1987);
we used Science Citation Index Cited Reference Search for forward tracking of important articles; and
for the first version of this review (1998) we contacted the pharmaceutical company Pharmacia and Upjohn, formerly Kabi, manufacturer and license holder of the antifibrinolytic drug tranexamic acid. We identified no additional (unpublished) studies.
Data collection and analysis
Selection of studies
Two authors (MIB, MRG) independently screened the titles and abstracts of the records obtained from the electronic searches and excluded obviously irrelevant studies. We obtained the full‐text articles for the remaining studies and the same two authors independently selected the trials that met the predefined criteria for inclusion in the review. The authors resolved disagreements by discussion and when necessary in consultation with a third review author (YBWEMR).
Data extraction and management
Two authors (MIB, MRG) independently reviewed all eligible studies and extracted details on the number of participants in the treated and the placebo or control group, the randomisation method, blinding method, the definitions for diagnosis and complications, and also ascertained whether an intention‐to‐treat analyses was done or could be reconstructed from the published data. Whenever consensus could not be reached, the review authors consulted a third review author (YBWEMR).
Assessment of risk of bias in included studies
Three authors (MIB, MRG, YBWEMR) independently assessed the risk of bias of each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion. We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear and provided information from each study in the 'Risk of bias' tables.
Measures of treatment effect
We processed data in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For the primary outcome we converted the outcome scales between good and poor outcome for the analysis. We scored death from any cause and rates of rebleeding, cerebral ischaemia, and hydrocephalus per treatment group. We expressed effects as risk ratios (RR) with 95% confidence intervals (CI).
Unit of analysis issues
The unit of analysis were individual participants, since we only included individually conducted randomised trials with a parallel design.
Dealing with missing data
In trials without intention‐to‐treat analysis, we tried to reconstruct such an analysis based only on the published data. For two trials (Hillman 2002; Tsementzis 1990) we tried to contact the principal investigator to retrieve data on the number of participants in whom rebleeding or cerebral ischaemia were proven on CT scan or at autopsy. We received a reply from one of the authors. We made no effort to obtain additional information for studies published 15 or more years ago.
Assessment of heterogeneity
For each outcome we assessed heterogeneity using the Chi² test and I² index (Higgins 2011). Because we suspected substantial heterogeneity, we used random‐effects models for all analyses.
Assessment of reporting biases
To investigate possible publication bias in this review, we planned to perform funnel plots when possible.
Data synthesis
We used the Review Manager software, RevMan 5.2, for statistical analysis (RevMan 2012), and used Mantel‐Haenszel random‐effects models for pooled analyses.
Subgroup analysis and investigation of heterogeneity
To assess whether masked studies showed different results compared with unmasked studies we divided all analyses into two groups: trials with control treatment (open studies) and trials with placebo treatment (blind studies). We pre‐planned an additional subgroup analysis to evaluate the effectiveness of antifibrinolytic therapy in trials with and without additional measures to prevent or reverse cerebral ischaemia and to evaluate the effectiveness of antifibrinolytic therapy according to treatment duration. We divided the included trials into one of three groups: (1) trials without ischaemia prevention and treatment duration > 72 hours, (2) trials with ischaemia prevention and treatment duration > 72 hours, and (3) trials with ischaemia prevention and treatment duration < 72 hours. We did not include a fourth group (trials without ischaemia prevention and treatment duration < 72 hours) as there were no trials that met these criteria.
Additionally we checked whether data were available on aneurysm treatment (i.e. clipping or coiling) according to antifibrinolytic treatment, to evaluate whether a subgroup analysis on clipping versus coiling was possible.
Sensitivity analysis
Because our secondary outcome measures were more prone to bias, we carried out sensitivity analyses on confirmed (rather than reported) rebleeding, cerebral ischaemia, and hydrocephalus rates.
Results
Description of studies
Results of the search
The updated search of the bibliographic databases yielded a total of 1521 records, from which we removed 476 duplicates, leaving a total of 1045 records. After screening titles and abstracts we excluded 931 records, leaving 114 records for which we obtained the full‐text articles. Further assessment resulted in 32 potentially relevant trials. Ten trials met the inclusion criteria for the review and we excluded 21 trials. We identified one ongoing trial from the searches of the trial registers. For an overview of the search results see Figure 1.
1.
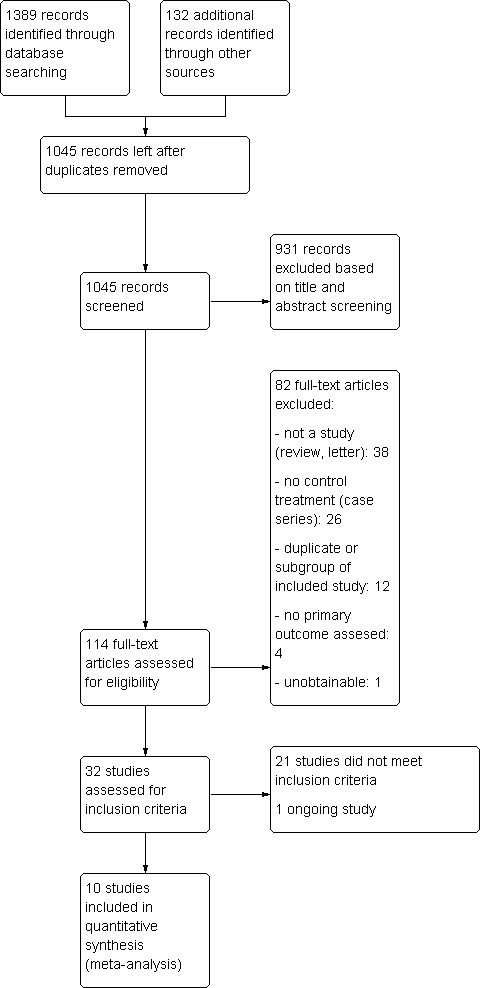
Study flow diagram.
Included studies
We included 10 studies (Chandra 1978; Fodstad 1981; Girvin 1973; Hillman 2002; Kaste 1979; Maurice 1978; Roos 2000a; Tsementzis 1990; Van Rossum 1977; Vermeulen 1984), which are described in detail in the Characteristics of included studies table. These 10 studies included 1904 participants of whom 959 were randomised to receive antifibrinolytic drugs, 597 received placebo treatment and 348 received control treatment. The Girvin 1973 study used epsilon‐amino‐caproic acid (39 participants), in all other studies tranexamic acid was used. In two studies participants were concomitantly treated with measures to prevent or reverse cerebral ischaemia (Hillman 2002; Roos 2000a). The duration of antifibrinolytic treatment differed considerably between studies and ranged from less than 72 hours up to six weeks. Short‐term treatment was used in only one study (Hillman 2002). In this study, participants were treated up to 72 hours after SAH. All other studies treated participants for at least 10 days (or until occlusion of the aneurysm) after SAH.
Clinical outcome was reported in four studies (Hillman 2002; Roos 2000a; Tsementzis 1990; Vermeulen 1984) and the number of participants with poor outcomes could be reconstructed. Death from all causes and rebleeding rates were reported in all 10 studies. In six studies, episodes of rebleeding were defined primarily by clinical symptoms.
Two trials (Fodstad 1981; Hillman 2002) reported CT scan or autopsy confirmed episodes of rebleeding. The Dutch‐British trial (Vermeulen 1984) and the STAR study (Roos 2000a) defined and reported rebleeding in two ways: as 'probable' if the diagnosis was suspected solely on clinical grounds and as 'definite' when proven by CT (after comparison with an earlier CT) or at autopsy.
Cerebral ischaemia was reported in six studies but defined in only three: in two studies (Roos 2000a; Vermeulen 1984) cerebral ischaemia was defined and reported in the same manner as episodes of rebleeding; as 'probable' and as 'definite' (CT‐scan or autopsy confirmed) cerebral ischaemia. Again, as for rebleeding, Fodstad 1981 reported on CT‐scan or autopsy confirmed cerebral ischaemia. Hillman 2002 reported percentage of transient and permanent delayed ischaemic neurological deficit. No clear definition was given and CT scans were not routinely used for confirmation of cerebral ischaemia. Because other studies did not make a distinction between permanent or transient cerebral ischaemia, we decided to combine these outcomes. In Hillman 2002, visible infarction on CT scan was reported; however, after contact with the author, we decided not to use this as a measure for cerebral ischaemia since this was not correlated to clinical signs of cerebral ischeamia.The other two studies (Girvin 1973; Tsementzis 1990) made no distinction between participants with episodes clinically suggestive of cerebral ischaemia or participants with confirmed cerebral ischaemia, and reported only an all‐inclusive number of participants. One study reported on 'delayed cerebral ischaemia' and 'post operative ischaemia' (Roos 2000a). Since all other studies did not make this distinction and reported an overall number of participants with cerebral ischaemia, we grouped these subgroups of ischaemia together for the analysis on cerebral ischaemia.
Five trials reported on hydrocephalus. Hydrocephalus was defined in only one study by means other than clinical grounds and could therefore be included in the sensitivity analysis concerning 'confirmed hydrocephalus' (Roos 2000a). No trials reported on aneurysm treatment modality (i.e. coiling versus clipping) according to antifibrinolytic or control treatment and thus a subgroup analysis based on modality was not possible.
Excluded studies
We excluded 21 studies for various reasons (see Characteristics of excluded studies): some were comparative trials between two or more antifibrinolytic agents, others used unconcealed randomisation methods, and some were not randomised but used historical controls. We excluded Fodstad 1978 as 23% of the participants in the trial were later excluded and an intention‐to‐treat analysis could not be reconstructed on the basis of the published data.
Risk of bias in included studies
For a summary of the risk of bias please see Figure 2 and Figure 3. Five of the 10 included trials used an intention‐to‐treat analysis; in one of the remaining trials (Maurice 1978) this analysis could be completely reconstructed using the available follow‐up data. In the other trials (Fodstad 1981; Hillman 2002; Tsementzis 1990; Van Rossum 1977) there was no follow‐up information available for participants who were excluded after randomisation. In three studies only very few participants (Fodstad 1981: n = 1; Tsementzis 1990: n = 4; Van Rossum 1977: n = 3) were excluded from the final analysis and therefore these studies could still be included in our review. In one study (Hillman 2002) 91 (15%) of the 596 participants were excluded after randomisation. Because this was less than the prespecified proportion of 20% we included this study in our analysis. We rated this study as high risk of bias for incomplete outcome data.
2.
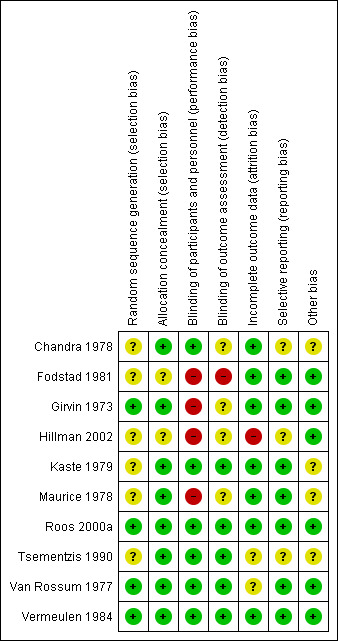
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Six studies used a double‐blind method (placebo controlled) and four used a control group with standard treatment without placebo.The control group studies were open label and we rated them as high risk of performance bias (all four studies) and attrition bias (one study).
Girvin 1973 used 'flip‐of‐a‐coin' randomisation as the method of allocation, which is more susceptible to bias compared with, for instance, the sealed envelope method. Nevertheless, we decided to include this study since this is an accepted form of randomisation with allocation concealment according to the Cochrane guidelines. All other studies used a method of concealed allocation that was considered appropriate at time of conducting the trial, although it was not always mentioned whether or not the sealed envelopes that were used were opaque.
Effects of interventions
The pooled RR for our primary outcome measure, 'poor outcome', was 1.02 (95% CI 0.91 to 1.15) (Analysis 1.1). The pooled RR for our second primary outcome measure, 'death from all causes', was 1.00 (95% CI 0.85 to 1.18) (Analysis 1.2).
1.1. Analysis.
Comparison 1 Antifibrinolytic treatment versus control treatment with or without placebo, Outcome 1 Poor outcome (death, vegetative or severe disability on Glasgow Outcome Scale at end of follow‐up): open versus blind studies.
1.2. Analysis.
Comparison 1 Antifibrinolytic treatment versus control treatment with or without placebo, Outcome 2 Death from all causes at end of follow up: open versus blind studies.
In the analysis on rebleeding rates (Analysis 1.3), antifibrinolytic therapy significantly reduced the risk of rebleeding (RR 0.65, 95% CI 0.44 to 0.97; 78 per 1000 people). Considerable heterogeneity was detected for this comparison (I² = 62%). Similar results were found in the subgroup analysis of the six double‐blind and placebo controlled studies (RR 0.64, 95% CI 0.43 to 0.97), the sensitivity analysis including four studies with CT scan or autopsy confirmed rebleeding (RR 0.44, 95% CI 0.26 to 0.75) (Analysis 1.4), as well as in the subgroup analysis of studies with and without ischaemia prevention according to duration of treatment (< 72 hours > 72 hours) (RR 0.65, 95% CI 0.44 to 0.97) (Analysis 1.5).
1.3. Analysis.
Comparison 1 Antifibrinolytic treatment versus control treatment with or without placebo, Outcome 3 Rebleeding reported at end of follow up: open versus blind studies.
1.4. Analysis.
Comparison 1 Antifibrinolytic treatment versus control treatment with or without placebo, Outcome 4 Confirmed rebleeding at end of follow‐up (sensitivity analysis): open versus blind studies.
1.5. Analysis.
Comparison 1 Antifibrinolytic treatment versus control treatment with or without placebo, Outcome 5 Rebleeding reported at end of follow‐up: trials with and without ischaemia prevention according to treatment duration.
In the six trials that reported cerebral ischaemia rates, antifibrinolytic treatment significantly increased the risk of cerebral ischaemia (RR 1.41, 95% CI 1.04 to 1.91; 83 per 1000 people) (Analysis 1.6). Similar (though non‐significant) results were seen in the subgroup analysis of the three placebo controlled trials (RR 1.38, 95% CI 0.87 to 2.19) as well as in the three trials with CT scan or autopsy confirmed cerebral ischaemia (RR 1.34, 95% CI 0.88 to 2.03) (Analysis 1.7). In these analyses there was considerable heterogeneity between the results from the four older studies and the results from the most recent studies (Hillman 2002; Roos 2000a), in which specific treatments to prevent cerebral ischaemia were used (I² = 52%).
1.6. Analysis.
Comparison 1 Antifibrinolytic treatment versus control treatment with or without placebo, Outcome 6 Cerebral ischaemia reported at end of follow‐up: open versus blind studies.
1.7. Analysis.
Comparison 1 Antifibrinolytic treatment versus control treatment with or without placebo, Outcome 7 Confirmed cerebral ischaemia at end of follow‐up (sensitivity analysis): open versus blind studies.
In five trials hydrocephalus was reported. Antifibrinolytic treatment overall had no effect on the reported rates of hydrocephalus (RR 1.11, 95% CI 0.90 to 1.36) (Analysis 1.8). In the subgroup analysis on placebo versus open studies the RR of antifibrinolytic treatment for hydrocephalus was 1.19 (95% CI 0.95 to 1.48) in the placebo controlled studies, while it was 0.64 (95% CI 0.34 to 1.18) in the open label trials. The sensitivity analysis on one trial with CT scan or autopsy confirmed hydrocephalus showed no effect of therapy on hydrocephalus rate (RR 1.17, 95% CI 0.87 to 1.55) (Analysis 1.9). Ischaemia prevention did not appear to have an effect on hydrocephalus in the subgroup analysis of studies with and without ischaemia prevention according to duration of treatment. The RR of hydrocephalus in people receiving antifibrinolytic treatment during > 72 hours with cerebral ischaemia prevention was 1.17 (95% CI 0.87 to 1.55) and for people receiving antifibrinolytic treatment during > 72 hours without ischaemia prevention the RR was 1.03 (95% CI 0.74 to 1.43) (Analysis 1.10).
1.8. Analysis.
Comparison 1 Antifibrinolytic treatment versus control treatment with or without placebo, Outcome 8 Hydrocephalus reported at end of follow‐up: open versus blind studies.
1.9. Analysis.
Comparison 1 Antifibrinolytic treatment versus control treatment with or without placebo, Outcome 9 Confirmed hydrocephalus at end of follow up (sensitivity analysis): open versus blind studies.
1.10. Analysis.
Comparison 1 Antifibrinolytic treatment versus control treatment with or without placebo, Outcome 10 Hydrocephalus reported at end of follow‐up: trials with and without ischaemia prevention according to treatment duration.
In the subgroup analysis of studies with ischaemia prevention according to duration of treatment (< 72 hours versus > 72 hours), the RR for people receiving antifibrinolytic treatment during < 72 hours with cerebral ischaemia prevention was 0.85 (95% CI 0.64 to 1.14) for 'poor outcome' (Analysis 1.11) and 0.83 (95% CI 0.52 to 1.35) for 'death from all causes' (Analysis 1.12). Furthermore, cerebral ischaemia did not significantly increase with antifibrinolytic treatment in trials where participants received concomitant ischaemia preventative treatment (Hillman 2002 and Roos 2000a combined: RR 1.09, 95% CI 0.78 to 1.51) (Analysis 1.13).
1.11. Analysis.
Comparison 1 Antifibrinolytic treatment versus control treatment with or without placebo, Outcome 11 Poor outcome (death, vegetative or severe disability on Glasgow Outcome Scale at end of follow‐up): trials with and without ischaemia prevention according to treatment duration.
1.12. Analysis.
Comparison 1 Antifibrinolytic treatment versus control treatment with or without placebo, Outcome 12 Death from all causes at end of follow‐up: trials with and without ischaemia prevention according to treatment duration.
1.13. Analysis.
Comparison 1 Antifibrinolytic treatment versus control treatment with or without placebo, Outcome 13 Cerebral ischaemia reported at end of follow‐up: trials with and without ischaemia prevention according to treatment duration.
Discussion
Summary of main results
Although this systematic review shows that antifibrinolytic treatment reduces the rate of rebleeding by approximately 35%, there is no evidence of benefit from antifibrinolytic treatment on case fatality or poor overall outcome. This analysis further shows that the lack of effect on clinical outcome, despite a reduction in rebleeding, may be caused by an increase in cerebral ischaemia with antifibrinolytic treatment, which was also shown in the sensitivity analysis in trials with data on the number of participants with CT or post‐mortem proven cerebral ischaemia (two double‐blind, one with open control group). Any possible beneficial effect of the reduction in the rebleeding‐rate on clinical outcome was offset by an increase in the rate of cerebral ischaemia. This was also seen in the most recent study (Hillman 2002) in which short‐term antifibrinolytic therapy was given with ischaemia preventative measures, although permanent delayed neurological deficit was decreased with antifibrinolytic treatment. This finding could suggest that short‐term antifibrinolytic treatment (compared to long‐term treatment in the study by Roos 2000a) does not influence recovery from initial ischaemia or development of secondary ischaemia and may, therefore, lead to improved clinical outcome. However, confirmatory evidence is needed.
Overall completeness and applicability of evidence
Trials in which people were included with symptoms and signs of SAH of suspected or proven aneurysmal cause, with confirmation of the diagnosis by the presence of subarachnoid blood on CT or on CSF examination were eligible for inclusion in the review. We chose this pragmatic approach and did not restrict our review to people with angiographically proven aneurysms, because in contrast to angiography, CT scan or CSF examination can be done instantaneously, independent of the clinical condition of the patient. One could argue that currently people with suspected aneurysmal SAH will undergo CT angiography (CT‐A) immediately to confirm the presence of an aneurysm. We chose not to limit our analysis to proven aneurysmal SAH, because CT‐A was not available at the time most of the trials were conducted. Moreover, CT‐A is not always available in referring hospitals without specialised units. Since antifibrinolytic treatment might be most beneficial when started as soon as possible, it is likely that treatment will start in the referring hospital without CT‐A confirmation of the presence of an aneurysm. An analysis on oral versus intravenous treatments or a comparison between treatment dosages could not be performed. Only one study used oral antifibrinolytic treatment, whereas other studies used intravenous therapy alone or a combination of intravenous and oral treatment in varying dosages. Furthermore, in the past decade treatment of cerebral aneurysms has changed considerably: whereas most studies were performed in the era where surgical treatment was the only method of occlusion of the aneurysm, currently endovascular coiling is an accepted alternative to surgical occlusion. Because no data on treatment modality were available from the included trials, an analysis according to aneurysm treatment modality was not possible. Such an analysis could, however, prove worthwhile in the future.
Quality of the evidence
In this systematic review we only included true randomised controlled trials and performed a subgroup analysis on masked versus unmasked studies in an attempt to minimise allocation and performance bias. However, many of the included studies were older (1970s and 1980s) and most did not report on many sources of potential bias, such as how the randomisation sequence was generated and if participant outcome was assessed by individuals who were blinded to the intervention given. Therefore, we scored many potential sources of bias as 'unclear risk' as can be seen in Figure 2 and Figure 3. However, most of these studies were small and in total comprised 24% of all included participants. Most included participants originated from three large studies (Hillman 2002; Roos 2000a; Vermeulen 1984). For two of these studies, comprising 50% of all data, we could find no potential sources of bias so we rated them as low risk of bias for each source (Roos 2000a; Vermeulen 1984). For Hillman 2002, however, many potential sources of bias could not be scored and were unclear, such as sequence generation, allocation concealment, and blinded outcome assessment. Moreover, 15% of included participants were later excluded from the analysis and the baseline characteristics and outcome of these participants were not reported. Since this was the only study included in the subgroup analysis for treatment duration of less than 72 hours with ischaemia prevention these results are far from conclusive. We know of only one other case series that evaluated short‐term antifibrinolytic treatment (Harrigan 2010). In this study all participants received antifibrinolytic treatment until occlusion of the aneurysm (average 56.5 hours after SAH ictus). Harrigan 2010 reported low rebleeding rates and permanent neurological deficit due to cerebral ischaemia (1.4% and 7.2% respectively). However, no follow‐up was done to evaluate the participants' clinical outcomes and no control group was available making it impossible to draw conclusions based on this study.
Potential biases in the review process
We searched all major medical electronic databases and other sources, using sensitive and validated search strategies. However, it is possible that we did not find all relevant publications. Furthermore, we only included studies that were published in medical literature and did not search the grey literature, such as government reports and unpublished information. Although all included trials met the predefined inclusion criteria, the studies differed considerably in participant selection, disease severity at baseline, start of treatment after diagnosis, dosage and type of trial medication, classification of events, and outcome and duration of follow‐up. This clinical heterogeneity may in part account for the statistical heterogeneity found in the analyses on rebleeding and cerebral ischaemia.
Agreements and disagreements with other studies or reviews
Our results are comparable to the results from a recent meta‐analysis (Gaberel 2012). However, Gaberel 2012 included all studies in which antifibrinolytic treatment was compared with control treatment, including retrospective analyses and studies with historical control groups. Retrospective analyses are known to be much more susceptible to selection and reporting bias, since the decision to treat a patient is made subjectively. Moreover, the other review did not compare placebo controlled versus open studies, which is another potential source of bias, since all outcomes, except for death, are made on subjective clinical grounds and can be easily biased when the outcome assessor is not blinded to the treatment given. In our review we included only randomised clinical trials and performed several sensitivity analyses, which should yield less biased results. Since most studies did not describe whether outcomes were assessed blinded to treatment, sensitivity analyses are essential. Despite the fact that all authors reported that confirmation of rebleeding was sought, in most instances this was done by examination of the cerebrospinal fluid. Because CSF examination is an unreliable test to confirm rebleeding (Vermeulen 1983) the diagnosis of rebleeding could often not be regarded as proven. The sensitivity analyses we performed on reported versus confirmed (with confirmatory evidence on CT scan or at autopsy) rebleeding, cerebral ischaemia, or hydrocephalus showed similar results to the overall analyses. Lack of confirmatory evidence of these complications thus appears to be of minor importance in our analyses. The analyses we performed on trials with control treatment compared with those given placebo treatment showed similar reducing effects of antifibrinolytic treatment on rebleeding and similar increasing effects on cerebral ischaemia, whereas hydrocephalus trials with control treatment showed a tendency towards a beneficial effect compared with trials with placebo treatment, demonstrating a tendency towards increased hydrocephalus rates.
Authors' conclusions
Implications for practice.
Antifibrinolytic treatment does not improve clinical outcome in people after SAH, although it does reduce the risk of rebleeding after SAH. Based on the currently available data, treatment with antifibrinolytic drugs cannot be recommended for people with subarachnoid haemorrhage from a presumed or proven aneurysmal origin.
Implications for research.
The available data suggest that short‐term (less than 72 hours) antifibrinolytic treatment, combined with cerebral ischaemia preventative measures, may reduce rebleeding rates without an increase in the proportion of people with cerebral ischaemia. Thus, short‐term treatment with antifibrinolytic therapy may be effective for people with aneurysmal SAH, but confirmatory evidence is lacking and a possible harmful effect can also not be excluded. Based on these findings new randomised trials of short‐term antifibrinolytic agents versus control in people with SAH are needed. Recently, a new trial comparing ultra early and short antifibrinolytic treatment (less than 24 hours) with control treatment has been registered and is planned (Verbaan 2012).
What's new
| Date | Event | Description |
|---|---|---|
| 5 September 2013 | Amended | Minor layout changes |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 3, 1998
| Date | Event | Description |
|---|---|---|
| 4 February 2013 | New citation required and conclusions have changed | A new subgroup analysis was added and the conclusions changed. |
| 4 February 2013 | New search has been performed | Updated searches completed and a new study added. We included one new study (Hillman 2002) with 505 participants. This study is different in treatment timing and duration of therapy and contributes 26.5% of all participants. The review now includes 10 trials and 1904 participants in total. |
| 21 July 2008 | Amended | Converted to new review format. |
| 7 February 2003 | New search has been performed | The results of the recently published STAR‐study, the second largest placebo‐controlled randomised trial of antifibrinolytic treatment in subarachnoid haemorrhage, has been added to the review. This study contributes 33% of all included patients in this review and 39% of the patients included in the comparison of antifibrinolytic treatment versus placebo. Furthermore, a Plain language summary of the review has been added. |
Acknowledgements
Previous versions of this review were supported by research grants from the Netherlands Heart Foundation (Nederlandse Hart Stichting). We would like to thank Brenda Thomas for her help in developing the new electronic database search. We would like to thank Hazel Fraser for her editorial advice.
Appendices
Appendix 1. MEDLINE search strategy
1. Subarachnoid Hemorrhage/ 2. intracranial hemorrhages/ or cerebral hemorrhage/ 3. Intracranial Aneurysm/ 4. Rupture, Spontaneous/ 5. 3 and 4 6. Aneurysm, Ruptured/ 7. exp brain/ or exp meninges/ 8. 6 and 7 9. ((subarachnoid or arachnoid) adj6 (haemorrhage$ or hemorrhage$ or bleed$ or blood$)).tw. 10. Vasospasm, Intracranial/ 11. ((cerebral or intracranial or cerebrovascular) adj6 (vasospasm or spasm)).tw. 12. sah.tw. 13. 1 or 2 or 5 or 8 or 9 or 10 or 11 or 12 14. exp Antifibrinolytic Agents/ 15. (anti‐fibrinolytic$ or antifibrinolytic$ or antifibrinolysin$ or anti‐fibrinolysin$ or antiplasmin$ or anti‐plasmin$ or (plasmin adj3 inhibitor$)).tw. 16. (fibrinolysis adj3 (prevent$ or inhib$ or antag$)).tw. 17. tranexamic acid/ 18. (4 amino methylcyclohexane carboxylate or 4 aminomethylcyclohexanecarbonic acid or 4 aminomethylcyclohexanecarboxylic acid or amca or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or anexan or antivoff or anvitoff orcaprilon or cis 4 aminomethylcyclohexanecarboxylic acid or cis aminomethyl cyclohexanecarboxylic acid or cl 65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or cyklokapron or exacyl or fibrinon or frenolyse or hemostan or hexacapron or hexakapron or kabi 2161 or kalnex or micranex or para aminomethylcyclohexane carboxylic acid or rikaparin or ronex or spotof or theranex or tramic or tranex or tranexam or tranexamic acid or tranexanic acid or tranexic or trans 1 aminomethylcyclohexane 4 carboxylic acid or trans 4 aminomethylcyclohexane 1 carboxylic acid or trans 4 aminomethylcyclohexane carboxylic acid or trans 4 aminomethylcyclohexanecarboxylic acid or trans achma or trans amcha or trans aminomethyl cyclohexane carboxylic acid or trans aminomethylcyclohexane carboxylic acid or trans aminomethylcyclohexanecarboxylic acid or transamin or transaminomethylcyclohexane carboxylic acid or transexamic acid or traxamic or trenaxin or ugurol).tw,nm. 19. exp Aminocaproic Acids/ 20. (acikaprin or afibrin or amicar or amino caproic acid or aminocaproic acid or aminohexanoic acid or capracid or capramol or caproamin or caprocid or caprogel or caprolest or caprolisin or caprolisine or caprolysin orcapromol or cl 10304 or cl10304 or cy 116 or cy116 or e aminocaproic acid or EACA or ecapron or ekaprol or epsamon or epsicaprom or epsicapron or epsikapron or epsilcapramin or epsilon amino caproate or epsilon amino caproic aci or depsilon aminocaproate or epsilon aminocaproic acid or epsilonaminocaproic acid or epsilonaminocapronsav or etha aminocaproic acid or ethaaminocaproic acid or gamma aminocaproic acidor gamma aminohexanoic acidor hemocaprol or hepin or hexalense or ipsilon or jd 177 or jd177 or neocaprol or nsc 26154 or nsc26154 or resplamin or tachostyptan).tw,nm. 21. Aprotinin/ 22. (9921 rp or antagosan or antilysin or antilysine or apronitin or apronitine or apronitrine or aprotimbin or aprotinin bovine or aprotinine or aprotonin or bayer a 128 or bayer a128 or bovine pancreatic secretory trypsin inhibitor or contrical or contrycal or contrykal or frey inhibitor or gordox or haemoprot or iniprol or kallikrein trypsin inhibitor or kazal type trypsin inhibitor or kontrikal or kontrycal or Kunitz inhibitor or Kunitz trypsin inhibitor or midran or pancreas antitrypsin or pancreas secretory trypsin inhibitor or pancreas trypsin inhibitor or pancreatic antitrypsin or pancreatic secretory trypsin inhibitor or pancreatic trypsin inhibitor or protinin orriker 52g or rivilina or rp 9921 or rp9921 or tracylol or trascolan or trasilol or traskolan or trasylol or trazylol or tumor associated trypsin inhibitor or zymofren).tw,nm. 23.( 4 aminomethylbenzoic acid or 4 amino methylbenzoic acid or alpha amino para toluic acid or amino methylbenzoic acid or aminomethyl benzoic acid or aminomethylbenzoic acid or PAMBA or para aminomethylbenzoic acid or para amino methylbenzoic acid or styptopur or styptosolut).tw,nm 24. Fibrinolysis/ai, de [Antagonists & Inhibitors, Drug Effects] 25. or/14‐24 26. 13 and 25 27. exp animals/ not humans.sh. 28. 26 not 27
Appendix 2. EMBASE search strategy
1. Subarachnoid Hemorrhage/ 2. brain hemorrhage/ or brain artery aneurysm rupture/ 3. Brain Vasospasm/ 4. exp Intracranial Aneurysm/ 5. exp rupture/ 6. 4 and 5 7. Aneurysm Rupture/ 8. exp brain/ or exp meninx/ 9. 7 and 8 10. ((subarachnoid or arachnoid) adj6 (haemorrhage$ or hemorrhage$ or bleed$ or blood$)).tw. 11. ((cerebral or intracranial or cerebrovascular) adj6 (vasospasm or spasm)).tw. 12. sah.tw. 13. 1 or 2 or 3 or 6 or 9 or 10 or 11 or 12 14. exp antifibrinolytic agent/ 15. (anti‐fibrinolytic$ or antifibrinolytic$ or antifibrinolysin$ or anti‐fibrinolysin$ or antiplasmin$ or anti‐plasmin$ or (plasmin adj3 inhibitor$)).tw. 16. (fibrinolysis adj3 (prevent$ or inhib$ or antag$)).tw. 17. tranexamic acid/ 18. (4 amino methylcyclohexane carboxylate or 4 aminomethylcyclohexanecarbonic acid or 4 aminomethylcyclohexanecarboxylic acid or amca or AMCHA or amchafibrin or amikapron or aminomethyl cyclohexane carboxylic acid or aminomethyl cyclohexanecarboxylic acid or aminomethylcyclohexane carbonic acid or aminomethylcyclohexane carboxylic acid or aminomethylcyclohexanecarbonic acid or aminomethylcyclohexanecarboxylic acid or aminomethylcyclohexanocarboxylic acid or aminomethylcyclohexanoic acid or amstat or anexan or antivoff or anvitoff orcaprilon or cis 4 aminomethylcyclohexanecarboxylic acid or cis aminomethyl cyclohexanecarboxylic acid or cl 65336 or cl65336 or cyclocapron or cyclokapron or cyklocapron or cyklokapron or exacyl or fibrinon or frenolyse or hemostan or hexacapron or hexakapron or kabi 2161 or kalnex or micranex or para aminomethylcyclohexane carboxylic acid or rikaparin or ronex or spotof or theranex or tramic or tranex or tranexam or tranexamic acid or tranexanic acid or tranexic or trans 1 aminomethylcyclohexane 4 carboxylic acid or trans 4 aminomethylcyclohexane 1 carboxylic acid or trans 4 aminomethylcyclohexane carboxylic acid or trans 4 aminomethylcyclohexanecarboxylic acid or trans achma or trans amcha or trans aminomethyl cyclohexane carboxylic acid or trans aminomethylcyclohexane carboxylic acid or trans aminomethylcyclohexanecarboxylic acid or transamin or transaminomethylcyclohexane carboxylic acid or transexamic acid or traxamic or trenaxin or ugurol).tw. 19. aminocaproic acid/ 20. (acikaprin or afibrin or amicar or amino caproic acid or aminocaproic acid or aminohexanoic acid or capracid or capramol or caproamin or caprocid or caprogel or caprolest or caprolisin or caprolisine or caprolysin orcapromol or cl 10304 or cl10304 or cy 116 or cy116 or e aminocaproic acid or EACA or ecapron or ekaprol or epsamon or epsicaprom or epsicapron or epsikapron or epsilcapramin or epsilon amino caproate or epsilon amino caproic aci or depsilon aminocaproate or epsilon aminocaproic acid or epsilonaminocaproic acid or epsilonaminocapronsav or etha aminocaproic acid or ethaaminocaproic acid or gamma aminocaproic acidor gamma aminohexanoic acidor hemocaprol or hepin or hexalense or ipsilon or jd 177 or jd177 or neocaprol or nsc 26154 or nsc26154 or resplamin or tachostyptan).tw. 21. aprotinin/ 22. (9921 rp or antagosan or antilysin or antilysine or apronitin or apronitine or apronitrine or aprotimbin or aprotinin bovine or aprotinine or aprotonin or bayer a 128 or bayer a128 or bovine pancreatic secretory trypsin inhibitor or contrical or contrycal or contrykal or frey inhibitor or gordox or haemoprot or iniprol or kallikrein trypsin inhibitor or kazal type trypsin inhibitor or kontrikal or kontrycal or Kunitz inhibitor or Kunitz trypsin inhibitor or midran or pancreas antitrypsin or pancreas secretory trypsin inhibitor or pancreas trypsin inhibitor or pancreatic antitrypsin or pancreatic secretory trypsin inhibitor or pancreatic trypsin inhibitor or protinin orriker 52g or rivilina or rp 9921 or rp9921 or tracylol or trascolan or trasilol or traskolan or trasylol or trazylol or tumor associated trypsin inhibitor or zymofren).tw. 23. 4 aminomethylbenzoic acid/ 24. (4 aminomethylbenzoic acid or 4 amino methylbenzoic acid or alpha amino para toluic acid or amino methylbenzoic acid or aminomethyl benzoic acid or aminomethylbenzoic acid or PAMBA or para aminomethylbenzoic acid or para amino methylbenzoic acid or styptopur or styptosolut).tw. 25. fibrinolysis/pc [Prevention] 26. or/14‐25 27. 13 and 26 28. limit 27 to human
Data and analyses
Comparison 1. Antifibrinolytic treatment versus control treatment with or without placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Poor outcome (death, vegetative or severe disability on Glasgow Outcome Scale at end of follow‐up): open versus blind studies | 4 | 1546 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.91, 1.15] |
| 1.1 Trials with control treatment (open studies) | 1 | 505 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.14] |
| 1.2 Trials with placebo treatment (blind studies) | 3 | 1041 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.93, 1.21] |
| 2 Death from all causes at end of follow up: open versus blind studies | 10 | 1904 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.85, 1.18] |
| 2.1 Trials with control treatment (open studies) | 4 | 709 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.56, 1.42] |
| 2.2 Trials with placebo treatment (blind studies) | 6 | 1195 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.87, 1.19] |
| 3 Rebleeding reported at end of follow up: open versus blind studies | 10 | 1904 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.97] |
| 3.1 Trials with control treatment (open studies) | 4 | 709 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.25, 1.74] |
| 3.2 Trials with placebo treatment (blind studies) | 6 | 1195 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.43, 0.97] |
| 4 Confirmed rebleeding at end of follow‐up (sensitivity analysis): open versus blind studies | 4 | 1505 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.26, 0.75] |
| 4.1 Trials with control treatment (open studies) | 2 | 564 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.11, 1.59] |
| 4.2 Trials with placebo treatment (blind studies) | 2 | 941 | Risk Ratio (M‐H, Random, 95% CI) | 0.46 [0.25, 0.86] |
| 5 Rebleeding reported at end of follow‐up: trials with and without ischaemia prevention according to treatment duration | 10 | 1904 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.44, 0.97] |
| 5.1 Trials without ischaemia prevention, treatment duration > 72 hours | 8 | 937 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.47, 1.31] |
| 5.2 Trials with ischaemia prevention treatment duration > 72 hours | 1 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.42, 0.80] |
| 5.3 Trials with ischaemia prevention, treatment duration < 72 hours | 1 | 505 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.09, 0.52] |
| 6 Cerebral ischaemia reported at end of follow‐up: open versus blind studies | 6 | 1671 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.04, 1.91] |
| 6.1 Trials with control treatment (open studies) | 3 | 630 | Risk Ratio (M‐H, Random, 95% CI) | 1.46 [0.99, 2.14] |
| 6.2 Trials with placebo treatment (blind studies) | 3 | 1041 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.87, 2.19] |
| 7 Confirmed cerebral ischaemia at end of follow‐up (sensitivity analysis): open versus blind studies | 3 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 1.34 [0.88, 2.03] |
| 7.1 Trials with control treatment (open studies) | 1 | 59 | Risk Ratio (M‐H, Random, 95% CI) | 2.58 [0.76, 8.77] |
| 7.2 Trials with placebo treatment (blind studies) | 2 | 941 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.82, 1.89] |
| 8 Hydrocephalus reported at end of follow‐up: open versus blind studies | 5 | 1179 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.90, 1.36] |
| 8.1 Trials with control treatment (open studies) | 2 | 138 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.34, 1.18] |
| 8.2 Trials with placebo treatment (blind studies) | 3 | 1041 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.95, 1.48] |
| 9 Confirmed hydrocephalus at end of follow up (sensitivity analysis): open versus blind studies | 1 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.87, 1.55] |
| 9.1 Trials with control treatment (open studies) | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 Trials with placebo treatment (blind studies) | 1 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.87, 1.55] |
| 10 Hydrocephalus reported at end of follow‐up: trials with and without ischaemia prevention according to treatment duration | 5 | 1179 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.90, 1.36] |
| 10.1 Trials without ischaemia prevention, treatment duration > 72 hours | 4 | 717 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.74, 1.43] |
| 10.2 Trials with ischaemia prevention, treatment duration > 72 hours | 1 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.87, 1.55] |
| 10.3 Trials with ischaemia prevention, treatment duration < 72 hours | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Poor outcome (death, vegetative or severe disability on Glasgow Outcome Scale at end of follow‐up): trials with and without ischaemia prevention according to treatment duration | 4 | 1546 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.91, 1.15] |
| 11.1 Trials without cerebral ischaemia prevention, treatment duration > 72 hours | 2 | 579 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.86, 1.22] |
| 11.2 Trials with ischaemia prevention, treatment duration > 72 hours | 1 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.91, 1.34] |
| 11.3 Trials with cerebral ischaemia prevention, treatment duration < 72 hours | 1 | 505 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.64, 1.14] |
| 12 Death from all causes at end of follow‐up: trials with and without ischaemia prevention according to treatment duration | 10 | 1904 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.85, 1.18] |
| 12.1 Trials without ischaemia prevention, treatment duration > 72 hours | 8 | 937 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.78, 1.35] |
| 12.2 Trials with ischaemia prevention with treatment duration > 72 hours | 1 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.79, 1.34] |
| 12.3 Trials with ischaemia prevention with treatment duration < 72 hours | 1 | 505 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.52, 1.35] |
| 13 Cerebral ischaemia reported at end of follow‐up: trials with and without ischaemia prevention according to treatment duration | 6 | 1671 | Risk Ratio (M‐H, Random, 95% CI) | 1.41 [1.04, 1.91] |
| 13.1 Trials without ischaemia prevention, treatment duration > 72 hours | 4 | 704 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.30, 2.40] |
| 13.2 Trials with ischaemia prevention, treatment duration > 72 hours | 1 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.75, 1.23] |
| 13.3 Trials with ischaemia prevention, treatment duration < 72 hours | 1 | 505 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.89, 2.04] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chandra 1978.
| Methods | Single centre study Random allocation (method of randomisation not described) Double‐blind treatment ITT analysis |
|
| Participants | Clinical diagnosis of aneurysmal SAH confirmed in CSF and on angiography Male:female: treatment group 11:9; placebo group 10:9 Mean age 51 years (range 20 to 65) Excluded: SAH > 7 days, 'relevant associated illness' |
|
| Interventions | Tranexamic acid (6 g per day iv in 6 doses) versus identical‐appearing placebo treatment for a treatment duration of 3 weeks No report on surgical interventions |
|
| Outcomes | Outcome: deaths from all causes at 3‐week follow‐up Events: rebleeding: reported, not defined; cerebral ischaemia: not reported; hydrocephalus: not reported | |
| Notes | No report on how many rebleeding were established on CT scan or at necropsy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | In the text, participants were reported to be randomly allocated, but how this was achieved is not reported |
| Allocation concealment (selection bias) | Low risk | Identical medication; however, unclear how medication was coded |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Neither the investigators nor the patients knew which subjects received which substance" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in text |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | More male than female participants included, which could point to some form of bias since people with SAH are more often female |
Fodstad 1981.
| Methods | Single‐centre study Random allocation (identical sequentially numbered sealed envelope) No blind treatment Not strictly ITT: 1 of the 30 control participants was excluded after randomisation because he had received tranexamic acid before admission | |
| Participants | Clinical diagnosis of aneurysmal SAH verified with CSF, CT scan and angiography Male:female: treatment group 13:17; control group 12:17 Mean age: treatment group 50 years (range 19 to 72); control group 53 years (range 27 to 70) Excluded: SAH > 3 days and known thrombotic disease | |
| Interventions | Tranexamic acid (6 g per day iv in 6 doses during the first week, 4 g in 4 doses iv in week 2 and 6 g orally in 4 doses in week 3 to 6) for a maximum duration of 6 weeks versus control group. Treatment continued until rebleeding, operation, discharge or death | |
| Outcomes | Outcome: deaths from all causes at 6‐week follow‐up Events: rebleeding: reported, confirmed by CSF examination/spectrophotometry, CT‐scan or at necropsy; cerebral ischaemia: reported, not defined; hydrocephalus: reported, not defined | |
| Notes | Of the participants with rebleeding, 5 of 6 in the treatment group and 5 of 7 in the control group died. All had necropsy. In the remaining participants rebleeding was confirmed by CT. 6 of 8 participants with cerebral ischaemia in the treatment group and 2 of 3 in the control group died. All had necropsy, in the remaining participants cerebral ischaemia was established on CT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in the text |
| Allocation concealment (selection bias) | Unclear risk | "Patients were assigned randomly" and "the sealed envelope technique was used" It was not mentioned whether these envelopes were opaque or not |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded (open study) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Part of recurrent haemorrhages were confirmed by lumbar puncture |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant was excluded from final analysis |
| Selective reporting (reporting bias) | Low risk | Protocol was previously published |
| Other bias | Low risk | None suspected based on study design and outcome |
Girvin 1973.
| Methods | Single‐centre study Random allocation (flip‐of‐a‐coin) No blind treatment ITT | |
| Participants | Clinical diagnosis of aneurysmal SAH probably confirmed on angiography Male:female ratio not described ("in control group were a relatively greater number of female patients") Age distribution not described Excluded: SAH > 7 days | |
| Interventions | Epsilon amino‐caproic acid, dosage probably 24 g per day (6 times 4 g) orally versus control group, duration on average in treatment group 7.7 days versus 5.5 in control group Treatment continued until operation or death | |
| Outcomes | Outcome: deaths from all causes (at unknown week) Events: rebleeding: reported, not defined; ischaemia: reported, not defined; hydrocephalus: not reported | |
| Notes | Poor description of study methods, definitions and results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The cases were randomised on admission, by the flip of a coin" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in the text |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Primary outcome described as "raison d'etre" of the study |
| Other bias | Low risk | None suspected based on study design and outcome |
Hillman 2002.
| Methods | Multi‐centre study Random allocation (sequence generation not specified) with sealed envelopes (not specified if opaque or not) No blind treatment Not strictly ITT: 91 participants excluded after randomisation because no aneurysm was found | |
| Participants | People suffering from CT‐verified SAH within 48 hours prior to hospital admission Male:female ratio: 1.89 in both groups Age distribution similar in both groups Excluded: SAH > 48 hours, age < 15 years, pregnancy, and history of thromboembolic disease. People in whom no aneurysm was demonstrated on angiographic studies were excluded after randomisation | |
| Interventions | Tranexamic acid (1 g iv immediately before transport, 1 g iv after 2 hours, and then 1 g every 6th hour until aneurysm occlusion up to 72 hours after SAH) versus control treatment | |
| Outcomes | Outcome: Glasgow Outcome Scale at 6 months Events: rebleeding confirmed on CT or during operation until 72 hours after admission; clinically established delayed ischaemic neurological deficit at 6 months, not confirmed by imaging |
|
| Notes | Poor description of delayed ischaemic neurological deficit and how this was scored No mention of follow‐up for rebleeding after 72 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described in the text |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes were used that contained a randomisation document; however, it was not reported how these were ordered and if they were opaque or not |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded (open study) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in the text |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 15% of included participants not evaluated in final analysis, which might have resulted in an overestimation of the effect estimate |
| Selective reporting (reporting bias) | Unclear risk | Previously published protocol was not mentioned, clinical outcome reported although not described in introduction or methods |
| Other bias | Low risk | None suspected based on study design and outcome |
Kaste 1979.
| Methods | Single‐centre study Random allocation (identical sequentially numbered treatment boxes) Double‐blind treatment Code broken after final evaluation ITT | |
| Participants | Clinical diagnosis of aneurysmal SAH verified in the CSF Male:female: active treatment group 16:16; placebo group 14:18 Age distribution similar in both groups Excluded: SAH > 72 hours, myocardial infarction within 6 months, unconsciousness, coagulation disorders or thrombotic disease, renal failure and pregnancy | |
| Interventions | Tranexamic acid (6 g per day iv in 6 doses) versus identical‐appearing placebo treatment for a maximum treatment duration of 3 weeks Treatment discontinued at operation |
|
| Outcomes | Outcome: deaths from all causes at 3 months. Events: rebleeding: suspected when 2 of sudden deterioration of consciousness, increase of neck rigidity, headache or focal signs ‐ rebleeding verified in CSF or at necropsy; ischaemia: reported, not defined; hydrocephalus: reported, not defined | |
| Notes | No definition on cerebral ischaemia or hydrocephalus: 'vasospasm and ventricular dilatation were seen on angiography' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described in the text |
| Allocation concealment (selection bias) | Low risk | Tranexamic acid and placebo were prepared in identical vials and were coded; the code was only broken after final evaluation of the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Tranexamic acid and placebo were prepared in identical vials and were coded; the code was only broken after final evaluation of the trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The code identifying each substance was broken only after the final evaluation of all 64 patients" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Protocol was previously published |
| Other bias | Unclear risk | Unconscious people not included causing the results to be less generalisable |
Maurice 1978.
| Methods | Single‐centre study Random allocation (identical sequentially numbered sealed envelop) No double‐blind treatment Not published as ITT, but ITT analysis could be reconstructed | |
| Participants | Clinical diagnosis of aneurysmal SAH probably verified in the CSF No description of age or sex ratios ('treatment group matched in age and sex control group') Excluded: SAH > 4 days, person > 65 years, unconsciousness | |
| Interventions | Tranexamic acid (6 g per day iv in 6 doses during the first week, 6 g orally in 4 doses in week 2 until week 6) for a maximum duration of 6 weeks versus control group; treatment continued until operation or death | |
| Outcomes | Outcome: deaths from all causes at 3 months Events: rebleeding: reported and defined; 'confirmed in CSF or at necropsy'; ischaemia: reported, not defined; hydrocephalus: reported, not defined | |
| Notes | Rebleeding 'confirmed' by CSF examination are not useable (see text), not reported how many rebleedings were established on CT scan or at necropsy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequentially numbered, sealed envelopes were used, but no description was made of how these numbers were created |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, sealed envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded (open study) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described in the text |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data, ITT analysis could be reconstructed |
| Selective reporting (reporting bias) | Low risk | Protocol was previously published |
| Other bias | Unclear risk | People "under 65" and "relatively little disturbed by the first bleed" not included in study, causing the results to be less generalisable |
Roos 2000a.
| Methods | Multicentre study Random allocation (identical sequentially numbered treatment boxes), blocked per centre Double‐blind treatment: code broken after all events and outcomes had been recorded ITT | |
| Participants | Clinical diagnosis of aneurysmal SAH verified on CT or in the CSF: if negative CT, angiography had to confirm an aneurysm before randomisation Male:female: treatment group 89:140; placebo group 73:160 Mean age: treatment group 56 years; placebo group 55 years Excluded: SAH > 96 hours or operation planned < 48 hours, coagulation disorders or thrombotic disease, renal failure, pregnancy, previous tranexamic acid treatment or people in whom death appeared imminent | |
| Interventions | Tranexamic acid (6 g per day iv in 6 doses in week 1, and 6 g orally per day in 4 doses in week 2 and 3) versus identical‐appearing placebo treatment for a maximum treatment duration of 3 weeks All participants received standard anti‐ischaemic treatment with nimodipine and hypervolaemia | |
| Outcomes | Outcome: Glasgow Outcome Scale at 3 months Events: rebleeding‐definite: confirmed by CT scan or at necropsy; rebleeding‐possible: sudden deterioration and death; infarction‐definite: confirmed by CT scan or at necropsy; infarction‐probable: gradual development of focal neurologic signs with or without deterioration in the level of consciousness; hydrocephalus: gradual deterioration of consciousness with on CT hydrocephalus and no other explanation Postoperative ischaemia: deterioration of consciousness or development of focal neurological signs immediately after recovery from anaesthesia compared to preoperative status, without rebleeding, infarction or hydrocephalus on CT or at autopsy Poor outcome caused by the initial bleeding: impaired consciousness or focal neurological signs from the time of the initial bleeding, without rebleeding, infarction or hydrocephalus on CT or at autopsy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence was generated by use of random number tables |
| Allocation concealment (selection bias) | Low risk | Identical, sequentially numbered treatment boxes, blocked per centre were used and boxes were consecutively numbered and administered in the same order to each following participant |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical and sequentially numbered treatment boxes were used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Only after recording of all events and outcomes, the trial code was broken" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Low risk | Previously published protocol |
| Other bias | Low risk | None suspected based on study design and outcome |
Tsementzis 1990.
| Methods | Single‐centre study Random allocation (identical sequentially numbered medication boxes) Double‐blind treatment Not strictly ITT: 4 participants excluded after randomisation who missed a few doses medication | |
| Participants | Clinical diagnosis of aneurysmal SAH verified on CT or in the CSF Male:female: treatment group 20:30; placebo group 26:24 Age distribution similar in both groups Excluded: SAH > 72 hours, antihypertensives or medication known to affect the fibrinolytic or coagulation systems, acute myocardial infarction, coagulation disorders or thrombotic disease, renal failure, pregnancy, previous tranexamic acid‐treatment or people in whom death seemed imminent | |
| Interventions | Tranexamic acid (9 g per day, iv in 6 doses in week 1, orally in 4 doses in week 2, 3 and 4) versus identical‐appearing placebo treatment for a maximum treatment duration of 4 weeks# Treatment was discontinued if an operation for the aneurysm began or if deep vein thrombosis or pulmonary infarction developed |
|
| Outcomes | Outcome: Glasgow Outcome Scale at discharge, 1, 3 and 6 months Events: rebleeding: reported and defined ‐ 'clinical signs confirmed on CT, in the CSF or at necropsy'; ischaemia: reported and defined ‐ 'clinical signs combined with the absence of evidence of rebleeding on CT or CSF'; hydrocephalus: reported, not defined | |
| Notes | No report of how many participants' rebleedings or cerebral ischaemia were established on CT scan or at necropsy No definition on hydrocephalus other then 'ventricular dilatation was seen on angiography' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation was not described |
| Allocation concealment (selection bias) | Low risk | Placebo controlled trial with sequentially numbered boxes with trial medication was used |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Boxes with either tranexamic acid or placebo were used that were numbered consecutively by the pharmacist |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported in the text, but because of control with placebo, not thought to have influenced results |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 participants were excluded and no mention is made on their outcome |
| Selective reporting (reporting bias) | Unclear risk | Not reported in the text |
| Other bias | Unclear risk | 20 participants with protocol violations were excluded from the analysis after randomisation |
Van Rossum 1977.
| Methods | Multicentre study Random allocation ("drug or placebo randomly administered in a sequence prescribed by and only known to the statistician") Double‐blind treatment Not strictly ITT: 3 participants excluded, because of other diagnosis, after randomisation |
|
| Participants | Clinical diagnosis of aneurysmal SAH verified in the CSF Male:female ratio or age distribution not described Excluded: SAH > 14 days (in 94% of the participants, treatment started within 1 week) | |
| Interventions | Tranexamic acid (4 g per day iv in 4 doses) versus identically appearing placebo treatment for a maximum treatment duration of 10 days Treatment was discontinued after the aneurysm operation | |
| Outcomes | Outcome: all‐cause mortality at 3 months Events: rebleeding: reported and defined ‐ 'clinical signs confirmed in the CSF or at necropsy'; ischaemia: not reported; hydrocephalus: not reported | |
| Notes | Rebleeds 'confirmed' by CSF examination are not useable (see text), not reported how many rebleedings were established on CT scan or at necropsy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequence was generated by an independent statistician |
| Allocation concealment (selection bias) | Low risk | Sequence was only known to the statistician and "it was impossible for medical staff or patients to distinguish between drug‐ and placebo‐containing ampoules" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "It was impossible for medical staff or patients to distinguish between drug‐ and placebo‐containing ampoules" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not reported in the text, but because of control with placebo not thought to have influenced results |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3 participants were excluded and not mentioned further |
| Selective reporting (reporting bias) | Low risk | Protocol was previously published |
| Other bias | Low risk | None suspected based on study design and outcome |
Vermeulen 1984.
| Methods | Multicentre study Random allocation (identical sequentially numbered treatment boxes), blocked per centre Double‐blind treatment Code broken after all events and outcomes had been recorded ITT | |
| Participants | Clinical diagnosis of aneurysmal SAH verified on CT or in the CSF: if negative CT, angiography had to confirm an aneurysm before randomisation Male:female: treatment group 95:146; placebo group 94:144 Mean age in both groups 50 years Excluded: SAH > 72 hours, coagulation disorders or thrombotic disease, renal failure, pregnancy, previous tranexamic acid‐treatment or people in whom death appeared imminent |
|
| Interventions | Tranexamic acid (6 g per day iv in 6 doses in week 1 and 2, 4 g iv or 6 g orally per day in 4 doses in week 3 and 4) versus identical‐appearing placebo treatment for a maximum treatment duration of 4 weeks Treatment was discontinued when an aneurysm‐operation began, if the angiography was negative, if venous thrombosis or pulmonary infarction developed or another diagnosis than aneurysm was established |
|
| Outcomes | Outcome: Glasgow Outcome Scale at 3 months Events: rebleeding‐definite: confirmed by CT scan or at necropsy; rebleeding‐possible: sudden deterioration and death; infarction‐definite: confirmed by CT scan or at necropsy; infarction‐probable: gradual development of focal neurologic signs with or without deterioration in the level of consciousness; hydrocephalus: reported, not defined | |
| Notes | No definition on hydrocephalus other than 'requiring shunting' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number tables were used |
| Allocation concealment (selection bias) | Low risk | Consecutively‐numbered boxes were used and were administered in that order to included participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo was used as control and the trial code was unbroken until the trial was finished |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The trial code was broken in May 1983, after all events and outcomes had been recorded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Low risk | Protocol was previously published |
| Other bias | Low risk | Not suspected based on study design and outcome |
CSF: cerebrospinal fluid CT: computed tomography ITT: intention‐to‐treat iv: intravenous SAH: subarachnoid haemorrhage
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adams 1981 | Comparative trial between antifibrinolytic agents and blood pressure reduction or fluid restriction |
| Ameen 1981 | Not a randomised trial; historic control group used |
| Brzecki 1974 | All participants received epsikapron, while being randomised between trasylol or placebo; also historical control group |
| Buchner 1985 | Not a randomised trial; historical control group |
| Chowdhary 1979 | Not strictly randomised; allocation to treatment or placebo according to days of the week |
| Chowdhary 1981 | Comparative trial between tranexamic acid and EACA |
| Chowdhary 1986 | No clinical outcome reported, only data on rebleeding and cerebral ischaemia |
| Fodstad 1978 | No ITT analysis possible; too many participants (14 of a of total 60 randomised) excluded from final analysis after randomisation |
| Gelmers 1980 | Not strictly randomised; allocation to treatment or placebo according to days of the week |
| Gibbs 1971 | Not a randomised trial; significant treatment differences, for instance concerning the timing of surgery, between the EACA‐treated participants and the control group |
| Kassell 1984 | Not a randomised trial; case‐control study |
| Knuckey 1982 | Not a randomised trial; case‐control study |
| Marchel 1992 | Not a randomised trial; historic control group used |
| Nibbelink 1975 | Not a randomised trial, no control group |
| Profeta 1975 | Not a randomised trial; historic control group used |
| Rosenorn 1988 | Not a randomised trial; case‐control study |
| Salaschek 1983 | Not a randomised trial; historical control group |
| Sengupta 1976 | Not a randomised trial; physicians preferred allocation |
| Shucart 1980 | Not a randomised trial; physicians preferred allocation |
| Starke 2008 | Not a randomised trial; case control study |
| Wijdicks 1989 | Not a randomised trial; case‐control study with historical control group |
EACA: epsilon amino‐caproic acid ITT: intention‐to‐treat
Characteristics of ongoing studies [ordered by study ID]
Verbaan 2012.
| Trial name or title | Ultra‐early tranexamic acid after subarachnoid haemorrhage. A prospective, randomised, multicenter study |
| Methods | Multicentre study Random allocation Open treatment with blind endpoint assessment Intention‐to‐treat |
| Participants | Clinical diagnosis of aneurysmal SAH verified on CT with inclusion within 24 hours of start of symptoms Excluded: no loss of consciousness after the haemorrhage with WFNS grade 1 or 2 on admission in combination with a perimesencephalic haemorrhage; bleeding pattern on CT compatible with a traumatic SAH; treatment for deep vein thrombosis; history of blood coagulation disorder; pregnancy or breastfeeding; severe renal (serum creatinine > 150 mmol/L) or liver failure (AST > 150 U/l or ALT > 150 U/l or AF > 150 U/l or gamma‐GT > 150 U/l); imminent death within 24 hours |
| Interventions | Standard treatment with additional administration of 1 g tranexamic acid intravenously in 10 minutes, immediately after the diagnosis SAH, succeeded by continuous infusion of 1 g per 8 hours until a maximum of 24 hours |
| Outcomes | Primary outcome: Modified Rankin Scale score dichotomised as a favourable (0 to 3) or unfavourable (4 to 6) outcome at 6 months after SAH Secondary: case fatality, cause of poor outcome, rebleeding rate, thromboembolic complications, delayed cerebral ischaemia, other complications (hydrocephalus, thromboembolic events, extracranial thrombosis or haemorrhagic complications), care cost |
| Starting date | January 2013 |
| Contact information | d.verbaan@amc.uva.nl |
| Notes |
CT: computed tomography SAH: subarachnoid haemorrhage WFNS: World Federation of Neurological Societies
Contributions of authors
Irem Baharoglu: co‐ordinated the updated review; collected data; undertook all aspects of the searches; was responsible for data management and entering data into RevMan; analysis of the data; and writing the updated review.
Menno Germans: participated in data collection; reviewed all data and participated in writing this update.
Yvo Roos: co‐ordinated and wrote previous versions of this review and oversaw the data collection and writing of the present update.
Gabriel Rinkel: participated in writing the current and previous versions of the review as well as securing funding.
Ale Algra: participated in writing the current and previous versions of the review and has also performed previous work which was the foundation of the current review.
Marinus Vermeulen: secured funding and participated in writing previous versions of the review and has also performed previous work which was the foundation of the current review.
Van Gijn: participated in writing previous versions of the review; and has also performed previous work which was the foundation of the current review.
All the authors participated in conceiving and designing the review; developing the search strategy; interpreting the data; providing methodological, clinical policy and consumer perspectives; and providing general advice on the review.
Sources of support
Internal sources
No sources of support supplied
External sources
Nederlandse Hartstichting (Netherlands Heart Foundation), Netherlands.
Declarations of interest
Some authors (YBWEMR, GJER, MV) participated in one of the two largest trials included in this review (Roos 2000a; Vermeulen 1984), which were co‐ordinated by two of the current review authors. MRG has written a protocol for a new trial (Verbaan 2012). None of the review authors have any financial or other commercial conflict of interest to disclose.
Edited (no change to conclusions)
References
References to studies included in this review
Chandra 1978 {published data only}
- Chandra B. Treatment of subarachnoid hemorrhage from ruptured intracranial aneurysm with tranexamic acid: a double‐blind clinical trial. Annals of Neurology 1978;3:502‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fodstad 1981 {published data only}
- Fodstad H, Forssell A, Liliequist B, Schannong M. Antifibrinolysis with tranexamic acid in aneurysmal subarachnoid hemorrhage: a consecutive controlled clinical trial. Neurosurgery 1981;8:158‐65. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Girvin 1973 {published and unpublished data}
- Girvin JP. The use of antifibrinolytic agents in the preoperative treatment of ruptured intracranial aneurysms. Transactions of the American Neurological Association 1973;98:150‐2. [MEDLINE: ] [PubMed] [Google Scholar]
Hillman 2002 {published data only}
- Hillman J, Fridriksson S, Nillson O, Yu Z, Saveland H, Jakobsson KE. Immediate administration of tranexamic acid and reduced incidence of early rebleeding after aneurysmal subarachnoid hemorrhage: a prospective randomized study. Journal of Neurosurgery 2002;97:771‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kaste 1979 {published data only}
- Kaste M, Ramsay M. Tranexamic acid in subarachnoid hemorrhage. A double‐blind study. Stroke 1979;10:519‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maurice 1978 {published data only}
- Maurice‐Williams RS. Prolonged antifibrinolysis: an effective non‐surgical treatment for ruptured intracranial aneurysms?. British Medical Journal 1978;1:945‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roos 2000a {published and unpublished data}
- Roos Y, for the STAR‐study group. Antifibrinolytic treatment in aneurysmal subarachnoid haemorrhage: a randomized placebo‐controlled trial. Neurology 2000;54:77‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tsementzis 1990 {published data only}
- Tsementzis SA, Hitchcock ER, Meyer CH. Benefits and risks of antifibrinolytic therapy in the management of ruptured intracranial aneurysms. A double‐blind placebo‐controlled study. Acta Neurochirurgica 1990;102:1‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tsementzis SA, Honan WP, Nightingale S, Hitchcock ER, Meyer CH. Fibrinolytic activity after subarachnoid haemorrhage and the effect of tranexamic acid. Acta Neurochirurgica 1990;103:116‐21. [DOI] [PubMed] [Google Scholar]
Van Rossum 1977 {published data only}
- Rossum J, Wintzen AR, Endtz LJ, Schoen JH, Jonge H. Effect of tranexamic acid on rebleeding after subarachnoid hemorrhage: a double‐blind controlled clinical trial. Annals of Neurology 1977;2:238‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vermeulen 1984 {published data only}
- Vermeulen M, Lindsay KW, Murray GD, Cheah F, Hijdra A, Muizelaar JP, et al. Antifibrinolytic treatment in subarachnoid hemorrhage. New England Journal of Medicine 1984;311:432‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adams 1981 {published data only}
- Adams HP Jr, Nibbelink DW, Torner JC, Sahs AL. Antifibrinolytic therapy in patients with aneurysmal subarachnoid hemorrhage. A report of the cooperative aneurysm study. Archives of Neurology 1981;38:25‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ameen 1981 {published data only}
- Ameen AA, Illingworth R. Anti‐fibrinolytic treatment in the pre‐operative management of subarachnoid haemorrhage caused by ruptured intracranial aneurysm. Journal of Neurology, Neurosurgery and Psychiatry 1981;44:220‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brzecki 1974 {published data only}
- Brzecki A, Misztal S. Results of Trasylol treatment of subarachnoid hemorrhage [Wyniki leczenia Trasylolem krwotoku podpajeczynowkowego]. Polski Tygodnik Lekarski 1974;29(2):53‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Buchner 1985 {published data only}
- Buchner H, Hacke W, Kugel I, Zeumer H, Ferbert A. Antifibrinolytic therapy following an aneurysmal subarachnoid hemorrhage [Antifibrinolytika in der konservativen Therapie der aneurysmatischen Subarachnoidalblutung]. Intensivmed 1985;22:424‐7. [Google Scholar]
Chowdhary 1979 {published data only}
- Chowdhary UM, Carey PC, Hussein MM. Prevention of early recurrence of spontaneous subarachnoid haemorrhage by epsilon‐aminocaproic acid. Lancet 1979;1:741‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chowdhary 1981 {published data only}
- Chowdhary UM, Sayed K. Comparative clinical trial of epsilon amino‐caproic acid and tranexamic acid in the prevention of early recurrence of subarachnoid haemorrhage. Journal of Neurology, Neurosurgery and Psychiatry 1981;44:810‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chowdhary 1986 {published data only}
- Chowdhary UM, Sayed K. Prevention of early recurrence of aneurysmal subarachnoid haemorrhage by tranexamic acid: a controlled clinical trial. Vascular Surgery 1986;1:8‐13. [EMBASE: 1986195828] [Google Scholar]
Fodstad 1978 {published data only}
- Fodstad H, Liliequist B, Schannong M, Thulin CA. Tranexamic acid in the preoperative management of ruptured intracranial aneurysms. Surgical Neurology 1978;10:9‐15. [MEDLINE: ] [PubMed] [Google Scholar]
Gelmers 1980 {published data only}
- Gelmers HJ. Prevention of recurrence of spontaneous subarachnoid haemorrhage by tranexamic acid. Acta Neurochirurgica 1980;52:45‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gibbs 1971 {published data only}
- Gibbs JR, Corkill AG. Use of an anti‐fibrinolytic agent (tranexamic acid) in the management of ruptured intracranial aneurysms. Postgraduate Medical Journal 1971;47:199‐200. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kassell 1984 {published data only}
- Kassell NF, Torner JC, Adams HP. Antifibrinolytic therapy in the acute period following aneurysmal subarachnoid hemorrhage. Preliminary observations from the Cooperative Aneurysm Study. Journal of Neurosurgery 1984;61:225‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Knuckey 1982 {published data only}
- Knuckey NW, Stokes BA. Medical management of patients following a ruptured cerebral aneurysm, with epsilon‐aminocaproic acid, kanamycin, and reserpine. Surgical Neurology 1982;17:181‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marchel 1992 {published data only}
- Marchel A. Antifibrinolytic treatment after subarachnoid hemorrhage. Neurologia i Neurochirurgia Polska 1992;26:502‐10. [MEDLINE: ] [PubMed] [Google Scholar]
Nibbelink 1975 {published data only}
- Nibbelink DW, Torner JC, Henderson WG. Intracranial aneurysms and subarachnoid hemorrhage. A cooperative study. Antifibrinolytic therapy in recent onset subarachnoid hemorrhage. Stroke 1975;6:622‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Profeta 1975 {published data only}
- Profeta G, Castellano F, Guarnieri L, Cigliano A, Amborsio A. Antifibrinolytic therapy in the treatment of subarachnoid haemorrhage caused by arterial aneurysms. Journal of Neurosurgical Sciences 1975;19:77‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Rosenorn 1988 {published data only}
- Rosenorn J, Eskesen V, Espersen JO, Haase J, Schmidt K. Antifibrinolytic therapy in patients with aneurysmal subarachnoid haemorrhage. British Journal of Neurosugery 1988;2:447‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salaschek 1983 {published data only}
- Salaschek M, Weinrich W. Epsilon aminocaproic acid (FACA) in the treatment of spontaneous subarachnoid haemorrhage [Antifibrinolytische behandlung spontaner subarachnoidalblutungen mit epsilon‐aminocapronaure]. Aktuelle Neurologie 1983;10:65‐8. [EMBASE: 1983183434] [Google Scholar]
Sengupta 1976 {published data only}
- Sengupta RP, So SC, Villarejo Ortega FJ. Use of epsilon aminocaproic acid (EACA) in the preoperative management of ruptured intracranial aneurysms. Journal of Neurosurgery 1976;44:479‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shucart 1980 {published data only}
- Shucart WA, Hussain SK, Cooper PR. Epsilon‐aminocaproic acid and recurrent subarachnoid hemorrhage: a clinical trial. Journal of Neurosurgery 1980;53:28‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Starke 2008 {published data only}
- Starke RM, Kim GH, Fernandez A, Komotar RJ, Hickman ZL, Otten ML, et al. Impact of a protocol for acute antifibrinolytic therapy on aneurysm rebleeding after subarachnoid hemorrhage. Stroke 2008;39:2617‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wijdicks 1989 {published data only}
- Wijdicks EF, Hasan D, Lindsay KW, Brouwers PJ, Hatfield R, Murray GD, et al. Short‐term tranexamic acid treatment in aneurysmal subarachnoid hemorrhage. Stroke 1989;20:1674‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Verbaan 2012 {published data only}
- Verbaan D. Ultra‐early tranexamic acid after subarachnoid hemorrhage. Netherlands Trial Register 2012. [DOI] [PMC free article] [PubMed]
Additional references
Adams 1982
- Adams HP. Current status of antifibrinolytic therapy for treatment of patients with aneurysmal subarachnoid hemorrhage. Stroke 1982;13:256‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Adams 1987
- Adams HP. Antifibrinolytics in aneurysmal subarachnoid hemorrhage. Do they have a role? Maybe. Archives of Neurology 1987;44:114‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Biller 1988
- Biller J, Godersky JC, Adams HP. Management of aneurysmal subarachnoid hemorrhage. Stroke 1988;19:1300‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carley 2005
- Carley S, Sen A. Best evidence topic report. Antifibrinolytics for the initial management of sub arachnoid haemorrhage. Emergency Medicine Journal 2005;22:274‐5. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chawjol 2008
- Chawjol M, Starke RM, Kim GH, Majer SA, Connolly ES. Antifibrinolytic therapy to prevent early rebleeding after subarachnoid hemorrhage. Neurocritical Care 2008;8:418‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Connolly 2012
- Connolly ES, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2012;43:1711‐37. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fodstad 1982
- Fodstad H. Antifibrinolytic treatment in subarachnoid haemorrhage: present state. Acta Neurochirurgica 1982;63:233‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gaberel 2012
- Gaberel T, Magheru C, Emery E, Derlon J. Antifibrinolytic therapy in the management of aneurismal subarachnoid hemorrhage revisited. A meta‐analysis. Acta Neurochirurgica 2012;154:1‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gibbs 1967
- Gibbs JR, O'Gorman P. Fibrinolysis in subarachnoid haemorrhage. Postgraduate Medical Journal 1967;43(506):779‐84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guo 2011
- Guo L, Zhou H, Xu J, Wang Y, Qiu Y, Jiang J. Risk factors related to aneurysmal rebleeding. World Neurosurgery 2011;76:292‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harrigan 2010
- Harrigan MR, Rajneesh KF, Ardelt AA, Fisher WS. Short‐term antifibrinolytic therapy before early aneurysm treatment in subarachnoid hemorrhage: effects on rehemorrhage, cerebral ischemia, and hydrocephalus. Neurosurgery 2010;67:935‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lindsay 1987
- Lindsay KW. Antifibrinolytic agents in subarachnoid haemorrhage. Journal of Neurology 1987;234:1‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Locksley 1966
- Locksley HB. Natural history of subarachnoid hemorrhage, intracranial aneurysms and arteriovenous malformations. Journal of Neurosurgery 1966;25:321‐68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Maira 2006
- Maira G, Albanese A, Pentimalli L, Tirpakova B. Treatment of intracranial aneurysms. Clinical and Experimental Hypertension 2006;28:371‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mayberg 1994
- Mayberg MR, Batjer HH, Dacey R, Diringer M, Haley EC, Heros RC, et al. Guidelines for the management of aneurysmal subarachnoid hemorrhage. A statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Circulation 1994;90:2592‐605. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Naidech 2005
- Naidech AM, Janjua N, Kreiter KT, Ostapkovich ND, Fitzsimmons BF, Parra A, et al. Predictors and impact of aneurysm rebleeding after subarachnoid hemorrhage. Archives of Neurology 2005;62:410‐6. [DOI] [PubMed] [Google Scholar]
Ramirez 1981
- Ramirez‐Lassepas M. Antifibrinolytic therapy in subarachnoid hemorrhage caused by ruptured intracranial aneurysm. Neurology 1981;31:316‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The NordicCochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The NordicCochrane Centre, The Cochrane Collaboration, 2012.
Rinkel 2008
- Rinkel GJ. Medical management of patients with aneurysmal subarachnoid haemorrhage. International Journal of Stroke 2008;3:193‐204. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Roos 2000b
- Roos YB, Haan RJ, Beenen LF, Groen RJ, Albrecht KW, Vermeulen M. Complications and outcome in patients with aneurysmal subarachnoid haemorrhage: a prospective hospital based cohort study in the Netherlands. Journal of Neurology, Neurosurgery and Psychiatry 2000;68:337‐41. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Gijn 2001
- Gijn J, Rinkel GJ. Subarachnoid haemorrhage: diagnosis, causes and management. Brain 2001;124:249‐78. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van Gijn 2007
- Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007;369:306‐18. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vermeulen 1980
- Vermeulen M, Muizelaar JP. Do antifibrinolytic agents prevent rebleeding after rupture of a cerebral aneurysm? A review. Clinical Neurology and Neurosurgery 1980;82:25‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vermeulen 1983
- Vermeulen M, Gijn J, Blijenberg BG. Spectrophotometric analysis of CSF after subarachnoid hemorrhage: limitations in the diagnosis of rebleeding. Neurology 1983;33:112‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vermeulen 1996
- Vermeulen M. Subarachnoid haemorrhage: diagnosis and treatment. Journal of Neurology 1996;243:496‐501. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weaver 1994
- Weaver JP, Fisher M. Subarachnoid hemorrhage: an update of pathogenesis, diagnosis and management. Journal of the Neurological Sciences 1994;125:119‐31. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weir 1987
- Weir B. Antifibrinolytics in subarachnoid hemorrhage. Do they have a role? No. Archives of Neurology 1987;44:116‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Roos 1998
- Roos YB, Vermeulen M, Rinkel GJ, Algra A, Gijn J, Algra A. Systematic review of antifibrinolytic treatment in aneurysmal subarachnoid haemorrhage. Journal of Neurology, Neurosurgery and Psychiatry 1998;65:942‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Roos 2003
- Roos YB, RinkelGJE, VermeulenM, Algra A, vanGijn J. Antifibrinolytic therapy for aneurysmal subarachnoid haemorrhage. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD001245] [DOI] [PubMed] [Google Scholar]


