Abstract
The timing and magnitude of muscle responses to perturbations are critical for acting in uncertain environments. A planned movement can strongly influence average muscle responses to perturbations, but certainty in when a perturbation will arrive changes this effect. The objective of this study was to investigate how uncertainty in perturbation timing influences the preparation and release of involuntary, perturbation-triggered responses. We hypothesized that uncertainty would influence the average magnitude of triggered responses and how they develop in time. We investigated three levels of uncertainty in when a proprioceptive cue to move would arrive by changing the duration and variability of the time between a preparation and movement cue. Participants performed ballistic elbow extension movements in response to the movement cue. Unexpected, large perturbations that flexed the elbow were delivered at various times between the preparation and movement cues to evaluate how cue uncertainty influenced the development of triggered responses. We found that this uncertainty strongly influences how a motor response is prepared, and the efficacy of triggering that response by a postural perturbation. When timing was certain, the motor plan was prepared within 150 ms of the expected disturbance, and consistently released earlier by a perturbation than could be done voluntarily. Less predictable stimuli led to much earlier planning and a lower probability of releasing the plan early. These results clarify how uncertainty in when to move influences the planning and early release of perturbation-triggered responses, demonstrating an effect similar to previous reports on the planning of volitional movements.
Keywords: Triggered reactions, temporal uncertainty, motor planning, stretch reflex, startle
Introduction
The performance of many tasks depends on our ability to respond to external disturbances of posture. In some cases, these disturbances are an external cue to initiate a planned movement. In other instances, they need to be counteracted to maintain limb posture or stability. Sometimes, these dual responses to a disturbance are contained in a single task. Consider an adult lightly holding a child learning to climb a tree. The adult will be prepared to grasp the child quickly in the event of proprioceptive or other cues indicating a fall. The weight of a falling child becomes a disturbance to the posture of the arms, which needs to be counteracted to maintain stability and ensure safety. The timing and magnitude of motor actions elicited in response to external disturbances are critical for the effective execution of many tasks. Some responses, such as the short- and long-latency stretch reflexes that can act to stabilize limb posture have consistent timing, but a magnitude that scales with task (Doemges and Rack 1992; Krutky et al. 2010; Perreault et al. 2008; Pruszynski et al. 2009). In contrast, responses associated with a planned movement cued by a disturbance can vary in timing and magnitude (Crago et al. 1976; Forgaard et al. 2015; Rothwell et al. 1980). Though uncertainty in when a disturbance will arrive, such as when a child might fall, does not affect the more stereotyped responses that act to stabilize limb posture (Forgaard et al. 2016), uncertainty may affect the preparation of a movement that is cued by a disturbance. The purpose of this study was to investigate how uncertainty in the time when a perturbation will arrive influences the preparation of a movement plan, and the associated timing and magnitude of the motor actions elicited in response to a perturbation.
A planned movement can strongly influence the average response to a perturbation, but certainty about when a perturbation will arrive changes this response. Muscle activity associated with the triggering of a planned motor action can begin as early as ~75 ms after the perturbation is applied (Crago et al. 1976; Ravichandran et al. 2013). Many factors that influence the planning and execution of movements also seem to influence these triggered responses, or more generally modulation of the perturbation-elicited response that depends on the existence of a motor plan. In paradigms that allow for planning, it is obvious that a motor plan needs to exist before it can modulate the response to a perturbation. There is no response modulation when instructions that permit planning are given at the time of the perturbation (Colebatch et al. 1979), though it appears that a response can be prepared as quickly as ~70 ms prior to the onset of a perturbation (Yang et al. 2011). When given ample time to plan, the average magnitude of the triggered response measured across trials is greatest when the time of the perturbation is predictable (Rothwell et al. 1980). However, it is unknown if this increased average response reflects the existence of a more complete motor plan, or the increased probability of triggering the plan early.
The preparation of voluntary movements has been studied extensively and is known to be influenced by uncertainty in the timing of an external cue to move. Neural activity recorded with electroencephalography (EEG) shows an increase in movement-related preparatory activity beginning 1500 ms before a cue to move that is predictable in time (Cui and MacKinnon 2009; Jahanshahi et al. 1995; Jankelowitz and Colebatch 2002; MacKinnon et al. 2013). A startling acoustic stimulus can elicit a prepared movement early and involuntarily (Valls-Sole et al. 1999; Valls-Sole et al. 1995), and this technique has also been used to demonstrate that voluntary movements are prepared up to 1500 ms before a predictable cue to move. The probability with which a prepared movement is elicited increases as startling stimuli are applied closer in time to the cue to move (Carlsen and Mackinnon 2010; MacKinnon et al. 2013). This involuntary elicitation of a prepared movement evolves differently when continuous temporal information is provided such that the time of the cue to move can be accurately predicted. In this case, the probability of eliciting the movement early does not begin to increase until 500 ms before the cue to move (Carlsen and Mackinnon 2010). Furthermore, when a cue to move is unpredictable in time, both the movement-related preparatory activity measured by EEG (Cui and MacKinnon 2009; Jankelowitz and Colebatch 2002; MacKinnon et al. 2013) and the probability with which a prepared movement can be elicited by startling stimuli (MacKinnon et al. 2013) remain low and constant in the 1500 ms preceding the cue to move. These studies have elucidated how uncertainty in when a cue to move will arrive influences the planning of volitional movements, but it is unclear if uncertainty in when a perturbation will arrive similarly influences triggered responses.
The objective of this study was to investigate how uncertainty in when a perturbation will arrive influences the preparation and release of triggered responses. We hypothesized that uncertainty in perturbation timing would influence the average magnitude of triggered responses and how they develop in time. We also evaluated if observed changes in the average magnitude could be explained by changes in the probability of triggering the prepared response early. To test our hypothesis, we investigated three levels of uncertainty in when a cue to move would arrive. These levels of uncertainty were achieved by changing the duration and variability of the time between a preparation cue and the movement cue. Participants were instructed to perform ballistic elbow extension movements in response to the movement cue. Unexpected, large perturbations that flexed the elbow were delivered at various times between the preparation and movement cues to evaluate how cue uncertainty influenced the development of triggered responses. Our findings have implications for understanding how our ability to prepare for and respond effectively to perturbations is influenced by uncertainty in when they will arrive.
Methods
Participants
Twelve, right-hand dominant individuals with no history of neurological disorders or musculoskeletal impairments participated in the study. There were 8 male and 4 female participants with an average age of 26 years old (range: 19–32 years old). The study was approved by the Northwestern University Institutional Review Board (IRB protocol STU00009204) and participants gave informed consent.
Equipment
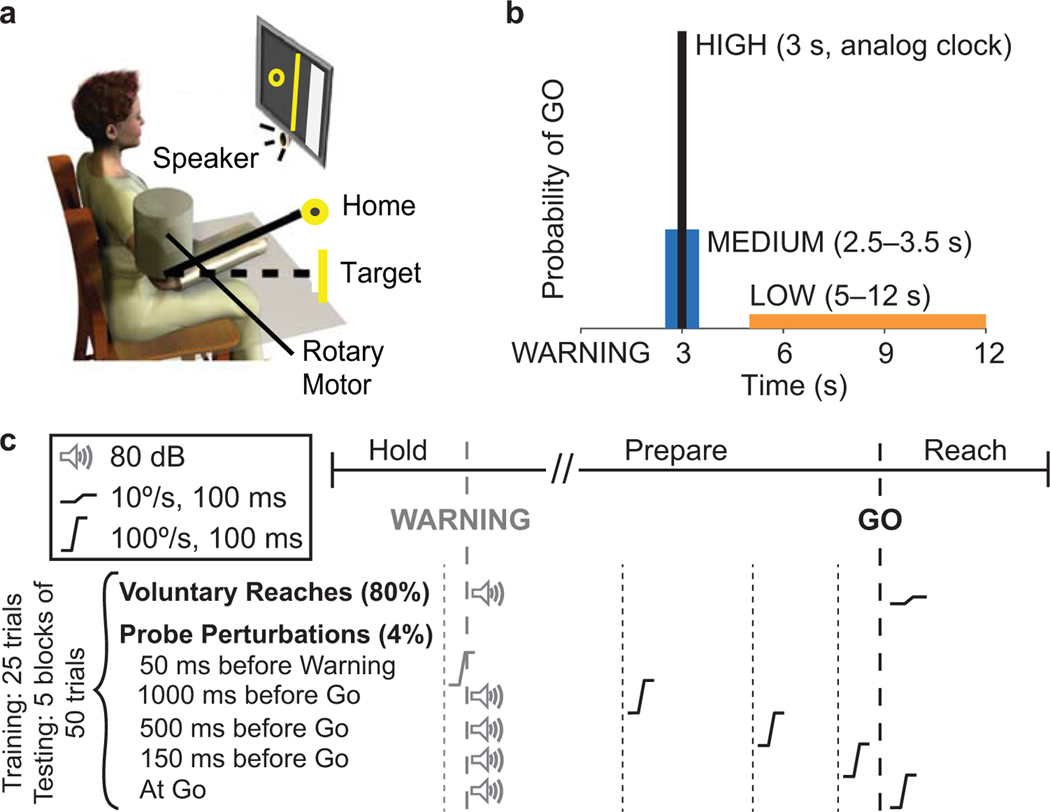
Participants were seated in an adjustable chair (Biodex); a lap belt and straps placed over each shoulder were used to secure the participant to the chair. Each participant was positioned with their dominant arm in 65° shoulder abduction, 35° shoulder flexion, and 100° elbow flexion. The participant’s forearm was coupled to a rotary motor (BSM90N-3150, Baldor Electric Company) via a custom-made plastic cast that held the forearm pronated and the wrist in a neutral position. The cast was attached to the rotary motor with the center of rotation of the motor aligned to the flexion/extension axis of the elbow (Fig. 1a). Physical stops and software limits were implemented to prevent elbow movement outside of the participant’s comfortable range of motion.
Fig. 1.
(a) Participants were seated and attached to a rotary motor aligned to the elbow joint. Visual feedback of current and target elbow position was provided on a LCD monitor. (b) A schematic representation of how the probability of receiving the proprioceptive GO cue following the WARNING cue was varied in each of the three temporal certainty conditions. The range of times between WARNING and GO cues is shown in parentheses. Participants performed all experimental conditions on separate days. (c) The testing protocol included voluntary reaches cued by a proprioceptive GO cue with high-intensity probe perturbations delivered in 20% of the trials to investigate the influence of certainty condition on the development and release of triggered responses. Probe perturbations were delivered at 50 ms before the WARNING cue and at 1000, 500, 150, and 0 ms before the GO cue.
The rotary motor was connected to a 10:1 planetary gear (AD140–010-PO, Apex Dynamics) to provide an angular measurement resolution of 3.6×10−3 deg. Forces and torques were measured using a six degrees-of-freedom load cell (630N80, JR3 Inc.). The motor was controlled using an admittance servo using custom software written in MATLAB xPC (Mathworks). Elbow movements were performed when the servo was compliant, simulating a mass with a 0.2 kgm2/rad moment of inertia. The motor was transiently switched to a stiff position servo with a 30 kNm/rad stiffness to apply postural perturbations, as described in the protocol below.
Surface electromyography (EMG) was recorded from the triceps lateral head (TRI) and left and right sternocleidomastoid neck muscles (SCM) using disposable, bipolar electrodes (Noraxon). EMG activity was amplified and conditioned using a Bortec AMT-8 (Bortec Biomedical Ltd) with a band-pass filter of 10–1,000 Hz. The resulting signals were anti-alias filtered using fifth order Bessel filters with a cutoff frequency of 500 Hz and then sampled at 2,500 Hz (PCI-DAS1602/16; Measurement Computing).
Visual feedback of the participant’s elbow angle was provided on a computer monitor placed in front of the participant. An auditory stimulus was used as a WARNING to cue the beginning of each trial. Auditory stimuli were presented with an intensity of 80 dB and duration of 40 ms via a Sonalert SC628ND speaker (Mallory Sonalert Products Inc) beneath the feedback monitor.
Protocol
At the beginning of each experimental condition, the maximum voluntary isometric contraction (MVC) was recorded for each muscle. For the arm muscles, the MVC was obtained with the arm secured to the stiff rotary motor. To obtain the MVC for each SCM muscle, participants were positioned with their neck in ipsilateral lateral flexion and contralateral rotation and the experimenter manually resisted contralateral neck rotation. EMG recorded during these tests was used to normalize EMG reported for the TRI and SCM muscles.
Our main objective was to investigate how uncertainty in when a perturbation will arrive influences muscle responses. We used two types of perturbations in this protocol. A small proprioceptive GO cue which guided motor planning and a larger probe perturbation to investigate the motor planning process and responses. Three levels of temporal uncertainty were studied during a simple reaction time task. An auditory WARNING cue signaled the initiation of each trial. The small perturbation acted as a proprioceptive GO cue for participants to initiate movement. A 25° elbow extension target position appeared with the WARNING cue. Participants were instructed to make ballistic movements past the target in response to the proprioceptive GO cue. The time between the WARNING and GO cues was determined by the level of temporal uncertainty. After each ballistic reach, participants were given feedback on the time required to reach the target. The motor exerted a constant bias torque requiring participants to generate 5% of their maximum voluntary elbow extension torque to help control the background activation in the TRI across participants and experimental conditions.
The auditory WARNING cue was delivered once a participant maintained an elbow position of 100±1° flexion for 1–1.5 s. This position was indicated by a home circle displayed on the feedback monitor. The target position was indicated by a vertical line. The GO cue was a 10°/s ramp perturbation with a duration of 100 ms in the direction of elbow flexion (1° displacement); it was insufficient to elicit a consistent long-latency stretch reflex in the agonist TRI (Lewis et al. 2005). Participants were instructed to respond as quickly as possible and to move as fast as possible once the GO cue was received.
The certainty with which participants knew the timing of the proprioceptive GO cue was varied by changing the characteristics of the time between the WARNING and GO cues (Fig. 1b). In the LOW certainty condition the GO cue was delivered between 5 and 12 s after the WARNING. In the medium certainty condition (MED), this period ranged from 2.5–3.5 s. In the HIGH certainty condition the GO cue was delivered precisely 3 s after the WARNING; an analog clock was displayed in the HIGH certainty condition to visually display the elapse of time between the WARNING and GO. Each certainty condition was tested on a separate day. The order of testing was randomized across participants.
A training phase was used at the start of each experiment to familiarize participants with the task. This involved having participants perform blocks of 10 voluntary reaches until they were responding accurately and quickly to the cues which occurred after one to three blocks. This was followed by a block of 25 reaches to become familiar with the large probe perturbations. Five probe perturbations were applied during this portion of training. The probe perturbations were 100°/s ramp perturbations with a duration of 100 ms (10° displacement). The average time required to reach the target during the training phase was used to encourage consistency throughout the later testing phase.
The testing phase involved having participants perform five blocks of 50 trials for each certainty condition. To characterize the effect of motor planning on the development of triggered responses, we applied the large probe perturbations unexpectedly in 20% of the trials. Probe perturbations were applied at one of four times between the WARNING and GO: 1000 ms, 500 ms, or 150 ms before the GO cue, or at the GO cue (GO-1000, GO-500, GO-150, GO-0). Probe perturbations were applied at one time, as a control, 50 ms before motor planning was cued by the WARNING (WARN-50). Each perturbation was applied in 4% of the trials (Fig. 1c). Perturbations were randomized but did not occur in consecutive trials. Participants rested 1–2 minutes in between blocks of trials and as needed during blocks.
Data Processing
The mean across each trial was removed from the TRI and SCM EMG recordings, which were then rectified and normalized by the average rectified EMG (0.5 s average) recorded during MVCs for each muscle. Recordings were aligned to the onset of the GO cue for voluntary reaching trials or the probe perturbation for the remaining trials. The magnitude of the muscle response elicited in the TRI agonist muscle was quantified by the average rectified EMG within four time windows relative to the perturbation: 25–50 ms, 50–75 ms, 75–100 ms, and 100–125 ms. These windows were chosen to be consistent with prior work on stretch reflexes and triggered reactions (Forgaard et al. 2015; Pruszynski et al. 2009; Ravichandran et al. 2013; Yang et al. 2011). Background muscle activity (BKG) was computed by the average rectified TRI EMG activity from 0 to 100 ms prior to the GO cue (voluntary reaches) or the probe perturbation.
The onset of SCM EMG activity was used to detect the early release of movement. This was accomplished by examining when the SCM EMG amplitude exceeded 3 standard deviations of the background response, and manually adjusting the onset to the initial rise of EMG above background. Many previous studies have used fixed SCM latencies to detect the release of a movement plan related to startle. Most have used a time threshold of 120 ms (Carlsen et al. 2004; Carlsen and Mackinnon 2010; Carlsen et al. 2011; Honeycutt and Perreault 2012; Ravichandran et al. 2013). Those studies, including some of our own, used protocols that did not exhibit early SCM activity in voluntary movement trials. In contrast, we frequently observed SCM activity within 120 ms of the proprioceptive GO cue during voluntary reaching trials (see Results for more details). Since we could not use the SCM to detect the startle-related, involuntary release of a prepared movement, we instead used a participant-specific time threshold for detecting SCM activity that occurred faster than that observed during the ballistic voluntary reaches used in our protocol. The participant-specific detection threshold was set as the lower fifth percentile of SCM latencies observed during all voluntary reaching trials in the MED certainty condition. Though both SCM muscles were commonly activated during volitional reaching, the left SCM tended to be faster and larger, so only results for the left SCM are reported. This participant-specific detection threshold was used to classify trials with and without early SCM activity during the probe perturbation trials. The onset of TRI EMG was used to examine the relationship between SCM and TRI activity during voluntary reaches. Here we used an amplitude detection threshold of five standard deviations above background for 25 ms, which was more robust to the sustained activity in this muscle during the baseline period.
Trials were excluded from analysis if the participant did not stay in the home position as instructed. Excluded conditions were those in which a change in position greater than one degree was observed in the 0.5 s before the GO cue or probe perturbation. Voluntary reaching trials were excluded if participants did not reach the target, reflecting a non-response to the GO cue. Trials with TRI BKG greater or less than two standard deviations from the average for each data set were excluded from further analysis (3.7% average exclusion rate). Four participants had mean BKG in one certainty condition that differed by >2% MVC from the remaining two certainty conditions; one of these participants reported fatigue due to recent exercise. This resulted in two LOW and two HIGH certainty data sets being removed from the analysis. All three temporal uncertainty conditions were included for the other eight participants. The WARN-50 probe perturbation elicited early SCM activity in only 1 trial across all participants; this trial was excluded from analysis.
Statistical Analysis
Our primary hypothesis was that uncertainty in when a proprioceptive cue will arrive would influence the average muscle response. We tested this by fitting a linear mixed-effects model with condition certainty (LOW, MED, HIGH), probe perturbation time (WARN-50, GO-1000, GO-500, GO-150, GO-0), and their interaction treated as fixed factors. Participant and each combination of participant and experimental condition were treated as random factors. The magnitude of the muscle response was analyzed at five time windows relative to perturbation onset (BKG, 25–50 ms, 50–75 ms, 75–100 ms, and 100–125 ms). We used a log-transformation of the EMG data to improve the normality of the muscle response magnitudes. Post-hoc comparisons are therefore presented as the ratio of the mean response between conditions. These ratios are expressed as a percentage along with the 95% confidence interval (CI). Performing these analyses without the log-transformation did not alter any of our primary conclusions. In the LOW certainty condition, the time of the probe perturbations was highly variable relative to when motor planning was cued by the WARNING. We evaluated if the actual amount of time available for movement preparation in the LOW certainty condition would influence the magnitude of the muscle response. We did this using a linear mixed-effects model with the amount of time between the WARNING cue and the probe perturbation treated as a continuous factor. Participant was treated as a random factor.
We also evaluated if uncertainty in when a proprioceptive cue will arrive would influence the probability of triggering the prepared response early. We did this using a linear mixed-effects model with certainty condition, probe perturbation time, and their interaction treated as fixed factors. Participant was treated as a random factor. A single probability of early SCM activity was computed for each experimental condition, so it was not necessary to include the combination of participant and experimental condition. Additionally, we evaluated if changes in the average magnitude could be explained by early triggering of prepared responses by including SCM classification (with or without early SCM activity) as a fixed factor, along with the first-order interactions, in the linear mixed-effects model of the magnitude of the TRI muscle. The WARN-50 probe perturbation time included only responses without early SCM activity, so it was excluded from this model. The inclusion of second-order interactions did not significantly improve the statistical model or alter our primary conclusions.
Statistical significance was evaluated against a p-value of 0.05 for all models. Post-hoc comparisons were made with the multcomp package in R. Post-hoc comparisons were made with the Z-scores reported by the multcomp package in R because degrees of freedom are not clearly defined for mixed-effects models (Hothorn et al. 2008). The mean and confidence intervals of statistical comparisons are reported with p-values adjusted for multiple comparisons (Hothorn et al. 2008). All statistical comparisons were performed using the nlme and multcomp packages in R (The R Foundation for Statistical Computing, 2013).
Results
Perturbation certainty influences the elicited motor response
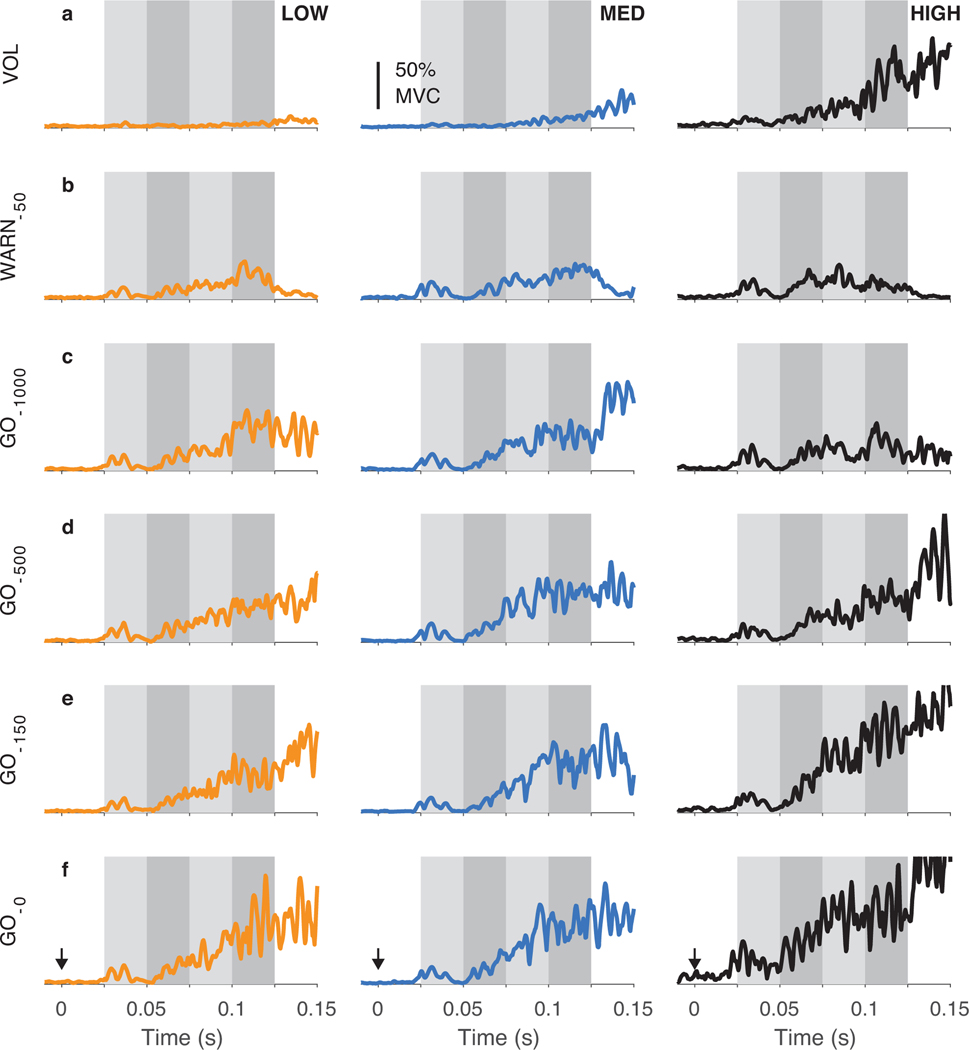
TRI muscle responses for a typical participant show that certainty in the time of the GO cue influences the development of triggered responses (Fig. 2). Probe perturbations elicited short-latency (25–50-ms time window) and long-latency (50–100-ms time window) stretch reflex responses, as expected. Under certain experimental conditions, a large magnitude triggered response was also observed approximately 75 ms following the perturbation onset. This triggered response increased in magnitude as the probe perturbation was applied closer in time to the GO cue (moving downwards from row c to row f). The influence of perturbation time was most dramatic for the HIGH certainty condition, in which the GO cue could be accurately predicted.
Fig. 2.
Representative data from one participant during the LOW (left column) MED (center column) and HIGH (right column) temporal certainty conditions. Traces show the average triceps EMG during voluntary reaching (row a) and in response to the five probe perturbation times used in these experiments (rows b–f). Each trace is the average of ten trials; trials for the voluntary reaching were selected randomly from all available trials. Responses were later analyzed in the four time windows illustrated (25–50 ms, 50–75 ms, 75–100 ms, 100–125 ms).
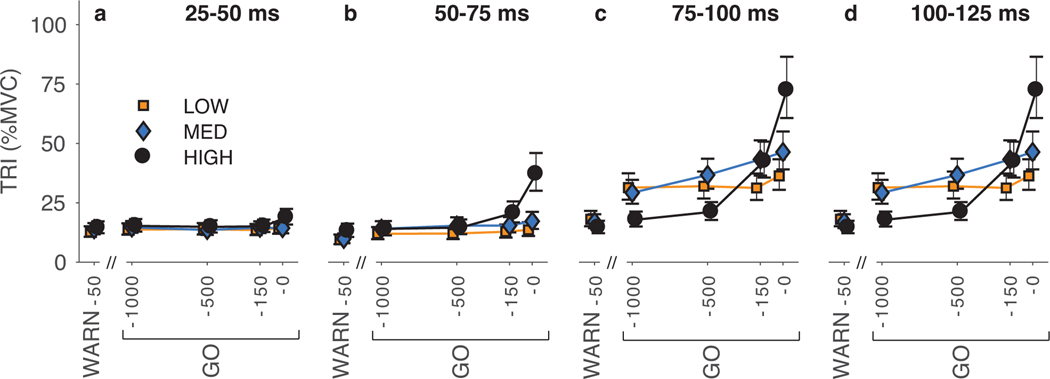
Across all participants, TRI responses to probe perturbations applied prior to the development of a motor plan (WARN-50) were consistent for the three temporal certainty conditions. There were no significant differences across certainty conditions for all time windows less than 100 ms (all p>0.56; Fig. 3a–c). The TRI response to the WARN-50 perturbation was larger in the LOW than the HIGH certainty condition for the 100–125-ms time window (207%, CI=[137%, 313%], p=0.021; Fig. 3d). This resulted from a small influence of certainty condition on the duration of the muscle response, observed in some participants.
Fig. 3.
The magnitude of the average TRI muscle response elicited by a perturbation is affected by temporal certainty condition and probe perturbation time. Responses are quantified in four time windows: (a) 25–50 ms, (b) 50–75 ms, (c) 75–100 ms, and (d) 100–125 ms as the fitted mean ± standard error from the statistical analysis. Differences in the response magnitude are most notable beginning in the period from 75–100 ms following the perturbation. Some differences are seen from 50–75 ms. See Results section for statistical comparisons.
Temporal certainty of the GO cue had minimal influence on the magnitude of the TRI muscle response in the first 75 ms following the perturbation. The magnitude of the TRI response in the 25–50-ms time window did not differ across temporal certainty condition (all p>0.19) or probe perturbation times (all p>0.44; Fig. 3a). With one exception, there was no statistically significant effect of certainty condition on the TRI response in the 50–75-ms time window (all p>0.066; Fig. 3b). The exception was a larger response to the GO-0 perturbation in the HIGH compared to the MED (216%, CI=[161%, 288%], p<0.001) and LOW (273%, CI=[202%, 370%], p<0.001) certainty conditions. This likely reflects anticipation of the GO cue in the HIGH certainty condition.
Temporal certainty of the GO cue influenced the magnitude of the average TRI muscle response approximately 75 ms after the probe perturbation was applied, a time consistent with when triggered responses have been reported previously (Crago et al. 1976; Hammond 1956). By applying probe perturbations at various times relative to the GO cue, we were able to characterize the development of triggered responses. In the 75–100-ms time window, we found differences in the average TRI muscle response across temporal certainty conditions and probe perturbation times (Fig. 3c). In the LOW certainty condition, the magnitude of the TRI response remained constant in advance of the GO cue (all p~1 for comparisons between consecutive probe perturbations and between GO-1000 and GO-0; Fig. 3c). In contrast, in the MED certainty condition, responses increased steadily in advance of the GO cue such that the response to GO-0 was larger than GO-1000 (159%, CI=[123%, 205%], p=0.016) but there was not a significant difference between consecutive probe perturbations (all p>0.85). In the HIGH certainty condition, a change in response magnitude was not observed until just prior to the GO cue. There was no significant difference in the response to GO-1000 and GO-500 probe perturbations (p~1), but the TRI response increased for the GO-150 (201% the response to GO-500, CI=[152%, 265%], p<0.001) and GO-0 (170% the response to GO-150, CI=[129%, 226%], p=0.008) probe perturbations. The effect of certainty condition on the development of the average TRI response from the GO-1000 to the GO-0 probe perturbations in the 100–125-ms time window was similar to the 75–100-ms time window. Although, in the 100–125-ms time window there was a notable increase in the average magnitude of the response for the LOW and MED certainty conditions, and for the GO-150 perturbation in the HIGH certainty condition. (Fig. 3d).
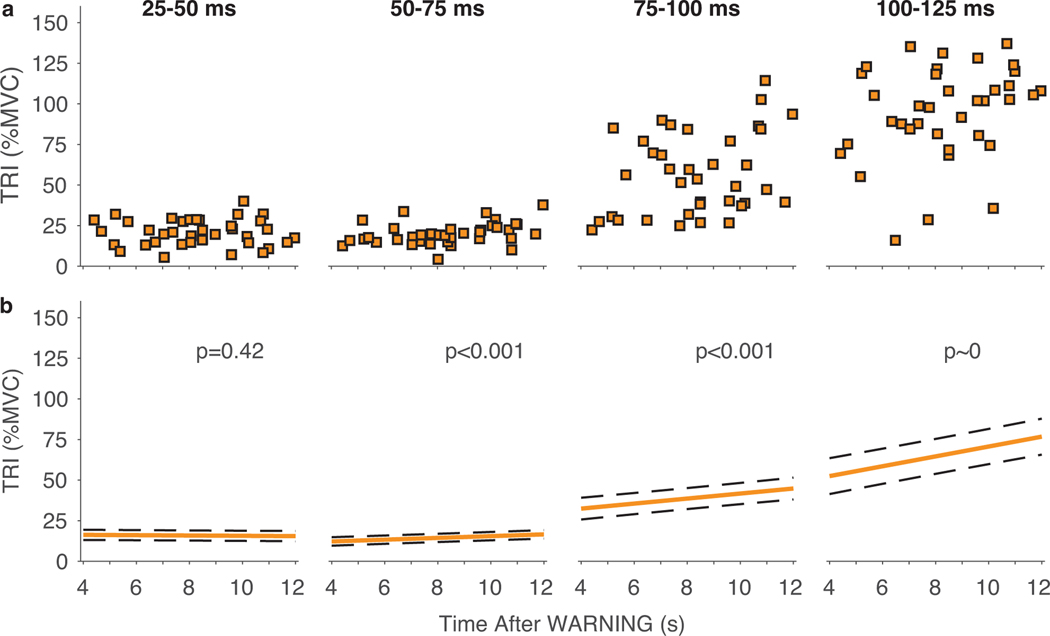
In addition to investigating the influence of uncertainty on the motor response elicited by a probe perturbation, we evaluated how the time between the WARNING cue and the probe influenced the elicited response at the highest level of uncertainty (Fig. 4a). This allowed us to describe how the magnitude of the TRI muscle response changed with the actual time available for movement preparation in contrast to our primary analysis which allowed us to describe how the average response to a perturbation changed with uncertainty in when a cue to move would arrive. For the HIGH certainty condition, there was a fixed relationship between the WARNING and GO cues, so this analysis would replicate the results shown in Fig. 3. In contrast, the relationship between the time available for movement preparation and the probe perturbation times was highly variable in the LOW certainty condition. The average magnitude of the response slowly increased with the amount of time available following the WARNING cue beginning in the 50–75-ms time window (Fig. 4b). While the magnitude of the average TRI response remained constant in advance of the GO cue when probe perturbations were referenced to the GO cue (Fig. 3), this analysis demonstrates that the response increased slowly as the time for preparation approached the longest time at which a perturbation could be expected.
Fig. 4.
(a) Representative data for a typical participant in the LOW certainty condition. Data are the magnitude of the TRI muscle response elicited by the probe perturbations in individual trials. Responses are quantified in four time windows: 25–50 ms, 50–75 ms, 75–100 ms, and 100–125 ms. All responses are plotted against the time after the WARNING when the probe perturbation was delivered. (b) Modeled group results across all subjects. Solid lines represent the mean response for the modeled fixed effects. Dashed lines represent the confidence intervals on these effects. P-values are for the slope of the relationship between the TRI response magnitude and time between the WARNING cue and perturbation onset.
We verified that differences in TRI muscle responses were not due to differences in the pre-activation level of the muscle. To do this, we compared the magnitude of background EMG across experimental conditions. For the group of participants, there were no significant differences across temporal certainty condition or probe perturbation time (all p>0.87).
The probability of an early response changes with perturbation certainty
We observed activation of the SCM muscles during the ballistic volitional reaches. Across the group of participants, SCM muscle activity was elicited in 84% of voluntary reaches within 0.5 s of the GO cue. The latency of SCM muscle activity was often faster than the threshold of 120 ms that is commonly used to detect the early release of a motor plan (Carlsen et al. 2011; Valls-Sole et al. 2008). SCM onset was less than 120 ms in 27% of all recorded voluntary reaches; percentages ranged from 11–63% across participants. All participant-specific detection thresholds were less than 120 ms, ranging from 78–111 ms. These thresholds represent the fifth percentile of the voluntary SCM latencies recorded for each participant, as described in the Methods.
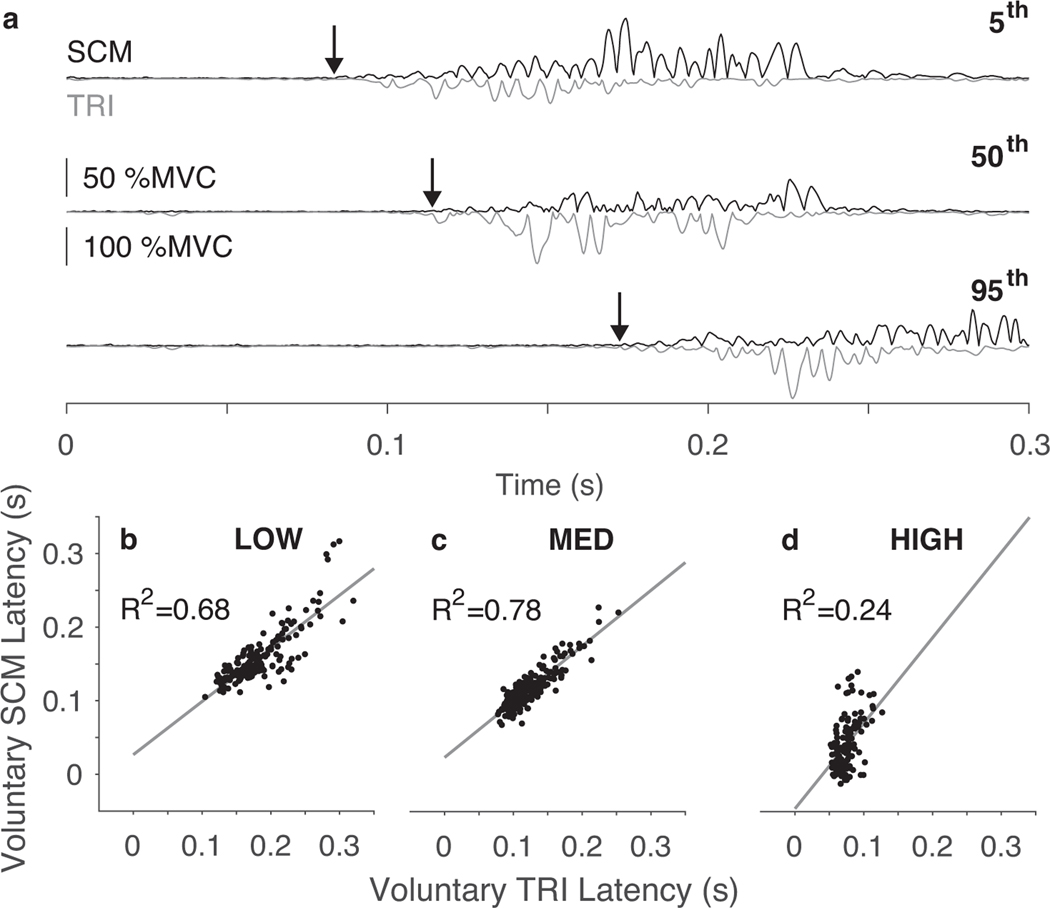
SCM activity observed during voluntary reaches was linked to the ballistic elbow extension motor plan. Individual trials show the onsets of SCM and TRI muscle activity were closely related across the distribution of SCM latencies observed across all voluntary reach trials (Fig. 5a). There was a linear relationship between the onset of activity in the SCM and TRI muscles in the MED and LOW certainty conditions (Fig. 5b, c). Across participants, the average R2 for both conditions was 0.68. We did not observe a strong relationship in the HIGH certainty condition, since there was little variation in onset times of the TRI muscle (Fig. 5d); the average R2 across participants was 0.36.
Fig. 5.
The SCM muscle activity was linked to the ballistic elbow extension movement during voluntary reaches. (a) SCM EMG (top trace) and TRI EMG (bottom trace) corresponding to individual trials at the fifth, 50th, and 95th percentile of voluntary SCM latencies observed in the MED certainty condition for a typical participant. (b) For a representative participant, SCM latency was linearly related to TRI muscle latency on a trial-by-trial basis for all voluntary reaching trials in the (b) LOW and (c) MED certainty conditions, but not in the (d) HIGH certainty condition.
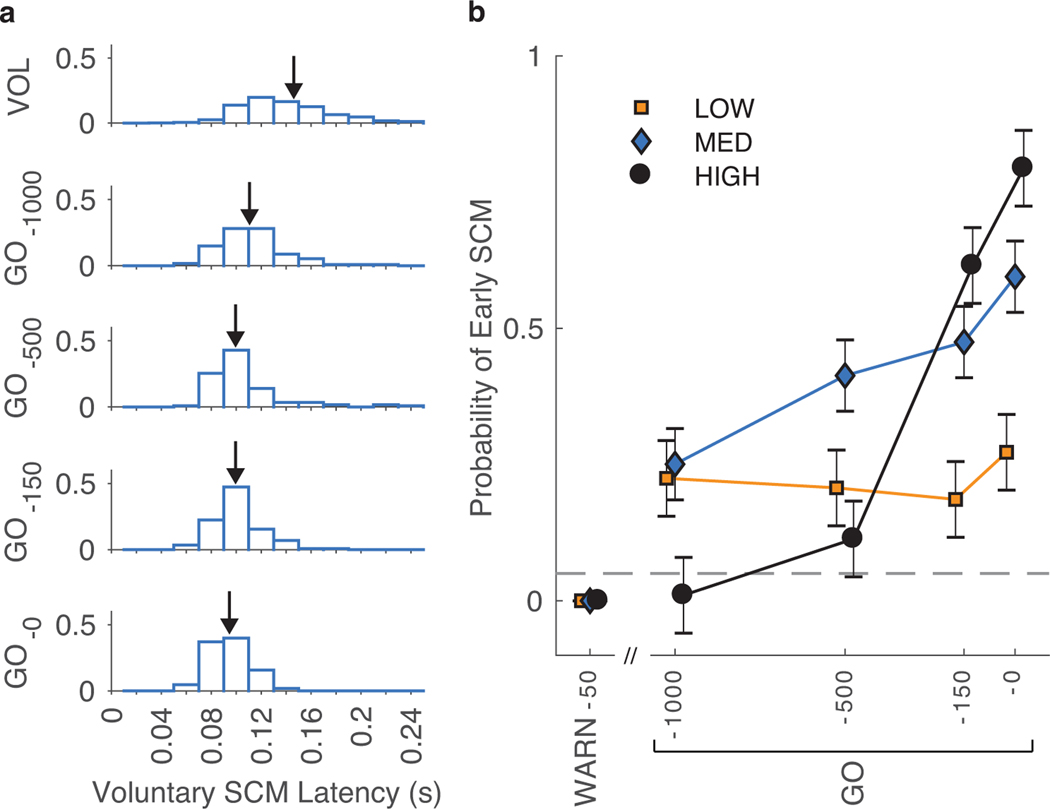
Perturbations often elicited SCM activity that was earlier than in the voluntary reaches. However, the timing of SCM onset depended strongly on when the perturbation arrived relative to the GO cue (Fig. 6a), and the certainty of when the GO cue would be given. We summarized the change in SCM onset latency by quantifying the probability of observing early SCM activity in perturbation trials relative to the voluntary reaches using the participant-specific latency thresholds for SCM activity. This probability of observing early SCM activity in the perturbation trials (Fig. 6b) evolved with a time course similar to the average TRI muscle activity in the 75–100-ms time window (shown in Fig. 3c). In the LOW certainty condition the probability of early SCM responses remained constant across all perturbation time points (all p~1). In the MED certainty condition, the probability increased steadily in advance of the GO cue with a significant increase from GO-1000 to GO-0 (p<0.0001), but no significant difference between consecutive probe perturbations (all p>0.30). In the HIGH certainty condition, the probability increased sharply between the GO-500 and GO-150 probe perturbations (Dprobability=0.50, CI=[0.35, 0.65], p~0), but there was no significant difference in the probability between the remaining consecutive probe perturbations (p>0.30). When the probe perturbation was applied prior to the instructions to prepare the elbow extension movement (WARN-50), early SCM activity was observed in only a single trial across all participants and certainty conditions.
Fig. 6.
(a) For a typical participant, the latency of SCM muscle activity was shifted earlier than voluntary reaches as probe perturbations were applied closer to the GO. Arrows point to the median SCM latency for each probe perturbation time. (b) The probability of early SCM muscle activity was affected by temporal certainty condition and probe perturbation time. In each temporal certainty condition, the probability of early SCM activity evolved across probe perturbation times similar to the development of the average TRI muscle response from 75–100 ms following the perturbation. The detection threshold for early SCM activity was set from voluntary reaching trials in the MED certainty condition at 0.05 (dashed line). Note: The WARN-50 probe perturbation was delivered prior to the beginning of motor planning, and the probability of early SCM activity was zero for all certainty conditions as defined in the Methods section. Data are the fitted mean ± standard error. See Results section for statistical comparisons.
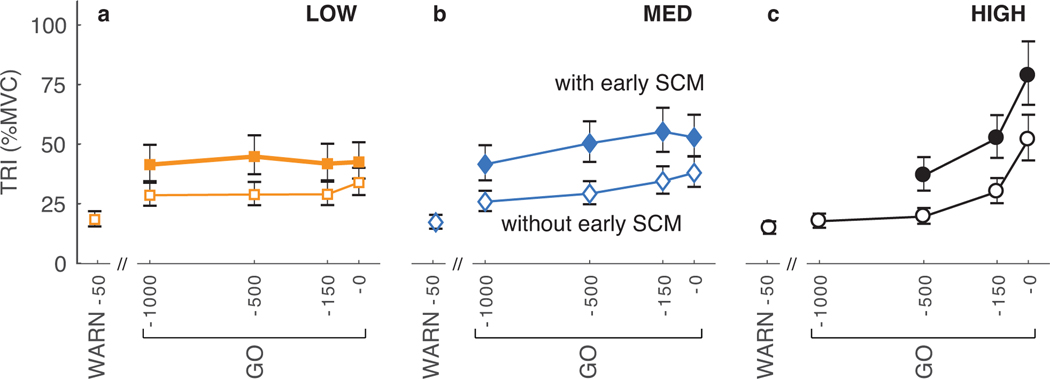
The early onset of SCM activity coincided with an increased magnitude of the TRI response in the probe trials, consistent with our finding that the SCM and TRI latencies were closely linked in the volitional motor plan (Fig. 7). We were unable to reliably detect the onset of TRI muscle activity related to only the planned reach because the probe perturbation also elicited a strong stretch reflex in this muscle. However, our observation of the coupling between SCM and TRI onset during voluntary reaches suggests that perturbation trials with an early onset of SCM activity should also have an early onset of the TRI activity related to the planned movement. This early onset for the TRI motor plan should be reflected in a larger TRI magnitude during the fixed time windows used for our analysis. We compared TRI magnitude in the 75–100-ms time window for responses with and without early SCM activity; this window was selected as it is when triggered responses became most notable (Fig. 3). The average TRI magnitude of responses with early SCM activity was significantly greater than the average magnitude of responses without early SCM activity for all but one possible comparison. Even the least significant change (MED certainty, GO-0) had a TRI magnitude with early SCM activity that was 139% (CI=[119%, 162%], p=0.001) of that without early SCM activity. The exception was at GO-0 in the LOW certainty condition (p=0.25).
Fig. 7.
TRI muscle responses elicited with early SCM muscle activity (filled markers) were larger than those responses without early SCM muscle activity (unfilled markers) for all temporal certainty conditions (a-c) and probe perturbation times preceding the GO cue, in the 75–100-ms time window. Larger TRI responses are consistent with the instructed ballistic reach. Data are the fitted mean ± standard error. The WARN-50 probe perturbation was not included in the analysis of SCM muscle activity but the average magnitude of the TRI response elicited is shown for reference. See Results section for statistical comparisons.
Discussion
This study investigated how uncertainty in the time when a proprioceptive cue to move will arrive influences the preparation and release of triggered responses. Our results demonstrate that uncertainty influences the time course of motor planning, and how the plan is triggered by a perturbation. We studied these phenomena using two muscles that were part of the motor plan: the TRI muscle stretched by the perturbation and the SCM muscle, which was not stretched but was activated in near synchrony with the TRI. Using both muscles allowed us to distinguish motor activity related to the plan from stretch reflex activity in the TRI. We focused mainly on TRI activity in the 75–100-ms time window after perturbation onset, which is when uncertainty-dependent changes in the TRI activity were first noted. We found that uncertainty in when a perturbation will arrive strongly influences how a motor response is prepared, and the efficacy of triggering that response by a postural perturbation. With uncertainty, long hold times also influenced the magnitude of the motor response. When timing was certain, the motor plan was prepared within 150 ms of the expected disturbance, and consistently released earlier by a perturbation than could be done voluntarily. In contrast, less predictable stimuli led to much earlier planning but also a lower probability of releasing the plan early. These effects of uncertainty on the motor plan were closely related to the average magnitude of the TRI activity in the 75–100-ms time window after perturbation onset. These results clarify how uncertainty in when a proprioceptive cue to move will arrive influences the planning and early release of triggered responses, demonstrating an effect that is very similar to previous reports on the planning of volitional movements.
Limitations of approach
One limitation of the study is our ability to interpret the response to the HIGH certainty GO-0 probe perturbation. Though we included the data points in our presentation of the results, this response was considerably larger than all other responses. This was likely because participants could anticipate the movement cue. Although the average TRI muscle activity was matched before perturbation onset and 25–50 ms after perturbation onset, the average response was larger in the 50–75-ms time window only for the GO-0 probe perturbation applied in the HIGH certainty condition. We cannot distinguish premature voluntary movement in anticipation of the cue from early triggering of the prepared movement or modulation of the response elicited by the probe perturbation. Hence, we refrain from discussing or interpreting these data below.
In many previous studies of voluntary motor planning, the latency of SCM muscle activity has been used as a physiological indicator of the brainstem-mediated contributions to the release of a prepared movement. Animal studies have demonstrated the contributions of brainstem nuclei to the startle response (Davis et al. 1982), and human studies have shown that the SCM muscle is activated as part of the generalized flexion response to a startling stimulus (Brown et al. 1991; Landis and Hunt 1939). In the presence of a motor plan, a startling acoustic stimulus can evoke the early release of the prepared movement along with activation of the SCM muscle within 120 ms (Carlsen et al. 2004; Carlsen et al. 2003; Carlsen et al. 2011). It has been suggested this SCM activity is related to the startle response, and that the early release of a prepared motor plan is therefore mediated by the brainstem (Carlsen et al. 2004; Valls-Sole et al. 1999; Valls-Sole et al. 1995). Multiple sensory stimuli, not solely auditory, have been shown to activate regions of the brainstem involved in the response to startle (Yeomans et al. 2002), and also to trigger brainstem-mediated postural responses in cats (Stapley and Drew 2009). We previously used SCM muscle activity as an indicator of startle to investigate the involvement of brainstem pathways in the response to postural perturbations in humans (Ravichandran et al. 2013). Since the SCM was active as part of the voluntary reach in our current protocol, we could not unambiguously interpret its activation in the context of startle-evoked brainstem activity. However, the approach we used, recording from a muscle involved in the motor plan but not influenced by the postural perturbation, was useful for distinguishing preparation-related activity from the stabilizing response of the stretch reflex.
Uncertainty influences the development of triggered responses
Our results demonstrate that uncertainty in when a proprioceptive cue to move will arrive influences the timing and magnitude of the average muscle response elicited by a perturbation. This is consistent with previous reports in the literature. We found the influence of temporal uncertainty on the average TRI response first becomes notable in the 75–100-ms time window. This is consistent with previous studies of the stretch reflex that noted preparation-related changes in the stretched muscle EMG occurring ~75 ms after perturbation onset (Crago et al. 1976; Hammond 1956; Ravichandran et al. 2013). We also found that introducing uncertainty in the planned motor action alters the response elicited by a perturbation. When perturbation frequency (Rothwell et al. 1980), direction (Crago et al. 1976), or intensity (Aimola et al. 2014) have been varied, the magnitude of the response elicited by a perturbation has been shown to be altered. The previous work by Rothwell and colleagues (Rothwell et al. 1980) is the closest to ours. They introduced uncertainty by varying the predictability of when the perturbation would arrive, and found that the average magnitude of the response in the stretched muscle was larger for more predictable perturbations. Our results support these findings, but extend them in two important ways. First, we used separate proprioceptive stimuli to cue the prepared movement and to elicit an involuntary response in the stretched muscle. Second, we recorded from the SCM muscle, which was part of the voluntary motor plan in our task yet not stretched by the proprioceptive stimuli. This allowed us to identify when the motor plan was released by monitoring a muscle that did not also have a stretch reflex response to our probe perturbation. These changes allowed us to describe the influence of uncertainty on the development and early release of triggered responses. In the condition with the highest level of uncertainty, we found a slow increase in the TRI response as the time available for preparation approached the longest time at which a perturbation could be expected. This result is consistent with previous data for the 75–100-ms time window, (Forgaard et al. 2016) and provides additional insight into how motor preparation evolves when there is uncertainty in when a perturbation will arrive.
The influence of uncertainty on the development of triggered responses is similar to the influence of uncertainty on the preparation of voluntary movements. The preparation of voluntary movements has been studied using startling acoustic stimuli capable of eliciting a planned movement involuntarily. These studies have quantified the early release of the movement to indicate when a motor plan is prepared in advance of a movement cue (Carlsen and Mackinnon 2010; MacKinnon et al. 2013). For each of the three temporal certainty conditions used in our study, we observed a time course for early movement onset and the development of the average TRI response that resembled the time course of volitional movement planning shown for similar movement uncertainty conditions cued by visual (Carlsen and Mackinnon 2010) or auditory (MacKinnon et al. 2013) stimuli. First, the preparation of a volitional motor plan was invariant in the time preceding an unpredictable movement cue delivered 4–12 s after a warning cue (MacKinnon et al. 2013). In our LOW certainty condition, there was a similar invariance in the probability of observing early SCM activity and in the magnitude of the average TRI response in advance of the cue to move. Second, the preparation of a volitional motor plan increased over a span of 1500 ms before a predictable movement cue delivered 2–3 s after a warning cue (Carlsen and Mackinnon 2010). This was similar to our observations in the MED certainty condition, for which the probability of observing early SCM activity and the average magnitude of the TRI muscle activity increased in the 1500-ms period preceding the proprioceptive cue to move. Finally, the preparation of a volitional motor plan was delayed when the cue to move could be accurately predicted (Carlsen and Mackinnon 2010), much like we found in our HIGH certainty condition. Thus, it seems that the motor planning we observed is similar to that reported in previous studies of volitional movement, and that this planning influenced perturbation-triggered responses in a manner similar to the early release of voluntary movements triggered by a startling acoustic stimulus. These results may suggest a shared planning process for voluntary and involuntary movement control.
Context-dependent timing of the response to perturbation
Uncertainty in when a proprioceptive cue would arrive influenced the average time when a motor plan was triggered by a perturbation, as measured by the probability of observing early activity in the SCM. When the motor plan was released directly influenced the magnitude of the observed TRI response beginning at approximately 75 ms. TRI activity was significantly larger when SCM activity was detected early. We believe that this provides evidence that the modulation of the TRI activity triggered by the perturbation can be attributed to an early release of the prepared motor response. This is in distinct contrast to the many studies reporting stretch reflex modulation in the absence of a prepared movement, including that which occurs with changes in background muscle activation (Matthews 1986) and perturbation magnitude (Lewis et al. 2005), or which serves to regulate limb stability (Doemges and Rack 1992; Krutky et al. 2010) or the dynamics of interjoint coupling (Kurtzer et al. 2008; Pruszynski et al. 2011). These examples have all been suggested to represent amplitude modulation of the stretch reflex. Our findings suggest that modulation coupled to the existence of a planned movement arises from a shift in movement onset rather than a change in the gain of the stretch reflex.
Our results point to the significance of movement preparation for the response to postural perturbations. A disturbance to arm posture can elicit muscle responses aimed to oppose the disturbance or to initiate a planned motor action. However, uncertainty in perturbation timing influences the preparation of a motor plan and the timing of its release. Overall, we suggest that shared planning processes may be an important link between voluntary movement control and the sophisticated involuntary responses elicited by postural perturbations.
Acknowledgements:
The authors would like to thank Tim Goetz-Haswell for his technical expertise. This work was supported by the National Institutes of Health Grants R01 NS05813 and T32 EB009406.
Contributor Information
Rosalind L. Heckman, Department of Biomedical Engineering, Northwestern University, Evanston, IL, USA; Shirley Ryan AbilityLab, 355 E Erie St, Chicago, IL 60611, USA; Department of Physical Therapy, Creighton University, Omaha, NE, USA.
Eric J. Perreault, Department of Biomedical Engineering, Northwestern University, Evanston, IL, USA Shirley Ryan AbilityLab, 355 E Erie St, Chicago, IL 60611, USA; Department of Physical Medicine and Rehabilitation, Northwestern University, Chicago, IL, USA.
References
- Aimola E, Valle MS, Casabona A (2014) Effects of predictability of load magnitude on the response of the Flexor Digitorum Superficialis to a sudden fingers extension PLoS One 9:e109067 doi: 10.1371/journal.pone.0109067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown P, Rothwell JC, Thompson PD, Britton TC, Day BL, Marsden CD (1991) New observations on the normal auditory startle reflex in man Brain 114 ( Pt 4):1891–1902 [DOI] [PubMed] [Google Scholar]
- Carlsen A, Chua R, Inglis JT, Sanderson DJ, Franks IM (2004) Prepared movements are elicited early by startle J Mot Behav 36:253–264 doi: 10.3200/JMBR.36.3.253-264 [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM (2003) Startle response is dishabituated during a reaction time task Exp Brain Res 152:510–518 doi: 10.1007/s00221-003-1575-5 [DOI] [PubMed] [Google Scholar]
- Carlsen AN, Mackinnon CD (2010) Motor preparation is modulated by the resolution of the response timing information Brain Res 1322:38–49 doi: 10.1016/j.brainres.2010.01.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen AN, Maslovat D, Lam MY, Chua R, Franks IM (2011) Considerations for the use of a startling acoustic stimulus in studies of motor preparation in humans Neurosci Biobehav Rev 35:366–376 doi: 10.1016/j.neubiorev.2010.04.009 [DOI] [PubMed] [Google Scholar]
- Colebatch JG, Gandevia SC, McCloskey DI, Potter EK (1979) Subject instruction and long latency reflex responses to muscle stretch J Physiol 292:527–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crago PE, Houk JC, Hasan Z (1976) Regulatory Actions of Human Stretch Reflex J Neurophysiol 39:925–935 [DOI] [PubMed] [Google Scholar]
- Cui R, MacKinnon CD (2009) The effect of temporal accuracy constraints on movement-related potentials Exp Brain Res 194:477–488 doi: 10.1007/s00221-009-1725-5 [DOI] [PubMed] [Google Scholar]
- Davis M, Gendelman DS, Tischler MD, Gendelman PM (1982) A primary acoustic startle circuit: lesion and stimulation studies J Neurosci 2:791–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doemges F, Rack PM (1992) Task-dependent changes in the response of human wrist joints to mechanical disturbance J Physiol 447:575–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgaard CJ, Franks IM, Maslovat D, Chin L, Chua R (2015) Voluntary reaction time and long-latency reflex modulation J Neurophysiol 114:3386–3399 doi: 10.1152/jn.00648.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forgaard CJ, Franks IM, Maslovat D, Chua R (2016) Perturbation Predictability Can Influence the Long-Latency Stretch Response PLoS One 11:e0163854 doi: 10.1371/journal.pone.0163854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond PH (1956) The influence of prior instruction to the subject on an apparently involuntary neuro-muscular response J Physiol 132:17–18P [PubMed] [Google Scholar]
- Honeycutt CF, Perreault EJ (2012) Planning of ballistic movement following stroke: insights from the startle reflex PLoS One 7:e43097 doi: 10.1371/journal.pone.0043097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models Biom J 50:346–363 doi: 10.1002/bimj.200810425 [DOI] [PubMed] [Google Scholar]
- Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ (1995) Self-initiated versus externally triggered movements [DOI] [PubMed] [Google Scholar]
- I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects Brain 118:913–933 [DOI] [PubMed] [Google Scholar]
- Jankelowitz SK, Colebatch JG (2002) Movement-related potentials associated with self-paced, cued and imagined arm movements Exp Brain Res 147:98–107 doi: 10.1007/s00221-002-1220-8 [DOI] [PubMed] [Google Scholar]
- Krutky MA, Ravichandran VJ, Trumbower RD, Perreault EJ (2010) Interactions between limb and environmental mechanics influence stretch reflex sensitivity in the human arm J Neurophysiol 103:429–440 doi: 10.1152/jn.00679.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzer IL, Pruszynski JA, Scott SH (2008) Long-latency reflexes of the human arm reflect an internal model of limb dynamics Curr Biol 18:449–453 doi: 10.1016/j.cub.2008.02.053 [DOI] [PubMed] [Google Scholar]
- Landis C, Hunt WA (1939) The Startle Pattern. Farrar & Rinehart, New York [Google Scholar]
- Lewis GN, Perreault EJ, MacKinnon CD (2005) The influence of perturbation duration and velocity on the long-latency response to stretch in the biceps muscle Exp Brain Res 163:361–369 doi: 10.1007/s00221-004-2182-9 [DOI] [PubMed] [Google Scholar]
- MacKinnon CD, Allen DP, Shiratori T, Rogers MW (2013) Early and unintentional release of planned motor actions during motor cortical preparation PLoS One 8:e63417 doi: 10.1371/journal.pone.0063417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews PB (1986) Observations on the automatic compensation of reflex gain on varying the preexisting level of motor discharge in man J Physiol 374:73–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault EJ, Chen K, Trumbower RD, Lewis G (2008) Interactions with compliant loads alter stretch reflex gains but not intermuscular coordination J Neurophysiol 99:2101–2113 doi: 10.1152/jn.01094.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Lillicrap TP, Scott SH (2009) Temporal evolution of “automatic gain-scaling” J Neurophysiol 102:992–1003 doi: 10.1152/jn.00085.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszynski JA, Kurtzer I, Nashed JY, Omrani M, Brouwer B, Scott SH (2011) Primary motor cortex underlies multi-joint integration for fast feedback control Nature 478:387–390 doi: 10.1038/nature10436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravichandran VJ, Honeycutt CF, Shemmell J, Perreault EJ (2013) Instruction-dependent modulation of the long-latency stretch reflex is associated with indicators of startle Exp Brain Res 230:59–69 doi: 10.1007/s00221-013-3630-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell JC, Traub MM, Marsden CD (1980) Influence of voluntary intent on the human long-latency stretch reflex Nature 286:496–498 [DOI] [PubMed] [Google Scholar]
- Stapley PJ, Drew T (2009) The pontomedullary reticular formation contributes to the compensatory postural responses observed following removal of the support surface in the standing cat J Neurophysiol 101:1334–1350 doi: 10.1152/jn.91013.2008 [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Kumru H, Kofler M (2008) Interaction between startle and voluntary reactions in humans Exp Brain Res 187:497–507 doi: 10.1007/s00221-008-1402-0 [DOI] [PubMed] [Google Scholar]
- Valls-Sole J, Rothwell JC, Goulart F, Cossu G, Munoz E (1999) Patterned ballistic movements triggered by a startle in healthy humans J Physiol 516 ( Pt 3):931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valls-Sole J, Sole A, Valldeoriola F, Munoz E, Gonzalez LE, Tolosa ES (1995) Reaction time and acoustic startle in normal human subjects Neurosci Lett 195:97–100 [DOI] [PubMed] [Google Scholar]
- Yang L, Michaels JA, Pruszynski JA, Scott SH (2011) Rapid motor responses quickly integrate visuospatial task constraints Exp Brain Res 211:231–242 doi: 10.1007/s00221-011-2674-3 [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Li L, Scott BW, Frankland PW (2002) Tactile, acoustic and vestibular systems sum to elicit the startle reflex Neurosci Biobehav Rev 26:1–11 [DOI] [PubMed] [Google Scholar]