Abstract
Simian virus 40 large T antigen is a multifunctional protein which has been shown to modulate the expression of genes transcribed by RNA polymerase I (Pol I), II, and III. In all three transcription systems, a key step in the activation process is the recruitment of large T antigen to the promoter by direct protein-protein interaction with the TATA binding protein (TBP)-TAF complexes, namely, SL1, TFIID, and TFIIIB. However, our previous studies on large T antigen stimulation of Pol I transcription also revealed that the binding to the TBP-TAFI complex SL1 is not sufficient to activate transcription. To further define the molecular mechanism involved in large T antigen-mediated Pol I activation, we examined whether the high-mobility group box-containing upstream binding factor (UBF) plays any role in this process. Here, using cell labeling experiments, we showed that large T antigen expression induces an increase in UBF phosphorylation. Further biochemical analysis demonstrated that UBF is phosphorylated by a kinase activity that is strongly associated with large T antigen, and that the carboxy-terminal activation domain of UBF is required for the phosphorylation to occur. Using in vitro reconstituted transcription assays, we demonstrated that the inability of alkaline phosphatase treated UBF to efficiently activate transcription can be rescued by large T antigen. Moreover, we showed that large T antigen-induced UBF phosphorylation promotes the formation of a stable UBF-SL1 complex. Together, these results provide strong evidence for an important role for the large T antigen-associated kinase in mediating the stimulation of RNA Pol I transcription.
Simian virus 40 (SV40), a small DNA virus belonging to the polyomavirus family, has been extensively studied as a model system for understanding the regulation of mammalian cell growth and proliferation. The viral gene A product, the large tumor antigen (large T antigen), has a crucial role in viral DNA replication and disruption of host cell growth control (13, 22). In addition, large T antigen can transform cultured cells and induce tumors in rodents (7, 18, 33, 38, 55). The oncogenic properties of large T antigen are mediated, in part, by direct interactions with a number of cellular proteins, including the tumor suppressor gene products pRb and p53 (17, 19, 41, 51). Large T antigen has also been found to associate with cellular kinases; however, the specific roles and, in many cases, the identity of these kinases have not yet been defined (1, 27, 46, 53). In addition, large T antigen can act as a transcriptional activator by stimulating the transcription of a variety of viral and cellular genes (2, 8, 10, 36, 43, 52). Although large T antigen has a specific as well as a nonspecific DNA binding activity, this function is not essential for transcriptional activation (23, 37). Rather, large T antigen appears to mediate its transactivation function through protein-protein interactions with a number of transcription factors such as TEF-1 (4), sp1 (29), p300 and CREB binding protein (21, 42), TFIIA (15), and TATA binding protein (TBP)-TAF complexes (15, 16, 63). Recent studies have suggested that large T antigen is capable of activating simple RNA polymerase II (Pol II) promoters consisting of a TATA box or an initiator element with at least one upstream transcription factor binding site (25). By using a TAFII250 temperature-sensitive cell line, it has been shown that large T antigen copurifies with the TBP-TAFII complex TFIID and performs a TAF-like function by substituting for TAFII250 in RNA Pol II transcription at the cyclin A promoter (14).
Recently, large T antigen has also been shown to activate the RNA Pol III U6 RNA gene (16). The mechanism of this activation appears to involve specific interactions with the TBP-containing factor BRF (TFIIB-related factor) as well as with two of the three TAFs in the Pol III-specific small nuclear RNA-activating protein complex (16).
We have recently shown that large T antigen, in addition to activating Pol II-transcribed genes, stimulates RNA Pol I transcription (63). Our studies revealed that large T antigen is recruited to the rRNA promoter through direct protein-protein interactions with the TBP-TAFI complex, SL1 (63). Interestingly, our data also indicated that the binding of large T antigen to SL1 is not sufficient for stimulating Pol I transcription, suggesting that other functional steps are required for this process to occur (63).
Taken together, the experimental results from all three systems strongly suggest that transcriptional activation by large T antigen is mediated, in part, by its ability to interact with diverse TBP-TAF complexes. However, the mechanism by which these multitudes of protein interactions regulate promoter activity remains to be elucidated. As large T antigen is recruited to the Pol I transcription complex, we were intrigued by the possibility that it influences the activity of another Pol I factor within this complex, such as the upstream binding factor (UBF). UBF is a high-mobility group (HMG) box-containing protein which binds to the upstream control element (UCE) and core regions of the rDNA promoter and recruits SL1 through protein-protein interactions (34, 49). Functional studies have indicated that the carboxy-terminal tail of UBF is necessary for transactivation (35). In addition, the carboxy-terminal domain contains several serine residues that can be phosphorylated both in vivo and in vitro, and work from several laboratories has shown a correlation between UBF phosphorylation and its transcriptional activity. These observations suggest that the phosphorylation state of UBF may modulate its transcriptional activity (26, 48, 60). Although it has been previously demonstrated that serine residues on UBF can be phosphorylated both in vivo and in vitro, the role of these phosphorylated residues in the regulation of UBF activity has not been elucidated.
In this work, we focus our analysis on the functional relationship between large T antigen and UBF. First, we show that expression of large T antigen induces UBF phosphorylation in primary cell cultures. Next, we demonstrate that the kinase activity that phosphorylates UBF is strongly associated with purified large T antigen. Furthermore, two-dimensional (2D) phosphopeptide analysis demonstrates that large T antigen phosphorylates UBF in vitro at sites that correspond to those observed in cells expressing large T antigen. Then, using an in vitro protein-protein interaction assay, we provide evidence that the phosphorylation on UBF promotes the formation of a stable protein complex between UBF and SL1. Finally, using in vitro reconstituted transcription assays, we show that phosphorylation of UBF by the large T antigen-associated kinase activity enhances UBF-dependent transcriptional activation.
MATERIALS AND METHODS
Plasmids.
The following plasmids were constructed for expressing the various N-terminal flag epitope-tagged UBF deletion mutant proteins in Escherichia coli. pAF670C (encoding flag-UBF670C [human UBF amino acids {aa} 1 to 670]), pAF489C (encoding flag-UBF489C [human UBF aa 1 to 489]), pAF284C (encoding flag-UBF284C [human UBF aa 1 to 284]), pAF182N (encoding flag-UBF182N [human UBF aa 182 to 764]), and pAF284N (encoding flag-UBF284N [human UBF aa 284 to 764]) were generated by inserting into NdeI-EcoRI-digested pAED4-flag vector an NdeI-EcoRI-cleaved fragment derived from pTβ670C, pTβ489C, pTβ284C, pTβ182N, or pTβ284N, respectively. The constructs pTβ670C, pTβ489C, pTβ284C, pTβ182N, and pTβ284N have been described previously (35). Plasmid prHu3, which contains the human rRNA promoter and plasmids containing the human TAFIs and TBP have been described previously (11, 12, 40). The UBF mutant construct pUBF-at, which contains point mutations at several serine residues that are potential casein kinase II (CKII) target sites was obtained from R. Voit and I. Grummt (60).
Cell culture and labeling.
Human skin fibroblast cell lines Hs68 and Hs68-LT (gifts from A. Schönthal) were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS), penicillin (100 U/ml), and streptomycin (0.1 mg/ml) (Irvine Scientific). The cells were plated on a 10-cm-diameter dish at a density of ∼80% confluency at the start of phosphate deprivation. Phosphate deprivation was performed in sodium phosphate- and sodium pyruvate-free DMEM (GIBCO-BRL) containing 0.5% dialyzed FBS (GIBCO-BRL) for 2 h. After phosphate deprivation, cells were metabolically labeled with [32P]orthophosphate (ICN) at a concentration of 0.25 mCi/ml in phosphate-free medium containing 0.5% dialyzed serum for 6 h. After labeling, cells were rapidly washed twice with ice-cold phosphate-buffered saline and lysed in 1 ml of RIPA buffer (50 mM Tris-HCl [pH 7.9], 150 mM NaCl, 1 mM EDTA, 0.1% sodium dodecyl sulfate [SDS], 0.5% deoxylcholate, 1% Nonidet P-40 [NP-40]) containing protease inhibitors (final concentrations: 10 μg of aprotinin per ml, 1 μg of leupeptin per ml, 1 μg of pepstatin per ml, and 1 mM phenylmethylsulfonyl fluoride) and phosphatase inhibitors (final concentrations: 20 mM NaF, 20 mM β-glycerolphosphate, 1 mM sodium orthovanadate, 1 μM okadaic acid). Sf9 insect cells were propagated and maintained in Hink’s TNM-FH insect medium (JRH Biosciences) supplemented with 10% heat-inactivated FBS.
Protein expression and purification.
All the baculoviruses used in this study have been described previously (63). For protein expression using recombinant baculovirus, Sf9 cells were infected at a multiplicity of infection of 5 and harvested 42 to 48 h after infection. Protein lysates were prepared by sonication of infected cells in TM10 (50 mM Tris-HCl [pH 7.9], 12.5 mM MgCl2, 1 mM EDTA, 10% glycerol) containing 0.1 M KCl with protease inhibitors unless indicated otherwise. Proteins expressed in E. coli BL21 (Lyss) containing the appropriate expression plasmid were induced with 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) for 2 h at 37°C. Cell lysates were prepared by sonication of induced cells in TM10 buffer containing 0.1 M KCl. All UBF protein lysates were prepared in TM10 buffer containing 0.5 M KCl instead. Purification of UBF protein used for cross-linking has been described previously (3). For producing a UBF antigen column, 2 ml of UBF protein (0.5 mg/ml) was mixed with 1 ml of equilibrated Affi-Gel 10 (Bio-Rad), and coupling was performed at 4°C for 4 h. The reaction was then stopped by washing the beads in 0.2 M ethanolamine (pH 8.0). Polyclonal antibodies against UBF were produced by immunizing rabbits with full-length UBF protein and were affinity purified with a UBF-Affi-Gel 10 column as described by Harlow and Lane (30). Two hundred micrograms of purified antibodies was then cross-linked to 0.1 ml (packed volume) of protein A-Sepharose resin as described previously (30). RNA Pol I used in the reconstituted transcription reaction was prepared as followed: nuclear extracts from HeLa cells were first applied to a heparin-agarose column. Fractions eluted with KCl at 250 mM were pooled, dialyzed against TM (50 mM Tris-HCl [pH 7.9], 12.5 mM MgCl2, 1 mM EDTA, 20% glycerol) buffer containing 0.1 M KCl and fractionated on a Sepharose 300 (Pharmacia) gel filtration column. Active fractions were then loaded onto a Q-Sepharose column (Poros) equilibrated against TM containing 0.1 M KCl. Proteins were eluted with a salt gradient from 0.1 to 0.7 M KCl. The active fractions were pooled, dialyzed to 0.125 M KCl, aliquoted, and stored at −80°C. This RNA Pol I preparation contained no detectable UBF and SL1 activity. SL1 was purified from HeLa cells as previously described (3, 11). Protein concentration was determined with a Bradford assay kit (Bio-Rad).
Kinase assay.
Cell lysates containing the protein of interest (substrates) were first mixed with either anti-flag M2 beads or glutathione-Sepharose beads at 4°C for 1 h. Beads were then washed three times with RIPA buffer and once with alkaline phosphatase buffer (20 mM Tris-HCl [pH 8.8], 10 mM MgCl2). Approximately 200 ng of each immobilized protein was treated with 1.0 U of shrimp alkaline phosphatase at 30°C for 15 min in 100 μl of alkaline phosphatase buffer with proteinase inhibitors. After phosphatase treatment, the beads were incubated with dissociation buffer (50 mM Tris-HCl [pH 8.0], 250 mM LiCl, 1% SDS, 0.5% NP-40) at 4°C for 15 min followed by two washes each with RIPA buffer and protein kinase buffer (20 mM Tris-HCl [pH 8.0], 150 mM NaCl, 5 mM MgCl2, 0.5% NP-40). The kinase reaction was allowed to proceed at room temperature for 30 min in 100 μl of protein kinase buffer containing approximately 100 ng of purified large T antigen, acetylated bovine serum albumin (1 mg/ml), 1 μM cold ATP, 10 μCi of [γ-32P]ATP, 50 mM NaF, 1 mM sodium orthovanadate, and protease inhibitors. The kinase reaction was stopped by adding EDTA to 20 mM. Beads were washed with ice-cold RIPA buffer plus 50 mM NaF and 1 mM sodium orthovanadate four times before being boiled in SDS sample buffer. In some reaction mixtures, 0.1 U of CKII (Sigma) was used in place of purified large T antigen.
Determination of the stoichiometry of the in vitro phosphorylation of UBF.
About 100 ng of UBF immobilized on anti-flag M2 beads was subjected to in vitro phosphorylation in the presence of purified large T antigen as described above. After extensive washing, the reaction mixture was resolved by SDS-polyacrylamide gel electrophoresis (PAGE), and the gels were stained with Coomassie blue to further estimate the amount of UBF. The number of moles of UBF used was obtained from the estimated amount of UBF and its known molecular weight. After destaining, the gel was dried and the amount of incorporated [32P]phosphate was quantitated with a PhosphoImager. An aliquot of the diluted kinase reaction mixture (including 10 μCi of [γ-32P]ATP and 1 μM cold ATP) was spotted on a 3-MM Whatman paper and used as a reference for the quantitation. According to the quantitation data, the number of moles of phosphate being transferred to UBF was obtained. The stoichiometry of UBF phosphorylation was calculated as mole(s) of phosphate transferred per mole of UBF.
Tryptic phosphopeptide analysis.
Tryptic phosphopeptide analysis was performed essentially as described previously (5, 60). Briefly, labeled UBF was cut out from an SDS-polyacrylamide gel and eluted into solution with 50 mM ammonium bicarbonate. Following trichloroacetic acid precipitation, labeled UBF protein was oxidized and then digested with trypsin (sequencing grade; Promega) in 50 mM ammonium bicarbonate. Digested peptides were resolved by electrophoresis for 40 min at 1,000 V on thin-layer cellulose plates (EM Science) in pH 1.9 buffer in the first dimension, followed by ascending chromatography in isobutyric acid buffer in the second dimension.
In vitro transcription assay.
In vitro transcription reactions were carried out as described previously in the presence of α-amanitin (100 μg/ml) and 30 ng of template plasmid DNA, prHu3 (11, 40). In vitro-synthesized RNA was detected by S1 nuclease analysis using 5′-end-labeled single-strand DNA oligonucleotides. Transcriptions in the presence of wild type and mutant large T antigen (200 ng/reaction) were done as previously described (63).
Immunoprecipitation and Western blot analysis.
Whole-cell lysates prepared from [32P]orthophosphate labeled Hs68 and Hs68-LT cells were precleared by centrifugation at 15,000 × g for 30 min at 4°C. The lysates were then mixed with 15 μl of protein A-Sepharose beads cross-linked with anti-UBF antibodies (approximately 1 μg of affinity-purified α-UBF antibodies) for 2 h at 4°C with constant mixing on a nutator. Immunoprecipitated proteins were then washed four times with RIPA buffer, boiled in SDS sample buffer, and resolved by SDS-PAGE. After electrophoresis, proteins were transferred to nitrocellulose membranes and exposed to film. To detect the amount of UBF, the same membrane was probed with affinity-purified anti-UBF antibodies (0.1 μg/ml) followed by detection with alkaline-phosphatase conjugated secondary antibody (Promega) and nitroblue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolylphosphate toluidinium (BCIP) as substrates. For interaction studies with SL1, 200 ng of flag-tagged UBF expressed from recombinant baculovirus-infected Sf9 cells was immobilized on anti-flag M2 beads, washed three times with TM10–0.3 M KCl, and washed one time with TM10–0.1 M KCl. The immobilized UBF was incubated with 50 μl of an SL1-containing fraction (purified from HeLa cells) in TM10–0.1 M KCl plus 0.1% NP-40. After overnight incubation, beads were washed four times in binding buffer and eluted with 50 μl of BCO buffer (20 mM Tris-HCl [pH 8.0], 0.5 mM EDTA, 20% glycerol, 1 M KCl, 1% deoxylcholate) for 30 min at 4°C. Eluted proteins were then precipitated with trichloroacetic acid for 20 min on ice and collected by centrifugation at 15,000 × g for 20 min. Pellets were washed twice with ice-cold acetone and air dried. Samples were boiled in SDS sample buffer, resolved by SDS-PAGE, and transferred to nitrocellulose membranes. The SL1 subunits were detected by incubation with the appropriate primary antibodies and visualized by the alkaline-phosphatase detection method.
RESULTS
Expression of large T antigen in human fibroblasts induces UBF phosphorylation.
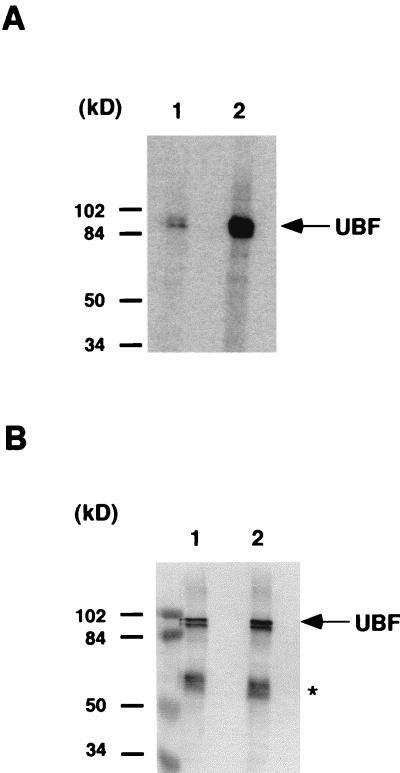
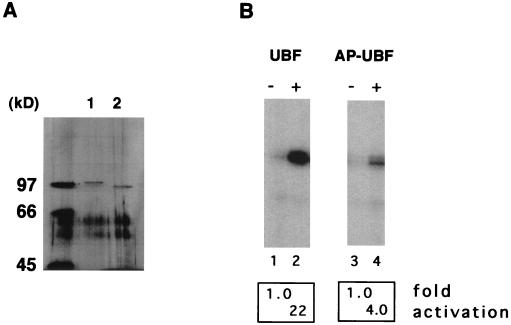
A large number of experimental data indicate that in human cells the formation of a productive transcription initiation complex requires the cooperative interaction of UBF and SL1 at the rRNA promoter (3, 31, 39). It has also been suggested that the phosphorylation status of UBF plays an important role in the activation process, yet no biochemical evidence for the role of this posttranslational modification has been provided (26, 47, 48, 60). Recently, however, results from our laboratory indicate that phosphorylation of UBF by one or more cellular kinases regulates the protein-protein interaction between UBF and SL1 (59). The observation that the binding of large T antigen to SL1 is required but not sufficient for RNA Pol I transcriptional activation (63), led us to examine whether the phosphorylation of UBF could be affected by the expression of large T antigen. To address this issue, we compared the level of UBF phosphorylation in two low passages, primary human skin fibroblast cell lines, Hs68 and Hs68-LT. Hs68-LT was derived from Hs68 by transforming the parental cells with large T antigen. Expression of large T antigen in Hs68-LT cells was confirmed by Western blotting (data not shown). The cells were metabolically labeled with [32P]orthophosphate, and endogenous UBF was immunoprecipitated from whole cell lysates with affinity-purified anti-UBF antibody cross-linked to protein A-Sepharose beads. The immunoprecipitation products were then separated by SDS-PAGE, and proteins were transferred to nitrocellulose membranes. Phosphorylation of UBF was detected by autoradiography of the membrane. As shown in Fig. 1A, phosphorylation of UBF in Hs68-LT cells (lane 2) was substantially higher than that in Hs68 cells (lane 1). Quantitation by a PhosphorImager revealed a more-than-fivefold increase in the level of UBF phosphorylation in Hs68-LT cells. To exclude the possibility that the difference of the phosphorylation signal was due to variation in the amount of UBF immunoprecipitated, the same membrane was immunoblotted with antibodies against UBF (Fig. 1B). The results indicated that approximately equal amounts of UBF protein were precipitated from each cell line, confirming that UBF was phosphorylated to a greater extent in Hs68-LT than in Hs68 cells. Similar results were also obtained with CV-1 and COS-7 (CV1-SV40 transformed) cells (data not shown). Therefore, our results suggest that the expression of large T antigen has little or no effect on the total amount of UBF expressed in the cell but rather greatly enhances the phosphorylation level of UBF.
FIG. 1.
Elevated UBF phosphorylation in large T antigen-expressing cells. (A) Human skin fibroblast cell line Hs68 and the large T antigen transformed cell line Hs68-LT (which is derived from Hs68) were labeled with 1.25 mCi of [32P]orthophosphate for 6 h in a 10-cm-diameter dish. Cells were then lysed in 1 ml of RIPA buffer in the presence of a phosphatase inhibitor cocktail. Endogenous UBF was immunoprecipitated with approximately 1 μg of affinity-purified anti-UBF antibodies cross-linked to protein A-Sepharose resin. Immunoprecipitated UBF proteins from Hs68 cells (lane 1) and Hs68-LT cells (lane 2) were resolved by SDS-PAGE and transferred to nitrocellulose membranes. Phosphorylation of UBF was determined by autoradiography. (B) The amount of immunoprecipitated UBF from Hs68 (lane 1) and Hs68-LT (lane 2) was determined by immunoblotting of the same membrane with affinity-purified anti-UBF antibodies. Molecular mass markers are as indicated. Immunoglobulin heavy chain is indicated by an asterisk.
UBF is extensively phosphorylated by a large T antigen-associated kinase activity.
SV40 large T antigen is a multifunctional protein that has been shown to interact with many cellular proteins such as transcription factors, tumor suppressor gene products, and protein kinases (22, 44, 46, 53, 54). However, the functional consequences of many of these interactions, especially with cellular protein kinases, remain unclear. To determine whether the phosphorylation of UBF is the result of a large T antigen-associated kinase activity, we performed an in vitro kinase assay with purified large T antigen. To control the specificity of the reaction, in addition to UBF, each of the SL1 subunits was also used as a substrate in the in vitro kinase assay. UBF and the three TAFIs were expressed as flag epitope-tagged proteins in Sf9 cells by using the baculovirus expression system, while TBP was expressed as a glutathione S-transferase-tagged protein in E. coli. The proteins were immobilized on either anti-flag M2 affinity resin (for UBF and TAFIs) or glutathione-Sepharose beads (for TBP), washed with dissociation buffer, and incubated in kinase buffer containing [γ-32P]ATP, in either the absence or presence of purified large T antigen. The large T antigen used was isolated from COS-7 cells by immunopurification with monoclonal antibodies cross-linked to protein A-Sepharose beads (56). After incubation, the beads were washed extensively and the bound proteins were resolved by SDS-PAGE. The phosphorylation of each protein was then assessed by autoradiography (Fig. 2A). In the presence of large T antigen, UBF became phosphorylated (lanes 8 and 14), while little or no phosphorylation was detected on TAFI110, TAFI63, and TBP (lanes 2, 4, and 10, respectively). Phosphorylation of UBF is dependent on the addition of large T antigen to the kinase reaction, since no phosphorylation was observed in its absence (lanes 1, 3, 5, 7, 9, 11, and 13). The calculated phosphorylation stoichiometry was ∼0.5 mol of phosphate/mol of UBF (see Materials and Methods for calculations). A very low level of phosphorylation was observed in TAFI48 (lane 6), which was also detectable upon large T antigen-mediated phosphorylation of a native immunopurified SL1 complex (lane 12). The role and significance of TAFI48 phosphorylation are currently unknown. The amount of each protein used in the assay was assessed by Coomassie blue or silver staining of the same SDS-polyacrylamide gel (Fig. 2B). Identical results were obtained with large T antigen expressed and purified from baculovirus-infected Sf9 cells (data not shown). In summary, these results indicate that a kinase activity, strongly associated with purified large T antigen, can efficiently phosphorylate UBF but not TBP or the TAFIs.
FIG. 2.
Phosphorylation of UBF by large T antigen-associated kinase activity. (A) flag-tagged TAFI110 (lanes 1 and 2), TAFI63 (lanes 3 and 4), TAFI48 (lanes 5 and 6), and UBF (lanes 7, 8, 13, and 14) and glutathione S-transferase-tagged TBP (lanes 9 and 10) were immobilized on anti-flag M2 beads and glutathione-Sepharose beads, respectively. Immobilized proteins were treated with alkaline phosphatase, washed extensively with dissociation buffer, and used as substrates in the kinase reactions. The substrate was incubated in either the absence (−) or presence (+) of large T antigen affinity purified from COS-7 cells. Phosphorylation of the Pol I factors was detected by autoradiography. The Pol I factor used in each set of kinase reactions and the molecular mass markers are as indicated. In lanes 11 and 12, human SL1 was immunoprecipitated with antibodies against TAFI110 and used as the substrate in a kinase assay. (B) The gels used for autoradiography in panel A were stained with either Coomassie blue (lanes 1 to 10) or silver (lanes 11 to 14) to show the amount of substrate used in each experiment. The silver-stained gel of large T antigen affinity purified from COS7 cells is shown in lane 15. Diamonds indicate positions of respective proteins. Immunoglobulin heavy chain is indicated by an asterisk.
In vitro phosphorylation of UBF by large T antigen-associated kinase occurs on sites that are also phosphorylated in vivo.
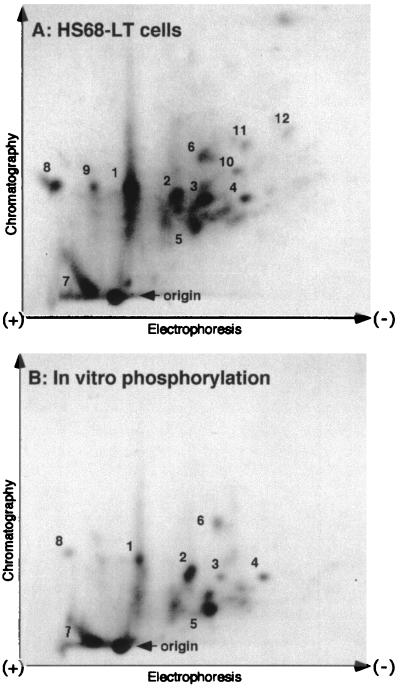
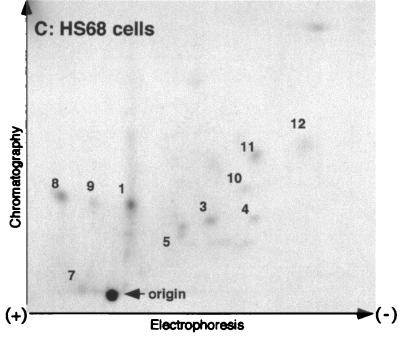
To determine the relationship between the in vitro and the in vivo phosphorylation of UBF induced by large T antigen, we carried out a comparative phosphopeptide analysis. As shown in Fig. 3A and B, tryptic phosphopeptide maps generated from UBF phosphorylated by the large T antigen-associated kinase in vitro closely resembled those obtained with UBF immunopurified from Hs68-LT cells labeled in vivo. Moreover, the in vitro-phosphorylated UBF peptide map also suggests that UBF is phosphorylated at multiple sites. A few peptides were detected in the UBF peptide maps from Hs68 cells (Fig. 3C). Several of the peptides from Hs68 cells, although weaker, appear to be shared with the Hs68-LT cells (Fig. 3C, peptides 1, 3 to 5, and 7 to 11). Interestingly, we observed several major peptides common to both the in vivo Hs68-LT and in vitro maps (Fig. 3A and B, peptides 1 to 8), whereas a few minor peptides were present in the in vivo samples only (peptides 9 to 12). The fact that phosphorylation of UBF by highly purified large T antigen and its associated kinase activity affected many of the same sites that were phosphorylated in vivo strongly suggests an important role for this kinase in the phosphorylation of UBF. The lack of a few peptides in the in vitro maps may either be due to technical limitations or it may reflect the existence of other enzymes that phosphorylate UBF.
FIG. 3.
2D tryptic phosphopeptide maps of UBF. Endogenous UBF was isolated by immunoprecipitation from [32P]orthophosphate (1 mCi/ml)-labeled Hs68-LT (A) and Hs68 (C) cells. Phosphorylation of UBF was analyzed by tryptic phosphopeptide mapping as described in Materials and Methods. (B) Tryptic phosphopeptide map of recombinant human UBF1 phosphorylated in vitro by large T antigen-associated kinase activity. The electrode position and the direction of thin-layer electrophoresis and ascending chromatography are as marked. The sample origin is as indicated in each panel.
RNA Pol I transcriptional activation by large T antigen is tightly linked to the phosphorylation of UBF by large T antigen-associated kinase activity.
To investigate the role of UBF phosphorylation in the large T antigen-mediated transcriptional activation process, we analyzed a set of large T antigen deletion mutants for their ability to activate transcription and to phosphorylate UBF. Large T antigen mutants were expressed and purified as previously described (63). In each assay, the same molar amount of large T antigen mutant protein was used, as judged by silver-stained SDS-polyacrylamide gels. The purified large T antigen deletion mutants were first tested in an in vitro reconstituted transcription assay for their ability to activate Pol I transcription (Fig. 4A). The results indicated that only wild-type large T antigen (aa 1 to 708) and the mutant containing the N-terminal 538 aa (aa 1 to 538) can efficiently activate transcription (lanes 3 and 4). Further deletions into the amino terminus resulted in a sharp loss of transactivation function (lanes 5 and 6). The amino-terminal deletion mutants and a mutant containing the region between aa 362 and 538, in addition to small t antigen, were transcriptionally inactive (lanes 7 to 11), which is in agreement with our previous results (63). The same set of large T antigen mutants were then tested for their ability to phosphorylate UBF in an in vitro kinase assay (Fig. 4B). As expected, the presence of purified wild-type large T antigen (aa 1 to 708) yielded a strong phosphorylation of UBF (lane 4). A mutant containing the first 538 aa (aa 1 to 538) stimulated UBF phosphorylation as efficiently as the wild-type large T antigen (lane 6), while further deletions into the carboxy-terminal region of large T antigen (aa 1 to 436) resulted in a dramatic decrease in UBF phosphorylation. Similarly, mutants with progressive deletions of the amino-terminal region of large T antigen (aa 362 to 708, aa 411 to 708, and aa 538 to 708), a mutant which contains only the internal region (aa 362 to 538), and small t antigen, had little or no effect on the phosphorylation of UBF (lanes 16, 10, 14, 12, and 18, respectively). In summary, our data indicate that the amino-terminal region of large T antigen, from aa 1 to 538, is required for the phosphorylation of UBF, suggesting that this region is tightly associated with a kinase activity. Importantly, the same region of large T antigen is also necessary for the stimulation of RNA Pol I transcription, further supporting the concept that UBF phosphorylation may be an important step in the transcriptional activation process mediated by large T antigen.
FIG. 4.
Functional analysis of UBF phosphorylation and Pol I transactivation by large T antigen mutants. (A) Purified large T antigen mutants were tested for their ability to activate Pol I transcription in an in vitro reconstituted transcription assay as described in Materials and Methods. Lanes 1 and 2 contain no purified large T antigen protein. The arrow indicates the position of the protected oligonucleotide fragment generated by S1 analysis. Fold activation was calculated by quantitation of radioactivity on the dried gel using a PhosphorImager (Molecular Dynamics). (B) Kinase assays were performed as described in Materials and Methods to determine the phosphorylation of UBF in either the absence (−) or presence (+) of purified large T antigen mutant proteins. The control reaction (−CTR) contains no large T antigen mutant protein. The corresponding amino acid numbers for each large T antigen mutant used are as indicated. (C) Schematic representation of large T antigen mutants used in the assays depicted in panels A and B. Data on SL1 binding properties of large T antigen mutants are from Zhai et al. (63). ++, strong binding to SL1; −, no significant binding to SL1.
The transactivation domain of UBF is necessary for phosphorylation by the large T antigen-associated kinase activity.
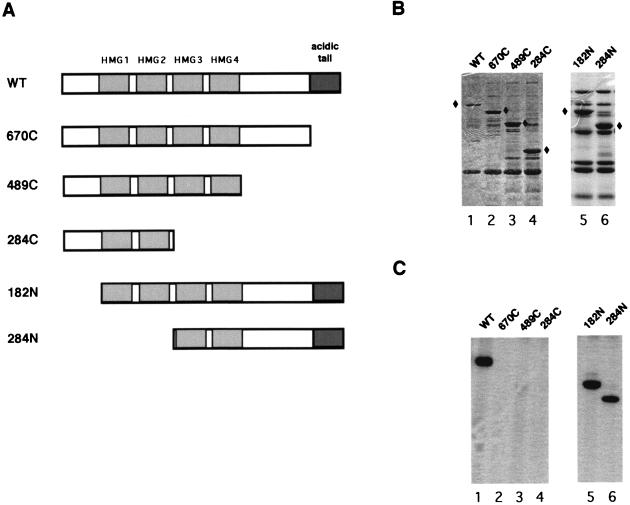
UBF contains multiple domains, each of which appears to have its own distinct biochemical function (34, 35). To better understand the nature of UBF phosphorylation and its role in transcriptional activation, we wanted to identify the domain of UBF required for phosphorylation by the large T antigen-associated kinase. For this purpose, we generated a set of UBF mutants missing either the carboxy-terminal (670C, 489C, and 284C) or amino-terminal (182N and 284N) region, as schematically represented in Fig. 5A. All proteins were engineered with a flag epitope tag at the amino terminus and expressed in E. coli, except for the wild-type UBF, which was expressed in Sf9 cells by using a recombinant baculovirus. Full-length human UBF cannot be expressed in E. coli (64). Each protein was then immobilized on anti-flag M2 beads and used as the substrate in kinase reactions containing purified large T antigen and [γ-32P]ATP. The immobilized proteins were extensively washed, resolved by SDS-PAGE, and then stained with Coomassie blue (Fig. 5B). In vitro phosphorylation of each UBF mutant was then assessed by autoradiography of the stained gel. As shown in Fig. 5C, wild-type UBF (lane 1) and the two amino-terminal deletion mutants (182N and 284N [lanes 5 and 6, respectively]) were efficiently phosphorylated in the presence of purified large T antigen. On the other hand, removal of the carboxy-terminal tail (UBF 670C [lane 2]) or further deletion of the carboxy-terminal region of UBF (489C and 284C [lanes 3 and 4, respectively]) did not result in any detectable level of phosphorylation. Therefore, we conclude that the carboxy-terminal acidic domain of UBF, from aa 670 to 764, is required for UBF phosphorylation by the large T antigen-associated kinase activity.
FIG. 5.
Mapping of the region of UBF required for phosphorylation by large T antigen-associated kinase activity. (A) Schematic representation of full-length (wild-type [WT]) and mutant UBF proteins. The four HMG domains (HMG1 to -4) and acidic tail are indicated by shaded boxes. (B) The amount and size of full-length and mutant UBF proteins used in the kinase assay were determined by SDS-PAGE and Coomassie blue staining. Bands representing each UBF mutant protein are indicated by diamonds. (C) Full-length and mutant UBF proteins were expressed as flag epitope-tagged proteins and used as substrates for large T antigen-associated kinase in the in vitro kinase reactions. Phosphorylation of UBF mutant proteins was detected by SDS-PAGE and subsequent autoradiography. Mutant UBF proteins used in each reaction are as indicated.
Phosphorylation of UBF by the large T antigen-associated kinase facilitates the formation of a stable UBF-SL1 complex.
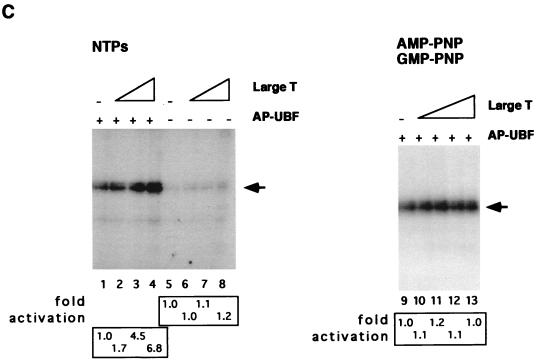
Recent results from our laboratory indicate that phosphorylation of UBF plays an important role in the establishment of a stable UBF-SL1 complex at the rDNA promoter (59). To determine whether phosphorylation of UBF by the large T antigen-associated kinase activity is involved in the regulation of SL1 binding to UBF, we performed a series of protein binding assays using alkaline phosphatase-treated UBF (AP-UBF) which was preincubated either with or without purified large T antigen. Flag epitope-tagged UBF was expressed in Sf9 cells, immobilized on anti-flag M2 beads, treated with alkaline phosphatase, and incubated with either purified large T antigen or buffer alone prior to the addition of SL1. The coimmunoprecipitated products were separated by SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with antibodies against TBP and TAFI110 to detect the presence of SL1. As shown in Fig. 6, untreated UBF (lane 1) interacted strongly with SL1, as indicated by the presence of TBP and TAFI110 in the immunoprecipitation product. The coimmunoprecipitation is specific for UBF, since a flag-tagged hepatitis C virus Pol did not pull down any detectable amount of SL1 (data not shown). Treatment of UBF with alkaline phosphatase resulted in a dramatic decrease in SL1 binding (lane 2). Importantly, when AP-UBF was preincubated with purified large T antigen in the presence of ATP, the binding to SL1 was restored (lane 3). On the other hand, preincubation of large T antigen with AP-UBF in the presence of the ATP analogue AMP-PNP (lane 4) or preincubation of AP-UBF with ATP but without large T antigen (lane 5) did not restore SL1 binding. Since there is no direct interaction between UBF and large T antigen (63), we can exclude the possibility that large T antigen functions as a bridge between UBF and SL1. Taken together, these results demonstrate that large T antigen, through an associated kinase activity, phosphorylates UBF and induces a strong interaction between UBF and SL1.
FIG. 6.
UBF phosphorylation by the large T antigen-associated kinase activity stimulates the interaction between UBF and SL1. Immobilized UBF (lane 1), AP-UBF (lane 2), AP-UBF rephosphorylated by incubation with purified large T antigen in the presence of ATP (lane 3), AMP-PNP (lane 4), and AP-UBF incubated with ATP in the absence of large T antigen (lane 5) were used to coimmunoprecipitate the SL1 complex from an SL1-containing fraction partially purified from HeLa cells. The presence of SL1 in the immunoprecipitate was detected by immunoblotting with antibodies against TAFI110 and TBP. Arrows indicate the position of TAFI110 and TBP. An aliquot (10%) of input SL1 in each reaction mixture is shown in lane 6.
Phosphorylation of UBF by the large T antigen-associated kinase activity enhances its transactivation properties.
An important biochemical function of UBF is its ability to stimulate rRNA transcription (34, 35). To examine whether phosphorylation of UBF by the large T antigen-associated kinase activity could enhance its transactivation activity, we performed in vitro transcription assays using purified large T antigen and UBF. Recombinant flag epitope-tagged UBF was expressed in baculovirus-infected Sf9 cells and immobilized on anti-flag M2 resin. One portion of the flag-tagged UBF was then subjected to alkaline phosphatase treatment. After elution from the resin, an aliquot of the purified proteins was separated by SDS-PAGE and silver stained to determine the protein amount and purity (Fig. 7A). UBF and AP-UBF appeared as a single band on silver-stained gels. Also, AP-UBF migrates faster in the SDS-polyacrylamide gel, denoting a change in its molecular mass. Equal amounts of purified UBF and AP-UBF, as judged by the silver-stained gels, were then tested for their ability to activate Pol I transcription in an in vitro transcription assay (Fig. 7B, lanes 1 to 4). In the absence of UBF, SL1 supported a basal level of transcription (lanes 1 and 3). As expected, the addition of purified UBF to the reaction mixture activated transcription ∼22-fold relative to reactions that did not contain UBF (lane 2). On the other hand, UBF that had been pretreated with alkaline phosphatase displayed only a fourfold activation (lane 4). We then asked whether large T antigen could rescue the transcriptional activity of dephosphorylated UBF. Transcription assays were performed as described above, either without large T antigen (Fig. 7C, lane 1) or with the addition of purified large T antigen (lanes 2 to 4). Clearly, addition of increasing amounts of large T antigen resulted in a proportional increase in rRNA synthesis by AP-UBF. Moreover, when UBF was excluded from the transcription assay, large T antigen did not stimulate the basal level SL1-dependent transcription (lanes 5 to 8), thus indicating that UBF is required for the stimulatory effect of large T antigen. In contrast, transcriptional activation by large T antigen was completely abolished when the transcription assays contained the nucleotide analogues AMP-PNP and GMP-PNP in place of ATP and GTP (lanes 9 to 13), to prevent phosphorylation of UBF. To further confirm that the phosphorylation of UBF by an associated kinase activity was responsible for large T antigen stimulation of transcription, AP-UBF was preincubated with large T antigen either in the absence of ATP, in the presence of ATP, or in the presence of AMP-PNP. After extensive washing, each UBF moiety was used in a transcription assay in the presence of partially purified RNA Pol I and SL1. As shown in Fig. 7D, transcription is strongly stimulated when AP-UBF is preincubated with large T antigen in the presence of ATP (lanes 4 and 5) but not in the absence of ATP or in the presence of AMP-PNP (lanes 3 and 6, respectively). Taken together, these results show that phosphorylation of UBF is important for its transactivation property and that the regulation of UBF phosphorylation by a large T antigen-associated kinase(s) is a crucial step in large T antigen-mediated Pol I transcriptional activation.
FIG. 7.
Large T antigen stimulates UBF-dependent transcription in the presence of AP-UBF. (A) UBF was expressed as a flag epitope-tagged protein by using recombinant baculovirus. Untreated UBF (lane 1) and AP-UBF (lane 2) were affinity purified by using anti-flag M2 beads. The concentration of each purified protein was determined by silver staining following SDS-PAGE. (B) In vitro transcription assays with untreated UBF (lane 2) and AP-UBF (lane 4). Transcription assays contained no UBF (lanes 1 and 3), approximately 0.25 ng of UBF (lane 2), or 0.25 ng of AP-UBF (lane 4). (C) Large T antigen stimulation of Pol I transcription in the absence of UBF (lanes 5 to 8) or in the presence of 0.25 ng of AP-UBF (lanes 1 to 4) as determined by in vitro reconstituted transcription assays. Each transcription reaction mixture contained either no large T antigen (lanes 1 and 5) or increasing amounts of purified large T antigen (50 to 250 ng; lanes 2 to 4 and 6 to 8). In lanes 9 to 13, nucleotide analogs AMP-PNP and GMP-PNP replaced ATP and GTP, respectively, in the transcription assay with 0.25 ng of AP-UBF. Each transcription reaction mixture contains either no large T antigen (lane 9) or increasing amounts of purified large T antigen (50 to 250 ng; lanes 10 to 13). The arrow indicates the position of the protected oligonucleotide fragment. (D) In vitro transcriptional activation by differentially treated UBF. Baculovirus expressed flag-tagged UBF immobilized on anti-flag M2 beads was either mock treated (lane 2), treated with alkaline phosphatase only (lane 3), or first treated with alkaline phosphatase and then incubated with purified large T antigen in the presence of either ATP (lanes 4 and 5) or AMP-PNP (lane 6). Following extensive washing, UBF proteins were then eluted from the affinity resin and dialyzed. An equal amount of protein, as determined by silver staining (data not shown), was tested for the ability to activate transcription in vitro. Transcription data were quantitated, and the fold activations are as indicated.
DISCUSSION
Several recent studies have made significant progress toward elucidating the molecular mechanism by which large T antigen stimulates cellular gene expression. Our laboratory and others have shown that a common theme in the activation of RNA Pol I-, II-, and III-transcribed genes is the binding of large T antigen to the TBP-TAF complexes SL1, TFIID, and TFIIIB (14, 16, 63). Thus, it appears that each TBP-TAF complex serves as a large T antigen-docking site for the subsequent regulation of promoter activity. Interestingly, these interactions appear to be mediated by direct protein-protein contacts between large T antigen and one or more TAFs. In addition, the lack of amino acid homology between the different classes of TAFs suggests that large T antigen most likely recognizes structural motifs that are conserved among these three complexes rather than the primary amino acid sequences. However, our previous studies on the regulation of human RNA Pol I transcription suggest that the binding of large T antigen to SL1, although necessary, is not sufficient for transcriptional activation (63). In fact, the minimal domain of large T antigen which retains the ability to efficiently bind to SL1, can only weakly activate Pol I transcription. These results prompted us to investigate the role of UBF in the molecular processes that lead to large T antigen stimulation of RNA Pol I transcription. In this work, we show that UBF is heavily phosphorylated in human primary fibroblasts expressing large T antigen. Additionally, highly purified large T antigen is associated with a kinase activity that can readily and specifically phosphorylate UBF in vitro. Moreover, we demonstrate by tryptic phosphopeptide mapping that large T antigen-associated kinase phosphorylates in vitro many of the same sites that are specifically phosphorylated in large T antigen-expressing cells. The most straightforward interpretation of these results is that UBF is a direct in vivo substrate of the large T antigen-associated kinase. The difference between the data from the 2D phosphopeptide mapping, which suggests that phosphorylation of UBF by the large T antigen-associated kinase occurs at multiple sites, and the estimated phosphorylation stoichiometry (approximately 0.5 mol of phosphate/mol of UBF) could be due to suboptimal phosphorylation conditions in the in vitro reaction. Future studies will address in more detail the biochemical requirements of this kinase activity.
An interesting aspect of large T antigen is its well-known ability to associate with a variety of cellular protein kinases (1, 9, 27, 46, 53). Other viral oncogenic proteins such as the adenovirus E1A protein and the product of the human papillomavirus E7 gene also associate with cellular kinases (20, 32, 50, 58). Recently, another viral protein, human immunodeficiency virus Tat, has been shown to influence human immunodeficiency virus gene expression by modulating the phosphorylation of the RNA Pol II carboxyl-terminal domain through protein-protein interactions with TFIIH, a multisubunit factor containing kinase activity (24). These findings suggest that cellular kinases fulfill an important function for the viruses. Many of the substrates of large T antigen kinase(s) and the physiological role of this association remain to be identified. Here, we describe UBF as a likely in vivo substrate of the large T antigen-associated kinase(s). Moreover, we provide evidence indicating that at least one of the functions of the large T antigen-associated kinase(s) is to stimulate rRNA gene expression through phosphorylation of UBF. It remains to be determined whether a similar mechanism is also involved in the activation of Pol II and Pol III genes by large T antigen.
Our findings indicate that UBF is the primary functional target of large T antigen and activation of Pol I transcription is achieved by UBF phosphorylation. To further establish the link between UBF phosphorylation and large T antigen stimulation of Pol I transcription, we carried out in vitro reconstituted transcription assays with SL1, dephosphorylated UBF, and purified large T antigen. The results of these experiments demonstrated that transcriptional activation by large T antigen is mediated by phosphorylation of UBF. Therefore, large T antigen, by binding to SL1, induces the phosphorylation of UBF and, consequently, the activation of rRNA synthesis. The phosphorylation status of UBF is modulated in response to a variety of physiological stimuli, and several studies have shown a correlation between UBF’s phosphorylation and its transcriptional activity (26, 48, 60). However, these studies failed to provide any evidence for the role of this modification. Attempts to determine whether UBF phosphorylation affects its DNA binding ability showed no effect (60). In this study, we show that the large T antigen-induced phosphorylation of UBF facilitates the formation of a stable complex between UBF and SL1. By preincubation of dephosphorylated UBF with purified large T antigen, we were able to restore the interaction between UBF and SL1, demonstrating the important role of the large T antigen-associated kinase activity in the maintenance of a stable UBF-SL1 complex.
These biochemical data provide further evidence for the functional relationship between UBF phosphorylation and Pol I transcriptional activation mediated by large T antigen. Further analysis shows that the carboxy-terminal tail of UBF is required for its phosphorylation induced by large T antigen. This region of UBF has been previously shown to be necessary for RNA Pol I transcriptional activation, and it is extensively phosphorylated at various serine residues in exponentially growing cells (48, 60). Moreover, mutational analysis shows that the region between aa 1 and 538 of large T antigen is necessary to support efficient phosphorylation of UBF. Interestingly, our data indicate that the same domain is also required for full stimulation of rRNA transcription (63). The strong correlation between its ability to activate transcription and to induce UBF phosphorylation suggests that the two properties of large T antigen are functionally linked.
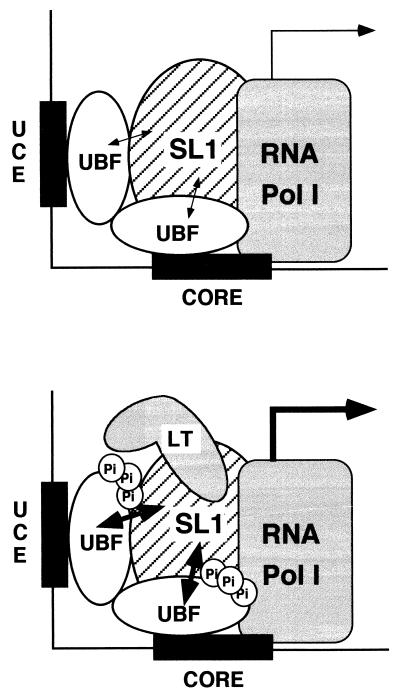
Taken together, the data presented here suggest a model whereby RNA Pol I transcription can be activated by the recruitment of large T antigen and an associated kinase activity to the rRNA promoter (Fig. 8). In this model, phosphorylation of UBF modulates its ability to interact with SL1. Based on our recent results, when UBF is hypophosphorylated, the interaction with SL1 is weak and unstable (59). This results in a low level of transcription. However, hyperphosphorylation of UBF augments the strength of UBF-SL1 interaction, leading to a stabilization of the initiation complex and higher transcriptional activity (59). In large T antigen-expressing cells, the interaction between SL1 and large T antigen serves as a mechanism to target large T antigen and its associated kinase activity to the rRNA promoter. Large T antigen does not bind by itself to UBF (63); therefore, it can stimulate UBF phosphorylation only when it is brought to the proximity of UBF through interaction with SL1. UBF is then phosphorylated at multiple sites by a large T antigen-associated kinase activity(ies). The phosphorylation of UBF stabilizes the cooperative interactions between UBF and SL1, resulting in enhanced transcriptional activity. The notion that large T antigen functions to activate transcription by stabilizing preinitiation complex formation has been recently suggested for the RNA Pol II-transcribed hsp70 and c-fos promoters (15). Their work shows that large T antigen stimulates transcription by stabilizing the TBP-TFIIA preinitiation complex on the promoter element through direct interactions with TBP and TFIIA. Thus, stabilization of a preinitiation complex may be a common mechanism used by large T antigen to activate Pol I and Pol II transcription. Importantly, stimulation of RNA Pol I transcription by large T antigen appears to be mediated by a cellular kinase that promotes strong protein interactions between UBF and SL1.
FIG. 8.
Model of a potential mechanism of Pol I transcriptional activation by large T antigen. Large T antigen activates Pol I transcription as follows: SL1, through direct protein-protein interactions, serves as a docking site for the recruitment of large T antigen to the rRNA promoter. This allows a kinase(s) associated with large T antigen to come in proximity and phosphorylate UBF. The phosphorylation of UBF stabilizes the SL1-UBF complex at the rRNA promoter, resulting in a higher transcriptional activity. The thickness of the single-headed and double-headed arrows corresponds to the transcriptional activity from the rRNA promoter and the strength of UBF-SL1 interaction, respectively.
Preliminary studies addressing the nature of this kinase activity indicate that it is tightly associated with affinity-purified large T antigen and remains bound even after washing under rather stringent conditions. Our analysis also indicates that the kinase activity remains associated with large T antigen after several column chromatography purification steps (64). Early work suggested that large T antigen exhibits a protein kinase activity (28, 57). However, later studies indicated that this activity most likely is not an intrinsic property of large T antigen but rather is due to a tightly associated protein kinase (6, 62). Analysis of purified large T antigen by SDS-PAGE and silver staining reveals a few substoichiometric bands whose relevance is currently under investigation (Fig. 2B, lane 15).
Although the concept that UBF can be modified by phosphorylation has been well established, very little is known about the cellular kinases involved. CKII, an abundant cellular kinase thought to be involved in some aspects of cell proliferation, has been shown to be able to modify the carboxy-terminal region of UBF at several serine residues in vitro (47, 61). Additional experiments have been performed to test whether phosphorylation of UBF by CKII is responsible for establishing a strong interaction between UBF and SL1; however, CKII phosphorylation of AP-UBF failed to promote the association with SL1 (data not shown). In addition, a UBF mutant (pUBF-at) with substitutions in serine residues that are putative targets of CKII can still be efficiently phosphorylated by the large T antigen-associated kinase (data not shown [60]). These results indicate that UBF phosphorylation by CKII is not directly involved in the regulation of UBF-SL1 interaction and that the kinase associated with large T antigen is distinct from CKII. In addition, since cdk2 has been shown to copurify with large T antigen, we tested whether the cdk2 inhibitor roscovitine blocks the large T antigen-dependent phosphorylation of UBF (1, 45). However, addition of roscovitine at concentrations that inhibit cdk2 kinase activity failed to block the phosphorylation of UBF (data not shown). Therefore, the cellular kinase(s) associated with large T antigen capable of phosphorylating UBF and stimulating the binding of UBF to SL1 remains to be identified. The isolation and characterization of this protein kinase(s) will lead to the dissection of an important cellular pathway that regulates Pol I transcription in response to not only SV40 infection but also a variety of physiological stimuli.
ACKNOWLEDGMENTS
We are grateful to the members of the Gene Expression Group at the University of Southern California for advice and discussions throughout the course of this work. We thank H.-M. Jantzen for constructs, A. Schönthal for Hs68 and Hs68-LT cell lines, R. Voit and I. Grummt for the pUBF-at construct, and N. Bernt for valuable advice on 2D phosphopeptide mapping. We are also thankful to Joann Tuan and Tiffany Bui for their technical support.
W.Z. is partially supported by the Heidelberger Predoctoral Scholarship Award in Cancer Research. This work was supported by a grant to L.C. (R01 GM53949) from the National Institutes of Health.
REFERENCES
- 1.Adamczewski J, Gannon J, Hunt T. Simian virus 40 large T antigen associates with cyclin A and p33cdk2. J Virol. 1993;67:6551–6557. doi: 10.1128/jvi.67.11.6551-6557.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson M M, Chen J, Cole C N, Conrad S E. Activation of the human thymidine kinase (TK) promoter by simian virus 40 large T antigen requires both the T antigen pRb family-binding domain and TK promoter sequences resembling E2F-binding sites. J Virol. 1996;70:6304–6313. doi: 10.1128/jvi.70.9.6304-6313.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bell S P, Learned R M, Jantzen H-M, Tjian R. Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science. 1988;241:1192–1197. doi: 10.1126/science.3413483. [DOI] [PubMed] [Google Scholar]
- 4.Berger L C, Smith D B, Davidson I, Hwong J J, Fanning E, Wildeman A G. Interaction between T antigen and TEA domain of the factor TEF-1 derepresses simian virus 40 late promoter in vitro: identification of T-antigen domains important for transcription control. J Virol. 1996;70:1203–1212. doi: 10.1128/jvi.70.2.1203-1212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle W J, van der Geer P, Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- 6.Bradley M K, Griffin J D, Livingston D M. Relationship of oligomerization to enzymatic and DNA-binding properties of the SV40 large T antigen. Cell. 1982;28:125–134. doi: 10.1016/0092-8674(82)90382-8. [DOI] [PubMed] [Google Scholar]
- 7.Brinster R L, Chen H Y, Messing A, van Dyke T, Levine A J, Palmiter R D. Transgenic mice harboring SV40 T-antigen genes develop characteristic brain tumors. Cell. 1984;37:367–379. doi: 10.1016/0092-8674(84)90367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao S X, Mishoe H, Elion J, Berg P E, Schechter A N. Activation of the human epsilon- and beta-globin promoters by SV40 T antigen. Biochem J. 1989;258:769–776. doi: 10.1042/bj2580769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cegielska A, Moarefi I, Fanning E, Virshup D M. T-antigen kinase inhibits simian virus 40 DNA replication by phosphorylation of intact T antigen on serines 120 and 123. J Virol. 1994;68:269–275. doi: 10.1128/jvi.68.1.269-275.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen H, Campisi J, Padmanabhan R. SV40 large T antigen transactivates the human cdc2 promoter by inducing a CCAAT box binding factor. J Biol Chem. 1996;271:13959–13967. [PubMed] [Google Scholar]
- 11.Comai L, Tanese N, Tjian R. The TATA-binding protein and associated factors are integral components of the RNA polymerase I transcription factor, SL1. Cell. 1992;68:965–976. doi: 10.1016/0092-8674(92)90039-f. [DOI] [PubMed] [Google Scholar]
- 12.Comai L, Zomerdijk J C B M, Beckmann H, Zhou S, Admon A, Tjian R. Reconstitution of transcription factor SL1: exclusive binding of TBP by SL1 or TFIID subunits. Science. 1994;266:1966–1972. doi: 10.1126/science.7801123. [DOI] [PubMed] [Google Scholar]
- 13.Conzen S D, Cole C N. The transforming proteins of simian virus 40. Semin Virol. 1994;5:349–356. [Google Scholar]
- 14.Damania B, Alwine J C. TAF-like function of SV40 large-T antigen. Genes Dev. 1996;10:1369–1381. doi: 10.1101/gad.10.11.1369. [DOI] [PubMed] [Google Scholar]
- 15.Damania B, Lieberman P, Alwine J C. Simian virus 40 large T antigen stabilizes the TATA-binding protein–TFIIA complex on the TATA element. Mol Cell Biol. 1998;18:3926–3935. doi: 10.1128/mcb.18.7.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Damania B, Mital R, Alwine J C. Simian virus 40 large T antigen interacts with human TFIIB-related factor and small nuclear RNA-activating protein complex for transcriptional activation of TATA-containing polymerase III promoters. Mol Cell Biol. 1998;18:1331–1338. doi: 10.1128/mcb.18.3.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeCaprio J A, Ludlow J W, Figge J, Shew J Y, Huang C M, Lee W H, Marsilio E, Paucha E, Livingston D M. SV40 large tumor antigen forms a specific complex with the product of retinoblastoma susceptibility gene. Cell. 1988;54:275–283. doi: 10.1016/0092-8674(88)90559-4. [DOI] [PubMed] [Google Scholar]
- 18.Dunnick W, Elenich L, Cunningham K, Chris C, Claflin L. Tumorigenesis in mice with an SV40 T antigen transgene driven by the immunoglobulin gamma 1 heavy chain germline promoter. Curr Top Microbiol Immunol. 1995;194:163–169. doi: 10.1007/978-3-642-79275-5_20. [DOI] [PubMed] [Google Scholar]
- 19.Dyson N, Buchkovich K, Whyte P, Harlow E. The cellular 107k protein that binds to adenovirus E1A also associates with the large T antigen of SV40 and JC virus. Cell. 1989;58:249–255. doi: 10.1016/0092-8674(89)90839-8. [DOI] [PubMed] [Google Scholar]
- 20.Dyson N, Guida P, Munger K, Harlow E. Homologous sequences in adenovirus E1A and human papillomavirus E7 proteins mediate interaction with the same set of cellular proteins. J Virol. 1992;66:6893–6902. doi: 10.1128/jvi.66.12.6893-6902.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckner R, Ludlow J W, Lill N L, Oldread E, Arany Z, Modjtahedi N, Decaprio J A, Livingston D M, Morgan J A. Association of p300 and CBP with simian virus 40 large T antigen. Mol Cell Biol. 1996;16:3454–3464. doi: 10.1128/mcb.16.7.3454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fanning E, Knippers R. Structure and function of simian virus 40 large tumor antigen. Annu Rev Biochem. 1992;61:55–85. doi: 10.1146/annurev.bi.61.070192.000415. [DOI] [PubMed] [Google Scholar]
- 23.Gallo G J, Gruda M C, Manuppello J R, Alwine J C. Activity of simian DNA-binding factors is altered in the presence of simian virus 40 (SV40) early proteins: characterization of factors binding to elements involved in activation of the SV40 late promoter. J Virol. 1990;64:173–184. doi: 10.1128/jvi.64.1.173-184.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Martinez L F, Mavankal G, Neveu J M, Lane W S, Ivanov D, Gaynor R B. Purification of a tat-associated kinase reveals a TFIIH complex that modulates HIV-1 transcription. EMBO J. 1997;16:2836–2850. doi: 10.1093/emboj/16.10.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilinger G, Alwine J C. Transcriptional activation by simian virus 40 large T antigen: requirements for simple promoter structures containing either TATA or initiator elements with variable upstream factor binding sites. J Virol. 1993;67:6682–6688. doi: 10.1128/jvi.67.11.6682-6688.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glibetic M, Taylor L, Larson D, Hannan R, Sells B, Rothblum L. The RNA polymerase I transcription factor UBF is the product of a primary response gene. J Biol Chem. 1995;270:4209–4212. doi: 10.1074/jbc.270.9.4209. [DOI] [PubMed] [Google Scholar]
- 27.Gotz C, Koenig M G, Issinger O-G, Montenarh M. A casein-kinase-2 related protein kinase is tightly associated with the large-T antigen of simian virus 40. Eur J Biochem. 1995;233:327–334. doi: 10.1111/j.1432-1033.1995.327_1.x. [DOI] [PubMed] [Google Scholar]
- 28.Griffin J D, Spangler G, Livingston D M. Protein kinase activity associated with simian virus 40 T antigen. Proc Natl Acad Sci USA. 1979;76:2610–2614. doi: 10.1073/pnas.76.6.2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gruda M, Alwine J C. Simian virus 40 (SV40) transcriptional activation mediated through the Oct/SPH region of the SV40 late promoter. J Virol. 1991;65:3553–3558. doi: 10.1128/jvi.65.7.3553-3558.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1988. [Google Scholar]
- 31.Hempel W M, Canvanaugh A H, Hannan R D, Taylor L, Rothblum L I. The species-specific RNA polymerase I transcription factor SL1 binds to the upstream binding factor. Mol Cell Biol. 1996;16:557–563. doi: 10.1128/mcb.16.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herrmann C H, Su L K, Harlow E. Adenovirus E1A is associated with a serine/threonine protein kinase. J Virol. 1991;65:5848–5859. doi: 10.1128/jvi.65.11.5848-5859.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inoue T, Hirabayashi Y, Mitsui H, Furuta Y, Suda Y, Aizawa S I, Ikawa Y. Experimental model for MDS-like myelodysplasia in transgenic mice harboring the SV40 large T antigen under an immunoglobulin enhancer. Leukemia. 1994;8:S202–S205. [PubMed] [Google Scholar]
- 34.Jantzen H-M, Admon A, Bell S P, Tjian R. Nuclear transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature. 1990;344:830–836. doi: 10.1038/344830a0. [DOI] [PubMed] [Google Scholar]
- 35.Jantzen H-M, Chow A M, King D S, Tjian R. Multiple domains of the RNA polymerase I activator hUBF interacts with the TATA-binding protein complex hSL1 to mediate transcription. Genes Dev. 1992;6:1950–1963. doi: 10.1101/gad.6.10.1950. [DOI] [PubMed] [Google Scholar]
- 36.Keller J M, Alwine J C. Activation of the SV40 late promoter: direct effects of T antigen in the absence of viral DNA replication. Cell. 1984;36:381–389. doi: 10.1016/0092-8674(84)90231-9. [DOI] [PubMed] [Google Scholar]
- 37.Keller J M, Alwine J C. Sequences in the simian virus 40 late promoter required for T-antigen-mediated trans activation. Mol Cell Biol. 1985;5:1859–1869. doi: 10.1128/mcb.5.8.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kumar T R, Graham K E, Asa S L, Low M J. Simian virus 40 T antigen induced gonadotroph adenomas: a model of human null cell adenomas. Endocrinology. 1998;139:3342–3351. doi: 10.1210/endo.139.7.6100. [DOI] [PubMed] [Google Scholar]
- 39.Learned R M, Learned T K, Haltiner M M, Tjian R. Human rRNA transcription is modulated by the coordinate binding of two factors to an upstream control element. Cell. 1986;45:847–857. doi: 10.1016/0092-8674(86)90559-3. [DOI] [PubMed] [Google Scholar]
- 40.Learned R M, Tjian R. In vitro transcription of human ribosomal RNA genes by RNA polymerase I. J Mol Appl Genet. 1982;1:575–584. [PubMed] [Google Scholar]
- 41.Levine A J. The p53 protein and its interactions with the oncogene products of the small DNA tumor viruses. Virology. 1990;177:419–426. doi: 10.1016/0042-6822(90)90505-l. [DOI] [PubMed] [Google Scholar]
- 42.Lill N, Tevethia M J, Eckner R, Livingston D M, Modjtahedi N. p300 family members associate with the carboxyl terminus of simian virus 40 large tumor antigen. J Virol. 1997;71:129–137. doi: 10.1128/jvi.71.1.129-137.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loeken M R, Khoury G, Brady J. Stimulation of the adenovirus E2 promoter by simian virus 40 T antigen or E1A occurs by different mechanisms. Mol Cell Biol. 1986;6:2020–2026. doi: 10.1128/mcb.6.6.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ludlow J W. Interactions between SV40 large-tumor antigen and the growth suppressor proteins pRB and p53. FASEB J. 1993;7:866–871. doi: 10.1096/fasebj.7.10.8344486. [DOI] [PubMed] [Google Scholar]
- 45.Meijer L. Chemical inhibitors of cyclin-dependent kinases. Trends Cell Biol. 1996;6:393–397. doi: 10.1016/0962-8924(96)10034-9. [DOI] [PubMed] [Google Scholar]
- 46.Muller E, Scheidtmann K H. Purification and characterization of a protein kinase which is activated by SV40 large T antigen and phosphorylates the tumor suppressor protein p53. Oncogene. 1995;10:1175–1185. [PubMed] [Google Scholar]
- 47.O’Mahony D J, Smith S D, Xie W, Rothblum L I. Analysis of the phosphorylation, DNA-binding and dimerization properties of the RNA polymerase I transcription factors UBF1 and UBF2. Nucleic Acids Res. 1992;20:1301–1308. doi: 10.1093/nar/20.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O’Mahony D J, Xie W, Smith S D, Singer H A, Rothblum L I. Differential phosphorylation and localization of the transcription factor UBF in vivo in response to serum deprivation. J Biol Chem. 1992;267:35–38. [PubMed] [Google Scholar]
- 49.Paule M R. Transcription of ribosomal RNA genes by eukaryotic RNA polymerase I. New York, N.Y: Springer-Verlag Inc.; 1998. [Google Scholar]
- 50.Pines J, Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990;346:760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- 51.Prives C, Bargonetti J, Freidman P N, Manfredi J J, Wang E H. Functional consequences of the interactions of the p53 tumor suppressor protein and SV40 large tumor antigen. Cold Spring Harbor Symp Quant Biol. 1991;56:227–235. doi: 10.1101/sqb.1991.056.01.028. [DOI] [PubMed] [Google Scholar]
- 52.Rice P W, Cole C N. Efficient transcriptional activation of many simple modular promoters by simian virus 40 large T antigen. J Virol. 1993;67:6689–6697. doi: 10.1128/jvi.67.11.6689-6697.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheidtmann K H, Haber A. Simian virus 40 large T antigen induces or activates a protein kinase which phosphorylates the transformation-associated protein p53. J Virol. 1990;64:672–679. doi: 10.1128/jvi.64.2.672-679.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schreier A A, Gruber J. Viral T antigen interacts with cellular proto-oncogene and anti-oncogene products. J Natl Cancer Inst. 1990;82:354–360. doi: 10.1093/jnci/82.5.354. [DOI] [PubMed] [Google Scholar]
- 55.Shibata M A, Jorcyk C L, Liu M L, Yoshidome K, Gold L G, Green J E. The C3(1)/SV40 T antigen transgenic mouse model of prostate and mammary cancer. Toxicol Pathol. 1998;26:177–182. doi: 10.1177/019262339802600121. [DOI] [PubMed] [Google Scholar]
- 56.Simanis V, Lane D P. An immunopurification procedure for SV40 large-T antigen. Virology. 1985;144:88–100. doi: 10.1016/0042-6822(85)90308-3. [DOI] [PubMed] [Google Scholar]
- 57.Tjian R, Robbins A. Enzymatic activities associated with a purified simian virus 40 T antigen-related protein. Proc Natl Acad Sci USA. 1979;76:610–614. doi: 10.1073/pnas.76.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tommasino M, Adamczewski J P, Carlotti F, Barth C F, Manetti R, Contorni M, Cavalieri F, Hunt T, Crawford L. HPV16 E7 protein associates with the protein kinase p33CDK2 and cyclin A. Oncogene. 1993;8:195–202. [PubMed] [Google Scholar]
- 59.Tuan J, Zhai W, Comai L. Recruitment of TATA-binding protein–TAF1 complex SL1 to the human ribosomal DNA promoter is mediated by the carboxy-terminal activation domain of upstream binding factor (UBF) and is regulated by UBF phosphorylation. Mol Cell Biol. 1999;19:2872–2879. doi: 10.1128/mcb.19.4.2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voit R, Kuhn A, Sander E E, Grummt I. Activation of mammalian ribosomal gene transcription requires phosphorylation of the nuclear transcription factor UBF. Nucleic Acids Res. 1995;23:2593–2599. doi: 10.1093/nar/23.14.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Voit R, Schnapp A, Kuhn A, Rosenbauer H, Hirschmann P, Stunnenberg H G, Grummt I. The nucleolar transcription factor mUBF is phosphorylated by casein kinase II in the C-terminal hyperacidic tail which is essential for transactivation. EMBO J. 1992;11:2211–2218. doi: 10.1002/j.1460-2075.1992.tb05280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Walser A, Deppert W. The kinase activity of SV40 large T antigen is mediated by a cellular kinase. EMBO J. 1986;5:883–889. doi: 10.1002/j.1460-2075.1986.tb04299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhai W, Tuan J, Comai L. SV40 large T antigen binds to the TBP-TAFI complex SL1 and coactivates ribosomal RNA transcription. Genes Dev. 1997;11:1605–1617. doi: 10.1101/gad.11.12.1605. [DOI] [PubMed] [Google Scholar]
- 64.Zhai, W., and L. Comai. Unpublished data.