Abstract
There is major concern about the impact of the COVID‐19 pandemic on adolescent suicidal ideation (SI) and peer relationships. We investigated (1) rates of SI and (2) the extent to which peer connectedness and pre‐existing neural activation to social reward predicted SI during the initial stay‐at‐home orders of the pandemic (April–May 2020) in a longitudinal sample of adolescent girls (N = 93; M age = 15.06; 69% White non‐Hispanic). Daily diary and fMRI methods were used to assess peer connectedness and neural activation to social reward, respectively. Nearly 40% of girls endorsed SI during the initial stay‐at‐home orders. Greater peer connectedness and neural responsivity to anticipated social reward were associated with a reduced odds of SI during the pandemic among girls.
Keywords: suicidal ideation, COVID‐19, neural processes
The novel coronavirus (COVID‐19) pandemic has dramatically disrupted adolescents’ lives across the globe, particularly their social experiences and activities (Rogers, Ha, & Ockey, 2021; Silk et al., 2021). School closures and government‐mandated social distancing guidelines forced many adolescents to severely restrict their in‐person interactions with their peers and miss out on key social milestones (e.g., school dances, graduation). According to surveys conducted during the pandemic, U.S. adolescents reported being most concerned about spending less time in‐person with their peers (Rogers et al., 2021; Silk et al., 2021) and teen girls, in particular, reported feeling lonelier during the pandemic compared to prior to the pandemic (Hinkelman, 2020; Wronski, 2020). These disruptions to adolescents’ daily experiences are concerning because peer relationships are paramount during this period of development (Dahl, Allen, Wilbrecht, & Suleiman, 2018). Further, greater difficulties with peers (e.g., isolation, rejection, and victimization) have been linked to increased suicide risk (Cheek, Goldston, Erkanli, Massing‐Schaffer, & Liu, 2020; Geoffroy et al., 2016; Miller et al., 2018). In the years leading up to the pandemic, suicide has been the second leading cause of death worldwide among individuals 10‐ to 24‐year‐old and one in five adolescents seriously considers suicide each year (Substance Abuse and Mental Health Services Administration, 2020). In light of the unprecedented changes to adolescents’ daily peer experiences due to the COVID‐19 pandemic, many have speculated about how the pandemic might impact adolescent suicidal thoughts and behaviors (e.g., Gunnell et al., 2020; Hoekstra, 2020; Szlyk, Berk, Peralta, & Miranda, 2020).
Suicidal ideation (SI), thoughts of or the desire to end one’s own life (Nock et al., 2008), is a risk factor for adolescent suicide. Prior to the pandemic, prevalence rates for SI among youth ranged from approximately 18% to 24% (Cha et al., 2018; Lindsey, Sheftall, Xiao, & Joe, 2019). Evidence for changes in SI during the pandemic has been mixed. Early studies suggested increased rates of SI in adolescents and young adults during the pandemic (Czeisler et al., 2020, 2021; Isumi, Doi, Yamaoka, Takahashi, & Fujiwara, 2020; Kaparounaki et al., 2020). More recent work has shown no increase in SI among adolescents during the pandemic (Fortgang et al., 2021). Considering that about one‐third of adolescent girls who report SI will attempt suicide during their lifetime (Nock et al., 2013), it is critical to determine which girls may be most at risk for SI during the pandemic, an event characterized by major disruptions to social relationships.
Multiple leading theories of suicide highlight the role of limited rewarding social experiences and social isolation, two characteristics of the COVID‐19 pandemic, in the emergence of suicidal thoughts and behaviors (for a review see Miller & Prinstein, 2019). Specifically, the Interpersonal Theory of Suicide (IPTS; Joiner, 2007; Van Orden et al., 2010) and Three‐Step Theory of Suicide (3ST; Klonsky & May, 2015) both emphasize that feeling disconnected from others confers risk for SI. The IPTS and 3ST draw on Baumeister and Leary’s (1995) seminal theory arguing that forming and maintaining positive social bonds is critical to well‐being. Further, forming and maintaining social bonds is a key developmental task during adolescence (Dahl et al., 2018). Prior to the pandemic, evidence had been growing that low social connectedness broadly (i.e., across peers, parents, and school) is associated with increased SI among adolescents (for a review see Whitlock, Wyman, & Moore, 2014). How peer connectedness may confer risk for SI during the pandemic, however, remains largely unknown. Two studies have demonstrated that SI during the pandemic is linked to greater loneliness among adolescents and adults (Fortgang et al., 2021; Killgore, Cloonan, Taylor, & Dailey, 2020). Research has also shown that adolescents are concerned about feeling connected to their peers during the pandemic (Silk et al., 2021), yet it is unclear if feeling disconnected from peers is associated with SI. To best understand how peer connectedness may influence SI, attention must be paid to the measurement of peer connectedness. Previous research examining the link between peer connectedness and SI has largely relied on one‐time questionnaire assessments of peer connectedness (e.g., Arango, Opperman, Gipson, & King, 2016; Czyz, Liu, & King, 2012) despite evidence that adolescents’ sense of connectedness with peers fluctuates (Silk et al., under review). Repeated assessments over short periods of time are more accurate in capturing experiences as they naturally occur with reduced retrospective bias compared to single assessments (Shiffman, Stone, & Hufford, 2007).
One additional individual‐level factor that might influence both perceptions of peer connectedness and SI during the COVID‐19 pandemic is neural responsivity to social reward. Prior evidence has linked neural responses to social feedback, both positive (e.g., acceptance by peers, compliments) and negative (e.g., rejection, criticism), to SI (Auerbach, Pagliaccio, Allison, Alqueza, & Alonso, 2021), and social connectedness (Fareri & Delgado, 2014; Silk et al., under review) among adolescents. Alterations in social reward processing may be particularly relevant for the pandemic due to the limitations on traditional opportunities for social interactions with peers (e.g., school, extracurricular activities) and positive feedback from peers, which becomes highly valued during adolescence (Dahl et al., 2018). Work from our group recently showed that neural responses to positive peer feedback, but not to negative peer feedback, among adolescent girls at high risk for depression were predictive of depressive symptoms during the pandemic (Sequeira, Silk, Hutchinson, Jones, & Ladouceur, in press). Reduced neural responsivity to social reward, particularly anticipated social reward, may contribute to reduced motivation to seek out potentially pleasurable interactions with peers during the pandemic. Prior research and theory on adolescent depression suggest that blunted neural responses to reward anticipation and feedback contribute to reduced pleasure and approach‐related behaviors, which are key features of depression (Forbes & Dahl, 2012; Keren et al., 2018). Therefore, we examined how peer connectedness during the pandemic and pre‐existing neural activation to social reward (anticipation and receipt) contributed to SI during the pandemic among a sample of adolescent girls using a daily diary and fMRI design.
Social reward processing is supported by social‐affective and motivational brain networks. These networks are comprised of subcortical and prefrontal cortical structures, including the dorsal striatum (e.g., putamen and caudate), ventral striatum (e.g., nucleus accumbens; NAcc), anterior insula (AI), ventral prefrontal cortex, and amygdala (for reviews see (Guyer, Silk, & Nelson, 2016; Nelson, Jarcho, & Guyer, 2016; Schriber & Guyer, 2016; Silverman et al., 2015; Somerville, 2013). The striatum and amygdala are implicated in encoding salience for social stimuli (Di Martino et al., 2008). Additionally, the striatum (ventral and dorsal) and amygdala are thought to guide social behavior based on developmentally relevant social cues (e.g., positive peer feedback) via projections with perceptual regions in the temporal lobe and executive systems in the prefrontal cortex (Nelson et al., 2016). The AI also supports the encoding of emotional salience of social cues (Craig & Craig, 2009; Menon & Uddin, 2010). The ventral medial prefrontal cortex (vmPFC), as well as other prefrontal cortical structures (medial prefrontal cortex, orbital prefrontal cortex), receive projections from the amygdala and striatum and are implicated in modulating the salience of social cues and updating social behaviors to respond flexibly (Nelson et al., 2016). Several dual‐systems models have suggested that the increased valuation of social reward during adolescence is driven, in part, by the protracted development of prefrontal cortical regions relative to subcortical social‐affective regions (Casey, Galván, & Somerville, 2016; Crone & Dahl, 2012; Nelson et al., 2016; Somerville, 2013). Neuroimaging studies in non‐clinical adolescent samples have found activation in these regions, including the dorsal and ventral striatum, AI, and vmPFC, to the anticipation and receipt of positive peer feedback (e.g., Gunther Moor, van Leijenhorst, Rombouts, Crone, & Van der Molen, 2010; Guyer, Choate, Pine, & Nelson, 2012).
Research examining whether alterations in social reward processing predict SI among adolescents is limited, with prior work largely focusing on social threat processing (for reviews see Auerbach et al., 2021; Ballard, Gilbert, Wusinich, & Zarate, 2021; Schmaal et al., 2020). However, some work on the association between neural activation to social reward and SI does exist. Using the Cyberball task (Williams, Cheung, & Choi, 2000), Harms et al. (2019) found that adolescents (approximately 15 years old) with a history of SI and suicide attempt exhibited reduced activation in social‐affective brain regions, including the putamen and insula, during both peer inclusion and exclusion relative to youth with low SI and youth with no history of SI or suicide attempt. These findings parallel prior neuroimaging work showing that reduced striatal activation to monetary reward is associated with higher SI in adults (Minzenberg et al., 2015). Additionally, adults with depression and a history of suicide attempt exhibited blunted activation in the vmPFC to non‐social positive feedback compared to adults with depression and no history of suicide attempt (Dombrovski, Szanto, Clark, Reynolds, & Siegle, 2013). Alterations in social reward‐related and motivational regions may be important neural markers of adolescent SI that warrant further investigation.
The present study investigated how peer connectedness during the pandemic and neural responsivity to positive peer feedback (assessed prior to the pandemic) contributed to SI during the initial stay‐at‐home orders of the COVID‐19 pandemic among adolescent girls. Additionally, we aimed to characterize rates of SI in the present sample during the pandemic. We hypothesized that feeling more connected to peers and greater neural activation to positive peer feedback (anticipated and received) in social‐affective and motivational networks would be associated with a reduced odds of reporting SI during the initial stay‐at‐home orders. The following a priori reward‐related regions were selected: caudate, putamen, AI, NAcc, and vmPFC. Reward‐related neural activation has adequate stability over a 1‐ to 2‐year period (Baranger et al., 2021), supporting the use of neural activation to reward as a trait‐level predictor in short‐term longitudinal analysis. Finally, we explored the association between peer connectedness during the pandemic and pre‐existing neural activation to positive peer feedback, based on work showing a link between neural activation to social feedback and daily peer connectedness (Silk et al., under review).
METHODS
Participants and Procedures
Participants were 93 adolescent girls ages 12–17 recruited from a larger longitudinal study (n = 129) focused on the neurodevelopment of social processing and risk for depression (Kaurin et al., in press; Sequeira et al., in press). Participants between the age of 11‐ and 13‐year‐old were recruited based on shy and/or fearful temperament as determined by the fear and/or shyness subscales of the Early Adolescent Temperament Questionnaire—Revised Version (EAT‐Q; Ellis & Rothbart, 2001). Two‐thirds of the sample was at high risk (+0.75 SDs) and one‐third of the sample was at low‐risk (−0.75 SDs) for depression based on the published age and sex‐matched mean shy and/or fearful EAT‐Q scores (for more details see Ellis & Rothbart, 2001). Participants were excluded from the larger study if they met criteria for current or past anxiety disorders (except specific phobia), mood disorders, psychotic disorders, or autism spectrum disorder at the first time point of the larger study (baseline). Data from larger study that were examined in the present analyses included questionnaire assessments and functional magnetic imaging (fMRI) data that were collected at baseline prior to the pandemic from 2016 to 2019.
A total of 113 participants from the larger study who provided written informed parental consent and child assent for future contact were sent information about the present COVID‐19 follow‐up study approximately 20 days after the start of the initial stay‐at‐home orders in the state of Pennsylvania (March 2020), where data were collected. Stay‐at‐home orders mandated residents to stay at home and only leave the house to perform essential duties (e.g., work, grocery shopping). Participants who had moved outside of the study’s region (n = 2) were required to be under similar stay‐at‐home orders in their respective locations to participate. Of the 113 participants who were initially contacted, 93 participants provided written informed parental consent and child assent online to participate in the COVID‐19 follow‐up study (M = 15.06, SD = 1.21; 59 high risk). The racial and ethnic makeup of the present sample was as follows: White non‐Hispanic (69.8%; n = 65), Black non‐Hispanic (17.2%, n = 16), Asian (2.2%, n = 2), Biracial (9.7%, n = 9), and Other (1.1%, n = 1). Mean total family income across the present sample was approximately $107,859 (SD = $605,554.41; Range = $15,000–$300,000). Data collected during the COVID‐19 follow‐up included 10‐day daily diary assessments and pre‐diary and post‐diary questionnaire assessments during the initial stay‐at‐home orders (April–May 2020). All procedures from the larger study and the COVID‐19 follow‐up were approved by the university’s Human Research Protection Office. On average, approximately 34 months (SD = 9.60; range = 16–52 months; median = 32 months) had passed between the baseline and the COVID‐19 follow‐up. Table 1 contains additional demographic information about the present sample. See the Supplemental Materials for a timeline of the present study’s assessments and procedures completed at each timepoint.
Table 1.
Participant Demographics by Groups Based on Suicidal Ideation (SI) During the Initial Stay‐At‐Home Orders of the COVID‐19 Pandemic Across the Full Sample (N = 93)
| Variable |
SI Group M (SD)/n (%) |
No SI Group M (SD)/n (%) |
|---|---|---|
| Age at baseline | 12.14 (0.74) | 12.34 (0.84) |
| Age at COVID‐19 follow‐up | 14.85 (1.05) | 15.20 (1.30) |
| White, non‐Hispanic | 27 (75%) | 38 (66.7%) |
| Black, non‐Hispanic | 8 (22.2%) | 8 (14%) |
| Biracial | 1 (2.8%) | 8 (14%) |
| Asian | 0 (0%) | 2 (3.5%) |
| Other | 0 (0%) | 1 (1.8%) |
| Baseline approximate total family income | $104,654 (61,540) | $109,711 (60,599) |
| Months from baseline COVID‐19 follow‐up | 32.1 (9.39) | 33.7 (9.76) |
| MFQ‐C Baseline | 10.70 (7.37) | 7.30 (6.47) |
| MFQ‐C COVID‐19 follow‐up pre‐diary* | 18.00 (8.64) | 8.72 (6.61) |
| MFQ‐C COVID‐19 follow‐up post‐diary* | 15.00 (8.72) | 7.42 (6.69) |
| MFQ‐SI COVID‐19 follow‐up pre‐diary* | 0.58 (0.97) | 0 (0) |
| MFQ‐SI COVID‐19 follow‐up post‐diary* | 0.56 (1.00) | 0 (0) |
| SIQ‐JR COVID‐19 follow‐up pre‐diary* | 8.89 (7.89) | 1.33 (2.86) |
| SIQ‐JR COVID‐19 follow‐up post‐diary* | 7.47 (7.19) | 1.00 (2.50) |
MFQ‐C = Mood and Feelings Questionnaire—Child Version (Angold et al., 1987) with SI items removed. MFQ‐SI = Mood and Feelings Questionnaire—Suicidal Ideation composite (Angold et al., 1987). SIQ‐JR = Suicidal Ideation Questionnaire—Junior Version (Reynolds, 1988).
Participants in the SI group were required to have endorsed at least one item on the SIQ‐JR in the past month (rating of two or more on any SIQ‐JR item) and/or one item on the MFQ‐SI sometimes (rating of one or more on any MFQ‐SI item) on either the pre‐/post‐diary questionnaires.
Groups means significantly different at p < .05.
To be included in the present study’s imaging analyses, participants were required to have completed the baseline fMRI scan and have useable imaging data based on scanning contraindications (e.g., head motion, scanner error, and poor task performance). Therefore, the sample for the imaging analyses was comprised of 65 of the 113 participants. Participants included in the imaging analyses did not significantly differ in age at baseline, t(91) = −0.28, p = .78, age at COVID‐19 follow‐up, t(91) = 0.04, p = .97, race, X 2(4, N = 93) = 1.45, p = .84, or income, t(69) = −1.15, p = .25, compared to participants not included in the imaging analyses.
Suicidal Ideation
To assess SI at the COVID‐19 follow‐up, we used two self‐report questionnaires: (1) the Suicidal Ideation Questionnaire—Junior Version (SIQ‐JR; Reynolds, 1987) and (2) the Mood and Feelings Questionnaire—Suicidal Ideation composite (MFQ‐SI; Angold et al., 1987). Both questionnaires were administered on the pre‐diary and post‐diary questionnaire assessments during the COVID‐19 follow‐up, but these measures have different reporting timeframes. The 15‐item SIQ‐JR assessed SI over the past month (for full item details see Supplemental Materials) and each item was rated using a 7‐point scale (0 = I never had this thought, 1 = I had this thought before but not in the past month, 2 = About once a month, 3 = Couple times a month, 4 = About once a week, 5 = Couple times a week, and 6 = Almost every day). Previous research has shown the SIQ‐JR has strong reliability (Reynolds & Mazza, 1999) and convergent predictive validity for suicide attempts (King, Jiang, Czyz, & Kerr, 2014) among adolescent samples. Cronbach’s alphas for the SIQ‐JR during the pandemic (pre‐diary assessment α = .91; post‐diary assessment α = .90) exceeded the recommended cutoff of .70 (Nunnally, 1978).
The MFQ‐SI is a subscale of the larger Mood and Feelings Questionnaire—Child Version (Angold et al., 1987) and is comprised of four items that assess SI over the past 2 weeks on a zero to two scale (0 = not true, 1 = sometimes true, and 2 = true). Previous research has used the MFQ‐SI subscale to measure SI (Oppenheimer et al., 2020), and the MFQ‐SI has demonstrated strong reliability and validity (Hammerton, Zammit, Potter, Thapar, & Collishaw, 2014). Cronbach’s alphas for the MFQ‐SI during the pandemic was adequate (pre‐diary assessment α = .67; post‐diary assessment α = .70). Because some questions asked about lethal behaviors, we used a detailed safety protocol and risk attenuation procedures (for more detail see Supplemental Materials).
The distribution of SI on the SIQ‐JR and MFQ‐SI in the present sample was highly zero‐inflated (for details see Supplemental Materials), indicating a restricted range of SI in the present sample. We transformed SI into a dichotomous dependent variable, which is consistent with prior work (e.g., Lemaire & Graham, 2011; Sheftall et al., 2021). Participants were divided into two groups based on their SIQ‐JR and MFQ‐SI scores at the COVID‐19 follow‐up. Participants in the SI group (n = 36) were required to have endorsed at least one item on the SIQ‐JR with a rating of two or more and/or one item on the MFQ‐SI with a rating of one or more. Participants in the no SI group (n = 57) were required to endorse all items on both the SIQ‐JR and MFQ‐SI below each of the previously stated thresholds. See the Supplemental Materials for rates of SI at the COVID‐19 follow‐up based on each measure alone.
The full sample did not complete the SIQ‐JR prior to the pandemic since this measure was not included in the larger study as suicidality was not a primary focus. Therefore, we were unable to examine changes in SI from before the pandemic to during the pandemic. However, information on rates of SI prior to the pandemic based on the MFQ‐SI only can be found in the Supplemental Materials.
Peer Connectedness During the COVID‐19 Pandemic
Peer connectedness was assessed via an online 10‐day daily diary protocol during the COVID‐19 follow‐up. On each daily diary assessment, participants were instructed to indicate how close and/or connected to their peers they had felt that day using a zero to one hundred sliding scale (0 = not at all, 100 = extremely). Daily peer connectedness ratings were summed and averaged across the total number of completed daily diary assessments to measure average closeness and/or connectedness to their peers in daily life during the initial stay‐at‐home orders. This method is consistent with prior research on adolescent peer experiences in daily life (Kaurin et al., in press; Oppenheimer et al., 2020). Participants completed 88% of daily diary assessments on average. Participants who completed less than 50% of the daily diary assessments (n = 3) were excluded from analyses using daily diary data.
Peer Social Incentive Delay task
To examine pre‐existing neural activation to positive peer feedback, we used the Peer Social Incentive Delay Task (P‐SID; Kaurin et al., in press), a virtual peer interaction task adapted from the original Social Incentive Delay task (Cremers, Veer, Spinhoven, Rombouts, & Roelofs, 2015; Spreckelmeyer et al., 2009). All participants completed the P‐SID during the baseline fMRI scan of the larger study. At a laboratory visit prior to the scan, participants selected and ranked same‐aged girls at purported affiliated universities whom they wished to interact with online during the fMRI scan. Participants made their selections based on the photographs and biographical profiles of the other girls. Participants were led to believe that the other girls would be watching them virtually and giving them feedback on their performance during the task. In reality, the other girls were fictitious “virtual peers” with standardized profiles (for more details see Supplement Materials).
The P‐SID fMRI task consisted of one run of 72 trials (27 social reward, 27 social punishment, 18 neutral). Each trial proceeded in the following order: cue (500 ms), fixation cross (1500–3500 ms), target (160–500 ms), black screen (1000 ms), virtual peer feedback (1650 ms), and black screen (2500–5000 ms). Participants were instructed to press a button with their right index finger when the target (white square) appeared on the black screen. Target presentation was variable (160–500 ms) to ensure that the hit rate in different conditions was similar across participants. The total duration of the P‐SID was 12 min and 2 s (480 volumes).
At the start of each trial, a cue (500 ms) indicated the potential feedback (positive, negative, neutral) that participants could receive from the virtual peer that reflected the three conditions of the task (social reward, social threat, and neutral). In the social reward condition, a circle cued possible positive peer feedback (virtual peer’s happy face) for a fast response or neutral feedback (virtual peer’s scrambled face) for a slow response. In the social punishment condition, a square cued possible negative peer feedback (virtual peer’s angry face) for a slow response or neutral feedback (virtual peer’s scrambled face) for a fast response. In the neutral condition, a triangle cued a neutral outcome (virtual peer’s scrambled face) regardless of whether the participant’s response was fast enough. Following the cue, a white fixation cross (1500–3500 ms) was displayed. The combination of the cue and fixation cross was referred to as the anticipation period. Virtual peer feedback (1650 ms) was displayed following the presentation of the fixation cross during the anticipation period and was comprised of facial expressions taken from the NIMH ChEFS photograph set (Egger et al., 2011). The presentation of virtual peer feedback was referred to as the receipt period. Only neural activation during the social reward and neutral conditions were examined in the present study.
Neuroimaging data acquisition & preprocessing
fMRI data were collected using a Siemens 3T PRISMA at the University of Pittsburgh MRRC with a 64‐channel phased array coil. Anatomical images, field maps, and functional images during the completion of the P‐SID were acquired (for more details see Supplemental Materials). A PC running E‐Prime (www.pstnet.com) was used to control stimulus display.
Data were pre‐preprocessed and analyzed using standard procedures in Statistical Parametric Mapping—Version 12 (SPM12; http://www.fil.ion.ucl.ac.uk/spm). Scans with >0.5 mm of incremental motion, >3 mm from the baseline image, and/or three standard deviations intensity shifts were considered outliers; outlier scans were replaced with a linear interpolation between the two nearest non‐outlier scans. Participants with more than 25% of volumes with excess movement were excluded from analyses. See the Supplemental Materials for more details.
First‐level analyses estimated individual effects using the general linear model approach implemented in SPM12. At the first level, the following task conditions were modeled: anticipation of peer feedback (i.e., cues), positive peer feedback receipt (i.e., smiling face following social reward cue), negative peer feedback receipt (i.e., angry face following social punishment cue), positive peer feedback miss (scrambled face following social reward cue), negative peer feedback hit (scrambled face following social punishment cue), and neutral feedback (scrambled face following neutral cue), with six motion parameters included as nuisance regressors. Regions of interests (ROI) analyses were conducted based on bilateral task activation in a priori regions (putamen, caudate, AI, NAcc, and vmPFC) for the social reward conditions (anticipation and receipt of positive peer feedback) of the P‐SID task. Images for all ROI masks can be found in the Supplemental Materials. The striatal masks were defined anatomically using the AAL atlas in the WFU PickAtlas toolbox for SPM12. The AI mask has been used previously in similar work (Silk et al., 2014). The vmPFC mask was a 10 mm sphere centered on MNI coordinates [2 46 −8], identified from a meta‐analysis on reward processing and subjective value (Bartra, McGuire, & Kable, 2013), and created using the PickAtlas toolbox. Mean parameter estimates were extracted from the ROIs for the contrast positive peer feedback anticipation>neutral anticipation and/or positive peer feedback receipt>neutral receipt using MarsBaR (Brett, Anton, Valabregue, & Poline, 2002) for subsequent statistical analyses.
Covariates
Age and time between baseline and the COVID‐19 follow‐up were included as covariates in all analyses. Additionally, depressive symptoms at baseline and the COVID‐19 follow‐up were assessed via the Mood and Feelings Questionnaire—Child Version (MFQ‐C; Angold et al., 1987) for sensitivity analyses described below. Items on the MFQ‐C pertaining to SI were excluded from total MFQ‐C scores.
Data Analytic Plan
All analyses were conducted in RStudio 4.0.3 (RStudio Team, 2020). We computed the percentage of girls who endorsed SI during the COVID‐19 follow‐up. Logistic regression analyses were conducted to model the odds of SI among girls at the COVID‐19 follow‐up given: (1) peer connectedness at the COVID‐19 follow‐up and (2) pre‐existing neural activation to positive peer feedback (anticipated and received). Age was entered as a covariate in all analyses. Time between baseline and the COVID‐19 follow‐up was entered as a covariate in imaging analyses, as fMRI data were collected at baseline of the larger study. Regression coefficients were used to calculate odds ratios (OR) and adjusted relative risk ratios (RR) for each independent variable included in each model. Separate models were conducted for each of the five a priori ROIs for positive peer feedback (anticipated and received). We applied a false discovery rate (FDR) correction for the number of tests conducted for each condition of the task (five tests for positive peer feedback anticipation; five tests for positive peer feedback receipt). Sensitivity analyses were conducted to determine if the effects of peer connectedness and neural activation to positive peer feedback remained significant when adding depressive symptoms into each model. Finally, Pearson correlations were computed to examine the associations between peer connectedness and neural activation to positive peer feedback (anticipated and received).
RESULTS
SI During the COVID‐19 Pandemic
Of the full sample (N = 93), 36 girls (39%) endorsed SI and 57 girls (61%) did not endorse SI at the COVID‐19 follow‐up.
Peer Connectedness Predicting Adolescent SI During the COVID‐19 Pandemic
In line with our hypotheses, greater feelings of peer connectedness were significantly associated with a reduced odds of reporting SI at the COVID‐19 follow‐up (full sample: OR = .96, RR = .98, p = .001; imaging subsample: OR = .94, RR = .97, p = .001), controlling for age. There was no significant main effect of age (p > .05). Sensitivity analyses revealed that the association between peer connectedness and odds of reporting SI at the COVID‐19 follow‐up remained significant when depressive symptoms at the COVID‐19 follow‐up was entered into the models (full sample: OR = .97, RR = .98, p = .034; imaging subsample: OR = .95, RR = .98, p = .026; see Table 2). Post hoc analyses failed to reveal a significant interaction between age and peer connectedness in predicting the odds of SI at the COVID‐19 follow‐up (p > .05).
Table 2.
Logistic regression coefficients, likelihood ratio 95% confidence intervals, odds ratios (OR), relative risk ratios (RR) predicting the likelihood of adolescent girls’ suicidal ideation during the initial stay‐at‐home orders of the COVID‐19 pandemic based on peer connectedness and neural activation to rewarding peer feedback (anticipated and received) during the Peer Social Incentive Delay Task (P‐SID) controlling for age and time since baseline
| Variables |
SI Group M (SD) |
No SI Group M (SD) |
β (SE) | Z | p | 95% CI | OR | RR |
|---|---|---|---|---|---|---|---|---|
| Peer connectedness | 43.65 (17.56) | 58.74 (19.33) | −.04 (.01) | −3.32 | .001*,†,▲ | 0.93, 0.98 | 0.96 | 0.98 |
| Social Reward Anticipation | ||||||||
| Putamen | −0.53 (1.61) | 0.50 (1.72) | −.37 (.16) | −2.24 | .025* | 0.48, 0.94 | 0.69 | 0.82 |
| Caudate | −0.21 (1.40) | 0.87 (1.82) | −.42 (.18) | −2.35 | .019*,† | 0.45, 0.91 | 0.66 | 0.79 |
| Anterior Insula | −0.32 (1.02) | 0.50 (1.40) | −.56 (.24) | −2.37 | .018*,† | 0.35, 0.88 | 0.57 | 0.73 |
| NAcc | 0.42 (1.80) | 0.42 (2.07) | .00 (.13) | 0.03 | .974 | 0.77, 1.30 | 1.00 | 1.00 |
| vmPFC | 0.33 (2.13) | 1.05 (2.41) | −.14 (.12) | −1.24 | .216 | 0.68, 1.08 | 0.87 | 0.91 |
| Social Reward Outcome | ||||||||
| Putamen | 0.30 (0.70) | 0.20 (0.58) | .21 (.41) | 0.52 | .606 | 0.55, 2.83 | 1.23 | 1.12 |
| Caudate | 0.14 (0.62) | 0.09 (0.63) | .12 (.41) | 0.30 | .767 | 0.51, 2.58 | 1.13 | 1.07 |
| Anterior Insula | 0.11 (0.51) | 0.03 (0.39) | .40 (.58) | 0.69 | .490 | 0.48, 4.83 | 1.24 | 1.14 |
| NAcc | 0.42 (0.97) | 0.32 (0.95) | .07 (.27) | 0.28 | .779 | 0.63, 1.89 | 1.08 | 1.05 |
| vmPFC | 0.23 (0.63) | 0.53 (0.90) | −.47 (.33) | −1.40 | .161 | 0.31, 1.18 | 0.63 | 0.77 |
NAcc = nucleus accumbens; vmPFC = ventromedial prefrontal cortex.
*p <.05. † p <.05 after controlling for depressive symptoms. ▲ = p <.05 for analyses conducted in full sample (n = 93) and imaging sample (n = 65).
Pre‐Existing Neural Activation to Positive Peer Feedback Predicting Adolescent SI During the COVID‐19 Pandemic
In line with our hypotheses, greater neural activation to anticipated positive peer feedback (relative to anticipated neutral peer feedback; P > N) in the caudate (OR = .66, RR = .79, putamen (OR = .69, RR = .82, p = .025) p = .019) and AI (OR = .57, RR = .73, p = .016) was significantly associated with a reduced odds of reporting SI at the COVID‐19 follow‐up, controlling for age and time between baseline and the COVID‐19 follow‐up (see Table 2). These effects were observed after applying a multiple comparison correction (FDR; ps = .042). There were no significant main effects of age or time since baseline in any of the models (ps > .05). Neural activation to anticipated positive peer feedback (P > N) in the NAcc and vmPFC was not significantly associated with the odds of reporting SI at the COVID‐19 follow‐up (ps > .05).
Sensitivity analyses revealed that caudate (OR = .67, RR = .81, p = .030) and AI (OR = .61, RR = .76, p = .044) activation to anticipated positive peer feedback (P > N) remained significantly associated with the odds of reporting SI at the COVID‐19 follow‐up, after controlling for depressive symptoms at baseline. The association between putamen activation to anticipated positive peer feedback (P > N) and the odds of reporting SI at the COVID‐19 follow‐up fell to non‐significant when depressive symptoms were included in the model (p > .05).
No significant associations were found between neural activation to the receipt of positive peer feedback (relative to neutral peer feedback) and the odds of reporting SI at the COVID‐19 follow‐up in any of the a priori regions (ps > .05; see Table 2). Post hoc analyses failed to reveal a significant interaction between age and neural activation to positive peer feedback (anticipation or receipt) in predicting the odds of reporting SI at the COVID‐19 follow‐up (ps > .05).
Exploratory Correlations Between Peer Connectedness During the Pandemic and Pre‐Existing Neural Activation to Positive Peer Feedback
No significant associations were found between peer connectedness during the pandemic and neural activation to positive peer feedback (anticipated and received) (ps > .05; Figure 1).
Figure 1.
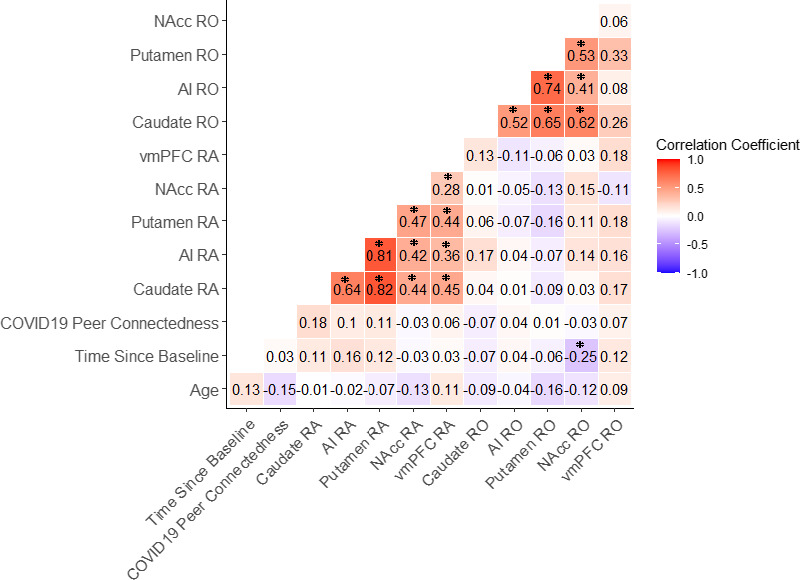
Pearson correlations examining the association between peer connectedness during the initial stay‐at‐home orders during COVID‐19, neural activation to positive peer feedback (anticipation and received) during the Peer Social Incentive Delay task (P‐SID) in all a priori regions, and covariates. Note. *p < .05. RA = neural activation during the social reward anticipation condition of the SID; RO = neural activation during the social reward receipt condition of the P‐SID
DISCUSSION
Public health officials and social scientists have questioned how disruptions to daily peer experiences during the COVID‐19 pandemic have impacted rates of SI among adolescents (Gunnell et al., 2020; Hoekstra, 2020; Szlyk et al., 2020). Due to the present study’s design, we were unable to directly examine changes in the prevalence of SI among adolescent girls during the pandemic compared to before the pandemic. However, nearly 40% of adolescent girls in the present sample reported SI during the initial stay‐at‐home orders of the pandemic, which is higher than rates found in epidemiological work on adolescents prior to the pandemic (18–24%; Cha et al., 2018; Lindsey et al., 2019). Thus, our findings suggest that adolescent SI during the pandemic may be a major concern demanding attention. Our findings also support our hypotheses that greater peer connectedness and neural activation to anticipated social reward, separately, are associated with a reduced odds of reporting SI during the initial stay‐at‐home orders of the pandemic. Thus, reduced peer connectedness and blunted neural activation to anticipated social reward may be risk factors for SI during the pandemic. Despite the small sample size, this study represents an important preliminary investigation of how both pandemic‐specific (e.g., reduced connectedness) and pre‐existing neurobiological (e.g., reduced neural activation to anticipated social reward) risk factors impact adolescent SI during the pandemic.
Our findings indicate that feeling less connected with peers may be an important risk factor for SI during the pandemic. The association between peer connectedness and the odds of reporting SI during the pandemic remained significant after controlling for depressive symptoms, suggesting a unique link between peer connectedness and SI. This finding is consistent with multiple theories of suicide risk (Klonsky & May, 2015; Van Orden et al., 2010). Although a recent study demonstrated that loneliness did not account for changes in SI during the pandemic compared to before the pandemic among adolescents (Fortgang et al., 2021), our findings are in line with work showing a significant association between feeling disconnected from others during the pandemic and SI (Killgore et al., 2020). Social restrictions related to the pandemic have largely limited the opportunities for youth to engage with their peers, during a developmental period in which peer support and experiences are of utmost importance (Dahl et al., 2018). It is also possible that youth who reported SI during the initial stay‐at‐home orders may seek out fewer opportunities for positive peer experiences or interpret interactions with their peers more negatively, thus, more work examining the direction of this finding is needed.
Greater caudate and AI activation to anticipated positive (vs. neutral) peer feedback were significantly associated with a reduced odds of reporting SI during the initial stay‐at‐home orders of the pandemic above and beyond the effects of depressive symptoms in the present sample. Thus, blunted caudate and AI activation to anticipated social reward may be a risk factor for SI during the pandemic. This is in line with work prior to the pandemic showing that adolescents with a history of SI and suicide attempt exhibited blunted caudate activation to social interaction (inclusion and exclusion) during the Cyberball task (Harms et al., 2019). Blunted caudate activation to anticipated social reward could support disruptions in multiple reward‐related processes, given the role of the caudate in supporting reward valuation, reward prediction error, reward learning (Featherstone & McDonald, 2004; Nelson et al., 2016). Limited prior research has examined AI activation to social reward among adolescents with SI, though prior work has shown higher AI activation to social rejection feedback in adolescents reporting high levels of SI (Oppenheimer et al., 2020). Blunted AI activation to anticipated social reward could contribute to reduced valuation of future social experiences due to the AI’s role in representing subjective salience (Menon & Uddin, 2010), though this remains speculative. More generally, adolescent girls with lower neural responsivity to potentially rewarding peer experiences may be more vulnerable to anhedonia (Forbes & Dahl, 2012) and/or loneliness (Cacioppo, Norris, Decety, Monteleone, & Nusbaum, 2009) during the pandemic, both risk factors for SI. More work is needed to replicate these findings in a larger sample and further investigate how caudate and AI functioning may impact subsequent social behaviors that may confer risk for SI.
The association between putamen activation to anticipated positive peer feedback and the odds of reporting SI during the initial stay‐at‐home orders did not remain significant when controlling for depressive symptoms. Therefore, this association may be accounted for by depressive symptoms and reduced putamen activation to anticipated social reward could be a shared neural marker with depression. This is consistent with prior work demonstrating that youth with depression exhibit blunted putamen activation to reward (Fischer et al., 2019; Forbes & Dahl, 2012; Keren et al., 2018).
Contrary to our hypotheses, no significant associations emerged between neural activation during receipt (rather than anticipation) of positive peer feedback and the odds of reporting SI during the initial stay‐at‐home orders of the pandemic. This could suggest that alterations in social reward anticipation are particularly important for understanding adolescent SI. Emerging research among adults suggests that the anticipatory period (vs. receipt period) during reward processing may be important in the etiology of SI (Tsypes, Owens, & Gibb, 2019), although findings have been mixed (Gallyer et al., 2020), and research on social reward processing among adolescents with SI is sparse. Blunted neural activation to reward anticipation may be particularly detrimental during the pandemic, when increased motivation and effort are needed to seek out rewarding social experiences, given the severe social restrictions. Our results highlight the need for further investigation in how different components of social reward processing impact adolescent SI. It is worth noting that there were non‐significant associations between neural activation to social reward (anticipated and received) and peer connectedness. One possibility is that the true effect size of these associations are very small (Lovakov & Agadullina, 2021), and more work is needed to understand how neural response to social reward impacts social functioning among youth with SI.
Limitations of the present study should be noted. The sample of adolescent girls was small, and the majority were White and of high‐income families. Findings from the present study may not generalize across gender, race, ethnicity, or culture; more work is needed to understand risk for SI among a larger sample of youth who may be disproportionately impacted by the pandemic (Tai, Shah, Doubeni, Sia, & Wieland, 2021). Additionally, this sample was not recruited to be at high risk for SI, thus, the range of SI severity was restricted, which led us to dichotomize SI. We recognize that the present findings may not generalize to girls with more severe levels of SI or other forms of psychopathology. Finally, the time between baseline and the COVID‐19 follow‐up differed across participants, although time between baseline and the COVID‐19 follow‐up was included as a covariate and was not significantly associated with the odds of reporting SI.
Despite these limitations, this study has several strengths. The use of a longitudinal design allowed us to examine how both concurrent social and pre‐existing neural factors prospectively predict the odds of SI during the pandemic. Examining how different stages of reward processing (anticipation, receipt) are associated with the odds of reporting SI during the pandemic is another strength of this study. Additionally, this study used novel multimodal assessments (fMRI, daily diary) of daily peer connectedness and neural response to social reward.
Since the time of data collection, the COVID‐19 pandemic has continued to impact adolescents’ daily lives. Moving forward, and in the future crises, it will be important for policy makers to carefully weigh the benefits and cost of limiting in‐person interactions among adolescents, an age group at lower risk for serious physical health issues but increased risk for SI (Glenn et al., 2020). Our findings indicate that some youth (i.e., girls with reduced peer connectedness, girls with reduced neural activation to anticipated social reward) may be at an elevated risk for SI during prolonged periods absent of in‐person peer interactions. Time‐ and cost‐effective behavioral proxies for blunted neural activation to anticipated social reward that can be used to identify adolescents most at risk are needed. Further, adolescents with reduced peer connectedness and neural responsivity to potential social rewards may be less motivated to reconnect with their peers after the pandemic. Parents and school officials may need to actively encourage and facilitate reconnecting adolescents with their peers, which may serve as one strategy to mitigate suicide risk after the pandemic.
Given the small sample size and restricted range of SI severity, results should be viewed as a preliminary investigation of both social and neurobiological risk factors for SI among adolescent girls during the pandemic. It should be noted that several robust predictors of SI do not reliably predict which youth will go on to attempt suicide (McHugh, Corderoy, Ryan, Hickie, & Large, 2019), and more research is needed to understand risk factors for both suicidal thoughts and behaviors. However, identifying risk factors for SI during adolescence is crucial, as risk for SI increases dramatically during adolescence (Nock et al., 2013) and SI is associated with increased distress, impaired functioning, greater health‐related costs, and worsening developmental trajectories characterized by persistent and escalating SI and other suicidal behaviors into adulthood (Copeland, Goldston, & Costello, 2017). Identifying early risk factors for SI may help alter these pathways toward greater health and well‐being. Our findings contribute to a growing body of literature aimed at better understanding biopsychosocial risk factors for adolescent SI. Findings from the present study may also extend to adolescent SI outside of the pandemic context, though this remains to be tested.
Supporting information
Supplementary Material. See for additional information on methods used in the present study.
This work was supported by the National Institute of Mental Health grant R01 MH103241 (PI: J.S. Silk).
The authors declare they have no competing or potential conflicts of interest. The authors thank the research staff for their help in conducting assessments and data acquisition for this project, and the participants of the study for their time and willingness to provide data.
References
- Angold, A. , Weissman, M. M. , John, K. , Merikangas, K. R. , Prusoff, B. A. , Wickramaratne, P. , … Warner, V. (1987). Parent and child reports of depressive symptoms in children at low and high risk of depression. Journal of Child Psychology and Psychiatry, 28(6), 901–916. 10.1111/j.1469-7610.1987.tb00678.x [DOI] [PubMed] [Google Scholar]
- Arango, A. , Opperman, K. J. , Gipson, P. Y. , & King, C. A. (2016). Suicidal ideation and suicide attempts among youth who report bully victimization, bully perpetration and/or low social connectedness. Journal of Adolescence, 51, 19–29. 10.1016/j.adolescence.2016.05.003 [DOI] [PubMed] [Google Scholar]
- Auerbach, R. P. , Pagliaccio, D. , Allison, G. O. , Alqueza, K. L. , & Alonso, M. F. (2021). Neural correlates associated with suicide and nonsuicidal self‐injury in youth. Biological Psychiatry, 89(2), 119–133. 10.1016/j.biopsych.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard, E. D. , Gilbert, J. R. , Wusinich, C. , & Zarate, C. A. (2021). New methods for assessing rapid changes in suicide risk. Frontiers in Psychiatry, 12, 1–9. 10.3389/fpsyt.2021.598434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranger, D. A. A. , Lindenmuth, M. , Nance, M. , Guyer, A. E. , Keenan, K. , Hipwell, A. E. , … Forbes, E. E. (2021). The longitudinal stability of fMRI activation during reward processing in adolescents and young adults. NeuroImage, 232, 117872, 10.1016/j.neuroimage.2021.117872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra, O. , McGuire, J. T. , & Kable, J. W. (2013). The valuation system: A coordinate‐based meta‐analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage, 76, 412–427. 10.1016/j.neuroimage.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumeister, R. F. , & Leary, M. R. (1995). The need to belong: Desire for interpersonal attachments as a fundamental human motivation. Psychological Bulletin, 117, 497–529. 10.1037/0033-2909.117.3.497 [DOI] [PubMed] [Google Scholar]
- Brett, M. , Anton, J. L. , Valabregue, R. , & Poline, J. B. (2002, June). Region of interest analysis using an SPM toolbox. In 8th International conference on functional mapping of the human brain, (2nd end., Vol. 16, pp. 497). Chicago, IL. [Google Scholar]
- Cacioppo, J. T. , Norris, C. J. , Decety, J. , Monteleone, G. , & Nusbaum, H. (2009). In the eye of the beholder: individual differences in perceived social isolation predict regional brain activation to social stimuli. Journal of Cognitive Neuroscience, 21(1), 83–92. 10.1162/jocn.2009.21007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey, B. J. , Galván, A. , & Somerville, L. H. (2016). Beyond simple models of adolescence to an integrated circuit‐based account: A commentary. Developmental Cognitive Neuroscience, 17, 128–130. 10.1016/j.dcn.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha, C. B. , Franz, P. J. , M. Guzmán, E. , Glenn, C. R. , Kleiman, E. M. , & Nock, M. K. (2018). Annual Research Review: Suicide among youth–epidemiology,(potential) etiology, and treatment. Journal of Child Psychology and Psychiatry, 59(4), 460–482. 10.1111/jcpp.12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheek, S. M. , Goldston, D. B. , Erkanli, A. , Massing‐Schaffer, M. , & Liu, R. T. (2020). Social rejection and suicidal ideation and attempts among adolescents following hospitalization: A prospective study. Journal of Abnormal Child Psychology, 48(1), 123–133. 10.1007/s10802-019-00580-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copeland, W. E. , Goldston, D. B. , & Costello, E. J. (2017). Adult associations of childhood suicidal thoughts and behaviors: A prospective, longitudinal analysis. Journal of the American Academy of Child & Adolescent Psychiatry, 56, 958–965.e4. 10.1016/j.jaac.2017.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig, A. D. , & Craig, A. D. (2009). How do you feel–now? The anterior insula and human awareness. Nature Reviews Neuroscience, 10(1), 59–70. 10.1038/nrn2555 [DOI] [PubMed] [Google Scholar]
- Cremers, H. R. , Veer, I. M. , Spinhoven, P. , Rombouts, S. A. R. B. , & Roelofs, K. (2015). Neural sensitivity to social reward and punishment anticipation in social anxiety disorder. Frontiers in Behavioral Neuroscience, 8, 1–9. 10.3389/fnbeh.2014.00439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone, E. A. , & Dahl, R. E. (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–650. 10.1038/nrn3313 [DOI] [PubMed] [Google Scholar]
- Czeisler, M. É. , Lane, R. I. , Petrosky, E. , Wiley, J. F. , Christensen, A. , Njai, R. , … Rajaratnam, S. M. W. (2020). Mental health, substance use, and suicidal ideation during the COVID‐19 pandemic — United States, June 24–30, 2020. MMWR Morbidity and Mortality Weekly Report, 69(32), 1049–1057. 10.15585/mmwr.mm6932a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler, M. É. , Lane, R. I. , Wiley, J. F. , Czeisler, C. A. , Howard, M. E. , & Rajaratnam, S. M. W. (2021). Follow‐up survey of US adult reports of mental health, substance use, and suicidal ideation during the COVID‐19 pandemic, September 2020. JAMA Network Open, 4(2), e2037665. 10.1001/jamanetworkopen.2020.37665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyz, E. K. , Liu, Z. , & King, C. A. (2012). Social connectedness and one‐year trajectories among suicidal adolescents following psychiatric hospitalization. Journal of Clinical Child & Adolescent Psychology, 41(2), 214–226. 10.1080/15374416.2012.651998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl, R. E. , Allen, N. B. , Wilbrecht, L. , & Suleiman, A. B. (2018). Importance of investing in adolescence from a developmental science perspective. Nature, 554, 441–450. 10.1038/nature25770 [DOI] [PubMed] [Google Scholar]
- Di Martino, A. , Scheres, A. , Margulies, D. S. , Kelly, A. M. C. , Uddin, L. Q. , Shehzad, Z. , … Milham, M. P. (2008). Functional connectivity of human striatum: A resting state FMRI study. Cerebral Cortex, 18, 2735–2747. 10.1093/cercor/bhn041 [DOI] [PubMed] [Google Scholar]
- Dombrovski, A. Y. , Szanto, K. , Clark, L. , Reynolds, C. F. , & Siegle, G. J. (2013). Reward signals, attempted suicide, and impulsivity in late‐life depression. JAMA Psychiatry, 70(10), 1020–1030. 10.1001/jamapsychiatry.2013.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger, H. L. , Pine, D. S. , Nelson, E. , Leibenluft, E. , Ernst, M. , Towbin, K. E. , & Angold, A. (2011). The NIMH Child Emotional Faces Picture Set (NIMH‐ChEFS): A new set of children’s facial emotion stimuli. International Journal of Methods in Psychiatric Research, 20(3), 145–156. 10.1002/mpr.343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, L. , & Rothbart, M. (2001). Revision of the early adolescent temperament questionnaire. 2001 Biennial Meeting for the Society for Research in Child Development.
- Fareri, D. S. , & Delgado, M. R. (2014). Social rewards and social networks in the human brain. The Neuroscientist, 20(4), 387–402. 10.1177/1073858414521869 [DOI] [PubMed] [Google Scholar]
- Featherstone, R. E. , & McDonald, R. J. (2004). Dorsal striatum and stimulus‐response learning: Lesions of the dorsolateral, but not dorsomedial, striatum impair acquisition of a stimulus‐response‐based instrumental discrimination task, while sparing conditioned place preference learning. Neuroscience, 124(1), 23–31. 10.1016/j.neuroscience.2003.10.038 [DOI] [PubMed] [Google Scholar]
- Fischer, A. S. , Ellwood‐Lowe, M. E. , Colich, N. L. , Cichocki, A. , Ho, T. C. , & Gotlib, I. H. (2019). Reward‐circuit biomarkers of risk and resilience in adolescent depression. Journal of Affective Disorders, 246, 902–909. 10.1016/j.jad.2018.12.104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes, E. E. , & Dahl, R. E. (2012). Research review: Altered reward function in adolescent depression: What, when and how? Journal of Child Psychology and Psychiatry, 53(1), 3–15. 10.1111/j.1469-7610.2011.02477.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortgang, R. G. , Wang, S. B. , Millner, A. J. , Reid‐Russell, A. , Beukenhorst, A. L. , Kleiman, E. M. , … Nock, M. K. (2021). Increase in suicidal thinking during COVID‐19. Clinical Psychological Science, 9(3), 482–488. 10.1177/2167702621993857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallyer, A. J. , Burani, K. , Mulligan, E. M. , Santopetro, N. , Dougherty, S. P. , Jeon, M. E. , … Hajcak, G. (2020). Blunted neural reward responsiveness and recent suicidal ideation in children and adolescents: Failure to replicate across two independent samples. BioRxiv, 10.1101/2020.05.19.104208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffroy, M.‐C. , Boivin, M. , Arseneault, L. , Turecki, G. , Vitaro, F. , Brendgen, M. , … Côté, S. M. (2016). Associations between peer victimization and suicidal ideation and suicide attempt during adolescence: Results from a prospective population‐based birth cohort. Journal of the American Academy of Child & Adolescent Psychiatry, 55(2), 99–105. 10.1016/j.jaac.2015.11.010 [DOI] [PubMed] [Google Scholar]
- Glenn, C. R. , Kleiman, E. M. , Kellerman, J. , Pollak, O. , Cha, C. B. , Esposito, E. C. , … Boatman, A. E. (2020). Annual research review: A meta‐analytic review of worldwide suicide rates in adolescents. Journal of Child Psychology and Psychiatry, 61(3), 294–308. 10.1111/jcpp.13106 [DOI] [PubMed] [Google Scholar]
- Gunnell, D. , Appleby, L. , Arensman, E. , Hawton, K. , John, A. , Kapur, N. , … Yip, P. S. F. (2020). Suicide risk and prevention during the COVID‐19 pandemic. The Lancet Psychiatry, 7, 468–471. 10.1016/S2215-0366(20)30171-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther Moor, B. , van Leijenhorst, L. , Rombouts, S. A. R. B. , Crone, E. A. , & Van der Molen, M. W. (2010). Do you like me? Neural correlates of social evaluation and developmental trajectories. Social Neuroscience, 5(5–6), 461–482. 10.1080/17470910903526155 [DOI] [PubMed] [Google Scholar]
- Guyer, A. E. , Choate, V. R. , Pine, D. S. , & Nelson, E. E. (2012). Neural circuitry underlying affective response to peer feedback in adolescence. Social Cognitive and Affective Neuroscience, 7(1), 81–92. 10.1093/scan/nsr043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer, A. E. , Silk, J. S. , & Nelson, E. E. (2016). The neurobiology of the emotional adolescent: From the inside out. Neuroscience and Biobehavioral Reviews, 70, 74–85. 10.1016/j.neubiorev.2016.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerton, G. , Zammit, S. , Potter, R. , Thapar, A. , & Collishaw, S. (2014). Validation of a composite of suicide items from the Mood and Feelings Questionnaire (MFQ) in offspring of recurrently depressed parents. Psychiatry Research, 216(1), 82–88. 10.1016/j.psychres.2014.01.040 [DOI] [PubMed] [Google Scholar]
- Harms, M. B. , Casement, M. D. , Teoh, J. Y. , Ruiz, S. , Scott, H. , Wedan, R. , & Quevedo, K. (2019). Adolescent suicide attempts and ideation are linked to brain function during peer interactions. Psychiatry Research: Neuroimaging, 289, 1–9. 10.1016/j.pscychresns.2019.05.001 [DOI] [PubMed] [Google Scholar]
- Hinkelman, L. (2020). Research brief: Findings from 1,273 U.S. Girls on school, technology, relationships and stress since COVID‐19. Retrieved October 1, 2020 https://static1.squarespace.com/static/597249b6d7bdcec54c7fdd10/t/5ef647de701c53068afb3c31/1593198563282/ROX+Research+Brief_Findings+from+1%2C273+US+Girls+on+School%2C+Technology%2C+Relationships+%26+Stress+Since+COVID‐19.pdf [Google Scholar]
- Hoekstra, P. J. (2020). Suicidality in children and adolescents: Lessons to be learned from the COVID‐19 crisis. European Child & Adolescent Psychiatry, 29, 737–738. 10.1007/s00787-020-01570-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isumi, A. , Doi, S. , Yamaoka, Y. , Takahashi, K. , & Fujiwara, T. (2020). Do suicide rates in children and adolescents change during school closure in Japan? The acute effect of the first wave of COVID‐19 pandemic on child and adolescent mental health. Child Abuse & Neglect, 110, 104680. 10.1016/j.chiabu.2020.104680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner, T. (2007). Why people die by suicide. Harvard University Press. [Google Scholar]
- Kaparounaki, C. K. , Patsali, M. E. , Mousa, D.‐P.‐V. , Papadopoulou, E. V. K. , Papadopoulou, K. K. K. , & Fountoulakis, K. N. (2020). University students’ mental health amidst the COVID‐19 quarantine in Greece. Psychiatry Research, 290, 113111. 10.1016/j.psychres.2020.113111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaurin, A. , Sequeira, S. L. , Ladouceur, C. , McKone, K. M. P. , Rosen, D. , Jones, N. P. , … Silk, J. (in press). Modeling sensitivity to social threat in adolescent girls: A psychoneurometric approach. Journal of Abnormal Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren, H. , O’Callaghan, G. , Vidal‐Ribas, P. , Buzzell, G. A. , Brotman, M. A. , Leibenluft, E. , … Stringaris, A. (2018). Reward processing in depression: A conceptual and meta‐analytic review across fMRI and EEG studies. American Journal of Psychiatry, 175(11), 1111–1120. 10.1176/appi.ajp.2018.17101124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore, W. D. S. , Cloonan, S. A. , Taylor, E. C. , & Dailey, N. S. (2020). Loneliness: A signature mental health concern in the era of COVID‐19. Psychiatry Research, , 290, 113117. 10.1016/j.psychres.2020.113117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- King, C. A. , Jiang, Q. , Czyz, E. K. , & Kerr, D. C. R. (2014). Suicidal ideation of psychiatrically hospitalized adolescents has one‐year predictive validity for suicide attempts in girls only. Journal of Abnormal Child Psychology, 42(3), 467–477. 10.1007/s10802-013-9794-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonsky, E. D. , & May, A. M. (2015). The Three‐Step Theory (3ST): A new theory of suicide rooted in the “ideation‐to‐action” framework. International Journal of Cognitive Therapy, 8(2), 114–129. 10.1521/ijct.2015.8.2.114 [DOI] [Google Scholar]
- Lemaire, C. M. , & Graham, D. P. (2011). Factors associated with suicidal ideation in OEF/OIF veterans. Journal of Affective Disorders, 130(1), 231–238. 10.1016/j.jad.2010.10.021 [DOI] [PubMed] [Google Scholar]
- Lindsey, M. A. , Sheftall, A. H. , Xiao, Y. , & Joe, S. (2019). Trends of suicidal behaviors among high school students in the United States: 1991–2017. Pediatrics, 144(5), e20191187. 10.1542/peds.2019-1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovakov, A. , & Agadullina, E. R. (2021). Empirically derived guidelines for effect size interpretation in social psychology. European Journal of Social Psychology, 1–20. 10.1002/ejsp.2752 [DOI] [Google Scholar]
- McHugh, C. M. , Corderoy, A. , Ryan, C. J. , Hickie, I. B. , & Large, M. M. (2019). Association between suicidal ideation and suicide: Meta‐analyses of odds ratios, sensitivity, specificity and positive predictive value. BJPsych Open, 5(2), 1–12. 10.1192/bjo.2018.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon, V. , & Uddin, L. Q. (2010). Saliency, switching, attention and control: A network model of insula function. Brain Structure and Function, 214(5–6), 655–667. 10.1007/s00429-010-0262-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A. B. , Linthicum, K. P. , Helms, S. W. , Giletta, M. , Rudolph, K. D. , Hastings, P. D. , … Prinstein, M. J. (2018). Reciprocal associations between adolescent girls’ chronic interpersonal stress and nonsuicidal self‐injury: A multi‐wave prospective investigation. Journal of Adolescent Health, 63, 694–700. 10.1016/j.jadohealth.2018.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, A. B. , & Prinstein, M. J. (2019). Adolescent suicide as a failure of acute stress‐response systems. Annual Review of Clinical Psychology, 15(1), 425–450. 10.1146/annurev-clinpsy-050718-095625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg, M. J. , Lesh, T. A. , Niendam, T. A. , Yoon, J. H. , Cheng, Y. , Rhoades, R. N. , & Carter, C. S. (2015). Control‐related frontal‐striatal function is associated with past suicidal ideation and behavior in patients with recent‐onset psychotic major mood disorders. Journal of Affective Disorders, 188, 202–209. 10.1016/j.jad.2015.08.049 [DOI] [PubMed] [Google Scholar]
- Nelson, E. E. , Jarcho, J. M. , & Guyer, A. E. (2016). Social re‐orientation and brain development: An expanded and updated view. Developmental Cognitive Neuroscience, , 17, 118–127. 10.1016/j.dcn.2015.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock, M. K. , Borges, G. , Bromet, E. J. , Alonso, J. , Angermeyer, M. , Beautrais, A. , … Williams, D. (2008). Cross‐national prevalence and risk factors for suicidal ideation, plans and attempts. British Journal of Psychiatry, 192(2), 98–105. 10.1192/bjp.bp.107.040113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock, M. K. , Green, J. G. , Hwang, I. , McLaughlin, K. A. , Sampson, N. A. , Zaslavsky, A. M. , & Kessler, R. C. (2013). Prevalence, correlates, and treatment of lifetime suicidal behavior among adolescents: Results from the national comorbidity survey replication adolescent supplement. JAMA Psychiatry, 70(3), 300–310. 10.1001/2013.jamapsychiatry.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunnally, J. C. (1978). Psychometric theory, 2nd edn. New York, NY: McGraw‐Hill. 10.1037/018882 [DOI] [Google Scholar]
- Oppenheimer, C. W. , Silk, J. S. , Lee, K. H. , Dahl, R. E. , Forbes, E. , Ryan, N. , & Ladouceur, C. D. (2020). Suicidal ideation among anxious youth: A preliminary investigation of the role of neural processing of social rejection in interaction with real world negative social experiences. Child Psychiatry and Human Development, 51, 163–173. 10.1007/s10578-019-00920-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds, W. M. (1987). Suicidal ideation questionnaire (SIQ). Odessa, FL: Psychological Assessment Resources. [Google Scholar]
- Reynolds, W. M. (1988). Suicidal ideation questionnaire: Professional manual. Psychological Assessment Resources. [Google Scholar]
- Reynolds, W. M. , & Mazza, J. J. (1999). Assessment of suicidal ideation in inner‐city children and young adolescents: Reliability and validity of the Suicidal Ideation Questionnaire‐JR. School Psychology Review, 28(1), 17–30. 10.1080/02796015.1999.12085945 [DOI] [Google Scholar]
- Rogers, A. A. , Ha, T. , & Ockey, S. (2021). Adolescents’ perceived socio‐emotional impact of COVID‐19 and implications for mental health: Results from a U.S.‐based mixed‐methods study. Journal of Adolescent Health, 68(1), 43–52. 10.1016/j.jadohealth.2020.09.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RStudio Team (2020). RStudio: Integrated development for R. Boston, MA: RStudio, PBC. Retrieved from July 1, 2020 http://www.rstudio.com/ [Google Scholar]
- Schmaal, L. , van Harmelen, A.‐L. , Chatzi, V. , Lippard, E. T. C. , Toenders, Y. J. , Averill, L. A. , … Blumberg, H. P. (2020). Imaging suicidal thoughts and behaviors: A comprehensive review of 2 decades of neuroimaging studies. Molecular Psychiatry, 25(2), 408–427. 10.1038/s41380-019-0587-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schriber, R. A. , & Guyer, A. E. (2016). Adolescent neurobiological susceptibility to social context. Developmental Cognitive Neuroscience, 19, 1–18. 10.1016/j.dcn.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira, S. L. , Silk, J. S. , Hutchinson, E. , Jones, N. P. , & Ladouceur, C. D. (in press). Neural responses to social reward predict depressive symptoms in adolescent girls during the COVID‐19 pandemic. Journal of Pediatric Psychology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheftall, A. H. , Vakil, F. , Armstrong, S. E. , Rausch, J. R. , Feng, X. , Kerns, K. A. , … Bridge, J. A. (2021). Clinical risk factors, emotional reactivity/regulation and suicidal ideation in elementary school‐aged children. Journal of Psychiatric Research, 138, 360–365. 10.1016/j.jpsychires.2021.04.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman, M. H. , Jedd, K. , & Luciana, M. (2015). Neural networks involved in adolescent reward processing: An activation likelihood estimation meta‐analysis of functional neuroimaging studies. NeuroImage, 122, 427–439. 10.1016/j.neuroimage.2015.07.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman, S. , Stone, A. A. , & Hufford, M. R. (2008). Ecological momentary assessment. Annual Review of Clinical Psychology, 4(1), 1–32. 10.1146/annurev.clinpsy.3.022806.091415 [DOI] [PubMed] [Google Scholar]
- Silk, J. S. , Nelson, E. , Dahl, R. E. , Stroud, L. , Lee, K. H. , & Siegle, G. J. (2014). Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive and Affective Neuroscience, 9, 1798–1807. 10.1093/scan/nst175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk, J. S. , Scott, L. , Hutchinson, E. A. , Lu, C. , Sequeira, S. , McKone, K. M. , … Ladouceur, C. (2021, February 2). Storm clouds and silver linings: Impacts of COVID‐19 and daily emotional health in adolescent girls. PsyArXiv. 10.31234/osf.io/hmsj8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk, J. S. , Sequeira, S. S. , Jones, N. P. , Lee, K. H. , Dahl, R. E. , Forbes, E. E. , … Ladouceur, C. D. (under review). Neural sensitivity to peer rejection predicts increases in depressive symptoms among anxious adolescents.
- Somerville, L. H. (2013). Special issue on the teenage brain: Sensitivity to social evaluation. Current Directions in Psychological Science, 22(2), 121–127. 10.1177/0963721413476512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreckelmeyer, K. N. , Krach, S. , Kohls, G. , Rademacher, L. , Irmak, A. , Konrad, K. , … Gründer, G. (2009). Anticipation of monetary and social reward differently activates mesolimbic brain structures in men and women. Social Cognitive and Affective Neuroscience, 4(2), 158–165. 10.1093/scan/nsn051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . (2020). Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health.
- Szlyk, H. S. , Berk, M. , Peralta, A. O. , & Miranda, R. (2020). COVID‐19 takes adolescent suicide prevention to less charted territory. Journal of Adolescent Health, 67(2), 161–163. 10.1016/j.jadohealth.2020.05.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai, D. B. G. , Shah, A. , Doubeni, C. A. , Sia, I. G. , & Wieland, M. L. (2021). The disproportionate impact of COVID‐19 on racial and ethnic minorities in the United States. Clinical Infectious Diseases, 72(4), 703–706. 10.1093/cid/ciaa815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsypes, A. , Owens, M. , & Gibb, B. E. (2019). Blunted neural reward responsiveness in children with recent suicidal ideation. Clinical Psychological Science, 7(5), 958–968. 10.1177/2167702619856341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Orden, K. A. , Witte, T. K. , Cukrowicz, K. C. , Braithwaite, S. R. , Selby, E. A. , & Joiner, T. E. (2010). The interpersonal theory of suicide. Psychological Review, 117(2), 575–600. 10.1037/a0018697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitlock, J. , Wyman, P. A. , & Moore, S. R. (2014). Connectedness and suicide prevention in adolescents: Pathways and implications. Suicide and Life‐Threatening Behavior, 44(3), 246–272. [DOI] [PubMed] [Google Scholar]
- Williams, K. D. , Cheung, C. K. T. , & Choi, W. (2000). Cyberostracism: Effects of being ignored over the internet. Journal of Personality and Social Psychology, 79, 748–762. 10.1037/0022-3514.79.5.748 [DOI] [PubMed] [Google Scholar]
- Wronski, L. (2020). Common sense media/surveymonkey poll: Coronavirus and teenagers. Retrieved from October 1, 2020 https://www.surveymonkey.com/curiosity/common‐sense‐media‐coronavirus/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material. See for additional information on methods used in the present study.


