Abstract
Background
Pressure ulcers (also known as pressure injuries, pressure sores and bed sores) are localised injuries to the skin or underlying soft tissue, or both, caused by unrelieved pressure, shear or friction. Specific kinds of beds, overlays and mattresses are widely used with the aim of preventing and treating pressure ulcers.
Objectives
To summarise evidence from Cochrane Reviews that assess the effects of beds, overlays and mattresses on reducing the incidence of pressure ulcers and on increasing pressure ulcer healing in any setting and population.
To assess the relative effects of different types of beds, overlays and mattresses for reducing the incidence of pressure ulcers and increasing pressure ulcer healing in any setting and population.
To cumulatively rank the different treatment options of beds, overlays and mattresses in order of their effectiveness in pressure ulcer prevention and treatment.
Methods
In July 2020, we searched the Cochrane Library. Cochrane Reviews reporting the effectiveness of beds, mattresses or overlays for preventing or treating pressure ulcers were eligible for inclusion in this overview. Two review authors independently screened search results and undertook data extraction and risk of bias assessment using the ROBIS tool. We summarised the reported evidence in an overview of reviews. Where possible, we included the randomised controlled trials from each included review in network meta‐analyses. We assessed the relative effectiveness of beds, overlays and mattresses for preventing or treating pressure ulcers and their probabilities of being, comparably, the most effective treatment. We assessed the certainty of the evidence using the GRADE approach.
Main results
We include six Cochrane Reviews in this overview of reviews, all at low or unclear risk of bias.
Pressure ulcer prevention: four reviews (of 68 studies with 18,174 participants) report direct evidence for 27 pairwise comparisons between 12 types of support surface on the following outcomes: pressure ulcer incidence, time to pressure ulcer incidence, patient comfort response, adverse event rates, health‐related quality of life, and cost‐effectiveness. Here we focus on outcomes with some evidence at a minimum of low certainty.
(1) Pressure ulcer incidence: our overview includes direct evidence for 27 comparisons that mostly (19/27) have very low‐certainty evidence concerning reduction of pressure ulcer risk. We included 40 studies (12,517 participants; 1298 participants with new ulcers) in a network meta‐analysis involving 13 types of intervention. Data informing the network are sparse and this, together with the high risk of bias in most studies informing the network, means most network contrasts (64/78) yield evidence of very low certainty. There is low‐certainty evidence that, compared with foam surfaces (reference treatment), reactive air surfaces (e.g. static air overlays) (risk ratio (RR) 0.46, 95% confidence interval (CI) 0.29 to 0.75), alternating pressure (active) air surfaces (e.g. alternating pressure air mattresses, large‐celled ripple mattresses) (RR 0.63, 95% CI 0.42 to 0.93), and reactive gel surfaces (e.g. gel pads used on operating tables) (RR 0.47, 95% CI 0.22 to 1.01) may reduce pressure ulcer incidence. The ranking of treatments in terms of effectiveness is also of very low certainty for all interventions. It is unclear which treatment is best for preventing ulceration.
(2) Time to pressure ulcer incidence: four reviews had direct evidence on this outcome for seven comparisons. We included 10 studies (7211 participants; 699 participants with new ulcers) evaluating six interventions in a network meta‐analysis. Again, data from most network contrasts (13/15) are of very low certainty. There is low‐certainty evidence that, compared with foam surfaces (reference treatment), reactive air surfaces may reduce the hazard of developing new pressure ulcers (hazard ratio (HR) 0.20, 95% CI 0.04 to 1.05). The ranking of all support surfaces for preventing pressure ulcers in terms of time to healing is uncertain.
(3) Cost‐effectiveness: this overview includes direct evidence for three comparisons. For preventing pressure ulcers, alternating pressure air surfaces are probably more cost‐effective than foam surfaces (moderate‐certainty evidence).
Pressure ulcer treatment: two reviews (of 12 studies with 972 participants) report direct evidence for five comparisons on: complete pressure ulcer healing, time to complete pressure ulcer healing, patient comfort response, adverse event rates, and cost‐effectiveness. Here we focus on outcomes with some evidence at a minimum of low certainty.
(1) Complete pressure ulcer healing: our overview includes direct evidence for five comparisons. There is uncertainty about the relative effects of beds, overlays and mattresses on ulcer healing. The corresponding network meta‐analysis (with four studies, 397 participants) had only three direct contrasts and a total of six network contrasts. Again, most network contrasts (5/6) have very low‐certainty evidence. There was low‐certainty evidence that more people with pressure ulcers may heal completely using reactive air surfaces than using foam surfaces (RR 1.32, 95% CI 0.96 to 1.80). We are uncertain which surfaces have the highest probability of being the most effective (all very low‐certainty evidence).
(2) Time to complete pressure ulcer healing: this overview includes direct evidence for one comparison: people using reactive air surfaces may be more likely to have healed pressure ulcers compared with those using foam surfaces in long‐term care settings (HR 2.66, 95% CI 1.34 to 5.17; low‐certainty evidence).
(3) Cost‐effectiveness: this overview includes direct evidence for one comparison: compared with foam surfaces, reactive air surfaces may cost an extra 26 US dollars for every ulcer‐free day in the first year of use in long‐term care settings (low‐certainty evidence).
Authors' conclusions
Compared with foam surfaces, reactive air surfaces may reduce pressure ulcer risk and may increase complete ulcer healing. Compared with foam surfaces, alternating pressure air surfaces may reduce pressure ulcer risk and are probably more cost‐effective in preventing pressure ulcers. Compared with foam surfaces, reactive gel surfaces may reduce pressure ulcer risk, particularly for people in operating rooms and long‐term care settings. There are uncertainties for the relative effectiveness of other support surfaces for preventing and treating pressure ulcers, and their efficacy ranking.
More high‐quality research is required; for example, for the comparison of reactive air surfaces with alternating pressure air surfaces. Future studies should consider time‐to‐event outcomes and be designed to minimise any risk of bias.
Plain language summary
What are the benefits and risks of beds, mattresses and overlays for preventing and treating pressure ulcers?
The overview presents a lot of data from randomised controlled trials and contains an advanced analysis called 'network meta‐analysis'. The analysis allows comparisons of all types of support surfaces for preventing or treating pressure ulcers. This interactive tool may help with navigation of the datahttps://stopthepressure.shinyapps.io/Cochrane_support_surface_reviews/.
Key messages
Static air mattresses or overlays, alternating pressure air mattresses or overlays, and gel pads used on operating tables may be better than foam mattresses for preventing pressure ulcers.
Compared with foam mattresses, alternating pressure air mattresses or overlays probably result in health benefits that outweigh their costs in preventing pressure ulcers.
Static air mattresses or overlays may be better than foam mattresses for ulcer healing, but may cost more.
It is unclear what the best treatment is for either preventing or treating pressure ulcers; what the effects of these treatment options are on people’s comfort and quality of life; and whether or not there are any unwanted effects.
What are pressure ulcers?
Pressure ulcers (also known as pressure sores or bed sores) are wounds to the skin and underlying tissue caused by prolonged pressure or rubbing. People who have mobility problems or who lie in bed for long periods are at risk of developing pressure ulcers.
What did we want to find out?
There are many types of beds, mattresses and overlays specifically designed for people with pressure ulcers. These can be made from a range of materials (such as foam, air cells and gel pads) and are divided into two groups:
‐ reactive (static) surfaces that apply a constant pressure to the skin; and
‐ active (alternating pressure) surfaces that regularly redistribute the pressure under the body.
We wanted to find out if different types of reactive and active surfaces:
‐ prevent pressure ulcers;
‐ help ulcers to heal;
‐ are comfortable and improve people’s quality of life;
‐ have health benefits that outweigh their costs; and
‐ have any unwanted effects.
We also wanted to find out what the best treatment options are for either preventing or healing pressure ulcers.
What did we do?
We searched for Cochrane Reviews that summarised the results of all available carefully designed studies (controlled trials) evaluating different beds, mattresses and overlays in preventing and treating pressure ulcers. A Cochrane Review provides a high level of evidence on the effectiveness of healthcare interventions. We summarised the results of these reviews in a single document (called an overview of reviews).
We also collected studies included in these reviews and compared all available treatments at the same time in a single analysis (called network meta‐analysis). We then summarised these results, and rated our confidence in the evidence, based on factors such as study methods and sizes.
What did we find?
Effects in preventing pressure ulcers
We found four reviews on the use of beds, mattresses and overlays for preventing pressure ulcers. From these, we included 40 studies (12,517 people) in a network meta‐analysis evaluating reduction of pressure ulcer risk. The network meta‐analysis evidence suggests that static (reactive) air overlays, alternating pressure air mattresses, and (reactive) gel pads used on operating tables may reduce pressure ulcer risk compared with foam mattresses.
We also included 10 studies (7211 people) in a network meta‐analysis evaluating the time taken for new ulcers to develop. The network meta‐analysis evidence suggests that reactive air surfaces may reduce the chances of developing new ulcers compared with foam surfaces.
Effects in treating pressure ulcers
We found two reviews on pressure ulcer healing. From these, we included four studies (397 people) in a network meta‐analysis. The network meta‐analysis evidence suggests that more people with ulcers may heal completely using reactive air surfaces than foam surfaces.
The overview evidence suggests that, if the time needed to completely heal an ulcer is looked at, reactive air surfaces may improve the chances of pressure ulcers healing when compared with foam mattresses.
However, it is unclear which treatment is best for either preventing or treating pressure ulcers.
Other effects in preventing and treating pressure ulcers
The overview evidence suggests that:
‐ compared with foam mattresses, alternating pressure air surfaces probably result in health benefits that outweigh their costs in preventing pressure ulcers;
‐ reactive air‐filled surfaces may cost more than foam mattresses in healing ulcers; and
‐ the other benefits and risks of these beds, mattresses and mattress overlays are unclear.
What are the limitations of the evidence?
Although the reviews we found used reliable methods, most of the studies in them were small and used methods likely to introduce errors in their results.
How up‐to‐date is this evidence?
The evidence in this overview is current to July 2020.
Background
Description of the condition
Pressure ulcers (also known as pressure injuries, pressure sores, decubitus ulcers and bed sores) are localised injuries to the skin or underlying soft tissue, or both, caused by unrelieved pressure, shear (i.e. forces moving in opposite directions) or friction (NPIAP 2016). Pressure ulcer severity is generally classified using the National Pressure Injury Advisory Panel (NPIAP) system (NPIAP 2016).
Stage 1: intact skin with a local appearance of non‐blanchable erythema (i.e. skin redness)
Stage 2: partial‐thickness skin loss with exposed dermis
Stage 3: full‐thickness skin loss
Stage 4: full‐thickness skin and tissue loss with visible fascia (i.e. the soft connective tissue that holds structures in place), muscle, tendon, ligament, cartilage or bone
Unstageable pressure injury: full‐thickness skin and tissue loss that is obscured by slough or eschar (i.e. dead tissue) so that the severity of injury cannot be confirmed
A deep tissue pressure injury: local injury of persistent, non‐blanchable deep red, maroon, purple discolouration or epidermal separation revealing a dark wound bed or blood‐filled blister.
The stages of pressure ulceration described above are consistent with those described in the International Classification of Diseases for Mortality and Morbidity Statistics of the World Health Organization 2019.
Pressure ulcers are relatively common, complex wounds that affect people across different populations and different care settings. A systematic review found that prevalence estimates for people affected by pressure ulcers in communities of the United Kingdom (UK), USA, Ireland and Sweden ranged from 5.6 to 2300 per 10,000, depending on the nature of the population surveyed and the denominators used for calculating these prevalence estimates (Cullum 2016). A subsequent, large, UK cross‐sectional survey of people receiving community health services in one area of the UK estimated that 1.8 people per 10,000 have a pressure ulcer. This study used a total population figure denominator for the community services where the survey was undertaken (Gray 2018).
Pressure ulcers confer a heavy burden in terms of personal impact and health service resource use. Having a pressure ulcer may impair physical, social and psychological activities (Gorecki 2009). Ulceration impairs health‐related quality of life (Essex 2009); can result in longer institution stays (Theisen 2012); and may increase the risk of systemic infection (Espejo 2018). Pressure ulceration also has substantial impacts on health systems: a 2015 systematic review of 14 studies across a range of care settings in Europe and North America showed that pressure ulcer‐related treatment costs ranged between EUR 1.71 and 470.49 per person, per day (Demarré 2015). In the UK, the annual average National Health Service (NHS) cost attributable to managing one person with a pressure ulcer in the community was estimated to be GBP 1400 for a Stage 1 pressure ulcer and more than GBP 8500 for more severe stages (2015/2016 prices; Guest 2018). In Australia, the annual cost of treating pressure ulcers was estimated to be AUD 983 million (95% confidence interval (CI) 815 to 1151 million) at 2012/2013 prices (Nguyen 2015). The serious consequences of pressure ulceration have led to an intensive focus on their prevention.
Description of the interventions
Pressure ulcers are considered to be largely preventable via the use of pressure‐relieving processes in those considered at risk. Additionally, pressure relief is part of the treatment offered to those with ulceration. Support surfaces are specialised medical devices designed to relieve or redistribute pressure on the body, or both, in order to prevent and treat pressure ulcers (NPIAP S3I 2019). Types of support surface include, but are not limited to, integrated bed systems, mattresses and overlays (NPIAP S3I 2019).
There are a number of different types of support surface, which can now be classified using the NPIAP Support Surface Standards Initiative (S3I) 'Terms and Definitions Related to Support Surfaces' (NPIAP S3I 2019). According to the NPIAP S3I terms and definitions, support surfaces may:
be powered (i.e. require electrical power to function) or non‐powered;
passively redistribute body weight (i.e. reactive pressure redistribution), or mechanically vary pressure on the body to reduce the duration of pressure on any one point (i.e. active pressure redistribution);
be made of a range of materials including, but not limited to: air cells, foam materials, fibre materials, gel materials, sheepskin for medical use, and water‐bags;
be constructed of air‐filled cells which have small holes on the surface through which air blows onto skin (i.e. low‐air‐loss feature) or have fluid‐like characteristics via forcing filtered air through ceramic beads (i.e. air‐fluidised feature), or have neither of these features.
Full details of support‐surface classifications are listed in Appendix 1. Various types of beds, overlays and mattresses are available, with the aim of promoting pressure ulcer prevention and treatment, including alternating pressure (active) air surfaces, reactive air surfaces, foam surfaces and alternative reactive support surfaces that are made neither of foam materials nor air cells.
How the intervention might work
Support surfaces used with the aim of preventing and treating pressure ulcers aim to redistribute pressure beneath the skin of the body, in order to increase blood flow to tissues and relieve skin and soft tissue distortion (Wounds International 2010). Powered support surfaces are operated by electricity, unlike non‐powered surfaces. Active support surfaces achieve pressure redistribution by frequently changing the points of contact between the surface and body, reducing the duration of the pressure applied to specific anatomical sites (Clark 2011; NPIAP S3I 2019). This contrasts with reactive support surfaces' mode of action, which is passive and includes immersion (i.e. 'sinking' of the body into a support surface) and envelopment (i.e. conforming of a support surface to the irregularities in the body). Reactive support surfaces distribute the pressure over a greater area, thereby reducing the magnitude of the pressure at specific sites (Clark 2011). Additionally, support surfaces with low‐air‐loss features are designed to improve the skin microclimate with the aim of maintaining skin and tissue integrity, particularly in people with incontinence (Wounds International 2010).
Why it is important to do this overview
Specific kinds of beds, mattresses and overlays are widely used for pressure ulcer prevention and treatment and are the focus of recommendations in key international and national guidelines, including the 2019 guideline published by a consortium of pressure ulcer organisations (EPUAP/NPIAP/PPPIA 2019), and the UK National Institute for Health and Care Excellence (NICE) 2014 guidelines (NICE 2014). These two guidelines both recommend using foam surfaces for preventing pressure ulcers. However, the EPUAP/NPIAP/PPPIA 2019 guideline also recommends considering the use of other support surface options but does not specify further options.
Several Cochrane Reviews evaluate the evidence for different beds, overlays and mattresses in preventing and treating pressure ulcers. These Cochrane Reviews include:
'Alternating pressure (active) air surfaces for preventing pressure ulcers' (Shi 2021a);
'Foam surfaces for preventing pressure ulcers' (Shi 2021b);
'Reactive air surfaces for preventing pressure ulcers' (Shi 2021c);
'Alternative reactive support surfaces (non‐foam and non‐air‐filled) for preventing pressure ulcers' (Shi 2021d) (Differences between protocol and review);
'Beds, overlays and mattresses for treating pressure ulcers' (Shi 2021e) (Differences between protocol and review).
In this overview, we draw together key findings from these reviews into a single document for decision‐makers. We summarise relevant Cochrane Reviews and the results of head‐to‐head comparisons of beds, overlays and mattresses in preventing and treating pressure ulcers. Where data are available, we also conduct network meta‐analyses which simultaneously compare all alternative beds, overlays and mattresses to investigate which may be most effective for preventing and treating pressure ulcers.
Objectives
To summarise evidence from Cochrane Reviews that assess the effects of beds, overlays and mattresses on reducing the incidence of pressure ulcers and on increasing pressure ulcer healing in any setting and population.
To assess the relative effects of different types of beds, overlays and mattresses for reducing the incidence of pressure ulcers and increasing pressure ulcer healing in any setting and population.
To cumulatively rank the different treatment options of beds, overlays and mattresses in order of their effectiveness in pressure ulcer prevention and treatment.
Methods
This section describes the methods for this overview of reviews and the network meta‐analysis. We largely focus on describing the overview process. Where required, we briefly describe specific methods related to the network meta‐analysis, for reference. Full details of the network meta‐analysis can be found in Appendix 2.
Criteria for considering reviews for inclusion
Types of reviews and studies
We included current versions of published Cochrane Reviews, and Cochrane Reviews which are now published but were ongoing when the electronic search was run, where only randomised controlled trials (RCTs) evaluating beds, overlays and mattresses in pressure ulcer prevention and treatment were eligible (Differences between protocol and review). All studies included in these reviews are part of the overview and were considered for inclusion in the network meta‐analysis.
We also re‐screened RCTs that were excluded from the eligible systematic reviews for the network meta‐analysis to assess if they could contribute data (an RCT may have been excluded due to comparison with an ineligible intervention but may still contribute information to the network meta‐analysis if the ineligible intervention is a common comparator to link eligible support surfaces into a network) (see Appendix 2). We excluded the ongoing studies and studies awaiting assessment identified in eligible reviews.
Types of participants
For pressure ulcer prevention, Cochrane Reviews involving any population, in any setting, were eligible.
For pressure ulcer treatment, Cochrane Reviews involving people with existing pressure ulcers, of any stage and in any setting, were eligible.
Types of interventions
We included reviews that assessed the effects of specific kinds of beds, overlays and mattresses (see Description of the interventions). Based on the NPIAP S3I terms and definitions related to support surfaces (NPIAP S3I 2019), eligible interventions included but were not limited to:
alternating pressure (active) air surfaces;
foam surfaces;
reactive air surfaces;
reactive fibre surfaces;
reactive gel surfaces;
reactive sheepskin surfaces; and
reactive water surfaces.
We excluded reviews that evaluated limb protectors, chair cushions, seat cushion overlays, traditional Chinese herb‐filled surfaces, homemade surfaces, and turning beds.
Types of outcome measures
Primary outcomes
For pressure ulcer prevention, the primary outcome was pressure ulcer incidence, reported as proportion of participants developing a new pressure ulcer of any stage or time to pressure ulcer development.
For pressure ulcer treatment, the primary outcome was complete healing of existing pressure ulcers, reported as the proportion of participants with healed pressure ulcers or time to pressure ulcer healing.
Secondary outcomes
We included the following secondary outcomes in this overview of reviews. Our network meta‐analyses only included primary outcomes.
Support surface‐associated patient comfort. The definition and measurement of this outcome varied from one review to another and were not restricted by included reviews; for example, the proportion of participants who report comfort, or comfort measured by a scale with continuous (categorical) numbers.
All reported adverse events (measured using surveys, questionnaires, data capture process or visual analogue scales). We considered the assessment of any event in general defined as adverse by participants, health professionals, or both.
Health‐related quality of life (HRQOL) (measured using a standardised generic questionnaire such as EQ‐5D (Herdman 2011), or pressure ulcer‐specific questionnaires such as the PURPOSE Pressure Ulcer Quality of Life (PU‐QOL) questionnaire (Gorecki 2013)). We present evidence on overall scores of questionnaires used rather than reporting multiple domain scores from the same measure.
Cost‐effectiveness. Data extracted were incremental mean cost per incremental gain in benefit (incremental cost‐effectiveness ratio (ICER)) and other measures of relative cost‐effectiveness (e.g. net monetary benefit, net health benefit).
We recorded outcome data from any time points specified in eligible reviews. Eligible reviews considered evidence on an outcome measure at multiple time points. We considered outcome measures at three months, or those closest to three months, as the primary interest of this overview (Schoonhoven 2007), regardless of the time points specified as being of primary interest by the review itself. Where a review only reported a single time point or did not specify a time point for its outcome measurement, we nevertheless included these data in this overview. For all outcomes, we classed:
one week or less up to eight weeks as short‐term follow‐up;
more than 8 weeks to 16 weeks as medium‐term follow‐up;
more than 16 weeks as long‐term follow‐up.
Search methods for identification of reviews
Electronic searches
We searched the Cochrane Database of Systematic Reviews (CDSR) in the Cochrane Library for any reviews with search terms related to 'support surfaces' and 'pressure ulcers' in the title, abstract or keyword fields. We identified studies to include in the network meta‐analysis by screening the reviews that met our inclusion criteria.
Data collection and analysis
Selection of reviews
Two overview authors (CS and ELG) independently assessed the titles and abstracts of the search results for relevance. These authors then independently inspected the full texts of all potentially eligible reviews for the overview. They resolved disagreements by discussion to reach consensus.
Once decisions had been made on the included reviews, two overview authors (CS and ELG) independently screened the included and excluded studies from each review for inclusion in the network meta‐analysis. The two reviewers resolved disagreements by discussion to reach consensus.
Data extraction and management
For this overview, we extracted the following data from each included review onto a pre‐prepared and piloted data extraction form.
Review identification and the review author.
Review titles and objectives.
Search date.
Review inclusion and exclusion criteria.
Number of included trials and participants.
Settings included.
Participant characteristics including mean age, proportions of participants by gender, and participants’ baseline skin status if available.
All comparisons of beds, overlays and mattresses.
Methods and results of risk of bias of the included trials.
Outcomes presented and time points of outcome data.
Narrative summary of data and meta‐analysis results (e.g. effect sizes and 95% confidence intervals (CIs)).
Details of heterogeneity assessment.
GRADE assessments.
Details of subgroup and sensitivity analyses where available.
One overview author extracted data, which a second author independently checked.
For network meta‐analyses, we extracted the following data for each relevant study, ideally from the review and, where required, from the trial publication itself.
Study design.
Care setting.
Characteristics of participants (average age, proportions of participants by gender, and participants’ baseline skin status).
Beds, overlays and mattresses or other interventions being compared.
Follow‐up duration.
Number of participants randomised and analysed.
Number of participants lost to follow‐up.
Number of participants developing new ulcers or healing rates of existing pressure ulcers.
We found that several trials of beds, overlays and mattresses for pressure ulcer prevention appeared in more than one review. In this case we reconciled data across these reviews to avoid duplication of evidence in the overview and double‐counting of trial data in the network meta‐analysis.
Assessment of methodological quality of included reviews
Assessment of risk of bias in included reviews for overview
Two overview authors (CS and ELG; or ELG and GN) who were not authors of the included Cochrane Review independently assessed risk of bias using the ROBIS tool (Whiting 2016). ROBIS assesses risk of bias in three phases: first, assessing relevance (optional); second, identifying concerns with the review process; and third, forming an overall judgement of the risk of bias. In the second phase, concerns with the review process can be identified for four specific domains:
Study eligibility criteria: assessing whether eligibility criteria of included reviews were pre‐specified, clear and appropriate to the review question.
Identification and selection of studies: assessing whether any trials that would have met the inclusion criteria of a review were not included in the review.
Data collection and study appraisal: assessing whether bias may have been introduced during the data collection or risk of bias assessment processes.
Synthesis and findings: assessing whether, when the review authors decided to pool data from the included trials (either in a quantitative or qualitative synthesis), the review authors have used appropriate methods to do so (Whiting 2016).
Concerns can be graded as 'low', 'high' or 'unclear'. We noted the rationale for decisions at each stage. As this overview only included Cochrane Reviews and relevance has been considered as part of our screening and selection process, we did not assess relevance using the ROBIS tool (an optional first phase).
Any disagreements between two overview authors were resolved by discussion; involvement of a third overview author was not required.
Assessment of risk of bias in included studies for network meta‐analyses
For RCTs in the network meta‐analyses, we used the results of the overall risk of bias of the included trials that had already been assessed by the review authors.
One RCT in the network meta‐analyses had not previously undergone risk of bias assessment. Two overview authors (CS and ELG) independently undertook this using Cochrane's risk of bias tool (Higgins 2017).
We present overall risk of bias judgements for the included studies based on (1) all risk of bias domains; and (2) all but the performance bias domain (Differences between protocol and review). We note further details about risk of bias assessment in Appendix 3.
Assessment of risk of bias for direct comparisons, each network contrast and a network as a whole
We used the approach proposed in Salanti 2014 to assess the overall risk of bias for direct evidence (i.e. evidence from the head‐to‐head comparison of two interventions), each network contrast (any pair of interventions in the network), and each network as a whole, as follows.
We used the study‐level overall risk of bias judgement without considering the performance bias domain to assess the risk of bias for each direct comparison of two interventions.
We assessed risk of bias for each contrast in the network, taking into account the study‐level risk of bias and their percentage contributions to the network estimate.
We also calculated the overall risk of bias in the network as a whole by considering the risk of bias for each direct comparison and their percentage contributions to the whole network.
For changes to this section, please see Differences between protocol and review.
Data synthesis
Measures of treatment effect
For dichotomous outcome data (e.g. pressure ulcer incidence), we present the risk ratio (RR) with its 95% confidence interval (CI). For continuous outcome data, we present the mean difference (MD) with 95% CIs for studies that used the same assessment scale. If studies reporting continuous data used different assessment scales, we report the standardised mean difference (SMD) with 95% CIs.
For time‐to‐event data (e.g. time to pressure ulcer development), we present the hazard ratio (HR) with its 95% CI. For those included studies reporting time‐to‐event data that did not report an HR, we estimated this using other reported outcomes, such as numbers of events, through employing available statistical methods (Parmar 1998; Tierney 2007).
For network meta‐analyses, we also present the relative ranking of each bed, overlay and mattress support surface based on the estimated probability of that surface being the most effective (in terms of ulcer prevention or healing). These values are a cumulative probability called the Surface Under the Cumulative RAnking (SUCRA) (Salanti 2011). A SUCRA value can range from 0% to 100% and the larger the SUCRA value, the higher the ranking of a bed, overlay or mattress support surface for the outcome of interest (Chaimani 2013; Salanti 2011).
Methods for overview data presentation and synthesis
The aim of this overview was to present a detailed summary of evidence on beds, overlays and mattresses for pressure ulcer prevention and treatment. We present all eligible comparisons grouped by intervention type. We used tabular formats and narrative techniques to present evidence summaries alongside the GRADE assessment for each comparison; and if the included reviews did not undertake GRADE assessment then we undertook it. Where possible, we also present results of meta‐analyses, along with details of effects models and measures of statistical heterogeneity (i.e. Chi² tests and relevant P values, and I² statistics). Where meta‐analyses had not been undertaken, we report study‐level effects narratively. We did not present results of subgroup and sensitivity analyses as the included reviews did not have sufficient data for any planned subgroup analysis, and sensitivity analyses reported in included reviews did not suggest any differences (Differences between protocol and review).
We present the certainty of evidence for eligible outcomes and comparisons from each included Cochrane Review in a summary of findings table. The table is designed according to the summary of findings table template proposed in Yepes‐Nuñez 2019 for network meta‐analysis. The table includes participants, interventions, comparators, outcomes, settings, the number of studies, the number of total participants, effect sizes and 95% CIs, anticipated absolute effects and 95% CIs of each group and difference between groups, certainty of evidence, and interpretation of findings.
Methods for network meta‐analyses
We conducted three separate network meta‐analyses: one for the proportion of participants developing a new pressure ulcer; one for time to pressure ulcer development; and one for the proportion of participants with healed pressure ulcers. We did not identify sufficient data for the pre‐planned analysis for time to pressure ulcer healing outcome.
Unit of analysis, missing data, homogeneity and transitivity assumptions, and reporting bias have impacts on the validity of network meta‐analysis. Prior to carrying out network meta‐analysis, we addressed those issues using methods described in Appendix 2. Here, transitivity means that important clinical and methodological characteristics (effect modifiers) at the comparison (rather than study) level are similar enough that we can assume that intervention effects are transitive across network contrasts.
We synthesised RCT data using the published network commands and network graph packages of STATA for network meta‐analysis and graphically present results (Chaimani 2013; Chaimani 2015; White 2015). We estimated the relative effectiveness of any two interventions as a function of each intervention relative to the reference intervention (foam surfaces).
Using STATA (networkplot), we produced a network plot of the included beds, overlays and mattresses for each network meta‐analysis, to understand the geometry of the evidence base and to inform the analysis. The term ‘networkplot’, as well as ‘network meta’ and ‘sucra’ in the paragraphs below, refer to commands developed for the STATA software package. We excluded studies with one eligible arm where the arm could not be connected to the network in any way.
We performed network meta‐analyses using multivariate meta‐regression models in STATA (network meta) to estimate the relative effects for network contrasts. This modelling approach addresses correlations between the effect sizes from multi‐arm studies. We fitted a consistency model that assumes an agreement between direct and indirect evidence, and we assumed that a bed, overlay or mattress support surface has the average effect size for a range of similar populations (random‐effects model). Note that indirect evidence is obtained from comparisons of treatments via a common comparator.
Methods for network meta‐analyses' relative rankings
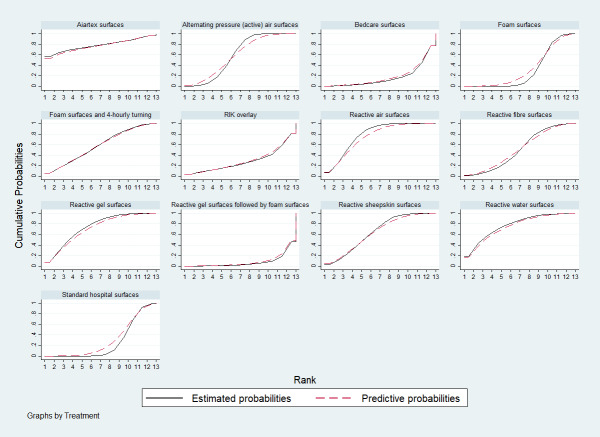
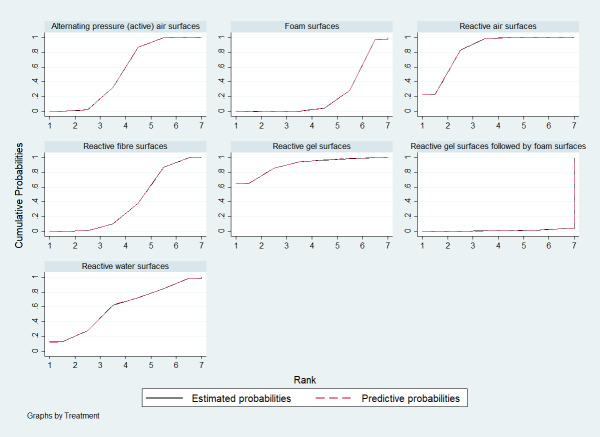
On the basis of relative effect estimates of each bed, overlay and mattress, we calculated the SUCRA percentages to estimate the relative rankings of bed, overlay and mattress support surfaces in STATA (sucra), and also presented a cumulative rank probability plot (Differences between protocol and review). We estimated the relative rankings for each network meta‐analysis, presenting a cumulative rank probability plot for each bed, overlay and mattress ‒ this is a plot of the cumulative rank probabilities that each intervention is less than or equal to a specific rank order against the possible rankings (Chaimani 2013).
Assessing the certainty of evidence and summary of findings tables for network meta‐analyses
We assessed the certainty of evidence for the network meta‐analyses using the GRADE approach proposed by Salanti 2014 via the Confidence in the Results of Network Meta‐Analysis (CINeMA) tool (Nikolakopoulou 2020). We assessed the certainty of evidence in two ways: (1) for each network contrast; and (2) for the network as a whole (assessing the certainty of the relative ranking).
The GRADE assessment using CINeMA involves consideration of six domains: within‐study bias; across‐studies bias (publication bias); indirectness; imprecision; heterogeneity; and incoherence (inconsistency). To make the within‐study bias judgement, CINeMA evaluates the contributions of the included studies to each network contrast, producing the percentage contribution matrix (Nikolakopoulou 2020). These contributions are then used to weight study‐level risk of bias results to estimate the network contrast‐level within‐study bias (Nikolakopoulou 2020). The certainty of evidence can be assessed as being high, moderate, low or very low. RCT evidence has the potential to be high certainty.
We present a separate summary of findings table for each network meta‐analysis undertaken. We present the following primary outcomes in the summary of findings tables.
Proportion of participants developing a new pressure ulcer.
Time to pressure ulcer development.
Proportion of participants with healed pressure ulcers.
Time to pressure ulcer healing.
Subgroup analysis and investigation of heterogeneity in the network meta‐analyses
Assessment of statistical heterogeneity and inconsistency
We assessed the presence of the common network statistical heterogeneity in network meta‐analyses using the I² statistic and its 95% CIs or Tau², or both (see Appendix 2 for further details). We also assessed inconsistency at levels of local loops, and the whole network using methods described in Appendix 2. Inconsistency refers to statistical disagreement between direct and indirect evidence and is a manifestation of non‐transitivity (Cipriani 2013).
Investigation of heterogeneity (including subgroup analysis)
Where a network had important heterogeneity, we followed steps proposed by Cipriani 2013 to investigate this further. We performed subgroup analyses for binary and categorical factors (or meta‐regression for continuous factors) for the following four study‐level characteristics.
Overall risk of bias (binary: low or unclear risk of bias; and high risk of bias) (Schulz 1995).
Settings (categorical: acute care and other hospital settings; long‐term care settings; operating theatre setting; and intensive care unit).
Baseline skin status (categorical: participants at risk, other skin status or non‐reporting; non‐blanchable erythema; existing ulcers of stage 2 or more severe) (Shi 2018b).
Follow‐up duration (categorical: short‐term; medium‐term; and long‐term) (Schoonhoven 2007) (Differences between protocol and review).
Sensitivity analysis for the network meta‐analyses
We assessed the robustness of our findings via a sensitivity analysis to assess the impact of missing data. We also undertook a post hoc sensitivity analysis to assess the impact of including only well‐defined support surfaces in the pressure ulcer incidence network meta‐analysis.
Further details about heterogeneity investigation and sensitivity analysis are given in Appendix 2.
Results
See Characteristics of included reviews (Appendix 4).
Description of included reviews
Overview of reviews: description of included reviews
The search generated 22 records. We excluded 13 records based on the title and abstract, and assessed nine in full text. Of these, we considered six Cochrane Reviews eligible for this overview: McGinnis 2014 and another five reviews (Shi 2021a; Shi 2021b; Shi 2021c; Shi 2021d; Shi 2021e) that were ongoing when the electronic search was run but are published now (See Figure 1).
1.
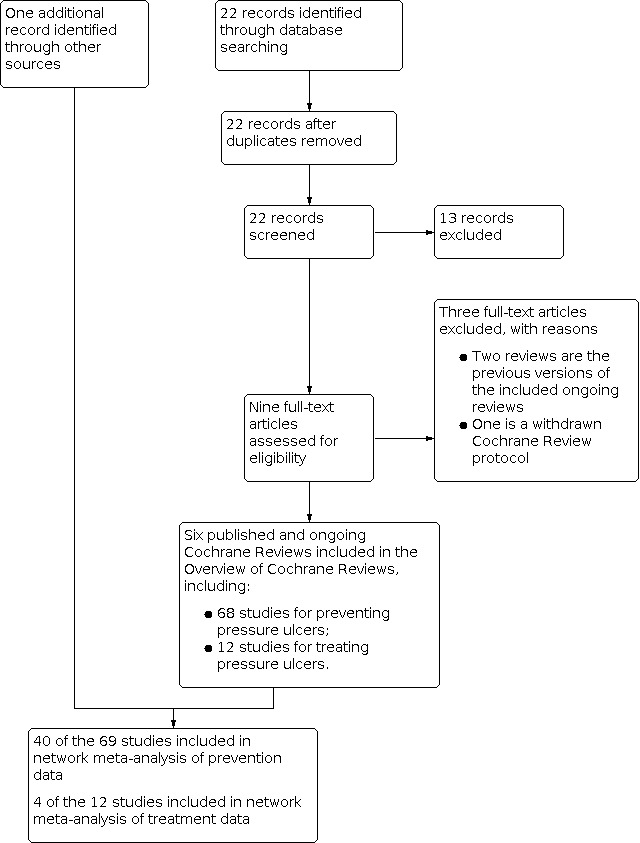
Study flow diagram
We excluded two reviews as they are previous versions of the included reviews (McInnes 2015; McInnes 2018). We also excluded a withdrawn Cochrane Review protocol (Greenwood 2017).
See Appendix 4 for a summary of characteristics of the six included reviews.
Of the six reviews, four focused on the use of beds, mattresses or overlays for preventing pressure ulcers (Shi 2021a; Shi 2021b; Shi 2021c; Shi 2021d). These four reviews report evidence on pressure ulcer incidence for 27 pairwise comparisons, time to pressure ulcer incidence for seven comparisons, patient comfort responses for 11 comparisons, all reported adverse events for six comparisons, health‐related quality of life for two comparisons, and cost‐effectiveness for four comparisons (see Appendix 5). These comparisons involved 12 types of beds, mattresses and overlays:
alternating pressure (active) air surfaces;
foam surfaces;
reactive gel surfaces followed by foam surfaces;
reactive air surfaces;
reactive gel surfaces;
reactive fibre surfaces;
reactive sheepskin surfaces;
reactive water surfaces;
reactive foam and gel surfaces;
Aiartex surfaces, a brand of support surfaces that could not be classified using the NPIAP S3I support surface terms and definitions;
Bedcare surfaces, a brand of support surfaces that could not be classified; and
'standard hospital surfaces' that could not be classified.
Two of the six reviews focused on the use of beds, mattresses or overlays for treating pressure ulcers (McGinnis 2014; Shi 2021e). These reviews report evidence on the proportion of participants with pressure ulcers completely healed for five comparisons, time to complete pressure ulcer healing for one comparison, support surface‐associated patient comfort for five comparisons, all reported adverse events for six comparisons, and cost‐effectiveness for one comparison (see Appendix 6). These comparisons involved seven types of beds, mattresses or overlays:
alternating pressure (active) air surfaces;
reactive air surfaces;
foam surfaces;
reactive gel surfaces;
reactive water surfaces;
a type of reactive surface (Aiartex) that we could not define using NPIAP S3I 2007 support surface terms; and
'standard hospital surfaces' that could not be classified.
Methodological quality of included reviews
ROBIS quality of included reviews
See Appendix 7.
We judged the overall risk of bias of included reviews using the ROBIS tool to be low for five reviews (Shi 2021a; Shi 2021b; Shi 2021c; Shi 2021d; Shi 2021e), and unclear for one review (McGinnis 2014).
Our judgements of the risk of bias for the four domains in ROBIS were as follows:
we judged the domain of 'study eligibility' to be of low concern for all included reviews;
we judged the domain of 'study identification and selection' to be of low concern for all included reviews;
we judged the domain of 'data collection and study appraisal' to be of low concern for five included reviews (Shi 2021a; Shi 2021b; Shi 2021c; Shi 2021d; Shi 2021e), but unclear for McGinnis 2014 because it evaluated the ulcer healing outcome but appeared not to collect data on key baseline characteristics of pressure ulcers from its included studies;
we judged the domain of 'synthesis and findings' to be of low concern for five included reviews (Shi 2021a; Shi 2021b; Shi 2021c; Shi 2021d; Shi 2021e), but unclear for McGinnis 2014 because it did not consider certainty of evidence assessment.
Certainty of evidence in included reviews
Of the six included reviews, five reported GRADE assessment, whilst McGinnis 2014 did not assess the certainty of evidence. For this latter review, we undertook GRADE assessment of relative treatment‐effect data.
Overall, the GRADE certainty of evidence was mainly low or very low, as summarised in Appendix 5 and Appendix 6. Of the 27 comparisons with pressure ulcer incidence data, we judged 3.7% of findings (1/27) to be of moderate certainty, 25.9% (7/27) of low certainty, and 63.0% (17/27) of very low certainty. The remaining two (7.4%) comparisons included studies that had no analysable data or results for evidence synthesis, thus no GRADE assessment.
Of the five comparisons presenting pressure ulcer healing outcome data, we judged 20.0% (1/5) of findings to be of low certainty and 80.0% (4/5) to be of very low certainty.
Common reasons for downgrading the certainty of evidence were risk of bias of included studies, imprecision and inconsistency.
Effect of interventions
We report evidence on pressure ulcer prevention in Section 1 and treatment evidence in Section 2. In each section, we summarise the key findings of the overview of reviews for each available outcome: we present full review findings in Appendix 5 (for prevention evidence) and Appendix 6 (for treatment evidence). For primary outcomes, we also report findings of network meta‐analysis, following their overview summaries.
Section 1. Beds, overlays and mattresses for preventing pressure ulcers
See Appendix 5 for full details of evidence for all outcomes as reported in included reviews.
For pressure ulcer incidence and time to ulcer development, we give a summary of overviews of reviews and then focus on findings from the relevant network meta‐analyses.
Pressure ulcer incidence
Overview of reviews
Four reviews reported evidence on 27 specific direct comparisons of beds, overlays and mattresses for preventing pressure ulcers (Shi 2021a, Shi 2021b, Shi 2021c, Shi 2021d; Appendix 5). Of the 27 comparisons,
21 are between two different types of support surface and their results are reported in Table 1;
four are between two support surfaces of the same type;
two have no data on this outcome for any synthesis (foam surfaces versus reactive water surfaces; and reactive water surfaces versus reactive fibre surfaces), thus no corresponding results are presented.
1. Summary of review results for overview of reviews.
| Pressure ulcer incidence | Standard hospital surfaces | Narrative results; reactive sheepskin surfaces may decrease the hazard of having new ulcers (3 studies, 1424 participants; Shi 2021d) ⊕⊕⊝⊝ LOWa |
Narrative results; Uncertain evidence (3 studies, 3072 participants; Shi 2021b) |
Time to pressure ulcer development | ||||||||||
| Aiartex surfaces | ||||||||||||||
| RIK microfluid static overlays | ||||||||||||||
| Bedcare surfaces | ||||||||||||||
| Reactive foam and gel surfaces | ||||||||||||||
| Reactive fibre surfaces | ||||||||||||||
| Reactive gel surfaces followed by foam surfaces | ||||||||||||||
| RR
0.35 (0.15 to 0.79) reactive water surfaces may reduce ulcer risk (1 study, 316 participants; Shi 2021d) ⊕⊕⊝⊝ LOWa |
Reactive water surfaces | |||||||||||||
| Narrative results: reactive sheepskin surfaces may reduce pressure ulcer risk (3 studies, 1424 participants; Shi 2021d) | Reactive sheepskin surfaces | |||||||||||||
| Narrative synthesis: reactive gel surfaces probably reduce pressure ulcer risk (2 studies, 446 participants; Shi 2021d) ⊕⊕⊕⊝ MODERATEa |
Narrative results; uncertain evidence (2 studies, 122 participants; Shi 2021d) ⊕⊝⊝⊝ VERY LOWa,b |
Narrative results; uncertain evidence (1 study, 166 participants; Shi 2021d) ⊕⊝⊝⊝ VERY LOWa,b |
Reactive gel surfaces | |||||||||||
| Narrative synthesis: alternating pressure (active) air surfaces may reduce pressure ulcer incidence (4 studies, 830 participants; Shi 2021a) ⊕⊕⊝⊝ LOWa |
RR 0.90 (0.68 to 1.19) Uncertain evidence for this comparison (3 studies, 285 participants; Shi 2021a; Shi 2021d) ⊕⊝⊝⊝ VERY LOWa,b |
RR 0.22 (0.06 to 0.76) Alternating pressure (active) air surfaces may reduce pressure ulcer risk (2 studies, 415 participants; Shi 2021a, Shi 2021d) ⊕⊕⊝⊝ LOWa,b |
RR 1.21 (0.52 to 2.83) Uncertain evidence (2 studies, 358 participants; Shi 2021a; Shi 2021d) ⊕⊝⊝⊝ VERY LOWa,b |
Alternating pressure (active) air surfaces | HR 2.25
(1.05 to 4.83) Alternating pressure (active) air surfaces may increase the hazard of having new ulcers (one study, 308 participants; Shi 2021a; Shi 2021c) ⊕⊕⊝⊝ LOWa |
HR 0.41 (0.10 to 1.64) Uncertain evidence (2 studies, 2105 participants; Shi 2021a; Shi 2021b) ⊕⊝⊝⊝ VERY LOWa,b |
||||||||
| Narrative results: uncertain evidence (2 studies, 216 participants; Shi 2021c) ⊕⊝⊝⊝ VERY LOWa,b |
RR 0.33 (0.07 to
1.58) Uncertain evidence (1 study, 110 participants; Shi 2021c) ⊕⊝⊝⊝ VERY LOWa,b |
RR 0.43 (0.04 to 4.29) Uncertain evidence (1 study, 37 participants; Shi 2021c; Shi 2021d) ⊕⊝⊝⊝ VERY LOWa,b |
RR 1.25 (0.56 to 2.77) Uncertain evidence (1 study, 66 participants; Shi 2021c; Shi 2021d) ⊕⊝⊝⊝ VERY LOWa,b |
RR 1.61 (0.90 to 2.88) Uncertain evidence (6 studies, 1648 participants; Shi 2021a; Shi 2021c) ⊕⊝⊝⊝ VERY LOWa,b |
Reactive air surfaces | |||||||||
| Narrative results: uncertain evidence (8 studies, 4066 participants; Shi 2021b) ⊕⊝⊝⊝ VERY LOWa,b |
RR 0.56 (0.19 to 1.60) Uncertain evidence (1 study, 206 participants; Shi 2021b) ⊕⊝⊝⊝ VERY LOWa,b |
Narrative results: uncertain evidence for this comparison (1 study, 91 participants; Shi 2021b; Shi 2021d) ⊕⊝⊝⊝ VERY LOWa,b |
RR 1.17
(0.64 to 2.14) Uncertain evidence for this comparison (1 study, 68 participants; Shi 2021b; Shi 2021d) ⊕⊝⊝⊝ VERY LOWa,b |
Narrative results: uncertain evidence for this comparison (1 study, 135 participants; Shi 2021b; Shi 2021d) ⊕⊝⊝⊝ VERY LOWa,b |
RR 0.63 (0.34 to 1.17) Alternating pressure (active) air surfaces may reduce pressure ulcer risk (4 studies, 2247 participants; Shi 2021a, Shi 2021b) ⊕⊕⊝⊝ LOWa,b |
RR 0.42 (0.18 to 0.96) Reactive air surfaces may reduce pressure ulcer incidence (4 studies, 229 participants; Shi 2021b; Shi 2021c) ⊕⊕⊝⊝ LOWa,b |
Foam surfaces |
This table presents summaries of direct evidence results from the individual reviews referred to. Blank cells indicate the lack of direct evidence in the included individual reviews.
aDowngraded for risk of bias. bDowngraded for imprecision.
As Table 1 indicates, of the 21 comparisons between different types of support surface, 14 comparisons yield very low‐certainty evidence regarding their relative effects (downgraded mainly for risk of bias and imprecision). The remaining seven comparisons have moderate‐certainty or low‐certainty evidence, suggesting that:
reactive air surfaces may reduce pressure ulcer risk compared with foam surfaces for people in acute and long‐term care settings;
alternating pressure (active) air surfaces may reduce pressure ulcer risk compared with foam surfaces for people in acute and long‐term care settings;
alternating pressure (active) air surfaces applied on both operating tables and hospital beds may reduce pressure ulcer risk compared with reactive gel surfaces used on operating tables followed by foam surfaces applied on hospital beds;
alternating pressure (active) air surfaces may reduce pressure ulcer risk compared with standard hospital surfaces for people in acute and long‐term care settings;
in operating rooms, reactive gel surfaces probably reduce pressure ulcer risk compared with 'standard hospital surfaces' that were not well described;
reactive water surfaces may reduce pressure ulcer risk compared with 'standard hospital surfaces' for people in acute care settings; and
reactive sheepskin surfaces may reduce pressure ulcer risk compared with 'standard hospital surfaces' for people in acute and long‐term care settings.
For the four comparisons between two support surfaces of the same type, there may be little to no difference in pressure ulcer incidence between different alternating pressure (active) air surfaces. However, it is unclear if there is a difference in pressure ulcer incidence between different types of reactive air surfaces, foam surfaces or reactive gel surfaces. These comparisons each yield very low‐certainty evidence.
Network meta‐analysis (prevention network)
Descriptions of included studies for network meta‐analysis
We identified 68 potentially eligible studies (18,174 participants) for the network meta‐analysis from the four reviews noted above (Shi 2021a; Shi 2021b; Shi 2021c; Shi 2021d). These studies compared two or more eligible beds, mattresses or overlays.
Of these 68 studies:
39 compared two or more eligible interventions and were included in the NMA; and
29 compared two or more eligible interventions but could not be joined into the NMA: 22 of these studies compared two or more of the same type of support surface and the remaining seven studies did not report any relevant data for analysis.
By screening the reference lists of four included reviews, we identified one more eligible study (Vanderwee 2005): it compared alternating pressure air surfaces (an eligible intervention) with foam surfaces plus four‐hourly turning (an ineligible intervention) and could connect to the network (see Figure 1). We also identified an ongoing RCT (NCT03351049): it compared reactive support surfaces with low air loss (i.e. reactive air surfaces) with reactive support surfaces without low air loss but provided no data for analysis. Overall, there was a total of 69 studies (18,621 participants). See Table 1 in Appendix 8 for the characteristics of these studies. See Appendix 9 for the references of these 69 studies.
Of the 69 studies, 40 connected to a network (with 12,517 participants having available data; median study sample size: 119 participants). See Table 2 in Appendix 8 for the summary characteristics across the 40 studies. The average participant age, specified in 38 studies, ranged from 37.2 to 87.0 years (median: 72.5 years). Among the 38 studies with participant sex specified, 4702 (45.1%) of participants were male and 5730 (54.9%) were female. Most of the studies (34/40) recruited people at risk of having a new ulcer, with risk assessed largely using the Waterlow, Norton or Braden scales (n = 11,845), and most of these participants were free of pressure ulcers at baseline (n = 9018). The median follow‐up duration of the included studies was 14 days (range: 5 days to 7 months). Most of the 27 studies with specified details were funded by industry. Most of the studies were conducted at acute care settings (21/40).
Descriptions of base‐case network
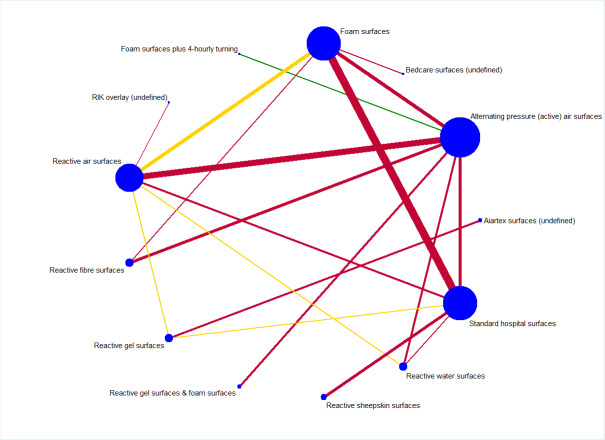
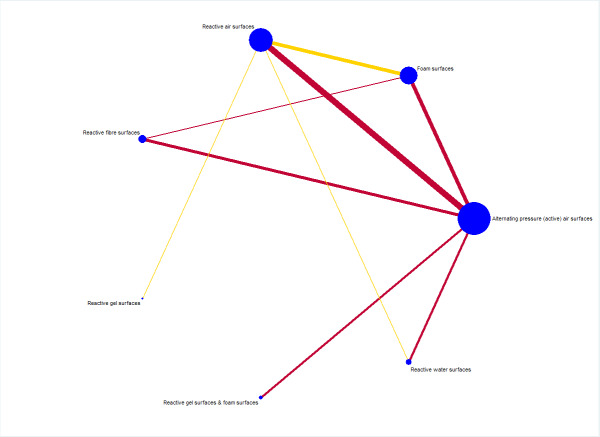
As Appendix 8 notes, 40 of the 69 studies connect into a network involving 13 interventions (termed base‐case network hereafter) (Figure 2).
2.
Prevention network: network plot for the base‐case network for pressure ulcer incidence outcome. We weighted node (circle) size by the number of studies reporting each intervention and weighted the thickness of the edge lines according to the inverse variance of the treatment effect estimates for the direct evidence contrast. We indicated the overall risk of bias for each direct comparison in the network diagram, using colour for three risk of bias ratings: low (green), unclear (yellow), and high (red).
The network has nine triangular loops (i.e. a set of three interventions that are connected to make a triangle in a network). This network has 78 network contrasts and 19 of these are direct contrasts: 42.1% (8/19) of direct contrasts were informed by only one study (see Table 3 in Appendix 8). Of the total 12,517 participants, 1298 (10.5%) participants developed new pressure ulcers. The 19 direct contrasts have a median of 316 participants (range: 37 to 4042), and 73.7% (14/19) of direct contrasts had fewer than 500 participants. The average number of events per network contrast was around 17 (1298/78).
Risk of bias for direct comparisons, each network contrast and the whole base‐case network
We summarise risk of bias assessments for the included studies in the topic of prevention (see Figure 7 in Appendix 8). Of the 40 studies contributing data to network meta‐analysis, two were rated low risk of bias, 16 were rated unclear, and 22 were rated high.
We have indicated the overall risk of bias for each direct comparison in the network diagram in Figure 2: five of the 19 direct comparisons were at low (green) or unclear (yellow) risk of bias whilst the remaining 14 were at high risk (red). Therefore, the overall risk of bias in the network as a whole was high.
We report risk of bias for each network contrast (see Figure 8 in Appendix 8). We considered eight of all 78 contrasts having data at low risk of bias (less than 25% of study data being of high risk of bias), 32 having data at unclear risk of bias (25% to 50% of study data being of high risk of bias), and 38 having data at high risk of bias (more than 50% of study data being of high risk of bias).
Network meta‐analysis results
See Table 2 summary of findings table for key comparisons with foam surfaces from the prevention network. Here we report relative effectiveness evidence for network contrasts between each eligible and well‐defined support surface versus foam surfaces. We did not include in the table support surfaces that could not be classified and the ineligible intervention.
2. Prevention network: summary of findings for key comparisons with foam surfaces as the reference.
| Outcome: pressure ulcer incidence | |||||||
|
Patient or population: people at risk of having pressure ulcers Setting: any care settings Intervention: reactive air surfaces; alternating pressure (active) air surfaces; reactive gel surfaces; reactive sheepskin surfaces; reactive fibre surfaces; reactive water surfaces; reactive gel surfaces followed by foam surfaces Comparator (reference): foam surfaces Follow‐up durations: median 14 days (range: 3 days to 12 months) | |||||||
| Total studies: 40 RCTs Total participants: 12,517 |
Anticipated absolute effects* (95% CI) | Relative effect from network meta‐analysis (95% CI) | Certainty of the evidence (GRADE) | Surface Under the Cumulative RAnking (SUCRA) | Comments | ||
| Interventions (numbers of studies and participants comparing the named intervention with foam surfaces) | Risk with foam surfaces | Risk with a type of support surface | Difference | ||||
| Reactive air surfaces (4 RCTs, 229 participants) | 106 per 1000 | 49 per 1000 (31 to 80) | 57 fewer per 1000 (26 fewer to 75 fewer) |
RR 0.46 (0.29 to 0.75) | ⨁⨁◯◯ LOWa,b | 78.1% ⨁◯◯◯ VERY LOWa,b,c |
Reactive air surfaces may reduce pressure ulcer incidence compared with foam surfaces. It is uncertain how likely it is that reactive air surfaces are the best intervention in reducing pressure ulcer incidence. |
| Alternating pressure (active) air surfaces (4 RCTs, 2247 participants) | 106 per 1000 | 67 per 1000 (44 to 98) | 39 fewer per 1000 (8 fewer to 62 fewer) |
RR 0.63 (0.42 to 0.93) | ⨁⨁◯◯ LOWa,b | 59.3% ⨁◯◯◯ VERY LOWa,b,c |
Alternating pressure (active) air surfaces may reduce pressure ulcer incidence compared with foam surfaces. It is uncertain how likely it is that alternating pressure (active) air surfaces are the best intervention in reducing pressure ulcer incidence. |
| Reactive gel surfaces (no analysable data for this direct comparison) | 106 per 1000 | 51 per 1000 (23 to 108) | 56 fewer per 1000 (83 fewer to 1 more) |
RR 0.47 (0.22 to 1.01) | ⨁⨁◯◯ LOWc | 74.6% ⨁◯◯◯ VERY LOWa,b,c |
Reactive gel surfaces may reduce pressure ulcer incidence compared with foam surfaces. It is uncertain how likely it is that reactive gel surfaces are the best intervention in reducing pressure ulcer incidence. |
| Reactive sheepskin surfaces (no analysable data for this direct comparison) | 106 per 1000 | 61 per 1000 (34 to 111) | 45 fewer per 1000 (72 fewer to 5 more) |
RR 0.58 (0.32 to 1.05) | ⨁◯◯◯ VERY LOWc,d | 64.1% ⨁◯◯◯ VERY LOWa,b,c |
It is uncertain if there is a difference between reactive sheepskin surfaces and foam surfaces in reducing pressure ulcer risk. It is uncertain how likely it is that reactive sheepskin surfaces are the best intervention in reducing pressure ulcer incidence. |
| Reactive fibre surfaces (1 RCT, 68 participants) | 106 per 1000 | 75 per 1000 (40 to 142) | 31 fewer per 1000 (66 fewer to 36 more) |
RR 0.71 (0.38 to 1.34) | ⨁◯◯◯ VERY LOWc,d | 50.6% ⨁◯◯◯ VERY LOWa,b,c |
It is uncertain if there is a difference between reactive fibre surfaces and foam surfaces in reducing pressure ulcer risk. It is uncertain how likely it is that reactive fibre surfaces are the best intervention in reducing pressure ulcer incidence. |
| Reactive water surfaces (no analysable data for this direct comparison) | 106 per 1000 | 46 per 1000 (18 to 115) | 60 fewer per 1000 (88 fewer to 9 fewer) |
RR 0.43 (0.17 to 1.09) | ⨁◯◯◯ VERY LOWc,d | 77.7% ⨁◯◯◯ VERY LOWa,b,c |
It is uncertain if there is a difference between reactive water surfaces and foam surfaces in reducing pressure ulcer risk. It is uncertain how likely it is that reactive water surfaces are the best intervention in reducing pressure ulcer incidence. |
| Reactive gel surfaces followed by foam surfaces (no analysable data for this direct comparison) | 106 per 1000 | 305 per 1000 (74 to 1000) | 199 more per 1000 (32 fewer to 894 more) |
RR 2.88 (0.70 to 11.83) | ⨁◯◯◯ VERY LOWa,c | 7.6% ⨁◯◯◯ VERY LOWa,b,c |
It is uncertain if there is a difference between reactive gel surfaces followed by foam surfaces and foam surfaces in reducing pressure ulcer risk. It is uncertain how likely it is that reactive gel surfaces followed by foam surfaces are the best intervention in reducing pressure ulcer incidence. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded once for some concerns about within‐study bias (risk of bias). bDowngraded once for heterogeneity. cDowngraded twice for imprecision. dDowngraded twice for risk of bias.
We undertook random‐effects network meta‐analyses. We assessed the transitivity, homogeneity and consistency assumptions for the base‐case network and considered that transitivity and consistency assumptions held (see Appendix 8). In terms of transitivity, the 19 direct contrasts – though heterogeneous in terms of risk of bias, and follow‐up duration – are homogeneous in terms of care settings, and participants' characteristics summarised at the level of direct contrasts (i.e. the proportions of sex, age and baseline skin status). Regarding consistency, the estimates of treatment effects from direct and indirect evidence are consistent globally (global design‐by‐treatment interaction model: χ2 statistic = 3.853, P value = 0.921) and locally (no inconsistency resulting from the tests of separating indirect from direct evidence). We present the results of the heterogeneity assessment below.
We report the analysis results in two ways:
Relative effectiveness results
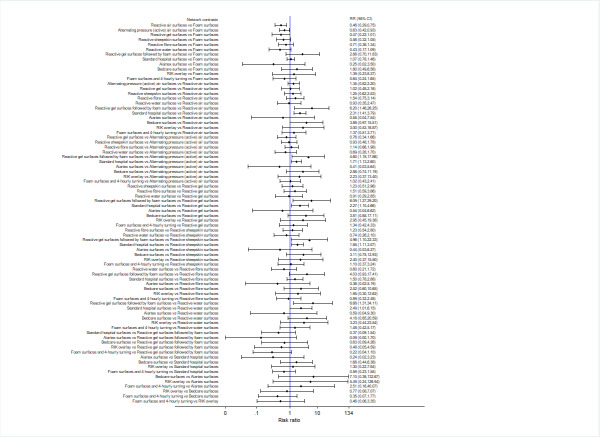
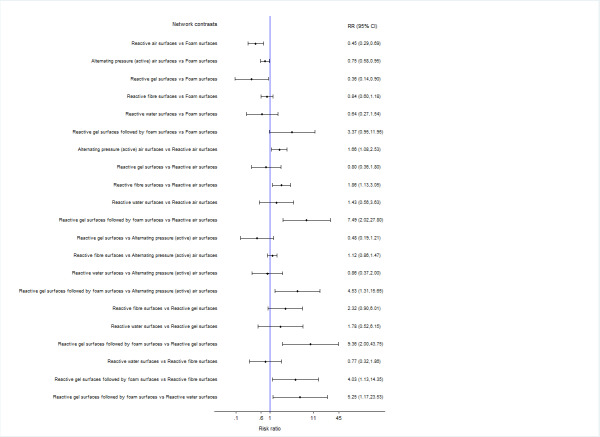
We report risk ratios (RRs) with their 95% CIs for each network contrast. See Figure 3.
3.
Prevention network: relative effectiveness results for 78 network contrasts
The relative effectiveness estimates of almost all network contrasts had wide confidence intervals and crossed RR = 1, meaning imprecise estimates that do not rule out the possibility of no‐effect. Nine contrasts had moderate or substantial heterogeneity (Appendix 8) and the majority of network contrasts were informed by studies at high risk of bias (see Figure 8 in Appendix 8). For GRADE assessment, the wide confidence intervals, heterogeneity and high risk of bias resulted in:
82.1% of contrasts (64/78) having very low‐certainty evidence (downgraded three times);
14.1% (11/78) having low‐certainty evidence (downgraded twice);
3.8% (3/78) having moderate‐certainty evidence (downgraded once).
Full details of the GRADE assessment can be found in Appendix 8.
Because of the volume of available data presented in this network analysis, we will focus on contrasts involving the five key types of support surface, chosen post hoc as likely to be the most informative for practice: alternating pressure (active) air surfaces, reactive air surfaces, foam surfaces, reactive sheepskin surfaces, and reactive gel surfaces (these support surfaces are probably more widely used than other options). None of the comparisons between these surfaces yields moderate‐certainty evidence. (The only contrasts where the evidence was of moderate certainty were those involving reactive gel surfaces on operating tables followed by foam surfaces on ward beds. However, the sequential use of these two types of support surfaces is relevant specifically to operating rooms and we did not consider this combination as a key intervention here). We have low‐certainty evidence (downgraded once for within‐study bias and once for heterogeneity, or twice for imprecision) for four key comparisons:
compared with foam surfaces, reactive air surfaces may reduce pressure ulcer incidence (RR 0.46, 95% CI 0.29 to 0.75). Extrapolating from the data, if 106 people per 1000 on foam surfaces will develop a new pressure ulcer by an average of 14 days' follow‐up, then 57 fewer people per 1000 will develop a pressure ulcer on reactive air surfaces (95% CI 26 to 75 fewer) (see Table 2);
compared with foam surfaces, alternating pressure (active) air surfaces may reduce pressure ulcer incidence (RR 0.63, 95% CI 0.42 to 0.93). Extrapolating from the data, if 106 people per 1000 on foam surfaces will develop a new pressure ulcer by an average of 14 days' follow‐up, then 39 fewer people per 1000 will develop a pressure ulcer on alternating pressure (active) air surfaces (95% CI 8 to 62 fewer) (see Table 2);
compared with foam surfaces, reactive gel surfaces may reduce pressure ulcer incidence (RR 0.47, 95% CI 0.22 to 1.01). Extrapolating from the data, if 106 people per 1000 on foam surfaces will develop a new pressure ulcer by an average of 14 days' follow‐up, then 56 fewer people per 1000 will develop a pressure ulcer on reactive gel surfaces (95% CI 83 fewer to 1 more) (see Table 2); and
the difference between reactive gel surfaces and reactive air surfaces is unclear in terms of their effectiveness on reducing pressure ulcer risk as the point estimate of RR suggests no difference but the very wide CI includes a ulcer risk reduction of 52% and a risk increase of 116% (RR 1.02, 95% CI 0.48 to 2.16).
We note that all study data on reactive gel surfaces, regardless of the comparators, were from operating rooms or long‐term care settings.
For the remaining comparisons, it is unclear whether there are differences in pressure ulcer incidence at an average of 14 days' follow‐up (all yielding very low‐certainty evidence, downgraded for within‐study bias, heterogeneity and/or imprecision):
between reactive sheepskin surfaces and foam surfaces (RR 0.58, 95% CI 0.32 to 1.05), reactive air surfaces (RR 1.25, 95% CI 0.62 to 2.53), alternating pressure (active) air surfaces (RR 0.93, 95% CI 0.48 to 1.78), or reactive gel surfaces (RR 1.23, 95% CI 0.51 to 2.96); and
between alternating pressure (active) air surfaces and reactive air surfaces (RR 1.35, 95% CI 0.82 to 2.20) or reactive gel surfaces (RR 0.76, 95% CI 0.34 to 1.66).
Ranking of interventions
We consider the network as a whole and report full results of ranking evidence in Appendix 8.
Here we present the cumulative probability plot for each support surface (see Figure 9 in Appendix 8), and the corresponding SUCRA values (higher values = higher probabilities of being the most effective), ordered from the highest to the lowest probability:
Aiartex surfaces: 78.3%;
reactive air surfaces: 78.1%;
reactive water surfaces: 77.7%;
reactive gel surfaces: 74.6%;
reactive sheepskin surfaces: 64.1%;
alternating pressure (active) air surfaces: 59.3%;
foam surfaces and four‐hourly turning: 57.5%;
reactive fibre surfaces: 50.6%;
foam surfaces: 30.2%;
a brand of overlay (RIK overlay): 29.0%;
standard hospital surfaces: 25.7%;
Bedcare surfaces: 17.3%; and
reactive gel surfaces followed by foam surfaces: 7.6%.
However, it is important to emphasise that all SUCRA values are lower than 80.0% and the ranking probabilities and rank order are highly uncertain (the ranking evidence is of very low certainty; downgraded once for risk of bias, once for both heterogeneity and inconsistency together, and twice for imprecision; see Appendix 8).
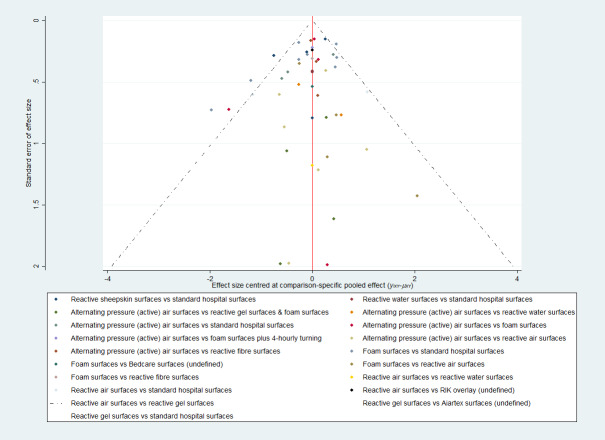
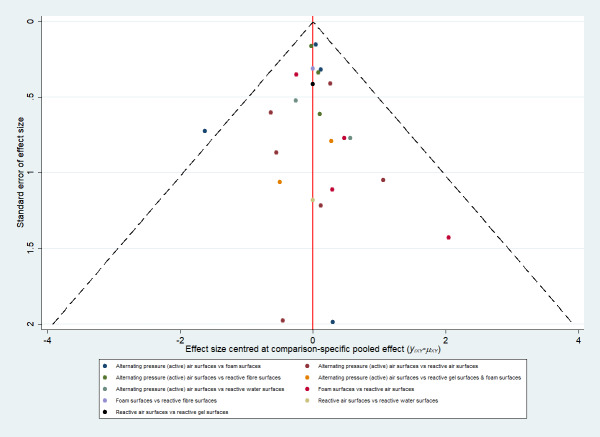
Whilst the Aiartex surface has the highest SUCRA value, and is ranked highest, this is probably artificially high as there is very sparse data for the direct evidence and the NMA estimates all have very wide CIs (consequently, the ranking is highly uncertain). There is no strong evidence of publication bias (see Figure 4).
4.
Prevention network: funnel plot of the base‐case analysis for pressure ulcer incidence outcome
Subgroup analyses
We performed pre‐planned subgroup analyses for four factors (see Homogeneity assumption tests and subgroup analysis section of Appendix 8). We found that the analyses for the factors of care settings and follow‐up duration reduced the Tau2 of the base‐case analysis (0.146) to be 0.075 and 0.117, respectively, meaning these factors could explain some heterogeneity whilst study‐level risk of bias (resulting in a Tau2 of 0.146) and baseline skin status (with a Tau2 of 0.202) did not explain heterogeneity. Therefore, the care setting and follow‐up duration may be important effect modifiers for the network meta‐analysis. However, due to the small number of included studies, we did not undertake analyses for individual care settings or categorised follow‐up durations and these exploratory analyses may be under‐powered.
Sensitivity analyses
We performed two sensitivity analyses to assess the robustness of the base‐case analysis: one pre‐planned sensitivity analysis using complete case data (40 studies and 12,183 available participants) and one post hoc sensitivity analysis assessing seven well‐defined support surfaces (24 studies with 5686 participants). See Appendix 8 for results of both analyses. These two sensitivity analysis networks shared similar limitations in terms of risk of bias and data sparseness with the base‐case analysis. Neither of these two sensitivity analyses resulted in substantial difference in relative effectiveness results for all network contrasts where available. The complete case sensitivity analysis did not change the rank order of interventions. The post hoc sensitivity analysis, which only included the seven well‐defined surfaces and only 24 studies, inevitably changed the SUCRA values (increased in general); however, the rank order did not change substantially (see Appendix 8). Therefore, the base‐case network meta‐analysis appears to be insensitive to missing data and the restriction of the analysis to the data from evaluations of well‐defined support surfaces.
Comparison of results from standard (pairwise) meta‐analysis with NMA findings
We compared the results of the base‐case analysis with the pairwise analysis results for the 12 of 19 direct comparisons that had data pooled in the included reviews (see Table 7 in Appendix 8). The NMA findings agree with the results of corresponding pairwise analyses for all 12 comparisons.
Time to pressure ulcer development
Overview of reviews
Four reviews included data on this outcome for seven direct comparisons of beds, overlays and mattresses (Shi 2021a, Shi 2021b, Shi 2021c, Shi 2021d; Appendix 5).
There are four comparisons of two different types of support surface (see Table 1 for full details). In nursing home settings, reactive air surfaces may reduce the hazard of having new pressure ulcers over 14 days' follow‐up compared with alternating pressure (active) air surfaces (low‐certainty evidence). In acute and long‐term care settings, reactive sheepskin surfaces may decrease the hazard of having new ulcers up to six months compared with 'standard hospital surfaces' (low‐certainty evidence). However, it is uncertain if there is a difference in the hazard of having new ulcers between alternating pressure (active) air surfaces and foam surfaces, or between foam surfaces and 'standard hospital surfaces' that were not well described: both have very low‐certainty evidence.
There are three comparisons of different types of the same category of surfaces (alternating pressure (active) air surfaces, foam surfaces and reactive air surfaces). In acute and long‐term care settings, there may be little to no difference in the risk of developing new pressure ulcers over 60 days' follow‐up between different types of alternating pressure (active) air surfaces (low‐certainty evidence). In intensive care units, viscoelastic foam surfaces with a density of 40 to 60 kg/m3 may decrease the risk of having new pressure ulcers over 11.5 days' follow‐up compared with foam surfaces with a density of 33 kg/m3 (low‐certainty evidence). In acute and long‐term care settings, solid foam surfaces may decrease the risk of having new pressure ulcers over one month's follow‐up compared with convoluted foam surfaces (low‐certainty evidence). It is unclear whether there is a difference in the hazard of having new pressure ulcers between two different brands (EHOB and KinAir) of reactive air surfaces (very low‐certainty evidence).
Network meta‐analysis (time‐to‐event network)
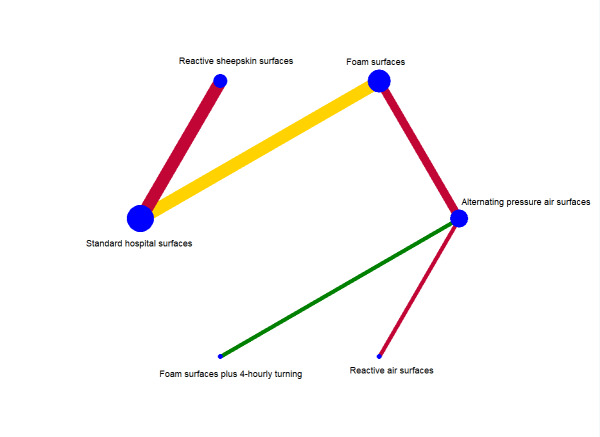
Descriptions of the network
The network meta‐analysis for this outcome comprised 10 studies evaluating six interventions (Figure 5). This network had five direct contrasts, a total of 15 network contrasts, but no triangular or quadratic loops. Of the five direct contrasts, two (40%) were informed by one study, one (20%) by two studies, and two (40%) by three studies. The network included a total of 7211 participants, 699 (9.6%) of whom developed new pressure ulcers. The five direct contrasts have a median of 1281 participants (range: 308 to 3072; see Appendix 10). The average number of events per network contrast was around 47 (699/15).
5.
Time‐to‐event network: network diagram for time to pressure ulcer development outcome
Risk of bias for direct comparisons, each network contrast and the whole base‐case network
Four of the 10 studies included for this network meta‐analysis were at low or unclear risk of bias whilst the remaining six were at high risk of bias (see Figure 7 in Appendix 8).
The overall risk of bias for direct comparisons is reported in Figure 5: one is at low risk of bias (green), one is at unclear (yellow), and three are at high risk (red). Therefore, the overall risk of bias in the network as a whole was high.
We assessed risk of bias for all 15 network contrasts: two of the 15 contrasts were at low risk of bias (less than 25% of study data being of high risk of bias), six at unclear risk of bias (25% to 50% of study data being of high risk of bias), and seven at high risk of bias (more than 50% of study data being of high risk of bias).
Network meta‐analysis results
See Table 3 summary of findings table for three key comparisons versus foam surfaces (reference) from the time‐to‐event network. Here we report relative effectiveness evidence for network contrasts between well‐defined support surfaces versus foam surfaces. We did not include support surfaces that could not be classified in the table.
3. Time‐to‐event network: summary of findings for key comparisons with foam surfaces as the reference.
| Outcome: time to pressure ulcer development | |||||||
|
Patient or population: people at risk of having pressure ulcers Setting: any care settings Intervention: reactive air surfaces; alternating pressure (active) air surfaces; reactive sheepskin surfaces Comparator (reference): foam surfaces Follow‐up durations: median 14 days (range: 3 days to 12 months) | |||||||
| Total studies: 10 RCTs Total participants: 7211 |
Anticipated absolute effects* (95% CI) | Relative effect from network meta‐analysis (95% CI) | Certainty of the evidence (GRADE) | SUCRA values | Comments | ||
| Interventions (numbers of studies and participants comparing the named intervention with foam surfaces) | Risk with foam surfaces | Risk with a type of support surface | Difference | ||||
| Reactive air surfaces (no analysable data for this direct comparison) | 106 per 1000 | 22 per 1000 (4 to 111) | 84 fewer per 1000 (102 fewer to 5 more) |
HR 0.20 (0.04 to 1.05) | ⨁⨁◯◯ LOWa | 89.7% ⨁◯◯◯ VERY LOWa,e,f |
Reactive air surfaces may reduce the hazard of having new pressure ulcers compared with foam surfaces. It is uncertain how likely it is that reactive air surfaces are the best intervention in reducing pressure ulcer risk. |
| Alternating pressure (active) air surfaces (2 RCTs, 2105 participants) |
106 per 1000 | 49 per 1000 (19 to 122) | 57 fewer per 1000 (87 fewer to 16 more) |
HR 0.45 (0.17 to 1.16) | ⨁◯◯◯ VERY LOWa,b,c | 62.1% ⨁◯◯◯ VERY LOWa,e,f |
It is uncertain if there is a difference between alternating pressure (active) air surfaces and foam surfaces in reducing the hazard of having new pressure ulcers. It is uncertain how likely it is that alternating pressure (active) air surfaces are the best intervention in reducing pressure ulcer risk. |
| Reactive sheepskin surfaces (no analysable data for this direct comparison) | 106 per 1000 | 69 per 1000 (24 to 190) | 37 fewer per 1000 (82 fewer to 84 more) |
HR 0.64 (0.22 to 1.88) | ⨁◯◯◯ VERY LOWc,d | 50.6% ⨁◯◯◯ VERY LOWa,e,f |
It is uncertain if there is a difference between reactive sheepskin surfaces and foam surfaces in reducing pressure ulcer risk. It is uncertain how likely it is that reactive sheepskin surfaces are the best intervention in reducing pressure ulcer risk. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; HR: hazard ratio | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded twice for risk of bias. bDowngraded twice for heterogeneity. cDowngraded twice for imprecision. dDowngraded once for risk of bias. eDowngraded once for heterogeneity. fDowngraded once for imprecision.
We undertook random‐effects network meta‐analyses. We assessed the transitivity, homogeneity and consistency assumptions for the network and considered that these assumptions held (see Appendix 10).
Relative effectiveness results
We report relative effectiveness results for all 15 network contrasts alongside their GRADE assessment in Table 4. There is low‐certainty evidence that reactive air surfaces may reduce the hazard of developing new pressure ulcers compared with foam surfaces (HR 0.20, 95% CI 0.04 to 1.05). Extrapolating from these data, if 106 people per 1000 develop a new ulcer on foam surfaces, 84 fewer per 1000 may develop them on reactive air surfaces (95% CI 102 fewer to 5 more). It is not clear whether there is a difference in the time to pressure ulcer development for all the other comparisons.
4. League table for time‐to‐event network: results of network meta‐analysis with GRADE assessment (below diagonal) and available pairwise meta‐analyses (above diagonal)a.
| Alternating pressure (active) air surfaces | 2.25 (1.05 to 4.83) | 0.41 (0.10 to 1.64) | 1.12 (0.70 to 1.79) | ||
| 2.25 (0.58 to 8.75) ⊕⊝⊝⊝ VERY LOWb,f |
Reactive air surfaces | ||||
| 0.71 (0.17 to 2.95) ⊕⊝⊝⊝ VERY LOWb,d |
0.31 (0.04 to 2.26) ⊕⊝⊝⊝ VERY LOWb,d |
Reactive sheepskin surfaces | 0.43 (0.30 to 0.61) | ||
| 0.45 (0.17 to 1.16) ⊕⊝⊝⊝ VERY LOWb,c,d |
0.20 (0.04 to 1.05) ⊕⊕⊝⊝ LOWb |
0.64 (0.22 to 1.88) ⊕⊝⊝⊝ VERY LOWd,e |
Foam surfaces | 0.68 (0.49 to 0.96) | |
| 1.12 (0.33 to 3.77) ⊕⊕⊝⊝ LOWd |
0.50 (0.08 to 3.08) ⊕⊝⊝⊝ VERY LOWd,e |
1.58 (0.24 to 10.36) ⊕⊝⊝⊝ VERY LOWd,e |
2.49 (0.53 to 11.68) ⊕⊝⊝⊝ VERY LOWd,e |
Foam surfaces plus four‐hourly turning | |
| 0.31 (0.09 to 1.06) ⊕⊝⊝⊝ VERY LOWe,f |
0.14 (0.02 to 0.87) ⊕⊝⊝⊝ VERY LOWb,d |
0.45 (0.21 to 0.94) ⊕⊝⊝⊝ VERY LOWb,d |
0.70 (0.32 to 1.54) ⊕⊝⊝⊝ VERY LOWc,d |
0.28 (0.05 to 1.58) ⊕⊝⊝⊝ VERY LOWd,e |
Standard hospital surfaces |
a Results of available pairwise meta‐analyses are presented in the upper right triangle above the diagonal, results of network meta‐analysis with GRADE assessment are presented in the lower left triangle. All results are HRs and their 95% CIs. For each cell, the HR and CI correspond to support surfaces in the same row and column, with the top‐left support surface compared to the lower right support surface in each case. bDowngraded twice for risk of bias. cDowngraded twice for heterogeneity. dDowngraded twice for imprecision. eDowngraded once for risk of bias. fDowngraded once for imprecision.
Half of network contrasts included data from studies that were mainly at high risk of bias (reasons for downgrading in Table 4). The data sparseness resulted in most relative effectiveness estimates for most network contrasts (13/15) having wide confidence intervals, crossing HR = 1. The network had no triangular loop and therefore inconsistency was not identified. The network had substantial heterogeneity (Tau2 = 0.329) and we identified two contrasts with heterogeneity: alternating pressure (active) air surfaces versus foam surfaces, P value = 0.009; and foam surfaces versus standard hospital surfaces, P value = 0.029 (see Appendix 10).
Ranking of interventions
Here we present SUCRA values for each support surface (higher = better), showing how likely it is for each intervention to be the most effective:
reactive air surfaces: 89.7%;
foam surfaces plus four‐hourly turning: 65.9%;
alternating pressure (active) air surfaces: 62.1%;
reactive sheepskin surfaces: 50.6%;
foam surfaces: 24.4%; and
standard hospital surfaces: 7.2%.
However, these ranking probabilities and rank order are highly uncertain (very low‐certainty evidence, downgraded twice for risk of bias, once for both heterogeneity and inconsistency together, and once for imprecision). The funnel plot did not strongly suggest publication bias (see Figure 14 in Appendix 10).
Subgroup and sensitivity analyses
The small number of studies in the network precluded any of the pre‐specified subgroup analyses.
Due to the nature of the outcome of time to pressure ulcer development, we did not consider missing data as an issue for this analysis and therefore did not undertake the related sensitivity analysis.
Comparison of results from standard (pairwise) meta‐analysis with NMA findings
There is no substantial difference between the results (for available comparisons) of the network meta‐analysis and pairwise analyses (see Table 4). However, we note that among those network contrasts with uncertain evidence, the important network contrast of reactive air surfaces versus alternating pressure air surfaces has a spuriously wide confidence interval (HR 2.25, 95% CI 0.58 to 8.75) as a result of data sparseness in the network. We consider the direct evidence to be more reliable: reactive air surfaces may reduce the hazard of having new pressure ulcers over 14 days' follow‐up compared with alternating pressure air surfaces in nursing home settings (HR 2.25, 95% CI 1.05 to 4.83).
Support surface‐associated patient comfort
The four included reviews report evidence for this outcome in 12 comparisons; all data were deemed at low or very low‐certainty (see Appendix 5). It is unclear whether there is a difference in support surface‐associated patient comfort between:
alternating pressure (active) air surfaces and foam surfaces, reactive air surfaces, reactive fibre surfaces, or between types of alternating pressure (active) air surface;
foam surfaces and reactive air surfaces, standard hospital surfaces, or between types of foam surface;
reactive air surfaces and either alternating pressure (active) air surfaces or RIK overlay, or between types of reactive air surface;
reactive gel surfaces and Aiartex surfaces; and between reactive sheepskin surfaces and standard hospital surfaces.
All reported adverse events
The four included reviews report evidence for this outcome for six comparisons; all data were deemed to be low or very low‐certainty evidence (see Appendix 5). It is unclear if there is a difference in adverse event rates between:
alternating pressure (active) air surfaces and foam surfaces, reactive gel surfaces used in the operating room followed by foam surfaces used on the ward bed, or between types of alternating pressure (active) air surface;
foam surfaces and reactive air surfaces, or Bedcare (undefined surfaces);
reactive gel surfaces and Aiartex surfaces.
Health‐related quality of life
Three included reviews report evidence on this outcome for two comparisons (Shi 2021a; Shi 2021b; Shi 2021d). See Appendix 5. There is low‐certainty evidence (downgraded twice for risk of bias) suggesting that there may be little or no difference between reactive sheepskin surfaces and standard hospital surfaces in health‐related quality of life, measured using a 100‐point visual analogue scale (higher = better), in long‐term care settings. It is unclear if there is a difference in health‐related quality of life measured using EQ‐5D‐5L or PU‐QoL‐UI at 90‐day follow‐up between alternating pressure (active) air surfaces and foam surfaces (low‐certainty evidence, downgraded twice for imprecision).
Cost‐effectiveness
Three reviews report evidence on this outcome for four comparisons (Shi 2021a; Shi 2021b; Shi 2021c). See Appendix 5. There is moderate‐certainty evidence (downgraded once for imprecision or risk of bias), suggesting that alternating pressure (active) air surfaces are probably more cost‐effective than foam surfaces for preventing pressure ulcers in acute and long‐term care settings; and that alternating pressure air mattresses are probably more cost‐effective than alternating pressure air overlays in acute and long‐term care settings.
There is low‐certainty evidence (downgraded twice for risk of bias, or downgraded once for risk of bias and once for indirectness) that foam surfaces may be more cost‐effective than standard hospital surfaces in preventing pressure ulceration in an acute care setting; and that reactive air surfaces are more cost‐effective than standard hospital surfaces in an acute care setting.
Section 2. Beds, overlays and mattresses for treating pressure ulcers
In this section, we summarise review evidence, alongside network meta‐analysis, where available, on the outcomes of the proportion of participants with pressure ulcers completely healed, time to complete pressure ulcer healing, support surface‐associated patient comfort, all reported adverse events, and cost‐effectiveness separately below. See Appendix 6 for full details of the evidence for these outcomes reported in included reviews.
For the first outcome, we also report (and focus on) findings of the associated network meta‐analyses following the summary of the overview of reviews.
Proportion of participants with pressure ulcers completely healed
Overview of reviews
Two reviews report evidence on this outcome for five comparisons (Shi 2021e; McGinnis 2014). However, evidence for all these comparisons is of low or very low certainty, mainly downgraded for risk of bias and imprecision. It is uncertain whether there is a difference in the proportion of participants with pressure ulcers completely healed between:
foam surfaces and alternating pressure (active) air surfaces, reactive air surfaces, or reactive water surfaces;
different types of alternating pressure (active) air surface;
reactive gel surfaces and Aiartex reactive surfaces.
Network meta‐analysis (treatment network)
See Table 5 summary of findings table for interventions compared with foam surfaces.
5. Treatment network: summary of findings for key comparisons with foam surfaces as the reference.
| Outcome: proportion of participants with pressure ulcers completely healed | |||||||
|
Patient or population: people with pressure ulcers Setting: any care settings Intervention: reactive air surfaces; alternating pressure (active) air surfaces; reactive water surfaces Comparator (reference): foam surfaces Follow‐up durations: median 37.5 days (range: 7 days to 18 months). | |||||||
| Total studies: 4 RCTs Total participants: 397 |
Anticipated absolute effects* (95% CI) | Relative effect from network meta‐analysis (95% CI) | Certainty of the evidence (GRADE) | Surface Under the Cumulative RAnking (SUCRA) | Comments | ||
| Interventions (numbers of studies and participants comparing the named intervention with foam surfaces) | Risk with foam surfaces | Risk with a type of support surface | Difference | ||||
| Reactive air surfaces (2 RCTs, 156 participants) | 410 per 1000 | 541 per 1000 (393 to 783) | 131 more per 1000 (17 fewer to 373 more) |
RR 1.32 (0.96 to 1.80) |
⨁⨁◯◯ LOWa | 83.9% ⨁◯◯◯ VERY LOWa,c |
Reactive air surfaces may increase pressure ulcer healing compared with foam surfaces. It is uncertain how likely it is that reactive air surfaces are the best intervention in healing pressure ulcers. |
| Alternating pressure (active) air surfaces (1 RCT, 49 participants) | 410 per 1000 | 397 per 1000 (107 to 1000) | 13 fewer per 1000 (303 fewer to 590 more) |
RR 0.97 (0.26 to 3.58) | ⨁◯◯◯ VERY LOWa,b | 43.0% ⨁◯◯◯ VERY LOWa,c |
It is uncertain if there is a difference in complete pressure ulcer healing between alternating pressure (active) air surfaces and foam surfaces. It is uncertain how likely it is that alternating pressure (active) air surfaces are the best intervention in healing pressure ulcers. |
| Reactive water surfaces (1 RCT, 101 participants) | 410 per 1000 | 410 per 1000 (295 to 565) | 0 fewer per 1000 (115 fewer to 155 more) |
RR 1.00 (0.72 to 1.38) | ⨁◯◯◯ VERY LOWa,b,c | 37.9% ⨁◯◯◯ VERY LOWa,c |
It is uncertain if there is a difference between reactive water surfaces and foam surfaces in complete pressure ulcer healing. It is uncertain how likely it is that reactive water surfaces are the best intervention in healing pressure ulcers. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate. The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited. The true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate. The true effect is likely to be substantially different from the estimate of effect. | |||||||
aDowngraded twice for imprecision. bDowngraded twice for risk of bias. cDowngraded once for risk of bias.
Descriptions of included studies for network meta‐analysis
From the included reviews (McGinnis 2014; Shi 2021e), we identified 12 studies that compared two or more eligible beds, mattresses or overlays for the network meta‐analysis of treatment data. We did not identify any studies from other resources. See Appendix 9 for the references of these 12 studies.
See Appendix 11 for the characteristics of the included studies.
There were two types of included studies:
studies which compared two or more eligible interventions and which were included in the NMA (n = 4); and
studies which compared two or more eligible interventions but which could not be joined into the NMA (n = 8): four studies evaluated the same interventions; three did not report relevant data for any analysis; and one reported a comparison that does not connect to the network.
A total of 12 studies with 972 randomised participants (median study sample size: 72 participants) was included in one or more of these categories. Each included RCT had two arms. The median duration of follow‐up of these included studies was 37.5 days (range: 7 days to 18 months). Most of the studies (11/12) were completely or partly funded by industry or received mattresses under evaluation from industries.
Participants enrolled were older adults (median of average participant age: 82.7 years; range: 64.0 to 86.5 years). Among those studies which specified sex, 284 (46.3%) of participants were male and 329 (53.7%) were female.
All 12 studies recruited people with existing pressure ulcers and participants were from either acute care settings (6/12 studies), or community and long‐term care settings (6/12 studies). For studies which reported these details, the average size of pressure ulcers at baseline ranged from 4.2 to 18.6 cm2 (median: 6.6 cm2).
Descriptions of base‐case network
As Appendix 11 notes, four studies (with 397 participants) connected into a network with four interventions (termed base‐case network hereafter; see Figure 6). This network had three direct contrasts, a total of six network contrasts, but no triangular or quadratic loop. Two of the three direct contrasts were informed by only one study and the third was informed by two studies. Of the total of 397 participants, 143 (36.0%) participants had complete ulcer healing. There were fewer than 200 participants in all direct contrasts (median: 120, range: 49 to 186). The average number of events per network contrast was 24 (143/6).
6.
Treatment network: network diagrams for pressure ulcer healing outcome
Risk of bias for direct comparisons, each network contrast and the whole network
We summarise risk of bias assessments for the included studies in the topic of treatment (see Figure 15 in Appendix 11). Of the four studies connecting to the network, two were at unclear risk of bias and the other two were at high risk of bias.
We have indicated the overall risk of bias for each direct comparison in the network diagram in Figure 6: one with unclear risk of bias (yellow), and two with high (red). Therefore, the overall risk of bias in the network as a whole was high.
We assessed risk of bias for each of the six contrasts in the network:
one has data at low risk of bias (less than 25% of data being of high risk of bias): foam surfaces versus reactive air surfaces;
two have unclear risk of bias (25% to 50% of data being of high risk of bias): alternating pressure active air surfaces versus reactive air surfaces, and reactive air surfaces versus reactive water surfaces; and
the remaining three have high risk of bias (more than 50% of data being of high risk of bias).
Network meta‐analysis results
We undertook random‐effects network meta‐analyses. Given the small number of included studies, we considered the transitivity assumption held. The network has no heterogeneity and no inconsistency and we considered these two assumptions held too.
Relative effectiveness results
See Table 6 for relative effectiveness results of all network contrasts and their GRADE assessment. As a result of data sparseness in the network, the relative effectiveness estimates for all network contrasts had wide confidence intervals, crossing RR = 1. Considering the limitations of data sparseness and risk of bias, we judged all but one network contrast as being of very low certainty evidence.
6. League table for treatment network: results of network meta‐analysis with GRADE assessment (below diagonal) and available pairwise meta‐analyses (above diagonal)a.
| Reactive water surfaces | 1.07 (0.70 to 1.64) | ||
| 1.03 (0.27 to 3.98) ⊕⊝⊝⊝ VERY LOWb,c |
Alternating pressure (active) air surfaces | 0.97 (0.26 to 3.58) | |
| 0.76 (0.48 to 1.19) ⊕⊝⊝⊝ VERY LOWc,d |
0.74 (0.19 to 2.82) ⊕⊝⊝⊝ VERY LOWc,d |
Reactive air surfaces | 1.32 (0.96 to 1.80) |
| 1.00 (0.72 to 1.38) ⊕⊝⊝⊝ VERY LOWb,c |
0.97 (0.26 to 3.58) ⊕⊝⊝⊝ VERY LOWb,c |
1.32 (0.96 to 1.80) ⊕⊕⊝⊝ LOWc |
Foam surfaces |
a Results of available pairwise meta‐analyses are presented in the upper right triangle above the diagonal, results of network meta‐analysis with GRADE assessment are presented in the lower left triangle. All results are RRs and their 95% CIs. For each cell, the RR and CI correspond to support surfaces in the same row and column, with the top‐left support surface compared to the lower right support surface in each case. bDowngraded twice for risk of bias. cDowngraded twice for imprecision. dDowngraded once for risk of bias.
There is low‐certainty evidence that more people with pressure ulcers using reactive air surfaces may heal completely than those using foam surfaces (RR 1.32, 95% CI 0.96 to 1.80). However, it is uncertain if there is a difference in pressure ulcer healing for all other comparisons (alternating pressure (active) air surfaces, foam surfaces, reactive air surfaces, and reactive water surfaces).
Ranking of interventions
Here we present SUCRA values, indicating the probability of each intervention being the most effective in healing pressure ulcers (see Appendix 11):
reactive air surfaces: 83.9%;
alternating pressure (active) air surfaces: 43.0%;
reactive water surfaces: 37.9%;
foam surfaces: 35.3%.
Whilst reactive air surfaces have the highest estimated probability of being the most effective, the ranking evidence is of very low certainty (downgraded twice for risk of bias, once for imprecision (see Appendix 11).
Subgroup analysis
Given the very limited number of included studies in the network, we did not undertake any pre‐specified subgroup analyses.
Sensitivity analysis
The sensitivity analysis using complete case data included the same four studies and 378 available participants (removing 19 cases from the base‐case analysis of 397 participants). The sensitivity analysis had the same network as the base‐case analysis, and shared the same issues of risk of bias, heterogeneity, inconsistency, and imprecision as the base‐case analysis. There is no substantial difference in relative effectiveness results and rank orders of interventions between the base‐case analysis and this sensitivity analysis. We therefore did not report full results of this sensitivity analysis network. We considered the base‐case meta‐analysis to be robust to missing data.
Comparison of results from standard (pairwise) meta‐analysis with NMA findings
The NMA findings agree with the results of the corresponding pairwise analyses for all three direct comparisons (see Table 6).
Time to complete pressure ulcer healing
Only Shi 2021e reports evidence on this outcome, for one comparison (see Appendix 6). There is low‐certainty evidence (downgraded twice for imprecision) that people using reactive air surfaces may be more likely to experience pressure ulcer healing than those using foam surfaces in long‐term care settings (HR 2.66, 95% CI 1.34 to 5.17; 1 study, 84 participants).
Support surface‐associated patient comfort
Two reviews report evidence on this outcome for five comparisons (Shi 2021e; McGinnis 2014). However, evidence for all these comparisons is of very low certainty, downgraded mainly for risk of bias and imprecision. See Appendix 6. It is uncertain if there is a difference in patient comfort responses between:
alternating pressure (active) air surfaces and foam surfaces or between different types of alternating pressure (active) air surface;
reactive air surfaces and foam surfaces or standard hospital surfaces;
reactive gel surfaces and Aiartex reactive surfaces.
All reported adverse events
Two reviews report evidence for this outcome for six comparisons (Shi 2021e; McGinnis 2014). However, evidence for all these comparisons is of low or very low certainty, downgraded mainly for risk of bias and imprecision. See Appendix 6. It is uncertain whether there is a difference in adverse event rates between:
alternating pressure (active) air surfaces and foam surfaces, or between different types of alternating pressure (active) air surface;
reactive air surfaces and foam surfaces, or standard hospital surfaces;
foam surfaces and reactive water surfaces;
reactive gel surfaces and Aiartex reactive surfaces.
Cost‐effectiveness
Only Shi 2021e reports cost‐effectiveness evidence for one comparison: reactive air surfaces may cost USD 26 per additional day without an ulcer in the first year in long‐term care settings (low‐certainty evidence, downgraded twice for imprecision; see Appendix 6).
Discussion
Summary of main results
In this overview of reviews, we have synthesised the main results of six Cochrane Reviews regarding the effects of beds, mattresses and overlays for preventing and treating pressure ulcers. We also used data from these reviews to conduct three network meta‐analyses: one network of 13 interventions for pressure ulcer incidence (informed by 40 studies with 12,517 participants); one examining the effect of six support surfaces on time to pressure ulcer development (informed by 10 studies with 7211 participants); and one examining the effects of four support surfaces on pressure ulcer healing (informed by four studies with 397 participants).
The results of our overview of direct evidence, where it was available, are consistent with our network analyses. Results generally indicate that the evidence is unclear regarding the relative effectiveness of the majority of available comparisons regarding pressure ulcer prevention (both risk and time to pressure ulcer development) and ulcer healing (all with very low‐certainty evidence). Additionally, it is unclear which support surface is the most effective in preventing and treating pressure ulcers (all ranking evidence is of very low certainty).
However, some important support surface comparisons have better quality evidence (i.e. those with better than very low‐certainty evidence):
compared with foam surfaces, reactive air surfaces may reduce the risk and hazard of developing new pressure ulcers (low‐certainty evidence from the network meta‐analysis). Furthermore, people with open pressure ulcers using reactive air surfaces may be more likely to heal completely than those using foam surfaces (low‐certainty evidence from the network meta‐analysis). In comparison with foam surfaces, reactive air surfaces may cost USD 26 per additional day without an ulcer in the first year in long‐term care settings (low‐certainty cost‐effectiveness evidence).
alternating pressure (active) air surfaces may reduce pressure ulcer incidence compared with foam surfaces (low‐certainty evidence from the network meta‐analysis). Alternating pressure (active) air surfaces are probably more cost‐effective than foam surfaces in preventing pressure ulcers (moderate‐certainty cost‐effectiveness evidence).
reactive gel surfaces may reduce pressure ulcer incidence compared with foam surfaces (low‐certainty evidence from the network meta‐analysis).
We note the evidence on reactive gel surfaces appears to be particularly applicable to operating room and long‐term care settings, where the studies involving gel surfaces were conducted.
There is also low‐certainty direct evidence that:
alternating pressure (active) air surfaces used on both operating tables and hospital beds may reduce pressure ulcer risk compared with reactive gel surfaces used on operating tables followed by foam surfaces applied on hospital beds.
reactive air surfaces may reduce the hazard of having new pressure ulcers over 14 days' follow‐up compared with alternating pressure air surfaces at a nursing home.
Included reviews also show that, irrespective of using surfaces for prevention or treatment, there is little known about any differences in support surface‐associated patient comfort and adverse event rates (all with low or very low‐certainty evidence). There is low‐certainty evidence on the health‐related quality of life for two comparisons in terms of preventing pressure ulcers, suggesting (1) little or no difference in health‐related quality of life between reactive sheepskin surfaces and standard hospital surfaces, but (2) uncertainty between alternating pressure (active) air surfaces and foam surfaces.
Overall completeness and applicability of evidence
The completeness of an overview is necessarily affected by how up to date the evidence of the reviews it includes is. This overview covers evidence from all eligible Cochrane Reviews. One of the six included reviews, McGinnis 2014, although the latest version, was published in 2014. However, we are still confident in the completeness of this overview. As for the other five included reviews, new searches were conducted in November 2019, and they contain all the evidence which was included in McGinnis 2014. Cochrane overviews tend not to include non‐Cochrane Reviews and so we only included Cochrane Reviews.
The completeness of a network meta‐analysis is related to its included studies and the related characteristics. The studies included in our three network meta‐analyses may not represent all potential data sources and relevant interventions. This is because, for example, seven of the 69 studies identified for the network of pressure ulcer incidence did not report the ulcer incidence outcome data and were excluded. As a result, among these seven studies, Hoshowsky 1994 (see Appendix 9), the only study evaluating reactive foam and gel surfaces, was excluded and reactive foam and gel surfaces disconnected from the network. Consequently, the network for pressure ulcer incidence only covers 13 interventions rather than all 14 interventions identified.
The impact of disconnecting reactive foam and gel surface on evidence completeness is clear: there was no evidence from network contrasts on these surfaces. However, we can see the direct evidence for these comparisons in the overview, as well as evidence on other direct comparisons between two support surfaces of the same type that could not be included in any network meta‐analysis. The availability of this evidence in the overview, alongside network meta‐analysis, means that all potential evidence is identifiable.
We examined the stability of the pressure ulcer incidence network by undertaking a post hoc sensitivity analysis. In this sensitivity analysis, we excluded five interventions that could not be classified, or were ineligible (Aiartex surfaces, Bedcare surfaces, RIK overlay, standard hospital surfaces, and foam surfaces plus four‐hourly turning) and as a result of these removals, reactive sheepskin surfaces also disconnected from the network. We included only seven support surfaces that could be classified and are widely accessible in practice. The sensitivity analysis did not substantially change the evidence for most network contrasts, indicating the stability of the network. We therefore have confidence in the full network of 13 interventions and report it as the primary analysis. We believe reporting the full network is likely to maximise its relevance to clinical decision‐making.
Regardless of the overview of reviews or network meta‐analysis, the populations in the included studies appear representative of the people who probably use the majority of pressure ulcer‐related care services in terms of care settings (acute and long‐term care settings), age (older adults), gender (almost half of participants were male) and pressure ulcer risk (or ulcer characteristics) at enrolment.
Evidence we report here appears to be largely applicable for people from any care setting. Indeed, we had evidence from exploratory subgroup analysis that care settings could be an effect modifier for the use of support surfaces in preventing pressure ulcers. However, due to the small number of included studies, we did not undertake analysis for individual care settings. Future reviews may want to identify evidence for separate care settings.
Quality of the evidence
We used the ROBIS tool to assess the risk of bias of the included reviews. With this assessment, we judged whether the original reviewers’ conclusions were appropriate and based on the available data. We judged five of the six included reviews to be at low overall risk of bias across the ROBIS assessment and the remaining one at unclear risk of bias. This is likely to be because we only included Cochrane Reviews, which are expected to follow the stringent guidance required for their publication.
We reported the certainty of the evidence reported in our included reviews and used the generic GRADE approach to assess the evidence certainty for McGinnis 2014, which has no GRADE assessment. The evidence presented in the majority of comparisons (65.6%) was rated very low certainty. The main reasons for downgrading the certainty of evidence included high risk of bias in the included studies and imprecision, caused by small sample sizes or low event rates, and/or heterogeneity.
We also assessed the certainty of evidence for all network meta‐analyses using the GRADE approach (with the CINeMA tool). We judged most of the network contrasts from each network meta‐analysis as having low or very low‐certainty evidence and judged ranking evidence from each network as having very low‐certainty. We mainly downgraded evidence certainty for risk of bias, heterogeneity and imprecision.
We downgraded for risk of bias for the results of most network contrasts and all the evidence relating to the rank ordering. This is mainly because we considered a high proportion of the included studies to be at high risk of bias for one or more domains. One reason for the high risk of bias judgement was lack of blinding of participants and personnel. However, if we disregarded risk of performance bias in the overall risk of bias assessment, most of the included studies were still at high risk of bias overall: 14 of the 40 studies contributing data to network meta‐analysis had unblinded outcome assessment. As a result, we judged the majority of the direct evidence (e.g. 14/19 for the pressure ulcer incidence network) to be at high risk of bias, and judged most of the network contrasts and whole networks as being at high risk of bias.
We downgraded for imprecision due to the sparse data in each network. For example, the network for pressure ulcer incidence has 42.1% of direct contrasts (8/19) informed by only one study. The network has 73.7% (14/19) of direct contrasts with fewer than 500 participants; and its average number of events per network contrast was only 17 (1298/78). The data sparseness means imprecision of estimates in terms of the relative effectiveness estimates (i.e. the very wide confidence intervals for most network contrasts) and ranking (i.e. the overlaps of rank orders between different support surfaces).
We note that the network contrast of reactive air surfaces versus alternating pressure air surfaces in the time‐to‐event network (for the pressure ulcer development outcome) needs careful GRADE assessment (particularly in terms of downgrading for imprecision). The confidence interval for the mixed estimate from network meta‐analysis (HR 2.25, 95% CI 0.58 to 8.75) appears to be spuriously wide whilst the corresponding direct estimate from pairwise meta‐analysis has a narrower interval (HR 2.25, 95% CI 1.05 to 4.83), meaning that pooling direct and indirect evidence in this network did not improve its precision. This situation has been previously acknowledged in the case of sparse networks (Brignardello‐Petersen 2019), and may be related to high heterogeneity as in our time‐to‐event network (Brignardello‐Petersen 2019). Given the greater imprecision in the mixed estimate, we downgraded the certainty of the evidence for imprecision and regard the direct evidence for this comparison as being more reliable.
We downgraded the certainty of the evidence for heterogeneity for network contrasts and networks' ranking under different considerations. The networks for both pressure ulcer incidence and time to pressure ulcer development have substantial heterogeneity but have no global or local inconsistency. We considered downgrading the results from these two networks for heterogeneity on the basis of network contrast by network contrast. The substantial heterogeneity in both networks was related to specific direct comparisons or evidence. We therefore identified those comparisons and downgraded evidence certainty for these network contrasts only (rather than for all network contrasts). We mainly downgraded the evidence certainty once for heterogeneity, though substantial, as no inconsistency was identified.
Regarding the rankings from these two networks, we considered the whole network heterogeneity in downgrading evidence certainty. We downgraded for heterogeneity only once as no inconsistency was identified and the heterogeneity, though substantial, has limited impact on the rank order for each network: the predicted probabilities that incorporated heterogeneity into probability estimates and estimated probabilities matched for all support surfaces (see Figure 9 and Figure 12 in Appendix 8). The ulcer healing network has no heterogeneity, thus no downgrading for heterogeneity for any evidence.
We did not downgrade evidence certainty for indirectness or publication bias (across‐studies bias). The funnel plots for two networks appear to indicate some evidence of small‐study effects (see Figure 4 and Figure 14 in Appendix 10). However, we did not consider the evidence for small‐study effects to be strong, given the small number of included studies per each direct contrast in each network; and we therefore did not downgrade for publication bias.
Potential biases in the overview process
The overview has some limitations. We only searched the Cochrane Library and only included Cochrane Reviews, and may have missed non‐Cochrane Reviews. However, to our knowledge, these included reviews probably cover all potential RCT evidence and, importantly, Cochrane Reviews are considered as higher‐quality and reliable evidence sources due to their quality criteria (Pollock 2017). Therefore, we are confident in the findings of this overview.
Five of the six included reviews involved authors of this overview which may introduce bias, particularly in study selection and risk of bias assessment. In order to reduce risk of these biases, two authors who were not authors of the included reviews, were invited to assess the risk of bias in the five reviews. The lead author (CS) and another author (ELG), not involved in McGinnis 2014, assessed the risk of bias of this review.
We identified primary studies from the included reviews of the overview for our network meta‐analysis rather than running separate literature searches. We may have missed some potentially eligible studies, particularly those comparing eligible support surfaces with ineligible interventions. However, the co‐authors of this overview have been researching this topic for decades and we believe that it is unlikely that we have missed eligible studies.
Agreements and disagreements with other studies or reviews
This is the first summary of evidence of Cochrane Reviews on preventing and treating pressure ulcers. We are not aware of any similar overviews of evidence on pressure ulcer prevention and treatment.
To our knowledge, Shi 2018a is the only network meta‐analysis evaluating all types of beds, mattresses and overlays for preventing pressure ulcers. This overview and network meta‐analysis are different from Shi 2018a in how specific support surfaces are classified and termed. In line with the included reviews (Shi 2021a; Shi 2021b; Shi 2021c; Shi 2021d; Shi 2021e), we classified support surfaces using the NPIAP S3I approach (NPIAP S3I 2007), whilst the network in Shi 2018a used specific terms. For example, reactive air‐fluidised surfaces, reactive air surfaces, and reactive low‐air‐loss surfaces were separate groups in Shi 2018a but all grouped to be 'reactive air surfaces' here. Similarly, the separate groups of alternating pressure (active) low‐air‐loss surfaces, alternating pressure (active) air surfaces, and hybrid air surfaces in Shi 2018a are all grouped into the classification of 'alternating pressure (active) air surfaces' here. Additionally, we re‐defined four interventions in our networks here: alternating pressure (active) air surfaces or RIK overlay, Aiartex surfaces, and reactive gel surfaces followed by foam surfaces were all classified as reactive gel surfaces in Shi 2018a, but further investigation suggested they are distinct interventions and all different from reactive gel surfaces. Foam surfaces plus four‐hourly turning were classified as 'foam surfaces' in Shi 2018a but we now consider turning as part of the intervention protocol rather than as a co‐intervention. We identified one new support surface (Bedcare) as a result of including a new study. We considered the factorial design study, Laurent 1998 (see Appendix 9), having two different, parallel comparisons in this network meta‐analysis, on the basis of the Cochrane guidance (Higgins 2020), rather than being a study with four parallel arms as used in Shi 2018a. As a result of these re‐classifications of support surfaces, the inclusion of new interventions, and the new treatment of the factorial trial, the network in Shi 2018a with 14 interventions/nodes became a network with 13 interventions here. We note that we also retained the term 'foam surfaces' in the overview and network meta‐analysis, as the included reviews did, rather than using 'high specification foam' surfaces which is not a classification in NPIAP S3I.
Shi 2018a used the term 'standard hospital surfaces' in their network, by grouping some ill‐defined interventions (e.g. 'standard care', 'standard mattress'). Whilst the definition of 'standard' surface is likely to have varied over time and by place, and NPIAP S3I 2007 discourages the term 'standard hospital surfaces,' we acknowledged the efforts to define standard hospital surfaces made in the included reviews (as originally described in primary studies). Consequently, we only termed things as 'standard' if no detail was provided with which to further classify the type of surface. We therefore kept using 'standard hospital surfaces' in our network meta‐analyses, but in order to assess the stability of the network, we excluded some interventions including 'standard hospital surfaces' for a post hoc sensitivity analysis. Exclusion of those surfaces did not result in substantial difference in network estimates.
Shi 2018a ran literature searches in August 2016 for all search databases and sources considered in the included reviews. Shi 2018a also searched the Chinese Biomedical Literature Database, which was not searched for the included reviews, due to concern about the validity of RCTs from China (Woodhead 2016). Searches for the included reviews were run in November 2019 with revised search strategies. The reviews included in the overview and network meta‐analysis did not include those trials from China (n = 7) but identified eight new studies (Allman 1987; Beeckman 2019; Berthe 2007; Cassino 2013; Nixon 2019; Park 2017; Rosenthal 2003; Sauvage 2017; see Appendix 9).
Given the differences in network constructions and included studies between Shi 2018a and this work, we compared the results for relative effectiveness. We found that this new analysis, with an improved network and the inclusion of more data, is consistent with Shi 2018a for key comparisons, but improves the precision of effect estimates.
A Cochrane Review (McInnes 2015), international guidelines (EPUAP/NPIAP/PPPIA 2019), and the UK's NICE pressure ulcer guidelines (NICE 2014), all appear to favour the use of foam surfaces in preventing pressure ulcers. McInnes 2015 concluded by noting the benefits of using 'higher‐specification foam mattresses rather than standard hospital foam mattresses' and stated that the 'relative merits of higher‐specification constant low‐pressure and alternating‐pressure support surfaces for preventing pressure ulcers are unclear...'. In this review, 'constant low‐pressure' surfaces include what we now term 'reactive air surfaces', and 'alternating‐pressure support surfaces' are what we now call 'alternating pressure (active) air surfaces'. Foam surfaces are widely recommended in pressure ulcer guidelines (EPUAP/NPIAP/PPPIA 2019; NICE 2014), and foam surfaces are probably widely used routinely for pressure ulcer prevention. However, our network evidence suggests both reactive air surfaces and alternating pressure (active) air surfaces have an advantage over foam surfaces in preventing pressure ulcers, and that alternating pressure (active) air surfaces are more cost‐effective than foam surfaces in preventing pressure ulcers. When looking at the relative effectiveness of alternating pressure (active) air surfaces and reactive air surfaces, we found the evidence is unclear, which is consistent with McInnes 2015. This result, however, tends to favour the benefits of using reactive air surfaces, which requires further evaluation. Additionally, there is cost‐effectiveness evidence on reactive air surfaces compared with foam surfaces, and reactive air surfaces compared with alternating pressure air surfaces, in preventing pressure ulcers. McInnes 2015 also suggests 'sheepskin overlays are effective in reducing the incidence of pressure ulcers' whilst our network meta‐analysis suggests uncertain evidence.
Our overview and network meta‐analysis also suggest that reactive air surfaces may be superior to foam surfaces in treating pressure ulcers. This is new evidence that the previous pairwise meta‐analysis of McInnes 2018 did not identify.
Authors' conclusions
Implications for practice.
This overview of current Cochrane Reviews provides the most up‐to‐date evidence on the use of beds, mattresses or overlays in preventing and treating pressure ulcers. Generally, we found the evidence to be insufficient or of very low certainty for both prevention and treatment. However, compared with foam surfaces (which are recommended and routinely used surfaces in most countries), reactive air surfaces may reduce pressure ulcer risk and may improve complete ulcer healing (although they may be associated with higher costs in long‐term care settings). Compared with foam surfaces, alternating pressure (active) air surfaces may reduce pressure ulcer risk and are probably more cost‐effective in preventing pressure ulcers. Additionally, reactive air surfaces may reduce the hazard of developing new pressure ulcers over 14 days' follow‐up compared with alternating pressure air surfaces in nursing homes. Finally, compared with foam surfaces, reactive gel surfaces may reduce pressure ulcer risk, particularly for people in operating room and long‐term care settings.
Implications for research.
The individual reviews and this overview have highlighted the lack of high‐certainty evidence on the use of most types of beds, mattresses or overlays for preventing and treating pressure ulcers. The scarcity of ulcer healing‐related evidence is particularly serious. The networks, all with sparse and low‐quality data, suggest that more high‐quality research is required. More RCT evidence is important for reactive air surfaces versus alternating pressure (active) air surfaces as both these types of support surface may reduce pressure ulcer risk but the direct and network evidence is still uncertain. Future research reports must fully describe the evaluated support surfaces.
The care setting might be a source of heterogeneity in results and future reviews may want to explore the influence of care settings.
Many of the existing RCTs in this field are poorly designed or reported, or both, and future research needs to be much more rigorous. Whilst it is challenging to avoid the risk of performance bias in studies of some types of support surfaces, as blinding of participants and personnel is seldom possible, stringent protocols (e.g. in terms of encouraging consistent care) can help minimise the risk of bias. It is also important to fully describe co‐interventions (e.g. repositioning) and ensure protocols mandate balanced use of co‐interventions across trial arms. The risk of detection bias can also be minimised with the use of digital photography and by masking adjudicators of the photographs to the types of support surfaces being evaluated (Baumgarten 2009). As most pressure ulcers occur in the first two to four weeks after admission, follow‐up periods should be longer than 14 days and clinically relevant in different settings.
One key limitation in included studies is small sample size. Future research should consider that pressure ulcer incidence can be low in certain settings for sample size calculations, and could incorporate relative effectiveness results produced here.
Trialists should carefully consider the choice of outcomes. Future research should use and report time‐to‐event data for pressure ulcer incidence and ulcer healing. Future research could nest cost‐effectiveness analysis in the conduct of trials where possible. Cost‐effectiveness evidence on reactive air surfaces compared with foam surfaces and alternating pressure air surfaces is particularly needed. If trialists consider measuring a quality of life outcome, then a validated health‐related quality of life measurement tool could be used to detect important change in individuals' status. The public sector should be encouraged to invest in further research.
History
Protocol first published: Issue 10, 2020
Acknowledgements
The authors are grateful to the following peer reviewers: Duncan Chambers and Una Adderley for feedback on the protocol, Zena Moore and Gill Worthy for feedback on the overview, and Janet Wale for feedback on both. Thanks are also due to Cochrane's Copy Edit Support team for copy‐editing the protocol and the overview, and to Cochrane Musculoskeletal, Oral, Skin and Sensory Network Editors Peter Tugwell and Jennifer Hilgart for feedback and final approval of the overview for publication.
Appendices
Appendix 1. Full details of support surfaces classifications
| Overarching class of support surface (as used in this review) | Corresponding subclasses of support surfaces used inShi 2018a | Descriptions of support surfaces | Selected examples (with example brands where possible) |
| Reactive air surfaces | Powered/non‐powered reactive air surfaces | A group of support surfaces constructed of air cells, which redistribute body weight over a maximum surface area (i.e. has reactive pressure redistribution mode), with or without the requirement for electrical power | Static air mattress overlay, dry flotation mattress (e.g. Roho, Sofflex), static air mattress (e.g. EHOB), and static mode of Duo 2 mattress |
| Powered/non‐powered reactive low‐air‐loss air surfaces | A group of support surfaces made of air cells, which have reactive pressure redistribution modes and a low‐air‐loss function, with or without the requirement for electrical power | Low‐air‐loss hydrotherapy | |
| Powered reactive air‐fluidised surfaces | A group of support surfaces made of air cells, which have reactive pressure redistribution modes and an air‐fluidised function, with the requirement for electrical power | Air‐fluidised bed (e.g. Clinitron) | |
| Foam surfaces | Non‐powered reactive foam surfaces | A group of support surfaces made of foam materials, which have a reactive pressure redistribution function, without the requirement for electrical power | Convoluted foam overlay (or pad), elastic foam overlay (e.g. Aiartex, microfluid static overlay), polyether foam pad, foam mattress replacement (e.g. MAXIFLOAT), solid foam overlay, viscoelastic foam mattress/overlay (e.g. Tempur, CONFOR‐Med, Akton, Thermo) |
| Alternative reactive support surfaces (non‐foam or air‐filled): reactive fibre surfaces | Non‐powered reactive fibre surfaces | A group of support surfaces made of fibre materials, which have a reactive pressure redistribution function, without the requirement for electrical power | Silicore (e.g. Spenco) overlay/pad |
| Alternative reactive support surfaces (non‐foam or air‐filled): reactive gel surfaces | Non‐powered reactive gel surfaces | A group of support surfaces made of gel materials, which have a reactive pressure redistribution function, without the requirement for electrical power | Gel mattress, gel pad used in operating theatre |
| Alternative reactive support surfaces (non‐foam or air‐filled): reactive sheepskin surfaces | Non‐powered reactive sheepskin surfaces | A group of support surfaces made of sheepskin, which have a reactive pressure redistribution function, without the requirement for electrical power | Australian Medical Sheepskins overlay |
| Alternative reactive support surfaces (non‐foam or air‐filled): reactive water surfaces | Non‐powered reactive water surfaces | A group of support surfaces based on water, which has the capability of a reactive pressure redistribution function, without the requirement for electrical power | Water mattress |
| Alternating pressure (active) air surfaces | Powered active air surfaces | A group of support surfaces made of air cells, which mechanically alternate the pressure beneath the body to reduce the duration of the applied pressure (mainly via inflating and deflating to alternately change the contact area between support surfaces and the body) (i.e. alternating pressure, or active, mode), with the requirement for electrical power | Alternating pressure‐relieving air mattress (e.g. Nimbus II, Cairwave, Airwave, MicroPulse), large‐celled ripple mattresses |
| Powered active low‐air‐loss air surfaces | A group of support surfaces made of air cells, which have the capability of alternating pressure redistribution as well as low air loss for drying local skin, with the requirement for electrical power | Alternating pressure low‐air‐loss air mattress | |
| Powered hybrid system air surfaces | A group of support surfaces made of air cells, which offer both reactive and active pressure redistribution modes, with the requirement for electrical power | Foam mattress with dynamic and static modes (e.g. Softform Premier Active) | |
| Powered hybrid system low‐air‐loss air surfaces | A group of support surfaces made of air cells, which offer both reactive and active pressure redistribution modes as well as a low‐air‐loss function, with the requirement for electrical power | Stand‐alone bed unit with alternating pressure, static modes and low air‐loss (e.g. TheraPulse) | |
| Standard hospital surfaces | Standard hospital surfaces | A group of support surfaces made of any materials, used as‐usual in a hospital and without reactive or active pressure redistribution capabilities, nor any other functions (e.g. low air loss, or air‐fluidised). | Standard hospital (foam) mattress, National Health Service Contract hospital mattress, standard operating theatre surface configuration, standard bed unit and usual care |
Appendix 2. Network meta‐analysis and relevant methods
1. Network meta‐analysis
Network meta‐analysis is an advanced meta‐analysis technique that can simultaneously compare multiple competing interventions for a given condition in a single statistical model, whilst maintaining randomisation (Caldwell 2005). Network meta‐analysis can produce 'indirect evidence' for a potential comparison where interventions are not evaluated in head‐to‐head RCTs. A network can link the direct evidence of A vs C and the direct evidence of B vs C, via a common comparator (i.e. C). Due to this, an indirect relative treatment effect for A vs B can be calculated (Glenny 2005; Riley 2017). Where there is also direct evidence for a comparison, the direct and indirect evidence can be combined to obtain a mixed estimate, which improves the precision of effect estimates (Higgins 1996; Lu 2004). Effect estimates from network meta‐analysis can be linked to probabilistic modelling to allow the ranking of treatments in terms of which is likely to be the most effective for the outcome of interest, which is likely to be the second best and so on (Salanti 2008).
2. Methods for network meta‐analysis
Unit of analysis issues
Some RCTs in the overview of reviews had unit of analysis issues identified as part of the review process. We did not report these as these RCTs were not included in any analysis. For the single RCT that was excluded from the Cochrane Reviews but was eligible for the network meta‐analysis, we noted there were no unit of analysis issues as the study presented outcomes at the level of participants (i.e. the unit of randomisation). We noted also that the included reviews had recorded whether the same participant was reported having multiple pressure ulcers. Where studies randomised at the participant level and outcomes were measured at the level of the ulcer, we would have considered the participant as the unit of analysis if the number of ulcers observed appeared to be equal to the number of participants (e.g. one pressure ulcer per person). However, we did not include any such RCTs for analysis.
Unit of analysis issues could have occurred if studies randomised at the participant level but outcomes of interest (e.g. the incidence of multiple pressure ulcers) were observed and data were presented and analysed at the level of the ulcer (clustered data). We noted that the included reviews had recorded whether data regarding multiple ulcers on a participant were (incorrectly) treated as independent within a study, or were analysed using within‐participant analysis methods. If clustered data were incorrectly analysed, the included reviews had recorded this as part of the risk of bias assessment.
Where a cluster trial was not incorrectly analysed, we planned to use available information to adjust for clustering ourselves, if possible, in accordance with the guidance described in Chapter 23 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2020). We planned to focus on the following.
The number of clusters randomly assigned to each intervention; and the average (mean) number of participants per cluster.
Outcome data ignoring the cluster design for the total number of participants.
Estimate of the intra‐cluster (or intra‐class) correlation coefficient (ICC).
However, we did not include any clustered trials in our analyses. We did note that included reviews recorded clustering issues for their included RCTs where appropriate.
Dealing with missing data
RCTs from the reviews included in the overview of reviews had missing data issues identified as part of the review process. We noted that the included reviews had addressed and reported these. For any RCTs that were excluded from the Cochrane Reviews but that were eligible for the network meta‐analysis, where there were missing data, and where relevant, we planned to contact study authors to pose specific queries about missing data. However, the only such RCT had no missing data issue. In the absence of other information, for pressure ulcer incidence we assumed that participants with missing data did not develop new pressure ulcers for the main analysis (i.e. we added missing data to the denominator but not the numerator). We examined the impact of this assumption through undertaking a sensitivity analysis (see Sensitivity analysis). For healing rates of pressure ulcers, we assumed that participants with missing data had unhealed pressure ulcers for the main analysis (i.e. we added missing data to the denominator but not the numerator). Again, we examined the impact of this assumption using a sensitivity analysis.
Assessment of heterogeneity
Assessment of heterogeneity was considered for network meta‐analysis as below.
Assessment of clinical and methodological heterogeneity within pairwise meta‐analysis
We noted that the included Cochrane Reviews had undertaken pairwise meta‐analyses based on their included trials where possible and assessed clinical and methodological heterogeneity within each analysis. We recorded the results of such assessments.
For the only trial that was previously excluded from the included Cochrane Reviews but was re‐considered as eligible in network meta‐analysis, we conducted pairwise meta‐analysis and compared information in terms of study design, participant, intervention and outcome measurement characteristics and overall risk of bias judgement at study level across the included studies in each meta‐analysis, in order to assess the presence of clinical and methodological heterogeneity.
Assessment of transitivity across network contrasts
Transitivity is an important concept when considering the validity of conducting a network meta‐analysis. It means that important clinical and methodological characteristics (effect modifiers) at the comparison (rather than study) level are similar enough that we can assume that intervention effects are transitive across network contrasts. This assumption means that it is then valid to calculate indirect treatment effects from direct data (Cipriani 2013). Transitivity can be considered an extension of clinical and methodological homogeneity at the comparison level (Cipriani 2013).
We did not have prior knowledge about potential effect modifiers from previous literature except for:
type of funding (e.g. industry, academic, government); we dichotomised this into not‐for‐profit and other (Lexchin 2003);
risk of bias; we considered the three judgements for the overall risk of bias judgement: low risk of bias, unclear risk of bias, and high risk of bias (Schulz 1995);
baseline skin status (pressure ulcer risk); we considered this as a categorical variable: participants at (high) risk of pressure ulcer development or with mixed skin status, participants with non‐blanchable erythema, participants with existing ulcers of stage 2 or serious, and non‐reporting. (Shi 2018a).
Also, we summarised information in terms of study designs, participants, interventions and outcome measurements and overall risk of bias judgements for each pairwise meta‐analysis and compared such information across all direct comparisons of a network to assess the transitivity assumption (Kew 2014). When data were insufficient for this assessment and the above assessment did not suggest any effect modification, we assumed that the transitivity assumption held. See Appendix 8; Appendix 10.
Assessment of reporting biases
For network meta‐analysis, we followed the systematic framework recommended by Page 2020 to assess risk of bias due to missing results (non‐reporting bias) in the network meta‐analysis of specific primary outcome data. To make an overall judgement about risk of bias due to missing results, we:
identified whether primary outcome data were unavailable by comparing the details of outcomes in trials registers, protocols or statistical analysis plans, if available, with the reported results. If the above information sources were unavailable, we compared outcomes in the conference abstracts or in the Methods section of the publication, or both, with the reported results. If non‐reporting of study results was found, we then planned to judge whether the non‐reporting was associated with the nature of findings by using the “Outcome Reporting Bias In Trials” (ORBIT) system (Kirkham 2018). However, following this approach, we did not identify substantial non‐reporting of study results.
assessed the influence of definitely missing primary outcome data on network meta‐analysis (see Effects of interventions).
assessed the likelihood of bias where a study had been conducted but not reported in any form. For this assessment, we considered whether the literature search was comprehensive and produced a comparison‐adjusted funnel plot for network meta‐analysis for seeking more evidence about the extent of missing results, provided there were at least 10 included studies (Peters 2008; Salanti 2014). To obtain a meaningful comparison‐adjusted funnel plot, we ordered the support surfaces by assuming that small studies were likely to favour advanced support surfaces (Chaimani 2015): Aiartex surfaces, Bedcare surfaces, alternating pressure (active) air surfaces, reactive air surfaces, reactive sheepskin surfaces, reactive gel surfaces followed by foam surfaces, reactive gel surfaces, reactive fibre surfaces, reactive water surfaces, reactive foam and gel surfaces, foam surfaces, and 'standard hospital surfaces'.
3. Subgroup analysis and investigation of heterogeneity
Assessment of statistical heterogeneity
We assessed the presence of common network statistical heterogeneity in network meta‐analysis using the I² measure and its 95% CIs and Tau² measure. We considered a P value less than 0.10 to indicate statistically significant heterogeneity given that the Chi² test has low power. We explored the common network heterogeneity via R (decomp.design) to locate potential sources of heterogeneity (Krahn 2013). Very broadly, we considered that I² values of 25% or less may indicate a low level of heterogeneity and values of 75% or more may indicate very high heterogeneity (Higgins 2003).
Assessment of inconsistency
Inconsistency refers to statistical disagreement between direct and indirect evidence and is a manifestation of non‐transitivity (Cipriani 2013). In a simple network, for example one with three interventions A, B and C, where there are studies comparing each of these treatments in a pairwise way, they can be linked in a network to form a single loop: A vs B, B vs C, C vs A trials. Inconsistency occurs when indirect evidence values do not statistically agree with available direct evidence (Cipriani 2013).
A complex network, however, often consists of multiple independent loops of evidence, and inconsistency could occur in each of the loops as well as in a whole network. Therefore, we assessed inconsistency at two levels: local inconsistency; and inconsistency in the whole network.
Local approaches to assessing inconsistency
Firstly, we used a loop‐specific approach (inconsistency plot test) to evaluate local inconsistency via STATA (ifplot). The approach focuses on each independent loop in isolation and calculates the difference between direct evidence and indirect evidence for a comparison, and tests whether the difference is significant (Song 2011). We assumed a common loop‐specific heterogeneity for this approach (Veroniki 2013).
We present the difference between direct and indirect evidence as the ratio of risk ratios with its 90% CI ‒ using its 90% CI is due to the low power of the inconsistency plot test (Song 2011). If the CI covers the value of one, there is no statistical difference between direct and indirect evidence (i.e. consistency assumption holds); otherwise, there may be the presence of local inconsistency. We particularly considered the ratio of risk ratio of 2 or more (or 0.5 or less) as an indication of inconsistency; a value of 2 or more means the treatment effects estimated from direct evidence may be at least twice as large as the indirect evidence (Chaimani 2013).
Secondly, we used the node‐splitting approach in STATA (network sidesplit) to also explore local inconsistency. This approach focuses on the network contrasts with direct evidence available (termed “node” by Dias 2014). Mixed estimate of such contrasts can be split into 'direct' and 'indirect' evidence, and the approach tests whether the difference between direct and indirect evidence is significant (Dias 2014).
Global approach to evaluating inconsistency
To assess global inconsistency, we ran a consistency and an inconsistency model and, from these, calculated the total inconsistency of all independent loops by comparing both models. Finally, we tested whether the total inconsistency was null using a global Wald test in the design‐by‐treatment interaction model (White 2015).
Investigation of heterogeneity
When important inconsistency or heterogeneity, or both, occurred, we followed steps proposed by Cipriani 2013 to investigate further:
checked the data extraction and data entry for errors and possible outlying studies;
planned to perform sensitivity analysis by removing outliers, if they had existed;
performed sub‐group analyses for study‐level characteristics (see below) if heterogeneity was still present, in order to explain heterogeneity as far as possible.
Sub‐group analysis
We investigated heterogeneity using the methods described in Chapter 10 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2020). We performed subgroup analyses/meta‐regression to determine whether the size of treatment effects was influenced by the following four study‐level characteristics.
Risk of bias (binary: low or unclear risk of bias; and high risk of bias) (Schulz 1995).
Settings (categorical: acute care and other hospital settings; long‐term care settings; operating theatre setting; and intensive care unit).
Baseline skin status (categorical: participants at risk, mixed skin status or non‐reporting; non‐blanchable erythema; existing ulcers of stage 2 or above) (Shi 2018a).
Follow‐up duration (categorical: short‐term; medium‐term; and long‐term as Criteria for considering reviews for inclusion specified) (Schoonhoven 2007) (Differences between protocol and review).
We did not perform subgroup analysis/meta‐regression when the number of studies included in the network meta‐analysis was not reasonable (i.e. fewer than 10).
4. Sensitivity analysis
We assessed the robustness of our findings via the following sensitivity analysis.
Impact of missing data
For pressure ulcer incidence outcome, we did this by repeating the analysis using complete case data (instead of assuming that participants with missing data did not develop new pressure ulcers, as in the main analysis).
For healing rates of pressure ulcers, we did this by repeating the analysis using complete case data (instead of assuming that participants with missing data had ulcers healed, as in the main analysis).
Post hoc sensitivity analysis of using eligible and well‐defined support surfaces in the prevention network
For the network meta‐analysis of pressure ulcer incidence data, we undertook a post hoc sensitivity analysis that involved seven well‐defined support surfaces but excluded interventions that were not well described and could not be classified, or were ineligible: Aiartex surfaces, Bedcare surfaces, RIK overlay, 'standard hospital surfaces', and foam surfaces plus four‐hourly turning.
Appendix 3. Risk of bias
1. Risk of bias assessment in individually randomised controlled trials
1. Was the allocation sequence randomly generated?
Low risk of bias
The study authors describe a random component in the sequence generation process, such as referring to a random number table, using a computer random number generator, coin tossing, shuffling cards or envelopes, throwing dice, drawing of lots.
High risk of bias
The study authors describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach: for example, sequence generated by odd or even date of birth, sequence generated by some rule based on date (or day) of admission, sequence generated by some rule based on hospital or clinic record number.
Unclear
Insufficient information about the sequence generation process to permit judgement of low or high risk of bias.
2. Was the treatment allocation adequately concealed?
Low risk of bias
Participants and study authors enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation: central allocation (including telephone, web‐based and pharmacy‐controlled randomisation); sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes.
High risk of bias
Participants or study authors enrolling participants could possibly foresee assignments and thus introduce selection bias, e.g. allocation was based on: using an open random allocation schedule (e.g. a list of random numbers); or assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered), alternation or rotation, date of birth, case record number, any other explicitly unconcealed procedure.
Unclear
Insufficient information to permit judgement of low or high risk of bias. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement, for example if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque and sealed.
3. Blinding: was knowledge of the allocated interventions by participants and personnel adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding.
Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding.
Blinding of key study participants and personnel attempted, but likely that the blinding could have been broken.
Either participants or some key study personnel were not blinded, and the non‐blinding of others is likely to introduce bias.
Unclear
Any one of the following.
Insufficient information to permit a judgement of low or high risk of bias.
The study did not address this outcome.
4. Blinding: was knowledge of the allocated interventions by outcome assessors adequately prevented during the study?
Low risk of bias
Any one of the following.
No blinding, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding.
Blinding of outcome assessment ensured, and unlikely that the blinding could have been broken.
High risk of bias
Any one of the following.
No blinding or incomplete blinding, and the outcome measurement is likely to be influenced by lack of blinding.
Blinding of outcome assessment attempted, but likely that the blinding could have been broken.
Unclear
Any one of the following.
Insufficient information to permit a judgement of low or high risk of bias.
The study did not address this outcome.
5. Were incomplete outcome data adequately addressed?
Low risk of bias
Any one of the following.
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is not sufficient to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes is not sufficient to have a clinically relevant impact on observed effect size.
Missing data have been imputed using appropriate methods.
High risk of bias
Any one of the following.
Reason for missing outcome data is likely to be related to the true outcome, with either imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, the proportion of missing outcomes compared with observed event risk is sufficient to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, the plausible effect size (difference in means or standardised difference in means) among missing outcomes is sufficient to induce clinically relevant bias in the observed effect size.
‘As‐treated’ analysis done, with substantial departure of the intervention received from that assigned at randomisation.
Potentially inappropriate application of simple imputation.
Unclear
Any one of the following.
Insufficient reporting of attrition/exclusions to permit judgement of low or high risk of bias (e.g. number randomised not stated; no reasons for missing data provided).
The study did not address this outcome.
6. Are reports of the study free of suggestion of selective outcome reporting?
Low risk of bias
Any of the following.
The study protocol is available and all of the study’s prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way.
The study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk of bias
Any one of the following.
Not all of the study’s prespecified primary outcomes have been reported.
One or more primary outcomes are reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not prespecified.
One or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect).
One or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis.
The study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear
Insufficient information to permit judgement of low or high risk of bias. It is likely that the majority of studies will fall into this category.
7. Other sources of potential bias
Low risk of bias
The study appears to be free of other sources of bias.
High risk of bias
There is at least one important risk of bias. For example, the study:
had a potential source of bias related to the specific study design used; or
has been claimed to have been fraudulent; or
had some other problem.
Unclear
There may be a risk of bias, but there is either:
insufficient information to assess whether an important risk of bias exists; or
insufficient rationale or evidence that an identified problem will introduce bias.
2. Risk of bias assessment in cluster‐randomised controlled trials (cluster‐RCTs)
1. Recruitment bias
Recruitment bias (or identification bias) is the bias that occurs in cluster‐RCTs if the personnel recruiting participants know individuals’ allocation, even when the allocation of clusters has been concealed appropriately. The knowledge of the allocation of clusters may lead to bias because the individuals' recruitment in cluster trials is often behind the clusters' allocation to different interventions; and the knowledge of allocation can determine whether individuals are recruited selectively.
This bias can be judged through considering the following questions.
Were all the individual participants identified/recruited before randomisation of clusters?
Is it likely that selection of participants was affected by knowledge of the intervention?
Were there baseline imbalances that suggest differential identification or recruitment of individual participants between arms?
2. Baseline imbalance
Baseline imbalance between intervention groups can occur due to chance, problems with randomisation, or identification/recruitment bias. The issue of recruitment bias has been considered above.
In terms of study design, the risk of chance baseline imbalance can be reduced by the use of stratified or pair‐matched randomisation. Minimisation — an equivalent technique to randomisation — can be used to achieve better balance in cluster characteristics between intervention groups if there is a small number of clusters.
Concern about the influence of baseline imbalance can be reduced if trials report the baseline comparability of clusters, or statistical adjustment for baseline characteristics.
3. Loss of clusters
Similar with missing outcome data in individually randomised trials, bias can occur if clusters are completely lost from a cluster trial, and are omitted from the analysis.
The amount of missing data, the reasons for missingness and the way of analysing data given the missingness should be considered in assessing the possibility of bias.
4. Incorrect analysis
Data analyses which do not take the clustering into account in cluster trials will be incorrect. Such analyses lead to a "unit of analysis error" and over‐precise results (overly small standard error) and overly small P values. Though these analyses will not result in biased estimates of effect, if not correctly adjusted, they will lead to too much weight allocated to cluster trials in a meta‐analysis.
Note that the issue of analysis may not lead to concern any more and will not be considered substantial if approximate methods are used by reviewers to address clustering in data analysis.
5. Comparability with individually randomised trials
In the case that a meta‐analysis includes, for example, both cluster‐randomised and individually randomised trials, potential differences in the intervention effects between different trial designs should be considered. This is because the "contamination" of intervention effects may occur in cluster‐randomised trials, which would lead to underestimates of effect. The contamination could be known as a "herd effect", i.e. within clusters, individuals' compliance with using an intervention may be enhanced, which in return affects the estimation of effect.
Appendix 4. Characteristics of the included reviews
| Review title (reference) | Search date | Eligibility criteria | Number of included trials and participants | Study sample size (median, range) | Settings included | Mean age | Proportions of participants by gender | Baseline skin status | Risk of bias of the included trials | Comparisons | Outcomes presented (including time points) |
| Pressure ulcer prevention | |||||||||||
| Alternating pressure (active) air surfaces for preventing pressure ulcers (Shi 2021a) | 14 Nov. 2019 | Randomised controlled trials that allocated participants to alternating pressure (active) air beds, overlays or mattresses. Comparators were any beds, overlays or mattresses that were applied for preventing pressure ulcers. | 32 studies (9058 participants) | 83 | A mixture of secondary and community in‐patient facilities (one study), acute care settings (19 studies), intensive care units (3 studies), and community and long‐term care settings (9 studies). | 37.2 to 87.0 years (median: 69.1 years) | 3654 (44.4%) male and 4571 (55.6%) female (26 studies) | 27 studies (8620 participants) had people at risk of having a new ulcer; 18 studies had 3812 (44.2%) participants free of pressure ulcers at baseline; nine studies had 4808 (55.8%) participants with superficial ulcers); one study (10 participants) had participants without risk of developing a pressure ulcer; and two studies (116 participants) recruited people with severe full‐thickness pressure ulcers alone | 26 (81.3%) of 32 studies rated at high overall risk of bias | (1) alternating pressure (active) air surfaces versus foam surfaces (six studies with 2427 participants) |
|
| (2) alternating pressure (active) air surfaces versus reactive air surfaces (seven studies with 1728 participants) |
|
||||||||||
| (3) alternating pressure (active) air surfaces versus reactive water surfaces (three studies with 414 participants) | Proportion of participants developing a new pressure ulcer follow‐up: median 10 days | ||||||||||
| (4) alternating pressure (active) air surfaces versus reactive fibre surfaces (four studies with 384 participants) |
|
||||||||||
| (5) alternating pressure (active) air surfaces versus reactive gel surfaces used in operating room followed by foam surfaces used on ward bed (two studies with 415 participants) |
|
||||||||||
| Foam surfaces for preventing pressure ulcers (Shi 2021b) | 14 Nov. 2019 | Randomised controlled trials that allocated participants to foam beds, overlays or mattresses. Comparators were any beds, overlays or mattresses that were applied for preventing pressure ulcers | 29 studies (9566 participants) | 101 | A mixture of secondary and community in‐patient facilities (2 studies), acute care settings (16 studies), intensive care units (3 studies), operating rooms (2 studies), and community and long‐term care settings (6 studies) | 47.0 to 85.3 years (median: 76.0 years) | 2659 (43.4%) male and 3466 (56.6%) female (24 studies) | 25 studies (8601 participants) had people at risk of having a new ulcer; 21 studies had 5512 (64.1%) participants free of pressure ulcers at baseline; four studies had 3089 (35.9%) participants with superficial ulcers); and two studies (148 participants) had people with severe full‐thickness pressure ulcers alone | 17 (58.6%) of 29 included studies rated at high overall risk of bias | (1) foam surfaces versus alternating pressure air surfaces (six studies, 2427 participants) |
|
| (2) foam surfaces versus reactive air surfaces (four studies, 236 participants) |
|
||||||||||
| (3) foam surfaces versus reactive fibre surfaces (two studies, 228 participants) | Proportion of participants developing a new pressure ulcer follow‐up: mean 17.7 days | ||||||||||
| (4) foam surfaces versus reactive gel surfaces (one study, 135 participants) | Proportion of participants developing a new pressure ulcer | ||||||||||
| (5) foam surfaces versus reactive foam and gel surfaces (one study, 91 participants) | Proportion of participants developing a new pressure ulcer | ||||||||||
| Reactive air surfaces for preventing pressure ulcers (Shi 2021c) | 14 Nov. 2019 | Randomised controlled trials that allocated participants to reactive air beds, overlays or mattresses. Comparators were any beds, overlays or mattresses that were applied for preventing pressure ulcers. | 17 studies (2604 participants) | 83 | A mixture of acute care and long‐term care settings (two studies), acute care settings (seven studies), intensive care units (four studies), and community and long‐term care settings (four studies) | 56 to 87 years (median: 72 years) | 1125 (44.8%) male and 1386 (55.2%) female (17 studies) | 13 studies (2335 participants) had people at risk of having a new ulcer; 10 studies had 2033 (87.1%) participants free of pressure ulcers at baseline; three studies had 302 (12.9%) participants with superficial ulcers; one study (57 participants; Sideranko 1992) had all participants free of ulcers; and two studies (112 participants) had people with severe full‐thickness pressure ulcers alone | eight (47.1%) of the 17 studies rated at high overall risk of bias | (1) reactive air surfaces versus alternating pressure (active) air surfaces (seven studies with 1728 participants) |
|
| (2) reactive air surfaces versus foam surfaces (four studies with 236 participants) |
|
||||||||||
| (3) reactive air surfaces versus reactive water surfaces (one study with 37 participants) | Proportion of participants developing a new pressure ulcer follow‐up: mean 9.5 days | ||||||||||
| (4) reactive air surfaces versus reactive gel surfaces (one study with 74 participants) | Proportion of participants developing a new pressure ulcer follow‐up: mean 6 months | ||||||||||
| Alternative reactive support surfaces (non‐foam and non‐air‐filled) for preventing pressure ulcers (Shi 2021d) | 14 Nov. 2019 | Randomised controlled trials that allocated participants to non‐foam and non‐air filled reactive beds, overlays or mattresses. Comparators were any beds, overlays or mattresses that were applied for preventing pressure ulcers | 21 studies (4693 participants) | 192.5 | Acute care settings (11 studies), intensive care units (one study), operating rooms (two studies), and long‐term care settings (seven studies) | 37.2 to 85.4 years (median: 72.5 years) | 1729 (43.2%) male and 2274 (56.8%) female (18 studies) | 16 studies (4152 participants) recruited people at risk of having a new ulcer; 13 studies had 3087 (76.4%) participants free of pressure ulcers at baseline; three had 953 (23.6%) participants with superficial ulcers; two studies (112 participants) had people with severe full‐thickness pressure ulcers alone | 17 (80.9%) of the 21 studies at high overall risk of bias | (1) reactive water surfaces versus alternating pressure (active) air surfaces (three studies with 414 participants) | Proportion of participants developing a new pressure ulcer follow‐up: median 10 days |
| (2) reactive water surfaces versus foam surfaces (one study with 117 participants) | |||||||||||
| (3) reactive water surfaces versus reactive air surfaces (one study with 37 participants) | Proportion of participants developing a new pressure ulcer follow‐up: mean 9.5 days | ||||||||||
| (4) reactive water surfaces versus reactive fibre surfaces (one study with 87 participants) | |||||||||||
| (5) reactive fibre surfaces versus alternating pressure (active) air surfaces (four studies with 384 participants) |
|
||||||||||
| (6) reactive fibre surfaces versus foam surfaces (two studies with 228 participants) | Proportion of participants developing a new pressure ulcer follow‐up: 17.7 days | ||||||||||
| (7) reactive gel surfaces on operating tables followed by foam surfaces on ward beds versus alternating pressure (active) air surfaces on operating tables and subsequently on ward beds (two studies with 415 participants) |
|
||||||||||
| (8) reactive gel surfaces versus reactive air surfaces (one study with 74 participants) | Proportion of participants developing a new pressure ulcer follow‐up: mean 6 months | ||||||||||
| (9) reactive gel surfaces versus foam surfaces (one study with 135 participants) | Proportion of participants developing a new pressure ulcer | ||||||||||
| (10) reactive gel surfaces versus reactive gel surfaces (one study with 113 participants) | |||||||||||
| (11) reactive foam and gel surfaces versus foam surfaces (one study with 91 participants) | Proportion of participants developing a new pressure ulcer | ||||||||||
| (12) reactive foam and gel surfaces versus reactive gel surfaces (one study with 166 participants) | Proportion of participants developing a new pressure ulcer | ||||||||||
| Pressure ulcer treatment | |||||||||||
| Beds, overlays and mattresses for treating pressure ulcers (Shi 2021e) | 14 Nov. 2019 | Randomised controlled trials that allocated participants to pressure redistributing beds, overlays or mattresses. Comparators were any beds, overlays or mattresses that were applied for treating pressure ulcers. | 13 studies (972 participants) | 72 | Acute care settings (including hospitals in general; six studies), and community and long‐term care settings (including community, nursing homes, long‐term facilities, geriatric unit; seven studies) | 64.0 to 86.5 years (median: 82.7 years) | 284 (46.3%) male and 329 (53.7%) female (10 studies) | All people with existing pressure ulcers; average size of pressure ulcers in seven studies (353 participants) median 6.6 cm2 (range 4.2 to 18.6 cm2) | 6 (46.2%) of the 13 included studies at high overall risk of bias | (1) alternating pressure (active) air surfaces versus foam surfaces (two studies with 132 participants) |
|
| (2) reactive air surfaces versus foam surfaces (three studies with 156 participants) |
|
||||||||||
| (3) foam surfaces versus reactive water surfaces (one study with 120 participants) |
|
||||||||||
| Pressure‐relieving devices for treating heel pressure ulcers (McGinnis 2014) | May 2013 | Randomised controlled trials that compared the effects of pressure‐relieving devices on the healing of pressure ulcers of the heel. Participants were treated in any care setting. Interventions were any pressure‐relieving devices including mattresses and specific heel devices. | One study (141 participants) | 141 | Long‐term care setting (elderly care setting) | Average 84.7 years | No data available | All people with heel pressure ulcers; the average severity score of ulcers 2.46 (SD 0.49) in Nimbus 3; 2.57 (0.48) in Cairwave | One study at high overall risk of bias | (1) alternating pressure (active) air surfaces (Nimbus 3) versus alternating pressure (active) air surfaces (Cairwave) (one study with 141 participants) |
|
Appendix 5. Summary of findings table: overview of prevention evidence
| Interventions versus comparators (included reviews; the number of studies with total participants) | Care setting | Relative effect (RR, 95% CI) | Anticipated absolute effect (95% CI) | Certainty of evidence | Interpretation of findings | ||
| Risk with comparators | Risk with interventions | Difference | |||||
|
|||||||
| Alternating pressure (active) air surfaces versus foam surfaces (Shi 2021a; Shi 2021b; 4 RCTs with 2247 participants) | Acute and long‐term care settings | RR 0.63, 0.34 to 1.17 | 104 per 1000 | 66 per 1000 (35 to 122) |
38 fewer per 1000 (69 fewer to 18 more) | ⊕⊕⊝⊝ LOW (downgraded once for risk of bias and once for moderate imprecision) |
Alternating pressure (active) air surfaces may reduce the number of incident pressure ulcer compared with foam surfaces; however, the evidence is low certainty. |
| Alternating pressure (active) air surfaces versus reactive air surfaces (Shi 2021a; Shi 2021c; 6 RCTs with 1648 participants) | Acute and long‐term care settings | RR 1.61, 0.90 to 2.88 | 22 per 1000 | 36 per 1000 (20 to 64) |
14 more per 1000 (2 fewer to 42 more) | ⊕⊝⊝⊝ VERY LOW (downgraded twice for high risk of bias and once for moderate imprecision) |
It is uncertain if the proportion of people developing a new pressure ulcer is decreased or increased when alternating pressure (active) air surfaces are compared with reactive air surfaces. |
| Alternating pressure (active) air surfaces versus reactive water surfaces (Shi 2021a; Shi 2021d; 2 RCTs with 358 participants) | Acute and long‐term care settings | RR 1.21, 0.52 to 2.83 | 52 per 1000 | 63 per 1000 (27 to 148) |
11 more per 1000 (25 fewer to 96 more) | ⊕⊝⊝⊝ VERY LOW (downgraded twice for high risk of bias and twice for substantial imprecision) |
It is uncertain if the proportion of people developing a new pressure ulcer is decreased or increased when alternating pressure (active) air surfaces are compared with reactive water surfaces. |
| Alternating pressure (active) air surfaces versus reactive fibre surfaces (Shi 2021a; Shi 2021d; 3 RCTs with 285 participants) | Acute care setting | RR 0.90, 0.68 to 1.19 | 424 per 1000 | 381 per 1000 (288 to 504) |
43 fewer per 1000 (136 fewer to 80 more) | ⊕⊝⊝⊝ VERY LOW (downgraded twice for high risk of bias and once for imprecision). |
It is uncertain if the proportion of people developing a new pressure ulcer is decreased or increased when alternating pressure (active) air surfaces are compared with reactive fibre surfaces. |
| Alternating pressure (active) air surfaces on operating tables and subsequently on ward beds versus reactive gel surfaces used in operating room followed by foam surfaces used on ward bed (Shi 2021a; Shi 2021d; 2 RCTs with 415 participants) | Operating room | RR 0.22, 0.06 to 0.76 | 68 per 1000 | 15 per 1000 (4 to 52) |
53 fewer per 1000 (64 fewer to 16 more) | ⊕⊕⊝⊝ LOW (downgraded once for risk of bias and once for imprecision) |
Alternating pressure (active) air surfaces applied on both operating tables and hospital beds may reduce the proportion of people developing a new pressure ulcer compared with reactive gel surfaces used on operating tables followed by foam surfaces applied on hospital beds. |
| Comparison between two types of alternating pressure (active) air surfaces (Shi 2021a; seven studies with 2833 participants) | Acute and long‐term care settings | None of the seven studies showed a difference in the proportion of people with incident pressure ulcers between different types of alternating pressure (active) air surfaces. | No pooling | No pooling | No pooling | ⊕⊕⊝⊝ LOW (downgraded once for risk of bias and once for imprecision) |
There may be little to no difference in the proportion of people with incident pressure ulcers between different types of alternating pressure (active) air surfaces. |
| Alternating pressure (active) air surfaces versus undefined 'standard hospital surfaces' (Shi 2021a; 4 RCTs with 830 participants) | Acute and long‐term care settings | All four studies consistently showed that alternating pressure (active) air surfaces could reduce the proportion of participants developing a new pressure ulcer compared with the undefined 'standard hospital surfaces'. | No pooling | No pooling | No pooling | ⊕⊕⊝⊝ LOW (downgraded twice for risk of bias) |
Alternating pressure (active) air surfaces may reduce the proportion of participants developing a new pressure ulcer compared with undefined standard hospital surfaces. |
| Foam surfaces versus reactive air surfaces (Shi 2021b; Shi 2021c; 4 RCTs with 229 participants) | Acute and long‐term care settings | RR 2.40, 1.04 to 5.54 | 106 per 1000 | 255 per 1000 (110 to 588) | 149 more per 1000 (4 more to 482 more) |
⊕⊕⊝⊝ LOW (downgraded once for risk of bias and once for imprecision) |
Foam surfaces may increase the proportion of participants developing a new pressure ulcer compared with reactive air surfaces. |
| Foam surfaces versus reactive fibre surfaces (Shi 2021b; Shi 2021d; one RCT with 68 participants) | Acute care setting | RR 1.17, 0.64 to 2.14 | 353 per 1000 | 413 per 1000 (226 to 755) | 60 more per 1000 (127 fewer to 402 more) |
⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias, and once for imprecision). |
It is uncertain if there is a difference in the proportion of participants developing a new pressure ulcer between foam surfaces with reactive fibre surfaces. |
| Foam surfaces versus reactive gel surfaces (Shi 2021b; Shi 2021d; one RCT with 135 participants) | Operating room | One study involving a totality of 135 individuals (270 halves of bodies) indicated no pressure ulcers developed in either group. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias, and twice for imprecision) |
It is uncertain if there is a difference in the proportion of participants developing a new pressure ulcer between foam surfaces and reactive gel surfaces. |
| Foam surfaces versus reactive foam and gel surfaces (Shi 2021b; Shi 2021d; one RCT with 91 participants) | Operating room | One study compared foam surfaces and reactive foam and gel surfaces in 91 participants (with 182 halves of bodies) using a split body design and found no pressure ulcers developed in either group. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias, and twice for imprecision) |
It is uncertain if there is a difference in the proportion of participants developing a new pressure ulcer between foam surfaces and reactive foam and gel surfaces. |
| Foam surfaces versus reactive water surfaces (Shi 2021b; Shi 2021d; one RCT with 117 participants) | Acute care setting | One RCT with 117 participants did not report any outcomes that were directly relevant to this review and so none of its data were analysable. | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
| Foam surfaces versus other types of foam surfaces (Shi 2021b; six RCTs with 733 participants) | Acute and long‐term care setting | The six studies reported heterogeneous ulcer incidence data between studies and data are hard to interpret. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias, twice for heterogeneity and once for imprecision) |
It is uncertain if there is a difference in the proportion of participants developing a new pressure ulcer between two types of foam surface. |
| Foam surfaces versus undefined surfaces (Bedcare; Shi 2021b; one RCT with 206 participants) | Long‐term care setting | RR 0.56, 0.19 to 1.60 | 87 per 1000 | 49 per 1000 (17 to 140) |
38 fewer per 1000 (70 fewer to 53 more) | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias and twice for imprecision) |
It is uncertain if there is a difference in the proportion of participants developing a new pressure ulcer between foam surfaces and undefined reactive surfaces. |
| Foam surfaces versus undefined standard hospital surfaces (Shi 2021b; eight studies with 4066 participants) | Acute care setting | Eight studies reported inconsistent results: five (3485 participants) reported no difference in the proportion of participants developing a new pressure ulcer between groups; two (168 participants) suggested foam surfaces reduced the risk of having new pressure ulcers; one (413 participants) suggested foam surfaces increased the risk. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias, and twice for inconsistency) |
It is uncertain if there is a difference in the proportion of participants developing a new pressure ulcer between foam surfaces and undefined standard hospital surfaces. |
| Reactive air surfaces versus reactive water surfaces (Shi 2021c; Shi 2021d; one RCT with 37 participants) | Intensive care unit | RR 0.43, 0.04 to 4.29 | 118 per 1000 | 51 per 1000 (5 to 505) |
67 fewer per 1000 (113 fewer to 387 more) |
⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias, and twice for imprecision) |
It is uncertain if there is a difference in the proportion of participants developing a new ulcer between reactive air surfaces and reactive water surfaces. |
| Reactive air surfaces versus reactive gel surfaces (Shi 2021c; Shi 2021d; one RCT with 66 participants) | Long‐term care setting | RR 1.25, 0.56 to 2.77 | 242 per 1000 | 302 per 1000 (136 to 670) |
60 more per 1000 (106 fewer to 428 more) |
⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias, and twice for imprecision) |
It is uncertain if there is a difference in the proportion of participants developing a new ulcer between reactive air surfaces and reactive gel surfaces. |
| Reactive foam and gel surfaces versus reactive gel surfaces (Shi 2021d; one RCT with 166 participants) | Operating room | The only study reported no pressure ulcers developed. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias and once for imprecision) |
It is uncertain if there is a difference in the proportion of participants developing a new pressure ulcer between reactive foam and gel surfaces and reactive gel surfaces. |
| Reactive air surfaces versus another type of reactive air surfaces (Shi 2021c; two studies with 223 participants) | Acute care setting | Neither study found a difference in the proportions of participants developing a new pressure ulcer between two different brands (EHOB and KinAir) of reactive air surface or between another two different brands (Sofflex and Roho) of reactive air surface. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias and twice for imprecision) |
It is uncertain if there is a difference in the proportions of participants developing a new pressure ulcer between two different brands (EHOB and KinAir) of reactive air surface or between another two different brands (Sofflex and Roho) of reactive air surface. |
| Reactive air surfaces versus undefined surfaces (alternating pressure (active) air surfaces or RIK microfluid static overlay) (Shi 2021c; one RCT with 110 participants) | Acute care setting | RR 0.33, 0.07 to 1.58 | 109 per 1000 | 36 per 1000 (8 to 172) |
73 fewer per 1000 (101 fewer to 63 more) | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias and twice for imprecision) |
It is uncertain if there is a difference in the proportion of participants developing a new ulcer between reactive air surfaces and undefined reactive surfaces. |
| Reactive air surfaces versus undefined standard hospital surfaces (Shi 2021c; two RCTs with 216 participants) | Acute care setting | Two studies (216 participants) reported inconsistent results: one study (116 participants) suggested no difference in the proportion of participants developing a new ulcer between groups whilst another study (100 participants) suggested reactive air surfaces reduced the risk of having new pressure ulcers (RR 0.21, 95% CI 0.07 to 0.70). | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias, twice for inconsistency and twice for imprecision) |
It is uncertain if there is a difference in the proportion of participants developing a new ulcer between reactive air surfaces and standard hospital surfaces. |
| Reactive water surfaces versus undefined 'standard hospital surfaces' (Shi 2021d; one RCT with 316 participants) | Acute care setting | RR 0.35, 0.15 to 0.79 | 130 per 1000 | 46 per 1000 (20 to 103) |
84 fewer per 1000 (110 fewer to 27 fewer) | ⊕⊕⊝⊝ LOW (downgraded twice for risk of bias) |
Reactive water surfaces may reduce the proportion of participants developing a new pressure ulcer compared with undefined standard hospital surfaces. |
| Reactive gel surfaces versus undefined 'standard hospital surfaces' (Shi 2021d; two RCTs with 446 participants) | Operating room | One study reported that reactive gel surfaces significantly reduced the incidence rates of sacral pressure ulcers compared with standard hospital surfaces (P = 0.01). Another study reported 10.7% (22/205) of people using reactive gel surfaces developed new pressure ulcers and the proportion was 20.4% (43/211) for those using standard hospital surfaces (RR 0.53, 95% CI 0.33 to 0.85). | No pooling | No pooling | No pooling | ⊕⊕⊕⊝ MODERATE (downgraded once for risk of bias) |
Reactive gel surfaces probably reduce the proportion of participants developing a new pressure ulcer. |
| Reactive gel surfaces versus undefined surfaces (Aiartex; Shi 2021d; two RCTs with 122 participants) | Long‐term care setting | Of the two studies, one reported one of 37 participants using reactive gel surfaces developed new pressure ulcers whilst none of the participants developed new ulcers when using undefined surfaces; another study reported none of 25 participants in each study arm developed new ulcers. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias, and twice for imprecision) |
It is uncertain if there is a difference between reactive gel surfaces and undefined reactive surfaces in the proportion of participants developing a new pressure ulcer. |
| Reactive sheepskin surfaces versus undefined 'standard hospital surfaces' (Shi 2021d; three RCTs with 1424 participants) | Acute and long‐term care settings | Three studies (1424 participants) all suggested that reactive sheepskin surfaces were associated with lower proportions of participants developing a new pressure ulcer than 'standard hospital surfaces'. | No pooling | No pooling | No pooling | ⊕⊕⊝⊝ LOW (downgraded twice for risk of bias) |
Reactive sheepskin surfaces may reduce the proportion of participants developing a new pressure ulcer compared with standard hospital surfaces. |
| Reactive gel surfaces versus reactive gel surfaces (Shi 2021d; one study, 113 participants) | Operating room | One study reported this outcome but indicated no pressure ulcers developed. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias and twice for imprecision) |
It is uncertain if there is a difference in the proportion of participants developing a new pressure ulcer between these two types of use of reactive gel surfaces. |
| Reactive water surfaces versus reactive fibre surfaces (Shi 2021d; one study, 87 participants) | Acute care setting | One study reported no outcomes directly relevant to this review and so none of its data were analysable. | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable |
|
|||||||
| Alternating pressure (active) air surfaces versus foam surfaces (Shi 2021a; Shi 2021b; 2 RCTs with 2105 participants) | Acute and long‐term care setting | HR 0.41, 0.10 to 1.64 | 98 per 1000 | 41 per 1000 (10 to 156) |
57 fewer per 1000 (88 fewer to 58 more) | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias, once for moderate imprecision, and twice for substantial inconsistency) |
It is uncertain if there is a difference between alternating pressure (active) air surfaces and foam surfaces in the hazard of developing new pressure ulcers. |
| Alternating pressure (active) air surfaces versus reactive air surfaces (Shi 2021a; Shi 2021c; 1 RCT with 308 participants) | Long‐term care setting | HR 2.25, 1.05 to 4.83 | 52 per 1000 | 113 per 1000 (54 to 227) |
61 more per 1000 (2 more to 175 more) | ⊕⊕⊝⊝ LOW (downgraded twice for high risk of detection bias) |
People treated with alternating pressure (active) air surfaces may have a higher risk of developing an incident pressure ulcer than those treated with reactive air surfaces over 14 days' follow‐up at nursing home. |
| Alternating pressure (active) air surfaces versus another type alternating pressure (active) air surfaces (Shi 2021a; two studies with 2581 participants) | Acute and long‐term care setting | Both of the two included studies suggested no clear difference in the risk of developing an incident pressure ulcer at time up to 60 days' follow‐up between these support surfaces. | No pooling | No pooling | No pooling | ⊕⊕⊝⊝ LOW (downgraded once for risk of bias and once for imprecision) |
There may be little to no difference in the risk of developing an incident pressure ulcer over 60 days' follow‐up between these support surfaces. |
| Foam surfaces versus other types of foam surfaces (Shi 2021b; two RCTs with 146 participants) | Acute care and long‐term care setting | One study reported an unadjusted HR of 0.33 (95% CI 0.17 to 0.64) for the comparison of the viscoelastic foam surfaces with a density of 40 to 60 kg/m3 versus foam surfaces with a density of 33 kg/m3 in intensive care unit setting whilst another study reported an adjusted HR of 0.40 (95% CI 0.20 to 0.80) for the comparison of solid foam surfaces versus convoluted foam surfaces at acute care and long‐term care settings. | No pooling | No pooling | No pooling | ⊕⊕⊝⊝ LOW (downgraded once for risk of bias and once for imprecision) |
Viscoelastic foam surfaces with a density of 40 to 60 kg/m3 and solid foam surfaces may decrease the risk of developing incident pressure ulcers at any point in time up to one month compared with the control foam surfaces. |
| Foam surfaces compared with undefined standard hospital surfaces (Shi 2021b; three studies with 3072 participants) | Acute care setting | One study (1729 participants) suggested foam surfaces reduced the hazard of developing a new ulcer, whilst another two studies (1343 participants) suggested no difference between foam surfaces and 'standard hospital surfaces'. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias and twice for inconsistency) |
It is uncertain whether there is a difference in the time to pressure ulcer incidence between foam surfaces and undefined standard hospital surfaces. |
| Reactive air surfaces versus another type of reactive air surfaces (Shi 2021c; one RCT with 123 participants) | Acute care setting | The study reported no statistically significant difference in survival analysis between the two types of reactive air surfaces (EHOB versus KinAir). | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias and twice for imprecision) |
It is uncertain if there is a difference between EHOB reactive air surfaces and KinAir reactive air surfaces in reducing the number of incident pressure ulcers. |
| Reactive sheepskin surfaces versus undefined 'standard hospital surfaces' (Shi 2021d; three RCTs with 1424 participants) | Acute and long‐term care settings | Three studies (1424 participants) all suggested that the use of reactive sheepskin surfaces was associated with a lower hazard of having new ulcers than using standard hospital surfaces at any particular time up to six months. | No pooling | No pooling | No pooling | ⊕⊕⊝⊝ LOW (downgraded twice for high risk of bias) |
Reactive sheepskin surfaces may decrease the hazard of having new ulcers at any particular time up to six months compared with standard hospital surfaces. |
|
|||||||
| Alternating pressure (active) air surfaces versus foam surfaces (Shi 2021a; Shi 2021b; one RCT with 76 participants) | Long‐term care setting | One study reported no significant difference in the overall satisfaction between study groups (P = 0.21). | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for high risk of bias and once for imprecision) |
It is uncertain whether there is any difference in support surface‐associated patient comfort between alternating pressure (active) air surfaces and foam surfaces. |
| Alternating pressure (active) air surfaces versus reactive air surfaces (Shi 2021a; Shi 2021c; 4 RCTs with 1364 participants) | Acute and long‐term care setting | Three studies appeared to report equivalent comfort between their study arms whilst a fourth study seemed to suggest that the use of alternating pressure (active) air surfaces was associated with better comfort than reactive air surfaces. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias and twice for substantial inconsistency) |
It is uncertain if there is any difference in support surface‐associated patient comfort between alternating pressure (active) air surfaces and reactive air surfaces. |
| Alternating pressure (active) air surfaces versus reactive fibre surfaces (Shi 2021a; Shi 2021d; one RCT with 187 participants) | Acute care setting | 19 dropouts among 93 people using alternating pressure (active) air surfaces; and 17 of 94 using reactive fibre surfaces due to the reason of discomfort. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias, once for imprecision, and once for indirectness) |
It is uncertain if there is any difference in support surface‐associated patient comfort between alternating pressure (active) air surfaces and reactive fibre surfaces. |
| Alternating pressure (active) air surfaces versus another type alternating pressure (active) air surfaces (Shi 2021a; seven studies with 2705 participants) | Acute and long‐term care setting | The studies report a range of different measures and outcome data cannot be easily interpreted. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias, once for imprecision, and once for publication bias) |
It is uncertain if there is a difference in support surface‐associated patient comfort between different types of alternating pressure (active) air surfaces. |
| Foam surfaces versus reactive air surfaces (Shi 2021b; Shi 2021c; one RCT with 72 participants) | Acute care setting | More people using reactive air surfaces had increased comfort than those using foam surfaces on top of an alternating pressure (active) air surface; fewer people had decreased comfort (P = 0.04). | No pooling | No pooling | No pooling | ⊕⊕⊝⊝ LOW (downgraded once for risk of bias, and once for imprecision) |
It is unclear if there is a difference in patient comfort responses between reactive air surfaces and foam surfaces on top of an alternating pressure (active) air surface. |
| Foam surfaces versus other types of foam surfaces (Shi 2021b; four studies with 669 participants) | Acute care settings | The studies report a range of different measures and outcome data cannot be easily interpreted. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias, twice for heterogeneity and once for imprecision) |
It is uncertain if there is a difference in positive patient comfort responses between different types of foam surfaces. |
| Foam surfaces versus undefined standard hospital surfaces (Shi 2021b; two RCTs with 1269 participants) | Acute care setting | One study measured comfort using a 5‐point scale (higher score = better comfort) and reported a mean comfort rating of 4.2 for foam surfaces and 4.0 for standard hospital mattress. Another study measured comfort using a 10‐point scale (higher score = poorer comfort) but reported no significant differences in comfort between foam mattresses (mean 2.33 and SD 0.98) and standard hospital mattress (mean 2.46 and SD 1.0). | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias and once for heterogeneity) |
It is uncertain if there is a difference in support surface‐associated patient comfort between foam surfaces and undefined standard hospital surfaces. |
| Reactive air surfaces versus undefined surfaces (alternating pressure (active) air surfaces or RIK microfluid static overlay) (Shi 2021c; one RCT with 110 participants) | Acute care setting | 68 participants rated comfort: 27 of 30 participants using undefined reactive surfaces and 29 of 34 using reactive air surfaces had comfort responses. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias and once for imprecision) |
It is uncertain if there is a difference in support surface‐associated patient comfort between reactive air surfaces and undefined reactive surfaces. |
| Reactive air surfaces versus another type of reactive air surface (Shi 2021c; one RCT with 84 participants) | Acute care setting | None of 84 participants gave a 'very uncomfortable' response in either reactive air surfaces; 5 gave an 'uncomfortable' response (all using Roho); 8 gave an 'adequate' response (4 in each group), 48 gave a 'comfortable' response (24 in each group), and 23 responded 'very comfortable' (13 using Sofflex and 10 using Roho). | No pooling | No pooling | No pooling | ⊕⊕⊝⊝ LOW (downgraded once for risk of bias and once for imprecision) |
It is unclear if there is a difference in the support surface‐associated patient comfort between the two specific reactive air surfaces under evaluation. |
| Reactive gel surfaces compared with undefined surfaces (Aiartex; Shi 2021d; one RCT with 50 participants) | Long‐term care setting | 20 people using undefined reactive surfaces responded with 'good' and 5 with 'excellent'; and 24 people using reactive gel surfaces responded with 'good' and 1 with 'excellent'. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias and once for imprecision) |
It is uncertain if there is a difference between reactive gel surfaces and undefined reactive surfaces in support surface‐associated patient comfort. |
| Reactive sheepskin surfaces versus undefined 'standard hospital surfaces' (Shi 2021d; one RCT with 297 participants) | Acute care setting | The study reported that people using reactive sheepskin surfaces rated comfort significantly higher than those using standard hospital surfaces (Z value of the Mann‐Whitney U test = ‐7.74, P < 0.001). | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias and once for imprecision) |
It is uncertain if there is a difference between reactive sheepskin surfaces and standard hospital surfaces in support surface‐associated patient comfort. |
|
|||||||
| Alternating pressure (active) air surfaces versus foam surfaces (Shi 2021a; Shi 2021b; three RCTs with 2181 participants) | Acute and long‐term care setting | Two studies reported similar rates of adverse events between their study arms; and a third study reported one death but did not specify which study group the death was associated with. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias and twice for substantial inconsistency). |
Available evidence was from 3 RCTs (2181 participants) that reported a variety of adverse events but data were not pooled. It is uncertain if there is any difference in reported adverse events between alternating pressure (active) air surfaces and foam surfaces. |
| Alternating pressure (active) air surfaces on operating tables and subsequently on ward beds versus reactive gel surfaces used in operating room followed by foam surfaces used on ward bed (Shi 2021a; Shi 2021d; one RCT with 198 participants) | Operating room | Approximately one half of people in each group reported adverse events. No difference in adverse events between groups was reported. No adverse events were related to the mattresses assigned. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias and twice for imprecision). |
It is uncertain if there is any difference in all reported adverse events between alternating pressure (active) air surfaces applied on both operating tables and hospital beds and reactive gel surfaces used on operating tables followed by foam surfaces applied on hospital beds. |
| Alternating pressure (active) air surfaces versus another type of alternating pressure (active) air surface (Shi 2021a; one RCT with 1971 participants) | Acute and long‐term care settings | The study reported that 377 adverse events were observed among 308 participants within 60 days. However, the study authors did not report these data by study groups. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias and twice for imprecision). |
It is uncertain if there is a difference in the adverse effects between the two formats of alternating pressure (active) air surfaces. |
| Foam surfaces versus reactive air surfaces (Shi 2021b; Shi 2021c; one study with 72 participants) | Acute care setting | One study reported counts of adverse events for each group and these data could not be pooled. | No pooling | No pooling | No pooling | ⊕⊕⊝⊝ LOW (downgraded once for risk of bias and once for imprecision) |
It is unclear if there is a difference in adverse event rates between foam surfaces and reactive air surfaces. |
| Foam surfaces versus undefined surfaces (Bedcare; Shi 2021b; one RCT with 206 participants) | Long‐term care setting | One study reported this outcome and stated no reported adverse events in either study group. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias and once for imprecision). |
It is uncertain if there is a difference in the adverse effects between foam surfaces and the undefined reactive surfaces. |
| Reactive gel surfaces compared with undefined surfaces (Aiartex; Shi 2021d; one RCT with 50 participants) | Long‐term care setting | One study reported this outcome but indicated no adverse events. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias and twice for imprecision). |
It is uncertain if there is a difference in the adverse effects between reactive gel surfaces and the undefined surfaces (Aiartex). |
|
|||||||
| Alternating pressure (active) air surfaces versus foam surfaces (Shi 2021a; Shi 2021b; one RCT with 2029 participants; the EQ‐5D‐5L measured 267 participants; and PU‐QoL‐UI measured 233 participants) | Acute and long‐term care setting | MD in the 90‐day EQ‐5D‐5L of 0.00 (95% CI ‐0.05 to 0.05); and MD in 90‐day PU‐QoL‐UI of 0.00 (95% CI ‐0.03 to 0.03) |
|
‐ |
|
⊕⊕⊝⊝ LOW (downgraded twice for substantial imprecision) |
It is unclear if there is a difference in health‐related quality of life measured using EQ‐5D‐5L or PU‐QoL‐UI at 90‐day follow‐up between alternating pressure (active) air surfaces and foam surfaces. |
| Reactive sheepskin surfaces versus undefined 'standard hospital surfaces' (Shi 2021d; one RCT with 588 participants; outcome measured on a 100‐point visual analogue scale, higher = better) | Long‐term care setting | The quality of life for those with ulcers using reactive sheepskin surfaces had a mean of 62.1 on a 100‐point visual analogue scale (higher = better) compared with 61.3 for those using standard hospital surfaces (Student’s t test P = 0.71). | No pooling | No pooling | No pooling | ⊕⊕⊝⊝ LOW (downgraded twice for risk of bias) |
There may be little to no difference between reactive sheepskin surfaces and standard hospital surfaces in the health‐related quality of life. |
|
|||||||
| Alternating pressure (active) air surfaces versus foam surfaces (Shi 2021a; Shi 2021b; one RCT with 2029 participants) | Acute and long‐term care setting | Incremental cost‐effectiveness ratio (ICER) = GBP –101,699 and Net Monetary Benefit (NMB) = GBP –2114 in the probabilistic analysis, meaning alternating pressure (active) air surfaces have lower costs and higher QALY (quality‐adjusted life‐years) values. Alternating pressure (active) air surfaces had a 99% probability of being cost‐effective at a threshold of GBP 20,000 and alternating pressure (active) air surfaces dominated reactive foam surfaces. | Not applicable | Not applicable | Not applicable | ⊕⊕⊕⊝ MODERATE (downgraded once for imprecision). |
Alternating pressure (active) air surfaces probably dominate reactive foam surfaces, meaning they are the cost‐effective option. |
| Alternating pressure (active) air surfaces versus another type of alternating pressure (active) air surface (Shi 2021a; one RCT with 1971 participants) | Acute and long‐term care settings | The cost effectiveness acceptability curve indicated that, on average, alternating pressure mattresses were associated with an 80% probability of being cost‐saving compared with alternating pressure overlays. | Not applicable | Not applicable | Not applicable | ⊕⊕⊕⊝ MODERATE (downgraded once for risk of bias) |
Alternating pressure air mattresses are probably more cost‐effective than alternating pressure air overlays. |
| Foam surfaces versus standard hospital surfaces (Shi 2021b; one RCT with 1168 participants) | Acute care setting | Foam surfaces have an 88% probability of being cost‐effective compared with standard hospital surfaces in preventing any pressure ulcer (including blanching erythema); and have a 95% probability of being cost‐effective in preventing non‐blanching erythema or worse. | Not applicable | Not applicable | Not applicable | ⊕⊕⊝⊝ LOW (downgraded twice for risk of bias) |
Foam surfaces may be more cost‐effective than standard hospital surfaces in preventing pressure ulceration. |
| Reactive air surfaces versus standard hospital surfaces (Shi 2021c; one RCT with 100 participants) | Acute care setting | One study, that did not express the outcome as the incremental cost per health benefit gained, reported that, when reactive air surfaces were used, the cost saved per 100 participants at risk was Canadian dollars 6302.6; pressure ulcers prevented per 100 participants at risk were 64; and therefore, reactive air surfaces dominated standard hospital surfaces. | Not applicable | Not applicable | Not applicable | ⊕⊕⊝⊝ LOW (downgraded once for risk of bias and once for indirectness) |
Reactive air surfaces are more cost‐effective than standard hospital surfaces. |
Appendix 6. Summary of findings table: overview of treatment evidence
| Interventions versus comparators (included reviews; the number of studies with total participants) | Care setting | Relative effect (RR, 95% CI) | Anticipated absolute effect (95% CI) | Certainty of evidence | Interpretation of findings | ||
| Risk with comparators | Risk with interventions | Difference | |||||
|
|||||||
| Alternating pressure (active) air surfaces versus foam surfaces (Shi 2021e; 2 RCTs with 132 participants) | Acute and long‐term care setting | Two studies reported this outcome: one study reported analysable data and the RR was 0.97 (95% CI 0.26 to 3.58); another study stated that the analysis of covariance showed no statistically significant difference in the healing of pressure ulcers between alternating pressure (active) air surfaces versus foam surfaces. |
No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for high risk of bias and twice for substantial imprecision) |
It is uncertain if there is a difference in the proportion of participants with healed pressure ulcers between alternating pressure (active) air surfaces and foam surfaces. |
| Reactive air surfaces versus foam surfaces (Shi 2021e; 2 RCTs with 156 participants) | Acute and long‐term care setting | RR 1.32, 0.96 to 1.80 | 442 per 1000 | 583 per 1000 (424 to 795) |
141 more per 1000 (18 fewer to 353 more) | ⊕⊕⊝⊝ LOW (downgraded twice for imprecision) |
It is unclear if there is a difference in the proportion of participants with pressure ulcers completely healed between reactive air surfaces and foam surfaces. |
| Foam surfaces versus reactive water surfaces (Shi 2021e; one RCT with 68 participants) | Long‐term care setting | RR 0.93, 0.61 to 1.42 | 481 per 1000 | 447 per 1000 (293 to 683) |
37 fewer per 1000 (191 fewer to 199 more) | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias, and twice for imprecision). |
It is uncertain if there is a difference in the proportion of participants with healed pressure ulcers between foam surfaces and reactive water surfaces. |
| Alternating pressure (active) air surfaces compared with the another type of alternating pressure (active) air surface (Shi 2021e; McGinnis 2014; 3 RCTs with 73 participants) | Acute and long‐term care setting | All studies reported no statistical difference between Nimbus alternating pressure air systems and Pegasus systems for improvement or healing of sacral pressure sores; no significant difference when using either mattress system in the number of heel ulcers healed (RR 1.49; 95% CI 0.90 to 2.45). |
No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias and twice for imprecision). |
It is unclear if there is a difference in the proportion of participants with pressure ulcers completely healed between different types of alternating pressure (active) air surfaces (Nimbus vs. Pegasus). |
| Reactive gel surfaces versus undefined reactive surfaces (Aiartex; Shi 2021e; one RCT with 72 participants) | Long‐term care setting | RR 1.58, 0.41 to 6.11 | 86 per 1000 | 136 per 1000 (35 to 525) |
50 more 1000 (51 fewer to 439 more) | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias and twice for imprecision). |
It is uncertain if there is a difference in the proportions of participants with pressure ulcers completely healed between people using reactive gel surfaces and those using undefined reactive surfaces. |
|
|||||||
| Reactive air surfaces versus foam surfaces (Shi 2021e; 1 RCT with 84 participants) | Long‐term care setting | HR 2.66, 1.34 to 5.17 | 463 per 1000 | 809 per 1000 (566 to 960) |
346 more per 1000 (103 more to 497 more) | ⊕⊕⊝⊝ LOW (downgraded twice for imprecision) |
People using reactive air surfaces may be more likely to have healed pressure ulcers compared with people using foam surfaces. |
|
|||||||
| Alternating pressure (active) air surfaces versus foam surfaces (Shi 2021e; one RCT with 39 participants) | Acute care setting | ‐ | The mean support surface‐associated patient comfort was 0. | ‐ | MD 0.4 higher (0.42 lower to 1.22 higher) |
⊕⊝⊝⊝ VERY LOW (downgraded twice for high risk of bias and once for imprecision) |
It is uncertain whether there is any difference between alternating pressure (active) air surfaces and foam surfaces in patient comfort responses. |
| Reactive air surfaces versus foam surfaces (Shi 2021e; one RCT with 72 participants) | Acute care setting | One study reported this outcome as the number of participants having changes in comfort from baseline and reported that 8 participants using reactive air surfaces had increased comfort, 4 had no change, and 1 had decreased comfort; whilst 3 participants using foam surfaces had increased comfort, 4 had no change and 6 reported decreased comfort (P value = 0.04). | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias and twice for substantial imprecision) |
We are uncertain whether there is any difference between reactive air surfaces and foam surfaces in patient comfort responses. |
| Alternating pressure (active) air surfaces compared with another type of alternating pressure (active) air surface (Shi 2021e; McGinnis 2014; 4 RCTs with 256 participants) | Acute and long‐term care setting | Two small studies reported a statistical significance favouring Nimbus system whilst two larger studies reported no significance. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias, once for heterogeneity and once for imprecision) |
It is uncertain if there is a difference in patient comfort responses between different types of alternating pressure (active) air surfaces (Nimbus vs Pegasus). |
| Reactive air surfaces versus undefined 'standard hospital surfaces' (Shi 2021e; one RCT with 40 participants) | Acute care setting | The study reported this outcome defined as self‐rated participant satisfaction measured using an 8‐item scale with a totality of 11 points ranging from 'total dissatisfaction' to 'complete satisfaction'. In total, 18 of 40 participants responded on the scale. No data analysis reported. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias, and once for imprecision) |
It is uncertain if there is a difference in patient comfort responses between these support surfaces. |
| Reactive gel surfaces versus undefined reactive surfaces (Aiartex; Shi 2021e; one RCT with 72 participants) | Long‐term care setting | 18 participants using reactive gel surfaces responded with 'Poor', 12 with 'Fair', six with 'Good', and one with 'Excellent', whilst 8 participants using the undefined reactive surfaces responded with 'Poor', 13 with 'Fair', 10 with 'Good', and 4 with 'Excellent'. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias, and once for imprecision) |
It is uncertain if there is a difference in patient comfort responses between these support surfaces. |
|
|||||||
| Alternating pressure (active) air surfaces versus foam surfaces (Shi 2021e; one RCT with 49 participants) | Acute care setting | One study (49 participants) reported there were no major adverse events that could be attributed to the support surfaces used. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias and twice for substantial inconsistency). |
It is uncertain if there is a difference in adverse events between alternating pressure (active) air surfaces and foam surfaces. |
| Foam surfaces versus reactive water surfaces (Shi 2021e; one RCT with 120 participants) | Long‐term care setting | One study reported this outcome, defined as the percentages of participants with one or more of the following types of adverse events: eczema, maceration, and pain. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias and twice for imprecision). |
It is uncertain if there is any difference in all reported adverse events between alternating pressure (active) air surfaces applied on both operating tables and hospital beds and reactive gel surfaces used on operating tables followed by foam surfaces applied on hospital beds. |
| Reactive air surfaces versus foam surfaces (Shi 2021e; two studies with 156 participants) | Acute care setting | Two studies (156 participants) reported this outcome and both did not clearly suggest any difference in adverse events. | No pooling | No pooling | No pooling | ⊕⊕⊝⊝ LOW (downgraded once for imprecision and once for indirectness) |
It is unclear if there is a difference in adverse events between reactive air surfaces and foam surfaces. |
| Alternating pressure (active) air surfaces compared with the another type of alternating pressure (active) air surfaces (Shi 2021e; McGinnis 2014; 4 RCTs with 256 participants) | Acute and long‐term care setting | The studies largely reported death data but did not state if there were other adverse events and outcome data were not pooled. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias, once for imprecision and once for indirectness) |
It is uncertain if there is a difference in adverse events between different types of alternating pressure (active) air surface. |
| Reactive air surfaces versus undefined 'standard hospital surfaces' (Shi 2021e; two RCTs with 152 participants) | Acute and long‐term care setting | We did not pool these data as the definitions of adverse events varied between studies. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded once for risk of bias, and twice for inconsistency) |
It is uncertain if there is any difference in adverse events between reactive air surfaces and standard hospital surfaces. |
| Reactive gel surfaces versus undefined reactive surfaces (Aiartex; Shi 2021e; one RCT with 72 participants) | Long‐term care setting | We did not pool these data as the definitions of adverse events varied between studies. | No pooling | No pooling | No pooling | ⊕⊝⊝⊝ VERY LOW (downgraded twice for risk of bias, and once for imprecision) |
It is uncertain if there is any difference in adverse events between reactive gel surfaces and undefined reactive surfaces. |
|
|||||||
| Reactive air surfaces compared with foam surfaces (Shi 2021e; one RCT with 87 participants) | Long‐term care setting | One study reported the extra cost due to the use of reactive air surfaces, compared with foam surfaces, divided by the ulcer‐free days and suggested that people using reactive air surfaces may cost an extra 26 US dollars for every ulcer‐free day in the first year of use. | Not applicable | Not applicable | Not applicable | ⊕⊕⊝⊝ LOW (downgraded twice for imprecision) |
Compared with foam surfaces, reactive air surfaces may cost an extra 26 US dollars for every ulcer‐free day in the first year of use in long‐term care settings. |
Appendix 7. ROBIS quality of included reviews
| Included reviews | Domain 1: Study eligibility criteria | Domain 2: Identification & search of studies | Domain 3: Data collection & study appraisal | Domain 4: Synthesis & findings | Risk of bias in the review |
| McGinnis 2014 | Low | Low | Unclear | Unclear | Unclear |
| Shi 2021a | Low | Low | Low | Low | Low |
| Shi 2021b | Low | Low | Low | Low | Low |
| Shi 2021c | Low | Low | Low | Low | Low |
| Shi 2021d | Low | Low | Low | Low | Low |
| Shi 2021e | Low | Low | Low | Low | Low |
Appendix 8. Prevention network: characteristics and data in the included studies and additional results of the prevention network analysis
Characteristics and data in the 69 potentially eligible studies
In the table below, we report the characteristics and data of the 69 RCTs identified in the topic of pressure ulcer prevention. We note that not all studies connected to a network and studies with asterisks contributed data to network meta‐analyses.
Table 1. Characteristics and data in the 69 potentially eligible studies
| Study ID | Study design | Number of arms | Follow‐up duration | Country | Funding source | Care setting | Sample size (male/ female) | Average age (years) | Baseline skin status | Study arm 1 (event/ total/ missing data) | Study arm 2 (event/ total/ missing data) | Effect estimates used for the time‐to‐event network meta‐analysis (expressed as natural logarithm of hazard ratio, that is, lnhr) | Standard errors used for the time‐to‐event network meta‐analysis (expressed as selnhr) |
| Allman 1987 * | RCT | 2 | 13 days | USA | Industries | Hospital | 72 (27/38) | 66.6 | Severe ulcers | Foam surfaces (15/34/2) | Reactive air surfaces (9/31/5) | ||
| Andersen 1982 * | RCT | 3 | 10 days | Denmark | ND | Hospital | 482 (206/276) | ND | At risk; free of ulcers | Alternating pressure (active) air surfaces (7/166/0) | Standard hospital surfaces (21/161/0); Reactive water surfaces (7/155/0) | ||
| Aronovitch 1999 * | RCT | 2 | 7 days | USA | Industries | Hospital (operation theatre and wards) | 217 (156/58) | 64.08 | At risk; free of ulcers | Alternating pressure (active) air surfaces (1/112/ND) | Reactive gel surfaces & Foam surfaces (7/105/ND) | ||
| Ballard 1997 | Cross‐over | 2 | 3 days | UK | Industries | Community & long‐term care | 10 (5/5) | 84 | No risk | Alternating pressure (active) air surfaces (ND) | Alternating pressure (active) air surfaces (ND) | ||
| Beeckman 2019 * | RCT | 2 | 14 days | Belgium | Industries | Community & long‐term care | 308 (71/237) | 87 | At risk; free of ulcers | Alternating pressure (active) air surfaces (18/154) | Reactive air surfaces (8/154) | 0.81 | 0.39 |
| Bennett 1998 * | RCT | 2 | 60 days | USA | Industries & Public | Hospital & community mixture | 116 (45/71) | ≥ 80 | Mixed skin status | Reactive air surfaces (8/42/16) | Standard hospital surfaces (4/56/2) | ||
| Berthe 2007 * | RCT | 2 | 7 months | Belgium | None | Hospital | 1729 | ND | At risk; free of ulcers | Foam surfaces (21/657) | Standard hospital surfaces (21/1072) | ‐1.05 | 0.32 |
| Bliss 1967 | RCT | 2 | 16 days | UK | Public | Hospital | 83 (27/56) | 81.2 | Mixed skin status | Alternating pressure (active) air surfaces (ND) | Standard hospital surfaces (ND) | ||
| Bliss 1995 | Multi‐arm multi‐stage | 8 | 17.7 days | UK | Industries | Hospital | 457 | ND | Mixed skin status | Alternating pressure (active) air surfaces (ND) | Reactive fibre surfaces (ND); Reactive water surfaces (ND); Foam surfaces (ND) | ||
| Bueno de Camargo 2018 (NCT02844166) | RCT | 2 | 11.5 days | Brazil | Industries | ICU | 62 (33/29) | 67.9 | At risk; free of ulcers | Foam surfaces (25/31) | Foam surfaces (10/31) | ||
| Cassino 2013 * | RCT | 2 | 12 weeks | Italy | Industries | Community & long‐term care | 72 (17/55) | 85.4 | Severe ulcers | Reactive gel surfaces (1/37) | Aiartex surfaces (0/35) | ||
| Cavicchioli 2007 * | RCT | 2 | 2 weeks | Italy | ND | Hospital | 170 (40/100) | 77.5 | Mixed skin status | Alternating pressure (active) air surfaces (2/69/17) | Reactive air surfaces (1/71/13) | ||
| Cobb 1997 | RCT | 2 | 40 days | USA | ND | Hospital | 123 (53/70) | 58 | At risk; free of ulcers | Reactive air surfaces (12/61) | Reactive air surfaces (8/62) | ||
| Collier 1996 | RCT | 8 | ND | UK | ND | Hospital | 90 (40/59) | ND | At risk; free of ulcers | Foam surfaces (ND) | Foam surfaces (ND) | ||
| Conine 1990 * | RCT | 2 | 3 months | Canada | Charity | Community & long‐term care | 187 (60/88) | 37.16 | At risk; free of ulcers | Alternating pressure (active) air surfaces (39/72/21) | Reactive fibre surfaces (45/76/18) | ||
| Cooper 1998 | RCT | 2 | 7 days | UK | Industries | Hospital | 100 (16/84) | 83 | At risk; free of ulcers | Reactive air surfaces (3/41/10) | Reactive air surfaces (5/43/6) | ||
| Daechsel 1985 * | RCT | 2 | 3 months | Canada | Industries | Community & long‐term care | 32 (16/16) | 40.55 | At risk; free of ulcers | Alternating pressure (active) air surfaces (4/16) | Reactive fibre surfaces (4/16) | ||
| Demarre 2012 | RCT | 2 | 14 days | Belgium | Public | Hospital | 610 (241/369) | 76.3 | At risk; free of ulcers | Alternating pressure (active) air surfaces (17/298/0) | Alternating pressure (active) air surfaces (18/312/0) | ||
| Ewing 1964 | RCT | 2 | 6 months | Australia | ND | Hospital | 36 | 72.5 | ND | Reactive sheepskin surfaces (ND) | Standard hospital surfaces (ND) | ||
| Feuchtinger 2006 * | RCT | 2 | 5 days | Germany | ND | Operating room | 175 (125/50) | 67.79 | At risk; free of ulcers | Foam surfaces (15/85) | Standard hospital surfaces (10/90) | 0.48 | 0.66 |
| Finnegan 2008 * | RCT | 2 | 8 days | USA | Industries | Hospital | 40 (21/12) | 56 | Severe ulcers | Alternating pressure (active) air surfaces (0/15/4) | Reactive air surfaces (0/18/3) | ||
| Gray 1994 | RCT | 2 | 10 days | UK | Industries | Hospital | 170 (66/104) | 76 | At risk; free of ulcers | Foam surfaces (ND) | Foam surfaces (ND) | ||
| Gray 2000 | RCT | 2 | 10 days | UK | Industries | Hospital | 100 (61/39) | 65 | At risk; free of ulcers | Foam surfaces (1/50) | Foam surfaces (1/50) | ||
| Gray 2008 | RCT | 2 | 6 months | UK | ND | Hospital | 100 | 83.2 | At risk; free of ulcers | Alternating pressure (active) air surfaces (4/50/0) | Alternating pressure (active) air surfaces (4/50/0) | ||
| Grindley 1996 | Cross‐over | 2 | 3 days | UK | Industries | Community & long‐term care | 20 (8/12) | 69.05 | Mixed skin status | Alternating pressure (active) air surfaces (ND) | Alternating pressure (active) air surfaces (ND) | ||
| Gunningberg 2000 * | RCT | 2 | 14 days | Sweden | Industries | Hospital | 101 (20/81) | 84.5 | At risk; free of ulcers | Foam surfaces (12/48) | Standard hospital surfaces (17/53) | ||
| Hampton 1997 | RCT | 2 | 4 months | UK | ND | Hospital | ND | ND | ND | Alternating pressure (active) air surfaces (0/36/0) | Alternating pressure (active) air surfaces (0/ND/ND) | ||
| Hofman 1994 * | RCT | 2 | 2 weeks | Netherlands | ND | Hospital | 46 (6/38) | 84.45 | At risk; free of ulcers | Foam surfaces (4/17/6) | Standard hospital surfaces (13/19/4) | ||
| Hoshowsky 1994 | RCT (split body design) | 2 | ND | USA | ND | Hospital | 505 | 47 | ND | Foam surfaces (ND) | Reactive gel surfaces (ND); Reactive foam and gel surfaces (ND) | ||
| Inman 1993 * | RCT | 2 | 18.8 days | Canada | Industries | ICU | 100 (51/47) | 64.4 | ND | Reactive air surfaces (3/49/1) | Standard hospital surfaces (14/49/1) | ||
| IRCT2015110619919N3 | RCT | 2 | ND | Iran | ND | Operating room | ND | ND | ND | Reactive gel surfaces (ND) | Standard hospital surfaces (ND) | ||
| Jiang 2014 * | RCT | 2 | 5 days | China | Public | Hospital | 1074 (621/453) | 57.94 | At risk; free of ulcers | Alternating pressure (active) air surfaces (5/512) | Reactive air surfaces (6/562) | ||
| Jolley 2004 * | RCT | 2 | ND | Australia | Industries & Public | Hospital | 539 (223/218) | 62.14 | At risk; free of ulcers | Reactive sheepskin surfaces (21/218/52) | Standard hospital surfaces (37/223/46) | ‐0.94 | 0.29 |
| Kemp 1993 | RCT | 2 | 1 month | USA | Charity | Hospital & community mixture | 84 (26/58) | 81 | At risk; free of ulcers | Foam surfaces (21/45) | Foam surfaces (12/39) | ||
| Laurent 1998 * | Factorial design | 4 | 15.04 days | Belgium | ND | Hospital | 312 (214/98) | 64 | ND | Alternating pressure (active) air surfaces (20/157) | Standard hospital surfaces (25/155) | ||
| Foam surfaces (21/152) | Standard hospital surfaces (24/160) | ||||||||||||
| Lazzara 1991 * | RCT | 2 | 6 months | USA | Industries | Community & long‐term care | 74 (6/21) | 83.6 | At risk; free of ulcers | Reactive air surfaces (10/33/4) | Reactive gel surfaces (8/33/4) | ||
| Malbrain 2010 * | RCT | 2 | 13.6 days | Belgium | ND | ICU | 16 (8/8) | 64.7 | Mixed skin status | Alternating pressure (active) air surfaces (2/8) | Reactive air surfaces (2/8) | ||
| McGowan 2000 * | RCT | 2 | ND | Australia | Industries & Public | Hospital | 297 (127/170) | 73.79 | At risk; free of ulcers | Reactive sheepskin surfaces (14/155) | Standard hospital surfaces (43/142) | ‐1.17 | 0.31 |
| Mistiaen 2009 * | RCT | 2 | 30 days | Netherlands | Public | Community & long‐term care | 588 (183/405) | 78 | At risk; free of ulcers | Reactive sheepskin surfaces (60/271/24) | Standard hospital surfaces (73/272/21) | ‐0.27 | 0.36 |
| Nixon 1998 * | RCT | 2 | 8 days | UK | Public | Operating room | 446 (235/208) | 55 | Mixed skin status | Reactive gel surfaces (22/205) | Standard hospital surfaces (43/211) | ||
| Nixon 2006 | RCT | 2 | 60 days | UK | Public | Hospital | 1972 (711/1260) | 75.2 | Mixed skin status | Alternating pressure (active) air surfaces (101/982/0) | Alternating pressure (active) air surfaces (106/989/0) | ||
| Nixon 2019 * | RCT | 2 | 90 days | UK | Public | Hospital & community mixture | 2029 (907/1119) | 78 | Mixed skin status | Alternating pressure (active) air surfaces (70/1016) | Foam surfaces (90/1013) | ‐0.27 | 0.16 |
| Ozyurek 2015 | RCT | 2 | 17.36 days | Turkey | Public | ICU | 357 (55/50) | 64.99 | At risk; free of ulcers | Foam surfaces (22/53/125) | Foam surfaces (23/52/127) | ||
| Park 2017 * | RCT | 2 | 2 weeks | South Korea | ND | Hospital | 122 (65/45) | 69.56 | At risk; free of ulcers | Foam surfaces (2/55/4) | Standard hospital surfaces (15/55/8) | ||
| Phillips 1999 | N‐of‐1 | 2 | 12 weeks | UK | ND | Community & long‐term care | 37 (11/26) | 87 | At risk; free of ulcers | Alternating pressure (active) air surfaces (2/ND/ND/ 37 randomised) | Alternating pressure (active) air surfaces (0/ND/ND/ 37 randomised) | ||
| Price 1999 | RCT | 2 | 14 days | UK | Industries & Public | Hospital | 80 (16/64) | 82.2 | At risk; free of ulcers | Alternating pressure (active) air surfaces (ND) | Reactive air surfaces (ND) | ||
| Pring 1998 | RCT | 3 | 7 days | UK | ND | Community & long‐term care | 40 | 40 | At risk; free of ulcers | Alternating pressure (active) air surfaces (ND) | Alternating pressure (active) air surfaces (ND) | ||
| Rafter 2011 | RCT | 2 | 1 month | UK | Industries | Hospital | 10 (4/6) | 74.9 | Mixed skin status | Alternating pressure (active) air surfaces (0/5/0) | Alternating pressure (active) air surfaces (2/5/0) | ||
| Ricci 2013 * | RCT | 2 | 4 weeks | Italy | Industries | Community & long‐term care | 50 (8/42) | 84.7 | Mixed skin status | Reactive gel surfaces (0/25) | Aiartex surfaces (0/25) | ||
| Rosenthal 2003 * | RCT | 2 | 6 months | USA | ND | Community & long‐term care | 76 | 68.8 | Severe ulcers | Alternating pressure (active) air surfaces (0/38) | Foam surfaces (0/38) | ||
| Russell 2000 * | RCT | 2 | 7 days | Canada | Industries | Hospital | 198 (150/48) | 65.2 | At risk; free of ulcers | Alternating pressure (active) air surfaces (2/98) | Reactive gel surfaces & Foam surfaces (7/100) | ||
| Russell 2003 * | RCT | 2 | 11.5 days | UK | Industries | Hospital | 1168 (391/777) | 83 | At risk; free of ulcers | Foam surfaces (48/562/2) | Standard hospital surfaces (66/604/0) | ‐0.16 | 0.22 |
| Sanada 2003 * | RCT | 3 | ND | Japan | ND | Hospital | 108 (42/40) | 71.3 | At risk; free of ulcers | Alternating pressure (active) air surfaces (6/55/18) | Standard hospital surfaces (10/27/8) | ||
| Santy 1994 | RCT | 6 | 12 days | UK | Public | Hospital | 552 | 80.24 | Mixed skin status | Foam surfaces (ND) | Foam surfaces (ND) | ||
| Sauvage 2017 * | RCT | 2 | 30 days | France | ND | Community & long‐term care | 76 (22/54) | 85.3 | At risk; free of ulcers | Alternating pressure (active) air surfaces (2/39) | Foam surfaces (13/37) | ‐1.7 | 0.52 |
| Schultz 1999 * | RCT | 2 | 6 days | USA | Industries & Public | Operating room | 413 (266/147) | 65.7 | At risk; free of ulcers | Foam surfaces (55/206) | Standard hospital surfaces (34/207) | ||
| Sideranko 1992 * | RCT | 3 | 9.5 days | USA | ND | ICU | 57 (33/24) | 65.9 | At risk; free of ulcers | Alternating pressure (active) air surfaces (5/20) | Reactive water surfaces (2/17); Reactive air surfaces (1/20) | ||
| Stapleton 1986 * | RCT | 2 | 3 months | UK | Public | Hospital | 100 (0/100) | 81 | At risk; free of ulcers | Alternating pressure (active) air surfaces (11/32) | Reactive fibre surfaces (12/34); Foam surfaces (14/34) | ||
| Takala 1996 * | RCT | 2 | 14 days | Finland | Industries | ICU | 40 (25/15) | 61.4 | At risk; free of ulcers | Foam surfaces (7/19) | Reactive air surfaces (0/21) | ||
| Taylor 1999 | RCT | 2 | 11.1 days | UK | ND | Hospital | 44 (25/19) | 68.4 | At risk; free of ulcers | Alternating pressure (active) air surfaces (0/22/0) | Alternating pressure (active) air surfaces (2/22/0) | ||
| Theaker 2005 | RCT | 2 | 14 days | UK | Industries | ICU | 62 (39/23) | 65 | At risk; free of ulcers | Alternating pressure (active) air surfaces (3/30) | Alternating pressure (active) air surfaces (6/32) | ||
| Van Leen 2011 * | RCT | 2 | 6 months | Netherlands | None | Community & long‐term care | 83 (16/67) | 82.1 | At risk; free of ulcers | Foam surfaces (7/42) | Reactive air surfaces (2/41) | ||
| Van Leen 2013 * | Cross‐over | 2 | 6 months | Netherlands | ND | Community & long‐term care | 41 (32/9) | 79.9 | At risk; free of ulcers | Foam surfaces (3/21) | Reactive air surfaces (1/20) | ||
| Van Leen 2018 * | RCT | 2 | 12 weeks | Netherlands | Industries | Community & long‐term care | 206 (60/146) | 82.4 | At risk; free of ulcers | Foam surfaces (5/103) | Bedcare surfaces (9/103) | ||
| Vanderwee 2005 * | RCT | 2 | 20 weeks | Belgium | ND | Hospital | 447 (163/283) | 82 | At risk; free of ulcers | Alternating pressure (active) air surfaces (34/222) | Foam surfaces plus 4‐h turning (35/225) | 0.11 | 0.24 |
| Vermette 2012 * | RCT | 2 | 14 days | Canada | None | Hospital | 110 (44/66) | 77.8 | At risk; free of ulcers | Reactive air surfaces (2/55) | Alternating pressure (active) air surfaces or RIK overlay (6/55) | ||
| Vyhlidal 1997 | RCT | 2 | 21 days | USA | Industries | Hospital | 40 (18/22) | 77.2 | At risk; free of ulcers | Foam surfaces (ND) | Foam surfaces (ND) | ||
| Whitney 1984 | RCT | 2 | 8.2 days | USA | ND | Hospital | 51 | 63.2 | Mixed skin status | Alternating pressure (active) air surfaces (ND) | Foam surfaces (ND) | ||
| Whittingham 1999 | RCT | 6 | 12 months | UK | ND | Community & long‐term care | 309 | ND | At risk; free of ulcers | Foam surfaces (ND) | Foam surfaces (ND) |
ND = no data available
Summary characteristics of 40 studies connecting to a network
Of the above 69 studies, 40 connected to a network. We report the summary characteristics of the 40 studies in the table below. This information reflects the applicability or generalisability of the network evidence.
Table 2. Summary characteristics of 40 studies connecting to a network
| Study characteristics | Details of studies |
| Types of studies | Two arms (n = 35): Allman 1987; Aronovitch 1999; Beeckman 2019; Bennett 1998; Berthe 2007; Cassino 2013; Cavicchioli 2007; Conine 1990; Daechsel 1985; Feuchtinger 2006; Finnegan 2008; Gunningberg 2000; Hofman 1994; Inman 1993; Jiang 2014; Jolley 2004; Lazzara 1991; Malbrain 2010; McGowan 2000; Mistiaen 2009; Nixon 1998; Nixon 2019; Park 2017; Ricci 2013; Rosenthal 2003; Russell 2000; Russell 2003; Sauvage 2017; Schultz 1999; Takala 1996; Van Leen 2011; Van Leen 2013; Van Leen 2018; Vanderwee 2005; Vermette 2012. Three or more arms (n = 5): Andersen 1982; Laurent 1998; Sanada 2003; Sideranko 1992; Stapleton 1986.
|
| Follow‐up duration | Median 14 days (range: 5 days to 7 months) |
| Funding sources |
|
| Age of participants | Average participant age: median 72.5 years (range 37.2 to 87.0 years) |
| Sex proportions | Male: 4702 (45.1%) in 38 studies Female: 5730 (54.9%) in 38 studies |
| Skin status at baseline | 38 studies specified the skin status at baseline
|
| Care settings |
|
Risk of bias assessment for the included studies and network evidence from the base‐case analysis
We summarise risk of bias assessments for the included studies in the topic of prevention in Figure 7.
7.
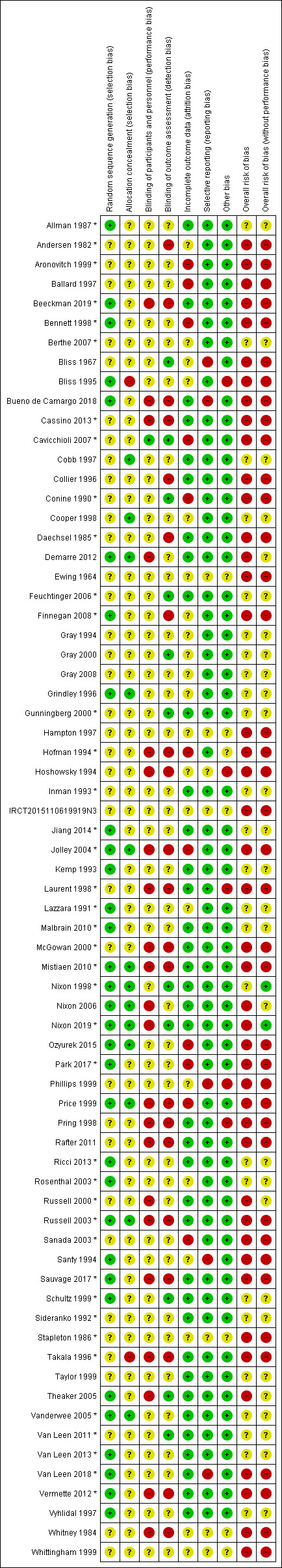
Prevention network: risk of bias summary of review authors' judgements about each risk of bias item for each study. Studies with asterisk contributed data for network meta‐analyses
Based on overall risk of bias in the 40 included studies, we assessed overall risk of bias for each of 78 network contrasts of the base‐case analysis. See Figure 8.
8.
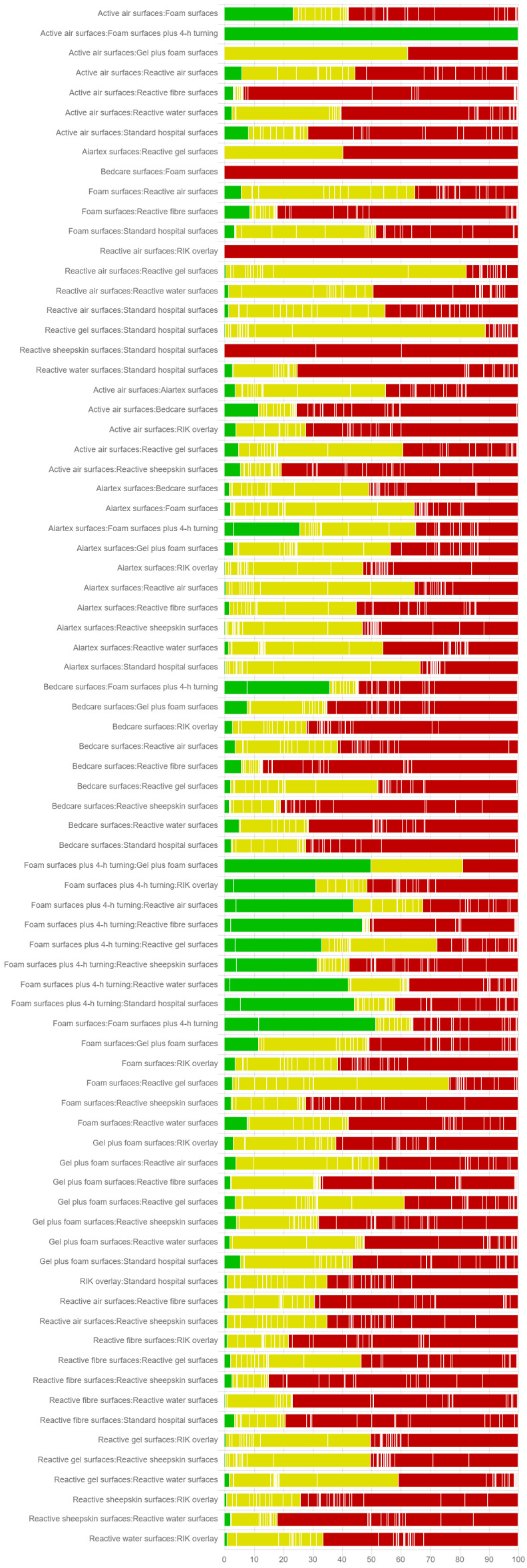
Risk of bias assessment for each network contrast. The CINeMA tool uses the percentage contribution matrix and combines this with the risk of bias assessments of the included studies (n = 40). Each row represents a network contrast of the prevention network. For each network contrast, the CINeMA tool computes the percentage contribution from studies judged to be at low, moderate, and high risk of bias. Green bars within each row indicate the percentage contribution from studies at low risk of bias, yellow for unclear risk of bias, and red for high risk of bias. Note not all of the 40 studies contributed data to each contrast's effectiveness estimation.
Assessing transitivity, homogeneity and consistency assumptions for the base‐case analysis
We assessed the transitivity, homogeneity and consistency assumptions for the base‐case analysis below.
(1) Transitivity assumption assessment
We summarised the characteristics of the included studies for each direct contrast and compared these direct contrast‐level summary characteristics across these direct contrasts (see the table below). This aims to assess if there are systematic differences between the direct contrasts other than the treatments under evaluations. Thus, data from direct contrasts can be used to calculate indirect evidence (transitivity). The 19 direct contrasts are heterogeneous in terms of risk of bias (also see Figure 2), and follow‐up duration (11/19 of direct contrasts with short‐term follow‐up, 6/19 with medium‐term follow‐up, and 2/19 with long‐term follow‐up). We considered these direct contrasts homogeneous in terms of care settings (mainly acute and long‐term care settings), and participants' characteristics: generally equal proportions of sex in 14/19 direct contrasts contributing the majority of data, age (mainly older adults), and baseline skin status (mainly at risk but free of existing ulcers). We assumed the transitivity assumption held.
Table 3. Summary characteristics of studies for 19 direct comparisons
| Direct comparisons | Number of studies | Study design, no. of multi‐arm studies (% of total participants) | Median of follow‐up | Care settings (% of total participants) | Number of events/ number of total participants (%) | Male (%) | Female (%) | Median of average age reported (years) | Baseline skin status (% of total participants) | Overall risk of bias level (% of total participants) |
| Alternating pressure (active) air surfaces vs foam surfaces | 4 | RCTs including one three‐arm study (3%) | 90 days | Acute care setting (93%), Long‐term care setting (7%) | 200/2247 (9%) | 929 (43%) | 1239 (57%) | 80 | At risk, free of ulcers (97%); Existing ulcers (3%) | Low and unclear (93%); High (7%) |
| Alternating pressure (active) air surfaces vs foam surfaces plus 4‐hourly turning | 1 | RCT | 20 weeks | Acute care setting (100%) | 69/447 (15%) | 163 (37%) | 283 (63%) | 82 | At risk, free of ulcers (100%) | Low (100%) |
| Alternating pressure (active) air surfaces vs reactive air surfaces | 6 | RCTs including one three‐arm study (2%) | 11.5 days | Acute care setting (77%) ICU (4%); Long‐term care setting (19%) |
50/1611 (3%) | 794 (49%) | 834 (51%) | 65 | At risk, free of ulcers (98%); Existing ulcers (2%) |
Unclear (67%); High (33%) |
| Alternating pressure (active) air surfaces vs reactive fibre surfaces | 3 | RCTs | 3 months | Acute care setting (23%); Long‐term care setting (73%) | 115/246 (47%) | 76 (27%) | 204 (73%) | 41 | At risk, free of ulcers (100%) | High (100%) |
| Alternating pressure (active) air surfaces vs reactive gel surfaces followed by foam surfaces | 2 | RCTs | 7 days | Operating room followed by wards (100%) | 17/415 (4%) | 306 (74%) | 106 (26%) | 65 | At risk, free of ulcers (100%) | Unclear (48%); High (52%) |
| Alternating pressure (active) air surfaces vs reactive water surfaces | 2 | Both RCTs with three arms (100%) | 10 days | Acute care setting (90%); ICU (10%) |
21/358 (6%) | 239 (44%) | 300 (56%) | 66 | At risk, free of ulcers (100%) | Unclear (10%); High (90%) |
| Alternating pressure (active) air surfaces vs 'standard hospital surfaces' | 3 | All multi‐arm studies (100%), including one factorial design (43%) | 12.5 days | Acute care setting (100%) | 89/721 (12%) | 462 (53%) | 414 (47%) | 68 | At risk, free of ulcers (100%) | High (100%) |
| Foam surfaces vs reactive air surfaces | 4 | Cross‐over design (18%) and parallel RCTs (82%) | 3 months | Acute care setting (28%); ICU (17%); Long‐term care setting (54%) |
44/229 (19%) | 100 (44%) | 129 (56%) | 73 | At risk, free of ulcers (69%); Existing ulcers (31%) |
Unclear (83%); High (17%) |
| Foam surfaces vs reactive fibre surfaces | 1 | RCT | 3 months | Acute care setting (100%) | 26/68 (38%) | 0 | 68 (100%) | 81 | At risk, free of ulcers (100%) | High (100%) |
| Foam surfaces vs 'standard hospital surfaces' | 8 | Factorial design (8%), and parallel RCTs | 35 days | Acute care setting (85%); Operating room (15%) |
378/4042 (9%) | 1087 (47%) | 1236 (53%) | 74 | At risk, free of ulcers (100%) | Unclear (59%); High (41%) |
| Foam surfaces vs Bedcare surfaces (undefined) | 1 | RCT | 12 weeks | Long‐term care setting (100%) | 14/206 (7%) | 60 (29%) | 146 (71%) | 82 | At risk, free of ulcers (100%) | High (100%) |
| Reactive air surfaces vs 'standard hospital surfaces' | 2 | RCTs | 39.4 days | ICU (50%); Acute care setting (50%) |
29/196 (15%) | 96 (45%) | 118 (55%) | 72 | Mixed skin status (100%) | Unclear (46%); High (54%) |
| Reactive air surfaces vs reactive gel surfaces | 1 | RCT | 6 months | Long‐term care setting (100%) | 18/66 (27%) | 6 (22%) | 21 (78%) | 84 | At risk, free of ulcers (100%) | Unclear (100%) |
| Reactive air surfaces vs reactive water surfaces | 1 | One three‐arm study (100%) | 9.5 days | ICU (100%) | 3/37 (8%) | 33 (58%) | 24 (42%) | 66 | At risk, free of ulcers (100%) | Unclear (100%) |
| Reactive air surfaces vs alternating pressure (active) air surfaces or RIK overlay | 1 | RCT | 14 days | Acute care setting (100%) | 8/110 (7%) | 44 (40%) | 66 (60%) | 78 | At risk, free of ulcers (100%) | High (100%) |
| Reactive gel surfaces vs 'standard hospital surfaces' | 1 | RCT | 8 days | Operating room (100%) | 65/416 (16%) | 235 (53%) | 208 (47%) | 55 | Mixed skin status (100%) | Unclear (100%) |
| Reactive gel surfaces vs Aiartex surfaces (undefined) | 2 | RCTs | 8 weeks | Long‐term care setting (100%) | 1/122 (1%) | 25 (20%) | 97 (80%) | 85 | Mixed skin status (41%); Existing ulcers (59%) |
Unclear (41%); High (59%) |
| Reactive sheepskin surfaces vs 'standard hospital surfaces' | 3 | RCTs | 30 days | Acute care setting (58%); Long‐term care setting (42%) |
248/1281 (19%) | 533 (40%) | 793 (60%) | 73 | At risk, free of ulcers (100%) | High (100%) |
| Reactive water surfaces vs 'standard hospital surfaces' | 1 | One three‐arm study (100%) | 10 days | Acute care setting (100%) | 28/316 (9%) | 206 (43%) | 276 (57%) | ND | At risk, free of ulcers (100%) | High (100%) |
ND = no data available
(2) Homogeneity assumption tests and subgroup analyses
Heterogeneity tests
The between‐study variance in this network (Tau2) is 0.146 which is much larger than median Tau2 = 0.056 (IQR 0.005 to 0.651), reference values for non‐pharmacological interventions used in semi‐objective outcomes (Turner 2012). We therefore considered this network had substantial heterogeneity. This resulted in downgrading for heterogeneity in assessing the certainty of evidence using the GRADE approach.
We considered downgrading for the following nine network contrasts.
Four network contrasts had moderate heterogeneity:
alternating pressure (active) air surfaces versus foam surfaces: between‐study heterogeneity I2 = 43.2%, Tau2 = 0.117;
alternating pressure (active) air surfaces versus reactive gel surfaces followed by foam surfaces: prediction interval extends into clinically important effects (RR = 1) in both directions;
alternating pressure (active) air surfaces versus standard hospital surfaces: between‐study heterogeneity I2 = 60.7%, Tau2 = 0.229;
foam surfaces versus reactive air surfaces: prediction interval extends into clinically important effects (RR = 1) in both directions.
Four network contrasts with substantial heterogeneity:
reactive air surfaces versus standard hospital surfaces: between‐study heterogeneity I2 = 85.9%, Tau2 = 2.142;
reactive gel surfaces versus standard hospital surfaces: prediction interval extends into clinically important effects (RR = 1) in both directions;
reactive sheepskin surfaces versus standard hospital surfaces: between‐study heterogeneity I2 = 79.8%, Tau2 = 0.206; and
reactive water surfaces versus standard hospital surfaces: prediction interval extends into clinically important effects (RR = 1) in both directions.
Subgroup analysis
We performed subgroup analyses to investigate further the heterogeneity of the base‐case analysis (Tau2 = 0.146) by considering four study‐level characteristics:
risk of bias (binary: low or unclear risk of bias as the reference versus high risk of bias): this regression resulted in a Tau2 of 0.146, showing that the factor could not explain the heterogeneity. Therefore, risk of bias factor may not be an important effect modifier for the base‐case analysis.
care settings (categorical: acute care setting as the reference versus long‐term care settings, operating theatre setting, and intensive care unit): this exploratory regression resulted in a Tau2 of 0.075 and suggested that the care setting may be an important effect modifier for the network meta‐analysis. However, due to the small number of included studies, we did not undertake analyses for individual care settings.
baseline skin status (categorical: participants at risk as the reference versus other skin status or non‐reporting, existing ulcers of stage 2 or more severe): this regression resulted in a Tau2 of 0.202, suggesting that baseline skin status could not explain the heterogeneity of the base‐case analysis.
follow‐up duration in weeks (categorical: short‐term duration as the reference versus medium‐term duration, long‐term duration, and no reporting): this exploratory regression resulted in a Tau2 of 0.117, suggesting that this factor only explained a small amount of heterogeneity of the base‐case analysis.
Note that there is a small number of studies in these exploratory analyses and they may be under‐powered.
(3) Consistency tests
We considered the estimates of treatment effects from direct and indirect evidence consistent globally (global design‐by‐treatment interaction model: χ2 statistic: 3.853, P value: 0.921) and locally (no inconsistency resulting from the tests of separating indirect from direct evidence): that is, the consistency assumption held in the network.
GRADE assessment for network evidence from the base‐case analysis
We assessed the certainty of the evidence for all network contrasts in the base‐case analysis. See the table below.
Table 4. GRADE assessment for network evidence from the base‐case analysis
| Comparison | Number of studies | Within‐study bias | Reporting bias | Indirectness | Imprecision | Heterogeneity | Incoherence | Confidence rating |
| Alternating pressure (active) air surfaces:Aiartex surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Alternating pressure (active) air surfaces:Bedcare surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Alternating pressure (active) air surfaces:Foam surfaces | 4 | Some concerns | Undetected | No concerns | No concerns | Some concerns | No concerns | Low |
| Alternating pressure (active) air surfaces:Foam surfaces plus 4‐h turning | 1 | No concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Low |
| Alternating pressure (active) air surfaces:Reactive gel surfaces followed by foam surfaces | 2 | Some concerns | Undetected | No concerns | No concerns | Some concerns | No concerns | Low |
| Alternating pressure (active) air surfaces:Reactive air surfaces | 6 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Alternating pressure (active) air surfaces:Reactive fibre surfaces | 3 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Alternating pressure (active) air surfaces:Reactive gel surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Alternating pressure (active) air surfaces:Reactive sheepskin surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Alternating pressure (active) air surfaces:Reactive water surfaces | 2 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Alternating pressure (active) air surfaces:RIK overlay | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Alternating pressure (active) air surfaces:Standard hospital surfaces | 3 | Major concerns | Undetected | No concerns | No concerns | Some concerns | No concerns | Very low |
| Aiartex surfaces:Bedcare surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Aiartex surfaces:Foam surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Aiartex surfaces:Foam surfaces plus 4‐h turning | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Aiartex surfaces:Reactive gel surfaces followed by foam surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Aiartex surfaces:Reactive air surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Aiartex surfaces:Reactive fibre surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Aiartex surfaces:Reactive gel surfaces | 2 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Aiartex surfaces:Reactive sheepskin surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Aiartex surfaces:Reactive water surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Aiartex surfaces:RIK overlay | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Aiartex surfaces:Standard hospital surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Bedcare surfaces:Foam surfaces | 1 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Bedcare surfaces:Foam surfaces plus 4‐h turning | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Bedcare surfaces:Reactive gel surfaces followed by foam surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Bedcare surfaces:Reactive air surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Bedcare surfaces:Reactive fibre surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Bedcare surfaces:Reactive gel surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Bedcare surfaces:Reactive sheepskin surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Bedcare surfaces:Reactive water surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Bedcare surfaces:RIK overlay | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Bedcare surfaces:Standard hospital surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Foam surfaces plus 4‐h turning:Reactive gel surfaces followed by foam surfaces | 0 | No concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Low |
| Foam surfaces plus 4‐h turning:Reactive air surfaces | 0 | No concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Low |
| Foam surfaces plus 4‐h turning:Reactive fibre surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Foam surfaces plus 4‐h turning:Reactive gel surfaces | 0 | No concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Low |
| Foam surfaces plus 4‐h turning:Reactive sheepskin surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Foam surfaces plus 4‐h turning:Reactive water surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Foam surfaces plus 4‐h turning:RIK overlay | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Foam surfaces plus 4‐h turning:Standard hospital surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Foam surfaces:Foam surfaces plus 4‐h turning | 0 | No concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Low |
| Foam surfaces:Reactive gel surfaces followed by foam surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Foam surfaces:Reactive air surfaces | 4 | Some concerns | Undetected | No concerns | No concerns | Some concerns | No concerns | Low |
| Foam surfaces:Reactive fibre surfaces | 1 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Foam surfaces:Reactive gel surfaces | 0 | No concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Low |
| Foam surfaces:Reactive sheepskin surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Foam surfaces:Reactive water surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Foam surfaces:RIK overlay | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Foam surfaces:Standard hospital surfaces | 8 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive gel surfaces followed by foam surfaces:Reactive air surfaces | 0 | Some concerns | Undetected | No concerns | No concerns | No concerns | No concerns | Moderate |
| Reactive gel surfaces followed by foam surfaces:Reactive fibre surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive gel surfaces followed by foam surfaces:Reactive gel surfaces | 0 | Some concerns | Undetected | No concerns | No concerns | No concerns | No concerns | Moderate |
| Reactive gel surfaces followed by foam surfaces:Reactive sheepskin surfaces | 0 | Major concerns | Undetected | No concerns | No concerns | Some concerns | No concerns | Very low |
| Reactive gel surfaces followed by foam surfaces:Reactive water surfaces | 0 | Some concerns | Undetected | No concerns | No concerns | No concerns | No concerns | Moderate |
| Reactive gel surfaces followed by foam surfaces:RIK overlay | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive gel surfaces followed by foam surfaces:Standard hospital surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive air surfaces:Reactive fibre surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive air surfaces:Reactive gel surfaces | 1 | No concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Low |
| Reactive air surfaces:Reactive sheepskin surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive air surfaces:Reactive water surfaces | 1 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive air surfaces:RIK overlay | 1 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive air surfaces:Standard hospital surfaces | 2 | Some concerns | Undetected | No concerns | No concerns | Major concerns | No concerns | Very low |
| Reactive fibre surfaces:Reactive gel surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive fibre surfaces:Reactive sheepskin surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive fibre surfaces:Reactive water surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive fibre surfaces:RIK overlay | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive fibre surfaces:Standard hospital surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive gel surfaces:Reactive sheepskin surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive gel surfaces:Reactive water surfaces | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive gel surfaces:RIK overlay | 0 | Some concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive gel surfaces:Standard hospital surfaces | 1 | No concerns | Undetected | No concerns | No concerns | Major concerns | No concerns | Low |
| Reactive sheepskin surfaces:Reactive water surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive sheepskin surfaces:RIK overlay | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive sheepskin surfaces:Standard hospital surfaces | 3 | Major concerns | Undetected | No concerns | No concerns | Major concerns | No concerns | Very low |
| Reactive water surfaces:RIK overlay | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
| Reactive water surfaces:Standard hospital surfaces | 1 | Major concerns | Undetected | No concerns | No concerns | Major concerns | No concerns | Very low |
| RIK overlay:Standard hospital surfaces | 0 | Major concerns | Undetected | No concerns | Major concerns | No concerns | No concerns | Very low |
Ranking probabilities of each intervention in the base‐case analysis
We present the cumulative probability plot for each support surface in Figure 9.
9.
Prevention network: cumulative probability plot for each support surface evaluated in the base‐case network. Cumulative probability plots show cumulative rank probabilities of each intervention being less than or equal to a given rank order. Note that SUCRA is the area under the plot for each support surface: higher SUCRA value = higher probability of being the best intervention. The closer the probability of a rank to 100% and the narrower the overlap with the ranks of other interventions, the greater the confidence in the ranking. Note predictive probabilities incorporate heterogeneity into probability estimates.
We estimated values of three ranking measures for each intervention in the base‐case analysis. See full results in the table below.
Table 5. Ranking probabilities of each intervention in the base‐case analysis
| Interventions | SUCRA (estimated % (predicted %)) | Probability of being the best (estimated % (predicted %)) | Mean rank (estimated values (predicted values)) |
| Foam surfaces | 30.2 (33.5) | 0.0 (0.0) | 9.4 (9.0) |
| Reactive air surfaces | 78.1 (74.5) | 6.0 (7.8) | 3.6 (4.1) |
| Alternating pressure (active) air surfaces | 59.3 (59.0) | 0.1 (1.4) | 5.9 (5.9) |
| Reactive gel surfaces | 74.6 (71.7) | 7.0 (7.8) | 4.0 (4.4) |
| Reactive sheepskin surfaces | 64.1 (62.7) | 2.6 (3.5) | 5.3 (5.5) |
| Reactive fibre surfaces | 50.6 (51.5) | 0.7 (1.5) | 6.9 (6.8) |
| Reactive water surfaces | 77.7 (75.0) | 18.4 (16.7) | 3.7 (4.0) |
| Reactive gel surfaces followed by foam surfaces | 7.6 (9.2) | 0.1 (0.1) | 12.1 (11.9) |
| Standard hospital surfaces | 25.7 (29.9) | 0.0 (0.0) | 9.9 (9.4) |
| Aiartex surfaces | 78.3 (77.4) | 56.5 (52.7) | 3.6 (3.7) |
| Bedcare surfaces | 17.3 (18.9) | 0.4 (0.4) | 10.9 (10.7) |
| RIK overlay | 29.0 (29.7) | 3.3 (2.9) | 9.5 (9.4) |
| Foam surfaces and 4‐hourly turning | 57.5 (57.0) | 4.9 (5.2) | 6.1 (6.2) |
In the Table, SUCRA value is a numerical summary of the cumulative probability plot for each support surface, and the plot shows cumulative rank probabilities that each intervention is less than or equal to a specific rank order against all possible ranks. The higher the SUCRA value, the higher the probability of being the best intervention. Probability of being the best value is the proportion of an intervention being ranked at the first place among a great number of repeated estimations for the intervention (simulations). Mean rank is the mean value of the distribution for the rank of each intervention. In the above table, all predicted values incorporate heterogeneity into value estimates.
All SUCRA values were less than 80.0% and they show considerable overlap, reflecting uncertainties in ranking. There are also overlaps between interventions for other two ranking measures (probability values of being the best interventions, and mean ranks). Here, Aiartex surfaces had the highest probability of being the best intervention. However, the ranking is probably artificially high: the direct evidence for the Aiartex surfaces involves only two small studies with a total of 122 participants (one event) and Aiartex surfaces correspond to 60 participants (no events). The NMA results of network contrasts involving Aiartex surfaces all have very wide CIs. These data limitations mean that it is unlikely that Aiartex surfaces are the most effective intervention.
We assessed the certainty of evidence regarding ranking and considered the ranking evidence of very low certainty. We downgraded once for risk of bias as the majority of data contributing to the base‐case network (73.5%) were at high risk of bias. We downgraded only once for both heterogeneity and inconsistency together. This is because the network had an overall substantial heterogeneity (Tau2 = 0.146) but the heterogeneity has limited impact on the rank order and the network had no global inconsistency.
We downgraded the evidence twice for imprecision: the network had sparse data and there are overlaps between interventions in terms of all rank order measures. We did not downgrade for indirectness. We did not downgrade for publication bias. This is because Figure 4 appears not to strongly suggest small‐studies effects and the small number of included studies per each direct contrast (40/13 = 3).
Full results of sensitivity analyses
Sensitivity analysis using complete case data
This sensitivity analysis included the same number of studies (n = 40) and 12,183 available participants. The analysis removed 334 cases from the total number of participants for the base‐case analysis (12,517 participants). The sensitivity analysis had the same network as the base‐case analysis. As with the base‐case analysis, the sensitivity analysis had limitations in terms of risk of bias, heterogeneity (Tau2 = 0.165), inconsistency (global test P value = 0.918), and imprecision. However, both analyses had no difference in relative effectiveness results for all but one network contrast, and no difference in rank orders of interventions. The exception is the comparison of standard hospital surfaces with reactive water surfaces. Its RR is 2.49 (95% CI 1.01 to 6.15), not crossing RR = 1 in the base‐case analysis, but in the sensitivity analysis, its RR is 2.46 (95% CI 0.97 to 6.20), crossing RR = 1. However, we considered this difference is not substantial and therefore the base‐case network meta‐analysis is not sensitive to missing data. Therefore, we did not report the details and results of this sensitivity analysis network.
Post hoc sensitivity analysis using eligible and well‐defined support surfaces
The post hoc sensitivity analysis contained 24 studies assessing seven interventions in 5686 participants with 494 events. It excluded interventions that were not well described and could not be classified, or were ineligible: Aiartex surfaces, Bedcare surfaces, RIK overlay, 'standard hospital surfaces', and foam surfaces plus four‐hourly turning. As a result of these removals, reactive sheepskin surfaces that were linked to standard hospital surfaces only were also removed. This sensitivity analysis explored the impact of restricting the network to the set of support surfaces that could be classified and widely accessible in practice: foam surfaces, reactive air surfaces, alternating pressure (active) air surfaces, reactive gel surfaces, reactive fibre surfaces, reactive water surfaces, and reactive gel surfaces followed by foam surfaces.
Network diagram and risk of bias assessment
The network diagram for this sensitivity analysis is shown in Figure 10. This network had nine direct contrasts and three triangular loops. Three of the nine direct contrasts included only one study: foam surfaces versus reactive fibre surfaces; reactive air surfaces versus reactive gel surfaces; and reactive air surfaces versus reactive water surfaces. The comparison of alternating pressure (active) air surfaces versus foam surfaces included four studies; the comparison of alternating pressure (active) air surfaces versus reactive air surfaces included six studies; alternating pressure (active) air surfaces versus reactive fibre surfaces included three studies; alternating pressure (active) air surfaces versus reactive gel surfaces followed by foam surfaces included two studies; alternating pressure (active) air surfaces versus reactive water surfaces included two studies; and foam surfaces versus reactive air surfaces included four studies.
10.
Prevention network: network diagram of post hoc sensitivity analysis of seven eligible and well defined support surfaces
The sensitivity analysis network has 21 network contrasts. The average number of events per network contrast was around 24 (494/21). The data were sparse and the relative effectiveness estimates of almost all network contrasts had wide confidence intervals and crossed RR = 1.
Figure 10 shows that three of nine direct comparisons were at unclear risk of bias whilst the remaining six direct comparisons were at high risk. Considering the risk of bias for each direct comparison and their percentage contributions to the whole network, we found that 23.4% of data were at unclear risk of bias and the remaining 76.6% were at high risk of bias.
Network meta‐analysis results
We undertook a random‐effects network meta‐analysis and report the relative effectiveness results for all 21 network contrasts with RRs and their 95% CIs in Figure 11. In comparison with the base‐case analysis (Figure 3), the post hoc sensitivity analysis resulted in no substantial difference in the relative effectiveness results for 19 of these 21 network contrasts (Figure 11). There are two exceptions, however: alternating pressure (active) air surfaces versus reactive air surfaces had a RR of 1.66 (95% CI 1.08 to 2.53), and reactive fibre surfaces versus reactive air surfaces had a RR of 1.83 (95% CI 1.13 to 3.05) in the sensitivity analysis. The base‐case analysis reported similar results for these two comparisons but their CIs crossed RR = 1 in the base‐case analysis. Therefore, the base‐case analysis is not sensitive to the exclusion of the above five interventions despite the fact that this sensitivity analysis appears to suggest that reactive air surfaces result in a lower pressure ulcer risk compared with alternating pressure (active) air surfaces and reactive fibre surfaces. We should be cautious about interpretations of these two contrasts' results in the base‐case analysis.
11.
Prevention network: relative effectiveness results expressed in RRs and 95% CIs for the post hoc sensitivity analysis
We report the rank order for all seven support surfaces in the network and the cumulative probability of a particular intervention being the best, second best, third best (etc.) intervention for preventing pressure ulcers in Figure 12. The SUCRA probabilities of being the best, and mean rank for each support surface are below.
12.
Prevention network: cumulative probability plot for each support surface evaluated in the post hoc sensitivity analysis. Cumulative probability plots show cumulative rank probabilities of each intervention being less than or equal to a given rank order. Note that SUCRA is the area under the plot for each support surface: higher SUCRA value = higher probability of being the best intervention. Note predictive probabilities incorporate heterogeneity into probability estimates.
Table 6. Ranking probabilities of each intervention in the sensitivity analysis
| Interventions | SUCRA (estimated % (predicted %)) | Probability of being the best (estimated % (predicted %)) | Mean rank (estimated values (predicted values)) |
| Foam surfaces | 21.9 (21.9) | 0.0 (0.0) | 5.7 (5.7) |
| Reactive air surfaces | 84.1 (84.1) | 22.6 (22.5) | 2.0 (2.0) |
| Alternating pressure (active) air surfaces | 53.7 (53.6) | 0.1 (0.1) | 3.8 (3.8) |
| Reactive gel surfaces | 89.9 (90.0) | 64.4 (64.7) | 1.6 (1.6) |
| Reactive fibre surfaces | 39.3 (39.3) | 0.1 (0.1) | 4.6 (4.6) |
| Reactive water surfaces | 59.9 (59.9) | 12.7 (12.5) | 3.4 (3.4) |
| Reactive gel surfaces followed by foam surfaces | 1.3 (1.2) | 0.1 (0.0) | 6.9 (6.9) |
This table suggests that, numerically, reactive air surfaces, reactive water surfaces and reactive gel surfaces still have the highest estimated SUCRA values (84.1%, 59.9% and 89.9%, respectively; Figure 12). However, the overlaps in terms of rank orders between these support surfaces are smaller in the sensitivity analysis than in the base‐case analysis. Therefore, interpretations of their rank orders for the base‐case analysis should be cautious.
The sensitivity analysis network presents no heterogeneity (Tau2 = 0). Regarding publication bias, Figure 13 also appears to indicate a slight small‐studies‐effect, which is consistent with the base‐case analysis.
13.
Prevention network: funnel plot of the sensitivity analysis network for pressure ulcer incidence outcome
Comparison of results from standard (pairwise) meta‐analysis with NMA findings
We reproduced results of standard (pairwise) meta‐analysis using reported data for 12 direct comparisons and compared these with the corresponding results from the base‐case network meta‐analysis. The table below shows that no statistical difference was identified for these 12 comparisons.
Table 7. Comparison of results from standard (pairwise) meta‐analysis with NMA findings
| Interventions | Pairwise meta‐analysis results, RR (95% CI) | NMA results, RR (95% CI) | Difference between two types of analysis results |
| Alternating pressure (active) air surfaces vs foam surfaces | 0.63 (0.34 to 1.17) | 0.63 (0.42 to 0.93) | No statistical difference |
| Alternating pressure (active) air surfaces vs reactive air surfaces | 1.61 (0.90 to 2.88) | 1.35 (0.82 to 2.20) | No statistical difference |
| Reactive water surfaces vs alternating pressure (active) air surfaces vs | 0.83 (0.35 to 1.92) | 0.69 (0.28 to 1.70) | No statistical difference |
| Reactive fibre surfaces vs alternating pressure (active) air surfaces | 1.11 (0.84 to 1.47) | 1.14 (0.66 to 1.98) | No statistical difference |
| Reactive gel surfaces followed by foam surfaces vs alternating pressure (active) air surfaces | 4.55 (1.28 to 16.67) | 4.60 (1.18 to 17.86) | No statistical difference |
| Reactive air surfaces vs foam surfaces | 0.42 (0.18 to 0.96) | 0.46 (0.29 to 0.75) | No statistical difference |
| Reactive fibre surfaces vs foam surfaces | 0.85 (0.47 to 1.56) | 0.71 (0.38 to 1.34) | No statistical difference |
| Bedcare surfaces vs foam surfaces | 1.79 (0.62 to 5.26) | 1.80 (0.49 to 6.58) | No statistical difference |
| Reactive water surfaces vs reactive air surfaces | 2.33 (0.22 to 25.00) | 0.93 (0.35 to 2.47) | No statistical difference |
| Reactive gel surfaces vs reactive air surfaces | 0.80 (0.36 to 1.79) | 1.02 (0.48 to 2.16) | No statistical difference |
| Alternating pressure (active) air surfaces or RIK overlay vs reactive air surfaces vs | 3.03 (0.64 to 14.29) | 3.00 (0.53 to 16.87) | No statistical difference |
| Standard hospital surfaces vs reactive water surfaces | 2.86 (1.25 to 6.67) | 1.50 (0.78 to 2.88) | No statistical difference |
Appendix 9. References of the primary studies included in network meta‐analyses
References of studies for the topic of pressure ulcer prevention
We identified 69 potentially eligible studies in the topic of pressure ulcer prevention. Here is the list of references for these studies. We use an asterisk to indicate the primary reference where a study has multiple references.
Allman 1987
Allman RM, Walker JM, Hart MK, Laprade CA, Noel LB, Smith CR. Air‐fluidized beds or conventional therapy for pressure sores. A randomised trial. Annals of Internal Medicine 1987;107(5):641‐8.
Andersen 1982
Andersen KE, Jensen O, Kvorning SA, Bach E. Decubitus prophylaxis: a prospective trial on the efficacy of alternating‐pressure air‐mattresses and water‐mattresses. Acta Dermatovener (Stockholm) 1982;63:227‐30.
Aronovitch 1999
* Aronovitch SA, Wilber M, Slezak S, Martin T, Utter D. A comparative study of an alternating air mattress for the prevention of pressure ulcers in surgical patients. Ostomy/Wound Management 1999;45(3):34‐44.
Aronovitch SA. A comparative, randomised, controlled study to determine safety and efficacy of preventive pressure ulcer systems: preliminary analysis. Advances in Wound Care 1998;11 (3 Suppl):15‐6.
Ballard 1997
Ballard K. Pressure‐relief mattresses and patient comfort. Professional Nurse 1997;13(1):27‐32.
Beeckman 2019
Anrys C, Van Tiggelen H, Verhaeghe S, Van Hecke A, Beeckman D. Independent risk factors for pressure ulcer development in a high‐risk nursing home population receiving evidence‐based pressure ulcer prevention: results from a study in 26 nursing homes in Belgium. International Wound Journal 2019;16(2):325‐33.
* Beeckman D, Serraes B, Anrys C, Van Tiggelen H, Van Hecke A, Verhaeghe S. A multicentre prospective randomised controlled clinical trial comparing the effectiveness and cost of a static air mattress and alternating air pressure mattress to prevent pressure ulcers in nursing home residents. International Journal of Nursing Studies 2019;97:105‐13.
NCT03597750. Comparison of static air support devices (Repose®) and alternating‐pressure devices in the prevention of pressure ulcers. ClinicalTrials.gov/show/NCT03597750.
Bennett 1998
Bennett RG, Baran PJ, DeVone LV, Bacetti H, Kristo B, Tayback M, et al. Low airloss hydrotherapy versus standard care for incontinent hospitalized patients. Journal of the American Geriatrics Society 1998;46(5):569‐76.
Berthe 2007
Berthe JV, Bustillo A, Mélot C, De Fontaine D. Does a foamy‐block mattress system prevent pressure sores? A prospective randomised clinical trial in 1729 patients. Acta Chirurgica Belgica 2007;107(2):155‐61.
Bliss 1967
Bliss MR, McLaren R, Exton‐Smith AN. Preventing pressure sores in hospital: controlled trial of a large‐celled ripple mattress. BMJ 1967;1(5537):394‐7.
Bliss 1995
* Bliss MR. Preventing pressure sores in elderly patients: a comparison of seven mattress overlays. Age and Ageing 1995;24:297‐302.
Bliss MR. Randomised controlled trial of seven pressure relieving mattress overlays for preventing pressure sores in elderly patients. Tissue Viability Society Conference 1994:5.
Bueno de Camargo 2018
* Bueno de Camargo WH, Pereira RD, Tanita MT, Heko L, Grion IC, Festti J, et al. The effect of support surfaces on the incidence of pressure injuries in critically ill patients: a randomised clinical trial. Critical Care Research and Practice 2018;2018:Article ID 3712067.
NCT02844166. Support surfaces to prevent pressure injuries. clinicaltrials.gov/show/NCT02844166.
Cassino 2013
Cassino R, Ippolito AM, Ricci E. Comparison of two mattress overlays in the prevention of pressure ulcers. Acta Vulnologica 2013;11(1):15‐21.
Cavicchioli 2007
Cavicchioli A, Carella G. Clinical effectiveness of a low‐tech versus high‐tech pressure‐redistributing mattress. Journal of Wound Care 2007;16(7):285‐9.
Cobb 1997
Cobb GA, Yoder LH, Warren JB. Pressure ulcers: patient outcomes on a KinAir bed or EHOB waffle mattress. TriService Nursing Research Program (TSNRP) 1997.
Collier 1996
Collier ME. Pressure‐reducing mattresses. Journal of Wound Care 1996;5(5):207‐11.
Conine 1990
* Conine TA, Daechsel D, Choi AK, Lau MS. Costs and acceptability of two special overlays for the prevention of pressure sores. Rehabilitation Nursing 1990;15(3):133‐7.
Conine TA, Daechsel D, Lau MS. The role of alternating air and Silicore overlays in preventing decubitus ulcers. International Journal of Rehabilitation Research 1990;13(1):133‐7.
Cooper 1998
Cooper PJ, Gray DG, Mollison J. A randomised controlled trial of two pressure‐reducing surfaces. Journal of Wound Care 1998;7(8):374‐6.
Daechsel 1985
Daechsel D, Conine TA. Special mattresses: effectiveness in preventing decubitus ulcers in chronic neurologic patients. Archives of Physical Medicine and Rehabilitation 1985;66(4):246‐8.
Demarre 2012
* Demarre L, Beeckman D, Vanderwee K, Defloor T, Grypdonck M, Verhaeghe S. Multi‐stage versus single‐stage inflation and deflation cycle for alternating low pressure air mattresses to prevent pressure ulcers in hospitalised patients: a randomised‐controlled clinical trial. International Journal of Nursing Studies 2012;49(4):416‐26.
Demarre L, Vanderwee K, Beeckman D, Defloor T. Pressure ulcer prevention: randomised controlled trial comparing the effect of a standard alternating pressure air mattress and a alternating low pressure air mattress with gradual inflation and deflation. EWMA Journal 2010;10(2):44.
Demarre L, Vanderwee K, Beeckman D, Defloor T. The effectiveness of a multistage low pressure air mattress in pressure ulcer prevention: an RCT Fourth European Nursing Congress. Journal of Clinical Nursing 2010;19:41.
Demarre L, Verhaeghe S, Van Hecke A, Grypdonck M, Clays E, Vanderwee K, et al. The effectiveness of three types of alternating pressure air mattresses in the prevention of pressure ulcers in Belgian hospitals. Research in Nursing & Health 2013;36(5):439‐52.
Ewing 1964
Ewing MR, Garrow C, Presley TA, Ashley C, Kisella NM. Further experiences in the use of sheep skins as an aid in nursing. Australian Nurses' Journal 1964;1964 September:215‐9.
Feuchtinger 2006
* Feuchtinger J, De Bie R, Dassen T, Halfens R. A 4‐cm thermoactive viscoelastic foam pad on the operating room table to prevent pressure ulcer during cardiac surgery. Journal of Clinical Nursing 2006;15(2):162‐7.
Feuchtinger J. [Preventing decubitus ulcer in heart surgery interventions: visco‐elastic foam layer on the operating room table‐‐a study] [Dekubituspravention wahrend kardiochirurgischer Eingriffe: Viskoelastische Schaumstoffauflage auf dem Operationstisch‐‐eine Studie]. Pflege Zeitschrift 2006;59(8):498‐501.
Finnegan 2008
Finnegan MJ, Gazzerro L, Finnegan JO, Lo P. Comparing the effectiveness of a specialized alternating air pressure mattress replacement system and an air‐fluidized integrated bed in the management of post‐operative flap patients: a randomized controlled pilot study. Journal of Tissue Viability 2008;17(1):2‐9.
Gray 1994
Gray D. A randomised clinical trial of two foam mattresses. Aberdeen Royal Hospitals NHS Trust. Medical Support System 1994:1‐4.
Gray D. A randomised controlled trial of two foam mattresses. Journal of Tissue Viability 1994;4(3):92.
* Gray DG, Campbell M. A randomised clinical trial of two types of foam mattresses. Journal of Tissue Viability 1994;4(4):128‐32.
Gray DG, Cooper PJ, Campbell M. A study of the performance of a pressure reducing foam mattress after three years of use. Journal of Tissue Viability 1998;8(3):9‐13.
Gray 2000
Gray D, Smith M. A randomized controlled trial of two pressure‐reducing foam mattresses. European Wound Management Association Conference; 1998 November; Harrogate (UK) 1998:4.
* Gray DG, Smith M. Comparison of a new foam mattress with the standard hospital mattress. Journal of Wound Care 2000;9(1):29‐31.
Gray 2008
Gray D, Cooper P, Bertram M, Duguid K, Pirie G. A clinical audit of the Softform Premier Active™ mattress in two acute care of the elderly wards. Wounds UK 2008;4(4):124‐8.
Grindley 1996
Grindley A, Acres J. Alternating pressure mattresses: comfort and quality of sleep. British Journal of Nursing (Mark Allen Publishing) 1996;5(21):1303‐10.
Gunningberg 2000
Gunningberg L, Lindholm C, Carlsson M, Sjoden PO. Effect of visco‐elastic foam mattresses on the development of pressure ulcers in patients with hip fractures. Journal of Wound Care 2000;9(10):455‐60.
Hampton 1997
Hampton S. Evaluation of the new Cairwave Therapy System in one hospital trust. British Journal of Nursing (Mark Allen Publishing) 1997;6(3):167‐70.
Hofman 1994
Hofman A, Geelkerken RH, Wille J, Hamming JJ, Hermans J, Breslau PJ. Pressure sores and pressure‐decreasing mattresses: controlled clinical trial. Lancet (London, England) 1994;343(8897):568‐71.
Hoshowsky 1994
Hoshowsky VM, Schramm CA. Intraoperative pressure sore prevention: an analysis of bedding materials. Research in Nursing & Health 1994;17(5):333‐9.
Inman 1993
Inman KJ, Sibbald WJ, Rutledge FS, Clark BJ. Clinical utility and cost‐effectiveness of an air suspension bed in the prevention of pressure ulcers. JAMA 1993;269(9):1139‐43.
IRCT2015110619919N3
IRCT2015110619919N3. The effect of silicone protective pad on pressure ulcer. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2015110619919N3 2016.
Jiang 2014
Jiang Q, Li X, Zhang A, Guo Y, Liu Y, Liu H, et al. Multicenter comparison of the efficacy on prevention of pressure ulcer in postoperative patients between two types of pressure‐relieving mattresses in China. International Journal of Clinical and Experimental Medicine 2014;7(9):2820‐7.
Jolley 2004
* Jolley DJ, Wright R, McGowan S, Hickey MB, Campbell DA, Sinclair RD, et al. Preventing pressure ulcers with the Australian Medical Sheepskin: an open‐label randomised controlled trial. Medical Journal of Australia 2004;180(7):324‐7.
Jolley DJ. A multilevel analysis of three randomised controlled trials of the Australian Medical Sheepskin in the prevention of sacral pressure ulcers. Medical Journal of Australia 2011;194(2):104.
Kemp 1993
Kemp MG, Kopanke D, Tordecilla L, Fogg L, Shott S, Matthiesen V, et al. The role of support surfaces and patient attributes in preventing pressure ulcers in elderly patients. Research in Nursing & Health 1993;16(2):89‐96.
Laurent 1998
Laurent S. Effectiveness of pressure decreasing mattresses in cardiovascular surgery patients: a controlled clinical trial. 3rd European Conference for Nurse Managers, 1997 Oct; Brussels (Belgium) 1998.
Lazzara 1991
Lazzara DJ, Buschmann MT. Prevention of pressure ulcers in elderly nursing home residents: are special support surfaces the answer? Decubitus 1991;4(4):42‐4, 46, 48.
Malbrain 2010
Malbrain M, Hendriks B, Wijnands P, Denie D, Jans A, Vanpellicom J, et al. A pilot randomised controlled trial comparing reactive air and active alternating pressure mattresses in the prevention and treatment of pressure ulcers among medical ICU patients. Journal of Tissue Viability 2010;19(1):7‐15.
McGowan 2000
McGowan S, Montgomery K, Jolley D, Wright R. The role of sheepskins in preventing pressure ulcers in elderly orthopaedic patients. Primary Intention 2000:127‐34.
Mistiaen 2010
Mistiaen P, Achterberg W, Ament A, Halfens R, Huizinga J, Montgomery K, et al. Cost‐effectiveness of the Australian Medical Sheepskin for the prevention of pressure ulcers in somatic nursing home patients: study protocol for a prospective multi‐centre randomised controlled trial (ISRCTN17553857). BMC Health Services Research 2008;8:4.
* Mistiaen P, Achterberg W, Ament A, Halfens R, Huizinga J, Montgomery K, et al. The effectiveness of the Australian Medical Sheepskin for the prevention of pressure ulcers in somatic nursing home patients: a prospective multicenter randomized‐controlled trial (ISRCTN17553857). Wound Repair and Regeneration 2010;18(6):572‐9.
Mistiaen P, Ament A, Francke AL, Achterberg W, Halfens R, Huizinga J, et al. An economic appraisal of the Australian Medical Sheepskin for the prevention of sacral pressure ulcers from a nursing home perspective. BMC Health Services Research 2010;10:226.
Mistiaen P, Francke A, Achterberg W, Ament A, Halfens R, Huizinga J. Australian Medical Sheepskin is effective for the prevention of pressure ulcers. Tijdschrift voor Ouderengeneeskunde 2009;5:186‐90.
Nixon 1998
Bridel‐Nixon J, McElvenny D, Brown J, Mason S. A randomized controlled trial using a double‐triangular sequential design: methodology and management issues. European Wound Management Association Conference; 1997 April 27‐29; Milan (Italy) 1997:65‐6.
Bridel‐Nixon J, McElvenny D, Brown J, Mason S. Findings from a double‐triangular sequential‐design randomized clinical trial of a dry polymer gel pad. European Wound Management Association Conference; 1997 April 27‐29; Milan (Italy) 1997:20‐1.
Brown J, McElvenny D, Nixon J, Bainbridge J, Mason S. Some practical issues in the design, monitoring and analysis of a sequential randomized trial in pressure sore prevention. Statistics in Medicine 2000;19(24):3389‐400.
ISRCTN43076542. Pressure sore risk in the operating department. www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN43076542.
* Nixon J, McElvenny D, Mason S, Brown J, Bond S. A sequential randomised controlled trial comparing a dry visco‐elastic polymer pad and standard operating table mattress in the prevention of post‐operative pressure sores. International Journal of Nursing Studies 1998;35(4):193‐203.
Nixon 2006
ISRCTN78646179. Randomised controlled trial comparing alternating pressure overlays with alternating pressure mattresses for pressure sore prevention and treatment. isrctn.com/ISRCTN78646179.
Nelson EA, Nixon J, Mason S, Barrow H, Phillips A, Cullum N. A nurse‐led randomised trial of pressure‐relieving support surfaces. Professional Nurse (London, England) 2003;18(9):513‐6.
Nixon J, Cranny G, Iglesias C, Nelson EA, Hawkins K, Phillips A, et al. Randomised, controlled trial of alternating pressure mattresses compared with alternating pressure overlays for the prevention of pressure ulcers: PRESSURE (pressure relieving support surfaces) trial. BMJ 2006;332(7555):1413‐5.
Nixon J, Cranny G, Nelson A, Iglesias C, Phillips A, Hawkins K, et al. Pressure trial clinical and patient outcomes. European Wound Management Association; 1998 November; Harrogate, UK 2005:157.
Nixon J, Cranny G, Nelson A, Iglesias C, Phillips A, Hawkins K, et al. The NHS Health Technology Assessment Programme. Pressure trial clinical and patient outcomes. EWMA Journal 2006;6(1):38.
* Nixon J, Nelson EA, Cranny G, Iglesias CP, Hawkins K, Cullum NA, et al. Pressure relieving support surfaces: a randomised evaluation. Health Technology Assessment (Winchester, England) 2006;10(22):1‐163.
Nixon J, Thorpe H, Barrow H, Phillips A, Nelson EA, Mason SA, et al. Reliability of pressure ulcer classification and diagnosis. Journal of Advanced Nursing 2005;50(6):613‐23.
Nixon J. Erratum: randomised, controlled trial of alternating pressure mattresses compared with alternating pressure overlays for the prevention of pressure ulcers: PRESSURE (pressure relieving support surfaces) trial (British Medical Journal (July 1, 2006) 333 (30)). BMJ 2006;333:30.
Nixon 2019
Brown S, Smith I, Brown J, Hulme C, Nixon J. Pressure relieving support surfaces: a randomised evaluation 2 (PRESSURE 2). Trials 2013;14(Suppl 1):P68.
Brown S, Smith IL, Brown JM, Hulme C, McGinnis E, Stubbs N, et al. Pressure RElieving Support SUrfaces: a Randomised Evaluation 2 (PRESSURE 2): study protocol for a randomised controlled trial. Trials 2016;17(1):604.
ISRCTN01151335. Pressure RElieving Support SUrfaces: a Randomised Evaluation 2. isrctn.com/ISRCTN01151335.
McGinnis E, Brown S, Collier H, Faulks P, Gilberts R, Greenwood C, et al. Pressure RElieving Support SUrfaces: a Randomised Evaluation 2 (PRESSURE 2) photographic validation sub‐study: study protocol for a randomised controlled trial. Trials 2017;18(1):132.
* Nixon J, Brown S, Smith IL, McGinnis E, Vargas‐Palacios A, Nelson EA, et al. Comparing alternating pressure mattresses and high‐specification foam mattresses to prevent pressure ulcers in high‐risk patients: the PRESSURE 2 RCT. Health Technology Assessment (Winchester, England) 2019;23(52):1‐176.
Nixon J, Smith IL, Brown S, McGinnis E, Vargas‐Palacios A, Nelson EA, et al. Pressure relieving support surfaces for pressure ulcer prevention (PRESSURE 2): clinical and health economic results of a randomised controlled trial. E Clinical Medicine 2019;14:42‐52.
Ozyurek 2015
Ozyurek P, Yavuz M. Prevention of pressure ulcers in the intensive care unit: a randomized trial of 2 viscoelastic foam support surfaces. Clinical Nurse Specialist CNS 2015;29(4):210‐7.
Park 2017
Park KH, Park J. The efficacy of a viscoelastic foam overlay on prevention of pressure injury in acutely ill patients: a prospective randomized controlled trial. Journal of Wound, Ostomy, and Continence Nursing 2017;44(5):440‐4.
Phillips 1999
Phillips L. Providing correct pressure‐relieving devices for optimum outcome. British Journal of Nursing (Mark Allen Publishing) 1999;8(21):1447‐52.
Price 1999
Price P, Bale S, Newcombe R, Harding K. Challenging the pressure sore paradigm. Journal of Wound Care 1999;8(4):187‐90.
Pring 1998
Pring J, Millman P. Evaluating pressure‐relieving mattresses. Journal of Wound Care 1998;7(4):177‐9.
Rafter 2011
Rafter L. Evaluation of patient outcomes: pressure ulcer prevention mattresses. British Journal of Nursing 2011;20(11):32.
Ricci 2013
Ricci E, Roberto C, Ippolito A, Bianco A, Scalise MT. A randomized study on the effectiveness of a new pressure‐relieving mattress overlay for the prevention of pressure ulcers in elderly patients at risk. EWMA Journal 2013;13(1):27‐32.
Rosenthal 2003
Rosenthal MJ, Felton RM, Nastasi AE, Naliboff BD, Harker J, Navach JH. Healing of advanced pressure ulcers by a generic total contact seat: 2 randomized comparisons with low air loss bed treatments. Archives of Physical Medicine and Rehabilitation 2003;84(12):1733‐42.
Russell 2000
Dunlop V. Preliminary results of a randomized, controlled study of a pressure ulcer prevention system. Advances in Wound Care 1998;11(3 Suppl):14.
Lichtenstein S. A 7 day comparative randomized parallel single centre study to determine the safety and efficacy of the Micropulse system for the prevention of pressure ulcers. Micropulse 1997.
* Russell JA, Lichtenstein SL. Randomized controlled trial to determine the safety and efficacy of a multi‐cell pulsating dynamic mattress system in the prevention of pressure ulcers in patients undergoing cardiovascular surgery. Ostomy/Wound Management 2000;46(2):46‐51, 54‐5.
Russell 2003
Russell LJ, Reynolds TM, Park C, Rithalia S, Gonsalkorale M, Birch J, et al. Randomized clinical trial comparing 2 support surfaces: results of the prevention of pressure ulcers study. Advances in Skin & Wound Care 2003;16(6):317‐27.
Sanada 2003
Matsui Y, Miyake S, Kawasaki T, Konya C, Sugama J, Sanada H. Randomized controlled trial of a two layer type air cell mattress in the prevention of pressure ulcers. Japanese Journal of Pressure Ulcers 2001;3(3):331‐7.
* Sanada H, Sugama J, Matsui Y, Konya C, Kitagawa A, Okuwa M, et al. Randomised controlled trial to evaluate a new double‐layer air‐cell overlay for elderly patients requiring head elevation. Journal of Tissue Viability 2003;13(3):112‐4, 116, 118.
Santy 1994
Santy JE, Butler MK, Whyman JD. A comparison study of 6 types of hospital mattress to determine which most effectively reduces the incidence of pressure sores in elderly patients with hip fractures in a District General Hospital. Report to Northern & Yorkshire Regional Health Authority 1994.
Sauvage 2017
Sauvage P, Touflet M, Pradere C, Portalier F, Michel JM, Charru P, et al. Pressure ulcers prevention efficacy of an alternating pressure air mattress in elderly patients: E(2)MAO a randomised study. Journal of Wound Care 2017;26(6):304‐12.
Schultz 1999
* Schultz A, Bien M, Dumond K, Brown K, Myers A. Etiology and incidence of pressure ulcers in surgical patients. AORN Journal 1999;70(3):434, 437‐40, 443‐9.
Schultz AA. Study results: prediction and prevention of pressure ulcers in surgical patients. Advances in Wound Care 1998;11(3):11.
Sideranko 1992
Sideranko S, Quinn A, Burns K, Froman RD. Effects of position and mattress overlay on sacral and heel pressures in a clinical population. Research in Nursing & Health 1992;15(4):245‐51.
Stapleton 1986
Stapleton M. Preventing pressure sores‐‐an evaluation of three products. Geriatric Nursing (London, England) 1986;6(2):23‐5.
Takala 1996
Takala J, Varmavuo S, Soppi E. Prevention of pressure sores in acute respiratory failure: a randomised controlled trial. Clinical Intensive Care 1996;7(5):228‐35.
Taylor 1999
Taylor L. Evaluating the Pegasus Trinova: a data hierarchy approach. British Journal of Nursing (Mark Allen Publishing) 1999;8(12):771‐4, 776‐8.
Theaker 2005
Theaker C, Kuper M, Soni N. Pressure ulcer prevention in intensive care ‐ a randomised control trial of two pressure‐relieving devices. Anaesthesia 2005;60(4):395‐9.
Vanderwee 2005
Vanderwee K, Grypdonck MH, Defloor T. Effectiveness of an alternating pressure air mattress for the prevention of pressure ulcers. Age and Ageing 2005;34(3):261‐7.
Van Leen 2011
Van Leen M, Hovius S, Neyens J, Halfens R, Schols J. Pressure relief, cold foam or static air? A single center, prospective, controlled randomized clinical trial in a Dutch nursing home. Journal of Tissue Viability 2011;20(1):30‐4.
Van Leen 2013
Van Leen M, Hovius S, Halfens R, Neyens J, Schols J. Pressure relief with visco‐elastic foam or with combined static air overlay? A prospective, crossover randomized clinical trial in a Dutch nursing home. Wounds 2013;25(10):287‐92.
Van Leen 2018
NTR4557. Is lowering of shear and friction forces (cost)effective for prevention of pressure ulcers? trialregister.nl/trial/4435.
* Van Leen M, Halfens R, Schols J. Preventive effect of a microclimate‐regulating system on pressure ulcer development: a prospective, randomized controlled trial in Dutch nursing homes. Advances in Skin & Wound Care 2018;31(1):1‐5.
Vermette 2012
Vermette S, Reeves I, Lemaire J. Cost effectiveness of an air‐inflated static overlay for pressure ulcer prevention: a randomized, controlled trial. Wounds 2012;24(8):207‐14.
Vyhlidal 1997
Vyhlidal SK, Moxness D, Bosak KS, Van Meter FG, Bergstrom N. Mattress replacement or foam overlay? A prospective study on the incidence of pressure ulcers. Applied Nursing Research 1997;10(3):111‐20.
Whitney 1984
Whitney JD, Fellows BJ, Larson E. Do mattresses make a difference? Journal of Gerontological Nursing 1984;10(9):20‐1, 24‐5.
Whittingham 1999
Whittingham K. Randomized control trial of six pressure‐redistributing foam mattresses. Journal of Tissue Viability 1999;9(3):104.
References of studies in the topic of treatment
We identified 12 potentially eligible studies in the topic of pressure ulcer treatment. Here is the list of studies. We use an asterisk to indicate the primary reference where a study has multiple references.
Allman 1987
Allman RM, Walker JM, Hart MK, Laprade CA, Noel LB, Smith CR. Air‐fluidized beds or conventional therapy for pressure sores. A randomized trial. Annals of Internal Medicine 1987;107(5):641‐8.
Cassino 2013
Cassino R, Ippolito AM, Cuffaro C, Corsi A, Ricci E. A controlled, randomized study on the effectiveness of two overlays in the treatment of decubitus ulcers. Minerva Chirurgica 2013;68(1):105‐16.
Day 1993
Day A, Leonard F. Seeking quality care for patients with pressure ulcers. Decubitus 1993;6(1):32‐43.
Devine 1995
Devine B. Alternating pressure air mattresses in the management of established pressure sores. Journal of Tissue Viability 1995;5(3):94‐8.
Evans 2000a
Evans D, Land L, Geary A. A clinical evaluation of the Nimbus 3 alternating pressure mattress replacement system. Journal of Wound Care 2000;9(4):181‐6.
Evans 2000b
Evans D, Land L, Geary A. A clinical evaluation of the Nimbus 3 alternating pressure mattress replacement system. Journal of Wound Care 2000;9(4):181‐6.
Ferrell 1993
Ferrell BA, Osterweil D, Christenson P. A randomized trial of low‐air‐loss beds for treatment of pressure ulcers. JAMA 1993;269(4):494‐7.
Groen 1999
Groen HW, Groenier KH, Schuling J. Comparative study of a foam mattress and a water mattress. Journal of Wound Care 1999;8(7):333‐5.
Mulder 1994
Mulder GD, Taro N, Seeley J, Andrews K. A study of pressure ulcer response to low air loss beds vs. conventional treatment. Journal of Geriatric Dermatology 1994;2(3):87‐91.
Munro 1989
Munro BH, Brown L, Heitman BB. Pressure ulcers: one bed or another? How does an air‐fluidized bed compare with pads and other devices on a standard bed? Geriatric Nursing 1989;10:190‐2.
Russell 2000
* Russell L, Reynolds T, Carr J, Evans A, Holmes M. A comparison of healing rates on two pressure‐relieving systems. British Journal of Nursing (Mark Allen Publishing) 2000;9(22):2270‐80.
Russell L, Reynolds TM, Carr J, Evans A, Holmes M. Randomised controlled trial of two pressure‐relieving systems. Journal of Wound Care 2000;9(2):52‐5.
Russell L. Randomized comparative clinical trial of Pegasus Cairwave mattress and Proactive seating cushion and Huntleigh Nimbus 3 & Aura seating cushion. Journal of Tissue Viability 1999;9(3):103‐4.
Russell L. Randomized comparative clinical trial of the Pegasus Cairwave mattress and Proactive seating cushion and the Huntleigh Nimbus III mattress and Alpha Transcell seating cushion. European Wound Management Association and Journal of Wound Care Autumn Conference; 1998 November; Harrogate (UK) 1998:4.
Strauss 1991
Strauss MJ, Gong J, Gary BD, Kalsbeek WD, Spear S. The cost of home air‐fluidized therapy for pressure sores. A randomized controlled trial. Journal of Family Practice 1991;33(1):52‐9.
Appendix 10. Time‐to‐event network: assessing transitivity, homogeneity and consistency assumptions
We assessed the transitivity, homogeneity and consistency assumptions for the time‐to‐event network analysis below.
(1) Transitivity assumption assessment
We summarised the characteristics of the included studies for each direct contrast and compared these direct contrast‐level summary characteristics across these direct contrasts. See the table below. This aims to assess if there are systematic differences between the direct contrasts other than the treatments under evaluations. Thus, data from direct contrasts can be used to calculate indirect evidence (transitivity). The five direct contrasts are heterogeneous in terms of risk of bias, and follow‐up duration (3/5 of direct contrasts with short‐term follow‐up, 1/5 with medium‐term follow‐up, and 1/5 with long‐term follow‐up). We considered these direct contrasts homogeneous in terms of care settings (mainly acute care settings), and participants' characteristics: proportions of sex, age (mainly older adults), and baseline skin status (mainly at risk but free of existing ulcers). We assumed the transitivity assumption held.
Table 1. Summary characteristics of included studies for each direct comparison in the network of time to pressure ulcer development
| Direct comparisons | Number of studies | Study design, no. of multi‐arm studies (% of total participants) | Median of follow‐up | Care settings (% of total participants) | Number of events/ number of total participants (%) | Male (%) | Female (%) | Median of average age reported (years) | Baseline skin status (% of total participants) | Overall risk of bias level (% of total participants) |
| Alternating pressure (active) air surfaces vs foam surfaces | 2 | Two‐arm RCTs | 60 days | Acute care setting (96%), Long‐term care setting (4%) | 175/2105 (8%) | 929 (44%) | 1173 (56%) | 82 | At risk, free of ulcers (100%) | Low (96%); High (4%) |
| Alternating pressure (active) air surfaces vs foam surfaces plus 4‐hourly turning | 1 | Two‐arm RCT | 20 weeks | Acute care setting (100%) | 69/447 (15%) | 163 (37%) | 283 (63%) | 82 | At risk, free of ulcers (100%) | Low (100%) |
| Alternating pressure (active) air surfaces vs reactive air surfaces | 1 | Two‐arm RCT | 14 days | Long‐term care setting (100%) | 26/308 (8%) | 71 (23%) | 237 (77%) | 87 | At risk, free of ulcers (100%) | High (100%) |
| Foam surfaces vs 'standard hospital surfaces' | 3 | Two‐arm RCTs | 11.5 days | Acute care setting (94%); Operating room (6%) |
181/3072 (6%) | 516 (38%) | 827 (62%) | 75 | At risk, free of ulcers (100%) | Unclear (62%); High (38%) |
| Reactive sheepskin surfaces vs 'standard hospital surfaces' | 3 | Two‐arm RCTs | 30 days | Acute care setting (58%); Long‐term care setting (42%) |
248/1281 (19%) | 533 (40%) | 793 (60%) | 73 | At risk, free of ulcers (100%) | High (100%) |
(2) Homogeneity assumption tests
The network has Tau2 = 0.329. This indicates the existence of heterogeneity. This resulted in downgrading for heterogeneity in assessing the certainty of evidence using the GRADE approach.
We considered downgrading for these two network contrasts:
alternating pressure (active) air surfaces versus foam surfaces: P value = 0.009; and
foam surfaces versus standard hospital surfaces: P value = 0.029.
(3) Consistency tests
The network has no closed loop. We did not identify any evidence of global or local inconsistency: design‐by‐treatment interaction model Chi2(1) = 1.36; probably > Chi2 = 0.243. The consistency assumption held in the network.
Publication bias
We report the funnel plot for the time‐to‐event network analysis in Figure 14. The plot did not strongly suggest publication bias.
14.
Time‐to‐event network: funnel plot of the analysis for time to pressure ulcer development outcome
Appendix 11. Treatment network: characteristics and data in the included studies and full ranking evidence
Characteristics and data of the 12 potentially eligible studies
In the table below, we report the characteristics and data of the 12 RCTs identified for the topic of pressure ulcer healing. We note that not all studies connected to a network and studies with an asterisk contributed data to network meta‐analyses.
Table 1. Characteristics and data of the 12 potentially eligible studies
| Study ID | Study design | Number of arms | Country | Being fully or partly funded by industry | Follow‐up duration | Care setting | Total participants (male/female, only those with available data ) | Age | Ulcer stage at baseline | Average ulcer surface area (cm2) | Study arm 1 (healing/total/ missing) | Study arm 2 (healing/total/ missing) |
| Allman 1987 * | RCT | 2 | USA | Partly | 13 days | Hospital | 72 (27/38) | 66.6 | superficial & deep | 9.4 | Reactive air surfaces (20/36) | Foam surfaces (15/36) |
| Cassino 2013 | RCT | 2 | Italy | Fully | 12 weeks | Community & long‐term care | 72 (17/55) | 85.4 | 1 to 4 | ND | Reactive gel surfaces (5/37) | Standard hospital surfaces (3/35) |
| Day 1993 | RCT | 2 | USA | Partly | 7 days | Hospital | 83 (35/48) | 76 | 2 to 4, unstageable | ND | Alternating pressure (active) air surfaces (ND) | Foam surfaces (ND) |
| Devine 1995 | RCT | 2 | UK | Partly | 4 weeks | Hospital | 41 (17/24) | 82.5 | 2 to 4 | 12.8 | Alternating pressure (active) air surfaces (10/16/6) | Alternating pressure (active) air surfaces (5/14/5) |
| Evans 2000a | RCT | 2 | UK | Fully | 20.5 days | Hospital | 12 (6/6) | 72.2 | 2 to 4 | 4.2 | Alternating pressure (active) air surfaces (3/7/0) | Alternating pressure (active) air surfaces (3/5/0) |
| Evans 2000b | RCT | 2 | UK | Fully | 53.5 days | Community & long‐term care | 20 (1/19) | 86.5 | 2 to 4 | 6.6 | Alternating pressure (active) air surfaces (1/10/0) | Alternating pressure (active) air surfaces (5/10/0) |
| Ferrell 1993 * | RCT | 2 | USA | Partly | 37.5 days | Community & long‐term care | 84 (42/42) | 84.5 | 2 to 4 | 4.2 | Reactive air surfaces (26/43) | Foam surfaces (19/41) |
| Groen 1999 * | RCT | 2 | Netherlands | ND | 4 weeks | ND | 120 | ND | 3 to 4 | ND | Foam surfaces (22/49) | Reactive water surfaces (25/52) |
| Mulder 1994 * | RCT | 2 | USA | Fully | 12 weeks | Community & long‐term care | 49 | ND | 3 to 4 | ND | Alternating pressure (active) air surfaces (5/31) | Foam surfaces (3/18) |
| Munro 1989 | RCT | 2 | USA | Fully | 15 days | Hospital | 40 (40/0) | ND | 2 to 3 | 18.62 | Reactive air surfaces (ND) | Standard hospital surfaces (ND) |
| Russell 2000 | RCT | 2 | UK | Fully | 18 months | Hospital | 183 | 84.2 | 2 or above | ND | Alternating pressure (active) air surfaces (24/55) | Alternating pressure (active) air surfaces (17/58) |
| Strauss 1991 | RCT | 2 | USA | Fully | 36 weeks | Community & long‐term care | 112 (57/55) | 64 | 3 to 4 | ND | Reactive air surfaces (29/47) | Standard hospital surfaces (ND) |
ND = no data available
Risk of bias assessment for the included studies connecting to the treatment network
We summarise risk of bias assessments for the included studies in the topic of prevention in Figure 15.
15.
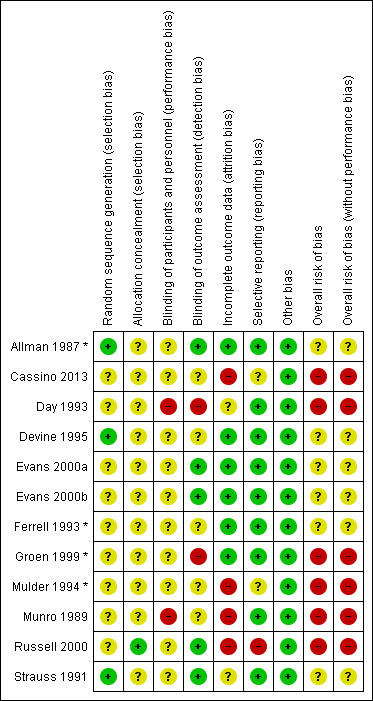
Treatment network: risk of bias summary of review authors' judgements about each risk of bias item for each study. Studies with asterisk contributed data for network meta‐analysis.
Ranking probabilities of each support surface in the treatment network meta‐analysis
We estimated values of three ranking measures for each type of support surface in the treatment network meta‐analysis. See full results in the Table below.
Table 2. Ranking probabilities of each support surface in the treatment network meta‐analysis
| Interventions | SUCRA (estimated % (predicted %)) | Probability of being the best (estimated % (predicted %)) | Mean rank (estimated values (predicted values)) |
| Foam surfaces | 35.3 (35.2) | 1.2 (1.1) | 2.9 (2.9) |
| Reactive air surfaces | 83.9 (83.8) | 59.9 (59.8) | 1.5 (1.5) |
| Alternating pressure (active) air surfaces | 43.0 (43.1) | 31.8 (32.2) | 2.7 (2.7) |
| Reactive water surfaces | 37.9 (37.8) | 7.1 (7.0) | 2.9 (2.9) |
SUCRA value is a numerical summary of the cumulative probability plot for each support surface and the plot shows cumulative rank probabilities that each intervention is less than or equal to a specific rank order against all possible ranks. The higher the SUCRA value, the higher the probability of being the best intervention. Probability of being the best value is the proportion of an intervention being ranked at the first place among a great number of repeated estimations for the intervention (simulations). Mean rank is the mean value of the distribution for the rank of each intervention. In the above table, all predicted values incorporate heterogeneity into value estimates.
We considered the ranking evidence is of very low certainty: we downgraded twice for risk of bias as 66.7% of data contributing to the base‐case network were at high risk of bias. We did not downgrade for both heterogeneity and inconsistency as the network had no substantial heterogeneity (Tau2 = 0.0), the ranking curves based on both estimated and predicted probabilities agree with each other, and the network had no global inconsistency.
We downgraded the evidence once for imprecision: the network had sparse data and there are overlaps between interventions in terms of rank orders. We did not downgrade for indirectness. We did not downgrade for publication bias too: the analysis included only four studies and the funnel plot did not strongly suggest small‐studies effects (Figure 16).
16.
Treatment network: funnel plot of the analysis for pressure ulcer healing outcome
Differences between protocol and review
We clarified the eligibility criteria further and included ongoing Cochrane Reviews in this overview, which was not specified in our protocol.
We assessed overall risk of bias for the included studies based on (1) all risk of bias domains; and (2) all but the performance bias domain. This was not specified in our protocol.
We clarified the methods used for assessing risk of bias for direct comparisons, each network contrast and the whole base‐case network. This was not specified in our protocol.
We undertook a post hoc sensitivity analysis to assess the impact of including only well‐defined support surfaces in the pressure ulcer incidence network meta‐analysis.
We did not report the results of subgroup analysis or sensitivity analyses of the included reviews in this overview.
We present cumulative rank probability plots rather than rankograms.
In exploring network heterogeneity in network meta‐analysis, we categorised the continuous values of follow‐up duration factor into three‐level indicators (i.e. short‐term; medium‐term; and long‐term) and performed a subgroup analysis for this rather than meta‐regression for a continuous factor.
Contributions of authors
Chunhu Shi: conceived the overview; designed the overview; coordinated the overview; extracted data; analysed or interpreted data; undertook quality assessment; performed statistical analysis; produced the first draft of the overview; contributed to writing or editing the overview; wrote to study authors/experts/companies; approved the final overview prior to publication; and is guarantor of the overview.
Jo Dumville: conceived the overview; designed the overview; coordinated the overview; analysed or interpreted data; produced the first draft of the overview; contributed to writing or editing the overview; advised on the overview; secured funding; performed previous work that was the foundation of the current overview; and approved the final overview prior to publication.
Nicky Cullum: conceived the overview; designed the overview; analysed or interpreted data; contributed to writing or editing the overview; advised on the overview; performed previous work that was the foundation of the current overview; and approved the final overview prior to publication.
Sarah Rhodes: analysed or interpreted data; performed statistical analysis; checked quality of statistical analysis; contributed to writing or editing the overview; advised on the overview; approved the final overview prior to publication.
Elizabeth McInnes: coordinated the overview; performed previous work that was the foundation of the current overview; approved the final overview prior to publication; and was the lead author of the previous version of the related reviews.
En Lin Goh: extracted data; checked quality of data extraction; undertook quality assessment; checked quality assessment; and approved the final overview prior to publication.
Gill Norman: analysed or interpreted data; checked quality assessment; contributed to writing or editing the overview; advised on the overview; and approved the final overview prior to publication.
Contributions of the editorial base
Gill Norman (Editor): edited the protocol; advised on methodology, interpretation and content; approved the final protocol prior to publication.
Gill Rizzello (Managing Editor): coordinated the editorial process; advised on content; edited the overview.
Sophie Bishop (Information Specialist): edited the search methods sections.
Tom Patterson (Editorial Assistant): edited the reference sections of the overview.
Sources of support
Internal sources
Division of Nursing, Midwifery and Social Work, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, UK
External sources
-
National Institute for Health Research, UK
This project is funded by the National Institute for Health Research (NIHR) under its Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB‐PG‐1217‐20006). The views expressed are those of the author(s) and not necessarily those of the NIHR or the Department of Health and Social Care.
-
NIHR Manchester Biomedical Research Centre (BRC), UK
This research was co‐funded by the NIHR Manchester BRC. The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the NIHR or the Department of Health and Social Care.
-
National Institute for Health Research (NIHR), UK
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Wounds. The views expressed are those of the authors and not necessarily those of the NIHR or the Department of Health and Social Care.
-
National Institute for Health Research Applied Research Collaboration (ARC) Greater Manchester, UK
Nicky Cullum and Jo Dumville’s work on this project was partially funded by the National Institute for Health Research Applied Research Collaboration Greater Manchester. The views expressed in this publication are those of the authors and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care.
Declarations of interest
Chunhu Shi: I received research funding from the National Institute for Health Research (Research for Patient Benefit, Evidence synthesis for pressure ulcer prevention and treatment, PB‐PG‐1217‐20006). I received support from the Tissue Viability Society to attend conferences unrelated to this work. The Doctoral Scholar Awards Scholarship and Doctoral Academy Conference Support Fund (University of Manchester) also supported a PhD and conference attendance respectively; both were unrelated to this work.
Jo Dumville: I am Chief Investigator on a National Institute for Health Research grant that funded the conduct of this review (Research for Patient Benefit, Evidence synthesis for pressure ulcer prevention and treatment, PB‐PG‐1217‐20006). This research was co‐funded by the National Institute for Health Research Manchester Biomedical Research Centre, and partly funded by the National Institute for Health Research Applied Research Collaboration Greater Manchester.
Nicky Cullum: I am Co‐investigator on a National Institute for Health Research grant that funded the conduct of this review (Research for Patient Benefit, Evidence synthesis for pressure ulcer prevention and treatment, PB‐PG‐1217‐20006). This research was co‐funded by the National Institute for Health Research Manchester Biomedical Research Centre, and partly funded by the National Institute for Health Research Applied Research Collaboration Greater Manchester.
My previous and current employers received research grant funding from the NHS Research and Development Programme, and subsequently the NIHR, for my participation in reviews contained in this work. The funders had no role in the conduct of these reviews. My previous employer received research grant funding from the NIHR for an RCT comparing different alternating pressure air surfaces for pressure ulcer prevention.
Sarah Rhodes: my salary is funded from three National Institute for Health Research grants and a grant from Greater Manchester Cancer.
Elizabeth McInnes: none known.
En Lin Goh: none known.
Gill Norman: my employment at the University of Manchester is funded by the National Institute for Health Research (NIHR). This research was co‐funded by the National Institute for Health Research Manchester Biomedical Research Centre.
Gill Worthy (peer reviewer) states: I have previously worked with three of the authors and performed the analysis of one of the included trials (PRESSURE).
New
References
References to included reviews
McGinnis 2014
- McGinnis E, Stubbs N. Pressure-relieving devices for treating heel pressure ulcers. Cochrane Database of Systematic Reviews 2014, Issue 2. Art. No: CD005485. [DOI: 10.1002/14651858.CD005485.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2021a
- Shi C, Dumville JC, Cullum N, Rhodes S, Jammali-Blasi A, McInnes E. Alternating pressure (active) air surfaces for preventing pressure ulcers. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD013620. [DOI: 10.1002/14651858.CD013620.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2021b
- Shi C, Dumville JC, Cullum N, Rhodes S, McInnes E. Foam surfaces for preventing pressure ulcers. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD013621. [DOI: 10.1002/14651858.CD013621.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2021c
- Shi C, Dumville JC, Cullum N, Rhodes S, Leung V, McInnes E. Reactive air surfaces for preventing pressure ulcers. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD013622. [DOI: 10.1002/14651858.CD013622.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2021d
- Shi C, Dumville JC, Cullum N, Rhodes S, McInnes E. Alternative reactive support surfaces (non-foam and non-air-filled) for preventing pressure ulcers. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD013623. [DOI: 10.1002/14651858.CD013623.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2021e
- Shi C, Dumville JC, Cullum N, Rhodes S, Jammali-Blasi A, Ramsden V, McInnes E. Beds, overlays and mattresses for treating pressure ulcers. Cochrane Database of Systematic Reviews 2021, Issue 5. Art. No: CD013624. [DOI: 10.1002/14651858.CD013624.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to excluded reviews
Greenwood 2017
- Greenwood CE, Nelson EA, Nixon J, McGinnis E. Pressure-relieving devices for preventing heel pressure ulcers. Cochrane Database of Systematic Reviews 2017, Issue 5. Art. No: CD011013. [DOI: 10.1002/14651858.CD011013.pub2] [DOI] [Google Scholar]
McInnes 2015
- McInnes E, Jammali-Blasi A, Bell-Syer SE, Dumville JC, Middleton V, Cullum N. Support surfaces for pressure ulcer prevention. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD001735. [DOI: 10.1002/14651858.CD001735.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
McInnes 2018
- McInnes E, Jammali-Blasi A, Bell-Syer SE, Leung V. Support surfaces for treating pressure ulcers. Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD009490. [DOI: 10.1002/14651858.CD009490.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Brignardello‐Petersen 2019
- Brignardello-Petersen R, Murad MH, Walter SD, McLeod S, Carrasco-Labra A, Rochwerg B, et al. GRADE approach to rate the certainty from a network meta-analysis: avoiding spurious judgments of imprecision in sparse networks. Journal of Clinical Epidemiology 2019;105:60-7. [DOI] [PubMed] [Google Scholar]
Caldwell 2005
- Caldwell DM, Ades AE, Higgins JP. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ 2005;331(7521):897-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2013
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in STATA. PLoS One 2013;8:e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaimani 2015
- Chaimani A, Salanti G. Visualizing assumptions and results in network meta-analysis: the network graphs package. Stata Journal 2015;15(4):905–50. [Google Scholar]
Cipriani 2013
- Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. Annals of Internal Medicine 2013;159(2):130-7. [DOI] [PubMed] [Google Scholar]
Clark 2011
- Clark M. Technology update: understanding support surfaces. Wounds International 2011;2(3):17-21. [Google Scholar]
Cullum 2016
- Cullum N, Buckley H, Dumville J, Hall J, Lamb K, Madden M, et al. Wounds Research for Patient Benefit: A 5-year Programme of Research. Southampton (UK): NIHR Journals Library, 2016. [PubMed] [Google Scholar]
Deeks 2020
- Deeks JJ, Higgins JP, Altman DG (editors). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Demarré 2015
- Demarré L, Van Lancker A, Van Hecke A, Verhaeghe S, Grypdonck M, Lemey J, et al. The cost of prevention and treatment of pressure ulcers: a systematic review. International Journal of Nursing Studies 2015;52(11):1754-74. [DOI] [PubMed] [Google Scholar]
Dias 2014
- Dias S, Welton NJ, Sutton AJ, Caldwell DM, Lu G, Ades AE. NICE DSU Technical Support Document 4: Inconsistency in Networks of Evidence Based on Randomised Controlled Trials. London: National Institute for Health and Care Excellence (NICE) 2014. [PubMed]
EPUAP/NPIAP/PPPIA 2019
- European Pressure Ulcer Advisory Panel, National Pressure Injury Advisory Panel, Pan Pacific Pressure Injury Alliance (EPUAP/NPIAP/PPPIA). Prevention and Treatment of Pressure Ulcers/Injuries: Quick Reference Guide. Emily Haesler (Ed.). EPUAP/NPIAP/PPPIA, 2019. [Google Scholar]
Espejo 2018
- Espejo E, Andrés M, Borrallo RM, Padilla E, Garcia-Restoy E, Bella F, Complex Wounds Working Group. Bacteremia associated with pressure ulcers: a prospective cohort study. European Journal of Clinical Microbiology & Infectious Diseases: Official Publication of the European Society of Clinical Microbiology 2018;37(5):969-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Essex 2009
- Essex HN, Clark M, Sims J, Warriner A, Cullum N. Health-related quality of life in hospital inpatients with pressure ulceration: assessment using generic health-related quality of life measures. Wound Repair and Regeneration 2009;17(6):797-805. [DOI] [PubMed] [Google Scholar]
Glenny 2005
- Glenny AM, Altman DG, Song F, Sakarovitch C, Deeks JJ, D’Amico R, et al. Indirect comparisons of competing interventions. Health Technology Assessment (Winchester, England) 2005;9:1-134, iii-iv. [DOI] [PubMed] [Google Scholar]
Gorecki 2009
- Gorecki C, Brown JM, Nelson EA, Briggs M, Schoonhoven L, Dealey C, et al. Impact of pressure ulcers on quality of life in older patients: a systematic review. Journal of the American Geriatrics Society 2009;57(7):1175-83. [DOI] [PubMed] [Google Scholar]
Gorecki 2013
- Gorecki C, Brown JM, Cano S, Lamping DL, Briggs M, Coleman S, et al. Development and validation of a new patient-reported outcome measure for patients with pressure ulcers: the PU-QOL instrument. Health and Quality of Life Outcomes 2013;11:95. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gray 2018
- Gray TA, Rhodes S, Atkinson RA, Rothwell K, Wilson P, Dumville JC, et al. Opportunities for better value wound care: a multiservice, cross-sectional survey of complex wounds and their care in a UK community population. BMJ Open 2018;8(3):e019440. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guest 2018
- Guest JF, Fuller GW, Vowden P, Vowden KR. Cohort study evaluating pressure ulcer management in clinical practice in the UK following initial presentation in the community: costs and outcomes. BMJ Open 2018;8(7):e021769. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herdman 2011
- Herdman M, Gudex C, Lloyd A, Janssen M, Kind P, Parkin D, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Quality of Life Research 2011;21(10):1727-36. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 1996
- Higgins JP, Whitehead A. Borrowing strength from external trials in a meta-analysis. Statistics in Medicine 1996;15:2733-49. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017). The Cochrane Collaboration, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2020
- Higgins JP, Eldridge S, Li T (editors). Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Kew 2014
- Kew KM, Dias S, Cates CJ. Long-acting inhaled therapy (beta-agonists, anticholinergics and steroids) for COPD: a network meta‐analysis. Cochrane Database of Systematic Reviews 2014, Issue 3. Art. No: CD010844. [DOI: 10.1002/14651858.CD010844.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkham 2018
- Kirkham JJ, Altman DG, Chan AW, Gamble C, Dwan KM, Williamson PR. Outcome reporting bias in trials: a methodological approach for assessment and adjustment in systematic reviews. BMJ 2018;362:k3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krahn 2013
- Krahn U, Binder H, König J. A graphical tool for locating inconsistency in network meta-analyses. BMC Medical Research Methodology 2013;13:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lexchin 2003
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ 2003;326(7400):1167-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lu 2004
- Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Statistics in Medicine 2004;23:3105-24. [DOI] [PubMed] [Google Scholar]
NCT03351049
- NCT03351049. An RCT on support surfaces for pressure ulcer prevention. ClinicalTrials.gov/show/NCT03351049 (first received 22 November 2017).
Nguyen 2015
- Nguyen KH, Chaboyer W, Whitty JA. Pressure injury in Australian public hospitals: a cost-of-illness study. Australian Health Review 2015;39(3):329-36. [DOI] [PubMed] [Google Scholar]
NICE 2014
- National Institute for Health and Care Excellence (NICE). Pressure ulcers: prevention and management. www.nice.org.uk/guidance/cg179 (accessed 08 October 2019). [PubMed]
Nikolakopoulou 2020
- Nikolakopoulou A, Higgins JP, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, et al. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Medicine 2020;17:e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
NPIAP 2016
- National Pressure Injury Advisory Panel (NPIAP). NPUAP Pressure Injury Stages; 2016. Available at cdn.ymaws.com/npuap.site-ym.com/resource/resmgr/npuap_pressure_injury_stages.pdf.
NPIAP S3I 2019
- National Pressure Injury Advisory Panel (NPIAP) Support Surface Standards Initiative (S3I). Terms and Definitions Related to Support Surfaces; November 2019. Available at cdn.ymaws.com/npiap.com/resource/resmgr/website_version_terms_and_de.pdf.
Page 2020
- Page MJ, Higgins JP, Sterne JA. Chapter 13: Assessing risk of bias due to missing results in a synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815-34. [DOI] [PubMed] [Google Scholar]
Peters 2008
- Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L. Contour-enhanced meta-analysis funnel plots help distinguish publication bias from other causes of asymmetry. Journal of Clinical Epidemiology 2008;61(10):991-6. [DOI] [PubMed] [Google Scholar]
Pollock 2017
- Pollock A, Campbell P, Brunton G, Hunt H, Estcourt L. Selecting and implementing overview methods: implications from five exemplar overview. Systematic Reviews 2017;6:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Riley 2017
- Riley RD, Jackson D, Salanti G, Burke DL, Price M, Kirkham J, et al. Multivariate and network meta-analysis of multiple outcomes and multiple treatments: rationale, concepts, and examples. BMJ 2017;358:j3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salanti 2008
- Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Statistical Methods in Medical Research 2008;17:279-301. [DOI] [PubMed] [Google Scholar]
Salanti 2011
- Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. Journal of Clinical Epidemiology 2011;64:163-71. [DOI] [PubMed] [Google Scholar]
Salanti 2014
- Salanti G, Giovane CD, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS One 2014;9(7):e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schoonhoven 2007
- Schoonhoven L, Bousema Mente T, Buskens E, on behalf of the prePURSE-study group. The prevalence and incidence of pressure ulcers in hospitalised patients in the Netherlands: a prospective inception cohort study. International Journal of Nursing Studies 2007;44(6):927-35. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408-12. [DOI] [PubMed] [Google Scholar]
Shi 2018a
- Shi C, Dumville JC, Cullum N. Support surfaces for pressure ulcer prevention: a network meta-analysis. PLoS One 2018;13:e0192707. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shi 2018b
- Shi C, Dumville JC, Cullum N. Skin status for predicting pressure ulcer development: a systematic review and meta-analyses. International Journal of Nursing Studies 2018;87:14-25. [DOI] [PubMed] [Google Scholar]
Song 2011
- Song F, Xiong T, Parekh-Bhurke S, Loke YK, Sutton AJ, Eastwood AJ, et al. Inconsistency between direct and indirect comparisons of competing interventions: meta-epidemiological study. BMJ 2011;343:d4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
Theisen 2012
- Theisen S, Drabik A, Stock S. Pressure ulcers in older hospitalised patients and its impact on length of stay: a retrospective observational study. Journal of Clinical Nursing 2012;21(3-4):380-7. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Turner 2012
- Turner RM, Davey J, Clarke MJ, Thompson SG, Higgins JP. Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane Database of Systematic Reviews. International Journal of Epidemiology 2012;41(3):818-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vanderwee 2005
- Vanderwee K, Grypdonck MH, Defloor T. Effectiveness of an alternating pressure air mattress for the prevention of pressure ulcers. Age and Ageing 2005;34(3):261-7. [DOI] [PubMed] [Google Scholar]
Veroniki 2013
- Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. International Journal of Epidemiology 2013;42(1):332-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2015
- White IR. Network meta-analysis. Stata Journal 2015;15:951–85. [Google Scholar]
Whiting 2016
- Whiting P, Savović J, Higgins JP, Caldwell DM, Reeves BC, Shea B, et al. ROBIS: a new tool to assess risk of bias in systematic reviews was developed. Journal of Clinical Epidemiology 2016;69:225-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woodhead 2016
- Woodhead M. 80% of China's clinical trial data are fraudulent, investigation finds. BMJ 2016;355:i5396. [DOI] [PubMed] [Google Scholar]
World Health Organization 2019
- World Health Organization. EH90 Pressure ulceration. ICD-11 for Mortality and Morbidity Statistics (Version: 04/2019). Available at icd.who.int/browse11/l-m/en#/http%3a%2f%2fid.who.int%2ficd%2fentity%2f455330172 (accessed 17 February 2020).
Wounds International 2010
- Wounds International. International Review. Pressure Ulcer Prevention: Pressure, Shear, Friction and Microclimate in Context. A Consensus Document. London (UK): Wounds International, 2010. [Google Scholar]
Yepes‐Nuñez 2019
- Yepes-Nuñez JJ, Li SA, Guyatt G, Jack SM, Brozek JL, Beyene J, et al. Development of the summary of findings table for network meta-analysis. Journal of Clinical Epidemiology 2019;115:1-3. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Shi 2020f
- Shi C, Dumville JC, Cullum N, Rhodes S, McInnes E. Beds, overlays and mattresses for preventing and treating pressure ulcers: an overview of Cochrane reviews and network meta-analysis. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD013761. [DOI: 10.1002/14651858.CD013761] [DOI] [PMC free article] [PubMed] [Google Scholar]