Abstract
Background
This is an updated Cochrane Review, first published in 2006 and updated in 2014. Gout is one of the most common rheumatic diseases worldwide. Despite the use of colchicine as one of the first‐line therapies for the treatment of acute gout, evidence for its benefits and harms is relatively limited.
Objectives
To update the available evidence of the benefits and harms of colchicine for the treatment of acute gout.
Search methods
We updated the search of CENTRAL, MEDLINE, Embase, Clinicaltrials.gov and WHO ICTRP registries to 28 August 2020. We did not impose any date or language restrictions in the search.
Selection criteria
We considered published randomised controlled trials (RCTs) and quasi‐randomised controlled trials (quasi‐RCTs) evaluating colchicine therapy compared with another therapy (placebo or active) in acute gout; low‐dose colchicine at clinically relevant doses compared with placebo was the primary comparison. The major outcomes were pain, participant global assessment of treatment success (proportion with 50% or greater decrease in pain from baseline up to 32 to 36 hours), reduction of inflammation, function of target joint, serious adverse events, total adverse events and withdrawals due to adverse events.
Data collection and analysis
We used standard methodological procedures as expected by Cochrane in this review update.
Main results
We included four trials (803 randomised participants), including two new trials, in this updated review. One three‐arm trial compared high‐dose colchicine (52 participants), low‐dose colchicine (74 participants) and placebo (59 participants); one trial compared high‐dose colchicine with placebo (43 participants); one trial compared low‐dose colchicine with non‐steroidal anti‐inflammatory drugs (NSAIDs) (399 participants); and one trial compared low‐dose colchicine with Chuanhu anti‐gout mixture (traditional Chinese Medicine compound) (176 participants). We did not identify any trials comparing colchicine to glucocorticoids (by any route).
The mean age of participants ranged from 51.2 to 70 years, and trial duration from 48 hours to 12 weeks. Two trials were at low risk of bias, one was possibly susceptible to selection bias (random sequence generation), reporting bias and other bias, and one open‐label trial was at high risk of performance and detection bias.
For the primary comparison, low‐quality evidence from one trial (103 participants, downgraded for imprecision and bias) suggests low‐dose colchicine may improve treatment outcome compared to placebo with little or no increased risk of adverse events. The number of people who reported treatment success (50% or greater pain reduction) at 32 to 36 hours was slightly larger with low‐dose colchicine (418 per 1000) compared with placebo (172 per 1000; risk ratio (RR) 2.43, 95% confidence interval (CI) 1.05 to 5.64; absolute improvement 25% more reported success (7% more to 42% more, the 95% CIs include both a clinically important and unimportant benefit); relative change of 143% more people reported treatment success (5% more to 464% more). The incidence of total adverse events was 364 per 1000 with low‐dose colchicine compared with 276 per 1000 with placebo: RR 1.32, 95% CI 0.68 to 2.56; absolute difference 9% more events with low‐dose colchicine (9% fewer to 43% more, the 95% CIs include both a clinically important effect and no effect); relative change of 32% more events (32% fewer to 156% more). No participants withdrew due to adverse events or reported any serious adverse events. Pain, inflammation and function were not reported.
Low‐quality evidence (downgraded for imprecision and bias) from two trials (124 participants) suggests that high‐dose colchicine compared to placebo may improve symptoms, but with increased risk of harms. More participants reported treatment success at 32 to 36 hours with high‐dose colchicine (518 per 1000) compared with placebo (240 per 1000): RR 2.16, 95% CI 1.28 to 3.65, absolute improvement 28% (8% more to 46% more); more also had reduced inflammation at this time point with high‐dose colchicine (504 per 1000) compared with placebo (48 per 1000): RR 10.50, 95% CI 1.48 to 74.38; absolute improvement 45% greater (22% greater to 68% greater); but more adverse events were reported with high‐dose colchicine (829 per 1000 compared with 260 per 1000): RR 3.21, 95% CI 2.01 to 5.11, absolute difference 57% (26% more to 74% more). Pain and function were not reported.
Low‐quality evidence from a single trial comparing high‐dose to low‐dose colchicine indicates there may be little or no difference in benefit in terms of treatment success at 32 to 36 hours but more adverse events associated with the higher dose. Similarly, low‐quality evidence from a single trial indicates there may also be little or no benefit of low‐dose colchicine over NSAIDs in terms of treatment success and pain reduction at seven days, with a similar number of adverse events reported at four weeks follow‐up. Reduction of inflammation, function of target joint and withdrawals due to adverse events were not reported in either of these trials, and pain was not reported in the high‐dose versus low‐dose colchicine trial.
We were unable to estimate the risk of serious adverse events for most comparisons as there were few events reported in the trials. One trial (399 participants) reported three serious adverse (one in a participant receiving low‐dose colchicine and two in participants receiving NSAIDs), due to reasons unrelated to the trial (low‐quality evidence downgraded for bias and imprecision).
Authors' conclusions
We found low‐quality evidence that low‐dose colchicine may be an effective treatment for acute gout when compared to placebo and low‐quality evidence that its benefits may be similar to NSAIDs. We downgraded the evidence for bias and imprecision. While both high‐ and low‐dose colchicine improve pain when compared to placebo, low‐quality evidence suggests that high‐dose (but not low‐dose) colchicine may increase the number of adverse events compared to placebo, while low‐quality evidence indicates that the number of adverse events may be similar with low‐dose colchicine and NSAIDs.
Further trials comparing colchicine to placebo or other treatment will likely have an important impact on our confidence in the effect estimates and may change the conclusions of this review. There are no trials reporting the effect of colchicine in populations with comorbidities or in comparison with other commonly used treatments, such as glucocorticoids.
Plain language summary
Colchicine for treating acute gout flares
Background
Gout is a very common cause of inflammatory arthritis (pain, redness, warmth and swelling of affected joints) and is caused by urate crystals forming either within or around joints. Uric acid is a normal waste product that is usually excreted with urine. However, in the case of gout, there is either excessive production of uric acid, or the body is not able to excrete it quickly enough, or a combination of both. An attack of gout usually occurs rapidly and usually resolves within 7 to 10 days.
Colchicine is a drug that is used mainly in gout to treat an acute attack or to prevent an attack while starting uric acid‐lowering therapy.
Study characteristics
This Cochrane Review is current to August 2020. We included four trials (803 randomised participants) in this updated review, including two new trials. One three‐arm trial compared high‐dose colchicine (52 participants), low‐dose colchicine (74 participants) and placebo (fake medicine) (59 participants); one trial compared high‐dose colchicine with placebo (43 participants); one trial compared low‐dose colchicine with non‐steroidal anti‐inflammatory drugs (NSAIDs) (399 participants); and one trial compared low‐dose colchicine with Chuanhu anti‐gout mixture (traditional Chinese Medicine compound) (176 participants). We selected low‐dose colchicine compared with placebo as the primary comparison; the low dose is consistent with doses used in practice. Trials were performed in hospital and multicentre settings in four countries, the majority of participants were male, and mean age ranged between 51.2 and 70 years. One trial received funding from a pharmaceutical company that was involved in the study design, data collection, data analysis, and manuscript writing.
Key results
Compared with placebo medication, low‐dose colchicine for people with acute gout may slightly improve treatment outcomes with little or no increased risk of adverse events at 32 to 36 hours.
Proportion of participants reporting treatment success (defined as 50% or more reduction in pain)
25% more people (7% more to 42% more) reported success, or 25 more out of 100
‐ 42 people out of 100 reported treatment success with colchicine
‐ 17 people out of 100 reported treatment success with placebo
Total adverse events (diarrhoea, vomiting or nausea)
6% more people reported adverse events (13% more to 23% more), or 6 more out of 100
‐ 26 people out of 100 reported side effects with colchicine
‐ 20 people out of 100 reported side effects with placebo
Pain, reduction of inflammation, function of target joint, serious adverse events or withdrawals due to adverse events were not reported for this comparison.
Other comparisons are briefly summarised here:
High‐dose colchicine may improve symptoms compared with placebo, but with more adverse events. High‐dose may have little or no benefit over low‐dose colchicine but more adverse events. There may also be little or no benefit of low‐dose colchicine over NSAIDs in terms of treatment success and pain reduction, with a similar number of adverse events.
There were no trials that compared colchicine to glucocorticoids.
Quality of the evidence
We found low‐quality evidence that low‐dose colchicine may be an effective treatment for acute gout when compared to placebo and low‐quality evidence that its benefits may be similar to NSAIDs. We downgraded the evidence for bias and imprecision. While both high‐ and low‐dose colchicine improve treatment success when compared to placebo, high‐dose (but not low‐dose) colchicine may increase the number of adverse events compared to placebo while the number of adverse events may be similar with low‐dose colchicine compared with NSAIDs.
Further research is very likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Summary of findings
Background
Description of the condition
Gout is a disease in which monosodium urate crystals form deposits in and around the joints, which may result in painful, swollen and tender joints or soft tissues and/or tophi. The onset of acute gout is often sudden and dramatic, often beginning at night, and characterised by severe pain (Schumacher 2005). Uric acid is a normal waste product that is usually excreted from the body. However, in gout, there is either excessive production of uric acid (in a minority of people with gout) or the body is not able to excrete it quickly enough (in most people with gout), or both. This results in the formation of monosodium urate crystals. The lower extremity joints, particularly the first metatarsophalangeal joint, are most commonly affected. Other joints that can be affected include the ankle, knee, foot, hand, wrist and elbow. Usually, only one joint is affected (monoarthritis), but oligoarticular or polyarticular acute gout may also occur and is more prevalent in male hypertensive people and in postmenopausal women (Kasper 2005).
Gout is the most common form of inflammatory joint disease in men greater than 40 years of age. Its prevalence varies widely, since both genetic and environmental factors contribute to its incidence (Schumacher 2005). The highest prevalence is among older men, with rates as high as 7% in men over the age of 65 years (Mikuls 2005). Risk factors traditionally associated with gout include obesity, alcohol consumption, renal dysfunction, diuretic use and hyperuricaemia (Wortmann 2002). Gout is historically perceived as a disease afflicting men, while women represent only 5% to 17% of all people with the disease. Premenopausal gout is rare and seen mostly in women with strong family histories of the disorder (Kasper 2005). Menopause increases the risk of gout among women, whereas postmenopausal hormone therapy modestly reduces gout risk (Hak 2010). Some people recover from an acute gout flare without intervention within seven days, but the clinically significant pain associated with acute gout flares creates a need for agents that accelerate the rate of improvement (Bellamy 1987).
Resting the afflicted joint for one to two days may aid in the resolution of a flare, and less medication may be needed to bring about the resolution (Agudelo 1972; Schumacher 2005). Ice therapy has been suggested to reduce the pain associated with acute flares; cold application may be a useful adjunct to the pharmacological treatment of acute gout (Moi 2013; Schlesinger 2002). Heat application to an inflamed joint may exacerbate inflammation and is not recommended (Dorwart 1974). Avoidance of triggers such as alcohol may reduce the frequency of flares (Tugwell 2004). Dietary interventions, such as limitation of carbohydrate intake, and increased proportional intake of protein and the use of unsaturated fat, may also limit the frequency of acute gout flares (Tugwell 2004). Pharmacological treatments that are commonly used in the treatment of acute gouty flares include oral non‐steroidal anti‐inflammatory drugs (NSAIDs), colchicine, oral glucocorticoids and injection of glucocorticoids into the affected joint (Arnold 1988; Janssens 2008a; Janssens 2008b; Schlesinger 2004; Wallace 1998; Wechalekar 2013). The use of adrenocorticotropic hormone may also aid in the resolution of acute gout flares (Schlesinger 2001). In this review we focus on the evidence for colchicine in the treatment of acute gout.
Description of the intervention
In gout, monosodium urate crystals are phagocytosed and additional leucocytes are attracted to the site of inflammation. Colchicine is the main alkaloid of the poisonous autumn crocus plant and has been used to treat acute gout for over 2000 years (Schlesinger 2004).
Colchicine is available in oral and intravenous forms. The absorption of oral colchicine is rapid but can be highly variable. Relief of pain usually occurs 24 hours after colchicine is given orally (Schlesinger 2004).
Serious systemic reactions, including bone marrow suppression, renal failure, alopecia, disseminated intravascular coagulation, hepatic necrosis, diarrhoea, seizures and death, have all been associated with intravenous colchicine (Wallace 1998).
The most common side effects of oral colchicine are nausea, vomiting and diarrhoea. These may be particularly difficult to endure when people are in pain and incapacitated by acute gouty arthritis (Morris 2003).
The recommended dosage of oral colchicine has been debated in the literature, and the optimal dose is not well defined (Cox 2004; Morris 2003; Sivagnanam 2004). International guidelines recommend using a low‐dose colchicine regimen because of the harms of higher doses, but vary in their specification of what the low‐dose regimen entails. The 2020 American College of Rheumatology recommends the US Food and Drug Administration (FDA) approved dosing of 1.2 mg immediately followed by 0.6 mg an hour later, with ongoing anti‐inflammatory therapy until the flare resolves (FitzGerald 2020). The updated 2016 European League Against Rheumatism (EULAR) guidelines also recommend a loading dose of 1 mg (within 12 hours of flare onset) followed 1 hour later by 0.5 mg on day 1 along with or without an NSAID (Richette 2017). The British Society of Rheumatology recommends 0.5 mg twice to four times daily, noting that the higher dose is often precluded by gastrointestinal side effects, most commonly diarrhoea (Hui 2017). The Australian Rheumatology Association recommends 0.5 mg to 1 mg initially, followed by 0.5 mg one hour later and waiting 12 hours before taking the next 0.5 mg dose (Australian Rheumatology Association 2019). Thereafter, 0.5 mg to 1 mg can be taken daily for a couple of days to completely settle the flare. Multinational recommendations from 14 countries from Europe, North and South America and Australasia published in 2014, also recommended up to 2 mg daily of colchicine (van der Heijde 2014).
The guidelines also indicate that a reduced dose is recommended for people with renal impairment (e.g. estimated glomerular filtration rate (eGFR) 10 mL/min/1.73 m2 to50 mL/min/1.73 m2) and the elderly, and avoidance in people with more severe renal impairment (GFR < 10 mL/min/1.73 m2). It should also be used with caution and at low doses in people taking potent inhibitors of cytochrome P450 3A4 (e.g. cimetidine, clarithromycin, erythromycin, fluoxetine, ketoconazole, protease inhibitors, tolbutamide) or P‐glycoprotein (e.g. clarithromycin, ciclosporin, erythromycin). Some also advise caution when using colchicine in people receiving statins, particularly in those with renal impairment, as there are case reports of myopathy and rhabdomyolysis following their combined use (Hui 2017; Richette 2017).
How the intervention might work
The exact mechanism by which colchicine relieves the pain of an acute flare is not presently known. The major pharmacological action of colchicine is its ability to bind to tubulin dimers (Schlesinger 2004). It prevents the polymerisation of microtubules by binding their protein subunits and preventing conglomeration, thereby disrupting membrane‐dependent functions, such as chemotaxis and phagocytosis (Schumacher 1993). Colchicine is also suspected to interfere with leukocyte function, preventing diapedesis, mobilisation, lysosomal degranulation and chemotaxis (Schlesinger 2004).
Why it is important to do this review
The natural course of untreated gouty arthritis flares can last from several hours to several weeks (Schlesinger 2004). Although resting the affected joint and cold therapy may provide some relief of symptoms, non‐pharmacological measures often fall short (Agudelo 1972; Moi 2013; Schlesinger 2002). Therefore it is important to understand the benefits and harms of pharmacological agents.
Colchicine is commonly used to treat people with mono‐ or oligoarticular acute gout but has potential side effects. In addition, people with gout may have clinically significant comorbidities that may influence the choice of therapy. While rheumatologic guidelines recommend using low‐dose oral colchicine to treat people with acute gout, the exact regimen varies between guidelines. This Cochrane Review was last updated in 2014. It is therefore timely to update it to include any relevant new evidence that may alter guidance about the use of colchicine for acute gout.
Objectives
To update the available evidence of the benefits and harms of colchicine for the treatment of acute gout.
Methods
Criteria for considering studies for this review
Types of studies
We included published randomised and quasi‐randomised controlled trials (RCTs or quasi‐RCTs).
Types of participants
We included trials that enrolled adults (aged 18 years or older) with a diagnosis of acute gout (author defined or presence of monosodium crystals in joint aspirate, or people fulfilling the American College of Rheumatology (Wallace 1977), or the Rome (1963) or New York (1966) criteria for gout (O'Sullivan 1972)). We excluded populations that included a mix of people with acute gout and other musculoskeletal pain unless results for the acute gout population could be separated out from the analysis.
Types of interventions
We included trials that evaluated the benefits or harms (or both) of colchicine administered by any route and at any dose.
We included any comparator, such as:
placebo or no treatment;
non‐steroidal anti‐inflammatory drugs (NSAIDs; via any route);
glucocorticoids (via any route);
other pharmacologic treatment (e.g. paracetamol);
complementary and alternative medicines;
one dose of colchicine versus another;
combination therapy (any of the above combinations).
Types of outcome measures
There is considerable variation in the outcome measures reported in trials of interventions for acute gout (Araújo 2015). For the purpose of this review, we aimed to include outcome measures that are considered to be of greatest importance to patients with acute gout and the clinicians who care for them.
OMERACT (Outcome Measures in Rheumatology Clinical Trials) has endorsed outcome measures to be used in evaluation of resolution of acute flares (Dalbeth 2014; Singh 2014). Intense pain is the hallmark of an acute gout flare and hence pain has been endorsed by OMERACT as an outcome measure; it has also been a consistent outcome measure in trials involving acute gout flares, although the instruments and time intervals used to measure pain vary (Araújo 2015). The additional outcome measures endorsed by OMERACT include joint swelling, joint tenderness and patient global assessment (Singh 2014).
Major outcomes
Pain (mean or mean change from baseline), measured by visual analogue scales (VAS), numerical or categorical ratings scales or other scales
Participant global assessment of treatment success, e.g. proportion reporting 50% or greater decrease in pain, completely improved, etc.
Reduction of inflammation (e.g. proportion reporting a reduction in joint swelling/erythema/tenderness or categorical scales)
Function of target joint (mean or mean change from baseline), measured by function scales
Serious adverse events (proportion of participants reporting the event in each group)
Total adverse events (proportion of participants reporting the event in each group)
Withdrawals due to adverse events (proportion of participants reporting the event in each group)
Minor outcome
Health‐related quality of life (mean or mean change from baseline), measured by generic quality of life scales such as SF‐36
We considered inclusion of all endpoints as measured in the included trials and combined data into short (up to 2 weeks), medium (2 to 6 weeks) and long‐term (more than 6 weeks). The primary time point for benefits was up to two weeks or the earliest time point if this time point was not reported. For harms the primary time point was end of trial.
Search methods for identification of studies
Electronic searches
We searched a registry of all RCTs in gout, established by Cochrane Musculoskeletal to facilitate the updates of a series of reviews of interventions for gout, including this review update.
The search for the gout registry was designed not to include terms for any interventions, in order to establish a registry of all randomised trials in this condition, regardless of the intervention.
The following electronic databases were searched to establish the registry. The search strategy combined standard Cochrane search filters for 'gout' and 'randomised trial', with no language restrictions.
Cochrane Central Register of Controlled Trials (CENTRAL) via OVID to 28 Aug 2020 (Appendix 1).
OVID MEDLINE 1948 to 28 Aug 2020 (Appendix 2).
Embase via OVID 1980 to 28 Aug 2020 (Appendix 3).
We also searched the clinical trials register ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform (WHO ICTRP) for relevant trials using the search term, 'gout'.
Details of the search strategies used for the previous version of the review are given in van Echteld 2014.
Searching other resources
We inspected the reference lists of included articles for potentially eligible additional trials.
Data collection and analysis
Selection of studies
Editorial staff from Cochrane Musculoskeletal initially screened titles and abstracts in the gout registry and retrieved the full text for all records they identified as RCTs of an intervention for people with gout.
Editorial staff annotated the population, intervention and comparator for each full‐text article, and assigned it to the appropriate gout review.
Review authors (BM and RJ) imported these records into Covidence to select trials eligible for this review update (Covidence 2021).
Data extraction and management
One review author (BM) extracted data regarding trial design, trial duration, characteristics of trial population, interventions, co‐interventions, adverse effects and loss to follow‐up of included trials, using a standardised data extraction form, and another review author (RJ or RB) checked the extraction. Outcome data were extracted in duplicate by BM and RJ. We resolved differences in data extraction by referring back to the original articles and establishing consensus, disagreements were resolved with a third review author (RB). Outcomes for this review were updated from those specified in the protocol (see Differences between protocol and review).
Comparisons
We included the following comparisons.
Low‐dose colchicine versus placebo
High‐dose colchicine versus placebo
High‐dose versus low‐dose colchicine
Colchicine versus NSAIDs
Colchicine versus glucocorticoids
Assessment of risk of bias in included studies
Two authors (BM and RJ) independently assessed the risk of bias of each included trial with regard to random sequence generation; allocation concealment; blinding of participants, care provider and outcome assessor for each outcome measure (see Types of outcome measures); incomplete outcome data; selective outcome reporting; and other potential sources of bias (baseline imbalance and inappropriate administration of a co‐intervention), conforming to the methods recommended by Cochrane (Higgins 2011). To determine the risk of bias of a trial, we evaluated the presence of sufficient information and the likelihood of potential bias for each criterion. We rated each criterion as low risk of bias, high risk of bias or unclear risk of bias (either lacking information or uncertainty over the potential for bias). In a consensus meeting of review authors, we discussed and resolved disagreements with a third review author (RB).
Measures of treatment effect
To assess benefits, if available, we extracted raw data for outcomes of interest as well as number of participants. If reported data needed to be converted or imputed, we recorded this in the notes section of the table Characteristics of included studies.
We plotted the results of each trial as risk ratios (RRs) with corresponding 95% confidence intervals (CIs) for dichotomous outcomes and as mean differences (MDs) between the intervention and comparator group, with corresponding 95% CIs for continuous data. The MD between treated group and control group is weighted by the inverse variance in the pooled treatment estimate. However, if different scales were used to measure the same conceptual outcome (e.g. functional status or pain), we calculated standardised mean differences (SMDs) instead, with corresponding 95% CIs. We calculated SMDs by dividing the MD by the standard deviation (SD), resulting in a unitless measure of treatment effect (Deeks 2021).
In the Effects of interventions section and the 'comments' column of the summary of findings table, we provided the absolute percentage difference, the relative percentage change from baseline, and the number needed to treat for an additional beneficial (NNTB) outcome, or the number needed to treat for an additional harmful outcome (NNTH) (NNTB or NNTH only for those outcomes with a clinically significant difference). For dichotomous outcomes, we calculated the absolute percentage change from the difference in the risks between the intervention and control group using GRADEpro GDT (GRADEpro 2015), and expressed this as a percentage. We calculated the NNTB or the NNTH from the control group event rate and the RR using the Visual Rx NNT calculator (Cates 2008).
For continuous outcomes, we calculated the absolute benefit as the improvement in the intervention group minus the improvement in the control group, in the original units, expressed as a percentage. We calculated the relative percentage change for dichotomous data as the RR ‐ 1 and expressed this as a percentage. For continuous outcomes, we calculated the relative difference in the change from baseline as the absolute benefit divided by the baseline mean of the control group, expressed as a percentage. For continuous outcomes, we calculated the NNTB or NNTH using the Wells calculator software available at the Cochrane Musculoskeletal Group (CMSG) editorial office. We determined the minimal clinically important difference for each outcome for input into the calculator.
Unit of analysis issues
In the event that we identified cross‐over trials in which the reporting of continuous outcome data precluded paired analysis, we did not plan to include these data in a meta‐analysis, in order to avoid unit of analysis error. Where carry‐over effects were thought to exist, and where sufficient data existed, we planned to only include data regarding the first period in the analysis (Higgins 2021b). However, we identified no cross‐over trials.
For trials containing more than two intervention groups, to make multiple pair‐wise comparisons between all possible pairs of intervention groups possible, if data were available we planned to include the same group of participants only once in the same meta‐analysis.
Dealing with missing data
In cases where individuals were missing from the reported results, we assumed the missing values to have a poor outcome. For dichotomous outcomes (e.g. number of withdrawals due to adverse events), we calculated the withdrawal rate using the number of participants randomised in the group as the denominator (worst case scenario). For continuous outcomes (e.g. mean change in pain score), we calculated the MD or SMD based on the number of participants analysed at that time point. If the number of participants analysed was not presented for each time point, we used the number of randomised participants in each group at baseline.
Where possible, we computed missing SDs from other statistics such as standard errors (SEs), CIs or P values, according to the methods recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021c). If we could not calculate SDs, we imputed them, for example from other trials in the meta‐analysis.
Assessment of heterogeneity
Prior to meta‐analysis we assessed trials for clinical homogeneity with respect to type of therapy, control group and outcomes. For any trials judged as clinically homogeneous, we assessed statistical heterogeneity using I2 statistics (Deeks 2021), using the following as an approximate guide for interpretation:
0% to 40% may not be important heterogeneity;
30% to 60% may represent moderate heterogeneity;
50% to 90% may represent substantial heterogeneity;
75% to 100% may represent considerable heterogeneity.
In cases of considerable heterogeneity (defined as I2 > 75%), we planned to explore the data further, including subgroup analyses, in an attempt to explain the heterogeneity.
Assessment of reporting biases
We planned an assessment of reporting bias through the screening of the International Clinical Trials Registry Platform of the World Health Organization (apps.who.int/trialsearch; De Angelis 2004), to determine whether the protocols for included RCTs were published before recruitment of participants into the trial had started. We planned to evaluate whether selective reporting of outcomes was present (outcome reporting bias).
We planned to compare the fixed‐effect estimate against the random‐effects model. In the event of the possible presence of small sample bias in the published literature (i.e. in which the intervention effect is more beneficial in smaller trials), the random‐effects estimate of the intervention is more beneficial than the fixed‐effect estimate (Deeks 2021). We planned to further explore the potential for reporting bias by funnel plots if more than 10 trials were included.
Data synthesis
Where trials were sufficiently homogeneous that it remained clinically meaningful for them to be pooled, we performed meta‐analysis using a random‐effects model, regardless of the I2 results. We performed analyses using Review Manager 5.4 (RevMan 2020), and produced forest plots for all analyses.
Subgroup analysis and investigation of heterogeneity
We planned to perform a subgroup analysis if data were available comparing treatment response for those with or without comorbidities.
If data were available in the trials, we planned to extract data on subgroups and present data with subgroup totals. We planned to compare the magnitudes of the effects informally between the subgroups by means of assessing the overlap of the CIs of the effect estimate, however, there were insufficient data for this subgroup analysis.
Sensitivity analysis
We had planned sensitivity analyses for trials with regard to allocation concealment, blinding of outcome assessor and loss to follow‐up, comparing trials with limitations (low risk of bias versus high risk of bias or unclear risk of bias), however, there were insufficient data to perform sensitivity analyses.
Summary of findings and assessment of the certainty of the evidence
The main results of the review are presented in a summary of findings table, which includes an overall grading of the evidence using the GRADE approach (GRADEpro 2015), as described in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2021). We produced a summary of the available data for the main outcomes (pain, participant global assessment of treatment success, reduction in inflammation, function of target joint, serious adverse events, total adverse events and total number of withdrawals due to adverse events).
Two review authors (BM and RJ) independently assessed the quality of the evidence using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it related to the trials which contributed data to the meta‐analyses for the prespecified outcomes, and reported the quality of evidence as high, moderate, low, or very low. We used methods and recommendations described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2021c; Schünemann 2021). We justified all decisions to downgrade the quality of trials using footnotes and provided comments to aid the reader's understanding of the review where necessary.
Results
Description of studies
Results of the search
Cochrane Musculoskeletal updated the search for all gout review updates on 28 August 2020, searching for trials from July 2011 to August 2020. The new search identified 4511 new records from Cochrane Central Register of Controlled Trials (CENTRAL) (849), MEDLINE (2125) and EMBASE (1537) databases. After screening titles and abstracts, we retrieved and reviewed the full text of 564 reports of studies potentially eligible for our gout reviews.
Of these 564 full‐text reports, we excluded 562 (168 due to ineligible study design, 70 due to ineligible population, 315 due to ineligible intervention, three trials used an ineligible comparator, two were already included in the previous version of this review, and one was in an ineligible setting).
We reviewed the remaining trials for eligibility for this review update and included two new trials (Roddy 2020; Wang 2014) in addition to two trials from the original review (Ahern 1987; Terkeltaub 2010). One additional trial is ongoing (TCTR20180608001), and one trial that was awaiting assessment in the previous review has still not been published (Wu 2014).
A flow diagram summarising the trial selection process is shown in Figure 1.
1.
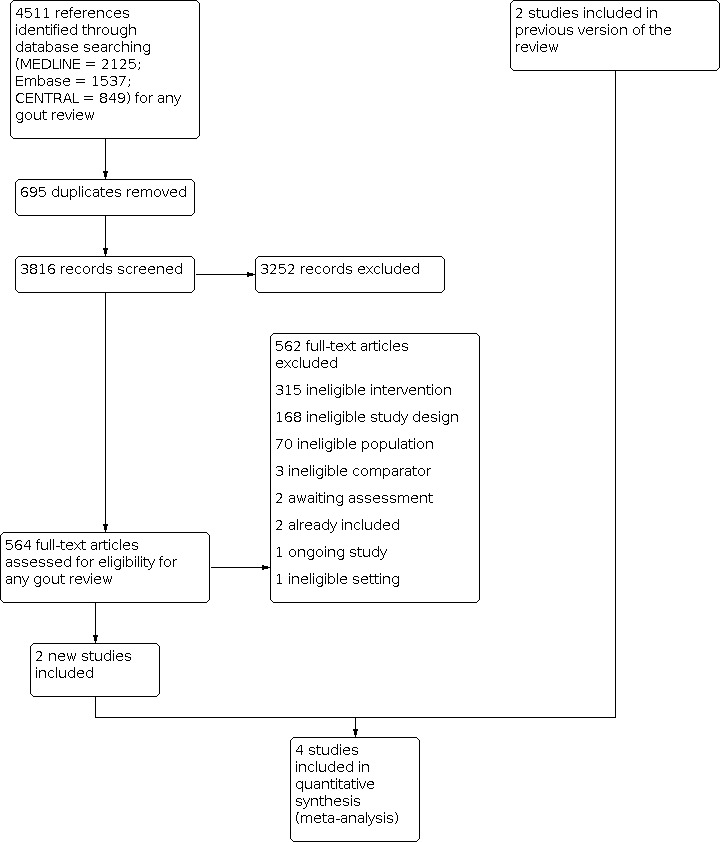
Study flow diagram.
Included studies
The four trials included in this updated review are described in the Characteristics of included studies table.
Study design, setting and characteristics
Two trials compared high‐dose colchicine to placebo (Ahern 1987; Terkeltaub 2010); one three‐arm trial also compared low‐dose colchicine to placebo and high‐dose to low‐dose colchicine (Terkeltaub 2010); one trial compared low‐dose colchicine to NSAIDs (Roddy 2020); and one trial compared low‐dose colchicine to Chuanhu anti‐gout mixture (Wang 2014). The four included trials were conducted in different countries: Australia (Ahern 1987), USA (Terkeltaub 2010), China (Wang 2014), and England (Roddy 2020). Total duration of the trials varied from 48 hours in Ahern 1987 to 12 weeks in Wang 2014. One trial received funding from a pharmaceutical company who reported roles in the study design, data collection and writing of the manuscript (Terkeltaub 2010). One trial was funded by the hospital where the trial was conducted and reported a role in the study design, but no role in data collection, data analysis, data interpretation or report writing (Wang 2014).
Study participants
The four trials included 803 randomised participants recruited from hospital and multicentre settings with trial sizes varying from 43 in Ahern 1987 to 399 participants in Roddy 2020. The majority of all trial participants were male, with mean age ranging from 51.2 to 70 years.
Inclusion criteria varied across the trials but all four trials required a diagnosis of acute gout in adults, either by the identification of monosodium urate crystals in synovial fluid from joint aspirate assessed under polarised light microscopy (Ahern 1987); diagnosis according to preliminary classification criteria of the American College of Rheumatology (ACR) with two flares within the past year (Terkeltaub 2010); a new diagnosis according to the 1977 preliminary classification criteria of the ACR and acute onset of gout of less than 48 hours (Wang 2014); or a clinical diagnosis of acute gout made by a general practitioner (GP) without joint aspiration, blood tests, imaging or diagnostic criteria (Roddy 2020). Terkeltaub 2010 specified that women needed to be postmenopausal for eligibility (5% of trial participants).
Interventions
Details of interventions for each trial are presented in the Characteristics of included studies tables.
Treatments were administered orally for all trials and consisted of high‐dose colchicine (1 mg followed by 0.5 mg every 2 hours until complete response or adverse events) versus placebo (total dose not specified) (Ahern 1987); high‐dose colchicine (4.8 mg over 6 hours) versus placebo (Terkeltaub 2010); low‐dose colchicine (1.8 mg over 1 hour) versus placebo (Terkeltaub 2010); high‐dose colchicine versus low‐dose colchicine (Terkeltaub 2010); low‐dose colchicine (0.5 mg 3 times per day for 4 days) versus NSAIDs (750 mg of naproxen at baseline then 250 mg every 8 hours for 7 days) (Roddy 2020); or low‐dose colchicine (0.5 mg twice daily for 3 days, then 0.5 mg daily for 7 days) versus Chuanhu anti‐gout mixture (250 mL) (Wang 2014).
Outcomes
Pain
One trial measured pain using an 11‐point Numerical Rating Scale (0 no pain to 10 worst possible pain), which was self‐reported by participants daily from baseline to seven days and at trial endpoint (4 weeks) (Roddy 2020).
Participant global assessment of treatment success: proportion of participants with 50% or greater decrease in pain score from baseline ‐ 32 to 36 hours
Two trials measured pain on a 100‐point VAS (Ahern 1987), and an 11‐point Likert scale (0 no pain to 10 worst possible pain; Terkeltaub 2010), and these data were used to report participant global assessment of treatment success. Ahern 1987 reported pain as a proportion of participants with 50% or greater improvement in pain from baseline at 12, 24, 36 and 48 hours. Terkeltaub 2010 reported the proportion of participants who responded to treatment, defined as a reduction in pain of 50% or more from baseline within 24 hours of the first trial dose. Alternate definitions of response for this trial included: (i) treatment response based on the target joint pain score 32 hours after the first dose, (ii) treatment response based on at least a two‐unit reduction in the target joint pain score 24 hours after the first dose, and (iii) treatment response based on at least a two‐unit reduction in the target joint pain score 32 hours after the first dose (Terkeltaub 2010).
Reduction of inflammation (joint swelling/erythema/tenderness)
One trial used a compound clinical score comprised of pain, tenderness, redness and swelling (4‐point scale, 0 to 3 score recorded for each symptom with a maximum score of 12 per joint) to present inflammation as the proportion of participants with 50% or greater decrease in inflammation score from baseline to 32 to 26 hours (Ahern 1987).
Wang 2014 reported biochemical marker changes for each study arm between baseline and 12‐week endpoint.
Function of target joint
No trials reported function as an outcome.
Serious and total adverse events
All trials reported on adverse events. All trials considered gastrointestinal events (diarrhoea, vomiting or nausea). Roddy 2020 also considered headache, skin rash, and 'other' participant nominated adverse events. Wang 2014 reported liver damage as an adverse event. Terkeltaub 2010 graded adverse events as mild, moderate, or severe based on a trial physician's clinical judgement in cases of acute gout flare.
Withdrawals due to adverse events
Terkeltaub 2010 and Wang 2014 reported the number of participants who withdrew from each study arm and the reason for withdrawal (including adverse events). Roddy 2020 reported the number of participant withdrawals from the trial but did not provide reasons. Ahern 1987 did not report withdrawals due to adverse events.
Health‐related quality of life
Roddy 2020 measured participants' quality of life at baseline for each study arm using the EQ‐5D‐5L instrument, however, these data were used to calculate quality‐adjusted life‐years to determine the cost‐effectiveness of colchicine versus NSAIDs. Follow‐up data for quality of life using the EQ‐5D‐5L instrument were not reported for this trial.
No other trials reported quality of life as an outcome.
Excluded studies
A full description of excluded studies is provided in the Characteristics of excluded studies tables. Two studies were not RCTs (Blank 2010; Moon 2011), five did not include participants with acute gout (ChiCTR1800020315; Karimzadeh 2006; Paulus 1974; Schlesinger 2011; Yamanaka 2018), and five did not include colchicine as the intervention (Hill 2013; Liu 2015; Rapado 1975; Schlesinger 2002; Yang 2018).
Risk of bias in included studies
See Characteristics of included studies tables.
A risk of bias graph and a risk of bias summary can be seen in Figure 2 and Figure 3, respectively.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We deemed three trials to have low risk of selection bias due to adequate random sequence generation, while Terkeltaub 2010 failed to describe the method of randomisation and so we deemed it unclear risk of selection bias. Allocation concealment was adequately reported for three trials, while we deemed Wang 2014 at unclear risk of selection bias for not explicitly reporting the method of allocation concealment.
Blinding
We judged three trials at low risk of performance and detection bias (Ahern 1987; Terkeltaub 2010; Wang 2014), where blinding of trial participants and all trial personnel were adequately described for the comparison of high‐dose colchicine to placebo, low‐dose colchicine to placebo, high‐dose colchicine to low‐dose colchicine and low‐dose colchicine to Chuantu anti‐gout mixture. We judged Roddy 2020 at high risk of performance and detection bias as participants and trial personnel were not blinded for the comparison between low‐dose colchicine and NSAIDs.
Incomplete outcome data
Risk of attrition bias was low in four trials (Ahern 1987; Roddy 2020; Terkeltaub 2010; Wang 2014). In Ahern 1987, two out of 43 randomised participants were excluded from the trial due to difficulties understanding VAS, although the author did not specify their allocated treatment group.
In Terkeltaub 2010, there were similar numbers of participants across three treatment groups with incomplete data due to lack of benefit, loss to follow‐up or for other unclear reasons (7/52 (13.5%) in the high‐dose colchicine group, 3/74 (4%) in the low‐dose colchicine group and 4/59 (6.8%) in the placebo group).
In Wang 2014, three out of 88 participants (3.4%) in the Chuanhu group and nine out of 88 participants (10.2%) in the low‐dose colchicine group withdrew or were lost to follow‐up for reasons including diarrhoea, bitter taste, liver damage, broken blinding and poor compliance.
Roddy 2020 reported a small number of participant withdrawals (0/199 in the low‐dose colchicine group and 3/200 (1.5%) in the NSAIDs group) and clinical trial unit withdrawals (4/199 (2%) in the low‐dose colchicine group and 4/200 (2%) in the NSAIDs group), however, reasons for withdrawal were not reported. The proportion of non‐responding participants was similar between treatment groups for data collected at eight time points throughout the trial.
Selective reporting
We deemed three trials at low risk of reporting bias (Ahern 1987; Roddy 2020; Wang 2014), and one trial at unclear risk of reporting bias (Terkeltaub 2010).
We judged Terkeltaub 2010 to be at unclear risk of reporting bias due to pain scores not being presented, despite the methods stating that these data were collected. Additionally, a responder's analysis was presented for benefit. This trial was registered in 23 July 2007 but recruitment commenced in April 2007.
Trial registration for Wang 2014 was applied for retrospectively on 5 July 2013 and the trial ran from 1 September 2011 to 30 September 2012. All reported outcomes were included in the trial registration without modification. Data for two outcomes (scores for joint swelling and limitation of joint activity) were not reported, but were provided by the corresponding author upon request.
Roddy 2020 did not provide any prespecified secondary outcome measures when registering the trial (NCT01994226), however, secondary outcomes were reported for time‐to‐treatment effect, complete pain resolution, self‐reported side effects, patient global assessment of treatment response, the use of additional medication for gout pain, self‐reported treatment adherence, relapse/recurrence of gout flare, quality of life (EQ‐5D‐5L), consultation/re‐attendance with health or medical professional for gout, and absence from work or education because of gout.
Other potential sources of bias
We judged three trials to be at low risk of other potential sources of bias (Ahern 1987; Roddy 2020; Wang 2014).
We judged other potential sources of bias to be at unclear risk for Terkeltaub 2010 as the site of flare and number of joints affected was not presented, and if baseline differences existed this may have affected the results. The number of participants treated at each site was not specified but it is stated that some sites did not have participants in all treatment groups. While the number of randomised participants in each group was approximately equal, the number of participants in each group who had an acute flare of gout and therefore had outcome data available was not equal, which may have biased the data estimates.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings 1. Low‐dose colchicine versus placebo for acute gout.
| Low‐dose colchicine versus placebo for acute gout | ||||||
| Patient or population: people with acute gout Settings: outpatient Intervention: low‐dose colchicine versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (placebo) | Low‐dose colchicine | |||||
| Pain ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Participant global assessment of treatment success: proportion with 50% or greater decrease in pain score from baseline ‐ 32 to 36 hours | 172 per 1000 | 418 per 1000 (181 to 970) | RR 2.43 (1.05 to 5.64) | 103 (1 study) | ⊕⊕⊝⊝ Lowa,b | Low‐dose colchicine may slightly increase the number of people who report treatment success. Absolute difference: 25% more reported success (7% more to 42% more); relative percentage change: 143% (5% more to 464% more) NNTB 5 (2 to 39)3 |
| Reduction of inflammation ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Function of target joint ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Serious adverse events | ‐ | ‐ | Not estimable | 133 (1 study) |
See comment | Participants reported no serious adverse events. |
| Total adverse events | 276 per 1000 | 364 per 1000 (188 to 706) | RR 1.32 (0.68 to 2.56) | 103 (1 study) | ⊕⊕⊝⊝ Lowa,b | Low‐dose colchicine may have little or no effect on the number of people reporting adverse events. Absolute difference: 9% more events with low‐dose colchicine (11% fewer to 29% more), compared to placebo. Relative percentage change: 32% more events (32% fewer to 156% more) |
| Withdrawals due to adverse events | ‐ | ‐ | Not estimable | 133 (1 study) |
See comment | No participants withdrew due to adverse events in either study arm. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to a risk of selection bias from unclear reporting of the method of randomisation, and a risk of reporting bias. bDowngraded one level for imprecision as the number of events was small (< 200) and 95% CI includes no effect, or appreciable benefit. cNote: number needed to treat for an additional beneficial (NNTB) or harmful outcome (NNTH) is not reported when result is not statistically significant. Number needed to treat for dichotomous outcomes calculated using 1/risk difference for single studies and Cates NNT calculator for meta‐analyses (www.nntonline.net/visualrx).
Summary of findings 2. High‐dose colchicine versus placebo for acute gout.
| High‐dose colchicine versus placebo for acute gout | ||||||
| Patient or population: people with acute gout Settings: hospital and outpatient Intervention: high‐dose colchicine versus placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (Placebo) | High‐dose colchicine | |||||
| Pain ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Participant global assessment of treatment success: proportion with 50% or greater decrease in pain score from baseline ‐ 32 to 36 hours | 240 per 1000 | 518 per 1000 (307 to 876) | RR 2.16 (1.28 to 3.65) | 124 (2 studies) | ⊕⊕⊝⊝ Lowa,b | High‐dose colchicine may increase the number of people reporting treatment success. Absolute difference: 28% more reported success (8% more to 46% more); relative percentage change: 116% (28% more to 265% more). NNTB: 4 (3 to 12)c |
| Reduction of inflammation: proportion with 50% or greater decrease in inflammation score from baseline ‐ 32 to 36 hours | 48 per 1000 | 504 per 1000 (278 to 1000) | RR 10.50 (1.48 to 74.38) | 43 (1 study) | ⊕⊕⊝⊝ Lowa,b | High‐dose colchicine may increase the number of people with reduced inflammation. Absolute difference: 45% greater reduction of inflammation with colchicine (22% greater to 68% greater). Relative percentage change: 950% (48% more to 7338% more) NNTB: 3 (2 to 19)c |
| Function of target joint ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Serious adverse events | ‐ | ‐ | Not estimable | 111 (1 study) |
See comment | One study reported that there were no serious adverse events and one study did not report whether any serious adverse events occurred or not. |
| Total adverse events | 260 per 1000 | 829 per 1000 (520 to 1000) | RR 3.21 (2.01 to 5.11) | 124 (2 studies) | ⊕⊕⊝⊝ Lowa,b | High‐dose colchicine may increase the number of people reporting adverse events. Absolute difference: 57% more events with high‐dose colchicine (26% more to 74% more), compared to placebo. Relative percentage change: 221% (101% more to 411% more). NNTH: 2 (2 to 10)3 |
| Withdrawals due to adverse events | ‐ | ‐ | Not estimable | 111 (1 study) |
See comment | One study reported that there were no withdrawals due to adverse events and one study did not report whether withdrawals due to adverse events occurred or not. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to a risk of selection bias from unclear reporting of the method of randomisation, and a risk of reporting bias in one study. bDowngraded one level for imprecision as the total number of participants is small and the total number of events is small. cNote: number needed to treat for an additional beneficial (NNTB) or harmful outcome (NNTH) is reported when the difference between treatment and control is statistically significant, calculated using Cates NNT calculator for dichotomous outcomes (www.nntonline.net/visualrx).
Summary of findings 3. High‐dose versus low‐dose colchicine for acute gout.
| High‐dose versus low‐dose colchicine for acute gout | ||||||
| Patient or population: people with acute gout Settings: outpatient Intervention: high‐dose versus low‐dose colchicine | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (low‐dose colchicine) | High‐dose colchicine | |||||
| Pain ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Not reported |
| Participant global assessment of treatment success: proportion with 50% or greater decrease in pain score from baseline ‐ 32 to 36 hours | 419 per 1000 | 365 per 1000 (235 to 570) | RR 0.87 (0.56 to 1.36) | 126 (1 study) | ⊕⊕⊝⊝ Lowa,b | The evidence suggests little to no difference for the proportion of people reporting treatment success between high‐dose colchicine and low‐dose colchicine. Absolute difference: 5% fewer reported success (23% fewer to 12% more). Relative percentage change: 13% fewer (44% fewer to 136% more) |
| Reduction of inflammation ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Function of target joint ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Serious adverse events | ‐ | ‐ | Not estimable | 126 (1 study) | See comment | One study reported that there were no serious adverse events in either study arm. |
| Total adverse events | 365 per 1000 | 770 per 1000 (551 to 1000) | RR 2.11 (1.51 to 2.95) | 126 (1 study) | ⊕⊕⊝⊝ Lowa,b | High‐dose colchicine may increase the number of adverse events compared to low‐dose colchicine. Absolute difference: 41% more events with high‐dose (19% more to 71% more), compared to low‐dose colchicine. Relative percentage change: 111% more events (51% more to 195% more). NNTH: 3 (2 to 5)c |
| Withdrawals due to adverse events | ‐ | ‐ | Not estimable | 126 (1 study) | See comment | One study reported that there were no withdrawals due to adverse events in either study arm. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTH: number needed to treat for an additional harmful outcome; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to a risk of selection bias from unclear reporting of the method of randomisation, and a risk of reporting bias. bDowngraded one level for imprecision as the number of events was small (< 200) and 95% CI includes no effect, or appreciable benefit. cNote: number needed to treat for an additional beneficial (NNTB) or harmful outcome (NNTH) is reported when the difference between treatment and control is statistically significant, calculated using Cates NNT calculator for dichotomous outcomes (www.nntonline.net/visualrx).
Summary of findings 4. Low‐dose colchicine versus NSAIDs.
| Low‐dose colchicine versus NSAIDs | ||||||
| Patient or population: people with acute gout Settings: 100 GPs across England Intervention: low‐dose colchicine versus naproxen | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control (NSAIDs) | Low‐dose colchicine | |||||
|
Pain NRS Scale from 0 to 10, 0 is no pain. Follow‐up: 7 days |
1.4 points | Mean pain was 0.1 points worse (0.29 better to 0.49 worse) | Not estimable | 399 (1 study) |
⊕⊕⊝⊝ Lowa,b | Low‐dose colchicine may have little or no effect on pain when compared to NSAIDs. Absolute difference: 1% worse pain (3% better to 5% worse). Relative percentage change: 1.4% worse (4% better to 7% worse)c |
|
Participant global assessment of treatment success: Completely better/much better Follow‐up: 7 days |
570 per 1000 | 553 per 1000 (467 to 655) | RR 0.97 (0.82 to 1.15) | 399 (1 study) |
⊕⊕⊝⊝ Lowa,b | Low‐dose colchicine may have little or no effect on the number of people reporting treatment success. Absolute difference: 1.7% fewer people reported treatment success as completely better or much better with low‐dose colchicine (10.3% fewer to 8.5% more), compared to NSAIDs. Relative percentage change: 3% fewer (18% fewer to 15% more) |
| Reduction of inflammation ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
| Function of target joint ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured |
|
Serious adverse events Follow‐up: 4 weeks |
See comment | See comment | Not estimable | ‐ | See comment | There were three serious adverse events (2 from the NSAIDs group and 1 from low‐dose colchicine group), none related to study interventions, and no deaths. |
|
Total number of participants reporting adverse events Follow‐up: 4 weeks |
500 per 1000 | 535 per 1000 (440 to 645) | RR 1.07 (0.88 to 1.29) | 399 (1 study) |
⊕⊕⊝⊝ Lowa,b | Low‐dose colchicine may have little or no difference to the number of adverse events compared to NSAIDs. Absolute difference: 3.5% more people reported adverse events (6% fewer to 14.5% more), compared to NSAIDs. Relative percentage change: 7% (12% fewer to 29% more) |
| Withdrawals due to adverse events | See comment | See comment | Not estimable | ‐ | See comment | There was one withdrawal from the low‐dose colchicine group with the reason unreported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; GPs: general practices; NRS: numeric rating scale; NSAIDs: non‐steroidal anti‐inflammatory drugs; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
aDowngraded one level due to a risk of performance and detection bias. bDowngraded one level for imprecision as the number of events was small (< 200) and 95% CI includes no effect, or appreciable benefit. Data were from a single study only, which may have been inadequately powered to detect a clinically important change in pain between groups (defined as 1.5 points on the 0 to 10 NRS pain scale). cRelative change was calculated as absolute change (mean difference) divided by mean at baseline in the NSAIDs group from Roddy 2020 (mean NRS baseline value was 7.1).
We performed five comparisons: low‐dose colchicine versus placebo, high‐dose colchicine versus placebo, high‐dose versus low‐dose colchicine, low‐dose colchicine versus NSAIDs and low‐dose colchicine versus Chuanhu anti‐gout mixture.
Low‐dose colchicine versus placebo
Low‐quality evidence from one trial (103 participants) suggests that compared to placebo, low‐dose colchicine may slightly increase the number of people who report treatment success (pain reduction of 50% or more from baseline) at 32 to 36 hours; 31/74 in the low‐dose colchicine group reported treatment success compared to 5/29 in the placebo group (risk ratio (RR) 2.43, 95% confidence interval (CI) 1.05 to 5.64; 1 trial, 103 participants; absolute increase of 25% (7% more to 42% more), relative increase of 143% (5% more to 464% more), number needed to treat for an additional beneficial outcome (NNTB) 5 (2 to 39); low‐quality evidence; Table 1; Analysis 1.1)). We downgraded the evidence for bias and imprecision.
1.1. Analysis.

Comparison 1: Low‐dose colchicine versus placebo, Outcome 1: Proportion with 50% or greater decrease in pain score from baseline
Low‐dose colchicine may have little or no effect on the number of people reporting adverse events. Up to 72 hours from baseline, there were 27/74 adverse events in the low‐dose colchicine group compared with 8/29 adverse events in the placebo group (RR 1.32, 95% CI 0.68 to 2.56; 1 trial; 103 participants; absolute difference 9% (9% fewer to 43% more), relative difference 32% (32% fewer to 156% more; low‐quality‐evidence; Table 1; Analysis 1.2)). We downgraded the evidence for bias and imprecision.
1.2. Analysis.

Comparison 1: Low‐dose colchicine versus placebo, Outcome 2: Total adverse events
The Terkeltaub 2010 trial did not measure pain, reduction of inflammation or function of the target joint. The authors reported that there were no withdrawals due to adverse events and no serious adverse events in either treatment group.
High‐dose colchicine versus placebo
We judged two trials to be clinically similar, facilitating the pooling of data for meta‐analysis. Participant global assessment of treatment success was presented by both trials as a proportion of participants with 50% or greater decrease in pain, where data were collected at 32 to 36 hours from baseline. Based upon data from the two trials (Ahern 1987; Terkeltaub 2010), there is low‐quality evidence (downgraded for selection and reporting bias and imprecision) that high‐dose colchicine may increase the number of people reporting treatment success when compared to placebo, with a clinically relevant proportion of participants reporting a reduction in pain of 50% or more from baseline at 32 to 36 hours; 35/74 reported treatment success in the high‐dose colchicine group compared with 12/50 (24%) in the placebo group (RR 2.16, 95% CI 1.28 to 3.65; I2 = 0%; 2 trials, 124 participants; absolute difference 28% more success (8% more to 46% more), relative difference 116% (28% more to 265% more), NNTB 4 (3 to 12); low‐quality evidence; Table 2; Analysis 2.1)).
2.1. Analysis.

Comparison 2: High‐dose colchicine versus placebo, Outcome 1: Proportion with 50% or greater decrease in pain score from baseline
Low‐quality evidence (downgraded for bias and imprecision) from Ahern 1987 demonstrates that high‐dose colchicine may increase the number of people with reduced inflammation. Reduction of inflammation was scored using a composite measure that included pain, tenderness, swelling and erythema; each graded on a 4‐point scale (0 = none to 3 = severe) to derive a maximum score of 12 in any one joint. At 36 hours, 11/22 people in the high‐dose colchicine reported 50% or greater decrease in inflammation score from baseline compared to 1/21 (5%) in the placebo group (RR 10.50, 95% CI 1.48 to 74.38; 1 trial, 43 participants; absolute difference 45% (22% greater to 68% greater), relative difference 950% (48% more to 7338% more), NNTB 3 (2 to 19); low‐quality evidence; Table 2; Analysis 2.2)).
2.2. Analysis.

Comparison 2: High‐dose colchicine versus placebo, Outcome 2: Proportion with 50% or greater decrease in inflammation from baseline
Although the risk of adverse events was likely to have been inflated in the Ahern 1987 trial due to the colchicine arm continually dosing participants every 2 hours until complete response or adverse events occurred, we pooled data from the two trials for total adverse events. Compared to placebo, there is low‐quality evidence that high‐dose colchicine may increase the number of people reporting adverse events; 62/74 (83%) in the high‐dose colchicine group reported adverse events compared to 13/50 (26%) in the placebo group (RR 3.21, 95% CI 2.01 to 5.11; 2 trials, 124 participants; absolute difference 57% (26% more to 74% more), relative difference 221% (101% more to 411% more), and number needed to treat for an additional harmful outcome (NNTH) 2 (2 to 10); low‐quality evidence; Table 2; Analysis 2.3)). Assessing the Terkeltaub 2010 trial independently also provides low‐quality evidence that high‐dose colchicine may increase the number of adverse events compared with placebo; high‐dose colchicine: 40/52, placebo: 8/29 (RR 2.79, 95% CI 1.52 to 5.12; low‐quality evidence; Analysis 2.3). We downgraded evidence for bias and imprecision.
2.3. Analysis.

Comparison 2: High‐dose colchicine versus placebo, Outcome 3: Total adverse events
Two trials comparing high‐dose colchicine and placebo did not report trial participant withdrawal due to serious adverse events, or withdrawals due to adverse events (Ahern 1987; Terkeltaub 2010). All 22 participants receiving high‐dose colchicine in Ahern 1987 developed diarrhoea or vomiting, or both. Terkeltaub 2010 reported diarrhoea, nausea and vomiting for total adverse events (124 participants). Terkeltaub 2010 reported that there were no serious adverse events and Ahern 1987 did not report whether adverse events occurred or not.
The major outcomes, pain and function of target joint were not reported in the two trials comparing high‐dose colchicine to placebo.
High‐dose versus low‐dose colchicine
Data from one trial (Terkeltaub 2010), found no evidence of important differences between high‐ and low‐dose colchicine for the proportion of participants who reported treatment success (reduction in pain of 50% or more from baseline). At 32 to 36 hours 19/52 (36%) (95% CI 23% to 57%) in the high‐dose colchicine group reported treatment success compared to 31/74 (42%) in the low‐dose colchicine group (RR 0.87, 95% CI 0.56 to 1.36; 1 trial, 126 participants; absolute difference 5% (23% fewer to 12% more), relative difference 13% (44% fewer to 136% more; low‐quality evidence; Table 3; Analysis 3.1)). We downgraded evidence for bias and imprecision.
3.1. Analysis.

Comparison 3: High‐dose versus low‐dose colchicine, Outcome 1: Proportion with 50% or greater decrease in pain score from baseline
Based on low‐quality evidence (downgraded for bias and imprecision) from one trial (Terkeltaub 2010), the number of participants reporting total adverse events may worsen with high‐dose colchicine (40/52 or 70%) compared to low‐dose colchicine (27/74 or 36%): RR 2.11, 95% CI 1.51 to 2.95; 1 trial, 126 participants; low‐quality evidence; absolute difference 41% (19% more to 71% more), relative difference 111% (51% more to 195% more), and NNTH 3 (2 to 5); Table 3; Analysis 3.2.
3.2. Analysis.

Comparison 3: High‐dose versus low‐dose colchicine, Outcome 2: Total adverse events
There were no withdrawals due to adverse events and no serious adverse events were reported in either arm of this trial.
Outcomes for pain, reduction of inflammation or function of target joint were not measured.
Low‐dose colchicine versus NSAIDs
Based on one trial with 399 participants (Roddy 2020), colchicine may have little or no effect on pain when compared to NSAIDs at seven days (Analysis 4.1) Mean pain on a scale of 0 to 10 (0 is no pain) was 1.4 points on those taking NSAIDs and 1.5 points on those taking opioids; absolute difference 1% worse pain (3% better to 5% worse); relative change 1.4% worse (4% better to 7% worse). We downgraded the evidence to low quality for bias and imprecision.
4.1. Analysis.

Comparison 4: Low‐dose colchicine versus NSAIDs, Outcome 1: Absolute mean pain (NRS 0 to 10)
Low‐dose colchicine may have little or no effect on the number of people reporting treatment success compared to NSAIDs. At seven days 110/199 (55%, 95% CI 47% to 65%) in the low‐dose colchicine group reported treatment success compared to 114/200 in the NSAIDs group: RR 0.97, 95% CI 0.82 to 1.15; 1 trial, 399 participants; absolute difference 1.7% fewer people reported treatment success as completely better or much better with low‐dose colchicine (10.3% fewer to 8.5% more), compared to NSAIDs relative difference 3% (18% fewer to 15% more); low‐quality evidence; Analysis 4.2; Table 4. We downgraded the evidence for bias and imprecision.
4.2. Analysis.

Comparison 4: Low‐dose colchicine versus NSAIDs, Outcome 2: Participant global assessment of treatment success (Completely better/much better)
This trial reported three serious adverse events (one in the low‐dose colchicine group and two in the NSAIDs group), however, none were related to trial interventions. There is little evidence of important differences for total adverse events between study arms (Analysis 4.3). There was one participant withdrawal in this trial, with reason not reported.
4.3. Analysis.

Comparison 4: Low‐dose colchicine versus NSAIDs, Outcome 3: Total adverse events at 4 weeks
Reduction of inflammation and function of target joint were not measured.
Low‐dose colchicine versus Chuanhu anti‐gout mixture
Very low‐quality evidence from one trial (176 participants, downgraded two levels for imprecision and one level for risk of bias) found no evidence of important differences between low‐dose colchicine and Chuanhu anti‐gout mixture for recurrence rate of acute gout (Wang 2014); 13/88 in the low‐dose colchicine group reported recurrence compared to 11/88 in the Chuanhu anti‐gout mixture: RR 1.18, 95% CI 0.56 to 2.49; 1 trial, 176 participants; very low‐quality evidence; absolute difference 2.2% (5.5% fewer to 18.6% more), relative difference 18% (44% fewer to 149% more); Analysis 5.1.
5.1. Analysis.

Comparison 5: Low‐dose colchicine versus Chuanhu anti‐gout mixture, Outcome 1: Recurrence rate of gout at 12 weeks
Based upon very low‐quality evidence, there were no important differences for withdrawal due to adverse events over 12 weeks between low‐dose colchicine (8/88) and Chunahu anti‐gout mixture (2/88): RR 4.00, 95% CI 0.87 to 18.31; absolute difference 6.8% (0.3% fewer to 39% more), relative difference 300% (13% fewer to 1731% more); Analysis 5.2. Based upon very low‐quality evidence, the total number of adverse events may significantly increase for participants taking low‐dose colchicine (27/88) versus Chuanhu anti‐gout mixture (2/88): RR 13.50, 95% CI 3.31 to 55.05, absolute difference 28% (5% more to 123% more), relative difference 1250% (231% more to 5405% more); Analysis 5.3.
5.2. Analysis.

Comparison 5: Low‐dose colchicine versus Chuanhu anti‐gout mixture, Outcome 2: Withdrawals due to adverse events
5.3. Analysis.

Comparison 5: Low‐dose colchicine versus Chuanhu anti‐gout mixture, Outcome 3: Total adverse events
Lack of raw data for adverse reactions prevented comparisons to be made between low‐dose colchicine and Chuanhu anti‐gout mixture groups.
No trials comparing low‐dose colchicine and Chuanhu anti‐gout mixture reported pain, participant global assessment of treatment success, reduction of inflammation or function of target joint.
There were no trials that compared colchicine to glucocorticoids, which are known to be an effective treatment for acute gout.
Discussion
Summary of main results
Compared to placebo, there was low‐quality evidence that low‐dose colchicine may slightly increase the number of people who report treatment success at 32 to 36 hours from baseline, and low‐quality evidence suggesting that low‐dose colchicine may have little or no effect on the number of people reporting adverse events from treatment. It is unclear whether low‐dose colchicine increases the number of people with serious adverse events or the number of people withdrawing due to adverse events due to no events being reported for these outcomes (see Table 1). None of the trials comparing low‐dose colchicine to placebo reported pain, reduction of inflammation or function of target joint. We downgraded the evidence due to the risk of selection or reporting bias, as well as imprecision.
There was low‐quality evidence that compared to placebo, high‐dose colchicine may increase the number of people reporting treatment success, and increase the number of people with reduced inflammation at 32 to 36 hours from baseline. Low‐quality evidence also suggests that high‐dose colchicine may increase the number of people reporting adverse events when compared to placebo, which mostly consisted of diarrhoea, vomiting or nausea in two trials. There were no trials comparing high‐dose colchicine to placebo that reported pain or function of target joint. There were no serious adverse events or withdrawals due to adverse events reported in one trial, making it unclear whether high‐dose colchicine increases the number of people with these outcomes. We downgraded the evidence due to the risk of selection or reporting bias, as well as imprecision.
Low‐quality evidence found that there may be no important differences for the proportion of people reporting treatment success between high‐dose colchicine and low‐dose colchicine, and suggests that high‐dose colchicine may increase the number of adverse events compared to low‐dose colchicine. It is uncertain whether high‐dose colchicine increases the number of people with serious adverse events or the number of people withdrawing due to adverse events compared to low‐dose colchicine, as one trial reported no events for these outcomes (see Table 3). Trials comparing high‐dose colchicine to low‐dose colchicine did not report pain, reduction of inflammation or function of target joint outcomes. We downgraded the evidence due to the risk of selection or reporting bias, as well as imprecision.
Based upon low‐quality evidence, low‐dose colchicine may have little or no effect on pain or the number of people reporting treatment success at seven days when compared to NSAIDs. One trial reported serious adverse events that were not related to any trial interventions. There was low‐quality evidence that low‐dose colchicine may make little or no difference to the number of adverse events compared to NSAIDs. We are uncertain whether low‐dose colchicine increases the number of withdrawals due to adverse events compared to NSAIDs, as low‐quality evidence from one trial reported one withdrawal in the low‐dose colchicine group without providing a reason. Trials comparing low‐dose colchicine to NSAIDs did not measure reduction of inflammation or function of target joint. We downgraded evidence from one trial due to risk of performance and detection bias in the trial's open‐label design.
Very low‐quality evidence found no important differences for withdrawal due to adverse events between low‐dose colchicine and Chuanhu anti‐gout mixture over 12 weeks. Based upon very low‐quality evidence, low‐dose colchicine may increase the number of people reporting adverse events when compared to Chuanhu anti‐gout mixture. No trials comparing low‐dose colchicine and Chuanhu anti‐gout mixture reported pain, participant global assessment of treatment success, reduction of inflammation or function of target joint. We downgraded the evidence due to the risk of selection bias and imprecision.
Overall completeness and applicability of evidence
There are several treatment options for acute gout. When it comes to the use of colchicine, the overall balance of benefits and harms is key. We identified four trials investigating the benefits and harms of colchicine. One trial compared low‐dose colchicine to placebo, two trials compared high‐dose colchicine to placebo and one trial compared high‐dose to low‐dose colchicine. One trial compared low‐dose colchicine to NSAIDs, and one trial compared low‐dose colchicine to Chuanhu anti‐gout mixture. The data suggest that compared to placebo, low‐dose colchicine is as effective as higher doses of colchicine but with lower risk of adverse events. When compared to NSAIDs, low‐dose colchicine may have little or no effect on pain intensity, and may have little or no difference in the number of adverse events.
Colchicine and its metabolites are excreted through the urinary and biliary tracts, and the presence of renal or liver disease increases the time needed for clearance of colchicine two‐ to three‐fold (Ben‐Chetrit 1998). This suggests that people with either liver or renal disease may need to be closely monitored even while on low doses of colchicine. Three trials included in this review did not provide baseline participant hepatic or renal function data (Ahern 1987; Roddy 2020; Terkeltaub 2010), however, one trial reported biochemical markers for liver and kidney function at baseline and 12‐week follow‐up (Wang 2014). Terkeltaub 2010 reported that the risk of experiencing gastrointestinal events was similar when comparing demographic characteristics, concomitant allopurinol use or estimated creatinine clearance.
The small number of included trials and trial participants means that uncommon but potentially serious adverse events may not have been detected. There have been reports of bone marrow and neuromuscular adverse events with the use of low‐dose colchicine (0.5 mg twice a day) (Kuncl 1987).
We found no published trials of colchicine compared with oral or intra‐articular glucocorticoids.
Colchicine can be administered intravenously to treat flares of acute gout. However, there is a narrow margin between an effective dose of the drug and a harmful dose that can result in serious health risks, including death. In February 2008, the US Food and Drug Administration (FDA) ruled that the manufacture or distribution of intravenous colchicine was no longer approved in the USA due to its adverse events (FDA 2008).
Colchicine is metabolised by cytochrome P450 3A4 (CYP3A4) and is also a substrate for the P‐glycoprotein transporter and serious (including fatal) colchicine adverse events have been reported in people taking standard therapeutic doses of colchicine and concomitantly prescribed medications that inhibit CYP3A4 or P‐glycoprotein (these include clarithromycin, erythromycin, ketoconazole, ritonavir, verapamil and diltiazem, among others). The US FDA recommends that these drugs not be used in people taking colchicine (particularly when there is coexisting renal of hepatic impairment) and, relatively recently, a dose reduction algorithm to predict and prevent colchicine adverse events in the presence of these drugs has been proposed (Terkeltaub 2010).
In two of the included trials (Ahern 1987; Terkeltaub 2010), low‐dose colchicine consisted of a total of 1.8 mg given over one hour. For one trial (Wang 2014), the low‐dose colchicine consisted of 0.5 mg taken orally two times per day for three days, then 0.5 mg once per day for an additional seven days; and one trial provided participants with a 0.5 mg colchicine tablet (low‐dose) every eight hours for four days (Roddy 2020).
Quality of the evidence
The overall quality of the evidence for the benefits and harms of colchicine for acute gout is low. Major outcomes for this review that were measured across four trials include: pain, patient global assessment of treatment success, reduction of inflammation and adverse events. Due to the small number of trials published on colchicine for acute gout and the variability of outcome measures used in the trials, pooling of data for meta‐analysis was only possible for two outcomes in two trials. There was the potential for selection bias and the risk of selective reporting in one trial, a high risk of performance and detection bias in one trial, and imprecision due to a small number of participants and events in the four trials.
Potential biases in the review process
We believe that our broad search strategy and database search identified all relevant trials, and minimised the likelihood of having missed any relevant trials. Two review authors independently assessed the trials for inclusion in the review and their risk of bias.
Agreements and disagreements with other studies or reviews
Our review updates a previous Cochrane Review (van Echteld 2014), and our conclusions are broadly in agreement with the previous update.
Two systematic reviews synthesising evidence for the management of gout have been published since our previous update, although they examined slightly different questions (Shekelle 2017; Stewart 2020). Shekelle 2017 reviewed the evidence for colchicine, NSAIDs and glucocorticoids in the management of acute gout flares. Stewart 2020 synthesised adverse event data for oral colchicine used for any indication, including both chronic and acute gout.
Shekelle 2017 included 28 studies evaluating pharmacological treatments for acute gout, including only two of the four trials investigating the use of colchicine that we included (Ahern 1987; Terkeltaub 2010). It is unclear why Wang 2014 was excluded as it is not listed as an excluded trial. Stewart 2020 also included two of the four trials we included (Terkeltaub 2010; Wang 2014), but it was unclear why Ahern 1987 was not included. Neither review identified Roddy 2020, as their search dates preceded publication of the trial.
The conclusions of both reviews in relation to colchicine are broadly in keeping with our updated review. Shekelle 2017 did not perform meta‐analyses and assessed the overall strength of evidence as high, moderate, low or insufficient but did not perform GRADE assessments. They also applied criteria proposed by Sir Austin Bradford Hill for causality when judging strength of evidence (Hill 1965), although these criteria are usually applied to observational studies. Stewart 2020 did not present data for the adverse events of colchicine for acute gout separately, but similarly to our review, reported that colchicine use was associated with an increased risk of diarrhoea and other gastrointestinal events.
Authors' conclusions
Implications for practice.
Based upon only four published trials, two with a low risk of bias, one with an unclear risk of bias and one with a high risk of bias, there is low‐quality evidence that low‐dose colchicine may be an effective treatment for acute gout. We downgraded the evidence because of a potential risk of selection and reporting biases, the high risk of performance and detection bias in one open‐label trial, and the small number of trial participants and the low event rates indicating imprecision. Both high‐ and low‐dose colchicine improve pain when compared to placebo, however, low‐dose colchicine may be the preferred treatment option for acute gout as low‐quality evidence suggests that high‐dose (but not low‐dose) colchicine may increase the number of adverse events compared to placebo.
From a clinical perspective, first‐line treatment of acute gout is a choice between low‐dose colchicine, NSAIDs or glucocorticoids, and guidelines do not differentiate between them except to note that a patient's risk profile and comorbidities will likely influence the choice of therapy in clinical practice. New data presented in this review indicates that there may be no difference in efficacy between low‐dose colchicine and NSAIDs.
Implications for research.
RCTs comparing low‐dose colchicine to glucocorticoids may further benefit clinical decision‐making. Such studies should ideally be pragmatic in the way that they include people with gout and all their typical comorbidities.
Planned trials should include participants with a range of gout manifestations (e.g. tophi, nephrolithiasis), comorbidities that influence pharmacotherapy choice for gout treatment (e.g. renal impairment, cardiovascular disease) and assess the outcomes recommended by OMERACT for studies of acute gout, including a reduction in gout flare frequency, joint pain, serum urate concentration, tophus burden, physical function and quality of life (Singh 2011). The CONSORT statement should also be used to guide both designing and reporting studies (Boutron 2008).
Trial reporting should include the method of randomisation and treatment allocation concealment, blinding of trial participants, trial personnel and outcome assessment, follow‐up of all participants who entered the trial and complete reporting of outcomes. Sample sizes should be reported and have adequate power to answer the research question; ideally trials should assess both the benefits and risks of lifestyle interventions. To enable comparison and pooling of the results of RCTs, we suggest that future trials report means with standard deviations (SDs) for continuous measures or number of events and total numbers analysed for dichotomous measures, and use standardised measurement tools for reporting relevant outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 14 December 2020 | New citation required and conclusions have changed | We added new evidence comparing colchicine to NSAIDs. |
| 28 August 2020 | New search has been performed | Updated search 28 August 2020, with two new trials included (Roddy 2020; Wang 2014). |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 24 September 2019 | Amended | Published note added after a request from the funding arbiter |
| 8 April 2014 | New search has been performed | New search. Added one trial, Terkeltaub 2010. |
| 8 April 2014 | New citation required and conclusions have changed | Conclusions changed and change in authors. |
| 24 September 2008 | Amended | CMSG ID: C097‐R |
Acknowledgements
Thanks to Louise Falzon for developing the search strategy and Dr Irene van Echteld and Professor Daniel Aletaha who were authors on the previous version of this review.
Thanks also to Dr Edward Roddy for providing unpublished Roddy 2020 trial data (participant adverse events) and Professor YouXin Wang for providing supplementary data for Wang 2014 upon email request.
Thanks to Jordi Pardo Pardo for establishing the gout registry, which we used to select studies for this review update, and to Emma Murphy, Elizabeth Houlding‐Braunberger, Humaira Mahfuz, Nicholas Lebel, Kaitlyn Brethour and Sheila Cyril for uploading full‐text articles and tagging the studies by intervention.
We would also like to thank Professor Jasvinder Singh, Lara Maxwell and Catherine Hofstetter for their constructive peer‐review feedback.
Appendices
Appendix 1. Cochrane CENTRAL search strategy
EBM Reviews ‐ Cochrane Central Register of Controlled Trials <July 2020>
1 exp Gout/
2 gout$.tw.
3 1 or 2
4 tophus.tw.
5 tophi.tw.
6 tophaceous.tw.
7 or/3‐6
Appendix 2. MEDLINE search strategy
Ovid MEDLINE(R) ALL <1946 to August 27, 2020>
1 exp Gout/
2 gout$.tw.
3 1 or 2
4 tophus.tw.
5 tophi.tw.
6 tophaceous.tw.
7 or/3‐6
8 randomized controlled trial.pt.
9 controlled clinical trial.pt.
10 randomized.ab.
11 placebo.ab.
12 drug therapy.fs.
13 randomly.ab.
14 trial.ab.
15 groups.ab.
16 or/8‐15
17 exp animals/ not humans.sh.
18 16 not 17
19 7 and 18
Appendix 3. Embase search strategy
Embase Classic+Embase <1947 to 2020 August 27>
1 exp Gout/
2 gout$.tw.
3 1 or 2
4 tophus.tw.
5 tophi.tw.
6 tophaceous.tw.
7 or/3‐6
8 random$.tw.
9 factorial$.tw.
10 crossover$.tw.
11 cross over.tw.
12 cross‐over.tw.
13 placebo$.tw.
14 (doubl$ adj blind$).tw.
15 (singl$ adj blind$).tw.
16 assign$.tw.
17 allocat$.tw.
18 volunteer$.tw.
19 crossover procedure/
20 double blind procedure/
21 randomized controlled trial/
22 single blind procedure/
23 or/8‐22
24 3 and 7 and 23
Data and analyses
Comparison 1. Low‐dose colchicine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Proportion with 50% or greater decrease in pain score from baseline | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.1 24 hours | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1.2 32 hours | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.2 Total adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.3 Gastrointestinal adverse events (diarrhoea, vomiting or nausea) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.4 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.5 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
1.3. Analysis.

Comparison 1: Low‐dose colchicine versus placebo, Outcome 3: Gastrointestinal adverse events (diarrhoea, vomiting or nausea)
1.4. Analysis.

Comparison 1: Low‐dose colchicine versus placebo, Outcome 4: Serious adverse events
1.5. Analysis.

Comparison 1: Low‐dose colchicine versus placebo, Outcome 5: Withdrawals due to adverse events
Comparison 2. High‐dose colchicine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Proportion with 50% or greater decrease in pain score from baseline | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1.1 12 hours | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 2.39 [0.52, 10.99] |
| 2.1.2 24 hours | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 2.88 [1.28, 6.48] |
| 2.1.3 32 to 36 hours | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 2.16 [1.28, 3.65] |
| 2.1.4 48 hours | 1 | 43 | Risk Ratio (M‐H, Random, 95% CI) | 1.91 [1.05, 3.49] |
| 2.1.5 Last time point | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 1.97 [1.20, 3.24] |
| 2.2 Proportion with 50% or greater decrease in inflammation from baseline | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2.1 12 hours | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2.2 24 hours | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2.3 36 hours | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.2.4 48 hours | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.3 Total adverse events | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 3.21 [2.01, 5.11] |
| 2.4 Gastrointestinal adverse events (diarrhoea, vomiting or nausea) | 2 | 124 | Risk Ratio (M‐H, Random, 95% CI) | 3.81 [2.28, 6.38] |
| 2.5 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.6 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
2.4. Analysis.

Comparison 2: High‐dose colchicine versus placebo, Outcome 4: Gastrointestinal adverse events (diarrhoea, vomiting or nausea)
2.5. Analysis.

Comparison 2: High‐dose colchicine versus placebo, Outcome 5: Serious adverse events
2.6. Analysis.

Comparison 2: High‐dose colchicine versus placebo, Outcome 6: Withdrawals due to adverse events
Comparison 3. High‐dose versus low‐dose colchicine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Proportion with 50% or greater decrease in pain score from baseline | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.1 24 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1.2 32 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.2 Total adverse events | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 3.3 Gastrointestinal adverse events (diarrhoea, vomiting or nausea) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.4 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.5 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
3.3. Analysis.

Comparison 3: High‐dose versus low‐dose colchicine, Outcome 3: Gastrointestinal adverse events (diarrhoea, vomiting or nausea)
3.4. Analysis.

Comparison 3: High‐dose versus low‐dose colchicine, Outcome 4: Serious adverse events
3.5. Analysis.

Comparison 3: High‐dose versus low‐dose colchicine, Outcome 5: Withdrawals due to adverse events
Comparison 4. Low‐dose colchicine versus NSAIDs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Absolute mean pain (NRS 0 to 10) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1.1 7 days | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.1.2 4 weeks | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4.2 Participant global assessment of treatment success (Completely better/much better) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.2.1 7 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.2.2 4 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.3 Total adverse events at 4 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.4 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
4.4. Analysis.

Comparison 4: Low‐dose colchicine versus NSAIDs, Outcome 4: Serious adverse events
Comparison 5. Low‐dose colchicine versus Chuanhu anti‐gout mixture.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Recurrence rate of gout at 12 weeks | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.2 Withdrawals due to adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.3 Total adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5.4 Serious adverse events | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
5.4. Analysis.

Comparison 5: Low‐dose colchicine versus Chuanhu anti‐gout mixture, Outcome 4: Serious adverse events
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahern 1987.
| Study characteristics | ||
| Methods |
Study design: randomised, double‐blind, two‐arm, parallel placebo‐controlled trial Setting: Repatriation General Hospital, Daw Park, South Australia Time period: not reported Intervention: oral colchicine taken at baseline (1 mg) and every 2 hours (0.5 mg) until complete response or adverse events versus matched placebo given in the same way Sample size: not described in the published report Analysis: not described whether ITT or per protocol analysis |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics
High‐dose colchicine group (n = 22)
Placebo group (n = 21)
Pre‐treatment group differences: none |
|
| Interventions |
High‐dose colchicine
Control (placebo)
Co‐interventions
|
|
| Outcomes | Outcomes evaluated every 6 hours for 48 hours by a single assessor Primary outcome
Secondary outcomes
Outcomes used in this review
Time pointsused in this review
|
|
| Source of funding | Not reported | |
| Notes |
Trial registration: not done as the trial was conducted prior to establishment of trial registries. Competing interests: the authors declared no competing interests for this study. Withdrawals: 2 participants withdrew but their treatment groups were not specified Total adverse events
Serious adverse events
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers (information received after communication with authors) |
| Allocation concealment (selection bias) | Low risk | Allocation was concealed and held in pharmacy (information received after communication with authors) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Matching placebo control was used, implying that participants were blinded. It was not stated in the report whether or not study personnel were blinded, however in fact both study personnel and participants were blinded (information received after communication with authors) |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (pain, treatment success, reduction of inflammation, function, serious adverse events, total adverse events, withdrawals due to adverse events) | Low risk | Participants were blinded for this trial (information received after communication with authors) |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes (reduction of inflammation, function) | Low risk | Outcome assessors were blinded (information received after communication with authors) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants were excluded because of inability to understand VAS ‐ it is not clear whether or not these participants were randomised but it is unlikely that this would have affected the study outcome |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported although it is not clear if the criterion for improvement (50% reduction from baseline score) was prespecified |
| Other bias | Low risk | No other potential sources of bias identified |
Roddy 2020.
| Study characteristics | ||
| Methods |
Study design: multicentre, parallel‐arm, open‐label randomised controlled trial Setting: 100 GP practices across England Time period: 29 January 2014 to 31 December 2015 Intervention: naproxen (NSAID) 750 mg immediately then 250 mg every 8 hours for 7 days, or low‐dose colchicine 500 mcg three times per day for 4 days. Sample size: a sample size of 200 participants per arm was required to detect a small standardised effect size of 0.3 for the primary outcome of change in pain intensity from baseline measured over the first 7 days, allowing for the repeated measures structure (assumed autocorrelation 0.6), 20% loss to follow‐up, 1:1 allocation ratio, 90% power and two‐sided type 1 error of 0.05. This trial aimed to assess the superiority of naproxen or colchicine (two‐tailed hypothesis testing). Analysis: ITT |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Colchicine group (n=199)
Naproxen group (n=200)
Pretreatment group differences: none |
|
| Interventions |
Low‐dose colchicine group
Control (naproxen group)
Co‐intervention
|
|
| Outcomes | Outcomes were measured at days 0 to 7 and week 4 Primary outcome
Mean change from baseline was compared between groups over days 1 to 7. Secondary outcomes
Outcome measures were collected by self‐completed daily diary (days 1–7) and a questionnaire at week 4. Outcomes used in this review
Time pointsused in this review
|
|
| Source of funding | This trial was funded by the National Institute for Health Research School for Primary Care Research (NIHR SPCR). | |
| Notes |
Trial registration: this trial was prospectively registered at ISRCTN (identifier: 69836939) on 21 November 2013 and at ClinicalTrials.gov (NCT01994226) on 25 November 2013. The total number of participants in each treatment arm (colchicine = 199, NSAIDs = 200) was less than sample size calculated a priori for an adequately powered study (200 per arm), allowing for a 20% loss to follow‐up. Competing interests: the authors declared no competing interests for this trial. Withdrawals: 6/199 (3%) in the colchicine group and 10/200 (5%) in the naproxen group. No reasons for withdrawal given. Total number of participants reporting adverse events*
*The corresponding author was contacted via email requesting unpublished data for number of participants who experienced "any side effects" over the 4‐week follow‐up period in this trial. These data (the number of participants who reported "any side effect") were provided and are reported as total adverse events. The type of adverse events that participants reported include nausea and/or vomiting, dyspepsia, abdominal pain, headache, constipation, diarrhoea, skin rash and "other" (free nominated text). Serious adverse events
None of the three serious adverse events outlined above were related to study interventions, and there were no deaths. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated 1:1 to naproxen or low‐dose colchicine groups using simple randomisation, which was undertaken by a healthcare professional using web access to a secure remote allocation system or, if this could not be accessed, a telephone randomisation service. |
| Allocation concealment (selection bias) | Low risk | Clinicians and participants did not know which treatment a participant would receive prior to randomisation, ensuring allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The GP prescribed the allocated medication. Participants and treating clinicians were aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (pain, treatment success, reduction of inflammation, function, serious adverse events, total adverse events, withdrawals due to adverse events) | High risk | This trial had an open‐label design without blinded outcome assessment or placebo. Baseline data were collected by self‐complete questionnaire prior to randomisation. Outcome measures were collected by self‐completed daily diary (days 1–7) and a questionnaire at week 4. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes (reduction of inflammation, function) | Low risk | No assessor reported outcomes were measured in this trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 399 eligible participants were randomised into naproxen group (n = 200), and low‐dose colchicine group (n = 199). ITT was used for the main analysis, evaluating participants as per allocation assignment. |
| Selective reporting (reporting bias) | Low risk | This trial was prospectively registered with ClinicalTrials.gov (NCT01994226) and ISRCTN registry (ISRCTN69836939). The registered primary and secondary outcomes in the ISRCTN registry match the collected outcomes data of the trial, except for the secondary outcomes time‐to‐treatment effect and complete pain resolution, which were added to the trial using data that was collected for other outcome measures. All measured outcomes from baseline to week 4 follow‐up reported descriptive statistics (mean, SD and number of participants) for continuous outcomes, and the number of events and number of participants for dichotomous outcomes. Days to complete pain resolution and number of days taken off work because of gout during 4‐week follow‐up were reported as median (IQR). |
| Other bias | Low risk | No other potential bias detected. |
Terkeltaub 2010.
| Study characteristics | ||
| Methods |
Study design: double‐blind, three‐arm parallel placebo controlled, randomised trial Setting: multicentre (54 centres in the USA) Time period: between April 2007 and October 2008 Intervention: low‐dose colchicine (1.2 mg followed by 0.6 mg in 1 hour followed by placebo doses every hour for 5 hours) versus high‐dose colchicine (1.2 mg followed by 0.6 mg every hour for 6 hours) versus placebo (2 placebo capsules initially, followed by 1 placebo capsule every hour for 6 hours) Sample size: not described Analysis: ITT analysis planned |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics (provided by 185 participants with a gout flare only) High‐dose colchicine group (n = 52)
Low‐dose colchicine group (n = 74)
Placebo group (n = 59)
Groups were similar at baseline. |
|
| Interventions |
Intervention group 1 (low‐dose colchicine)
Intervention group 2 (high‐dose colchicine)
Control group (placebo)
Co‐intervention
|
|
| Outcomes | Outcomes recorded by participants at prespecified time points for pain using a standardised diary: pain was recorded at baseline, hourly for the first 8 hours and every 8 hours thereafter (while awake) until 72 hours following the initial dose or symptom resolution; recording at 24 hours mandatory. Study physicians at each site assessed participants within 48 hours after onset of symptoms and up to 3 more visits, the last being 7 days after flare onset (they reviewed the participant diaries and recorded pertinent data on standardised case report forms). Primary outcome
Secondary outcomes
Outcomes used in this review
Time points used in this review
|
|
| Source of funding | The trial was sponsored by URL Pharma. The Chief Medical Officer for URL Pharma had key roles in the study design data collection, data analysis and writing of the manuscript. Prior to the start of the trial, URL Pharma agreed that the authors had full rights to submit the manuscript for publication; URL Pharma approval of the content of the submitted manuscript was not required, and publication of the manuscript was not contingent upon the approval of URL Pharma. | |
| Notes |
Trial registration: this trial was registered at ClinicalTrials.gov (NCT00506883) on 23 July 2007. Withdrawals: 1 participant in the placebo group withdrew without recording any data ("non‐responder") and was not included in the ITT analysis. The number of participants in each group included as the "safety population" was not equal due to the method of inclusion in the trial (many participants were randomised but only those who had a gout flare were included). Only a responder analysis was presented ‐ mean (SD) pain scores at each time point were not presented. Competing interests: "Dr. Terkeltaub has received consulting fees from Altus, Ardea, BioCryst, Novartis, Pfizer, Procter & Gamble, Regeneron, Savient, EnzymeRx, Takeda, URL Pharma, and UCB (less than $10,000 each) and has received Research Service grants from the VA (more than $10,000). Dr. Furst has received consulting fees from Abbott, Actelion, Amgen, Bristol‐Myers Squibb, Biogen Idec, Centocor, Gilead, Genentech, GlaxoSmithKline, Merck, Nitec, Novartis, UCB, Wyeth, and Xoma (less than $10,000 each), speaking fees from Abbott, Actelion, and UCB (less than $10,000 each), and honoraria from Abbott, Actelion, Amgen, Bristol‐Myers Squibb, Biogen Idec, Centocor, Genentech, Gilead, Merck, and Nitec (less than $10,000 each); he has received grants from Abbott, Actelion, Amgen, Bristol‐ Myers Squibb, Genentech, Gilead, GlaxoSmithKline, the NIH, Nitec, Novartis, Roche, UCB, Wyeth, and Xoma. Salamandra, LLC (employer of Drs. Bennett and Kook) is a regulatory and clinical consulting firm contracted by URL Pharma. D.A.T.A. Inc. (employer of Dr. Crockett) is a contract statistics company retained by United Bio‐ source (a contract research organization) to provide statistical services for this clinical trial. Dr. Davis owns stock options in URL Pharma, and he holds 2 patents pertaining to the dosing of colchicine with clarithromycin." Total adverse events
Serious adverse events
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not described |
| Allocation concealment (selection bias) | Low risk | At the randomisation visit the investigator dispensed a blister card containing 8 identical‐looking capsules (in a combination of active drug and placebo capsules) for use during their next gout flare. Quote: "over encapsulated (to preserve double‐blindedness) colchicine and matching over encapsulated placebo were provided" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both participants and study personnel were blinded to treatment |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (pain, treatment success, reduction of inflammation, function, serious adverse events, total adverse events, withdrawals due to adverse events) | Low risk | The participant assessed pain, symptoms, adverse events and rescue medication by using a standardised diary; and were unaware of treatment. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes (reduction of inflammation, function) | Low risk | The study physicians at each site were blinded to treatment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/52 (13.5%) in the high‐dose colchicine group, 3/74 (4%) in the low‐dose colchicine group and 4/59 (6.8%) in the placebo group did not complete full follow‐up, either because of lack of benefit, loss to follow‐up or for other unclear reasons (ClinicalTrials.gov: NCT00506883 on 23 July 2007) |
| Selective reporting (reporting bias) | Unclear risk | This trial was registered 23 July 2007 but recruitment began in April 2007 and final data collection occurred in October 2007. For benefit only a responders analysis was presented. The mean (SD) pain scores were not presented. The methods also state that symptoms were collected but this is not reported. |
| Other bias | Unclear risk | Site of flare and number of joints affected were not presented. If baseline differences existed this may have affected the results. The numbers of participants by group treated at each site was not specified but it is stated that some sites did not have participants in all treatment groups. While the number of randomised participants in each group was approximately equal, the numbers of participants in each group who had an acute flare of gout and therefore had outcome data available was not equal. |
Wang 2014.
| Study characteristics | ||
| Methods |
Study design: double‐blind, double‐dummy, non‐inferiority randomised controlled trial Setting: Affiliated Hospital of Qingdao University Medical College Time period: between 1 September 2011 and 30 September 2012 Intervention: Chuanhu anti‐gout mixture (a Chinese traditional herbal compound) versus colchicine Sample size: to test whether the Chuanhu anti‐gout mixture 250 mL orally daily was inferior to colchicine for the treatment of acute gouty arthritis, a prespecified non‐inferiority margin of 15% was used. A sample size of 77 participants per treatment group was needed to reach a power of 80% and a one‐sided type I error of 5%. Analysis: per‐protocol and ITT analysis were both used in the trial. |
|
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics Chuanhu group (n=88)
Colchicine group (n=88)
Pre‐treatment group differences: none |
|
| Interventions |
Intervention (low‐dose colchicine)
Control (Chuanhu anti‐gout mixture)
Co‐interventions
|
|
| Outcomes | Outcomes were measured at 3 months Primary outcome
Secondary outcome
Adverse events ‐ pain, swelling, and activity limitation of the joints (measured on a 1‐4 point scale, for absent, mild, moderate, or severe, respectively) were recorded in the participant diaries, and biochemical indicators were detected. Outcomes included in this review
Time points included in this review
|
|
| Source of funding | The trial was supported by the Affiliated Hospital of Qingdao University Medical College. The sponsor of the trial had a role in the study design, but not in data collection, data analysis and data interpretation, or writing of the report. | |
| Notes |
Trial registration: the trial was registered retrospectively at ISRCTN.org (ISRCTN65219941). Withdrawals: 3/88 (3%) in the Chuanhu group discontinued intervention (diarrhoea = 1, unable to tolerate bitter taste = 1, blinding was broken = 1) and 9/88 (10%) in the colchicine group discontinued intervention (severe diarrhoea = 6, liver damage = 2, poor compliance = 1) Total adverse events
The corresponding author was contacted via email requesting unpublished data for number of participants who experienced "any side effects" over the 4‐week follow‐up period in this trial. These data (the number of participants who reported "any side effect") were provided and are reported as adverse events. The type of adverse events that participants reported include nausea and/or vomiting, dyspepsia, abdominal pain, headache, constipation, diarrhoea, skin rash and "other" (free nominated text). Serious adverse events
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table was computer‐generated by SAS statistical software. |
| Allocation concealment (selection bias) | Unclear risk | It is unclear whether allocation to groups was adequately concealed or not. Quote: “YG Wang was in charge of the participants’ assignment to interventions”. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The Chuanhu group received a 250 mL oral liquid Chuanhu anti‐gout mixture and placebo tablets that mimicked colchicine, and the low‐dose colchicine group received colchicine tablets as per study design and a placebo oral liquid that mimicked the Chuanhu anti‐gout mixture. There is low risk of performance bias due to participants and study personnel being blinded to treatment allocation. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes (pain, treatment success, reduction of inflammation, function, serious adverse events, total adverse events, withdrawals due to adverse events) | Low risk | Participants were blinded from knowing whether they received low‐dose colchicine or Chuanhu anti‐gout mixture. |
| Blinding of outcome assessment (detection bias) Assessor‐reported outcomes (reduction of inflammation, function) | Low risk | Data collectors were blinded to group allocations hence there is a low risk of bias in the measurement of study outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/88 (3%) (diarrhoea = 1, bitter taste = 1, broken blinding = 1) in the Chuanhu group and 9/88 (10%) (diarrhoea = 6, liver damage = 2, poor compliance = 1) in the colchicine group. ITT was performed in both groups. |
| Selective reporting (reporting bias) | Low risk | Trial was registered retrospectively at ISRCTN.org (ISRCTN65219941). All outcomes published in the journal were included in the trial registration without modification. |
| Other bias | Low risk | None apparent. |
ACR: American College of Rheumatology BMI: body mass index CI: confidence interval GP: general practitioner IQR: interquartile range ITT: intention‐to‐treat MCP: metacarpophalangeal joint MTP: metatarsophalangeal joint NRS: numeric rating scale NSAID: non‐steroidal anti‐inflammatory drug PIP: proximal interphalangeal joint SD: standard deviation VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blank 2010 | Ineligible study design; not RCT |
| ChiCTR1800020315 | Ineligible participants; did not include participants with acute gout |
| Hill 2013 | Ineligible intervention; did not include colchicine as the intervention |
| Karimzadeh 2006 | Ineligible participants; did not include participants with acute gout |
| Liu 2015 | Ineligible intervention; did not include colchicine as the intervention |
| Moon 2011 | Ineligible study design; not RCT |
| Paulus 1974 | Ineligible participants; did not include participants with acute gout |
| Rapado 1975 | Ineligible intervention; did not include colchicine as the intervention |
| Schlesinger 2002 | Ineligible intervention ‐ both groups treated with similar doses of prednisolone and colchicine; did not include colchicine as the intervention |
| Schlesinger 2011 | Ineligible participants; did not include participants with acute gout |
| Yamanaka 2018 | Ineligible participants; did not include participants with acute gout |
| Yang 2018 | Ineligible intervention; did not include colchicine as the intervention |
Characteristics of studies awaiting classification [ordered by study ID]
Wu 2014.
| Methods |
Design: parallel two‐arm randomised controlled trial Setting: China Interventions: acupoint therapy plus half‐dose colchicine versus usual dose colchicine Sample size: sample size calculation not provided Analysis: ITT |
| Participants |
Number of participants
Inclusion criteria
Exclusion criteria
Baseline characteristics
|
| Interventions |
Intervention ‐ acupoint therapy plus half‐dose colchicine
Control ‐ usual dose colchicine
Co‐interventions
|
| Outcomes |
Study outcomes
|
| Notes |
Trial registration: not reported Withdrawals: none Adverse events
|
ITT: intention‐to‐treat
Characteristics of ongoing studies [ordered by study ID]
TCTR20180608001.
| Study name | Comparing efficacy of loading and non‐loading colchicine in acute crystal induced arthritis |
| Methods | Randomised controlled trial |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions | Study arm 1: 2 tablets of colchicine (0.6 mg) taken orally at 0 hour and 1 tablet of colchicine (0.6 mg) taken orally at 1 hour Study arm 2: 1 tablet of colchicine (0.6 mg) taken orally at 0 hour |
| Outcomes |
Primary outcome
Secondary outcome
|
| Starting date | Date of registration: 7 June 2018 |
| Contact information | Ployrung Vechpanich 110 Intawarorod rd T.Sriphume A.Muang Chiang Mai 50200 Thailand Email: gemmyrainbow@hotmail.com |
| Notes | Estimated trial completion date: 31 January 2019 No source of funding reported |
ACR/EULAR: American College of Rheumatology/European Alliance of Associations for Rheumatology CPPD: calcium pyrophosphate dihydrate eGFR: estimated glomerular filtration rate VAS: visual analogue scale
Differences between protocol and review
We modified the PICO to include any type of comparator. We created an additional subheading 'complementary and alternative medicines' and removed 'non‐pharmacological treatment'. We replaced comparator group subheadings for 'paracetamol' and 'interleukin (IL‐1) inhibitors' with 'other pharmacological treatment'. We added 'no treatment' to the placebo subheading to create 'placebo or no treatment'.
We changed the major outcomes to 'pain', 'participant global assessment of treatment success', 'reduction of inflammation', 'function of target joint', 'serious adverse events', 'total adverse events' and 'withdrawals due to adverse events' to align with the core outcome domains established by the OMERACT Gout Special Interest Group for gout studies (Araújo 2015; Schumacher 2009).
Contributions of authors
BM and RB wrote the updated review; RB and RJ screened initial search results and identified studies that fulfilled the inclusion criteria; BM and RJ extracted data and entered data into Review Manager 5; and BM and RJ performed risk of bias assessment. MW and NS contributed to the revisions of this manuscript. All authors contributed to the interpretation of the review and approved the final draft.
Sources of support
Internal sources
No sources of support provided
External sources
-
National Health and Medical Research Council (NHMRC), Australia
Funding for Cochrane Musculoskeletal ‐ Australia and Cochrane Australia
Declarations of interest
Bayden J McKenzie has no declarations of interest.
Mihir D Wechalekar has no declaration of interest for this review; he has received research grants for unrelated work in a different disease area from Janssen Research (Philadelphia, USA).
Renea V Johnston is the Managing Editor of Cochrane Musculoskeletal, but was not involved in editorial decisions regarding this review. She is a recipient of a NHMRC (Australia) Cochrane Collaboration Round 7 Funding Program Grant, which supports the Cochrane Musculoskeletal Australian Editorial base, but the funders do not participate in the conduct of this review. RJ has no declarations of interest.
Naomi Schlesinger has no declaration of interest for this review; she has a research grant from AMGEN and has received honorarium from Horizon pharmaceutics.
Rachelle Buchbinder is the Co‐ordinating Editor of Cochrane Musculoskeletal, but was not involved in editorial decisions regarding this review. She is a recipient of a National Health and Medical Research Council (NHMRC) Cochrane Collaboration Round 7 Funding Program Grant, which supports the activities of Cochrane Musculoskeletal Australia and Cochrane Australia, but the funders do not participate in the conduct of reviews. She has no declarations of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Ahern 1987 {published data only}
- Ahern MJ, Reid C, Gordon TP. Does colchicine work? Results of the first controlled study in gout. Australian and New Zealand Journal of Medicine 1987;17:301-4. [DOI] [PubMed] [Google Scholar]
Roddy 2020 {published data only}69836939
- Roddy E, Clarkson K, Blagojevic-Bucknall M, Mehta R, Oppong R, Avery A, et al. Open-label randomised pragmatic trial (CONTACT) comparing naproxen and low-dose colchicine for the treatment of gout flares in primary care. Annals of the Rheumatic Diseases 2020;79:276-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Terkeltaub 2010 {published data only}
- Furst D, Maranian E, Davis P, Wason MW, Khanna S, Terkeltaub D. Chronologic age, renal function, and comorbid conditions (physiologic age) of patients with gout did not increase likelihood of adverse events (AEs): AGREE study post hoc analyses. Arthritis and Rheumatism 2009;62(Suppl 10):147. [Google Scholar]
- Terkeltaub R, Furst D, Bennett E, Kook K, Crockett K, Davis RS, et al. Colchicine efficacy assessed by time to 50 reduction of pain is comparable in low dose and high dose regimens: secondary analyses of the agree trial. Arthritis and Rheumatism 2010;60(Suppl 10):1103. [Google Scholar]
- Terkeltaub RA, Furst DE, Bennett K, Kook KA, Crockett RS, Davis MW. High versus low dosing of oral colchicine for early acute gout flare: twenty-four-hour outcome of the first multicenter, randomised, double-blind, placebo-controlled, parallel-group, dose-comparison colchicine study. Arthritis and Rheumatism 2010;62(4):1060-8. [DOI] [PubMed] [Google Scholar]
- Wason S, Lauterio T, Crockett S, Davis, M. Colchicine, as assessed by target joint pain scores, is effective at 16 hours in patients with acute gout flares. Arthritis and Rheumatism 2012;64(Suppl 10):S812. [Google Scholar]
Wang 2014 {published data only}
- Wang Y, Wang L, Li E, Li Y, Wang Z, Sun X, et al. Chuanhu anti-gout mixture versus colchicine for acute gouty arthritis: a randomized, double-blind, double-dummy, non-inferiority trial. International Journal of Medical Sciences 2014;11(9):880-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Blank 2010 {published data only}
- Blank N. Acute gouty arthropathy: treatment of acute gout attack with colchicine. Medizinische Klinik 2010;105(9):669-70. [Google Scholar]
ChiCTR1800020315 {published data only}
- ChiCTR1800020315. Study for the effectiveness of different courses of low-dose colchicine for preventing acute flares during urate lowering therapy by febuxostat. www.chictr.org.cn/showproj.aspx?proj=32164 (first received 23 December 2018).
Hill 2013 {published data only}
- Hill E, Higgs JB, Sky K, Sit M, Collamer AN. Does starting allopurinol prolong acute treated gout? Arthritis and Rheumatism 2013;65(Suppl 10):S730. [DOI] [PubMed] [Google Scholar]
Karimzadeh 2006 {published data only}
- Karimzadeh H, Nazari J, Mottaghi P, Kabiri P. Different duration of colchicine for preventing recurrence of gouty arthritis. Journal of Research in Medical Sciences 2006;11(2):104-7. [Google Scholar]
Liu 2015 {published data only}
- Liu Y, Li Z-C, Chen J-B, Yu T-T, Cui C, Zhang H-B. Therapeutic efficacy of small doses of colchicine combined with glucocorticoid for acute gouty arthritis. People's Military Medical Press 2015;40(8):652. [Google Scholar]
Moon 2011 {published data only}
- Moon KT. Low-dose colchicine effective for acute gout flare-ups. American Family Physician 2011;83(3):316. [Google Scholar]
Paulus 1974 {published data only}
- Paulus HE, Schlosstein LH, Godfrey RG, Klinenberg JR, Bluestone R. Prophylactic colchicine therapy of intercritical gout. A placebo controlled study of probenecid treated patients. Arthritis and Rheumatism 1974;17(5):609-14. [DOI] [PubMed] [Google Scholar]
Rapado 1975 {published data only}
- Rapado A. Influence of atmospheric pressure on acute attacks of gout: negative findings. Revista Espanola de Reumatologia 1975;2(4):188-93. [Google Scholar]
Schlesinger 2002 {published data only}
- Schlesinger N, Baker DG, Beutler AM, Hoffman BI, Schumacher Hr Jr. Local ice therapy during bouts of acute gouty arthritis. Journal of Rheumatology 2002;29(2):331-4. [PubMed] [Google Scholar]
Schlesinger 2011 {published data only}
- Schlesinger N, Lin H-Y, De Meulemeester M, Nasonov E, Rovensky J, Arulmani U, et al. Efficacy of canakinumab (ACZ885) in the prevention of flares in gout patients initiating allopurinol therapy. NDT Plus 2010;3:iii3-4.
- Schlesinger N, Mysler E, Lin H, De Meulemeester M, Rovensky J, Arulmani U, et al. Canakinumab reduces the risk of acute gouty arthritis flares during initiation of allopurinol treatment: results of a double-blind, randomised study. Annals of the Rheumatic Diseases 2011;70(7):1264-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yamanaka 2018 {published data only}
- Yamanaka H, Tamaki S, Ide Y, Kim H, Inoue K, Sugimoto M. Stepwise dose increase of febuxostat is comparable with colchicine prophylaxis for the prevention of gout flares during the initial phase of urate-lowering therapy: results from FORTUNE-1, a prospective, multicentre randomised study. Annals of the Rheumatic Diseases 2018;77(2):270-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2018 {published data only}
- Yang D-H, Chen H-C, Wei JC-C. Early lowering of serum uric acid levels in the treatment of gouty arthritis with an acute attack. International Journal of Rheumatic Diseases 2018;21(Suppl 1):218. [Google Scholar]
References to studies awaiting assessment
Wu 2014 {published data only}
- Wu YQ, Hu JH, Zhao ZY, Zhou C, Yu X, Zheng Y, et al. Clinical observation on senile patients with acute gouty arthritis treated by acupoint application. Journal of the American Geriatrics Society 2014;62(Suppl 2):S381. [Google Scholar]
References to ongoing studies
TCTR20180608001 {published data only}
- TCTR20180608001. Comparing efficacy of loading and non-loading colchicine in acute crystal induced arthritis. www.thaiclinicaltrials.org 7 June 2018.
Additional references
Agudelo 1972
- Agudelo CA, Schumacher HR Jr, Phelps P. Effect of exercise on urate crystal-induced inflammation in canine joints. Arthritis and Rheumatism 1972;15:609-16. [DOI] [PubMed] [Google Scholar]
Araújo 2015
- Araújo F, Cordeiro I, Ramiro S, Falzon L, Branco JC, Buchbinder R. Outcomes assessed in trials of gout and accordance with OMERACT-proposed domains: a systematic literature review. Rheumatology 2015;54(6):981-93. [DOI] [PubMed] [Google Scholar]
Arnold 1988
- Arnold MH, Preston SJ, Buchanan WW. Comparison of the natural history of untreated acute gouty arthritis vs acute gouty arthritis treated with non-steroidal-anti-inflammatory drugs. British Journal of Clinical Pharmacology 1988;26:488-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Australian Rheumatology Association 2019
- Australian Rheumatology Association. Patient information on colchicine (last updated April 2019). rheumatology.org.au/patients/documents/Colchicine_2019v2.pdf (accessed 16 November 2020).
Bellamy 1987
- Bellamy N, Downie WW, Buchanan WW. Observations on spontaneous improvement in patients with podagra: implications for therapeutic trials of non-steroidal anti-inflammatory drugs. British Journal of Clinical Pharmacology 1987;24(1):33-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ben‐Chetrit 1998
- Ben-Chetrit E, Levy M. Colchicine: 1998 update. Seminars in Arthritis and Rheumatism 1998;28:48-59. [DOI] [PubMed] [Google Scholar]
Boutron 2008
- Boutron I, Moher D, Altman DG, Schulz K, Ravaud P, CONSORT Group. Methods and processes of the CONSORT Group: example of an extension for trials assessing nonpharmacologic treatments. Annals of Internal Medicine 2008;148:295-309. [DOI] [PubMed] [Google Scholar]
Cates 2008 [Computer program]
- Visual Rx. Version 3. Dr. Chris Cates' EBM website, 2008. www.nntonline.net.
Covidence 2021 [Computer program]
- Veritas Health Innovation Covidence. Melbourne, Australia: Veritas Health Innovation, accessed 27 August 2020. Available at covidence.org.
Cox 2004
- Cox AR. Colchicine in acute gout: optimal dose of colchicine is still elusive. BMJ 2004;328(7434):288. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dalbeth 2014
- Dalbeth N, Zhong CS, Grainger R, Khanna D, Khanna PP, Singh JA, et al. Outcome measures in acute gout: a systematic literature review. Journal of Rheumatology 2014;41(3):558-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Angelis 2004
- De Angelis CD, Drazen JM, Frizelle FA, Haug C, Hoey J, Horton R, et al. Clinical Trial Registration: a statement from the International Committee of Medical Journal Editors. JAMA 2004;292(11):1363-4. [DOI] [PubMed] [Google Scholar]
Deeks 2021
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Dorwart 1974
- Dorwart BB, Hansell JR, Schumacher HR Jr. Effects of cold and heat on urate-induced synovitis in the dog. Arthritis and Rheumatism 1974;17(5):563-71. [DOI] [PubMed] [Google Scholar]
FDA 2008
- Food and Drug Administration. Drug products containing colchicine for injection; enforcement action dates. Federal Register (updated February 2008). Department of Health and Human Services, 2008. Available at www.federalregister.gov/documents/2008/02/08/08-564/drug-products-containing-colchicine-for-injection-enforcement-action-dates.
FitzGerald 2020
- FitzGerald JD, Dalbeth N, Mikuls T, Brignardello-Petersen R, Guyatt G, Abeles AM, et al. 2020 American College of Rheumatology Guideline for the management of gout. Arthritis Care Research 2020;0(0):1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro 2015 [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Version accessed 8 September 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Hak 2010
- Hak AE, Curhan GC, Grodstein F, Choi HK. Menopause, postmenopausal hormone use and risk of incident gout. Annals of the Rheumatic Diseases 2010;69(7):1305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Higgins 2021b
- Higgins JP, Eldridge S. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Higgins 2021c
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Hill 1965
- Hill AB. The environment and disease: association or causation? Proceedings of the Royal Society of Medicine 1965;58(5):295-300. [PMC free article] [PubMed] [Google Scholar]
Hui 2017
- Hui M, Carr A, Cameron S, Davenport G, Doherty M, Forrester H, et al, British Society for Rheumatology Standards, Audit and Guidelines Working Group. The British Society for Rheumatology Guideline for the management of gout. Rheumatology 2017;56:e1-e20. [DOI] [PubMed] [Google Scholar]
Janssens 2008a
- Janssens HJ, Janssen M, Van de Lisdonk EH, Van Riel PL, Van Weel C. Use of oral prednisolone or naproxen for the treatment of gout arthritis: a double-blind, randomised equivalence trial. Lancet 2008;371:1854-60. [DOI] [PubMed] [Google Scholar]
Janssens 2008b
- Janssens HJ, Lucassen PL, Van de Laar FA, Janssen M, Van de Lisdonk EH. Systemic corticosteroids for acute gout. Cochrane Database of Systematic Reviews 2008, Issue 2. Art. No: CD005521. [DOI: 10.1002/14651858.CD005521.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kasper 2005
- Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson DL, et al. Harrison's Principles of Internal Medicine. 16th edition. McGraw-Hill Professional, 2005. [Google Scholar]
Kuncl 1987
- Kuncl R, Duncan G, Watson D, Alderson K, Rogawski MA, Peper M. Colchicine myopathy and neuropathy. New England Journal of Medicine 1987;316(25):1562-8. [DOI] [PubMed] [Google Scholar]
Mikuls 2005
- Mikuls TR, Farrar JT, Fernandes S, Schumacher HR Jr, Saag KG. Gout epidemiology: results from the UK General Practice Research Database, 1990-1999. Annals of the Rheumatic Diseases 2005;64:267-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moi 2013
- Moi JH, Sriranganathan MK, Edwards CJ, Buchbinder R. Lifestyle interventions for chronic gout. Cochrane Database of Systematic Reviews 2013, Issue 5. Art. No: CD010039. [DOI: 10.1002/14651858.CD010039.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2003
- Morris I, Varughese G, Mattingly P. Colchicine in acute gout. BMJ 2003;327(7426):1275-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Sullivan 1972
- O'Sullivan JB. Gout in a New England Town. A prevalence study in Sudbury, Massachusetts. Annals of the Rheumatic Diseases 1972;31(3):166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Richette 2017
- Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castañeda-Sanabria J, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Annals of the Rheumatic Diseases 2017;76:29-42. [DOI] [PubMed] [Google Scholar]
Schlesinger 2001
- Schlesinger N, Schumacher HR Jr. Gout: can management be improved? Current Opinion in Rheumatology 2001;13(3):240-4. [DOI] [PubMed] [Google Scholar]
Schlesinger 2004
- Schlesinger N. Management of acute and chronic gouty arthritis: present state-of-the-art. Drugs 2004;64(21):2399-416. [DOI] [PubMed] [Google Scholar]
Schumacher 1993
- Schumacher H, Klippel J, Koopman W. Primer on the Rheumatic Diseases. 10th edition. Arthritis Foundation, 1993. [Google Scholar]
Schumacher 2005
- Schumacher HR, Edwards LN, Perez-Ruiz F, Becker M, Chen LX, Furst DE. Outcome measures for acute and chronic gout. Journal of Rheumatology 2005;32(12):2452-5. [PubMed] [Google Scholar]
Schumacher 2009
- Schumacher HR, Taylor W, Edwards L, Grainger R, Schlesinger N, Dalbeth N, et al. Outcome domains for studies of acute and chronic gout. Journal of Rheumatology 2009;36(10):2342-5. [DOI] [PubMed] [Google Scholar]
Schünemann 2021
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.2 (updated February 2021). Cochrane, 2021. Available from training.cochrane.org/handbook.
Shekelle 2017
- Shekelle PG, Newberry SJ, FitzGerald JD, Motala A, O’Hanlon CE, Tariq A, et al. Management of gout: A systematic review in support of an American College of Physicians Clinical Practice Guideline. Annals of Internal Medicine 2017;166:37-51. [DOI: 10.7326/M16-0461] [DOI] [PubMed] [Google Scholar]
Singh 2011
- Singh JA, Taylor WJ, Simon LS, Khanna PP, Stamp LK, McQueen FM, et al. Patient-reported outcomes in chronic gout: a report from OMERACT 10. Journal of Rheumatology 2011;38:1452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2014
- Singh JA, Taylor WJ, Dalbeth N, Simon LS, Sundy J, Grainger R, et al. OMERACT endorsement of measures of outcome for studies of acute gout. Journal of Rheumatology 2014;41(3):569-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sivagnanam 2004
- Sivagnanam G. Colchicine in acute gout: low dose colchicine was started after usual dose. BMJ 2004;328(7434):288-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2020
- Stewart S, Yang KC, Atkins K, Dalbeth N, Robinson PC. Adverse events during oral colchicine use: a systematic review and meta-analysis of randomised controlled trials. Arthritis Research & Therapy 2020;22:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tugwell 2004
- Tugwell P, Shea B, Boers M, Brooks P, Simon LS, Strand V, et al. Evidence-Based Rheumatology. BMJ Books, 2004. [Google Scholar]
van der Heijde 2014
- Sivera F, Andrés M, Carmona L, Kydd AS, Moi J, Seth R, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systematic literature review and expert opinion of abroad panel of rheumatologists in the 3e initiative. Annals of the Rheumatic Diseases 2014;73:328–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wallace 1977
- Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu T-F. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis and Rheumatism 1977;20:895-900. [DOI] [PubMed] [Google Scholar]
Wallace 1998
- Wallace SL, Singer JZ. Therapy in gout. Rheumatic Diseases Clinics of North America 1988;14:441-57. [PubMed] [Google Scholar]
Wechalekar 2013
- Wechalekar MD, Vinik O, Schlesinger N, Buchbinder R. Intra-articular glucocorticoids for acute gout. Cochrane Database of Systematic Reviews 2013, Issue 4. Art. No: CD009920. [DOI: 10.1002/14651858.CD009920.pub2] [DOI] [PubMed] [Google Scholar]
Wortmann 2002
- Wortmann RL. Gout and hyperuricemia. Current Opinion in Rheumatology 2002;14:281-6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Schlesinger 1997
- Schlesinger N, Baker DDB, Schumacher R. Interventions for hyperuricemia gout.. Cochrane Database of Systematic Reviews 1997, Issue 3. Art. No: CD000462. [DOI: 10.1002/14651858.CD000462.pub2] [DOI] [Google Scholar]
Schlesinger 2006
- Schlesinger N, Schumacher R, Catton M, Maxwell L. Colchicine for acute gout. Cochrane Database of Systematic Reviews 2006, Issue 4. Art. No: CD006190. [DOI: 10.1002/14651858.CD006190] [DOI] [PubMed] [Google Scholar]
van Echteld 2014
- Echteld I, Wechalekar MD, Schlesinger N, Buchbinder R, Aletaha D. Colchicine for acute gout. Cochrane Database of Systematic Reviews 2014, Issue 8. Art. No: CD006190. [DOI: 10.1002/14651858.CD006190.pub2] [DOI] [PubMed] [Google Scholar]


