Abstract
This study aimed to determine pain characteristics in patients with persistent headache after COVID-19 and to investigate the role of increased intracranial pressure (ICP) in the pathogenesis of this headache. This is a case-control study comparing the parameters and measurements indicating increased ICP based on magnetic resonance imaging between COVID-19–diagnosed patients with persistent headache and a control group. Optic nerve sheath diameter (ONSD) and eyeball transverse diameter (ETD) were performed on the left eye of each participant. Seventeen of the patients (53.12%) met the diagnostic criteria for new daily persistent headache. Seven patients (21.87%) had migraine, and eight (25%) had tension headache characteristics. No significant difference was observed between the patient and control groups in terms of the ONSD and ETD values. It is possible that the etiopathogenesis is multifactorial. We consider that future studies that will evaluate ICP measurements in large patient groups can present a different perspective for this subject.
Key Words: COVID-19, headache, intracranial hypertension, optic nerve sheath diameter, magnetic resonance imaging
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, first emerged in a cluster of cases presenting with pneumonia of an unknown cause in Wuhan, China in December 2019 before being recognized as a global pandemic by the World Health Organization on March 11, 2020 (Huang et al., 2020). A large subset of patients with SARS-CoV-2 infection present with neurological symptoms, including headache, dizziness, cerebrovascular disease, seizures, altered consciousness, anosmia, ageusia, visual loss, neuropathic pain, Guillain-Barré syndrome, and skeletal muscle involvement (Altunisik et al., 2021; Mao et al., 2020). Although many prevalence studies exist in the literature concerning the headache observed in COVID-19 patients, information on the characteristics and etiopathogenesis of this headache remains limited. Conflicting results from prevalence studies have been obtained; some suggested the prevalence of new-onset headache during the acute phase of infection to be 59%, which is high relative to that observed in concert with other viral infections (Megemont et al., 2020). The prevalence of headache has been reported to be high, especially in patients with mild and moderate SARS-CoV-2 infections (Wu and McGoogan, 2020). In a case series evaluating the clinical manifestations of COVID-19 in China, the frequency of headache ranged from 8% to 23% (Mao et al., 2020). Based on clinical observations and a small case series, the headache associated with COVID-19 is described as acute and was reported to often have different characteristics relative to prior headaches (Toptan et al., 2020).
Headache associated with systemic viral infections has been included in the third edition of the International Classification of Headache Disorders (ICHD-3). Headache attributed to systemic viral infection is diagnosed if there is an association between the onset of pain and systemic viral infection in the absence of encephalitic or meningitic signs. In this context, the underlying mechanism remains unclear, but it has been defined as moderate or severe diffuse headache that may be accompanied by fever (Headache Classification Committee of the International Headache Society, 2018). Headache is a very frequently reported symptom among patients with COVID-19, and recently, cases of persistent headache have been reported even weeks after recovery from the disease. This type of headache meets the diagnostic criteria for new daily persistent headache (NDPH) and has been reported to have holocranial, pressure-like pain characteristics that start within 2 weeks after SARS-CoV-2 infection, especially in outpatients without a preexisting primary headache diagnosis (Liu et al., 2020). In a previous study, 37.8% of patients who experienced headache in the acute phase of their SARS-CoV-2 infection were reported to develop persistent headache, and 60.7% of these patients met the NDPH criteria (Caronna et al., 2020). The pathophysiology of NDPH is not yet clearly known, but it is thought that pain may arise from cytokine production and persistent glial activation (Yamani and Olesen, 2019). To the best of our knowledge, only a limited number of comprehensive studies reviewing persistent symptoms, such as headache, after the acute stage of COVID-19 exist.
Although brain imaging has previously been used for the exclusion of other pathologies that may cause increased cerebrospinal fluid (CSF) pressure, some of the findings of modern neuroimaging methods are now included in the diagnostic criteria of intracranial hypertension (Friedman et al., 2013). Many parameters that can be evaluated by magnetic resonance imaging (MRI) of the brain can provide clues concerning increased intracranial pressure (ICP). Among these measurements, the optic nerve sheath diameter (ONSD) and eyeball transverse diameter (ETD) are considered the main parameters of interest. The optic nerve sheath anatomically continues with the dura mater and has a trabecular arachnoid space through which CSF slowly drains (Ossoinig, 1979). The optic nerve and the surrounding sheath can be visualized on MRI using a fat-suppressed T2-weighted sequence (Weigel et al., 2006).
The detection of an increased ONSD has become a valued method to indicate increased ICP in the last 15 years. The principle underlying this analysis is the connection of the subarachnoid space under the optic nerve sheath with the cerebral CSF spaces and its anatomical expansion due to increased ICP (Rohr et al., 2010). The calculation of ONSD is a useful and noninvasive measure of ICP, and an enlarged ONSD has been accepted as an indisputable imaging marker suggesting increased ICP (Zheng et al., 2020). In a study of patients with traumatic brain injury, a significant relationship was found between ONSD as measured by MRI and ICP confirmed with an invasive method. In addition, ONSD has been shown to be increased in patients with idiopathic intracranial hypertension and decreased in patients with intracranial hypotension, respectively (Kimberly and Noble, 2008). ONSD can also be measured on ultrasound, but it is known that MRI provides more precise measurements (Lagreze et al., 2007). It has also been reported that there is a strong correlation between ETD and ONSD in MRI (Kim et al., 2018).
In addition to ONSD, other MRI markers that suggest an increase in ICP include the buckling of the optic nerve, perioptic nerve sheath distention, flattening or anterior bulging of the posterior sclera, partial or total empty sella, Meckel cave enlargement, tonsillar herniation, ventricular thinning, and filling defect in the venous sinuses (Bidot et al., 2015). The current study sought to determine the pain characteristics of patients who developed persistent headache after COVID-19 and to investigate the role of intracranial hypertension in the pathogenesis of this headache.
METHODS
This was a case-control study that compared MRI parameters and measurements indicating increased ICP between patients diagnosed with COVID-19 who developed persistent headache and a control group. The patient group included people aged 18 to 60 years who were confirmed to have COVID-19 by the polymerase chain reaction test based on nasopharyngeal swab sampling at the time of displaying the first symptoms of the disease and who presented to the neurology outpatient clinic of our hospital due to persistent headache. Meanwhile, individuals who presented to the neurology outpatient clinic for a different reason (e.g., tinnitus, positional vertigo, or somatization) and did not have a history of COVID-19 and contact with someone with COVID-19 or headache, who underwent a cranial MRI examination, and who were reported to have normal examination findings were selected for the control group. The MRI examinations of all patients were performed at 2 to 12 weeks after their initial diagnosis of COVID-19. Examinations that did not clearly show the optic nerve sheath were not included in this study. Analyses were performed by a radiologist with at least 10 years of professional experience in head and neck radiology. Patients who were diagnosed with intracranial or intraorbital masses; those with previous head trauma, hydrocephalus, idiopathic intracranial hypertension, brain atrophy, thyroid ophthalmopathy, severe myopia, or hyperopia; and those who used medical agents that may alter the ICP (e.g., acetazolamide, furosemide, and steroids) were excluded from this study. Also excluded were those patients who exhibited any clinical or laboratory evidence for meningitis or meningoencephalitis, such as neck stiffness, altered consciousness, and focal neurological findings.
Morphometric measurements, including ONSD and ETD, were collected from the left eye of each study participant. In addition, intracranial hypertension markers, such as buckling of the optic nerve, perioptic nerve sheath distention, flattening or anterior bulging of the posterior sclera, partial or total empty sella, Meckel cave enlargement, tonsillar herniation, ventricular thinning, and filling defect in the venous sinuses, were recorded. Considering that no significant differences in the measurements between the right and left eyes of individuals were found in previous studies (Ballantyne et al., 2002), we only evaluated the left eye in this study. ONSD was measured at a point 3 mm behind the optic disc, The cursors were placed on the outer contour of the optic nerve, perpendicular to its longitudinal axis (Fig. 1), whereas ETD was measured using the longest diameter of the eyeball (Fig. 2). The past headache characteristics of the patients were reviewed, and their headache diagnoses were confirmed according to the ICHD-3 criteria. Neurological examination findings, such as papillary edema, visual field loss, motor deficit, and cranial nerve paralysis (especially abducens), were not detected in any of the included patients. This study was approved by the local ethics committee and conducted in accordance with the principles of the Declaration of Helsinki.
FIGURE 1.
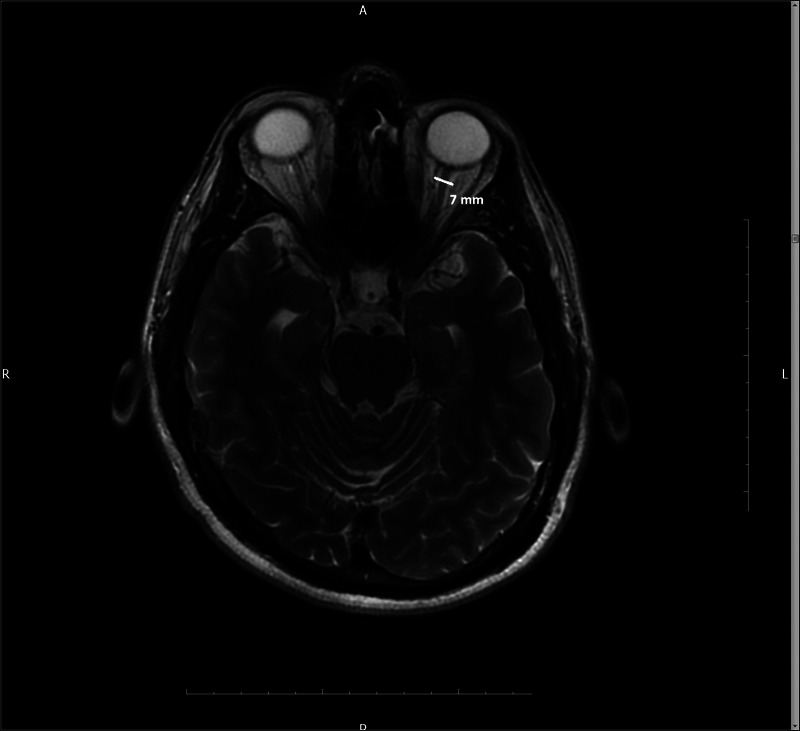
The optic nerve sheath diameter was measured 3 mm behind the optic disc on MRI using a fat-suppressed T2-weighted axial sequence.
FIGURE 2.
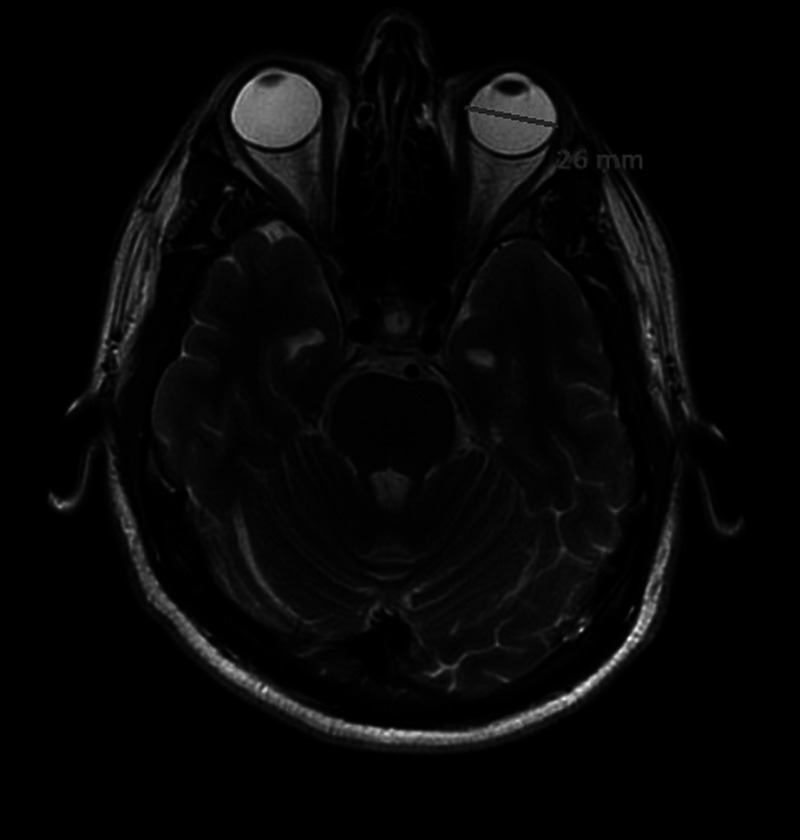
Eyeball transverse diameter was measured from the highest diameter of the eyeball on MRI using a fat-suppressed T2-weighted axial sequence.
MRI Protocol
Brain MRI scans were obtained on a 1.5-T GE Signa scanner (General Electric Medical Systems, Milwaukee, WI) using the standard head coil. Examination sequences were composed of T2-weighted images in the axial plane (TR, 6556 milliseconds; TE, 111.6 milliseconds; field of view, 230 × 230 mm; NSA [number of signal averages], 2; thickness, 5 mm; gap, 1 mm; slices; matrix, 192 × 288 mm) and sagittal T1-weighted images (TR, 490 milliseconds; TE, 4.0 milliseconds; field of view, 240 × 240 mm; NSA, 2; thickness, 6 mm; gap, 1 mm; matrix, 192 × 288 mm).
Statistical Analysis
Categorical data were expressed as numbers and percentages. The Shapiro-Wilk test was used to evaluate the distribution of numerical data. Mean, standard deviation, median, and minimum-maximum (range) values were used when presenting descriptive statistics. The Student's t-test was used for the comparison of numerical data, whereas the chi-square test was used for the comparison of categorical data. Also, the Mann-Whitney U-test was used when comparing nonparametric variables between two groups. Results with a p-value of less than 0.05 were considered to be statistically significant.
RESULTS
A total of 106 people between the ages of 18 and 60 years, including 32 diagnosed with COVID-19 and 74 controls, were included in this study. The patient group contained 19 women (59.4%), and the control group contained 44 women (59.5%). The mean age of the patient group was 39.94 ± 7.59 (range, 24–55) years, and that of the control group was 37.24 ± 8.70 (range, 19–57) years. No statistically significant difference was found between the mean ages of the patient and control groups (p = 0.132). The sociodemographic characteristics of the patient and control groups are summarized in Table 1. Seventeen of the patients (53.12%) met the NDPH diagnostic criteria. Seven patients (21.87%) presented with migraine, and eight (25%) had tension headache (TTH) characteristics. None of the patients had a history of a primary headache diagnosis meeting the NDPH diagnostic criteria in the pre–COVID-19 period. Four patients diagnosed with migraine began to experience migraine attacks after COVID-19, whereas three patients who had previously been diagnosed with migraine reported an increase in the frequency, duration, and/or severity of their migraines after the disease. Of the patients diagnosed with TTH, five were newly diagnosed with headache and three had a history of TTH diagnosis before COVID-19 but reported an increase in the frequency, duration, and/or severity of pain after the disease. There were also reports made of additional neurological symptoms, including loss of smell in 26 patients (81.25%), loss of taste in 14 patients (43.75%), and dizziness in 12 patients (37.5%). Pulmonary involvement was observed in thorax computed tomography during the disease in three patients (9.37%). The clinical characteristics of the patients are summarized in Table 2.
TABLE 1.
Distribution of Sex and Mean Age in the Patient and Control Groups
| Patient | Control | p | ||||
|---|---|---|---|---|---|---|
| n | % | n | % | |||
| Sex | Male | 13 | (40.6) | 30 | (40.5) | 0.946a |
| Female | 19 | (59.4) | 44 | (59.5) | ||
| Age, y | 39.94 ± 7.59 | 37.24 ± 8.70 | 0.132b | |||
n replaced by mean + standard deviation, % replaced by median values.
aChi-square test. bMann-Whitney U-test.
TABLE 2.
Clinical Features of the Patients
| Olfactory dysfunction | 26 (81.25%) |
| Taste disorder | 14 (43.75%) |
| Dizziness | 12 (37.5%) |
| Pulmonary involvement | 3 (9.37%) |
| Migraine | 7 (21.87%) |
| Tension-type headache | 8 (25%) |
| NDPH | 17 (53.12%) |
The mean ONSD values were measured as 5.14 ± 0.75 mm in the patient group and 5.28 ± 0.83 mm in the control group; thus, there was no significant difference between the patient and control groups in terms of ONSD values (p = 0.416). The ETD values were measured as 22.88 ± 0.88 mm in the patient group and 23.16 ± 0.91 mm in the control group, also revealing that no significant difference existed between the two groups (p = 0.136). The patient and control groups were also compared in terms of intracranial hypertension markers, such as buckling of the optic nerve, perioptic nerve sheath distention, flattening or anterior bulging of the posterior sclera, partial or total empty sella, Meckel cave enlargement, tonsillar herniation, thinning of the ventricles, and filling defect in the venous sinuses, and there was no significant difference apparent between the patient and control groups in relation to the frequency of buckling of the optic nerve, perioptic nerve sheath distention, partial or total empty sella, and ventricular thinning. Also, none of the participants in the patient and control groups experienced flattening or anterior bulging of the posterior sclera, enlargement of the Meckel cave enlargement, tonsillar herniation, or filling defect in the venous sinuses. The data of the MRI measurement parameters of the patient and control groups are summarized in Table 3.
TABLE 3.
Comparison of the MRI Findings Between the Patient and Control Groups
| MRI Finding | Patient (n = 32) | Control (n = 74) | p |
|---|---|---|---|
| Optic nerve sheath diameter, mean ± SD | 5.14 ± 0.75 | 5.28 ± 0.83 | 0.416a |
| Eyeball transverse diameter, mean ± SD | 22.88 ± 0.88 | 23.16 ± 0.91 | 0.136a |
| Perioptic nerve sheath distention, n (%) | 7 (21.9%) | 11 (14.9%) | 0.378b |
| Bulking of the optic nerve, n (%) | 6 (18.8%) | 10 (13.5%) | 0.690b |
| Empty sella, n (%) | 10 (31.3%) | 12 (17.6%) | 0.189b |
| Ventricular thinning, n (%) | 10 (31.3%) | 20 (27%) | 0.658b |
aStudent's t-test. bChi-square test.
DISCUSSION
Increasing evidence suggests that neurotropism is a common feature of human coronaviruses. Headache has become a common symptom that can assist physicians in the diagnosis of COVID-19. However, the underlying mechanisms of COVID-19–associated headache remain unclear. In this study, we investigated the hypothesis that reasons other than increased ICP also play a role in persistent headache that develops after COVID-19.
Current knowledge about cerebral hemodynamics, such as changes in ICP in patients with COVID-19, is extremely limited. In a previous study, ultrasonographic OSND measurements and transcranial Doppler ultrasonography were performed in COVID-19 cases followed up within the intensive care unit, and increased ICP values from more than half of the included patients were confirmed. In addition, increased ICP was associated with a longer length of stay in the intensive care unit (Battaglini et al., 2020). However, there are also case reports suggesting the presence of secondary idiopathic intracranial hypertension based on the detection of papilledema, abducens paralysis, and high CSF pressure after lumbar puncture performed due to headache in children followed up with after the diagnosis of multisystem inflammatory syndrome (Baccarella et al., 2021; Verkuil et al., 2020). In a different case report, the opening pressure of CSF was found to be 400 mm/water in a patient who underwent lumbar puncture due to headache and impaired consciousness, and it was noted that there was subarachnoid space that was more apparent around the optic nerves and vertical bulking of the optic nerves on brain MRI scan (Noro et al., 2020). Considering that previous studies were conducted with a wide range of cases presenting with pulmonary involvement, intubation, severe course of COVID-19, and systemic inflammatory response, it does not seem possible to attribute increased ICP to infection with SARS-CoV-2 alone. Instead, carbon dioxide retention and changes in cerebral hemodynamics caused by systemic, metabolic, and inflammatory complications that may develop under intensive care conditions are more conceivable. On the other hand, however, in a study evaluating 13 patients with persistent headache after COVID-19, it was found that the opening pressure of CSF after lumbar puncture was above 250 mm/water in 46% of the included patients (Silva et al., 2020).
In our study, we did not observe any MRI findings suggestive of increased ICP in patients with persistent headache after COVID-19. Current data on increased ICP findings are mostly presented at this time in the form of small series and case reports of patients with a severe disease spectrum; therefore, we can consider that, in addition to increased ICP, there may be other factors with roles in the persistence of headache. In addition, because we did not collect measurements from our participants at the beginning of their disease course, it is also possible that the ICP may have increased in the acute period before returning to normal levels through compensatory mechanisms. None of the patients included in our study had clinical, laboratory, or pulmonary involvement findings indicating a severe disease spectrum nor presented with any indication for hospitalization. From this perspective, we consider that parameters and measurements indicating ICP changes in MRI can contribute to the prediction of the disease course.
Angiotensin-converting enzyme 2 (ACE2), the functional receptor of SARS-CoV-2 in the body, is also expressed by cerebral vascular endothelial cells. Therefore, the presence of ACE2, which causes trigeminovascular sensitivity in the meningeal endothelium, has triggered the suggestion that endotheliitis may be a potential pathophysiological mechanism responsible for COVID-19 headache (Baig et al., 2020). Factors such as the neurotoxic effect of cytokine release syndrome associated with systemic inflammatory response and the resulting meningeal sensitization and local inflammatory peptide release that stimulate the trigeminal terminals may also play a role in the pathogenesis of this headache (Caronna et al., 2020; Huang et al., 2020). It has been reported that viral RNA can be detected in body fluids for more than 6 weeks, and circulating proinflammatory cytokine levels may remain high for a few weeks after infection in some patient subgroups (Guan et al., 2020). Cytokine production and persistent glial activation induced by SARS-CoV-2 may also contribute to the pathogenesis of headache. Many similar mechanisms, whether individual or multifactorial, may cause headache in both acute and persistent disease stages. However, it remains unclear to what extent increased ICP contributes to this clinical situation.
Headache associated with COVID-19 has been the subject of many studies, yet our knowledge of headache characteristics remains limited. In a previous study, while NDPH was detected in 60.7% of patients, migraine-like headache was observed in only one fourth of the sample, and none of these patients had a history of migraine (Caronna et al., 2020). In another study, the majority (79.5%) of COVID-19 cases presented with headache, and this symptom that newly appeared during the infection period was described to have different characteristics relative to previously experienced headaches (Uygun et al., 2020). In contrast, in a study from Spain, it was stated that the presence of previous primary headache was determinant of the headache phenotypes seen in COVID-19 (Porta-Etessam et al., 2020). Eighty-one percent of the patients in our study did not have any primary headache diagnosis before COVID-19, and 53.12% met the NDPH criteria. Four patients developed migraine, and five developed TTH after their disease. Based on these results, we consider that the presence of a primary headache is not a determining factor for the headache that develops after COVID-19. Meningeal sensitization secondary to inflammatory response and the resulting activation of the trigeminovascular system may play a role in the pathogenesis of this newly developed headache.
In the literature, the loss of taste and smell was significantly more frequent in the COVID-19 patient group with headache in comparison with in the COVID-19 group without headache (Caronna et al., 2020). In addition, it has been suggested that, in patients with headache and olfactory dysfunction, activation of the trigeminovascular system through stimulation of the trigeminal nerve branches in the nasal cavity may play a role in the pathogenesis of headache (Varga et al., 2020). In our study, loss of smell was reported by 81% of the included patients. This remarkable combination of olfactory dysfunction and headache may bring to mind the idea that the virus uses olfactory structures as an alternative route to the central nervous system in addition to stimulating the trigeminal nerve endings in the nasal cavity. Therefore, the presence of postviral headache may also be an indicator of central nervous system involvement.
The COVID-19 pandemic and the quarantine process have created a psychosocial and stressor burden on society. Studies have shown that patients have increased depression and anxiety scores and stress levels (Elbay et al., 2020; Mazza et al., 2020). The close relationship of headaches such as migraine, TTH, and NDPH with psychosocial factors suggests that emotional factors could also be involved in the etiopathogenesis of headache due to COVID-19. In addition, the adverse effects of therapeutic agents used in the treatment of infection and problems experienced during the treatment and follow-up course of patients with headache both due to the contamination risk and disrupted services in health care institutions are likely to lead to the development of headache.
Our study has certain limitations. The number of patients in the study was relatively small. Also, although the fundus was evaluated as part of the neurological examination performed on all patients, this task was not undertaken by an ophthalmologist. In addition, patients with a clinical manifestation of severe SARS-CoV-2 infection who were hospitalized for a long time were not included in this study. Lastly, CSF pressure was not monitored among our patient population by performing a lumbar puncture.
CONCLUSIONS
The ongoing COVID-19 pandemic that has affected the world for the last year has brought many unknowns into our lives. The neurotoxic effects of SARS-CoV-2 have grown more observable with each passing day. It is possible that the pathogenesis of headache associated with COVID-19 is multifactorial; however, it is not yet clear to what extent increased ICP is responsible for this headache. Using MRI, which is an easily accessible and noninvasive imaging modality, clinicians can evaluate ICP changes and make predictions concerning the course of the disease and length of stay in the hospital and intensive care unit, respectively. We consider that future longitudinal studies may evaluate ICP measurements with invasive and noninvasive methods in large patient groups to offer a different perspective on this subject.
DISCLOSURE
The authors declare no conflict of interest.
Contributor Information
Suat Kamil Sut, Email: sut_kml@hotmail.com.
Sukru Sahin, Email: sukrumirza@gmail.com.
Ali Haydar Baykan, Email: drbaykan@hotmail.com.
REFERENCES
- Altunisik E, Sayiner HS, Aksoz S, Cil E, Ozgenc G. (2021) Neurological symptoms in COVID-19 patients. Bratisl Lek Listy. 122:39–44. [DOI] [PubMed] [Google Scholar]
- Baccarella A, Linder A, Spencer R, Jonokuchi AJ, King PB, Maldonado-Soto A, Boneparth A, Hooe BS, Schweickert AJ, Carlin RF, Kingery F, Vargas WS, Sewell TB, Silver WG. (2021) Increased intracranial pressure in the setting of multisystem inflammatory syndrome in children, associated with COVID-19. Pediatr Neurol. 115:48–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baig AM, Khaleeq A, Ali U, Syeda H. (2020) Evidence of the COVID-19 virus targeting the CNS: Tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Nerosci. 11:995–998. [DOI] [PubMed] [Google Scholar]
- Ballantyne SA, O'Neill G, Hamilton R, Hollman AS. (2002) Observer variation in the sonographic measurement of optic nerve sheath diameter in normal adults. Eur J Ultrasound. 15:145–149. [DOI] [PubMed] [Google Scholar]
- Battaglini D, Santori G, Chandraptham K, Iannuzzi F, Bastianello M, Tarantino F. (2020) Neurological complications and noninvasive multimodal neuromonitoring in critically ill mechanically ventilated COVID-19 patients. Front Neurol. 11:602114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidot S, Saindane AM, Peragallo JH, Bruce BB, Newman NJ, Biousse V. (2015) Brain imaging in idiopathic intracranial hypertension. J Neuroophthalmol. 35:400–411. [DOI] [PubMed] [Google Scholar]
- Caronna E, Ballvé A, Llauradó A, Gallardo VJ, Ariton DM, Lallana S, López Maza S, Olivé Gadea M, Quibus L, Restrepo JL, Rodrigo-Gisbert M, Vilaseca A, Hernandez Gonzalez M, Martinez Gallo M, Alpuente A, Torres-Ferrus M, Pujol Borrell R, Alvarez-Sabin J, Pozo-Rosich P. (2020) Headache: A striking prodromal and persistent symptom, predictive of COVID-19 clinical evolution. Cephalalgia. 40:1410–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbay RY, Kurtulmuş A, Arpacioğlu S, Karadere E. (2020) Depression, anxiety, stress levels of physicians and associated factors in Covid-19 pandemics. Psychiatry Res. 290:113130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DI, Liu GT, Digre KB. (2013) Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 81:1159–1165. [DOI] [PubMed] [Google Scholar]
- Guan W-J Ni Z-Y Hu Y Liang WH Ou CQ He JX Liu L Shan H Lei CL Hui DSC Du B Li LJ Zeng G Yuen KY Chen RC Tang CL Wang T Chen PY Xiang J Li SY Wang JL Liang ZJ Peng YX Wei L Liu Y Hu YH Peng P Wang JM Liu JY Chen Z Li G Zheng ZJ Qiu SQ Luo J Ye CJ Zhu SY Zhong NS, China Medical Treatment Expert Group for Covid-19 (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society (2018) The international classification of headache disorders, 3rd edition. Cephalalgia. 38:1–211. [DOI] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Jun JS, Kim R. (2018) Measurement of the optic nerve sheath diameter with magnetic resonance imaging and its association with eyeball diameter in healthy adults. J Clin Neurol. 14:345–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberly HH, Noble VE. (2008) Using MRI of the optic nerve sheath to detect elevated intracranial pressure. Crit Care. 12:181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagreze WA, Lazzaro A, Weigel M, Hansen HC, Hennig J, Bley TA. (2007) Morphometry of the retrobulbar human optic nerve: Comparison between conventional sonography and ultrafast magnetic resonance sequences. Invest Ophthalmol Vis Sci. 48:1913–1917. [DOI] [PubMed] [Google Scholar]
- Liu JW, De Luca RD, Neto HOM, Barcellos I. (2020) Post-COVID-19 syndrome? New daily persistent headache in the aftermath of COVID-19. Arq Neuropsiquiatr. 78:11. [DOI] [PubMed] [Google Scholar]
- Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 77:683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza MG De Lorenzo R Conte C Poletti S Vai B Bollettini I Melloni EMT Furlan R Ciceri F Rovere-Querini P; COVID-19 BioB Outpatient Clinic Study group, Benedetti F (2020) Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. Brain Behav Immun. 89:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megemont LP, Paris P, Tronchere A, Salazard JP, Pereira B, Dallel R, Aumeran C, Beytout J, Jacomet C, Laurichesse H, Lesens O, Mrozek N, Vidal M, Moisset X. (2020) High prevalence of headaches during Covid-19 infection: A retrospective cohort study. Headache. 60:2578–2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noro F, Cardoso FM, Marchiori E. (2020) COVID-19 and benign intracranial hypertension: A case report. Rev Soc Bras Med Trop. 53:e20200325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossoinig KC. (1979) Standardized echography: Basic principles, clinical applications, and results. Int Ophthalmol Clin. 19:127–210. [PubMed] [Google Scholar]
- Porta-Etessam J, Matías-Guiu JA, González-García N, Gómez Iglesias P, Santos-Bueso E, Arriola-Villalobos P, García-Azorín D, Matías-Guiu J. (2020) Spectrum of headaches associated with SARS-CoV-2 infection: Study of healthcare professionals. Headache. 60:1697–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohr A, Jensen U, Riedel C, van Baalen A, Fruehauf MC, Bartsch T, Hedderich J, Doerner L, Jansen O. (2010) MR imaging of the optic nerve sheath in patients with craniospinal hypotension. AJNR Am J Neuroradiol. 31:1752–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MT, Lima MA, Torezani G, Soares CN, Dantas C, Brandao CO, Espindola O, Siqueira MM, Araujo AQC. (2020) Isolated intracranial hypertension associated with COVID-19. Cephalalgia. 40:1452–1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toptan T, Aktan C, Basari A, Bolay H. (2020) Case series of headache characteristics in COVID-19; headache can be an isolated symptom. Headache. 60:1788–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uygun O, Ertaş M, Ekizoğlu E, Bolay H, Özge A, Orhan EK, Çağatay AA, Baykan B. (2020) Headache characteristics in COVID-19 pandemic-a survey study. J Headache Pain. 21:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Mocha H. (2020) Endothelial cell infection and endotheliitis in COVID-19. Lancet. 395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkuil LD, Liu GT, Brahma VL, Avery RA. (2020) Avery Pseudotumor cerebri syndrome associated with MIS-C: A case report. Lancet. 396:532. [DOI] [PubMed] [Google Scholar]
- Weigel M, Lagreze WA, Lazzaro A, Hennig J, Bley TA. (2006) Fast and quantitative high-resolution magnetic resonance imaging of the optic nerve at 3.0 Tesla. Invest Radiol. 41:83–86. [DOI] [PubMed] [Google Scholar]
- Wu Z, McGoogan JM. (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 323:1239–1242. [DOI] [PubMed] [Google Scholar]
- Yamani N, Olesen J. (2019) New daily persistent headache: A systematic review on an enigmatic disorder. J Headache Pain. 20:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Hao D, Tang G, Zhou R, Pang J, Dong C. (2020) High-resolution MRI assessment of optic nerve sheath diameter in adults: Optic nerve sheath variation and a new diagnostic tool for intracranial hypertension. Acta Radiol. 284185120966715. doi: 10.1177/0284185120966715. [DOI] [PubMed] [Google Scholar]


