Abstract
Background
Helium and oxygen mixtures (heliox), have been used sporadically in respiratory medicine for decades. Their use in acute respiratory emergencies such as asthma has been the subject of considerable debate. Despite the lapse of more than 60 years since it was first proposed, the role of heliox in treating patients with severe acute asthma remains unclear.
Objectives
To determine the effect of the addition of heliox to standard medical care on the course of acute asthma, as measured by pulmonary function testing and clinical endpoints.
Search methods
Randomised controlled trials were identified from the Cochrane Airways Group Specialised Register. In addition, we contacted primary authors and experts and searched reference lists of articles. Searches are current to August 2010.
Selection criteria
1) Randomised, single or double blind, controlled trials; 2) children or adults with a clinical diagnosis of acute asthma seen in emergency departments or equivalent acute care settings; and 3) compared treatment with inhaled heliox to placebo (oxygen or air).
Data collection and analysis
Two review authors independently assessed the studies for inclusion and quality assessment; disagreement was resolved by a third review author and consensus.
Main results
This review includes a total of ten trials involving 544 acute asthma patients. Seven studies involved adults and three studies dealt solely with children. Three were assessed as high quality (Jadad score > 3). Pulmonary function tests were recorded during heliox administration (15 to 60 min). Pooling of the eight trials contributing data to this review showed no significant group differences (standardised mean differences ‐0.28; 95% confidence interval (CI) ‐0.56 to 0.01). There was significant heterogeneity among the studies. Heliox use did improve pulmonary function only in the subgroup of patients with the most severe baseline pulmonary function impairment; however, this conclusion is based on a small number of studies. There were no significant differences between groups when adults versus children, and high versus low heliox dose studies were compared. Finally, at the end of treatment, participants treated with heliox showed no significant different risk of admission to hospital (RR 0.83 (95%CI 0.66 to 1.08, P = 0.17, I2 = 0%).
Authors' conclusions
The existing evidence does not provide support for the administration of helium‐oxygen mixtures to all ED patients with acute asthma. At this time, heliox treatment does not have a role to play in the initial treatment of patients with acute asthma. Nevertheless, new evidence suggests certain beneficial effects in patients with more severe obstruction. Since these conclusions are based upon between‐group comparisons and small studies, they should be interpreted with caution.
Plain language summary
Heliox (mixture of helium and oxygen) compared to either oxygen or air for people with acute asthma
Acute asthma is a common disease presentation to the emergency department (ED) in many countries. Treatment of acute asthma is based on rapid reversal of bronchospasm and arresting airway inflammation. The main agents employed to treat acute asthma include combined treatments with bronchodilating agents and corticosteroids. However, there is evidence that helium and oxygen mixtures (heliox) may provide additional benefits to patients with acute asthma. This review examines the evidence from ten randomised controlled trials involving 544 patients which compared heliox to oxygen or air, when used in conjunction with the other standard acute treatments. The reviewers conclude that the evidence does not support routine use of heliox in patients with acute asthma.
Background
Helium and oxygen mixtures (heliox), have been used sporadically in respiratory medicine for decades. For example, as early as 1935 heliox was introduced to the medical community for treatment of upper and lower airway obstruction (Barach 1935). The interest in heliox for treatment of asthma became prominent in the 1980's when deaths from asthma began to rise. Due to their low density with respect to air (80% helium/20% oxygen mixture has a density approximately one third that of air), heliox mixtures have the potential to decrease airway resistance and therefore decrease work of breathing in those situations associated with increased airway resistance. Thus, they may provide benefit to patients suffering from obstructive lesions of the larynx, trachea, and airways. Additionally, research using a heliox mixture, has demonstrated a greater percentage of lung particle retention (Anderson 1993). This suggests that one of the beneficial effects of heliox use in reactive airway diseases may be improved deposition of aerosolized bronchodilators in ventilated acute asthma patients (Goode 2001). Heliox has also been recommended as a useful adjunct in the adult patient with severe asthma, both during spontaneous ventilation as well as during mechanical ventilation (Shiue 1989; Gluck 1990; Kass 1995; Manthous 1995). Conversely, other research has concluded that the inhaled mass of albuterol decreased significantly when a nebulizer was powered with heliox rather than air (Hess 1999). Reports describing the use of heliox in children with asthma also provide conflicting results, with some failing (Carter 1996) and others showing a benefit (Kudukis 1997). However, much of the evidence arises from either small trials or uncontrolled studies. Fortunately, larger trials comparing the effectiveness of heliox to oxygen for beta‐agonist therapy have recently been performed (Henderson 1999).
In summary, much is unknown regarding the use of heliox in acute asthma. First, without controlled studies, the effect of heliox is difficult to assess. Second, the duration of administration and optimal helium/oxygen mixture remain undetermined. Finally, the cost of treatment is relatively high and the number needed to treat for an acceptable benefit has not been established. Given the above controversies, the need for a systematic review exists. Prior to the publication of the first version if this review in 2002, no systematic reviews on this topic had been published, and it is not surprising that heliox use is variable and institution specific. Despite the lapse of more than 60 years since its use was first proposed, the role of heliox in treating patients with acute severe asthma is unclear.
Objectives
To determine the effect of the addition of heliox to standard medical care on the course of acute asthma, as measured by pulmonary function and clinical endpoints.
Methods
Criteria for considering studies for this review
Types of studies
Only randomised, single or double blind, controlled trials were considered for inclusion. Both parallel group and crossover designs were considered.
Types of participants
Studies including either children or adult (> 18 years of age) patients presenting to an emergency department or equivalent care settings for treatment of acute asthma were considered for inclusion in the review. Age formed one of the sub‐groups examined in the review. All study participants had a clinical diagnosis of asthma exacerbation (according to accepted criteria such as those published by the American Thoracic Society (ATS); studies involving solely patients with chronic obstructive pulmonary disease (COPD) were excluded. Studies including both COPD and asthmatics patients were to be considered if patients with acute asthma could be separately analysed by reviewing the study or through correspondence with the authors. Studies involving acute asthma patients requiring mechanical ventilation at presentation were also excluded.
Types of interventions
Only studies comparing treatment with inhaled heliox to control (oxygen or air) were considered. Study co‐interventions such as corticosteroids and other drugs were monitored and formed planned subgroup comparisons when possible. Different helium‐oxygen mixtures (80/20, 70/30, 60/40), and duration of heliox administration were considered in subgroup analysis.
Types of outcome measures
Primary outcomes
Changes in peak expiratory flow (PEF; absolute and per cent of predicted [% PEF]), forced expiratory volume in the first second (FEV1; absolute and per cent of predicted [% FEV1])
Secondary outcomes
Symptom score/symptoms: wheezing, shortness of breath, dyspnoea, accessory muscle use;
physiological measures: PaO2, SaO2, and vital signs;
side effects/adverse effects; and
clinical outcomes: need for mechanical ventilation and admissions to the hospital.
The timing of assessment was during breathing heliox (15 to 60 min) and assessments included up to six hours of treatment in the Emergency Department (ED).
Search methods for identification of studies
Electronic searches
We identified trials using the Cochrane Airways Group Specialised Register of trials, which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and CINAHL, and handsearching of respiratory journals and meeting abstracts (please see the Airways Group Module for further details). All records in the Specialised Register coded as 'asthma' were searched using the following terms:
helium OR heliox OR oxygen*
An additional search of CENTRAL, the Cochrane Controlled Trials Register, was completed using the same search strategy. Searches are current to August 2010.
Other sources
We contacted authors of all studies to locate other unpublished or "in progress" studies which met the inclusion criteria. We searched references from included studies, reviews and texts for citations.
Data collection and analysis
Selection of trials
Titles, abstracts, and citations were independently reviewed by the two reviewers (GR, CR) to assess potential relevance for full review. From the full text, both reviewers independently assessed studies for inclusion based on the criteria for population, intervention, study design and outcomes. Agreement was measured using kappa statistics and any disagreement over study inclusion was resolved by a third reviewer (CVP) and consensus.
Quality assessment
Two reviewers (GR,CR) assessed the methodological quality of the included trials using two methods. First, using the Cochrane approach to assessment of allocation concealment: 1) Grade A: Adequate concealment 2) Grade B: Uncertain 3) Grade C: Clearly inadequate concealment. Second, each study was assessed for validity on a 0‐5 scale, method developed by Jadad (Jadad 1996). Inter‐rater reliability was measured for both quality scales by using kappa statistics.
Data extraction
Two review authors (GR, CR) independently extracted data from included trials and entered results into the Cochrane Collaboration software program (Review Manager 5). Data extraction included the following items: 1) population: age, gender, number of patients studied, patient demographics, withdrawals; 2) Intervention: agent, dose, route of delivery, and duration of therapy; 3) control: concurrent treatments; 4) outcomes; and 5) design: method of randomisation and allocation concealment.
Statistical considerations
All included trials were combined using Review Manager 5. For continuous variables, the results of individual studies were calculated as a random‐effects weighted mean (WMD) or standardised mean differences (SMD), with 95% confidence intervals (CIs). To adjust for differences in baseline spirometric measures between the control and treatment groups, the baseline difference between groups was added to the control group at the time of outcome assessment. The contribution of each trial to the pooled estimate was proportional to the inverse of the variance. Homogeneity of effect sizes were tested with the Dersimonian and Laird method with P < 0.1 as the cut point for significance. We also measured heterogeneity by using the I‐squared test (Higgins 2003). Values of 25%, 50% and 75% represent low, moderate and high heterogeneity respectively. Sensitivity analysis was performed using: age (adults versus children), different helium‐oxygen mixtures (80/20, versus 70/30 or 60/40), methodological quality (concealment Grade A versus other Grades; Jadad score (> 3 versus ≤ 3) and the method of heliox use (studies designed to washout air in the lungs and replace it with heliox versus studies that used heliox to deliver nebulized therapy). Finally, differences between estimates were tested according to methods described by Altman and Bland 2003.
Results
Description of studies
The initial search produced 94 potentially relevant citations. Of these, 13 studies were reviewed in full text for possible inclusion, all identified from the literature search. Five studies were excluded because they were non‐randomised trials and one because it included out‐of‐hospital patients (L'Her 2000). One randomised trial (Kudukis 1997) was not included. There were a number of reasons for this: 1) The primary outcome measure was pulsus paradoxus; 2) FEV1 and PEF data could not be obtained from the authors; 3) an unvalidated "dyspnoea score" was used; this was composed of: SaO2, inspired FIO2, inspiratory breath sounds, accessory muscle use, expiratory wheeze and cerebral function, and 4) SaO2 data were provided; however, it was unclear what FIO2 when those measurements were made. After two updates (2002 and 2006), a total of ten randomised controlled trials (544 participants) were selected for inclusion (seeTable 1). There was excellent agreement between the two review authors´ quality scores for the six trials (kappa = 1.0).
1. Characteristics of trials included in the review.
| Trial | Mean age | Baseline severity | Quality score(Jadad) | Helium‐O2 mixture |
| Dorfman et al | 28.5 | 43% (PEF) | 1 | 80:20 |
| Carter et al. | 12.3 | 49% (FEV1) | 4 | 70:30 |
| Henderson et al. | 44.5 | 1.07 L (FEV1) | 2 | 70:30 |
| Kass et al. | 33.2 | 29% (PEF) | 2 | 70:30 |
| Kress et al. | 32.5 | 32% (FEV1) | 1 | 80:20 |
| Rose et al. | 37.0 | 127 L/m (PEF) | 4 | 70:30 |
| Kim et al. | 7.3 | Pulmonary index > 8 | 3 | 70:30 |
| Lin Lee et al | 34 | 35% (PEF) | 4 | 80:20 |
| Xie et al | 1.35 l (FEV1) | 3 | 79:21 | |
| Rivera et al | 8 | Dyspne index 4 or higher | 3 | 70:30 |
A total of four papers were initially included for this review (Carter 1996; Henderson 1999; Kass 1999; Dorfman 2000) and two further papers were added in 2002 (Kress 2002; Rose 2002). A third update in 2006 has added an additional four studies (Xie 2003a; Kim 2005; Lin Lee 2005a; Rivera 2006).
A total of ten randomised controlled trials were selected for inclusion. Seven studies involved adults (Dorfman 2000; Henderson 1999; Kass 1999; Kress 2002; Rose 2002; Xie 2003a; Lin Lee 2005b) and three recruited children (Carter 1996; Kim 2005; Rivera 2006). Eight studies were conducted in North America, one in Taiwan, and one in China. The studies enrolled patients with mild to severe reductions in the mean pulmonary function measures at presentation. For example, mean pre‐treatment PEFs or FEV1s were reported as: 43% predicted (Dorfman 2000), 39 to 42% predicted (Henderson 1999), 26 to 30% predicted (Kass 1999), 49% predicted (Carter 1996), and 32.6% (Kress 2002), 1.35 L (Xie 2003a) and 35% (Lin Lee 2005b). Eight studies include patients presenting to an ED (Henderson 1999; Kass 1999; Dorfman 2000; Kress 2002; Rose 2002; Kim 2005; Lin Lee 2005a; Rivera 2006); on the other hand, one study included patients that required hospital admission (Carter 1996) and one trial administered treatment in a respiratory outpatient department (Xie 2003a). Inhaled albuterol and corticosteroids were used in almost all the trials; One study used fenoterol as a bronchodilator. In three studies patients received a helium‐oxygen mixture of 80:20 (Dorfman 2000; Kress 2002; Lin Lee 2005a), six used the 70:30 mixture (Carter 1996; Henderson 1999; Kass 1999; Rose 2002; Kim 2005; Rivera 2006), and one used a 79:21 mixture (Xie 2003a). The comparison driving gas for the studies were air (Dorfman 2000; Xie 2003a) or oxygen (Carter 1996; Henderson 1999; Kass 1999; Kress 2002; Rose 2002; Kim 2005; Lin Lee 2005a; Rivera 2006). Two studies were designed to washout the air in the lungs and replace it with heliox (Carter 1996; Kass 1999). On the other hand, eight studies used heliox to deliver nebulised therapy (Henderson 1999; Dorfman 2000; Kress 2002; Rose 2002; Xie 2003a; Kim 2005; Lin Lee 2005a; Rivera 2006). The duration of heliox therapy was between 15 and 480 min. The main reported outcome variable in eight trials was spirometric measurements (PEF as % predicted, FEV1 as % predicted, PEF L/min, FEV1 L). Two studies included two different randomised trials each (Xie 2003a; Xie 2003b;Lin Lee 2005a; Lin Lee 2005b). However, only one trial from each study contributed data. Reasons for the exclusion of both remaining trials were: 1) Heliox was administered for two days (Xie 2003b), and no data were available to pool (Lin Lee 2005b).
Risk of bias in included studies
All studies were randomised, controlled trials; three were double‐blind, four were single‐blind, and three were unblinded. Using the Jadad method, three studies had a score > 3 (Carter 1996; Rose 2002; Lin Lee 2005a). The concealment of allocation was adequate (A) in two studies (Rose 2002;Lin Lee 2005a), and unclear (B) in eight (Carter 1996; Kass 1999; Henderson 1999; Kass 1999; Dorfman 2000 ; Kress 2002; Xie 2003a; Kim 2005; Rivera 2006). Overall, the methodological quality was rated as moderate.
Effects of interventions
Eight trials examined response to treatment using pulmonary function tests. Four studies included PEF (% predicted) (Carter 1996; Kass 1999; Dorfman 2000; Lin Lee 2005a), two studies reported PEF (L/min) (Henderson 1999; Rose 2002), and two studies included FEV1 (L) (Kress 2002; Xie 2003a). In one study SD were estimated from SEM (Kass 1999) and in another trial FEV1 (L) and SD were estimated and confirmed by authors (Kress 2002). There do appear to be unresolved issues concerning PEF measurements in patients breathing helium‐oxygen mixtures, because helium is lighter than nitrogen. In one of the papers included here (Kass 1999), PEF was measured with a peak flow meter and the authors did not report correction for gas density. By contrast, in a study that was not included here (Kudukis 1997), PEF measurements made while breathing heliox needed to be corrected by a factor of 1.32 when measured using a Wright Peak Flow Meter. Spirometers were used for the other four trials in this review which should not require correction since they are volumetric devices. Pooling of the eight trials contributing data to this review showed no significant group differences (SMD ‐0.28; 95% CI ‐0.56 to 0.01, Analysis 1.1). However this effect showed heterogeneity between the studies (I2 = 45.8%). There were no significant differences between groups when we compared adults versus children, high dose versus low heliox dose and breath heliox versus nebulized with heliox (see MetaView graphs). When we compared studies stratified by methodological quality, high quality studies showed a significant improvement in pulmonary function (SMD ‐0.56; 95%CI ‐1.04 to ‐0.08); however, there was no statistically significant difference when the high and low quality subgroups were compared with each other (SMD ‐0.46; 95%CI‐2.12 to 1.20). Finally, the three studies with a moderate to severe decrease in baseline pulmonary function showed a significant difference compared with the five studies with a mild‐moderate decrease (SMD 0.61;95%CI 0.21 to 1.00). Statistical heterogeneity remained, albeit to a lower degree (I2 = 27.2%); however, with subgroup comparisons based on baseline pulmonary function, there is a danger of regression to the mean.
1.1. Analysis.
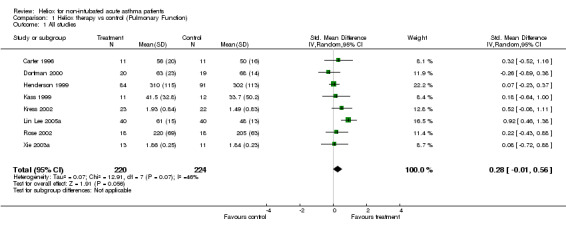
Comparison 1 Heliox therapy vs control (Pulmonary Function), Outcome 1 All studies.
With regard to additional outcomes, seven studies reported admission to hospital (Dorfman 2000; Henderson 1999; Kress 2002; Lin Lee 2005a; Rose 2002; Kim 2005; Rivera 2006), five reported dyspnoea or pulmonary index (Carter 1996; Kass 1999; Rose 2002; Kim 2005; Rivera 2006), four reported heart rate (Carter 1996; Kass 1999; Dorfman 2000; Kress 2002), and three reported oxygen saturation (Carter 1996; Dorfman 2000; Rose 2002). There was no significant reduction in admission to hospital in favour of heliox (RR 0.83; 95%CI 0.63 to 1.08, Analysis 2.4). This effect was statistically homogeneous (I2 = 0%). There were no significant differences between groups in the remaining secondary variables (dyspnoea, heart rate and oxygen saturation). Two studies reported need for mechanical ventilation; there were no intubations in the first (Henderson 1999) and one patient in the control group required endotracheal intubation in the second (Kass 1999). Finally, adverse effects were reported in two trials. In the Henderson 1999 study, one patient became hypoxic while receiving the 70:30 heliox mix, and the Dorfman 2000 study reported only one heliox‐treated patient that experienced dizziness during the intervention. Overall, heliox appears to be safe and well tolerated in the mixtures used in these studies to treat acute asthma.
2.4. Analysis.
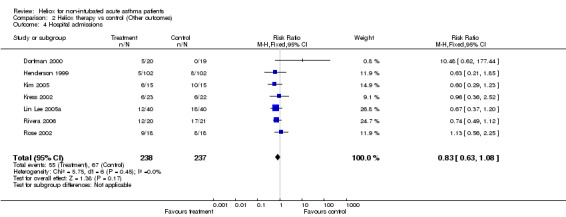
Comparison 2 Heliox therapy vs control (Other outcomes), Outcome 4 Hospital admissions.
Discussion
This systematic review has attempted to incorporate the best available evidence on heliox use in patients with acute asthma. We found ten randomised placebo‐controlled clinical trials that compared heliox to other forms of standard care. Several important conclusions arise from the analysis. Firstly, the addition of heliox to standard medical care during the course of acute asthma was not found to be more effective than a comparison delivery with air or oxygen. The overall pooled analysis reveals that there are not significant differences between groups. Furthermore, the point estimate is associated with narrow confidence intervals, including clinically unimportant changes. These results are similar irrespective of the age (children versus adults), methodological quality (high versus low) and mixture of heliox (e.g. high or low helium‐oxygen mixtures). However, the studies characterised by moderate to severe pulmonary function impairment showed a significant improvement compared with the studies with mild to moderate pulmonary function impairment. These findings suggest that heliox could be more effective than oxygen/air in delivering inhaled particles of beta‐agonists to distal airways, particularly in the most severe patients. Lastly, there was insufficient information to pool other outcomes or side effects, so no firm conclusions regarding adverse effects cannot be drawn. In one study one patient became hypoxic while receiving the 70:30 heliox mixture. Overall, heliox appears to be safe and well tolerated in the mixtures used in these studies to treat acute asthma.
Strengths and limitations
Our analysis is subject to the general problems of meta‐analysis. There is a possibility of publication bias in this meta‐analysis. For example, by missing unpublished trials we may be providing an inaccurate estimation of the effect of heliox treatment. However, a comprehensive search of the published literature for potentially relevant studies was conducted, using a systematic strategy to avoid bias. This was followed by attempts to contact corresponding and first authors. Despite these efforts the funnel plot (for admissions) appears asymmetrical (Figure 1). There was no statistical heterogeneity for this outcome, and the 'outlying' study with the point estimate favouring control was available as a letter to a journal (Dorfman 2000). We cannot exclude the possibility that other negative, unpublished studies exist which would alter the pooled effect estimate. There is also a possibility of study selection bias; however, we employed two independent review authors, and feel confident that the studies excluded were done so for consistent and appropriate reasons.
1.
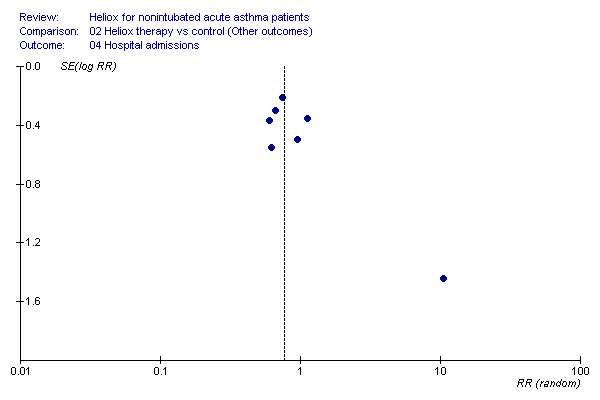
Funnel plot of the admission data.
Like all systematic reviews, this meta‐analysis is limited by the quality of existing research and how the data are reported. Only three of ten included trials were considered "high quality". In fact, comparison of trials with high methodological quality to low did affect the conclusions. Nevertheless, the number and size of studies included remains small. So, the current conclusions may be modified by the publication of results from larger trials.
Authors' conclusions
Implications for practice.
The existing evidence fails to demonstrate a clear benefit from the administration of helium‐oxygen mixtures to all ED patients with acute asthma.
Treatment with heliox may improve pulmonary function in the most severe acute asthma patients; however, clinicians must ensure other evidence‐based treatments are employed.
Implications for research.
Questions regarding the treatment of acute asthma with heliox remain unanswered:
Additional studies are needed to confirm the sub‐group findings from this review suggesting a beneficial effect of heliox in severe acute asthma. In future studies, severity must be clearly defined and based on presenting pulmonary function results and response to initial beta‐agonist therapy whenever possible.
Studies involving very young children need to be performed to determine the effect of heliox in this age group
Further studies are required to examine the effect of heliox based on the prior inhaled steroid use in patients presenting to the ED with an asthma exacerbation. The effect of treatment may differ based on inhaled steroid use, and the answer to this question remains unclear. Inhaled steroids are increasingly employed and the development of high dose inhaled steroids with lower systemic activity suggests that this would be an important area for future research.
Future research on acute asthma must concentrate on well defined outcomes which may lead to more informative reviews. More specifically, criteria for discharge and reporting of lung function test data in a systematic fashion would assist in further work. Finally, better description of the methodology would also be beneficial.
What's new
| Date | Event | Description |
|---|---|---|
| 5 June 2014 | Amended | PLS title amended |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 2, 2003
| Date | Event | Description |
|---|---|---|
| 25 August 2010 | New search has been performed | New literature search run, no new included studies found. |
| 19 August 2009 | New search has been performed | Literature search re‐run; no new studies found |
| 1 August 2008 | New search has been performed | Literature search re‐run; no new studies found. |
| 25 May 2008 | Amended | Converted to new review format. |
| 2 August 2006 | New citation required and conclusions have changed | 2006 update 1) The analysis included four new trials: Xie 2003; Kim 2005; Lin Lee 2005; Rivera 2006. 2) In the sensitivity analyses we included the baseline pulmonary function impairment (mild to moderate versus moderate to severe). 3) The studies that presented a moderate to severe pulmonary function reduction showed a significant improvement compared with the studies with mild to moderate baseline obstruction. 4) Overall, the conclusions remain unchanged. |
Acknowledgements
The authors wish to acknowledge the assistance of Toby Lasserson, Liz Arnold and Karen Blackhall of the Cochrane Airways Group (CAG). We would also like to acknowledge the assistance of the following corresponding authors: Stephen Mette, Jesse B. Hall and David Lin Lee. Finally, the assistance of Professor Paul Jones and Dr Chris Cates was greatly appreciated.
Data and analyses
Comparison 1. Heliox therapy vs control (Pulmonary Function).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All studies | 8 | 444 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.01, 0.56] |
| 2 By Age | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Adults | 7 | 422 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [‐0.04, 0.58] |
| 2.2 Children | 1 | 22 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.52, 1.16] |
| 3 By Methodological Quality | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 High | 3 | 138 | Std. Mean Difference (IV, Random, 95% CI) | 0.56 [0.08, 1.04] |
| 3.2 Low | 5 | 306 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.12, 0.33] |
| 4 By Dose | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 High (80:20) | 4 | 188 | Std. Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.19, 0.90] |
| 4.2 Low (70:30) | 4 | 256 | Std. Mean Difference (IV, Random, 95% CI) | 0.12 [‐0.12, 0.37] |
| 5 Method of heliox use | 8 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Airway resistance | 2 | 45 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.34, 0.83] |
| 5.2 Drug delivery | 6 | 399 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.07, 0.63] |
| 6 By baseline severity | 8 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Mild‐Moderate | 5 | 296 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.16, 0.29] |
| 6.2 Moderate‐Severe | 3 | 148 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.67 [0.34, 1.00] |
1.2. Analysis.
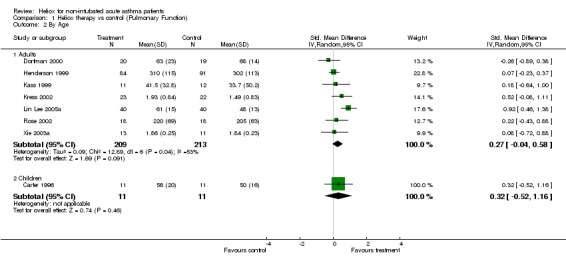
Comparison 1 Heliox therapy vs control (Pulmonary Function), Outcome 2 By Age.
1.3. Analysis.
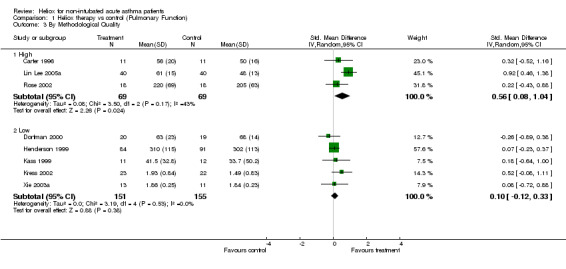
Comparison 1 Heliox therapy vs control (Pulmonary Function), Outcome 3 By Methodological Quality.
1.4. Analysis.
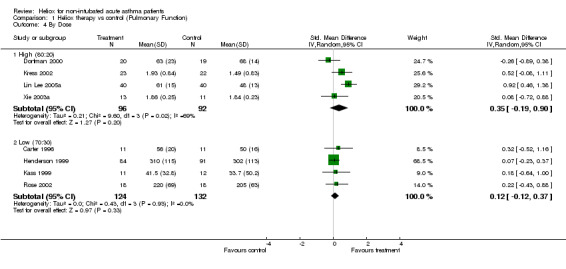
Comparison 1 Heliox therapy vs control (Pulmonary Function), Outcome 4 By Dose.
1.5. Analysis.
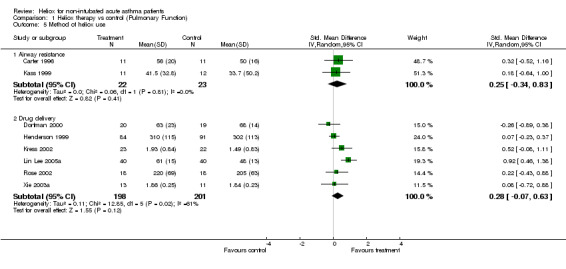
Comparison 1 Heliox therapy vs control (Pulmonary Function), Outcome 5 Method of heliox use.
1.6. Analysis.
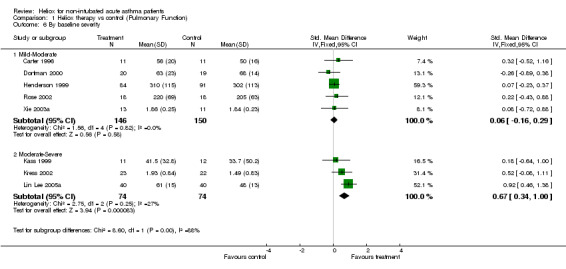
Comparison 1 Heliox therapy vs control (Pulmonary Function), Outcome 6 By baseline severity.
Comparison 2. Heliox therapy vs control (Other outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Heart rate | 4 | 127 | Mean Difference (IV, Random, 95% CI) | 7.63 [‐8.22, 23.49] |
| 2 SaO2 | 3 | 97 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐1.01, 1.09] |
| 3 Dyspnea or Pulmonary index | 5 | 152 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐1.19, 0.48] |
| 4 Hospital admissions | 7 | 475 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.63, 1.08] |
2.1. Analysis.
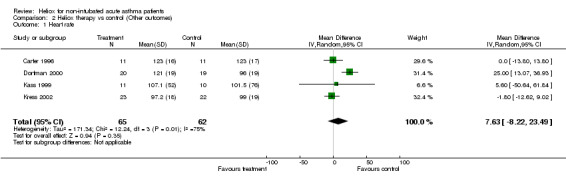
Comparison 2 Heliox therapy vs control (Other outcomes), Outcome 1 Heart rate.
2.2. Analysis.
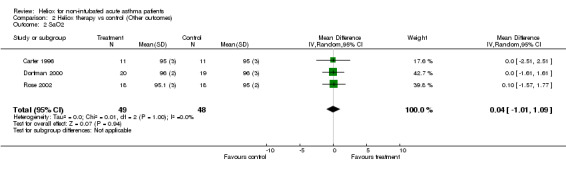
Comparison 2 Heliox therapy vs control (Other outcomes), Outcome 2 SaO2.
2.3. Analysis.
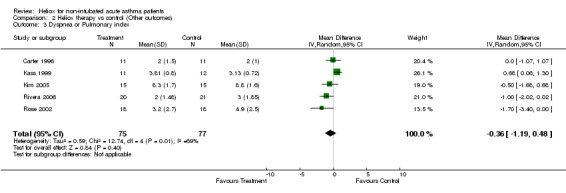
Comparison 2 Heliox therapy vs control (Other outcomes), Outcome 3 Dyspnea or Pulmonary index.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carter 1996.
| Methods | Randomization: no details. Blinding: double‐blind, crossover Withdrawals: two | |
| Participants | 11 hospitalised, children (5‐18 y) Mean age = 12.3 ± 3.6. Baseline FEV‐1 = 49%. | |
| Interventions | Heliox (70:30) or oxygen (30%) during 15 min. All received albuterol 5 mg and systemic corticosteroids | |
| Outcomes | FEV1 and PEF % of predicted RR HR SaO2 Dyspnea | |
| Notes | Authors did not respond. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
Dorfman 2000.
| Methods | Randomization: no details. Blinding: no blinding parallel study Withdrawals: one | |
| Participants | 39 ED children and adult acute asthmatics (8‐55 y) Mean age = 12.3 ± 3.6 Baseline FEV‐1 = 49% | |
| Interventions | Heliox (70:30) or oxygen (30%) during 15 min. All received albuterol 5 mg and systemic corticosteroids | |
| Outcomes | FEV1 and PEF % of predicted. RR HR SaO2 Dyspnea | |
| Notes | Authors did not respond | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
Henderson 1999.
| Methods | Randomization: no details Blinding: single‐blinded, parallel study Withdrawals: not stated | |
| Participants | 204 ED adult acute asthmatics (18‐65 y) Mean age = 44.5 ±11.8. Baseline FEV‐1 = 1.1 L | |
| Interventions | Albuterol 5 mg in heliox (70:30) or oxygen every 15 min x 3 (45 min) All received systemic corticosteroids (prednisone) | |
| Outcomes | PEF (L/min) or FEV1 (L) Admission rate | |
| Notes | Authors did not respond | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
Kass 1999.
| Methods | Randomization: no details Blinding: non‐blinded, parallel study Withdrawals: five | |
| Participants | 23 ED adult acute asthmatics (18‐50 y) Mean age = 33.1 ± 2.8 Baseline PEF = 29% | |
| Interventions | Albuterol nebulized 5 mg with heliox (70:30) or oxygen (30%) All received systemic corticosteroids | |
| Outcomes | PEF % of predicted or L/min RR HR Dyspnea | |
| Notes | Authors did not respond | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
Kim 2005.
| Methods | Randomization: no details Blinding: single‐blinded, parallel study. Withdrawals: one | |
| Participants | 30 ED children (2‐18 y) with acute asthma. Mean age = 7.3 ± 4.3. Baseline Pulmonary Index > 8 (moderate to severe) | |
| Interventions | Albuterol 5 mg nebulized continuously with heliox (70:30) or oxygen (100%) for 60 min. All patients received albuterol 5 mg nebulized and systemic corticosteroids | |
| Outcomes | Pulmonary index, Admission rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
Kress 2002.
| Methods | Randomization: no details Blinding: single blind, parallel study Withdrawals: not stated | |
| Participants | 45 ED adult acute asthmatics (18‐50 y) Mean age = 32.5 Baseline FEV1 = 32% | |
| Interventions | Albuterol 5 mg in heliox (80:20) or oxygen every 30 min x 3 (90 min) | |
| Outcomes | FEV1 % of change HR SpO2 Admission rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
Lin Lee 2005a.
| Methods | Randomization: computer‐generated numbers Blinding: double blind, parallel study Withdrawals: no withdrawals | |
| Participants | 80 ED adult acute asthmatics (18‐50 y) Mean age = 34 ± 8 y Baseline PEF = 35% | |
| Interventions | Albuterol 2.5 mg in heliox (80:20) or air/oxygen every 20 min x 3 | |
| Outcomes | PEF % of change Admission rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Computer‐generated numbers |
Rivera 2006.
| Methods | Randomization: block randomisation. Blinding: single‐blinded, parallel study No withdrawals | |
| Participants | 41 ED children with acute asthma (3‐16 y). Mean age = 8 y. Baseline dyspnoea index of 4 or higher (moderate‐severe) | |
| Interventions | Albuterol nebulized continuously (0.45 mg/kg, max. 15 mg/h) with heliox (70:30) or air/oxygen. All patients received albuterol 2.5 mg nebulized x 3 and systemic corticosteroids | |
| Outcomes | Dyspnea index Admission rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
Rose 2002.
| Methods | Randomization: block randomisation by using a computer generated method Blinding: double blind, parallel study Withdrawals:not stated | |
| Participants | 32 ED adult acute asthmatics (18‐55 y) Mean age = 37 Baseline PEF = 127 L/min | |
| Interventions | Albuterol nebulized continuously (12.5 mg/h) with heliox (70:30) or oxygen (120 min) | |
| Outcomes | PEF (L/min), FEV1 (L), RR Dyspnea SpO2 | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | 'block randomisation by using a computer generated method' |
Xie 2003a.
| Methods | Randomization: no details. Blinding: non‐blinded, parallel study. Withdrawals: not stated | |
| Participants | 24 ED adult acute asthmatics (26‐68 y). Mean age: not stated. Baseline FEV1 = 1.35 L | |
| Interventions | Fenoterol 0.5 mg nebulized with heliox (79:21) or air | |
| Outcomes | FEV1 (L) FVC (L) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
ED: Emergency department; FEV1: Forced expiratory volume in one second; HR: Heart rate; PEF: Peak expiratory flow
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bag 2002 | Stable asthma patients |
| Gluck 1990 | Non‐randomised trial; ventilated patients |
| Kass 1995 | Non‐randomised trial; ventilated patients |
| Kudukis 1997 | Pulsus paradoxus as main outcome Absolute or per cent of predicted PEF or FEV1 values were not reported. Correspondence with author did not provide additional data |
| L'Her 2000 | Out‐of‐hospital patients |
| Lin Lee 2005b | Second randomised trial. No data available to pool |
| Manthous 1995 | Non‐randomized trial |
| Schaeffer 1999 | Non‐randomized trial; ventilated patients |
| Shiue 1989 | Non‐randomized trial |
| Verbeek 1998 | Non‐randomized trial |
| Xie 2003b | Second randomised trial. Use of heliox during 48 hours |
Contributions of authors
GUS initiated the review, wrote the protocol, performed the searches, contributed to selection for inclusion, quality assessment, extracted data, converted the review to RevMan 4.0, and is the principle author. CAR contributed to the protocol development, performed selection for inclusion and quality assessments, and helped edit. CVP originally developed the idea for the review, assisted with protocol writing and edited. BHR was the assigned ARG editor and assisted with protocol writing and edited.
Sources of support
Internal sources
Departamento de Emergencia. Hospital Central de las FF.AA., Uruguay.
Department of Emergency Medicine, University of Alberta, Canada.
External sources
Canadian Institute of Health Research (CIHR), Canada.
Declarations of interest
The authors who have been involved in this review have done so without any known conflicts of interest. They are neither involved with the primary studies nor affiliated with any companies that produces heliox.
Edited (no change to conclusions)
References
References to studies included in this review
Carter 1996 {published data only}
- Carter ER, Webb CR, Moffitt DR. Evaluation of heliox in children hospitalized with acute severe asthma. A randomized crossover trial. Chest 1996;109:1256‐61. [DOI] [PubMed] [Google Scholar]
Dorfman 2000 {unpublished data only}
- Dorfman TA, Burton JH, Jones P, Shipley ER, Mette SA. Inhaled heliox does not benefit emergency department patients with moderate to severe asthma. American Journal of Emergency Medicine 2000;18:495‐7. [DOI] [PubMed] [Google Scholar]
Henderson 1999 {published data only}
- Henderson SO, Acharya P, Kilaghbian T, Perez J, Korn CS, Chan LS. Use of heliox‐driven nebulizer therapy in the treatment of acute asthma. Annals of Emergency Medicine 1999;33:141‐6. [DOI] [PubMed] [Google Scholar]
Kass 1999 {published data only}
- Kass EJ, Terregino CA. The effect of heliox in acute severe asthma. A randomized controlled trial. Chest 1999;116:296‐300. [DOI] [PubMed] [Google Scholar]
Kim 2005 {published data only}
- Kim IK, Phrampus E, Venkataraman S, et al. Helium/oxygen‐driven albuterol nebulization in the treatment of children with moderate to severe asthma exacerbations: A randomized, controlled trial. Pediatrics 2005;116:1127‐33. [DOI] [PubMed] [Google Scholar]
Kress 2002 {published data only}
- Kress JP, Noth I, Gehlbach BK, Barman N, Pohlman AS, Miller A, et al. The utility of albuterol nebulized with heliox during acute asthma exacerbations. American Journal of Respiratory and Critical Care Medicine 2002;165:1317‐21. [DOI] [PubMed] [Google Scholar]
Lin Lee 2005a {published data only}
- Lin Lee D, Wel Hsu C, Lee H, et al. Beneficial effects of albuterol therapy driven by heliox versus by oxygen in severe asthma exacerbation. Academic Emergency Medicine 2005;12(9):820‐7. [DOI] [PubMed] [Google Scholar]
Rivera 2006 {published data only}
- Rivera ML, Kim TY, Stewart GM, et al. Albuterol nebulized in heliox in the initial ED treatment of pediatric asthma: a blinded, randomized controlled trial. American Journal of Emergency Medicine 2006;24:38‐42. [DOI] [PubMed] [Google Scholar]
Rose 2002 {published data only}
- Rose JS, Panacek EA, Miller P. Prospective randomized trial of heliox‐driven continuous nebulizers in the treatment of asthma in the emergency department. Journal of Emergency Medicine 2002;22:133‐7. [DOI] [PubMed] [Google Scholar]
Xie 2003a {published data only}
- Xie L, Liu Y, Chen L, et al. Inhaling B2‐agonist with heliox‐driven in bronchial asthma. Chinese Medical Journal 2003;116:1011‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Bag 2002 {published data only}
- Bag R, Bandi V, Fromm RE, Guntupalli KK. The effect of heliox‐driven bronchodilator aerosol therapy on pulmonary function tests in patients with asthma. Journal of Asthma 2002;39:659‐65. [DOI] [PubMed] [Google Scholar]
Gluck 1990 {published data only}
- Gluck EH, Onorato DJ, Castriotta R. Helium‐oxygen mixtures in intubated patients with status asthmaticus and respiratory acidosis. Chest 1990;98:693‐8. [DOI] [PubMed] [Google Scholar]
Kass 1995 {published data only}
- Kass JE, Castriotta RJ. Heliox therapy in acute severe asthma. Chest 1995;107:757‐60. [DOI] [PubMed] [Google Scholar]
Kudukis 1997 {published data only}
- Kudukis TM, Manthous CA, Schmidt GA, Hall JB, Wylam ME. Inhaled helium‐oxygen revisted: effect of inhaled helium‐oxygen during the treatment of status asthmaticus in children. Journal of Pediatrics 1997;130:217‐24. [DOI] [PubMed] [Google Scholar]
L'Her 2000 {published data only}
- L'Her E, Monchi M, Joly B, Marichy J, Lejay M, Dhainaut JF. Helium‐oxygen breathing in the early emergency care of acute severe asthma: a randomized pilot study. European Journal of Emergency Medicine 2000;7:271‐5. [DOI] [PubMed] [Google Scholar]
Lin Lee 2005b {published data only}
- Lin Lee D, Wel Hsu C, Lee H, et al. Beneficial effects of albuterol therapy driven by heliox versus by oxygen in severe asthma exacerbation. Academic Emergency Medicine 2005;12(9):820‐7. [DOI] [PubMed] [Google Scholar]
Manthous 1995 {published data only}
- Manthous CA, Hall JB, Melmed A, Caputo MA, Walter J, Klocksieben JM, et al. Heliox improves pulsus paradoxus and peak expiratory flow in non‐intubated patients with severe asthma. American Journal of Respiratory and Critical Care Medicine 1995;151:310‐4. [DOI] [PubMed] [Google Scholar]
Schaeffer 1999 {published data only}
- Schaeffer EM, Pohlman A, Morgan S, Hall JB. Oxygenation in status asthmaticus improves during ventilation with helium‐oxygen. Critical Care Medicine 1999;27:2666‐70. [DOI] [PubMed] [Google Scholar]
Shiue 1989 {published data only}
- Shiue ST, Gluck EH. The use of helium‐oxygen mixtures in the support of patients with status asthmaticus and respiratory acidosis. Journal of Asthma 1989;26:177‐80. [DOI] [PubMed] [Google Scholar]
Verbeek 1998 {published data only}
- Verbeek PR, Chopra A. Heliox does not improve FEV1 in acute asthma patients. Journal of Emergency Medicine 1998;16:545‐8. [DOI] [PubMed] [Google Scholar]
Xie 2003b {published data only}
- Xie L, Liu Y, Chen L, Hao F, Jin G, Zhao H. Inhaling beta(2)‐agonist with heliox‐driven in bronchial asthma. Chinese Medical Journal 2003;116(7):1011‐5. [PubMed] [Google Scholar]
Additional references
Altman and Bland 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 1993
- Anderson M, Svartengren M, Bylin G, Philipson K, Camner P. Deposition in asthmatics of particles inhaled in air or in helium‐oxygen. American Review of Respiratory Disease 1993;147:524‐8. [DOI] [PubMed] [Google Scholar]
Barach 1935
- Barach AL. The use of helium in the treatment of asthma and obstructive lesions in the larynx and trachea. Annals of Internal Medicine 1935;9:739‐65. [Google Scholar]
Goode 2001
- Goode ML, Fink JB, Dhand R, Tobin M. Improvement in aerosol with helium‐oxygen mixtures during mechanical ventilation. American Journal of Respiratory and Critical Care Medicine 2001;163:109‐14. [DOI] [PubMed] [Google Scholar]
Hess 1999
- Hess DR, Acosta FI, Ritz RH, Kacmareck RM, Camargo CA. The effect of heliox on nebulizer function using a beta‐agonist bronchodilator. Chest 1999;115:184‐9. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deecks JJ, et al. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized controlled trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Manthous 1997
- Manthous CA, Morgan S, Pohlman A, Hall JB. Heliox in the treatment of airflow obstruction: a critical review of the literature. Respiratory Care 1997;42:1034‐42. [Google Scholar]
References to other published versions of this review
Rodrigo 2001
- Rodrigo G, Rodrigo C, Pollack C, Travers A. Helium‐oxygen mixture for nonintubated acute asthma patients. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD002884] [DOI] [PubMed] [Google Scholar]


