ABSTRACT
Living systems, from micro- to macro-scales, are strongly impacted by physical factors such as temperature, pH, and the concentration of compounds in their surrounding environment. In the macro-world, it is obvious that small changes in these parameters can have profound, and even devastating, impacts on an ecosystem. For example, in the case of global warming, a change in climate, and specifically a few degrees in temperature, has taken one million species of animals to the brink of extinction. Scale things down 6 orders of magnitude, our gut microbiota also experiences similar changes in temperature due to disease. In this highly competitive environment, physical perturbations inflict long-term consequences on the microbiota ecosystem and, in turn, on the host organism. My laboratory is exploring the feedback between the gut’s physical environment, the microbiota, and disease. Our research highlights the importance of measuring physical parameters for the prediction of microbial dynamics and microbiota therapies.
KEYWORDS: gut biogeography, microbiota, physical perturbations
COMMENTARY
When I think of the physical environment and its effect on living systems, I cannot avoid reflecting on global warming, and how many animal and plant species we are at risk of losing (the estimate is a staggering one-third [1]). As a naive biophysicist entering the microbiology field during my Ph.D., I realized that living systems at all scales, from plants and animals down to microbes, are impacted by the physical environment. For example, we normally think of a fever as a short, transient illness, but it affects the trillions of microorganisms in our gut over tens of their generations, similar generational timescales as those on which global warming impacts mammals. In the macro-scale world, global warming affects climate and weather; could this be akin to a fever’s impact on gut motility and host responses? Following the analogy, could a fever lead to the loss of microbial species in our gut?
Our gut microbiota functions as a personalized pharmacy in our gut—anything our microbes excrete has the potential to make it through our blood system and impact all organs, just like the compounds in a pill taken orally. Can major changes in the gut environment modify the availability of these compounds, and hence human health? My laboratory is addressing these questions by investigating how physical factors due to natural physiology, disease, and industrialization affect the gut microbiota and host health.
PHYSICAL FACTORS SHAPE OUR GUT-ASSOCIATED MICROBIOTA
Physical factors are important to microbes living in host-associated environments but are often overlooked. Here I am referring to physical factors as features of an ecosystem that lead to physical forces. For example, a sugar such as glucose has a biochemical impact on a microbial ecosystem, but it’s also a solute that impacts the osmotic pressure of the habitat. Similarly, while short chain fatty acids are crucial microbial metabolites, they are also key molecules affecting gut pH balance and hydrogen potential.
We usually focus on the biochemical interactions between microbes and their host. These are of course critical—understanding metabolic function is essential to achieving predictability in these complex communities. However, just like at the macro-scale, the physical environment sets fundamental ground rules that can supersede biochemical interactions.
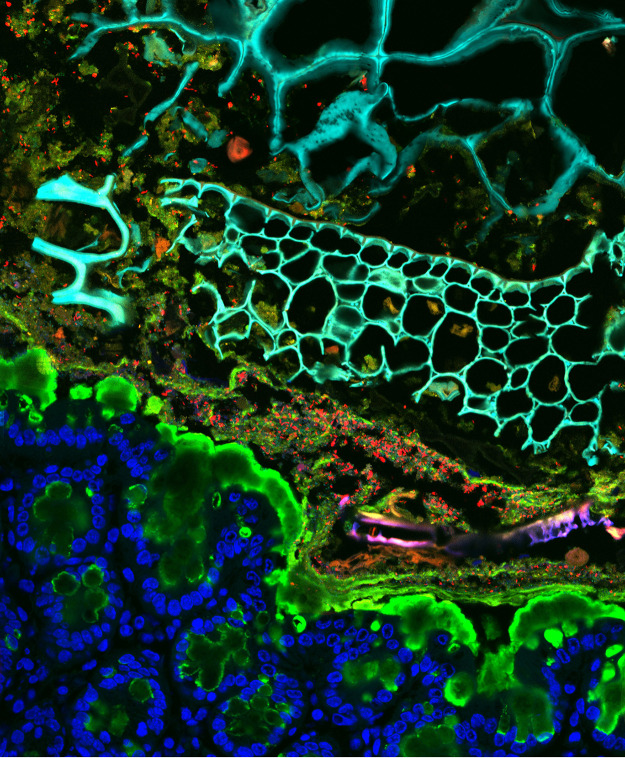
The intestinal gut environment is determined by multiple physical parameters, including osmolality, pH, flow, and mucus stiffness, among many others; these parameters vary along the length of the gut and are tightly regulated by gut epithelial absorption and secretion. The importance of gut biogeography and physical factors in the establishment of bacterial communities is well documented (2–4), but it has been minimally explored as a driver of community composition. The physical environment is particularly important in the limiting cases—e.g., bacteria that are unable to grow at low pH won’t be able to take advantage of the high nutrient concentration in the stomach. Physical niches in the intestines are carefully modulated by a myriad of host secretions such as bile acids, gastric fluid, and bicarbonate (5–7). The microbiota itself is also able to feedback on the physical environment via fermentation products and excretion of compounds such as short-chain fatty acids. Thanks to all of these factors and feedbacks, the pH of a healthy gut naturally varies along the digestive system by as much as 4 logs (5). Therefore, even without considering the changing biochemical and immunological niches, microbes are in steep competition to grow in selected locations in their journey through the gut. By the time bacteria are excreted as stool, this rich history of exploration through multiple complex environments is all but lost. Only via in situ measurements can we start to appreciate the complexity of these ecosystems, comprising multiple habitats within a single gastrointestinal tract. In our lab we leverage physical measurements of the gut environment paired with imaging and spatial measurements to explore this complexity at the single-cell level (Fig. 1). If the gut environment is heterogeneous in health, what happens during disease?
FIG 1.
In situ imaging of the gastrointestinal tract highlights a complex environment. Intestinal sections were stained with 4′,6-diamidino-2-phenylindole (DAPI) (false colored blue in tissue and cyan in lumen) and labeled with fluorescence in situ hybridization (FISH) probes (red, Bacteroidaceae; green, Enterococcus) and UEA-1 (green) to label mucus.
DISEASE RESHAPES THE GUT ENVIRONMENT
As one might expect, while the heterogeneity of the gut environment is tightly regulated in health, this control can be largely lost during host illness. Disease can cause local and systemic changes to the gut physical environment such as changes in pH in inflammatory bowel disease (IBD) (5), changes in osmolality and flow due to malabsorption and diarrhea (8), or changes in temperature due to inflammation (9, 10). These changes in the physical environment naturally impact the resident microbiota and apply strong selective pressures. Beyond constraining the ability of organisms to grow within an environment, physical stressors also greatly impact cellular physiology even in regimes where growth can occur. For example, hyperosmotic stress, as caused by malabsorption in the gut, leads to the outflow of water into the gut lumen (from epithelial and bacterial cells alike) to compensate for the increased external molecule concentration. Water excretion mechanically impacts the bacterial envelope due to reduced turgor pressure inside the cell. Furthermore, reduced water in the cytoplasm changes molecular interactions because of increased crowding. Which microbes best survive these changes? Unfortunately for the host, it’s the pathogens, which tend to have a wider arsenal of stress response pathways than commensal bacteria. Infectious agents can even reinforce the disrupted habitat, further hampering recovery of the microbiota and gut health (which we recently reviewed in the context of gut biogeography [11]). Diseased states can become stable and lead to chronic conditions where homeostasis is not retained by the host. Unsurprisingly, loss of the natural gut equilibrium feeds back on the microbiota. Importantly, our lab is finding that the impact of chronic diseases cannot be unraveled without comprehending how the changed physical environment sets up changed habitats affecting drug delivery and the ability of microbes to colonize, grow, and evolve.
EVOLUTION AND ERADICATION IN THE MICROBIOTA
When physical stresses are prolonged, microbes may need to modify complex cellular components, such as the cell wall in the case of osmotic stress, to adapt. These adaptations are harder to achieve than modifying individual proteins to develop resistance (e.g., an uptake channel in the case of response to an antibiotic). This means that the physical environment can strongly limit the capacity of an organism to persist within a competitive habitat because of the complexity of the adaptations that are required.
Importantly, even short perturbation timescales from the perspective of the host affect many generation cycles for the microbiota. Supporting this idea, during my postdoc, as I was analyzing my first microbiome data set, I found that increasing gut osmolality by inducing malabsorption over a period of days led a single family comprising almost 50% of the bacteria to disappear (12). Had I made a mistake? How could such a short perturbation lead to such a devastating effect? After repeating this experiment in multiple different communities, I realized that we were witnessing the wiping out of an entire family of bacteria from an ecosystem due to a change in the physical environment. I remember trying to explain to my family how big of an effect this is. Think of taking away all felines or primates from a habitat—this is what eradicating a family of organisms is like at the macro-scale.
We later demonstrated that members of this family, called Muribaculaceae (or S24-7), are highly sensitive to increased osmolality in vitro, leading them to disappear during osmotic laxative treatment in vivo and not return postrecovery (12). Interestingly, upon external reintroduction, we were able to reestablish this family in the gut, but only provided that the osmolality levels were normalized (12), highlighting the importance of the physical environment for microbiota colonization as well as maintenance. As we started following up on this work, we wondered, what happens if lost microbes are not externally reintroduced?
ARE WE CHANGING OUR MICROBIOTA FOR THE WORSE?
A major driver for changes in the physical environment at the macro-scale is industrialization. Over the past 150 years, industrialization has impacted living systems at all scales, with lasting effects. We have heavily modified our gut habitat because we have changed the way we eat, fight disease, and interact with our environment. Parallel to macro-scale physical environment changes such as global warming and acidification, modern drugs such as antacids and laxatives can significantly disrupt the micro-scale physical environment within our bodies. Nearly all efforts to study the impact of the gut microbiota in health and disease have focused on the effect of microbes present in the microbiota; however, recent strong evidence also points to the critical role for foundational microbial members missing due to lifestyle changes and industrialization (13, 14). Reduced gut microbial diversity has been linked to numerous “modern” diseases such as inflammatory bowel disease, obesity, allergies, and autoimmune disorders (15). My lab is deeply interested in understanding how changes to the physical environment due to modern diseases lead to the disappearance of beneficial microbes, and how to counteract those changes.
As an example, the Muribaculaceae family, which we found to be so negatively impacted by osmotic laxatives, was named as the most prevalent proximal gut bacterium in homeothermic animals (16); however, it was found in only 10% of individuals in industrialized human populations (17). Interestingly, Muribaculaceae are highly prevalent in traditional uncontacted Amerindians (18) as well as the Hadza in Tanzania (19), indicating that they may be vanishing in industrialized countries due to changes in lifestyle. There is accumulating evidence that Muribaculaceae presence is anticorrelated from certain industrialized world diseases such as diabetes (20, 21), and its disappearance correlates with the increasing prevalence and incidence of these diseases (13). While we do not yet know the mechanisms and involvement of these disappearing species in our health, we may have a fleeting time window of opportunity to do so prior to their complete disappearance in natural ecosystems.
As with global warming on our planet, we have begun showing how chronic environmental changes in our gut can drive living organisms to vanish. We still know so little about basic effects of the physical environment on our microbiota, and yet they shape our health and wellbeing in countless ways. Mirroring traditional ecology, where pH, temperature, and other factors have always been at the very heart of understanding, my goal is to bring these fundamentals to the forefront of microbiome science as we seek to understand, predict, modify, and, importantly, safeguard the incredible microbial ecosystem we harbor within us. We were lucky to catch Muribaculaceae before it disappeared—what else are missing that may be on the brink of extinction?
ACKNOWLEDGMENTS
I would like to thank Sean Gibbons for nominating me for this commentary and Kevin Foster, Thad Hughes, and Katharine Ng for helpful conversations and feedback.
Our work on the impact on the physical environment could not be possible without support from the NSERC Discovery Grant, CIHR Team Grant: Canadian Microbiome Initiative 2, Crohn’s and Colitis Canada, CIFAR, Michael Smith Foundation for Health Research Scholar Award, the Weston Foundation: Weston Family Microbiome Initiative, Paul Allen Distinguished Investigator Award, Scialog, Research Corporation for Science Advancement, Frederick Gardner Cottrell Foundation, Paul G. Allen Frontiers Group (27872), Johnson & Johnson Women in STEM2D Award, and Canada Foundation for Innovation.
The views expressed in this article do not necessarily reflect the views of the journal or of ASM.
This article is part of a special series sponsored by Floré.
REFERENCES
- 1.Román-Palacios C, Wiens JJ. 2020. Recent responses to climate change reveal the drivers of species extinction and survival. Proc Natl Acad Sci USA 117:4211–4217. doi: 10.1073/pnas.1913007117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tropini C, Earle KA, Huang KC, Sonnenburg JL. 2017. The gut microbiome: connecting spatial organization to function. Cell Host Microbe 21:433–442. doi: 10.1016/j.chom.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R. 2009. Bacterial community variation in human body habitats across space and time. Science 326:1694–1697. doi: 10.1126/science.1177486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Donaldson GP, Lee SM, Mazmanian SK. 2016. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol 14:20–32. doi: 10.1038/nrmicro3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nugent SG, Kumar D, Rampton DS, Evans DF. 2001. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut 48:571–577. doi: 10.1136/gut.48.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vernia P, Caprilli R, Latella G, Barbetti F, Magliocca FM, Cittadini M. 1988. Fecal lactate and ulcerative colitis. Gastroenterology 95:1564–1568. doi: 10.1016/S0016-5085(88)80078-7. [DOI] [PubMed] [Google Scholar]
- 7.Hove H, Mortensen PB. 1995. Influence of intestinal inflammation (IBD) and small and large bowel length on fecal short-chain fatty acids and lactate. Dig Dis Sci 40:1372–1380. doi: 10.1007/BF02065554. [DOI] [PubMed] [Google Scholar]
- 8.Juckett G, Trivedi R. 2011. Evaluation of chronic diarrhea. Am Fam Physician 84:1119–1126. [PubMed] [Google Scholar]
- 9.Kluger MJ. 1989. Body temperature changes during inflammation: their mediation and nutritional significance. Proc Nutr Soc 48:337–345. doi: 10.1079/pns19890049. [DOI] [PubMed] [Google Scholar]
- 10.Cunha BA. 2007. Fever of unknown origin: focused diagnostic approach based on clinical clues from the history, physical examination, and laboratory tests. Infect Dis Clin North Am 21:1137–1187. doi: 10.1016/j.idc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen J, Pepin DM, Tropini C. 2021. Cause or effect? The spatial organization of pathogens and the gut microbiota in disease. Microbes Infect 23:104815. doi: 10.1016/j.micinf.2021.104815. [DOI] [PubMed] [Google Scholar]
- 12.Tropini C, Moss EL, Merrill BD, Ng KM, Higginbottom SK, Casavant EP, Gonzalez CG, Fremin B, Bouley DM, Elias JE, Bhatt AS, Huang KC, Sonnenburg JL. 2018. Transient osmotic perturbation causes long-term alteration to the gut microbiota. Cell 173:1742–1754.e17. doi: 10.1016/j.cell.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonnenburg JL, Sonnenburg ED. 2019. Vulnerability of the industrialized microbiota. Science 366:eaaw9255. doi: 10.1126/science.aaw9255. [DOI] [PubMed] [Google Scholar]
- 14.Sonnenburg ED, Smits SA, Tikhonov M, Higginbottom SK, Wingreen NS, Sonnenburg JL. 2016. Diet-induced extinctions in the gut microbiota compound over generations. Nature 529:212–215. doi: 10.1038/nature16504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mosca A, Leclerc M, Hugot JP. 2016. Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front Microbiol 7:455. doi: 10.3389/fmicb.2016.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson LR, Sanders JG, McDonald D, Amir A, Ladau J, Locey KJ, Prill RJ, Tripathi A, Gibbons SM, Ackermann G, Navas-Molina JA, Janssen S, Kopylova E, Vázquez-Baeza Y, González A, Morton JT, Mirarab S, Zech Xu Z, Jiang L, Haroon MF, Kanbar J, Zhu Q, Jin Song S, Kosciolek T, Bokulich NA, Lefler J, Brislawn CJ, Humphrey G, Owens SM, Hampton-Marcell J, Berg-Lyons D, McKenzie V, Fierer N, Fuhrman JA, Clauset A, Stevens RL, Shade A, Pollard KS, Goodwin KD, Jansson JK, Gilbert JA, Knight R, Earth Microbiome Project Consortium. 2017. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 551:457–463. doi: 10.1038/nature24621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagkouvardos I, Lesker TR, Hitch TCA, Gálvez EJC, Smit N, Neuhaus K, Wang J, Baines JF, Abt B, Stecher B, Overmann J, Strowig T, Clavel T. 2019. Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 7:28. doi: 10.1186/s40168-019-0637-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clemente JC, Pehrsson EC, Blaser MJ, Sandhu K, Gao Z, Wang B, Magris M, Hidalgo G, Contreras M, Noya-Alarcón Ó, Lander O, McDonald J, Cox M, Walter J, Oh PL, Ruiz JF, Rodriguez S, Shen N, Song SJ, Metcalf J, Knight R, Dantas G, Dominguez-Bello MG. 2015. The microbiome of uncontacted Amerindians. Sci Adv 1:e1500183. doi: 10.1126/sciadv.1500183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ormerod KL, Wood DLA, Lachner N, Gellatly SL, Daly JN, Parsons JD, Dal’Molin CGO, Palfreyman RW, Nielsen LK, Cooper MA, Morrison M, Hansbro PM, Hugenholtz P. 2016. Genomic characterization of the uncultured Bacteroidales family S24-7 inhabiting the guts of homeothermic animals. Microbiome 4:36. doi: 10.1186/s40168-016-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Livanos AE, Greiner TU, Vangay P, Pathmasiri W, Stewart D, McRitchie S, Li H, Chung J, Sohn J, Kim S, Gao Z, Barber C, Kim J, Ng S, Rogers AB, Sumner S, Zhang X-S, Cadwell K, Knights D, Alekseyenko A, Bäckhed F, Blaser MJ. 2016. Antibiotic-mediated gut microbiome perturbation accelerates development of type 1 diabetes in mice. Nat Microbiol 1:16140. doi: 10.1038/nmicrobiol.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X-S, Li J, Krautkramer KA, Badri M, Battaglia T, Borbet TC, Koh H, Ng S, Sibley RA, Li Y, Pathmasiri W, Jindal S, Shields-Cutler RR, Hillmann B, Al-Ghalith GA, Ruiz VE, Livanos A, van ‘T Wout AB, Nagalingam N, Rogers AB, Sumner SJ, Knights D, Denu JM, Li H, Ruggles KV, Bonneau R, Williamson RA, Rauch M, Blaser MJ. 2018. Antibiotic-induced acceleration of type 1 diabetes alters maturation of innate intestinal immunity. Elife 7:e37816. doi: 10.7554/eLife.37816. [DOI] [PMC free article] [PubMed] [Google Scholar]