Abstract
Background
This is an update of a Cochrane Review first published in The Cochrane Library in Issue 2, 2006.
Allergic rhinitis represents a global health problem. Non‐specific nasal hyper‐responsiveness is an important feature of allergic and non‐allergic rhinitis. This phenomenon is believed to result from the effect of allergic inflammation on the sensory nerves that supply the upper airway mucosa. A pharmacological agent that has proved useful in the investigation of effects of neuronal stimulation is capsaicin, the pungent component of hot pepper. Intranasal capsaicin specifically stimulates afferent nerves consisting mostly of unmyelinated C fibers and some myelinated A‐delta fibers. As a result it can trigger central and axonal reflexes, the latter being putatively mediated by the release of neuropeptides. Capsaicin, as a blocking agent of neuropeptides, blocks the axon reflex and may exert a curative effect on allergic rhinitis.
Objectives
To assess the effectiveness of capsaicin for allergic rhinitis in adults.
Search methods
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL); PubMed; EMBASE; CINAHL; Web of Science; BIOSIS Previews; Cambridge Scientific Abstracts; mRCT and additional sources for published and unpublished trials. The date of the most recent search was 2 September 2009.
Selection criteria
Randomized controlled trials of capsaicin for allergic rhinitis in adults.
Data collection and analysis
Three authors read each paper, blind to its identity. Decisions concerning inclusion were made by simple majority. We all performed quality assessment independently.
Main results
One small trial did not find evidence that intranasal capsaicin had a therapeutic effect in allergic rhinitis. A small pharmacological effect on clinical histamine dose response was found. After treatment, leukotriene levels in nasal lavage did not increase in the capsaicin group.
Authors' conclusions
There is insufficient evidence to assess the use of capsaicin in clinical practice.
Plain language summary
Capsaicin for allergic rhinitis
Allergic rhinitis is a common health problem affecting between 10% and 25% of the population, and its prevalence is increasing. The symptoms include a running or blocked nose, itching and sneezing. Several drug therapies are available including antihistamines and steroids. Capsaicin, which is the pungent component of hot pepper, has also been used. With repeated doses applied topically it may desensitize the lining of the nose and have a therapeutic effect on allergic rhinitis. The review found one small, low quality randomized controlled trial which did not demonstrate a therapeutic effect of capsaicin on allergic rhinitis symptoms. Further trials are needed.
Background
This is an update of a Cochrane Review first published in The Cochrane Library in Issue 2, 2006.
Allergic rhinitis represents a global health problem. It is an extremely common disease worldwide affecting 10% to 25% of the population (IRMWG 1994) and an increasing prevalence in the last few decades has been recognized (Aberg 1996).
Allergic rhinitis is clinically defined as a symptomatic disorder of the nose induced by an IgE‐mediated inflammation following exposure of the membranes lining the nose to allergens such as dust mites, moulds and pollens. Symptoms of rhinitis include rhinorrhea, nasal obstruction, nasal itching and sneezing, which are reversible spontaneously or with treatment.
Allergic rhinitis was previously classified, based on time of exposure, as seasonal, perennial or occupational. A new classification has been developed which is based on duration of symptoms and distinguishes allergic rhinitis as 'intermittent' or 'persistent' (ARIA 2008). According to this classification, intermittent allergic rhinitis (symptoms for less than four days per week, or for less than four weeks) corresponds most closely with seasonal allergic rhinitis (hay fever). Persistent (symptoms for more than four days per week, or for more than four weeks) would correspond most closely with perennial and occupational allergic rhinitis.
A diagnosis of allergic rhinitis is made by:
positive history;
positive skin test for prevalent aeroallergen and/or by allergen specific serum IgE;
demonstration that the symptoms are the results of IgE‐mediated inflammation (Skoner 2001).
Patients with non‐allergic rhinitis with eosinophilia syndrome (NARES), where nasal eosinophilia is present yet skin tests or serum IgE levels reveal no evidence of an allergic cause, will not be considered in this review.
The concept of 'minimal persistent inflammation' is a new but important hypothesis that was proposed by Ciprandi et al (Ciprandi 1995) and is a feature of perennial and seasonal allergy (Ricca 2000). It is characterized by an inflammation made up of different cells. The symptoms are currently considered to be caused mainly by the accumulation and activation of infiltrating cells, such as mast cells, basophils and eosinophils, which release mediators and cytokines and result in allergic inflammation.
Non‐specific nasal hyper‐responsiveness is also an important feature of allergic and non‐allergic rhinitis. It can be defined as increased nasal responsiveness to a normal stimulus resulting in sneezing, nasal congestion and secretion (Gerth van Wijk 1999). This phenomenon is believed to result from the effect of allergic inflammation on the sensory nerves that supply the upper airway mucosa. The nerves present in nasal mucosa include cholinergic nerves and nerves of the non‐adrenergic, non‐cholinergic system (NANC). Sensory C fibers contain neuropeptides, such as substance P, neurokinin A and K, and calcitonin gene‐related peptide (Bousquet 2001). In vitro studies have indicated sensory neuropeptides induce neutrophil adhesion, stimulate proliferation of human T lymphocytes and increase the production of inflammatory cytokines by human monocytes (Stjarne 1998). Various non‐allergic triggers have been shown to act on the nasal mucosa through sensorineural stimulation. Also, in allergic rhinitis stimulation of sensory nerves per se can produce inflammatory changes, a phenomenon known as neurogenic inflammation (Togias 2000).
Management options for allergic rhinitis include allergen avoidance, medication, immunotherapy and education. Medications include oral and topical H1‐antihistamines, decongestants, leukotriene antagonists and chromones, topical glucocorticosteroids and anti‐cholinergics. These drugs may provide immediate control of symptoms, however, they have no long‐lasting effect when stopped (Bousquet 2001).
A pharmacologic agent that has proved useful in the investigation of effects of neuronal stimulation is capsaicin (8‐methyl‐n‐vanillyl‐6‐nomamide), the pungent component of hot pepper. Intranasal capsaicin specifically stimulates afferent nerves consisting mostly of unmyelinated C fibers and some myelinated A‐delta fibers. As a result it can trigger central and axonal reflexes, the latter being putatively mediated by the release of neuropeptides (Sanico 1997). Capsaicin, as a blocking agent of neuropeptides, especially substance P, blocks the axon reflex and exerts a curative effect on allergic rhinitis (Zhang 1995). Capsaicin pretreatment of the nasal mucosa may result in a long‐lasting amelioration of symptoms upon allergen challenge in patients with allergic rhinitis (Stjarne 1998) and non‐allergic rhinitis (Lacroix 1991). The duration of the treatment may be one to two months. Neurogenic mechanisms play a role in nasal allergic inflammation and repeated topical capsaicin applications induce desensitization of the human nasal mucosa in vivo, with a reduction in the tissue content of neuropeptides (Lacroix 1991).
We have systematically reviewed the effectiveness and safety of capsaicin in the treatment of allergic rhinitis in adults.
Objectives
To assess the effectiveness of capsaicin for allergic rhinitis in adults.
To determine the incidence of side effects.
To ascertain a suitable protocol for capsaicin administration.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs), irrespective of publication status, date of publication or language.
Types of participants
Adult patients with allergic rhinitis in general practice or outpatient departments.
Types of interventions
Comparisons sought were:
topical nasal capsaicin versus topical placebo.
We also considered comparisons between different topical nasal capsaicin preparations.
Types of outcome measures
Primary outcomes
Improvement of symptoms (global symptoms).
Secondary outcomes
Visual analogue scales (VAS).
Rhinitis quality of life (RQL).
Nasal lavage leukotrienes.
Follow‐up time was to be a year after treatment if possible.
Search methods for identification of studies
We conducted systematic searches for randomised controlled trials. There were no language, publication year or publication status restrictions. The date of the last search was 2 September 2009.
Electronic searches
We searched the Cochrane Ear, Nose and Throat Disorders Group Trials Register; the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library Issue 3, 2009); PubMed; EMBASE; AMED; CINAHL; LILACS; KoreaMed; IndMed; PakMediNet; CAB Abstracts; Web of Science; BIOSIS Previews; mRCT (Current Controlled Trials); Clinicaltrials.gov;ICTRP (International Clinical Trials Registry Platform) and Google.
We modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, we combined subject strategies with adaptations of the highly sensitive search strategy designed by the Cochrane Collaboration for identifying randomised controlled trials and controlled clinical trials (as described in The Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1, Box 6.4.b. (Handbook 2008)).
CENTRAL search strategy
#1 MeSH descriptor Rhinitis, Allergic, Perennial explode all trees #2 MeSH descriptor Rhinitis, Allergic, Seasonal explode all trees #3 MeSH descriptor Rhinitis, this term only #4 rhiniti* #5 (#3 OR #4) #6 MeSH descriptor Hypersensitivity explode all trees #7 allerg* or hypersensitiv* #8 (#6 OR #7) #9 (#5 AND #8) #10 (perennial:ti or persistent:ti or nonseasonal:ti or nose:ti or nasal:ti or cat*:ti or fur:ti or hair*:ti or dander:ti or dust*:ti or mite*:ti or pet*:ti or dog*:ti or cockroach*:ti #11 (seasonal:ti or intermittent:ti or spring:ti or summer:ti or pollen:ti or grass*:ti or birch:ti or ragweed:ti or tree*:ti or weed*:ti or mugwort:ti or willow:ti or alder:ti) #12 (#10 OR #11) #13 (( #5 OR #8 ) AND #12) #14 (hayfever OR "hay fever" OR pollenosis OR pollinosis OR SAR) #15 (#1 OR #2 OR #9 OR #13 OR #14) #16 MeSH descriptor Capsaicin explode all trees #17 (Capsaicin* OR Axsain OR Zacin OR Capsicum OR Capsidol OR Zostrix OR Capzasin OR Gelcen OR Katrum OR Capsin) #18 #16 OR #17 #19 #15 AND #20
Search strategies for other key databases including PubMed are shown in Appendix 1.
Searching other resources
We scanned reference lists of identified studies for further trials. We searched PubMed, TRIPdatabase, NHS Evidence ‐ ENT and audiology, and Google to retrieve existing systematic reviews possibly relevant to this systematic review, in order to search their reference lists for additional trials. Abstracts from conference proceedings were sought via the Cochrane Ear, Nose and Throat Disorders Group Trials Register.
For the original review, the principal authors of the identified randomized controlled trials were contacted for additional studies. Pharmaceutical companies involved in the production of topical nasal capsaicin were contacted in order to obtain unpublished randomized controlled trials.
Data collection and analysis
Study selection
The titles and abstracts of all reports identified through the searches were scanned by two authors. Disagreement as to which papers to include was resolved by consensus. Full reports were obtained for trials appearing to meet the inclusion criteria or for which there was insufficient information in the title and abstract to make a clear decision.
Quality assessment
Methodological quality assessment was performed independently by all three authors. All used two different methods of assessment. Firstly the Cochrane approach was used to assess allocation concealment. All trials were scored and entered using the following principles:
Grade A: adequate concealment; Grade B: unclear concealment; Grade C: obviously not adequate concealment.
In addition, each study was assessed using a 0 to 5 scale described by Jadad 1996 and summarized as follows:
Was the study described as randomized? (1 = Yes, 0 = No) Was the study described as being double‐blind? (1 = Yes, 0 = No) Was there a description of withdrawals and drop‐outs? (1 = Yes, 0 = No) Was the method of randomization well described and appropriate? (1 = Yes, 0 = No) Was the method of double‐blinding well described and appropriate? (1 = Yes, 0 = No) Deduct one point if methods for randomization or blinding were inappropriate.
Data extraction
Data were independently extracted by two authors (Cheng J and Yang XN) using specially designed data extraction forms. The characteristics of the trial participants, interventions and outcomes for the included trial were presented in a study table. Authors were contacted for clarification or further information.
Data analysis
Had sufficient trials been identified we had planned to calculate a weighted treatment effect across trials using the Cochrane statistical package in RevMan 5.0 (RevMan 2008). For continuous outcomes, a weighted mean difference (WMD) or a standardized mean difference (SMD) would have been calculated as appropriate. Should suitable trials be identified for updates of this review we will employ the following methods.
For dichotomous outcomes a relative risk (RR) will be calculated. Pooled treatment effects will be expressed with their 95% confidence intervals (95% CI). Heterogeneity of effect size across pooled studies will be calculated, and the I2 statistic will be calculated in RevMan. Sensitivity analyses will be carried out on the basis of methodological quality.
Results
Description of studies
Following the update searches in September 2009, a total of 13 references were obtained. From these we identified no studies which met the inclusion criteria for this review. We listed one study as ongoing (White 2008) and excluded a further two studies (Ciabatti 2009; Sanico 1998). See 'Characteristics of excluded studies' for reasons for the exclusion of studies.
For the original review, electronic and manual searches identified 48 potential trials and reviews. Of these, a total of eight articles were identified for possible inclusion. After reviewing the methods section of each of these studies, we excluded seven from the review (see 'Characteristics of excluded studies' for details). One trial met the inclusion criteria (Gerth van Wijk 2000). For a full description please see the table of 'Characteristics of included studies'.
Study design
Gerth van Wijk 2000 was a randomized, placebo‐controlled, double‐blind parallel study.
Participants
Twenty‐six participants were recruited to the study. Inclusion criteria were a history of perennial rhinitis and intradermal skin reaction of at least one plus‐sign to 3 BU/mL house dust mite extract. In the case of a concomitant seasonal allergic rhinitis, patients were tested outside the season. Exclusion criteria were patients with nasal polyposis, nasal surgery less than three months before the study and nasal infection during the two weeks before the study.
Interventions
The nasal airway was anaesthetized by three applications (10 mg/puff) of lidocaine base (100 mg/ml) in each nostril. During treatment 0.5 mL solution was sprayed into each nostril (0.15 mg capsaicin). In 14 days, seven applications of capsaicin or placebo were given at two‐day intervals.
Outcome measures
The following outcomes were assessed in the study: rhinitis quality of life (RQL); visual analogue scales (VAS); nasal challenge with house dust mites (HDM) and histamine; symptom scores; nasal lavage and mediator assays. The outcomes were measured before and six weeks after treatment. Visual analogue scores and rhinitis quality of life were additionally assessed three months after treatment. Nasal challenges with house dust mite extract (100 BU/ml, 1000 BU/ml, 10000 BU/ml) were performed at 10‐minute intervals after a challenge with phosphate‐buffered saline (PBS). Nasal responsiveness was monitored using a symptom score from Lebel 1988. The area of the curve (AUC) was calculated as the sum of the three scores. Nasal challenges with histamine (0.25, 0.50, 1.0, 2.0 and 4.0 mg/ml) were performed at five‐minute intervals after challenge with phosphate‐buffered saline. Nasal responsiveness was again monitored using a symptom score from Lebel 1988. The area of the curve was calculated as the sum of the five scores.
Risk of bias in included studies
The included study was randomized, controlled and double‐blinded. The method of randomization was not reported. After the experimental period, there had been drop‐out of three patients from the treatment group and two patients from the placebo group. The study was graded three according to the Jadad Scale.
Effects of interventions
Of the 26 patients recruited in Gerth van Wijk 2000, there were five drop‐outs. One patient treated with capsaicin was lost to follow up because of a long‐lasting stay abroad. Four patients (two in the capsaicin group and two in the placebo group) could not complete the second series of nasal challenges.
With analysis of covariance using the baseline measurement of the outcome variable as covariance, a significant treatment effect on mean rhinitis quality of life scores could not be detected at six weeks or three months, on visual analogue scales at six weeks or three months, or on the area of the curve (house dust mite) at six weeks after treatment (Figure 1). In contrast, a significant effect of capsaicin on the area of the curve for histamine dose response at six weeks (Figure 2) was demonstrated using the outcome of the first histamine challenge as covariance (P = 0.03).
1.
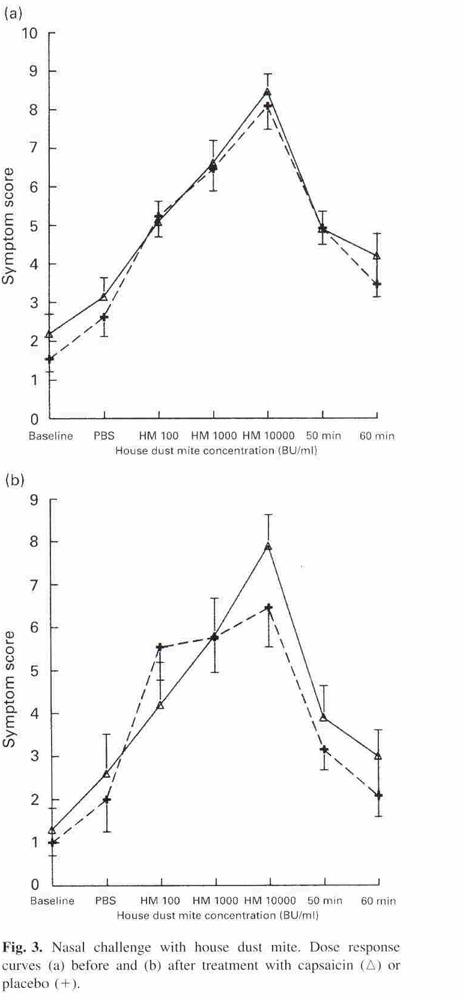
Copyright © [2000] [Blackwell Science Ltd. Gerth Van Wijk R, Terreehorst IT, Mulder PG, Garrelds IM, Blom HM, Popering S. Intranasal capsaicin is lacking therapeutic effect in perennial allergic rhinitis to house dust mite. A placebo‐controlled study. Clinical and Experimental Allergy; 30: 1792‐8]: reproduced with permission.
2.
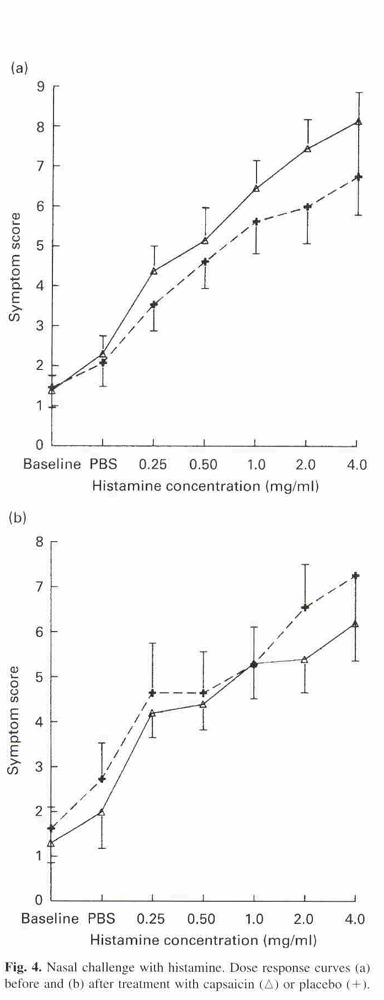
Copyright © [2000] [Blackwell Science Ltd. Gerth Van Wijk R, Terreehorst IT, Mulder PG, Garrelds IM, Blom HM, Popering S. Intranasal capsaicin is lacking therapeutic effect in perennial allergic rhinitis to house dust mite. A placebo‐controlled study. Clinical and Experimental Allergy; 30: 1792‐8]: reproduced with permission.
Leukotrienes in nasal lavage are reported in Table 1. After treatment, leukotriene levels did not increase in the capsaicin group. No adverse effects were reported from this intervention.
1. Leukotrienes in nasal lavage (pg/ml) (Gerth van Wijk 2000).
| Baseline | HDM Challenge | |
| Before treatment placebo group | 83.1 | 453.8 |
| Before treatment capsaicin group | 85.3 | 110.6 |
| After treatment placebo group | 144.7 | 194.3 |
| After treatment capsaicin group | 92.7 | 66.6 |
Discussion
We attempted to identify all randomized controlled trials which studied the use of capsaicin in the treatment of allergic rhinitis. However, despite an exhaustive search of the literature only one study was identified that met the inclusion criteria. The quality of this study was not high; the number of subjects in the trial was small (n = 26) and there were five drop‐outs (19%). After treatment, leukotriene levels decreased in the capsaicin group because inflammation mediators (leukotrienes) are released and depleted by capsaicin.
We also identified seven non‐randomized controlled trials including five before and after studies. Some data from the excluded studies are summarized in Table 2.
2. Data from the excluded studies.
| Study | Subjects' gender M/F | Anaesthesia | Intervention | Dosages | Adverse effect | Follow‐up times | Ages (years) (mean) | Duration of rhinitis | Therapeutic effect |
| Ge 2001 | 58 (38/20) | Diocaine | Spread in inferior turbinate | Not reported Application once a week, total 4 times |
Not reported | Immediately after treatment | 20 to 61 (33) | 2 to 20 ears (7.9) | Positive |
| He 2003 | 41 (21/20) | Not reported | Spread in inferior turbinate and nasal septum | Not reported Application once a day, total 14 times |
Not reported | 9 months | 8 to 50 (33) | 1 to 18 years (5.6) | Positive |
| Liu 2003 | 53 (19/34) | Diocaine | Spread in inferior and middle turbinate and nasal septum | Not reported Application twice a week, total 8 times |
Not reported | 1 year | 14 to 45 (31) | 1 to 17 years | Positive |
| Liu 2004 | 104 (62/42) 84 treatment/20 control | Diocaine | Spread in inferior turbinate and nasal septum | Not reported Application once a week, total 4 times |
Not reported | 1 month | 18 to 60 (36) | Not reported | Positive |
| Stjarne 1998 | 9 (5/4) 5 (treatment)/4 (control) |
Lidocaine | Capsaicin and saline‐soaked cotton wool in the nose | 30 uM Application once a day, total 2 times |
Pain reported; unclear in what proportion of participants | 2 months | 25 to 33 (28) | Not reported | Positive |
| Zhang 1995 | 50 (18/32) | Diocaine | Spread in inferior and middle turbinate and nasal septum | Not reported Application once a week, total 4 times |
Not reported | Immediately after treatment | (30.7) | (7.9 years) | Positive |
| Zhang 1999 | 100 (72/28) | Diocaine | Spread in inferior and middle turbinate | 0.2 mg | Not reported | 1 year | (35.4) | (5.6 years) | Positive |
Further well‐constructed randomized controlled trials will be required to determine whether capsaicin is effective in the treatment of allergic rhinitis. Two factors which could be considered in future studies are method of administration and location of treatment.
In the Gerth van Wijk 2000 study the capsaicin (0.5 mL solution/0.15 mg capsaicin) was sprayed into each nostril. This may have resulted in a large amount of discharge and dilution of the drug. It has been suggested that capsaicin at higher doses may be more effective. Sanico 1997 studied capsaicin treatment with 1 ug, 10 ug and 100 ug; changes in symptom scores, leukocyte counts and albumin and lysozyme levels indicated generally increasing effects with higher capsaicin doses. Sanico 1998 concluded that high‐dose capsaicin induces plasma extravasation in the human nose and that this effect is neuronally mediated. Application by means of, for example, capsaicin‐soaked cotton wool in the nose may achieve administration of a higher dose by prolonging contact of the drug with the nasal mucosa.
Location of treatment may also be a relevant factor: an abundance of sensory neuropeptides above the middle turbinate may suggest that delivery of capsaicin to that area would be more beneficial.
Authors' conclusions
Implications for practice.
One small trial did not find evidence of a therapeutic effect of intranasal capsaicin in allergic rhinitis but did find evidence of a small effect on the area of the curve for histamine dose response. After treatment, leukotriene levels in nasal lavage did not increase in the capsaicin group. There is insufficient evidence to assess the use of capsaicin in clinical practice.
Implications for research.
This appears to be a field of treatment for allergic rhinitis that warrants further research. This would require larger randomized trials, standardized rating scales and outcome measures (improvement of symptoms, visual analogue scales and rhinitis quality of life) and similar time periods (one or two months). It would be interesting to study the effects of increasing the length of time capsaicin is in contact with the nasal mucosa and the application of the drug to the top of the nose.
What's new
| Date | Event | Description |
|---|---|---|
| 3 December 2009 | New search has been performed | New searches were run in September 2009. No new studies were identified for inclusion. |
History
Protocol first published: Issue 4, 2003 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 20 October 2008 | Amended | Converted to new review format. |
Acknowledgements
We thank Martin Burton, Jenny Bellorini, Carolyn Doree and Annette Foley for their insightful comments and editorial assistance. We especially thank Jenny Bellorini from the Cochrane ENT Group for encouragement, expert critique and technical support.
Appendices
Appendix 1. Search strategies
| PubMed | EMBASE (Ovid) | CINAHL (EBSCO) |
| #1 "rhinitis, allergic, perennial" [Mesh] #2 "rhinitis, allergic, seasonal" [Mesh] #3 "rhinitis" [Mesh single term] #4 rhinit* [tiab] OR hypersensitivit* [tiab] #5 #3 OR #4 #6 allerg* [tiab] OR "hypersensitivity" [Mesh] #7 #5 AND #6 #8 (perennial [ti] OR persistent [ti] OR nonseaosnal [ti] OR nose [ti] OR nasal [ti] OR cat*[ti] OR fur [ti] OR hair* [ti] OR dander [ti] OR dust* [ti] OR mite* [ti] OR pet* [ti] OR dog* [ti] OR cockroach* [ti]) #9 (seasonal [ti] OR intermittent [ti] spring [ti] OR summer [ti] OR pollen [ti] OR grass* [ti] OR birch [ti] OR ragweed [ti] OR tree* [ti] OR weed* [ti] OR mugwort [ti] OR willow [ti] OR alder [ti]) #10 #8 OR #9 #11 (#5 OR #6) AND #10 #12 (hayfever [tiab] OR "hay fever" [tiab] OR pollenosis [tiab] OR pollinosis [tiab] OR SAR [tiab]) #13 #1 OR #2 OR #7 OR #11 OR 12 #14 "Capsaicin" [Mesh] #15 (Capsaicin* [tiab] OR Axsain [tiab] OR Zacin [tiab] OR Capsicum [tiab] OR Capsidol [tiab] OR Zostrix [tiab] OR Capzasin [tiab] OR Gelcen [tiab] OR Katrum [tiab] OR Capsin [tiab]) #16 #14 OR #15 #17 #13 AND #16 | 1 exp Allergic Rhinitis/ 2 Rhinitis/ 3 Rhinit*.tw. 4 3 or 2 5 exp Hypersensitivity/ 6 (allerg* or hypersensitiv*).tw. 7 6 or 5 8 4 and 7 9 (perennial or persistent or nonseaosnal or nose or nasal or cat* or fur or hair* or dander or dust* or mite* or pet* or dog* or cockroach*).ti. 10 (seasonal or intermittent or spring or summer or pollen or grass* or birch or ragweed or tree* or weed* or mugwort or willow or alder).ti. 11 10 or 9 12 4 or 7 13 11 and 12 14 (hayfever or "hay fever" or pollenosis or pollinosis or SAR).tw. 15 8 or 1 or 13 or 14 16 Capsaicin/ 17 Capsaicin derivative/ 18 (Capsaicin* or Axsain or Zacin or Capsicum or Capsidol or Zostrix or Capzasin or Gelcen or Katrum or Capsin).tw. 19 16 OR 17 OR 18 19 15 AND 19 | S1 (MH "Rhinitis, Allergic, Perennial") or (MH "Rhinitis, Allergic, Seasonal") S2 (MH "Rhinitis") S3 TX rhinit* S4 S2 or S3 S5 TX allerg* S6 (MH "Hypersensitivity") S7 S5 or S6 S8 S4 and S7 S9 TI perennial or persistent or nonseasonal or nose or nasal or cat* or fur or hair* or dander or dust* or mite* or pet* or dog* or cockroach* S10 TI seasonal or intermittent or spring or summer or pollen or grass* or birch or ragweed or tree* or weed* or mugwort or willow or alder S11 S9 or S10 S12 S4 or S7 S13 S11 and S12 S14 TX hayfever OR "hay fever" OR pollenosis OR pollinosis OR SAR S15 S1 or S8 or S13 or S14 S16 (MH "Capsaicin") S17 TX Capsaicin* or Axsain or Zacin or Capsicum or Capsidol or Zostrix or Capzasin or Gelcen or Katrum or Capsin S18 S16 OR S17 S19 S15 AND S18 |
| Web of Science | CAB Abstracts/BIOSIS Previews (Ovid) | mRCT |
| #1 TS=rhiniti* #2 TS=(allerg* OR hypersensitiv*) #3 #2 AND #1 #4 TI=(perennial or persistent or nonseasonal or nose or nasal or cat* or fur or hair* or dander or dust* or mite* or pet* or dog* or cockroach*) #5 TI=(seasonal or intermittent or spring or summer or pollen or grass* or birch or ragweed or tree* or weed* or mugwort or willow or alder) #6 #5 OR #4 #7 #6 AND #1 #8 #6 AND #2 #9 TS=(hayfever OR "hay fever" OR pollenosis OR pollinosis OR SAR) #10 #9 OR #8 OR #7 OR #3 #11 TS=(Capsaicin* or Axsain or Zacin or Capsicum or Capsidol or Zostrix or Capzasin or Gelcen or Katrum or Capsin) #12 #10 AND #11 | 1 Rhinitis/ 2 Rhinit*.tw. 3 2 or 1 4 exp Hypersensitivity/ 5 (allerg* OR hypersensitivit*).tw. 6 5 or 4 7 3 and 6 8 (perennial or persistent or nonseasonal or nose or nasal or cat* or fur or hair* or dander or dust* or mite* or pet* or dog* or cockroach*).ti. 9 (seasonal or intermittent or spring or summer or pollen or grass* or birch or ragweed or tree* or weed* or mugwort or willow or alder).ti. 10 9 or 8 11 3 or 6 12 10 and 11 13 (hayfever or "hay fever" or pollenosis or pollinosis or SAR).tw. 14 Capsaicin* or Axsain or Zacin or Capsicum or Capsidol or Zostrix or Capzasin or Gelcen or Katrum or Capsin).tw. | (Capsaicin% or Axsain or Zacin or Capsicum or Capsidol or Zostrix or Capzasin or Gelcen or Katrum or Capsin) |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gerth van Wijk 2000.
| Methods | Design: double‐blind, placebo‐controlled parallel study Allocation: not described Concealment: not described Loss to follow up: 5 of 26 (19%) Jadad scale: 1‐1‐1 |
|
| Participants | Country: Netherlands Subjects: n = 26 (58% female) Age range: 20 to 46 years (mean 30.5) To prevent interference with capsaicin treatment, symptomatic medication for rhinitis had to be withdrawn: corticosteroids were withdrawn 2 months, astemizole 6 weeks, cromoglycate nedocromil 3 weeks and antihistamines 3 days before entering the study. Patients with nasal polyposis, nasal surgery less than 3 months before the study and nasal infection during the 2 weeks before the study were excluded. |
|
| Interventions | The capsaicin solution (0.1 mmol/L) consisted of pelargonic acid vanillylamide dissolved in 3 ml alcohol (96) and diluted in 1L NaCl solution (0.9%). During provocation, 0.5 mL solution was sprayed into each nostril (0.15 mg capsaicin). In 14 days, 7 applications of capsaicin or placebo were given at 2‐day intervals |
|
| Outcomes | Immediately before treatment, baseline visual analogue scores and rhinitis quality of life scores were obtained from the patients
In addition, a nasal challenge with house dust mite and 24 hours later, a nasal challenge with histamine, was performed Adverse events: not reported |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ciabatti 2009 | ALLOCATION: Randomized, placebo‐controlled trial of capsicum oleous nasal spray PARTICIPANTS: 208 patients with idiopathic rhinitis |
| Ge 2001 | ALLOCATION: Not randomized (before and after study) |
| He 2003 | ALLOCATION: Not randomized (before and after study) |
| Liu 2003 | ALLOCATION: Not randomized (before and after study) |
| Liu 2004 | ALLOCATION: Not randomized (controlled clinical trial) |
| Sanico 1998 | ALLOCATION: Not randomized (experimental study: capsaicin spray challenge testing) |
| Stjarne 1998 | ALLOCATION: Not randomized (controlled clinical trial) |
| Zhang 1995 | ALLOCATION: Not randomized (before and after study) |
| Zhang 1999 | ALLOCATION: Not randomized (before and after study) |
Characteristics of ongoing studies [ordered by study ID]
White 2008.
| Trial name or title | Sinol + MucoAd® (SMAN) evaluation trial: a double‐blind, cross‐over comparison of Sinol and SMAN in subjects with congestion due to allergic rhinitis |
| Methods | Randomized, double‐blind (subject, caregiver, investigator, outcomes assessor), active control, cross‐over assignment, safety/efficacy study |
| Participants | Patients 12 years and older with a history of allergic rhinitis for at least 2 years and a positive skin prick and/or intradermal test |
| Interventions | Sinol versus Sinol‐M nasal spray |
| Outcomes | Primary outcome measures: daily total nasal symptom score (congestion, rhinorrhea, sneezing, nasal itching) Secondary outcome measures: number of sprays of study drug used |
| Starting date | September 2008 |
| Contact information | Martha White, MD, Institute for Allergy and Asthma, Wheaton, Maryland, United States, 20902 |
| Notes | — |
Contributions of authors
Cheng Jing: Searching for studies; initial screening and study selection; quality assessment; writing to study authors; data extraction; data analysis; drafting protocol and review text.
Yang Xiang Ning: Initial screening and study selection; quality assessment.
Liu Xian: Data extraction; writing to study authors; data analysis; drafting protocol and review text.
Zhang Shi Ping: Content expertise; resolution of disagreements on study inclusion/exclusion etc.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Gerth van Wijk 2000 {published data only}
- Gerth Van Wijk R, Terreehorst IT, Mulder PG, Garrelds IM, Blom HM, Popering S. Intranasal capsaicin is lacking therapeutic effect in perennial allergic rhinitis to house dust mite. A placebo‐controlled study. Clinical and Experimental Allergy 2000;30:1792‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ciabatti 2009 {published data only}
- Ciabatti PG, D'Ascanio L. Intranasal capsicum spray in idiopathic rhinitis: a randomized prospective application regimen trial. Acta Oto‐Laryngologica 2009;129(4):367‐71. [DOI] [PubMed] [Google Scholar]
Ge 2001 {published data only}
- Ge HQ. Clinical study of CAP in the treatment of perennial allergic rhinitis. Zhong Guo Chang Kuang Yi Xue 2001;14:187‐8. [Google Scholar]
He 2003 {published data only}
- He R, Chen YG, Guo X. Clinical study of CAP in the treatment of perennial allergic rhinitis. Si Chuan Yi Xue 2003;24:1238‐9. [Google Scholar]
Liu 2003 {published data only}
- Liu K, Luo Y. Clinical study of capsaicin in the treatment of 53 cases patients with allergic rhinitis. Xian Dai Yi Yao Wei Sheng 2003;19:1658. [Google Scholar]
Liu 2004 {published data only}
- Liu JJ, Qu SP, Nan ZY. Clinical study of CAP plus incision of anterior ethmoid nerve in the treatment of perennial allergic rhinitis. Chinese Journal of Otorhinolaryngology of Integrated Traditional and Western Medicine 2004;12:89. [Google Scholar]
Sanico 1998 {published data only}
- Sanico AM, Philip G, Proud D, Naclerio RM, Togias A. Comparison of nasal mucosal responsiveness to neuronal stimulation in non‐allergic and allergic rhinitis: effects of capsaicin nasal challenge. Clinical and Experimental Allergy 1998;28(1):92‐100. [DOI] [PubMed] [Google Scholar]
Stjarne 1998 {published data only}
- Stjarne P, Rinder J, Heden‐Blomquist E, Cardell LO, Lundberg J, Zetterstrom O, et al. Capsaicin desensitization of the nasal mucosa reduces symptoms upon allergen challenge in patients with allergic rhinitis. Acta Oto‐Laryngologica 1998;118:235‐9. [DOI] [PubMed] [Google Scholar]
Zhang 1995 {published data only}
- Zhang RX, Jiang DS, Li ZJ, Zhou SM, Ding JC, Qiao DB, et al. Clinical observation and therapeutic mechanism of blocking agent of substance P nerves in the treatment of perennial allergic rhinitis. Zhonghua Er Bi Yan Hou Ke Za Zhi 1995;30:163‐5. [PubMed] [Google Scholar]
Zhang 1999 {published data only}
- Zhang FY, Zhu XN, Han DM, Wang H, Wang LY. Clinical study of capsaicin in the treatment of allergic rhinitis. Lin Chuang Er Bi Yan Hou Ke Za Zhi 1999;13:499‐500. [PubMed] [Google Scholar]
References to ongoing studies
White 2008 {published data only}
- Comparative study of Sinol and Sinol‐M in patients with congestion due to allergic rhinitis (SMAN). http://clinicaltrials.gov/ (accessed 8 December 2009).
Additional references
Aberg 1996
- Aberg N, Sundell J, Eriksson B, Hesselmar B, Aberg B. Prevalence of allergic diseases in schoolchildren in relation to family history, upper respiratory infections and residential characteristics. Allergy 1996;51:232‐7. [DOI] [PubMed] [Google Scholar]
ARIA 2008
- Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008;63(Suppl 86):8‐160. [DOI] [PubMed] [Google Scholar]
Bousquet 2001
- Bousquet J, Cauwenberge P, Khalaer N. Allergic rhinitis and its impact on asthma. Journal of Allergy and Clinical Immunology 2001;108(Suppl 15):s147‐s334. [DOI] [PubMed] [Google Scholar]
Ciprandi 1995
- Ciprandi G, Buscaglia S, Pesce G, Pronzato C, Ricca V, Parmiani S, et al. Minimal persistent inflammation is present at mucosal level in patients with asymptomatic rhinitis and mite allergy. Journal of Allergy and Clinical Immunology 1995;96:971‐9. [DOI] [PubMed] [Google Scholar]
Gerth van Wijk 1999
- Gerth van Wijk RG, deGraaf‐int Veld C, Garreids IM. Nasal hyperresponsiveness. Rhinology 1999;37:50‐5. [PubMed] [Google Scholar]
Handbook 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane‐handbook.org.
IRMWG 1994
- International Rhinitis Management Working Group. International consensus report on diagnosis and management of rhinitis. Allergy 1994;49 (Suppl 19):1‐34. [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore A, Carrol D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17:1‐12. [DOI] [PubMed] [Google Scholar]
Lacroix 1991
- Lacroix JS, Buvelot JM, Polla BS, Lundberg JM. Improvement of symptoms of non‐allergic chronic rhinitis by local treatment with capsaicin. Clinical and Experimental Allergy 1991;21:595‐600. [DOI] [PubMed] [Google Scholar]
Lebel 1988
- Lebel BB, Morel J, Chanal A, Godard I, Michel PFB. Correlation between symptoms and the threshold for release of mediators in nasal secretions during nasal challenge with grass‐pollen grains. Journal of Allergy and Clinical Immunology 1988;82:869‐77. [DOI] [PubMed] [Google Scholar]
RevMan 2008 [Computer program]
- The Nordic Cochrane Centre. The Cochrane Collaboration. Review Manager (RevMan). Version 5.0. Copenhagen: The Nordic Cochrane Centre. The Cochrane Collaboration, 2008.
Ricca 2000
- Ricca V, Landi M, Ferrero P, Bairo A, Tazzer C, Canonica GW, et al. Minimal persistent inflammation is also present in patients with seasonal allergic rhinitis. Journal of Allergy and Clinical Immunology 2000;105:54‐7. [DOI] [PubMed] [Google Scholar]
Sanico 1997
- Sanico AM, Atsuta S, Proud D, Togias A. Dose‐dependent effects of capsaicin nasal challenge: in vivo evidence of human airway neurogenic inflammation. Journal of Allergy and Clinical Immunology 1997;100:632‐41. [DOI] [PubMed] [Google Scholar]
Sanico 1998
- Sanico AM, Atsuta S, Proud D, Togias A. Plasma extravasation through neuronal stimulation in human nasal mucosa in the setting of allergic rhinitis. Journal of Applied Physiology 1998;84:537‐43. [DOI] [PubMed] [Google Scholar]
Skoner 2001
- Skoner DP. Allergic rhinitis: definition, epidemiology, pathophysiology, detection and diagnosis. Journal of Allergy and Clinical Immunology 2001;108:s2‐s8. [DOI] [PubMed] [Google Scholar]
Togias 2000
- Togias A. Unique mechanistic features of allergic rhinitis. Journal of Allergy and Clinical Immunology 2000;105:S599‐604. [DOI] [PubMed] [Google Scholar]


