Abstract
Background
Surgical resection (usually lobectomy) is considered the treatment of choice for many individuals with early stage non‐small cell lung cancer (NSCLC) . However much of the evidence is observational.
Objectives
To determine whether, in patients with early stage NSCLC, surgical resection of cancer improves disease‐specific and all‐cause mortality compared with no treatment, radiotherapy or chemotherapy.
Search methods
For this update we ran a new search in October 2009, using the following search strategy designed in the original review: Cochrane Central Register of Controlled Trials (CENTRAL) (accessed through The Cochrane Library, 2009, Issue 3), MEDLINE (accessed through PubMed), and EMBASE (accessed through Ovid).
Selection criteria
Randomised controlled trials comparing surgery alone (or in combination with other therapy) with non‐surgical therapy and randomised trials comparing different surgical approaches.
Data collection and analysis
A pooled hazard ratio was calculated where possible. Tests for statistical heterogeneity were performed.
Main results
Thirteen trials were included with a total of 2290 patients. Some of the included studies were judged as having a high risk of bias. There were no studies with an untreated control group. In a pooled analysis of three trials, overall survival was superior in patients with resectable stage I to IIIA NSCLC who underwent resection and complete mediastinal lymph node dissection compared with those undergoing resection and lymph node sampling (hazard ratio 0.63, 95% CI 0.51 to 0.78, P ≤ 0.0001) and there was no statistically significant heterogeneity. A further trial found an increased rate of local recurrence in patients with stage I NSCLC treated with limited resection compared with lobectomy. One small trial found a survival advantage in favour of chemotherapy followed by surgery compared to chemotherapy followed by radiotherapy in patients with stage IIIA NSCLC. However none of the other trials in the review demonstrated a significant improvement in overall survival in patients treated with surgery compared with non surgical therapy.
Authors' conclusions
Conclusions about the efficacy of surgery in NSCLC are limited by the volume and quality of the current evidence base, however lung cancer resection combined with complete mediastinal lymph node dissection is associated with a modest improvement in survival compared with lung cancer resection combined with systematic sampling of mediastinal nodes in patients with stage I to IIIA NSCLC. Current evidence suggests that in stage IIIA N2 NSCLC, chemotherapy followed by surgery is as effective as chemotherapy followed by radical radiotherapy, and radical concurrent chemotherapy and radiotherapy is as effective as induction chemoradiation followed by surgery in terms of overall survival.
Plain language summary
Surgery may improve survival rates for non‐small cell lung cancer limited to the lung and surrounding affected glands
Surgical resection is currently considered to be the best treatment for some types of lung cancer limited to the lung and surrounding glands with tumour cells (lymph nodes). There is no compelling evidence to show that lung cancer surgery improves survival compared with other types of therapy such as radiotherapy or chemotherapy. Surgery is often performed in combination with removal of lymph nodes draining the lung with the tumour. There is some evidence that complete removal of all lymph nodes may improve survival compared with only removing a limited number of nodes. Individuals with small cancers localised to the lung appear to have an increased risk of local recurrence if treated with a limited resection rather than a more extensive resection of the involved lung. More research is needed to better understand the types of patients that might benefit most from surgery.
Background
Lung cancer is one of the leading causes of cancer deaths and its five‐year survival is 15% in the United States (Gloeckler Ries 2003). However most individuals with lung cancer present with symptoms only once the cancer has become locally advanced or spread to distant sites. Observational studies show improved survival in individuals with earlier stage disease who undergo resection and this (usually lobectomy) is considered the treatment of choice for individuals with stage I and II non‐small cell lung cancer (NSCLC) (Detterbeck 2001; Jones 2001; Scott 2007). Most surgical series have shown five‐year survival in those with localised (stage I) non‐small cell lung cancer (NSCLC) to be from 55 % to 72% (Nesbitt 1995; Thomas 2002) with even more favourable results reported for individuals with small (< 3 cm) localised cancers (stage IA) (Nesbitt 1995; Reif 2000; Ost 2008). For individuals with stage II NSCLC surgical series report five‐year survival rates of 29% to 51% with more favourable results for individuals with small (< 3 cm) primary lesions in some series (Nesbitt 1995; Martini 1992). By contrast the five‐year survival of individuals with stage I lung cancer not treated surgically is reported to be from 4% to 14% (Flehinger 1992; Sobue 1992; Rowell 2001). Current guidelines suggest the role of surgery is more limited in stage IIIA NSCLC (Robinson 2007). In some patients, occult microscopic tumour involvement of nodes in the mediastinum is detected at the time of surgery and for these patients adjuvant chemotherapy is recommended (Robinson 2007). In individuals with prospectively identified stage IIIA NSCLC multi‐modality treatment is recommended, preferably with concurrent chemotherapy and radiotherapy (Robinson 2007). However recent guidelines also acknowledge that the evidence is not compelling and the recommendations might change as the results of future and ongoing trials become available (Robinson 2007; Rowell 2004). In particular there might be a role for surgery as part of a multi‐modality approach in some subsets of patients with stage IIIA NSCLC, for example those with low volume or microscopic N2 mediastinal disease that is technically resectable (Farray 2005).
Lederle and Niewoehner have argued that the negative results of previous lung cancer screening trials have provided indirect evidence against a benefit from surgery and they highlight that much of the data supporting surgery is observational (Lederle 1994). Although there have been several reviews examining the evidence in relation to surgery for NSCLC, to our knowledge, there have been no prior systematic reviews of randomised controlled trials (Detterbeck 2001; Lederle 1994; Reif 2000; Smythe 2003; Scott 2007).
This is an update of the review published in 2005. The purpose of this review was to determine the effectiveness of surgery for early stage NSCLC. In endeavouring to address this we have considered randomised controlled trials comparing surgical resection for early stage lung cancer with no intervention, radiotherapy or chemotherapy. In addition we have considered trials comparing different surgical approaches, for example, lobectomy or pneumonectomy with systematic mediastinal nodal dissection versus lobectomy or pneumonectomy with mediastinal lymph node sampling. These trials might provide further indirect evidence about the overall efficacy of surgery. The aim of this review was not to address the efficacy of neo‐adjuvant or adjuvant therapy, therefore trials comparing surgery alone with surgery plus chemotherapy or radiotherapy have not been included in this review.
Objectives
To determine whether, in patients with early stage non‐small cell lung cancer, surgical resection of cancer improves five‐year disease‐specific and all‐cause mortality compared with no treatment, radiotherapy or chemotherapy.
To compare the effectiveness of different surgical approaches (e.g. lobectomy versus limited resection) in improving five‐year disease specific or all‐cause mortality in patients with early stage lung cancer.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials (RCT).
Types of participants
(1) individuals with pathologically (histopathology) confirmed non‐small cell lung cancer; (2) individuals with stage I to IIIA lung cancer at the time of trial entry (on clinical examination or diagnostic imaging or other diagnostic/staging procedures).
Types of interventions
The main intervention was surgical resection of lung cancer including lobectomy, sleeve resection, pneumonectomy, segmentectomy or wedge resection (with or without mediastinal node dissection) alone or in combination with other therapy. We considered the following comparison groups: no treatment, sham surgery, radiotherapy or chemotherapy alone or in combination. We also considered studies comparing different types of surgery, for example lobectomy compared to limited resection.
We recorded whether surgical resection was complete or not for each study (where reported). The following definitions were applied:
Complete surgical resection (R0): bronchial and pleural resection margins are clear (microscopically) and if hilar or mediastinal lymph nodes are positive then the anatomically highest lymph node above the positive node should be clear of microscopic disease. Residual microscopic disease (R1): microscopic disease present in the bronchial or pleural resection margins or at the highest anatomical lymph node station resected. Residual macroscopic disease (R2): macroscopically incomplete resection at either the bronchial or pleural resection margin or the highest anatomical lymph node station.
We excluded trials comparing surgery alone with surgery plus chemotherapy or radiotherapy.
Types of outcome measures
Primary outcomes
Primary outcome measures were:
(1) overall survival;
(2) survival (all causes) at two, three, four of five years;
(3) lung cancer specific survival at two, three, four of five years.
Secondary outcomes
Secondary outcome measures considered (where reported) were:
(1) 30‐day mortality
(2) treatment‐related deaths;
(3) progression‐free survival;
(4) 5‐year disease‐free survival;
(5) loco‐regional recurrence rates at two, three, four or five years;
(6) respiratoy function, including forced expiratory volume in one second (FEV1), forced vital capacity (FVC), and maximum voluntary ventilation (MVV) at one and two years.
We also considered quality of life and performance status but none of the trials included in the review reported on these outcomes.
Adverse effects, such as chemotherapy or radiotherapy‐related toxicity and postoperative morbidity and 30‐day mortality were also recorded. Toxic events, where recorded, were classified according to the World Health Organization (WHO) scale and only grades three and four were considered. The number and causes of withdrawals and drop outs were extracted from the trials and described.
Trials with less than two years of patient follow up post‐treatment were excluded from the review.
Search methods for identification of studies
We ran a search in October 2009 to update the original completed review. We searched Cochrane Central Register of Controlled Trials (CENTRAL) (accessed through The Cochrane Library, 2009, Issue 3), MEDLINE (accessed through PubMed), and EMBASE (accessed through Ovid). We also searched the Cochrane Lung Cancer Specialised Register. We slightly modified the original search strategies as shown in Appendix 1. In the same appendix we include the search methods published in the previous version of the review and the original searches.We also searched for additional citations of the relevant papers.
Data collection and analysis
Selection of studies
Two independent authors (RM & DH in the for the original review and RM & ZW for the 2010 update) searched the titles and abstracts obtained from the initial computerised search for potentially relevant trials for full review. Initially studies were categorised into the following groups:
(1) include: RCT meeting the described inclusion criteria and those where it was impossible to tell from the abstract, title, MeSH headings or key words; (2) exclude: non RCT or RCT examining interventions not relevant to the review.
The full texts of those studies in category one were then examined independently by two authors (GW and RM for the original review and RM and ZW for the 2009 update) to determine whether they met the study inclusion criteria. Disagreements were resolved by consensus.
Data extraction and management
Data was extracted by one of the authors (RM) and entered in the Cochrane Collaboration software (Review Manager Version 5.0). Authors of included studies were asked to confirm the data extracted where possible. Data extracted from graphs was also extracted by a second author for the main study outcomes (GW).
Different staging criteria have been used to stage individuals between different studies because staging criteria have been revised in the last few decades (Mountain 1986; Mountain 1997). Where stated in the primary studies, the staging criteria used were recorded in the review. In addition the number and type of investigations conducted for staging differs between studies. For each study included in the review we recorded, where possible, the number and type of investigations used for staging. The Certainty Factor was used to classify the method of staging used (Sobin 1997). This classification is used to reflect the validity of the TNM classification reported. We used the following C‐factor definitions (Sobin 1997): C1: evidence from standard diagnostic means (e.g. inspection, palpation and standard radiography). C2: evidence obtained by special diagnostic means (e.g. computerised tomography, magnetic resonance imaging (MRI), positron emission tomography (PET), endoscopy, biopsy and cytology). C3: evidence from surgical exploration, including biopsy and cytology (e.g. mediastinoscopy). C4: evidence of the extent of disease following definitive surgery and pathological examination of the resected specimen.
We reported the performance status of individuals in primary studies where mentioned.
Assessment of risk of bias in included studies
Two independent authors (RM and ST) assessed the risk of bias of included studies according to the Cochrane Handbook (Higgins 2008). We examined the adequacy of the methods used to generate the allocation sequence, the concealment of allocation, and the level of blinding (clinician, participants, and outcome assessors). We also evaluated the risk associated with dropouts, as estimated by the percentage of participants lost. We used the following definitions:
Generation of the allocation sequence • Adequate, if the allocation sequence was generated by a computer or random number table. Drawing of lots, tossing of a coin, shuffling of cards, or throwing dice were considered as adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure. • Unclear, if the trial was described as randomised, but the method used for the allocation sequence generation was not described. • Inadequate, if a system involving dates, names, or admittance numbers was used for the allocation of patients.
Allocation concealment • Adequate, if the allocation of patients involved a central independent unit, on‐site locked computer, identically appearing numbered drug bottles or containers prepared by an independent pharmacist or investigator, or sealed envelopes. • Unclear, if the trial was described as randomised, but the method used to conceal the allocation was not described. • Inadequate, if the allocation sequence was known to the investigators who assigned participants or if the study was quasi‐randomised.
Blinding (or masking). Blinding of outcome assessors was assessed for all main outcomes together and was characterised as: • Adequate, if the outcome assessors of the trial were blinded to the intervention. • Unclear, if there was no information on blinding. • Not performed, if the outcome assessors were not blinded to the intervention.
Incomplete outcome data • Adequate, if the numbers and reasons for drop‐outs and withdrawals in all intervention groups were described and were comparable between groups. • Unclear, if the report gave the impression that there had been no drop‐outs or withdrawals, but it was unclear whether the analysis included missing data in an adequate manner • Inadequate, if the number or reasons for drop‐outs and withdrawals was either unbalanced between groups, differ in reason or was high enough to alter the effect of the intervention. To judge the latter, we compared the proportion of dropouts to the event rate.
Measures of treatment effect
Treatment effect was measured with hazard ratios (HR) for time‐to‐event variables, risk ratios (RR) for dichotomous variables and mean differences for continuous variables. To extract time‐to‐event data from the included trials, we applied the methods described by Parmar (Parmar 1998) implemented in a public available Excel spreadsheet (Tierney 2007).
Dealing with missing data
Where possible the statistical analysis was conducted in accordance with the intention to treat principle, i.e. where possible, patients were analysed in the groups to which they were randomised to, regardless of whether they received the treatment they were assigned or whether they were observed until the completion of the follow‐up period.
Assessment of heterogeneity
Homogeneity of effect sizes among studies being pooled was assessed with the I2 statistic. Meta‐analysis was conducted only if the data was sufficiently homogeneous both clinically and statistically (I2 < 60%).
Data synthesis
For time‐to‐event outcomes (overall survival and progression‐free survival), pooled hazard ratios were computed with an inverse‐variance method under a fixed‐effects model (Parmar 1998; Whitehead 1991). A fixed‐effects metanalysis was conducted since the inter‐study variance was less than would be expected under the fixed‐effects assumption (Whitehead 1991).
Dichotomous and continuous outcomes were pooled using the Mantel‐Haenzsel method under a random‐effects model. Pooled effect measures were calculated with 95% confidence intervals. All statistical analyses were done with Review Manager software.
Sensitivity analysis
In the case of meta‐analysis, sensitivity analyses were planned on the basis of trial quality and the methods of meta‐analysis but because of the small number of studies available for meta‐analysis these were not performed.
Results
Description of studies
In the original review there were 1181 citations identified by the MEDLINE search, 70 citations identified by the search of the Cochrane Central Register of Controlled Trials and approximately 430 citations identified by the EMBASE search. After review of abstracts selected from the search of electronic databases, bibliographies and handsearches, 27 studies were selected for full text review. Eleven trials (some with multiple citations) were selected for inclusion in the review (Albain 2003; Izbicki 1998; Ginsberg 1995; Johnstone 2002; Morrison 1963; NCI 1975; Shepherd 1998; Stathopoulos 1996; Sugi 1998; Sugi 2000; Wu 2002). The two authors (RM & GW) agreed on the studies to be included in all but one study (Kappa = 0.93). One ongoing trial was also identified but results are not available as yet (ACOSOG Z0030). There were no additional studies identified by contacting authors of primary studies or experts in the field.
When the search was updated in 2009 there were a further 1048 abstracts identified and searched independently by two authors (RM and ZW), seven citations were selected for full text review and two additional trials (Stephens 2005; van Meerbeeck 2007) were included in the review. In addition a further article identified provided more up to date results for one of the trials included in the original review (Albain 2003). The four other citations selected for review were duplicate publications or reports of the trials selected for inclusion. The trials have been grouped into the following categories:
Surgery alone compared with radiotherapy alone for local and loco‐regional stage (I to III) NSCLC In one early study, individuals with lung cancer (including squamous cell carcinoma, adenocarcinoma and 'oat cell' (or small cell) and anaplastic carcinoma) without clinical evidence of spread of the tumour outside the chest and without evidence of gross mediastinal involvement either clinically or radiologically, were randomised to surgical resection (pneumonectomy or lobectomy) or to radiotherapy (Morrison 1963).
Radiotherapy was given by an 8‐million‐volt linear accelerator. It was planned to give a mean dose of 45 Gy to the tumour with daily fractionated treatments over a period of four weeks. The tumour and 2 cm of normal surrounding lung and the hilar and mediastinal areas were included in the fields. All patients tolerated the full prescribed treatment.
The surgical group underwent radical resection of the tumour and associated hilar and mediastinal lymph nodes. If complete resection was not possible at the time of thoracotomy, palliative resections were not performed. Thirty per cent of individuals in the surgical group and 36% in the radiotherapy group had some evidence of mediastinal involvement at the original examination. Sixty‐seven percent of patients in the surgical group and 61% in the radiotherapy group had squamous cell carcinoma. Nine patients (32%) in the radiotherapy group and 10 (33%) in the surgical group had 'oat cell' or anaplastic carcinoma.
Chemotherapy plus surgery compared with radiotherapy alone in stage IIIA NSCLC There was one study in which chemotherapy and surgery was compared with radiotherapy in the treatment of individuals with stage IIIA NSCLC with biopsy proven mediastinal node involvement (Shepherd 1998). Individuals were eligible for the trial if they were able to tolerate the planned surgery and had a predicted postoperative FEV1 of more than 0.8 L and an ECOG performance status of two or less.
In the chemotherapy/surgery group, chemotherapy consisted of cisplatin (120 mg /m2) on days 1 and 29 and vinblastine (6 mg/m2) on days 1,15,22, 29 and 43. Patients proceeded to surgery between days 51 and 64 if they had stable disease or a partial or complete response. Resection with radical lymph node dissection were performed. Those who had a complete resection received the same chemotherapy commencing six weeks postoperatively.
In the radiotherapy arm a total dose of 60 Gy was planned to be given as 2 Gy daily five days a week. The trial was terminated prematurely after other trials had shown that chemoradiotherapy was superior to radiotherapy alone in the management of patients with stage IIIA and it was no longer considered appropriate to have a radiotherapy alone control arm.
Another study was included in this category in the 2010 update (Stephens 2005). Patients were eligible if they had microscopically confirmed NSCLC stage T3, N1, M0 or T1‐3, N2, M0 disease, considered by the local thoracic surgeon to be unresectable but to have the potential to become resectable following chemotherapy.
Radiotherapy patients received thoracic radiotherapy according to the site and extent of tumour and local practice and following the recommendations of the 1994 Department of Health Standing Medical Advisory Committee (Standing Medical Advisory Committee 1994), which stated that patients should receive 50‐60 Gy to their tumour over a period of 3‐6 weeks.
Patients in the chemotherapy/surgery group received four cycles of chemotherapy (either a combination of mitomycin, vinblastine and cisplatin or a combination of mitomycin, ifosfamide, with mesna, and cisplatin) at 3‐week intervals. Surgical resection, if considered feasible, was carried out between four and six weeks after the final cycle of chemotherapy. The surgical technique was decided by the local surgeon according to the site and extent of the tumour and local practice.
Although it had been estimated that 350 patients could be recruited in 3 years, only 48 from 12 centres were recruited. Some changes to the protocol were suggested but there was no common agreement about those and the Data and Monitoring and Ethics Committee recommended closing the trial in 1999.
Surgery versus no surgery in patients with initially inoperable loco‐regional cancer treated with radiotherapy In one early collaborative trial, 425 individuals with lung cancer who were initially considered to be inoperable because of regional spread were given a course of radiotherapy (40 Gy over 4 weeks to primary tumour and mediastinum) (NCI 1975). After radiotherapy there were 152 individuals with cancer who were subsequently considered resectable and these individuals were randomised to either surgical resection or no surgery. Patients were initially classified as inoperable if they had 1) mediastinal, supraclavicular, or scalene lymph node involvement, 2) chest wall invasion, or 3) encroachment of tumour upon the carina. The exact proportion in each category was not described in the trial report . Histological or cytological diagnosis of lung cancer was confirmed after central pathological review. Twenty‐two percent of participants in the surgery group and 27% in the no surgery group had 'oat cell' lung cancer.
Chemotherapy followed by surgery versus chemotherapy followed by radiotherapy in stage IIIA NSCLC There were two studies in this category (Johnstone 2002; Stathopoulos 1996). A further trial was added at that time of the 2010 update (van Meerbeeck 2007).
In one small study chemotherapy followed by surgery was compared with chemotherapy followed by radiotherapy in patients with stage IIIA NSCLC (Stathopoulos 1996). Participants over age 75 or with active cardiac disease were excluded. The participants included in this study appear to have been classified as inoperable prior to inclusion in the study but the criteria used to make this assessment and the TNM status of participants was not described. Fifty percent of participants were staged at thoracotomy and the remainder were staged by bronchoscopy, computed tomography of the chest, abdomen and brain and bone scan.
The intervention group were assigned to four cycles of chemotherapy followed by surgical resection and the control group were assigned to six cycles of chemotherapy followed by radiotherapy. Chemotherapy consisted of cisplatinum (90 mg/m2), vindesine (3 mg/m2) and epirubicin (40 mg/m2), administered once every three weeks. Radiotherapy consisted of 50 Gy in the primary site of the tumour and in the mediastinum. The radiation applied was by parallel opposed fields encompassing the primary lesion with a 2 cm margin of normal appearing lung when possible. Treatment volume was defined using computerised tomography. The daily treatment fraction was 2 Gy. Participants in the surgical group underwent either lobectomy or pneumonectomy but it was not described whether this was accompanied by mediastinal lymph node dissection or sampling. According to one of the investigators the trial was terminated on the basis of a preliminary analysis.
In the Radiation Oncology Group (RTOG) trial 89‐01, chemotherapy and radiotherapy was compared with preoperative chemotherapy and surgical resection in patients with stage IIIA (T1‐T3 N2 M0) NSCLC (Johnstone 2002). In this trial all patients were required to have histological documentation of N2 disease. Initially participants were randomised prior to induction chemotherapy, but the protocol was later modified to randomise participants after induction chemotherapy.
Chemotherapy consisted of cisplatin 120 mg/m2 on days 1 and 29, vinblastine 4.5 mg/m2 on days 1, 15, 29, and 43, and mitomycin‐C 8 mg/m2 on days 1 and 29. Mitomycin‐C was removed from the induction chemotherapy regimen after randomisation of the first 16 participants. Participants were randomised to surgery on day 71 followed by cisplatin on days 99 and 127 and vinblastine on days 99, 113, 127, and 141 or to radiotherapy commencing on day 71 and given to 64 Gy in 2.0 Gy fractions, followed by cisplatin on days 141 and 169 and vinblastine on days 141, 155, 169, and 183. The trial was terminated prematurely because phase II trials had demonstrated the feasibility of preoperative concurrent chemoradiation in this group of patients and the study was superseded by the North American Intergroup trial 0139 (RTOG 93‐09).
In the 2010 update one additional study was identified. The study was conducted on behalf of the European Organisation for Research and Treatment of Cancer‐Lung Cancer Group (EORTC‐LCG) (van Meerbeeck 2007). The EORTC‐LCG trial compared induction chemotherapy followed by surgery with induction chemotherapy followed by definitive radiotherapy. Only patients showing a complete, partial or minor response to induction chemotherapy were eligible for random assignment to either surgery or radiotherapy. Patients included in the study had histologic or cytologic proven N2 disease that was considered to be unresectable. Eighty seven percent of patients received three cycles of chemotherapy, consisting of a platinum, either cisplatin at a dosage of at least 80mg/m2 or carboplatin on target AUC of at least 5, combined with at least one additional chemotherapeutic agent including gemcitabine in 40% of patients and taxane in 21% of patients. Further details of dosing or additional chemotherapeutic agents were not described in the publication of the trial. Randomisation occurred after completion of induction chemotherapy, as only patients demonstrating a degree of response to chemotherapy were included. Radiotherapy was commenced no later than ten weeks after completion of chemotherapy. Treatment dose consisted of 60‐62.6 Gy to the primary tumour and involved mediastinum and 40‐46 Gy to uninvolved mediastinum with a fraction size of 1.95‐2.05 and number of fractions of 30‐32 and a total treatment duration of 40‐46 days. Surgery included lobectomy and pneumonectomy and was considered complete based on pathological report of both the surgical margins and the highest mediastinal lymph node being free of tumour. Patients underwent follow up visits every three months for two years and six months thereafter.
Concurrent chemotherapy and full course radiotherapy versus induction chemotherapy and radiotherapy followed by surgery
One trial was included in this category (Albain 2003). The RTOG 93‐09 (North American Intergroup trial 0139) compared concurrent chemotherapy and full course radiotherapy with concurrent chemotherapy/radiotherapy induction followed by surgical resection in individuals with stage IIIA NSCLC. Participants with technically resectable (at randomisation) T1‐3, cyto‐histologically proven N2, M0 tumours were included. If CT scan showed contralateral nodes of greater than 1 cm then biopsy was needed to exclude N3 disease. For participation patients were required to have a predicted post resection forced expiratory volume in 1s (FEV1) of at least 800 cm2 on quantitative perfusion scan if FEV1 overall was less than 2000 cm2. The Karnofsky performance status was 90 or 100; or, if 70 or 80, the albumin was at least 85% of the normal value, with less than 10% weight loss with in the previous 3 months.
All patients had induction therapy with cisplatin 50 mg/m2 on days 1,8, 29 and 36, and etoposide 50 mg/m2 on days 1 to 5 and 29‐33 and daily radiotherapy to 45 Gy starting day 1 in 1.8 Gy fractions. The intervention group then underwent resection (with mediastinal lymph node sampling or dissection) 3‐ 5 weeks after completion of radiotherapy if there had been no disease progression. The control group received uninterrupted radiotherapy to 61 Gy if they had not progressed after initial induction treatment. Both groups received two cycles of consolidative chemotherapy (same doses and schedule as during induction).
Limited resection (wedge excision or segmentectomy) versus lobectomy for stage IA peripheral NSCLC There was one study which compared limited resection with lobectomy in individuals with stage I NSCLC (Ginsberg 1995). In this study individuals with T1 N0 peripheral tumours that were suspected or proven to be lung cancer were randomised to either limited resection (thoracotomy with wedge resection or segmentectomy) or thoracotomy with lobectomy. All patients were able to tolerate a lobectomy as assessed by cardiopulmonary function (details were not provided, but 93% of participants in both groups had a preoperative FEV1 of 50% or greater). Preoperative staging was clinical, including examination findings and biochemistry and chest x‐ray but computed tomography was performed only as indicated. The study was performed at multiple institutions in North America.
The technique of segmental resection required isolation, division, and suture of the appropriate segmental bronchus, artery, and vein and up to two adjacent segments could be removed as part of a limited resection. Large wedge resections were also performed when appropriate in the limited resection group and in this case at least 2 cm of normal lung tissue was required to be resected beyond the tumour. In both segmental resection and wedge excision, surgeons were allowed latitude in surgical technique for division of pulmonary tissue. At the time of thoracotomy, but before randomisation, it was required that the pathology was confirmed by frozen section, if not done prior to surgery, and that disease was confirmed to be N0 by sampling the relevant lymph nodes and submitting for frozen section analysis. The appropriateness of limited resection was also assessed at this time.
Eligible participants were then randomised intraoperatively. After completion of the resection the surgeon was required to confirm that clinically the tumour had been completely resected and all required lymph node stations had been sampled and, by frozen section analysis, confirmed to be negative for metastatic disease. If the resection was incomplete or the tumour was found to be greater than T1 or N0, the protocol specified that the surgeon complete the lobectomy. There were 771 participants registered for the study and 276 were entered into the study at the time of surgery. There were 29 patients excluded after randomisation and 247 considered eligible for the analysis. Recurrence rates, cancer related deaths and all cause mortality were examined at follow up. In addition, pulmonary function testing was performed preoperatively and postoperatively at 6 and 12 to 18 months (FEV1, FVC, MMEFR (maximum mid‐expiratory flow rate), MVV). However only 60% and 66% underwent pulmonary function testing at 6 months and 12‐18 months respectively. Video‐assisted thoracoscopic lobectomy versus conventional lobectomy for stage I NSCLC In one study video‐assisted thoracoscopic lobectomy was compared with conventional lobectomy in individuals with stage IA NSCLC (Sugi 2000). In this study 100 consecutive patients with clinical stage IA NSCLC were randomised to either open thoracotomy with conventional lobectomy or video‐assisted thoracoscopic (VATS) lobectomy. Participants were staged with bone scan and computed tomography (CT) of the abdomen, in addition to CT of the chest and the head preoperatively. Mediastinoscopy was not performed preoperatively. Individuals with mediastinal lymph nodes of more than 10 mm in maximal diameter on CT were not included in the study.
In the open group, participants underwent a posterolateral thoracotomy via the fifth intercostal space and lobectomy was performed with complete mediastinal lymph node dissection. Participants in the VATS group underwent lobectomy through an 8 cm‐access axillary incision through the fourth or fifth intercostal space, with two or three ports for the application of thoracoscopic instruments. The authors stated that hilar and mediastinal lymph node dissections were performed in a manner similar to that used in the open group. Intraoperatively 11 % of participants had more advanced disease than stage I (13% in the open group and 8% in the VATS group) and two patients in the VATS group had small cell cancer but none of these were excluded from the analysis. Distal and local recurrence rates and overall survival were described in the report.
Complete mediastinal lymph node dissection versus mediastinal lymph node systematic sampling in patients with resectable NSCLC There were three studies that compared complete mediastinal lymph node dissection with conventional mediastinal lymph node sampling in patients with resectable NSCLC (Izbicki 1998; Sugi 1998; Wu 2002). For this review the terminology recommended by Keller has been used, that is systematic sampling (SS) refers to the routine biopsy of lymph nodes at the levels specified by the authors and complete mediastinal lymph node dissection (CMLND) refers to the routine removal (at the levels specified by the authors) of all ipsilateral lymph node containing tissue (Keller 2002).
Sugi et al reported a study in which participants with peripheral NSCLC less than two cm in diameter and without clinical or radiological evidence of intrapulmonary, hilar, mediastinal or metastatic disease were randomised to thoracotomy and lobectomy (or bi‐lobectomy) with CMLND or thoracotomy and lobectomy (or bi‐lobectomy) and mediastinal SS (Sugi 1998). Participants with hilar or mediastinal lymph nodes of greater than one cm on CT examination were excluded and mediastinoscopy was not performed preoperatively. In the mediastinal SS group, interlobar, peribronchial, and hilar nodes representing nodes 10, 11, and 12 (as defined in the map by the American Thoracic Society) were dissected (Martini 1983). Mediastinotomy was performed by longitudinal incision of the mediastinal pleura, and the nodes of regions 2 to 9 were explored and nodes suspected of harbouring cancer were removed and sent for histopathological analysis. The nodes of regions 4,5, and 7 were removed routinely from all patients. In the CMLND group radical en bloc mediastinal lymphadenectomy was performed as described by Naruke et al and Martini & Flehinger (Martini 1987; Naruke 1976). In the group undergoing CMLND, 7% were found to have N1 disease and 12% N2 disease after pathological staging. In the mediastinal SS group, 5% were found to have N1 disease and 14% N2 disease after pathological staging (Sugi 1998). One tumour in each group was found to be a small cell carcinoma after resection and pathological evaluation and four participants in the CMLND group and three in the SS group were found to have secondary lung cancer from other sites rather than primary lung cancer. Patients with involvement of any N2 nodes received 50 Gy of radiation to the entire mediastinum postoperatively.
In another study, Izbicki and co‐workers compared CMLND with conventional SS (Izbicki 1998). In this study individuals with curatively resectable NSCLC were randomised at thoracotomy. Preoperative staging consisted of chest radiography, bronchoscopy, computed tomography scan of the thorax and abdomen, abdominal ultrasound and bone scan. Mediastinoscopy was performed only in individuals with enlarged mediastinal lymph nodes (> 1 cm in short‐axis diameter). Individuals with distant metastasis, N3 disease or extensive N2 disease were excluded. Resection of the primary lung tumour via anterolateral thoracotomy (fourth intercostal space) was similar in both groups consisting of classic lobectomy, pneumonectomy and in some cases with bronchoplastic procedures or sleeve resection. Extended resections were performed for some tumours. In the SS group resection was accompanied by regional lymphadenectomy of interlobular, peribronchial, and hilar nodes representing nodes 10, 11, and 12 according to the American Thoracic Society lymph node mapping (Martini 1983). Mediastinotomy was performed and exploration of nodes of stations 2 to 9 performed. Nodes suspicious for cancer were removed and sent for histopathological analysis. Nodes of stations 4,5, and 7 were routinely removed in all patients. In the group assigned to CMLND en bloc mediastinal lymphadenectomy was performed as described by Naruke et al and Martini and Flehinger (Martini 1987; Naruke 1976). Adjuvant radiotherapy was administered for patients with pathological stage T3 or T4 tumour (stage IIIA or IIIB) to the tumour bed and patients with involvement of N2 nodes on histopathology received radiation to the mediastinum.
In a further study comparing CMLND to conventional mediastinal SS, individuals with resectable clinical stage I to IIIA NSCLC who were 70 years of age or less were enrolled (Wu 2002). Preoperatively individuals were staged with bronchoscopy, chest radiography, CT scan of the thorax, and abdominal ultrasound. Operated patients were re‐staged according to pathological findings and patients meeting the eligibility criteria were followed up. In both groups surgical resection, including lobectomy or pneumonectomy or resection combined with bronchoplastic procedures or sleeve resection was performed via posterolateral thoracotomy in the fifth intercostal space. In some cases extended resections were performed for T3 disease. In the group assigned to CMLND, nodal dissection was performed as described by Naruke et al (Naruke 1976). In the group assigned to conventional SS, hilar lymph node dissection was undertaken and mediastinotomy was performed and nodes of stations 1 to 9 were explored. Nodes with suspected metastases (larger than one cm in diameter or hard) were excised and submitted for histopathological examination. Nodes of station 7 were removed routinely in all patients. In this study there was no statement about whether or not participants received any adjuvant therapy, but one of the investigators on this study informed us that individuals with stage III disease were referred for adjuvant radiotherapy but compliance was about 30% in both groups.
Risk of bias in included studies
Risk of bias in included studies is described below and in the risk of bias tables. See also Figure 1 and Figure 2.
1.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
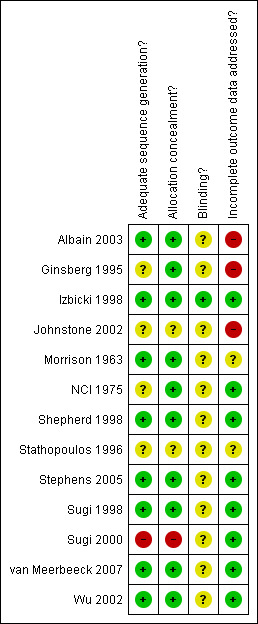
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Randomisation
In the only trial included in the Surgery alone compared with radiotherapy alone for local and loco‐regional stage (I to III) NSCLC group (Morrison 1963) allocation concealment and the method used to generate the randomisation sequence were adequate. In the studies of the Chemotherapy plus surgery compared with radiotherapy alone in stage IIIA NSCLC category, allocation was concealed in both of them and the sequence properly generated (Shepherd 1998; Stephens 2005). Allocation concealment was considered adequate and sequence generation was not described in the only trial in the category Surgery versus no surgery in patients with initially inoperable loco‐regional cancer treated with radiotherapy (NCI 1975). In the Chemotherapy followed by surgery versus chemotherapy followed by radiotherapy in stage IIIA NSCLC group, one study had adequate concealment of allocation and proper sequence generation (van Meerbeeck 2007) and the other two had inadequate concealment and sequence generation was not described (Johnstone 2002; Stathopoulos 1996). The only study that belongs to the Concurrent chemotherapy and full course radiotherapy versus induction chemotherapy and radiotherapy followed by surgery group (Albain 2003) had proper concealment of allocation and sequence generation. In the following comparison Limited resection (wedge excision or segmentectomy) versus lobectomy for stage IA peripheral NSCLC the only study included had adequate concealment of allocation and the method to generate the randomisation sequence was not described (Ginsberg 1995). In the following category Video‐assisted thoracoscopic lobectomy versus conventional lobectomy for stage I NSCLC the only study included (Sugi 2000) had inadequate concealment of allocation and inadequate method to generate the randomisation sequence. The studies in the last comparison Complete mediastinal lymph node dissection versus mediastinal lymph node systematic sampling in patients with resectable NSCLC had an adequate concealment of allocation and proper sequence generation (Izbicki 1998; Sugi 1998; Wu 2002).
Description of withdrawals and losses to follow up
In the only study that belongs to the Surgery alone compared with radiotherapy alone for local and loco‐regional stage (I to III) NSCLC group (Morrison 1963), there was no statement about losses to follow up. In one of the studies (Shepherd 1998) of the Chemotherapy plus surgery compared with radiotherapy alone in stage IIIA NSCLC comparison there were no losses to follow up and they were appropriately described in the other one (Stephens 2005): 1 patient was withdrawn from the study in the chemotherapy/surgery arm, 39 out of 48 were known to have died and of the remaining 9 survivors median follow up was 14 months (range 5 to 68 months). Regarding the comparison Surgery versus no surgery in patients with initially inoperable loco‐regional cancer treated with radiotherapy all patients randomised were followed until death or for at least five years in the only study included (NCI 1975). In the Chemotherapy followed by surgery versus chemotherapy followed by radiotherapy in stage IIIA NSCLC group there was no clear statement about follow up in one of the studies (Stathopoulos 1996), there were no losses to follow up in another one (van Meerbeeck 2007) and the description of withdrawals and follow up was not complete in another study (Johnstone 2002). In the only study pertaining to the Concurrent chemotherapy and full course radiotherapy versus induction chemotherapy and radiotherapy followed by surgery comparison (Albain 2003), description of withdrawals and losses to follow up was not complete. In this trial 8% of participants were excluded after randomisation because they did not meet the inclusion criteria and these were excluded from the analysis however the rates of ineligibility and reasons for exclusion did not differ between the two study groups (Albain 2003). Withdrawals and losses to follow up were very high (18% in both groups) and probably affect the results in the study (Ginsberg 1995) belonging to the Limited resection (wedge excision or segmentectomy) versus lobectomy for stage IA peripheral NSCLC comparison. In the category Video‐assisted thoracoscopic lobectomy versus conventional lobectomy for stage I NSCLC the only study included (Sugi 2000) had no losses to follow up. The studies from the group Complete mediastinal lymph node dissection versus mediastinal lymph node systematic sampling in patients with resectable NSCLC had adequate description of withdrawals and follow up (Izbicki 1998; Sugi 1998; Wu 2002).
Please find further information about incomplete outcome data of the included studies in the Appendix 2.
Blinding of outcome assessment None of the trials described any blinding of investigators who were assessing outcomes such as cause of death or disease recurrence. After contacting one of the authors of one study we were told that investigators undertaking the follow up were blind to the type of operation (Izbicki 1998). In some circumstances it would have been technically difficult to blind investigators. For example where the cause of death relates directly to the intervention, e.g. postoperative death or death from radiation fibrosis.
Effects of interventions
Statistical considerations
Time‐to‐event analysis could be conducted with ten trials. In three trials hazard ratios and confidence intervals were provided in the study reports (Albain 2003; van Meerbeeck 2007;Stephens 2005). In six trials (Ginsberg 1995; Izbicki 1998; Johnstone 2002; Stathopoulos 1996; Sugi 2000; Wu 2002), the hazard ratio and its variance were calculated from information reported in the primary studies (number of events and logrank test p‐value). In the last trial, hazard ratios were computed extracting data from the Kaplan‐Meier curve (Sugi 1998).
It was only feasible to conduct a pooled analysis for three trials that were sufficiently homogeneous (those comparing mediastinal lymph node sampling with mediastinal lymphadenectomy) (Izbicki 1998; Sugi 1998; Wu 2002).
For the other trials included in the review survival at two, three, four of five years (depending on the data reported for the primary studies) was described by entering the number of participants surviving at two, three, four of five years in Review Manager but a pooled analysis was not conducted.
Please note that in the results graphs, n refers to the number of outcome events and N to the number of participants. Trials selected for full text review but excluded from the review are outlined (with reasons for exclusion) in the table of excluded trials.
Surgery alone compared with radiotherapy alone for local and loco‐regional stage (I to III) NSCLC The results of the one small study comparing surgery with radiotherapy are inconclusive (Morrison 1963). At four years of follow up, seven out of 30 patients treated with surgery were still alive compared with two out of 28 patients treated with radiotherapy (RR = 3.27, 95% CI 0.74 to 14.42, P = 0.12). However one‐year survival was worse in the surgical group compared with the radiotherapy group (43% versus 64%). In a subgroup analysis of patients with squamous cell carcinoma there were one out of 17 (6%) patients in the radiotherapy group and six out of 20 (30%) patients in the surgery group still alive at four years (RR = 5.10, 95% CI 0.68 to 38.29, P = 0.11). In the paper this difference was reported to be significant at the 5% level but the exact P value and method of analysis were not described. In the surgical group there were three patients who died within two months of the operation from complications related to surgery. There was no comment about whether resection was complete in those assigned to surgery who underwent resection. There were two patients who died from treatment related complications in the radiotherapy group (one at 14 months following haemorrhage at the time of dilatation of an oesophageal stricture and one at 57 months from radiation fibrosis).
Chemotherapy plus surgery compared with radiotherapy alone in stage IIIA NSCLC The results of the one small study comparing chemotherapy and surgery with radiotherapy alone in stage IIIA NSCLC are inconclusive because of early closure of the study (Shepherd 1998). Thirteen of the 16 patients randomised to the chemotherapy and surgery arm underwent thoracotomy and 10 had complete resections. A definition for complete resection was not described in the study report. Three patients did not proceed to surgery, one due to progressive disease and two due to toxicity related to chemotherapy. Only eight patients had postoperative chemotherapy. In the radiotherapy arm of the study the response rate to radiotherapy was 53% (five partial and three complete responses), only one patient discontinued treatment early because of progressive disease. Survival at two years was 44% in the surgical group and 40% in the radiotherapy group (RR 1.09, CI 0.48 to 2.51). It was reported that grade three and four haematological toxicity and nausea and vomiting was limited to patients who had chemotherapy (exact proportion not described). There were three patients who had febrile neutropenia but no deaths related to chemotherapy. One patient had grade three radiation pneumonitis but none had grade three or four oesophagitis. Two patients had prolonged ventilation postoperatively and one prolonged air leak, infection and atelectasis. There were no perioperative deaths described in the report.
Another trial was also closed with a small number of patients (Stephens 2005). Twenty four participants were randomised to the chemotherapy/surgery arm of this study, 1 was withdrawn, 21 patients received all 4 cycles of chemotherapy and 2 received 3 cycles, however only 4 were treated surgically (2 pneumonectomies, 1 lobectomy and 1 sleeve resection). Three further patients had thoracotomies without resection and the remaining 16 had progressive disease post chemotherapy. Of the 19 patients that did not have resection 13 received radiotherapy. Twenty of the 24 patients randomised to radiotherapy received radiotherapy, the commonest schedules were 50Gy/20f, 50 Gy/15f, 40Gy/20f, 37Gy/26f and 28 Gy/8f. Four patients in the radiotherapy arm did not receive treatment (one patient refused treatment, one was considered unsuitable for radiotherapy, the diagnosis for one patient was changed to SCLC and for the remaining patient the reason is not known). Of the 48 patients, 39 were known to have died (19 in the radiotherapy arm and 20 in the chemotherapy/surgery arm). The median follow‐up for the nine survivors was 14 months (range 5‐68 months). The cause of death was lung cancer in 35 patients (19 in the radiotherapy arm/16 in the chemotherapy/surgery arm). Overall survival was similar in the two groups (HR 0.91, 95% CI 0.49 to 1.72, P = 0.78). Median survival was 11.2 and 13.8 months, 1‐year survival 43% and 54%, and 2‐year survival 16% and 15% for the radiotherapy and chemotherapy/surgery groups, respectively. The authors reported no statistically significant differences in quality of life (SF‐36 questionnaires) between the 2 groups but qualitative data was provided in the study report only. There were 2 perioperative deaths, both in patients who underwent pneumonectomy.
These two studies were not meta‐analysed but their results (RR and HR) are shown on a single graph (Analysis 2.1)
2.1. Analysis.
Comparison 2 Chemotherapy plus surgery versus radiotherapy for stage IIIA NSCLC, Outcome 1 2‐year survival.
Surgery versus no surgery in patients with initially inoperable loco‐regional cancer treated with radiotherapy Amongst patients with initially inoperable lung cancer (without distant metastases) who were considered to be operable after a course of radiotherapy there was no difference in five‐year survival between those assigned to surgery versus those assigned to no surgery (NCI 1975). Eight percent of participants in the surgery group survived five years compared with 6% in the no surgery group (RR 1.42, 95% CI 0.42 to 4.84, P = 0.57). Disease free survival was also similar between the two groups at five years (RR 1.58, 95% CI 0.39 to 6.38, P = 0.52). It was stated in the study report that subgroup analyses were conducted according to pre‐treatment characteristics (e.g. type of lymph node involvement) and that differences between subgroups were small and no pattern to the variation was evident but further details were not provided. Respiratory complications (respiratory infection, radiation pneumonitis, respiratory insufficiency) were more common in the group undergoing surgery (RR 3.0, 95% CI 1.27 to 7.11, P = 0.01). There was no information provided about what proportion of participants in the surgery group had a complete resection. Chemotherapy followed by surgery versus chemotherapy followed by radiotherapy in stage IIIA NSCLC There were two studies included in this category (Johnstone 2002; Stathopoulos 1996) . A further trial was added at the time of the 2010 update (van Meerbeeck 2007). These trials however were clinically and statistically heterogeneous (chi squared for homogeneity 5.18, P = 0.08) and a pooled analysis was not performed. The results are described separately. Of particular note the treatment protocols in the chemotherapy/radiotherapy groups differed somewhat between these two studies.
In one study which compared chemotherapy and surgery with chemotherapy and radiotherapy there was no significant difference in survival at four years (Johnstone 2002). In this study 19 (73%) of the participants in the surgery group had complete resections (R0) and four had pathologic residual disease (R1‐2). After more than four years of follow up there were 21 deaths (out of 29) in the chemotherapy/surgery group and 27 deaths (out of 32 participants) in the chemotherapy/radiotherapy group. The hazard ratio was 0.8 (95% CI 0.45 to 1.42, P = 0.456) for overall survival, indicating a lower chance of dying in the chemotherapy/surgery treatment arm. The details of all grade three and four toxicities were not described in the report of this trial, however the authors stated that there were no cases of grade four acute radiation toxicity in the chemo/radiotherapy group. The incidences of postinduction chemotherapy and radiation toxicity were said to be equivalent across treatment arms. Grade four toxicity was noted to be more common in patients receiving mitomycin‐C. There were two treatment‐related deaths in the chemotherapy/surgery group and one in the chemotherapy/radiotherapy group (RR 2.21, 95% CI 0.21 to 23.08, P = 0.51).
In the small study reported by Stathopoulos et al there was a significant improvement in survival in the intervention group (Stathopoulos 1996). Sixty‐seven percent of patients in the intervention group had a complete resection after chemotherapy but the criteria used to classify the adequacy of the resection were not described. Five‐year survival was 29% in the chemotherapy/surgery group compared with 0% in the chemotherapy/radiotherapy group (P < 0.01). The hazard ratio was 0.39 (95% CI 0.19 to 0.81, P = 0.010). Toxicity and treatment‐related complications were not described.
The EORTC‐LCG study identified in the 2010 review (van Meerbeeck 2007) was a large multi‐institutional trial. This study found no statistically significant difference in five‐year overall survival between the surgery or radiotherapy arm post induction chemotherapy for stage IIIA‐N2 disease. Seventy seven participants (50%) in the surgery group had complete resection. Complete resection versus incomplete resection had a hazard ratio = 0.46 (95% CI 0.32 to 0.67). Acute grade 3‐4 oesophageal toxicity was observed in one (<1%) patient out of the 154 patients who underwent radiotherapy, with five (4%) patients in this treatment arm experiencing acute grade 3‐4 pulmonary toxicity. The study reported late pulmonary fibrosis in 11 patients (7%) and one patient died from radiation pneumonitis. Eleven patients (4%) died within thirty days of surgery. At five years of follow up there were 138 deaths (out of 167) in the chemotherapy surgery group and 141 deaths (out of 165) in the chemotherapy/radiotherapy group. The hazard ratio for overall survival for surgery versus radiotherapy was 0.94 (95% CI 0.74 to 1.19, P = 0.596). Progression‐free survival also did not differ significantly between the 2 treatment groups, hazard ratio 0.94 (95% CI 0.74 to 1.19, P = 0.605).
Concurrent chemotherapy and full course radiotherapy versus induction chemotherapy and radiotherapy followed by surgery
In the North American Intergroup trial 0139 there was no significant difference in overall survival between the two treatment groups (Albain 2003). (Hazard Ratio 0.87 (95% CI 0.69 to 1.10), P = 0.24). Progression‐free survival was improved in the group receiving induction chemoradiation followed by surgery compared with those receiving full course chemoradiation alone (Hazard Ratio 0.77 (95% CI 0.62 to 0.96), P = 0.017). At 5 years, 22% of participants in the chemoradiation/surgery arm were disease‐free compared with 11% of participants in the chemoradiation arm. During induction chemotherapy/radiotherapy the amount of chemotherapy delivered was similar in both groups. However fewer patients in the surgical group completed consolidative chemotherapy compared with the chemoradiation alone group (55% versus 74%, P < 0.0001). Radiotherapy was administered per protocol (or with acceptable variation) in 96% of patients in the surgical group and 79% in the chemoradiation alone group (P < 0.0001). Of the 202 participants in the chemoradiation/surgery group 155 underwent resection (3 wedge resections, 98 lobectomies and 54 pneumonectomies). Eight percent of participants died from treatment related causes in the chemo/radiation/surgery group compared with 2% in the chemoradiation group. The majority of treatment‐related deaths in the surgical group occurred after pneumonectomy (14 out of 16), with only one death occurring after lobectomy. Grade 3 or 4 oesophagitis was more common in the chemoradiation group (23%) compared with the chemoradiation/surgery group (10%), P = 0.0006. However other toxicities such as pneumonitis, neutropenia, nausea or emesis, were not significantly different between the two groups. Haematological toxicity was reportedly greater in the chemoradiation group during consolidative chemotherapy (56% vs 36%) (Albain 2003). Limited resection (wedge excision or segmentectomy) versus lobectomy for stage IA peripheral NSCLC In the one study that compared limited resection with lobectomy in patients with peripheral stage I NSCLC, limited resection was associated with an increased risk of local recurrence (Ginsberg 1995). In this study there was also a trend to improved overall survival, the five‐year survival was 74% in the lobectomy group and 55% in the limited resection group. The hazard ratio was 0.67 (95% CI 0.44 to 1.02, P = 0.062). The rate of recurrence per person/year was 0.054 in the limited resection group versus 0.019 in the lobectomy group (RR 2.84, 95% CI 1.32 to 6.1, P = 0.007). The non‐local recurrence rates were not significantly different between the two groups (RR 1.07, 95% CI 0.56 to 2.06, P = 0.83). There was a trend to an increased rate of deaths with cancer in the limited resection group compared with the lobectomy group (0.063 per person/years versus 0.043 per person/year). The relative risk for death with cancer was 1.46 (95% CI 0.87 to 2.45, P = 0.15).
The investigators also conducted an analysis which included all patients randomised. They stated that the magnitude of the increase in overall death rate and death with cancer fell from 41% to 26% for the overall death rate and from 47% to 28% for deaths with cancer and lost statistical significance but the actual results and statistics were not reported. In the limited resection group there was less of a fall (from baseline preoperative level) in FEV1 at 12 to 18 months (mean % difference) compared with the lobectomy group. The mean difference between groups was 5.91 (95% CI 0.29 to 11.53, P = 0.04). However this difference is of doubtful clinical significance and the results are difficult to interpret because less than 67% of participants had lung function results available at 12 to 18 months. For FVC, MMEFR and MVV, the mean % difference in the change from baseline was not significantly different between the groups at 12 to 18 months. However limited data were also available for these outcomes. The authors stated that there were no significant differences in the types and number of postoperative complications except respiratory failure requiring postoperative ventilation for more than 24 hours. Six patients in the lobectomy group required postoperative ventilation for more than 24 hours and none in the limited resection group (RR 0.08, 95% CI 0.0 to 1.38, P = 0.08). There were two postoperative deaths in the lobectomy group and one in the limited resection group but these figures were for all 276 individuals randomised (including those exclusions after randomisation discussed above) and it was not clear what the denominator was for each group from the report.
Video‐assisted thoracoscopic lobectomy versus conventional lobectomy for stage I NSCLC In the only study included in this category there was no difference in survival between those treated with resection via open thoracotomy and those treated with VATS (Sugi 2000). There was no comment in the study report about whether resection was complete in all participants or not. The three‐year survival was 93% in the open group and 90% in the VATS group (RR 0.97, 95% CI 0.86 to 1.10, P = 0.64). The five‐year survival rate was 85% in the open group and 90% in the VATS group (RR 1.09, 95% CI 0.91 to 1.23, P = 0.46). The authors did not comment on post operative morbidity or mortality, quality of life, pain, duration of surgery or length of stay (Sugi 2000).
Complete mediastinal lymph node dissection versus mediastinal lymph node systematic sampling in patients with resectable NSCLC The results of the individual trials included in this analysis differ. Izbicki et al reported no significant difference in overall survival between those undergoing CMLND compared with those undergoing SS with a median follow up of 47 months (Izbicki 1998). Sugi and co‐workers also reported no difference in five‐year survival (Sugi 1998). However Wu et al conducted a survival analysis in which some participants were followed for 10 years or more and found significantly better overall survival in those undergoing mediastinal lymph node dissection after adjustment for stage (Wu 2002). In one study there was no comment about whether resection was complete in all cases or not (Sugi 1998). In the remaining two trials participants with residual tumour at the resection margin were excluded after randomisation, but there was no statement about whether this included both macroscopic and microscopic residual disease (Izbicki 1998; Wu 2002).
In the study reported by Izbicki et al, 32% of individuals assigned to CMLND had squamous cell carcinoma compared with 53% of those assigned to SS and this difference was statistically significant (P = 0.032). However the groups were reasonably well balanced for other characteristics. In the study by Wu et al the groups were well balanced for baseline characteristics, although 48% of individuals in the CMLND group had stage IIIA disease compared with 28% in the SS group and this probably reflects more accurate pathological staging in the dissection group rather than a real difference (Wu 2002).
A pooled analysis (fixed‐effects model) was conducted for overall survival for the three studies included in this category. There was a significant reduction in the risk of death in the group undergoing CMLND, the pooled hazard ratio was estimated to be 0.63 (95% CI 0.51 to 0.78, P ≤ 0.0001) and there was no significant statistical heterogeneity between studies being pooled (I2 = 0%, chi2 1.30, P = 0.52). In the reports of two of these primary studies, subgroup analyses by stage were performed. However this type of analysis could be misleading because stage migration in the group undergoing mediastinal lymph node dissection could affect the survival results (Will Rogers phenomenon) (Feinstein 1985; Izbicki 1998; Wu 2002). Therefore we did not conduct a subgroup analysis by stage.
In one trial there was a non‐significant trend to improved disease‐free survival in the CMLND group with a median follow up of 47.5 months, the hazard ratio was reported to be 0.82 (95% CI 0.54 to 1.27) (Izbicki 1998). The remaining trials did not report time to event data for disease recurrence and so a meta‐analysis was not performed. The percentage of patients developing local or distant recurrences was reported in the trials. Meta‐analysis was conducted on these data although it is important to note that the follow‐up periods for each of the studies differ. There was a significant reduction in any cancer recurrence (local or distant) in the CMLND group (RR 0.79, 95% CI 0.66 to 0.95, P = 0.01) and there was no significant statistical heterogeneity (P = 0.64). This appears to be mainly due to a reduction in the number of distant recurrences (RR 0.78, 95% CI 0.61 to 1.00, P = 0.05) and again there was no significant heterogeneity detected (P = 0.7).
None of the trials individually found a significant difference between the groups in terms of 30‐day operative mortality. In the pooled analysis, the relative risk was 0.86 (95% CI 0.19 to 3.77, P = 0.84) and there was no significant statistical heterogeneity between studies being pooled (P = 0.39). In the study by Wu et al, morbidity by treatment group was not described (Wu 2002). Postoperative complications were reported in the studies by Izbicki et al and Sugi et al (Izbicki 1998; Sugi 1998). Air leak lasting more than five days was significantly more common in patients assigned to CMLND (RR 2.94, 95% CI 1.01 to 8.54, P = 0.05) and there was no significant heterogeneity detected (P = 0.74). For all other postoperative complications including retained bronchial secretions requiring more than two bronchoscopies, recurrent laryngeal nerve lesions, repeat thoracotomies, postoperative pneumonia and cardiac arrhythmias there were no significant differences between the sampling and dissection groups (P > 0.25). However because of the relatively small number of complications, larger sample sizes would be needed to detect modest or small differences in the rates of these complications between the CMLND and SS groups.
Discussion
Summary of main results
This is an update of the original systematic review of randomised controlled trials of surgery for NSCLC first published in 2005. Thirteen trials with a total of 2290 patients were included in the review. There were no studies comparing surgery alone with a no‐treatment arm identified by the literature search. There was only one small trial in which surgery alone was compared with radiotherapy alone in individuals with bronchogenic carcinoma limited to the thorax but the trial included some patients with 'oat cell' lung cancer (Morrison 1963). In this study there was a trend to improved four‐year survival in individuals treated with surgery particularly those with squamous cell carcinoma, however because of the small numbers included in this study the results are imprecise and fail to reach significance at the conventional 5% level (RR 3.27, 95% CI 0.74 to 14.42).
The Lung Cancer Study Group trial showed that in patients with stage I NSCLC there was a significant increase of almost three fold in local recurrence in the limited resection group, the trend to a reduction in the rate of death with cancer and death from all causes in the lobectomy group did not reach statistical significance at the conventional 5% level (Ginsberg 1995). The study was designed to show equivalence between the two groups and therefore a priori a more conservative P value > 0.1 was considered to be acceptable evidence of equivalence. However the 95% confidence intervals for the hazard ratio for five‐year overall survival are wide (0.44 to 1.02) and encompass values of equivalence, but also do not exclude a clinically important difference between the two groups. A further study conducted in patients with stage I NSCLC found no difference in survival in between those treated with VATS lobectomy compared with those treated with open lobectomy (Sugi 2000). The results of studies comparing CMLND with SS are also of interest with respect to the efficacy of surgery in general (Sugi 1998; Wu 2002; Izbicki 1998). In the pooled analysis of the three studies there was a significant reduction in death from all causes in the group undergoing CMLND. These results suggest that the CMLND group have approximately 63% as great a risk of dying on any given day, given survival to that point, compared to the lymph node sampling group. However the true hazard ratio could be between 0.51 to 0.78 at the 95% confidence level.
In patients with initially inoperable loco‐regional lung cancer one small study found no difference in survival between those treated with radiotherapy followed by surgery compared with radiotherapy alone (NCI 1975 ). Overall the results of studies included in this review suggest that the role of surgery in stage IIIA NSCLC is limited. Two studies comparing radiotherapy alone with chemotherapy plus surgery in patients with stage IIIA NSCLC were also inconclusive because of small numbers of participants due to the premature closure of these trials (Shepherd 1998; Stephens 2005). There were three trials that compared chemotherapy followed by surgery with chemotherapy followed by radiotherapy however these trials were both clinically and statistically heterogeneous (Johnstone 2002 ;Stathopoulos 1996; van Meerbeeck 2007). One of these studies was inconclusive because of very small numbers and premature closure of the trial (Johnstone 2002). One very small study found a significant improvement in survival in favour of chemotherapy/surgery compared with sequential chemotherapy and radiotherapy in stage IIIA disease (Stathopoulos 1996). The largest of the studies in this category, the EORTC 08941 trial, that compared surgery with radiotherapy in individuals with stage IIIA (N2) NSCLC who had responded to neoadjuvant/induction chemotherapy did not show any significant difference in overall survival or progression‐free survival between the treatment groups (van Meerbeeck 2007). The North American Intergroup trial 0139 (RTOG 93‐09) reported that in patients with stage IIIA N2 NSCLC progression‐free survival was better in those treated with induction chemotherapy/radiotherapy followed by surgery compared with those treated with concurrent chemotherapy and full course radiotherapy (Albain 2003). However, treatment‐related deaths were more common in the surgical group and overall survival was not significantly different between the two groups.
In the North American Intergroup trial 0139, the majority of post operative deaths in the surgical group occurred in those requiring pneumonectomy and post hoc subgroup analysis suggested there may be an improvement in overall survival in those who are judged to be suitable for lobectomy at the outset of treatment. In addition, within the surgical arms of both the EORTC 08941 trial and the North American Intergroup trial 0139 subgroup analysis showed poorer survival amongst those who had persistent pathological N2 disease compared with those who had no pathological residual mediastinal disease (van Meerbeeck 2007; Albain 2003). However any potential improvements in such subgroups of patients would need to be assessed in further randomised controlled trials. The results of the studies in this review suggest that overall, any survival benefit of chemoradiation plus surgery over radical chemoradiation alone in stage IIIA NSCLC is likely to be small.
Overall completeness and applicability of evidence
Several of the studies included in this review were conducted many years ago and therefore given the changing epidemiology of lung cancer and changes in the accuracy of staging the results may not be generalisable to current practice (Morrison 1963; NCI 1975; Janssen‐Heijnen 2003). In addition the radiation dose used in some of studies might be considered suboptimal by contemporary standards (Morrison 1963; Stathopoulos 1996; Hensing 2001).
Few of the trials included in this review have described the experience of the surgeons involved in performing surgery. However this information is important for interpreting the results of these trials. The efficacy of the intervention may be influenced by the experience of the surgeons. Furthermore this information is required when making judgements about the generalisability of any findings. For example, mediastinal lymph node dissection is routinely practised in some countries whereas mediastinal lymph node sampling is performed more often in others. Variation also exists between different institutions and in some cases within institutions. The results of trials performed by experienced surgeons may not be easily generalised to those with less experience with the technique.
Quality of the evidence
The results of this review should be interpreted taking into account the risk of bias in the primary studies included. Important methodological weaknesses were identified in some of the studies. The risk of bias was difficult to assess in some studies because of a lack of information in the study reports about methodology and/or follow up (Johnstone 2002; Stathopoulos 1996; Albain 2003;NCI 1975; Ginsberg 1995). Blinding of outcome assessment was only described for one trial (Izbicki 1998). However it is not always feasible to blind or use placebos in studies involving surgery or comparing complex interventions. Several studies in this review have some methodological weaknesses that represent serious threats to the internal validity of the findings (Ginsberg 1995; Sugi 2000). In particular the Lung Cancer Study Group trial reported high rates of losses to follow up in both groups and did not clearly state whether patients were analysed according to treatment received or treatment assigned (Ginsberg 1995). It should also be noted that blinded assessment of outcome was not undertaken in this study and the high local recurrence rate in the limited resection group could to some extent reflect a detection bias. In the only trial that compared VATS lobectomy with open lobectomy the analysis was not by intention‐to‐treat and the randomisation was not concealed and therefore this trial has a high risk of bias (Sugi 2000) (Schulz 1995). The three studies included in the meta‐analysis comparing complete mediastinal lymph node dissection with mediastinal lymph node sampling in individuals with resectable NSCLC were all judged to have a relatively low risk of bias (Sugi 1998; Wu 2002 ; Izbicki 1998).
The largest of the three studies comparing chemotherapy followed by surgery with chemotherapy followed by radiotherapy in stage IIIA disease (van Meerbeeck 2007) was judged to have a low risk of bias and therefore the results of this study are likely to have greater internal validity than the two smaller trials in this category that were assessed as having a potentially higher risk of bias (Johnstone 2002, Stathopoulos 1996). In the only study in this category that found a significant improvement in survival in the surgical group, the risk of bias was unclear because of a lack of information in the study report (Stathopoulos 1996). The results were not based on an intention to treat analysis. In addition in such a small study it is possible that imbalance between unknown prognostic factors could have arisen and the actual TNM status of individuals was not described (Stathopoulos 1996). From the description of follow up data provided in the North American Intergroup trial 0139 (RTOG 93‐09) it was not possible to assess whether losses to follow up might have introduced any unacceptable risk of bias (Albain 2003).
Potential biases in the review process
Systematic reviews can be limited by selection bias or publication bias and by the quality of primary studies in the review. In the present review the authors have been careful not to draw conclusions that go beyond the strength of the evidence in the primary studies. The majority of studies identified in this review are negative and it is therefore unlikely that the results of any unpublished small negative trials would alter the conclusions of the review. However the possibility that publication bias could affect the results of the meta‐analysis of trials comparing CMLND with SS in resectable NSCLC cannot be discounted completely.
Agreements and disagreements with other studies or reviews
To our knowledge there have been no other systematic reviews of surgery for non‐small cell lung cancer in the literature.
Authors' conclusions
Implications for practice.
Surgical resection has long been considered to provide the best chance for cure for patients with early stage NSCLC. Current evidence from randomised controlled trials neither supports nor discounts this contention. There are no randomised controlled trials comparing surgery for lung cancer with a non‐intervention group. The results of trials comparing surgery with radiotherapy in potentially operable NSCLC are inconclusive (Morrison 1963). There is some indirect evidence to support the role of surgery in local or loco‐regional lung cancer. In particular lobectomy as compared with limited resection was shown to reduce the rate of local recurrence in individuals with stage I NSCLC in one study (Ginsberg 1995). In addition, mediastinal lymph node dissection appears to improve survival compared with mediastinal lymph node sampling in individuals with stages I to IIIA NSCLC (Izbicki 1998; Sugi 1998; Wu 2002). However the strength of this evidence is limited by the small number of participants studied to date. Furthermore interpretation is hampered by the methodological weaknesses of some of the primary studies. Patients being offered surgery for NSCLC need to be fully informed about the potential risks and benefits of this therapy. Current evidence suggests that in stage IIIA N2 NSCLC, chemotherapy followed by surgery is as effective as chemotherapy followed by radical radiotherapy, and radical concurrent chemotherapy and radiotherapy is as effective as induction chemoradiation followed by surgery in terms of overall survival (Albain 2003; van Meerbeeck 2007; Johnstone 2002).
The results of the ACOSOG Z30 trial (yet to be published) will be important to further clarify the benefit of CMLND dissection relative to SS in patients with T1‐2, N0‐1(less than hilar), M0 NSCLC.
Implications for research.
The findings of this review have implications for the conduct of future lung cancer surgery trials. Some common methodological problems were identified. Guidelines for the conduct of thoracic surgical oncological trials would be a useful resource for those contemplating this type of research. Recommendations for the conduct of clinical trials in other procedural fields have been published (Qureshi 2004). Issues such as how to handle exclusions after randomisation, outcome evaluation, and the construct and analysis of studies conducted across multiple institutions should be considered. The types of outcome measures also need to be given careful consideration. For example preventing local recurrence even in the absence of an overall survival benefit may be an important outcome, but should be assessed by measuring additional outcomes such as quality of life.
The current evidence suggests that surgery does not significantly improve survival after induction chemotherapy plus or minus radiotherapy in patients with stage IIIA (N2) NSCLC and therefore it may be reasonable to conduct further randomised controlled trials comparing surgery (plus or minus chemotherapy) with radiotherapy or chemoradiation in selected groups of patients with earlier stage NSCLC. For example in older patients in whom the perioperative mortality of surgery is on average 6% for patients aged 70 to 79 years and 8% for those 80 years and older (Kiser 2001) or in patients with reduced respiratory reserve. Future studies might also be able to clarify whether there are subgroups of patients (for example those who are technically suitable for lobectomy at presentation) with stage IIIA N2 disease who may benefit from induction chemoradiation followed by surgery compared with chemoradiation alone. Further well conducted randomised controlled trials are required to determine whether there are any differences in long term survival and quality of life between patients with stage I NSCLC resected using VATS versus open thoracotomy.
What's new
| Date | Event | Description |
|---|---|---|
| 10 February 2010 | New search has been performed | A search was run and two new studies were identified. In addition a further article identified provided more up to date results for one of the trials included in the original review. Conclusions did not change. |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 1, 2005
| Date | Event | Description |
|---|---|---|
| 18 September 2008 | Amended | Converted to new review format. |
| 10 October 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We wish to thank the Iberoamerican Cochrane Centre for assistance with database searches, and Dr Sera Tort, Dr Marta Roque and Dr Elinor Thompson (Co‐ordinators of the Cochrane Lung Cancer Group) for assistance with protocol development and editing of the review. We would like to acknowledge the help provided by authors of primary studies who have responded to our correspondence and provided additional information. At the beginning of this project Renée Manser was supported by an Australian National Health and Medical Research Council postgraduate scholarship (scholarship number 201713).
Appendices
Appendix 1. Search strategies
Search strategies performed (October 2nd 2009)
MEDLINE (PubMed)
#1 Surgical resection[tw] 19772
#2 "Thoracic Surgery"[Mesh] 8810
#3 Pneumonectomy[Mesh] 17180
#4 "Lymph Node Excision"[Mesh] 26539
#5 Lobectom*[tw] 10590
#6 Wedge resection[tw] 1889
#7 Lymph node sampling[tw] 401
#8 Limited resection[tw] 571
#9 Segmentectomy[tw] 1311
#10 Sleeve resection[tw] 379
#11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 79637
#12 "Carcinoma, Non‐Small‐Cell Lung"[Mesh] 19874
#13 non small cell lung cancer[tw] 16130
#14 nsclc[tw] 10401
#15 ((#12) OR #13) OR #14 23861
#16 (#11) AND #15 2682
#17 (randomized controlled trial[pt] OR controlled clinical trial[pt] OR randomized[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab]) NOT (animals[mh] NOT (humans[mh] AND animals[mh])) 2156252
#18 (#16) AND #17 975
#19 ("2003"[Publication Date] : "3000"[Publication Date]) AND ((#16) AND #17) 571
CENTRAL (The Cochrane Library 2009, Issue 3)
#1 MeSH descriptor Carcinoma, Non‐Small‐Cell Lung explode all trees 1521
#2 non small cell cancer in Clinical Trials 3093
#3 nsclc in Clinical Trials 1649
#4 (#1 OR #2 OR #3) 4081
#5 surgical resection in Clinical Trials 1850
#6 lobectom* in Clinical Trials 213
#7 wedge resection in Clinical Trials 58
#8 lymph node sampling in Clinical Trials 158
#9 limited resection in Clinical Trials 252
#10 segmentectomy in Clinical Trials 24
#11 sleeve resection in Clinical Trials 4
#12 MeSH descriptor Thoracic Surgery explode all trees 139
#13 MeSH descriptor Pneumonectomy explode all trees 333
#14 MeSH descriptor Lymph Node Excision explode all trees 773
#15 (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) 4165
#16 (#4 AND #15) 343
#17 (#16), from 2003 to 2009 72
EMBASE (Ovid)
1 exp thorax surgery/ 198201
2 exp lung resection/ 9076
3 exp lymphadenectomy/ 19347
4 lobectom*.mp. 11843
5 (surgical adj3 resection).mp. 18629
6 Wedge resection.mp. 1472
7 Lymph node sampling.mp. 370
8 (Limited adj3 resection).mp. 972
9 segmentectomy.mp. 1023
10 sleeve resection.mp. 327
11 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 240414
12 exp lung non small cell cancer/ 23350
13 Non small Cell Lung Cancer.mp. 15503
14 nsclc.mp. 9898
15 13 or 12 or 14 25699
16 11 and 15 3316
17 Clinical trial/ 557163
18 Randomized controlled trials/ 174001
19 Random Allocation/ 27071
20 Single‐Blind Method/ 8542
21 Double‐Blind Method/ 74144
22 Cross‐Over Studies/ 21774
23 Placebos/ 131701
24 Randomi?ed controlled trial$.tw. 35063
25 RCT.tw. 2931
26 Random allocation.tw. 646
27 Randomly allocated.tw. 10509
28 Allocated randomly.tw. 1367
29 (allocated adj2 random).tw. 565
30 Single blind$.tw. 7675
31 Double blind$.tw. 86799
32 ((treble or triple) adj blind$).tw. 141
33 Placebo$.tw. 113115
34 Prospective Studies/ 85878
35 33 or 32 or 21 or 26 or 17 or 22 or 18 or 30 or 23 or 29 or 25 or 27 or 28 or 20 or 34 or 24 or 19 or 31 731476
36 Case study/ 6386
37 Case report.tw. 123217
38 Abstract report/ or letter/ 512027
39 38 or 36 or 37 639194
40 35 not 39 705947
41 animal/ not human/ 14494
42 40 not 41 705851
43 42 and 16 864
44 43 864
45 limit 43 to yr="2003 ‐Current" 599
Search methods published in the previous version of the review
The MEDLINE (1966 to December 2003) (Pub Med) database was searched using the recommended Cochrane search strategy for randomised controlled trials in addition to the following:
#1. Surgical resection
#2. "Surgery"[Subheading]
#3. "Thoracic Surgery"[MESH]
#4. "Pneumonectomy"[MESH]
#5. "Lymph Node Excision"[MESH]
#6. Lobectomy
#7. Wedge resection
#8. Lymph node sampling
#9. Limited resection
#10. Segmentectomy
#11. Sleeve resection
#12. or/1‐11
#13. "Carcinoma, Non‐Small‐Cell Lung"[MESH]
#14. NSCLC
#15. Non small Cell Lung Cancer
#16. or/13‐15
An advanced search of the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Plus, Issue 4, 2003) was also undertaken using the following strategy:
1 LUNG AND CANCER
2 BRONCHOGENIC AND CARCINOMA
3 LUNG AND NEOPLASM*
4 1 OR 2 OR 3
5 LUNG AND SURGERY*
6 LUNG AND RESECTION
7 PNEUMONECTOMY
8 LOBECTOMY
9 WEDGE AND RESECTION
10 LIMITED AND RESECTION
11 5 OR 6 OR 7 OR 8 OR 9 OR 10
12 11 AND 4
EMBASE (1974 to December 2003) was also searched using the following search strategy:
#1. Surgical ADJ resection
#2. Surgery#.DE.
#3. Thorax‐Surgery#.DE.
#4. Lung‐Resection#.DE.
#5. Lymph‐Node‐Dissection.MJ.
#6. Lobectomy
#7. Wedge ADJ resection
#8. Lymph ADJ node ADJ sampling
#9. Limited ADJ resection
#10. Segmentectomy
#11. Sleeve ADJ resection
#12. or/1‐11
#13. Lung‐Non‐Small‐Cell‐Cancer#.DE.
#14. NSCLC
#15. Nonsmall ADJ cell ADJ lung ADJ cancer
#16. Non ADJ small ADJ cell ADJ lung ADJ cancer
#17. or/13‐16
The journal Lung Cancer was handsearched from 1995 to March 2004 including abstracts from scientific meetings of the International Association for the Study of Lung Cancer. Abstracts from the annual scientific meeting of the American Association for Thoracic Surgery were searched for the year 2002. Abstracts from the annual scientific meeting of the European Association for Cardiothoracic Surgery were handsearched for the years 1999 to 2003. The bibliographies of identified studies and narrative reviews were searched for additional citations. Authors of primary studies and experts in the field were contacted
Appendix 2. Additional information on incomplete outcome data
In four trials, participants were analysed in the groups to which they were assigned regardless of whether they received the treatment or not (NCI 1975; Albain 2003; Stephens 2005; van Meerbeeck 2007). In the NCI study three patients assigned to no surgery requested and underwent surgery and 13 patients assigned to the surgery group did not undergo surgery (NCI 1975). None of the remaining studies included in the review contained a clear statement that they had conducted an intention‐to‐treat analysis.
In the study by Shepherd et al, all patients received the treatment which they were randomised to and were included in the analysis (confirmed by contacting one of the authors) (Shepherd 1998). In one study, two patients randomised to VATS lobectomy were converted to open lobectomy due to intraoperative bleeding and these two were then subsequently analysed as part of the open lobectomy group, so the analysis was not intention‐to‐treat (Sugi 2000).
In the study by Johnstone et al, there was one participant in the radiotherapy group who was ineligible and excluded after randomisation (Johnstone 2002). In the only study comparing surgery alone with radiotherapy alone, 30 participants were randomised to surgery and 28 to radiotherapy. All patients in the radiotherapy group tolerated the full course of treatment (Morrison 1963). Only 17 patients assigned to surgery underwent resection (82% of these had squamous cell carcinoma) the remainder were inoperable at the time of thoracotomy (Morrison 1963). However all patients assigned to the surgical group were included in the analysis regardless of whether or not they received treatment (although there was no statement about losses to follow up)(Morrison 1963). An intention‐to‐treat analysis was not conducted by Stathopoulos et al in their study (Stathopoulos 1996). Three patients (two from the intervention group) were excluded after entering the study, because of very early disease progression or patient choice to discontinue treatment before completion (Stathopoulos 1996).
In the only study comparing limited resection with lobectomy, 276 patients were randomised intraoperatively, but 29 were excluded from the analysis after randomisation (Ginsberg 1995). There were eight exclusions due to ineligible cell types, eight patients did not have T1 N0 disease, eight patients had benign disease, one patient had a middle lobe tumour, two patients had metastases that appeared within one week of randomisation, one patient had a non‐pulmonary primary and one patient had a previous malignancy (Ginsberg 1995). Of those who were included in the analysis (n=247), the authors stated that 122 received a limited resection and 125 received a lobectomy. Eight additional patients were unable to receive the assigned form of operation as a result of complications during the operation while 11 of the 139 "limited resection" patients required complete lobectomy because of either greater than T1 N0 disease or incomplete resection (Ginsberg 1995). It is not entirely clear if these additional 19 patients were excluded after randomisation in addition to the 29 exclusion described above. An additional analysis was conducted on all 276 patients randomised in terms of overall survival and recurrence rates however it was not clear in the report if this excludes the eight additional patients who did not receive the assigned operation because of complications or the 11 out of 139 limited resection patients who required completion lobectomy (Ginsberg 1995).
In one study comparing CMLND with SS, 201 participants were randomised, 100 to the intervention group and 101 to the SS group but 32 patients were excluded after randomisation, 12 because of residual tumour, 10 because they had small cell lung cancer, 5 because of N3 disease and 5 because they had metastatic disease in the resected lobe of the lung (Izbicki 1998). There were a greater number of exclusions in the CMLND group compared with the SS group (24 versus 8). Apart from these exclusions, individuals appear to have been analysed in the groups to which they were assigned but strictly speaking this does not constitute an intention‐to‐treat analysis (Izbicki 1998; Fergusson 2002). In another study comparing CMLND with SS, all patients analysed appear to have been analysed in the groups to which they were assigned (Sugi 1998). In this study, there were nine individuals with histology other than primary non‐small cell lung cancer and these appear to have been included in the analysis (Sugi 1998). In the study by Wu et al, participants appear to have been analysed in the groups to which they were assigned, however there were 61 exclusions after randomisation and one surgical death was censored in the survival analysis (Wu 2002). After randomisation 28 patients were excluded from the CMLND group and 33 from the SS group. Of these, 36 were excluded because of incomplete resection, 15 because they had small cell lung cancer or other type of pathology and 10 with stage IIIB or IV disease (Wu 2002).
Data and analyses
Comparison 1. Surgical resection alone versus radiotherapy alone for clinical stage I to III lung cancer.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 2‐year survival | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 4‐year survival | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 30‐day mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Subgroup analysis: 4‐year survival in patients with squamous cell carcinoma | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
1.1. Analysis.
Comparison 1 Surgical resection alone versus radiotherapy alone for clinical stage I to III lung cancer, Outcome 1 2‐year survival.
1.2. Analysis.
Comparison 1 Surgical resection alone versus radiotherapy alone for clinical stage I to III lung cancer, Outcome 2 4‐year survival.
1.3. Analysis.
Comparison 1 Surgical resection alone versus radiotherapy alone for clinical stage I to III lung cancer, Outcome 3 30‐day mortality.
1.4. Analysis.
Comparison 1 Surgical resection alone versus radiotherapy alone for clinical stage I to III lung cancer, Outcome 4 Subgroup analysis: 4‐year survival in patients with squamous cell carcinoma.
Comparison 2. Chemotherapy plus surgery versus radiotherapy for stage IIIA NSCLC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 2‐year survival | 2 | Effect of intervention (Random, 95% CI) | Totals not selected |
Comparison 3. Surgery versus no surgery in patients with initially inoperable loco‐regional cancer treated with radiotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 5‐year survival | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 5‐year disease free survival | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Respiratory complications | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
3.1. Analysis.
Comparison 3 Surgery versus no surgery in patients with initially inoperable loco‐regional cancer treated with radiotherapy, Outcome 1 5‐year survival.
3.2. Analysis.
Comparison 3 Surgery versus no surgery in patients with initially inoperable loco‐regional cancer treated with radiotherapy, Outcome 2 5‐year disease free survival.
3.3. Analysis.
Comparison 3 Surgery versus no surgery in patients with initially inoperable loco‐regional cancer treated with radiotherapy, Outcome 3 Respiratory complications.
Comparison 4. Chemotherapy plus surgery versus chemotherapy plus radiotherapy for stage IIIA NSCLC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | Hazard ratio (Random, 95% CI) | Totals not selected | |
| 2 Progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 3 Treatment‐related deaths | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
4.1. Analysis.
Comparison 4 Chemotherapy plus surgery versus chemotherapy plus radiotherapy for stage IIIA NSCLC, Outcome 1 Overall survival.
4.2. Analysis.
Comparison 4 Chemotherapy plus surgery versus chemotherapy plus radiotherapy for stage IIIA NSCLC, Outcome 2 Progression‐free survival.
4.3. Analysis.
Comparison 4 Chemotherapy plus surgery versus chemotherapy plus radiotherapy for stage IIIA NSCLC, Outcome 3 Treatment‐related deaths.
Comparison 5. Concurrent chemotherapy and full course radiotherapy versus induction concurrent chemoradiation and surgery.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 2 Progression‐free survival | 1 | Hazard Ratio (Random, 95% CI) | Totals not selected | |
| 3 Treatment‐related deaths | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Grade 3 or 4 oesophagitis | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only |
5.1. Analysis.
Comparison 5 Concurrent chemotherapy and full course radiotherapy versus induction concurrent chemoradiation and surgery, Outcome 1 Overall survival.
5.2. Analysis.
Comparison 5 Concurrent chemotherapy and full course radiotherapy versus induction concurrent chemoradiation and surgery, Outcome 2 Progression‐free survival.
5.3. Analysis.
Comparison 5 Concurrent chemotherapy and full course radiotherapy versus induction concurrent chemoradiation and surgery, Outcome 3 Treatment‐related deaths.
5.4. Analysis.
Comparison 5 Concurrent chemotherapy and full course radiotherapy versus induction concurrent chemoradiation and surgery, Outcome 4 Grade 3 or 4 oesophagitis.
Comparison 6. Limited resection (wedge excision or segmentectomy) versus lobectomy for stage IA peripheral NSCLC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in FEV1 (from baseline) at 12 to 18 months (mean % difference) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Change in FVC (from baseline) at 12 to 18 months (mean % difference) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Change in MMEFR (from baseline) at 12 to 18 months (mean % difference) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Change in MVV (from baseline) at 12 to 18 months (mean % difference) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 Loco‐regional recurrence rate (per person/year) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Non‐local recurrence rate (per person/year) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Death with cancer rate (per person/year) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8 Post operative respiratory failure requiring ventilation for more than 24 hours | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
6.1. Analysis.
Comparison 6 Limited resection (wedge excision or segmentectomy) versus lobectomy for stage IA peripheral NSCLC, Outcome 1 Change in FEV1 (from baseline) at 12 to 18 months (mean % difference).
6.2. Analysis.
Comparison 6 Limited resection (wedge excision or segmentectomy) versus lobectomy for stage IA peripheral NSCLC, Outcome 2 Change in FVC (from baseline) at 12 to 18 months (mean % difference).
6.3. Analysis.
Comparison 6 Limited resection (wedge excision or segmentectomy) versus lobectomy for stage IA peripheral NSCLC, Outcome 3 Change in MMEFR (from baseline) at 12 to 18 months (mean % difference).
6.4. Analysis.
Comparison 6 Limited resection (wedge excision or segmentectomy) versus lobectomy for stage IA peripheral NSCLC, Outcome 4 Change in MVV (from baseline) at 12 to 18 months (mean % difference).
6.5. Analysis.
Comparison 6 Limited resection (wedge excision or segmentectomy) versus lobectomy for stage IA peripheral NSCLC, Outcome 5 Loco‐regional recurrence rate (per person/year).
6.6. Analysis.
Comparison 6 Limited resection (wedge excision or segmentectomy) versus lobectomy for stage IA peripheral NSCLC, Outcome 6 Non‐local recurrence rate (per person/year).
6.7. Analysis.
Comparison 6 Limited resection (wedge excision or segmentectomy) versus lobectomy for stage IA peripheral NSCLC, Outcome 7 Death with cancer rate (per person/year).
6.8. Analysis.
Comparison 6 Limited resection (wedge excision or segmentectomy) versus lobectomy for stage IA peripheral NSCLC, Outcome 8 Post operative respiratory failure requiring ventilation for more than 24 hours.
Comparison 7. Video‐assisted thoracoscopic lobectomy versus open lobectomy for stage I NSCLC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 3‐year survival | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 5‐year survival | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
7.1. Analysis.
Comparison 7 Video‐assisted thoracoscopic lobectomy versus open lobectomy for stage I NSCLC, Outcome 1 Overall survival.
7.2. Analysis.
Comparison 7 Video‐assisted thoracoscopic lobectomy versus open lobectomy for stage I NSCLC, Outcome 2 3‐year survival.
7.3. Analysis.
Comparison 7 Video‐assisted thoracoscopic lobectomy versus open lobectomy for stage I NSCLC, Outcome 3 5‐year survival.
Comparison 8. Mediastinal lymph node sampling versus systematic nodal dissection.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 3 | Hazard ratio (Fixed, 95% CI) | 0.63 [0.51, 0.78] | |
| 2 30‐day surgical mortality | 3 | 829 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.19, 3.77] |
| 3 Retained bronchial secretions requiring more than 2 bronchoscopies | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.72, 3.49] |
| 4 Air leak persisting for more than 5 days | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 2.94 [1.01, 8.54] |
| 5 Recurrent laryngeal nerve lesions | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 2.30 [0.23, 22.88] |
| 6 Repeat thoracotomies | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.29, 4.24] |
| 7 Postoperative pneumonia | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.33, 3.18] |
| 8 Cardiac arrhythmias | 2 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.71, 2.21] |
| 9 Local recurrence rates | 3 | 755 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.54, 1.19] |
| 10 Distant recurrence rate | 3 | 755 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.60, 1.00] |
| 11 Any disease recurrence | 3 | 755 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.66, 0.95] |
8.1. Analysis.
Comparison 8 Mediastinal lymph node sampling versus systematic nodal dissection, Outcome 1 Overall survival.
8.2. Analysis.
Comparison 8 Mediastinal lymph node sampling versus systematic nodal dissection, Outcome 2 30‐day surgical mortality.
8.3. Analysis.
Comparison 8 Mediastinal lymph node sampling versus systematic nodal dissection, Outcome 3 Retained bronchial secretions requiring more than 2 bronchoscopies.
8.4. Analysis.
Comparison 8 Mediastinal lymph node sampling versus systematic nodal dissection, Outcome 4 Air leak persisting for more than 5 days.
8.5. Analysis.
Comparison 8 Mediastinal lymph node sampling versus systematic nodal dissection, Outcome 5 Recurrent laryngeal nerve lesions.
8.6. Analysis.
Comparison 8 Mediastinal lymph node sampling versus systematic nodal dissection, Outcome 6 Repeat thoracotomies.
8.7. Analysis.
Comparison 8 Mediastinal lymph node sampling versus systematic nodal dissection, Outcome 7 Postoperative pneumonia.
8.8. Analysis.
Comparison 8 Mediastinal lymph node sampling versus systematic nodal dissection, Outcome 8 Cardiac arrhythmias.
8.9. Analysis.
Comparison 8 Mediastinal lymph node sampling versus systematic nodal dissection, Outcome 9 Local recurrence rates.
8.10. Analysis.
Comparison 8 Mediastinal lymph node sampling versus systematic nodal dissection, Outcome 10 Distant recurrence rate.
8.11. Analysis.
Comparison 8 Mediastinal lymph node sampling versus systematic nodal dissection, Outcome 11 Any disease recurrence.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albain 2003.
| Methods | Randomised controlled trial (1994‐2001) | |
| Participants | Individuals with stage IIIA pathologically confirmed N2 non‐small cell lung cancer. Patients with performance status 0‐1 were eligible if resection was technically feasible at randomisation | |
| Interventions | All patients first received induction chemotherapy consisting of cisplatin 50mg/m2 d1,8 and etoposide 50 mg/m2 d1‐5(PE) X2 and daily radiotherapy to 45 Gy starting day 1. After induction, individuals were treated according to the following: Intervention group: underwent resection if no progression, followed by PE X2; Control group: received uninterrupted radiotherapy to 61 Gy and PE X2 | |
| Outcomes | Toxicity, morbidity and mortality associated with treatment. Progression‐free and overall survival | |
| Notes | C factor was C3 ‐ all patients had CT scan chest and had mediastinoscopy and biopsy to confirm N2 status and exclude N3 disease (if contralateral nodes were present) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated |
| Allocation concealment? | Low risk | Random allocation schedule generated at the Radiation Therapy Oncology Group statistical centre |
| Blinding? All outcomes | Unclear risk | Information not reported |
| Incomplete outcome data addressed? All outcomes | High risk | Description of withdrawals and follow up was incomplete. Median follow up for all patients was 22.5 months (range 0.9 to 125.1) and for those still alive at the final analysis was 69.3 months (6.2 to 125.1). Only 92% of patients randomised were eligible for the analysis, mainly due to factors such as wrong stage or incomplete staging. |
Ginsberg 1995.
| Methods | Randomised controlled trial (1982 to 1988) | |
| Participants | Individuals with T1 N0 peripherally based, suspected or proven lung cancer and able to tolerate lobectomy as assessed by cardiopulmonary function | |
| Interventions | Intervention group: limited resection (wedge or segmentectomy); Control group: lobectomy Randomisation was stratified according to age, pulmonary function, and whether the intended limited resection would be a wedge or segment. | |
| Outcomes | Post operative morbidity and mortality. Pulmonary function (FEV1, FVC, MMEFR and MVV) at 6 and 12 months. Overall survival and local and distant cancer recurrence rates. | |
| Notes | C‐factor staging : C4 (after thoracotomy); C1 (prior to thoracotomy) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not reported |
| Allocation concealment? | Low risk | 'Randomisation occurred intraoperatively by telephone communication to the Operations Office' |
| Blinding? All outcomes | Unclear risk | Information not reported |
| Incomplete outcome data addressed? All outcomes | High risk | Description of withdrawals and losses to follow up were reported in a subsequent letter to the editor (18% in both groups). The magnitude of losses to follow up were judged to be sufficiently large that they might affect the results of the study |
Izbicki 1998.
| Methods | Randomised controlled trial (1989 to 1991) | |
| Participants | Individuals with curatively resectable non‐small cell lung cancer. Individuals with N3, or M1 or R1 or R2 disease or small cell, excluded after randomisation and resection (for survival analysis) | |
| Interventions | Intervention group: Thoracotomy and lung resection with CMLND. Control group: Thoracotomy and lung resection with mediastinal SS (regional lymph node dissection including hilar nodes (10,11,12), mediastinotomy with exploration of nodes 2‐9 and routine removal of nodes of stations 4,5 and 7) | |
| Outcomes | Surgical mortality and morbidity, intra‐operative parameters and post‐operative parameters, local recurrence and distant recurrence rates, cancer‐related survival and overall survival | |
| Notes | C‐factor staging: C4 (after thoracotomy) Tumours were classified using staging classification suggested by UICC 1987. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Information not reported in the paper. Classified as adequate after contacting the authors. |
| Allocation concealment? | Low risk | Allocation concealment was unclear from the paper, but after contacting the authors, concealment of allocation was reclassified as adequate |
| Blinding? All outcomes | Low risk | Information not reported in the paper. After contacting the authors, we were told that investigators undertaking the follow up were blind to the type of operation |
| Incomplete outcome data addressed? All outcomes | Low risk | Three patients from each group were lost to follow up |
Johnstone 2002.
| Methods | Randomised controlled trial (1990 to 1994) | |
| Participants | Individuals with stage IIIA (T1‐T3N2MO) NSCLC. Histologically confirmed N2 disease at mediastinoscopy or anterior mediastinotomy | |
| Interventions | Intervention group: induction chemotherapy followed by surgical resection; Control group: Induction chemotherapy followed by radiotherapy (64Gy). Patients stratified according bulky N2 disease (visible on plain chest radiography) versus other N2 disease | |
| Outcomes | Toxicity, treatment related morbidity and mortality. Overall survival at 4 years. | |
| Notes | C‐factor staging : C3
Staging classification criteria not described Trial terminated early. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Information not reported |
| Allocation concealment? | Unclear risk | Information not reported |
| Blinding? All outcomes | Unclear risk | Information not reported |
| Incomplete outcome data addressed? All outcomes | High risk | Description of withdrawals and losses to follow up not complete. 75 patients entered in the study including 12 patients entered but then not randomised after induction. 2 patients ineligible and not included, leaving 29 in surgical group and 32 in the radiotherapy group that were included in the analysis. 2 patients lost to follow up but it was not stated which group or groups they were lost from however all others were followed for at least 48 months. |
Morrison 1963.
| Methods | Randomised controlled trial (1954 to 1958) | |
| Participants | Individuals with histologically confirmed lung cancer (including small cell cancer) and clinically confined to the chest. | |
| Interventions | Intervention group: thoracotomy and radical resection of tumour and associated hilar and mediastinal lymph nodes Control group: Radiotherapy (planned mean dose of 45 Gy). | |
| Outcomes | Morbidity and mortality associated with treatment. Overall survival at 1, 2, 3, and 4 years. | |
| Notes | C‐factor staging: C1 Predates modern staging criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'The method of treatment was decided by random selection cards, prepared by the Medical Research Council’s statistical unit. |
| Allocation concealment? | Low risk | 'Cards on which the treatment was indicated were drawn from sealed envelopes' |
| Blinding? All outcomes | Unclear risk | None described |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No statement about losses to follow up |
NCI 1975.
| Methods | Randomised controlled trial (1963 to 1966) | |
| Participants | Patients with locally advanced lung cancer (NSCLC and small cell) who were initially classified as inoperable but were thought to be potentially operable after radiotherapy . Patients were classified as inoperable if they had 1) mediastinal, supraclavicular, or scalene lymph node involvement, 2) chest wall invasion, or 3) encroachment of tumour upon the carina. | |
| Interventions | After radiotherapy individuals with cancer that was subsequently considered resectable were randomised to either surgical resection or no surgery. | |
| Outcomes | 5‐year overall survival, treatment related complications | |
| Notes | C‐factor staging: C1 Predates modern staging criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | 'Separate lists of random assignments were prepared for each institution and kept at the statistical center' |
| Allocation concealment? | Low risk | 'The assignment for each new patient was obtained by phone call to the statistical center' |
| Blinding? All outcomes | Unclear risk | None described |
| Incomplete outcome data addressed? All outcomes | Low risk | 'All patients in the study groups were followed until death or through 5 full years of survival' |
Shepherd 1998.
| Methods | Randomised controlled trial (prior to 1997) | |
| Participants | Individuals with Stage IIIA NSCLC with biopsy proven mediastinal node involvement and fit for surgery with predicted post‐operative FEV1 of > 0.8L. ECOG performance status of less than or equal to 2. | |
| Interventions | Intervention group: induction chemotherapy followed by surgical resection; Control group: radiotherapy (60Gy) | |
| Outcomes | Response rates, toxicity and treatment related morbidity and mortality. Survival at 2 years. | |
| Notes | Trial closed prematurely C‐factor staging: C2/3 (biopsy proven mediastinal node involvement) Staging classification criteria not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Information not reported. After contacting the authors, generation of sequence was reclassified as adequate (computer generated) |
| Allocation concealment? | Low risk | Information not reported. After contacting the authors, allocation concealment was reclassified as adequate |
| Blinding? All outcomes | Unclear risk | Information not reported |
| Incomplete outcome data addressed? All outcomes | Low risk | Information not reported. After contacting the authors it was confirmed that there were no losses to follow up |
Stathopoulos 1996.
| Methods | Randomised controlled trial (1988 to 1991) | |
| Participants | Individuals with histologically confirmed stage IIIA NSCLC (on surgical specimen). TNM status of participants was not specified in the report. In both groups the Karnofsky performance status ranged between 70 to 90. | |
| Interventions | Intervention group: 4 courses of chemotherapy followed by surgical resection (either lobectomy or pneumonectomy). Control group: 6 courses of chemotherapy followed by radiotherapy (50Gy to primary site and mediastinum). Chemotherapy consisted of cis‐platinum, vindesine and epirubicin administered once every 3 weeks. | |
| Outcomes | Response rate, toxicity and 5 year overall survival | |
| Notes | C‐factor for staging: 50% C4 and 50% C2 Staging criteria used were not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | This trial was not described as randomised but after contacting the author it was classified as randomised, however method used to generate the allocation sequence was not supplied. |
| Allocation concealment? | Unclear risk | Information not reported |
| Blinding? All outcomes | Unclear risk | None described |
| Incomplete outcome data addressed? All outcomes | Unclear risk | No description of withdrawals and follow up |
Stephens 2005.
| Methods | Randomised controlled trial (1995 to 1999) | |
| Participants | Patients had microscopically confirmed NSCLC stage T3, N1, M0 or T1‐3, N2, M0 disease, considered by the local thoracic surgeon to be unresectable but to have the potential to become resectable following chemotherapy. | |
| Interventions | Radiotherapy group: Thoracic radiotherapy (to be decided by the local radiation oncologist according to the site and extent of the tumour and local practice and around 50‐60Gy over 3‐6 weeks). Chemotherapy group: 4 cycles of chemotherapy at 3‐week intervals followed by surgical resection, if feasible, between 4 and 6 weeks after the final cycle of chemotherapy. |
|
| Outcomes | Survival, adverse effects and quality of life. | |
| Notes | Although it had been estimated that 350 patients could be recruited in 3 years, only 48 from 12 centres were recruited. Some changes to the protocol were suggested but there was no common agreement about those and the Data and Monitoring and Ethics Committee recommended closing the trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Generated by minimisation |
| Allocation concealment? | Low risk | 'Clinicians telephoned the Cancer Division of the Medical Research Council Clinical Trials Unit' |
| Blinding? All outcomes | Unclear risk | Information not reported |
| Incomplete outcome data addressed? All outcomes | Low risk | Description of withdrawals and losses to follow up adequate |
Sugi 1998.
| Methods | Randomised controlled trial (1985 to 1992) | |
| Participants | Individuals with peripheral NSCLC less than 2 cm in diameter & hilar or mediastinal lymph nodes less than 1cm on CT. No pre‐operative mediastinoscopy performed. | |
| Interventions | Intervention group: thoracotomy with lobectomy or bilobectomy and mediastinal lymph node dissection (n=59) Control: thoracotomy with lobectomy or bilobectomy and mediastinal lymph node sampling | |
| Outcomes | Surgical mortality and morbidity, duration of surgery and blood loss. Overall 3 and 5 year survival, local and distant recurrence rate | |
| Notes | C‐factor staging C4 (after thoracotomy) Lymph nodes were classified using the scheme of the American Thoracic Society (Martini 1983) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | 'Patients were randomly divided into two groups using computer‐generated random numbers’ |
| Allocation concealment? | Low risk | Information not reported. After contacting the authors, allocation concealment was reclassified as adequate |
| Blinding? All outcomes | Unclear risk | None described |
| Incomplete outcome data addressed? All outcomes | Low risk | Description of withdrawals and losses to follow up adequate (two patients, one in each group, were lost to follow up) |
Sugi 2000.
| Methods | Randomised controlled trial (1993 to 1994) | |
| Participants | Individuals with clinical stage IA NSCLC. No preoperative mediastinoscopy | |
| Interventions | Intervention group: video‐assisted thoracoscopic lobectomy; Control group: conventional lobectomy. Both groups had hilar and mediastinal lymph node dissections performed in a similar manner | |
| Outcomes | Cancer recurrence rates, overall survival at 3 and 5 years. | |
| Notes | C‐factor staging C2 prior to surgery and C4 after resection Staging classification criteria not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | High risk | 'Patients were randomised into the 2 groups according to their ID numbers' |
| Allocation concealment? | High risk | Information not reported. If randomisation was generated by alternation it is very probable that allocation was not concealed |
| Blinding? All outcomes | Unclear risk | None described |
| Incomplete outcome data addressed? All outcomes | Low risk | Information not reported. After contacting study authors, it was confirmed that there were no losses to follow up |
van Meerbeeck 2007.
| Methods | Randomised controlled trial (1994‐2002) | |
| Participants | Patients had histologic or cytologic proven stage IIIA N2 disease. | |
| Interventions | Intervention:Three cycles of platinum‐based induction chemotherapy followed by surgery Control group: Three cycles of platinum‐based induction chemotherapy followed by definitive radiotherapy |
|
| Outcomes | Five‐year overall survival, progression‐free survival, toxicity and mortality | |
| Notes | C factor for staging C3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Computer generated |
| Allocation concealment? | Low risk | Central allocation (European Organisation and Treatment of Cancer Data Centre) |
| Blinding? All outcomes | Unclear risk | None described |
| Incomplete outcome data addressed? All outcomes | Low risk | There were no losses to follow up for the outcome of overall survival in those patients who were randomly assigned. |
Wu 2002.
| Methods | Randomised controlled trial (1989 to 1995) | |
| Participants | Individuals with pathologically confirmed NSCLC, cTNM stage I‐IIIA, age <71 Patients with stage IIIB and IV cancer after re‐staging from resection were excluded after randomisation as were those with incomplete resection and cancer other than NSCLC (total exclusions =61) | |
| Interventions | Intervention group: lung resection plus systematic nodal dissection. Control group: Lung resection plus mediastinal lymph node sampling (hilar lymph node dissection, mediastinotomy & nodes of stations 1‐9 were explored, nodes of station 7 were routinely removed) | |
| Outcomes | Surgical mortality. Overall survival (5 years). Proportion of participants experiencing tumour recurrence. | |
| Notes | C‐factor staging C4 (after thoracotomy) Pathological stages were classified using the 1997 UICC revisions of the international system for staging lung cancer (Mountain 1997) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Information not reported. After contacting study authors, sequence generation was reclassified as adequate (computer generated) |
| Allocation concealment? | Low risk | Information not reported. After contacting study authors, concealment of allocation was reclassified as adequate |
| Blinding? All outcomes | Unclear risk | None described |
| Incomplete outcome data addressed? All outcomes | Low risk | Description of withdrawals and losses to follow up were adequate (There were six cases lost to follow‐up and the rate of lost to follow‐up was 1.88%) |
NSCLC = non‐small cell lung cancer FEV1= Forced expiratory volume in 1 second; FVC = Forced vital capicity; MMEFR= maximum midexpiratory flow rate; MVV= maximum voluntary ventilation SS = systematic sampling CMLND = complete mediastinal lymph node dissection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bretel 1997 | Report of uncontrolled trial of patients with stage IIIB NSCLC treated with chemotherapy and bifractionated radiotherapy followed by repeat evaluation and surgical excision. |
| Cox 1991 | Review of studies examining outcomes in patients with stage IIIA (N2) NSCLC treated with radiotherapy. |
| Durci 1991 | Large case series of patients with stage III disease treated with surgery or radiotherapy |
| Ferguson 2000 | Large retrospective case series of patients undergoing major lung resection at a single institution. |
| Harpole 1995 | Report on a large series of patients with stage I NSCLC. |
| Keller 2000 | Non randomised comparison of systematic sampling versus complete mediastinal lymph node dissection in 373 patients with resected stage II‐IIIa NSCLC who had been randomised to a phase III trial of adjuvant therapy by the Eastern Co‐operative Oncology Group. Complete mediastinal lymph node dissection was associated with an improved survival in patients with right sided tumours. |
| Kirby 1995 | Randomised controlled trial comparing video‐assisted thoracic surgery with muscle sparing thoracotomy in patients with stage I non‐small cell lung cancer. Outcomes assessed included intraoperative and post‐operative complications, length of hospital stay and post‐thoracotomy pain. This study was excluded from the systematic review because there was no long term follow up (average length of follow up 13 months) and no survival analysis at two or five years. |
| MRC Trial small cell | Randomised controlled trial of surgery versus radiotherapy in patients with potentially operable (bronchoscopically accessible) small cell or 'oat cell' carcinoma of the bronchus. |
| Pitz 2002 | Phase II multicentre study of neoadjuvant chemotherapy followed by either surgical resection (if mediastinal lymph nodes were clear of metastases) or radiotherapy if mediastinoscopy demonstrated mediastinal lymph node metastases. |
| Sugiura 1999 | Non randomised study comparing quality of life outcomes in individuals with clinical stage I NSCLC in a consecutive series of patients treated with thoracotomy and lobectomy with a consecutive series of patients treated with VATS lobectomy. |
| Taylor 2004 | Large retrospective case series comparing outcomes in individuals with clinical stage IIIA non‐small cell lung cancer treated with concurrent chemoradiation compared with induction chemotherapy followed by surgical resection |
| Van Kooten 2002 | Trial of neoadjuvant/induction chemotherapy with cisplatin and gemcitabine in patients with stage IIIA/B NSCLC followed by either surgical resection or radiotherapy. Assignment to surgery or radiotherapy was based on assesment of oncologist and surgeon with respect to resectability (not randomised). |
| Yano 1995 | Retrospective case series comparing limited resection with radiotherapy for stage I non‐small cell lung cancer. |
| Yim 2000 | Case series comparing cytokine responses in patients undergoing video‐assisted thoracic surgery with those undergoing conventional thoracotomy |
Characteristics of ongoing studies [ordered by study ID]
ACOSOG Z0030.
| Trial name or title | American College of Surgeons Oncology Group Z0030 Trial |
| Methods | |
| Participants | Patients with NSCLC T1‐2, N0‐1(less than hilar), M0. ECOG performance status 0‐2. |
| Interventions | Eligible participants are assessed intra‐operatively with mediastinal lymph node sampling and frozen section. Those with T1‐2, N0‐1(less than hilar), M0 disease are randomised to either lymph node sampling or complete lymph node dissection intraoperatively |
| Outcomes | Overall survival. Operative time, post‐operative complications, duration of chest tube drainage and length of hospitalisation. Local recurrence free survival and local‐regional recurrence free survival |
| Starting date | 1999 |
| Contact information | http://www.acosog.org |
| Notes | Closed to recruitment |
Contributions of authors
Renée Manser initiated the review and developed the protocol. She also carried out the literature search, reviewed abstracts and full text studies for inclusion, participated in the quality assessments, data extraction, analysis and drafted the final version of the review. Gavin Wright reviewed full text articles identified by the initial literature search for inclusion in the review. He also participated in the assessment of study quality and data extraction from graphs in the original review. He assisted with revisions of the final version of the review. David Hart searched the abstracts and titles and reviews for articles for inclusion in the review and helped with revision of the final version of the review. Graham Byrnes provided advice on the statistical analysis, interpretation of findings and the write up of the review. Donald Campbell helped with protocol development and assisted with revisions of the final version. In the update of the review, Zoe Wainer helped search the abstracts and assess the full text studies for inclusion in the review and also assisted with writing some of the update and Sera Tort participated in the risk of bias assessment and the editing of the review.
Sources of support
Internal sources
Department of Respiratory Medicine, ST Vincent's Hospital, Melbourne, Victoria, Australia.
Department of Medical Oncology and Haematology, Peter MacCallum Cancer Centre, Melbourne, Victoria, Australia.
External sources
-
National Institute for Health Research (2009 Cochrane Review Incentive Scheme), UK.
Funding awarded to the completion of the update this review
Declarations of interest
Gavin Wright has contributed patients to the ACOSOG Z30 trial (ACOSOG Z0030) which has yet to be published.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Albain 2003 {published data only}
- Albain KS, Scott CB, Rusch VR, Turrisi AT, Shepherd FA, Smith C, et al. SWOG, NCIC, ECOG, CALGB, NCCTG. Phase III comparison of concurrent chemotherapy plus radiotherapy (CT/RT) and CT/RT followed by surgical resection for stage IIIA(pN2) non‐small cell lung cancer (NSCLC): Initital results from intergroup trial 0139 (RTOG 93‐09). Proceedings of American Society of Clinical Oncology 2003;22:621 (abstract no 2497). [Google Scholar]
- Albain KS, Scott CB, Rusch VR, Turrisi AT, Shepherd SA, Smith C, et al. Phase III study of concurrent chemotherapy and full course radiotherapy (CT/RT) versus CT/RT induction followed by surgical resection for stage IIIA (pN2) non‐small cell lung cancer (NSCLC): First outcome analysis of North American Intergroup trial 0139 (RTOG 93‐09). Lung Cancer 2003;41(Suppl 2):4. [Google Scholar]
- Albain KS, Swann RS, Rusch VW, Turrisi AT, Shepherd FA, Smith C, Che Y, Livingston RB, Feins RH, Gandara DR, Fry WA, Darling G, Johnson DH, Green M, Miller RC, Ley J, Sause WT, Cox JD. Radiotherapy plus chemotherapy with or without surgical resection for stage III non‐small cell lung cancer: a phase III randomised controlled trial. Lancet 2009;374:379‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ginsberg 1995 {published data only}
- Ginsberg RJ, Rubinstein LV for the Lung Cancer Study Group. Randomized trial of lobectomy versus limited resection for T1 N0 non‐small cell lung cancer. Annals of Thoracic Surgery 1995;60:615‐23. [DOI] [PubMed] [Google Scholar]
- Rubinstein LV, Ginsberg RJ. Lobectomy versus limited resection in T1 N0 lung cancer. Annals of Thoracic Surgery 1996;62:1249‐50. [DOI] [PubMed] [Google Scholar]
Izbicki 1998 {published data only}
- Izbicki JR, Passlick B, Pantel K, Pichlmeier U, Hosch SB, Karg O, et al. Effectiveness of radical systematic mediastinal lymphadenectomy in patients with resectable non‐small cell lung cancer: Results of a prospective randomized controlled trial. Annals of Surgery 1998;227(1):138‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izbicki JR, Thetter O, Habekost M, Karg O, Passlick B, Kubuschok B, et al. Radical systematic mediastinal lymphadenectomy in non‐small cell lung cancer: a randomized controlled trial. British Journal of Surgery 1994;81(2):229‐35. [DOI] [PubMed] [Google Scholar]
Johnstone 2002 {published data only}
- Johnstone DW, Byhardt RW, Ettinger D, Scott CB. Phase III study comparing chemotherapy and radiotherapy with preoperative chemotherapy and surgical resection in patients with non‐small cell lung cancer spread to mediastinal lymph nodes (N2); final report of RTOG 89‐01. International Journal of Radiation Oncology, Biology, Physics 2002;54(2):365‐9. [DOI] [PubMed] [Google Scholar]
Morrison 1963 {published data only}
- Morrison R, Deeley TJ, Cleland WP. The treatment of carcinoma of the bronchus: A clinical trial to compare surgery and supervoltage radiotherapy. Lancet 1963;1:683‐4. [Google Scholar]
NCI 1975 {published data only}
- Warram J, on behalf of collaborating investigators. Preoperative irradiation of cancer of the lung: Preliminary report of a therapeutic trial (a collaborative study). Cancer 1969;23:419‐29. [DOI] [PubMed] [Google Scholar]
- Warram J, on behalf of collaborating investigators. Preoperative irradiation of cancer of the lung: final report of a therapeutic trial (a collaborative study). Cancer 1975;36(3):914‐25. [DOI] [PubMed] [Google Scholar]
Shepherd 1998 {published data only}
- Shepherd FA, Johnston MR, Payne D, Burkes R, Deslauriers J, Cormier Y, et al. Randomized study of chemotherapy and surgery versus radiotherapy for stage IIIA non‐small cell lung cancer: a National Cancer Institute of Canada Clinical Trials Group Study. British Journal of Cancer 1998;78(5):683‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stathopoulos 1996 {published data only}
- Stathopoulos GP, Papakostas P, Malamos NA, Samelis GF, Moschopoulos N. Chemo‐radiotherapy versus chemo‐surgery in stage IIIA non‐small cell lung cancer. Oncology Reports 1996;3:673‐6. [DOI] [PubMed] [Google Scholar]
Stephens 2005 {published data only}
- Stephens RJ, Girling DJ, Hopwood P, Thatcher N on behalf of the Medical Research Council Lung Cancer Working Party. A randomised controlled trial of pre‐operative chemotherapy followed, if feasible, by resection versus radiotherapy in patients with inoperable stage T3, N1, M0 or T1‐3, N2, M0 non‐small cell lung cancer. Lung Cancer 2005;49:395‐400. [DOI] [PubMed] [Google Scholar]
Sugi 1998 {published data only}
- Sugi K, Nawata K, Fujita N, Ueda K, Tanaka T, Matsuoka T, et al. Systematic lymph node dissection for clinically diagnosed peripheral non‐small cell lung cancer less than 2 cm in diameter. World Journal of Surgery 1998;22:290‐5. [DOI] [PubMed] [Google Scholar]
Sugi 2000 {published data only}
- Sugi K, Kaneda Y, Esato K. Video‐assisted thoracoscopic lobectomy achieves a satisfactory long‐term prognosis in patients with clinical stage IA lung cancer. World Journal of Surgery 2000;24:27‐31. [DOI] [PubMed] [Google Scholar]
van Meerbeeck 2007 {published data only}
- Splinter TAW, Kirkpatrick A, Meerbeeck J, Vanteenkiste J, Postmus P, Giaccone G on behalf of the EORTC Lung Cancer Cooperative group and VKSL. Randomised trial of surgery versus radiotherapy in patients with stage IIIA non‐small cell lung cancer after response to induction chemotherapy, intergroup study 08941 (abstract). Proceedings of American Society of Clinical Oncology 1998;17:453a. [Google Scholar]
- Splinter TAW, Schil PE, Kramer GWPM, Meerbeeck J, Gregor A, Rocmans P, et al. Randomized trial of surgery versus radiotherapy in patients with stage IIIA (N2) non‐small cell lung cancer after response to induction chemotherapy (EORTC 08941). Clinical Lung Cancer 2000;2(1):69‐72. [DOI] [PubMed] [Google Scholar]
- Meerbeeck JP, Vansteenkiste JF, Schil PEY, Weynants P, Scagliotti G, Darwish S, et al. Neoadjuvant chemotherapy (CT) followed by surgical resection (SR) or thoracic radiotherapy (TRT) in stage IIIA‐N2 non‐small cell lung cancer (NSCLC): A multicentric randomised European experience. European Respiratory Journal 1997;10(Supplement 25):153 (abstract). [Google Scholar]
- van Meerbeeck, JP, Kramer GWPM, Schil PEY, Legrand C, Smit EF, Schramel F, et al. Randomized controlled trial of resection versus radiotherapy after induction chemotherapy in stage IIIA‐N2 Non‐small cell lung cancer. J Natl Cancer Inst 2007;99:442‐50. [DOI] [PubMed] [Google Scholar]
Wu 2002 {published data only}
- Wu YL, Huang ZF, Wang SY, Yang XN, Ou W. A randomized trial of systematic nodal dissection in resectable non‐small cell lung cancer. Lung Cancer 2002;36:1‐6. [DOI] [PubMed] [Google Scholar]
- Wu YL, Wang S, Huang Z, et al. Extent of lymphadenectomy in stage I‐IIIA non‐small cell lung cancer: a randomised clinical trial. Zhonghua Zhong Liu Za Zhi 2001;23(1):43‐5. [PubMed] [Google Scholar]
References to studies excluded from this review
Bretel 1997 {published data only}
- Bretel JJ, Arrigada R, Chevalier T, Baldeyrou P, Grunenwald D, Pechoux C, et al. Optimisation of combination radiotherapy and chemotherapy in non‐small cell lung cancer [Optimisation de l'association radiotherapie et chimiotherapie dans les carcinomes bronchiques non a petites cellules]. Cancer Radiotherapie 1997;1:148‐53. [DOI] [PubMed] [Google Scholar]
Cox 1991 {published data only}
- Cox JD, Azarnia N, Byhardt RW, Shin KH, Emami B, Perez CA. N2 (clinical) non‐small cell carcinoma of the lung : prospective trials of radiation therapy with total doses 60 GY by the Radiation Therapy Oncology Group. Int J Radiation Oncology Biol. Phys. 1991;20(1):7‐12. [DOI] [PubMed] [Google Scholar]
Durci 1991 {published data only}
- Durci ML, Komaki R, Oswald MJ, Mountain CF. Comparison of surgery and radiation therapy for non‐small cell carcinoma of the lung with mediastinal metastasis. International Journal of Radiation Oncology, Biology, Physics 1991;21(3):629‐36. [DOI] [PubMed] [Google Scholar]
Ferguson 2000 {published data only}
- Ferguson MK, Karrison T. Does pneumonectomy for lung cancer adversely influence long‐term survival?. The Journal of Thoracic and Cardiovascular Surgery 2000;119(3):440‐8. [DOI] [PubMed] [Google Scholar]
Harpole 1995 {published data only}
- Harpole DH, Herndon JE, Young WG, Wolfe WG, Sabiston DC. Stage I Nonsmall cell lung cancer: A multivariate analysis of treatment methods and patterns of recurrence. Cancer 1995;76(5):788‐96. [DOI] [PubMed] [Google Scholar]
Keller 2000 {published data only}
- Keller SM, Adak S, Wagner H, Johnson DH. Mediastinal lymph node dissection improves survival in patients with stages II and IIIa non‐small cell lung cancer. Annals of Thoracic Surgery 2000;70(2):358‐65. [DOI] [PubMed] [Google Scholar]
Kirby 1995 {published data only}
- Kirby TJ, Mack MJ, Landreneau RJ, Rice TW. Lobectomy‐ video‐assisted thoracic surgery versus muscle sparing thoracotomy: A randomized trial. Journal of Thoracic and Cardiovascular Surgery 1995;109(5):997‐1002. [DOI] [PubMed] [Google Scholar]
MRC Trial small cell {published data only}
- Anonymous. Comparative trial of surgery and radiotherapy for the primary treatment of small‐celled or oat‐celled carcinoma of the bronchus. First report to the Medical Research Council by the working‐party on the evaluation of different methods of therapy in carcinoma of the bronchus. Lancet 1966;2(7471):979‐86. [PubMed] [Google Scholar]
- Fox W, Scalding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small‐celled or oat‐celled carcinoma of bronchus. Ten‐year follow‐up. Lancet 1973;2(7820):63‐5. [DOI] [PubMed] [Google Scholar]
- Miller AB, Fox W, Tall R. Five‐year follow‐up of the Medical Research Council comparative trial of surgery and radiotherapy for the primary treatment of small‐celled or oat‐celled carcinoma of the bronchus. Lancet 1969;2(7619):501‐5. [DOI] [PubMed] [Google Scholar]
Pitz 2002 {published data only}
- Pitz CCM, Maas KW, Swieten HA, Riviere AB, Hofman P, Schramel FMNH. Surgery as part of combined modality treatment in stage IIIB non‐small cell lung cancer. Annals of Thoracic Surgery 2002;74(1):164‐9. [DOI] [PubMed] [Google Scholar]
Sugiura 1999 {published data only}
- Sugiura H, Morikawa T, Kaji M, Sasamura Y, Kondo S, Katoh H. Long‐term benefits for the quality of life after video‐assisted thoracoscopic lobectomy in patients with lung cancer. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques 1999;9(6):403‐8. [PubMed] [Google Scholar]
Taylor 2004 {published data only}
- Taylor NA, Liao ZX, Cox JD, Stevens C, Roth J, Walsh G, et al. Equivalent outcome of patients with clinical stage IIIA non‐small cell lung cancer treated with concurrent chemoradiation compared with induction chemotherapy followed by surgical resection. International Journal of Radiation Oncology, Biology, Physics 2004;58(1):204‐12. [DOI] [PubMed] [Google Scholar]
Van Kooten 2002 {published data only}
- Kooten M, Rosenberg M, Orlando M, Morero J, Vilanova M, Rojas O, et al. Neoadjuvant chemotherapy with gemcitabine and cisplatin in stage IIIA/B non‐small cell lung cancer. Investigational New Drugs 2002;20:439‐46. [DOI] [PubMed] [Google Scholar]
Yano 1995 {published data only}
- Yano T, Yokoyama H, Yoshino I, Tayama K, Asoh H, Hata K, Ichinose Y. Results of a limited resection for compromised or poor‐risk patients with clinical stage I non‐small cell carcinoma of the lung. Journal of the American College of Surgeons 1995;181:33‐7. [PubMed] [Google Scholar]
Yim 2000 {published data only}
- Yim APC, Wan S, Lee PW, Arifi AA. VATS lobectomy reduces cytokine responses compared with conventional surgery. Annals of Thoracic Surgery 2000;70:243‐7. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACOSOG Z0030 {published data only}
- Allen M (study chair) for the American College of Surgeons Oncology Group (Thoracic Organ Site Committee). Randomized trial of mediastinal lymph node sampling versus complete lymphadenectomy during pulmonary resection in patients with N0 or N1 (less than hilar) non‐small cell lung cancer. http://www.acosog.org/studies/synopses/Z0030_Synopsis.pdf accessed 12th July 2004. [DOI] [PMC free article] [PubMed]
Additional references
Detterbeck 2001
- Detterbeck FC, Egan TM. Surgery for stage II non‐small cell lung cancer. In: Detterbeck FC, Rivera MP, Socinski MA, Rosenman JG editor(s). Diagnosis and treatment of lung cancer ‐ an evidence based guide. Philadelphia: W.B. Saunders Company, 2001:191‐7. [Google Scholar]
Farray 2005
- Farray D, Mirkovic N, Albain KS. Multimodality Therapy for Stage III Non‐Small‐Cell Lung Cancer. Journal of Clinical Oncology 2005;23(14):3257‐69. [DOI] [PubMed] [Google Scholar]
Feinstein 1985
- Feinstein AR, Sosin DM, Wells CK. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. New England Journal of Medicine 1985;312(25):1604‐08. [DOI] [PubMed] [Google Scholar]
Fergusson 2002
- Fergusson D, Aaron SD, Guyatt G, Hebert P. Post‐randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 2002;325:652‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flehinger 1992
- Flehinger BJ, Kimmel M, Melamed MR. The effect of surgical treatment on survival from early lung cancer: Implications for screening. Chest 1992;101:1013‐18. [DOI] [PubMed] [Google Scholar]
Gloeckler Ries 2003
- Gloeckler Ries LA, Reichman ME, Reidel Lewis D, Hankey BJ, Edwards BK. Cancer survival and incidence from the Surveillence Epidemiology, and End Results (SEER) Program. The Oncologist 2003;8(6):541‐52. [DOI] [PubMed] [Google Scholar]
Hensing 2001
- Hensing, TA, Halle JS, Socinski MA. Chemotherapy for stage IIIA,B Non‐small cell lung cancer. In: Detterbeck FC, Rivera MP, Socinski MA, Rosenman JG editor(s). Diagnosis and treatment of lung cancer ‐ an evidence based guide. Philadelphia: W.B. Saunders Company, 2001:291‐303. [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2 [updated September 2009]. The Cochrane Collaboration. Available from www.cochrane‐handbook.org, 2008. [Google Scholar]
Janssen‐Heijnen 2003
- Janssen‐Heijnen ML, Coebergh JW. The changing epidemiology of lung cancer in Europe. Lung Cancer 2003;41(3):245‐58. [DOI] [PubMed] [Google Scholar]
Jones 2001
- Jones DR, Detterbeck FC. Surgery for stage I non‐small cell lung cancer. In: Detterbeck FC, Rivera MP, Socinski MA, Rosenman JG editor(s). Diagnosis and treatment of lung cancer‐ an evidence based guide. Philadelphia: W.B. Saunders Company, 2001:177‐90. [Google Scholar]
Keller 2002
- Keller SM. Complete mediastinal lymph node dissection ‐ does it make a difference?. Lung Cancer 2002;36:7‐8. [DOI] [PubMed] [Google Scholar]
Kiser 2001
- Kiser AC, Detterbeck FC. Chapter 8: General aspects of surgical treatment. In: Detterbeck FC, Rivera MP, Socinski MA, Rosenman JG editor(s). Diagnosis and treatment of lung cancer ‐ an evidence based guide. Philadelphia: W.B. Saunders Company, 2001:133‐47. [Google Scholar]
Lederle 1994
- Lerdele FA, Niewoehner DE. Lung cancer surgery: a critical review of the evidence. Archives of Internal Medicine 1994;154(21):2397‐401. [DOI] [PubMed] [Google Scholar]
Martini 1983
- Martini N, Flehinger BJ, Zaman MB, Beattie EJ. Results of resection in non‐oat cell carcinoma of the lung with mediastinal lymph node metastases. Annals of Surgery 1983;198:386‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martini 1987
- Martini N, Flehinger BJ. The role of surgery in N2 lung cancer. Surgical Clinics of North America 1987;67:1037. [DOI] [PubMed] [Google Scholar]
Martini 1992
- Martini N, Burt ME, Bains MS, McCormack PM, Rusch VW, Ginsberg RJ. Survival after resection of stage II non‐small cell lung cancer. Annals of Thoracic Surgery 1992;54:460‐6. [DOI] [PubMed] [Google Scholar]
Mountain 1986
- Mountain CF. A new international staging system for lung cancer. Chest 1986;89(Supplement 4):225‐33. [DOI] [PubMed] [Google Scholar]
Mountain 1997
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest 1997;111(6):1710‐17. [DOI] [PubMed] [Google Scholar]
Naruke 1976
- Naruke T, Suemasu K, Ishikawa S. Surgical treatment for lung cancer with metastasis to mediastinal lymph nodes. The Journal of Thoracic and Cardiovascular Surgery. 1976;71(2):279‐85. [PubMed] [Google Scholar]
Nesbitt 1995
- Nesbitt JC, Putman JB, Walsh GL, Roth JA, Mountain CF. Survival in early stage non‐small cell lung cancer. Annals of Thoracic Surgery 1995;60:466‐72. [DOI] [PubMed] [Google Scholar]
Ost 2008
- Ost D, Goldberg J, Rolnitzky L, Rom WN. Survival after Surgery in Stage IA and IB Non Small Cell Lung Cancer. American Journal of Respiratory and Critical Care Medicine 2008;177:516‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. [DOI] [PubMed] [Google Scholar]
Qureshi 2004
- Qureshi AI, Hutson AD, Harbaugh RE, Stieg PE, Hopkins LN. Methods and design considerations for randomised clinical trials evaluating surgical or endovascular treatments for cerebrovascular diseases. Neurosurgery 2004;54:248‐67. [DOI] [PubMed] [Google Scholar]
Reif 2000
- Reif MS, Socinski MA, Rivera MP. Evidence‐based medicine in the treatment of non‐small cell lung cancer. Clinics in Chest Medicine 2000;21(1):107‐20. [DOI] [PubMed] [Google Scholar]
Robinson 2007
- Robinson LA, Ruckdeschel JC, Wagner H Jr, Stevens CW. Treatment of non‐small cell lung cancer‐stage IIIA: ACCP evidence‐based clinical practice guidelines (2nd edition). Chest 2007;123(3 Suppl):243S‐265S. [DOI] [PubMed] [Google Scholar]
Rowell 2001
- Rowell NP, Williams C. Radical radiotherapy for stage I/II non‐small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable). Cochrane Database of Systematic Reviews 2001, Issue 1. [Art. No.: CD002935. DOI: 10.1002/14651858.CD002935] [DOI] [PubMed] [Google Scholar]
Rowell 2004
- Rowell NP, O'Rourke N. Concurrent chemoradiotherapy in non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2004, Issue 4. [Art. No.: CD002140. DOI: 10.1002/14651858.CD002140.pub2] [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Scott 2007
- Scott WJ, Howington J, Feigenberg S, Movsas B, Pisters K. Treatment of Non‐small Cell Lung Cancer Stage I and Stage II: ACCP Evidence Based Clinical Practice Guidelines (2nd Edition). Chest 2007;132:234S‐242S. [DOI] [PubMed] [Google Scholar]
Smythe 2003
- Smythe WR. Treatment of stage I non‐small cell lung carcinoma. Chest 2003;123(Supplement 1):181‐7. [PubMed] [Google Scholar]
Sobin 1997
- Sobin LH, Wittekind CH. UICC TNM classification of malignant tumours. 5th Edition. New York: Wiley‐Liss, 1997:12‐3. [Google Scholar]
Sobue 1992
- Sobue T, Suzuki T, Matsuda M, Kuroishi T, Ikeda S, Naruke T, et al. Survival for clinical stage I lung cancer not surgically treated: Comparison between screen‐detected and symptom‐detected cases. Cancer 1992;69:685‐92. [DOI] [PubMed] [Google Scholar]
Standing Medical Advisory Committee 1994
- Department of Health Standing Medical Advisory Committee. The management of lung cancer: current clinical practices; report of a working group to the Standing Medical Advisory Committee. Department of Health, 1994. [Google Scholar]
Thomas 2002
- Thomas P, Doddoli C, Thirion X, Ghez O, Payan‐Defais MJ, Giudicelli R, et al. Stage I Non‐Small Cell Lung Cancer: A Pragmatic Approach to Prognosis After Complete Resection. Annals of Thoracic Surgery 2002;73:1065‐70. [DOI] [PubMed] [Google Scholar]
Tierney 2007
- Tierney, JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whitehead 1991
- Whitehead A, Whitehead J. A general parametric approach to the meta‐analysis of randomised clinical trials. Statistics in Medicine 1991;10:1665‐77. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Manser 2005
- Manser R, Wright G, Hart D, Byrnes G, Campbell D. Surgery for early stage non‐small cell lung cancer. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD004699.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


