Abstract
Background
Maternal complications, including psychological/mental health problems and neonatal morbidity, have commonly been observed in the postpartum period. Home visits by health professionals or lay supporters in the weeks following birth may prevent health problems from becoming chronic, with long‐term effects. This is an update of a review last published in 2017.
Objectives
The primary objective of this review is to assess the effects of different home‐visiting schedules on maternal and newborn mortality during the early postpartum period. The review focuses on the frequency of home visits (how many home visits in total), the timing (when visits started, e.g. within 48 hours of the birth), duration (when visits ended), intensity (how many visits per week), and different types of home‐visiting interventions.
Search methods
For this update, we searched the Cochrane Pregnancy and Childbirth Group's Trials Register, ClinicalTrials.gov, the WHO International Clinical Trials Registry Platform (ICTRP) (19 May 2021), and checked reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCTs) (including cluster‐, quasi‐RCTs and studies available only as abstracts) comparing different home‐visiting interventions that enrolled participants in the early postpartum period (up to 42 days after birth) were eligible for inclusion. We excluded studies in which women were enrolled and received an intervention during the antenatal period (even if the intervention continued into the postnatal period), and studies recruiting only women from specific high‐risk groups (e.g. women with alcohol or drug problems).
Data collection and analysis
Two review authors independently assessed trials for inclusion and risk of bias, extracted data and checked them for accuracy. We used the GRADE approach to assess the certainty of the evidence.
Main results
We included 16 randomised trials with data for 12,080 women. The trials were carried out in countries across the world, in both high‐ and low‐resource settings. In low‐resource settings, women receiving usual care may have received no additional postnatal care after early hospital discharge.
The interventions and controls varied considerably across studies. Trials focused on three broad types of comparisons, as detailed below. In all but four of the included studies, postnatal care at home was delivered by healthcare professionals. The aim of all interventions was broadly to assess the well‐being of mothers and babies, and to provide education and support. However, some interventions had more specific aims, such as to encourage breastfeeding, or to provide practical support.
For most of our outcomes, only one or two studies provided data, and results were inconsistent overall. All studies had several domains with high or unclear risk of bias.
More versus fewer home visits (five studies, 2102 women)
The evidence is very uncertain about whether home visits have any effect on maternal and neonatal mortality (very low‐certainty evidence). Mean postnatal depression scores as measured with the Edinburgh Postnatal Depression Scale (EPDS) may be slightly higher (worse) with more home visits, though the difference in scores was not clinically meaningful (mean difference (MD) 1.02, 95% confidence interval (CI) 0.25 to 1.79; two studies, 767 women; low‐certainty evidence). Two separate analyses indicated conflicting results for maternal satisfaction (both low‐certainty evidence); one indicated that there may be benefit with fewer visits, though the 95% CI just crossed the line of no effect (risk ratio (RR) 0.96, 95% CI 0.90 to 1.02; two studies, 862 women). However, in another study, the additional support provided by health visitors was associated with increased mean satisfaction scores (MD 14.70, 95% CI 8.43 to 20.97; one study, 280 women; low‐certainty evidence). Infant healthcare utilisation may be decreased with more home visits (RR 0.48, 95% CI 0.36 to 0.64; four studies, 1365 infants) and exclusive breastfeeding at six weeks may be increased (RR 1.17, 95% CI 1.01 to 1.36; three studies, 960 women; low‐certainty evidence). Serious neonatal morbidity up to six months was not reported in any trial.
Different models of postnatal care (three studies, 4394 women)
In a cluster‐RCT comparing usual care with individualised care by midwives, extended up to three months after the birth, there may be little or no difference in neonatal mortality (RR 0.97, 95% CI 0.85 to 1.12; one study, 696 infants). The proportion of women with EPDS scores ≥ 13 at four months is probably reduced with individualised care (RR 0.68, 95% CI 0.53 to 0.86; one study, 1295 women). One study suggests there may be little to no difference between home visits and telephone screening in neonatal morbidity up to 28 days (RR 0.97, 95% CI 0.85 to 1.12; one study, 696 women). In a different study, there was no difference between breastfeeding promotion and routine visits in exclusive breastfeeding rates at six months (RR 1.47, 95% CI 0.81 to 2.69; one study, 656 women).
Home versus facility‐based postnatal care (eight studies, 5179 women)
The evidence suggests there may be little to no difference in postnatal depression rates at 42 days postpartum and also as measured on an EPDS scale at 60 days. Maternal satisfaction with postnatal care may be better with home visits (RR 1.36, 95% CI 1.14 to 1.62; three studies, 2368 women). There may be little to no difference in infant emergency health care visits or infant hospital readmissions (RR 1.15, 95% CI 0.95 to 1.38; three studies, 3257 women) or in exclusive breastfeeding at two weeks (RR 1.05, 95% CI 0.93 to 1.18; 1 study, 513 women).
Authors' conclusions
The evidence is very uncertain about the effect of home visits on maternal and neonatal mortality. Individualised care as part of a package of home visits probably improves depression scores at four months and increasing the frequency of home visits may improve exclusive breastfeeding rates and infant healthcare utilisation. Maternal satisfaction may also be better with home visits compared to hospital check‐ups. Overall, the certainty of evidence was found to be low and findings were not consistent among studies and comparisons. Further well designed RCTs evaluating this complex intervention will be required to formulate the optimal package.
Plain language summary
Home visits in the early period after the birth of a baby
What is the issue?
Health problems for mothers and babies commonly occur or become apparent in the weeks following the birth. For the mothers these include postpartum haemorrhage (substitute excessive blood loss), fever and infection, abdominal and back pain, abnormal discharge (heavy or smelly vaginal discharge), thromboembolism (a blood clot), and urinary tract complications (being unable to control the urge to pee), as well as psychological and mental health problems such as postnatal depression. Mothers may also need support to establish breastfeeding. Babies are at risk of death related to infections (babies may be badly affected by infections), asphyxia (difficulties in breathing, caused by lack of oxygen), and preterm birth (being born prematurely).
Why is this important?
Home visits by health professionals or lay supporters in the early postpartum period may prevent health problems from becoming long‐term, with effects on women, their babies, and their families. This review looked at different home‐visiting schedules in the weeks following the birth.
What evidence did we find?
We included 16 randomised trials with data for 12,080 women. Some trials focused on physical checks of the mother and newborn, while others provided support for breastfeeding, and one included the provision of practical support with housework and childcare. They were carried out in both high‐resource countries and low‐resource settings where women receiving usual care may not have received additional postnatal care after early hospital discharge.
The trials focused on three broad types of comparisons: schedules involving more versus fewer postnatal home visits (five studies), schedules involving different models of care (three studies), and home versus facility postnatal check‐ups (eight studies). In all but four of the included studies, postnatal care at home was delivered by healthcare professionals. For most of our outcomes, only one or two studies provided data. Overall, our results were inconsistent.
The evidence was very uncertain about whether home visits reduced newborn deaths or serious health problems with the mother. Women's physical and psychological health were not improved with more intensive schedules of home visits although more individualised care improved women's mental health in one study and maternal satisfaction was slightly better in two studies. Overall, babies may be less likely to have additional medical care if their mothers received more postnatal home visits. More home visits may have encouraged more women to exclusively breastfeed their babies and women to be more satisfied with their postnatal care. The different outcomes reported in different studies, how the outcomes were measured, and the considerable variation in the interventions and control conditions across studies were limitations of this review. The certainty of the evidence was generally found to be low or very low according to the GRADE criteria.
What does this mean?
Increasing the number of postnatal home visits may promote infant health and exclusive breastfeeding and more individualised care may improve outcomes for women. More research is needed before any particular schedule of postnatal care can be recommended.
Summary of findings
Summary of findings 1. Schedules involving more versus fewer home visits in the early postpartum period.
| Schedules involving more compared to fewer postpartum visits for home visits in the early postpartum period | ||||||
| Patient or population: mothers and infants receiving home visits in the early postpartum period Setting: Canada, Denmark, Iran, Syria, Turkey, UK, USA, Zambia Intervention: schedules with more postpartum home visits Comparison: schedules with fewer postpartum home visits | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with fewer postpartum visits | Risk with more postpartum visits | |||||
| Maternal mortality within 42 days post‐birth | Study population | RR 0.39 (0.02 to 9.41) | 225 (1 RCT) | ⊕⊝⊝⊝ VERY LOW1 2 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Neonatal mortality | Study population | RR 0.99 (0.26 to 3.69) | 1281 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW3 4 | Data were provided from a 3‐arm trial ‐ with data eligible for 2 comparisons. | |
| 8 per 1000 | 8 per 1000 (2 to 30) | |||||
| Postnatal depression (last assessment up to 42 days postpartum) | Study population | 767 (2 RCTs) |
⊕⊕⊝⊝ LOW5 |
Postnatal depression measured on Edinburgh Postnatal Depression Scale. The maximum score is 30 and any score above 10 is considered to indicate depression. | ||
| The mean postnatal depression score in the group with fewer postpartum visits ranged from 4.5 to 6.7 | MD 1.02 higher (0.25 higher to 1.79 higher) | |||||
| Maternal satisfaction with postnatal care | Study population | RR 0.96 (0.90 to 1.02) | 862 (2 RCTs) | ⊕⊕⊝⊝ LOW6 | Data were provided from a 3‐arm trial ‐ with data eligible for 2 comparisons | |
| 842 per 1000 | 809 per 1000 (758 to 859) | |||||
| Maternal satisfaction with postnatal care | Study population | 280 (1 RCT) |
LOW7,8 ⊕⊕⊝⊝ |
Mean satisfaction score at 8 weeks postpartum. Satisfaction questionnaire with possible range of 0‐170. Higher score indicates greater satisfaction. | ||
| The mean postnatal satisfaction score in the group with fewer postpartum visits was 139.9 | MD 14.70 higher (8.43 higher to 20.97 higher) | |||||
| Serious neonatal morbidity up to 6 months | Study population | ‐ | 0 (0 RCTs) | ‐ | No RCT reported this outcome | |
| ‐ | ‐ | |||||
| Exclusive breastfeeding (last assessment up to 6 weeks) | Study population | RR 1.17 (1.01 to 1.36) | 960 (2 RCTs) | ⊕⊕⊝⊝ LOW6 | ||
| 483 per 1000 | 565 per 1000 (488 to 657) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; MD: Mean difference; RCT: Randomised controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1We downgraded 1 level for serious limitations in study design: unclear risk for detection, attrition and other bias and high risk for performance bias.
2We downgraded 2 levels for very serious imprecision: a single trial with wide 95%CI and small number of events.
3We downgraded 1 level for serious limitations in study design: unclear selection bias and high risk of bias for performance, detection, attrition and selective reporting bias.
4We downgraded 2 levels for very serious limitations in imprecision: wide 95% CI and small number of events.
5We downgraded 2 levels for very serious limitations in study design: performance, detection and attrition bias were at high risk.
6We downgraded 2 levels for very serious limitations in study design: selection bias was unclear risk and performance, detection, attrition and selective reporting were at high risk of bias.
7We downgraded 1 level for serious limitations in study design: performance bias was at high risk and detection, attrition and other bias were at unclear risk.
8We downgraded 1 level for serious imprecision due to a small sample size.
Background
Description of the condition
The postpartum period, defined by the World Health Organization (WHO) as the period from childbirth to the 42nd day following delivery (WHO 2005), is critical for both mothers and newborns. An estimated 295,000 maternal deaths occur worldwide each year because of pregnancy‐related complications in the antenatal, intrapartum, and postpartum periods, especially in resource‐limited settings (WHO 2019). These deaths are often sudden and unpredictable, with 11% to 17% occurring during childbirth itself and 50% to 71% occurring during the postpartum period (WHO 2005). Maternal health problems commonly observed in the postpartum period include postpartum haemorrhage, fever, abdominal and back pain, abnormal discharge, puerperal genital infection, thromboembolic disease, and urinary tract complications (Bashour 2008), as well as psychological and mental health problems, such as postnatal depression. The postpartum period is also critical for newborns. Every year, approximately 3.7 million babies die in the first four weeks of life. Most of these infants are born in developing countries and most die at home. Nearly 40% of all deaths of children younger than five years old occur within the first 28 days of life (neonatal or newborn period). Just three causes ‐ infections, asphyxia, and preterm birth ‐ account for nearly 80% of these deaths (WHO/UNICEF 2009). Moreover, the postpartum period is a time of transition for women and their families, who are adjusting on physical, psychological, and social levels (Shaw 2006). In most developed countries, postpartum hospital stays are often shorter than 48 hours following a vaginal birth; thus, most postpartum care is provided in community and ambulatory‐care settings. Early intervention in the postpartum period may prevent health problems from becoming chronic with long‐term effects on women, their babies, and their families.
Description of the intervention
The purpose of a home‐visiting program is to provide support at home for mothers, babies, and families by health professionals or skilled attendants. However, a single clearly defined methodology for this intervention does not exist. Further, the term "home visiting" is used differently in various contexts (AAP 2009). Since the 1970s, the length of hospital stay after childbirth has fallen dramatically in many high‐resource settings. Early postnatal discharge of healthy mothers and term infants does not appear to have adverse effects on breastfeeding or maternal depression when women are offered at least one nurse‐midwife home visit after discharge (Brown 2002). Home‐visiting programs provide breastfeeding and hygiene education, parenting and child health instruction, and general support to families; they successfully address many of the barriers to access, including transportation issues, initiation of timely care, and completeness of services (AAP 1998; AAP 2009). Several trials have assessed the impact of home‐visiting programs, especially effects on child abuse and neglect in vulnerable families (Donovan 2007; Olds 1997; Quinlivan 2003). Others focused on the effectiveness and cost‐effectiveness of intensive home‐visiting programs (Barlow 2007; Carabin 2005; McIntosh 2009). Some home‐visiting programs have specifically targeted high‐risk groups such as women suffering domestic abuse (intimate partner violence) or families that are economically or socially disadvantaged. Home‐visiting programs for high‐risk groups or those by child health nurses may include components during pregnancy and may continue over many months or years; such programs are outside the scope of this review and have been addressed in other Cochrane Reviews (Bennett 2008; Jahanfar 2013; Macdonald 2008; Turnbull 2012). In this review, we focus on the early postnatal period, following discharge from hospital.
In 2009, WHO and the United Nations Children's Fund (UNICEF) recommended home visits by skilled attendants in resource‐limited settings. In high‐mortality settings and where access to facility‐based care is limited, at least two home visits are recommended for all home births: the first visit should occur within 24 hours of the birth, the second visit on day three, and if possible, a third visit should be made before the end of the first week of life (day seven). For babies born in a healthcare facility, the first home visit was recommended to be made as soon as possible after the mother and baby return home, with remaining visits following the same schedule as for home births (WHO/UNICEF 2009).
A Cochrane Review demonstrated the effectiveness of community‐based intervention packages in improving neonatal outcomes and reducing maternal and neonatal morbidity and mortality in resource‐limited settings; home visiting is the one of the main components in each of these intervention packages. This review offers encouraging evidence of the value of integrating maternal and newborn care in community settings (Lassi 2015). Therefore, we did not include intervention packages of continuous care, with components of antenatal or hospital care, in our review.
How the intervention might work
In high‐resource settings, healthy women and babies are frequently discharged from hospital within one or two days of the birth, and in low‐resource settings women may be discharged within hours of the birth, or may give birth at home (Brown 2002). Potentially, home visits by healthcare professionals or trained support workers within the first few days of the birth may offer opportunities for assessment of the mother and newborn, health education, infant feeding support, emotional or practical support and, if necessary, referral to other health professionals or agencies (Carabin 2005; Donovan 2007; Lassi 2015; Shaw 2006). Postpartum visits may prevent health problems developing or reduce their impact by early intervention or referral. Home visits have improved coverage of key maternal and newborn care practices such as early initiation of breastfeeding, exclusive breastfeeding, skin‐to‐skin contact, delayed bathing, attention to hygiene (e.g. hand washing and water quality), umbilical cord care, infant skin care. In addition, home visits may identify conditions that require additional care or check‐up, as well as counselling regarding when to take the mother and newborn to a healthcare facility (WHO/UNICEF 2009). Home visits may involve not only the assessment of the mother and newborn for physical problems but also assessment of maternal mental health, family circumstances and the home environment.
Depending on the context, home visits may take a non‐judgmental and supportive role or a more directive approach in which the goals are to monitor family compliance with standards of parenting care and ensure the newborn's health and welfare. The type of approach used can influence the ability of the carers to engage mothers and newborns, resulting in acceptance or rejection of the help offered and potential for further disengagement (Doggett 2005).
Why it is important to do this review
Despite many studies and reviews, evidence regarding the effectiveness of different types of home‐visiting programs in the early postnatal period is not sufficient. In some contexts once women have been discharged from hospital there may be no postnatal follow‐up, or very limited postnatal follow‐up. In higher‐resource settings, once women are at home, services may be provided by a range of health and social care agencies (newborn health visitors, social workers, paediatricians and general practitioners) and may be fragmented; postnatal home visits potentially allow continuity of care after hospital discharge and for the assessment and referral of the mother and newborn.
This Cochrane Review addresses the following questions: do different schedules of postpartum home‐visiting programs reduce maternal/neonatal mortality and morbidities, and if they do, what is the optimal schedule for postpartum home visits? This Cochrane Review includes reports evaluating the frequency, timing, duration and intensity of home visits. The optimal schedule has been set out by WHO/UNICEF 2009, however, there was no clear evidence underpinning recommendations. This is an update of a review last published in 2017.
Objectives
The primary objective of this review is to assess the effects of different home‐visiting schedules on maternal and newborn mortality during the early postpartum period. The review focuses on the frequency of home visits (how many home visits in total), the timing (when visits started, e.g. within 48 hours of the birth), duration (when visits ended), intensity (how many visits per week), and different types of home‐visiting interventions.
Methods
Criteria for considering studies for this review
Types of studies
We included studies that compared outcomes of participants after home visits with outcomes of those who received no home visits, or received different types of home‐visiting interventions. We included studies that used random or quasi‐random allocations of participants. The unit of allocation in eligible studies could be the individual or the group (i.e. cluster‐randomised). We also planned to include studies available only as abstracts, noting that these studies were awaiting assessment, pending publication of the full report. However, we did not identify any such study.
Types of participants
Eligible studies enrolled participants in the early postpartum period (up to 42 days after birth). We excluded studies in which women were enrolled and received an intervention during the antenatal period, even those in which the intervention continued into the postnatal period.
We planned to exclude studies that only recruited women from specific high‐risk groups (e.g. women identified with alcohol or drug problems), as interventions to support such women have been addressed elsewhere (Turnbull 2012).
Types of interventions
Interventions included scheduled home visiting in the postpartum period (excluding studies with antenatal home visiting in which the visits continued over many months). Interventions were home visits with various frequency, timings, duration and intensity.
We planned to include studies with co‐intervention(s). Home visits could include outreach visits to non‐healthcare facilities. Trials including a group that did not receive home visits would have been eligible.
Types of outcome measures
Primary outcomes
Maternal mortality at 42 days post‐birth.
Neonatal mortality.
Secondary outcomes
Maternal outcomes
Maternal morbidities (postpartum haemorrhage, puerperal fever, abdominal and back pain, abnormal discharge, puerperal genital infection, thromboembolic disease, and urinary tract complications) within 42 days after birth.
Maternal mental health (depression, anxiety) and related problems (intimate partner violence, drug use) at 42 days after birth.
Satisfaction with overall care and service at 42 days after birth.
Contraceptive use (this outcome was not pre‐specified).
Neonatal outcomes
Neonatal morbidities (pneumonia, upper respiratory tract infection, diarrhoea, septic meningitis, encephalopathy or cerebral injury, and jaundice) within 28 days after birth.
Established feeding regimen (e.g. exclusive breastfeeding) at 28 days after birth.
Incomplete immunisation.
Failure to thrive, abuse, neglect, domestic violence from parents for any reason within 28 days after birth.
Infant health care utilisation (this outcome was not pre‐specified).
Serious neonatal morbidity up to six months (this outcome was not pre‐specified).
Search methods for identification of studies
The following methods section is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
For this update, we searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (19 May 2021).
The Register is a database containing over 27,000 reports of controlled trials in the field of pregnancy and childbirth. It represents over 30 years of searching. For full current search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set that has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
In addition, we searched ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform (ICTRP) for unpublished, planned and ongoing trial reports (19 May 2021) using the search methods detailed in Appendix 1.
Searching other resources
(1) References from published studies
We searched the reference lists of relevant trials and reviews identified.
(2) Unpublished literature
We contacted authors for more details about the published trials/ongoing trials.
We did not apply any language restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Yonemoto 2017.
For this update, the following methods were used for assessing 190 reports that were identified as a result of the updated search.
The following methods section is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors (NY and SN) independently assessed eligibility for inclusion for all studies identified as a result of the search strategy. We resolved discrepancies by discussion and by consulting a third review author (RM).
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager 5 software (RevMan 2020) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019). Any disagreement was resolved by discussion or by involving a third review author.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2019). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis).
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio (RR) with 95% confidence intervals (CI).
Continuous data
We used the mean difference (MD) if outcomes were measured in the same way between trials. In future updates, as appropriate, we will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
We included cluster‐randomised trials in the analyses along with individually randomised trials. When including cluster‐RCTs, we adjusted their sample sizes with methods described in the Handbook (Higgins 2019), using an estimate of the intra‐cluster correlation co‐efficient (ICC) derived from the trial, from a similar trial or from a study of a similar population. Where we used ICCs from other sources, we reported this and planned to conduct sensitivity analyses to investigate the effect of variation in the ICC. We identified both cluster‐randomised trials and individually‐randomised trials, and we synthesised the relevant information provided there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
Trials with multiple treatment arms
One trial with three arms has been included in this review as two separate studies (Bashour 2008a; Bashour 2008b); to avoid double counting, the control group data (events and sample) were shared between the two study comparisons.
Dealing with missing data
For included studies, we noted levels of attrition as:
low risk of bias (indicates no missing data, or a low level of missing data, on an intention‐to‐treat (ITT) basis);
high risk of bias (indicates high level of missing data);
unclear risk of bias.
We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by conducting sensitivity analyses (see Sensitivity analysis).
For all outcomes, we carried out analyses, as far as possible, on an ITT basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised, minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager 5 software (RevMan 2020). We planned to use fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. However due to the diversity of interventions in the trials, we used random‐effects meta‐analysis for all outcomes. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we did not combine trials. If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We planned to perform a subgroup analysis according to the following clinically logical predefined groups.
Initiation of the intervention (within 48 hours after birth or later).
Duration of the intervention (less than three weeks versus three or more weeks).
Intensity or frequency of the intervention (less than one visit/week versus one or more visits/week.).
Person doing the visit: medical professional versus skilled attendant.
Parity: primiparity versus multiparity.
However, interventions in included trials were too heterogeneous to conduct the subgroup analyses planned as above. We therefore decided to conduct subgroup analyses by intensity/frequency of the intervention only, in the comparison of more home visits versus fewer home visits, as outlined below:
1. Any number of home visits versus no home visit.
2. Four or more home visits versus fewer than four home visits.
3. More home visits versus fewer home visits (both groups had more than four home visits).
We planned to assess differences between subgroups using interaction tests available in Review Manager 5 (RevMan 2020).
Sensitivity analysis
We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result. There were too few trials included in any analysis, and so we were unable to carry out sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
We assessed the certainty of the evidence using the GRADE approach, as outlined in the GRADE handbook. We assessed the certainty of the body of evidence relating to the following outcomes for the main comparison (schedules involving more versus fewer postpartum visits).
Maternal mortality at 42 days post‐birth
Neonatal mortality
Postnatal depression (last assessment up to 42 days postpartum)
Maternal satisfaction with postnatal care
Serious neonatal morbidity up to six months (this outcome was not pre‐specified)
Exclusive breastfeeding
We used GRADEpro GDT to import data from Review Manager 5 (RevMan 2020), and to create a 'Summary of findings' table. The GRADE approach uses five considerations (study limitations, inconsistency of effect, imprecision, indirectness, and publication bias) to assess the certainty of the body of evidence for each outcome. RCT data are initially considered to provide high‐certainty evidence, but based on assessment of these five domains, the certainty of evidence for a given outcome may be downgraded to moderate, low or very low. For each of these domains, the certainty of evidence may be downgraded by one level for serious concerns, or by two levels for very serious concerns.
Results
Description of studies
Results of the search
See: Figure 1
1.
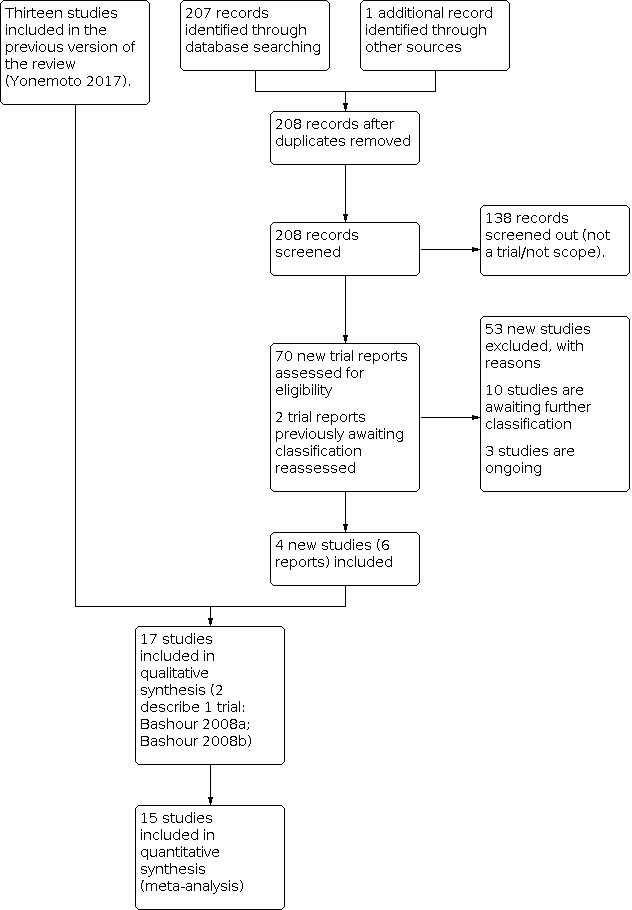
Study flow diagram.
We assessed 69 new trial reports from the updated search, and one report from checking reference lists. We reassessed the two reports that were awaiting classification in the previous version of the review (Furnieles‐Paterna 2011; Salazar 2011). We included four new trials (six reports) and excluded 53 reports. Ten trials are awaiting classification and three trials are ongoing (Kristensen 2018; NCT04226807; NCT04257552).
Included studies
After assessing eligibility, we included 16 randomised trials with a total of 12,080 women (Aksu 2011; Bashour 2008a; Bashour 2008b; Christie 2011; Escobar 2001; Furnieles‐Paterna 2011; Gagnon 2002; Kronborg 2007; Lieu 2000; MacArthur 2002; Milani 2017; Mirmolaei 2014; Morrell 2000; Paul 2012; Ransjo‐Arvidson 1998; Salazar 2011; Steel 2003).
Design
Three of the trials (Christie 2011; Kronborg 2007; MacArthur 2002) were cluster‐randomised, with health centres or healthcare staff as the units of randomisation. For these trials, event rates and/or sample sizes have been adjusted in the analysis to take account of cluster design effect. One trial (Furnieles‐Paterna 2011) was quasi‐randomised, depending on the women's addresses.
One of the trials included three arms; women in the intervention groups received either four home visits or one home visit, while the control group received no home visits. In order for us to set out the results for all three groups, we have reported this trial as though it were two studies (Bashour 2008a; Bashour 2008b). In the Data and analyses, women receiving four home visits versus no home visits are entered under Bashour 2008a; whereas those receiving one home visit versus no home visits are compared in Bashour 2008b. The control group's number of events and number of participants in the sample have been divided between these comparisons, to avoid double counting.
Setting
The studies were carried out in countries across the globe in both high‐ and low‐resource settings. Three studies were carried out in the UK (Christie 2011; MacArthur 2002; Morrell 2000), three in the USA (Escobar 2001; Lieu 2000; Paul 2012), two in Canada (Gagnon 2002; Steel 2003), two in Iran (Milani 2017; Mirmolaei 2014), two in Spain (Furnieles‐Paterna 2011; Salazar 2011), and one each in Denmark (Kronborg 2007), Syria (Bashour 2008a; Bashour 2008b), Turkey (Aksu 2011), and Zambia (Ransjo‐Arvidson 1998). It is important to take the time and setting into account when interpreting results, as routine practice varied across time and in different settings. For example, in the UK, usual care may have involved up to seven home visits, whereas in other settings there may have been no postnatal care after hospital discharge.
Interventions and comparisons
The number and type of visits examined varied considerably across these trials, and control conditions also varied. Broadly, trials examined three types of comparisons: schedules involving more versus fewer postnatal home visits; schedules involving different models of care; and home versus hospital clinic postnatal follow‐up. In view of the complexity of interventions, we have set out the main components of interventions, and a description of control conditions in Table 2.
1. Description of interventions and control conditions.
| Name of study | Intervention | Control |
| STUDIES COMPARING MORE VS FEWER HOME VISITS | ||
|
Ransjo‐Arvidson 1998 4 home visits vs 1 home visit |
208 women randomised. Women were visited at home 4 times, at 3, 7, 28 and 42 days postpartum by a midwife. Each visit lasted about an hour. Women were asked about their own and their babies’ health but there was no formal health education. | 200 women received 1 visit by a midwife at about 42 days postpartum. |
|
Bashour 2008a; Bashour 2008b 4 vs 1 vs 0 home visits |
2 intervention groups: (1) Women (301) received 4 home visits on the first, 3rd, 7th day and 4 weeks after delivery. The aim of visits was to provide emotional support, assess maternal and infant health, assess the home, educate re breastfeeding and to discuss family planning. The visits were carried out by midwives. (2) Women (301) received a home visit on the first day only. The aim was to support and educate the woman and assess condition of mother and newborn. The visit was carried out by a midwife. |
301 women received normal care in Syria which was early discharge (as early as 2 hours following delivery) and no planned postnatal care. |
|
Christie 2011 6 health visitor home visits vs 1 |
136 women completed the pre‐test in the intervention group (referred by 39 health visitors). The intervention group received 6 health visitor visits between 10‐14 days and 8 weeks postpartum (approximately weekly visits). Health visitors provided advice and support, carried out assessments and offered health promotion. First visit 10‐14 days, 6 visits up to 8 weeks (weekly). |
159 women completed the pre‐test (nominated by 40 health visitors). The control group received 1 health visitor visit at 10‐14 days. Health visitors provided advice and support, carried out assessments and offered health promotion. Any further visits were discretionary. All women received usual postnatal care (midwife visits at home). |
|
Aksu 2011 Single postpartum visit vs no visit |
Women in both groups received standard care which included in‐hospital breastfeeding education. 33 women. Women were visited once at home 3 days after delivery by a trained supporter who provided advice and support. Visits lasted about 30 minutes. |
33 women received standard care which included breastfeeding education before hospital discharge. |
|
Morrell 2000 10 additional home visits vs 0 additional home visits |
All women received routine postnatal care at home from midwives and health visitors. 311 women received additional support from trained community support workers. Women received up to 10 visits lasting up to 3 hours between hospital discharge up to 28 days. Community workers helped with housework, caring for the baby and provided emotional support and reinforced midwife advice re breastfeeding. |
312 women received routine postnatal care which included home visits from community midwives and health visitors. Women received no additional visits from support workers Women in both groups received routine pp care with approximately 7 midwife visits and a health visitor visit. |
| STUDIES COMPARING DIFFERENT WAYS OF OFFERING CARE | ||
|
Steel 2003 Up to 2 home visits versus telephone screen by nurse and discretionary home visits |
353 women were telephoned on the first working day following discharge and arrangements were made for 2 home visits to take place within 10 days postpartum with the first visit being scheduled as soon as possible. The visits were structured to include infant assessment and public health nurse carrying out the visits could refer for other care if necessary. |
380 women were allocated to the telephone screen group. On the first working day following discharge women were phone by a public health nurse with a structured screening questionnaire and to elicit any concerns about feeding or the mother or infant’s health. A home visit was made if the nurse or mother thought one was needed (in 1 of the sites where home visits were routine care before the trial 54% of the women allocated to telephone screen had at least 1 home visit). |
|
Kronborg 2007 1‐3 structured postnatal visits by specially trained health visitors vs 1 or more unstructured health visitor visits. In this trial the intervention group did receive slightly more visits (mean 2.5 vs 2.1) but the main thrust of this intervention seemed to be the special HV training and the focus on promotion of breastfeeding through more structured visits. |
11 areas; 780 women recruited. The intervention included special health visitor training (18 hours) focusing on promoting breastfeeding. Health visitors then visited women at home on 1‐3 occasions and the visits were structured, focusing on breastfeeding continuation. Women received the first visit soon after hospital discharge and mothers with limited or no breastfeeding experience were offered up to 2 further visits focusing on breastfeeding (mean number of visits 2.5). It was not clear whether health visitors also offered standard care (i.e. covered content of visits as per control group). | 11 areas; 815 women. Health visitors received no additional training. Women were offered standard care which was 1 or more unstructured visits by health visitors up to 5 weeks postpartum. Women tended to receive approximately 2 home visits (mean 2.1); the content of visits was not specified. |
|
MacArthur 2002 Flexible visits vs routine care (scheduled visits) |
18 intervention practices (1 dropped out before recruitment of women) 1087 women recruited. Midwives were trained to provide a more flexible model of postnatal care responsive to women’s needs. There was no fixed schedule or number of postnatal visits. The number and content of visits at home was determined by midwives in consultation with women. After the initial visit a symptoms checklist was used and visits could take place up to 10‐12 weeks. (Midwife records suggest the mean number of visits was 6). It was not clear if women also received health visitor care. | 19 clusters, 977 women recruited. Routine care which “generally consists of 7 midwife home visits to 10‐14 days (can continue to day 28)” with care from health visitors thereafter. Mean number of midwife visits was approximately 4, it was not clear how many health visitor visits women received. |
| STUDIES COMPARING HOME VS FACILITY POSTNATAL CARE | ||
|
Furnieles‐Paterna 2011 Single home visit in first 48 hours after discharge plus routine check up at hospital vs single hospital visit |
100 women allocated to one puerperal home visit during the first 48 hours after discharge, and then the usual check‐up carried out in the health centre. | 100 women allocated to the usual check up at health centre. |
|
Lieu 2000 Single home visit vs single hospital visit |
580 women were allocated to receive a single home visit within 48 hours of hospital discharge by a nurse. Visits were scheduled to last 60‐90 minutes with some educational component. (This single visit was INSTEAD of rather than in additional to usual care; women received home visit rather than attending clinic visit in the hospital). | 583 women attended a 20 minute paediatric clinic visit within 48 hours of the birth. This visits may also have included some guidance and education. |
|
Escobar 2001 Single home visit vs single hospital (group or individual visit) |
508 women were allocated to receive a single home visit within 48 hours of hospital discharge by a nurse. Visits were scheduled to last 60‐90 minutes with some educational component. (This single visit was INSTEAD of rather than in additional to usual care; women received home visit rather than attending clinic visit in the hospital. 96% received a home visit as allocated although 75 women also attended for a hospital visit). |
506 women allocated to attend a 1‐2 hour group based visit where women (in groups of 5‐8). Women were offered newborn checks and guidance as part of group sessions. Multiparous women could opt for a 15‐minute paediatric clinic visit within 48 hours of the birth. This visit may also have included some guidance and education (157 had the group visit only, 264 the individual visit only, 64 both and 4 both home and hospital). |
|
Gagnon 2002 Single home visit vs single hospital visit |
All women received a nurse telephone contact at 48 hours post‐birth. 283 women were allocated to receive follow‐up at home at 3‐4 days postpartum. Home visits were by a community nurse. Visits were planned to last 1 hour and included newborn examination and guidance on infant care and breastfeeding. Women did not attend for hospital clinic visit at 3‐4 days (usual care). | All women received a nurse telephone contact at 48 hours post‐birth. 282 women were randomised to receive usual care which included a hospital clinic visit at 3‐4 days for newborn check and guidance on infant care and breastfeeding. Visits lasted up to 45 minutes (no home visit). |
|
Paul 2012 Single home visits vs single hospital visit |
576 women. Single visit by health visiting nurses within 48 hours of hospital discharge (typically 3‐5 days after the birth). The nurse had special training in promoting and supporting breastfeeding. | 578 women. Usual care. Clinic based postnatal follow‐up arranged by obstetricians. Women in both groups also had an office based visit for the baby approximately 1 week after the nurse visit or 5‐14 days after birth arranged by the hospital newborn nursery doctor). |
|
Milani 2017 2 home visits vs routine care (hospital based care) |
92 women recruited. The intervention was the postpartum health care providing at home on the 3–5th and 13–15th day after delivery according to the designed guideline. Healthcare providers were educated midwives. The average visit time was 30–45 minutes which would change with mothers' request. The intervention included greeting and recording checklists which were filled by midwives after interviewing and examining the mother and infant on each visit. | 184 women recruited. Usual hospital‐based care, if requested. It was only stated "lack of home visit". |
|
Mirmolaei 2014 2 home visits vs routine care (referral health service center) |
200 women recruited. Mothers and their neonates received the first postpartum care at health service centers in both groups. The intervention were second (10‐15 days), and third (42‐60 days) cares were provided by a trained midwife at home. Postpartum home visiting includes greeting and establishing an intimate relationship with the mother, identifying mother's SES and lifestyle, assessing vital signs, and recording checklists which were filled by midwives after interviewing and examining the mother and infant on each visit. | The control group received second and third cares provided by healthcare providers (mostly midwives) at a referral health service center. |
|
Salazar 2011 Attention at home within first week vs routine care (out‐patient clinic) |
213 women allocated to receive attention at home during the first week postpartum by a specialised nursing unit. | 217 women allocated to receive routine checks at days 7 and 30 at the out‐patient clinic. |
vs: versus
1. Schedules involving more versus fewer home visits
In five of our included studies, the main comparison was between women receiving more versus fewer home visits in the postnatal period.
Aksu 2011 examined the effect of one postnatal visit by a trained supporter versus no postnatal visits; Bashour 2008a; Bashour 2008b compared four or one postnatal home visits from midwives versus no home visits following hospital discharge. Ransjo‐Arvidson 1998 compared four midwife home visits versus one midwife home visit. In these three studies, carried out in low‐resource settings, women may have received no additional postnatal care.
In contrast, Christie 2011 and Morrell 2000 examined the impact of additional care in settings where women already received more than four postnatal visits from midwives as part of usual care. Christie 2011 compared groups receiving six health visitor visits versus one health visitor visit (in addition to midwifery care) and Morrell 2000 examined the impact of up to 10 visits from lay supporters; again, visits were provided in addition to routine midwifery care, which was available to women in both intervention and control groups. (In the Data and analyses tables, we have separated studies where women in both groups received more than four home visits, as the impact of interventions is likely to have been different from that in settings where women received no, or very limited postnatal care.)
2. Schedules comparing different models of postnatal care at home
Three studies examined different ways of providing postnatal care.
Steel 2003 compared the effects of two visits by public health nurses in the early postnatal period, compared with a telephone screening interview, with discretionary nurse home visits.
In a cluster‐RCT, Kronborg 2007 looked at the effects of more structured postnatal visits; women in the intervention group were visited between one and three times by health visitors who had attended special training on promoting and supporting breastfeeding. Women in the control group received usual care by health visitors who had not attended the breastfeeding courses.
MacArthur 2002 compared postnatal care that was adapted to the individual needs of women and home visits extended beyond the usual period of care (flexible visits up to 10 to 12 weeks postpartum). This was compared with usual care which involved a more rigid schedule of midwife home visits confined to the early postnatal period.
3. Home versus facility postnatal care
Eight of the included studies compared outcomes in women attending hospital clinics or for postnatal checks and follow‐up (usual care) versus home visits by nurses (Escobar 2001; Furnieles‐Paterna 2011; Gagnon 2002; Lieu 2000; Paul 2012; Salazar 2011) and educated midwives (Milani 2017; Mirmolaei 2014).
For all types of comparisons the purpose of visits was broadly similar: to assess the physical health and well being of mothers and babies (with referral for further care where necessary), to promote and support breastfeeding, to assess maternal emotional well being and to offer health education and support. In some cases the intervention focused on a particular aspect of care (e.g. breastfeeding), whereas other interventions were more general.
Outcomes
The outcomes measured in different studies varied. Most studies included some measure of maternal and infant health (although the particular outcomes measured, the way they were measured, and the time of follow‐up varied considerably between studies). Infant healthcare utilisation was also reported in a number of trials. Maternal emotional well being and rates of breastfeeding were reported in some of the studies, and a minority reported maternal satisfaction with postnatal care. For Mirmolaei 2014, data were not available for any of our outcomes.
Dates of the study
Results of trials were published between 1998 and 2017, although study data may have been collected some years before publication (e.g. in Ransjo-Arvidson 1998, women were recruited between 1989 and 1992).
Sources of trial funding
Eleven studies reported the source of trial funding and three studies had no information about funding source (please see details in Characteristics of included studies).
Trial authors' declarations of interest
Nine studies declared no conflict of interests, and seven studies had no information about conflict of interest (please see details in Characteristics of included studies).
Excluded studies
Fifty‐eight studies identified by the searches were excluded after assessing the full trial reports. Thirteen studies did not specifically examine postnatal home visits (Adachi 2016; Bagherinia 2017; Gunn 1997; Gunn 1998; Hannan 2013; Laliberte 2016; NCT00298311 2006; NCT01620723 2012; NCT03715218 2018; NCT03887910 2019; ; Pluym 2021; Roberts 2016; Simons 2001). Two studies were excluded as the intervention was for contraception (NCT02769676 2016; NCT03165838 2017). NCT03880032 2019 was excluded as the intervention was a cognitive behavioral therapy intervention for anxiety. Two studies were excluded as they focused on outcomes in women following early hospital discharge after the birth rather than on different schedules of home visits for women discharged at the same time (Boulvain 2004; Carty 1990). Eight studies were excluded as the intervention was given during antenatal period (Baur 2012; Goldfeld 2019; Gonzalez 2018; Gupta 2019; Harrison 2019; NCT02069782 2014; Tandon 2018; Tomlinson 2016). Three studies were excluded because they examined complex interventions that included components delivered during the antenatal period (Korfmacher 1999; Lumley 2006; Olds 2002). Park Himes 2017 examined complex interventions and did not separately analyse the home visit intervention. Six studies were excluded as the intervention was after six months (Dodge 2013a; Dodge 2019; Goodman 2019; Hutton 2017; Kilburn 2017; Olds 2002). Eight studies were excluded because they recruited mothers before birth (Catherine 2016; Hodgins 2020; Kikuchi 2015; Lakin 2015; McConnell 2016; Mohd Shukri 2019; Rotheram‐Borus 2017; Var 2015; Rotheram‐Borus 2014). Two studies were excluded because they randomised mothers 42 days after birth (Modi 2017; Sawyer 2017). Three studies were of observational design with random sampling or convenient sample (Dodge 2013b; Dodge 2014; Ghodsbin 2012). Hannan 2014 was a secondary analysis of a trial that did not include home visits. Paul 2013 was a secondary analysis of a trial with no comparison between groups. Two studies were a pre‐post test design (Jiao 2019; Navidian 2017). NCT03448289 2018 was an intervention trial with a single arm. One study, which recruited high‐risk women, involved intervention by child health nurses, rather than more general care of the mother and baby in the early postnatal period (Izzo 2005). Quinlivan 2003 focused on a high‐risk group rather than on the impact of different schedules of care. Finally, Stanwick 1982 was excluded for methodological reasons; there were major protocol deviations in this study, with many women in the intervention group failing to receive the intervention as planned, and analysis was carried out according to treatment received rather than by randomisation group (data were not available to allow us to restore women to their original randomisation groups).
Risk of bias in included studies
The included studies were mixed in terms of risk of bias; we were unable to carry out planned sensitivity analysis (temporarily excluding studies at high or unclear risk of bias for allocation concealment) as too few studies contributed data to allow any meaningful additional analysis.
We have set out the 'Risk of bias' assessments for individual studies in Figure 2 and for overall bias across all studies for different bias domains in Figure 3.
2.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We judged 11 of the 16 included studies to be at low risk of bias because they used adequate methods to generate the randomisation sequence. Seven used computer‐generated sequences or external trial randomisation services (Aksu 2011; Escobar 2001; Gagnon 2002; Kronborg 2007; Lieu 2000; MacArthur 2002; Paul 2012) and five used random number tables (Christie 2011; Morrell 2000; Steel 2003; Mirmolaei 2014; Salazar 2011). In three trials (Bashour 2008aBashour 2008bMilani 2017Ransjo‐Arvidson 1998), it was not clear how the randomisation sequence was decided. Furnieles‐Paterna 2011 used a quasi‐randomised method, so we assessed this study to be at high risk of bias.
Concealment of group allocation at the point of randomisation was assessed as being at low risk of bias in nine studies; five trials reported using sequentially numbered, sealed envelopes to conceal allocation (Bashour 2008a; Bashour 2008b; Escobar 2001; Lieu 2000; Morrell 2000; Steel 2003) and four used external randomisation services (Christie 2011; Gagnon 2002; Furnieles‐Paterna 2011; Kronborg 2007; MacArthur 2002). In the trials by Aksu 2011, Paul 2012, Ransjo‐Arvidson 1998, Milani 2017, Mirmolaei 2014, and Salazar 2011, the methods used to conceal allocation were not described, or were not clear.
Blinding
Blinding women and care providers to this type of intervention is not generally feasible and no attempts to achieve blinding for these groups were described. All studies were judged to be at high risk of bias for this domain. It is possible that lack of blinding may have been an important source of bias.
In eight of the trials, it was reported that outcome assessors were blind to group allocation (Bashour 2008a; Bashour 2008b; Escobar 2001; Furnieles‐Paterna 2011; Gagnon 2002; Lieu 2000; MacArthur 2002; Paul 2012; Steel 2003). However, with the exception of Gagnon 2002, who took extra precautions to ensure blinding (where outcome data were assessed by interview), women may have revealed their treatment group, and it was not clear whether or not blinding was successful; none of the trialists reported checking the success of blinding. Blinding of outcome assessors was either not attempted or not mentioned in the remaining eight trials (Aksu 2011; Christie 2011; Kronborg 2007; Morrell 2000; Ransjo‐Arvidson 1998; Milani 2017; Mirmolaei 2014; Salazar 2011).
Incomplete outcome data
In nine of the included trials, sample attrition and missing data did not appear to be important sources of bias (assessed as low or unclear risk of bias) (Bashour 2008a; Bashour 2008b; Christie 2011; Escobar 2001; Furnieles‐Paterna 2011; Lieu 2000; Paul 2012; Steel 2003; Mirmolaei 2014; Salazar 2011). In some trials, although attrition was balanced across groups, there was more than 10% loss to follow‐up. In the Aksu 2011 trial, the response rate at four months postpartum was 82%; 16% were lost to follow‐up in the Kronborg 2007 study and 15% were lost to follow‐up in the trials by Gagnon 2002 and Ransjo‐Arvidson 1998. By four months postpartum, more than 20% of the sample were lost to follow‐up in the MacArthur 2002 trial, and a whole cluster was also excluded post‐randomisation in the home visit group; this trial was judged to be at high risk of attrition bias. Loss to follow‐up was not balanced in the intervention and control groups in the Morrell 2000 study. In this study, while the response rate was 83% for those women receiving additional postnatal visits, it was only 75% in the control group.
Selective reporting
Assessing selective reporting bias is not easy without access to study protocols, and for all studies included in the review, risk of bias was assessed from published study reports. In most, but not all of the studies, the primary outcomes were specified in the methods section and trialists reported results for these outcomes. We were unable to carry out planned investigation of possible publication bias by generating funnel plots as too few studies contributed data. We assessed whether appropriate outcomes were reported in the trial, if the trial registration number was available.
Other potential sources of bias
In most of the studies there were no other obvious sources of bias. In four of the trials, there was some imbalance between groups at baseline (Escobar 2001; Gagnon 2002; Lieu 2000; Morrell 2000). In the Steel 2003 study, women were recruited in two study areas and usual practice was different in each area and this led to protocol deviations; again, it is not clear how this would have affected results. Finally, in the Ransjo‐Arvidson 1998 trial, much of the analysis related to the intervention group only. In addition, the nature of the intervention may have affected findings. Midwives asked women about their health as part of the intervention, so women in the intervention group were asked repeatedly to identify health problems; whereas women in the control group were only asked as part of follow‐up assessments. This may have affected recall and introduced a risk of response bias. This trial may also have had the potential for publication bias, because the publication date was more than six years after study completion.
The three cluster‐randomised trials included in the review (Christie 2011; Kronborg 2007; MacArthur 2002) appeared to have comparable groups at baseline, and adjusted their results to take account of the cluster effect in their analyses. In the cluster trial reported by Christie 2011, health visitors were the unit of randomisation and it appeared that there were differences between health visitors in terms of the number of women recruited to the trial and in their practices; the impact of these differences in individual practices is unclear.
Effects of interventions
See: Table 1
Schedules involving more versus fewer home visits (five trials with 2102 women)
In five included studies, the main comparison was between women receiving more versus fewer home visits in the postnatal period (Aksu 2011; Bashour 2008a; Bashour 2008b; Christie 2011; Morrell 2000; Ransjo‐Arvidson 1998). One trial included three arms, and in order to report findings for its two different intervention groups, we treated this trial as though it were two separate studies (Bashour 2008a; Bashour 2008b). One of the trials (Christie 2011) was a cluster‐randomised trial, and in the data and analyses tables we have used the effective sample size and event rates (adjusted for cluster design effect). See Table 3 for details of these adjustments.
2. Adjustments for cluster trials.
| Trial | Outcome | Average cluster size | ICC | DE | Original sample | Adjusted sample |
GIV data |
| Christie 2011 | Analysis 1.1 Maternal mortality |
3.7 (reported in trial) |
Based on data below as not reported for this outcome | 1.243 | I = 129 C = 151 |
I = 104 C = 121 Cant do this for number of events as only one event |
Not reported |
| Analysis 1.11 Depression |
3.7 (reported in trial) |
0.09 (reported in trial) | 1.243 | I = 129 C = 151 |
I = 104 C = 121 |
1.26 (0.16, 2.36) | |
| Analysis 1.12 Anxiety |
3.7 (reported in trial) |
0 | 1 | I = 129 C = 151 |
NA | 2.11 (‐1.64, 5.86) | |
| Analysis 1.14 Satisfaction |
3.7 (reported in trial) |
0 | 1 | I = 129 C = 151 |
NA | 14.51 (7.7, 21.28) | |
| Analysis 1.20 Any breastfeeding |
3.7 (reported in trial) |
0.03 (reported in trial) |
1.081 | I = 29/129 C = 36/151 |
I = 27/119 C = 33/140 |
0.94 (0.33, 1.01) | |
| Analysis 1.25 Infant health utilisation |
3.7 (reported in trial) |
0.02 (reported in trial) |
1.054 | I = 9/129 C = 23/151 |
I = 9/122 C = 22/143 |
0.36 (0.15, 0.85) |
Aksu 2011 examined the effect of one postnatal visit versus no postnatal visits; Bashour 2008a; Bashour 2008b examined four home visits or one home visit versus no home visits; Ransjo‐Arvidson 1998 examined four home visits versus one home visit. Christie 2011 and Morrell 2000 examined the impact of additional care in settings where women already received more than four visits as part of usual care. Christie 2011 compared groups receiving six health visitor visits versus one health visitor visit (in addition to midwifery care). Morrell 2000 examined up to 10 lay supporter visits versus no additional visits, with routine midwifery care available to women in both the intervention and control groups. (In the Data and analyses tables, we have separated studies where women in both groups received more than four home visits.)
For many of our prespecified outcomes, only one or two studies contributed data, and results were not always available for all women randomised. For each result, we have specified the number of studies and women for whom data were available (for cluster‐randomised trials, these are the adjusted figures). We anticipated that the treatment effect might differ in trials comparing different numbers of visits; we therefore used a random‐effects model for all analyses in this comparison.
Primary outcomes
Maternal mortality up to 42 days postpartum
Only one trial reported this outcome (Christie 2011). The evidence is very uncertain about whether there is any difference in maternal mortality between groups receiving additional health visitor visits, compared with controls. Only one death reported in the additional health visitor visits group (RR 0.39, 95% CI 0.02 to 9.41; one study with 225 women, very low‐certainty evidence;Analysis 1.1).
1.1. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 1: Maternal mortality within 42 days post‐birth
Neonatal mortality
Two trials reported on neonatal death (Bashour 2008a; Bashour 2008b; Ransjo‐Arvidson 1998); there was no strong evidence that more visits were associated with fewer deaths. The evidence was assessed to be very uncertain about the effects of the intervention on neonatal mortality (RR 0.99, 95% CI 0.26 to 3.69; three studies, 1281 women; very low‐certainty evidence;Analysis 1.2). Similarly, women receiving one or four home visits versus no home visits, or four or more home visits versus one home visit, showed very uncertain results for neonatal deaths (RR 3.06, 95% CI 0.37 to 25.39; one study, 873 women; and RR 0.48, 95% CI 0.09 to 2.60; one study, 408 women; respectively).
1.2. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 2: Neonatal mortality
Secondary outcomes
Severe maternal morbidity
Two studies reported this outcome. Bashour 2008a; Bashour 2008b reported the number of women seeking medical help for a health problem and Ransjo‐Arvidson 1998 reported the number of women in whom a doctor had identified a problem up to 42 days. The numbers of women with problems were very similar in intervention and control groups, and the evidence suggested that there may be little to no difference between groups either overall, or for women receiving different patterns of visits (overall RR 0.96, 95% CI 0.81 to 1.15, two studies, 1228 women; four visits or one visit versus no visits RR 0.97, 95% CI 0.80 to 1.17, one study, 876 women; and, four visits versus one visit RR 0.90, 95% CI 0.52 to 1.54, one study, 352 women; Analysis 1.3).
1.3. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 3: Severe maternal morbidity
Maternal health problems up to 42 days
Only one study reported results for most of our pre‐specified outcomes relating to maternal postpartum health problems up to 42 days after the birth (Bashour 2008a; Bashour 2008b). There may be little to no difference between women receiving four or one postnatal home visits versus no postnatal home visits for secondary postpartum haemorrhage (RR 0.78, 95% CI 0.49 to 1.26; two studies, 873 women; Analysis 1.4); abdominal pain (RR 1.06, 95% CI 0.83 to 1.34; two studies, 869 women; Analysis 1.5); back pain (RR 0.96, 95% 0.83 to 1.11; two studies, 871 women; Analysis 1.6); urinary tract complications (RR 0.83, 95% CI 0.63 to 1.10; two studies, 876 women; Analysis 1.7); fever (RR 1.30, 95% CI 0.93 to 1.82; two studies, 876 women; Analysis 1.8) or dyspareunia (RR 1.18, 95% CI 0.90 to 1.55; two studies, 869 women; Analysis 1.9). No studies reported on thromboembolic disease or puerperal genital tract infections.
1.4. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 4: Secondary postpartum haemorrhage
1.5. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 5: Abdominal pain up to 42 days postpartum
1.6. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 6: Back pain up to 42 days postpartum
1.7. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 7: Urinary tract complications up to 42 days postpartum
1.8. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 8: Maternal fever up to 42 days postpartum
1.9. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 9: Dyspareunia
One study reported mean scores on a scale measuring maternal perceptions of their general health at six weeks postpartum (Morrell 2000). The evidence suggested that there were little or no differences between women receiving additional postnatal support and controls (MD ‐1.60, 95% CI ‐4.72 to 1.52; one study, 539 women; Analysis 1.10). The score was measured using the SF‐36 general perception domain. A high score on this instrument means good general heath perception.
1.10. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 10: Maternal perception of general health at 6 weeks (mean SF36)
Postnatal depression and anxiety
None of the studies included in this comparison reported the number of women with a diagnosis of depression in the postnatal period. Two studies looked at mean scores on the Edinburgh Postnatal Depression Scale (EPDS) at six weeks (Morrell 2000) and eight weeks postpartum (Christie 2011). In the Morrell 2000 study, women received additional support from lay people, and in Christie 2011, women received additional health visitor support, as well as routine midwife home visits. The intervention did not appear to have a positive effect in either study, and overall, women receiving the additional visits had slightly higher mean depression scores (MD 1.02, 95% CI 0.25 to 1.79; two studies, 767 women; low‐certainty evidence; Analysis 1.11). Higher scores on the EPDS relates to a bad outcome for women, with the maximum score being 30 and anything above 10 considered to indicate depression. Christie 2011 reported mean anxiety scores at eight weeks postpartum; there were no differences between groups (MD 3.80, 95% CI ‐0.18 to 7.78; one study, 280 women; Analysis 1.12).
1.11. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 11: Mean postnatal depression score (last assessment up to 42 days postpartum)
1.12. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 12: Mean maternal anxiety score (last assessment up to 42 days postpartum)
Maternal satisfaction with care in the postnatal period
Women were asked about their satisfaction with postnatal care in two studies. In one study the number of women saying they were "happy" with their postnatal experience was reported (Bashour 2008a; Bashour 2008b) (Analysis 1.13). Women receiving no formal postnatal care were slightly more satisfied with their experience, although the CI crossed the line of no effect, and so there may be no real effect (RR 0.96, 0.90 to 1.02; two studies, 862 women; low‐certainty evidence). In a second study (Christie 2011), the additional support provided by health visitors was associated with increased mean satisfaction scores (MD 14.70, 95% CI 8.43 to 20.97; one study, 280 women; low‐certainty evidence; Analysis 1.14).
1.13. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 13: Maternal satisfaction with postnatal care
1.14. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 14: Mean satisfaction score with postnatal care
Neonatal morbidity
Two studies reported infant respiratory tract infections up to eight weeks postpartum, although each trial defined the condition differently. In Bashour 2008a; Bashour 2008b, the number of babies suffering a cough or cold was reported, whereas in the Ransjo‐Arvidson 1998 trial the infants appeared to have more serious illness. Overall, and in individual studies, there was little to no evidence of differences between groups (RR 0.99, 95% CI 0.84 to 1.17; three studies, 1217 infants; Analysis 1.16).
1.16. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 16: Infant respiratory tract infection within 42 days
A single study reported on the number of infants with jaundice (not defined), with no effect between groups (RR 1.04, 95% CI 0.85 to 1.26; 861 infants; Analysis 1.15). In the same study, approximately half of the babies were reported to have had diarrhoea; however, more infants in the group receiving no visits were reported to suffer from diarrhoea, compared to those whose mothers received postnatal home visits (RR 0.85, 95% CI 0.74 to 0.98; two studies, 861 infants; Analysis 1.17).
1.15. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 15: Infant jaundice
1.17. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 17: Infant diarrhoea up to 42 days postpartum
Breastfeeding
Exclusive breastfeeding at up to six weeks was reported in three studies (Aksu 2011; Morrell 2000; Ransjo‐Arvidson 1998); and exclusive breastfeeding up to six months was also reported in three studies (Aksu 2011; Bashour 2008a; Bashour 2008b; Morrell 2000). Women receiving additional support at home may be more likely to exclusively breastfeed their babies at six weeks postpartum (RR 1.17, 95% CI 1.01 to 1.36; three studies, 960 women; low‐certainty evidence; Analysis 1.18), and at the last assessment up to six months postpartum (RR 1.38, 95% CI 1.10 to 1.73; three studies, 1309 women; low‐certainty evidence;Analysis 1.19).
1.18. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 18: Exclusive breastfeeding (last assessment up to 6 weeks)
1.19. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 19: Exclusive breastfeeding (last assessment up to 6 months)
For any breastfeeding, there may be little to no difference between women receiving additional postnatal visits and controls at either six weeks or up to six months postpartum (RR 1.05, 95% CI 0.88 to 1.25; two studies, 807 women; and RR 1.01, 95% CI 0.99 to 1.03; two studies, 1315 women, respectively; Analysis 1.20; Analysis 1.21).
1.20. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 20: Any breastfeeding (up to 6 weeks)
1.21. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 21: Any breastfeeding (last assessment up to 6 months)
Aksu 2011 reported mean duration of breastfeeding (months) in 54 women who had received one postnatal visit versus no postnatal visits at home. In both groups, women breastfed their babies for approximately a year or more on average, but the mean duration was increased by three months in women receiving a home visit (MD 3.00, 95% CI 2.33 to 3.67; one study, 54 women; Analysis 1.22).
1.22. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 22: Mean duration of any breastfeeding (months)
Incomplete immunisation
The evidence suggests that the intervention has no effect on the number of infants receiving immunisations; the vast majority of infants were immunised whether or not their mothers received postnatal care at home (RR 0.99, 95% CI 0.96 to 1.01; two studies, 868 women; Analysis 1.23).
1.23. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 23: Infant immunisation took place
Failure to thrive, abuse, neglect, and domestic violence from parents for any reason within 28 days after birth were not reported in any of the trials.
Outcomes that were not pre‐specified
Infant healthcare utilisation
Three studies reported the number of babies requiring urgent health care during the postnatal period, although the way this outcome was defined varied in the three studies (Bashour 2008a; Bashour 2008b reported hospital visits up to four months; Ransjo‐Arvidson 1998 reported referrals to paediatricians made by midwives at six weeks; and Christie 2011 reported use of emergency medical services up to eight weeks). Bashour 2008a, Bashour 2008b, and Christie 2011 described self‐referrals by parents of the infant, and Ransjo‐Arvidson 1998 described referrals made by midwives at a routine appointment. Overall, babies may be less likely to have additional medical care if their mothers received more postnatal home visits (RR 0.48, 95% CI 0.36 to 0.64; four studies, 1365 infants; Analysis 1.25).
1.25. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 25: Non prespecified ‐ Infant health care utilisation
Serious neonatal morbidity at six weeks was not reported in any trial.
One study reported on contraceptive use at 42 days postpartum; the evidence suggests that home visits have no effect on contraceptive use (RR 0.98, 95% CI 0.82 to 1.16; two studies, 856 women; Analysis 1.24).
1.24. Analysis.

Comparison 1: Schedules involving more versus fewer home visits, Outcome 24: Non prespecified ‐ Contraceptive use
Schedules comparing different models of postnatal care (three studies with 4394 women)
Three studies are included in this comparison. Each examined a different type of intervention and control condition, and we have not pooled findings in meta‐analyses. In brief, Steel 2003 compared two home visits compared with a telephone screening interview, with discretionary nurse home visits. In Kronborg 2007, health visitors (HVs) were randomised, and women were visited between one and three times by HVs who had attended special training on supporting breastfeeding, compared with usual care by HVs who had not been specially trained. MacArthur 2002 compared individualised postnatal care up to 10 to 12 weeks postpartum with usual care, which involved a more rigid schedule of midwife home visits in the early postnatal period.
For most of our prespecified outcomes, no data were reported in any of the three trials.
Primary outcomes
Maternal mortality up to 42 days postpartum
None of the studies reported on maternal mortality.
Neonatal mortality
In the study by MacArthur 2002, there were only three neonatal deaths from a sample of 2064 women. The study indicated that there may be little to no difference between treatment groups (RR 1.80, 95% CI 0.16 to 19.79; one study, 2064 women; Analysis 2.1).
2.1. Analysis.

Comparison 2: Schedules comparing different models of postnatal care at home, Outcome 1: Neonatal mortality
Secondary outcomes
None of the studies reported on maternal general morbidity, although MacArthur 2002 reported on the number of women with EPDS scores greater than 12 (the cut‐off used to denote high risk of postnatal depression) at four months postpartum. Women receiving individualised extended postnatal care were less likely to have EPDS scores ≥ 13 compared with women receiving routine care (RR 0.68, 95% CI 0.53 to 0.86; one study, 2064 women; Analysis 2.2).
2.2. Analysis.

Comparison 2: Schedules comparing different models of postnatal care at home, Outcome 2: Postnatal depression (EPDS ≥ 13 at 4 months postpartum)
Steel 2003 reported the number of babies with health problems up to four weeks; there appeared to be no difference between groups (RR 0.97, 95% CI 0.85 to 1.12; one study, 696 women; Analysis 2.3).
2.3. Analysis.

Comparison 2: Schedules comparing different models of postnatal care at home, Outcome 3: Neonatal morbidity up to 28 days
Breastfeeding
The cluster‐randomised trial by Kronborg 2007 examined the impact of care from HVs with special training to promote and support breastfeeding. The study suggested there may be little to no difference in the number of women who had stopped exclusive breastfeeding at six weeks (RR 0.81, 95% CI 0.58 to 1.14; one study, 647 women; Analysis 2.4). Few women in either group continued to exclusively breastfeed at six months and there was little to no evidence of difference between groups identified (RR 1.47, 95% CI 0.81 to 2.69; one study, 656 women; Analysis 2.5).
2.4. Analysis.

Comparison 2: Schedules comparing different models of postnatal care at home, Outcome 4: Stopped exclusive breastfeeding (last assessment up to 6 weeks)
2.5. Analysis.

Comparison 2: Schedules comparing different models of postnatal care at home, Outcome 5: Exclusive breastfeeding (last assessment up to 6 months)
In the study comparing home visits versus telephone screening (Steel 2003), most women in both groups were breastfeeding their babies at six weeks postpartum (any breastfeeding) and there was evidence of no difference between groups (RR 1.03, 95% CI 0.99 to 1.08; one study, 558 women; Analysis 2.6).
2.6. Analysis.

Comparison 2: Schedules comparing different models of postnatal care at home, Outcome 6: Any breastfeeding (up to 6 weeks)
None of our other pre‐specified infant outcomes were reported in any of these studies.
Home versus facility postnatal care (eight studies with 5179 women)
Eight studies compared women attending hospital clinics or a referral health service center for postnatal checks (usual care) versus home visits by nurses (Escobar 2001; Furnieles‐Paterna 2011; Gagnon 2002; Milani 2017; Mirmolaei 2014; Lieu 2000; Paul 2012; Salazar 2011).
Primary outcomes
Maternal mortality up to 42 days postpartum
None of these studies reported on maternal mortality.
Neonatal mortality
None of these studies reported on neonatal mortality.
Secondary outcomes
Maternal morbidity
Four studies reported on maternal use of emergency health care in the postnatal period, although there were some differences in definitions; Escobar 2001 and Lieu 2000 reported on the number of women making an urgent hospital visit up to two weeks, and Paul 2012 reported the number of women seeking unplanned emergency health care up to two weeks (RR 1.04, 95% CI 0.82 to 1.33, three studies, 3242 women; Analysis 3.1); Gagnon 2002 reported hospital admissions up to eight weeks postpartum and Lieu 2000 and Escobar 2001 hospital admissions within two weeks (RR 1.33, 95% CI 0.46 to 3.81, three studies, 2690 women; Analysis 3.2). Pooled results from these studies revealed little to no difference between women receiving home postnatal care versus hospital clinic postnatal care.
3.1. Analysis.

Comparison 3: Home versus facility postnatal care, Outcome 1: Severe maternal morbidity (emergency health care visits)
3.2. Analysis.

Comparison 3: Home versus facility postnatal care, Outcome 2: Severe maternal morbidity (hospital readmissions)
Maternal anxiety and depression
Three studies reported on the number of women with depressive symptoms at two weeks postpartum; there was little to no difference in numbers of women in the intervention and control groups who had symptoms (RR 1.10, 95% CI 0.93 to 1.30; two studies with 2177 women; Analysis 3.3). Milani 2017 reported on the number of women with severe postpartum depressive symptom defined by EPDS score at sixty days after delivery; and found little to no difference between groups (RR 0.86, 95% CI 0.34 to 2.16; Analysis 3.4).
3.3. Analysis.

Comparison 3: Home versus facility postnatal care, Outcome 3: Postnatal depression (last assessment up to 42 days postpartum)
3.4. Analysis.

Comparison 3: Home versus facility postnatal care, Outcome 4: Postpartum depression based EPDS at 60 days
Gagnon 2002 reported mean scores on the State Trait Anxiety Inventory (STAI) at two weeks. There was little to no difference between groups (MD 0.30, 95% CI ‐1.08 to 1.68, 513 women; Analysis 3.5).
3.5. Analysis.

Comparison 3: Home versus facility postnatal care, Outcome 5: Mean maternal anxiety score (last assessment up to 42 days postpartum)
Salazar 2011 reported anxiety and depression through the Hospital Anxiety and Depression Scale (HADS). The results appear to favour the home visit group; however, there may be little or no difference between the groups, as the wide confidence intervals do cross the line of no effect (RR 0.25, 95% CI 0.05 to 1.19; 430 women; Analysis 3.6)
3.6. Analysis.

Comparison 3: Home versus facility postnatal care, Outcome 6: Maternal anxiety and depression (HADS score)
Data on depression and anxiety were also collected in the Paul 2012 study. However, while the MDs between groups were set out, mean scores for women in the home and hospital groups were not reported and we were unable to enter data from this trial in our data and analyses tables. The authors reported no clear differences in mean EPDS or STAI scores at two weeks, two months and six months postpartum.
Satisfaction with care
In three studies (Escobar 2001; Furnieles‐Paterna 2011; Lieu 2000), women seemed to prefer home care rather than hospital clinic care; postnatal care was rated as good or excellent by 70% of women in the home care group compared with 54% in the clinic group (unweighted percentages). Satisfaction may be higher with home visits rather than hospital clinic care (there was high heterogeneity for this outcome: Tau² = 0.02; Chi² = 10.95, df = 2 (P = 0.004); I² = 82%); (RR 1.36, 95% CI 1.14 to 1.62; three studies; 2368 women; Analysis 3.7). Gagnon 2002 identified little to no difference in mean scores for satisfaction with postnatal care at eight weeks (MD ‐0.10, 95% CI ‐0.88 to 0.68; one study, 513 women; Analysis 3.8).
3.7. Analysis.

Comparison 3: Home versus facility postnatal care, Outcome 7: Maternal satisfaction with postnatal care
3.8. Analysis.

Comparison 3: Home versus facility postnatal care, Outcome 8: Mean satisfaction score with postnatal care
Breastfeeding
Five studies examined at least one outcome relating to breastfeeding. Gagnon 2002 reported the number of women exclusively breastfeeding at two weeks. There was little to no difference between groups (RR 1.05, 95% CI 0.93 to 1.18; one study, 513 women; Analysis 3.9). Escobar 2001 and Lieu 2000 reported the number of women who had discontinued any breastfeeding at two weeks; again, there was little to no difference between groups (RR 0.93, 95% CI 0.78 to 1.12; two studies, 2177 women; Analysis 3.10). Furnieles‐Paterna 2011 reported there may be little or no difference between home visits and hospital visits in the number of women who had discontinued breastfeeding within 30 days (RR 0.78, 95% CI 0.45 to 1.35; 185 women; Analysis 3.12). Paul 2012 examined the number of women breastfeeding at eight weeks postpartum, and slightly more women in the home visit group were still breastfeeding at this time (RR 1.09, 95% CI 1.00 to 1.18; one study, 1000 women; Analysis 3.11).
3.9. Analysis.

Comparison 3: Home versus facility postnatal care, Outcome 9: Exclusive breastfeeding (last assessment up to 6 weeks)
3.10. Analysis.

Comparison 3: Home versus facility postnatal care, Outcome 10: Discontinued breastfeeding (up to 6 weeks)
3.12. Analysis.

Comparison 3: Home versus facility postnatal care, Outcome 12: Discontinuation breastfeeding (up to 30 days)
3.11. Analysis.

Comparison 3: Home versus facility postnatal care, Outcome 11: Any breastfeeding (last assessment up to 6 months)
Outcomes that were not pre‐specified
Infant healthcare utilisation
Four studies reported on infant use of emergency health care; Escobar 2001 and Lieu 2000 reported on the number of infants re‐hospitalised within two weeks of initial discharge, and Gagnon 2002 reported infant hospital admissions up to eight weeks postpartum (RR 1.05, 95% CI 0.57 to 1.92, three studies, 2690 infants; Analysis 3.13). Escobar 2001, Lieu 2000 and Paul 2012 also reported the number of infants requiring urgent clinic visits or unplanned emergency health care up to two weeks (RR 1.15, 95% CI 0.95 to 1.38, three studies, 3257 infants,Analysis 3.12). Pooled results revealed little to no difference in infant health service use for women receiving hospital clinic versus home postnatal care.
3.13. Analysis.

Comparison 3: Home versus facility postnatal care, Outcome 13: Non prespecified ‐ Infant emergency health care visits
Serious neonatal morbidity at six months was not reported in any trial.
Planned subgroup and sensitivity analyses
It was not possible to conduct planned subgroup analyses due to few studies contributing data to any comparison, and the interventions in the included trials being too heterogenous. In future updates of the review, as more data become available, we will carry out planned additional analyses.
Similarly, planned sensitivity analysis by risk of bias was not performed; again, too few studies contributed data to any particular analysis to make such additional analyses meaningful.
Discussion
Summary of main results
In this review, we have included 16 randomised trials with data for 12,080 women. The trials were carried out in countries across the world, and in both high‐ and low‐resource settings. In low‐resource settings, women receiving usual care may have received no additional postnatal care after early hospital discharge.
The interventions and control conditions varied considerably across studies, with trials focusing on three broad types of comparisons: schedules involving more versus fewer postnatal home visits (five studies), schedules involving different models of care (three studies), and home versus hospital clinic postnatal check‐ups (eight studies). In all but two of the included studies, postnatal care at home was delivered by healthcare professionals. The broad aims of all interventions were to assess the well being of mothers and babies, and to provide education and support, although some interventions had more specific aims such as to encourage breastfeeding or to provide practical support.
For most of our outcomes, only one or two studies provided data, and overall results were inconsistent.
Schedules involving more versus fewer home visits
In the five studies comparing more versus less postnatal home visits, the evidence was very uncertain about the effects of home visits on maternal and neonatal mortality (Table 1). Only one study (which reported a large number of outcomes overall) reported results for most of our outcomes relating to maternal morbidity, and there was little evidence to suggest that more postnatal visits at home were associated with any improvements in maternal health.
Two studies examining maternal depression compared mean scores on the EPDS. Results suggested that women receiving more visits had higher mean scores, denoting an increased risk of depression, although the difference in score is probably not clinically meaningful. The reason for this finding is not clear. It is possible that women who had more contact with healthcare professionals may have been more willing to disclose their feelings. The authors of one trial (Morrell 2000) also speculated that increased provision of support may somehow disrupt women's usual support networks, or that the withdrawal of services may result in increased depression.
Two studies reported on maternal satisfaction with postnatal care. In one of these, additional health visitor support was associated with increased satisfaction scores, whilst in another, fewer visits were associated with slightly increased satisfaction. There was some evidence that postnatal care at home may reduce infant healthcare utilisation in the weeks following the birth, and that more home visits may encourage more women to exclusively breastfeed their babies. The evidence regarding any breastfeeding was less clear, although one study with a small sample size suggested that a home visit may encourage women to continue to breastfeed for a longer period. There was no strong evidence that infant morbidity, including jaundice and respiratory tract infections, was affected by home visits. In a single study, however, episodes of diarrhoea were reported less often by women in the groups receiving visits. This study reported a large number of outcomes, and as findings were not consistent, it is possible that this finding occurred by chance.
Schedules comparing different models of postnatal care at home
For the three studies comparing different ways of offering care involving postnatal home visits, it was not clear that interventions had a consistent effect, and many of our pre‐specified outcomes were not reported. There did not appear to be strong evidence from two studies that experimental interventions increased the number of women breastfeeding their babies. In one study, women in the experimental groups receiving an extended programme of home visits by midwives appeared to have lower EPDS scores at four months postpartum.
Home versus facility postnatal care
Eight studies examined home versus facility postnatal checks. There were no data reported for most of our outcomes. There was little or no difference between groups for maternal anxiety or depression. In two studies, women seemed to prefer home rather than hospital care, while a third study examining satisfaction with care did not identify any clear difference between groups. There was no strong evidence that home care was associated with an increase in breastfeeding, or that infant healthcare utilisation differed between groups.
Overall completeness and applicability of evidence
The studies included in the review examined different sorts of interventions in different types of settings, and drawing clear conclusions is not simple. The trials had a variety of aims, with some focusing on physical checks of the mother and newborn, while others specifically aimed to provide support for breastfeeding. One study included the provision of more practical support with housework and childcare. Under these circumstances, it is not surprising that results from the studies were not entirely consistent. This variation in aims was reflected in the choice of outcomes reported in different studies, and for most of our outcomes, there were very few data. Further, for outcomes such as breastfeeding there were differences in how and when outcomes were measured. Important clinical outcomes relating to maternal and infant health were mostly not reported, and for these outcomes results were dominated by a single study. Perhaps surprisingly, not all of the studies reported maternal satisfaction with different schedules or ways of offering care; those studies that did report maternal satisfaction provided some evidence that women preferred care at home. Improved maternal satisfaction with care involving home visits may be related to women's increased health awareness, support for behavioural change, and improved access to healthcare services; however, the evidence on maternal views is still limited. There was some evidence from two studies carried out in high‐resource settings that maternal depression scores were increased in women receiving more postnatal visits; the reasons for this finding are not clear, and this finding warrants further research attention in future trials and qualitative research.
Quality of the evidence
We assessed the certainty of the evidence for six outcomes for the main comparison (schedules involving more versus fewer postpartum visits) using GRADE (Table 1).
Overall, we assessed the certainty of evidence to range from low to very low. There is very low‐certainty evidence for the outcomes of maternal mortality and neonatal mortality, with our downgrading decisions relating to limitations in study design (risk of bias) and imprecision (wide 95% CIs and small numbers of events). There is low‐certainty evidence for the outcome of postnatal depression, with our downgrading decisions relating to very serious limitations in study design. There is low‐certainty evidence for the outcome of maternal satisfaction, with downgrading for very serious limitations in study design. Serious neonatal morbidity up to six months was not reported. There is low‐certainty evidence for the outcome of exclusive breastfeeding (last assessment up to six weeks), with downgrading again for very serious limitations in study design, due to the high risk of both selection bias and attrition bias.
Most of the results in the review are derived from one or two studies, and several of the studies had small sample sizes. We were unable to pool many of the data in meta‐analysis; there was a lack of consistency among studies in terms of the outcomes reported, and the time and manner in which outcomes were measured. In addition, there was considerable diversity in terms of the aims of interventions and the ways they were delivered. These differences mean that for any one outcome, there were few data, and most of our results were inconclusive.
Potential biases in the review process
We are aware that authors carrying out a review may themselves introduce bias. We took a number of measures to try to reduce bias; at least two review authors carried out data extraction and assessed risk of bias. All data were checked after entry. Nevertheless, assessing risk of bias (for example) requires individual judgements, and it is possible that a different review team may have made different assessments. We also acknowledge that we should have used the generic inverse variance method of analyses for one of the cluster trials (Christie 2011), since the trial had correctly adjusted their data. However, since the data from Christie 2011 was being combined in meta‐analysis, we decided to make adjustments to the sample size and/or events and combine the data with other parallel trials.
Agreements and disagreements with other studies or reviews
Generally, postnatal home visits seem likely to increase maternal satisfaction, promote breastfeeding, and reduce infant morbidities, but these effects are very much dependent upon the aims of the package of the postnatal interventions. The findings are in line with what a previous Cochrane Review has shown (Lassi 2015).
Authors' conclusions
Implications for practice.
The evidence is very uncertain about the effect of home visits on maternal and neonatal mortality. Individualised care as part of a package of home visits may improve depression scores at four months. Increasing the frequency of home visits may improve exclusive breastfeeding rates and reduce infant healthcare utilisation. Maternal satisfaction may also be better with home visits, compared to hospital check‐ups. Overall, the certainty of evidence was found to be low to very low, and findings were not consistent among studies and comparisons. The frequency, timing, duration and intensity of such home visits needs to be based upon local and individual needs.
Implications for research.
Further well‐designed randomised controlled trials or any other studies evaluating this complex intervention will be required to formulate the optimal package. The design of interventions in such a trial should be based upon postpartum health priorities in each context, which would determine the intensity and content of postnatal care visits. A core outcome set will be needed for future research.
Feedback
Feedback from MacArthur and Bick, March 2015
Summary
The findings of our study (MacArthur 2002) have been included in this review in the opposite direction to the results reported in our Lancet paper. The review states that the intervention group had worse (higher) EPDS scores than the control group, which is opposite to the actual findings.
The review concludes "Significantly more women receiving extended postnatal care had high EPDS scores (RR 1.47, 95% CI 1.13 to 1.92) (Analysis 2.9)". This is completely wrong as it was significantly FEWER, not more.
Similarly, the Discussion states “For the three studies comparing different ways of offering care involving postnatal home visits it was not clear that interventions had a consistent or positive effect, and many of our prespecified outcomes were not reported. There did not appear to be strong evidence from two studies that experimental interventions increased the number of women breastfeeding their babies. In one study, women in the experimental groups receiving an extended programme of home visits by health visitors appeared to have higher EPDS scores. The reason for this finding is not clear". Again this is incorrect.
This serious inaccuracy should be rectified, and the conclusions of the overall review amended.
Comment submitted by Christine MacArthur and Debra Bick, March 2015
Reply
Thanks to Professors MacArthur and Bick for this feedback. The feedback is correct ‐ in a previous version of this review data on postnatal depression scores for the MacArthur 2002 trial was entered the wrong way around and appeared to favour the control group. The review team apologises for this serious data entry mistake and the data have now been corrected. The text has also been amended in the abstract, the plain language summary, the main results section and the discussion, so that it is now clear that results from this trial show a reduction in depression scores in the group receiving an extended programme of home visits by health visitors.
Contributors
Reply from Naohiro Yonemo, Therese Dowswell, Shuko Nagai, and Rintaro Mori, April 2017
What's new
| Date | Event | Description |
|---|---|---|
| 19 May 2021 | New citation required but conclusions have not changed | Conclusions remain unchanged. |
| 19 May 2021 | New search has been performed | Search updated and four new studies included (Furnieles‐Paterna 2011; Milani 2017; Mirmolaei 2014; Salazar 2011). A GRADE 'Summary of findings' table has been incorporated and the evidence has been assessed using the GRADE approach. |
History
Protocol first published: Issue 9, 2011 Review first published: Issue 7, 2013
| Date | Event | Description |
|---|---|---|
| 6 April 2017 | Feedback has been incorporated | The authors have added a response to Feedback 1 |
| 6 April 2017 | Amended | In a previous version of this review data on postnatal depression scores for the MacArthur 2002 trial was entered the wrong way around and appeared to favour the control group. This has now been corrected (see Analysis 2.2). The text has also been amended in the abstract, the plain language summary, the main results section and the discussion, so that it is now clear that results from this trial show a reduction in depression scores in the group receiving an extended programme of home visits by health visitors. |
| 6 April 2017 | New citation required but conclusions have not changed | The review has been amended to correct an error in the data entered for the MacArthur 2002 trial. |
| 9 April 2015 | Feedback has been incorporated | Feedback 1 from Christine MacArthur and Deborah Bick. |
Acknowledgements
The authors would like to acknowledge the help received from the Cochrane Pregnancy and Childbirth Group and Thai Cochrane Network.
As part of the prepublication editorial process, this updated review has been commented on by three peers (an editor and two referees who are external to the editorial team), a member of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser. The authors are grateful to the following peer reviewers for their time and comments: Prof Valerie Smith, Prof in Midwifery, Trinity College Dublin, Ireland, and Rachel Plachcinski, Cochrane Consumer.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Evidence Synthesis Programme, the NIHR, National Health Service (NHS) or the Department of Health and Social Care.
We thank Therese Dowswell for her contribution as an author on previous versions of this review. We also thank Dr Seyedeh Tahereh Mirmolaei of Tehran University of Medical Sciences to translated and shared the information about her study in English. We also thank Erika Ota for her help with assessing the studies, contributing to analyses and preparing the manuscript for the latest update (2021). Finally we would like to thank Kerry Dwan, Statistical Editor, Cochrane Editorial and Methods Department, for her help in checking and adjusting the data for one of the cluster‐randomised trials (Christie 2011).
Appendices
Appendix 1. Search methods for ICTRP and ClinicalTrials.gov
ICTRP
postpartum AND home
postnatal AND home
postpartum AND visit*
postnatal AND visit*
postpartum AND midwife
postnatal AND midwife
postpartum AND nurse
postnatal AND nurse
ClinicalTrials.gov
Advanced search
postpartum | home
postnatal | home
midwife | home
nurse | home
Data and analyses
Comparison 1. Schedules involving more versus fewer home visits.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Maternal mortality within 42 days post‐birth | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.02, 9.41] |
| 1.1.1 More vs fewer visits (both groups had more than 4 visits) | 1 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.02, 9.41] |
| 1.2 Neonatal mortality | 3 | 1281 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.26, 3.69] |
| 1.2.1 Home visits vs no home visits | 2 | 873 | Risk Ratio (M‐H, Random, 95% CI) | 3.06 [0.37, 25.39] |
| 1.2.2 4 or more visits vs less than 4 | 1 | 408 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.09, 2.60] |
| 1.3 Severe maternal morbidity | 3 | 1228 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.81, 1.15] |
| 1.3.1 Home visits vs no home visits | 2 | 876 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.80, 1.17] |
| 1.3.2 4 or more visits vs less than 4 | 1 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.52, 1.54] |
| 1.4 Secondary postpartum haemorrhage | 2 | 873 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.49, 1.26] |
| 1.4.1 Home visits vs no home visits | 2 | 873 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.49, 1.26] |
| 1.5 Abdominal pain up to 42 days postpartum | 2 | 869 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.83, 1.34] |
| 1.5.1 Home visits vs no home visits | 2 | 869 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.83, 1.34] |
| 1.6 Back pain up to 42 days postpartum | 2 | 871 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.11] |
| 1.6.1 Home visits vs no home visits | 2 | 871 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.83, 1.11] |
| 1.7 Urinary tract complications up to 42 days postpartum | 2 | 876 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 1.7.1 Home visits vs no home visits | 2 | 876 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.63, 1.10] |
| 1.8 Maternal fever up to 42 days postpartum | 2 | 876 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.93, 1.82] |
| 1.8.1 Home visits vs no home visits | 2 | 876 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.93, 1.82] |
| 1.9 Dyspareunia | 2 | 869 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.90, 1.55] |
| 1.9.1 Home visits vs no home visits | 2 | 869 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.90, 1.55] |
| 1.10 Maternal perception of general health at 6 weeks (mean SF36) | 1 | 539 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐4.72, 1.52] |
| 1.10.1 More vs fewer visits (both groups had 4+ visits) | 1 | 539 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐4.72, 1.52] |
| 1.11 Mean postnatal depression score (last assessment up to 42 days postpartum) | 2 | 767 | Mean Difference (IV, Random, 95% CI) | 1.02 [0.25, 1.79] |
| 1.11.1 More vs fewer visits (both groups had 4+ visits) | 2 | 767 | Mean Difference (IV, Random, 95% CI) | 1.02 [0.25, 1.79] |
| 1.12 Mean maternal anxiety score (last assessment up to 42 days postpartum) | 1 | 280 | Mean Difference (IV, Random, 95% CI) | 3.80 [‐0.18, 7.78] |
| 1.12.1 More visits vs fewer than 4 (both groups had more than four visits) | 1 | 280 | Mean Difference (IV, Random, 95% CI) | 3.80 [‐0.18, 7.78] |
| 1.13 Maternal satisfaction with postnatal care | 2 | 862 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.90, 1.02] |
| 1.13.1 Home visits vs no home visits | 2 | 862 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.90, 1.02] |
| 1.14 Mean satisfaction score with postnatal care | 1 | 280 | Mean Difference (IV, Random, 95% CI) | 14.70 [8.43, 20.97] |
| 1.14.1 More visits vs fewer (both groups had more than 4 visits) | 1 | 280 | Mean Difference (IV, Random, 95% CI) | 14.70 [8.43, 20.97] |
| 1.15 Infant jaundice | 2 | 861 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.85, 1.26] |
| 1.15.1 Home visits vs no home visits | 2 | 861 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.85, 1.26] |
| 1.16 Infant respiratory tract infection within 42 days | 3 | 1217 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.84, 1.17] |
| 1.16.1 Home visits vs no home visits | 2 | 865 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.89, 1.15] |
| 1.16.2 4 or more visits vs less than 4 | 1 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.12, 1.22] |
| 1.17 Infant diarrhoea up to 42 days postpartum | 2 | 861 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.74, 0.98] |
| 1.17.1 Home visits vs no home visits | 2 | 861 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.74, 0.98] |
| 1.18 Exclusive breastfeeding (last assessment up to 6 weeks) | 3 | 960 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.01, 1.36] |
| 1.18.1 Home visits vs no home visits | 1 | 60 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [1.00, 3.23] |
| 1.18.2 4 or more visits vs less than 4 | 1 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [1.05, 1.22] |
| 1.18.3 More vs fewer visits (both groups had more than 4 visits) | 1 | 548 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.89, 1.51] |
| 1.19 Exclusive breastfeeding (last assessment up to 6 months) | 4 | 1309 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [1.10, 1.73] |
| 1.19.1 Home visits vs no home visits | 3 | 816 | Risk Ratio (M‐H, Random, 95% CI) | 1.50 [1.15, 1.94] |
| 1.19.2 More vs fewer visits (both groups had more than 4 visits) | 1 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.66, 1.69] |
| 1.20 Any breastfeeding (up to 6 weeks) | 2 | 807 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.88, 1.25] |
| 1.20.1 More vs fewer visits (both groups had more than 4 visits) | 2 | 807 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.88, 1.25] |
| 1.21 Any breastfeeding (last assessment up to 6 months) | 3 | 1315 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.99, 1.03] |
| 1.21.1 Home visits vs no home visits | 2 | 822 | Risk Ratio (M‐H, Random, 95% CI) | 1.01 [0.99, 1.04] |
| 1.21.2 More vs fewer visits (both groups had more than 4 visits) | 1 | 493 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.68, 1.38] |
| 1.22 Mean duration of any breastfeeding (months) | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 3.00 [2.33, 3.67] |
| 1.22.1 Home visits vs no home visits | 1 | 54 | Mean Difference (IV, Random, 95% CI) | 3.00 [2.33, 3.67] |
| 1.23 Infant immunisation took place | 2 | 868 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.96, 1.01] |
| 1.24 Non prespecified ‐ Contraceptive use | 2 | 856 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.82, 1.16] |
| 1.25 Non prespecified ‐ Infant health care utilisation | 4 | 1365 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.36, 0.64] |
| 1.25.1 Home visits vs no home visits | 2 | 748 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.38, 1.24] |
| 1.25.2 4 or more visits vs less than 4 | 1 | 352 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.28, 0.60] |
| 1.25.3 More vs fewer visits (both groups had more than 4 visits) | 1 | 265 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.23, 1.00] |
Comparison 2. Schedules comparing different models of postnatal care at home.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Neonatal mortality | 1 | 2064 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.16, 19.79] |
| 2.1.1 Flexible schedule vs routine visits | 1 | 2064 | Risk Ratio (M‐H, Random, 95% CI) | 1.80 [0.16, 19.79] |
| 2.2 Postnatal depression (EPDS ≥ 13 at 4 months postpartum) | 1 | 1295 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.53, 0.86] |
| 2.2.1 Flexible schedule vs routine visits | 1 | 1295 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.53, 0.86] |
| 2.3 Neonatal morbidity up to 28 days | 1 | 696 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.12] |
| 2.3.1 Home visit vs telephone screen | 1 | 696 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.85, 1.12] |
| 2.4 Stopped exclusive breastfeeding (last assessment up to 6 weeks) | 1 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.58, 1.14] |
| 2.4.1 Breastfeeding promotion vs routine visits | 1 | 647 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.58, 1.14] |
| 2.5 Exclusive breastfeeding (last assessment up to 6 months) | 1 | 656 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.81, 2.69] |
| 2.5.1 Breastfeeding promotion vs routine visits | 1 | 656 | Risk Ratio (M‐H, Random, 95% CI) | 1.47 [0.81, 2.69] |
| 2.6 Any breastfeeding (up to 6 weeks) | 1 | 558 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.99, 1.08] |
| 2.6.1 Home visit vs telephone screen | 1 | 558 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.99, 1.08] |
Comparison 3. Home versus facility postnatal care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Severe maternal morbidity (emergency health care visits) | 3 | 3242 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.82, 1.33] |
| 3.2 Severe maternal morbidity (hospital readmissions) | 3 | 2690 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.46, 3.82] |
| 3.3 Postnatal depression (last assessment up to 42 days postpartum) | 2 | 2177 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.93, 1.30] |
| 3.4 Postpartum depression based EPDS at 60 days | 1 | 276 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.34, 2.16] |
| 3.5 Mean maternal anxiety score (last assessment up to 42 days postpartum) | 1 | 513 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐1.08, 1.68] |
| 3.6 Maternal anxiety and depression (HADS score) | 1 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.05, 1.19] |
| 3.7 Maternal satisfaction with postnatal care | 3 | 2368 | Risk Ratio (M‐H, Random, 95% CI) | 1.36 [1.14, 1.62] |
| 3.8 Mean satisfaction score with postnatal care | 1 | 513 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.88, 0.68] |
| 3.9 Exclusive breastfeeding (last assessment up to 6 weeks) | 1 | 513 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.93, 1.18] |
| 3.10 Discontinued breastfeeding (up to 6 weeks) | 2 | 2177 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.78, 1.12] |
| 3.11 Any breastfeeding (last assessment up to 6 months) | 1 | 1000 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [1.00, 1.18] |
| 3.12 Discontinuation breastfeeding (up to 30 days) | 1 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.45, 1.35] |
| 3.13 Non prespecified ‐ Infant emergency health care visits | 3 | 3257 | Risk Ratio (M‐H, Random, 95% CI) | 1.15 [0.95, 1.38] |
| 3.14 Non prespecified ‐ Infant hospital readmissions | 3 | 2690 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.57, 2.36] |
3.14. Analysis.

Comparison 3: Home versus facility postnatal care, Outcome 14: Non prespecified ‐ Infant hospital readmissions
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aksu 2011.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial carried out in Aydin,Turkey. Duration of study: from March to July 2008. |
|
| Participants | 66 women who gave birth at Zübeyde Hanim Maternity Hospital located in Aydin,Turkey. Inclusion criteria: Mothers: (1) Being primiparous(giving birth to a live infant for the first time). (2) Giving birth by vaginal delivery. (3) Delivering a healthy newborn. (4) Birth occurring at gestational age of 37 weeks or more. (5) Giving birth to a singleton baby. (6) Providing informed consent. (7) Living in the city of Aydin (to make home visits more convenient). (8) Being able to communicate/speak in Turkish. (9) Not using any drugs that would be likely to affect breast milk. (10) Having an intention to breast feed. (11) Not having history of chronic diseases. (12) Not smoking. Exclusion criteria: Infants: (1) Lower than 2500 g at birth. (2) With an Apgar score of 7 or lower. (3) With congenital anomalies or serious disease. (4) Those needing intensive care. |
|
| Interventions | (1) Intervention group (n = 33): A single home visit 3 days after delivery from trained supporters and focusing on breastfeeding education. All women received standard breastfeeding education in the first few hours (within 24 hours) after delivery. (2) Control group (n = 33): Routine care which included breastfeeding education in the first few hours (within 24 hours) after delivery (no breastfeeding education at home on day 3 postpartum from supporters). |
|
| Outcomes | (1) Exclusive breastfeeding at 2 weeks.
(2) Exclusive breastfeeding at 6 weeks.
(3) Exclusive breastfeeding at 6 months.
(4) Duration of breastfeeding reported by the participants at 18 months after delivery.
(5) Duration of exclusive breastfeeding (months).
(6) Duration of breastfeeding (months).
(7) Breastfeeding knowledge scores at 2 weeks. (8) Breastfeeding knowledge scores at 6 weeks. |
|
| Notes | The study was carried out in a developing country. Dates of study: March and July 2008 Funding sources: unclear as not reported Declarations of interest: unclear as not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of computer‐generated random numbers. |
| Allocation concealment (selection bias) | Unclear risk | After the baseline interview, participants were randomly allocated to 1 of the 2 groups; the method used at the point of randomisation was not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible, but the authors state, "obviously, the intervention could not be blinded, however, those obtaining the outcomes could have been blinded to the patient groups". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The authors state, "obviously, the intervention could not be blinded, however, those obtaining the outcomes could have been blinded to the patient groups". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not ITT analysis. There was some loss to follow‐up (Intervention group: 3+3 /33, Control group: 3+3 /33). 54/66 were followed up at 18 months (82%). |
| Selective reporting (reporting bias) | High risk | The primary outcome was not pre‐defined in the methods. |
| Other bias | Low risk | Groups appeared comparable at baseline. No other bias apparent. |
Bashour 2008a.
| Study characteristics | ||
| Methods | This was a 3‐arm trial involving 2 intervention groups. in order to include all the data from the trial we have treated it as 2 studies with the control group data shared between each study. In the Bashour 2008a arm women received 4 visits (vs no visits). In Bashour 2008b women received 1 visit (vs no visits). Study design: randomised controlled trial carried out in Damascus, Syria. Women were recruited between June and December 2004. |
|
| Participants | 903 women who had recently given birth at the Maternity Teaching Hospital in Damascus, Syria. Inclusion criteria: (1) Women who delivered a healthy newborn whether by vaginal delivery or caesarean section. (2) Women who lived within 30 km from the hospital. (3) Women who were available for the follow‐up for the coming 6 months. Exclusion criteria: (1) Women who delivered prematurely. (2) Women who delivered babies with low birthweight (< 2500 g). (3) Women who delivered babies with apparent congenital anomalies. |
|
| Interventions | The intervention consisting of home visits aimed to examine, follow‐up, educate, support, and counsel women who had recently given birth. (1) Group A (n = 301): (Initiation: ≤ 48 hours, Duration: ≥ 3 weeks, Intensity: > 1/week). 4 postnatal home visits by registered midwives; on days 1, 3, 7, and 30. (2) Group B (n = 301): (Initiation: > 48 hours, Duration: < 3 weeks, Intensity: < 1/week) 1 postnatal home visit by registered midwives; on day 3. (3) Group C (n = 301): (No home visit: Initiation: NA, Duration: NA, Intensity: NA). Current standard of care in Syria (no visit following hospital discharge). |
|
| Outcomes | Primary outcomes: (1) Maternal postpartum morbidities at 4 months postpartum. (2) Postnatal care uptake at 4 months postpartum. (3) Contraceptive uptake and type at 4 months postpartum. (4) Infant morbidities at 4 months of life. (5) Infant immunisation according to the national schedule at 3 months. (6) Infant feeding, namely exclusive breastfeeding during the first 4 months of life. Secondary outcomes: (7) Women's perceptions of their health, their impressions about the home visit/s and perceptions of the quality of care. |
|
| Notes | The study was carried out in a developing country. Dates of study: not reported Funding sources: Regional Changing Childbirth Research Program at Faculty of Health Sciences, American University of Beirut; supported by Wellcome Trust grant, Beirut, Lebanon Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that randomisation was in blocks of 7, but it was not clear how the sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Numbered opaque and sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were reported to be blinded to the group assignment. However, it was evident that the assessors were able to tell whether women had received home visits or not from the interviews. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not ITT analysis. 16 of Group A, 7 Group B, 4 Group C were excluded. |
| Selective reporting (reporting bias) | High risk | Assessment from published study report. Primary outcomes were pre‐defined with 6 measures, but sample size calculation was based on maternal morbidity only. |
| Other bias | Low risk | Other bias not apparent. |
Bashour 2008b.
| Study characteristics | ||
| Methods | This was a 3‐arm trial involving 2 intervention groups. in order to include all the data from the trial we have treated it as 2 studies with the control group data shared between each study. In the Bashour 2008a arm women received 4 visits (vs no visits). In Bashour 2008b women received 1 visit (vs no visits). Study design: randomised controlled trial carried out in Damascus, Syria. Women were recruited between June and December 2004. |
|
| Participants | 903 women who had recently given birth at the Maternity Teaching Hospital in Damascus, Syria. Inclusion criteria: (1) Women who delivered a healthy newborn whether by vaginal delivery or caesarean section. (2) Women who lived within 30 km from the hospital. (3) Women who were available for the follow‐up for the coming 6 months. Exclusion criteria: (1) Women who delivered prematurely. (2) Women who delivered babies with low birthweight (< 2500 g). (3) Women who delivered babies with apparent congenital anomalies. |
|
| Interventions | The intervention consisting of home visits aimed to examine, follow‐up, educate, support, and counsel women who had recently given birth. (1) Group A (n = 301): (Initiation: ≤ 48 hours, Duration: ≥ 3 weeks, Intensity: > 1/week) 4 postnatal home visits by registered midwives; on days 1, 3, 7, and 30. (2) Group B (n = 301): (Initiation: > 48 hours, Duration: < 3 weeks, Intensity: < 1/week) 1 postnatal home visit by registered midwives; on day 3. (3) Group C (n = 301): (No home visit: Initiation: NA, Duration: NA, Intensity: NA) Current standard of care in Syria (no visit following hospital discharge). |
|
| Outcomes | Primary outcomes: (1) Maternal postpartum morbidities at 4 months postpartum. (2) Postnatal care uptake at 4 months postpartum. (3) Contraceptive uptake and type at 4 months postpartum. (4) Infant morbidities at 4 months of life. (5) Infant immunisation according to the national schedule at 3 months. (6) Infant feeding, namely exclusive breastfeeding during the first 4 months of life. Secondary outcomes: (7) Women's perceptions of their health, their impressions about the home visit/s and perceptions of the quality of care. |
|
| Notes | The study was carried out in a developing country. Dates of study: not reported Funding sources: Regional Changing Childbirth Research Program at Faculty of Health Sciences, American University of Beirut; supported by Wellcome Trust grant, Beirut, Lebanon Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated that randomisation was in blocks of 7, but it was not clear how the sequence was generated. |
| Allocation concealment (selection bias) | Low risk | Numbered opaque and sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome assessors were reported to be blinded to the group assignment. However, it was evident that the assessors were able to tell whether women had received home visits or not from the interviews. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not ITT analysis in all tables. 16 of Group A, 7 Group B, 4 Group C were excluded. |
| Selective reporting (reporting bias) | High risk | Assessment from published study report. Primary outcomes were pre‐defined with 6 measures, but sample size calculation was based on maternal morbidity only. |
| Other bias | Low risk | Other bias not apparent. |
Christie 2011.
| Study characteristics | ||
| Methods | Cluster‐randomised controlled trial in Northern Ireland, United Kingdom. | |
| Participants | 102 eligible health visitors, 976 first‐time 'low risk' mothers were recruited, and 295 mothers agreed to take part and completed baseline assessment. Inclusion criteria: (1) Given birth during 2002–2004. (2) Agreed to take part in the study (visited by a health visitor). Exclusion criteria: (1) History of family violence. (2) Parent indifference towards baby. (3) Lone parent. (4) Mother under 19 years old. (5) History/current mental illness or physical illness/disability‐parent. (6) Drug or alcohol addiction. (7) Parent abused or neglected as a child. (8) Infant premature (pre 37 weeks). (9) Infant learning difficulty or severe physical illness. (10) Low‐birth weight baby (under 2500 g) or multiple birth. (11) Previous stillbirth. (12) Pressures on family unit (intense). (13) Dfficulty understanding English. |
|
| Interventions | (1) Intervention group: (n = 453 first‐time 'low risk' mothers were recruited, and 136 mothers agreed to take part). (Initiation: > 48 hours, Duration: ≥ 3 weeks, Intensity: ≥ 1/week) 6 home visits from 10–14 days to 8 weeks postpartum, i.e. weekly home contacts by health visitors who provided support, carried out assessments and offered health promotion. At 8 weeks data were available for 129 women; at 7 months postpartum data were available for 115 women. (2) Control group: (n = 523 first‐time 'low risk' mothers were recruited, and 159 mothers agreed to take part). (Initiation: > 48 hours, Duration: < 3 weeks, Intensity: < 1/ week) 1 home visit from 10 to 14 days postpartum home visit (the standard frequency of home visits). Any further visits were discretionary. At 8 weeks data were available for 151 women; at 7 months postpartum data were available for 141 women. |
|
| Outcomes | Primary outcomes: (1) The EPDS at 8 weeks and 7 months postpartum. Secondary outcomes: (2) Role restriction sub‐scale of Parenting Stress Index at 8 weeks and 7 months postpartum. (3) Perceived stress index at 8 weeks and 7 months postpartum. (4) Maternal physical health wellbeing rating at 8 weeks and 7 months postpartum. (5) Baby nurture (parenting difficulty with baby's crying, sleeping, physical health and feeding, and breastfeeding) at 8 weeks and 7 months postpartum. (6) Satisfaction with health visiting service ‐ surgery satisfaction questionnaire at 8 weeks and 7 months postpartum. (7) Attending family doctor for baby at 8 weeks and 7 months postpartum. (8) Use of emergency medical services for baby at 8 weeks and 7 months postpartum. (9) Self‐efficacy (Parenting Expectations Survey ‐ PES) at 8 weeks and 7 months postpartum. |
|
| Notes | Dates of study: 2002‐4 Funding sources: this research was funded by a Special Nursing Fellowship awarded from the Research and Development Office of the Department of Health, Social Services and Public Safety, Northern Ireland Declarations of interest: unclear as not reported Sample size and or events were adjusted for the data from this trial with help from Kerry Dwan, Cochrane Central Methods (see table) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random number table was used for randomisation of health visitor. All families with newborn infants in Northern Ireland are systematically and routinely allocated to a health visitor according to each family's doctor, geographical location and/or last name. |
| Allocation concealment (selection bias) | Low risk | Cluster‐randomised trial. Each health visitor was assigned a code' and this code was used to conceal identity during the randomisation process. Allocation was undertaken by clerical staff associated with a computerised Child Health System which directly receives notification of all births directly from maternity services. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. Due to ethical and program considerations, it was not possible to hide study allocation from mothers or health visitors once randomisation had occurred. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not ITT analysis. Of 102 eligible health visitors 3 were excluded and a further 20 did not refer any women to the trial. In addition of 976 eligible women, 295 completed baseline assessment and 256 were followed up at 7 months. |
| Selective reporting (reporting bias) | Low risk | The primary outcome was pre‐defined in the methods. |
| Other bias | Unclear risk | Baseline characteristics of women of women in the 2 groups appeared similar. Cluster design effect was considered in the analysis. There was some variation amongst health visitors in terms of the way the intervention was delivered and women's outcomes. |
Escobar 2001.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial carried out in a private hospital in Santa Clara, California, USA. (Duration of the study: a 17‐month period in 1998 to 1999.) |
|
| Participants | 1014 mother–infant pairs. Eligibility criteria: (1) Mother–infant pairs whose hospital length of stay (LOS) was expected to be 48 hours or less. (2) Based on the hospital's clinical protocol for selecting mothers and newborns at low medical and social risk. Exclusion criteria: (1) Infant weighed < 2500 or > 4600 g at birth. (2) Infant was < 36 or > 42 weeks' gestation. (3) Infant was admitted to the intensive care nursery. (4) Mother or the infant had a medical problem that warranted special follow‐up by a paediatrician or a nurse practitioner. (5) By clinical protocol, paediatricians ordered complete blood counts only for newborns with medical problems (e.g. “rule out sepsis”). (6) Newborns with a haematocrit of < 40 or an absolute neutrophil count of < 7000 at any time. (7) Mothers and newborns whose anticipated LOS was > 48 hours,usually because of caesarean delivery. (8) Mother was 14 years old or younger; was 15 to 17 years old without a parent or a guardian available for informed consent. (9) Mother had a positive toxicology screen for drugs of abuse after admission to labour and delivery. (10) A social worker had requested, before eligibility assessment for the study, that a home visit be done. (11) Mother did not speak English. (12) Infant did not have Kaiser Foundation Health Plan, Inc, coverage. (13) Infant was being adopted. (14) Family lived outside the area served by the home health nurses. (15) Family was not reachable by telephone. (16) Family was in the process of moving. * For those mother–infant pairs with multiple reasons for exclusion, we recorded only the first reason for exclusion on a hierarchically ordered list of exclusions. |
|
| Interventions | (1) Intervention (home nurse visit) group (n = 508): (Initiation: > 48 hours, Duration: < 3 weeks, Intensity: < 1/week) A single home health visit within 48 hours after hospital discharge by a registered nurse from the KPMCP Home Health department. (2) Control (hospital‐based follow‐up) group (n = 506): (No home visit: Initiation: NA, Duration: NA, Intensity: NA) Usual hospital‐based follow‐up care. Women were allocated to receive a 1‐2 hour group‐based visit (in groups of 5‐8). Women were offered newborn checks and guidance. Multiparous women could opt for a 15 minute paediatric clinic visit within 48 hours of the birth. This visit may also have included some guidance and education. |
|
| Outcomes | Primary outcomes: (1) Combined clinical outcome measure that was considered present if either the mother or the newborn experienced any of the following. a) Re‐hospitalisation, emergency department use, or urgent clinic visit use within 10 days after delivery. b) Occurrence of maternal depressive symptoms as documented by a telephone interview 2 weeks after delivery; and/or c) Discontinuation of breastfeeding as documented by a telephone interview 2 weeks after delivery. Secondary outcomes: (2) Maternal satisfaction was assessed. (3) The average regional costs of these services were derived using the KPMCP's computerised Cost Management Information System, which estimates the costs of each unit of service. |
|
| Notes | Data from the Kaiser Permanente Medical Care Program (KPMCP). Dates of study: 1998‐1999 Funding sources: Grant MCJ #R40 MC 0010303 from the Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services; Grant #9003 from the Sidney Garfield Memorial Fund; Grant #970005 from the Innovation Program of The Permanente Medical Group, Inc.; and Grant #1998‐6861 from the David and Lucile Packard Foundation's Center for the Future of Children. Declarations of interest: unclear by no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study group assignments determined in advance by a random number generator. |
| Allocation concealment (selection bias) | Low risk | A series of sealed, opaque, sequentially numbered envelopes containing allocations. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | 1 of investigators who was kept blinded to group assignment, reviewed all re‐hospitalisations using objective criteria. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was less than 5% attrition. |
| Selective reporting (reporting bias) | Unclear risk | The primary outcome was pre‐defined in the methods. Data source of cost analysis was unclear. |
| Other bias | Unclear risk | Baseline (household income) was unbalanced. |
Furnieles‐Paterna 2011.
| Study characteristics | ||
| Methods | "Randomised comparative study" Appears to be quasi randomised depending on addresses of women | |
| Participants | 200 Primiparous women discharged in the first 72 hours after birth | |
| Interventions | (1) Experimental group (n = 100): Puerperal home visit during the first 48 hours after discharge, and then the usual check‐up carried out in the Health Center (2) Control group (n = 100): Puerperal Visit in their Health Center |
|
| Outcomes | Telelphone survey conducted between 30 and 40 days postpartum. Data were collected on the mother's clinical course and the neonate (appearance of complications, use of emergency services and other assistance services, care postpartum workshops, type of breastfeeding, duration and reason for abandonment of BF) and on the degree of mother satisfaction | |
| Notes | Study dates: Not reported Study funding sources: Not reported Study authors’ declarations of interest: Not reported Ethical approval obtained? Not reported Study prospectively registered? Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomised dependent on address of woman |
| Allocation concealment (selection bias) | Unclear risk | Not reported in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported in paper |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "In order to avoid observation bias, the telephone survey was carried out by a different midwife than the one carried out by the VP." Not clear if the midwife was aware of the allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3% lost to follow‐up in intervention group. 0% lost in intervention group. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available to assess |
| Other bias | Unclear risk | Not known |
Gagnon 2002.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial carried out at a university teaching hospital (3700 births/year)
and affiliated community health centres in Montreal, Quebec, Canada. Duration of the study: from January 1997 to September 1998. |
|
| Participants | 586 healthy mother‐infant pairs. Inclusion criteria: (1) Infant breast fed at least once in the hospital. (2) Living in a defined catchment area proximal to the hospital. (3) Mothers and newborn infants participated in the short stay program when certain health and psychosocial criteria were met. The program included discharge within 36 hours of birth, telephone follow‐up, and a hospital nurse clinic visit. Exclusions criteria: (1) Women not eligible for the short stay program including those having caesarean birth. (2) Parity ≥ 5. (3) Blood loss at birth ≥ 500 mL. (4) More than second‐degree perineal tear. (5) Maternal inability to void adequately. (6) Non receipt of indicated RhoGAM. (7) Mother unable to care for self or infant. (8) Multiple birth. (9) Birthweight < 2500 g. (10) Gestational age < 37 weeks. (11) Abnormal neonatal examination. (12) Infant unable to maintain body temperature. (13) Breastfeeding not tolerated in hospital. (14) Language barrier. (15) The need for social services referral. *The only exclusion criterion for this study was non‐participation in the short‐stay program. |
|
| Interventions | (1) Experimental (Community follow‐up) group (n = 292): (Initiation: > 48 hours, Duration: < 3 weeks, Intensity: < 1/week) Women in both the experimental and control groups received a 48‐hour postpartum telephone contact. In addition women in the experimental group received a community nurse visit at 3 to 4 days postpartum in the woman's home. Nurse contacts continued when community follow‐up was judged to be required. (2) Control (Hospital follow‐up) group (n = 294): (No home visit: Initiation: NA, Duration: NA, Intensity: NA) 48‐hour postpartum telephone contact and a hospital clinic visit at 3 to 4 days postpartum. |
|
| Outcomes | Primary outcomes: (1) Breastfeeding frequency at 2 weeks' postpartum by maternal diary. (2) Infant weight gain at 2 weeks postpartum by research assistants using digital scales. (3) Maternal anxiety at 2 weeks postpartum using the STAI. (4) Post discharge service satisfaction at 2 weeks postpartum using the Client Satisfaction Questionnaire. (5) Health and community services use at 2 months postpartum using a diary and medical record review. Secondary outcomes: (6) Insufficient breastfeeding(defined by us as < 4.5 feeds per day). (7) Type of feeding (breastfeeding, formula, or mixed). (8) Birthweight not regained at follow‐up. |
|
| Notes | Dates of study: between January 1997 and September 1998. Funding sources: the Fonds de la recherche en santé au Québec (FRSQ), Canada. Drs Gagnon and Dougherty are research scholars of the FRSQ. At the time of this study, Dr Gagnon was also a research scholar of the Fondation de recherche en sciences infirmières du Québec. Dr Leduc is a research scholar of the National Health Research and Development Program of Health Canada. Declarations of interest: unclear (no information) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were stratified by parity in blocks of 8 using a computer‐generated table of random numbers. |
| Allocation concealment (selection bias) | Low risk | By telephone within 24 hours of hospital discharge with notification of group assignment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible, given the nature of the intervention, masking of the women and health professionals was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Research assistants, blind to both treatment group and research questions, collected all data. Different research assistants collected outcome data and notified women and clinicians of group assignment; outcome assessors were blind to group assignment and during any contact with subjects were instructed to ask subjects not to divulge their group status. Furthermore, research hypotheses were not divulged to the research assistants. Outcome data were not collected by clinical staff. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were some missing data (about 15%), but it was relatively balanced. |
| Selective reporting (reporting bias) | Unclear risk | The main outcomes measured were breastfeeding frequency and infant weight gain assessed at 2 weeks postpartum (abstract only). |
| Other bias | High risk | There were differences between groups for some baseline characteristics. |
Kronborg 2007.
| Study characteristics | ||
| Methods | A community‐based cluster‐randomised trial in western Denmark. | |
| Participants | 22 municipalities clusters randomised, 1597 mothers recruited. | |
| Interventions | (1) Intervention group (n = 781, 11 clusters). (Initiation: < 48 hours, Duration: ≥ 3 weeks, Intensity: < 1/week) The intervention included special health visitor training focusing on promoting breastfeeding. Mothers received 1–3 home visits during the first 5 weeks postpartum. The intervention addressed maternal psychosocial factors. The first visit was scheduled as soon as possible after coming home from the hospital. Mothers who were primipara and multipara with previously short breastfeeding experience more likely to be selected for further support; the 2 additional visits within the first 5 weeks after delivery. In addition, mothers received an informative booklet about breastfeeding. (2) control group (n = 816, 11 clusters). (No home visit: Initiation: NA, Duration: NA, Intensity: NA) (health visitors received no additional training.) Mothers offered the health visitor's usual practice consisting of 1 or more non‐standardised visits. |
|
| Outcomes | Primary outcome: (1) Duration of exclusive breastfeeding during 6 months of follow‐up. Secondary outcome: (2) Mother's satisfaction with the breastfeeding period. |
|
| Notes | Dates of study: between January 2004 and August 2004 Funding sources: The Health Insurance Foundation, The Lundbeck Foundation and The Counties of Ribe and Ringkjobing in Denmark. Declarations of interest: unclear by no information Clinical Trial.gov (ClinicalTrials.gov Identifier: 00145834). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation was computerised. |
| Allocation concealment (selection bias) | Low risk | Cluster randomisation was carried out by staff not involved in the project. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible (the randomisation was computerised and done independently of the investigators, and the identity of the health visitors was blinded to the investigators). |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly described. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were some discrepancies in the numbers reported in the text and tables. There was no loss of clusters reported. Response rate was 84%. |
| Selective reporting (reporting bias) | Low risk | 2 primary outcomes were pre‐defined in the methods. |
| Other bias | Low risk | Groups appeared similar at baseline and analysis took account of the cluster design effect. |
Lieu 2000.
| Study characteristics | ||
| Methods | Randomised controlled trial carried out in the Kaiser Foundation hospital linked 5 outpatient clinics in California, USA between 1996‐1997 | |
| Participants | 1163 mother‐newborn pairs. Inclusion criteria: (1) Hospital length of stay was expected to be 48 hours or less based on the hospital's clinical protocol for selecting mothers and newborns at low medical and social risk. Exclusion criteria: (1) If mother‐newborn pairs planned to receive follow‐up at non‐study clinics. (2) Medical reasons if the infant weighed < 2500 or > 4600 g at birth, or had stayed in the intensive care nursery. (3) If the mother or infant had a medical problem that warranted follow‐up by a paediatrician or nurse practitioner. (4) Mothers and newborns whose anticipated length of stay was > 48 hours, usually due to caesarean delivery. (5) Potential participants for social reasons if the mother was 14 years old or younger; was 15 to 17 years old without a parent or guardian available for informed consent; had a positive toxicology screen for drugs of abuse after admission to labour and delivery; or if a social worker had requested, before eligibility assessment for the study, that a home visit be done. (6) If the mother spoke a language other than English or Spanish, the newborn was not covered by the health maintenance organisation (HMO) or was being adopted. (7) The family lived outside the area served by the home health nurses, was not reachable by telephone, or was in the process of moving. |
|
| Interventions | (1) Home visit group A (n = 580): (Initiation: > 48 hours, Duration: < 3 weeks, Intensity: < 1/week) A home visit within 48 hours after hospital discharge by a registered nurse or public health nurse from the HMO's home health department. The clinical protocol and a standardised charting form specified the recommended elements of history, physical examination, and anticipatory guidance for the home visits, which were intended to last 60 to 90 minutes. Women were not offered follow‐up at the hospital clinic. (2) Control group B (n = 583): (Initiation: NA, Duration: NA, Intensity: NA) Usual follow‐up care, a 20‐minute paediatric clinic visit within 48 hours after hospital discharge. Nurse practitioners and paediatricians at 4 clinics conducted the visits, which included history and physical examination of the newborn, anticipatory guidance, and laboratory testing if indicated. |
|
| Outcomes | (1) Re‐hospitalisation, urgent clinic visit by the mother or newborn within 2 weeks. (2) Maternal urgent clinic visit within 6 weeks. (3) Breastfeeding discontinuation at 2 weeks. (4) Breastfeeding discontinuation at 12 weeks. (5) Maternal depressive symptoms at 2 weeks. (6) Maternal satisfaction at 2 weeks. (7) Costs of home visits and paediatric clinic follow‐up visits given on the third or 4th postpartum day to low‐risk mothers and newborns with postpartum hospital stays of 48 hours or less. |
|
| Notes | Dates of study: between July 1996 and September 1997 Funding sources: the Innovation Program of Kaiser Permanente, Northern California, Grant MCJ 067951 from the Maternal and Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services, and the Agency for Healthcare Research and Quality Declarations of interest: unclear by no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study group assignments determined in advance by a random number generator. |
| Allocation concealment (selection bias) | Low risk | Using a series of sealed, opaque, sequentially numbered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | 2 paediatrician investigators assigned severity of illness for each newborn re‐hospitalisation after blinded review other data were collected by interviewers and may have been susceptible to bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing cases are 6 at 2 weeks and 11 at 12 weeks in home visit group, 10 at 2 weeks and 18 at 12 weeks in control group (< 5% attrition). |
| Selective reporting (reporting bias) | High risk | The primary outcome was not pre‐defined in the methods. |
| Other bias | High risk | Many baseline characteristics were unbalanced (race, education, household income, initiated prenatal care in first trimester). |
MacArthur 2002.
| Study characteristics | ||
| Methods | Cluster‐randomised controlled trial carried out in the general practice from the West Midlands health region in United Kingdom. | |
| Participants | 37 general practice clusters randomised, 36 clusters recruited, 3580 women eligible, and 2064 women recruited. Eligible criteria: (1) If they had postnatal care in the recruited practices between October 1997 and April 1999. (2) Women were informed about the study between 34 weeks' gestation and the first home visit. (3) Written informed consent was obtained. Exclusion criteria: (1) Women who expected to move out of the general practice in the postnatal period. |
|
| Interventions | (1) Home visits group A (clusters = 18 randomised, 17 recruited, n = 1830 eligible, 1087 recruited): (Initiation: > 48 hours, Duration: ≥ 3 weeks, Intensity: < 1/week) Community‐based postnatal care meant that care could be tailored flexibly to individual needs. Care was led by midwives, with contact with general practitioners based on referral, including home visits and the final discharge consultation. To ensure that specific needs could be identified, even if not spontaneously reported by the women or observed by the midwife, a symptom checklist was used at the first visit (immediate symptoms only), at days 10 and 28, and at the discharge consultation and at 10–12 weeks. "The Edinburgh Postnatal Depression Scale (EPDS) was also used to screen for depression at day 28 and at the discharge consultation. Care plans were made and visits scheduled on the basis of these results so that care could be tailored to individual needs rather than based on a predetermined schedule. we extended care so that the last home visit was routinely at 28 days and women had their discharge consultation at 10–12 weeks." (2) Home visits group B (Cluster = 19 randomised and recruited, n = 1750 eligible, 977 recruited): (Initiation: > 48 hours, Duration: ≥ 3 weeks, Intensity: ≥ 1/week) Usulal postnatal care generally consists of about 7 midwife home visits up to 10–14 days (can continue to day 28) after birth, and care from health visitors thereafter. General practitioners did routine home visits and a final 6–8 week check. |
|
| Outcomes | Primary outcome: (1) The women's health and wellbeing (summary physical and mental component scores (PCS and MCS)) of the short form 36 (SF36) general health questionnaire at 4 months. (2) EPDS at 4 months. Secondary outcome: (3) Women' views about care (overall satisfaction and others). |
|
| Notes | Dates of study: between Oct 1997 to April 1999 Funding sources: UK national health service research and Development HTA programme Declarations of interest: written as "None declared" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A member of the Birmingham Clinical Trials Unit who was independent of the trial team, with a customised computer program, Minimisation method. |
| Allocation concealment (selection bias) | Low risk | A member of the Birmingham Clinical Trials Unit who was independent of the trial team, with a customised computer program, Minimisation method. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible, masking of health professionals or participants was not possible. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Questionnaires were returned to the study office in prepaid envelopes. Masking of participants to the interventions was not possible. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not ITT analysis, 1 cluster was excluded following randomisation in the home visit group, 743 not recruited of 1830, 286 of 1087 not returned at 4 month in the home visit group, 773 not recruited of 1750, 255 of 977 not returned at 4 month in the control group. |
| Selective reporting (reporting bias) | Low risk | 2 primary outcomes were pre‐defined in the methods. |
| Other bias | Low risk | Groups appeared comparable at baseline. Analysis took account of the cluster design effect. |
Milani 2017.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial carried out in 4 affiliated hospitals (Taleghani, Shohada, Mahdie, and Imam Hossein) of Shahid Beheshti University in Iran. | |
| Participants | 276 women who had delivered in these hospitals. (282 women randomised.) Eligible criteria: (1) Not having a chronic disease. (2) A single, normal weight neonate without congenital disorders. (3) EPDS score of < 10 (having depression), not having a history of depression, and not taking antidepressants. If EPDS scores were of < 10 or had suicidal thoughts, they were excluded the study and referred to a psychiatrist. (4) Iranian nationality. Exclusion criteria: (1) Unwillingness to continue the study. (2) Migration from the area of study. |
|
| Interventions | (1) Intervention group (n = 92) (94 women randomised) (Intiaion: > 48 hours, Duration: < 3 week, Intensity: ≥ 1/week) The intervention was the postpartum health care providing at home on the 3–5th and 13–15th day after delivery according to the designed guideline. Healthcare providers were educated midwives. The average visit time was 30–45 minutes which would change with mothers' request. The intervention included greeting and recording checklists which were filled by midwives after interviewing and examining the mother and infant on each visit. (2) Control group (n = 184) (188 women randomised) (No home visit: Intiation: NA, Duration: NA, Intensity: NA) Usual hospital‐based care, if requested. (It was only stated "lack of home visit".) |
|
| Outcomes | EPDS, cut‐off of score was set as mild (< 10), moderate (10‐13) and severe (> 13). | |
| Notes | The study was carried out in a developing country. The trial was registered as IRCT 2013060313565N1. Dates of study: between July 2013 and October 2013 Funding sources: none Declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | It was stated "randomly" but it was not clear how the sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | It was not clear how to do the allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | It was not clearly stated, but carried out ITT analysis. 2 women were with loss to follow‐up in both groups and 2 women was with missing data for outcome in control group. |
| Selective reporting (reporting bias) | Unclear risk | Assessment from published study report. Primary outcome was not clearly stated, but an outcome (EPDS) was only reported and the sample size was calculated in the report. |
| Other bias | Unclear risk | Proportion of Level of education and delivery type in baseline were slightly unbalanced. |
Mirmolaei 2014.
| Study characteristics | ||
| Methods | Study design: randomised controlled trial carried out in a reference center for screening infant hypothyroidism in Tehran, Iran. | |
| Participants | 200 mothers were recruited who had recently given birth during September‐December 2010. Eligible criteria: (1) A woman who had a healthy and term newborn in her recent low‐risk pregnancy (2) Recruited between 3‐5 days after delivery, received the first postpartum care in health service centers by a general physician and a dentist (3) Ability to discuss and understand the Persian language, and being a resident of any of 10, 11 or 17 zones of Tehran metropolitan and the first or second birth order for her infant. Exclusion criteria: (1) Have physical or mental disorder in each mother or neonate. (2) Divorce. (3) Mother or infant hospitalisation for more than 72 hours. |
|
| Interventions | (1) Home visits group (n = 88 eligible for analysis, 100 recruited): 2 home visits (Intiaion: > 48 hours, Duration: ≥ 3 week, Intensity: < 1/week) Mothers and their neonates received the first postpartum care at health service centers in both groups. Second (10‐15 days), and third (42‐60 days) cares were provided by a trained midwife at home. (2) Referral health service center (control) group (n = 86 eligible for analysis, 100 recruited): (No home visit: Intiation: NA, Duration: NA, Intensity: NA) Secondary and tertiary care were provided by healthcare providers (who are mostly midwives) at a referral health service center. |
|
| Outcomes | Primary outcomes: maternal healthy behaviours, maternal quality of life Secondary outcomes: maternal practices in infant care. |
|
| Notes | Dates of study: between September 2010 and 2011 Funding sources: Master of Science thesis in midwifery funded by Tehran University of Medical Sciences Declarations of interest: written as "None declared" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Using a table of random numbers, subjects were manually assigned to intervention and control groups." |
| Allocation concealment (selection bias) | Unclear risk | It was not clearly described how the allocation concealment conducted. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clearly described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 12 in intervention group and 14 in control group dropped out with reasons described. |
| Selective reporting (reporting bias) | Unclear risk | The protocol or trial registry numbers were not available. |
| Other bias | Low risk | Groups appeared comparable at baseline. |
Morrell 2000.
| Study characteristics | ||
| Methods | Randomised controlled trial carried out in United Kingdom (Duration of the study: from October 1996 to November 1997). |
|
| Participants | 623 postnatal women. Inclusion criteria: (1) Aged 17 years or over. (2) Who delivered a live baby. (3) Who lived in the area served by community midwives at the recruiting hospital. * Information on the trial was given to women from the 32nd week of pregnancy Exclusion criteria: (1) Participant could not give informed consent. (2) Participant could not communicate in English. (3) Participant had a baby in the special care baby unit for more than 48 hours. |
|
| Interventions | (1) Intervention group (n = 311) (Initiation: > 48 hours, Duration: ≥ 3 week, Intensity: ≥ 1/week) Offered postnatal care at home by community midwives (usual care which involved up to 7 visits), also offered 10 visits from a support worker for up to 3 hours per day in the first 28 postnatal days. Community workers helped with housework, caring for the baby, provided emotional support and reinforced midwife advice on breastfeeding. (2) Control group (n = 312) (Initiation: > 48 hours, Duration: not written clearly, Intensity: not written clearly) Offered postnatal care at home by community midwives which involved up to 7 visits. Details were not written clearly. |
|
| Outcomes | Primary outcome: (1) The short form36 (SF36) general health perception domain measured at 6 weeks. Secondary outcomes: (2) The other SF36 domains at 6 weeks and 6 months. (3) The EPDS at 6 weeks and 6 months. (4) The Duke functional social support scale at 6 weeks and 6 months. (5) Breastfeeding rates at 6 weeks and 6 months. (6) Satisfaction with care at 6 weeks and 6 months. (7) Use of services at 6 weeks and 6 months. (8) Personal costs at 6 weeks and 6 months. |
|
| Notes | Dates of study: between October 1996 to November 1997 Funding sources: NHS research and development, Health Technocolgy Asessment programme Declarations of interest: unclear as not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random digit tables prepared in advance. |
| Allocation concealment (selection bias) | Low risk | By sequentially numbered, sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible, and not clearly described. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Postal questionnaire follow‐up. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intervention group (260/311) vs control group (233/312) at 6 month, control group was relatively high attrition proportion. |
| Selective reporting (reporting bias) | Low risk | The primary outcome measure was pre‐defined the SF‐36 general health perception domain measured at 6 weeks, secondary outcomes were pre‐defined the other of SF‐36, EPDS, social support, and breastfeeding rates. |
| Other bias | High risk | Many baseline were unbalanced (twin, used TENS machine, 1 or more adults aged 18 and over living with mother). |
Paul 2012.
| Study characteristics | ||
| Methods | Randomised controlled trial with 2 arms at a medical centre in Pennsylvania, USA, between September 2006 and August 2009. | |
| Participants | 1154 women intending to breastfeed. Inclusion criteria: (1) Women able to speak English, with access to telephones. (2) Living in study area. (3) After normal discharge (after vaginal delivery or caesarean) with no serious morbidities. (4) Not referred for social care visit and with healthy newborn (e.g. without jaundice or needing neonatal intensive care unit stay). Exclusion criteria: (1) Women or babies with atypical hospital stay (2 nights or longer after vaginal birth or 4 nights after caesarean). |
|
| Interventions | (1) Experimental intervention group (n = 576): (Initiation: < 48 hours, Duration: < 3 week, Intensity: < 1/week) Single visit by health visiting nurses within 48 hours of hospital discharge (typically 3‐5 days after the birth). The nurse had special training in promoting and supporting breastfeeding. (2) Control/Comparison intervention group (n = 578): (No home visit: Initiation: NA, Duration: NA, Intensity: NA) Usual care. (Clinic based postnatal follow‐up arranged by obstetricians.) (Women in both groups also had an office based visit for the baby approximately 1 week after the nurse visit or 5‐14 days after birth arranged by the hospital newborn nursery doctor.) |
|
| Outcomes | Primary outcome: Unplanned health service utilisation (inpatient, emergency, urgent or acute care) up to 14 days and up to 2 months following hospital discharge. Secondary outcomes: (1) Breastfeeding duration and exclusivity. (2) Maternal depression (EPDS). (3) Anxiety (STAI). (4) Perceived social support. (5) Parenting self‐efficacy at 2 weeks, 2 months and 6 months post delivery. (6) Maternal satisfaction with postnatal care. |
|
| Notes | Dates of study: between September 2006 and August 2009 Funding sources: grant R40 MC 06630 from the Maternal Child Health Bureau (Title V, Social Security Act), Health Resources and Services Administration, Department of Health and Human Services. Additional support was provided by the Children’s Miracle Network. Declarations of interest: written in "None reported" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence stratified for type of delivery. |
| Allocation concealment (selection bias) | Unclear risk | Not described. Follow‐up was arranged by the hospital but it was not clear how this was done. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Women and care providers would be aware of treatment allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | It was stated that telephone follow‐up was by blind study co‐ordinators. It was not clear whether this attempted blinding was successful. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It was stated that an ITT analysis was carried out. Loss to follow‐up was reported to be similar between group. Follow‐up at 2 weeks was 92%. There was further loss to follow‐up at the 2 months interview and at 6 months. |
| Selective reporting (reporting bias) | Low risk | Primary outcome measure was pre‐defined meternal and infant use of unplanned healthcare services in the 14 days after delivery. Secondary outcomes measure were healthcare utilisation, breastfeeding duration and exclusively, postpartum depression, anxiety, and satisfaction. |
| Other bias | Unclear risk | Other bias not apparent. Sex, late preterm and birthweight (< 2500 g) were slightly unbalanced. |
Ransjo‐Arvidson 1998.
| Study characteristics | ||
| Methods | Randomised controlled trial carried out in the University Teaching Hospital in Lusaka, the capital city of Zambia Duration of the study: 2 year and 10 month period from May 1989 to February 1992. |
|
| Participants | A total of 408 mothers who had a normal delivery and gave birth to a healthy term infant. Inclusion criteria: (1) The labour was assessed as“normal” by the attending midwife; gestational age 37–42 weeks, singleton birth, spontaneous vaginal delivery, vertex presentation, Apgar score > 8 points at 1 minute after birth, no visible malformations of the newborn, and mother and newborn assessed by the midwife as being "healthy". Exclusion criteria: none. |
|
| Interventions | Home visit Group A (n = 208): (Initiation: > 48 hours, Duration: ≥ 3 weeks, Intensity: ≥ 1/week) Mother/infant dyads who were visited by a midwife in their homes at days 3, 7, 28, and 42 after delivery. Home visit Group B (n = 200): Initiation: > 48 hours, Duration: < 3 week, Intensity: < 1/week). Mother/infant dyads who were only visited at day 42 after delivery. |
|
| Outcomes | (1) Maternal morbidity (abdominal pain, body pain, fever, excessive bleeding, pain from broken suture line, cough and other) (engorged breast, broken episiotomy, offensive lochia, hypertension, fever 37.6+, other). (2) Infant mortality. Infant morbidity (cord infection, eye discharge, cough and/or cold, skin infection, baby warm or cold, other) at 42 days (end of puerperium) iInfected cord, infected eyes, acute respiratory infection, skin infection, fever 37.6+, other). |
|
| Notes | The study was carried out in a developing country. Dates of study: between May 1989 and February 1992. Funding sources: the Swedish Agency for Research Collaboration with Developing Countries (SAREC), the Norwegian Aid Development (NORAD), the Ministry of Health (MoH) in Zambia, University Teaching Hospital (UTH), Lusaka, School of Medicine, University of Zambia (UNZA) and Stockholm University College of Health Sciences. Declarations of interest: unclear by no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described well. On defined days of week 6 women were selected at random for participation. 3 women were allocated to each group. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Data collection instruments were tested for reliability by an independent research midwife, but the blinding is not clear. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 172 (86%) of the mothers and infants in Group A, 168 (84%) in Group B attended the postnatal clinic after 42 days. |
| Selective reporting (reporting bias) | High risk | Primary outcome was not pre‐defined in the methods. |
| Other bias | High risk | Much of the analysis related to the intervention group only and there may have been risk of response bias as midwives asked women about their health as part of the intervention; women in the intervention group were asked repeatedly to identify health problems. Publication of findings was more than 6 years after completion of the trial. |
Salazar 2011.
| Study characteristics | ||
| Methods | RCT | |
| Participants | Inclusion criteria: "Gestation at term No major toxic habits Primipara or multipara The non‐existence of puerperal pathology that requires assistance in the puerperium: · HT‐Preeclampsia‐eclampia · Gestational diabetes · Grades 2‐3‐4 heart disease · Rh isoimmunization · Endocrinopathies · PROM · HIV · Maternal infection (TORCH, Listeria) Risk pregnancies are included, if once finished successfully, do not require further specific control: · threatened preterm laborb Placenta previa · Mild moderate anemia · Excessive or decreased weight gain · Previous caesarean section · Short stature · Poor control of pregnancy Vaginal delivery with: · Cephalic or breech presentation · Spontaneous or induced · Dilation with or without medication (oxytocin‐epidural) · Termination: spontaneous or instrumental (suction cup or spatula) · Spontaneous delivery or manual extraction, always with complete placenta Puerperium: · Hematic loss less than 500 ml · Adequate uterine involution · Normal vital signs · Episiotomy and normal breasts Healthy NB, at term with adequate weight for gestational age" |
|
| Interventions | Experimental group (home) (n = 213): Attention at home within the first week postpartum by a specialized nursing unit Control group (out‐patient clinic) (n = 217): Routine checks in out‐patient clinic at 7 and 30 days |
|
| Outcomes | Anxiety and depression rates measured with HAD Scale at 7 and 30 days postpartum. Measured as: ‐ NO (scores 0 to 7) ‐ Probable (8‐10) ‐ Pathologic (> 10) ‐ Severe (> 32) ‐ Missing |
|
| Notes | Study dates: Not reported (“over two years...”) Study funding sources: Not reported Study authors’ declarations of interest: The authors declare that they have no conflict of interest. Ethical approval obtained? Not reported Study prospectively registered? Not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "women were randomized according to a randomization table" |
| Allocation concealment (selection bias) | Unclear risk | Not reported in paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not reported in paper |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported in paper |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 8/213 (3.76%) lost to follow up in intervention group; 14/217 (6.45%) lost in comparison group |
| Selective reporting (reporting bias) | Unclear risk | No protocol available to assess |
| Other bias | Unclear risk | Not known |
Steel 2003.
| Study characteristics | ||
| Methods | Randomised controlled trial carried out in 2 tertiary centres in southeastern Ontario, Canada. Duration of the study: during the study recruited period, 27 January 1997 to 31 January 1999. |
|
| Participants | 733 participants with primiparas delivering a singleton infant and were discharged within 2 days of the birth of their infants. Inclusion criteria: 1) Delivered a singleton infant vaginally. 2) Discharged within 2 days of the birth of their infants. 3) Resided in the areas served by the local Community Care Access Centre (CCAC). 4) Capability which understand English well enough to give informed consent. Excclusion criteria: none. |
|
| Interventions | Home visit Group A (n = 380): (Initiation: > 48 hours, Duration: < 3 weeks, Intensity: ≥ 1/week) Consisted of 2 home visits by a Public Health Nurse (PHN). Mothers allocated to this group were telephoned on the first working day following discharge, and arrangements were made for the first PHN visit as soon as possible. The second visit was scheduled to take place within 10 days of discharge, although in some cases it was delayed by a few days. The visits were structured to include a thorough infant and postpartum assessment. Referrals to other support services, primary medical care or community support services were made if needs for these services were identified by either the mother or PHN. Telephone screen Group B (n = 353): (Initiation: > 48 hours, Duration: < 3 weeks, Intensity: < 1/week) Consisted of a telephone screening call to the new mother on the first working day following her discharge from hospital. The content of the call was structured to elicit the mother's concerns in the areas of infant feeding, her baby's general health and her emotional status. A home visit was made if either the mother or PHN identified a need. Referrals to other support services provided by the Health Unit, primary medical care or community support services were made if a need was identified. Otherwise no further contact was initiated by the PHN, although the mother was provided with the Health Unit telephone number and encouraged to call if she wished further support. |
|
| Outcomes | Primary (main) outcome: breastfeeding rates (and duration) at 6 months (Mothers who were not breastfeeding at discharge were excluded from the analysis of breastfeeding outcomes) Secondary outcomes: (1) Maternal confidence (Maternal Confidence Scale of Carty and Bradley) at 2 weeks. (2) Infants morbilities within 4 weeks (at 2 weeks and 4 weeks). (Concerns about weight, feeding difficulties, dehydration, jaundice, breathing problems, cold, congenital problems, concerns with cord, gastrointestinal/colic, infection, injury, rash, other problems). (3) Costs of the 2 models. |
|
| Notes | The 2 sites differed slightly in the provision of service. Dates of study: between January 1997 and January 1999 Funding sources: unclear by no information Declarations of interest: unclear by no information |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocations determined by random numbers. |
| Allocation concealment (selection bias) | Low risk | A sequential set of sealed envelopes, prepared in advance by the research associate. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible. The study was carried out in 2 sites and routine care was very different in the 2 sites and protocol deviations reflected normal practice in each of the sites. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Research assistants who were reported to be blinded to the allocation of the mothers. It was not clear whether this attempted blinding was successful. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported using an ITT approach, but not analysed as ITT. 733 women were randomised in 2 sites and in both sites loss to follow‐up at 2 and 4 weeks was less than or approximately 5%. Only those women who were breastfeeding were followed up at 6 months (here we have used the 4 week denominators for breastfeeding outcomes at 6 months). |
| Selective reporting (reporting bias) | Low risk | The main outcome was the rate of breastfeeding at 6 months, but other outcomes not described in methods. |
| Other bias | High risk | The 2 sites differed slightly in the provision of service. |
EPDS: Edinburgh Postnatal Depression Scale ITT: intention to treat MCS: mental health score PCS: physical health score NA: not applicable RhoGAM: Rh (D) immunoglobulin SF‐36: short 36 STAI: State‐Trait Anxiety Inventory vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adachi 2016 | The study had no intervention of home visits. |
| Bagherinia 2017 | The study had no intervention of home visits. |
| Baur 2012 | The study had included an intervention during antenatal period. |
| Boulvain 2004 | The study recruited mothers before birth and focused on early hospital discharge. |
| Carty 1990 | The study recruited mothers before birth and focused on early hospital discharge. |
| Catherine 2016 | The study recruited mothers before birth. |
| Dodge 2013a | The study had included an intervention after 6 months. |
| Dodge 2013b | The study was a random representative subsample design. The study was not a randomised control trial |
| Dodge 2014 | The study was a random representative subsample design.The study was not a randomised control trial |
| Dodge 2019 | The study had included an intervention after 6 months. |
| Ghodsbin 2012 | The study was a convenience sample design. The study was not a randomised control trial. |
| Goldfeld 2019 | The study had included an intervention during antenatal period. |
| Gonzalez 2018 | The study had included an intervention during antenatal period. |
| Goodman 2019 | The study had included an intervention after 6 months. |
| Gunn 1997 | The study was not a randomised control trial and had no intervention of home visits. |
| Gunn 1998 | The study had no intervention of home visits. |
| Gupta 2019 | The study only included a special group and included an intervention after 6 months. |
| Hannan 2013 | The study had no intervention of home visits. |
| Hannan 2014 | The study was secondary analysis in a randomised control trial and had no intervention of home visits. |
| Harrison 2019 | The study included an intervention during antenatal care period |
| Hodgins 2020 | Women were enrolled in the antenatal period and the intervention was not home visits, but a screening tool for detecting low birth weight infants in rural Nepal. |
| Hutton 2017 | The study only included a special group (low‐SES) and included an intervention after 6 months |
| Izzo 2005 | The study included specific high‐risk group (low‐income, young and unmarried at the time of the birth of their first child) and focused on visits by a child health nurse rather than on early postnatal care. |
| Jiao 2019 | The study was a pre‐test and post‐test design. |
| Kikuchi 2015 | The study recruited mothers before birth. |
| Kilburn 2017 | The study included an intervention after 6 months. |
| Korfmacher 1999 | The study included an intervention during antenatal period. |
| Lakin 2015 | The study recruited mothers before birth. |
| Laliberte 2016 | The study had no intervention of home visits. |
| le Roux 2013 | The study only included a special group (women living with HIV) . |
| Lumley 2006 | The study examined a complex intervention which included components offered during the antenatal period. |
| McConnell 2016 | The study recruited mothers before birth. |
| Modi 2017 | The study allocated mothers after 42 days at birth. |
| Mohd Shukri 2019 | The study recruited mothers before birth. |
| Navidian 2017 | The study was a pre‐test and post‐test design. |
| NCT00298311 2006 | The study had no intervention of home visits. |
| NCT01620723 2012 | The study had no intervention of home visits. |
| NCT02069782 2014 | The study included an intervention during antenatal period. |
| NCT02769676 2016 | The study only included an intervention for contraception. |
| NCT03165838 2017 | The study only included an intervention for contraception. |
| NCT03448289 2018 | The study was not a randomised control trial. |
| NCT03715218 2018 | The study had no intervention of home visits. |
| NCT03880032 2019 | The study had intervention with cognitive behavioral therapy intervention for anxiety. |
| NCT03887910 2019 | The study had no intervention of home visits. |
| Olds 2002 | The study included an intervention during antenatal period. |
| Park Himes 2017 | The study examined a complex intervention which included components, but not analysed separately home visits. |
| Paul 2013 | The study was a secondary analysis in a randomised controlled trial and no comparison between groups. |
| Pluym 2021 | No home visits ‐ study compared clinic visits in the intervention group at 2 and 6 weeks postpartum versus a single visit at 6 weeks postpartum. Women also at high risk of comorbidities: obesity, diabetes mellitus, mental health disorders and hypertensive disorders. |
| Quinlivan 2003 | The study only included a special group (teenage mothers). |
| Roberts 2016 | The study had no intervention of home visits. |
| Rotheram‐Borus 2014 | The study included an intervention during antenatal period. |
| Rotheram‐Borus 2017 | The study recruited mothers before birth. |
| Sawyer 2017 | The study allocated mothers after 42 day at birth. |
| Simons 2001 | The study had no intervention of home visits. |
| Stanwick 1982 | There were major protocol deviations in this study and results were not reported according to randomisation group. |
| Tandon 2018 | The study included an intervention during antenatal period. |
| Tomlinson 2016 | The study included an intervention during antenatal period. |
| Var 2015 | The study recruited mothers before birth. |
Characteristics of studies awaiting classification [ordered by study ID]
ACTRN12618000491268.
| Methods | Randomised controlled trial Setting: 2 hospitals in Victoria, Australia: The Royal Women's Hospital, Parkville and Bendigo Health Care Group, Bendigo Hospital, Bendigo |
| Participants | Recruitment target: 150 Inclusion criteria: eligibility will be ascertained in two stages: first during pregnancy where if eligible they will be recruited and screened and then at birth for all follow up after birth even if they have been randomised. During Pregnancy: Nulliparous; up to 36 weeks gestation; able to speak English and to respond to a written questionnaire; live within 40 minutes’ drive of the hospital from which they are recruited At Birth: Baby born at term without a severe disability. Exclusion criteria: During pregnancy: Multiparous; > 36 weeks gestation; under the age of 20; unable to speak or respond to a questionnaire in English; if live more than 40 minutes’ drive from the hospital. At birth: baby is born <37 weeks gestation or with severe disability. |
| Interventions |
Intervention: the intervention group (G2), will be offered three newborn behavioural observations coinciding with episodes of routine post‐natal care in the first month of parenthood (in addition to being offered treatment as usual (TAU) in the hospital or in the community for their mental health as required). Comparison: Recruits randomized to clinical comparison group G1 will be offered referral for ‘treatment as usual' (TAU) for their mental health either through the hospital they are recruited from or within the community. |
| Outcomes | Quality of mother‐infant interaction; diagnosis of postnatal depression; infant development; parenting stress; acceptability and usefulness of the NBO; psychosocial functioning; At relationship satisfaction; parental reflective functioning; parenting self‐efficacy; infant temperament; prenatal attachment; parental attachment ‐ at 4 months postpartum. |
| Notes | Registered: 4/04/2018 ‐ retrospectively registered. Trial reported as having taken place from 10/08/2017 to 31/03/2018. No published results available. Principle Investigator: Dr Susan Nicolson ‐ Susan.Nicolson@thewomens.org.au Emailed trial authors 28/06/21 ‐ no response received |
Baghersad 2020.
| Methods | Randomised controlled trial Setting: Isfahan, Iran |
| Participants | 62 mothers in the postpartum stage in Isfahan in 2015 Inclusion criteria: not reported Exclusion criteria: not reported |
| Interventions |
Intervention: Postpartum home care ‐ 3 visits, no further details Comparator: Not reported |
| Outcomes | Infection, pain and swelling at the suture site; pain in the abdomen, thighs, breasts, teeth. Mean score of knowledge of the correct pattern of breastfeeding; satisfaction with the performance of the health care team. |
| Notes | Registered: no details No published results available. Principle Investigator: no details, to follow‐up at next update |
IRCT201403152324N14.
| Methods | Randomised controlled trial Setting: Barhaloo Hospital (recruiting hospital) and Tehran Univerisity of Medical Sciences, Iran |
| Participants | Target sample size: 86 Inclusion criteria: postpartum women with indications as follows ‐ live, healthy and term infant; no history of physical and mental illness in the mother; lack of postpartum complication such as postpartum hemorrhage, preeclampsia, infection; no incidence of adverse events, including death and in the last 3 months; willingness to breast feeding; ability to read and write Exclusion criteria: Mother or infant hospitalization during the study; baby and infant deaths during the study; psychological problems after childbirth; incidence of adverse events during the study, including the death of relatives; lack of exclusive breastfeeding |
| Interventions |
Intervention: In addition to routine care, participants to receive continuous care model program ‐ which includes following: before hospital discharge, demographic questionnaire, postpartum depression, postpartum QOL questionnaire and the Pittsburgh Sleep Quality Index will be administered, followed by a meeting for 30 to 60 minutes to explain and give a training manual for mothers, then follow‐up (weekly phone calls and in person at home if necessary) will be conducted in the first 12 weeks postpartum. Phone calls will last for 20 minutes and be based on client needs and changed as needed. There is possibility for women to have 24 hour calls with the researcher. The questionnaires will be completed again after 12 weeks. Comparator: The control group will receive usual care after giving birth. |
| Outcomes | Quality of life and Quality of Sleep measured by Edinburgh Postnatal Depression questionnaire, Pittsburgh Sleep Quality Index (PSQI) and Specific Postnatal quality of Life questionnaire |
| Notes | Trial registered: 20/07/2014 ‐ registered while recruiting Recruitment start date: 13/04/2014 Recruitment end date: 21/08/2014 Recruitment status: reported to be 'complete' Corresponding author: Maryam Keshavarz, Keshavarz.m@iums.ac.ir; m_keshir@yahoo.com No published results available Emailed trial authors 28/06/21 ‐ no response received |
IRCT2016092529965N1.
| Methods | Unclear ‐ details from Cochrane Central Register of Controlled Trials, but no description of methods reported. Study aim was reported to be the effect of home‐based supportive educational counselling on parental expectations and postpartum stress in primiparous women |
| Participants |
Inclusion criteria: ability to read and write; no history of mental illnesses, addiction, and the lack of maternal and neonatal hospital readmission; pregnancy of 37‐42 weeks with vaginal delivery; living with the husband Exclusion criteria: unwillingness to the presence of midwife or the researcher at home after holding the meeting at health centers; incidence of any acute problems for mother, baby or family process. |
| Interventions |
Intervention: home‐based supportive educational counselling, which will be held during 3 occasions for 45 minutes. The first will be held in clinic, while the second and third will be held at home of participant. The training is through pamphlets and videos. The researcher’s telephone number will be distributed to the mothers to be able to call the researcher if any problem occurs. Comparator: No action will be taken in this group. They will only receive post childbirth routine care within 10‐15 and 42‐45 days after childbirth, which will be contributed by personnel of Health Care Center (doesn't report where or define 'routine care'). |
| Outcomes | Parental expectations; stress; mental and behavioural disorders associated with the puerperium, not elsewhere classified; reaction to severe stress; and adjustment disorders |
| Notes | Date added to Central: 31 March 2019 No other details provided about this trial No published results available ‐ to follow up at next update |
IRCT2016121431416N1.
| Methods | A semi‐experimental two‐group four‐stage clinical trial, conducted on 64 mothers at the postpartum ward Setting: Martyr Beheshti Hospital, Isfahan, Iran |
| Participants | Target sample: 62 Inclusion criteria: 18 years old to 48 years old; accessibility to the residence of the participants; providing written consent form; having a natural delivery with no complications; having a healthy normal neonate; does not require any special care; mother’s, families and house provides appropriate conditions; the presence of one of the family members during home visits Exclusion criteria: reported to be 'The lack of care on two occasions completely' |
| Interventions | A questionnaire was completed at 'the 1st to 3rd, 10st to 15th and 42nd to 60th days for both groups. Each visit lasted about an hour and conducted by at least 2 or 3 midwives with a master’s degree. The training package included “physical examination of mother and infant, evaluating mother’s getting back to normal during the postpartum period and Training in the mother, infant and family members and etc”'. Intervention: The care of the intervention group was conducted at three stages by two expert midwives for 45 to 90 minutes. The educational package contained physical examination of the mother and the infant; evaluating the condition of getting back to normal after delivery and necessary educations about the mother; the infant and the family members. The correct method of performing postpartum exercises and breastfeeding were educated through simulation to the mother and her companion. Comparator: Routine care performed by midwives in health centers in accordance with the national standards. |
| Outcomes | Breastfeeding pattern, awareness of maternal health, awareness of child health, husband’s behavior, and mother’s satisfaction. . |
| Notes | Trial registered: 11/05/2017 Recruitment start date: 6/08/2015 Recruitment end date: 19/02/2016 Corresponding author: Parvin Bahadoran, bahadoran@nm.mui.ac.ir No published results available. Emailed trial authors 28/06/21 ‐ no response received |
IRCT20200108046055N1.
| Methods | A randomised controlled trial Setting: Imam Khomeini Hospital in Falavarjan, Iran |
| Participants | Target sample: 100 Normal vaginal delivery mothers who were hospitalized for less than 48 hours Inclusion criteria: Willingness to participate in the study, uncomplicated normal delivery, pregnancy age between 37 to 42 weeks, infant weight between 2500 and 4000 g, hospital stay less than 48 hours, home distance to hospital less than 20 km, Iranian race, completing of informed consent to participate in the study Exclusion criteria: Mothers with high‐risk pregnancies such as: diabetes, hypertension, vaginal bleeding, neonatal premature, neonatal polycythemia, cephalohumatoma, mother does not wish to continue with the project |
| Interventions |
Intervention: after delivery intervention group to receive 5 home visits with education package according the program and the patient will be followed up for problems, visits on day 1 (before hospital discharge) 3 ‐ 7 ‐ 14 ‐ 42 Comparator: control group to receive postpartum care through health centers ‐ phone calls are made to them on days 3‐7 ‐ 14 ‐ 42, their status is checked and questionnaires are completed by telephone |
| Outcomes | knowledge and attitude about exclusive breastfeeding |
| Notes | Trial registered: 22/02/2020 Recruitment start date: 4/02/2020 Recruitment end date: 15/03/2020 Corresponding author: Dr. Mahnaz Zarshenas mahnaz_zarshenas@yahoo.com Emailed Dr Mahnaz Zarshenas 28/06/21 ‐ response received to state that the study was conducted by a student as a thesis and some of the results are still under review ‐ to follow up at next update |
ISRCTN45202278.
| Methods | A randomized controlled trial (Three arms) Setting: A public tertiary hospital in Singapore |
| Participants | 204 first‐time mothers during the early postpartum period |
| Interventions |
Intervention 1: the web‐based psychoeducation group Intervention 2: the home‐based psychoeducation group Comparator: control group receiving standard care |
| Outcomes | Maternal parental self‐efficacy, social support, psychological well‐being (anxiety and postnatal depression), and cost evaluation. Data will be collected at baseline, 1 month, 3 months, and 6 months post‐delivery |
| Notes | Recruitment started: October 2016 Recruitment completed: February 2017, with 68 mothers in each group The 6‐month follow‐up data collection was completed in August 2017 Corresponding author: Honggu He, National University of Singapore, Singapore Protocol only available ‐ no contact details of corresponding author ‐ to follow up at next update |
Liu 2020.
| Methods | A randomized controlled trial Setting: tertiary hospital, China |
| Participants | Postpartum women at a tertiary hospital |
| Interventions | 124 eligible postpartum women recruited and 108 of them (54 intervention group, 54 control group) completed this study Intervention: received evidence‐based health education within 1 week after returning home and received a second visit 1 month later Comparator: control group received routine postpartum home visits |
| Outcomes | Adherence to doing the month was measured by the Adherence to Doing‐the‐Month Practices questionnaire (ADP). Maternal physical health was measured by the Chair Stand Test and Postpartum Symptom Checklist. Maternal psychological health was measured by the Edinburgh Postnatal Depression Scale (EPDS). |
| Notes | Recruitment: December 2016 to July 2017 No numerical results reported: 'The ADP score of the intervention group was significantly lower than that of the control group (p < 0.001). The number of participants in the experimental group with poor appetite and indigestion was significantly lower than that of control group. No significant differences were found in numbers of symptoms and average EPDS scores between the 2 study groups (p > 0.05).' Corresponding author: YanQun Liu, Wuhan University School of Health Sciences, China Only email address available from trial report: yuyun7169@163.com Emailed trial authors 28/06/21 ‐ no response received |
NCT04084275.
| Methods | RCT Setting: Turkey |
| Participants | 117 healthy gravida 1 postpartum women who had given birth vaginally Inclusion criteria: puerpera who
|
| Interventions |
Intervention: "Levine's conservation model was used as the theoretical framework for this study. A literature review was used to determine the contents of the intervention program. A nursing care program which consisted of 8 sessions, the first of which was at the hospital and the others at the homes of puerpera and which were held at different times and lasted 12 weeks in total, based on Levine's Conservation Model was provided to the women in the intervention group. Each session lasted approximately 60‐120 minutes, according to the educational and practical contents.The puerpera were given training on different subjects based on the module during each session." Comparator: standard nursing care given after birth which can be solely breastfeeding training. |
| Outcomes | Quality of life, fatigue, sleep quality |
| Notes | Dates: July 2016 ‐ June 2017 Registered prospectively in 2019 Principle investigator: ŞADİYE ÖZCAN, Erzincan university faculty of health sciences No other contact details available ‐ unable to locate an email address ‐ to follow up again at next update |
TCTR20190206004.
| Methods | Reported to be randomized controlled trial ‐ but no details of methods or groups Setting: Thailand |
| Participants | Adolescent mothers Inclusion criteria: 1) age 10‐19 years old, 2) first time adolescent mother, 3) normal delivery and being the 1st day hospitalization at postpartum unit 4) having the EPDS score < 11 which is considered to not have PPD before participating in the program, 5) having primary family members such as husband or other family members (e.g. mother, father, grandmother, or friend) to provide care and social support during postpartum period, and 6) ability to communicate in Thai language |
| Interventions | Nurse‐Led Social Support Program to prevent postpartum depression among adolescent mothers ‐ not clear whether there is more than one intervention group Intervention: In the program, the researcher will provide informational support about postpartum depression and social support to adolescent mother and significant providers of adolescent mother, train adolescent mother to ask for the need of social support after childbirth that necessary and consistent with own needs, and the researcher also train significant providers of adolescent mothers to provide social support that necessary and consistent with the needs of adolescent mothers. The NLSS program consists of four components into two individual implementation phases over a period of 4 weeks by providing support to adolescent mothers and significant providers of adolescent mother at postpartum unit and the participants home via 1‐time home visit and 2‐time telephone contact. |
| Outcomes | Postpartum depression |
| Notes | No other details available in Central, Cochrane Library as of 31/05/21 ‐ to follow up at next update |
Characteristics of ongoing studies [ordered by study ID]
Kristensen 2018.
| Study name | What are the effects of supporting early parenting by increasing the understanding of the infant? A randomised community based trial |
| Methods | Cluster‐randomised trial |
| Participants | 2566 participants. The primary study population is formed by new families, mothers and fathers and their infant/s. |
| Interventions | Intervention group: NBO, Newborn behavioral observation In the intervention group new parents will receive the NBO delivered in connection with the examination of the newborn in a shared observation with the parents in the home visit of the health visitor 3 weeks postpartum Control group: practice as usual In the comparison group new parents will receive practice as usual due to the examination of their newborn in the home visit of the health visitor 3 weeks post part |
| Outcomes | Karitane parenting confidence scale, KPCS. Change is being assessed. (Time frame: measured at 2 weeks, 3 and 9 months postpartum) Infant Care Index (Time frame: measured 4 months postpartum) Ages & States questionnaire, ASQ‐SE. Change is being assessed. (Time frame: measured at 2 weeks, 3 and 9 months postpartum) The Major Depression Inventory (MDI10) (Time frame: measured at 2 weeks, 3 and 9 months postpartum) Breast‐feeding period in weeks (Time frame: measured at 3 and 9 months) |
| Starting date | 1 January 2017 |
| Contact information | Hanne Kronborg, Associate Professor, University of Aarhus |
| Notes |
NCT 03070652 What Are the Effects of Supporting Early Parenting by Increasing the Understanding of the Infant? https://clinicaltrials.gov/ct2/show/NCT03070652 |
NCT04226807.
| Study name | Re‐imagining the Postpartum Process: The Impact of Earlier Postpartum Contact on Attendance at Postpartum Visits and Maternal Wellbeing |
| Methods | A randomised trial Setting: Montefiore medical centre, New York, USA |
| Participants |
Inclusion criteria: Had a vaginal or cesarean delivery at the Jack D. Weiler Hospital Gestational age greater than or equal to 36 weeks Received prenatal care at the Comprehensive Family Care Center (CFCC) clinic Exclusion criteria: Fetal or neonatal death or demise |
| Interventions |
Intervention: Patient to receive a phone call from research staff 2‐3 weeks after giving birth asking about overall wellbeing, screening for complications and postpartum depression and referring to lactation or WIC as needed in addition to routine postpartum visit Comparator: Patient receives routine postpartum visit only |
| Outcomes | Attendance at comprehensive postpartum care visit at 12 weeks postpartum, breastfeeding rates at 6 months (percentage of women exclusively breastfeeding, using combined breastfeeding and formula feeding or exclusively using formula), need for family planning met (percentage of women who want to be using a birth control method who are using one), social work referral (percentage of women who attend a social work appointment), repeat pregnancy within 6 months of delivery (percentage of women with a positive pregnancy test since delivery), readmission to the hospital ( percentage of women admitted to any Montefiore hospital), acceptability of early visit (percentage of women who were "Very Satisfied" or "Satisfied" with the early phone call) |
| Starting date | Study start date: January 31, 2021 |
| Contact information | Principal Investigator: Talitha Bruney, mailto:talmarti%40montefiore.org?subject=NCT04226807, 2019‐10372, Impact of Earlier Postpartum Contact on Postpartum Visit Compliance and Maternal Wellbeing |
| Notes | Ongoing Estimate Completion date: October 2021 |
NCT04257552.
| Study name | |
| Methods | A randomised trial Setting: Magee Womens Hospital, University of Pittsburgh, Pennsylvania, United States |
| Participants | Postpartum women Target sample: 273 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention 1: Attention Control Participants will receive texts related to infant care to mirror the attention participants receive in the Healthy Beyond Pregnancy intervention. Intervention 2: Pre‐Scheduled Postpartum Visit Participants will have their postpartum visit scheduled while they are still in the hospital after delivery. Intervention 3: Behavioral Healthy Beyond Pregnancy Web‐based application grounded in tenants of behavioral economics. |
| Outcomes | Effective contraception (non‐barrier method), rates of breast feeding, diabetes screening includes either 2 hour GTT OR fasting blood sugar and Hgba1c, Follow up includes documented resolution or treatment of persistent hypertension |
| Starting date | Study start date: February 3, 2020 |
| Contact information | Principal Investigator: Katherine Himes, mailto:himekp%40upmc.edu?subject=NCT04257552, STUDY19040312, Care After Pregnancy Study (CAPS): Engaging Women in Postpartum Care |
| Notes | Ongoing Estimate Completion date: December 2021 |
Differences between protocol and review
In the 'Objectives' and 'Types of interventions' sections, we changed the description of the interventions. The interventions and control conditions varied considerably across studies with trials focusing on three broad types of comparisons: schedules involving more versus fewer postnatal home visits, schedules involving different models of care, and home versus hospital clinic postnatal check‐ups.
We added GRADE 'Summary of findings' tables to this update (2021).
It was not possible to conduct planned subgroup analyses due to the interventions in the included trials being too heterogenous.
We used random‐effects models in all analyses because of heterogeneity in interventions (2021).
The following are non prespecified outcomes that have been added since the publication of the protocol: contraceptive use; infant health care utilisation, and serious neonatal morbidity up to six months.
In response to peer review comments, the data for one of the included studies (Christie 2011) has been checked and re‐analysed as some of the data had not been entered correctly (e.g. for control group and intervention group ‐ incorrect denominators had been used; cluster trial adjustments have also been recalculated for this 2021 update).
Contributions of authors
Naohiro Yonemoto, Shuko Nagai and Rintaro Mori contributed to conceptualisation of this review and development of the protocol. Naohiro Yonemoto and Shuko Nagai screened and reviewed the identified studies, and contributed to data entry. Naohiro Yonemoto contributed to the analyses. In this update, Rintaro Mori also contributed to writing and advised on the analyses. All the review authors approved the final version of the review.
Sources of support
Internal sources
Juntendo University School of Medicine, Japan
National Center of Neurology and Psychiatry, Japan
External sources
Health and Labour Sciences Research Grants, Japan
-
NIHR, UK
TD is supported by the NIHR NHS Cochrane Collaboration Programme grant scheme award for NHS‐prioritised centrally‐managed, pregnancy and childbirth systematic reviews: CPGS 10/4001/02
Declarations of interest
Naohiro Yonemoto: none known.
Shuko Nagai: none known.
Rintaro Mori: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Aksu 2011 {published data only}
- Aksu H, Kucuk M, Duzgun G. The effect of postnatal breastfeeding education/support offered at home 3 days after delivery on breastfeeding duration and knowledge: a randomized trial. Journal of Maternal-Fetal and Neonatal Medicine 2011;24(2):354-61. [DOI] [PubMed] [Google Scholar]
Bashour 2008a {published data only}
- Bashour HN, Kharouf MH, Abdulsalam AA, El Asmar K, Tabbaa MA, Cheikha SA. Effect of postnatal home visits on maternal/infant outcomes in Syria: a randomized controlled trial. Public Health Nursing 2008;25(2):115-25. [DOI] [PubMed] [Google Scholar]
Bashour 2008b {published data only}
- Bashour HN, Kharouf MH, Abdulsalam AA, El Asmar K, Tabbaa MA, Cheikha SA. Effect of postnatal home visits on maternal/infant outcomes in Syria: a randomized controlled trial. Public Health Nursing 2008;25(2):115-25. [DOI] [PubMed] [Google Scholar]
Christie 2011 {published data only}
- Christie J, Bunting B. The effect of health visitors' postpartum home visit frequency on first-time mothers: cluster randomised trial. International Journal of Nursing Studies 2011;48(6):689-702. [DOI] [PubMed] [Google Scholar]
Escobar 2001 {published data only}
- Escobar G, Braveman P, Ackerson L, Odouli R, Coleman-Phox K, Lieu T. Home visits vs. hospital-based group follow-up visits after early postpartum discharge [abstract]. In: Pediatric Academic Societies Annual Meeting; 2001 April 28-May 1; Baltimore, USA. 2001:Abstract no: 1111. [DOI] [PubMed]
- Escobar GJ, Braveman PA, Ackerson L, Odouli R, Coleman-Phox K, Capra AM, et al. A randomized comparison of home visits and hospital-based group follow-up visits after early postpartum discharge. Pediatrics 2001;108:719-27. [DOI] [PubMed] [Google Scholar]
Furnieles‐Paterna 2011 {published data only}
- Furnieles-Paterna E, Hoyuelos-Camara H, Montiano-Ruiz I, Penalver-Julve N, Fitera-Lamas L. Randomized comparative study of the puerperal visits in the mothers house and in the health center [Spanish] [Estudio comparativo y aleatorizado de la visita puerperal en el domicilio de la madre y en el centro de salud]. Matronas Profesion 2011;12(3):65-73. [Google Scholar]
Gagnon 2002 {published data only}
- Gagnon AJ, Dougherty G, Jimenez V, Leduc N. Randomized trial of postpartum care after hospital discharge. Pediatrics 2002;109(6):1074-80. [DOI] [PubMed] [Google Scholar]
Kronborg 2007 {published data only}
- Kronborg H, Vaeth M, Olsen J, Iversen L, Harder I. Effect of early postnatal breastfeeding support: a cluster-randomized community based trial. Acta Paediatrica 2007;96(7):1064-70. [DOI] [PubMed] [Google Scholar]
Lieu 2000 {published data only}
- Lieu TA, Braveman PA, Escobar GJ, Fischer AF, Jensvold NG, Capra AM. A randomized comparison of home and clinic follow-up visits after early postpartum hospital discharge. Pediatrics 2000;105:1058-65. [DOI] [PubMed] [Google Scholar]
MacArthur 2002 {published data only}
- MacArthur C, Winter HR, Bick DE, Knowles H, Lilford R, Henderson C, et al. Effects of redesigned community postnatal care on womens' health 4 months after birth: a cluster randomised trial. Lancet 2002;359:378-85. [DOI] [PubMed] [Google Scholar]
Milani 2017 {published data only}
- Milani HS, Amiri P, Mohsey M, Monfared ED, Vaziri SM, Malekkhahi A, et al. Effect of health care as the "home visiting" on postpartum depression: a controlled clinical trial. International Journal of Preventive Medicine 2017;8:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mirmolaei 2014 {published data only}
- Mirmolaei ST, AmelValizadeh M, Mahmoodi M, Tavakol Z. The effect of postpartum home care on maternal practices in infant care. Journal of Urmia Nursing and Midwifery Faculty 2012;10(3):1-10. [CENTRAL: CN-02004121] [Google Scholar]
- Mirmolaei ST, Amelvalizadeh M, Mahmoudi M, Tavakol Z. Effect of home postpartum care on quality of life of low risk mothers. HAYAT 2011;17(2):42-51. [CENTRAL: CN-02004119] [Google Scholar]
- Mirmolaei ST, Valizadeh MA, Mahmoodi M, Tavakol Z. Comparison of effects of home visits and routine postpartum care on the healthy behaviors of Iranian low-risk mothers. International Journal of Preventive Medicine 2014;5(1):61-8. [PMID: ] [PMC free article] [PubMed] [Google Scholar]
Morrell 2000 {published data only}
- Morrell CJ, Spiby H, Stewart P, Walters S, Morgan A. Costs and effectiveness of community postnatal support workers: randomised controlled trial. BMJ 2000;321(7261):593-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Paul 2012 {published data only}
- Paul IM, Beiler JS, Schaefer EW, Hollenbeak CS, Alleman N, Sturgis SA, et al. A randomized trial of single home nursing visits vs office-based care after nursery/maternity discharge: the Nurses for Infants Through Teaching and Assessment After the Nursery (NITTANY) Study. Archives of Pediatrics & Adolescent Medicine 2012;166(3):263-70. [DOI] [PubMed] [Google Scholar]
Ransjo‐Arvidson 1998 {published data only}
- Ransjo-Arvidson AB, Chintu K, Ng'andu N, Eriksson B, Susu B, Christensson K, et al. Maternal and infant health problems after normal childbirth: a randomised controlled study in Zambia. Journal of Epidemiology & Community Health 1998;52:385-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Salazar 2011 {published data only}
- Salazar I, Sainz JA, Garcia E, Marrugal V, Garrido R. Influence of early postpartum home visits on the detection and clinical course of postpartum depression. Progresos de Obstetricia y Ginecologia 2011;54(2):65-70. [Google Scholar]
Steel 2003 {published data only}
- Steel O'Connor KO, Mowat DL, Scott HM, Carr PA, Dorland JL, Young Tai KF. A randomized trial of two public health nurse follow-up programs after early obstetrical discharge: an examination of breastfeeding rates, maternal confidence and utilization and costs of health services. Canadian Journal of Public Health. Revue Canadienne de Sante Publique 2003;94(2):98-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young Tai KFW, O'Connor KO, Scott HM, Mowat DL, Carr PA. Do community follow-up programs improve infant outcome after early obstetrical discharge? A randomized controlled trial. [abstract]. Pediatric Research 2000;47(4):161A. [Google Scholar]
References to studies excluded from this review
Adachi 2016 {published data only}
- Adachi K, Petrini MA, Shrestha S, Rana Khagi B. Development and evaluation of a newborn care education programme in primiparous mothers in Nepal. Midwifery 2016;42:21-8. [CENTRAL: CN-01368595] [EMBASE: 615941823] [PMID: ] [DOI] [PubMed] [Google Scholar]
Bagherinia 2017 {published data only}
- Bagherinia M, Mirghafourvand M, Shafaie FS. The effect of educational package on functional status and maternal self-confidence of primiparous women in postpartum period: a randomized controlled clinical trial. Journal of Maternal-fetal & Neonatal Medicine 2017;30(20):2469-75. [CENTRAL: CN-01600836] [PMID: ] [DOI] [PubMed] [Google Scholar]
Baur 2012 {published data only}
- Baur L. Effectiveness of a home-based early intervention on children's BMI at age two years: Randomised controlled trial. Obesity Facts 2012;5(Suppl 1):34. [CENTRAL: CN-01005199] [EMBASE: 70781742] [Google Scholar]
Boulvain 2004 {published data only}
- Boulvain M, Perneger TV, Othenin-Girard V, Petrou S, Berner M, Irion O. Home-based versus hospital-based postnatal care: a randomised trial. BJOG: an international journal of obstetrics and gynaecology 2004;111(8):807-13. [DOI] [PubMed] [Google Scholar]
Carty 1990 {published data only}
- Carty EM, Bradley CF. A randomized, controlled evaluation of early postpartum hospital discharge. Birth 1990;17:199-204. [DOI] [PubMed] [Google Scholar]
Catherine 2016 {published data only}
- Catherine NL, Gonzalez A, Boyle M, Sheehan D, Jack SM, Hougham KA, et al. Improving children's health and development in British Columbia through nurse home visiting: a randomized controlled trial protocol. BMC Health Services Research 2016;16(a):349. [CENTRAL: CN-01414003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catherine NLA, Boyle M, Zheng Y, McCandless L, Xie H, Lever R, et al. Nurse home visiting and prenatal substance use in a socioeconomically disadvantaged population in British Columbia: analysis of prenatal secondary outcomes in an ongoing randomized controlled trial. CMAJ Open 2020;8(4):E667-75. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodge 2013a {published data only}
- Dodge KA, Goodman WB, Murphy RA, O'Donnell K, Sato J. Randomized controlled trial of universal postnatal nurse home visiting: impact on emergency care. Pediatrics 2013;132 Suppl 2:S140-S146. [CENTRAL: CN-00962434] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodge 2013b {published data only}
- Dodge A, Goodman WB, Murphy R, O'Donnell K, Sato J. Toward population impact from home visiting. Zero to Three 2013;33(3):17-23. [PMC free article] [PubMed] [Google Scholar]
Dodge 2014 {published data only}
- Dodge KA, Goodman WB, Murphy RA, O'Donnell K, Sato J, Guptill S. Implementation and randomized controlled trial evaluation of universal postnatal nurse home visiting. American Journal of Public Health 2014;104(Suppl 1):S136-S143. [CENTRAL: CN-00973333] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dodge 2019 {published data only}
- Dodge KA, Goodman WB, Bai Y, O'Donnell K, Murphy RA. Effect of a community agency-administered nurse home visitation program on program use and maternal and infant health outcomes: a randomized clinical trial. JAMA Network Open 2019;2(11):e1914522. [CENTRAL: CN-02004348] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ghodsbin 2012 {published data only}
- Ghodsbin F, Yazdani K, Jahanbin I, Keshavarzi S. The effect of home visit during the first six weeks of postpartum on the quality of life of primiparous women referred to Shiraz health centers of Shiraz University of Medical Sciences. Investigacion & Educacion en Enfermeria 2012;30(3):339-45. [CENTRAL: CN-02004117] [Google Scholar]
Goldfeld 2019 {published data only}
- Goldfeld S, Price A, Smith C, Bruce T, Bryson H, Mensah F, et al. Nurse home visiting for families experiencing adversity: a randomized trial. Pediatrics 2019;143(1):e20181206. [CENTRAL: CN-01793793] [EMBASE: 625807740] [PMID: ] [DOI] [PubMed] [Google Scholar]
Gonzalez 2018 {published data only}
- Gonzalez A, Catherine N, Boyle M, Jack SM, Atkinson L, Kobor M, et al. Healthy Foundations Study: a randomised controlled trial to evaluate biological embedding of early-life experiences. BMJ Open 2018;8(1):e018915. [CENTRAL: CN-01627433] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodman 2019 {published data only}
- Goodman WB, Dodge KA, Bai Y, O'Donnell KJ, Murphy RA. Randomized controlled trial of Family Connects: effects on child emergency medical care from birth to 24 months. Development and Psychopathology 2019;31(5):1863-72. [CENTRAL: CN-01986161] [EMBASE: 629210350] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gunn 1997 {published data only}
- Gunn JM. The Role Of The General Practitioner In Postnatal Care: An Early Intervention Study [thesis]. Melbourne: University of Melbourne, 1997. [Google Scholar]
Gunn 1998 {published data only}
- Gunn J, Lumley J, Chondros P, Young D. Does an early postnatal check-up improve maternal health: results from a randomised trial in Australian general practice. British Journal of Obstetrics and Gynaecology 1998;105(9):991-7. [DOI] [PubMed] [Google Scholar]
Gupta 2019 {published data only}
- Gupta A, Dadhich JP, Ali SM, Thakur N. Skilled counseling in enhancing early and exclusive breastfeeding rates: an experimental study in an urban population in India. Indian Pediatrics 2019;56(2):114-8. [CENTRAL: CN-01981881] [PMID: ] [PubMed] [Google Scholar]
Hannan 2013 {published data only}
- Hannan J. APN telephone follow up to low-income first time mothers. Journal of Clinical Nursing 2013;22(1/2):262-70. [CENTRAL: CN-00915791] [EMBASE: 22845337] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hannan 2014 {published data only}
- Hannan J. Newborn morbidities and health charges: the first eight weeks. Pediatric Nursing 2014;40(3):121-6. [CENTRAL: CN-01041375] [EMBASE: 25134225] [PMID: ] [PubMed] [Google Scholar]
Harrison 2019 {published data only}
- Harrison MS, Bunge-Montes S, Rivera C, Jimenez-Zambrano A, Heinrichs G, Scarbro S, et al. Delivery of home-based postpartum contraception in rural Guatemalan women: a cluster-randomized trial protocol. Trials 2019;20(1):639. [CENTRAL: CN-02004347] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodgins 2020 {published data only}
- Hodgins S, Rajbhandari B, Joshi D, Ban B, Khatry S, Mullany LC. Community-based cluster randomized controlled trial: empowering households to identify and provide appropriate care for low-birthweight newborns in Nepal. BMC Public Health 2020;20(1):1274. [CENTRAL: CN-02177382] [EMBASE: 632691083] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hutton 2017 {published data only}
- Hutton JS, Gupta R, Gruber R, Berndsen J, DeWitt T, Ollberding NJ, et al. Randomized trial of a children's book versus brochures for safe sleep knowledge and adherence in a high-risk population. Academic Pediatrics 2017;17(8):879-86. [CENTRAL: CN-01447053] [PMID: ] [DOI] [PubMed] [Google Scholar]
Izzo 2005 {published data only}
- Izzo CV, Eckenrode JJ, Smith EG, Henderson CR, Cole R, Kitzman H, et al. Reducing the impact of uncontrollable stressful life events through a program of nurse home visitation for new parents. Prevention Science 2005;6(4):269-74. [DOI] [PubMed] [Google Scholar]
Jiao 2019 {published data only}
- Jiao N, Zhu L, Chong YS, Chan WS, Luo N, Wang W, et al. Web-based versus home-based postnatal psychoeducational interventions for first-time mothers: a randomised controlled trial. International Journal of Nursing Studies 2019;99:103385. [CENTRAL: CN-02004349] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kikuchi 2015 {published data only}
- Kikuchi K, Ansah E, Okawa S, Shibanuma A, Gyapong M, Owusu-Agyei S, et al. Ghana's Ensure Mothers and Babies Regular Access to Care (EMBRACE) program: study protocol for a cluster randomized controlled trial. Trials 2015;16:22. [CENTRAL: CN-01256192] [EMBASE: 602649509] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kilburn 2017 {published data only}
- Kilburn MR, Cannon JS. Home visiting and use of infant health care: a randomized clinical trial. Pediatrics 2017;139(1):220161274. [CENTRAL: CN-01381985] [EMBASE: 614049581] [PMID: ] [DOI] [PubMed] [Google Scholar]
Korfmacher 1999 {published data only}
- Korfmacher J, O'Brien R, Hiatt S, Olds D. Differences in program implementation between nurses and paraprofessionals providing home visits during pregnancy and infancy: a randomized trial. American Journal of Public Health 1999;89(12):1847-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lakin 2015 {published data only}
- Lakin A, Sutter MB, Magee S. Newborn well-child visits in the home setting: a pilot study in a family medicine residency. Family Medicine 2015;47(3):217-21. [CENTRAL: CN-01109869] [PMID: ] [PubMed] [Google Scholar]
Laliberte 2016 {published data only}
- Laliberte C, Dunn S, Pound C, Sourial N, Yasseen AS, Millar D, et al. A randomized controlled trial of innovative postpartum care model for mother-baby dyads. PloS One 2016;11(2):e0148520. [CENTRAL: CN-01136989] [EMBASE: 608920617] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
le Roux 2013 {published data only}
- le Roux IM, Tomlinson M, Harwood JM, O'Connor MJ, Worthman CM, Mbewu N, et al. Outcomes of home visits for pregnant township mothers and their infants in South Africa: a cluster randomised controlled trial. AIDS (London, England) 2013;27(9):1461-71. [CENTRAL: CN-00861192] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lumley 2006 {published data only}
- Lumley J, Small R, Brown S, Watson L, Gunn J, Mitchell C, et al. Prism (program of resources, information and support for mothers) protocol for a community-randomised trial [isrctn03464021]. BMC Public Health 2003;3:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumley J, Watson L, Small R, Brown S, Mitchell C, Gunn J. Prism (program of resources, information and support for mothers): a community-randomised trial to reduce depression and improve women's physical health six months after birth. BMC Public Health 2006;6:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis K, Small R, Brown S. Using documents to investigate links between implementation and sustainability in a complex community intervention: the PRISM study. Social Science & Medicine 2012;75(7):1222-9. [DOI] [PubMed] [Google Scholar]
McConnell 2016 {published data only}
- McConnell M, Ettenger A, Rothschild C W, Muigai F, Cohen J. Can a community health worker administered postnatal checklist increase health-seeking behaviors and knowledge?: evidence from a randomized trial with a private maternity facility in Kiambu County, Kenya. BMC Pregnancy and Childbirth 2016;16(1):136. [CENTRAL: CN-01426733] [EMBASE: 610583576] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Modi 2017 {published data only}
- Modi D, Desai S, Dave K, Shah S, Desai G, Shah P, et al. Cluster randomized trial of a mHealth intervention "ImTeCHO" to improve delivery of proven maternal, neonatal, and child care interventions through community-based Accredited Social Health Activists (ASHAs) by enhancing their motivation and strengthening supervision in tribal areas of Gujarat, India: study protocol for a randomized controlled trial. Trials 2017;18(1):270. [CENTRAL: CN-01600312] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohd Shukri 2019 {published data only}
- Mohd Shukri NH, Wells J, Eaton S, Mukhtar F, Petelin A, Jenko-Praznikar Z, et al. Randomized controlled trial investigating the effects of a breastfeeding relaxation intervention on maternal psychological state, breast milk outcomes, and infant behavior and growth. American Journal of Clinical Nutrition 2019;110(1):121-30. [CENTRAL: CN-01960615] [EMBASE: 628476897] [PMID: ] [DOI] [PubMed] [Google Scholar]
Navidian 2017 {published data only}
- Navidian A, Sarasiyabi AS, Koochakzai M. The effect of home-based supportive-educational counseling on primigravidas' postpartum stress. International Journal of Women's Health and Reproduction Sciences 2017;5(2):112-8. [CENTRAL: CN-01328332] [EMBASE: 614488119] [Google Scholar]
NCT00298311 2006 {published data only}
- NCT00298311. Effect of home-based peer support on maternal-infant interaction and postpartum depression [An RCT to evaluate the effect of home-based peer support on maternal-infant interaction, infant health outcomes, and postpartum depression]. Available from: clinicaltrials.gov/show/NCT00298311 (first received 2006 March 02). [CENTRAL: CN-01481181]
NCT01620723 2012 {published data only}
- NCT01620723. Evaluation of breastfeeding support after short time hospitalization [Effect evaluation of a theory and evidence based programme for establishing effective breastfeeding after short time hospitalization post partum]. Available from: clinicaltrials.gov/show/NCT01620723 (first received 2012 June 15). [CENTRAL: CN-01595738]
NCT02069782 2014 {published data only}
- NCT02069782. Mother and infant home visiting program evaluation. Available from: clinicaltrials.gov/show/NCT02069782 (first received 2014 February 24).
NCT02769676 2016 {published data only}
- NCT02769676. A comparison of short interval vs. routine postpartum visit on contraceptive initiation. Available from: clinicaltrials.gov/show/NCT02769676 (first received 2016 May 11). [CENTRAL: CN-01558193]
NCT03165838 2017 {published data only}
- NCT03165838. Effectiveness of shortened time interval to postpartum visit in Iiproving postpartum attendance. Available from: clinicaltrials.gov/show/NCT03165838 (first received 2017 May 24). [CENTRAL: CN-01575281]
NCT03448289 2018 {published data only}
- NCT03448289. Use of a reproductive life planning tool at the pediatric well-baby visit with postpartum women. Available from: clinicaltrials.gov/show/NCT03448289 (first received 2018 February 28). [CENTRAL: CN-01483511]
NCT03715218 2018 {published data only}
- NCT03715218. Impact of mothers touch program to improve maternal health after birth [Indian postpartum care: a randomised trial to improve maternal health six weeks after birth]. Available from: clinicaltrials.gov/show/NCT03715218 (first received 2018 October 23). [CENTRAL: CN-01664337]
NCT03880032 2019 {published data only}
- NCT03880032. Happy Mother-Healthy Baby: an anxiety-focused early prenatal intervention [Happy Mother-Healthy Baby: an anxiety-focused early prenatal intervention for the prevention of common mental disorders in Pakistan]. Available from: clinicaltrials.gov/show/NCT03880032 (first received 2019 March 19). [CENTRAL: CN-01930983]
NCT03887910 2019 {published data only}
- NCT03887910. Northwell health visits: a Family Connects Pilot implementation at Northwell Health. Available from: clinicaltrials.gov/show/NCT03887910 (first received 2019 March 25). [CENTRAL: CN-01931053]
Olds 2002 {published data only}
- Olds DL, Robinson J, O'Brien R, Luckey DW, Pettitt LM, Henderson CR Jr, et al. Home visiting by paraprofessionals and by nurses: a randomized, controlled trial. [Comment]. Pediatrics 2002;110(3):486-96. [DOI] [PubMed] [Google Scholar]
Park Himes 2017 {published data only}
- Park Himes K, Donovan H, Wang S, Weaver C, Grove JR, Facco FL. Healthy beyond pregnancy, a web-based intervention to improve adherence to postpartum care: randomized controlled feasibility trial. JMIR Human Factors 2017;4(4):e26. [CENTRAL: CN-02004118] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paul 2013 {published data only}
- Paul IM, Downs DS, Schaefer EW, Beiler JS, Weisman CS. Postpartum anxiety and maternal-infant health outcomes. Pediatrics 2013;131(4):e1218-24. [DOI] [PubMed] [Google Scholar]
Pluym 2021 {published data only}
- NCT03733405. Postpartum care timing: a randomized trial [Randomized control trial of postpartum visits at two or six weeks to evaluate clinic attendance and emergency department usage]. Available from: clinicaltrials.gov/show/NCT03733405 (first received 2018 November 07). [CENTRAL: CN-01918332]
- Pluym ID, Mok T, Holliman K, Afshar Y, Rao R, Tandel MD, et al. Randomized Control Trial of Postpartum Visits at Two and Six Weeks. American Journal of Obstetrics & Gynecology MFM 2021:100363. [CENTRAL: CN-02257474] [EMBASE: 634692347] [DOI] [PubMed] [Google Scholar]
- Pluym ID, Tandel M, Kwan L, Mok T, Holliman K, Afshar Y, et al. 1105 Edinburgh postnatal depression scores and postpartum healthcare utilization. American Journal of Obstetrics and Gynecology (first received 2021);224(2):S681-2. [CENTRAL: CN-02247497] [EMBASE: 2010868449]
- Pluym ID, Tandel M, Kwan L, Mok T, Holliman K, Afshar Y, et al. 57 Randomized Control Trial of Postpartum Visits at 2 weeks and 6 weeks. American Journal of Obstetrics and Gynecology 2021;224(2):S40. [CENTRAL: CN-02247498] [EMBASE: 2010868436] [DOI] [PubMed] [Google Scholar]
Quinlivan 2003 {published data only}
- Quinlivan JA, Box H, Evans SF. Postnatal home visits in teenage mothers: a randomised controlled trial. Lancet 2003;361(9361):893-900. [DOI] [PubMed] [Google Scholar]
Roberts 2016 {published data only}
- Roberts RP, Blackwell SC, Brown KM, Pedroza C, Sibai BM, Tyson JE. Early compared with delayed physician rounds on patient satisfaction of postpartum women: a randomized controlled trial. Obstetrics and Gynecology 2016;128(2):381-6. [CENTRAL: CN-01379692] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rotheram‐Borus 2014 {published data only}
- Rotheram-Borus MJ, Tomlinson M, le Roux IM, Harwood JM, Comulada S, O'Connor MJ, et al. A cluster randomised controlled effectiveness trial evaluating perinatal home visiting among South African mothers/infants. Plos One 2014;9(10):e105934. [CENTRAL: CN-01076843] [EMBASE: 600311324] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rotheram‐Borus 2017 {published data only}
- Rotheram-Borus MJ, Le Roux K, Le Roux IM, Christodoulou J, Laurenzi C, Mbewu N, et al. To evaluate if increased supervision and support of South African Government health workers' home visits improves maternal and child outcomes: study protocol for a randomized controlled trial. Trials 2017;18(1):368. [CENTRAL: CN-01599959] [EMBASE: 617646882] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sawyer 2017 {published data only}
- Sawyer MG, Reece CE, Bowering K, Jeffs D, Sawyer ACP, Mittinty M, et al. Nurse-moderated internet-based support for new mothers: non-inferiority, randomized controlled trial. Journal of Medical Internet Research 2017;19(7):e258. [CENTRAL: CN-01446870] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Simons 2001 {published data only}
- Simons J, Reynolds J, Morison L. Randomised controlled trial of training health visitors to identify and help couples with relationship problems following a birth. British Journal of General Practice 2001;51:793-9. [PMC free article] [PubMed] [Google Scholar]
Stanwick 1982 {published data only}
- Stanwick RS, Moffat MEK, Robitaille Y, Edmond A, Dok C. An evaluation of the routine postnatal public health nurse home visit. Canadian Journal of Public Health 1982;73:200-5. [PubMed] [Google Scholar]
Tandon 2018 {published data only}
- Tandon SD, Ward EA, Hamil JL, Jimenez C, Carter M. Perinatal depression prevention through home visitation: a cluster randomized trial of mothers and babies 1-on-1. Journal of Behavioral Medicine 2018;41(5):641-52. [CENTRAL: CN-01915399] [EMBASE: 626711953] [PMID: ] [DOI] [PubMed] [Google Scholar]
Tomlinson 2016 {published data only}
- Tomlinson M, Rotheram-Borus MJ, le Roux IM, Youssef M, Nelson SH, Scheffler A, et al. Thirty-six-month outcomes of a generalist paraprofessional perinatal home visiting intervention in South Africa on maternal health and child health and development. Prevention Science 2016;17(8):937-48. [CENTRAL: CN-01441564] [EMBASE: 619829399] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Var 2015 {published data only}
- Var C, Bazzano AN, Srivastav SK, Welty JC, Ek NI, Oberhelman RA. Newborn Infection Control and Care Initiative for health facilities to accelerate reduction of newborn mortality (NICCI): study protocol for a randomized controlled trial. Trials 2015;16:257. [CENTRAL: CN-01257757] [EMBASE: 604884093] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Var C, Oberhelman RA, Shu T, Leang S, Duggal R, Le J, et al. A Linked Community and Health Facility Intervention to Improve Newborn Health in Cambodia: the NICCI Stepped-Wedge Cluster-Randomized Controlled Trial. International Journal of Environmental Research and Public Health 2020;17(5). [CENTRAL: CN-02101970] [EMBASE: 2003908820] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
ACTRN12618000491268 {published data only}
- ACTRN12618000491268. Understanding your Newborn and Adapting to parenthood (UNA): a randomised clinical effectiveness trial of the Newborn Behavioural Observations (NBO) for new families [Understanding your Newborn and Adapting to parenthood (UNA): A randomised clinical effectiveness trial of the Newborn Behavioural Observations (NBO) on mother-infant relationship, and maternal postnatal depression and stress in new families at-risk of postnatal depression]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12618000491268 (first received 2018). [CENTRAL: CN-01905765]
Baghersad 2020 {published data only}
- Baghersad Z, Mokhtari F, Bahadoran P. Effect of postpartum home care on mothers' health [Effect of postpartum home care on mothers' health]. Iranian Journal of Obstetrics, Gynecology and Infertility 2020;23(4):45-53. [CENTRAL: CN-02250307] [EMBASE: 2004921923] [Google Scholar]
IRCT201403152324N14 {published data only}
- IRCT201403152324N14. Quality of Life in Postpartum [Effect of Training Based on Continuous Care Model on Quality of Life and Quality of Sleep in the Postpartum Period]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201403152324N14 (first received 2014). [CENTRAL: CN-01809101]
IRCT2016092529965N1 {published data only}
- IRCT2016092529965N1. The effect of Educational Counseling on parental expectations and postpartum stress [The effect of home-based Supportive Educational Counseling on parental expectations and postpartum stress in primiparous women]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016092529965N1 2016. [CENTRAL: CN-01878137]
IRCT2016121431416N1 {published data only}
- IRCT2016121431416N1. Effect Postpartum care at home on postpartum outcome [Aimed to investigate the effect of Postpartum care at home on postpartum outcome]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT2016121431416N1 (first received 2017). [CENTRAL: CN-01889935]
IRCT20200108046055N1 {published data only}
- IRCT20200108046055N1. The effect of postpartum home visit on mothers' knowledge and attitude about exclusive breastfeeding, incidence of some breastfeeding complications [The effect of postpartum home visit on mothers' knowledge and attitude about exclusive breastfeeding, incidence of some complications of lactation and physiological jaundice in neonates in hospital]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=IRCT20200108046055N1 (first received 2020). [CENTRAL: CN-02171268]
ISRCTN45202278 {published data only}
- ISRCTN45202278. Web-based and home-based postnatal psychoeducational interventions for first-time mothers [The effectiveness and cost-effectiveness of web-based and home-based postnatal psychoeducational interventions for first-time mothers: A randomized controlled trial]. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN45202278 (first received 2016). [CENTRAL: CN-01804044]
Liu 2020 {published data only}
- Liu Y, Hu J, Chen X, Yu Y, Bai J. Effects of a health education program targeted to Chinese women adhering to their cultural practice of doing the month: a randomized controlled trial. Midwifery 2020 Jul 18;90:102796. [CENTRAL: CN-02160376] [EMBASE: 632485916] [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT04084275 {published data only}
- NCT04084275. The effect of care given using Levine's Conservation Model on postpartum quality of life in primiparas. Available from: clinicaltrials.gov/show/NCT04084275 (first received 2019 September 10). [CENTRAL: CN-01984172]
TCTR20190206004 {published data only}
- TCTR20190206004. The effectiveness of a nurse-led social support program to prevent postpartum depression among adolescent mothers: a randomized controlled trial. Http://www.who.int/trialsearch/Trial2.aspx?TrialID=TCTR20190206004 (first received 2019). [CENTRAL: CN-01993127]
References to ongoing studies
Kristensen 2018 {unpublished data only}
- Kristensen IH, Kronborg H. What are the effects of supporting early parenting by enhancing parents' understanding of the infant? Study protocol for a cluster-randomized community-based trial of the Newborn Behavioral Observation (NBO) method. BMC Public Health 2018;18(1):832. [CENTRAL: CN-01935208] [EMBASE: 627710416] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04226807 {published data only}
- NCT04226807. Impact of Earlier Postpartum Contact on Postpartum Visit Compliance and Maternal Wellbeing [Re-imagining the Postpartum Process: The Impact of Earlier Postpartum Contact on Attendance at Postpartum Visits and Maternal Wellbeing]. Https://clinicaltrials.gov/show/NCT04226807 (first received 2020 Jan 13). [CENTRAL: CN-02079327]
NCT04257552 {published data only}
- NCT04257552. Care After Pregnancy Study (CAPS): Engaging Women in Postpartum Care [Care After Pregnancy Study (CAPS): Randomized Controlled Trial of Patient Engagement After Pregnancy]. Https://clinicaltrials.gov/show/NCT04257552 (first received 2020 February 06). [CENTRAL: CN-02080137]
Additional references
AAP 1998
- American Academy of Pediatrics Council on Child and Adolescent Health. The role of home-visitation programs in improving health outcomes for children and families. Pediatrics 1998;101(3):486-9. [DOI] [PubMed] [Google Scholar]
AAP 2009
- American Academy of Pediatrics Council on Child and Adolescent Health. The role of preschool home-visiting programs in improving children's developmental and health outcomes. Pediatrics 2009;123(2):598-603. [DOI] [PubMed] [Google Scholar]
Barlow 2007
- Barlow J, Davis H, McIntosh E, Jarrett P, Mockford C, Stewart-Brown S. Role of home visiting in improving parenting and health in families at risk of abuse and neglect: results of a multicentre randomised controlled trial and economic evaluation. Archives of Disease in Childhood 2007;92(3):229-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bashour 2008
- Bashour HN, Kharouf MH, Abdulsalam AA, El Asmar K, Tabbaa MA, Cheikha SA. Effect of postnatal home visits on maternal/infant outcomes in Syria: a randomized controlled trial. Public Health Nursing 2008;25(2):115-25. [DOI] [PubMed] [Google Scholar]
Bennett 2008
- Bennett C, Macdonald G, Dennis JA, Coren E, Patterson J, Astin M, et al. Home-based support for disadvantaged adult mothers. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD003759. [DOI: 10.1002/14651858.CD003759.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2002
- Brown S, Small R, Faber B, Krastev A, Davis P. Early postnatal discharge from hospital for healthy mothers and term infants. Cochrane Database of Systematic Reviews 2002, Issue 3. Art. No: CD002958. [DOI: 10.1002/14651858.CD002958] [DOI] [PubMed] [Google Scholar]
Carabin 2005
- Carabin H, Cowan LD, Beebe LA, Skaggs VJ, Thompson D, Agbangla C. Does participation in a nurse visitation programme reduce the frequency of adverse perinatal outcomes in first-time mothers? Paediatric and Perinatal Epidemiology 2005;19(3):194-205. [DOI] [PubMed] [Google Scholar]
Doggett 2005
- Doggett C, Burrett S, Osborn DA. Home visits during pregnancy and after birth for women with an alcohol or drug problem. Cochrane Database of Systematic Reviews 2005, Issue 4. Art. No: CD004456. [DOI: 10.1002/14651858.CD004456.pub2] [DOI] [PubMed] [Google Scholar]
Donovan 2007
- Donovan EF, Ammerman RT, Besl J, Atherton H, Khoury JC, Altaye M, et al. Intensive home visiting is associated with decreased risk of infant death. Pediatrics 2007;119(6):1145-51. [DOI] [PubMed] [Google Scholar]
Higgins 2019
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews. The Cochrane Collaboration, 2019. Available from www.cochrane-handbook.org.
Jahanfar 2013
- Jahanfar S, Janssen PA, Howard LM, Dowswell T. Interventions for preventing or reducing domestic violence against pregnant women. Cochrane Database of Systematic Reviews 2013, Issue 2. Art. No: CD009414. [DOI: 10.1002/14651858.CD009414.pub2] [DOI] [PubMed] [Google Scholar]
Lassi 2015
- Lassi ZS, Bhutta ZA. Community-based intervention packages for reducing maternal and neonatal morbidity and mortality and improving neonatal outcomes. Cochrane Database of Systematic Reviews 2015, Issue 3. Art. No: CD007754. [DOI: 10.1002/14651858.CD007754.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Macdonald 2008
- Macdonald G, Bennett C, Dennis JA, Coren E, Patterson J, Astin M, et al. Home-based support for disadvantaged teenage mothers. Cochrane Database of Systematic Reviews 2008, Issue 1. Art. No: CD006723. [DOI: 10.1002/14651858.CD006723.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
McIntosh 2009
- McIntosh E, Barlow J, Davis H, Stewart-Brown S. Economic evaluation of an intensive home visiting programme for vulnerable families: a cost-effectiveness analysis of a public health intervention. Journal of Public Health 2009;31(3):423-33. [DOI] [PubMed] [Google Scholar]
Olds 1997
- Olds DL, Eckenrode J, Henderson CR Jr, Kitzman H, Powers J, Cole R, et al. Long-term effects of home visitation on maternal life course and child abuse and neglect. Fifteen-year follow-up of a randomized trial. JAMA 1997;278(8):637-43. [PubMed] [Google Scholar]
RevMan 2020 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.4.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Shaw 2006
- Shaw E, Levitt C, Wong S, Kaczorowski J, McMaster University Postpartum Research Group. Systematic review of the literature on postpartum care: effectiveness of postpartum support to improve maternal parenting, mental health, quality of life, and physical health. Birth 2006;33(3):210-20. [DOI] [PubMed] [Google Scholar]
Turnbull 2012
- Turnbull C, Osborn DA. Home visits during pregnancy and after birth for women with an alcohol or drug problem. Cochrane Database of Systematic Reviews 2012, Issue 1. Art. No: CD004456. [DOI: 10.1002/14651858.CD004456.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2005
- World Health Organization. Make every mother and child count. The World Health Report 2005.
WHO 2019
- WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. Maternal mortality: Levels and trends 2000 to 2017. WHO, 2019. [Google Scholar]
WHO/UNICEF 2009
- WHO/UNICEF. Joint statement. Home visits for the newborn child: a strategy to improve survival. WHO/FCH/CAH/09.02 2009. [PubMed]
References to other published versions of this review
Yonemoto 2011
- Yonemoto N, Nagai S, Mori R. Schedules for home visits in the early postpartum period. Cochrane Database of Systematic Reviews 2011, Issue 9. Art. No: CD009326. [DOI: 10.1002/14651858.CD009326] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yonemoto 2017
- Yonemoto N, Dowswell T, Nagai S, Mori R. Schedules for home visits in the early postpartum period. Cochrane Database of Systematic Reviews 2017, Issue 8. Art. No: CD009326. [DOI: 10.1002/14651858.CD009326.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


