Abstract
Background
Most persons with type 2 diabetes are overweight and obesity worsens the metabolic and physiologic abnormalities associated with diabetes.
Objectives
The objective of this review is to assess the effectiveness of lifestyle and behavioral weight loss and weight control interventions for adults with type 2 diabetes.
Search methods
Studies were obtained from computerized searches of multiple electronic bibliographic databases, supplemented with hand searches of selected journals and consultation with experts in obesity research.
Selection criteria
Studies were included if they were published or unpublished randomized controlled trials in any language, and examined weight loss or weight control strategies using one or more dietary, physical activity, or behavioral interventions, with a follow‐up interval of at least 12 months.
Data collection and analysis
Effects were combined using a random effects model.
Main results
The 22 studies of weight loss interventions identified had a 4,659 participants and follow‐up of 1 to 5 years. The pooled weight loss for any intervention in comparison to usual care among 585 subjects was 1.7 kg (95 % confidence interval [CI] 0.3 to 3.2), or 3.1% of baseline body weight among 517 subjects. Other main comparisons demonstrated non significant results: among 126 persons receiving a physical activity and behavioral intervention, those who also received a very low calorie diet lost 3.0 kg (95% CI ‐0.5 to 6.4), or 1.6% of baseline body weight, more than persons receiving a low‐calorie diet. Among 53 persons receiving identical dietary and behavioral interventions, those receiving more intense physical activity interventions lost 3.9 kg (95% CI ‐1.9 to 9.7), or 3.6% of baseline body weight, more than those receiving a less intense or no physical activity intervention. Comparison groups often achieved significant weight loss (up to 10.0 kg), minimizing between‐group differences. Changes in glycated hemoglobin generally corresponded to changes in weight and were not significant when between‐group differences were examined. No data were identified on quality of life and mortality.
Authors' conclusions
Weight loss strategies using dietary, physical activity, or behavioral interventions produced small between‐group improvements in weight. These results were minimized by weight loss in the comparison group, however, and examination of individual study arms revealed that multicomponent interventions including very low calorie diets or low calorie diets may hold promise for achieving weight loss in adults with type 2 diabetes.
Plain language summary
Long‐term non‐pharmacological weight loss interventions for adults with type 2 diabetes mellitus
Most persons with type 2 diabetes are overweight, and the health of these persons can be improved with weight loss. Weight loss is very difficult to achieve in the long‐term, however, particularly among persons with diabetes. This systematic review of diet, physical activity, and behavioral interventions for weight loss, revealed a decrease in weight of 1.7 kg at one year or more. These results were minimized by weight loss in the comparison group, however. No data were identified on quality of life or mortality.
Background
Description of the condition
Diabetes mellitus is a metabolic disorder resulting from a defect in insulin secretion, insulin action, or both. A consequence of this is chronic hyperglycaemia (i.e. elevated levels of plasma glucose) with disturbances of carbohydrate, fat and protein metabolism. Long‐term complications of diabetes mellitus include retinopathy, nephropathy and neuropathy. The risk of cardiovascular disease is increased. For a detailed overview of diabetes mellitus, please see under 'Additional information' in the information on the Metabolic and Endocrine Disorders Group in The Cochrane Library (see 'About the Cochrane Collaboration', 'Collaborative Review Groups (CRGs)'). For an explanation of methodological terms, see the main Glossary in The Cochrane Library.
The prevalence of obesity and diabetes continues to increase in the developed world, with recent self‐reported, survey data indicating that more than 56% of adults are overweight, and about 20% are obese (Mokad 2001). Overweight is defined as a body mass index (BMI) of 25 to 29.9 kg/m2, and obesity as a BMI of ≥30 kg/m2 (NHLBI 1998). Of U.S. adults over the age of 20 years, 8.6% have diabetes, of which one third are undiagnosed (CDC 2002). Both obesity and weight gain are major risk factors for diabetes (Maggio 1997; Pi‐Sunyer 2000) and every 1‐kg increase in average self‐reported weight is associated with a 9% relative increase in the prevalence of diabetes (Mokdad 2000). Eighty to ninety percent of persons with type 2 diabetes are obese (Wing 2000) and obesity worsens the metabolic and physiologic abnormalities associated with diabetes, particularly hyperglycemia, hyperlipidemia, and hypertension (Maggio 1997; Wing 2000).
Description of the intervention
One of the cornerstones of diabetes care for overweight persons is weight loss, as insulin sensitivity and glycemic control improve (Pi‐Sunyer 2000), and moderate, intentional weight loss is associated with a reduced mortality (Williamson 2000). For persons with diabetes, weight loss improves lipid profiles, decreasing triglycerides and low‐density lipoprotein (LDL) cholesterol levels, and improves blood pressure (Maggio 1997), mental health, and quality of life (Wing 1987; Wing 1991). However, these benefits are thought to be clinically meaningful only if weight loss is sustained over time (Wing 1985).
Dietary and behavioral treatment for weight loss can produce an average loss of 8% of initial body weight over 3 to 12 months (NHLBI 1998). However, it is difficult to define effective weight control measures for the long term in the general population (NHLBI 1998; O'Meara 1998). The majority of obese patients regain most of the weight initially lost in successful interventions (Maggio 1997; Wadden 1989; Wing 1985). Skender and colleagues note that most of the weight lost in the early phase (16 to 20 weeks) is regained within 2 to 5 years (Skender 1996). Even when weight loss is effectively achieved using consistent, multi‐factorial behavioral therapy for 6 months or more, one‐third of weight loss is usually regained over the subsequent year (Perri 1993).
Obese or overweight persons with diabetes may face different and additional issues from non‐diabetic persons trying to achieve weight loss. Studies suggest that persons with diabetes lose less weight than nondiabetic persons and they regain their weight more rapidly (Wing 2000), although the mechanisms responsible are unclear and the validity of this observation has not been systematically examined. (Wing 2000). Complex treatment regimens for diabetes, hypertension, and hyperlipidemia complicate behavioral change aimed at weight reduction.
It is thus important to evaluate the scope of our knowledge on the effectiveness of dietary, physical activity, and behavioral interventions for weight loss and weight control in type 2 diabetes. Behavioral strategies are based on behavioral and learning principles and provide tools for overcoming barriers to improving and optimizing diet and physical activity levels. Most importantly, effective long‐term interventions must be defined for these populations. In addition, it is important to determine areas of uncertainty where further research is needed.
Why it is important to do this review
We have identified two relevant systematic reviews in the English literature. Brown et al. (Brown 1996) examined the effectiveness of weight loss interventions in obese persons with type 2 diabetes. They searched the published and unpublished English language literature prior to 1995. Outlier studies were removed to achieve homogeneity, interventions were stratified very broadly (all behavioral interventions in one stratum, for example), and pooled effects included all types of study designs (i.e. pre versus post and randomized controlled trials). These researchers found that diet alone had the largest effect on weight loss and metabolic control, while behavioral therapies alone and exercise alone produced the smallest changes in weight. They found few studies with follow‐up intervals longer than 6 months.
Ciliska et al. (Ciliska 1995) also reviewed the literature on the results of weight loss in obese persons with non‐insulin dependent diabetes. They searched the published, English language literature from 1985 forward and included only studies with a comparison group. They synthesized studies in a narrative fashion, and concluded that weight loss and metabolic control can be achieved, but not maintained.
These two reviews provide useful information but are outdated. Another useful summary is that of Boulé and colleagues (Boule 2001) in a meta‐analysis (based on a Cochrane review) of the effects of exercise on glycemic control and BMI in type 2 diabetes. They focused on predetermined programs of physical activity, and did not focus on weight loss as a goal for studies included in their review.
The aims of this review are to provide a comprehensive examination of the effectiveness of long‐term weight loss and weight control interventions in adults with type 2 diabetes. This review will be updated at regular intervals to reflect the addition of new research.
Objectives
The objective of this study is to assess the effectiveness of long‐term weight loss and weight control interventions for adults type 2 diabetes.
Primary research questions
which intervention strategies achieve or maintain weight loss, and what characteristics of those strategies correlate with weight loss or maintenance?
what characteristics of populations correlate with weight loss?
how does follow‐up interval relate to weight loss?
Secondary research question
what intervention strategies affect lipids, blood pressure, glycemic control, morbidity and mortality, and quality of life?
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs) with a follow‐up period of 12 months or greater as long‐term weight loss is needed to produce effects on health outcomes such as cardiovascular disease events (Wing 1985). We define follow‐up period as the time from randomization until the last measurement in the study. The intervention itself may be of any duration. We had initially planned to include all types of study designs, however, since we identified an adequate number of RCTs, we decided to restrict this review to RCTs only.
Types of participants
Participants were persons ≥18 years of age with type 2 diabetes. If the type of diabetes was not specified, studies were included if they involved adults with diabetes, with or without insulin treatment. Studies of mixed type 1 and type 2 populations were included if outcomes were reported separately for persons with type 2 diabetes. Persons labelled with "non‐insulin‐dependent diabetes" (NIDDM) were assumed to have type 2 diabetes. Studies involving only "insulin‐dependent diabetes" (IDDM) participants were excluded unless there was information to indicate that they had type 2 disease (e.g., concurrent use of oral hypoglycemic agents and insulin).
The acceptable diagnostic criteria for diabetes included those described by the National Diabetes Data Group standards (NDDG 1979), the World Health Organization standards (Alberti 1998; WHO 1980; WHO 1985) or the American Diabetes Association standards (ADA 1997). If the diagnostic criteria were not given in the study, the author's statement of the diagnosis of diabetes among study participants was accepted.
Study participants were of any weight or BMI at baseline; they did not have to be overweight or obese. Weight loss or weight maintenance decreases cardiovascular risk in persons with a BMI less than 25.0 kg/m2 (Eriksson 1991; Kuller 2001; Ornish 1998) (the lower limit for overweight). In addition, when Brown and colleagues reviewed weight loss interventions in diabetes (Brown 1996) they found that many studies did not clearly identify participants as overweight or obese, and when they used overweight/obese as an inclusion criteria they had to eliminate many studies.
Types of interventions
Interventions included in the review had weight loss or weight control as one of the primary stated goals of the intervention. Interventions where weight loss is a secondary or unexpected result were not included. Interventions focused on the patient, rather than the provider or health care system. Interventions which focus on changing provider behavior were excluded, even when outcomes are measured in the patient (e.g., an intervention to educate providers about weight loss counselling, with patient weight the main outcome).
Interventions were classified as dietary, physical activity, or behavioral strategies. Dietary programs involve providing recommendations and/or material support for achieving a specific dietary regime where the goal is weight loss or weight control. All types of dietary programs initiated for the purpose of weight loss or control were examined in this review, including low‐calorie diets (800 to 1,500 kcal/day) and very low calorie diets (<800 kcal/day) (NHLBI 1998). Studies were excluded if the sole purpose of the intervention was nutrition education or to teach about diet (e.g., carbohydrate and fat exchanges, food consumption and the relationship to glycemia), but not explicitly to achieve weight loss or weight control.
Physical activity programs were included if one of the primary goals of the program was to achieve weight loss or weight control by increased physical activity. Physical activity interventions included a specific approach to increasing activity levels, including counseling, an exercise prescription, or participation in either a supervised or unsupervised exercise program. When it was simply stated that participants were advised to increase their level of exercise, with no details of the intervention, was not considered a physical activity program.
Behavioral strategies are based on behavioral and learning principles and address barriers to diet or physical activity (NHLBI 1998). These strategies include one or more of the following interventions: education, cognitive‐behavioral therapy (e.g., stimulus control, reinforcement, goal setting), social support, or psychotherapy.
Studies were included that examined the combined effect of two or more of the interventions discussed above.
Excluded interventions
We excluded pharmacologic therapy, surgery, acupuncture, and hypnosis for the purpose of weight loss as these interventions have very different mechanisms of action and are best dealt with by a separate review. We also excluded herbal remedies and dietary supplements. Provider‐focused interventions will be excluded, as mentioned above.
Types of comparison interventions
We included studies with a range of comparison groups in order to determine which interventions were more effective than others.
The comparison group could receive
no intervention;
usual care;
the same intervention at a different intensity (frequency, duration, time frame for delivery);
any other weight loss or weight control intervention: behavioral strategy, dietary program, physical activity program, drug therapy, surgery).
Effect modifiers
We planned on examining the following effect modifiers, If there were sufficient data: adherence to treatment, initial weight, comorbidities, change in smoking status, physical activity, baseline glycemic control, change in hypoglycemic medications, and medications for comorbidities such as hypertension and hyperlipidemia.
Types of outcome measures
Primary outcomes
weight, body mass index (BMI), % weight loss from baseline weight, abdominal fat distribution;
mortality;
quality of life.
Secondary outcomes
morbidity;
cardiovascular disease events;
glycated hemoglobin (GHb);
fasting blood sugar;
serum lipids;
blood pressure;
adverse events;
cardiovascular fitness;
incidence of hypertension;
biliary tract disease.
Timing of outcome assessment
Studies with a follow‐up period of 12 months or greater were included in this review. In order for weight loss to produce long‐term effects on health outcomes such as cardiovascular disease events, the demonstration of long‐term weight loss is needed (Wing 1985). Therefore, we excluded interventions for which follow‐up was less than 12 months, and data from all follow‐up intervals greater than or equal to 12months were obtained.
Search methods for identification of studies
Electronic searches
Using Medical Subject Headings (MeSH) and text words, we searched the following databases between the date indicated and May 2004: MEDLINE (1966), EMBASE (1980), CINAHL (1982), ERIC (1980), PsychInfo (1967), Web of Science (1981), Biosis (1969), Nutrition Abstracts and Review (1980), The Cochrane Library (2004, issue 2), and the Cochrane Central Register of Controlled Trials (2004, issue 2). We manually searched journals expected to have the highest relevance, from 1980: Diabetes Care, International Journal of Obesity and Related Metabolic Disorders, Obesity Research (commenced in 1993), American Journal of Clinical Nutrition, and Journal of the American Dietetic Association. Lists of all included studies and relevant reviews were examined and experts in obesity were consulted for additional citations. The National Heart, Lung, and Blood Institute 1998 review (NHLBI 1998) and the University of York, National Health Centre for Reviews and Dissemination review (UYCRD 1997) were reviewed for relevant citations.
There were no language restrictions on our searches.
Search strategies: MEDLINE search strategy is displayed under Appendix 1. Search strategies for other databases can be obtained from the authors.
Searching other resources
Conference proceedings and abstracts were included in a sensitivity analysis, but not in the primary review, because there was insufficient detail available in these to evaluate the intervention and the quality of the study. Dissertations were excluded, as these are difficult to locate in full text.
Data collection and analysis
Selection of studies
The titles and abstracts were obtained from the searches of the electronic databases noted above and potentially relevant full‐text articles were then screened. Two people screened the MEDLINE titles and abstracts.
Data extraction and management
For studies that fulfilled inclusion criteria, two reviewers independently abstracted relevant population and intervention characteristics using a standardized template and consensus was achieved through discussion. Outcomes examined included weight loss, percent weight loss (based on individual data), BMI, fasting blood glucose, glycated hemoglobin, blood pressure, and lipid concentrations. Abstraction of data was not blinded, as there is no evidence that blinding of this process decreases bias in the conduct of systematic reviews and meta‐analyses (Berlin 1997; Irwig 1994). We attempted to contact the authors for missing data or when we needed clarification of the data presented.
For continuous outcomes we abstracted for each study group the baseline sample size, pre‐ and post‐ intervention mean and measure of dispersion (SD [standard deviation], standard error of the mean (SEM), or 95% confidence interval) for the intervention and comparison groups. When necessary, mean and SD were approximated from figures using an image scanner to optimize resolution. If only range (Rhiel 1986) for interquartile range (Hoaglin 1983) were given as the measure of variation, the standard deviation (SD) was estimated.
Assessment of risk of bias in included studies
We assessed internal validity by examining each study for potential selection, attrition, and detection bias (Clarke 2001) factors thought to have significant effects on measured outcomes in intervention studies (Feinstein 1985). Studies were not excluded on the basis of poor quality; a sensitivity analysis was planned to compare results between studies with potential bias and those without.
Measures of treatment effect
Glycated hemoglobin [either hemoglobin A1 (HbA1) or hemoglobin A1c (HbA1c)] was measured with a variety of techniques. We converted studies reporting HbA1 to HbA1c values using a formula (Little 1991), and conducted a sensitivity analysis using only studies which reported HbA1c in the original data.
Assessment of heterogeneity
Heterogeneity was examined using a standard chi‐squared test and a significance level of alpha=0.1, in view of the low power of such tests. Heterogeneity was also examined with I2 , where I2 values of 75% indicate a high level of heterogeneity (Higgins 2003). When heterogeneity was found, we attempted to determine potential reasons for it by examining individual study characteristics and those of subgroups of the main body of evidence.
We analyzed the effects of treatment in individual study arms so as to examine within‐group changes for both intervention and comparison groups. These effects were also pooled using the DerSimonian and Laird random‐effects model (DerSimonian 1954).
Assessment of reporting biases
Funnel plots were used in exploratory data analysis to assess for the potential existence of small sample bias. There are a number of explanations for the asymmetry of a funnel plot, including true heterogeneity of effect with respect to study size, poor methodological design of small studies (Sterne 2001), and publication bias. Thus this exploratory data tool may be misleading (Tang 2000; Thornton 2000) and we did not place undue emphasis on this tool (Tang 2000).
Data synthesis
When data were available which were sufficiently similar with respect to interventions and outcomes, pooled estimates of effect were obtained using Review Manager software. For continuous variables reported in the same scale, the absolute differences in outcome between each follow‐up and the baseline measure was reported for each study group (delta I and delta C). The estimate of variance of (delta I) and (delta C) was calculated from the outcome measures in each study group using the formula Vpre+ Vpost ‐ 2r(SDpre*SDpost), where Vpre is the variance of the mean baseline outcome, Vpost is the variance of the mean follow‐up outcome, r is the correlation between the baseline and follow‐up values, and SDpre and SDpost are the standard errors of the baseline and follow‐up groups, respectively. The variance of (delta) was then calculated as the sum of the variance of (delta I) and the variance of (delta C). Because studies do not report r, and its true value is unknown, a sensitivity analysis was performed using values of 0.25, 0.5, 0.75 and 1.0. Tabular data and the figure are presented using r=0.75, unless otherwise noted.
Data were pooled using the random‐effects model, with the DerSimonian and Laird formula for calculating between‐study variance (DerSimonian 1954). Each study was weighted by the inverse of the study variance.
Meta‐regression was performed to determine whether various study‐level characteristics (follow‐up interval, duration of the intervention, number of intervention contacts, total attrition, year of publication) affected the between‐group change in weight. We examined interaction terms for all models. We used SAS for the meta‐regression (version 8.02, SAS Institute Inc., Cary, N.C.).
Subgroup analysis and investigation of heterogeneity
Should the quantity of data permit, we planned to examine subgroups based on intervention components, care delivered to the comparison group, and demographic characteristics.
Sensitivity analysis
We compared the results of fixed‐ and random‐effects models and different values of the correlation coefficient between pre‐and post‐values. Should the quantity of data permit, we planned to perform a sensitivity analysis based on attrition rates, use of last‐outcome‐carried‐forward, and language of publication.
Results
Description of studies
Results of the search
The study flow diagram is presented in Figure 1; no unpublished studies were identified. Two studies were excluded from our meta‐analysis: one (Heitzmann 1987) fulfilled our inclusion criteria but did not provide any measure of dispersion for outcomes, and another (Ash 2003) combined intervention and control groups.
1.
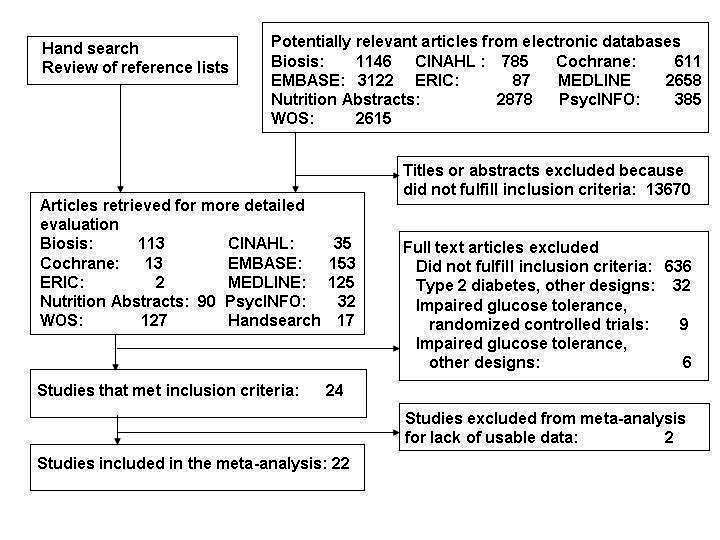
Study flow diagram
Included studies
Characteristics of the 22 eligible studies (in 21 publications) (Wing 1991a; Wing 1985 (BM); Heller 1988; Korhonen 1987; Pissarek 1980; Zapotoczky 2001; Hanefeld 1991; Sone 2002; Trento 2002; Uusitupa 1993; Hockaday 1978; Metz 2000; Milne 1994; Muchmore 1994; Kaplan 1987 (D+PA); Wing 1988a (Study 1); Wing 1988a (Study 2); Pascale 1995; Wing 1986; Wing 1988b; Wing 1991b; Wing 1994) are shown in the Table of Included Studies. (Wing reported two studies in one publication (Wing 1988a (Study 1)). All studies focused on weight loss interventions, with or without subsequent interventions to maintain weight; no study addressed interventions for weight control in persons with diabetes who were not overweight or obese. The 22 studies had a total of 4,659 participants (range 20 to 2,205). Follow‐up intervals ranged between 1 and 5 years, and the interventions lasted 10 weeks to 5 years. Mean age was 55 years and mean duration of diabetes 6.5 years (means not weighted). Mean baseline weight for the comparison groups was 91.8 kg (range, 76.4 to 106.8 kg) and mean body mass index was 33.2 kg/m2 (range, 23.0 to 38.1 kg/m2). The only study with a BMI ≤25 kg/m2 involved a weight loss intervention where the goal was a BMI ≤22 kg/m2 (Sone 2002).
The interventions included in this review are heterogeneous with respect to their components (Figure 2). There were considerable differences in the care provided to the comparison group. In nine studies this group received usual care (Wing 1985 (BM); Heller 1988; Korhonen 1987; Pissarek 1980; Zapotoczky 2001; Hanefeld 1991; Sone 2002; Trento 2002; Uusitupa 1993), but in six studies the comparison group received a dietary, physical activity, and a behavioral intervention, differing from the intervention group only in the number of calories delivered (very low calorie diet versus low calorie diet) (Wing 1991a; Wing 1994), the type of behavioral intervention (specifically self‐monitoring blood glucose (Wing 1986; Wing 1988b) or spousal involvement in education (Wing 1991b), or the type of diet (Pascale 1995). Four studies examined different intensities and methods of delivery of a low calorie diet (Hockaday 1978; Metz 2000; Milne 1994; Muchmore 1994).
2.
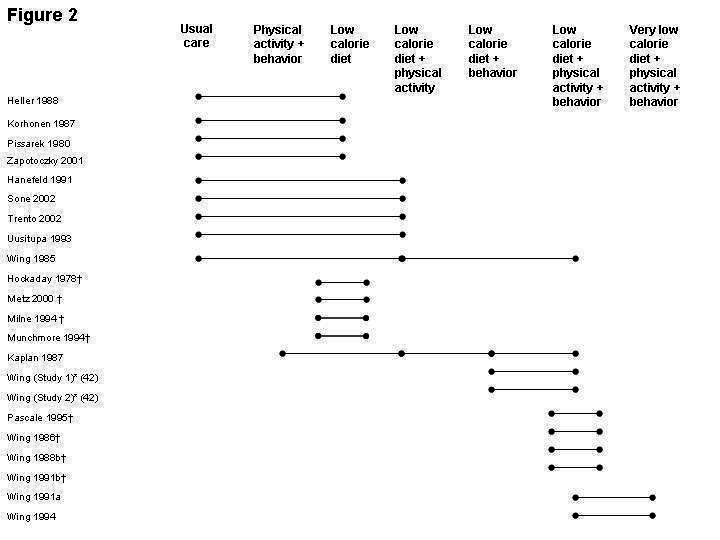
Risk of bias in included studies
Most participants were from a clinic population, and participants either self‐selected (n=8), were selected by providers (n=2), or the sampling method was unclear (n=6). Three studies obtained participants by probability sampling (Pissarek 1980; Trento 2002; Wing 1991b). In another two studies the entire accessible population was examined (29,33). The randomization procedure was described in only two studies (Trento 2001; Metz 2000) and allocation concealment was adequately reported in only one (Metz 2000). Attrition ranged between 0% and 30%, and five studies had completion rates less than 80%. Blinding of the assessor was reported in only one study (Metz 2000) and blinding of the provider in just one as well (Trento 2001).
Effects of interventions
Weight
The effects of interventions on between‐group change in weight are shown in Figure 3 and Appendix 2. Due to heterogeneity of interventions and comparisons, we believed it appropriate to obtain pooled estimates for only three groups of studies (Appendix 2; Appendix 3) any intervention versus usual care, very low calorie diet versus low calorie diet, and physical activity versus no or less intensive physical activity. In the first group (seven studies with available data; one study had two intervention groups (Wing 1985 (BM)) the intervention group received a dietary intervention with or without a behavioral or activity intervention (Wing 1985 (BM);Heller 1988; Korhonen 1987; Pissarek 1980; Zapotoczky 2001; Uusitupa 1993; Trento 2001), and the pooled effect for interventions with a follow‐up between 1 and 2 years was a reduction in weight of 1.7 kg (95% CI, 0.3 to 3.2) (585 subjects) (3.1% of baseline weight, 517 subjects). In the two studies that compared very low calorie diets with low calorie diets (Wing 1991a; Wing 1994) the pooled effect was a nonsignificant reduction of 3.0 kg (95% CI, ‐0.5 to 6.4) (1.6% of baseline weight) (126 subjects) at 72 and 104 weeks of follow‐up. In the two studies by Wing et al. reported in a single article (Wing 1988a (Study 1); Wing 1988a (Study 2)), in which a combination of dietary, physical activity, and behavioral interventions was compared to identical interventions with either no or less physical activity, the pooled effect among 53 subjects was also not significant: a loss of 3.9 kg (95% CI, ‐1.9 to 9.7) (3.6%). Within‐group changes in weight for the intervention and comparison groups are presented in Appendix 3. Effects are pooled by intervention type, regardless of randomization (i.e., each study arm is treated as a pre‐ versus post‐design study). Weight change for control groups ranged from a gain of 2.1 kg (a group receiving usual care) (Uusitupa 1993) to a loss of 8.2 kg (a 1000 kcal/day deficit diet with 20 visits over 52 weeks (Wing 1986)). In the intervention groups, weight loss ranged from 0.6 kg (a 5‐visit intervention with group counseling on a high‐carbohydrate, low‐fat diet (39)) to 8.6 kg (a combined in‐patient out‐patient 800 kcal/day diet (Pissarek 1980)). More marked weight loss was noted in an intervention group (14.5 kg) as well as in the control group (10.0 kg) at preliminary (1 year) follow‐up with a very low calorie diet combined with a physical activity and a behavioral intervention (Wing 1995). The pooled effects for six intervention types, including usual care (Appendix 3), revealed that all produced significant (p<0.05) weight loss, with a very low calorie diet combined with physical activity and behavioral therapy producing the largest effect.
3.
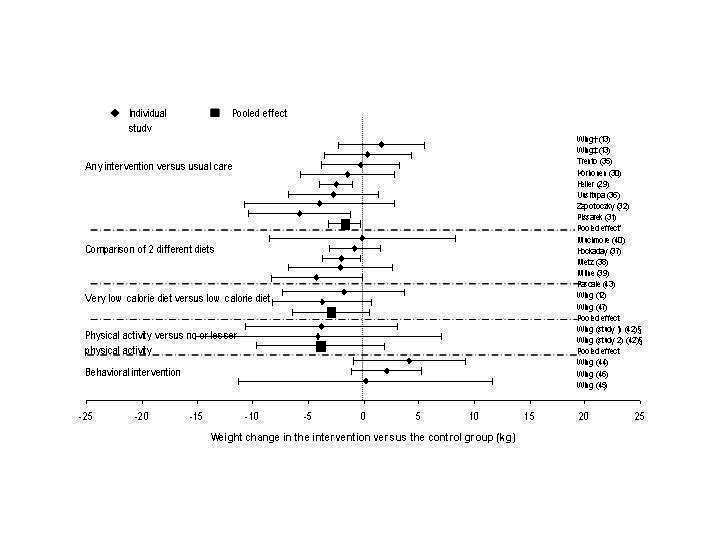
Between‐group change in weight (kg) stratified by intervention type.
Other outcomes
Between‐group changes in HbA1c (Figure 4; Appendix 2) (range, ‐2.6% to 1.0%) generally corresponded to changes in weight, and between‐group pooled estimates were generally not significant (Appendix 4; Appendix 5), although several included studies did have a significant decrease in HbA1c (Wing 1991a; Wing 1991a; Kaplan 1987 (D+PA) (diet and physical activity (Trento 2001). Between‐study heterogeneity was significant (P<0.05) for these small groups of studies.
4.
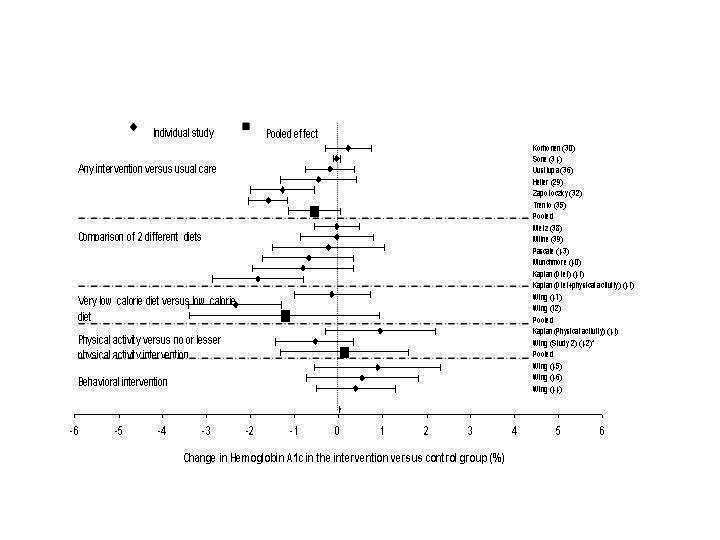
Between‐group change in hemoglobin A1c (%), stratified by intervention type.
Systolic blood pressure was examined in six studies (Zapotoczky 2001; Sone 2002; Trento 2002; Uusitupa 1993; Metz 2000; Wing 1994), with a between‐group change ranging between 1 mm Hg and ‐4 mm Hg; similar results were noted for diastolic blood pressure. Thirteen studies reported between‐group change in total cholesterol (Wing 1991a; Pissarek 1980; Zapotoczky 2001; Hanefeld 1991; Sone 2002; Trento 2002; Uusitupa 1993; Hockaday 1978; Metz 2000; Milne 1994; Wing 1988a (Study 1); Pascale 1995; Wing 1994) (range, ‐0.4 mmol/l [‐7.2 mg/dl] to 0.3 mmol/l [5.9 mg/dl]). No data were identified on mortality, morbidity, adverse events, or quality of life among the studies included in this review.
Regression and sensitivity analyses
The funnel plot was asymmetric, indicating potential small sample bias (Figure 5). For net change in weight, we found no significant interactions with follow‐up interval, duration of the intervention, number of intervention contacts, or year of publication and weight. Higher total attrition rates were correlated with increased weight loss (p=0.028).
5.
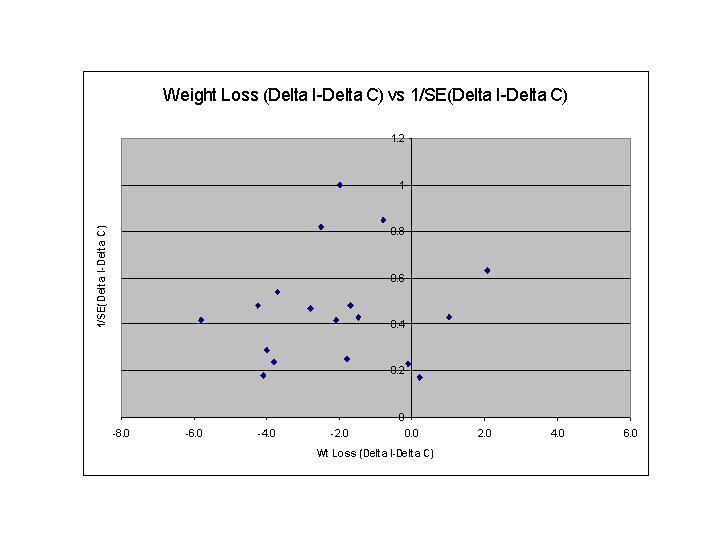
Sensitivity analyses showed only minor changes in pooled estimates and 95% CI when the correlation between pre‐ and post‐ values was assigned different values. Because most studies did not report components of quality that were assessed (method of randomization, allocation concealment, and blinding of the assessor), we could not examine the effects of these variables on outcomes.
Pooled effects for studies reporting only HbA1c produced no significant differences from the outcomes achieved by converting HbA1 measures to HbA1c. The performance of stratified analyses was limited by sparse data and there were not enough studies to examine whether a physical activity intervention increased the effectiveness of dietary interventions.
Discussion
Summary of main results
Randomized, controlled trials of weight loss interventions using diet with or without physical activity or behavioral interventions report only small additional declines in weight and HbA1c over those achieved by comparison groups. A partial explanation for these small changes is that the comparison group often had moderate weight loss (up to 10.0 kg), minimizing between‐group differences. Cointerventions and contamination of the comparison group, as well as the "intervention effect" of study participation likely contributed to weight loss in comparison groups.
Our examination of weight change in individual study arms (intervention and comparison) produced somewhat more promising results. Although not large, the magnitude of weight loss demonstrated from combined dietary, physical activity, and behavioral interventions is associated with improved health outcomes (NHLBI 1998). Sustained, intensive interventions (Pissarek 1980; Zapotoczky 2001) and combined initial (Wing 1991a) or intermittent (Wing 1994) very low calorie diet appear to have the most effect on weight loss, and a low calorie diet combined with physical activity and behavioral interventions was also effective (Wing 1988a (Study 1)). However, these conclusions are based on a small number of studies and it is well established that healthy changes in diet and physical activity are difficult to maintain in the long‐term (NHLBI 1998; Glenny 1997).
The results of our analysis of single arms of studies must be interpreted with caution. The observed effects are not confined to the effect of the intervention, as in our analysis of between‐group change, where the groups were obtained through randomization. Extraneous factors, both known and unknown, may be responsible for the observed effects.
At a population level, a decrease in weight of 2 to 3 kg and a decrease in HbA1c of 0.3% may well have significant health benefits, whether through small improvements in a large segment of the population, or through larger benefits achieved among the population subset with more substantial weight or HbA1c reductions (Khaw 2001).
Potential biases in the review process
Our review has limitations. First, the studies examined were very heterogeneous with respect to the interventions, limiting quantitative syntheses. Second, we had insufficient power to examine either the effectiveness of specific component interventions, or the relationships between intervention characteristics and outcomes. Third, this review is limited to published studies; although we contacted experts for additional unpublished data, none was obtained. Fourth, attrition is an important issue in weight loss studies because selective loss to follow‐up has been demonstrated; higher attrition occurs among those who do not achieve a weight loss goal (Kramer 1989), as demonstrated here with a positive relationship between higher attrition rates and increased weight loss among completers. Participants were most commonly self‐selected and thus possibly more motivated to complete the study; applicability to general populations may thus be limited. Fifth, the quality of individual studies in this review varied. Adequate randomization procedures and concealment of allocation were reported in only a few studies and blinding of the assessor was rarely implemented. Sixth, the funnel plot indicated potential small sample bias; in other words, the published studies identified in this review, with relatively small sample sizes, may represent a subset of the total body of literature. The identified studies may represent a select group reporting more weight loss than might be found if the total body of literature was available.
Agreements and disagreements with other studies or reviews
Our findings are consistent with those found in non‐diabetic populations, where comprehensive, intensive group dietary and behavioral programs produced similar mean losses in weight: 8 kg to 10 kg at 6 months with a regain of 30% to 35% of weight loss at 1 year (Wadden 2000; Kramer 1989). Our finding that very low calorie diets are not significantly more effective than low calorie diets in a small number of studies is not inconsistent with the conclusions of a prior review that found the two interventions equally effective in general populations at greater than 1 year of follow‐up (NHLBI 1998). A comprehensive review of the effectiveness of weight‐loss interventions in persons with diabetes (Brown 1996) indicated that dietary interventions alone produced an average weight loss of 9 kg and behavioral programs alone a loss of 3 kg at short‐term follow‐up; few studies examined outcomes beyond 6 months.
Healthy lifestyle changes with weight loss and a decrease in diabetes incidence have been demonstrated in the long‐term among persons with impaired glucose tolerance or impaired fasting glucose, who are at high risk of developing diabetes (DPPRG 2002; Tuomilehto 2001 ). Whether the lifestyle interventions in these two large trials can be translated to diabetic populations has yet to be determined, although we also found that more intensive and sustained interventions may be more effective.
Authors' conclusions
Implications for practice.
In conclusion, weight loss and control in the long term appear to be difficult to achieve for adults with type 2 diabetes employing currently used lifestyle and behavioral strategies, although we found a clinically meaningful decrease in weight with some interventions. Perhaps other strategies in conjunction with lifestyle interventions should be considered for weight loss or control. Pharmacotherapy achieves modest, but statistically significant, weight loss over 26 to 52 weeks in general populations (Norris 2004). Surgical interventions can produce substantial weight loss at up to 10 years of follow‐up in general populations (Pories 1992; Sjostrom 1999) and among persons with diabetes (Pories 1992; ADA 1997). Much remains to be learned about how to implement dietary, physical activity, and behavioral interventions designed to achieve weight loss and weight control in the long term and whether intensive interventions such as those successfully used in persons with impaired glucose tolerance are effective among diabetic populations.
Implications for research.
Future research needs to focus on sustained interventions for weight loss and control and long‐term outcomes for health and quality of life. The economic efficiency of effective long‐term weight loss interventions needs to be established. Effective interventions for weight control in persons not currently overweight need further attention. Methodologic standards need to be followed with rigorous application of good study design principles: allocation concealment, minimization of attrition, follow‐up of dropouts, comparison of dropouts to completers at baseline, and intention‐to‐treat analyses.
What's new
| Date | Event | Description |
|---|---|---|
| 28 October 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors wish to thank Nathalie Bousader, MD, Florence J. Dallo, MPH, and Rolanda Watkins, MPH for assistance with abstracting data from studies. Jan Stansell, MSc, and Karla Bergerhoff, MD, were invaluable in their assistance in devising and running search strategies. Mary Serdula, MD, MPH, and Barabara A Bowman, PhD, provided thoughful input and comments on the Cochrane protocol and on the related journal publication.
Appendices
Appendix 1. Search strategy
| ELECTRONIC SEARCHES: |
| Unless otherwise stated, search terms were free text terms; exp = exploded MeSH: Medical subject heading (Medline medical index term); the dollar sign ($) stands for any character(s); the question mark (?) = to substitute for one or no characters; tw = text word; pt = publication type; sh = MeSH: Medical subject heading (Medline medical index term); adj = adjacency. 1. diabetes mellitus, non insulin dependent[MeSH Terms] 2. insulin resistance[MeSH Terms] 3. obesity in diabetes[MeSH Terms] 4. impaired glucose toleranc*[Title/Abstract] 5. impaired fasting glucose 6. glucose intoleranc*[Title/Abstract] 7. insulin resist*[Title/Abstract] 8. MODY[Title/Abstract] 9. dm2[Title/Abstract] 10. niddm[Title/Abstract] 11. iidm[Title/Abstract] 12. non insulin depend*[Title/Abstract] 13. noninsulin depend*[Title/Abstract] 14. noninsulindepend*[Title/Abstract] 15. non insulin?depend*[Title/Abstract] 16. type 2 diab*[Title/Abstract] 17. type II diab*[Title/Abstract] 18. keto* resist* diabet*[Title/Abstract] 19. non keto* diabet*[Title/Abstract] 20. nonketo* diabet*[Title/Abstract] 21. adult onset diabet*[Title/Abstract] 22. late onset diabet*[Title/Abstract] 23. matur* onset diabet*[Title/Abstract] 24. slow onset diabet*[Title/Abstract] 25. stabl* onset diabet*[Title/Abstract 26. metabolic* syndrom*[Title/Abstract] 27. plurimetabolic* syndrom*[Title/Abstract] 28. pluri metabolic* syndrom*[ Title/Abstract] 29. or/1‐28 30. dermatomyositis[MeSH Terms] 31. Myotonic dystrophy[MeSH Terms] 32. Diabetes insipidus[MeSH Terms] 33. dermatomyositis[Title/Abstract] 34. myotonic dystroph*[Title/Abstract] 35. diabet* insipidus[Title/Abstract] 36. or/30‐35 37. 29 not 36 38. obesity[MeSH Terms] 39. obes*[Title/Abstract] 40. weight gain*[Title/Abstract] 41. weight gain[MeSH Terms] 42. weight loss[Title/Abstract] 43. weight loss[MeSH Terms] 44. body mass index [Title/Abstract] 45. body mass index[MeSH Terms] 46. adipos* [Title/Abstract] 47. overweight[Title/Abstract] 48. over weight [Title/Abstract] 49. overload syndrom*[Title/Abstract] 50. overeat*[Title/Abstract] 51. over eat*[Title/Abstract] 52. overfeed*[Title/Abstract] 53. over feed*[Title/Abstract] 54. weight cycling[Title/Abstract] 55. weight reduc*[Title/Abstract] 56. weight losing[Title/Abstract] 57. weight maint*[Title/Abstract] 58. weight decreas*[Title/Abstract] 59. weight watch*[Title/Abstract] 60. weight control*[Title/Abstract] 61. or/38‐60 62. exercise[MeSH Terms] 63. "physical education and training"[MeSH Terms] 64. "physical fitness"[MeSH Terms] 65. exercis*[Title/Abstract] 66. exertion*[Title/Abstract] 67. sport*[Title/Abstract] 68. walking[Title/Abstract] 69. jogging[Title/Abstract] 70. swimming[Title/Abstract] 71. strength train*[Title/Abstract] 72. resistance train*[Title/Abstract] 73. aerobic train*[Title/Abstract] 74. physical education*[Title/Abstract] 75. physical fitness[Title/Abstract] 76. training[Title/Abstract] 77. Life style[MeSH Terms] 78. Health education[MeSH Terms] 79. health behavior[MeSH Terms] 80. health promotion[MeSH Terms] 81. sports[MeSH Terms] 82. exertion[MeSH Terms] 83. exercise‐therapy[MeSH Terms] 84. nutrition[MeSH Terms] 85. nutrition*[Title/Abstract] 86. diet therapy[MeSH Terms] 87. feeding‐behavior[MeSH Terms] 88. life style[Title/Abstract] 89. lifestyle[Title/Abstract] 90. health* behav*[Title/Abstract] 91. health* educ*[Title/Abstract] 92. health* promot*[Title/Abstract] 93. physic* activ*[Title/Abstract] 94. bicyc*[Title/Abstract] 95. cycling[Title/Abstract] 96. weight lift*[Title/Abstract] 97. gymnastic*[Title/Abstract] 98. danc*[Title/Abstract] 99. diabetic diet[MeSH Terms] 100. diet*[Title/Abstract] 101. diet therapy[MeSH Terms] 102. or/62‐101 103. 37 and 61 and 102 |
Appendix 2. Outcomes summary
| Study | Follow‐up | Intervention | Weight Di‐Dc (95%CI) | GHb Di‐Dc (95%CI) |
| Hanefeld 1991 | 260 | LCD + PA | NR | NR |
| Heller 1988 | 52 | LCD | ‐2.5 (‐4.0, ‐1.0) | ‐0.5 (‐1.3, 0.4) |
| Hockaday 1978 | 52 | LCD | ‐0.8 (‐3.1, 1.5) | NR |
| Kaplan 1987 (D) | 78 | LCD | NR | ‐0.8 (‐2.0, 0.3) |
| Kaplan 1987 (D + PA) | 78 | LCD + PA | NR | ‐1.8 (‐2.9, ‐0.8) |
| Kaplan 1987 (PA) | 78 | PA | NR | 0.9 (‐0.3, 2.2) |
| Korhonen 1987 | 52 | LCD | ‐1.5 (‐5.7, 2.8) | 0.2 (‐0.3, 0.7) |
| Metz 2000 | 52 | LCD | ‐2.0 (‐3.7, ‐0.3) | ‐0.04 (‐0.6, 0.5) |
| Milne 1994 | 78 | LCD | ‐2.1 (6.8, 2.6) | ‐0.04 (‐0.9, 0.8) |
| Muchmore 1994 | 52 | LCD | ‐0.1 (‐8.5, 8.3) | ‐0.7 (‐1.7, 0.3) |
| Pascale 1995 | 52 | LCD + PA + B | ‐4.2 (‐8.6, ‐0.1) | ‐0.2 (‐1.5, 1.0) |
| Pissarek 1980 | 108 | LCD | ‐5.8 (‐10.4, ‐1.2) | NR |
| Sone 2002 | 156 | LCD + PA | NR | ‐0.1 (‐0.1, 0.0) |
| Trento 2002 | 52/104/225 | LCD + PA | ‐0.6 (‐3.6, 2.4) ‐0.3 (‐4.2, 3.6) ‐0.3 (‐3.8, 3.2) | ‐0.2 (‐0.6, 0.2) ‐0.8 (‐1.2, ‐0.4) ‐1.6 (‐2.0, ‐1.2) |
| Uusitupa 1993 | 52/104 | LCD + PA | ‐2.8 (‐4.5, ‐1.1) ‐2.7 (‐6.8, 1.3) | ‐0.2 (‐0.7, 0.3) ‐0.2 (‐0.8, 0.4) |
| Wing 1986 | 62 | LCD + PA + B | 4.1 (‐0.9, 9.2) | 0.4 (‐0.5, 1.3) |
| Wing 1988a (Study 1) | 62 | LCD + PA + B | ‐3.8 (‐10.6, 3.0) | NR |
| Wing 1988a (Study 2) | 62 | LCD + PA + B | ‐4.1 (‐15.2, 7.0) | ‐0.5 (‐1.4, 0.4) |
| Wing 1985 (NE) | 68 | LCD + PA | 0.4 (‐3.5, 4.3) | NR |
| Wing 1985 (BM) | 68 | LCD + PA + B | 1.6 (‐2.3, 5.6) | NR |
| Wing 1988b | 68 | LCD + PA + B | 0.2 (‐11.2, 11.6) | 0.9 (‐0.6, 2.3) |
| Wing 1991b | 72 | LCD + PA + B | 2.1 (‐1.1, 5.2) | 0.5 (‐0.7, 1.8) |
| Wing 1991a | 72 | VLCD + PA + B | ‐1.8 (‐7.3, 3.7) | ‐2.4 (‐3.4, ‐1.3) |
| Wing 1994 | 52/104 | VLCD + PA + B | ‐3.7 (‐8.2, 0.8) ‐1.5 (‐5.1, 2.1) | ‐0.2 (‐1.0, 0.7) ‐0.2 (‐1.2, 0.9) |
| Zapotoczky 2001 | 52 | LCD | ‐4.0 (‐10.7, 2.8) | ‐1.3 (‐2.0, ‐0.6) |
| B, behavioral intervention C, control group CI, confidence interval D, diet I, intervention group LCD, low calorie diet NR, not reported PA, physical activity VLCD, very low calorie diet | ||||
| Di‐Dc is the between‐group difference |
Appendix 3. Meta‐analysis results for weight change for single study arms
| Intervention | Number studies | N | Estimate effect | Heterogeneity (p) |
| Usual care | 7 | 564 | ‐2.0 (‐3.5 to ‐0.6) | 0.05 |
| Low calorie diet | 12 | 917 | ‐3.7 (‐5.1 to ‐2.3) | <0.0001 |
| Low calorie diet, physical activity | 3 | 232 | ‐1.8 (‐3.2 to ‐0.3) | 0.61 |
| Low calorei diet, behavioral intervention intervention | 2 | 53 | ‐4.0 (‐7.2 to ‐0.7) | 1.00 |
| Low calorie diet, activity, behavioral intervention | 13 | 485 | ‐4.1 (‐5.4 to ‐2.9) | 0.10 |
| Very low calorie diet, activity, behavioral intervention | 2 | 126 | ‐7.7 (‐9.8 to ‐5.5) | 0.55 |
Appendix 4. Summary of pooled estimates, fixed‐effects model: Intervention versus usual care
| Outcome | Number studies | N | rho = 0.25 | rho = 0.50 | rho = 0.75 | rho = 1.00 |
| Weight (kg) | 7 | 585 | ‐2.4 (95%CI ‐3.2 to ‐0.3) | ‐2.2 (95%CI ‐3.5 to ‐1.0) | ‐1.9 (95%CI ‐3.0 to ‐0.8) | ‐1.5 (95%CI ‐1.5 to ‐1.5) |
| % wt loss (%) | 6 | 517 | ‐2.9 (95%CI ‐4.5 to ‐1.4) | ‐3.0 (95%CI ‐4.5 to ‐1.5) | ‐3.1 (95%CI ‐4.5 to ‐1.7) | ‐1.9 (95%CI ‐1.9 to ‐1.9) |
| Body mass index (mg/m2) | 2 | 192 | ‐0.6 (95%CI ‐2.1 to 0.9) | ‐0.6 (95%CI ‐1.8 to 0.7) | ‐0.6 (95%CI ‐1.4 to 0.3) | ‐0.7 (95%CI ‐0.7 to ‐0.6) |
| Fasting glucose (mmol/l) | 3 | 272 | 0.3 (95%CI ‐0.5 to 1.1) | 0.3 (95%CI ‐0.3 to 1.0) | 0.3 (95%CI ‐0.1 to 0.8) | 0.2 (95%CI 0.2 to 0.2) |
| Glycated hemoglobin (%) | 5 | 381 | ‐0.4 (95%CI ‐0.8 to 0.1) | ‐0.4 (95%CI ‐0.7 to 0) | ‐0.4 (95%CI ‐0.6 to ‐0.1) | 0.2 (95%CI 0.2 to 0.2) |
| Systolic blood pressure (mmHg) | 2 | 114 | ‐1.9 (95%CI ‐9.5 to 5.7) | ‐1.9 (95%CI ‐8.2 to 4.4) | ‐1.9 (95%CI ‐6.4 to 2.7) | ‐1.5 (95%CI ‐2.8 to ‐0.2) |
| Diastolic blood pressure(mmHg) | 2 | 114 | 0 (95%CI ‐4.2 to 4.2) | 0 (95%CI ‐3.5 to 3.5) | 0 (95%CI ‐2.5 to 2.5) | 0 (95%CI ‐0.2 to 0.2) |
| Total cholesterol (mmol/l) | 4 | 344 | ‐0.2 (95%CI ‐0.5 to 0.2) | ‐0.2 (95%CI ‐0.4 to 0.1) | 0.1 (95%CI 0 to 0.1) | ‐0.1 (95%CI ‐0.1 to ‐0.1) |
| LDL cholesterol (mmol/l) | 1 | 34 | ‐0.1 (95%CI ‐0.9 to 0.7) | ‐0.1 (95%CI ‐0.8 to 0.5) | ‐0.1 (95%CI ‐0.6 to 0.4) | ‐0.1 (95%CI ‐0.3 to 0.0) |
| HDL cholesterol (mmol/l) | 3 | 226 | 0.1 (95%CI 0.0 to 0.2) | 0.1 (95%CI 0.0 to 0.2) | 0.1 (95%CI 0.1 to 0.2) | 0.0 (95%CI 0.0 to 0.0) |
| Triglycerides (mmol/l) | 3 | 226 | ‐0.4 (95%CI ‐0.7 to 0) | ‐0.4 (95%CI ‐0.7 to ‐0.1) | ‐0.4 (95%CI ‐0.6 to ‐0.1) | ‐0.2 (95%CI ‐0.2 to ‐0.1) |
Appendix 5. Summary of pooled estimates, random‐effect model: Intervention versus usual care
| Outcome | Number studies | N | rho = 0.25 | rho = 0.50 | rho = 0.75 | rho = 1.00 |
| Weight (kg) | 7 | 585 | ‐ 2.4 ( 95% CI ‐3.7 to ‐1.1) Q = 4.7 p = 0.7 I squared = 0% | ‐ 2.2 ( 95% CI ‐3.5 to ‐1.0) Q = 6.0 p = 0.5 I squared = 0% | ‐ 1.7 ( 95% CI ‐3.2 to ‐0.3) Q = 9.2 p = 0.2 I squared = 24.0% | ‐ 2.1 ( 95% CI ‐3.0 to ‐1.3) Q = 66.2 p < 0.00001 I squared = 92.3% |
| % wt loss (%) | 6 | 517 | ‐ 2.9 ( 95% CI ‐4.5 to ‐1.4) Q = 0.8 p = 1.0 I squared = 0% | ‐ 3.0 ( 95% CI ‐4.5 to ‐1.5) Q = 1.2 p = 1.0 I squared = 0% | ‐ 3.1 ( 95% CI ‐4.5 to ‐1.7) Q = 2.2 p = 0.8 I squared = 0% | ‐ 3.5 ( 95% CI ‐4.6 to ‐2.5) Q = 90.5 p < 0.00001 I squared = 94.5% |
| Body mass index (mg/m2) | 2 | 192 | ‐ 0.6 ( 95% CI ‐2.1 to 0.9) Q = 0.0 p = 0.9 I squared = 0% | ‐ 0.6 ( 95% CI ‐1.8 to 0.7) Q = 0.0 p = 0.9 I squared = 0% | ‐ 0.6 ( 95% CI ‐1.4 to 0.3) Q = 0.1 p = 0.8 I squared = 0% | ‐ 0.6 ( 95% CI ‐0.8 to ‐0.4) Q = 4.8 p = 0.03 I squared = 79.3% |
| Fasting glycose (mmol/l) | 3 | 272 | 0.3 ( 95% CI ‐0.5 to 1.1) Q = 1.5 p = 0.5 I squared = 0% | 0.3 ( 95% CI ‐0.4 to 1.0) Q = 2.3 p = 0.3 I squared = 11.5% ‐ 0.6 ( 95% CI ‐2.1 to 0.9) Q = 0.0 p = 0.9 I squared = 0% | 0.3 ( 95% CI ‐0.4 to 1.0) Q = 4.4 p = 0.1 I squared = 54.6% | 0.3 ( 95% CI ‐0.2 to 0.8) Q = 166.1 p < 0.00001 I squared = 98.8% |
| Glycated hemoglobin (%) | 5 | 381 | ‐0.4 ( 95% CI ‐0.9 to 0.2) Q = 3.6 p = 0.3 I squared = 15.5% | ‐0.3 ( 95% CI ‐0.8 to 0.2) Q = 5.3 p = 0.2 I squared = 43.0% | ‐0.3 ( 95% CI ‐0.8 to 0.2) Q = 10.2 p = 0.02 I squared = 70.7% | ‐0.3 ( 95% CI ‐0.8 to 0.2) Q = 266.7 p < 0.00001 I squared = 98.9% |
| Systolic blood pressure (mmHg) | 2 | 114 | ‐1.9 ( 95% CI ‐9.5 to 5.7) Q = 0.1 p = 0.8 I squared = 0% | ‐1.9 ( 95% CI ‐8.2 to 4.4) Q = 0.1 p = 0.8 I squared = 0% | ‐1.9 ( 95% CI ‐6.4 to 2.7) Q = 0.2 p = 0.7 I squared = 0% | ‐1.7 ( 95% CI ‐3.5 to 0.2) Q = 1.6 p = 0.2 I squared = 37.0% |
| Diastolic blood pressure (mmHg) | 2 | 114 | 0 ( 95% CI ‐4.2 to 4.2) Q = 0 p = 1.0 I squared = 0% | 0 ( 95% CI ‐3.5 to 3.5) Q = 0 p = 1.0 I squared = 0% | 0 ( 95% CI ‐2.5 to 2.5) Q = 0 p = 1.0 I squared = 0% | 0 ( 95% CI ‐0.2 to 0.2) Q = NA p = NA I squared = NA |
| Total cholesterol (mmol/l) | 4 | 344 | ‐0.2 ( 95% CI ‐0.5 to 0.2) Q = 1.1 p = 0.8 I squared = 0% | ‐0.2 ( 95% CI ‐0.4 to 0.1) Q = 1.6 p = 0.7 I squared = 0% | ‐0.1 ( 95% CI ‐0.4 to 0.2) Q = 8.7 p = 0.03 I squared = 65.7% | ‐0.2 ( 95% CI ‐0.5 to 0.1) Q = 99.5 p < 0.00001 I squared = 97.0% |
| LDL cholesteorl (mmol/l) | 1 | 34 | ‐0.1 ( 95% CI ‐0.9 to 0.7) Q = NA p = NA I squared = NA | ‐0.1 ( 95% CI ‐0.8 to 0.5) Q = NA p = NA I squared = NA | ‐0.1 ( 95% CI ‐0.6 to 0.4) Q = NA p = NA I squared = NA | ‐0.1 ( 95% CI ‐0.3 to 0.0) Q = NA p = NA I squared = NA |
| HDL cholestorel (mmol/l) | 3 | 226 | 0.1 ( 95% CI 0.0 to 0.3) Q = 4.3 p = 0.1 I squared = 52.9% | 0.1 ( 95% CI ‐0.1 to 0.2) Q = 6.3 p = 0.04 I squared = 68.3% | 0.1 ( 95% CI ‐0.1 to 0.2) Q = 11.9 p = 0.003 I squared = 83.1% | 0.1 ( 95% CI ‐0.2 to 0.4) Q = 154.6 p < 0.00001 I squared = 99.4% |
| Triglycerides (mmol/l) | 3 | 226 | ‐0.4 ( 95% CI ‐0.7 to 0) Q = 1.0 p = 0.8 I squared = 0% | ‐0.4 ( 95% CI ‐0.7 to ‐0.1) Q = 1.4 p = 0.7 I squared = 0% | ‐0.4 ( 95% CI ‐0.6 to ‐0.1) Q = 2.5 p = 0.5 I squared = 0% | ‐0.2 ( 95% CI ‐0.4 to ‐0.1) Q = 7.3 p = 0.06 I squared = 58.6% |
Data and analyses
Comparison 1. VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 weight loss (kg) | 2 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐2.95 [‐6.42, 0.53] |
| 2 BMI | 2 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐1.08 [‐2.33, 0.17] |
| 3 FBS | 2 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐1.42 [‐2.30, ‐0.54] |
| 4 GHb | 2 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐1.03 [‐1.71, ‐0.36] |
| 5 Total Cholesterol | 2 | 126 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.01, 0.51] |
| 6 HDL | 2 | 126 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [0.00, 0.11] |
| 7 Triglycerides | 2 | 126 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [‐0.14, 0.48] |
| 8 weight loss (kg) | 2 | 126 | Mean Difference (IV, Random, 95% CI) | ‐2.95 [‐6.42, 0.53] |
| 9 BMI | 2 | 126 | Mean Difference (IV, Random, 95% CI) | ‐1.08 [‐2.33, 0.17] |
| 10 FBS | 2 | 126 | Mean Difference (IV, Random, 95% CI) | ‐2.51 [‐6.42, 1.39] |
| 11 GHb | 2 | 126 | Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐3.38, 0.93] |
| 12 Total Cholesterol | 2 | 126 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.06, 0.54] |
| 13 HDL | 2 | 126 | Mean Difference (IV, Random, 95% CI) | 0.06 [0.00, 0.11] |
| 14 Triglycerides | 2 | 126 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐0.14, 0.48] |
| 15 % weight loss | 2 | 126 | Mean Difference (IV, Fixed, 95% CI) | ‐1.61 [‐4.25, 1.03] |
| 16 % weight loss (random) | 2 | 126 | Mean Difference (IV, Random, 95% CI) | ‐1.61 [‐4.25, 1.03] |
1.1. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 1 weight loss (kg).
1.2. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 2 BMI.
1.3. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 3 FBS.
1.4. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 4 GHb.
1.5. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 5 Total Cholesterol.
1.6. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 6 HDL.
1.7. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 7 Triglycerides.
1.8. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 8 weight loss (kg).
1.9. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 9 BMI.
1.10. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 10 FBS.
1.11. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 11 GHb.
1.12. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 12 Total Cholesterol.
1.13. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 13 HDL.
1.14. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 14 Triglycerides.
1.15. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 15 % weight loss.
1.16. Analysis.
Comparison 1 VLCD vs different intervention (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 16 % weight loss (random).
Comparison 2. PA (1‐10: fixed models. 11‐20: random models, rho=0.75).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 weight loss (kg) | 2 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐3.88 [‐9.71, 1.94] |
| 2 GHb | 2 | 61 | Mean Difference (IV, Fixed, 95% CI) | ‐0.04 [‐0.77, 0.69] |
| 3 weight loss (kg) | 2 | 53 | Mean Difference (IV, Random, 95% CI) | ‐3.88 [‐9.71, 1.94] |
| 4 GHb | 2 | 61 | Mean Difference (IV, Random, 95% CI) | 0.13 [‐1.31, 1.58] |
| 5 % weight loss | 2 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐3.58 [‐9.89, 2.74] |
| 6 % weight loss (random) | 2 | 53 | Mean Difference (IV, Random, 95% CI) | ‐3.58 [‐9.89, 2.74] |
2.1. Analysis.
Comparison 2 PA (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 1 weight loss (kg).
2.2. Analysis.
Comparison 2 PA (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 2 GHb.
2.3. Analysis.
Comparison 2 PA (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 3 weight loss (kg).
2.4. Analysis.
Comparison 2 PA (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 4 GHb.
2.5. Analysis.
Comparison 2 PA (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 5 % weight loss.
2.6. Analysis.
Comparison 2 PA (1‐10: fixed models. 11‐20: random models, rho=0.75), Outcome 6 % weight loss (random).
Comparison 3. Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 weight loss (kg) | 8 | 585 | Mean Difference (IV, Fixed, 95% CI) | ‐1.91 [‐3.00, ‐0.82] |
| 2 BMI | 2 | 192 | Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐1.44, 0.31] |
| 3 FBS | 3 | 272 | Mean Difference (IV, Fixed, 95% CI) | 0.34 [‐0.11, 0.80] |
| 4 GHb | 5 | 381 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐0.99, ‐0.48] |
| 5 SBP | 2 | 114 | Mean Difference (IV, Fixed, 95% CI) | ‐1.85 [‐6.41, 2.70] |
| 6 DBP | 2 | 114 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐2.49, 2.49] |
| 7 Total Cholesterol | 4 | 344 | Mean Difference (IV, Fixed, 95% CI) | 0.06 [‐0.02, 0.14] |
| 8 HDL | 3 | 226 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [0.06, 0.17] |
| 9 Triglycerides | 4 | 344 | Mean Difference (IV, Fixed, 95% CI) | ‐0.36 [‐0.58, ‐0.14] |
| 10 % weight loss | 6 | 517 | Mean Difference (IV, Fixed, 95% CI) | ‐3.10 [‐4.48, ‐1.72] |
| 11 weight loss (kg) | 8 | 585 | Mean Difference (IV, Random, 95% CI) | ‐1.72 [‐3.15, ‐0.29] |
| 12 BMI | 2 | 192 | Mean Difference (IV, Random, 95% CI) | ‐0.57 [‐1.44, 0.31] |
| 13 FBS | 3 | 272 | Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.37, 1.00] |
| 14 GHb | 5 | 381 | Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐1.44, 0.10] |
| 15 SBP | 2 | 114 | Mean Difference (IV, Random, 95% CI) | ‐1.85 [‐6.41, 2.70] |
| 16 DBP | 2 | 114 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐2.49, 2.49] |
| 17 Total Cholesterol | 4 | 344 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.41, 0.15] |
| 18 HDL | 3 | 226 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.05, 0.23] |
| 19 Triglycerides | 4 | 344 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐0.58, ‐0.14] |
| 20 % weight loss | 6 | 517 | Mean Difference (IV, Random, 95% CI) | ‐3.10 [‐4.48, ‐1.72] |
3.1. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 1 weight loss (kg).
3.2. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 2 BMI.
3.3. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 3 FBS.
3.4. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 4 GHb.
3.5. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 5 SBP.
3.6. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 6 DBP.
3.7. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 7 Total Cholesterol.
3.8. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 8 HDL.
3.9. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 9 Triglycerides.
3.10. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 10 % weight loss.
3.11. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 11 weight loss (kg).
3.12. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 12 BMI.
3.13. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 13 FBS.
3.14. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 14 GHb.
3.15. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 15 SBP.
3.16. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 16 DBP.
3.17. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 17 Total Cholesterol.
3.18. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 18 HDL.
3.19. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 19 Triglycerides.
3.20. Analysis.
Comparison 3 Any intervention vs usual care (F/U</=2y) (1‐11: fixed models. 12‐22: random models, rho=0.75), Outcome 20 % weight loss.
Comparison 4. Any intervention vs usual care (1‐2y follow‐up) (HbA1c in original paper).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 GHb (fixed model) | 4 | 306 | Mean Difference (IV, Fixed, 95% CI) | ‐0.76 [‐1.03, ‐0.50] |
| 2 GHb (random model) | 4 | 306 | Mean Difference (IV, Random, 95% CI) | ‐0.71 [‐1.63, 0.20] |
4.1. Analysis.
Comparison 4 Any intervention vs usual care (1‐2y follow‐up) (HbA1c in original paper), Outcome 1 GHb (fixed model).
4.2. Analysis.
Comparison 4 Any intervention vs usual care (1‐2y follow‐up) (HbA1c in original paper), Outcome 2 GHb (random model).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hanefeld 1984.
| Methods | Companion to Hanefeld 1991 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hanefeld 1988.
| Methods | Companion to Hanefeld 1991 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hanefeld 1991.
| Methods | Follow‐up: 260w No. study arms: one Setting: Germany, diabetes clinic Number: 1139 Comparison: Usual care | |
| Participants | Age: 47y Sex: 43%F Duration DM: NR Treatment: Diet only BL wt, BMI: NR, 28.9 BL GHb: NR | |
| Interventions | Duration: 260 w Frequency: q3m No. contacts: 20 Group/individual: Group Medium: In‐person Facilitator: Unclear Diet: Individualized low calorie diet (3350‐6281KJ/d); fat<35% intake; structured intensive general health education PA: Group exercise sessions and unsupervised program; including aerobic training Behavioral: None reported | |
| Outcomes | Weight: BMI: Y >5% loss (%): FBS: Y GHb: Cholesterol: Y LDL: HDL: TG: Y SBP: Y DBP: Y Other: smoking, change DM medications, PA | |
| Notes | Sampling method: Entire accessible population Jadad Score: 1,0,0,B Randomization procedure: NR Allocation concealment: Unclear Attrition: 12% Blinding pt: NR Blinding assessor: No Blinding provider: No BL comparable: FBS higher in C, but controlled for in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hartwell 1986.
| Methods | Companion to Kaplan 1987 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Heitzmann 1987.
| Methods | Follow‐up: 78w No. study arms: Three Setting: USA, setting unclear Number: 44 Comparison: Muscle relaxation | |
| Participants | Age: 53y Sex: 52%F Duration DM: NR Treatment: 60% insulin or oral agents BL wt, BMI: 81.7, NR BL GHb: 10.7 | |
| Interventions | Duration: Unclear Frequency: 7 weekly meetings, 2 home visits No. contacts: 9 Group/individual: Group Medium: In‐person Facilitator: Nutritionist Diet: All 3 intervention groups got dietary advice; details unclear PA: Three intervention groups told to monitor PA; details unclear Behavioral: I1: self‐monitoring wt and PA; I2: cognitive modification group: positive self‐statements, goal‐setting; I3: combined intervention | |
| Outcomes | Weight: Y BMI: >5% loss (%): FBS: GHb: Cholesterol: LDL: HDL: TG: SBP: DBP: | |
| Notes | Sampling method: referred by doc or through public service announcements Jadad Score: 2,0,0,A Randomization procedure: Adequate Allocation concealment: Adequate Attrition: 20% Blinding pt: No Blinding assessor: NR Blinding provider: No BL comparable: Yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Heller 1988.
| Methods | Follow‐up: 52w No. study arms: Two Setting: UK, University Hospital Number: 87 Comparison: Usual care | |
| Participants | Age: 56y Sex: 45%F Duration DM: NR Treatment: Diet only BL wt, BMI: 86.1, 32.0 BL GHb: 12.7 | |
| Interventions | Duration: 26w Frequency: Weekly x 3; then 3m and 6m No. contacts: 5 visits Group/individual: Group Medium: In‐person Facilitator: Nurse and dietician Diet: Exclude sugar, high fiber; other details unclear PA: None Behavioral: Self‐monitoring | |
| Outcomes | Weight: Y BMI: >5% loss (%): FBS: GHb: Y Cholesterol: LDL: HDL: TG: SBP: DBP: | |
| Notes | Sampling method: Entire accessible population Jadad Score: 1,0,1,B Randomization procedure: NR Allocation concealment: Unclear Attrition: 14% Blinding pt: No Blinding assessor: NR Blinding provider: No BL comparable: Yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Hockaday 1978.
| Methods | Follow‐up: 52w No. study arms: Two Setting: UK, diabetes clinic Number: 93 Comparison: Low CHO, calorie restricted diet | |
| Participants | Age: 52y Sex: 44%F Duration DM: NR Treatment: Diet only BL wt, BMI: 76.4, NR BL GHb: NR | |
| Interventions | Duration: 52w Frequency: One month then q3m No. contacts: 4 visits Group/individual: Individual Medium: In‐person Facilitator: Dietician Diet: I: 54% CHO, increased polyunsaturated fatty acids; C: 40% CHO; both got restricted calorie diets PA: None Behavioral: None | |
| Outcomes | Weight: Y BMI: >5% loss (%): FBS: Y GHb: Cholesterol: Y LDL: HDL: TG: SBP: DBP: | |
| Notes | Sampling method: Unclear Jadad Score: 1,0,0,B Randomization procedure: NR Allocation concealment: Unclear Attrition: NR Blinding pt: No Blinding assessor: NR Blinding provider: No BL comparable: No; increased wt in I | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Julius 1993.
| Methods | Companion to Hanefeld 1991 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Kaplan 1985.
| Methods | Companion to Kaplan 1987 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Kaplan 1987 (D+PA).
| Methods | Follow‐up: 78w No. study arms: Three Setting: intervention groups (diet, PA, diet + PA) USA; setting unclear Number: 76 Study design: Quasi‐randomized trial Comparison: Weekly didactic presentations on general diabetes care | |
| Participants | Age: 55y Sex: 58%F Duration DM: NR Treatment: 37% diet only, 25% insulin BL wt, BMI: 92.2, NR BL GHb: 8.2 | |
| Interventions | Duration: 10w Frequency: weekly No. contacts: 10 Group/individual: Group Medium: In‐person Facilitator: Dietician, psychology graduate student, physical education graduate student Diet: All 4 groups got low calorie diet; diet only group got behavioral modification treatment PA: PA group: 1 supervised, and 2 unsupervised sessions q week Behavioral: All 3 treatment groups: self‐monitoring, goal setting, planning, feedback | |
| Outcomes | Weight: Y (no data by group) BMI: >5% loss (%): FBS: GHb: Y Cholesterol: LDL: HDL: TG: SBP: DBP: Other: Quality of life, cost effectiveness | |
| Notes | Sampling method: Self (patient) selected Jadad Score: 1,0,1,C Randomization procedure: By group attended Allocation concealment: No Attrition: 3% Blinding pt: No Blinding assessor: NR Blinding provider: No BL comparable: Yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Kaplan 1987 (diet).
| Methods | Follow‐up: 78w No. study arms: Three Setting: intervention groups (diet, PA, diet + PA) USA; setting unclear Number: 76 Study design: Quasi‐randomized trial Comparison: Weekly didactic presentations on general diabetes care | |
| Participants | Age: 55y Sex: 58%F Duration DM: NR Treatment: 37% diet only, 25% insulin BL wt, BMI: 92.2, NR BL GHb: 8.2 | |
| Interventions | Duration: 10w Frequency: weekly No. contacts: 10 Group/individual: Group Medium: In‐person Facilitator: Dietician, psychology graduate student, physical education graduate student Diet: All 4 groups got low calorie diet; diet only group got behavioral modification treatment PA: PA group: 1 supervised, and 2 unsupervised sessions q week Behavioral: All 3 treatment groups: self‐monitoring, goal setting, planning, feedback | |
| Outcomes | Weight: Y (no data by group) BMI: >5% loss (%): FBS: GHb: Y Cholesterol: LDL: HDL: TG: SBP: DBP: Other: Quality of life, cost effectiveness | |
| Notes | Sampling method: Self (patient) selected Jadad Score: 1,0,1,C Randomization procedure: By group attended Allocation concealment: No Attrition: 3% Blinding pt: No Blinding assessor: NR Blinding provider: No BL comparable: Yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Kaplan 1987 (PA).
| Methods | Follow‐up: 78w No. study arms: Three Setting: intervention groups (diet, PA, diet + PA) USA; setting unclear Number: 76 Study design: Quasi‐randomized trial Comparison: Weekly didactic presentations on general diabetes care | |
| Participants | Age: 55y Sex: 58%F Duration DM: NR Treatment: 37% diet only, 25% insulin BL wt, BMI: 92.2, NR BL GHb: 8.2 | |
| Interventions | Duration: 10w Frequency: weekly No. contacts: 10 Group/individual: Group Medium: In‐person Facilitator: Dietician, psychology graduate student, physical education graduate student Diet: All 4 groups got low calorie diet; diet only group got behavioral modification treatment PA: PA group: 1 supervised, and 2 unsupervised sessions q week Behavioral: All 3 treatment groups: self‐monitoring, goal setting, planning, feedback | |
| Outcomes | Weight: Y (no data by group) BMI: >5% loss (%): FBS: GHb: Y Cholesterol: LDL: HDL: TG: SBP: DBP: Other: Quality of life, cost effectiveness | |
| Notes | Sampling method: Self (patient) selected Jadad Score: 1,0,1,C Randomization procedure: By group attended Allocation concealment: No Attrition: 3% Blinding pt: No Blinding assessor: NR Blinding provider: No BL comparable: Yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Korhonen 1987.
| Methods | Follow‐up: 52w No. study arms: Two Setting: Finland, university clinic Number: 71 Comparison: Written materials from doctor at first visit | |
| Participants | Age: 56y Sex: 48%F Duration DM: NR Treatment: Diet only BL wt, BMI: 89.7, 32.2 BL GHb: 10.9 | |
| Interventions | Duration: 52w Frequency: 1,2,3,6,12m No. contacts: 5 visits Group/individual: Individual Medium: In person Facilitator: Nurse Diet: Individualized LCD, low saturated fat, increased complex CHOs and vegetables PA: None Behavioral: None | |
| Outcomes | Weight: Y BMI: >5% loss (%): FBS: Y GHb: Y Cholesterol: Y (narrative only) LDL: HDL: TG: SBP: Y (narrative only) DBP: | |
| Notes | Sampling method: Unclear Jadad Score: 1,0,1,B Randomization procedure: NR Allocation concealment: Unclear Attrition: 11.2% Blinding pt: No Blinding assessor: NR Blinding provider: No BL comparable: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Laitinen 1993.
| Methods | Compansion to Uusitupa 1993 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Laitinen 1994a.
| Methods | Compansion to Uusitupa 1993 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Laitinen 1994b.
| Methods | Compansion to Uusitupa 1993 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lindahl 1999.
| Methods | Compansion to Uusitupa 1993 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Lindner 1992.
| Methods | Companion to Hanefeld 1991 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
McCarron 1997.
| Methods | Compansion to Metz 2000 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
McCarron 2001.
| Methods | Compansion to Metz 2000 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Metz 1997.
| Methods | Companion to Metz 2000 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Metz 2000.
| Methods | Follow‐up: 52w No. study arms: Two Setting: USA, multicenter academic centers Number: 119 Comparison: Same diet, self‐selected foods | |
| Participants | Age: 54y Sex: 58%F Duration DM: NR Treatment: No insulin BL wt, BMI: NR, 34.5 BL GHb: 8.8 | |
| Interventions | Duration: 52w Frequency: Monthly No. contacts: 12 visits Group/individual: Individual Medium: In‐person and telephone Facilitator: Nutritionist Diet: Reduced calories using pre‐prepared meals; 22% fat; calories not specified PA: None Behavioral: None | |
| Outcomes | Weight: Y BMI: >5% loss (%): Y FBS: Y GHb: Y Cholesterol: Y LDL: Y HDL: Y TG: Y SBP: Y DBP: Y | |
| Notes | Sampling method: Self (patient) selected Jadad Score: 2,2,1,A Randomization procedure: Adequate Allocation concealment: Adequate Attrition: 23% total Blinding pt: No Blinding assessor: Yes Blinding provider: No BL comparable: Yes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Milne 1994.
| Methods | Follow‐up: up to 78w, average 52w No. study arms: Two Setting: New Zealand, community hospital Number: 64 Comparison: 500kcal/d deficit diet | |
| Participants | Age: 59y Sex: 55%F Duration DM: 4.8y Treatment: Diet or oral agents BL wt, BMI: 78.3, 29.0 BL GHb: 9.0 | |
| Interventions | Duration: 78w Frequency: No. contacts: 5 visits Group/individual: Group Medium: In‐person Facilitator: NR Diet: Modified fat; high CHO/fiber PA: None Behavioral: Social support | |
| Outcomes | Diet: Modified fat; high CHO/fiber PA: None Behavioral: Social support | |
| Notes | Weight: Y BMI: >5% loss (%): FBS: GHb: Y Cholesterol: Y LDL: Y HDL: Y TG: Y SBP: DBP: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Muchmore 1994.
| Methods | Follow‐up: 52w No. study arms: Two Setting: USA, large group practice Number: 23 Comparison: Identical amount of attention; focused on ADA guidelines of general nutrition principles | |
| Participants | Age: 59y Sex: 61%F Duration DM: 5.5y Treatment: 26% diet only; 76 % oral agents BL wt, BMI: 99.1, 33.3 BL GHb: 10.5 | |
| Interventions | Duration: 32w Frequency: weekly X 8, then irregular No. contacts: 11 Group/individual: Combined Medium: In‐person Facilitator: dietician; nurse educator Diet: Individual dietary counseling: low calorie diet; focus on CHO counting and SMBG PA: None Behavioral: Unclear | |
| Outcomes | Diet: Individual dietary counseling: low calorie diet; focus on CHO counting and SMBG PA: None Behavioral: Unclear | |
| Notes | Weight: Y BMI: >5% loss (%): FBS: GHb: Y Cholesterol: LDL: HDL: TG: SBP: DBP: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Pascale 1992.
| Methods | Companion to Wing 1995 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pascale 1995.
| Methods | Follow‐up: 52w No. study arms: Two Setting: USA, academic center Number: 44 Comparison: 1000‐1500kcal/d; same behavioral sessions | |
| Participants | Age: 56y Sex: 100%F Duration DM: NR Treatment: No insulin BL wt, BMI: 93.1, 36.4 BL GHb: 10.9 | |
| Interventions | Duration: 40w Frequency: weekly for 16w, then 1,2,4,6m No. contacts: 20 visits Group/individual: Both group and individual Medium: In‐person Facilitator: Trained therapist Diet: Low fat (20% total calories) and 1000‐1500kcal/d diet PA: Graded goals for programmed activity Behavioral: 16w course: stimulus control, problem solving, social skills training, goal setting | |
| Outcomes | Diet: Low fat (20% total calories) and 1000‐1500kcal/d diet PA: Graded goals for programmed activity Behavioral: 16w course: stimulus control, problem solving, social skills training, goal setting | |
| Notes | Weight: Y BMI: Y >5% loss (%): FBS: Y GHb: Y Cholesterol: Y LDL: Y HDL: Y TG: Y SBP: DBP: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Pissarek 1980.
| Methods | Follow‐up: 108w No. study arms: Two Setting: Germany, academic center Number: 150 Comparison: One session routine dietary advice | |
| Participants | Age: 51y Sex: 60%F Duration DM: NR Treatment: No insulin, 53% diet only BL wt, BMI: 82.8, NR BL GHb: NR | |
| Interventions | Duration: 12w Frequency: 2w in‐patient, then weekly No. contacts: 24 visits Group/individual: Individual Medium: In‐person Facilitator: physician Diet: 800 kcal/diet for persons >120% IBW PA: None Behavioral: None | |
| Outcomes | Diet: 800 kcal/diet for persons >120% IBW PA: None Behavioral: None | |
| Notes | Weight: Y BMI: >5% loss (%): FBS: GHb: Cholesterol: Y LDL: HDL: TG: Y SBP: DBP: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Smith 1991.
| Methods | Companion to wing 1994 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Sone 2002.
| Methods | Follow‐up: 156w No. study arms: Two Setting: Japan, multicenter, diabetes Clinics Number: 2205 Comparison: Usual care | |
| Participants | Age: 59y Sex: 45%F Duration DM: 11.3y Treatment: 21% diet only, 20% insulin BL wt, BMI: NR, 23.0 BL GHb: 7.8 | |
| Interventions | Duration: 156w Frequency: clinic visits (uncertain frequency), telephone call q2w No. contacts: 78 visits Group/individual: Individual Medium: In‐person, written, telephone Facilitator: nurse, other Diet: Weight loss counseling (details unclear); goal BMI<22 PA: PA counseling, (details unclear), pedometer Behavioral: Self‐monitoring, feedback | |
| Outcomes | Diet: Weight loss counseling (details unclear); goal BMI<22 PA: PA counseling, (details unclear), pedometer Behavioral: Self‐monitoring, feedback | |
| Notes | Weight: BMI: Y >5% loss (%): FBS: Y GHb: Y Cholesterol: Y LDL: HDL: Y TG: Y SBP: Y DBP: Y | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Trento 2001.
| Methods | Compansion to Trento 1998 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Trento 2002.
| Methods | Compansion to Trento 1998 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Trento 1998.
| Methods | Follow‐up: 52, 108 and 225w No. study arms: Two Setting: Italy, academic center Number: 112 Comparison: Usual one‐on‐one clinic visits q3m | |
| Participants | Age: 62y Sex: 46%F Duration DM: 9.6y Treatment: 14% diet only, 86% oral agents BL wt, BMI: 77.8, 27.9 BL GHb: 7.4 | |
| Interventions | Duration: 52, 108 and 225w Frequency: 11/4.3y No. contacts: 11 visits Group/individual: Group Medium: In‐person Facilitator: Physicians Diet: Educational sessions on overweight, meal planning, food choices, BS control, smoking PA: Counseled to increase PA Behavioral: SMBG, SM wt, role playing, problem‐solving | |
| Outcomes | Weight: Y BMI: Y >5% loss (%): FBS: Y GHb: Y Cholesterol: Y LDL: HDL: Y TG: Y SBP: Y DBP: Y Other: Quality of life, economic outcomes | |
| Notes | Sampling method: Probability sampling accessible population Jadad Score: 2,1,1,B Randomization procedure: Random number table Allocation concealment: Unclear Attrition: 20% at 4y Blinding pt: No Blinding assessor: Unclear Blinding provider: Yes, at regular clinic visits BL comparable: No: control better educated and more DM knowledge | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Uusitupa 1996.
| Methods | Compansion to Uusitupa 1993 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Uusitupa 1993.
| Methods | Follow‐up: 52 and 104w No. study arms: Two Setting: Finland, academic diabetes center Number: 86 Comparison: Usual care in community clinic q3m | |
| Participants | Age: 53y Sex: 43%F Duration DM: 2y Treatment: Diet only BL wt, BMI: 88.8, 31.6 BL GHb: 7.8 | |
| Interventions | Duration: 12w run‐in, then 104w Frequency: 11 visits in 2y No. contacts: 11 visits Group/individual: Individual Medium: In‐person and written Facilitator: Dietician, physician, nurse Diet: Individualized energy restricted, low fat (<30% total energy), increased unrefined CHOs PA: Encouraged to walk, jog, other, 3‐4 times a week Behavioral: None | |
| Outcomes | Diet: Individualized energy restricted, low fat (<30% total energy), increased unrefined CHOs PA: Encouraged to walk, jog, other, 3‐4 times a week Behavioral: None | |
| Notes | Weight: Y BMI: Y >5% loss (%): FBS: Y GHb: Y Cholesterol: Y LDL: Y HDL: Y TG: Y SBP: Y DBP: Y | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Vanninen 1993.
| Methods | Compansion to Uusitupa 1993 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Vanninen 1994.
| Methods | Compansion to Uusitupa 1993 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Vanninen 1992.
| Methods | Compansion to Uusitupa 1993 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1987.
| Methods | Companion to Wing 1985 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1988b.
| Methods | Follow‐up: 68w No. study arms: Setting: USA, academic center Number: 20 Comparison: Same education; SMBG but no goals or teaching on how to adjust intake and activity | |
| Participants | Age: 53y Sex: 65%F Duration DM: NR Treatment: 20% diet only, 80% oral agents BL wt, BMI: 90.5, NR BL GHb: 10.5 | |
| Interventions | Duration: 68w Frequency: 22 meetings No. contacts: 22 Group/individual: Combined Medium: In‐person Facilitator: NR Diet: Both groups: Low calorie (1000‐1500kcal/w) with increased complex CHO and fiber PA: Both groups: Walking, goal to 10 miles/w Behavioral: Both groups: goal setting, controlling eating cues, planning, self‐reinforcement, problem solving. Intervention: SMBG with instruction how to adjust intake and activity in response to BG | |
| Outcomes | Diet: Both groups: Low calorie (1000‐1500kcal/w) with increased complex CHO and fiber PA: Both groups: Walking, goal to 10 miles/w Behavioral: Both groups: goal setting, controlling eating cues, planning, self‐reinforcement, problem solving. Intervention: SMBG with instruction how to adjust intake and activity in response to BG | |
| Notes | Weight: Y BMI: >5% loss (%): FBS: GHb: Y Cholesterol: LDL: HDL: TG: SBP: DBP: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1985 (BM).
| Methods | Follow‐up: 68w No. study arms: Three Setting: groups: behavioral modification, nutrition education; usual care USA, academic center Number: 53 Comparison: Standard care, monthly meetings | |
| Participants | Age: 55y Sex: 62%F Duration DM: 5.9y Treatment: 25% diet only; 75% oral agents BL wt, BMI: 96.4, 34.8 BL GHb: 9.3 | |
| Interventions | Duration: 16w Frequency: weekly No. contacts: Group/individual: Combined Medium: In‐person, written materials Facilitator: behavioral psychologist, nutritionist Diet: All groups: (wt x 12)‐1000 calories/d; nutrition education: exchange lists plan, basic information PA: Behavioral modification: goal 1000 kcal/w via walking Behavioral: Behavioral modification: stimulus control, goal setting, self‐monitoring, change cognitions, financial incentives | |
| Outcomes | Diet: All groups: (wt x 12)‐1000 calories/d; nutrition education: exchange lists plan, basic information PA: Behavioral modification: goal 1000 kcal/w via walking Behavioral: Behavioral modification: stimulus control, goal setting, self‐monitoring, change cognitions, financial incentives | |
| Notes | Weight: Y BMI: >5% loss (%): FBS: Y, for whole group GHb: Y, for whole group Cholesterol: Y, for whole group LDL: HDL: Y, for whole group TG: Y, for whole group SBP: Y, for whole group DBP: Y, for whole group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1985 (NE).
| Methods | Follow‐up: 68w No. study arms: Three Setting: groups: behavioral modification, nutrition education; usual care USA, academic center Number: 53 Comparison: Standard care, monthly meetings | |
| Participants | Age: 55y Sex: 62%F Duration DM: 5.9y Treatment: 25% diet only; 75% oral agents BL wt, BMI: 96.4, 34.8 BL GHb: 9.3 | |
| Interventions | Duration: 16w Frequency: weekly No. contacts: Group/individual: Combined Medium: In‐person, written materials Facilitator: behavioral psychologist, nutritionist Diet: All groups: (wt x 12)‐1000 calories/d; nutrition education: exchange lists plan, basic information PA: Behavioral modification: goal 1000 kcal/w via walking Behavioral: Behavioral modification: stimulus control, goal setting, self‐monitoring, change cognitions, financial incentives | |
| Outcomes | Diet: All groups: (wt x 12)‐1000 calories/d; nutrition education: exchange lists plan, basic information PA: Behavioral modification: goal 1000 kcal/w via walking Behavioral: Behavioral modification: stimulus control, goal setting, self‐monitoring, change cognitions, financial incentives | |
| Notes | Weight: Y BMI: >5% loss (%): FBS: Y, for whole group GHb: Y, for whole group Cholesterol: Y, for whole group LDL: HDL: Y, for whole group TG: Y, for whole group SBP: Y, for whole group DBP: Y, for whole group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1986.
| Methods | Follow‐up: 62w No. study arms: Two Setting: USA academic center Number: 50 Comparison: 1000kcal/d diet with 20 visits | |
| Participants | Age: 54y Sex: 78%F Duration DM: NR Treatment: 50% oral agents, 50% insulin BL wt, BMI: 96.4, NR BL GHb: 10.9 | |
| Interventions | Duration: 52w Frequency: 20 visits/52w No. contacts: 20 visits Group/individual: Combined Medium: In‐person Facilitator: NR Diet: Both groups: 1000kcal/d deficit PA: Both groups, walking encouraged Behavioral: Intervention: SMBG with adjusting of intake and activity; both groups: self‐monitoring intake, planning, decrease stimuli, social support | |
| Outcomes | Diet: Both groups: 1000kcal/d deficit PA: Both groups, walking encouraged Behavioral: Intervention: SMBG with adjusting of intake and activity; both groups: self‐monitoring intake, planning, decrease stimuli, social support | |
| Notes | Weight: Y BMI: >5% loss (%): FBS: Y GHb: bY Cholesterol: for combined group LDL: HDL: TG: SBP: for combined group DBP: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1988a (Study 1).
| Methods | Follow‐up: 62w No. study arms: Two Setting: USA, setting NR Number: 25 Comparison: Same diet and behavioral interventions; light calisthetics and flexibility exercises | |
| Participants | Age: 54y Sex: 84%F Duration DM: 4.6y Treatment: 52% diet, 48% oral agents BL wt, BMI: 98.9, 37.5 BL GHb: 9.6 | |
| Interventions | Duration: 62w Frequency: 2 times a week for 10w, then monthly times 6 No. contacts: 26 Group/individual: Group Medium: In‐person Facilitator: NR Diet: Caloric deficit for goal 1kg/w loss PA: Supervised walking 2 times a week, 1 time unsupervised Behavioral: Rate of eating, stimulus control, coping with social pressure, pre‐planning, relapse prevention; both groups | |
| Outcomes | Diet: Caloric deficit for goal 1kg/w loss PA: Supervised walking 2 times a week, 1 time unsupervised Behavioral: Rate of eating, stimulus control, coping with social pressure, pre‐planning, relapse prevention; both groups | |
| Notes | Weight: Y BMI: >5% loss (%): FBS: GHb: Cholesterol: LDL: HDL: TG: SBP: DBP: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1988a (Study 2).
| Methods | Follow‐up: 62w No. study arms: Two Setting: USA, setting NR Number: 30 | |
| Participants | Age: 56y Sex: 70%F Duration DM: 7.0y Treatment: 27% insulin, 63% oral agents BL wt, BMI: 102, 37.9 BL GHb: 10.9 | |
| Interventions | Duration: 62w Frequency: 3 times a week for 10w, then weekly for 10 weeks, then monthly No. contacts: Group/individual: Group Medium: In‐person Facilitator: NR Comparison: Same diet and behavioral interventions; no PA Diet: Caloric deficit for goal 1kg/w loss PA: Supervised walking 3 times a week Behavioral: Rate of eating, stimulus control, coping with social pressure, pre‐planning, relapse prevention; both groups | |
| Outcomes | Diet: Caloric deficit for goal 1kg/w loss PA: Supervised walking 3 times a week Behavioral: Rate of eating, stimulus control, coping with social pressure, pre‐planning, relapse prevention; both groups | |
| Notes | Weight: Y BMI: Y >5% loss (%): FBS: Y GHb: Y Cholesterol: Y LDL: HDL: Y TG: Y SBP: DBP: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1991a.
| Methods | Follow‐up: 72w No. study arms: Two Setting: USA academic center Number: 36 Comparison: LCD 4200‐6300J/d | |
| Participants | Age: 51y Sex: 76%F Duration DM: 7.0y Treatment: 18% diet only, 21% using insulin BL wt, BMI: 104.5, 38.1 BL GHb: 10.4 | |
| Interventions | Duration: 72w Frequency: No. contacts: 24 visits Group/individual: Group Medium: In‐person Facilitator: Team Diet: VLCD for 8 weeks, followed by low calorie diet (4200‐6300/d PA: Both groups: walking, advance to 10 miles/w Behavioral: Both groups: self‐monitoring BG and intake, relapse prevention, stimulus control, modify cognitions | |
| Outcomes | Diet: VLCD for 8 weeks, followed by low calorie diet (4200‐6300/d PA: Both groups: walking, advance to 10 miles/w Behavioral: Both groups: self‐monitoring BG and intake, relapse prevention, stimulus control, modify cognitions | |
| Notes | Weight: Y BMI: Y >5% loss (%): FBS: GHb: Y Cholesterol: Y LDL: HDL: Y TG: Y SBP: DBP: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1991b.
| Methods | Follow‐up: 72w No. study arms: Two Setting: USA, setting unclear Number: 49 Comparison: No spouse attending meetings | |
| Participants | Age: 52y Sex: 58%F Duration DM: NR Treatment: 26% diet only, 21% insulin BL wt, BMI: 102.4, 35.7 BL GHb: 10.3 | |
| Interventions | Duration: 78w Frequency: 20 meetings total both groups No. contacts: 20 Group/individual: Group Medium: In‐person Facilitator: Team Diet: Both groups: 20w behavioral weigh loss program with 1200‐1500 kcal/d diet PA: Both groups: walking to 1000 Kcal/w Behavioral: Spouse attended with intervention group; both groups: goal setting, problem solving, stimulus control | |
| Outcomes | Diet: Both groups: 20w behavioral weigh loss program with 1200‐1500 kcal/d diet PA: Both groups: walking to 1000 Kcal/w Behavioral: Spouse attended with intervention group; both groups: goal setting, problem solving, stimulus control | |
| Notes | Weight: Y BMI: Y >5% loss (%): FBS: Y GHb: Y Cholesterol: LDL: HDL: TG: SBP: DBP: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1994.
| Methods | Follow‐up: 52 and 104w No. study arms: Two Setting: USA, academic medical center Number: 93 Comparison: 1000‐1200kcal/d, weekly meetings | |
| Participants | Age: 52y Sex: 65%F Duration DM: 6.8y Treatment: 25% on insulin; stopped at beginning of study BL wt, BMI: 107.7, 38.3 BL GHb: 10.5 | |
| Interventions | Duration: 52w Frequency: weekly No. contacts: Group/individual: Group Medium: In‐person Facilitator: Team Diet: 400‐500kcal/d for weeks 1‐12, 24‐36, otherwise 1000‐1200kcal/d PA: Both groups, walk 10 miles/w Behavioral: Both groups: goal‐setting, self‐monitoring, SMBG, stimulus control, pre‐planning, relapse prevention | |
| Outcomes | Diet: 400‐500kcal/d for weeks 1‐12, 24‐36, otherwise 1000‐1200kcal/d PA: Both groups, walk 10 miles/w Behavioral: Both groups: goal‐setting, self‐monitoring, SMBG, stimulus control, pre‐planning, relapse prevention | |
| Notes | Weight: Y BMI: >5% loss (%): FBS: Y GHb: Y Cholesterol: Y LDL: Y HDL: Y TG: Y SBP: Y DBP: Y | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1995.
| Methods | Follow‐up: 52w No. study arms: Two Setting: USA, community recruitment, setting unclear Number: 93 Comparison: 1000‐1200 kcal/d, low fat | |
| Participants | Age: 51.8y Sex: 68%F Duration DM: 6.8y Treatment: 23% insulin, 60% oral agents BL wt, BMI: 106.8, 37.9 BL GHb: NR | |
| Interventions | Duration: 52w Frequency: weekly, 52 visits No. contacts: 52 visits Group/individual: Group Medium: In‐person Facilitator: Team of therapists Diet: VLCD weeks 1‐12 and 24‐36 (approx. 500kcal/d); otherwise 1000‐1200 kcal/d, low fat PA: Both groups: encouraged to walk 10 miles/w Behavioral: Both groups: self‐monitoring, stimulus control, goal setting | |
| Outcomes | Diet: VLCD weeks 1‐12 and 24‐36 (approx. 500kcal/d); otherwise 1000‐1200 kcal/d, low fat PA: Both groups: encouraged to walk 10 miles/w Behavioral: Both groups: self‐monitoring, stimulus control, goal setting | |
| Notes | Weight: Y BMI: >5% loss (%): FBS: GHb: Cholesterol: LDL: HDL: TG: SBP: DBP: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1996.
| Methods | Companion to Wing 1995 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wing 1997.
| Methods | Companion to Wing 1994 | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Zapotoczky 2001.
| Methods | Follow‐up: 52w No. study arms: Two Setting: Austria, academic internal medicine clinic Number: 36 | |
| Participants | Age: 58y Sex: 68%F Duration DM: NR Treatment: 56% diet only, 44% oral agents BL wt, BMI: 82.3, NR BL GHb: 8.0 | |
| Interventions | Duration: 12m Frequency: Monthly No. contacts: 12 visits Group/individual: Group Medium: In‐person Facilitator: Dietician Comparison: Usual care Diet: Educational program using ADA nutrition guidelines PA: One group exercise session Behavioral: One "psychoeducation" session | |
| Outcomes | Diet: Educational program using ADA nutrition guidelines PA: One group exercise session Behavioral: One "psychoeducation" session | |
| Notes | Weight: Y BMI: >5% loss (%): FBS: GHb: Y Cholesterol: Y LDL: Y HDL: Y TG: Y SBP: Y DBP: Y | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
BL, baseline BMI, body mass index mg/m2 CHO, carbohydrate DBP, diastolic blood pressure DM diabetes mellitus F, female FBS, fasting blood sugar m, month NR, not reported SBP, systolic blood pressure w, week y, year Y, yes
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agurs‐Collins 1997 | Follow‐up 6 months |
| Altmann 1987 | Study includes persons with diabetes, but no outcomes for this subgroup |
| Arauz 2001 | General diabetes education; follow‐up 4 months |
| Bloomgarden 1987 | General diabetes education; weight loss not specific focus |
| Boehm 1993 | General diabetes education; weight loss was the focus for only some participants and no details were given of the dietary intervention |
| Brown 2002 | General diabetes education; no information on caloric restriction or specific diet |
| Caplan 1995 | Weight loss was not goal; no dietary intervention |
| Currey 1977 | Persons with diabetes were included, but no outcomes for this subgroup |
| Dalmau Llorca 2003 | Intervention was not focused on weight loss |
| De Luis 2001 | No weight outcomes |
| Di Loreto 2003 | Not primarily a weight loss intervention; focuses on physical activity |
| Fernandez Suarez1995 | Not a dietary program |
| Frost 1989 | Follow‐up 12 weeks |
| Gaede 2001 | General diabetes education; goal was not weight loss |
| Gallagher 1984 | Goal was not weight loss |
| Gallagher 1987 | Goal was not weight loss |
| Garcia 1996 | Goal was not weight loss |
| Genuth 1979 | No weight data at follow‐up |
| Giacco 2000 | Not a weight loss intervention |
| Gilliland 2002 | Lifestyle intervention; goal was not weight loss; no explicit calorie restricted diet |
| Henry 1986 | Intervention was for 60 to 365 days and no average given; assume less than 1 year |
| Hughes 1999 | Type 1 and type 2 diabetes combined; no type 2 specific data |
| Jayasuriya 2000 | No weight data at follow‐up |
| Kanders 1989 | 18 month weight loss intervention with persons wtih a subset; but no weight or other specific outcomes |
| Karlsson 1998 | Reports data from other studies with no original data |
| Keyserling 2002 | Weight loss was not the goal |
| Kirk 2001 | Weight loss was not the goal; main focus was an exercise consultation |
| Kirkman 1994 | General diabetes education; primary goal was improved glycemic control |
| Luscombe 2002 | Follow‐up 12 weeks |
| Mancini 1981 | Persons with diabetes were included, but no diabetes‐specific outcomes were presented |
| Manning 1998 | Type 1 and type 2 diabetes were combined |
| Mengham 1999 | Type 1 and type 2 diabetes were combined |
| Murphy 1999 | Not a weight loss intervention |
| Nothwehr 2001 | Not a weight loss intervention |
| Reaven 1985 | Follow‐up interval unclear, but appears to be less than 1 year |
| Ridgeway 1999 | General diabetes education |
| Schafer 1987 | Type 1 and type 2 diabetes combined |
| Simmons 1998 | Type 1 and type 2 diabetes combined |
| Simonen 2000 | Outcomes for both randomized groups were combined |
| Solerte 1989 | Type 1 and type 2 diabetes combined |
| Szybinski 1978 | Follow‐up 24 days |
| Toobert 2003 | Follow‐up 6 months |
| Tudor‐Locke 2004 | Follow‐up 16 weeks; primary goal not weight loss |
| Turnin 1992 | Type 1 and type 2 diabetes combined |
| Ueda 1999 | No weight outcomes |
| Werdier 1984 | Unclear if wt loss is the goal; not weight outcomes at 12 months or more |
Characteristics of ongoing studies [ordered by study ID]
Look AHEAD 2003.
| Trial name or title | Look AHEAD (Action for Health in Diabetes) |
| Methods | |
| Participants | Overweight and obese persons wtih type 2 diabetes |
| Interventions | Intensive weight loss program delivered over 4 years |
| Outcomes | Time to incidence of a major cardiovascular disease event, cost effectiveness, quality of life |
| Starting date | 2001 |
| Contact information | Mark Espeland PhD; mespelan@wfubmc.edu |
| Notes |
Contributions of authors
SUSAN L. NORRIS: Study supervision, study concept and design, acquisition of data, analysis and interpretation of the data, drafting and critical revision of the manuscript
XUANPING ZHANG: Study concept and design, acquisition of data, analysis and interpretation of the data, critical revision of the manuscript, technical support
ALISON AVENELL: Study concept and design, analysis and interpretation of the data, critical revision of the manuscript
EDWARD GREGG: Study concept and design, analysis and interpretation of the data, critical revision of the manuscript
TAMARA J BROWN: Acquisition of data, analysis and interpretation of the data
CHRISTOPHER SCHMID: Analysis and interpretation of the data, critical revision of the manuscript
JOSEPH LAU: Study concept and design, analysis and interpretation of the data, critical revision of the manuscript
Sources of support
Internal sources
Centers for Disease Control and Pevention, USA.
External sources
No sources of support supplied
Declarations of interest
None identified
With regard to the contribution of S. Norris, the views expressed in this review are those of the author, and no official endorsement by the Agency for Healthcare Research and Quality or the U.S. Department of Health and Human Services is intended or should be inferred.
With regard to the contributions of A. Avenell, the Health Services Research Unit is core funded by the Chief Scientist Office of the Scottish Executive Health Department; however, the views expressed here are those of the authors.
Edited (no change to conclusions)
References
References to studies included in this review
Hanefeld 1984 {published data only}
- Hanefeld M, Haller H, Schulze J, Julius U, Fischer S, Rothe G. The diabetes intervention study ‐ a multicentre, multi‐intervention study in type II diabetics. 1. Conception, diagnostic, therapeutic programme, outcome of screening and epidemiological results on entrance to the study [German]. Deutsche Gesundheitswesen 1984;39(48):1889‐94. [Google Scholar]
Hanefeld 1988 {published data only}
- Hanefeld M, Schulze J, Fischer S, Rossger G, Lindner J, Groh G, et al. Intensified basic treatment reduces the need for drugs and improves glucose control: an important result of the Diabetes Intervention Study. Zeitschrift fur die Gesamte Innere Medizin und Ihre Grenzgebiete 1988;43(24):700‐2. [PubMed] [Google Scholar]
Hanefeld 1991 {published data only}
- Hanefeld M, Fischer S, Schmechel H, Rothe G, Schulze J, Dude H, et al. Diabetes Intervention Study: Multi‐intervention trial in newly diagnosed NIDDM. Diabetes Care 1991;14:308‐17. [DOI] [PubMed] [Google Scholar]
Hartwell 1986 {published data only}
- Hartwell SL, Kaplan RM, Wallace JP. Comparison of behavioral interventions for control of type II diabetes mellitus. Behavior Therapy 1986;17:447‐61. [Google Scholar]
Heitzmann 1987 {published data only}
- Heitzmann CA, Kaplan RM, Wilson DK, Sandler J. Sex differences in weight loss among adults with type II diabetes mellitus. Journal of Behavioral Medicine 1987;10:197‐211. [DOI] [PubMed] [Google Scholar]
Heller 1988 {published data only}
- Heller SR, Clarke P, Daly H, Davis I, McCulloch DK, Allison SP, et al. Group education for obese patients with type 2 diabetes: greater success at less cost. Diabetic Medicine 1988;5:552‐6. [DOI] [PubMed] [Google Scholar]
Hockaday 1978 {published data only}
- Hockaday TD, Hockaday JM, Mann JI, Turner RC. Prospective comparison of modified fat‐high‐carbohydrate with standard low‐carbohydrate dietary advice in the treatment of diabetes: one year follow‐up study. British Journal of Nutrition 1978;39(2):357‐62. [DOI] [PubMed] [Google Scholar]
Julius 1993 {published data only}
- Julius U, Gross P, Hanefeld M. Work absenteeism in type 2 diabetes mellitus: results of the prospective diabetes intervention study. Diabete Metabolisme 1993;19:202‐6. [PubMed] [Google Scholar]
Kaplan 1985 {published data only}
- Kaplan RM, Wilson DK, Hartwell SL, Merino KL, Wallace JP. Prospective evaluation of HDL cholesterol changes after diet and physical conditioning programs for patients with type II diabetes mellitus. Diabetes Care 1985;8:343‐8. [DOI] [PubMed] [Google Scholar]
Kaplan 1987 (D+PA) {published data only}
- Kaplan RM, Hartwell SL, Wilson DK, Wallace JP. Effects of diet and exercise interventions on control and quality of life in non‐insulin‐dependent diabetes mellitus. Journal of General Internal Medicine 1987;2:220‐7. [DOI] [PubMed] [Google Scholar]
Kaplan 1987 (diet) {published data only}
- Kaplan RM, Hartwell SL, Wilson DK, Wallace JP. Effects of diet and exercise interventions on control and quality of life in non‐insulin‐dependent diabetes mellitus. Journal of General Internal Medicine 1987;2:220‐7. [DOI] [PubMed] [Google Scholar]
Kaplan 1987 (PA) {published data only}
- Kaplan RM, Hartwell SL, Wilson DK, Wallace JP. Effects of diet and exercise interventions on control and quality of life in non‐insulin‐dependent diabetes mellitus. Journal of General Internal Medicine 1987;2:220‐7. [DOI] [PubMed] [Google Scholar]
Korhonen 1987 {published data only}
- Korhonen T, Uusitupa M, Aro A, Kumpulainen T, Siitonnen O, Voutilainen E, et al. Efficacy of dietary instructions in newly diagnosed non‐insulin‐dependent diabetic patients. Acta Medica Scandinavica 1987;222:323‐31. [DOI] [PubMed] [Google Scholar]
Laitinen 1993 {published data only}
- Laitinen JH, Ahola IE, Sarkkinen ES, Winberg RL, Harmaakorph‐Livonen PA, Uusitupa MI. Impact of intensified dietary therapy on energy and nutrient intakes and fatty acid composition of serum lipids in patients with recently diagnosed non‐insulin‐dependent mellitus. Journal of American Dietetic Association 1993;93:276‐83. [DOI] [PubMed] [Google Scholar]
Laitinen 1994a {published data only}
- Laitinen J, Uusitupa M, Ahola I, Laakso M, Siitonen O. Metabolic and dietary variables associated with glycaemic control in patients with recently diagnosed type II diabetes mellitus. Diabetes, Nutrition and Metabolism ‐ Clinical and Experimental 1994;7(2):77‐87. [Google Scholar]
Laitinen 1994b {published data only}
- Laitinen J, Uusitupa M, Ahola I, Siitonen O. Metabolic and dietary determinants of serum lipids in obese patients with recently diagnosed non‐insulin‐dependent diabetes. Annals of Medicine 1994;26(2):119‐24. [DOI] [PubMed] [Google Scholar]
Lindahl 1999 {published data only}
- Lindahl B, Nilsson TK, Jansson JH, Asplund K, Hallmans G. Improved fibrinolysis by intense lifestyle intervention. A randomized trial in subjects with impaired glucose tolerance. Journal of Internal Medicine 1999;246(1):105. [DOI] [PubMed] [Google Scholar]
Lindner 1992 {published data only}
- Lindner J, Schmechel H, Hanefeld M, Schwanebeck U, Bauch K. Coronary heart disease and insulin concentration in type II diabetic patients‐‐results of a diabetes intervention dy. [German]. Zeitschrift fur die Gesamte Innere Medizin und Ihre Grenzgebiete 1992;47(6):246‐50. [PubMed] [Google Scholar]
McCarron 1997 {published data only}
- McCarron DA, Oparil S, Chait A, Haynes RB, Kris‐Etherton P, Stern JS, et al. Nutritional management of cardiovascular risk factors. A randomized clinical trial. Archives of Internal Medicine 1997;157(2):169‐77. [PubMed] [Google Scholar]
McCarron 2001 {published data only}
- McCarron DA, Reusser ME. Reducing cardiovascular disease risk with diet. Obesity Research 2001;9:335S‐40S. [DOI] [PubMed] [Google Scholar]
Metz 1997 {published data only}
- Metz JA, Kris‐Etherton PM, Morris CD, Mustad VA, Stern JS, Oparil S, et al. Dietary compliance and cardiovascular risk reduction with a prepared meal plan compared with a self‐selected diet. American Journal of Clinical Nutrition 1997;66:373‐385. [DOI] [PubMed] [Google Scholar]
Metz 2000 {published data only}
- Metz JA, Stern JS, Kris‐Etherton P, Reusser RB, Morris CD, Hatton DC, et al. A randomized trial of improved weight loss with a prepared meal plan in overweight and obese patients‐impact on cardiovascular risk reduction. Archives of Internal Medicine 2000;160(14):215‐‐8. [DOI] [PubMed] [Google Scholar]
Milne 1994 {published data only}
- Milne RM, Mann JI, Chisholm AW, Williams SM. Long‐term comparison of three dietary prescriptions in the treatment of NIDDM. Diabetes Care 1994;17(1):74‐80. [DOI] [PubMed] [Google Scholar]
Muchmore 1994 {published data only}
- Muchmore DB, Springer J, Miller M. Self‐monitoring of blood glucose in overweight type 2 diabetic patients. Acta Diabetologica 1994;31:215‐9. [DOI] [PubMed] [Google Scholar]
Pascale 1992 {published data only}
- Pascale RW, Wing RR, Blair EH, Harvey JR, Guare JC. The effect of weight‐loss on change in waist‐to‐hip ratio in patients with type 2 diabetes. International Journal of Obesity 1992;16(1):59‐65. [PubMed] [Google Scholar]
Pascale 1995 {published data only}
- Pascale R, Wing RR, Butler B, Mullen M, Bononi P. Effects of a behavioral weight loss program stressing calorie restriction verus calorie plus fat restriction in obese individuals with NIDDM or a family history of diabetes. Diabetes Care 1995;18:1241‐8. [DOI] [PubMed] [Google Scholar]
Pissarek 1980 {published data only}
- Pissarek D, Panzram G, Lundershausen R, Adolph W, Senf L. Intensified therapy of newly detected maturity onset diabetes [German]. Endokrinologie 1980;75(1):105‐15. [PubMed] [Google Scholar]
Smith 1991 {published data only}
- Smith DE, Wing RR. Diminished weight loss and behavioral compliance during repeated diets in obese patients with type II diabetes. Health Psychology 1991;10(6):378‐83. [DOI] [PubMed] [Google Scholar]
Sone 2002 {published data only}
- Sone H, Katagiri A, Ishibashi S, Abe R, Saito Y, Murase T, Yamashita H, et al. Effects of lifestyle modifications on patients with type 2 diabetes: the Japan Diabetes Complications Study (JDCS) study design, baseline analysis and three year‐interim report. Hormone and Metabolic Research 2002;34(9):509. [DOI] [PubMed] [Google Scholar]
Trento 2001 {published data only}
- Trento M, Passera P, Tomalino M, Bajardi M, Pomero F, Allione A, et al. Group visits improve metabolic control in type 2 diabetes: a 2‐year follow‐up. Diabetes Care 2001;24(6):995‐1000. [DOI] [PubMed] [Google Scholar]
Trento 2002 {published data only}
- Trento M, Passera P, Bajardi M, Tomalino M, Grassi G, Borgo E, et al. Lifestyle intervention by group care prevents deterioration of Type II diabetes: a 4‐year randomized controlled clinical trial. Diabetologia 2002;45(9):1231‐9. [DOI] [PubMed] [Google Scholar]
Trento 1998 {published data only}
- Trento M, Passera P, Tomalino M, Pagnozzi F, Pomero F, Vaccari P, et al. Therapeutic group education in the follow‐up of patients with non‐insulin treated, non‐insulin dependent diabetes mellitus. Diabetes, Nutrition and Metabolism 1998;11:212‐6. [Google Scholar]
Uusitupa 1996 {published data only}
- Uusitupa MI. Early lifestyle intervention in patients with non‐insulin‐dependent diabetes mellitus and impaired glucose tolerance. Annals of Medicine 1996;28(5):445‐9. [DOI] [PubMed] [Google Scholar]
Uusitupa 1993 {published data only}
- Uusitupa M, Laitinen J, Siitonen O, Vanninen E, Pyorala K. The maintenance of improved metabolic control after intensified diet therapy in recent type 2 diabetes. Diabetes Research and Clinical Practice 1993;19:227‐38. [DOI] [PubMed] [Google Scholar]
Vanninen 1993 {published data only}
- Vanninen E, Uusitupa M, Lansimies E, Siitonen O, Laitinen J. Effect of metabolic control on autonomic function in obese patients with newly diagnosed type 2 diabetes. Diabetic Medicine 1993;10(1):66‐73. [DOI] [PubMed] [Google Scholar]
Vanninen 1994 {published data only}
- Vanninen E, Laitinen J, Uusitupa M. Physical activity and fibrinogen concentration in newly diagnosed NIDDM. Diabetes Care 1994;17(9):1031‐8. [DOI] [PubMed] [Google Scholar]
Vanninen 1992 {published data only}
- Vanninen E, Uusitupa M, Siitonen O, Laitinen J, Lansimies E. Habitual physical activity, aerobic capacity and metabolic control in patients with newly‐diagnosed type 2 (non‐insulin‐dependent) diabetes mellitus: effect of 1‐year diet and exercise intervention. Diabetologia 1992;35:340‐6. [DOI] [PubMed] [Google Scholar]
Wing 1987 {published data only}
- Wing R, Koeske R, Epstein L, Nowalk M, Gooding W, Becker D. Long‐term effects of modest weight loss in type II diabetic patients. Archives of Internal Medicine 1987;147:1749‐53. [PubMed] [Google Scholar]
Wing 1988b {published data only}
- Wing RR, Epstein LH, Nowalk MP, Scott N. Self‐regulation in the treatment of Type II diabetes. Behavior Therapy 1988;19:11‐23. [Google Scholar]
Wing 1985 (BM) {published data only}
- Wing RR, Epstein LH, Nowalk MP, Koeske R, Hagg S. Behavior change, weight loss, and physiological improvements in type II diabetic patients. Journal of Consulting and Clinical Psychology 1985;53(1):111‐22. [DOI] [PubMed] [Google Scholar]
Wing 1985 (NE) {published data only}
- Wing RR, Epstein LH, Nowalk MP, Koeske R, Hagg S. Behavior change, weight loss, and physiological improvements in type II diabetic patients. Journal of Consulting and Clinical Psychology 1985;53(1):111‐22. [DOI] [PubMed] [Google Scholar]
Wing 1986 {published data only}
- Wing RR, Epstein LH, Nowalk MP, Scott N, Koeske R, Hagg S. Does self‐monitoring of blood glucose levels improve dietary compliance for obese patients with type II diabetes?. American Journal of Medicine 1986;81(5):830‐6. [DOI] [PubMed] [Google Scholar]
Wing 1988a (Study 1) {published data only}
- Wing RR, Epstein LH, Paternostro‐Bayles M, Kriska A, Nowalk MP, Gooding W. Exercise in a behavioral weight control programme for obese patients with type 2 diabetes. Diabetologia 1988;31:902‐9. [DOI] [PubMed] [Google Scholar]
Wing 1988a (Study 2) {published data only}
- Wing RR, Epstein LH, Paternostro‐Bayles M, Kriska A, Nowalk MP, Gooding W. Exercise in a behavioral weight control programme for obese patients with type 2 diabetes. Diabetologia 1988;31:902‐9. [DOI] [PubMed] [Google Scholar]
Wing 1991a {published data only}
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair EH. Effects of a very‐low‐calorie diet on long‐term glycemic control in obese type 2 diabetic subjects. [see comments.]. Archives of Internal Medicine 1991;151(7):1334‐40. [PubMed] [Google Scholar]
Wing 1991b {published data only}
- Wing RR, Marcus MD, Epstein LH, Jawad A. A "family‐based" approach to the treatment of obese type II diabetic patients. Journal of Consulting and Clinical Psychology 1991;59(1):156‐62. [DOI] [PubMed] [Google Scholar]
Wing 1994 {published data only}
- Wing RR, Blair E, Marcus M, Epstein LH, Harvey J. Year‐long weight loss treatment for obese patients with type II diabetes: does including an intermittent very‐low‐calorie diet improve outcome?. American Journal of Medicine 1994;97(4):354‐62. [DOI] [PubMed] [Google Scholar]
Wing 1995 {published data only}
- Wing RR, Shiffman S, Drapkin RG, Grilo CM, McDermott M. Moderate versus restrictive diets: Implications for relapse. Behavior Therapy 1995;26(1):5‐24. [Google Scholar]
Wing 1996 {published data only}
- Wing RR, Anglin K. Effectiveness of a behavioral weight control program for blacks and white with NIDDM. Diabetes Care 1996;19(5):409‐13. [DOI] [PubMed] [Google Scholar]
Wing 1997 {published data only}
- Wing RR. Insulin sensitivity as a predictor of weight regain. Obesity Research 1997;5(1):24‐9. [DOI] [PubMed] [Google Scholar]
Zapotoczky 2001 {published data only}
- Zapotoczky H, Semlitsch B, Herzog G, Bahadori B, Siebenhofer A, Pieber TR, Zapotoczky HG. A controlled study of weight reduction in type 2 diabetics treated by two reinforcers. International Journal of Behavioral Medicine 2001;8(1):42‐9. [Google Scholar]
References to studies excluded from this review
Agurs‐Collins 1997 {published data only}
- Agurs‐Collins TD, Kumanyika SK, Have TR, Adams‐Campbell LL. A randomized controlled trial of weight reduction and exercise for diabetes managment in older African‐American subjects. Diabetes Care 1997;20:1503‐11. [DOI] [PubMed] [Google Scholar]
Altmann 1987 {published data only}
- Altmann J, Kornhuber AW, Kornhuber HH. Stroke: cardiovascular risk factors and the quantitative effects of dietary treatment on them. European Neurology 1987;26(2):90‐9. [DOI] [PubMed] [Google Scholar]
Arauz 2001 {published data only}
- Arauz AG, Sanchez G, Padilla G, Fernandez M, Rosello M, Guzman S. Community diabetes educational intervention at the primary care level. Revista Panamericana de Salud Publica 2001;9(3):145‐53. [DOI] [PubMed] [Google Scholar]
Bloomgarden 1987 {published data only}
- Bloomgarden ZT, Karmally W, Metzger MJ, Brothers M, Nechemias C, Bookman J, et al. Randomized, controlled trial of diabetic patient education: improved knowledge without improved metabolic status. Diabetes Care 1987;10(3):263‐72. [DOI] [PubMed] [Google Scholar]
Boehm 1993 {published data only}
- Boehm S, Schlenk EA, Raleigh E, Ronis D. Behavioral analysis and behavioral strategies to improve self‐management of Type II diabetes. Clinical Nursing Research 1993;2:327‐44. [DOI] [PubMed] [Google Scholar]
Brown 2002 {published data only}
- Brown SA, Garcia AA, Kouzekanani K, Hanis CL. Culturally competent diabetes self‐management education for mexican Americans; the Starr County border health Initiative. Diabetes Care 2002;25:259‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caplan 1995 {published data only}
- Caplan GA, Colagiuri R, Lord SR, Colagiuri S. Exercise in older people with type II diabetes maintains bone density despite weight loss. Australian Journal on Ageing 1995;14(2):71‐5. [Google Scholar]
Currey 1977 {published data only}
- Currey H, Malcolm R, Riddle E, Schachte M. Behavioral treatment of obesity. Limitations and results with the chronically obese. Journal of the American Medical Association 1977;237(26):2829‐31. [DOI] [PubMed] [Google Scholar]
Dalmau Llorca 2003 {published data only}
- Dalmau Llorca MR, Garcia BG, Aguilar MC, Palau GA. Group versus individual education for type‐2 diabetes patients. Aten Primaria 2003;32(1):36‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
De Luis 2001 {published data only}
- Luis RD, Izaola O, Aller R. Assessment of compliance with a 1500 calorie diet in an overweight population of type 2 diabetics [Spanish]. Nutricion Hospitalaria 2001;16(4):122‐5. [PubMed] [Google Scholar]
Di Loreto 2003 {published data only}
- Loreto C, Fanelli C, Lucidi P, Murdolo G, Cicco A, Parlanti N, et al. Validation of a counseling strategy to promote the adoption and the maintenance of physical activity by type 2 diabetic subjects. Diabetes Care 2003;26(2):404‐408. [DOI] [PubMed] [Google Scholar]
Fernandez Suarez1995 {published data only}
- Fernandez Suarez F, Trueba A, Ferrus JA, Olloqui J, Lorente N, Leoz A. The effect of a program of care for the diabetic on control of the disease [Spanish]. Atencion Primaria 1995;15(6):341‐2. [PubMed] [Google Scholar]
Frost 1989 {published data only}
- Frost C. Comparison of two methods of energy prescription for obese non‐insulin‐dependent diabetics. Practical Diabetes 28, 401‐411. Practical Diabetes 1989;28:410‐411. [Google Scholar]
Gaede 2001 {published data only}
- Gaede P, Beck M, Vedal P, Pedersen O. Limited impact of lifestyle education on patients with Type 2 diabetes mellitus and microalbuminuria: results from a randomized intervention study. Diabetic Medicine 2001;18(2):104‐8. [DOI] [PubMed] [Google Scholar]
Gallagher 1984 {published data only}
- Gallagher AM, Abraira C, Henderson WG. A four‐year prospective trial of unmeasured diet in lean diabetic adults. Diabetes Care 1984;7(6):557‐65. [DOI] [PubMed] [Google Scholar]
Gallagher 1987 {published data only}
- Gallagher AM, Henderson W, Abraira C. Dietary patterns and metabolic control in diabetic diets: a prospective study of 51 outpatient men on unmeasured and exchange diets. Journal of the American College of Nutrition 1987;6(6):525‐32. [DOI] [PubMed] [Google Scholar]
Garcia 1996 {published data only}
- Garcia R, Suarez R. Diabetes education in the elderly: A 5‐year follow‐up of an interactive approach. Patient Education and Counseling 1996;29(1):87‐97. [DOI] [PubMed] [Google Scholar]
Genuth 1979 {published data only}
- Genuth S. Supplemented fasting in the treatment of obesity and diabetes. The American Journal of Clinical Nutrition 1979;32:2579‐86. [DOI] [PubMed] [Google Scholar]
Giacco 2000 {published data only}
- Giacco R, Parillo M, Rivellese AA, Lasorella G, Giacco A, D'Episcopo L, et al. Long‐term dietary treatment with increased amounts of fiber‐rich low‐glycemic index natural foods improves glood glucose control and reduces the number of hypoglycemic events in type 2 diabetic patients. Diabetes Care 2000;23:1461‐6. [DOI] [PubMed] [Google Scholar]
Gilliland 2002 {published data only}
- Gilliland SS, Azen SP, Perez GE, Carter JS. Strong in body and spirit: lifestyle intervention for native American adults with diabetes in New Mexico. Diabetes Care 2002;25(1):78‐83. [DOI] [PubMed] [Google Scholar]
Henry 1986 {published data only}
- Henry RR, Wallace P, Olefsky JM. Effects of weight loss on mechanisms of hyperglycemia in obese non‐in sulin‐dependent diabetes mellitus. Diabetes 1986;35:990‐8. [DOI] [PubMed] [Google Scholar]
Hughes 1999 {published data only}
- Hughes J, Todorovic V, Kemp H. 'The sugar buddies': an intervention programme for obese patients with poorly controlled diabetes. Journal of Human Nutrition and Dietetics 1999;12(Suppl):71‐8. [Google Scholar]
Jayasuriya 2000 {published data only}
- Jayasuriya R, Griffiths R, Cheung J. Outcome assessment of a community based model of general practitioner care of diabetes patients. Practical Diabetes International 2000;17(6):179‐82. [Google Scholar]
Kanders 1989 {published data only}
- Kanders BS, Blackburn GI, Avin PI, Norton D. Weight loss outcome and health benefits associated with the optifast program in the treatment of obesity. International Journal of Obesity 1989;13(Suppl 2):131‐4. [PubMed] [Google Scholar]
Karlsson 1998 {published data only}
- Karlsson J, Sjöström L, Sullivan M. Swedish obese subjects (SOS): an intervention study of obesity. Two year follow‐up of health‐related quality of life (HRQL) and eating behavior after gastric bypass surgery for severe obesity. International Journal of Obesesity 1998;22:113‐26. [DOI] [PubMed] [Google Scholar]
Keyserling 2002 {published data only}
- Keyserling TC, Samuel‐Hodge CD, Ammerman AS, Ainsworth BE, Henriquez‐Roldan CF, Elasy TA, et al. A randomized trial of an intervention to improve self‐care behaviors of African‐American women with type 2 diabetes: impact of physical activity. Diabetes Care 2002;25(9):1576‐83. [DOI] [PubMed] [Google Scholar]
Kirk 2001 {published data only}
- Kirk AF, Higgins LA, Hughes AR, Fisher BM, Mutrie N, Hillis S, et al. A randomized, controlled trial to study the effect of exercise consultation on the promotion of physical activity in people with type 2 diabetes: a pilot study. Diabetic Medicine 2001;18(11):877‐82. [DOI] [PubMed] [Google Scholar]
Kirkman 1994 {published data only}
- Kirkman MS, Weinberger M, Landsman PB, Samsa GP, Shortliffe EA, Simei DL. A telephone‐delivered intervention for patients with NIDDM. Diabetes Care 1994;17(8):840‐6. [DOI] [PubMed] [Google Scholar]
Luscombe 2002 {published data only}
- Luscombe ND, Clifton PM, Noakes M, Parker B, Wittert G. Effects of energy‐restricted diets containing increased protein on weight loss, resting energy expenditure, and the thermic effect of feeding of type 2 diabetes. Diabetes Care 2002;25(4):652‐657. [DOI] [PubMed] [Google Scholar]
Mancini 1981 {published data only}
- Mancini M, DiDiase G, Contaldo F, Fischetti A, Grasso L, Mattioli P. Medical complications of severe obesity; importance of treatment by very‐low calorie diets: Iintermediate and long‐term effects. International Journal of Obesity 1981;5:341‐52. [PubMed] [Google Scholar]
Manning 1998 {published data only}
- Manning R, Jung R, Leese G, Newton R. The comparison of four weight reduction strategies aimed at overweight patients with diabetes melllitus: four‐year follow‐up. Diabetic Medicine 1998;15:497‐502. [DOI] [PubMed] [Google Scholar]
Mengham 1999 {published data only}
- Mengham LH, Morris BF, Palmer CR, White AJS. Is intensive dietetic intervention effective for overweight patients with diabetes mellitus? A randomised controlled study in a general practice. Practical Diabetes International 1999;16(1):5‐8. [Google Scholar]
Murphy 1999 {published data only}
- Murphy C, Simkins M, Helowicz R. Diabetes exercise project. Journal of Human Nutrition and Dietetics 1999;12(Suppl 1):90. [Google Scholar]
Nothwehr 2001 {published data only}
- Nothwehr FK. Sequencing diet and exercise programs for African American women with diabetes. Diabetes Educator 2001;27(2):245‐51. [DOI] [PubMed] [Google Scholar]
Reaven 1985 {published data only}
- Reaven GM. Beneficial effect of moderate weight loss in older patients with non‐insulin‐dependent diabetes mellitus poorly controlled with insulin. Journal of the American Geriatrics Society 1985;33(2):93‐5. [DOI] [PubMed] [Google Scholar]
Ridgeway 1999 {published data only}
- Ridgeway NA, Harvill DR, Harvill LM, Falin TM, Forester GM, Gose OD. Improved control of type 2 diabetes mellitus: a practical education/behavior modification program in a primary care clinic. Southern Medical Journal 1999;92(7):667‐72. [DOI] [PubMed] [Google Scholar]
Schafer 1987 {published data only}
- Schafer RG. Effectiveness of exchange diet vs. basic diet teaching in achieving treatment goals. Diabetes Care 1987;19:666‐7. [DOI] [PubMed] [Google Scholar]
Simmons 1998 {published data only}
- Simmons D, Fleming C, Voyle J, Fou F, Feo S, Gatland B. A pilot urban church‐based programme to reduce risk factors for diabetes among Western Samoans in New Zealand. Diabetic Medicine 1998;15(2):136‐42. [DOI] [PubMed] [Google Scholar]
Simonen 2000 {published data only}
- Simonen P, Gylling H, Howard AN, Miettinen TA. Introducing a new component of the metabolic syndrome: low cholesterol absorption. American Journal of Clinical Nutrition 2000;72(1):82‐8. [DOI] [PubMed] [Google Scholar]
Solerte 1989 {published data only}
- Solerte SB, Fioravanti M, Schifino N, Ferrari E. Effects of diet‐therapy on urinary protein excretion albuminuria and renal haemodynamic function in obese diabetic patients with overt nephropathy. International Journal of Obesity 1989;13(2):203‐11. [PubMed] [Google Scholar]
Szybinski 1978 {published data only}
- Szybinski Z, Sieradzki J, Mruk K, Kucharski K, Poskuta W, Korzeniowska D, Jastrzebska E. Effect of reducing diet on carbohydrate tolerance and therapeutic management of obese diabetics. Polskie Archiwum Medycyny Wewnetrznej 1978;60(5):423‐31. [PubMed] [Google Scholar]
Toobert 2003 {published data only}
- Toobert DJ, Glasgow RE, Stycker LA, Barrera Jr M, Radcliffe JL, Wander RC, Bagdade, J.D. Biologic and quality‐of‐life outcomes from the Mediterranean lifestyle program. Diabetes Care 2003;26:2288‐2293. [DOI] [PubMed] [Google Scholar]
Tudor‐Locke 2004 {published data only}
- Tudor‐Locke C, Bell RC, Myers AM, Harris SB, Ecclestone NA, Lauzon N, et al. Controlled outcome evaluation of the First Step Program: a daily physical activity intervention for individuals with type II diabetes. International Journal of Obesesity 2004;28(1):113‐9. [DOI] [PubMed] [Google Scholar]
Turnin 1992 {published data only}
- Turnin M‐CG, Beddok RH, Clottes JP, Martini PF, Abadie RG, Buisson J‐C, et a. Telematic expert system Diabeto: new tool for diet self‐monitoring for diabetic patients. Diabetes Care 1992;15(2):204‐12. [DOI] [PubMed] [Google Scholar]
Ueda 1999 {published data only}
- Ueda K, Sumitani S, Yamada H. Effectiveness of the two‐step diabetes education program. Japanese Journal of Nutrition 1999;57(2):71‐80. [Google Scholar]
Werdier 1984 {published data only}
- Werdier D, Jesdinsky HJ, Helmich P. A randomized, controlled study on the effect of diabetes counseling in the offices of 12 general practitioners. Revue d Epidemiologie et de Sante Publique 1984;32(3‐4):225‐9. [PubMed] [Google Scholar]
References to ongoing studies
Look AHEAD 2003 {published data only}
- The Look AHEAD Research Group. Look AHEAD (Action for Health in Diabetes): Design and methods for a clinical trial of weight loss for the prevention of cardiovascular disease in type 2 diabetes. Controlled Clinical Trials 2003;24:610‐628. [DOI] [PubMed] [Google Scholar]
Additional references
ADA 1997
- American Diabetes Association. Report on the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20(Suppl 1):S5‐20. [DOI] [PubMed] [Google Scholar]
Alberti 1998
- Alberti KM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part I: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic Medicine 1998;15:539‐53. [DOI] [PubMed] [Google Scholar]
Ash 2003
- Ash S, Reeves MM, Yeo S, Morrison G, Carey D, Capra S Ash S, Reeves MM, Yeo S, Morrison G, Carey D, Capra S. Effect of intensive dietetic interventions on weight and glycaemic control in overweight men with Type II diabetes: A randomised trial [Ash S; Reeves MM; Yeo S; Morrison G; Carey D; Capra S]. International Journal of Obesity 2003;27:797‐802. [DOI] [PubMed] [Google Scholar]
Berlin 1997
- Berlin J, Miles C, Crigilano M, Conill A, Goldmann D, Horowitz D, et al. Does blinding of readers affect the results of meta‐analyses? Results of a randomized trial.. Online Journal of Current Clinical Trials 1997;Document no. 205. [Google Scholar]
Boule 2001
- Boule MG, Haddad E, Kenny GP, Wells GA, Sigal RJ. Effects of exercise on glycemic control and body mass in type 2 diabetes mellitus: a meta‐analysis of controlled clinical trials. Journal of American Medical Association 2001;286:1218‐27. [DOI] [PubMed] [Google Scholar]
Brown 1996
- Brown SA, Upchurch S, Anding R, Winter M, Ramirez G. Promoting weight loss in type II diabetes. Diabetes Care 1996;19:613‐24. [DOI] [PubMed] [Google Scholar]
CDC 2002
- U. S. Department of Health and Human Services, Centers for Disease Control and Prevention. National diabetes fact sheet: general information and national estimates on diabetes in the United States,. http://www.cdc.gov/diabetes/pubs/factsheet.htm (Accessed May 7, 2002) 2002.
Ciliska 1995
- Ciliska D, Kelly C, Petrov N, Chalmers J. A review of weight loss interventions for obese people with non‐insulin‐dependent diabetes mellitus. Canadian Journal of Diabetes Care 1995;19:10‐15. [Google Scholar]
Clarke 2001
- Clarke M, Oxman AD. Cochrane reviewers handbook. Update Software 2001. [Google Scholar]
DerSimonian 1954
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1954;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
DPPRG 2002
- Diabetes Prevention Program Research Group. Reduction in the indicence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine 2002;346:393‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eriksson 1991
- Eriksson K‐F, Lindgarde F. Prevention of type 2 (non‐insulin‐dependent‐diabetes) mellitus by diet and physical exercise. Diabetologia 1991;34:891‐8. [DOI] [PubMed] [Google Scholar]
Feinstein 1985
- Feinstein AR. Clinical Epidemiology: The Architecture of Clinical Research. New Haven, CT: W.B. Clinical Epidemiology: The Architecture of Clinical Research. New Haven, CT: W.B. New Haven, CT, USA: W.B. Saunders, 1985. [Google Scholar]
Glenny 1997
- Glenny AM, O'Meara S, Melville A, Sheldon TA, Wilson C. The treatment and prevention of obesity: A systematic review of the literature. International Journal of Obesity 1997;21:715‐37. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. British Medical Journal 2003;327:556‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hoaglin 1983
- Hoaglin DC, Mosteller F, Tukey JW. Understanding Robust and Exploratory Data Analysis. 1st Edition. New York: John Wiley & Sons, Inc, 1983. [Google Scholar]
Irwig 1994
- Irwig L, Toteson A, Gatsonis C, Lau J, Colditz G, Chalmers T, et al. Guidelines for meta‐analyses evaluating diagnostic tests. Annals of Internal Medicine 1994;120:667‐76. [DOI] [PubMed] [Google Scholar]
Khaw 2001
- Khaw K‐T, Wareham N, Luben R, Bingham S, Oakes S, Welch A, et al. Glycated heamoglobin, diabetes, and mortality in men in Norfolk cohort of European prospective investigation of cancer and nutrition (EPIC‐Norfolk). BMJ 2001;322(15):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kramer 1989
- Kramer FM, Jeffery RW, Forster JL, Snell MK. Long‐term follow‐up of behavioral treatment for obesity: patterns of weight regain in men and women. International Journal of Obesity 1989;13:123‐36. [PubMed] [Google Scholar]
Kuller 2001
- Kuller JL, Simkin‐Silverman LRsi, Wing RR, Meilahn EN, Ives DG. Womens Healthy lifestyle project: a randomized clincial trial: results at 54 months. Circulation 2001;103:32‐7. [DOI] [PubMed] [Google Scholar]
Little 1991
- Little RR, Wiedmeyer H‐M, England JD, Naito HK, Goldstein DE. Interlaboratory comparison of glycohemoglobin results: College of American Pathologists survey data. Clinical Chemistry 1991;37:1725‐9. [PubMed] [Google Scholar]
Maggio 1997
- Maggio CA, Pi‐Sunyer FX. The prevention and treatment of obesity. Application to type 2 diabetes. Diabetes Care 1997;20(11):1744‐66. [DOI] [PubMed] [Google Scholar]
Mokad 2001
- Mokad AH, Bowman BA, Ford ES, Vinicor F, Marks J, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286(10):1195‐7. [DOI] [PubMed] [Google Scholar]
Mokdad 2000
- Mokdad A, Ford E, Bowman B, Nelson D, Engelgau M, Vinicor, et al. Diabetes trends in the US.: 1990‐1998. Diabetes Care 2000;23:1278‐83. [DOI] [PubMed] [Google Scholar]
NDDG 1979
- National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039‐57. [DOI] [PubMed] [Google Scholar]
NHLBI 1998
- National Heart Lung and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overwieght and obesity in adults: the evidence report. National Institutes of Health, Washington DC 1998. [PubMed] [Google Scholar]
Norris 2004
- Norris SL, Zhang X, Avenell A, Gregg E, Schmidt CH, Kim C, et al. Efficacy of pharmacotherapy for weight loss in adults with type 2 diabetes mellitus: a meta‐analysis. Archives Internal Medicine 2004;164:1395‐404. [DOI] [PubMed] [Google Scholar]
O'Meara 1998
- O'Meara S, Glenny A‐M, Sheldon T, Melville A, Wilson C. Systematic review of the effectiveness of interventions used in the management of obesity. Journal of Human Nutrition & Dietetics 1998;11:203‐6. [Google Scholar]
Ornish 1998
- Ornish D, Scherwitz LW, Billings JH, et al. Intensive lifestyle changes for reversal of coronary heart disease. Journal American Medical Association 1998;280:2001‐7. [DOI] [PubMed] [Google Scholar]
Perri 1993
- Perri MG, Sears SFJ, Clark JE. Strategies for improving maintenance of weight loss. Toward a continuous care model of obesity management. [Review] [39 refs]. Diabetes Care 1993;16:200‐9. [DOI] [PubMed] [Google Scholar]
Pi‐Sunyer 2000
- Pi‐Sunyer EX. Weight loss and mortality in type 2 diabetes. Diabetes Care 2000;23:1451‐2. [DOI] [PubMed] [Google Scholar]
Pories 1992
- Pories WJ, MacDonald KG, Jr, Morgan EJ, Sinha MK, Dohm GL, Swanson MS, et al. Surgical treatment of obesity and its effect on diabetes: 10‐year follow‐up. American Journal of Clinical Nutrition 1992;55(2:Suppl):Suppl‐585S. [DOI] [PubMed] [Google Scholar]
Rhiel 1986
- Rhiel GS. The effect of non‐normality on the use of the range in estimating the population standard deviation. Journal Statistical Computing 1986;24:71‐82. [Google Scholar]
Sjostrom 1999
- Sjostrom CD, Lissner L, Wedel H, Sjostrom L. Reduction in incidence of diabetes, hypertension and lipid disturbances after intentional weight loss induced by bariatric surgery: the SOS Intervention Study. Obesity Research 1999;7(5):477‐84. [DOI] [PubMed] [Google Scholar]
Skender 1996
- Skender M, Goodrick G, Junco DD, et al. Comparison of 2 year weight loss trends in behavioral treatments of obesity: diet, exercise and combination interventions. Journal of the American Dietetic Association 1996;96:342‐6. [DOI] [PubMed] [Google Scholar]
Sterne 2001
- Sterne JAC, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman DG, editors. Systematic Reviews in Health Care; Meta‐analysis in Context. Meta‐analysis in Context. London: BMJ Publishing Group, 2001:189‐208. [Google Scholar]
Tang 2000
- Tang JL, Liu JLY. Misleading funnel plot for detection of bias in meta‐analysis. Journal of Clinical Epidemiology 2000;53:477‐84. [DOI] [PubMed] [Google Scholar]
Thornton 2000
- Thornton A, Lee P. Publication bias in meta‐analysis: its causes and consequences. Journal of Clinical Epidemiology 2000;53:207‐16. [DOI] [PubMed] [Google Scholar]
Tuomilehto 2001
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne‐Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. New England Journal of Medicine 2001;344(18):1343‐50. [DOI] [PubMed] [Google Scholar]
UYCRD 1997
- The University of York Centre for Reviews and Dissemination. The prevention and treatment of obesity. London: The Royal Society of Medicine Press Ltd., 1997. [Google Scholar]
Wadden 1989
- Wadden TA, Sternberg JA, Letizia KA, et al. Treatment of obesity by very low calorie diet, behavior therapy, and their combination: a five‐year perspective. International Journal of Obesity 1989;13:39‐46. [PubMed] [Google Scholar]
Wadden 2000
- Wadden TA, Foster GD. Behavioral treatment of obesity. Medical Clinics of North America 2000;84(2):441‐61. [DOI] [PubMed] [Google Scholar]
WHO 1980
- WHO Expert Committee on Diabetes Mellitus. World Health Organization Technical Report. World Health Organization Technical Report Series 1980. [PubMed] [Google Scholar]
WHO 1985
- WHO Expert Committee on Diabetes Mellitus. World Health Organization Technical Report.. World Health Organization Technical Report Series 727 1985. [Google Scholar]
Williamson 2000
- Williamson DF, Thompson TJ, Thun M, Flanders D, Pamuk E, Byers T. Intentional weight loss and mortality among overweight individuals with diabetes. Diabetes Care 2000;23:1499‐504. [DOI] [PubMed] [Google Scholar]
Wing 1985
- Wing R, Epstein L, Nowalk M, Koeske R, Hagg S. Behavior change, weight loss, and physiological improvements in type II diabetic patients. Journal of Consulting & Clinical Psychology 1985;53(1):111‐22. [DOI] [PubMed] [Google Scholar]
Wing 1987
- Wing RR, Koeske R, Epstein LH, Nowalk MP, Gooding W, Becker D. Long‐term effects of modest weight loss in type II diabetic patients. Archives of Internal Medicine 1987;147:1749‐53. [PubMed] [Google Scholar]
Wing 1991
- Wing RR, Marcus MD, Salata R, Epstein LH, Miaskiewicz S, Blair E. Effects of a very‐low‐calorie diet on long‐term glycemic control in obese type 2 diabetic subjects. Archives of Internal Medicine 1991;151:1334‐40. [PubMed] [Google Scholar]
Wing 2000
- Wing RR. Weight loss in the management of type 2 diabetes. In: Gerstein HC, Haynes RB editor(s). Evidence‐Based Diabetes Care. Ontario, Canada: B.C. Decker, Inc, 2000:252‐76. [Google Scholar]


