Abstract
Background
Depression is common in young people. It has a marked negative impact and is associated with self‐harm and suicide. Preventing its onset would be an important advance in public health. This is an update of a Cochrane review that was last updated in 2011.
Objectives
To determine whether evidence‐based psychological interventions (including cognitive behavioural therapy (CBT), interpersonal therapy (IPT) and third wave CBT)) are effective in preventing the onset of depressive disorder in children and adolescents.
Search methods
We searched the specialised register of the Cochrane Common Mental Disorders Group (CCMDCTR to 11 September 2015), which includes relevant randomised controlled trials from the following bibliographic databases: The Cochrane Library (all years), EMBASE (1974 to date), MEDLINE (1950 to date) and PsycINFO (1967 to date). We searched conference abstracts and reference lists of included trials and reviews, and contacted experts in the field.
Selection criteria
We included randomised controlled trials of an evidence‐based psychological prevention programme compared with any comparison control for young people aged 5 to 19 years, who did not currently meet diagnostic criteria for depression.
Data collection and analysis
Two authors independently assessed trials for inclusion and rated their risk of bias. We adjusted sample sizes to take account of cluster designs and multiple comparisons. We contacted trial authors for additional information where needed. We assessed the quality of evidence for the primary outcomes using GRADE.
Main results
We included 83 trials in this review. The majority of trials (67) were carried out in school settings with eight in colleges or universities, four in clinical settings, three in the community and four in mixed settings. Twenty‐nine trials were carried out in unselected populations and 53 in targeted populations.
For the primary outcome of depression diagnosis at medium‐term follow‐up (up to 12 months), there were 32 trials with 5965 participants and the risk of having a diagnosis of depression was reduced for participants receiving an intervention compared to those receiving no intervention (risk difference (RD) ‐0.03, 95% confidence interval (CI) ‐0.05 to ‐0.01; P value = 0.01). We rated this evidence as moderate quality according to the GRADE criteria. There were 70 trials (73 trial arms) with 13,829 participants that contributed to the analysis for the primary outcome of depression symptoms (self‐rated) at the post‐intervention time point, with results showing a small but statistically significant effect (standardised mean difference (SMD) ‐0.21, 95% CI ‐0.27 to ‐0.15; P value < 0.0001). This effect persisted to the short‐term assessment point (up to three months) (SMD ‐0.31, 95% CI ‐0.45 to ‐0.17; P value < 0.0001; 16 studies; 1558 participants) and medium‐term (4 to 12 months) assessment point (SMD ‐0.12, 95% CI ‐0.18 to ‐0.05; P value = 0.0002; 53 studies; 11,913 participants); however, the effect was no longer evident at the long‐term follow‐up. We rated this evidence as low to moderate quality according to the GRADE criteria.
The evidence from this review is unclear with regard to whether the type of population modified the overall effects; there was statistically significant moderation of the overall effect for depression symptoms (P value = 0.0002), but not for depressive disorder (P value = 0.08). For trials implemented in universal populations there was no effect for depression diagnosis (RD ‐0.01, 95% CI ‐0.03 to 0.01) and a small effect for depression symptoms (SMD ‐0.11, 95% CI ‐0.17 to ‐0.05). For trials implemented in targeted populations there was a statistically significantly beneficial effect of intervention (depression diagnosis RD ‐0.04, 95% CI ‐0.07 to ‐0.01; depression symptoms SMD ‐0.32, 95% CI ‐0.42 to ‐0.23). Of note were the lack of attention placebo‐controlled trials in targeted populations (none for depression diagnosis and four for depression symptoms). Among trials implemented in universal populations a number used an attention placebo comparison in which the intervention consistently showed no effect.
Authors' conclusions
Overall the results show small positive benefits of depression prevention, for both the primary outcomes of self‐rated depressive symptoms post‐intervention and depression diagnosis up to 12 months (but not beyond). Estimates of numbers needed to treat to benefit (NNTB = 11) compare well with other public health interventions. However, the evidence was of moderate to low quality using the GRADE framework and the results were heterogeneous. Prevention programmes delivered to universal populations showed a sobering lack of effect when compared with an attention placebo control. Interventions delivered to targeted populations, particularly those selected on the basis of depression symptoms, had larger effect sizes, but these seldom used an attention placebo comparison and there are practical difficulties inherent in the implementation of targeted programmes. We conclude that there is still not enough evidence to support the implementation of depression prevention programmes.
Future research should focus on current gaps in our knowledge. Given the relative lack of evidence for universal interventions compared with attention placebo controls and the poor results from well‐conducted effectiveness trials of universal interventions, in our opinion any future such trials should test a depression prevention programme in an indicated targeted population using a credible attention placebo comparison group. Depressive disorder as the primary outcome should be measured over the longer term, as well as clinician‐rated depression. Such a trial should consider scalability as well as the potential for the intervention to do harm.
Keywords: Adolescent; Child; Child, Preschool; Female; Humans; Male; Young Adult; Depression; Depression/diagnosis; Depression/prevention & control; Depressive Disorder; Depressive Disorder/diagnosis; Depressive Disorder/prevention & control; Program Evaluation; Psychotherapy; Psychotherapy/methods; Randomized Controlled Trials as Topic
Plain language summary
Evidence‐based psychological interventions for preventing depression in children and adolescents
The aim of this review was to assess the efficacy of evidence‐based psychological interventions designed to prevent the onset of a depressive disorder and to reduce any existing symptoms of depression.
Who may be interested in this review?
People involved in public health initiatives, school personnel and mental health clinicians.
Why is this review important?
Depressive disorder is common. It is associated with a negative impact on the functioning of young people and is expensive to society at large. Finding a way to prevent the onset of depressive disorder has the potential to make an important impact on the burden of depression in young people.
What questions does this review aim to answer?
Whether psychological depression prevention programmes designed to prevent the onset of depressive disorder in children and adolescents are effective.
Which trials were included in the review?
We included 83 studies (in particular randomised controlled trials) of evidence‐based psychotherapy interventions (cognitive behavioural therapy (CBT) and third wave CBT, interpersonal therapy) that had the specific aim of preventing the onset of depressive disorder. For the primary outcome of depression diagnosis at medium‐term follow‐up (up to 12 months), there were 32 trials with 5965 participants and for the primary outcome of depression symptoms (self‐rated) there were 73 trials with 13,829 participants.
What does the evidence from the review tell us?
We found that, compared with any comparison group, psychological depression prevention programmes have small positive benefits on depression prevention. There were some problems with the way the trials were done and in particular the results showed that compared to an attention placebo comparison group (a control intervention that controls for non‐specific factors like involvement in a trial and attention from researchers), these programmes had no effect. There is still not enough evidence to support the implementation of depression prevention programmes. However, based on the effects seen for targeted depression prevention programmes (albeit with inadequate control groups), we recommend that further research be undertaken to test the effectiveness of depression prevention programmes in populations of young people who already have some symptoms of depression. Such trials should compare the intervention to an attention placebo comparison group and measure whether depressive diagnosis is prevented in the long term. They also need to consider whether the approach is something that can be implemented in the real world. In addition, they should consider and measure whether the intervention produces harmful outcomes.
Summary of findings
Summary of findings for the main comparison. Evidence‐based psychological interventions versus any comparator for depression diagnosis at the medium‐term follow‐up.
| Evidence‐based psychological interventions compared to any comparator for depression diagnosis at the medium‐term follow‐up | |||||
| Patient or population: children and adolescents Settings: various Intervention: evidence‐based psychological interventions (targeted and universal) Comparison: any | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | ||||
| Any comparator | Evidence‐based psychological interventions | ||||
|
Evidence‐based psychological interventions versus any comparator (Overall) ‐ effect on diagnosis of depression The assumed risk is based on control group rates of depression diagnosis at medium‐term follow‐up (from a rank ordering of control group rates of each included study). |
Study population |
RR 0.84 (0.72 to 0.97) |
⊕⊕⊕⊝ Moderate1 | — | |
| 193 per 1000 |
162 per 1000 (139 to 187) |
||||
| Low (0%) | |||||
|
0 per 1000 (0 to 0) |
|||||
| Moderate (18.5%) | |||||
| 185 per 1000 |
155 per 1000 (133 to 180) |
||||
| High (70.7%) | |||||
| 707 per 1000 |
594 per 1000 (509 to 685) |
||||
|
Evidence‐based psychological interventions versus any comparator (Targeted programmes) ‐ effect on diagnosis of depression The assumed risk is based on control group rates of depression diagnosis at medium‐term follow‐up (from a rank ordering of control group rates of each included study). |
Study population | RR 0.82 (0.68 to 0.99) | ⊕⊝⊝⊝ Very low1,2,3 | — | |
| 243 per 1000 | 199 per 1000 (165 to 240) | ||||
| Low (0%) | |||||
|
0 per 1000 (0 to 0) |
|||||
| Moderate (20.4%) | |||||
| 204 per 1000 |
167 per 1000 (139 to 202) |
||||
| High (76.7%) | |||||
| 767 per 1000 |
629 per 1000 (521 to 759) |
||||
|
Evidence‐based psychological interventions versus any comparator (Universal programmes) ‐ effect on diagnosis of depression The assumed risk is based on control group rates of depression diagnosis at medium‐term follow‐up (from a rank ordering of control group rates of each included study). |
Study population | RR 0.87 (0.66 to 1.14) | ⊕⊕⊕⊝ Moderate4 | — | |
| 99 per 1000 | 86 per 1000 (65 to 113) | ||||
| Low (1.0%) | |||||
| 10 per 1000 |
9 per 1000 (7 to 12) |
||||
| Moderate (14.5%) | |||||
| 144 per 1000 |
125 per 1000 (95 to 164) |
||||
| High (30.8%) | |||||
| 308 per 1000 |
268 per 1000 (203 to 351) |
||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1We downgraded quality owing to lack of clarity over allocation concealment and presence of other bias. 2Heterogeneity (I2 = 53%). 3Omitting trials in which the outcome was measured indirectly (i.e. using cut‐points from self‐rated depression symptom inventories) caused the treatment effect for targeted depression prevention programmes to become non‐significant (RD ‐0.04, 95% CI ‐0.08 to 0.00; k = 15; n = 2783). 4We downgraded quality owing to a lack of clarity over random sequence generation and allocation concealment.
Summary of findings 2. Evidence‐based psychological interventions versus any comparator for self‐reported depression scores at the post‐intervention assessment.
| Evidence‐based psychological interventions versus any comparator for self‐rated depression scores at the post‐intervention assessment | ||||||
|
Patient or population: children and adolescents Settings: various Intervention: evidence‐based psychological interventions (targeted and universal) Comparison: any | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Any comparator | Evidence‐based psychological interventions | |||||
|
Evidence‐based psychological interventions versus any comparator (Overall) ‐ self‐rated depression scores (higher score is equivalent to a poorer outcome) |
The mean self‐reported depression score ranged across control groups from 0.66 to 105.51 points. | The mean self‐rated depression score in the intervention group was 0.21 standard deviations lower (0.27 to 0.15 lower) | — | 13,829 (73 trials) |
⊕⊕⊝⊝ Low1,2 |
— |
|
Evidence‐based psychological interventions versus any comparator (Targeted ‐ self‐rated depression scores (higher score is equivalent to a poorer outcome)) |
The mean self‐reported depression score ranged across control groups from 4.30 to 105.51 points. | The mean self‐rated depression score in the intervention group was 0.32 standard deviations lower (0.42 to 0.23 lower) | — | 4816 (42 trials) | ⊕⊕⊕⊝ Moderate3 |
— |
|
Evidence‐based psychological interventions versus any comparator (Universal programmes) ‐ self‐rated depression scores (higher score is equivalent to a poorer outcome) |
The mean self‐reported depression score ranged across control groups from 0.66 to 50.49 points. | The mean self‐rated depression score in the intervention group was 0.11 standard deviations lower (0.17 to 0.05 lower) | — | 9013 (31 trials) | ⊕⊕⊕⊝ Moderate1 | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1We downgraded quality owing to a lack of clarity about random sequence generation and allocation concealment and the presence of other bias.
2Heterogeneity (I2 = 57%).
3We downgraded quality owing to a lack of clarity over allocation concealment and the presence of other bias.
Background
Description of the condition
Depression is a common problem in young people. Overall prevalence rates from a large meta‐analysis, measured from point prevalence up to 12‐month period prevalence, were estimated at 2.8% for children under the age of 13 and 5.6% for young people aged 13 to 18 years (Costello 2006). Rates rise steeply in adolescence (Fergusson 2001), with the peak period for the emergence of new cases of depression being during adolescence and young adulthood (Kessler 2005). By the age of 19, between a fifth and a quarter of young people have suffered from a depressive disorder (Lewinsohn 1993; Lewinsohn 1998). Depression in young people is associated with poor academic performance and vocational attainment and achievement, difficulties with interpersonal relationships, substance abuse, and attempted and completed suicide (Birmaher 1996; Birmaher 1996a; Brent 1986; Brent 2002; Fleming 1993; Gould 1998; Rao 1995; Rhode 1994). The Global Burden of Disease study, first published in 1997, ranked depressive disorder fourth in its estimate of disease burden, ahead of ischaemic heart disease, cerebrovascular disease and tuberculosis (Murray 1997). By 2002, depressive disorders ranked second in developed countries and first in developing countries with low mortality (Mathers 2004). Young people account for the greatest global burden of disease (Gore 2011). This means that reducing the incidence of depression has become a major focus, with a large number of trials of preventative interventions being published in the last three decades.
Description of the intervention
Prevention can be universal, where the intervention is implemented for a designated population regardless of risk, or targeted to a population at high risk for the disorder. Targeted interventions can be further classified into selective interventions that focus on populations with a risk factor for the disorder (e.g. family history) and indicated interventions that focus on populations with symptoms or signs suggestive of incipient disorder (Mrazek 1994). Early intervention may be considered prevention or treatment. The Institute of Medicine Report (Mrazek 1994), and the updated report (O'Connell 2009), recommend that prevention is defined as those interventions that occur prior to the onset of a clinically diagnosed disorder.
There are many psychological treatments for depression, which include psychodynamic, humanistic, integrative, systemic, behavioural and cognitive behavioural therapies (CBT) (including 'third wave' cognitive behavioural therapies). In a previous version of this review we had included both psychological interventions (broadly) and psychoeducational interventions; however, this review highlighted the fact that the vast majority of depression interventions developed thus far have been based on CBT and interpersonal therapy (IPT) (Callahan 2012; Merry 2011). The most robust evidence for the treatment of depression is for cognitive behavioural therapy (CBT) and interpersonal therapy (IPT) (which is an integrative therapy) (e.g. NICE 2005; McDermott 2011). They therefore represent a 'good best bet' in terms of depression prevention.
Depression prevention interventions are often delivered to a group within a school setting. This is because young people spend a significant amount of time at school so disseminating a programme within a school or classroom, and to groups of young people is likely to be cost‐effective. Delivery to a group may also reinforce efficacy by providing positive peer experiences. Both group and individual interventions usually take place on a weekly basis with 8 to 12 sessions delivered (Merry 2011).
How the intervention might work
The aetiology of depressive disorder is complex and includes biological, psychological and social factors (Cicchetti 1998; Davidson 2002; Goodyer 2000; Lewinsohn 1994; McCauley 2001). There are clear theories regarding the individual factors that create a predisposition to developing depression, which may alternatively provide a model for promoting resilience in the face of stress. These underlie the currently available evidence‐based interventions for depression. These theories provide a basis for the development of prevention programmes and an understanding of the mechanisms by which they achieve a reduction in the rates of the onset of depression.
Beck developed cognitive behavioural therapy based on his cognitive model of depression (Beck 1976). He proposed that individuals prone to depression have cognitive distortions that result in a negative view of themselves, the world and the future. In CBT, people learn to identify, explore and modify relationships between negative thinking, behaviour and depressed mood. This is achieved by learning to identify and monitor the intensity of different moods in themselves, recognising thoughts and behaviours that have contributed to this mood, and learning how to address these by evaluating and challenging unhelpful thoughts and engaging in behaviour that contributes to improved mood. The associated concepts of 'attributional style' (Abramson 1978) and 'learned helplessness' (Petersen 1993; Seligman 1979) have also contributed to components of CBT. Those with a pessimistic attributional style see negative events as a stable and enduring part of themselves, while positive events are seen as transient occurrences in which they have played no part. Learned helplessness is a phenomenon of withdrawal with depression the result of a perceived failure or inability to control aversive events. Both are associated with a sense of helplessness and hopelessness, which leads to passivity in the face of challenges and contributes to low mood (McCauley 2001). People who are prone to depression are then less likely to take an active approach to dealing with difficulties. CBT also tends to include a component of effective problem‐solving.
Interpersonal conflict, difficulty with role transitions and experiences of loss are all well known as risk factors in the development of depressive disorder in young people (Birmaher 1996; Lewinsohn 1994; Lipsitz 2013; McCauley 2001). IPT helps a person resolve interpersonal problems through a range of techniques and thereby increases a person's access to social support and decreases interpersonal stress, which improves emotional processing and interpersonal skills and ultimately improves symptoms via a range of mechanisms. There are a number of specific techniques that can be used, such as helping the client to express and explore different emotions within social situations, encouraging them to develop supportive relationships with others outside of the therapeutic context, and using role play to allow the client to 'test out' and improve on their communication style (Lipsitz 2013).
While evidence is yet to be clearly established in young people, 'third wave CBT' approaches are becoming popular. These approaches are characterised (in comparison with CBT) by techniques that target the process, rather than content of thoughts, helping people to become aware of and accept their thoughts in a non‐judgemental way (Hofmann 2010). They include such interventions as acceptance and commitment therapy (ACT) (Hayes 2003), mindfulness‐based cognitive therapy (MBCT) (Teasdale 1995), dialectical behaviour therapy (DBT) (Linehan 1993), and the expanded model of behavioural activation (BA) (Martell 2001).
Why it is important to do this review
Since the previous update of the review was published in 2011 (Merry 2011), there have been a large number of trials of preventive interventions for depression in children and adolescents. Between publication of the original review in 2004 (Merry 2004b) and the publication of the update in 2011, the findings changed slightly from showing that targeted programmes were potentially effective in preventing depression for young people, with more mixed results from universal programmes, to supporting both targeted and universal depression prevention programmes as having the potential to prevent depression. With so many new trials published, it is possible that the results could change. It is also the case that many of the most promising approaches to depression prevention have been difficult to replicate in large‐scale pragmatic efficacy trials (e.g. Araya 2013; Stallard 2012). Governments are keen to take action to address the burden of depression on society, and its relationship to suicide attempts and completed suicide. It is critical that depression prevention programmes that are implemented are based on the evidence and represent the best possible approach of all those that have been tested to data for maximum benefit. It is timely to re‐evaluate the evidence currently available for the efficacy of depression prevention programmes as well as to explore how different therapeutic approaches may modify overall prevention effects. This version of the review therefore includes a more homogeneous group of trials by excluding trials of psychoeducational interventions, interventions delivered to those who have suffered trauma and interventions that are primarily aimed at preventing anxiety. It is important that this review, with more limited inclusion criteria, is undertaken in order to help direct governments to the most promising approach to depression prevention.
Objectives
To determine whether evidence‐based psychological interventions (including cognitive behavioural therapy (CBT), interpersonal therapy (IPT) and third wave CBT)) are effective in preventing the onset of depressive disorders in children and adolescents.
We included:
universal interventions; and
targeted interventions aimed at young people at risk of developing a depressive disorder. Within these targeted intervention trials we investigated the impact of the type of targeted approach (indicated versus selected) on the overall treatment effect of targeted interventions.
We also separately investigated the impact of the type of control group on the overall treatment effect within the targeted and universal trials.
Finally, we undertook exploratory analyses, using meta‐regression, to further investigate whether the type and intensity or other components of the intervention or baseline severity of depression impacted on the overall treatment effect.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), including cluster‐RCTs. We also considered cross‐over designs eligible for inclusion. (They will be eligible in future updates, but none were located for this update).
Types of participants
Participant characteristics
We included trials if participants were children and adolescents whose mean aged fell within the range of 5.0 to 19.9 years There were no restrictions on gender or ethnicity.
Diagnosis
We included studies if participants did not currently meet the criteria for a clinical diagnosis of depressive disorder.
There were three ways of selecting participants for trial inclusion that were eligible for this review:
trials that recruited an unselected population of participants regardless of their level of depressive symptoms (i.e. 'universal' programmes);
trials that recruited participants on the basis of a specific risk factor for depression, such as the death of a parent, parental conflict or a family history of depression (i.e. 'selected' programmes);
trials in which participants had elevated levels of depressive symptoms according to scores on standardised, validated scales of depression (i.e. 'indicated' programmes).
We included studies that included participants with a history of a depression if the intervention was aimed at the prevention of depression in a non‐clinical setting, and where the participants were not being currently treated for depression. Although this is not a purist definition of prevention, in fact the majority of included trials did not rigorously assess whether or not participants had a history of depressive disorder while some of the best designed included trials that we identified did do this. It was illogical to exclude those trials that did assess whether or not participants had a history of depressive disorder, given that the participants in the other trials were likely to have also included young people with past episodes of depressive disorder that had been unrecognised and untreated.
We excluded studies if they lacked a clear definition of participants, included children and adolescents who met the Diagnostic and Statistical Manual of Mental Disorders (DSM) IV (DSM‐IV‐TR) (American Psychiatric Association 2000) or International Classification of Diseases(ICD‐10) (World Health Organization 2007) criteria for depressive disorder, were clearly designed to be treatment trials or where there was no adequate assessment of participants.
Co‐morbidities
We excluded trials in which participants were recruited primarily with respect to other psychological problems (e.g. post‐traumatic stress disorder, anxiety, substance use, insomnia) or physical illnesses (e.g. diabetes or HIV) but where depression was a potential comorbid issue and was therefore measured as a secondary outcome measure.
Setting
We included trials regardless of the setting within which the intervention took place (e.g. school, primary care setting).
Types of interventions
Experimental intervention
As recommended in the Institute of Medicine Report (Mrazek 1994; O'Connell 2009), we classified prevention as those interventions that occurred prior to the onset of a clinically diagnosable disorder and this could include interventions for individuals with elevated symptoms of disorder but who did not currently meet the criteria for a clinical disorder.
In a previous version of this review, we included both psychological and educational interventions. However, in this version of the review we have only included evidence‐based psychological interventions. CBT and IPT have been established as efficacious in treatment and most prevention researchers have built on this in prevention. Indeed, the majority of trials included in the previous version were CBT‐based and restricting inclusion as we have done in this version ensures greater homogeneity, therefore enabling us to explore the impact of different approaches within these broad categories on the overall treatment effect. Therefore, we only included trials investigating the efficacy of CBT‐oriented (including problem‐solving interventions (PST) and third wave CBT) or IPT‐oriented (or combination) or other similar approaches in the review.
We had an inclusive approach to these included therapies so that, for example, an intervention might only include one of a range of the CBT techniques in isolation (e.g. only cognitive restructuring, or only monitoring mood in relation to activities), but was still included as being CBT‐based. Problem‐solving techniques are often included in CBT interventions, but can also be delivered in isolation, for example problem‐solving therapy (PST). Third wave therapies included mindfulness, acceptance and commitment therapy (ACT), dialectical behavioural therapy (DBT), positive psychology and any purely behavioural approaches.
The number of sessions delivered and the way interventions were delivered could vary. In most cases, interventions were delivered in groups, but given the increasing use of new media to deliver interventions these modes of delivery as well as traditional face‐to‐face individual and group‐based interventions are included. Trials had to implement the majority of an intervention primarily to the child/adolescent themselves, either in individual or group‐based sessions. We therefore excluded trials that delivered an intervention for parents with the aim of impacting on parenting practices and, subsequently, depressive symptoms in their child, without any sessions delivered to the child themselves.
We excluded interventions targeted at helping children to manage the consequences of a specific event or situation (e.g. divorce). However, we included trials in cases where the intervention was sufficiently broad and participants were taught skills that could be applied to a wide variety of problematic situations.
We excluded secondary and tertiary interventions, including relapse prevention and pharmacological interventions for depression.
Comparator intervention
The comparison groups that were eligible for inclusion in this review, in order of increasing rigorousness, were:
treatment as usual (TAU), defined as the normal healthcare curriculum, physical education classes or the ability to access any school‐based and/or external mental health care as required;
no treatment (NT);
wait‐list (WL);
attention placebo (AP), defined by Merry 2006 (p.178) as “controlling for non‐specific factors….which we would not expect to affect factors specifically implicated in the aetiology of depression.” These non‐specific factors could include participating in a trial with a prescribed curriculum and materials and having time off regular classes. Attention placebo may include psychoeducation about general mental and/or physical health, however, the programmes would not target mood specifically. We rated the ‘credibility’ of the AP (i.e. how well‐matched it is to the intervention and how well it would control for likely non‐specific factors of the intervention); and
other, which may include brief psychoeducation and/or information and support but does not include another psychological intervention.
We excluded head‐to‐head trials where CBT, IPT or third wave CBT was only compared to another type of psychological intervention as our primary aim was to examine the efficacy of these interventions in preventing depression.
Types of outcome measures
In the first update of the review, Merry 2011, we excluded general adjustment, academic/work function, social adjustment, cognitive style and suicidal ideation/attempts outcomes given the paucity of data that existed for these outcomes in the first version of the review (Merry 2004b). In this version of the review we have included clinician‐rated depression symptoms because, while the majority of trials use self‐rated measures, a good minority of trials also use a clinician‐rated measure and it is important to assess the impact on depression according to different raters. We have been able to reinstate an outcome related to our early functioning outcomes, but have only included general functioning, again due to the paucity of outcomes for more specific categories of functioning. We have also now included anxiety because of the high co‐morbidity between depression and anxiety.
Primary outcomes
Our primary outcomes were:
Prevalence of depression diagnosis at medium‐term follow‐up (i.e. between four and 12 months), measured using a recognised diagnostic system such as DSM‐IV‐TR (American Psychiatric Association 2000) or ICD‐10 (World Health Organization 2007), or tools that yield diagnoses of depressive disorder according to these systems (e.g. the Kiddie Schedule for Affective Disorders Scale (K‐SADS; Kaufman 1997)) or, where this was not available, using a predesignated cut‐off point on a continuous measure of depression symptoms likely to be correlated with the presence of a depressive disorder therefore indicating 'caseness' (e.g. the Children's Depression Inventory (CDI; Kovacs 1992).
Depression symptoms at the post‐intervention assessment point assessed using a standardised, validated self‐report measure of depression symptoms (e.g. CDI; Kovacs 1992).
Our primary outcome of depression symptoms at post‐intervention and medium‐term depression diagnosis was based on the literature that shows that subsyndromal depressive symptoms are predictive of the onset of major depressive disorder (Cuijpers 2004).
Where more than one outcome measure was used, we entered the highest quality outcome measure into the analyses. For this we used a hierarchy based on psychometric properties and appropriateness for use with children and adolescents, following the method described by Hazell 2002 (see Appendix 1).
Secondary outcomes
Our secondary outcomes were:
Depression diagnosis and depression symptoms (self rated) at other time points (see 'Timing of outcome assessment' below).
Depression symptoms (clinician‐rated)using a standardised validated measure (e.g. Children's Depression Rating Scale‐Revised; Poznanski 1996).
Anxiety symptoms at post‐intervention and follow‐up, measured using a standardised, validated measure of anxiety symptoms (e.g. the Revised Children's Manifest Anxiety Scale (RCMAS; Reynolds 1985), the Spence Anxiety Scale (SCAS; Spence 2003), the Beck Anxiety Inventory (BAI; Beck 1988), the State Anxiety Inventory for Children (SAIC; Spielberger 1970) or the Revised Child Anxiety and Depression Scale (RCADS; Chorpita 2005)).
General and social functioning at post‐intervention and follow‐up, measured using a standardised, validated measure of general or social functioning (e.g. Children's Global Assessment Scale (CGAS; Shaffer 1983), the Child and Adolescent Social and Adaptive Functioning Scale (CASAFS; Price 2002), the Paediatric Quality of Life Enjoyment and Satisfaction Questionnaire (PQ‐LES‐Q; Endicott 2006), or the Social Adjustment Scale‐Self‐Report for Youth (SAS‐SR‐Y; Weissman 1980)).
The above is not intended to represent an exhaustive list of validated psychometric scales for the assessment of anxiety or functioning. Instead, these are the scales that we, as review authors, would expect to encounter as outcome measures in this particular field of research.
Timing of outcome assessment
We analysed all outcomes at four time points:
post‐intervention;
short‐term follow‐up (up to three months);
medium‐term follow‐up (four to 12 months); and
long‐term follow‐up (over 12 months).
If there was more than one follow‐up within a specified time frame we used the data for the longest follow‐up point within that time frame, except for long‐term follow‐up, where we only used data measured up to 36 months (three years) to ensure some consistency.
Hierarchy of outcome measures
Where more than one outcome measure was used, we entered the highest quality outcome measure into the analyses. For this we used a hierarchy based on psychometric properties and appropriateness for use with children and adolescents, following the method described by Hazell 2002.
The hierarchy of measurement tools for each is as follows:
Clinician‐based assessments
Children's Depression Rating Scale (CDRS)
Hamilton Depression Rating Scale (HAM‐D)
Montgomery‐Asberg Depression Rating Scale (MADRS)
Schedule for Affective Disorders and Schizophrenia for School Aged Children (K‐SADS)
Bellevue Index of Depression (BID)
Self‐report measures:
Beck Depression Inventory (BDI)
Children's Depression Inventory (CDI)
Mood and Feeling Questionnaire (MFQ)
Reynolds Adolescent Depression Scale (RADS)
Kutcher Adolescent Depression Scale (KADS)
Depressive Adjective Checklist (DACL)
Child Depression Scale (CDS)
Centre for Epidemiologic Studies Depression Scale (CESD)
Search methods for identification of studies
The Cochrane Common Mental Disorders Group maintains a specialised register of randomized controlled trials, the CCMDCTR. This register contains over 40,000 reference records (reports of RCTs) for anxiety disorders, depression, bipolar disorder, eating disorders, self‐harm and other mental disorders within the scope of this Group. The CCMDCTR is a partially studies based register with >50% of reference records tagged to c12,500 individually PICO coded study records. Reports of trials for inclusion in the register are collated from (weekly) generic searches of Medline (1950‐), Embase (1974‐) and PsycINFO (1967‐), quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL) and review specific searches of additional databases. Reports of trials are also sourced from international trial registries, drug companies, the hand‐searching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses. Details of CCMD's core search strategies (used to identify RCTs) can be found on the Group's website with an example of the core Medline search displayed in Appendix 2.
Electronic searches
1. Cochrane Common Mental Disorders Group's Specialised Register (CCMDCTR)
The Group's Information Specialist cross‐searched the CCMDCTR‐Refs and CCMDCTR‐Studies registers (11 September 2015) using the following updated search strategy:
#1. (prevent* NEAR2 (depress* or "mental health")):ti,ab,kw,ky,emt,mh,mc #2. ((stress or trauma or disaster*) and depress* and symptom*):ti,ab #3. ((psycholog* or problem* or symptom or symptoms) NEAR1 (adjust* or adaptat* or externali* or internali*)):ti,ab,kw,ky,emt,mh,mc #4. (depression or depressive or dysthymi* or “depressed mood” or “low mood*” or “mood *regulation” or “mood disorder*” or “mental health”):ti,ab,kw,ky,emt,mh,mc #5. ((prevent* or primary or targeted or universal* or selective or selected or indicated) NEAR2 (intervention* or program*)):ti,ab,kw,ky,emt,mh,mc #6. ("early intervention*" or risk or at‐risk or vulnerab* or (health NEAR3 promot*) or "health literacy" or educat* or psychoeducat* or training or "life skill*" or *school* or classroom* or campus or internet* or online or divorce* or death or bereave* or bullied or bully*):ti,ab,kw,ky,emt,mh,mc #7. (adolesc* or preadolesc* or pre‐adolesc* or child* or boys or girls or juvenil* or minors or pre‐school or preschool or paediatric* or pediatric* or pubescen* or puberty or *school* or campus or teen* or (young next (adult* or people or patient* or men* or women* or mother* or male or female or survivor* or offender* or minorit*)) or youth* or (student* and (college or universit*)) or undergraduate* or peer or peers):ti,ab #8. ((#1 or #2) and #7) #9. (((#3 OR #4) AND (#5 OR #6)) AND #7) #10. (#8 or #9)
Key to CRS search tags: ti:title; ab:abstract; kw:keywords; emt:EMTREE headings; mc:MeSH checkwords; mh:MeSH Headings
Earlier update searches (conducted in June 2010 and July 2013) when the register was called the CCDANCTR (reflecting the Group's earlier name of the Cochrane Depression, Anxiety and Neurosis Group) can be found in Appendix 3.
2. International trial registries
We searched international trial registries via the World Health Organization's trials portal (ICTRP) and ClinicalTrials.gov to identify unpublished or ongoing studies (to 11 September 2015).
3. Additional electronic database searches
The original version of this review (Merry 2004b) also incorporated additional searches of MEDLINE (to Dec 2002), EMBASE (to January 2003), PsycINFO (to January 2003) and ERIC to (Dec 2002). The original search terms for all databases were updated (16 September 2009) (see Appendix 4; Appendix 5; Appendix 6; Appendix 7) and searches of these four databases were re‐run at this time.
All bibliographic database searches post‐2009, however, were conducted in the CCDAN/CCMD‐CTR only as this database is regularly updated with reports of relevant randomized trials from CENTRAL, MEDLINE, EMBASE and PsycINFO.
This review was first updated and re‐published in December 2011 (Merry 2011).
Searching other resources
The reference lists of articles and other reviews retrieved in the search were searched;
For this update of the search, conference abstracts from all relevant conferences in the field of depression prevention in children and adolescents were handsearched. For previous updates of this review, we specifically handsearched conference abstracts, 1994, 1996 and 1998‐2001, for the American Academy of Child and Adolescent Psychiatry;
Authors of the included trials were also consulted to find out if they knew of any published or unpublished RCTs in the area, which had not yet been identified.
Data collection and analysis
Selection of studies
At least two of the review authors independently performed the selection of trials for inclusion in this update of the review. Where a title or abstract appeared to describe a trial eligible for inclusion, we obtained the full article and two authors independently inspected it to assess its relevance to this review based on the inclusion criteria outlined in the Criteria for considering studies for this review section. A third review author resolved any discrepancies between the two authors.
Data extraction and management
Two review authors (two of either SH, GC or KW) and one research assistant (one of either AS, KL or AH) independently extracted data. A third review author (SH, GC or SM) resolved discrepancies. To ensure accurate data entry, we double‐checked the data after entry for analysis. We extracted the following details from the included trials and the information is presented in the Characteristics of included studies section:
Methods
Study design (i.e. RCT or cluster‐RCT).
If the intervention was conducted by the team who developed it.
Characteristics of the trial participants
Focus of intervention (i.e. universal or targeted).
If there was a cut‐point (and what this was; for indicated prevention trials) used on a validated depression measurement scale to include participants.
What aspect of risk (for selected prevention trials) was the basis for inclusion into a trial.
Whether a diagnostic interview was used to exclude participants with a current or previous depressive episode, and the % of participants included who had experienced a previous episode.
Baseline severity of depression.
Age and sex of participants.
Location of intervention programmes, e.g. school or community.
What psychiatric diagnoses were excluded.
Whether participants were at risk of suicide.
Whether parents with a history of schizophrenia or bipolar disorder were excluded.
Interventions used
Description of intervention including intervention type (e.g. CBT, IPT, CBT + IPT, PST, third wave), focus of CBT (i.e. included both cognitive and behavioural techniques, or was focused mainly on cognitive techniques, or was focused mainly on behavioural techniques; and the specific types of key techniques that were included), the components of the intervention (e.g. cognitive restructuring, behavioural techniques, problem‐solving, social skills training, relaxation techniques, third wave techniques, anxiety management techniques, component/s focusing on management of specific problems, whether there were parent sessions) whether it was manualised, if it was online.
The number and length of sessions (delivered), intended intensity (intended total time in hours), treatment duration, size of group (where group‐based), who delivered the intervention and assessment of fidelity.
Type of comparison condition (e.g. treatment as usual, wait‐list, attention placebo).
We coded interventions as containing cognitive restructuring if they mentioned participants identifying and learning about thinking errors/dysfunctional thoughts or the impact of negative emotional states on thoughts, and as containing behavioural techniques if they included any technique that aimed to activate people, encouragement to engage in pleasant events or activities, activity monitoring and/or scheduling, monitoring mood in relation to activities, bringing to mind pleasant activities and distraction techniques. Interventions that we coded as containing elements of problem‐solving described teaching participants ways in which to identify problems, generate potential solutions and evaluate solutions. We classed an intervention as containing social skills training if it reported teaching participants assertiveness, negotiation strategies or positive ways in which to respond in social settings that were culturally appropriate. We coded interventions as containing relaxation techniques if they mentioned employing 'relaxation' strategies or training, and we coded them as containing third wave techniques if they reported teaching mindfulness, yoga, meditation and/or distancing techniques.
Outcomes
The tool and method used to establish depression diagnosis.
The tool and method used to measure depression symptoms.
The tool and method used for anxiety symptoms.
The tool and method used for general and/or social functioning.
Assessment time points.
When aspects of methodology were unclear, or when the data were in a form unsuitable for meta‐analysis and trials appeared to meet the eligibility criteria, we sought additional information from the corresponding author. We also sought the treatment manual or, if this was not available, details of the components of the intervention that were delivered as part of the intervention from every corresponding author. We have indicated in the notes section of the Characteristics of included studies table if an author supplied additional data.
Main comparison
We planned one main comparison (i.e. any evidence‐based psychological intervention compared with any comparator). Within this we subgrouped by the type of population (i.e. universal versus targeted).
Assessment of risk of bias in included studies
For the original version of this review, two independent authors assessed methodological quality using the quality rating scale devised by Moncrieff and colleagues (Moncrieff 2006); those trials scoring 30 or more were deemed 'high' quality, those scoring 23 or more were deemed 'adequate' with sensitivity analysis undertaken on this basis.
For the current version of the review, we updated our methods to conform to the current version of the Cochrane Handbook for Systematic Review of Interventions (Higgins 2011). Specifically, we examined each trial for random sequence generation method, allocation concealment, blinding of participants and assessors, the methods of addressing incomplete outcome data and potential selective reporting. For the domain of 'other potential sources of bias' we assessed the independence of the investigators (were the investigators independent of those who developed the intervention) and implementation integrity (were sessions taped and rated, was the integrity reported and was it adequate).
Two review authors (two of either SH, JB or KW) independently performed all assessments of the risk of bias. A third author (GC) resolved any discrepancies.
A description of the assessment of risk of bias is provided in the 'Risk of bias' tables in the Characteristics of included studies section.
Measures of treatment effect
Dichotomous data
We pooled dichotomous data using the risk difference (RD) with a 95% confidence interval (CI). We have used the risk difference as we consider that this is the most relevant measure for this analysis. Our primary question is whether the onset of an episode of a depressive disorder is lower following an intervention. If an intervention is successful, the absolute number of participants with a diagnosis of depressive disorder following the intervention will be lower than those with a diagnosis of a depressive disorder in the control group. The risk difference is easy to interpret and can be converted to number needed to treat to benefit (NNTB), which is meaningful when considering whether or not depression prevention is likely to be an effective public health intervention. We made an a priori decision to include the NNTB for the primary outcome of depression diagnosis at medium‐term follow‐up as a way of interpreting the results for the reader.
Continuous data
For continuous outcomes, we pooled the means and standard deviations using the standardised mean difference with a 95% CI.
Typically SMD effect sizes of 0.20 are considered small, 0.50 are considered medium and 0.80 are considered large (Pace 2011); however, given that most SMDs reported in meta‐analyses conducted in the social sciences range between ‐0.08 and 1.08 (Lipsey 1993), and in the prevention science field effects sizes are smaller, we made the decision prior to commencing work on this version of the review to consider effect sizes of 0.20 or less as small, effect sizes that approached 0.30 as medium; and effect sizes that approached 0.50 as large.
Unit of analysis issues
Cluster‐randomised trials
For all cluster‐randomised trials we adjusted for the effects of clustering following the procedure outlined in Higgins 2008b, section 16.3.4. Where information on the interclass correlation (ICC) was not reported within the text of a trial, we contacted trial authors to request this information. Where we were unable to obtain this information from the trial authors, we used an ICC estimate of 0.0282, as this represents the average of the ICCs obtained from the other trials included in the analysis. Where a trial/s presented a range of ICC or design effect values, and where it was unclear which value applied to a given outcome or time point, we used the higher value in order to calculate the most conservative sample size.
Multi‐arm trials
Where a trial/s had more than one evidence‐based intervention arm compared with a single control group, we chose the intervention arm that was the most active. Where there was more than one eligible intervention within a class (i.e. CBT, IPT), we chose the most intense in terms of the intervention that had the most intervention components or was longer, or both.
Where a trial/s included multiple comparator arms, we extracted data from the most rigorous control group to ensure the estimated treatment effect was not inflated, following the hierarchy of comparator rigorousness outlined in the Types of interventions section.
As the previous version of this review found that the magnitude of effect for depression prevention programmes is similar between boys and girls, we have combined data by gender where a trial/s presented data separately for boys and girls. Additionally, as other work has found no evidence of moderation by ethnicity (Marchand 2010), we have also combined data where a trial/s presented data separately for members of different ethnicity. Further, our interest was in examining factors related to the type of intervention and methods that might impact on the overall treatment effects (see Subgroup analysis and investigation of heterogeneity section).
Dealing with missing data
It is common for authors to report using intention‐to‐treat (ITT) analyses to account for missing data, although it is generally the case that the data presented and extracted for the meta‐analysis were raw data. Where it is not clear whether an ITT analysis was undertaken, or where data were reported based on adjusted means (or it was unclear), we contacted trial authors to obtain the raw mean and standard deviations (SDs) based on available information. In most cases, therefore, data extracted and used within meta‐analyses are based on observed case data. Where adjusted means have been used, we undertook sensitivity analyses (see Sensitivity analysis section).
Where data on the primary outcomes reported in this review were missing, we requested these from the trial authors by letter or email, or both. We have noted in the Characteristics of included studies section whether these data were supplied. In some cases we sought secondary outcome data where available.
Where SDs for continuous outcomes were not reported and we as review authors were unable to obtain the information from corresponding authors, we imputed a pooled SD using the method outlined in Townsend 2001.
Assessment of heterogeneity
We assessed heterogeneity visually by inspecting the forest plots and identifying those trials with 95% CIs outside the general pattern of the others and, more formally, by checking the results of the I2 statistic. We took into account: (i) the magnitude and direction of effects, and (ii) the strength of evidence for heterogeneity (e.g. the width of the 95% CI for the I2).
We used the following guide as recommended in the Cochrane Handbook for Systematic Reviews of Interventions to the interpretation of the I2 statistic (Higgins 2008a; Higgins 2008b):
0% to 40%: heterogeneity might not be important;
30% to 60%: may represent moderate heterogeneity*;
50% to 90%: may represent substantial heterogeneity*;
75% to 100%: considerable heterogeneity.
Should any meta‐analysis be associated with substantial or considerable heterogeneity (i.e. I2 ≥ 75%), we triple‐checked the data to ensure these had been entered correctly.
Assessment of reporting biases
We assessed trial reports, and protocols where available, to assess whether trial authors reported all prespecified outcome(s).
We assessed publication bias by inspecting funnel plots for the primary outcomes of the review where there were more than 10 trials included in the analysis as recommended.
Data synthesis
We carried out statistical analyses in accordance with the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions(Higgins 2008a; Higgins 2008b). We used the random‐effects model with 95% CIs to pool data. Specifically, for dichotomous outcomes we used the Mantel‐Haenszel method whilst, for continuous outcomes, we used the inverted variance method.
Subgroup analysis and investigation of heterogeneity
Subgroup analyses
We analysed trials separately based on one main prespecified subgroup: universal versus targeted interventions.
For the primary outcome measures of self‐rated depression scores at post‐intervention and depression diagnosis at medium‐term follow‐up, we also undertook an additional subgroup analysis to investigate whether the type of control group modified the pooled effect size. For the group of trials that we classified as targeted interventions, we undertook a further subgroup analysis for selected versus indicated versus combined approaches.
Meta‐regression analyses
For the primary outcome measures of depression diagnosis at the medium‐term assessment and self‐reported depression scores at the post‐intervention assessment, we undertook a series of random‐effects univariate meta‐regression models to examine whether the type and aspects of the intervention approach and population characteristics (severity of baseline depression) modified the pooled effect size of universal and targeted interventions separately as follows:
baseline depression severity (subthreshold; mild; moderate; severe);
intended intervention intensity (number of hours);
type of therapist (mental health expert; non‐mental health expert; student);
broad intervention focus (CBT; IPT; CBT plus IPT; third wave)
CBT focus (CBT with an equal emphasis on cognitive and behavioural components; CBT with a cognitive focus; CBT with a behavioural focus);
inclusion of a relaxation component (for CBT interventions only);
inclusion of a problem‐solving skills component (for CBT interventions only);
inclusion of a social skills training component (for CBT interventions only);
method of delivery (face‐to‐face (group and individual) versus online/telephone).
We performed the random‐effects meta‐regression analyses in Comprehensive Meta‐Analysis for Windows, version 3.2.1 (Biostat 2014).
Sensitivity analysis
For the primary outcomes reported in this review, we also checked the robustness of the results by conducting the following sensitivity analyses:
use of adjusted, rather than raw, mean scores (for outcomes measured on a continuous scale only);
adequacy of allocation concealment (high and unclear risk versus low risk);
inclusion of participants with a previous depression;
use of a cut‐point to establish depressive disorder.
'Summary of findings' tables
We prepared 'Summary of findings' tables for the primary outcome measures of depression diagnosis at the medium‐term follow‐up and self‐rated depression scores at the post‐intervention assessment following the recommendations outlined in the Cochrane Handbook for Systematic Reviews of Interventions, section 11.5 (Higgins 2008b), Guyatt 2013a (for dichotomous outcomes) and Guyatt 2013b (for continuous outcomes). For both outcomes we based our estimates of risk on a range of control group rates at medium‐term follow‐up. To do this we extracted the proportion diagnosed for each included trial and rank ordered them from the lowest to the highest proportion, then took the lowest, the median and the highest proportion to determine the risk categories (lowest, median, highest). We prepared 'Summary of findings' tables using GRADE profiler for Windows, version 3.6.1 (GRADE profiler).
Two review authors (GC and KW) independently appraised the quality of evidence following the recommendations in section 12.2 of the Cochrane Handbook for Systematic Reviews of Interventions(Higgins 2008b).
Timeline
We will carry out a new search for RCTs and update the review when it is likely that new trials have been published that may change the conclusions of the review.
Results
Description of studies
For a full description of each trial, see the Characteristics of included studies section.
Results of the search
2011 version of this review
Sixty‐eight trials (from 66 publications) were included in the 2011 version of this review. For the 2015 update, we combined the data referenced in the 2011 update as Cardemil 2002a and Cardemil 2002b (now Cardemil 2002) and Clarke 1993a and 1993 (now Clarke 1993). We received further information with regards to the randomisation procedure used in two previously excluded trials (Jaycox 1994; Kowalenko 2005), and one trial previously classified as awaiting assessment (Gallegos 2008). In all cases the authors confirmed that they did randomise schools or individuals (or both) to intervention and control group. As a result, these three trials have now been included in the current review.
As the inclusion criteria for this update were changed to ensure a more homogeneous group of trials specifically targeting the prevention of depression, we have now excluded 26 previously included trials. Please see the Excluded studies section for more details on this.
In total, we included 43 independent trials from the 2011 version of the review in this update.
Results of the search for the 2015 update of the review
In total there were 1855 articles retrieved from the updated searches (from June 2010 to September 2015), with 1825 remaining after de‐duplication. We obtained six further articles either by correspondence with trial authors or from handsearching key references. Two review authors (SH, GC) read the titles and abstracts of all those articles retrieved and a third author (SM) resolved discrepancies. In total, we excluded 1486 articles on this basis with 343 retained for inspection of the full article text for eligibility. We excluded a total of 216 studies; only studies that one would expect to be included in the review but did not quite meet the inclusion criteria have been included in the Excluded studies section. A total of 40 trials were eligible for inclusion from this search in the update of this review.
In total, we included 83 independent trials in this update of the review (Figure 1). However, when describing the characteristics of these trials in the Description of studies section below, the total numbers often exceed 83 as some trials contained multiple intervention arms (Horowitz a2007; Horowitz b2007; Sheffield a2006; Sheffield b2006; Sheffield c2006).
1.
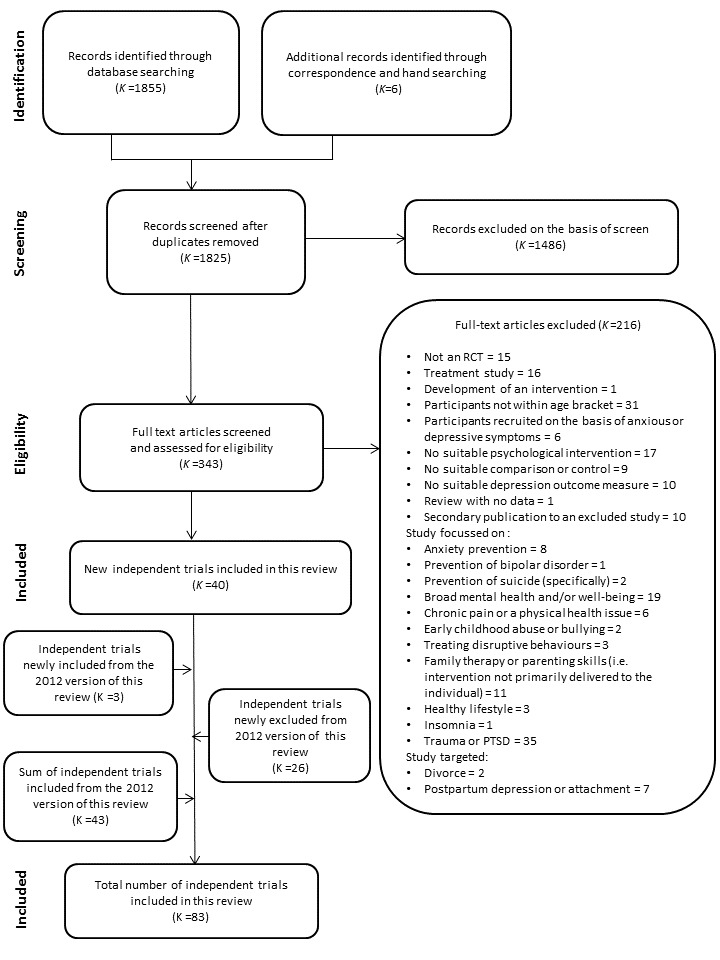
PRISMA diagram
Included studies
Eighty‐three trials were eligible for inclusion. We obtained data suitable for pooling for meta‐analysis from 76 trials for at least one of the included primary or secondary outcomes either from the published paper or via correspondence with trial authors. For the primary outcomes, we obtained data suitable for pooling for self‐reported depression scores at the post‐intervention assessment from 70 independent trials (73 independent trial arms) and, for diagnosis of depressive disorder at the medium‐term assessment, we obtained data suitable for pooling from 32 trials.
Seven trials did not provide data suitable for meta‐analysis (Karami 2012; Khalsa 2012; Lillevoll 2014; Noël 2013; Petersen 1997; Stoppelbein 2003; Wong 2014). For Karami 2012, Lillevoll 2014 and Petersen 1997, only summary statistics were presented, rather than raw data. For Khalsa 2012, change scores were reported with no information on baseline scores for the depression measure provided. In the case of Noël 2013, although the authors provided information on the number of treatment dropouts for the overall sample, the number of participants remaining in the intervention and control groups at the post‐intervention assessment was unclear. For Stoppelbein 2003, data were only reported for the total sample and subsamples, rather than for the intervention and control groups specifically. For Wong 2014, 72.8% of data were missing at post‐intervention, due to either attrition or data corruption. Given this, we decided not to include data from the trial in any analysis. The implications for this decision are that there are missing data and it is unclear what the impact on the results of the review would be if we had these data and could include them in the meta‐analyses.
The inclusion of two trials from the original review, Hyun 2005 and Schmiege 2006, involved substantial discussion between the two authors that screened the trials (GC and SH) and a third co‐author (SM). While the aim of these trials was not depression prevention per se, they were clearly targeting factors closely associated with onset of depression in groups who are at high risk of depression (in the case of Schmiege 2006 this was preventing grief with the aim of preventing depression, and in the case of Hyun 2005, participants were youths residing in a shelter), and depression was one of the key outcomes in each of these trials. Significant discussion also occurred with regards to the inclusion of Kauer 2012. This intervention involved daily monitoring of activity and mood, however it was unclear whether this information was fed back to young people themselves, in addition to GPs, and therefore whether it constituted a behavioural intervention. We felt that the act of monitoring one's actions in and of itself was sufficient to be classed as a behavioural intervention, particularly given evidence of the efficacy of this self‐management behaviour for preventing relapse of depression in adults (Ekers 2014; Ludman 2003).
There were a number of trials with multiple eligible intervention arms. For Cardemil 2002, Clarke 1993, Gillham 2007, Pössel 2008, and Schmiege 2006 we combined data across gender or ethnicity or schools as we considered that there was no strong a priori reason to suspect the efficacy of these interventions would be moderated by these factors, nor were we investigating these potential modifiers in this particular update. For a further three (Gilham 1994‐Study 2; Lillevoll 2014; Pattison 2001), although trial authors tested different components or approaches to the same intervention, we also combined data across these arms as the evaluation of the efficacy of treatment components is beyond the scope of this review.
Four trials reported data for more than one eligible arm within an intervention class (i.e. CBT, IPT) (Rohde 2014a; Rohde 2014b; Rose 2014; Sethi 2010). For Sethi 2010, we included data from the combined face‐to‐face and online therapy arm, for Rohde 2014a and Rohde 2014b, we included data from the CBT rather than bibliotherapy arms, and for Rose 2014 we included data from the combined RAP and interpersonal therapy arm.
Design
Most trials (k = 52) employed a simple randomisation procedure whereby individual participants were randomly allocated to the intervention or control groups (Arnarson 2009; Cardemil 2002; Castellanos 2006; Chaplin 2006; Charbonneau 2012; Clarke 1995; Clarke 2001; Cova 2011‐Targeted; Dobson 2010; Ellis 2011; Fleming 2012; Fresco 2009; Garber 2009; Garcia 2011; Gillham, Hamilton 2006a; Gillham 2007; Gillham 2012; Horowitz a2007; Horowitz b2007; Hyun 2005; Karami 2012; Kauer 2012; Liehr 2010; Lillevoll 2014; Makarushka 2012; Manicavasagar 2014; McCarty 2011; McCarty 2013; McLaughlin 2011; Merry 2004; Mirzamani 2012; Noël 2013; Pattison 2001; Petersen 1997; Puskar 2003; Quayle 2001; Rohde 2014a; Rohde 2014b; Seligman 1999; Seligman 2007; Sethi 2010; Shatte 1997; Snyder 2010; Stice 2006; Stice 2008; Whittaker 2012; Wijnhoven 2014; Woods 2011; Young 2006; Young 2010a; Yu 2002‐study 3). In one further trial we only used data from the Australian female sample as neither data for males nor for the Swedish sub‐sample were randomised (Livheim 2014‐study 1(girls).
The remaining 34 trials employed cluster‐randomisation whereby schools, classes or families rather than the individual were the unit of randomisation (Araya 2013; Bella‐Awusah 2015; Calear 2009; Clarke 1993; Compas 2009; Cowell 2009; Gallegos 2008; Gilham 1994‐Study 2; Gillham, Reivich 2006b; Jaycox 1994; Khalsa 2012; Kindt 2014; Kowalenko 2005; Mendelson 2010; O'Leary‐Barrett 2013; Pössel 2004; Pössel 2008; Pössel 2013; Reynolds 2011; Rivet‐Duval 2010; Roberts 2003; Roberts 2010; Rooney 2006; Rooney 2013; Rose 2014; Sawyer 2010; Schmiege 2006; Sheffield a2006; Sheffield b2006; Sheffield c2006; Stallard 2012a; Spence 2003; Stoppelbein 2003; Wong 2014).
Sample size
Sample size varied from 18 participants (Liehr 2010) to 5634 participants (Sawyer 2010).
For two trials the number of participants included in analyses was unclear (Compas 2009; Mendelson 2010). For the first trial, we calculated the sample size on the basis of the percentage of families retained by the post‐intervention assessment. For the 12‐month (medium‐term) assessment, we have used the same sample size as reported for the depression diagnosis outcome. For the second trial, as the number of participants with information on all post‐intervention outcomes was presented as a range of values (i.e. 42 to 47 in the intervention group and 40 to 43 in the control group), we have used the middle value (i.e. 45 and 42) as the sample size.
Setting
Of the trials included in this review, the majority (k = 42) had been conducted in the United States of America (Cardemil 2002; Chaplin 2006; Charbonneau 2012; Clarke 1993; Clarke 1995; Clarke 2001; Compas 2009; Cowell 2009; Fresco 2009; Garber 2009; Gilham 1994‐Study 2; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Gillham 2007; Gillham 2012; Horowitz a2007; Horowitz b2007; Jaycox 1994; Khalsa 2012; Liehr 2010; Makarushka 2012; McCarty 2011; McCarty 2013; McLaughlin 2011; Mendelson 2010; Noël 2013; Petersen 1997; Puskar 2003; Pössel 2013; Reynolds 2011; Rohde 2014a; Rohde 2014b; Schmiege 2006; Seligman 1999; Seligman 2007; Shatte 1997; Snyder 2010; Stice 2006; Stice 2008; Stoppelbein 2003; Young 2006; Young 2010a), followed by Australia (Calear 2009; Ellis 2011; Kauer 2012; Kowalenko 2005; Livheim 2014‐study 1(girls); Manicavasagar 2014; Pattison 2001; Quayle 2001; Roberts 2003; Roberts 2010; Rooney 2006; Rooney 2013; Rose 2014; Sawyer 2010; Sethi 2010; Sheffield a2006; Sheffield b2006; Sheffield c2006; Wong 2014), New Zealand (Fleming 2012; Merry 2004; Whittaker 2012; Woods 2011), the United Kingdom (Castellanos 2006; O'Leary‐Barrett 2013; Spence 2003; Stallard 2012a), Chile (Araya 2013; Cova 2011‐Targeted), Germany (Pössel 2004; Pössel 2008), the Islamic Republic of Iran (Karami 2012; Mirzamani 2012), Mexico (Gallegos 2008; Garcia 2011), The Netherlands (Kindt 2014; Wijnhoven 2014), and one each from Canada (Dobson 2010), China (Yu 2002‐study 3), Iceland (Arnarson 2009), Mauritius (Rivet‐Duval 2010), Nigeria (Bella‐Awusah 2015), Norway (Lillevoll 2014), and South Korea (Hyun 2005).
The majority of these trials (k = 67) were conducted in school settings (Araya 2013; Arnarson 2009; Bella‐Awusah 2015; Calear 2009; Cardemil 2002; Castellanos 2006; Chaplin 2006; Clarke 1993; Clarke 1995; Cova 2011‐Targeted; Cowell 2009; Dobson 2010; Fleming 2012; Gallegos 2008; Garcia 2011; Gilham 1994‐Study 2; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Gillham 2007; Gillham 2012; Horowitz a2007; Horowitz b2007; Karami 2012; Khalsa 2012; Kindt 2014; Kowalenko 2005; Lillevoll 2014; McCarty 2011; McCarty 2013; McLaughlin 2011; Mendelson 2010; Merry 2004; Mirzamani 2012; Noël 2013; O'Leary‐Barrett 2013; Pattison 2001; Petersen 1997; Pössel 2004; Pössel 2008; Pössel 2013; Puskar 2003; Quayle 2001; Rivet‐Duval 2010; Roberts 2003; Roberts 2010; Rohde 2014a; Rohde 2014b; Rooney 2006; Rooney 2013; Rose 2014; Sawyer 2010; Shatte 1997; Sheffield a2006; Sheffield b2006; Sheffield c2006; Snyder 2010; Spence 2003; Stallard 2012a; Stice 2006; Stice 2008; Stoppelbein 2003; Whittaker 2012; Wijnhoven 2014; Wong 2014; Woods 2011; Young 2006; Young 2010a; Yu 2002‐study 3). Eight were conducted in college or university settings (Charbonneau 2012; Ellis 2011; Fresco 2009; Reynolds 2011; Rohde 2014b; Seligman 1999; Seligman 2007; Sethi 2010), four were conducted in clinical settings (Clarke 2001; Compas 2009; Gillham, Hamilton 2006a; Kauer 2012), three were conducted in community settings (Hyun 2005; Liehr 2010; Schmiege 2006), and the remaining four trials were conducted in mixed settings (Garber 2009; Livheim 2014‐study 1(girls); Makarushka 2012; Manicavasagar 2014).
Participants
The age of participants at intake ranged from 8.0 years through to 24.0 years.
Twenty‐nine trials investigated the efficacy of a universal depression prevention programme delivered to unselected populations (Araya 2013; Calear 2009; Cardemil 2002; Chaplin 2006; Clarke 1993; Gallegos 2008; Horowitz a2007; Horowitz b2007; Khalsa 2012; Lillevoll 2014; Manicavasagar 2014; Merry 2004; Pattison 2001; Pössel 2004; Pössel 2008; Pössel 2013; Quayle 2001; Reynolds 2011; Rivet‐Duval 2010; Roberts 2010; Rooney 2006; Rooney 2013; Rose 2014; Sawyer 2010; Shatte 1997; Snyder 2010; Spence 2003; Whittaker 2012; Wong 2014). We coded a further four trials as universal interventions based on the trial authors' original description (Garcia 2011; Gillham 2007; Liehr 2010; Shatte 1997).
A total of 53 trials investigated the efficacy of targeted depression prevention programmes (Arnarson 2009; Bella‐Awusah 2015; Castellanos 2006; Charbonneau 2012; Clarke 1995; Clarke 2001; Compas 2009; Cowell 2009; Dobson 2010; Ellis 2011; Fleming 2012; Fresco 2009; Garber 2009; Hyun 2005; Karami 2012; Kauer 2012; Kindt 2014; Kowalenko 2005; Livheim 2014‐study 1(girls); Makarushka 2012; McCarty 2011; McCarty 2013; McLaughlin 2011; Mendelson 2010; Mirzamani 2012; Noël 2013; O'Leary‐Barrett 2013; Puskar 2003; Rohde 2014a; Rohde 2014b; Schmiege 2006; Seligman 1999; Seligman 2007; Sethi 2010; Stallard 2012a; Stice 2006; Stice 2008; Stoppelbein 2003; Wijnhoven 2014; Woods 2011; Young 2006; Young 2010a; Yu 2002‐study 3). For one additional trial (Cova 2011‐Targeted), although both a targeted and universal programme was evaluated, allocation to the universal programme was not randomised. We have therefore only presented data for the targeted intervention arm in this review. We coded seven further trials as a targeted based on the trial authors' original description of the intervention despite the fact that although the programmes originally aimed to recruit only children with high depression scores, in the case of there being small classes all children were included regardless of their current levels of depression symptoms (Gilham 1994‐Study 2; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Gillham 2012; Jaycox 1994; Petersen 1997; Roberts 2003).
For the majority of these trials the population was selected on the basis of elevated depression symptoms and we therefore classed them as indicated programmes; in some the population was selected on the basis of a risk factor for depression, and in some they were selected on the basis of both elevated symptoms and some risk factor as described below. Risk was defined on the basis of:
elevated depression symptoms (k = 36: Arnarson 2009; Bella‐Awusah 2015; Clarke 1995; Charbonneau 2012; Cova 2011‐Targeted; Dobson 2010; Ellis 2011; Gilham 1994‐Study 2; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Gillham 2012; Kauer 2012; Kowalenko 2005; Livheim 2014‐study 1(girls); Makarushka 2012; McCarty 2011; McCarty 2013; McLaughlin 2011; Mirzamani 2012; Petersen 1997; Puskar 2003; Roberts 2003; Rohde 2014a; Rohde 2014b; Seligman 2007; Sethi 2010; Sheffield a2006; Sheffield b2006; Stallard 2012a; Stice 2006; Stice 2008; Stoppelbein 2003; Wijnhoven 2014; Woods 2011; Young 2006; Young 2010a);
elevated depressive symptoms and poor family relationships or perceived family conflict (k = 2: Jaycox 1994; Yu 2002‐study 3);
elevated depression symptoms and a parent with a history of depression (k = 2: Clarke 2001; Garber 2009);
elevated depression symptoms and living in a rural area (k = 1: Noël 2013);
elevated personality symptoms (e.g. negative thinking, hopelessness) (k = 2: Castellanos 2006; O'Leary‐Barrett 2013);
a pessimistic attributional style (k = 2: Seligman 1999; Fresco 2009);
a parent with current depression (k = 1: Compas 2009);
recent (≤ 2 years) parental bereavement (k = 1: Schmiege 2006);
recent (duration unclear) parental divorce (k = 1: Karami 2012);
being the child of a Mexican immigrant woman (k = 1: Cowell 2009);
excluded or at risk of being excluded from mainstream education (k = 1: Fleming 2012);
residing in a shelter for homeless and runaway youth (k = 1: Hyun 2005);
residing in a disadvantaged/underserved urban neighbourhood (k = 1: Mendelson 2010);
attending a school located in a low‐income neighbourhood (k = 1: Kindt 2014).
In one further trial, one arm of the prevention programme was implemented in a targeted population (Sheffield a2006), one arm was implemented in a mixed population (Sheffield b2006), and one arm was delivered to an unselected population (Sheffield c2006).
Most trials (k = 46) included participants with subthreshold symptoms of depression at baseline (Calear 2009; Cardemil 2002; Chaplin 2006; Cowell 2009; Fresco 2009; Gallegos 2008; Gilham 1994‐Study 2; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Gillham 2007; Gillham 2012; Horowitz a2007; Horowitz b2007; Jaycox 1994; Kindt 2014; Liehr 2010; Lillevoll 2014; Livheim 2014‐study 1(girls); Manicavasagar 2014; McCarty 2011; McCarty 2013; Merry 2004; Noël 2013; Pattison 2001; Pössel 2004; Pössel 2008; Quayle 2001; Reynolds 2011; Rivet‐Duval 2010; Roberts 2003; Roberts 2010; Rohde 2014a; Rohde 2014b; Rooney 2013; Rose 2014; Sawyer 2010; Schmiege 2006; Seligman 1999; Seligman 2007; Sheffield c2006; Snyder 2010; Spence 2003; Stallard 2012a; Stoppelbein 2003; Whittaker 2012; Young 2010a). While still designed as prevention trials, in 14 trials participants were experiencing mild depressive symptoms at baseline (Araya 2013; Charbonneau 2012; Clarke 1993; Clarke 1995; Clarke 2001; Cova 2011‐Targeted; Garber 2009; Garcia 2011; Hyun 2005; McLaughlin 2011; Puskar 2003; Rooney 2006; Shatte 1997; Young 2006). Three trials included participants with mild‐to‐moderate symptoms of depression at baseline (Arnarson 2009; Stice 2006; Stice 2008), 10 included participants with moderate symptoms at baseline (Bella‐Awusah 2015; Compas 2009; Dobson 2010; Ellis 2011; Fleming 2012; Kauer 2012; Makarushka 2012; Sethi 2010; Woods 2011; Yu 2002‐study 3), and four included participants with severe symptoms of depression at baseline (Kowalenko 2005; Sheffield a2006; Sheffield b2006; Wijnhoven 2014). For the remaining nine trials, severity of depression symptoms at baseline could not be ascertained either because scores at baseline were not provided (Karami 2012; Khalsa 2012; Mendelson 2010; Petersen 1997; Pössel 2013), or because there are no established cut‐offs for the depression measure used (Castellanos 2006; Mirzamani 2012; O'Leary‐Barrett 2013; Wong 2014).
In five trials, participants were reported as having had either depression (Compas 2009; Garber 2009; Seligman 1999), or a mental health disorder (Garcia 2011; Roberts 2010), at some point in their lifetime; however, the majority of trials did not report on how many participants had previously suffered from depression. In the trials by Garcia 2011 and Roberts 2010, 5.1% and between 7% and 9% of participants were reported to have suffered from a mental health condition at some point in their lives. Compas 2009 reported that 13% of participants in the intervention condition and 23% of participants in the control condition had previously been diagnosed with depression, for Roberts 2010, between 7% and 9% of participants had previously been diagnosed with a depressive disorder, and in the trial by Garber 2009 55.3% of the of participants in the intervention condition and 55.41% of participants in the control condition reported a previous episode.
Interventions
See Table 3 for a description of what intervention components were included in each intervention tested in the trials included in this review. For one trial, due to time and resource limitations, we were unable to arrange for the treatment manual to be translated. We have therefore not categorised the components of this treatment in this review (Mirzamani 2012).
1. Classification of intervention components.
| Study |
Cognitive restructuring (Y/N) |
Behavioural techniques (Y/N) |
Problem‐solving (Y/N) |
Social skills training (Y/N) |
Relaxation techniques (Y/N) |
Third wave techniques (Y/N) |
Anxiety management techniques (Y/N) |
Component/s focusing on management of specific problems (Y/N) |
Parental component/s (Y/N) |
Predominant therapeutic focus |
| Araya 2013 | Y | N | Y | N | N | N | N | N | Y | CBT (cognitive) |
| Arnarson 2009 | Y | Y | Y | Y | Y | N | N | N | N | CBT plus IPT |
| Bella‐Awusah 2015 | Y | Y | N | N | Y | N | N | N | N | CBT (behavioural) |
| Calear 2009 | Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) |
| Cardemil 2002 | Y | N | Y | Y | N | N | N | N | N | CBT (cognitive) |
| Castellanos 2006 | Y | N | N | N | N | N | N | N | N | CBT (cognitive) |
| Chaplin 2006 | Y | N | Y | Y | Y | N | N | Y1 | N | CBT (cognitive) |
| Charbonneau 2012 | N | N | N | N | Y | Y | N | N | N | Third wave |
| Clarke 1993 | N | Y | N | N | N | N | N | N | N | Behaviour therapy (third wave) |
| Clarke 1995 | Y | N | N | N | N | N | N | N | N | CBT (cognitive) |
| Clarke 2001 | Y | N | N | N | N | N | N | Y2 | Y | CBT (cognitive) |
| Compas 2009 | Y | Y | N | N | N | Y | N | Y2 | Y | CBT (cognitive and behavioural) |
| Cova 2011‐Targeted | Y | N | Y | Y | Y | Y | Y | Y3 | N | CBT (cognitive) |
| Cowell 2009 | N | N | Y | Y | N | N | N | Y4 | Y | |
| Dobson 2010 | Y | N | N | N | N | N | N | N | N | CBT (cognitive) |
| Ellis 2011 | Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) |
| Fleming 2012 | Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) |
| Fresco 2009 | Y | N | N | N | N | N | N | N | N | CBT (cognitive) |
| Gallegos 2008 | Y | N | Y | N | Y | N | Y | N | Y | CBT (cognitive) |
| Garber 2009 | Y | N | N | N | N | N | N | N | Y | CBT (cognitive) |
| Garcia 2011 | Y | N | Y | Y | Y | Y | Y | Unclear | Unclear | Third wave |
| Gilham 1994‐Study 2 | Y | N | Y | Y | Y | N | N | Y | N5 | CBT (cognitive) |
| Gillham, Hamilton 2006a | Y | N | Y | Y | Y | N | N | Unclear | N | CBT (cognitive) |
| Gillham, Reivich 2006b | Y | N | Y | Y | Y | N | N | Unclear | Y | CBT (cognitive) |
| Gillham 2007 | Y | N | Y | Y | Y | N | N | Unclear | N | CBT (cognitive) |
| Gillham 2012 | Y | N | N | Y | Y | N | N | Y | N | CBT (cognitive) |
| Horowitz a2007 | Y | N | N | N | N | N | N | N | N | CBT (cognitive) |
| Horowitz b2007 | N | N | N | Y | N | N | N | N | N | IPT |
| Hyun 2005 | Y | Y | N | N | Y | N | N | Y6 | N | CBT (cognitive and behavioural) |
| Jaycox 1994 | Y | N | Y | Y | Y | N | N | Y7 | N | CBT (cognitive) |
| Karami 2012 | Y | N | Y | Y | Y | N | N | Y8 | N | CBT (cognitive) |
| Kauer 2012 | N | Y | N | N | N | N | N | N | N | Behaviour therapy (third wave) |
| Khalsa 2012 | N | N | N | N | Y | Y | N | N | N | Third wave |
| Kindt 2014 | Y | N | N | Y | Y | N | N | N | N | CBT (cognitive) |
| Kowalenko 2005 | Y | Y | Y | Y | N | N | N | N | N | CBT (cognitive and behavioural) |
| Liehr 2010 | N | N | N | N | N | Y | N | N | N | Third wave |
| Lillevoll 2014 | Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) |
| Livheim 2014‐study 1(girls) | N | N | N | N | N | Y | N | N | N | Third wave |
| Makarushka 2012 | Y | Y | N | N | N | N | N | N | N | CBT (cognitive and behavioural) |
| Manicavasagar 2014 | Unclear | Unclear | N | N | Y | Y | N | N | N | Third wave |
| McCarty 2011 | Y | Y | Y | Y | Y | N | N | N | Y | CBT (cognitive and behavioural) |
| McCarty 2013 | Y | Y | Y | Y | Y | N | N | N | Y | CBT (cognitive and behavioural) |
| McLaughlin 2011 | Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) |
| Mendelson 2010 | N | N | N | N | N | Y | N | N | N | Third wave |
| Merry 2004 | Y | Y | Y | Y | Y | N | Y | N | N | CBT plus IPT |
| Mirzamani 2012 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Noël 2013 | Y | Y | Y | Y | N | N | N | Y9 | N | CBT (cognitive and behavioural) |
| O'Leary‐Barrett 2013 | Y | N | N | N | N | N | N | N | N | CBT (cognitive) |
| Pattison 2001 | Y | N | Y | Y | Y | N | N | Unclear | N | CBT (cognitive) |
| Petersen 1997 | Y | N | Y | Y | Y | N | N | N | N | Problem‐solving |
| Pössel 2004 | Y | N | N | Y | N | N | N | N | N | CBT (cognitive) |
| Pössel 2008 | Y | Y | N | Y | N | N | N | N | N | CBT (cognitive and behavioural) |
| Pössel 2013 | Y | Y | N | Y | N | N | N | N | N | CBT (cognitive and behavioural) |
| Puskar 2003 | Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) |
| Quayle 2001 | Y | N | Y | Y | N | N | N | Y1 | N | CBT (cognitive) |
| Reynolds 2011 | N | Y | N | N | N | N | N | N | N | Behaviour therapy (third wave) |
| Rivet‐Duval 2010 | Y | Y | Y | Y | Y | N | Y | N | N | CBT plus IPT |
| Roberts 2003 | Y | N | Y | Y | N | N | N | Y1 | N | CBT (cognitive) |
| Roberts 2010 | Y | Y | Y | Y | N | N | Unclear | Unclear | N | CBT (cognitive) |
| Rohde 2014a | Y | Y | N | N | N | N | N | N | N | CBT (cognitive and behavioural) |
| Rohde 2014b | Y | Y | N | N | N | N | N | N | N | CBT (cognitive and behavioural) |
| Rooney 2006 | Y | N | N | N | Y | N | N | N | N | CBT (cognitive) |
| Rooney 2013 | Y | Y | N | N | Y | N | Y | N | N | CBT (cognitive and behavioural) |
| Rose 2014 | Y | Y | Y | Y | N | N | Unclear | N | N | CBT plus IPT |
| Sawyer 2010 | Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) |
| Schmiege 2006 | Y | Y | Y | Y | N | N | N | Y10 | Y | CBT (cognitive) |
| Seligman 1999 | Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) |
| Seligman 2007 | Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) |
| Sethi 2010 | Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) |
| Shatte 1997 | Y | N | Y | Y | Y | N | N | Y6 | N | CBT (cognitive) |
| Sheffield a2006 | Y | Y | Y | Y | N | N | N | N | N | CBT (cognitive and behavioural) |
| Sheffield b2006 | Y | Y | Y | Y | N | N | N | N | N | CBT (cognitive and behavioural) |
| Sheffield c2006 | Y | N | Y | N | N | N | N | N | N | CBT (cognitive) |
| Snyder 2010 | N | N | N | N | N | Y | N | N | N | Third wave |
| Spence 2003 | Y | N | Y | N | N | N | N | N | N | CBT (cognitive and behavioural) |
| Stallard 2012a | Y | Y | Y | Y | Y | N | Y | N | N | CBT plus IPT |
| Stice 2006 | Y | Y | N | N | N | N | N | N | N | CBT (cognitive and behavioural) |
| Stice 2008 | Y | Y | N | N | N | N | Y | N | N | CBT (cognitive and behavioural) |
| Stoppelbein 2003 | Y | Y | N | N | Y | N | N | N | N | CBT (cognitive and behavioural) |
| Whittaker 2012 | Y | Y | Y | N | Y | N | N | N | N | CBT (cognitive and behavioural) |
| Wijnhoven 2014 | Y | N | N | N | N | N | N | N | N | CBT (cognitive) |
| Wong 2014 | Y | Y | Y | Y | Y | N | N | N | N | CBT (cognitive and behavioural) |
| Woods 2011 | Y | Y | Y | Y | N | N | N | N | N | CBT (cognitive and behavioural) |
| Young 2006 | N | N | N | Y | N | N | N | N | N | IPT |
| Young 2010a | N | N | N | Y | N | N | N | N | N | IPT |
| Yu 2002‐study 3 | Y | N | Y | Y | Y | N | N | Y1 | N | CBT (cognitive) |
1Penn Resiliency programmes place some emphasis on resolution of family conflict.
2Addresses beliefs related to or coping with a parent diagnosed with depression, or both.
3Addresses resolving conflict with family and friends.
4 Addresses being an immigrant
5Although for some participants there was a parental component, this was not controlled. Instead only the feasibility of offering parental sessions was evaluated.
6Addresses factors involved in the participants' decision to run away from home.
7Addresses coping with parental conflict.
8Addresses coping with parental divorce.
9Addresses coping with rural living.
10Addresses coping with grief after the death of a parent.
The majority of prevention programmes were CBT‐based (k = 65). However, within this broad class of intervention there was significant variation in terms of the emphasis of the components delivered. We considered 32 of these CBT‐based interventions to have an equal emphasis on both the cognitive and behavioural components (Calear 2009; Compas 2009; Cova 2011‐Targeted; Ellis 2011; Fleming 2012; Hyun 2005; Kowalenko 2005; Lillevoll 2014; Makarushka 2012; McCarty 2011; McCarty 2013; McLaughlin 2011; Noël 2013; Pössel 2008; Pössel 2013; Puskar 2003; Rohde 2014a; Rohde 2014b; Rooney 2013; Sawyer 2010; Seligman 1999; Seligman 2007; Sethi 2010; Sheffield a2006; Sheffield b2006; Spence 2003; Stice 2006; Stice 2008; Stoppelbein 2003; Whittaker 2012; Wong 2014; Woods 2011), while we considered some (k = 31) to have a greater emphasis on the cognitive components (Araya 2013; Cardemil 2002; Chaplin 2006; Clarke 1995; Clarke 2001; Dobson 2010; Fresco 2009; Gallegos 2008; Garber 2009; Gilham 1994‐Study 2; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Gillham 2007; Gillham 2012; Horowitz a2007; Jaycox 1994; Karami 2012; Kindt 2014; Mirzamani 2012; O'Leary‐Barrett 2013; Pattison 2001; Pössel 2004; Quayle 2001; Roberts 2003; Roberts 2010; Rooney 2006; Schmiege 2006; Shatte 1997; Sheffield c2006; Wijnhoven 2014; Yu 2002‐study 3). In one of the included trials we considered that the intervention placed a greater emphasis on the behavioural components (Bella‐Awusah 2015). Some of these trials also combined elements of psychoeducation (e.g. Castellanos 2006).
Six combined CBT with interpersonal therapy (IPT) (Arnarson 2009; Merry 2004; Horowitz b2007; Rivet‐Duval 2010; Rose 2014; Stallard 2012a), three trials evaluated IPT only (Horowitz b2007; Young 2006; Young 2010a), two trials evaluated problem‐solving therapy (PST) only (Cowell 2009; Petersen 1997), and 10 evaluated third wave interventions (Charbonneau 2012; Clarke 1993; Garcia 2011; Kauer 2012; Khalsa 2012; Liehr 2010; Livheim 2014‐study 1(girls); Mendelson 2010; Reynolds 2011; Snyder 2010). For one trial, Manicavasagar 2014, correspondence with trial authors suggested that the intervention contained elements of CBT, however, we coded this intervention as third wave in the present review based on our interpretation of the website components. All authors of this review were in agreement with this categorisation.
A number of trials evaluated specific depression prevention programmes, including the Penn Resiliency Programme (PRP) (Cardemil 2002; Chaplin 2006; Gilham 1994‐Study 2; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Gillham 2007; Gillham 2012; Jaycox 1994; Pattison 2001; Roberts 2003; Shatte 1997), or modifications of the PRP (Quayle 2001; Yu 2002‐study 3), the Coping with Stress programme (Clarke 1995; Clarke 2001; Dobson 2010; Garber 2009), or a modified version of this programme (Horowitz a2007), the Problem Solving for Life programme (Sheffield a2006; Sheffield b2006; Sheffield c2006), the Blues Group (Stice 2006; Stice 2008; Rohde 2014a; Rohde 2014b), the Adolescents Coping with Emotion programme (Kowalenko 2005), or modified versions of this programme (McLaughlin 2011; Woods 2011), the Resourceful Adolescent programme (Rivet‐Duval 2010; Rose 2014; Stallard 2012a) or modified versions of this programme (Merry 2004), and MoodGYM (Calear 2009; Ellis 2011; Lillevoll 2014; Sethi 2010). Other prevention programmes evaluated included LISA‐T with or without the LARS component (Pössel 2004; Pössel 2008; Pössel 2013), Apex (Seligman 1999; Seligman 2007), Op Volle Kracht (Kindt 2014; Wijnhoven 2014), the Positive Thoughts and Actions programme (McCarty 2011; McCarty 2013), the Aussie Optimism programme with or without the Positive Thinking programme (Roberts 2010; Rooney 2013), the Interpersonal Psychotherapy‐Adolescent Skills Training programme (IPT‐AST; Young 2006; Young 2010a), I Think, Feel and Act programme (Araya 2013), Women and Relaxation, Openness, Contemplation and Kindness programme (Charbonneau 2012), the Mexican American Problem Solving programme (Cowell 2009), the SPRAX programme (Fleming 2012), Self‐Administered Optimism Training (Fresco 2009), the FRIENDS for Life programme (Gallegos 2008), Project Wings (Garcia 2011), MOBILETYPE (Kauer 2012), Yoga Ed (Khalsa 2012), Mindfulness Schools (Liehr 2010), Acceptance and Commitment Therapy (Livheim 2014‐study 1(girls)), Blues Blaster (Makarushka 2012), Bite Back (Manicavasagar 2014), the Positive Thinking programme (Rooney 2006), Talk 'n' Time (Noël 2013), Teaching Kids to Cope (Puskar 2003), the Brief Behavioural Activation Treatment for Depression programme (Reynolds 2011), the beyondblue Secondary Schools Research Initiative (Sawyer 2010), the Family Bereavement programme (Schmiege 2006), the Positive Psychoeducation programme (Snyder 2010), Problem Solving for Life (Spence 2003), the Coping with Depression programme (Stoppelbein 2003), MEMO (Whittaker 2012), and the Thiswayup Schools programme (Wong 2014).
Others were not so formally described but stated that they were based on principles of cognitive behavioural therapy (Arnarson 2009; Bella‐Awusah 2015; Castellanos 2006; Compas 2009; Cova 2011‐Targeted; Clarke 1993; Cova 2011‐Targeted; Hyun 2005; Karami 2012; Mirzamani 2012; O'Leary‐Barrett 2013; Petersen 1997), interpersonal therapy (Horowitz b2007), or third wave therapy (Mendelson 2010). Most interventions were manualised. For five trials it was unclear if the intervention was manualised (Hyun 2005; Karami 2012; Khalsa 2012; Petersen 1997; Young 2010a).
The majority of these interventions were delivered face‐to‐face (only 10 trials used a purely individual approach and the remainder were group approaches); only eight were delivered in an online format (Calear 2009; Ellis 2011; Fleming 2012; Lillevoll 2014; Makarushka 2012; Manicavasagar 2014; Sethi 2010; Wong 2014). Two programmes were delivered using a telephone format (Kauer 2012; Whittaker 2012), and two combined face‐to‐face sessions with online coaching, self‐monitoring or both (Fresco 2009; Seligman 2007).
The number of intervention sessions that were delivered ranged from one (Fresco 2009) to 48 (Mendelson 2010). Just over one‐half of all trials (k = 47) comprised between eight and 12 sessions (Cardemil 2002; Chaplin 2006; Charbonneau 2012; Compas 2009; Cowell 2009; Gallegos 2008; Gilham 1994‐Study 2; Gillham 2007; Gillham 2012; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Horowitz a2007; Horowitz b2007; Hyun 2005; Jaycox 1994; Karami 2012; Kowalenko 2005; Liehr 2010; Livheim 2014‐study 1(girls); Quayle 2001; McCarty 2011; McCarty 2013; McLaughlin 2011; Noël 2013; Pattison 2001; Puskar 2003; Pössel 2004; Pössel 2008; Pössel 2013; Roberts 2003; Rooney 2006; Rooney 2013; Sawyer 2010; Schmiege 2006; Seligman 1999; Seligman 2007; Shatte 1997; Sheffield a2006; Sheffield c2006; Spence 2003; Stallard 2012a; Stoppelbein 2003; Wijnhoven 2014; Woods 2011; Young 2006; Young 2010a; Yu 2002‐study 3).
For one further trial (Cowell 2009), the duration of the treatment period was unclear. We as review authors were therefore unable to determine which time point/s indicated the post‐intervention and/or follow‐up assessments. Finally, in Lillevoll 2014 the intervention consisted of delivery of the five modules of MoodGYM. However, only 8.54% of those allocated to MoodGym actually logged in.
The intervention called the beyondblue Secondary Schools Research Initiative is notable because it was delivered during a single school term over a three‐year period in conjunction with school‐wide intervention components including, for example, interventions to improve the quality of social interactions among all members of the school community, interventions to facilitate adolescents’ access to support and professional services at school and in the wider community and community forums to provide information about how to identify potential problems and seek help for these (Sawyer 2010).
The intervention programme was delivered by mental health professionals in one‐third of trials (k= 28) (Araya 2013; Arnarson 2009; Bella‐Awusah 2015; Castellanos 2006; Clarke 1995; Clarke 2001; Compas 2009; Garber 2009; Garcia 2011; Gillham, Hamilton 2006a; Hyun 2005; Kowalenko 2005; Livheim 2014‐study 1(girls); McCarty 2013; McLaughlin 2011; Puskar 2003; Reynolds 2011; Rooney 2006; Rooney 2013; Schmiege 2006; Seligman 1999; Seligman 2007; Snyder 2010; Stoppelbein 2003; Wijnhoven 2014; Woods 2011; Young 2006; Young 2010a). For 17 trials the intervention was delivered by non‐mental health professionals (Clarke 1993; Cowell 2009; Gallegos 2008; Gillham 2012; Khalsa 2012; Kindt 2014; Liehr 2010; Mendelson 2010; Merry 2004; Noël 2013; O'Leary‐Barrett 2013; Rivet‐Duval 2010; Roberts 2010; Spence 2003; Sawyer 2010; Sheffield c2006; Yu 2002‐study 3), or a combination of mental health and non‐mental health professionals in nine trials (Chaplin 2006; Gillham 2007; Petersen 1997; Pössel 2004; Pössel 2008; Roberts 2003; Shatte 1997; Sheffield a2006; Sheffield b2006). For a further 10 trials, graduate students with experience in mental health delivered the intervention (Cardemil 2002; Charbonneau 2012; Cova 2011‐Targeted; Dobson 2010; Gilham 1994‐Study 2; Gillham, Reivich 2006b; Pössel 2013; Rohde 2014a; Rohde 2014b; Stallard 2012a), whilst for a further seven trials students (unclear if they had experience in mental health) delivered the intervention (Horowitz a2007; Horowitz b2007; Jaycox 1994; Quayle 2001; Rose 2014; Stice 2006; Stice 2008). In five trials, it is unclear who delivered the intervention (Fresco 2009; Karami 2012; McCarty 2011; Mirzamani 2012; Pattison 2001). The remaining 10 trials involved self‐monitoring (Calear 2009; Ellis 2011; Fleming 2012; Kauer 2012; Lillevoll 2014; Makarushka 2012; Manicavasagar 2014; Sethi 2010; Whittaker 2012; Wong 2014).
Comparison conditions
Various comparison conditions were used. Many trials (k= 30) compared the intervention programmes with treatment as usual (TAU), variously described as "normal teaching activities" (Araya 2013), the ability to seek any school‐based and/or external mental health care as required (Arnarson 2009; Clarke 1995; Clarke 2001; Garber 2009; Gillham, Hamilton 2006a; McCarty 2011; Woods 2011; Young 2006; Young 2010a), the usual health care and/or wellness curriculum (Clarke 1993; Horowitz a2007; Horowitz b2007; Kindt 2014; Liehr 2010; Pössel 2013; Reynolds 2011; Roberts 2003; Roberts 2010; Rooney 2006; Rooney 2013; Stallard 2012a; Wong 2014), the usual physical education curriculum (Khalsa 2012), provision of brochures describing treatment options (Rohde 2014a; Rohde 2014b), didactic lectures on various topics in psychology (Stoppelbein 2003), or the usual coping skills and problem‐solving course (McLaughlin 2011). No further details on TAU content were provided in two trials (Livheim 2014‐study 1(girls); Puskar 2003). Twenty‐seven trials compared the intervention to a "no treatment" or assessment only condition (Cardemil 2002; Castellanos 2006; Chaplin 2006; Cova 2011‐Targeted; Cowell 2009; Ellis 2011; Fresco 2009; Gallegos 2008; Gilham 1994‐Study 2; Gillham, Reivich 2006b; Hyun 2005; Lillevoll 2014; O'Leary‐Barrett 2013; Petersen 1997; Pössel 2004; Pössel 2008; Sawyer 2010; Seligman 1999; Seligman 2007; Sethi 2010; Sheffield a2006; Sheffield b2006; Sheffield c2006; Spence 2003; Stice 2008; Wijnhoven 2014; Yu 2002‐study 3), nine used a wait‐list condition (Bella‐Awusah 2015; Calear 2009; Fleming 2012; Jaycox 1994; Kowalenko 2005; Mendelson 2010; Noël 2013; Quayle 2001; Rivet‐Duval 2010), 14 trials compared the intervention to an attention placebo condition (Dobson 2010; Garcia 2011; Gillham 2007; Kauer 2012; Makarushka 2012; Manicavasagar 2014; Merry 2004; Pattison 2001; Rose 2014; Schmiege 2006; Shatte 1997; Snyder 2010; Stice 2006; Whittaker 2012), and two used another condition (e.g. psychoeducation, brief information or both) as the comparison (Compas 2009; McCarty 2013). For four trials, the comparison condition was not adequately described. For two of these trials, the comparison condition was probably a no treatment condition (Charbonneau 2012; Mirzamani 2012), whilst for the remaining two the comparison condition was probably TAU (Gillham 2012; Karami 2012).
Outcomes
Times for follow‐up varied. Eighteen trials limited follow‐up to immediately post‐intervention (Castellanos 2006; Chaplin 2006; Ellis 2011; Fleming 2012; Fresco 2009; Hyun 2005; Kowalenko 2005; Liehr 2010; Lillevoll 2014; Livheim 2014‐study 1(girls); Manicavasagar 2014; McCarty 2013; McLaughlin 2011; Mendelson 2010; Mirzamani 2012; Reynolds 2011; Sethi 2010; Wijnhoven 2014). Ten trials reported post‐intervention and short‐term outcomes up to three months (Bella‐Awusah 2015; Clarke 1993; Clarke 1995; Jaycox 1994; Kauer 2012; Snyder 2010; Stice 2006; Stice 2008; Young 2006; Yu 2002‐study 3). Twenty‐seven reported post‐intervention and medium‐term outcomes between four and 12 months (Araya 2013; Arnarson 2009; Calear 2009; Clarke 2001; Compas 2009; Gallegos 2008; Gillham 2012; Gillham, Reivich 2006b; Horowitz a2007; Horowitz b2007; Kindt 2014; Makarushka 2012; Pattison 2001; Puskar 2003; Pössel 2008; Pössel 2013; Quayle 2001; Rivet‐Duval 2010; Rohde 2014a; Rohde 2014b; Rose 2014; Schmiege 2006; Shatte 1997; Sheffield a2006; Sheffield b2006; Sheffield c2006; Whittaker 2012); five trials reported post‐intervention, short‐term, and medium‐term outcomes (Charbonneau 2012; Dobson 2010; Garcia 2011; Gilham 1994‐Study 2; Pössel 2004); one trial reported post‐intervention, short‐term, medium‐term and long‐term outcomes of greater than 12 months (Cardemil 2002); and 15 reported post‐intervention, medium‐term and long‐term outcomes (Garber 2009; Gillham 2007; Gillham, Hamilton 2006a; McCarty 2011; Merry 2004; Roberts 2003; Roberts 2010; Rooney 2006; Rooney 2013; Sawyer 2010; Seligman 1999; Seligman 2007; Spence 2003; Woods 2011; Young 2010a). Two trials reported medium‐term outcomes only (Cova 2011‐Targeted; Stallard 2012a), whilst one reported medium‐ and long‐term outcomes only (O'Leary‐Barrett 2013).
For five trials, data for outcomes measured on a continuous scale were adjusted (Compas 2009; McCarty 2011; Mendelson 2010; Seligman 1999; Seligman 2007). Four of these trials adjusted for baseline depression scores only. For Mendelson 2010, adjustment was made for baseline depression scores, gender, age and grade level. We included these trials in meta‐analysis, however we undertook sensitivity analyses to investigate what impact, if any, inclusion of these trials had on the overall pooled effect size.
Depression diagnosis
Diagnosis of depressive disorder was determined from computerised medical records in one trial (Gillham, Hamilton 2006a), from the Diagnostic Interview Schedule for Children, version four (DISC‐IV; Shaffer 2000) in three further trials (Gillham 2012; Rooney 2006; Rooney 2013), the major depression subscale of Diagnostic Interview Schedule for Children, Adolescents, and Parents (DISCAP; Holland 1997) in one trial (Rose 2014), the Kiddie‐Schedule for Affective Disorders and Schizophrenia‐Present and Lifetime version (K‐SADS‐PL; Kaufman 1997) in four trials (Arnarson 2009; Compas 2009; Young 2006; Young 2010a), the Kiddie‐Schedule for Affective Disorders and Schizophrenia for School‐Age Children (K‐SADS; Puig‐Antich 1983) in three trials (Rohde 2014a; Stice 2008; Whittaker 2012), the Longitudinal Interval Follow‐up Evaluation (LIFE; Shapiro 1979) in three trials (Seligman 1999; Seligman 2007; Spence 2003), the Kiddie‐Schedule for Affective Disorders and Schizophrenia‐Epidemiological version (K‐SADS‐E; Orvaschel 1986) in combination with the LIFE in three trials (Clarke 1995; Garber 2009; Makarushka 2012), the Anxiety Disorders Interview Schedule for Children (ADIS‐C; Silverman 1996) in one trial (Spence 2003), the ADIS‐C in combination with the LIFE in one trial (Sheffield b2006), the Structured Clinical Interview for DSM‐IV (SCID‐1; First 1997) in one trial (Charbonneau 2012), or from cut‐points on either the Brief Symptom Inventory (BSI; Derogatis 1983) in one trial (O'Leary‐Barrett 2013: cut‐point not specified), the Beck Depression Inventory‐second revision (BDI‐II; Beck 1996) in two trials (Araya 2013: BDI ≥ 17.0; Stice 2006: BDI ≥ 30.0), the Children's Depression Inventory (CDI; Kovacs 1992) in 10 trials (Cardemil 2002: CDI ≥ 30.0; Gallegos 2008: CDI ≥ 19.0; Gilham 1994‐Study 2: CDI ≥ 15.0; Gillham, Reivich 2006b: CDI ≥ 19.0; Jaycox 1994: CDI ≥ 15.0; Quayle 2001: CDI ≥ 13.0; Roberts 2003: CDI ≈ 15.0; Rose 2014: CDI ≥ 19.0; Shatte 1997: CDI ≥ 12.0; Yu 2002‐study 3: CDI ≥ 15.0), the Center for Epidemiologic Studies Depression Scale (CES‐D; Radloff 1977) in two trials (Calear 2009: CES‐D ≥ 24.0; Clarke 1993: CES‐D ≥ 24.0), or the Short Mood and Feelings Questionnaire (SMFQ; Angold 1995) in one trial (Stallard 2012a: SMFQ ≥ 5.0).
Self‐reported depression
Self‐reported depression symptomatology was assessed using the Beck Depression Inventory (BDI‐I; Beck 1961) in four trials (Fresco 2009; Hyun 2005; Seligman 1999; Seligman 2007), the BDI‐II in six trials (Araya 2013; Bella‐Awusah 2015; Cova 2011‐Targeted; Merry 2004; Stice 2006; Stice 2008), the BDI‐Youth version (BDI‐Y; Beck 2005) in one trial (McLaughlin 2011), a modified version of the BDI‐II in one trial (Spence 2003), the CDI in 28 trials (Arnarson 2009; Cardemil 2002; Chaplin 2006; Dobson 2010; Gallegos 2008; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Gillham 2007; Gillham 2012; Horowitz a2007; Horowitz b2007; Jaycox 1994; Kindt 2014; Kowalenko 2005; Pattison 2001; Quayle 2001; Roberts 2003; Roberts 2010; Rooney 2006; Rooney 2013; Rose 2014; Schmiege 2006; Shatte 1997; Sheffield a2006; Sheffield b2006; Sheffield c2006; Wijnhoven 2014; Woods 2011), the CES‐D in 19 trials (Calear 2009; Charbonneau 2012; Clarke 1993; Compas 2009; Dobson 2010; Garber 2009; Horowitz a2007; Horowitz b2007; Lillevoll 2014; Makarushka 2012; Pössel 2004; Sawyer 2010; Sheffield a2006; Sheffield b2006; Snyder 2010; Wijnhoven 2014; Young 2006; Young 2010a; Yu 2002‐study 3), Reynold's Adolescent Depression Scale, version one (RADS; Reynolds 1989) in three trials (Jaycox 1994; Merry 2004; Puskar 2003), Reynold's Adolescent Depression Scale, version two (RADS‐2; Reynolds 2002) in five trials (Fleming 2012; Gillham 2012; Livheim 2014‐study 1(girls); Rivet‐Duval 2010; Whittaker 2012), the Mood and Feelings Questionnaire (MFQ; Angold 1987) in two trials (McCarty 2013; Whittaker 2012), the Short Mood Feeling Questionnaire (SMFQ) in three trials (Liehr 2010; Mendelson 2010; Stallard 2012a), the Brief Symptom Inventory (BSI) in two trials (Castellanos 2006; O'Leary‐Barrett 2013) from a continuous scale created by summing depression items on the K‐SADS in one trial (Stice 2008), from depression subscale scores on the Depression Anxiety Stress Scale (DASS‐d; Lovibond 1995) in four trials (Ellis 2011; Kauer 2012; Manicavasagar 2014; Reynolds 2011), from depression subscale scores on the Selbstbeurteilungsbogen‐Depressive Störungen (Self‐Report Questionnaire‐Depression; SBB‐DES; Döpfner 2000) in one trial (Pössel 2008), or from a Persian translation of the Children's Depression Scale (CDS; Tisher 1983; Najarian 1994) in one study (Mirzamani 2012).
For one trial (Rose 2014), data on self‐reported depression symptoms were assessed using both the CDI and the RADS‐2. Following the hierarchy of outcome measures outlined in Appendix 1, we preferentially extracted data from the CDI in this review.
For one further trial standard deviations (SDs) for self‐reported depression scores were not reported in the text (Compas 2009). We therefore estimated pooled SDs for this trial from F tests using the formula in Townsend 2001.
Clinician‐rated depression
Clinician‐rated depression symptomatology was assessed using the modified 14‐item Hamilton Depression Rating Scale (HAM‐D; Hamilton 1960; Endicott 1981) in three trials (Clarke 1995; Clarke 2001; Seligman 1999), or from the Children's Depression Rating Scale‐Revised (CDRS‐R; Poznanski 1996) in five trials (Fleming 2012; Garber 2009; Gillham 2007; McCarty 2011; Whittaker 2012).
Anxiety
Anxiety symptomatology was assessed using the Beck Anxiety Inventory (BAI; Beck 1988) in four trials (Cova 2011‐Targeted; Dobson 2010; Seligman 1999; Seligman 2007), the Revised Child Anxiety and Depression Scale (RCADS; Chorpita 2005) in two trials (Araya 2013; Stallard 2012a), the Revised Children's Manifest Anxiety Scale (RCMAS; Reynolds 1985) in seven trials (Calear 2009; Gillham, Hamilton 2006a; Gillham 2012; Roberts 2003; Roberts 2010; Rooney 2006; Schmiege 2006), the Spence Children's Anxiety Scale (SCAS; Spence 2003) in five trials (Fleming 2012; Gallegos 2008; Sheffield a2006; Sheffield b2006; Sheffield c2006), the State Anxiety Inventory for Children (SAI‐C; Spielberger 1970) in one trial (Liehr 2010), the trait anxiety subscale of the SAI‐C in one trial (Pattison 2001), the anxiety subscale of the DASS (DASS‐a; Lovibond 1995) in three trials (Ellis 2011; Kauer 2012; Manicavasagar 2014), the anxiety subscale of the Mood and Anxiety Symptom Questionnaire (MASQ; Watson 1991) in one trial (Dobson 2010), the anxiety subscale of the Youth Self‐Report (YSR; Achenbach 2001) in one trial (Compas 2009), or from anxiety subscale scores on the Selbstbeurteilungsbogen‐Angststörungen (Self‐Report Questionnaire‐Depression) (SBB‐ANG; Döpfner 2000) in one trial (Pössel 2008).
Functioning
Functioning was assessed using the Child and Adolescent Social and Adaptive Functioning Scale (CASAFS; Price 2002) in four trials (Sheffield a2006; Sheffield b2006; Sheffield c2006; Spence 2003), the Children's Global Assessment Scale (CGAS; Shaffer 1983) in two trials (Young 2006; Young 2010a), the Paediatric Quality of Life Enjoyment and Satisfaction Questionnaire (PQ‐LES‐Q; Endicott 2006) in one trial (Fleming 2012), the Student Adaption to College Questionnaire (SACQ; Baker 1989) in one trial (Charbonneau 2012), and the Short Warwick‐Edinburgh Mental Well‐Being Scale (SWEMWBS; Tennant 2007) in one trial (Manicavasagar 2014). Three trials, Rohde 2014a, Rohde 2014b and Stice 2008, measured functioning using the Social Adjustment Scale‐Self‐Report for Youth (SAS‐SR‐Y; Weissman 1980). As higher scores on this instrument are indicative of worse, rather than better functioning, we inversed mean scores for the intervention and control groups for these three trials.
Excluded studies
After consultation with the Trials Search Co‐ordinator (TSC), and because of the large number of trials, we have only provided references for those trials that one would reasonably expect to be included in the review but, on closer inspection, did not meet inclusion criteria (see Characteristics of excluded studies for further information on the reasons for exclusion for these references). However, we have included the full list of exclusion reasons for trials, and the numbers associated with this, for transparency both in this section and within the PRISMA flow chart (see Figure 1).
Trials included in the original review that are now excluded
Twenty‐six trials from the original review are excluded from this update for the following reasons: six focused primarily on trauma (Berger 2008; Layne 2008; Raider 2008; Shen 2002; Tol 2008; Zehnder 2010), four focused on the prevention of anxiety (Balle 2009; Berry 2009; Lock 2003; Lowry‐Webster 2003), four employed a parenting or family intervention where the focus was on family issues, such as divorce, rather than depression (Barnet 2007; Mason 2007; McLaughlin 2007; Wolchik 2000), three focused on reducing stress, anxiety and anger (Hains 1990; Hains 1992; Hains 1994), two focused on treating disruptive behaviours (Cabiya 2008; King 1990), two focused on broad mental health or wellbeing (Bond 2004; Kumakech 2009), one focused on stress management (Kraag 2009), one recruited participants with either depressive or anxious symptoms (Simpson 2008), one was a treatment study (Lamb 1998), one focused on chronic pain (Palermo 2009), and one focused on vocational preparedness (Vuori 2008).
Trials excluded from the updated search
Of the 175 full‐text articles retrieved, we consolidated references into respective studies for which there were multiple references (Buhler 2011; Ishikawa 2010; Van Voorhees 2009; Wasserman 2010; Williford 2012), after which we excluded 206 studies from the review. We excluded 31 studies as the mean age of participants was not within our specified age bracket, 35 contained an intervention that focused on trauma or PTSD, 19 contained an intervention that focused on broad mental health and/or wellbeing, 15 were not RCTs, 17 did not employ a suitable psychological intervention (e.g. equine therapy), 16 were targeted to the treatment, rather than prevention of depression, 11 did not deliver the intervention primarily to the individual child/adolescent (e.g. employed family therapy or focused on parenting skills), nine did not contain a suitable comparison condition, 10 did not contain a specific depression outcome measure, seven employed interventions targeted at postpartum depression or dysfunctional attachment styles, eight employed interventions focused on anxiety, six employed interventions focused on either chronic pain, or a physical health issue, four recruited participants on the basis of depressive or anxious symptoms, three studies focused on promoting a healthy lifestyle, three employed interventions primarily targeting disruptive behaviours, two focused on dealing with the effects of divorce, one described the development of an intervention, one was targeted to prevent bipolar disorder, one was a treatment for insomnia, one intervention focused on early childhood abuse, one focused on bullying and one was a review for which there were no data.
Some trials received significant discussion between the authors as to whether they should be included in the review. The study by McBride 2012 used “cognitively based psychoeducation” in which participants were taught about cognitive distortions (as well as depression symptoms) and asked to identify these distortions in vignettes. The study by Marcotte 1993 was also similar in that children listened to a scenario, and then discussed the irrational beliefs contained within it. We excluded these two studies on the basis that within the traditional CBT approach, individuals are required to work with their own thoughts and to engage in cognitive restructuring or some technique that will result in changing their own behaviours and/or cognitions.
The study by Mason 2012 was primarily a family intervention, and many of the components focused on families identifying and managing depression and co‐occurring substance use. As the intervention contained just one session (out of 10) that was delivered to the adolescent (the remaining nine were either delivered to the entire family or to the parent alone), we excluded it on the basis of that it did not focus sufficiently on the adolescent themselves.
The FRIENDS programme was originally designed to prevent childhood anxiety. Although the previous version of this review included trials of the FRIENDS programme as the programme's effects on depression symptoms is also typically assessed, in this update of the review we sought to include only those interventions in which the primary focus was to prevent depression. Therefore we also excluded several previously included studies (Balle 2009; Lock 2003; Lowry‐Webster 2003), and subsequent studies identified in the updated search.
Ongoing studies
We identified 12 ongoing studies that appear eligible for inclusion in the review once they are completed and data are available. (See Characteristics of ongoing studies).
Studies awaiting assessment
We identified eight studies that we were unable to obtain or translate, meaning that we could not assess whether or not they were eligible for inclusion in the review. (See Characteristics of studies awaiting classification).
New studies found in this assessment
This focused update of the review retained 40 of the previously included trials, two trials that had been previously excluded and one trial that was awaiting assessment (Merry 2011) (43 in all). We also included an additional 40 trials from the updated search. These 83 trials represent a more homogeneous group, particularly in terms of intervention type (primarily targeting the prevention of depression and only including CBT, third wave CBT and IPT interventions).
Risk of bias in included studies
For the complete risk of bias for each trial, please see the 'Risk of bias' tables in the Characteristics of included studies. See Figure 2 for an overview of the proportion of trials rated as 'low', 'unclear' and 'high' risk of bias for each aspect of trial design, and Figure 3 for a summary of risk of bias judgements for each aspect of trial design cross‐tabulated by trial.
2.
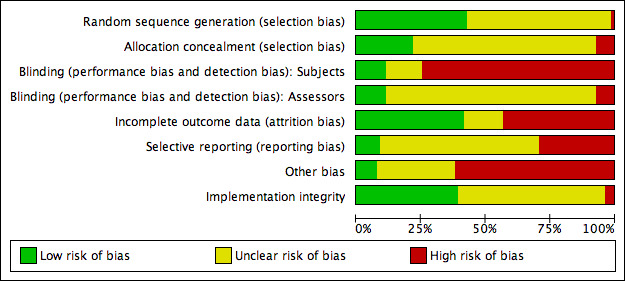
'Risk of bias' graph: Review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The assessment of risk of selection bias requires consideration of both the adequacy of random sequence generation and allocation concealment.
Random sequence generation
With respect to adequacy of randomisation, we rated 38 trials as at low risk of bias for this item as an unbiased method of sequence generation was used, including: computer‐generated lists of random numbers (Araya 2013; Calear 2009; Chaplin 2006; Clarke 2001; Compas 2009; Dobson 2010; Fleming 2012; Garber 2009; Garcia 2011; Gillham, Hamilton 2006a; Gillham 2007; Gillham 2012; Kindt 2014; Lillevoll 2014; Manicavasagar 2014; O'Leary‐Barrett 2013; Schmiege 2006; Stice 2006; Whittaker 2012; Wijnhoven 2014; Woods 2011), random numbers tables/lists (Gilham 1994‐Study 2; Horowitz a2007; Horowitz b2007; Livheim 2014‐study 1(girls); Merry 2004; Noël 2013; Young 2006; Young 2010a), picking envelopes out of hats/containers (Jaycox 1994; Sheffield a2006; Sheffield b2006; Sheffield c2006), using random number generators/sequences (unclear if computerised generators) (Kauer 2012; McCarty 2013; McLaughlin 2011; Stallard 2012a), or using a permuted block randomisation (Puskar 2003). We rated the remaining 47 as unclear risk of bias for this item as the method of generating the randomisation sequence was not specified (Arnarson 2009; Cardemil 2002; Castellanos 2006; Charbonneau 2012; Clarke 1993; Clarke 1995; Cova 2011‐Targeted; Cowell 2009; Ellis 2011; Fresco 2009; Gallegos 2008; Gillham, Reivich 2006b; Hyun 2005; Karami 2012; Khalsa 2012; Kowalenko 2005; Liehr 2010; Makarushka 2012; McCarty 2011; Mendelson 2010; Mirzamani 2012; Pattison 2001; Petersen 1997; Pössel 2004; Pössel 2008; Pössel 2013; Quayle 2001; Reynolds 2011; Rivet‐Duval 2010; Roberts 2003; Roberts 2010; Rohde 2014a; Rohde 2014b; Rooney 2006; Rooney 2013; Rose 2014; Sawyer 2010; Seligman 1999; Seligman 2007; Sethi 2010; Shatte 1997; Snyder 2010; Spence 2003; Stice 2006; Stoppelbein 2003; Wong 2014; Yu 2002‐study 3). We rated one trial as high risk of bias for this item as although schools were allocated via ballot, only two schools were included (Bella‐Awusah 2015).
Allocation concealment
For 19 trials, we rated this item as low risk of bias as appropriate methods to conceal allocation were used. A variety of methods of concealment were used, including: using a independent researcher/statistician to generate the randomisation sequence (Calear 2009; Kindt 2014; Manicavasagar 2014; McCarty 2013; Merry 2004; Noël 2013; Sawyer 2010; Stallard 2012a; Wijnhoven 2014), sealed, opaque envelopes (Clarke 2001; Compas 2009; Jaycox 1994; McCarty 2011), unique ID numbers (Fleming 2012), sequentially numbered mobile phones (Kauer 2012), or a computerised randomisation procedure (Whittaker 2012). For three further trials, although sequence generation was descried as adequately concealed, the method used was not stated (Sheffield a2006; Sheffield b2006; Sheffield c2006). We also rated these trials as at low risk of bias for this item. The majority (k= 62) provided no information on allocation concealment and we therefore rated them as at unclear risk of bias for this item (Arnarson 2009; Bella‐Awusah 2015; Cardemil 2002; Castellanos 2006; Chaplin 2006; Charbonneau 2012; Clarke 1993; Clarke 1995; Cova 2011‐Targeted; Cowell 2009; Dobson 2010; Ellis 2011; Fresco 2009; Gallegos 2008; Garber 2009; Garcia 2011; Gilham 1994‐Study 2; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Gillham 2007; Gillham 2012; Horowitz a2007; Horowitz b2007; Hyun 2005; Karami 2012; Khalsa 2012; Kowalenko 2005; Liehr 2010; Makarushka 2012; Mendelson 2010; Mirzamani 2012; O'Leary‐Barrett 2013; Pattison 2001; Petersen 1997; Pössel 2004; Pössel 2008; Pössel 2013; Puskar 2003; Quayle 2001; Reynolds 2011; Roberts 2003; Roberts 2010; Rooney 2006; Rooney 2013; Rose 2014; Schmiege 2006; Seligman 1999; Seligman 2007; Sethi 2010; Shatte 1997; Snyder 2010; Spence 2003; Stice 2006; Stice 2008; Stoppelbein 2003; Wong 2014; Woods 2011; Young 2006; Young 2010a; Yu 2002‐study 3). We rated one further trial as unclear risk of bias as it could not be determined if allocation was adequately concealed (Araya 2013). For a second, it was unclear if the project co‐ordinators were independent of the research team and, by extension, whether allocation could have been adequately concealed (Rohde 2014b). We rated five as at high risk of bias for this item, as authors, clinicians, project co‐ordinators or teachers involved in the research project undertook allocation (Lillevoll 2014; McLaughlin 2011; Rivet‐Duval 2010; Rohde 2014a), or because correspondence with trial authors revealed that although students' names were concealed, the allocation sequence itself was not (Livheim 2014‐study 1(girls)).
Blinding
Using the Cochrane 'Risk of bias' tool, we assessed blinding separately for participants and outcome assessors.
Blinding of participants
We rated a total of 12 trials as low risk of bias for this item as either a credible attention placebo was used as the control condition (Dobson 2010; Garcia 2011; Gillham 2007; Makarushka 2012; Pattison 2001; Rose 2014; Stallard 2012a), little information as to the content of either the intervention or control conditions was provided to participants (Snyder 2010), both the intervention and control conditions were described to participants as equally effective (Pössel 2013; Shatte 1997), or because participants were blinded to treatment allocation, although no details on how this was achieved were provided (Manicavasagar 2014; Whittaker 2012). We rated 11 as unclear risk of bias for this item as either not enough details were provided (Charbonneau 2012; Karami 2012; Liehr 2010; Mirzamani 2012), some participants were able to correctly identify to which group, intervention or control they had been allocated (Merry 2004), the attention placebo control condition was not credible (Schmiege 2006), parents were informed of their child's allocation to the intervention or control condition (Roberts 2003), or although adequate participant blinding could have been achieved, without access to the participant information sheets and plain language statements (PLS) we cannot verify this (Clarke 1993; Cowell 2009; McLaughlin 2011; Stoppelbein 2003). For the majority (k= 52), however, the nature of the intervention and the control group meant that it was unlikely participants could have remained blind to treatment allocation (Araya 2013; Arnarson 2009; Bella‐Awusah 2015; Cardemil 2002; Castellanos 2006; Chaplin 2006; Clarke 1995; Clarke 2001; Compas 2009; Cova 2011‐Targeted; Ellis 2011; Fresco 2009; Gallegos 2008; Garber 2009; Gilham 1994‐Study 2; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Gillham 2012; Hyun 2005; Jaycox 1994; Kowalenko 2005; Lillevoll 2014; Livheim 2014‐study 1(girls); McCarty 2011; McCarty 2013; Mendelson 2010; Noël 2013; O'Leary‐Barrett 2013; Petersen 1997; Puskar 2003; Pössel 2004; Quayle 2001; Reynolds 2011; Rivet‐Duval 2010; Roberts 2010; Rohde 2014a; Rohde 2014b; Rooney 2006; Rooney 2013; Sawyer 2010; Seligman 1999; Seligman 2007; Sethi 2010; Spence 2003; Stice 2006; Stice 2008; Wijnhoven 2014; Wong 2014; Woods 2011; Young 2006; Young 2010a; Yu 2002‐study 3). We therefore rated these trials as high risk of bias for this item. We also rated a further 11 trials as high risk of bias for this item as it was stated in the trial report that participants were not blind to allocation (Calear 2009; Fleming 2012; Horowitz a2007; Horowitz b2007; Kauer 2012; Khalsa 2012; Kindt 2014; Pössel 2008; Sheffield a2006; Sheffield b2006; Sheffield c2006).
Blinding of outcome assessors
Trials with assessor‐rated outcomes (25)
We rated 16 trials as low risk of bias for this item as outcome assessors were blind to treatment allocation (Arnarson 2009; Charbonneau 2012; Clarke 2001; Compas 2009; Garber 2009; Gillham 2007; Gillham 2012; McCarty 2011; Rohde 2014a; Rohde 2014b; Rose 2014; Seligman 1999; Seligman 2007; Stice 2008; Whittaker 2012; Young 2006; Young 2010a).
We rated eight trials as unclear as insufficient details on assessor blinding were provided (Clarke 1995; Gallegos 2008; Gillham, Hamilton 2006a; Makarushka 2012; Noël 2013; Rooney 2006; Rooney 2013; Spence 2003).
We rated three trials as high risk of bias as the assessors were not blind to allocation (Fleming 2012; Sheffield a2006; Sheffield b2006; Sheffield c2006).
We mostly rated the risk of bias for assessment of the self‐report depression outcomes in these trials as unclear or high risk of bias given in the majority of cases the participants (who were self‐rating their own depression scores) were not blind to treatment allocation or it was unclear if they were blind to treatment allocation. In only one case were the participants blind to allocation and so the risk of bias for the self‐rated outcome in this trial is rated low (Whittaker 2012).
Trials with only self‐report outcomes
The majority of the trials included in this review included only self‐reported outcome measures, therefore we rated most as unclear risk of bias for this item because it was not clear if the participants (who were rating their own depression symptoms) were blind to treatment allocation (k = 56) (Araya 2013; Bella‐Awusah 2015; Calear 2009; Cardemil 2002; Castellanos 2006; Chaplin 2006; Clarke 1993; Cova 2011‐Targeted; Cowell 2009; Dobson 2010; Ellis 2011; Fresco 2009; Garcia 2011; Gilham 1994‐Study 2; Gillham, Reivich 2006b; Horowitz a2007; Horowitz b2007; Hyun 2005; Jaycox 1994; Karami 2012; Kauer 2012; Khalsa 2012; Kindt 2014; Kowalenko 2005; Liehr 2010; Lillevoll 2014; Livheim 2014‐study 1(girls); Manicavasagar 2014; McCarty 2013; Mendelson 2010; McLaughlin 2011; Mirzamani 2012; Merry 2004; O'Leary‐Barrett 2013; Pattison 2001; Petersen 1997; Pössel 2004; Pössel 2008; Pössel 2013; Puskar 2003; Quayle 2001; Reynolds 2011; Rivet‐Duval 2010; Roberts 2003; Roberts 2010; Sawyer 2010; Schmiege 2006; Sethi 2010; Shatte 1997; Snyder 2010; Stallard 2012a; Stice 2006; Stoppelbein 2003; Wijnhoven 2014; Wong 2014; Woods 2011; Yu 2002‐study 3).
There are 36 trials that report data on diagnosis of a depressive disorder at any time point. For 14 of these trials this was established by cut‐points on a self‐report rating scale: either the Brief Symptom Inventory (BSI; Derogatis 1983) in one trial (O'Leary‐Barrett 2013: cut‐point not specified), the Beck Depression Inventory‐second revision (BDI‐II; Beck 1996) in two trials (Araya 2013: BDI ≥ 17.0; Stice 2006: BDI ≥ 30.0), the Children's Depression Inventory (CDI; Kovacs 1992) in 10 trials (Cardemil 2002: CDI ≥ 30.0; Gallegos 2008: CDI ≥ 19.0; Gilham 1994‐Study 2: CDI ≥ 15.0; Gillham, Reivich 2006b: CDI ≥ 19.0; Jaycox 1994: CDI ≥ 15.0; Quayle 2001: CDI ≥ 13.0; Roberts 2003: CDI ≈ 15.0; Rose 2014: CDI ≥ 19.0; Shatte 1997: CDI ≥ 12.0; Yu 2002‐study 3: CDI ≥ 15.0), the Center for Epidemiologic Studies Depression Scale (CES‐D; Radloff 1977) in two trials (Calear 2009: CES‐D ≥ 24.0; Clarke 1993: CES‐D ≥ 24.0), or the Short Mood and Feelings Questionnaire (SMFQ; Angold 1995) in one trial (Stallard 2012a: SMFQ ≥ 5.0). Of these trials, we rated 12 as high risk of bias. For the remaining 22 trials in which diagnosis of a depressive disorder was established from a diagnostic instrument, we rated 15 as at unclear risk of bias, seven at low risk of bias and one at high risk of bias for this item.
Incomplete outcome data
We rated a total of 36 trials as low risk of bias for this item because less than 10% of data were missing for the post‐intervention assessment (Bella‐Awusah 2015; Clarke 2001; Dobson 2010; Fleming 2012; Garber 2009; Gilham 1994‐Study 2; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Gillham 2007; Gillham 2012; Horowitz a2007; Horowitz b2007; Liehr 2010; McCarty 2013; McLaughlin 2011; Mirzamani 2012; Noël 2013; Pattison 2001; Petersen 1997; Puskar 2003; Reynolds 2011; Rivet‐Duval 2010; Roberts 2003; Rooney 2013; Rose 2014; Schmiege 2006; Seligman 1999; Seligman 2007; Sethi 2010; Shatte 1997; Snyder 2010; Stice 2008; Whittaker 2012; Young 2006; Young 2010a; Yu 2002‐study 3). We rated 11 as unclear risk of bias for this item either because the number of participants included in post‐intervention analyses was unclear (Castellanos 2006; Compas 2009; Cowell 2009; Ellis 2011; Karami 2012; Lillevoll 2014; Livheim 2014‐study 1(girls); McCarty 2011; Sawyer 2010), no data on post‐intervention analyses were reported (O'Leary‐Barrett 2013), or because for continuous outcome measures missing item scores were imputed, however, missing total scores were not (Charbonneau 2012). Although 15 trials with greater than 10% missing data reported using imputed data to perform ITT analyses, as information included in the present review was based on raw data from fewer participants than the number randomised, we rated all 15 trials as high risk of bias for this item (Araya 2013; Fresco 2009; Kauer 2012; Kindt 2014; Kowalenko 2005; Makarushka 2012; Pössel 2008; Rohde 2014a; Rohde 2014b; Sheffield a2006; Sheffield b2006; Sheffield c2006; Stallard 2012a; Stice 2006; Wijnhoven 2014). We rated the remaining 25 trials as at high risk of bias for this item as greater than 10% of data at the post‐intervention assessment was missing and appropriate methods for imputing these data were not attempted (Arnarson 2009; Calear 2009; Cardemil 2002; Chaplin 2006; Clarke 1993; Clarke 1995; Cova 2011‐Targeted; Gallegos 2008; Garcia 2011; Gillham, Hamilton 2006a; Hyun 2005; Jaycox 1994; Khalsa 2012; Manicavasagar 2014; Mendelson 2010; Merry 2004; Pössel 2004; Pössel 2013; Quayle 2001; Roberts 2010; Rooney 2006; Spence 2003; Stoppelbein 2003; Wong 2014; Woods 2011).
Selective reporting
We assessed whether selective reporting may have been present by considering whether trial authors reported results for outcomes that were pre‐specified in the subgroups pre‐specified.
We rated eight trials as low risk of bias for this item either because they were theses (Gallegos 2008; McLaughlin 2011; Rivet‐Duval 2010; Snyder 2010), or because all outcomes listed in the trial protocol were reported (Lillevoll 2014; Sawyer 2010; Stallard 2012a; Whittaker 2012). We rated the majority (k = 50), however, as unclear risk of bias for this item as we were unable to locate a trial protocol (Bella‐Awusah 2015; Calear 2009; Charbonneau 2012; Clarke 2001; Compas 2009; Cova 2011‐Targeted; Cowell 2009; Dobson 2010; Ellis 2011; Fleming 2012; Fresco 2009; Garber 2009; Garcia 2011; Gillham, Hamilton 2006a; Gillham 2012; Horowitz a2007; Horowitz b2007; Hyun 2005; Karami 2012; Khalsa 2012; Liehr 2010; Livheim 2014‐study 1(girls); McCarty 2013; Mendelson 2010; Merry 2004; Mirzamani 2012; Pattison 2001; Puskar 2003; Pössel 2004; Pössel 2008; Pössel 2013; Roberts 2003; Roberts 2010; Rohde 2014a; Rohde 2014b; Rooney 2006; Rooney 2013; Rose 2014; Sethi 2010; Sheffield a2006; Sheffield b2006; Sheffield c2006; Spence 2003; Stice 2006; Stoppelbein 2003; Wijnhoven 2014; Woods 2011; Young 2006; Young 2010a; Yu 2002‐study 3). We rated one further trial as at unclear risk of bias as some relevant outcomes were reported in a secondary report (Schmiege 2006). We rated the remaining 27 trials as at high risk of bias for this item as information from the trial protocol indicates that key outcomes may not have been reported (Araya 2013; Kindt 2014; Manicavasagar 2014; O'Leary‐Barrett 2013; Wong 2014), information on some outcomes listed in the methods section were not reported (Arnarson 2009; Chaplin 2006; Gilham 1994‐Study 2; Gillham 2007; Gillham, Reivich 2006b; Kauer 2012; Makarushka 2012; McCarty 2011; Noël 2013; Petersen 1997; Reynolds 2011), some post‐hoc analyses were undertaken (Cardemil 2002; Clarke 1993; Jaycox 1994; Kowalenko 2005; Quayle 2001; Seligman 1999; Seligman 2007; Shatte 1997; Stice 2008), information on some participants was not reported (Castellanos 2006), or because data on an outcome not mentioned in the methods section were presented (Clarke 1995).
Other potential sources of bias
Independence of investigators
We rated eight trials as low risk of bias for this item as they were conducted by an independent research team (Rivet‐Duval 2010; Sethi 2010; Sheffield a2006; Sheffield b2006; Sheffield c2006; Snyder 2010; Stallard 2012a; Stoppelbein 2003). We rated 19 trials as unclear risk of bias for this item either because insufficient details on whether those who developed the intervention also conducted the trial were provided (Araya 2013; Clarke 1993; Dobson 2010; Ellis 2011; Gallegos 2008; Liehr 2010; Lillevoll 2014; Mendelson 2010; Merry 2004; Mirzamani 2012; Pattison 2001; Petersen 1997; Spence 2003; Young 2010a), or because, although trial authors did not develop the intervention, it was adapted to the trial setting by the trial authors (McLaughlin 2011; Noël 2013; Quayle 2001; Woods 2011). We rated one further trial as unclear risk of bias for this item as correspondence with trial authors revealed that although trial authors developed the intervention, as it was an online intervention the development team had no input in the administration of the intervention. Participants were instead instructed to navigate the website independently (Manicavasagar 2014). However, as most trials were conducted by those that developed the intervention, we rated bias for this item as high risk for the majority of the included trials (k = 59) (Arnarson 2009; Bella‐Awusah 2015; Calear 2009; Cardemil 2002; Castellanos 2006; Chaplin 2006; Charbonneau 2012; Clarke 1995; Clarke 2001; Compas 2009; Cova 2011‐Targeted; Cowell 2009; Fleming 2012; Fresco 2009; Garber 2009; Garcia 2011; Gilham 1994‐Study 2; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Gillham 2007; Gillham 2012; Horowitz a2007; Horowitz b2007; Hyun 2005; Jaycox 1994; Karami 2012; Kauer 2012; Khalsa 2012; Kindt 2014; Kowalenko 2005; Livheim 2014‐study 1(girls); Makarushka 2012; McCarty 2011; McCarty 2013; O'Leary‐Barrett 2013; Pössel 2004; Pössel 2008; Pössel 2013; Puskar 2003; Reynolds 2011; Roberts 2003; Roberts 2010; Rohde 2014a; Rohde 2014b; Rooney 2006; Rooney 2013; Rose 2014; Sawyer 2010; Schmiege 2006; Seligman 1999; Seligman 2007; Shatte 1997; Stice 2006; Stice 2008; Whittaker 2012; Wijnhoven 2014; Wong 2014; Young 2010a; Yu 2002‐study 3).
Implementation integrity
We rated a total of 24 trials as low risk of bias as implementation integrity was assessed as adequate (Bella‐Awusah 2015; Clarke 1993; Clarke 1995; Clarke 2001; Compas 2009; Cowell 2009; Dobson 2010; Garber 2009; Gillham 2007; Gillham 2012; McCarty 2011; McCarty 2013; Puskar 2003; Roberts 2003; Roberts 2010; Rohde 2014a; Rohde 2014b; Rooney 2013; Rose 2014; Sawyer 2010; Shatte 1997; Snyder 2010; Stallard 2012a; Stice 2008). We also rated all 10 trials in which in the intervention was delivered remotely as low risk of bias for this item as the nature of the intervention would suggest that the intervention would have been delivered with equal fidelity to all participants (Calear 2009; Ellis 2011; Fleming 2012; Kauer 2012; Lillevoll 2014; Makarushka 2012; Manicavasagar 2014; Sethi 2010; Whittaker 2012; Wong 2014). We rated most trials (k = 42), however, as unclear risk of bias for this item as it was unclear whether implementation integrity was assessed (Araya 2013; Arnarson 2009; Cardemil 2002; Castellanos 2006; Charbonneau 2012; Cova 2011‐Targeted; Fresco 2009; Garcia 2011; Gilham 1994‐Study 2; Gillham, Reivich 2006b; Horowitz a2007; Horowitz b2007; Hyun 2005; Jaycox 1994; Karami 2012; Khalsa 2012; Kowalenko 2005; Liehr 2010; Livheim 2014‐study 1(girls); McLaughlin 2011; Mendelson 2010; Merry 2004; Mirzamani 2012; Pattison 2001; Petersen 1997; Quayle 2001; Reynolds 2011; Rivet‐Duval 2010; Rooney 2006; Schmiege 2006; Seligman 1999; Seligman 2007; Sheffield a2006; Sheffield b2006; Sheffield c2006; Spence 2003; Stice 2006; Stoppelbein 2003; Wijnhoven 2014; Woods 2011; Young 2006; Yu 2002‐study 3). We also rated an additional seven trials as unclear risk of bias as although implementation integrity was assessed, it was unclear if this was assessed as adequate (Gallegos 2008; Gillham, Hamilton 2006a; Noël 2013; O'Leary‐Barrett 2013; Pössel 2004; Pössel 2008; Young 2010a). For two trials implementation integrity was not assessed (Kindt 2014; Pössel 2013), whilst for a further trial, although fidelity was assessed, it was not assessed adequately (Chaplin 2006). We therefore rated all these trials as high risk of bias for this item.
Effects of interventions
Outcome 1. Depression diagnosis
Thirty‐six trials (n = 6963) evaluated the effects of an evidence‐based psychological therapy on depression diagnoses. Overall, there was evidence of a small effect in favour of the intervention at post‐intervention assessment (k = 20; n = 3232; risk difference (RD) ‐0.05, 95% confidence interval (CI) ‐0.08 to ‐0.02; I2 = 43.0%) and medium‐term follow‐up (the primary outcome; see Figure 4) (k = 32; n = 5965; RD ‐0.03, 95% CI ‐0.05 to ‐0.01; I2 = 47.0%), but there was no evidence of an effect at the short‐ or long‐term follow‐up assessments.
4.
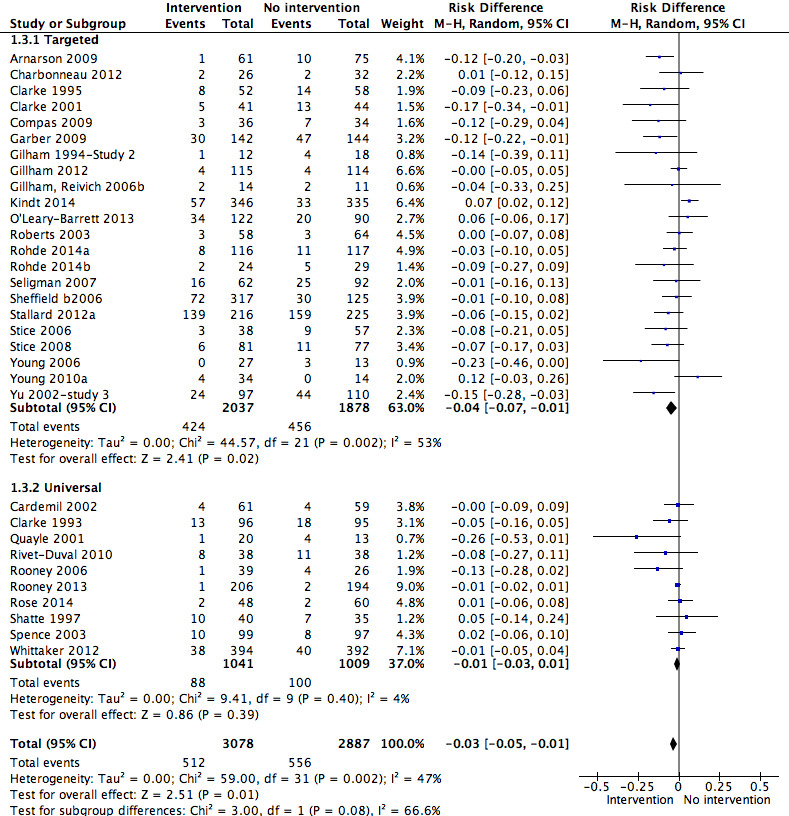
Forest plot of comparison: 1 Psychological intervention versus any comparison post‐intervention, outcome: 1.3 Depressive disorder medium‐term follow‐up (primary outcomes).
The effect size for reduction of depression diagnosis overall at medium‐term follow‐up (primary outcome), translates to a number needed to treat to benefit (NNTB) of 33 (22 to 148).
For the medium‐term follow‐up assessment (the primary outcome) the quality of the evidence, as assessed using the GRADE criteria, was moderate (see Table 1).
There was no evidence that the way in which populations were selected for intervention (i.e. universal versus targeted) modified the efficacy of these prevention programmes at either post‐intervention (Chi² = 0.68; df = 1; P value = 0.41; I² = 0%), medium‐term (Chi² = 3.00; df = 1; P value = 0.08; I² = 66.6%) or long‐term follow‐up (Chi² = 0.63; df = 1; P value = 0.43; I² = 0%) assessments. However, there was some evidence to suggest that the way in which populations were selected for intervention did modify the efficacy of these programmes at the short‐term assessment, with targeted populations showing greater response compared with universal populations (Chi² = 5.92; df = 1; P value = 0.01; I² = 83.1%).
1.1 Targeted depression prevention programmes
There was evidence of a small effect in favour of targeted depression programmes compared with any comparator at the post‐intervention (RD ‐0.06, 95% CI ‐0.10 to ‐0.02; k = 13; n = 2022) (Analysis 1.1), short‐term (RD ‐0.11, 95% CI ‐0.19 to ‐0.02; k = 4; n = 360) (Analysis 1.2) and medium‐term (RD ‐0.04, 95% CI ‐0.07 to ‐0.01; k = 22; n = 3915) (Analysis 1.3) assessments, but the effect was not statistically significant at the long‐term follow‐up (Analysis 1.4).
1.1. Analysis.
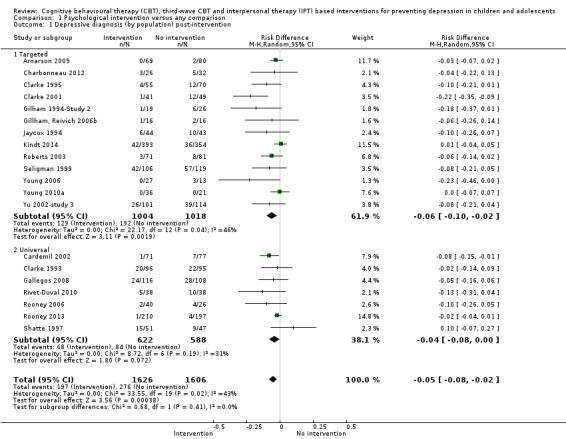
Comparison 1 Psychological intervention versus any comparison, Outcome 1 Depressive diagnosis (by population) post‐intervention.
1.2. Analysis.
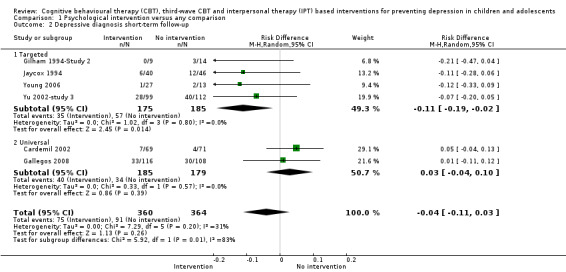
Comparison 1 Psychological intervention versus any comparison, Outcome 2 Depressive diagnosis short‐term follow‐up.
1.3. Analysis.
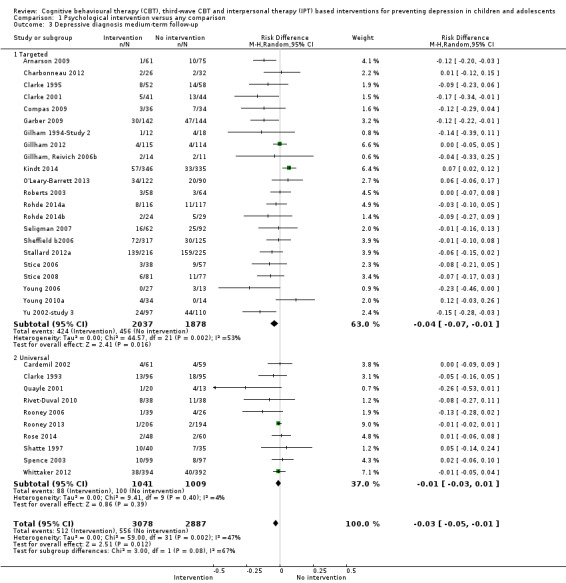
Comparison 1 Psychological intervention versus any comparison, Outcome 3 Depressive diagnosis medium‐term follow‐up.
1.4. Analysis.
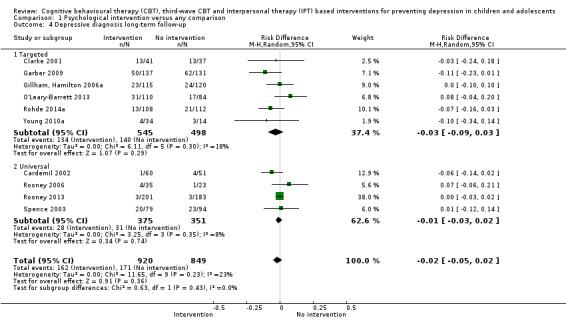
Comparison 1 Psychological intervention versus any comparison, Outcome 4 Depressive diagnosis long‐term follow‐up.
For the medium‐term follow‐up assessment (the primary outcome), however, the quality of the evidence was very low (see Table 1).
1.2 Universal depression prevention programmes
There was no evidence of a statistically significant effect in favour of universal depression prevention programmes compared with any comparator at either the post‐intervention (Analysis 1.1), short‐term (Analysis 1.2), medium‐term (Analysis 1.3) or long‐term (Analysis 1.4) follow‐up assessments.
The quality of the evidence, as assessed using the GRADE criteria, was moderate for the medium‐term follow‐up assessment (the primary outcome) (see Table 1).
Outcome 2. Depression symptoms (self‐reported)
A total of 76 trials (n = 14,660) evaluated the efficacy of an evidence‐based depression prevention programme on self‐reported symptoms of depression. Overall, there was evidence of a small effect in favour of these interventions compared with any comparator at the post‐intervention assessment (the primary outcome) (k = 73; n = 13,829; standardised mean difference (SMD) ‐0.21, 95% CI ‐0.27 to ‐0.15; I2 = 57.0%; see Figure 5), a small to moderate effect at short‐term follow‐up (k = 16; n = 1558; SMD ‐0.31, 95% CI ‐0.45 to ‐0.17; I2 = 38.0%) and a small effect at medium‐term follow‐up (k = 53; n = 11,913; SMD ‐0.12, 95% CI ‐0.18 to ‐0.05; I2 = 57.0%); however, this effect was no longer evident at the long‐term follow‐up.
5.
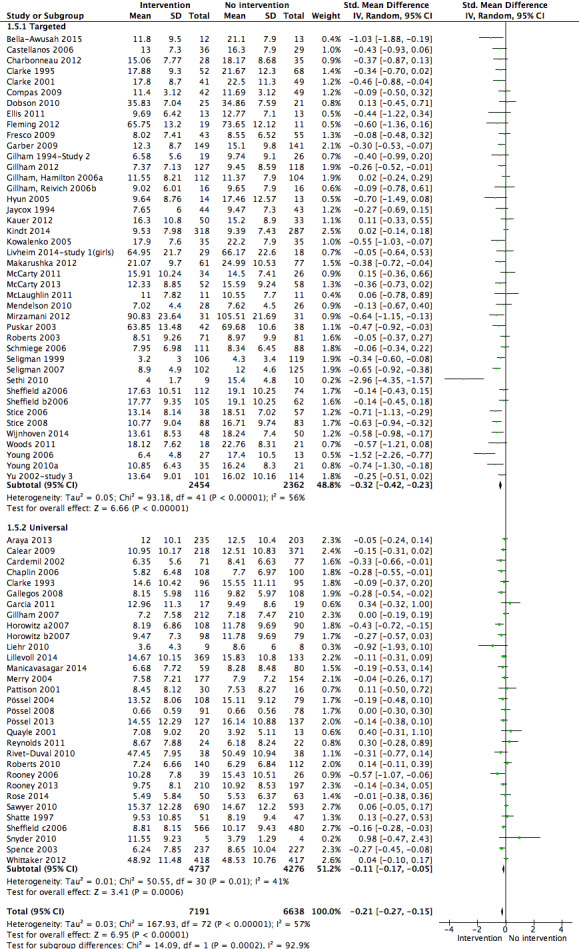
Forest plot of comparison: 1 Psychological intervention versus any comparison post‐intervention, outcome: 1.5 Depression scores (self‐report) post‐intervention follow‐up (primary outcome).
For the post‐intervention assessment (the primary outcome), however, the quality of the evidence was low (see Table 2).
There was evidence that the way in which populations were selected for intervention (i.e. universal versus targeted) modified the efficacy of these prevention programmes at the post‐intervention assessments with targeted interventions more effective than universal (Chi² = 14.09; df = 1; P value = 0.0002; I² = 92.9%) at medium‐term follow‐up (Chi² = 10.91; df = 1; P value = 0.001; I² = 90.8%), but not at short‐term (Chi² = 2.19; df = 1; P value = 0.14; I² = 54.4%) or long‐term (Chi² = 0.56; df = 1; P value = 0.45; I² = 0%) follow‐up.
2.1 Targeted depression prevention programmes
There was evidence of a small to moderate effect in favour of targeted depression prevention programmes compared with any comparator at the post‐intervention assessment (SMD ‐0.32, 95% CI ‐0.42 to ‐0.23; k = 42; n = 4816) (Analysis 1.5), a moderate effect in favour of these programmes at the short‐term assessment (SMD ‐0.37, 95% CI ‐0.54 to ‐0.20; k = 11; n = 999) (Analysis 1.6), and a small effect in favour of these programmes at the medium‐term assessment (SMD ‐0.23, 95% CI ‐0.33 to ‐0.12; k = 29; n = 4448) (Analysis 1.7). However, there was no evidence of a statistically significant treatment effect for these programmes at the long‐term assessment (Analysis 1.8).
1.5. Analysis.
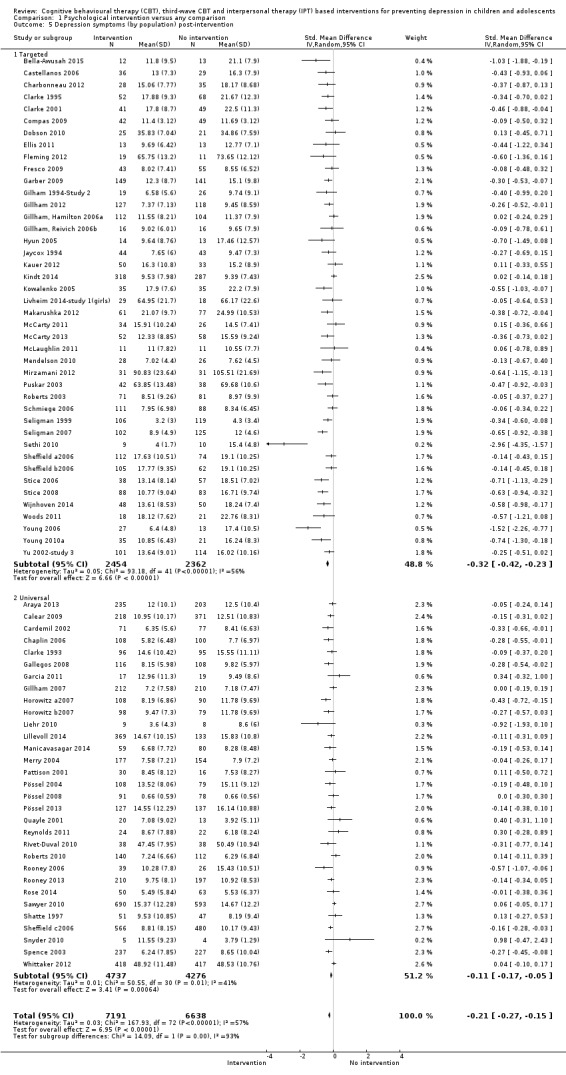
Comparison 1 Psychological intervention versus any comparison, Outcome 5 Depression symptoms (by population) post‐intervention.
1.6. Analysis.
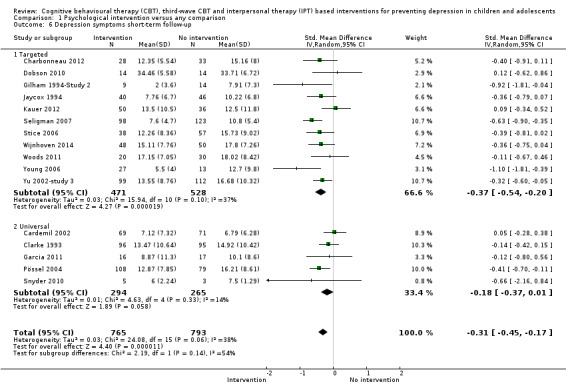
Comparison 1 Psychological intervention versus any comparison, Outcome 6 Depression symptoms short‐term follow‐up.
1.7. Analysis.
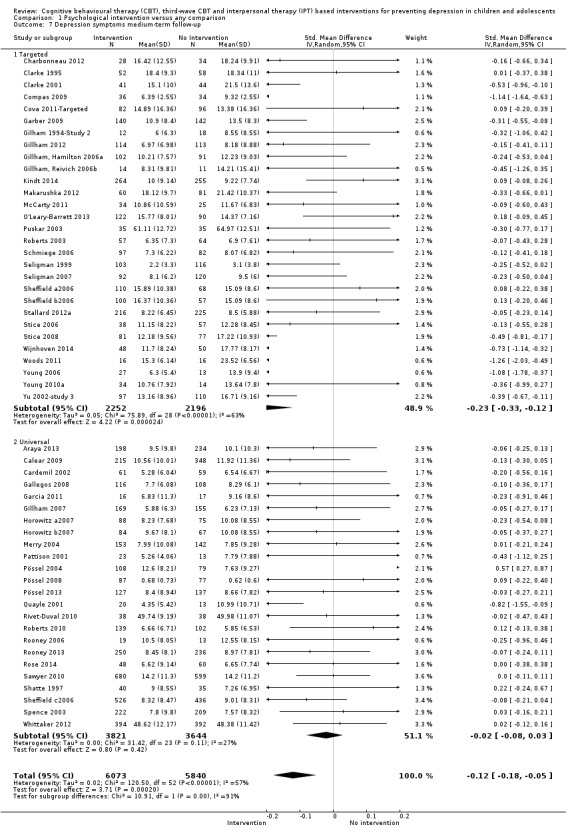
Comparison 1 Psychological intervention versus any comparison, Outcome 7 Depression symptoms medium‐term follow‐up.
1.8. Analysis.

Comparison 1 Psychological intervention versus any comparison, Outcome 8 Depression symptoms long‐term follow‐up.
The quality of the evidence was moderate for the post‐intervention assessment (the primary outcome) (see Table 2).
2.2 Universal depression prevention programmes
There was evidence of a small effect in favour of universal depression prevention programmes compared with any comparator at the post‐intervention assessment (SMD ‐0.11, 95% CI ‐0.17 to ‐0.05; k = 31; n = 9013) (Analysis 1.5), but not at the short‐term follow‐up (SMD ‐0.18, 95% CI ‐0.37 to 0.01; k = 5; n = 559) (Analysis 1.6), or at the medium‐term (Analysis 1.7) or long‐term (Analysis 1.8) follow‐up.
For the post‐intervention assessment (the primary outcome), the quality of the evidence was moderate (Table 2).
Outcome 3. Depression symptoms (clinician‐rated)
Eleven trials (n = 2175) investigated the efficacy of an evidence‐based depression programme on clinician‐rated depression scores. Overall, there was evidence of a small treatment effect in favour of these interventions at the post‐intervention assessment (k = 11; n = 2175; SMD ‐0.23, 95% CI ‐0.41 to ‐0.05; I2 = 70.0%), but not at the medium‐term or long‐term follow‐up and there were no data reported at short‐term follow‐up.
There was evidence that the way in which populations were selected for intervention (i.e. universal versus targeted) modified the effect of these programmes at the post‐intervention assessment (Chi² = 10.45; df = 1; P value = 0.001; I² = 90.4%), but not at the medium‐term follow‐up (Chi² = 0.79; df = 1; P value = 0.37; I² = 0%). The test for subgroup differences could not be undertaken at the long‐term follow‐up, however, as only targeted depression prevention programmes reported data for this outcome at this time point.
3.1 Targeted depression prevention programmes
There was evidence of a small effect in favour of targeted depression prevention programmes compared with any comparator at the post‐intervention assessment (SMD ‐0.28, 95% CI ‐0.44 to ‐0.11; k = 10; n = 1340) (Analysis 1.9), but not at the medium‐term (Analysis 1.10) or long‐term (Analysis 1.11) follow‐up. No trial of a targeted depression prevention programme reported outcomes for clinician‐rated depression scores at the short‐term follow‐up.
1.9. Analysis.
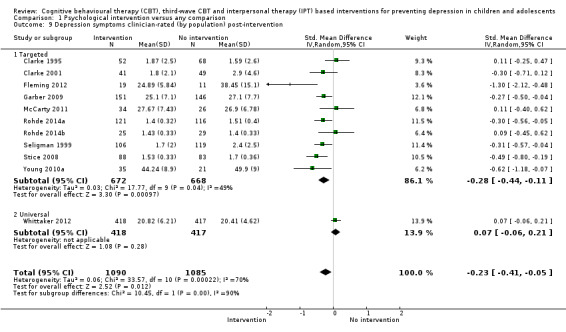
Comparison 1 Psychological intervention versus any comparison, Outcome 9 Depression symptoms clinician‐rated (by population) post‐intervention.
1.10. Analysis.
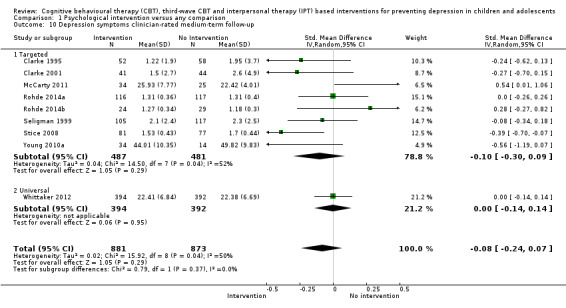
Comparison 1 Psychological intervention versus any comparison, Outcome 10 Depression symptoms clinician‐rated medium‐term follow‐up.
1.11. Analysis.
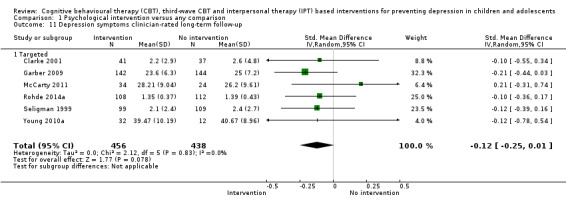
Comparison 1 Psychological intervention versus any comparison, Outcome 11 Depression symptoms clinician‐rated long‐term follow‐up.
3.2 Universal depression prevention programmes
Only one trial, Whittaker 2012 (n = 835), assessed the efficacy of a universal depression prevention programme on clinician‐rated depression scores. There was no evidence of a significant treatment effect for this programme at either the post‐intervention assessment (Analysis 1.9) or medium‐term (Analysis 1.10) follow‐up. No data were available for either the short‐term or long‐term follow‐up.
Outcome 4. Anxiety symptoms
Twenty‐four trials (n = 4490) evaluated the efficacy of an evidence‐based depression prevention programme on anxiety scores. Overall, there was no evidence of an effect in favour of these programmes at either the post‐intervention assessment or long‐term follow‐up. There was, however, evidence of a small to moderate effect favouring these programmes at the short‐term follow‐up (k = 3; n = 334; SMD ‐0.33, 95% CI ‐0.59 to ‐0.07; I2 = 19.0%) and a small effect at the medium‐term follow‐up (k = 18; n = 4957; SMD ‐0.08, 95% CI ‐0.14 to ‐0.01; I2 = 16.0%).
There was no evidence that the way in which populations were selected for intervention (i.e. universal versus targeted) modified the effect of these programmes at either the post‐intervention assessment, medium‐term or long‐term follow‐up. The test for subgroup differences could not be undertaken at the short‐term follow‐up assessment, however, as only targeted depression prevention programmes reported data for this outcome at this time point.
4.1 Targeted depression prevention programmes
There was no evidence of an effect for targeted depression prevention programmes compared with any comparator at the post‐intervention assessment (Analysis 1.12), medium‐term (Analysis 1.14), or long‐term (Analysis 1.15) follow‐up. There was, however, evidence of a small to moderate treatment effect in favour of these programmes at the short‐term follow‐up (SMD ‐0.33, 95% CI ‐0.59 to ‐0.07; k = 3; n = 334) (Analysis 1.13).
1.12. Analysis.
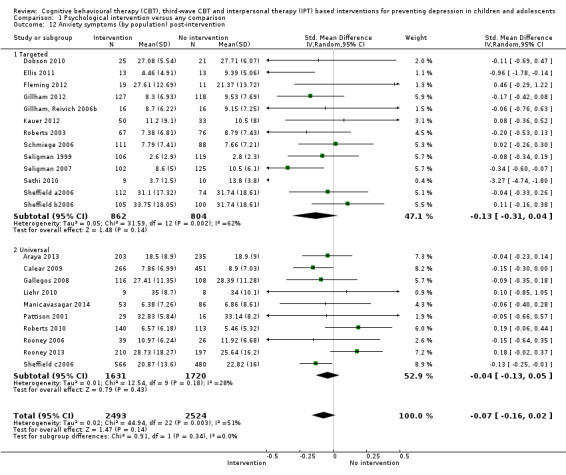
Comparison 1 Psychological intervention versus any comparison, Outcome 12 Anxiety symptoms (by population) post‐intervention.
1.14. Analysis.
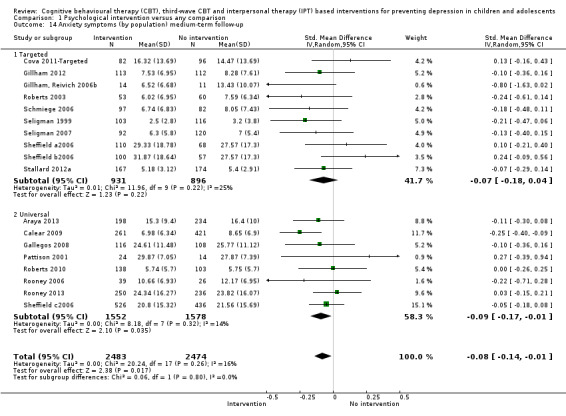
Comparison 1 Psychological intervention versus any comparison, Outcome 14 Anxiety symptoms (by population) medium‐term follow‐up.
1.15. Analysis.

Comparison 1 Psychological intervention versus any comparison, Outcome 15 Anxiety symptoms (by population) long‐term follow‐up.
1.13. Analysis.
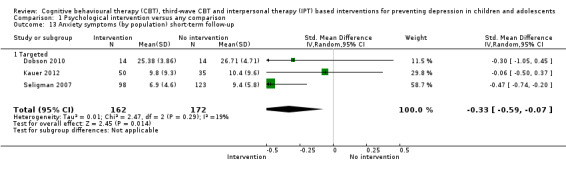
Comparison 1 Psychological intervention versus any comparison, Outcome 13 Anxiety symptoms (by population) short‐term follow‐up.
4.2 Universal depression prevention programmes
There was also no evidence of an effect for universal depression prevention programmes compared with any comparator at the post‐intervention assessment (Analysis 1.12) or long‐term (Analysis 1.15) follow‐up. At the medium‐term follow‐up, however, there was evidence of a small treatment effect in favour of these interventions (SMD ‐0.09, 95% CI ‐0.17 to ‐0.01; k = 8; n = 3130) (Analysis 1.14). As no trial of a universal depression prevention programme reported data on anxiety scores at the short‐term follow‐up, data for this outcome at this time point were unavailable.
Outcome 5. General and social functioning
Only 11 of the 83 included trials evaluated the efficacy of an evidence‐based depression prevention programme on functioning scores (n = 1554). Overall, there was evidence of a small effect in favour of these programmes at the post‐intervention assessment (k = 10; n = 2067; SMD 0.24, 95% CI 0.06 to 0.41; I2 = 61.0%), a large effect at the short‐term follow‐up (k = 1; n = 40; SMD 0.81, 95% CI 0.12 to 1.49; I2 = not estimable) and small effect at the medium‐term follow‐up (k = 11; n = 2449; SMD 0.15, 95% CI 0.02 to 0.28; I2 = 45.0%). However, there was no evidence of any effect by the long‐term follow‐up.
There was no evidence that the way in which populations were selected for intervention (i.e. universal versus targeted) modified the effect of these programmes at either the post‐intervention assessment, medium‐term or long‐term follow‐up. As only one trial of a targeted depression prevention programme reported data on functioning scores at the short‐term follow‐up, subgroup analyses could not be undertaken at this time point.
5.1 Targeted depression prevention programmes
There was evidence of a small treatment effect in favour of targeted depression prevention programmes compared with any comparator at the post‐intervention assessment (SMD 0.27, 95% CI 0.04 to 0.50; k = 9; n = 1021) (Analysis 1.16), and a large effect for these interventions at the short‐term follow‐up (SMD 0.81, 95% CI 0.12 to 1.49; k = 1; n = 40) (Analysis 1.17). However, there was no evidence of an effect for these interventions by the medium‐term (Analysis 1.18) or long‐term (Analysis 1.19) follow‐up.
1.16. Analysis.
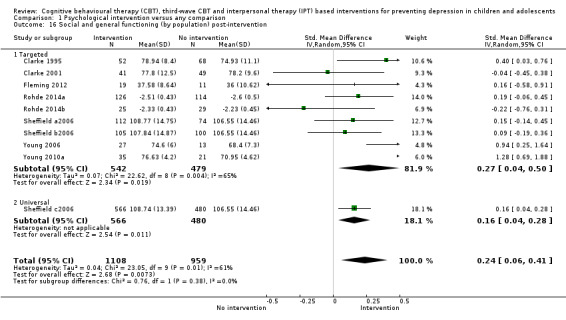
Comparison 1 Psychological intervention versus any comparison, Outcome 16 Social and general functioning (by population) post‐intervention.
1.17. Analysis.
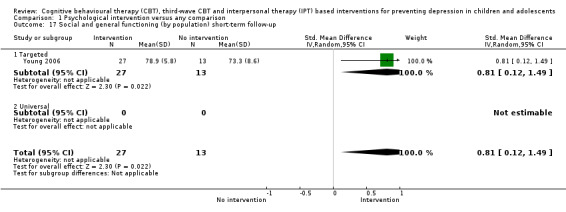
Comparison 1 Psychological intervention versus any comparison, Outcome 17 Social and general functioning (by population) short‐term follow‐up.
1.18. Analysis.
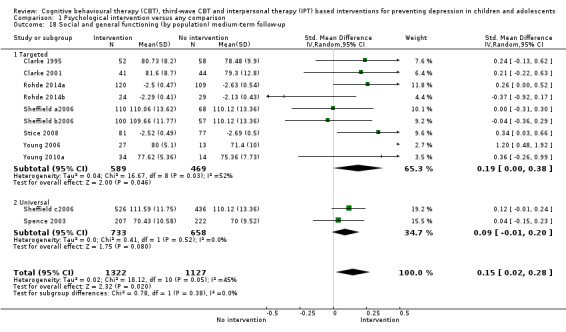
Comparison 1 Psychological intervention versus any comparison, Outcome 18 Social and general functioning (by population) medium‐term follow‐up.
1.19. Analysis.
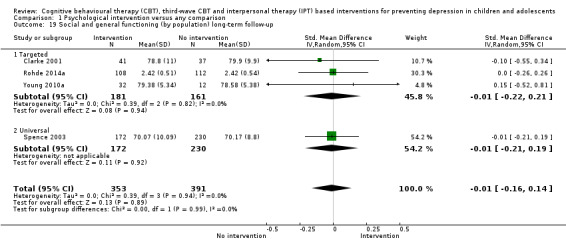
Comparison 1 Psychological intervention versus any comparison, Outcome 19 Social and general functioning (by population) long‐term follow‐up.
5.2 Universal depression prevention programmes
Only two trials evaluated the efficacy of a universal depression prevention programme on functioning scores. For these trials there was evidence of a small effect at the post‐intervention assessment (SMD 0.16, 95% CI 0.04 to 0.28; k = 1; n = 1046) (Analysis 1.16), however, there was no evidence of an effect by the medium‐ (Analysis 1.18) or long‐term (Analysis 1.19) follow‐up. As neither trial reported information on functioning scores at the short‐term follow‐up, data for this outcome were unavailable at this time point.
3. Sensitivity analyses
We undertook a series of sensitivity analyses for the primary outcome measures of depression diagnosis at the medium‐term follow‐up period and for self‐rated depression scores at the post‐intervention assessment point as outlined in the Assessment of heterogeneity section.
3.1 Use of adjusted, rather than raw, mean scores
A total of five trials reported adjusted, rather than raw, mean scores for outcomes measured on a continuous scale (Compas 2009; McCarty 2011; Mendelson 2010; Seligman 1999; Seligman 2007).
3.1.1 Depression diagnosis at the medium‐term follow‐up assessment
3.1.1.1 Targeted interventions
As none of the five trials that reported adjusted, rather than raw, mean scores evaluated the efficacy of a targeted intervention on depression diagnosis, we did not conduct sensitivity analyses.
3.1.1.2 Universal interventions
As none of the five trials that reported adjusted, rather than raw, mean scores evaluated the efficacy of a universal intervention on depression diagnosis, we did not conduct sensitivity analyses.
3.1.2 Self‐reported depression scores at the post‐intervention assessment
3.1.2.1 Targeted interventions
The omission of these trials resulted in no material change to either the magnitude or significance of the effect of targeted interventions on self‐rated depression scores at the post‐intervention assessment point.
3.1.2.2 Universal interventions
As none of the five trials that reported adjusted, rather than raw, mean scores evaluated the efficacy of a universal programme, we did not conduct sensitivity analyses.
3.2 Adequacy of allocation concealment
To assess the impact of allocation concealment adequacy, we carried out a sensitivity analysis to investigate the effect of excluding trials where allocation concealment had not been done or where this was unclear (i.e. those trials rated as high or unclear risk of bias for this item) and compared these to trials rated as low risk of bias. A total of 67 trials were rated as either unclear or high risk of bias for allocation concealment (Araya 2013; Arnarson 2009; Bella‐Awusah 2015; Cardemil 2002; Castellanos 2006; Chaplin 2006; Charbonneau 2012; Clarke 1993; Clarke 1995; Cova 2011‐Targeted; Cowell 2009; Dobson 2010; Ellis 2011; Fresco 2009; Gallegos 2008; Garber 2009; Garcia 2011; Gilham 1994‐Study 2; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Gillham 2007; Gillham 2012; Horowitz a2007; Horowitz b2007; Hyun 2005; Karami 2012; Khalsa 2012; Kowalenko 2005; Liehr 2010; Lillevoll 2014; Livheim 2014‐study 1(girls); Makarushka 2012; McLaughlin 2011; Mendelson 2010; Mirzamani 2012; O'Leary‐Barrett 2013; Pattison 2001; Petersen 1997; Pössel 2004; Pössel 2008; Pössel 2013; Puskar 2003; Quayle 2001; Reynolds 2011; Rivet‐Duval 2010; Roberts 2003; Roberts 2010; Rohde 2014a; Rohde 2014b; Rooney 2006; Rooney 2013; Rose 2014; Schmiege 2006; Seligman 1999; Seligman 2007; Sethi 2010; Shatte 1997; Snyder 2010; Spence 2003; Stice 2006; Stice 2008; Stoppelbein 2003; Wong 2014; Woods 2011; Young 2006; Young 2010a; Yu 2002‐study 3).
3.2.1 Depression diagnosis at the medium‐term follow‐up assessment
3.2.1.1 Targeted interventions
When these trials were omitted, the results for depressive diagnoses remained significant for targeted interventions at the medium‐term follow‐up.
3.2.1.2 Universal interventions
The omission of these trials also resulted in no material change to the effect of universal interventions on depression diagnosis at the medium‐term follow‐up.
3.2.2 Self‐reported depression scores at the post‐intervention assessment
3.2.2.1 Targeted interventions
Omitting these trials did not materially affect the significance or magnitude of the results for targeted interventions at the post‐intervention assessment.
3.2.2.2 Universal interventions
The omission of these trials caused the effect of universal programmes on self‐rated depression scores to become non‐significant at the post‐intervention assessment (SMD ‐0.04, 95% CI ‐0.12 to 0.04; k = 7; n = 4828).
3.3 Inclusion of participants with previous depression
A total of nine trials clearly stated that they included participants with previous depression (Clarke 1995; Clarke 2001; Compas 2009; Fresco 2009; Garber 2009; Roberts 2003; Seligman 1999; Stice 2008; Young 2006). We therefore conducted sensitivity analyses to investigate the impact of the inclusion of these trials on the pooled estimates of treatment efficacy.
3.3.1 Depression diagnosis at the medium‐term follow‐up assessment
3.3.1.1 Targeted interventions
At the medium‐term follow‐up the omission of these trials reduced the magnitude of the effect for targeted intervention programmes to non‐significance (SMD ‐0.02, 95% CI ‐0.06 to 0.02; k = 15; n = 3044).
3.3.1.2 Universal interventions
No trials that included participants with previous depression tested the impact of interventions for universal populations.
3.3.2 Self‐reported depression scores at the post‐intervention assessment
3.3.2.1 Targeted interventions
The omission of these trials did not result in any material change to the overall treatment effect for targeted programmes on self‐reported depression scores at the post‐intervention assessment.
3.3.2.2 Universal interventions
No trials that included participants with previous depression tested the impact of interventions for universal populations.
3.4 Depression diagnosis made from cut‐points
Thirty‐two trials reported data on diagnosis of a depressive disorder at the medium‐term assessment point. For 12 of these trials this was established by cut‐points on a self‐report rating scale (Cardemil 2002: CDI ≥ 30.0; Clarke 1993: CES‐D ≥ 24.0; Gilham 1994‐Study 2: CDI ≥ 15.0; Gillham, Reivich 2006b: CDI ≥ 19.0; O'Leary‐Barrett 2013: BSI: cut‐point not specified; Quayle 2001: CDI ≥ 13.0; Roberts 2003: CDI ≈ 15.0; Rose 2014: CDI ≥ 19.0; Shatte 1997: CDI ≥ 12.0; Stallard 2012a: SMFQ ≥ 5.0; Stice 2006: BDI ≥ 30.0; Yu 2002‐study 3: CDI ≥ 15.0).
3.2.1 Depression diagnosis at the medium‐term follow‐up assessment
3.2.1.1 Targeted interventions
The omission of these trials caused the treatment effect for targeted depression prevention programmes to become non‐significant (RD ‐0.04, 95% CI ‐0.08 to 0.00; k = 15; n = 2783).
3.2.1.2 Universal interventions
For universal interventions, however, the omission of these trials resulted in no material difference to either the magnitude or significance of the overall result.
4. Subgroup analyses
As we anticipated that there would be considerable heterogeneity between trials in this review, we planned to conduct several subgroup analyses at the outset on type of control condition (treatment as usual (TAU), wait‐list, attention placebo) and, for targeted interventions specifically, on the way in which the population were targeted for intervention (selected, indicated, combined). As outlined in the Subgroup analysis and investigation of heterogeneity section, we have only conducted these for the primary outcome measures of depression diagnoses at the medium‐term follow‐up and self‐rated depression scores at the post‐intervention assessment point.
4.1 Type of control condition
For both universal and targeted interventions we undertook a subgroup analysis to ascertain whether the type of control condition impacted on treatment efficacy.
4.1.1 Targeted interventions
Of the 53 trials that evaluated the efficacy of a targeted depression prevention programme, 20 compared the intervention to no form of alternative treatment (Castellanos 2006; Charbonneau 2012; Cova 2011‐Targeted; Cowell 2009; Ellis 2011; Fresco 2009; Gilham 1994‐Study 2; Gillham, Reivich 2006b; Hyun 2005; Mirzamani 2012; O'Leary‐Barrett 2013; Petersen 1997; Seligman 1999; Seligman 2007; Sethi 2010; Sheffield a2006; Sheffield b2006; Stice 2008; Wijnhoven 2014; Yu 2002‐study 3), a further 20 compared the intervention to treatment as usual (Arnarson 2009; Clarke 1995; Clarke 2001; Garber 2009; Gillham, Hamilton 2006a; Gillham 2012; Karami 2012; Kindt 2014; Livheim 2014‐study 1(girls); McCarty 2011; McLaughlin 2011; Puskar 2003; Roberts 2003; Rohde 2014a; Rohde 2014b; Stallard 2012a; Stoppelbein 2003; Woods 2011; Young 2006; Young 2010a), four compared the intervention to an attention placebo condition (Dobson 2010; Kauer 2012; Makarushka 2012; Schmiege 2006), two compared the intervention to another (undefined) condition (Compas 2009; McCarty 2013), and the remaining seven compared the intervention to a wait‐list control condition (Bella‐Awusah 2015; Fleming 2012; Jaycox 1994; Kowalenko 2005; Mendelson 2010; Noël 2013; Stice 2006).
4.1.1.1 Depression diagnosis at the medium‐term follow‐up
There was no evidence that the type of control group modified the overall efficacy (Chi² = 1.36; df = 3; P value = 0.71; I² = 0%) (Analysis 2.1).
2.1. Analysis.
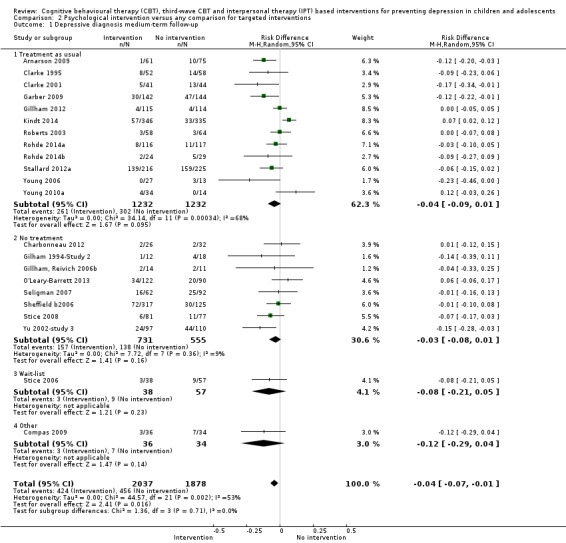
Comparison 2 Psychological intervention versus any comparison for targeted interventions, Outcome 1 Depressive diagnosis medium‐term follow‐up.
4.1.1.2 Self‐reported depression scores at the post‐intervention assessment
Again, there was no evidence that the type of control group modified the overall efficacy (Chi² = 6.96; df = 4; P value = 0.14; I² = 42.5%) (Analysis 2.2).
2.2. Analysis.
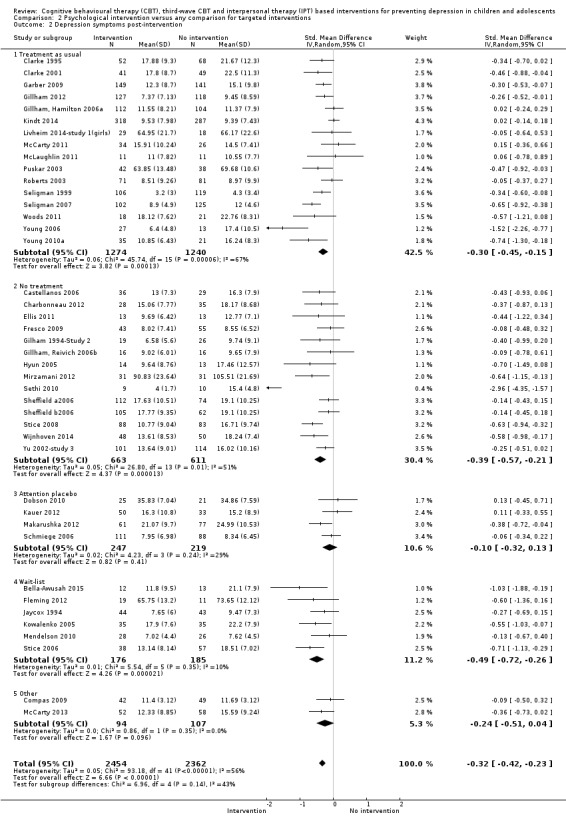
Comparison 2 Psychological intervention versus any comparison for targeted interventions, Outcome 2 Depression symptoms post‐intervention.
4.1.2 Universal interventions
Of the 33 trials that evaluated the efficacy of a universal depression prevention programme, nine compared the intervention against no form of alternative treatment (Cardemil 2002; Chaplin 2006; Gallegos 2008; Lillevoll 2014; Pössel 2004; Pössel 2008; Sawyer 2010; Sheffield c2006; Spence 2003), 11 compared the intervention to treatment as usual (Araya 2013; Clarke 1993; Horowitz a2007; Horowitz b2007; Khalsa 2012; Liehr 2010; Reynolds 2011; Roberts 2010; Rooney 2006; Rooney 2013; Wong 2014), nine compared the intervention against an attention placebo condition (Garcia 2011; Gillham 2007; Manicavasagar 2014; Merry 2004; Pattison 2001; Pössel 2013; Shatte 1997; Snyder 2010; Whittaker 2012), and four compared the intervention to a wait‐list control (Calear 2009; Quayle 2001; Rivet‐Duval 2010; Rose 2014).
4.1.2.1 Depression diagnosis at the medium‐term follow‐up
There was also no evidence that the type of control group modified the overall efficacy of universal depression prevention programmes (Chi² = 1.57; df = 3; P value = 0.67; I² = 0%) (Analysis 3.1).
3.1. Analysis.
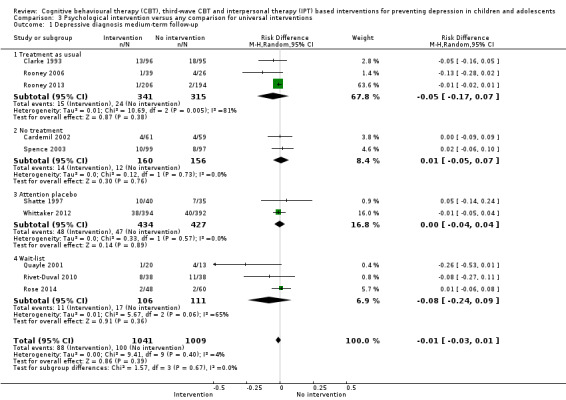
Comparison 3 Psychological intervention versus any comparison for universal interventions, Outcome 1 Depressive diagnosis medium‐term follow‐up.
4.1.2.2 Self‐reported depression scores at the post‐intervention assessment
There was also no evidence of a significant subgroup difference for these programmes by type of control group for self‐rated depression scores at post‐intervention assessment (Chi² = 5.99; df = 3; P value = 0.11; I² = 49.9%) (Analysis 3.2).
3.2. Analysis.
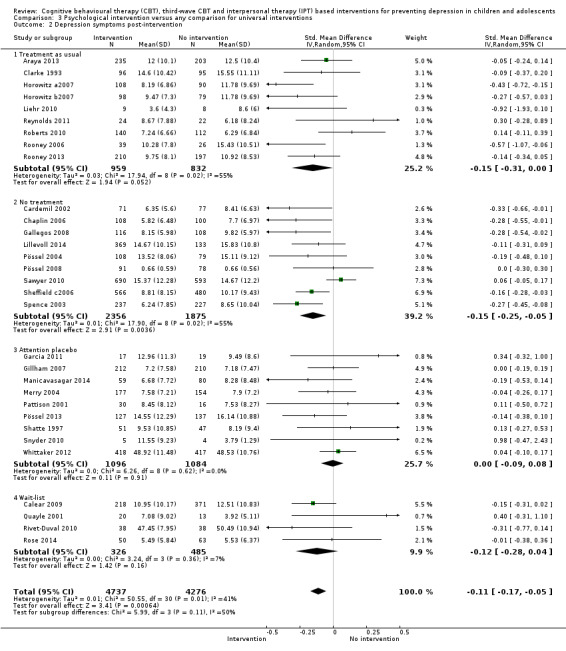
Comparison 3 Psychological intervention versus any comparison for universal interventions, Outcome 2 Depression symptoms post‐intervention.
4.3 Method of selecting targeted population (targeted interventions)
For the 53 trials that evaluated the efficacy of a targeted depression prevention programme, the majority (k = 36) were classified as indicated as inclusion was on the basis of elevated depression symptomatology (Arnarson 2009; Bella‐Awusah 2015; Clarke 1995; Charbonneau 2012; Cova 2011‐Targeted; Dobson 2010; Ellis 2011; Gilham 1994‐Study 2; Gillham, Hamilton 2006a; Gillham, Reivich 2006b; Gillham 2012; Kauer 2012; Kowalenko 2005; Livheim 2014‐study 1(girls); Makarushka 2012; McCarty 2011; McCarty 2013; McLaughlin 2011; Mirzamani 2012; Petersen 1997; Puskar 2003; Roberts 2003; Rohde 2014a; Rohde 2014b; Seligman 2007; Sethi 2010; Sheffield a2006; Sheffield b2006; Stallard 2012a; Stice 2006; Stice 2008; Stoppelbein 2003; Wijnhoven 2014; Woods 2011; Young 2006; Young 2010a). Twelve were classified as selected as inclusion in the trial was determined by the presence of some putative risk factor for depression (Castellanos 2006; Compas 2009; Cowell 2009; Fleming 2012; Fresco 2009; Hyun 2005; Karami 2012; Kindt 2014; Mendelson 2010; O'Leary‐Barrett 2013; Schmiege 2006; Seligman 1999). The remaining five were classified as combined as inclusion was on the basis of elevated depression score and presence of a putative risk factor for depression (Clarke 2001; Garber 2009; Noël 2013; Jaycox 1994; Yu 2002‐study 3).
4.3.1 Depression diagnosis at the medium‐term follow‐up
There was evidence that the efficacy of targeted depression prevention programmes may differ depending on the way in which the target population was selected (Chi² = 9.10; df = 2; P value = 0.01; I² = 78.0%). Both indicated (RD ‐0.03, 95% CI ‐0.06 to ‐0.01; k = 16; n = 2374) (Analysis 4.1) and combined (RD ‐0.14, 95% CI ‐0.21 to ‐0.07; k = 3; n = 578) (Analysis 4.1) programmes were associated with a small but significant treatment effect whereas selected approaches were not (Analysis 4.1).
4.1. Analysis.
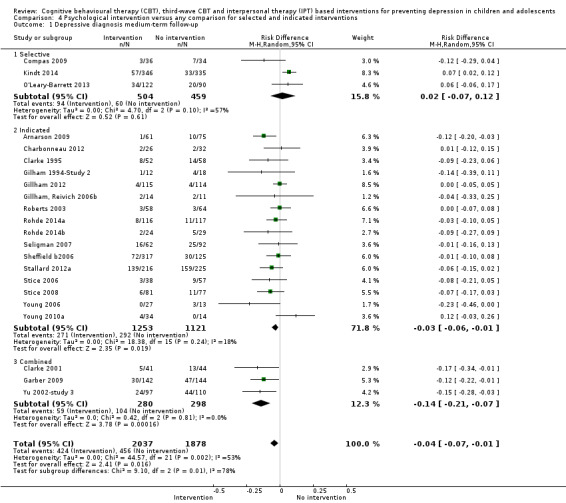
Comparison 4 Psychological intervention versus any comparison for selected and indicated interventions, Outcome 1 Depressive diagnosis medium‐term follow‐up.
4.3.2 Self‐reported depression scores at the post‐intervention assessment
However, for self‐rated depression scores at post‐intervention assessment, there was no evidence that the method of selecting the targeted population modified the overall treatment effect for these programmes (Chi² = 4.98; df = 2; P value = 0.08; I² = 59.9%) (Analysis 4.2).
4.2. Analysis.
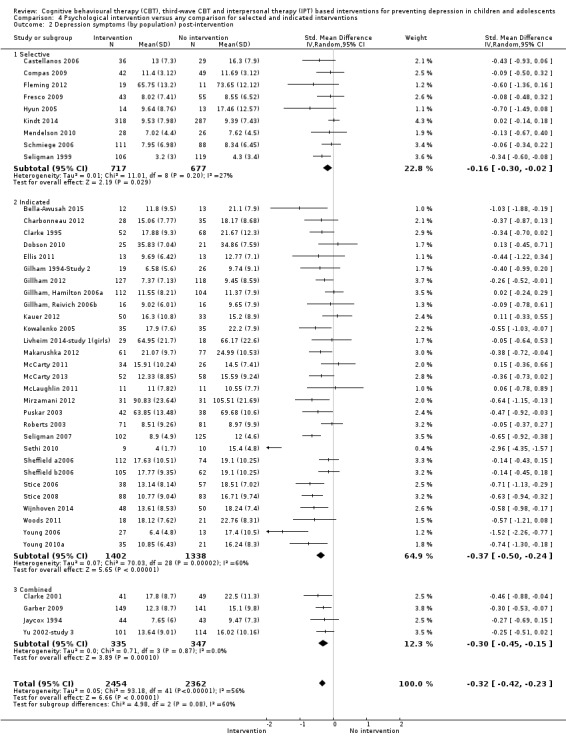
Comparison 4 Psychological intervention versus any comparison for selected and indicated interventions, Outcome 2 Depression symptoms (by population) post‐intervention.
5 Meta‐regression analyses
We investigated potential sources of clinical and methodological heterogeneity using meta‐regression for the primary outcome measures of self‐reported depression scores at the post‐intervention assessment and depression diagnosis at the medium‐term follow‐up, as outlined in the Subgroup analysis and investigation of heterogeneity section.
5.1 Targeted interventions
5.1.1 Depression diagnosis at the medium‐term follow‐up
There is some suggestion that baseline depression severity may modify the estimate of treatment efficacy for these interventions on depression diagnosis at the medium‐term assessment with the trial that included severely depressed participants at baseline indicating no evidence of a statistically significant treatment effect compared to those that included mildly or moderately depressed participants at baseline (Table 4; overall P value = 0.02). However, as only one targeted depression prevention programme included severely depressed children at baseline, spurious associations cannot be ruled out.
2. Univariate meta‐regression analyses for self‐reported depression diagnosis at the medium‐term assessment (targeted interventions).
| k | RR | (95% CI) | β | (95% CI) |
P value (moderator) |
Adjusted R2 (%) | I2 (Res) (%) | P value (overall) | |
| Overall effect | 22 | 0.82 | (0.68 to 0.99) | ‐0.20 | (‐0.40 to 0.01) | 0.06 | 0 | 37.0 | 0.04 |
| Continuous | |||||||||
| Intensity of intervention (hours) | 21 | — | — | ‐0.02 | (‐0.04 to 0.01) | 0.08 | 92.0 | 0.9 | 0.08 |
| Binary | |||||||||
| Focus of intervention | |||||||||
| CBT (reference) | 17 | 0.81 | (0.65 to 1.01) | — | — | — | 0 | 44.9 | 0.95 |
| CBT + IPT | 2 | 0.44 | (0.07 to 2.90) | ‐0.03 | (‐0.74 to 0.68) | 0.93 | — | — | — |
| IPT | 2 | 0.53 | (0.01 to 26.34) | ‐0.39 | (‐2.64 to 1.85) | 0.72 | — | — | — |
| Third wave | 1 | 1.23 | (0.19 to 8.15) | 0.44 | (‐1.71 to 2.59) | 0.67 | — | — | — |
| Depression severity at baseline | |||||||||
| Subthreshold (reference) | 10 | 1.01 | (0.81 to 1.27) | — | — | — | 99.0 | 0.5 | 0.02 |
| Mild | 8 | 0.57 | (0.43 to 0.77) | ‐0.52 | (‐0.86 to ‐0.17) | 0.01 | — | — | — |
| Moderate | 2 | 0.59 | (0.40 to 0.88) | ‐0.48 | (‐0.93 to ‐0.03) | 0.04 | — | — | — |
| Severe | 1 | 0.95 | (0.65 to 1.37) | ‐0.01 | (‐0.44 to 0.41) | 0.95 | — | — | — |
| Focus of CBT (for CBT studies only) | |||||||||
| CBT – cognitive and behavioural (reference) | 9 | 0.87 | (0.76 to 1.01) | — | — | — | 0 | 41.8 | 0.62 |
| CBT ‐ cognitive | 10 | 0.83 | (0.59 to 1.18) | 0.10 | (‐0.33 to 0.54) | 0.62 | |||
| CBT ‐ behavioural | 0 | — | — | — | — | — | — | — | — |
| Inclusion of relaxation component (for CBT studies only) | |||||||||
| No mention of relaxation component (reference) | 11 | 0.77 | (0.63 to 0.95) | — | — | — | 0 | 37.2 | 0.28 |
| Relaxation component described as included | 8 | 0.90 | (0.65 to 1.24) | 0.22 | (‐0.20 to 0.63) | 0.28 | — | — | — |
| Inclusion of problem‐solving skills training component (for CBT studies only) | |||||||||
| No mention of problem‐solving component (reference) | 11 | 0.77 | (0.55 to 1.08) | — | — | — | 0 | 41.8 | 0.99 |
| Problem‐solving component described as included | 8 | 0.86 | (0.74 to 1.01) | ‐0.01 | (‐0.43 to 0.43) | 0.99 | — | — | — |
| Inclusion of social skills training (for CBT studies only) | |||||||||
| No mention of social skills component (reference) | 9 | 0.70 | (0.54 to 0.91) | — | — | — | 11.0 | 32.9 | 0.13 |
| Social skills component described as included | 10 | 0.93 | (0.73 to 1.18) | 0.30 | (‐0.09 to 0.70) | 0.13 | — | — | — |
| Type of facilitator | |||||||||
| Mental health expert (reference group) | 9 | 0.64 | (0.45 to 0.90) | — | — | — | 0 | 21.2 | 0.12 |
| Students | 8 | 0.89 | (0.78 to 1.01) | 0.18 | (‐0.34 to 0.70) | 0.48 | — | — | — |
| Non‐mental health expert | 5 | 1.05 | (0.73 to 1.53) | 0.49 | (0.01 to 0.98) | 0.05 | — | — | — |
| Mode of delivery | |||||||||
| Face‐to‐face (group or individual) | 22 | 0.82 | (0.68 to 0.99) | ‐0.20 | (‐0.40 to 0.01) | 0.06 | 0 | 37.0 | 0.04 |
| Online/telephone | 0 | — | — | — | — | — | — | — | — |
k refers to number of trials.
CBT: cognitive behavioural therapy CI: confidence interval IPT: interpersonal therapy
There were no important differences in the magnitude of intervention effects on the basis of the intensity of intervention or of any of the other binary components of intervention (Table 4).
5.1.2 Self‐reported depression symptoms at the post‐intervention assessment
There was some suggestion that the focus of the intervention may modify the estimate of treatment efficacy, with interpersonal therapy (IPT) associated with greater reductions in self‐reported depression scores at post‐intervention than third wave approaches (Table 5; overall P value = 0.03).
3. Univariate meta‐regression analyses for self‐reported depression scores at the post‐intervention assessment (targeted interventions).
| k | SMD | (95% CI) | β | (95% CI) |
P value (moderator) |
Adjusted R2 (%) |
I2 (Res) (%) |
P value (overall) | |
| Overall effect | 42 | ‐0.32 | (‐0.42 to ‐0.23) | ‐0.33 | (‐0.44 to ‐0.22) | > 0.001 | 0 | 56.0 | > 0.001 |
| Continuous | |||||||||
| Intensity of intervention (hours) | 37 | — | — | 0.02 | (‐0.01 to 0.03) | 0.06 | 15.0 | 50.5 | 0.06 |
| Binary | |||||||||
| Focus of intervention | |||||||||
| CBT (reference) | 36 | ‐0.32 | (‐0.42 to ‐0.22) | — | — | — | 17.0 | 54.2 | 0.03 |
| CBT + IPT | 0 | — | — | — | — | — | — | — | — |
| IPT | 2 | ‐1.11 | (‐1.89 to ‐0.33) | ‐0.75 | (‐1.35 to ‐0.15) | 0.02 | — | — | — |
| Third wave | 4 | ‐0.10 | (‐0.35 to 0.15) | 0.21 | (‐0.16 to 0.59) | 0.26 | — | — | — |
| Depression severity at baseline | |||||||||
| Subthreshold (reference) | 15 | ‐0.20 | (‐0.33 to ‐0.07) | — | — | — | 12.0 | 56.0 | 0.20 |
| Mild | 10 | ‐0.51 | (‐0.69 to ‐0.33) | ‐0.31 | (‐0.60 to ‐0.02) | 0.03 | — | — | — |
| Moderate | 10 | ‐0.41 | (‐0.71 to ‐0.11) | ‐0.14 | (‐0.45 to 0.16) | 0.35 | — | — | — |
| Severe | 4 | ‐0.31 | (‐0.54 to ‐0.07) | ‐0.12 | (‐0.49 to 0.25) | 0.52 | — | — | — |
| Focus of CBT (for CBT studies only) | |||||||||
| CBT – cognitive and behavioural (reference) | 18 | ‐0.42 | (‐0.58 to ‐0.27) | — | — | — | 31.0 | 47.4 | 0.06 |
| CBT ‐ cognitive | 17 | ‐0.20 | (‐0.30 to ‐0.10) | 0.20 | (‐0.01 to 0.40) | 0.05 | — | — | — |
| CBT ‐ behavioural | 1 | ‐1.07 | (‐1.91 to ‐0.23) | ‐0.66 | (‐1.68 to 0.37) | 0.20 | — | — | — |
| Inclusion of relaxation component (for CBT studies only) | |||||||||
| No mention of relaxation component (reference) | 17 | ‐0.30 | (‐0.41 to‐0.91) | — | — | — | 0 | 57.2 | 0.93 |
| Relaxation component described as included | 18 | ‐0.33 | (‐0.50 to ‐0.17) | ‐0.01 | (‐0.24 to 0.22) | 0.93 | — | — | — |
| Inclusion of problem‐solving skills training component (for CBT studies only) | |||||||||
| No mention of problem‐solving component (reference) | 15 | ‐0.35 | (‐0.49 to 0.20) | — | — | — | 0 | 57.7 | 0.59 |
| Problem‐solving component described as included | 20 | ‐0.29 | (‐0.43 to ‐0.15) | 0.06 | (‐0.17 to 0.29) | 0.59 | — | — | — |
| Inclusion of social skills training component (for CBT studies only) | |||||||||
| No mention of social skills component (reference) | 13 | ‐0.40 | (‐0.54 to ‐0.27) | — | — | — | 13.0 | 52.5 | 0.19 |
| Social skills component described as included | 22 | ‐0.26 | (‐0.39 to ‐0.13) | 0.15 | (‐0.08 to 0.38) | 0.19 | |||
| Type of facilitator | |||||||||
| Mental health expert (reference group) | 20 | ‐0.39 | (‐0.52 to ‐0.26) | — | — | — | 30.0 | 43.5 | 0.08 |
| Students | 7 | ‐0.40 | (‐0.62 to ‐0.19) | ‐0.02 | (‐0.29 to 0.24) | 0.85 | — | — | — |
| Non‐mental health expert | 7 | ‐0.11 | (‐0.21 to ‐0.01) | 0.24 | (0.02 to 0.46) | 0.03 | — | — | — |
| Mode of delivery | |||||||||
| Face‐to‐face (group or individual) (reference group) | 36 | ‐0.32 | (‐0.42 to ‐0.23) | — | — | — | 0 | 59.2 | 0.87 |
| Online/telephone | 6 | ‐0.45 | (‐0.98 to ‐0.02) | ‐0.03 | (‐0.39 to 0.33) | 0.87 | — | — | — |
k refers to number of trials.
CBT: cognitive behavioural therapy CI: confidence interval IPT: interpersonal therapy
For other potential moderators, there were no important differences in the magnitude of intervention effects (see Table 5).
5.2 Universal interventions
5.2.1 Depression diagnosis at the medium‐term follow‐up
There were no material differences in the magnitude of intervention effects on the basis of the intensity of intervention or any of the other potential moderators investigated (see Table 6).
4. Univariate meta‐regression analyses for self‐reported depression diagnosis at the medium‐term assessment (universal interventions).
| k | RR | (95% CI) | β | (95% CI) |
P value (moderator) |
AdjustedR2 (%) |
I2 (Res) (%) |
P value (overall) | |
| Overall effect | 10 | 0.87 | (0.66 – 1.14) | ‐0.14 | (‐0.45 to 0.17) | 0.33 | 0 | 0 | 0.30 |
| Continuous | |||||||||
| Intensity of intervention (hours) | 9 | — | — | 0.02 | (‐0.04 to 0.08) | 0.38 | 0 | 0 | 0.38 |
| Binary | |||||||||
| Focus of intervention | |||||||||
| CBT (reference) | 7 | 0.92 | (0.64 to 1.31) | — | — | — | 0 | 0 | 0.76 |
| CBT + IPT | 2 | 0.79 | (0.38 to 1.64) | ‐0.16 | (‐1.13 to 0.80) | 0.70 | — | — | — |
| IPT | 0 | ||||||||
| Third wave | 1 | 0.72 | (0.37 to 1.38) | ‐0.26 | (‐1.14 to 0.62) | 0.51 | — | — | — |
| Depression severity at baseline | |||||||||
| Subthreshold (reference) | 7 | 0.90 | (0.65 to 1.23) | — | — | — | 0 | 0 | 0.73 |
| Mild | 3 | 0.77 | (0.37 to 1.58) | ‐0.11 | (‐0.81 to 0.59) | 0.73 | — | — | — |
| Moderate | 0 | — | — | — | — | — | — | — | — |
| Severe | 0 | — | — | — | — | — | — | — | — |
| Focus of CBT (for CBT studies only) | |||||||||
| CBT – cognitive and behavioural (reference) | 5 | 0.93 | (0.67 to 1.30) | — | — | — | 0 | 0 | 0.70 |
| CBT ‐ cognitive | 4 | 0.61 | (0.23 to 1.64) | ‐0.15 | (‐1.03 to 0.73) | 0.70 | — | — | — |
| CBT ‐ behavioural | 0 | — | — | — | — | — | — | — | — |
| Inclusion of relaxation component (for CBT studies only) | |||||||||
| No mention of relaxation component (reference) | 4 | 0.93 | (0.47 to 1.86) | — | — | — | 0 | 0 | 0.87 |
| Relaxation component described as included | 5 | 0.89 | (0.64 to 1.24) | ‐0.06 | (‐0.95 to 0.82) | 0.87 | — | — | — |
| Inclusion of problem‐solving skills training component (for CBT studies only) | |||||||||
| No mention of problem‐solving component (reference) | 2 | 0.26 | (0.05 to 1.30) | — | — | — | 0 | 0 | 0.17 |
| Problem‐solving component described as included | 7 | 0.94 | (0.70 to 1.28) | 1.27 | (‐0.68 to 3.23) | 0.17 | — | — | — |
| Inclusion of social skills training component (for CBT studies only) | |||||||||
| No mention of social skills component (reference) | 4 | 0.91 | (0.60 to 1.39) | — | — | — | 0 | 0 | 0.85 |
| Social skills component described as included | 5 | 0.87 | (0.53 to 1.43) | ‐0.06 | (‐0.81 to 0.68) | 0.85 | — | — | — |
| Type of facilitator | |||||||||
| Mental health expert (reference group) | 2 | 0.26 | (0.05 to 1.30) | — | — | — | 0 | 0 | 0.39 |
| Students | 3 | 0.68 | (0.22 to 2.04) | 0.97 | (‐1.36 to 3.30) | 0.35 | — | — | — |
| Non‐mental health expert | 4 | 0.90 | (0.61 to 1.32) | 1.22 | (‐0.83 to 3.27) | 0.19 | — | — | — |
| Mode of delivery | |||||||||
| Face‐to‐face (group or individual) | 9 | 0.82 | (0.57 to 1.16) | — | — | — | 0 | 0 | 0.62 |
| Online/telephone | 1 | 0.94 | (0.62 to 1.44) | 0.15 | (‐0.50 to 0.79) | 0.62 | — | — | — |
k refers to number of trials.
CBT: cognitive behavioural therapy CI: confidence interval IPT: interpersonal therapy
5.1.2 Self‐reported depression symptoms at the post‐intervention assessment
There was some suggestion that the intensity of the intervention may modify the estimate of treatment effectiveness for these interventions with those of longer duration (hours) associated with less efficacy (Table 7; overall P value > 0.001). There were no other material differences in the magnitude of the intervention effect on the basis of any of the other potential moderators investigated (see Table 7).
5. Univariate meta‐regression analyses for self‐reported depression scores at the post‐intervention assessment (universal interventions).
| k | SMD | (95% CI) | β | (95% CI) |
P value (moderator) |
AdjustedR2 (%) |
I2 (Res) (%) |
P value (overall) |
|
| Overall effect | 31 | ‐0.11 | (‐0.17 to ‐0.05) | ‐0.11 | (‐0.17 to ‐0.04) | >0.001 | 0 | 41.0 | > 0.001 |
| Continuous | |||||||||
| Intensity of intervention (hours) | 29 | — | — | 0.01 | (0.00 to 0.02) | > 0.001 | 68.0 | 18.0 | > 0.001 |
| Binary | |||||||||
| Focus of intervention | |||||||||
| CBT (reference) | 21 | ‐0.11 | (‐0.18 to ‐0.04) | — | — | — | 0 | 46.5 | 0.79 |
| CBT + IPT | 3 | ‐0.08 | (‐0.25 to 0.10) | 0.02 | (‐0.24 to 0.28) | 0.87 | — | — | — |
| IPT | 1 | ‐0.27 | (‐0.57 to 0.02) | ‐0.16 | (‐0.58 to 0.25) | 0.43 | — | — | — |
| Third wave | 6 | ‐0.01 | (‐0.31 to 0.30) | 0.07 | (‐0.19 to 0.33) | 0.57 | — | — | — |
| Depression severity at baseline | |||||||||
| Subthreshold (reference) | 25 | ‐0.11 | (‐0.18 to ‐0.04) | — | — | — | 0 | 45.9 | 0.62 |
| Mild | 5 | ‐0.06 | (‐0.26 to 0.14) | 0.05 | (‐0.16 to 0.27) | 0.62 | |||
| Moderate | 0 | — | — | — | — | — | — | — | — |
| Severe | 0 | — | — | — | — | — | — | — | — |
| Focus of CBT (for CBT studies only) | |||||||||
| CBT – cognitive and behavioural (reference) | 11 | ‐0.08 | (‐0.15 to ‐0.01) | — | — | — | 2.0 | 42.7 | 0.42 |
| CBT ‐ cognitive | 13 | ‐0.14 | (‐0.24 to ‐0.03) | ‐0.05 | (‐0.19 to 0.08) | 0.42 | |||
| CBT ‐ behavioural | 0 | — | — | — | — | — | — | — | — |
| Inclusion of relaxation component (for CBT studies only) | |||||||||
| No mention of relaxation component (reference) | 11 | ‐0.13 | (‐0.23 to ‐0.04) | — | — | — | 9.0 | 40.8 | 0.45 |
| Relaxation component described as included | 13 | ‐0.08 | (‐0.16 to ‐0.01) | 0.05 | (‐0.09 to 0.19) | 0.45 | — | — | — |
| Inclusion of problem‐solving skills training component (for CBT studies only) | |||||||||
| No mention of problem‐solving component (reference) | 6 | ‐0.20 | (‐0.34 to ‐0.07) | — | — | — | 14.0 | 40.4 | 0.13 |
| Problem‐solving component described as included | 18 | ‐0.08 | (‐0.15 to ‐0.01) | 0.12 | (‐0.04 to 0.28) | 0.13 | — | — | — |
| Inclusion of social skills training component (for CBT studies only) | |||||||||
| No mention of social skills component (reference) | 8 | ‐0.18 | (‐0.29 to ‐0.07) | — | — | — | 13.0 | 39.5 | 0.11 |
| Social skills component described as included | 16 | ‐0.06 | (‐0.13 to 0.01) | 0.11 | (‐0.03 to 0.24) | 0.11 | |||
| Type of facilitator | |||||||||
| Mental health expert (reference group) | 11 | ‐0.11 | (‐0.23 to 0.02) | — | — | — | 0 | 48.0 | 0.57 |
| Students | 6 | ‐0.21 | (‐0.38 to ‐0.05) | ‐0.10 | (‐0.35 to 0.14) | 0.38 | — | — | — |
| Non‐mental health expert | 8 | ‐0.09 | (‐0.22 to 0.03) | 0.01 | (‐0.19 to 0.22) | 0.88 | — | — | — |
| Mode of delivery | |||||||||
| Face‐to‐face (group or individual) | 27 | ‐0.11 | (‐0.19 to ‐0.04) | — | — | — | 0 | 43.9 | 0.76 |
| Online/telephone | 4 | ‐0.07 | (‐0.18 to 0.03) | 0.03 | (‐0.15 to 0.20) | 0.76 | — | — | — |
k refers to number of trials.
CBT: cognitive behavioural therapy CI: confidence interval IPT: interpersonal therapy
6 Self‐rated versus clinician‐rated outcome
While we could not formally investigate whether ratings made by clinicians or participants impacted on the results, we have extracted data from trials that measured depression using both self‐rated and clinician‐rated measurement tools and carried out a meta‐analysis that only provides subtotals. Where data were available, the effect sizes for depression rated by the participant (self‐rated) were consistently larger than the clinician‐rated outcome (Analysis 5.1; Analysis 5.2; Analysis 5.3).
5.1. Analysis.
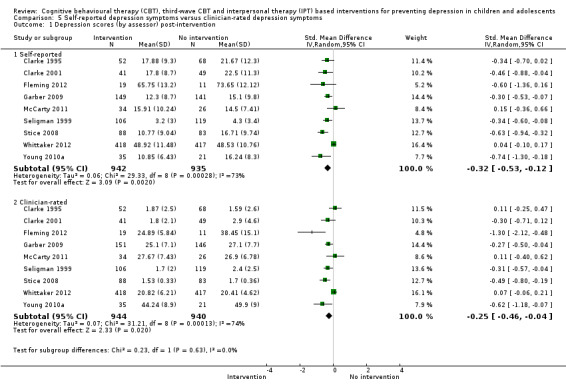
Comparison 5 Self‐reported depression symptoms versus clinician‐rated depression symptoms, Outcome 1 Depression scores (by assessor) post‐intervention.
5.2. Analysis.
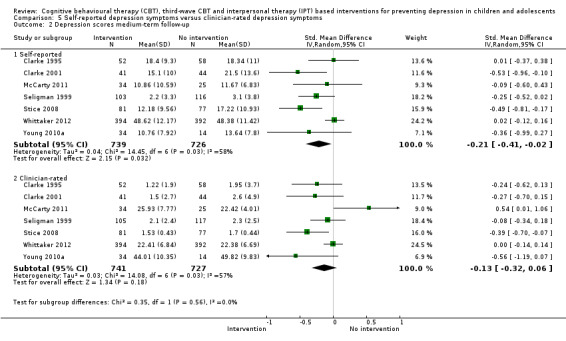
Comparison 5 Self‐reported depression symptoms versus clinician‐rated depression symptoms, Outcome 2 Depression scores medium‐term follow‐up.
5.3. Analysis.

Comparison 5 Self‐reported depression symptoms versus clinician‐rated depression symptoms, Outcome 3 Depression scores long‐term follow‐up.
7 Publication bias
The presence and impact of publication bias is minimised in the present review through the use of an exhaustive systematic review procedure and ongoing contact with a large number of trial authors in this field. Nevertheless, we inspected funnel plots for the primary outcomes measures to assess the likely presence of publication bias.
There was no evidence of major funnel plot asymmetry for either primary outcome measure (see Figure 6 and Figure 7). However, it should be noted that both RDs and SMDs are naturally correlated with their respective standard errors and therefore this can result in spurious funnel plot asymmetry (see Higgins 2008b, section 10.4.3).
6.
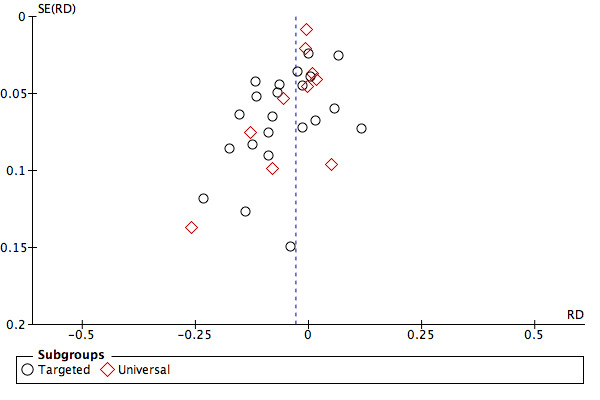
Funnel plot of analysis 1.4: Psychological intervention versus any comparison post‐intervention for depressive disorder at the medium‐term follow‐up.
7.
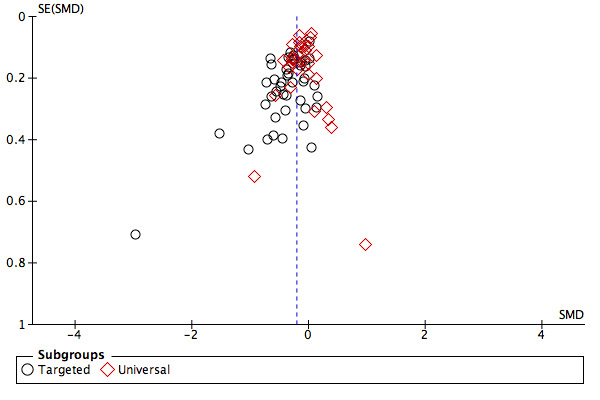
Funnel plot of analysis 1.6: Psychological intervention versus any comparison post‐intervention for depression scores at the post‐intervention assessment.
Discussion
Summary of main results
In this more specific review we attempted to focus on studies likely to be more homogeneous in their approach and findings, and to identify some of the factors that might inform attempts at implementation. The studies were all of cognitive behavioural therapy (CBT), interpersonal therapy (IPT) or third wave CBT approaches, and the majority were conducted in schools and delivered to groups.
The overall results again showed small positive benefits in terms of depression prevention, for both the primary outcomes of self‐rated depressive symptoms post‐intervention and depression diagnosis up to 12 months (but not beyond). The results for the secondary outcomes, including anxiety and general functioning, were broadly consistent with these findings, again with little effect seen beyond 12 months; however it should be noted that for anxiety and functioning there were limited data. Estimates of numbers needed to treat to benefit compare well with other public health interventions. However, which programmes should be implemented, and how, is not clear. In addition, the evidence was of moderate to low quality using the GRADE framework and the results were heterogeneous.
A key consideration in the field is whether to provide interventions to universal or targeted populations. There were significantly larger effects for targeted programmes in terms of reduced depression symptom severity but not for reduction in depressive disorder. A larger effect for indicated targeted interventions is, of course, to be expected because of the higher levels of depression at the start of the intervention. However, the evidence from this review is unclear with regard to whether the type of population modified the overall effects, with significant differences when comparing targeted and universal interventions for depression symptoms but not for depressive disorder. In terms of the practical implementation of programmes, we therefore summarise the results separately for universal and targeted interventions:
For universal interventions there was no evidence of an effect:
in reduction of depressive disorder at medium‐term follow‐up (primary outcome) or at other time points (post‐intervention assessment, or at short‐ medium‐ or long‐term follow‐up);
in reduction of depression symptoms beyond post‐intervention assessment (primary outcome) (i.e. at short‐, medium‐ or long‐term follow‐up).
For targeted approaches there is evidence of an effect:
in reduction of depressive disorder at medium‐term follow‐up (primary outcome), post‐intervention assessment and short‐term follow‐up, but not at long‐term follow‐up;
in reduction of depression symptoms at post‐intervention assessment (primary outcome) and at short‐ and medium‐term but not long‐term follow‐up.
However, when clinicians rated the effect on depression symptoms for targeted interventions (data were available at post‐intervention assessment, and medium‐ and long‐term follow‐up), rather than participants themselves, there was little evidence of an effect beyond post‐intervention assessment.
The issue of the lack of attention placebo comparisons is important. Although statistical analyses did not show a moderation effect by comparison group for either of our primary outcomes, this may reflect differences in comparison groups. There were a number of studies of universal interventions with an attention placebo control group and the analyses showed a lack of heterogeneity and a complete lack of effect. There were far fewer trials of targeted interventions using an attention placebo condition and none for our primary outcome of depressive disorder. There were trials using an attention placebo control for our other primary outcome, depression symptoms, and again this individual subgroup showed no effect.
Within the targeted studies our analysis generates an interesting hypothesis that interventions targeted to those with elevated symptoms, rather than risk alone, will result in larger effects but, again, the lack of attention placebo control comparisons for these studies should be noted.
Effect size in comparison with the previous version of this review
Overall the risk of a diagnosis of depression was reduced from 19.3% to 16.2%. The effect size for reduction in a diagnosis of depression overall at medium‐term follow‐up translates to a number needed to treat to benefit (NNTB) of 33 (22 to 148). These results are different from the NNTB of 11 shown in the previous version of this review (Merry 2011). However, a recent review of aspirin to prevent cardiovascular events reported that the NNTB to prevent one major cardiovascular event over a mean follow‐up of 6.8 years was 284 and the NNTB to prevent one stroke over 6.8 years was 614 (Xie 2014). For antihypertensives for those with hyper‐ or pre‐hypertensive blood pressure the NNTB to prevent one stroke over a median of 4.3 years was 169(Sipahi 2012). In both cases, the conclusions of the respective studies were that aspirin and antihypertensives were of benefit. Given the burden of depression, prevention programmes could still be a worthwhile investment in public health terms, particularly those aimed at targeted populations. The skills taught in these programmes are also useful life skills and have applicability throughout the life course for a range of psychological problems. However, of the trials that measured depression diagnosis, only two used an attention placebo comparison and, consistent with the findings for depressive symptoms, there was no effect of the intervention in these trials.
Following the large trial conducted by Stallard’s team (Stallard 2012a), there has been concern that these interventions may do harm because the group that received the classroom‐based CBT had more negative thinking at 12 months follow‐up than those in the usual school provision arm. As is often the case with trials of psychotherapy, little attention has been paid to this possibility (Berk 2009). There is no evidence from the studies to date that these interventions cause worsening symptoms.
Issue of attention placebo comparisons
As we have noted in previous versions of this review (Merry 2004b; Merry 2011), few trials have compared the intervention with an attention placebo. The placebo effect is high in studies of depression (Howick 2013). The placebo‐controlled trials in this review showed no evidence of effect. The significant effects were in those studies that compared intervention to treatment as usual, wait‐list and 'other' comparison groups. There is also significant heterogeneity, except in the attention placebo subgroup analyses where heterogeneity is low. This all points to the worrying concern that the apparent effects of prevention may be placebo. Addressing this possibility is a priority for future studies.
Presence of previous depression
It is also important to consider whether the presence of previous episodes of depression modifies the treatment effect. Most trials do not consider whether participants have already had an episode of depressive disorder. From our previous work, the most effective trial reported was the targeted programme by Clarke 2001, where the initial effect size equalled ‐0.46, resulting in a risk difference of ‐0.22 and a NNTB of five (Merry 2011). Effects in this trial persisted to 12 months with an effect size of ‐0.53, a risk difference of ‐0.17 and a NNTB of six. This finding was replicated by Garber 2009, with a NNTB of nine. The design of the trials by Clarke 2001 and Garber 2009 included populations selected on the basis of elevated symptoms and risk. Garber 2009 found that around 55% of participants had a lifetime episode of depressive episode prior to the intervention meaning that prevention had effectively been combined with relapse prevention in this trial. We therefore conducted sensitivity analyses and found that the small effects on depression diagnosis were reduced to non‐significance following the omission of those trials in which participants had had a previous episode of depressive disorder. The effects that we are seeing may therefore represent early intervention or even relapse prevention effects, a possibility supported by the fact that participants' baseline severity of depression in many of the included trials indicated at least mild symptoms of depression. This may not matter in practice. The majority of young people with a depressive disorder do not receive professional help from clinical services (Fergusson 1993; Fergusson 2001; Merikangas 2011; Patton 2007). Interventions rolled out in settings such as school may therefore help to address this unmet need.
What to implement?
Scalability of these interventions is an important consideration. Many programmes designed for scalability have failed to show an effect (e.g. Araya 2013; Spence 2003; Stallard 2012a), while, for example, the two‐stage screening used by Clarke 2001 and Garber 2009 would be difficult to implement in a community‐based setting.
Various technology advances offer promise with regard to rolling out efficacious interventions with fidelity. Already there are a number of preventive interventions that have been delivered via the internet. However, the potential for poor adherence with interventions delivered online and the challenges of delivering software over multiple devices and platforms must also be considered (Christensen 2009). In this review, one of the included trials of an online programme (MoodGYM) found that only 8.54% of those allocated to the intervention actually logged into the service. Innovative approaches such as the incorporation of gaming elements (Fleming 2014) and social networking (Rice 2014) may increase engagement and adherence. However, even with adequate adherence to either face‐to‐face or online interventions, attending/completing sessions does not necessarily mean that a young person is acquiring or mastering the skills taught. Few trials of psychological treatments or interventions have thus far examined the potential mediators of benefit on depressive symptoms (Weersing 2009).
Of interest to us was a novel intervention approach tested in two of the included trials (Castellanos 2006; O'Leary‐Barrett 2013). In these trials a CBT‐based intervention was used but was adapted to particular personality factors that defined four high‐risk groups (hopelessness, impulsive, sensation seeking and anxiety sensitive). Due to the strict inclusion criteria for this review we could only include data for the participants considered at risk of depression. However, the intervention reduced depression scores in all four high‐risk groups, suggesting that effects were not specific. This suggests that there is room for innovation in terms of targeting prevention programmes in this way to prevent depression. Depressed populations are heterogeneous for risk factors and precipitating events that may have increased the risk of developing depression, suggesting that it may be unrealistic to expect that a single intervention approach would be beneficial for everyone.
In this update of the review we have taken a more targeted approach to try to identify the most efficacious approaches to depression prevention. We showed that neither the mode of delivery (i.e. face‐to‐face including group or individual combined versus online/telephone) nor the type of facilitator who delivered the intervention had any material impact on the magnitude of the overall treatment effect. In future updates it may be important to consider the impact of group compared with individually delivered interventions, bearing in mind the implications in terms of scalability. IPT interventions had the largest effect sizes but there were very few trials of IPT, and the trial by Horowitz 2007 showed no difference between CBT and IPT (Horowitz a2007; Horowitz b2007). The vast majority of included trials employ a CBT‐based intervention, with few trials of IPT or third wave CBT. This is also true of the treatment trials for depression in young people (Callahan 2012), and is surprising given that IPT is recommended in guidelines (McDermott 2011; NICE 2005). Overall, given the paucity of trials of different approaches, it is challenging to conclude which is the most promising approach to roll out in a public health intervention. Our findings suggest that further studies of IPT‐based preventive interventions would be worthwhile.
Meta‐regression analyses showed little reduction in the risk of a diagnosis of depression for those with more severe depressive symptoms at baseline. The magnitude of intervention effects for depression symptoms in universal trials was modified by the intensity of intervention (hours) with longer interventions associated with smaller effect sizes. We also visually inspected the effect sizes of self‐rated and clinician‐rated depression in those trials that reported both and at each time point the effect sizes for self‐rated depression were larger. Such factors should be tested in further trials.
Overall completeness and applicability of evidence
Generally the data in the trials were clearly reported or authors were happy to provide us with extra data, or both.
Of the 83 trials included in this review, only 36 had data for the primary outcome of depression diagnosis at medium‐term follow‐up assessment, and there were even fewer trials that provided data for the long‐term follow‐up assessment (> 12 months). Given that the presence of a depressive disorder has been most robustly linked to disability and cost (Fergusson 2007; Gore 2011; Murray 1997), it is the prevention of depressive disorder, with its resulting morbidity and mortality, that is critical. Two trials did measure very long‐term outcomes at six and four years respectively (Garber 2009; Spence 2003). Although these results were not included in this review, it is promising to note that in the case of Garber 2009 (as referred to in Brent 2015) the positive impact of the depression prevention programme on incidents of depressive disorder was maintained at the six‐year follow‐up. This is in contrast to Spence 2003, who did not find an effect. Measuring depressive disorder over longer periods of follow‐up is important given that the incident rate climbs during adolescence and into young adulthood Kessler 2005 and many of the interventions in this review are initially delivered in children or younger adolescents.
As discussed above, there are a much larger number of trials that use a credible attention placebo and little difference is shown between intervention and placebo. However, the majority of these were trials in universal populations and so it is harder to ensure that the findings from trials in targeted populations are not the result of the attention itself. The changes post‐intervention could clearly represent a placebo effect. As the effects persist for up to 12 months, a placebo effect may be less likely, especially as nearly all the interventions are completed in 12 weeks or less. Well‐designed, large trials of targeted interventions with an attention control group, particularly those that measure depression diagnosis at longer‐term follow‐up, are needed before we can settle this question.
It was pleasing to be able to include one trial undertaken in a low‐ to middle‐income country (Nigeria; Bella‐Awusah 2015). In screening the results of our search, we noticed a number of trials undertaken in low‐ and middle‐income countries, most of which we had to exclude; for example, a trial of a life‐skills programme for those at risk of suicide undertaken in Cambodia (Jegannathan 2014). Interventions tested in high‐income and Western countries may not be suitable or relevant for low‐ and middle‐income countries (Carnevale 2012), but it is also the case that cost‐effective school and community‐based interventions are likely to be more relevant in these countries that have large services gaps for mental health provision (Patel 2007a; Patel 2007b). A recent systematic review examined the degree to which the small but positive effects of trials in universal depression interventions could be implemented in real world practice and highlighted a lack of generalisability given that all trials were conducted in high‐income countries (Carnevale 2012).
We were unable to examine the efficacy of trials that incorporated cultural adaptations, but this would be critical in determining the nature of public health interventions that should be implemented.
Quality of the evidence
There are some fundamental issues that limit our confidence in the findings from these trials.
We rated less than half of the trials included in this review as low risk of bias for random sequence generation or allocation concealment, suggesting that selection bias may be high. Sensitivity analyses excluding trials at high or unclear risk of allocation concealment bias resulted in no material change to outcomes, except that the effect for universal programmes on self‐rated depression scores was reduced to non‐significance at the post‐intervention assessment.
Due to the nature of the intervention and comparison conditions, performance and detection bias (for self‐reported depression symptoms) is likely to be high in the majority of trials given that blinding of participants in most trials was not possible and that most trials relied on participant self‐report depression symptoms as the outcome. For the primary outcome of depressive disorder at the medium‐term follow‐up assessment, data were determined by diagnostic assessment in around two‐thirds of the included trials, of which we considered only a quarter as low risk of bias for this item. A diagnosis of depressive disorder was estimated by a cut‐point on a self‐rated depression symptom rating scale in the remaining trials and, given the range of tools and cut‐points measured, with little clear literature to guide comparisons across tools and measures, there is some concern about how meaningful a cut‐point on a self‐rated scale is as a proxy measure for depression diagnosis (Stockings 2015).
We rated over half of the trials included in this review as high or unclear risk of bias for the domain of incomplete outcome data and we rated very few trials as low risk of bias for the domain of selective reporting. We were also only able to obtain trial protocols for 25.3% of trials and only eight trials had low risk of selective reporting.
In terms of other risks of bias, most trials were conducted by those that developed the intervention and therefore we rated these trials as high risk of bias for this item. Very few trials assessed intervention integrity adequately and few trials systematically reported on participant adherence to the planned intervention.
In summary, there are a number of serious short‐comings in the studies.
Potential biases in the review process
Several of the review authors (SM and JB) are involved in two of the trials on adolescent depression prevention included in the current review (Merry 2004; Whittaker 2012). However, the structure of the review, with multiple authors and reviewers, is likely to have protected against biased reporting or reviewing of these trials.
We did not systematically extract data on adverse outcomes; these are seldom measured in these types of trials. In future updates we will include systematic extraction and analysis of these data.
Agreements and disagreements with other studies or reviews
This review used an extensive search strategy resulting in more studies than those uncovered by other recent reviews and it includes only randomised controlled trials (RCTs) that use evidence‐based psychological treatments for depression with a primary focus on preventing depression. Our analyses have taken into account the cluster‐randomised design employed in many of the trials and ours is the largest review to date that has formally explored whether various factors such as how the population was selected for intervention (targeted versus universal), the comparison group (no treatment (NT), wait‐list (WL), treatment as usual (TAU), attention placebo (AP)), baseline severity of depression, and the intervention approach and techniques modify the magnitude of the overall intervention effects.
Findings with regard to depression symptoms are consistent with a number of previous reviews (Gladstone 2009; Horowitz 2006; Merry 2004b; Spence 2007), including a recently published review (Corrieri 2014). While reviews have consistently shown evidence that depression prevention interventions lead to reduction in symptoms, earlier reviews were cautious in their conclusions because few trials measured the impact on depression diagnosis. Our previous update highlighted a continuing paucity of studies that measured depression diagnosis. We now have 36 trials that measured diagnosis of a depressive disorder, or a proxy based on cut‐off scores at medium‐term follow‐up showing significant effects, consistent with two meta‐analyses of trials in participants older than in our review, which also showed a reduction in depression diagnosis (Cuijpers 2008; Stice 2009). However, in this review we have carefully considered the issue of the type of comparison group and note that there is no effect in trials in universal populations compared with attention placebo comparisons and there are no trials of interventions delivered to targeted populations that have used an attention placebo comparison.
A number of reviews employing a variety of methods (meta‐analyses, narrative reviews) have shown that interventions delivered to targeted populations have a greater effect than those delivered to universal populations (Gladstone 2009; Gladstone 2011; Horowitz 2006; Stice 2009), although statistical testing of the modification of results by the type of population (targeted versus universal) has mostly not been done. We found evidence that the type of population modified the results for depression symptoms but not for depressive disorder. Two previous reviews have shown that while the effect sizes for targeted interventions were generally larger, the type of population did not significantly impact on the overall results (Cuijpers 2008; Jane‐Llopis 2003). In these studies little consideration was given to the type of control group. There have been few investigations of the impact of indicated targeted approaches compared to selective targeted approaches. Our findings that selective approaches are less effective than indicated approaches are in contrast to those of Cuijpers 2008, who found no differences and Horowitz 2006, who found that selected, but not indicated, trials were significantly different from universal trials.
We included additional analyses of potential modifiers of treatment effect. We have shown that the length of intervention significantly modifies the magnitude of treatment effect in some analyses, with longer interventions less effective in reducing symptoms in universal interventions. Findings from other analyses are contradictory with longer interventions associated with larger effect sizes (Jane‐Llopis 2003), and shorter interventions associated with larger effects (Stice 2009).
Our findings, which show that who delivered the intervention did not moderate outcome, are consistent with the findings of Stice 2009 but different from those of Jane‐Llopis 2003, who showed that delivery by healthcare professionals produced larger effects.
Our finding that IPT approaches have the largest effect (albeit only tested in two trials) was also found by Cuijpers 2008, albeit that IPT was only tested in two trials.
Finally, we investigated whether the mode of delivery modified the overall treatment effects, given the increasing popularity of internet and other new media means to deliver interventions. A recent narrative review that included eight RCTs and non‐randomised trials of four internet‐based treatment programmes for anxiety or depression highlighted that these interventions showed promise (Calear 2010). Our analysis shows that the mode of delivery does not modify the overall intervention effects, although there were a small number of trials classified as online or phone‐based, and there are potentially issues with adherence and engagement (Christensen 2009).
Authors' conclusions
Implications for practice.
There is still not enough evidence to support the implementation of depression prevention programmes. While depression prevention programmes overall are associated with a reduction in depression diagnosis and depressive symptoms at up to 12 months follow‐up, with promising numbers needed to treat to benefit (NNTBs), prevention programmes delivered to universal populations have a sobering lack of effect when compared with an attention placebo control. Interventions delivered to targeted populations, particularly those selected on the basis of depression symptoms, have larger effect sizes, but these studies have seldom used an attention placebo comparison. Further, there are practical difficulties inherent in the implementation of a targeted programme.
Where this has been investigated, trials in this review include participants with a past history of depressive episodes, so the effects being seen may be more properly considered to include treatment or relapse prevention rather than primary prevention. This is probably not of practical importance, given that the majority of young people with depressive disorder do not receive any intervention and are likely to benefit from the interventions. A real world reduction in diagnosis of a depressive disorder, be it primary prevention, treatment or relapse prevention, would be a worthwhile achievement given the burden of depression.
Implications for research.
Are we there yet? Disappointingly no, despite the number of studies. The results from this review show that for approaches based on cognitive behavioural therapy (CBT) and interpersonal therapy (IPT), there is a relative lack of evidence for universal interventions compared with attention placebo controls and well‐conducted effectiveness trials of universal interventions have shown no evidence of effect (Araya 2013; Stallard 2012a). Targeted approaches appear the most likely to succeed, notwithstanding Rose's maxim that this approach is likely to miss a larger proportion of those at risk of depression than a targeted approach is likely to find (Rose 1992). However, this review has revealed a gap in the knowledge base in that depression prevention programmes have not been tested in targeted populations in trials with an appropriate attention placebo comparison group. We believe that depression prevention programmes should be tested in an indicated targeted population using an attention placebo group. CBT, and particularly IPT, approaches are worth pursuing; however, it may be worth thinking more widely about different approaches to tackling this important problem, such as approaches targeting personality risk factors, for example. We did not investigate age as a modifier, but it is likely that consideration of the age of onset is important in designing trials; it is possible that preventive interventions earlier in life may be efficacious in preventing depression during adolescence (Rapee 2013), and it is important to measure the long‐term outcomes of any prevention programme that is studied, preferably into early adulthood. Intensity of intervention was not found to be a significant modifier of intervention effect for targeted approaches and this requires further investigation.
Further, methodological weaknesses identified in this review should be addressed in future research including:
measurement of depressive disorder as a primary outcome and over the long term (at least 12 months or more);
use of a clinician‐rated measure, as well as self‐rated measures;
consideration of scalability; and
-
consideration of the potential to do harm, such as:
increasing depressive symptoms;
increasing negative cognitions; and
the potential for increasing self‐harm and or suicidal thoughts/behaviours.
The estimates of numbers needed to treat to benefit and the cost of depression to society mean that this is worth further study.
What's new
| Date | Event | Description |
|---|---|---|
| 1 August 2016 | New citation required and conclusions have changed | Some conclusions have changed due to the inclusion of new studies in the analysis. |
| 1 August 2016 | New search has been performed | New update to the review completed |
History
Protocol first published: Issue 3, 2001 Review first published: Issue 1, 2004
| Date | Event | Description |
|---|---|---|
| 11 September 2015 | New search has been performed | Updated search to September 2015 and new studies added |
| 29 March 2011 | New search has been performed | Updated search and new studies added. |
| 15 January 2011 | New citation required and conclusions have changed | The conclusions have changed due to the inclusion of new studies in the analysis. |
| 8 May 2010 | Amended | Converted to new review format. |
| 23 July 2008 | New citation required and conclusions have changed | Substantive amendment. |
Notes
During the course of this review update the authors have recognised that the review topic might now be better addressed in a series of separate intervention‐specific reviews, including, but not limited to, a review of psychoeducation and education programmes for preventing depression in children and adolescents, a review of prevention trials undertaken in the aftermath of trauma and a review of trials where the primary aim is the prevention of anxiety.
Acknowledgements
There have been a number of people who have made valuable contributions to this review since the first version was prepared. The advice of Prof Philip Hazell is gratefully acknowledged. The 2002 Review Completion Workshop run at the Cochrane Centre in Melbourne was extremely helpful and we would like to thank Dr Sally Green and the centre staff for organising this. We would like to thank Jane Dennis (the previous Managing Editor of CCDAN) and Jessica Sharp (current Managing Editor of CCMD) and other CCMD group staff and editors who have advised us on many aspects of the review over the years. We would like to thank Sarah Dawson (Cochrane Information Specialist) for updating and running the searches for the previous and current version of the review, as well as helping with screening. We have had help from various people with screening and data extraction for the previous and current version of the review including Matt Gillard, Magenta Simmons, Gemma Colhoun, Alysha Simonson, Kate Lovey and Amy Hamington, who contributed to data extraction. For translation of non‐English language papers for this current version of the review we are grateful to Sam Irving, Yusuke Ogawa, Farhad Shokraneh, Katy Sivyer and Abel Toledano. For providing us with pre‐published data, we are grateful to Tolulope Bella‐Awusah.
Nellie Muller contributed to the drafting of the original published protocol but is no longer available to assist with writing the review. Heather McDowell contributed to the original and first update of the review, and Tessa Brudevold‐Iversen to the first update of the review, but they are no longer available to assist. We are very grateful to them as co‐authors of the previous versions.
Appendices
Appendix 1. Hierarchy of outcome measures
Most studies used several depression rating scales or diagnostic interviews as outcome measures. For the purposes of pooling results to obtain an aggregate outcome, a single 'best available' outcome measure was chosen for each study. The order of selection was determined by the rating of each instrument over the following five criteria: appropriateness to children and adolescents; reliability; construct validity; agreement with clinical interview; track record in psychopharmacological research. Most of the data for this rating were obtained from a review by Petti (Petti 1985).
The hierarchy of selection for analysis, and the number of criteria met by each rating scale (in parentheses), were as follows:
Schedule for Affective Disorders and Schizophrenia for School‐Age Children (Kiddie‐SADS), combined child and parent report;
Children's Depression Rating Scale (CDRS);
Bellevue Index of Depression (BID);
Children's Depression Inventory (CDI);
Hamilton Depression Rating Scale (HAM‐D);
Depressive Adjective Checklist (DACL).
Appendix 2. Cochrane Specialised Register ‐ core MEDLINE search strategy
Core search strategy used to inform the Cochrane Common Mental Disorders Group's specialised register: OVID Medline A weekly search alert based on condition + RCT filter only 1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/
2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf.
3. [RCT filter]: (controlled clinical trial.pt. or randomized controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomized controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
4. (1 and 2 and 3)
Records are screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs are tagged to the appropriate study record. Similar weekly search alerts are also conducted on OVID EMBASE and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
Appendix 3. Cochrane search strategies to 2013 (CCDANCTR)
The Specialised Register of the Cochrane Depression, Anxiety and Neurosis Group was searched using the following terms:
Update 1: (June 2010)
CCDANCTR‐Studies Register Diagnosis = (depress* or dysthymi*) and Age Group = (child* or adolescen* or unclear or "not stated") and Free‐Text = (prevent* or "early intervention*" or risk or at‐risk or vulnerab* or (health and promot*) or "health literacy" or educat* or psychoeducat* or training or "life skill*" or school* or classroom* or internet* or divorce* or death or bereave*) CCDANCTR‐References Register Title/Abstract = (depression or depressive or dysthymi* or “depressed mood" or “mental health”) and Free‐text = (adolesc* or preadolesc* or pre‐adolesc* or child* or boys or girls or juvenil* or minors or pre‐school or preschool or paediatric* or pediatric* or pubescen* or puberty or school* or high‐school or teen* or young or youth* or (student* and (college or universit*)) or undergraduate*) and Free‐text = (prevent* or "early intervention*" or risk or at‐risk or vulnerab* or (health and promot*) or "health literacy" or educat* or psychoeducat* or training or "life skill*" or school* or classroom* or internet* or divorce* or death or bereave*)
Update 2: (June 2010 to July 2013) CCDANCTR‐References Register
#1 (prevent* NEAR2 (depress* or "mental health")) #2 (psycholog* or problem* or symptom or symptoms) NEAR1 (adjust* or adaptat* or externali* or internali*) #3 (depression or depressive or dysthymi* or “depressed mood” or “low mood*” or “mood *regulation” or “mood disorder*” or “mental health”):ti,ab #4 ((prevent* or primary or targeted or universal* or selective or selected or indicated) NEAR2 (intervention* or programmes*)) #5 ("early intervention*" or risk or at‐risk or vulnerab* or (health NEAR3 promot*) or "health literacy" or educat* or psychoeducat* or training or "life skill*" or *school* or classroom* or campus or internet* or online or divorce* or death or bereave* or bullied or bully*) #6 (adolesc* or preadolesc* or pre‐adolesc* or child* or boys or girls or juvenil* or minors or pre‐school or preschool or paediatric* or pediatric* or pubescen* or puberty or *school* or campus or teen* or young or youth* or (student* and (college or universit*)) or undergraduate* or peer or peers):ti,ab,kw,ky,emt,mc,mh #7 ((#1 or ((#2 or #3) and (#4 or #5))) and #6) Key to CRS search tags: ti:title; ab:abstract; kw:keywords; emt:EMTREE headings; mc:MeSH checkwords; mh:MeSH Headings
Appendix 4. MEDLINE search strategy
Ovid MEDLINE was searched using the following terms:
1. Affective Symptoms/ or Depression/ or Behavioral Symptoms/ 2. exp Depressive Disorder/ 3. (depressi$ adj3 disorder$).tw. 4. (depressi$ adj3 symptom$).tw. 5. (depressi$ adj3 episode$).tw. 6. subclinical depress$.tw. 7. depressed mood.tw. 8. or/1‐7 9. early intervention$.tw. 10. (early onset or recent onset or (prevent$ adj3 onset)).tw. 11. (sub‐threshold or subthreshold).tw. 12. (sub‐syndrom$ or subsyndrom$).tw. 13. indicat$ prevention.tw. 14. select$ prevention.tw. 15. (targeted prevention or targeted intervention$).tw. 16. (universal prevention or universal intervention$).tw. 17. (prevention programmes$ or prevention intervention$).tw. 18. Primary Prevention/ 19. Preventive Health Services/ 20. or/9‐19 21. 8 and 20 22. exp Health Education/ 23. (educat$ adj3 pack$).tw. 24. (educat$ adj3 interv$).tw. 25. (educat$ adj3 programmes$).tw. 26. Counseling/ 27. group counsel$.tw. 28. exp Psychotherapy/ 29. exp Behavior Therapy/ 30. cognitive behav$ intervention$.tw. 31. group intervention$.tw. 32. or/22‐31 33. 8 and 32 34. Depression/pc, th [Prevention & Control, Therapy] 35. Depressive Disorder/pc, th [Prevention & Control, Therapy] 36. 34 or 35 37. 21 or 33 or 36 38. limit 37 to "all child (0 to 18 years)" 39. clinical trial.pt. 40. clinical trial$.mp. 41. random$.mp. 42. placebo$.ti,ab. 43. or/39‐42 44. 38 and 43
Appendix 5. PsycINFO search strategy
Ovid PsycINFO was searched using the following terms:
1."Depression (Emotion)"/ or Major Depression/ or Affective Disorder/ or Dysthymic Disorder/ 2. (depressi$ adj3 disorder$).tw. 3. (depressi$ adj3 symptom$).tw. 4. (depressi$ adj3 episode$).tw. 5. subclinical depress$.tw. 6. depressed mood.tw. 7. or/1‐6 8. early intervention$.tw. 9. (early onset or recent onset or (prevent$ adj3 onset)).tw. 10. (sub‐threshold or subthreshold).tw. 11. (sub‐syndrom$ or subsyndrom$).tw. 12. indicat$ prevention.tw. 13. select$ prevention.tw. 14. (targeted prevention or targeted intervention$).tw. 15. (universal prevention or universal intervention$).tw. 16. (prevention programmes$ or prevention intervention$).tw. 17. Primary Prevention/ 18. Preventive Health Services/ 19. or/8‐18 20. 7 and 19 21. exp Health Education/ 22. (educat$ adj3 pack$).tw. 23. (educat$ adj3 interv$).tw. 24. (educat$ adj3 programmes$).tw. 25. Counseling/ 26. group counsel$.tw. 27. exp Psychotherapy/ 28. exp Behavior Therapy/ 29. cognitive behav$ intervention$.tw. 30. group intervention$.tw. 31. or/21‐30 32. 7 and 31 33. Depression/pc, th [Prevention & Control, Therapy] 34. Depressive Disorder/pc, th [Prevention & Control, Therapy] 35. 33 or 34 36. 20 or 32 or 35 37. limit 37 to (100 childhood <birth to age 12 yrs> or 200 adolescence <age 13 to 17 yrs>) 38. clinical trial.pt. 39. clinical trial$.mp. 40. random$.mp. 41. placebo$.ti,ab. 42. or/38‐41 43. 37 and 42
Appendix 6. EMBASE search strategy
Ovid EMBASE was searched using the following terms:
1. Depression/ or Major Depression/ or Dysthymia/ or Mood Disorder/ 2. (depressi$ adj3 disorder$).tw. 3. (depressi$ adj3 disorder$).tw. 4. (depressi$ adj3 symptom$).tw. 5. (depressi$ adj3 episode$).tw. 6. subclinical depress$.tw. 7. depressed mood.tw. 8. or/1‐7 9. early intervention$.tw. 10. (early onset or recent onset or (prevent$ adj3 onset)).tw. 11. (sub‐threshold or subthreshold).tw. 12. (sub‐syndrom$ or subsyndrom$).tw. 13. indicat$ prevention.tw. 14. select$ prevention.tw. 15. (targeted prevention or targeted intervention$).tw. 16. (universal prevention or universal intervention$).tw. 17. (prevention programmes$ or prevention intervention$).tw. 18. Primary Prevention/ 19. Preventive Health Services/ 20. or/9‐19 21. 8 and 20 22. exp Health Education/ 23. (educat$ adj3 pack$).tw. 24. (educat$ adj3 interv$).tw. 25. (educat$ adj3 programmes$).tw. 26. Counseling/ 27. group counsel$.tw. 28. exp Psychotherapy/ 29. exp Behavior Therapy/ 30. cognitive behav$ intervention$.tw. 31. group intervention$.tw. 32. or/22‐31 33. 8 and 32 34. Depression/pc, th [Prevention & Control, Therapy] 35. Depressive Disorder/pc, th [Prevention & Control, Therapy] 36. 34 or 35 37. 21 or 33 or 36 38. limit 37 to (preschool child <1 to 6 years> or school child <7 to 12 years> or adolescent <13 to 17 years>) 39. clinical trial.pt. 40. clinical trial$.mp. 41. random$.mp. 42. placebo$.ti,ab. 43. or/39‐42 44. 38 and 43
Appendix 7. ERIC search strategy
((Thesaurus Descriptors: "Depression (psychology)") or (Thesaurus Descriptors: Dysthymia) or (Keywords: "depressive disorder" or "Keywords: depression disorder") or (Keywords: "depressive symptoms" or "Keywords: depression symptoms" or Keywords: "symptoms of depression")) and (Education Level: "Elementary Education" or Education Level: "Elementary Secondary Education" or Education Level: "Primary Education" or Education Level: "Secondary Education")
Data and analyses
Comparison 1. Psychological intervention versus any comparison.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depressive diagnosis (by population) post‐intervention | 20 | 3232 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.08, ‐0.02] |
| 1.1 Targeted | 13 | 2022 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.10, ‐0.02] |
| 1.2 Universal | 7 | 1210 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.08, 0.00] |
| 2 Depressive diagnosis short‐term follow‐up | 6 | 724 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.11, 0.03] |
| 2.1 Targeted | 4 | 360 | Risk Difference (M‐H, Random, 95% CI) | ‐0.11 [‐0.19, ‐0.02] |
| 2.2 Universal | 2 | 364 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.04, 0.10] |
| 3 Depressive diagnosis medium‐term follow‐up | 32 | 5965 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.05, ‐0.01] |
| 3.1 Targeted | 22 | 3915 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.07, ‐0.01] |
| 3.2 Universal | 10 | 2050 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 4 Depressive diagnosis long‐term follow‐up | 10 | 1769 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.05, 0.02] |
| 4.1 Targeted | 6 | 1043 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.09, 0.03] |
| 4.2 Universal | 4 | 726 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.02] |
| 5 Depression symptoms (by population) post‐intervention | 73 | 13829 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.27, ‐0.15] |
| 5.1 Targeted | 42 | 4816 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.42, ‐0.23] |
| 5.2 Universal | 31 | 9013 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.17, ‐0.05] |
| 6 Depression symptoms short‐term follow‐up | 16 | 1558 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.45, ‐0.17] |
| 6.1 Targeted | 11 | 999 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.54, ‐0.20] |
| 6.2 Universal | 5 | 559 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.37, 0.01] |
| 7 Depression symptoms medium‐term follow‐up | 53 | 11913 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.18, ‐0.05] |
| 7.1 Targeted | 29 | 4448 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.33, ‐0.12] |
| 7.2 Universal | 24 | 7465 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.08, 0.03] |
| 8 Depression symptoms long‐term follow‐up | 15 | 3836 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.06, 0.06] |
| 8.1 Targeted | 7 | 847 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.21, 0.11] |
| 8.2 Universal | 8 | 2989 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.06, 0.09] |
| 9 Depression symptoms clinician‐rated (by population) post‐intervention | 11 | 2175 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.41, ‐0.05] |
| 9.1 Targeted | 10 | 1340 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.44, ‐0.11] |
| 9.2 Universal | 1 | 835 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.06, 0.21] |
| 10 Depression symptoms clinician‐rated medium‐term follow‐up | 9 | 1754 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.24, 0.07] |
| 10.1 Targeted | 8 | 968 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.30, 0.09] |
| 10.2 Universal | 1 | 786 | Std. Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.14, 0.14] |
| 11 Depression symptoms clinician‐rated long‐term follow‐up | 6 | 894 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.25, 0.01] |
| 11.1 Targeted | 6 | 894 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.25, 0.01] |
| 12 Anxiety symptoms (by population) post‐intervention | 23 | 5017 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.16, 0.02] |
| 12.1 Targeted | 13 | 1666 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.31, 0.04] |
| 12.2 Universal | 10 | 3351 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.13, 0.05] |
| 13 Anxiety symptoms (by population) short‐term follow‐up | 3 | 334 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.59, ‐0.07] |
| 13.1 Targeted | 3 | 334 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.59, ‐0.07] |
| 14 Anxiety symptoms (by population) medium‐term follow‐up | 18 | 4957 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.14, ‐0.01] |
| 14.1 Targeted | 10 | 1827 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.07 [‐0.18, 0.04] |
| 14.2 Universal | 8 | 3130 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.17, ‐0.01] |
| 15 Anxiety symptoms (by population) long‐term follow‐up | 5 | 971 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.44, 0.14] |
| 15.1 Targeted | 2 | 293 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.43, 0.03] |
| 15.2 Universal | 3 | 678 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.61, 0.40] |
| 16 Social and general functioning (by population) post‐intervention | 10 | 2067 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [0.06, 0.41] |
| 16.1 Targeted | 9 | 1021 | Std. Mean Difference (IV, Random, 95% CI) | 0.27 [0.04, 0.50] |
| 16.2 Universal | 1 | 1046 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [0.04, 0.28] |
| 17 Social and general functioning (by population) short‐term follow‐up | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.81 [0.12, 1.49] |
| 17.1 Targeted | 1 | 40 | Std. Mean Difference (IV, Random, 95% CI) | 0.81 [0.12, 1.49] |
| 17.2 Universal | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Social and general functioning (by population) medium‐term follow‐up | 11 | 2449 | Std. Mean Difference (IV, Random, 95% CI) | 0.15 [0.02, 0.28] |
| 18.1 Targeted | 9 | 1058 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [0.00, 0.38] |
| 18.2 Universal | 2 | 1391 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.01, 0.20] |
| 19 Social and general functioning (by population) long‐term follow‐up | 4 | 744 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.16, 0.14] |
| 19.1 Targeted | 3 | 342 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.22, 0.21] |
| 19.2 Universal | 1 | 402 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.21, 0.19] |
Comparison 2. Psychological intervention versus any comparison for targeted interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depressive diagnosis medium‐term follow‐up | 22 | 3915 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.07, ‐0.01] |
| 1.1 Treatment as usual | 12 | 2464 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 1.2 No treatment | 8 | 1286 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.08, 0.01] |
| 1.3 Wait‐list | 1 | 95 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.21, 0.05] |
| 1.4 Other | 1 | 70 | Risk Difference (M‐H, Random, 95% CI) | ‐0.12 [‐0.29, 0.04] |
| 2 Depression symptoms post‐intervention | 42 | 4816 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.42, ‐0.23] |
| 2.1 Treatment as usual | 16 | 2514 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.45, ‐0.15] |
| 2.2 No treatment | 14 | 1274 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.57, ‐0.21] |
| 2.3 Attention placebo | 4 | 466 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.32, 0.13] |
| 2.4 Wait‐list | 6 | 361 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.72, ‐0.26] |
| 2.5 Other | 2 | 201 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.51, 0.04] |
| 3 Depression symptoms medium‐term follow‐up | 29 | 4448 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.33, ‐0.12] |
| 3.1 Treatment as usual | 15 | 2315 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.42, ‐0.13] |
| 3.2 No treatment | 9 | 1207 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.30, 0.09] |
| 3.3 Attention placebo | 3 | 761 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.26, 0.03] |
| 3.4 Wait‐list | 1 | 95 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.55, 0.28] |
| 3.5 Other | 1 | 70 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.14 [‐1.64, ‐0.63] |
2.3. Analysis.

Comparison 2 Psychological intervention versus any comparison for targeted interventions, Outcome 3 Depression symptoms medium‐term follow‐up.
Comparison 3. Psychological intervention versus any comparison for universal interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depressive diagnosis medium‐term follow‐up | 10 | 2050 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.03, 0.01] |
| 1.1 Treatment as usual | 3 | 656 | Risk Difference (M‐H, Random, 95% CI) | ‐0.05 [‐0.17, 0.07] |
| 1.2 No treatment | 2 | 316 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.05, 0.07] |
| 1.3 Attention placebo | 2 | 861 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.04, 0.04] |
| 1.4 Wait‐list | 3 | 217 | Risk Difference (M‐H, Random, 95% CI) | ‐0.08 [‐0.24, 0.09] |
| 2 Depression symptoms post‐intervention | 31 | 9013 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.17, ‐0.05] |
| 2.1 Treatment as usual | 9 | 1791 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.31, 0.00] |
| 2.2 No treatment | 9 | 4231 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.25, ‐0.05] |
| 2.3 Attention placebo | 9 | 2180 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.09, 0.08] |
| 2.4 Wait‐list | 4 | 811 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.28, 0.04] |
| 3 Depression symptoms medium‐term follow‐up | 24 | 7465 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.08, 0.03] |
| 3.1 No treatment | 7 | 3367 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.10, 0.16] |
| 3.2 Treatment as usual | 6 | 1505 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.16, 0.05] |
| 3.3 Attention placebo | 7 | 1813 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.10, 0.09] |
| 3.4 Wait‐list | 4 | 780 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.34, 0.07] |
| 3.5 Other | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.3. Analysis.
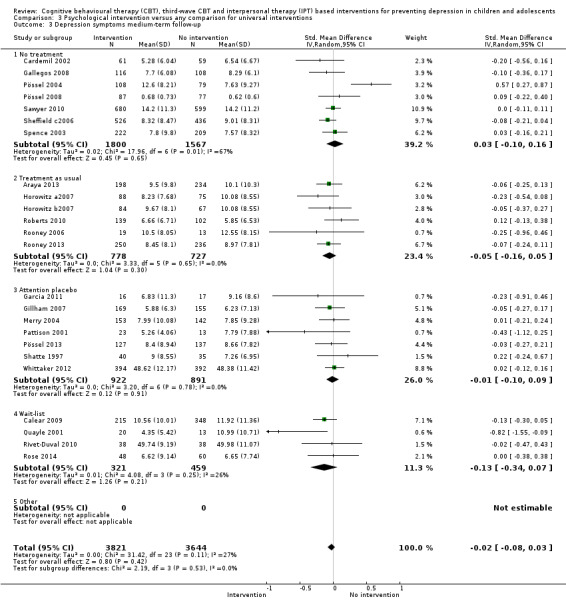
Comparison 3 Psychological intervention versus any comparison for universal interventions, Outcome 3 Depression symptoms medium‐term follow‐up.
Comparison 4. Psychological intervention versus any comparison for selected and indicated interventions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depressive diagnosis medium‐term follow‐up | 22 | 3915 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.07, ‐0.01] |
| 1.1 Selective | 3 | 963 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.07, 0.12] |
| 1.2 Indicated | 16 | 2374 | Risk Difference (M‐H, Random, 95% CI) | ‐0.03 [‐0.06, ‐0.01] |
| 1.3 Combined | 3 | 578 | Risk Difference (M‐H, Random, 95% CI) | ‐0.14 [‐0.21, ‐0.07] |
| 2 Depression symptoms (by population) post‐intervention | 42 | 4816 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.42, ‐0.23] |
| 2.1 Selective | 9 | 1394 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.30, ‐0.02] |
| 2.2 Indicated | 29 | 2740 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.50, ‐0.24] |
| 2.3 Combined | 4 | 682 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.45, ‐0.15] |
Comparison 5. Self‐reported depression symptoms versus clinician‐rated depression symptoms.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression scores (by assessor) post‐intervention | 9 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Self‐reported | 9 | 1877 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.53, ‐0.12] |
| 1.2 Clinician‐rated | 9 | 1884 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.46, ‐0.04] |
| 2 Depression scores medium‐term follow‐up | 7 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Self‐reported | 7 | 1465 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.41, ‐0.02] |
| 2.2 Clinician‐rated | 7 | 1468 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.32, 0.06] |
| 3 Depression scores long‐term follow‐up | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Self‐reported | 4 | 390 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.37, 0.16] |
| 3.2 Clinician‐rated | 4 | 388 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.27, 0.14] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Araya 2013.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: BDI‐II: 13.5 (mild) Mean age: 14.5 Age range: not specified Percentage male: 55.5% Setting: school Psychiatric diagnoses excluded: unclear Suicide risk excluded: unclear Parents with history of schizophrenia/bipolar disorder excluded: unclear Country: Chile |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: I Think, Feel, and Act Number of sessions: 11 sessions plus 2 booster sessions Length of sessions: 1 hour Intensity (total number of hours): 13 hours Duration of treatment period: unclear. Booster sessions were conducted at 2 and 7 months. Group size: unclear Delivered by: trained mental health research workers, including: psychologists, teachers, social workers and others Fidelity: not assessed Type of comparison: TAU comprising normal teaching activities and assessments which, according to school curriculum, were described as ‘counselling’. Teachers advised to place more emphasis on emotional problems, provide better information to students, to allow students to exchange experiences, and provide mutual support to one another. |
|
| Outcomes | Diagnosis: established from cut‐points on the BDI of 17.0 for the overall sample; 14.0 for boys and 20.0 for girls (however, we were unable to obtain these data from the authors) Name of self‐report depression measure: BDI‐II Name of clinician report depression measure: N/A Name of anxiety measure: RCADS (omitting the depression and separation anxiety subscales) Name of general functioning measure: N/A Assessment points: post‐intervention and 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...a computer‐generated list of random numbers..." (p.1005) |
| Allocation concealment (selection bias) | Unclear risk | "The trial statistician..." (p.1005) Unclear whether the sequence was concealed from the other researchers involved in the trial, however |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the intervention suggests that it is likely participants were aware to which group they had been allocated. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 17.7% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: sensitivity analyses were conducted by imputing missing data using multiple imputations. However, the authors state that results did not differ from those using observed cases and therefore chose to present outcomes based on observed cases only. |
| Selective reporting (reporting bias) | High risk | Trial protocol (i.e. Araya 2011) would suggest that scores on the "Self‐Harm Questionnaire" and that clinically significant depression (as established from cut scores on the BDI‐II) were also assessed |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Arnarson 2009.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: 75th to 90th percentile on the CDI What risk was basis of inclusion for selected studies: 75th percentile or higher on the negative attribution style composite of the CSAQ Diagnostic interview to exclude those with current or previous depression: those with current depression excluded as well as those scoring above the 90th percentile of the CDI, and those with a past episode of a depressive disorder Baseline severity of depression: CDI: 14.9 (mild‐moderate) Mean age: not specified Age range: 14 to 15 Percentage male: 49.4% Setting: school State what psychiatric diagnoses were excluded: dysthymia, cyclothymia, anorexia, bulimia, any psychotic disorder, bipolar disorder (types I or II), comorbid substance use/disorder, conduct disorder, oppositional defiance disorder and attention deficit hyperactivity disorder Suicide risk excluded: unclear Parents with history of schizophrenia/bipolar disorder excluded: unclear Country: Iceland |
|
| Interventions | Broad category: CBT and IPT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: not specified Number of sessions: 14 sessions Length of sessions: unclear. As sessions were delivered during usual class time, assumption is 1 hour. Intensity (total number of hours): 14 hours (on assumption each session has a duration of 1 hour) Duration of treatment period: 11 weeks Group size: 6 to 8 Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU comprising the ability to seek any school‐based or other services as necessary except those associated with systematic interventions |
|
| Outcomes | Diagnosis: Hodges' Child Assessment Scale, the A‐Life (for follow‐up interviews between 2003‐2005), or the K‐SADS (between 2004‐2005) Name of self‐report depression measure: CDI (data not reported in a usable format) Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term) for depression diagnosis only |
|
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no Coding for depression severity at baseline: where baseline severity was mild to moderate as in this trial, it was rated as mild. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants...were randomly assigned..." (p.581) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to an assessment only control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Low risk | "All interviewers were uninformed as to the intervention condition of participants at all interviews" (p.580) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 12.87% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken. |
| Selective reporting (reporting bias) | High risk | Scores on the CDI at follow‐up not reported |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Bella‐Awusah 2015.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: BDI‐II ≥ 18.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current episodes of depression included. Additionally, as no student self‐disclosed past psychiatric treatment for any mental illness, it is likely that those with past episodes of depression were also included. Baseline severity of depression: BDI‐II: 24.7 (moderate) Mean age: 15.7 Age range: 14 to 17 Percentage male: 30.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified. However, no student self‐disclosed past psychiatric treatment for any mental illness. Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Nigeria |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: not specified Number of sessions: 5 sessions Length of sessions: 45 to 60 minutes Intensity (total number of hours): up to 5 hours Duration of treatment period: 5 weeks Group size: 20 Delivered by: Child and Adolescent Psychiatrist Fidelity: assessed as adequate Type of comparison: WL |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: BDI‐II Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and approx. 4 months (short‐term) |
|
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: yes Author contacted for outcome data: yes |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "...randomly designated...by ballot" (manuscript p.6) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 2.5% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: LOCF |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes (only 2 of the 5 sessions, however) Implementation integrity adequate: yes Implementation integrity reported: yes |
Calear 2009.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. Those with current and/or past episodes of depression were not excluded, however. Baseline severity of depression: CES‐D: 11.8 (subthreshold) Mean age: 14.3. Age range: 12 to 17 Percentage male: 44.1% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: N/A as MoodGYM freely available to access Online: yes Name of programme: MoodGYM Number of sessions: 5 sessions Length of sessions: 20 to 40 minutes Intensity (total number of hours): up to 3.3 hours Duration of treatment period: 5 weeks Group size: N/A as MoodGYM individual‐based programme Delivered by: N/A Fidelity: online, therefore standardised Type of comparison: WL |
|
| Outcomes | Diagnosis: established from cut‐points on the CES‐D of ≥ 24 Name of self‐report depression measure: CES‐D Name of clinician report depression measure: N/A Name of anxiety measure: RCMAS Name of general functioning measure: N/A Assessment points: post‐intervention and 6 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: N/A as MoodGYM freely available to access Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...a computerized random number generator..." (p.1023) |
| Allocation concealment (selection bias) | Low risk | "An independent statistician randomly allocated schools... The identity of the schools was concealed from the statistician during this process." (p.1023) |
| Blinding (performance bias and detection bias) Subjects | High risk | "Information and consent forms outlining the details of the trial and the school's assignment to either intervention or control were distributed to all participating students and their parents" (p.1023) |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 13.3% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Cardemil 2002.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. Those with current and/or past episodes of depression not excluded, however. Baseline severity of depression: CDI: 9.5 (subthreshold) Mean age: 11.1 Age range: 10 to 12 Percentage male: 47.4% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes. Penn Resiliency Program manual is freely available on request. Online: no Name of programme: Penn Resiliency Program Number of sessions: 12 sessions Length of sessions: 90 minutes Intensity (total number of hours): 18 hours Duration of treatment period: 12 weeks Group size: 10 Delivered by: masters‐level graduate students (clinical psychology, educational psychology, counselling) Fidelity: not assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: established from cut‐points on the CDI of ≥ 30 Name of self‐report depression measure: CDI Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 3 months (short‐term), 12 months (medium‐term) and 24 months (long‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no. Penn Resiliency Program manual is freely available on request. Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 13.5% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, the authors did undertake post‐hoc analyses of Latino versus African children and high symptomatic versus low symptomatic children. |
| Other bias | Unclear risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Castellanos 2006.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: scoring one standard deviation above the school mean on the negative thinking subscale of the Substance Use Risk Profile Scale (SURPS; Conrod 2002) Diagnostic interview to exclude those with current or previous depression: not undertaken. Those with current and/or past depression not excluded, however. Baseline severity of depression: BSI: 16.0 (unclear) Mean age: 14.0 Age range: 13 to 16 Percentage male: 35.7% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: UK |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: not specified Number of sessions: 2 sessions Length of sessions: 90 minutes Intensity (total number of hours): 3 hours Duration of treatment period: unclear Group size: 2 to 9 Delivered by: mental health experts Fidelity: not assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: BSI Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 36.89% Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: authors state they use LOCF method, however, the number of participants included in this analysis is unclear |
| Selective reporting (reporting bias) | High risk | Protocol not available. Numbers of participants included in analyses are unclear, however, and yet there is a high proportion of treatment drop‐outs. |
| Other bias | Unclear risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Chaplin 2006.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. Those with current and/or past depression not excluded, however. Baseline severity of depression: CDI: approximately 8.0 (subthreshold) Mean age: 12.2 Age range: 11 to 14 Percentage male: 50.5% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes. Penn Resiliency Program manual is freely available on request. Online: no Name of programme: Penn Resiliency Program Number of sessions: 12 sessions Length of sessions: 90 minutes Intensity (total number of hours): 18 hours Duration of treatment period: 12 weeks Group size: 9 to 14 Delivered by: both non‐mental health and mental health experts Fidelity: assessed but only for the purposes of supervision Type of comparison: NT |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no. Penn Resiliency Program manual is freely available on request. Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... randomly assigned... using a computer‐generated random numbers table" (p.114) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 23.80% Means and SDs used in meta‐analysis based on what data: observed cases (girls only) Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | High risk | Protocol not available. Data on 12‐month outcomes not presented as too few participants had been assessed by this time point. |
| Other bias | Unclear risk | Trial conducted by those who developed the intervention |
| Implementation integrity | High risk | Implementation integrity assessed: yes (only for purposes of supervision, however) Implementation integrity adequate: no Implementation integrity reported: N/A |
Charbonneau 2012.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: scoring above the average on the negative reactivity and negative intensity subscales of the Affect Intensity Measure (AIM; Larsen 1986). Diagnostic interview to exclude those with current or previous depression: not undertaken. Unclear whether those with current and/or past episodes of depression were excluded. Baseline severity of depression: CES‐D: 19.9 (mild) Mean age: 18.0 Age range: 17 to 19 Percentage male: 0.0% Setting: university Psychiatric diagnoses excluded: unclear Suicide risk excluded: unclear Parents with history of schizophrenia/bipolar disorder excluded: unclear Country: USA |
|
| Interventions | Broad category: third wave (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Women and Relaxation, Openness Contemplation and Kindness (ROCK) Number of sessions: 8 sessions Length of sessions: 1 hour Intensity (total number of hours): 8 hours Duration of treatment period: 8 weeks Group size: 10 to 12 Delivered by: masters‐level graduate student (clinical psychology) Fidelity: not assessed. However, researcher who developed the intervention also delivered all 8 intervention sessions. Type of comparison: unclear. Described as a “control group”. |
|
| Outcomes | Diagnosis: SCID‐I Name of self‐report depression measure: CES‐D Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: SACQ Assessment point: post‐intervention, short‐term and medium‐term |
|
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Unclear risk | Content of the control condition was not adequately described, therefore difficult to determine whether participants would have been able to determine to which group they had been allocated or not. |
| Blinding (performance bias and detection bias) Assessors | Low risk | "Interviewers were blinded to participant condition" (p.31) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 8.00% dropped out whilst a further 12.50% did not complete the post‐intervention assessment. Means and SDs used in meta‐analysis based on what data: for outcomes measured on a continuous scale (e.g. self‐reported depression scores), trial authors imputed missing item scores for those participants with fewer than 3 items missing. However, scores for those participants who missed more than 3 items on a scale, or who missed the scale entirely, were not imputed. Data for continuous outcomes therefore may contain some imputed values. Data for categorical outcomes (e.g. depression diagnosis) are based on observed cases (defined as those who attended at least 1 session). Intention‐to‐treat analyses: hierarchical linear modelling |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial and therapy sessions conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Clarke 1993.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. Those with current and/or past depression were not excluded, however. Baseline severity of depression: CES‐D: 16.3 (mild) Mean age: 15.1. Age range: 14 to 16 Percentage male: 53.9% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: BT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: not specified Number of sessions: 5 sessions Length of sessions: 50 minutes Intensity (total number of hours): 4.2 hours Duration of treatment period: 5 weeks Group size: unclear Delivered by: non‐mental health experts Fidelity: assessed as adequate Type of comparison: TAU comprising the usual health class curriculum delivered on the same days as the intervention, however, assessment of the control class curriculum revealed no overlap in content with regard to depressive disorders and/or related mental health issues. |
|
| Outcomes | Diagnosis: established from cut‐points on the CES‐D of ≥ 24 Name of self‐report depression measure: CES‐D Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 12 weeks (short‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes. Correspondence with authors revealed manual was no longer available. Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Unclear risk | The nature of the intervention suggests it may have been possible to blind participants to allocation. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 21.05% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, the authors did undertake post‐hoc analyses of males versus females. |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Low risk | Implementation integrity assessed: research assistants observed classes and rated fidelity Implementation integrity adequate: yes Implementation integrity reported: yes |
Clarke 1995.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 24 and K‐SADS What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current depression excluded. Those with past episodes of depression, however, were not excluded. Baseline severity of depression: CES‐D: 22.9 (mild) Mean age: 15.3 Age range: 14 to 16 Percentage male: 30.0% Setting: school State what psychiatric diagnoses were excluded: dysthymia, bipolar disorder Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Coping with Stress Number of sessions: 15 sessions Length of sessions: 45 minutes Intensity (total number of hours): 11.25 hours Duration of treatment period: 5 weeks Group size: unclear Delivered by: mental health experts Fidelity: assessed as adequate Type of comparison: TAU comprising freedom to continue with any pre‐existing treatment or to seek new assistance during the study period |
|
| Outcomes | Diagnosis: K‐SADS and LIFE Name of self‐report depression measure: CES‐D Name of clinician report depression measure: modified 14‐item version of the HAM‐D Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 5 weeks (short‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes. Correspondence with authors revealed no manual was available. Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | No information specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 16.67% Means and SDs used in meta‐analysis based on what data: observed cases (defined as those who completed at least one of the follow‐up assessments) Intention‐to‐treat analyses: unclear whether intention‐to‐treat analyses were undertaken |
| Selective reporting (reporting bias) | High risk | Data on General Assessment of Functioning scores presented, which is not indicated as an outcome measure within the methods section |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
Clarke 2001.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 24 What risk was basis of inclusion for selected studies: parental depression Diagnostic interview to exclude those with current or previous depression: those with current depression excluded. Those with past episodes of depression, however, were not excluded. Baseline severity of depression: CES‐D: 24.4 (mild) Mean age: 14.6 Age range: 13 to 18 Percentage male: 35.6% Setting: HMO State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Coping with Stress Number of sessions: 15 sessions Length of sessions: 60 minutes Intensity (total number of hours): 15 hours Duration of treatment period: unclear Group size: 6 to 10 Delivered by: mental health experts Fidelity: assessed as adequate Type of comparison: TAU comprising freedom to continue with any pre‐existing treatment or to seek new assistance during the study period provided by the HMO and/or by outside healthcare providers |
|
| Outcomes | Diagnosis: K‐SADS Name of self‐report depression measure: CES‐D Name of clinical report depression measure: modified 14‐item version of the HAM‐D Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... assignment was preprinted using a computer program..." (p.1129) |
| Allocation concealment (selection bias) | Low risk | "... sealed in sequentially numbered envelopes, which were opened in sequential order by the project coordinator..." (p.1129) |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Low risk | "Assessors were unaware of the experimental condition of interviewed subjects" (p.1128) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 4.30% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using random‐effects regression |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
Compas 2009.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: parental depression Diagnostic interview to exclude those with current or previous depression: those with current depression excluded. Those with past episodes of depression were not excluded (13% of intervention group and 23% of control group). Baseline severity of depression: YSR depression/anxiety subscale: 55.9 (moderately elevated) Mean age: 11.5 Age range: 9 to 15 Percentage male: 54.8% Setting: mental health clinics/practices, family and general medical practices State what psychiatric diagnoses were excluded: autism spectrum disorders, mental retardation, bipolar I, schizophrenia, conduct disorder, comorbid substance use/disorder, Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: those with parents diagnosed with bipolar I, schizophrenia, schizoaffective disorder, substance use/abuse excluded as were those whose parents were currently suicidal Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: not specified Number of sessions: 8 sessions plus 4 booster sessions Length of sessions: unclear. As sessions delivered during visits, assumption is 1 hour. Intensity (total number of hours): 12 hours (on assumption each session has a duration of 1 hour) Duration of treatment period: 8 weeks plus 1 booster session per month for an additional 4 months Group size: 4 families per group Delivered by: mental health experts Fidelity: assessed as adequate Type of comparison: other |
|
| Outcomes | Diagnosis: K‐SADS‐PL Name of self‐report depression measure: CES‐D Name of clinician report depression measure: N/A Name of anxiety measure: YSR anxiety subscale Name of general functioning measure: N/A Assessment points: post‐intervention and 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (not provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The order of randomization was determined by a random number generator..." (p.1012) |
| Allocation concealment (selection bias) | Low risk | "...the assignment order was kept in a series of sealed envelopes that were opened by research assistants who were blind to assignment until the envelopes were opened..." (p.1012) |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Low risk | "Doctoral candidates in clinical psychology, who were blind to condition, conducted the structured diagnostic interviews..." (p.1011) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 29.68% Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: using multivariate mixed‐effects models with maximum likelihood estimation |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
Cova 2011‐Targeted.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: BDI‐II ≥ 7.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current depression could participate in the intervention, but they were excluded from all subsequent analyses. Unclear if those with past episodes of depression were excluded, however. Baseline severity of depression: BDI‐II: 17.85 (intervention group) and 16.80 (control group) (mild) Mean age: not specified Age range: 14 to 15 Percentage male: 0% Setting: schools State what psychiatric diagnoses were excluded: exclusion criteria not stated Suicide risk excluded: exclusion criteria not stated Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not stated Country: Chile |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: unclear Online: no Name of programme: not specified Number of sessions: approx. 11 sessions. The intervention programme was, however, adapted to fit with students' timetables so some students may have received fewer sessions. Length of sessions: approx. 1.5 hours. The intervention programme was, however, adapted to fit with students' timetables so some students may have received shorter sessions. Intensity (total number of hours): approx. 16.5 hours (on assumption each participants received 11 sessions of 1.5 hours duration) Duration of treatment period: unclear as frequency of sessions not stated Group size: 15 to 23 Delivered by: mental health experts (graduate‐level psychologists) Fidelity: unclear if assessed Type of comparison: correspondence with study authors suggests NT |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: BDI‐II Name of clinician report depression measure: N/A Name of anxiety measure: BAI Name of general functioning measure: N/A Assessment points: 7 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: yes Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation "by chance" Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 14.8% Means and SDs used in meta‐analysis based on what data: observed cases (girls only) Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Cowell 2009.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: being the child of a Mexican immigrant woman Diagnostic interview to exclude those with current or previous depression: those with current depression were excluded. Unclear whether those with past episodes of depression were also excluded, however. Baseline severity of depression: CDI: 9.2 (sub‐threshold) Mean age: 10.4 Age range: not specified Percentage male: not specified Setting: school State what psychiatric diagnoses were excluded: depression. Children attending special education classes were also excluded. Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: mothers with current depression were excluded. Unclear whether those with a past history of any mental illness were also excluded, however. Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Mexican American Problem Solving Program (Stop, Think, and Act) Number of sessions: 10 sessions Length of sessions: unclear. As sessions delivered during visits, assumption is 1 hour. Intensity (total number of hours): 10 hours (on assumption each session has a duration of 1 hour) Duration of treatment period: unclear Group size: 4 to 5 Delivered by: non‐mental health experts (nurses) Fidelity: assessed but unclear if assessed as adequate Type of comparison: NT |
|
| Outcomes | Diagnosis: established from cut‐points on the CDI of ≥ 12 (numbers not reported in manuscript, however) Name of self‐report depression measure: CDI Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 10 weeks (short‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Schools were randomised to intervention and control groups" (p.179) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Unclear risk | The clustered nature of allocation suggests it is possible participants could have been blind to treatment allocation. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | No information specified |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 11.3% Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: using "Ruben's Hot deck imputation (1987)..." (p.187) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
Dobson 2010.
| Methods | Design: RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 24 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current and/or past episodes of depression were excluded Baseline severity of depression: CES‐D: 32.1 (moderate) Mean age: 15.3 Age range: 13 to 18 Percentage male: 30.4% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Canada |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Coping with Stress Number of sessions: 15 sessions Length of sessions: 45 minutes Intensity (total number of hours): 11.25 hours Duration of treatment period: unclear. Typically the Coping with Stress programme is delivered as 2 sessions per week. Assumption, therefore, is that the duration of the treatment period was 8 weeks. Group size: unclear Delivered by: doctoral students in clinical psychology Fidelity: assessed as adequate Type of comparison: AP entitled “Let’s Talk” |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CES‐D and CDI Name of clinician report depression measure: N/A Name of anxiety measure: BAI and MASQ. Name of general functioning measure: N/A Assessment points: post‐intervention, 3 months (short‐term) and 6 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no. Coping with Stress manual is freely available on request. Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomly generated list of the two conditions was generated by a computer program..." (p.296) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Low risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | No information specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: sensitivity analyses were conducted by imputing missing data using the expectation‐maximisation algorithm. However, the authors state that results did not differ from those using observed cases and therefore chose to present outcomes based on observed cases only. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
Ellis 2011.
| Methods | Design: RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: those with "low to moderate levels of psychological distress" on the K‐10. No cut‐point is specified, however. What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. However, those with K‐10 ≥ 30 were excluded. Those with past episodes of depression were not excluded, however. Baseline severity of depression: DASS depression subscale: 15.0 (moderate) Mean age: 19.7 Age range: not specified Percentage male: 23.0% Setting: university State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: N/A as MoodGYM freely available to access Online: yes Name of programme: MoodGYM Number of sessions: 3 sessions Length of sessions: 60 minutes Intensity (total number of hours): 3 hours Duration of treatment period: 3 weeks Group size: N/A as MoodGYM is an individual‐based programme Delivered by: N/A (self‐monitoring) Fidelity: online, therefore standardised Type of comparison: NT |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: DASS depression subscale (DASS‐21‐d) Name of clinician report depression measure: N/A Name of anxiety measure: DASS anxiety subscale (DASS‐21‐a) Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: N/A as MoodGYM freely available to access Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomly allocated..." (p.462) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: unclear. Abstract suggests 39 participants were included, and outcome data are available for 39 participants. However, gender breakdown in the methods section seems to indicate 40 participants were included. Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Fleming 2012.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: students excluded, or at risk of being excluded, from mainstream education due to behavioural problems Diagnostic interview to exclude those with current or previous depression: not undertaken, although those with “extreme depression” were excluded (p.531). Unclear whether those with past episodes of depression were also excluded Baseline severity of depression: CDRS‐R: 39.6 (moderate) Mean age: 14.9 Age range: 13 to 16 Percentage male: 56.0% Setting: schools (alternative education) State what psychiatric diagnoses were excluded: "Only those judged not to be safe using the computerized program were excluded" (p.531) Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: no Country: New Zealand |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: N/A Online: yes Name of programme: SPARX Number of sessions: 7 modules Length of sessions: 30 minutes Intensity (total number of hours): 3.5 hours Duration of treatment period: 5 weeks Group size: unclear Delivered by: online Fidelity: online, therefore standardised Type of comparison: WL |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: RADS‐2 Name of clinician report depression measure: CDRS‐R Name of anxiety measure: SAS Name of general functioning measure: PQ‐LES‐Q Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: yes (provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was carried out in a 1:1 ratio using a computer generated randomization sequence. Allocation was stratified by study site and arranged in permuted blocks" (p.533) |
| Allocation concealment (selection bias) | Low risk | "Allocation concealment was ensured by allocating each participant a unique study number..." (p.533) |
| Blinding (performance bias and detection bias) Subjects | High risk | "It was not possible to blind participants to their treatment allocation" (p.533) |
| Blinding (performance bias and detection bias) Assessors | High risk | "The researcher was unblinded after the baseline assessment" (p.533) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 6.30%; 0% (for depression diagnosis) Means and SDs used in meta‐analysis based on what data: observed cases (defined as those who completed at least one SPARX module and completed the 5 week post‐treatment assessment Intention‐to‐treat analyses: ITT analyses were undertaken, however, the sample was not large enough to form the primary outcome |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Fresco 2009.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: scoring in the top percentile on the Expanded Attributional Style Questionnaire (Pessimism) Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: BDI‐I: 9.4 (sub‐threshold) Mean age: 19.2 Age range: not specified Percentage male: 22.0% Setting: university State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Self Administered Optimism Training (SOT) Number of sessions: 1 session plus daily monitoring Length of sessions: 10 minute session plus daily monitoring of unclear duration Intensity (total number of hours): unclear Duration of treatment period: 28 days Group size: N/A as monitoring was individual‐based intervention Delivered by: unclear Fidelity: unclear if assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: BDI‐I Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "... participants were...randomly assigned..." (p.354) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 12.5% (unbalanced) Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: expectation‐maximisation imputation algorithm |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Gallegos 2008.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 9.4 (sub‐threshold) Mean age: 9.9 Age range: 9 to 11 Percentage male: 47.4% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Mexico |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: AMISTAD (Mexican version of the FRIENDS for Life program) Number of sessions: 10 sessions plus 2 booster sessions Length of sessions: 60 to 75 minutes Intensity (total number of hours): up to 12.5 hours (including booster sessions) Duration of treatment period: 10 weeks (booster sessions at 1 month and 3 months post‐intervention) Group size: unclear Delivered by: non‐mental health experts Fidelity: assessed as adequate Type of comparison: NT |
|
| Outcomes | Diagnosis: established from CDI of ≥ 19.0 Name of self‐report depression measure: CDI (Spanish version) Name of clinician report depression measure: N/A Name of anxiety measure: SAS (Spanish version) Name of general functioning measure: N/A Assessment points: post‐intervention and 6 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Schools... were randomly assigned..." (p.62) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | No information specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 10.8% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Low risk | As this trial was reported in a thesis, it is unlikely selective outcome reporting was present |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: yes (for 17.00% of cases) Implementation integrity adequate: unclear Implementation integrity reported: N/A |
Garber 2009.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 20.0 What risk was basis of inclusion for selected studies: parental depression Diagnostic interview to exclude those with current or previous depression: those with current episodes of depression excluded. However, inclusion criteria allowed for those who had experienced a previous episode, but were currently in remission for at least 2 months. 55.3% of intervention group and 55.4% of control group had experienced a previous episode. Baseline severity of depression: CES‐D: 18.6 (mild) Mean age: 14.8 Age range: 13 to 17 Percentage male: 58.5% Setting: HMO, university medical centres, schools State what psychiatric diagnoses were excluded: bipolar I disorder, schizophrenia Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: yes Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Coping with Stress Number of sessions: 14 sessions Length of sessions: 90 minutes Intensity (total number of hours): 21.0 hours Duration of treatment period: overall 8 months (first 8 weeks acute) Group size: 3 to 10 Delivered by: mental health experts Fidelity: assessed as adequate Type of comparison: TAU comprising freedom to initiate or continue any non‐intervention mental health care |
|
| Outcomes | Diagnosis: established from K‐SADS‐PL and LIFE ≥ 4.0 Name of self‐report depression measure: CES‐D Name of clinician report depression measure: CDRS‐R Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term), 33 months (long‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... randomized using the Begg and Iglewicz modification of the Efron biased coin toss... by a computer program" (p.2217) |
| Allocation concealment (selection bias) | Low risk | "Participants were randomized centrally at the Pittsburgh site..." (p.2217) |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Low risk | "Independent evaluators were blinded to experimental condition throughout the study..." (p.2116) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 8.20% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: mixed models including LOCF |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
Garcia 2011.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. Reported that 5.1% of participants had experienced a previous mental health condition. Baseline severity of depression: DASS‐d: 10.9 (mild) Mean age: 14.8 Age range: 14 to 16 Percentage male: 0.0% Setting: school State what psychiatric diagnoses were excluded: none Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: no Country: Mexico |
|
| Interventions | Broad category: third wave (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Project Wings Number of sessions: 16 sessions Length of sessions: 3 hours Intensity (total number of hours): 48 hours Duration of treatment period: 16 weeks plus booster sessions at 3 and 7 months Group size: unclear Delivered by: mental health workers (youth workers) Fidelity: not assessed Type of comparison: AP |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: DASS‐d Name of clinical report depression measure: N/A Name of anxiety measure: DASS‐a Name of general functioning measure: N/A Assessment points: post‐intervention, 3 months (short‐term), 9 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computerised permuted block randomisation schedule was created..." (p.439) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Low risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported and there is no report of blinding. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 14.30% Means and SDs used in meta‐analysis based on what data: observed cases (those with at least 2 post‐intervention assessments) Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Gilham 1994‐Study 2.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: not specified What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 7.7 (sub‐threshold) Mean age: not specified Age range: 10 to 12 Percentage male: 53.4% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Depression Prevention Program plus a parental component (now referred to as Penn Resiliency Program) Number of sessions: 12 sessions Length of sessions: 2 hours Intensity (total number of hours): 24 hours Duration of treatment period: 12 weeks Group size: 8 to 12 Delivered by: Doctoral students in clinical psychology Fidelity: not assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: established from CDI of ≥ 15.0 Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 2 months (short‐term), 6 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no We have assumed that there were 4 clusters ‐ 2 intervention and 2 in control ‐ for ICC and sample size adjustment |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Correspondence with study authors indicated that randomisation was conducted using a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 9.59% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, longer‐term follow‐up data have not been reported and follow‐up data have not been reported for those children recruited in year 2. |
| Other bias | Unclear risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Gillham 2007.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current depression excluded. Unclear whether those with past depression were also excluded, however. Baseline severity of depression: CDI: 8.4 (subthreshold) Mean age: 12.1 Age range: 11 to 14 Percentage male: 54.1% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Penn Resiliency Program Number of sessions: 12 sessions Length of sessions: 90 minutes Intensity (total number of hours): 18 hours Duration of treatment period: 12 weeks Group size: 6 to 14 Delivered by: all Fidelity: assessed as satisfactory to good Type of comparison: AP |
|
| Outcomes | Diagnosis: established from CDI of ≥ 13.0 or from clinically significant symptoms on the CDRS‐R of ≥ 65.0 Name of self‐report depression measure: CDI Name of clinician report depression measure: CDRS‐R Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term) and 36 months (long‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no (manual freely available on request) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... computer‐generated random numbers sequence" (p.10) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Low risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Low risk | "Interviewers and coders were not informed of participants'... assignments" (p.13) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 8.86% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: undertaken, but based on only those who completed baseline and at least one post‐intervention assessment |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, CDRS‐R raw scores are not presented nor are the proportion of children with elevated, high, and clinically significant levels of depression. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: described as "adequate to good" Implementation integrity reported: yes |
Gillham 2012.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: not specified What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: although a diagnostic interview was undertaken, those with current and/or past episodes of depression were not excluded Baseline severity of depression: CDI: 10.6 (sub‐threshold) Mean age: not stated Age range: 10 to 15 Percentage male: 52.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Penn Resiliency Program Number of sessions: 10 sessions plus 6 booster sessions offered once every 6 months post‐intervention Length of sessions: 90 minutes Intensity (total number of hours): 15 hours (length of booster sessions unclear) Duration of treatment period: 10 weeks Group size: unclear Delivered by: non‐mental health experts Fidelity: fidelity of first 2 to 3 sessions assessed and descried as satisfactory. However, the authors note that fidelity for this trial was lower than that observed for other trials of PRP. Type of comparison: described as “control group”; probably TAU |
|
| Outcomes | Diagnosis: computer‐assisted DISC‐IV Name of self‐report depression measure: CDI and RADS‐2 Name of clinician report depression measure: N/A Name of anxiety measure: RCMAS Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no (manual freely available on request) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... computer‐generated random number sequence..." (p.625) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Low risk | "Interviewers were not informed of students' condition assignment" (p.627) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 7.90% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: described as "satisfactory" but less than that achieved in other trials of PRP Implementation integrity reported: yes |
Gillham, Hamilton 2006a.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CDI ≥ 7.0 for girls and ≥ 9.0 for boys What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current depression excluded Baseline severity of depression: CDI: 12.9 (subthreshold) Mean age: not specified Age range: 11 to 12 Percentage male: 46.9% Setting: HMO State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Penn Resiliency Program Number of sessions: 12 sessions Length of sessions: 90 minutes Intensity (total number of hours): 18 hours Duration of treatment period: 12 weeks Group size: unclear Delivered by: mental health experts Fidelity: assessed but unclear if adequate fidelity (therapist compliance scores ranged between 88.1% and 95.8% according to a measure of fidelity developed by the study authors) Type of comparison: TAU comprising usual care in an HMO setting |
|
| Outcomes | Diagnosis: established from computerised HMO databases across the 2‐year follow‐up period Name of self‐report depression measure: CDI Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term), 24 months (long‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no (manual freely available on request) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer generated random number sequence..." (p.207) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | High risk | No information specified with respect to blinding of those extracting diagnostic information from the HMO database |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 20.30% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: undertaken but unclear what method used |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: yes Implementation integrity adequate: unclear (64% to ‐95%) Implementation integrity reported: yes |
Gillham, Reivich 2006b.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 10.8 (subthreshold) Mean age: not specified Age range: 11 to 13 Percentage male: 70.5% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT‐parent adaption (for further information on intervention components, see Table 3) Manualised: yes (manual freely available on request) Online: no Name of programme: Penn Resiliency Program‐Parent Number of sessions: 8 sessions Length of sessions: 90 minutes Intensity (total number of hours): 12 hours Duration of treatment period: 8 weeks Group size: 10 to 12 Delivered by: research associates with at least an undergraduate degree in psychology (1 had a doctorate in psychology) Fidelity: not assessed Type of comparison: NT. Families were free to pursue counselling and/or other psychological therapies. |
|
| Outcomes | Diagnosis: CDI ≥ 19.0 Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: RCMAS Name of general functioning measure: N/A Assessment points: post‐intervention and 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no (manual freely available on request) Author contacted for outcome data: no There were 44 clusters and we assume 22 for each group. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned to one of two study conditions" (p.330) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 9.10% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: undertaken but unclear what method used |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. However, data on parental outcomes are not reported due to a high level of non‐response from parents and therefore large amounts of missing data. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Horowitz a2007.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 9.7 (sub‐threshold) Mean age: 14.4 Age range: 14 to 15 Percentage male: 46.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: CB programme (based on Coping with Stress programme) Number of sessions: 8 sessions Length of sessions: 90 minutes Intensity (total number of hours): 12 hours Duration of treatment period: 8 weeks Group size: 8 to 15 (median 11) Delivered by: students Fidelity: not assessed Type of comparison: TAU comprising normal health classes in which students were taught the standard wellness curriculum |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI and CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "A random number list was used...to assign participants..." (p.695) "Within class periods, participants were randomly assigned to condition unless there were fewer than 15 students participating. This occurred for only two classes...for those two classes, randomization was done at the class level rather than at the individual level" (p.695). Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | "Participants and group leaders were aware of group assignment..." (p.695) |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 1.32% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: the authors undertook sensitivity analyses using an unspecified method. However, the authors state that results did not differ from those using observed cases. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Horowitz b2007.
| Methods | See Horowitz a2007 | |
| Participants | See Horowitz a2007 | |
| Interventions | Broad category: IPT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: IPT‐AST Number of sessions: 8 sessions Length of sessions: 90 minutes Intensity (total number of hours): 12 hours Duration of treatment period: 8 weeks Group size: 8 to 15 (median 11) Delivered by: students Fidelity: not assessed Type of comparison: TAU comprising normal health classes in which students were taught the standard wellness curriculum |
|
| Outcomes | See Horowitz a2007 | |
| Notes | See Horowitz a2007 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | See Horowitz a2007 |
| Allocation concealment (selection bias) | High risk | See Horowitz a2007 |
| Blinding (performance bias and detection bias) Subjects | High risk | See Horowitz a2007 |
| Blinding (performance bias and detection bias) Assessors | Low risk | See Horowitz a2007 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | See Horowitz a2007 |
| Selective reporting (reporting bias) | Unclear risk | See Horowitz a2007 |
| Other bias | Unclear risk | See Horowitz a2007 |
| Implementation integrity | Unclear risk | See Horowitz a2007 |
Hyun 2005.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: residing in a shelter for homeless and runaway youth Diagnostic interview to exclude those with current or previous depression: unclear whether a diagnostic interview was undertaken. Those with elevated depression scores and/or past depression were not excluded. Baseline severity of depression: BDI: 15.3 (mild) Mean age: 15.5 Age range: not specified Percentage male: 100% Setting: homeless shelter State what psychiatric diagnoses were excluded: those diagnosed with any psychiatric disorder were excluded Suicide risk excluded: unclear Parents with history of schizophrenia/bipolar disorder excluded: no Country: South Korea |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: unclear. Session by session information on intervention components available in the publication. Online: no Name of programme: not specified Number of sessions: 8 sessions Length of sessions: 50 minutes Intensity (total number of hours): 6.7 hours Duration of treatment period: 8 weeks Group size: 6 to 8 Delivered by: mental health experts Fidelity: not assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: BDI‐I Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Research participants were randomly assigned..." (p.162) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 15.63% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Jaycox 1994.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: ≥ 0.50 on a composite score based on the z score of the CDI and the Child’s Perception Questionnaire of parental conflict. Those scoring below this composite score, however, were included in the trial subject to availability and space in the groups. What risk was basis of inclusion for selected studies: child’s perception of parental conflict Diagnostic interview to exclude those with current or previous depression: not undertaken. Unclear whether those with current and/or past episodes of depression were excluded. Baseline severity of depression: CDI: 9.5 (sub‐threshold) Mean age: 11.4 Age range: 10 to 13 Percentage male: 53.8% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Penn Prevention Program (Penn Resiliency Program) Number of sessions: 12 sessions Length of sessions: 90 minutes Intensity (total number of hours): 18 hours Duration of treatment period: 12 weeks Group size: 10 to 12 Delivered by: students Fidelity: not assessed Type of comparison: WL |
|
| Outcomes | Diagnosis: established from CDI of ≥ 15 Name of self‐report depression measure: CDI, RADS and a composite measure Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 12 weeks (short‐term) |
|
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no There were 8 clusters and we assumed 4 in each |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Correspondence with study authors revealed that the randomisation sequence was generated by pulling envelopes were picked out of a hat |
| Allocation concealment (selection bias) | Low risk | Correspondence with study authors revealed that the person generating the allocation sequence could not see what was written in each envelope |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 15.38% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: unclear if undertaken |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, the trial commenced with 3 separate intervention groups that were subsequently combined in a post‐hoc manner as there were no differences between them in terms of main outcomes at post‐test. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Karami 2012.
| Methods | Design: RCT Conducted by the team who developed the intervention: unclear |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: those children that were reported to be "depressed". No cut‐point specified, however. What risk was basis of inclusion for selected studies: parental divorce Diagnostic interview to exclude those with current or previous depression: not undertaken. Unclear whether those with current and/or past episodes of depression were excluded. Baseline severity of depression: unclear as means and SDs on CDI at baseline not specified Mean age: 12.0 Age range: 10 to 13 Percentage male: not specified Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Islamic Republic of Iran |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: unclear Online: no Name of programme: not specified Number of sessions: 8 sessions Length of sessions: 60 minutes Intensity (total number of hours): 8 hours Duration of treatment period: 12 weeks Group size: unclear Delivered by: not specified Fidelity: not assessed Type of comparison: unclear; presumably TAU comprising usual care from the welfare centre |
|
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A |
|
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (not provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.78) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: unclear as numbers of participants included in final analyses not specified Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: unclear if undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear if trial undertaken by those that developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Kauer 2012.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: K10 ≥ 16.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: unclear whether a diagnostic interview was undertaken and whether those with current and/or past episodes of depression were excluded Baseline severity of depression: DASS‐21 depression subscale: 20.0 (moderate) Mean age: 18.1 Age range: 14 to 24 Percentage male: 28.0% Setting: GP clinics State what psychiatric diagnoses were excluded: those diagnosed with any severe psychiatric or medical condition (e.g. current psychosis) and those requiring imminent hospitalisation Suicide risk excluded: unclear Parents with history of schizophrenia/bipolar disorder excluded: no Country: Australia |
|
| Interventions | Broad category: BT (for further information on intervention components, see Table 3) Manualised: N/A Online: telephone Name of programme: MOBILETYPE Number of sessions: recommended 2 entries per day Length of sessions: 1 to 3 minutes Intensity (total number of hours): 2.5 hours (based on reported average number of messages sent per day being 3, for an average of 17 days, assuming 3 minutes per message) Duration of treatment period: 2 to 4 weeks Group size: N/A (individual) Delivered by: N/A (self‐monitoring) Fidelity: not assessed Type of comparison: AP |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: DASS‐d Name of clinical report depression measure: N/A Name of anxiety measure: DASS‐a Name of general functioning measure: N/A Assessment points: post‐intervention, between 2 to 4 weeks (short‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...a random seed generators to allocate each program to the 200 identification numbers in at the individual level..." (no pagination specified) |
| Allocation concealment (selection bias) | Low risk | "A research assistant downloaded each program by selecting the next consecutive link for the next study mobile and was blinded to allocation..." (no pagination specified) |
| Blinding (performance bias and detection bias) Subjects | High risk | "Participants...became aware of the group allocation at the post‐test..." (no pagination specified) |
| Blinding (performance bias and detection bias) Assessors | High risk | "...GPs because aware of the group allocation at the post‐test..." (no pagination specified) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 26.3% (numbers from Reid 2011, Figure 1) Means and SDs used in meta‐analysis based on what data: unclear, assume observed cases Intention‐to‐treat analyses: maximum likelihood estimation (based on 114 rather than 118 participants, however) |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, information on the SF‐12 Health Survey, the AUDIT, the Adolescent Coping Scale, and a range of other outcome measures no reported in this paper or in a related publication (i.e. Reid 2011). In addition, 6‐month follow‐up data are also not reported. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Khalsa 2012.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: not specified Mean age: 16.8. Age range: 15 to 19 Percentage male: 57.9% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: third wave (for further information on intervention components, see Table 3) Manualised: unclear Online: no Name of programme: Yoga Ed Number of sessions: 23 to 32 sessions Length of sessions: 30 to 40 minutes Intensity (total number of hours): up to 21.3 hours Duration of treatment period: 11 weeks Group size: unclear Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: TAU comprising normal physical education classes |
|
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A |
|
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (not provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomly assigned by class..." (p.82) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | "...lack of blinding of subjects..." (p.88) |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 17.30% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Kindt 2014.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: low income. To be included schools had to have at least 30% of their pupils living in low‐income areas, however, all young people attending these schools were then eligible for inclusion. Diagnostic interview to exclude those with current or previous depression: not undertaken. Those with current and/or past episodes of depression were not excluded, however. Baseline severity of depression: CDI: 8.5 (sub‐threshold) Mean age: 13.4 Age range: 11 to 16 Percentage male: unclear Setting: school State what psychiatric diagnoses were excluded: none Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: no Country: The Netherlands |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Op Volle Kracht ("At Full Strength") Number of sessions: 16 sessions Length of sessions: unclear Intensity (total number of hours): unclear Duration of treatment period: 5 months Group size: 25 Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: TAU comprising usual school curriculum which, in some schools, did include social skills training |
|
| Outcomes | Diagnosis: Established from CDI of ≥ 19.0 Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: yes (provided) Author contacted for outcome data: yes (provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was conducted within schools at the class level…with an allocation ratio of 1:1…[using] a computerized random number generator with a blocked randomization scheme (block size 2)…" (p.5277) |
| Allocation concealment (selection bias) | Unclear risk | "An independent researcher from the research institute…" (p.5277) generated the randomization sequence. However, the "list of classes that were allocated to control or intervention condition…was communicated to the school by the first author." (p.5277), suggesting that allocation may not have been adequately concealed from study authors. |
| Blinding (performance bias and detection bias) Subjects | High risk | "...the study was not blind...adolescents knew whether they received the program or not" (p.5288) |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 13.00% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: multiple imputations |
| Selective reporting (reporting bias) | High risk | Trial protocol (i.e. Kindt 2012) would suggest that scores on the Children's Negative Cognitive Errors Questionnaire‐Revised, the Children's Response Styles Questionnaire, the Adolescent Cognitive Style Questionnaire, and the substance use and happiness sub‐scales of the Adolescent Life Event Schedule will also be assessed |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | High risk | Implementation integrity assessed: "We decided not to check... program integrity and adherence to the program..." (p.5289) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Kowalenko 2005.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CDI ≥ 18.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. Those with current and/or past episodes of depression not excluded, however. Baseline severity of depression: CDI: 21.6 (severe) Mean age: 14.6 Age range: 13 to 16 Percentage male: 0.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Adolescents Coping with Emotions (ACE) Number of sessions: 8 sessions Length of sessions: 90 minutes Intensity (total number of hours): 12 hours Duration of treatment period: 8 weeks Group size: 8 to 10 Delivered by: mental health experts Fidelity: not assessed Type of comparison: WL |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Correspondence with study authors indicates that 16 of the 17 schools included in this trial were allocated randomly. One "non‐compliant" school changed its allocation from that determined by the randomisation sequence. Method of randomisation also not clear. |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 11.83% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: LOCF |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, although a complete dataset is available for 126 participants, only 9 of the participants with complete data are male. Therefore, a post hoc decision was made to present data for females only. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Liehr 2010.
| Methods | Design: RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: those form an ethnic minority group in the USA recruited during a summer camp Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: SMFQ: 8.8 (sub‐threshold) Mean age: 9.5 Age range: not specified Percentage male: 71.0% Setting: summer camps State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: third wave (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Mindful Schools (mindfulschools.org) Number of sessions: 10 sessions Length of sessions: 15 minutes Intensity (total number of hours): 2.5 hours Duration of treatment period: 2 weeks Group size: unclear Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: TAU comprising lessons prepared by a health educator on the importance of activity, healthy eating and stress management |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: SMFQ Name of clinician‐report depression measure: N/A Name of anxiety measure: SAI‐C Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.70) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 5.55% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Brief report. |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Lillevoll 2014.
| Methods | Design: RCT Conducted by the team who developed the intervention: no. However, the intervention was translated into Norwegian by the research team. |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CES‐D: 11.2 (sub‐threshold) Mean age: 16.8 Age range: 15 to 20 Percentage male: 43.2% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Norway |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: N/A as MoodGYM freely available to access Online: yes Name of programme: MoodGYM Number of sessions: 5 sessions Length of sessions: 45 minutes Intensity (total number of hours): 3.75 hours Duration of treatment period: 6 weeks Group size: N/A as MoodGYM individual‐based programme Delivered by: N/A (self‐monitoring) Fidelity: online, therefore standardised Type of comparison: NT |
|
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A |
|
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: no (MoodGYM freely available) Author contacted for outcome data: yes (not provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "…randomization was undertaken using the SPSS program to generate the random numbers, which then were ordered in ascending order and allocated numbers from 1‐4." (p.4) |
| Allocation concealment (selection bias) | High risk | "…randomization…was undertaken by the first author." (p.4) |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Correspondence with study authors confirmed all outcomes were self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 30.00%. Note that only 8.54% of those randomised actually registered with the MoodGYM programme. Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: mean substitution |
| Selective reporting (reporting bias) | Low risk | Trial protocol indicates that all proposed outcome measures were reported |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Livheim 2014‐study 1(girls).
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: those who, according to school counsellors, were experiencing mild to moderate depression symptoms. Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken but unclear whether those with current and/or past episodes of depression were excluded. Baseline severity of depression: RADS‐2: 65.21 (subthreshold) Mean age: 14.6 Age range: 12.5 ‐to 17.75 Percentage male: 0% Setting: mixed State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: third wave (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Acceptance and Commitment Therapy Number of sessions: 8 sessions Length of sessions: unclear Intensity (total number of hours): unclear Duration of treatment period: 8 weeks Group size: unclear Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: RADS‐2 Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...using a random number table..." (no pagination specified) |
| Allocation concealment (selection bias) | High risk | Correspondence with study authors revealed that although students' names were concealed, the allocation sequence itself was not concealed. Instead, the number table included the condition next to the number sequence. |
| Blinding (performance bias and detection bias) Subjects | High risk | Correspondence with study authors revealed that participants were not blind to allocation |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 12.1% Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: mixed model repeated measures |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Makarushka 2012.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D > 13. Symptoms were also required to be persistent as eligible participants were also required to have a score on the CES‐D of > 16.0 at first screen. What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current depression excluded. Those with past episodes of depression were not excluded, however. Baseline severity of depression: CES‐D: 27.0 (moderate) Mean age: 12.7 Age range: not specified Percentage male: 44.0% Setting: mixed State what psychiatric diagnoses were excluded: dysthymia and mania Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: N/A Online: yes Name of programme: Blues Blaster Number of sessions: 6 modules Length of sessions: unclear Intensity (total number of hours): unclear Duration of treatment period: 6 weeks Group size: N/A (individual‐based intervention) Delivered by: N/A (self‐monitoring) Fidelity: online, therefore standardised Type of comparison: AP |
|
| Outcomes | Diagnosis: established from K‐SADS and LIFE Name of self‐report depression measure: CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 6 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.33) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Low risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported but no detail of blinding. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 14.3% (unbalanced) Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: estimation maximisation algorithm |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, the data on the proportion of participants diagnosed with a depressive disorder at the 6‐month follow‐up period not reported. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Manicavasagar 2014.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: DASS‐21: 8.9 (sub‐threshold) Mean age: 15.4 Age range: 15 to 18 Percentage male: 32.5% Setting: schools and youth centres State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: third wave (for further information on intervention components, see Table 3) Manualised: N/A as website freely available Online: yes Name of programme: Bite Back Number of sessions: 6 sessions Length of sessions: 60 minutes Intensity (total number of hours): 6 hours Duration of treatment period: 6 weeks Group size: N/A (individual‐based intervention) Delivered by: N/A (self‐monitoring) Fidelity: online, therefore standardised Type of comparison: AP |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: DASS‐21 Name of clinician report depression measure: N/A Name of anxiety measure: DASS‐21 Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomly allocated...through a block randomization method…[using] a random number generator in Excel to allocate blocks of 10 participants to one of [the] two conditions" (no pagination specified) |
| Allocation concealment (selection bias) | Low risk | "An independent researcher not associated with this study... " (no pagination specified) |
| Blinding (performance bias and detection bias) Subjects | Low risk | "It was important to conceal...participants' allocated condition..." (no pagination specified) |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 34.5% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken. Instead non‐compliant participants and non‐completers were excluded from subsequent analyses. |
| Selective reporting (reporting bias) | High risk | Trial protocol would suggest that scores on the Student Life Satisfaction Scale (SLSS), the Scale of Positive and Negative Experience (SPANE), modified General Self‐Efficacy Scale (GSE), the modified Rosenberg's Self‐Esteem scale and a number of Wellbeing indicators, as measured by the modified Life Orientation Test ‐ Revised (LOT‐R), were also assessed |
| Other bias | Unclear risk | Trial conducted by those who developed the intervention. Although correspondence with study authors revealed that the research team provided no involvement to participants during the trial. Participants were instead instructed to navigate the website on their own. |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
McCarty 2011.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: MFQ ≥ 14.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: unclear whether diagnostic interview undertaken and whether those with current and/or past episodes of depression were excluded. Those with high scores on the PHQ were, however, excluded. Baseline severity of depression: MFQ: 14.6 (sub‐threshold) Mean age: 13.0 Age range: 12 to 13 Percentage male: 49.2% Setting: school State what psychiatric diagnoses were excluded: none Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: no Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Positive Thoughts and Actions Program Number of sessions: 12 sessions Length of sessions: 50 minutes Intensity (total number of hours): 10 hours Duration of treatment period: 12 weeks Group size: unclear Delivered by: unclear Fidelity: correspondence with authors confirmed fidelity was assessed as adequate Type of comparison: TAU comprising freedom to seek school‐based or other services but not any systematic interventions |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: MFQ. However, data on this outcome could not be included in meta‐analyses because scores were adjusted for baseline depression symptoms as measured by the CDRS‐R. Name of clinician report depression measure: CDRS‐R. However, data on this outcome could not be included in meta‐analyses because scores were adjusted for baseline depression symptoms as measured by the CDRS‐R. Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term), 18 months (long‐term) |
|
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: yes (provided) Author contacted for outcome data: yes (provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Low risk | Correspondence with study authors indicated that parents were given 2 opaque manilla envelopes and were instructed to open or the other at the end of the baseline interview. Each set of envelopes included one with a card indicating "PTA Group" and one indicating "Control". RAs had no way of knowing which card was in which envelope. They were also instructed to return the sealed envelope at the end of the interview for verification. |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Low risk | Correspondence with study authors indicated that the assessors who conducted interviews, including the administration of the CDRS‐R, were blinded to group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 10.5% Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: undertaken but method unclear |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, mean and SD scores on the CDRS‐R were not reported |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes (via correspondence) Implementation integrity adequate: yes (via correspondence) Implementation integrity reported: yes (via correspondence) |
McCarty 2013.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: MFQ ≥ 14.0 (top 25%) What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: unclear whether diagnostic interview undertaken and whether those with current and/or past episodes of depression were excluded. Those with high scores on the PHQ‐9, indicative of current probable MDD, however, were excluded. Baseline severity of depression: MFQ: 14.7 (sub‐threshold) Mean age: 12.7 Age range: 11 to 15 Percentage male: 39.2% Setting: school State what psychiatric diagnoses were excluded: intellectual disability. Unclear whether other psychiatric diagnoses were excluded, however. Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: no Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Positive Thoughts and Actions Program Number of sessions: 12 sessions Length of sessions: 50 minutes Intensity (total number of hours): 10 hours Duration of treatment period: 12 weeks Group size: unclear Delivered by: mental health experts Fidelity: assessed as adequate Type of comparison: other |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: MFQ Name of clinician report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...random number sequences..." (p.556) |
| Allocation concealment (selection bias) | Low risk | "A statistician applied [the] random number sequences..." (p.556) |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to the control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 8.3% Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: general linear model repeated measures analysis |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes (via correspondence) Implementation integrity adequate: yes. Described as excellent for 92.00% of classes. Implementation integrity reported: yes (via correspondence) |
McLaughlin 2011.
| Methods | Design: RCT Conducted by the team who developed the intervention: no. However, the team were involved in adapting the intervention for this setting. |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: "Clinically significant" scores on either the BDI‐II or CES‐D. No cut‐point specified, however. What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: unclear whether diagnostic interview undertaken and whether those with current and/or past depression were excluded. Those with elevated depression scores were, however, excluded. Baseline severity of depression: CES‐D: 19.4 (mild) Mean age: 11.8 Age range: 10 to 15 Percentage male: 59.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: modified version of the Adolescent Coping with Depression programme Number of sessions: 10 sessions Length of sessions: 50 minutes Intensity (total number of hours): 8.2 hours Duration of treatment period: 10 weeks Group size: unclear Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU comprising the Vernon 1998 curriculum (a school‐based coping skills and problem‐solving curriculum) |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: BDI‐Y Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...random number generator..." (p.70) |
| Allocation concealment (selection bias) | High risk | "...school psychologist and school psychology intern...The first halves of numbers generated were assigned to the experimental group and the last half of numbers generated were assigned to the treatment as usual group" (p.70) |
| Blinding (performance bias and detection bias) Subjects | Unclear risk | The nature of the intervention suggests it may have been possible to blind participants to allocation. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | High risk | "The school psychologist, school psychology intern, and a school counsellor were...the data collectors...The school psychology intern is also the author...the experimenter had knowledge of the hypotheses for the study..." (p.77) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 4.0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Low risk | As this trial was reported in a thesis, it is unlikely selective outcome reporting was present |
| Other bias | Unclear risk | Trial not conducted by those who developed the intervention. However, intervention was adapted (reduced to 10 sessions) by the author for this setting. |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Mendelson 2010.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: those living in disadvantaged and/or underserved urban communities Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: SMFQ: score not specified Mean age: 10.1 Age range: 9 to 11 Percentage male: 39.2% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: third wave (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: none specified Number of sessions: 48 sessions Length of sessions: 45 minutes Intensity (total number of hours): 36 hours Duration of treatment period: 12 weeks Group size: 25 Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: WL |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: SMFQ Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 11.4% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Merry 2004.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes. The team were involved in adapting the intervention for this setting. |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current depression, as established from: i) total BDI‐II scores of ≥ 23 and scores of 2 or 3 on items 2 or 9 of the BDI‐II; ii) total BDI‐II scores of ≥ 30; iii) a score of 3 on item 9 of the BDI‐II; iv) total RADS scores of ≥ 77; v) a positive score on any of the "critical items" on the RADS were excluded from all analyses (although they were still eligible to participate in the programme). Unclear whether those with past episodes of depression were also excluded. Baseline severity of depression: BDI‐II: 8.9 (sub‐threshold) Mean age: 14.2 Age range: 13 to 15 Percentage male: 48.4% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: excluded from all analyses (although they were still eligible to participate in the programme) Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: New Zealand |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: RAP‐Kiwi Number of sessions: 11 sessions Length of sessions: 60 minutes Intensity (total number of hours): 11 hours Duration of treatment period: in one school sessions were conducted twice a week for 6 weeks, whilst in the second sessions were conducted once a week for 11 weeks Group size: unclear Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: AP |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: BDI‐II, RADS Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term), 18 months (long‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomization tables..." (p.539) |
| Allocation concealment (selection bias) | Low risk | "Participating students were given a study number. A research assistant who did not know the pupils used these numbers and randomization tables to assign students..." (p.539) |
| Blinding (performance bias and detection bias) Subjects | Unclear risk | Participants were tested to determine whether they were aware to which group they had been allocated. Some were able to correctly determine to which group they had been allocated (11% guess correctly; 14% in the intervention group). |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 15.6% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: although ITT analyses were also undertaken, data presented are based on observed cases |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Mirzamani 2012.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CDS between 96 and 140 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDS: 109.4 (unclear) Mean age: 16.0 Age range: not specified Percentage male: not specified Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Islamic Republic of Iran |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: not specified Number of sessions: not specified Length of sessions: not specified Intensity (total number of hours): not specified Duration of treatment period: not specified Group size: not specified Delivered by: not specified Fidelity: not assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDS Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly..." assigned Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | No information specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 6.1%. Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Unclear if trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Noël 2013.
| Methods | Design: RCT Conducted by the team who developed the intervention: no. However, the team were involved in adapting the intervention for this setting. |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: ≥ 10.0 on CES‐D What risk was basis of inclusion for selected studies: living in a rural community Diagnostic interview to exclude those with current or previous depression: those with current depression not excluded. Unclear whether those with past episodes of depression were excluded Baseline severity of depression: CES‐D: 14.9 (sub‐threshold) Mean age: 13.8 Age range: 13 to 15 Percentage male: 0.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Talk 'n' Time Number of sessions: 12 sessions Length of sessions: 90 minutes Intensity (total number of hours): 18 hours Duration of treatment period: 12 weeks Group size: 8 Delivered by: non‐mental health experts Fidelity: assessed, but unclear if assessed as adequate Type of comparison: WL |
|
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A |
|
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (not provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "…a random number table [was used]..." (p.11) |
| Allocation concealment (selection bias) | Low risk | "…a research assistant who did not do any of the assessments..." (p.11) |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | "...a trained interviewer [undertook assessments]..." (p.11) Unclear whether this interviewer was blind to treatment allocation, however |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: the authors state that "[a]pproximately 8 percent of participants dropped out before providing complete data..." (p.1) Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: possibly LOCF |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, follow‐up data are not reported. |
| Other bias | Unclear risk | Trial not conducted by those who developed the intervention. However, intervention was adapted by the author for this setting. |
| Implementation integrity | Unclear risk | Implementation integrity assessed: yes Implementation integrity adequate: no reported Implementation integrity reported: N/A |
O'Leary‐Barrett 2013.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: those scoring 1 SD above the school average on the hopelessness subscale of the SURPS What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: BSI‐depression: 17.4 (unclear) Mean age: unclear for this sub‐sample Age range: unclear for this sub‐sample Percentage male: unclear for this sub‐sample Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: UK |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: not specified Number of sessions: 2 sessions Length of sessions: 90 minutes Intensity (total number of hours): 3 hours Duration of treatment period: unclear Group size: 6 Delivered by: non‐mental health experts Fidelity: assessed, but not unclear if assessed as adequate Type of comparison: NT |
|
| Outcomes | Diagnosis: established from BSI. Cut‐point, however, unclear. Name of self‐report depression measure: BSI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: 12 months (medium‐term), 24 months (long‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: yes (provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "…a computerized randomization procedure." (p.912) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Unclear risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: data at post‐intervention not available Means and SDs used in meta‐analysis based on what data: unclear Intention‐to‐treat analyses: full information maximum likelihood estimation |
| Selective reporting (reporting bias) | High risk | Protocol implies that data on binge drinking frequency, drinking frequency, drinking quality, drinking problems, as assessed by an abbreviated version of Rutger's Alcohol Problem Index, illicit drug use frequency, emotional and behavioural problems, school attendance, grade attainment, coping skills, motives for drinking, and antisocial behaviours assessed using the BSI were also assessed |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: yes Implementation integrity adequate: no reported Implementation integrity reported: N/A |
Pattison 2001.
| Methods | Design: RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 7.9 (sub‐threshold) Mean age: 10.4 Age range: 9 to 12 Percentage male: 48.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Penn Resiliency Program Number of sessions: 10 sessions Length of sessions: 120 minutes Intensity (total number of hours): 20 hours Duration of treatment period: 11 weeks Group size: 16 Delivered by: unclear Fidelity: not assessed Type of comparison: AP |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: trait anxiety subscale of the STAIC Name of general functioning measure: N/A Assessment points: post‐intervention, 8 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Unclear risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported but there is no detail about blinding. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 4.2% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Petersen 1997.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: not specified Mean age: not specified Age range: 11 to 13 Percentage male: not specified Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: unclear Online: no Name of programme: not specified Number of sessions: 16 sessions Length of sessions: 40 minutes Intensity (total number of hours): 10.7 hours Duration of treatment period: 3 months Group size: unclear Delivered by: mental health experts and students Fidelity: not assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 8.7% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, diagnostic data not presented. |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Puskar 2003.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: RADS ≥ 60.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: RADS: 70.3 (mild) Mean age: 16.0 Age range: 14.1 to 18.3 Percentage male: 18.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Teaching Kids to Cope Number of sessions: 10 sessions Length of sessions: 45 minutes Intensity (total number of hours): 7.5 hours Duration of treatment period: 10 weeks Group size: unclear Delivered by: mental health experts Fidelity: assessed as adequate Type of comparison: TAU. No further description provided. |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: RADS Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "... equal allocation using permuted block randomization within school sites..." (p.74) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 7.9% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: repeated measures analysis using mixed modelling methods |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
Pössel 2004.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CES‐D: 8.6 (sub‐threshold). Mean age: 14.0 Age range: not specified Percentage male: 52.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Germany |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: LISA‐T Number of sessions: 10 sessions Length of sessions: 90 minutes Intensity (total number of hours): 15 hours Duration of treatment period: 10 weeks Group size: 8 to 24 Delivered by: Mental health experts and students Fidelity: assessed but unclear if assessed as adequate Type of comparison: NT |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 3 months (short‐term), 6 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...classes were to be randomly assigned...We tried to recruit both training and control groups in each school; however, there was one school with only one class, which we assigned to the training group. In another school with three classes, we randomly assigned two classes to the training group" (p.1005) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 27.4% Means and SDs used in meta‐analysis based on what data: observed cases (based on those who did not miss more than 2 assessments) Intention‐to‐treat analyses: unclear if undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: yes Implementation integrity adequate: no Implementation integrity reported: N/A |
Pössel 2008.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: SBB‐DES: 0.6 (sub‐threshold) Mean age: 13.7 Age range: not specified Percentage male: 53.5% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Germany |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: LARS and LISA‐T Number of sessions: 10 sessions Length of sessions: 90 minutes Intensity (total number of hours): 15 hours Duration of treatment period: 10 weeks Group size: 8 to 18 (median 14) Delivered by: mental health experts and students Fidelity: assessed but unclear if assessed as adequate Type of comparison: NT |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: SBB‐DES Name of clinical report depression measure: N/A Name of anxiety measure: SBB‐ANG Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...classes were randomly assigned to the intervention and control groups..." (p.108) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | "Adolescents, parents, and teachers of the intervention and control groups were informed about the program’s objectives...It was explained that having a control group is essential in order to study the program’s effects" (p.109) |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 10.0% Means and SDs used in meta‐analysis based on what data: observed cases (based on 163 and 138 rather than 163 and 136) Intention‐to‐treat analyses: hierarchical linear model analyses |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: yes Implementation integrity adequate: N/A Implementation integrity reported: no |
Pössel 2013.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: not specified Mean age: 15.1 Age range: not specified Percentage male: 37.3% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: LARS and LISA Number of sessions: 10 sessions Length of sessions: unclear Intensity (total number of hours): unclear Duration of treatment period: 10 weeks Group size: unclear Delivered by: masters' level clinical psychology students Fidelity: assessed but unclear if assessed as adequate Type of comparison: TAU comprising usual wellness classes |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: SBB‐ANG Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Participants were randomly assigned…" (p.433) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Low risk | "Both interventions were described to students... as probably efficacious" (p.434) |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 12.0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. Brief report. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | High risk | Implementation integrity assessed: no. Only assessed via self‐ratings of the material covered within each session. Implementation integrity adequate: unclear Implementation integrity reported: no |
Quayle 2001.
| Methods | Design: RCT Conducted by the team who developed the intervention: no. However, the team were involved in adapting the intervention for this setting. |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 7.4 (sub‐threshold) Mean age: not specified Age range: 11 to 12 Percentage male: 0.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: The Optimism and Lifeskills Program (adapted from the Penn Resiliency Program) Number of sessions: 8 sessions Length of sessions: 80 minutes Intensity (total number of hours): 10.7 hours Duration of treatment period: 8 weeks Group size: 12 Delivered by: students Fidelity: not assessed Type of comparison: WL |
|
| Outcomes | Diagnosis: established from CDI ≥ 13.0 Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 29.8% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | High risk | Protocol not available. Analyses of those scoring above the clinical cut‐point for depression appear post hoc. |
| Other bias | High risk | Trial not conducted by those who developed the intervention. However, intervention was adapted by the author for this setting. |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Reynolds 2011.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: DASS‐d: 5.0 (subthreshold) Mean age: 17.9 Age range: not specified Percentage male: 45.7% Setting: college State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: BT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Brief Behavioral Activation Treatment for Depression (BATD) Number of sessions: 15 sessions Length of sessions: 120 minutes Intensity (total number of hours): 30 hours Duration of treatment period: 15 weeks Group size: unclear Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU comprising classes to facilitate student adjustment, including: academic skills, career exploration, library resources, campus safety, sexuality, diversity and responsible decision making. Students were encouraged to make contact with a faculty advisor and to keep diaries reflecting on the process of adjusting to college life. |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: DASS‐d Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | "Research assistants were not affiliated in any way with the courses...Research assistants were blind to the class condition as well as the study hypotheses" (p.557). However, primary outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 9.6% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: generalised estimating equations |
| Selective reporting (reporting bias) | High risk | Protocol not available. Outcomes assessed with the DASS, which includes an anxiety subscale. Data on this outcome not reported, however. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Rivet‐Duval 2010.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: RADS: 15.2 (sub‐threshold) Mean age: 14.0 Age range: 12 to 16 Percentage male: 50.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Mauritius |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: RAP Number of sessions: 11 sessions Length of sessions: 60 minutes Intensity (total number of hours): 11 hours Duration of treatment period: 11 weeks Group size: 8 to 12 Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: WL |
|
| Outcomes | Diagnosis: unclear how this was established Name of self‐report depression measure: RADS‐2 Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.70) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | High risk | "Teachers running the RAP‐A program randomly assigned the students..." (p.70) |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | "All forms were scored by the primary researcher (not blinded to group allocation" (p.88) However, primary outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: N/A as 0% dropouts |
| Selective reporting (reporting bias) | Low risk | As this trial was reported in a thesis, it is unlikely selective outcome reporting was present |
| Other bias | Low risk | Trial not conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Roberts 2003.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: children in each class were rank ordered on the basis of CDI scores. The 13 children with the highest score from each class were invited to participate. In classes with fewer than 13 students, all were eligible to participate. Diagnostic interview to exclude those with current or previous depression: those with current and/or past depression not excluded Baseline severity of depression: CDI: 11.1 (sub‐threshold) Mean age: 11.9 Age range: 11 to 13 Percentage male: 50.3% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Penn Resiliency Program Number of sessions: 12 sessions Length of sessions: 120 minutes Intensity (total number of hours): 24 hours Duration of treatment period: 12 weeks Group size: unclear Delivered by: mental health experts and school nurses Fidelity: assessed as adequate Type of comparison: TAU comprising monitoring of symptoms and regular health curriculum |
|
| Outcomes | Diagnosis: CDI ≈ 15.0 Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: RCMAS Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term), 30 months (long‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.623) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | "Parents were informed of their child's school group status..." (p.623). Parents therefore could have communicated allocation to their children. |
| Blinding (performance bias and detection bias) Assessors | High risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 5.3% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Trial not conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: "With only one exception, facilitators achieved a high level of program integrity..." (p.623) Implementation integrity reported: yes |
Roberts 2010.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken. Reported that between 5% to 7% of participants had experienced a mental health condition at some point in their life. Baseline severity of depression: CDI: 7.8 (sub‐threshold) Mean age: 12.0 Age range: 11 to 13 Percentage male: 45.6% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Aussie Optimism Program Number of sessions: 20 sessions Length of sessions: 60 minutes Intensity (total number of hours): 20 hours Duration of treatment period: 20 weeks Group size: unclear Delivered by: non‐mental health experts Fidelity: assessed as adequate Type of comparison: TAU comprising 20 regular health education classes relating to self‐improvement and interpersonal skills. Lessons had similar learning outcomes to the intervention. |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: RCMAS Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term), 18 months (long‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | "...trained research assistants [who were] blind to group allocation" (p.70) However, primary outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 13.9% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: assessed as high fidelity Implementation integrity reported: yes |
Rohde 2014a.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: endorsed 2 or more symptoms on the CES‐D What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken but unclear if those with current and/or past episodes of depression were excluded Baseline severity of depression: CES‐D: 1.40 (subthreshold) Mean age: 15.5 Age range: 13 to 19 Percentage male: 32.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: none specified Number of sessions: 6 sessions Length of sessions: 60 minutes Intensity (total number of hours): 6 hours Duration of treatment period: 6 weeks Group size: 4 to 8 Delivered by: female masters‐level graduate students Fidelity: assessed as adequate Type of comparison: TAU comprising an NIMH educational brochure describing symptoms of MDD and treatment options |
|
| Outcomes | Diagnosis: established from the K‐SADS Name of self‐report depression measure: N/A Name of clinician report depression measure: K‐SADS Name of anxiety measure: N/A Name of general functioning measure: SAS‐SR‐Y Assessment points: post‐intervention, 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: yes (provided) Author contacted for outcome data: yes (provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐generated random numbers" (p.67) |
| Allocation concealment (selection bias) | High risk | Correspondence with study authors indicated the project co‐ordinator who derived the random sequence was not independent of the research team |
| Blinding (performance bias and detection bias) Subjects | High risk | Correspondence with study authors revealed that participants were not blind to treatment allocation |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | "Assessors...were blind to condition..." (p.67) However, social functioning outcomes are self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 4.0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using multiple imputation |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: all sessions assessed as delivered with competence Implementation integrity reported: yes |
Rohde 2014b.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: endorsed 2 or more symptoms on the CES‐D What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken but unclear if those with current and/or past episodes of depression were excluded Baseline severity of depression: CES‐D: 1.47 (subthreshold) Mean age: 19.0 Age range: 17‐22 Percentage male: 30.5% Setting: college State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: none specified Number of sessions: 6 sessions Length of sessions: 60 minutes Intensity (total number of hours): 6 hours Duration of treatment period: 6 weeks Group size: 4 to 8 Delivered by: female masters‐level graduate students Fidelity: assessed as adequate Type of comparison: TAU comprising an NIMH educational brochure describing symptoms of MDD and treatment options |
|
| Outcomes | Diagnosis: established from the K‐SADS Name of self‐report depression measure: N/A Name of clinician report depression measure: K‐SADS. Please note that mean and SDs for this outcome variable obtained through correspondence differ modestly from the published values. There is no material difference in terms of direction or magnitude, however. Name of anxiety measure: N/A Name of general functioning measure: adapted from 17 items from the SAS‐SR‐Y Assessment points: post‐intervention, 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: yes (provided) Author contacted for outcome data: yes (provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐generated random numbers…" (p.49) |
| Allocation concealment (selection bias) | Unclear risk | "...assigned by the project coordinator..." (p.49) Unclear if the project co‐ordinator was independent from the research team, however |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | "Assessors were blind to condition..." (p.49) However, social functioning outcomes are self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 10.0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using imputed data in 20 data sets |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: described as "...good or very good fidelity..." (p.51) Implementation integrity reported: yes |
Rooney 2006.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken but unclear whether those with current depression excluded. Those with past episodes of depression not excluded. Baseline severity of depression: CDI: 13.9 (mild) Mean age: 9.1 Age range: 8 to 9 Percentage male: 56.7% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Positive Thinking Program Number of sessions: 8 sessions Length of sessions: 60 minutes Intensity (total number of hours): 8 hours Duration of treatment period: 8 weeks Group size: unclear Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU comprising regular health education curriculum |
|
| Outcomes | Diagnosis: established from the DICA‐IV Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: RCMAS Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term), 18 months (long‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly allocated..." (p.79) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 11.8% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Rooney 2013.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken but unclear whether those with current and/or past depression excluded Baseline severity of depression: CDI: 12.0 (subthreshold) Mean age: 8.8 Age range: 9 to 10 Percentage male: 51.4% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Aussie Optimism: Positive Thinking Program Number of sessions: 10 sessions Length of sessions: 60 minutes Intensity (total number of hours): 10 hours Duration of treatment period: 10 weeks Group size: unclear Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU comprising regular health education curriculum |
|
| Outcomes | Diagnosis: established from the DICA‐IV Name of self‐report depression measure: CDI (minus item 9) Name of clinical report depression measure: Name of anxiety measure: SCAS Name of general functioning measure: N/A Assessment points: post‐intervention, 6 months (medium‐term), 18 months (long‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly allocated..." (p.847) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | "...clinicians were blind to the school conditions and they were not aware of the intervention effects on the students" (p.852) However, primary outcomes are self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 2.4% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using GLMM (i.e. LOCF) |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
Rose 2014.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes (specifically, PIR component) |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 8.2 (sub‐threshold) Mean age: 12.2 Age range: 9‐14 Percentage male: 56.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: RAP plus Peer Interpersonal Relatedness (PIR) Number of sessions: 20 sessions Length of sessions: 50 minutes Intensity (total number of hours): 16.7 hours Duration of treatment period: 20 weeks Group size: 6 to 12 Delivered by: students Fidelity: assessed as adequate Type of comparison: AP |
|
| Outcomes | Diagnosis: established from scores on the major depression subscale of the DISCAP Name of self‐report depression measure: CDI and RADS‐2. Scores on the CDI will be extracted in preference in the present review. Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and approx. 9 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (not provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.512) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Low risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Diagnostic interviews were "...administered by a senior clinical psychologist who was unaware of the experimental conditions" (p.513). However, primary outcomes are self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 1.4% (for RAP‐PIR and control groups) Means and SDs used in meta‐analysis based on what data: hierarchical linear modelling which the authors explain "...can accommodate missing data..." (p.514) Intention‐to‐treat analyses: N/A |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the PIR intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
Sawyer 2010.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 14.4 (sub‐threshold) Mean age: 13.1 Age range: not specified Percentage male: 47.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: beyondblue Secondary Schools Research Initiative Number of sessions: 10 sessions over 3 years Length of sessions: 45 minutes Intensity (total number of hours): 22.5 hours Duration of treatment period: 3 years Group size: unclear Delivered by: non‐mental health experts Fidelity: assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term), 24 months (long‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (not provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly allocated..." (p.202) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Low risk | "...by a research assistant who was blind to the groups to which schools were being allocated" (p.202) |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | All outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: unclear Means and SDs used in meta‐analysis based on what data: adjusted conditional model using a dummy variable coded as 1 if the assessment was incomplete and as 0 if compete Intention‐to‐treat analyses: N/A |
| Selective reporting (reporting bias) | Low risk | Protocol not available. However, supplementary files on the beyondblue website would indicate that all intended outcomes were reported. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
Schmiege 2006.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: families in which a parent had recently died Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken but those with current and/or past episodes of depression not excluded Baseline severity of depression: CDI: 9.8 (subthreshold) Mean age: 11.4 Age range: not specified Percentage male: 53.0% Setting: mail solicitation, media outlets, agencies in contact with recently bereaved families (e.g. churches, schools, hospitals) State what psychiatric diagnoses were excluded: conduct disorder, oppositional defiance disorder, ADHD (unmedicated), aggressive and/or delinquent children Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: no Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: The Family Bereavement Program Number of sessions: 12 sessions Length of sessions: 120 minutes Intensity (total number of hours): 24 hours Duration of treatment period: 12 weeks (3 months) Group size: 5 to 9 Delivered by: mental health experts Fidelity: not assessed Type of comparison: AP |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: RCMAS Name of general functioning measure: N/A Assessment points: post‐intervention and 11 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...computer program..." (p.589, Sandler 2003) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, attention placebo condition was not credible. Without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 3.7% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: modelling approaches undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. However, many outcomes are also reported in Sandler 2003. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Seligman 1999.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: scoring in the bottom quartile on the ASQ Diagnostic interview to exclude those with current or previous depression: those with current depression (indicated by a score of ≥ 19.0 on the BDI) were excluded. Those with past episodes of depression not excluded (7.5%). Baseline severity of depression: BDI: 7.3 (subthreshold) Mean age: not stated (19.0) Age range: All participants were 19 Percentage male: 48.0% Setting: university State what psychiatric diagnoses were excluded: those with current anxiety disorders, substance use/disorder, mania, cyclothymia, psychosis, somatisation disorder, hypochondriasis, undifferentiated somatoform disorder, anorexia, and bulimia Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: no Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Prevention Program (APEX) Number of sessions: 8 sessions Length of sessions: 120 minutes Intensity (total number of hours): 16 hours Duration of treatment period: 8 weeks Group size: 10 to 12 Delivered by: mental health experts Fidelity: not assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: LIFE Name of self‐report depression measure: BDI Name of clinical report depression measure: HAM‐D Name of anxiety measure: BAI Name of general functioning measure: N/A Assessment points: post‐intervention, 12 months (medium‐term), and 36 months (long‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | "To determine if diagnostic interviewers were blind as to which condition participants were in, following each interview, we had them guess...At all the evaluations but one...interviewers were unable to accurately guess which condition participants were in" (no pagination specified). Some outcomes, however, were self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 3.5% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using survival analysis |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, the authors did undertake post‐hoc analyses of those with moderate versus severe depression symptomatology. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Seligman 2007.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: BDI score between 9 and 24 Diagnostic interview to exclude those with current and/or past episodes of depression: those with current and/or past episodes of depression not excluded Baseline severity of depression: BDI: 10.1 (subthreshold) Mean age: not stated (19.0) Age range: All participants were 19 Percentage male: 35.0% Setting: university State what psychiatric diagnoses were excluded: exclusion criteria not stated Suicide risk excluded: exclusion criteria not stated Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not stated Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: partly. Included ongoing web‐based materials such as email coaching. Name of programme: APEX Number of sessions: 8 sessions Length of sessions: 120 minutes Intensity (total number of hours): 16 hours Duration of treatment period: 8 weeks Group size: 10 to 12. Email coaching individual. Delivered by: mental health experts Fidelity: not assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: LIFE Name of self‐report depression measure: BDI Name of clinical report depression measure: HAM‐D Name of anxiety measure: BAI Name of general functioning measure: N/A Assessment points: post‐intervention and between 4 to 6 months (medium term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | "...research assistants asked participants not to tell the interviewer which condition they were in" (p.1118) Primary outcomes, however, were self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 5.4% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using survival analysis |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, the authors did undertake post‐hoc analyses of those with moderate versus severe depression symptomatology. Additionally, follow‐up data were not reported. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Sethi 2010.
| Methods | Design: RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: not specified What risk was basis of inclusion for selected studies: mild to moderate depression according to DASS‐21 scores Diagnostic interview to exclude those with current and/or past episodes of depression: those with past episodes of depression not excluded, however, those with current depression, as indicated by extremely high scores on the DASS‐21, were excluded Baseline severity of depression: DASS‐21‐d: 18.20 (moderate) Mean age: 19.5 Age range: 18 to 23 Percentage male: 21% Setting: university State what psychiatric diagnoses were excluded: exclusion criteria not stated Suicide risk excluded: exclusion criteria not stated Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not stated Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: N/A as MoodGYM freely available to access Online: yes Name of programme: MoodGYM Number of sessions: 3 sessions plus 2 assessment‐only sessions Length of sessions: between 20 to 40 minutes Intensity (total number of hours): up to 3.33 hours Duration of treatment period: 3 weeks Group size: N/A (individual‐based intervention) Delivered by: N/A (self‐monitoring) Fidelity: online, therefore standardised Type of comparison: NT |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: DASS‐21‐d Name of clinical report depression measure: N/A Name of anxiety measure: DASS‐21‐a Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: N/A as MoodGYM freely available to access Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: N/A as 0% dropouts |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Trial not conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Shatte 1997.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current and/or past episodes of depression not excluded Baseline severity of depression: CDI: 12.3 (mild) Mean age: 12.7 Age range: 12 to 14 Percentage male: 53.3% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Penn Resiliency Program Number of sessions: 12 sessions Length of sessions: 120 minutes Intensity (total number of hours): 24 hours Duration of treatment period: 12 weeks Group size: 9 Delivered by: non‐mental health experts and students Fidelity: assessed as adequate Type of comparison: AP |
|
| Outcomes | Diagnosis: CDI ≥ 12.0 Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Low risk | Each programme "...was presented to parents and teachers as based on established psychological theory..." (p.17) |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Outcomes self‐reported and no detail of blinding of participants. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 6.6% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: undertaken, but method unclear |
| Selective reporting (reporting bias) | High risk | Protocol not available. However, the authors did undertake post‐hoc analyses of those completing 3 of the initial 4 sessions versus those who completed 8 of the 12 sessions. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
Sheffield a2006.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: scoring in the top 20% on the combined CDI and CES‐D What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interviews not undertaken to exclude those with current depression. Those with past episodes of depression not excluded. Baseline severity of depression: CDI: 22.0 (severe) Mean age: 14.3 Age range: 13 to 15 Percentage male: 31.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Problem‐Solving for Life Number of sessions: 8 sessions Length of sessions: 45 minutes Intensity (total number of hours): 6 hours Duration of treatment period: 2 school terms Group size: unclear Delivered by: non‐mental health experts and students Fidelity: not assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI and CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: SCAS Name of general functioning measure: CASAFS Assessment points: post‐intervention and 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...randomly allocated [...]using a number drawn by the researchers at random from a container..." (p.67) |
| Allocation concealment (selection bias) | Low risk | "...the sequence [was] concealed until assignment..." (p.67) |
| Blinding (performance bias and detection bias) Subjects | High risk | "...participants...were not blind to experimental condition" (p.70) |
| Blinding (performance bias and detection bias) Assessors | High risk | "...participants...and assessors were not blind to experimental condition" (p.70) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 10.2% (universal intervention) and 7.3% (targeted intervention) Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using hierarchical linear modelling |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Trial not conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Sheffield b2006.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: scoring in the top 20% on the combined CDI and CES‐D What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interviews not undertaken to exclude those with current depression. Those with past episodes of depression not excluded. Baseline severity of depression: CDI: 22.0 (severe) Mean age: 14.3 Age range: 13 to 15 Percentage male: 31.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Problem‐Solving for Life Number of sessions: 16 sessions Length of sessions: 8 sessions of 45 minutes plus 8 sessions of 90 minutes Intensity (total number of hours): 18 hours Duration of treatment period: 2 school terms Group size: 8 to 10 Delivered by: non‐mental health experts and students Fidelity: not assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: ADIS‐C MDD, DYS and LIFE Name of self‐report depression measure: CDI and CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: SCAS Name of general functioning measure: CASAFS Assessment points: post‐intervention and 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Sheffield a2006 |
| Allocation concealment (selection bias) | Low risk | See Sheffield a2006 |
| Blinding (performance bias and detection bias) Subjects | High risk | See Sheffield a2006 |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | See Sheffield a2006 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | See Sheffield a2006 |
| Selective reporting (reporting bias) | Unclear risk | See Sheffield a2006 |
| Other bias | Unclear risk | See Sheffield a2006 |
| Implementation integrity | Unclear risk | See Sheffield a2006 |
Sheffield c2006.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interviews not undertaken to exclude those with current depression. Those with past episodes of depression not excluded. Baseline severity of depression: CDI: 11.1 (subthreshold) Mean age: 14.3 Age range: 13 to 15 Percentage male: 46.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Problem‐Solving for Life Number of sessions: 8 sessions Length of sessions: 45 minutes Intensity (total number of hours): 6 hours Duration of treatment period: one school term Group size: unclear Delivered by: non‐mental health professionals Fidelity: not assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI and CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: SCAS Name of general functioning measure: CASAFS Assessment points: post‐intervention and 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes(not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | See Sheffield a2006 |
| Allocation concealment (selection bias) | Low risk | See Sheffield a2006 |
| Blinding (performance bias and detection bias) Subjects | High risk | See Sheffield a2006 |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | See Sheffield a2006 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | See Sheffield a2006 |
| Selective reporting (reporting bias) | Unclear risk | See Sheffield a2006 |
| Other bias | Unclear risk | See Sheffield a2006 |
| Implementation integrity | Unclear risk | See Sheffield a2006 |
Snyder 2010.
| Methods | Design: RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview not undertaken to exclude those with current depression, those with past episodes of depression not excluded Baseline severity of depression: CES‐D: 8.4 (subthreshold) Mean age: not specified Age range: 13 to 14 Percentage male: 40.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: USA |
|
| Interventions | Broad category: third wave (positive psychology) (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Positive Psychoeducation Number of sessions: 7 sessions Length of sessions: 40 minutes Intensity (total number of hours): 4.7 hours Duration of treatment period: 7 weeks Group size: 5 Delivered by: mental health experts Fidelity: assessed as adequate Type of comparison: AP |
|
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.30) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Low risk | "To keep parents and participants blind to the hypotheses, limited detail about the content of the groups was provided" (p.30) |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Outcomes self‐reported but unclear detail about blinding. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 7.1% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Low risk | As this trial was reported in a thesis, it is unlikely selective outcome reporting was present |
| Other bias | Low risk | Trial not conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
Spence 2003.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current depression excluded. Those with past episodes of depression not excluded. Baseline severity of depression: BDI: 7.8 (subthreshold) Mean age: 12.8 Age range: 12 to 14 Percentage male: 48.5% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Problem Solving for Life Number of sessions: 8 sessions Length of sessions: 45 minutes Intensity (total number of hours): 6 hours Duration of treatment period: 8 weeks Group size: unclear Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: ADIS‐C and LIFE Name of self‐report depression measure: BDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: CASAFS Assessment points: post‐intervention, 12 months (medium‐term) and 36 months (long‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 15.6% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: unclear if undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Stallard 2012a.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: MFQ ≥ 2.0 over 2 assessments approx. 2 weeks apart What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview not used to exclude those with current depression. Those with past episodes of depression not excluded. Baseline severity of depression: SMFQ: 10.6 (subthreshold) Mean age: not specified Age range: 8 to 11 Percentage male: 34.9% Setting: school State what psychiatric diagnoses were excluded: none Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: no Country: UK |
|
| Interventions | Broad category: CBT with elements of IPT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: RAP Number of sessions: 9 sessions and 2 booster sessions Length of sessions: 50 to 60 minutes Intensity (total number of hours): up to 9 hours (not including booster sessions) Duration of treatment period: unclear Group size: unclear Delivered by: students with at least an undergraduate degree in a relevant discipline Fidelity: assessed as adequate Type of comparison: TAU comprising usual personal health and social education classes provided by the school |
|
| Outcomes | Diagnosis: SMFQ ≥ 5.0 Name of self‐report depression measure: SMFQ Name of clinical report depression measure: N/A Name of anxiety measure: RCADS Name of general functioning measure: N/A Assessment points: 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...we allocated year groups on a 1:1:1 ratio. We balanced the trial arms for key characteristics by calculating an imbalance statistic for a large random sample of possible allocation sequences" (no pagination specified). |
| Allocation concealment (selection bias) | Low risk | "A statistician with no other involvement in the study randomly selected one sequence from a subset..." (no pagination specified) |
| Blinding (performance bias and detection bias) Subjects | Unclear risk | The nature of the trial suggests it is likely participants could have remained blind to the fact they were allocated to a placebo control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Outcomes self‐reported but there is no detail about blinding. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 21.1% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: multiple imputation |
| Selective reporting (reporting bias) | Low risk | Trial protocol (i.e. Stallard 2010) would suggest that all intended outcomes were assessed |
| Other bias | Low risk | Trial not conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes (5% of sessions) Implementation integrity adequate: 89.00% of sessions covered core tasks, with at least 75% of the core tasks covered in the remaining 11% of sessions Implementation integrity reported: yes |
Stice 2006.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 20.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken and those with current depression indicated by BDI ≥ 30.0 were excluded. Those with previous episodes of depression not excluded. Baseline severity of depression: BDI: 19.9 (mild‐moderate) Mean age: 18.4 Age range: 15 to 22 Percentage male: 30.0% Setting: school State what psychiatric diagnoses were excluded: none Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: no Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: The Blues Group Number of sessions: 4 sessions Length of sessions: 60 minutes Intensity (total number of hours): 4 hours Duration of treatment period: 4 weeks Group size: 6 to 10 Delivered by: students Fidelity: not assessed Type of comparison: WL |
|
| Outcomes | Diagnosis: BDI ≥ 30.0 Name of self‐report depression measure: BDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 6 months (short‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no Coding for depression severity at baseline: where baseline severity was mild to moderate as in this trial, it was rated as mild. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a wait‐list control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 18.0% by 6 months Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: full information maximum likelihood ratio based on expectation‐maximisation algorithm |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Stice 2008.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 20.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken and those with current and/or previous episodes of depression excluded Baseline severity of depression: BDI: 19.8 (mild‐moderate) Mean age: 15.6 Age range: 14 to 19 Percentage male: 44.0% Setting: school State what psychiatric diagnoses were excluded: none Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: no Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: not specified Number of sessions: 6 sessions Length of sessions: 60 minutes Intensity (total number of hours): 6 hours Duration of treatment period: 6 weeks Group size: 6 to 10 Delivered by: students Fidelity: assessed as adequate Type of comparison: NT |
|
| Outcomes | Diagnosis: 16 item version of the K‐SADS Name of self‐report depression measure: BDI and a continuous score created by summing items from the K‐SADS Name of clinical report depression measure: 16 item version of the K‐SADS Name of anxiety measure: N/A Name of general functioning measure: SAS‐SR‐Y Assessment points: post‐intervention and 6 months (short‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (provided) Author contacted for outcome data: no Coding for depression severity at baseline: where baseline severity was mild to moderate as in this trial, it was rated as mild. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐generated random numbers..." (p.597) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | "...assessors...were blind to condition..." (p.597) Primary outcomes, however, were self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 1.2% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: full information maximum likelihood ratio based on expectation‐maximisation algorithm |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available. However, depression outcomes at 2 years not reported. Additionally, the authors did undertake post‐hoc analyses of clinically significant change in depression symptomatology. |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: yes Implementation integrity adequate: yes Implementation integrity reported: yes |
Stoppelbein 2003.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: no |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: a score of 50 to 70 on the CDI What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those with current and/or past episodes of depression not excluded Baseline severity of depression: CDI: 10.6 (subthreshold) Mean age: 15.0 Age range: not specified Percentage male: 41.0% Setting: school State what psychiatric diagnoses were excluded: those with any “current psychiatric diagnosis” excluded from analyses Suicide risk excluded: unclear Parents with history of schizophrenia/bipolar disorder excluded: no Country: USA |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Coping with Depression Number of sessions: 10 sessions Length of sessions: 50 minutes Intensity (total number of hours): 8.3 hours Duration of treatment period: 10 weeks Group size: 20 Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU comprising didactic lectures about general topics in psychology |
|
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A |
|
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: no Author contacted for outcome data: yes (not provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly assigned..." (p.41) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | Unclear risk | The clustered nature of allocation suggests it is possible participants could have been unable to determine to which group they had been allocated. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 11.9% Means and SDs used in meta‐analysis based on what data: observed cases (based on those who completed 8 or more sessions) Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Low risk | Trial not conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Whittaker 2012.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview was not undertaken, however, those with RADS scores ≥ 76.0 or those with current depression according to the CRDS‐R were excluded. Unclear whether those with past episodes of depression were also excluded. Baseline severity of depression: RADS‐II: 53.5 (subthreshold) Mean age: 14.3 Age range: 13 to 17 Percentage male: 31.7% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: New Zealand |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: N/A Online: telephone Name of programme: MEMO Number of sessions: 2 mobile telephone messages per day (mixture of SMS messages and links to videos and/or external websites) Length of sessions: unclear Intensity (total number of hours): unclear Duration of treatment period: 9 weeks Group size: telephone messages (individual) Delivered by: N/A (self‐monitoring) Fidelity: N/A, although only three‐quarters of participants viewed at least half of the messages sent Type of comparison: AP |
|
| Outcomes | Diagnosis: K‐SADS Name of self‐report depression measure: RADS‐2 and MFQ Name of clinical report depression measure: CDRS‐R Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: yes (provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐based randomisation..." (no pagination specified) |
| Allocation concealment (selection bias) | Low risk | "...allocation concealment was maintained by computer‐based randomisation so that researchers were unaware of possible allocation" (no pagination specified) |
| Blinding (performance bias and detection bias) Subjects | Low risk | "Participants were not aware of which program was the intervention and which was the control..." (no pagination specified) |
| Blinding (performance bias and detection bias) Assessors | Low risk | "The interviews were conducted by research assistants blinded to allocation..." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 2.3% Means and SDs used in meta‐analysis based on what data: observed cases (via correspondence) Intention‐to‐treat analyses: LOCF (via correspondence) |
| Selective reporting (reporting bias) | Low risk | Trial protocol would suggest that all intended outcomes were assessed |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Wijnhoven 2014.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CDI ≥ 16.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: although diagnostic interviews were not undertaken, those with CDI score of ≥ 19.0 were excluded. Those with past episodes of depression not excluded. Baseline severity of depression: CDI: 20.9 (severe) Mean age: 13.3 Age range: 11 to 15 Percentage male: 0% Setting: school State what psychiatric diagnoses were excluded: those currently receiving mental health care were excluded Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: no Country: The Netherlands |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Op Volle Kracht ("At Full Strength") Number of sessions: 8 sessions Length of sessions: 50 minutes Intensity (total number of hours): 6.7 hours Duration of treatment period: 8 weeks Group size: unclear Delivered by: mental health experts Fidelity: not assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDU and CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...using a computerized random number generator..." (p.3) |
| Allocation concealment (selection bias) | Low risk | "An independent researcher performed the randomization..."(p.3) |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a non‐treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 15.3% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: full information maximum likelihood ratio based on expectation‐maximisation algorithm |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Wong 2014.
| Methods | Design: cluster‐RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: PHQ‐5: 2.9 (unclear) Mean age: not specified Age range: 14 to 15 Percentage male: 30.0% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not reported Suicide risk excluded: exclusion criteria not reported Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not reported Country: Australia |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: N/A (online) Online: yes Name of programme: Thiswayup Schools Number of sessions: 7 sessions Length of sessions: 40 minutes Intensity (total number of hours): 4.7 hours Duration of treatment period: 7 weeks Group size: individual‐based therapy Delivered by: N/A (self‐monitoring) Fidelity: N/A. Online, therefore standardised. Type of comparison: TAU comprising regular health and personal development classes |
|
| Outcomes | Diagnosis: (no useable data) Name of self‐report depression measure: (no useable data) Name of clinical report depression measure: (no useable data) Name of anxiety measure: (no useable data) Name of general functioning measure: (no useable data) Assessment points: N/A |
|
| Notes | Author contacted for methodological detail: yes (not provided) Author contacted for treatment manual: yes (not provided) Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...randomly allocated..." (p.91) Method of randomisation not specified, however |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 72.8% Means and SDs used in meta‐analysis based on what data: N/A Intention‐to‐treat analyses: linear mixed‐model repeated measures analysis |
| Selective reporting (reporting bias) | High risk | Protocol indicates knowledge gained with respect to causes and symptoms of depression will also be assessed |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Low risk | Implementation integrity assessed: N/A (standardised) Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Woods 2011.
| Methods | Design: RCT Conducted by the team who developed the intervention: no. However, the team were involved in adapting the intervention for this setting. |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CDI ≥ 63.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not undertaken Baseline severity of depression: CDI: 24.5 (moderate) Mean age: 14.0 Age range: not specified Percentage male: not specified Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: New Zealand |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: ACE‐Kiwi Number of sessions: 8 sessions Length of sessions: 90 minutes Intensity (total number of hours): 12 hours Duration of treatment period: 8 weeks Group size: 8 to 12 Delivered by: mental health experts Fidelity: unclear if assessed Type of comparison: TAU comprising ongoing counselling with the school counsellor and/or referral to mental health services as required |
|
| Outcomes | Diagnosis: N/A Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention, 2 months (short‐term), 12 months (medium‐term) |
|
| Notes | Author contacted for methodological detail: yes (provided) Author contacted for treatment manual: yes (provided) Author contacted for outcome data: yes (provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer‐generated random assignment..." (p.43) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 57.0% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | Trial not conducted by those who developed the intervention. However, intervention was adapted by the author for this setting. |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Young 2006.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 16.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview undertaken and those with current depression and/or those with CES‐D scores ≥ 40.0 were excluded. Those with past episodes of depression were not excluded. Baseline severity of depression: CES‐D: 25.2 (mild) Mean age: 13.4 Age range: 11 to 16 Percentage male: 14.6% Setting: school State what psychiatric diagnoses were excluded: panic disorder, obsessive‐compulsive disorder, post‐traumatic stress disorder, conduct disorder, oppositional defiance disorder, bipolar disorder, psychosis and ADHD (untreated) Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not reported Country: USA |
|
| Interventions | Broad category: IPT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Interpersonal Psychotherapy‐Adolescent Skills Training Number of sessions: 8 sessions Length of sessions: 90 minutes Intensity (total number of hours): 12 hours Duration of treatment period: 8 weeks Group size: 3 to 7 Delivered by: mental health experts Fidelity: not assessed Type of comparison: TAU comprising referral to school counsellors and/or social worker as required. Additional psychotherapy and/or medication was also available as required. |
|
| Outcomes | Diagnosis: K‐SADS‐PL Name of self‐report depression measure: CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: CGAS Assessment points: post‐intervention and 6 months (short‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...using a table of random numbers..." (p.1257) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | "...interviews were performed by a clinical evaluator...who was blind to treatment condition" (p.1257) Primary outcomes, however, were self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 2.4% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: LOCF |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Young 2010a.
| Methods | Design: RCT Conducted by the team who developed the intervention: unclear |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: CES‐D ≥ 16.0 What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: those who met diagnostic criteria for depression were excluded. Unclear whether those with past episodes of depression were also excluded. Baseline severity of depression: CES‐D: 15.2 (subthreshold) Mean age: 14.5 Age range: 11 to 17 Percentage male: 40.3% Setting: school State what psychiatric diagnoses were excluded: panic disorder, obsessive‐compulsive disorder, post‐traumatic stress disorder, conduct disorder, oppositional defiance disorder, bipolar disorder, psychosis and ADHD (untreated) Suicide risk excluded: yes Parents with history of schizophrenia/bipolar disorder excluded: no Country: USA |
|
| Interventions | Broad category: IPT (for further information on intervention components, see Table 3) Manualised: unclear Online: no Name of programme: Interpersonal Psychotherapy‐Adolescent Skills Training Number of sessions: 8 sessions Length of sessions: 90 minutes Intensity (total number of hours): 12 hours Duration of treatment period: unclear Group size: 4 to 6 Delivered by: mental health experts Fidelity: assessed but unclear if assessed as adequate Type of comparison: TAU comprising referral to school counsellors and/or social worker as required |
|
| Outcomes | Diagnosis: K‐SADS Name of self‐report depression measure: CES‐D Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: CGAS Assessment points: post‐intervention, 12 months (medium‐term) and 18 months (long‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: yes (provided) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...table of random numbers..." (p.428) |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have been blind to the fact they were allocated to treatment as usual. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | "The evaluations were conducted by independent evaluators..." (p.429) Primary outcomes, however, were self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 2.8% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: using hierarchical linear modelling and LOCF |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No information specified |
| Implementation integrity | Unclear risk | Implementation integrity assessed: yes Implementation integrity adequate: N/A Implementation integrity reported: N/A |
Yu 2002‐study 3.
| Methods | Design: RCT Conducted by the team who developed the intervention: yes |
|
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: those with scores in the top 25% for their age bracket on combined z scores on the CDI and on the perception of family relationships item of the Cohesion and Conflict subscale of the FES What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: diagnostic interview not undertaken, however, those with current and/or past episodes of depression not excluded Baseline severity of depression: CDI: 17.1 (moderate) Mean age: 11.8 Age range: 8 to 15 Percentage male: 55.5% Setting: school State what psychiatric diagnoses were excluded: exclusion criteria not specified Suicide risk excluded: exclusion criteria not specified Parents with history of schizophrenia/bipolar disorder excluded: exclusion criteria not specified Country: China |
|
| Interventions | Broad category: CBT (for further information on intervention components, see Table 3) Manualised: yes Online: no Name of programme: Penn Resiliency Program, Chinese adaption Number of sessions: 10 sessions Length of sessions: 120 minutes Intensity (total number of hours): 20 hours Duration of treatment period: 10 weeks Group size: 10 to 14 Delivered by: non‐mental health experts Fidelity: not assessed Type of comparison: NT |
|
| Outcomes | Diagnosis: CDI ≥ 15.0 (moderate depression) and CDI ≥ 20.0 (severe depression) Name of self‐report depression measure: CDI Name of clinical report depression measure: N/A Name of anxiety measure: N/A Name of general functioning measure: N/A Assessment points: post‐intervention and 6 months (short‐term) |
|
| Notes | Author contacted for methodological detail: no Author contacted for treatment manual: no Author contacted for outcome data: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information specified |
| Allocation concealment (selection bias) | Unclear risk | No information specified |
| Blinding (performance bias and detection bias) Subjects | High risk | The nature of the trial suggests it is unlikely participants could have remained blind to the fact they were allocated to a no treatment control group. However, without access to the participant information sheets and PLS, level of blinding cannot be ascertained. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Outcomes self‐reported. Assessor blinding therefore not applicable. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportion of participants with incomplete post‐intervention self‐reported depression scores: 2.3% Means and SDs used in meta‐analysis based on what data: observed cases Intention‐to‐treat analyses: N/A |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | High risk | Trial conducted by those who developed the intervention |
| Implementation integrity | Unclear risk | Implementation integrity assessed: unclear if assessed Implementation integrity adequate: N/A Implementation integrity reported: N/A |
ADHD: attention deficit hyperactivity disorder ADIS‐C: Anxiety Disorders Interview Schedule for Children ASQ: Attribution Style Questionniare
AP: attention placebo BAI: Beck Anxiety Inventory BDI: Beck Depression Inventory BDI‐II: Beck Depression Inventory‐second revision BSI: Brief Symptom Inventory
BT: behavioural therapy CASAFS: Child and Adolescent Social and Adaptive Functioning Scale
CATCH‐IT: Competent Adulthood Transition with Cognitive‐behavioral and Interpersonal Training CBT: cognitive behavioural therapy CDI: Children's Depression Inventory CDRS‐R: Children's Depression Rating Scale‐Revised CDS: Children's Depression Scale CES‐D: Center for Epidemiologic Studies Depression Scale CGAS: Children's Global Assessment Scale
CSAQ: Cognitive Somatic Anxiety Questionniare
CURB: DASS: Depression Anxiety Stress Scale
DICA‐IV: Diagnostic Interviewfor Children and Adolescents, version four DISC‐IV: Diagnostic Interview Schedule for Children, version four DISCAP: Diagnostic Interview Schedule for Children, Adolescents, and Parents
FES: Family Environment Scale
GLMM: Generalised Linear Mixed Model GP: general practitioner
HAM‐D: Hamilton Depression Rating Scale HMO: health maintenance organisation IPT: interpersonal therapy IPT‐AST: interpersonal psychotherapy‐adolescent skills training ITT: intention‐to‐treat K‐SADS: Kiddie‐Schedule for Affective Disorders and Schizophrenia for School‐Age Children K‐SADS‐E: Kiddie‐Schedule for Affective Disorders and Schizophrenia‐Epidemiological version K‐SADS‐PL: Kiddie‐Schedule for Affective Disorders and Schizophrenia‐Present and Lifetime version
LARS&LISA‐T: Ease of Handling Social Aspects in Everyday Life‐Training (English Translation) LIFE: Longitudinal Interval Follow‐up Evaluation LOCF: last observation carried forward MASQ: Mood and Anxiety Symptom Questionnaire MDD: major depressive disorder MFQ: Mood and Feeling Questionnaire N/A: not available NIMH: National Institute of Mental Health NT: no treatment PIR: Peer Interpersonal Relatedness
PHQ‐9: Patient Health Questionniare‐9 item version. PLS: plain language statement PQ‐LES‐Q: Paediatric Quality of Life Enjoyment and Satisfaction Questionnaire PRP: Penn Resilience Program
RAP‐PIR: Resourceful Adolescent Program‐Peer Interpersonal Relatedness RADS: Reynold's Adolescent Depression Scale RADS‐2: Reynold's Adolescent Depression Scale, version two RCADS: Revised Child Anxiety and Depression Scale RCMAS: Revised Children's Manifest Anxiety Scale RCT: randomised controlled trial SACQ: Student Adaption to College Questionnaire SAI‐C: State Anxiety Inventory for Children SAS‐SR‐Y: Social Adjustment Scale‐Self‐Report for Youth SAS: Social Adjustment Scale SBB‐DES: Selbstbeurteilungsbogen‐Depressive Störungen (Self‐Report Questionnaire‐Depression) SCAS: Spence Children's Anxiety Scale SCID‐I: Structured Clinical Interview for DSM‐IV SD: standard deviation SMFQ: Short Mood Feeling Questionnaire SWEMWBS: Short Warwick‐Edinburgh Mental Well‐Being Scale TAU: treatment as usual WL: wait‐list YSR: Youth Self‐Report
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abbott 2014 | Participants recruited on the basis of depressive or anxious symptoms |
| Attwood 2012 | Not a RCT |
| Balle 2009 | Not primarily a depression prevention programme ‐ the focus is on anxiety prevention |
| Bannink 2012 | Not primarily a depression prevention programme ‐ th focus is instead on broad mental health or well‐being, or both |
| Barnet 2007 | Not primarily a depression prevention programme ‐ parenting/family intervention or focus on family problems (e.g. divorce) |
| Barrett 2001 | Focus is on anxiety prevention |
| Berger 2008 | Not primarily a depression prevention programme ‐ the focus is on trauma |
| Berry 2009 | Not primarily a depression prevention programme ‐ the focus is on anxiety prevention |
| Bond 2004 | Not primarily a depression prevention programme ‐ the focus is on broad mental health or well‐being, or both |
| Boogar 2012 | Treatment study |
| Boring 2012 | Not primarily a depression prevention programme ‐ the focus is instead on improving relationships, parenting and coping in children with divorced parents |
| Bourque 2013 | No suitable validated depression outcome measure |
| Britton 2014 | No suitable validated depression outcome measure |
| Brody 2012 | Not primarily a depression prevention programme ‐ the focus is instead on broad mental health or well‐being, or both |
| Buttigieg 2015 | Intervention not primarily delivered to the individual (e.g. family therapy or parenting skills) |
| Cabiya 2008 | Not primarily a depression prevention programme ‐ the focus is on treating disruptive behaviours |
| Cook 2015 | Focus is on broad mental health or well‐being, or both |
| Davidson 2014 | Focus is on trauma or PTSD |
| Day 2013 | Participants not within the age bracket specified for this review |
| Gerson 2013 | Study 1: use of alternate allocation. Study 2: participants not within the age bracket specified for this review |
| Hains 1990 | Not primarily a depression prevention programme ‐ the focus is on anxiety, stress and anger |
| Hains 1992 | Not primarily a depression prevention programme ‐ the focus is on anxiety, stress and anger |
| Hains 1994 | Not primarily a depression prevention programme ‐ the focus is instead on anxiety, stress and anger |
| Healy 2014 | Intervention not primarily delivered to the individual (e.g. family therapy or parenting skills) |
| Hoek 2012 | Not primarily a depression prevention programme ‐ the focus is instead on depression or anxiety, or both |
| Hyun 2010 | Not primarily a depression prevention programme ‐ the focus is instead on broad mental health or well‐being, or both. |
| Ishikawa 2010 | Not primarily a depression prevention programme ‐ the focus is instead on improving social skills |
| Ishimura 2014 | Participants not within the age bracket specified for this review |
| King 1990 | Not primarily a depression prevention programme ‐ the focus is on treating disruptive behaviours |
| Klein 2011 | Participants recruited on the basis of depressive or anxious symptoms |
| Kraag 2009 | Not primarily a depression prevention programme ‐ the focus is on broad mental health or well‐being, or both |
| Kumakech 2009 | Not primarily a depression prevention programme ‐ the focus is on broad mental health or well‐being, or both |
| Lamb 1998 | Treatment study |
| Layne 2008 | Not primarily a depression prevention programme ‐ the focus is on trauma |
| Lock 2003 | Not primarily a depression prevention programme ‐ the focus is on anxiety prevention |
| Lowry‐Webster 2003 | Not primarily a depression prevention programme ‐ the focus is on anxiety prevention |
| Manassis 2010 | Not primarily a depression prevention programme ‐ the focus is instead on depression and/or anxiety |
| Manz 2001 | Participants recruited on the basis of depressive or anxious symptoms |
| Marcotte 1993 | Does not contain a suitable psychological intervention |
| Mason 2007 | Not primarily a depression prevention programme ‐ parenting/family intervention or focus on family problems (e.g. divorce) |
| Mason 2012 | Not primarily a depression prevention programme ‐ parenting/family intervention or focus on family problems (e.g. divorce) |
| Mateu‐Martínez 2013 | Not a RCT |
| McBride 2012 | Intervention not focused on addressing participants' own cognitions to reduce depressive symptomatology. Focus is instead on generic psychoeducation to increase awareness of the link between depressive symptoms and perceptions. |
| McLaughlin 2007 | Not primarily a depression prevention programme ‐ parenting/family intervention or focus on family problems (e.g. divorce) |
| Muriungi 2013 | No suitable psychological intervention |
| Palermo 2009 | Not primarily a depression prevention programme ‐ the focus is on chronic pain |
| Parker 2011 | Treatment study |
| Peters 2014 | No suitable comparison or control group |
| Raider 2008 | Not primarily a depression prevention programme ‐ the focus is on trauma |
| Reid 2011 | Not primarily a depression prevention programme ‐ the focus is instead on broad mental health or well‐being, or both |
| Sankaranarayanan 2014 | No suitable depression outcome measure |
| Shen 2002 | Not primarily a depression prevention programme ‐ the focus is on trauma |
| Sibinga 2013 | Not primarily a depression prevention programme ‐ the focus is instead on broad mental health or well‐being, or both |
| Simpson 2008 | Not primarily a depression prevention programme ‐ participants recruited on the basis of depressive or anxious symptoms |
| Singhal 2014 | Not a RCT |
| Stallard 2014 | Focus is on anxiety prevention |
| Stallard 2015 | Focus is on trauma or PTSD |
| Stasiak 2014 | Treatment study |
| Tol 2008 | Not primarily a depression prevention programme ‐ the focus is on trauma |
| Treutiger 2013 | Not a RCT |
| van de Weijer‐Bergsma 2014 | No suitable validated depression outcome measure |
| Van Voorhees 2009 | Not a suitable control/comparison condition ‐ head to head trial of 2 active interventions instead |
| Vuori 2008 | Not primarily a depression prevention programme ‐ the focus is on vocational preparedness |
| Wahl 2014 | No suitable comparison or control group |
| Wolchik 2000 | Not primarily a depression prevention programme ‐ parenting/family intervention or focus on family problems (e.g. divorce) |
| Zehnder 2010 | Not primarily a depression prevention programme ‐ the focus is on trauma |
PTSD: post‐traumatic stress disorder RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Baramkoohi 2009.
| Methods | Design: no information available Description: no information available |
| Participants | Age range: no information available Country: no information available |
| Interventions | Broad category: no information available Name of programme: no information available Comparison group: no information available |
| Outcomes | Diagnosis: no information available Name of self‐report depression measure: no information available |
| Notes | — |
Cohen 2014.
| Methods | Design: no information available Description: no information available |
| Participants | Age range: no information available Country: no information available |
| Interventions | Broad category: no information available Name of programme: none Comparison group: no information available |
| Outcomes | Diagnosis: no information available Name of self‐report depression measure: no information available |
| Notes | — |
Ehring 2010.
| Methods | Design: RCT Description: targeted |
| Participants | Age range: 15 to 22 years Country: The Netherlands |
| Interventions | Broad category: CBT Name of programme: rumination focused CBT Comparison group: no treatment |
| Outcomes | Diagnosis: no Name of self‐report depression measure: Beck Depression Inventory II |
| Notes | — |
La Greca 2013.
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: question if this is included: participants must report symptoms of social anxiety and/or depression that exceed clinical cut‐offs on the Social Anxiety Scale for Adolescents (SAS‐A > or = to 50) or the Center for Epidemiologic Studies‐Depression Scale (CES‐D > or = to 16) What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not reported Baseline severity of depression: N/A Mean age: N/A Age range: 13 to 18 years Percentage male: N/A Setting: school Psychiatric diagnoses excluded: social anxiety, depression, PTSD, bipolar disorder, psychosis, eating disorder, substance use disorder, conduct disorder Suicide risk excluded: yes. Must not endorse active suicidal items on the Columbia Suicide Severity Rating Scale (C‐SSRS). Parents with history of schizophrenia/bipolar disorder excluded: not reported Country: USA |
| Interventions | Broad category: IPT Manualised: not reported Online: no Name of programme: PEERS/UTalk Comparison: education/support (ES) |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: Centre of Epidemiological Studies Depression Scale (CES‐D; Radloff 1977). This is a secondary outcome measure. Name of clinician report depression measure: not reported Name of anxiety measure: Anxiety Disorder Interview Schedule ‐ Children (ADIS‐C) Name of general functioning measure: Clinicians Global Impression Scale Assessment points: baseline, 12 weeks, 6 months |
| Notes | — |
Levin 2014.
| Methods | Design: RCT Description: universal |
| Participants | Age range: 18 to 20 years Country: USA |
| Interventions | Broad category: 3rd wave CBT Name of programme: ACT on college life (ACT‐CL) Comparison group: wait‐list |
| Outcomes | Diagnosis: no Name of self‐report depression measure: DASS |
| Notes | — |
McCauly 2003.
| Methods | Design: RCT Description: targeted |
| Participants | Age range: 12 to 15 years Country: USA |
| Interventions | Broad category: CBT Name of programme: Coping and Support Training for the Transition (CAST‐T) Comparison group: TAU |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: not reported |
| Notes | — |
Rasing 2013.
| Methods | Design: RCT |
| Participants | Description: indicated and selective Cut‐point for inclusion for indicated studies: Children’s Depression Inventory 2 (CDI 2; Kovacs 1992) and Spence Children Anxiety Scale (SCAS; Spence 2003) What risk was basis of inclusion for selected studies: a parent with elevated levels of depression or anxiety as determined by the Brief Symptom Inventory (BSI; De Beurs 2005) Diagnostic interview to exclude those with current or previous depression: not reported Baseline severity of depression: N/A Mean age: N/A Age range: 11 to 15 years Percentage male: N/A Setting: school Psychiatric diagnoses excluded: not reported Suicide risk excluded: prominence of suicidal ideation excluded (score 2 on CDI item: a desire to kill oneself, if given the chance) Parents with history of schizophrenia/bipolar disorder excluded: not reported Country: The Netherlands |
| Interventions | Broad category: CBT Manualised: not reported Online: no Name of programme: ‘Een Sprong Vooruit’ (A Jump Forward) Comparison: no intervention |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: (CDI 2; Kovacs 1992) Name of clinician report depression measure: not reported Name of anxiety measure: (SCAS; Spence 2003) Name of general functioning measure: not reported Assessment points: baseline, T2 (after session 2), T3 (after session 4), post‐intervention, 6 months follow‐up, 12 months follow‐up |
| Notes | — |
Redzic 2014.
| Methods | Design: no information available Description: no information available |
| Participants | Age range: no information available Country: no information available |
| Interventions | Broad category: no information available Name of programme: no information available Comparison group: no information available |
| Outcomes | Diagnosis: no information available Name of self‐report depression measure: no information available |
| Notes | — |
Saulsberry 2013.
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: not reported What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not reported Baseline severity of depression: N/A Mean age: N/A Age range: not reported Percentage male: N/A Setting: primary care clinic, school clinic, hospital Psychiatric diagnoses excluded: not reported Suicide risk excluded: not reported Parents with history of schizophrenia/bipolar disorder excluded: not reported Country: USA |
| Interventions | Broad category: CBT, BT, IPT plus additional motivational component Manualised: yes Online: yes Name of programme: CURB (modification of CATCH‐IT) Comparison: wait‐list |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: not reported Name of clinician report depression measure: not reported Name of anxiety measure: not reported Name of general functioning measure: not reported Assessment points: not reported |
| Notes | — |
Tak 2012.
| Methods | Design: cluster‐RCT |
| Participants | Description: universal Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: not reported Baseline severity of depression: N/A Mean age: N/A Age range: 12 to 14 years Percentage male: N/A Setting: school Psychiatric diagnoses excluded: not reported Suicide risk excluded: not reported Parents with history of schizophrenia/bipolar disorder excluded: not reported Country: The Netherlands |
| Interventions | Broad category: CBT and social problem‐solving Manualised: yes Online: no Name of programme: Op Volle Kracht Comparison: usual school curriculum, which in some schools does include social skills |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: CDI Name of clinician report depression measure: none Name of anxiety measure: Revised Children’s Manifest Anxiety Scale (RCMAS) Name of general functioning measure: none Assessment points: baseline, post‐intervention |
| Notes | — |
Tang 2013.
| Methods | Design: RCT Description: targeted |
| Participants | Age range: not reported Country: Taiwan |
| Interventions | Broad category: IPT Name of programme: none Comparison group: TAU |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: not reported |
| Notes | — |
van Voorhees 2010.
| Methods | Design: RCT Description: targeted |
| Participants | Age range: 13 to 17 years Country: USA |
| Interventions | Broad category: CBT and IPT Name of programme: CATCH‐IT Comparison group: Attention Monitoring Psycho‐education (AMPE) Arm |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: not reported |
| Notes | — |
CATCH‐IT: Competent Adulthood Transition with Cognitive Behavioral Humanistic and Interpersonal Training CBT: cognitive behavioural therapy CDI: Children's Depression Inventory CES‐D: Center for Epidemiologic Studies Depression Scale DASS: Depression Anxiety Stress Scale IPT: interpersonal therapy N/A: not available RCT: randomised controlled trial SAS: Social Adjustment Scale SCAS: Spence Children's Anxiety Scale TAU: treatment as usual
Characteristics of ongoing studies [ordered by study ID]
Chim 2013.
| Trial name or title | Adapted and Translated, Adolescent Depression, Internet Intervention |
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: 16 on the CES‐D What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: unclear Baseline severity of depression: N/A Mean age: N/A Age range: 13 to 21 years Percentage male: N/A Setting: community Psychiatric diagnoses excluded: Depression, Schizophrenia and Bipolar Affective Disorder Suicide risk excluded: imminent suicide risk excluded Parents with history of schizophrenia/bipolar disorder excluded: yes Country: Hong Kong |
| Interventions | Broad category: CBT and IPT Manualised: yes Online: yes Name of programme: AT‐CATCH (adaption of CATCH‐IT) Comparison: interactive anti‐smoking website |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: Centre of Epidemiological Studies Depression Scale (CES‐D; Radloff 1977) and Depression Anxiety Stress Scale (DASS; Lovibond 1995) Name of clinician report depression measure: not reported Name of anxiety measure: not reported Name of general functioning measure: not reported Assessment points: baseline, 3 months, 6 months, 12 months |
| Starting date | 31 January 2013 |
| Contact information | Dr David Chim, The University of Hong Kong |
| Notes | — |
Garber 2013.
| Trial name or title | A Family Depression Prevention Program (FDP) |
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: parent with a current or history of a depressive disorder within child's life Diagnostic interview to exclude those with current or previous depression: yes Baseline severity of depression: N/A Mean age: N/A Age range: 9 to 15.6 years Percentage male: N/A Setting: community Psychiatric diagnoses excluded: schizophrenia, bipolar I disorder, current depressive disorder, developmental disability, substance use disorder Suicide risk excluded: not reported Parents with history of schizophrenia/bipolar disorder excluded: yes Country: USA |
| Interventions | Broad category: CBT Manualised: not reported Online: no Name of programme: Family Depression Prevention (FDP) program Comparison: written information. Families receive written materials about depression and the effects of parental depression on children. |
| Outcomes | Diagnosis: Longitudinal Interval Follow‐up Evaluation (LIFE) Name of self‐report depression measure: Youth Self‐report (YSR) depression subscale Name of clinician report depression measure: not reported Name of anxiety measure: Youth Self‐report (YSR) anxiety subscale Name of general functioning measure: not reported Assessment points: baseline, post‐intervention, 6 months, 12 months, 18 months and 24 months |
| Starting date | December 2014 |
| Contact information | Robin Weersing (robin.weersing@mail.sdsu.edu); San Diego University Judy Garber (jgarber.vanderbilt@gmail.com); Vanderbilt University Bruce Compas (Bruce.Compas@Vanderbilt.edu); Vanderbilt University |
| Notes | — |
Geisner 2013.
| Trial name or title | Web based personalized intervention for risky drinking college students with depressed mood: examining the moderating effect of drinking motives |
| Methods | Design: RCT |
| Participants | Description: Targeted Cut‐point for inclusion for indicated studies: BDI score of 14 or more What risk was basis of inclusion for selected studies: elevated depression and risk drinking Diagnostic interview to exclude those with current or previous depression: not reported Baseline severity of depression: not reported Mean age: not reported Age range: university students Percentage male: 35% Setting: community Psychiatric diagnoses excluded: not reported Suicide risk excluded: not reported Parents with history of schizophrenia/bipolar disorder excluded: not reported Country: not reported |
| Interventions | Broad category: CBT Manualised: yes Online: yes Name of programme: unnamed Comparison: assessment only |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: not reported Name of clinician report depression measure: not reported Name of anxiety measure: not reported Name of general functioning measure: not reported Assessment points: not reported |
| Starting date | not reported |
| Contact information | not reported |
| Notes |
Nauta 2012.
| Trial name or title | Screening and Training: Enhancing Resilience in Kids |
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: 80th percentile of either the subscale for depression or the cluster of subscales for anxiety What risk was basis of inclusion for selected studies: at least one parent has a current or in the last 5 years has had a unipolar mood or anxiety disorder; meet 2 of the 3 High Risk Index criteria i.e. being female, have 2 affected parents, having a parent with a history of past suicidal behaviour Diagnostic interview to exclude those with current or previous depression: yes Baseline severity of depression: N/A Mean age: N/A Age range: 8 to 17 years Percentage male: N/A Setting: mental health services, GP, media (including digital) Psychiatric diagnoses excluded: mental retardation; current diagnosis of a mental disorder that warrants regular treatment but included those with e.g. a diagnosis like ADHD that was sufficiently treated (stable) Suicide risk excluded: no Parents with history of schizophrenia/bipolar disorder excluded: yes Country: The Netherlands |
| Interventions | Broad category: CBT Manualised: not reported Online: no Name of programme: none Comparison: attention placebo (minimal written information) |
| Outcomes | Diagnosis: unclear Name of self‐report depression measure: Revised Child Anxiety and Depression Scale (RCADS; Chorpita 2005) Name of clinician report depression measure: not reported Name of anxiety measure: Revised Child Anxiety and Depression Scale (RCADS; Chorpita 2005) Name of general functioning measure: not reported Assessment points: baseline, 4 months, 12 months, 24 months |
| Starting date | 1 October 2010 |
| Contact information | Maaike Nauta (m.h.nauta@rug.nl); University of Groningen |
| Notes | — |
Platt 2014a.
| Trial name or title | The PRODO trial |
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: at least one parent who meets diagnostic criteria for a current (or past, during the child's lifetime) diagnosis of depression Diagnostic interview to exclude those with current or previous depression: yes Baseline severity of depression: N/A Mean age: N/A Age range: 8 to 17 years Percentage male: N/A Setting: recruitment from adult psychiatric clinics and advertisements Psychiatric diagnoses excluded: any current or previous psychiatric disorder, or has undergone treatment or is receiving treatment for depression Suicide risk excluded: not reported Parents with history of schizophrenia/bipolar disorder excluded: N/A Country: Germany |
| Interventions | Broad category: CBT Manualised: yes Online: no Name of programme: Raising Healthy Children Comparison: no intervention |
| Outcomes | Diagnosis: Diagnostic Interview for Psychiatric Disorders for Children and Adolescents (K‐DIPS; Unnewehr 2008) Name of self‐report depression measure: Depression Inventory for Children and Adolescents (DIKJ; children aged 8 to 12; Stiensmeier‐Pelster 2000) and the German‐version of the revised Beck Depression Inventory (BDI‐II; children aged 13 and over; Hautzinger 1994) Name of clinician report depression measure: not reported Name of anxiety measure: not reported Name of general functioning measure: not reported Assessment points: baseline, 6 months, 9 months, 15 months |
| Starting date | 7 April 2014 |
| Contact information | Belinda Platt (belinda.platt@med.uni‐muenchen.de); Department of Child and Adolescent Psychiatry, Psychosomatics, and Psychotherapy, Munich |
| Notes | — |
Toth 2011.
| Trial name or title | Interpersonal Psychotherapy for Adolescent Girls (IPT‐A) |
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: N/A What risk was basis of inclusion for selected studies: child maltreatment Diagnostic interview to exclude those with current or previous depression: not reported Baseline severity of depression: N/A Mean age: N/A Age range: 13 to 15 years Percentage male: 0% Setting: community Psychiatric diagnoses excluded: not reported Suicide risk excluded: yes ‐ actively suicidal excluded Parents with history of schizophrenia/bipolar disorder excluded: N/A Country: USA |
| Interventions | Broad category: IPT Manualised: not reported Online: no Name of programme: none Comparison: enhanced care that they would typically receive in a community‐based setting |
| Outcomes | Diagnosis: not reported Name of self‐report depression measure: not reported Name of clinician report depression measure: not reported Name of anxiety measure: not reported Name of general functioning measure: not reported Assessment points: baseline, mid‐intervention (6 weeks), post‐intervention (12 weeks), 12 months and 18 months |
| Starting date | July 2011 |
| Contact information | Sheree Toth (sheree.toth@rochester.edu). |
| Notes | — |
Van Voorhees 2012.
| Trial name or title | Competent Adulthood Transition with Cognitive Behavioral Humanistic and Interpersonal Training (CATCH‐IT) |
| Methods | Design: RCT |
| Participants | Description: targeted Cut‐point for inclusion for indicated studies: must score between 8 to 17 on the CES‐D What risk was basis of inclusion for selected studies: N/A Diagnostic interview to exclude those with current or previous depression: past depression and dysthymia included Baseline severity of depression: N/A Mean age: 14.87 years (based on those currently enrolled at 2015) Age range: 13 to 18 years Percentage male: N/A Setting: primary care clinic Psychiatric diagnoses excluded: current MDD, schizophrenia, bipolar disorder Suicide risk excluded: yes ‐ if at serious imminent risk of suicide Parents with history of schizophrenia/bipolar disorder excluded: not reported Country: USA |
| Interventions | Broad category: CBT and IPT Manualised: yes Online: yes Name of programme: CATCH‐IT Comparison: Health Education‐attention control |
| Outcomes | Diagnosis: K‐SADS Name of self‐report depression measure: CES‐D Name of clinician report depression measure: not reported Name of anxiety measure: the Screen for Child Anxiety Related Emotional Disorders (SCARED; Birmaher 1997) Name of general functioning measure: Pediatric Quality of Life and Enjoyment and Satisfaction Questionnaire ‐ parent and child versions (PQ‐LES‐Q; Endicott 1981) Assessment points:baseline and at 2, 6, 12, 18 and 24 months post‐intake |
| Starting date | 2012 |
| Contact information | Benjamin Van Voorhees (bvanvoor@uic.edu) |
| Notes | — |
ADHD: attention deficit hyperactivity disorder
BT: Behavioural therapy CATCH‐IT: Competent Adulthood Transition with Cognitive Behavioral Humanistic and Interpersonal Training
CURB: Adaptation of CATCH‐IT CBT: cognitive behavioural therapy CES‐D: Center for Epidemiologic Studies Depression Scale GP: general practitioner IPT: interpersonal therapy K‐SADS: Kiddie‐Schedule for Affective Disorders and Schizophrenia for School‐Age Children MDD: major depressive disorder N/A: not available RCT: randomised controlled trial
Differences between protocol and review
In the first version of the review, the protocol indicated that uncontrolled and controlled clinical trials, open trials, case‐controlled trials and cohort trials (e.g. Altman 1991; Myles 2000; SIGN 2000) would be included if there were no, very few, or only poor quality RCTs. However, given the large number of RCTs retrieved both for the first version and for this updated version of the review, only RCTs have been included.
In the first update of the review, we excluded general adjustment, academic/work function, social adjustment, cognitive style and suicidal ideation/attempts outcomes given the paucity of data that existed for these outcomes. In this version of the review we have included clinician‐rated depression symptoms as a secondary outcome and have specified that the primary outcome of depression symptoms will be measured using validated self‐report measures. We added the clinician‐rated depression symptom outcome because while the majority of trials use self‐rated measures, a good minority of trials now included also used a clinician‐rated measure and it is important to assess the impact on depression according to different raters. We have been able to reinstate our early outcomes related to functioning but have only included general functioning, again due to paucity of outcomes for more specific categories of functioning. We have also now included anxiety because of the high co‐morbidity between depression and anxiety.
Assessment of the risk of bias was first updated in the previous update of the review and has been updated again in line with new guidance.
We made the decision prior to this version of the review to consider effect sizes of 0.20 or less as small, effect sizes that approached 0.30 as medium and effect sizes that approached 0.50 as large.
In this update of the review, we have aimed to have a more homogeneous group of included studies and thus have altered the inclusion criteria in the following ways:
We have only included psychological interventions (rather than educational).
We have only included evidence‐based psychological interventions; the vast majority of studies in the previous versions of this review were CBT‐based and continue to be so. Evidence‐based interventions also include IPT interventions and we have included third wave CBT interventions.
Given the lack of significant findings with regard to gender and risk group, we have not undertaken subgroup analysis for these variables. We sought in this review to further the field of depression prevention by seeking to explore which of the many depression prevention programmes might be the most useful and this concentrated our subgroup analysis on how the populations for these trials were selected (universal, targeted: indicated and selected). Our meta‐regression complemented this by looking at other salient features of the interventions that might impact on efficacy. Our other main concern was with regard to the important issue of comparison group, which time and time again has been shown to have an impact on effect sizes (e.g. Weisz 2006). Thus we introduced a new subgroup analysis to investigate this.
Contributions of authors
Sally Merry co‐ordinated the original review and first update, extracted and entered data, ran the analyses, took a lead role in writing the review and has continued to provide input on design and data analysis, and has contributed to the writing of the review.
Sarah Hetrick ran searches, screened trials for inclusion, extracted data and assisted with the write‐up of the original review and first update, and has co‐ordinated this update including guiding methodological updates, extracting and entering all the data, running the analyses and taking a lead role in the writing of the review.
Georgina Cox ran searches, screened trials for inclusion, extracted and entered data and has contributed to the write‐up in this and the previous update of the review.
Julliet Bir screened trials for inclusion, extracted and entered data, checked drafts of the review for the original and previous version of the review, and has assisted with 'Risk of bias' assessment for the majority of the trials included in this current update of the review.
For this update, Katrina Witt screened some trials for inclusion, extracted and entered some data, double‐checked all data, assisted with data analysis and with writing up some aspects of the review, and checked drafts of the review.
Sources of support
Internal sources
University of Auckland, New Zealand.
External sources
Health Research Council, New Zealand.
Declarations of interest
Professor Merry and Ms J Bir have been involved in a trial of a depression prevention programme (Merry 2004). The results of this trial have been included in this update.
Sarah Hetrick is an invesitgator on a range of trials of interventions for the treatment of youth depression.
None of the other authors have any declarations of interest to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Araya 2013 {published data only}
- Araya R, Fritsch R, Spears M, Rojas G, Martinez V, Barroilhet S, et al. School intervention to improve mental health of students in Santiago, Chile: a randomized clinical trial [ISRCTN19466209]. JAMA Pediatrics 2013;167:1004‐10. [DOI] [PubMed] [Google Scholar]
- Araya R, Montgomery AA, Fritsch R, Gunnell D, Stallard P, Noble S, et al. School‐based intervention to improve the mental health of low‐income, secondary school students in Santiago, Chile (YPSA): Study protocol for a randomized controlled trial [ISRCTN19466209]. Trials 2011;12:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyp SS. School‐based interventions for student mental health disorders. Journal of Developmental and Behavioral Pediatrics 2014;35:233. [Google Scholar]
Arnarson 2009 {published and unpublished data}
- Arnarson EÖ, Craighead WE. Prevention of depression among Icelandic adolescents. Behaviour Research and Therapy 2009;47(7):577‐85. [DOI] [PubMed] [Google Scholar]
- Arnarson EÖ, Craighead WE. Prevention of depression among Icelandic adolescents: a 12‐month follow‐up. Behaviour Research and Therapy 2011;49(3):170‐4. [DOI] [PubMed] [Google Scholar]
Bella‐Awusah 2015 {unpublished data only}
- Bella‐Awusah T, Ani C, Ajuwon A, Omigbodun O. Effectiveness of brief school‐based, group cognitive behavioural therapy for depressed adolescents in South West Nigeria. Child and Adolescent Mental Health 2016;21(1):44‐50. [DOI: 10.1111/camh.12104; Identified Sept. 2015 as an ePub ahead of print] [DOI] [PubMed] [Google Scholar]
Calear 2009 {unpublished data only}
- Calear AL, Christensen H, Mackinnon A, Griffiths KM. Adherence to the MoodGYM program: outcomes and predictors for an adolescent school‐based population. Journal of Affective Disorders 2013;147:338‐44. [DOI] [PubMed] [Google Scholar]
- Calear, AL, Christensen, H, Mackinnon, A, Griffiths, KM, O'Kearney, R. The YouthMood Project: a cluster randomised controlled trial of an online cognitive‐behavioural program with adolescents. Journal of Consulting and Clinical Psychology 2009;77(6):1021‐32. [DOI] [PubMed] [Google Scholar]
- Neil AL, Batterham P, Christensen H, Bennett K, Griffiths KM. Predictors of adherence by adolescents to a cognitive behavior therapy website in school and community‐based settings. Journal of Medical Internet Research 2009;11(1):e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cardemil 2002 {published and unpublished data}
- Cardemil EV, Reivich KJ, Beevers CG, Seligman MEP, James J. The prevention of depressive symptoms in low‐income, minority children: two‐year follow‐up. Behaviour Research and Therapy 2007;45(2):313‐27. [DOI] [PubMed] [Google Scholar]
- Cardemil EV, Reivich KJ, Seligman M. The prevention of depressive symptoms in low‐income minority middle school students. Prevention and Treatment 2002;5(1):ArtID 8. [Google Scholar]
Castellanos 2006 {published data only}
- Castellanos N, Conrod P. Brief interventions targeting personality risk factors for adolescent substance misuse reduce depression, panic and risk‐taking behaviours. Journal of Mental Health 2006;15(6):645‐58. [Google Scholar]
Chaplin 2006 {published data only}
- Chaplin TM, Gillham JE, Reivich K, Elkon AGL, Samuels B, Freres DR, et al. Depression prevention for early adolescent girls. A pilot study of all girls versus co‐ed groups. Journal of Adolescence 2006;26(1):110‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Charbonneau 2012 {published data only}
- Charbonneau AM. Managing stress in the transition to college: the effects of a relaxation intervention on emotional reactivity, depressive symptoms, and adjustment to college in female first year undergraduates. Dissertation Abstracts International Section B: The Sciences and Engineering 2012;72(9‐B):5566. [Google Scholar]
Clarke 1993 {published data only}
- Clarke GN, Hawkins W, Murphy N, Sheeber L. School‐based primary prevention of depressive symptomatology in adolescents: findings from two studies. Journal of Adolescent Research 1993;8(2):183‐204. [Google Scholar]
Clarke 1995 {published data only}
- Clarke GN, Hawkins W, Murphy M, Sheeber LB, Lewinsohn PM, Seeley JR. Targeted prevention of unipolar depressive disorder in an at‐risk sample of high school adolescents: a randomized trial of a group cognitive intervention. Journal of the American Academy of Child and Adolescent Psychiatry 1995;34(3):312‐21. [DOI] [PubMed] [Google Scholar]
Clarke 2001 {published data only}
- Clarke GN, Hornbrook M, Lynch F, Polen M, Gale J, Beardslee W, et al. A randomized trial of a group cognitive intervention for preventing depression in adolescent offspring of depressed parents. Archives of General Psychiatry 2001;58:1127‐34. [DOI] [PubMed] [Google Scholar]
Compas 2009 {published data only}
- Compas BE. Family cognitive behavioral prevention of depression in children of parents with a history of major depressive disorder [NCT00183482]. http://clinicaltrials.gov/show/NCT00183482 2004.
- Compas BE, Champion JE, Forehand R, Cole DA, Reeslund KL, Fear J, et al. Coping and parenting: Mediators of 12‐month outcomes of a family group cognitive‐behavioral preventive intervention with families of depressed parents. Journal of Consulting and Clinical Psychology 2010;78(5):623‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Forehand R, Keller G, Champion JE, Rakow A, Reeslund KL, et al. Randomized controlled trial of a family cognitive‐behavioral preventive intervention for children of depressed parents. Journal of Consulting and Clinical Psychology 2009;77(6):1007‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Forehand R, Thigpen J, Hardcastle E, Garai E, McKee L, et al. Efficacy and moderators of a family group cognitive‐behavioral preventive intervention for children of parents with depression. Journal of consulting and clinical psychology 2015;83:541‐553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Forehand R, Thigpen JC, Keller G, Hardcastle EJ, Cole DA, et al. Family group cognitive‐behavioral preventive intervention for families of depressed parents: 18‐ and 24‐month outcomes. Journal of Consulting and Clinical Psychology 2011a;79(4):488‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compas BE, Keller G, Forehand R. Preventive intervention in families of depressed parents: a family cognitive‐behavioral intervention. In: Strauman TJ, Costanzo PR, Garber J editor(s). Depression in Adolescent Girls: Science and Prevention. New York, NY: Guildford Press, 2011b:318‐39. [Google Scholar]
- McKee LG, Parent J, Forehand R, Rakow A, Watson KH, Dunbar JP, et al. Reducing youth internalizing symptoms: effects of a family‐based preventive intervention on parental guilt induction and youth cognitive style. Development and Psychopathology 2014;26(2):319‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cova 2011‐Targeted {published data only}
- Cova F, Rincón P, Melipillán R. Evaluation of the efficacy of a prevention program for depression in female adolescents [Evaluación de la eficacia de un programa preventivo para la depresión en adolescentes de sexo femenino]. Terapia Psicológica 2011;29(2):245‐50. [Google Scholar]
Cowell 2009 {published data only}
- Cowell JM, McNaughton D, Ailey S, Gross D, Fogg L. Clinical trial outcomes of the Mexican American Problem Solving program (MAPS). Hispanic Health Care International 2009;7(4):178‐89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dobson 2010 {published data only}
- Dobson KS, Hopkins JA, Fata L, Scherrer M, Allan LC. The prevention of depression and anxiety in a sample of high‐risk adolescents: a randomized controlled trial. Canadian Journal of School Psychology 2010;25(4):291‐310. [Google Scholar]
Ellis 2011 {published data only}
- Ellis LA, Campbell AJ, Sethi S, O'Dea BM. Comparative randomized trial of an online cognitive‐behavioral therapy program and an online support group for depression and anxiety. Journal of Cyber Therapy and Rehabilitation 2011;4(4):461‐7. [Google Scholar]
Fleming 2012 {published data only}
- Fleming T, Dixon R, Frampton C, Merry S. A pragmatic randomized controlled trial of computerized CBT (SPARX) for symptoms of depression among adolescents excluded from mainstream education. Behavioural and Cognitive Psychotherapy 2012;40(5):529‐41. [DOI] [PubMed] [Google Scholar]
Fresco 2009 {published data only}
- Fresco DM, Moore MT, Walt L, Craighead LW. Self‐administered optimism training: mechanisms of change in a minimally supervised psychoeducational intervention. Journal of Cognitive Psychotherapy 2009;23(4):350‐67. [Google Scholar]
Gallegos 2008 {published data only}
- Gallegos J. Preventing Childhood Anxiety and Depression: Testing the Effectiveness of a School‐Based Program in México [PhD thesis]. Austin, TX: The University of Texas at Austin, 2008. [Google Scholar]
- Gallegos J. Preventing childhood anxiety and depression: testing the effectiveness of a school‐based program in Mexico. Dissertation Abstracts International Section A: Humanities and Social Sciences 2009;69(12‐A):4686. [Google Scholar]
Garber 2009 {published and unpublished data}
- Beardslee W. Organizing & coordinating resources & supports to promote resilience & reduce risk for children whose parents have mental illnesses. Neuropsychiatrie de l'Enfance et de l'Adolescence. 2012:S62.
- Beardslee WR, Brent DA, Weersing VR, Clarke GN, Porta G, Hollon SD, et al. Prevention of depression in at‐risk adolescents: longer‐term effects. JAMA Psychiatry 2013;70:1161‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent DA, Brunwasser SM, Hollon SD, Weersing VR, Clarke GN, Dickerson JF, et al. Effect of a cognitive‐behavioral prevention program on depression 6 years after implementation among at‐risk adolescents: a randomized clinical trial. JAMA Psychiatry 2015;30:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber J, Clarke GN, Weersing VR, Beardslee WR, Brent DA, Gladstone TRG, et al. Prevention of depression in at‐risk adolescents: a randomized controlled trial. JAMA 2009;301(21):2215‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Garcia 2011 {published data only}
- Garcia C, Pintor J, Vazquez G, Alvarez‐Zumarraga E. Project Wings, a coping intervention for Latina adolescents: a pilot study. Western Journal of Nursing Research 2011;35(4):434‐58. [DOI] [PubMed] [Google Scholar]
- Gracia C, Pintor JK, Lindgren S. Feasibility and acceptability of a school‐based coping intervention for Latina adolescents. Journal of School Nursing 2010;26(1):42‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gilham 1994‐Study 2 {published data only}
- Gillham JE. Preventing depressive symptoms in school children. Dissertation Abstracts International 1994;55(9‐B):4119. [Google Scholar]
Gillham, Hamilton 2006a {published data only}
- Gillham JE, Hamilton J, Freres DR, Patton K, Gallop R. Preventing depression among early adolescents in the primary care setting: Erratum. Journal of Abnormal Child Psychology 2008;36(2):297‐8. [DOI] [PubMed] [Google Scholar]
- Gillham JE, Hamilton J, Freres DR, Patton P, Gallop R. Preventing depression among early adolescents in the primary care setting: a randomized controlled study of the Penn Resiliency Program. Journal of Abnormal Child Psychology 2006;34(2):203‐19. [DOI] [PubMed] [Google Scholar]
Gillham, Reivich 2006b {published data only}
- Gillham JE, Reivich KJ, Freres DR, Lascher M, Litzinger S, Shatte A, et al. School‐based prevention of depression and anxiety symptoms in early adolescence: a pilot of a parent intervention component. School Psychology Quarterly 2006;21(3):323‐48. [Google Scholar]
Gillham 2007 {published data only}
- Cutuli JJ, Chaplin TM, Gillham JE, Reivich KJ, Seligman ME. Preventing co‐occurring depression symptoms in adolescents with conduct problems: the Penn Resiliency Program. Annals of the New York Academy of Sciences 2006;1094:282‐6. [DOI] [PubMed] [Google Scholar]
- Gillham JE, Reivich KJ, Freres DR, Chaplin TM, Shatte AJ, Samuels B, et al. School‐based prevention of depressive symptoms: a randomized controlled study of the effectiveness and specificity of the Penn Resiliency Program. Journal of Consulting and Clinical Psychology 2007;75(1):9‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillham 2012 {published data only}
- Gillham JE, Reivich KJ, Brunwasser SM, Freres DR, Chajon ND, Kash‐Macdonald VM, et al. Evaluation of a group cognitive‐behavioral depression prevention program for young adolescents: a randomized effectiveness trial. Journal of Clinical Child and Adolescent Psychology 2012;41(5):621‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seligman MEP, Gillham JE, Reivich KJ. Preventing depression in school children [NCT00360451]. http://clinicaltrials.gov/show/NCT00360451 2002.
Horowitz a2007 {published data only}
- Garber J. Promoting well‐being in teens [NCT00374439]. http://clinicaltrials.gov/show/NCT00374439 2004.
- Horowitz J. Preventing depression in adolescents: a prospective trial of two universal prevention programs. Dissertation Abstracts 2008;68(12‐B):8399. [Google Scholar]
- Horowitz JL, Garber J, Ciesla JA, Young JF, Mufson L. Prevention of depressive symptoms in adolescents: a randomized trial of cognitive‐behavioral and interpersonal prevention programs. Journal of Consulting and Clinical Psychology 2007;75(5):693‐706. [DOI] [PubMed] [Google Scholar]
Horowitz b2007 {published data only}
- Horowitz J. Preventing depression in adolescents: a prospective trial of two universal prevention programs. Dissertation Abstracts International 2008;68(12‐B):8399. [Google Scholar]
Hyun 2005 {published and unpublished data}
- Hyun M, Hyang‐In C, Young‐Ja L. The effect of cognitive‐behavioral group therapy on the self‐esteem, depression, and self‐efficacy of runaway adolescents in a shelter in South Korea. Applied Nursing Research 2005;18:160‐6. [DOI] [PubMed] [Google Scholar]
Jaycox 1994 {published data only}
- Gillham J, Reivich K. Prevention of depressive symptoms in school‐children. Psychological Science 1999;10(5):461‐2. [DOI] [PubMed] [Google Scholar]
- Gillham J, Reivich K, Jaycox L, Seligman M. Prevention of depressive symptoms in school‐children: two year follow‐up. Psychological Science 1995;6(6):343‐51. [Google Scholar]
- Gillham JE. Preventing depressive symptoms in school children. Dissertation Abstracts International 1995;55(9‐B):4119. [Google Scholar]
- Gillham JE. Preventing depressive symptoms in school children. Dissertation Abstracts International 1995;55(9‐B):4119. [Google Scholar]
- Jaycox LH, Reivich KJ, Gillham J, Seligman M. Preventing depressive symptoms in school children. Behavior Research and Therapy 1994;32:801‐16. [DOI] [PubMed] [Google Scholar]
- Zubernis L, Cassidy K, Gillham J, Reivich K, Jaycox L. Prevention of depressive symptoms in preadolescent children of divorce. Journal of Divorce and Remarriage 1999;30(1/2):11‐36. [Google Scholar]
Karami 2012 {published data only}
- Karami S, Ghasemzadeh A, Saadat M, Mazaheri E, Zandipour T. Effects of group counseling with cognitive‐behavioral approach on reducing divorce children's depression. Procedia ‐ Social and Behavioral Sciences 2012;46:77‐81. [Google Scholar]
Kauer 2012 {published data only}
- Kauer S, Reid S, Crooke A, Khor A, Patton G, Jorm A, et al. P01‐307‐Emotional self‐awareness: preliminary analyses of a RCT using a cellular phone self‐monitoring program (mobiletype) to decrease early symptoms of depression. European Psychiatry 2011;26(Suppl 1):309. [Google Scholar]
- Kauer SD, Reid SC, Crooke AH, Khor A, Hearps SJ, Jorm AF, et al. Self‐monitoring using mobile phones in the early stages of adolescent depression: randomized controlled trial. Journal of Medical Internet Research 2012;14(3):e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khalsa 2012 {published data only}
- Khalsa SB, Hickey‐Schultz L, Cohen D, Steiner N, Cope S. Evaluation of the mental health benefits of yoga in a secondary school: a preliminary randomized controlled trial. Journal of Behavioral Health Services and Research 2012;39(1):80‐90. [DOI] [PubMed] [Google Scholar]
Kindt 2014 {published data only}
- Kindt KC, Zundert R, Engels RC. Evaluation of a Dutch school‐based depression prevention program for youths in high risk neighborhoods: study protocol of a two‐armed randomized controlled trial. BMC Public Health 2012;12:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindt KCM, Kleinjan M, Janssens JMAM, Scholte RHJ. Evaluation of a school‐based depression prevention program among adolescents from low‐income areas: a randomized controlled effectiveness trial. International Journal of Environmental Research and Public Health 2014;11(5):5273‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kowalenko 2005 {published data only}
- Kowalenko N, Rapee RM, Simmons J, Wignall A, Hoge R, Whitefield K, et al. Short‐term effectiveness of a school‐based early intervention program for adolescent depression. Clinical Child Psychology and Psychiatry 2005;10(4):493‐507. [Google Scholar]
Liehr 2010 {published data only}
- Liehr P, Diaz N. A pilot study examining the effect of mindfulness on depression and anxiety for minority children. Archives of Psychiatric Nursing 2010;24(1):69‐71. [DOI] [PubMed] [Google Scholar]
Lillevoll 2014 {published data only}
- Lillevoll KR, Vangberg HCB, Griffiths KM, Waterloo K, Eisemann MR. Uptake and adherence of a self‐directed Internet‐based mental health intervention with tailored e‐mail reminders in senior high schools in Norway. BMC Psychiatry 2014;14:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Livheim 2014‐study 1(girls) {published data only}
- Livheim F, Hayes L, Ghaderi A, Magnusdottir T, Högfeldt A, Rowse J, et al. The effectiveness of acceptance and commitment therapy for adolescent mental health: Swedish and Australian pilot outcomes. Journal of Child and Family Studies 2015;24:1016‐30. [Google Scholar]
Makarushka 2012 {published data only}
- Makarushka MM. Efficacy of an Internet‐based intervention targeted to adolescents with subthreshold depression. Dissertation Abstracts International Section A: Humanities and Social Sciences 2012;73(3‐A):977. [Google Scholar]
Manicavasagar 2014 {published data only}
- Manicavasagar V, Horswood D, Burckhardt R, Lum A, Hadzi‐Pavlovic D, Parker G. Feasibility and effectiveness of a web‐based positive psychology program for youth mental health: randomized controlled trial. Journal of Medical Internet Research 2014;16(6):e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarty 2011 {published data only}
- McCarty C. Prevention of depression within salient adolescent contexts [NCT01220635]. http://clinicaltrials.gov/ct2/show/NCT01220635 2010.
- McCarty CA, Violette HD, McCauley E. Feasibility of the Positive Thoughts and Actions Prevention Program for middle schoolers at risk for depression. Depression Research and Treatment 2011;2011:Article ID 241386. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCarty 2013 {published data only}
- McCarty CA, Violette HD, Duong MT, Cruz RA, McCauley E. A randomized trial of the Positive Thoughts and Action Program for depression among early adolescents. Journal of Clinical Child and Adolescent Psychology 2013;42(4):554‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
McLaughlin 2011 {published data only}
- McLaughlin CL. Evaluating the effect of an empirically‐supported group intervention for students at‐risk for depression in a rural school district. Dissertation Abstracts International Section B: The Sciences and Engineering 2011;71(9‐B):5820. [Google Scholar]
Mendelson 2010 {published data only}
- Mendelson T, Greenberg MT, Dariotis JK, Gould LF, Rhoades BL, Leaf PJ. Feasibility and preliminary outcomes of a school‐based mindfulness intervention for urban youth. Journal of Abnormal Child Psychology 2010;38(7):985‐94. [DOI] [PubMed] [Google Scholar]
Merry 2004 {published and unpublished data}
- Merry S, McDowell H, Wild C, Bir J, Cunliffe R. A randomized placebo‐controlled trial of a school‐based depression prevention program. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(5):538‐47. [DOI] [PubMed] [Google Scholar]
Mirzamani 2012 {published data only}
- Mirzamani SM, Azvar F, Dolatshahi B, Askari A. Efficacy of life skills training on reduce depressive symptoms in student population [اثربخشي آموزش مهارت هاي زندگي بر كاهش علايم افسردگي دانش آموزان]. Journal of Research in Behavioural Sciences 2012;10(2):124‐32. [Google Scholar]
Noël 2013 {published data only}
- Noël LT, Rost K, Gromer J. Depression prevention among rural preadolescent girls: a randomized controlled trial. School Social Work Journal 2013;38:No pagination specified. [Google Scholar]
O'Leary‐Barrett 2013 {published data only}
- O'Leary‐Barrett M, Topper L, Al‐Khudhairy N, Pihl RO, Castellanos‐Ryan N, Mackie CJ, et al. Two‐year impact of personality‐targeted, teacher‐delivered interventions on youth internalizing and externalizing problems: a cluster‐randomized trial. Journal of the American Academy of Child and Adolescent Psychiatry 2013;52:911‐20. [DOI] [PubMed] [Google Scholar]
Pattison 2001 {published data only}
- Pattison C, Lynd‐Stevenson R. The prevention of depressive symptoms in children: the immediate and long‐term outcomes of a school‐based program. Behaviour Change 2001;18(2):92‐102. [Google Scholar]
Petersen 1997 {published data only}
- Petersen A, Leffert A, Graham B, Alwin J, Ding S. Promoting mental health during the transition into adolescence. In: Schulenberg J, Maggs JL, Hierrelmann AK editor(s). Health Risks and Developmental Transitions During Adolescence. New York, NY: Cambridge University Press, 1997:471‐97. [Google Scholar]
Pössel 2004 {published and unpublished data}
- Pösell P, Horn AB, Hautzinger M. Preliminary results of a school‐based depression prevention program for adolescents [Erst ergebnisse eines programms zur schulbasierten pravention von depressiven symptomen bei jugendlichen]. Zeitschrift fur Gesundheitpsychologie 2003;11(1):10‐20. [Google Scholar]
- Pössel P, Baldus C, Horn AB, Groen G, Hautzinger M. Influence of general self‐efficacy on the effects of a school‐based universal primary prevention program: a randomized and controlled follow‐up study. Journal of Child Psychology and Psychiatry 2005;46(9):982‐94. [DOI] [PubMed] [Google Scholar]
- Pössel P, Horn AB, Groen G, Hautzinger M. School‐based prevention of depressive symptoms in adolescents: a 6‐month follow‐up. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(8):1003‐10. [DOI] [PubMed] [Google Scholar]
- Pössel P, Horn AB, Hautzinger M. Comparison of two school based depression prevention programs for adolescents. Zeitschrift fur Klinische Psychologie und Psychotherapie 2006;35(2):109‐16. [Google Scholar]
- Wahl MS, Patak, MA, Pössel P, Hautzinger M. A school‐based universal programme to prevent depression and to build up life skills. Journal of Public Health 2011;19(4):349‐56. [Google Scholar]
Pössel 2008 {published and unpublished data}
- Pössel P, Adelson JL, Hautzinger M. A randomized trial to evaluate the course of effects of a program to prevent adolescent depressive symptoms over 12 months. Behaviour Research and Therapy 2011;49(12):838‐51. [DOI] [PubMed] [Google Scholar]
- Pössel P, Seemann S, Hautzinger M. Impact of comorbidity in prevention of adolescent depressive symptoms. Journal of Counseling Psychology 2008;55(1):106‐17. [Google Scholar]
Pössel 2013 {published data only}
- Pössel P, Martin NC, Garber J, Hautzinger M. A randomized controlled trial of a cognitive‐behavioral program for the prevention of depression in adolescents compared with nonspecific and no‐intervention control conditions. Journal of Counseling Psychology 2013;60(3):432‐8. [DOI] [PubMed] [Google Scholar]
Puskar 2003 {published and unpublished data}
- Puskar K, Lamb J, Tusai‐Mumford K. Teaching kids to cope: a preventive mental health nursing strategy for adolescents. Journal of Child and Adolescent Psychiatric Nursing 1997;10(3):18‐28. [DOI] [PubMed] [Google Scholar]
- Puskar K, Sereika S, Tusaie‐Mumford K. Effect of the Teaching Kids to Cope (TKC) Program on outcomes of depression and coping among rural adolescents. Journal of Child and Adolescent Psychiatric Nursing 2003;16(2):71‐80. [DOI] [PubMed] [Google Scholar]
Quayle 2001 {published data only}
- Quayle D, Dziuraweic S. The effect of an optimism and lifeskills program on depressive symptoms in preadolescence. Behaviour Change 2001;18(4):194‐203. [Google Scholar]
Reynolds 2011 {published data only}
- Reynolds EK, MacPherson L, Tull MT, Baruch DE, Lejuez CW. Integration of the brief Behavioral Activation Treatment for Depression (BATD) into a college orientation program: depression and alcohol outcomes. Journal of Counseling Psychology 2011;58(4):555‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rivet‐Duval 2010 {unpublished data only}
- Rivet‐Duval E. Preventing Adolescent Depression and Suicide in Mauritius: The Efficacy of a Universal School‐based Program [MSc thesis]. Sydney, NSW: University of Sydney, 2005. [Google Scholar]
- Rivet‐Duval E, Heriot S, Hunt C. Preventing adolescent depression in Mauritius: a universal school‐based program. Child and Adolescent Mental Health 2010;16(2):86‐91. [DOI] [PubMed] [Google Scholar]
Roberts 2003 {published and unpublished data}
- Roberts C, Kane R, Bishop B, Matthews H, Thomson H. The prevention of depressive symptoms in rural school children: a follow‐up study. International Journal of Mental Health Promotion 2004;6(3):4‐16. [Google Scholar]
- Roberts C, Kane R, Thomson H, Bishop B, Hart B. The prevention of depressive symptoms in rural school children: a randomized controlled trial. Journal of Consulting and Clinical Psychology 2003;71(3):622‐8. [DOI] [PubMed] [Google Scholar]
Roberts 2010 {published data only}
- Roberts CM, Kane R, Bishop B, Cross D, Fenton J, Hart B. The prevention of anxiety and depression in children from disadvantaged schools. Behaviour Research and Therapy 2010;48(1):68‐73. [DOI] [PubMed] [Google Scholar]
Rohde 2014a {published data only}
- Brière FN, Rohde P, Shaw H, Stice E. Moderators of two indicated cognitive‐behavioral depression prevention approaches for adolescents in a school‐based effectiveness trial. Behaviour Research and Therapy 2014;53:55‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde P, Stice E, Shaw H, Brière FN. Indicated cognitive behavioral group depression prevention compared to bibliotherapy and brochure control: acute effects of an effectiveness trial with adolescents. Journal of Consulting and Clinical Psychology 2014;82(1):65‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde P, Stice E, Shaw H, Gau JM. Effectiveness trial of an indicated cognitive‐behavioral group adolescent depression prevention program versus bibliotherapy and brochure control at 1‐ and 2‐year follow‐up. Journal of Consulting and Clinical Psychology 2015;83(4):736‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rohde 2014b {published data only}
- Rohde P, Stice E, Shaw H, Gau JM. Cognitive‐behavioral group depression prevention compared to bibliotherapy and brochure control: nonsignificant effects in pilot effectiveness trial with college students. Behaviour Research and Therapy 2014;55:48‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde PD. Effectiveness trial of an adolescent depression prevention program [NCT00904891]. http://clinicaltrials.gov/show/NCT00904891 2009.
Rooney 2006 {published data only (unpublished sought but not used)}
- Rooney R, Roberts C, Kane R, Pike L, Winsor A, White J, Brown A. The prevention of depression in 8‐ to 9‐year‐old children: a pilot study. Australian Journal of Guidance and Counselling 2006;16(1):76‐90. [Google Scholar]
Rooney 2013 {published data only}
- Johnstone J, Rooney RM, Hassan S, Kane RT. Prevention of depression and anxiety symptoms in adolescents: 42 and 54 months follow‐up of the Aussie Optimism Program‐Positive Thinking Skills. Frontiers in Psychology 2014;5:364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney R, Hassan S, Kane R, Roberts CM, Nesa M. Reducing depression in 9‐10 year old children in low SES schools: a longitudinal universal randomized controlled trial. Behaviour Research and Therapy 2013;51(12):845‐54. [DOI] [PubMed] [Google Scholar]
Rose 2014 {published data only}
- Rose K, Hawes DJ, Hunt CJ. Randomized controlled trial of a friendship skills intervention on adolescent depressive symptoms. Journal of Consulting and Clinical Psychology 2014;82(3):510‐20. [DOI] [PubMed] [Google Scholar]
Sawyer 2010 {published data only}
- Sawyer MG, Harchak TF, Spence SH, Bond L, Graetz B, Kay D, et al. School‐based prevention of depression: A 2‐year follow‐up of a randomized controlled trial of the beyondblue schools research initiative. Journal of Adolescent Health 2010;47(3):297‐304. [DOI] [PubMed] [Google Scholar]
- Sawyer MG, Pfeiffer S, Spence SH, Bond L, Graetz B, Kay D, et al. School‐based prevention of depression: a randomised controlled study of the beyondblue schools research initiative. Journal of Child Psychology and Psychiatry 2010;51(2):199‐209. [DOI] [PubMed] [Google Scholar]
- Spence SH, Sawyer MG, Sheffield J, Patton G, Bond L, Graetz B, et al. Does the absence of a supportive family environment influence the outcome of a universal intervention for the prevention of depression?. International Journal of Environmental Research and Public Health 2014;11(5):5113‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmiege 2006 {published data only}
- Schmiege SJ, Khoo ST, Sandler IN, Ayers TS, Wolchik SA. Symptoms of internalizing and externalizing problems: modelling recovery curves after the death of a parent. American Journal of Preventive Medicine 2006;31(6 Suppl 1):s152‐60. [DOI] [PubMed] [Google Scholar]
Seligman 1999 {published data only}
- Seligman ME, Schulman P, DeRubies RJ, Hollon SD. The prevention of depression and anxiety. Prevention and Treatment 1999; Vol. 2:ArtID8.
Seligman 2007 {published data only}
- Seligman ME, Schulman P, Tryon AM. Group prevention of depression and anxiety symptoms. Behaviour Research and Therapy 2007;45(6):1111‐26. [DOI] [PubMed] [Google Scholar]
Sethi 2010 {published data only}
- Sethi S, Campbell AJ, Ellis LA. The use of computerized self‐help packages to treat adolescent depression and anxiety. Journal of Technology in Human Services 2010;28:144‐60. [Google Scholar]
Shatte 1997 {published data only}
- Shatte AJ. Prevention of Depressive Symptoms in Adolescents: Issues of Dissemination and Mechanisms of Change. Philadelphia, PA: University of Pennsylvania, 1996. [Google Scholar]
- Shatte AJ. Prevention of depressive symptoms in adolescents: issues of dissemination and mechanisms of change. Dissertation Abstracts International Section B: The Sciences and Engineering 1997;57(11‐B):7236. [Google Scholar]
Sheffield a2006 {published and unpublished data}
- Sheffield JK, Spence SH, Rapee RM, Kowalenko N, Wignall A, Davis A, et al. Evaluation of universal, indicated and combined cognitive‐behavioral approaches to the prevention of depression among adolescents. Journal of Consulting and Clinical Psychology 2006;74(1):66‐79. [DOI] [PubMed] [Google Scholar]
Sheffield b2006 {published data only}
- Sheffield JK, Spence SH, Rapee RM, Kowalenko N, Wignall A, Davis A, et al. Evaluation of universal, indicated and combined cognitive‐behavioral approaches to the prevention of depression among adolescents. Journal of Consulting and Clinical Psychology 2006;74(1):66‐79. [DOI] [PubMed] [Google Scholar]
Sheffield c2006 {published data only}
- Sheffield JK, Spence SH, Rapee RM, Kowalenko N, Wignall A, Davis A, et al. Evaluation of universal, indicated and combined cognitive‐behavioral approaches to the prevention of depression among adolescents. Journal of Consulting and Clinical Psychology 2006;74(1):66‐79. [DOI] [PubMed] [Google Scholar]
Snyder 2010 {published data only}
- Snyder S. School‐based positive psychoeducation in early adolescents: effects on happiness, depression, anxiety, school engagement, and persistence. Dissertation Abstracts International Section B: The Sciences and Engineering 2010;71(4‐B):2727. [Google Scholar]
Spence 2003 {published and unpublished data}
- Spence S, Sheffield J, Donovan C. Preventing adolescent depression: an evaluation of the Problem Solving for Life Program. Journal of Consulting and Clinical Psychology 2003;71(1):3‐13. [DOI] [PubMed] [Google Scholar]
- Spence SH, Sheffield J, Donovan C. Problem Solving for Life: evaluation of a program to prevent depression among adolescents. Presented at the 28th Annual Conference of the British Association for Behavioural and Cognitive Psychotherapies; 19‐22 July 2000; London, UK 2000.
- Spence SH, Sheffield JK, Donovan CL. Long‐term outcome of a school‐based, universal approach to prevention of depression in adolescents. Journal of Consulting and Clinical Psychology 2005;73(1):160‐7. [DOI] [PubMed] [Google Scholar]
Stallard 2012a {published data only}
- Anderson R, Ukoumunne OC, Sayal K, Phillips R, Taylor JA, Spears M, et al. Cost‐effectiveness of classroom‐based cognitive behaviour therapy in reducing symptoms of depression in adolescents: a trial‐based analysis. Journal of Child Psychology and Psychiatry 2014;55(12):1390‐7. [DOI] [PubMed] [Google Scholar]
- Phillips R, Spears MR, Montgomery AA, Millings A, Sayal K, Stallard P. Could a brief assessment of negative emotions and self‐esteem identify adolescents at current and future risk of self‐harm in the community? A prospective cohort analysis. BMC Public Health 2013;22(13):604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallard P. A single blind randomised controlled trial to determine the effectiveness of group cognitive behaviour therapy (CBT) in the prevention of depression in high risk adolescents [ISRCTN19083628]. Health Technology Research Projects (www.hta.ac.uk/1667) 2010 (accessed 7 August 2013).
- Stallard P. The promise randomised controlled trial: school based CBT for the prevention of depression in young adolescents abstract. European Child & Adolescent Psychiatry [abstracts of the 15th International Congress of European Society for Child and Adolescent Psychiatry, ESCAP; 2013 Jul 6‐10; Dublin Ireland] 2013;22:S131. [DOI] [PubMed] [Google Scholar]
- Stallard P, Buck R. Preventing depression and promoting resilience: feasibility study of a school‐based cognitive‐behavioural intervention. British Journal of Psychiatry Supplementum 2013;54:s18‐23. [DOI] [PubMed] [Google Scholar]
- Stallard P, Montgomery AA, Araya R, Anderson R, Lewis G, Sayal K, et al. Protocol for a randomised controlled trial of a school based cognitive behaviour therapy (CBT) intervention to prevent depression in high risk adolescents (PROMISE) [ISRCTN19083628]. Trials 2010;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallard P, Phillips R, Montgomery AA, Spears M, Anderson R, Taylor J, et al. A cluster randomised controlled trial to determine the clinical effectiveness and cost‐effectiveness of classroom‐based cognitive‐behavioural therapy (CBT) in reducing symptoms of depression in high‐risk adolescents. Health Technology Assessment 2013;17(47):No pagination specified. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallard P, Sayal K, Phillips R, Taylor JA, Spears M, Anderson R, et al. Classroom based cognitive behavioural therapy in reducing symptoms of depression in high risk adolescents: pragmatic cluster randomised controlled trial [ISRCTN19083628]. BMJ (Clinical research ed) 2012;345(7878):e6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor JA, Phillips R, Cook E, Georgiou L, Stallard P, Sayal K. A qualitative process evaluation of classroom‐based cognitive behaviour therapy to reduce adolescent depression. International Journal of Environmental Research and Public Health 2014;11(6):5951‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stice 2006 {published data only (unpublished sought but not used)}
- Marchand E, Ng J, Rohde P, Stice E. Effects of an indicated cognitive‐behavioral depression prevention program are similar for Asian American, Latino, and European American adolescents. Behaviour Research and Therapy 2010;48(8):821‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Burton E, Bearman SK, Rhode P. Randomized trial of a brief depression prevention program: an elusive search for a psychosocial placebo control condition. Behaviour Research and Therapy 2006;45(5):863‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stice 2008 {published and unpublished data}
- Gau JM, Stice E, Rohde P, Seeley JR. Negative life events and substance use moderate cognitive behavioral adolescent depression prevention intervention. Cognitive Behaviour Therapy 2012;41(3):241‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritschel LA. School‐based group psychotherapy for at‐risk adolescents. International Journal of Group Psychotherapy 2011;61(2):311‐7. [DOI] [PubMed] [Google Scholar]
- Rohde P, Stice E, Gau JM. Effects of three depression prevention interventions on risk for depressive disorder onset in the context of depression risk factors. Prevention Science 2012;13(6):584‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde P, Stice E, Gau JM, Marti CN. Reduced substance use as a secondary benefit of an indicated cognitive‐behavioral adolescent depression prevention program. Psychology of Addictive Behaviours 2012;26(3):599‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Rohde P, Gau J, Ochner C. Relation of depression to perceived social support: results from a randomized adolescent depression prevention trial. Behaviour Research and Therapy 2011;49(5):361‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Rohde P, Gau JM, Wade E. Efficacy trial of a brief cognitive–behavioral depression prevention program for high‐risk adolescents: effects at 1‐ and 2‐year follow‐up. Journal of Consulting and Clinical Psychology 2010;78(6):856‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Rohde P, Seeley JR, Gau JM. Brief cognitive‐behavioral depression prevention program for high‐risk adolescents outperforms two alternative interventions: a randomized efficacy trial. Journal of Consulting and Clinical Psychology 2008;76(4):595‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Rohde P, Seeley JR, Gau JM. Testing mediators of intervention effects in randomized controlled trials: an evaluation of three depression prevention programs. Journal of Consulting and Clinical Psychology 2010;78(2):273‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice EM. Depression prevention program for high‐risk adolescents [NCT00183417]. http://clinicaltrials.gov/show/NCT00183417 2004.
Stoppelbein 2003 {published and unpublished data}
- Stoppelbein L. Primary Prevention: an evaluation of a high‐school based cognitive‐behavioral program. Dissertation Abstracts International Section B: The Sciences and Engineering 2003;64(8‐B):4066. [Google Scholar]
Whittaker 2012 {published and unpublished data}
- Whittaker R. A randomised controlled trial of a multimedia mobile phone programme to reduce depressive symptoms in adolescents compared to an attention control mobile phone programme [ADAPT] [ACTRN12609000405213]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN = 12609000405213 2009.
- Whittaker R, Merry S, Stasiak K, McDowell H, Doherty I, Shepherd M, et al. MEMO‐‐A mobile phone depression prevention intervention for adolescents: development process and postprogram findings on acceptability from a randomized controlled trial. Journal of Medical Internet Research 2012;14(1):e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wijnhoven 2014 {published data only}
- Wijnhoven LA, Creemers DH, Vermulst AA, Scholte RH, Engels RC. Randomized controlled trial testing the effectiveness of a depression prevention program ('Op Volle Kracht') among adolescent girls with elevated depressive symptoms. Journal of Abnormal Child Psychology 2014;42(2):217‐28. [DOI] [PubMed] [Google Scholar]
Wong 2014 {published data only}
- Wong N, Kady L, Mewton L, Sunderland M, Andrews G. Preventing anxiety and depression in adolescents: a randomised controlled trial of two school based Internet‐delivered cognitive behavioural therapy programmes. Internet Interventions 2014;1(2):90‐4. [Google Scholar]
Woods 2011 {published data only}
- Woods B, Jose PE. Effectiveness of a school‐based indicated early intervention program for Maori and Pacific adolescents. Journal of Pacific Rim Psychology 2011;5(1):40‐50. [Google Scholar]
Young 2006 {published data only}
- Young JF, Gallop R, Mufson L. Mother‐child conflict and its moderating effects on depression outcomes in a preventive intervention for adolescent depression. Journal of Clinical Child and Adolescent Psychology 2009;38(5):696‐704. [DOI] [PubMed] [Google Scholar]
- Young JF, Kranzler A, Gallop RT, Mufson L. Interpersonal psychotherapy‐adolescent skills training: effects on school and social functioning. School Mental Health 2012;4(4):254‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JF, Makover HB, Cohen JR, Mufson L, Gallop RJ, Benas JS. Interpersonal psychotherapy‐adolescent skills training: anxiety outcomes and impact of comorbidity. Journal of Clinical Child and Adolescent Psychology 2012;41(5):640‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JF, Mufson L, Davies M. Efficacy of interpersonal psychotherapy‐adolescent skills training: an indicated preventive intervention for depression. Journal of Child Psychology and Psychiatry and Allied Disciplines 2006;47(12):1254‐62. [DOI] [PubMed] [Google Scholar]
- Young JF, Mufson L, Gallop R. Preventing depression: a randomized trial of Interpersonal Psychotherapy‐Adolescent Skills Training. Depression and Anxiety 2010;27(5):426‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Young 2010a {published data only}
- Young J. Prevention of depression in adolescents [NCT00258752]. http://clinicaltrials.gov/show/NCT00258752 2005.
- Young JF, Makover HB, Cohen JR, Mufson L, Gallop RJ, Benas JS. Interpersonal psychotherapy‐adolescent skills training: anxiety outcomes and impact of comorbidity. Journal of Clinical Child and Adolescent Psychology 2012;41(5):640‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JF, Mufson L, Gallop R. Preventing depression: a randomized trial of interpersonal psychotherapy‐adolescent skills training. Depression and Anxiety 2010;27(5):426‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2002‐study 3 {published data only}
- Yu DL, Seligman MEP. Preventing depressive symptoms in Chinese children. Prevention and Treatment 2002;5(1):No pagination specified. [Google Scholar]
- Yu L. Preventing depressive symptoms in Chinese children [thesis]. Dissertation Abstracts International 2000; Vol. 60, issue 12‐B:6389.
References to studies excluded from this review
Abbott 2014 {published data only}
- Abbott JA, Klein B, McLaren S, Austin DW, Molloy M, Meyer D, et al. Out & Online; effectiveness of a tailored online multi‐symptom mental health and wellbeing program for same‐sex attracted young adults: study protocol for a randomised controlled trial. Trials 2014;15:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
Attwood 2012 {published data only}
- Attwood M, Meadows S, Stallard P, Richardson T. Universal and targeted computerised cognitive behavioural therapy (Think, Feel, Do) for emotional health in schools: results from two exploratory studies. Child and Adolescent Mental Health 2012;17(3):173‐8. [DOI] [PubMed] [Google Scholar]
Balle 2009 {published data only}
- Balle M, Tortella‐Feliu M. Efficacy of a brief school‐based program for selective prevention of childhood anxiety. Anxiety, Stress and Coping: An International Journal 2009;23(1):71‐85. [DOI] [PubMed] [Google Scholar]
Bannink 2012 {published data only}
- Bannink R, Broeren S, Zwanenburg EJ, As E, Looij‐Jansen P, Raat H. Effectiveness of a web‐based tailored intervention (E‐health4Uth) and consultation to promote adolescents’ health: randomized controlled trial. Journal of Medical Internet Research 2014;16(5):e143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannink R, Zwanenburg EJ, Looij JP, As E, Raat H. Evaluation of computer‐tailored health education ('E‐health4Uth') combined with personal counselling ('E‐health4Uth + counselling') on adolescents' behaviours and mental health status: design of a three‐armed cluster randomised controlled trial. BMC Public Health 2012;12:1083‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnet 2007 {published data only}
- Barnet B, Liu J, DeVoe M, Alperovitz‐Bichell K, Duggan AK. Home visiting for adolescent mothers: effects on parenting, maternal life course, and primary care linkage. Annals of Family Medicine 2007;5(3):224‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrett 2001 {published data only}
- Barrett P, Turner C. Prevention of anxiety symptoms in primary school children: preliminary results from a universal school‐based trial. British Journal of Clinical Psychology 2001;40(399):410. [DOI] [PubMed] [Google Scholar]
Berger 2008 {published data only}
- Berger R, Gelkopf M. School‐based intervention for the treatment of tsunami‐related distress in children: a quasi‐randomized controlled trial. Psychotherapy for Psychosomatics 2008;78(6):364–71. [DOI] [PubMed] [Google Scholar]
Berry 2009 {published data only}
- Berry K, Hunt CJ. Evaluation of an intervention program for anxious adolescent boys who are bullied at school. Journal of Adolescent Health 2009;45(4):376‐82. [DOI] [PubMed] [Google Scholar]
Bond 2004 {published data only}
- Bond L, Patton G, Glover S, Carlin JB, Butler H, Thomas L, et al. The Gatehouse Project: can a multilevel school intervention affect emotional wellbeing and health risk behaviours?. Journal of Epidemiology and Community Health 2004;58:997‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boogar 2012 {published data only}
- Boogar IR. Effectiveness of the Teasdale Cognitive Therapy on depression reduction in guidance and high school students [عنوان: اثربخشي درمان شناختي تيزديل بر كاهش افسردگي دانش آموزان دوره هاي راهنمايي و متوسطه]. Psychological Research 2012;14(2):25‐40. [Google Scholar]
Boring 2012 {published data only}
- Boring J. Children of Divorce Coping with Divorce (CoD‐CoD): evaluating the efficacy of an Internet‐based preventative intervention for children of divorce. Dissertation Abstracts International Section B: The Sciences and Engineering 2012;73(3B):1841. [Google Scholar]
Bourque 2013 {published data only}
- Bourque J, Beaton A, Mainville L, Chalifoux M, LeBlanc J. Effect of a dialectical behavioural therapy‐based intervention on the developmental assets of grade 9 and 10 youths: results of a randomized trial [Effect d'une intervention basée sur la thérapie comportementale dialectique sur les acquis développementaux de junes de 9e et 10e années: Résultats d'un essai randomisé]. Revue de Psychoéducation 2013;42(2):333‐55. [Google Scholar]
Britton 2014 {published data only}
- Britton WB, Lepp NE, Niles HF, Rocha T, Fisher NE, Gold JS. A randomized controlled pilot trial of classroom‐based mindfulness meditation compared to an active control condition in sixth‐grade children. Journal of School Research 2014;52(3):263‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brody 2012 {published data only}
- Brody GH, Chen YF, Kogan SM, Yu T, Molgaard VK, DiClemente RJ, et al. Family‐centered program deters substance use, conduct problems, and depressive symptoms in black adolescents. Pediatrics 2012;129(1):108‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buttigieg 2015 {published data only}
- Buttigieg JP, Shortt AL, Slaviero TM, Hutchinson D, Kremer P, Toumbourou JW. A longitudinal evaluation of the Resilient Families randomized trial to prevent early adolescent depressive symptoms. Journal of Adolescence 2015;44:204‐13. [DOI] [PubMed] [Google Scholar]
Cabiya 2008 {published data only}
- Cabiya JJ, Padilla‐Cotto L, Gonzalez K, Sanchez‐Cestero J, Martinez‐Taboas A, Sayers S. Effectiveness of a cognitive‐behavioral intervention for Puerto Rican children. Interamerican Journal of Psychology 2008;42(2):195‐202. [Google Scholar]
Cook 2015 {published data only}
- Cook CR, Frye M, Slemrod T, Lyon AR, Renshaw TL, Zhang Y. An integrated approach to universal prevention: independent and combined effects of PBIS and SEL on youths' mental health. School Psychology Quarterly 2015;30:166‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davidson 2014 {published data only}
- Davidson TM, Yuen EK, Felton JW, McCauley J, Gros KS, Ruggiero KJ. Feasibility assessment of a brief, web‐based behavioral activation intervention for adolescents with depressed mood. International Journal of Psychiatry Medicine 2014;48:69‐82. [DOI] [PubMed] [Google Scholar]
Day 2013 {published data only}
- Day V, McGrath PJ, Mojtowicz M. Internet‐based guided self‐help for university students with anxiety, depression and stress: a randomized controlled clinical trial. Behavior Research and Therapy 2013;51(7):344‐51. [DOI] [PubMed] [Google Scholar]
Gerson 2013 {published data only}
- Gerson MW, Fernandez N. PATH: A program to build resilience and thriving in undergraduates. Journal of Applied Social Psychology 2013;43(11):2169‐84. [Google Scholar]
Hains 1990 {published data only}
- Hains AA, Szyjakowski M. A cognitive stress‐reduction intervention program for adolescents. Journal of Counseling Psychology 1990;37(1):79‐84. [Google Scholar]
Hains 1992 {published data only}
- Hains AA. Comparison of cognitive‐behavioral stress management techniques with adolescent boys. Journal of Counseling & Development 1992;70:600‐5. [Google Scholar]
Hains 1994 {published data only}
- Hains A, Ellman S. Stress inoculation training as a preventative intervention for high school youths. Journal of Cognitive Psychotherapy: An International Quarterly 1994;8(3):219‐32. [Google Scholar]
Healy 2014 {published data only}
- Healy KL, Sanders MR. Randomized controlled trial of a family intervention for children bullied by peers. Behavior Therapy 2014;45:760‐77. [DOI] [PubMed] [Google Scholar]
Hoek 2012 {published data only}
- Hoek W, Aarts F, Schuurmans J, Cuijpers P. Who are we missing: non‐participation in an Internet intervention trial for depression and anxiety in adolescents. European Child and Adolescent Psychiatry 2012;21(10):593‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek W, Schuumans J, Koot H, Cuijpers P. Prevention of depression and anxiety in adolescents: a randomized controlled trial testing the efficacy and mechanisms of Internet‐based self‐help problem‐solving therapy. Trials 2009;10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoek W, Schuurmans J, Koot HM, Cuijpers P. Effects of Internet‐based guided self‐help problem‐solving therapy for adolescents with depression and anxiety: a randomized controlled trial. PLoS One 2012;7(8):e43485. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hyun 2010 {published data only}
- Hyun MS, Nam KA, Kim MA. Randomized controlled trial of a cognitive‐behavioral therapy for at‐risk Korean male adolescents. Archives of Psychiatric Nursing 2010;24(3):202‐11. [DOI] [PubMed] [Google Scholar]
Ishikawa 2010 {published data only}
- Ishikawa S, Iwanaga M, Yamashita B, Sato H, Sato S. Long‐term effects of social skills training on depressive symptoms in children [社会的ス キ ル訓練に よ る児童の抑う つ症状への長期的効果]. Japanese Journal of Educational Psychology 2010;58(3):372‐84. [Google Scholar]
Ishimura 2014 {published data only}
- Asano K, Hatori K, Ishimura I, Koganei K, Nomura T, Sukigara N, et al. Psycho‐educational intervention on self‐compassion attitude and its effects on depressive symptoms. International Journal of Psychiatry in Clinical Practice [Abstracts of the 12th International Forum on Mood and Anxiety Disorders (IFMAD); Barcelona, Spain: 7 November, 2012 to 9 November, 2012] 2012;16:34‐5. [Google Scholar]
- Ishimura I, Yamaguchi M, Nomura T, Sukigara N. Effective self‐compassionate task for enhancing mental health in Japanese college students. Annual International Conference on Cognitive and Behavioral Psychology 2014:48. [Google Scholar]
King 1990 {published data only}
- King CA, Kirschenbaum DS. An experimental evaluation of a school‐based program for children at risk: Wisconsin early intervention. Journal of Community Psychology 1990;18(2):167‐77. [Google Scholar]
Klein 2011 {published data only}
- Klein B. Evaluation of a tailored online same‐sex attracted youth focused transdiagnostic/multi‐symptom mental health and wellbeing program. http://www.anzctr.org.au/ACTRN12611000700932.aspx 2011.
Kraag 2009 {published data only}
- Kraag G, Breukelen GJP, Kok G, Hosman C. 'Learn Young, Learn Fair', a stress management program for fifth and sixth graders: longitudinal results from an experimental study. Journal of Child Psychology and Psychiatry 2009;50(9):1185‐95. [DOI] [PubMed] [Google Scholar]
Kumakech 2009 {published data only}
- Kumakech E, Cantor‐Graae E, Maling S, Bajunirwe F. Peer‐group support intervention improves the psychosocial well‐being of AIDS orphans: cluster randomized trial. Social Science and Medicine 2009;68(6):1038‐43. [DOI] [PubMed] [Google Scholar]
Lamb 1998 {published data only}
- Lamb JM, Puskar KR, Sereika SM, Corcoran M. School‐based intervention to promote coping in rural teens. American Journal of Maternal Child Nursing 1998;23(4):187‐94. [DOI] [PubMed] [Google Scholar]
Layne 2008 {published data only}
- Layne CM, Saltzman WR, Poppleton L, Burlingame GM, Pasalić A, Duraković E, et al. Effectiveness of a school‐based group psychotherapy program for war‐exposed adolescents: a randomized controlled trial. Journal of the American Academy of Child and Adolescent Psychiatry 2008;47(9):1048‐62. [DOI] [PubMed] [Google Scholar]
Lock 2003 {published data only}
- Lock S, Barrett PM. A longitudinal study of developmental differences in universal preventive intervention for child anxiety. Behaviour Change 2003;20(4):183‐99. [Google Scholar]
Lowry‐Webster 2003 {published data only}
- Lowry‐Webster HM, Barrett PM, Lock S. A universal prevention trial of anxiety symptomology during childhood: results at 1‐year follow‐up. Behaviour Change 2003;20(1):25‐43. [Google Scholar]
Manassis 2010 {published data only}
- Manassis K, Wilansky‐Traynor P, Farzan N, Kleiman V, Parker K, Sanford M. The feelings club: randomized controlled evaluation of school‐based CBT for anxious or depressive symptoms [ISRCTN88858028]. Depression & Anxiety 2010;27(10):945‐52. [DOI] [PubMed] [Google Scholar]
Manz 2001 {published data only}
- Manz R, Junge J, Margraf J. Primary prevention of anxious and depressive symptoms in adolescents. First results from a quasi‐experimental study. Zeitschrift fur Gesundheitswissenschafeten 2001;9:229‐30. [Google Scholar]
Marcotte 1993 {published data only}
- Marcotte D, Baron P. The efficacy of a school‐based rational‐emotive intervention strategy with depressive adolescents [L'efficacite d'une strategie d'intervention emotivo‐rationnelle aupres d'adolescents depressifs du milieu scolaire]. Canadian Journal of Counselling 1993;27(2):77‐92. [Google Scholar]
Mason 2007 {published data only}
- Mason WA, Kosterman R, Hawkins JD, Haggerty KP, Spoth RL, Redmond C. Influence of a family‐focused substance use preventive intervention on growth in adolescent depressive symptoms. Journal of Research on Adolescence 2007;17(3):541‐64. [Google Scholar]
Mason 2012 {published data only}
- Mason WA, Haggerty KP, Fleming AP, Casey‐Goldstein M. Family intervention to prevent depression and substance use among adolescents of depressed parents. Journal of Child and Family Studies 2012;21(6):891‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mateu‐Martínez 2013 {published data only}
- Mateu‐Martínez O, Piqueras JA, Jiménez‐Albiar I, Espada JP, Carballo JL, Orgilés M. Effectiveness of a brief cognitive‐behavioral prevention program for social rejection in children [Eficacia de un programa de prevención cognitivo‐conductual breve del rechazo social en niños]. Terapia Psicológica 31;2:187‐95. [Google Scholar]
McBride 2012 {published data only}
- McBride MC. The effects of brief psychoeducation on adolescents' depressive symptoms and perceptions of parenting. Dissertation Abstracts International Section B: The Sciences and Engineering 2012;72(8‐B):4989. [Google Scholar]
McLaughlin 2007 {published data only}
- McLaughlin AE, Campbell FA, Pungello EP, Skinner M. Depressive symptoms in young adults: the influences of the early home environment and early educational child care. Child Development 2007;78(3):746‐56. [DOI] [PubMed] [Google Scholar]
Muriungi 2013 {published data only}
- Muriungi SK, Ndetei DM. Effectiveness of psycho‐education on depression, hopelessness, suicidality, anxiety and substance use among basic diploma students at Kenya Medical Training College. South African Journal of Psychiatry 2013;19(2):41‐50. [Google Scholar]
Palermo 2009 {published data only}
- Palermo TM, Wilson AC, Peters M, Lewandowski A, Somhegyi H. Randomized controlled trial of an Internet‐delivered family cognitive‐behavioral therapy intervention for children and adolescents with chronic pain. Pain 2009;146:205‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Parker 2011 {published data only}
- Parker AG, Hetrick SE, Jorm AF, Yung AR, McGorry PD, Mackinnon AJ, et al. The effectiveness of simple psychological and exercise interventions for high prevalence mental health problems in young people: a factorial randomised controlled trial [ACTRN12608000550303]. Trials 2011;12:76‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Peters 2014 {published data only}
- Peters HO. Social connections: Internet prevention of loneliness and depression in first year university students PhD dissertation. Dissertation Abstracts International: Section B: The Sciences and Engineering 2014.
Raider 2008 {published data only}
- Raider MC, Steele W, Delillo‐Storey M, Jacobs J, Kuban C. Structured sensory therapy (SITCAP‐ART) for traumatized adjudicated adolescents in residential treatment. Residential Treatment For Children and Youth 2008;25(2):167‐85. [Google Scholar]
Reid 2011 {published data only}
- Reid SC, Kauer SD, Hearps SJC, Crooke ADA, Khor AS, Sanci LA, et al. A mobile phone application for the assessment and management of youth mental health problems in primary care: a randomised controlled trial [NCT00794222]. BMC Family Practice 2011;12:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sankaranarayanan 2014 {published data only}
- Sankaranarayanan A, Cycil C. Resiliency training in Indian children: a pilot investigation of the Penn Resiliency Program. International Journal of Environmental Research and Public Health 2014;11(4):4125‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shen 2002 {published data only}
- Shen Y‐J. Short‐term group play therapy with Chinese earthquake victims: effects on anxiety, depression and adjustment. International Journal of Play Therapy 2002;11(1):43‐63. [Google Scholar]
Sibinga 2013 {published data only}
- Sibinga EM, Perry‐Parrish C, Chung SE, Johnson SB, Smith M, Ellen JM. School‐based mindfulness instruction for urban male youth: a small randomized controlled trial. Preventive Medicine 2013;57(6):799‐801. [DOI] [PubMed] [Google Scholar]
Simpson 2008 {published data only}
- Simpson AT. The Roles of Self‐Regulation and Coping in a Preventative Cognitive‐Behavioural Intervention for School‐Age Children At‐Risk for Internalizing Disorders [Thesis]. Toronto, ON: University of Toronto, 2007. [Google Scholar]
Singhal 2014 {published data only}
- Singhal M, Manjula M, Vijay Sagar KJ. Development of a school‐based program for adolescents at‐risk for depression in India: results from a pilot study. Asian Journal of Psychiatry 2014;10:56‐61. [DOI] [PubMed] [Google Scholar]
Stallard 2014 {published data only}
- Stallard P, Skryabina E, Taylor G, Phillips R, Daniels H, Anderson R, et al. Classroom‐based cognitive behaviour therapy (FRIENDS): a cluster randomised controlled trial to Prevent Anxiety in Children through Education in Schools (PACES). Lancet Psychiatry 2014;1:185‐92. [DOI] [PubMed] [Google Scholar]
Stallard 2015 {published data only}
- Stallard P, Skryabina E, Taylor G, Phillips R, Daniels H, Anderson R, et al. Can school‐based CBT programmes reduce anxiety in children? Results from the preventing anxiety in children through education in schools (PACES) randomised controlled trial. European Psychiatry 2015:190. [Google Scholar]
Stasiak 2014 {published data only}
- Stasiak K, Hatcher S, Frampton C, Merry SN. A pilot double blind randomized placebo controlled trial of a prototype computer‐based cognitive behavioural therapy program for adolescents with symptoms of depression. Behavioural and Cognitive Psychotherapy 2014;42(4):385‐401. [DOI] [PubMed] [Google Scholar]
Tol 2008 {published data only}
- Tol WA, Komproe IH, Susanty D, Jordans MJ, Macy RD, Jong JT. School‐based mental health intervention for children affected by political violence in Indonesia: a cluster randomized trial. JAMA 2008;300(6):655‐62. [DOI] [PubMed] [Google Scholar]
Treutiger 2013 {published data only}
- Treutiger B‐M, Lindberg L. Prevention of depressive symptoms among adolescent girls. In: Andershed A‐K editor(s). Girls at Risk: Advancing Responsible Adolescent Development. New York, NY: Springer New York, 2013:57‐78. [Google Scholar]
van de Weijer‐Bergsma 2014 {published data only}
- Weijer‐Bergsma E, Langenberg G, Brandsma R, Oort FJ, Bögels SM. The effectiveness of a school‐based mindfulness training as a program to prevent stress in elementary school children. Mindfulness 2014;5:238‐48. [Google Scholar]
Van Voorhees 2009 {published data only}
- Hoek W, Marko M, Fogel J, Schuurmans J, Gladstone T, Bradford N, et al. Randomized controlled trial of primary care physician motivational interviewing versus brief advice to engage adolescents with an Internet‐based depression prevention intervention: 6‐month outcomes and predictors of improvement. Translational Research 2011;158(6):315‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iloabachie C, Wells C, Goodwin B, Baldwin M, Vanderplough‐Booth K, Gladstone T, et al. Adolescent and parent experiences with a primary care/Internet‐based depression prevention intervention (CATCH‐IT). General Hospital Psychiatry 2011;33(6):543‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulsberry A, Marko‐Holguin M, Blomeke K, Hinkle C, Fogel J, Gladstone T, et al. Randomized clinical trial of a primary care Internet‐based intervention to prevent adolescent depression: one‐year outcomes. Journal of the Canadian Academy of Child and Adolescent Psychiatry 2013;22(2):106‐17. [PMC free article] [PubMed] [Google Scholar]
- Voorhees BW. A randomized controlled trial of a primary care Internet‐based depression prevention intervention for adolescents (CATCH‐IT): 12‐month outcomes. Journal of Investigative Medicine [Abstracts of the 2010 Combined Annual Meeting of the Central Society for Clinical Research and the Midwestern Section American Federation for Medical Research; Chicago, IL: 22 April, 2010 to 23 April, 2010] 2010;58(4):654. [Google Scholar]
- Voorhees BW, Fogel J, Pomper BE, Marko M, Reid N, Watson N, et al. Adolescent dose and ratings of an Internet‐based depression prevention program: a randomized trial of primary care physician brief advice versus a motivational interview. Journal of Cognitive and Behavioral Psychotherapies 2009;9(1):1‐19. [PMC free article] [PubMed] [Google Scholar]
- Voorhees BW, Fogel J, Reinecke MA, Gladstone T, Stuart S, Gollan J, et al. Randomized clinical trial of an Internet‐based depression prevention program for adolescents (Project CATCH‐IT) in primary care: 12‐week outcomes. Journal of Developmental and Behavioral Pediatrics 2009;30:123‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees BW, Vanderplough‐Booth K, Fogel J, Gladstone T, Bell C, Stuart S, et al. Integrative Internet‐based depression prevention for adolescents: a randomized clinical trial in primary care for vulnerability and protective factors. Journal of the Canadian Academy of Child and Adolescent Psychiatry 2008;17(4):184‐96. [PMC free article] [PubMed] [Google Scholar]
Vuori 2008 {published data only}
- Vuori J, Koivisto P, Mutanen P, Jokisaari M, Salmela‐Aro K. Towards working life: effects of an intervention on mental health and transition to post‐basic education. Journal of Vocational Behavior 2008;72(1):67‐80. [Google Scholar]
Wahl 2014 {published data only}
- Wahl MS, Adelson JL, Patak MA, Pössel P, Hautzinger M. Teachers or psychologists: who should facilitate depression prevention programs in schools?. International Journal of Environmental Research and Public Health 2014;11(5):5294‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolchik 2000 {published data only}
- Luecken LJ, Hagan MJ, Mahrer NE, Wolchik SA, Sandler IN, Tein JY. Effects of a prevention program for divorced families on youth cortisol reactivity 15 years later. Psychology & Health 2015;30:751‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolchik SA, West SG, Sandler IN, Tein JY, Coatsworth D, Lengua L, et al. An experimental evaluation of theory‐based mother and mother‐child programs for children of divorce. Journal of Consulting and Clinical Psychology 2000;68(5):843‐56. [PubMed] [Google Scholar]
Zehnder 2010 {published data only}
- Zehnder D, Meuli M, Landolt MA. Effectiveness of a single‐session early psychological intervention for children after road traffic accidents: a randomised controlled trial. Child and Adolescent Psychiatry and Mental Health 2010;4:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Baramkoohi 2009 {published data only}
- Baramkoohi AA. Training in life skills for decreasing depression [آموزش مهارتهاي زندگي براي كاهش افسردگي]. Journal of Iranian Psychologists 2009;5(20):297‐306. [Google Scholar]
Cohen 2014 {published data only}
- Cohen EN. Effects of self‐administered rosy glow optimism training on mental health in dysphoric college students. Dissertation Abstracts International Section B: The Sciences and Engineering 2014; Vol. 74:No pagination specified.
Ehring 2010 {published data only}
- Ehring T. Randomized controlled trial evaluating two versions of a rumination‐focused CBT training for the presence of depression and anxiety in adolescents and young adults. http://clinicaltrials.gov/show/NCT01223677 2010.
La Greca 2013 {published data only}
- Greca A. Coping with adolescent peer victimization and reducing anxious/depressed symptoms. http://clinicaltrials.gov/show/NCT02011438 2013.
Levin 2014 {published data only}
- Levin ME. Evaluating a prototype acceptance and commitment training web‐based prevention program for depression and anxiety in college students. Dissertation Abstracts International Section B: The Sciences and Engineering 2014; Vol. 75:No pagination specified.
- Levin ME, Pistorello J, Seeley JR, Hayes SC. Feasibility of a prototype web‐based acceptance and commitment therapy prevention program for college students. Journal of American College Health 2014;62:20‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
McCauly 2003 {published data only}
- McCauly E, Stoep A. Middle school to high school transition project. http://clinicaltrials.gov/show/NCT00071513 2003.
Rasing 2013 {published data only}
- Rasing SPA, Creemers DHM, Janssens JMAM, Scholte RHJ. Effectiveness of depression and anxiety prevention in adolescents with high familial risk: study protocol for a randomized controlled trial. BMC Psychiatry 2013;13:316‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Redzic 2014 {published data only}
- Redzic NM. The effects of an Internet‐based psychoeducational program on reducing depressive symptoms in adolescents. Dissertation Abstracts International Section B: The Sciences and Engineering 2014;74:No pagination specified. [Google Scholar]
Saulsberry 2013 {published data only}
- Saulsberry A, Corden ME, Taylor‐Crawford K, Crawford TJ, Johnson M, Froemel J, et al. Chicago Urban Resiliency Building (CURB): an Internet‐based depression‐prevention intervention for urban African‐American and Latino adolescents. Journal of Child and Family Studies 2013;22(1):150‐60. [Google Scholar]
Tak 2012 {published data only}
- Tak YR, Kleinjan M, Lichtwarck‐Aschoff A, Engels RC. Secondary outcomes of a school‐based universal resiliency training for adolescents: a cluster randomized controlled trial. BMC Public Health 2014;14:1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak YR, Lichtwarck‐Aschoff A, Gillham JE, Zundert RMP, Engels RCME. Universal school‐based depression prevention 'op volle kracht': a longitudinal cluster randomized controlled trial. Journal of Abnormal Child Psychology 2015 Sep 25 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- Tak YR, Zundert RM, Kuijpers RCWM, Vlokhoven BS, Rensink HFW, Engels RCME. A randomized controlled trial testing the effectiveness of a universal school‐based depression prevention program 'Op Volle Kracht' in the Netherlands. BMC Public Health 2012;12:21‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2013 {published data only}
- Tang T‐C, Huang S‐Y. Interpersonal psychotherapy for treating psychological disturbances in adolescents who experienced bullied experiences: randomized case control study [Abstract 426]. European Psychiatry 2013;28(Suppl 1):1. [Google Scholar]
van Voorhees 2010 {published data only}
- Voorhees B. Primary Care Internet‐Based Depression Prevention for Adolescents (CATCH‐IT). http://clinicaltrials.gov/show/NCT01228890 2010.
References to ongoing studies
Chim 2013 {published data only}
- Chim D. Adaptation of a US Internet tool to prevent depression and addiction in Hong Kong adolescents. http://clinicaltrials.gov/show/NCT01783652 2013.
Garber 2013 {published data only}
- Garber J. Family cognitive behavioral prevention of depression in youth and parents. http://clinicaltrials.gov/show/NCT02021578 2013.
Geisner 2013 {published data only}
- Geisner IM, Mittman A, Kilmer JR. Web based personalized intervention for risky drinking college students with depressed mood: examining the moderating effect of drinking motives. Alcoholism, Clinical and Experimental Research [Abstracts of the 36th Annual Scientific Meeting of the Research Society on Alcoholism (RSA) Annual Conference; Orlando, FL: 22 June, 2013 to 26 June, 2013]. 2013:81A.
Nauta 2012 {published data only}
- Nauta MH, Festen H, Reichart CG, Nolen WA, Stant AD, Bockting CL, et al. Preventing mood and anxiety disorders in youth: a multi‐centre RCT in the high risk offspring of depressed and anxious patients. BMC Psychiatry 2012;12:31‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Platt 2014a {published data only}
- Platt B, Pietsch K, Krick K, Oort F, Schulte‐Körne G. Study protocol for a randomised controlled trial of a cognitive‐behavioural prevention programme for the children of parents with depression: the PRODO trial. BMC Psychiatry 2014;14:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt BJ, Schulte‐Körne G. Primary Prevention Of Depression in Offspring of Depressed Parents (PRODO). http://clinicaltrials.gov/show/NCT02115880 2014.
Toth 2011 {published data only}
- Toth S, Cicchetti D, Todd Manly J. Prevention of depression in maltreated and nonmaltreated adolescents. https://clinicaltrials.gov/ct2/show/NCT01534377 2011.
Van Voorhees 2012 {published data only}
- Gladstone G, Marko‐Holguin M, Rothberg P, Nidetz J, Diehl A, DeFrino DT, et al. An internet‐based adolescent depression preventive intervention: study protocol for a randomized control trial. Trials 2015;16:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees B, Marko M. Primary Care Internet Based Depression Prevention for Adolescents (CATCH‐IT) (also known as 'Promoting AdolescenT Health' or 'PATH'). http://clinicaltrials.gov/show/NCT01893749 2012.
Additional references
Abramson 1978
- Abramson LY, Seligman MEP, Teasdale I. Learned helplessness in humans: critique and reformulation. Journal of Abnormal Psychology 1978; Vol. 87, issue 1:49‐59. [PubMed]
Achenbach 2001
- Achenbach TM, Rescorla LA. Manual for Achenbach System of Empirically Based Assessments (ASEBA) School‐Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, and Families, 2001. [Google Scholar]
Altman 1991
- Altman DG. Practical Statistics for Medical Research. London: Chapman and Hall, 1991. [Google Scholar]
American Psychiatric Association 2000
- American Psychiatric Association. Diagnostic and Statistical Manual (4th edition text revision). Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
Angold 1987
- Angold A, Costello EJ. Mood and Feelings Questionnaire (MFQ). Durham, NC: Duke University, Developmental Epidemiology Program, 1987. [Google Scholar]
Angold 1995
- Angold A, Costello EJ, Messer SC, Pickles A, Winder F, Silver D. Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. International Journal of Methods in Psychiatric Research 1995;5:237‐49. [Google Scholar]
Araya 2011
- Araya R, Montgomery AA, Fritsch R, Gunnell D, Stallard P, Noble S, et al. School‐based intervention to improve the mental health of low‐income, secondary school students in Santiago, Chile (YPSA): study protocol for a randomized controlled trial. Trials 2011;12:49‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Araya 2013
- Araya R, Fritsch R, Soears M, Rojas G, Martinez V, Barroihet S, et al. School intervention to improve mental health of students in Santiago, Chile. JAMA Pediatrics 2013;167(11):1004‐10. [DOI] [PubMed] [Google Scholar]
Baker 1989
- Baker RW, Siryk B. The Student Adaption to College Questionnaire (SACQ). Los Angeles, CA: Western Psychological Services, 1989. [Google Scholar]
Beck 1961
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [DOI] [PubMed] [Google Scholar]
Beck 1976
- Beck AT. Cognitive Therapy and the Emotional Disorders. New York, NY: International Universities Press, 1976:356. [Google Scholar]
Beck 1988
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. Journal of Consulting and Clinical Psychology 1988b;56:893‐7. [DOI] [PubMed] [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory‐II. San Antonio, TX: Psychological Corporation, 1996. [Google Scholar]
Beck 2005
- Beck JS, Beck AT, Jolly JB. Beck Youth Inventories. New York, NY: Pearson, Inc, 2005. [Google Scholar]
Berk 2009
- Berk M, Parker G. The elephant on the couch: side‐effects of psychotherapy. Australian and New Zealand Journal of Psychiatry 2009;43(9):787‐94. [DOI] [PubMed] [Google Scholar]
Biostat 2014 [Computer program]
- Biostat. Comprehensive Meta‐Analysis, Version 3.2.1. Englewood, NJ: Biostat, 2014.
Birmaher 1996
- Birmaher B, Ryan N, Williamson D, Brent D, Kaufman J, Dahl R, et al. Childhood and adolescent depression: a review of the past 10 years. Part I. Journal of the American Academy of Child and Adolescent Psychiatry 1996;35(11):1427‐39. [DOI] [PubMed] [Google Scholar]
Birmaher 1996a
Birmaher 1997
- Birmaher B, Khetarpal S, Brent D. The Screen for Child Anxiety Related Emotional Disorders (SCARED): scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry 1997;37:906‐14. [DOI] [PubMed] [Google Scholar]
Brent 1986
Brent 2002
Buhler 2011
- Buhler A, Kotter C, Jaursch S, Losel F. Prevention of familial transmission of depression: EFFEKT‐E, a selective program for emotionally burdened families. Journal of Public Health 2011:321‐7.
Calear 2010
- Calear AL, Christensen H. Review of Internet‐based prevention and treatment programs for anxiety and depression in children and adolescents. Medical Journal of Australia 2010;192(11):s12. [DOI] [PubMed] [Google Scholar]
Callahan 2012
- Callahan P, Liu P, Purcell R, Parker AG, Hetrick SE. Evidence map of prevention and treatment interventions for depression in young people. Depression Research and Treatment 2012; Vol. 2012, issue Article ID 820735:11 pages. [DOI] [PMC free article] [PubMed]
Carnevale 2012
- Carnevale TD. Universal adolescent depression prevention: programs a review. Journal of School Nursing 2012;29(3):181‐95. [DOI] [PubMed] [Google Scholar]
Chorpita 2005
- Chorpita BF, Moffitt C, Gray J. Psychometric properties of the Revised Child Anxiety and Depression Scale in a clinical sample. Behavior Research and Therapy 2005;43:309‐22. [DOI] [PubMed] [Google Scholar]
Christensen 2009
- Christensen H, Griffiths KM, Farrer L. Adherence in Internet interventions for anxiety and depression. Journal of Medical Internet Research 2009;11(2):e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cicchetti 1998
- Cicchetti D, Toth SL. The development of depression in children and adolescents. American Psychologist 1998;53(2):221‐41. [DOI] [PubMed] [Google Scholar]
Conrod 2002
- Conrod PJ, Woicik P. Validation of a four‐factor model of personality risk for substance abuse and examination of a brief instrument for assessing personality risk. Addiction Biology 2002;7:329‐46. [Google Scholar]
Corrieri 2014
- Corrieri S, Heider D, Conrad I, Blume A, König HH, Riedel‐Heller SG. School‐based prevention programs for depression and anxiety in adolescence: a systematic review. Health Promotion International 2014;29(3):427‐41. [DOI] [PubMed] [Google Scholar]
Costello 2006
- Costello JE, Erkanli A, Angold A. Is there an epidemic of child or adolescent depression. Journal of Child Psychology and Psychiatry 2006;47(12):1263‐71. [DOI] [PubMed] [Google Scholar]
Cuijpers 2004
- Cuijpers P, Smit F. Subthreshold depression as a risk indicator for major depressive disorder: a systematic review of prospective studies. Acta Psychiatrica Scandinavica 2004;109:325‐31. [DOI] [PubMed] [Google Scholar]
Cuijpers 2008
- Cuijpers P, Straten A, Smit F, Mihalopoulos C, Beekman A. Preventing the onset of depressive disorders: a meta‐analytic review of psychological interventions. American Journal of Psychiatry 2008;165(10):1272‐80. [DOI] [PubMed] [Google Scholar]
Davidson 2002
- Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, et al. Neural and behavioral substrates of mood and mood regulation. Biological Psychiatry 2002;52:478‐502. [DOI] [PubMed] [Google Scholar]
De Beurs 2005
- Beurs E, Zitman F. The Brief Symptom Inventory. The reliability and validity of a practical alternative to SCL‐90 [De betrouwbaarheid envaliditeit van een handzaam alternatief voor de SCL‐90]. Maandblad GeestelijkeVolksge 2005;61(2):120. [Google Scholar]
Derogatis 1983
- Derogatis LR, Melisaratos N. The Brief Symptom Inventory: an introductory report. Psychological Medicine 1983;13(3):595‐605. [PubMed] [Google Scholar]
Döpfner 2000
- Döpfner M, Lehmkuhl G. Diagnostic System for Mental Disorders in Children and Adolescents Following ICD‐10 and DSM‐IV [DISYPS‐KJ: Diagnostik‐System für psychische Störungen im Kindes‐ und Jugendalter nach ICD‐10 und DSM‐IV]. Bern, Switzerland: Huber, 2000. [Google Scholar]
Ekers 2014
- Ekers D, Webster L, Straten A, Cuijpers P, Richards D, Gilbody S. Behavioural activation for depression: an update of meta‐analysis of effectiveness and sub‐group analysis. PLoS One 2014;9(6):e100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Endicott 1981
- Endicott J, Cohen J, Nee J, Fleiss J, Sarantakos S. Hamilton Depression Rating Scale: extracted from regular and change versions of the Schedule for Affective Disorders and Schizophrenia. Archives of General Psychiatry 1981;38:98‐103. [DOI] [PubMed] [Google Scholar]
Endicott 2006
- Endicott J, Nee J, Yang R, Wohlberg C. Pediatric Quality of Life Enjoyment and Satisfaction Questionnaire (PQ‐LES‐Q): reliability and validity. Journal of the American Academy of Child and Adolescent Psychiatry 2006;45:401‐7. [DOI] [PubMed] [Google Scholar]
Fergusson 1993
- Fergusson DM, Horwood L, Lynskey MT. Prevalence and comorbidity of DSM‐III‐R diagnoses in a birth cohort of 15 year olds. Journal of the American Academy of Child and Adolescent Psychiatry 1993;32(6):1127‐34. [DOI] [PubMed] [Google Scholar]
Fergusson 2001
- Fergusson DM, Horwood LJ. The Christchurch health and development study: review of findings on child and adolescent mental health. Australian and New Zealand Journal of Psychiatry 2001;35:287‐96. [DOI] [PubMed] [Google Scholar]
Fergusson 2007
- Fergusson D, Boden JM, Horwood LJ. Recurrence of major depression in adolescence and early adulthood, and later mental health, educational, and economic outcomes. British Journal of Psychiatry 2007;191(14):335‐42. [DOI] [PubMed] [Google Scholar]
First 1997
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM‐IV Axis I Disorders. Arlington, VA: American Psychiatric Publishing, Inc, 1997. [Google Scholar]
Fleming 1993
- Fleming JE, Boyle MH, Offord DR. The outcome of adolescent depression in the Ontario child health study follow‐up. Journal of the American Academy of Child and Adolescent Psychiatry 1993;32(1):28‐33. [DOI] [PubMed] [Google Scholar]
Fleming 2014
- Fleming T, Cheek C, Merry SN, Thabrew H, Bridgman H, Stasiak K, et al. Serious games for the treatment or prevention of depression: a systematic review. Revista de Psicopatología y Psicología Clínica ‐ Spanish Journal of Clinical Psychology 19;3:227‐42. [Google Scholar]
Gladstone 2009
- Gladstone T, Beardslee W. The prevention of depression in children and adolescents: a review. Canadian Journal of Psychiatry 2009;54(4):212‐21. [DOI] [PubMed] [Google Scholar]
Gladstone 2011
- Gladstone TR, Beardslee WR, O’Connor EE. The prevention of adolescent depression. Psychiatric Clinics of North America 2011;34(1):35‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goodyer 2000
- Goodyer IM, Tamplin A, Herbert J, Altham PME. Recent life events, cortisol, dehydroepiandrosterone and the onset of major depression in high‐risk adolescents. British Journal of Psychiatry 2000;177(6):499‐504. [DOI] [PubMed] [Google Scholar]
Gore 2011
- Gore FM, Bloem PJ, Patton GC, Ferguson J, Joseph V, Coffey C, et al. Global burden of disease in young people aged 10‐24 years: a systematic analysis. Lancet 2011;377(9783):2093‐102. [DOI] [PubMed] [Google Scholar]
Gould 1998
- Gould MS, King R, Greenwald S, Fisher P, Schwab‐Stone M, Kramer R, et al. Psychopathology associated with suicidal ideation and attempts among children and adolescents. Journal of the American Academy of Child and Adolescent Psychiatry 1998;37(9):915‐23. [DOI] [PubMed] [Google Scholar]
GRADE profiler [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 20 April 2015. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Guyatt 2013a
- Guyatt GH, Oxman AD, Santesso N, Helfand M, Vist G, Kunz R, et al. GRADE guidelines 12: Preparing summary of findings tables ‐ binary outcomes. Journal of Clinical Epidemiology 2013;66:158‐72. [DOI] [PubMed] [Google Scholar]
Guyatt 2013b
- Guyatt GH, Thorlund K, Oxman AD, Walter SD, Patrick D, Furukawa TA, et al. GRADE guidelines 13: Preparing summary of findings tables and evidence profiles ‐ continuous outcomes. Journal of Clinical Epidemiology 2013;66:173‐83. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology Neurosurgery and Psychiatry 1960;12:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hautzinger 1994
- Hautzinger M, Bailer M, Worall H, Keller F. Beck‐Depressions‐Inventar (BDI). Test manual [Bearbeitung der deutschen Ausgabe. Testhandbuch]. German. Göttingen: Huber, 1994. [Google Scholar]
Hayes 2003
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and Commitment Therapy: an Experiential Approach to Behavior Change. London: The Guilford Press, 2003. [Google Scholar]
Hazell 2002
- Hazell P, O'Connell D, Heathcote D, Henry D. Tricyclic drugs for depression in children and adolescents. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD002317] [DOI] [PubMed] [Google Scholar]
Higgins 2008a
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Higgins 2008b
- Higgins JPT, Altman DG (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester (UK): John Wiley & Sons, 2008. [Google Scholar]
Higgins 2011
- Higgins J P, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmann 2010
- Hofmann SG, Sawyer AT, Fang A. The empirical status of the "new wave" of cognitive behavioral therapy. Psychiatric Clinics of North America 2010;33(3):701‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Holland 1997
- Holland D, Dadds M. The Diagnostic Interview Schedule for Children, Adolescents, and Parents. Brisbane, QLD: Griffith University, 1997. [Google Scholar]
Horowitz 2006
- Horowitz J, Garber J. The prevention of depressive symptoms in children and adolescents: a meta‐analytic review. Journal of Consulting and Clinical Psychology 2006;74:401–15. [DOI] [PubMed] [Google Scholar]
Horowitz 2007
- Horowitz JL, Garber J, Ciesla JA, Young JF, Mufson L. Prevention of depressive symptoms in adolescents: a randomized trial of cognitive‐behavioral and interpersonal prevention programs. Journal of Consulting and Clinical Psychology 2007;75(5):693‐706. [DOI] [PubMed] [Google Scholar]
Howick 2013
- Howick J, Friedemann, C, Tsakok M, et al. Are treatments more effective than placebos? A systematic review and meta‐analysis. PLoS One 2013;8(5):e62599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jane‐Llopis 2003
- Jane‐Llopis EVA, Hosman C, Jenkins, R, Anderson P. Predictors of efficacy in depression prevention programmes. Meta‐analysis. British Journal of Psychiatry 2003;183(5):384‐97. [DOI] [PubMed] [Google Scholar]
Jegannathan 2014
- Jegannathan B, Dahlblomc K, Kullgren G. Outcome of a school‐based intervention to promote life‐skills among young people in Cambodia. Asian Journal of Psychiatry 2014;9:78‐84. [DOI] [PubMed] [Google Scholar]
Kaufman 1997
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, et al. Schedule for affective disorders and schizophrenia for school‐age children ‐ present and lifetime version: Initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry 1997;36:980‐9. [DOI] [PubMed] [Google Scholar]
Kessler 2005
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age‐of‐onset distributions of DSM‐IV disorders in the National Comorbidity Survey replication. Archives of General Psychiatry 2005;62:593‐602. [DOI] [PubMed] [Google Scholar]
Kindt 2012
- Kindt KCM, Zundert R, Engles RCME. Evaluation of a Dutch school‐based depression prevention program for youths in high risk neighborhoods: study protocol of a two armed randomized controlled trial. BMC Public Health 2012;12:212. [Netherlands Trials Registry: NTR3110] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kovacs 1992
- Kovacs M. Children's Depression Inventory Manual. North Tonawanda, NY: Multi‐Health Systems, 1992. [Google Scholar]
Larsen 1986
- Larsen R, Diener E, Emmons RA. Affect intensity and reactions to daily life events. Journal of Personality and Social Psychology 1986;51:803‐14. [Google Scholar]
Lewinsohn 1993
- Lewinsohn PM, Hops H, Poberts RE, Seeley JR, Andrews JA. Prevalence and incidence of depression and other DSM‐IIIR disorders in high school students. Journal of Abnormal Psychology 1993;102:133‐4. [DOI] [PubMed] [Google Scholar]
Lewinsohn 1994
- Lewinsohn PM, Roberts RE, Seeley JR, Rohde P, Gotlib IH, Hops H. Adolescent psychopathology, II: Psychosocial risk factors for depression. Journal of Abnormal Psychology 1994;103(2):302‐15. [DOI] [PubMed] [Google Scholar]
Lewinsohn 1998
- Lewinsohn PM, Rohde P, Seely JR. Major depressive disorder in older adolescents: prevalence, risk factors, and clinical implications. Clinical Psychology Review 1998;18(7):765‐94. [DOI] [PubMed] [Google Scholar]
Linehan 1993
- Linehan M. Cognitive‐behavioral Treatment of Borderline Personality Disorder. New York: The Guilford Press, 1993. [Google Scholar]
Lipsey 1993
- Lipsey MW, Wilson DB. The efficacy of psychological, educational, and behavioral treatment: confirmation from meta‐analysis. American Psychologist 1993;48:1181‐209. [DOI] [PubMed] [Google Scholar]
Lipsitz 2013
- Lipsitz JD, Markowitz JC. Mechanisms of change in interpersonal therapy (IPT). Clinical Psychology Review 2013;33(8):1134‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lovibond 1995
- Lovibond SH, Lovibond PF. Manual for the Depression Anxiety Stress Scales. 2nd Edition. Sydney: Psychology Foundation, 1995. [Google Scholar]
Ludman 2003
- Ludman E, Katon W, Bush T, Rutter C, Lin E, Simon G, et al. Behavioural factors associated with symptom outcomes in a primary care‐based depression prevention trial. Psychological Medicine 2003;33(6):1061‐70. [DOI] [PubMed] [Google Scholar]
Marchand 2010
- Marchand E, Ng J, Rohde P, Stice E. Effects of an indicated cognitive‐behavioral depression prevention program are similar for Asian American, Latino, and European American adolescents. Behavior Research and Therapy 2010;48(8):821‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martell 2001
- Martell CR, Addis ME, Jacobson NS. Depression in Context: Strategies for Guided Action. New York: WW Norton, 2001. [Google Scholar]
Mathers 2004
- Mathers CD, Bernard C, Iburg KM, Inoue M, Fat DM, Shibuya K, et al. Global burden of disease in 2002: data sources, methods and results. Global programme on evidence for health policy discussion paper no. 54. World Health Organization 2004.
McCauley 2001
- McCauley E, Pavlidis K, Kendall K. Developmental precursors of depression: the child and the social environment [Cambridge Child and Adolescent Psychiatry]. In: Goodyer IM editor(s). The Depressed Child and Adolescent. 2nd Edition. Cambridge: Cambridge University Press, 2001:46‐78. [Google Scholar]
McDermott 2011
- McDermott B, Baigent M, Chanen A, Fraser L, Graetz B, Hayman N, et al. Clinical Practice Guidelines: Depression in Adolescents and Young Adults. Melbourne: beyondblue: the National Depression Initiative, 2011. [Google Scholar]
Merikangas 2011
- Merikangas KR, He JP, Burstein M, Swendsen J, Avenevoli S, Case B, et al. Service utilization for lifetime mental disorders in US adolescents: results of the National Comorbidity Survey‐Adolescent Supplement (NCS‐A). Journal of the American Academy of Child and Adolescent Psychiatry 2011;50(1):32‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Merry 2004b
- Merry SN, Hetrick S, McDowell H, Bir J. Psychological and/or educational interventions for the prevention of depression in children and adolescents. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003380.pub2] [DOI] [PubMed] [Google Scholar]
Merry 2006
- Merry SN. Population‐based Approaches to Reducing Depression in Adolescents in New Zealand [Doctor of Medicine thesis]. Auckland: The University of Auckland, 2006. [Google Scholar]
Merry 2011
- Merry S N, Hetrick SE, Cox GR, Brudevold‐Iversen T, Bir JJ, McDowell H. Psychological and educational interventions for preventing depression in children and adolescents. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD003380.pub3] [DOI] [PubMed] [Google Scholar]
Moncrieff 2006
- Moncrieff J, Churchill R, Drummond C, McGuire H. Development of a quality assessment instrument for trials of treatments for depression and neurosis. International Journal of Psychiatric Research Methods 2006;10(3):126‐33. [Google Scholar]
Mrazek 1994
- Mrazek PJ, Haggerty RJ. Reducing Risks for Mental Disorders. Frontiers for Preventive Research. Washington DC: National Academy Press, 1994. [PubMed] [Google Scholar]
Murray 1997
Myles 2000
- Myles PS, Gin T. Statistical Methods for Anaesthesia and Intensive Care. Oxford: Butterworth Heinemann, 2000. [Google Scholar]
Najarian 1994
- Najarian B. The construction and validation of the short form of Children Depression Scale (CDS‐A) by factor analysis [ساخت و اعتباريابی فرم کوتاه مقياس افسردگی کودکان (CDS‐A) به وسيله تحليل عوامل]. Psychological Research 1994;2(3‐4):24‐44. [Google Scholar]
NICE 2005
- National Institute for Health and Clinical Excellence, NICE. Depression in Children and Young People: Identification and management in primary, community and secondary care. National Clinical Practice Guideline 28. Leicester, UK: The British Psychological Society, 2005. [PubMed] [Google Scholar]
O'Connell 2009
- O'Connell ME, Boat T, Warner KE. Preventing Mental, Emotional, and Behavioral Disorders Among Young People. Progress and Possibilities. Washington DC: The National Academies Press, 2009. [PubMed] [Google Scholar]
Orvaschel 1986
- Orvaschel H, Puig‐Antich J. Schedule for Affective Disorders and Schizophrenia for School‐Age Children, Epidemiologic Version: Kiddie‐SADS‐E (K‐SADS‐E), 4th version. Pittsburgh, PA: Western Psychiatric Institute and Clinic, 1986. [Google Scholar]
Pace 2011
- Pace NL. Research methods for meta‐analyses: best practice and research. Clinical Anaesthesiology 2011;25(4):523‐33. [DOI] [PubMed] [Google Scholar]
Patel 2007a
- Patel V, Araya R, Chatterjee S, Chisholm D, Cohen A, Silva M, et al. Treatment and prevention of mental disorders in low‐income and middle‐income countries. Lancet 370;9591:991‐1005. [DOI] [PubMed] [Google Scholar]
Patel 2007b
- Patel V, Flisher AJ, Hetrick S, McGorry P. Mental health of young people: a global public‐health challenge. Lancet 2007;369(9569):1302–13. [DOI] [PubMed] [Google Scholar]
Patton 2007
- Patton GC, Hetrick SE, McGorry P. Service responses for youth onset mental disorders. Current Opinion in Psychiatry 2007;20:319‐24. [DOI] [PubMed] [Google Scholar]
Petersen 1993
- Petersen AC, Compas BE, Brooks‐Gunn J, Stemmler M, Ey S, Grant KE. Depression in adolescence. American Psychologist 1993;48(2):155‐68. [DOI] [PubMed] [Google Scholar]
Petti 1985
- Petti T. Scales of potential use in the psychopharmacological treatment of depressed children and adolescents. Psychopharmacology Bulletin 1985;21:951‐77. [PubMed] [Google Scholar]
Poznanski 1996
- Poznanski EO, Mokros HB. Children's Depression Rating Scale‐Revised Manual. Los Angeles, CA: Western Psychological Services, 1996. [Google Scholar]
Price 2002
- Price CS, Spence SH, Sheffield J, Donovan C. The development and psychometric properties of a measure of social and adaptive functioning for children and adolescents. Journal of Clinical Child and Adolescent Psychology 2002;31:111‐22. [DOI] [PubMed] [Google Scholar]
Puig‐Antich 1983
- Puig‐Antich J, Chambers WJ. Schedule for Affective Disorders and Schizophrenia for School‐Age Children (6–18 years). Pittsburgh, PA: Western Psychiatric Institute, 1983. [Google Scholar]
Radloff 1977
- Radloff LS. The CES–D Scale: a self‐report depression scale for research in the general population. Applied Psychological Measurement 1977;1:385‐401. [Google Scholar]
Rao 1995
Rapee 2013
- Rapee RM. The preventative effects of a brief, early intervention for preschool‐aged children at risk for internalising: follow‐up into middle adolescence. Journal of Child Psychology and Psychiatry 2013;54(7):780‐8. [DOI] [PubMed] [Google Scholar]
Reid 2011
- Reid SC, Kauer SD, Hearps SJ, Crooke AH, Khor AS, Sanci LA, et al. A mobile phone application for the assessment and management of youth mental health problems in primary care: a randomised controlled trial. BMC Family Practice 2011;12:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reynolds 1985
- Reynolds CR, Richmond BO. Revised Children's Manifest Anxiety Sale (RCMAS) manual. Los Angeles, CA: Western Psychological Services, 1985. [Google Scholar]
Reynolds 1989
- Reynolds WM. Reynold's Child Depression Scale. Odessa, FL: Psychological Assessment Resources, 1989. [Google Scholar]
Reynolds 2002
- Reynolds WM. Reynolds Adolescent Depression Scale, 2nd edition (RADS‐2): Professional Manual. Lutz: Psychological Assessment Resources, 2002. [Google Scholar]
Rhode 1994
Rice 2014
- Rice SM, Goodall J, Hetrick SE, Parker AG, Gilbertson T, Amminger GP, et al. Online and social networking interventions for the treatment of depression in young people: a systematic review. Journal of Medical Internet Research 2014;16(9):e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rose 1992
- Rose G. The Strategy of Preventive Medicine. 1st Edition. Oxford: Oxford University Press, 1992. [Google Scholar]
Sandler 2003
- Sandler IN, Ayers TS, Wolchik SA, Tein J, Kwok O, Haine RA, et al. The Family Bereavement Program: efficacy evaluation of a theory‐based prevention program for parentally bereaved children and adolescents. Journal of Consulting and Clinical Psychology 2003;70(3):587‐600. [DOI] [PubMed] [Google Scholar]
Seligman 1979
Shaffer 1983
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S. A Children’s Global Assessment Scale (CGAS). Archives of General Psychiatry 1983;40:1228‐31. [DOI] [PubMed] [Google Scholar]
Shaffer 2000
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab‐Stone ME. NIMH Diagnostic Interview Schedule for Children version IV (NIMH DISC–IV): description, differences from previous versions, and reliability of some common diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry 2000;39:28‐38. [DOI] [PubMed] [Google Scholar]
Shapiro 1979
- Shapiro R, Keller M. Longitudinal Interval Follow‐up Evaluation (LIFE). Boston, MA: Massachusetts General Hospital, 1979. [Google Scholar]
SIGN 2000
- Scottish Intercollegiate Guidelines Network (SIGN). Grading system for recommendations in evidence based clinical guidelines: report of a review of the system of grading recommendations for SIGN. http://www.sign.ac.uk 2000.
Silverman 1996
- Silverman WK, Albano AM. Anxiety Disorders Interview Schedule for DSM–IV—Child Version: Parent Interview Schedule. San Antonio, TX: The Psychological Corporation–Harcourt Brace, 1996. [Google Scholar]
Sipahi 2012
- Sipahi I, Swaminathan A, Natesan V, Debanne SM, Simon DI, Fang JC. Effect of antihypertensive therapy on incident stroke in cohorts with prehypertensive blood pressure levels: a meta‐analysis of randomized controlled trials. Stroke 2012;43:432‐40. [DOI] [PubMed] [Google Scholar]
Spence 2003
- Spence SH, Barrett PM, Turner CM. Psychometric properties of the Spence Children's Anxiety Scale with young adolescents. Journal of Anxiety Disorders 2003;17:605‐25. [DOI] [PubMed] [Google Scholar]
Spence 2007
- Spence SH, Shortt AL. Research review: Can we justify the widespread dissemination of universal, school‐based interventions for the prevention of depression among children and adolescents?. Journal of Child Psychology and Psychiatry 2007;48(6):526‐42. [DOI] [PubMed] [Google Scholar]
Spielberger 1970
- Spielberger CD, Gorsuch RL, Lushene RE. Manual for the State‐Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press, 1970. [Google Scholar]
Stallard 2010
- Stallard P, Montgomery AA, Araya R, Anderson R, Lewis G, Sayal K, et al. Protocol for a randomised controlled trial of a school based cognitive behaviour therapy (CBT) intervention to prevent depression in high risk adolescents (PROMISE). Trials 2010;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stallard 2012
- Stallard P, Sayal K, Phillips R, Taylor JA, Spears M, Anderson R, et al. Classroom based cognitive behavioural therapy in reducing symptoms of depression in high risk adolescents: pragmatic cluster randomised controlled trial. BMJ 2012;345:e6058. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stice 2006
- Stice E, Burton E, Bearman SK, Rhode P. Randomized trial of a brief depression prevention program: an elusive search for a psychosocial placebo control condition. Behaviour Research and Therapy 2006;45(5):863‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stice 2008
- Stice E, Rohde P, Seeley JR, Gau JM. Brief cognitive‐behavioral depression prevention program for high‐risk adolescents outperforms two alternative interventions: a randomized efficacy trial. Journal of Consulting and Clinical Psychology 2008;76(4):595‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stice 2009
- Stice E, Shaw H, Bohon C, Marti CN, Rohde P. A meta‐analytic review of depression prevention programs for children and adolescents: factors that predict magnitude of intervention effects. Journal of Consulting Clinical Psychology 2009;77(3):486‐503. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stiensmeier‐Pelster 2000
- Stiensmeier‐Pelster J, Schürmann M, Duda K. Depressionsinventar für Kinder und Jugendliche (DIKJ). Göttingen: Hogrefe, 2000. [Google Scholar]
Stockings 2015
- Stockings E, Degenhardt L, Lee YY, Mihalopoulos C, Liu A, Hobbs M, Patton G. Symptom screening scales for detecting major depressive disorder in children and adolescents: a systematic review and meta‐analysis of reliability, validity and diagnostic utility. Journal of Affective Disorders 2015;174:447‐63. [DOI] [PubMed] [Google Scholar]
Teasdale 1995
- Teasdale JDS, Segal Z, Williams JMG. How does cognitive therapy prevent depressive relapse and why should attentional control (mindfulness) training help?. Behaviour Research and Therapy 1995;33(1):25‐39. [DOI] [PubMed] [Google Scholar]
Tennant 2007
- Tennant R, Hiller L, Fishwick R, Platt S, Joesph S, Weich S, et al. The Warwick‐Edinburgh Mental Well‐being Scale (WEMWBS): development and UK validation. Health Quality of Life Outcomes 2007;5:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tisher 1983
- Tisher M, Lang M. The Children’s Depression Scale: review and further developments. In: Cantwell DP, Carlson GA editor(s). Affective Disorders in Childhood and Adolescence: an Update. New York, NY: Spectrum Publications, Inc, 1983:181‐210. [Google Scholar]
Townsend 2001
- Townsend E, Hawton K, Altman DG, Gunnell D, Hazell P, House A, et al. The efficacy of problem‐solving treatments after deliberate self‐harm: meta‐analysis of randomized controlled trials with respect to depression, hopelessness and improvement in problems. Psychological Medicine 2001;13(6):979‐88. [DOI] [PubMed] [Google Scholar]
Unnewehr 2008
- Unnewehr S, Schneider S, Margraf J. Kinder‐DIPS ‐ Diagnostisches Interview bei Psychischen Störungen im Kindes‐ und Jugendalter. Heidelberg: SpringerMedizin, 2008. [Google Scholar]
Vernon 1998
- Vernon A. A Journey Through Emotional, Social, Cognitive, and Self‐development. Champaign, IL: Research Press, 1998. [Google Scholar]
Wasserman 2010
- Wasserman D, Carli V, Wasserman C, Apter A, Balazs J, Bobes J, et al. Saving and empowering young lives in Europe (SEYLE): a randomized controlled trial. BMC Public Health 2010;10:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watson 1991
- Watson D, Clark LA. The Mood and Anxiety Symptom Questionnaire. Iowa City, IA: Department of Psychology, University of Iowa, 1991. [Google Scholar]
Weersing 2009
- Weersing VR, Rozenman M, Gonzalez A. Core components of therapy in youth: do we know what to disseminate?. Behavior Modification 2009;33(1):24‐47. [DOI] [PubMed] [Google Scholar]
Weissman 1980
- Weissman MM, Orvaschel H, Padian N. Children’s symptom and social functioning self‐report scales: comparison of mothers’ and children’s reports. Journal of Nervous and Mental Disease 1980;168:736‐40. [DOI] [PubMed] [Google Scholar]
Weisz 2006
- Weisz JR, McCarty CA, Valeri SM. Effects of psychotherapy for depression in children and adolescents: a meta‐analysis. Psychological Bulletin 2006;132(1):132‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williford 2012
- Williford A, Boulton A, Noland B, Little TD, Karna A, Salmivalli C. Effects of the KiVa anti‐bullying program on adolescents' depression, anxiety, and perception of peers: Erratum. Journal of Abnormal Child Psychology 2012;40:301‐2. [DOI] [PubMed] [Google Scholar]
World Health Organization 2007
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems, 10th revision. Geneva, Switzerland: World Health Organization, 2007. [Google Scholar]
Xie 2014
- Xie M, Shan Z, Chen S, Yang W, Bao W, Rong Y, et al. Aspirin for primary prevention of cardiovascular events: meta‐analysis of randomized controlled trials and subgroup analysis by sex and diabetes status. PLoS One 2014;9(10):e90286. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Merry 2004a
- Merry SN, Hetrick S, McDowell H, Bir J, Muller N. The effectiveness of psychological and/or educational interventions for the prevention of depression in children and adolescents. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD003380.pub2] [DOI] [PubMed] [Google Scholar]
Merry 2011b
- Merry SN, Hetrick SE, Cox GR, Brudevold‐Iversen T, Bir JJ, McDowell H. Psychological and educational interventions for preventing depression in children and adolescents. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD003380.pub3] [DOI] [PubMed] [Google Scholar]


