Abstract
Background
Treatment guidelines for asthma recommend inhaled corticosteroids (ICS) as first‐line therapy for children with persistent asthma. Although ICS treatment is generally considered safe in children, the potential systemic adverse effects related to regular use of these drugs have been and continue to be a matter of concern, especially the effects on linear growth.
Objectives
To assess the impact of ICS on the linear growth of children with persistent asthma and to explore potential effect modifiers such as characteristics of available treatments (molecule, dose, length of exposure, inhalation device) and of treated children (age, disease severity, compliance with treatment).
Search methods
We searched the Cochrane Airways Group Specialised Register of trials (CAGR), which is derived from systematic searches of bibliographic databases including CENTRAL, MEDLINE, EMBASE, CINAHL, AMED and PsycINFO; we handsearched respiratory journals and meeting abstracts. We also conducted a search of ClinicalTrials.gov and manufacturers' clinical trial databases to look for potential relevant unpublished studies. The literature search was conducted in January 2014.
Selection criteria
Parallel‐group randomised controlled trials comparing daily use of ICS, delivered by any type of inhalation device for at least three months, versus placebo or non‐steroidal drugs in children up to 18 years of age with persistent asthma.
Data collection and analysis
Two review authors independently performed study selection, data extraction and assessment of risk of bias in included studies. We conducted meta‐analyses using the Cochrane statistical package RevMan 5.2 and Stata version 11.0. We used the random‐effects model for meta‐analyses. We used mean differences (MDs) and 95% CIs as the metrics for treatment effects. A negative value for MD indicates that ICS have suppressive effects on linear growth compared with controls. We performed a priori planned subgroup analyses to explore potential effect modifiers, such as ICS molecule, daily dose, inhalation device and age of the treated child.
Main results
We included 25 trials involving 8471 (5128 ICS‐treated and 3343 control) children with mild to moderate persistent asthma. Six molecules (beclomethasone dipropionate, budesonide, ciclesonide, flunisolide, fluticasone propionate and mometasone furoate) given at low or medium daily doses were used during a period of three months to four to six years. Most trials were blinded and over half of the trials had drop out rates of over 20%.
Compared with placebo or non‐steroidal drugs, ICS produced a statistically significant reduction in linear growth velocity (14 trials with 5717 participants, MD ‐0.48 cm/y, 95% CI ‐0.65 to ‐0.30, moderate quality evidence) and in the change from baseline in height (15 trials with 3275 participants; MD ‐0.61 cm/y, 95% CI ‐0.83 to ‐0.38, moderate quality evidence) during a one‐year treatment period.
Subgroup analysis showed a statistically significant group difference between six molecules in the mean reduction of linear growth velocity during one‐year treatment (Chi² = 26.1, degrees of freedom (df) = 5, P value < 0.0001). The group difference persisted even when analysis was restricted to the trials using doses equivalent to 200 μg/d hydrofluoroalkane (HFA)‐beclomethasone. Subgroup analyses did not show a statistically significant impact of daily dose (low vs medium), inhalation device or participant age on the magnitude of ICS‐induced suppression of linear growth velocity during a one‐year treatment period. However, head‐to‐head comparisons are needed to assess the effects of different drug molecules, dose, inhalation device or patient age. No statistically significant difference in linear growth velocity was found between participants treated with ICS and controls during the second year of treatment (five trials with 3174 participants; MD ‐0.19 cm/y, 95% CI ‐0.48 to 0.11, P value 0.22). Of two trials that reported linear growth velocity in the third year of treatment, one trial involving 667 participants showed similar growth velocity between the budesonide and placebo groups (5.34 cm/y vs 5.34 cm/y), and another trial involving 1974 participants showed lower growth velocity in the budesonide group compared with the placebo group (MD ‐0.33 cm/y, 95% CI ‐0.52 to ‐0.14, P value 0.0005). Among four trials reporting data on linear growth after treatment cessation, three did not describe statistically significant catch‐up growth in the ICS group two to four months after treatment cessation. One trial showed accelerated linear growth velocity in the fluticasone group at 12 months after treatment cessation, but there remained a statistically significant difference of 0.7 cm in height between the fluticasone and placebo groups at the end of the three‐year trial.
One trial with follow‐up into adulthood showed that participants of prepubertal age treated with budesonide 400 μg/d for a mean duration of 4.3 years had a mean reduction of 1.20 cm (95% CI ‐1.90 to ‐0.50) in adult height compared with those treated with placebo.
Authors' conclusions
Regular use of ICS at low or medium daily doses is associated with a mean reduction of 0.48 cm/y in linear growth velocity and a 0.61‐cm change from baseline in height during a one‐year treatment period in children with mild to moderate persistent asthma. The effect size of ICS on linear growth velocity appears to be associated more strongly with the ICS molecule than with the device or dose (low to medium dose range). ICS‐induced growth suppression seems to be maximal during the first year of therapy and less pronounced in subsequent years of treatment. However, additional studies are needed to better characterise the molecule dependency of growth suppression, particularly with newer molecules (mometasone, ciclesonide), to specify the respective role of molecule, daily dose, inhalation device and patient age on the effect size of ICS, and to define the growth suppression effect of ICS treatment over a period of several years in children with persistent asthma.
Plain language summary
Do inhaled corticosteroids reduce growth in children with persistent asthma?
Review question: We reviewed the evidence on whether inhaled corticosteroids (ICS) could affect growth in children with persistent asthma, that is, a more severe asthma that requires regular use of medications for control of symptoms.
Background: Treatment guidelines for asthma recommend ICS as first‐line therapy for children with persistent asthma. Although ICS treatment is generally considered safe in children, parents and physicians always remain concerned about the potential negative effect of ICS on growth.
Search date: We searched trials published until January 2014.
Study characteristics: We included in this review trials comparing daily use of corticosteroids, delivered by any type of inhalation device for at least three months, versus placebo or non‐steroidal drugs in children up to 18 years of age with persistent asthma.
Key results: Twenty‐five trials involving 8471 children with mild to moderate persistent asthma (5128 treated with ICS and 3343 treated with placebo or non‐steroidal drugs) were included in this review. Eighty percent of these trials were conducted in more than two different centres and were called multi‐centre studies; five were international multi‐centre studies conducted in high‐income and low‐income countries across Africa, Asia‐Pacifica, Europe and the Americas. Sixty‐eight percent were financially supported by pharmaceutical companies.
Meta‐analysis (a statistical technique that combines the results of several studies and provides a high level of evidence) suggests that children treated daily with ICS may grow approximately half a centimeter per year less than those not treated with these medications during the first year of treatment. The magnitude of ICS‐related growth reduction may depend on the type of drug. Growth reduction seems to be maximal during the first year of therapy and less pronounced in subsequent years of treatment. Evidence provided by this review allows us to conclude that daily use of ICS can cause a small reduction in height in children up to 18 years of age with persistent asthma; this effect seems minor compared with the known benefit of these medications for asthma control.
Quality of evidence: Eleven of 25 trials did not report how they guaranteed that participants had an equal chance of receiving ICS or placebo or non‐steroidal drugs. All but six trials did not report how researchers were kept unaware of the treatment assignment list. However, this methodological limitation may not significantly affect the quality of evidence because the results remained almost unchanged when we excluded these trials from the analysis.
Summary of findings
for the main comparison.
| Inhaled corticosteroids compared with placebo or non‐steroidal drugs for children with persistent asthma: effects on growth | ||||||
|
Patient or population: children up to 18 years of age with persistent asthma Settings: outpatient Intervention: inhaled corticosteroids Comparison: placebo or non‐steroidal drugs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Mean difference (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or non‐steroidal drugs | Inhaled corticosteroids1 | |||||
| Linear growth velocity in first year of treatment (cm/y) | Mean linear growth velocity ranged across control groups from 5.5 to 8.5 cm/y | Mean reduction in linear growth velocity was 0.48 cm/y | ‐0.48 cm/y (‐0.65 to ‐0.30) less growth in the ICS group | 5717 (14 trials) |
⊕⊕⊕⊝ moderate2, 3 | |
| Change from baseline in height over first year of treatment (cm) | Mean change from baseline in height over a 1‐year period ranged across the control groups from 4.7 to 8.6 cm/y | Mean reduction in change from baseline in height over a 1‐year period was 0.61 cm | ‐0.61 cm (‐0.83 to ‐0.38) less growth in the ICS group | 3275 (15 trials) |
⊕⊕⊕⊝ moderate2,3 | |
| Change in height standard deviation score (SDS) in first year of treatment | Mean change in height SDS score across control groups from ‐0.09 to 0.5 | Mean reduction in change in height SDS score was 0.13 | ‐0.13 (‐0.24 to ‐0.01) less growth in the ICS group | 258 (4 trials) |
⊕⊕⊕⊝ moderate2,3 | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Inhaled corticosteroids included beclomethasone dipropionate, budesonide, ciclesonide, flunisolide, fluticasone propionate and mometasone fumarate.
2A considerable number of included trials did not report the methods of random sequence generation and allocation concealment and had high withdrawal rates, especially in the control groups (deduct 1 point for limitations)
3Significant heterogeneity was noted in results across studies that may be expected because of differences in the molecule, daily dose and age group across trials. However, all trials showed negative effects of ICS on growth, suggesting that the heterogeneity is quantitative but not qualitative and may not significantly affect the conclusions of this review (do not deduct point for inconsistency)
Background
Description of the condition
According to the Global Initiative for Asthma (GINA) (GINA 2014), asthma is operationally defined as "a chronic inflammatory disorder of the airways in which many cells and cellular elements play a role. The chronic inflammation is associated with airway hyperresponsiveness that leads to recurrent episodes of wheezing, breathlessness, chest tightness, and coughing, particularly at night or in the early morning. These episodes are usually associated with widespread, but variable, airflow obstruction within the lung that is often reversible either spontaneously or with treatment." During childhood, asthma is a phenotypically heterogeneous condition with a wide spectrum of symptoms and severity (Bush 2004; Stein 2004). This fact makes the diagnosis and treatment of childhood asthma challenging.
In developed countries, the prevalence of asthma has markedly increased over the past 40 to 50 years, especially among the paediatric population (Asher 2010; ISAAC 1998; Masoli 2004). In these countries, asthma has emerged as a major public health problem because of high prevalence, associated morbidity and substantial healthcare costs and societal burden. However, evidence recently provided by the International Study of Asthma and Allergies in Childhood (ISAAC) suggests that the prevalence of asthma may have reached a plateau in many developed countries (Asher 2010; Lai 2009). In contrast, asthma prevalence is sharply increasing in developing countries (Africa, Central and South America, Asia and the Pacific region), probably as the result of rapid and ongoing urbanisation and westernisation (Asher 2010; Braman 2006). The global burden of childhood asthma is continuing to rise.
Description of the intervention
Chronic airway inflammation is the primary underlying pathology of asthma, regardless of phenotype and disease severity (Busse 2001; GINA 2014). Through complex interaction between various cells and inflammatory mediators, the inflammatory process in the airways leads to vascular leakage, bronchoconstriction, hypersecretion of mucus, inflammatory cell infiltration, airway hyperresponsiveness and ultimately airway remodelling (GINA 2014; NHLBI 2007). These pathophysiological changes are responsible for clinical and functional manifestations of asthma. Thus, airway inflammation is the most important target of therapy in long‐term asthma management.
Inhaled corticosteroids (ICS) are currently considered first‐line treatment for persistent asthma, both in adults and in children (BTS & SIGN 2012; GINA 2014; Lougheed 2012; NHLBI 2007). Studies have demonstrated clinical benefits of ICS in controlling asthma symptoms, reducing exacerbations and hospitalisations, decreasing airway hyperresponsiveness and airway inflammation, improving pulmonary function, improving quality of life and reducing asthma‐related deaths (Adams 2011a; Adams 2011b; Adams 2011c; Covar 2003; Juniper 1990; Olivieri 1997; Suissa 2000; Van Essen‐Zandvliet 1992; Van Rens 1999). Seven ICS are currently available for clinical use worldwide: beclomethasone dipropionate, budesonide, ciclesonide, flunisolide, fluticasone propionate, mometasone furoate and triamcinolone acetate. Each ICS has different pharmacokinetic and pharmacodynamic properties and biologic characteristics; however, all ICS can achieve similar therapeutic benefits when given at equipotent doses (BTS & SIGN 2012; GINA 2014; Lougheed 2012). Optimal doses of ICS for persistent childhood asthma remain unclear. The most recent asthma guidelines recommend low‐dose ICS (<200 μg/d of hydrofluoroalkane (HFA)‐beclomethasone or equivalent) for children with mild to moderate persistent asthma; however, children with more severe asthma and those with poor response to low doses of ICS may require higher doses to achieve satisfactory asthma control (BTS & SIGN 2012; GINA 2014; Lougheed 2012).
Although ICS are generally considered safe as treatment for children with asthma, the potential systemic adverse effects related to long‐term use of these drugs, especially the effects on growth, have been and continue to be a matter of concern (Allen 1998; Allen 2002; Pedersen 2001). In 1998, based on a report of the panel of experts, the US Food and Drug Administration (FDA) stated that labels warning of a potential reduction in growth in children are required on all ICS products (FDA 1998). Since that time, the relationship between ICS and growth impairment in children with asthma has been extensively discussed in the literature (Allen 2006; Brand 2001; Carlsen 2002; Creese 2001; Price 2002; Salvatoni 2003; Sizonenko 2002; Witzmann 2000; Wolthers 2001).
How the intervention might work
Currently, ICS are the most potent anti‐inflammatory drugs available for the long‐term treatment of persistent asthma. The therapeutic benefits of ICS have been directly related to a decrease in airway inflammation (Djukanovic 1992).
The molecular mechanisms by which ICS exert anti‐inflammatory effects are not entirely understood. ICS are believed to bind with cytoplasmic glucocorticoid receptors in target cells (Barnes 2006; Colice 2000; Leung 2003; Sobande 2008). The corticosteroid‐receptor complexes translocate to the cell nucleus, acting as a transcriptional modulator to repress expression of inflammatory genes. However, the interaction with corticosteroid response genes may not explain entirely the anti‐inflammatory effects of ICS. Corticosteroids have recently been found to interact with other cytoplasmic factors, such as activator protein‐1 and nuclear factor‐kB, which also affect genomic transcription (Colice 2000).
The mechanism of corticosteroid‐induced growth impairment also is not yet clearly understood. Corticosteroids are known to inhibit growth hormone (GH) secretion, insulin‐like growth factor‐1 (IGF‐1) bioactivity, collagen synthesis and adrenal androgen production (Allen 1998; Wolthers 1997). In addition to altering GH output, corticosteroids may reduce GH receptor expression and uncouple the receptors from their signal transduction mechanisms (Allen 1998; Pedersen 2001). Furthermore, corticosteroids may exert a direct growth‐retarding effect on the growth plates (Allen 1998). However, results regarding the association between ICS and alterations in production or activity of GH and IGF‐1 have been inconsistent (Hedlin 1998; Wolthers 1997).
Why it is important to do this review
One Cochrane systematic review with five randomised trials (Sharek 2000a) suggests that moderate doses of inhaled beclomethasone and fluticasone cause a decrease in linear growth velocity of 1.51 cm/y and 0.43 cm/y, respectively. This Cochrane review has been converted to a journal article (Sharek 2000b). Over the past 10 years, several newly undertaken randomised trials have used various new and old inhaled corticosteroid molecules (Becker 2006; Bensch 2011; Gillman 2002; Guilbert 2006; Martinez 2011; Pedersen 2010; Skoner 2008; Skoner 2011; Sorkness 2007; Wasserman 2006). We therefore decided to conduct this systematic review with the goal of evaluating the adverse effects of all currently available ICS on growth in children with persistent asthma. Factors that may influence ICS‐induced growth suppression, such as molecule, dosage, inhalation device, duration of exposure, compliance with treatment, disease severity and participant age, were explored.
Objectives
To assess the impact of ICS on the linear growth of children with persistent asthma and to explore potential effect modifiers such as characteristics of available treatments (molecule, dose, length of exposure, inhalation device) and of treated children (age, disease severity, compliance with treatment).
Methods
Criteria for considering studies for this review
Types of studies
Parallel‐group randomised controlled trials.
Types of participants
Children up to 18 years of age with the diagnosis of persistent asthma.
Types of interventions
Daily use of ICS, delivered by any type of inhalation device for at least three months, compared with placebo or non‐steroidal drugs.
Comparisons are as follows.
ICS alone versus placebo.
ICS alone versus non‐steroidal drugs, such as long‐acting beta2‐agonists (LABA) and leukotriene receptor antagonists (LTRA).
ICS associated with non‐steroidal drugs versus same dose of non‐steroidal drugs.
Types of outcome measures
Primary outcomes
Linear growth velocity, obtained by measuring height at a number of time points during the study and performing linear regression of height against time (Price 2002). The slope of the regression gives linear growth velocity, expressed in cm/year (y) or mm/week (wk).
Secondary outcomes
Change in height standard deviation score (SDS) over time, defined as the difference between an individual's growth velocity and predicted normal growth velocity divided by the predicted normal growth velocity standard deviation (SD) for individuals of the same age, sex and ethnicity, if available (Pedersen 2001).
Change from baseline in height (cm) over time.
Change in height z‐score over time.
We did not intend to include lower leg length measured by knemometry as the outcome because this measurement correlates poorly with statural height and tends to overestimate potential effects of ICS on growth (Allen 1999; Efthimiou 1998). We added adult height (cm) and catch‐up growth after cessation of ICS as post hoc secondary outcomes.
Search methods for identification of studies
Electronic searches
We identified trials from the Cochrane Airways Group Specialised Register of trials (CAGR), which is derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, CINAHL, AMED and PsycINFO, and we handsearched respiratory journals and meeting abstracts (please see Appendix 1 for further details). All records in the CAGR coded as 'asthma' were searched using the following terms:
(((steroid* or corticosteroid* or glucocorticoid* ) and inhal*) or budesonide or Pulmicort or fluticasone or Flixotide or Flovent or ciclesonide or Alvesco or triamcinolone or Kenalog or beclomethasone or Becotide or Becloforte or Becodisk or QVAR or Flunisolide or AeroBid or mometasone or Asmanex or Symbicort or Advair or Inuvair)
AND
(grow* or height* or SDS)
AND
(child* or paediat* or pediat* or adolesc* or teen* or prepubertal* or pre‐pubertal* or puberty or pubertal* or infan* or toddler* or bab* or young*)
We also conducted a search of ClinicalTrials.gov. All databases were searched from their inception to the present time, and language of publication was not restricted. The initial searches were conducted in November 2011, and an updated search was conducted in February 2013 and January 2014.
Searching other resources
We checked reference lists of all primary studies and review articles to look for additional references. We also searched manufacturers' clinical trial databases to uncover potential relevant unpublished studies.
Data collection and analysis
Selection of studies
Two review authors independently assessed the titles and abstracts of all potential studies identified by the search strategy. Full‐text articles were retrieved when studies appeared to meet the inclusion criteria, or when data in the title and abstract were insufficient to allow a clear decision regarding their inclusion. We resolved disagreements through discussion; if required, we consulted the third review author.
Data extraction and management
Two review authors (LZ, SOMP) independently extracted data from the included trials using specially designed and pilot‐tested data extraction forms. For trials with multiple reports, we extracted data from each report separately and combined information across multiple data collection forms afterwards. We resolved disagreements by discussion and entered the extracted data into RevMan version 5.1 (Review Manager 5).
We extracted the following data.
Study characteristics: year of publication, name of first author, country of origin, setting and source/sponsorship.
Methods: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective reporting and other sources of bias.
Participants: sample size, demographics and inclusion and exclusion criteria.
Intervention: type of inhaled corticosteroid, dosage, frequency of administration, inhalation device, treatment duration and compliance with treatment.
Comparator: placebo or non‐steroidal drugs (same details as for the intervention).
Co‐interventions.
Results: mean value of outcome measures in each group, SD or other metrics for uncertainty (standard errors (SE), confidence intervals (CI), t values or P values for differences in means) of outcome measurements in each group, number of participants who underwent randomisation and number of participants in each group for whom outcomes were measured.
We converted SE or 95% CI to SD using the calculator of RevMan (Review Manager 5). We used Engauge digitising software (digitizer.sourceforge.net) to extract data from figures for linear growth velocity (CAMP 2000; Guilbert 2006; Skoner 2011), change from baseline in height (Becker 2006; Bisgaard 2004; Guilbert 2006; Martinez 2011; Skoner 2008) and change in height SDS (Kannisto 2000; Turpeinen 2008; Verberne 1997) in the first year of treatment. We also extracted data from figures to determine change from baseline in height during three to eight months of treatment (Allen 1998; Becker 2006; Bensch 2011; Bisgaard 2004; Doull 1995; Martinez 2011; Skoner 2008; Skoner 2011) and linear growth velocity in the second year (CAMP 2000; Guilbert 2006) and the third year of treatment (CAMP 2000). Data from each figure were extracted twice by the same review author (LZ), at points at least one week apart. The mean of two measurements was used.
Assessment of risk of bias in included studies
Two review authors (LZ, SOMP) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Disagreements were resolved by discussion or by involving the third review author. We assessed risk of bias according to the following domains.
Allocation sequence generation.
Concealment of allocation.
Blinding of participants and investigators.
Incomplete outcome data.
Selective outcome reporting.
We also noted other sources of bias. We graded each potential source of bias as yes, no or unclear, on the basis of whether the potential for bias was low, high or unknown, respectively.
Measures of treatment effect
Measurements of growth are continuous outcomes, so we used mean differences (MDs) and 95% CIs as the metrics to determine treatment effects. A negative value for MD indicates that ICS have suppressive effects on linear growth compared with controls. The lower limit of the 95% CI corresponds to the maximum potential reduction in growth.
Unit of analysis issues
We considered each individual comparison as the unit of analysis. For comparison between participants treated with ICS and controls, we combined different doses of the same molecule into a single corticosteroid group (Allen 1998; Skoner 2008; Skoner 2011). We also combined different age groups (Gillman 2002) and different gender groups (Roux 2003) into a single treatment group. The methods used for combining groups were based on recommendations provided by the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). When both placebo and non‐steroidal drugs were used as controls (Becker 2006; CAMP 2000; Simons 1997), we used only the data from the placebo group for comparison with data from the intervention group. For subgroup analyses, when trials compared two or more ICS molecules (Gillman 2002; Kannisto 2000) or different doses of the same molecule (Allen 1998; Skoner 2008; Skoner 2011) versus the control group, we considered the comparison between each molecule or each dose and controls as a unit of analysis. In this case, we split the original control group into two or more small control groups with equal numbers of participants. One trial (Gradman 2010) contributed data for the subgroup analysis of change from baseline in height at six and eight months, so we split each treatment group into two small groups consisting of equal numbers of participants.
Dealing with missing data
Seven trials did not report SDs or other metrics of uncertainty for growth measurements. For five trials (Guilbert 2006; Roux 2003; Simons 1997; Skoner 2011; Tinkelman 1993), we obtained SDs from P values, SEs or 95% CIs to determine mean differences between groups. For two trials, we imputed missing SDs of mean change from baseline in height (Becker 2006) and missing SDs of linear growth velocity (Gillman 2002) by using SDs of linear growth velocity and mean changes from baseline in height, respectively, because the two measurements had similar values. In another trial (CAMP 2000), we imputed missing SDs of linear growth rate using SDs from the other trial (Jonasson 2000) in the same corticosteroid subgroup.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis. The I² statistic ranges from 0% to 100% and measures the degree of inconsistency across studies, with values of 25%, 50% and 75% corresponding to low, moderate and high heterogeneity, respectively (Higgins 2003). We conducted prespecified subgroup analyses and sensitivity analyses to explore potential sources of heterogeneity and the possibility of effect modifiers.
Assessment of reporting biases
When we suspected reporting bias (see 'Selective reporting bias' above), we attempted to contact study authors to ask them to provide missing outcome data. When this was not possible, and the missing data were thought to introduce serious bias, we explored the impact of excluding such studies from the overall assessment of results by performing a sensitivity analysis. We used funnel plots and Egger's test to assess potential publication bias (Higgins 2003).
Data synthesis
Effects of ICS on linear growth were assessed at five time points of treatment: three to five months, six to eight months, one or nearly one year, two years and three years. In one trial (Turpeinen 2008) with 18‐month treatment, growth data obtained between seven and 18 months were used for analysis at the one‐year time point because ICS was given at a constant dose during this period rather than on a step‐down schedule during the first six months of treatment. In another trial involving 75 children (Kannisto 2000), growth data from 32 children treated with the same drug at a constant dose throughout the whole year were used for the analysis of change in height SDS at the one‐year time point. In the trial of Pauwels 2003, a total of 3195 children five to17 years of age were recruited, but data on linear growth were available for only 1974 children five to 10 years of age.
We performed meta‐analyses using the Cochrane statistical package RevMan 5 (Review Manager 5). We used the random‐effects model for meta‐analyses because it is more appropriate than the fixed‐effect model and provides more conservative estimates with wider CIs when heterogeneity across studies is significant. Otherwise, the two models generate similar results.
When trials reported summarised data on growth measurement such as MD and SE between treatment groups, rather than mean and uncertainty of measurement in each treatment group, we included such trials in the meta‐analysis using MD and SE between treatment groups.
We used the number of participants who contributed data for analysis rather than the intention‐to‐treat population for meta‐analysis because the main aim of this review was to answer the question of whether use of ICS would cause growth suppression in ICS‐treated children with persistent asthma.
We evaluated the quality of the evidence using GRADE methodology and prepared a summary of findings table using the outcomes Linear growth velocity in first year of treatment (cm/y), change from baseline in height over first year of treatment (cm), change in height standard deviation score (SDS) in first year of treatment. We decided to do this post hoc.
Subgroup analysis and investigation of heterogeneity
We planned, a priori, to carry out seven subgroup analyses to explore potential sources of heterogeneity.
Type of ICS: beclomethasone dipropionate, budesonide, ciclesonide, flunisolide, fluticasone propionate, mometasone fumarate, triamcinolone acetate.
Inhalation device: chlorofluorocarbon (CFC)‐metered‐dose inhaler (CFC‐MDI), HFA‐MDI, dry powder inhaler (DPI), nebuliser.
Daily dose of ICS: low, medium and high daily doses of seven ICS based on GINA criteria (GINA 2014): CFC‐beclomethasone: 100 to 200 μg, > 200 to 400 μg, > 400 μg; HFA‐beclomethasone: 50 to 100 μg, > 100 to 200 μg, > 200 μg; budesonide (DPI): 100 to 200 μg, > 200 to 400 μg, > 400 μg; nebulised‐budesonide: 250 to 500 μg, > 500 to 1000 μg, > 1000 μg; ciclesonide: 100 μg, > 100 to 200 μg, > 200 μg; fluticasone propionate: 100 to 200 μg, > 200 to 500 μg, > 500 μg; mometasone furoate: 110 μg, ≥ 220 μg, ≥ 440 μg; triamcinolone acetonide: 400 to 800 μg, > 800 to 1200 μg, > 1200 μg. Dose categories of flunisolide were defined according to GINA 2012 criteria as low (500 to 750 μg), medium (> 750 to 1250 μg) and high (> 1250 μg). All doses of ICS were reported on the basis of ex‐valve rather than ex‐inhaler values. This classification does not refer to dose equivalence but rather to estimated clinical comparability. GINA dose categories of ICS are based on published information and available studies, including direct comparisons when available. We estimated equivalent doses of ICS according to British Thoracic Society (BTS) criteria (BTS & SIGN 2012).
Duration of exposure: three to six months, > six to 12 months, > 12 months.
Asthma severity: mild, moderate and severe persistent asthma.
Age of participants: preschoolers (two to five years), prepubertal children (> five to 12 years), adolescent (> 12 to 18 years).
Concomitant use of non‐steroidal antiasthmatic drugs: ICS alone, ICS combined with non‐steroidal drugs.
We did not perform the planned subgroup analysis on duration of exposure, as the meta‐analysis was conducted at five time points of treatment that were different from those specified a priori. Data derived from the included trials were suitable only for conducting subgroup analyses on type of ICS, inhalation device, daily dose of ICS (low vs medium) and participant age (toddlers and preschoolers vs prepubertal children). We conducted post hoc subgroup analyses on molecules, devices and doses, selecting trials in which only one factor varied. Given that the number of subgroups is generally small, a P value < 0.10 rather than the conventional level of 0.05 was considered statistically significant for the Chi² test in detecting differences between subgroups.
We did not perform the planned meta‐regression analysis to explore the respective role of molecule, daily dose, inhalation device and participant age on the effect size of ICS‐induced growth suppression because all four co‐variates are highly correlated. In this case, it is impossible to untangle the independent effect of each co‐variate (Higgins 2011). Moreover, such analysis has low statistical power because of the relatively small number of included trials.
Sensitivity analysis
Sensitivity analysis was used to assess the potential impact of particular decisions or missing information on the findings of the review (Higgins 2011). We conducted, as planned a priori, the following sensitivity analyses.
Exclusion from the analysis of trials with high risk of bias due to missing data or unbinding, or both.
Exclusion from the analysis of trials in which the compliance rate with ICS was lower than 75%, or in which no data regarding compliance with treatment were provided.
Exclusion from the analysis of pharmaceutical industry–sponsored trials.
We also conducted post hoc sensitivity analyses and excluded trials in which withdrawal rates were higher than 20%, trials in which non‐steroidal drugs rather than placebo were used as controls, trials in which participants previously receiving ICS for longer than one month before study entry were included and trials in which growth data used for analysis were extracted from the figures or were obtained over a portion of the treatment period, rather than over the entire treatment period.
Results
Description of studies
Results of the search
The initial search of electronic databases in November 2011 yielded 435 citations, and the update search in February 2013 and in January 2014 revealed 11 and five citations, respectively. After screening the titles and abstracts, we identified 39 papers as potentially relevant, each of which we reviewed in full text. A total of 13 articles were excluded after full review, leaving 26 articles reporting 24 trials for inclusion in this review. We found one additional trial by checking reference lists of primary studies and review articles. Thus 25 trials were included in this review (Figure 1).
1.
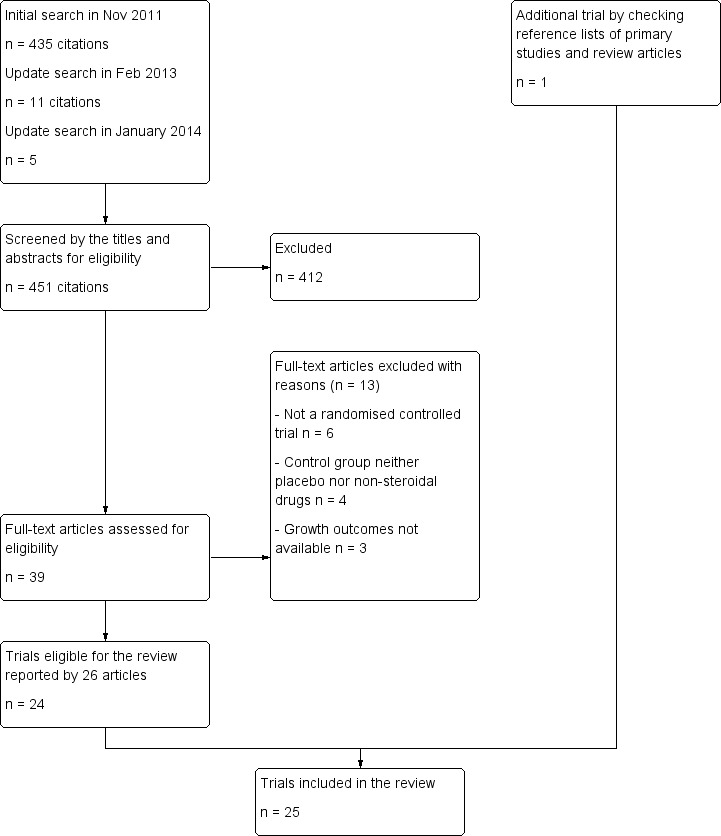
Flow diagram of trial selection.
Included studies
See Characteristics of included studies.
We included 25 trials involving 8471 children with mild to moderate persistent asthma, of whom 5128 were treated with ICS and 3343 with placebo or non‐steroidal drugs.
Design
All 25 studies were randomised, parallel‐group, controlled trials. All but five (Gradman 2010; Jonasson 2000; Kannisto 2000; Storr 1986; Turpeinen 2008) were multi‐centre trials. Five (Becker 2006; Bisgaard 2004; Pauwels 2003; Pedersen 2010; Skoner 2008) were international multi‐centre studies. With the exception of eight studies (CAMP 2000; Gradman 2010; Guilbert 2006; Jonasson 2000; Kannisto 2000; Martinez 2011; Sorkness 2007; Storr 1986), all included trials were sponsored by pharmaceutical companies.
Participants
Four trials (Bisgaard 2004; Guilbert 2006; Storr 1986; Wasserman 2006) involved toddlers and preschoolers one to five years of age, 13 trials (Becker 2006; Bensch 2011; CAMP 2000; Doull 1995; Gillman 2002; Gradman 2010; Pauwels 2003; Pedersen 2010; Price 1997; Skoner 2008; Skoner 2011; Tinkelman 1993; Turpeinen 2008) involved prepubertal children four to 12 years of age and eight trials (Jonasson 2000; Kannisto 2000; Martinez 2011; Roux 2003; Simons 1997; Sorkness 2007; Tinkelman 1993; Verberne 1997) involved prepubertal and pubertal children five to 18 years of age. All trials described gender (male) ratios from 46% to 75%. Diagnosis of asthma was based on American Thoracic Society (ATS), National Heart, Lung and Blood Institute (NHLBI) or GINA criteria (Allen 1998; Becker 2006; Gradman 2010; Jonasson 2000; Martinez 2011; Pedersen 2010; Skoner 2008; Skoner 2011; Turpeinen 2008; Verberne 1997), on both symptoms and spirometry test results (Bensch 2011; CAMP 2000; Gillman 2002; Pauwels 2003; Roux 2003; Simons 1997; Tinkelman 1993) or on symptoms alone (Doull 1995; Price 1997). Three trials (Kannisto 2000; Sorkness 2007; Wasserman 2006) did not report the criteria used for diagnosis of asthma. Another three trials involved toddlers/preschoolers with recurrent wheezing (Bisgaard 2004; Storr 1986) or recurrent wheezing and a positive asthma predictive index (Guilbert 2006). Twenty trials used frequency of asthma symptoms and baseline forced expiratory volume in one second (FEV1) as the criteria for classification of asthma severity. Eleven trials (Becker 2006; Bensch 2011; Gradman 2010; Jonasson 2000; Pauwels 2003; Price 1997; Roux 2003; Simons 1997; Skoner 2008; Skoner 2011; Turpeinen 2008) involved participants with mild persistent asthma, seven (CAMP 2000; Doull 1995; Gillman 2002; Pedersen 2010; Sorkness 2007; Tinkelman 1993; Verberne 1997) involved participants with mild to moderate persistent asthma and five (Bisgaard 2004; Guilbert 2006; Kannisto 2000; Storr 1986; Wasserman 2006) failed to report the severity of asthma or asthma‐like symptoms.
Eight trials (Becker 2006; Doull 1995; Jonasson 2000; Kannisto 2000; Price 1997; Roux 2003; Skoner 2011; Storr 1986) included only steroid‐naïve participants, that is, those with no previous regular use of ICS or duration of use less than two weeks before study entry. Six trials included only participants who had previously used ICS for less than one month (Pauwels 2003; Simons 1997; Tinkelman 1993), two months (Bensch 2011) and four months (Guilbert 2006; Turpeinen 2008). In the remaining trials, no restriction was placed on previous use of ICS, and the prevalence of such use ranged from 15.6% to 88% among included participants.
Interventions
The ICS molecule used was beclomethasone dipropionate (Becker 2006; Doull 1995; Gillman 2002; Martinez 2011; Simons 1997; Storr 1986; Tinkelman 1993; Verberne 1997), budesonide (CAMP 2000; Gradman 2010; Jonasson 2000; Kannisto 2000; Pauwels 2003; Turpeinen 2008), ciclesonide (Pedersen 2010; Skoner 2008), flunisolide (Bensch 2011; Gillman 2002), fluticasone propionate (Allen 1998; Bisgaard 2004; Guilbert 2006; Price 1997; Roux 2003; Sorkness 2007; Wasserman 2006) or mometasone furoate (Skoner 2011). Different inhaler devices used included CFC‐MDI, HFA‐MDI, DPI (Diskhaler or Turbuhaler) and nebuliser (further details are available in Table 2 and under Characteristics of included studies). Durations of intervention included 12 weeks (Pedersen 2010; Wasserman 2006), 26 to 30 weeks (Doull 1995; Storr 1986), 44 to 48 weeks (Martinez 2011; Sorkness 2007), 52 to 56 weeks (Allen 1998; Becker 2006; Bensch 2011; Bisgaard 2004; Gillman 2002; Gradman 2010; Kannisto 2000; Price 1997; Simons 1997; Skoner 2008; Skoner 2011; Tinkelman 1993; Verberne 1997), two years (Guilbert 2006; Jonasson 2000; Roux 2003), three years (Pauwels 2003) and four to six years (CAMP 2000). Treatment compliance was not measured or reported in nine trials (Gillman 2002; Kannisto 2000; Martinez 2011; Pauwels 2003; Pedersen 2010; Price 1997; Roux 2003; Storr 1986; Wasserman 2006). In the remaining 16 trials, treatment compliance was measured by self‐reporting and/or by more objective methods (counting the number of used drug blisters, checking the drug canister weight or using a dose counter). The compliance rate was higher than 75% in all but three trials for which these data were available (CAMP 2000; Guilbert 2006; Jonasson 2000).
1. Description of interventions: molecule, daily dose, inhalation device and treatment duration of ICS.
| Study | Molecule | Daily dose (μg/d) | Inhalation device | Treatment duration |
| Allen 1998 | Fluticasone | 100, twice daily 200, twice daily |
Diskhaler | 52 weeks |
| Becker 2006 | Beclomethasone | 400, twice daily | CFC‐MDI | 56 weeks |
| Bensch 2011 | Flunisolide | 400, twice daily | HFA‐MDI | 52 weeks |
| Bisgaard 2004 | Fluticasone | 200, twice daily | HFA‐MDI | 52 weeks |
| CAMP 2000 | Budesonide | 400, twice daily | Turbuhaler | 4‐6 years |
| Doull 1995 | Beclomethasone | 400, twice daily | Diskhaler | 7 months |
| Gillman 2002 | Flunisolide Beclomethasone |
400, twice daily 400, twice daily |
HFA‐MDI CFC‐MDI |
52 weeks |
| Gradman 2010 | Budesonide | 200, twice daily | DPI | 52 weeks |
| Guilbert 2006 | Fluticasone | 200, twice daily | CFC‐MDI | 2 years |
| Jonasson 2000 | Budesonide | 100, once daily 200, once daily 200, twice daily |
Turbuhaler | 27 months |
| Kannisto 2000 | Fluticasone Budesonide |
500, 2 months; 200, thereafter 800, 2 months; 400, thereafter |
Diskus Turbuhaler |
12 months |
| Martinez 2011 | Beclomethasone | 100, twice dailya | HFA‐DMI | 44 weeks |
| Pauwels 2003 | Budesonide | 200, once daily | Turbuhaler | 3 years |
| Pedersen 2010 | Ciclesonide | 50, once daily 100, once daily 200, once daily |
HFA‐MDI | 12 weeks |
| Price 1997 | Fluticasone | 100, twice daily | Diskhaler | 12 months |
| Roux 2003 | Fluticasone | 200, twice daily | Diskus | 2 years |
| Simons 1997 | Beclomethasone | 400, twice daily | Diskhaler | 12 months |
| Skoner 2008 | Ciclesonide | 50, once daily 200, once daily |
HFA‐MDI | 12 months |
| Skoner 2011 | Mometasone | 100, once daily 200, once daily 200, twice daily |
DPI | 52 weeks |
| Sorkness 2007 | Fluticasone | 200, twice daily | Diskus | 48 weeks |
| Storr 1986 | Beclomethasone | 300, 3 times a day | Jet nebuliser | 6 months |
| Tinkelman 1993 | Beclomethasone | 400, 4 times a day | CFC‐MDI | 52 weeks |
| Turpeinen 2008 | Budesonide | 800, twice daily, 1 month; then 400, twice daily, 5 months; 200, thereafterb |
Turbuhaler | 18 months |
| Verberne 1997 | Beclomethasone | 400, twice daily | Diskhaler | 54 weeks |
| Wasserman 2006 | Fluticasone | 100, twice daily 200, twice daily |
CFC‐MDI | 12 weeks |
Abbreviations:
CFC: chlorofluorocarbon.
DPI: dry powder inhaler.
HFA: hydrofluoroalkane.
ICS: inhaled corticosteroids.
MDI: metered‐dose inhaler.
aOnly‐daily beclomethasone group was used as the intervention group.
bOnly continuous budesonide group was used as the intervention group.
Co‐intervention with additional antiasthmatic drugs such as long‐acting beta2‐agonists, antileukotrienes or theophylline was allowed in seven trials (Allen 1998; Bensch 2011; CAMP 2000; Guilbert 2006; Simons 1997; Skoner 2011; Wasserman 2006).
Outcome measures
Fifteen trials (Allen 1998; Becker 2006; Bensch 2011; Bisgaard 2004; CAMP 2000; Gillman 2002; Guilbert 2006; Jonasson 2000; Pauwels 2003; Pedersen 2010; Price 1997; Roux 2003; Skoner 2008; Skoner 2011; Tinkelman 1993) used linear growth velocity as the outcome measure, alone or in combination with other growth measurements. Seventeen trials used change from baseline in absolute height over time as the outcome measure (Allen 1998; Becker 2006; Bensch 2011; Bisgaard 2004; Doull 1995; Gillman 2002; Gradman 2010; Guilbert 2006; Martinez 2011; Roux 2003; Simons 1997; Skoner 2008; Sorkness 2007; Storr 1986; Tinkelman 1993; Turpeinen 2008; Verberne 1997). Three trials (Kannisto 2000; Price 1997; Verberne 1997) used change in height SDS as the outcome measure.
Excluded studies
We excluded 13 studies from the review. The reasons for exclusion are summarised under Characteristics of excluded studies.
Risk of bias in included studies
Full details of the risk of bias for each trial can be found under Characteristics of included studies. A graphical summary of our 'Risk of bias' judgements can be found in Figure 2 and Figure 3.
2.
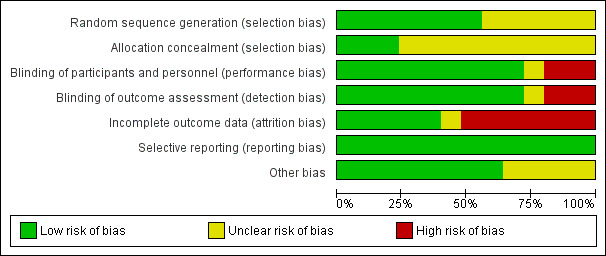
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
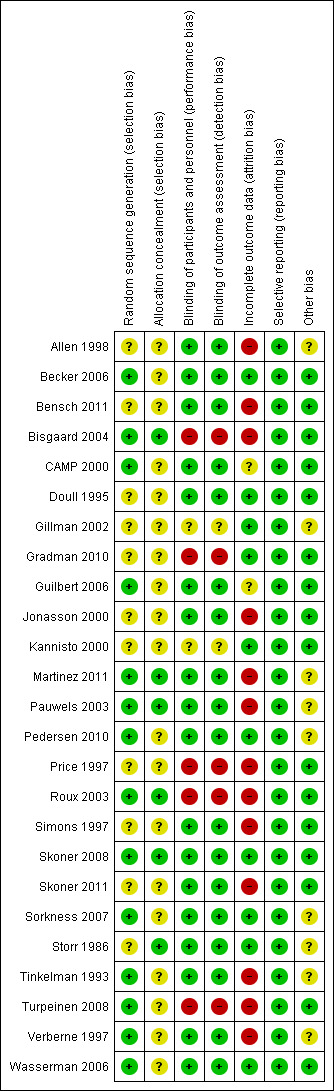
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Fourteen trials used adequate methods of random sequence generation, and 11 trials did not provide details about allocation sequence generation. All but six trials (Bisgaard 2004; Martinez 2011; Pauwels 2003; Roux 2003; Skoner 2008; Storr 1986) failed to report the method of allocation concealment.
Blinding
Eighteen trials (72%) used placebo or non‐steroidal drugs that matched the appearance of ICS for blinding, five trials (Bisgaard 2004; Gradman 2010; Price 1997; Roux 2003; Turpeinen 2008) had an open‐label design and two trials (Gillman 2002; Kannisto 2000) did not report sufficient information to allow ascertainment of blinding.
Incomplete outcome data
All trials reported numbers of and reasons for withdrawals by group. The withdrawal rate was higher than 20% in 14 trials (Allen 1998; Bensch 2011; Bisgaard 2004; Jonasson 2000; Martinez 2011; Pauwels 2003; Pedersen 2010; Price 1997; Roux 2003; Simons 1997; Skoner 2011; Tinkelman 1993; Turpeinen 2008; Verberne 1997). In nine trials, the control group had a higher withdrawal rate than the ICS group, mainly as the result of poor asthma control.
Selective reporting
Twenty‐five trials reported all outcomes mentioned in the methods section with no apparent bias. The funnel plots (Figure 4) showed slight asymmetry on visual inspection, but Egger's test did not show statistically significant small‐study effects, suggesting no publication bias.
4.
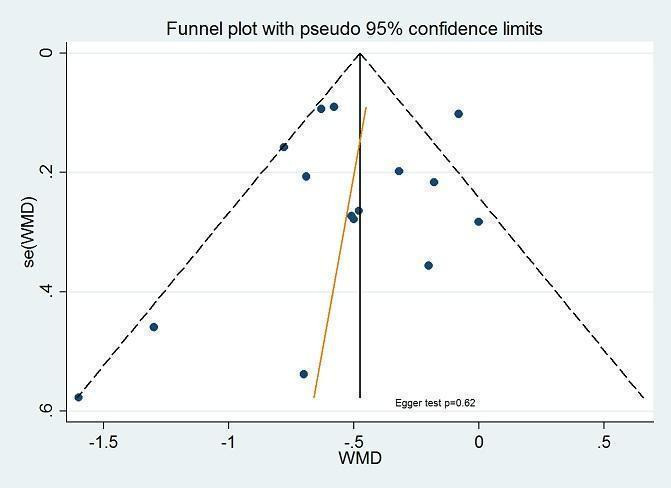
Funnel plot of comparison: inhaled corticosteroids vs placebo or non‐steroidal drugs: 1‐year (or nearly 1‐year) treatment, outcome: linear growth velocity (cm/y). Funnel plot with Egger's test for small‐study effects conducted in Stata.
Other potential sources of bias
Nine trials (Allen 1998; Gillman 2002; Martinez 2011; Pauwels 2003; Pedersen 2010; Sorkness 2007; Storr 1986; Tinkelman 1993; Verberne 1997) did not report the method used for height measurement. No other potential sources of bias were observed in the included trials.
Effects of interventions
See: Table 1
Three‐ to five‐month treatment
Linear growth velocity (mm/wk)
One 12‐week trial including 904 participants (Pedersen 2010) did not show a statistically significant difference in mean linear growth velocity between the ciclesonide 50, 100 and 200 μg/d and placebo groups (mean ± SE, 0.82 ± 0.16, 0.97 ± 0.10, 0.95 ± 0.12 and 0.96 ± 0.18, P value > 0.05; data extracted from the figure).
Change from baseline in height (cm)
One 12‐week trial including 332 participants (Wasserman 2006) showed that mean change from baseline in height (cm) was similar between the CFC‐fluticasone 100 μg/d and placebo groups (MD 0.055 cm, 95% CI, ‐0.275 to 0.386, P value 0.74) and between the CFC‐fluticasone 200 μg/d and placebo groups (MD ‐0.012 cm, 95% CI, ‐0.347 to 0.324, P value 0.95). Eleven trials including 3332 participants with treatment duration longer than or equal to six months reported mean change from baseline in height or mean height in each treatment group at the three‐ to five‐month time point; however, all 11 trials used figures to present the means but not uncertainty of measurement (SD, SE or 95% CI). In five trials (Bensch 2011; Bisgaard 2004; CAMP 2000; Gradman 2010; Skoner 2008), the authors explicitly stated that no statistically significant difference in mean increase in height was observed during the first three months of treatment between ICS and control groups. In another five trials (Allen 1998; Becker 2006; Doull 1995; Martinez 2011; Skoner 2011), the MD between ICS and control groups in change from baseline in height (data extracted from the figures) during the first three months of treatment was 0 cm, ‐0.1 cm, ‐0.4 cm, ‐0.18 cm and ‐0.04 cm, respectively. Only one trial (Simons 1997) reported that effect of beclomethasone 400 μg/d on height appeared to be greatest during months one through three, with an MD of 1.3 cm in height increase between beclomethasone and placebo groups.
Change in height SDS
None of the trials reported this outcome.
Change in height z‐score
None of the trials reported this outcome.
Six‐ to eight‐month treatment
Linear growth velocity (cm/y)
Two trials including 369 participants (Doull 1995; Guilbert 2006) showed that seven‐ and eight‐month treatment with ICS was associated with decreased linear growth velocity compared with placebo, with a pooled MD of ‐1.23 cm/y (95% CI ‐2.32 to ‐0.13, P value 0.03, I2 = 92%) (Analysis 1.1).
1.1. Analysis.
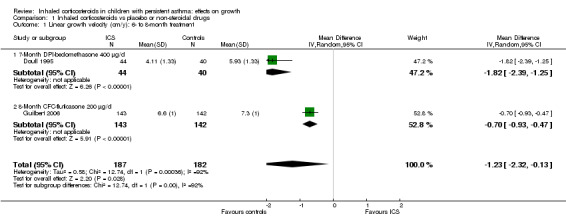
Comparison 1 Inhaled corticosteroids vs placebo or non‐steroidal drugs, Outcome 1 Linear growth velocity (cm/y): 6‐ to 8‐month treatment.
Change from baseline in height (cm)
Three trials including 167 participants (Doull 1995; Gradman 2010; Storr 1986) provided data on the increase in height at the six‐ to eight‐month time point. Pooled results of three trials showed a statistically significant difference in mean change from baseline in height between ICS and control groups (MD ‐0.77 cm, 95% CI ‐1.10 to ‐0.43, P value < 0.00001, I2 = 24%) (Analysis 1.2). Nine trials with treatment duration longer than eight months presented partial data on the increase in height at the six‐ to eight‐month time point as the figures could not be pooled because of lack of SD. In two trials (Guilbert 2006; Simons 1997), the authors explicitly stated that a statistically significant suppressive effect of ICS on height increase was observed. In the remaining seven trials (Allen 1998; Becker 2006; Bensch 2011; Bisgaard 2004; Martinez 2011; Skoner 2008; Skoner 2011) in which growth data were extracted from the figures, the MD between ICS and control groups in change from baseline in height was ‐0.11 cm, ‐0.52 cm, ‐0.20 cm, ‐0.33 cm, ‐0.51 cm, ‐0.22 cm and ‐0.54 cm, respectively.
1.2. Analysis.
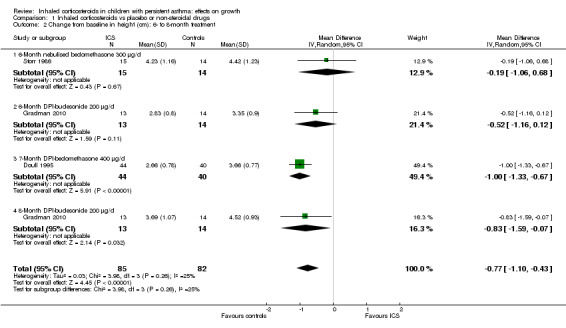
Comparison 1 Inhaled corticosteroids vs placebo or non‐steroidal drugs, Outcome 2 Change from baseline in height (cm): 6‐ to 8‐month treatment.
Change in height SDS
None of the trials reported this outcome.
Change in height z‐score
None of the trials reported this outcome.
One‐year (or nearly one‐year) treatment
Linear growth velocity (cm/y)
Thirteen trials with 14 comparisons (Allen 1998; Becker 2006; Bensch 2011; Bisgaard 2004; CAMP 2000; Gillman 2002; Guilbert 2006; Jonasson 2000; Price 1997; Roux 2003; Skoner 2008; Skoner 2011; Tinkelman 1993) among 3743 participants provided data on mean linear growth velocity in each treatment group. Meta‐analysis of these 13 trials showed that participants treated with ICS had a statistically significant reduction in linear growth velocity compared with the control group, with an MD of ‐0.47 cm/y (95% CI ‐0.66 to ‐0.27, P value < 0.00001) (Analysis 1.3). Significant heterogeneity in results was noted between studies (I2 = 60%). One trial (Pauwels 2003) provided MD and 95% CI of linear growth velocity between corticosteroid and placebo groups among 1974 prepubertal children. We included this trial in the meta‐analysis using MD and SE of linear growth velocity, and the results remained almost unchanged (14 trials with 15 comparisons among 5717 participants; MD ‐0.48 cm/y, 95% CI ‐0.65 to ‐0.30, P value < 0.0001) (Figure 5).
1.3. Analysis.
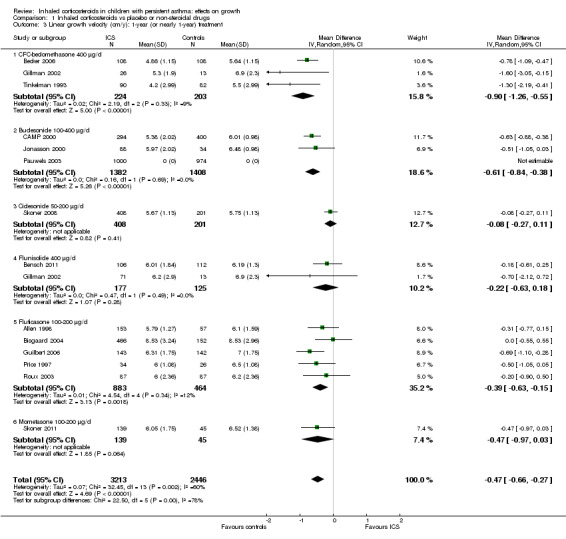
Comparison 1 Inhaled corticosteroids vs placebo or non‐steroidal drugs, Outcome 3 Linear growth velocity (cm/y): 1‐year (or nearly 1‐year) treatment.
5.
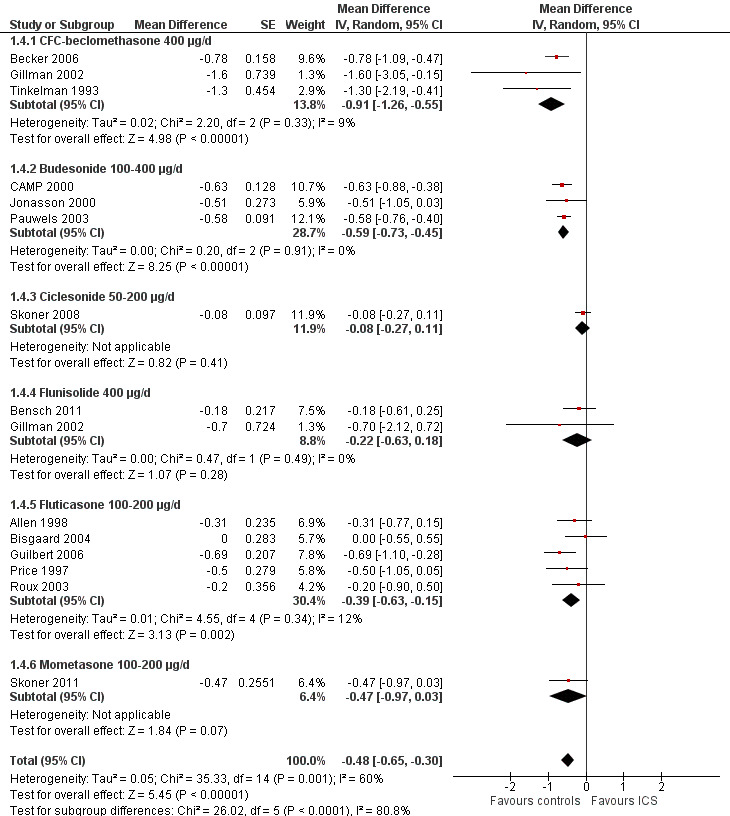
Forest plot of comparison: 1: inhaled corticosteroids versus placebo or non‐steroidal drugs, outcome: 1.4: linear growth velocity (cm/y): 1‐year (or nearly 1‐year) treatment—use of MD and SE for meta‐analysis.
The subgroup analysis on molecules showed a statistically significant difference between six ICS regarding effects on linear growth velocity during a one‐year treatment period (Chi² = 26.1, df = 5, P value < 0.0001) (Figure 5). In a post hoc analysis in which only trials using doses equivalent to 200 μg/d HFA‐beclomethasone were selected, the group difference in suppressive effect on linear growth velocity was also statistically significant (Chi² = 15.3, df = 4, P value 0.004) (Figure 6).
6.
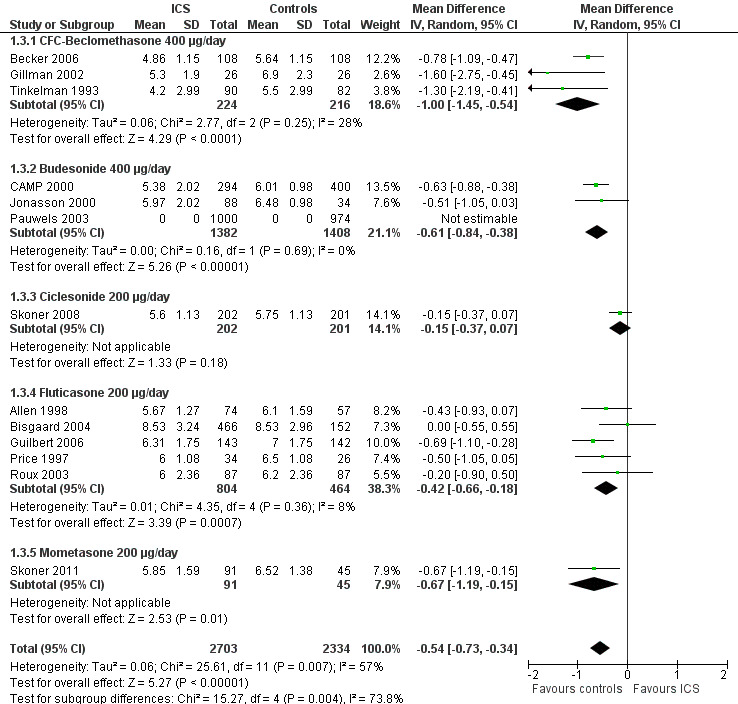
Post hoc subgroup analysis on molecule selecting trials using similar dose equivalence of 200 μg/d HFA‐beclomethasone: linear growth velocity (cm/y) during 1‐year treatment.
A post hoc subgroup analysis for inhalation devices within the molecule fluticasone propionate 200 μg/d did not show a statistically significant difference between CFC‐MDI, DPI and HFA‐MDI (Chi² = 4.31, df = 2, P value 0.12) regarding the effects of ICS on linear growth velocity during a one‐year treatment period (Figure 7).
7.
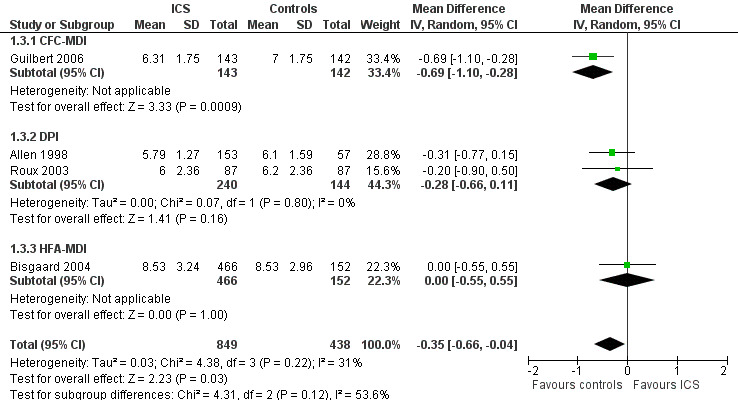
Post hoc subgroup analysis for inhalation device within the molecule fluticasone propionate 200 μg/d: linear growth velocity (cm/y) during 1‐year treatment.
The subgroup analysis on daily ICS dose did not show a statistically significant difference in mean reduction of linear growth velocity during one‐year treatment between low and medium doses (Chi² = 2.59, df = 1, P value 0.11) (Figure 8). The post hoc subgroup analysis within the molecule budesonide also did not show a statistically significant difference between 100 to 200 μg/d and 400 μg/d in terms of suppressive effects on linear growth velocity (Figure 9).
8.
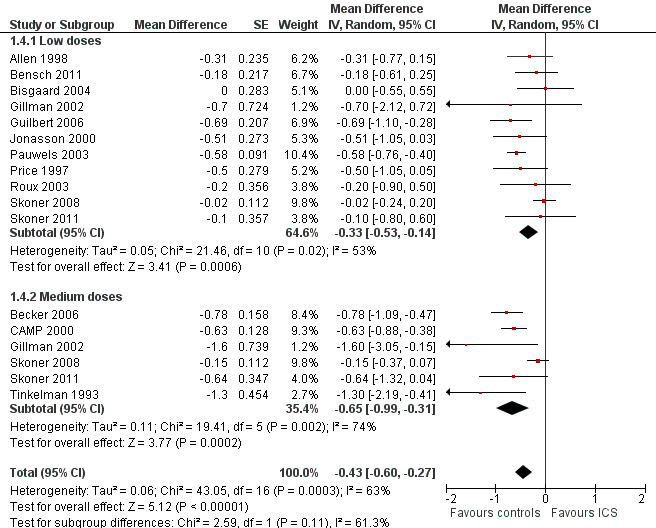
Post hoc subgroup analysis on the ICS dose: linear growth velocity (cm/y) during 1‐year treatment.
9.
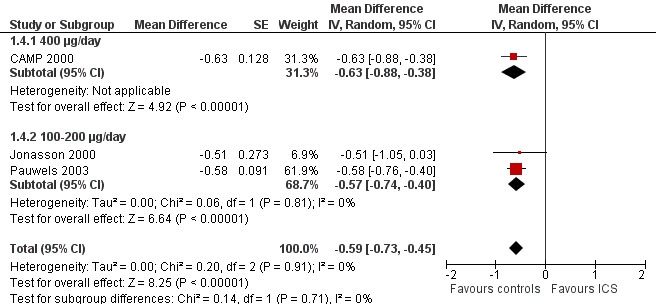
Post hoc subgroup analysis on ICS doses within the molecule budesonide: linear growth velocity (cm/y) during 1‐year treatment.
No impact of age group on magnitude of effect was apparent (Chi² = 0.05, df = 1, P value 0.82), that is, between toddlers and preschoolers and prepubertal children (Figure 10). The post hoc group analysis within the molecule fluticasone propionate 200 μg/d yielded similar results (toddlers and preschoolers: Bisgaard 2004; Guilbert 2006, MD ‐0.37 cm/y, 95% CI ‐1.05 to 0.30; prepubertal children: Allen 1998, MD ‐0.31 cm/y, 95% CI ‐0.77 to 0.15) (Chi² = 0.02, df = 1, P value 0.88).
10.
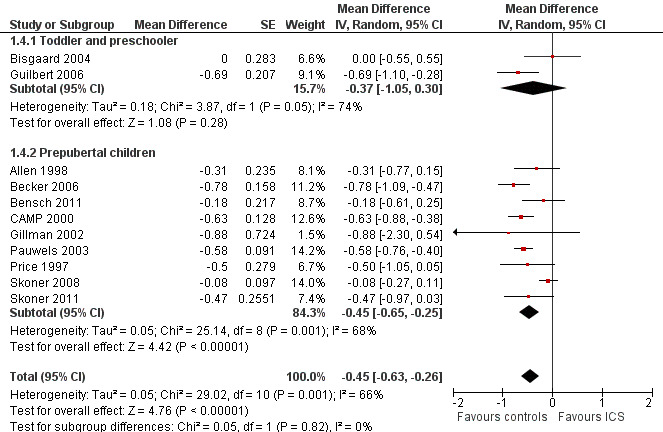
Post hoc subgroup analysis on participant age: linear growth velocity (cm/y) during 1‐year treatment.
Ten sensitivity analyses were conducted to explore potential effect modifiers; these analyses did not substantially change the results (Table 3).
2. Results of sensitivity analyses: effect of inhaled corticosteroids (ICS) on linear growth velocity in the first year of treatment.
| Analyses (no. of trials) | Exclusion of trials (reasons) | Exclusion of trials (studies) | Effect size (MD, 95% confidence interval (CI)) |
| Overall analysis (n = 14) |
‐0.48 cm/y (‐0.65 to ‐0.30) | ||
| Sensitivity analysis 1 | Open‐label trials | Bisgaard 2004; Price 1997; Roux 2003 | ‐0.52 cm/y (‐0.71 to ‐0.33) |
| Sensitivity analysis 2 | Random sequence generation not reported |
Allen 1998; Bensch 2011; Gillman 2002; Jonasson 2000; Skoner 2011 |
‐0.48 cm/y (‐0.74 to ‐0.22) |
| Sensitivity analysis 3 | Imputation of missing standard deviation (SD) | CAMP 2000; Gillman 2002 | ‐0.44 cm/y (‐0.62 to ‐0.25) |
| Sensitivity analysis 4 | Treatment compliance rate < 75% or no data available |
CAMP 2000; Gillman 2002; Guilbert 2006; Jonasson 2000; Pauwels 2003; Roux 2003 |
‐0.39 cm/y (‐0.65 to ‐0.13) |
| Sensitivity analysis 5 | Withdrawal rate > 20% |
Allen 1998; Bensch 2011; Bisgaard 2004; Pauwels 2003; Roux 2003; Skoner 2011; Tinkelman 1993 |
‐0.48 cm/y (‐0.71 to ‐0.25) |
| Sensitivity analysis 6 | Non‐steroidal drugs rather than placebo used as controls |
Bisgaard 2004; Gillman 2002; Roux 2003; Tinkelman 1993 |
‐0.47 cm/y (‐0.65 to ‐0.29) |
| Sensitivity analysis 7 | Trials included participants receiving previous ICS for longer than 1 month |
Allen 1998; Bensch 2011; Bisgaard 2004; CAMP 2000; Gillman 2002; Guilbert 2006; Skoner 2008 |
‐0.61 cm/y (‐0.74 to ‐0.48) |
| Sensitivity analysis 8 | Trial in which only a subset of data was analysed |
Allen 1998 | ‐0.49 cm/y (‐0.67 to ‐0.31) |
| Sensitivity analysis 9 | Growth data extracted from the figures |
CAMP 2000; Guilbert 2006; Skoner 2011 | ‐0.44 cm/y (‐0.65 to ‐0.23) |
| Sensitivity analysis 10 | Trials sponsored by pharmaceutical industry |
Allen 1998; Becker 2006; Bensch 2011; Bisgaard 2004; Gillman 2002; Pauwels 2003; Price 1997; Roux 2003; Skoner 2008; Skoner 2011; Tinkelman 1993 |
‐0.63 cm/y (‐0.83 to ‐0.43) |
Change from baseline in height (cm)
Fifteen trials including 16 comparisons among 3314 participants (Allen 1998; Becker 2006; Bensch 2011; Bisgaard 2004; Gillman 2002; Gradman 2010; Guilbert 2006; Martinez 2011; Roux 2003; Simons 1997; Skoner 2008; Sorkness 2007; Tinkelman 1993; Turpeinen 2008; Verberne 1997) provided data on mean change from baseline in height over a one‐year (nearly one‐year) treatment period in each treatment group. Meta‐analysis of 15 trials showed that participants treated with ICS had a statistically significantly lower mean increase in height compared with the control group, with an MD of ‐0.61 cm (95% CI ‐0.83 to ‐0.38, P value < 0.00001) (Analysis 1.5). Significant heterogeneity in results was noted between studies (I2 = 63%).
1.5. Analysis.
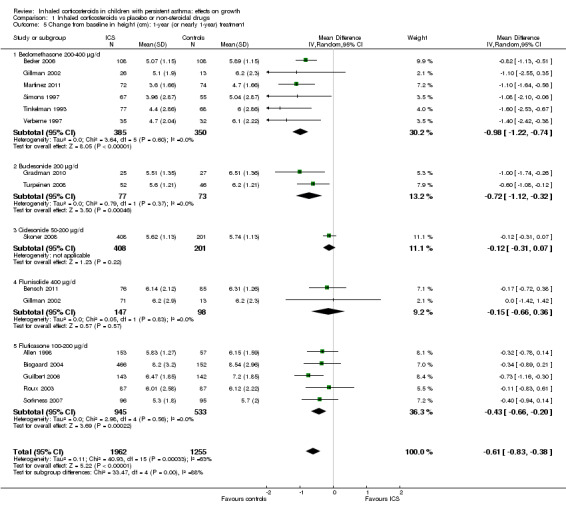
Comparison 1 Inhaled corticosteroids vs placebo or non‐steroidal drugs, Outcome 5 Change from baseline in height (cm): 1‐year (or nearly 1‐year) treatment.
The subgroup analysis on molecules yielded results similar to those obtained for linear growth velocity (Analysis 1.5). In a post hoc subgroup analysis of data from trials using doses equivalent to 200 μg/d of HFA‐beclomethasone, the difference in suppressive effect on the increase in height during one‐year treatment was also statistically significant between beclomethasone, ciclesonide and fluticasone propionate (Chi² = 20.5, df = 2, P value < 0.0001) (Figure 11).
11.
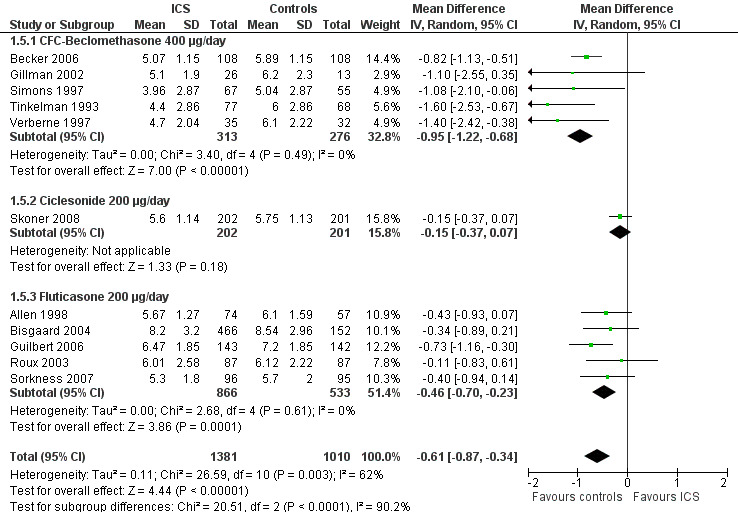
Post hoc subgroup analysis on molecule selecting trials using doses equivalent to 200 μg/d HFA‐beclomethasone: change from baseline in height (cm) during 1‐year treatment.
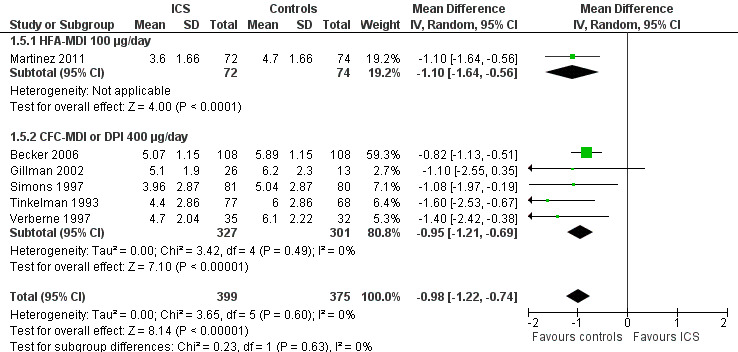
The post hoc subgroup analysis on devices within the molecule beclomethasone (dose equivalence of CFC formulation 400 μg/d) did not find a statistically significant difference between CFC‐MDI and DPI regarding the magnitude of effect of ICS on increase in height (Chi² = 0.34, df = 2, P value 0.56) (Figure 12). Another post hoc subgroup analysis within the molecule fluticasone propionate 200 μg/d also did not find a statistically significant impact of device (CFC‐MDI, DPI or HFA‐MDI) on growth‐suppressive effect of ICS (Chi² = 2.57, df = 2, P value 0.28) (Figure 13).
12.
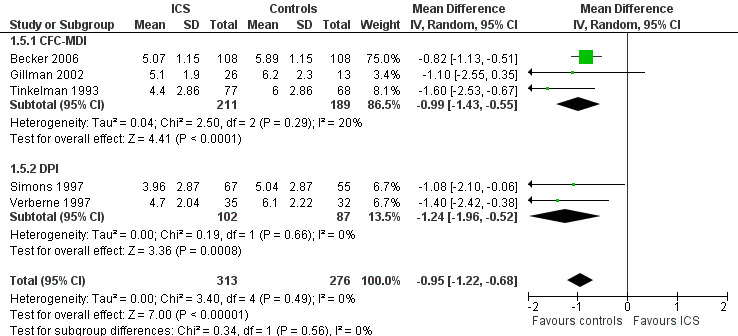
Post hoc subgroup analysis on device within the molecule beclomethasone (dose equivalence of CFC‐formulation 400 μg/d): change from baseline in height (cm) during 1‐year treatment.
13.
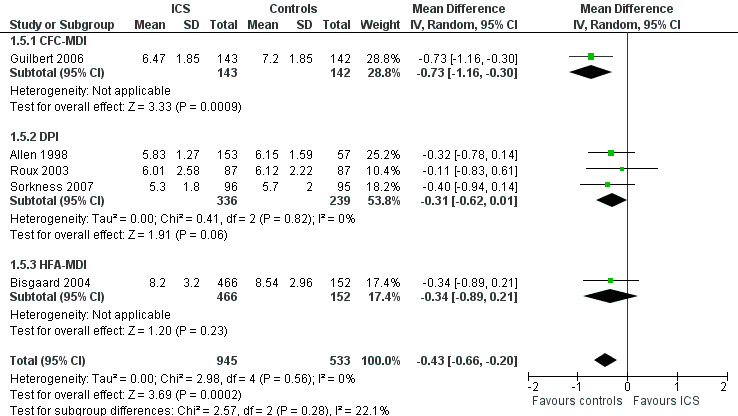
Post hoc subgroup analysis on device within the molecule fluticasone propionate 200 μg/d: change from baseline in height (cm) during 1‐year treatment.
The subgroup analysis on daily ICS dose showed that medium doses produced a statistically significantly greater reduction in mean change from baseline in height compared with low doses (Chi² = 3.94, df = 1, P value 0.05) (Figure 14). However, a post hoc subgroup analysis within the molecule beclomethasone did not show a statistically significant difference between low and medium doses in terms of growth‐suppressive effect (Chi² = 0.23, df = 1, P value 0.63) (Figure 15).
14.
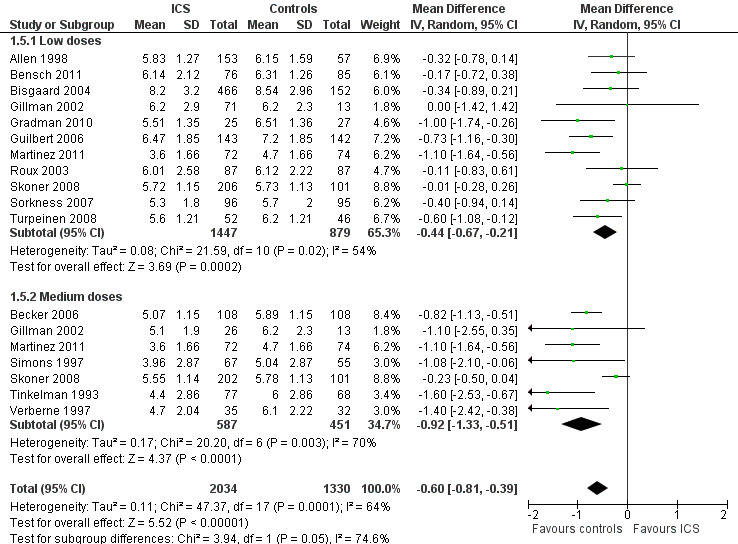
Post hoc subgroup analysis on ICS dose: change from baseline in height (cm) during 1‐year treatment.
15.
Post hoc subgroup analysis on ICS dose within the molecule beclomethasone: change from baseline in height (cm) during 1‐year treatment.
Finally, no statistically significant impact of age group on the effect of ICS was noted, that is, between toddlers and preschoolers and prepubertal children (Chi² = 0.24, df = 2, P value 0.63) (Figure 16).
16.
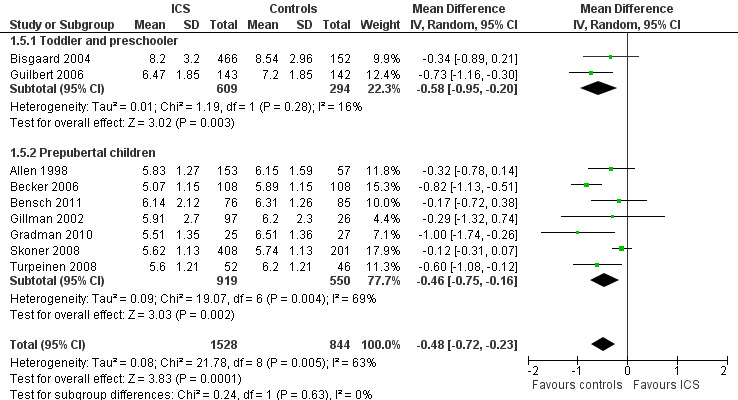
Post hoc subgroup analysis on participant age: change from baseline in height (cm) during 1‐year treatment.
Sensitivity analyses yielded similar results to those obtained for linear growth velocity (Table 4).
3. Results of sensitivity analyses: effect of inhaled corticosteroids (ICS) on change from baseline in height in first year of treatment.
| Analyses (no. of trials) | Exclusion of trials (reasons) | Exclusion of trials (studies) | Effect size (mean difference (MD), 95% confidence interval (CI)) |
| Overall analysis (n = 15) | ‐0.61 cm/y (‐0.83 to ‐0.38) | ||
| Sensitivity analysis 1 | Open‐label trials |
Bisgaard 2004; Gradman 2010; Roux 2003; Turpeinen 2008 |
‐0.65 cm/y (‐0.94 to ‐0.37) |
| Sensitivity analysis 2 | Random sequence generation not reported |
Allen 1998; Bensch 2011; Gillman 2002; Gradman 2010; Simons 1997 |
‐0.64 cm/y (‐0.94 to ‐0.35) |
| Sensitivity analysis 3 | Imputation of missing standard deviation (SD) | Becker 2006; Gillman 2002 | ‐0.59 cm/y (‐0.83 to ‐0.34) |
| Sensitivity analysis 4 | Treatment compliance rate < 75% or no data available |
Gillman 2002; Guilbert 2006; Roux 2003 | ‐0.64 cm/y (‐0.90 to ‐0.38) |
| Sensitivity analysis 5 | Withdrawal rate > 20% |
Allen 1998; Bensch 2011; Bisgaard 2004; Martinez 2011; Roux 2003; Simons 1997 |
‐0.70 cm/y (‐1.02 to ‐0.38) |
| Sensitivity analysis 6 | Non‐steroidal drugs rather than placebo used as controls |
Bisgaard 2004; Gillman 2002; Gradman 2010; Roux 2003; Tinkelman 1993; Turpeinen 2008; Verberne 1997 |
‐0.55 cm/y (‐0.84 to ‐0.26) |
| Sensitivity analysis 7 | Trials that included participants receiving previous regular use of ICS for longer than 1 month before study entry |
Allen 1998; Bensch 2011; Bisgaard 2004; Gillman 2002; Gradman 2010; Guilbert 2006; Martinez 2011; Skoner 2008; Sorkness 2007; Turpeinen 2008; Verberne 1997 |
‐0.83 cm/y (‐1.35 to ‐0.32) |
| Sensitivity analysis 8 | Growth data extracted from the figures |
Becker 2006; Bisgaard 2004; Guilbert 2006; Martinez 2011; Skoner 2008 |
‐0.60 cm/y (‐0.87 to ‐0.33) |
| Sensitivity analysis 9 | 18‐Month trial in which the growth data used for the analysis were obtained between 7 and 18 months when ICS was given at a constant dose |
Turpeinen 2008 | ‐0.61 cm/y (‐0.85 to ‐0.37) |
| Sensitivity analysis 10 | Trial in which only a subset of data was analysed |
Allen 1998 | ‐0.64 cm/y (‐0.88 to ‐0.39) |
| Sensitivity analysis 11 | Trials sponsored by pharmaceutical industry |
Allen 1998; Becker 2006; Bensch 2011; Bisgaard 2004; Gillman 2002; Roux 2003; Simons 1997; Skoner 2008; Tinkelman 1993; Turpeinen 2008; Verberne 1997 |
‐0.78 cm/y (‐1.08 to ‐0.48) |
Change in height SDS
Four trials including 258 participants (Kannisto 2000; Price 1997; Turpeinen 2008; Verberne 1997) provided mean change in height SDS and uncertainty of measurement in each treatment group. Meta‐analysis of four trials showed that participants treated with ICS had a statistically significantly lower mean change in height SDS compared with those treated with placebo, with an MD of ‐0.13 (95% CI ‐0.24 to ‐0.01, P value 0.03) (Analysis 1.6). Significant heterogeneity in results was noted between studies (I2 = 68%).
1.6. Analysis.
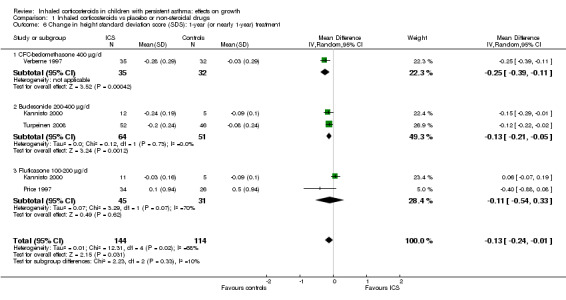
Comparison 1 Inhaled corticosteroids vs placebo or non‐steroidal drugs, Outcome 6 Change in height standard deviation score (SDS): 1‐year (or nearly 1‐year) treatment.
Change in height z‐score
None of the trials reported this outcome.
Two‐year treatment
Linear growth velocity (cm/y)
Five trials including 3174 participants (CAMP 2000; Guilbert 2006; Jonasson 2000; Pauwels 2003; Roux 2003) provided data on linear growth velocity in the second year of treatment. Meta‐analysis of five trials showed no statistically significant differences in linear growth velocity between ICS and control groups (MD ‐0.19 cm/y, 95% CI ‐0.48 to 0.11, P value 0.22, I2 = 75%) (Analysis 1.7; Analysis 1.8). In contrast, meta‐analysis of these five trials showed that participants treated with ICS had a statistically significant reduction in linear growth velocity in the first year of treatment compared with the control group, with an MD of ‐0.58 cm/y (95% CI ‐0.71 to ‐0.44, P value < 0.00001). Meta‐analysis of three trials (Jonasson 2000; Pauwels 2003; Roux 2003) consisting of ICS‐naïve participants yielded similar results regarding effects of ICS‐induced suppression on linear growth velocity in the first year of treatment (MD ‐0.55 cm/y, 95% CI ‐0.72 to ‐0.39, P value < 0.00001).
1.7. Analysis.
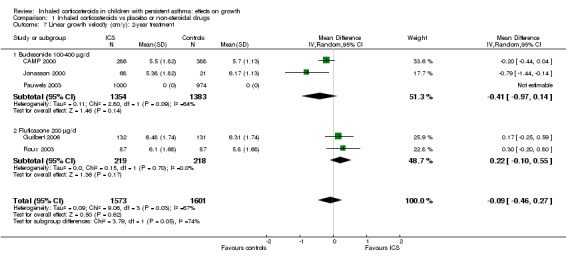
Comparison 1 Inhaled corticosteroids vs placebo or non‐steroidal drugs, Outcome 7 Linear growth velocity (cm/y): 2‐year treatment.
1.8. Analysis.
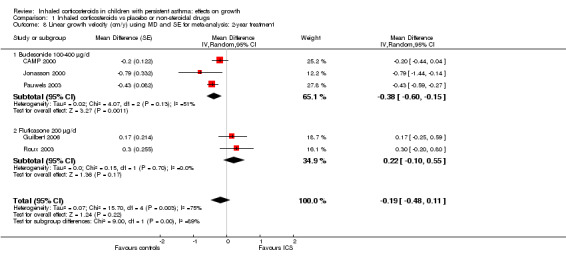
Comparison 1 Inhaled corticosteroids vs placebo or non‐steroidal drugs, Outcome 8 Linear growth velocity (cm/y) using MD and SE for meta‐analysis: 2‐year treatment.
Change from baseline in height (cm)
Two trials including 437 participants (Guilbert 2006; Roux 2003) provided data on mean change from baseline in height over two years of treatment. Meta‐analysis of two trials showed no statistically significant differences in the mean increase in height between ICS and control groups (MD ‐0.30 cm, 95% CI ‐2.09 to 1.49, P value 0.74) (Analysis 1.9).
1.9. Analysis.
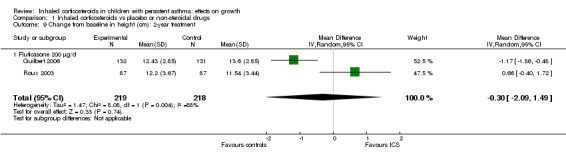
Comparison 1 Inhaled corticosteroids vs placebo or non‐steroidal drugs, Outcome 9 Change from baseline in height (cm): 2‐year treatment.
Change in height SDS
None of the trials reported this outcome.
Change in height z‐score
None of the trials reported this outcome.
Three‐year treatment
Linear growth velocity (cm/y)
Two trials (CAMP 2000; Pauwels 2003) presented the results of linear growth velocity in the third year of treatment, but the data were not suitable for meta‐analysis. In the trial of CAMP 2000, mean linear growth velocity was 5.34 cm/y (data extracted from the figure) in both budesonide (n = 288) and placebo groups (n = 379). The trial of Pauwels 2003 including 1974 prepubertal children reported lower linear growth velocity in the budesonide group during the third year of treatment compared with the placebo group (MD ‐0.33 cm/y, 95% CI ‐0.52 to ‐0.14, P value 0.0005), but the difference was less than that seen in the first year of treatment (MD ‐0.58 cm/y, 95% CI ‐0.76 to ‐0.40, P value < 0.0001).
Change from baseline in height (cm)
The trial of CAMP 2000 reported that, at the end of treatment with a mean duration of 4,3 years, the mean increase in height in the budesonide group was 1.1 cm less than the mean increase in the placebo group (22.7 vs 23.8 cm, P value 0.005).
Change in height SDS
None of the trials reported this outcome.
Change in height z‐score
None of the trials reported this outcome.
Off‐treatment follow‐up (two‐ to four‐month)
Linear growth velocity (cm/y)
Two trials (Skoner 2008; Skoner 2011) reported the results of linear growth velocity during two‐ and three‐month off‐treatment follow‐up periods, but the data were not suitable for meta‐analysis. The trial of Skoner 2008, which included 566 participants, showed similar linear growth velocity during two‐month follow‐up between ciclesonide 40 μg/d and 160 μg/d and placebo, with mean values (SE) of 6.06 cm/y (0.3), 5.64 cm/y (0.24) and 5.75 cm/y (0.23), respectively. We combined the data from two ciclesonide groups and found no statistically significant difference in linear growth velocity between the ciclesonide and control groups (MD 0.10 cm/y, 95% CI ‐0.49 to 0.69, P value 0.74) (Analysis 1.10). The trial of Skoner 2011 showed lower linear growth velocity during three‐month follow‐up in the mometasone 200 μg once daily group (n = 34) compared with the placebo group (n = 30) (MD ‐2.42 cm/y, SE 1.18, P value 0.05), but no statistically significant difference in linear growth velocity was found between groups given mometasone 100 μg/d once daily (n = 40), 100 μg/d twice daily (n = 36) and placebo (n = 30).
1.10. Analysis.
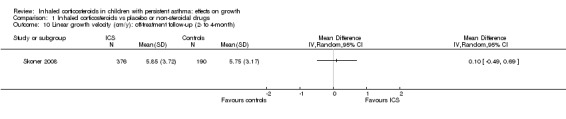
Comparison 1 Inhaled corticosteroids vs placebo or non‐steroidal drugs, Outcome 10 Linear growth velocity (cm/y): off‐treatment follow‐up (2‐ to 4‐month).
Increase in height (cm)
One trial (Doull 1995) including 104 participants found no statistically significant difference between beclomethasone and placebo groups in the mean increase in height during a four‐month off‐treatment follow‐up period (MD 0.11 cm/y, 95% CI ‐0.12 to 0.34, P value 0.36) (Analysis 1.11)
1.11. Analysis.
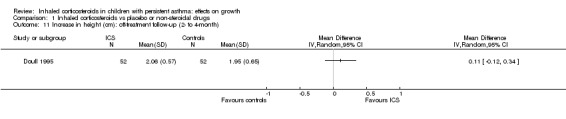
Comparison 1 Inhaled corticosteroids vs placebo or non‐steroidal drugs, Outcome 11 Increase in height (cm): off‐treatment follow‐up (2‐ to 4‐month).
Change in height SDS
None of the trials reported this outcome.
Change in height z‐score
None of the trials reported this outcome.
Off‐treatment follow‐up (12‐month)
Linear growth velocity (cm/y)
One trial (Guilbert 2006) including 285 participants showed greater linear growth velocity in the fluticasone propionate group compared with the placebo group during a 12‐month off‐treatment follow‐up period (MD 0.60 cm/y, 95% CI 0.40 to 0.80, P value < 0.00001) (Analysis 1.12).
1.12. Analysis.
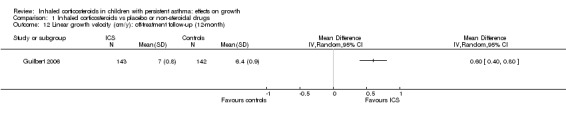
Comparison 1 Inhaled corticosteroids vs placebo or non‐steroidal drugs, Outcome 12 Linear growth velocity (cm/y): off‐treatment follow‐up (12‐month).
Increase in height (cm)
None of the trials reported this outcome.
Change in height SDS
None of the trials reported this outcome.
Change in height z‐score
None of the trials reported this outcome.
Off‐treatment follow‐up (adulthood)
Adult height (cm)
Kelly 2012 reported the results of long‐term follow‐up of 658 participants in the CAMP 2000 trial, showing that participants treated with budesonide 400 μg/d for a mean duration of 4.3 years at a prepubertal age had a mean reduction of 1.20 cm (95% CI ‐1.90 to ‐0.50, P value 0.001) in adult height compared with those treated with placebo (Analysis 1.13).
1.13. Analysis.
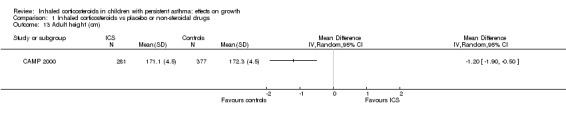
Comparison 1 Inhaled corticosteroids vs placebo or non‐steroidal drugs, Outcome 13 Adult height (cm).
Discussion
This systematic review showed that regular use of ICS at low or medium daily doses was associated with statistically significant growth suppression measured by linear growth velocity, change from baseline in height and change in height SDS during a one‐year treatment period in children with mild to moderate persistent asthma. The subgroup analysis indicated that the effect size of ICS on linear growth velocity appeared to be associated more strongly with the ICS molecule than with the device or dose. ICS‐induced growth suppression seemed to be maximal during the first year of therapy and less pronounced during subsequent years of treatment.
In contrast to the effect on lower leg growth velocity measured by knemometry, which was observed within a few weeks after treatment (Wolthers 1993; Wolthers 1997), a detectable suppressive effect of ICS on the patient's statural height may occur months later. This review did not find an overall effect of ICS during the first three months of treatment. A statistically significant suppressive effect of ICS on both linear growth velocity and change from baseline in height was observed during a six‐ to eight‐month treatment period. Although lower leg length measured by knemometry is more sensitive in detecting ICS‐induced suppressive effects on growth, this measurement correlates poorly with statural height and tends to overestimate potential effects of ICS on growth (Allen 1999; Efthimiou 1998).
Most growth trials were of one‐year duration, and 15 trials showed a consistent overall suppressive effect of about 0.5 cm. The meaningful effect was unaffected by 11 sensitivity analyses underlying the robustness of findings. However, extrapolation of findings of one‐year growth studies to subsequent years has been questioned because growth‐suppressive effects of ICS appear to be time dependent (CAMP 2000; Guilbert 2006; Karlberg 1993; Pauwels 2003; Pedersen 2001). Five trials (CAMP 2000; Guilbert 2006; Jonasson 2000; Pauwels 2003; Roux 2003) included treatment periods longer than one year. In data from these five trials, we explored the growth‐suppressive effects of ICS after the first year of treatment; no statistically significant difference or a smaller difference in linear growth velocity than that observed during the first year of treatment was found between participants given ICS and controls during the second and third years of treatment. It remains unclear why ICS‐induced growth suppression in asthmatic children is less pronounced during subsequent years of treatment than during the first year of treatment.
This review included four trials that provided data on linear growth after treatment cessation for periods ranging from two to 12 months. Three trials did not find statistically significant catch‐up growth two to four months after treatment with ICS (beclomethasone, ciclesonide or mometasone) was stopped. One trial showed accelerated linear growth velocity in the fluticasone group compared with the placebo group 12 months after treatment cessation, but there remained a statistically significant difference of 0.7 cm in height between the fluticasone and placebo groups at the end of the three‐year trial. The relationship between prepubescent growth suppression as estimated by one‐year trials and final adult height also remains to be better defined. Long‐term follow‐up of participants in the CAMP 2000 trials showed that those treated with budesonide 400 μg/d for a mean duration of 4.3 years during prepubertal age had a mean reduction of 1.20 cm (95% CI ‐1.90 to ‐0.50) in adult height compared with those treated with placebo. This is the largest randomised prospective study conducted so far to investigate the potential impact of ICS‐induced growth suppression on adult height in prepubertal children with asthma. In contrast, another long‐term prospective follow‐up study of participants in a randomised trial showed that children with asthma who had received long‐term treatment with budesonide attained normal adult height (Agertoft 2000). However, caution should be taken in interpreting the findings of this study because only 47% (142/300) of participants in the budesonide group and 56% (18/32) of those in the control group contributed data for the analysis.
Available ICS vary in their therapeutic index (risk‐benefit ratio) based on relative receptor affinity, pulmonary bioavailability and oral bioavailability (Colice 2000; Hogger 2003). The subgroup analysis of this review showed a statistically significant group difference between six molecules in mean reduction of linear growth velocity during a one‐year treatment period. The group difference persisted even when the analysis was restricted to trials using similar ICS doses. However, the clinical relevance of this statistically significant difference between ICS molecules in terms of growth‐suppressive effect remains to be defined. Delivery characteristics of inhalers may affect therapeutic index and risk of clinically important systemic adverse effects of ICS. ICS delivered via a clinically very effective inhaler with high intrapulmonary drug deposition would be expected to have a greater systemic effect than those delivered through a less effective inhaler because drug absorption from the lung is greater in the former (Pedersen 2001). However, subgroup analysis of trials using the same molecule given at equivalent doses did not show a statistically significant impact of the inhalation device on the magnitude of ICS‐induced growth suppression.
Subgroup analyses on the daily ICS dose showed that medium doses produced a statistically significantly greater reduction in mean change from baseline in height but not in linear growth velocity during a one‐year treatment period compared with low doses. Moreover, the difference between low and medium doses in terms of reduction in the mean change from baseline in height did not persist when the analysis was restricted to trials using the molecule beclomethasone, albeit with lower power. We acknowledge that head‐to‐head comparisons are better suited than subgroup analyses for use in identifying determinants of response. Hence, another review (Pruteanu 2012) of a series of three Cochrane reviews exploring the safety profile of ICS in children with persistent asthma, which compared ICS doses in head‐to‐head comparisons, demonstrated a small but statistically significant group difference (0.20 cm/y) in growth velocity between low and low to medium doses in favour of low‐dose ICS, confirming a dose‐response growth suppression.
Given the complex interaction between ICS molecule, inhalation device and dose, any definitive conclusions regarding the influence of each factor on the magnitude of growth‐suppressive effects of ICS should be derived from head‐to‐head trials comparing the same molecule at the same dose delivered by different inhalers, or comparing different molecules at equivalent doses delivered by the same inhaler. A limited number of such trials have showed that fluticasone propionate has a more favourable therapeutic index compared with beclomethasone or budesonide (Ferguson 1999; Hoekx 1996; Yiallouros 1997). The effects of different drugs and delivery devices will be investigated by the third of a series of three Cochrane reviews, which will address the effects of ICS on growth in children with persistent asthma (Axelsson 2013).
Growth studies with ICS are generally conducted in prepubertal children because the growth velocity is relatively constant and linear during this so‐called growth hormone–dependent phase (FDA 2007; Pedersen 2001). However, the applicability of results obtained in this age group to older children has been questioned because of the possibility of different sensitivity to the adverse effects of ICS on growth between prepubertal and pubertal children (Agertoft 2000; Pedersen 2001; Verberne 1998). The trials included in this review recruited participants with a wide age range, including toddlers/preschoolers, prepubertal children and pubertal children. Eight trials included children five to 18 years of age, but no separate data were available for pubertal children. Thus, this review did not provide an estimate of ICS‐induced growth‐suppressive effects in adolescents. The subgroup analysis of this review showed no statistically significant differences in ICS‐induced suppression in linear growth velocity between two trials in toddlers and preschoolers and nine trials in prepubertal children.
Summary of main results
Twenty five trials involving 8471 (5128 ICS‐treated and 3343 control) children with mild to moderate persistent asthma were included in this review. Six molecules (beclomethasone, budesonide, ciclesonide, flunisolide, fluticasone and mometasone) given at low or medium daily doses were used during a period from three months to four to six years. Compared with placebo or non‐steroidal drugs, ICS produced a statistically significant reduction in linear growth velocity (14 trials including 5717 participants; MD ‐0.48 cm/y, 95% CI ‐0.65 to ‐0.30, P value < 0.0001) and in change from baseline in height (15 trials including 3275 participants; MD ‐0.61 cm/y, 95% CI ‐0.83 to ‐0.38, P value < 0.00001) during a one‐year treatment period. The subgroup analysis showed a statistically significant difference between six molecules in mean reduction of linear growth velocity during a one‐year treatment period (Chi² = 26.1, df = 5, P value < 0.0001). CFC‐beclomethasone 400 μg/d (three trials including 439 participants) and budesonide via DPI (three trials including 2790 participants) produced a relatively greater reduction in mean linear growth velocity, with an MD (95% CI) of ‐0.91 cm/y (‐1.26 to ‐0.55) and ‐0.59 cm/y (‐0.73 to ‐0.45), respectively, compared with HFA‐ciclesonide 50 to 200 μg/d (one trial including 609 participants; MD ‐0.08 cm/y, 95% CI ‐0.27 to 0.11), HFA‐flunisolide 400 μg/d (two trials including 314 participants; MD ‐0.22 cm/y, 95% CI ‐0.63 to 0.18), fluticasone propionate 100 to 200 μg/d (five trials including 1405 participants; MD ‐0.39 cm/y, 95% CI ‐0.63 to ‐0.15) and mometasone via DPI 100 to 200 μg/d (one trial including 184 participants; MD ‐0.47 cm/y, 95% CI ‐0.97 to 0.03). The effects persisted with restriction to doses equivalent to 200 μg/d HFA‐beclomethasone. The subgroup analysis of daily ICS doses showed that medium doses produced a statistically significantly greater reduction in mean change from baseline in height (Chi² = 3.95, df = 1, P value 0.05) but not in linear growth velocity (Chi² = 2.59, df = 1, P value 0.11) during a one‐year treatment period compared with low doses. The difference between low and medium doses in terms of reduction in mean change from baseline in height did not persist when the analysis was restricted to a few trials using the molecule beclomethasone. Subgroup analyses did not show a statistically significant impact of inhalation device and participant age on the magnitude of ICS‐induced suppression of linear growth velocity during a one‐year treatment period. No statistically significant difference in linear growth velocity was found between participants given ICS and controls during the second year of treatment (five trials with 3174 participants; MD ‐0.19 cm/y, 95% CI ‐0.48 to 0.11, P value 0.22) in contrast to an MD of ‐0.58 cm/y (95% CI ‐0.71 to ‐0.44, P value < 0.00001) in favour of placebo during the first year of treatment. No statistically significant difference (one trial including 667 participants; MD 5.34 cm/y in both treatment groups) or a smaller difference in linear growth velocity (MD ‐0.33 cm/y, 95% CI ‐0.52 to ‐0.14, P value 0.0005) than that observed during the first year of treatment was found between ICS and control groups during the third year of treatment. Among four trials reporting data on linear growth during two‐ to 12‐month off‐treatment follow‐up, three trials did not report statistically significant catch‐up growth in the ICS group two to four months after treatment cessation. One trial showed accelerated linear growth velocity in the fluticasone group 12 months after treatment cessation, but there remained a statistically significant difference of 0.7 cm in height between fluticasone and placebo groups at the end of the three‐year trial. One trial with follow‐up into adulthood showed that participants of prepubertal age treated with budesonide 400 μg/d for a mean duration of 4.3 years had a mean reduction of 1.20 cm (95% CI ‐1.90 to ‐0.50) in adult height compared with those treated with placebo.
Overall completeness and applicability of evidence
All but five of the 25 trials included in this review are multi‐centre trials, and five are international multi‐centre trials conducted in high‐income and low‐income countries across Africa, Asia‐Pacifica, Europe and the Americas. The trials included in this review involved participants with a wide age range, including toddlers/preschoolers and prepubertal and pubertal children. Thus evidence derived from this review may have wide applicability. However, only participants with mild to moderate persistent asthma were included, and a relatively narrow dose range (low or medium doses) of ICS was used in these trials, so caution should be taken when extrapolating the findings of this review to patients with more severe asthma, who may need a higher dose of ICS. We explored the influence of molecule, daily dose, inhalation device and participant age on the growth‐suppressive effect of ICS by performing indirect comparisons using subgroup analyses; data from head‐to‐head randomised trials are needed to confirm these findings. This review identified only two trials conducted by the same group to assess the effects of new molecules such as ciclesonide and mometasone on linear growth in asthmatic children. Further studies are warranted to compare potential adverse effects on growth between new and older molecules in children with persistent asthma. A limited number of trials have reported effects of ICS on linear growth beyond two years of treatment, thus the growth‐suppressive effect of ICS during a longer period of treatment in children with persistent asthma remains to be better defined by additional studies.
Quality of the evidence
The evidence provided by this review was derived from 25 randomised, parallel‐group, controlled trials with a total of 8471 participants (5128 ICS‐treated and 3343 control). Twelve trials were specially designed to assess the effects of ICS on linear growth in children with asthma. Most trials (72%) used adequate methods for blinding. Demographic and baseline characteristics of participants were comparable between treatment groups in all included trials, despite lack of information or uncertainty about randomisation methods used in many trials. This review included three different growth measurements (linear growth velocity, change in height over a one‐year period and change in height SDS) and yielded a similar conclusion with respect to the growth‐suppressive effects of ICS.
Given that a considerable number of included trials did not report methods of random sequence generation and allocation concealment, had high withdrawal rates, used an open‐label design and were sponsored by the pharmaceutical industry, selection bias, attrition bias, performance and detection bias and sponsorship bias might have occurred. However, sensitivity analyses showed that these potential biases did not significantly affect the results of this review, underlying the robustness of the findings. We also used sensitivity analyses to assess the potential impact of compliance with treatment, previous use of ICS and missing data on the results; no significant influence of these factors was found.
Heterogeneity among results of the trials included in this review may be expected because of differences in the molecule, daily dose and age group across trials. However, all trials showed negative effects of ICS on growth, suggesting that the heterogeneity is quantitative but not qualitative and may not significantly affect the conclusions of this review.
Children with asthma are more likely to be atopic and to receive concomitant corticosteroids for other indications, such as allergic rhinitis or atopic dermatitis. However, given the expected balance of concomitant use of other forms of corticosteroids, the observed data are unlikely to overestimate or underestimate the impact of ICS on linear growth. Systemic corticosteroids used for exacerbations may also cause a negative impact on linear growth. Only nine trials (Allen 1998; Becker 2006; Bisgaard 2004; Guilbert 2006; Pauwels 2003; Price 1997; Roux 2003; Skoner 2008; Tinkelman 1993) reported use of systemic corticosteroids in some participants, and all trials reported a higher incidence of the use of such drugs in control groups compared with ICS groups. In the remaining trials, it may be expected that participants in the control groups were more likely to receive systemic corticosteroids than those in the ICS groups. However, use of systemic corticosteroids is usually infrequent during a one‐year treatment period in children with mild to moderate persistent asthma, as shown in the trial of Guilbert 2006 (mean number of courses of systemic corticosteroids/100 child‐years in the placebo group 89.4, 95% CI 78.3 to 102.2). The potential impact of such infrequent short‐term use of systemic corticosteroids on linear growth probably is minimal and may not significantly underestimate the ICS‐induced growth‐suppressive effect.
Potential biases in the review process
A relatively restrictive literature search strategy for this review might fail to identify efficacy trials in which adverse effects of ICS including effects on growth have been collected as secondary outcomes but not reported. Moreover, in the screening stage by titles and abstracts for eligibility, we cannot rule out the possibility that we missed efficacy trials that might have included growth data in the main text (tables and figures) but did not describe them in the abstract. No data were available to allow assessment of the potential impact on this review of exclusion of such trials.
Agreements and disagreements with other studies or reviews
Two previous systematic reviews (Allen 1994; Sharek 2000b) were conducted to assess growth‐suppressive effects of corticosteroids in children with asthma. Allen 1994 showed that oral corticosteroids, but not inhaled beclomethasone, were associated with growth impairment in children with asthma. However, caution should be taken in interpreting the findings of this review, given that most of the included studies were not randomised trials, and that non‐standard statistical methods were used for pooling the results of included studies. The meta‐analysis of Sharek 2000b, including five randomised trials with 633 participants, showed that moderate doses of inhaled beclomethasone (four trials) and fluticasone (one trial) caused a decrease in linear growth velocity of 1.51 cm/y and 0.43 cm/y, respectively. However, all but one trial of beclomethasone included in this review used mean change from baseline in height over time as the outcome measure; thus a beclomethasone‐induced decrease of 1.51 cm/y should refer to reduction in the mean increase in height over a one‐year period rather than linear growth velocity. The growth‐suppressive effects of inhaled beclomethasone on increase in height over a one‐year period as shown by Sharek 2000b (four trials with 450 participants) and by our review (six trials with 870 participants) were concordant at ‐1.09 cm/y (95% CI ‐1.18 to ‐1.00) and ‐1.08 cm/y (95% CI ‐1.17 to ‐0.99), respectively.
Authors' conclusions
Implications for practice.
This systematic review shows that regular use of ICS at low or medium daily doses is associated with an overall unadjusted mean reduction of 0.48 cm/y in linear growth velocity and a 0.61‐cm change from baseline in height during a one‐year treatment period in children with mild to moderate persistent asthma. The effect size of ICS on linear growth appears to be associated more strongly with the ICS molecule than with the device or dose (low to medium dose range). ICS‐induced suppression of linear growth seems to be maximal during the first year of therapy and less pronounced during subsequent years of treatment. Although catch‐up growth up to 12 months after ICS cessation has been documented, limited evidence suggests that ICS‐induced growth suppression in children of prepubertal age may persist until they reach adult height. Growth suppression appears neither progressive nor regressive, and it is not cumulative beyond the first year of therapy. Although the well‐established benefits of regular use of ICS may outweigh the potential risks of a relatively small and non‐cumulative suppression in linear growth in children with persistent asthma, one would suggest that ICS should be prescribed at the lowest effective dose. Moreover, it is prudent to monitor linear growth in children treated with ICS, given that individual susceptibility to these drugs may vary considerably.
Implications for research.
Current evidence suggests that regular use of ICS at low or medium daily doses is associated with linear growth suppression in children with mild to moderate persistent asthma. However, further research is warranted to discover more definitive answers to the following questions.
Do ICS given at medium to high daily doses have a greater effect on linear growth in children with moderate to severe persistent asthma compared with ICS at low doses associated with non‐steroidal drugs, such as long‐acting beta‐agonists or leukotriene receptor antagonists?
Does intermittent use of ICS have comparable efficacy and less growth‐suppressive effect in children with mild persistent asthma compared with regular use of these drugs? This has been looked at in Chauhan 2013.
Additional trials using different combinations of factors are needed to specify the respective effects of molecule, daily dose, inhalation device, patient age and duration of treatment on the effects of ICS on linear growth in children with persistent asthma.
Which factors are associated with catch‐up growth versus persistent growth impairment until final adult height in children with asthma?
What is the potential growth‐suppressive effect of ICS during a longer period of treatment in children with asthma?
Are different ICS molecules associated with variable catch‐up growth?
More data are needed to explore the molecule dependency of growth suppression, particularly with newer molecules (mometasone, ciclesonide).
Acknowledgements
Thanks to Elizabeth Stovold for help in defining the search strategy. Thanks also to Chris Cates for useful comments, and to Emma Welsh for commenting and providing ongoing assistance. Thanks to Inge Axelsson for providing input in drafting the review protocol. Thanks to Joseph Lau for constructive criticism and useful suggestions regarding the review draft.
Chris Cates was the Editor for this review and commented critically on the review.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| CENTRAL (The Cochrane Library) | Monthly |
| PSYCINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and the RCT filter are adapted to identify trials in other electronic databases.
Data and analyses
Comparison 1. Inhaled corticosteroids vs placebo or non‐steroidal drugs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Linear growth velocity (cm/y): 6‐ to 8‐month treatment | 2 | 369 | Mean Difference (IV, Random, 95% CI) | ‐1.23 [‐2.32, ‐0.13] |
| 1.1 7‐Month DPI‐beclomethasone 400 μg/d | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐1.82 [‐2.39, ‐1.25] |
| 1.2 8‐Month CFC‐fluticasone 200 μg/d | 1 | 285 | Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐0.93, ‐0.47] |
| 2 Change from baseline in height (cm): 6‐ to 8‐month treatment | 3 | 167 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.10, ‐0.43] |
| 2.1 6‐Month nebulised beclomethasone 300 μg/d | 1 | 29 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐1.06, 0.68] |
| 2.2 6‐Month DPI‐budesonide 200 μg/d | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.16, 0.12] |
| 2.3 7‐Month DPI‐beclomethasone 400 μg/d | 1 | 84 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.33, ‐0.67] |
| 2.4 8‐Month DPI‐budesonide 200 μg/d | 1 | 27 | Mean Difference (IV, Random, 95% CI) | ‐0.83 [‐1.59, ‐0.07] |
| 3 Linear growth velocity (cm/y): 1‐year (or nearly 1‐year) treatment | 14 | 5659 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.66, ‐0.27] |
| 3.1 CFC‐beclomethasone 400 μg/d | 3 | 427 | Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.26, ‐0.55] |
| 3.2 Budesonide 100‐400 μg/d | 3 | 2790 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.84, ‐0.38] |
| 3.3 Ciclesonide 50‐200 μg/d | 1 | 609 | Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.27, 0.11] |
| 3.4 Flunisolide 400 μg/d | 2 | 302 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.63, 0.18] |
| 3.5 Fluticasone 100‐200 μg/d | 5 | 1347 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.63, ‐0.15] |
| 3.6 Mometasone 100‐200 μg/d | 1 | 184 | Mean Difference (IV, Random, 95% CI) | ‐0.47 [‐0.97, 0.03] |
| 4 Linear growth velocity (cm/y): 1‐year (or nearly 1‐year) treatment—use of MD and SE for meta‐analysis | 14 | Mean Difference (Random, 95% CI) | ‐0.48 [‐0.65, ‐0.30] | |
| 4.1 CFC‐beclomethasone 400 μg/d | 3 | Mean Difference (Random, 95% CI) | ‐0.91 [‐1.26, ‐0.55] | |
| 4.2 Budesonide 100‐400 μg/d | 3 | Mean Difference (Random, 95% CI) | ‐0.59 [‐0.73, ‐0.45] | |
| 4.3 Ciclesonide 50‐200 μg/d | 1 | Mean Difference (Random, 95% CI) | ‐0.08 [‐0.27, 0.11] | |
| 4.4 Flunisolide 400 μg/d | 2 | Mean Difference (Random, 95% CI) | ‐0.22 [‐0.63, 0.18] | |
| 4.5 Fluticasone 100‐200 μg/d | 5 | Mean Difference (Random, 95% CI) | ‐0.39 [‐0.63, ‐0.15] | |
| 4.6 Mometasone 100‐200 μg/d | 1 | Mean Difference (Random, 95% CI) | ‐0.47 [‐0.97, 0.03] | |
| 5 Change from baseline in height (cm): 1‐year (or nearly 1‐year) treatment | 15 | 3217 | Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐0.83, ‐0.38] |
| 5.1 Beclomethasone 200‐400 μg/d | 6 | 735 | Mean Difference (IV, Random, 95% CI) | ‐0.98 [‐1.22, ‐0.74] |
| 5.2 Budesonide 200 μg/d | 2 | 150 | Mean Difference (IV, Random, 95% CI) | ‐0.72 [‐1.12, ‐0.32] |
| 5.3 Ciclesonide 50‐200 μg/d | 1 | 609 | Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.31, 0.07] |
| 5.4 Flunisolide 400 μg/d | 2 | 245 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.66, 0.36] |
| 5.5 Fluticasone 100‐200 μg/d | 5 | 1478 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.66, ‐0.20] |
| 6 Change in height standard deviation score (SDS): 1‐year (or nearly 1‐year) treatment | 4 | 258 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.24, ‐0.01] |
| 6.1 CFC‐beclomethasone 400 μg/d | 1 | 67 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.39, ‐0.11] |
| 6.2 Budesonide 200‐400 μg/d | 2 | 115 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.21, ‐0.05] |
| 6.3 Fluticasone 100‐200 μg/d | 2 | 76 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.54, 0.33] |
| 7 Linear growth velocity (cm/y): 2‐year treatment | 5 | 3174 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.46, 0.27] |
| 7.1 Budesonide 100‐400 μg/d | 3 | 2737 | Mean Difference (IV, Random, 95% CI) | ‐0.41 [‐0.97, 0.14] |
| 7.2 Fluticasone 200 μg/d | 2 | 437 | Mean Difference (IV, Random, 95% CI) | 0.22 [‐0.10, 0.55] |
| 8 Linear growth velocity (cm/y) using MD and SE for meta‐analysis: 2‐year treatment | 5 | Mean Difference (Random, 95% CI) | ‐0.19 [‐0.48, 0.11] | |
| 8.1 Budesonide 100‐400 μg/d | 3 | Mean Difference (Random, 95% CI) | ‐0.38 [‐0.60, ‐0.15] | |
| 8.2 Fluticasone 200 μg/d | 2 | Mean Difference (Random, 95% CI) | 0.22 [‐0.10, 0.55] | |
| 9 Change from baseline in height (cm): 2‐year treatment | 2 | 437 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.09, 1.49] |
| 9.1 Fluticasone 200 μg/d | 2 | 437 | Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐2.09, 1.49] |
| 10 Linear growth velocity (cm/y): off‐treatment follow‐up (2‐ to 4‐month) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 Increase in height (cm): off‐treatment follow‐up (2‐ to 4‐month) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 12 Linear growth velocity (cm/y): off‐treatment follow‐up (12‐month) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13 Adult height (cm) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
1.4. Analysis.
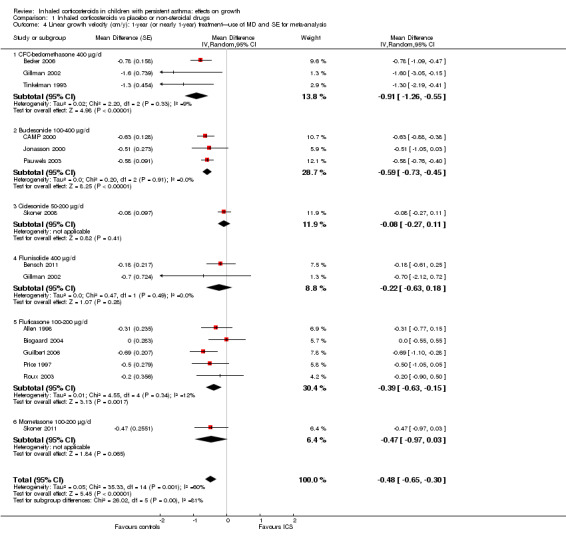
Comparison 1 Inhaled corticosteroids vs placebo or non‐steroidal drugs, Outcome 4 Linear growth velocity (cm/y): 1‐year (or nearly 1‐year) treatment—use of MD and SE for meta‐analysis.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Allen 1998.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: GlaxoSmithKline |
|
| Participants | Setting: 19 clinical centres in the USA
Eligible: 344
Randomly assigned: 111 (fluticasone 50 μg); 108 (fluticasone 100 μg); 106 (placebo) Analysed: 85 (fluticasone 50 μg); 96 (fluticasone 100 μg); 87 (placebo) Gender (male): 75% Age, years, mean (range): 8.0 (4.0‐11.9) Inclusion criteria: prepubescent children with persistent asthma for at least 3 months, diagnosed according to American Thoracic Society criteria; normal growth rates as defined by height measurements between the 5th and 95th centiles and growth velocity between the 10th and 97th centiles Exclusion criteria: had received systemic, intranasal or ophthalmic corticosteroids within the month before study entry; cataracts, glaucoma; any other significant concurrent disease or condition Previous regular use of ICS: 46% in the fluticasone groups; 45% in the placebo group |
|
| Interventions | Test group:
Control group: matching placebo Treatment was delivered twice daily via a Diskhaler. Treatment duration was 52 weeks |
|
| Outcomes |
Growth was measured monthly throughout the 52‐week treatment period |
|
| Notes | Fifty‐seven participants showing signs of puberty during the period of treatment were excluded from the growth analysis. The number of participants in each treatment group included in the analysis is as follows: 74 (fluticasone 50 μg); 79 (fluticasone 100 μg); 57 (placebo). Treatment compliance was measured at each visit by counting the number of package blisters that were used and dividing by the number of blisters that should have been used during the interval. Compliance rates ranged between 90% and 96% and were similar across treatment groups Use of systemic corticosteroids for exacerbations: fluticasone 11/181 (6.1%); placebo 25/87 (28.7%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal rate was < 20% in the fluticasone group and 34% in the placebo group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Unclear risk | Method of height measure not reported |
Becker 2006.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: Merck Research Laboratories |
|
| Participants | Setting: 30 medical centres worldwide (Asia, Africa, Europe, North America and South America)
Eligible: 575
Randomly assigned: 120 (montelukast 5 mg); 119 (CFC‐beclomethasone 200 μg); 121 (placebo) Analysed: 109 (montelukast 5 mg); 108 (CFC‐beclomethasone 200 μg); 108 (placebo) Gender (male): 64.4% Age, years, mean ± SD: boys 7.71 ± 0.85; girls 7.35 ± 0.56 Inclusion criteria: mild, persistent asthma at step 2 of the Global Initiative for Asthma guidelines and a 6‐month or longer history of asthma; Tanner stage I, with heights and weights in the 5th to 95th percentile range for age as described in the National Center for Health Statistics guidelines; bone age within 2 years of chronological age; pre‐bronchodilator FEV1 at least 75% of predicted Exclusion criteria: severe chronic sinus disease, nasal polyposis, pulmonary disease other than asthma or upper or lower respiratory tract infection; use of the following medications before visit 1: antileukotrienes (within 1 month); nasal, ocular and inhaled corticosteroids (2 weeks‐1 month); oral corticosteroids (4 months); more than 2 courses of inhaled corticosteroids (no course exceeded 14 days) for asthma (12 months); astemizole (3 months); theophylline, nedocromil, cromolyn, long‐acting beta2‐agonists and antimuscarinics (4 weeks); methylphenidate, thyroid hormone, growth hormone, anabolic corticosteroids, calcitonin, estrogens, progestins, bisphosphonates, anticonvulsants and phosphate‐binding antacids (any time before visit 1) Previous regular use of ICS: < 14 days |
|
| Interventions | Test group:
Control group: matching placebos Beclomethasone and matching placebo were delivered twice daily via a CFC‐MDI with spacer. Montelukaste was given once daily. Treatment duration was 56 weeks |
|
| Outcomes |
Height was measured in triplicate in the morning at the same time of day during each visit using a standard stadiometer, every 8 weeks during 56 weeks |
|
| Notes | Treatment compliance was measured by tablet counts; canister weight was > 95% in each treatment group Use of systemic corticosteroids for exacerbations: CFC‐beclomethasone 28/119 (23.5%); montelukast 30/120 (25.0%); placebo 42/121 (34.7%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, randomised schedule |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal rate was 11/119 (9.2%) in the beclomethasone group and 13/121 (10.7%) in the placebo group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Bensch 2011.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: Forest Laboratories, Inc. |
|
| Participants | Setting: 45 centres in the USA Eligible: not reported Randomly assigned: 122 (HFA‐flunisolide); 127 (placebo) Analysed: 106 (HFA‐flunisolide); 112 (placebo) Gender (male): 59.2% Age, years, mean ± SD: flunisolide 6.5 ± 1.57; placebo 6.4 ± 1.57 Inclusion criteria: prepubescent (4‐9.5 years of age) children with mild persistent asthma; had not used an ICS for 60 days, oral steroids for 90 days or nasal steroids for 30 days before the 2‐week run‐in period; Tanner stage ≤ 1; screening height between 5th and 95th percentiles for age and sex; able to use an MDI; could perform spirometry and complete diary cards Exclusion criteria: clinically significant pulmonary disease other than asthma; medical or psychiatric illness that could interfere with study assessments; hospitalisation or emergency room or office visit for exacerbation of asthma within 2 months before the start of the run‐in; immunotherapy other than an established maintenance programme; treatment with an investigational drug within 30 days of the run‐in; hypersensitivity to albuterol, flunisolide or HFA propellant; use of or intent to use any other steroid; and use of or intent to use any concomitant medication that could affect growth, such as methylphenidate Previous regular use of ICS: < 60 days |
|
| Interventions | Test group: HFA‐flunisolide, 400 μg/d Control group: matching placebo Treatment was given twice daily via HFA‐MDI. Treatment duration was 52 weeks |
|
| Outcomes |
Height was measured by trained personnel in triplicate using a stadiometer at weeks 4, 8, 12, 20, 28, 36, 44 and 52 |
|
| Notes | Compliance with study medication was monitored by self‐recording on the diary card of the number of puffs administered daily, and by weighing of drug canisters returned to the study site. Compliance rate was 86.1% in the flunisolide group and 87.7% in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal rate was 41/119 (34.5%) in the flunisolide group and 38/123 (30.9%) in the placebo group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Bisgaard 2004.
| Methods | Design: multi‐centre, randomised, open‐label, parallel‐group, controlled trial Sponsor: GlaxoSmithKline |
|
| Participants | Setting: multi‐centre, Bulgaria (13%), Czech Republic (8%), Croatia (5%), Hungary (12%), Israel (4%), New Zealand (4%), Poland (16%), Russia (20%), Slovakia (9%) and South Africa (10%) Eligible: 668 Randomly assigned: 471 (HFA‐fluticasone 100 μg); 154 (sodium cromoglycate 5 mg) Analysed: 381 (HFA‐fluticasone 100 μg); 122 (sodium cromoglycate 5 mg) Gender (male): 64% (fluticasone); 71% (cromoglycate) Age, months, mean (range): fluticasone 31.1 (11‐47); SCG 30.7 (11‐47) Inclusion criteria: children aged 12‐47 months with documented history of recurrent cough or wheeze and between 5th and 95th centiles for height and weight on growth charts provided by the Child Growth Foundation, London, UK Exclusion criteria: had received systemic corticosteroid therapy for > 5 days within 8 weeks or ICS at doses > FP 100 mcg/d or other ICS of 200 mcg/d within 4 weeks of visit 1; hospitalised or altered medication within 4 weeks of visit 1 or hospitalised on > 2 occasions for recurrent wheeze within 12 months of visit 1; systemic disease likely to affect growth; low birth weight (< 1.5 kg) or born before 32 weeks’ gestation Previous regular use of ICS: 36% of participants |
|
| Interventions | Test group: HFA‐fluticasone, 200 μg/d Control group: sodium cromoglycate, 20 mg/d Fluticasone was given twice daily via HFA‐MDI with Babyhaler, and cromoglycate was given 4 times daily via MDI with Nebuhaler. Treatment duration was 52 weeks |
|
| Outcomes |
Participant height and/or length was measured at each visit in triplicate by the same observer (between 6:00 AM and 10:00 AM) using a calibrated stadiometer and/or an infantometer |
|
| Notes | No formal assessment of treatment compliance was made. Compliance rate was > 90% according to participant's diary, in which use of prescribed medications was recorded Use of systemic corticosteroids for exacerbations: fluticasone 6%; sodium cromoglycate 12% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, randomised schedule |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal rate was 19% in the fluticasone group and 21% in the cromoglycate group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
CAMP 2000.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: National Heart, Lung and Blood Institute, USA |
|
| Participants | Setting: 8 clinical centres in the USA Eligible: 1041 Randomly assigned: 311 (budesonide 200 μg); 312 (nedocromil sodium 8 mg); 418 (placebo) Analysed: 98,4% (budesonide 200 μg); 98.4% (nedocromil sodium 8 mg); 98.3% (placebo) Gender (male): 58.2% (budesonide); 66% (nedocromil); 56% (placebo) Age, years, mean ± SD: budesonide 9.0 ± 2.1; nedocromil 8.8 ± 2.1; placebo 9.0 ± 2.2 Inclusion criteria: children 5 to 12 years of age with mild to moderate asthma (defined by presence of symptoms or by use of an inhaled bronchodilator at least twice weekly or use of daily medication for asthma) at least 6 months in the year before the interview; methacholine reactivity (FEV1, PC20) no greater than 12.5 mg/mL Exclusion criteria: severe asthma (2 or more hospitalisations for asthma in the past year, 6 or more steroid bursts in the past year, intubation for asthma at any time in the past); presence of 1 or more of the following confounding or complicating conditions: other active pulmonary disease; pulmonary function suggesting a ventilatory defect or evidence of irreversible lung disease; severe chronic sinusitis or nasal polyposis; introduction of or change in allergen immunotherapy in the month before the interview; use of more than 4 sprays of nasal steroids daily (only beclomethasone allowed) at the time of random assignment; current use of cimetidine, metoclopramide, ranitidine or other treatment for gastroesophageal reflux; participation in another pharmaceutical, immunotherapy, respiratory or asthma study; pregnancy; inability to perform acceptable spirometry; inability to complete the methacholine challenge Previous regular use of ICS: 40.5% in the budesonide group; 36.5% in the nedocromil group; 35.9% in the placebo group |
|
| Interventions | Test group:
Control group: matching placebos Budesonide was delivered via Turbuhaler, and nedocromil was delivered via Tilade‐MDI, twice daily. Treatment duration ranged from 4‐6 years |
|
| Outcomes |
Children’s height was measured by Harpenden stadiometer at every visit using a standard protocol |
|
| Notes | Treatment compliance was measured by percentage of days child reported to take prescribed dose of study medication as recorded in a dairy. Compliance rate was 73.7% in the budesonide group and 76.2% in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, randomised schedule |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No discontinuations due to asthma or to any clinical or laboratory adverse experiences. Percentage of participants who completed the study was similar in the 3 groups |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Doull 1995.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: Allen and Hanbury (UK) and the Wessex Medical Trust |
|
| Participants | Setting: 5 health centres in the Southampton area Eligible: 104 Randomly assigned: 52 (beclomethasone 200 μg); 52 (placebo) Analysed: 44 (beclomethasone 200 μg); 40 (placebo) Gender (male): 70.1% Age, months, mean ± SE: beclomethasone 100.3 ± 1.4; placebo 99.4 ± 1.6 Inclusion criteria: children 7 to 9 years of age, with 5 or more wheezing episodes in the preceding year or an episode of wheezing lasting for 3 days or longer in the preceding year Exclusion criteria: use of inhaled or oral corticosteroids; severe respiratory disease such as cystic fibrosis Previous regular use of ICS: not allowed |
|
| Interventions | Test group:
Control group: matching placebo Treatment was delivered twice daily via Diskhaler. Treatment duration was 7 months, followed by a washout period of 4 months |
|
| Outcomes |
Height was measured by a single observer in triplicate at each visit, using a Raven monitor |
|
| Notes | Treatment compliance was measured by counting the used Diskhaler blisters. Compliance rate was 75.2% in the beclomethasone group and 75.9% in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal rate was 2/52 (3.8%) in the beclomethasone group and 8/52 (15.4%) in the placebo group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Gillman 2002.
| Methods | Design: multi‐centre, randomised, open‐label, parallel‐group, controlled trial Sponsor: Forest Laboratories, Inc., New York, NY |
|
| Participants | Setting: 24 clinical centres in the USA Eligible: 241 Randomly assigned: 152 (HFA‐flunisolide 200 μg); 39 (CFC‐beclomethasone 200 μg); 44 (cromolyn 1600 μg) Gender (male): 61.7% Age, years, mean ± SD: 8.3 ± 2.1 Inclusion criteria: boys and premenarchal girls 4‐11 years of age with diagnosis of mild to moderate persistent asthma requiring pharmacotherapy (inhaled beta2‐agonist, leukotriene antagonist, nedocromil, cromolyn, inhaled corticosteroid); FEV1 ≥ 60% of predicted value after washout period (capable children only); ≥ 12% increase in FEV1 after albuterol inhalation at or within 12 months of visit 1 (capable children only); use of inhaled prescription antiasthma medication at approved doses for a minimum of 30 days before visit 1, or asthma symptoms requiring use of an inhaled bronchodilator at least 3 times a week for a minimum of 30 days before visit 1; ability to comply with protocol instructions with the help of a parent or guardian; ability to provide verbal informed consent Exclusion criteria: clinically significant pulmonary disease other than asthma; history of a significant medical or psychiatric condition that could interfere with efficacy and safety assessment; upper or lower respiratory tract infection within 30 days of visit 1; hospitalisation or urgent visit for an acute exacerbation of asthma within 3 months of visit 1; treatment with an investigational drug other than HFA‐flunisolide within 30 days of visit 1; hypersensitivity to study medications Previous regular use of ICS: 66.5% of participants |
|
| Interventions | Test group:
Control group: cromolyn, 6400 μg/d Flunisolide was given via HFA‐MDI, and beclomethasone was given via CFC‐MDI, twice daily. Cromolyn was given via MDI, 4 times daily. Treatment duration was 52 weeks |
|
| Outcomes |
Height of children was measured by a stadiometer at each visit |
|
| Notes | Treatment compliance was not measured during the trial period. Compliance rate was > 80% in the run‐in period All children 4‐5 years of age (n = 29) received HFA‐flunisolide and were excluded from the analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal reported in the study groups |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Unclear risk | Method of height measure not reported |
Gradman 2010.
| Methods | Design: single‐centre, randomised, open‐label, parallel‐group, controlled trial Sponsor: Fonden til faglig udvikling af speciallægepraksis, Toyota‐Fonden Denmark, Else & Helene Alstrup’s Fond, Lily Benthine Lund’s Fond, Johannes M. Klein’s Fond and A. and J. Rasmussens Fond |
|
| Participants | Setting: Children's Clinic Randers, Randers, Denmark Eligible: 52 Randomly assigned: 22 (budesonide 200 μg); 21 (montelukast 5 mg) Gender (male): 71.15% Age, years, mean (range): budesonide 9.2 (5–11); montelukast 8.8 (5–11) Inclusion criteria: prepubertal (5‐11 years) children with mild persistent asthma according to GINA guidelines; Tanner stage 1, with height and weight in the 3rd–97th percentile range for age and gender according to Danish standard charts; no use of systemic corticosteroids within the previous 4 weeks; no concurrent disease or medications that might affect growth; appropriate inhaler technique and ability to cooperate to knemometry Exclusion criteria: individuals required to use intranasal, oral or parenteral corticosteroids during the trial Previous regular use of ICS: 88% in the budesonide group; 78% in the montelukast group |
|
| Interventions | Test group:
Control group:
Budesonide was given via DPI, and treatment was given once daily for 52 weeks |
|
| Outcomes |
Height was measured in triplicate by the same experienced observer with a wall‐mounted Harpenden stadiometer |
|
| Notes | Adherence to study treatment was recorded in a diary and confirmed by counting the number of remaining tablets (Singulair) or by reading the dose counter (Pulairmax). Compliance rate was 95%‐98% in the budesonide group and 95%‐96% in the montelukast group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Guilbert 2006.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: National Institutes of Health and National Jewish Medical and Research Center, USA |
|
| Participants | Setting: 5 clinical centres in the USA Eligible: 456 Randomly assigned: 143 (CFC‐fluticasone); 142 (placebo) Gender (male): 61.5% (CFC‐fluticasone); 62.7% (placebo) Age, years, mean ± SD: fluticasone 3.0 ± 0.6; placebo 3.0 ± 0.6 Inclusion criteria: preschool children with no clinically significant medical disorders apart from wheezing or allergy; high risk for persistence of asthma‐like symptoms according to a positive modified asthma predictive index; had received not more than 4 months of treatment with inhaled corticosteroids before enrolment; asthma symptoms not requiring inhaled corticosteroids during a run‐in month Exclusion criteria: See above Previous regular use of ICS: < 4 months |
|
| Interventions | Test group:
Control group: matching placebo Treatment was given twice daily via MDI with a valved spacer (AeroChamber). Treatment duration was 2 years, followed by a 1‐year washout period |
|
| Outcomes |
Height was measured at every visit with an upright stadiometer (Harpenden, Holtain) by established procedures |
|
| Notes | Adherence to treatment, defined as percentage of days in which a child took the prescribed dose of study medication as measured by an electronic meter. Compliance rate was 74% in the fluticasone group and 69% in the placebo group Use of systemic corticosteroids for exacerbations (number of courses/100 child‐years): fluticasone 57.4 (95% CI 49.0 to 67.3); placebo 89.4 (95% CI 78.3 to 102.2) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Withdrawal rate was 11/143 (7.7%) in the fluticasone group and 12/142 (8.5%) in the placebo group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Jonasson 2000.
| Methods | Design: single‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: AstraZeneca AS |
|
| Participants | Setting: Ulleval University Hospital, Oslo, Norway Eligible: not reported Randomly assigned: 28 (BUD 100 μg+placebo); 32 (BUD 200 μg+placebo); 28 (BUD 100 μg+BUD 100 mcg); 34 (placebo+placebo) Gender (male): 65.6% Age, years, mean: 9.7 Inclusion criteria: children with mild asthma, diagnosed according to International Consensus report and Nordic Consensus report; 3 previous obstructive episodes or 1 previous obstructive episode with atopy; at least 1 of these episodes had to have occurred within the last year before randomisation Exclusion criteria: patients who had used inhaled steroids within 2 months, or cromoglycate and/or nedocromil within 4 weeks, of entry Previous regular use of ICS: not allowed |
|
| Interventions | Test group:
Control group:
Treatment was given twice daily via Turbuhaler inhaler for 27 months |
|
| Outcomes |
Participant height was measured at every visit throughout the study period by a wall‐fixed stadiometer. 3 trained persons carried out all height measurements during the study |
|
| Notes | Study was the direct continuation of a previous 12‐week trial. Treatment compliance was assessed by counting the remaining doses in the inhaler device, which initially contained 200 doses. This was done at every 6‐month interval or when the inhalers were returned. Compliance rate ranged from 52%‐59% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal rate was 20/88 (25%) in the budesonide groups and 13/34 (38.2%) in the placebo group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Kannisto 2000.
| Methods | Design: single‐centre, randomised, open‐label, parallel‐group, controlled trial Sponsor: Finnish Foundation for Pediatric Research and Kuopio University Hospital |
|
| Participants | Setting: Allergy Unit of the Department of Pediatrics, Kuopio University Hospital, Finland Eligible: not reported Randomly assigned: 30 (fluticasone); 30 (budesonide); 15 (cromones) Gender (male): 48% Age, years, mean (range): 9.5 (5.5–14.7) Inclusion criteria: children with newly diagnosed asthma who had started their first period of maintenance medication Exclusion criteria: not reported Previous regular use of ICS: none of the participants |
|
| Interventions | Test group:
Control group:
Fluticasone was given via Diskus and budesonide was given via Turbuhaler, twice daily. Cromones were given via MDI with a large volume spacer or via DPI. Treatment duration was 12 months |
|
| Outcomes |
Height was measured by an experienced asthma nurse using a calibrated Harpenden stadiometer |
|
| Notes | Compliance was assessed by a home monitoring diary in which participants had recorded used medication doses. Compliance rate was not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawal reported |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Martinez 2011.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: National Heart, Lung and Blood Institute, USA |
|
| Participants | Setting: 5 clinical centres in the USA Eligible: 843 Randomly assigned: 71 (combined group); 72 (daily beclomethasone); 71 (rescue beclomethasone); 74 (placebo) Gender (male): 159/288 (55.2%) Age, years, mean ± SD: combined 11.4 ± 3.1; daily beclomethasone 10.8 ± 3.5; rescue beclomethasone 10.4 ± 2.8; placebo: 10.4 ± 3.2 Inclusion criteria: children and adolescents between 6 and 18 years of age; history of mild persistent asthma during the previous 2 years; naive to controller treatment with a history of 1 to 2 exacerbations in the previous year; treated for the previous 8 weeks with a monotherapy other than inhaled corticosteroids; illness controlled for the previous 8 weeks on low‐dose corticosteroids as monotherapy (≤ 160 μg daily with a beclomethasone equivalent) Exclusion criteria: participants excluded from the study if they had a pre‐bronchodilator FEV1 less than 60% predicted at first visit; admitted to hospital for asthma in the previous year; any asthma exacerbation in the previous 3 months or more than 2 in the previous year; history of life‐threatening asthma exacerbations that required intubation or mechanical ventilation, or that resulted in a hypoxic seizure Previous regular use of ICS: ranged from 72%‐82% among treatment groups |
|
| Interventions | Test group:
Control group: placebo: twice‐daily placebo with placebo plus albuterol as rescue Daily treatment was given twice daily via HFA‐MDI. Rescue treatment was given via HFA‐MDI for symptom relief. Treatment duration was 44 weeks |
|
| Outcomes |
Method for height measure was not reported |
|
| Notes | Treatment compliance was not measured | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Data Coordinating Center (DCC; Penn State Hershey College, PA, USA) generated the random allocation sequence |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal rate was 30/214 (14.0%) in the active treatment groups and 24/74 (32.4%) in the placebo group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Unclear risk | Method of height measure not reported |
Pauwels 2003.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: AstraZeneca Research and Development |
|
| Participants | Setting: 499 sites in 32 countries
Eligible: 7241
Randomly assigned: 3642 (budesonide); 3599 (placebo) Analysed: 3597 (budesonide); 3568 (placebo) (5‐10 years old: 1000 [budesonide]; 974 [placebo] Gender (male): 46% Age, years (range): 5‐17 Inclusion criteria: mild asthma defined as wheeze, cough, dyspnoea or chest tightening at least once per week, but not as often as daily; reversible airway obstruction defined as an increase in FEV1 > 12% after use of a short‐acting bronchodilator; a fall in FEV1 > 15% on exercise testing, or variation > 15% between the 2 highest and the 2 lowest peak expiratory flow rates during 14 days Exclusion criteria: symptoms of asthma or asthma treatment for longer than 2 years before entry to the study; more than 30 days of treatment with a glucocorticosteroid; more than 1 depot glucocorticosteroid injection per year; decision by treating physician that delay in inhaled glucocorticosteroid treatment was inappropriate; pre‐bronchodilator FEV1 < 60% of predicted; postbronchodilator FEV1 < 80% of predicted; other clinically significant disease Previous regular use of ICS: < 30 days |
|
| Interventions | Test group:
Control group: matching placebo Treatment was given once daily via Turbuhaler for 3 years |
|
| Outcomes |
We measured participants' height to calculate percentage of predicted FEV1. Because we did not define growth as an outcome in our protocol, height measurements were not standardised to the quality and accuracy needed for an analysis of this variable |
|
| Notes | About 30% of participants left the study during follow‐up. No significant difference between budesonide and placebo groups. Treatment compliance was not measured. Only participants 5‐10 years of age had available data on linear growth Use of systemic corticosteroids for exacerbations: at 12 months: budesonide 2.0%, placebo 3.1%; at 24 months: budesonide 1.7%, placebo 3.3%; at 36 months: budesonide 1.5%, placebo 2.0% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done at the sponsor’s site by a person not involved in analysis of data |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | About 30% of participants left the study during follow‐up. No significant difference was noted between budesonide and placebo groups |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Unclear risk | Height was measured for calculation of predicted value of FEV1; method of height measure was not reported |
Pedersen 2010.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: Nycomed |
|
| Participants | Setting: 110 sites in Bulgaria, Germany, Hungary, Poland, Romania, Russia, South Africa, Spain and Ukraine Eligible: 1335 Randomly assigned: 305 (ciclesonide 50 μg); 312 (ciclesonide 100 μg); 313 (ciclesonide 200 μg); 150 (placebo) Analysed: 255 (ciclesonide 50 μg); 255 (ciclesonide 100 μg); 255 (ciclesonide 200 μg); 110 (placebo) Gender (male): 65.8% Age, years, median (range): CIC40 8.0 (6‐11); CIC80 8.0 (6‐11); CIC160 9.0 (6‐11); placebo 8.0 (6‐11) Inclusion criteria: children 6‐11 years of age with persistent asthma for ≥ 6 months; able to perform reproducible lung function tests and have an acceptable MDI inhalation technique; mean PEF value (over last week) of 40%‐90% of predicted value, as well as FEV1 reversibility ≥ 12% predicted after inhalation of 200‐400 mg salbutamol at the end of the run‐in period Exclusion criteria: history of near fatal asthma; respiratory tract infection or asthma exacerbation within the past 30 days; 2 or more inpatient hospitalisations for asthma in the previous year; use of systemic glucocorticosteroids within 30 days before study entry or for > 60 days in the previous 2 years Previous regular use of ICS: ranged from 64.2%‐69.9% among treatment groups |
|
| Interventions | Test group:
Control group: matching placebo Treatment was given once daily via HFA‐MDI (2 subgroups: with and without use of a spacer) for 12 weeks |
|
| Outcomes |
Height was measured at start and end of treatment period using a stadiometer |
|
| Notes | Treatment compliance was not formally monitored. Participants and/or caregivers were told to report in their diaries deviations from intended treatment schedule Compliance rate was not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation scheme |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Unclear risk | Method of height measure not reported |
Price 1997.
| Methods | Design: multi‐centre, randomised, open‐label, parallel‐group, controlled trial Sponsor: Allen and Hanburys Ltd. (Glaxo Wellcome UK Limited) |
|
| Participants | Setting: 15 hospital centres in the UK Eligible: not reported Randomly assigned: 52 (fluticasone 50 μg); 70 (sodium cromoglycate 20 mg) Analysed: 34 (fluticasone 50 μg); 26 (sodium cromoglycate 20 mg) Gender (male): 61.5% Age, years, mean ± SD (range): FP 6.0 ± 1.4 (4.2–9.6); SCG 6.4 ± 1.6 (4.0–10.0) Inclusion criteria: prepubertal children between 4 and 10 years of age; history of asthma with recurrent episodes of wheeze and cough; on at least 6 days of the 2‐week baseline period, experienced either PEF measurements < 80% of maximum value, or daytime or night‐time symptom scores of 1 or higher (scale 0–3) and requirement for extra bronchodilator medication during the same 24‐hour period; satisfactory inhaler and peak flow meter technique Exclusion criteria: use of inhaled prophylactic therapy for asthma within the past year; use of oral corticosteroids within the past 3 months; acute respiratory tract infection in the past 2 weeks; concurrent disease or medications that might affect growth Previous regular use of ICS: not allowed |
|
| Interventions | Test group:
Control group:
Fluticasone was given twice daily via Diskhaler, and cromoglycate was given 4 times a day via Spinhaler. Treatment duration was 12 months |
|
| Outcomes |
Height was measured with Holtain stadiometers at the start and end of baseline, after 8 weeks of treatment and at 6‐weekly intervals thereafter. 3 height measurements were recorded at each visit |
|
| Notes | Treatment compliance was not formally measured Use of systemic corticosteroids for exacerbations: fluticasone 5/52 (9.6%); sodium cromoglycate 5/70 (7.1%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal rate was 16/52 (30.8%) in the fluticasone group and 43/71 (60.6%) in the cromoglycate group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Roux 2003.
| Methods | Design: multi‐centre, randomised, open‐label, parallel‐group, controlled trial Sponsor: GlaxoSmithKline |
|
| Participants | Setting: 52 specialist clinics in France Eligible: 207 Randomly assigned: 87 (fluticasone); 87 (nedocromil sodium) Analysed: 87 (fluticasone); 87 (nedocromil sodium) Gender (male): 74.7% Age, years, mean ± SD: fluticasone 9.1 ± 2.5: nedocromil 9.4 ± 2.4 Inclusion criteria: children 6‐14 years of age; weighed ≥ 13 kg, with persistent asthma defined as exacerbations occurring at least once a week but less often than daily, or chronic symptoms requiring daily treatment with a short‐acting β2‐agonist Exclusion criteria: treatment during the previous month with an oral, inhaled or intranasal corticosteroid, a chromone theophylline or a long‐acting β2‐agonist; uncontrolled serious concurrent disease Previous regular use of ICS: not allowed |
|
| Interventions | Test group:
Control group:
Fluticasone was given twice daily via Diskus/Accuhaler. Nedocromil sodium was given twice daily via MDI. Treatment duration was 24 months |
|
| Outcomes |
Height was measured at the end of the run‐in period and at months 12 and 24, using the standard methods in place at each centre |
|
| Notes | Treatment compliance was not measured Use of systemic corticosteroids for exacerbations: fluticasone 26%; nedocromil sodium 43% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Balanced, block randomisation with gender stratification |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal rate was 13/87 (14.9%) in the fluticasone group and 28/87 (32.2%) in the nedocromil sodium group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Simons 1997.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: GlaxoSmithKline |
|
| Participants | Setting: multi‐centres in Canada Eligible: 315 Randomly assigned: 81 (beclomethasone); 80 (salmeterol); 80 (placebo) Analysed: 81 (beclomethasone); 80 (salmeterol); 80 (placebo) Gender (male): 58% Age, years, mean ± SD: 9.3 ± 2.4 Inclusion criteria: children 6‐14 years of age with clinically stable asthma; less than 1 month of treatment at any time with inhaled or oral glucocorticoids for asthma; no glucocorticoid treatment for asthma within 3 months before enrolment; FEV1 > 70% after bronchodilator had been withheld for 6 hours; 10% increase in FEV1 30 minutes after inhalation of 400 mcg of albuterol; requirement of < 8 mg of methacholine/mL to decrease the FEV1 by 20% (PC20); ability to refrain from using study medications for 36 hours and from using rescue albuterol for 6 hours before visits Exclusion criteria: any emergency department visits or hospitalisations for asthma within the prior 3 months; history of life‐threatening asthma; history of adverse reactions to medications used in the study Previous regular use of ICS: < 1 month |
|
| Interventions | Test group:
Control group: matching placebo Treatment was given twice daily via Diskhaler for 12 months |
|
| Outcomes |
Height was measured by the same trained observer at each site. A calibrated stadiometer was used at most study sites |
|
| Notes | Treatment compliance was monitored by counting the number of medication blisters used. Compliance rate was 100% in the beclomethasone group and 99% in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal rate was 17/81 (20.9%) in the beclomethasone group and 31/80 (38.8%) in the placebo group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Skoner 2008.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: Sanofi‐aventis US and Altana Pharma US, Inc, a Nycomed company |
|
| Participants | Setting: 85 centres in 4 countries (United States [63 centres], Argentina [12 centres], Venezuela [6 centres] and Chile [4 centres]) Eligible: 1127 Randomly assigned: 221 (ciclesonide 50); 219 (ciclesonide 200); 221 (placebo) Analysed: 206 (ciclesonide 50); 202 (ciclesonide 200); 201 (placebo) Gender (male): 67.2% Age, years, mean ± SD: ciclesonide 50: 6.6 ± 0.97; ciclesonide 200: 6.7 ± 0.93; placebo: 6.7 ± 0.95 Inclusion criteria: female and male children 5.0 to 7.5 and 5.0 to 8.5 years of age, respectively; diagnosis of mild persistent asthma ≥ 3 months before screening; FEV1 ≥ 80% predicted (after ≥ 4‐hour albuterol withheld); effective use of MDI devices Exclusion criteria: ICS within 30 days before screening, at a dosage exceeding fluticasone propionate 100 mcg/d or equivalent; previous daily or alternate‐day oral corticosteroid treatment for a total of 60 days within 2 years before visit 3 or within 30 days before screening; unable or refused to use study devices as required Previous regular use of ICS: 19.5% in the ciclesonide 50 group; 21% in the ciclesonide 200 group; 19% in the placebo group |
|
| Interventions | Test group:
Control group: matching placebo Treatment was given once daily via HFA‐MDI without spacer for 12 months |
|
| Outcomes |
Height was measured at each visit by a trained technician using a Harpenden stadiometer. Median of 4 acceptable serial measurements used in the analysis |
|
| Notes | Treatment compliance to study medication was monitored via participant diaries and by canister weight. Compliance rate ranged from 79.6%‐81.9% by canister weight and from 97.7%‐99.1% by diary Use of systemic corticosteroids for exacerbations: ciclesonide 50: 10/221 (4.5%); ciclesonide 200: 4/219 (1.8%); placebo: 11/221 (5.0%) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule was generated by the Biostatistics Department of Quintiles, Inc (Kansas City, MO) and was stratified according to age‐gender classification |
| Allocation concealment (selection bias) | Low risk | Central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal rate was 18.1% in the CIC 40 μg group, 14.2% in the CIC 160 μg group and 18.1% in the placebo group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Skoner 2011.
| Methods | Design: phase III, multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: Merck & Co, Inc |
|
| Participants | Setting: multi‐centres in the USA Eligible: not reported Randomly assigned: 48 (mometasone 100 μg QD); 44 (mometasone 100 μg BID); 50 (mometasone 200 μg QD); 45 (placebo) Analysed: 48 (mometasone 100 μg QD); 44 (mometasone 100 μg BID); 50 (mometasone 200 μg QD); 45 (placebo) Gender (male): 70.1% Age, years, mean: 6.5 Inclusion criteria: children 4–9 years of age with history of asthma ≥ 6 months; FEV1 ≥ 75% predicted value at both screening visit and baseline visit (for children 4–5 years of age, FEV1 ≥ 75% predicted value for any single measurement, or average morning PEF ≥ 75% predicted normal at screening or baseline); normal height (5th–95th percentiles on standard growth charts) upon measurement with a stadiometer; skeletal age within 2 years of chronological age; morning plasma cortisol levels ≥ 5 μg/dL; no greater than stage 1 in the Tanner Classification of Sex Maturity Exclusion criteria: increase or decrease in FEV1 ≥ 20% between screening and baseline visits; ≥ 12 puffs per day of albuterol on any 2 consecutive days between screening and baseline visits; inpatient hospitalisation for asthma control within the previous 3 months; ventilator support for respiratory failure secondary to asthma within the previous 5 years; hospital admission for management of airway obstruction on 2 or more occasions over the past 6 months; asthma requiring daily use of nebulised short‐acting β2‐agonist or any use of long‐acting β2‐agonists; asthma requiring long‐term use of inhaled or systemic corticosteroids; inability to use a DPI device or a peak flow meter; history or evidence of abnormal growth; presence of any disease or condition with the potential to substantially affect growth or requiring concomitant corticosteroid therapy; evidence of gross malnutrition; history of any disease that could have interfered with study evaluations; upper or lower respiratory tract infection within 2 weeks of screening and baseline visits Previous regular use of ICS: not allowed |
|
| Interventions | Test group:
Control group: matching placebo Treatment was given via DPI for 52 weeks |
|
| Outcomes |
Height was measured using a Harpenden stadiometer, and a mean of 3 values was recorded. When possible, the same study personnel performed height measurements for a given participant throughout the study, at approximately the same time of day for each visit |
|
| Notes | Treatment compliance was assessed by diary. Compliance rate ranged from 75%‐83% in the mometasone groups and 89% in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal rate was 52/187 (27.8%) |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Sorkness 2007.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: National Heart, Lung and Blood Institute |
|
| Participants | Setting: multi‐centres in the USA Eligible: 648 Randomly assigned: 96 (fluticasone 100 μg); 94 (fluticasone 100 μg/salmeterol 50 μg); 95 (montelukast 5 mg) Analysed: 86 (fluticasone 100 μg); 81 (fluticasone 100 μg/salmeterol 50 μg); 83 (montelukast 5 mg) Gender (male): 61.4% Age, years, mean ± SD: fluticasone 9.8 ± 2; fluticasone+salmeterol 10.3 ± 2.1; montelukast 9.6 ± 2.2 Inclusion criteria: children 6 to younger than 14 years of age with physician‐diagnosed mild to moderate persistent asthma; ability to perform reproducible spirometry; FEV1 ≥ 80% predicted normal at screening and ≥ 70% predicted normal at random assignment; methacholine FEV1 PC20 ≤ 12.5 mg/mL Exclusion criteria: respiratory tract infection, asthma exacerbation or systemic corticosteroid use within 4 weeks; 2 or more asthma hospitalisations in the past year; history of a life‐threatening asthma exacerbation; ≥ 4 courses of systemic corticosteroids in the past year; cigarette smoking within the past year; pregnancy or lactation; failure to practice abstinence or to use a medically acceptable birth control method; history of adverse reactions to PACT medications Previous regular use of ICS: 60.4% in the fluticasone group; 51.1% in the fluticasone+salmeterol group; 57.9% in the montelukast group |
|
| Interventions | Test group:
Control group:
Fluticasone and fluticasone/salmeterol were given via Diskus. Treatment duration was 48 weeks |
|
| Outcomes |
|
|
| Notes | Treatment compliance was measured by dose indicator. Compliance rate ranged from 86% to 97.7% | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified by study centre, and within each centre, a stratified randomisation scheme was applied on the basis of bronchodilator response, race (white or non‐white) and methacholine test |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal rate was 10/96 (10.4%) in the fluticasone group and 12/95 (12.6%) in the montelukast group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Unclear risk | Method of height measure not reported |
Storr 1986.
| Methods | Design: single‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: SETRHA and The Royal Alexandra Hospital Centenary Fund |
|
| Participants | Setting: Royal Alexandra Hospital for Sick Children, Brighton, UK Eligible: 29 Randomly assigned: 15 (beclomethasone); 14 (placebo) Analysed: 15 (beclomethasone); 13 (placebo) Gender (male): 62.1% Age, years, mean (range): 3.6 (1.6‐5.6) Inclusion criteria: During the 6 months before the study, all had had severe recurrent wheezing episodes and had responded well to treatment with nebulised bronchodilator agents. The number of previous hospital admissions for asthma ranged from 4 to 14, with at least 2 in the previous 6 months. None of the participants were satisfactorily controlled before the study began Exclusion criteria: not reported Previous regular use of ICS: none of the participants |
|
| Interventions | Test group:
Control group: matching placebo Treatment was given 3 times daily via jet nebuliser for 6 months |
|
| Outcomes |
|
|
| Notes | Treatment compliance was not measured | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details provided |
| Allocation concealment (selection bias) | Low risk | Allocation code was kept by the hospital pharmacist and by Allen and Hanburys Ltd |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 participant did not complete the study |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Unclear risk | Method of height measure not reported |
Tinkelman 1993.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, placebo‐controlled trial Sponsor: American Academy of Allergy and Immunology and Glaxo Inc |
|
| Participants | Setting: multi‐centres in the USA Eligible: 195 Randomly assigned: 102 (beclomethasone); 93 (theophylline) Analysed: 76 (beclomethasone); 69 (theophylline) Gender (male): 62.6% Age, years, mean (range): 11.9 (6‐17) Inclusion criteria: children 6 to 18 years of age with episodes of dyspnoea, cough and wheezing requiring intermittent or frequent bronchodilator treatment; FEV1 > 50% of predicted value and reversibility of ≥ 15% of baseline following an inhaled bronchodilator Exclusion criteria: currently or in the past 6 months regularly smoked cigarettes, cigars, pipe or marijuana; acute respiratory infection within 3 weeks of start of the study; systemic glucocorticoid treatment within the month before the study or for longer than 30 days in the past 2 years; theophylline and aerosol glucocorticoid treatment received together for longer than 1 month in the past year; regular aerosol cromolyn treatment within the past 60 days; topical nasal corticosteroids in the past 30 days; receiving immunotherapy and had not reached a maintenance dose; serious adverse reactions to theophylline or glucocorticoids in the past; other illnesses that would be an absolute or relative contraindication to theophylline or glucocorticoid treatment; attention deficit disorder, behavioral disorder, legal or mental incapacity, mental retardation, alcohol or drug abuse or other psychological or emotional disorder requiring treatment. Females excluded if pregnant, lactating or sexually active and not using reliable birth control Previous regular use of ICS: less than 1 month |
|
| Interventions | Test group:
Control group:
Beclomethasone was given 4 times a day via CFC‐MDI. Theophylline capsule was given twice daily. Treatment duration was 52 weeks |
|
| Outcomes |
|
|
| Notes | Treatment compliance was measured by diary. Compliance rate was 90.2% in the beclomethasone group and 86% in the theophylline group Use of systemic corticosteroids for exacerbations: beclomethasone 18.6%; theophylline 36.6% |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified by clinical centre and previous theophylline or BDP use separately for participants older or younger than 16 years of age |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal rate was 25.5% in the beclomethasone group and 25.8% in the theophylline group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Unclear risk | Method of height measure not reported |
Turpeinen 2008.
| Methods | Design: single‐centre, randomised, open‐label, parallel‐group, controlled trial Sponsor: Helsinki University Central Hospital and AstraZeneca |
|
| Participants | Setting: Helsinki University Central Hospital Eligible: 193 Randomly assigned: 59 (continuous budesonide); 58 (budesonide/placebo); 61 (disodium cromoglycate) Analysed (for growth): 52 (continuous budesonide); 46 (budesonide/placebo); 44 (disodium cromoglycate) Gender (male): 59.7% Age, years, mean (range): 6.9 (5‐10) Inclusion criteria: children 5‐10 years of age with symptoms such as wheezing, prolonged cough or shortness of breath, suggesting asthma for at least 1 month before study entry and with significant bronchial reversibility. According to symptoms and lung function tests, most children could be categorised as having mild persistent asthma Exclusion criteria: children with acute asthma; an FEV1 < 50% predicted value; treatment during preceding 2 months with ICS, cromones, leukotriene modifiers or long‐acting beta2‐agonists; total cumulative doses of previously used ICS > 36 mg, nasal corticosteroids > 12 mg or > oral doses equivalent to 200 mg prednisolone Previous regular use of ICS: cumulative doses < 36 mg |
|
| Interventions | Test group:
Control group:
Budesonide was given via Turbuhaler, and cromoglycate was given via MDI. Treatment duration was 18 months |
|
| Outcomes |
Height was measured at each clinic visit using a stadiometer (Holtain, Crymych, UK) following a standardised procedure |
|
| Notes | For the budesonide treatment groups, treatment compliance was recorded using a home spirometer, which recorded peak inspiratory flow via Turbuhaler each time a dose of the drug was taken. In the DSCG group, the returned MDI drug canisters were counted and weighed every 3 months. Compliance rate was not reported. Mean treatment compliance for the 3 treatment groups decreased linearly from an initial level of approximately 90% to a mean level of approximately 60% towards the end of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by a computer programme |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal rate was 6/59 (10.2%) in the budesonide group and 16/61 (26.2%) in the cromoglycate group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Verberne 1997.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, controlled trial Sponsor: Glaxo Wellcome B.V., Zeist, The Netherlands |
|
| Participants | Setting: outpatient paediatric clinics of 9 hospitals, 6 university hospitals and 3 general hospitals, The Netherlands Eligible: 67 Randomly assigned: 35 (beclomethasone); 32 (salmeterol) Analysed : 35 (beclomethasone); 32 (salmeterol) Gender (male): 67.2% Age, years, mean ± SD: beclomethasone 10.5 ± 2.3; salmeterol 10.6 ± 2.9 Inclusion criteria: children 6 to 16 years of age with mild to moderate asthma according to American Thoracic Society criteria; FEV1 55%–90% of predicted value and/or ratio of FEV1 to FVC 50%–75%; increase of at least 10% in FEV1 after inhalation of 0.8 mg salbutamol; airway hyperresponsiveness to methacholine (i.e. 20% fall in FEV1 after inhalation of 150 mcg or less methacholine (PD20 methacholine)); ability to produce reproducible lung function tests; history of stable asthma for at least 1 month with no exacerbations or respiratory tract infections Exclusion criteria: use of inhaled corticosteroids in the previous 6 months or cromoglycate in the previous 2 weeks Previous regular use of ICS: 17.6% in the beclomethasone group; 15.6% in the salmeterol group |
|
| Interventions | Test group:
All drugs were given twice daily via Diskhaler for 54 weeks |
|
| Outcomes |
Method for height measure was not reported |
|
| Notes | Treatment compliance was measured by counting used blisters. Compliance rate was 92% in the beclomethasone group and 91% in the placebo group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Generated by a computer programme |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching control |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching control |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawal rate was 3/35 (8.6%) in the beclomethasone group and 7/32 (21.9%) in the salmeterol group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but published reports include all expected outcomes, including those that were prespecified |
| Other bias | Unclear risk | Method of height measure not reported |
Wasserman 2006.
| Methods | Design: multi‐centre, randomised, double‐blind, parallel‐group, controlled trial Sponsor: GlaxoSmithKline Inc |
|
| Participants | Setting: 77 study sites in the USA Eligible: 493 Randomly assigned: 111 (CFC‐fluticasone 50 μg); 108 (CFC‐fluticasone 100 μg); 113 (placebo) Analysed: 111 (CFC‐fluticasone 50 μg); 108 (CFC‐fluticasone 100 μg); 113 (placebo) Gender (male): 61.3% Age, months, mean (range): 35.6 (24–47) Inclusion criteria: children 24‐47 months of age; at least 2 exacerbations in the year before screening; regular maintenance therapy for asthma required during the 6 weeks before screening and/or short‐acting β‐agonist therapy for relief of respiratory symptoms at least twice weekly during the 3 weeks before screening Exclusion criteria: history of life‐threatening asthma; upper or lower respiratory tract infection; systemic or moderate to high doses of inhaled corticosteroids within 8 weeks; more than 2 courses of systemic corticosteroids during the previous 6 months; an investigational drug within 30 days of screening Previous regular use of ICS: 24% in the fluticasone 50 group; 29% in the fluticasone 100 group; 35% in the placebo group |
|
| Interventions | Test group:
Control group: matching placebo All drugs were given twice daily via CFC‐MDI with a valved holding chamber. Treatment duration was 12 weeks |
|
| Outcomes |
Height was measured in triplicate at approximately the same time of day using a calibrated stadiometer at each visit |
|
| Notes | Treatment compliance was not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was stratified by age |
| Allocation concealment (selection bias) | Unclear risk | No details provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Use of matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Use of matching placebo |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Withdrawal rate was 18/219 (8.2%) in the fluticasone group and 18/113 (15.9%) in the placebo group |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but the published reports include all expected outcomes, including those that were pre‐specified |
| Other bias | Low risk | Study appears to be free of other sources of bias |
BDP: budesonide dipropionate.
BUD: budesonide.
CFC: chlorofluorocarbon.
CIC: ciclesonide.
DPI: dry powder inhaler.
DSCG: disodium cromoglycate
DXA: dual‐energy x‐ray absorptiometry.
FENO: fractional exhaled nitric oxide.
FEV1: forced expiratory volume in one second.
FP: fluticasone propionate.
HFA: hydrofluoroalkane.
HPA: hypothalamic‐pituitary‐adrenal.
ICS: inhaled corticosteroids.
MDI: metered‐dose inhaler.
PC20: 20%.
PEF: peak expiratory flow.
SCG: sodium cromoglycate.
SD: standard deviation.
SDS: standard deviation score.
SE: standard error.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agertoft 1994 | Not a randomised controlled trial |
| Baxter‐Jones 2000 | Control group neither placebo nor non‐steroidal drugs |
| Brand 2011 | Growth outcomes not available |
| CAMP 1999 | Not a randomised controlled trial |
| Heuck 1998 | Control group neither placebo nor non‐steroidal drugs |
| Kerrebijn 1976 | Not a randomised controlled trial |
| Martinati 1998 | Not a randomised controlled trial |
| Merkus 1993 | Not a randomised controlled trial |
| Rao 1999 | Growth outcomes not available |
| Teper 2004 | Growth outcomes not available |
| Tinkelman 1996 | Not a randomised controlled trial |
| Verberne 1998 | Control group neither placebo nor non‐steroidal drugs |
| Xu 2005 | Control group neither placebo nor non‐steroidal drugs |
Differences between protocol and review
We reworded the objectives, but they retained their original meaning.
We added adult height (cm) as a post hoc secondary outcome.
We planned to use the following subgroups for dose: dosage of ICS (HFA‐beclomethasone equivalent): low daily dose (≤ 200 μg), medium daily dose (> 200 to 400 μg) and high daily dose (> 400 μg). However, we eventually used the dose recommendations provided by the GINA guidelines.
We also conducted post hoc sensitivity analyses while excluding trials in which withdrawal rates were higher than 20%, those in which non‐steroidal drugs rather than placebo were used as controls and trials in which the growth data used for analysis were extracted from figures or obtained from a portion of the treatment period or participant group rather than from the entire treatment period or participant group. We also conducted post hoc subgroup analyses at equivalent doses and within molecules.
We included a summary of findings table post hoc.
Contributions of authors
Linjie Zhang (LZ) conceived of the study and wrote the protocol and the review. Sílvio OM Prietsch (SOMP) and Francine M Ducharme (FMD) provided input in writing the protocol and the review, and approved the final draft of the review.
LZ and SOMP were responsible for study selection, quality assessment, data collection and data analysis.
Sources of support
Internal sources
-
Faculty of Medicine, Federal University of Rio Grande, Brazil.
Part time for research
External sources
No sources of support supplied
Declarations of interest
No conflicts of interest are known.
Edited (no change to conclusions)
References
References to studies included in this review
Allen 1998 {published data only}
- Allen DB, Bronsky EA, LaForce CF, Nathan RA, Tinkelman DG, Vandewalker ML, et al. Growth in asthmatic children treated with fluticasone propionate. Fluticasone Propionate Asthma Study Group. The Journal of Pediatrics 1998;132(3 Pt 1):472‐7. [DOI] [PubMed] [Google Scholar]
Becker 2006 {published data only}
- Becker AB, Kuznetsova O, Vermeulen J, Soto‐Quiros ME, Young B, Reiss TF, et al. Linear growth in prepubertal asthmatic children treated with montelukast, beclomethasone, or placebo: a 56‐week randomized double‐blind study. Annals of Allergy, Asthma & Immunology 2006;96(6):800‐7. [DOI] [PubMed] [Google Scholar]
Bensch 2011 {published data only}
- Bensch GW, Greos LS, Gawchik S, Kpamegan E, Newman KB. Linear growth and bone maturation are unaffected by 1 year of therapy with inhaled flunisolide hydrofluoroalkane in prepubescent children with mild persistent asthma: a randomized, double‐blind, placebo‐controlled trial. Annals of Allergy, Asthma & Immunology 2011;107(4):323‐9. [DOI] [PubMed] [Google Scholar]
Bisgaard 2004 {published data only}
- Bisgaard H, Allen D, Milanowski J, Kalev I, Willits L, Davies P. Twelve‐month safety and efficacy of Inhaled fluticasone propionate in children aged 1 to 3 years with recurrent wheezing. Pediatrics 2004;113:e87. [DOI] [PubMed] [Google Scholar]
CAMP 2000 {published data only}
- Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, et al. Effect of inhaled glucocorticoids in childhood on adult height. New England Journal of Medicine 2012;367:904‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The childhood asthma management program research group. Long‐term effects of budesonide or nedocromil in children with asthma. The New England Journal of Medicine 2000;343(15):1054‐63. [DOI] [PubMed] [Google Scholar]
Doull 1995 {published data only}
- Doull I, Freezer N, Holgate S. Osteocalcin, growth, and inhaled corticosteroids: a prospective study. Archives of Disease in Childhood 1996;74:497‐501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doull IJ, Freezer NJ, Holgate ST. Growth of prepubertal children with mild asthma treated with inhaled beclomethasone dipropionate. American Journal of Respirtory and Critical Care Medicine 1995;151(1):1715‐9. [DOI] [PubMed] [Google Scholar]
Gillman 2002 {published data only}
- Gillman SA, Anolik R, Schenkel E, Newman K. One‐year trial on safety and normal linear growth with flunisolide HFA in children with asthma. Clinical Pediatrics 2002;41(5):333‐40. [DOI] [PubMed] [Google Scholar]
Gradman 2010 {published data only}
- Gradman J, Wolthers OD. A randomized trial of lower leg and height growth in children with asthma treated with inhaled budesonide from a new dry powder inhaler. Pediatric Allergy and Immunology 2010;21(1 Pt 2):e206‐12. [DOI] [PubMed] [Google Scholar]
Guilbert 2006 {published data only}
- Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, et al. Long‐term inhaled corticosteroids in preschool children at high risk for asthma. The New England Journal of Medicine 2006;354(19):1985‐97. [DOI] [PubMed] [Google Scholar]
Jonasson 2000 {published data only}
- Jónasson G, Carlsen KH, Jonasson C, Mowinckel P. Low‐dose inhaled budesonide once or twice daily for 27 months in children with mild asthma. Allergy 2000;55(8):740‐8. [DOI] [PubMed] [Google Scholar]
Kannisto 2000 {published data only}
- Kannisto S, Korppi M, Remes K, Voutilainen R. Adrenal suppression, evaluated by a low dose adrenocorticotropin test, and growth in asthmatic children treated with inhaled steroids. The Journal of Clinical Endocrinology and Metabolism 2000;85(2):652‐7. [DOI] [PubMed] [Google Scholar]
- Kannisto S, Voutilainen R, Remes K, Korppi M. Efficacy and safety of inhaled steroid and cromone treatment in school‐age children: a randomized pragmatic pilot study. Pediatric Allergy and Immunology 2001;13:24‐30. [DOI] [PubMed] [Google Scholar]
Martinez 2011 {published data only}
- Martinez FD, Chinchilli VM, Morgan WJ, Boehmer SJ, Lemanske RF Jr, Mauger DT, et al. Use of beclomethasone dipropionate as rescue treatment for children with mild persistent asthma (TREXA): a randomised, double‐blind, placebo‐controlled trial. Lancet 2011;377(9766):650‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pauwels 2003 {published data only}
- Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double‐blind trial. Lancet 2003;361(9363):1071‐6. [DOI] [PubMed] [Google Scholar]
Pedersen 2010 {published data only}
- Pedersen S, Potter P, Dachev S, Bosheva M, Kaczmarek J, Springer E, et al. Efficacy and safety of three ciclesonide doses vs placebo in children with asthma: the RAINBOW study. Respiratory Medicine 2010;104:1618‐28. [DOI] [PubMed] [Google Scholar]
Price 1997 {published data only}
- Price JF, Russell G, Hindmarsh PC, Weller P, Heaf DP, Williams J. Growth during one year of treatment with fluticasone propionate or sodium cromoglycate in children with asthma. Pediatric Pulmonology 1997;24(3):178‐86. [DOI] [PubMed] [Google Scholar]
Roux 2003 {published data only}
- Roux C, Kolta S, Desfougères JL, Minini P, Bidat E. Long‐term safety of fluticasone propionate and nedocromil sodium on bone in children with asthma. Pediatrics 2003;111(6 Pt 1):e706‐13. [DOI] [PubMed] [Google Scholar]
Simons 1997 {published data only}
- Simons FE. A comparison of beclomethasone, salmeterol, and placebo in children with asthma. Canadian Beclomethasone Dipropionate‐Salmeterol Xinafoate Study Group. New England Journal of Medicine 1997;337(23):1659‐65. [DOI] [PubMed] [Google Scholar]
Skoner 2008 {published data only}
- Skoner DP, Maspero J, Banerji D, Ciclesonide Pediatric Growth Study Group. Assessment of the long‐term safety of inhaled ciclesonide on growth in children with asthma. Pediatrics 2008;121(1):e1‐14. [DOI] [PubMed] [Google Scholar]
Skoner 2011 {published data only}
- Skoner DP, Meltzer EO, Milgrom H, Stryszak P, Teper A, Staudinger H. Effects of inhaled mometasone furoate on growth velocity and adrenal function: a placebo‐controlled trial in children 4‐9 years old with mild persistent asthma. Journal of Asthma 2011;48(8):848‐59. [DOI] [PubMed] [Google Scholar]
Sorkness 2007 {published data only}
- Sorkness CA, Lemanske RF Jr, Mauger DT, Boehmer SJ, Chinchilli VM, Martinez FD, et al. Long‐term comparison of 3 controller regimens for mild‐moderate persistent childhood asthma: the Pediatric Asthma Controller Trial. Journal of Allergy and Clinical Immunology 2007;119(1):64‐72. [DOI] [PubMed] [Google Scholar]
Storr 1986 {published data only}
- Storr J, Lenney CA, Lenney W. Nebulised beclomethasone dipropionate in preschool asthma. Archives of Disease in Childhood 1986;61:270‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tinkelman 1993 {published data only}
- Tinkelman DG, Reed CE, Nelson HS, Offord KP. Aerosol beclomethasone dipropionate compared with theophylline as primary treatment of chronic, mild to moderately severe asthma in children. Pediatrics 1993;92(1):64‐77. [PubMed] [Google Scholar]
Turpeinen 2008 {published data only}
- Turpeinen M, Nikander K, Pelkonen AS, Syvänen P, Sorva R, Raitio H, et al. Daily versus as‐needed inhaled corticosteroid for mild persistent asthma (the Helsinki early intervention childhood asthma study). Archives of Disease in Childhood 2008;93(8):654‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verberne 1997 {published data only}
- Verberne AA, Frost C, Roorda RJ, Laag H, Kerrebijn KF. One year treatment with salmeterol compared with beclomethasone in children with asthma. The Dutch Paediatric Asthma Study Group. American Journal of Respiratory and Critical Care Medicine 1997;156(3 Pt 1):688‐95. [DOI] [PubMed] [Google Scholar]
Wasserman 2006 {published data only}
- Wasserman RL, Baker JW, Kim KT, Blake KV, Scott CA, Wu W, et al. Efficacy and safety of inhaled fluticasone propionate chlorofluorocarbon in 2‐ to 4‐year‐old patients with asthma: results of a double‐blind, placebo‐controlled study. Annals of Allergy, Asthma & Immunology 2006;96(6):808‐18. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agertoft 1994 {published data only}
- Agertoft L, Pedersen S. Effects of long‐term treatment with an inhaled corticosteroid on growth and pulmonary function in asthmatic children. Respiratory Medicine 1994;88:373‐81. [DOI] [PubMed] [Google Scholar]
Baxter‐Jones 2000 {published data only}
- Baxter‐Jones AD, Helms PJ, Russell G, Grant A, Ross S, Cairns JA, et al. Early asthma prophylaxis, natural history, skeletal development and economy (EASE): a pilot randomised controlled trial. Health Technology Assessment 2000;4(28):1‐89. [PubMed] [Google Scholar]
Brand 2011 {published data only}
- Brand PL, Luz García‐García M, Morison A, Vermeulen JH, Weber HC. Ciclesonide in wheezy preschool children with a positive asthma predictive index or atopy. Respiratory Medicine 2011;105:1588‐95. [DOI] [PubMed] [Google Scholar]
CAMP 1999 {published data only}
- CAMP Group. The Childhood Asthma Management Program (CAMP): design, rationale, and methods. Childhood Asthma Management Program Research Group. Controlled Clinical Trials 1999;20(1):91‐120. [PubMed] [Google Scholar]
Heuck 1998 {published data only}
- Heuck C, Wolthers OD, Kollerup G, Hansen M, Teisner B. Adverse effects of inhaled budesonide (800 micrograms) on growth and collagen turnover in children with asthma: a double‐blind comparison of once‐daily versus twice‐daily administration. Journal of Pediatrics 1998;133(5):608‐12. [DOI] [PubMed] [Google Scholar]
Kerrebijn 1976 {published data only}
- Kerrebijn KF. Beclomethasone dipropionate in long‐term treatment of asthma in children. Journal of Pediatrics 1976;89(5):821‐6. [DOI] [PubMed] [Google Scholar]
Martinati 1998 {published data only}
- Martinati LC, Bertoldo F, Gasperi E, Fortunati P, Lo Cascio V, Boner AL. Longitudinal evaluation of bone mass in asthmatic children treated with inhaled beclomethasone dipropionate or cromolyn sodium. Allergy 1998;53:705‐8. [DOI] [PubMed] [Google Scholar]
Merkus 1993 {published data only}
- Merkus PJ, Essen‐Zandvliet EE, Duiverman EJ, Houwelingen HC, Kerrebijn KF, Quanjer PH. Long‐term effect of inhaled corticosteroids on growth rate in adolescents with asthma. Pediatrics 1993;91(6):1121‐6. [PubMed] [Google Scholar]
Rao 1999 {published data only}
- Rao R, Gregson RK, Jones AC, Miles EA, Campbell MJ, Warner JO. Systemic effects of inhaled corticosteroids on growth and bone turnover in childhood asthma: a comparison of fluticasone with beclomethasone. European Respiratory Journal 1999;13(1):87‐94. [DOI] [PubMed] [Google Scholar]
Teper 2004 {published data only}
- Teper AM, Colom AJ, Kofman CD, Maffey AF, Vidaurreta SM, Bergadá I. Effects of inhaled fluticasone propionate in children less than 2 years old with recurrent wheezing. Pediatric Pulmonology 2004;37:111‐5. [DOI] [PubMed] [Google Scholar]
Tinkelman 1996 {published data only}
- Tinkelman D. Theophylline therapy for children with asthma. European Respiratory Review 1996;6(34):79‐83. [Google Scholar]
Verberne 1998 {published data only}
- Verberne AA, Frost C, Duiverman EJ, Grol MH, Kerrebijn KF. Addition of salmeterol versus doubling the dose of beclomethasone in children with asthma. The Dutch Asthma Study Group. American Journal of Respiratory and Critical Care Medicine 1998;158(1):213‐9. [DOI] [PubMed] [Google Scholar]
Xu 2005 {published data only}
- Xu Z, Chen HY, Zhang SY, Wang XL, He CR, Qiao YX. Efficacy and safety of corticosteroid inhalation in the treatment of childhood asthma. Chinese Journal of Contemporary Pediatrics 2005;7(1):47‐50. [Google Scholar]
Additional references
Adams 2011a
- Adams NP, Bestall JC, Lasserson TJ, Jones P, Cates CJ. Fluticasone versus placebo for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003135.pub4] [DOI] [Google Scholar]
Adams 2011b
- Adams NP, Bestall JB, Malouf R, Lasserson TJ, Jones PW. Beclomethasone versus placebo for chronic asthma. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD002738.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Adams 2011c
- Adams NP, Bestall JB, Jones PW. Budesonide versus placebo for chronic asthma in children and adults. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003274] [DOI] [Google Scholar]
Agertoft 2000
- Agertoft L, Pedersen S. Effect of long‐term treatment with inhaled budesonide on adult height in children with asthma. New England Journal of Medicine 2000;343:1064‐9. [DOI] [PubMed] [Google Scholar]
Allen 1994
- Allen DB, Mullen M, Mullen B. A meta‐analysis of the effect of oral and inhaled corticosteroids on growth. Journal of Allergy and Clinical Immunology 1994;93(6):967‐76. [DOI] [PubMed] [Google Scholar]
Allen 1999
- Allen DB. Limitations of short‐term studies in predicting long‐term adverse effects of inhaled corticosteroids. Allergy 1999;54:29‐34. [DOI] [PubMed] [Google Scholar]
Allen 2002
- Allen DB. Safety of inhaled corticosteroids in children. Pediatric Pulmonology 2002;33:208‐20. [DOI] [PubMed] [Google Scholar]
Allen 2006
- Allen DB. Effects of inhaled steroids on growth, bone metabolism, and adrenal function. Advances in Pediatrics 2006;53:101‐10. [DOI] [PubMed] [Google Scholar]
Asher 2010
- Asher MI. Recent perspectives on global epidemiology of asthma in childhood. Allergologia et Immunopathologia 2010;38(2):83‐7. [DOI] [PubMed] [Google Scholar]
Axelsson 2013
- Inge A, Prietsch SOM, Zhang L. Inhaled corticosteroids in children with persistent asthma: effects of different drugs and delivery devices on growth. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD010126] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barnes 2006
- Barnes PJ. How corticosteroids control inflammation: Quintiles Prize Lecture 2005. British Journal of Pharmacology 2006;148(3):245‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braman 2006
- Braman SS. The global burden of asthma. Chest 2006;130:4S‐12S. [DOI] [PubMed] [Google Scholar]
Brand 2001
- Brand PLP. Inhaled corticosteroids reduce growth. Or do they?. European Respiratory Journal 2001;17:287‐94. [DOI] [PubMed] [Google Scholar]
BTS & SIGN 2012
- British Thoracic Society and Scottish Intercollegiate Guidelines Network. British Guideline on the Management of Asthma. http://www.brit‐thoracic.org.uk/Portals/0/Guidelines/ AsthmaGuidelines/ sign101%20Jan%202012.pdf. (accessed 26 April 2013).
Bush 2004
- Bush A. Phenotype specific treatment of asthma in children. Paediatric Respiratory Reviews 2004;5(Supplement 1):S93‐S101. [DOI] [PubMed] [Google Scholar]
Busse 2001
- Busse WW, Lemanske RF. Asthma. New England Journal of Medicine 2001;344(5):350‐62. [DOI] [PubMed] [Google Scholar]
Carlsen 2002
- Carlsen KH, Gerritsen J. Inhaled steroids in children: adrenal suppression and growth impairment. European Respiratory Journal 2002;19:985‐8. [DOI] [PubMed] [Google Scholar]
Chauhan 2013
- Chauhan BF, Chartrand C, Ducharme FM. Intermittent versus daily inhaled corticosteroids for persistent asthma in children and adults. Cochrane Database of Systematic Reviews 2013, Issue 2. [DOI: 10.1002/14651858.CD009611.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Colice 2000
- Colice GL. Comparing inhaled corticosteroids. Respiratory Care 2000;45(7):846‐53. [PubMed] [Google Scholar]
Covar 2003
- Covar RA, Szefler SJ, Martin RJ, Sundstrom DA, Silkoff PE, Murphy J, et al. Relations between exhaled nitric oxide and measures of disease activity among children with mild‐to‐moderate asthma. Journal of Pediatrics 2003;142(5):469‐75. [DOI] [PubMed] [Google Scholar]
Creese 2001
- Creese KH, Doull IJ. Effects of inhaled corticosteroids on growth in asthmatic children. Current Allergy and Asthma Reports 2001;1(2):122‐6. [DOI] [PubMed] [Google Scholar]
Djukanovic 1992
- Djukanović R, Wilson JW, Britten KM, Wilson SJ, Walls AF, Roche WR, et al. Effect of an inhaled corticosteroid on airway inflammation and symptoms in asthma. American Review of Respiratory Disease 1992;145(3):669‐74. [DOI] [PubMed] [Google Scholar]
Efthimiou 1998
- Efthimiou J, Barnes PJ. Effect of inhaled corticosteroids on bones and growth. European Respiratory Journal 1998;11:1167‐77. [DOI] [PubMed] [Google Scholar]
FDA 1998
- US Food, Drug Administration (FDA). FDA requires new pediatric labelling for inhaled, intranasal corticosteroids. FDA Talk Paper November 9, 1998.
FDA 2007
- FDA. Guidance for Industry. Orally inhaled and intranasal corticosteroids: evaluation of the effects on growth in children. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm071968.pdf (accessed 16 December 2013).
Ferguson 1999
- Ferguson AC, Spier S, Manjra A, Versteegh FG, Mark S, Zhang P. Efficacy and safety of high‐dose inhaled steroids in children with asthma: a comparison of fluticasone propionate with budesonide. Journal of Pediatrics 1999;134(4):422‐7. [DOI] [PubMed] [Google Scholar]
GINA 2012
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention 2012 (update). www.ginasthma.com.org (accessed 22 April 2012).
GINA 2014
- Global Initiative for Asthma (GINA). Global strategy for asthma management and prevention, revised 2014. www.ginasthma.com.org (accessed 01 May 2014).
Hedlin 1998
- Hedlin G, Ingemansson M, Brännegord M, Marcus C, Stierna P. A study of children with asthma treated with inhaled corticosteroids (ICS) with or without growth retardation. Journal of Allergy and Clinical Immunology 1998;101:S13. [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analysis. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. www.cochrane‐handbook.org, 2011. [Google Scholar]
Hoekx 1996
- Hoekx JCM, Hedlin G, Pedersen W, Sorva R, Hollingworth K, Efthimiou J. Fluticasone propionate compared with budesonide: a double‐blind trial in asthmatic children using powder devices at a dosage of 400 μg/day. European Respiratory Journal 1996;9:2263‐72. [DOI] [PubMed] [Google Scholar]
Hogger 2003
- Hogger P. Dose response and therapeutic index of inhaled corticosteroids in asthma. Current Opinion in Pulmonary Medicine 2003;9:1‐8. [DOI] [PubMed] [Google Scholar]
ISAAC 1998
- ISAAC Steering Committee. Worldwide variations in the prevalence of asthma symptoms: the International Study of Asthma and Allergies in Childhood (ISAAC). European Respiratory Journal 1998;12:315‐35. [DOI] [PubMed] [Google Scholar]
Juniper 1990
- Juniper EF, Kline PA, Vanzieleghem MA, Ramsdale EH, O'Byrne PM, Hargreave FE. Effect of long‐term treatment with an inhaled corticosteroid (budesonide) on airway hyperresponsiveness and clinical asthma in nonsteroid‐dependent asthmatics. American Review of Respiratory Disease 1990;142(4):832‐6. [DOI] [PubMed] [Google Scholar]
Karlberg 1993
- Karlberg J, Gelander L, Albertsson‐Wikland K. Distinctions between short‐ and long‐term human growth studies. Acta Paediatrics 1994;83:777‐8. [DOI] [PubMed] [Google Scholar]
Kelly 2012
- Kelly HW, Sternberg AL, Lescher R, Fuhlbrigge AL, Williams P, Zeiger RS, et al. Effect of inhaled glucocorticoids in childhood on adult height. New England Journal of Medicine 2012;367(10):904‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 2009
- Lai C, Beasley R, Crane J, Foliaki S, Shah J, Weiland S. Global variation in the prevalence and severity of asthma symptoms: Phase Three of the International Study of Asthma and Allergies in Childhood (ISAAC). Thorax 2009;64(6):476‐83. [DOI] [PubMed] [Google Scholar]
Leung 2003
- Leung DY, Bloom JW. Update on glucocorticoid action and resistance. Journal of Allergy and Clinical Immunology 2003;111(1):3‐22. [DOI] [PubMed] [Google Scholar]
Lougheed 2012
- Lougheed MD, Lemiere C, Ducharme FM, Licskai C, Del SD, Rowe BH, et al. Canadian Thoracic Society 2012 guideline update: diagnosis and management of asthma in preschoolers, children and adults. Canadian Respiratory Journal 2012;19(2):127‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Masoli 2004
- Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma (GINA) Program. The global burden of asthma: executive summary of the GINA Dissemination Committee report. Allergy 2004;59(5):469‐78. [DOI] [PubMed] [Google Scholar]
NHLBI 2007
- National Heart Lung and Blood Institute. Guidelines for the diagnosis and management of asthma (EPR‐3). www.nhlbi.nih.gov/guidelines/asthma (accessed 20 June 2011).
Olivieri 1997
- Olivieri D, Chetta A, Donno M, Bertorelli G, Casalini A, Pesci A, et al. Effect of short‐term treatment with low‐dose inhaled fluticasone propionate on airway inflammation and remodeling in mild asthma: a placebo‐controlled study. American Journal of Respiratory and Critical Care Medicine 1997;155(6):1864‐71. [DOI] [PubMed] [Google Scholar]
Pedersen 2001
- Pedersen S. Do inhaled corticosteroids inhibit growth in children?. Americian Journal of Respiratory and Critical Care Medicine 2001;164(4):521‐35. [DOI] [PubMed] [Google Scholar]
Price 2002
- Price J, Hindmarsh P, Hughes S, Efthimiou J. Evaluating the effects of asthma therapy on childhood growth: what can be learnt from the published literature?. European Respiratory Journal 2002;19:1179‐93. [DOI] [PubMed] [Google Scholar]
Pruteanu 2012
- Pruteanu A, Chauhan BF, Zhang L, Prietsch SOM, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: dose‐response effects on growth. Cochrane Database of Systematic Reviews In press. [10.1002/14651858.CD009878] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan) Version 5.0. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2008.
Salvatoni 2003
- Salvatoni A, Piantanida E, Nosetti L, Nespoli L. Inhaled corticosteroids in childhood asthma: long‐term effects on growth and adrenocortical function. Paediatric Drugs 2003;5(6):351‐61. [DOI] [PubMed] [Google Scholar]
Sharek 2000a
- Sharek PJ, Bergman D, Francine D. Beclomethasone for asthma in children: effects on linear growth. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD001282] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sharek 2000b
- Sharek PJ, Bergman DA. The effect of inhaled steroids on the linear growth of children with asthma: a meta‐analysis. Pediatrics 2000;106:e8. [DOI] [PubMed] [Google Scholar]
Sizonenko 2002
- Sizonenko PC. Effects of inhaled or nasal glucocorticosteroids on adrenal function and growth. Journal of Pediatric Endocrinology and Metabolism 2002;15(1):5‐26. [DOI] [PubMed] [Google Scholar]
Sobande 2008
- Sobande PO, Kercsmar CM. Inhaled corticosteroids in asthma management. Respiratory Care 2008;53(5):625‐33. [PubMed] [Google Scholar]
Stein 2004
- Stein RT, Martinez FD. Asthma phenotypes in childhood: lessons from epidemiological approach. Paediatric Respiratory Reviews 2004;5(2):155‐61. [DOI] [PubMed] [Google Scholar]
Suissa 2000
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low‐dose inhaled corticosteroids and the prevention of death from asthma. New England Journal of Medicine 2000;343(5):332‐6. [DOI] [PubMed] [Google Scholar]
Van Essen‐Zandvliet 1992
- Essen‐Zandvliet EE, Hughes MD, Waalkens HJ, Duiverman EJ, Pocock SJ, Kerrebijn KF. Effects of 22 months of treatment with inhaled corticosteroids and/or beta‐2‐agonists on lung function, airway responsiveness, and symptoms in children with asthma. The Dutch Chronic Non‐specific Lung Disease Study Group. American Review of Respiratory Disease 1992;146(3):547‐54. [DOI] [PubMed] [Google Scholar]
Van Rens 1999
- Rensen EL, Straathof KC, Veselic‐Charvat MA, Zwinderman AH, Bel EH, Sterk PJ. Effect of inhaled steroids on airway hyperresponsiveness, sputum eosinophils, and exhaled nitric oxide levels in patients with asthma. Thorax 1999;54(5):403‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witzmann 2000
- Witzmann KB, Fink RJ. Inhaled corticosteroids in childhood asthma: growing concerns. Drugs.2000;59 2000;59(Suppl 1):9‐14. [DOI] [PubMed] [Google Scholar]
Wolthers 1993
- Wolthers OD, Pedersen S. Short‐term growth during treatment with inhaled fluticasone propionate and beclomethasone dipropionate. Archives of Disease in Childhood 1993;68(5):673‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolthers 1997
- Wolthers O, Hansen M, Juul A, Nielsen HK, Pedersen S. Knemometry, urine cortisol excretion and measures of the insulin‐like growth factor axis and collagen turnover in the assessment of systemic activity of inhaled corticosteroids. Pediatric Research 1997;41:44‐50. [DOI] [PubMed] [Google Scholar]
Wolthers 2001
- Wolthers OD. Inhaled corticosteroids and growth. Journal of Pediatric Endocrinology and Metabolism 2001;14(Suppl 6):1487‐90. [PubMed] [Google Scholar]
Yiallouros 1997
- Yiallouros PK, Milner AD, Conway E, Honour JW. Adrenal function and high dose inhaled corticosteroids for asthma. Archives of Disease in Childhood 1997;76:405‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]


