Abstract
Background
Toremifene (TOR) and tamoxifen (TAM) can both be used as treatments for advanced breast cancer.
Objectives
To compare the efficacy and safety of TOR with TAM in patients with advanced breast cancer.
Search methods
The Cochrane Breast Cancer Group's Specialised Register was searched (1 July 2011) using the codes for "toremifene", "fareston", "tamoxifen, "nolvadex, and "breast cancer". We also searched MEDLINE (via PubMed) (from inception to 1 July 2011), EMBASE (via Ovid) (from inception to 1 July 2011), The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 7, 2011), and the WHO International Clinical Trials Registry Platform search portal (1 July 2011). In addition, we screened the reference lists of relevant trials or reviews.
Selection criteria
Randomised controlled trials (RCTs) that compared the efficacy and safety, or both of TOR with TAM in women with advanced breast cancer. Trials that provided sufficient data on one of the following items: objective response rate (ORR), time to progression (TTP), overall survival (OS), and adverse events, were considered eligible for inclusion.
Data collection and analysis
Studies were assessed for eligibility and quality. Two review authors independently extracted the following details: first author, publication year, country, years of follow‐up, treatment arms, intention‐to‐treat (ITT) population size, menopausal status of patients, hormone receptor status, response criteria, efficacy and safety outcomes of TOR and TAM arms. Hazard ratios (HR) were derived for time‐to‐event outcomes, where possible, and response and adverse events were analysed as dichotomous variables. We used a fixed‐effect model for meta‐analysis unless there was significant between‐study heterogeneity.
Main results
A total of 2061 patients from seven RCTs were included for final analysis, with 1226 patients in the TOR group and 835 patients in the TAM group. The ORR for the TOR group was 25.8% (316/1226) whereas, the ORR for the TAM group was 26.9% (225/835). The pooled risk ratio (RR) suggested that the ORRs were not statistically different between the two groups (RR 1.02, 95% confidence interval (CI) 0.88 to 1.18, P = 0.83). The median TTP was 6.1 months for the TOR group and 5.8 months for the TAM group. The median OS was 27.8 months for the TOR group and 27.6 months for the TAM group. There were no significant differences in TTP and OS between the two therapeutic groups (for TTP: HR 1.08, 95% CI 0.94 to 1.24; for OS: HR 1.02, 95% CI 0.86 to 1.20). The frequencies of most adverse events were also similar in the two groups, while headache seemed to occur less in the TOR group than in the TAM group (RR 0.14, 95% CI 0.03 to 0.74, P = 0.02). There was no significant heterogeneity between studies in most of the above meta‐analyses. Sensitivity analysis did not alter the results.
Authors' conclusions
TOR and TAM are equally effective and the safety profile of the former is at least not worse than the latter in the first‐line treatment of patients with advanced breast cancer. Thus, TOR may serve as a reasonable alternative to TAM when anti‐oestrogens are applicable but TAM is not the preferred choice for some reason.
Plain language summary
Toremifene versus tamoxifen for advanced breast cancer
Breast cancer is the most common cancer in women. When it has spread beyond the breast, it is called advanced breast cancer. Treatments for advanced breast cancer include chemotherapy, endocrine therapy and possibly surgery and radiation therapy. Of endocrine therapy, tamoxifen (TAM) is the oldest and most‐prescribed selective oestrogen‐receptor modulator. However, several significant adverse effects have been described after long‐term TAM treatment. Toremifene (TOR), which can also be used to treat advanced breast cancer, has a mechanism similar to that of TAM. The objective of this review was to compare TOR with TAM in terms of overall survival, response to treatment, time to progression, and adverse effects.
Seven eligible studies were identified, all of which provided information on response to treatment (in 2061 patients), five on progression‐free survival (in 1436 patients) and four on overall survival (in 1374 patients). The trials were generally old (conducted between late 1980s and early 1990s) and were of modest quality.
Based on the data from these trials, 25.8% of the patients in the TOR group responded to the treatment, compared with 26.9% in the TAM group. The cancers of 50% of the patients in the TOR group had progressed after 6.1 months, compared with 5.8 months in the TAM group. Half of the patients in the TOR group survived longer than 27.8 months, compared with 27.6 months in the TAM group. The risk for progression and death in the TOR group was not significantly different from that in the TAM group. The frequencies of most adverse events were also similar in the two groups, except that the number of headaches occurring in the TOR group was only about one‐seventh of that in the TAM group. However, considering the results of other large trials, we cannot exclude the possibility that this is purely a play of chance. Due to the lack of data, no conclusions can be made as to the long‐term adverse effects achieved with either treatment.
The evidence from this review suggests that TOR and TAM are equally effective and the safety profile of the former is at least not worse than the latter in the first‐line treatment of patients with advanced breast cancer. Thus, TOR may serve as a reasonable alternative to TAM when anti‐oestrogens are applicable but TAM is not the preferred choice for some reason.
Background
Description of the condition
Breast cancer is the most common cancer among women worldwide with over one million new cases each year (Cox 2006). It causes an average of 400,000 deaths each year (Anderson 2008). In 2010, the new cases were projected as 1.5 million globally (Anderson 2008). Despite advances in treatment, breast cancer remains a leading cause of death worldwide (Jemal 2008; Parkin 2002).
Approximately 70% of the breast cancers, when diagnosed, are steroid hormone receptor positive. Some breast cancer therapies are targeted at the oestrogen receptor alpha (ERα), including tamoxifen, toremifene, and fulvestrant. Other hormone therapies target oestrogen production either by aromatase inhibitors, such as letrozole, anastrozole, and exemestane, or by ovarian suppression (Lange 2011). Of note, both the therapeutic efficacy and adverse effects of these drugs vary dramatically from patient to patient. Nearly half of steroid receptor‐positive breast cancers will acquire resistance to these therapies so that effective stabilisation might require complementary therapies rather than single agent hormone therapies.
Description of the intervention
Tamoxifen (TAM), a non‐steroidal, triphenylethylene‐based anti‐oestrogen with tissue specific oestrogenic (agonist) and anti‐oestrogenic (antagonist) activity, is used to treat advanced oestrogen receptor‐positive breast cancer following appropriate chemotherapy, surgery, radiation or combinations of treatment (Clemons 2002; EBCTCG 1992; Jaiyesimi 1995). Currently, this agent has proved to be effective as an adjuvant treatment for breast cancer after mastectomy or conservative surgery (EBCTCG 1998; EBCTCG 2005) and it has also been approved for the chemoprevention of breast cancer in women at high risk of developing the disease (Fisher 1998; Powles 1989). However, several significant adverse effects such as thromboembolic events, ocular changes and endometrial carcinoma have been described after long‐term TAM treatment (EBCTCG 2005; Osborne 1998). During the last three decades, several alternative hormonal therapies have become available as first‐line or adjuvant treatments for breast cancer.
Toremifene (TOR), like TAM, is a non‐steroidal triphenylethylene selective oestrogen receptor‐modulator. This agent binds to the oestrogen receptor (ER) with oestrogenic or anti‐oestrogenic properties depending on the dose, duration of treatment, target organ or endpoint used (Kangas 1986; Kangas 1992). Preclinical data suggest that TOR has a lower oestrogenic to anti‐oestrogenic ratio than TAM, which might explain some of the differences between these two agents (di Salle 1990). TOR has been found to have an efficacy similar to that of TAM in advanced breast cancer and a generally similar side‐effect profile.
How the intervention might work
TAM works by competing with oestrogen to bind to ERs in breast cancer cells. The mechanism of anti‐oestrogens is thought to involve genomic and non‐genomic actions. Actions involving genes, including autocrine and paracrine growth factor secretion, oncogene expression and regulation of apoptosis, are considered to be mediated by ERs (Jordan 1978; Lahti 1994; Ramkumar 1995; Warri 1993). The inhibition of calcium metabolism and high‐conductivity chloride channels (Su 1985; Valverde 1993), inhibition of protein kinases and glycoprotein p170 (Chatterjee 1990; DeGregorio 1989), decline of membrane fluidity (Wiseman 1994) and influence on lipid peroxidation and anti‐oxidative enzymes (Ahotupa 1994; Thangaraju 1995) are non‐genomic actions.
Toremifene (TOR) is an oestrogen‐receptor modulator similar to TAM. It induces apoptosis and inhibits human breast cancer cells from entering mitosis (Warri 1993). TOR differs from TAM by the addition of a single chloride ion on a side chain. Preclinical data suggest that TOR has a lower oestrogenic to anti‐oestrogenic ratio than TAM (Hirsimaki 2002) resulting in different metabolism and a potentially more favourable toxicity profile.
Why it is important to do this review
To date, a number of independent randomised trials have compared the efficacy and safety of TOR with those of TAM in patients with advanced breast cancer (Gershanovich 1997; Hayes 1995; Milla‐Santos 2001; Nomura 1993; Pyrhonen 1997; Stenbygaard 1993). Due to the differences in agent doses, treatment settings and study designs of the studies, we will perform a meta‐analysis to synthesise available evidence to compare the efficacy and safety of TOR with TAM in patients with advanced breast cancer. The results of this meta‐analysis should be of value to decision‐makers when they are faced with the choice of TOR versus TAM for patients with advanced breast cancer.
Objectives
To compare the efficacy and safety of toremifene (TOR) with tamoxifen (TAM) in patients with advanced breast cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) that compared toremifene (TOR) with tamoxifen (TAM) were eligible for inclusion.
We excluded the following studies.
Studies that involved mixed populations of women with early or advanced breast cancer for which data cannot be extracted only for women with advanced cancer (however, these studies were still eligible to be included in our discussion regarding the efficacy and safety of TOR and TAM).
Studies with outcomes that did not match the predefined outcomes.
Types of participants
Participants were women with a diagnosis of advanced breast cancer. The inclusion criteria was: histologically verified inoperable primary, metastatic, or recurrent breast cancer; measurable or evaluable disease according to World Health Organization (WHO) criteria.
Types of interventions
We included any studies that compared the efficacy and safety of TOR with TAM in women with advanced breast cancer. Other therapies were allowed as long as the participants were randomised to receive TOR or TAM. The doses of TOR ranged from 40 to 240 mg/day while the doses of TAM ranged from 20 to 40 mg/day.
Types of outcome measures
Primary outcomes
Overall survival (OS), defined as the length of time from the date of randomisation to death from any cause.
Secondary outcomes
Objective response rate (ORR), defined as the sum of the complete response (CR) and partial response (PR).
Time to progression (TTP), defined as the time between randomisation and the onset of relapse or disease progression.
Adverse effects such as hot flushes, sweating, nausea, voice changes, vaginal discharge, vaginal bleeding, vaginal dryness, vomiting, headache, thromboembolic events, cardiac events, ocular disorders and endometrial cancer.
Search methods for identification of studies
See: Breast Cancer Group methods used in reviews.
Systematic searches were performed using the following search terms: "toremifene", "fareston", "tamoxifen", "nolvadex", and "breast cancer". The search was limited to human studies. Studies published in all languages were included. All eligible studies were retrieved, and their bibliographies were checked for other relevant publications. When the same patient population was used in several publications, only the most recent, largest or complete study report was included in the meta‐analysis.
Electronic searches
We searched the following bibliographic database sources. (a) The Cochrane Breast Cancer Group's (CBCG) Specialised Register, which identified studies and codes references as outlined in the CBCG's module (www.mrw.interscience.wiley.com/cochrane/clabout/articles/BREASTCA/frame.html). Trials coded with the key words "toremifene", "fareston", "tamoxifen", "nolvadex", and "breast cancer" were extracted and considered for inclusion in the review. (b) MEDLINE (via PubMed) (from July 2008 to 1 July 2011). (c) EMBASE (via Ovid) (from July 2008 to 1 July 2011). (d) The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 7, 2011). (e) The WHO International Clinical Trials Registry Platform (ICTRP) search portal (http://apps.who.int/trialsearch/Default.aspx) for all prospectively registered and ongoing trials on 1 July 2011.
Searching other resources
Bibliographic searching We tried to identify further studies from reference lists of identified relevant trials or reviews. A copy of the full article for each reference reporting a potentially eligible trial was obtained, where possible. Where this was not possible, we initially planned to contact the trial authors for additional information. In the current version of this review, this did not actually happen as no additional studies other than those included were considered potentially eligible.
Data collection and analysis
Selection of studies
Two review authors (Chen Mao and Zu‐Yao Yang) independently scanned the title, abstract, or both sections of all publications retrieved using the above selection criteria to determine which studies were to be assessed further. All potentially relevant articles were investigated as full text. Where discrepancies in opinion existed, these were resolved by a third review author (Jin‐Ling Tang).
Data extraction and management
Two review authors (Zu‐Yao Yang and Jun‐Hua Zhou) independently extracted data from the included studies using forms designed by the author group. We reached consensus on all items. Whenever studies pertained to populations of patients that overlapped, only the study with the longest follow‐up and the largest number of patients was used in the final analysis. Where possible, the following data were collected from each study: first author, publication year, country, years of follow‐up, treatment arms, intention‐to‐treat (ITT) population size, menopausal status of patients, hormone receptor status, response criteria, efficacy outcomes of TOR and TAM arms (ORR, TTP, and OS) (see Characteristics of included studies). We resolved any differences in the extracted data by discussion with a third review author (Jin‐Ling Tang).
Assessment of risk of bias in included studies
Only randomised controlled trials were included. Further, the methodological quality of included randomised trials was assessed and reported using the criteria specified in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). The assessments were performed independently by two review authors (Chen Mao and Zu‐Yao Yang). We followed The Cochrane Collaboration's recommendation by using the 'Risk of bias' assessment tool for assessing risk of bias in each included study. 'Risk of bias' assessment comprised a description and a judgement for each entry in a 'Risk of bias' table, where each entry addressed a specific feature of the study.
We assessed the following methodological features: (1) sequence generation, (2) allocation sequence concealment, (3) blinding, (4) incomplete outcome data, (5) selective outcome reporting, and (6) other potential sources of bias.
If the selected studies had different levels of risk of bias, we performed a sensitivity analysis to assess the stability of the results based on 'Risk of bias' assessments.
Measures of treatment effect
For dichotomous data, the effect measures were expressed as risk ratios (RR) with 95% confidence intervals (CIs). The comparisons between the TOR and TAM arms with respect to CR, PR and ORR were measured by overall RRs with 95% CIs. A RR equal to one indicated no significant differences in the CR, PR or ORR between the two therapeutic groups; a RR greater than one indicated that the TOR group had a higher CR, PR or ORR than the TAM group; a RR less than one indicated that the TOR group had a lower CR, PR or ORR than the TAM group.
For time‐to‐event data, the effect measure was expressed as a hazard ratio (HR) with 95% CI. The comparisons with respect to TTP and OS were measured by overall HRs with 95% CI. An HR equal to one indicated no significant differences in the TTP and OS between the two therapeutic groups; an HR greater than one indicated that the TOR group had a shorter TTP and OS than the TAM group; an HR less than one indicated that the TOR group had a longer TTP and OS than the TAM group. If the HR and associated variances were not reported, we extracted data from Kaplan‐Meier curves.
Unit of analysis issues
We took into account the level at which randomisation occurred, such as for cross‐over trials, cluster‐randomised trials and multiple observations for the same outcome. We originally planned to re‐analyse studies with potential unit of analysis errors by calculating effective sample sizes, where possible (Higgins 2008). Such re‐analysis did not take place as no unit‐of analysis errors were identified in the included studies.
Dealing with missing data
Where possible, we planned to contact the original investigators to obtain relevant missing data. However, despite the presence of some missing data, we did not actually contact the original investigators as planned because all eligible studies turned out to be "old" ones (see Characteristics of included studies). No email addresses were provided, and it was highly possible that the mailing addresses have changed over the past two decades. Thus, we alternatively tried to complement our datasets with studies that were duplicates of the included ones. We carefully evaluated important numerical data such as screened, randomised patients; and ITT, as‐treated and per‐protocol (PP) populations.
Assessment of heterogeneity
Heterogeneity was checked by the Chi2 test (Cochran 1954) and the I2 statistic (Higgins 2003). The criteria for identification of heterogeneity was a P value less than 0.10 for the Chi2 test and an I2 statistic greater than 50%. When there was no statistical evidence for heterogeneity in effect sizes, we used the fixed‐effect model (Mantel 1959). When significant heterogeneity was identified, we used the random‐effects model (DerSimonian 1986) and explored sources of significant heterogeneity.
Assessment of reporting biases
An estimate of potential publication bias was carried out using a funnel plot. An asymmetric plot suggested a possible publication bias. Funnel plot asymmetry was assessed by the method of Egger's linear regression test using the standardised estimate of the size effect as the dependent variable and the inverse of the standard error as the independent variable. The significance of the intercept was determined by the t‐test suggested by Egger and P < 0.05 was considered representative of a statistically significant publication bias (Egger 1997).
Data synthesis
Data were summarised statistically, if available and of sufficient quality. We performed statistical analyses in accordance with the statistical guidelines referenced in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). For dichotomous outcomes, such as response rate, the Mantel‐Haenszel method was used to combined data from different studies using Review Manager software (RevMan). The pooled RR was estimated by a fixed‐effect model unless between‐study heterogeneity was found and could not be explained. In the case that heterogeneous results were combined, the random‐effects model (Dersimonian‐Laird method) was used. Similar principles applied to the combination of time‐to‐event data (OS and PFS), for which the effect measure was expressed as HR, using the Exp [(O‐E)/Var] method in Review Manager.
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses to assess the source of heterogeneity and the effects of related factors on clinical outcomes. Where there were sufficient studies, we performed subgroup analyses on the following:
effect of menopausal status on outcome measures;
effect of hormone receptor status on outcome measures;
effect of agent doses on outcome measures;
impact of line of treatment on outcome measures; and
impact of study quality on outcome measures.
Sensitivity analysis
If adequate data were available, we performed sensitivity analyses to assess the robustness of our results by repeating the analysis with the following adjustments:
repeating the analysis excluding studies with high risk of bias;
repeating the analysis each time excluding a single study to determine the influence of the individual data set on the pooled results (Tobias 1999).
We also tested the robustness of the results by repeating the analysis using different measures of effect size (risk ratio, odds ratio etc) and different statistical models (fixed‐effect and random‐effects models).
Results
Description of studies
Results of the search
The literature search was conducted on 1 July 2011. Our search strategy identified 697 relevant references from PubMed, EMBASE and CENTRAL. Among them, 26 references, according to the inclusion criteria, were considered as potentially eligible for the present review (see Figure 1 for details of the selection process).
1.
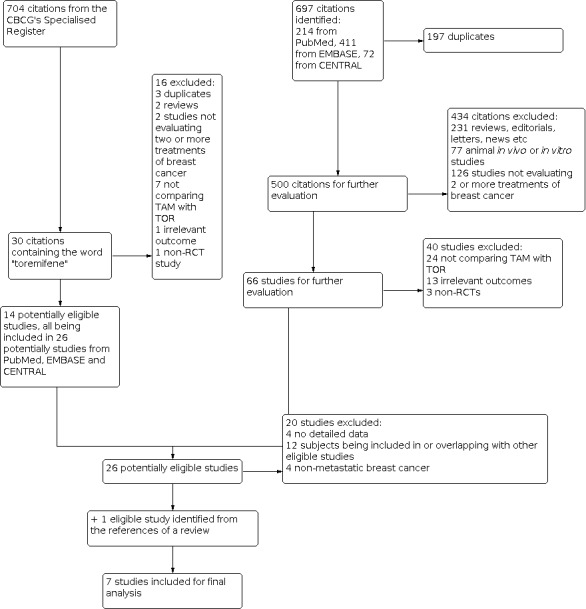
Study flow diagram
When the Cochrane Breast Cancer Group's Specialised Register was searched on 1 July 2011, it contained 704 references pertaining to clinical trials in breast cancer, of which 30 references were coded as "toremifene". Fourteen references were considered potentially eligible for the present meta‐analysis. All of them have been included in the 26 potentially eligible references identified from PubMed, EMBASE and CENTRAL.
Twenty of the aforementioned 26 references were further excluded, including four title‐only or abstract‐only references that were without extractable information, 12 studies whose data overlapped with or had been fully included in other studies, and four studies that were not investigating metastatic breast cancer patients (see: Characteristics of excluded studies), which left six studies eligible for this meta‐analysis. By checking the reference lists of relevant reviews, one more eligible study (Kaufmann 1993) was identified (from Pyrhonen 1999). Hence, seven studies were included in the final meta‐analysis.
Two studies (Hayes 1995; Gershanovich 1997) performed randomised three‐arm comparisons of two separate doses of TOR with TAM. For the analysis of response and adverse events data, we combined the low‐ and high‐dose TOR groups and presented a single comparison with TAM. However, this was not possible for the analysis of TTP and OS due to the lack of relevant data. Thus, we used only a single‐dose group (60 mg/day) from TOR. We chose this group because 60 mg/day was more often used in other studies. Using this dose group could help reduce the clinical heterogeneity across different studies.
Included studies
See: Characteristics of included studies; Table 1: Main characteristics of studies included in the meta‐analysis.
1. Main characteristics of studies included in the meta‐analysis.
| First author | Year | Country | Year of follow‐up | Treatment arms | Number of patients | Response criteria |
Menopausal status |
Receptor status |
||
| TOR | TAM | TOR | TAM | |||||||
| Gershanovich | 1997 | Russia, Latvia, Estonia | 1987‐1992 | 60 or 240 mg/day | 40 mg/day | 314 | 149 | WHO/ECOG | post‐ | pos or un |
| Hayes | 1995 | US, South Africa, and 4 other countries | 1988‐1991 | 60 or 200 mg/day | 20 mg/day | 433 | 215 | WHO/ECOG | post‐ or peri‐ | pos or un |
| Kaufmann | 1993 | Germany | NR | 60 mg/day | 30 mg/day | 71 | 71 | WHO/ECOG | post‐ | pos or un |
| Milla‐Santos | 2001 | Spain | 1996‐1999 | 60 mg/day | 40 mg/day | 106 | 111 | WHO/ECOG | post‐ | pos |
| Nomura | 1993 | Japan | NR | 40 mg/day | 20 mg/day | 57 | 57 | NR | NR | NR |
| Pyrhonen | 1997 | Finland, Sweden, Norway, Poland, Hungary and the Czech Republic | 1986‐1992 | 60 mg/day | 40 mg/day | 214 | 201 | WHO | post‐ | pos or un |
| Stenbygaard | 1993 | Denmark | 1987‐1989 | 240 mg/day | 40 mg/day | 31 | 31 | WHO | post‐ | pos or un |
NR: not reported Pos or un: positive or unknown TAM: tamoxifen TOR: toremifene
The studies were generally old with most being conducted in the late 1980s or early 1990s. Six studies adopted a parallel design. The one remaining study randomised 66 patients to TAM (40 mg orally once daily) or TOR (120 mg orally twice daily), with a cross‐over planned following disease progression.
In total, 2065 patients were randomised. One study (Stenbygaard 1993) excluded four randomised patients in its final analysis. Thus, data of 2061 patients were included in the present review, with 1226 patients in the TOR group and 835 patients in the TAM group. The median or mean age of these patients ranged from 60 to 65 years. There were five studies performed in post‐menopausal women and one study performed in pre‐ or post‐menopausal women. The majority of the patients were either ER‐positive or of unknown status.
TOR or TAM was given as first‐line treatment for advance breast cancer in six studies. In the study of Nomura 1993, the line of treatment was unclear due to an absence of the full report. The dosage of TOR administered in individual studies was 40 mg/day, 60 mg/day, 200 mg/day or 240 mg/day, while that of TAM was 20 mg/day, 30 mg/day or 40 mg/day. The median length of follow‐up was reported in three studies (Gershanovich 1997; Pyrhonen 1997Stenbygaard 1993), which were 20.5, 25.2, and 19 months, respectively.
All studies provided data on ORR, five studies on TTP and four studies on OS (see: Characteristics of included studies). Four studies reported adverse events with data suitable for meta‐analysis (Gershanovich 1997; Hayes 1995; Milla‐Santos 2001Pyrhonen 1997). One study (Nomura 1993) provided information on adverse events in its full report, but the details were not available from the abstract included in this review (see: Characteristics of included studies).
Excluded studies
Risk of bias in included studies
Most studies were considered as "low or unclear risk" of bias, because the baseline characteristics were homogeneous between treatment arms, the outcomes were objective indicators, relevant data were reported completely, and data analysis was done in an ITT manner (see: table of Characteristics of included studies and Figure 2).
2.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Two studies (Pyrhonen 1997Stenbygaard 1993) may be at high risk of bias; in both of these studies, some important baseline characteristics were unevenly distributed between the two treatment groups. In addition, Stenbygaard 1993 did not conduct an ITT analysis.
Allocation
See table of Characteristics of included studies.
Blinding
See table of Characteristics of included studies.
Incomplete outcome data
See table of Characteristics of included studies.
Selective reporting
See table of Characteristics of included studies.
Other potential sources of bias
See table of Characteristics of included studies.
Effects of interventions
Response rates
All eligible studies presented the data of objective response rate (ORR) which included 1226 patients in the TOR group and 835 patients in the TAM group. Table 2 lists the response rates for the two therapeutic groups. There were 80 versus 50 complete responses and 223 versus 162 partial responses in the TOR and TAM groups, respectively. The ORR for the TOR group was 25.8 % (316/1226) whereas the ORR for the TAM group was 26.9% (225/835). The pooled risk ratio (RR) suggested that the ORRs were not statistically different between the two therapeutic groups (RR 1.02, 95% confidence interval (CI) 0.88 to 1.18, P = 0.83) with no heterogeneity between the studies (P = 0.55, I2 = 0%; Figure 3). Egger's test did not detect the possible existence of publication bias (t = 0.23, P = 0.82; Figure 4).
2. The response rates for the toremifene and tamoxifen groups.
| Treatment Groups | TOR group vs. TAM group | Heterogeneity | |||||
| TOR | TAM | RR (95% CI) | P | Q | P | I2 (%) | |
| CR, n (%) | 80 (6.9) | 50 (6.5) | 1.20 (0.85 to 1.70) | 0.30 | 6.26 | 0.28 | 20 |
| PR, n (%) | 223 (19.3) | 162 (21.2) | 0.96 (0.80 to 1.16) | 0.68 | 4.16 | 0.53 | 0 |
| SD, n (%) | 264 (24.0) | 165 (23.3) | 1.04 (0.87 to 1.23) | 0.69 | 1.94 | 0.75 | 0 |
| PD, n (%) | 450 (41.0) | 275 (38.9) | 1.04 (0.84 to 1.28) | 0.72 | 10.83 | 0.03 | 63 |
| ORR, n (%) | 316 (25.8) | 225 (26.9) | 1.02 (0.88 to 1.18) | 0.83 | 4.94 | 0.55 | 0 |
CR: complete response ORR: objective response rate PD: progressive disease PR: partial response RR: risk ratio SD: stable disease TAM: tamoxifen TOR: toremifene
3.

Forest plot of comparison: 1 Toremifene (TOR) vs tamoxifen (TAM), outcome: 1.5 Objective response.
4.

Funnel plot of comparison: 1 Toremifene (TOR) vs tamoxifen (TAM), outcome: 1.5 Objective response.
Time to progression
Five studies provided data on time to progression (TTP) (see: Characteristics of included studies). The median TTP was 6.1 months for patients treated with TOR and 5.8 months for those treated with TAM. Three of these studies (Gershanovich 1997; Milla‐Santos 2001Pyrhonen 1997) presented the data on the hazard ratio (HR) with 95% CIs for TTP. The other studies (Hayes 1995; Stenbygaard 1993) did not provide HR values or data for calculating HRs. Therefore, only three studies were included in the final analysis, with 477 patients in the TOR group and 461 patients in the TAM group. There was no significant difference in TTP between the two therapeutic groups (P = 0.28). The pooled HR was 1.08 (95% CI 0.94 to 1.24; Table 3; Figure 5) with no heterogeneity between studies (P = 0.26, I2 = 26%). Significant publication bias was found by Egger's test (t = 31.40, P = 0.02). Two studies reported only the median TTP for different treatment groups which were 5.6 versus 5.8 months (TOR versus TAM, similarly hereinafter; Hayes 1995) and 28 versus 33 months (Stenbygaard 1993), respectively. It was unknown whether there were significant differences between the treatment groups.
3. The time to progression and overall survival for the toremifene and tamoxifen groups.
| Median | TOR group vs. TAM group | Heterogeneity | |||||
| TOR | TAM | HR (95% CI) | P | Q | P | I2 (%) | |
| Time to progression | 6.1 | 5.8 | 1.08 (0.94 to 1.24) | 0.28 | 2.72 | 0.26 | 26(%) |
| Overall survival | 27.8 | 27.6 | 1.02 (0.86 to 1.20) | 0.85 | 0.21 | 0.98 | 0(%) |
HR: Hazard ratio TAM: tamoxifen TOR: toremifene
5.

Forest plot of comparison: 1 Toremifene (TOR) vs tamoxifen (TAM), outcome: 1.6 Time to progression.
Overall survival
Four studies provided data on overall survival (OS) (Gershanovich 1997; Hayes 1995; Milla‐Santos 2001; Pyrhonen 1997) (see: Characteristics of included studies). The median OS was 27.8 months for patients treated with TOR and 27.6 months for those treated with TAM. We directly extracted HRs with 95% CIs for OS from three of these studies (Gershanovich 1997; Hayes 1995; Milla‐Santos 2001) and calculated the HR with 95% CIs for the fourth study (Pyrhonen 1997). In total, there were 698 patients in the TOR group and 676 patients in the TAM group. There was no significant difference in the OS between the two therapeutic groups (P = 0.85).The pooled HR was 1.02 (95% CI 0.86 to 1.20; Table 3; Figure 6) with no heterogeneity between studies (P = 0.98, I2 = 0%). No publication bias was found by Egger's test (t = 3.77, P = 0.06).
6.

Forest plot of comparison: 1 Toremifene (TOR) vs tamoxifen (TAM), outcome: 1.7 Overall survival.
Adverse events
Table 4 lists the frequencies of adverse events in the TOR and TAM groups. There were no significant differences in the frequencies between the two groups except for headaches (P = 0.02) which were much less in the TOR group. All studies included in the relevant meta‐analyses reported less frequent cardiac events and endometrial cancer in the TOR group. However, the difference between the TOR group and the TAM group did not reach statistical significance (0.69 [0.35, 1.37] for cardiac events and 0.22 [0.04, 1.33] for endometrial cancer).
4. The frequencies of adverse events in toremifene and tamoxifen groups.
| Adverse events | No. of studies % |
TOR group | TAM group | P value | ||
| No. | % | No. | % | |||
| Hot flushes | 4 | 190 | 17.8 | 103 | 15.2 | 0.58 |
| Sweating | 3 | 48 | 7.6 | 39 | 8.5 | 0.82 |
| Nausea | 3 | 97 | 10.1 | 52 | 9.2 | 0.71 |
| Voice changes | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Vaginal discharge | 3 | 79 | 8.2 | 42 | 7.4 | 0.84 |
| Vaginal bleeding | 4 | 22 | 2.1 | 33 | 4.9 | 0.23 |
| Vaginal dryness | 0 | ‐ | ‐ | ‐ | ‐ | ‐ |
| Vomiting | 3 | 22 | 2.3 | 8 | 1.4 | 0.28 |
| Headache | 2 | 1 | 0.2 | 8 | 3.1 | 0.02 |
| Thromboembolic events | 4 | 30 | 2.8 | 27 | 4.0 | 0.47 |
| Cardiac events | 4 | 16 | 1.5 | 16 | 2.4 | 0.29 |
| Ocular disorders | 3 | 8 | 1.3 | 14 | 3.0 | 0.10 |
| Endometrial cancer | 3 | 0 | 0 | 4 | 0.9 | 0.10 |
TAM: tamoxifen TOR: toremifene
Subgroup analysis
As specified in our protocol, we planned to conduct a series of subgroups analyses. However, because of the homogeneity of menopausal status, ER‐status and line of treatment in most studies and the highly heterogeneous dose comparisons in different studies, we could not divide the eligible studies into clinically relevant subgroups according to these factors to examine their effect on outcome measures. Thus, no subgroup analyses were actually conducted.
Sensitivity analysis
We performed sensitivity analysis for study quality (low or unclear risk versus high risk). Significant subgroup differences were seen in the analysis of complete response, progressive disease, objective response and nausea. Studies with high risk of bias appeared to favour TOR in these outcomes. Repeating the analysis each time excluding a single study other than those studies with a high risk of bias did not result in a change in conclusions (data not shown).
Discussion
Tamoxifen (TAM), a non‐steroidal anti‐oestrogen, was developed more than 30 years ago for the treatment of postmenopausal women with advanced breast cancer. Unfortunately, the use of TAM has several significant adverse effects such as thromboembolic events, ocular changes and endometrial carcinoma (EBCTCG 2005; Osborne 1998). During the last three decades, several alternative hormonal therapies have become available as first‐line or adjuvant treatment for breast cancer. Toremifene (TOR), another triphenylethylene anti‐oestrogen, has been compared with TAM efficacy in a number of randomised trials.
Summary of main results
According to the data included in this review, the objective response rate (ORR) for the TOR group was 25.8% whereas the ORR for the TAM group was 26.9%. The pooled risk ratio (RR) showed that the ORR was not statistically different between the two groups. Similarly, there was no significant difference in time to progression (TTP) or overall survival (OS) between the TOR and TAM groups. TOR is comparable to TAM in terms of efficacy. As for adverse events, the two agents were generally similar, except that the frequency of headaches seemed to be lower in the TOR group than in the TAM group. There was no significant heterogeneity across studies in most of our meta‐analyses. Sensitivity analysis did not materially alter these results.
Overall completeness and applicability of evidence
The completeness of the evidence summarised in this review is unsatisfactory. First, long‐term follow‐up data on adverse events of TOR are lacking. The use of TAM raised concern because long‐term treatment with this agent was associated with an increased risk of several significant adverse events. Some of the adverse events, e.g., endometrial cancer, and the impact of this drug on overall mortality, may take a long time to be observed. Recently, an Early Breast Cancer Trialists' Collaborative Group (EBCTCG) study (EBCTCG 2011) with a median follow‐up of 13 years reported that among women who had taken TAM over five years, non‐breast cancer mortality was not significantly increased, although there was a 2.4 fold increase in the risk of uterine cancer. Thus, although we found no significant difference in most adverse events between TAM and TOR, there is no guarantee that TOR is necessarily safer than TAM, as the maximum median follow‐up of the included studies was only 25.2 months. Second, the information on TTP, OS and adverse events of Kaufmann 1993 were not available. The complete data on response and adverse events of Nomura 1993 were also absent. In addition, several studies reported the length of TTP or OS, or both, without hazard ratio (HR) values. It is not impossible that the lack of these data has introduced some bias.
In the past decade, it has been established that aromatase inhibitors have better efficiency and safety than TAM (Gradishar 2010). Aromatase inhibitors are likely to be chosen over TOR as well in many cases. However, there are still situations where anti‐oestrogens can be used. In these cases, TOR can be considered as an available alternative when TAM cannot be chosen for some reason, e.g. drug resistance. The outcomes used in this meta‐analysis are all important ones for patients. The majority of patients included in this review were post‐menopausal and ER‐positive, which can be easily identified in clinical practice. However, caution should still be taken applying the evidence summarised by this review to current practice, because the doses of TOR versus TAM were highly inconsistent across studies, which warrants further investigation before a standard regimen is developed.
Quality of the evidence
All studies included in this review were randomised controlled trials. Although the information needed to fully assess the methodological quality was generally not reported in detail, most studies achieved good balance in baseline characteristics. The outcome data were properly reported and an ITT analysis was used in most studies. However, we did identify two studies with a high risk of bias. It seems that the results of these two studies were more favourable for TOR than were the other studies. Fortunately, sensitivity analysis excluding these studies resulted in no alteration in our main conclusions. Thus, the overall quality of the evidence was deemed modest.
Potential biases in the review process
Potentially, there may be two major sources of potential bias. First, although reported, some outcome data were not available because we could not obtain the full reports of the original studies (Kaufmann 1993; Nomura 1993). Several studies investigated the TTP or OS, but did not provide HR values or data for calculating HR so we excluded them from the meta‐analyses. The lack of relevant data might have affected the comparison of headaches between TAM and TOR groups, which was based on only two studies with 680 patients. The IBIS‐I trial (Cuzick 2007) comparing TAM with placebo for preventing breast cancer in more than 7000 participants showed that the incidence of headaches was higher in the placebo group than in the TAM group (28.8% versus 24.5%). Although this trial was quite different from the studies included in this review in terms of participants, it does have important implications for our understanding of the safety of TAM treatment. Since there were only nine cases of headaches out of 680 patients in this review, the difference we observed between TOR and TAM might result purely from chance. The possible publication bias in the meta‐analyses of TTP detected by Egger's tests is another issue of concern.
Agreements and disagreements with other studies or reviews
Our findings are generally consistent with published reviews (e.g. Zhou 2011) and most of the related studies, as summarised in our meta‐analysis. Studies comparing TOR and TAM in early‐stage breast cancer patients, such as Pagani 2004 and Lewis 2010, also found that the efficacy (disease‐free survival and overall survival) and toxicity of TOR and TAM were similar.
Authors' conclusions
Implications for practice.
In view of the equal objective response rate, time to progression and overall survival benefits and the possibly similar safety profile of the two agents, toremifene is a reasonable alternative to tamoxifen for the treatment of post‐menopausal women with ER‐positive advanced breast cancer when anti‐oestrogens are applicable but TAM is not the preferred choice for some reason.
Implications for research.
1. As mentioned above, the maximum follow‐up of the included studies was only 25.2 months, which might not be enough for some important adverse effects to be observed. Thus, to draw a firm conclusion on whether toremifene is better than tamoxifen in terms of safety, studies with long‐term follow‐up are needed.
2. Most studies included in this review were concerned with post‐menopausal women. Only two studies included peri‐menopausal women. Further trials are justified to examine the impact of menopausal status on the effect of toremifene versus tamoxifen. The most suitable doses of toremifene versus tamoxifen used in trials as well as in daily practice also need further investigation.
3. Studies (Asaishi 1993; Jönsson 1991; Pyrhönen 1994; Vogel 1993) have shown that among tamoxifen‐failed breast cancer patients, the objective response rate of high‐dose toremifene treatment (120 to 240 mg) varied from 0% to 12%, with another 17% to 26% patients having stable disease, and the adverse events were generally mild to moderate. It would be clinically relevant to know the efficacy and safety of toremifene in tamoxifen‐resistant patients as compared with other regimens.
What's new
| Date | Event | Description |
|---|---|---|
| 6 February 2018 | Review declared as stable | No new studies have been conducted on this topic since review publication. Therefore we do not expect to update this review. |
History
Protocol first published: Issue 1, 2011 Review first published: Issue 7, 2012
Acknowledgements
We are grateful to the Cochrane Breast Cancer Group for their help and comments on the protocol and review.
Data and analyses
Comparison 1. Toremifene (TOR) vs tamoxifen (TAM).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Complete response | 6 | 1919 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.85, 1.70] |
| 1.1.1 Low or unclear risk | 4 | 1442 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.98, 2.40] |
| 1.1.2 High risk | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.45, 1.39] |
| 1.2 Partial response | 6 | 1919 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.80, 1.16] |
| 1.2.1 Low or unclear risk | 4 | 1442 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.82, 1.30] |
| 1.2.2 High risk | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.61, 1.13] |
| 1.3 Stable disease | 5 | 1805 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.87, 1.23] |
| 1.3.1 Low or unclear risk | 3 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.89, 1.35] |
| 1.3.2 High risk | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.66, 1.23] |
| 1.4 Progressive disease | 5 | 1805 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.84, 1.28] |
| 1.4.1 Low or unclear risk | 3 | 1328 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.81, 1.07] |
| 1.4.2 High risk | 2 | 477 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [1.11, 1.85] |
| 1.5 Objective response | 7 | 2061 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.88, 1.18] |
| 1.5.1 Low or unclear risk | 5 | 1584 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.94, 1.36] |
| 1.5.2 High risk | 2 | 477 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.64, 1.05] |
| 1.6 Time to progression | 3 | 938 | Hazard Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.08 [0.94, 1.24] |
| 1.7 Overall survival | 4 | 1374 | Hazard Ratio (Exp[(O‐E) / V], Fixed, 95% CI) | 1.02 [0.86, 1.20] |
| 1.8 Nausea | 3 | 1526 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.47] |
| 1.8.1 Low or unclear risk | 2 | 1111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.88, 1.85] |
| 1.8.2 High risk | 1 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.30, 1.19] |
| 1.9 Voice changes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.10 Vaginal discharge | 3 | 1526 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.73, 1.47] |
| 1.10.1 Low or unclear risk | 2 | 1111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.65, 1.38] |
| 1.10.2 High risk | 1 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [0.65, 4.01] |
| 1.11 Vaginal bleeding | 4 | 1743 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.19, 1.50] |
| 1.11.1 Low or unclear risk | 3 | 1328 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.14, 2.19] |
| 1.11.2 High risk | 1 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.11, 3.71] |
| 1.12 Hot flushes | 4 | 1743 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.86, 1.32] |
| 1.12.1 Low or unclear risk | 3 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.84, 1.34] |
| 1.12.2 High risk | 1 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.62, 1.87] |
| 1.13 Vomiting | 3 | 1526 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [0.69, 3.59] |
| 1.13.1 Low or unclear risk | 2 | 1111 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.70, 4.96] |
| 1.13.2 High risk | 1 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.19, 4.60] |
| 1.14 Headache | 2 | 680 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.74] |
| 1.15 Thromboembolic events | 4 | 1743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.48, 1.39] |
| 1.15.1 Low or unclear risk | 3 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.37, 1.50] |
| 1.15.2 High risk | 1 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.42, 2.12] |
| 1.16 Cardiac events | 4 | 1743 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.35, 1.37] |
| 1.16.1 Low or unclear risk | 3 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.22, 1.36] |
| 1.16.2 High risk | 1 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.34, 2.63] |
| 1.17 Vaginal dryness | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.18 Ocular disorders | 3 | 1095 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.19, 1.16] |
| 1.18.1 Low or unclear risk | 2 | 680 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.15, 1.84] |
| 1.18.2 High risk | 1 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.11, 1.54] |
| 1.19 Endometrial cancer | 3 | 1095 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.04, 1.33] |
| 1.19.1 Low or unclear risk | 2 | 680 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.02, 1.69] |
| 1.19.2 High risk | 1 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.64] |
| 1.20 Sweating | 3 | 1095 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.70, 1.57] |
| 1.20.1 Low or unclear risk | 2 | 680 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.38, 1.51] |
| 1.20.2 High risk | 1 | 415 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.76, 2.10] |
1.1. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 1: Complete response
1.2. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 2: Partial response
1.3. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 3: Stable disease
1.4. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 4: Progressive disease
1.5. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 5: Objective response
1.6. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 6: Time to progression
1.7. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 7: Overall survival
1.8. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 8: Nausea
1.9. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 9: Voice changes
1.10. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 10: Vaginal discharge
1.11. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 11: Vaginal bleeding
1.12. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 12: Hot flushes
1.13. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 13: Vomiting
1.14. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 14: Headache
1.15. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 15: Thromboembolic events
1.16. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 16: Cardiac events
1.17. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 17: Vaginal dryness
1.18. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 18: Ocular disorders
1.19. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 19: Endometrial cancer
1.20. Analysis.

Comparison 1: Toremifene (TOR) vs tamoxifen (TAM), Outcome 20: Sweating
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Gershanovich 1997.
| Study characteristics | ||
| Methods | Design: A multicentre, randomised, open‐label, phase III trial Centres: Moscow (Russia), St. Petersburg (Russia), Riga (Latvia), and Tallinn (Estonia) Accrual: From February 1987 to March 1992 Randomisation: The treatments were randomised and the study drug containers were pre‐numbered separately for measurable and non‐measurable strata. The patients were given a study number with respective study drug in the order of accrual Baseline comparability: The pretreatment characteristics of the patients are evenly balanced among the treatment arms |
|
| Participants | 463 postmenopausal women with histologically or cytologically verified, previously untreated, inoperable primary, residual, metastatic, or recurrent breast cancer that was ER‐positive or ER‐unknown ER‐status: 142 (30.7%) positive; 13 (2.8%) negative; 308 (66.5%) unknown Age (median and range): Arm A, 60.9 (38.0 to 85.0); arm B, 62.2 (35.0 to 82.0); arm C, 59.6 (31.0 to 90.0) |
|
| Interventions | Arm A (n = 157): one toremifene 60 mg tablet daily (TOR60) Arm B (n = 157): two toremifene 60 mg tablets twice a day (TOR240) Arm C (n = 149): one tamoxifen 40 mg tablet daily (TAM40) |
|
| Outcomes | Response Time to progression Overall survival Time to treatment failure Response duration Safety Quality of life |
|
| Notes | Median follow‐up time was 20.5 months. 404 (87.3%) patients were evaluable and eligible ITT analysis was used. The results of ITT analysis and that of evaluable patients analysis were similar in terms of response rate and time to progression. For other outcomes, no mention was made in this respect 27 (17%) patients in TOR60, 30 (19%) patients in TOR240, and 32 (22%) patients in TAM40 discontinued the study prematurely 122 (77.7%) patients in TOR60, 116 (73.9%) patients in TOR240, and 115 (77.2%) patients in TAM40 progressed at the time of data cut‐off. Median time to progression was 4.9 (3.8 to 7.3) months in TOR60, 6.1 (4.5 to 8.0) months in TOR240, and 5.0 (3.7 to 6.2) months in TAM40. The HR for TOR60 vs TAM was 0.99 (95% CI: 0.77 to 1.27). The HR for TOR240 vs TAM40 was 0.89 (95% CI: 0.69 to 1.19) 97 (61.8%) patients in TOR60, 96 (31.4%) patients in TOR240, and 89 (59.7%) patients in TAM40 died at the time of data cut‐off. Deaths on study, including the causes of deaths, were not significantly different among the treatment arms. Median overall survival was 25.4 (20.8 to 31.0) months in TOR60, 23.8 (20.9 to 29.7) months in TOR240, and 23.4 (18.4 to 34.2) months in TAM40. The HR for TOR60 vs TAM40 was 1.04 (95% CI: 0.78 to 1.39). The HR for TOR240 vs TAM40 was 0.98 (95% CI: 0.73 to 1.31) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Although no information was provided on how the random sequence was generated, the baseline characteristics were comparable between the treatment arms |
| Allocation concealment (selection bias) | Low risk | Although no information was provided on whether the allocation was concealed, the baseline characteristics were comparable between the treatment arms |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although this is an open‐label trial, all the efficacy outcomes and most of the safety outcomes used in the present meta‐analysis are objective, not subjective. They were unlikely to have been biased by patients' awareness of their treatment status |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | This is an open‐label trial. It is unknown whether the outcome assessment had been biased by the assessors' awareness of the treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for every prespecified outcome were completely reported |
| Selective reporting (reporting bias) | Low risk | Data for every prespecified outcome were completely reported |
| Other bias | Low risk | No other apparent source of bias was identified |
Hayes 1995.
| Study characteristics | ||
| Methods | Design: A multicentre, randomised, phase III trial Centres: 129 sites in six countries, including US, South Africa and four other countries (no detail) Accrual: From November 11, 1988 to August 31, 1991 Randomisation: pre‐stratified by whether patients had bone‐only metastases (with or without other non‐measurable disease) or nonbony assessable disease Baseline comparability: The treatment arms were similar regarding race, ER‐ and PgR‐ content, site of dominant disease, disease‐free interval between primary diagnosis and first recurrence, and performance status |
|
| Participants | 648 postmenopausal or perimenopausal women with a histologically documented prior history of breast cancer that was ER‐ and/or PgR‐positive or ER/PgR‐unknown Age (median and range): Arm A, 63 (37 to 88); arm B, 62 (40 to 85); arm C, 61 (35 to 85) |
|
| Interventions | Arm A (n = 221): TOR 60 mg/day (TOR60) Arm B (n = 212): TOR 200 mg/day (TOR200) Arm C (n = 215): TAM 20 mg/day (TAM) |
|
| Outcomes | Response Time to progression Overall survival Response duration Tumour Flare Adverse events Quality of life |
|
| Notes | Median follow‐up time was unknown. 546 (84%) patients were assessable Response rates were not statistically different between treatment arms, whether they were evaluated by ITT or by assessable patients only. All other data are presented for all patients on study by ITT only 160 (72.4%) patients in TOR60, 155 (73.1%) patients in TOR200, and 150 (69.8%) patients in TAM progressed at time of analysis. Median time to progression was 5.6 months in TOR60, 5.6 months in TOR200, and 5.8 months in TAM, the differences not statistically different 76 (34.4%) patients in TOR60, 95 (44.8%) patients in TOR200, and 81 (37.7%) patients in TAM died. Median overall survival was 38.3 months in TOR60, 30.1 months in TOR200, and 31.7 months in TAM. The HR for TOR60 vs TAM was 0.96 (95% CI: 0.70 to 1.31). The HR for TOR200 vs TAM was 1.23 (95% CI: 0.91 to 1.68) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Although no information was provided on how the random sequence was generated, the baseline characteristics were comparable between the treatment arms |
| Allocation concealment (selection bias) | Low risk | Although no information was provided on whether the allocation was concealed, the baseline characteristics were comparable between the treatment arms |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Whether the trial was blinded or not is unknown. However, all the efficacy outcomes and most of the safety outcomes used in the present meta‐analysis were objective, not subjective. They were unlikely to have been biased by patients' awareness of their treatment status |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whether the trial was blinded or not is unknown |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for every prespecified outcome were completely reported |
| Selective reporting (reporting bias) | Low risk | Data for every prespecified outcome were completely reported |
| Other bias | Low risk | No other apparent source of bias was identified |
Kaufmann 1993.
| Study characteristics | ||
| Methods | Design: A phase III trial Centre: Germany Time of accrual: Not reported Randomisation: The patients were allocated to treatments in order of accrual either by central randomisation or individually by study sites according to preexisting randomisation lists depending on the study Baseline comparability: Not reported |
|
| Participants | 142 postmenopausal women, with histologically or cytologically verified, previously untreated, locally advanced and/or metastatic, measurable or evaluable breast cancer that was ER‐positive or ER‐unknown Age: Not reported |
|
| Interventions | Arm A (n = 71): one TOR 60 mg tablet daily (TOR) Arm B (n = 71): one TAM 30 mg tablet daily (TAM) |
|
| Outcomes | Response Time to treatment failure Survival Adverse events |
|
| Notes | This study was identified by reviewing the reference list of a meta‐analysis (Pyrhonen 1999). The original report was not available. The meta‐analysis included data on 4 outcomes as listed above, among which only the data for response from individual studies were reported separately. Data for other outcomes of this study were not available. Median follow‐up time was unknown Individual studies in this overview report no difference in toxicity between TOR and TAM |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided on how the random sequence was generated. Baseline comparability was unknown |
| Allocation concealment (selection bias) | Unclear risk | No information was provided on whether the allocation was concealed. Baseline comparability was unknown |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Whether the trial was blinded or not is unknown. However, response was an objective outcome, not subjective. It was unlikely to have been biased by patients' awareness of their treatment status |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Whether the trial was blinded or not is unknown |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is difficult to evaluate this item due to unavailability of the original report |
| Selective reporting (reporting bias) | Unclear risk | It is difficult to evaluate this item due to unavailability of the original report |
| Other bias | Unclear risk | It is difficult to evaluate this item due to unavailability of the original report |
Milla‐Santos 2001.
| Study characteristics | ||
| Methods | Design: A prospective, randomised, double‐blind, phase III trial Centre: Spain Accrual: From January 1996 to January 1999 Randomisation: Following the Meinert's methodology Baseline comparability: The treatment groups were comparable with regard to age, metastatic sites, and baseline parameters. No statistically significant differences were detected that might indicate a lack of homogeneity between groups |
|
| Participants | 217 postmenopausal women with histopathological documented advanced breast cancer with positive ER. Age (mean and range): Arm A, 61.3 (56 to 75); arm B, 60.8 (55 to 75) |
|
| Interventions | Arm A (n =106): TOR 60 mg/daily/o.r. (TOR) Arm B (n = 111): TAM 40 mg/daily/o.r. (TAM) |
|
| Outcomes | Response Toxicity Time to progression (equals to duration of response in this study) Survival |
|
| Notes | Median follow‐up time was unknown. All patients were eligible 87 (82.1%) patients in TOR and 100 (90.1%) patients in TAM progressed at the moment of data cut‐off. Median time to progression was 11.9 (9.7 to 13.3) months in TOR and 9.2 (6.2 to 10.8) months in TAM. HR (TOR vs TAM) was 0.98 (95% CI: 0.78 to 1.28) 72 (67.9%) patients in TOR and 89 (80.2%) patients in TAM died at the moment of data cut‐off. Median overall survival was 15.4 (12.6 to 19.4) months in TOR and 12.3 (9.8 to 14.5) months in TAM. HR (TOR vs TAM) was 1.03 (95% CI 0.79 to 1.37) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Although no details was provided on how the random sequence was generated, the baseline characteristics were comparable between the treatment arms |
| Allocation concealment (selection bias) | Low risk | Although no information was provided on whether the allocation was concealed, the baseline characteristics were comparable between the treatment arms |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This is a double‐blind trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This is a double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for every prespecified outcome were completely reported |
| Selective reporting (reporting bias) | Low risk | Data for every prespecified outcome were completely reported |
| Other bias | Low risk | No other apparent source of bias was identified |
Nomura 1993.
| Study characteristics | ||
| Methods | Design: A double blind trial Centre: Japan Time of accrual: Not reported Randomisation: Not reported Baseline comparability: No significant difference was observed in patient characteristics between the two groups |
|
| Participants | 114 patients with advanced or recurrent breast cancer Menopausal and ER‐status and age of patients were not reported |
|
| Interventions | Arm A (n = 57): NK 622 (toremifene citrate) 40 mg daily, o.r. for 12 weeks (TOR) Arm B (n = 57): TAM 20 mg daily, o.r. for 12 weeks (TAM) |
|
| Outcomes | Response Adverse events "usefulness" (no definition was provided) |
|
| Notes | The study was published in a Japanese journal. Only the abstract was written in English, so that detailed information was limited Median follow‐up time was unknown Median duration to onset of CR were 91 days in the NK 622 group and 169 days in the TAM group. Median duration of efficacy in CR and PR cases was 155 days in the NK 622 group and 154.5 days in the TAM group Adverse effects were encountered in 7 patients (12.3%) of each of the 2 groups. The number of each adverse effect was not reported. Administration was discontinued in one patient with eruption and another patient with abnormal values of liver function tests in the TAM group, while there was no such case in the NK 622 group There was no significant difference between the 2 groups in the above results except the duration to onset of CR |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Although details on how the random sequence was generated was not available, the baseline characteristics were comparable between the treatment arms |
| Allocation concealment (selection bias) | Low risk | Although details on whether the allocation was concealed was not available, the baseline characteristics were comparable between the treatment arms |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This is a double‐blind trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This is a double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | It is difficult to evaluate this item due to unavailability of the original report |
| Selective reporting (reporting bias) | Unclear risk | It is difficult to evaluate this item due to unavailability of the original report |
| Other bias | Unclear risk | It is difficult to evaluate this item due to unavailability of the original report |
Pyrhonen 1997.
| Study characteristics | ||
| Methods | Design: A randomised, multicentre, double‐blind, phase III trial Centres: 26 centres in 6 countries (Finland, Sweden, Norway, Poland, Hungary and the Czech Republic) Accrual: From June 1986 to May 1992 Randomisation: The patients were stratified by whether or not they had measurable or only evaluable disease and were randomly assigned to treatment with TOR or TAM. Randomisation was performed centrally, using computer generated lists that were prepared separately for both stratification groups and for each participating centre. Baseline comparability: Patient characteristics are evenly balanced between the two arms except for the levels of ER: in the TAM group a larger proportion of patients had high ER‐levels (mean ER‐concentrations 119 and 171 fmol mg‐1 cytosol protein for TOR and TAM patients, respectively) |
|
| Participants | 415 post‐menopausal patients with histologically or cytologically verified inoperable primary, metastatic or recurrent breast cancer that was ER‐positive or ER‐unknown Age (mean and range): Arm A, 65.5 (33.6 to 87.6); arm B, 65.9 (44.8 to 90.2) |
|
| Interventions | Arm A (n = 214): TOR 60 mg (TOR) Arm B (n = 201): TAM 40 mg (TAM) |
|
| Outcomes | Response Time to progression Time to treatment failure Duration of response Overall survival Toxicity |
|
| Notes | Median follow‐up time was 25.2 months. 379 (91.3%) patients were eligible. The performance status and body weights of the patients were similar in the treatment groups throughout the study ITT analysis was used. The response rates were calculated for all randomised patients as well as for all eligible and all evaluable patients separately. The results appeared to be similar 176 (82.2%) patients in TOR and 147 (73.1%) patients in TAM progressed at the time of data cut‐off. HR of time to progression (TOR vs TAM) was 1.25 (95% CI: 1.00 to 1.56) Median time to treatment failure was 6.3 months in TOR and 8.5 months in TAM. HR (TOR vs TAM) was 1.12 (95% CI: 0.92 to 1.38) 123 (57.5%) in TOR and 115 (57.2%) in TAM died at the time of data cut‐off. Median overall survival was 33.0 (28.5 to 37.0) months in TOR and 38.7 (31.8 to 41.9) months in TAM. The difference was not statistically significant (P = 0.645) (HR not reported) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The random sequence was generated by computer |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This is a double‐blind trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This is a double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for every prespecified outcome were completely reported |
| Selective reporting (reporting bias) | Low risk | Data for every prespecified outcome were completely reported |
| Other bias | High risk | The baseline ER‐levels were significantly different between the two groups |
Stenbygaard 1993.
| Study characteristics | ||
| Methods | Design: A double‐blind cross‐over trial Centre: Denmark Accrual: From September 1987 to March 1989 Randomisation: No details was provided Baseline comparability: Nine patients starting treatment with TOR and 1 starting with TAM had liver metastases (P = 0.01). None of the other patient characteristics including performance status showed any statistically significant difference |
|
| Participants | 66 patients with histologically verified inoperable primary, metastatic, or recurrent breast cancer that was ER‐positive or ER‐unknown Age (median and range): Arm A, 64 (42 to 82); Arm B, 61 (38 to 75) |
|
| Interventions | Arm A (n = 31): TAM, 40 mg orally o.d. (TOR) Arm B (n = 31): TOR, 120 mg orally b.i.d. (TAM) |
|
| Outcomes | Response Time to progression |
|
| Notes | Median follow‐up time was 19 months One patient was excluded due to adverse reactions and was evaluable for toxicity only, one did not have histologically verified breast cancer, one received irradiation of the only evaluable parameter, and one had previously received TAM for advanced breast cancer, leaving 62 patients evaluable for response to first‐line treatment. Only the 62 patients were analysed The median survival time of TAM was longer, but the difference was not significant (P = 0.16). No detailed data were reported 44 patients completed the cross‐over and are evaluable for response to second‐line treatment. Of these patients, 21 initially received TOR and crossed over to TAM and 23 initially received TAM and crossed to TOR. Prognostic factors did not differ significantly between the two groups None of the patients reported adverse reactions when receiving TAM or TOR as second‐line treatment. Seven of the 44 patients died due to PD within 8 weeks after the cross‐over. No responses were observed in the 37 patients who completed at least 8 weeks treatment after the cross‐over |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided |
| Allocation concealment (selection bias) | Unclear risk | No information was provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | This is a double‐blind trial |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | This is a double‐blind trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for every prespecified outcome were completely reported |
| Selective reporting (reporting bias) | Low risk | Data for every prespecified outcome were completely reported |
| Other bias | High risk | Nine patients starting treatment with TOR and 1 starting with TAM had liver metastases (P = 0.01) |
b.i.d.: twice daily ER: oestrogen receptor HR: hazard ratio ITT: intention‐to‐treat o.d.: once daily o.r.: orally PD: progressive disease PgR: progesterone receptor TAM: tamoxifen TOR: toremifene
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bonneterre 1996 | Non‐extractable |
| Ellmen 2003 | Duplicate of Hayes 1995 |
| Garin 1989 | Duplicate of Gershanovich 1997 |
| Gershanovich 1997b | Pooled analysis of two randomised, three‐arm clinical trials, of which the original data were not available; probably duplicate of Gershanovich 1997 and Hayes 1995 included in the present meta‐analysis |
| Gershanovich 1997c | Duplicate of Gershanovich 1997 |
| Gianni 2006 | Overlaps with Holli 2000b; early stage breast cancer |
| Gianni 2009 | Overlaps with Pagani 2004; non‐extractable |
| Herrstedt 1989 | Duplicate of Stenbygaard 1993 |
| Holli 1998a | Duplicate of Holli 2000b; early stage breast cancer |
| Holli 1998b | Non‐metastatic breast cancer |
| Holli 2000a | Duplicates with Holli 2000b; early stage breast cancer |
| Holli 2000b | Early stage breast cancer |
| Holli 2002 | Duplicate of Holli 2000b early stage breast cancer |
| Konstantinova 1990 | Non‐extractable |
| Lewis 2010 | Early stage breast cancer |
| Pagani 2003 | Duplicate of Pagani 2004; early stage breast cancer |
| Pagani 2004 | Early stage breast cancer |
| Pyrhonen 1990 | Duplicates with Pyrhonen 1997 |
| Simoncini 1996 | Non‐extractable |
| Stenbygaard 1990 | Duplicate of Stenbygaard 1993 |
Differences between protocol and review
None known.
Contributions of authors
Developing search strategy: Chen Mao, Zu‐Yao Yang and Shan Liu Study assessment: Chen Mao and Zu‐Yao Yang Data extraction: Zu‐Yao Yang and Jun‐Hua Zhou Resolution of disagreements in study assessment and data extraction: Qing Chen and Jin‐Ling Tang Data entry and analysis: Chen Mao, Zu‐Yao Yang and Jin‐Ling Tang Clinical expertise and suggestions: Ben‐Fu He and Rong‐Cheng Luo Writing protocol and review: Chen Mao, Zu‐Yao Yang, Ben‐Fu He, Shan Liu, Qing Chen and Jin‐Ling Tang
Sources of support
Internal sources
No sources of support supplied
External sources
-
211 Project, China
This study was supported by Guangdong Province "211 Project" (Grant number GW201004).
Declarations of interest
None to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Gershanovich 1997 {published data only}
- Gershanovich M, Garin A, Baltina D, Kurvet A, Kangas L, Ellmen J, and Eastern European Study Group. A phase III comparison of two toremifene doses to tamoxifen in postmenopausal women with advanced breast cancer. Breast Cancer Research and Treatment 1997;45:251-62. [DOI] [PubMed] [Google Scholar]
Hayes 1995 {published data only}
- Hayes DF, Van Zyl JA, Hacking A, Goedhals L, Bezwoda WR, Mailliard JA et al. Randomized comparison of tamoxifen and two separate doses of toremifene in postmenopausal patients with metastatic breast cancer. Journal of Clinical Oncology 1995;13:2556-66. [DOI] [PubMed] [Google Scholar]
Kaufmann 1993 {published data only}
- Pyrhonen S, Ellmen J, Vuorinen J, Gershanovich M, Tominaga T, Kaufmann M et al. Meta-analysis of trials comparing toremifene with tamoxifen and factors predicting outcome of antiestrogen therapy in postmenopausal women with breast cancer. Breast Cancer Research and Treatment 1999;56:133-43. [DOI] [PubMed]
Milla‐Santos 2001 {published data only}
- Milla-Santos A, Milla L, Rallo L, Solano V. Phase III randomized trial of toremifene vs tamoxifen in hormonodependant advanced breast cancer. Breast Cancer Research and Treatment 2001;65:119-24. [DOI] [PubMed] [Google Scholar]
Nomura 1993 {published data only}
- Nomura Y, Tominaga T, Abe O, Izuo M, Ogawa N. Clinical evaluation of NK 622 (toremifene citrate) in advanced or recurrent breast cancer--a comparative study by a double blind method with tamoxifen. Japanese Journal of Cancer and Chemotherapy (Gan to Kagaku Ryoho) 1993;20:247-58. [PubMed] [Google Scholar]
Pyrhonen 1997 {published data only}
- Pyrhonen S, Valavaara R, Modig H, Pawlicki M, Pienkowski T, Gundersen S et al. Comparison of toremifene and tamoxifen in post-menopausal patients with advanced breast cancer: a randomized double-blind, the 'nordic' phase III study. British Joumal of Cancer 1997;76:270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stenbygaard 1993 {published data only}
- Stenbygaard LE, Herrstedt J, Thomsen JF, Svendsen KR, Engelholm SA, Dombernowsky P. Toremifene and tamoxifen in advanced breast cancer--a double-blind cross-over trial. Breast Cancer Research and Treatment 1993;25:57-63. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bonneterre 1996 {published data only}
- Bonneterre J. Comparative phase III studies of toremifene and tamoxifen in advanced breast cancer. Bulletin de la Société Française de Cancérologie Privée 1996;15:45-6. [Google Scholar]
Ellmen 2003 {published data only}
- Ellmen J, Hakulinen P, Partanen A, Hayes DF. Estrogenic effects of toremifene and tamoxifen in postmenopausal breast cancer patients. Breast Cancer Research and Treatment 2003;82:103-11. [DOI] [PubMed] [Google Scholar]
Garin 1989 {published data only}
- Garin AM, Perevodchikova ML, Gershanovich M, Baltina D, Zharkov SA, Trofimova NB et al. A comparative study of toremifene for two dose levels and tamoxifen in the treatment of advanced breast cancer. Proceedings of the 12th Annual San Antonio Breast Cancer Symposium; 1988 Dec 7-9. In: Breast Cancer Research and Treatment. Vol. 14. 1989:137.
Gershanovich 1997b {published data only}
- Gershanovich M, Hayes DF, Ellmen J, Vuorinen J. High-dose toremifene vs tamoxifen in postmenopausal advanced breast cancer. Oncology (Williston Park) 1997;11 Suppl 4:S29-36. [PubMed] [Google Scholar]
Gershanovich 1997c {published data only}
- Gershanovich ML, Garin AM, Baltinia D, Kurvet A, Kangas L, Ellmen Iu. Results of phase II clinical trial of tamoxifen and toremifen in two different doses in advanced breast cancer in postmenopausal women. Voprosy Onkologii 1997;43:587-95. [PubMed] [Google Scholar]
Gianni 2006 {published data only}
- Gianni L, Panzini I, Li S, Gelber RD, Collins J, Holmberg SB et al. Ocular toxicity during adjuvant chemoendocrine therapy for early breast cancer: results from International Breast Cancer Study Group trials. Cancer 2006;106:505-13. [DOI] [PubMed] [Google Scholar]
Gianni 2009 {published data only}
- Gianni L, Gelber S, Ravaioli A, Price KN, Panzini I, Fantini M et al. Second non-breast primary cancer following adjuvant therapy for early breast cancer: a report from the International Breast Cancer Study Group. European Journal of Cancer 2009;45:561-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrstedt 1989 {published data only}
- Herrstedt J, Stenbygaard LE, Thomsen JF, Svendsen KR, Engelholm SA, Dombernowsky P. Double blind crossover study of toremifene versus tamoxifen in advanced breast cancer. Proceedings of the 12th Annual San Antonio Breast Cancer Symposium; 1988 Dec 7-9. In: Breast Cancer Research and Treatment. Vol. 14. 1989:137.
Holli 1998a {published data only}
- Holli K, Joensuu H, Valavaara R, Blanco G, Kataja V, Pukkala E. Interim results of the Finnish toremifene vs tamoxifen adjuvant trial. Breast Cancer Research and Treatment 1998;50:283. [Google Scholar]
Holli 1998b {published data only}
- Holli K. Adjuvant trials of toremifene vs tamoxifen: the European experience. Oncology 1998;12 Suppl 4:S23-7. [PubMed] [Google Scholar]
Holli 2000a {published data only}
- Holli K. Toxicity and early survival results of a prospective, randomized adjuvant trial comparing toremifene and tamoxifen in node-positive breast cancer [abstract]. In: Proceedings of the American Society of Clinical Oncology. Vol. 19. 2000:87a, Abstract 334.
Holli 2000b {published data only}
- Holli K, Valavaara R, Blanco G, Kataja V, Hietanen P, Flander M et al for the Finnish Breast Cancer Group. Safety and efficacy results of a randomized trial comparing adjuvant toremifene and tamoxifen in postmenopausal patients with node-positive breast cancer. Journal of Clinical Oncology 2000;20:3487-94. [DOI] [PubMed] [Google Scholar]
Holli 2002 {published data only}
- Holli K. Tamoxifen versus toremifene in the adjuvant treatment of breast cancer. European Journal of Cancer 2002;38 Suppl 6:S37-8. [DOI] [PubMed] [Google Scholar]
Konstantinova 1990 {published data only}
- Konstantinova MM, Gershanovich MA. The results of a comparative clinical study of the antiestrogenic preparations toremifene and tamoxifen in locally advanced and disseminated breast cancer. Voprosy Onkologii 1990;36:1182-6. [PubMed] [Google Scholar]
Lewis 2010 {published data only}
- Lewis JD, Chagpar AB, Shaughnessy EA, Nurko J, McMasters K, Edwards MJ. Excellent outcomes with adjuvant toremifene or tamoxifen in early stage breast cancer. Cancer 2010;116:2307-15. [DOI] [PubMed] [Google Scholar]
Pagani 2003 {published data only}
- Pagani O, Gelber S, Simoncini E, Holmberg SB, Colleoni M, Crivellari D et al. In: Proceedings of American Society of Clinical Oncology. Vol. 22. 2003:20, Abstract 79.
Pagani 2004 {published data only}
- Pagani O, Gelber S, Price K, Zahrieh D, Gelber R, Simoncini E et al. Toremifene and tamoxifen are equally effective for early-stage breast cancer: first results of International Breast Cancer Study Group Trials 12-93 and 14-93. Annals of Oncology 2004;15:1749-59. [DOI] [PubMed] [Google Scholar]
Pyrhonen 1990 {published data only}
- Pyrhonen SO. Phase III studies of toremifene in metastatic breast cancer. Breast Cancer Research and Treatment 1990;16 Suppl:S41-6. [DOI] [PubMed] [Google Scholar]
Simoncini 1996 {published data only}
- Simoncini E. Phase III randomised study of adjuvant endocrine therapy (tamoxifen vs toremifene) with vs without anthracycline/cyclophosphamide (AC) in peri- and postmenopausal women with node-positive breast cancer, with chemotherapy started either concurrently with or prior to endocrine therapy. Physician Data Query (PDQ). In: http://onlinelibrary.wiley.com/o/cochrane/clcentral/articles/492/CN-00302492/frame.html.
Stenbygaard 1990 {published data only}
- Stenbygaard LE, Herrstedt J, Thomsen JF, Svendsen K, Engelholm SA, Dombernowsky P. Clinical cross resistance in patients with advanced breast cancer. A double-blind cross-over study of toremifene (Tor) and tamoxifen (tam). Annals of Oncology 1990;1 Suppl:17. [Google Scholar]
Additional references
Ahotupa 1994
- Ahotupa M, Hirsimaki P, Parssinen R, Mantyla E. Alterations of drug metabolizing and antioxidant enzyme activities during tamoxifen-induced hepatocarcinogenesis in the rat. Carcinogenesis 1994;15(5):863-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Anderson 2008
- Anderson BO, Yip CH, Smith RA, Shyyan R, Sener SF, Eniu A et al. Guideline implementation for breast healthcare in low-income and middle-income countries: overview of the Breast Health Global Initiative Global Summit 2007. Cancer 2008;113 Suppl(8):2221-43. [PMID: ] [DOI] [PubMed] [Google Scholar]
Asaishi 1993
- Asaishi K, Tominaga T, Abe O, Izuo M, Nomura Y. Efficacy and safety of high dose NK 622 (toremifene citrate) in tamoxifen failed patients with breast cancer. Gan To Kagaku Ryoho 1993;20(1):91-9. [PubMed] [Google Scholar]
Chatterjee 1990
- Chatterjee M, Harris AL. Enhancement of adriamycin cytotoxicity in a multidrug resistant Chinese hamster ovary (CHO) subline, CHO-Adrr, by toremifene and its modulation by alpha 1 acid glycoprotein. European Journal of Cancer 1990;26(4):432-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Clemons 2002
- Clemons M, Danson S, Howell A. Tamoxifen ("Nolvadex"): a review. Cancer Treatment Reviews 2002;28(4):165-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cochran 1954
- Cochran WG. The combination of estimates from different experiments. Biometrics 1954;10(1):101-29. [PMID: 11318188]11318188 [Google Scholar]
Cox 2006
- Cox MC, Dan TD, Swain SM. Emerging drugs to replace current leaders in first-line therapy for breast cancer. Expert Opinion on Emerging Drugs 2006;11(3):489-501. [PMID: 16939387 ] [DOI] [PubMed] [Google Scholar]
Cuzick 2007
- Cuzick J, Forbes JF, Sestak I, Cawthorn S, Hamed H, Holli K, et al. Long-term results of tamoxifen prophylaxis for breast cancer--96-month follow-up of the randomized IBIS-I trial. Journal of the National Cancer Institute 2007;99(4):272-82. [DOI] [PubMed] [Google Scholar]
DeGregorio 1989
- DeGregorio MW, Ford JM, Benz CC, Wiebe VJ. Toremifene: pharmacologic and pharmacokinetic basis of reversing multidrug resistance. Journal of Clinical Oncology 1989;7(9):1359-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177-88. [PMID: ] [DOI] [PubMed] [Google Scholar]
di Salle 1990
- di Salle E, Zaccheo T, Ornati G. Antiestrogenic and antitumor properties of the new triphenylethylene derivative toremifene in the rat. Journal of Steroid Biochemistry 1990;36(3):203-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
EBCTCG 1992
- Early Breast Cancer Trialists' Collaborative Group. Systemic treatment of early breast cancer by hormonal, cytotoxic, or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Early Breast Cancer Trialists' Collaborative Group. Lancet 1992;339(8785):71-85. [PMID: ] [PubMed] [Google Scholar]
EBCTCG 1998
- Early Breast Cancer Trialists' Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet 1998;351(9114):1451-67. [PMID: ] [PubMed] [Google Scholar]
EBCTCG 2005
- Early Breast Cancer Trialists' Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;365(9472):1687-717. [PMID: ] [DOI] [PubMed] [Google Scholar]
EBCTCG 2011
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG), Davies C, Godwin J, Gray R, Clarke M, Cutter D et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011;378(9793):771-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315(7109):629-34. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fisher 1998
- Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. Journal of the National Cancer Institute 1998;90(18):1371-88. [PMID: ] [DOI] [PubMed] [Google Scholar]
Gradishar 2010
- Gradishar WJ. Adjuvant endocrine therapy for early breast cancer: the story so far. Cancer Investigation 2010;28(4):433-42. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 [updated September 2008]. The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org.
Hirsimaki 2002
- Hirsimaki P, Aaltonen A, Mantyla E. Toxicity of antiestrogens. The Breast Journal 2002;8(2):92-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jaiyesimi 1995
- Jaiyesimi IA, Buzdar AU, Decker DA, Hortobagyi GN. Use of tamoxifen for breast cancer: twenty-eight years later. Journal of Clinical Oncology 1995;13(2):513-29. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jemal 2008
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T et al. Cancer statistics, 2008. CA: A Cancer Journal for Clinicians 2008;58(2):71-96. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jönsson 1991
- Jönsson PE, Malmberg M, Bergljung L, Ingvar C, Ericsson M, Ryden S et al. Phase II study of high dose toremifene in advanced breast cancer progressing during tamoxifene treatment. Anticancer Research 1991;11:873-5. [PubMed] [Google Scholar]
Jordan 1978
- Jordan VC, Dix CJ, Naylor KE, Prestwich G, Rowsby L. Nonsteroidal antiestrogens: their biological effects and potential mechanisms of action. Journal of Toxicology and Environmental Health 1978;4(2-3):363-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kangas 1986
- Kangas L, Nieminen AL, Blanco G, Gronroos M, Kallio S, Karjalainen A et al. A new triphenylethylene compound, Fc-1157a. II. Antitumor effects. Cancer Chemotherapy and Pharmacology 1986;17(2):109-13. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kangas 1992
- Kangas L. Agonistic and antagonistic effects of antiestrogens in different target organs. Acta Oncologica (Stockholm, Sweden) 1992;31(2):143-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lahti 1994
- Lahti EI, Knip M, Laatikainen TJ. Plasma insulin-like growth factor I and its binding proteins 1 and 3 in postmenopausal patients with breast cancer receiving long term tamoxifen. Cancer 1994;74(2):618-24. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lange 2011
- Lange CA, Yee D. Killing the second messenger: targeting loss of cell cycle control in endocrine-resistant breast cancer. Endocrine-Related Cancer 2011;18(4):C19-C24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mantel 1959
- Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute 1959;22(4):719-48. [PMID: ] [PubMed] [Google Scholar]
Osborne 1998
- Osborne CK. Tamoxifen in the treatment of breast cancer. The New England Journal of Medicine 1998;339(22):1609-18. [PMID: ] [DOI] [PubMed] [Google Scholar]
Parkin 2002
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA: A Cancer Journal for Clinicians 2005;55(2):74-108. [PMID: 15761078 ] [DOI] [PubMed] [Google Scholar]
Powles 1989
- Powles TJ, Hardy JR, Ashley SE, Farrington GM, Cosgrove D, Davey JB et al. A pilot trial to evaluate the acute toxicity and feasibility of tamoxifen for prevention of breast cancer. British Journal of Cancer 1989;60(1):126-31. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pyrhönen 1994
- Pyrhönen S, Valavaara R, Vuorinen J, Hajba A. High dose toremifene in advanced breast cancer resistant to or relapsed during tamoxifen treatment. Breast Cancer Research and Treatment 1994;29:223-8. [DOI] [PubMed] [Google Scholar]
Pyrhonen 1999
- Pyrhonen S, Ellmen J, Vuorinen J, Gershanovich M, Tominaga T, Kaufmann M et al. Meta-analysis of trials comparing toremifene with tamoxifen and factors predicting outcome of antiestrogen therapy in postmenopausal women with breast cancer. Breast Cancer Research and Treatment 1999;56(2):133-43. [DOI] [PubMed] [Google Scholar]
Ramkumar 1995
- Ramkumar T, Adler S. Differential positive and negative transcriptional regulation by tamoxifen. Endocrinology 1995;136(2):536-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
RevMan [Computer program]
- The Nordic Cochane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochane Centre, The Cochrane Collaboration, 2011.
Su 1985
- Su HD, Mazzei GJ, Vogler WR, Kuo JF. Effect of tamoxifen, a nonsteroidal antiestrogen, on phospholipid/calcium-dependent protein kinase and phosphorylation of its endogenous substrate proteins from the rat brain and ovary. Biochemical Pharmacology 1985;34(20):3649-53. [PMID: ] [DOI] [PubMed] [Google Scholar]
Thangaraju 1995
- Thangaraju M, Ezhilarasi R, Sachdanandam P. Effect of tamoxifen on erythrocyte membrane lipids, lipid peroxides, and antioxidative enzymes in breast cancer women. Cancer Biochemistry Biophysics 1995;14(4):297-302. [PMID: ] [PubMed] [Google Scholar]
Tobias 1999
- Tobias A. Assessing the influences of a single study in the meta-analysis estimate. Stata Technical Bulletin 1999;8(17):15-7. [Google Scholar]
Valverde 1993
- Valverde MA, Mintenig GM, Sepulveda FV. Differential effects of tamoxifen and I- on three distinguishable chloride currents activated in T84 intestinal cells. Pflugers Archiv: European Journal of Physiology 1993;425(5-6):552-4. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vogel 1993
- Vogel CL, Shemano I, Schoenfelder J, Gams RA, Green MR. Multicenter phase II efficacy trial of toremifene in tamoxifen-refractory patients with advanced breast cancer. Journal of Clinical Oncology 1993;11:345-50. [DOI] [PubMed] [Google Scholar]
Warri 1993
- Warri AM, Huovinen RL, Laine AM, Martikainen PM, Harkonen PL. Apoptosis in toremifene-induced growth inhibition of human breast cancer cells in vivo and in vitro. Journal of the National Cancer Institute 1993;85(17):1412-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wiseman 1994
- Wiseman H. Tamoxifen: new membrane-mediated mechanisms of action and therapeutic advances. Trends in Pharmacological Sciences 1994;15(3):83-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Zhou 2011
- Zhou WB, Ding Q, Chen L, Liu XA, Wang S. Toremifene is an effective and safe alternative to tamoxifen in adjuvant endocrine therapy for breast cancer: results of four randomized trials. Breast Cancer Research and Treatment 2011;128(3):625-31. [DOI] [PubMed] [Google Scholar]


