Abstract
Most studies indicate no benefit of adjuvant therapy with VEGFR tyrosine kinase inhibitors in advanced renal cell carcinoma (RCC). PROTECT (NCT01235962) was a randomized, double-blind, placebo-controlled phase 3 study to evaluate adjuvant pazopanib in patients with locally advanced RCC at high risk of relapse after nephrectomy (pazopanib, n = 769; placebo, n = 769). The results of the primary analysis showed no difference in disease-free survival between pazopanib 600 mg and placebo. Here we report the final overall survival (OS) analysis (median follow-up: pazopanib, 76 mo, interquartile range [IQR] 66–84; placebo, 77 mo, IQR 69–85). There was no significant difference in OS between the pazopanib and placebo arms (hazard ratio 1.0, 95% confidence interval 0.80–1.26; nominal p > 0.9). OS was worse for patients with T4 disease compared to those with less advanced disease and was better for patients with body mass index (BMI) ≥30 kg/m2 compared to those with lower BMI. OS was significantly better for patients who remained disease-free at 2 yr after treatment compared with those who relapsed within 2 yr. These findings are consistent with the primary outcomes from PROTECT, indicating that adjuvant pazopanib does not confer a benefit in terms of OS for patients following resection of locally advanced RCC.
Keywords: Pazopanib, Renal cell carcinoma, Tyrosine kinase inhibitor
Patient summary:
In the randomized, double-blind, placebo-controlled phase 3 PROTECT study, overall survival was similar for patients with locally advanced renal cell carcinoma (RCC) at high risk of relapse after nephrectomy who received adjuvant therapy with pazopanib or placebo. Pazopanib is not recommended as adjuvant therapy following resection of locally advanced RCC.
VEGFR tyrosine kinase inhibitors are successfully used in the treatment of advanced renal cell carcinoma (RCC). However, most studies indicate no benefit of these agents in terms of overall survival (OS) in the adjuvant setting. Of the five studies reported (ASSURE [1,2], SORCE [3], ATLAS [4], S-TRAC [5], and PROTECT [6]), only S-TRAC reported a significant improvement in disease-free survival (DFS) associated with adjuvant sunitinib [5]. PROTECT (NCT01235962) was a randomized, double-blind, placebo-controlled phase 3 study to evaluate adjuvant pazopanib in patients with locally advanced RCC at high risk of relapse after nephrectomy [6]. The results of the primary analysis (data cutoff October 15, 2015) showed no difference in DFS between pazopanib 600 mg and placebo (hazard ratio [HR] 0.86, 95% confidence interval [CI] 0.70–1.1; p = 0.17). Higher pazopanib trough plasma concentrations (Ctrough) levels were associated with better DFS but did not increase treatment discontinuations or grade 3/4 adverse events (AEs), with the exception of hypertension [7]. In this report, we present the final overall survival (OS) analysis from the PROTECT trial (data cutoff April 15, 2019).
The design and primary outcomes from PROTECT have been reported previously [6]. Patients with nonmetastatic RCC and predominant clear-cell histology at high risk of recurrence (advanced T stage, nodal positive status, and high tumor grade) were randomly assigned to receive pazopanib or placebo for 1 yr. A pazopanib starting dose of 800 mg/d was initially administered (pazopanib, n = 198; placebo, n = 205), but this was reduced to 600 mg/d following a blinded safety review that indicated a higher than expected discontinuation rate with the higher dose. The protocol was approved by institutional review boards and independent ethics committees, and all patients provided written informed consent.
The primary endpoint was DFS in the intent-to-treat pazopanib 600 mg (ITT600 mg) population; OS was evaluated as a secondary endpoint. Survival was assessed every 6 mo until death, study completion, or study termination. In this analysis, OS is summarized for the overall intent-to-treat population (ITTALL) using the Kaplan-Meier method and compared between treatment arms using a stratified log-rank test (stratification factors were pathologically determined TNM stage and Fuhrman grades). Multivariable Cox regression analysis was used to assess the impact of baseline characteristics on OS. Multivariable analyses were exploratory and were not prespecified in the protocol.
A total of 1538 patients were enrolled (pazopanib, n = 769; placebo, n = 769). At April 15, 2019, all patients had completed the study or withdrawn (Supplementary Fig. 1). As previously reported, patient characteristics were well balanced between the treatment arms and the primary analysis showed no difference in DFS between placebo and pazopanib (data cutoff October 15, 2016; Supplementary Table 1 and Supplementary Table 2) [6].
Analysis of OS for a median follow-up duration of 76 mo (interquartile range [IQR] 66–84) in the pazopanib arm and 77 mo (IQR 69–85) in the placebo arm for patients without a death event showed no significant difference between the treatment arms (ITTALL: HR 1.0, 95% CI 0.80–1.26; nominal p > 0.9; Fig. 1A). Deaths were reported for 145 participants in the pazopanib arm and 150 in the placebo arm, and median OS was not estimable because of the small number of events. OS was worse for patients with T4 disease compared to those with less advanced T stage (T1/2 and T3), and was better for patients with body mass index (BMI) ≥30 kg/m2 compared with those with lower BMI (Fig. 1B,C). OS was better for patients who remained disease-free at 2 yr after treatment compared with those who relapsed within 2 yr (Fig. 1D). OS according to BMI and T stage in each treatment arm is shown in Supplementary Figure 2 and Supplementary Figure 3. Among patients receiving pazopanib, there was no difference in OS between those with high and low Ctrough levels (>25 or ≤20.5 μg/ml) at weeks 3–5 or weeks 16–20 (Supplementary Fig. 4). There was also no difference in OS between the pazopanib and placebo arms for the subgroup of patients in the ITT800 population who were randomly assigned before the reduction in pazopanib starting dose to 600 mg/d (Supplementary Fig. 5).
Fig. 1 –
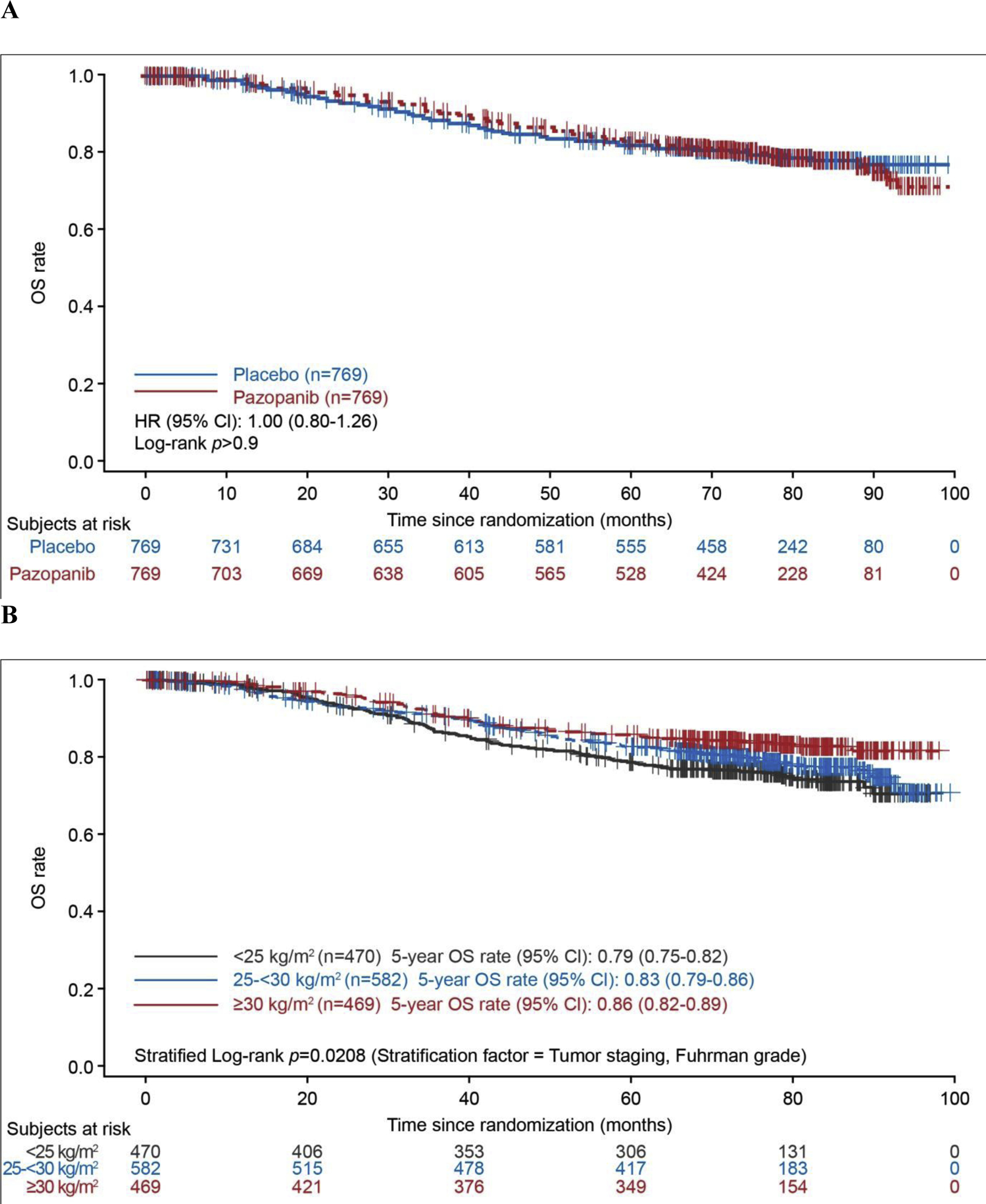
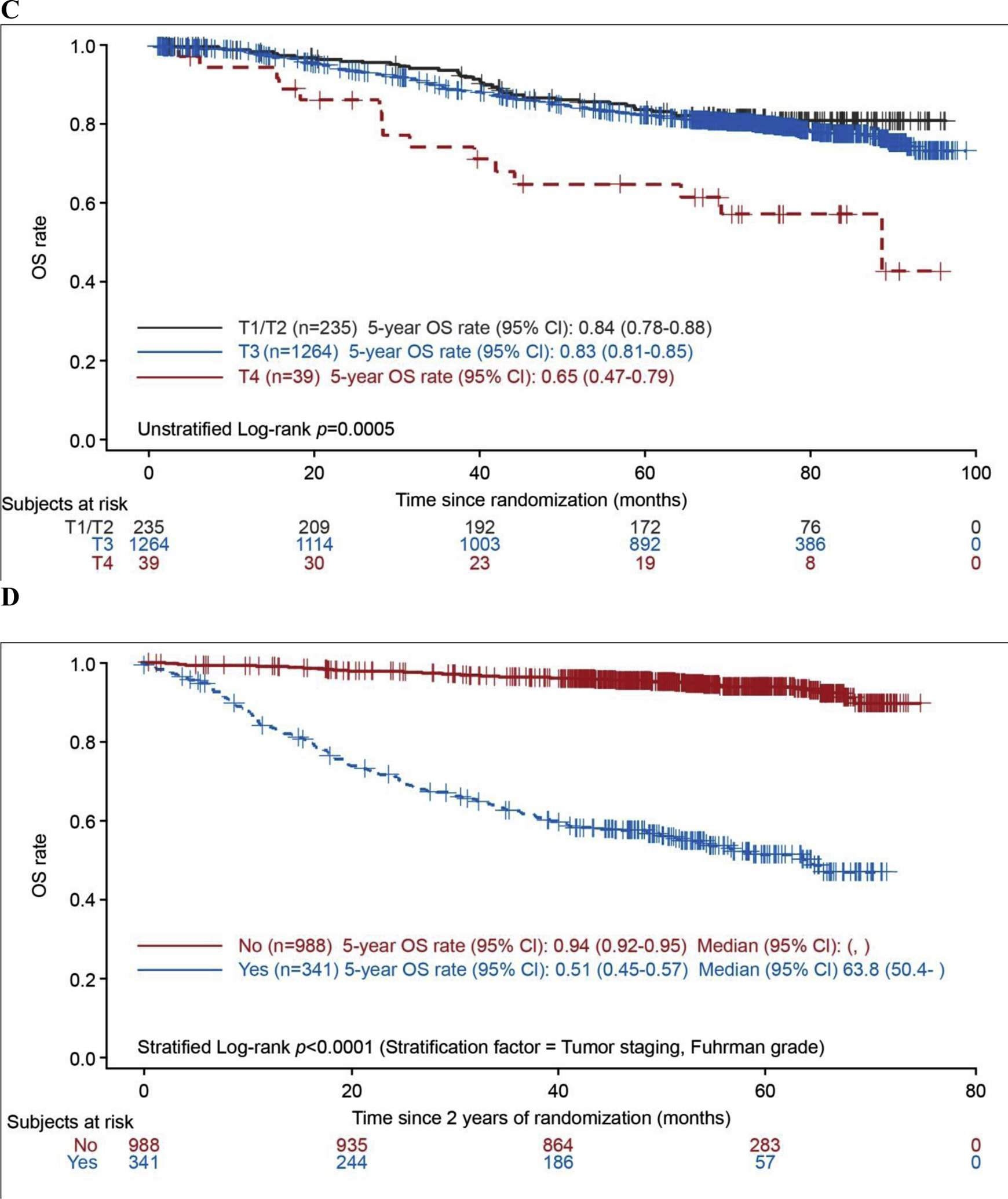
Overall survival in the pooled intent-to-treat group (all patients from the ITT pazopanib 600 mg, and ITT pazopanib 800 mg populations) according to (A) treatment arm, (B) body mass index, (C) T stage, and (D) disease relapse within 2 yr (landmark analysis). BMI = body mass index; CI = confidence interval; DFS = disease-free survival; HR = hazard ratio; OS = overall survival; ITT = intent to treat.
On multivariable analysis, T4 disease, high Fuhrman grade, stage T3 disease, and Latin America location were associated with a higher risk of death, and BMI ≥30 kg/m2 was associated with a lower risk of death (Table 1).
Table 1 –
Multivariable Cox modela of analysis of overall survival
| Pazopanib (n = 769) |
Placebo (n = 769) |
Multivariable Cox model | ||
|---|---|---|---|---|
| HR | p value | |||
| BMI, n (%) | ||||
| <25 kg/m2 | 234 (30.4) | 236 (31) | Reference | |
| ≥25 to <30 kg/m2 | 281 (37) | 301 (39) | 0.802 | 0.11 |
| ≥30 kg/m2 | 246 (32) | 223 (29) | 0.592 | 0.001 |
| T stage, n (%) | ||||
| T1/T2 | 117 (15) | 118 (15) | Reference | |
| T3 | 634 (82) | 630 (82) | 1.417 | 0.050 |
| T4 | 18 (2.3) | 21 (2.7) | 3.312 | <0.001 |
| Fuhrman grade, n (%)b | ||||
| High (grade 3/4) | 534 (69) | 485 (63) | 1.641 | <0.001 |
| Low (grade 1/2) | 235 (31) | 282 (37) | Reference | |
| Region, n (%) | ||||
| Asia Pacific | 86 (11) | 86 (11) | 0.745 | 0.2 |
| Europe | 432 (56) | 447 (58) | 1.243 | 0.14 |
| Latin America | 44 (5.7) | 34 (4.4) | 1.784 | 0.025 |
| North America | 207 (27) | 202 (26) | Reference | |
BMI = body mass index; HR = hazard ratio.
Sex, BMI, T stage, Fuhrman grade, region, race (Asian vs non-Asian)c, and treatment were entered in a Cox model to build a final model to best fit the data using a forward selection algorithm with entry criterion of p ≤ 0.05. The final Cox model includes treatment group (forced to the model), BMI group, T stage, Fuhrman grade, and region. Interaction effects of BMI and sex on overall survival were not significant and were therefore not included in the final model. Overall survival is defined as the time from randomization until death due to any cause. The length of this interval is calculated as the date of death minus the date of randomization plus 1 d. For subjects who do not die, time to death will be censored at the last date of known contact (as recorded in the electronic case report form).
Two patients in the placebo arm had missing Fuhrman grade.
Twenty-nine patients (pazopanib n = 19; placebo n = 10) had missing race data.
Variables that are significantly associated with OS are indicated using bold font.
The most frequent subsequent systemic treatments were VEGFR or mTOR inhibitors (pazopanib, 25% [191/769]; placebo, 26% [201/769]). Sixty-five (8.5%) of the patients receiving pazopanib and 56 (7.3%) of those receiving placebo received a subsequent immunotherapy regimen.
Since the cutoff date for the primary analysis, nine additional AEs have been reported in six patients receiving pazopanib 600 mg and 11 AEs in ten patients receiving placebo; none were considered treatment-related. Serious AEs included cardiac failure in the pazopanib arm, and Kaposi’s sarcoma, thrombocytopenia, breast cancer, and cerebrovascular accident in the placebo arm.
In summary, these analyses indicate that adjuvant pazopanib confers no OS benefit for patients with localized or locally advanced RCC following nephrectomy. AE reporting was consistent with the known safety profile of pazopanib in advanced RCC. These findings are also consistent with the updated analysis of the ASSURE study, which failed to show a significant difference in OS between adjuvant sunitinib or sorafenib and placebo in patients with clear cell RCC [2].
Multivariable Cox model analyses from our study suggest that patients with BMI ≥30 kg/m2 have a 41% lower risk of death compared with those with BMI of <25 kg/m2. This observation supports the “obesity paradox”, whereby overweight or obese patients are at higher risk of clear cell RCC but, conversely, also have better prognosis than normal-weight patients with RCC [8,9]. It has been suggested that although BMI is not an independent predictor of mortality, tumors that develop in obese patients with RCC may be more indolent, possibly related to differences in the expression of genes involved in metabolic and fatty acid pathways, such as fatty acid synthase [8]. Although they are unlikely to inform clinical decision-making, these data suggest that body weight should be considered as a stratification factor in future clinical trials. The present data also indicate that the risk of death was less than three times higher for patients with T4 disease and 42% higher for those with T3 disease compared to patients with T1/2 disease. Landmark analysis using the Kaplan-Meier method showed that patients remaining disease free at 2 yr after randomization had significantly better OS than those with disease recurrence within 2 yr. This finding highlights the poor outcome—median OS of 63.8 mo—for patients who have relapses relatively early, within 2 yr of nephrectomy.
In conclusion, these findings add to the primary outcomes from the PROTECT study, which indicated that adjuvant pazopanib 600 mg does not prolong DFS following resection of locally advanced RCC. Survival analysis showed no difference in OS between the treatment arms. Pazopanib is not recommended as adjuvant therapy following resection of locally advanced RCC.
Supplementary Material
Acknowledgments:
We thank the patients and their families, the investigators and site staff, and the study teams who participated in the PROTECT study. Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by a Memorial Sloan Kettering Cancer Center Support Grant/Core Grant (P30 CA008748). Editorial assistance was provided by ApotheCom and funded by Novartis.
Financial disclosures:
Robert J. Motzer certifies that all conflicts of interest, including specific financial interests and relationships and affiliations relevant to the subject matter or materials discussed in the manuscript (eg, employment/affiliation, grants or funding, consultancies, honoraria, stock ownership or options, expert testimony, royalties, or patents filed, received, or pending), are the following: Robert J. Motzer has a consulting or advisory role with Pfizer, Novartis, Eisai, Exelixis, Merck, Genentech, Incyte, Lilly, and Roche; has received institutional research funding from Pfizer, Bristol-Myers Squibb, Eisai, Novartis, Genentech, and Roche; and has received travel and accommodation expenses from Bristol-Myers Squibb. Naomi Haas is a consultant for Pfizer, Exelixis, and Leidos. Christian Doehn is a consultant for Apogepha, Bristol-Myers Squibb, Eisai, EUSA Pharm, Ipsen, Merck, MSD, Novartis, Pfizer, and Roche; and participates in lecture/speaker bureaus for Bristol-Myers Squibb Ipsen, MSD, Novartis, Pfizer, and Roche. Frede Donskov has received grants from Pfizer, Ipsen, and MSD. Marine Gross-Goupil has received advisory board fees and honoraria from Pfizer, Ipsen, Roche, Novartis, Bristol-Myers Squibb, and MSD; and travel support from Pfizer, Ipsen, Roche, Novartis, Bristol Myers Squibb, MSD, AstraZeneca, and Amgen. Jae Lyun Lee is a consultant for Pfizer Korea, Sanofi Aventis Korea, MSD Korea, AstraZeneca, LG Chemical, and BMS Korea; and has received grants from Pfizer Korea and Ipsen Korea. Ho Yeong Lim is a consultant for Eisai, Bayer, Bristol-Myers Squibb, AstraZeneca, Ipsen, Merck Serono, and Roche. Bohuslav Melichar is a consultant for Novartis, Pfizer, Bristol-Myers Squibb, Astellas, Bayer, MSD, Merck Serono, Sanofi, Servier, AstraZeneca, Amgen, Janssen, Eisai, Eli Lilly, Pierre Fabre, and Ipsen; participates in lecture/speaker bureaus for Novartis, Pfizer, Bristol-Myers Squibb, and Roche; has received travel and accommodation expenses from Merck Serono and Bristol-Myers Squibb; and has received writing assistance, medicines, equipment, and administrative support from Novartis. Milada Zemanova is a consultant for Bristol-Myers Squibb, AstraZeneca, MSD, Novartis, Pfizer, and Roche; participates in lecture/speaker bureaus for Bristol-Myers Squibb, AstraZeneca, MSD, Roche, and Novartis; and has received travel and accommodation expenses from Bristol-Myers Squibb and AstraZeneca. Brian Rini has received institutional research funding from Pfizer, MSD, Genentech/Roche, Peloton, Aveo, AstraZeneca, and Bristol-Myers Squibb; is a consultant for Bristol-Myers Squibb, Pfizer, Genentech/Roche, Aveo, Novartis, Synthorx, Peloton, Compugen, MSD, Corvus, Surface Oncology, 3D Medicines, Aravive, and Alkermes; and owns stock in PTC Therapeutics. Toni K. Choueiri has participated in advisory boards, consultation, and manuscript preparation for and has received travel and accommodation expenses and clinical trial grants from Bristol-Myers Squibb, Exelixis, Pfizer, Merck, AstraZeneca, Lilly, Eisai, Novartis, GSK, and EMD Serono; owns stock in Pionyr and Tempest; and is a member of the NCCN kidney panel. Medical writing and editorial assistance support may have been provided by communications companies funded by pharmaceutical companies; the Dana-Farber Cancer Institute may have received additional independent funding from drug companies or/and royalties potentially involved in research. Lori Wood has participated in clinical trials for Novartis, Roche, BMS, AstraZeneca, and Merck. M. Neil Reaume has received consulting fees/honoraria from Bayer, Pfizer, Bristol-Myers Squibb, AstraZeneca, Novartis, Eisai, Ipsen, Merck, and Roche; and is a consultant for Astellas. Arnulf Stenzl is a consultant for Ipsen Pharma, Janssen, Alere, Bristol-Myers Squibb, Steba Biotech, Synergo, Ferring, and Astellas; participates in a speaker bureau for Janssen, Ipsen Pharma, CureVac, Astellas, Amgen, and Sanofi Aventis; has received research grants from Amgen, Immatics Biotechnologies, Novartis AG, and Karl Storz AG; and has participated in clinical studies for Johnson & Johnson, Roche, Cepheid, Bayer AG, Immatics Biotechnologies, and GemeDX Biosciences. Simon Chowdhury has a consulting/advisory role and participated in speaker bureaus for Clovis Oncology, Astellas Pharma, Bayer, BeiGene Janssen, Pfizer, and Sanofi; has received honoraria from GlaxoSmithKline and Novartis; and has received research funding from Clovis Oncology, Johnson & Johnson, and Sanofi. Ray McDermott has received personal fees from Bristol-Myers Squibb, MSD, and Novartis. Agnieszka Michael is a board member for Clovis, Eisai, Ipsen, Roche, and Tesaro; participates in lecturer/speaker bureaus for Bristol-Myers Squibb, Ipsen, Novartis, and Pfizer; has conducted educational presentations for GlaxoSmithKline; and has received travel and accommodation expenses from Clovis, Ipsen, Roche, and Tesaro. Miguel Izquierdo and Paola Aimone are employees of and have stock options in Novartis. Hong Zhang is employed by Novartis. Cora N. Sternberg is a consultant for Pfizer, MSD, Merck, AstraZeneca, Astellas, Sanofi-Genzyme, Roche-Genentech, Incyte, Medscape, and UroToday. Paul Russo, Sergei Varlamov, and Evgeny Kopyltsov have nothing to disclose.
Funding/Support and role of the sponsor:
The PROTECT study was funded by Novartis. The sponsor played a role in the design and conduct of the study; data collection, management, analysis and interpretation; and preparation, review, and approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This trial is registered at Clinicaltrials.gov as NCT01235962.
References
- 1.Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, nonmetastatic renal-cell carcinoma (ECOG-ACRIN E2805): a double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016;387:2008–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haas NB, Manola J, Dutcher JP, et al. Adjuvant treatment for high-risk clear cell renal cancer: updated results of a high-risk subset of the ASSURE randomized trial. JAMA Oncol 2017;3:1249–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eisen TQG, Frangou E, Smith B, et al. Primary efficacy analysis results from the SORCE trial (RE05): adjuvant sorafenib for renal cell carcinoma at intermediate or high risk of relapse: an international, randomised double-blind phase III trial led by the MRC CTU at UCL. Ann Oncol 2019;30(Suppl 5):v851–934. [Google Scholar]
- 4.Gross-Goupil M, Kwon TG, Eto M, et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann Oncol 2018;29:2371–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant sunitinib in high-risk renal-cell carcinoma after nephrectomy. N Engl J Med 2016;375:2246–54. [DOI] [PubMed] [Google Scholar]
- 6.Motzer RJ, Haas NB, Donskov F, et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma. J Clin Oncol 2017;35:3916–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sternberg CN, Donskov F, Haas NB, et al. Pazopanib exposure relationship with clinical efficacy and safety in the adjuvant treatment of advanced renal cell carcinoma. Clin Cancer Res 2018;24:3005–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi Y, Park B, Jeong BC, et al. Body mass index and survival in patients with renal cell carcinoma: a clinical-based cohort and meta-analysis. Int J Cancer 2013;132:625–34. [DOI] [PubMed] [Google Scholar]
- 9.Hakimi AA, Furberg H, Zabor EC, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst 2013;105:1862–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


