Abstract
Background
Degarelix is a gonadotropin‐releasing hormone antagonist that leads to medical castration used to treat men with advanced or metastatic prostate cancer, or both. It is unclear how its effects compare to standard androgen suppression therapy.
Objectives
To assess the effects of degarelix compared with standard androgen suppression therapy for men with advanced hormone‐sensitive prostate cancer.
Search methods
We searched multiple databases (CENTRAL, MEDLINE, Embase, Scopus, Web of Science, LILACS until September 2020), trial registries (until October 2020), and conference proceedings (until December 2020). We identified other potentially eligible trials by reference checking, citation searching, and contacting study authors.
Selection criteria
We included randomized controlled trials comparing degarelix with standard androgen suppression therapy for men with advanced prostate cancer.
Data collection and analysis
Three review authors independently classified studies and abstracted data from the included studies. The primary outcomes were overall survival and serious adverse events. Secondary outcomes were quality of life, cancer‐specific survival, clinical progression, other adverse events, and biochemical progression. We used a random‐effects model for meta‐analyses and assessed the certainty of evidence for the main outcomes according to GRADE.
Main results
We included 11 studies with a follow‐up of between three and 14 months. We also identified five ongoing trials.
Primary outcomes
Data to evaluate overall survival were not available.
Degarelix may result in little to no difference in serious adverse events compared to standard androgen suppression therapy (risk ratio (RR) 0.80, 95% confidence interval (CI) 0.62 to 1.05; low‐certainty evidence; 2750 participants). Based on 114 serious adverse events in the standard androgen suppression group, this corresponds to 23 fewer serious adverse events per 1000 participants (43 fewer to 6 more). We downgraded the certainty of evidence for study limitations and imprecision.
Secondary outcomes
Degarelix likely results in little to no difference in quality of life assessed with a variety of validated questionnaires (standardized mean difference 0.06 higher, 95% CI 0.05 lower to 0.18 higher; moderate‐certainty evidence; 2887 participants), with higher scores reflecting better quality of life. We downgraded the certainty of evidence for study limitations.
Data to evaluate cancer‐specific survival were not available.
The effects of degarelix on cardiovascular events are very uncertain (RR 0.15, 95% CI 0.04 to 0.61; very low‐certainty evidence; 80 participants). We downgraded the certainty of evidence for study limitations, imprecision, and indirectness as this trial was conducted in a unique group of high‐risk participants with pre‐existing cardiovascular morbidities.
Degarelix likely results in an increase in injection site pain (RR 15.68, 95% CI 7.41 to 33.17; moderate‐certainty evidence; 2670 participants). Based on 30 participants per 1000 with injection site pain with standard androgen suppression therapy, this corresponds to 440 more injection site pains per 1000 participants (192 more to 965 more). We downgraded the certainty of evidence for study limitations.
We did not identify any relevant subgroup differences for different degarelix maintenance doses.
Authors' conclusions
We did not find trial evidence for overall survival or cancer‐specific survival comparing degarelix to standard androgen suppression, but serious adverse events and quality of life may be similar between groups. The effects of degarelix on cardiovascular events are very uncertain as the only eligible study had limitations, was small with few events, and was conducted in a high‐risk population. Degarelix likely results in an increase in injection site pain compared to standard androgen suppression therapy. Maximum follow‐up of included studies was 14 months, which is short. There is a need for methodologically better designed and executed studies with long‐term follow‐up evaluating men with metastatic prostate cancer.
Plain language summary
Degarelix for newly diagnosed advanced prostate cancer
Review question
How does degarelix, a newer drug that treats prostate cancer by lowering male sex hormone levels, compare to existing medications for newly diagnosed advanced prostate cancer?
Background
There is no cure if prostate cancer has spread outside of the prostate gland to lymph nodes or to the bones. In such a situation, hormonal therapy that lowers levels of the male sex hormone testosterone can slow down cancer growth. Testosterone levels are regulated by complicated mechanisms that involve a hormone known as gonadotropin‐releasing hormone (GnRH), which is present in men at different levels at different times of the day. It is understood that giving men with prostate cancer high levels of medications that increase GnRH levels first raises testosterone levels, and then drops them to very low levels. These medications are commonly used to treat men with prostate cancer that has spread outside the prostate. Degarelix is a newer drug known as a GnRH antagonist, which blocks receptors in the brain and thereby lowers testosterone levels immediately.
Study characteristics
We included randomized controlled trials (studies in which participants are assigned to one of two or more treatment groups using a random method) comparing degarelix and standard hormonal therapy in men with newly diagnosed advanced prostate cancer. The evidence is current to September 2020 for electronic databases, to October 2020 for trial registries, and to December 2020 for conference proceedings.
Key results
We found 11 studies that were eligible for inclusion in the review, but none of these studies evaluated the risk of dying from any cause or dying from prostate cancer. There may be no difference between degarelix and standard hormonal therapy in serious unwanted effects and quality of life. The effects of degarelix on cardiovascular issues such as the risk of a heart attack or stroke are uncertain; while one study suggested that the risk may be reduced with degarelix, it had major issues, in particular that it was conducted in men at high risk for such problems. We found that degarelix therapy likely results in an increase in the occurrence of pain at the injection site.
Certainty of the evidence
The certainty of evidence for the various outcomes ranged from moderate to very low. There is a need for additional, better designed studies to further understand the effects of degarelix for newly diagnosed advanced prostate cancer.
Summary of findings
Summary of findings 1. Degarelix compared to standard androgen suppression therapy for treating advanced hormone‐sensitive prostate cancer.
| Degarelix compared to standard androgen suppression therapy for treating advanced hormone‐sensitive prostate cancer | ||||||
| Patient or population: hormone‐sensitive prostate cancer Setting: outpatient Intervention: degarelix Comparison: standard androgen suppression therapy | ||||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) |
What happens |
|
| Risk with standard androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy) | Risk difference with degarelix | |||||
| Overall survival | ‐ | ‐ | ‐ | ‐ | N/A | We do not know the effect of degarelix on overall survival. |
| Serious adverse events Follow‐up: range 1 month to 14 months | 2750 (9 RCTs) | ⊕⊕⊝⊝ LOW 1 2 | RR 0.80 (0.62 to 1.05) | Study population | Degarelix may have little to no effect on serious adverse events. | |
| 114 per 1000 | 23 fewer per 1000 (43 fewer to 6 more) | |||||
| Quality of life assessed with: FACT‐P, EORTC QLQ‐C30, SF‐36 Follow‐up: 14 months | 2887 (3 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ‐ | The mean quality of life was 0. | SMD 0.06 higher (0.05 lower to 0.18 higher) | Degarelix probably has little to no effect on quality of life. |
| Cancer‐specific survival | ‐ | ‐ | ‐ | ‐ | N/A | We do not know the effect of degarelix on cancer‐specific survival. |
| Cardiovascular events Follow‐up: 12 months | 80 (1 RCT) | ⊕⊕⊝⊝ VERY LOW 1 2 3 | RR 0.15 (0.04 to 0.61) | General population4 | The effect of degarelix on cardiovascular events is very uncertain. | |
| 300 per 1000 | 255 fewer per 1000 (288 fewer to 117 fewer) | |||||
| Injection site pain Follow‐up: range 1 month to 14 months | 2670 (8 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | RR 15.68 (7.41 to 33.17) | Study population | Degarelix probably increases the occurrence of injection site pain. | |
| 30 per 1000 | 440 more per 1000 (192 more to 965 more) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FACT‐P: Functional Assessment of Cancer Therapy‐Prostate; GnRH: gonadotropin‐releasing hormone; RCT: randomized controlled trial; RR: risk ratio; SMD: standardized mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by one level for study limitations (performance or detection bias, or both). 2Downgraded by one level for imprecision. 3Downgraded by one level for indirectness, as the Margel 2019 (0102‐15‐RMC) study was based on participants with pre‐existing cardiovascular morbidity (“high risk population”). 4The control event rate was taken from Cardwell 2020, which enrolled 20,216 prostate cancer patients from the Scottish Cancer Registry.
Background
Description of the condition
Worldwide, prostate cancer is the second most common cancer in men, with 1.3 million newly diagnosed people in 2018 (GLOBOCAN 2018). This tumor type is associated with significant mortality, leading to an estimated 359,000 prostate cancer deaths in 2018, making it the fifth‐leading cause of death from cancer in men (GLOBOCAN 2018). Prostate cancer that is limited to the prostate gland, or that has spread locally outside it but not to more distant organs, is considered to be a potentially curable disease. However, prostate cancer that is disseminated to regional lymph nodes or that has metastasized to bones or to other areas is currently only amenable for palliative therapy such as androgen suppression therapy (EAU 2020).
The androgen testosterone is important for the growth and survival of the prostate as well as prostate cancer cells. This dependency forms the basis for systemic androgen deprivation therapy, which is the mainstay of treatment for metastatic prostate cancer (EAU 2020). Androgen suppression therapy inhibits or eliminates testicular testosterone production and decreases circulating testosterone in the blood to very low, so‐called castrate levels. The suppression of testosterone slows prostate cancer disease progression and leads to a decrease in prostate‐specific antigen (PSA).
There are different therapy options available to achieve androgen suppression.
Standard systemic androgen suppression therapy includes surgical or medical castration, an antiandrogen monotherapy, or a combination of both treatment options. While surgical castration (bilateral orchiectomy or subcapsular orchiectomy) removes the source of testicular androgen production, medical castration using gonadotropin‐releasing hormone (GnRH) agonists (e.g. leuprorelin, goserelin, buserelin, and triptorelin) induces castration by drug, administered as depot preparations subcutaneously or intramuscularly at defined intervals (e.g. four weeks, three months, or six months) (EAU 2020). GnRH agonists bind to the GnRH receptors on gonadotropin‐producing cells in the pituitary, causing an initial release of both luteinizing hormone (LH) and follicle stimulating hormone (FSH), which causes a subsequent temporary increase in testosterone production from testicular Leydig cells. In the long term, GnRH receptors are downregulated on the gonadotropin‐producing cells, resulting in a decline in pituitary production of LH and FSH and a reduction of serum testosterone to castration levels.
Surgical and medical castrations are recommended as standard initial treatment options for advanced stages of prostate cancer (EAU 2020).
Antiandrogens are administered orally or as depot preparations and work by blockade of the androgen receptor. A Cochrane Review has demonstrated the reduced effectiveness of this drug class when compared to systemic androgen deprivation therapy in the form of surgical or medical castration (Kunath 2014). While its use in combination with surgical or medical castration is not recommended due to increased side effects and costs at only marginal benefits, it is used as a first‐line form of secondary hormonal treatment for men who progress to systemic androgen therapy (EAU 2020).
Description of the intervention
Degarelix is a GnRH antagonist that competitively binds to receptors in the pituitary gland, leading to immediate castration (Damber 2012b). Degarelix is administered subcutaneously as a depot preparation with a starting dose of 240 mg, and 80 mg or 160 mg maintenance doses every four weeks thereafter or tri‐monthly 480 mg subcutaneous maintenance doses. The standard dosage is 240 mg in the first month, followed by monthly injections of 80 mg (EAU 2020). Abarelix is another GnRH antagonist which is not part of this review.
Adverse effects of the intervention
Surgical castration achieves fast androgen suppression. However, it might cause psychological distress, and some men consider it to be unacceptable because of its irreversibility (EAU 2020). For this reason, more attention has been paid to the medical use of androgen suppression therapies, especially with the evolvement of GnRH antagonists, GnRH agonists, and antiandrogens. However, these therapies have potential adverse events such as injection side effects, gynecomastia, breast pain, hot flushes, and cardiovascular events. A pooled analysis of individual participant data of five randomized controlled trials found differences regarding survival and PSA progression, as well as musculoskeletal and urinary tract events, favoring degarelix when compared to GnRH agonists (Klotz 2014). Furthermore, degarelix may also decrease the risk of death and the incidence of cardiovascular events in men with pre‐existing cardiovascular disease (Klotz 2014).
How the intervention might work
Androgens are necessary for the growth of prostate cancer cells. The secretion of the androgen testosterone is regulated by the hypothalamic‐pituitary‐gonadal axis. The hypothalamus secretes gonadotropin‐releasing hormone (GnRH; also known as luteinizing hormone‒releasing hormone (LHRH)), which stimulates the release of luteinizing hormone (LH) and follicle‐stimulating hormone (FSH) from the anterior pituitary gland. The distribution of LH stimulates the Leydig cells of the testes to secrete testosterone, which is then converted within the prostate cells by 5‐α‐reductase enzyme to dihydrotestosterone (Gibbs 1996). Dihydrotestosterone is important for the development, growth and differentiation of cells of the prostate gland, as well as prostate cancer. Androgen suppression therapy aims to reduce or prevent testosterone secretion, thereby slowing down disease progression (Huggins 2002). The suppression of testosterone also leads to a decrease of prostate‐specific antigen (PSA).
Surgical castration (bilateral orchiectomy or subcapsular orchiectomy) removes the source of testicular androgen production, leading to immediate castration.
GnRH agonists suppress androgen production through a negative feedback mechanism. The continuous exposure of GnRH from the hypothalamus leads to a desensitization of GnRH receptors in the anterior pituitary gland causing a downregulation of LH and testosterone production. The initial exposure of GnRH results in a surge of LH and testosterone levels (also known as flare phenomenon). This surge can induce an exacerbation of clinical symptoms, such as bone pain, ureteral obstruction, and spinal cord compression in men with advanced prostate cancer. The simultaneous short‐term administration of antiandrogens can prevent this testosterone surge. A combination of GnRH agonists with antiandrogens is known as maximal androgen suppression therapy.
Non‐steroidal antiandrogens (e.g. bicalutamide, flutamide, and nilutamide) or steroidal antiandrogens (e.g. cyproterone acetate) compete with testosterone and dihydrotestosterone at the receptor level in the prostate cell nucleus, leading to an androgen suppression.
GnRH antagonists bind competitively to GnRH receptors in the pituitary gland leading to an immediate reduction of LH and testosterone levels without provoking an LH or testosterone surge (Broqua 2002; Damber 2012b).
Why it is important to do this review
A former meta‐analysis on individual patient data including five randomized controlled trials suggested that degarelix is an alternative to standard androgen suppression therapies (Klotz 2014). The GnRH antagonist may have beneficial effects on lower urinary tract symptoms, testosterone suppression, and PSA progression compared to standard androgen suppression (Klotz 2014; Kunath 2015). However, the current European guideline on prostate cancer indicates surgical castration as the 'gold standard' for androgen suppression, and long‐acting GnRH agonists are currently the main forms of androgen suppression therapy (EAU 2020). The current American Urological Association guideline strongly recommends that clinicians should offer androgen suppression therapy with either GnRH agonists or antagonists or surgical castration in men with metastatic hormone‐sensitive prostate cancer (AUA 2020). However, the effect of degarelix compared to standard androgen suppression therapy remains unclear (EAU 2020). Since publication of the systematic review of Kunath 2015, further randomized controlled trials have been published. We therefore expect this review to yield meaningful new insights into the effects of this agent to inform clinical and health policy decision‐making.
Objectives
To assess the effects of degarelix compared with standard androgen suppression therapy for men with advanced hormone‐sensitive prostate cancer.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐group randomized controlled trials comparing degarelix with standard androgen suppression therapy for men with advanced prostate cancer. There was no restriction on publication status or language of publication.
Types of participants
We initially planned to include men with advanced stages of prostate cancer who were not previously treated with androgen suppression therapy. We defined advanced prostate cancer as any of the following diagnoses.
Men with documented disease spread outside the prostate either to the lymph nodes or other organs (N+/M0 or M1a‐c) (TNM 2005).
Men with locally advanced disease who have not undergone surgery or radiation with no spread outside the prostate either to the lymph nodes or other organs (T3‐4/N0 or Nx/M0) (TNM 2005).
Men who have undergone local treatment with curative intent (such as local radiation therapy, radical surgery, or cryotherapy) with biochemical evidence of failure as documented by an elevated or rising PSA in the absence of spread outside the prostate either to the lymph nodes or other organs (T3‐4/N0 or Nx/M0) (TNM 2005).
We post hoc included men with localized disease (defined as prostate cancer within the prostate gland; T1‐2 N0 M0; TNM 2005; see Differences between protocol and review).
There were no restrictions on age or ethnicity of men.
Types of interventions
We included trials with the following comparisons of experimental versus comparator intervention.
Experimental intervention
Degarelix 240 mg subcutaneous (s.c.) given as a starting dose and 80 mg s.c. maintenance doses every four weeks thereafter (or the following maintenance doses: 160 mg s.c. monthly, 480 mg s.c. tri‐monthly).
Comparator interventions
Standard androgen suppression therapy included surgical or medical castration monotherapy, non‐steroidal or steroidal antiandrogen monotherapy, or maximal androgen blockade (combination therapy of surgical or medical castration with antiandrogens).
Bilateral surgical castration included total and subcapsular techniques.
Medical castration monotherapy was defined as an androgen suppression therapy using leuprorelin, goserelin, buserelin, or triptorelin. Antiandrogen therapy included non‐steroidal antiandrogens (e.g. bicalutamide, flutamide, and nilutamide) or steroidal antiandrogens (e.g. cyproterone acetate).
Androgen suppression therapies using estrogens or 5‐α‐reductase inhibitors or combination therapies of medical/surgical castration and newer androgen suppression therapies such as abiraterone, enzalutamide, darolutamide, or apalutamide were not part of this review.
Comparisons
Degarelix versus standard androgen suppression therapy.
Minimum duration of intervention
We included studies evaluating degarelix therapy with at least one administration.
Minimum duration of follow‐up
We included studies evaluating degarelix therapy with a minimum follow‐up of at least 30 days, because androgen suppression arises after this time in almost all men.
Types of outcome measures
Measurement of outcomes assessed in this review was not an eligibility criterion.
Primary outcomes
Overall survival
Serious adverse events
Secondary outcomes
Quality of life
Cancer‐specific survival
Clinical progression
Other adverse events
Biochemical progression
Method and timing of outcome measurement
Overall survival: defined as the time from randomization to the date of death.
Serious adverse events: defined as adverse events during the study requiring hospitalization or that were life‐threatening or fatal, or that were reported as serious adverse events by the authors of the original publication, measured at six months, one year, two years, or at the longest reported follow‐up.
Cancer‐specific survival: defined as the time from randomization to the date of cancer‐related death.
Clinical progression: defined as the date from randomization to disease progression, determined by the appearance of new—or an increase in existing—bone or extraskeletal metastases confirmed by imaging or physical examination.
Quality of life: assessed using validated generic and disease‐specific questionnaires, measured at baseline, six months, one year, two years, or at the longest reported follow‐up.
Other adverse events: injection site pain, cardiovascular events, total non‐serious adverse events, back pain, gynecomastia, constipation, diarrhea, vomiting, cardiac arrest, hypertension, myocardial infarction, libido decrease, erectile dysfunction, fatigue, hot flushes, anemia, hepatic enzyme increase, hepatic failure, dyspnea, gastritis, urinary tract infection, hematuria and urinary retention, defined as any new adverse events during the study (after the first dose of study medication until 30 days after the last dose), measured at six months, one year, two years, or at the longest reported follow‐up.
Biochemical progression: defined as the date from randomization to PSA progression; determined by an increase of more than 25% in the serum PSA concentration from the nadir value on two evaluations.
Post hoc analyses
We included the following outcomes post hoc; for details see Differences between protocol and review.
Mortality during study conduction, as a further adverse event outcome
Discontinuation due to adverse events
Total non‐serious adverse events
Main outcomes for summary of findings table
We have presented a summary of findings table reporting the following outcomes.
Overall survival
Serious adverse events
Quality of life
Cancer‐specific survival
Cardiovascular events
Injection site pain (see Differences between protocol and review)
Search methods for identification of studies
We performed a comprehensive systematic search with no restrictions on language of publication or publication status.
Electronic searches
We searched the following sources from inception of each database.
The Cochrane Central Register of Controlled Trials (CENTRAL; last searched 15 September 2020)
MEDLINE (via OvidSP; 1946 onwards to 15 September 2020)
Embase (initial search in March 2017 via Elsevier's Embase.com, update searches via OvidSP, 1947 onwards to 15 September 2020)
Web of Science (Clarivate Analytics; 1970 onwards to 15 September 2020)
Scopus (last update search on 15 September 2020)
LILACS (Latin American and Caribbean Health Science Information database; 1982 onwards to 15 September 2020)
Two review authors (FK, SS) developed the search strategy after input and feedback from the research team. The search strategy is adapted from the version of the previous published systematic review (Kunath 2015). We used controlled vocabulary, such as Medical Subject Headings (MeSH) and Emtree terms, in combination with keywords for the concepts of prostatic neoplasms, degarelix and androgen suppression therapies, including specific drug names. We made an effort to account for plurals, acronyms, and synonyms. For details on the search strategy, see Appendix 1.
We also searched the following trial registries (last searched 20 October 2020).
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov/)
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/)
We used the following keywords for this search: ‘degarelix,’ ‘firmagon,’ 'FE200486,' 'FE 200486.' We checked every included study for a trial registry entry (see Characteristics of included studies tables).
Searching other resources
We identified other potentially eligible trials or ancillary publications by searching the reference lists of retrieved included trials and reviews. We contacted the study authors of trials and representatives of the manufacturing company Ferring Pharmaceuticals for further studies and missing information. We included correspondence information in the Characteristics of included studies tables.
We searched the electronically available abstract books of the following conferences for unpublished studies.
American Society of Clinical Oncology (ASCO; jco.ascopubs.org/; 2004 until 2020; last searched 4 December 2020)
European Association of Urology (EAU; www.sciencedirect.com/journal/european-urology-supplements/issues; Annual EAU Congress; 2004 until 2020; last searched 4 December 2020)
American Urological Association (AUA; www.auajournals.org/; 2008 until 2020; last searched 4 December 2020)
We used the following keywords for this search: ‘degarelix,’ ‘firmagon,’ 'FE200486,' 'FE 200486.'
Data collection and analysis
Selection of studies
We used EndNote reference management software to collate references and remove potential duplicate records (EndNote 2019). Three review authors (JJJ, FK, FZ) independently screened the abstracts or titles (or both) of the remaining records for studies that were considered to be potentially eligible and assessed as full texts. The same three review authors assessed the full texts, mapped records to studies, and classified studies as included studies, excluded studies, or ongoing studies in accordance with the criteria for each provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Any discrepancies were resolved through consensus or recourse to a fourth review author (CS or SS). We documented the reasons for exclusion of studies in a Characteristics of excluded studies table. A PRISMA flow diagram illustrating the process of study selection is shown in Figure 1 (Liberati 2009).
1.
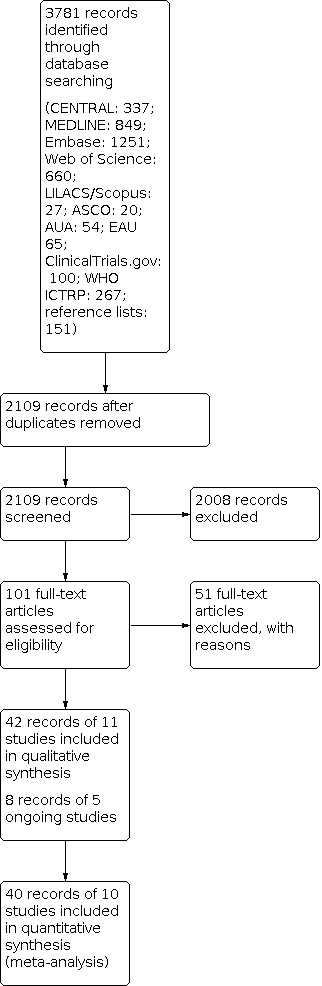
Study flow diagram.
Data extraction and management
We used a data abstraction form that had been pilot tested (Kunath 2015).
Three review authors (JJJ, FK, FZ) independently abstracted the following information from the included studies, which is presented in the Characteristics of included studies table.
Study design
Study dates
Study settings and country
Participant inclusion and exclusion criteria
Participant details, such as baseline demographics and disease characteristics
Number of participants by study and by study arm
Details of relevant experimental and comparator interventions such as dose, route, frequency, and duration
Definitions of relevant outcomes, method and timing of outcome measurement, as well as any relevant subgroups
Study funding sources
Declarations of interest by primary investigators
We extracted outcome data relevant to this review as needed for calculation of summary statistics and measures of variance. We did not assess time‐to‐event outcomes because no studies reported the respective endpoints. For dichotomous outcomes, we used numbers of events and totals for population of a 2 × 2 table, as well as summary statistics with corresponding measures of variance. For continuous outcomes, we used means and standard deviations or data necessary to calculate this information. Any disagreements were resolved by discussion or by consultation with a fourth review author (AB) if required.
We contacted authors of the included studies to obtain key missing data as needed; we included information on any correspondence in the Characteristics of included studies tables.
Information regarding any potentially relevant ongoing studies, including trial identifier, is provided in the Characteristics of ongoing studies table.
Dealing with duplicate and companion publications
In the event of duplicate publications, companion documents, or multiple reports of a primary study, we maximized yield of information by mapping all publications to unique studies and collating all available data. We used the most complete data set aggregated across all known publications. In case of doubt, we gave priority to the publication reporting the longest follow‐up associated with our primary or secondary outcomes.
Assessment of risk of bias in included studies
Three review authors (JJJ, FK, FZ) independently assessed the risk of bias of each included study. Any disagreements were resolved by consensus or by consultation with a fourth review author (SS, JJM, or CS) if required.
We assessed risk of bias using Cochrane's risk of bias tool for randomized controlled trials (Higgins 2017). We assessed the following risk of bias domains.
Random sequence generation (selection bias)
Allocation concealment (selection bias)
Blinding of participants and personnel (performance bias)
Blinding of outcome assessment (detection bias)
Incomplete outcome data (attrition bias)
Selective reporting (reporting bias)
Other sources of bias
We judged risk of bias domains as 'low risk,' 'high risk,' or 'unclear risk' as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017), and present a risk of bias summary figure to illustrate these findings.
For performance bias (blinding of participants and personnel) and detection bias (blinding of outcome assessment), we evaluated risk of bias separately for each outcome and grouped outcomes according to whether they were measured subjectively or objectively, as described in Blinding (performance bias and detection bias).
We assessed attrition bias (incomplete outcome data) on an outcome‐specific basis, and grouped outcomes with judgments when reporting our findings in the Characteristics of included studies tables.
We further summarized the risk of bias across domains for each outcome in each included study, as well as across studies and domains for each outcome.
We defined the following endpoints as subjective outcomes as determined by their susceptibility to detection bias and the importance of blinding of outcome assessors.
Serious adverse events
Cancer‐specific survival
Clinical progression
Quality of life
Other adverse events
Biochemical progression
We defined the following endpoint as an objective outcome.
Overall survival
Concomitant interventions had to be the same in the experimental and comparator groups to establish valid comparisons.
Measures of treatment effect
We did not assess data for time‐to‐event outcomes.
We expressed dichotomous data as risk ratios (RRs) with 95% confidence intervals (CIs).
We expressed quality of life data (continuous data) as standardized mean difference (SMD) with 95% CIs. Before standardization, we multiplied the mean values from Crawford 2013 (CS37) by −1 to correct for differences in the direction of the scale.
Unit of analysis issues
The unit of analysis is the individual participant. In the case of trials with more than two intervention groups, we handled these in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Dealing with missing data
We obtained missing data from study authors and included information regarding any correspondence with study authors in the Characteristics of included studies tables. We investigated attrition rates (e.g. dropouts, losses to follow‐up, and withdrawals) and critically appraised issues regarding missing data. We did not impute missing data.
Assessment of heterogeneity
We identified heterogeneity (inconsistency) through visual inspection of the forest plots to assess the amount of overlap of CIs, and the I² statistic, which quantifies heterogeneity across studies (Higgins 2002; Higgins 2003). We interpreted I² as follows:
0% to 40%: may not be important;
30% to 60%: may indicate moderate heterogeneity;
50% to 90%: may indicate substantial heterogeneity;
75% to 100%: considerable heterogeneity.
When we found heterogeneity, we attempted to determine possible reasons for it by examining individual study and subgroup characteristics.
Assessment of reporting biases
We obtained study protocols to assess for selective outcome reporting. We included fewer than 10 studies investigating any given outcome, and therefore did not use funnel plots to assess small‐study effects.
Data synthesis
We summarized data using a random‐effects model. We interpreted random‐effects meta‐analyses with consideration of the whole distribution of effects. In addition, we performed statistical analyses according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We used the Mantel‐Haenszel method for dichotomous outcomes, and the inverse variance method for continuous outcomes. We did not assess time‐to‐event outcomes. We used Review Manager 5 software to perform analyses (Review Manager 2020).
Subgroup analysis and investigation of heterogeneity
We performed the following subgroup analysis.
Degarelix 240 mg s.c. given as a starting dose and 80 mg s.c. maintenance doses every four weeks thereafter versus degarelix 240 mg s.c. given as a starting dose and 160 mg s.c. maintenance doses every four weeks thereafter versus degarelix 240 mg s.c. given as a starting dose and tri‐monthly 480 mg maintenance doses s.c.
We were not able to perform the following subgroup analyses due to lack of data for the predefined subgroups.
Different standard androgen suppression therapies (surgical castration versus medical castration versus antiandrogen monotherapy versus combination of medical castration and antiandrogen therapy).
Different stages of advanced hormone‐sensitive prostate cancer (non‐metastatic versus metastatic disease).
Sensitivity analysis
We performed sensitivity analyses to explore the influence of the following factors on effect sizes.
Restricting the analysis by taking into account risk of bias, by excluding studies at 'high risk' or 'unclear risk' (one of the criteria 'high risk' or two of the criteria 'unclear risk').
Summary of findings and assessment of the certainty of the evidence
We assessed the overall certainty of the evidence for each outcome according to the GRADE approach, which takes into account five criteria not only related to internal validity (risk of bias, inconsistency, imprecision, publication bias) but also to external validity, such as directness of results (Guyatt 2008). For each comparison, two review authors (JJJ, FZ/FK) independently rated the certainty of evidence for each outcome as 'high,' 'moderate,' 'low,' or 'very low' using GRADEpro GDT (GRADEpro GDT), with any discrepancies resolved by consensus or through arbitration by a third review author (AB or CS) if required. We have presented a summary of the evidence for the main outcomes in a summary of findings table, which provides key information about the best estimate of the magnitude of the effect in relative terms and absolute differences for each relevant comparison of alternative management strategies; numbers of participants and studies addressing each important outcome; and the rating of the overall confidence in effect estimates for each outcome (Guyatt 2011; Schünemann 2019a).
Interpreting results and drawing conclusions
We used the recommendations of Schünemann 2019b for drawing and phrasing conclusions according to the individual GRADE domains.
Results
Description of studies
We included 11 randomized controlled trials (for details see Characteristics of included studies; Table 2). We additionally identified five ongoing studies (for details see Characteristics of ongoing studies).
1. Baseline characteristics.
| Study name | Intervention(s) and comparators (s) | Follow‐up | Number of participants | Study dates | Stage of disease |
| Anderson 2013 (CS28) | Degarelix 240/80 mg1 | 12 weeks | 27 | 2009 to 2010 | Localized/locally advanced: 9 (22.5%) Metastatic: 14 (35%) Unclear: 17 (42.5%) |
| GnRH agonist with flare protection (goserelin 3.6 mg s.c. every 28 days with bicalutamide 50 mg orally per day for 14 days) | 13 | ||||
| Axcrona 2012 (CS31) | Degarelix 240/80 mg1 | 12 weeks | 82 | 2009 to 2011 | Localized: 56 (31%) Advanced: 106 (59%) Unclear: 17 (9%) |
| GnRH agonist with flare protection (goserelin 3.6 mg s.c. every 28 days with bicalutamide 50 mg orally per day for 28 days) | 97 | ||||
| Crawford 2013 (CS37) | Degarelix 240/80 mg2 (intermittent; data not included) | 14 months | 175 | 2009 to 2012 | Unclear (not reported) |
| Degarelix 240/80 mg1 | 50 | ||||
| GnRH agonist with flare protection (leuprolide 7.5 mg i.m. monthly, maintenance dose 22.5 mg i.m. 3‐monthly with bicalutamide 50 mg orally per day for 28 days on Investigator's discretion) | 178 | ||||
| Klotz 2008 (CS21) | Degarelix 240/160 mg (degarelix starting dose of 240 mg s.c. with maintenance doses of 80 mg s.c. every 28 days) | 364 days | 202 | 2006 to 2007 | Localized: 191 (31%) Locally advanced: 178 (29%) Metastatic: 125 (20%) Not classifiable: 116 (19%) |
| Degarelix 240/80 mg1 | 207 | ||||
| GnRH agonist (leuprolide 7.5 mg i.m. monthly) | 201 | ||||
| Margel 2019 (0102‐15‐RMC) | Degarelix 240/80 mg s.c.1 | 12 months | 41 | 2015 to 2019 | Localized: 59 (74%) Metastatic: 21 (26%) |
| GnRH agonist 3‐monthly (at the discretion of the treating urologist/oncologist) | 28 | ||||
| Mason 2013 (CS30) | Degarelix 240/80 mg1 | 12 weeks | 180 | 2009 to 2011 | Localized: 152 (62%) Advanced: 83 (34%) Unclear: 9 (4%) |
| GnRH agonist with flare protection (goserelin 3.6 mg s.c. every 28 days with bicalutamide 50 mg orally per day for 14 days) | 64 | ||||
| Ozono 2018 (3550‐CL‐0010) | Degarelix 240/480 mg (starting dose of 240 mg s.c. with maintenance doses of 480 mg s.c. every 84 days) | 12 months | 117 | 2013 to 2016 | Localized: 124 (53%) Locally advanced: 63 (27%) Metastatic: 44 (19%) Unclear: 3 (1%) |
| GnRH agonist (goserelin 3.6 mg s.c. with maintenance dose 10.8 mg s.c. every 84 days) | 117 | ||||
| Sawazaki 2019 | Degarelix 240/80 mg1 | 6 months | 50 | 2016 to 2018 | Localized: 76 (76%) Locally advanced and/or metastatic: 24 (24%) |
| GnRH agonist (leuprolide 3.75 mg every 28 days) | 50 | ||||
| Sayyid 2017 (DEG_PRE‐OP) | Degarelix 240/80 mg1 | 12 weeks | 13 | 2012 to 2015 | Localized: 10 (26%) Locally advanced: 15 (60%) Node positive: 6 (24%) PSA failure (> 0.2 ng/mL) or use of adjuvant androgen suppression/radiotherapy: 8 (21%)3 |
| Degarelix 240/80 mg s.c. 2‐monthly + bicalutamide 50 mg orally per day (data not included) | 14 | ||||
| GnRH agonist + bicalutamide (leuprorelin 22.5 mg, leuprolide 22.5 mg, or goserelin acetate 10.8 mg 3‐monthly and bicalutamide 50 mg orally per day) | 12 | ||||
| Shore 2012 (CS35) | Degarelix 240/480 mg (starting dose of 240 mg s.c. with maintenance doses of 480 mg s.c. every 3 months) | 13 months | 565 | 2009 to 2011 | Unclear (not reported) |
| GnRH agonist (goserelin 3.6 mg s.c. with maintenance doses of 10.8 mg s.c. 3‐monthly) | 283 | ||||
| Xie 2016 (PANDA) | Degarelix 240/80 mg1 | 364 days | 143 | 2013 to 2015 | Unclear (not reported) |
| GnRH agonist (goserelin 3.6 mg s.c. monthly) | 142 |
Abbreviations: GnRH: gonadotropin‐releasing hormone; i.m.: intramuscular; PSA: prostate‐specific antigen; s.c.: subcutaneous
1Degarelix starting dose of 240 mg s.c. with maintenance doses of 80 mg s.c. every 28 days. 2Degarelix starting dose of 240 mg s.c. with maintenance doses of 80 mg s.c. every 28 days. Six maintenance doses of degarelix 80 mg per month at Days 28 to 168 were administered. If a participant had PSA ≥ 2 ng/mL at any visit, additional doses of degarelix 240 mg followed by 80 mg maintenance dose(s) were administered. Degarelix treatment provided for first seven months (one starting dose and six maintenance doses) followed by no treatment for next seven‐month period. 3Multiple entries possible.
Results of the search
We identified 3781 records through electronic database searching. After removal of duplicates, we screened the titles and abstracts of 2109 records, and excluded 2008 records. We reviewed 101 full‐text articles and excluded 51 with reasons (see Characteristics of excluded studies). We included 50 records of 16 studies: 42 records of 11 included studies and 8 records of 5 ongoing studies (see Characteristics of included studies; Characteristics of ongoing studies). The flow of literature through the assessment process is shown in the PRISMA flow chart (Figure 1).
Included studies
Source of data
All trials were identified through the literature search. We identified multiple abstracts and conference proceedings for most of the included trials.
Study design and settings
We included 11 parallel‐group randomized controlled trials (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Sawazaki 2019; Sayyid 2017 (DEG_PRE‐OP); Shore 2012 (CS35); Xie 2016 (PANDA)). None of the included trials had a cross‐over design. The included studies were reported as 'open‐label' with no blinding of participants or personnel, and were multicenter studies that included outpatients. Countries contributing to the enrollment of study participants are summarized in the Characteristics of included studies tables.
Study duration with outcome assessment was less than 14 months in all trials, as follows: 3 months: Anderson 2013 (CS28); Axcrona 2012 (CS31); Mason 2013 (CS30); Sayyid 2017 (DEG_PRE‐OP); 6 months: Sawazaki 2019; 12 months: Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Ozono 2018 (3550‐CL‐0010); Shore 2012 (CS35); Xie 2016 (PANDA); and 14 months: Crawford 2013 (CS37).
We found five ongoing studies (000108 (PRONOUNCE); JPRN‐UMIN000014243; NCT01542021; NCT02799706; NCT04182594). For details, see Characteristics of ongoing studies.
Participants
We included a total of 2777 randomized participants: 1629 participants received degarelix, and 1148 received standard androgen suppression therapy. All studies included men aged over 18 years. In the Anderson 2013 (CS28) trial, the percentage of participants with locally advanced or metastatic prostate cancer was less than 80%. All of the other included trials involved mainly men with localized prostate cancer (percentage of participants with advanced prostate cancer: Axcrona 2012 (CS31) 59%, Klotz 2008 (CS21) 50%, Margel 2019 (0102‐15‐RMC) 26%, Mason 2013 (CS30) 35%, Ozono 2018 (3550‐CL‐0010) 46%, Sawazaki 2019 24%, Sayyid 2017 (DEG_PRE‐OP) 24%). Three trials did not report the stage of disease of the included participants (Crawford 2013 (CS37); Shore 2012 (CS35); Xie 2016 (PANDA)). Margel 2019 (0102‐15‐RMC) included participants with pre‐existing cardiovascular morbidity.
Interventions and comparators
Degarelix was administered as a subcutaneous (s.c.) starting dose of 240 mg (two 120 mg s.c. injections), followed by monthly maintenance doses of 80 mg, in the following trials: Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Mason 2013 (CS30); Sawazaki 2019; Sayyid 2017 (DEG_PRE‐OP); Xie 2016 (PANDA). In the Ozono 2018 (3550‐CL‐0010) trial, participants received an initial degarelix dose of 240 mg s.c. followed by maintenance doses of 480 mg s.c. every 84 days. Klotz 2008 (CS21) had an additional treatment arm with starting dose of 240 mg s.c., followed by a monthly intensified maintenance dose of 160 mg s.c. Participants in Shore 2012 (CS35) received starting degarelix dose of 240 mg s.c. followed by a maintenance dose of 480 mg s.c. after one month with further administrations after 4, 7, and 10 months. In Crawford 2013 (CS37), degarelix was administered continuously (group 1) or intermittently (group 2); only participants treated continuously were included in the review. Sayyid 2017 (DEG_PRE‐OP) had an additional treatment arm with starting dose of 240 mg s.c. followed by two monthly maintenance doses of 80 mg each combined with the non‐steroidal antiandrogen bicalutamide once daily 50 mg. We did not include this treatment arm in our analyses.
Standard androgen suppression therapy was performed using: goserelin 3.6 mg s.c. with maintenance therapy using goserelin 10.8 mg s.c. every 84 days (Ozono 2018 (3550‐CL‐0010), Shore 2012 (CS35)); goserelin 3.6 mg s.c. every 28 days (Anderson 2013 (CS28); Axcrona 2012 (CS31); Mason 2013 (CS30); Xie 2016 (PANDA)); leuprolide 7.5 mg intramuscular (i.m.) every 28 days (Klotz 2008 (CS21)); leuprolide 7.5 mg i.m. with maintenance therapy using leuprolide 22.5 mg i.m. every 3 months (Crawford 2013 (CS37)); leuprorelin 22.5 mg every 3 months or goserelin 10.8 mg every 3 months (Sayyid 2017 (DEG_PRE‐OP)); and leuprolide 3.75 mg every 28 days (Sawazaki 2019).
The following studies combined gonadotropin‐releasing hormone (GnRH) agonist therapy with bicalutamide 50 mg orally for flare protection: Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Mason 2013 (CS30); Sawazaki 2019. One study used maximum androgen suppression therapy (Sayyid 2017 (DEG_PRE‐OP)). One trial did not further specify androgen suppression therapy and stated that men were treated using a GnRH agonist at the discretion of the treating urologist/oncologist (Margel 2019 (0102‐15‐RMC)). We identified no trials comparing degarelix with surgical castration or antiandrogen monotherapy.
Outcomes
We did not find data for overall survival, cancer‐specific survival, or clinical progression. One study reported survival data, but this outcome was not prespecified in the protocol, was post hoc analyzed, and follow‐up of study was 12 months (Klotz 2008 (CS21)). We considered these data as a further adverse event outcome and referred to it as 'mortality during study conduction' (see analysis of adverse events: Analysis 1.20; Types of outcome measures; Differences between protocol and review).
1.20. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 20: Mortality during study conduction (post hoc)
The co‐primary outcome 'serious adverse events' was reported in the following trials: Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Shore 2012 (CS35); Xie 2016 (PANDA). Sayyid 2017 (DEG_PRE‐OP) reported treatment‐emergent adverse events; however, it was unclear which of the reported adverse events met the definition of serious adverse events according to our predefined definition, therefore we did not include the results of this trial in the review.
Two trials reported data for biochemical progression (Klotz 2008 (CS21); Xie 2016 (PANDA)).
The following trials evaluated adverse event outcomes: Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Sayyid 2017 (DEG_PRE‐OP); Shore 2012 (CS35); Xie 2016 (PANDA).
We included the quality of life assessment of three studies (Crawford 2013 (CS37); Klotz 2008 (CS21); Shore 2012 (CS35)), using data from the following scales: EORTC QLQ‐C30 mapped to EORTC‐8D (Klotz 2008 (CS21)), 36‐item Short Form Health Survey (SF‐36; Shore 2012 (CS35)), and Functional Assessment of Cancer Therapy‐Prostate (FACT‐P) (Crawford 2013 (CS37)). Further studies evaluated quality of life, but we did not include their assessments as data were not relevant to this review (scale used: Anderson 2013 (CS28); Mason 2013 (CS30): International Prostate Symptom Score (IPSS); Axcrona 2012 (CS31): Benign Prostatic Hyperplasia Impact Index (BII)).
We did not include outcomes from Sawazaki 2019 because none of the reported outcomes were relevant to this review.
Funding
All studies reporting outcomes relevant to this review were sponsored by Ferring Pharmaceuticals or Astellas Pharma Inc. Conflicts of interest with pharmaceutical companies were reported in all studies. For details, see Characteristics of included studies table.
Excluded studies
We excluded 51 records after full‐text evaluation. Reasons for exclusion are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
See Figure 2; Figure 3 for details of risk of bias assessment, and Characteristics of included studies for judgments of the individual risk of bias domains.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Five studies reported an adequate method of sequence generation and were rated as at low risk of bias (Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Ozono 2018 (3550‐CL‐0010); Sayyid 2017 (DEG_PRE‐OP); Xie 2016 (PANDA)). Random sequence generation was unclear in six studies (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Mason 2013 (CS30); Sawazaki 2019; Shore 2012 (CS35)).
Allocation concealment
Three studies reported an adequate method of allocation concealment and were rated as at low risk of bias (Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Sayyid 2017 (DEG_PRE‐OP)). Allocation concealment was unclear in eight studies (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Sawazaki 2019; Shore 2012 (CS35); Xie 2016 (PANDA)).
Blinding
Blinding of participants and personnel
Overall survival: no data were available.
Serious adverse events, biochemical progression, other adverse events, quality of life: all included trials were open‐label studies without blinding of participants and personnel, leading to high risk of bias.
Blinding of outcome assessment
Overall survival: no data were available.
Serious adverse events, biochemical progression, other adverse events, quality of life: two studies blinded outcome assessment, resulting in a judgment of low risk of bias (Margel 2019 (0102‐15‐RMC); Sayyid 2017 (DEG_PRE‐OP)). All other trials reported insufficient information to permit judgment.
Incomplete outcome data
We grouped outcomes with similar susceptibility to attrition bias given the reporting characteristics of the studies, as follows.
Oncological outcomes
Overall survival, cancer‐specific survival, clinical progression: no data were available.
Biochemical progression: two studies reported data for this outcome with no missing outcome data, resulting in a judgment of low risk of bias (Klotz 2008 (CS21); Xie 2016 (PANDA)). The remaining studies did not address this outcome, leading to unclear risk of bias.
Adverse events
We judged the risk of attrition bias as low for 10 included trials (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Sayyid 2017 (DEG_PRE‐OP); Shore 2012 (CS35); Xie 2016 (PANDA)). The remaining included trial did not address this outcome (Sawazaki 2019).
Quality of life
We judged the risk of attrition bias as low for the three studies which provided quality of life data included in this review (Crawford 2013 (CS37); Klotz 2008 (CS21); Shore 2012 (CS35)). A further three studies reported quality of life data using scales not relevant to this review; we did not include these data in the review, leading to unclear risk of bias (Anderson 2013 (CS28); Axcrona 2012 (CS31); Mason 2013 (CS30)). The remaining included studies did not address this outcome, resulting in a judgment of unclear risk of bias.
Selective reporting
We judged two studies as at high risk of reporting bias: Margel 2019 (0102‐15‐RMC) reported no data for quality of life, although this outcome was prespecified in their protocol, and Sawazaki 2019 did not report data for adverse events when evaluation of this outcome could have been expected.
We judged the risk of reporting bias as unclear for four studies. We did not identify full‐text publications for Crawford 2013 (CS37) and Shore 2012 (CS35), or a protocol for Xie 2016 (PANDA), and information was insufficient to permit a judgment for Sayyid 2017 (DEG_PRE‐OP).
We identified the study protocols of all remaining studies, and all outcomes of interest were reported.
Other potential sources of bias
We identified no other potential sources of other bias in any of the included studies.
Effects of interventions
See: Table 1
For details, see Table 1; Characteristics of included studies; Data and analyses.
Degarelix versus standard androgen suppression therapy
Overall survival
No data were available for this outcome.
Serious adverse events
We included nine trials evaluating serious adverse events in 2750 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Shore 2012 (CS35); Xie 2016 (PANDA)). Degarelix versus standard androgen suppression therapy may result in little to no difference in serious adverse events (risk ratio (RR) 0.80, 95% confidence interval (CI) 0.62 to 1.05; I² = 9%; low‐certainty evidence). This corresponds to 23 fewer serious adverse events per 1000 participants after maximum 14 months (43 fewer to 6 more). We downgraded the certainty of evidence for study limitations and imprecision (Analysis 1.1; Table 1; Figure 4).
1.1. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 1: Serious adverse events
4.

Forest plot of comparison: 1 Degarelix 240 mg induction dose/80 mg maintenance dose versus standard androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy), outcome: 1.1 Serious adverse events.
Quality of life
We included three studies measuring quality of life (Crawford 2013 (CS37); Klotz 2008 (CS21); Shore 2012 (CS35)). Degarelix likely results in little to no clinically meaningful difference in quality of life after maximum 14 months (standardized mean difference (SMD) 0.06, 95% CI −0.05 to 0.18; I² = 39%; moderate‐certainty evidence). We downgraded the certainty of evidence for study limitations (Analysis 1.2; Table 1).
1.2. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 2: Quality of life
Cancer‐specific survival
No data were available for this outcome.
Clinical progression
No data were available for this outcome.
Other adverse events
Injection site pain
We identified eight studies including 2670 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Shore 2012 (CS35); Xie 2016 (PANDA)). Degarelix therapy likely increases injection site pain compared to standard androgen suppression therapy (RR 15.68, 95% CI 7.41 to 33.17; I² = 63%; moderate‐certainty evidence; Analysis 1.3; Figure 5). This corresponds to 440 more injection site pains per 1000 participants after maximum 14 months (192 more to 965 more). We downgraded the certainty of evidence for study limitations (Table 1).
1.3. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 3: Injection site pain
5.

Forest plot of comparison: 1 Degarelix 240 mg induction dose/80 mg maintenance dose versus standard androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy), outcome: 1.3 Injection site pain.
Cardiovascular events
Cardiovascular events were assessed in one study (80 men) that predominantly enrolled participants with pre‐existing cardiovascular morbidity (Margel 2019 (0102‐15‐RMC)). The effects of degarelix on cardiovascular events in a general population in clinical routine when compared with standard androgen suppression therapy are very uncertain (RR 0.15, 95% CI 0.04 to 0.61; very low‐certainty evidence; Analysis 1.4). This corresponds to 255 fewer cardiovascular events per 1000 participants after 12 months (288 fewer to 117 fewer). We downgraded for study limitations, imprecision, and indirectness for the patient population (Table 1).
1.4. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 4: Cardiovascular events
Back pain
We identified five studies including 2102 men (Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Ozono 2018 (3550‐CL‐0010); Shore 2012 (CS35)). Degarelix may reduce back pain slightly when compared with standard androgen suppression therapy (RR 0.66, 95% CI 0.46 to 0.96; I² = 0%; Analysis 1.5).
1.5. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 5: Back pain
Gynecomastia
We identified one study including 25 men (Sayyid 2017 (DEG_PRE‐OP)). Degarelix may result in little to no difference in gynecomastia when compared with standard androgen suppression therapy (RR 0.31, 95% CI 0.01 to 6.94; I² = not applicable; Analysis 1.6).
1.6. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 6: Gynecomastia
Constipation
We identified four studies including 1112 men (Anderson 2013 (CS28); Crawford 2013 (CS37); Klotz 2008 (CS21); Ozono 2018 (3550‐CL‐0010)). Degarelix may result in little to no difference in constipation when compared with standard androgen suppression therapy (RR 0.75, 95% CI 0.39 to 1.46; I² = 26%; Analysis 1.7).
1.7. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 7: Constipation
Diarrhea
We identified two studies including 253 men (Crawford 2013 (CS37); Sayyid 2017 (DEG_PRE‐OP)). Degarelix may result in little to no difference in diarrhea when compared with standard androgen suppression therapy (RR 1.56, 95% CI 0.47 to 5.18; I² = 0%; Analysis 1.8).
1.8. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 8: Diarrhea
Vomiting
We identified two studies including 837 men (Crawford 2013 (CS37); Klotz 2008 (CS21)). Degarelix may result in little to no difference in vomiting when compared with standard androgen suppression therapy (RR 1.56, 95% CI 0.79 to 3.08; I² = 0%; Analysis 1.9).
1.9. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 9: Vomiting
Loss of sexual interest
We identified two studies including 270 men (Mason 2013 (CS30); Sayyid 2017 (DEG_PRE‐OP)). Degarelix may result in little to no difference in loss of sexual interest when compared with standard androgen suppression therapy (RR 1.06, 95% CI 0.35 to 3.17; I² = not applicable; Analysis 1.10).
1.10. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 10: Loss of sexual interest
Loss of sexual function
We identified two studies including 427 men (Axcrona 2012 (CS31); Mason 2013 (CS30)). Degarelix may result in little to no difference in loss of sexual interest when compared with standard androgen suppression therapy (RR 0.82, 95% CI 0.39 to 1.69; I² = 0%; Analysis 1.11).
1.11. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 11: Loss of sexual function
Fatigue
We identified six studies including 1996 men (Anderson 2013 (CS28); Crawford 2013 (CS37); Klotz 2008 (CS21); Mason 2013 (CS30); Sayyid 2017 (DEG_PRE‐OP); Shore 2012 (CS35)). Degarelix likely results in little to no difference in fatigue when compared with standard androgen suppression therapy (RR 0.83, 95% CI 0.60 to 1.16; I² = 0%; Analysis 1.12).
1.12. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 12: Fatigue
Hot flushes
We identified eight studies including 2412 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Sayyid 2017 (DEG_PRE‐OP); Shore 2012 (CS35)). Degarelix likely results in little to no difference in hot flushes when compared with standard androgen suppression therapy (RR 0.99, 95% CI 0.86 to 1.14; I² = 21%; Analysis 1.13).
1.13. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 13: Hot flushes
Anemia
We identified five studies including 1914 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Klotz 2008 (CS21); Ozono 2018 (3550‐CL‐0010); Shore 2012 (CS35)). Degarelix likely reduces the occurrence of anemia when compared with standard androgen suppression therapy (RR 0.31, 95% CI 0.13 to 0.74; I² = 0%; Analysis 1.14).
1.14. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 14: Anemia
Hepatic enzyme increase (alanine aminotransferase)
We identified four studies including 1014 men (Klotz 2008 (CS21); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Sayyid 2017 (DEG_PRE‐OP)). Degarelix likely increases the occurrence of hepatic enzyme increase (measured: alanine aminotransferase) when compared with standard androgen suppression therapy (RR 2.15, 95% CI 1.26 to 3.66; I² = 0%; Analysis 1.15).
1.15. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 15: Hepatic enzyme increase (alanine aminotransferase)
Dyspnea
We identified one study including 182 men (Axcrona 2012 (CS31)). Degarelix may result in little to no difference in dyspnea when compared with standard androgen suppression therapy (RR 0.39, 95% CI 0.02 to 9.41; I² = not applicable; Analysis 1.16).
1.16. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 16: Dyspnea
Urinary tract infection
We identified five studies including 1908 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Shore 2012 (CS35)). Degarelix likely reduces the occurrence of urinary tract infection when compared with standard androgen suppression therapy (RR 0.47, 95% CI 0.25 to 0.87; I² = 0%; Analysis 1.17).
1.17. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 17: Urinary tract infection
Hematuria
We identified two studies including 636 men (Crawford 2013 (CS37); Klotz 2008 (CS21)). Degarelix may result in little to no difference in hematuria when compared with standard androgen suppression therapy (RR 1.69, 95% CI 0.58 to 4.94; I² = 0%; Analysis 1.18).
1.18. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 18: Hematuria
Urinary retention
We identified five studies including 1925 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Klotz 2008 (CS21); Mason 2013 (CS30); Shore 2012 (CS35)). Degarelix may result in little to no difference in urinary retention when compared with standard androgen suppression therapy (RR 0.43, 95% CI 0.13 to 1.40; I² = 0%; Analysis 1.19).
1.19. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 19: Urinary retention
Mortality during study conduction (post hoc)
We added this outcome post hoc. We identified four studies including 1821 men (Klotz 2008 (CS21); Margel 2019 (0102‐15‐RMC); Shore 2012 (CS35); Xie 2016 (PANDA)). Degarelix probably reduces mortality during study conduction slightly when compared with standard androgen suppression therapy (RR 0.45, 95% CI 0.21 to 0.97; I² = 0%; Analysis 1.20).
Discontinuation due to adverse events (post hoc)
We added this outcome post hoc. We identified eight studies including 2666 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Shore 2012 (CS35); Xie 2016 (PANDA)). Degarelix may result in little to no difference in discontinuation due to adverse events when compared with standard androgen suppression therapy (RR 1.11, 95% CI 0.79 to 1.56; I² = 0%; Analysis 1.21).
1.21. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 21: Discontinuation due to adverse events (post hoc)
Total non‐serious adverse events (post hoc)
We added this outcome post hoc. We identified eight studies including 2412 men (Anderson 2013 (CS28); Axcrona 2012 (CS31); Crawford 2013 (CS37); Klotz 2008 (CS21); Mason 2013 (CS30); Ozono 2018 (3550‐CL‐0010); Sayyid 2017 (DEG_PRE‐OP); Shore 2012 (CS35)). Degarelix likely increases total non‐serious adverse events slightly when compared with standard androgen suppression therapy (RR 1.08, 95% CI 1.01 to 1.15; I² = 49%; Analysis 1.22).
1.22. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 22: Total non‐serious adverse events (post hoc)
Other
No data were available for the following outcomes: rash, pruritus, hemorrhage, nocturia, urinary frequency, edema, anorexia, and gastrointestinal disorders.
Biochemical progression
Two studies assessed biochemical progression (Klotz 2008 (CS21); Xie 2016 (PANDA)). The effects of degarelix on biochemical progression when compared with standard androgen suppression therapy are very uncertain (RR 0.61, 95% CI 0.43 to 0.87; I² = 0%; low‐certainty evidence). This corresponds to 75 fewer biochemical progressions per 1000 participants after 12 months (110 fewer to 25 fewer). We downgraded the certainty of evidence for study limitations and imprecision. We additionally downgraded by one level for indirectness because the percentage of men with locally advanced or metastatic prostate cancer was < 80% (Analysis 1.23; Figure 6).
1.23. Analysis.

Comparison 1: Degarelix versus standard androgen suppression therapy, Outcome 23: Biochemical progression
6.

Forest plot of comparison: 1 Degarelix 240 mg induction dose/80 mg maintenance dose versus standard androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy), outcome: 1.2 Biochemical progression.
Subgroup analysis
We attempted to perform subgroup analyses for the main outcomes included in the summary of findings table, as follows.
Overall survival
We were not able to perform a subgroup analysis for this outcome.
Serious adverse events
The risk of suffering serious adverse events was RR 0.66, 95% CI 0.39 to 1.14 with degarelix 240 mg induction dose/80 mg maintenance dose monthly s.c.; RR 0.85, 95% CI 0.51 to 1.42 with degarelix 240 mg induction dose/160 mg maintenance dose monthly s.c.; and RR 0.90, 95% CI 0.64 to 1.26 with degarelix 240 mg induction dose/480 mg maintenance dose every 3 months s.c. The test for interaction was not significant (P = 0.65; I² = 0%; Analysis 2.1).
2.1. Analysis.

Comparison 2: Degarelix versus androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy): subgroup analysis based on different doses, Outcome 1: Serious adverse events
Quality of life
The SMD for participants receiving degarelix 240 mg induction dose/80 mg maintenance dose monthly s.c. was −0.03, 95% CI ‐0.33 to 0.28; the SMD for participants receiving degarelix 240 mg induction dose/480 mg maintenance dose every 3 months s.c. was 0.10, 95% CI −0.04 to 0.24. The test for interaction was not significant (P = 0.46; I² = 0%; Analysis 2.2).
2.2. Analysis.

Comparison 2: Degarelix versus androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy): subgroup analysis based on different doses, Outcome 2: Quality of life
Injection site pain
The risk of suffering injection site pain was RR 14.94, 95% CI 4.48 to 49.81 with 240 mg induction dose/80 mg maintenance dose monthly s.c.; RR 61.20, 95% CI 3.82 to 979.36 with degarelix 240 mg induction dose/160 mg maintenance dose monthly s.c.; and RR 15.24, 95% CI 8.50 to 27.31 with degarelix 240 mg induction dose/480 mg maintenance dose every 3 months s.c. The test for interaction was not significant (P = 0.63; I² = 0%; Analysis 2.3).
2.3. Analysis.

Comparison 2: Degarelix versus androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy): subgroup analysis based on different doses, Outcome 3: Injection site pain
Cardiovascular events
We were not able to perform a subgroup analysis for this outcome.
Sensitivity analysis
Because of substantial heterogeneity, we performed a sensitivity analysis for the outcome injection site pain by excluding the following trials: Anderson 2013 (CS28), Mason 2013 (CS30), Axcrona 2012 (CS31), and Crawford 2013 (CS37) (see Sensitivity analysis). The effect estimate remained stable favoring standard androgen suppression therapy (RR 44.28, 95% CI 10.99 to 178.38; I² = 0%; not shown).
Discussion
Summary of main results
We identified 11 randomized controlled trials and included data from 10 studies in meta‐analyses. We additionally identified five ongoing trials.
No data were available for the outcomes overall survival, cancer‐specific survival, and clinical progression. Degarelix likely results in no clinically meaningful difference in quality of life compared to standard androgen suppression therapy, and the two treatment groups may be similar in terms of serious adverse events. Degarelix likely increases the occurrence of injection site pain. The effects of degarelix on cardiovascular events are very uncertain.
Overall completeness and applicability of evidence
Several limitations to this review deserve consideration by the reader.
We did not find data on patient‐relevant oncological outcomes because no study prospectively planned to assess outcomes such as 'overall survival,' 'cancer‐specific survival,' or 'clinical progression.'
Participants enrolled in the included trials differed substantially from our predefined patient characteristics, as the percentage of participants with locally advanced or metastatic prostate cancer was less than 80% in most trials.
We were unable to evaluate long‐term oncological outcomes (i.e. survival) because none of the included studies had a follow‐up greater than 365 days. While some studies reported mortality during study conduction, we considered this as an adverse event outcome because with a short‐term follow‐up of less than one year, no survival/mortality data could be mature.
Data were insufficient to conduct all of the intended subgroup analyses, so we are uncertain whether the different standard androgen suppression therapies (surgical castration versus medical castration versus antiandrogen monotherapy versus combination of medical castration and antiandrogen therapy) or the different stages of advanced hormone‐sensitive prostate cancer (non‐metastatic versus metastatic disease) impacts the effectiveness of degarelix.
All of the included trials reporting outcomes relevant to this review were funded by Ferring Pharmaceuticals or Astellas Pharma Inc, and many study authors had industry relationships.
Quality of the evidence
We rated the certainty of the evidence as moderate, low, or very low for the reasons described below.
-
We consistently downgraded the evidence for study limitations for at least one of the following reasons:
performance bias, as none of the included trials blinded participants or personnel. This might have impacted the intensity of follow‐up and the type of care men received;
detection bias, as most of the included trials did not blind outcome assessors (or this was not reported). This might have impacted information relating to whether the intervention or control treatment was effective;
reporting bias, as one study did not report quality of life data although this outcome was prespecified in their protocol (Margel 2019 (0102‐15‐RMC)). Another study did not report data for adverse events when evaluation of this outcome could have been expected (Sawazaki 2019);
we furthermore had concerns about insufficient reporting resulting in an unclear risk of reporting and attrition bias.
We downgraded the evidence for imprecision in the setting of wide confidence intervals and low numbers of events.
We downgraded the evidence for indirectness when the participant population of the included studies did not correspond to our predefined study population.
Potential biases in the review process
We employed a comprehensive search strategy of multiple data sources to search for randomized controlled trials without any publication or language restrictions. However, there remains the possibility that we may have missed studies published in a language other than English, those published in non‐indexed journals, or studies that were not published at all, resulting in potential publication bias. We contacted the authors of all of the included trials to seek further information and data, but only received a response from the authors of two studies.
Agreements and disagreements with other studies or reviews
We are very uncertain as to the effect of degarelix on cardiovascular events in a general population in clinical routine. However, there is considerable evidence available from observational studies including a large number of participants for evaluation of cardiovascular events in patients receiving GnRH antagonists (Cardwell 2020; Davey 2020; George 2020; Perrone 2020). Both degarelix and GnRH agonists increase the risks of cardiovascular disease in prostate cancer patients (Cardwell 2020; George 2020). George 2020 evaluated data from five countries including 48,757 men receiving GnRH agonists and 2144 men receiving GnRH antagonists. Study authors found no difference between groups in risk of any cardiovascular disease, but there may be an increased risk of acute myocardial infarction and arrhythmia in men receiving GnRH antagonists. Cardwell 2020 identified 20,216 prostate cancer patients followed for 73,570 person‐years from the Scottish Cancer Registry. GnRH antagonists and agonists were associated with a 30% increase in cardiovascular events. Data from the UK primary care setting suggest there is a decreased risk of experiencing cardiac events with degarelix. However, patients that received degarelix switched treatment more frequently to a GnRH agonist than the other way round (Davey 2020). It has been suggested that patients receiving androgen suppression therapy in any form should be stratified based on level of cardiovascular disease and monitored accordingly (Davey 2020). Whether degarelix offers any benefit to the subset of individuals at increased risk, as suggested by one included trial (Margel 2019 (0102‐15‐RMC)), remains to be seen.
The results of this Cochrane Review are largely consistent with those of other previously published reviews. Kunath 2015 performed a very similar rigorous systematic review evaluating how GnRH antagonists compared with standard androgen suppression therapy. However, we were able to provide an updated search and include additional trial data. Other reviews did not use a rigorous methodology (i.e. predefined methodology, published protocol, comprehensive search strategy, risk of bias assessment, evaluation of evidence certainty using GRADE) or even consider risk of bias assessments in their conclusions (Abufaraj 2020; Cui 2014; Hosseini 2016; Klotz 2014; Kunath 2015; Sciarra 2016). This Cochrane Review includes data that were not previously included in systematic reviews and is therefore the most up‐to‐date.
The current guideline of the American Urological Association does not make a distinction between the different types of androgen suppression therapy in advanced hormone‐sensitive prostate cancer, but recommends that the use of non‐steroidal antiandrogens (i.e. bicalutamide) should be restricted to testosterone flare protection only (AUA 2020). Also, the guideline of the European Association of Urology determines that there is no high‐level evidence available favoring one specific type of androgen suppression therapy (EAU 2020). The guideline recommends that GnRH antagonist and bilateral surgical castration are the preferred treatment options for men with impending spinal cord compression (EAU 2020).
The National Institute for Health and Care Excellence (NICE) invited Ferring Pharmaceuticals to submit evidence for the clinical and cost‐effectiveness (Uttley 2017). Uttley 2017 published a review of the evidence contained within the company's submission to NICE. They identified that the GnRH antagonist degarelix was non‐inferior to standard androgen suppression therapy regarding the reduction of testosterone levels, but achieved a more rapid suppression of PSA. Degarelix also decreased the incidence of testosterone flare that is typically associated with GnRH agonists (Uttley 2017). However, there was no testosterone flare protection in the control groups of the included trials, and Uttley 2017 stated that this was not in accordance with current UK clinical practice. This evaluation on behalf of NICE suggested that degarelix was not cost‐effective for the subgroup with metastatic disease, but could be cost‐effective for the subgroup with spinal metastases (Uttley 2017). However, it should be considered that the recommendation for degarelix in patients with impending spinal cord compression is based on the results of small (post hoc defined) subgroup analyses and on reflection that a rapid androgen suppression with prevention of testosterone flare might be clinically useful. Most participants included in randomized controlled trials had a non‐advanced disease stage, and the studies were not predefined to evaluate degarelix for this purpose.
Authors' conclusions
Implications for practice.
It is unclear if degarelix has any effect on overall survival, cancer‐specific survival, or clinical progression because we did not identify data for these outcomes. Degarelix likely results in no clinically meaningful difference in quality of life, and may result in similar serious adverse events compared to standard androgen suppression therapy. Injection site pain is likely increased with the use of degarelix. The effects of degarelix on cardiovascular events in a general population in clinical routine and on biochemical progression are very uncertain. While degarelix likely increases the total number of non‐serious adverse events slightly, there were similar discontinuations due to adverse events. Degarelix probably reduces the rate of fatal adverse events, as it reduced mortality during study conduction slightly. Degarelix may reduce back pain slightly; likely reduces anemia and urinary tract infections; but also likely increases hepatic enzyme increase compared to standard androgen suppression therapy. Subgroup analyses for different maintenance doses showed no difference between groups for serious adverse events, quality of life, and injection site pain. It remains unclear if different standard androgen suppression therapies or different stages of advanced hormone‐sensitive prostate cancer (non‐metastatic versus metastatic disease) affect these findings. We rated the certainty of evidence as low or moderate because the estimates of effect may be biased due to lack of blinding of participants and personnel and outcome assessment. We are uncertain if the results of biochemical progression directly apply to patients in clinical routine because most trials included predominantly men with localized or locally advanced disease. The long‐term effect of degarelix is still unclear, as the included studies did not evaluate long‐term outcomes. All of the trials reporting outcomes relevant to this review were funded by Ferring Pharmaceuticals or Astellas Pharma Inc, and many study authors had industry relationships. Patients receiving degarelix or other types of androgen suppression therapy should be monitored regularly for cardiovascular events.
Implications for research.
There is a need for methodologically better designed and executed studies, as well as for studies evaluating men with metastatic prostate cancer. Future studies should assess patient‐relevant oncological outcomes such as overall survival, cancer‐specific survival, clinical progression, and should evaluate if different standard androgen suppression therapies or different stages of advanced hormone‐sensitive prostate cancer (non‐metastatic versus metastatic disease) have an effect on the results. There is a need for studies with long‐term follow‐up to evaluate efficacy and safety outcomes and for studies with more participants to reach optimal information size.
What's new
| Date | Event | Description |
|---|---|---|
| 10 August 2021 | Amended | Minor typographical error correction. |
History
Protocol first published: Issue 2, 2017 Review first published: Issue 8, 2021
Notes
Parts of the Methods section and Appendix 1 of this review are based on a standard template developed by the Cochrane Metabolic and Endocrine Disorders Group, which has been modified and adapted for use by the Cochrane Urology Group.
Acknowledgements
We thank the Cochrane Urology editorial team for their help and support, especially Philipp Dahm and Robert Lane. We thank Annemarie Uhlig from UroEvidence for her support and methodological advice. We thank Miran Kenk and Neil Fleshner for additional information on DEG_PRE‐OP, and Bo‐Eric Persson for additional information on PANDA. The replies of Tsutomu Nishiyama, Motohide Uemura, Shin Egawa, and Kenta Miki helped us to clearly exclude the following studies: JPRN‐UMIN000013151; JPRN‐UMIN000015519; JPRN‐UMIN000021806. We also would like to thank Akira Yokomizo for information on the status of the ongoing study JPRN‐UMIN000014243. We also thank the following reviewers for their time and feedback: Thean Hsian Tan, Ganesh Raj, Chris Warlick, Jonathan Wright.
Appendices
Appendix 1. Search strategies
| The Cochrane Library |
| 1 MeSH descriptor: [Prostatic Neoplasms] explode all trees 2 (prostat* near (cancer* or tumo* or neoplas* or carcinom* or malign*)) 3 (#1 or #2) 4 (LHRH antagonist* or LH RH antagonist* or GNRH antagonist* or GN RH antagonist*) 5 (FE200486* or FE 200486*) 6 (firmagon* or degarelix*) 7 (#4 or #5 or #6) 8 (#3 and #7) |
| MEDLINE (via OvidSP) |
| 1 Prostatic Neoplasms/ 2 (prostat* adj3 (cancer* or tumo* or neoplas* or carcinom* or malign*)).tw. 3 1 or 2 4 (LHRH antagonist* or LH RH antagonist* or GNRH antagonist* or GN RH antagonist*).tw. 5 (FE200486* or FE 200486*).mp. 6 (firmagon* or degarelix*).mp. 7 4 or 5 or 6 8 3 and 7 |
| Web of Science (Clarivate Analytics) |
| 1 TS=(prostat* same (cancer* or tumo* or neoplas* or carcinom* or malign*)) 2 TS=((LHRH same antagonist*) or (LH same RH same antagonist*)) 3 TS=((gnrh same antagonist*) OR (gn same rh same antagonist*)) 4 TS=(FE200486*) 5 TS=(FE same 200486*) 6 TS=(firmagon* OR degarelix*) 7 #6 OR #5 OR #4 OR #3 OR #2 8 #7 AND #1 |
| Trial registers: ClinicalTrials.gov, World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal |
| Keywords: ‘degarelix’, ‘firmagon’, 'FE200486', 'FE 200486' |
| Scopus |
| degarelix OR firmagon OR FE200486 OR FE 200486 |
| LILACS |
| Keywords: ‘degarelix’, ‘firmagon’, 'FE200486', 'FE 200486' |
| Embase (via OvidSP) |
| 1 exp prostate tumor/ 2 (prostat* adj3 (cancer* or tumo* or neoplas* or carcinoma* or malign*)).tw. 3 1 or 2 4 exp gonadorelin antagonist/ 5 (LHRH antagonist* or LH RH antagonist or GNRH antagonist* or GN RH antagonist*).tw. 6 (FE200486* or FE 200486*).tw. 7 (firmagon* or degarelix*).tw. 8 4 or 5 or 6 or 7 9 3 and 8 |
Data and analyses
Comparison 1. Degarelix versus standard androgen suppression therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Serious adverse events | 9 | 2750 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.62, 1.05] |
| 1.2 Quality of life | 3 | 2887 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.05, 0.18] |
| 1.3 Injection site pain | 8 | 2670 | Risk Ratio (M‐H, Random, 95% CI) | 15.68 [7.41, 33.17] |
| 1.4 Cardiovascular events | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.04, 0.61] |
| 1.5 Back pain | 5 | 2102 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.46, 0.96] |
| 1.6 Gynecomastia | 1 | 25 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.01, 6.94] |
| 1.7 Constipation | 4 | 1112 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.39, 1.46] |
| 1.8 Diarrhea | 2 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.47, 5.18] |
| 1.9 Vomiting | 2 | 837 | Risk Ratio (M‐H, Random, 95% CI) | 1.56 [0.79, 3.08] |
| 1.10 Loss of sexual interest | 2 | 270 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.35, 3.17] |
| 1.11 Loss of sexual function | 2 | 427 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.39, 1.69] |
| 1.12 Fatigue | 6 | 1996 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.60, 1.16] |
| 1.13 Hot flushes | 8 | 2412 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.86, 1.14] |
| 1.14 Anemia | 5 | 1914 | Risk Ratio (M‐H, Random, 95% CI) | 0.31 [0.13, 0.74] |
| 1.15 Hepatic enzyme increase (alanine aminotransferase) | 4 | 1014 | Risk Ratio (M‐H, Random, 95% CI) | 2.15 [1.26, 3.66] |
| 1.16 Dyspnea | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.39 [0.02, 9.41] |
| 1.17 Urinary tract infection | 5 | 1908 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.25, 0.87] |
| 1.18 Hematuria | 2 | 636 | Risk Ratio (M‐H, Random, 95% CI) | 1.69 [0.58, 4.94] |
| 1.19 Urinary retention | 5 | 1925 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.13, 1.40] |
| 1.20 Mortality during study conduction (post hoc) | 4 | 1821 | Risk Ratio (M‐H, Random, 95% CI) | 0.45 [0.21, 0.97] |
| 1.21 Discontinuation due to adverse events (post hoc) | 8 | 2666 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.79, 1.56] |
| 1.22 Total non‐serious adverse events (post hoc) | 8 | 2412 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [1.01, 1.15] |
| 1.23 Biochemical progression | 2 | 691 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.43, 0.87] |
Comparison 2. Degarelix versus androgen suppression therapy (GnRH agonists or maximum androgen suppression therapy): subgroup analysis based on different doses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Serious adverse events | 9 | 2951 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.63, 1.03] |
| 2.1.1 Degarelix 240 mg/80 mg | 7 | 1466 | Risk Ratio (M‐H, Random, 95% CI) | 0.66 [0.39, 1.14] |
| 2.1.2 Degarelix 240 mg/160 mg | 1 | 403 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.42] |
| 2.1.3 Degarelix 240 mg/480 mg | 2 | 1082 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.64, 1.26] |
| 2.2 Quality of life | 3 | 2887 | Std. Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.05, 0.18] |
| 2.2.1 Degarelix 240 mg/80 mg | 2 | 2040 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.33, 0.28] |
| 2.2.2 Degarelix 240 mg/480 mg | 1 | 847 | Std. Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.04, 0.24] |
| 2.3 Injection site pain | 8 | 2670 | Risk Ratio (M‐H, Random, 95% CI) | 15.68 [7.41, 33.17] |
| 2.3.1 Degarelix 240 mg/80 mg | 6 | 1286 | Risk Ratio (M‐H, Random, 95% CI) | 14.94 [4.48, 49.81] |
| 2.3.2 Degarelix 240 mg/160 mg | 1 | 302 | Risk Ratio (M‐H, Random, 95% CI) | 61.20 [3.82, 979.36] |
| 2.3.3 Degarelix 240 mg/480 mg | 2 | 1082 | Risk Ratio (M‐H, Random, 95% CI) | 15.24 [8.50, 27.31] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anderson 2013 (CS28).
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled clinical trial Study dates: 2009 to 2010 Setting: multicenter, outpatient, international Country: Germany, Spain, United Kingdom Official title: a randomized, parallel‐arm, open‐label trial comparing degarelix with goserelin plus antiandrogen flare protection (bicalutamide), in terms of reduction in international prostate symptom score (IPSS), in patients with lower urinary tract symptoms (LUTS) secondary to locally advanced prostate cancer Follow‐up: 12 weeks |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Sample size: 42 (randomized)/40 (treated) Stage of disease n (%): localized/locally advanced 9 (22.5%); metastatic 14 (35%); unclear 17 (42.5%) |
|
| Interventions | Group 1 (n = 27): degarelix 240 mg/80 mg administered into the abdominal wall every 28 days. A starting dose of 240 mg (40 mg/mL) degarelix was administered on Day 0 as two 3 mL s.c. injections. The second and third doses of 80 mg (20 mg/mL) degarelix were administered as single 4 mL s.c. injections on Days 28 and 56, respectively. Group 2 (n = 13): goserelin (3.6 mg) + bicalutamide (50 mg); goserelin implants (3.6 mg) were inserted s.c. into the abdominal wall every 28 days. The second and third doses of goserelin were administered on Days 31 and 59, respectively. On Day 0, 3 days before the first dose of goserelin on Day 3, men began once‐daily oral treatment with bicalutamide (50 mg) as antiandrogen flare protection; this treatment was continued for 14 days after the first dose of goserelin. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | Ferring Pharmaceuticals | |
| Declarations of interest | Authors had industry relationships. | |
| Notes | Due to low recruitment rate, the inclusion criteria were modified, and the trial was prematurely stopped. 40 of 280 expected participants received at least 1 dose of study treatment and had at least 1 postdose efficacy assessment, and so were included in the full analysis set. Trial ID: NCT00831233, EUCTR2008‐004338‐26‐ES |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: “Patients were randomised 3:1” Comment: insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: “Patients were randomised 3:1” Comment: insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: “open‐label study”; there was no blinding (or it was not reported) Comment: we judge that subjective outcomes are influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote from publication: “open‐label study”; there was no blinding of outcome assessment (or it was not reported) Comment: insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) Biochemical progression | Unclear risk | Comment: the study did not address this outcome. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: two of 42 randomized participants (4.8%) were excluded from analysis because they were never treated. The proportion of missing outcomes is not enough to have a clinically relevant impact on the intervention effect estimate. |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Comment: quality of life assessment was not included because data were not relevant to this review (scale used: IPSS). |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available, and all outcomes of interest have been reported. |
| Other bias | Low risk | Comment: we did not identify other sources of bias. |
Axcrona 2012 (CS31).
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled clinical trial Study dates: 2009 to 2011 Setting: multicenter, outpatient, international Country: Belgium, Denmark, Finland, Italy, Norway, Portugal, Sweden, Turkey Official title: a randomized, parallel‐arm, open‐label trial comparing degarelix with goserelin plus antiandrogen flare protection (bicalutamide), in terms of volume reduction of the prostate in patients with prostate cancer being candidates for medical castration Follow‐up: 12 weeks |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Sample size: 182 (randomized)/179 (treated) Stage of disease, n (%): localized 56 (31%); advanced 106 (59%); unclear 17 (9%) |
|
| Interventions | Group 1 (n = 82): degarelix 240 mg/80 mg administered into the abdominal wall every 28 days. A starting dose of 240 mg (40 mg/mL) degarelix was administered on Day 0 as two 3 mL s.c. injections. The second and third doses of 80 mg (20 mg/mL) degarelix were administered as single 4 mL s.c. injections on Days 28 and 56, respectively. Group 2 (n = 97): goserelin (3.6 mg) + bicalutamide (50 mg); goserelin implants (3.6 mg) were inserted s.c. into the abdominal wall every 28 days. The first dose was administered on Day 0. The second and third doses of goserelin were administered on Days 28 and 56, respectively. On Day 0, participants began once‐daily oral treatment with bicalutamide (50 mg) as antiandrogen flare protection; this treatment continued for 28 days after the first dose of goserelin. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | Ferring Pharmaceuticals | |
| Declarations of interest | Authors had industry relationships. | |
| Notes | Trial ID: NCT00884273, EUCTR2008‐008604‐40‐SE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: “patients were randomized” Comment: insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: “patients were randomized” Comment: insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: “open‐label trial”; there was no blinding (or it was not reported) Comment: we judge that subjective outcomes are influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote from publication: “open‐label trial”; there was no blinding of outcome assessment (or it was not reported) Comment: insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) Biochemical progression | Unclear risk | Comment: the study did not address this outcome. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no missing outcome data. |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Comment: quality of life assessment was not included because data were not relevant to this review (scale used: BPHII). |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available, and all outcomes that are of interest have been reported. |
| Other bias | Low risk | Comment: we did not identify other sources of bias. |
Crawford 2013 (CS37).
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled clinical trial
Study dates: 2009 to 2012 Setting: multicenter, outpatients, national Country: United States Official title: a randomized, controlled, open‐label study investigating the safety and efficacy of degarelix given intermittently vs continuous androgen deprivation therapy with Lupron or degarelix in patients with prostate cancer with prior treatment failure after localized treatment Follow‐up: 14 months |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Sample size: 409 (randomized)/403 (treated) Stage of disease: unclear |
|
| Interventions | Group 1 (Degarelix Intermittent) (n = 175): Phase A: degarelix 240/80 mg; Phase B: degarelix paused; men in this arm received degarelix with a starting dose of 240 mg at a concentration of 40 mg/mL on Day 0 administered s.c. into the anterior abdominal wall via 2 equivalent injections of 120 mg (3 mL) each. 6 maintenance doses of degarelix 80 mg per month at a concentration of 20 mg/mL (4 mL) at Days 28 to 168 were administered. During Phase B of the trial, if a participant had PSA ≥ 2 ng/mL at any visit, additional doses of degarelix 240 mg followed by 80 mg maintenance dose(s) were administered. Degarelix treatment provided for first 7 months (1 starting dose and 6 maintenance doses) followed by no treatment for next 7‐month period. Group 2 (Degarelix Continuous) (n = 50): degarelix 240/80 mg; men in this arm received degarelix with a starting dose of 240 mg at a concentration of 40 mg/mL administered on Day 0 (Visit 1) s.c. into the anterior abdominal wall via 2 equivalent injections of 120 mg (3 mL) each. 13 maintenance doses of degarelix 80 mg per month at a concentration of 20 mg/mL (4 mL) at Days 28 to 364 administered s.c. into the anterior abdominal wall. Degarelix treatment provided for complete study period (1 starting dose and 13 maintenance doses). Group 3 (Leuprolide Continuous) (n = 178): leuprolide 7.5/22.5 mg; men in this arm received leuprolide 7.5 mg 1‐month depot injection on Day 0, administered i.m. into a large muscle, as per manufacturer's labeling directions. 1 injection of 22.5 mg leuprolide 3‐month depot was administered i.m. as per manufacturer's labeling directions at Day 28 and every 3 months afterwards for 4 additional doses (i.e. at Days 112, 196, 280, and 364, respectively). On Investigator's discretion, men in the arm could take bicalutamide (Casodex) for a maximum of 28 days to alleviate increased signs and symptoms due to initial upsurge in testosterone levels. Leuprolide treatment for complete study period (1 starting dose and 5 maintenance doses of 3‐month depot each) |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | Ferring Pharmaceuticals | |
| Declarations of interest | None reported; clinical development support Ferring Pharmaceuticals. | |
| Notes | No full‐text publication available. We did not include data for the degarelix intermittent arm as this information was not relevant to this review. Trial ID: NCT00928434 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: “men were randomized” Comment: insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: “men were randomized” Comment: insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from ClinicalTrials.gov: “This was an open‐label, randomized, parallel‐arm, multicenter study” Comment: we judge that subjective outcomes are influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote from publication: “open‐label trial”; there was no blinding of outcome assessment (or it was not reported) Comment: insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) Biochemical progression | Unclear risk | Comment: the study did not address this outcome. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no missing outcome data. |
| Incomplete outcome data (attrition bias) Quality of life | Low risk | Comment: missing outcome data balanced in numbers across intervention groups (Group 2 18.0% vs Group 3 15.7%). |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is available, but we did not identify full‐text publications. |
| Other bias | Low risk | Comment: we did not identify other sources of bias. |
Klotz 2008 (CS21).
| Study characteristics | ||
| Methods | Study design: parallel‐group (3‐arm) randomized controlled clinical trial Study dates: 2006 to 2007 Setting: multicenter, outpatient, international Country: Canada, Czech Republic, Germany, Hungary, Mexico, Netherlands, Puerto Rico, Romania, Russian Federation, Ukraine, United Kingdom, United States Official title: an open‐label, multicenter, randomized, parallel‐group study, investigating the efficacy and safety of degarelix 1‐month dosing regimens, 160 mg (40 mg/mL) and 80 mg (20 mg/mL), in comparison to LUPRON DEPOT 7.5 mg in men with prostate cancer requiring androgen ablation therapy Follow‐up: 364 days |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Sample size: 620 (randomized)/610 (treated) Stage of disease, n (%): localized 191 (31%); locally advanced 178 (29%); metastatic 125 (20%); not classifiable 116 (19%) |
|
| Interventions | Group 1 (n = 202): degarelix 240/160 mg; initial dose of 240 mg s.c. on Day 0. Maintenance dose of 160 mg s.c. given every 28 days for 364 days. Group 2 (n = 207): degarelix 240/80 mg; initial dose of 240 mg s.c. on Day 0. Maintenance dose of 80 mg s.c. given every 28 days for 364 days. Group 3 (n= 201): leuprolide 7.5 mg; leuprolide (Lupron Depot) 7.5 mg i.m. every 28 days starting at Day 0. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | Ferring Pharmaceuticals | |
| Declarations of interest | Authors had industry relationships. | |
| Notes | Trial ID: NCT00295750, EUCTR2005‐005595‐33‐DE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: “Randomization lists were prepared centrally (...), using validated computer program” Comment: randomization was adequately performed. |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: “Central allocation” Comment: adequate allocation concealment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: “open‐label study” Comment: we judge that subjective outcomes are influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote from publication: “Open‐label study”; “personnel were unaware of blood values” Comment: insufficient information to permit judgment. The “personnel were unaware of blood values,” but it remained unclear if outcome assessment was blinded to PSA values for evaluation of biochemical progression, and there was no information for assessment of adverse events. |
| Incomplete outcome data (attrition bias) Biochemical progression | Low risk | Comment: no relevant missing outcome data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no missing outcome data. |
| Incomplete outcome data (attrition bias) Quality of life | Low risk | Comment: the return rate of questionnaires used in the study was minimum 90.6%. Plausible effect size among missing outcomes not enough to have a clinically relevant impact on observed effect size. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available, and all outcomes of interest have been reported. |
| Other bias | Low risk | Comment: we did not identify other sources of bias. |
Margel 2019 (0102‐15‐RMC).
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized open‐label controlled clinical trial Study dates: 2015 to 2019 Setting: multicenter (2 centers), national, outpatient Country: Israel Official title: a pilot study on endothelial function and cardiovascular biomarkers in prostate cancer (PCa) patients, with pre‐existing cardiovascular disease, treated with degarelix vs luteinizing hormone‐releasing hormone (LHRH) agonists Follow‐up: 12 months |
|
| Participants | Men with advanced (high‐risk or metastatic) prostate cancer and pre‐existing cardiovascular disease Inclusion criteria:
Exclusion criteria:
Sample size: 80 (randomized)/80 (treated) Stage of disease, n (%): localized 59 (74%); metastatic 21 (26%) |
|
| Interventions | Group 1 (n = 41): degarelix; initial loading dose of 240 mg degarelix followed by 11 monthly injections of 80 mg Group 2 (n = 39): GnRH agonist; 4 injections of 3‐month depot at the discretion of the treating urologist/oncologist |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | Ferring Pharmaceuticals | |
| Declarations of interest | Authors had industry relationships. | |
| Notes | Trial ID: NCT02475057 The following patient‐relevant predefined outcome has not been reported as of yet: change in quality of life score as assessed by the Functional Assessment of Cancer Therapy‐Prostate (FACT‐P) quality of life questionnaire. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: “Randomization was done by minimization using MINIM software” Comment: we assume that randomization was adequately performed. |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: “The allocation sequence was created and coordinated at the study central office” Comment: adequate allocation concealment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: “open‐label study” Comment: we judge that subjective outcomes are influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote from publication: “A cardiologist blinded to treatment assignment reviewed all medical records and categorized all cardiac events” Comment: adequate outcome assessment. |
| Incomplete outcome data (attrition bias) Biochemical progression | Unclear risk | Comment: the study did not address this outcome. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no missing outcome data |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Comment: the study did not address this outcome. |
| Selective reporting (reporting bias) | High risk | Comment: the study protocol is available. Quality of life is prespecified in the protocol but not reported in the results. |
| Other bias | Low risk | Comment: we did not identify other sources of bias. |
Mason 2013 (CS30).
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled clinical trial Study dates: 2009 to 2011 Setting: multicenter, outpatient, international Country: France, Germany, Greece, Netherlands, Spain, United Kingdom, United States Official title: a randomized, parallel‐arm, open‐label trial comparing degarelix with goserelin plus antiandrogen flare protection (bicalutamide), in terms of prostate size reduction in prostate cancer patients of intermediate‐to‐high risk, who require neoadjuvant hormone therapy prior to radiotherapy (curative intent) Follow‐up: 12 weeks |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Sample size: 246 (randomized)/244 (treated) Stage of disease, n (%): localized 152 (62%); advanced 83 (34%); not classifiable 9 (4%) |
|
| Interventions | Group 1 (n = 180): degarelix 240 mg/80 mg administered into the abdominal wall every 28 days. A starting dose of 240 mg (40 mg/mL) degarelix was administered on Day 0 as two 3 mL s.c. injections. The second and third doses of 80 mg (20 mg/mL) degarelix were administered as single 4 mL s.c. injections on Days 28 and 56, respectively. Group 2 (n = 64): goserelin (3.6 mg) + bicalutamide (50 mg); on Day 0, men began once‐daily oral treatment with bicalutamide as antiandrogen flare protection. This treatment continued for 2 weeks after the first dose of goserelin (i.e. 17 days in total). On Day 3, the first goserelin implant was inserted s.c. into the abdominal wall. The second and third doses of goserelin were administered on Days 31 and 59, respectively. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | Ferring Pharmaceuticals | |
| Declarations of interest | Authors had industry relationships. | |
| Notes | Trial ID: NCT00833248, EUCTR2008‐005232‐33‐NL | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: “patients were randomised in a 3:1 ratio” Comment: insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: “patients were randomised in a 3:1 ratio” Comment: insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: “open‐label trial”; there was no blinding (or it was not reported) Comment: we judge that subjective outcomes are influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote from publication: “open‐label trial”; there was no blinding of outcome assessment (or it was not reported) Comment: insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) Biochemical progression | Unclear risk | Comment: the study did not address this outcome. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no missing outcome data. |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Comment: quality of life assessment was not included because data were not relevant to this review (scale used: IPSS). |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available, and all outcomes of interest have been reported. |
| Other bias | Low risk | Comment: we did not identify other sources of bias. |
Ozono 2018 (3550‐CL‐0010).
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled clinical trial Study dates: 2013 to 2016 Setting: multicenter, national, outpatient Country: Japan Official title: ASP3550 Phase III study ‐ an open‐label, active‐controlled, parallel‐arm study, comparing ASP3550 with goserelin acetate in patients with prostate cancer Follow‐up: 12 months |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Sample size: 234 (randomized)/234 (treated) Stage of disease, n (%): localized 124 (53%); locally advanced 63 (27%); advanced (metastasized) 44 (19%); not classifiable 3 (1%) |
|
| Interventions | Group 1 (n = 117): degarelix (ASP3550) 240 mg/480 mg; an initial dose of 240 mg (40 mg/mL) degarelix was s.c. administered; after Day 28, a maintenance dose of 480 mg (60 mg/mL) was given once every 84 days. Group 2 (n = 117): goserelin (3.6 mg); an initial dose of 3.6 mg goserelin was s.c. administered; after Day 28, a maintenance dose of 10.8 mg was given once every 84 days. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | Astellas Pharma Inc | |
| Declarations of interest | Authors had industry relationships. | |
| Notes | This study consisted of 2 parts: PART 1: ASP3550 or goserelin acetate administered for 1 year; PART 2: men assigned to receive ASP3550 and who completed the treatment in PART 1 were eligible for the treatment in PART 2, and received ASP3550 maintenance dose s.c. for long‐term safety and efficacy. We did not include data for PART 2 because of the single‐arm design. Trial ID: NCT01964170 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: “subjects were randomly allocated into a degarelix or goserelin group using a minimization method of adjusting age, cancer stage, pretreatment, and serum PSA” Comment: we assume that randomization was adequately performed. |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: “subjects were randomly allocated into a degarelix or goserelin group using a minimization method of adjusting age, cancer stage, pretreatment, and serum PSA” Comment: insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: “open‐label, parallel‐arm study”, “For the safety analysis, the incidence of AEs, SAEs, and ADRs were collected and graded according to Common Terminology Criteria for Adverse Events version 4.0.” Comment: we judge that subjective outcomes are influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote from publication: “open‐label trial”; there was no blinding of outcome assessment (or it was not reported) Comment: insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) Biochemical progression | Unclear risk | Comment: the study did not address this outcome. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk |
Quote from publication: “degarelix group: withdrawals 19/117 (=16.2 %); goserelin group: withdrawals 23/117 (=19.7 %)” Comment: missing outcome data are balanced in numbers across intervention groups with similar reasons for missing data across groups. |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Comment: the study did not address this outcome. |
| Selective reporting (reporting bias) | Low risk | Comment: the study protocol is available, and all outcomes of interest have been reported. |
| Other bias | Low risk | Comment: we did not identify other sources of bias. |
Sawazaki 2019.
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized open‐label controlled clinical trial
Study dates: 2016 to 2018
Setting: single‐center trial, national, outpatient
Country: Japan Official title: metabolic changes with degarelix vs leuprolide plus bicalutamide in patients with prostate cancer Follow‐up: 6 months |
|
| Participants |
Inclusion criteria:
Exclusion criteria:
Sample size: 100 Stage of disease, n (%): localized 76 (76%); locally advanced or metastatic, or both: 24 (24%) |
|
| Interventions | Group 1 (n = 50): degarelix starting dose of 240 mg s.c. followed by a maintenance dose of 80 mg every 28 days Group 2 (n = 50): leuprolide 3.75 mg dose every 28 days. Men in the leuprolide arm were given prophylactic 80 mg bicalutamide once daily to prevent the flare phenomenon; this was continued throughout the initial dosing period of 14 days. |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Funding sources | Not reported | |
| Declarations of interest | None reported. | |
| Notes | No outcomes from this study were included in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: “prospective randomized, parallel‐arm, open‐label, single‐center trial” Comment: insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: “prospective randomized, parallel‐arm, open‐label, single‐center trial” Comment: insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: “Open‐label study” Comment: none of the reported outcomes were relevant to this review, therefore none were included in the review. Evaluation of adverse events could have been expected, and we judge that subjective outcomes are influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote from publication: “open‐label study”; there was no blinding of outcome assessment (or it was not reported) Comment: insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) Biochemical progression | Unclear risk | Comment: the study did not address this outcome. |
| Incomplete outcome data (attrition bias) Adverse events | Unclear risk | Comment: the study did not address this outcome. |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Comment: the study did not address this outcome. |
| Selective reporting (reporting bias) | High risk | Comment: adverse events were not reported, although evaluation of this outcome could have been expected. |
| Other bias | Low risk | Comment: we did not identify other sources of bias. |
Sayyid 2017 (DEG_PRE‐OP).
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized open‐label controlled clinical trial
Study dates: 2012 to 2015
Setting: 2 centers, national, outpatients
Country: Canada Official title: phase II randomized open‐label study of neo‐adjuvant degarelix vs LHRH agonist in prostate cancer patients prior to radical prostatectomy Follow‐up: 12 weeks |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Sample size: 39 (randomized)/39 (treated) Stage of disease, n (%; multiple entry): localized 10 (26%); locally advanced 15 (60%); node positive 6 (24%); PSA failure (> 0.2 ng/mL) or use of adjuvant androgen suppression/radiotherapy 8 (21%) |
|
| Interventions | Group 1 (n = 13): degarelix 240 mg/80 mg; 1 degarelix 240 mg s.c. injection (starting dose), followed by 2 monthly maintenance doses of 80 mg each Group 2 (n = 14): degarelix 240 mg/80 mg + bicalutamide; 1 degarelix 240 mg s.c. injection (starting dose), followed by 2 monthly maintenance doses of 80 mg each; bicalutamide as a once‐daily 50 mg tablet Group 3 (n = 12): GnRH agonist + bicalutamide; LHRH as a 3‐month injectable dose of leuprorelin 22.5 mg, leuprolide 22.5 mg, or goserelin acetate 10.8 mg; bicalutamide as a once‐daily 50 mg tablet |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | Ferring Pharmaceuticals | |
| Declarations of interest | Authors had industry relationships. | |
| Notes | We did not include Group 2 in our analyses because combined degarelix and non‐steroidal antiandrogen was not predefined in our protocol. Trial ID: NCT01674270 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from publication: “patients were block‐randomized 1:1:1” Quote from correspondence: “This study followed block randomization and was stratified by study site using a computer‐generated list of random numbers.” Comment: adequate random sequence generation. |
| Allocation concealment (selection bias) | Low risk |
Quote from publication: not reported Quote from correspondence: “The allocation sequence was created and coordinated centrally, through the University Health Network Uro‐Oncology Research Unit in Toronto. Participant enrolment and assignment to intervention was performed at each site utilizing prefilled sequential randomisation envelopes which contained a 4‐digit code (2‐digit centre code followed by a 2‐digit patient code plus the treatment assignment listed as Arm A, B, or C). This 4‐digit randomisation number was recorded in the site enrolment log, the subject's eCRF and on the study medication page.” Comment: adequate allocation concealment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: “Open‐label study” Quote from correspondence: “This was an open label randomized study; therefore all study investigators, participants and research coordination staff were unblinded to the treatment allocation for the duration of the study.” Comment: we judge that subjective outcomes are influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Low risk |
Quote from correspondence: “Tissue and data handlers and analysts were blinded to the treatment allocation.” Comment: adequate outcome assessment. |
| Incomplete outcome data (attrition bias) Biochemical progression | Unclear risk | Comment: the study did not address this outcome. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no missing outcome data. |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Comment: the study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk |
Quote from correspondence: “While safety was not pre‐specified as an outcome, toxicity of study treatments was monitored throughout the study, with regular reporting...” Comment: insufficient information to permit judgment. |
| Other bias | Low risk | Comment: we did not identify other sources of bias. |
Shore 2012 (CS35).
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized controlled clinical trial Study dates: 2009 to 2011 Setting: multicenter, international, outpatient Country: Belgium, Canada, Czech Republic, Finland, Germany, Hungary, Mexico, Netherlands, Poland, Romania, Russian Federation, Ukraine, United Kingdom, United States Official title: an open‐label, multicenter, randomized, parallel‐arm 1‐year trial comparing the efficacy and safety of degarelix 3‐month dosing regimen with goserelin acetate in patients with prostate cancer requiring androgen deprivation therapy Follow‐up: 13 months |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Sample size: 859 (randomized)/848 (treated) Stage of disease, n (%): unclear |
|
| Interventions | Group 1 (n = 565): degarelix 240 mg/480 mg administered by s.c. injections into the abdominal wall. A starting dose of 240 mg degarelix was administered on Day 0. 1 month later a maintenance dose of 480 mg was administered; this was repeated after 4, 7, and 10 months (i.e. a total of 5 administrations). Group 2 (n = 283): goserelin 3.6 mg/10.8 mg administered by s.c. implants into the abdominal wall. An initial dose of 3.6 mg goserelin was administered on Day 0. 1 month later a subsequent dose of 10.8 mg was administered; this was repeated after 4, 7, and 10 months (i.e. a total of 5 implants). |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | Ferring Pharmaceuticals | |
| Declarations of interest | None reported. | |
| Notes | Trial ID: NCT00946920, EUCTR2008‐005276‐27‐HU | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
Quote from publication: “open‐label, randomised study” Comment: insufficient information to permit judgment. |
| Allocation concealment (selection bias) | Unclear risk |
Quote from publication: “open‐label, randomised study” Comment: insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from publication: “open‐label study”; there was no blinding (or it was not reported) Comment: we judge that subjective outcomes are influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote from publication: “open‐label study”; there was no blinding of outcome assessment (or it was not reported) Comment: insufficient information to permit judgment. |
| Incomplete outcome data (attrition bias) Biochemical progression | Unclear risk | Comment: the study did not address this outcome. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk | Comment: no missing outcome data. |
| Incomplete outcome data (attrition bias) Quality of life | Low risk | Comment: exclusion rate 1 of 848 (0.1%). |
| Selective reporting (reporting bias) | Unclear risk | Comment: the study protocol is available, but we did not identify full‐text publications. |
| Other bias | Low risk | Comment: we did not identify other sources of bias. |
Xie 2016 (PANDA).
| Study characteristics | ||
| Methods | Study design: parallel‐group randomized open‐label controlled clinical trial
Study dates: 2013 to 2015 Setting: multicenter, national, outpatient Country: China Official title: an open‐label, multicenter, randomized, parallel‐group trial comparing the efficacy and safety of degarelix 1‐month dosing regimen with goserelin in Chinese patients with prostate cancer requiring androgen ablation therapy Follow‐up: 364 days |
|
| Participants | Inclusion criteria:
Exclusion criteria:
Sample size: 285 (randomized)/283 (treated) Stage of disease, n (%): unclear |
|
| Interventions | Group 1 (n = 143): degarelix 240 mg/80 mg. Starting dose of 240 mg degarelix at a concentration of 40 mg/mL, administered as deep s.c. injections on Day 0 in the abdominal region via 2 equivalent injections of 120 mg each; 12 maintenance doses of 80 mg degarelix at a concentration of 20 mg/mL, administered at monthly (28‐day) intervals as deep s.c. injections in the abdominal region via 1 injection of 80 mg. Group 2 (n = 142): goserelin 3.6 mg. 13 doses of 3.6 mg goserelin sustained‐release depot (Zoladex 3.6 mg), administered at monthly (28‐day) intervals s.c. into the anterior abdominal wall according to the directions for use per the manufacturer’s labeling. |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Funding sources | Ferring Pharmaceuticals | |
| Declarations of interest | Authors had industry relationships. | |
| Notes | Trial ID: NCT01744366 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
Quote from correspondence: “Computer‐generated randomisation lists allocating patients to one of the two treatments in a 1:1 ratio per stratum. The randomisation lists were stratified into groups of patients having had previous therapy with 5‐alpha reductase inhibitors within the last year, and those patients that did not.” Comment: adequate random sequence generation. |
| Allocation concealment (selection bias) | Unclear risk |
Quote from correspondence: “The treatment allocation was open‐label.” Comment: insufficient information to permit judgment. |
| Blinding of participants and personnel (performance bias) Subjective outcomes | High risk |
Quote from correspondence: “An open‐label design was chosen as blinding was not feasible due to the formulation differences between degarelix and goserelin.” Comment: we judge that subjective outcomes are influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Subjective outcomes | Unclear risk |
Quote from correspondence: “Testosterone and PSA levels (with the exception of the screening samples) were masked for Sponsor personnel directly involved in the trial.” Comment: blood values are not likely to being influenced by lack of blinding, but insufficient reporting regarding outcome assessment of adverse events. |
| Incomplete outcome data (attrition bias) Biochemical progression | Low risk | Comment: no relevant missing outcome data. |
| Incomplete outcome data (attrition bias) Adverse events | Low risk |
Quote from correspondence: “There were two patients withdrawing consent after randomisation and before first trial product administration ('first dose'); otherwise no exclusions were made.” Comment: the proportion of missing outcomes is not enough to have a clinically relevant impact on the intervention effect estimate. |
| Incomplete outcome data (attrition bias) Quality of life | Unclear risk | Comment: the study did not address this outcome. |
| Selective reporting (reporting bias) | Unclear risk | Comment: no study protocol is available. |
| Other bias | Low risk | Comment: we did not identify other sources of bias. |
GnRH: gonadotropin‐releasing hormone
FSH: follicle‐stimulating hormone
i.m.: intramuscular
IPSS: International Prostate Symptom Score
PSA: prostate‐specific antigen
QoL: quality of life
s.c.: subcutaneous
CT scan: computed tomography scan
ABPI: Ankle Brachial Pressure Index
WHO: World Health Organization
UICC: Union for International Cancer Control
LHRH: luteinizing hormone releasing hormone
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abrahamsson 2014 | Wrong comparator |
| Abrahamsson 2015 | Wrong comparator |
| Albertsen 2013a | Wrong study design (review) |
| Albertsen 2013b | Wrong study design (review) |
| Albertsen 2014 | Wrong study design (review) |
| Ammannagari 2016 | Wrong study design |
| Augustovski 2006 | Wrong study design (HTA) |
| Aust Prescr 2010 | Wrong study design (review) |
| AWMSG 2009 | Wrong study design (HTA) |
| AWMSG 2012 | Wrong study design (HTA) |
| Borre 2015 | Wrong study design (review) |
| Borsellino 2014 | Wrong study design |
| Chan 2014 | Wrong study design |
| ChoungSoo 2012 | Wrong study design (single‐arm degarelix) |
| Crawford 2013 | Wrong study design (review) |
| Crehange 2015 | Wrong study design |
| Damber 2012a | Wrong study design (reply to editorial letter) |
| Dearnaley 2016 | Wrong comparator |
| Degarelix Study Grp. 2005 | Wrong comparator |
| Guerif 2017 | Wrong comparator |
| Iversen 2013 | Wrong study design (review) |
| JPRN‐UMIN000013151 | Wrong study design (non‐randomized trial) |
| JPRN‐UMIN000015519 | Wrong co‐intervention (brachytherapy) |
| JPRN‐UMIN000021806 | Trial discontinued |
| Medical Letter 2009 | Wrong study design (review) |
| NCT01786265 | Wrong study design |
| NCT02234089 | Wrong study design |
| NCT02278185 | Wrong comparator (enzalutamide) |
| Nosov 2016 | Wrong study design |
| Nozawa 2015 | Wrong comparator (degarelix + bicalutamide) |
| Nozawa 2016 | Wrong comparator (degarelix + bicalutamide) |
| Prescrire Int 2010 | Wrong study design (review) |
| Shore 2010 (CS21A) | No comparator |
| Sokolakis 2014 | Wrong study design (non‐randomized trial) |
| Touijer 2014 | Wrong comparator |
| Van Poppel 2006 | Wrong comparator |
| Van Poppel 2007 | Wrong comparator |
| Weston 2005 | Wrong study design (reply) |
HTA: health technology assessment
Characteristics of ongoing studies [ordered by study ID]
000108 (PRONOUNCE).
| Study name | A multi‐centre, randomised, assessor‐blind, controlled trial comparing the occurrence of major adverse cardiovascular events (MACEs) in patients with prostate cancer and cardiovascular disease receiving degarelix (gonadotropin‐releasing hormone (GnRH) receptor antagonist) or leuprolide (GnRH receptor agonist) |
| Methods |
Study design: parallel‐group randomized open‐label controlled clinical trial Setting: multicenter, international, outpatient Country: United States (majority of sites), Canada, Czech Republic, France, Germany, Greece, Poland, Russian Federation, Slovakia, South Africa, United Kingdom Follow‐up: 364 days |
| Participants |
Inclusion criteria:
Exclusion criteria:
Target sample size: 900 |
| Interventions |
Group 1: degarelix Group 2: leuprolide |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | April 2016 |
| Contact information | Contact: Clinical Development Support Email: DK0‐Disclosure@ferring.com Study Director: Clinical Development Support Ferring Pharmaceuticals Principal Investigator: Howard Scher, MD Sidney Kimmel Center for Urologic and Prostate Cancers, Memorial Sloan Kettering Cancer Center Principal Investigator: Matthew Roe, MD, MHS Division of Cardiovascular Medicine, Duke Clinical Research Institute |
| Notes | Sponsors and collaborators:
Estimated study completion date: October 2021 Estimated primary completion date: October 2021 (final data collection date for primary outcome measure) Trial ID: NCT02663908 |
JPRN‐UMIN000014243.
| Study name | Randomised controlled study of GnRH antagonist monotherapy and CAB with GnRH agonist plus bicalutamide for patients with metastatic prostate cancer |
| Methods |
Study design: parallel‐group randomized open‐label controlled clinical trial Setting: multicenter, national Country: Japan |
| Participants |
Inclusion criteria:
Exclusion criteria:
Target sample size: 200 Age (years): ≥ 20 years (no upper limit) Sex (M/F): male only |
| Interventions |
Group 1: degarelix 240 mg, s.c. at Day 1; degarelix 240 mg, s.c. every 4 weeks; bicalutamide 80 mg daily (as deferred CAB therapy in the case of PSA recurrence) Group 2: leuprorelin or goserelin: s.c. injection (according to usage and administration of package insert); bicalutamide 80 mg daily |
| Outcomes |
Primary outcomes:
Secondary outcomes:
|
| Starting date | July 2014 |
| Contact information | Akira Yokomizo Kyushu University Department of Urology, Graduate School of Medical Sciences 3‐1‐1, Maidashi, Higashi‐Ku, Fukuoka, Japan, 812‐8582 Telephone: +81 (0)92‐642‐5378 Email: yokoa@uro.med.kyushu‐u.ac.jp |
| Notes | Funded by Astellas Pharma Inc Recruitment will be closed by July 2017. Anticipated last follow‐up date: March 2019 Corresponding author (Akira Yokomizo) quote: “No clinical data will be available until the last patient's follow up after two years.” Trial ID: JPRN‐UMIN000014243, KYUCOG‐1401 |
NCT01542021.
| Study name | Establishing a neo‐adjuvant platform for developing targeted agents: androgen deprivation therapy prior to prostatectomy for patients with intermediate and high risk prostate cancer |
| Methods |
Study design: randomized, parallel assignment, open‐label Setting: single center; Memorial Sloan Kettering Cancer Center (MSKCC) Country: United States Follow‐up: 2 years |
| Participants |
Inclusion criteria:
Exclusion criteria:
Target sample size: 41 |
| Interventions |
Group 1: degarelix Group 2: androgen deprivation therapy |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | February 2012 |
| Contact information | Dana Rathkopf, MD; Memorial Sloan Kettering Cancer Center |
| Notes | Sponsors and collaborators: Memorial Sloan Kettering Cancer Center and Ferring Pharmaceuticals Recruitment status: active, not recruiting (checked on 5 November 2020) Estimated primary completion date: February 2021 Trial ID: NCT01542021 |
NCT02799706.
| Study name | Phase IIIb randomised trial comparing irradiation plus long term adjuvant androgen deprivation with GnRH antagonist versus GnRH agonist plus flare protection in patients with very high risk localized or locally advanced prostate cancer |
| Methods |
Study design: parallel‐group randomized open‐label controlled clinical trial Setting: multicenter Country: Europe Follow‐up: unclear |
| Participants |
Inclusion criteria:
Exclusion criteria:
Target sample size: 885 Age (years): 18 to 80 Sex (M/F): male only |
| Interventions |
Group 1 (sham comparator): GnRH agonist + radiation therapy (RT) As the study investigates the effect of a drug given concomitantly to radiotherapy, all men will be treated with the same treatment technique and target dose. The preferred treatment technique is intensity modulated radiotherapy + a GnRH agonist will be given for the duration selected for each participant. A non‐steroidal antiandrogen (e.g. flutamide, bicalutamide) will be given orally 1 week before the first injection of the GnRH agonist and will be continued for no longer than 8 weeks to protect against flare. Dose may vary due to availability of different brand names and pharmaceutical forms. The start of antiandrogen must be registered as Day 1 of treatment in the GnRH agonist arm. Group 2 (active comparator): degarelix + RT As the study investigates the effect of 2 drugs given concomitantly to radiotherapy, all men will be treated with the same treatment technique and target dose. The preferred treatment technique is intensity modulated radiotherapy + a GnRH antagonist will be given for a predefined duration of 18, 24, or 36 months as per institution policy. Each institution must adhere to the chosen duration of treatment for all participants throughout the study. |
| Outcomes |
Primary outcomes:
Where
Secondary outcomes:
|
| Starting date | April 2017 |
| Contact information | Piotr Banski, PhD Telephone: 003227741553 Email: piotr.banski@eortc.be |
| Notes | Sponsors and collaborators: European Organisation for Research and Treatment of Cancer (EORTC) Principal Investigator: Dirk Boehmer, MD, PhD Charité ‐ Universitaetsmedizin Berlin ‐ Campus Benjamin Franklin Recruitment status: recruiting (checked on 14 August 2020) Estimated primary completion date: June 2024 (final data collection date for primary outcome measure) Trial ID: NCT02799706 |
NCT04182594.
| Study name | A phase‐II, randomised, assessor‐blind, controlled trial comparing the occurrence of cardiovascular events in patients with prostate cancer and cardiovascular risk factors receiving degarelix or GnRH agonist |
| Methods |
Study design: randomized phase II, open‐label superiority study of the use of androgen suppression therapy combined with second‐line hormonal or chemotherapy in men with advanced prostate cancer and pre‐existing cardiovascular risks Setting: Rabin Medical Center ‐ Beilinson Hospital Country: Israel Follow‐up: 1 year |
| Participants |
Inclusion criteria:
Exclusion criteria:
Target sample size: 80 |
| Interventions |
Group 1: degarelix Group 2: GnRH agonist |
| Outcomes |
Primary outcome:
Secondary outcomes:
|
| Starting date | 17 January 2020 |
| Contact information | Rabin Medical Center ‐ Beilinson Hospital Yaara Ber, PhD 972‐3‐9376553 yaaraba1@clalit.org.il |
| Notes | Sponsor: Rabin Medical Center and Ferring Pharmaceuticals Recruitment status: not yet recruiting (checked on 4 November 2020) Estimated primary completion date: 17 January 2023 Trial ID: NCT04182594 |
BMI: body mass index
FACT‐P: Functional Assessment of Cancer Therapy‐Prostate
GnRH: gonadotropin‐releasing hormone
PSA: prostate‐specific antigen
s.c.: subcutaneous
WHO: World Health Organization
CAB: complete androgen blockade
TURP: transurethral resection of the prostate
PTEN: Phosphatase and tensin homolog
MRI: Magnetic resonance imaging
ICH/GCP: International Conference on Harmonisation of technical requirements for registration of pharmaceuticals for human use/ Good Clinical Practice
AST: aspartate transaminase
ALT: alanine transaminase
PCI: percutaneous coronary intervention
CABG: coronary artery bypass grafting BP: blood pressure
CTCAE: common terminology criteria for adverse events
ABPI: ankle brachial pressure index
MACCE: major adverse cardiac and cerebrovascular events
NTproBNP: N terminales pro brain natriuretic peptide
Differences between protocol and review
This review was based on a published protocol; any differences between the protocol and the review are as follows.
We included 'mortality during study conduction' as a further adverse event outcome because no study prospectively planned to assess our predefined primary outcome of overall survival.
We included the patient‐relevant outcome 'discontinuation due to adverse events' as a further adverse event outcome.
We included the outcome 'total non‐serious adverse events' for better interpretation of the other serious and non‐serious adverse events.
We initially planned to assess the outcome 'injection site events.' However, the included studies did not assess this outcome, and we post hoc specified this patient‐relevant event and instead assessed 'injection site pain.'
We specified our predefined outcome 'pain,' and assessed data for 'back pain.'
We specified our predefined outcome 'infections,' and assessed data for 'urinary tract infection.'
We also included data for men with localized disease (defined as prostate cancer within the prostate gland; T1‐2 N0 M0) because the percentage of participants with locally advanced or metastatic prostate cancer was less than 80% in all included trials (TNM 2005). We downgraded the certainty of evidence for indirectness where appropriate.
We initially developed our search strategy to search Embase via DIMDI. However, we changed the search strategy because we searched Embase via OvidSP because of license problems.
Contributions of authors
Friedemann Zengerling (FZ): title/abstract screening, acquiring trial reports, full‐text screening, data extraction, data analysis, review drafting.
Joachim J Jakob (JJJ): critical review of protocol draft, title/abstract screening, acquiring trial reports, full‐text screening, data extraction, data analysis, data interpretation, review drafting.
Stefanie Schmidt (SS): critical review of protocol and manuscript, risk of bias assessment, methodological advice.
Joerg J Meerpohl (JJM): protocol and review drafting, data analysis, data interpretation, critical review of manuscript, methodological advice.
Anette Blümle (AB): data extraction, data interpretation, review drafting.
Christine Schmucker (CS): fourth reviewer for the selection of studies/evaluation of adverse events, risk of bias assessment, review drafting.
Benjamin Mayer (BM): data analysis, data interpretation, critical review of manuscript, methodological advice.
Frank Kunath (FK): protocol drafting, search strategy development, risk of bias assessment, data interpretation, data analysis, review drafting, critical review of manuscript.
Sources of support
Internal sources
-
Frank Kunath, Germany
University Hospital Erlangen, Germany, Salary support for Frank Kunath
-
Joachim J Jakob, Germany
University Hospital Ulm, Germany, Salary support for Joachim J Jakob
-
Stefanie Schmidt, Germany
UroEvidence@Deutsche Gesellschaft für Urologie, Berlin, Salary support for Stefanie Schmidt
-
Joerg J Meerpohl, Germany
University of Freiburg Faculty of Medicine Freiburg, Institute for Evidence in Medicine (for Cochrane Germany Foundation), Salary support for Joerg J Meerpohl
-
Friedemann Zengerling, Germany
University Hospital Ulm, Salary support for Friedemann Zengerling
-
Anette Blümle, Germany
University of Freiburg Faculty of Medicine Freiburg, Institute for Evidence in Medicine (for Cochrane Germany Foundation), Salary support for Anette Blümle
-
Christine Schmucker, Germany
University of Freiburg Faculty of Medicine Freiburg, Institute for Evidence in Medicine (for Cochrane Germany Foundation), Salary support for Christine Schmucker
-
Benjamin Mayer, Germany
University Ulm, Institute of Epidemiology and Medical Biometry, Salary support for Benjamin Mayer
External sources
-
Frank Kunath, Germany
None
-
Joachim J Jakob, Germany
None
-
Stefanie Schmidt, Germany
None
-
Joerg J Meerpohl, Germany
None
-
Friedemann Zengerling, Germany
None
-
Anette Blümle, Germany
None
-
Christine Schmucker, Germany
None
-
Benjamin Mayer, Germany
None
Declarations of interest
Friedemann Zengerling: none known
Joachim J Jakob: none known
Stefanie Schmidt: none known
Joerg J Meerpohl: none known
Anette Blümle: none known
Christine Schmucker: none known
Benjamin Mayer: none known
Frank Kunath: none known
These authors contributed equally to this work.
These authors contributed equally to this work.
Edited (no change to conclusions)
References
References to studies included in this review
Anderson 2013 (CS28) {published data only}
- Anderson J, Al-Ali G, Wirth M, Gual JB, Gomez Veiga F, Colli E, et al. Degarelix versus goserelin (+ antiandrogen flare protection) in the relief of lower urinary tract symptoms secondary to prostate cancer: results from a phase IIIb study (NCT00831233). Urologia Internationalis 2013;90(3):321-8. [DOI: 10.1159/000345423] [DOI] [PubMed] [Google Scholar]
Axcrona 2012 (CS31) {published data only}
- Axcrona K, Aaltomaa S, da Silva CM, Ozen H, Damber JE, Tankó LB, et al. Androgen deprivation therapy for volume reduction, lower urinary tract symptom relief and quality of life improvement in patients with prostate cancer: degarelix vs goserelin plus bicalutamide. BJU International 2012;110(11):1721-8. [DOI: 10.1111/j.1464-410X.2012.11107.x] [DOI] [PubMed] [Google Scholar]
- Axcrona K, Aaltomaa S, Da Silva CM, Ozen H, Damber JE, Tanko LB, et al. ADT for volume reduction, symptom relief and quality of life improvement in men with prostate cancer: degarelix versus goserelin plus bicalutamide. European Urology, Supplements 2012;11(1):e985-e985a. [DOI] [PubMed] [Google Scholar]
Crawford 2013 (CS37) {published data only}
- Crawford D, Shore N, Higano C, Neijber A, Yankov V. PD27-05 Intermittent androgen deprivation with the gonadotropin-releasing hormone antagonist degarelix. Journal of Urology 2014;191(4):e766. [Google Scholar]
- Crawford ED, Shore N, Higano C, Neijber A, Yankov V. Intermittent androgen deprivation with the gonadotropin-releasing hormone (GnRH) antagonist degarelix: results of study CS37. In: 14th Annual Meeting of the Society of Urologic Oncology; 2013 Dec 4-6; Bethesda (MD). Bethesda (MD): Society of Urologic Oncology Inc, 2013:214. [Poster #185] [suonet.org/docs/meetings/suo1312/2013-suo-program-book.aspx]
- Higano CS, Crawford ED, Shore ND, Bosnyak Z, Malmberg A, Neijber A, et al. Risk of cardiovascular events with degarelix versus leuprolide after biochemical relapse of prostate cancer: exploratory analysis of a randomized controlled trial. Journal of Clinical Oncology 2015;33(7 Suppl):151. [DOI: 10.1200/jco.2015.33.7_suppl.151] [DOI] [Google Scholar]
Klotz 2008 (CS21) {published data only}
- Boccon-Gibod L, Klotz L, Schroder FH, Andreou C, Persson B, Cantor P, et al. Efficiacy and safety of degarelix versus leuprolide depot (7,5 mg) in a 12-month, randomized, open-label, phase III study in patients with prostate cancer. Annals of Oncology 2008;19(Suppl 7):vii198. [DOI] [PubMed] [Google Scholar]
- Boccon-Gibod L, Klotz L, Schroder H, Andreou C, Persson BE, Cantor P, et al. Degarelix compared to leuprolide depot 7.5 mg in a 12-month randomised, open-label, parallel-group phase III study in prostate cancer patients. European Urology Supplements 2008;7(3):205. [Google Scholar]
- Crawford ED, Moul JW, Shore ND, Olesen TK, Persson BE. Prostate-specific antigen and serum alkaline phosphatase levels in prostate cancer patients receiving degarelix or leuprolide. Journal of Urology 2010;183(4):e338. [DOI] [PubMed] [Google Scholar]
- Crawford ED, Tombal B, Keane TT, Boccardo F, Miller K, Shore N, et al. FSH suppression and tumour control in patients with prostate cancer during androgen deprivation with a GnRH agonist or antagonist. Scandinavian Journal of Urology 2018;52(5-6):349-57. [DOI: 10.1080/21681805.2018.1522372] [DOI] [PubMed] [Google Scholar]
- Damber J, Tammela TLJ, Iversen P, Abrahamsson P, Boccon-Gibod L, Olesen TK, et al. The effect of baseline testosterone on the efficacy of degarelix and leuprolide: further insights from a 12-month, comparative, phase III study in prostate cancer patients. Urology 2012;80(1):174-80. [DOI] [PubMed] [Google Scholar]
- Damber JE, Tammela TLJ, Abrahamsson PA, Iversen P, Jensen JK, Olesen TK, et al. Comparing testosterone and PSA for different baseline testosterone concentrations during initiation of degarelix and leuprolide treatment. European Urology Supplements 2009;8(4):130. [Google Scholar]
- Gittelman M, Shore N, Jensen J, Persson B, Olesen T. Degarelix versus leuprolide treatment in patients with advanced prostate cancer (Pca): PSA failures during a randomized, phase III trial (CS21). In: ASCO Genitourinary Cancers Symposium; 2009 Feb 26-28. Orlando: American Society of Clinical Oncology (ASCO), 2009. [Abstr #209]
- Iversen P, Karup C, Meulen E, Tanko LB, Huhtaniemi I. Hot flushes in prostatic cancer patients during androgen-deprivation therapy with monthly dose of degarelix or leuprolide. Prostate Cancer and Prostatic Diseases 2011;14(2):184-90. [DOI] [PubMed] [Google Scholar]
- Iversen P, Karup C, Van Der Meulen EA, Tankó LB, Huhtaniemi I. Hot flushes (HF) during androgen deprivation therapy: direct comparison of monthly degarelix and leuprolide in a phase 3 trial. Annals of Oncology 2010;21(Suppl 8):viii278. [Google Scholar]
- Klotz L, Boccon-Gibod L, Shore ND, Andreou C, Persson BE, Cantor P, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU International 2008;102(11):1531-8. [DOI: 10.1111/j.1464-410X.2008.08183.x.] [DOI] [PubMed] [Google Scholar]
- Klotz L, Smith M, Persson BE, Olesen TK, Wilde A. Cardiovascular safety of degarelix: results from a 12-month, comparative, randomized, open-label, parallel-group phase iii trial in prostate cancer patients. Journal of Urology 2010;183(4):e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D, Nielsen SK, Van Keep M, Andersson F, Greene D. Quality of life improvement in patients treated with degarelix versus leuprorelin for advanced prostate cancer. Journal of Urology 2015;193(3):839-46. [DOI] [PubMed] [Google Scholar]
- Moul JW, Crawford E, Shore N, Olesen T, Jensen J, Persson B. PSA and serum alkaline phosphatase (S-ALP) control in patients with prostate cancer (PCa) receiving degarelix or leuprolide. In: ASCO Genitourinary Cancers Symposium; 2010 March 5-7. Chicago: American Society of Clinical Oncology (ASCO), 2010. [Abstr #111]
- Persson BE, Keane T, Damber JE, Boccardo F, Collette L, Crawford ED. Effect of gonadotrophin-releasing hormone antagonist induced follicle-stimulating hormone suppression on prostate-specific antigen response. In: European Urology 9th European Multidisciplinary Meeting on Urological Cancers. Barcelona (ESP): European Society for Medical Oncology, 2017:e2726.
- Schröder FH, Boccon-Gibod L, Tombal B, Miller K, Jensen JK, Olesen TK, et al. Degarelix versus leuprolide in patients with prostate cancer: effect in metastatic patients as assessed by serum alkaline phosphatase. European Urology Supplements 2009;8(4):130. [Google Scholar]
- Schröder FH, Tombal B, Miller K, Boccon-Gibod L, Shore ND, Crawford ED, et al. Changes in alkaline phosphatase levels in patients with prostate cancer receiving degarelix or leuprolide: results from a 12-month, comparative, phase III study. BJU International 2010;106(2):182-7. [DOI] [PubMed] [Google Scholar]
- Schroeder F, Boccon-Gibod L, Tombal B, Miller K, Olesen TK, Persson BE. Degarelix versus leuprolide in patients with metastatic prostate cancer: assessment of serum alkaline phosphatase over time. European Journal of Cancer 2009;7(2-3 Suppl):411. [Google Scholar]
- Shore ND, Moul JW, Crawford E, Meulen E, Olesen T, Persson B. Prostate-specific antigen (PSA) progression-free survival (PFS): a comparison of degarelix versus leuprolide in patients with prostate cancer. Journal of Clinical Oncology 2011;29(7 Suppl):12. [Google Scholar]
- Smith MR, Klotz L, Persson BE, Olesen TK, Wilde AA. Cardiovascular safety of degarelix: results from a 12-month, comparative, randomized, open label, parallel group phase III trial in patients with prostate cancer. Journal of Urology 2010;184(6):2313-9. [DOI] [PMC free article] [PubMed]
- Sommerauer M, Doehn C. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. Onkologe 2009;15(8):799-801. [DOI] [PubMed] [Google Scholar]
- Tombal B, Miller K, Boccon-Gibod L, Schröder F, Jensen JK, Olesen TK, et al. Degarelix vs. leuprolide treatment in patients with advanced prostate cancer: PSA failures during a randomised, phase III trial (CS21). European Urology Supplements 2009;8(4):130. [Google Scholar]
- Tombal B, Miller K, Boccon-Gibod L, Schröder F, Olesen TK, Persson BE. Degarelix versus leuprolide in prostate cancer patients: new prostate-specific antigen data from a phase III trial (CS21). European Journal of Cancer 2009;7(2-3 Suppl):411. [Google Scholar]
- Tombal B, Miller K, Boccon-Gibod L, Schröder F, Shore N, Crawford ED, et al. Additional analysis of the secondary end point of biochemical recurrence rate in a phase 3 trial (CS21) comparing degarelix 80 mg versus leuprolide in prostate cancer patients segmented by baseline characteristics. European Urology 2010;57(5):836-42. [DOI] [PubMed] [Google Scholar]
Margel 2019 (0102‐15‐RMC) {published data only}
- Margel D, Peer A, Ber Y, Shavit-Grievink L, Tabachnik T, Sela S, et al. Cardiovascular morbidity in a randomized trial comparing GnRH agonist and GnRH antagonist among patients with advanced prostate cancer and preexisting cardiovascular disease. Journal of Urology 2019;202(6):1199-208. [DOI] [PubMed] [Google Scholar]
Mason 2013 (CS30) {published data only}
- Mason M, Maldonado Pijoan X, Steidle C, Guerif S, Wiegel T, Meulen E, et al. Neoadjuvant androgen deprivation therapy for prostate volume reduction, lower urinary tract symptom relief and quality of life improvement in men with intermediate- to high-risk prostate cancer: a randomised non-inferiority trial of degarelix versus goserelin plus bicalutamide. Clinical Oncology (Royal College of Radiologists) 2013;25(3):190-6. [DOI: 10.1016/j.clon.2012.09.010] [DOI] [PubMed] [Google Scholar]
- Mason M, Steidle CP, Deliveliotis C, Guerif S, Maldonado X, Khoo V, et al. Degarelix as neoadjuvant hormone therapy in patients with prostate cancer: results from a phase IIIb randomized, comparative trial versus goserelin plus bicalutamide. Journal of Clinical Oncology 2012;30(15):e15199. [Google Scholar]
Ozono 2018 (3550‐CL‐0010) {published data only}
- Ozono S, Tsukamoto T, Naito S, Horie S, Ohashi Y, Uemura H, et al. Efficacy and safety of 3-month dosing regimen of degarelix in Japanese subjects with prostate cancer: a phase III study. Cancer Science 2018;109(6):1920-9. [DOI: 10.1111/cas.13600] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozono S, Ueda T, Hoshi S, Yamaguchi A, Maeda H, Fukuyama Y, et al. The efficacy and safety of degarelix, a GnRH receptor antagonist: a multicenter, randomized, maintenance dose-finding phase II study with Japanese prostate cancer patients. Journal of Clinical Oncology 2011;29(7 Suppl):154. [DOI] [PubMed] [Google Scholar]
Sawazaki 2019 {published data only}
- Sawazaki H, Araki D, Kitamura Y, Yagi K. Metabolic changes with degarelix vs leuprolide plus bicalutamide in patients with prostate cancer: a randomized clinical study. World Journal of Urology 2020;38(6):1465-71. [DOI] [PubMed] [Google Scholar]
- Sawazaki H, Araki D. Influence of different modalities of androgen deprivation therapy on lipid and glucose metabolism and fat accumulation in patients with prostate cancer: degarelix vs leuprolide plus bicalutamide. Journal of Urology 2019;201(Suppl 4):e497. [DOI: 10.1097/01.JU.0000555937.43866.d7] [DOI] [Google Scholar]
Sayyid 2017 (DEG_PRE‐OP) {published data only}
- Sayyid R, Evans A, Hersey K, Maloni R, Hurtado-Coll A, Kulkarni G, et al. A phase II, randomized, open-label study of neoadjuvant degarelix versus LHRH agonist in prostate cancer patients prior to radical prostatectomy. Clinical Cancer Research 2017;23(8):1974-80. [DOI: 10.1158/1078-0432.CCR-16-1790] [DOI] [PubMed] [Google Scholar]
- Sayyid R, Gleave M, Hersey K, Maloni R, Hurtado-Coll A, Evans A, et al. A phase II, randomized, open label study of neoadjuvant degarelix versus LHRH agonist in prostate cancer patients prior to radical prostatectomy. Journal of Urology 2016;195(4):e856, PD37-04. [DOI] [PubMed] [Google Scholar]
Shore 2012 (CS35) {published data only}
- Shore N, Crawford ED, Gittelman M, Tombal B, Tammela T, Wolff J, et al. Efficacy and safety of a 3-monthly depot formulation of degarelix compared with goserelin in prostate cancer. In: 13th Annual Meeting of the Society of Urologic Oncology; 2012 Nov 28-30; Bethesda (MD). Bethesda (MD): Society of Urologic Oncology Inc, 2012:116. [suonet.org/docs/meetings/suo1211/2012_suo_program_book.aspx]
- Tombal B, Tammela TLJ, Wolff JM, Payne H, Crawford ED, Shore N, et al. Efficacy and safety of a 3-monthly depot formulation of degarelix compared with goserelin in prostate cancer. European Urology Supplements 2012;11(5):228. [1569-9056 (ISSN)] [Google Scholar]
Xie 2016 (PANDA) {published data only}
- Sun Y, Xie L, Xu T, Jakobsen JS, Han W, Sorensen PS, et al. Efficacy and safety of degarelix in patients with prostate cancer: results from a phase III study in China. Asian Journal of Urology 2020;7:301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Bosnyák Z, Sun Y, Malmberg A, Neijber A, Fen WX. Degarelix is well tolerated and effective for the treatment of prostate cancer: results from a phase III study in China. European Urology Supplements 2016;15(3):e848. [Google Scholar]
References to studies excluded from this review
Abrahamsson 2014 {published data only}
- Abrahamsson PA, Albers P, Morote Robles J, Malmberg A, Neijber AN, Boccon-Gibod L. Disease characteristics influencing the duration of the off-treatment period during intermittent androgen deprivation therapy with degarelix in prostate cancer. European Urology Supplements 2014;13(5):141. [Google Scholar]
Abrahamsson 2015 {published data only}
- Abrahamsson PA, Boccon-Gibod L, De Jong IJ, Morote J, Malmberg A, Neijber A, et al. Factors predicting the off-treatment duration during intermittent androgen deprivation (IAD) therapy with degarelix in prostate cancer. European Urology Supplements 2015;14(2):e565-e565a. [DOI] [PubMed] [Google Scholar]
Albertsen 2013a {published data only}
- Albertsen P, Tombal B, Wiegel T, Van Der Meulen E, Persson BE, Olesen TK, et al. Androgen deprivation therapy by a gonadotropin releasing hormone antagonist, degarelix, lowers the risk of cardiovascular events or death when compared to luteinising hormone-releasing agonists. Journal of Urology 2013;189(4):e322. [Google Scholar]
Albertsen 2013b {published data only}
- Albertsen PC, Nilsson J, Klotz L, Payne H, Van Der Meulen E, Persson BE, et al. Comparision of the risk of cardiovascular events and death in patients treated with degarelix compared with LHRH agonists. Journal of Clinical Oncology 2013;31(6 Suppl):42. [Google Scholar]
Albertsen 2014 {published data only}
- Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J. Cardiovascular morbidity associated with gonadotropin releasing hormone agonists and an antagonist. European Urology 2014;65(3):565-73. [DOI] [PubMed] [Google Scholar]
Ammannagari 2016 {published data only}
- Ammannagari N, Javvaji C, Danchaivijitr P, George S. Dramatic PSA increase with tumor shrinkage after initiating degarelix in advanced prostate cancer. Clinical Genitourinary Cancer 2016;14(1):e123-5. [DOI] [PubMed] [Google Scholar]
Augustovski 2006 {published data only}
- Augustovski F, Colantonio L, Pichon Riviere A. Androgen deprivation treatment (hormonal therapy) for the management of prostate cancer. In: Informe Tecnico Breve. Vol. No. 30. Buenos Aire: Institute for Clinical Effectiveness and Health Policy (IECS), 2006. [CRD 32006001093]
Aust Prescr 2010 {published data only}
- No authors listed. Degarelix. Australian Prescriber 2010;33(6):193-198. [Google Scholar]
AWMSG 2009 {published data only}
- All Wales Medicines Strategy Group (AWMSG). Degarelix (Firmagon®) for the treatment of advanced hormone-dependent prostate cancer. In: AWMSG Secretariat Assessment Report Advice. Vol. 2109. Penarth: All Wales Therapeutics and Toxicology Centre (AWTTC), secretariat of the All Wales Medicines Strategy Group (AWMSG), 2009. [Google Scholar]
AWMSG 2012 {published data only}
- All Wales Medicines Strategy Group (AWMSG). Degarelix (Firmagon®) 80 mg and 120 mg subcutaneous injection (Structured abstract). In: AWMSG Secretariat Assessment Report Advice. Vol. Record ID 32013000039. Penarth: All Wales Therapeutics and Toxicology Centre, Academic Centre, University Hospital Llandough, 2012. [Google Scholar]
Borre 2015 {published data only}
- Borre M, Keane T, Bosnyak Z, Malmberg A, Neijber A. Regional differences in cardiovascular status and events in prostate cancer patients treated with a luteinising hormone-releasing hormone agonist vs antagonist: results of a pooled analysis. Journal of Urology 2015;193(4):e929-30. [Google Scholar]
Borsellino 2014 {published data only}
- Borsellino N, Lo Mauro M, Nicastro R, Macaluso S, Vitale ME, Di Trapani D, et al. Pattern of progression during castration with GnRH agonists GnRH (A) and rescue with GnRH antagonist degarelix (D) in patients (PTS) with metastatic prostate cancer (MPC). Journal of Clinical Oncology 2014;32(15):e16072. [Google Scholar]
Chan 2014 {published data only}
- Chan CK, Chan MTY, Ma WK, Chu PSK, Cheung FK, Man CW. Preliminary results on the prospective serum prostate specific antigen (PSA) and testosterone monitoring in patients requiring hormonal treatment by Degarelix (Firmagon ®) injection or surgical castration for prostate cancer. BJU International 2014;113:14. [Google Scholar]
ChoungSoo 2012 {published data only}
- ChoungSoo K, Sang E, Byung H, Sung J, Ingab J, David F, et al. An open-label, multi-centre bridging trial of the efficacy and safety of degarelix one-month-dosing regimen in Korean patients with prostate cancer requiring androgen ablation therapy. International Journal of Urology 2012;19:153. [Google Scholar]
Crawford 2013 {published data only}
- Crawford ED, Shore N, Miller K, Tombal B, Kathrup C, Van Der Meulen E, et al. Degarelix versus LHRH agonists: differential skeletal and urinary tract outcomes from an analysis of six comparative randomized clinical trials. Journal of Clinical Oncology 2013;31(6):68. [Google Scholar]
Crehange 2015 {published data only}
- Crehange G, Cochet A, Cueff A, Martin E, Maingon P, Brunotte F, et al. Early radiation response between GnRH agonist versus antagonist combined with radiotherapy in high-risk prostate cancer. Journal of Clinical Oncology 2015;33(7 Suppl 1).
Damber 2012a {published data only}
- Damber JE. The effect of baseline testosterone on the efficacy of degarelix and leuprolide: further insights from a 12-month, comparative, phase III study in prostate cancer patients. Urology 2012;80(1):181. [DOI] [PubMed] [Google Scholar]
Dearnaley 2016 {published data only}
- Dearnaley D, Saltzstein DR, Sylvester JE, Karsh L, Mehlhaff BA, Pieczonka C, et al. Neo/adjuvant ADT to EBRT: randomized phase 2 trial of the oral GnRH antagonist, TAK-385 (relugolix, RGX) and degarelix (DGX) in patients (pts) with prostate cancer (PC). Annals of Oncology 2016;27(Suppl 6):vi243-65. [Google Scholar]
Degarelix Study Grp. 2005 {published data only}
- Degarelix Study Group. Degarelix—a phase II multicentre, randomized dose-escalating study testing a novel GnRH receptor blocker in prostate cancer patients. European Urology Supplements 2005;4(3):228. [Google Scholar]
Guerif 2017 {published data only}
- Guerif SG, Latorzeff I, Roca L, Allouache D, Supiot S, Lagneau E, et al. The acute toxicity results of the GETUG-AFU 22 study: a multicenter randomized phase II trial comparing the efficacy of a short hormone therapy in combination with radiotherapy to radiotherapy alone as a salvage treatment for patients with detectable PSA after radical prostatectomy. Journal of Clinical Oncology 2017;35(6 Suppl):16. [Google Scholar]
Iversen 2013 {published data only}
- Iversen P, Damber JE, Malmberg A, Persson BE, Klotz L. Improved outcomes with degarelix monotherapy compared with luteinizing hormone-releasing hormone (LHRH) agonists plus antiandrogen flare protection in the treatment of men with advanced prostate cancer. Scandinavian Journal of Urology 2013;47:7. [Google Scholar]
JPRN‐UMIN000013151 {published data only (unpublished sought but not used)}
- Kawashima A, Uemura M, Nagahara A, Yamamoto Y, Takada S, Inagaki Y, et al. GnRH antagonist plus bicalutamide might be an effective therapy as initial combined androgen blockade for patients with high grade prostate cancer. Annals of Oncology 2015;26:ix75. [Google Scholar]
- Uemura M, Nagahara A, Yamamoto Y, Takada S, Inagaki Y, Kinouchi T, et al. GnRH antagonist plus bicalutamide may be an effective therapy as initial combined androgen blockade for patients with high grade prostate cancer. European Journal of Cancer 2015;51:S493. [Google Scholar]
- Uemura M. An analytical study of laboratory tests and clinical effectiveness for combination therapies using GnRH antagonist/antiandrogen and GnRH agonist/antiandrogen in prostate cancer patients. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000015335 (first received 13 February 2014).
JPRN‐UMIN000015519 {published data only (unpublished sought but not used)}
- Miki K, Sasaki H, Kido M, Takahashi H, Aoki M, Egawa S. A comparative study on the efficacies of gonadotropin-releasing hormone (GnRH) agonist and GnRH antagonist in neoadjuvant androgen deprivation therapy combined with transperineal prostate brachytherapy for localized prostate cancer. BMC Cancer 2016;16(1):708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K. A comparative study on the efficacies of gonadotropin-releasing hormone (GnRH) agonist and GnRH antagonist in neoadjuvant androgen deprivation therapy combined with transperineal prostate brachytherapy for localized prostate cancer. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000018030 (first received 24 October 2014). [DOI] [PMC free article] [PubMed]
JPRN‐UMIN000021806 {published data only (unpublished sought but not used)}
- Nishiyama T. Study of the difference in clinical efficacy by the difference between the GnRH agonist and GnRH antagonist when adding short-term androgen deprivation therapy to definitive radiation therapy for localized intermediate-risk prostate cancer. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000025146 (first received 6 April 2016).
Medical Letter 2009 {published data only}
- No authors listed. Degarelix (Firmagon) for prostate cancer. Medical Letter on Drugs and Therapeutics 2009;51(1323):82-3. [PubMed] [Google Scholar]
NCT01786265 {published data only}
- NCT01786265. Finite androgen ablation vs. finite androgen ablation in combination with abiraterone acetate and prednisone. clinicaltrials.gov/show/NCT01786265 (first received 7 February 2013).
NCT02234089 {unpublished data only}
- NCT02234089. Therapy pathways in the treatment of hormone naïve prostate cancer patients with and without comorbidities treated with degarelix or luteinizing-hormone-releasing-hormone (LHRH) agonists (ProComD). clinicaltrials.gov/ct2/show/NCT02234089?term=NCT02234089&draw=2 (first received 9 September 2014).
NCT02278185 {published data only}
- NCT02278185. Enzalutamide versus standard androgen deprivation therapy for the treatment hormone sensitive prostate cancer. clinicaltrials.gov/ct2/show/NCT02278185 (first received 29 October 2014).
Nosov 2016 {published data only}
- Nosov A, Reva S, Protsenko S, Buevich N, Veliev E, Petrov S. Safety and efficacy of neoadjuvant chemohormonal and hormonal treatment followed by radical prostatectomy for patients with high- and very high risk prostate cancer: initial results of prospective, randomized, phase III clinical trial. European Urology Supplements 2016;15(5):e1193. [Google Scholar]
Nozawa 2015 {published data only}
- Nozawa M, Sugiyama T, Sugimoto K, Nagai T, Hatanaka Y, Oki T, et al. Phase II study of degarelix/bicalutamide combination for prostate cancer. Journal of Clinical Oncology 2015;33(15):e16005. [Google Scholar]
Nozawa 2016 {published data only}
- Nozawa M, Sugimoto K, Nagai Y, Sugiyama T, Hatanaka Y, Oki T, et al. Degarelix/bicalutamide combination versus degarelix alone as initial androgen-deprivation therapy. Journal of Clinical Oncology 2016;34(2):183. [Google Scholar]
Prescrire Int 2010 {published data only}
- No authors listed. Degarelix: more rapid medical castration, nothing more. Prescrire International 2010;19(107):106-8. [PubMed] [Google Scholar]
Shore 2010 (CS21A) {published data only (unpublished sought but not used)}
- Crawford ED, Moul JW, Shore N, Van Der Meulen E, Persson BE. Time to progression in patients with prostate cancer: a comparison of continuous degarelix versus degarelix following leuprolide treatment. Journal of Urology 2011;185(4):e289-90. [Google Scholar]
- Crawford ED, Moul JW, Shore ND, Olesen TK, Persson BE. Switching from leuprolide to degarelix vs continuous degarelix treatment—effects on long-term prostate-specific antigen control. Journal of Urology 2010;183(4):e262. [Google Scholar]
- Crawford ED, Shore ND, Moul JW, Tombal B, Schröder FH, Miller K, et al. Long-term tolerability and efficacy of degarelix: 5-year results from a phase III extension trial with a 1-arm crossover from leuprolide to degarelix. Urology 2014;83(5):1122-8. [DOI] [PubMed]
- Crawford ED, Tombal B, Miller K, Boccon-Gibod L, Schroder F, Shore N, et al. A phase III extension trial with a 1-arm crossover from leuprolide to degarelix: comparison of gonadotropin-releasing hormone agonist and antagonist effect on prostate cancer. Journal of Urology 2011;186(3):889-97. [DOI] [PubMed] [Google Scholar]
- EUCTR2006-006913-34-HU. An open-label, multi-centre, extension study, evaluating the long-term safety and tolerability of degarelix one-month dosing regimen in patients with prostate cancer requiring androgen ablation therapy. www.clinicaltrialsregister.eu/ctr-search/search?query=EUCTR2006-006913-34-HU (first received 23 March 2007).
- NCT00451958. A long-term extension study evaluating a one-month dosing regimen of degarelix in prostate cancer requiring androgen ablation therapy. clinicaltrials.gov/show/NCT00451958 (first received 26 March 2007).
- Persson B, Olesen TK, Jensen J, Mason M. Effects of switching from leuprolide to degarelix on long-term prostate-specific antigen (PSA) and serum alkaline phosphatase (S-ALP) control. Annals of Oncology 2010;21:viii280. [Google Scholar]
- Plekhanov A, Crawford ED, Olesen TK, Van Der Meulen EA, Persson BE. Switching from leuprolide to degarelix vs continuous degarelix treatment—effects on long-term PSA control. European Urology Supplements 2010;9(6):538. [Google Scholar]
- Shore ND, Moul JW, Crawford E, Olesen T, Persson B. Long-term prostate-specific antigen (PSA) control in prostate cancer (PCa) patients switched from leuprolide to degarelix or receiving continuous degarelix treatment. Journal of Clinical Oncology 2010;28(15):4673. [Google Scholar]
- Shore ND, Moul JW, Crawford E, Van Der Meulen E, Olesen T, Persson B. Prostate-specific antigen (PSA) progression-free survival (PFS): a comparison of degarelix versus leuprolide in patients with prostate cancer. Journal of Clinical Oncology 2011;29(7):12. [Google Scholar]
- Tombal B, Schröder F, Miller K, Van Der Meulen E, Persson BE. Long term prostate-specific antigen (PSA) control in prostate cancer: continuous degarelix or degarelix following leuprolide. European Urology Supplements 2011;10(2):335. [Google Scholar]
Sokolakis 2014 {published data only}
- Sokolakis I, Zarkadoulias A, Kazanas K, Georgopoulos P, Xouplidis K, Hatzimouratidis K. Comparing the frequency and severity of hot flashes in prostate cancer patients under androgen deprivation therapy with an LHRH agonist or antagonist. European Urology Supplements 2014;13(7):e1567. [Google Scholar]
Touijer 2014 {published data only}
- Touijer KA, Chen Y, Carver BS, Coleman JA, Laudone VP, Hullings M, et al. Establishing a neoadjuvant platform for developing targeted agents: degarelix prior to prostatectomy for patients with intermediate- and high-risk prostate cancer. Journal of Clinical Oncology 2014;32(15 Suppl):TPS5105. [Google Scholar]
Van Poppel 2006 {published data only}
- Van Poppel H. A one-year, multicentre, randomised study of degarelix, a gonadatrophin-releasing hormone (GnRH) receptor blocker, in prostate cancer patients. European Urology Supplements 2006;5(2):251. [Google Scholar]
Van Poppel 2007 {published data only}
- Van Poppel H, De La Rosette JJ, Persson BE, Olesen TK, Degarelix Study Group. Long-term evaluation of degarelix, a gonadotrophin-releasing hormone (GNRH) receptor blocker, investigated in a multicentre randomised study in prostate cancer (CAP) patients. European Urology Supplements 2007;6(2):28. [Google Scholar]
Weston 2005 {published data only}
- Weston P, Persson BE. Hormone therapy in prostate cancer: LHRH antagonists versus LHRH analogues. European Urology 2005;47(3):422. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
000108 (PRONOUNCE) {published data only}
- Margel D, Pe'er A, Ber Y, Shaparberg M, Sela S, Duivenvoorde W, et al. Cardiovascular events biomarkers in a randomized trial comparing LHRH agonist and antagonist among patients with advanced prostate cancer. Journal of Urology 2018;199(4S):e702. [Google Scholar]
- Margel D, Peer A, Ber Y, Shaparberg M, Sela S, Ozalvo R, et al. Early cardiovascular morbidity in a pilot prospective randomized trial comparing LHRH agonist and antagonist among patients with advanced prostate cancer. Journal of Urology 2017;197(4S):e768. [DOI: 10.1016/j.juro.2017.02.1787] [DOI] [Google Scholar]
- Scher H, Roe M. A trial comparing cardiovascular safety of degarelix versus leuprolide in patients with advanced prostate cancer and cardiovascular disease (PRONOUNCE) [A multi-center, randomized, assessor-blind, controlled trial comparing the occurrence of major adverse cardiovascular events (MACEs) in patients with prostate cancer and cardiovascular disease receiving degarelix (gonadotropin-releasing hormone (GnRH) receptor antagonist) or leuprolide (GnRH receptor agonist)]. clinicaltrials.gov/show/NCT02663908 (first received 26 January 2016).
- Slovin SF, Melloni C, Mansor-Lefebvre S, Neijber A, Roe M. A multicenter, randomized, controlled trial comparing the occurrence of major adverse cardiovascular events (MACEs) in patients (PTS) with prostate cancer (pc) and cardiovascular disease (CVD) receiving degarelix (GnRH receptor antagonist) or leuprolide (GnRH receptor agonist). Journal of Clinical Oncology 2018;36(6 Suppl 1):TPS395. [DOI: 10.1200/JCO.2018.36.6_suppl.TPS395] [DOI] [Google Scholar]
JPRN‐UMIN000014243 {published data only}
- UMIN000014243. Randomized controlled study of GnRH antagonist monotherapy and CAB with GnRH agonist plus bicalutamide for patients with metastatic prostate cancer. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000016585 (first received 12 June 2014). [KYUCOG-1401]
NCT01542021 {unpublished data only}
- NCT01542021. Establishing a neo-adjuvant platform for developing targeted agents: androgen deprivation therapy prior to prostatectomy for patients with intermediate and high risk prostate cancer. clinicaltrials.gov/ct2/show/NCT01542021?term=nct01542021&draw=2&rank=1 (first received 1 March 2012). [11-182]
NCT02799706 {published data only}
- NCT02799706. Trial comparing irradiation plus long term adjuvant androgen deprivation with GnRH antagonist versus GnRH agonist plus flare protection in patients with very high risk localized or locally advanced prostate cancer (PEGASUS) [Phase IIIb randomized trial comparing irradiation plus long term adjuvant androgen deprivation with GnRH antagonist versus GnRH agonist plus flare protection in patients with very high risk localized or locally advanced prostate cancer. A joint study of the EORTC ROG and GUCG]. clinicaltrials.gov/show/NCT02799706 (first received 15 June 2016). [EORTC-1414 (PEGASUS)]
NCT04182594 {unpublished data only}
- NCT04182594. A phase-II, randomized, assessor-blind, controlled trial comparing the occurrence of cardiovascular events in patients with prostate cancer and cardiovascular risk factors receiving degarelix or GnRH agonist. clinicaltrials.gov/ct2/show/NCT04182594?term=NCT04182594&draw=2&rank=1 (first received 2 December 2019).
Additional references
Abufaraj 2020
- Abufaraj M, Iwata T, Kimura S, Haddad A, Al-Ani H, Abusubaih L, et al. Differential impact of gonadotropin-releasing hormone antagonist versus agonist on clinical safety and oncologic outcomes on patients with metastatic prostate cancer: a meta-analysis of randomized controlled trials. European Urology 2021;79(1):44-53. [DOI: 10.1016/j.eururo.2020.06.002] [DOI] [PubMed] [Google Scholar]
AUA 2020
- Lowrance W, Breau R, Chou R, Chapin BF, Crispino T, Dreicer R, et al. Advanced prostate cancer: AUA/ASTRO/SUO guideline. www.auanet.org/guidelines/advanced-prostate-cancer#x14936 (accessed 2 October 2020).
Broqua 2002
- Broqua P, Riviere PJ, Conn PM, Rivier JE, Aubert ML, Junien JL. Pharmacological profile of a new, potent, and long-acting gonadotropin-releasing hormone antagonist: degarelix. Journal of Pharmacology and Experimental Therapeutics 2002;301(1):95-102. [DOI] [PubMed] [Google Scholar]
Cardwell 2020
- Cardwell CR, O'Sullivan JM, Jain S, Harbinson MT, Cook MB, Hicks BM, et al. The risk of cardiovascular disease in prostate cancer patients receiving androgen deprivation therapies. Epidemiology 2020;31(3):432-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cui 2014
- Cui Y, Zong H, Yan H, Li N, Zhang Y. Degarelix versus goserelin plus bicalutamide therapy for lower urinary tract symptom relief, prostate volume reduction and quality of life improvement in men with prostate cancer: a systematic review and meta-analysis. Urologia Internationalis 2014;93(2):152-9. [DOI] [PubMed] [Google Scholar]
Damber 2012b
- Damber JE, Tammela TL, Iversen P, Abrahamsson PA, Boccon-Gibod L, Olesen TK, et al. The effect of baseline testosterone on the efficacy of degarelix and leuprolide: further insights from a 12-month, comparative, phase III study in prostate cancer patients. Urology 2012;80(1):174-80. [DOI] [PubMed] [Google Scholar]
Davey 2020
- Davey P, Kirby MG. Cardiovascular risk profiles of GnRH agonists and antagonists: real-world analysis from UK general practice. World Journal of Urology 2021;39(2):307-315. [DOI: 10.1007/s00345-020-03433-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
EAU 2020
- Mottet N, Cornford P, den Bergh RCN, Briers E, De Santis M, Fanti S, et al. EAU – ESTRO – ESUR – SIOG Guidelines on Prostate Cancer. uroweb.org/guideline/prostate-cancer/ (accessed 2 October 2020).
EndNote 2019 [Computer program]
- EndNote. Philadelphia: Clarivate Analytics, 2019. Available at www.endnote.com.
George 2020
- George G, Garmo H, Scailteux LM, Balusson F, De Coster G, De Schutter H, et al. Risk of cardiovascular disease following gonadotropin-releasing hormone agonists vs antagonists in prostate cancer: real-world evidence from five databases. International Journal of Cancer 2021;148(9):2203-2211. [DOI: 10.1002/ijc.33397] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gibbs 1996
- Gibbs SJ, Plowman PN. Androgen deprivation and antagonism in the treatment of advanced prostatic carcinoma. Clinical Oncology 1996;8(6):346-52. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2018
- World Health Organization, International Agency for Research on Cancer, Global Cancer Observatory. GLOBOCAN 2018: Prostate. globocan.iarc.fr (accessed 5 October 2020).
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime) GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), (accessed 5 October 2020). Available at gradepro.org.
Guyatt 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Schünemann HJ, et al. GRADE: what is "quality of evidence" and why is it important to clinicians? BMJ (Clinical Research Ed.) 2008;336(7651):995-8. [DOI: 10.1136/bmj.39490.551019.BE] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [DOI: 10.1016/j.jclinepi.2010.04.026] [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine 2002;21(11):1539-58. [DOI: 10.1002/sim.1186] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557-60. [DOI: 10.1136/bmj.327.7414.557] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019. [Google Scholar]
Higgins 2011b
- Higgins JPT, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:569-594. [Google Scholar]
Higgins 2017
- Higgins JPT, Savovic J, Page MJ, Elbers RG, Sterne JAC. Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:205-228. [Google Scholar]
Hosseini 2016
- Hosseini SA, Rajabi F, Akbari Sari A, Ayati M, Heidari S, Ghamary F. Degarelix for the treatment of advanced prostate cancer compared with GnRh-agonists: a systematic review and meta-analysis. Medical Journal of the Islamic Republic of Iran 2016;30:317;eCollection. [PMC free article] [PubMed] [Google Scholar]
Huggins 2002
- Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. Journal of Urology 2002;167(2 Pt 2):948-51. [PubMed] [Google Scholar]
Klotz 2014
- Klotz L, Miller K, Crawford ED, Shore N, Tombal B, Karup C, et al. Disease control outcomes from analysis of pooled individual patient data from five comparative randomised clinical trials of degarelix versus luteinising hormone-releasing hormone agonists. European Urology 2014;66(6):1101-8. [DOI] [PubMed] [Google Scholar]
Kunath 2014
- Kunath F, Grobe HR, Rücker G, Motschall E, Antes G, Dahm P, et al. Non-steroidal antiandrogen monotherapy compared with luteinising hormone–releasing hormone agonists or surgical castration monotherapy for advanced prostate cancer. Cochrane Database of Systematic Reviews 2014, Issue 6. Art. No: CD009266. [DOI: 10.1002/14651858.CD009266.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunath 2015
- Kunath F, Borgmann H, Blümle A, Keck B, Wullich B, Schmucker C, et al. Gonadotropin-releasing hormone antagonists versus standard androgen suppression therapy for advanced prostate cancer A systematic review with meta-analysis. BMJ Open 2015;5(11):e008217. [DOI: 10.1136/bmjopen-2015-008217] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1371/journal.pmed.1000100] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perrone 2020
- Perrone V, Esposti LD, Giacomini E, Veronesi C, Blini V, Oderda M. Cardiovascular risk profile in prostate cancer patients treated with GnRH agonists versus antagonists: an Italian real-world analysis. Thrapeutics and Clinical Risk Management 2020;16:393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Schünemann 2019a
- Schünemann HJ, Higgins JPT, Vist GE, Glasziou P, Akl EA, Skoetz N, Guyatt GH. Chapter 14: Completing ‘Summary of findings' tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:375-402. [Google Scholar]
Schünemann 2019b
- Schünemann HJ, Vist GE, Higgins JPT, Santesso N, Deeks JJ, Glasziou P, et al. Chapter 15: Interpreting results and drawing conclusions. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors(s). Cochrane Handbook for Systematic Reviews of Interventions. 2nd edition. Chichester (UK): John Wiley & Sons, 2019:403-432. [Google Scholar]
Sciarra 2016
- Sciarra A, Fasulo A, Ciardi A, Petrangeli E, Gentilucci A, Maggi M, et al. A meta-analysis and systematic review of randomized controlled trials with degarelix versus gonadotropin-releasing hormone agonists for advanced prostate cancer (Erratum in: Medicine (Baltimore). 2016 Dec 09;95(49):e5671). Medicine (Baltimore) 2016;95(27):e3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
TNM 2005
- Wittekind CF, Klimpfinger M, Sobin L. TNM-Atlas. Vol. 5. Auflage. Berlin, Heidelberg: Springer-Verlag, 2005. [Google Scholar]
Uttley 2017
- Uttley L, Whyte S, Gomersall T, Ren S, Wong R, Chambers D, et al. Degarelix for treating advanced hormone-dependent prostate cancer: an evidence review group perspective of a NICE Single Technology Appraisal. PharmacoEconomics 2017;35:717-26. [DOI: 10.1007/s40273-016-0481-1] [DOI] [PubMed] [Google Scholar]


